Abstract
Positively charged amino acid side-chains play important roles in anion binding and permeation through the CFTR chloride channel. One pore-lining lysine residue in particular (K95) has been shown to be indispensable for anion binding, conductance, and selectivity. Here, we use functional investigation of CFTR to show that a nearby arginine (R134) plays a functionally analogous role. Removal of this positive charge (in the R134Q mutant) drastically reduces single-channel conductance, weakens binding of both permeant and blocking anions, and abolishes the normal anion conductance selectivity pattern. Each of these functional effects was reversed by a second-site mutation (S1141K) that introduces an ectopic positive charge to a nearby pore-lining residue. Substituted cysteine accessibility experiments confirm that R134—but not nearby residues in the same transmembrane helix—is accessible within the pore lumen. These results suggest that K95 and R134, which are very close together within the inner vestibule of the pore, play analogous, important roles, and that both are required for the normal anion binding and anion conductance properties of the pore. Nevertheless, that fact that both positive charges can be “transplanted” to other sites in the inner vestibule with little effect on channel permeation properties indicates that it is the overall number of charges—rather than their exact locations—that controls pore function.
Keywords: Cystic fibrosis transmembrane conductance regulator, Chloride channel, Channel pore, Anion permeation, Channel structure
Introduction
Cystic fibrosis (CF) is caused by loss-of-function mutations in the CF transmembrane conductance regulator (CFTR), an epithelial cell anion channel [1]. The atomic structure of human CFTR illustrates that the anion permeation pathway is decorated throughout by positively charged arginine and lysine side-chains [2, 3] (Fig. 1). This confirms and reinforces long-standing functional data suggesting that interactions between permeating anions and fixed positive charges are an important component of the normal anion permeation mechanism [4–6]. The CFTR pore is lined by multiple transmembrane helices (TMs) and has been functionally separated into a narrow selectivity filter/gate region flanked by a deep, wide inner vestibule and a shorter, narrower outer vestibule (Fig. 1C). In the open channel, the inner vestibule is connected to the cytoplasm by a lateral portal formed by the cytoplasmic extensions of several TMs (TMEs) (Fig. 1B,C). The narrow region is lined by uncharged side-chains; however, positively charged side-chains have been shown to make important functional contributions to each of the other pore regions [4] (Fig. 1). In the outer vestibule, positive charges [R104 (TM1), R334 (TM6), K335 (TM6)] act to attract extracellular Cl− ions to the pore; mutations that remove these positive charges reduce Cl− conductance and cause inward rectification of the current–voltage relationship [7–9]. Similarly, a number of positively charged side-chains [K190 (TME3), R248(TME4), R303(TME5), K370 (TME6)] have been shown to attract cytoplasmic Cl− ions to the pore [10–12]. Within the inner vestibule of the pore, a single positively charged residue, K95 (TM1), has been shown to play a very important role in the control of anion binding and anion conductance (see below). All of the above-mentioned positively charged side-chains have been shown to line the CFTR pore using cysteine-scanning mutagenesis [7, 9, 11, 13–15].
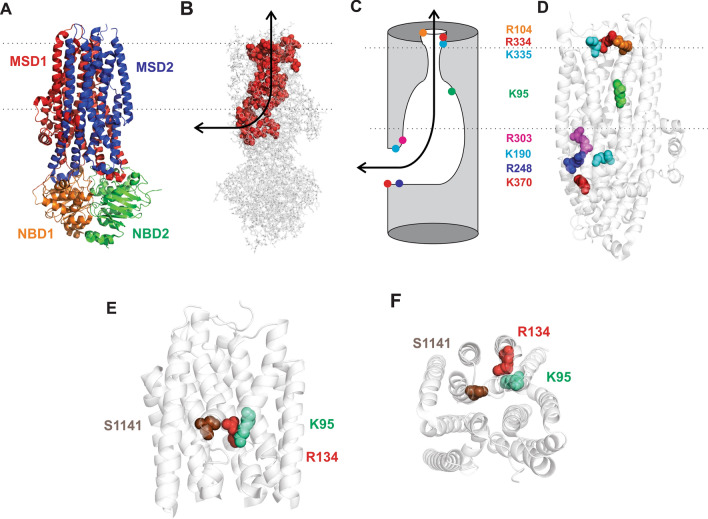
Fig. 1.
Structural views of the CFTR channel pore. A Atomic structure of human CFTR in a phosphorylated, ATP-bound state [3]. This state is expected to resemble the open channel conformation, however, the pore remains closed to Cl− permeation at its extracellular end [3]. Different colours reflect the domain organization of CFTR, representing two membrane-spanning domains (MSDs) and two cytoplasmic NBDs. The cytoplasmic regulatory domain is absent from this structure. B Same structure showing the location of putative pore-lining amino acid side-chains (as space-filling models in red) [4, 5, 34]. As reviewed recently [35], cytoplasmic anions access the central transmembrane pore via a lateral portal located on the intracellular side of the membrane (black arrow). C Cartoon model of the pore, based on earlier reviews [4, 35], illustrating the location of important positively charged pore-lining side-chains. Cytoplasmic anions are attracted to the lateral portal by several positive charges (K190, R248, R303, K370), before passing into the wide inner vestibule, where they interact with the positive charge associated with K95. Beyond this lies the narrow region of the pore, which is uncharged. The outer vestibule is also decorated with a number of positive charges, viz. R104, R334, K335. D Location of these positively charged side-chains within the MSDs. Same structure as in A, but with the NBDs removed for clarity. Same colour scheme as in C. Dotted lines indicate the approximate extent of the membrane in A–D. E, F Relative location of amino acids mutated in the present study, in a close view of the TMs from A. E Viewed from the side, as in A–D; F viewed from the extracellular side of the membrane. The distances between these side-chains (β carbon–β carbon distance) as measured from these structures are: K95-R134 6.7 Å; K95-S1141 12.1 Å; R134-S1141 11.6 Å
The inner vestibule of the pore plays an important role in the control of anion binding and anion conductance, and K95 is essential in this role. This region is thought to contain the binding site for a wide range of large blocking anions that enter the pore from its cytoplasmic end [16]. This same site has been proposed to be involved in the binding of permeant anions [17]. Mutagenesis of K95 has a charge-dependent effect on the binding affinity of many pore-blocking anions [18, 19] including permeant anions [17, 20], consistent with an electrostatic interaction between this residue and anions inside the pore. Mutations that remove this positive charge also greatly reduce single-channel Cl− conductance [15, 19, 20], suggesting that Cl− ion binding within the inner vestibule is important for normal Cl− permeation [17]. The charge-neutralizing mutation K95Q also disrupts the normal anion conductance selectivity pattern [21], suggesting that the relative affinity of different anions for binding within the inner vestibule controls the relative duration of their transit through the pore.
Interestingly, the functional role of K95 can be “transplanted” to other sites lining the inner vestibule, by co-mutagenesis to remove the endogenous positive charge at K95 and to introduce an ectopic positive charge at other sites [e.g. I344K or V345K (TM6), S1141K (TM12)] [19, 22]. Such “charge-transplant” co-mutations have been shown to restore the single-channel conductance [19, 22], anion binding [17, 19, 22, 23] and anion conductance selectivity [21] properties of the wild-type pore. These findings suggest that it is the presence of a pore-lining positive charge, rather than its exact position at K95 in TM1, that is important for these functional properties of the inner vestibule.
The availability of atomic structures of CFTR also allows in silico investigation of the mechanism of anion interaction with the CFTR pore, for example using molecular dynamics (MD) simulations [24]. One recent MD investigation of phosphorylated, ATP-bound zebrafish CFTR suggested that Cl− ions inside the pore most frequently interact with two positively charged residues within the inner vestibule (human residue numbering), K95 and R134 [25]. This might seem surprising since R134 is located in TM2, not normally included in the list of pore-lining TMs [4–6]. Nevertheless, analysis of the apparent solvent-accessibility of side-chains in the dephosphorylated, ATP-free structure of human CFTR [2] carried out by Hwang et al. [5] suggested that R134 does indeed make a “tiny” contribution to the inner vestibule of the pore. This recent review described R134 as a “yet-to-be studied residue” [5]. In fact, in 2006 this laboratory published data showing that the R134Q mutation alters the shape of the macroscopic current–voltage relationship, and reduces the single-channel conductance below measurable levels, suggesting a severe disruption of the Cl− permeation mechanism [10]. Here, we re-visit the functional role of R134, and show that it plays a remarkably similar role to that of its near-neighbour in the pore inner vestibule, K95 (Fig. 1E,F).
Materials and methods
Experiments were carried out on baby hamster kidney (BHK) cells transiently transfected with CFTR, as described previously [19]. In this study, we have used two different human CFTR variants as backgrounds for further mutagenesis: wild-type CFTR and “cys-less” CFTR in which all cysteines have been removed by mutagenesis [26] and that also includes a mutation in the first nucleotide binding domain (NBD) (V510A) to increase protein expression in the cell membrane [27]. Additional mutations were introduced into these two backgrounds using the QuikChange site-directed mutagenesis system (Agilent Technologies, Santa Clara, CA, USA) and verified by DNA sequencing. All macroscopic current recordings in a wild-type background (Figs. 5, 6, 7) were carried out on very high open probability channels bearing the E1371Q mutation that effectively isolates effects on Cl− permeation [17, 19, 21].
Fig. 5.
Block of Cl− permeation by intracellular Au(CN)2−. A Example I–V relationships for wild-type, R134Q, and R134Q/S1141K, recorded before (control) and after sequential addition of 100 µM and 1 mM Au(CN)2− to the intracellular solution. B, C Mean fraction of control current remaining after addition of different concentrations of Au(CN)2− at membrane potentials of − 100 mV (B) and − 40 mV (C) for these same channel variants (black circle wild-type; white circle R134Q; black down-pointing triangle R134Q/S1141K). Data have been fitted as described in “Materials and methods”. Mean of data from 3–6 patches. D Mean KD values for different channel variants obtained from such fits as a function of membrane potential (black circle wild-type; white circle R134Q; black down-pointing triangle R134Q/S1141K; white up-pointing triangle S1141K). Mean of data from 6–8 patches
Fig. 6.
Block of Cl− permeation by intracellular NPPB. A Example I–V relationships for wild-type, R134Q, and R134Q/S1141K, recorded before (control) and after addition of 50 µM NPPB to the intracellular solution. B Mean fraction of control current remaining after addition of this concentration of NPPB, at a membrane potential of − 100 mV. Asterisks indicate a significant difference from wild-type (*p < 0.002; **p < 10–6). Mean of data from 5 patches
Fig. 7.
Relative conductance of different intracellular anions in CFTR. A Example I–V relationships recorded from eight different inside-out patches containing wild-type, R134Q, R134Q/S1141K, or S1141K as indicated. In each case, currents were recorded during perfusion with the named anion in the intracellular (bath) solution. Note that changes in current amplitude following perfusion with different anions were fully reversible on returning to Cl−-containing perfusate (not shown). B Mean relative anion conductance (GX/GCl) for the different anions indicated, estimated from changes in the slope of macroscopic I–V relationships such as those shown in A, as described in “Materials and methods”. Mean of data from 3–7 patches
Western blotting for CFTR was carried out as described previously [28] with minor modifications. Cells were harvested 48 h after transfection, at ~ 80% confluency. Cells were washed twice with ice-cold PBS, scraped, then transferred to a 1.5-mL tube where they were resuspended in RIPA buffer supplemented with protease inhibitors. Cells were lysed on ice for 30 min. by repeatedly vortexing and passing through a pipette tip every 5 min. The lysate was then centrifuged (15,000g, 30 min., 4 °C) and the supernatant poured into a new tube. Protein concentration was determined using the Bradford protein assay method. Depending on the transfection, 5–30 µg of protein was pre-incubated with loading buffer for 30 min. at 37 °C then loaded onto a 7.5% acrylamide-SDS gel. The protein was then transferred to a BioTrace™ nitrocellulose membrane (Pall Corporation, Pensacola, FL, USA). Immunoblotting for CFTR was performed by incubating the membrane overnight at 4 °C with monoclonal mouse anti-CFTR antibody (M3A7) at 1:2000 dilution. After washing, the membrane was incubated for 1 h at room temperature with secondary antibody (horseradish peroxidase-conjugated goat anti-mouse, Abcam Inc., Toronto, ON, Canada) at 1:20,000 dilution. Detection was carried out using the Clarity™ Western ECL Substrate kit (Bio-Rad Laboratories, Mississauga, ON, Canada) following the manufacturer’s instructions. Relative expression of mature, complex glycosylated CFTR protein (“Band C”, ~ 175 kDa) and immature, core glycosylated protein (“Band B”, ~ 150 kDa) was assessed by densitometry analysis of scanned Western blot images using ImageJ (Version 1.48, National Institutes of Health, Bethesda, MD, USA).
Functional characterization of CFTR channels was carried out using single-channel and macroscopic patch-clamp recordings from inside-out membrane patches, as described in detail previously [19]. For most experiments, the intracellular (bath) solution contained (mM): 150 NaCl, 2 MgCl2, 10 N-tris[hydroxymethyl]methyl-2-aminoethanesulfonate (TES), pH 7.4. For experiments quantifying the relative conductance of different anions (Fig. 7), the intracellular solution contained (mM): 154 NaX (where X is the test anion, namely Cl−, Br−, I−, NO3−, SCN−, or formate), 2 Mg(OH)2, 10 TES, pH 7.4. For experiments on cys-less CFTR (Fig. 3), the extracellular (pipette) solution contained (mM): 150 NaCl, 2 MgCl2, 10 TES, pH 7.4; for all other experiments, 150 Na gluconate was substituted for NaCl to generate an outwardly directed [Cl−] gradient. Current traces were filtered at 150 Hz (for macroscopic currents) or 50 Hz (for single-channel currents) using an eight-pole Bessel filter, digitized at 1 kHz, and analysed using pCLAMP10 software (Molecular Devices, Sunnyvale, CA, USA). Measurement of single-channel and macroscopic current amplitudes, and construction of leak-subtracted current–voltage (I–V) relationships were carried out as described previously [19]. Membrane voltages were corrected for liquid junction potentials calculated using pCLAMP software.
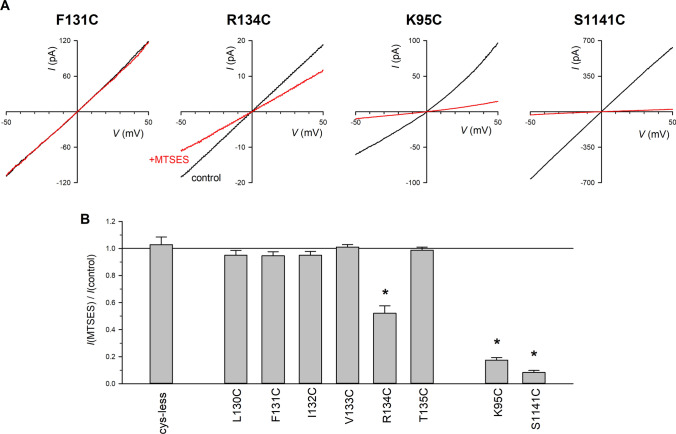
Fig. 3.
Modification of pore-lining cysteine side-chains by cytoplasmically applied MTSES. A Example I–V relationships for F131C, R134C, K95C and S1141C recorded before (black) and after (red) addition of MTSES (200 µM) to the intracellular solution. B Mean effect of MTSES on macroscopic current amplitude. Asterisk indicates a significant change in macroscopic current amplitude (p < 0.05). Mean of data from 3–7 patches
To monitor modification of cysteine side-chains by negatively charged [2-sulfonatoethyl] methanethiosulfonate (MTSES), macroscopic current amplitudes were measured during brief voltage ramps (to ± 50 mV) from a holding potential of 0 mV applied every 6 s [29]. MTSES was added to the cytoplasmic side of membrane patches at a high concentration (200 μM) that has no effect on Cys-less CFTR [27].
Block of Cl− permeation by low concentrations of Au(CN)2− and 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) was investigated by direct application of these substances to the intracellular face of inside-out patches from high-concentration stocks made up in normal intracellular solution. For Au(CN)2−, concentration–inhibition relationships were fitted by the equation:
where KD is the apparent blocker dissociation constant.
Relative conductance of different anions was estimated by perfusion of the intracellular face of inside-out patches containing constitutively active E1371Q-CFTR channels with solutions containing different anions (see above), as described previously [21]. Relative conductance was quantified by measuring the slope conductance of the relatively linear part of the current–voltage relationship between − 100 and 0 mV.
Experiments were carried out at room temperature, 21–24 °C. Values are presented as mean ± SEM. For graphical presentation of mean values, error bars represent SEM, and where no error bars are visible SEM is smaller than the size of the symbol. Tests of significance were carried out using Student’s two-tailed t test, with p < 0.05 being considered statistically significant. Unless otherwise stated, chemicals were from Sigma-Aldrich (Oakville, ON, Canada) except for KAu(CN)2 (Strem Chemicals, Newburyport, MA, USA).
Results
Membrane expression of R134 mutants
Previously we showed that expression of R134Q, but not R134E, in BHK cells led to the appearance of macroscopic CFTR Cl− currents in excised membrane patches [10]. This might suggest that the charge-reversing R134E mutant results in failure of CFTR to traffic to the cell membrane, perhaps consistent with a severe processing defect. However, Western blot analysis showed that both R134Q and R134E were able to generate mature, complex glycosylated (Band C) protein, although for R134E the relative abundance of this mature form was less than for wild-type CFTR (Fig. 2). This suggests that mutagenesis of R134 has only moderate effects on CFTR protein folding and trafficking to the membrane, and raises the possibility that the lack of functional expression we previously reported for R134E [10] reflects this mutant having negligible channel function.
Fig. 2.

Protein expression of different CFTR variants expressed in BHK cells. A Representative Western blot for CFTR using protein from BHK cells transfected with the named CFTR variant. The locations of Band B (~ 150 kDa) and Band C (~ 175 kDa) are indicated to the left. Cys-less was used as a control CFTR variant that fails to generate significant amounts of Band C protein in BHK cells cultured at 37 °C [27]. B Mean abundance of Band C protein (as a percentage of total) as determined by densitometric analysis. Asterisks indicate a significant difference from wild-type (p < 0.00001). Mean of data from five independent transfections
Accessibility of the R134 side-chain
Previously we used internal application of MTSES to identify introduced cysteine side-chains lining the inner vestibule of the pore in TM1 [14], TM6 [30], TM11 [31] and TM12 [32]. Using a similar approach, we found that application of MTSES caused a consistent decrease in macroscopic current amplitude in R134C (Fig. 3). However, introduction of cysteine at a number of nearby sites in TM2 did not result in macroscopic current sensitivity to MTSES (Fig. 3). As positive controls, we confirmed that nearby pore-lining cysteines K95C and S1141C (Fig. 1E, F) were potently inhibited by MTSES (Fig. 3), as previously reported by ourselves [14, 32] and others [15, 33].
Role of R134’s positive charge in channel functional properties
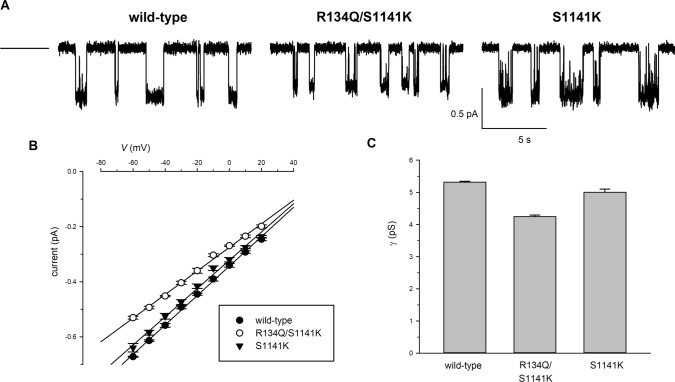
Previously we indicated that the single-channel conductance of R134Q was below the resolution of patch-clamp recording [10]. Consistent with this, we found that application of protein kinase A catalytic subunit and ATP to membrane patches excised from R134Q-expressing BHK cells routinely led to an increase in current and an increase in current noise, however, we were unable to distinguish single-channel opening and closing events in these patches (data not shown). Conservatively, we would estimate that the conductance of R134Q must be < 10% that of wild-type CFTR. In contrast to this, single-channel events were observed for R134Q/S1141K (Fig. 4), and the conductance of these channels was 80 ± 1% of wild-type conductance (n = 5). As reported previously [17, 19], the S1141K mutant itself had almost no effect on conductance (94 ± 2% of wild-type conductance, n = 5) (Fig. 4). Comparing R134Q/S1141K with S1141K (Fig. 4C) suggests that, in the presence of an ectopic positive charge at position 1141, neutralizing the native positive charge at position R134 caused only a 15 ± 2% reduction in conductance (n = 5).
Fig. 4.
Single channel current amplitude in different CFTR variants. A Example single-channel currents carried by the named channel variants at a membrane potential of − 50 mV. The closed state is indicated by the line to the far left. B Mean single-channel current–voltage relationships for wild-type (black circle), R134Q/S1141K (white circle), and S1141K (black down-pointing triangle). C Mean single-channel conductance (γ) measured from the slope of the current–voltage relationship from individual patches. Mean of data from 5 patches for each channel variant
The R134Q mutation was also associated with a decrease in the apparent binding affinity of the permeant anion Au(CN)2− (Fig. 5). As described recently [17], Au(CN)2− block of wild-type CFTR showed a complex voltage-dependence, with the calculated KD showing a minimum at around − 40 mV (Fig. 5C,D). At a membrane potential of − 100 mV, mean KD was increased from 54.3 ± 3.3 µM in wild-type (n = 8) to 2308 ± 168 µM in R134Q (n = 8), an increase of over 40-fold (p < 10–5); while at − 40 mV, mean KD increased from 40.0 ± 3.0 µM (n = 8) to 2478 ± 307 µM (n = 8), an increase of over 60-fold (p < 10–5) (Fig. 5D). These increases in KD were almost completely reversed in the R134Q/S1141K double mutant (Fig. 5), which showed mean KDs of 168 ± 20 µM at − 100 mV and 126 ± 15 µM at − 40 mV (n = 8) (Fig. 5D). As described recently [17], S1141K itself was associated with a slight weakening of Au(CN)2− block under these conditions (mean KD 87.1 ± 8.1 µM at − 100 mV (p < 0.002) and 63.6 ± 7.1 µM at − 40 mV (p < 0.01) (n = 6) (Fig. 5D).
Removal of the positive charge at R134 also greatly weakened the blocking effects of the organic blocking anion NPPB applied to the cytoplasmic face of inside-out patches (Fig. 6). Introduction of an ectopic positive charge (in R134Q/S1141K) not only restored NPPB block, but in fact led to stronger blocking effects than observed in wild-type (Fig. 6). The S1141K mutation itself has previously been shown to result in very strong NPPB block [19].
Finally, R134Q disrupted the normal anion conductance selectivity of macroscopic CFTR currents (Fig. 7). As described previously [21], in wild-type CFTR the conductance selectivity sequence (GX/GCl) is Cl− > NO3− > Br− > formate > SCN− > I− (Fig. 7). This same selectivity sequence was observed in R134Q/S1141K and in S1141K, although these mutants were associated with slight increases in GX/GCl for some anions (Fig. 7). In contrast, R134Q was associated with a relative loss of conductance selectivity and an overall GX/GCl sequence of SCN− ≥ NO3− ≥ Br− ≥ Cl− > I− > formate (Fig. 7).
Discussion
The positively charged side-chain of K95 in TM1 plays a key role in determining anion binding and anion conductance within the inner vestibule of the CFTR pore (see “Introduction”). The present results suggest that the positively charged side-chain of R134, in TM2, plays an equally important, functionally analogous role. Thus, removal of this positive charge (as in R134Q) results in a dramatic reduction in single-channel conductance (Fig. 4) that—while not possible to quantify—appears to be even more severe than that seen in K95Q [19, 20, 22]. The R134Q mutation also greatly weakens the binding of anions within the inner vestibule—both the high-affinity permeant anion Au(CN)2− (Fig. 5) and the large organic anion NPPB (Fig. 6). These effects are very similar to the well-known effect of mutations at K95 on binding of permeant [17, 20] and large blocking anions [18, 19]. Finally, R134Q disrupts the normal anion conductance selectivity pattern, resulting in a channel in which most permeant anions show a rather similar conductance (Fig. 7), suggesting a diminution in the ability of the channel to discriminate between different anions. This effect on conductance selectivity is, in fact, remarkably similar to that reported previously for K95Q [21], which was suggested to reflect the importance of permeant anion binding to a site involving K95 in determining overall anion residence time within the channel. In aggregate, the results presented here suggest that the presence of both these positively charged side-chains (K95 and R134) is required for the normal anion binding, anion conductance, and conductance selectivity properties of the channel.
Importantly for understanding the roles of these positive charges, we find that both R134 and K95 side-chains line the inner vestibule of the pore (Fig. 3). Unfortunately, R134C gave only extremely small currents when expressed in BHK cells, possibly due to a combination of low conductance and poor membrane expression in the trafficking-defective cys-less background. Nevertheless, these small currents, where they could be quantified, were consistently reduced in amplitude following exposure to MTSES (Fig. 3). Similarly, we found that exposure to positively charged [2-(trimethylammonium)ethyl] MTS (MTSET) (2 mM) caused a small, inconsistent reduction in current amplitude in R134C (data not shown), suggesting a relatively charge-independent effect of modification. However, nearby TM2 residues—specifically those on the extracellular side of R134 and so predicted to be located further into the inner vestibule—did not appear to be pore-lining, based on a lack of effect of MTSES on cysteines introduced into these positions (Fig. 3). This apparently minor contribution to the inner vestibule of the pore may reflect TM2’s position away from the central axis of the pore and close to the lipid membrane (Fig. 1F). In silico analyses of CFTR structures have led to the suggestion that the R134 side-chain might be accessible to the pore [5, 25]. Furthermore, those mutations at R134 that we have studied that remove (R134Q) or reverse (R134E) the charge on the side-chain at position 134 do not appear to have major impacts on CFTR protein folding or trafficking (Fig. 2), suggesting that this positive charge is not essential for the CFTR protein to obtain a relatively normal conformation.
Also importantly, all of the functional effects we describe in R134Q can be reversed by introduction of an ectopic positive charge nearby in the inner vestibule, as demonstrated by the wild type-like functional properties of R134Q/S1141K. Thus, in striking contrast to R134Q itself, R134Q/S1141K shows a single-channel conductance only slightly less than that of wild-type CFTR (Fig. 4); shows only very slightly weakened binding of permeant Au(CN)2− ions (Fig. 5), and slightly stronger binding of organic NPPB blocking anions (Fig. 6) compared to wild-type; and shows very similar anion conductance selectivity properties to wild-type (Fig. 7). These results closely resemble the “functional rescue” of each of these properties seen when the positive charge of K95 is “transplanted” either to S1141 in TM12 [17, 19] or to other, nearby pore-lining sites in the inner vestibule [17, 21–23]. These findings, which appear very similar for R134 (present results) as for K95, strongly suggest that it is the overall number of positively charged pore-lining side-chains—or overall positive charge density—that is important for controlling these functional properties of the inner vestibule of the pore. Conversely, the fact that these positive charges are located in TM1 (K95) and TM2 (R134)—rather than, for example, TM12 (S1141, present results and [17, 19]) or TM6 [17, 21–23] appears to be of relatively minor importance, suggesting that these positive charges do not make strongly location-dependent interactions, for example with residues in other TM helices.
Positively charged amino acid side-chains make important functional contributions throughout the CFTR anion permeation pathway (see “Introduction” and Fig. 1). However, we have previously assumed that K95 was the only functionally important positive charge located within the inner vestibule of the pore [4]. The finding that the R134 side-chain lines the pore and plays a very similar functional role as K95 therefore prompts some re-evaluation of our ideas of inner vestibule function. Clearly both of these positive charges are required for normal interactions with permeating anions, since neutralization of either (e.g. K95Q, R134Q) greatly weakens anion binding and greatly reduces conductance. This suggests that the presence of two relatively closely situated pore-lining positive charges (Fig. 1E,F) is necessary to attract anions to the inner vestibule and/or to constitute a functionally important anion binding site in this region of the pore. Anion binding to this putative site is then presumably important in determining anion residency time in the pore and thus anion conductance as discussed previously [21]. Perhaps most surprisingly, introduction of an additional positive charge—for example, in S1141K and also at other sites in the inner vestibule (e.g. I344K, V345K)—does not lead to a further increase in permeant anion binding affinity [17] (Fig. 5) or a further increase in conductance [17, 19, 22] (Fig. 4), but instead greatly increases the apparent binding affinity of divalent anions [17, 19, 22, 23]. This suggests that the positive charge density within the inner vestibule of the wild-type CFTR pore is well optimized for the binding of monovalent, rather than divalent anions. However, we may previously have underestimated the number of positively charged pore-lining side-chains required to achieve this optimal charge density.
Abbreviations
- BHK
Baby hamster kidney
- CF
Cystic fibrosis
- CFTR
CF transmembrane conductance regulator
- MD
Molecular dynamics
- MSD
Membrane-spanning domain
- MTSES
[2-Sulfonatoethyl] methanethiosulfonate
- MTSET
[2-(Trimethylammonium)ethyl] methanethiosulfonate
- NBD
Nucleotide binding domain
- NPPB
5-Nitro-2-(3-phenylpropylamino)benzoic acid
- TES
N-Tris[hydroxymethyl]methyl-2-aminoethanesulfonate
- TM
Transmembrane helix
- TME
Transmembrane helix extension
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by PL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (RGPIN/05124–2017).
Availability of data and material
All data generated or analysed during this study are included in this published article. The materials used in this study are available from the corresponding author, upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Castellani C, Assael BM. Cystic fibrosis: a clinical view. Cell Mol Life Sci. 2017;74:129–140. doi: 10.1007/s00018-016-2393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu F, Zhang Z, Csanády L, Gadsby DC, Chen J. Molecular structure of the human CFTR ion channel. Cell. 2017;169:85–95. doi: 10.1016/j.cell.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z, Liu F, Chen J. Molecular structure of the ATP-bound, phosphorylated human CFTR. Proc Natl Acad Sci USA. 2018;115:12757–12762. doi: 10.1073/pnas.1815287115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linsdell P. Architecture and functional properties of the CFTR channel pore. Cell Mol Life Sci. 2017;74:67–83. doi: 10.1007/s00018-016-2389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang T-C, Yeh J-T, Zhang J, Yu Y-C, Yeh H-I, Destefano S. Structural mechanisms of CFTR function and dysfunction. J Gen Physiol. 2018;150:539–570. doi: 10.1085/jgp.201711946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csanády L, Vergani P, Gadsby DC. Structure, gating, and regulation of the CFTR anion channel. Physiol Rev. 2019;99:707–738. doi: 10.1152/physrev.00007.2018. [DOI] [PubMed] [Google Scholar]
- 7.Smith SS, Liu X, Zhang Z-R, Sun F, Kriewall TE, McCarty NA, Dawson DC. CFTR: covalent and noncovalent modification suggests a role for fixed charges in anion conduction. J Gen Physiol. 2001;118:407–431. doi: 10.1085/jgp.118.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong X, Linsdell P. Molecular determinants and role of an anion binding site in the external mouth of the CFTR chloride channel pore. J Physiol. 2003;549:387–397. doi: 10.1113/jphysiol.2002.038232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J-J, Fatehi M, Linsdell P. Identification of positive charges situated at the outer mouth of the CFTR chloride channel pore. Pflügers Arch. 2008;457:351–360. doi: 10.1007/s00424-008-0521-6. [DOI] [PubMed] [Google Scholar]
- 10.St. Aubin CN, Linsdell P, (2006) Positive charges at the intracellular mouth of the pore regulate anion conduction in the CFTR chloride channel. J Gen Physiol 128:535–545 [DOI] [PMC free article] [PubMed]
- 11.El Hiani Y, Linsdell P. Functional architecture of the cytoplasmic entrance to the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Biol Chem. 2015;290:15855–15865. doi: 10.1074/jbc.M115.656181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M-S, Cowley EA, El Hiani Y, Linsdell P. Functional organization of cytoplasmic portals controlling access to the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel pore. J Biol Chem. 2018;293:5649–5658. doi: 10.1074/jbc.RA117.001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck EJ, Yang Y, Yaemsiri S, Raghuram V. Conformational changes in a pore-lining helix coupled to cystic fibrosis transmembrane conductance regulator channel gating. J Biol Chem. 2008;283:4957–4966. doi: 10.1074/jbc.M702235200. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, El Hiani Y, Linsdell P. Alignment of transmembrane regions in the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Gen Physiol. 2011;138:165–178. doi: 10.1085/jgp.201110605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X, Bai Y, Hwang T-C. Cysteine scanning of CFTR’s first transmembrane segment reveals its plausible roles in gating and permeation. Biophys J. 2013;104:786–797. doi: 10.1016/j.bpj.2012.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linsdell P. Cystic fibrosis transmembrane conductance regulator chloride channel blockers: pharmacological, biophysical and physiological relevance. World J Biol Chem. 2014;5:26–39. doi: 10.4331/wjbc.v5.i1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linsdell P. On the relationship between anion binding and chloride conductance in the CFTR anion channel. Biochim Biophys Acta. 2021;1863:183558. doi: 10.1016/j.bbamem.2021.183558. [DOI] [PubMed] [Google Scholar]
- 18.Linsdell P. Location of a common inhibitor binding site in the cytoplasmic vestibule of the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Biol Chem. 2005;280:8945–8950. doi: 10.1074/jbc.M414354200. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J-J, Li M-S, Qi J, Linsdell P. Regulation of conductance by the number of fixed positive charges in the intracellular vestibule of the CFTR chloride channel pore. J Gen Physiol. 2010;135:229–245. doi: 10.1085/jgp.200910327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge N, Muise CN, Gong X, Linsdell P. Direct comparison of the functional roles played by different transmembrane regions in the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Biol Chem. 2004;279:55283–55289. doi: 10.1074/jbc.M411935200. [DOI] [PubMed] [Google Scholar]
- 21.Linsdell P. Anion conductance selectivity mechanism of the CFTR chloride channel. Biochim Biophys Acta. 2016;1858:740–747. doi: 10.1016/j.bbamem.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 22.El Hiani P, Linsdell P. Tuning of CFTR chloride channel function by location of positive charges within the pore. Biophys J. 2012;103:1719–1726. doi: 10.1016/j.bpj.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linsdell P. Interactions between permeant and blocking anions inside the CFTR chloride channel pore. Biochim Biophys Acta. 2015;1848:1573–1590. doi: 10.1016/j.bbamem.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Callebaut I, Hoffmann B, Lehn P, Mornon J-P. Molecular modelling and molecular dynamics of CFTR. Cell Mol Life Sci. 2017;74:3–22. doi: 10.1007/s00018-016-2385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farkas B, Tordai H, Padányi R, Tordai A, Gera J, Paragi G, Hegedűs T. Discovering the chloride pathway in the CFTR channel. Cell Mol Life Sci. 2020;77:765–778. doi: 10.1007/s00018-019-03211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mense M, Vergani P, White DM, Altberg G, Nairn AC, Gadsby DC. In vivo phosphorylation of CFTR promotes formation of a nucleotide-binding domain heterodimer. EMBO J. 2006;25:4728–4739. doi: 10.1038/sj.emboj.7601373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M-S, Demsey AFA, Qi J, Linsdell P. Cysteine-independent inhibition of the CFTR chloride channel by the cysteine-reactive reagent sodium (2-sulphonatoethyl) methanethiosulphonate. Br J Pharmacol. 2009;157:1065–1071. doi: 10.1111/j.1476-5381.2009.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Hiani Y, Linsdell P. Role of the juxtamembrane region of cytoplasmic loop three in gating and conductance of the CFTR chloride channel. Biochemistry. 2012;51:3971–3981. doi: 10.1021/bi300065z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El Hiani Y, Negoda A, Linsdell P. Cytoplasmic pathway followed by chloride ions to enter the CFTR channel pore. Cell Mol Life Sci. 2016;73:1917–1925. doi: 10.1007/s00018-015-2113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Hiani Y, Linsdell P. Changes in accessibility of cytoplasmic substances to the pore associated with activation of the cystic fibrosis transmembrane conductance regulator chloride channel. J Biol Chem. 2010;285:32126–32140. doi: 10.1074/jbc.M110.113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, El Hiani Y, Rubaiy HN, Linsdell P. Relative contribution of different transmembrane segments to the CFTR chloride channel pore. Pflügers Arch. 2014;466:477–490. doi: 10.1007/s00424-013-1317-x. [DOI] [PubMed] [Google Scholar]
- 32.Qian F, El Hiani Y, Linsdell P. Functional arrangement of the 12th transmembrane region in the CFTR chloride channel based on functional investigation of a cysteine-less variant. Pflügers Arch. 2011;462:559–571. doi: 10.1007/s00424-011-0998-2. [DOI] [PubMed] [Google Scholar]
- 33.Bai Y, Li M, Hwang T-C. Structural basis for the channel function of a degraded ABC transporter, CFTR (ABCC7) J Gen Physiol. 2011;138:495–507. doi: 10.1085/jgp.201110705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negoda A, Hogan MS, Cowley EA, Linsdell P. Contribution of the eighth transmembrane segment to the function of the CFTR chloride channel pore. Cell Mol Life Sci. 2019;76:2411–2423. doi: 10.1007/s00018-019-03043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linsdell P. Cystic fibrosis transmembrane conductance regulator (CFTR): Making an ion channel out of an active transporter structure. Channels. 2018;12:284–290. doi: 10.1080/19336950.2018.1502585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article. The materials used in this study are available from the corresponding author, upon reasonable request.