Abstract
Alzheimer’s disease (AD) is a chronic neurodegenerative disease characterised by cognitive impairment, behavioural alteration, and functional decline. Over 130 AD-associated susceptibility loci have been identified by genome-wide association studies (GWAS), while whole genome sequencing (WGS) and whole exome sequencing (WES) studies have identified AD-associated rare variants. These variants are enriched in APOE, TREM2, CR1, CD33, CLU, BIN1, CD2AP, PILRA, SCIMP, PICALM, SORL1, SPI1, RIN3, and more genes. Given that aging is the single largest risk factor for late-onset AD (LOAD), the accumulation of somatic mutations in the brain and blood of AD patients have also been explored. Collectively, these genetic findings implicate the role of innate and adaptive immunity in LOAD pathogenesis and suggest that a systemic failure of cell-mediated amyloid-β (Aβ) clearance contributes to AD onset and progression. AD-associated variants are particularly enriched in myeloid-specific regulatory regions, implying that AD risk variants are likely to perturbate the expression of myeloid-specific AD-associated genes to interfere Aβ clearance. Defective phagocytosis, endocytosis, and autophagy may drive Aβ accumulation, which may be related to naturally-occurring antibodies to Aβ (Nabs-Aβ) produced by adaptive responses. Passive immunisation is providing efficiency in clearing Aβ and slowing cognitive decline, such as aducanumab, donanemab, and lecanemab (ban2401). Causation of AD by impairment of the innate immunity and treatment using the tools of adaptive immunity is emerging as a new paradigm for AD, but immunotherapy that boosts the innate immune functions of myeloid cells is highly expected to modulate disease progression at asymptomatic stage.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-021-03986-5.
Keywords: Neurodegeneration, Single nucleotide polymorphism, Polygenic risk score, Endo-lysosomal network, Amyloid plaques, Microglia, Monocytes
Overview of Alzheimer’s disease (AD)
AD is a chronic neurodegenerative disease, clinically characterised by deterioration in learning, memory, word-finding, spatial orientation, and problem-solving that gradually undermines the ability to perform daily activities [1]. AD is the leading form of dementia and has been prioritised as one of our top public health concerns [1]. Classical pathological features of AD include extracellular senile plaques, intracellular neurofibrillary tangles (NFT) and brain atrophy [1]. Senile plaques are the aggregated forms of amyloid-β (Aβ) peptides that are produced by sequential cleavage of the amyloid precursor protein (APP) by β- and γ-secretases [2]. β-secretase cleaves the juxta-membrane domain of APP to release the ectodomain and then γ-secretase cleaves multiple sites in the transmembrane domain of APP to release carboxy-terminal fragments and Aβ peptides (~ 4.5 kDa), ranging from 38 to 43 residues [2]. Compared with Aβ ending in residue 40 (Aβ1-40), Aβ ending in residue 42 (Aβ1–42) is more hydrophobic and amyloidogenic [2]. Intraneuronal NFTs are marked by hyperphosphorylated tau (p-tau) [3]. The NFT burden is more closely associated with synaptic loss and cognitive impairment than amyloid burden [3]. Abnormalities of innate immunity, such as, reactive astrogliosis and microgliosis, are being increasingly acknowledged as AD hallmarks, but whether these histopathological features are beneficial or detrimental remains inconclusive [4]. Microglia are the resident macrophages in the central nervous system (CNS) and serve as the first line of innate defence in brain injury. Microglia stay in a “resting” (but fully functional) state in the absence of noxious stimuli or structural damage [5]; however, aggregated Aβ, invading pathogens, and unwanted materials are potent microglial activators [6]. Co-localisation of reactive microglia with Aβ plaques has been shown in both the brains of AD patients and animal models of AD [7]. Moreover, reactive astrogliosis is also recognised in AD patients and transgenic animal models and activated astrocytes also contribute to Aβ clearance [8]. A better understanding of AD-associated innate immune responses may shed light on mechanisms driving Aβ clearance.
The diagnostic criteria of AD have evolved from the gold-standard postmortem examination of Aβ and tau deposition into the current suite of biofluid biomarker and molecular imaging [9]. Compelling evidence shows that aberrant Aβ accumulation starts 20–30 years before clinical onset, so AD dementia should be considered as a late stage in the continuum of AD-associated biological alterations [10, 11]. Aβ imaging by positron emission tomography (PET) and cerebrospinal fluid (CSF) measurements of Aβ, tau, and p-tau allow accurate estimates of these pathological hallmarks in preclinical and prodromal AD [11]. Although these advanced techniques facilitate the accurate detection of preclinical AD patients, their applications are mostly in research and clinical trials to enable an accurate enrolment of AD subjects [9, 11]. Molecular neuroimaging and CSF measurements are invasive and expensive methods that require trained personnel and advanced equipment, which limits their potential as population-based diagnostic techniques in a hospital setting [9]. Therefore, it is urgent to develop non-invasive population screening tests, such as blood biomarkers, for the early diagnosis of AD patients and the early intervention or disease management [9].
AD is classified into early-onset AD (EOAD), where symptoms appear before the age of 65 years, and late-onset AD (LOAD), where symptoms appear after the age of 65 years [12]. EOAD and LOAD share common pathological features and are both considered to be highly heritable [12]. While heritability estimates for EOAD are over 90%, heritability estimates for LOAD are in the range of 58–79% [13]. Aging is still the single largest risk factor for LOAD [13]. This review summarises the latest genomic findings of AD, including well-established monogenic causes of EOAD and the ongoing genetic studies of LOAD, including the accumulated somatic mutations during aging. These genetic-based findings may enhance our understanding of the biological basis of AD-associated variants and provide new insights into AD diagnosis, prognosis, and treatment.
Genetics of AD
Monogenic causes of EOAD
Proteolytic APP processing and the consequent Aβ production can be altered by autosomal dominant mutations in APP, presenilin 1 (PSEN1) and presenilin 2 (PSEN2) genes, which are the genetic hallmarks of EOAD that account for less than 1% of total AD cases. More than 50 highly penetrant mutations have been reported in the APP gene (chr21) and the overall effect of these mutations make APP more susceptible to be cleaved by β-secretase, resulting in more neurotoxic Aβ1-42, altered hydrophobicity and enhanced aggregation propensity [14]. Individuals with trisomy 21 (Down’s syndrome) carry three copies of wild-type APP and invariably develop AD neuropathologic features during their teens, supporting the key role of Aβ dyshomeostasis in initiating AD [2]. The other two EOAD genes are highly homologous members of the presenilin gene family, encoding subunits of γ-secretase [14]. EOAD mutations in the PSEN1 (chr14) and PSEN2 (chr1) genes alter the cleavage site specificity, favouring cleavage at position 42 rather than position 40, leading to the production of more aggregation-prone Aβ1-42 and more efficient Aβ deposition in the brain [15]. Over 350 mutations have been identified in PSEN1, making PSEN1 mutations the most common cause of EOAD.1PSEN1 mutation carriers usually develop dementia between 30 and 50 years of age [14]. While PSEN1 mutations contribute to 80% of EOAD cases, only approximately 30 mutations have been identified in the PSEN2 gene [12]. This strong genetic evidence in EOAD makes altered APP processing and Aβ overproduction the “cornerstone” of the “Aβ-amyloid theory”, in which the overproduced neurotoxic Aβ drives synaptic loss, microgliosis, astrocytosis, NFT formation, and neuritic dystrophy [2]. Given that similar neuropathological hallmarks are also observed in LOAD [12], the “Aβ-amyloid theory” has been widely accepted in the field to guide AD research and therapeutic development in the past 2 decades [2].
APOE: the strongest genetic risk factor of LOAD
LOAD accounts for over 95% of total AD cases worldwide [1]. Common polymorphisms in the apolipoprotein E (APOE) gene are the major genetic determinants for AD risk. ApoE is a lipoprotein (even if glycosylated) that is expressed in brain, liver, and myeloid cells and is involved in cholesterol and lipid transportation, neuronal growth and immunoregulation [16]. Three different alleles of APOE encode three isoforms, including ApoE ε2, ApoE ε3, and ApoE ε4 [16]. Compared with non-APOE ε4 carriers, APOE ε4 heterozygotes and APOE ε4 homozygotes have a 4.6-fold and 14.9-fold higher odds ratio (OR) of AD risk respectively, which can be further elevated to 25.4-fold by advanced age, while APOE ε2 confers protection against AD (OR = 0.6) [17]. Although three isoforms differ by only two amino acids, the structure and function of ApoE isoforms are significantly altered, influencing Aβ clearance, lipid metabolism, glucose metabolism, neuronal signalling, innate immune response, and mitochondrial function [12]. Of note, the precise mechanism by which APOE ε4 increases AD risk remains inconclusive, so further investigation of the APOE gene is critical for advancing our understanding of AD and for developing therapeutics.
Genome-wide association studies (GWAS) of AD
Background of GWAS
GWAS have endeavoured to reveal the underlying genetic variants in AD pathogenesis and elucidate their biological roles in determining an individual’s risk for AD. High-throughput genotyping allows the simultaneous genotyping of single nucleotide polymorphisms (SNP) across the whole genome, leading to the emergence of GWAS [18]. GWAS examines the association of millions of SNPs located in both coding and non-coding regions across the genome with a particular trait without prior assumptions about biological pathways, resulting in the identification of common polymorphisms (minor allele frequency [MAF]>1%) associated with a disease trait [18]. An important concept in GWAS is linkage disequilibrium (LD), referring to non-random segregation of nearby alleles leading to the inheritance of large blocks of variants [12, 18]. The identification of a lead SNP can be used as a surrogate tag for an entire region of the high LD, which means GWAS may not always identify the truly causal genes [12, 18]. Therefore, the subsequent mapping and functional characterisation of the SNP is needed to prioritise the candidate causal genes. The traditional case–control GWAS of AD recruits clinically confirmed AD cases and cognitively normal (CN) controls. The late onset of AD and the lack of standardised biomarkers make the recruitment, ascertainment, and genotyping of AD cases difficult. For instance, the UK Biobank (UKB) contains over 500,000 participants, but only around 1000 individuals are clinically confirmed AD cases [18]. To address this limitation, GWAS by proxy (GWAX) has been developed by including AD proxy cases and controls based on their parental history of AD (or other types of dementia) [18]. Compared with case–control AD, AD proxy cases with family history of AD are weakly genetically associated with AD, so that GWAX requires four times of proxy cases and controls for equivalent statistical power [18]. GWAX can therefore drastically increase statistical power due to its large sample size.
The first small-scale GWAS before 2013
AD GWAS were started in 2007 and nine small-scale GWAS were published up to 2009. Only SNPs within the APOE locus reached genome-wide significance and were replicated in independent studies [19]. These early studies recruited around 2,000 AD cases and controls, but GWAS required larger sample numbers for the detection of small-effect associations. In 2009, two large-scale GWAS replicated the established associations within the APOE locus and identified the first novel SNPs outside APOE locus that reached genome-wide significance, including CLU, PICALM, and CR1 [20–22]. Subsequent GWAS identified more AD susceptibility loci, including BIN1 [23, 24], ABCA7 [25], MS4A [24, 25], CD2AP [24, 25], CD33 [24, 25], and EPHA1 (later known as ZYX) [24, 25]. These early large-scale GWAS provided compelling evidence that susceptibility loci are distributed in established biological pathways involved in AD pathogenesis, including APP processing, endocytosis, and immunity.
Large-scale GWAS and GWAX between 2013 and 2021
The investigation into additional genetic risk factors would require larger sample sizes and meta-analysis with pre-existing GWAS datasets to increase the statistical power. In 2013, a meta-analysis of 17,008 cases and 37,154 controls, followed by replication in an independent cohort consisting of 8,572 cases and 11,312 controls, confirmed previous GWAS findings and identified 11 novel AD susceptibility loci: HLA-DRB5-DRB1, SORL1, PTK2B, SLC24A4, ZCWPW1, CELF1, NME8, FERMT2, CASS4, INPP5D, and MEF2C [26]. The subsequent pathway analyses identified the associated functional pathways, including the immune responses and regulation of endocytosis, while all genetic network modules identified by the correlated gene expression analysis were associated with the immune responses [27], particularly microglia in the CNS [28]. To further boost the statistical power, 314,278 proxy cases and controls from the UKB dataset were meta-analysed with this GWAS, leading to the identification of 27 AD susceptibility loci, three of which were novel: ADAM10, BCKDK/KAT8, and ACE [29]. As GWAS cannot identify the causality of genes, Marioni and colleagues used the expression quantitative trait loci (eQTL) analysis and prioritised TOMM40, KAT8, and CR1 as the candidate causal genes of AD [29]. In 2019, a larger GWAX, consisting of 534,403 individuals, identified 29 susceptibility loci, in which nine loci were novel: ADAMTS4, HESX1, CLNK, CNTNAP2, ADAM10, APH1B, KAT8, ALPK2, and AC074212.3 [30]. Jansen and colleagues examined genes located within ± 10 kb of the lead SNPs for eQTL gene mapping, and identified CLU, HLA-DRB5, HLA-DRB1, HLA-DQA, HLA-DQB1, KAT8, PRSS36, ZNF232, and CEACAM19 as candidate causal genes [30]. Later, Kunkle and colleagues conducted a larger GWAS, including 21,982 cases and 41,944 controls, and identified 24 susceptibility loci, including three novel risk loci: IQCK, ADAMTS1, and WWOX [31]. For candidate gene prioritisation, they examined protein-coding genes within ± 500 kb of the lead SNP and tested their associations with eQTL datasets of AD-related tissues, e.g., monocytes and macrophages, and further identified several candidate causal genes in the ADAM10, ADAMTS1, ACE, and IQCK loci [31]. Intriguingly, a significant association between AD GWAS and microglia in a mouse-based eQTL dataset was observed, accompanied by enrichments of common variants in microglial pathways, suggesting the direct involvement of microglia in AD [30, 31]. Pathway analyses in GWAS between 2018 and 2019 all implicated amyloid and tau processing, endocytosis (lipid metabolism), and immunity [29–31]. In 2021, Schwartzentruber and colleagues meta-analysed a GWAX dataset from the UKB and the stage I dataset of Kunkle’s study, including 53,042 AD proxy cases, 21,982 AD cases and 397,844 controls, and identified 37 risk loci, including 4 novel associations near CCDC6, TSPAN14, NCK2, and SPRED2 loci [32]. Given the importance of immunity and microglia implicated by previous GWAS, this study first employed a microglial eQTL dataset of human and successfully identified 80 distinct genes at 27 loci, expression of which might be altered by AD-associated risk variants in microglia [32]. Their integrative analysis generated a list of candidate causal genes, including newly discovered CCDC6, TSPAN14, NCK2, and SPRED2, and previously discovered BIN1, APH1B, PTK2B, PILRA, CASS4, ABCA7, SORL1, PICALM, SPI1, and CR1 [32]. Pathways related to endocytosis, immune responses, phagocytosis, and complement cascade were strongly implicated by gene enrichment analysis [32].
Strikingly, a massive GWAS/GWAX study in 2020 reported 75 AD risk loci, including 42 novel loci, by meta-analysing samples from the UKB and numerous European GWAS consortia, including 39,106 AD cases, 46,828 proxy cases, and 401,577 controls [33]. Their pathway analyses implicated the established AD functional pathways, including pathways related to amyloid and tau, endocytosis (lipid metabolism), and immunity [33]. The subsequent single-cell expression enrichment analysis solely suggested a significant relationship between microglial expressions and AD neuropathology [33]. OTULIN, SHARPIN, RHOH, BLNK, SIGLEC11, LILRB2, and GRN were exclusively expressed on microglia, while JAZF1, TSPAN14, NCK2, and RASGEF1C were primarily expressed on microglia [33]. More recently, the largest GWAS/GWAX to date identified another seven novel AD susceptibility loci by recruiting 90,338 (46,613 proxy) cases and 1,036,225 (318,246 proxy) controls from 13 cohorts: AGRN, FHL2, TNIP1, HAVCR2, TEME106B, GRN, and NTN5 [34]. By using tissue type and cell type enrichment analysis, this study demonstrated that human microglia were the only cell type significantly associated with AD [34]. The subsequent gene set analysis, chromatin enrichment analysis, eQTL enrichment analysis, and functional consequence enrichment analysis identified microglial pathways, amyloid and tau aggregation, and immunity as AD-associated functional pathways [34].
Overall, over 130 loci have been identified by large-scale AD GWAS and AD-associated variants are particularly enriched in APP processing, amyloid and tau aggregation, lipid and cholesterol metabolism, endocytosis, innate immune responses, and neuronal-synaptic signalling pathways in AD pathogenesis (Table 1; Supplementary Table 2). By breaking up these biological pathways at the cellular and molecular level, endocytosis, phagocytosis, and autophagy are the biological processes commonly shared by these AD-associated pathways. The myeloid-related innate immune pathways are argued to play a central role in AD pathogenesis and microglia in the brain are regarded as one of the key players [28, 32, 33].
Table 1.
Microglial genes and pathways contributing to AD risk implicated by AD genomics
| Gene | Protein description | Genetic link to AD | Type of variants | Microglial-related functions | References |
|---|---|---|---|---|---|
| APOE | Apolipoprotein; chaperone of Aβ | 3 Isomers | GWAS SNP | Chaperone Aβ and lipids; endocytosis; phagocytosis | Poirier (1993) |
| CLU | Clusterin; apolipoprotein; chaperone of Aβ | rs11787077; rs1532278; rs28834970; rs4236673; rs9331896 | GWAS SNP | Chaperone Aβ and lipids; inflammation; phagocytosis | Lambert (2013); Marioni (2018); Jansen (2019); Kunkle (2019) |
| I275T in exon5; R447W in exon8 | Rare variant | Zhang (2020) | |||
| TREM2 | Microglial PRR; binds apoptotic cells, debris, and Aβ | rs143332484; rs187370608; rs7748513 | GWAS SNP | ITAM signalling; actin cytoskeletal rearrangement; phagocytosis | Jansen (2019); Schwartzentruber (2021); Salih (2019) |
| R47H, R62H, H157Y, D87N, E151K, etc | GWAS SNP; rare variants | Jansen (2019); Kunkle (2019); Jonsson (2013); Guerrerio (2013); Sims (2017); Jiang (2016); Jin (2014); Jin (2015); Sirkis (2016); Zhang (2020) | |||
| SYK | Tyrosine kinases | – | Prioritised gene | TREM2 signalling; phagocytosis | Sierksma (2020) |
| LYN | Tyrosine kinases | – | Prioritised gene | TREM2 signalling; phagocytosis | Sierksma (2020) |
| INPP5D | Encode SHIP-1, a SH2-containing phosphatase | rs10933431; rs35349669; rs7421448 | GWAS SNP; prioritised gene | Bind to DAP12 to inhibit phagocytosis | Schwartzentruber (2021); Salih (2019) |
| PLCG2 | Encode PLC-γ2, a phospholipase | rs12444183; rs12446759 | GWAS SNP | TREM2 signalling; phagocytosis | Marioni (2018); Schwartzentruber (2021) |
| rs72824905 (P522R) | Rare variant | Sims (2017) | |||
| BLNK | SH2-containing protein | rs6584063 | GWAS SNP | Downstream TREM2 signalling | Sierksma (2020) |
| MS4A gene cluster | Transmembrane cell surface receptor | rs7933202; rs1582763; rs72924626; rs1582763; rs2081545; rs7935829; rs983392; rs636317 | GWAS SNP; prioritised gene | Intracellular protein trafficking; phagocytosis | Lambert (2013); Marioni (2018); Jansen (2019); Kunkle (2019); Schwartzentruber (2021); Novikova (2021); Salih (2019) |
| rs185080144 (A69V) | Rare variant | Zhang (2020) | |||
| ADAM10 | Metalloproteinase | rs442495; rs593742 | GWAS SNP | TREM2 shedding | Marioni (2018); Jansen (2019); Kunkle (2019); Schwartzentruber (2021) |
| CD33 | Siglec PRR that binds to sialic acids | rs3865444 | GWAS SNP | ITIM signalling; phagocytosis inhibition | Lambert (2013); Marioni (2018); Jansen (2019); Schwartzentruber (2021); Salih (2019) |
| E85V in exon2; I71M in exon2; T141A in exon 4 | Rare variants | Zhang (2020) | |||
| CLNK | SH2-containing adapter protein | rs4351014; rs6448451; rs6448453; rs6846529 | GWAS SNP | Phagocytosis | Jansen (2019); Schwartzentruber (2021) |
| SIGLEC11 | Siglec cell surface PRR | rs9304690 | GWAS SNP | ITIM signalling; | Schwartzentruber (2021) |
| LILRB4 | ITIM-containing transmembrane receptor | rs731170 | Prioritised gene | Putatively involved in ITIM signalling | Salih (2019) |
| CR1 | Complement receptor for C3b/C4b and C1q | rs2093760; rs4844610; rs6656401; rs679515 | GWAS SNP | Complement system cascade; mediate phagocytosis | Lambert (2013); Marioni (2018); Jansen (2019); Kunkle (2019); Schwartzentruber (2021) |
| chr1:207461994C>T | Somatic mutation | Helgadottir (2019) | |||
| TLR4 | Toll-like receptor | rs4986790 | Common variant | TLR signalling; mediate phagocytosis | Miron (2019) |
| SCIMP | Non-TIR-containing TLR adaptor protein | rs113260531; rs57402520; rs61182333; rs9916042 | GWAS SNP | TLR signalling to selectively produce IL-6 and IL12p; mediate phagocytosis | Jansen (2019); Schwartzentruber (2021) |
| BCL3 | Transcriptional coactivator | rs10401176; rs2927468; rs8103315 | GWAS SNP | NF-κB activation | Marioni (2018) |
| SHARPIN | Subunit of LUBAC regulating NF-κB and MAPK signalling | rs34173062 | GWAS SNP | NF-κB and MAPK activation | Schwartzentruber (2021) |
| ITGAM | Encode integrin CD11b; | rs79113991 | Prioritised gene | Integrin for focal adhesion | Salih (2019) |
| PTK2B | Protein kinase | rs1532278; rs28834970; rs34181358; rs4236673 | GWAS SNP | Focal adhesion; actin cytoskeletal rearrangement; phagocytosis | Lambert (2013); Marioni (2018); Jansen (2019) |
| chr8.27316070C>A | Somatic mutation | Helgadottir (2019) | |||
| CASS4 | Scaffolding protein | rs6014724; rs6024870; rs7274581 | GWAS SNP | Focal adhesion; cytoskeletal rearrangement | Lambert (2013); Jansen (2019); Kunkle (2019); Schwartzentruber (2021) |
| ABI3 | Actin binding protein | rs616338 (S209F) | Rare coding variant | Focal adhesion; actin polymerisation | Sims (2017) |
| CD2AP | Scaffolding protein co-localising with F-actin | rs10948363; rs1385742; rs7767350; rs9381563; rs9473117 | GWAS SNP; prioritised gene | Focal adhesion; pseudopod and phagosome formation; phagocytosis | Lambert (2013); Marioni (2018); Jansen (2019); Kunkle (2019); Schwartzentruber (2021); Novikova (2021) |
| ZYX/EPHA1 | Focal adhesion protein | rs10808026; rs11763230; rs11771145; rs12703526; rs7810606; rs35251323; rs9640386 | GWAS SNP; prioritised gene | Actin cytoskeletal rearrangement; focal adhesion | Lambert (2013); Marioni (2018); Jansen (2019); Kunkle (2019); Schwartzentruber (2021); Novikova (2021) |
| FERMT2 | Component of extracellular matrix structures | rs17125924 | GWAS SNP | Actin polymerisation; focal adhesion | Lambert (2013); Marioni (2018); Kunkle (2019); Schwartzentruber (2021) |
| H579D in exon14 | Rare variant | Zhang (2020) | |||
| BIN1 | Early endocytic protein | rs12989701; rs17014873; rs4663105; rs6733839 | GWAS SNP | Endo-lysosomal trafficking; phagocytosis | Lambert (2013); Marioni (2018); Jansen (2019); Kunkle (2019); Schwartzentruber (2021); Novikova (2021) |
| rs141119288 (P431L in exon15); rs117721706 (R263Q in exon10) | Rare variant | Zhang (2020) | |||
| rs61748157; chr2.128054946G>T | Somatic mutation | Parcerisas (2014); Helgadottir (2019) | |||
| RIN3/SLC24A4 | Early endocytic protein | rs12590654; rs10498633; rs12881735 | GWAS SNP | Recruit BIN1, CD2AP; endo-lysosomal trafficking; phagocytosis | Lambert (2013); Marioni 2018; Jansen (2019); Kunkle (2019); Schwartzentruber (2021) |
| RABEP1 | Rab-GTPase binding effector protein | rs7225151 | GWAS SNP | Endosome trafficking and fusion | Lambert (2013); Marioni 2018 |
| RAB10 | Rab superfamily member | rs142787485 | Rare variant | Endosomal trafficking and fusion | Ridge 2017 |
| PICALM | Endocytic adaptor | rs10792832; rs3844143; rs3851179; rs867611 | GWAS SNP | Endo-lysosomal trafficking; phagocytosis | Lambert (2013); Marioni 2018; Jansen (2019); Kunkle (2019); Schwartzentruber (2021) |
| SORL1 | Endosomal trafficking receptor | rs11218343; rs74685827 | GWAS SNP | Endo-lysosomal network; lipid metabolism; intracellular sorting via retromer pathway | Lambert (2013); Marioni 2018; Jansen (2019); Kunkle (2019); Schwartzentruber (2021) |
| Many rare variants | Zhang (2020); Campion (2019) | ||||
| Several somatic mutations | Nicolas 2018; Keogh 2018; Helgadottir (2019) | ||||
| APBB3 | Intracellular APP binding protein | – | Prioritised gene | Internalisation of APP; unclear in microglia | Novikova (2021) |
| SPPL2A | Aspartyl protease in late endosomes and lysosomes | rs10467994; rs12592778; rs59685680; rs8025980; rs8035452 | GWAS SNP | Intramembrane proteolysis of TNFα and CD74; unclear in microglia | Lambert (2013); Marioni 2018; Kunkle (2019); Schwartzentruber (2021) |
| HEXB | Lysosomal enzyme | – | Prioritised gene | Lysosomal function in microglia | Sierksma (2020) |
| LAPTM5 | Lysosomal protein | – | Prioritised gene | Activation of cysteine-type endopeptidase activity in lysosomes; unclear in microglia | Salih (2019) |
| GRN | Lysosomal protein | rs5848 | GWAS SNP | Lysosomal degradation | Schwartzentruber (2021) |
| KAT8 | Histone acetyltransferases | rs59735493; rs889555; rs2884738; | GWAS SNP | Reduce IRF3 transcriptions; suppress innate immunity | Marioni 2018; Jansen (2019); Schwartzentruber (2021) |
| RELB | Transcription factor | rs117612135; rs187270432 | GWAS SNP | Transcriptional activation of lymphokines | Marioni 2018 |
| SPI1 | Transcription factor PU.1 | rs1057233 | Common variant | Transcription factor regulating many microglial-specific gene expressions | Huang 2017; Sierksma (2020); Pimenova (2021); Novikova (2021) |
| HLA cluster | Human leukocyte antigen | rs36096565; rs6605556; rs6931277; rs9269853; rs9469112; rs9271058 | GWAS SNP | Antigen presentation to T cells; adaptive immune responses | Jansen (2019); Kunkle (2019); Schwartzentruber (2021); Sierksma (2020); Salih (2019) |
APOE stratified GWAS
Given that APOE genotype is the single largest genetic risk factor for LOAD, it has been hypothesised that APOE ε4 might modulate the expression of AD variants (epistasis), making them precluded in GWAS. Jun and colleagues re-analysed the GWAS study in 2013 [26] by stratifying the cohort into APOE ε4+ (10,352 cases and 9,207 controls) and APOE ε4− (7184 cases and 26,968 controls) sub-populations [35]. In the APOE ε4− cohort, 17 novel AD-associated SNPs near the MAPT locus was observed, which were associated with tau protein [35]. The downstream eQTL test illustrated that the SNP rs113986870 was associated with the transcription of KANSL1 and MAPT exons, suggesting that the regulation of KANSL1 and MAPT splicing event might modulate AD risk independently of APOE genotype [35]. Regarding the established AD susceptibility loci, the association signals in CR1, CLU, and PICALM loci were stronger in APOE ε4+ cohort, while those in MS4A region were stronger in APOE ε4− cohort [35]. More recently, Kang and colleagues analysed genotyping data of 2,291 Korean individuals (1119 AD cases and 1172 controls) and 1956 Japanese individuals (980 AD and 976 controls) in an APOE ε4-stratified manner to identify AD risk variants [36]. They reported three novel lead SNPs in LRIG1 and CACNA1A genes in APOE ε4− subpopulation, both of which were mainly expressed in the brain and involved in neurobiological pathways [36]. The association of SNPs in the established AD susceptibility loci, including APOE, PVRL2, TOMM40, ABCA7, and BIN1, were also replicated in the traditional GWAS analysis [36]. This study not only demonstrated the possible involvement of LRIG1 and CACNA1A genes in AD progression independent of APOE genotype but further proved that the investigation into non-European cohorts could provide novel insights into AD genetics.
Limitations and future evolution of GWAS
Although GWAS have revealed critical insights into AD genetics, there are still many underlying issues to be considered. Technically, case–control GWAS recruits clinically-confirmed AD cases and CN controls, but only 70–80% of AD cases were accurately diagnosed by laboratory biomarkers and around 30% of CN controls were preclinical AD cases. The case cohort of GWAX included AD proxy cases that relied on the self-report of their family members, so that the case cohort was contaminated by other types of dementia. These contaminated cohorts might result in the discovery of unreliable lead SNPs and the masking of the AD-associated SNPs. Moreover, recent GWAS were not independent because of the extensive overlapping in their participants and therefore the subsequent characterisation of susceptibility loci might be biased [18]. The UKB is the common dataset shared by all GWAX and a homogeneous population of European ancestry has been recruited, implying that population stratification may be a confounding factor that may result in the discovery of European-specific AD-associated SNPs [18]. Non-European GWAS of AD have identified novel genetic variants [12, 18, 36]. Ethnic diversity across populations confirms the established AD loci and offers the opportunity to discover novel genetic variants or the underlying molecular pathways because of differences in LD structure. Of note, differences in LD structure and allele frequencies are computational obstacles to multi-ethnic GWAS, and more comprehensive statistical models are required to address these limitations [18].
Another limitation is that GWAS is incapable of identifying the causal variant of AD. Early GWAS annotated susceptibility loci as the nearest genes to the lead SNPs, but different lead SNPs in the same locus can be annotated to different nearest genes by different studies [18]. Most lead SNPs identified in GWAS are located in non-coding regions and these variants are likely to regulate the expression of nearby or distant genes to confer disease risk, so the annotation of lead SNPs to the nearest genes may not be accurate. The implicated genes of lead SNPs may be up to 2Mbps away, which could potentially make some lead SNPs unmapped or incorrectly mapped because the upstream and downstream cut-offs of widely-used SNP databases, e.g., dbSNP, are lower than 2Mbps [37]. Many statistical methods have been used in recent GWAS to address this difficulty. Almost all large-scale GWAS have used eQTL association test between GWAS variants and gene expression levels (mRNA transcript or protein) in various AD-related tissue types, e.g. brain and blood, to prioritise the candidate causal genes in susceptibility loci [29–33]. These studies have further used methylation QTL, histone acetylation QTL, splicing QTL, and chromatin interaction mapping to identify the candidate genes involved in the interaction between SNPs and nearby or distant genes [30, 33]. However, current studies only consider the genomic range of at most 1Mbps flanking the lead SNPs using these fine-mapping methodologies, therefore further improvement is required for GWAS SNP-gene mapping. Furthermore, the investigation into the biological implication of the lead SNPs becomes a critical downstream analysis of GWAS data, such as pathway analysis/gene set analysis using MAGMA [30, 31, 33]. MAGMA can analyse multiple GWAS SNPs simultaneously and provide novel insights into the underlying functional and biological pathways. By using this technique, researchers have identified the involvement of the immune pathways, endocytosis, cholesterol transport, and amyloid and tau processing in AD pathogenesis.
Given the importance of myeloid cells (microglia) and immunity in AD, cell type-specific analysis becomes necessary downstream GWAS. The lack of sophisticated microglial datasets for QTL analysis was an ongoing problem until 2019, during which a transcriptional profile of human microglia was first used in AD GWAS eQTL association test, affirming that previously reported immune-related genes were highly expressed in microglia and SNPs were significantly enriched in microglial regulatory pathways [32]. This study further explored peaks of Assay for Transposase-Accessible Chromatin using sequencing (ATAC-seq) from microglia to elucidate how chromatin accessibility affected microglial gene expression, contributing to a deeper understanding of BIN1, TSPOAP1, and CASS4 in microglial gene regulation in AD [32]. Since most GWAS SNPs are located in non-coding regions, Nott and colleagues combined ATAC-seq, combining chromatin immunoprecipitation sequencing (ChIP-seq), and proximity ligation-assisted ChIP-seq (PLAC-seq) to study the non-coding regulatory regions in microglia, neurons, and oligodendrocytes from AD patients, which sketched an extended microglia-specific enhancer-promoter network in AD [38]. For instance, the XYZ (EPHA1), CLU, PICALM, MS4A, ABCA7, CR1, and BIN1 loci had microglia-specific enhancers harbouring AD risk variants [38]. The CR1, BIN1, TREM2, CLU, SPI1, PICALM, SORL1, MS4A, ABCA7, HLA-DRB1, KAT8, etc. loci contained promoters that were PLAC-linked to other AD risk SNPs [38]. These findings suggested long-range chromatin interactions between these loci and other GWAS SNPs and will be discussed in detail in the following section “Neuro-immune pathway involvements in AD”. These studies highlight the importance of cell type-specific analyses in polygenic disease, such as microglia in AD, and further demonstrate a useful methodology to study the regulatory roles of AD risk SNPs that reside in non-coding regions. Overall, it seems doubtful that GWAS will reach its full potential regarding discovering common variants associated with AD. The ongoing whole-exome sequencing (WES) and whole-genome sequencing (WGS) studies are likely to identify novel variants that are not detectable by GWAS.
Polygenic risk score (PRS) in AD
AD GWAS have recognised over 130 AD susceptibility loci, of which AD-associated genes are enriched in endocytosis and innate immune pathways. Given that all AD-associated genes other than the APOE gene have small effect sizes, the combination of many genetic variants across the whole genome is likely to determine an individual’s risk for AD. A PRS is an estimate of an individual’s genetic liability to a phenotype, e.g., AD, which is calculated as a sum of their genome-wide genotypes and weighted by the effect size estimates of these genotypes derived from GWAS summary statistic data [39]. The GWAS dataset analysed by Lambert and colleagues had been used as the reference summary statistic data by multiple PRS studies [26]. Escott-Price and colleagues developed a PRS model, including 20 GWAS SNPs identified before 2014 and the APOE genotype, the best prediction accuracy of which was area under curve (AUC) of 74.5% [40]. A similar PRS model containing 25 SNPs identified in the Lambert study was also reported to predict the age-specific risk for developing AD [41]. More recently, Daunt and colleagues studied the WGS data of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset and extracted 114,000 SNPs into their PRS algorithm [42]. They observed a clear association between PRS score, tau level, Aβ1-42 level, a cognitive decline from mild cognitive impairment (MCI) baseline, and clinical classification [42]. Rather than using PRS to simply differentiate between AD and CN, this study illustrated its potential capacity to identify individuals at high risk of cognition decline, further suggesting its possible application in a clinical setting. Furthermore, a more recent study alternatively used the GWAS summary statistic data from the Kunkle’s study, which is a larger GWAS than the Lambert’s study, to study the ADNI dataset [43]. Their PRS model including APOE was significantly associated with plasma tau-181 regardless of disease diagnosis or Aβ burden, while the PRS model excluding APOE was only associated with plasma tau-181 in Aβ+ and MCI participants [43]. Stocker and colleagues also used the summary statistics from Kunkle’s study and extracted 72 SNPs into their PRS model [44]. They stratified their PRS model by the APOE genotype and tested it against the basic model consisting of age, sex, and education [44]. The AUC of PRS with or without APOE was greater than that of the basic model (0.812, 0.810, and 0.772, respectively) [44]. PRS+ and APOE-ε4 carriers were 4.6-fold more likely to receive AD diagnosis within 17 years compared with PRS− and non-APOE-ε4 participants [44].
The investigations into AD PRS so far have demonstrated many benefits. While some studies simply combined SNPs with established association with AD into their PRS model, some studies expanded to SNPs that did not reach genome-wide significance in GWAS [40, 42]. The latter strategy is likely to capture genetic variants in loci where the specific functional variants remain unknown but causal to AD, and, therefore, contribute to a more comprehensive understanding of AD genetics. Additionally, one PRS study used a PRS model derived from GWAS to assess disease risk in familial EOAD, sporadic EOAD, familial LOAD, and sporadic LOAD [45]. This PRS model could predict disease risk and age of onset in both familial LOAD and sporadic EOAD cohorts, strengthening the notion that EOAD and LOAD share genetic risk factors [45]. Current PRS models have shown their capability to predict disease risk, age of onset, cognition decline, and biomarker changes, implying their promising role in a clinical setting and stratified medicine. Although it is a robust method, current studies are limited by their small sample sizes, vague LD between common variants and rare variants, and genetic differences in different ethnic populations. For instance, Zhou and colleagues showed that PRS model derived from GWAS were able to predict individual’s risk and cognitive decline in Hong Kong Chinese cohorts, but genetic differences still existed [46]. Besides expanding sample size to confirm PRS findings, future PRS models are expected to use the most recent large GWAS as the summary statistics and further consider the regulatory and post-translational impact of SNPs in non-coding regions, as hinted by eQTL, methylation QTL, histone acetylation QTL, splicing QTL tests.
Rare variants in LOAD
Well-established rare variants in TREM2, SORL1 and ABCA7
Traditional GWAS capture common genetic variants by direct assay or by genotype imputation using high-density reference panels, but enrichments of rare variants (MAF<1%) were observed in AD-relevant genes and pathways, which cannot be accurately detected by GWAS [31]. Kunkle and Jansen workgroups used genotype imputation methods, such as the Haplotype Reference Consortium, to address this limitation [30, 31], but the best way is by whole-exome/genome arrays, WES, or WGS. One of the most well-established rare genetic variants associated with elevated LOAD risk is R47H in the TREM2 gene. This rare variant was first reported in 2013, the effect size of which was as high as APOE ε4 while the MAF of which was much lower [47, 48]. The association of R47H with elevated LOAD risk was successfully replicated in European-American, Spanish, French-Caucasian, North American-Caucasian and African-American populations, but failed in a Han Chinese population [49]. R62H is another AD-associated rare variant in the TREM2 gene [50]. Sims and colleagues used whole-exome microarray and confirmed the strong associations between R47H and R62H and increased LOAD risks, while they also observed novel AD rare variants, including a protective variant in PLCG2 (P522R) and a risk variant in ABI3 (S209F) [51]. Compared with CN controls, a significant enrichment of rare variants in TREM2 has been observed in AD patients [52]. The combined analysis of multiple rare variants, such as the sequence kernel association test, offers the advantage of testing the association between multiple variants in a region with AD over the traditional single-marker association analysis [52]. The advances of sequencing techniques and analytic tool have led to the discovery of more TREM2 rare variants, including L211P, W191X, S31F, R47C, R136Q, H157Y, and S183C [52–54].
Besides TREM2, rare genetic variants in SORL1 and ABCA7 genes are also associated with LOAD risks. In 2007, six rare SNPs clustered in SORL1 locus were identified, in which variants in the 5′ cluster protected against AD while those in the 3′ cluster conferred LOAD risk [55]. Since then, more putative pathogenic rare variants in SORL1 have been identified in both LOAD case–control studies and EOAD family-based studies [56]. Furthermore, a significant enrichment of rare variants across the full ABCA7 locus in LOAD patients, compared with CN controls, has also been reported, including frameshift variants, nonsense variants, and variants resulting in out-of-frame splicing [57]. To study the effects of multiple ABCA7 rare variants, C-alpha tests have been introduced to analyse, collapse, and determine their effects and effect size, leading to the identification of a rare protective variant, G215S, associated with AD [58]. Meanwhile, common variants within SORL1 and ABCA7 loci were identified by GWAS [26, 29–31], suggesting their participation in both EOAD and LOAD.
Additional rare variants identified by WES and WGS
Early small-scale studies employed cost-effective approaches, such as targeted sequencing, to identify rare variants in established AD-related genes. To uncover rare variants in novel loci, more recent studies have invested significant financial and human resources to perform WES or WGS. The Alzheimer’s Disease Sequencing Project (ADSP) aimed at conducting a WGS family-based study and a WES case–control study of over 30,000 samples and they have already sequenced 600 patients in the family-based cohort and 11,000 individuals in the case–control cohort [59]. By analysing the ADSP dataset, a rare SORL1 loss-of-function (LOF) variant, TREM2 R47H, a common variant in PILRA, and a novel rare variant in the long non-coding RNA AC099552.4 were identified [60, 61]. At gene level, OPRL1 and GAS2L2 were significantly associated with AD and ZNF655 trended toward an association, implicating transcriptional regulation [61]. Another study recruited 7,252 non-APOE ε4 carriers from WES dataset of ADSP and revealed a novel variant in the NSF gene associated with AD [62]. In addition, weighted burden analysis was applied to over 10,000 WES subjects from ADSP and consolidated the participation of rare variants in TREM2, ABCA7, SORL1, and PSEN1 in AD [63]. PIK3R1, WNT7A, C1R, and EXOC5 were highly likely to harbour detrimental rare variants, while TIAF1 and NDRG2 might contain protective rare variants [63]. These rare variants were significantly enriched in the PI3K/Akt signalling pathway that was tightly associated with TREM2 (discussed below) [63]. The largest exome analysis to date sequenced over 25,000 samples from the ADSP cohort and a European consortium, which confirmed the established rare variants in TREM2, SORL1, and ABCA7 loci and identified novel rare variants in microglial gene ATP8B4 [64]. Additional deleterious rare variants in ADAM10, ABCA1, ORC6, PRSS3, B3GNT4, and possibly protective variants in CBX3 and SRC genes were also reported to show suggestive association with AD [64].
Besides WES, WGS is important for identifying rare variants in non-coding regions. By using the WGS data of ADSP dataset, rare variants in ABCA1, TMEM132A, and AKAP9 were reported to segregate with AD in families, in which AKAP9 was nominally associated with LOAD risk [65, 66]. These rare-variant association studies further identified rare variants in CR1, BIN1, FERMT2, and SLC24A4, in line with GWAS findings [65, 66]. Recently, a family-based WGS association study (partially from ADSP) identified 13 novel AD candidate loci, implicating FNBP1L, SEL1L, LINC00298, PRKCH, C15ORF41, C2CD3, KIF2A, APC, LHX9, NALCN, CTNNA2, SYTL3, and CLSTN2 genes [67]. Unlike previous studies that predominantly implicated innate immunity and Aβ clearance, these rare variants were significantly in enriched in neuroplastic, synaptic, and neurodevelopmental pathways [67]. These studies highlight the necessity of WGS regarding the identification of genetic variants in non-coding regions and in underestimated pathways underlying AD pathogenesis. Collectively, AD-associated genes identified by WES and WGS implicated APP metabolism, endocytosis, phagocytosis, and innate immune responses, which confirmed GWAS findings and provided novel insights into AD-related biological pathways. Further replication of these novel findings in independent cohorts is required because some variants are extremely rare.
Beyond inherited genetics: somatic mutations in LOAD
Somatic mutations and aging
Human genetic studies have now identified over 130 AD susceptibility loci, of which associated genes are enriched in endocytosis, phagocytosis, and innate immune pathways, but these germline variations cannot fully account for disease risks. The traditional notion of invariant genome throughout the lifespan has been overturned by genetic mosaicism [68, 69]. Mosaicism is defined as the presence of non-inherited post-zygotic mutations in genetically different cells within a single organism [70]. Their contribution to neurodegenerative diseases has long been hypothesized due to the complex interplay between somatic mutations and aging. Aging is accompanied by the accumulation of harmful biological alterations over time, in which DNA damage and DNA repair play an important role [71]. DNA damage may be caused by the exposure to reactive oxygen species (ROS), toxic agents, and ionising radiation, as well as by being generated by compromised DNA repair mechanisms. The imbalanced production and elimination of ROS results in oxidative stress that induces nuclear oxidative damage in preclinical AD, MCI, and AD brains, including oxidised DNA lesions, single-strand breaks (SSB), and double-strand breaks (DSB) [72]. Cells activate DNA damage response to detect and repair DNA damage. However, the levels of DNA repair enzymes have been reported to be abnormal, while genetic polymorphisms in these enzyme-encoding genes have been reported to compromise DNA repair in the CNS and periphery of AD patients [72]. Compromised DNA repair may un-repair or erroneously repair DNA damage, resulting in the loss of genetic stability and the emergence of somatic mutations [71], including single nucleotide variants (SNV), insertions and deletions (INDEL), copy number variants (CNV), structural variants, repeat expansions, insertions of transposable elements, and aneuploidy [68, 69]. Somatic mutations may be carried by post-mitotic neurons and may be expanded by replicative myeloid cells in the CNS and periphery.
Somatic mutations in healthy aging brains
An understanding of the prevalence and pattern of somatic mutations in “normal” aging brains will help set a baseline for the interpretation of somatic mutations in AD brains. SNVs are the most common type of somatic mutations in normal aging brains [73]. A deep single-cell WGS study identified roughly 1,500 somatic SNVs (80% C>T transitions) in neurons from the cerebral cortex of normal aging individuals, which were often caused by erroneous DNA replication [74]. Somatic SNVs also increased with age in neurons from prefrontal cortex (PFC) and hippocampus of normal aging individuals [75]. The concept of “mutational signature”, which is widely used in cancer genetics and identifies the related biochemical processes, has been examined in AD studies [75]. C>T and T>C mutations formed “signature A” that was positively associated with age regardless of brain region or disease status, which resembled a “clocklike” signature found in cancer genomics and may reflect a universal genomic aging mechanism [75]. C>T mutations alone formed “signature B” that did not correlate with age and were located predominantly in the hippocampal dentate gyrus [75]. “Signature C” solely consisted of C>A variants that were closely associated with oxidative DNA damage and were moderately associated with age [75]. These three mutational signatures behaved slightly differently in a transcriptomic study: somatic C>T and T>C mutations increased with age only in hypothalamus and basal ganglia respectively; somatic C>A mutations were significantly associated with age only in putamen and caudate basal ganglia rather than in cortex [76]. These studies revealed somatic SNV accumulation with age in normal human brains and the three mutational signatures suggested a valid role of somatic mutations in aging and neurological disorders. Some differences of the mutational pattern of SNVs in neurons of different brain regions still exist and more studies are needed to establish a constant pattern of somatic SNVs.
The landscape of somatic mutations in AD
Somatic mutations in EOAD-related genes
Somatic mutations in EOAD genes are of great interest. The first striking discovery of somatic and germline mosaicism in AD was reported by Beck in 2004 [77]. The mother (an EOAD case) was negative for PSEN1 mutations in DNA from peripheral lymphocytes, but the PSEN1 missense variant P436Q was detected in DNA from her cerebral cortex [77]. One of her daughters inherited the mutant haplotype and presented with dementia at the age of 27, which was much earlier than the age of disease onset of the mother [77]. This demonstrated a gene dosage effect on the age of disease onset and phenotypic severity [68, 77]. Later, somatic mutations in APP, PSEN1, and PSEN2 loci were reported [78–80]. These early studies confirmed the existence of somatic mutations at EOAD loci, which might contribute to the heterogenous genetic landscape in AD patients, but how these variants modulated AD remained to be determined. Recently, a novel mechanism for acquired genetic variability of APP was proposed [81]. APP mRNA from somatic cells was reverse-transcribed into genomic complementary DNA (cDNA) and missing introns; novel inter-exonic junctions, SNVs, and INDELs were found in the cDNA that was incorporated back into the genome [81]. Neurons from LOAD patients showed increased cDNA diversity, including 11 pathogenic SNVs associated with AD risk [81]. The brain genome was more plastic than formally thought.
Somatic studies in AD brain and blood
More studies have systemically investigated somatic mutations in LOAD patients by using WES and deep targeted sequencing. Early exome studies of AD have identified AD brain-specific somatic mutations in LRP2, LRP1B, RYR2, PION, SLC6A20, and SEMA5B genes, but their questionable methodologies made it doubtful whether these somatic mutations were involved in AD pathogenesis [82, 83]. More recently, a better study performed deep WES (read depth of 584-fold) of DNA from laser capture micro-dissected regions of hippocampus and from the matched blood tissues of 52 AD cases and 11 controls to study somatic SNVs (variant allele frequency [VAF]<5%) [84]. The burden of somatic SNVs was five times higher in blood than in hippocampus regardless of AD diagnosis, suggesting probable different acquired mutational processes underlying hippocampus and blood [84]. Two age-related mutational signatures (C>T, T>C) were the commonest in both hippocampus and blood, while the signature of oxidative DNA damages (C>A), accounting for 22% of brain somatic SNVs, were only significant in AD brains, indicating a remarkable role of oxidative stress in AD brain [75]. This study identified 175 hippocampal-specific, rare, pathogenic, somatic SNVs that were significantly enriched for the PI3K/Akt pathway, MAPK pathway, and AMP-activated protein kinase pathway [84]. Twenty-eight genes possessing putative pathogenic SNVs were directly associated with tau phosphorylation and PIN1 was the most promising candidate gene, followed by PLCG1 and LRP2 [84]. It was estimated that 0.53 somatic SNVs (VAF>0.52%) in neurons from hippocampus accumulated per exome per year and this rate was five times greater in blood [84].
Given that protein-coding regions only account for 1% of the human genome, many somatic mutations may reside in non-coding regions. Keogh and colleagues performed targeted sequencing (> 5,000-fold) of postmortem brains from 20 AD cases and 14 controls [85]. This study also concluded that that AD brain-specific somatic mutations were mainly explained by C>T transitions, which was consistent with previous findings [75, 85]. They detected a wide range of SNV-harbouring genes, including DNMT3A, TET2, EIF4G1, LRRK2, NOTCH3, SETX, SORL1, TAF15, UCHL1, and VPS35, implying blood cell lineages [85]. This study was the first report of the somatic mutational landscape across different brain regions, but a larger clean cohort excluding other forms of dementia is required to validate the findings. Another study applied targeted sequencing (~ 1027-fold) of 11 Aβ-related genes and identified nine candidate somatic mutations (VAF>0.2%) in APP, SORL1, NCSTN, and MARK4 genes [86]. This study failed to identify damaging somatic mutations in EOAD genes, indicating that EOAD-related somatic mutations may moderately contribute to LOAD [86]. More recently, another targeted sequencing study (~ 698-fold) studied 28 AD GWAS genes from the brain and blood of EOAD patients, LOAD patients, and controls [87]. They identified 11 candidate AD brain-specific somatic SNVs in CR1, PSEN2, BIN1, CLU, SORL1, and APP locus (VAF 0.7–2.6%) [87]. Only one LOAD brain-specific somatic mutation in CR1 region was validated, located in the regulatory region of the gene encoding CD55, potentially contributing to LOAD pathogenesis by inhibiting the complement pathway [87].
The rationale of somatic mutations in AD
The human genome is under constant threat by external and endogenous mutagens and genome maintenance can be imperfect, leading to the emergence and accumulation of somatic mutations during aging. During normal aging, 23 and 40 SNVs might accumulate in PFC and hippocampus respectively per genome per year [75, 88]. In LOAD, ~ 22 SNVs are estimated to accumulate in hippocampal neurons per exome per year, which is much higher than that in normal aging because the exome covers approximately 1% of the whole genome [84]. The accumulation rate of somatic mutations is tightly associated with age in all populations, but the contribution of oxidative DNA damage (C>A transitions) is significantly remarkable in the LOAD brains [75, 84]. Therefore, in contrast to normal aging, a distinct, designated genetic program may drive the process of genetic mosaicism in LOAD. The existence of somatic mutations in LOAD brains have been identified in established AD susceptibility loci or neurodegeneration-associated loci. The widespread accumulation of somatic mutations in AD-associated loci may predispose an individual to AD development. Additionally, there is an increased tendency of LOAD patients to accumulate somatic mutations due to increased DNA damage and impaired DNA repair [72, 84]. In both EOAD and LOAD cases, carrying somatic mutations in EOAD loci may be associated with an earlier disease onset [77, 85, 86]. On the contrary, the somatic gain of mutations in GWAS hits or unclassified loci would minimally increase disease risk, which might explain the sporadic occurrence, delayed onset, and slower spread of Aβ lesions in LOAD patients [77, 85, 86]. However, this hypothesis awaits further testing [88].
Although the existence of somatic mutations in AD brains and bloods has been reported by several preliminary studies, more replications and improvements are required to strengthen and clarify their involvement in AD pathogenesis. First, there are false-positive reads of somatic mutations due to DNA damage during cell lysis and due to DNA polymerases during amplification, which cannot be easily distinguished from naturally-occurring somatic variants. Second, many somatic mutation studies used the blood genome as the reference to call somatic mutations in the brain, which was inappropriate because the generation rate of somatic mutations in the blood may be five times higher than that in brain [84]. Another obvious limitation is the analysis of bulk DNA extracted from a specific brain region. This approach masks the potential different mutational profiles of neurons, microglia, and other glial cells in the CNS. As implicated by GWAS, the innate myeloid cells (microglia) and their biological pathways are highly associated with AD risks, so future studies should shift focus to the somatic spectrum of AD glial cells, such as microglia, which may reveal the underlying disease-causing mechanisms of AD. Meanwhile, the improvement in capturing, analytic, sequencing techniques and the recruitment of larger cohorts are expected to validate these preliminary results and to uncover the role of somatic mutations in AD pathogenesis.
Neuro-immune pathway involvements in AD
Implication for the immune system
The long, silent, preclinical phase of AD involves the abnormal aggregation and propagation of Aβ and tau, as well as the simultaneous innate responses and interactions between astrocytes, microglia, and vasculature, which maintain CNS homeostasis [10]. Mounting evidence supports that defective clearance of Aβ and tau during the cellular phase is the core (and may be initiating) contributor to LOAD [2]. Cerebral Aβ can be cleared by CSF absorption, brain-blood barrier (BBB) efflux, and enzyme or cell-mediated degradation [89]. GWAS, WES, and WGS studies have identified over 130 susceptibility loci, many of which are enriched in endocytosis, phagocytosis, and innate immune responses, e.g., BIN1, CR1, CD33, CD2AP, PICALM, CLU, TREM2, ABCA7, SORL1, ABI3, PLCG2, and RIN3 (Supplementary Table 2). They converge on microglia as the key participants in LOAD pathogenesis, suggesting the necessity of microglia/myeloid-specific cellular integrative analysis. A genome-wide survival analysis revealed the critical role of regulation of myeloid gene expression in AD, as implicated by the association between reduced SPI1 expression (rs1057233[G]) in myeloid cells and delayed onset of AD [90]. The SPI1 gene encodes PU.1, a critical transcription factor (TF) for myeloid and B cell development and function [90]. PU.1 binds to the cis-regulatory elements of many AD-associated genes in myeloid cells, including ABCA7, CD33, MS4A, PILRA, PILRB, TREM2, TREML2, and TYROBP, but not APOE [90]. An integrative analysis of GWAS and a transcriptomic profile of AD mouse models identified novel microglial-related AD risk genes, e.g., TREML2, SYK, GRN, SLC2A5, HEXB, LYN, and BLNK, all of which were upregulated in microglia via PU.1 [91]. Experimentally reduced PU.1 level attenuated the phagocytic activity of mouse microglia and reduced the expression of myeloid-associated AD risk genes, further confirming the role of transcriptional regulation of risk genes by PU.1 in AD pathogenesis [90, 92]. By integrating GWAS with myeloid epigenomic and transcriptomic datasets, more over-represented motifs in the active enhancers in myeloid cells were identified, e.g., MAF, SMAD, USF, and SP1, in which AD risk variants were specifically enriched [93]. This study incorporated these enhancer data with chromatic interactions and eQTL datasets to nominate candidate causal genes of AD, which were regulated by these AD variant-harbouring enhancers, including ZYX, TP53INP1, AP4E1, RIN3, APBB3, RABEP1, and CASS4 [93]. AD risk variants in non-coding region, particularly enhancer region, may interfere with TF binding and regulate gene expression in myeloid cells to modulate AD susceptibility and these variants implicate dysfunction of microglial and endo-lysosomal networks in LOAD.
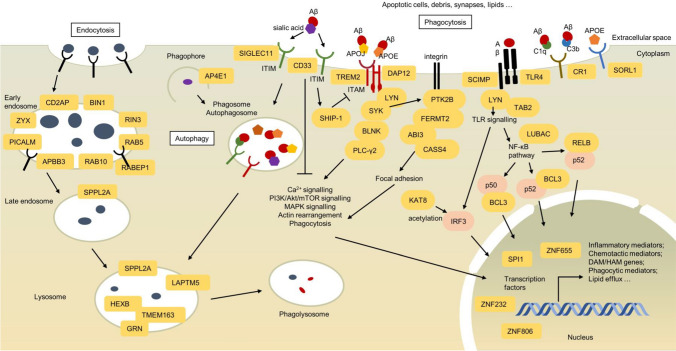
Microglia are brain-resident macrophages that act as the first-line innate immune sentinels of the brain parenchyma and perform classical macrophage functions, such as surveillance of environment, clearance of debris and invading pathogens, and maintenance of cellular homeostasis. Microglial clearance of Aβ involves several mechanisms: endocytosis, phagocytosis, micropinocytosis, enzymatic degradation, extracellular chaperone-mediated clearance, and autophagy [94]. The endo-lysosomal network of microglia, which has been extensively implicated by AD GWAS, is an integral part of receptor-mediated endocytosis, phagocytosis, and autophagy. Endocytosis is a cellular process where extracellular material is internalised, and this process can be categorised into phagocytosis, clathrin-mediated endocytosis, and pinocytosis. In receptor-mediated endocytosis, surface receptors bind extracellular cargo via clathrin-coated pits, followed by the recycling of receptors back to the plasma membrane or the fusion with late endosomes and lysosomes for degradation [95]. Neuronal endocytosis is also responsible for the amyloidogenic processing of APP. Many genes associated with this process have been well studied in neurons, in which enlarged endosomes and accelerated endocytosis result in Aβ overproduction [95]. Recent integrative genetic studies have intensively implicated the role of endo-lysosomal system in microglial phagocytosis of Aβ rather than the role in Aβ overproduction. In microglial phagocytosis, the engagement between phagocytic receptors and extracellular cargo activates actin cytoskeletal rearrangement to form phagosomes, which sequentially fuse with early endosomes, late endosomes, and lysosomes to form phagolysosomes for material degradation [95]. Furthermore, autophagy is also a homeostatic cellular pathway for degrading damaged organelles, pathogens, and abnormal protein aggregates, including Aβ [96]. This cellular process can be categorised into microautophagy, chaperone-mediated autophagy, and macroautophagy, in which cytoplasmic materials are directly absorbed into lysosome, targeted into lysosome, and encapsulated in autophagosome followed by fusion with lysosome, respectively [97]. This section will describe how AD risk variants might modulate microglial endocytosis, phagocytosis, and macroautophagy in both innate and adaptive immune systems in AD (Table 1; Fig. 1).
Fig. 1.
Microglial genes and pathways involved in AD pathogenesis implicated by AD genomics. Microglial cells maintain CNS homeostasis by sensing, endocytosing (left), phagocytosing (right) apoptotic cells, debris, synapses, and Aβ. GWAS, WGS, WES and integrative analyses with transcriptomics, epigenomics and proteomics have nominated many genes (circled in orange) involved in phagocytic recognition, focal adhesion, actin cytoskeletal rearrangement, endo-lysosomal network, phago-lysosomal network, and, auto-lysosomal network (mid), and transcriptional responses in microglia
Microglial clearance of Aβ by endo-lysosomal network
Phagocytic substrates: ApoE/ApoJ-chaperoned Aβ
Microglia transform from a “ramified” homeostatic state into “ameboid” activated state in response to myelin debris, apoptotic cells, unwanted synapses, dystrophic neurites, and oligomeric and fibrillar Aβ. Activated microglia recognise these substrates by expressing pattern recognition receptors (PRRs) [94, 95]. Oligomeric and fibrillar Aβ can be coated by apolipoproteins, complement proteins, or naturally-occurring anti-Aβ antibodies (NAbs-Aβ), enabling chaperone/opsonin-receptor interaction that activates microglial phagocytosis [95]. One of the major apolipoproteins that chaperone Aβ is ApoE, in which its opsonising capability may be associated with its lipidation status [98]. ApoE ε2 is highly lipidated and may coat more Aβ; while ApoE ε4 is poorly lipidated, possibly leading to weaker ApoE-Aβ binding [98]. Although ApoE lipidation is likely to promote ApoE-Aβ binding, microglial phagocytic receptors have been shown to bind to poorly-lipidated ApoE ε4 with higher affinity than non-lipidated ApoE ε3, suggesting a more complicated between ApoE, Aβ, and microglial phagocytotic receptors [99]. In APOE-deficient transgenic mice, there was a significant reduction in plaque–associated microgliosis and activated microglial gene expression accompanied by increased dystrophic neurites around fibrillar plaques, suggesting a critical role of APOE for stimulating innate immune responses [100]. Induced pluripotent stem cells-derived microglia (APOE ε4/ε4) also exhibited morphological alteration, inflammatory gene expression and less efficient phagocytotic behaviours compared to non-ApoE ε4 microglia [101].
Another apolipoprotein is CLU (clusterin, also known as apolipoprotein J, ApoJ), a well-recognised GWAS locus, which can also chaperone Aβ, promote Aβ solubility, and convey Aβ to lipoprotein receptor to initiate microglial endocytosis/phagocytosis [26, 29–31, 102]. In microglia, the enhancers of the CLU loci contained AD risk variants that not only upregulated the expression of CLU but also interacted with the active promoters of other genes, including PTK2B and SCARA3 [38]. Moreover, intracellular CLU may be tightly associated with macroautophagy, in which cytoplasmic cargo is encapsulated in autophagosome that subsequently fuses with lysosome to form autolysosome for material degradation [97]. CLU co-localises with LC3, an autophagy marker, from autophagosome to autolysosome, implying its potential involvement in autophagosome biogenesis in AD [103].
Microglial phagocytic receptors
Genetic studies have implicated multiple microglial phagocytic receptors and the most well-recognised PRR gene is TREM2 that is exclusively expressed on microglia. Transmembrane TREM2 binds to oligomeric Aβ and APOE/APOJ-chaperoned Aβ to induce efficient TREM2-dependent microglial phagocytosis [104–106]. After ligand-receptor engagement, the TREM2/DAP12 (TYROBP) complex induces the phosphorylation of immunoreceptor tyrosine‐based activation motif (ITAM) [107]. Phosphorylated ITAMs serve as docking sites for Src homology 2 (SH2)-containing proteins, such as SYK tyrosine-protein kinase, the phosphorylation of which is mediated by LYN [107]. Both SYK and LYN have been implicated by integrative GWAS analyses [91]. Activated SYK stimulates the MAPK signalling, Ca2+ signalling, PI3K/Akt signalling, actin remodelling, and phagocytosis [107]. In addition, CLNK – a candidate risk gene[30, 32–34]– also encodes a SH2-containing adapter protein in macrophages, suggesting a putative involvement in aiding immune signal transduction in microglia [108]. Regarding the common variants identified by GWAS, the microglia-specific enhancer, promoter, and open chromatin regions of the TREM2 loci harboured AD risk SNPs that were likely to manipulate TREM2 expression and contribute to microglial phagocytosis [38]. The TREM2 rare variants, R47H and R62H, sit within the basic ligand-binding batch in TREM2, destabilising the interaction between TREM2 and anionic ligands [50, 51, 107]. In transgenic AD mice, R47H impaired microglial activation and reduced plaque-associated microglia compared with its common variant [109]. Similarly, AD patients carrying R47H demonstrated reduced plaque-associated microglia and accelerated amyloidogenesis, leading to more severe axonal dystrophy around senile plaques, while TERM2 over-expression promoted microglial phagocytosis [110]. Additionally, a more recent study has reported that TREM2 variants in this basic batch subtly alter ApoE binding; however, TREM2 variants in novel hydrophobic patches could significantly inhibit TREM2 from binding the hinge region of ApoE (residues 192-238), which might be the major determinant modulating TREM2-ApoE interaction [99]. Multiple binding domains in TREM2 have been reported and the adequate activation of TREM2 enables microglia to properly respond to substrates, but AD-associated variants might disrupt microglial activities.
The adequate TREM2/DAP12 signal transduction is critical for TREM2-dependent microglial phagocytosis. INPP5D, also known as SHIP-1, encodes a SH2-containing phosphatase that binds to activated DAP12 to promote or inhibit kinase recruitment and signal transduction [107]. The common GWAS AD risk variants rs10933431 and rs35349669 in the INPP5D locus have been associated with higher SHIP-1 expression in myeloid cells, but the weaker expression in neurons, indicating its significant role in modulating myeloid cell functions in AD [26, 29–32]. The promotor of the INPP5D locus has also been identified to PLAC-link with distant AD risk SNPs, further strengthening the notion of abnormal SHIP-1 expression in microglia [38]. Moreover, one downstream target of SYK is PLC-γ2 (encoded by PLCG2) and its recruitment is mediated by BLNK, both of which are candidate AD risk genes, and their expressions are upregulated in microglia [91, 94]. The protective rare variant of PLCG2, P522R (rs72824905), is located near the auto-inhibitory domain and affects the enzyme activity in boosting microglial phagocytosis, further decelerating AD progression [29, 32, 51, 111].
The TREM2 ectodomain can be shed by ADAM proteases, encoded by GWAS AD risk genes ADAM10 and ADAM17 [29–31], to secrete soluble TREM2 (sTREM2), the CSF level of which has been associated with AD status [107]. Regarding proteases themselves, the ADAM10 locus contains promoters that not only harbour AD risk SNPs but also PLAC-link with distant AD risk SNPs, potentially modulating ADAM10 expression in microglia [38]. The ADAM10/17 cleavage site of TREM2 harbours a rare variant, H157Y (rs2234255) [112], which is prevalent in Han Chinese and elevates AD risk four-fold [113, 114]. In contrast to R47H that attenuates sTREM2 shedding, H157Y promotes TREM2 shedding and reduces full-length TREM2, further compromising phagocytosis [112]. Additionally, a common variant in the MS4A locus, rs1582763, has been associated with increased CSF sTREM2, reduced AD risks, and delayed disease onset [115]. The MS4A locus is another GWAS AD risk locus [24–26, 29] and TREM2 shedding could be reduced by introducing anti-MS4A4A antibodies in macrophages [115]. Genes in the MS4A gene cluster encode many transmembrane proteins that may facilitate TREM2-substrate binding and intracellular protein trafficking in microglia [115]. Overall, most genetic variants in TREM2, TREM2/DAP-12 signalling cascade, and TREM2 shedding procedures compromise TREM2-dependent phagocytosis in microglia, suggesting a protective role of TREM2 in the early stage of AD. The role of many other TREM2 rare variants in AD, including E151K, L211P, R52H, S183C, T223I, T66M, W191X, R136Q, R47C and S31F, await further characterisation by molecular and cellular experiments [52–54].
In contrast to ITAM, immunoreceptor tyrosine-based inhibitory motif (ITIM)-containing receptors inhibit immune signal transduction to reduce microglial phagocytosis in AD. One type of ITIM receptor is sialic acid-binding immunoglobulin-like lectin (Siglec) receptor, such as CD33, which is expressed on monocytes, macrophages, and microglia and is able to bind to sialic acids [24–26, 29, 30, 32]. Sialic acids can also decorate gangliosides and glycoproteins, which are the major components found on the surface of Aβ plaques [116]. These Aβ plaques decorated by ApoE and sialic acid-containing glycoproteins and glycolipids may activate CD33/ITIM signalling and thus “activate” its inhibition of microglial action [116]. ITIM phosphorylation leads to the docking of SH2-containing phosphatases that dephosphorylate signalling intermediates to inhibit ITAM signalling and microglial phagocytosis [94]. The SNP rs12459419, which is in exon 2 in the CD33 locus, is the causal SNP responsible for altered CD33 splicing and reduced AD risk [29]. The common variant of rs12459419 generates a full-length CD33M transcript containing exon 2, the sialic acid-binding domain, leading to higher CD33M levels that inhibit Aβ uptake and exacerbate AD [116]. Conversely, the alternative variant (T) encodes a shorter CD33m isoform lacking exon 2, representing a gain-of-function isoform that enhances microglial phagocytosis of Aβ [117]. The CD33m isoform may accumulate in intracellular peroxisomes and enhance Aβ clearance regardless of cell stimulation or activation in monocytes and microglial [118]. Additionally, another SNP rs3865444 is in strong LD with rs12459419 and is in the promoter region of the CD33 gene [116]. The rs12459419-T is often co-inherited with rs3865444-A, which further enhances exon 2 skipping, reduces CD33M expression, and ultimately protects against AD [116]. Moreover, rare coding variants in CD33 exons show suggestive association with AD risk, including E85V, I71M, and T141A [54].
Besides CD33, large-scale GWAS and integrative studies have identified more ITIM-containing transmembrane receptors, e.g., SIGLEC11, LILRB4, and PILRA, that putatively act similarly to CD33 in microglia [30, 32, 119]. The common missense variant G78R (rs1859788) of the PILRA gene is a likely causal variant protecting against AD at the 7q21 locus (rs1476679) [120]. The R78 variant (protective) reduces the engagement between sialic acid and PILRA binding site, resulting in reduced inhibitory signalling in microglia [120]. This variant is also in LD with a synonymous variant rs2405442:T>C in the PILRA gene that reduces the transcriptional efficiency in PILRA, further reducing the inhibition of microglial actions [121]. Additionally, PILRA is an entry receptor for Herpes simplex virus type 1 (HSV-1) that is proposed to be involved in AD pathogenesis [120, 122]. Recent studies reported that the R78 variant not only reduced HSV-1-PILRA interaction [120] but also increased the level of HSV-1-specific antibodies in AD and MCI patients, compared with those with G78 (risk) [122]. As a recently-identified AD-associated inhibitory receptor, more investigations into PILRA are required to explore their biological functions. In addition to that microglia are immunosuppressed by increased Siglec expression, Aβ plaques decorated by sialic acids may activate Siglec/ITIM signalling and then be masked against microglial recognition, leading to the evasion from immune surveillance and the accumulation of Aβ plaques [116]. It would be interesting to further study the interaction between gangliosides, glycoproteins, Aβ plaques, and microglial Siglec receptors in AD pathogenesis.
The complement system is also a powerful arm of the innate immune system, participating in recognition, trafficking, and phagocytosis, but its function in the brain is poorly understood. As implicated by GWAS, CR1 is a crucial regulator of innate immunity [26, 29–31]. CR1 (complement receptor 1, also known as CD35) is involved in the clearance of immune complexes opsonised by complement components C3b, C4b, C3bi, C1q, mannose-binding lectin, and ficolins in the classical and alternative pathways of the complement cascade [123]. Human transmembrane CR1 contains three-six long homologous repeats and different isomers of CR1 are characterised by different numbers of repeats and different ligand preferences [123]. The most common isomers are CR1-F (fast or CR1*1) and CR1-S (slow or CR1*2) isomer and the latter has been well associated with SNPs rs6656401 and rs3818361 in AD GWAS [20, 21, 24, 25, 29, 30]. The SNP rs6656401 is in the noncoding region of the CR1 locus and is associated with the isomer CR1-S that contains an extra C3b/C4b binding site [123]. Carrying CR1-S had been hypothesised to recognise C3b-opsonised fibrillar Aβ more efficiently and promote Aβ clearance, but rs6656401 was associated with insufficient Aβ clearance and increased AD risks [124]. Therefore, it could be speculated that CR1-S might act as the C3b-inactivating factor that cleaves C3b more efficiently to suppress complement activation and phagocytosis [123]. Moreover, a CNV of the CR1 gene was reported to increase the expression of CR1-S, leading to reduced total CR1 and compromised Aβ clearance, ultimately elevating 30% of AD risk in a Flanders–Belgian cohort [125]. Another coding variant rs4844609 (S1610T) in CR1 is also in strong LD with rs6656401 and this variant locates in the coding region of the CR1 gene [123]. This SNP has been associated with the generation of soluble CR1 (sCR1) that provides complement inhibitory activity [123]. Compared with the common variants, carrying minor variants of rs4844609 and/or rs6656401 could significantly increase sCR1 level in plasma, which might explain the compromised Aβ clearance in AD pathogenesis [123]. Strikingly, a LOAD brain-specific somatic SNV was also detected in the CR1 locus, situated in the regulatory region of the gene encoding CD55, which also acts as C3b/C4b-inactivating factor [87]. It was speculated that this somatic mutation could alter a TF binding site and CD55 transcriptional activity [87]. Moreover, the opsonin C4b maps to the HLA-DRB1 locus, which is a GWAS locus implicated by eQTL [26]. Therefore, strong genetic evidence affirms the role of complement system in AD pathogenesis and CR1 SNPs might inhibit complement activation and reduce CR1 expression to reduce Aβ clearance and increase AD risks.
Toll-like receptors (TLR) are another type of PRRs highly expressed on microglia in the brain and TLR2, TLR4, and co-receptor CD14 can bind to oligomeric and fibrillar Aβ to mediate microglial phagocytosis and produce inflammatory mediators [94, 126]. TLR4 SNP rs4986790 has been associated with attenuated TLR4 signalling and reduced AD risks [127, 128], but this association was not reported by any AD GWAS. After ligand-TLR engagement, TLR interacts with Toll/interleukin-1 receptor (TIR) domain-containing adaptor proteins to activate signalling cascade [129]. SCIMP is a universal non-TIR-containing TLR adaptor protein for TLR2, TLR3, TLR4, and TLR9 in macrophages and is encoded by a candidate AD risk gene with OR of 1.1 [29, 30, 32, 130]. The SCIMP-mediated TLR4 pathway is LYN-dependent, resulting in a selective pro-inflammatory output of IL-6 and IL-12p40 [131]. LYN is a novel AD risk gene that is highly expressed in microglia and regulated by TF PU.1, suggesting the potential hyperactive SCIMP-LYN-TLR signalling in AD [91]. Downstream TLR signalling, nuclear factor kappa light chain enhancer of activated B cells (NF-κB) and MAPK signalling is regulated by LUBAC protein, the components of which are encoded by the SHARPIN and RBCK1 genes, as identified by GWAS [32–34, 132]. Overall, TLR-mediated microglial phagocytosis may be associated with AD, but its association with AD is weaker than other phagocytic receptors.
Engulfment and cytoskeletal dynamics
After recognising phagocytic substrates, the formation of phagocytic cups and pseudopods via rearranging actin cytoskeleton is required, which is poorly understood in microglia. Cytoskeletal rearrangement allows for focal adhesion between phagocytic substrates and integrins to warrant subsequent phagocytosis. The ITGAM loci, encoding CD11b, reached genome-wide significance in early small-scale GWAS, but failed to be replicated in large-scale GWAS [24]. By comparing the transcriptional network of transgenic mouse model of AD with human GWAS, the ITGAM gene was predicted to be microglia-specific risk gene potentially associated with AD risks [119]. More recently, Nott and colleagues identified that the microglia-specific super enhancers, which harboured AD risk variants, were PLAC-linked to a subset of genes not implicated by GWAS, including ITGAM and ITGAX genes [38]. This novel discovery implicated that integrins might be upregulated in microglia during AD progression, most likely in microglial phagocytosis.
Common variants in PTK2B and CASS4 and rare coding variants in ABI3 have been associated with LOAD risks and these genes potentially participate in focal adhesion [26, 30, 31, 51]. PTK2B encodes PYK2, which belongs to the focal adhesion kinase family that directly binds and phosphorylates CAS proteins, such as CASS4, leading to actin cytoskeletal re-arrangement [133]. Intriguingly, TREM2/DAP12 signalling activates SYK that engages with PYK2 in a reciprocal manner [133]. Proteomic and transcriptomic analyses have revealed that the abundance of PYK2 containing alternative exon-exon junctions is significantly different in the brains of AD and preclinical AD cases, suggesting a role of alternative RNA splicing in PYK2 in AD progression [134, 135]. Two AD brain-specific somatic mutations in PTK2B have been identified, but how they alter protein function remains unclear [82, 87]. In addition, a rare coding variant S209F (rs616338) in ABI3, which is intensively expressed on microglia, has been recently identified, potentially interfering with actin polymerisation [51, 136, 137]. Recently, the promoters of the PTK2B, CASS4, and ABI3 locus have been identified to PLAC-link with distant AD risk SNPs, suggesting the altered regulation of this gene expression in microglia [38].
According to GWAS, several AD risk genes have been implicated in the regulation of actin cytoskeletal reorganisation, including CD2AP, ZYX (previously known as EPHA1), FERMT2, and NCK2 [23–26, 29, 32]. In macrophages and dendritic cells, CD2AP is a typical SH3-containing scaffolding molecule that co-localises with F-actin, polymerisation of which is essential for pseudopodia and phagosome formation [95]. Similarly, ZYX has also been associated with actin filaments [95]. Both CD2AP and ZYX loci contain promoters, enhancers, and open chromatin regions that harbour AD risk SNPs, indicating that the regulation of them might be altered in microglia [38]. The scaffolding protein FERMT2 not only enhances integrin-mediated focal adhesions, but also participants in actin polymerisation during migration of hepatocytes, implying a putative similar role in microglia [138]. A rare coding variant in the exon 14 of FERMT2, H579D, was identified to be associated with AD, but its effect remained unclear [54]. Moreover, NCK2 is a typical SH2-containing adaptor protein that regulates actin cytoskeletal rearrangement via the interaction with tyrosine kinases or phosphorylated signalling intermediates [139]. Overall, focal adhesion and actin-associated proteins are important contributors to LOAD pathogenesis, but more studies are needed to explore their impacts in microglia phagocytosis.
Catabolism: endo-lysosomal and phago-lysosomal pathways
The early endosomes are the primary sorting compartment of endocytosis. Many genes participating in early endosome function and trafficking, e.g., BIN1, CD2AP, PICALM, SORL1, and RIN3, have been repeatedly implicated by AD GWAS [20, 26, 30–32]. The microglia-specific enhancer of BIN1 contains a SNP, rs6733839, that has been ranked as the most significant casual variant after APOE, because it can alter an enhancer-binding site to regulate BIN1 expression specifically in microglia [32, 38]. Several rare variants and two rare somatic mutations within the BIN1 locus have been documented, but the further characterisation of them is needed [51, 54, 82, 87]. Secondly, RIN3 is located within the SLC24A4 locus and is regarded as the causal gene because it harbours a rare deleterious variant [31]. Upregulation of RIN3 in AD mouse model was observed, accompanied by early endosome enlargement and dysfunction [140]. RIN3 also recruits BIN1 and CD2AP to early endosomes for endosomal trafficking and signalling [140]. Furthermore, RIN3 has guanine nucleotide exchange factory activity for RAB5 GTPases while RABEP1 encodes a RAB5 effector protein, which are all required for early endosome and phagosome biogenesis, fusion, and trafficking [93, 140]. RAB10 is another key regulator of membrane trafficking and a rare variant rs142787485 in RAB10 protects against AD [141]. Moreover, the integrative analysis of GWAS with myeloid genomics has nominated a novel AD risk gene, APBB3, which is the downstream target gene of certain active enhancers associated with AD risk variants in myeloid cells [93]. APBB3 is responsible for sorting internalised APP into endosomes and promoting amyloidogenic pathway, but its role in myeloid cells remains unknown [93].
The role of SORL1 and PICALM in endocytosis, particularly endosome sorting, has been well characterised in APP processing and Aβ production in neurons (reviewed in [95]), but recent studies proposed that their AD-relevant functions are predominant in microglia [38]. The microglia-specific enhancers, promoters, and open chromatin regions of the PICALM and SORL1 loci have been reported to harbour many AD risk SNPs identified by GWAS [38]. Their regulatory regions are further PLAC-linked to many distant AD-risk variants in other loci, such as SC5D, CCDC83, and EED [38]. Although the PICALM and SORL1 genes are expressed in many cell types, they are only PLAC-linked to microglia-specific enhancers [38]. These discoveries pinpoint that the PICALM and SORL1 expression might be aberrantly regulated by local and distant AD risk SNPs specifically in microglia, illustrating that their role in microglial activities might surpass their participation in APP processing and Aβ metabolism in neurons.
The formation of early endosome or phagosome requires portions of plasma membrane and cell surface receptors, which should be partially replenished by the interaction between recycling endosomes and the trans-Golgi network (TGN). Novikova and colleagues integrated AD GWAS and myeloid epigenomics and transcriptomics and identified AD risk enhancers that might regulate myeloid-specific genes, e.g., AP4E1 and AP4M1 loci [93]. The AP4E1 and AP4M1 genes encode subunits of the adaptor protein complex 4 that is responsible for protein sorting between endosomes and the TGN [93]. AP4E1 is also reported to regulate the early steps of autophagosome formation, implying its potential involvement in endo-lysosomal, phago-lysosomal, and auto-lysosomal networks in AD myeloid cells [142]. Additionally, the WES of AD in non-APOE ε4 carriers has identified a novel variant, rs199533 in the NSF gene, which has not been assigned to GWAS locus [62]. NSF is a critical ATPase associated with intracellular membrane trafficking and Golgi morphology [62]. A variant in the AKAP9 gene, p.R434W, has also been associated with LOAD in a WGS study [66]. Th AKAP9 gene encodes a scaffolding protein that assembles protein kinases and phosphatases in the TGN to maintain its integrity and interact with centrosomes [66]. More recently, the massive, unpublished GWAS has reported novel GWAS loci that implicated the involvement of more TGN-associated proteins, e.g., SORT1, ICA1, and USP6NL, in LOAD pathogenesis [33]. USP6NL is also a GTPase-activating protein for RAB5 and involved in membrane trafficking between early endosome, recycling endosome, and the TGN [33]. However, more studies are required to validate their associations with LOAD and to clarify their roles in microglia activities.
Early endosomes mature to form the late endosomes that fuse with lysosomes, in which several AD risk genes have been identified. As implicated by GWAS, TMEM163 is a poorly understood lysosomal transporter that regulates Zn2+ accumulation in lysosome in endocytic/autophagic pathway in neurons, but its role in microglia remains unknown [32, 143]. Subsequent GWAS and integrative studies have nominated SPPL2A as a novel AD risk gene that encodes a transmembrane aspartyl protease localised to late endosomes and lysosomes [29, 31, 32, 93]. SPPL2A expression in microglia is regulated by an active enhancer element, which is regulated by AD risk alleles, resulting in the association between SPPL2A and AD risk, but its function in microglia remains unclear [93]. By comparing AD GWAS with AD mouse transcriptomics, the gene cluster highly responsive to Aβ has pinpointed a risk gene, HEXB [91]. HEXB has a binding site for TF PU.1 and its expression is upregulated in microglia when encountering Aβ [91]. In zebrafish, hexb knockout led to lysosomal abnormalities in early glial cells, mimicking neurological disease, further implicating a role of HEXB in microglial lysosomes [144]. LAPTM5 is another lysosomal protein that contains a binding site for TF PU.1 and is preferentially upregulated in immune cells [119]. High LAPTM5 expression might be associated with reduced microglial phagocytic ability, but the more functional analysis is needed to elucidate its impact in AD [119]. The recent, unpublished GWAS studies have identified many lysosome-associated proteins and strongly strengthened their role in microglia, including IDUA, TMEM106B, CTSB, TPCN1, and CTSH [33, 34]. IDUA is essential for the degradation of glycosaminoglycans [145], while CTSB and CTSH are lysosomal cysteine proteinases critical for the overall degradation of lysosomal proteins [146]. Lastly, TPCN1 and TMEM106B are critical for endo-lysosomal trafficking [147, 148].
GRN is the latest lysosomal marker implicated by GWAS and integrative microglial studies [32, 91]. In AD, rs5848-T of GRN has been associated with reduced GRN expression and increased AD risk [149]. GRN deficiency has been linked to enlarged lysosomes, dysregulation of innate immunity, and microglial-specific transcriptional upregulation of many lysosomal genes, such as previously discussed HEXB, in AD mouse models [149]. Compared with AD mouse models with heterozygous and homozygous GRN genotypes, GRN knockout mice demonstrated reduced plaque deposits, increased plaque-associated microgliosis, reduced microglial phagocytosis, and higher expressions of TREM2/DAP12-related and inflammation-related genes [149]. In contrast, GRN overexpression protected neurons from neurotoxic Aβ, attenuated hippocampal plaques, and rescued memory deficits [149]. In general, upregulation of GRN mRNA has been observed in AD cases and AD mouse models, which might confer anti-inflammatory impacts, better lysosomal regulation, and enhanced microglial phagocytosis [149]. GRN could be a promising therapeutic target to slow down AD progression. Overall, an increasing number of lysosomal proteins have been identified in the recent years, strongly implicating their role in endocytosis, phagocytosis, and autophagy in AD pathogenesis.
Cellular responses
Phagocytosis is not an isolated process but is accompanied by a wide range of cellular responses, including the generation of ROS, production of inflammatory cytokines and chemokines, and resolution of tissue damage. Recent genetic studies have identified many transcriptional regulators associated with LOAD. The TLR-agonist engagement activates downstream NF-κB, MAPK, and TF IRF3 to induce the production of IFN and pro-inflammatory cytokines [129]. IRF3 recruitment to IFN-I gene promotor is critical for IFN production and the activity of IRF3 is tightly regulated by post-translational modifications [150]. One of the modifiers of IRF3 is KAT8, which is a histone acetyltransferase highly expressed in macrophages [150]. KAT8 can acetylate IRF3 at lysine 359 to inhibit its binding to the IFN-I promoter region, leading to the reduced transcriptional activity of IRF3 and suppressed innate immune response [150]. The KAT8 locus has been repeatedly implicated by GWAS and its common variants, rs59735493 and rs889555, have been associated with altered KAT8 expression in AD hippocampus [29, 30, 32]. KAT8 level is regulated by KANSL1, which has also been associated with AD in APOE ε4 cohorts [35]. Another TF implicated by GWAS is RELB (encoded by RELB) that plays a role downstream TLR NF-κB signalling by dimerising with p52 to form p52:RELB heterodimers that would be translocated into the nucleus for transcriptional activation of homeostatic lymphokines [29, 151].
Marioni and colleagues conducted a large-scale GWAS in 2018 and identified a list of transcriptional regulators, including CLASRP, GEMIN7, and CSTF1, which may function as alternative splicing regulator, pre-mRNA splicing regulator, and 3’end scissor, respectively [29]. However, none of their corresponding loci were replicated in later GWAS, leaving their involvement in AD questionable. This study also implicated the role of zinc-finger proteins (ZNF) in AD, which were then repeated in WES and somatic mutation studies, including ZNF232, ZNF655, and ZNF806, further pinpointing transcriptional regulation and DNA-binding activity [29, 61, 82]. Later, the IKZF1 locus has been recently implicated by GWAS, and it encodes a TF associated with chromatin remodelling [32]. Wightman and colleagues have also identified the AD risk SNPs in the FHL2 locus that may link signalling pathways to transcriptional regulation [34]. The massive GWAS has also identified genes encoding several transcriptional regulators, e.g., EED, PRDM7, KLF16, and SLC2ARG [33]. EED maintains the transcriptional repression of genes and regulates integrin function.2 Interestingly, the regulatory regions of the EED gene not only harbour AD risk SNPs but also PLAC-link to distant AD risk SNPs in the PICALM locus in microglia specifically [38]. The investigations into transcriptional regulators in AD seem to be at an early stage, but there are sufficient evidence showing microglia-specific transcriptional regulation in AD progression.
Adaptive immune responses activated by microglia
Another important arm of the human immune system is the adaptive immune system that utilises antigen-specific receptors on lymphoid cells to eliminate specific pathogens and establish immunological memory [152]. In lymph nodes, T lymphocytes recognise antigens presented by antigen-presenting cells (APC), such as monocyte-derived dendritic cells and tissue-resident macrophages, leading to T cell activation and differentiation [152]. Whether microglia can behave as APC to initiate adaptive immune responses in the CNS is questionable, but recent evidence recognises the potential of microglia acting as APC [153]. The HLA-DRB1 locus has been repeatedly implicated by AD GWAS and subsequent eQTL has prioritised HLA-DRB1, HLA-DRB5, HLA-DQA1, and HLA-DPA as candidate causal genes [30–32]. Rare coding variants in HLA-DPA1 and HLA-DQA1 are possibly associated with AD risks [51, 61]. These genes encode subunits of major histocompatibility complex (MHC)-II molecules that are expressed on APCs and are responsible for presenting antigens to activate T cells [152], suggesting a role of adaptive immunity in AD pathogenesis. The integrative analysis of AD GWAS with AD mouse model transcriptomics has also identified the mouse ortholog locus of human HLA locus (mouse H2-ob) as a prioritised microglial gene significantly correlated with Aβ burden [91, 119]. Moreover, CD2AP may play a role in forming the specialised junction between APCs and T cells and CD2AP inactivation directed CD4+ T cells to the follicular helper lineage to enhance protective antibody responses in viral infection [26, 29–32, 154]. SCIMP (a previously-discussed transmembrane adapter protein) is also involved in antigen presentation between APCs, T cells, and B cells via interacting with LYN kinase [155]. The latest GWAS has reported an AD risk locus near IGHG1, and single-variant analyses have reported three rare coding variants in IGHG3 as suggestively associated with AD risks [32, 61]. IGHG1 and IGHG3 encode the constant region of immunoglobulin heavy chains, strongly pinpointing the role of naturally-occurring antibodies in AD. This evidence suggests that microglia are likely to engulf Aβ and present it via MHC II molecule to activate T cells and induce auto-antibody responses in AD, as shown by the NAbs-Aβ against N-terminus, C-terminus, and mid-domain of Aβ [156].
Characterisation of microglia transcriptomics in AD
Microglial functional states are not discrete. Some gene sets are differentially expressed and can be utilised to define subsets by single-cell RNA sequencing (scRNA-seq) and single-nucleus RNA sequencing (snRNA-seq). Keren-Shaul and colleagues have used transcriptional single-cell sorting and scRNA-seq to characterise immune populations in the brain of wild-type and transgenic mouse model of AD, resulting in the identification of disease-associated microglia (DAM) that co-localised with Aβ plaques [157]. Compared with homeostatic microglia, mouse DAM are transcriptionally distinct and characterised by unique signature of lipid metabolism and phagocytic genes, including downregulated microglial homeostatic genes (P2ry12, Cx3cr1, and Tmem119) and upregulated AD risk genes (Apoe, Clec7a, Lpl, Lilrb4, Cstd, Itgax, Tyrobp, and Trem2) [157]. DAM differentiation is a two-step process where the transition from stage 1 to stage 2 is Trem2-dependent accompanied by the upregulation of lysosomal and phagocytic pathways [157]. Trem2 LOF mutations or knockout disabled the transition into stage 2 DAM [157], which was associated with a compromised bioenergetic microglial state and reduced cerebral glucose metabolism via the PI3K/Akt/mTOR and MAPK signalling pathways, suggesting the possible protective role of DAM in AD [158]. In addition, scRNA-seq analysis of microglia throughout the mouse lifespan has revealed high microglial heterogeneity in healthy developing brains, followed by the emergence of inflammatory and interferon-responsive microglia (IRM) in the aged and injured brain [159]. IRMs might be detrimental because they downregulated homeostatic and phagocytic genes, such as P2ry12, Cx3xr1, and Trem2, but upregulated Apoe, Ifn, and cytokine genes [159]. The investigations into the transcriptional profile of myeloid cells in mouse models of AD further strengthen GWAS findings because endo-lysosomal and phagocytic genes are generally upregulated in DAM, reflecting the involvement of these pathways in AD microglia.
More evidence is required to determine whether DAM and IRM are protective or detrimental, however, it could be useful to evaluate whether these microglial phenotypes exist in human brains as well. After recognising DAM in mouse brain, Keren-Shaul and colleagues identified Lpl expression as a marker of mouse DAM and stained postmortem brains of AD and CN controls for Aβ and LPL [157]. LPL+ microglia co-localised with Aβ plaques, suggesting that DAM could be the conserved phagocytic cells between mouse and human [157]. The transcriptional profile of human AD microglia (actually “bulk” tissues) was subsequently compared with mouse DAM [160]. The neurodegeneration-related genes were enriched but moderately upregulated in AD brain tissues, but the classical inflammatory signalling and peripheral immune cell infiltration were more apparent in the transcriptional profile of human AD microglia [160]. Additionally, snRNA-seq was used to examine all cell types of PFC from 48 postmortem human brain samples (24 AD and 24 controls) [161]. The single-cell resolution detected cell-type-specific expression of APOE, which was upregulated in microglia but downregulated in astrocytes, supporting the notion that microglia are central players in AD [161]. Four microglial subclusters were identified in AD, but only one cluster showed partially overlapping gene expression pattern with mouse DAM and aged microglial signatures [157, 161]. The largest scRNA-seq project has analysed over 16,000 microglia isolated from AD brains and controls and has identified nine microglial subsets [162]. Over 80% of microglia were homeostatic cells, while other clusters were characterised by the cellular stress response, interferon response, anti-inflammatory genes, antigen-presenting genes (DAM-like), and proliferative genes, respectively [162]. To detect human microglia more accurately and achieve a better transcriptome coverage, cell sorting was used to isolate CD11b+ microglial cells from the superior frontal gyrus from ten AD patients and 15 controls [163]. Microglia of AD patients and controls displayed differential expression patterns, such as upregulation of APOE, ABCA7, PTK2B, SPI1, and ZYX and downregulation of MEF2C in AD [163], suggesting increased activity of endo-lysosomal networks in AD microglia. Human AD microglia tend to reflect a mixture of aging processes (upregulation of age-related genes) and an age-independent, disease-related activation process (stable expression of APOE) [163]. However, these studies about the transcriptional profile of human AD microglia have barely converged on a consistent profile, while the discovery of mouse DAM can be replicated by other studies. The inconsistency in human AD microglia may be because postmortem tissues may not reflect the long, initial phase of AD and individuals differ substantially. Overall, the recent studies about human AD microglia and DAM indeed strengthen the role of endo-lysosomal network in disease progression. Therefore, more studies targeted in this direction are expected to elucidate the role of microglia during disease progress.
Central and peripheral immune cell crosstalk in AD
The brain has long been considered an immune-privileged site because the BBB strictly “prevents” peripheral cells from entering the brain, but this concept is flawed as the infiltration of peripheral monocytes and lymphoid cells has been observed in mouse models of neurological disease [164, 165]. Cerebral Aβ can be cleared intracellularly in or near the CNS by microglia and infiltrative peripheral immune cells, and extracellularly by ISF and CSF bulk flow into the lymphatic system and the circulation [89]. The transcriptomics of meningeal lymphatic endothelial cells, brain-blood endothelial cells, and microglia of AD mouse models intriguingly identified overlapping AD-associated genetic signatures, especially Apoe [166]. The endothelial cells of meningeal lymphatic vessels and BBB both abnormally expressed Abca1, Adam10, Cd2ap, Fermt2, and Sppl2a, suggesting perturbated endo-lysosomal pathways in endothelial cells and possible defective Aβ drainage into the periphery [166]. Aβ efflux into circulation may be essential to clear cerebral Aβ because, in AD mice, 40–60% of cerebral Aβ is removed in the periphery by myeloid phagocytosis or endocytosis, excretion via bile or urine, and enzymatic degradation [167]. Several studies have assessed current AD GWAS with cell-type and tissue-type-specific datasets to evaluate whether AD risk variants are enriched in any specific cell or tissue types. Significant heritability enrichments in genes active in “immune/haematopoietic cell” and “liver” categories were noticeable in AD cases [168]. Primary peripheral mononuclear cells showed the most significant signal [168]. Integrating AD GWAS with open chromatin datasets of diverse tissue types has shown that AD risk SNPs were preferentially enriched in DNase hypersensitivity sites of primary monocytes [169]. These open chromatin regions harbouring AD-related SNPs contained specific TF motifs, including SPI1 (PU.1), EGR1, MER2A, and CEBPA [169]. These studies together demonstrate the immune cell-specific enrichment of AD risk variants and further hint that AD risk variants may act by locating in regulatory regions that regulate gene expression in myeloid cells, particularly monocytes in circulation and microglia in the CNS. The discovery of AD-associated SNPs at SPI.1and SPI.1 binding sites provides a plausible explanation that the disruption of critical TF binding motifs and altering the expression of critical TFs may have widespread effects on gene expression and cellular function [90, 169]. It can be further hypothesised that alternative epigenetic modifications, abnormal binding of chromatin remodelling factors or altered promoter-enhancer interactions caused by AD risk variants may result in similar widespread effects in AD. Overall, the monocyte/macrophage lineage has been repeatedly identified as the most significant AD-associated myeloid cells in the periphery and both monocytes and microglia have been associated with SPI1 (PU.1) [90]. Monocyte/macrophage lineages are very likely to interact with T cells, and potentially B cells, to eliminate peripheral Aβ, cooperating with central immune responses to combat AD progression.
Besides genetic associations, functional assays have been performed to evaluate the phagocytic ability and therapeutic potential of peripheral myeloid cells. In AD mouse models, the deficiency of SCARA1, a primary scavenger receptor for Aβ in the brain, compromised Aβ clearance by peripheral phagocytes and accelerated AD progression, which was rescued by pharmacological upregulation of SCARA1 [170]. In human studies, AD monocytes showed defective Aβ phagocytosis and high potency of apoptosis instead of proper degradation of Aβ compared with those of controls [171]. Monocytes from preclinical, prodromal (MCI), AD, and controls showed similar basal phagocytic ability, but pre-treating cells with phagocytosis activators and inhibitors gave rise to differential responses depending on Aβ burden, suggesting an altered innate phagocytic potential of myeloid cells in AD [172]. Furthermore, low-dose infusions of peripheral human umbilical cord blood cell-derived monocytes reduced Aβ and improved cognition in transgenic AD mice [173], suggesting that peripheral clearance of Aβ by monocytes may ameliorate AD. Considering the defective phagocytic function of myeloid cells in both the CNS and periphery, compounds and agents that boost the phagocytic activity and migration of myeloid cells are expected to facilitate Aβ clearance. For instance, the combination of boosting TREM2, scavenger receptors, TLRs and inhibiting SIGLEC receptors on microglia may facilitate Aβ clearance in the CNS. Moreover, boosting the phagocytosis of monocytes and macrophages may further improve Aβ elimination in the periphery.
Apart from innate immunity, NAbs-Aβ have been described since 1993 [174]. The early studies have demonstrated that NAbs-Aβ might enhance microglial migration to Aβ, promote microglial phagocytosis of Aβ, hydrolyse Aβ, and eventually reduce Aβ burden [174]. NAbs-Aβ have been found in the blood and CSF of AD patients and they can recognise linear epitopes in the Aβ monomers/dimers and conformation-specific epitopes in oligomeric/fibrillar Aβ aggregates [156]. During AD progression, levels of NAbs to the N-terminus of fibrillar Aβ (~ AA1-10) increased, which were the most effective antibodies promoting Aβ clearance, while levels of NAbs to the mid-domain of fibrillar Aβ (~ AA17-32) decreased, which reflected oligomerisation and toxicity of Aβ [156]. This dynamic pattern of NAbs-Aβ indicates the possible failure of Aβ clearance and toxicity inhibition by humoral responses in AD [156]. The N-terminal NAbs-Aβ might greatly contribute to immunoglobulin-mediated phagocytosis via the interaction between lymphoid and myeloid cells to facilitate Aβ clearance [167]. Among all AD disease-modifying treatments (DMT) under investigation, immunotherapy—the passive immunisation through administration of NAbs-Aβ—is most extensively explored and most clinically developed, including aducanumab and gantenerumab [175]. On 7th June 2021, aducanumab (Aduhelm™ by Biogen/Eisai/Neurimmune) has been given the accelerated approval as the first DMT for AD from the U.S. Food and Drug Administration (FDA).3 Aducanumab binds to the N-terminus (AA3-6) of Aβ oligomers and fibrils and recognises a conformational epitope that only presents in aggregated Aβ [175, 176]. It can significantly reduce the level of Aβ plaques by 70% and slow the cognition decline by 20–40% [176]. Although its side effects and high costs are controversial, its approval highlights that AD therapeutics finally enter mainstream. Similarly, gantenerumab (Roche/MorphoSys) is another instance of human NAbs-Aβ that binds a conformational epitope on fibrillar Aβ (N-terminus [AA3-12] and mid-domain [AA18-27]) [175]. In a randomised, placebo-controlled, multi-arm clinical trial of NAbs-Aβ, the administration of gantenerumab significantly reduced the level of Aβ plaques, CSF p-tau181, and CSF total tau, and attenuated increases of CSF neurofilament light chain, but there was no beneficial effect on cognition of patients [177]. Its beneficial effect on plaque reduction and biomarker in asymptomatic AD patients suggested that it might be an effective strategy at early stage of AD to postpone disease progression.
Another type of AD immunotherapy is the administration of humanised mAb-Aβ, such as solanezumab, donanemab, crenezumab, ponezumab, bapineuzumab, and lecanemab [175]. Solanezumab (Lily) has been tested in the same clinical trial with gantenerumab, but solanezumab significantly increased the level of Aβ1-42 and neurofilament light chain in CSF [177]. Additionally, donanemab (N3pG, Lilly) is another instance of humanised mAb-Aβ that recognises the pyroglutamate form of Aβ in Aβ plaques instead of soluble or insoluble Aβ.4 The recent phase 2 clinical trial of donanemab demonstrated a rapid reduction of Aβ load by 24 weeks, a dramatic reduction of plasma p-tau217 at 12 weeks, and a normalised cognition composite at 76 weeks [178]. On 24th June 2021, donanemab was granted Breakthrough Therapy Designation by the U.S. FDA and its accelerated approval is also promising.5 Moreover, lecanemab (Ban2401, Eisai/BioArctic), a humanised mAb-Aβ selectively against soluble Aβ protofibrils, was also granted Breakthrough Therapy Designation by U.S. FDA because it can reduce Aβ burden by 70%.6 Overall, NAbs-Aβ and humanised mAb-Aβ bind different epitopes and conformations to promote Aβ clearance and modify disease progression. There are some concerns about passive immunisation. For instance, it is not fully understood whether brain entry of NAbs-Aβ and humanised mAb-Aβ is necessary and whether peripheral clearance of Aβ is sufficient to modify AD progression. Secondly, the efficacy and safety of all candidate antibodies should be re-assessed in larger and longer clinical trials to achieve a more reliable clinical outcome.
Conclusions
Advanced genomic methodologies, sequencing techniques, and integrative bioinformatic analyses have dramatically enhanced our understanding of AD and implicate the role of both innate and adaptive immunity in AD pathogenesis. While EOAD is largely initiated by Aβ overproduction and age-related defective APOE-mediated clearance of Aβ, LOAD appears to be driven by the dysregulation of phagocytic clearance of Aβ by myeloid cells. Recent large-scale GWAS have discovered over 130 susceptibility loci associated with AD risks. The subsequent integrative analyses with cell and tissue-type-specific transcriptomic, proteomic, and epigenomic datasets have pinpointed candidate causal genes involved in monocytic/microglial phagocytosis, endocytosis, and autophagy in innate immune response, as well as antigen presentation in adaptive immune response. These data not only consolidate the central state of microglia in AD but also overturn the traditional notion that AD is a CNS-limited disease, leading to a new concept that the central, peripheral myeloid and lymphoid cells may work synergically to eliminate Aβ in AD. Besides inherited genetic polymorphisms, the emergence and accumulation of somatic mutations in AD brain and blood has been validated, which is distinct from that in normal aging processes, and turn out to be a plausible mechanism underlying the compromised myeloid phagocytosis in AD. Existing evidence from GWAS, WES, WGS, and somatic mutation studies have constructed a genetic landscape of AD but extrapolating these findings from susceptibility loci into causal genes is still challenging. The inability of mouse models to recapitulate the full phenotype of human AD, the difficulties of collecting enough human microglia, and the absence of somatic mutation models are of concern. It will be important to study the crosstalk between central and peripheral immune responses. Since GWAS has implicated the predominant role of innate immune responses in both the CNS and periphery, immunotherapy that boosts the phagocytic and endocytic function of microglia and monocytes could make a significant contribution to systemic Aβ elimination. Additionally, we watch with great anticipation for the current clinical trials using passive immunisation with NAbs-Aβ and humanised mAb-Aβ to show effective disease modification. Aducanumab has been granted the first DMT for AD by FDA, while donanemab, and lecanemab (ban2401) have been granted Breakthrough Therapy Designation recently. Causation of AD by deficient innate immunity and treatment by the adaptive immunity is emerging as a new paradigm for AD. The passive immunisation leads AD therapeutics into the mainstream, but more investigations into boosting innate immune responses at the early, asymptomatic stage is expected because these strategies may postpone the entry into a symptomatic stage.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors were grateful to the University of Melbourne for providing open access to PubMed for literature reading.
Abbreviations
- AD
Alzheimer's disease
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- ADSP
Alzheimer’s Disease Sequencing Project
- APC
Antigen-presenting cells
- APOE
Apolipoprotein E
- APP
Amyloid precursor protein
- ATAC-seq
Transposase-accessible chromatin using sequencing
- AUC
Area under curve
- Aβ
β-Amyloid
- Aβ1-40
Aβ ending in residue 40
- Aβ1-42
Aβ ending in residue 42
- BBB
Blood–brain barrier
- cDNA
Complementary DNA
- ChIP-seq
Chromatin immunoprecipitation sequencing
- CN
Cognitively normal
- CNS
Central nervous system
- CNV
Copy number variants
- CSF
Cerebrospinal fluid
- DAM
Disease-associated microglia
- DMT
Disease-modifying treatments
- DSB
Double-strand breaks
- EOAD
Early-onset Alzheimer's disease
- eQTL
Expression quantitative trait loci
- FDA
Food and Drug Administration
- GWAS
Genome-wide association studies
- GWAX
Genome-wide association studies by proxy
- HSV-1
Herpes simplex virus type 1
- INDEL
Insertions and deletions
- iPSC
Induced pluripotent stem cells
- IRM
Interferon-responsive microglia
- ISF
Interstitial fluid
- ITAM
Immunoreceptor tyrosine‐based activation motif
- ITIM
Immunoreceptor tyrosine-based inhibitory motif
- LD
Linkage disequilibrium
- LOAD
Late-onset Alzheimer's disease
- LOF
Loss-of-function
- MAF
Minor allele frequency
- MAPK
Mitogen-activated protein kinase
- MCI
Mild cognitive impairment
- MHC
Major histocompatibility complex
- NAbs-Aβ
Naturally-occurring antibodies to β-amyloid
- NFT
Neurofibrillary tangles
- NF-κB
Nuclear factor kappa light chain enhancer of activated B cells
- OR
Odds ratio
- PET
Positron emission tomography
- PFC
Prefrontal cortex
- PI3K/Akt
Phosphoinositide-3-kinase/Protein kinase B
- PLAC-seq
Proximity ligation-assisted ChIP-seq
- PRR
Pattern recognition receptors
- PRS
Polygenic risk score
- PSEN1
Presenilin 1
- PSEN2
Presenilin 2
- p-tau
Hyperphosphorylated tau
- ROS
Reactive oxygen species
- sCR1
Soluble CR1
- scRNA-seq
Single-cell RNA sequencing
- SH2
Src homology 2
- Siglec
Sialic acid-binding immunoglobulin-like lectin
- SNP
Single nucleotide polymorphisms
- snRNA-seq
Single-nucleus RNA sequencing
- SNV
Single nucleotide variants
- SSB
Single-strand breaks
- sTREM2
Soluble TREM2
- TF
Transcription factor
- TGN
Trans-Golgi network
- TIR
Toll/interleukin-1 receptor
- TLR
Toll-like receptors
- UKB
United Kingdom Biobank
- VAF
Variant allelic frequency
- WES
Whole-exome sequencing
- WGS
Whole-genome sequencing
Author contributions
All authors contributed to the writing of this review article.
Funding
This work was supported by the Melbourne Research Scholarship provided by the Florey Institute of Neuroscience and Mental Health, the University of Melbourne, and Qiankang Life Science Melbourne R&D Centre.
Availability of data and material
All data analysed and summarised during this study were included in this article and its supplementary information files.
Declarations
Conflict of interest
All authors declared no conflict of interests.
Ethics approval and consent to participate
No ethics approval was applicable to this work.
Consent for publication
All authors provided consent to the publication of this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer's disease. Lancet. 2021;397(10284):1577–1590. doi: 10.1016/s0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8(6):595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang CW, Shao E, Mucke L. Tau: enabler of diverse brain disorders and target of rapidly evolving therapeutic strategies. Science. 2021 doi: 10.1126/science.abb8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McQuade A, Blurton-Jones M. Microglia in Alzheimer's disease: exploring how genetics and phenotype influence risk. J Mol Biol. 2019;431(9):1805–1817. doi: 10.1016/j.jmb.2019.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calsolaro V, Edison P. Neuroinflammation in Alzheimer's disease: current evidence and future directions. Alzheimers Dement. 2016;12(6):719–732. doi: 10.1016/j.jalz.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer's disease. J Cell Biol. 2018;217(2):459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer's disease: molecular mechanisms. Int J Dev Neurosci. 2006;24(2–3):167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Phillips EC, Croft CL, Kurbatskaya K, O'Neill MJ, Hutton ML, Hanger DP, et al. Astrocytes and neuroinflammation in Alzheimer's disease. Biochem Soc Trans. 2014;42(5):1321–1325. doi: 10.1042/BST20140155. [DOI] [PubMed] [Google Scholar]
- 9.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Strooper B, Karran E. The cellular phase of Alzheimer's disease. Cell. 2016;164(4):603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 11.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuner SM, Tcw J, Goate AM. Genetic architecture of Alzheimer's disease. Neurobiol Dis. 2020;143:104976. doi: 10.1016/j.nbd.2020.104976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sims R, Hill M, Williams J. The multiplex model of the genetics of Alzheimer’s disease. Nat Neurosci. 2020;23(3):311–322. doi: 10.1038/s41593-020-0599-5. [DOI] [PubMed] [Google Scholar]
- 14.Cacace R, Sleegers K, Van Broeckhoven C. Molecular genetics of early-onset Alzheimer's disease revisited. Alzheimers Dement. 2016;12(6):733–748. doi: 10.1016/j.jalz.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283(44):29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993;342(8873):697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- 17.Saddiki H, Fayosse A, Cognat E, Sabia S, Engelborghs S, Wallon D, et al. Age and the association between apolipoprotein E genotype and Alzheimer disease: a cerebrospinal fluid biomarker-based case-control study. PLoS Med. 2020;17(8):e1003289. doi: 10.1371/journal.pmed.1003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews SJ, Fulton-Howard B, Goate A. Interpretation of risk loci from genome-wide association studies of Alzheimer's disease. Lancet Neurol. 2020;19(4):326–335. doi: 10.1016/S1474-4422(19)30435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen L, Jia J. An overview of genome-wide association studies in Alzheimer's disease. Neurosci Bull. 2016;32(2):183–190. doi: 10.1007/s12264-016-0011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41(10):1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41(10):1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 22.Jun G, Naj AC, Beecham GW, Wang LS, Buros J, Gallins PJ, et al. Meta-analysis confirms CR1, CLU, and PICALM as alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch Neurol. 2010;67(12):1473–1484. doi: 10.1001/archneurol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303(18):1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011;43(5):429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45(12):1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Convergent genetic and expression data implicate immunity in Alzheimer's disease (2015). Alzheimers Dement 11(6):658–671. 10.1016/j.jalz.2014.05.1757 [DOI] [PMC free article] [PubMed]
- 28.Skene NG, Bryois J, Bakken TE, Breen G, Crowley JJ, Gaspar HA, et al. Genetic identification of brain cell types underlying schizophrenia. Nat Genet. 2018;50(6):825–833. doi: 10.1038/s41588-018-0129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marioni RE, Harris SE, Zhang Q, McRae AF, Hagenaars SP, Hill WD, et al. GWAS on family history of Alzheimer's disease. Transl Psychiatry. 2018;8(1):99. doi: 10.1038/s41398-018-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansen IE, Savage JE, Watanabe K, Bryois J, Williams DM, Steinberg S, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer's disease risk. Nat Genet. 2019;51(3):404–413. doi: 10.1038/s41588-018-0311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, et al. Genetic meta-analysis of diagnosed Alzheimer's disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat Genet. 2019;51(3):414–430. doi: 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartzentruber J, Cooper S, Liu JZ, Barrio-Hernandez I, Bello E, Kumasaka N, et al. Genome-wide meta-analysis, fine-mapping and integrative prioritization implicate new Alzheimer's disease risk genes. Nat Genet. 2021;53(3):392–402. doi: 10.1038/s41588-020-00776-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellenguez C, Küçükali F, Jansen I, Andrade V, Moreno-Grau S, Amin N, et al. New insights on the genetic etiology of Alzheimer’s and related dementia. medRxiv. 2020 doi: 10.1101/2020.10.01.20200659. [DOI] [Google Scholar]
- 34.Wightman DP, Jansen IE, Savage JE, Shadrin AA, Bahrami S, Rongve A, et al. Largest GWAS (N=1,126,563) of Alzheimer’s disease implicates microglia and immune cells. medRxiv. 2020 doi: 10.1101/2020.11.20.20235275. [DOI] [Google Scholar]
- 35.Jun G, Ibrahim-Verbaas CA, Vronskaya M, Lambert JC, Chung J, Naj AC, et al. A novel Alzheimer disease locus located near the gene encoding tau protein. Mol Psychiatry. 2016;21(1):108–117. doi: 10.1038/mp.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang S, Gim J, Gunasekaran TI, Choi KY, Lee JJ, Seo EH, et al. APOE-stratified genome-wide association study suggests potential novel genes for late-onset Alzheimer’s disease in East-Asian descent. medRxiv. 2021 doi: 10.1101/2020.07.02.20145557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29(1):308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nott A, Holtman IR, Coufal NG, Schlachetzki JCM, Yu M, Hu R, et al. Brain cell type-specific enhancer-promoter interactome maps and disease-risk association. Science. 2019;366(6469):1134–1139. doi: 10.1126/science.aay0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi SW, Mak TS-H, O’Reilly PF. Tutorial: a guide to performing polygenic risk score analyses. Nat Protoc. 2020;15(9):2759–2772. doi: 10.1038/s41596-020-0353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Escott-Price V, Shoai M, Pither R, Williams J, Hardy J. Polygenic score prediction captures nearly all common genetic risk for Alzheimer's disease. Neurobiol Aging. 2017;49:214.e217–214.e211. doi: 10.1016/j.neurobiolaging.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Leonenko G, Sims R, Shoai M, Frizzati A, Bossù P, Spalletta G, et al. Polygenic risk and hazard scores for Alzheimer's disease prediction. Ann Clin Transl Neurol. 2019;6(3):456–465. doi: 10.1002/acn3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Logue MW, Panizzon MS, Elman JA, Gillespie NA, Hatton SN, Gustavson DE, et al. Use of an Alzheimer’s disease polygenic risk score to identify mild cognitive impairment in adults in their 50s. Mol Psychiatry. 2019;24(3):421–430. doi: 10.1038/s41380-018-0030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zettergren A, Lord J, Ashton NJ, Benedet AL, Karikari TK, Lantero Rodriguez J, et al. Association between polygenic risk score of Alzheimer’s disease and plasma phosphorylated tau in individuals from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimer's Res Therapy. 2021;13(1):17. doi: 10.1186/s13195-020-00754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stocker H, Perna L, Weigl K, Möllers T, Schöttker B, Thomsen H, et al. Prediction of clinical diagnosis of Alzheimer’s disease, vascular, mixed, and all-cause dementia by a polygenic risk score and APOE status in a community-based cohort prospectively followed over 17 years. Mol Psychiatry. 2020 doi: 10.1038/s41380-020-0764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cruchaga C, Del-Aguila JL, Saef B, Black K, Fernandez MV, Budde J, et al. Polygenic risk score of sporadic late-onset Alzheimer's disease reveals a shared architecture with the familial and early-onset forms. Alzheimers Dement. 2018;14(2):205–214. doi: 10.1016/j.jalz.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou X, Chen Y, Ip FCF, Lai NCH, Li YYT, Jiang Y, et al. Genetic and polygenic risk score analysis for Alzheimer's disease in the Chinese population. Alzheimer's Dement. 2020;12(1):e12074–e12074. doi: 10.1002/dad2.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013;368(2):107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, et al. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368(2):117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carmona S, Zahs K, Wu E, Dakin K, Bras J, Guerreiro R. The role of TREM2 in Alzheimer's disease and other neurodegenerative disorders. Lancet Neurol. 2018;17(8):721–730. doi: 10.1016/S1474-4422(18)30232-1. [DOI] [PubMed] [Google Scholar]
- 50.Jin SC, Benitez BA, Karch CM, Cooper B, Skorupa T, Carrell D, et al. Coding variants in TREM2 increase risk for Alzheimer's disease. Hum Mol Genet. 2014;23(21):5838–5846. doi: 10.1093/hmg/ddu277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sims R, van der Lee SJ, Naj AC, Bellenguez C, Badarinarayan N, Jakobsdottir J, et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer's disease. Nat Genet. 2017;49(9):1373–1384. doi: 10.1038/ng.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sirkis DW, Bonham LW, Aparicio RE, Geier EG, Ramos EM, Wang Q, et al. Rare TREM2 variants associated with Alzheimer's disease display reduced cell surface expression. Acta Neuropathol Commun. 2016;4(1):98. doi: 10.1186/s40478-016-0367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin SC, Carrasquillo MM, Benitez BA, Skorupa T, Carrell D, Patel D, et al. TREM2 is associated with increased risk for Alzheimer's disease in African Americans. Mol Neurodegener. 2015;10:19. doi: 10.1186/s13024-015-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang W, Jiao B, Xiao T, Liu X, Liao X, Xiao X, et al. Association of rare variants in neurodegenerative genes with familial Alzheimer's disease. Ann Clin Transl Neurol. 2020;7(10):1985–1995. doi: 10.1002/acn3.51197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39(2):168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campion D, Charbonnier C, Nicolas G. SORL1 genetic variants and Alzheimer disease risk: a literature review and meta-analysis of sequencing data. Acta Neuropathol. 2019;138(2):173–186. doi: 10.1007/s00401-019-01991-4. [DOI] [PubMed] [Google Scholar]
- 57.Pereira CD, Martins F, Wiltfang J, da Cruz ESOAB, Rebelo S. ABC transporters are key players in Alzheimer's disease. J Alzheimers Dis. 2018;61(2):463–485. doi: 10.3233/JAD-170639. [DOI] [PubMed] [Google Scholar]
- 58.Sassi C, Nalls MA, Ridge PG, Gibbs JR, Ding J, Lupton MK, et al. ABCA7 p. G215S as potential protective factor for Alzheimer's disease. Neurobiol Aging. 2016;46:235 e231–239. doi: 10.1016/j.neurobiolaging.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beecham GW, Bis JC, Martin ER, Choi SH, DeStefano AL, van Duijn CM, et al. The Alzheimer's Disease Sequencing Project: study design and sample selection. Neurology Genetics. 2017;3(5):e194. doi: 10.1212/NXG.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raghavan NS, Brickman AM, Andrews H, Manly JJ, Schupf N, Lantigua R, et al. Whole-exome sequencing in 20,197 persons for rare variants in Alzheimer's disease. Ann Clin Transl Neurol. 2018;5(7):832–842. doi: 10.1002/acn3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bis JC, Jian X, Kunkle BW, Chen Y, Hamilton-Nelson KL, Bush WS, et al. Whole exome sequencing study identifies novel rare and common Alzheimer's-associated variants involved in immune response and transcriptional regulation. Mol Psychiatry. 2020;25(8):1859–1875. doi: 10.1038/s41380-018-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan KH, Feingold E, Rosenthal SL, Demirci FY, Ganguli M, Lopez OL, et al. Whole-exome sequencing analysis of Alzheimer's disease in non-APOE*4 carriers. J Alzheimers Dis. 2020;76(4):1553–1565. doi: 10.3233/JAD-200037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Curtis D, Bakaya K, Sharma L, Bandyopadhyay S. Weighted burden analysis of exome-sequenced late-onset Alzheimer's cases and controls provides further evidence for a role for PSEN1 and suggests involvement of the PI3K/Akt/GSK-3beta and WNT signalling pathways. Ann Hum Genet. 2020;84(3):291–302. doi: 10.1111/ahg.12375. [DOI] [PubMed] [Google Scholar]
- 64.Holstege H, Hulsman M, Charbonnier C, Grenier-Boley B, Quenez O, Ahmad S, et al. Exome sequencing identifies three novel AD-associated genes. Alzheimers Dement. 2020;16(S2):e041592. doi: 10.1002/alz.041592. [DOI] [Google Scholar]
- 65.Beecham GW, Vardarajan B, Blue E, Bush W, Jaworski J, Barral S, et al. Rare genetic variation implicated in non-Hispanic white families with Alzheimer disease. Neurol Genet. 2018;4(6):e286. doi: 10.1212/NXG.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vardarajan BN, Barral S, Jaworski J, Beecham GW, Blue E, Tosto G, et al. Whole genome sequencing of Caribbean Hispanic families with late-onset Alzheimer's disease. Ann Clin Transl Neurol. 2018;5(4):406–417. doi: 10.1002/acn3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prokopenko D, Morgan SL, Mullin K, Hofmann O, Chapman B, Kirchner R, et al. Whole-genome sequencing reveals new Alzheimer's disease-associated rare variants in loci related to synaptic function and neuronal development. Alzheimers Dement. 2021 doi: 10.1002/alz.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leija-Salazar M, Piette C, Proukakis C. Review: somatic mutations in neurodegeneration. Neuropathol Appl Neurobiol. 2018;44(3):267–285. doi: 10.1111/nan.12465. [DOI] [PubMed] [Google Scholar]
- 69.Proukakis C. Somatic mutations in neurodegeneration: an update. Neurobiol Dis. 2020;144:105021. doi: 10.1016/j.nbd.2020.105021. [DOI] [PubMed] [Google Scholar]
- 70.Biesecker LG, Spinner NB. A genomic view of mosaicism and human disease. Nat Rev Genet. 2013;14(5):307–320. doi: 10.1038/nrg3424. [DOI] [PubMed] [Google Scholar]
- 71.Maynard S, Fang EF, Scheibye-Knudsen M, Croteau DL, Bohr VA. DNA damage, DNA repair, aging, and neurodegeneration. Cold Spring Harb Perspect Med. 2015 doi: 10.1101/cshperspect.a025130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin X, Kapoor A, Gu Y, Chow MJ, Peng J, Zhao K, et al. Contributions of DNA damage to Alzheimer's disease. Int J Mol Sci. 2020 doi: 10.3390/ijms21051666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McConnell MJ, Moran JV, Abyzov A, Akbarian S, Bae T, Cortes-Ciriano I, et al. Intersection of diverse neuronal genomes and neuropsychiatric disease: the brain somatic mosaicism network. Science. 2017 doi: 10.1126/science.aal1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lodato MA, Woodworth MB, Lee S, Evrony GD, Mehta BK, Karger A, et al. Somatic mutation in single human neurons tracks developmental and transcriptional history. Science. 2015;350(6256):94–98. doi: 10.1126/science.aab1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lodato MA, Rodin RE, Bohrson CL, Coulter ME, Barton AR, Kwon M, et al. Aging and neurodegeneration are associated with increased mutations in single human neurons. Science. 2018;359(6375):555–559. doi: 10.1126/science.aao4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia-Nieto PE, Morrison AJ, Fraser HB. The somatic mutation landscape of the human body. Genome Biol. 2019;20(1):298. doi: 10.1186/s13059-019-1919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beck JA, Poulter M, Campbell TA, Uphill JB, Adamson G, Geddes JF, et al. Somatic and germline mosaicism in sporadic early-onset Alzheimer's disease. Hum Mol Genet. 2004;13(12):1219–1224. doi: 10.1093/hmg/ddh134. [DOI] [PubMed] [Google Scholar]
- 78.Rovelet-Lecrux A, Charbonnier C, Wallon D, Nicolas G, Seaman MN, Pottier C, et al. De novo deleterious genetic variations target a biological network centered on Abeta peptide in early-onset Alzheimer disease. Mol Psychiatry. 2015;20(9):1046–1056. doi: 10.1038/mp.2015.100. [DOI] [PubMed] [Google Scholar]
- 79.Bushman DM, Kaeser GE, Siddoway B, Westra JW, Rivera RR, Rehen SK, et al. Genomic mosaicism with increased amyloid precursor protein (APP) gene copy number in single neurons from sporadic Alzheimer's disease brains. Elife. 2015 doi: 10.7554/eLife.05116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sala Frigerio C, Lau P, Troakes C, Deramecourt V, Gele P, Van Loo P, et al. On the identification of low allele frequency mosaic mutations in the brains of Alzheimer's disease patients. Alzheimers Dement. 2015;11(11):1265–1276. doi: 10.1016/j.jalz.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 81.Lee MH, Siddoway B, Kaeser GE, Segota I, Rivera R, Romanow WJ, et al. Somatic APP gene recombination in Alzheimer's disease and normal neurons. Nature. 2018;563(7733):639–645. doi: 10.1038/s41586-018-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parcerisas A, Rubio SE, Muhaisen A, Gomez-Ramos A, Pujadas L, Puiggros M, et al. Somatic signature of brain-specific single nucleotide variations in sporadic Alzheimer's disease. J Alzheimers Dis. 2014;42(4):1357–1382. doi: 10.3233/JAD-140891. [DOI] [PubMed] [Google Scholar]
- 83.Wei W, Keogh MJ, Aryaman J, Golder Z, Kullar PJ, Wilson I, et al. Frequency and signature of somatic variants in 1461 human brain exomes. Genet Med. 2019;21(4):904–912. doi: 10.1038/s41436-018-0274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park JS, Lee J, Jung ES, Kim MH, Kim IB, Son H, et al. Brain somatic mutations observed in Alzheimer's disease associated with aging and dysregulation of tau phosphorylation. Nat Commun. 2019;10(1):3090. doi: 10.1038/s41467-019-11000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keogh MJ, Wei W, Aryaman J, Walker L, van den Ameele J, Coxhead J, et al. High prevalence of focal and multi-focal somatic genetic variants in the human brain. Nat Commun. 2018;9(1):4257. doi: 10.1038/s41467-018-06331-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nicolas G, Acuna-Hidalgo R, Keogh MJ, Quenez O, Steehouwer M, Lelieveld S, et al. Somatic variants in autosomal dominant genes are a rare cause of sporadic Alzheimer's disease. Alzheimers Dement. 2018;14(12):1632–1639. doi: 10.1016/j.jalz.2018.06.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Helgadottir HT, Lundin P, Wallen Arzt E, Lindstrom AK, Graff C, Eriksson M. Somatic mutation that affects transcription factor binding upstream of CD55 in the temporal cortex of a late-onset Alzheimer disease patient. Hum Mol Genet. 2019;28(16):2675–2685. doi: 10.1093/hmg/ddz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lodato MA, Walsh CA. Genome aging: somatic mutation in the brain links age-related decline with disease and nominates pathogenic mechanisms. Hum Mol Genet. 2019;28(R2):R197–R206. doi: 10.1093/hmg/ddz191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11(8):457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang KL, Marcora E, Pimenova AA, Di Narzo AF, Kapoor M, Jin SC, et al. A common haplotype lowers PU.1 expression in myeloid cells and delays onset of Alzheimer's disease. Nat Neurosci. 2017;20(8):1052–1061. doi: 10.1038/nn.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sierksma A, Lu A, Mancuso R, Fattorelli N, Thrupp N, Salta E, et al. Novel Alzheimer risk genes determine the microglia response to amyloid-beta but not to TAU pathology. EMBO Mol Med. 2020;12(3):e10606. doi: 10.15252/emmm.201910606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pimenova AA, Herbinet M, Gupta I, Machlovi SI, Bowles KR, Marcora E, et al. Alzheimer's-associated PU.1 expression levels regulate microglial inflammatory response. Neurobiol Dis. 2021;148:105217. doi: 10.1016/j.nbd.2020.105217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Novikova G, Kapoor M, Tcw J, Abud EM, Efthymiou AG, Chen SX, et al. Integration of Alzheimer's disease genetics and myeloid genomics identifies disease risk regulatory elements and genes. Nat Commun. 2021;12(1):1610. doi: 10.1038/s41467-021-21823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ennerfelt HE, Lukens JR. The role of innate immunity in Alzheimer's disease. Immunol Rev. 2020;297(1):225–246. doi: 10.1111/imr.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Podlesny-Drabiniok A, Marcora E, Goate AM. Microglial phagocytosis: a disease-associated process emerging from Alzheimer's disease genetics. Trends Neurosci. 2020;43(12):965–979. doi: 10.1016/j.tins.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Daily KP, Amer A. Microglial autophagy-mediated clearance of amyloid-beta plaques is dysfunctional in Alzheimer’s disease mice. Alzheimers Dement. 2020;16(S3):e044120. doi: 10.1002/alz.044120. [DOI] [Google Scholar]
- 97.Uddin MS, Stachowiak A, Mamun AA, Tzvetkov NT, Takeda S, Atanasov AG, et al. Autophagy and Alzheimer’s disease: from molecular mechanisms to therapeutic implications. Front Aging Neurosci. 2018 doi: 10.3389/fnagi.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu J, Liu CC, Chen XF, Zhang YW, Xu H, Bu G. Opposing effects of viral mediated brain expression of apolipoprotein E2 (apoE2) and apoE4 on apoE lipidation and Abeta metabolism in apoE4-targeted replacement mice. Mol Neurodegener. 2015;10(1):6. doi: 10.1186/s13024-015-0001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kober DL, Stuchell-Brereton MD, Kluender CE, Dean HB, Strickland MR, Steinberg DF, et al. Functional insights from biophysical study of TREM2 interactions with apoE and Aβ1-42. Alzheimers Dement. 2021;17(3):475–488. doi: 10.1002/alz.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ulrich JD, Ulland TK, Mahan TE, Nystrom S, Nilsson KP, Song WM, et al. ApoE facilitates the microglial response to amyloid plaque pathology. J Exp Med. 2018;215(4):1047–1058. doi: 10.1084/jem.20171265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin YT, Seo J, Gao F, Feldman HM, Wen HL, Penney J, et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer's disease phenotypes in human iPSC-derived brain cell types. Neuron. 2018;98(6):1141–1154 e1147. doi: 10.1016/j.neuron.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.DeMattos RB, O'Dell MA, Parsadanian M, Taylor JW, Harmony JA, Bales KR, et al. Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2002;99(16):10843–10848. doi: 10.1073/pnas.162228299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang F, Kumano M, Beraldi E, Fazli L, Du C, Moore S, et al. Clusterin facilitates stress-induced lipidation of LC3 and autophagosome biogenesis to enhance cancer cell survival. Nat Commun. 2014;5(1):5775. doi: 10.1038/ncomms6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yeh FL, Wang Y, Tom I, Gonzalez LC, Sheng M. TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron. 2016;91(2):328–340. doi: 10.1016/j.neuron.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 105.Zhao Y, Wu X, Li X, Jiang LL, Gui X, Liu Y, et al. TREM2 is a receptor for β-amyloid that mediates microglial function. Neuron. 2018;97(5):1023–1031.e1027. doi: 10.1016/j.neuron.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lessard CB, Malnik SL, Zhou Y, Ladd TB, Cruz PE, Ran Y, et al. High-affinity interactions and signal transduction between Aβ oligomers and TREM2. EMBO Mol Med. 2018 doi: 10.15252/emmm.201809027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nizami S, Hall-Roberts H, Warrier S, Cowley SA, Di Daniel E. Microglial inflammation and phagocytosis in Alzheimer's disease: potential therapeutic targets. Br J Pharmacol. 2019;176(18):3515–3532. doi: 10.1111/bph.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Al-Fadhel SZ, Al-Ghuraibawi NHA, Mohammed Ali DM, Al-Hakeim HK. Serum cytokine dependent hematopoietic cell linker (CLNK) as a predictor for the duration of illness in type 2 diabetes mellitus. J Diabetes Metab Disord. 2020;19(2):959–966. doi: 10.1007/s40200-020-00588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Song WM, Joshita S, Zhou Y, Ulland TK, Gilfillan S, Colonna M. Humanized TREM2 mice reveal microglia-intrinsic and -extrinsic effects of R47H polymorphism. J Exp Med. 2018;215(3):745–760. doi: 10.1084/jem.20171529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yuan P, Condello C, Keene CD, Wang Y, Bird TD, Paul SM, et al. TREM2 haplodeficiency in mice and humans impairs the microglia barrier function leading to decreased amyloid compaction and severe axonal dystrophy. Neuron. 2016;92(1):252–264. doi: 10.1016/j.neuron.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 111.Kleineidam L, Chouraki V, Prochnicki T, van der Lee SJ, Madrid-Marquez L, Wagner-Thelen H, et al. PLCG2 protective variant p.P522R modulates tau pathology and disease progression in patients with mild cognitive impairment. Acta Neuropathol. 2020;139(6):1025–1044. doi: 10.1007/s00401-020-02138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schlepckow K, Kleinberger G, Fukumori A, Feederle R, Lichtenthaler SF, Steiner H, et al. An Alzheimer-associated TREM2 variant occurs at the ADAM cleavage site and affects shedding and phagocytic function. EMBO Mol Med. 2017;9(10):1356–1365. doi: 10.15252/emmm.201707672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang T, Hou JK, Gao Q, Yu JT, Zhou JS, Zhao HD, et al. TREM2 p.H157Y variant and the risk of Alzheimer's disease: a meta-analysis involving 14,510 subjects. Curr Neurovasc Res. 2016;13(4):318–320. doi: 10.2174/1567202613666160808095530. [DOI] [PubMed] [Google Scholar]
- 114.Li R, Wang X, He P. The most prevalent rare coding variants of TREM2 conferring risk of Alzheimer's disease: a systematic review and meta-analysis. Exp Ther Med. 2021;21(4):347. doi: 10.3892/etm.2021.9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deming Y, Filipello F, Cignarella F, Cantoni C, Hsu S, Mikesell R, et al. The MS4A gene cluster is a key modulator of soluble TREM2 and Alzheimer's disease risk. Sci Transl Med. 2019 doi: 10.1126/scitranslmed.aau2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao L. CD33 in Alzheimer's disease - biology, pathogenesis, and therapeutics: a mini-review. Gerontology. 2019;65(4):323–331. doi: 10.1159/000492596. [DOI] [PubMed] [Google Scholar]
- 117.Bhattacherjee A, Jung J, Zia S, Ho M, Eskandari-Sedighi G, St. Laurent CD, et al. The CD33 short isoform is a gain-of-function variant that enhances Aβ1–42 phagocytosis in microglia. Mol Neurodegener. 2021;16(1):19. doi: 10.1186/s13024-021-00443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Siddiqui SS, Springer SA, Verhagen A, Sundaramurthy V, Alisson-Silva F, Jiang W, et al. The Alzheimer's disease-protective CD33 splice variant mediates adaptive loss of function via diversion to an intracellular pool. J Biol Chem. 2017;292(37):15312–15320. doi: 10.1074/jbc.M117.799346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Salih DA, Bayram S, Guelfi S, Reynolds RH, Shoai M, Ryten M, et al. Genetic variability in response to amyloid beta deposition influences Alzheimer's disease risk. Brain Commun. 2019;1(1):fcz022. doi: 10.1093/braincomms/fcz022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rathore N, Ramani SR, Pantua H, Payandeh J, Bhangale T, Wuster A, et al. Paired Immunoglobulin-like Type 2 Receptor Alpha G78R variant alters ligand binding and confers protection to Alzheimer's disease. PLoS Genet. 2018;14(11):e1007427. doi: 10.1371/journal.pgen.1007427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miller J, McKinnon L, Murcia JDG, Kauwe J, Ridge PG. Synonymous variant rs2405442 in PILRA is associated with Alzheimer's disease and affects RNA expression by destroying a ramp sequence. Alzheimers Dement. 2020;16(S3):e045988. doi: 10.1002/alz.045988. [DOI] [Google Scholar]
- 122.Agostini S, Costa AS, Mancuso R, Guerini FR, Nemni R, Clerici M. The PILRA G78R variant correlates with higher HSV-1-specific IgG titers in Alzheimer’s disease. Cell Mol Neurobiol. 2019;39(8):1217–1221. doi: 10.1007/s10571-019-00712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fonseca MI, Chu S, Pierce AL, Brubaker WD, Hauhart RE, Mastroeni D, et al. Analysis of the putative role of CR1 in Alzheimer's disease: genetic association, expression and function. PLoS ONE. 2016;11(2):e0149792. doi: 10.1371/journal.pone.0149792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mahmoudi R, Kisserli A, Novella JL, Donvito B, Drame M, Reveil B, et al. Alzheimer's disease is associated with low density of the long CR1 isoform. Neurobiol Aging. 2015;36(4):1766 e1765–1766 e1712. doi: 10.1016/j.neurobiolaging.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 125.Brouwers N, Van Cauwenberghe C, Engelborghs S, Lambert JC, Bettens K, Le Bastard N, et al. Alzheimer risk associated with a copy number variation in the complement receptor 1 increasing C3b/C4b binding sites. Mol Psychiatry. 2012;17(2):223–233. doi: 10.1038/mp.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar Abeta-stimulated microglial activation. J Neurosci. 2009;29(38):11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Minoretti P, Gazzaruso C, Vito CD, Emanuele E, Bianchi M, Coen E, et al. Effect of the functional toll-like receptor 4 Asp299Gly polymorphism on susceptibility to late-onset Alzheimer's disease. Neurosci Lett. 2006;391(3):147–149. doi: 10.1016/j.neulet.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 128.Miron J, Picard C, Lafaille-Magnan M, Savard M, Labonté A, Breitner J, et al. Association of TLR4 with Alzheimer's disease risk and presymptomatic biomarkers of inflammation. Alzheimers Dement. 2019;15(7):951–960. doi: 10.1016/j.jalz.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 129.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5(461):461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luo L, Curson JEB, Liu L, Wall AA, Tuladhar N, Lucas RM, et al. SCIMP is a universal Toll-like receptor adaptor in macrophages. J Leukoc Biol. 2020;107(2):251–262. doi: 10.1002/JLB.2MA0819-138RR. [DOI] [PubMed] [Google Scholar]
- 131.Luo L, Bokil NJ, Wall AA, Kapetanovic R, Lansdaal NM, Marceline F, et al. SCIMP is a transmembrane non-TIR TLR adaptor that promotes proinflammatory cytokine production from macrophages. Nat Commun. 2017;8(1):14133. doi: 10.1038/ncomms14133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cohen P, Strickson S. The role of hybrid ubiquitin chains in the MyD88 and other innate immune signalling pathways. Cell Death Differ. 2017;24(7):1153–1159. doi: 10.1038/cdd.2017.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Beck TN, Nicolas E, Kopp MC, Golemis EA. Adaptors for disorders of the brain? The cancer signaling proteins NEDD9, CASS4, and PTK2B in Alzheimer's disease. Oncoscience. 2014;1(7):486–503. doi: 10.18632/oncoscience.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Raj T, Li YI, Wong G, Humphrey J, Wang M, Ramdhani S, et al. Integrative transcriptome analyses of the aging brain implicate altered splicing in Alzheimer's disease susceptibility. Nat Genet. 2018;50(11):1584–1592. doi: 10.1038/s41588-018-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Johnson ECB, Dammer EB, Duong DM, Yin L, Thambisetty M, Troncoso JC, et al. Deep proteomic network analysis of Alzheimer's disease brain reveals alterations in RNA binding proteins and RNA splicing associated with disease. Mol Neurodegener. 2018;13(1):52. doi: 10.1186/s13024-018-0282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Conway OJ, Carrasquillo MM, Wang X, Bredenberg JM, Reddy JS, Strickland SL, et al. ABI3 and PLCG2 missense variants as risk factors for neurodegenerative diseases in Caucasians and African Americans. Mol Neurodegener. 2018;13(1):53. doi: 10.1186/s13024-018-0289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Satoh JI, Kino Y, Yanaizu M, Tosaki Y, Sakai K, Ishida T, et al. Microglia express ABI3 in the brains of Alzheimer's disease and Nasu-Hakola disease. Intractable Rare Dis Res. 2017;6(4):262–268. doi: 10.5582/irdr.2017.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yasuda-Yamahara M, Rogg M, Frimmel J, Trachte P, Helmstaedter M, Schroder P, et al. FERMT2 links cortical actin structures, plasma membrane tension and focal adhesion function to stabilize podocyte morphology. Matrix Biol J Int Soc Matrix Biol. 2018;68–69:263–279. doi: 10.1016/j.matbio.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 139.Frese S, Schubert WD, Findeis AC, Marquardt T, Roske YS, Stradal TE, et al. The phosphotyrosine peptide binding specificity of Nck1 and Nck2 Src homology 2 domains. J Biol Chem. 2006;281(26):18236–18245. doi: 10.1074/jbc.M512917200. [DOI] [PubMed] [Google Scholar]
- 140.Shen R, Zhao X, He L, Ding Y, Xu W, Lin S, et al. Upregulation of RIN3 induces endosomal dysfunction in Alzheimer's disease. Transl Neurodegener. 2020;9(1):26. doi: 10.1186/s40035-020-00206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ridge PG, Karch CM, Hsu S, Arano I, Teerlink CC, Ebbert MTW, et al. Linkage, whole genome sequence, and biological data implicate variants in RAB10 in Alzheimer's disease resilience. Genome Med. 2017;9(1):100. doi: 10.1186/s13073-017-0486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Davies AK, Itzhak DN, Edgar JR, Archuleta TL, Hirst J, Jackson LP, et al. AP-4 vesicles contribute to spatial control of autophagy via RUSC-dependent peripheral delivery of ATG9A. Nat Commun. 2018;9(1):3958. doi: 10.1038/s41467-018-06172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cuajungco MP, Kiselyov K. The mucolipin-1 (TRPML1) ion channel, transmembrane-163 (TMEM163) protein, and lysosomal zinc handling. Front Biosci (Landmark Ed) 2017;22:1330–1343. doi: 10.2741/4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kuil LE, Lopez Marti A, Carreras Mascaro A, van den Bosch JC, van den Berg P, van der Linde HC, et al. Hexb enzyme deficiency leads to lysosomal abnormalities in radial glia and microglia in zebrafish brain development. Glia. 2019;67(9):1705–1718. doi: 10.1002/glia.23641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Poletto E, Pasqualim G, Giugliani R, Matte U, Baldo G. Worldwide distribution of common IDUA pathogenic variants. Clin Genet. 2018;94(1):95–102. doi: 10.1111/cge.13224. [DOI] [PubMed] [Google Scholar]
- 146.Patel S, Homaei A, El-Seedi HR, Akhtar N. Cathepsins: Proteases that are vital for survival but can also be fatal. Biomed Pharmacother. 2018;105:526–532. doi: 10.1016/j.biopha.2018.05.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ruas M, Chuang KT, Davis LC, Al-Douri A, Tynan PW, Tunn R, et al. TPC1 has two variant isoforms, and their removal has different effects on endo-lysosomal functions compared to loss of TPC2. Mol Cell Biol. 2014;34(21):3981–3992. doi: 10.1128/mcb.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Feng T, Mai S, Roscoe JM, Sheng RR, Ullah M, Zhang J, et al. Loss of TMEM106B and PGRN leads to severe lysosomal abnormalities and neurodegeneration in mice. EMBO Rep. 2020;21(10):e50219. doi: 10.15252/embr.202050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mendsaikhan A, Tooyama I, Walker DG. Microglial progranulin: involvement in Alzheimer's disease and neurodegenerative diseases. Cells. 2019 doi: 10.3390/cells8030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huai W, Liu X, Wang C, Zhang Y, Chen X, Chen X, et al. KAT8 selectively inhibits antiviral immunity by acetylating IRF3. J Exp Med. 2019;216(4):772–785. doi: 10.1084/jem.20181773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dorrington MG, Fraser IDC. NF-kappaB signaling in macrophages: dynamics, crosstalk, and signal Integration. Front Immunol. 2019;10(705):705. doi: 10.3389/fimmu.2019.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S3–23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Das R, Chinnathambi S. Microglial priming of antigen presentation and adaptive stimulation in Alzheimer's disease. Cell Mol Life Sci. 2019;76(19):3681–3694. doi: 10.1007/s00018-019-03132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Raju S, Kometani K, Kurosaki T, Shaw AS, Egawa T. The adaptor molecule CD2AP in CD4 T cells modulates differentiation of follicular helper T cells during chronic LCMV infection. PLoS Pathog. 2018;14(5):e1007053. doi: 10.1371/journal.ppat.1007053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Draber P, Vonkova I, Stepanek O, Hrdinka M, Kucova M, Skopcova T, et al. SCIMP, a transmembrane adaptor protein involved in major histocompatibility complex class II signaling. Mol Cell Biol. 2011;31(22):4550–4562. doi: 10.1128/MCB.05817-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu YH, Wang J, Li QX, Fowler CJ, Zeng F, Deng J, et al. Association of naturally occurring antibodies to beta-amyloid with cognitive decline and cerebral amyloidosis in Alzheimer's disease. Sci Adv. 2021 doi: 10.1126/sciadv.abb0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, et al. A unique microglia type associated with restricting development of Alzheimer's disease. Cell. 2017;169(7):1276–1290 e1217. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 158.Deczkowska A, Keren-Shaul H, Weiner A, Colonna M, Schwartz M, Amit I. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell. 2018;173(5):1073–1081. doi: 10.1016/j.cell.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 159.Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. 2019;50(1):253–271 e256. doi: 10.1016/j.immuni.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Friedman BA, Srinivasan K, Ayalon G, Meilandt WJ, Lin H, Huntley MA, et al. Diverse brain myeloid expression profiles reveal distinct microglial activation states and aspects of Alzheimer's disease not evident in mouse models. Cell Rep. 2018;22(3):832–847. doi: 10.1016/j.celrep.2017.12.066. [DOI] [PubMed] [Google Scholar]
- 161.Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, et al. Single-cell transcriptomic analysis of Alzheimer's disease. Nature. 2019;570(7761):332–337. doi: 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Olah M, Menon V, Habib N, Taga MF, Ma Y, Yung CJ, et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer's disease. Nat Commun. 2020;11(1):6129. doi: 10.1038/s41467-020-19737-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Srinivasan K, Friedman BA, Etxeberria A, Huntley MA, van der Brug MP, Foreman O, et al. Alzheimer's patient microglia exhibit enhanced aging and unique transcriptional activation. Cell Rep. 2020;31(13):107843. doi: 10.1016/j.celrep.2020.107843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Berchtold D, Priller J, Meisel C, Meisel A. Interaction of microglia with infiltrating immune cells in the different phases of stroke. Brain Pathol. 2020;30(6):1208–1218. doi: 10.1111/bpa.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Harms AS, Thome AD, Yan Z, Schonhoff AM, Williams GP, Li X, et al. Peripheral monocyte entry is required for alpha-synuclein induced inflammation and neurodegeneration in a model of Parkinson disease. Exp Neurol. 2018;300:179–187. doi: 10.1016/j.expneurol.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Da Mesquita S, Papadopoulos Z, Dykstra T, Brase L, Farias FG, Wall M, et al. Meningeal lymphatics affect microglia responses and anti-Abeta immunotherapy. Nature. 2021;593(7858):255–260. doi: 10.1038/s41586-021-03489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang J, Gu BJ, Masters CL, Wang YJ. A systemic view of Alzheimer disease - insights from amyloid-beta metabolism beyond the brain. Nat Rev Neurol. 2017;13(10):612–623. doi: 10.1038/nrneurol.2017.111. [DOI] [PubMed] [Google Scholar]
- 168.Gagliano SA, Pouget JG, Hardy J, Knight J, Barnes MR, Ryten M, et al. Genomics implicates adaptive and innate immunity in Alzheimer's and Parkinson's diseases. Ann Clin Transl Neurol. 2016;3(12):924–933. doi: 10.1002/acn3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tansey KE, Cameron D, Hill MJ. Genetic risk for Alzheimer's disease is concentrated in specific macrophage and microglial transcriptional networks. Genome Med. 2018;10(1):14. doi: 10.1186/s13073-018-0523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Frenkel D, Wilkinson K, Zhao L, Hickman SE, Means TK, Puckett L, et al. Scara1 deficiency impairs clearance of soluble amyloid-beta by mononuclear phagocytes and accelerates Alzheimer's-like disease progression. Nat Commun. 2013;4:2030. doi: 10.1038/ncomms3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fiala M, Lin J, Ringman J, Kermani-Arab V, Tsao G, Patel A, et al. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer's disease patients. J Alzheimers Dis. 2005;7(3):221–232. doi: 10.3233/jad-2005-7304. [DOI] [PubMed] [Google Scholar]
- 172.Gu BJ, Huang X, Ou A, Rembach A, Fowler C, Avula PK, et al. Innate phagocytosis by peripheral blood monocytes is altered in Alzheimer's disease. Acta Neuropathol. 2016;132(3):377–389. doi: 10.1007/s00401-016-1596-3. [DOI] [PubMed] [Google Scholar]
- 173.Darlington D, Li S, Hou H, Habib A, Tian J, Gao Y, et al. Human umbilical cord blood-derived monocytes improve cognitive deficits and reduce amyloid-beta pathology in PSAPP mice. Cell Transpl. 2015;24(11):2237–2250. doi: 10.3727/096368915X688894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bach JP, Dodel R. Naturally occurring autoantibodies against β-Amyloid. Adv Exp Med Biol. 2012;750:91–99. doi: 10.1007/978-1-4614-3461-0_7. [DOI] [PubMed] [Google Scholar]
- 175.van Dyck CH. Anti-amyloid-β monoclonal antibodies for Alzheimer's disease: pitfalls and promise. Biol Psychiatry. 2018;83(4):311–319. doi: 10.1016/j.biopsych.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature. 2016;537(7618):50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 177.Salloway S, Farlow M, McDade E, Clifford DB, Wang G, Llibre-Guerra JJ, et al. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Nat Med. 2021;27(7):1187–1196. doi: 10.1038/s41591-021-01369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691–1704. doi: 10.1056/NEJMoa2100708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analysed and summarised during this study were included in this article and its supplementary information files.