Abstract
Metastasis is the main culprit of cancer-associated mortality and involves a complex and multistage process termed the metastatic cascade, which requires tumor cells to detach from the primary site, intravasate, disseminate in the circulation, extravasate, adapt to the foreign microenvironment, and form organ-specific colonization. Each of these processes has been already studied extensively for molecular mechanisms focused mainly on protein-coding genes. Recently, increasing evidence is pointing towards RNAs without coding potential for proteins, referred to as non-coding RNAs, as regulators in shaping cellular activity. Since those first reports, the detection and characterization of non-coding RNA have explosively thrived and greatly enriched the understanding of the molecular regulatory networks in metastasis. Moreover, a comprehensive description of ncRNA dysregulation will provide new insights into novel tools for the early detection and treatment of metastatic cancer. In this review, we focus on discussion of the emerging role of ncRNAs in governing cancer metastasis and describe step by step how ncRNAs impinge on cancer metastasis. In particular, we highlight the diagnostic and therapeutic applications of ncRNAs in metastatic cancer.
Keywords: miRNAs, lncRNAs, circRNAs, Metastatic cancer, Cancer therapy
Introduction
Metastasis is defined as the spread of neoplastic cells from their original site to other regions of the body, thus resulting in the development of secondary tumors [1, 2]. It is now established that metastasis represents a complex and multi-step process termed metastatic cascade. This process can be parsed into distinct steps: local invasion at the primary site, entry into the vasculature (intravasation), transport and survival in the circulation, arrest, and exit from the distant capillary bed (extravasation), and colonization within the secondary organ parenchyma [1, 3]. Clinically, metastasis, accounting for more than 90% of all cancer mortality [1], causes not only direct damage to the affected organs, but also systemic syndromes including endocrine abnormalities, malignant effusions, hematologic disorders, and cachexia [4].
Previous studies have advanced to unveil the molecular basis of this life-threatening process. Several driving oncogenic mutations, including ERBB2 in breast cancer, BRAF in melanoma, ALK in lung cancers, and ZFP36L2 in colorectal cancer have been revealed to be crucial for metastatic disease [5, 6]. Epithelial–mesenchymal transition (EMT), an event during embryonic development, is exploited by cancer cells to acquire undifferentiated morphology and invasive traits, thereby facilitating metastasis [7]. Besides, extracellular matrix (ECM) degradation proteases, cell adhesion molecules, Rho/ROCK-mediated cytoskeleton motility, and angiogenetic process have also shown a substantial effect in programs enabling migration from primary sites [1]. What’s more, pro-survival signaling such as AKT pathways and signaling favoring immune evasion such as TGF-β pathways are key features of metastatic cancer that control the survival of disseminated tumor cells [5]. Aside from these general mediators of metastasis, context-dependent pathways mediating tumor-stroma interactions in the particular foreign microenvironments have essential roles for the adaptability and survival of metastatic tumor cells at ectopic sites. However, with the recent discovery of thousands of noncoding transcripts (noncoding RNAs, ncRNAs) which account for approximately 30% of the human genome, we must admit that there is still a long way to go to fully understand the mechanisms of cancer metastasis. In the human genome, only 1–2% of the DNA codes for proteins. In the past, the remaining 98% noncoding sequences were considered as “evolutionary junk”. Increasing evidence indicates that instead, these RNA transcripts have a tremendous impact on diverse physiological and pathological processes [8]. To date, high throughput transcriptome analyses have identified a myriad of ncRNAs that have surpassed the number of protein-coding genes in the human genome, and the number is still rapidly expanding [9, 10]. Indeed, many/most cancer-associated mutations reside within the non-coding regions rather than in the coding regions in the human genome [11]. Using the length of 200 nt as a criterion, those ncRNAs are divided into two subclasses: small ncRNAs (such as microRNAs (miRNAs), PIWI-Interacting RNAs (piRNAs)) and long noncoding RNAs (LncRNAs) [9].The characterization of such an expanded repertoire of ncRNAs is shedding new light on the molecular mechanisms of metastasis. For instance, the first metastasis-associated miRNA miR-10b, which promotes metastasis via regulating RhoC expression, was identified in breast cancer [12]. Moreover, the miR-200 family has been shown to play a key role in regulating EMT program [13]. Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), one of the first lncRNAs discovered, has been related to a high risk of metastatic progression in patients with lung cancer [14].
In this review, we mainly focus on recent findings of ncRNAs and their regulatory roles in distinct steps of the metastatic cascade to discuss how ncRNAs orchestrate a series of pathological events such as EMT, transendothelial migration, anoikis, immune evasion, dormancy, etc. We also provide a brief introduction to the biogenesis and function of ncRNAs and summarize the clinical value of ncRNAs in the diagnosis and treatment of cancer metastasis. Overall, we aim to provide a perspective for future research directions and hope that an understanding of the role of ncRNAs in cancer metastasis can help more bench discoveries be translated into clinical application.
Biogenesis and function of ncRNAs
Biogenesis and function of miRNAs
miRNA is a well-characterized class of small ncRNA, defined as single-stranded transcripts of ~ 20nt in length that regulate gene expression post-transcriptionally [15, 16].The biogenesis of miRNAs involves transcription by RNA polymerase II from corresponding miRNA-encoding genes [17], being cleaved by the Drosha and DGCR8 complex [18, 19], transported to the cytoplasm [20], processed by Dicer [21], and ultimately, formation of RNA-induced silencing complex (RISC) by association with Argonaute [22].
Mechanistically, the 5ʹ end (known as the seed sequence) of the miRNAs binds to the 3ʹ untranslated region (3ʹUTR) of target mRNAs by Watson–Crick pairing [22, 23]. This interaction can lead to inhibition of mRNAs of key metastasis genes by either blocking the translational machinery or promoting its degradation (Fig. 1). For instance, in prostate cancer, miR-34a binds to 3′ UTR of CD44 (an adhesion molecule) mRNA and result in its degradation, thereby inhibiting metastasis to the lung [24]. As another example, miR-506 targets the 3′-UTR of SNAI2 (a transcription factor) mRNA and inhibits its expression so as to upregulate metastasis-suppressing genes and attenuate cell migration and invasion in ovarian cancer [25]. miRNAs exert a far-reaching influence on gene regulation; it is estimated that as high as 60% of coding genes are affected by miRNAs [26].
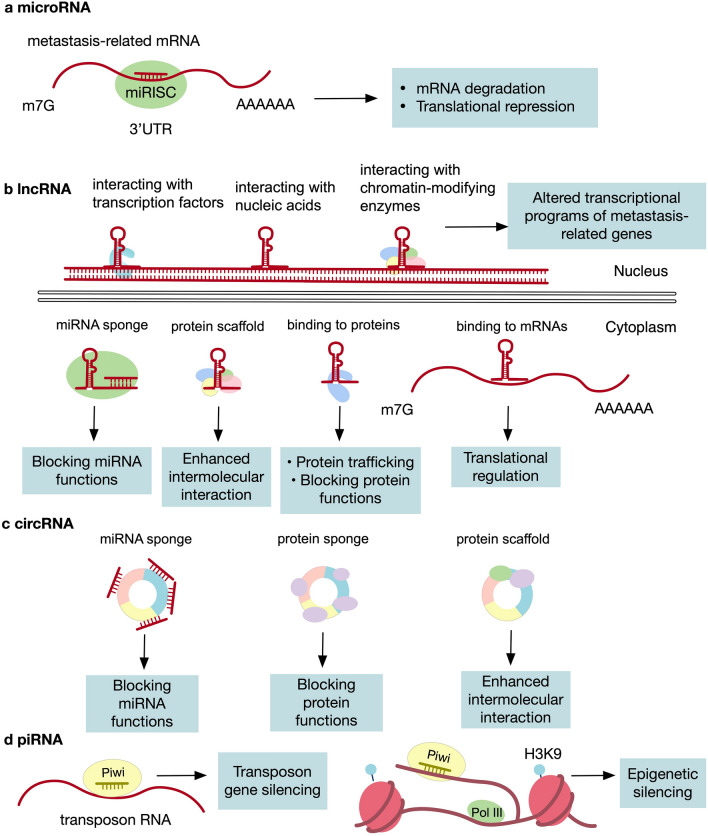
Fig. 1.
Functional mechanisms of ncRNA. a miRNAs bind to the 3ʹUTR of target mRNAs by Watson–Crick pairing via their 5’ end, leading to inhibition of mRNAs by either blocking the translational machinery or promoting its degradation. b In the nucleus, lncRNAs can interact with nucleic acids, chromatin-modifying enzymes, and transcription factors to influence transcriptional programs of genes. In the cytoplasm, they can sponge miRNAs and/or proteins to block their functions, bind to proteins and guide them to specific subcellular localization, bind to mRNAs to regulate their translation, and act as scaffolds for different molecules to favor their intermolecular interactions. c circRNAs bind to transcripts or proteins to suppress their function or to act as scaffolds for enhanced intermolecular interaction. d piRNAs bind to transposon RNA to mediate transposon gene silencing. They can also associate with target transcripts to favor heterochromatin formation, leading to their epigenetic silencing of the target loci
Biogenesis and function of lncRNAs
LncRNA is a subclass of non-coding transcripts that is longer than 200nt [27]. LncRNAs share something in common with their protein-coding counterparts exemplified by the fact that they are both transcribed by RNA polymerase II, capped and generally polyadenylated, and spliced [28]. Similar to mRNAs, lncRNAs can arise from almost anywhere in the genome and in different directions [10]. Besides, lncRNAs form unique secondary structures which to some extent determine their distinct functions [29]. After biogenesis, lncRNAs either remain in the nucleus or are exported to the cytoplasm [30].
Compared to other ncRNAs, the mechanisms by which lncRNAs exert their functions are more complex and diverse. LncRNAs can cis- or trans-regulate genes at both transcriptional and post-transcriptional levels. Mechanistically, lncRNAs exhibit four distinct modes of action (Fig. 1), including function as decoys to block biomolecular functions [9], guidance the specific subcellular localization of proteins, function as scaffolds for intermolecular interactions, and interaction with nucleic acids, chromatin-modifying enzymes as well as transcription factors to influence transcriptional programs of genes. These impact various aspects of tumor metastasis. For instance, lncRNA Gas5 acts as an endogenous sponge to bind to and inactivate miR-222 in glioblastoma to attenuate cell invasion and migration [31], while its role as a protein decoy for glucocorticoid receptor has also been shown in various cancer cell lines[32]. In addition, lncRNA HOTAIR recruits polycomb repressive complex 2 (PRC2) to alter the H3K27me3 patterns and facilitate transcription of genes involved in metastasis in breast cancer [33].
Biogenesis and function of other ncRNAs
Other ncRNAs include circular RNA (circRNA), piRNA, small nucleolar RNA (snoRNA), pseudogenes, tRNA-derived small RNAs (tsRNAs), etc. Here we will briefly describe a few of the relatively well-studied types in cancer biology.
Circular RNA (circRNA) is a kind of single-stranded RNA in which the 3' and 5' ends join together to form a covalently closed loop, with the length of 100nt ~ over 4 kb [34, 35]. Comparing with lncRNAs, circRNAs are more stable than linear transcripts due to the lack of 3ʹ and 5ʹ ends, indicating a huge potential for cancer detection [36, 37]. So far, several action modes of circRNAs have been identified (Fig. 1). As one mechanism of action, circRNAs can act as sponges directly binding to biomolecules [38, 39], resulting in titrating away proteins or miRNAs which are related to metastasis. For example, circCCDC66 serves as a sponge for a group of miRNAs to protect several oncogenic mRNAs (e.g., MYC) from degradation, thus promoting colon cancer metastasis [40]. CircRNAs may also function as scaffolds to assemble various molecules involved in classical metastatic pathways, such as Wnt signaling pathway [41]. Of note, some studies even have proposed the protein coding potential of a subset of circRNAs [42, 43]. One of such cases reported in cancer metastasis is circPPP1R12A, which was shown to be translated into a functional protein and promote liver metastasis of colon cancer [44].
piRNA is a type of small ncRNA of 24 ~ 32nt in length whose canonical function is to silence transposons in germline cells thus maintain genomic integrity [45]. While miRNAs bind to AGO of Argonaute proteins to regulate gene expression, as the name suggests, piRNAs associate with the PIWI subfamily of Argonaute proteins [46]. Although thought as germline-specific in the first place, recent studies have begun to reveal functions of piRNAs in epigenetic modification and genome rearrangement in somatic cells, and emerging evidence suggests that this subclass of ncRNAs is also aberrantly expressed in multiple metastatic carcinoma types [47]. However, understanding of the biological mechanisms of piRNAs in cancer metastasis currently remains in its infancy (Fig. 1). Recent publications have brought new insight into the underlying mechanisms. In colorectal cancer, piR-1245 favors metastasis probably via a piRNA-dependent mRNA degradation of a panel of tumor suppressor genes [48]. Whereas in breast cancer, piR-36712 interacts with RNAs of SEPW1P (pseudogene of SEPW1) to promote targeting of SEPW1 RNA by miRNAs, thus augmenting P53-mediated metastasis-suppressing signaling [49].
The role of ncRNAs in metastatic cascade
The metastatic cascade is a simplified description in sequential order of how primary cancer cells disseminate and colonize ectopic sites [2]. At the outset of this journey, cancer cells break through the confinement of the primary tumor, attributing to the initiation of EMT [50]. Next, after detachment from the primary site, cancer cells enter into the blood vessels, which involves angiogenesis and proteolytic function [51]. Subsequently in the circulation, cancer cells are present as either single cells or as emboli with platelets and/or leukocytes to protect them from shear forces, immune attack, and anoikis [52]. In the capillary of distant organs, cancer cells arrest via physical trapping or selective adhesion and extravasate [51]. Afterward, the vast majority of extravasated cells will succumb, while the rest of them can survive in a dormant state [3]. Ultimately, cancer cells must adapt to and remodel the microenvironment to establish a macrometastatic lesion from micrometastases [1].
This whole cascade is orchestrated by a cohort of molecular pathways such as RhoGTP and EMT signaling, together with genes including cell adhesion molecules, matrix degradation protease, cytoskeleton, chemokines, and angiogenic factors [3]. There is mounting evidence that ncRNAs play a critical role in the regulation of these pathways and genes [8, 9]. In this section, we will classify the related studies by specific steps to discuss the regulatory landscape of pathways and genes by ncRNAs in distinct metastatic processes.
ncRNAs-mediated local invasion
Local invasion involves detachment from the primary site, disruption of the basement membrane, and penetration to the adjacent parenchyma [1]. Epithelial cell–cell adhesions are mediated mainly by cadherin, particularly E-cadherin, whose loss is robustly correlated with enhanced metastatic potential in multiple carcinoma types [1]. The dissolution of E-cadherin can be attributed to the EMT program, which also leads to dramatic alterations in cell shape from the polarized and organized epithelial morphology to the nonpolarized shape with heightened invasiveness [53]. Along with the loss of intercellular adhesions, cancer cells switch the pattern of cell–matrix adhesion molecules (e.g., integrins), favoring their passage to the stroma [50]. Integrin, a family of transmembrane linkers composed of α and β subunits, acts as a context-dependent function during metastasis through its inside-out and outside-in signaling [54]. In addition, active proteolysis (e.g., by matrix metalloproteinases (MMPs) and serine proteinases) and cytoskeleton-mediated motility (e.g., by Rho/ROCK regulated actin contractility) are also requisite [3] (Fig. 2).
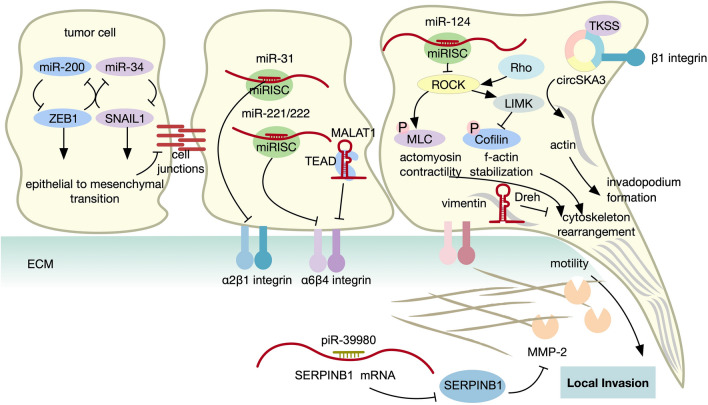
Fig. 2.
Role of ncRNA in local invasion. Multiple ncRNAs that regulate local invasion are shown, acting on diverse hallmarks of cancer metastasis including EMT, cell adhesion, ECM degradation, and cell motility
The most canonical mechanism by which miRNAs regulate the EMT program would be the double-negative feedback loop formed by pairs of miRNAs and EMT-TFs (transcription factors that promote EMT-associated gene expression). For instance, miR-200 suppressed the expression of EMT-TFs ZEB at the post-transcriptional level, in turn, ZEB targeted miR-200 and repressed its transcription [55]. Similar feedback loops also existed between miR-34a and Snail [56], miR‑1/miR‑200b and SNAIL2 [57], and miR‑203 and SNAIL1 [58]. In addition to these loops, it has long been shown that a mass of miRNAs, such as miR-8084 [59], miR-484 [60], miR-708-3p [61], miR-506 [25], miR-4521[62] can modulate the level of EMT-TFs or EMT markers in a great variety of metastatic cancers.
A plethora of lncRNAs has been implicated in regulating EMT either by impinging on EMT-TFs and/ or EMT-associated markers. A previous study demonstrated that oncogenic lncRNA ZEB1-AS1 could induce EMT in hepatocellular carcinoma (HCC) cells by stimulating the transcription of EMT-TFs ZEB1 [63]. At the same time, lncRNA MEG3 was shown to promote EMT by inhibiting the expression of E-cadherin through epigenetic mechanisms in lung cancer cells. MEG3 recruited PRC2, an essential epigenetic transcriptional repressor, to induce inactive H3K27me3 markers on the promoter of E-cadherin gene [64]. In addition, lncRNA JPX also mediated the metastatic potential of lung cancer, where it sponged miR-33a-5p to abrogate the inhibitory effect on Twist [65].
Recently, novel circRNAs have also been proved to be the underlying mechanism of EMT program [66]. For instance, in triple-negative breast cancer, CircANKS1B functioned as a sponge for miR-148a-3p and miR-152-3p, thereby augmenting the translation of transcription factor USF1. Then, USF1 induced the transcription of TGF-β1, triggering TGF-β1/Smad signaling to activate EMT program [67]. At present, increasing numbers of laboratory studies are revealing the roles of circRNAs in EMT in a variety of cancer types, such as circNEIL3 in pancreatic cancer [68], circCORO1C in laryngeal squamous cell carcinoma [69], and CircNR3C2 in triple-negative breast cancer [70]. Likewise, piRNAs were introduced as new players in EMT, such as piR-1037 in human oral squamous cell carcinoma cells [71] and piR-932 in breast cancer stem cells [72], though the underlying molecular mechanisms have yet to be elucidated.
What’s more, miRNA has been involved in the regulation of integrin pattern. For example, in breast cancer, miR-31 downregulated the expression of α2β1 integrin (a metastasis repressor [73]) and facilitated metastasis to the lung [74]. In another model of breast cancer, miR-221/222 suppressed the level of α6β4 integrin (a metastasis promoter) and inhibited invasion [75]. Recently, MALAT1, one of the most well-studied metastasis-promoting lncRNA, has been shown to repress transcription factor TEAD to inhibit the expression of α6β4 integrin, leading to suppression of breast cancer metastasis in a transgenic mouse model [76]. Other than MALAT1, H19 [77], CASC9 [78], HOXD-AS1 [79] have also been shown to affect ECM-integrin interactions in multiple metastatic carcinoma types. Besides, evidence from pan-cancer bioinformatics analysis demonstrated that circRNA CDR1as exerted its effects on matrix organization, integrin binding, and collagen binding, indicative of a pro-metastatic role [80].
ncRNAs can also act as modulators for MMPs. One of such cases was that transcription-regulating kinase CDK8 stimulated colorectal cancer metastasis through effects on TGFβ-regulated miR-181b expression. Mechanistically, miR-181b targeted and inhibited the expression of TIMP3, the MMP inhibitor, thus resulting in the upregulation of MMPs [81]. LncRNAs are also regulators of MMPs in local invasion exemplified by recent evidence including ODRUL [82], MATN1-AS1 [83], PRECSIT[84], LSAMP-AS1 [85], CASC2 [86], and others. In gastric cancer, an in vivo research showed that cirRNA 0,000,096 upregulated MMP-2 and MMP-9 to confer increased tumor invasiveness [87]. As another example, circ0001361, newly identified in bladder cancer, was proved to enhance the local invasion of cancer cells by sponging miR-491-5p and upregulating MMP-9 [88]. Furthermore, piR-39980 increased the expression of MMP-2 and downregulates SERPINB1(an endogenous inhibitor of serine proteases) to promote migration and invasion in human osteosarcoma cells [89].
It is worth mentioning that miRNAs also regulate cellular motility, for instance, miR-124 [90, 91], miR-138 [91], miR-BART2-5p [92] and miR-200b [93], and so forth. have been demonstrated to target RHO/ROCK cytoskeletal remodeling pathway in cancer metastasis. Similarly, lncRNAs can affect the motility of cancer cells by regulating cytoskeleton reorganization. In HBV-related HCC, Dreh could suppress cancer cell migration by directly binding to intermediate filament vimentin, leading to its inactivation, and further altered the cytoskeleton structure [94]. As another example, lncMER52A, newly identified exclusively in HCC, was shown to enhance metastasis by stabilizing p120-catenin and triggering the p120-ctn/Rac1/Cdc42 axis [95]. Circular RNAs have also been implicated in the enhanced cellular motility during metastasis. A previous study showed that circSKA3, directly binding to integrin β1 and Tks5, contributed to the formation of actin-based invadopodia, thereby facilitating breast cancer invasion [96]. In short, ncRNAs have been implicated in every aspect of local invasion.
ncRNAs-mediated intravasation
Intravasation involves cancer cells that have undergone local invasion entering into nearby capillaries or lymphatic vessels [97]. This active process requires intercellular adhesions between cancer cells and endothelial cells (ECs), disruption of the endothelial integrity, and augmented motility to cross the barriers of microvessel walls [98]. Equally important is the ability to stimulate the formation of neovasculature within the primary malignant lesion, which is mediated by several angiogenic factors and growth factors such as VEGFA, ANGPTL4, PDGFB, FGF2, and so on [99]. In general, tumor-associated microvasculature is tortuous and leaky compared with normal blood vessels; therefore, neoangiogenesis is believed to facilitate intravasation [100] (Fig. 3).
Fig. 3.
Role of ncRNA in intravasation and survival in the circulation. Direct or indirect regulations of intravasation and CTCs survival by several ncRNAs are shown. These include diverse hallmarks of cancer metastasis such as a. angiogenesis b. enhanced vascular permeability, c. immune evasion, and d. anoikis resistance
Accumulating evidence has suggested multifaceted roles of ncRNAs in intravasation by the regulation of vascular permeability. In the local microenvironment of lung cancer, miR-23a was revealed to dissolve the tight junction and promote vascular permeability via targeting Zonula occludens protein-1 (ZO-1) in ECs, thus facilitating entry into the bloodstream [101]. A similar mechanism has been functionally described in HCC, where miR-103 disrupted endothelial integrity to promote transendothelial migration by post-transcriptional repression of VE-Cadherin, p120-catenin, and ZO-1 in ECs [102]. Recently, miR-629-5p was shown to break down the endothelial barrier through inhibition of Cadherin EGF LAG seven-pass G-type receptor 1(CELSR1) in ECs within tumor microenvironment of invasive lung cancer [103]. LncRNAs also regulate the tight junctions of endothelial cells to influence tumor cells entry into the circulation. For example, in gastric cancer, depletion of lncRNA MALAT1 in human umbilical vein endothelial cells (HUVECs) resulted in elevated vascular permeability in the local environment by inhibiting the expression of VE-cadherin and β-catenin [104].
Growing number of studies suggested that circRNAs can also act as regulators of vascular permeability. A recent study showed that exosomal circ-IARS derived from pancreatic cancer cells was captured by HUVECs, resulting in increased endothelial permeability. This was achieved through competitive inhibition of miR-122, thus upregulating the expression of downstream target RhoA, which led to increased expression of F-actin and decreased expression of ZO-1. Clinical data showed that Circ-IARS expression was positively correlated with liver metastasis of pancreatic cancer [105]. In another model of cholangiocarcinoma, circ-CCAC1 was demonstrated to dissolve the tight junction between ECs. Mechanistic investigations revealed that circ-CCAC1 interacted with EZH2 to suppress its nuclear translocation, thereby alleviating its inhibitory effect on SH3GL2, which downregulated ZO-1 and Occludin in ECs [106].
Several miRNAs, termed as angio-miRNAs, were shown to play a vital role in neovasculature formation and dissemination into the circulation. For instance, miR-205 enhanced the growth of blood vessels in ovarian cancer through the PTEN-AKT pathway [107]. A previous study reported that miR-135b induced angiogenesis in gastric cancer via downregulating the expression of FOXO1 [108]. Furthermore, miR-130a was revealed to function as a driver of angiogenesis in gastric cancer by suppressing the expression of c-MYB [109]. In contrast, miR-125b and miR-100 attenuated intravasation and metastasis in HCC. Encapsulated tumor cluster (VETC), a vascular pattern that facilitated the penetration of tumor cells into the circulation, was ablated by miR125b and miR-100. Mechanistic investigations revealed that these miRNAs directly inhibit the expression of Angiopoietin 2 (Angpt2), the key factor for VETC formation [110].
Notably, tumor cells usually suffer from hypoxia in the local microenvironment to give rise to new vessels, attributing to the induction of a vast array of lncRNAs. In non-small-cell lung cancer, lncRNA-p21 was significantly upregulated under hypoxic conditions, followed by a global induction of pro-angiogenic genes, including VEGFA, MMP2, PDGFB, and FGF2 [111]. In addition, lncRNA RAB11B-AS1 was induced by HIF2-mediated transcription in hypoxic breast cancer, thus enhancing angiogenesis and metastasis. Mechanistically, RAB11B-AS1 recruited RNA polymerase II to the promoter of VEGFA and ANGPTL4 genes, thereby upregulating the expression of these angiogenic factors [112]. Besides, lncRNAs can also negatively modulate intravasation. Adenomatous polyposis coli (APC) gene is a well-known tumor suppressor gene in colorectal cancer (CRC), and it has been reported recently that lncRNA-APC1 activated by APC exerted its anti-metastasis function by attenuating MAPK pathway-induced angiogenesis in endothelial cells in CRC [113].
In addition, a newly identified circRNA circPRRC2A was revealed as a player in metastatic renal cell carcinoma. CircPRRC2A sponged miR-514a-5p and miR-6776-5p (the negative regulators of TRPM3) to protect TRPM3 mRNA from degradation. Subsequently, the upregulated TRPM3 enhanced tumor angiogenesis [114]. In gastric cancer, a recent research showed that circSHKBP1 upregulated Vascular Endothelial Growth Factor A(VEGF) to confer enhanced tumor invasiveness. Regarding the molecular mechanism, circSHKBP1 acted as a sponge for miR-582-3p to attenuate the inhibitory effect on HUR, an RNA-binding protein that stabilized VEGF mRNA [115]. Indeed, a plethora of novel angio-circRNA in cancer metastasis has been identified recently, such as circRNA-MYLK [116], hsa-circ-001653 [117], circRNA-100338 [118], and so on.
Several in vivo studies have shown prominent roles that ncRNAs play in intravasation. One study using transgenic mice model for mammary tumor exhibited that fewer circulating tumor cells (CTCs) were collected from the peripheral blood of miR-10b-deficient mice compared with control mice, suggesting a positive impact of miR10b on intravasation [119]. In an orthotopic HCC model of mice, lncRNA-ATB knockdown led to an obvious reduction in the number of CTCs, indicating that lncRNA-ATB contributed to intravasation of HCC cells [120].
ncRNAs-mediated survival in the circulation
Once entering the bloodstream, CTCs are present as either single cells or emboli [52]. Emboli can be formed by tumor cells or by tumor cells with leukocytes, platelets, fibrins, etc. [52]. In fact, emboli can protect tumor cells from the threats of the innate immune system (mainly natural killer (NK) cells) and shear stress, two major challenges that CTCs have to overcome in the circulation [121]. For example, tumor cells interact with platelets through different cell surface receptors and ligands such as tissue factor and/or L- and P-selectins, promoting platelet coats formation as a physical shield against shear stress [122]. Other than that, platelets also favor immune evasion. Adhered platelets secrete TGF-β1 to counteract NK granule mobilization and interferon-γ secretion, and transfer platelet MHC-I to tumor cells to evade recognition, thus synergizing to confer resistance to NK-mediated cytolytic activity [123]. Furthermore, in the absence of adhesion to ECM that is necessary for cell survival, cancer cells are subject to anoikis, a class of apoptosis induced by a loss of integrin-dependent anchorage [124]. Anoikis induces cell death through the canonical intrinsic and extrinsic apoptotic pathways [125]. CTCs are enabled to anoikis resistance by induction of many mechanisms, including alterations in integrins expression, reactive oxygen species (ROS)-induced pro-survival signaling, constitutively activation of anti-apoptotic, pro-survival pathways such as PI3K/Akt and the Ras-Raf-Mek-Erk signaling, and so forth [98, 126] (Fig. 3).
In the circulation, platelet-derived microparticles (PMPs) are the main sources of miRNAs [127]. Although there is no direct evidence, previous research has demonstrated that miRNAs may serve as protective signals for CTCs against NK cells. A study reported that miR-296-3p enhanced the tolerance of prostate cancer cells to NK cells via directly repressing the expression of intercellular adhesion molecule 1 (ICAM-1). Subsequent in vivo experiment confirmed that miR-296-3p-ICAM-1 pathway had a positive role in guiding prostate cancer metastasis to the lung, suggesting that miR-296-3p likely increased survival of CTCs from the threats of NK cell [128]. Previous studies have illustrated the important role of breast cancer stem-like cell (BCSC) during metastasis, yet the underlying mechanisms are still being unraveled. miR20a was demonstrated to endow BCSC with resistance to NK cell cytotoxicity and facilitate its spreading to the lung. The processes were achieved via the inhibitory effect of miR20a on MICA and MICB, two stimulators for NK cell activation [129]. In addition, a previous study identified miRNA to be a downstream effector of TGF-β1-mediated immunosuppressive signaling against tumor-associated NK cells. TGF-β-induced miR-183 abrogated the effectiveness of NK cells by directly targeting DNAX activating protein 12 kDa (DAP12), which is a key transmembrane protein for cytolytic signal transduction in NK cells [130].
LncRNAs are also important innate immune system regulators. HOTAIR, one of the most well-studied pro-metastasis lncRNAs, was shown to inhibit NK cell activity via activating Wnt/β-catenin pathway [131]. While another novel lncRNA, lnc-CD56, upregulated CD56 in NK cells and contributed to their differentiation [132]. Aberrantly expressed circRNAs are also involved in the regulation of CTCs survival, though the direct evidence remains scarce. One study pointed to a critical role of circ_0000977 in immune evasion of pancreatic cancer cells from NK cells. Circ_0000977 acted as a decoy for miR-153, which directly targeted and repressed the expression of HIF1A, thus triggering attenuated cytotoxicity of NK cells and immune escape [133]. Conversely, CDR1as/ciRS-7 [80] and CircARSP91 [134] had been described as NK cell stimulators in multiple carcinoma types.
Aside from immune evasion, miRNAs are also involved in anoikis. Recently, miR-141 was identified to function as a crucial regulator for anoikis resistance and peritoneal metastasis in ovarian cancer. Notably, miR-141 directly targeted KLF12 mRNA and suppressed its expression, followed by Sp1-mediated survivin upregulation, which inhibited intrinsic apoptotic pathway [135]. This study revealed a novel pathway in anoikis resistance and implied a possible mechanism by which CTCs escape anoikis. Emerging evidence has suggested that various lnRNAs are also implicated in anoikis. In ovarian cancer cells, depletion of the key metastasis-associated lncRNA MALAT1 decreased metastasis and increased anoikis via affecting the alternative splicing program of pro-apoptotic gene KIF1B. Mechanistically, MALAT1 silencing suppressed the expression of splicing factor RNA binding protein fox-1 homolog 2 (RBFOX2), and led to preferential splicing of the pro-apoptotic full-length isoform of KIF1B [136]. Other metastasis-associated lncRNAs, such as HULC [137], HOTAIR [138], and LINC00958 [139], have been identified to drive anoikis resistance; however, the underlying molecular mechanisms warrant further investigation.
Notably, circRNA may also contribute to anoikis resistance. One study showed that lung cancer cells upregulated circUBAP2 to limit their susceptibility to anoikis, possibly owing to the elevated level of c-IAP1, Bcl-2, and surviving [140]. The role of piRNA in resisting attacks from the immune system in CTCs is still being unraveled, while they appear to be involved in regulating anoikis/ cell survival and apoptosis. For example, piR-004800 was reported to promote myeloma cell survival via the regulation of PI3K/Akt/mTOR pathway [141]. piR-8041, a tumor suppressor of glioblastoma, was also revealed to induce apoptosis probably via the inhibition of survival pathway MAPK/ERK [142].
Besides, the notion that lnRNAs are potential regulators in CTCs survival was further supported by profiling of CTCs gene expression in patients with metastatic castrate-resistance prostate cancer, which uncovered upregulated lncRNA SChLAP1 in CTCs, suggesting its pro-survival role for metastatic tumor cells in the bloodstream [143].
ncRNAs-mediated extravasation
In the context of extravasation, CTCs arrest in small capillaries of distant organs via physically trapping or specific adhesive interactions between CTCs and ECs, then they overcome the endothelial barrier to invade into the parenchyma [3]. Notably, a pattern, that organ tropism for different carcinoma types to form metastases, is ubiquitous. This metastatic preference can be explained by the anatomical layout of the vasculature, the organ-specific circulation pattern, and tumor cell–autonomous traits [51]. The structures of microvessels vary largely depending on organ types. For instance, the sinusoidal capillaries in the liver and bone are highly permeable and seem to be permissive to CTCs, whereas the continuous capillaries in brain and lung together with surrounding cells create an impermeable barrier known as the blood–brain barrier (BBB) and blood–gas barrier [144]. Accordingly, CTCs exploit further strategies to overcome these obstacles. First, adhesion molecules such as E-Selectin, N-cadherin, Integrins, CD44, and MUC1 are expressed to enhance the attachment of cancer cells to ECs [145]. Next, transendothelial migration of CTCs is further facilitated by EC junction opening through disruption of intercellular adhesions or induction of EC apoptosis [146]. Ultimately, CTCs squeeze between ECs, which requires dynamic changes in cell shape mediated by Rho/ROCK-regulated actin contractility [51] (Fig. 4).
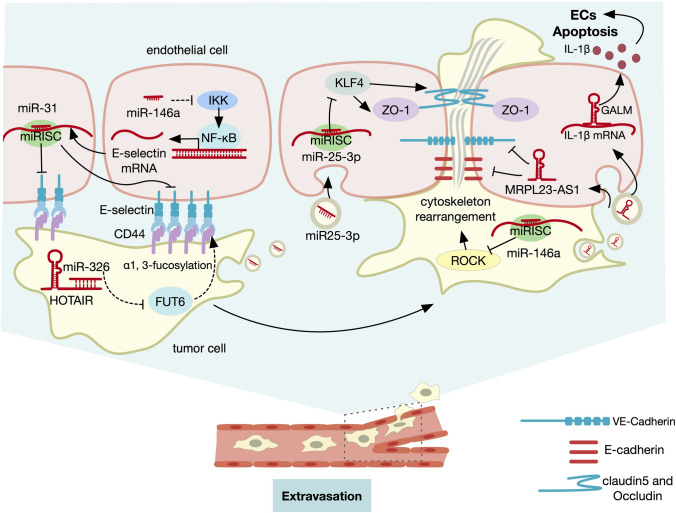
Fig. 4.
Role of ncRNA in extravasation. ncRNAs are involved in the process of extravasation, as shown above, specifically including endothelium permeability, tumor cells adhesion to ECs, and tumor cells motility
Emerging evidence has suggested that ncRNAs are involved in the process of extravasation through modulation the adhesion of tumor cells to the ECs. As mentioned above, E-Selectin, an adhesion receptor that is expressed by vascular endothelial cells and mediates leukocytes and cancer cells rolling on the endothelium, has been widely investigated in cancer metastasis [145, 147]. In CRC, metastatic potential of cancer cells was alleviated by miR-146a and miR181b, which downregulated E-selectin in ECs by regulation of NF-κB pathway [148]. LncRNA HOTAIR has been reported to regulate CRC liver metastasis through an E-Selectin-associated mechanism. HOTAIR functioned as a decoy for miR-326 and positively regulated Fucosyltransferase 6 (FUT6) expression to promote fucosylation modification of CD44, which was required as an E-selectin ligand during CRC spreading [149]. Accordingly, it can be inferred that HOTAIR may facilitate metastasis by favoring the attachment of cancer cells to the ECs at the target organ.
miRNAs can also exert their effect on EC junction opening. A recent study revealed a mechanism regarding how CRC-secreted exosomal miR-25-3p disrupted the endothelium integrity at metastasis in the liver and lung in vivo. In the study, miR-25-3p directly targeted and inhibited the expression of Kruppel-Like Factor 4(KLF4), a transcription factor that maintains vascular integrity. Decreased KLF4 then reduced the expression of tight junction proteins ZO-1, occludin, and Caludin5 of lung and liver endothelial cells, thus favoring the extravasation of CTCs [150]. In addition, miR-181c was reported to be conducive to the breakdown of BBB. miR-181c was secreted by brain metastatic cancer cells encapsulated in extracellular vesicles and taken by brain endothelial cells where it directly targeted PDPK1, which resulted in abnormal actin dynamics and subsequent BBB disruption [151].
lncRNAs are also important determinants of vascular integrity. In lung metastasis from adenoid cystic carcinoma, lncRNA MRPL23-AS1 promoted metastasis via enhancing permeability of pulmonary endothelium. Mechanistically, exosomal MRPL23-AS1 derived from primary tumor induced transcriptional silencing of E-cadherin through binding of EZH2 to enhance the H3k27me3 of the E-cadherin promoter region. The novel RNA–protein complex augmented the expression of VEGF in lung ECs, resulting in the breaching of EC intercellular adhesions [152]. As another model of liver metastasis from gallbladder cancer, lncGALM released by cancer cells facilitated extravasation by promoting the leakiness of endothelial barrier. Regarding the molecular mechanism, lncGALM can directly bind to IL-1β mRNAs to increase their stability, thus favoring the apoptosis of liver sinusoidal endothelial cells [153]. Furthermore, a study revealed that the exosomes containing lncRNA GS1-600G8.5, which secreted by brain metastatic breast cancer cells, were internalized by brain microvascular endothelial cells, consequently leading to increased permeability of BBB and infiltration of cancer cells [154]. Several other lncRNAs have also emerged as key molecules to facilitate BBB disruption, such as LOC102640519 via the induction of LOC102640519/HOXC13/ZO-1 axis [155] and Snhg3 via the regulation of TWEAK/Fn14/STAT3 pathway [156].
The exact roles of circRNAs and piRNAs in extravasation have yet to be observed. However, based on current evidence, their involvement in this process can be proposed. Recent studies provided some evidence that circRNAs contribute to vascular permeability regulation in different disease models. For example, circ-USP1 could regulate vascular integrity in glioma endothelial cells. Mechanistically, circ-USP1 acted as a decoy for miR-194-5p and suppressed its activity to alleviate its inhibitory effect on transcription factor FLI1. As a result, FLI1 promoted the expression of tight junction proteins including claudin-5, occludin, and ZO-1 to enhance endothelium function [157]. Furthermore, circHIPK3 was reported to regulate retinal vascular leakage through sponging miR-30a-3p, thereby elevating the expression of vascular endothelial growth factor-C(VEGFC), frizzled class receptor 4(FZD4), and Wnt family member 2 (WNT2) [158]. In addition, circDLGAP4 [159] and circHECW2 [160] were reported to affect BBB integrity by regulating tight junction protein and mesenchymal marker expression, though their exact roles in extravasation to form brain metastasis have not been explored yet. Notably, no piRNA has been reported to influence extravasation directly; however, it can regulate the maintenance of blood–retinal barrier (BRB) in human retinal pigment epithelium through reorganization of tight junctions [161], indicating its potential role on the modulation of endothelium integrity and extravasation.
Intriguingly, miRNAs are also likely to modulate extravasation by dynamically altering cytoskeleton-mediated cellular motility. In melanoma, miR-146a augmented primary tumor and lung metastasis growth, whereas impaired CTCs extravasation owing to miR-146a-dependent inhibition of ITGAV and ROCK1. Consistently, miR-146a was downregulated in CTCs compared with primary tumor and distant lung colonies. Hence, it was appealing to speculate that miR-146a was implicated in the dynamic change of cell states during metastasis, where CTCs lowered the level of miR-146a to favor cell extravasation through ROCK1 activation and subsequently restored miR-146a expression to enhance subsequent colonization [162]. Furthermore, miRNAs have been investigated for their involvement in extravasation in vivo. For example, several models using cell tracking technique observed different miRNAs including miR-214, miR-143, and miR-145 were involved in the extravasation of CTCs [163–165].
ncRNAs-mediated colonization
Colonization, the process of extravasated carcinoma cells surviving and proliferating at foreign sites, represents one of the most inefficient steps in the metastatic cascade [3]. First, in order to survive at distant microenvironments, primary tumors release systemic signals to convert incompatible sites into more hospitable ones, known as the establishment of a premetastatic niche [166]. Next, upon reaching the distant sites, the majority of carcinoma cells suffer high rates of attrition (exceeds 99%), while a small minority persist as microcolonies for a long period of state called dormancy [97, 167]. Subsequently, to develop into macroscopic metastases, the founding cells depend on two attributes: adaptability to specific foreign microenvironments and capacity for self-renewal [1]. The first one can be explained by the “seed and soil” hypothesis proposed by Stephen Paget more than 100 years ago, stating that metastases develop only when “seeds (cancer cells) fall on congenial soil (organ microenvironment)” [168]. This dictates complex mechanisms to modify ectopic microenvironments, such as secretory signals or mobilization and recruitment of bone marrow-derived cells [123, 169]. The second one can be conferred by a program called mesenchymal–epithelial transition (MET) [170]. As discussed above, during the early course of the metastatic cascade, EMT is activated to enhance local invasion. It is now recognized that primary cancer cells that have undergone EMT are in a state of low proliferation, therefore, at distant sites disseminated cancer cells are proposed to go through a phenotypic reversion, that is, MET to reinitiate outgrowth [171] (Fig. 5).
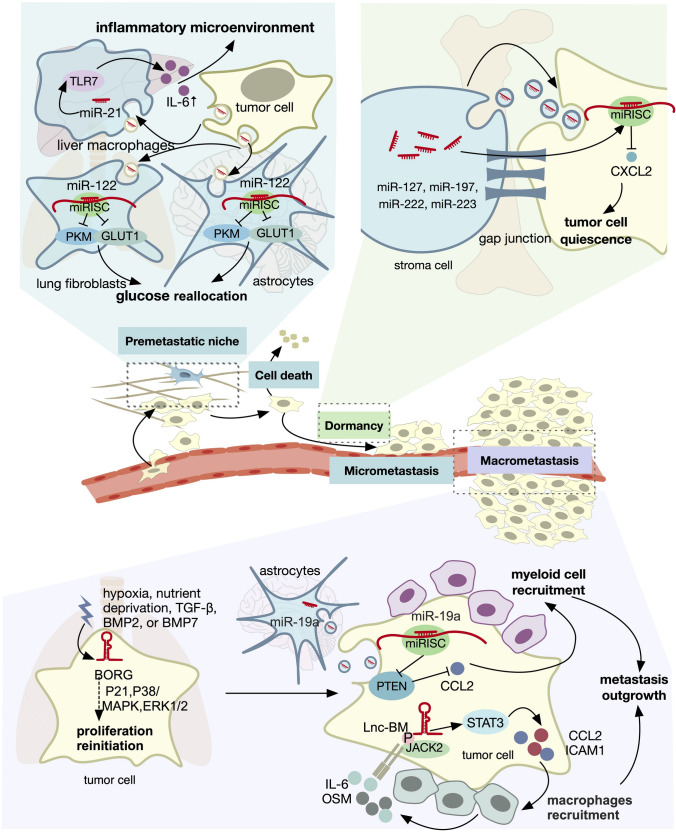
Fig. 5.
Role of ncRNA in colonization. Dysregulated ncRNAs promote colonization in different organs through distinct mechanisms. The different stages of colonization include a establishment of a premetastatic niche, b dormancy, and c metastasis outgrowth
In the phase of premetastatic, miRNAs remodel the foreign microenvironment to strengthen the settlement of disseminated cancer cells in several manners. For instance, miR-122 reprogrammed the metabolism of premetastatic niche cells to achieve this end. More specifically, miR-122 derived from breast cancer cells directly targeted mRNAs of pyruvate kinase (PKM), citrate synthase (CS), and glucose transporter 1(GLUT1), thus interfering with glucose metabolism and result in glucose reallocation in niche cells in brain and lung [172]. In addition, miR-21 established a proinflammatory premetastatic niche to facilitate CRC cells seeding to the liver via binding to toll-like receptor 7(TLR7) and enhance IL-6 production [173]. Moreover, a recent study uncovered that increased vascular permeability in the lung premetastatic niche required the downregulation of miR-30 family [174].
So far there is no solid evidence regarding the exact mechanism of how lncRNAs function in pre-metastatic niche establishment. However, a number of dysregulated lncRNAs have been identified to be involved in pulmonary pre-metastatic niche formation of breast cancer by high-throughput sequencing. Functional enrichment analysis revealed that these lncRNAs were correlated with the TGF-β signaling pathway, pentose phosphate pathway, Hedgehog signaling pathway, and so on, while the molecular mechanisms underlying this process awaited further study [175]. Moreover, a recent study uncovered that ovarian cancer-derived lncRNA SPOCD1-AS induced the establishment of peritoneal pre-metastatic niche. The proposed mechanisms of this included mesothelial-to-mesenchymal transition of mesothelial cells and enhanced adhesion of cancer cells to mesothelial cells, and the above processes were achieved through interaction between lncRNA SPOCD1-AS and G3BP stress granule assembly factor 1(G3BP1) [176].
The contribution of circRNAs and piRNAs to colonization has been largely elusive. Using paired-end RNA sequencing, it was revealed that the primary and metastatic sites of ovarian cancer exhibited different circRNAs expression pattern [177], indicating possible roles of circRNAs on adaptability to the foreign microenvironment. Furthermore, in the tail vein injection mouse models of metastatic CRC, circPTK2 was shown to promote the formation of lung metastasis, suggesting its potential in colonization. The underlying molecular mechanisms involve the interaction of circPTK2 and vimentin [178].
For the subsequent dormant state, the roles of ncRNAs have been widely investigated. During the formation of bone marrow metastasis of breast cancer, a number of miRNAs, including miR-127, -197, -222, and -223 in stromal cells were transferred to breast cancer cells via gap junctions to inhibit the expression of C–X–C motif chemokine ligand 12(CXCL12), therefore, inducing cell cycle arrest in breast cancer cells [179]. Recently, a novel regulatory mechanism of dormancy in pulmonary metastasis of breast cancer was posited. It is widely recognized that breast cancer cells could spread from the primary site at very early stages of tumorigenesis and remain in quiescence for long periods [180]. lncRNA BORG was identified as a metastasis-promoting gene whose upregulation can reinitiate proliferation in dormant disseminated breast cancer cells. It was shown that in dormant metastatic tissues, the level of BORG expression was low; however, when faced with environmental stresses and signals (e.g., hypoxia, nutrient deprivation, TGF-β, BMP2, or BMP7), BORG was induced to reactivate these quiescent cells into proliferative states presumably through effectors including P21, P38/MAPK, ERK1/2, and others. Thus, this state plasticity of dormant disseminated tumor cells highlighted that targeting BORG would be a potential therapy for breast cancer lung metastasis [181]. A large number of cirRNAs and piRNAs had been reported to mediate cell cycle arrest [142, 182–185], hence they could be involved in the mechanisms of microcolonies and the state of long-term dormancy.
Ultimately, to foster macrometastatic growth, miRNAs orchestrate distinct adaptive programs, including bidirectional intercellular crosstalk between cancer cells and microenvironment. On the one hand, normal fibroblasts of distant organ sites are converted into cancer-associated fibroblasts (CAFs) upon the infiltration of metastatic cells. This process was demonstrated to be regulated by miR-1247-3p when HCC colonized the lung. Exosomal miR-1247-3p shed by HCC cells directly targeted and inhibited the expression of B4GALT3 in fibroblasts, resulting in fibroblasts activation via B4GALT3-β1-integrin-NF-κB axis. In turn, transformed CAFs further favored metastasis outgrowth by increased secretion of IL-6 and IL-8 [186]. On the other hand, miR-19a derived from astrocytes could reshape the metastatic cancer cells, leading to adaptive PTEN loss and resultant C–C motif chemokine ligand 2(CCL2)-dependent myeloid cell recruitment that promoted brain metastasis outgrowth [187]. Recently, in a model of mutant p53 breast cancer, miR-30d was also demonstrated to mediate CAFs recruitment in the lung metastasis [188].
Likewise, lncRNAs are implicated in the crosstalk between disseminated tumor cells and secondary microenvironment to establish overt metastasis. In breast cancer brain metastasis, lnc-BM augmented colonization by forming a positive-feedback loop between breast cancer cells and brain microenvironment. Mechanistic investigation revealed that increased lnc-BM associated with JAK2 and induced its kinase activity, thus leading to STAT3 phosphorylation. Active STAT3 promoted macrophages recruitment via upregulation of CCL2. In turn, attracted macrophages secreted oncostatin M (OSM) and IL-6 to further reinforce the JAK2/STAT3 pathway in metastatic breast cancer cells [189]. Conversely, in another model of breast cancer brain metastasis, lnc-XIST exhibited a negative role in metastasis formation by inhibition of miRNA-503-mediated M1–M2 polarization of microglia. This reprograming of microglia increased the production of immune-suppressive cytokines and inactivated T-cells, thereby facilitating the development of brain metastasis [190].
Besides, ncRNAs also serve as signals to initiate the MET program. A recent study indicated that the aforementioned negative feedback loops formed by pairs of miRNAs and EMT-TFs during EMT are also implicated in MET [191].In addition, several lncRNAs were reported to modulate MET program, such as CILA1 via modulation of the Wnt/β-catenin signaling pathway [192] and H19 via regulation of Twist expression [193]. Besides, circ-0005585 was suggested to promote MET program in ovarian cancer cells in a recent study [194]. Currently, the roles of piRNAs on MET remain largely unexplored, although as discussed above, they have been implicated in the EMT program in the context of local invasion.
Clinical applications of ncRNAs
ncRNAs as biomarkers for cancer metastasis
Given that the expression patterns of ncRNAs differ substantially across diverse cancers, and differential expression signatures are linked to distinct cancer states, it raises the possibility that ncRNAs can be appealing diagnostic and prognostic biomarkers [195]. Indeed, a plethora of published studies have provided evidence that ncRNAs can serve as novel tools for predicting cancer metastasis. In breast cancer, the primary lesion with low levels of miR-335 and miR-126 had a higher tendency to form metastasis [196], while patients with prostate cancer were more prone to develop metastasis when the expression of lncRNA PCAT-14 was low in the primary tumor [197]. In laryngeal cancer, higher hsa_circRNA_100855 level in the primary tumor was correlated with metastasis or advanced clinical stage [198]. Similarly, piR-1245, a novel oncogenic mediator, was significantly increased in patients with advanced and metastatic CRC [48].
Notably, ncRNAs including miRNAs, lncRNAs, circRNAs piwiRNAs [195] can all be detected in body fluids, including blood [199], urine [200], saliva [201] and sputum [202] with high abundance and stability. In general, in the extracellular space, ncRNAs are released from cells and encapsulated inside extracellular vesicles (mainly exosomes) to avoid digestion by the nuclease [195]. Alternatively, ncRNAs interact and form complexes with protein Argonaute 2 [203] or high-density lipoproteins [204]. Comprehensive analyses of exosomal ncRNAs have confirmed their abundancy in exosomes and uncovered distinct expression profiles of miRNAs, piwiRNAs and circRNAs between healthy individuals and cancer patients, making them potential tools to discriminate between healthy people and patients, and even define cancer states [205, 206]. Moreover, the new diagnostic tools are much less invasive than conventional biopsies. A growing body of evidence has demonstrated the utility of circulating ncRNAs to predict metastasis. CRC patients with high levels of serum miR-203 had a greater probability of liver or systemic metastasis [207]. The well-characterized pro-metastasis lncRNAs such as H19 [208, 209], MALAT1 [210, 211] and HOTAIR [33, 212] were present at high levels in the circulation across a variety of metastasis cancers. Plasmas level of circ-KIAA1244 was found to be downregulated in gastric cancer patients with lymphatic metastasis [213].
The piles of evidence have proved ncRNAs as potent biomarkers in preclinical studies and some ncRNAs indeed have entered into clinical trials. PCA3, a prostate-specific marker which can be detected in urine non-invasively, was the first ncRNA to be approved by the FDA for cancer diagnosis [214]. At present, there are hundreds of ongoing clinical trials evaluating ncRNA-associated biomarkers in multiple metastatic carcinoma types, including detection for dysregulated miRNAs as biomarkers for bone metastases (NCT03895216), analysis of circulating exosomal RNAs in the lung metastases of osteosarcoma (NCT03108677), and assessment of miRNAs of metastatic breast cancer patients (NCT02338167), and so forth.
ncRNAs as therapeutic targets for cancer metastasis
ncRNAs play crucial roles in cancer progression as either oncogenes or tumor suppressors; therefore, the development of therapeutic interventions by targeting ncRNAs has represented a burgeoning field of cancer research. However, approaches to modulate ncRNAs expression are far different from traditional agents. The development of RNA-targeting techniques has opened up new strategies to manipulate ncRNAs in vivo. For miRNA, antisense RNAs (antimiRs) are used to exert inhibitory functions, while double-stranded miRNA mimics are employed to replenish their expressions [215, 216]. AntimiRs are single-stranded RNAs that are complementary to the target miRNA. Mechanistically, antimiRs bind to the target miRNA strand and inactivate its function. miRNA mimics are a synthetic duplex form of oligoribonucleotides that aim to re-introduce the functions of the lost tumor suppressor miRNA counterparts [215]. For lncRNA, antisense oligonucleotides (ASOs) and small interfering RNAs are utilized to modulate its expression. In brief, small interfering RNAs are synthetic double-stranded RNAs that are incorporated into RISC and interact with target lncRNA to direct its cleavage. ASOs, conversely, are synthetic short DNAs that hybridize to the target lncRNA and create a DNA-RNA duplex to be digested by RNase H [216]. Currently, there are two major issues in the field of ncRNA-based therapeutics: the stability and affinity of drug molecules and the establishment of delivery vehicles to the target sites [215]. For the former, to optimize the pharmacokinetic properties of therapeutic RNAs, chemical modifications are required before their delivery, such as 2’ -O-methyl (2’-O-Me) and locked nucleic acid (LNA) modifications [217–219]. For example, in diffuse large B-cell lymphoma (DLBCL), cobomarsen, an LNA-based anti-miR-155 compound, has shown potential therapeutic efficacy in vitro and in vivo, as well as in a patient [220]. For the latter, oligonucleotides need delivery systems to improve cellular uptake and reduce systemic toxicity, among which lipid-based nanocarriers are widely applied [219]. Meanwhile, novel delivery approaches are being developed. For instance, in non-small cell lung cancer (NSCLC), hydrophobically modified let-7b miRNAs (hmiRNAs) which could enable efficient delivery without the need for lipid formulation has been demonstrated as an effective strategy in pre-clinical mouse models [221].
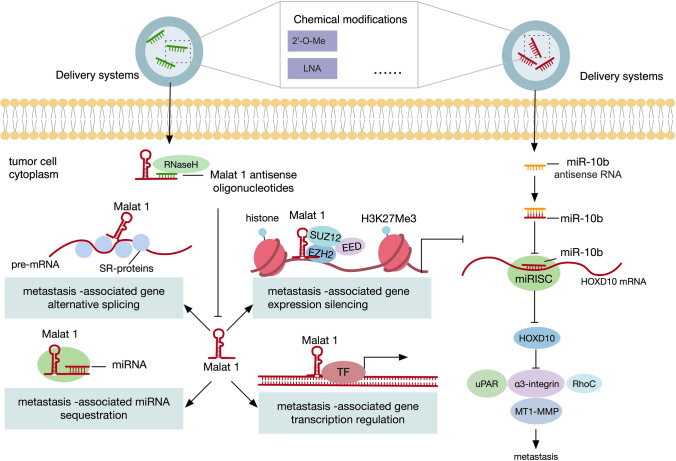
As most cases of metastasis cancer are unresectable at the time of diagnosis, the development of drugs that target ncRNAs provides promising therapeutic strategies. Pre-clinical studies manipulating a number of ncRNAs dysregulated during metastasis have been shown to exert prominent therapeutic effects (Fig. 6). Take miR-10b, the first recognized metastasis-associated miRNA for example. The addition of the nanodrug that contained miR-10b inhibitors significantly lowered the dose of doxorubicin, eliciting complete and persistent regression of metastatic lesions in mouse models of breast cancer [222]. Another well-studied pro-metastasis lncRNA MALAT1 has also been employed therapeutically. ASO-mediated silencing of lncRNA MALAT1 could greatly slow down tumor growth and impair metastasis formation in mouse models of lung and breast cancer [210, 223]. Of note, various miRNA-based therapeutics have entered clinical trials, with some of them have been under clinical development [224]. For instance, MRX34, a liposomal miR-34a mimic, had shown potent anticancer effectiveness in several preclinical studies, including mouse models of lung [225], liver [226], pancreatic [227], and prostate cancer [24]. Subsequently, MRX34 was approved for human trials in patients with advanced solid tumors, which was the first clinical trial for miRNA drug in history. However, whereas 4% overall response rate was achieved, recently the trial was terminated due to severe immune-related adverse events that led to four deaths. Although halted early, this study bought up the concern about toxic effects that come with miRNA drugs, which should catch the attention of drug development in the future [228].
Fig. 6.
ncRNAs as therapeutic targets for cancer metastasis. In mouse models of breast cancer, MALAT1 antisense oligonucleotides hybridize to MALAT1 and create a DNA–RNA duplex to be digested by RNase H, leading to impaired metastasis formation. Meanwhile, the addition of the nanodrug that contained single-stranded miR-10b antisense RNAs that are complementary to miR-10b counteracts the metastasis-promoting effect of miR-10b. Mechanistically, miR-10b antisense RNA directly binds to miR-10b and inactivate its function
Context-dependent roles of ncRNAs in metastasis
Various ncRNAs have been found to exhibit both tumor suppressive and oncogenic functions. A notable example is Malat1, which was originally identified as a predictor for metastasis in early stage non-small cell lung cancer [14]. Consistently, subsequent studies have reported the upregulation of Malat1 and its association with poor outcomes in a large number of metastatic cancer types [229]. Experimental studies also demonstrated metastasis-suppressive effect of Malat1 inactivation both in vitro and in vivo and the underlying mechanisms were proposed as the interaction of Malat1 with EZH2, SUZ12, and a number of miRNAs [230]. However, several recent studies have revealed a metastasis-suppressive role for Malat1 in CRC and breast cancer [76, 231, 232]. In contrast to previous studies, analysis of several large-scale data sets showed that Malat1 expression was significantly lower in human CRC and breast cancer, and particularly a reduction in metastatic than primary breast cancer tissues was observed [76, 231]. A positive correlation between MALAT1 expression and patient survival was also reported [76, 231]. Of note, using both transgenic and xenograft mouse models, knockout of Malat1 promoted lung metastasis of breast cancer and this phenotype could be reversed by re-expression of Malat1 [76]. Nevertheless, there were numerous methodological differences between these studies that may account for the divergent results [233]. The major difference appears to arise from the different strategies used for Malat1 gene deletion [76]. Indeed, manipulation of certain regions of Malat1-encoding gene could lead to significant upregulation of neighboring genes involving Neat1, Frmd8, Tigd3, and so on [234]. Therefore, it is unknown whether the phenotypes were specifically due to the loss of Malat1 lncRNA per se or the confounding effect of other gene expressions. In addition, it is also essential to validate the specific biological function of Malat1 through the performance of rescue experiments [76]. Furthermore, when it comes to clinical data, stratification of patient subgroups (e.g., TNM staging or molecular subtypes) and the source of the samples being analyzed (e.g., bulk sequencing that is affected by tumor purity and intratumor heterogeneity or single-cell sequencing) have emerged as potentially important considerations [233].
Similarly, multifaceted roles of circRNAs in distinct phases of cancer metastasis have been reported. A prime example of this is circHIPK3. While in CRC and cervical cancer, circHIPK3 displayed a pro-metastatic phenotype presumably through enhancing EMT [235], altering cell–ECM interactions [236], and generation of protruding actin structures [237] of cancer cells in the stage of local invasion, it suppressed bladder cancer metastasis via inhibition of angiogenesis [238]. Taken together, the context-depend effect of circHIPK3 may be largely determined by tumor types and/or downstream effector molecules. As ncRNAs are emerging as key therapeutic targets for many cancer types, it is of vital importance to consolidate the contradicting findings and comprehensively understand the underlying causes of the discrepancy in the role of ncRNAs in cancer metastasis.
Perspectives and conclusions
In the past decade, ncRNAs have been under vivid investigations in diverse physiological and pathological processes. Extensive studies support a prominent role of ncRNAs in orchestrating every step of the metastatic cascade (Table 1). Moreover, the expression profiling and identification of candidate ncRNAs in cancer metastasis have been dramatically promoted owing to the development of high-throughput sequencing technology. Although mounting evidence has revealed that ncRNAs are involved in the regulation of metastasis, their roles underlying specific steps of metastasis cascade warrant further investigation. Of note, it is important to realize that ncRNAs function pleiotropically in distinct steps. This notion is supported by many cases. miR-31 has been reported to exert its impact simultaneously on local invasion, intravasation, and colonization, and what’s more, even served as both positive and negative roles [74, 239]. Another prime example is the double negative feedback loops formed by pairs of miRNAs and EMT-TFs, where miRNAs hamper the EMT program at the early stage of metastasis cascade to inhibit local invasion, conversely favor the MET program at the late stage to promote colonization. This multifaceted nature of ncRNAs comes with many caveats. For example, this highlights the difficulty to target ncRNAs therapeutically because of the contradictory roles that ncRNAs play across distinct steps in metastatic cascade, as abrogate one process might lead to activation of another. Therefore, future work will be required to address the questions of the mechanisms by which ncRNAs regulate metastasis under more specific circumstances [240].
Table 1.
Some dysregulated ncRNAs and their putative roles in the metastasis cascade
| ncRNA | Targets | Cancer type | Regulation | Ref |
|---|---|---|---|---|
| Local invasion | ||||
| EMT | ||||
| miR-200 | ZEB | Multiple cancer types | Down | [55] |
| miR-34a | Snail | Multiple cancer types | Down | [56] |
| miR‑1 | SNAIL2 | Multiple cancer types | Down | [57] |
| miR‑203 | SNAIL1 | Multiple cancer types | Down | [58] |
| miR-4521 | AKT/GSK3β/Snai1 pathway | Gastric cancer | Down | [62] |
| ZEB1 AS1 | ZEB1 | Hepatocellular carcinoma | Up | [63] |
| MEG3 | E-cadherin and microRNA-200 family | Lung cancer | Up | [64] |
| JPX | miR-33a-5p /Twist1 axis | Lung cancer | Up | [65] |
| circANKS1B | TGF-β1/Smad signaling | Breast cancer | Up | [67] |
| circNEIL3 | miR-432-5p | Pancreatic cancer | Up | [68] |
| circCORO1C | let-7c-5p/PBX3 axis | Laryngeal squamous cell carcinoma | Up | [69] |
| circNR3C2 | miR-513a-3p | Breast cancer | Down | [70] |
| piR-1037 | – | Oral squamous cell carcinoma | Up | [71] |
| piR-932 | – | Breast cancer | Up | [72] |
| Cell adhesion | ||||
| miR-31 | Wnt/β-catenin signaling(integrins) | Breast cancer | Up | [74] |
| miR-221/222 | β4 integrin | Breast cancer | Down | [75] |
| MALAT1 | TEAD | Breast cancer | Down | [76] |
| H19 | β3 and β4 integrins | Prostate cancer | Up | [77] |
| CASC9 | LAMC2 | Esophageal squamous cell carcinoma | Up | [78] |
| HOXD-AS1 | HOXD3 | Colorectal cancer | Down | [79] |
| circSKA3 | β1 integrin | Breast cancer | Up | [96] |
| ECM degradation | ||||
| miR-181b | TIMP3 | Colorectal cancer | Up | [81] |
| ODRUL | miR-3182/MMP2 Axis | Osteosarcoma | Up | [82] |
| MATN1-AS1 | RELA and MAPK signaling | Glioblastoma | Down | [83] |
| PRECSIT | STAT3 Signaling | Cutaneous Squamous Cell Carcinoma | Up | [84] |
| LSAMP-AS1 | miR-183-5p and decorin | Prostate cancer | Down | [85] |
| CASC2 | MMP-2 | Gastric cancer | Down | [86] |
| Circ 0,000,096 | MMP-2 and MMP-9 | Gastric cancer | Up | [87] |
| Circ0001361 | miR-491-5p/MMP-9 Axis | Bladder cancer | Up | [88] |
| CircCDR1as | Associated with matrix organization, integrin binding, and collagen binding | Pan-cancer | Up | [80] |
| piR-39980 | MMP-2 | Osteosarcoma | Up | [89] |
| Cell motility | ||||
| miR-124 | ROCK2 | Hepatocellular carcinoma | Down | [90] |
| miR-138 | RhoC | Cholangiocarcinoma | Down | [91] |
| miR- BART2-5p | RND3 | Nasopharyngeal carcinoma | Up | [92] |
| miR-200b | ARHGAP18 | Breast cancer | Down | [93] |
| Dreh | Vimentin | Hepatocellular carcinoma | Down | [94] |
| MER52A | p120-ctn/Rac1/Cdc42 axis | Hepatocellular carcinoma | Up | [95] |
| Intravasation | ||||
| miR10b | – | Breast cancer | Down | [119] |
| lncRNA-ATB | – | Hepatocellular carcinoma | Up | [120] |
| Vascular permeability | ||||
| miR-23a | ZO-1 | Lung cancer | Up | [101] |
| miR-103 | VE-Cadherin, p120-catenin and ZO-1 | Hepatocellular carcinoma | Up | [102] |
| miR-629-5p | CELSR1 | Lung cancer | Up | [103] |
| circ-IARS | miR-122/RhoA/ZO-1 axis | Pancreatic cancer | Up | [105] |
| circ-CCAC1 | EZH2/ SH3GL2/ ZO-1 Occludin axis | Cholangiocarcinoma | Up | [106] |
| Angiogenesis | ||||
| miR-205 | PTEN-AKT pathway | Ovarian cancer | Up | [107] |
| miR-125b | angiopoietin 2 | Hepatocellular carcinoma | Down | [110] |
| miR-100 | MTOR-p70S6K signalling pathway | Hepatocellular carcinoma | Down | [110] |
| lncRNA-p21 | VEGFA | Lung cancer | Up | [111] |
| RAB11B-AS1 | VEGFA and ANGPTL4 | Breast cancer | Up | [112] |
| lncRNA-APC1 | MAPK pathway | Colorectal cancer | Down | [113] |
| CircPRRC2A | miR-514a-5p, miR-6776-5p and TRPM3 | Renal cell carcinoma | Up | [114] |
| CircMYLK | miR-29a and VEGFA/VEGFR2 | Bladder cancer | Up | [116] |
| circRNA-100338 | VE-cadherin and ZO-1 | Hepatocellular carcinoma | Up | [118] |
| circSHKBP1 | miR-582-3p/HUR/VEGF axis | Gastric cancer | Up | [115] |
| Survival in the circulation | ||||
| Immune evasion | ||||
| miR-296-3p | ICAM-1 | Prostate cancer | Up | [128] |
| miR-20a | MICA and MICB | Breast cancer | Up | [129] |
| Anoikis resista-nce | ||||
| miR-141 | KLF12/Sp1/survivin axis | Ovarian cancer | Up | [135] |
| MALAT1 | RBFOX2/KIF1B axis | Ovarian cancer | Up | [136] |
| SChLAP1 | – | Prostate cancer | Up | [143] |
| Extravasation | ||||
| miR-214 | – | Breast cancer, Pancreatic cancer, Melanoma | Up | [163, 164] |
| Endothelium permeability | ||||
| miR-143 | CREB1 and MEKK2 | Breast cancer | Up | [165] |
| miR-25-3p | KLF4/ZO-1, occludin, and Caludin5 axis | Colorectal cancer | Up | [150] |
| miR-181c | PDPK1 | Breast cancer | Up | [151] |
| MRPL23-AS1 |
E-cadherin VEGF |
Adenoid cystic carcinoma | Up | [152] |
| GALM | IL-1β | Gallbladder cancer | Up | [153] |
| GS1-600G8.5 | Associated with BBB permeability | Breast cancer | Up | [154] |
| Tumor cells adhesion to ECs | ||||
| miR-146a and miR181b | NF-κB/E-selectin axis | Colorectal cancer | Down | [148] |
| Tumor cells motility | ||||
| miR-146a | ITGAV and ROCK1 | Melanoma | Down | [162] |
| Colonization | ||||
| Establishment of a premetasta-tic niche | ||||
| miR-122 | glucose metabolism enzymes | Breast cancer | Up | [172] |
| miR-21 | TLR7-IL-6 axis | Colorectal cancer | Up | [173] |
| miR-30 | Skp2 | Melanoma | Down | [174] |
| TUC339, UCA1, etc. | Associated with pre-metastatic niche formation | Breast cancer | Up or Down | [175] |
| SPOCD1-AS | G3BP1 | Ovarian cancer | Up | [176] |
| Dormancy | ||||
| miR-127, -197, -222, and -223 | CXCL12 | Breast cancer | Up | [179] |
| BORG | P21, P38/MAPK, ERK1/2, etc. | Breast cancer | Up | [181] |
| Metastasis outgrowth | ||||
| miR-1247-3p | B4GALT3-β1-integrin-NF-κB axis | Hepatocellular carcinoma | Up | [186] |
| miR-19a | PTEN | Breast cancer | Up | [187] |
| miR-30d | Golgi apparatus | Breast cancer | Up | [188] |
| lnc-BM | JAK2/STAT3 pathway | Breast cancer | Up | [189] |
| lnc-XIST | miRNA-503 | Breast cancer | Down | [190] |
| circPTK2 | vimentin | Colorectal cancer | Up | [178] |
The diagnostic and therapeutic applications of ncRNAs are evolving at a rapid pace, and it is expected that those ncRNAs-based biomarkers and drugs will be put into clinical use, with FDA-approved lncRNA PCA3 being a good precedent. One of the major issues for ncRNA biomarkers when they are translated from bench into clinical rests on the low specificity among different types of patients. To address this challenge, combining various candidate ncRNA biomarkers as an integrated tool has been shown to enhance the diagnostic value. In the future, prospective trials with larger patient cohorts are needed to confirm the utility of ncRNA biomarkers in terms of specificity and sensitivity [195]. Concerning ncRNA-based therapy, in addition to those noted above, novel targeting approaches to manipulate ncRNAs expression in cancer cells show potential for improved specificity and reduced off-target effects, such as splice-switching oligonucleotides, steric blocking oligonucleotides, and genome editing tool CRISPR/Cas9 [216]. At the same time, chemical modifications to maintain the in vivo stability and bioactivity should be optimized, cancer-cell-specific delivery systems are required to be developed, to further improve the safety and effectiveness of ncRNA-based therapy strategy.
Abbreviations
- ncRNAs
Noncoding RNAs
- EMT
Epithelial–mesenchymal transition
- ECM
Extracellular matrix
- miRNAs
MicroRNAs
- piRNAs
PIWI-Interacting RNAs
- lncRNAs
Long noncoding RNAs
- MALAT1
Metastasis-associated lung adenocarcinoma transcript 1
- RISC
RNA-induced silencing complex
- 3ʹUTR
3ʹ Untranslated region
- Gas5
Growth Arrest Specific 5
- HOTAIR
HOX Transcript Antisense RNA
- PRC2
Polycomb repressive complex 2
- circRNA
Circular RNA
- snoRNA
Small nucleolar RNA
- tsRNAs
TRNA-derived small RNAs
- MMPs
Matrix metalloproteinases
- EMT-TFs
EMT-related transcription factors
- ZEB1-AS1
ZEB1 antisense 1
- MEG3
Maternally Expressed 3
- HCC
Hepatocellular carcinoma
- JPX
JPX Transcript, XIST Activator
- TEAD
TEA Domain Transcription Factor
- CASC9
Cancer Susceptibility 9
- MATN1-AS1
MATN1 Antisense RNA 1
- PRECSIT
P53 Regulated Carcinoma-Associated Stat3 Activating Long Intergenic Non-Protein Coding Transcript
- LSAMP-AS1
LSAMP Antisense RNA 1
- CASC2
Cancer Susceptibility 2
- SERPINB1
Serpin Family B Member 1
- ECs
Endothelial cells
- VEGFA
Vascular Endothelial Growth Factor A
- VEGF
Vascular Endothelial Growth Factor A
- ANGPTL4
Angiopoietin-Like 4
- PDGFB
Platelet-Derived Growth Factor Subunit B
- FGF2
Fibroblast Growth Factor 2
- ZO-1
Zonula occludens protein-1
- CELSR1
Cadherin EGF LAG seven-pass G-type receptor 1
- HUVECs
Human umbilical vein endothelial cells
- FOXO1
Forkhead Box O1
- VETC
Encapsulated tumor cluster
- Angpt2
Angiopoietin 2
- RAB11B-AS1
RAB11B Antisense RNA 1
- APC
Adenomatous polyposis coli
- CRC
Colorectal cancer
- TRPM3
Transient Receptor Potential Cation Channel Subfamily M Member 3
- CTCs
Circulating tumor cells
- ATB
lncRNA activated by TGF-β
- NK cells
Natural killer cells
- ROS
Reactive oxygen species
- PMPs
Platelet-derived microparticles
- ICAM-1
Intercellular adhesion molecule 1
- BCSC
Breast cancer stem-like cell
- MICA
MHC Class I Polypeptide-Related Sequence A
- MICB
MHC Class I Polypeptide-Related Sequence B
- DAP12
DNAX activating protein 12 kDa
- KLF12
Kruppel-Like Factor 12
- KIF1B
Kinesin Family Member 1B
- RBFOX2
RNA binding protein fox-1 homolog 2
- SChLAP1
SWI/SNF Complex Antagonist Associated With Prostate Cancer 1
- BBB
Blood–brain barrier
- FUT6
Fucosyltransferase 6
- KLF4
Kruppel-Like Factor 4
- MRPL23-AS1
MRPL23 Antisense RNA
- EZH2
Enhancer of Zeste 2 Polycomb Repressive Complex 2 Subunit
- VEGFC
Vascular endothelial growth factor-C
- FZD4
Frizzled class receptor 4
- WNT2
Wnt family member 2
- BRB
Blood–retinal barrier
- MET
Mesenchymal–epithelial transition
- PKM
Pyruvate kinase
- CS
Citrate synthase
- GLUT1
Glucose transporter 1
- TLR7
Toll-like receptor 7
- G3BP1
G3BP Stress Granule Assembly Factor 1
- CXCL12
C–X–C Motif Chemokine Ligand 12
- CAFs
Cancer-associated fibroblasts
- CCL2
C–C Motif Chemokine Ligand 2
- OSM
Oncostatin M
- XIST
X Inactive Specific Transcript
- PCAT-14
Prostate Cancer-Associated Transcript 14
- antimiRs
Antisense RNAs
- ASOs
Antisense oligonucleotides
- 2’-O-Me
2’ -O-methyl
- LNA
Locked nucleic acid
- PCA3
Prostate Cancer Associated 3
Authors’ contributions
ZGZ and XWW: designed the article, QLL and ZZ: performed the literature search and drafted the work, ZGZ and XWW: revised the work.
Funding
This work was supported by National Natural Science Foundation of China (81821002 and 82003113) and 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (2016105 and ZYGD20006).
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qiu-Luo Liu and Zhe Zhang have contributed equally to this work.
Contributor Information
Xiawei Wei, Email: xiaweiwei@scu.edu.cn.
Zong-Guang Zhou, Email: zhou767@163.com.
References
- 1.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 3.Obenauf AC, Massague J. Surviving at a distance: organ-specific metastasis. Trends Cancer. 2015;1(1):76–91. doi: 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson KA, Walsh D, Abdullah O, McDonnell F, Homsi J, Komurcu S, et al. Common complications of advanced cancer. Semin Oncol. 2000;27(1):34–44. [PubMed] [Google Scholar]
- 5.Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 2013;19(11):1450–1464. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- 6.Chen HN, Shu Y, Liao F, Liao X, Zhang H, Qin Y, et al. Genomic evolution and diverse models of systemic metastases in colorectal cancer. Gut. 2021 doi: 10.1136/gutjnl-2020-323703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nature Rev Cancer. 2018;18(2):128. doi: 10.1038/nrc.2017.118. [DOI] [PubMed] [Google Scholar]
- 8.Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18(1):5–18. doi: 10.1038/nrc.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beermann J, Piccoli M-T, Viereck J, Thum T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev. 2016;96(4):1297–1325. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 10.Wong C-M, Tsang FH-C, Ng IO-L. Non-coding RNAs in hepatocellular carcinoma: molecular functions and pathological implications. Nat Rev Gastroenterol Hepatol. 2018;15(3):137–151. doi: 10.1038/nrgastro.2017.169. [DOI] [PubMed] [Google Scholar]
- 11.Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106(23):9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 13.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10(5):593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 14.Ji P, Diederichs S, Wang W, Boing S, Metzger R, Schneider PM, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22(39):8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 15.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 16.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408(6808):86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 17.Cullen BR. Transcription and processing of human microRNA precursors. Mol Cell. 2004;16(6):861–865. doi: 10.1016/j.molcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432(7014):231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425(6956):415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 20.Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303(5654):95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 21.Diederichs S, Haber DA. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 2007;131(6):1097–1108. doi: 10.1016/j.cell.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 22.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 24.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17(2):211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang D, Sun Y, Hu L, Zheng H, Ji P, Pecot CV, et al. Integrated analyses identify a master microRNA regulatory network for the mesenchymal subtype in serous ovarian cancer. Cancer Cell. 2013;23(2):186–199. doi: 10.1016/j.ccr.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattick JS, Rinn JL. Discovery and annotation of long noncoding RNAs. Nat Struct Mol Biol. 2015;22(1):5–7. doi: 10.1038/nsmb.2942. [DOI] [PubMed] [Google Scholar]
- 28.Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316(5830):1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 29.Cruz JA, Westhof E. The dynamic landscapes of RNA architecture. Cell. 2009;136(4):604–609. doi: 10.1016/j.cell.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao X, Wang P, Liu J, Zheng J, Liu Y, Chen J, et al. Gas5 exerts tumor-suppressive functions in human glioma cells by targeting miR-222. Mol Ther. 2015;23(12):1899–1911. doi: 10.1038/mt.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3(107):8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 35.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE. 2012;7(2):e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao L, Zhang Y, et al. The Landscape of Circular RNA in Cancer. Cell. 2019;176(4):869–881. doi: 10.1016/j.cell.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Enuka Y, Lauriola M, Feldman ME, Sas-Chen A, Ulitsky I, Yarden Y. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 2016;44(3):1370–1383. doi: 10.1093/nar/gkv1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S, Huang V, Xu X, Livingstone J, Soares F, Jeon J, et al. Widespread and functional RNA circularization in localized prostate cancer. Cell. 2019;176(4):831–843. doi: 10.1016/j.cell.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 39.Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 40.Hsiao KY, Lin YC, Gupta SK, Chang N, Yen L, Sun HS, et al. Noncoding effects of circular RNA CCDC66 promote colon cancer growth and metastasis. Cancer Res. 2017;77(9):2339–2350. doi: 10.1158/0008-5472.CAN-16-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang F, Fang E, Mei H, Chen Y, Li H, Li D, et al. Cis-acting circ-CTNNB1 promotes beta-catenin signaling and cancer progression via DDX3-mediated transactivation of YY1. Cancer Res. 2019;79(3):557–571. doi: 10.1158/0008-5472.CAN-18-1559. [DOI] [PubMed] [Google Scholar]
- 42.Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, et al. Circ-ZNF609 is a circular rna that can be translated and functions in myogenesis. Mol Cell. 2017;66(1):22–37. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, et al. Translation of circRNAs. Mol Cell. 2017;66(1):9–21. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng X, Chen L, Zhou Y, Wang Q, Zheng Z, Xu B, et al. A novel protein encoded by a circular RNA circPPP1R12A promotes tumor pathogenesis and metastasis of colon cancer via Hippo-YAP signaling. Mol Cancer. 2019;18(1):47. doi: 10.1186/s12943-019-1010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siomi MC, Sato K, Pezic D, Aravin AA. PIWI-interacting small RNAs: the vanguard of genome defence. Nat Rev Mol Cell Biol. 2011;12(4):246–258. doi: 10.1038/nrm3089. [DOI] [PubMed] [Google Scholar]
- 46.Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442(7099):203–207. doi: 10.1038/nature04916. [DOI] [PubMed] [Google Scholar]
- 47.Ross RJ, Weiner MM, Lin H. PIWI proteins and PIWI-interacting RNAs in the soma. Nature. 2014;505(7483):353–359. doi: 10.1038/nature12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weng W, Liu N, Toiyama Y, Kusunoki M, Nagasaka T, Fujiwara T, et al. Novel evidence for a PIWI-interacting RNA (piRNA) as an oncogenic mediator of disease progression, and a potential prognostic biomarker in colorectal cancer. Mol Cancer. 2018;17(1):16. doi: 10.1186/s12943-018-0767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan L, Mai D, Zhang B, Jiang X, Zhang J, Bai R, et al. PIWI-interacting RNA-36712 restrains breast cancer progression and chemoresistance by interaction with SEPW1 pseudogene SEPW1P RNA. Mol Cancer. 2019;18(1):9. doi: 10.1186/s12943-019-0940-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samatov TR, Tonevitsky AG, Schumacher U. Epithelial-mesenchymal transition: focus on metastatic cascade, alternative splicing, non-coding RNAs and modulating compounds. Mol Cancer. 2013;12(1):107. doi: 10.1186/1476-4598-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reymond N, d'Agua BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13(12):858–870. doi: 10.1038/nrc3628. [DOI] [PubMed] [Google Scholar]
- 52.Keller L, Pantel K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat Rev Cancer. 2019;19(10):553–567. doi: 10.1038/s41568-019-0180-2. [DOI] [PubMed] [Google Scholar]
- 53.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18(9):533–548. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68(19):7846–7854. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- 56.Siemens H, Jackstadt R, Hunten S, Kaller M, Menssen A, Gotz U, et al. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle. 2011;10(24):4256–4271. doi: 10.4161/cc.10.24.18552. [DOI] [PubMed] [Google Scholar]
- 57.Liu YN, Yin JJ, Abou-Kheir W, Hynes PG, Casey OM, Fang L, et al. MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via Slug-independent mechanisms. Oncogene. 2013;32(3):296–306. doi: 10.1038/onc.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moes M, Le Bechec A, Crespo I, Laurini C, Halavatyi A, Vetter G, et al. A novel network integrating a miRNA-203/SNAI1 feedback loop which regulates epithelial to mesenchymal transition. PLoS ONE. 2012;7(4):e35440. doi: 10.1371/journal.pone.0035440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao Y, Ma H, Gao C, Lv Y, Chen X, Xu R, et al. Tumor-promoting properties of miR-8084 in breast cancer through enhancing proliferation, suppressing apoptosis and inducing epithelial-mesenchymal transition. J Transl Med. 2018;16(1):38. doi: 10.1186/s12967-018-1419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harquail J, LeBlanc N, Ouellette RJ, Robichaud GA. miRNAs 484 and 210 regulate Pax-5 expression and function in breast cancer cells. Carcinogenesis. 2019;40(8):1010–1020. doi: 10.1093/carcin/bgy191. [DOI] [PubMed] [Google Scholar]
- 61.Lee JW, Guan W, Han S, Hong DK, Kim LS, Kim H. MicroRNA-708-3p mediates metastasis and chemoresistance through inhibition of epithelial-to-mesenchymal transition in breast cancer. Cancer Sci. 2018;109(5):1404–1413. doi: 10.1111/cas.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xing S, Tian Z, Zheng W, Yang W, Du N, Gu Y, et al. Hypoxia downregulated miR-4521 suppresses gastric carcinoma progression through regulation of IGF2 and FOXM1. Mol Cancer. 2021;20(1):9. doi: 10.1186/s12943-020-01295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li T, Xie J, Shen C, Cheng D, Shi Y, Wu Z, et al. Upregulation of long noncoding RNA ZEB1-AS1 promotes tumor metastasis and predicts poor prognosis in hepatocellular carcinoma. Oncogene. 2016;35(12):1575–1584. doi: 10.1038/onc.2015.223. [DOI] [PubMed] [Google Scholar]
- 64.Terashima M, Tange S, Ishimura A, Suzuki T. MEG3 Long Noncoding RNA Contributes to the Epigenetic Regulation of Epithelial-Mesenchymal Transition in Lung Cancer Cell Lines. J Biol Chem. 2017;292(1):82–99. doi: 10.1074/jbc.M116.750950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pan J, Fang S, Tian H, Zhou C, Zhao X, Tian H, et al. lncRNA JPX/miR-33a-5p/Twist1 axis regulates tumorigenesis and metastasis of lung cancer by activating Wnt/beta-catenin signaling. Mol Cancer. 2020;19(1):9. doi: 10.1186/s12943-020-1133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shang BQ, Li ML, Quan HY, Hou PF, Li ZW, Chu SF, et al. Functional roles of circular RNAs during epithelial-to-mesenchymal transition. Mol Cancer. 2019;18(1):138. doi: 10.1186/s12943-019-1071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeng K, He B, Yang BB, Xu T, Chen X, Xu M, et al. The pro-metastasis effect of circANKS1B in breast cancer. Mol Cancer. 2018;17(1):160. doi: 10.1186/s12943-018-0914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen P, Yang T, Chen Q, Yuan H, Wu P, Cai B, et al. CircNEIL3 regulatory loop promotes pancreatic ductal adenocarcinoma progression via miRNA sponging and A-to-I RNA-editing. Mol Cancer. 2021;20(1):51. doi: 10.1186/s12943-021-01333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu Y, Zhang Y, Zheng X, Dai F, Lu Y, Dai L, et al. Circular RNA circCORO1C promotes laryngeal squamous cell carcinoma progression by modulating the let-7c-5p/PBX3 axis. Mol Cancer. 2020;19(1):99. doi: 10.1186/s12943-020-01215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fan Y, Wang J, Jin W, Sun Y, Xu Y, Wang Y, et al. CircNR3C2 promotes HRD1-mediated tumor-suppressive effect via sponging miR-513a-3p in triple-negative breast cancer. Mol Cancer. 2021;20(1):25. doi: 10.1186/s12943-021-01321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li G, Wang X, Li C, Hu S, Niu Z, Sun Q, et al. Piwi-interacting RNA1037 enhances chemoresistance and motility in human oral squamous cell carcinoma cells. Onco Targets Ther. 2019;12:10615–10627. doi: 10.2147/OTT.S233322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang H, Ren Y, Xu H, Pang D, Duan C, Liu C. The expression of stem cell protein Piwil2 and piR-932 in breast cancer. Surgical Oncology-Oxford. 2013;22(4):217–223. doi: 10.1016/j.suronc.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 73.Ramirez NE, Zhang Z, Madamanchi A, Boyd KL, O'Rear LD, Nashabi A, et al. The alpha(2)beta(1) integrin is a metastasis suppressor in mouse models and human cancer. J Clin Invest. 2011;121(1):226–237. doi: 10.1172/JCI42328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lv C, Li F, Li X, Tian Y, Zhang Y, Sheng X, et al. MiR-31 promotes mammary stem cell expansion and breast tumorigenesis by suppressing Wnt signaling antagonists. Nat Commun. 2017;8(1):1036. doi: 10.1038/s41467-017-01059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dentelli P, Traversa M, Rosso A, Togliatto G, Olgasi C, Marchio C, et al. miR-221/222 control luminal breast cancer tumor progression by regulating different targets. Cell Cycle. 2014;13(11):1811–1826. doi: 10.4161/cc.28758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim J, Piao HL, Kim BJ, Yao F, Han Z, Wang Y, et al. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat Genet. 2018;50(12):1705–1715. doi: 10.1038/s41588-018-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bacci L, Aiello A, Ripoli C, Loria R, Pugliese D, Pierconti F, et al. H19-dependent transcriptional regulation of beta3 and beta4 integrins upon estrogen and hypoxia favors metastatic potential in prostate cancer. Int J Mol Sci. 2019 doi: 10.3390/ijms20164012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liang Y, Chen X, Wu Y, Li J, Zhang S, Wang K, et al. LncRNA CASC9 promotes esophageal squamous cell carcinoma metastasis through upregulating LAMC2 expression by interacting with the CREB-binding protein. Cell Death Differ. 2018;25(11):1980–1995. doi: 10.1038/s41418-018-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang MH, Zhao L, Wang L, Ou-Yang W, Hu SS, Li WL, et al. Nuclear lncRNA HOXD-AS1 suppresses colorectal carcinoma growth and metastasis via inhibiting HOXD3-induced integrin beta3 transcriptional activating and MAPK/AKT signalling. Mol Cancer. 2019;18(1):31. doi: 10.1186/s12943-019-0955-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zou Y, Zheng S, Deng X, Yang A, Xie X, Tang H, et al. The role of circular RNA CDR1as/ciRS-7 in regulating tumor microenvironment: a pan-cancer analysis. Biomolecules. 2019 doi: 10.3390/biom9090429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liang J, Chen M, Hughes D, Chumanevich AA, Altilia S, Kaza V, et al. CDK8 selectively promotes the growth of colon cancer metastases in the liver by regulating gene expression of TIMP3 and matrix metalloproteinases. Cancer Res. 2018;78(23):6594–6606. doi: 10.1158/0008-5472.CAN-18-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu KP, Ma XL, Zhang CL. LncRNA ODRUL contributes to osteosarcoma progression through the miR-3182/MMP2 axis. Mol Ther. 2017;25(10):2383–2393. doi: 10.1016/j.ymthe.2017.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Han N, Yang L, Zhang X, Zhou Y, Chen R, Yu Y, et al. LncRNA MATN1-AS1 prevents glioblastoma cell from proliferation and invasion via RELA regulation and MAPK signaling pathway. Ann Transl Med. 2019;7(23):784. doi: 10.21037/atm.2019.11.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piipponen M, Nissinen L, Riihila P, Farshchian M, Kallajoki M, Peltonen J, et al. p53-regulated long noncoding RNA PRECSIT promotes progression of cutaneous squamous cell carcinoma via STAT3 signaling. Am J Pathol. 2020;190(2):503–517. doi: 10.1016/j.ajpath.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 85.Hua X, Liu Z, Zhou M, Tian Y, Zhao PP, Pan WH, et al. LSAMP-AS1 binds to microRNA-183-5p to suppress the progression of prostate cancer by up-regulating the tumor suppressor DCN. EBioMedicine. 2019;50:178–190. doi: 10.1016/j.ebiom.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Li Y, Jiang L, Lv S, Xu H, Fan Z, He Y, et al. E2F6-mediated lncRNA CASC2 down-regulation predicts poor prognosis and promotes progression in gastric carcinoma. Life Sci. 2019;232:116649. doi: 10.1016/j.lfs.2019.116649. [DOI] [PubMed] [Google Scholar]
- 87.Li P, Chen H, Chen S, Mo X, Li T, Xiao B, et al. Circular RNA 0000096 affects cell growth and migration in gastric cancer. Br J Cancer. 2017;116(5):626–633. doi: 10.1038/bjc.2016.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu F, Zhang H, Xie F, Tao D, Xiao X, Huang C, et al. Hsa_circ_0001361 promotes bladder cancer invasion and metastasis through miR-491-5p/MMP9 axis. Oncogene. 2020;39(8):1696–1709. doi: 10.1038/s41388-019-1092-z. [DOI] [PubMed] [Google Scholar]
- 89.Das B, Jain N, Mallick B. piR-39980 promotes cell proliferation, migration and invasion, and inhibits apoptosis via repression of SERPINB1 in human osteosarcoma. Biol Cell. 2020;112(3):73–91. doi: 10.1111/boc.201900063. [DOI] [PubMed] [Google Scholar]
- 90.Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH, Chen SP, et al. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut. 2012;61(2):278–289. doi: 10.1136/gut.2011.239145. [DOI] [PubMed] [Google Scholar]
- 91.Wang Q, Tang H, Yin S, Dong C. Downregulation of microRNA-138 enhances the proliferation, migration and invasion of cholangiocarcinoma cells through the upregulation of RhoC/p-ERK/MMP-2/MMP-9. Oncol Rep. 2013;29(5):2046–2052. doi: 10.3892/or.2013.2304. [DOI] [PubMed] [Google Scholar]
- 92.Jiang C, Li L, Xiang YQ, Lung ML, Zeng T, Lu J, et al. Epstein-barr virus miRNA BART2-5p promotes metastasis of nasopharyngeal carcinoma by suppressing RND3. Cancer Res. 2020;80(10):1957–1969. doi: 10.1158/0008-5472.CAN-19-0334. [DOI] [PubMed] [Google Scholar]
- 93.Humphries B, Wang Z, Li Y, Jhan JR, Jiang Y, Yang C. ARHGAP18 Downregulation by miR-200b suppresses metastasis of triple-negative breast cancer by enhancing activation of RhoA. Cancer Res. 2017;77(15):4051–4064. doi: 10.1158/0008-5472.CAN-16-3141. [DOI] [PubMed] [Google Scholar]
- 94.Huang JF, Guo YJ, Zhao CX, Yuan SX, Wang Y, Tang GN, et al. Hepatitis B virus X protein (HBx)-related long noncoding RNA (lncRNA) down-regulated expression by HBx (Dreh) inhibits hepatocellular carcinoma metastasis by targeting the intermediate filament protein vimentin. Hepatology. 2013;57(5):1882–1892. doi: 10.1002/hep.26195. [DOI] [PubMed] [Google Scholar]
- 95.Wu Y, Zhao Y, Huan L, Zhao J, Zhou Y, Xu L, et al. An LTR retrotransposon-derived long noncoding RNA lncMER52A promotes hepatocellular carcinoma progression by binding p120-catenin. Cancer Res. 2020;80(5):976–987. doi: 10.1158/0008-5472.CAN-19-2115. [DOI] [PubMed] [Google Scholar]
- 96.Du WW, Yang W, Li X, Fang L, Wu N, Li F, et al. The circular RNA circSKA3 binds integrin beta1 to induce invadopodium formation enhancing breast cancer invasion. Mol Ther. 2020;28(5):1287–1298. doi: 10.1016/j.ymthe.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 98.Strilic B, Offermanns S. Intravascular survival and extravasation of tumor cells. Cancer Cell. 2017;32(3):282–293. doi: 10.1016/j.ccell.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 99.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 100.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168(4):670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36(34):4929–4942. doi: 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- 102.Fang JH, Zhang ZJ, Shang LR, Luo YW, Lin YF, Yuan Y, et al. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology. 2018;68(4):1459–1475. doi: 10.1002/hep.29920. [DOI] [PubMed] [Google Scholar]
- 103.Li Y, Zhang H, Fan L, Mou J, Yin Y, Peng C, et al. MiR-629-5p promotes the invasion of lung adenocarcinoma via increasing both tumor cell invasion and endothelial cell permeability. Oncogene. 2020;39(17):3473–3488. doi: 10.1038/s41388-020-1228-1. [DOI] [PubMed] [Google Scholar]
- 104.Li Y, Wu Z, Yuan J, Sun L, Lin L, Huang N, et al. Long non-coding RNA MALAT1 promotes gastric cancer tumorigenicity and metastasis by regulating vasculogenic mimicry and angiogenesis. Cancer Lett. 2017;395:31–44. doi: 10.1016/j.canlet.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 105.Li J, Li Z, Jiang P, Peng M, Zhang X, Chen K, et al. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. J Exp Clin Cancer Res. 2018;37(1):177. doi: 10.1186/s13046-018-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu Y, Leng K, Yao Y, Kang P, Liao G, Han Y, et al. A novel circular RNA, circ-CCAC1, contributes to CCA progression, induces angiogenesis, and disrupts vascular endothelial barriers. Hepatology. 2020 doi: 10.1002/hep.31493. [DOI] [PubMed] [Google Scholar]
- 107.He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang J, et al. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9(26):8206–8220. doi: 10.7150/thno.37455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bai M, Li J, Yang H, Zhang H, Zhou Z, Deng T, et al. miR-135b delivered by gastric tumor exosomes inhibits FOXO1 expression in endothelial cells and promotes angiogenesis. Mol Ther. 2019;27(10):1772–1783. doi: 10.1016/j.ymthe.2019.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Yang H, Zhang H, Ge S, Ning T, Bai M, Li J, et al. Exosome-derived miR-130a activates angiogenesis in gastric cancer by targeting C-MYB in vascular endothelial cells. Mol Ther. 2018;26(10):2466–2475. doi: 10.1016/j.ymthe.2018.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 110.Zhou HC, Fang JH, Shang LR, Zhang ZJ, Sang Y, Xu L, et al. MicroRNAs miR-125b and miR-100 suppress metastasis of hepatocellular carcinoma by disrupting the formation of vessels that encapsulate tumour clusters. J Pathol. 2016;240(4):450–460. doi: 10.1002/path.4804. [DOI] [PubMed] [Google Scholar]
- 111.Castellano JJ, Navarro A, Vinolas N, Marrades RM, Moises J, Cordeiro A, et al. LincRNA-p21 impacts prognosis in resected non-small cell lung cancer patients through angiogenesis regulation. J Thorac Oncol. 2016;11(12):2173–2182. doi: 10.1016/j.jtho.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 112.Niu Y, Bao L, Chen Y, Wang C, Luo M, Zhang B, et al. HIF2-Induced long noncoding RNA RAB11B-AS1 promotes hypoxia-mediated angiogenesis and breast cancer metastasis. Cancer Res. 2020;80(5):964–975. doi: 10.1158/0008-5472.CAN-19-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang FW, Cao CH, Han K, Zhao YX, Cai MY, Xiang ZC, et al. APC-activated long noncoding RNA inhibits colorectal carcinoma pathogenesis through reduction of exosome production. J Clin Invest. 2019;129(2):727–743. doi: 10.1172/JCI122478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li W, Yang FQ, Sun CM, Huang JH, Zhang HM, Li X, et al. circPRRC2A promotes angiogenesis and metastasis through epithelial-mesenchymal transition and upregulates TRPM3 in renal cell carcinoma. Theranostics. 2020;10(10):4395–4409. doi: 10.7150/thno.43239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xie M, Yu T, Jing X, Ma L, Fan Y, Yang F, et al. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol Cancer. 2020;19(1):112. doi: 10.1186/s12943-020-01208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhong Z, Huang M, Lv M, He Y, Duan C, Zhang L, et al. Circular RNA MYLK as a competing endogenous RNA promotes bladder cancer progression through modulating VEGFA/VEGFR2 signaling pathway. Cancer Lett. 2017;403:305–317. doi: 10.1016/j.canlet.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 117.Shi H, Li H, Zhen T, Dong Y, Pei X, Zhang X. hsa_circ_001653 implicates in the development of pancreatic ductal adenocarcinoma by regulating microRNA-377-mediated HOXC6 axis. Mol Ther Nucleic Acids. 2020;20:252–264. doi: 10.1016/j.omtn.2019.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.Huang XY, Huang ZL, Huang J, Xu B, Huang XY, Xu YH, et al. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res. 2020;39(1):20. doi: 10.1186/s13046-020-1529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim J, Siverly AN, Chen D, Wang M, Yuan Y, Wang Y, et al. Ablation of miR-10b suppresses oncogene-induced mammary tumorigenesis and metastasis and reactivates tumor-suppressive pathways. Cancer Res. 2016;76(21):6424–6435. doi: 10.1158/0008-5472.CAN-16-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25(5):666–681. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 121.Leblanc R, Peyruchaud O. Metastasis: new functional implications of platelets and megakaryocytes. Blood. 2016;128(1):24–31. doi: 10.1182/blood-2016-01-636399. [DOI] [PubMed] [Google Scholar]
- 122.Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33(1):231–269. doi: 10.1007/s10555-014-9498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. 2012;30(5):525–532. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- 125.Gilmore AP. Anoikis. Cell Death Differ. 2005;12(Suppl 2):1473–1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- 126.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5(10):816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 127.Lazar S, Goldfinger LE. Platelet microparticles and miRNA transfer in cancer progression: many targets, modes of action, and effects across cancer stages. Front Cardiovasc Med. 2018;5:13. doi: 10.3389/fcvm.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu X, Chen Q, Yan J, Wang Y, Zhu C, Chen C, et al. MiRNA-296-3p-ICAM-1 axis promotes metastasis of prostate cancer by possible enhancing survival of natural killer cell-resistant circulating tumour cells. Cell Death Dis. 2013;4:e928. doi: 10.1038/cddis.2013.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang B, Wang Q, Wang Z, Jiang J, Yu SC, Ping YF, et al. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 2014;74(20):5746–5757. doi: 10.1158/0008-5472.CAN-13-2563. [DOI] [PubMed] [Google Scholar]
- 130.Donatelli SS, Zhou JM, Gilvary DL, Eksioglu EA, Chen X, Cress WD, et al. TGF-beta-inducible microRNA-183 silences tumor-associated natural killer cells. Proc Natl Acad Sci U S A. 2014;111(11):4203–4208. doi: 10.1073/pnas.1319269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li GJ, Ding H, Miao D. Long-noncoding RNA HOTAIR inhibits immunologic rejection of mouse leukemia cells through activating the Wnt/beta-catenin signaling pathway in a mouse model of leukemia. J Cell Physiol. 2019;234(7):10386–10396. doi: 10.1002/jcp.27705. [DOI] [PubMed] [Google Scholar]
- 132.Zhang R, Ni F, Fu B, Wu Y, Sun R, Tian Z, et al. A long noncoding RNA positively regulates CD56 in human natural killer cells. Oncotarget. 2016;7(45):72546–72558. doi: 10.18632/oncotarget.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ou ZL, Luo Z, Wei W, Liang S, Gao TL, Lu YB. Hypoxia-induced shedding of MICA and HIF1A-mediated immune escape of pancreatic cancer cells from NK cells: role of circ_0000977/miR-153 axis. RNA Biol. 2019;16(11):1592–1603. doi: 10.1080/15476286.2019.1649585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ma Y, Zhang C, Zhang B, Yu H, Yu Q. circRNA of AR-suppressed PABPC1 91 bp enhances the cytotoxicity of natural killer cells against hepatocellular carcinoma via upregulating UL16 binding protein 1. Oncol Lett. 2019;17(1):388–397. doi: 10.3892/ol.2018.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mak CS, Yung MM, Hui LM, Leung LL, Liang R, Chen K, et al. MicroRNA-141 enhances anoikis resistance in metastatic progression of ovarian cancer through targeting KLF12/Sp1/survivin axis. Mol Cancer. 2017;16(1):11. doi: 10.1186/s12943-017-0582-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gordon MA, Babbs B, Cochrane DR, Bitler BG, Richer JK. The long non-coding RNA MALAT1 promotes ovarian cancer progression by regulating RBFOX2-mediated alternative splicing. Mol Carcinog. 2019;58(2):196–205. doi: 10.1002/mc.22919. [DOI] [PubMed] [Google Scholar]
- 137.Zhu Y, Zhang X, Qi L, Cai Y, Yang P, Xuan G, et al. HULC long noncoding RNA silencing suppresses angiogenesis by regulating ESM-1 via the PI3K/Akt/mTOR signaling pathway in human gliomas. Oncotarget. 2016;7(12):14429–14440. doi: 10.18632/oncotarget.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Okugawa Y, Toiyama Y, Hur K, Toden S, Saigusa S, Tanaka K, et al. Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis. 2014;35(12):2731–2739. doi: 10.1093/carcin/bgu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seitz AK, Christensen LL, Christensen E, Faarkrog K, Ostenfeld MS, Hedegaard J, et al. Profiling of long non-coding RNAs identifies LINC00958 and LINC01296 as candidate oncogenes in bladder cancer. Sci Rep. 2017;7(1):395. doi: 10.1038/s41598-017-00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yin Y, Gao H, Guo J, Gao Y. Effect of circular RNA UBAP2 silencing on proliferation and invasion of human lung cancer A549 cells and its mechanism. Zhongguo Fei Ai Za Zhi. 2017;20(12):800–807. doi: 10.3779/j.issn.1009-3419.2017.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ma H, Wang H, Tian F, Zhong Y, Liu Z, Liao A. PIWI-interacting RNA-004800 is regulated by S1P receptor signaling pathway to keep myeloma cell survival. Front Oncol. 2020;10:438. doi: 10.3389/fonc.2020.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jacobs DI, Qin Q, Fu A, Chen Z, Zhou J, Zhu Y. piRNA-8041 is downregulated in human glioblastoma and suppresses tumor growth in vitro and in vivo. Oncotarget. 2018;9(102):37616–37626. doi: 10.18632/oncotarget.26331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Singhal U, Wang Y, Henderson J, Niknafs YS, Qiao Y, Gursky A, et al. Multigene profiling of CTCs in mCRPC identifies a clinically relevant prognostic signature. Mol Cancer Res. 2018;16(4):643–654. doi: 10.1158/1541-7786.MCR-17-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Augustin HG, Koh GY. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science. 2017 doi: 10.1126/science.aal2379. [DOI] [PubMed] [Google Scholar]
- 145.St Hill CA. Interactions between endothelial selectins and cancer cells regulate metastasis. Front Biosci (Landmark Ed) 2011;16:3233–3251. doi: 10.2741/3909. [DOI] [PubMed] [Google Scholar]
- 146.Miles FL, Pruitt FL, van Golen KL, Cooper CR. Stepping out of the flow: capillary extravasation in cancer metastasis. Clin Exp Metastasis. 2008;25(4):305–324. doi: 10.1007/s10585-007-9098-2. [DOI] [PubMed] [Google Scholar]
- 147.Laubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol. 2010;20(3):169–177. doi: 10.1016/j.semcancer.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 148.Zhong L, Huot J, Simard MJ. p38 activation induces production of miR-146a and miR-31 to repress E-selectin expression and inhibit transendothelial migration of colon cancer cells. Sci Rep. 2018;8(1):2334. doi: 10.1038/s41598-018-20837-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pan S, Liu Y, Liu Q, Xiao Y, Liu B, Ren X, et al. HOTAIR/miR-326/FUT6 axis facilitates colorectal cancer progression through regulating fucosylation of CD44 via PI3K/AKT/mTOR pathway. Biochim Biophys Acta Mol Cell Res. 2019;1866(5):750–760. doi: 10.1016/j.bbamcr.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 150.Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9(1):5395. doi: 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen CW, Fu M, Du ZH, Zhao F, Yang WW, Xu LH, et al. Long noncoding RNA MRPL23-AS1 promoteoid cystic carcinoma lung metastasis. Cancer Res. 2020 doi: 10.1158/0008-5472.CAN-19-0819. [DOI] [PubMed] [Google Scholar]
- 153.Li H, Hu Y, Jin Y, Zhu Y, Hao Y, Liu F, et al. Long noncoding RNA lncGALM increases risk of liver metastasis in gallbladder cancer through facilitating N-cadherin and IL-1beta-dependent liver arrest and tumor extravasation. Clin Transl Med. 2020;10(7):e201. doi: 10.1002/ctm2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lu Y, Chen L, Li L, Cao Y. Exosomes derived from brain metastatic breast cancer cells destroy the blood-brain barrier by carrying lncRNA GS1–600G8.5. Biomed Res Int. 2020;2020:7461727. doi: 10.1155/2020/7461727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wu L, Ye Z, Pan Y, Li X, Fu X, Zhang B, et al. Vascular endothelial growth factor aggravates cerebral ischemia and reperfusion-induced blood-brain-barrier disruption through regulating LOC102640519/HOXC13/ZO-1 signaling. Exp Cell Res. 2018;369(2):275–283. doi: 10.1016/j.yexcr.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 156.Zhang J, Dong B, Hao J, Yi S, Cai W, Luo Z. LncRNA Snhg3 contributes to dysfunction of cerebral microvascular cells in intracerebral hemorrhage rats by activating the TWEAK/Fn14/STAT3 pathway. Life Sci. 2019;237:116929. doi: 10.1016/j.lfs.2019.116929. [DOI] [PubMed] [Google Scholar]
- 157.Gao Y, Wu P, Ma Y, Xue Y, Liu Y, Zheng J, et al. Circular RNA USP1 regulates the permeability of blood-tumour barrier via miR-194-5p/FLI1 axis. J Cell Mol Med. 2020;24(1):342–355. doi: 10.1111/jcmm.14735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shan K, Liu C, Liu BH, Chen X, Dong R, Liu X, et al. Circular noncoding RNA HIPK3 mediates retinal vascular dysfunction in diabetes mellitus. Circulation. 2017;136(17):1629–1642. doi: 10.1161/CIRCULATIONAHA.117.029004. [DOI] [PubMed] [Google Scholar]
- 159.Bai Y, Zhang Y, Han B, Yang L, Chen X, Huang R, et al. Circular RNA dlgap4 ameliorates ischemic stroke outcomes by targeting miR-143 to regulate endothelial-mesenchymal transition associated with blood-brain barrier integrity. J Neurosci. 2018;38(1):32–50. doi: 10.1523/JNEUROSCI.1348-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang L, Han B, Zhang Y, Bai Y, Chao J, Hu G, et al. Engagement of circular RNA HECW2 in the nonautophagic role of ATG5 implicated in the endothelial-mesenchymal transition. Autophagy. 2018;14(3):404–418. doi: 10.1080/15548627.2017.1414755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Roy R, Pattnaik S, Sivagurunathan S, Chidambaram S. Small ncRNA binding protein, PIWI: a potential molecular bridge between blood brain barrier and neuropathological conditions. Med Hypotheses. 2020;138:109609. doi: 10.1016/j.mehy.2020.109609. [DOI] [PubMed] [Google Scholar]
- 162.Raimo M, Orso F, Grassi E, Cimino D, Penna E, De Pitta C, et al. miR-146a exerts differential effects on melanoma growth and metastatization. Mol Cancer Res. 2016;14(6):548–562. doi: 10.1158/1541-7786.MCR-15-0425-T. [DOI] [PubMed] [Google Scholar]
- 163.Dettori D, Orso F, Penna E, Baruffaldi D, Brundu S, Maione F, et al. Therapeutic silencing of miR-214 inhibits tumor progression in multiple mouse models. Mol Ther. 2018;26(8):2008–2018. doi: 10.1016/j.ymthe.2018.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Penna E, Orso F, Cimino D, Tenaglia E, Lembo A, Quaglino E, et al. microRNA-214 contributes to melanoma tumour progression through suppression of TFAP2C. EMBO J. 2011;30(10):1990–2007. doi: 10.1038/emboj.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Avalle L, Incarnato D, Savino A, Gai M, Marino F, Pensa S, et al. MicroRNAs-143 and -145 induce epithelial to mesenchymal transition and modulate the expression of junction proteins. Cell Death Differ. 2017;24(10):1750–1760. doi: 10.1038/cdd.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9(4):285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 168.Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989;8(2):98–101. [PubMed] [Google Scholar]
- 169.Hiratsuka S, Duda DG, Huang Y, Goel S, Sugiyama T, Nagasawa T, et al. C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (Gr-1)-positive cells. Proc Natl Acad Sci U S A. 2011;108(1):302–307. doi: 10.1073/pnas.1016917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brabletz T. To differentiate or not–routes towards metastasis. Nat Rev Cancer. 2012;12(6):425–436. doi: 10.1038/nrc3265. [DOI] [PubMed] [Google Scholar]
- 171.Brabletz T. EMT and MET in metastasis: where are the cancer stem cells? Cancer Cell. 2012;22(6):699–701. doi: 10.1016/j.ccr.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 172.Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17(2):183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shao Y, Chen T, Zheng X, Yang S, Xu K, Chen X, et al. Colorectal cancer-derived small extracellular vesicles establish an inflammatory premetastatic niche in liver metastasis. Carcinogenesis. 2018;39(11):1368–1379. doi: 10.1093/carcin/bgy115. [DOI] [PubMed] [Google Scholar]
- 174.Qi F, He T, Jia L, Song N, Guo L, Ma X, et al. The miR-30 family inhibits pulmonary vascular hyperpermeability in the premetastatic phase by direct targeting of Skp2. Clin Cancer Res. 2015;21(13):3071–3080. doi: 10.1158/1078-0432.CCR-14-2785. [DOI] [PubMed] [Google Scholar]
- 175.Feng T, Zhang P, Sun Y, Wang Y, Tong J, Dai H, et al. High throughput sequencing identifies breast cancer-secreted exosomal LncRNAs initiating pulmonary pre-metastatic niche formation. Gene. 2019;710:258–264. doi: 10.1016/j.gene.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 176.Wang C, Wang J, Shen X, Li M, Yue Y, Cheng X, et al. LncRNA SPOCD1-AS from ovarian cancer extracellular vesicles remodels mesothelial cells to promote peritoneal metastasis via interacting with G3BP1. J Exp Clin Cancer Res. 2021;40(1):101. doi: 10.1186/s13046-021-01899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ahmed I, Karedath T, Andrews SS, Al-Azwani IK, Mohamoud YA, Querleu D, et al. Altered expression pattern of circular RNAs in primary and metastatic sites of epithelial ovarian carcinoma. Oncotarget. 2016;7(24):36366–36381. doi: 10.18632/oncotarget.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang H, Li X, Meng Q, Sun H, Wu S, Hu W, et al. CircPTK2 (hsa_circ_0005273) as a novel therapeutic target for metastatic colorectal cancer. Mol Cancer. 2020;19(1):13. doi: 10.1186/s12943-020-1139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71(5):1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 180.van 't Veer LJ, Dai H, vandeVijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 181.Gooding AJ, Parker KA, Valadkhan S, Schiemann WP. The IncRNA BORG: a novel inducer of TNBC metastasis, chemoresistance, and disease recurrence. J Cancer Metastasis Treat. 2019 doi: 10.20517/2394-4722.2019.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44(6):2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xu L, Feng X, Hao X, Wang P, Zhang Y, Zheng X, et al. CircSETD3 (Hsa_circ_0000567) acts as a sponge for microRNA-421 inhibiting hepatocellular carcinoma growth. J Exp Clin Cancer Res. 2019;38(1):98. doi: 10.1186/s13046-019-1041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Karedath T, Ahmed I, Al Ameri W, Al-Dasim FM, Andrews SS, Samuel S, et al. Silencing of ANKRD12 circRNA induces molecular and functional changes associated with invasive phenotypes. BMC Cancer. 2019;19(1):565. doi: 10.1186/s12885-019-5723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sun M, Zhao W, Chen Z, Li M, Li S, Wu B, et al. Circular RNA CEP128 promotes bladder cancer progression by regulating Mir-145-5p/Myd88 via MAPK signaling pathway. Int J Cancer. 2019;145(8):2170–2181. doi: 10.1002/ijc.32311. [DOI] [PubMed] [Google Scholar]
- 186.Fang T, Lv H, Lv G, Li T, Wang C, Han Q, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. 2018;9(1):191. doi: 10.1038/s41467-017-02583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Capaci V, Bascetta L, Fantuz M, Beznoussenko GV, Sommaggio R, Cancila V, et al. Mutant p53 induces Golgi tubulo-vesiculation driving a prometastatic secretome. Nat Commun. 2020;11(1):3945. doi: 10.1038/s41467-020-17596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang S, Liang K, Hu Q, Li P, Song J, Yang Y, et al. JAK2-binding long noncoding RNA promotes breast cancer brain metastasis. J Clin Invest. 2017;127(12):4498–4515. doi: 10.1172/JCI91553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xing F, Liu Y, Wu SY, Wu K, Sharma S, Mo YY, et al. Loss of XIST in breast cancer activates MSN-c-Met and reprograms microglia via exosomal miRNA to promote brain metastasis. Cancer Res. 2018;78(15):4316–4330. doi: 10.1158/0008-5472.CAN-18-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Celia-Terrassa T, Bastian C, Liu DD, Ell B, Aiello NM, Wei Y, et al. Hysteresis control of epithelial-mesenchymal transition dynamics conveys a distinct program with enhanced metastatic ability. Nat Commun. 2018;9(1):5005. doi: 10.1038/s41467-018-07538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lin Z, Sun L, Xie S, Zhang S, Fan S, Li Q, et al. Chemotherapy-Induced Long Non-coding RNA 1 Promotes Metastasis and Chemo-Resistance of TSCC via the Wnt/beta-Catenin Signaling Pathway. Mol Ther. 2018;26(6):1494–1508. doi: 10.1016/j.ymthe.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang SH, Wu XC, Zhang MD, Weng MZ, Zhou D, Quan ZW. Upregulation of H19 indicates a poor prognosis in gallbladder carcinoma and promotes epithelial-mesenchymal transition. Am J Cancer Res. 2016;6(1):15–26. [PMC free article] [PubMed] [Google Scholar]
- 194.Deng G, Zhou X, Chen L, Yao Y, Li J, Zhang Y, et al. High expression of ESRP1 regulated by circ-0005585 promotes cell colonization in ovarian cancer. Cancer Cell Int. 2020;20:174. doi: 10.1186/s12935-020-01254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Anfossi S, Babayan A, Pantel K, Calin GA. Clinical utility of circulating non-coding RNAs - an update. Nat Rev Clin Oncol. 2018;15(9):541–563. doi: 10.1038/s41571-018-0035-x. [DOI] [PubMed] [Google Scholar]
- 196.Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451(7175):147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.White NM, Zhao SG, Zhang J, Rozycki EB, Dang HX, McFadden SD, et al. Multi-institutional analysis shows that low PCAT-14 expression associates with poor outcomes in prostate cancer. Eur Urol. 2017;71(2):257–266. doi: 10.1016/j.eururo.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 198.Xuan L, Qu L, Zhou H, Wang P, Yu H, Wu T, et al. Circular RNA: a novel biomarker for progressive laryngeal cancer. Am J Transl Res. 2016;8(2):932–939. [PMC free article] [PubMed] [Google Scholar]
- 199.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hanke M, Hoefig K, Merz H, Feller AC, Kausch I, Jocham D, et al. A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol Oncol. 2010;28(6):655–661. doi: 10.1016/j.urolonc.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 201.Park NJ, Zhou H, Elashoff D, Henson BS, Kastratovic DA, Abemayor E, et al. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res. 2009;15(17):5473–5477. doi: 10.1158/1078-0432.CCR-09-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Xie Y, Todd NW, Liu Z, Zhan M, Fang H, Peng H, et al. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer. 2010;67(2):170–176. doi: 10.1016/j.lungcan.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108(12):5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4):423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yuan T, Huang X, Woodcock M, Du M, Dittmar R, Wang Y, et al. Plasma extracellular RNA profiles in healthy and cancer patients. Sci Rep. 2016;6:19413. doi: 10.1038/srep19413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hur K, Toiyama Y, Okugawa Y, Ide S, Imaoka H, Boland CR, et al. Circulating microRNA-203 predicts prognosis and metastasis in human colorectal cancer. Gut. 2017;66(4):654–665. doi: 10.1136/gutjnl-2014-308737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ohtsuka M, Ling H, Ivan C, Pichler M, Matsushita D, Goblirsch M, et al. H19 noncoding RNA, an independent prognostic factor, regulates essential Rb-E2F and CDK8-beta-catenin signaling in colorectal cancer. EBioMedicine. 2016;13:113–124. doi: 10.1016/j.ebiom.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhou Y, Sheng B, Xia Q, Guan X, Zhang Y. Association of long non-coding RNA H19 and microRNA-21 expression with the biological features and prognosis of non-small cell lung cancer. Cancer Gene Ther. 2017;24(8):317–324. doi: 10.1038/cgt.2017.20. [DOI] [PubMed] [Google Scholar]
- 210.Gutschner T, Hammerle M, Eissmann M, Hsu J, Kim Y, Hung G, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73(3):1180–1189. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu SP, Yang JX, Cao DY, Shen K. Identification of differentially expressed long non-coding RNAs in human ovarian cancer cells with different metastatic potentials. Cancer Biol Med. 2013;10(3):138–141. doi: 10.7497/j.issn.2095-3941.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, et al. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32(13):1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tang W, Fu K, Sun H, Rong D, Wang H, Cao H. CircRNA microarray profiling identifies a novel circulating biomarker for detection of gastric cancer. Mol Cancer. 2018;17(1):137. doi: 10.1186/s12943-018-0888-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Day JR, Jost M, Reynolds MA, Groskopf J, Rittenhouse H. PCA3: from basic molecular science to the clinical lab. Cancer Lett. 2011;301(1):1–6. doi: 10.1016/j.canlet.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 215.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 216.Arun G, Diermeier SD, Spector DL. Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol Med. 2018;24(3):257–277. doi: 10.1016/j.molmed.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452(7189):896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 218.Lima WF, Wu H, Nichols JG, Sun H, Murray HM, Crooke ST. Binding and cleavage specificities of human Argonaute2. J Biol Chem. 2009;284(38):26017–26028. doi: 10.1074/jbc.M109.010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Adams BD, Parsons C, Walker L, Zhang WC, Slack FJ. Targeting noncoding RNAs in disease. J Clin Invest. 2017;127(3):761–771. doi: 10.1172/JCI84424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Anastasiadou E, Seto AG, Beatty X, Hermreck M, Gilles ME, Stroopinsky D, et al. Cobomarsen, an oligonucleotide inhibitor of miR-155, slows DLBCL tumor cell growth in vitro and in vivo. Clin Cancer Res. 2021;27(4):1139–1149. doi: 10.1158/1078-0432.CCR-20-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Segal M, Biscans A, Gilles ME, Anastasiadou E, De Luca R, Lim J, et al. Hydrophobically modified let-7b miRNA enhances biodistribution to NSCLC and downregulates HMGA2 in vivo. Mol Ther Nucleic Acids. 2020;19:267–277. doi: 10.1016/j.omtn.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yoo B, Kavishwar A, Ross A, Wang P, Tabassum DP, Polyak K, et al. Combining miR-10b-targeted nanotherapy with low-dose doxorubicin elicits durable regressions of metastatic breast cancer. Cancer Res. 2015;75(20):4407–4415. doi: 10.1158/0008-5472.CAN-15-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Arun G, Diermeier S, Akerman M, Chang KC, Wilkinson JE, Hearn S, et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016;30(1):34–51. doi: 10.1101/gad.270959.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Slack FJ, Chinnaiyan AM. The role of non-coding RNAs in oncology. Cell. 2019;179(5):1033–1055. doi: 10.1016/j.cell.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Trang P, Wiggins JF, Daige CL, Cho C, Omotola M, Brown D, et al. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol Ther. 2011;19(6):1116–1122. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wiggins JF, Ruffino L, Kelnar K, Omotola M, Patrawala L, Brown D, et al. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;70(14):5923–5930. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pramanik D, Campbell NR, Karikari C, Chivukula R, Kent OA, Mendell JT, et al. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol Cancer Ther. 2011;10(8):1470–1480. doi: 10.1158/1535-7163.MCT-11-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hong DS, Kang YK, Borad M, Sachdev J, Ejadi S, Lim HY, et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer. 2020 doi: 10.1038/s41416-020-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Goyal B, Yadav SRM, Awasthee N, Gupta S, Kunnumakkara AB, Gupta SC. Diagnostic, prognostic, and therapeutic significance of long non-coding RNA MALAT1 in cancer. Biochim Biophys Acta Rev Cancer. 2021;1875(2):188502. doi: 10.1016/j.bbcan.2021.188502. [DOI] [PubMed] [Google Scholar]
- 230.Chen Q, Zhu C, Jin Y. The Oncogenic and Tumor Suppressive Functions of the Long Noncoding RNA MALAT1: An Emerging Controversy. Front Genet. 2020;11:93. doi: 10.3389/fgene.2020.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kwok ZH, Roche V, Chew XH, Fadieieva A, Tay Y. A non-canonical tumor suppressive role for the long non-coding RNA MALAT1 in colon and breast cancers. Int J Cancer. 2018;143(3):668–678. doi: 10.1002/ijc.31386. [DOI] [PubMed] [Google Scholar]
- 232.Latorre E, Carelli S, Raimondi I, D'Agostino V, Castiglioni I, Zucal C, et al. The ribonucleic complex HuR-MALAT1 represses CD133 expression and suppresses epithelial-mesenchymal transition in breast cancer. Cancer Res. 2016;76(9):2626–2636. doi: 10.1158/0008-5472.CAN-15-2018. [DOI] [PubMed] [Google Scholar]
- 233.Arun G, Spector DL. MALAT1 long non-coding RNA and breast cancer. RNA Biol. 2019;16(6):860–863. doi: 10.1080/15476286.2019.1592072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang B, Arun G, Mao YS, Lazar Z, Hung G, Bhattacharjee G, et al. The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult. Cell Rep. 2012;2(1):111–123. doi: 10.1016/j.celrep.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Qian W, Huang T, Feng W. Circular RNA HIPK3 promotes EMT of cervical cancer through sponging miR-338-3p to Up-regulate HIF-1alpha. Cancer Manag Res. 2020;12:177–187. doi: 10.2147/CMAR.S232235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zeng K, Chen X, Xu M, Liu X, Hu X, Xu T, et al. CircHIPK3 promotes colorectal cancer growth and metastasis by sponging miR-7. Cell Death Dis. 2018;9(4):417. doi: 10.1038/s41419-018-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 237.Yan Y, Su M, Qin B. CircHIPK3 promotes colorectal cancer cells proliferation and metastasis via modulating of miR-1207-5p/FMNL2 signal. Biochem Biophys Res Commun. 2020;524(4):839–846. doi: 10.1016/j.bbrc.2020.01.055. [DOI] [PubMed] [Google Scholar]
- 238.Li Y, Zheng F, Xiao X, Xie F, Tao D, Huang C, et al. CircHIPK3 sponges miR-558 to suppress heparanase expression in bladder cancer cells. EMBO Rep. 2017;18(9):1646–1659. doi: 10.15252/embr.201643581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szasz AM, Wang ZC, et al. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137(6):1032–1046. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 240.Wu P, Gao W, Su M, Nice EC, Zhang W, Lin J, et al. Adaptive mechanisms of tumor therapy resistance driven by tumor microenvironment. Front Cell Dev Biol. 2021;9:641469. doi: 10.3389/fcell.2021.641469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.