Abstract
Purpose
Telerehabilitation systems have the potential to enable therapists to monitor and assist stroke patients in achieving high-intensity upper extremity exercise in the home environment. We adopted an iterative user-centred approach, including multiple data sources and meetings with end-users and stakeholders to define the user requirements for home-based upper extremity rehabilitation using wearable motion sensors for subacute stroke patients.
Methods
We performed a requirement analysis consisting of the following steps: 1) context & groundwork; 2) eliciting requirements; 3) modelling & analysis; 4) agreeing requirements. During these steps, a pragmatic literature search, interviews and focus groups with stroke patients, physiotherapists and occupational therapists were performed. The results were systematically analysed and prioritised into “must-haves”, “should-haves”, and “could-haves”.
Results
We formulated 33 functional requirements: eighteen must-have requirements related to blended care (2), exercise principles (7), exercise delivery (3), exercise evaluation (4), and usability (2); ten should-haves; and five could-haves. Six movement components, including twelve exercises and five combination exercises, are required. For each exercise, appropriate exercise measures were defined.
Conclusion
This study provides an overview of functional requirements, required exercises, and required exercise measures for home-based upper extremity rehabilitation using wearable motion sensors for stroke patients, which can be used to develop home-based upper extremity rehabilitation interventions. Moreover, the comprehensive and systematic requirement analysis used in this study can be applied by other researchers and developers when extracting requirements for designing a system or intervention in a medical context.
Keywords: Stroke, home-based, rehabilitation, upper extremity, requirements, wearables
IMPLICATIONS FOR REHABILITATION
This study provides an extensive overview of user requirements for home-based upper extremity rehabilitation using wearable motion sensors in stroke patients.
These requirements can be used as a basis for developing home-based UE telerehabilitation interventions.
Including these requirements may facilitate the clinical implementation of such telerehabilitation systems.
The comprehensive and systematic approach used in this sudy can be applied by other researchers and developers when extracting requirements for designing a system or intervention in a medical context.
Introduction
Stroke is the leading cause of adult disability worldwide, and the incidence is still rising [1]. Motor impairment is one of the most common symptoms, initially present in about 75% of stroke patients [2]. Upper extremity (UE) motor impairments restrict patients’ capacity and performance of daily life activities. Rehabilitation can improve UE capacity and regain independence in daily life activities [3], ideally starting within the first five weeks post-stroke to maximally utilise the potential of recovery of UE capacity [4]. Key elements of motor rehabilitation include a high number of repetitions, task-specific exercises, and context specificity [5].
Despite its efficacy, applying high-repetitive UE rehabilitation interventions in clinical practice remains problematic [6,7]. Currently, the intensity of task-related exercise is often low [8–10]. In clinical rehabilitation, the availability of therapists to provide intensive supervised therapy is limited [11]. Moreover, cognitive and motivational issues limit patients in performing exercises independently outside therapy sessions [12,13]. Literature showed that when patients were discharged home, the main reported barriers to exercise were related to fatigue and the availability and distance from the exercise places. Additional reported barriers were the absence of a person to assist with the exercises and a lack of knowledge on how to perform exercises [14]. The limited amount of exercise may induce suboptimal recovery of UE function [15] and may lead to deterioration of UE capacity after being discharged home from rehabilitation [16].
Telerehabilitation for stroke patients is evolving rapidly [17–19], and there is growing evidence of its efficacy and non-inferiority in improving UE capacity compared to usual care [17–20]. Telerehabilitation is an alternative method of providing conventional rehabilitation (diagnostics, treatment and monitoring) at a remote location, using information and communication technology [18]. Telerehabilitation includes technologies such as phone and video consultations and virtual reality, as well wearable motion sensors are increasingly being used [18,21]. Wearable motion sensors can consist of any type (e.g., accelerometers, gyroscopes and microelectromechanical systems) of sensors integrated into wearable objects or directly worn on the body to measure human movement [22]. Compared to usual care and other telerehabilitation technologies, wearable motion sensors have advantages, such as being relatively inexpensive and unobtrusive and allow real-time movement tracking and feedback in real-world settings [21]. With that, it enables therapists to monitor progress, personalise treatment, and assist patients in achieving high intensities of exercise therapy in the home environment without direct supervision. Therefore, wearable technologies are promising to overcome barriers experienced with current intensive therapy programs and may reduce healthcare costs [13,19].
Although telerehabilitation is compelling and shows advantages compared to conventional rehabilitation, patients and therapists experience barriers in using telerehabilitation in daily practice [19,23,24]. Common barriers are the unease of use, reliance on someone else’s help, exercises not being tailored to the preferred skills and level of abilities, lack of flexibility of schedule and location, and a lack of interaction with therapists [25–28]. To improve the adoption of new technology, end-user involvement is essential throughout the development and implementation process [23,24,27]. Nowadays, end-users are often involved in some way in the development of telerehabilitation. Still, the same barriers to using technology in clinical practice are present [29], and there is no evidence for an optimal single step-by-step approach to involve end-users in developing telerehabilitation systems [30]. Instead, researchers need to select the most suitable research methods for their research objectives, the research context, and the characteristics of the participants. Ideally, multiple data sources should be combined, such as scientific literature and qualitative data collected from various stakeholders [30].
Current literature generally provides user requirements for stroke telerehabilitation, regardless of the specific user context and characteristics [31–33]. There are no particular recommendations for home-based UE stroke rehabilitation using wearable motion sensors. This study aims to define the user requirements for home-based UE rehabilitation using wearable motion sensors for sub-acute stroke patients. To overcome previously reported limitations of current telerehabilitation systems, we follow an iterative user-centred approach using multiple data sources, including a pragmatic literature search, interviews and focus groups with end-users, and meetings with various stakeholders.
Materials and methods
We conducted a requirement analysis described by Nuseibeh & Easterbrook [34] to define user requirements of a home-based UE rehabilitation system for sub-acute stroke patients (seven days to six months post-stroke [35]) using wearable motion sensors. We distinguished three categories of user requirements: 1) functional requirements, 2) required exercises, and 3) required exercise measures. The functional requirements define the functions of a system, which are specifications of the system’s behaviour. The required exercises represent the exercises that must be included in the home-based UE rehabilitation system. The required exercise measures represent the constructs that need to be evaluated and given feedback on when patients perform the exercises without direct supervision in the home environment.
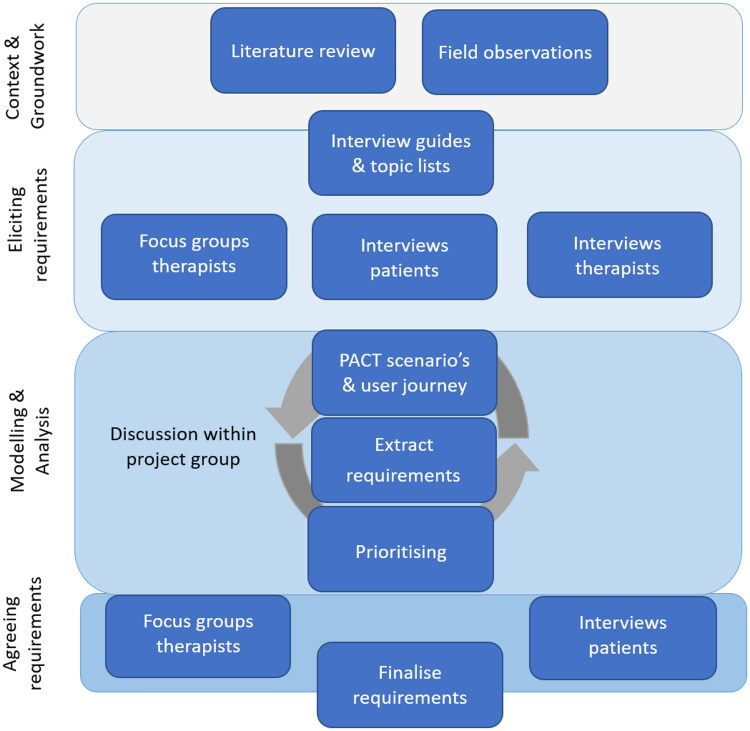
The requirement analysis included the following steps [34]: 1) Context and groundwork: to familiarise with the topic and provide a base for eliciting requirements. 2) Eliciting requirements: communicating with users to determine their requirements. 3) Modelling and analysing requirements: document the elicited information in an understandable and organised way (e.g., user scenarios and user journeys) to formulate precise, complete, unambiguous requirements and prioritise those requirements. 4) Agreeing requirements: verify that the requirements accurately present users’ needs. See Figure 1 for an overview of the methods.
Figure 1.
Overview of the requirement analysis. *PACT: People Activity Context Technology.
Participant recruitment
The users from whom we elicited and verified the system requirements were stroke patients, occupational therapists, and physiotherapists. We have used purposive criterion sampling to recruit these users.
In four rehabilitation centre stroke units and one primary physiotherapy practice in the Netherlands, we recruited occupational therapists and physiotherapists with more than three years of experience with UE stroke rehabilitation. Therapists did not need to have an affinity for using technology in rehabilitation. To verify requirements, we applied the same criteria.
We also included adult patients (> 18 years) with an ischemic or haemorrhagic unilateral stroke resulting in one-sided UE motor impairment. Participants receive rehabilitation for their UE impairments and can voluntarily lift the impaired UE against gravity (> 30 degrees shoulder anteflexion). We initially recruited sub-acute patients admitted to inpatient rehabilitation. However, we also included patients discharged home, possibly in the chronic phase post-stroke. We asked them to reflect retrospectively on their experiences during the sub-acute stage when transitioning from a rehabilitation centre to home. Patients were excluded if they had comprehensive aphasia resulting in the inability to provide informed consent or understand the interview questions. All participating patients provided written informed consent.
Context and groundwork
To familiarise with the users and the context of stroke rehabilitation, one researcher (AL) observed usual rehabilitation care, consisting of individual and group UE rehabilitation sessions, in the stroke ward of Rijndam Rehabilitation (Rotterdam, the Netherlands).
Next, we conducted a pragmatic literature search of guidelines, systematic reviews, and meta-analyses until September 2020 in PubMed to get acquainted with the literature on UE stroke rehabilitation interventions and treatment principles. We used combinations of the following Medical Subject Headings (Mesh): stroke, rehabilitation and upper extremity, and applied the following filters: systematic review, meta-analysis, guideline and practice guideline. One researcher (AL) screened the titles, abstracts and full texts when needed to select studies that met the following criteria: including adult stroke patients (> 18 years) and evaluating the effects of physical exercise interventions for the paretic UE on International Classification of Functioning, Disability and Health (ICF) function or activity level outcomes. Studies were excluded when evaluating medication, passive interventions (e.g., passive joint mobilisations), technical neurophysiologic interventions (e.g., transcranial brain stimulation), mental exercises (e.g., motor imagery), robotic interventions (e.g., robot-assisted therapy) or when solely including patients in the acute or chronic phase post-stroke. AL summarised the intervention characteristics, efficacy, treatment principles and the use of technology in the included studies. Next, studies were categorised based on their topics: Blended-care principles (principles related to the mixture of online therapy and in-person treatment), Exercise principles (principles related to an exercise intervention, e.g., task specificity), Exercise delivery (the specifications of an exercise intervention; e.g., the number of sets and repetitions), Exercise evaluation (the specification of measuring and evaluating exercise progress), and Usability (the degree to which the intervention (including technology) fit the intended use). Studies could be assigned to multiple categories. Using the categorised results as background information, two researchers (AL and GR) formulated interview questions related to the categories to interview therapists (Supplementary file A) and patients (Supplementary file B) and to conduct focus groups with therapists (Supplementary file C). We considered this a pragmatic search since the aim was to get acquainted with the literature, and only one researcher searched one database and used a limited number of search terms.
Eliciting requirements
To elicit the requirements, we conducted nine interviews and six focus groups with physiotherapists or occupational therapists, and nine interviews with stroke patients between September 2020 and July 2021. All participants were introduced to the research aims and the starting points of this research, which are providing home-based, UE exercise therapy using wearable motion sensors. To facilitate the selection of specific exercises, we made an inventory of the top ten most reported UE rehabilitation goals among patients and therapists. Two researchers (AL and GR) analysed the movement components in each of the ten goals, following the definitions of the movement components as reported by Lemmens et al. [36]. Next, focus groups were conducted by one (AL) or two researchers (AL and GR) with therapists about the most relevant exercises and exercise measures related to those movement components. Focus groups were conducted in the rehabilitation centre or online (due to COVID-19 restrictions). Patients were interviewed individually to allow more personal questions about the impact of stroke on their daily lives, goals, and personal experience with UE stroke rehabilitation. One researcher (AL) interviewed patients in the rehabilitation centre or at the participants’ houses when patients were discharged home. During the focus group, notes were made, and the interviews were recorded and transcribed verbatim.
Requirement modelling and analysis
Based on the interviews and focus group results, we developed user scenarios using the PACT (People, Activities, Contexts, and Technologies) framework. User scenarios provide detailed descriptions of the context, users, and motivations [37]. The PACT is a validated guideline to specifically construct a user scenario in the context of medical tele-treatment, from which functional requirements can be extracted [23]. In addition to the user scenarios, we developed a user journey, describing all actions the user should take before, during and after using a home-based UE rehabilitation system and the possible interactions between the user and the system. From the user scenario and journey, we extracted requirements.
Next, we prioritised requirements. Therapists individually ranked the selected exercises from one to three, indicating the most relevant exercises for the target patients. We selected the two most important exercises for each movement component based on the ranking. The exercise measures and the functional requirements were prioritised using the MoSCoW technique (“Must-have”, “Should-have,” “Could-have”, and “Won’t have”) [38]. Prioritisation was based on the qualifiers “importance” from the patient and therapist’s perspective and “risks (for therapy quality)” from the therapist’s perspective [38]. The prioritised requirements were listed in tables.
Preliminary results were presented and discussed during stakeholder group meetings, including representatives from rehabilitation technology companies, physiatrists, physical therapists and occupational therapists involved in stroke rehabilitation, biomechanical technicians, and stroke patients.
Agreeing requirements
To verify the validity of the requirements and requirement prioritisation generated from the focus groups and interviews, we conducted verification focus groups and interviews with therapists and patients who were not involved in the step of eliciting requirements. Two additional focus groups, including occupational therapists and physiotherapists providing UE rehabilitation to stroke patients in three rehabilitation facilities in the Netherlands, were conducted online by one researcher (AL). The patient’s perspective was also included in the verification. Eight stroke patients discharged home from a rehabilitation centre were interviewed by one researcher (AL) at their houses. During the focus groups, notes were made, and the interviews were recorded and transcribed verbatim.
Results
Pragmatic literature search
The pragmatic literature search resulted in 124 studies; 40 met the inclusion criteria (Supplementary file D) and were categorised into five topics.
Focus groups and interview descriptives
After nine interviews with patients, six focus groups and nine interviews with therapists, the five topics were discussed, and no new information regarding those topics occurred. Eight verification interviews with patients and two verification focus groups with therapists confirmed the previous findings and resulted in two additional ‘should haves’. Each focus group lasted 1 to 1.5 h, and the interviews took about 45 min each. Table 1 shows the numbers and characteristics of all participating patients and therapists (Table 1).
Table 1.
Characteristics of patients and therapists who participated in interviews or focus groups.
| Stroke patients |
Therapists |
||||
|---|---|---|---|---|---|
| Individual interviews (n = 9) | Verification interviews (n = 8) | Individual interviews (n = 9) | Focus group (n = 7) | Verification group (n = 5) | |
| Age (years; median, IQR) | 63 (48–76) | 60.5 (49–76) | 32.5 (31–59) | 33 (31–48) | 31 (24–59) |
| Gender (male/female) | 5/4 | 7/1 | 1/8 | 2/5 | 1/4 |
| Type of stroke (ischemic/hemorrargic) |
6/3 | 6/2 | |||
| Post-stroke phase (sub-acute/chronic) |
6/3 | 5/3 | |||
| Time since stroke (days) | 65 (53–214) | 150.5 (75–193.75) | |||
| Setting (primary practice/rehabilitation centre) | 6/3 | 0/8 | 1/8 | 0/7 | 1/4 |
| Profession (occupational therapist/ physiotherapist) | 5/4 | 3/4 | 2/3 | ||
| Stroke rehabilitation experience (years; median, IQR) | 10.5 (5–37) | 11 (5–25) | 8 (3–37) | ||
Functional requirements
We have formulated 33 functional requirements: eighteen must-haves, ten should-haves, and five could-haves. Each requirement is presented in Table 2 and described in more detail in the second column.
Table 2.
Functional requirements divided into must-haves, should-haves, and could-haves.
| Themes | Requirement | Specification | ||
|---|---|---|---|---|
| Must-haves | 1 | Exercise principles | The system measures and stimulates the activity of the paretic UE in daily life. |
|
| 2 | Exercise principles | The system provides and stimulates UE exercises. |
|
|
| 3 | Exercise principles | The system provides high repetitive UE training. |
|
|
| 4 | Exercise principles | The system provides task-related training. |
|
|
| 5 | Exercise principles | The exercises allow interaction with functional objects. |
|
|
| 6 | Exercise principles | The system allows patient-specific goal setting and provides feedback on those goals. |
|
|
| 7 | Exercise principles | The system motivates patients to accomplish pre-set goals and adhere to the intervention. a |
|
|
| 8 | Blended care principles | The system allows blending remote therapy (e-therapy) and face-to-face therapy. |
|
|
| 9 | Blended care principles | The system allows low-threshold contact between therapists and patients. |
|
|
| 10 | Exercise delivery | The system instructs the patient on performing the exercises safely and correctly. |
|
|
| 11 | Exercise delivery | The system allows adaptation of the intervention to patient-specific needs and goals. |
|
|
| 12 | Exercise delivery | The system allows adaptation of the exercise level during a session. |
|
|
| 13 | Exercise evaluation | The system provides feedback to the patient about the amount of paretic UE activity during daily life. |
|
|
| 14 | Exercise evaluation | The system provides feedback to the patient about the movement quantity during a session. |
|
|
| 15 | Exercise evaluation | The system provides feedback to the patient about the movement quality during a session. |
|
|
| 16 | Exercise evaluation | The system allows therapists to remotely evaluate the daily activity, quantity and quality of exercise sessions performed by patients. |
|
|
| 17 | Usability | The system is easy for patients to use; the patient can use the system preferably independent from a healthcare professional or caregiver. |
|
|
| 18 | Usability | The system is easy to use for therapists. |
|
|
| Should-haves | 19 | Exercise principles | The system provides shoulder stabilising exercises. |
|
| 20 | Exercise principles | The system provides strengthening exercises. |
|
|
| 21 | Exercise principles | The system provides total task practice with feedback on accomplished tasks. |
|
|
| 22 | Exercise delivery | The exercises allow interaction with daily life objects in the patient’s environment. |
|
|
| 23 | Exercise delivery | The system provides optional visual and auditive elements during the exercises. |
|
|
| 24 | Exercise evaluation | The system presents reference values on daily life activity by measuring the activity of the ipsilesional UE. |
|
|
| 25 | Exercise evaluation | The system summarises exercise performance results for patients after each exercise session. |
|
|
| 26 | Exercise evaluation | The system summarises daily UE activity results for patients. |
|
|
| 27 | Exercise evaluation | The system presents reference values for the exercise measures in the therapist overview. |
|
|
| 28 | Usability | The system allows use by people with foreign languages. |
|
|
| Could-haves | 29 | Exercise delivery | The system allows intervention adaptation to patient-specific needs and goals based on the patient’s previous performance during a session. |
|
| 30 | Exercise evaluation | The system allows video recording and video sharing with the therapists. |
|
|
| 31 | Exercise evaluation | The system provides feedback on movement quality during daily life. |
|
|
| 32 | Usability | The system proposes a set of exercises and quantitative and qualitative measures for each exercise based on the patient’s functional goals. |
|
|
| 33 | Usability | The therapist interface is available in the electronic patient record. |
|
|
UE: upper extremity; FMA: Fugel Meyer Assessment.
aItems require design-oriented research and are not described in detail in this paper.
Therapists indicated stimulating activity of the paretic UE in daily life and exercise training as important exercise principles (Table 2: 1, 2). They agreed on the importance of task-related and high repetitive exercise training using functional objects (Table 2: 3, 4, 5). Feedback on the exercises must be provided to the patient after completing an exercise session and in real-time on both quantitative and qualitative elements of movement (Table 2: 7, 13, 14, 15). The system must also include an easy-to-use therapist interface to allow remote evaluation of UE use and exercises (Table 2: 16, 18). Therapists acknowledge the lack of guidelines about the intensity and specific content of the exercises. They emphasised the heterogeneity in stroke patients and the need for personalised UE stroke rehabilitation (Table 2: 6, 11, 12). The therapist’s clinical expertise, experience, and knowledge are required to personalise exercise delivery and evaluation. Therefore, therapists stress a blended care system (Table 2: 8, 9) which enables the personalisation of the exercises, number of repetitions, exercise level, feedback content, and feedback frequency.
Patients also emphasised the importance of individualised, task-related exercises (Table 2: 4, 5). Exercises must match patients’ functional goals, and patients must be able to adjust the exercise level to their abilities (Table 2: 6, 11, 12). The system must be motivating (Table 2: 7), using feedback on the accomplishment of goals and the quality of the exercise performance (Table 2: 13, 14, 15). Patients experienced a gap between daily supervised treatment during rehabilitation admission and the lack of supervision in the home environment. They stressed that besides the system needs to be easy and safe to use independently at home (Table 2: 10, 17), their data of practising and recovery must be shared with a therapist, and there must be low-threshold contact options with a therapist (Table 2: 8, 9, 16).
Two new requirements (Table 2: 19, 28) were formulated based on the verification sessions with therapists and patients and classified as should-haves.
Exercises and exercise measures
In total, we identified seventeen exercises required for the home-based training system. The analysis of the ten most reported UE rehabilitation goals identified the movement components: reach, grasp, hold, release, displace, lift, positioning, push, pull, shove, and manipulate (26). Twelve exercises were identified regarding these movement components (Table 3A). In addition, five functional combinations of those exercises are included (Table 3B). Specific elements can be adjusted to induce variation in exercise performance and are reported in the table column ‘movement variation’. The measures and their prioritisation are exercise-dependent (Table 3). Must-haves for all exercise measures are the number of repetitions, the resting time between repetitions, the number of sets and the resting time between sets (Table 2: 14).
Table 3.
Exercises and exercise measures for each movement component (A) and functional combinations of movement components (B).
| Movement variation | Measures |
|||
|---|---|---|---|---|
| Must have | Should have | Could have | ||
| A. Movement components | ||||
| Reach | ||||
| Reach to a cylinder object and touch it. The object can be in different positions; the patient can reach in different directions: vertical ‘high’ (90–160 degrees shoulder flexion), vertical ‘low’ (< 90 degrees shoulder flexion), horizontal abduction (0–90 degrees horizontal shoulder abduction), horizontal adduction (90–130 degrees shoulder adduction). | reach distance; reach direction; velocity |
trunk and shoulder compensatory movements; reach distance |
touch accuracy; smoothness | ROM shoulder, (anteflexion, abduction, adduction); ROM elbow (flexion-extension) |
| Reach to a point and touch it. (Moving) targets are shown (on a digital screen). The patient touches the targets with preferably the index finger. Targets can appear and disappear in different sizes and speeds. | velocity; target size | trunk and shoulder compensatory movements; reach distance |
touch accuracy; smoothness | ROM shoulder, (anteflexion, abduction, adduction); ROM elbow (flexion-extension) |
| Grasp | ||||
| Grasp a cylinder (cylinder grasp). | position of the object; dosage of grip strength | timing of hand opening and closing; grip strength | ROM index finger and thumb; trunk and shoulder compensatory movements | |
| Grasp a small cube (three-point grasp). | position of the object; dosage of grip strength | timing of hand opening and closing; grip strength | ROM index finger and thumb; trunk and shoulder compensatory movements | |
| Hold & release | ||||
| Hold and release a cylinder (cylinder grasp) on the system-indicated moments, durations and force. | position of the object; velocity; dosage of grip strength | timing of hand opening; grip strength | trunk and shoulder compensatory movements | |
| Hold and release a small cube (three-point grasp) on the system-indicated moments, durations and force. | position of the object; velocity; dosage of grip strength | timing of hand opening; grip strength | trunk and shoulder compensatory movements | |
| Displace, lift & positioning | ||||
| Lifting a cylinder, transport it in different directions (< 90 degrees shoulder flexion, horizontal 0–90 degrees horizontal shoulder adduction, horizontal 90–130 degrees shoulder adduction) while keeping it in an upright position | transport direction; transport distance; velocity; dosage of grip strength | object tilt (in degrees); transport distance; smoothness; grip strength; trunk and shoulder compensatory movements | ROM shoulder, (anteflexion, abduction, adduction); ROM elbow (flexion-extension) | Velocity |
| Lifting a cylinder object, transport it in different directions (< 90 degrees shoulder flexion, horizontal 0–90 degrees horizontal shoulder abduction, horizontal 90–130 degrees shoulder adduction) while keeping it in an upright position and tilting the object on the by the tablet indicated place, moment and speed. | transport direction; transport distance; velocity; dosage grip strength; object tilt degrees; object tilt timing | object tilt (in degrees); transport distance; accuracy of object tilt; smoothness; grip strength; trunk and shoulder compensatory movements | ROM shoulder (anteflexion, abduction, adduction); ROM elbow (flexion-extension); ROM wrist (pronation-supination) | Velocity |
| Push/pull/shove | ||||
| Moving the palmar side of the hand over the back of the head | velocity | trunk and shoulder compensatory movements; smoothness | ROM shoulder (anteflexion, abduction, adduction); ROM elbow (flexion-extension); velocity of the hand | |
| Moving the palmar side of the hand over the back | velocity | trunk and shoulder compensatory movements smoothness | ROM shoulder (anteflexion, abduction, adduction); ROM elbow (flexion-extension); velocity of the hand | |
| Manipulate | ||||
| Hold a pen in hand and perform 'writing’ exercises: following lines straight and curled, writing the alphabet in block letters. | velocity, size of the lines and letters | smoothness; grip strength | Velocity | ROM index finger and thumb |
| Grasp small objects (small pegs) between fingers (optional: turn the pegs around) and put each of them separately in a small container | velocity | smoothness; grip strength | Velocity | ROM wrist (pronation- supination); ROM index finger and thumb |
| B. Combination Exercises | ||||
| Reach + grasp (cylinder or 3-point grasp) | ||||
| Reach to the object (cylinder or cube) and grasp it (cylinder or 3-point grasp). The object can be placed in different positions; the patient should reach in different directions (high (90–160 degrees shoulder flexion), low (< 90 degrees shoulder flexion), horizontal 0–90 degrees horizontal shoulder abduction), horizontal 90–130 degrees shoulder adduction) | reach distance; reach direction; dosage of grip strength |
timing of hand opening and closing, smoothness; grip strength; trunk and shoulder compensatory movements; reach distance | ROM shoulder (anteflexion, abduction, adduction); ROM elbow (flexion-extension) | ROM index finger and thumb |
| Reach + grasp + hold & release | ||||
| Reach to the object (cylinder or cube) and grasp it (cylinder or 3-point grasp). The object can be placed in different positions; the patient should reach in different directions (high (90–160 degrees shoulder flexion), low (< 90 degrees shoulder flexion), horizontal 0–90 degrees horizontal shoulder abduction), horizontal 90–130 degrees shoulder adduction). Hold and release a cylinder object on the tablet indicated moments, durations and force. | reach distance; reach direction; dosage of grip strength; velocity | timing of hand opening and closing; smoothness; grip strength; trunk and shoulder compensatory movements; reach distance | ROM shoulder (anteflexion, abduction, adduction); ROM elbow (flexion-extension) | ROM index finger and thumb |
| Reach + grasp + positioning, displace & lift | ||||
| Reach to the object (cylinder or cube) and grasp it (cylinder or 3-point grasp). The object can be placed in different positions; the patient should reach in different directions (high (90–160 degrees shoulder flexion), low (< 90 degrees shoulder flexion), horizontal 0–90 degrees horizontal shoulder adduction), horizontal 90–130 degrees shoulder abduction). Lift the object and transport it in different directions (< 90 degrees shoulder flexion, horizontal 0–90 degrees horizontal shoulder adduction, horizontal 90–130 degrees shoulder adduction) while keeping it upright. Tilt the object on the tablet, indicating place, moment and speed. | reach distance; reach direction; dosage of grip strength; transport direction; transport distance; velocity |
timing of hand opening and closing; object tilt (in degrees); smoothness; trunk and shoulder compensatory movements; grip strength; reach distance; object tilt (in degrees); transport distance; accuracy of object tilt | ROM shoulder (anteflexion, abduction, adduction); ROM elbow (flexion-extension) | ROM wrist (pronation-supination); ROM index finger and thumb |
| Reach + grasp + push, pull & shove | ||||
| Reach to the object (cylinder or cube) and grasp it (cylinder or 3-point grasp). The object can be placed in different positions; the patient should reach in different directions (high (90–160 degrees shoulder flexion), low (< 90 degrees shoulder flexion), horizontal 0–90 degrees horizontal shoulder abduction), horizontal 90–130 degrees shoulder adduction). Move the object over the back of the head. | reach distance; reach direction; dosage grip strength; velocity |
timing of hand opening and closing; trunk and shoulder compensatory movements; smoothness; grip strength | ROM shoulder (anteflexion, abduction, adduction); ROM elbow (flexion-extension) | ROM wrist (pronation-supination); ROM index finger and thumb; velocity of the hand |
| Reach to the object (cylinder or cube) and grasp it (cylinder or 3-point grasp). The object can be placed in different positions; the patient should reach in different directions (high (90–160 degrees shoulder flexion), low (< 90 degrees shoulder flexion), horizontal 0–90 degrees horizontal shoulder abduction), horizontal 90–130 degrees shoulder adduction). Move the object over the back. | reach distance; reach direction; dosage grip strength; velocity | timing of hand opening and closing; trunk and shoulder compensatory movements, smoothness; grip strength | ROM shoulder (anteflexion, abduction, adduction); ROM elbow (flexion-extension) | ROM wrist (pronation-supination); ROM index finger and thumb; velocity of the hand |
Reach: intentional movement of the arm towards an object; grasp: to make a motion of seizing, snatching or clutching; hold & release: Keeping an object in a fixed position in the hand without external support and freeing the object from grip; Displace, lift & positioning: moving an object without the object being in contact with a surface in the environment and maintaining a fixed position of the shoulder, arm and hand in space; push/pull/shove: to apply force against an object with the intention to move or stabilise; manipulate: to skilfully control the position of an object using the fingers [36]. ROM: Range of Motion.
Discussion
In this study, we defined requirements for home-based UE rehabilitation for stroke patients using wearable motion sensors based on a user-centred design approach. Requirements were categorised into functional requirements, required exercise and exercise measures. Eighteen must-have functional requirements were found related to blended care (2), exercise principles (7), exercise delivery (3), exercise evaluation (4), and usability (2). Six movement components, including twelve exercises and five combination exercises, were reported to be the most important. For each, relevant measures were found and classified as must-have, should-have, or could-have.
Our research started with a pragmatic search of the literature investigating UE rehabilitation interventions for stroke patients. Key elements we found in the literature regarding exercise principles and exercise delivery for UE therapy in stroke patients were task-related and high-intensity exercises and personalisation of the exercises, exercise level and intensity to a patient’s capacity and goals [5,39]. However, no specific recommendations could be extracted from the literature and integrated into our requirement overview. For example, although the literature suggests the non-inferiority of blended rehabilitation compared to usual rehabilitation and its potential cost-effectiveness [17–19,40], no specific recommendations regarding designing a blended care intervention (e.g., a minimum of face-to-face or digital contact moments) are provided. Furthermore, no consistent recommendations were made regarding the exercise delivery methods, application and required repetitions to improve UE capacity and stimulate the recovery of UE function in stroke patients [41].
Stroke patients’ UE training preferences contain UE skills used in daily activities [42]. To facilitate the transfer of the UE capacity acquired with training to daily life UE use, exercises require relation with patients’ functional goals and interaction with functional objects, preferably objects used in daily life involving grasping, holding or manipulating [42]. The combination of stimulating UE activity and exercises is more effective in improving UE capacity than forced use of the paretic UE alone or exercise alone [3] and may further improve the transfer from training to daily life situations. However, UE rehabilitation systems currently available for rehabilitation focus on stimulating UE exercises [43] or stimulating daily life activity alone [44–46]. Preferably one single system includes both functionalities.
Our study indicates the importance of high repetitive exercise and daily use. Still, we could not specify the required intensity of exercises or use. In line with the literature [27], therapists and patient panels in our study reported that stroke patients’ rehabilitation requires individualised treatment, including a tailored selection of exercises and intensity. This emphasises the need for a blended care approach. However, current literature describes telerehabilitation as supplementary to usual rehabilitation instead of having the potential to contribute equally [25]. Based on the results of this study, we presume accurate and real-time performance feedback to the patient, at least weekly evaluation of performance by the therapists, and the option of ad hoc contact between patient and therapist is mandatory to increase the contribution of telerehabilitation and reduce the need for face-to-face therapy sessions.
Although the mechanisms underlying motor recovery in stroke patients are still not fully understood, providing real-time performance feedback during training may enhance motor learning [47] and stimulates patients to continue exercising [48]. Individualised training programs require individualised feedback. Therefore, exercise measures for real-time performance feedback should be chosen in therapist-patient interactions. Movement measures can also be used to evaluate stroke patients’ UE recovery. Our study is in line with findings from the literature suggesting trunk and shoulder compensatory movements or movement smoothness may indicate recovery of UE function [49,50].
Other studies identifying requirements for telerehabilitation in stroke patients used comparable approaches, including multiple iterations of end-user sessions. Most studies did not provide an extensive overview of functional requirements for UE home-based rehabilitation systems, but focused on a specific element, such as the systems’ usability or feedback measures [51,52]. A recently published scoping review provides a comprehensive overview of design requirements for home-based UE rehabilitation robotics for stroke patients [28]. Still, we did not find studies explicitly focusing on UE telerehabilitation systems for stroke patients using wearable motion sensors. Since wearable motion sensors allow for real-time movement tracking and feedback in real-world settings, the application and the requirements of a sensor-based system may differ from the application of home-based robotics. In our study, some of the requirements for measuring paretic UE activity during daily life and the device’s usability seem typical for wearable motion sensors. Conversely, the remaining requirements might apply to home-based rehabilitation solutions using other types of technology for this patient population.
Another home-based upper limb training device using IMUs shows promising feasibility in chronic stroke patients [53]. This study did not report the user requirements. Some of the essential requirements from our research, such as using blended-care principles and allowing intervention adaptation to patient-specific needs and goals, seem not to be incorporated in this device. Including these requirements may facilitate the clinical implementation of such telerehabilitation systems. On the other hand, developing a system complying with all must-have user requirements can be challenging. To overcome this challenge, an iterative development process in which all stakeholders are actively involved in making trade-offs between the benefits of each requirement and the technical and financial boundaries is recommended [33].
Limitations
We did not adopt an in-depth qualitative software-supported analytic approach to the interview and focus group data we collected (e.g., thematic analysis of transcribed data). However, the user-centred approach, including multiple iterations of eliciting and analysing requirements, fitted the aims of defining user requirements for home-based UE rehabilitation using wearable motion sensors [33]. To ensure the validity and reliability of our results, we followed a well-established systematic method of requirement engineering described by Nuseibeh & Easterbrook [34], including verifying the elicited requirements in other therapists and patients. In addition, we followed the PACT guidelines, which allowed us to analyse the user requirements validly in the context of telerehabilitation [23]. The number of included therapists and patients was sufficient for the aim of this study [51,54]. Participants were purposefully selected for this study and provided rich data on the topics of interest. Data saturation on must-have requirements was reached; no new must-have requirements were derived during the validation phase.
An explicit understanding of the behaviour, needs and wishes that characterise the target group is the core of a user-centred design approach [54]. For this reason, the generalizability of our results to other populations or purposes may be limited. This study focused on requirements for stroke patients in the sub-acute phase who can voluntarily lift the impaired UE against gravity (> 30 degrees shoulder anteflexion). Within the population of stroke patients, there is a large variety of disease-related characteristics (e.g., motor impairment severity, recovery phase and prognosis) [5], which may result in a different set of requirements for home-based UE rehabilitation. However, the overview of requirements systematically derived from our study can be used as a basis for developing home-based UE rehabilitation interventions based on wearable motion sensors.
Conclusion
This study provides an overview of user requirements for home-based UE rehabilitation using wearable motion sensors for sub-acute stroke patients, subdivided into functional requirements, required exercises and exercise measures. The requirements are prioritised as must-haves, should-haves, and could-haves. They can be used to develop home-based UE rehabilitation interventions based on wearable motion sensors. Moreover, the comprehensive and systematic requirement analysis used in this study can be applied by other researchers and developers when extracting requirements for designing a system or intervention in a medical context.
Supplementary Material
Acknowledgements
The authors thank all participants involved in the study and the ArmCoach4Stroke project members for participating in the stakeholder meetings.
Funding Statement
This work was supported by the Dutch Heart Foundation, NWO and ZonMw, the Netherlands, under Grant [104021001].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Feigin VL, Abajobir AA, Abate KH, et al. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Neurol. 2017;16(11):877–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langhorne P, Coupar F, Pollock A.. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8(8):741–754. [DOI] [PubMed] [Google Scholar]
- 3.Kwakkel G, Veerbeek JM, van Wegen EE, et al. Constraint-induced movement therapy after stroke. Lancet Neurol. 2015;14(2):224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwakkel G, Winters C, van Wegen EE, EXPLICIT-Stroke Consortium, et al. Effects of unilateral upper limb training in two distinct prognostic groups early after stroke: the EXPLICIT-Stroke randomized clinical trial. Neurorehabil Neural Repair. 2016;30(9):804–816. Oct [DOI] [PubMed] [Google Scholar]
- 5.Langhorne P, Bernhardt J, Kwakkel G.. Stroke rehabilitation. Lancet. 2011;377(9778):1693–1702. [DOI] [PubMed] [Google Scholar]
- 6.Pedlow K, Lennon S, Wilson C.. Application of constraint-induced movement therapy in clinical practice: an online survey. Arch Phys Med Rehabil. 2014;95(2):276–282. [DOI] [PubMed] [Google Scholar]
- 7.Viana R, Teasell R.. Barriers to the implementation of Constraint-Induced movement therapy into practice. Topics in Stroke Rehabilitation. 2012;19(2):104–114. [DOI] [PubMed] [Google Scholar]
- 8.Hayward KS, Brauer SG.. Dose of arm activity training during acute and subacute rehabilitation post stroke: a systematic review of the literature. Clin Rehabil. 2015;29(12):1234–1243. [DOI] [PubMed] [Google Scholar]
- 9.Lang CE, MacDonald JR, Gnip C.. Counting repetitions: an observational study of outpatient therapy for people with hemiparesis post-stroke. J Neurol Phys Ther. 2007;31(1):3–10. [DOI] [PubMed] [Google Scholar]
- 10.Lang CE, Macdonald JR, Reisman DS, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009;90(10):1692–1698. Oct [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwakkel G. Impact of intensity of practice after stroke: issues for consideration. Disability Rehabil. 2006;28(13-14):823–830. [DOI] [PubMed] [Google Scholar]
- 12.Fleet A, Che M, Mackay-Lyons M, et al. Examining the use of constraint-induced movement therapy in canadian neurological occupational and physical therapy. Physiother Can. 2014;66(1):60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alarcón-Aldana AC, Callejas-Cuervo M, Bo APL.. Upper limb physical rehabilitation using serious videogames and motion capture systems: a systematic review. Sensors. 2020;20(21):5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Débora Pacheco B, Guimaraes Caetano LC, Amorim Samora G, et al. Perceived barriers to exercise reported by individuals with stroke, who are able to walk in the community. Disabil Rehabil. 2021;43(3):331–337. [DOI] [PubMed] [Google Scholar]
- 15.Kwakkel G, Kollen BJ, Wagenaar RC.. Long term effects of intensity of upper and lower limb training after stroke: a randomised trial. J Neurol Neurosurg Psychiatry. 2002;72(4):473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franck JA, Smeets R, Seelen HAM.. Changes in arm-hand function and arm-hand skill performance in patients after stroke during and after rehabilitation. PLoS ONE. 2017;12(6):e0179453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostrowska PM, Śliwiński M, Studnicki R, et al. Telerehabilitation of Post-Stroke patients as a therapeutic solution in the era of the covid-19 pandemic. Healthcare. 2021;9(6):654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laver KE, Adey-Wakeling Z, Crotty M, et al. Telerehabilitation services for stroke. Cochrane Database Syst Rev. 2020;1(1):Cd010255. Jan 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tchero H, Tabue Teguo M, Lannuzel A, et al. Telerehabilitation for stroke survivors: systematic review and Meta-Analysis. J Med Internet Res. 2018;20(10):e10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Abel KT, Janecek JT, et al. Home-based technologies for stroke rehabilitation: a systematic review. Int J Med Inform. 2019;123:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porciuncula F, Roto AV, Kumar D, et al. Wearable movement sensors for rehabilitation: a focused review of technological and clinical advances. Pm R. 2018;10(9 Suppl 2):S220–S232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markets Ra . Motion sensor market - growth, trends, COVID-19 impact, and forecasts (2022–2027). 2022. https://www.researchandmarkets.com/reports/5239646/motion-sensor-market-growth-trends-covid-19#product
- 23.Huis In 't Veld RM, Widya IA, Bults RG, et al. A scenario guideline for designing new teletreatments: a multidisciplinary approach. J Telemed Telecare. 2010;16(6):302–307. [DOI] [PubMed] [Google Scholar]
- 24.Jayasree-Krishnan V, Ghosh S, Palumbo A, et al. Developing a framework for designing and deploying Technology-Assisted rehabilitation after stroke: a qualitative study. Am J Phys Med Rehabil. 2021;100(8):774–779. Aug 1 [DOI] [PubMed] [Google Scholar]
- 25.Caughlin S, Mehta S, Corriveau H, et al. Implementing telerehabilitation after stroke: lessons learned from Canadian trials. Telemed J E Health. 2020;26(6):710–719. Jun [DOI] [PubMed] [Google Scholar]
- 26.Levy T, Jc L, Killington M, et al. Just that four letter word, hope": stroke survivors’ perspectives of participation in an intensive upper limb exercise program; a qualitative exploration. Physiother Theory Pract. 2021;12:1–15. [DOI] [PubMed] [Google Scholar]
- 27.Elnady A, Mortenson WB, Menon C.. Perceptions of existing wearable robotic devices for upper extremity and suggestions for their development: findings from therapists and people with stroke. JMIR Rehabil Assist Technol. 2018;5(1):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, Fu Q, Tyson S, et al. A scoping review of design requirements for a home-based upper limb rehabilitation robot for stroke. Top Stroke Rehabil. 2022;29(6):449–463. [DOI] [PubMed] [Google Scholar]
- 29.Schreiweis B, Pobiruchin M, Strotbaum V, et al. Barriers and facilitators to the implementation of eHealth services: systematic literature analysis. J Med Internet Res. 2019;21(11):e14197. Nov 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kip H, Keizer J, da Silva MC, et al. Methods for Human-Centered eHealth development: narrative scoping review. J Med Internet Res. 2022;24(1):e31858. Jan 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasr N, Leon B, Mountain G, et al. The experience of living with stroke and using technology: opportunities to engage and co-design with end users. Disabil Rehabil Assist Technol. 2016;11(8):653–660. Nov [DOI] [PubMed] [Google Scholar]
- 32.Wentink M, van Bodegom-Vos L, Brouns B, et al. How to improve eRehabilitation programs in stroke care? A focus group study to identify requirements of end-users. BMC Med Inform Decis Mak. 2019;19(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wentink MM, VAN Bodegom-Vos L, Brouns B, et al. What is important in E-health interventions for stroke rehabilitation? A survey study among patients, informal caregivers and health professionals. Int J Telerehabil. 2018;10(1):15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nuseibeh B, Easterbrook S, editors. Requirements engineering: a roadmap. Proceedings of the conference on the future of software engineering, New York, United States. 2000. [Google Scholar]
- 35.Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Neurorehabil Neural Repair. 2017;31(9):793–799. [DOI] [PubMed] [Google Scholar]
- 36.Lemmens RJM, Janssen-Potten YJM, Timmermans AAA, et al. Arm hand skilled performance in cerebral palsy: activity preferences and their movement components. BMC Neurol. 2014;14:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foundation ID. User Scenarios [cited 2021 14-Oct]. Available from: https://www.interaction-design.org/literature/topics/user-scenarios [Google Scholar]
- 38.Berander P, Andrews A.. Requirements prioritization. Eng Manag Softw Requir. 2005;69–94. [Google Scholar]
- 39.Van Peppen RP, Kwakkel G, Wood-Dauphinee S, et al. The impact of physical therapy on functional outcomes after stroke: what’s the evidence? Clin Rehabil. 2004;18(8):833–862. Dec [DOI] [PubMed] [Google Scholar]
- 40.Ballantyne R, Rea PM.. A game changer: 'The use of digital technologies in the management of upper limb rehabilitation. Adv Exp Med Biol. 2019;1205:117–147. [DOI] [PubMed] [Google Scholar]
- 41.Church G, Ali A, Smith CL, et al. Examining clinical practice guidelines for exercise and physical activity as part of rehabilitation for people with stroke: a systematic review. Int J Environ Res Public Health. 2022;19(3):1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timmermans AA, Seelen HA, Willmann RD, et al. Arm and hand skills: training preferences after stroke. Disabil Rehabil. 2009;31(16):1344–1352. [DOI] [PubMed] [Google Scholar]
- 43.Widmer M, Held JPO, Wittmann F, et al. Reward during arm training improves impairment and activity after stroke: a randomized controlled trial. Neurorehabil Neural Repair. 2022;36(2):140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Held JP, Klaassen B, van Beijnum BF, et al. Usability evaluation of a VibroTactile feedback system in stroke subjects. Front Bioeng Biotechnol. 2016;4:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Held JPO, Luft AR, Veerbeek JM.. Encouragement-Induced Real-World upper limb use after stroke by a tracking and feedback device: a study protocol for a Multi-Center, Assessor-Blinded, randomized controlled trial. Front Neurol. 2018;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore SA, Da Silva R, Balaam M, et al. Wristband accelerometers to motiVate arm exercise after stroke (WAVES): study protocol for a pilot randomized controlled trial. Trials. 2016;17(1):508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maier M, Ballester BR, Verschure P.. Principles of neurorehabilitation after stroke based on motor learning and brain plasticity mechanisms. Front Syst Neurosci. 2019;13:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida T, Otaka Y, Osu R, et al. Motivation for rehabilitation in patients with subacute stroke: a qualitative study [original research]. Front Rehabil Sci. 2021;2:664758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Kordelaar J, van Wegen E, Kwakkel G.. Impact of time on quality of motor control of the paretic upper limb after stroke. Arch Phys Med Rehabil. 2014;95(2):338–344. [DOI] [PubMed] [Google Scholar]
- 50.van Kordelaar J, van Wegen EE, Kwakkel G.. Unraveling the interaction between pathological upper limb synergies and compensatory trunk movements during reach-to-grasp after stroke: a cross-sectional study. Exp Brain Res. 2012;221(3):251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cavuoto LA, Subryan H, Stafford M, et al. Understanding user requirements for the design of a Home-Based stroke rehabilitation system. Proc Hum Factors Ergon Soc Annu Meet. 2018;62(1):1037–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Meulen FB, Klaassen B, Held J, et al. Objective evaluation of the quality of movement in daily life after stroke. Front Bioeng Biotechnol. 2015;3:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wittmann F, Held JP, Lambercy O, et al. Self-directed arm therapy at home after stroke with a sensor-based virtual reality training system. J Neuroeng Rehab. 2016;13(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Administration DgtftUSGS . User-Centered Design Basics. 2021. https://www.usability.gov/what-and-why/user-centered-design.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.