Abstract
The transforming growth factor β (TGF-β) family of cytokines comprises a group of proteins, their receptors, and effector molecules that, in a coordinated manner, modulate a plethora of physiological and pathophysiological processes. TGF-β1 is the best known and plausibly most active representative of this group. It acts as an immunosuppressant, contributes to extracellular matrix remodeling, and stimulates tissue fibrosis, differentiation, angiogenesis, and epithelial-mesenchymal transition. In recent years, this cytokine has been established as a vital regulator of organismal aging and cellular senescence. Finally, the role of TGF-β1 in cancer progression is no longer in question. Because this protein is involved in so many, often overlapping phenomena, the question arises whether it can be considered a molecular bridge linking some of these phenomena together and governing their reciprocal interactions. In this study, we reviewed the literature from the perspective of the role of various TGF-β family members as regulators of a complex mutual interplay between senescence and cancer. These aspects are then considered in a broader context of remaining TGF-β-related functions and coexisting processes. The main narrative axis in this work is centered around the interaction between the senescence of normal peritoneal cells and ovarian cancer cells. The discussion also includes examples of TGF-β activity at the interface of other normal and cancer cell types.
Keywords: Cancer, Cellular senescence, Cytokines, Transforming growth factor
Introduction
Cytokines constitute a broad and heterogeneous group of low-molecular-weight molecules that include interleukins, chemokines, interferons, hemopoietic agents, tumor necrosis factors (TNFs) and transforming growth factor-beta (TGF-β) superfamily members [1]. One of the most unique features of all cytokines is their pleiotropism, which refers to their ability to contribute to various cellular, molecular, and biochemical interactions and processes. Another striking feature of cytokines is their widespread distribution, resulting from the multiplicity of cellular sources, which, taking into account their diversified functionality, makes them, next to hormones, one of the most important regulators of cellular metabolism in both physiological and pathological conditions [2].
The current knowledge of cytokine biology is the most advanced with respect to cytokines' capacity to regulate the functioning of immune cells and to modulate—both promote and inhibit—immune responses [3]. Recently, much has been learned about the role of cytokines as mediators of intercellular interactions between cancer cells and the noncancerous tissue microenvironment (reactive stroma), especially in the context of the formation of cancer metastases and supporting events, such as angiogenesis and epithelial-mesenchymal transition (EMT) [4]. At the same time, there is considerable evidence pointing to the engagement of cytokines in the aging process, either at the organismal or cellular level (senescence) [5]. In both of the abovementioned areas of biomedical research, i.e., cancer and aging biology, TGF-β superfamily members, and TGF-β1 in particular, appear to be the key players [6]. Interestingly, these molecules also seem to operate at the interface of these processes, especially mediating the mutual interplay between senescent and cancer cells. This specific aspect of TGF-β superfamily member activity has never been comprehensively addressed, and earlier and elegant reviews ([6–8], for example) were focused on the role of TGF-β from an isolated perspective of one specific phenomenon or another. For this reason, the main goal and novelty of this study was to fill the obvious gap regarding the placement of TGF-β signaling as the joint and integral element of cancer–aging interactions. The narration is focused on but not limited to the intraperitoneal dissemination of ovarian cancer and pro-cancerogenic activity of senescent peritoneal cells.
TGF-β signaling and function
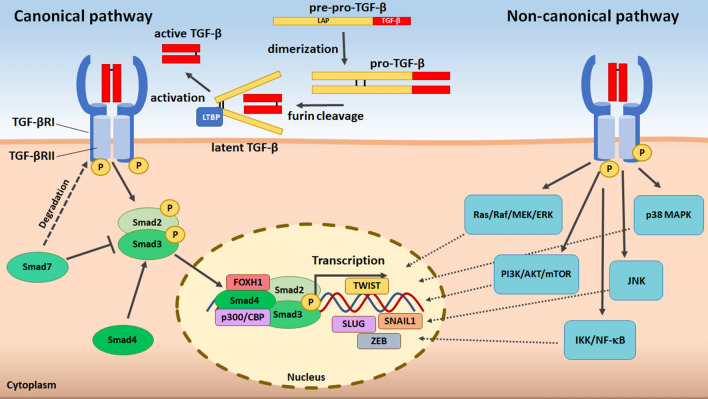
The TGF-β superfamily of cytokines comprises over 50 members, of which TGF-βs (in humans, isoforms TGF-β1, -β2, and -β3), bone morphogenetic proteins (BMPs), growth and differentiation factors (GDFs), activins and inhibin are the most prominent. Moreover, TGF-β signaling includes specific receptors (TGF-βRI and -RII), a latency-associated protein (LAP), latent TGF-β binding proteins (LTBPs), and a group of Smad-dependent (Smad-2, -3, -4) and Smad-independent downstream pathways [8–11].
TGF-β1 is the very first archetypic member of this large family of biomolecules. Discovered in 1983 [12], it is the most extensively studied representative of this group. This and the remaining TGF-βs are synthesized in an inactive form in a complex consisting of TGF-β and LAP (initially as monomeric pre-pro-TGF-β and then dimeric pro-TGF-β). Afterward, the TGF-β propeptide is cleaved by the enzyme furin to form latent (but still inactive) TGF-β, where mature TGF-β molecules remain attached to the LAP. The activation step is guided by LTBPs, which direct the complexed TGF-β toward the membrane compartment, where it is subjected to extracellular matrix (ECM) proteases, integrins, reactive oxygen species (ROS) or thrombospondin 1 (TSP-1) [13–16]. Once activated, mature TGF-βs gain the ability to interact with transmembrane TGF-βRII, triggering recruitment and heteromeric complexing with TGF-βRI and a further signal cascade. Canonically, TGF-β effector signaling utilizes the phosphorylation of Smad2 and Smad3 transcription factors, which are transferred to the nucleus in a complex with Smad4. Alternatively, TGF-β operates independently of Smad, engaging a variety of signal transducers, such as Ras/Raf/MEK/ERK, p38 MAPK, JNK, PI3K/AKT/mTOR and IKK/NF-κB [8, 17] (Fig. 1 with additional explanations in a legend).
Fig. 1.
Major signaling pathways of intracellular TGF-β activity. TGF-βs are produced as inactive molecules, initially monomers (pre-pro-TGF-β) and then dimers (pro-TGF-β), complexed with LAP. Upon the cleavage of the complex by the enzyme furin (the formation of latent TGF-β) and cytokine activation (guided by LTBP), mature TGF-βs become recognizable by their receptors. Active TGF-βs connect with TGF-βRII within the plasma membrane, which is followed by the recruitment and phosphorylation of TGF-βRI, leading to the formation of a heterotetrameric complex. The serine–threonine activity of TGF-βRI leads to the phosphorylation of Smad2 and Smad3, which assemble with Smad4 and are transferred to the nucleus. There, the Smads interact with other proteins, strengthening their affinity to DNA and driving the activation or repression of TGF-β target gene transcription. Within the cytoplasm, Smad7 restricts this signaling by stimulating turnover of TGF-β receptors. The alternate (noncanonical) pathway of TGF-β signaling starts from analogical cytokine processing, activation, and receptor binding; however, further intracellular signal transmission is Smad-independent. Instead, it engages other TGF-β downstream routes, such as Ras/Raf/MEK/ERK, p38 MAPK, JNK, PI3K/AKT/mTOR and IKK/NF-κB, and culminates in the nucleus modulating the transcription of TGF-β-related genes by interactions with other, context-dependent transcription factors
TGF-β is synthesized and released by inflammatory (neutrophils, macrophages) and noninflammatory cells (platelets, fibroblasts, endothelial cells) [18–22]. At the same time, those and other cells act as recipients of this cytokine signaling [23–25], demonstrating both an autocrine and a paracrine TGF-β activity mode. This activity is, in turn, very broad and includes the inhibition of the release of proinflammatory cytokines (immunosuppression) [26], regulation of cell proliferation (mainly anti-proliferative effects) [27], differentiation [28], apoptosis (both induction [29] and inhibition [30]), autophagy [31], and senescence [6]. In addition, TGF-β controls mitochondrial metabolism [32], induces the generation of ROS [33], mediates the secretion of angiogenic mediators (e.g., vascular endothelial growth factor [VEGF]) [34], and stimulates ECM component (e.g., fibronectin [35]) deposition, leading to cellular hypertrophy [36] and tissue fibrosis [37]. As per the remaining members of the family, BMPs are linked, among others, with osteogenesis (BMP-2, BMP-6, BMP-9) [38], chondrogenesis (BMP-1, BMP-2A, BMP-3) [39], angiogenesis (BMP-4) [40], and adipogenesis (BMP-2, BMP-4) [41].
Normal cells are not the sole sources of and targets for TGF-β activity. Cytokines play several roles in the development and progression of cancer cells [42]. In this context, TGF-β has been recognized—apart from the abovementioned behaviors typical of normal cells—as a trigger of EMT and stemness features [43] and as an agent determining chemoresistance [44]. The motility or invasiveness of multiple types of malignant cells has been found to be promoted by TGF-β [45, 46]. Another example of the pro-cancerogenic involvement of TGF-β is the development of reactive stroma characteristics (e.g., the formation of cancer-associated cells), as well as metabolic reprogramming of the tumor microenvironment (e.g., increased ROS efflux and α-ketoglutarate overproduction), leading to energetic fostering of cancer cells [47]. Years ago, Steiner and Barack provided an elegant summary of the role of TGF-β signaling in cancer. They injected TGF-β-overexpressing cells into animals transfected with prostate cancer cells, which resulted in a remarkable increase in the tumor size and an increased frequency of lung metastases compared with untreated animals [48]. At the same time, it must be emphasized that TGF-β exerts some clear anticancer activities, which allows TGF-β signaling overall to be treated as a tumor suppressor mechanism. One of the most obvious aspects of such activity is the ability of the cytokine to restrict normal cell proliferation [49], preventing the accumulation of potentially oncogenic mutations and malignant transformation. Under some circumstances, cytokines also contribute to the inhibition of cancer cell growth [50]. Finally, TGF-β acts as a repressor of human telomerase reverse transcriptase (hTERT), restricting the immortality of cancer cells [51] and likely leading to their senescence [52].
Cancer is also associated with aberrant expression and signaling of various BMPs. Intriguingly, BMPs seem to have bidirectional activity in cancer cells, contributing either to increased expansion or suppression of the disease [53]. The pro-cancerous activity of BMPs has been revealed in experiments using the BMP antagonist Noggin, whose overexpression translates to reduced tumor size and bone destruction in an animal model of prostate cancer [54] as well as to decreased EMT and invasive properties of melanoma cells [55]. BMP-2 was found, in turn, to contribute to the increased motility of cancer cells [56]. At the same time, BMP-4-treated cells displayed considerably smaller tumors in a xenograft model than control cells, suggesting some anticancer functions of this molecule, likely achieved through the induction of cancer cell senescence [57]. Tumors expressing BMP-3B appeared to develop slower than their counterparts, which were negative for this cytokine [58]. Generally, the activity of BMPs in cancer is complex, and multiple experiments performed in this regard clearly imply that their dichotomic, positive or negative impact may be strictly associated with the cancer type, other signaling pathways engaged, and a more detailed tissue context [59].
TGF-β signature in aging and senescence
Organismal aging and longevity
Aged organisms are more prone to several systemic disorders. To a large extent, this is caused by their decreased ability to cope with external and internal stressors of various (physical, chemical, biological) natures [60]. One of the critical culprits of this homeodynamic disequilibrium is the deterioration of defense responses, manifested by immunosenescence (declined capacity of immune cells to properly counteract antigen-associated challenges) and inflamm-aging (a chronic inflammation-like state at the cellular and tissue levels) [61, 62]. For obvious reasons, cytokines, including TGF-βs, play a role in both of the aforementioned phenomena, although reports regarding age-associated changes in the cytokine level are somewhat conflicting. For example, Zhao et al. reported that the concentration of TGF-β1 is lower in serum from elderly individuals [63], and others have suggested that the age-dependent TGF-β1 decline may start in earlier periods of life [64]. On the other hand, Forsey et al. revealed that the plasma level of TGF-β1 increases during aging [65], which is in line with findings by other authors [66]. Additionally, the signaling originating from BMP-4, another member of the TGF-β family, tends to increase with aging, which has been attributed to age-related impairment in neurogenesis and cognition [67].
The polymorphisms in the TGF-β1 coding gene seem, in turn, to contribute to human longevity. For instance, the G/C915 polymorphism appeared to correlate with an extraordinary lifespan in an Italian cohort study [68]. Another polymorphism in this gene (codon 10, T>C, rs1800471), which has also been found in centenarians [69] and which translates to altered plasma levels of TGF-β1 [70], may explain the increased production of this anti-inflammatory cytokine by elderly individuals.
Aging also determines some abnormalities at the level of TGF-β receptors. More precisely, it has been found to drive the conversion of TGF-β type I receptor activin-like kinase 5 (Alk5) to Alk1 in cartilage. Because Alk5 levels decrease at higher dynamics than Alk1, the Alk1/Alk5 ratio increases [71]. It is thought that the age-dependent decline in Alk5 levels may be associated with an altered Alk5 synthesis/degradation ratio [72]. Eventually, these changes result in a deterioration of TGF-β capacity to maintain cartilage homeostasis, its degradation, and osteoarthritis development [73].
Cellular senescence in vitro
Whereas the overproduction of TGF-β1 at the level of the whole organism (plasma) may be causatively linked with immunosenescence, increased secretion of this cytokine at the cellular level may be an important piece of the puzzle in the disease-promoting inflamm-aging phenomenon [74]. One of the most striking aspects of inflamm-aging is the cancer-promoting activity of senescent cells, which is mechanistically linked to the so-called senescence-associated secretory phenotype (SASP). SASP refers to hypersecretion by senescent cells of a myriad of cytokines, growth factors, and proteolytic enzymes that can support all critical aspects of cancer cell progression, such as proliferation, migration, invasion, EMT, and angiogenesis [75–77]. TGF-βs, and TGF-β1 in particular, are canonical cytokines whose synthesis and release by senescent cells are increased. This effect has been reported at the mRNA and/or protein levels in fibroblasts [78, 79], endothelial cells (TGF-β2 and TGF-βRII) [80, 81], keratinocytes [82], and peritoneal mesothelial cells (PMCs) [83].
Cytokines are also important players in the development of the senescence state itself. In fibroblasts, TGF-β appeared to mediate senescence elicited by overexpression of Polycomb protein chromobox 7 (CBX7)-regulated β3 integrins (ITGB3) in an oncogene-induced senescence (OIS) model, and the process occurred independently of their ligand-binding activity. When siRNA was administered against TGF-β receptor 2 (TGF-βRII), β3 integrin-dependent senescence was abolished [84]. In an in vitro model of paracrine cellular interactions between keratinocytes and fibroblasts, conditioned medium generated by the former induced senescence in the latter, and this activity was abolished upon neutralization of TGF-β [85]. Fibroblasts subjected to the keratinocyte-derived secretome also overproduced autologous TGF-β1 and TGF-β2 in a mechanism involving the activity of reactive oxygen species (ROS) released by keratinocytes [85]. The paracrine induction of senescence (SA-β-Gal/γ-H2A.X-positive phenotype) has also been observed in nonsenescent PMCs and fibroblasts subjected to conditioned medium generated by senescent PMCs. Notably, to acquire its pro-senescence potential, TGF-β1 released to an environment in a latent form had to be activated by TSP-1 [83]. It should be stressed that the pro-senescence activity of TGF-β1 is equivalent to the tumor-suppressive activity of this cytokine, as the cells that cannot divide are obviously resistant to neoplastic conversion [86].
TGF-β pro-senescence activity mechanisms are broad and diverse. In peritoneal mesothelial cells (PMCs) exposed to exogenous recombinant human (rh)TGF-β1, the cytokine inhibited DNA synthesis, upregulated the p27 cell cycle inhibitor, and stimulated protein synthesis, plausibly leading to the development of cellular hypertrophy [25]. Similar to other cell types [87], TGF-β1 activated senescence-associated β-galactosidase (SA-β-Gal) [25], a main biochemical marker of cellular senescence, and the neutralization of TGF-β1 in a conditioned medium translated to decreased activity of the enzyme in cells subjected to that medium [88]. Notably, the induction of SA-β-Gal is not a universal feature of TGF-β1. For instance, it failed to induce the enzyme in primary fibroblasts derived from the mouth, skin and colon [89], which may theoretically result from various concentrations of TGF-β1 in the environment, differences in its activation status, and different expressions of TGF-β1 receptors. Untergasser et al. showed that although TGF-β induces SA-β-Gal in prostate basal cells, this effect is a hallmark of their differentiation rather than senescence [90].
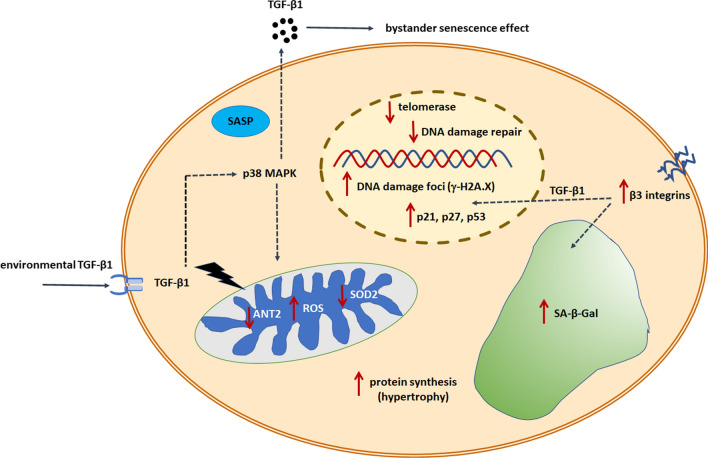
The role of ROS in the pro-senescence activity of TGF-β is unquestioned, although the relationship between these two agents is clearly bidirectional. On one hand, TGF-β1 increased the generation of oxidants in a dose- and time-dependent manner [83], but at the same time, cytokine secretion may be downstream of ROS. This behavior confirms the observation of reduced TGF-β1 content in PMC-derived conditioned medium upon preincubation with the spin-trap ROS scavenger PBN [88] and increased TGF-β1 secretion in cells treated with the strong, exogenous oxidant t-BHP [91]. Similar positioning of TGF-β1 relative to ROS was found in mesangial cells [92]. In addition to direct mitochondrial ROS targeting by TGF-β, the cytokine also affects other elements of mitochondrial metabolism, causally contributing to senescence. These effects include downregulation of adenine nucleotide translocase-2 (ANT2), a protein responsible for the exchange of ADP and ATP through the mitochondrial inner membrane [93], and decreased activity of mitochondrial superoxide dismutase 2 (SOD2) [94] (Fig. 2).
Fig. 2.
TGF-β signaling and molecular modulators of cellular senescence. Signals received by a cell through the stimulation of TGF-β receptors and the activation of downstream signaling cascades lead to the development of a senescence phenotype. This includes an inhibition of the cell cycle (upregulated p21, p27, p53 inhibitory proteins), an accumulation of DNA damage foci, and impaired DNA repair. TGF-β also acts as a suppressor of telomerase, which may contribute to telomere shortening and cancer cell senescence. TGF-β1 remains in a mutual, bidirectional relation with p38 MAPK, which contributes to the formation of SASP and the hypersecretion of the cytokine to the environment and the induction of senescence in nearby nonsenescent cells in an autocrine and paracrine manner. Causatively, TGF-β1-dependent senescence engages abnormal mitochondrial metabolism, manifested by increased generation of ROS, decreased activity of antioxidants, and decreased cell ability to generate ATP. TGF-β1 is also responsible for cellular hypertrophy due to its capacity to stimulate cellular protein synthesis as well as the activity of SA-β-Gal
An essential element of TGF-β1-ROS interplay is the upregulation of p38 mitogen-activated protein kinase (p38 MAPK) [83]. The blockade of this enzyme led to the inhibition of ROS-dependent induction of TGF-β1. At the same time, increased phosphorylation of p38 MAPK may be prevented not only by protecting cells against oxidative stress, but also by inhibiting TGF-β1 [91], which demonstrates the role of this cytokine as an extracellular stressor. Such relationships are not surprising given that p38 MAPK belongs to a group of stress-activated kinases, and its association with oxidative stress has been well documented [95].
Finally, TGF-β1 has also been recognized as a potential link between senescence observed at the cellular (in vitro) level and organismal (in vivo) aging [6]. A correlative analysis of TGF-β1 concentration as a function of omental tissue donor age showed that the older the tissue/PMC donor was, the higher the production of TGF-β1 by early-passage cells isolated from that tissue. At the same time, there was a negative relationship between cumulative population doubling (CPD) values and TGF-β1 levels, implying that this cytokine must contribute to the loss of replicative potential in these cells in vitro [96]. One of the explanations for the age-CPD-TGF-β1 relationship may be the age-dependent accumulation of senescent cells in vivo. There is strong evidence that senescent PMCs aggregate in the omentum, and their frequency in older individuals is higher than that in younger donors [97]. Taking this into account, one may theorize that tissues obtained from older patients contain a higher fraction of TGF-β1-hypersecreting senescent cells, and thus, the culture loses the capacity to divide earlier. Rapisarda et al. arrived at similar conclusions [84]. They observed that skin fibroblasts obtained from older donors display an increased mRNA level for different regulators of the TGF-β pathway, including TGF-β receptors (I and II) and the downstream transcription factors Smad3 and Smad4. They also confirmed that there exists a positive correlation between calendar aging and the cellular senescence phenotype manifested by decreased proliferative capacity of cells, upregulated p16 and p21 cell cycle inhibitors, and increased expression of β3 integrins that induce senescence through TGF-β signaling [84].
The role of BMPs in cellular senescence seems to counteract, at least to some extent, the unidirectional pro-senescence activity of TGF-β1. For example, BMP-7 activates PI3K/AKT signaling, which leads to the inhibition of senescence (decreased SA-β-Gal, p16, p53) in human nucleus pulposus cells [98]. The activation of BMP-SMAD signaling promoted proliferation and suppressed p16-dependent senescence during the generation of induced pluripotent stem cells generated from fibrodysplasia ossificans progressiva fibroblasts [99]. Contrary to these findings, BMP-4 was found to promote senescence of human retinal pigment epithelial cells in a mechanism cooperating with oxidative stress and p53-p21-Rb signaling [100]. The same molecule was found to mediate, through the Smad-dependent upregulation of p16 and p21, the senescence of lung cancer cells subjected to adriamycin [101].
Senescent–cancer cell interplay and the role of TGF-β
Cancer is among the most canonical age-associated diseases. From the cellular perspective, the age-dependent increase in cancer occurrence is based on two factors. The first is a long-term accumulation of oncogenic mutations, whereas the second is based on the accumulation of senescent cells in tissues and their ability to locally generate, mainly through the SASP phenomenon, a unique, tumor-promoting tissue microenvironment [76, 101–103]. These cells have also been recognized as an important element of tumor-associated, reactive stroma [104]. Over the past two decades, the pro-cancerous activity of senescent cells has been established for several cellular models, including melanoma [105], breast cancer [106], prostate cancer [107], lung cancer [108], pancreatic cancer [109], colorectal cancer [110], and ovarian cancer [111].
At the same time, the issue of a potential pro-cancerous activity of senescent cells with respect to childhood cancers is unclear and still underinvestigated. For example, pituitary stem cells whose senescence is triggered by oncogenic β-catenin display SASP, the inhibition of which translates to reduced tumorigenesis in mouse models of pediatric craniopharyngioma [112]. At the same time, Buhl and colleagues found that pilocytic astrocytoma cells, also undergoing oncogene-induced senescence, display SASP, the constituents of which, particularly IL-1β, cause growth arrest of proliferating cells instead of the hypothesized growth promotion [113]. These contrary findings imply that the pro-cancerous phenotype of senescent cells is not a universal phenomenon and that the restrictions may stem from either the cancer type or age specificity of the malignancy. It should also be emphasized that the possibility of SASP that may develop in children, theoretically contributing to altered tumor growth, is plausibly a result—as in the studies indicated above—of premature oncogene-induced senescence (OIS) rather than a proliferation-dependent replicative senescence that needs much more time and is more probable in adults.
Ovarian cancer is one of the most frequent and deadliest malignancies in women [114–116]. The breaking point in the pathogenesis of the disease is the dissemination of cancerous cells within the peritoneal cavity [117–119]. Intraperitoneal spread of ovarian cancer has been found in as many as 70% of patients in advanced stages of the disease [120]. PMCs that line the peritoneal cavity with a single layer of squamous cells play an overriding role in the development of peritoneal carcinomatosis, creating a hospitable metastatic niche for invading cells [121]. As mentioned before, their pro-cancerogenic potential increases when they adopt features of cellular senescence [76, 111], and TGF-β appears to be the key cytokine determining the variety of reciprocal interactions between senescent PMCs and ovarian cancer cells (Fig. 3). At the same time, as will be demonstrated in subsequent sections, such activity of TGF-β family members is not restricted to the pathophysiology of ovarian cancer, making this cytokine a key mediator in senescent–cancer cell interplay.
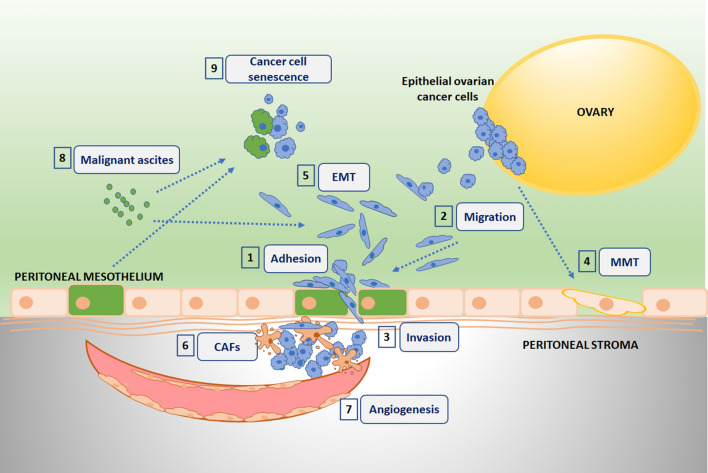
Fig. 3.
Ovarian cancer progression-associated phenomena engaging TGF-β-dependent interplay between cancer and normal senescent cells. 1 The cytokine stimulates increased adhesion of cancer cells to senescent PMCs (green cytoplasm) by the upregulation of surface-fibronectin production and intensified interplay between fibronectin and α5β1 integrins on cancer cells. 2 Migration of cancer cells is promoted by chemotactic activity of the cytokine hypersecreted to an environment by senescent PMCs. 3 Increased transmesothelial invasion of ovarian cancer cells is associated with TGF-β1-dependent deterioration of intercellular junctions in senescent PMCs. 4 Mesothelial-mesenchymal transition (MMT) of young PMCs is triggered by TGF-β1 released by high-grade serous ovarian cancer cells. 5 Epithelial-mesenchymal transition (EMT) of ovarian cancer cells, one of the most critical steps in the intraperitoneal spread of the disease, is causatively linked with the activity of TGF-β1 present in malignant ascites and/or secreted by peritoneal cells. 6 Cancer-associated fibroblasts (CAFs) may develop in the peritoneal cavity from normal stromal fibroblasts subjected to environmental TGF-β1. 7 TGF-β1 released by senescent PMCs contributes to reprogramming of ovarian cancer cell secretome toward the hypersecretion of various pro-angiogenic agents. 8 TGF-β1 present in malignant ascites may contribute to its immunosuppressive activity as well as to the ascites-dependent high aggressiveness of undifferentiated ovarian cancer. 9 TGF-β1 secreted by peritoneal mesothelial cells and fibroblasts, especially senescent cells, as well as present in malignant ascites, is able to promote senescence in ovarian cancer cells (senescent cells have green cytoplasm)
Adhesion
The solid anchoring of cancer cells to biocompatible cellular and/or acellular (ECM proteins) surfaces is critical for their temporary (e.g., during intra- and extravasation) or permanent (e.g., the formation of a distant metastasis) retention at a particular location [122]. For intraperitoneal ovarian cancer progression, the adhesion of cancer cells to normal PMCs, peritoneal fibroblasts or ECM proteins is one of the first steps in cancer metastasis, determining the effectiveness of this process [123, 124]. When PMCs adapt to the senescence phenotype, the effectiveness of ovarian cancer cell attachment is even more pronounced [91]. Mechanistic analysis of this improvement showed that it engages changes in alternative splicing (increased expression of the exon-spliced out transcript of the ED-B region) and an almost three-fold increase in cell surface fibronectin production by senescent cells, resulting in its intensified binding to cancer cell-derived α5β1 integrins. The overproduction of fibronectin was caused, in turn, by ROS-induced TGF-β1, which resembles the oxidant-dependent overproduction of this cytokine in mesangial cells [92] or TGFβ1-promoted fibronectin synthesis in fibroblasts whose premature senescence was elicited by exposure to exogenous oxidants [87]. TGF-β1 was also found to be one of the mediators responsible for increased adhesion of ovarian cancer cells to PMCs and peritoneal fibroblasts upon their exposure to malignant ascites [125], which is a proinflammatory fluid that accumulates in the peritoneum in the majority of patients with advanced peritoneal carcinomatosis [126].
The relationship between TGF-βs and fibronectin was also analyzed in the context of a dormant state (one of the reasons for cancer relapse [127]) in breast cancer cells. Research by Barney et al. revealed that a population of dormant cells contains a fraction of senescent cells, whose secretory phenotype may be one of the sources of TGF-β. In turn, this TGF-β controls fibronectin secretion and assembly, recognized as critical for interactions with αvβ3 and α5β1 integrins, allowing the formation and maintenance of dormancy [128].
There is also a second, more classic aspect of cancer cell adhesion that is necessary for reducing adhesion, facilitating morphological reorganization and increased motility [129]. The mode of the anti-adhesive activity of TGF-βs was described in oral cancer cells. TGF-β family members (TGF-β1 and TGF-β2) released by cancer-associated fibroblasts (CAFs) promote the malignant phenotype of genetically unstable oral squamous carcinoma cells by inhibiting the expression of multiple adhesion molecules (E-cadherin, desmoglein 1 and 3, desmoplakin, desmocollin), which eventually leads to epithelial cell dis-cohesion and increased invasion of keratinocytes in vitro. Notably, this effect was observed in CAFs originating from genetically unstable cells in which—conversely to their genetically stable counterparts—malignant keratinocytes were able to trigger senescence in CAFs, which translated to their paracrine stimulation of keratinocyte invasion [85]. The production of TGF-β1 and TGF-β2 by CAFs established from genetically unstable cells was considerably higher than that by CAFs established from genetically stable lines or normal fibroblasts. This effect, in combination with the activity of CAFs in weakening intercellular epithelial adhesion, explains, along with obvious genetic differences between these cells (TP53 and p16 status), the diversified aggression of these two cancer subtypes [130].
Motility
The motility of cancer cells is manifested by three separate but often overlapping phenomena: proliferation (mitotic activity), migration (chemotaxis), and invasion (chemotaxis-driven and proteolysis-supported cell movement) [131]. All three aspects of ovarian cancer cell motility have been found to be promoted by senescent PMCs to a larger degree than by proliferating, nonsenescent cells. Experiments using exogenous recombinant TGF-β1 showed that the cytokine is capable of stimulating the proliferation and migration of ovarian cancer cells (A2780, SKOV-3) at doses corresponding to the cytokine level in senescent PMC-derived conditioned medium. At the same time, however, interventions with neutralizing antibodies added to that medium did translate exclusively to the inhibition of cell migration (A2780), which implies that the activity of rhTGF-β1 does not precisely mimic all aspects of cell-derived TGF-β1 biological activity [111]. There is also a report emphasizing that the ability of ovarian cancer cells to respond to the promigratory activity of TGF-β may depend on the TP53 status in these cells [132].
Regarding the stimulatory effect of senescent PMCs on ovarian cancer cell invasion, TGF-β1 was found to participate in the senescence-dependent deterioration of intracellular junctions (connexin 43, occludin, desmoglein, E-cadherin), which was recognized as the prime reason for the increased propensity of PMCs to penetrate cancer cells. Indeed, when TGF-β1 signaling was neutralized in presenescent cells, the effectiveness of transmesothelial invasion of both established and primary ovarian cancer cells markedly declined. Regarding signaling pathways cooperating with TGF-β1 in the context of PMCs’ junctional protein expression, AKT, p38 MAPK, and NF-κB appeared to play a role [133].
TGF-β was also found to play a role in the intensified invasion of keratinocytes exposed to conditioned medium from senescent CAFs originating from genetically unstable oral squamous cell carcinomas but not from nonsenescent CAFs from genetically stable carcinomas. The disruption of keratinocyte adhesion followed by increased invasion was found to be controlled by a synergistic relationship between TGF-β1 and MMP-2 [134]. This pair of molecules is known to cooperate because TGF-β1 was found to upregulate endopeptidase [135], whereas MMP-2 has the ability to cleave and activate latent cytokines [136].
Gene expression profiling allows the use of human mammary epithelial cells (HMECs) as a model for studies of basal-like breast cancer, equivalent to triple-negative (estrogen receptor(-)/progesterone receptor(-)/Her2/Neu(-)) breast carcinomas [137]. It has been found that the disruption of TGF-β pathways by an intervention against TGF-βRII in immortalized HMECs bearing ectopically expressed H-Ras-V12 led to the suppression of oncogene-induced senescence and the promotion of tumorigenic and metastatic phenotypes, confirming the antitransformative activity of TGF-β signaling in these cells [138]. Senescent HMECs display augmented TGF-β signaling, as evidenced by the upregulated expression of several transcripts downstream of TGF-β. Importantly, these targets (e.g., MMP-2, uPAR, and CD44) are closely associated with the cell’s ability to invade and metastasize, which explains the relatively high migratory potential of these cells. Moreover, senescent HMECs are characterized by decreased expression of antimigratory Rac1b and increased expression of promigratory Rac1 [137], which suggests that although senescent HMECs with intensified TGF-β signaling are resistant to oncogene-related transformation, they display augmented motility and metastatic potential.
TGF-β-related control over cell proliferation and senescence has also been observed in nonsolid tumors. This is the case, e.g., for acute promyelocytic leukemia cells bearing Pml−/− characteristics, in which the lack of the intact promyelocytic leukemia tumor suppressor (PML) resulted in the inability of TGF-β to inhibit cell growth and elicit senescence [139]. Increased expression of TGF-β1 was also reported in BCR-ABL+CD41+CD150+ leukemic megakaryocyte-lineage cells as one of the measures of their senescence phenotype (along with increased expression of SA-β-Gal and p16 and p21 cell cycle inhibitors), and neutralization of the cytokine diminished chronic myeloid leukemia cell leukemogenic capacity both in vitro and in vivo [140].
EMT and MMT
A morphological rearrangement of cancer cells is usually an obligatory phenomenon allowing them to detach from a primary tumor and effectively proliferate, migrate, and invade surrounding tissues. Classically, the acquisition of a spindle-shaped morphology by cancer cells is called an EMT [141]. This transformation is not restricted, however, to malignancies. Mesothelial cells may also lose their typical epithelial-like appearance and adopt fibroblast-like morphology in the process of mesothelial-mesenchymal transition (MMT), which is causatively linked with the pathogenesis of peritoneal fibrosis [142].
TGF-β1 is one of the leading triggers of EMT, and its activity in this process relies on a network of related signaling molecules and effector pathways [143]. In a model of paracrine interactions between PMCs and colorectal cancer cells (SW480 line), neutralization of TGF-β1 in conditioned medium from senescent normal cells prevented senescence-dependent upregulation of vimentin and downregulation of E-cadherin (EMT markers) in cancer cells [110]. In another model of gastrointestinal tract tumors (gastric cancer), the upregulation of TGF-β signaling was found in cells that developed a senescence phenotype in response to a complete genetic depletion of microRNA 200 (miR-200), the negative regulators of the ZEB1 and ZEB2 transcription factors [144]. This effect was accompanied by an enrichment of the EMT pathway and the recruitment of stromal cells into the tumor microenvironment. At the same time, the targeting of TGF-βRI resulted in the efficient prevention of senescence phenotype development [145]. In ovarian cancer, TGF-β1 was recognized as the prime mediator of EMT [8]; however, direct links between the development of this phenotype and hypersecretion of this cytokine by senescent cells have not been proven.
The contribution of TGF-β1 to the development of the EMT-like state was also hypothesized in leukemia cells as an element of the bone marrow pro-malignant microenvironment [146].
TGF-β1 secreted by senescent PMCs into the environment and activated by TSP-1 has been found to mediate the spreading of this process (the so-called bystander senescence effect) in an autocrine (in young PMCs) and paracrine (in young fibroblasts) manner [83]. At the same time, young PMCs subjected to a TGF-β1-rich secretome of highly aggressive high-grade serous ovarian cancer cells (HGSOCs) are capable of developing MMT more effectively than cells exposed to a conditioned medium generated by less aggressive ovarian cancer histotypes. Targeting cytokines with specific neutralizing antibodies whose production by HGSOCs was higher than that by endometrioid or clear-cell ovarian cancers revealed that the reversal of the MMT phenotype (reduced vimentin combined with increased E-cadherin) was achievable upon the inhibition of TGF-β1 and its related effectors (Smad 2/3 and integrin-linked kinase, ILK) [147]. The role of TGF-β1 in MMT was also reported by Sandoval et al., who found that PMCs treated with conditioned medium generated by ovarian cancer cells acquire morphology similar to that elicited by 1 ng/mL of this cytokine [148]. Other authors were able, in turn, to connect TGF-β1-Smad 3 signaling with MMT [149].
It should also be pointed out that cellular senescence and EMT (plausibly also MMTs) seemingly cross paths and are clearly intertwined at some points. It has been postulated that activation of EMT translates to suppression of cellular senescence, as various transcription factors can inhibit senescence and induce EMT [150]. In some instances, however, the same stimuli (e.g., RASV12) may positively modulate both processes, as occurs in epithelial cells subjected to RASV12 cooperating with TGF-β1 and RASV12 fibroblasts [151, 152].
Cancer-associated fibroblasts
Another intensively studied aspect of senescent cell-derived TGF-β1 is the formation of cancer-associated fibroblasts (CAFs) and their tumorigenic activity. CAFs are defined as a population of cells in which a dominant subset of α-smooth muscle actin (αSMA)-positive myofibroblasts is mixed with fibroblasts with features typical of an original tissue, exhibiting well-pronounced proangiogenic and tumor-promoting activities [153, 154]. Recently, CAFs have been found to share some molecular features with senescent cells (e.g., SASP [89]), which may suggest that there is some affinity in causes and signaling in both phenomena. In this context, Mellone et al. elegantly showed that there is an overlap between the induction of senescence and myofibroblast differentiation. They found that fibroblasts undergoing senescence in response to triggers such as TGF-β1, replication, ionizing radiation and hydrogen peroxide display ultrastructural features of CAFs (upregulated αSMA, palladin, and phosphor-FAK) analogous to their counterparts exposed to TGF-β1. Notably, SA-β-Gal colocalized with αSMA, indicating that both processes (senescence and CAF formation) may be two sides of the same coin. Myofibroblastic transdifferentiation in cells forced into senescence was associated with the induction of TGF-β1 signaling, as its inhibition using Smad3 siRNA, a pan-TGF-β antibody and TGFβR1 inhibitor, reduced the αSMA increase elicited by some pro-senescence stimuli [89]. The picture of the mutual relationship between senescence and CAF formation was additionally expanded by Tan et al., who found that TGF-β1-induced fibroblast activation and senescence are preceded by intensified autophagic flux (reduced expression of p62) [155]. Conditioned medium from cells experiencing TGF-β1-related autophagy resulted in the release of agents intensifying the migration and invasion of oral squamous cell carcinoma cells, providing evidence that alterations in autophagy may determine the cancerogenic capacity of CAFs [155].
The TGF-β-dependent mechanism has also been implicated in the ability of CAFs to determine the susceptibility of adjacent epithelia (prostate, forestomach) to become oncogenic [156]. Stromal cell-derived TGF-β has also been found to stimulate the invasive potential of colorectal cancer cells, and the chemical neutralization of TGFβR1 suppressed metastasis formation in laboratory animals. This activity of TGF-β was associated with antiapoptotic GP130/STAT3 signaling and the GP130 ligand interleukin-11, which is secreted exclusively by TGF-β-treated CAFs [157]. In regard to ovarian cancer, activated fibroblasts, that is, CAFs obtained from omentum with ovarian carcinomatosis or normal peritoneal fibroblasts treated with TGF-β1, display higher pro-adhesive and pro-invasive capabilities than their normal counterparts [158].
Angiogenesis
The formation of new blood vessels and increased permeability of the existing vessels are the most critical phenomena supporting excessive growth and survival of tumor cells [159]. The efficiency of angiogenesis has been found to be additionally fueled by various kinds of senescent cells, including fibroblasts [160] and PMCs [161]. Stimulation of the production of proangiogenic agents, such as VEGF [34] and fibroblast growth factor [162], has been recognized as a feature of TGF-β1.
Improved tissue vascularization is an essential element during intraperitoneal ovarian cancer progression [163], and sites with a high density of blood vessels have been recognized as niches for the effective implantation of metastasizing cells [164]. For interactions between PMCs and ovarian cancer cells, TGF-β1 has been recognized as the mediator (along with IL-6) of the development of an angiogenic secretory phenotype in malignant cells subjected to conditioned medium generated by senescent PMCs. Increased production and release of the chemokines CXCL1, CXCL8, hepatocyte growth factor, and VEGF translated to proangiogenic behavior of the vascular endothelium, including increased proliferation and migration. Intriguingly, the level of TGF-β1 secreted by senescent PMCs correlated with the production of angiogenic agents by cancer cells. Mechanistically, TGF-β1 caused more acute angiogenic activity of cancer cells by a dose- and time-dependent induction of three transcription factors: HIF-1α, NF-κB/p50, and AP-1/c-Jun [161].
Bai and colleagues revealed, in turn, that the inhibition of TGF‐β signaling retards senescence of pluripotent stem cell-derived endothelial cells and allows their function to be maintained, including their proliferative capacity, tubulogenesis, acetylated low‐density lipoprotein uptake, and endothelial nitric oxide synthase (eNOS) expression. The TGF‐β1-dependent senescence response of the endothelium was associated with hyperexpression of the cell cycle inhibitory proteins p15, p16, and p21, and the inhibition of cytokines decreased the levels of these proteins [165]. Another study focusing on endoglin, an auxiliary TGF‐β1 receptor, showed that senescence of endothelial cells is associated with an increased short/long endoglin ratio and that a reduced proliferative index of endothelial cells may be conferred by short endoglin having the ability to interact with both TGF-βIRs, ALK5 and ALK1. The important role of short endoglin in vascular aging was confirmed by in vivo experiments in which mice overexpressing this molecule displayed hypertension, reduced hypertensive reaction to nitric oxide inhibition, suppressed vasodilatory response to TGF-β1, and decreased eNOS expression in some organs [166].
Malignant ascites
The role of an acellular microenvironment in cancer progression is unquestioned. The perfect example is the presence and activity of malignant ascites. This term refers to the fluid that accumulates in the peritoneal cavity in a subset of patients in advanced stages of intraperitoneal malignancy (especially ovarian cancer) and creates immunosuppressive and cancer-permissive conditions [167, 168]. It has been demonstrated that TGF-β1 belongs to a wide array of malignant soluble constituents of ascites, and its concentration in this fluid is higher than that in benign ascites from noncancerous patients [169]. Because malignant ascites are in continuous direct contact with PMCs, their effect on senescence induction was examined, and this research showed an acceleration of senescence development in cells maintained in medium supplemented with 10% fluid [169]. Under these conditions, TGF-β1 appeared to not contribute to senescence exacerbation, which may indicate that its pro-senescence activity toward PMCs may be restricted in malignant ascites by its limited concentration or low activity. In addition, some elements of malignant ascites’ composition may jeopardize receptors or TGF-β effector pathways in PMCs. At the same time, it should be emphasized that malignant ascites promote the proliferation and migration of ovarian cancer cells and that the TGF-β1 concentration is especially high in ascites from patients with an undifferentiated histotype [170], which is known for its relatively high aggressiveness [171]. It has been demonstrated that increased motility of ovarian cancer cells in response to malignant ascites may be inhibited by TGF-β1 targeting and that cytokines promote the migration of malignant cells by suppressing miR-125b [172].
Ascites-derived TGF-β1 also appeared to be responsible for the induction of EMT (upregulation of vimentin and downregulation of E-cadherin) in ovarian cancer cells. The TGF-β1-dependent changes in EMT markers were regulated at the transcriptional level by Smad 2/3, ILK, AP-1, and SP-1. Importantly, neutralization of TGF-β1 in ascites resulted in decreased transmesothelial invasiveness of cancer cells, proving the biological activity of this cytokine [173].
Cancer cell senescence
Despite the historical dogma that cancer cells do not undergo senescence and thus are literally immortal [174], it is now clear that they may undergo therapy-induced and spontaneous replicative senescence, similar to what occurs in normal somatic cells [52]. Although the biological and clinical significance of cancer cell senescence is still not fully understood, as it may dichotomously translate to either growth inhibition of some cells or growth promotion of others [52], several reports document that activation of TGF-β signaling may play a regulatory role in this process. Taking into account the engagement of telomeres in the induction of senescence, the inhibition of the hTERT subunit in breast cancer cells by the TGF-β-Smad 3 pathway may be considered the first element of this cytokine’s activity. Mechanistically, TGF-β induces Smad3 attachment to c-Myc, which appears to be critical for transcription factor binding to the hTERT promoter and effective telomerase inhibition [51]. The repression of telomerase, shortening of telomeres, and cellular senescence have also been identified in lung cancer cells [175] and in breast cancer cells in which TGF-β receptor signaling was provoked in response to one of the TGF-β family members, bone morphogenetic protein-7 (BMP7) [176]. BMP7 secreted by bone stromal cells has also been implicated as a trigger of senescence (SA-β-Gal) in prostate cancer stem cells (CSCs). BMP7 operates through its receptor 2 (BMPR2), leading to the activation of p38 MAPK and upregulation of the p21 cell cycle inhibitor and the metastasis suppressor gene NDRG1. Interestingly, in contrast to the classic view on senescence as an irreversible nonproliferative state, the BMP7-induced senescence of prostate CSCs appeared to be reversible upon BMP7 withdrawal. In this case, the phosphorylation of p38 MAPK was reversed with a subsequent restoration of ERK phosphorylation (a major positive cell cycle regulator) [177], indicating—according to the authors of the study—that the p38 MAPK/ERK ratio may be important for determining the proliferative/nonproliferative status of cancer cells [178].
B cell lymphoma is another model in which mutual cooperation between TGF-β1 and senescence was demonstrated [179]. The lymphoma sections in which TGF-β1 was abundant were characterized by low expression of the proliferation marker Ki67, implying the presence of senescence. Indeed, experiments using Myc-driven lymphoma cells with inhibited apoptosis by stable bcl2 transduction proved that exogenous TGF-β1 promotes senescence (SA-β-Gal, p21) in a histone methyltransferase (Suv39h1)-dependent manner. At the same time, genetic targeting of this enzyme reversed the senescence phenotype, allowing improved Myc-driven tumor development. Detailed analysis of TGF-β1 activity revealed a repression of several E2F target genes, including Cyclin A and MCM7, and increased expression of heterochromatinization-related transcripts, such as DNA methyltransferase 3B and HP1β. At the same time, TGF-β1 activity did not increase oxidative stress or DNA damage, showing that cytokines induce senescence solely via the Suv39h1 pathway. Because TGF-β1 is not a constituent of lymphoma’s SASP, it was hypothesized that it is derived from a noncancerous microenvironment. This assumption was positively verified when the ability of apoptotic lymphoma cells to force TGF-β1 release by lymphoma-infiltrating macrophages was demonstrated. This observation was in line with previous, well-grounded knowledge that macrophages generate TGF-β1 as a result of phosphatidylserine-dependent ingestion of apoptotic cells [180]. The apoptosis-induced macrophage-derived cytokine efficiently induced SA-β-Gal in lymphoma cells, and this effect was abrogated in the presence of a TGF-β receptor type I inhibitor [179].
Recently, spontaneous senescence was also demonstrated in vivo and in vitro in primary ovarian cancer cells from chemotherapy-naïve patients. Experiments aimed at identifying the role of the peritoneal environment in this process showed that senescence of cancer cells may be induced by products of senescent normal peritoneal cells’ secretome and malignant ascites, with a particular role of soluble TGF-β1. For cancer cells treated with conditioned medium from senescent PMCs, the cytokine induced the formation of γ-H2A.X foci, whereas in the case of exposure to fibroblast-derived medium, the activity of TGF-β1 translated to increased activity of SA-β-Gal and accumulation of 53BP1. As per senescence induction in response to malignant ascites, TGF-β1 appeared to be responsible for this effect in fluids from patients with serous and undifferentiated tumors [181].
Ovarian cancer cells are also prone to senescence elicited by various drugs. One of them is a poly (ADP-ribose) polymerase (PARP) inhibitor, olaparib, capable of promoting senescence (SA-β-Gal, G1 growth arrest) in several representative ovarian cancer cell lines through a p16- and p53-dependent mechanism. One of the hallmarks of this effect is the upregulated expression of TGF-β3 mRNA [182]. Taking into account the well-known cancer-promoting activity of normal somatic senescent cells [111], the overexpression of TGF-β3 by senescent cancer cells may indicate that some elements of nonsenescent cell progression related to this cytokine (e.g., improved cell migration [183]) may become intensified.
The contribution of TGF-β1 signaling to cancer cell senescence was also postulated in p53- and p16-deficient hepatocellular cancer cells (HCCs) [184], the spontaneous senescence of which was linked with the SIP1 gene, encoding a zinc-finger transcription factor engaged in TGF-β signaling [185]. Experimental inhibition of SIP1 led to a restoration of initially declined hTERT activity and to bypassing senescence [184]. Further research by the same group demonstrated that TGF-β1 directly induces senescence in HCC cells. In contrast to OC cells, TGF-β1 induced senescence independent of p53 status in an effector mechanism that elevated p21 and G1 growth arrest. The pro-senescence effect of TGF-β1 also involved oxidative stress and the NOX4 gene, as ROS production inhibition and NOX4 silencing prevented both p21 increase and growth arrest upon TGF-β1 exposure. In addition, experiments on mice revealed that the intratumoral administration of TGF-β1 may restrict tumor expansion, whereas inhibition of TGF-βR2 results in a reduced in vivo senescence response and intensified tumor progression [186].
Unique cell types, the senescence of which is also regulated by TGF-β signaling, are mesenchymal stem cells [94] and CSCs. In the latter, TGF-β released by CAFs can initiate the development of CSCs or the dedifferentiation of non-CSCs to CSCs via Wnt- or Zeb1-dependent signaling [187, 188]. At the same time, BMP-7, a member of the TGF-β superfamily, secreted by bone stromal cells appeared to trigger senescence of prostatic CSCs in a p38 MAPK-, p21-, and N-Myc downstream regulated 1 (NDRG1)-dependent mechanism. When BMP-7 signaling was inhibited in vitro and in vivo, the manipulation allowed the cells to resume their proliferative capacity [178]. Premature senescence was also found in the epithelial stem cell population subjected to ectopic expression of TGF-β1 [189]. Generally, the activation of TGF-β signaling is considered a brake signal suppressing the tumorigenic potential of CSCs, which was demonstrated using various models, such as breast [190] and gastric [191] carcinomas.
Conclusions and future directions
Knowledge pertaining to the constant and complex interplay between aging and cancer is expanding dynamically. Experimental data indicate that the pathophysiology of cancer is closely associated with the presence and activity of senescent cells, whereas TGF-β signaling seems to be a critical element of the senescent–cancer cell interactome. These discoveries have given rise to a variety of strategies to cope with the unwanted activity of this cytokine, including various kinds of antibodies (monoclonal, neutralizing, bifunctional antibodies), antisense oligonucleotides, TGF-β-related vaccines, and receptor kinase inhibitors [192] (Table 1).
Table 1.
Medicinal preparations tested in clinical trials to target TGF-β signaling in cancer patients
| Drug | Target | Application | Reference |
|---|---|---|---|
|
AP 12009 (Trabedersen) |
TGF-β2 | Improved survival of patients with high-grade glioma | [193] |
|
GC 1008 (Fresolimumab) |
TGF-β | Increased overall survival and immune response in patients with breast cancer | [194] |
|
Lucanix™ (Belagenpumatucel-L) |
TGF-β2 | Improved survival in patients with non-small-cell lung carcinoma | [195] |
|
LY2157299 (Galunisertib) |
TGF-βRI/ALK5* | Improved survival of patients with pancreatic cancer | [196] |
| LY3200882 | TGF-βRI | Potentially effective in patients with pancreatic cancer | [197] |
|
M7824 (Bintrafusp alfa) |
TGF-βRII | Potentially effective in patients with non-small-cell lung carcinoma | [198] |
|
TEW7179 (Vactosertib) |
TGF-βRI/ALK5 | Tested in patients with advanced solid tumors | [199] |
|
Vigil™ (Gemogenovatucel-T) |
TGF-β1 TGF-β2 |
Improved survival and relapse free time in patients with ovarian cancer | [200] |
*ALK5—activin receptor-like kinase 5
There is also a long list of natural compounds that potentially affect TGF-β signaling in normal and cancer cells. These include resveratrol [201], curcumin [202], emodin [202], lycopene [203], carnosol [204], eupatolide [205], and many others [206]. The effectiveness of these diversified approaches is, however, extremely limited, which is plausibly associated with the fact that TGF-β plays—apart from its pro-senescence and pro-cancerous activity—multiple beneficial, physiological roles, which, if blocked, may lead to other molecular and metabolic abnormalities and side effects.
One of the most important questions that has to be answered is whether effective anti-senescence/cancer TGF-β treatment should be directed against the cytokine itself or should precisely target some of the numerous downstream elements of TGF-β signaling. Generally, knowledge of these effectors is continually growing; however, the findings must be organized in a hierarchical manner to recognize those molecules and pathways that cannot be manipulated and those whose modifications may yield promising results. Another issue that should be experimentally addressed are the circumstances and context-dependent determinants of TGF-β superfamily cytokine activation from their latent forms, the interference with which may keep the cytokines inactive or adjust their rate of activation to the current needs of cells. Despite all objective limitations in the employment of anti-TGF-β strategies in clinical practice, we believe that the information provided in this review may help to define new experimental and/or clinical avenues whose exploration will translate to a more effective fight against aging and cancer.
Author contributions
All authors contributed to the study conception, design, and writing.
Funding
The authors of the study are supported by a grant from the National Science Centre, Poland (registration number 2020/37/B/NZ5/00100).
Availability of data and material
Not applicable.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dinarello CA. Historical insights into cytokines. Eur J Immunol. 2007;37(Suppl 1):S34–45. doi: 10.1002/eji.200737772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, Duan S, Eiwegger T, Eljaszewicz A, Ferstl R, Frei R, Garbani M, Globinska A, Hess L, Huitema C, Kubo T, Komlosi Z, Konieczna P, Kovacs N, Kucuksezer UC, Meyer N, Morita H, Olzhausen J, O'Mahony L, Pezer M, Prati M, Rebane A, Rhyner C, Rinaldi A, Sokolowska M, Stanic B, Sugita K, Treis A, van de Veen W, Wanke K, Wawrzyniak M, Wawrzyniak P, Wirz OF, Zakzuk JS, Akdis CA. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138:984–1010. doi: 10.1016/j.jaci.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 3.Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Lan T, Chen L, Wei X. Inflammatory cytokines in cancer: comprehensive understanding and clinical progress in gene therapy. Cells. 2021;10:100. doi: 10.3390/cells10010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tominaga K, Suzuki HI. TGF-beta signaling in cellular senescence and aging-related pathology. Int J Mol Sci. 2019;20:5002. doi: 10.3390/ijms20205002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roane BM, Arend RC, Birrer MJ. Review: targeting the transforming growth factor-beta pathway in ovarian cancer. Cancers (Basel) 2019;11:668. doi: 10.3390/cancers11050668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumari A, Shonibare Z, Monavarian M, Arend RC, Lee NY, Inman GJ, Mythreye K. TGFbeta signaling networks in ovarian cancer progression and plasticity. Clin Exp Metastasis. 2021;38:139–161. doi: 10.1007/s10585-021-10077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinck AP, Mueller TD, Springer TA. Structural biology and evolution of the TGF-beta family. Cold Spring Harb Perspect Biol. 2016;8:a022103. doi: 10.1101/cshperspect.a022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Namwanje M, Brown CW. Activins and inhibins: roles in development, physiology, and disease. Cold Spring Harb Perspect Biol. 2016;8:a021881. doi: 10.1101/cshperspect.a021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heldin CH, Moustakas A. Signaling Receptors for TGF-beta Family Members. Cold Spring Harb Perspect Biol. 2016;8:a022053. doi: 10.1101/cshperspect.a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258:7155–7160. doi: 10.1016/S0021-9258(18)32345-7. [DOI] [PubMed] [Google Scholar]
- 13.Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 14.Murphy-Ullrich JE, Suto MJ. Thrombospondin-1 regulation of latent TGF-beta activation: a therapeutic target for fibrotic disease. Matrix Biol. 2018;68–69:28–43. doi: 10.1016/j.matbio.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimura SL. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am J Pathol. 2009;175:1362–1370. doi: 10.2353/ajpath.2009.090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amarnath S, Dong L, Li J, Wu Y, Chen W. Endogenous TGF-beta activation by reactive oxygen species is key to Foxp3 induction in TCR-stimulated and HIV-1-infected human CD4+CD25- T cells. Retrovirology. 2007;4:57. doi: 10.1186/1742-4690-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzavlaki K, Moustakas A. TGF-beta signaling. Biomolecules. 2020;10:487. doi: 10.3390/biom10030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahl SM, McCartney-Francis N, Allen JB, Dougherty EB, Dougherty SF. Macrophage production of TGF-beta and regulation by TGF-beta. Ann N Y Acad Sci. 1990;593:188–196. doi: 10.1111/j.1749-6632.1990.tb16111.x. [DOI] [PubMed] [Google Scholar]
- 19.Grotendorst GR, Smale G, Pencev D. Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J Cell Physiol. 1989;140:396–402. doi: 10.1002/jcp.1041400226. [DOI] [PubMed] [Google Scholar]
- 20.Blakytny R, Ludlow A, Martin GE, Ireland G, Lund LR, Ferguson MW, Brunner G. Latent TGF-beta1 activation by platelets. J Cell Physiol. 2004;199:67–76. doi: 10.1002/jcp.10454. [DOI] [PubMed] [Google Scholar]
- 21.Huang M, Sharma S, Zhu LX, Keane MP, Luo J, Zhang L, Burdick MD, Lin YQ, Dohadwala M, Gardner B, Batra RK, Strieter RM, Dubinett SM. IL-7 inhibits fibroblast TGF-beta production and signaling in pulmonary fibrosis. J Clin Invest. 2002;109:931–937. doi: 10.1172/JCI0214685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying WZ, Sanders PW. Dietary salt increases endothelial nitric oxide synthase and TGF-beta1 in rat aortic endothelium. Am J Physiol. 1999;277:H1293–H1298. doi: 10.1152/ajpheart.1999.277.4.H1293. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Li Y, Li N, Teng W, Wang M, Zhang Y, Xiao Z. TGF-beta1 promotes scar fibroblasts proliferation and transdifferentiation via up-regulating MicroRNA-21. Sci Rep. 2016;6:32231. doi: 10.1038/srep32231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lev PR, Salim JP, Marta RF, Osorio MJ, Goette NP, Molinas FC. Platelets possess functional TGF-beta receptors and Smad2 protein. Platelets. 2007;18:35–42. doi: 10.1080/09537100600800743. [DOI] [PubMed] [Google Scholar]
- 25.Ksiazek K, Korybalska K, Jorres A, Witowski J. Accelerated senescence of human peritoneal mesothelial cells exposed to high glucose: the role of TGF-beta1. Lab Invest. 2007;87:345–356. doi: 10.1038/labinvest.3700519. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura A, Muto G. TGF-beta function in immune suppression. Curr Top Microbiol Immunol. 2011;350:127–147. doi: 10.1007/82_2010_87. [DOI] [PubMed] [Google Scholar]
- 27.Huang SS, Huang JS. TGF-beta control of cell proliferation. J Cell Biochem. 2005;96:447–462. doi: 10.1002/jcb.20558. [DOI] [PubMed] [Google Scholar]
- 28.Wang MK, Sun HQ, Xiang YC, Jiang F, Su YP, Zou ZM. Different roles of TGF-beta in the multi-lineage differentiation of stem cells. World J Stem Cells. 2012;4:28–34. doi: 10.4252/wjsc.v4.i5.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoo J, Ghiassi M, Jirmanova L, Balliet AG, Hoffman B, Fornace AJ, Jr, Liebermann DA, Bottinger EP, Roberts AB. Transforming growth factor-beta-induced apoptosis is mediated by Smad-dependent expression of GADD45b through p38 activation. J Biol Chem. 2003;278:43001–43007. doi: 10.1074/jbc.M307869200. [DOI] [PubMed] [Google Scholar]
- 30.Moon JR, Oh SJ, Lee CK, Chi SG, Kim HJ. TGF-beta1 protects colon tumor cells from apoptosis through XAF1 suppression. Int J Oncol. 2019;54:2117–2126. doi: 10.3892/ijo.2019.4776. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki HI, Kiyono K, Miyazono K. Regulation of autophagy by transforming growth factor-beta (TGF-beta) signaling. Autophagy. 2010;6:645–647. doi: 10.4161/auto.6.5.12046. [DOI] [PubMed] [Google Scholar]
- 32.Casalena G, Daehn I, Bottinger E. Transforming growth factor-beta, bioenergetics, and mitochondria in renal disease. Semin Nephrol. 2012;32:295–303. doi: 10.1016/j.semnephrol.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon YS, Lee JH, Hwang SC, Choi KS, Yoon G. TGF beta1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene. 2005;24:1895–1903. doi: 10.1038/sj.onc.1208262. [DOI] [PubMed] [Google Scholar]
- 34.Kariya T, Nishimura H, Mizuno M, Suzuki Y, Matsukawa Y, Sakata F, Maruyama S, Takei Y, Ito Y. TGF-beta1-VEGF-A pathway induces neoangiogenesis with peritoneal fibrosis in patients undergoing peritoneal dialysis. Am J Physiol Renal Physiol. 2018;314:F167–F180. doi: 10.1152/ajprenal.00052.2017. [DOI] [PubMed] [Google Scholar]
- 35.Hocevar BA, Brown TL, Howe PH. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim JY, Park SJ, Hwang HY, Park EJ, Nam JH, Kim J, Park SI. TGF-beta1 induces cardiac hypertrophic responses via PKC-dependent ATF-2 activation. J Mol Cell Cardiol. 2005;39:627–636. doi: 10.1016/j.yjmcc.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Frangogiannis N. Transforming growth factor-beta in tissue fibrosis. J Exp Med. 2020;217:e20190103. doi: 10.1084/jem.20190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki Y, Montagne K, Nishihara A, Watabe T, Miyazono K. BMPs promote proliferation and migration of endothelial cells via stimulation of VEGF-A/VEGFR2 and angiopoietin-1/Tie2 signalling. J Biochem. 2008;143:199–206. doi: 10.1093/jb/mvm215. [DOI] [PubMed] [Google Scholar]
- 41.Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ, Rosen V, Mundy GR, Harris SE. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol. 1998;142:295–305. doi: 10.1083/jcb.142.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim BN, Ahn DH, Kang N, Yeo CD, Kim YK, Lee KY, Kim TJ, Lee SH, Park MS, Yim HW, Park JY, Park CK, Kim SJ. TGF-beta induced EMT and stemness characteristics are associated with epigenetic regulation in lung cancer. Sci Rep. 2020;10:10597. doi: 10.1038/s41598-020-67325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhagyaraj E, Ahuja N, Kumar S, Tiwari D, Gupta S, Nanduri R, Gupta P. TGF-beta induced chemoresistance in liver cancer is modulated by xenobiotic nuclear receptor PXR. Cell Cycle. 2019;18:3589–3602. doi: 10.1080/15384101.2019.1693120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barrett CS, Millena AC, Khan SA. TGF-beta effects on prostate cancer cell migration and invasion require FosB. Prostate. 2017;77:72–81. doi: 10.1002/pros.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pang MF, Georgoudaki AM, Lambut L, Johansson J, Tabor V, Hagikura K, Jin Y, Jansson M, Alexander JS, Nelson CM, Jakobsson L, Betsholtz C, Sund M, Karlsson MC, Fuxe J. TGF-beta1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene. 2016;35:748–760. doi: 10.1038/onc.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Angioni R, Sanchez-Rodriguez R, Viola A, Molon B. TGF-beta in cancer: metabolic driver of the tolerogenic crosstalk in the tumor microenvironment. Cancers (Basel) 2021;13:401. doi: 10.3390/cancers13030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steiner MS, Barrack ER. Transforming growth factor-beta 1 overproduction in prostate cancer: effects on growth in vivo and in vitro. Mol Endocrinol. 1992;6:15–25. doi: 10.1210/mend.6.1.1738367. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura S, Kawai T, Kamakura T, Ookura T. TGF-beta3 is expressed in taste buds and inhibits proliferation of primary cultured taste epithelial cells. In Vitro Cell Dev Biol Anim. 2010;46:36–44. doi: 10.1007/s11626-009-9239-9. [DOI] [PubMed] [Google Scholar]
- 50.Liu H, Zhu Y, Zhu H, Cai R, Wang KF, Song J, Wang RX, Zhou RX. Role of transforming growth factor beta1 in the inhibition of gastric cancer cell proliferation by melatonin in vitro and in vivo. Oncol Rep. 2019;42:753–762. doi: 10.3892/or.2019.7190. [DOI] [PubMed] [Google Scholar]
- 51.Li H, Xu D, Li J, Berndt MC, Liu JP. Transforming growth factor beta suppresses human telomerase reverse transcriptase (hTERT) by Smad3 interactions with c-Myc and the hTERT gene. J Biol Chem. 2006;281:25588–25600. doi: 10.1074/jbc.M602381200. [DOI] [PubMed] [Google Scholar]
- 52.Mikula-Pietrasik J, Niklas A, Uruski P, Tykarski A, Ksiazek K. Mechanisms and significance of therapy-induced and spontaneous senescence of cancer cells. Cell Mol Life Sci. 2020;77:213–229. doi: 10.1007/s00018-019-03261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Ye Y, Long X, Xiao P, Ren X, Yu J. BMP signaling and its paradoxical effects in tumorigenesis and dissemination. Oncotarget. 2016;7:78206–78218. doi: 10.18632/oncotarget.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Virk MS, Petrigliano FA, Liu NQ, Chatziioannou AF, Stout D, Kang CO, Dougall WC, Lieberman JR. Influence of simultaneous targeting of the bone morphogenetic protein pathway and RANK/RANKL axis in osteolytic prostate cancer lesion in bone. Bone. 2009;44:160–167. doi: 10.1016/j.bone.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Busch C, Drews U, Eisele SR, Garbe C, Oppitz M. Noggin blocks invasive growth of murine B16–F1 melanoma cells in the optic cup of the chick embryo. Int J Cancer. 2008;122:526–533. doi: 10.1002/ijc.23139. [DOI] [PubMed] [Google Scholar]
- 56.Langenfeld EM, Calvano SE, Abou-Nukta F, Lowry SF, Amenta P, Langenfeld J. The mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cells. Carcinogenesis. 2003;24:1445–1454. doi: 10.1093/carcin/bgg100. [DOI] [PubMed] [Google Scholar]
- 57.Buckley S, Shi W, Driscoll B, Ferrario A, Anderson K, Warburton D. BMP4 signaling induces senescence and modulates the oncogenic phenotype of A549 lung adenocarcinoma cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L81–L86. doi: 10.1152/ajplung.00160.2003. [DOI] [PubMed] [Google Scholar]
- 58.Dai Z, Popkie AP, Zhu WG, Timmers CD, Raval A, Tannehill-Gregg S, Morrison CD, Auer H, Kratzke RA, Niehans G, Amatschek S, Sommergruber W, Leone GW, Rosol T, Otterson GA, Plass C. Bone morphogenetic protein 3B silencing in non-small-cell lung cancer. Oncogene. 2004;23:3521–3529. doi: 10.1038/sj.onc.1207441. [DOI] [PubMed] [Google Scholar]
- 59.Bach DH, Park HJ, Lee SK. The dual role of bone morphogenetic proteins in cancer. Mol Ther Oncolytics. 2018;8:1–13. doi: 10.1016/j.omto.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rattan SI. Aging, anti-aging, and hormesis. Mech Ageing Dev. 2004;125:285–289. doi: 10.1016/j.mad.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 62.Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, Ligotti ME, Zareian N, Accardi G. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front Immunol. 2019;10:2247. doi: 10.3389/fimmu.2019.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao H, Zhang H, Qin X. Age-related differences in serum MFGE8, TGFbeta1 and correlation to the severity of atherosclerosis determined by ultrasound. Mol Med Rep. 2017;16:9741–9748. doi: 10.3892/mmr.2017.7838. [DOI] [PubMed] [Google Scholar]
- 64.Okamoto Y, Gotoh Y, Uemura O, Tanaka S, Ando T, Nishida M. Age-dependent decrease in serum transforming growth factor (TGF)-beta 1 in healthy Japanese individuals; population study of serum TGF-beta 1 level in Japanese. Dis Markers. 2005;21:71–74. doi: 10.1155/2005/381215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Forsey RJ, Thompson JM, Ernerudh J, Hurst TL, Strindhall J, Johansson B, Nilsson BO, Wikby A. Plasma cytokine profiles in elderly humans. Mech Ageing Dev. 2003;124:487–493. doi: 10.1016/S0047-6374(03)00025-3. [DOI] [PubMed] [Google Scholar]
- 66.Salvioli S, Capri M, Bucci L, Lanni C, Racchi M, Uberti D, Memo M, Mari D, Govoni S, Franceschi C. Why do centenarians escape or postpone cancer? The role of IGF-1, inflammation and p53. Cancer Immunol Immunother. 2009;58:1909–1917. doi: 10.1007/s00262-008-0639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meyers EA, Gobeske KT, Bond AM, Jarrett JC, Peng CY, Kessler JA. Increased bone morphogenetic protein signaling contributes to age-related declines in neurogenesis and cognition. Neurobiol Aging. 2016;38:164–175. doi: 10.1016/j.neurobiolaging.2015.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carrieri G, Marzi E, Olivieri F, Marchegiani F, Cavallone L, Cardelli M, Giovagnetti S, Stecconi R, Molendini C, Trapassi C, De Benedictis G, Kletsas D, Franceschi C. The G/C915 polymorphism of transforming growth factor beta1 is associated with human longevity: a study in Italian centenarians. Aging Cell. 2004;3:443–448. doi: 10.1111/j.1474-9728.2004.00129.x. [DOI] [PubMed] [Google Scholar]
- 69.Ruberto S, Santovito A. Association of TGFbeta1 codon 10 (T>C) and IL-10 (G>C) cytokine gene polymorphisms with longevity in a cohort of Italian population. Am J Hum Biol. 2021;33:e23491. doi: 10.1002/ajhb.23491. [DOI] [PubMed] [Google Scholar]
- 70.Awad MR, El-Gamel A, Hasleton P, Turner DM, Sinnott PJ, Hutchinson IV. Genotypic variation in the transforming growth factor-beta1 gene: association with transforming growth factor-beta1 production, fibrotic lung disease, and graft fibrosis after lung transplantation. Transplantation. 1998;66:1014–1020. doi: 10.1097/00007890-199810270-00009. [DOI] [PubMed] [Google Scholar]
- 71.Blaney Davidson EN, Remst DF, Vitters EL, van Beuningen HM, Blom AB, Goumans MJ, van den Berg WB, van der Kraan PM. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J Immunol. 2009;182:7937–7945. doi: 10.4049/jimmunol.0803991. [DOI] [PubMed] [Google Scholar]
- 72.Hui W, Young DA, Rowan AD, Xu X, Cawston TE, Proctor CJ. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann Rheum Dis. 2016;75:449–458. doi: 10.1136/annrheumdis-2014-206295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hodgson D, Rowan AD, Falciani F, Proctor CJ. Systems biology reveals how altered TGFbeta signalling with age reduces protection against pro-inflammatory stimuli. PLoS Comput Biol. 2019;15:e1006685. doi: 10.1371/journal.pcbi.1006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xia S, Zhang X, Zheng S, Khanabdali R, Kalionis B, Wu J, Wan W, Tai X. An update on inflamm-aging: mechanisms, prevention, and treatment. J Immunol Res. 2016;2016:8426874. doi: 10.1155/2016/8426874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faget DV, Ren Q, Stewart SA. Unmasking senescence: context-dependent effects of SASP in cancer. Nat Rev Cancer. 2019;19:439–453. doi: 10.1038/s41568-019-0156-2. [DOI] [PubMed] [Google Scholar]
- 76.Mikula-Pietrasik J, Stryczynski L, Uruski P, Tykarski A, Ksiazek K. Procancerogenic activity of senescent cells: a case of the peritoneal mesothelium. Ageing Res Rev. 2018;43:1–9. doi: 10.1016/j.arr.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 77.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vande Berg JS, Rose MA, Haywood-Reid PL, Rudolph R, Payne WG, Robson MC. Cultured pressure ulcer fibroblasts show replicative senescence with elevated production of plasmin, plasminogen activator inhibitor-1, and transforming growth factor-beta1. Wound Repair Regen. 2005;13:76–83. doi: 10.1111/j.1067-1927.2005.130110.x. [DOI] [PubMed] [Google Scholar]
- 79.Pascal T, Debacq-Chainiaux F, Chretien A, Bastin C, Dabee AF, Bertholet V, Remacle J, Toussaint O. Comparison of replicative senescence and stress-induced premature senescence combining differential display and low-density DNA arrays. FEBS Lett. 2005;579:3651–3659. doi: 10.1016/j.febslet.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 80.Shelton DN, Chang E, Whittier PS, Choi D, Funk WD. Microarray analysis of replicative senescence. Curr Biol. 1999;9:939–945. doi: 10.1016/S0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- 81.Lee MY, Wang Y, Vanhoutte PM. Senescence of cultured porcine coronary arterial endothelial cells is associated with accelerated oxidative stress and activation of NFkB. J Vasc Res. 2010;47:287–298. doi: 10.1159/000265563. [DOI] [PubMed] [Google Scholar]
- 82.Tremain R, Marko M, Kinnimulki V, Ueno H, Bottinger E, Glick A. Defects in TGF-beta signaling overcome senescence of mouse keratinocytes expressing v-Ha-ras. Oncogene. 2000;19:1698–1709. doi: 10.1038/sj.onc.1203471. [DOI] [PubMed] [Google Scholar]
- 83.Mikula-Pietrasik J, Sosinska P, Janus J, Rubis B, Brewinska-Olchowik M, Piwocka K, Ksiazek K. Bystander senescence in human peritoneal mesothelium and fibroblasts is related to thrombospondin-1-dependent activation of transforming growth factor-beta1. Int J Biochem Cell Biol. 2013;45:2087–2096. doi: 10.1016/j.biocel.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 84.Rapisarda V, Borghesan M, Miguela V, Encheva V, Snijders AP, Lujambio A, O'Loghlen A. Integrin beta 3 regulates cellular senescence by activating the TGF-beta pathway. Cell Rep. 2017;18:2480–2493. doi: 10.1016/j.celrep.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hassona Y, Cirillo N, Lim KP, Herman A, Mellone M, Thomas GJ, Pitiyage GN, Parkinson EK, Prime SS. Progression of genotype-specific oral cancer leads to senescence of cancer-associated fibroblasts and is mediated by oxidative stress and TGF-beta. Carcinogenesis. 2013;34:1286–1295. doi: 10.1093/carcin/bgt035. [DOI] [PubMed] [Google Scholar]
- 86.Campisi J. Aging and cancer: the double-edged sword of replicative senescence. J Am Geriatr Soc. 1997;45:482–488. doi: 10.1111/j.1532-5415.1997.tb05175.x. [DOI] [PubMed] [Google Scholar]
- 87.Frippiat C, Chen QM, Zdanov S, Magalhaes JP, Remacle J, Toussaint O. Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem. 2001;276:2531–2537. doi: 10.1074/jbc.M006809200. [DOI] [PubMed] [Google Scholar]
- 88.Ksiazek K, Mikula-Pietrasik J, Jorres A, Witowski J. Oxidative stress-mediated early senescence contributes to the short replicative life span of human peritoneal mesothelial cells. Free Radic Biol Med. 2008;45:460–467. doi: 10.1016/j.freeradbiomed.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 89.Mellone M, Hanley CJ, Thirdborough S, Mellows T, Garcia E, Woo J, Tod J, Frampton S, Jenei V, Moutasim KA, Kabir TD, Brennan PA, Venturi G, Ford K, Herranz N, Lim KP, Clarke J, Lambert DW, Prime SS, Underwood TJ, Vijayanand P, Eliceiri KW, Woelk C, King EV, Gil J, Ottensmeier CH, Thomas GJ. Induction of fibroblast senescence generates a non-fibrogenic myofibroblast phenotype that differentially impacts on cancer prognosis. Aging (Albany NY) 2016;9:114–132. doi: 10.18632/aging.101127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Untergasser G, Gander R, Rumpold H, Heinrich E, Plas E, Berger P. TGF-beta cytokines increase senescence-associated beta-galactosidase activity in human prostate basal cells by supporting differentiation processes, but not cellular senescence. Exp Gerontol. 2003;38:1179–1188. doi: 10.1016/j.exger.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 91.Ksiazek K, Mikula-Pietrasik J, Korybalska K, Dworacki G, Jorres A, Witowski J. Senescent peritoneal mesothelial cells promote ovarian cancer cell adhesion: the role of oxidative stress-induced fibronectin. Am J Pathol. 2009;174:1230–1240. doi: 10.2353/ajpath.2009.080613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iglesias-De La Cruz MC, Ruiz-Torres P, Alcami J, Diez-Marques L, Ortega-Velazquez R, Chen S, Rodriguez-Puyol M, Ziyadeh FN, Rodriguez-Puyol D. Hydrogen peroxide increases extracellular matrix mRNA through TGF-beta in human mesangial cells. Kidney Int. 2001;59:87–95. doi: 10.1046/j.1523-1755.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 93.Kretova M, Sabova L, Hodny Z, Bartek J, Kollarovic G, Nelson BD, Hubackova S, Luciakova K. TGF-beta/NF1/Smad4-mediated suppression of ANT2 contributes to oxidative stress in cellular senescence. Cell Signal. 2014;26:2903–2911. doi: 10.1016/j.cellsig.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 94.Wu J, Niu J, Li X, Wang X, Guo Z, Zhang F. TGF-beta1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. BMC Dev Biol. 2014;14:21. doi: 10.1186/1471-213X-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct. 2011;2011:792639. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Książek K, Winckiewicz M, Staniszewski R, Breborowicz A, Witowski J. Correlation between the donor age and the proliferative lifespan of human peritoneal mesothelial cells in vitro: is TGF-beta1 a link? Exp Gerontol. 2007;42:840–843. doi: 10.1016/j.exger.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 97.Sosinska P, Mikula-Pietrasik J, Ryzek M, Naumowicz E, Ksiazek K. Specificity of cytochemical and fluorescence methods of senescence-associated beta-galactosidase detection for ageing driven by replication and time. Biogerontology. 2014;15:407–413. doi: 10.1007/s10522-014-9505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gong C, Pan W, Hu W, Chen L. Bone morphogenetic protein-7 retards cell subculture-induced senescence of human nucleus pulposus cells through activating the PI3K/Akt pathway. Biosci Rep. 2019;39:BSR20182312. doi: 10.1042/BSR20182312. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Hayashi Y, Hsiao EC, Sami S, Lancero M, Schlieve CR, Nguyen T, Yano K, Nagahashi A, Ikeya M, Matsumoto Y, Nishimura K, Fukuda A, Hisatake K, Tomoda K, Asaka I, Toguchida J, Conklin BR, Yamanaka S. BMP-SMAD-ID promotes reprogramming to pluripotency by inhibiting p16/INK4A-dependent senescence. Proc Natl Acad Sci USA. 2016;113:13057–13062. doi: 10.1073/pnas.1603668113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu D, Wu J, Spee C, Ryan SJ, Hinton DR. BMP4 mediates oxidative stress-induced retinal pigment epithelial cell senescence and is overexpressed in age-related macular degeneration. J Biol Chem. 2009;284:9529–9539. doi: 10.1074/jbc.M809393200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Su D, Zhu S, Han X, Feng Y, Huang H, Ren G, Pan L, Zhang Y, Lu J, Huang B. BMP4-Smad signaling pathway mediates adriamycin-induced premature senescence in lung cancer cells. J Biol Chem. 2009;284:12153–12164. doi: 10.1074/jbc.M807930200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 103.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 104.Marsh T, Pietras K, McAllister SS. Fibroblasts as architects of cancer pathogenesis. Biochim Biophys Acta. 2013;1832:1070–1078. doi: 10.1016/j.bbadis.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guan X, LaPak KM, Hennessey RC, Yu CY, Shakya R, Zhang J, Burd CE. Stromal senescence by prolonged CDK4/6 inhibition potentiates tumor growth. Mol Cancer Res. 2017;15:237–249. doi: 10.1158/1541-7786.MCR-16-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 107.Taddei ML, Cavallini L, Comito G, Giannoni E, Folini M, Marini A, Gandellini P, Morandi A, Pintus G, Raspollini MR, Zaffaroni N, Chiarugi P. Senescent stroma promotes prostate cancer progression: the role of miR-210. Mol Oncol. 2014;8:1729–1746. doi: 10.1016/j.molonc.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Papadopoulou A, Kletsas D. Human lung fibroblasts prematurely senescent after exposure to ionizing radiation enhance the growth of malignant lung epithelial cells in vitro and in vivo. Int J Oncol. 2011;39:989–999. doi: 10.3892/ijo.2011.1132. [DOI] [PubMed] [Google Scholar]
- 109.Wang T, Notta F, Navab R, Joseph J, Ibrahimov E, Xu J, Zhu CQ, Borgida A, Gallinger S, Tsao MS. Senescent carcinoma-associated fibroblasts upregulate IL8 to enhance prometastatic phenotypes. Mol Cancer Res. 2017;15:3–14. doi: 10.1158/1541-7786.MCR-16-0192. [DOI] [PubMed] [Google Scholar]
- 110.Mikula-Pietrasik J, Sosinska P, Maksin K, Kucinska MG, Piotrowska H, Murias M, Wozniak A, Szpurek D, Ksiazek K. Colorectal cancer-promoting activity of the senescent peritoneal mesothelium. Oncotarget. 2015;6:29178–29195. doi: 10.18632/oncotarget.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mikula-Pietrasik J, Uruski P, Sosinska P, Maksin K, Piotrowska-Kempisty H, Kucinska M, Murias M, Szubert S, Wozniak A, Szpurek D, Sajdak S, Piwocka K, Tykarski A, Ksiazek K. Senescent peritoneal mesothelium creates a niche for ovarian cancer metastases. Cell Death Dis. 2016;7:e2565. doi: 10.1038/cddis.2016.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gonzalez-Meljem JM, Haston S, Carreno G, Apps JR, Pozzi S, Stache C, Kaushal G, Virasami A, Panousopoulos L, Mousavy-Gharavy SN, Guerrero A, Rashid M, Jani N, Goding CR, Jacques TS, Adams DJ, Gil J, Andoniadou CL, Martinez-Barbera JP. Stem cell senescence drives age-attenuated induction of pituitary tumours in mouse models of paediatric craniopharyngioma. Nat Commun. 2017;8:1819. doi: 10.1038/s41467-017-01992-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Buhl JL, Selt F, Hielscher T, Guiho R, Ecker J, Sahm F, Ridinger J, Riehl D, Usta D, Ismer B, Sommerkamp AC, Martinez-Barbera JP, Wefers AK, Remke M, Picard D, Pusch S, Gronych J, Oehme I, van Tilburg CM, Kool M, Kuhn D, Capper D, von Deimling A, Schuhmann MU, Herold-Mende C, Korshunov A, Brummer T, Pfister SM, Jones DTW, Witt O, Milde T. The senescence-associated secretory phenotype mediates oncogene-induced senescence in pediatric pilocytic astrocytoma. Clin Cancer Res. 2019;25:1851–1866. doi: 10.1158/1078-0432.CCR-18-1965. [DOI] [PubMed] [Google Scholar]
- 114.Javadi S, Ganeshan DM, Qayyum A, Iyer RB, Bhosale P. Ovarian cancer, the revised FIGO staging system, and the role of imaging. AJR Am J Roentgenol. 2016;206:1351–1360. doi: 10.2214/AJR.15.15199. [DOI] [PubMed] [Google Scholar]
- 115.Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clin Obstet Gynecol. 2012;55:3–23. doi: 10.1097/GRF.0b013e31824b4611. [DOI] [PubMed] [Google Scholar]
- 116.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Farsinejad S, Cattabiani T, Muranen T, Iwanicki M. Ovarian cancer dissemination-a cell biologist's perspective. Cancers (Basel) 2019;11:1957. doi: 10.3390/cancers11121957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Amadori D, Sansoni E, Amadori A. Ovarian cancer: natural history and metastatic pattern. Front Biosci. 1997;2:g8–10. [PubMed] [Google Scholar]
- 120.Pickel H, Lahousen M, Girardi F, Tamussino H, Stettner H. Intraperitoneal and retroperitoneal spread of ovarian cancer. In: Sharp C, Mason W, Leake R, editors. Ovarian cancer: biologic and therapeutic challenges. London: Chapman and Hall; 1990. [Google Scholar]
- 121.Kenny HA, Chiang CY, White EA, Schryver EM, Habis M, Romero IL, Ladanyi A, Penicka CV, George J, Matlin K, Montag A, Wroblewski K, Yamada SD, Mazar AP, Bowtell D, Lengyel E. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. J Clin Invest. 2014;124:4614–4628. doi: 10.1172/JCI74778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Janiszewska M, Primi MC, Izard T. Cell adhesion in cancer: Beyond the migration of single cells. J Biol Chem. 2020;295:2495–2505. doi: 10.1074/jbc.REV119.007759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kenny HA, Krausz T, Yamada SD, Lengyel E. Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells to the omentum. Int J Cancer. 2007;121:1463–1472. doi: 10.1002/ijc.22874. [DOI] [PubMed] [Google Scholar]
- 124.Mikula-Pietrasik J, Sosinska P, Kucinska M, Murias M, Maksin K, Malinska A, Ziolkowska A, Piotrowska H, Wozniak A, Ksiazek K. Peritoneal mesothelium promotes the progression of ovarian cancer cells in vitro and in a mice xenograft model in vivo. Cancer Lett. 2014;355:310–315. doi: 10.1016/j.canlet.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 125.Uruski P, Mikula-Pietrasik J, Pakula M, Budkiewicz S, Drzewiecki M, Gaiday AN, Wierzowiecka M, Naumowicz E, Moszynski R, Tykarski A, Ksiazek K. Malignant ascites promote adhesion of ovarian cancer cells to peritoneal mesothelium and fibroblasts. Int J Mol Sci. 2021;22:4222. doi: 10.3390/ijms22084222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smolle E, Taucher V, Haybaeck J. Malignant ascites in ovarian cancer and the role of targeted therapeutics. Anticancer Res. 2014;34:1553–1561. [PubMed] [Google Scholar]
- 127.Damen MPF, van Rheenen J, Scheele C. Targeting dormant tumor cells to prevent cancer recurrence. FEBS J. 2021;288:6286–6303. doi: 10.1111/febs.15626. [DOI] [PubMed] [Google Scholar]
- 128.Barney LE, Hall CL, Schwartz AD, Parks AN, Sparages C, Galarza S, Platt MO, Mercurio AM, Peyton SR. Tumor cell-organized fibronectin maintenance of a dormant breast cancer population. Sci Adv. 2020;6:eaaz4157. doi: 10.1126/sciadv.aaz4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cavallaro U, Christofori G. Cell adhesion in tumor invasion and metastasis: loss of the glue is not enough. Biochim Biophys Acta. 2001;1552:39–45. doi: 10.1016/s0304-419x(01)00038-5. [DOI] [PubMed] [Google Scholar]
- 130.Cirillo N, Hassona Y, Celentano A, Lim KP, Manchella S, Parkinson EK, Prime SS. Cancer-associated fibroblasts regulate keratinocyte cell-cell adhesion via TGF-beta-dependent pathways in genotype-specific oral cancer. Carcinogenesis. 2017;38:76–85. doi: 10.1093/carcin/bgw113. [DOI] [PubMed] [Google Scholar]
- 131.Stuelten CH, Parent CA, Montell DJ. Cell motility in cancer invasion and metastasis: insights from simple model organisms. Nat Rev Cancer. 2018;18:296–312. doi: 10.1038/nrc.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oh E, Quartuccio SM, Cheng W, Ahmed RA, King SM, Burdette JE. Mutation or loss of p53 differentially modifies TGFbeta action in ovarian cancer. PLoS One. 2014;9:e89553. doi: 10.1371/journal.pone.0089553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pakula M, Witucka A, Uruski P, Radziemski A, Moszynski R, Szpurek D, Maksin K, Wozniak A, Sajdak S, Tykarski A, Mikula-Pietrasik J, Ksiazek K. Senescence-related deterioration of intercellular junctions in the peritoneal mesothelium promotes the transmesothelial invasion of ovarian cancer cells. Sci Rep. 2019;9:7587. doi: 10.1038/s41598-019-44123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hassona Y, Cirillo N, Heesom K, Parkinson EK, Prime SS. Senescent cancer-associated fibroblasts secrete active MMP-2 that promotes keratinocyte dis-cohesion and invasion. Br J Cancer. 2014;111:1230–1237. doi: 10.1038/bjc.2014.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin SW, Ke FC, Hsiao PW, Lee PP, Lee MT, Hwang JJ. Critical involvement of ILK in TGFbeta1-stimulated invasion/migration of human ovarian cancer cells is associated with urokinase plasminogen activator system. Exp Cell Res. 2007;313:602–613. doi: 10.1016/j.yexcr.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 136.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. doi: 10.1101/gad.14.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Melzer C, von der Ohe J, Hass R, Ungefroren H. TGF-beta-dependent growth arrest and cell migration in benign and malignant breast epithelial cells are antagonistically controlled by Rac1 and Rac1b. Int J Mol Sci. 2017;18:1574. doi: 10.3390/ijms18071574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lin S, Yang J, Elkahloun AG, Bandyopadhyay A, Wang L, Cornell JE, Yeh IT, Agyin J, Tomlinson G, Sun LZ. Attenuation of TGF-beta signaling suppresses premature senescence in a p21-dependent manner and promotes oncogenic Ras-mediated metastatic transformation in human mammary epithelial cells. Mol Biol Cell. 2012;23:1569–1581. doi: 10.1091/mbc.e11-10-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lin HK, Bergmann S, Pandolfi PP. Cytoplasmic PML function in TGF-beta signalling. Nature. 2004;431:205–211. doi: 10.1038/nature02783. [DOI] [PubMed] [Google Scholar]
- 140.Tanabe Y, Kawamoto S, Takaku T, Morishita S, Hirao A, Komatsu N, Hara E, Mukaida N, Baba T. Expansion of senescent megakaryocyte-lineage cells maintains CML cell leukemogenesis. Blood Adv. 2020;4:6175–6188. doi: 10.1182/bloodadvances.2020003117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 142.Terri M, Trionfetti F, Montaldo C, Cordani M, Tripodi M, Lopez-Cabrera M, Strippoli R. Mechanisms of peritoneal fibrosis: focus on immune cells-peritoneal stroma interactions. Front Immunol. 2021;12:607204. doi: 10.3389/fimmu.2021.607204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hao Y, Baker D, Ten Dijke P. TGF-beta-mediated epithelial-mesenchymal transition and cancer metastasis. Int J Mol Sci. 2019;20:2767. doi: 10.3390/ijms20112767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guan T, Dominguez CX, Amezquita RA, Laidlaw BJ, Cheng J, Henao-Mejia J, Williams A, Flavell RA, Lu J, Kaech SM. ZEB1, ZEB2, and the miR-200 family form a counterregulatory network to regulate CD8(+) T cell fates. J Exp Med. 2018;215:1153–1168. doi: 10.1084/jem.20171352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yu L, Cao C, Li X, Zhang M, Gu Q, Gao H, Balic JJ, Xu D, Zhang L, Ying L, Xu D, Yang Y, Wu D, He B, Jenkins BJ, Liu Y, Li J. Complete loss of miR-200 family induces EMT associated cellular senescence in gastric cancer. Oncogene. 2021;41(1):26–36. doi: 10.1038/s41388-021-02067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kidan N, Khamaisie H, Ruimi N, Roitman S, Eshel E, Dally N, Ruthardt M, Mahajna J. Ectopic expression of snail and twist in Ph+ leukemia cells upregulates CD44 expression and alters their differentiation potential. J Cancer. 2017;8:3952–3968. doi: 10.7150/jca.19633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pakula M, Uruski P, Niklas A, Wozniak A, Szpurek D, Tykarski A, Mikula-Pietrasik J, Ksiazek K. A unique pattern of mesothelial-mesenchymal transition induced in the normal peritoneal mesothelium by high-grade serous ovarian cancer. Cancers (Basel) 2019;11:662. doi: 10.3390/cancers11050662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sandoval P, Jimenez-Heffernan JA, Rynne-Vidal A, Perez-Lozano ML, Gilsanz A, Ruiz-Carpio V, Reyes R, Garcia-Bordas J, Stamatakis K, Dotor J, Majano PL, Fresno M, Cabanas C, Lopez-Cabrera M. Carcinoma-associated fibroblasts derive from mesothelial cells via mesothelial-to-mesenchymal transition in peritoneal metastasis. J Pathol. 2013;231:517–531. doi: 10.1002/path.4281. [DOI] [PubMed] [Google Scholar]
- 149.Rynne-Vidal A, Au-Yeung CL, Jimenez-Heffernan JA, Perez-Lozano ML, Cremades-Jimeno L, Barcena C, Cristobal-Garcia I, Fernandez-Chacon C, Yeung TL, Mok SC, Sandoval P, Lopez-Cabrera M. Mesothelial-to-mesenchymal transition as a possible therapeutic target in peritoneal metastasis of ovarian cancer. J Pathol. 2017;242:140–151. doi: 10.1002/path.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Smit MA, Peeper DS. Epithelial-mesenchymal transition and senescence: two cancer-related processes are crossing paths. Aging (Albany NY) 2010;2:735–741. doi: 10.18632/aging.100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, Beug H, Grunert S. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol. 2002;156:299–313. doi: 10.1083/jcb.200109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/S0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 153.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 154.Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front Oncol. 2014;4:62. doi: 10.3389/fonc.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tan ML, Parkinson EK, Yap LF, Paterson IC. Autophagy is deregulated in cancer-associated fibroblasts from oral cancer and is stimulated during the induction of fibroblast senescence by TGF-beta1. Sci Rep. 2021;11:584. doi: 10.1038/s41598-020-79789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 157.Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, Byrom D, Riera A, Rossell D, Mangues R, Massague J, Sancho E, Batlle E. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cai J, Tang H, Xu L, Wang X, Yang C, Ruan S, Guo J, Hu S, Wang Z. Fibroblasts in omentum activated by tumor cells promote ovarian cancer growth, adhesion and invasiveness. Carcinogenesis. 2012;33:20–29. doi: 10.1093/carcin/bgr230. [DOI] [PubMed] [Google Scholar]
- 159.Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77:1745–1770. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Coppe JP, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281:29568–29574. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 161.Mikula-Pietrasik J, Sosinska P, Naumowicz E, Maksin K, Piotrowska H, Wozniak A, Szpurek D, Ksiazek K. Senescent peritoneal mesothelium induces a pro-angiogenic phenotype in ovarian cancer cells in vitro and in a mouse xenograft model in vivo. Clin Exp Metastasis. 2016;33:15–27. doi: 10.1007/s10585-015-9753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kay EP, Lee MS, Seong GJ, Lee YG. TGF-beta s stimulate cell proliferation via an autocrine production of FGF-2 in corneal stromal fibroblasts. Curr Eye Res. 1998;17:286–293. doi: 10.1076/ceyr.17.3.286.5212. [DOI] [PubMed] [Google Scholar]
- 163.Heredia-Soto V, Lopez-Guerrero JA, Redondo A, Mendiola M. The hallmarks of ovarian cancer: Focus on angiogenesis and micro-environment and new models for their characterisation. EJC Suppl. 2020;15:49–55. doi: 10.1016/j.ejcsup.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gerber SA, Rybalko VY, Bigelow CE, Lugade AA, Foster TH, Frelinger JG, Lord EM. Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am J Pathol. 2006;169:1739–1752. doi: 10.2353/ajpath.2006.051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bai H, Gao Y, Hoyle DL, Cheng T, Wang ZZ. Suppression of transforming growth factor-beta signaling delays cellular senescence and preserves the function of endothelial cells derived from human pluripotent stem cells. Stem Cells Transl Med. 2017;6:589–600. doi: 10.5966/sctm.2016-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Blanco FJ, Grande MT, Langa C, Oujo B, Velasco S, Rodriguez-Barbero A, Perez-Gomez E, Quintanilla M, Lopez-Novoa JM, Bernabeu C. S-endoglin expression is induced in senescent endothelial cells and contributes to vascular pathology. Circ Res. 2008;103:1383–1392. doi: 10.1161/CIRCRESAHA.108.176552. [DOI] [PubMed] [Google Scholar]
- 167.Krugmann J, Schwarz CL, Melcher B, Sterlacci W, Ozalinskaite A, Lermann J, Agaimy A, Vieth M. Malignant ascites occurs most often in patients with high-grade serous papillary ovarian cancer at initial diagnosis: a retrospective analysis of 191 women treated at Bayreuth Hospital, 2006–2015. Arch Gynecol Obstet. 2019;299:515–523. doi: 10.1007/s00404-018-4952-9. [DOI] [PubMed] [Google Scholar]
- 168.Adam RA, Adam YG. Malignant ascites: past, present, and future. J Am Coll Surg. 2004;198:999–1011. doi: 10.1016/j.jamcollsurg.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 169.Mikula-Pietrasik J, Uruski P, Matuszkiewicz K, Szubert S, Moszynski R, Szpurek D, Sajdak S, Tykarski A, Ksiazek K. Ovarian cancer-derived ascitic fluids induce a senescence-dependent pro-cancerogenic phenotype in normal peritoneal mesothelial cells. Cell Oncol (Dordr ) 2016;39:473–481. doi: 10.1007/s13402-016-0289-1. [DOI] [PubMed] [Google Scholar]
- 170.Mikula-Pietrasik J, Uruski P, Szubert S, Moszynski R, Szpurek D, Sajdak S, Tykarski A, Ksiazek K. Biochemical composition of malignant ascites determines high aggressiveness of undifferentiated ovarian tumors. Med Oncol. 2016;33:94. doi: 10.1007/s12032-016-0810-4. [DOI] [PubMed] [Google Scholar]
- 171.Silva EG, Tornos C, Bailey MA, Morris M. Undifferentiated carcinoma of the ovary. Arch Pathol Lab Med. 1991;115:377–381. [PubMed] [Google Scholar]
- 172.Yang L, Zhang X, Ma Y, Zhao X, Li B, Wang H. Ascites promotes cell migration through the repression of miR-125b in ovarian cancer. Oncotarget. 2017;8:51008–51015. doi: 10.18632/oncotarget.16846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pakula M, Mikula-Pietrasik J, Witucka A, Kostka-Jeziorny K, Uruski P, Moszynski R, Naumowicz E, Sajdak S, Tykarski A, Ksiazek K. The epithelial-mesenchymal transition initiated by malignant ascites underlies the transmesothelial invasion of ovarian cancer cells. Int J Mol Sci. 2019;20:137. doi: 10.3390/ijms20010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Carrel A. On the permanent life of tissues outside of the organism. J Exp Med. 1912;15:516–528. doi: 10.1084/jem.15.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Katakura Y, Nakata E, Miura T, Shirahata S. Transforming growth factor beta triggers two independent-senescence programs in cancer cells. Biochem Biophys Res Commun. 1999;255:110–115. doi: 10.1006/bbrc.1999.0129. [DOI] [PubMed] [Google Scholar]
- 176.Cassar L, Nicholls C, Pinto AR, Chen R, Wang L, Li H, Liu JP. TGF-beta receptor mediated telomerase inhibition, telomere shortening and breast cancer cell senescence. Protein Cell. 2017;8:39–54. doi: 10.1007/s13238-016-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, Shimada Y, Ari-i S, Wada H, Fujimoto J, Kohno M. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999;18:813–822. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- 178.Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, Wilber A, Watabe K. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208:2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Reimann M, Lee S, Loddenkemper C, Dorr JR, Tabor V, Aichele P, Stein H, Dorken B, Jenuwein T, Schmitt CA. Tumor stroma-derived TGF-beta limits myc-driven lymphomagenesis via Suv39h1-dependent senescence. Cancer Cell. 2010;17:262–272. doi: 10.1016/j.ccr.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 180.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI0211638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pakula M, Maly E, Uruski P, Witucka A, Bogucka M, Jaroszewska N, Makowska N, Niklas A, Moszynski R, Sajdak S, Tykarski A, Mikula-Pietrasik J, Ksiazek K. Deciphering the molecular mechanism of spontaneous senescence in primary epithelial ovarian cancer cells. Cancers (Basel) 2020;12:296. doi: 10.3390/cancers12020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang Z, Guo J, Zhou J, Liu H, Xu C. Olaparib induced senescence under p16 or p53 dependent manner in ovarian cancer. J Gynecol Oncol. 2019;30:16. doi: 10.3802/jgo.2019.30.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gao J, Zhu Y, Nilsson M, Sundfeldt K. TGF-beta isoforms induce EMT independent migration of ovarian cancer cells. Cancer Cell Int. 2014;14:72. doi: 10.1186/s12935-014-0072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ozturk N, Erdal E, Mumcuoglu M, Akcali KC, Yalcin O, Senturk S, rslan-Ergul A, Gur B, Yulug I, Cetin-Atalay R, Yakicier C, Yagci T, Tez M & Ozturk M, Reprogramming of replicative senescence in hepatocellular carcinoma-derived cells. Proc Natl Acad Sci USA. 2006;103:2178–2183. doi: 10.1073/pnas.0510877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Verschueren K, Remacle JE, Collart C, Kraft H, Baker BS, Tylzanowski P, Nelles L, Wuytens G, Su MT, Bodmer R, Smith JC, Huylebroeck D. SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5'-CACCT sequences in candidate target genes. J Biol Chem. 1999;274:20489–20498. doi: 10.1074/jbc.274.29.20489. [DOI] [PubMed] [Google Scholar]
- 186.Senturk S, Mumcuoglu M, Gursoy-Yuzugullu O, Cingoz B, Akcali KC, Ozturk M. Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology. 2010;52:966–974. doi: 10.1002/hep.23769. [DOI] [PubMed] [Google Scholar]
- 187.Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F, D'Alessio AC, Young RA, Weinberg RA. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 2013;154:61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 189.Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G, Smith GH. Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev Biol. 1995;168:47–61. doi: 10.1006/dbio.1995.1060. [DOI] [PubMed] [Google Scholar]
- 190.Tang B, Yoo N, Vu M, Mamura M, Nam JS, Ooshima A, Du Z, Desprez PY, Anver MR, Michalowska AM, Shih J, Parks WT, Wakefield LM. Transforming growth factor-beta can suppress tumorigenesis through effects on the putative cancer stem or early progenitor cell and committed progeny in a breast cancer xenograft model. Cancer Res. 2007;67:8643–8652. doi: 10.1158/0008-5472.CAN-07-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ehata S, Johansson E, Katayama R, Koike S, Watanabe A, Hoshino Y, Katsuno Y, Komuro A, Koinuma D, Kano MR, Yashiro M, Hirakawa K, Aburatani H, Fujita N, Miyazono K. Transforming growth factor-beta decreases the cancer-initiating cell population within diffuse-type gastric carcinoma cells. Oncogene. 2011;30:1693–1705. doi: 10.1038/onc.2010.546. [DOI] [PubMed] [Google Scholar]
- 192.Ciardiello D, Elez E, Tabernero J, Seoane J. Clinical development of therapies targeting TGFbeta: current knowledge and future perspectives. Ann Oncol. 2020;31:1336–1349. doi: 10.1016/j.annonc.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 193.Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahapatra AK, Suri A, Balasubramaniam A, Nair S, Oliushine V, Parfenov V, Poverennova I, Zaaroor M, Jachimczak P, Ludwig S, Schmaus S, Heinrichs H, Schlingensiepen KH, Trabedersen Glioma Study G (2011) Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol 13: 132–142. [DOI] [PMC free article] [PubMed]
- 194.Formenti SC, Lee P, Adams S, Goldberg JD, Li X, Xie MW, Ratikan JA, Felix C, Hwang L, Faull KF, Sayre JW, Hurvitz S, Glaspy JA, Comin-Anduix B, Demaria S, Schaue D, McBride WH. Focal irradiation and systemic TGFbeta blockade in metastatic breast cancer. Clin Cancer Res. 2018;24:2493–2504. doi: 10.1158/1078-0432.CCR-17-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Giaccone G, Bazhenova LA, Nemunaitis J, Tan M, Juhasz E, Ramlau R, van den Heuvel MM, Lal R, Kloecker GH, Eaton KD, Chu Q, Dunlop DJ, Jain M, Garon EB, Davis CS, Carrier E, Moses SC, Shawler DL, Fakhrai H. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur J Cancer. 2015;51:2321–2329. doi: 10.1016/j.ejca.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 196.Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M, Trojan J, Kozloff M, Simionato F, Cleverly A, Smith C, Wang S, Man M, Driscoll KE, Estrem ST, Lahn MMF, Benhadji KA, Tabernero J. TGFbeta receptor inhibitor galunisertib is linked to inflammation- and remodeling-related proteins in patients with pancreatic cancer. Cancer Chemother Pharmacol. 2019;83:975–991. doi: 10.1007/s00280-019-03807-4. [DOI] [PubMed] [Google Scholar]
- 197.Yap TA, Vieito M, Baldini C, Sepulveda-Sanchez JM, Kondo S, Simonelli M, Cosman R, van der Westhuizen A, Atkinson V, Carpentier AF, Lohr M, Redman R, Mason W, Cervantes A, Le Rhun E, Ochsenreither S, Warren L, Zhao Y, Callies S, Estrem ST, Man M, Gandhi L, Avsar E, Melisi D. First-in-human phase I Study of a next-generation, oral, TGFbeta receptor 1 inhibitor, LY3200882, in patients with advanced cancer. Clin Cancer Res. 2021;27:6666–6676. doi: 10.1158/1078-0432.CCR-21-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Paz-Ares L, Kim TM, Vicente D, Felip E, Lee DH, Lee KH, Lin CC, Flor MJ, Di Nicola M, Alvarez RM, Dussault I, Helwig C, Ojalvo LS, Gulley JL, Cho BC. Bintrafusp Alfa, a bifunctional fusion protein targeting TGF-beta and PD-L1, in second-line treatment of patients with NSCLC: results from an expansion cohort of a phase 1 trial. J Thorac Oncol. 2020;15:1210–1222. doi: 10.1016/j.jtho.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jung SY, Hwang S, Clarke JM, Bauer TM, Keedy VL, Lee H, Park N, Kim SJ, Lee JI. Pharmacokinetic characteristics of vactosertib, a new activin receptor-like kinase 5 inhibitor, in patients with advanced solid tumors in a first-in-human phase 1 study. Invest New Drugs. 2020;38:812–820. doi: 10.1007/s10637-019-00835-y. [DOI] [PubMed] [Google Scholar]
- 200.Rocconi RP, Monk BJ, Walter A, Herzog TJ, Galanis E, Manning L, Bognar E, Wallraven G, Stanbery L, Aaron P, Senzer N, Coleman RL, Nemunaitis J. Gemogenovatucel-T (Vigil) immunotherapy demonstrates clinical benefit in homologous recombination proficient (HRP) ovarian cancer. Gynecol Oncol. 2021;161:676–680. doi: 10.1016/j.ygyno.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 201.Ashrafizadeh M, Najafi M, Orouei S, Zabolian A, Saleki H, Azami N, Sharifi N, Hushmandi K, Zarrabi A, Ahn KS. Resveratrol modulates transforming growth factor-beta (TGF-beta) signaling pathway for disease therapy: a new insight into its pharmacological activities. Biomedicines. 2020;8:261. doi: 10.3390/biomedicines8080261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Thacker PC, Karunagaran D. Curcumin and emodin down-regulate TGF-beta signaling pathway in human cervical cancer cells. PLoS One. 2015;10:e0120045. doi: 10.1371/journal.pone.0120045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jhou BY, Song TY, Lee I, Hu ML, Yang NC. Lycopene inhibits metastasis of human liver adenocarcinoma SK-Hep-1 cells by downregulation of NADPH oxidase 4 protein expression. J Agric Food Chem. 2017;65:6893–6903. doi: 10.1021/acs.jafc.7b03036. [DOI] [PubMed] [Google Scholar]
- 204.Giacomelli C, Daniele S, Natali L, Iofrida C, Flamini G, Braca A, Trincavelli ML, Martini C. Carnosol controls the human glioblastoma stemness features through the epithelial-mesenchymal transition modulation and the induction of cancer stem cell apoptosis. Sci Rep. 2017;7:15174. doi: 10.1038/s41598-017-15360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Boldbaatar A, Lee S, Han S, Jeong AL, Ka HI, Buyanravjikh S, Lee JH, Lim JS, Lee MS, Yang Y. Eupatolide inhibits the TGF-beta1-induced migration of breast cancer cells via downregulation of SMAD3 phosphorylation and transcriptional repression of ALK5. Oncol Lett. 2017;14:6031–6039. doi: 10.3892/ol.2017.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Avila-Carrasco L, Majano P, Sanchez-Tomero JA, Selgas R, Lopez-Cabrera M, Aguilera A, Gonzalez Mateo G. Natural plants compounds as modulators of epithelial-to-mesenchymal transition. Front Pharmacol. 2019;10:715. doi: 10.3389/fphar.2019.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.