Abstract
Inflammasomes are large immune multiprotein complexes that tightly regulate the production of the pro-inflammatory cytokines, being dependent on cell regulatory volume mechanisms. Aquaporins (AQPs) are protein channels that facilitate the transport of water and glycerol (aquaglyceroporins) through membranes, essential for cell volume regulation. Although these membrane proteins are highly expressed in monocytes and macrophages, their role in the inflammatory process is still unclear. Here, we investigated the role of aquaglyceroporin AQP3 in NLRP3-inflammasome activation by complementary approaches based either on shRNA silencing or on AQP3 selective inhibition. The latter has been achieved using a reported potent gold-based inhibitor, Auphen. AQP3 inhibition or silencing partially blocked LPS-priming and decreased production of IL-6, proIL-1β, and TNF-α, suggesting the possible involvement of AQP3 in macrophage priming by Toll-like receptor 4 engagement. Moreover, AQP3-dependent cell reswelling increased IL-1β release through caspase-1 activation. NLRP3-inflammasome activation induced by reswelling, nigericin, and ATP was also blocked when AQP3 was inhibited or silenced. Altogether, these data point towards AQPs as potential players in the setting of the inflammatory response.
Keywords: Aquaglyceroporin, Inflammasome, Inflammation, Interleukin-1, Macrophages, Water and glycerol permeability
Introduction
Inflammasomes are large immune multiprotein complexes that tightly regulate the production of the pro-inflammatory cytokines interleukin (IL)-1β and IL-18. Several studies have demonstrated that specifically the NLRP3 inflammasome can be involved in metabolic homeostasis dysregulation, such as type-2 diabetes, fatty liver disease, and gout [1–5]. The NLRP3 inflammasome is a major component of the innate immune system and is upregulated and activated in response to different pathogen-associated molecule patterns (PAMPs) [6], and/or danger-associated molecule patterns (DAMPs) [7, 8] that are host-derived signals of cellular stress or injury.
The NLRP3 inflammasome is constituted by a cytosolic sensor from the nucleotide-binding domain and leucine-rich repeat-containing receptor family, the protein NLRP3; an adaptor protein, apoptosis-associated speck-like protein containing a caspase activating recruitment domain (ASC); and an effector molecule, the cysteine protease pro-caspase-1. Two signals finely regulate the activation of NLRP3 inflammasome in macrophages. The first signal, cell priming, triggers the nuclear factor NF-kB enhancing the expression of the inflammasome components [9] and the synthesis of different pro-inflammatory cytokines as IL-6 or proIL-1β. This is initiated by the ligation of a pathogen recognition receptor (PRR) such as the Toll-like receptor 4 (TLR4) with its respective ligand, e.g., lipopolysaccharide (LPS). The second signal, the inflammasome activation, promotes the assembly of the inflammasome components. During activation, pro-caspase-1 is recruited to the inflammasome, generally facilitated by ASC, and processes proIL-1β and proIL-18 to their mature and active forms, IL-1β and IL-18. Caspase-1 also cleaves the cytosolic protein gasdermin D (GSDMD) to promote plasma membrane pore formation, leading to the release of cytokines and pyroptotic cell death [10]. The exact mechanisms enrolled with the activation of inflammasomes are not fully understood; however, evidences involving the generation reactive oxygen species (ROS), lysosomal damage, and intracellular potassium (K+) efflux have been reported [11–14]. More recently, cell reswelling has also been pointed as an event that precedes NLRP3 activation and consequent IL-1β secretion [11, 15, 16].
The regulatory volume mechanism described for NLRP3-inflammasome activation is not exclusive for macrophages; in fact, the ability to adjust and adapt its volume is vital for survival and proper functioning to any animal cell. Subsequently, proteins involved in the regulation of cell volume may have a key role in the onset of inflammation. Recently, aquaporins (AQPs) were proposed to be involved in the setting of inflammasome activation [11, 15–18]. AQPs are a family of highly conserved transmembrane protein channels that facilitate the transport of water and small uncharged solutes across cell membranes [19, 20]. So far, 13 human AQPs, AQP0–AQP12, have been described and were divided into three subfamilies based on their primary structure and pore selectivity. Orthodox AQPs are considered strict water channels (AQP0, 1, 2, 4, 5, 6, 8, although AQP6 and AQP8 also transport anions [21] and ammonia [22]; aquaglyceroporins (AQP3, 7, 9, 10) allow the transport of water and small uncharged molecules, such as glycerol and urea; and unorthodox AQPs (AQP11, 12) are subcellular isoforms whose selectivity and function still need clarification [23, 24]). Additionally, a few isoforms, including AQP3, also facilitate hydrogen peroxide membrane permeation and are called peroxiporins [25–28]. Aquaporins are important for numerous cellular functions, such as epithelial fluid transport, cell proliferation, brain water homeostasis, and adipocyte metabolism, and are considered potential drug targets for human diseases [29, 30]. Furthermore, by interacting with the cytoskeleton and signaling cascades, AQPs can influence cell morphology, volume, movement, and migration [27, 32]. Several AQPs are expressed in cells of both the innate and adaptive immunity [33, 34], while their dysregulation was demonstrated in various human diseases [29, 31, 35, 36]. AQPs seem to be involved in the phagocytosis, and in immune cell activation and migration [17, 37–40]. Furthermore, general AQPs’ blockage reduces IL-1β release from macrophages activated through a variety of NLRP3 stimuli [15]. Thus, inhibition of AQPs in macrophages specifically during the regulatory volume decrease process is sufficient to limit IL-1β release through the NLRP3-inflammasome axis [11].
In this study, we activated the NLRP3 inflammasome in monocytes and macrophages to investigate aquaporins expression in inflammation. AQPs contribution to cell volume changes was examined by measuring cell membrane water and glycerol permeability. Moreover, we took advantage of the selective AQP3 inhibitor Auphen [Au(phen)Cl2)Cl] (phen = 1,10-phenanthroline) [41–44] to study the role of AQP3, the aquaglyceroporin found most expressed in THP-1 monocytic cells, in the production of IL-6 and activation of NLRP3 inflammasome.
Results
AQP3 and AQP9 are the most abundant aquaglyceroporins in human monocytes and macrophages.
Initially, an expression screening to validate the presence of different AQPs in human primary monocytes and THP-1 cells was conducted, showing that AQP9 and AQP3 are the most abundant aquaglyceroporins in these cell types (Fig. 1a, b). In addition, expressions of AQP1, AQP6, AQP7, AQP8, AQP11, and AQP12 were also detected in these cells but in lower amounts (Fig. 1a, b). LPS stimulation increased AQP6 and AQP9 in primary human monocytes as well as AQP1, AQP3, AQP8, AQP9, and AQP11 in PMA-treated THP-1 cells (Fig. 1c, d) and, as expected, LPS also upregulated the expression of different pro-inflammatory cytokines (Fig. 1e). However, at the protein level, AQP3 expression was not induced by LPS in PMA-treated THP-1 cells (Fig. 1f). AQP3 expression was silenced around 50% using lentiviral shRNA (Fig. 1g) and LPS stimulation of AQP3-silenced cells resulted in a weaker upregulation of AQP3 gene expression (Fig. 1h).
Fig. 1.
Aquaporins and inflammatory markers expression and their modulation by LPS in monocytes and THP-1 cells. a Relative expression of AQPs in resting monocytes. b Relative expression of AQPs in resting THP-1 cells. c Expression of AQPs in LPS-treated monocytes normalized to non-LPS (dotted line) for each AQP. d Expression of AQPs in PMA and PMA + LPS-treated THP-1 cells normalized to non-PMA-treated cells (dotted line) for each AQP. e Expression of IL-6, TNFα, and IL-1β in LPS-treated monocytes normalized to non-LPS (dotted line). Gene expression values are relative to HPRT-1. f Representative blots and relative AQP3 protein expression in non-PMA, PMA, and PMA + LPS-treated THP-1 cells in relation to α-tubulin. g Expression of the AQP3 silencing in THP-1 cells by qPCR normalized to wild-type cells (dotted line). h Expression of AQP3 transfected THP-1 cells treated PMA or PMA + LPS. Data represent mean ± SD of three independent experiments. *P < 0.05, **P < 0.01; ***P < 0.001; *LPS vs resting cells
AQP3 is a functional glycerol channel in THP-1
To evaluate AQP3 channel activity in THP-1, we measured membrane water and glycerol permeability in THP-1 cells. We found an osmotic permeability coefficient (Pf) of 5.26 ± 0.69) × 10–4 cm s−1 and a glycerol permeability (Pgly) of 4.6 ± 0.6 × 10–6 cm s−1 (Fig. 2a), and respective activation energy (Ea) values of 9.1 ± 0.51 for water and 14.6 ± 1.29 for glycerol transport (Fig. 2b), consistent with active aquaporin channels in cell membranes [45].
Fig. 2.
AQP3 is a functional glycerol channel in THP-1. a Water and glycerol permeabilities measured by stopped-flow spectroscopy in THP-1 cells. b Activation energy for water and glycerol transport in THP-1 cells. c Glycerol permeability (Pgly) in THP-1 cells before and after 10 μM Auphen treatment. d, e Time course of cell volume change after an osmotic challenge with mannitol (d) or with glycerol (e), measured in adherent THP-1 cells by fluorescence microscopy, to evaluate permeability. f, h Representative water (f) and glycerol (h) permeability measurements in untreated, PMA-treated, or PMA + LPS-treated THP-1 before and after Auphen treatment. g Water permeability (Pf) in THP-1 treated as in (f). i Glycerol permeability (Pgly) in THP-1 treated as in (h). a–c, Bars show mean ± SD from 4 independent experiments. d–i Bars show mean ± SD from 10 cells analyzed on 3 coverslips in 2 different cell platings. ##P < 0.01, ###P < 0.001; #Auphen-treated vs non-treated cells
AQP3 was the main contributor to Pgly in THP-1, as the treatment with the specific AQP3 inhibitor Auphen [41, 43] significantly reduced cell glycerol permeability (Fig. 2c). Water permeability calculated from the rate of cell volume equilibration of THP-1 cells exposed to a hyperosmotic mannitol solution was similar among resting and LPS-activated cells (Fig. 2d). Also, glycerol permeability measured from the rate of cell volume re-equilibration after cell challenge with hyperosmotic glycerol solution, producing a fast cell shrinkage and cell reswelling due to glycerol entrance followed by water, was similar in LPS-activated and resting THP-1 cells (Fig. 2e), indicating that AQP3 protein expression was constant after LPS treatment (Fig. 1f). Treatment of THP-1 with 10 μM Auphen blocked glycerol, but not water permeability, in PMA and PMA + LPS-activated cells (Fig. 2f–i), supporting AQP3 as the major contributor for glycerol permeability in LPS-activated THP-1 cells.
AQP3 affects LPS-induced IL-6, TNF-α, and proIL-1β production
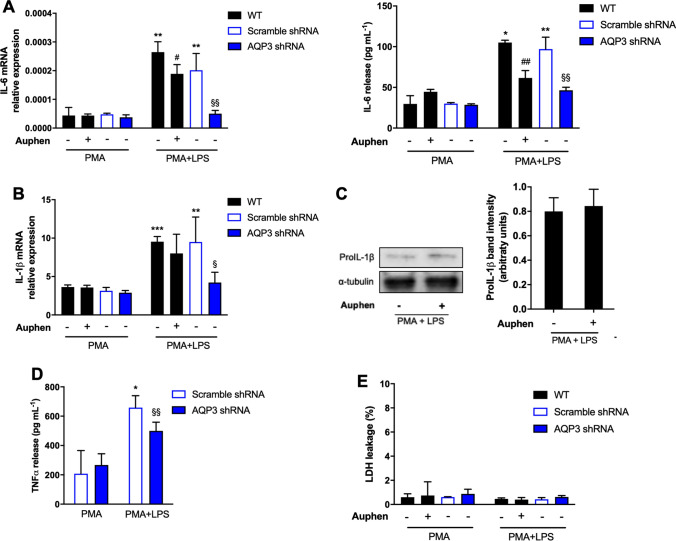
Since AQP3 protein expression and activity were not affected by LPS, we next investigated if AQP3 activity could interfere with LPS-induced cytokine production. We found that Auphen treatment was able to significantly decrease the production of IL-6 (Fig. 3a), but was not affecting the production of proIL-1β at both mRNA and protein level (Fig. 3b, c). To rule out possible toxic effects of Auphen that could be related to the decrease of IL-6 production, we measured extracellular LDH and found that Auphen was not toxic to macrophages (Fig. 3e). LPS-priming in AQP3-silenced cells produced a similar effect to Auphen treatment in IL6 expression and release. In addition, also TNFα release was diminished (Fig. 3d).
Fig. 3.
AQP3 involvement in macrophage priming. a IL6 mRNA expression (left) and protein release (right) in PMA and PMA + LPS-treated THP-1 in the absence and presence of 10 μM Auphen or in AQP3-silenced cells. b IL1β mRNA expression in THP-1 cells treated as in (a). c Representative blots (left) and relative pro-IL1β protein expression in relation to α-tubulin (right) in THP-1 treated with PMA + LPS in the presence and absence of Auphen or in AQP3-silenced cells. d TNFα release in PMA and PMA + LPS-treated THP-1 in AQP3-silenced cells. e Percentage of extracellular LDH in cells treated as in (a). Bars show mean ± SD from 3 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; *PMA + LPS vs PMA. #P < 0.05; ##P < 0.01; #Auphen vs without Auphen; §P < 0.05; §§P < 0.01; §AQP3 shRNA vs Scramble shRNA
AQP3 is involved in cell swelling induced NLRP3 activation, without affecting K+ efflux
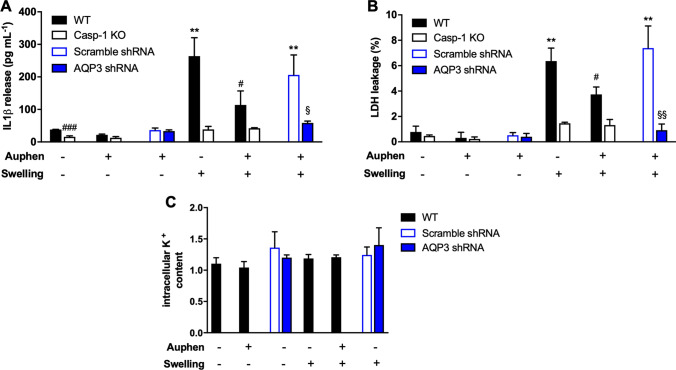
Previous studies reported that cell swelling induces the release of IL-1β upon NLRP3-inflammasome activation [11, 15, 16]. Here, we found that cell swelling triggered by glycerol uptake was also able to induce the release of IL-1β, and it was dependent on AQP3 activity (Fig. 4a). Similarly, AQP3 blockage or gene silencing significantly reduced inflammasome-mediated pyroptosis after cell swelling (Fig. 4b). However, neither AQP3-dependent cell swelling nor its blockage affected intracellular K+ concentration (Fig. 4c), making difficult to infer a direct relation of AQP3-glycerol uptake with K+ efflux. We hypothesize that although AQP3 activity favors glycerol entrance and cell swelling with possible dilution of cytosolic K+ at the single-cell level, this effect is not able to be detected when measuring the K+ intracellular content of the whole cellular population.
Fig. 4.
Swelling AQP3-dependent inflammasome activation. a IL-1β release and b percentage of cell death after imposing glycerol cell swelling in THP-1 cells non-treated and treated with 10 μM Auphen, or in AQP3 shRNA cells as indicated. Wild-type (WT), caspase-1 deficient (Casp-1 KO), and scramble shRNA THP-1 were used. c Intracellular K+ content of PMA + LPS-treated wild-type THP-1 cells after swelling or shrinking in the presence or absence of Auphen or in AQP3 shRNA cells as indicated. Values are normalized to control cells. Bars show mean ± SD from three independent experiments. **P < 0.01; *Swelling vs no challenge. #P < 0.05; ###P < 0.001; #Auphen vs without Auphen; §P < 0.05; §§P < 0.01; §AQP3 shRNA vs scramble shRNA
AQP3 affects nigericin and ATP induced IL-1β release
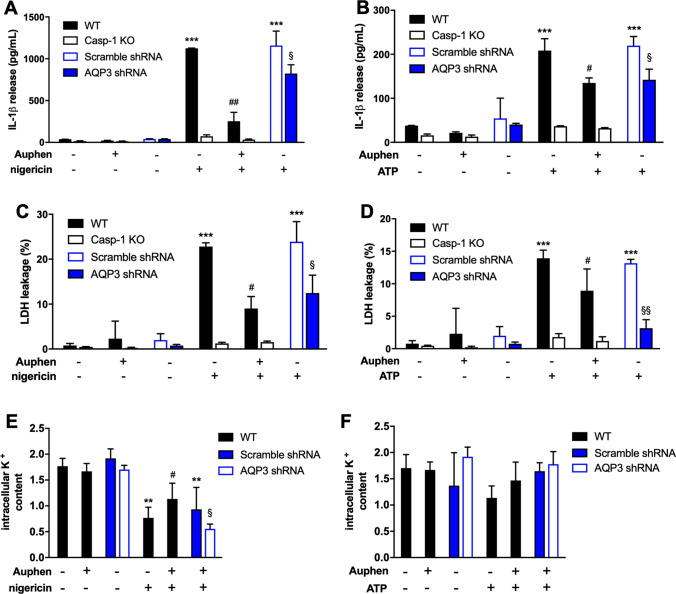
We next examined if AQP3 could be also involved in NLRP3 activation by the K+ ionophore nigericin or by the activation of the P2X7 receptor by extracellular ATP. We found that caspase-1 induced IL-1β release after nigericin or ATP treatment was also affected by Auphen and AQP3 silencing (Fig. 5a, b). In parallel, AQP3 blockage/silencing was also able to reduce pyroptosis induced by these cell treatments (Fig. 5c, d). These data suggest that AQP3 could be also involved in the canonical NLRP3 activation. Both, nigericin and P2X7 receptor activation induce cellular K+ efflux as the most probable step for NLRP3 activation [15, 46]. Auphen was able to partially recover K+ efflux induced by nigericin treatment (Fig. 5e), suggesting that AQP3 could favor NLRP3 activation by facilitating cellular K+ efflux. No variations in K+ efflux induced by ATP treatment were observed (Fig. 5f).
Fig. 5.
Contribution of AQP3 for inflammasome activation. a, b IL-1β release measured by ELISA, and c, d percentage of cell death after challenging PMA + LPS primed THP-1 cells with nigericin (a, c) and ATP (b, d). e Intracellular K+ content of PMA + LPS primed THP-1 cells after nigericin stimulation in non-treated and Auphen-treated cells; values were normalized to control cells. All experiments were done in wild-type (WT) and Caspase-1 knocked-out (Casp-1 KO) cells non-treated and treated with Auphen and AQP3 shRNA cells. Bars show mean ± SD from 3 independent experiments. **P < 0.01, ***P < 0.001; *Nigericin/ATP vs no challenge. #P < 0.05, ##P < 0.01; #Auphen vs without Auphen. §P < 0.05, §§P < 0.01; §AQP3 shRNA vs Scramble shRNA
Altogether, our study demonstrates a role for the aquaglyceroporin AQP3 in the production of IL-6 induced by LPS and the activation of the NLRP3 inflammasome in macrophages after glycerol-induced cell swelling, nigericin, or ATP treatment.
Discussion
Aquaglyceroporins are important channels that regulate cell volume and different physiological processes, as fat metabolism in adipose tissue and metabolic-related complications [36, 47, 48]. Our study identifies the aquaglyceroporin AQP3 as an active glycerol channel in macrophages, responsible for cytokine production after LPS treatment and further IL-1β release and pyroptosis induction after NLRP3-inflammasome activation by cell swelling, and ATP or nigericin treatment. NLRP3 inflammasome is a key signaling pathway of the innate immune system that induces the processing and release of the pro-inflammatory cytokines IL-1β and IL-18, as well as a specific type of cell death called pyroptosis [9, 10, 49]. Different water-permeable AQPs have been described to be involved in NLRP3 activation, and AQP1 deficiency reduces the release of IL-1β after NLRP3 activation by hypotonicity, nigericin, or ATP treatment [11]. Our study shows that AQP3 selective blockage or gene silencing is also able to reduce IL-1β release in response to these stimuli, and furthermore, it shows that AQP3 inhibition or silencing is also able to reduce the associated pyroptotic cell death upon NLRP3 activation. So far, aquaglyceroporins have not been associated with inflammasome activation, and the mechanism of AQP1-mediated NLRP3 activation was attributed to facilitated water transport during the cell swelling process [11, 17]. In our study, we found that AQP3 is involved in the decrease of intracellular K+ induced by nigericin, a well-known K+ ionophore, but with no effect when ATP was used or cell swelling was induced. The effect of AQPs in intracellular K+ decrease has been also studied in the activation of NLRP3 induced by MSU crystals, where the AQPs general blockers Hg2+ and phloretin decreased MSU-induced IL-1β release by diluting intracellular concentration of K+, without affecting K+ net levels [17]; however, it is not known which AQP isoform is important for MSU-induced NLRP3 activation. Since, in general, AQPs are not K+ permeable channels, the mechanism of intracellular K+ decrease could be related to cell swelling and entrance of water into the cell thus diluting intracellular K+ concentration, and not related to a direct K+ efflux [50]. This agrees with our results where we found that glycerol-induced cell swelling was not affecting intracellular K+ concentration and AQP3 only partially recovered intracellular K+ after nigericin treatment. In addition, AQP3 peroxiporin activity contributing to rising intracellular ROS with subsequent inflammasome activation cannot be disregarded, as the NLRP3 inflammasome could be also triggered by K+-efflux-independent mechanisms [51].
Our study also identifies AQP9 as another major aquaglyceroporin expressed in macrophages, in agreement with previous studies where the expression of AQP9 was found in several immune cells including monocytes and macrophages [39]. Since the treatment with the specific AQP3 inhibitor Auphen was not able to completely block IL-1β release, we cannot rule out AQP9 involvement in NLRP3-inflammasome activation, suggesting that different AQP isoforms may act together. However, while AQP3 expression is not affected by LPS-priming, AQP9 is significantly increased, and interestingly, LPS-induced IL-6, TNF-α, and proIL-1β are affected by AQP3 silencing. However, proIL-1β expression was not blocked by Auphen, suggesting that AQP3 blockage and silencing may impact differently on the TLR4 signaling pathway and NF-кB activation. The exact mechanism of IL-6 release is poorly understood and our data suggest that AQP3 could play a role in this pathway. It was previously demonstrated that IL-6 expression is induced by NF-кB activation in mouse primary cortical neurons; however, it is the cell depolarization that causes transport and release of IL-6 vesicles [52]. Interestingly, AQP7, the most representative aquaglyceroporin in pancreatic β-cells, has been shown to play an important role in the exocytosis pathway of insulin [53, 54]. The raise in blood glycerol induces pancreatic β-cell swelling, activating volume-regulated anion channels and subsequent Cl− efflux and cell membrane depolarization. The depolarization opens voltage-sensitive Ca2+ channels and Ca2+ influx, triggering a signalization cascade that results in insulin secretion [55]. Therefore, AQP3 could similarly affect the release of IL-6 containing vesicles during LPS activation of macrophages, since the reduction of IL-6 release was higher than its mRNA expression, although some effects of AQP3 in IL-6 gene expression cannot be excluded.
Overall, this study identifies AQP3 as an important player in the inflammatory response by modulating different signaling pathways in macrophages, LPS-induced IL-6 release, and activation of the NLRP3 inflammasome. This study warrants future studies to examine the use of specific AQP3 inhibitors, as Auphen or its derivates, as new therapeutic tools for inflammatory diseases.
Materials and methods
Reagents
Key reagents and their sources were as follows: Escherichia coli LPS serotype 055:B5, nigericin sodium salt, PMA, and ATP were from Sigma-Aldrich. The composition of the physiological buffer used in all experiments to treat cells with nigericin or ATP was as follows: 147 mM NaCl, 10 mM HEPES, 13 mM D-glucose, 2 mM KCl, 2 mM CaCl2, and 1 mM MgCl2; pH 7.4. The gold(III) compound Auphen was synthesized according to previously published procedures [56].
Human peripheral blood monocytes isolation and treatment
Monocytes were isolated from healthy donors’ whole peripheral blood (n = 3) by negative selection using the EasySep™ Cell Separation human monocyte enrichment kit (StemCell Technologies), according to the manufacturers’ instructions. The Institutional Review Board of the Hospital Clínico Universitario Virgen de la Arrixaca approved the use of these blood samples. Informed consent was obtained from all individuals enrolled in the study following the principles set out in the WMA Declaration of Helsinki. Monocytes were cultured in complete medium composed of RPMI 1640 medium (Biowest) supplemented with 1% l-glutamine, 10% fetal bovine serum (FBS, Hyclone), and no antibiotics. Monocytes were plated at a density of 0.5 × 106 monocytes/well in 24-well plates and treated or not with 100 ng/mL LPS for 2 h.
THP-1 culture and treatments
The human acute monocytic leukemia THP-1 cell line (ATCC® TIB-202) were cultured in complete medium composed of RPMI 1640 medium (Biowest) supplemented with 1% L-glutamine, 10% FBS (Hyclone), and no antibiotics, at 37 °C with 5% CO2. THP-1 knockout for caspase-1 (KO Casp-1) was a kind gift of Dr. V. Hournung (Ludwig Maximilians Universitat Munchen, Germany). For inflammasome priming, THP-1 cells were plated at a density of 2 × 106 cells/well in 12-well plates and treated with 0.5 μM of PMA for 30 min, washed with physiological buffer, and primed with 100 ng/mL of LPS for 24 h in OptiMEM (Gibco) or in complete medium for inflammasome activation. After LPS-priming, cells were washed twice with physiological buffer, and then, NLRP3 inflammasome was activated by 30 min treatment with 10 μM of nigericin or 3 mM of ATP, both in physiological buffer. To inhibit AQP3 function, 10 μM Auphen was added to the media either with LPS to investigate AQP role in macrophage pro-inflammatory priming or with ATP or nigericin to investigate AQP role in NLRP3-inflammasome activation.
AQP3 silencing
AQP3 silencing was performed in THP-1 cell line using commercial expression vectors (Tebu-bio, Madrid, Spain) encoding for three different short hairpin RNA (shRNA) and delivered by a lentiviral system, according to the manufacturers ‘protocol. Scramble shRNA was produced in parallel. For cell infection, cells were seeded with an inoculum of 100 000 cells/cm2 in 6-well plates and the instructions recommended by Tebu-bio were followed. Five-day post-infection cells were expanded and when cells reached confluence of 70–80% selection with 0.8 µg/mL puromycin (Sigma, Kawasaki, Kanagawa, Japan) was initiated. Infections were validated by fluorescent microscopy, quantitative PCR, and functional assays.
RNA extraction and quantitative PCR
Monocytes were washed twice with PBS before total RNA purification using the MicroRNeasy kit (Qiagen) and treated with Turbo™DNase (Invitrogen) for 30 min, according to the manufacturer’s recommendations. RNA was quantified on a NanoDrop 2000 (Thermo Fisher Scientific). Reverse transcription was performed using an iScript™ cDNA Synthesis Kit (BioRad). Quantitative PCR (qPCR) was performed using an iQTM 5 Optical System (BioRad) with an SYBR Green mix (Takara) and specific pre-designed primers for AQP3 (Hs_AQP1_1_SG QT00212996), AQP6.
(Hs_AQP6_1_SG QT00010633), AQP8 ( Hs_AQP8_1_SG QT00039123), AQP9.
(Hs_AQP9_1_SG QT00017710), AQP11 ( Hs_AQP11_1_SG QT00230636), AQP12.
(Hs_AQP12A_1_SG QT00236432), HPRT-1 (Hs_HPRT-1_1_SG QT00059066), IL6.
(Hs_IL6_1_SG QT00083720), IL1B (Hs_IL1B_1_SG QT00021385), TNFA.
(Hs_TNF_1_SG QT00029162) and NLRP3 (Hs_NLRP3_1_SG QT00029771) (Qiagen).
THP-1 were washed twice with PBS before total RNA purification using the RNeasy mini kit (Qiagen) and treated with RNase-free DNase I (Invitrogen) for 30 min, according to the manufacturer’s recommendations. Reverse transcription was performed as described for monocytes and qPCR reactions were carried out using a CFX96 Real-Time System C1000 (BioRad), the TaqMan Universal PCR Master Mix (Applied Biosystems) and the following specific TaqMan pre-designed primers for AQP0 (Hs0085175_m1), AQP1 (Hs01028916_m1), AQP2 (Hs00166640_m1), AQP3 (Hs01105469_g1), AQP4 (Hs00242342_m1), AQP5 (Hs00387048_m1), AQP6 (Hs00155808_m1), AQP7 (Hs00357359_m1), AQP8 (Hs01086280_g1), AQP9 (Hs00175573_m1), AQP10 (Hs00369738_m1), AQP11 (Hs005426181_m1), AQP12 (Hs01651303_m1), HPRT-1 (Hs02800695_m1), IL6 (Hs00174131_m1), and IL1B (Hs01555410_m1) (Applied Biosystems). All results were normalized to the level of the reference gene (HPRT-1) and relative quantification was calculated using a variation of the Livak method [57], described by Fleige and Pfaffl [58]. All samples were run in triplicate and the average values were calculated.
Western blotting
Proteins were separated by SDS-PAGE before semi-dry transfer as previously described [59]. After blocking, the following specific primary antibodies were used: anti-AQP1 and AQP3 antibody (1:100 and 1:200, respectively; Santa Cruz Biotechnology), anti-IL-1β and anti-α-tubulin (1:1000; Sigma-Aldrich) antibody. Secondary anti-rabbit and anti-mouse (1:5000; Jackson Immuno Research), and anti-goat (1:7500; Jackson Immuno Research) HRP-linked antibodies were used for sensitive ECL-mediated (Amersham Biosciences) detection. Band intensity was measured using the ImageJ software (https://imagej.nih.gov).
Permeability assays in cell suspensions
THP-1 cells were centrifuged at 150 × g to obtain a cellular pellet and were resuspended in isotonic medium for 10 min. The cell suspension was homogeneous with THP-1 cells showing a spherical shape, as observed under light microscopy. The diameter of cells was measured for all the preparations using ImageJ software.
Permeability assays in cell suspensions were performed by stopped-flow light scattering following the protocol described in [41]. Briefly, experiments were performed on a HI-TECH Scientific PQ/SF-53 stopped-flow apparatus, which has a 2 ms dead time and is temperature-controlled (24 °C), interfaced with an IBM PC/AT compatible 80386 microcomputer. This procedure was performed to measure water and glycerol permeability and the activation energy (Ea) for each transport, and to validate AQP3 as the representative glycerol channel in THP-1 using Auphen as inhibitor. Four-to-eight runs were stored and analyzed in each experimental condition. In each run, 0.1 mL cellular suspension was mixed with an equal amount of hyperosmotic solution to reach inwardly directed gradients of solute. For osmotic water permeability (Pf), a hyperosmotic shock solution containing a non-permeable solute was used (mannitol 480 mM in PBS 7.4) producing an inwardly directed gradient of solute and water outflux until an osmotic equilibrium is reached. To measure glycerol permeability (Pgly), a hyperosmotic shock solution containing glycerol (glycerol 480 mM in PBS 7.4) was used, creating an inwardly directed glycerol gradient. After the first fast cell shrinkage due to water outflow, glycerol influx in response to its chemical gradient is followed by water influx with subsequent cell reswelling. In all the permeability assays, the tonicity of the osmotic shocks (given by the ratio of the initial-to-final medium osmolarity after the applied osmotic challenges) is similar (tonicity of 1.6). Baselines were acquired using the respective incubation buffers as isotonic shock solutions.
The kinetics of cell shrinkage and reswelling were measured from the time course of 90° scattered light intensity at 530 nm until a stable light scatter signal was attained. Pf was calculated as Pf = k(Vo/A)(1/Vw(osmout)∞), where Vw is the molar volume of water, Vo/A is the initial cell volume-to-area ratio, (osmout)∞ is the final medium osmolarity after the applied osmotic gradient, and k (s−1) is the single exponential time constant fitted to the light scatter signal of cell shrinkage; Pgly was calculated by Pgly = k(V0/A), where Vo/A is the initial cell volume-to-area ratio and k (s−1) is the single exponential time constant fitted to the light scatter signal of glycerol influx. For inhibition assays, cells were incubated with Auphen (10 µM) 30 min prior to permeability experiments. The activation energy (Ea) of water and glycerol transport was calculated from the slope of the Arrhenius plot (lnPf or lnPgly as a function of 1/T) multiplied by the gas constant R.
Permeability assays in adherent cells
Water (Pf) and glycerol (Pgly) permeability were measured in individual adherent cells on a coverslip, as previously described [24, 60]. To perform this experiment in resting cells, coverslips were coated with poly-L-lysin for cell adherence. Briefly, cells were loaded with 5 mM calcein acetoxymethyl ester (calcein-AM; Sigma-Aldrich) for 30 min at 37 °C in 5% CO2. The coverslips with the adhered cells were mounted in a closed perfusion chamber (Warner Instruments, Hamden, USA) on the stage of a Zeiss Axiovert 200 inverted microscope. Fluorescence was excited at wavelength 495/10 nm and the emission fluorescence was collected with a 535/25 nm bandpass filter coupled with a 515 nm dichroic beam splitter. Images were captured using a × 40 epifluorescence oil immersion objective and a digital camera (CoolSNAP EZ, Photometrics, USA) and recorded by the Metafluor Software (Molecular Devices, USA).
Cells were perfused with 300 mM HEPES buffer (135 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 10 mM D-Glucose, 5 mM HEPES, pH 7.4, and 300 mOsM) for 60 s, after which 300 mM mannitol (for water permeability) or 300 mM glycerol (for glycerol permeability) was added to the buffer to achieve an external osmolarity of 600 mOsM. Cell volume (V) was measured at selected time points from 2D images obtained during the permeability assays to evaluate the initial volume (prior to the osmotic challenge Vo) and the final equilibrium volume. Pf and Pgly coefficients were evaluated from the measured time-dependent volume changes, Vrel = V/Vo, obtained by adding mannitol (Pf) or glycerol (Pgly) to the external media, using the model equations described in [61] using the Berkeley Madonna software (http://www.berkeleymadonna.com).
Lactate dehydrogenase (LDH)
The presence of LDH in cell-free supernatants was measured using the cytotoxicity detection kit (Roche, Barcelona, Spain) following the manufacturer’s instructions to evaluate treatment-dependent cell death. Cell death was defined as the percentage of released LDH compared with maximal LDH levels in cell lysates obtained using Lysis buffer on cells plated only with PMA (positive control). The percentage of the LDH release was calculated using ET buffer as negative control.
ELISA assays
Cell-free supernatants were collected and clarified by centrifugation. IL-6 and IL-1β were quantified by ELISA following the kit supplier’ protocol instructions (R&D Systems) and absorbances were measured in a Cobas 6000.
Intracellular K+ measurement
After stimulation, cells were washed briefly with molecular biology grade water (Sigma) to wash ions from the stimulating buffer. Cells were then scraped in 200 µL molecular biology grade water and lysed by three freeze–thaw cycles. Lysates were centrifuged at 13,200 rpm for 10 min at 4 ºC and supernatants were used to measure intracellular K+ content by direct potentiometry in a Cobas 6000 (Roche) according to the kit supplier’s protocol (Roche).
Statistical analysis
All the experiments were performed in biological and technical triplicates. Results were expressed as mean ± SD of n individual experiments. Statistical analysis between groups was performed by two-way ANOVA followed by an unpaired two-tailed Student’s test or non-parametric Mann–Whitney test. P values < 0.05 were considered statistically significant. Statistical analyses were performed using the Graph Prism software (GraphPad Software).
Acknowledgements
This research was funded by Fundação para a Ciência e Tecnologia (FCT), Portugal, through individual fellowship to IV da Silva (PD/BD/113634/2015), grant PTDC/BTM-SAL/28977/2017, and strategic projects UID/DTP/04138/2019, and by Ministerio de Economía, Industria y Competitividad, Spain (grant SAF2017‐88276‐R to P.P.), Fundación Séneca (grant 20859/PI/18 to P.P.), and the European Research Council (ERC‐2013‐CoG grant 614578 to P.P.). We appreciate Veit Hournung (Ludwig Maximilians Universitat Munchen, Germany) for THP-1 Casp-1 KO cells. We thank María Carmen Baños and Ana I. Gómez-Sánchez (IMIB-Arrixaca, Murcia, Spain) for technical assistance, and EU COST Action BM1406 for fruitful discussions and short-term scientific mission for I.V.S..
Abbreviations
- AQP
Aquaporin
- ATP
Adenosine triphosphate
- Casp-1
Caspase-1
- GSDMD
Gasdermin D
- IL
Interleukin
- NF-κB
Nuclear factor-κB
- NLRP3
Nucleotide-binding oligomerization domain-like receptors family pyrin domain containing 3
- Pf
Osmotic water permeability
- Pgly
Glycerol permeability
- TNF-α
Tumor necrosis factor alpha
Author contributions
IVS, PP, and GS contributed for the experimental planning; IVS, CC, and HM-B. performed the experiments; IVS analyzed the data; GS, PP, and AC contributed to reagents/materials/analysis tools; IVS wrote the main manuscript; PP and GS contributed to the intellectual input and scientific discussion, and edited the main manuscript; and, all authors read and approved the final manuscript.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pablo Pelegrín and Graça Soveral joint senior authors.
Contributor Information
Pablo Pelegrín, Email: pablo.pelegrin@imib.es.
Graça Soveral, Email: gsoveral@ff.ulisboa.pt.
References
- 1.Jourdan T, Godlewski G, Cinar R, Bertola A, Szanda G, Liu J, Tam J, Han T, Mukhopadhyay B, Skarulis MC, Ju C, Aouadi M, Czech MP, Kunos G. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. 2013;19(9):1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo B, Li B, Wang W, Liu X, Xia Y, Zhang C, Zhang M, Zhang Y, An F. NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS ONE. 2014;9(8):e104771. doi: 10.1371/journal.pone.0104771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehal WZ. The inflammasome in liver injury and non-alcoholic fatty liver disease. Dig Dis. 2014;32(5):507–515. doi: 10.1159/000360495. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Xia L, Zhang F, Zhu D, Xin C, Wang H, Zhang F, Guo X, Lee Y, Zhang L, Wang S, Guo X, Huang C, Gao F, Liu Y, Tao L. A novel mechanism of diabetic vascular endothelial dysfunction: hypoadiponectinemia-induced NLRP3 inflammasome activation. Biochim Biophys Acta 1863. 2017;6:1556–1567. doi: 10.1016/j.bbadis.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, He X, Yuan X, Hong J, Bhat O, Li G, Li PL, Guo J. NLRP3 Inflammasome formation and activation in nonalcoholic steatohepatitis: therapeutic target for antimetabolic syndrome remedy FTZ. Oxid Med Cell Longe. 2018;2018:2901871. doi: 10.1155/2018/2901871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13(1):11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 7.Ishii KJ, Suzuki K, Coban C, Takeshita F, Itoh Y, Matoba H, Kohn LD, Klinman DM. Genomic DNA released by dying cells induces the maturation of APCs. Journal of immunology. 2001;167(5):2602–2607. doi: 10.4049/jimmunol.167.5.2602. [DOI] [PubMed] [Google Scholar]
- 8.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 9.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. Journal of immunology. 2009;183(2):787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity. 2018;48(1):35–44. doi: 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Compan V, Baroja-Mazo A, Lopez-Castejon G, Gomez AI, Martinez CM, Angosto D, Montero MT, Herranz AS, Bazan E, Reimers D, Mulero V, Pelegrin P. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 2012;37(3):487–500. doi: 10.1016/j.immuni.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Lima H, Jr, Jacobson LS, Goldberg MF, Chandran K, Diaz-Griffero F, Lisanti MP, Brojatsch J. Role of lysosome rupture in controlling Nlrp3 signaling and necrotic cell death. Cell Cycle. 2013;12(12):1868–1878. doi: 10.4161/cc.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38(6):1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hafner-Bratkovic I, Pelegrin P. Ion homeostasis and ion channels in NLRP3 inflammasome activation and regulation. Curr Opin Immunol. 2018;52:8–17. doi: 10.1016/j.coi.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Schorn C, Frey B, Lauber K, Janko C, Strysio M, Keppeler H, Gaipl US, Voll RE, Springer E, Munoz LE, Schett G, Herrmann M. Sodium overload and water influx activate the NALP3 inflammasome. J Biol Chem. 2011;286(1):35–41. doi: 10.1074/jbc.M110.139048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyle JP, Bryant CE, Monie TP. Cell swelling and the NLRP3 inflammasome. Immunity. 2013;38(3):399. doi: 10.1016/j.immuni.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabolli V, Wallemme L, Lo Re S, Uwambayinema F, Palmai-Pallag M, Thomassen L, Tyteca D, Octave JN, Marbaix E, Lison D, Devuyst O, Huaux F. Critical role of aquaporins in interleukin 1beta (IL-1beta)-induced inflammation. J Biol Chem. 2014;289(20):13937–13947. doi: 10.1074/jbc.M113.534594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meli R, Pirozzi C, Pelagalli A. New perspectives on the potential role of aquaporins (AQPs) in the physiology of inflammation. Front Physiol. 2018;9:101. doi: 10.3389/fphys.2018.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5(9):687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 20.Carbrey JM, Agre P. Discovery of the aquaporins and development of the field. Handb Exp Pharmacol. 2009;190:3–28. doi: 10.1007/978-3-540-79885-9_1. [DOI] [PubMed] [Google Scholar]
- 21.Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P. Rapid gating and anion permeability of an intracellular aquaporin. Nature. 1999;402(6758):184–187. doi: 10.1038/46045. [DOI] [PubMed] [Google Scholar]
- 22.Soria LR, Fanelli E, Altamura N, Svelto M, Marinelli RA, Calamita G. Aquaporin-8-facilitated mitochondrial ammonia transport. Biochem Biophys Res Commun. 2010;393(2):217–221. doi: 10.1016/j.bbrc.2010.01.104. [DOI] [PubMed] [Google Scholar]
- 23.Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci. 2005;118(Pt 15):3225–3232. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- 24.Madeira A, Fernandez-Veledo S, Camps M, Zorzano A, Moura TF, Ceperuelo-Mallafre V, Vendrell J, Soveral G. Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obesity. 2014;22(9):2010–2017. doi: 10.1002/oby.20792. [DOI] [PubMed] [Google Scholar]
- 25.Miller EW, Dickinson BC, Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci USA. 2010;107(36):15681–15686. doi: 10.1073/pnas.1005776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertolotti M, Bestetti S, Garcia-Manteiga JM, Medrano-Fernandez I, Dal Mas A, Malosio ML, Sitia R. Tyrosine kinase signal modulation: a matter of H2O2 membrane permeability? Antioxid Redox Signal. 2013;19(13):1447–1451. doi: 10.1089/ars.2013.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigues C, Pimpao C, Mosca AF, Coxixo AS, Lopes D, da Silva IV, Pedersen PA, Antunes F, Soveral G. Human aquaporin-5 facilitates hydrogen peroxide permeation affecting adaption to oxidative stress and cancer cell migration. Cancers. 2019 doi: 10.3390/cancers11070932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe S, Moniaga CS, Nielsen S, Hara-Chikuma M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem Biophys Res Commun. 2016;471(1):191–197. doi: 10.1016/j.bbrc.2016.01.153. [DOI] [PubMed] [Google Scholar]
- 29.Soveral G, Nielsen S, Casini A. Aquaporins in health and disease: new molecular targets for drug discovery. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2016. [Google Scholar]
- 30.Soveral G, Casini A. Aquaporin modulators: a patent review (2010–2015) Expert Opin Ther Pat. 2017;27(1):49–62. doi: 10.1080/13543776.2017.1236085. [DOI] [PubMed] [Google Scholar]
- 31.Aikman B, de Almeida A, Meier-Menches SM, Casini A. Aquaporins in cancer development: opportunities for bioinorganic chemistry to contribute novel chemical probes and therapeutic agents. Metallomics. 2018;10(5):696–712. doi: 10.1039/c8mt00072g. [DOI] [PubMed] [Google Scholar]
- 32.Verkman AS. Aquaporins in clinical medicine. Annu Rev Med. 2012;63:303–316. doi: 10.1146/annurev-med-043010-193843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishibashi K, Kuwahara M, Gu Y, Tanaka Y, Marumo F, Sasaki S. Cloning and functional expression of a new aquaporin (AQP9) abundantly expressed in the peripheral leukocytes permeable to water and urea, but not to glycerol. Biochem Biophys Res Commun. 1998;244(1):268–274. doi: 10.1006/bbrc.1998.8252. [DOI] [PubMed] [Google Scholar]
- 34.Moon C, Rousseau R, Soria JC, Hoque MO, Lee J, Jang SJ, Trink B, Sidransky D, Mao L. Aquaporin expression in human lymphocytes and dendritic cells. Am J Hematol. 2004;75(3):128–133. doi: 10.1002/ajh.10476. [DOI] [PubMed] [Google Scholar]
- 35.da Silva IV, Rodrigues JS, Rebelo I, Miranda JPG, Soveral G. Revisiting the metabolic syndrome: the emerging role of aquaglyceroporins. Cell Mol Life Sci: CMLS. 2018;75(11):1973–1988. doi: 10.1007/s00018-018-2781-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.da Silva IV, Soveral G. Aquaporins in obesity. Adv Exp Med Biol. 2017;969:227–238. doi: 10.1007/978-94-024-1057-0_15. [DOI] [PubMed] [Google Scholar]
- 37.Jablonski EM, Webb AN, McConnell NA, Riley MC, Hughes FM., Jr Plasma membrane aquaporin activity can affect the rate of apoptosis but is inhibited after apoptotic volume decrease. Am J Physiol Cell Physiol. 2004;286(4):C975–985. doi: 10.1152/ajpcell.00180.2003. [DOI] [PubMed] [Google Scholar]
- 38.Zhu N, Feng X, He C, Gao H, Yang L, Ma Q, Guo L, Qiao Y, Yang H, Ma T. Defective macrophage function in aquaporin-3 deficiency. FASEB J: Off Publ Fed Am Soc Exp Biol. 2011;25(12):4233–4239. doi: 10.1096/fj.11-182808. [DOI] [PubMed] [Google Scholar]
- 39.Holm A, Karlsson T, Vikstrom E. Pseudomonas aeruginosa lasI/rhlI quorum sensing genes promote phagocytosis and aquaporin 9 redistribution to the leading and trailing regions in macrophages. Front Microbiol. 2015;6:915. doi: 10.3389/fmicb.2015.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holm A, Magnusson KE, Vikstrom E. Pseudomonas aeruginosa N-3-oxo-dodecanoyl-homoserine lactone elicits changes in cell volume, morphology, and AQP9 characteristics in macrophages. Front Cell infect Microbiol. 2016;6:32. doi: 10.3389/fcimb.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martins AP, Marrone A, Ciancetta A, Galan Cobo A, Echevarria M, Moura TF, Re N, Casini A, Soveral G. Targeting aquaporin function: potent inhibition of aquaglyceroporin-3 by a gold-based compound. PLoS ONE. 2012;7(5):e37435. doi: 10.1371/journal.pone.0037435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serna A, Galan-Cobo A, Rodrigues C, Sanchez-Gomar I, Toledo-Aral JJ, Moura TF, Casini A, Soveral G, Echevarria M. Functional inhibition of aquaporin-3 with a gold-based compound induces blockage of cell proliferation. J Cell Physiol. 2014;229(11):1787–1801. doi: 10.1002/jcp.24632. [DOI] [PubMed] [Google Scholar]
- 43.de Almeida A, Martins AP, Mosca AF, Wijma HJ, Prista C, Soveral G, Casini A. Exploring the gating mechanisms of aquaporin-3: new clues for the design of inhibitors? Mol BioSyst. 2016;12(5):1564–1573. doi: 10.1039/c6mb00013d. [DOI] [PubMed] [Google Scholar]
- 44.de Almeida A, Mosca AF, Wragg D, Wenzel M, Kavanagh P, Barone G, Leoni S, Soveral G, Casini A. The mechanism of aquaporin inhibition by gold compounds elucidated by biophysical and computational methods. Chem Commun. 2017;53(27):3830–3833. doi: 10.1039/c7cc00318h. [DOI] [PubMed] [Google Scholar]
- 45.Madeira A, Moura TF, Soveral G. Detecting aquaporin function and regulation. Front Chem. 2016;4:3. doi: 10.3389/fchem.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269(21):15195–15203. doi: 10.1016/S0021-9258(17)36591-2. [DOI] [PubMed] [Google Scholar]
- 47.Laforenza U, Bottino C, Gastaldi G. Mammalian aquaglyceroporin function in metabolism. Biochem Biophys Acta 1858. 2016;1:1–11. doi: 10.1016/j.bbamem.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 48.da Silva IV, Cardoso C, Mendez-Gimenez L, Camoes SP, Fruhbeck G, Rodriguez A, Miranda JP, Soveral G. Aquaporin-7 and aquaporin-12 modulate the inflammatory phenotype of endocrine pancreatic beta-cells. Arch Biochem Biophys. 2020;691:108481. doi: 10.1016/j.abb.2020.108481. [DOI] [PubMed] [Google Scholar]
- 49.Broz P, Pelegrin P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2019 doi: 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- 50.Day RE, Kitchen P, Owen DS, Bland C, Marshall L, Conner AC, Bill RM, Conner MT. Human aquaporins: regulators of transcellular water flow. Biochem Biophys Acta 1840. 2020;5:1492–1506. doi: 10.1016/j.bbagen.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 51.Gross CJ, Mishra R, Schneider KS, Medard G, Wettmarshausen J, Dittlein DC, Shi H, Gorka O, Koenig PA, Fromm S, Magnani G, Cikovic T, Hartjes L, Smollich J, Robertson AAB, Cooper MA, Schmidt-Supprian M, Schuster M, Schroder K, Broz P, Traidl-Hoffmann C, Beutler B, Kuster B, Ruland J, Schneider S, Perocchi F, Gross O. K(+) Efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity. 2016;45(4):761–773. doi: 10.1016/j.immuni.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Tsakiri N, Kimber I, Rothwell NJ, Pinteaux E. Mechanisms of interleukin-6 synthesis and release induced by interleukin-1 and cell depolarisation in neurones. Mol Cell Neurosci. 2008;37(1):110–118. doi: 10.1016/j.mcn.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Delporte C, Virreira M, Crutzen R, Louchami K, Sener A, Malaisse WJ, Beauwens R. Functional role of aquaglyceroporin 7 expression in the pancreatic beta-cell line BRIN-BD11. J Cell Physiol. 2009;221(2):424–429. doi: 10.1002/jcp.21872. [DOI] [PubMed] [Google Scholar]
- 54.Arsenijevic T, Perret J, Van Laethem JL, Delporte C. Aquaporins involvement in pancreas physiology and in pancreatic diseases. Int J Mol Sci. 2019 doi: 10.3390/ijms20205052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Louchami K, Best L, Brown P, Virreira M, Hupkens E, Perret J, Devuyst O, Uchida S, Delporte C, Malaisse WJ, Beauwens R, Sener A. A new role for aquaporin 7 in insulin secretion. Cellular Physiol Biochem: Int J Exp Cell Physiol Biochem Pharmacol. 2012;29(1–2):65–74. doi: 10.1159/000337588. [DOI] [PubMed] [Google Scholar]
- 56.Abbate F, Orioli P, Bruni B, Marcon G, Messori L. Crystal structure and solution chemistry of the cytotoxic complex 1,2-dichloro(o-phenanthroline) gold(III) chloride. Inorg Chim Acta. 2000;311(1–2):1–5. doi: 10.1016/S0020-1693(00)00299-1. [DOI] [Google Scholar]
- 57.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 58.Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006;27(2–3):126–139. doi: 10.1016/j.mam.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 59.da Silva IV, Barroso M, Moura T, Castro R, Soveral G. Endothelial aquaporins and hypomethylation: potential implications for atherosclerosis and cardiovascular disease. Int J Mol Sci. 2018 doi: 10.3390/ijms19010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Madeira A, Mosca AF, Moura TF, Soveral G. Aquaporin-5 is expressed in adipocytes with implications in adipose differentiation. IUBMB Life. 2015;67(1):54–60. doi: 10.1002/iub.1345. [DOI] [PubMed] [Google Scholar]
- 61.Madeira A, Camps M, Zorzano A, Moura TF, Soveral G. Biophysical assessment of human aquaporin-7 as a water and glycerol channel in 3T3-L1 adipocytes. PLoS ONE. 2013;8(12):e83442. doi: 10.1371/journal.pone.0083442. [DOI] [PMC free article] [PubMed] [Google Scholar]