Abstract
Characterized by a surplus of whole-body adiposity, obesity is strongly associated with the prognosis of atherosclerosis, a hallmark of coronary artery disease (CAD) and the major contributor to cardiovascular disease (CVD) mortality. Adipose tissue serves a primary role as a lipid-storage organ, secreting cytokines known as adipokines that affect whole-body metabolism, inflammation, and endocrine functions. Emerging evidence suggests that adipokines can play important roles in atherosclerosis development, progression, as well as regression. Here, we review the versatile functions of various adipokines in atherosclerosis and divide these respective functions into three major groups: protective, deteriorative, and undefined. The protective adipokines represented here are adiponectin, fibroblast growth factor 21 (FGF-21), C1q tumor necrosis factor-related protein 9 (CTRP9), and progranulin, while the deteriorative adipokines listed include leptin, chemerin, resistin, Interleukin- 6 (IL-6), and more, with additional adipokines that have unclear roles denoted as undefined adipokines. Comprehensively categorizing adipokines in the context of atherosclerosis can help elucidate the various pathways involved and potentially pave novel therapeutic approaches to treat CVDs.
Keywords: Cardiovascular diseases, Adipose tissue, Adiponectin, Leptin, Obesity
Introduction
Cardiovascular diseases (CVDs) are the leading cause of mortality, with approximately 17.9 million deaths reported in 2019 globally [1]. Coronary artery disease (CAD) is among the most common forms of CVDs. CAD is characterized by the formation of plaques along the arterial walls that are highly and chronically inflammatory, and this buildup is known as atherosclerosis [2]. Atherosclerosis is initiated by the retention of apolipoprotein-B containing lipoproteins in the subendothelial space of arteries that triggers an inflammatory response [3], promotes the migration and proliferation of smooth muscle cells, and forms a necrotic core [4]. Chronic inflammation has become an inevitable factor contributing to the formation of atherosclerotic plaque and participating in various stages of development. Inflammatory signaling in atherosclerosis coordinates the recruitment of monocyte-derived macrophages and T lymphocytes that heavily influence plaque stability, leading to rupture and thrombosis [5].
Obesity is one of the major risk factors for CVDs. Its primary co-morbidities, insulin resistance and type 2 diabetes (T2DM), increase the incidence and severity of atherosclerosis 2–4 folds. About 40% of deaths in T2DM patients are due to risk factors associated with CVDs [6]. Features in obesity, like adiposity, confer abnormalities in metabolism and are linked to CVDs and other metabolic diseases. Besides as the primary organ of energy storage, adipose tissue has been well recognized to produce adipokines that regulate metabolism, inflammation, and endocrine functions [7]. Moreover, the patterns associated with the secretion of adipokines can vary depending on the state of the adipose tissue. Adiposity in obesity can be classified into two key fates: hyperplasia, the de novo maturation of preadipocytes, and hypertrophy, an enlargement in adipocyte size. Hyperplasia obesity and hypertrophic obesity show distinct profiles in adipokine production, generally beneficial and detrimental, respectively [8]. Adipokines, such as adiponectin, FGF21, and CTRP9, can be protective in metabolic diseases like atherosclerosis, while other adipokines, such as leptin, chemerin, resistin, and pro-inflammatory cytokines that are secreted from hypertrophic adipose tissue, can further burden the progression of the disease.
In this review, we highlight the well-known and lesser known adipokines, extensively catalog the roles they play in atherosclerosis, and further explore adipokines as potential therapeutic targets for the treatment of atherosclerosis. Based on the rigorous assessments made, we labeled each adipokine into three major groups: protective, deteriorative, and undefined (Fig. 1 and Tables 1, 2, 3).
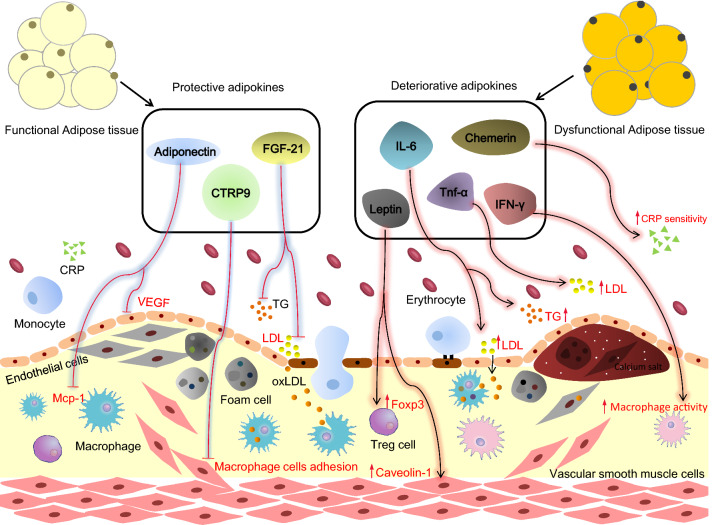
Fig. 1.
Representative adipokines in regulating atherosclerosis. Atherosclerosis is a chronic inflammatory cardiovascular disease impacting the arterial walls with lipid-laden plaque. Obesity is a major risk factor for atherosclerosis. Adipokines have been shown to play a myriad of roles in the progression and regression of atherosclerosis, with the former being exacerbated in obesity. Key representative adipokines that regulate atherosclerosis are described here. Protective adipokines depicted are adiponectin, CTRP9, and FGF-21. Adiponectin inhibits macrophage and endothelial cell (EC) activation via the inhibition of monocyte chemoattractant protein-1 (MCP-1) and vascular endothelial growth factor (VEGF), respectively. FGF-21 decreases circulating triglycerides (TG) and low-density lipoproteins (LDL). CTRP9 prevents monocyte adhesion to the vascular wall. Deteriorative adipokines depicted are leptin, chemerin, IL-6, TNF-α, and IFN-γ. Leptin upregulates the expression of FoxP3 in regulatory T cells (Treg) and caveolin-1 in ECs. Chemerin upregulates pathogenic C-Reactive Protein (CRP) levels. IL-6 and TNF-α activate the inflammatory response and influence the lipid profile. IFN-γ also induces inflammation and promotes the transformation of macrophages into cholesterol-loaded foam cells
Table 1.
Protective adipokines for atherosclerosis
| Adipokines | Major function | Rank of evidence | References |
|---|---|---|---|
| Adiponectin |
↓ cholesterol, lipid droplet MSR, VCAM-1, TNF-α, MCP-1 in macrophage, ↓ cAMP-PKA- TNF-α,IL-8, VEGF in ECs ↓adiponectin ∞ ↑CAC, TG, plaque volume, IMT, ∞ ↓ HDL-C |
**** | [12–23, 25] |
| FGF21 |
↑ insulin sensitivity and regulates lipid metabolism ↓the levels of plasma triglycerides, free fatty acids and cholesterol in genetically compromised diabetic and obese rodents ↓ the levels of TG and LDL,↑HDL ↑ ABCA1/ABCG1 expression at mRNA and protein level in macrophages ↑foam cells formation, macrophage migration, inflammatory response, and lipid metabolism in OxLDL-induced THP-1 macrophages ↓ proliferation and migration of smooth muscle cells ↓endothelial dysfunction ↓ conversion of macrophages to foam cells ↓oxidized LDL-C uptake by macrophages ↓sterol regulatory element-binding protein-2 ↓ apoptosis in cultured cardiac endothelial cells from male adult rats ↓∞ the cytotoxic and apoptotic effect of H2O2 in a dose-dependent manner |
**** | [26, 29–34, 192–202] |
| CTRP9 |
↓VSMCs’ proliferation and phenotype switch and cell dysfunction ↓neointimal formation, endothelial cell senescence and dysfunction ↓pro-inflammatory cytokines in macrophages and THP-1 cell adhesion to VSMCs; ↑ the autophagy level in atherosclerosis lesions ↓ serum glucose level and VSMC cholesterol uptake; ↑the expression of cholesterol efflux-related molecules ↑carotid plaque stability ↓atherosclerosis through AMPK-NLRP3 inflammasome singling pathway, activating AMP-dependent kinase, PGC-1α/AMPK-mediated antioxidant enzyme induction, the AMPKα/ KLF4 signaling pathway, or AMPK/ mTOR pathway |
****** | [36–42, 203] |
| PGRN |
↓inflammation and adhesion molecules, conversion of macrophages to foam cells and foam cell formation; PGRN degradation into GRNs ↑ inflammation; ↑endothelial nitric oxide synthase ↓cholesterol uptake ↓TNF-α |
*** | [45, 47, 49, 51] |
Rank of evidence: Weak (*), moderate (**), strong (***), stronger (****), Strongest (*****)
Table 2.
Deteriorative adipokines for atherosclerosis
| Adipokines | Major function | Rank of evidence | References |
|---|---|---|---|
| Leptin |
↑piHDL, Lp(a) and apoB100 ↓T-cell helper type 1 response ↑FoxP3 expression and Treg cell function ↑caveolin-1, ERK1/2, eNOS in ECs ↑AngII, ROS, JNK, caveolin-1 in smooth cells ↑TSP-1 |
**** | [53–59, 61, 204, 205] |
| Chemerin |
↑chemerin ∞ ↑ high-sensitivity CRP, IL6, TNF-α, resistin, leptin, BMI, TG, hypertension, ∞ ↓ HDL-C ↑chemerin ∞ ↑ Gensini score ↓chemerin → ↓atherosclerosis, TNFα,IL1β in Apoe−/− mice |
*** | [67–70] |
| Resistin |
↑ lipid profile,↑ insulin resistance, ↑TG ↑ macrophages polarization, ↑TNF-α, ↑IL-1β, ↑IL-6, ↑VCAM-1, ↑VSMCs, ↑MCP-1, ↑monocyte-endothelial adhesion |
**** | [71–78] |
| FABP4 |
∞ a cluster of metabolic and inflammatory risk factors ↓levels of the adipocyte fatty acid binding protein 4, insulin sensitivity ∞↓cholesterol ester accumulation and inflammatory responses |
*** | [90–92, 206–212] |
| IL-1β |
expression of various cytokines, chemokines, adhesion molecules ↑leukocytes↑ platelet adhesion to collagen and thrombin↑ VCAM-1↑MCP-1 recruitment↑∞IL-10 produce↓ SMC proliferation↑ and macrophage proliferation in plaques ↑ vascular smooth muscle cell calcium deposition ↑ smooth muscle markers ↑ intimal proliferation↑ advance atherosclerosis: outward remodeling, SMC- and collagen-fibrous cap↑ |
***** | [95, 97–106, 213, 214] |
| IL-18 |
cholesterol efflux (lipoprotein cholesterol↑ serum cholesterol↑) oxidative stress ↑ endothelial dysfunction |
* | [110, 111] |
|
IL18r no differences in atherogenesis induct MMP-9 to promote plaque rupture involve IFN-γ-dependent mechanism to develop atherosclerosis combine with IL-17 promote the diagnostic value of CT |
*** | [112–118] | |
| IL-6 |
↑IL-6 ∞ macrophage infiltration in plaque ↑ anti-inflammatory cytokines level in plaques ↑ recruitment of inflammatory cells to the atherosclerotic plaque↑ ↑IL-6 ∞ SMC↑ ↑IL-6 ∞ lipid content ↑ TG ↑ LDL ↑ lipid accumulation↓ |
**** | [121, 122, 126, 215] |
|
IL-6−/−∞lesion formation↑MMP-9↓pro-inflammatory cytokines↑ IL-6−/−∞serum cholesterol↑ |
** | [119, 120] | |
| IFN-γ |
↓ plaque destabilization,↑ foam cell,↑macrophage activity, ↑oxidative stress,↓IFN-γ∞↓ macrophage, ↓IFN-γ∞↓T lymphocyte ↑mini-TrpRS, ↑VSMC, ↑monocyte adhesion, ↓ECs glucose metabolism, ↑IFN-γ∞↑ECs dysfunction |
*** | [127, 128, 130, 131, 133, 135–138] |
| TNF-α |
↑pro-atherosclerotic factors, such as ICAM-1, VCAM-1, MCP-1 the paracrine ring between adipose cells and macrophages ↑the migration and proliferation of medial smooth muscle cells ↑the transcytosis of lipoproteins (e.g., LDL) across endothelial cells and macrophages ↑the intracellular cAMP level and the expression level of SRA co-activation of NF-κB and PPAR-γ ↑ DNA binding of Osf2, AP1, CREB and ↑vascular calcification |
**** | [141–149] |
| PAI-1 |
↑neointima formation ↑fibrin(ogen) accumulation; ↑thrombosis ↑cell proliferation and SMCs senescence ↑macrophage invasion |
**** | [151–155, 158] |
| RBP4 |
↑macrophage cholesterol uptake and foam cell formation ↑RBP4 serum levels in patients with established carotid atherosclerosis∞ the severity of atherosclerosis |
** | [161, 162] |
| LCN2 |
↓the stimulatory effect of lipopolysaccharide on cytokine gene expression ↑the development of aortic atherosclerotic lesions ↑intraplaque monocyte/macrophage infiltration and pentraxin-3 and collagen-1 expressions ↑ the production of IL6 ↑IL-8 ↑ monocyte chemotactic protein-1 in human macrophages ↑human coronary artery smooth muscle cells ↑ THP1 monocyte adhesion to HUVECs accompanied with upregulation of intercellular adhesion molecule-1 ↑vascular cell adhesion molecule-1 ↑E-selectin associated with nuclear factor-κB (NF-κB) upregulation |
*** | [163, 166, 167] |
| ∞↓Plaque size | [216] |
Rank of evidence: Weak (*), moderate (**), strong (***), stronger (****), Strongest (*****)
Table 3.
Adipokines with undefined roles in atherosclerosis
| Adipokines | Major function | References |
|---|---|---|
| Adipsin | ↑adipsin ∞ ↑ all-cause death and rehospitalization in CAD patients | [169] |
| adipsin do not impact atherosclerosis in Ldlr-/- mice | [170] | |
| IL-17 |
monocytes/chemokines/inflammatory cytokines produce ↑ monocyte chemotaxis ↑ macrophage differentiate↑ foam cell formation↓ immunization recruitment↑ |
[172–174] |
|
protective and regulatory role Inhibit pathogenic Th1 differentiate to Anti-inflammatory different gene backgrounds induce difference different atherosclerosis stage |
[175–178] | |
| Omentin |
circulating and EAT-derived omentin level ↓ in patients with CAD ↓macrophage accumulation, foam cell formation and mRNA expression of pro-inflammatory mediators (TNF-α, IL-6 and MCP-1); ↑anti-inflammatory M2 phenotype during macrophage phenotypic differentiation ↓lipid droplets and plasma total cholesterol levels ↓angiotensin II-induced VSMC migration and platelet-derived growth factor-BB-induced proliferation |
[179, 180] |
| an increased cardiovascular risk with high plasma omentin levels | [182] | |
| BMPs |
↑monocyte recruitment and chemoattraction through direct activation of BMPRII ↑endothelial inflammation and endothelial dysfunction ↑Hepatic Cholesterol Biosynthesis ↑vascular calcification |
[183, 184] |
| BMPRII knockdown ↑ endothelial inflammation, atherosclerosis | [185] | |
| NAMPT |
↑neutrophil infiltration; ↓collagen levels; ↑ MMP-9 content, CXCL1 levels, inflammation, macrophage number and apoptosis; ↓plasma HDL-C levels and cholesterol efflux ↑plaque area; |
[186, 187] |
| Ldlr−/− iNAMPThi ↓plaque burden; ↑ lesion stabilization; ↓macrophages to apoptosis | [188] | |
| Vaspin |
↓inflammatory phenotypes and foam cell formation; ↓migration and proliferation, ↑ collagen production Vaspin↑in macrophages/vascular smooth muscle cells (VSMCs) within human coronary atheromatous plaques |
[190] |
|
circulating vaspin and severity of AS: no association ↓vaspin serum concentrations ∞the recent presence of ischemic events in patients with carotid stenosis |
[189] | |
|
vaspin is linked to CV risk factors serum vaspin concentration is genetically modulated |
[191] |
Protective adipokines in atherosclerosis
Adiponectin
Adiponectin (Acrp30, AdipoQ, apM1) was identified as an adipokine by several independent groups, and has been initially shown to regulate lipid metabolism and insulin sensitivity [9–11]. A number of clinical studies have indicated that adiponectin could possibly be anti-atherogenic. A case–control study of 101 patients with type 1 diabetes revealed an inverse association with plasma adiponectin levels and the progression of coronary artery calcification (CAC) [12]. In patients with coronary heart disease, adiponectin was found to be positively associated with circulating high density lipoprotein-cholesterol (HDL-C), but negatively associated with plasma triglycerides (TG) [13, 14]. In another study, nondiabetic patients with low circulating adiponectin corresponded with intimal thickening, an increase in lipid-rich plaque, and elevated plasma lipoproteins [15]. Similar results were replicated in obese subjects in which adiponectin levels were inversely connected to intima-media thickness (IMT), serum triglycerides, fasting insulin, and insulin resistance using homeostasis model assessment-insulin resistance (HOMA-IR). Positive associations were found when evaluating large artery elasticity index (LAEI), small artery elasticity index (SAEI), and HDL-C [16]. Furthermore, a study in atherosclerotic patients suggested similar findings, as well as the discovery that adiponectin secretion from adipocytes was further dampened in patients who smoked [17]. Smoking has been identified as a risk factor for atherosclerosis. This study found adiponectin was decreased in smokers and proved that nicotine might reduce adiponectin expression via ATP-dependent potassium (KATP) channel in adipocytes.
In assessing inflammation, a clinical study in patients with CAD exhibited a decrease in adiponectin and an increase in IL-6, tumor necrosis factor-α (TNF-α), Toll-like receptor 4 (TLR4), and macrophage infiltration in epicardial adipose tissue [18]. Further strengthening the negative association between adiponectin and atherosclerosis, the Matsuzawa group at Osaka University uncovered a specific role for adiponectin that involves inhibiting the formation of foam cells by preventing lipid droplet accumulation and cholesterol loading in macrophages [19]. Mechanistically, this was achieved by inhibiting the expression and activity of the class A macrophage scavenger receptor (MSR) ligand [19]. An in vivo study from the same group showed a reduction in atherosclerotic lesion area in apolipoprotein E-deficient (apoE−/−) mice upon treatment with a recombinant adenovirus expressing adiponectin. Adiponectin downregulated the expression of vascular cell adhesion molecule-1(VCAM-1), MSR, and TNF-α, with no changes in CD36 [20]. Luo et al. overexpressed adiponectin in macrophages, resulting in decreased secretion of pro-inflammatory cytokines, such as monocyte chemotactic protein-1 (MCP-1) and TNF-α, prevented macrophage foam cell formation, and improved insulin sensitivity [21]. Kobashi et al. revealed that adiponectin could inhibit IL-8 through the cAMP–PKA–TNFα signaling pathway in human aortic endothelial cells (HAEC) [22]. Similarly, another study found that adiponectin could inhibit vascular endothelial growth factor (VEGF)-mediated endothelial cell (EC) migration through the cAMP–PKA signaling pathway in human coronary artery endothelial cells (HCAECs) [23]. To investigate the direct involvement of adiponectin with atherosclerosis outcome, the Scherer group employed adiponectin knockout mice and adiponectin overexpressing mice crossed with either low-density lipoprotein-deficient (Ldlr−/−) or apoE−/− mice. Surprisingly, the study conveyed no difference in lipoprotein profile, lesion area, and plaque morphology in either model [24]. Despite this, using mouse studies with T-cadherin and apoE double knockout mice, Fujishima et al. found an increase in atherosclerosis severity at 12 weeks on a high-cholesterol diet compared to control apoE−/− mice. The data further confirms adiponectin as an anti-atherogenic adipokine due to its required interactions with T-cadherin for proper functioning [25]. Overall, numerous studies indicate a protective role of adiponectin in atherosclerosis, although the undergoing molecular mechanism remains complex (Table 1, adiponectin).
FGF-21
FGF-21 is mainly secreted by the liver and skeletal muscle. FGF-21 has also been identified in adipose tissue as an adipokine and can enhance insulin sensitivity through regulating lipid metabolism [26–28]. In atherosclerosis, FGF-21 can alter the lipid profile by modulating transcription factors and key transporters involved in lipid metabolism. FGF-21 induces liver X receptors (LXR) to upregulate the expression of ABCA1 and ABCG1 in macrophages and promote cholesterol efflux [29]. Concurrently, FGF-21 lessens hypercholesterolemia by inhibiting the transcription factor sterol regulatory element-binding protein-2 (SREBP-2) in hepatocytes, which is involved in cholesterol biosynthesis [30]. In diabetic monkeys, FGF-21 treatment has been shown to reduce circulating TG and low-density lipoprotein (LDL), accompanied by an increase in HDL [31]. In oxidized low-density lipoprotein (oxLDL)-loaded THP-1 macrophages, FGF-21 can regulate foam cell formation, cell migration and death, inflammatory response, and lipid metabolism [32]. FGF-21 can further promote the secretion of the previously mentioned protective adipokine, adiponectin, which in turn can reduce endothelial dysfunction, suppress the proliferation of smooth muscle cells, and prevent the transformation of macrophages to foam cells [30]. In human umbilical vein endothelial cells (HUVECs), treatment with FGF-21 diminished the cytotoxic and apoptotic effects of hydrogen peroxide. Exogenous FGF-21 impeded the apoptosis of microvascular endothelial cells in rat hearts under atherosclerotic conditions, further suggesting a protective role in early atherosclerosis [33]. Another study presented FGF-21 to be protective against dyslipidemia in apoE−/− mice by inhibiting the inflammasome through NLRP3, preventing ROS buildup and production, and reducing ER stress [34]. As another well-established protective adipokine in atherosclerosis, FGF-21 is a promising therapeutic target (Table 1, FGF-21).
CTRP9
CTRP9, a newly discovered adipokine [35], can activate a variety of signaling pathways that exert anti-atherogenic effects, particularly in stabilizing carotid plaque. It is documented that CTRP9 attenuates vascular smooth muscle cell (VSMC) proliferation and VSMC phenotype switching by activating AMP-dependent kinase [36, 37]. CTRP9 decreases neointimal lesion formation [37], limits endothelial cell senescence through the AMPKα/KLF4 signaling pathway [38], and retards oxLDL-induced endothelial dysfunction through PGC-1α/AMPK-mediated antioxidant enzyme induction [39]. In the inflammatory response, CTRP9 downregulates pro-inflammatory cytokine secretion in macrophages [40] and upregulates the autophagy in atherosclerotic lesions through the AMPK/mTOR pathway [41]. In addition, the AMPK–NLRP3 inflammasome signaling pathway is involved in the atheroprotective function of CTRP9 [42]. Furthermore, CTRP9 lowers cholesterol uptake in VSMCs with an increase in the expression of cholesterol efflux-related molecules [36]. Besides these direct protections, CTRP9 may benefit atherosclerosis through improving glucose metabolism, particularly in the setting of T2DM [35, 43, 44] (Table 1, CTRP9).
Progranulin
Progranulin (PGRN) is a unique anti-inflammatory growth factor that regulates cell cycle and cell motility [45]. Progranulin is abundantly expressed in various cell types besides adipocytes, including immune cells, epithelial cells, neurons, and chondrocytes [46]. The anti-atherogenic effects of progranulin are mediated through influencing local and/or systemic inflammation and chemotaxis of VSMCs and macrophages, with the opposite occurring in studies with PGRN knockout mice [45, 47]. Kawase et al. proved that the protective effects of PGRN depended on anti-TNF-α [48]. There was a similar study showed that PGRN protected vascular endothelium countered with atherosclerotic inflammation and reduced TNF-α expression [49]. It is also demonstrated that PGRN directly binds to TNF receptors to affect the TNFα/TNFR interaction [46, 50]. Additionally, another mouse study by Nguyen et al. showed that hematopoietic deficiency of PGRN in Ldlr−/− mice promotes cholesterol uptake and foam cell formation [51] (Table 1, PGRN).
Deteriorative adipokines for atherosclerosis
Leptin
Leptin is the adipokine that declares adipose tissue as an endocrine organ [52]. Pathogenic leptin (in obesity) can accelerate atherogenesis [53]. A cross-sectional study involving 174 men and 26 women with T2DM found that plasma leptin levels were tightly correlated to coronary atherosclerosis [54]. In systemic lupus erythematosus (SLE) patients, leptin levels were also strongly associated with an increased risk of atherosclerosis, as well as lipid markers of inflammation, such as piHDL, Lp(a), and apoB100 [55]. In apoE−/− mice, 4 week administration of leptin (125 μg/day) significantly increased atherosclerosis and thrombosis after vascular injury [56]. Leptin-deficient mice (ob/ob) suppress atherogenesis when crossed with apoE−/− mice, independent of serum cholesterol, TNF-α, or adiponectin [57]. Consistent with the findings in apoE−/− mouse mode, ob/ob:Ldlr−/− mice are protected from atherosclerosis by reducing the T-cell helper type 1 (Th1) response and promoting regulatory T-cell (Treg) function [58]. Singh et al. showed that leptin could upregulate caveolin-1 and activate ERK1/2 and eNOS signaling in vascular endothelial cells [59]. Schroeter et al. found that apoE and caveolin-1 are critical in leptin-induced lesion development, ROS formation, and smooth muscle cell proliferation [60]. Raman et al. also demonstrated that leptin could induce atherosclerosis progression in apoE-/- mice, subsequently showing that this process can be reversed by knocking out thrombospondin-1 (TSP-1) [61]. TSP-1 deficiency inhibits leptin-induced atherosclerosis progression and reduces CREB activation and vimentin protein expression in aortic lysates without changing the plasma lipid profile [61].
However, the Multi-Ethnic Study of Atherosclerosis (MESA) revealed that leptin does not have a correlation with cardiovascular events. The study was conducted in men and women in different ethnic backgrounds, adjusted for multiple risk factors [62]. Additional animal studies with conflicting outcomes show varying roles of leptin in atherosclerosis. Severe hypercholesterolemia was observed in ob/ob:Ldlr−/− mice compared to Ldlr−/− mice despite chow diet feeding (0.075% cholesterol) [63]. Jun et al. found that a type 1 diabetes model, Ins2 + /Alkita:apoE−/− mouse, had 92% less leptin but an increased risk for atherosclerosis compared to nondiabetic Ins2 + / + :apoE−/− mice. Daily supplements of leptin reversed this risk in Ins2 + /Alkita:apoE−/− mice by significantly decreasing aortic arch lesion area, accompanied by upregulated hepatic sortilin-1, which is a receptor for LDL clearance [64]. Wei et al. showed that leptin receptor-mediated STAT3-independent signaling pathways offer protection against atherosclerosis in a model of obesity and hyperlipidemia using a selective leptin receptor-STAT3 signaling deficiency mouse model: Leprs/s:ApoE−/− [65]. Collectively, these data suggest that, although leptin can offer metabolism benefits, increased leptin levels are more likely to contribute to atherosclerosis progression through acting on multiple signaling pathways, including ROS, JNK, and STAT3, with obesity exacerbating the leptin-induced pathogenesis of CVDs (Table 2, leptin).
Chemerin
Similar to leptin, chemerin is a white-adipocyte-enriched adipokine [66]. A clinical study in patients with chest pain revealed a positive association between chemerin secretion and plasma levels of high-sensitivity C-reactive protein (CRP), IL-6, TNF-α, resistin, leptin, triglycerides, as well as body mass index (BMI) and hypertension. An inverse correlation was seen with circulating HDL-C. Despite this, after adjusting for established risk factors, chemerin is not a significant biomarker of atherosclerosis [67]. Another clinical study involving 367 hypertensive patients suggested plasma levels of chemerin to be an independent biomarker of arterial integrity and early stage atherosclerosis [68]. Chemerin mRNA levels in human epicardial adipose tissue are positively associated with TNF-α, BMI, waist circumference, fasting blood glucose, and Gensini score, which is an indication for the severity of atherosclerosis [69]. Adenovirus-mediated knockdown of chemerin in high-fat-diet-fed apoE−/− mice ameliorated atherosclerosis outcome, followed by decreasing pro-inflammatory cytokines, such as TNF-α and IL-1β [70] (Table 2, chemerin).
Resistin
Another representative white adipocyte-derived adipokine is resistin, which has multiple roles in the development of atherosclerosis, such as vascular inflammation, lipid accumulation, and plaque destabilization [71]. Clinical data imply that after an atherothrombotic ischemic stroke event, patients with high plasma resistin levels have an increased risk of 5-year mortality or disability [72]. Reilly et al. demonstrated that plasma resistin levels correlated with markers of inflammation and can predict coronary atherosclerosis in asymptomatic humans [73]. Animal studies also confirm the link between resistin and inflammation in CVDs. In obese and atherogenic albino rats, higher resistin levels are associated with worse pro-atherogenic lipid profile and inflammation [74]. In rabbits, resistin exacerbates atherosclerosis by inducing vascular inflammation [75]. Consistently, resistin overexpression in Ldlr−/− mice aggravates atherosclerosis burden, reduces brown fat tissue activity, and induces insulin resistance. These outcomes are attributed to resistin-mediated hypothalamic leptin resistance [76]. Resistin expression is notably increased in apoE−/− mice too. Additionally, Burnett et al. found that recombinant resistin treatment of murine aortic endothelial cells increased soluble vascular cell adhesion molecule (sVCAM) and monocyte chemoattractant protein (MCP)-1, two pro-atherogenic factors [77]. Resistin also significantly promotes the proliferation of rat VSMCs [78]. A study using patients’ samples showed that resistin inhibited neutrophil infiltration, likely contributing to the alleviated atherosclerotic plaque inflammation [79]. These studies conducted in various animal models and human samples are overall consistent in supporting a pro-atherogenic role of resistin (Table 2, resistin).
FABP4
Adipocyte fatty acid-binding protein (A-FABP; also known as FABP4 or aP2) is expressed in adipocytes and macrophages, influencing metabolic activity in a variety of ways. FABP4 was initially discovered in adipocytes as an intracellular protein activated by PPARγ to regulate lipid transport and fatty acid metabolism [80–83]. Early animal studies have shown that FABP4 deficiency in both adipocytes and macrophages improves hyperinsulinemia, hyperglycemia, insulin resistance, dyslipidemia, and fatty liver disease in the context of genetic and dietary obesity [84–86]. FABP4 was soon found to be secreted by adipocytes and abundantly present in the circulation and correlate with metabolic risks [87], macrovascular complications [88], and atherosclerosis [89, 90] in humans. From a large-cohort prospective study, serum FABP4 is a biomarker of higher risk of CVD mortality [91]. In another clinical study in a Chinese cohort, FABP4 was found to be positively associated with carotid atherosclerosis in Chinese women but not in men. This sex difference may be due to lower baseline serum FABP4 levels in men [90]. FABP4 also functions in macrophages to regulate the accumulation of cholesterol esters and inflammatory response [92]. Finally, atherosclerosis in apoE−/− mice was significantly reduced by FABP4 deficiency in macrophages [93]. (Table 2, FABP4).
IL-1β
Adipocytes also produce many nonexclusive cytokines that are expressed in the other tissues and types of cells. Among them, Interleukin-1β (IL-1β) is an innate inflammatory response factor that plays an important role in promoting the development of atherosclerosis [94, 95]. IL-1β is secreted upon the activation of the NLRP3 inflammasome. When stimulated, IL-1β triggers macrophages to release pro-inflammatory cytokines and activates T-helper cells. In atherosclerosis, IL-1β promotes immune cell recruitment and increases vascular permeability [96–98]. The size of aortic lesions in IL-1β knockout [99, 100] and neutralizing mice [101] are significantly reduced because of the dampened recruitment of monocytes and activation of macrophages to the intima. Serum IL-1β levels can serve as a biomarker of advanced stages of atherosclerosis [102], plaque calcification, and potentially fibrous caps formation [103].
Interestingly, IL-1β has an endogenous inhibitor, IL-1Ra. Deficiency in IL-1Ra promotes neointimal formation in mice after injury [104, 105]. Consistently, IL-1β inhibition with canakinumab significantly improved the reendothelialization of denuded carotid arteries and limited neointimal formation, an inflammatory response in the incidence of cardiovascular events [106]. It is thus plausible that targeting IL-1β offers therapeutic promise in atherosclerosis (Table 2, IL-1β).
IL-18
Interleukin-18 (IL-18) is a pro-inflammatory and pro-atherogenic cytokine modulating cholesterol efflux [107], plaque stabilization [108], and plaque rupture susceptibility [109, 110]. Genetic analysis of IL-18 variations in CAD patients suggests a causal role of IL-18 in atherosclerosis associated with higher mortality [111]. IL-18 inhibitors have been shown to prevent plaque progression and promote plaque stability [112]. It remains unclear whether IL-18 is an independent predictor of atherosclerosis or an indirect influencing factor. A more plausible consensus is that the pro-atherogenic effects of IL-18 are more likely to be dependent on IFN-γ [112–114] or other relevant factors [115–118] (Table 2, IL-18).
IL-6
Studies in IL-6 knockout atherogenic mouse models have shown that IL-6 can promote plaque formation, influence serum cholesterol, and upregulate matrix metalloprotein-9 (Mmp-9), which is associated with vulnerable plaques [119, 120]. Other work has shown that IL-6 is independently associated with the early onset of atherosclerosis [121]. IL-6 stimulation of VSMCs in vivo and in vitro activates the renin-angiotensin system, expands vascular oxidative stress and endothelial dysfunction, and impacts the migration and proliferation of VSMCs [122, 123]. In aged animals, elevated IL-6 levels induced vascular mitochondrial dysfunction and accelerated atherogenesis [124, 125]. Therapeutically, treatment of mice with an IL-6 inhibitor significantly suppressed endothelial activation, intimal smooth muscle cell infiltration, and monocyte recruitment, and subsequently impacted plaque progression [126]. The pathogenesis of IL-6 in atherosclerosis has been extensively studied in mice, potentially making it a desirable target for treatment (Table 2, IL-6).
IFN-γ
Interferon γ (IFN-γ) is a major inflammatory cytokine in atherosclerosis [127]. A prospective study of 2380 CAD patients followed for 56 months has revealed IFN-γ activity as a predictor for a long-term prognosis of major coronary events [128]. Both the pro- and anti-atherogenic effects of IFN-γ have been documented due to the complexities of its role in atherosclerosis [129, 130]. Previous studies have highlighted IFN-γ expression in lipid-laden macrophages of atherosclerosis lesions [131, 132] and at all stages of development [133]. In vitro, treatment of oxLDL-loaded THP-1 human macrophages with IFN-γ promoted foam cell formation and inhibited cholesterol 27-hydroxylase [134]. Endothelial cell function is imperative in maintaining normal vessel integrity. Lee et al. performed transcriptomic and metabolic analyses of HCAECs treated with IFN-γ and unraveled a metabolic shift in endothelial function with worsened glucose metabolism and increased fatty acid oxidation [135]. Sáez et al. validated these findings by linking IFN-γ and high glucose levels to endothelial dysfunction [136]. The plaque area was decreased by 75% in IFN-γ-deficient Ldlr−/− mice after 8 weeks on cholesterol-enriched diet feeding [137]. IFN-γ deficiency also decreased lesion size in apoE−/− mice fed with a cholesterol-enriched diet (0.15% cholesterol) for 12 weeks [138]. However, Niwa et al. found that IFN-γ produced by bone marrow-derived cells inhibited the advancement of atherosclerosis. After 6 weeks on a high-fat diet (HFD), Ldlr−/− mice received IFN-γ-deficient bone marrow developed larger lesions than those received control bone marrow without affecting lipid profiles [139]. The majority of studies on IFN-γ suggest a role of this cytokine in atherosclerosis progression and prove a benefit to consider IFN-γ therapies (Table 2, IFN-γ).
TNF-α
TNF-α is a cytokine of high biological value, and its production in adipose tissue is increased in obesity and T2DM [140]. Indeed, TNF-α is expressed by many cells, including adipocytes, monocytes, macrophages, endothelial cells, and VSMCs. It is appreciated that TNF-α promotes the progression of atherosclerosis through a variety of factors [141–149]. TNF-α upregulates the expression of intercellular cell adhesion molecule-1 (ICAM-1), scavenger receptor class A (SRA), and MCP-1 both in vitro and in vivo [143, 146, 149], and induces the migration and proliferation of medial smooth muscle cells in the vascular wall to the intima [145]. TNF-α also advances vascular calcification, mediated by the cAMP signaling pathway [141]. In mature bone marrow dendritic cell-derived exosomes, stimulating TNF-α can trigger the NF-κB pathway and elicit endothelial inflammation [148]. In regards to lipid and fatty acid metabolism, TNF-α can increase the transcytosis of lipoproteins (e.g., LDL) across endothelial cells and macrophages, eventually leading to LDL retention in the vascular wall [143, 147]. One study found no correlation between plaque progression and instability and the TNF-α receptor p55, suggesting that other receptors may mediate the TNF-α activity [150]. Altogether, TNF-α is a critical factor that warrants clinical significance for populations susceptible to CVDs. Meanwhile, the impact of its receptors and mediators need further characterization (Table 2, TNF-α).
PAI-1
Fibrinolytic imbalances in the progression of atherosclerosis have been observed in various experimental and clinical studies. Fibrous deposits in plaques can be removed by plasminogen activators. In advanced atherosclerosis, fibrin depositions are rampant, and plasminogen activators are downregulated [151–155]. Type 1 plasminogen activator inhibitor (PAI-1) is the primary inhibitor of plasminogen activators. Elevated expression levels of PAI-1 in the plasma and coronary plaques were found in metabolic syndrome patients [156]. It was also shown that male patients with metabolic syndrome were prone to thrombosis due to the increased PAI-1 [157]. The upregulation of PAI-1 induces neointima formation, fibrin(ogen) accumulation, and thrombosis [151–155]. Protection against atherosclerosis in PAI-1-deficient mice has ascertained its pro-atherogenic role, primarily improving fibrin clearance in plaques [151, 153]. Consistently, the expression of PAI-1 mRNA is found to increase in the arteries of patients with advanced atherosclerosis [158]. However, Sjoland et al. found that aortic PAI-1 expression has little to do with atherosclerosis progression [159]. Indeed, adipose tissue, particularly metabolically detrimental visceral fat, is a major source of PAI-1 in obesity and insulin resistance [160]. The effects of PAI-1 on neointimal lesion formation represent a previously unwitnessed role for the plasminogen activation system in the pathogenesis of atherosclerosis [151, 153] (Table 2, PAI-1).
RBP4
Retinol-binding protein 4 (RBP4), an adipokine mainly secreted from the liver and adipose tissue, negatively impacts glucose metabolism and insulin sensitivity [161]. Serum RBP4 levels positively correlated with the severity of carotid atherosclerosis in patients [162]. RBP4 invokes atherogenesis by promoting cholesterol uptake and inducing macrophage-derived foam cell formation. Elevated levels of circulating RBP4 can potentially be a predictor of atherosclerosis [161] (Table 2, RBP4).
LCN2
Lipocalin-2 (LCN2) is a complex bioactive hormone expressed in adipocytes, neutrophils, osteoblasts, and macrophages, primarily exhibiting antimicrobial effects, activating inflammatory cytokines, and regulating glucose homeostasis [163–165]. Serum LCN2 levels are positively correlated with the severity of CAD [166]. In apoE−/− mice, chronic administration of LCN2 accelerated the development of aortic lesions with increased monocyte and macrophage within plaques and increased plaque instability. LCN2 can also enhance the production of inflammatory cytokines such as IL-6, IL-8, and MCP-1 in macrophages and human coronary smooth muscle cells. In HUVECs co-cultured with THP1 monocytes, LCN2 treatment stimulates cell adhesion and increases gene expression of ICAM-1, VCAM-1, and NF-κB [167]. Additionally, LCN2 can impact endothelial cell and VSMC proliferation. Overall, LCN2 systemically contributes to atherosclerosis by activating inflammation, cell adhesion, foam cell formation, and plaque vulnerability [167] (Table 2, LCN2).
Adipokines with undefined roles in atherosclerosis
In addition to the adipokines mentioned above, other adipokines such as adipsin, Interleukin-17 (IL-17), omentin, bone morphogenetic proteins (BMPs), nicotinamide phosphoribosyl transferase (NAMPT), and Vaspin have been shown to somewhat be involved in atherosclerosis. Due to limited evidence or conflicting data, more work is needed to illustrate their explicit roles in atherosclerosis. In this review, we refer to these factors as undefined adipokines in atherosclerosis.
Adipsin
Adipsin (complement factor D) is the first cytokine identified to be produced in white adipose tissue, hence the discovery of adipocyte-derived cytokines: adipokines [168]. Ohtsuki et al. studied 370 patients with CAD and found plasma adipsin to be positively associated with mortality and rehospitalization, illuminating a potential role as a biomarker [169]. However, in animal studies, Adipisin−/−:Ldlr−/− double knockout mice displayed no significant differences in the aortic root and arch lesion area after 14 weeks on a western diet feeding [170]. Further studies are needed to establish a working model for adipsin in atherosclerosis (Table 3, adipsin).
IL-17
To date, there lacks consensus on whether IL-17 is protective or deteriorative in atherosclerosis [171]. Several mouse models and in vitro studies support a pro-atherogenic effect of IL-17 [172–174]. Here, IL-17 sustains an inflamed plaque microenvironment. Additional studies showed that IL-17 could be both pro- and anti-atherogenic [175, 176], whereas some studies stated that IL-17 has only protective effects. In the presence of well-known anti-inflammatory cytokines, IL-17 can be induced and may play a protective and regulatory role in atherogenesis. This may be due to anti-inflammatory Th17 cells that inhibit the differentiation of pathogenic Th1 cells. Taken together, IL-17 in the pathogenesis of atherosclerosis is unresolved and behaves differently based on the experimental models and context [175–178]. Moderate or severe atherosclerosis, a single gene or multiple gene knockouts, and patterns of dietary intervention all manifest varying outcomes. Establishing more consistent models to study IL-17 in atherosclerosis is thus needed (Table 3, IL-17).
Omentin
Omentin is a relatively new adipokine mainly expressed in visceral adipose tissue. It is documented that omentin can inhibit macrophage accumulation, foam cell formation, and the expression of pro-inflammatory genes (TNF-α, IL-6, and MCP-1) and promote an anti-inflammatory (M2-like) phenotype during macrophage differentiation in vitro and in vivo [179, 180]. Du et al. observed the down-regulation of omentin in the serum and epicardial adipose tissue (EAT) in patients with CAD [181]. On the contrary, Saely et al. found increased plasma omentin as a predictor of cardiovascular events in CAD patients [182]. Therefore, the role of omentin in CVDs remains uncertain [180] (Table 3, omentin).
BMPs
The expression of BMPs is known to increase in atherosclerosis. BMPs induce monocyte recruitment, endothelial inflammation, and endothelial dysfunction, particularly BMP4 and BMP2 [183, 184]. The balance between BMPs (2 and 4) and BMP antagonists influences these outcomes. Inhibition of BMPs in Ldlr−/− mice by a potent pharmacological BMP inhibitor (LDN-193189) influenced atherosclerosis regression [183, 184]. Simoes Sato et al. found that BMPs secreted by VSMCs in atherosclerotic lesions can induce monocyte chemotaxis via direct activation of BMP receptor II (BMPRII), while Kim et al. found that BMPRII down-regulation resulted in endothelial inflammation and atherosclerosis progression [184, 185]. The exact role of each individual BMP in atherosclerosis should be distinguished, so do their functioning mechanism (Table 3, BMPs).
NAMPT
NAMPT, also known for another name visfatin, is the key enzyme for NAD + biosynthesis from the precursor nicotinamide. It is produced by adipocytes and other inflammatory cells in adipose tissue, and has been connected to atherosclerosis and insulin resistance. Nencioni et al. used a pharmacological inhibitor of NAMPT to mitigate inflammation and downregulate neutrophil activation and recruitment in an atherosclerotic mouse model [186]. In cholesterol metabolism, NAMPT knockdown manifested protection by enhancing cholesterol efflux through the PPARα-LXRα- ABCA1/G1 pathway [187]. Notably, what is mentioned above involves the actions of extracellular NAMPT (eNAMPT). It has an intracellular isoform (iNAMPT). Bermudez et al. studied the leukocyte-specific overexpression of iNAMPT in mice and observed less plaque burden and increased lesion stabilization. The effects of iNAMPT are influenced by PPARγ and is independent of changes in eNAMPT [188]. Given the opposite functions of eNAMPT and iNAMPT in atherosclerosis, though the former is the true cytokine, we temporarily put NAMPT in this class of undefined adipokines (Table 3, NAMPT).
Vaspin
Visceral adipose tissue-derived serpin (vaspin) was initially identified as a novel adipokine related to obesity with insulin-sensitizing effects [189, 190]. Sato et al. indicated that vaspin is anti-atherosclerotic and improves plaque stability in apoE−/− mice [190]. In a large cohort of patients with axial spondylarthritis, serum vaspin is associated with CVD risk factors [191]. Another study found the down-regulation of serum vaspin levels to be a trace marker of recent ischemic events in patients with carotid stenosis [189]. The relation between circulating vaspin levels and the severity of atherosclerosis, therefore, needs further data both clinically and pre-clinically to be determined [189, 191] (Table 3, vaspin).
Discussion
Obesity can significantly increase the risk of T2DM and CAD. It is well known that besides the primary function in lipid storage, adipose tissue impacts the whole body via producing numerous adipokines. The secretion patterns of adipokines change in dysfunctional adipose tissue (such as in obesity) compared to normal functioning adipose tissue vary in depots, such as subcutaneous, visceral, and perivascular, and are also affected by nutrient status. Despite the keep-growing list of adipokines and new functions and mechanisms to be discovered, there is concrete evidence to conclude that adipose tissue can regulate atherosclerosis outcomes by means of adipokine.
Although different adipokines regulate the process of atherosclerosis in different ways, there is some commonality in the pathways shared by adipokines (Fig. 1). Representative protective adipokines such as adiponectin, CTRP9, and FGF-21 vary in their regulatory mechanisms. Adiponectin reduces MCP-1 expression in macrophages and VEGF in ECs; FGF-21 mainly impacts circulating levels of TG and LDL; and CTRP9 inhibits the adhesion of macrophages to VSMCs. Both adiponectin and FGF21 can reduce LDL-C and increase HDL-C to offer additional protection from atherosclerosis. Adipokines like leptin, chemerin, resistin, and LCN2 can activate pro-inflammatory cytokines, such as TNF-α and IL-1β, and thus accelerate the progression. The pro-inflammatory adipokines secreted from adipose tissue, including IL-1β, IL-18, IL-6, IFN-γ, and TNF-α, worsen the atherosclerosis burden and are exacerbated in obesity.
In summary, adipokines underlie the increased risk of atherosclerosis in obesity and T2DM and may serve as biomarkers of atherogenesis. However, investigating the exact roles of adipokines in atherosclerosis is warranted for future clinical applications. It should also be reminded that adipokines function in orchestration and changes in one adipokine may affect others. Categorizing adipokines into protective or deteriorative classes may incite synergetic strategies to treat atherosclerosis and CVDs.
Acknowledgements
We thank Dr. Bingxiang Xu from Shanghai University of Sport for his participating in designing the frame of this manuscript.
Author contributions
LL, ZS, XJ, WZ, JL, TZ, and LQ all contributed to the literature search, writing, and revising the manuscript. LL and LQ designed the frame of this manuscript. All authors contributed to this manuscript and approved the submitted version.
Funding
This work was supported by Shanghai Frontiers Science Research Base of Exercise and Metabolic Health, the research program of exercise and public health (0831) in Shanghai University of Sport, Shanghai higher education young teachers training funding program (A2-0213-22-0058-5) and the National Institutes of Health grants DK112943, DK128848, and HL087123 to L.Q.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors agree to publish this review.
Data availability
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Longhua Liu and Zunhan Shi have contributed equally to this work.
Contributor Information
Longhua Liu, Email: liulonghua@sus.edu.cn.
Li Qiang, Email: lq2123@cumc.columbia.edu.
References
- 1.World Health Organization (2020) World health statistics 2020: monitoring health for the SDGs, sustainable development goals. World Health Organization. https://apps.who.int/iris/handle/10665/332070. License: CC BY-NC-SA 3.0 IGO
- 2.Frostegard J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. doi: 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabas I, Lichtman AH. Monocyte-macrophages and T cells in atherosclerosis. Immunity. 2017;47(4):621–634. doi: 10.1016/j.immuni.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res. 2018;114(4):590–600. doi: 10.1093/cvr/cvy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby P, Ridker PM, Hansson GK. Leducq transatlantic network on A: inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giorda CB, Avogaro A, Maggini M, Lombardo F, Mannucci E, Turco S, Alegiani SS, Raschetti R, Velussi M, Ferrannini E, et al. Recurrence of cardiovascular events in patients with type 2 diabetes: epidemiology and risk factors. Diabetes Care. 2008;31(11):2154–2159. doi: 10.2337/dc08-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang W, Ye DD. The potential of adipokines as biomarkers and therapeutic agents for vascular complications in type 2 diabetes mellitus. Cytokine Growth Factor Rev. 2019;48:32–39. doi: 10.1016/j.cytogfr.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20(4):242–258. doi: 10.1038/s41580-018-0093-z. [DOI] [PubMed] [Google Scholar]
- 9.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270(45):26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 10.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271(18):10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 11.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose most abundant gene transcript 1) Biochem Biophys Res Commun. 1996;221(2):286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 12.Maahs DM, Ogden LG, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson JE, Ehrlich J, Eckel RH, Rewers M. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111(6):747–753. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 13.von Eynatten M, Hamann A, Twardella D, Nawroth PP, Brenner H, Rothenbacher D. Relationship of adiponectin with markers of systemic inflammation, atherogenic dyslipidemia, and heart failure in patients with coronary heart disease. Clin Chem. 2006;52(5):853–859. doi: 10.1373/clinchem.2005.060509. [DOI] [PubMed] [Google Scholar]
- 14.Patel JV, Abraheem A, Dotsenko O, Creamer J, Gunning M, Hughes EA, Lip GY. Circulating serum adiponectin levels in patients with coronary artery disease: relationship to atherosclerotic burden and cardiac function. J Intern Med. 2008;264(6):593–598. doi: 10.1111/j.1365-2796.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- 15.Marso SP, Mehta SK, Frutkin A, House JA, McCrary JR, Kulkarni KR. Low adiponectin levels are associated with atherogenic dyslipidemia and lipid-rich plaque in nondiabetic coronary arteries. Diabetes Care. 2008;31(5):989–994. doi: 10.2337/dc07-2024. [DOI] [PubMed] [Google Scholar]
- 16.Shargorodsky M, Boaz M, Goldberg Y, Matas Z, Gavish D, Fux A, Wolfson N. Adiponectin and vascular properties in obese patients: is it a novel biomarker of early atherosclerosis? Int J Obes (Lond) 2009;33(5):553–558. doi: 10.1038/ijo.2009.37. [DOI] [PubMed] [Google Scholar]
- 17.Fan LH, He Y, Xu W, Tian HY, Zhou Y, Liang Q, Huang X, Huo JH, Li HB, Bai L, et al. Adiponectin may be a biomarker of early atherosclerosis of smokers and decreased by nicotine through KATP channel in adipocytes. Nutrition. 2015;31(7–8):955–958. doi: 10.1016/j.nut.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Wei Y, Wang L, Wang X, Du X, Sun Z, Dong N, Chen X. Decreased adiponectin and increased inflammation expression in epicardial adipose tissue in coronary artery disease. Cardiovasc Diabetol. 2011;10:2. doi: 10.1186/1475-2840-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103(8):1057–1063. doi: 10.1161/01.CIR.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106(22):2767–2770. doi: 10.1161/01.CIR.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 21.Luo N, Liu J, Chung BH, Yang Q, Klein RL, Garvey WT, Fu Y. Macrophage adiponectin expression improves insulin sensitivity and protects against inflammation and atherosclerosis. Diabetes. 2010;59(4):791–799. doi: 10.2337/db09-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, Kihara S, Funahashi T, Takata M, Temaru R, Sato A, et al. Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res. 2005;97(12):1245–1252. doi: 10.1161/01.RES.0000194328.57164.36. [DOI] [PubMed] [Google Scholar]
- 23.Mahadev K, Wu X, Donnelly S, Ouedraogo R, Eckhart AD, Goldstein BJ. Adiponectin inhibits vascular endothelial growth factor-induced migration of human coronary artery endothelial cells. Cardiovasc Res. 2008;78(2):376–384. doi: 10.1093/cvr/cvn034. [DOI] [PubMed] [Google Scholar]
- 24.Nawrocki AR, Hofmann SM, Teupser D, Basford JE, Durand JL, Jelicks LA, Woo CW, Kuriakose G, Factor SM, Tanowitz HB, et al. Lack of association between adiponectin levels and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2010;30(6):1159–1165. doi: 10.1161/ATVBAHA.109.195826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujishima Y, Maeda N, Matsuda K, Masuda S, Mori T, Fukuda S, Sekimoto R, Yamaoka M, Obata Y, Kita S, et al. Adiponectin association with T-cadherin protects against neointima proliferation and atherosclerosis. Faseb j. 2017;31(4):1571–1583. doi: 10.1096/fj.201601064R. [DOI] [PubMed] [Google Scholar]
- 26.Wu X, Qi YF, Chang JR, Lu WW, Zhang JS, Wang SP, Cheng SJ, Zhang M, Fan Q, Lv Y, et al. Possible role of fibroblast growth factor 21 on atherosclerosis via amelioration of endoplasmic reticulum stress-mediated apoptosis in apoE(-/-) mice. Heart Vessels. 2015;30(5):657–668. doi: 10.1007/s00380-014-0557-9. [DOI] [PubMed] [Google Scholar]
- 27.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26(3):271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, Villarroya F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011;286(15):12983–12990. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabari FS, Karimian A, Parsian H, Rameshknia V, Mahmoodpour A, Majidinia M, Maniati M, Yousefi B. The roles of FGF21 in atherosclerosis pathogenesis. Rev Endocr Metab Disord. 2019;20(1):103–114. doi: 10.1007/s11154-019-09488-x. [DOI] [PubMed] [Google Scholar]
- 30.Jin L, Lin Z, Xu A. Fibroblast growth factor 21 protects against atherosclerosis via fine-tuning the multiorgan crosstalk. Diabetes Metab J. 2016;40(1):22–31. doi: 10.4093/dmj.2016.40.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shang W, Yu X, Wang H, Chen T, Fang Y, Yang X, Zhou P, Nie F, Zhou Q, Zhou J. Fibroblast growth factor 21 enhances cholesterol efflux in THP-1 macrophage-derived foam cells. Mol Med Rep. 2015;11(1):503–508. doi: 10.3892/mmr.2014.2731. [DOI] [PubMed] [Google Scholar]
- 32.Wang N, Li JY, Li S, Guo XC, Wu T, Wang WF, Li DS. Fibroblast growth factor 21 regulates foam cells formation and inflammatory response in Ox-LDL-induced THP-1 macrophages. Biomed Pharmacother. 2018;108:1825–1834. doi: 10.1016/j.biopha.2018.09.143. [DOI] [PubMed] [Google Scholar]
- 33.Wu L, Qian L, Zhang L, Zhang J, Zhou J, Li Y, Hou X, Fang Q, Li H, Jia W. Fibroblast growth factor 21 is related to atherosclerosis independent of nonalcoholic fatty liver disease and predicts atherosclerotic cardiovascular events. J Am Heart Assoc. 2020;9(11):e015226. doi: 10.1161/JAHA.119.015226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng Z, Zheng Q, Chen J, Tan X, Li Q, Ding L, Zhang R, Lin X. FGF21 mitigates atherosclerosis via inhibition of NLRP3 inflammasome-mediated vascular endothelial cells pyroptosis. Exp Cell Res. 2020;393(2):112108. doi: 10.1016/j.yexcr.2020.112108. [DOI] [PubMed] [Google Scholar]
- 35.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, Gimeno R, Lodish HF. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 2009;23(1):241–258. doi: 10.1096/fj.08-114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Q, Zhang H, Lin J, Zhang R, Chen S, Liu W, Sun M, Du W, Hou J, Yu B. C1q/TNF-related protein 9 inhibits the cholesterol-induced vascular smooth muscle cell phenotype switch and cell dysfunction by activating AMP-dependent kinase. J Cell Mol Med. 2017;21(11):2823–2836. doi: 10.1111/jcmm.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uemura Y, Shibata R, Ohashi K, Enomoto T, Kambara T, Yamamoto T, Ogura Y, Yuasa D, Joki Y, Matsuo K, et al. Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. Faseb j. 2013;27(1):25–33. doi: 10.1096/fj.12-213744. [DOI] [PubMed] [Google Scholar]
- 38.Wang G, Han B, Zhang R, Liu Q, Wang X, Huang X, Liu D, Qiao W, Yang M, Luo X, et al. C1q/TNF-related protein 9 attenuates atherosclerosis by inhibiting hyperglycemia-induced endothelial cell senescence through the ampkalpha/klf4 signaling pathway. Front Pharmacol. 2021;12:758792. doi: 10.3389/fphar.2021.758792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun H, Zhu X, Zhou Y, Cai W, Qiu L. C1q/TNF-related protein-9 ameliorates Ox-LDL-induced endothelial dysfunction via PGC-1alpha/AMPK-mediated antioxidant enzyme induction. Int J Mol Sci. 2017;18(6):1097. doi: 10.3390/ijms18061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Zhang P, Li T, Liu Y, Zhu Q, Chen T, Liu T, Huang C, Zhang J, Zhang Y, et al. CTRP9 enhances carotid plaque stability by reducing pro-inflammatory cytokines in macrophages. Biochem Biophys Res Commun. 2015;458(4):890–895. doi: 10.1016/j.bbrc.2015.02.054. [DOI] [PubMed] [Google Scholar]
- 41.Huang C, Zhang P, Li T, Li J, Liu T, Zuo A, Chen J, Guo Y. Overexpression of CTRP9 attenuates the development of atherosclerosis in apolipoprotein E-deficient mice. Mol Cell Biochem. 2019;455(1–2):99–108. doi: 10.1007/s11010-018-3473-y. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Gong X, Ni S, Wang Y, Zhu L, Ji N. C1q/TNF-related protein-9 attenuates atherosclerosis through AMPK-NLRP3 inflammasome singling pathway. Int Immunopharmacol. 2019;77:105934. doi: 10.1016/j.intimp.2019.105934. [DOI] [PubMed] [Google Scholar]
- 43.Peterson JM, Wei Z, Seldin MM, Byerly MS, Aja S, Wong GW. CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol. 2013;305(5):R522–533. doi: 10.1152/ajpregu.00110.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei Z, Lei X, Petersen PS, Aja S, Wong GW. Targeted deletion of C1q/TNF-related protein 9 increases food intake, decreases insulin sensitivity, and promotes hepatic steatosis in mice. Am J Physiol Endocrinol Metab. 2014;306(7):E779–790. doi: 10.1152/ajpendo.00593.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kojima Y, Ono K, Inoue K, Takagi Y, Kikuta K, Nishimura M, Yoshida Y, Nakashima Y, Matsumae H, Furukawa Y, et al. Progranulin expression in advanced human atherosclerotic plaque. Atherosclerosis. 2009;206(1):102–108. doi: 10.1016/j.atherosclerosis.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 46.Liu CJ, Bosch X. Progranulin: a growth factor, a novel TNFR ligand and a drug target. Pharmacol Ther. 2012;133(1):124–132. doi: 10.1016/j.pharmthera.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawase R, Ohama T, Matsuyama A, Matsuwaki T, Okada T, Yamashita T, Yuasa-Kawase M, Nakaoka H, Nakatani K, Inagaki M, et al. Deletion of progranulin exacerbates atherosclerosis in ApoE knockout mice. Cardiovasc Res. 2013;100(1):125–133. doi: 10.1093/cvr/cvt178. [DOI] [PubMed] [Google Scholar]
- 48.Wang BC, Liu H, Talwar A, Jian J. New discovery rarely runs smooth: an update on progranulin/TNFR interactions. Protein Cell. 2015;6(11):792–803. doi: 10.1007/s13238-015-0213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwang HJ, Jung TW, Hong HC, Choi HY, Seo JA, Kim SG, Kim NH, Choi KM, Choi DS, Baik SH, et al. Progranulin protects vascular endothelium against atherosclerotic inflammatory reaction via Akt/eNOS and nuclear factor-κB pathways. PLoS ONE. 2013;8(9):e76679. doi: 10.1371/journal.pone.0076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abella V, Scotece M, Conde J, Lopez V, Pirozzi C, Pino J, Gomez R, Lago F, Gonzalez-Gay MA, Gualillo O. The novel adipokine progranulin counteracts IL-1 and TLR4-driven inflammatory response in human and murine chondrocytes via TNFR1. Sci Rep. 2016;6:20356. doi: 10.1038/srep20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen AD, Nguyen TA, Singh RK, Eberle D, Zhang J, Abate JP, Robles A, Koliwad S, Huang EJ, Maxfield FR, et al. Progranulin in the hematopoietic compartment protects mice from atherosclerosis. Atherosclerosis. 2018;277:145–154. doi: 10.1016/j.atherosclerosis.2018.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 53.Peelman F, Waelput W, Iserentant H, Lavens D, Eyckerman S, Zabeau L, Tavernier J. Leptin: linking adipocyte metabolism with cardiovascular and autoimmune diseases. Prog Lipid Res. 2004;43(4):283–301. doi: 10.1016/j.plipres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Reilly MP, Iqbal N, Schutta M, Wolfe ML, Scally M, Localio AR, Rader DJ, Kimmel SE. Plasma leptin levels are associated with coronary atherosclerosis in type 2 diabetes. J Clin Endocrinol Metab. 2004;89(8):3872–3878. doi: 10.1210/jc.2003-031676. [DOI] [PubMed] [Google Scholar]
- 55.McMahon M, Skaggs BJ, Sahakian L, Grossman J, FitzGerald J, Ragavendra N, Charles-Schoeman C, Chernishof M, Gorn A, Witztum JL, et al. High plasma leptin levels confer increased risk of atherosclerosis in women with systemic lupus erythematosus, and are associated with inflammatory oxidised lipids. Ann Rheum Dis. 2011;70(9):1619–1624. doi: 10.1136/ard.2010.142737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bodary PF, Gu S, Shen Y, Hasty AH, Buckler JM, Eitzman DT. Recombinant leptin promotes atherosclerosis and thrombosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25(8):e119–122. doi: 10.1161/atvb.25.8.1634. [DOI] [PubMed] [Google Scholar]
- 57.Chiba T, Shinozaki S, Nakazawa T, Kawakami A, Ai M, Kaneko E, Kitagawa M, Kondo K, Chait A, Shimokado K. Leptin deficiency suppresses progression of atherosclerosis in apoE-deficient mice. Atherosclerosis. 2008;196(1):68–75. doi: 10.1016/j.atherosclerosis.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 58.Taleb S, Herbin O, Ait-Oufella H, Verreth W, Gourdy P, Barateau V, Merval R, Esposito B, Clement K, Holvoet P, et al. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(12):2691–2698. doi: 10.1161/ATVBAHA.107.149567. [DOI] [PubMed] [Google Scholar]
- 59.Singh P, Peterson TE, Sert-Kuniyoshi FH, Jensen MD, Somers VK. Leptin upregulates caveolin-1 expression: implications for development of atherosclerosis. Atherosclerosis. 2011;217(2):499–502. doi: 10.1016/j.atherosclerosis.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schroeter MR, Leifheit-Nestler M, Hubert A, Schumann B, Glückermann R, Eschholz N, Krüger N, Lutz S, Hasenfuss G, Konstantinides S, et al. Leptin promotes neointima formation and smooth muscle cell proliferation via NADPH oxidase activation and signalling in caveolin-rich microdomains. Cardiovasc Res. 2013;99(3):555–565. doi: 10.1093/cvr/cvt126. [DOI] [PubMed] [Google Scholar]
- 61.Ganguly R, Khanal S, Mathias A, Gupta S, Lallo J, Sahu S, Ohanyan V, Patel A, Storm K, Datta S, et al. TSP-1 (thrombospondin-1) deficiency protects ApoE(-/-) mice against leptin-induced atherosclerosis. Arterioscler Thromb Vasc Biol. 2021;41(2):e112–e127. doi: 10.1161/ATVBAHA.120.314962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin SS, Blaha MJ, Muse ED, Qasim AN, Reilly MP, Blumenthal RS, Nasir K, Criqui MH, McClelland RL, Hughes-Austin JM, et al. Leptin and incident cardiovascular disease: the multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2015;239(1):67–72. doi: 10.1016/j.atherosclerosis.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hasty AH, Shimano H, Osuga J, Namatame I, Takahashi A, Yahagi N, Perrey S, Iizuka Y, Tamura Y, Amemiya-Kudo M, et al. Severe hypercholesterolemia, hypertriglyceridemia, and atherosclerosis in mice lacking both leptin and the low density lipoprotein receptor. J Biol Chem. 2001;276(40):37402–37408. doi: 10.1074/jbc.M010176200. [DOI] [PubMed] [Google Scholar]
- 64.Jun JY, Ma Z, Pyla R, Segar L. Leptin treatment inhibits the progression of atherosclerosis by attenuating hypercholesterolemia in type 1 diabetic Ins2(+/Akita):apoE(-/-) mice. Atherosclerosis. 2012;225(2):341–347. doi: 10.1016/j.atherosclerosis.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo W, Bodary PF, Shen Y, Wickenheiser KJ, Ohman MK, Guo C, Bahrou KL, Myers MG, Jr, Eitzman DT. Leptin receptor-induced STAT3-independent signaling pathways are protective against atherosclerosis in a murine model of obesity and hyperlipidemia. Atherosclerosis. 2011;214(1):81–85. doi: 10.1016/j.atherosclerosis.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vernochet C, Peres SB, Davis KE, McDonald ME, Qiang L, Wang H, Scherer PE, Farmer SR. C/EBPalpha and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor gamma agonists. Mol Cell Biol. 2009;29(17):4714–4728. doi: 10.1128/MCB.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lehrke M, Becker A, Greif M, Stark R, Laubender RP, von Ziegler F, Lebherz C, Tittus J, Reiser M, Becker C, et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol. 2009;161(2):339–344. doi: 10.1530/EJE-09-0380. [DOI] [PubMed] [Google Scholar]
- 68.Gu P, Cheng M, Hui X, Lu B, Jiang W, Shi Z. Elevating circulation chemerin level is associated with endothelial dysfunction and early atherosclerotic changes in essential hypertensive patients. J Hypertens. 2015;33(8):1624–1632. doi: 10.1097/HJH.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 69.Gao X, Mi S, Zhang F, Gong F, Lai Y, Gao F, Zhang X, Wang L, Tao H. Association of chemerin mRNA expression in human epicardial adipose tissue with coronary atherosclerosis. Cardiovasc Diabetol. 2011;10:87. doi: 10.1186/1475-2840-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu H, Xiong W, Luo Y, Chen H, He Y, Cao Y, Dong S. Adipokine chemerin stimulates progression of atherosclerosis in ApoE(-/-) mice. Biomed Res Int. 2019;2019:7157865. doi: 10.1155/2019/7157865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou L, Li JY, He PP, Yu XH, Tang CK. Resistin: potential biomarker and therapeutic target in atherosclerosis. Clin Chim Acta. 2021;512:84–91. doi: 10.1016/j.cca.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 72.Efstathiou SP, Tsiakou AG, Tsioulos DI, Panagiotou TN, Pefanis AV, Achimastos AD, Mountokalakis TD. Prognostic significance of plasma resistin levels in patients with atherothrombotic ischemic stroke. Clin Chim Acta. 2007;378(1–2):78–85. doi: 10.1016/j.cca.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 73.Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111(7):932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 74.Sabry MM, Dawood AF, Rashed LA, Sayed SM, Hassan S, Younes SF. Relation between resistin, PPAR-gamma, obesity and atherosclerosis in male albino rats. Arch Physiol Biochem. 2020;126(5):389–398. doi: 10.1080/13813455.2018.1550094. [DOI] [PubMed] [Google Scholar]
- 75.Cho Y, Lee SE, Lee HC, Hur J, Lee S, Youn SW, Lee J, Lee HJ, Lee TK, Park J, et al. Adipokine resistin is a key player to modulate monocytes, endothelial cells, and smooth muscle cells, leading to progression of atherosclerosis in rabbit carotid artery. J Am Coll Cardiol. 2011;57(1):99–109. doi: 10.1016/j.jacc.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 76.Asterholm IW, Rutkowski JM, Fujikawa T, Cho YR, Fukuda M, Tao C, Wang ZV, Gupta RK, Elmquist JK, Scherer PE. Elevated resistin levels induce central leptin resistance and increased atherosclerotic progression in mice. Diabetologia. 2014;57(6):1209–1218. doi: 10.1007/s00125-014-3210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burnett MS, Lee CW, Kinnaird TD, Stabile E, Durrani S, Dullum MK, Devaney JM, Fishman C, Stamou S, Canos D, et al. The potential role of resistin in atherogenesis. Atherosclerosis. 2005;182(2):241–248. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 78.Hirai H, Satoh H, Kudoh A, Watanabe T. Interaction between resistin and adiponectin in the proliferation of rat vascular smooth muscle cells. Mol Cell Endocrinol. 2013;366(1):108–116. doi: 10.1016/j.mce.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 79.Liberale L, Bertolotto M, Carbone F, Contini P, Wust P, Spinella G, Pane B, Palombo D, Bonaventura A, Pende A, et al. Resistin exerts a beneficial role in atherosclerotic plaque inflammation by inhibiting neutrophil migration. Int J Cardiol. 2018;272:13–19. doi: 10.1016/j.ijcard.2018.07.112. [DOI] [PubMed] [Google Scholar]
- 80.Cook JS, Lucas JJ, Sibley E, Bolanowski MA, Christy RJ, Kelly TJ, Lane MD. Expression of the differentiation-induced gene for fatty acid-binding protein is activated by glucocorticoid and cAMP. Proc Natl Acad Sci U S A. 1988;85(9):2949–2953. doi: 10.1073/pnas.85.9.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amri EZ, Bertrand B, Ailhaud G, Grimaldi P. Regulation of adipose cell differentiation. I. Fatty acids are inducers of the aP2 gene expression. J Lipid Res. 1991;32(9):1449–1456. doi: 10.1016/S0022-2275(20)41912-1. [DOI] [PubMed] [Google Scholar]
- 82.Distel RJ, Robinson GS, Spiegelman BM. Fatty acid regulation of gene expression. Transcriptional and post-transcriptional mechanisms. J Biol Chem. 1992;267(9):5937–5941. doi: 10.1016/S0021-9258(18)42645-2. [DOI] [PubMed] [Google Scholar]
- 83.Kletzien RF, Foellmi LA, Harris PK, Wyse BM, Clarke SD. Adipocyte fatty acid-binding protein: regulation of gene expression in vivo and in vitro by an insulin-sensitizing agent. Mol Pharmacol. 1992;42(4):558–562. [PubMed] [Google Scholar]
- 84.Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274(5291):1377–1379. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- 85.Uysal KT, Scheja L, Wiesbrock SM, Bonner-Weir S, Hotamisligil GS. Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology. 2000;141(9):3388–3396. doi: 10.1210/endo.141.9.7637. [DOI] [PubMed] [Google Scholar]
- 86.Furuhashi M, Fucho R, Gorgun CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest. 2008;118(7):2640–2650. doi: 10.1172/JCI34750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang J, Wat NM, Wong WK, Lam KS. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem. 2006;52(3):405–413. doi: 10.1373/clinchem.2005.062463. [DOI] [PubMed] [Google Scholar]
- 88.Yeung DC, Xu A, Tso AW, Chow WS, Wat NM, Fong CH, Tam S, Sham PC, Lam KS. Circulating levels of adipocyte and epidermal fatty acid-binding proteins in relation to nephropathy staging and macrovascular complications in type 2 diabetic patients. Diabetes Care. 2009;32(1):132–134. doi: 10.2337/dc08-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yeung DC, Wang Y, Xu A, Cheung SC, Wat NM, Fong DY, Fong CH, Chau MT, Sham PC, Lam KS. Epidermal fatty-acid-binding protein: a new circulating biomarker associated with cardio-metabolic risk factors and carotid atherosclerosis. Eur Heart J. 2008;29(17):2156–2163. doi: 10.1093/eurheartj/ehn295. [DOI] [PubMed] [Google Scholar]
- 90.Hao Y, Ma X, Luo Y, Shen Y, Dou J, Pan X, Bao Y, Jia W. Serum adipocyte fatty acid binding protein levels are positively associated with subclinical atherosclerosis in Chinese pre- and postmenopausal women with normal glucose tolerance. J Clin Endocrinol Metab. 2014;99(11):4321–4327. doi: 10.1210/jc.2014-1832. [DOI] [PubMed] [Google Scholar]
- 91.von Eynatten M, Breitling LP, Roos M, Baumann M, Rothenbacher D, Brenner H. Circulating adipocyte fatty acid-binding protein levels and cardiovascular morbidity and mortality in patients with coronary heart disease: a 10-year prospective study. Arterioscler Thromb Vasc Biol. 2012;32(9):2327–2335. doi: 10.1161/ATVBAHA.112.248609. [DOI] [PubMed] [Google Scholar]
- 92.Hui X, Li H, Zhou Z, Lam KS, Xiao Y, Wu D, Ding K, Wang Y, Vanhoutte PM, Xu A. Adipocyte fatty acid-binding protein modulates inflammatory responses in macrophages through a positive feedback loop involving c-Jun NH2-terminal kinases and activator protein-1. J Biol Chem. 2010;285(14):10273–10280. doi: 10.1074/jbc.M109.097907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Layne MD, Patel A, Chen YH, Rebel VI, Carvajal IM, Pellacani A, Ith B, Zhao D, Schreiber BM, Yet SF, et al. Role of macrophage-expressed adipocyte fatty acid binding protein in the development of accelerated atherosclerosis in hypercholesterolemic mice. FASEB J. 2001;15(14):2733–2735. doi: 10.1096/fj.01-0374fje. [DOI] [PubMed] [Google Scholar]
- 94.Dinarello CA. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur J Immunol. 2011;41(5):1203–1217. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 95.Beaulieu LM, Lin E, Mick E, Koupenova M, Weinberg EO, Kramer CD, Genco CA, Tanriverdi K, Larson MG, Benjamin EJ, et al. Interleukin 1 receptor 1 and interleukin 1beta regulate megakaryocyte maturation, platelet activation, and transcript profile during inflammation in mice and humans. Arterioscler Thromb Vasc Biol. 2014;34(3):552–564. doi: 10.1161/ATVBAHA.113.302700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paramel Varghese G, Folkersen L, Strawbridge RJ, Halvorsen B, Yndestad A, Ranheim T, Krohg-Sorensen K, Skjelland M, Espevik T, Aukrust P, et al. NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc. 2016 doi: 10.1161/JAHA.115.003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qiao L, Ma J, Zhang Z, Sui W, Zhai C, Xu D, Wang Z, Lu H, Zhang M, Zhang C, et al. Deficient chaperone-mediated autophagy promotes inflammation and atherosclerosis. Circ Res. 2021;129(12):1141–1157. doi: 10.1161/CIRCRESAHA.121.318908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23(4):656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 100.Kamari Y, Shaish A, Shemesh S, Vax E, Grosskopf I, Dotan S, White M, Voronov E, Dinarello CA, Apte RN, et al. Reduced atherosclerosis and inflammatory cytokines in apolipoprotein-E-deficient mice lacking bone marrow-derived interleukin-1alpha. Biochem Biophys Res Commun. 2011;405(2):197–203. doi: 10.1016/j.bbrc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 101.Vromman A, Ruvkun V, Shvartz E, Wojtkiewicz G, Santos Masson G, Tesmenitsky Y, Folco E, Gram H, Nahrendorf M, Swirski FK, et al. Stage-dependent differential effects of interleukin-1 isoforms on experimental atherosclerosis. Eur Heart J. 2019;40(30):2482–2491. doi: 10.1093/eurheartj/ehz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gomez D, Baylis RA, Durgin BG, Newman AAC, Alencar GF, Mahan S, St Hilaire C, Muller W, Waisman A, Francis SE, et al. Interleukin-1beta has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat Med. 2018;24(9):1418–1429. doi: 10.1038/s41591-018-0124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ceneri N, Zhao L, Young BD, Healy A, Coskun S, Vasavada H, Yarovinsky TO, Ike K, Pardi R, Qin L, et al. Rac2 modulates atherosclerotic calcification by regulating macrophage interleukin-1beta production. Arterioscler Thromb Vasc Biol. 2017;37(2):328–340. doi: 10.1161/ATVBAHA.116.308507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Isoda K, Shiigai M, Ishigami N, Matsuki T, Horai R, Nishikawa K, Kusuhara M, Nishida Y, Iwakura Y, Ohsuzu F. Deficiency of interleukin-1 receptor antagonist promotes neointimal formation after injury. Circulation. 2003;108(5):516–518. doi: 10.1161/01.CIR.0000085567.18648.21. [DOI] [PubMed] [Google Scholar]
- 105.Chamberlain J, Evans D, King A, Dewberry R, Dower S, Crossman D, Francis S. Interleukin-1beta and signaling of interleukin-1 in vascular wall and circulating cells modulates the extent of neointima formation in mice. Am J Pathol. 2006;168(4):1396–1403. doi: 10.2353/ajpath.2006.051054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Viana-Huete V, Fuster JJ. Potential therapeutic value of interleukin 1b-targeted strategies in atherosclerotic cardiovascular disease. Rev Esp Cardiol (Engl Ed) 2019;72(9):760–766. doi: 10.1016/j.recesp.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 107.Bhat OM, Kumar PU, Giridharan NV, Kaul D, Kumar MJ, Dhawan V. Interleukin-18-induced atherosclerosis involves CD36 and NF-kappaB crosstalk in Apo E-/- mice. J Cardiol. 2015;66(1):28–35. doi: 10.1016/j.jjcc.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 108.Mallat Z, Corbaz A, Scoazec A, Besnard S, Leseche G, Chvatchko Y, Tedgui A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104(14):1598–1603. doi: 10.1161/hc3901.096721. [DOI] [PubMed] [Google Scholar]
- 109.Formanowicz D, Rybarczyk A, Radom M, Tanas K, Formanowicz P. A stochastic petri net-based model of the involvement of interleukin 18 in atherosclerosis. Int J Mol Sci. 2020;21(22):8574. doi: 10.3390/ijms21228574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hulthe J, McPheat W, Samnegard A, Tornvall P, Hamsten A, Eriksson P. Plasma interleukin (IL)-18 concentrations is elevated in patients with previous myocardial infarction and related to severity of coronary atherosclerosis independently of C-reactive protein and IL-6. Atherosclerosis. 2006;188(2):450–454. doi: 10.1016/j.atherosclerosis.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 111.Tiret L, Godefroy T, Lubos E, Nicaud V, Tregouet DA, Barbaux S, Schnabel R, Bickel C, Espinola-Klein C, Poirier O, et al. Genetic analysis of the interleukin-18 system highlights the role of the interleukin-18 gene in cardiovascular disease. Circulation. 2005;112(5):643–650. doi: 10.1161/CIRCULATIONAHA.104.519702. [DOI] [PubMed] [Google Scholar]
- 112.Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, Humbert Y, Chvatchko Y, Tedgui A. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res. 2001;89(7):E41–45. doi: 10.1161/hh1901.098735. [DOI] [PubMed] [Google Scholar]
- 113.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(-/-) mice through release of interferon-gamma. Circ Res. 2002;90(2):E34–38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 114.Tenger C, Sundborger A, Jawien J, Zhou X. IL-18 accelerates atherosclerosis accompanied by elevation of IFN-gamma and CXCL16 expression independently of T cells. Arterioscler Thromb Vasc Biol. 2005;25(4):791–796. doi: 10.1161/01.ATV.0000153516.02782.65. [DOI] [PubMed] [Google Scholar]
- 115.Wang J, Sun C, Gerdes N, Liu C, Liao M, Liu J, Shi MA, He A, Zhou Y, Sukhova GK, et al. Interleukin 18 function in atherosclerosis is mediated by the interleukin 18 receptor and the Na-Cl co-transporter. Nat Med. 2015;21(7):820–826. doi: 10.1038/nm.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Troseid M, Seljeflot I, Weiss TW, Klemsdal TO, Hjerkinn EM, Arnesen H. Arterial stiffness is independently associated with interleukin-18 and components of the metabolic syndrome. Atherosclerosis. 2010;209(2):337–339. doi: 10.1016/j.atherosclerosis.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 117.Zirlik A, Abdullah SM, Gerdes N, MacFarlane L, Schonbeck U, Khera A, McGuire DK, Vega GL, Grundy S, Libby P, et al. Interleukin-18, the metabolic syndrome, and subclinical atherosclerosis: results from the Dallas heart study. Arterioscler Thromb Vasc Biol. 2007;27(9):2043–2049. doi: 10.1161/ATVBAHA.107.149484. [DOI] [PubMed] [Google Scholar]
- 118.Suo F, Jiang F, Fang X, Ma A, Ma L. Contrast of diagnostic value between IL-17 combined with IL-18 and CT angiography in carotid atherosclerosis. Exp Ther Med. 2019;17(2):1400–1404. doi: 10.3892/etm.2018.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Madan M, Bishayi B, Hoge M, Amar S. Atheroprotective role of interleukin-6 in diet- and/or pathogen-associated atherosclerosis using an ApoE heterozygote murine model. Atherosclerosis. 2008;197(2):504–514. doi: 10.1016/j.atherosclerosis.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJ, Trautwein C, Luchtefeld M, Schmittkamp C, Heeneman S, et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110(22):3493–3500. doi: 10.1161/01.CIR.0000148135.08582.97. [DOI] [PubMed] [Google Scholar]
- 121.Lee WY, Allison MA, Kim DJ, Song CH, Barrett-Connor E. Association of interleukin-6 and C-reactive protein with subclinical carotid atherosclerosis (the Rancho Bernardo study) Am J Cardiol. 2007;99(1):99–102. doi: 10.1016/j.amjcard.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 122.Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Bohm M, Nickenig G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res. 2004;94(4):534–541. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- 123.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19(10):2364–2367. doi: 10.1161/01.ATV.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 124.Tyrrell DJ, Blin MG, Song J, Wood SC, Zhang M, Beard DA, Goldstein DR. Age-associated mitochondrial dysfunction accelerates atherogenesis. Circ Res. 2020;126(3):298–314. doi: 10.1161/CIRCRESAHA.119.315644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tyrrell DJ, Goldstein DR. Ageing and atherosclerosis: vascular intrinsic and extrinsic factors and potential role of IL-6. Nat Rev Cardiol. 2021;18(1):58–68. doi: 10.1038/s41569-020-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schuett H, Oestreich R, Waetzig GH, Annema W, Luchtefeld M, Hillmer A, Bavendiek U, von Felden J, Divchev D, Kempf T, et al. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32(2):281–290. doi: 10.1161/ATVBAHA.111.229435. [DOI] [PubMed] [Google Scholar]
- 127.Biros E, Reznik JE, Moran CS. Role of inflammatory cytokines in genesis and treatment of atherosclerosis. Trends Cardiovasc Med. 2021 doi: 10.1016/j.tcm.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 128.Pedersen ER, Midttun O, Ueland PM, Schartum-Hansen H, Seifert R, Igland J, Nordrehaug JE, Ebbing M, Svingen G, Bleie O, et al. Systemic markers of interferon-gamma-mediated immune activation and long-term prognosis in patients with stable coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;31(3):698–704. doi: 10.1161/ATVBAHA.110.219329. [DOI] [PubMed] [Google Scholar]
- 129.Harvey EJ, Ramji DP. Interferon-gamma and atherosclerosis: pro- or anti-atherogenic? Cardiovasc Res. 2005;67(1):11–20. doi: 10.1016/j.cardiores.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 130.McLaren JE, Ramji DP. Interferon gamma: a master regulator of atherosclerosis. Cytokine Growth Factor Rev. 2009;20(2):125–135. doi: 10.1016/j.cytogfr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 131.Voloshyna I, Littlefield MJ, Reiss AB. Atherosclerosis and interferon-gamma: new insights and therapeutic targets. Trends Cardiovasc Med. 2014;24(1):45–51. doi: 10.1016/j.tcm.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu XH, Zhang J, Zheng XL, Yang YH, Tang CK. Interferon-gamma in foam cell formation and progression of atherosclerosis. Clin Chim Acta. 2015;441:33–43. doi: 10.1016/j.cca.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 133.Elyasi A, Voloshyna I, Ahmed S, Kasselman LJ, Behbodikhah J, De Leon J, Reiss AB. The role of interferon-gamma in cardiovascular disease: an update. Inflamm Res. 2020;69(10):975–988. doi: 10.1007/s00011-020-01382-6. [DOI] [PubMed] [Google Scholar]
- 134.Kushiyama A, Sakoda H, Oue N, Okubo M, Nakatsu Y, Ono H, Fukushima T, Kamata H, Nishimura F, Kikuchi T, et al. Resistin-like molecule beta is abundantly expressed in foam cells and is involved in atherosclerosis development. Arterioscler Thromb Vasc Biol. 2013;33(8):1986–1993. doi: 10.1161/ATVBAHA.113.301546. [DOI] [PubMed] [Google Scholar]
- 135.Lee LY, Oldham WM, He H, Wang R, Mulhern R, Handy DE, Loscalzo J. Interferon-gamma impairs human coronary artery endothelial glucose metabolism by tryptophan catabolism and activates fatty acid oxidation. Circulation. 2021;144(20):1612–1628. doi: 10.1161/CIRCULATIONAHA.121.053960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saez JC, Contreras-Duarte S, Labra VC, Santibanez CA, Mellado LA, Inostroza CA, Alvear TF, Retamal MA, Velarde V, Orellana JA. Interferon-gamma and high glucose-induced opening of Cx43 hemichannels causes endothelial cell dysfunction and damage. Biochim Biophys Acta Mol Cell Res. 2020;1867(8):118720. doi: 10.1016/j.bbamcr.2020.118720. [DOI] [PubMed] [Google Scholar]
- 137.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23(3):454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 138.Whitman SC, Ravisankar P, Daugherty A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E-/- mice. J Interferon Cytokine Res. 2002;22(6):661–670. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 139.Niwa T, Wada H, Ohashi H, Iwamoto N, Ohta H, Kirii H, Fujii H, Saito K, Seishima M. Interferon-gamma produced by bone marrow-derived cells attenuates atherosclerotic lesion formation in LDLR-deficient mice. J Atheroscler Thromb. 2004;11(2):79–87. doi: 10.5551/jat.11.79. [DOI] [PubMed] [Google Scholar]
- 140.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 141.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102(21):2636–2642. doi: 10.1161/01.CIR.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 142.Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24(11):2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 143.Ohta H, Wada H, Niwa T, Kirii H, Iwamoto N, Fujii H, Saito K, Sekikawa K, Seishima M. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis. 2005;180(1):11–17. doi: 10.1016/j.atherosclerosis.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 144.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25(10):2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 145.Zhang L, Peppel K, Sivashanmugam P, Orman ES, Brian L, Exum ST, Freedman NJ. Expression of tumor necrosis factor receptor-1 in arterial wall cells promotes atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(5):1087–1094. doi: 10.1161/01.ATV.0000261548.49790.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xiao N, Yin M, Zhang L, Qu X, Du H, Sun X, Mao L, Ren G, Zhang C, Geng Y, et al. Tumor necrosis factor-alpha deficiency retards early fatty-streak lesion by influencing the expression of inflammatory factors in apoE-null mice. Mol Genet Metab. 2009;96(4):239–244. doi: 10.1016/j.ymgme.2008.11.166. [DOI] [PubMed] [Google Scholar]
- 147.Zhang Y, Yang X, Bian F, Wu P, Xing S, Xu G, Li W, Chi J, Ouyang C, Zheng T, et al. TNF-α promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: crosstalk between NF-κB and PPAR-γ. J Mol Cell Cardiol. 2014;72:85–94. doi: 10.1016/j.yjmcc.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 148.Gao W, Liu H, Yuan J, Wu C, Huang D, Ma Y, Zhu J, Ma L, Guo J, Shi H, et al. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-alpha mediated NF-kappaB pathway. J Cell Mol Med. 2016;20(12):2318–2327. doi: 10.1111/jcmm.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tay C, Liu YH, Hosseini H, Kanellakis P, Cao A, Peter K, Tipping P, Bobik A, Toh BH, Kyaw T. B-cell-specific depletion of tumour necrosis factor alpha inhibits atherosclerosis development and plaque vulnerability to rupture by reducing cell death and inflammation. Cardiovasc Res. 2016;111(4):385–397. doi: 10.1093/cvr/cvw186. [DOI] [PubMed] [Google Scholar]
- 150.Blessing E, Bea F, Kuo CC, Campbell LA, Chesebro B, Rosenfeld ME. Lesion progression and plaque composition are not altered in older apoE-/- mice lacking tumor necrosis factor-alpha receptor p55. Atherosclerosis. 2004;176(2):227–232. doi: 10.1016/j.atherosclerosis.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 151.Eitzman DT, Westrick RJ, Xu Z, Tyson J, Ginsburg D. Plasminogen activator inhibitor-1 deficiency protects against atherosclerosis progression in the mouse carotid artery. Blood. 2000;96(13):4212–4215. doi: 10.1182/blood.V96.13.4212. [DOI] [PubMed] [Google Scholar]
- 152.DeYoung MB, Tom C, Dichek DA. Plasminogen activator inhibitor type 1 increases neointima formation in balloon-injured rat carotid arteries. Circulation. 2001;104(16):1972–1971. doi: 10.1161/hc4101.097110. [DOI] [PubMed] [Google Scholar]
- 153.Zhu Y, Farrehi PM, Fay WP. Plasminogen activator inhibitor type 1 enhances neointima formation after oxidative vascular injury in atherosclerosis-prone mice. Circulation. 2001;103(25):3105–3110. doi: 10.1161/01.CIR.103.25.3105. [DOI] [PubMed] [Google Scholar]
- 154.Schafer K, Muller K, Hecke A, Mounier E, Goebel J, Loskutoff DJ, Konstantinides S. Enhanced thrombosis in atherosclerosis-prone mice is associated with increased arterial expression of plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol. 2003;23(11):2097–2103. doi: 10.1161/01.ATV.0000097766.36623.DF. [DOI] [PubMed] [Google Scholar]
- 155.Khoukaz HB, Ji Y, Braet DJ, Vadali M, Abdelhamid AA, Emal CD, Lawrence DA, Fay WP. Drug targeting of plasminogen activator inhibitor-1 inhibits metabolic dysfunction and atherosclerosis in a murine model of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2020;40(6):1479–1490. doi: 10.1161/ATVBAHA.119.313775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zorio E, Gilabert-Estelles J, Espana F, Ramon LA, Cosin R, Estelles A. Fibrinolysis: the key to new pathogenetic mechanisms. Curr Med Chem. 2008;15(9):923–929. doi: 10.2174/092986708783955455. [DOI] [PubMed] [Google Scholar]
- 157.Suehiro A, Wakabayashi I, Uchida K, Yamashita T, Yamamoto J. Impaired spontaneous thrombolytic activity measured by global thrombosis test in males with metabolic syndrome. Thromb Res. 2012;129(4):499–501. doi: 10.1016/j.thromres.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 158.Schneiderman J, Sawdey MS, Keeton MR, Bordin GM, Bernstein EF, Dilley RB, Loskutoff DJ. Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proc Natl Acad Sci U S A. 1992;89(15):6998–7002. doi: 10.1073/pnas.89.15.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sjoland H, Eitzman DT, Gordon D, Westrick R, Nabel EG, Ginsburg D. Atherosclerosis progression in LDL receptor-deficient and apolipoprotein E-deficient mice is independent of genetic alterations in plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol. 2000;20(3):846–852. doi: 10.1161/01.ATV.20.3.846. [DOI] [PubMed] [Google Scholar]
- 160.Kaji H. Adipose tissue-derived plasminogen activator inhibitor-1 function and regulation. Compr Physiol. 2016;6(4):1873–1896. doi: 10.1002/cphy.c160004. [DOI] [PubMed] [Google Scholar]
- 161.Liu Y, Zhong Y, Chen H, Wang D, Wang M, Ou JS, Xia M. Retinol-binding protein-dependent cholesterol uptake regulates macrophage foam cell formation and promotes atherosclerosis. Circulation. 2017;135(14):1339–1354. doi: 10.1161/CIRCULATIONAHA.116.024503. [DOI] [PubMed] [Google Scholar]
- 162.Kadoglou NP, Lambadiari V, Gastounioti A, Gkekas C, Giannakopoulos TG, Koulia K, Maratou E, Alepaki M, Kakisis J, Karakitsos P, et al. The relationship of novel adipokines, RBP4 and omentin-1, with carotid atherosclerosis severity and vulnerability. Atherosclerosis. 2014;235(2):606–612. doi: 10.1016/j.atherosclerosis.2014.05.957. [DOI] [PubMed] [Google Scholar]
- 163.Wu G, Li H, Zhou M, Fang Q, Bao Y, Xu A, Jia W. Mechanism and clinical evidence of lipocalin-2 and adipocyte fatty acid-binding protein linking obesity and atherosclerosis. Diabetes Metab Res Rev. 2014;30(6):447–456. doi: 10.1002/dmrr.2493. [DOI] [PubMed] [Google Scholar]
- 164.Mosialou I, Shikhel S, Luo N, Petropoulou PI, Panitsas K, Bisikirska B, Rothman NJ, Tenta R, Cariou B, Wargny M, et al. Lipocalin-2 counteracts metabolic dysregulation in obesity and diabetes. J Exp Med. 2020 doi: 10.1084/jem.20191261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mosialou I, Shikhel S, Liu JM, Maurizi A, Luo N, He Z, Huang Y, Zong H, Friedman RA, Barasch J, et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017;543(7645):385–390. doi: 10.1038/nature21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xiao Y, Xu A, Hui X, Zhou P, Li X, Zhong H, Tang W, Huang G, Zhou Z. Circulating lipocalin-2 and retinol-binding protein 4 are associated with intima-media thickness and subclinical atherosclerosis in patients with type 2 diabetes. PLoS ONE. 2013;8(6):e66607. doi: 10.1371/journal.pone.0066607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shibata K, Sato K, Shirai R, Seki T, Okano T, Yamashita T, Koide A, Mitsuboshi M, Mori Y, Hirano T, et al. Lipocalin-2 exerts pro-atherosclerotic effects as evidenced by in vitro and in vivo experiments. Heart Vessels. 2020;35(7):1012–1024. doi: 10.1007/s00380-020-01556-6. [DOI] [PubMed] [Google Scholar]
- 168.Cook KS, Min HY, Johnson D, Chaplinsky RJ, Flier JS, Hunt CR, Spiegelman BM. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237(4813):402–405. doi: 10.1126/science.3299705. [DOI] [PubMed] [Google Scholar]
- 169.Ohtsuki T, Satoh K, Shimizu T, Ikeda S, Kikuchi N, Satoh T, Kurosawa R, Nogi M, Sunamura S, Yaoita N, et al. Identification of adipsin as a novel prognostic biomarker in patients with coronary artery disease. J Am Heart Assoc. 2019;8(23):e013716. doi: 10.1161/JAHA.119.013716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu L, Chan M, Yu L, Wang W, Qiang L. Adipsin deficiency does not impact atherosclerosis development in Ldlr(-/-) mice. Am J Physiol Endocrinol Metab. 2021;320(1):E87–e92. doi: 10.1152/ajpendo.00440.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, et al. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31(7):1565–1572. doi: 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121(15):1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.de la Paz S-M, Blanco-Favela F, Mora-Ruiz MD, Chavez-Rueda AK, Bernabe-Garcia M, Chavez-Sanchez L. IL-17-differentiated macrophages secrete pro-inflammatory cytokines in response to oxidized low-density lipoprotein. Lipids Health Dis. 2017;16(1):196. doi: 10.1186/s12944-017-0588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Usui F, Kimura H, Ohshiro T, Tatsumi K, Kawashima A, Nishiyama A, Iwakura Y, Ishibashi S, Takahashi M. Interleukin-17 deficiency reduced vascular inflammation and development of atherosclerosis in Western diet-induced apoE-deficient mice. Biochem Biophys Res Commun. 2012;420(1):72–77. doi: 10.1016/j.bbrc.2012.02.117. [DOI] [PubMed] [Google Scholar]
- 175.Liuzzo G, Trotta F, Pedicino D. Interleukin-17 in atherosclerosis and cardiovascular disease: the good, the bad, and the unknown. Eur Heart J. 2013;34(8):556–559. doi: 10.1093/eurheartj/ehs399. [DOI] [PubMed] [Google Scholar]
- 176.Ghoreschi K, Laurence A, Yang XP, Hirahara K, O'Shea JJ. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 2011;32(9):395–401. doi: 10.1016/j.it.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen S, Crother TR, Arditi M. Emerging role of IL-17 in atherosclerosis. J Innate Immun. 2010;2(4):325–333. doi: 10.1159/000314626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 179.Hiramatsu-Ito M, Shibata R, Ohashi K, Uemura Y, Kanemura N, Kambara T, Enomoto T, Yuasa D, Matsuo K, Ito M, et al. Omentin attenuates atherosclerotic lesion formation in apolipoprotein E-deficient mice. Cardiovasc Res. 2016;110(1):107–117. doi: 10.1093/cvr/cvv282. [DOI] [PubMed] [Google Scholar]
- 180.Watanabe K, Watanabe R, Konii H, Shirai R, Sato K, Matsuyama TA, Ishibashi-Ueda H, Koba S, Kobayashi Y, Hirano T, et al. Counteractive effects of omentin-1 against atherogenesisdagger. Cardiovasc Res. 2016;110(1):118–128. doi: 10.1093/cvr/cvw016. [DOI] [PubMed] [Google Scholar]
- 181.Du Y, Ji Q, Cai L, Huang F, Lai Y, Liu Y, Yu J, Han B, Zhu E, Zhang J, et al. Association between omentin-1 expression in human epicardial adipose tissue and coronary atherosclerosis. Cardiovasc Diabetol. 2016;15:90. doi: 10.1186/s12933-016-0406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Saely CH, Leiherer A, Muendlein A, Vonbank A, Rein P, Geiger K, Malin C, Drexel H. High plasma omentin predicts cardiovascular events independently from the presence and extent of angiographically determined atherosclerosis. Atherosclerosis. 2016;244:38–43. doi: 10.1016/j.atherosclerosis.2015.10.100. [DOI] [PubMed] [Google Scholar]
- 183.Derwall M, Malhotra R, Lai CS, Beppu Y, Aikawa E, Seehra JS, Zapol WM, Bloch KD, Yu PB. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(3):613–622. doi: 10.1161/ATVBAHA.111.242594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Simoes Sato AY, Bub GL, Campos AH. BMP-2 and -4 produced by vascular smooth muscle cells from atherosclerotic lesions induce monocyte chemotaxis through direct BMPRII activation. Atherosclerosis. 2014;235(1):45–55. doi: 10.1016/j.atherosclerosis.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 185.Kim CW, Song H, Kumar S, Nam D, Kwon HS, Chang KH, Son DJ, Kang DW, Brodie SA, Weiss D, et al. Anti-inflammatory and antiatherogenic role of BMP receptor II in endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(6):1350–1359. doi: 10.1161/ATVBAHA.112.300287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nencioni A, da Silva RF, Fraga-Silva RA, Steffens S, Fabre M, Bauer I, Caffa I, Magnone M, Sociali G, Quercioli A, et al. Nicotinamide phosphoribosyltransferase inhibition reduces intraplaque CXCL1 production and associated neutrophil infiltration in atherosclerotic mice. Thromb Haemost. 2014;111(2):308–322. doi: 10.1160/TH13-07-0531. [DOI] [PubMed] [Google Scholar]
- 187.Li S, Wang C, Li K, Li L, Tian M, Xie J, Yang M, Jia Y, He J, Gao L, et al. NAMPT knockdown attenuates atherosclerosis and promotes reverse cholesterol transport in ApoE KO mice with high-fat-induced insulin resistance. Sci Rep. 2016;6:26746. doi: 10.1038/srep26746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bermudez B, Dahl TB, Medina I, Groeneweg M, Holm S, Paz SM, Rousch M, Otten J, Herias V, Varela LM, et al. leukocyte overexpression of intracellular NAMPT attenuates atherosclerosis by regulating PPARgamma-dependent monocyte differentiation and function. Arterioscler Thromb Vasc Biol. 2017;37(6):1157–1167. doi: 10.1161/ATVBAHA.116.308187. [DOI] [PubMed] [Google Scholar]
- 189.Aust G, Richter O, Rohm S, Kerner C, Hauss J, Kloting N, Ruschke K, Kovacs P, Youn BS, Bluher M. Vaspin serum concentrations in patients with carotid stenosis. Atherosclerosis. 2009;204(1):262–266. doi: 10.1016/j.atherosclerosis.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 190.Sato K, Shirai R, Yamaguchi M, Yamashita T, Shibata K, Okano T, Mori Y, Matsuyama TA, Ishibashi-Ueda H, Hirano T, et al. Anti-atherogenic effects of vaspin on human aortic smooth muscle cell/macrophage responses and hyperlipidemic mouse plaque phenotype. Int J Mol Sci. 2018 doi: 10.3390/ijms19061732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rueda-Gotor J, Lopez-Mejias R, Remuzgo-Martinez S, Pulito-Cueto V, Corrales A, Lera-Gomez L, Portilla V, Gonzalez-Mazon I, Blanco R, Exposito R, et al. Vaspin in atherosclerotic disease and cardiovascular risk in axial spondyloarthritis: a genetic and serological study. Arthritis Res Ther. 2021;23(1):111. doi: 10.1186/s13075-021-02499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Basurto L, Gregory MA, Hernandez SB, Sanchez-Huerta L, Martinez AD, Manuel-Apolinar L, Avelar FJ, Alonso LAM, Sanchez-Arenas R. Monocyte chemoattractant protein-1 (MCP-1) and fibroblast growth factor-21 (FGF-21) as biomarkers of subclinical atherosclerosis in women. Exp Gerontol. 2019;124:110624. doi: 10.1016/j.exger.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 193.Jia H, Cheng J, Zhou Q, Peng J, Pan Y, Han H. Fibroblast growth factor 21 attenuates inflammation and oxidative stress in atherosclerotic rat via enhancing the Nrf1-ARE signaling pathway. Int J Clin Exp Pathol. 2018;11(3):1308–1317. [PMC free article] [PubMed] [Google Scholar]
- 194.Kokkinos J, Tang S, Rye KA, Ong KL. The role of fibroblast growth factor 21 in atherosclerosis. Atherosclerosis. 2017;257:259–265. doi: 10.1016/j.atherosclerosis.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 195.Li E, Wang T, Wang F, Wang T, Sun LQ, Li L, Niu SH, Zhang JY. FGF21 protects against ox-LDL induced apoptosis through suppressing CHOP expression in THP1 macrophage derived foam cells. BMC Cardiovasc Disord. 2015;15:80. doi: 10.1186/s12872-015-0077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y, Jin L, Lian Q, Huang Y, Ding H, et al. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation. 2015;131(21):1861–1871. doi: 10.1161/CIRCULATIONAHA.115.015308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Maeng HJ, Lee GY, Bae JH, Lim S. Effect of fibroblast growth factor 21 on the development of atheromatous plaque and lipid metabolic profiles in an atherosclerosis-prone mouse model. Int J Mol Sci. 2020;21(18):6836. doi: 10.3390/ijms21186836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pan ZC, Wang SP, Ou TT, Liu H, Ma JW, Wang WX, Fang WY, Qu XK, Zhang M. A study on the expression of FGF-21 and NF-kappaB pathway in the tissues of atherosclerotic mice. Eur Rev Med Pharmacol Sci. 2017;21(3 Suppl):102–107. [PubMed] [Google Scholar]
- 199.Wu X, Lu Y, Fu K, Wang S, Zhao D, Peng H, Fan Q, Lu Y, Xin M, Liu J. Impact of exogenous fibroblast growth factor 21 on atherosclerosis in apolipoprotein E deficient mice. Zhonghua Xin Xue Guan Bing Za Zhi. 2014;42(2):126–131. [PubMed] [Google Scholar]
- 200.Yafei S, Elsewy F, Youssef E, Ayman M, El-Shafei M. Fibroblast growth factor 21 association with subclinical atherosclerosis and arterial stiffness in type 2 diabetes. Diabetes Metab Syndr. 2019;13(1):882–888. doi: 10.1016/j.dsx.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 201.Yan X, Gou Z, Li Y, Wang Y, Zhu J, Xu G, Zhang Q. Fibroblast growth factor 21 inhibits atherosclerosis in apoE-/- mice by ameliorating Fas-mediated apoptosis. Lipids Health Dis. 2018;17(1):203. doi: 10.1186/s12944-018-0846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhu W, Wang C, Liu L, Li Y, Li X, Cai J, Wang H. Effects of fibroblast growth factor 21 on cell damage in vitro and atherosclerosis in vivo. Can J Physiol Pharmacol. 2014;92(11):927–935. doi: 10.1139/cjpp-2014-0227. [DOI] [PubMed] [Google Scholar]
- 203.Yu XH, Zhang DW, Zheng XL, Tang CK. C1q tumor necrosis factor-related protein 9 in atherosclerosis: mechanistic insights and therapeutic potential. Atherosclerosis. 2018;276:109–116. doi: 10.1016/j.atherosclerosis.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 204.Chiu CZ, Wang BW, Shyu KG. Molecular regulation of the expression of leptin by hypoxia in human coronary artery smooth muscle cells. J Biomed Sci. 2015;22:5. doi: 10.1186/s12929-014-0109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schroeter MR, Leifheit-Nestler M, Hubert A, Schumann B, Gluckermann R, Eschholz N, Kruger N, Lutz S, Hasenfuss G, Konstantinides S, et al. Leptin promotes neointima formation and smooth muscle cell proliferation via NADPH oxidase activation and signalling in caveolin-rich microdomains. Cardiovasc Res. 2013;99(3):555–565. doi: 10.1093/cvr/cvt126. [DOI] [PubMed] [Google Scholar]
- 206.Boord JB, Maeda K, Makowski L, Babaev VR, Fazio S, Linton MF, Hotamisligil GS. Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation. 2004;110(11):1492–1498. doi: 10.1161/01.CIR.0000141735.13202.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Coleman SL, Park YK, Lee JY. Unsaturated fatty acids repress the expression of adipocyte fatty acid binding protein via the modulation of histone deacetylation in RAW 264.7 macrophages. Eur J Nutr. 2011;50(5):323–330. doi: 10.1007/s00394-010-0140-9. [DOI] [PubMed] [Google Scholar]
- 208.Hasan ST, Zingg JM, Kwan P, Noble T, Smith D, Meydani M. Curcumin modulation of high fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Atherosclerosis. 2014;232(1):40–51. doi: 10.1016/j.atherosclerosis.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 209.Hertzel AV, Xu H, Downey M, Kvalheim N, Bernlohr DA. Fatty acid binding protein 4/aP2-dependent BLT1R expression and signaling. J Lipid Res. 2017;58(7):1354–1361. doi: 10.1194/jlr.M074542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Krusinova E, Pelikanova T. Fatty acid binding proteins in adipose tissue: a promising link between metabolic syndrome and atherosclerosis? Diabetes Res Clin Pract. 2008;82(Suppl 2):S127–134. doi: 10.1016/j.diabres.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 211.Mankowska-Cyl A, Krintus M, Rajewski P, Sypniewska G. A-FABP and its association with atherogenic risk profile and insulin resistance in young overweight and obese women. Biomark Med. 2013;7(5):723–730. doi: 10.2217/bmm.13.61. [DOI] [PubMed] [Google Scholar]
- 212.Xiao Y, Xiao X, Xu A, Chen X, Tang W, Zhou Z. Circulating adipocyte fatty acid-binding protein levels predict the development of subclinical atherosclerosis in type 2 diabetes. J Diabetes Compl. 2018;32(12):1100–1104. doi: 10.1016/j.jdiacomp.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 213.Wang X, Chen L, Liu J, Yan T, Wu G, Xia Y, Zong G, Li F. In vivo treatment of rat arterial adventitia with interleukin1beta induces intimal proliferation via the JAK2/STAT3 signaling pathway. Mol Med Rep. 2016;13(4):3451–3458. doi: 10.3892/mmr.2016.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Roubille F, Busseuil D, Shi Y, Nachar W, Mihalache-Avram T, Mecteau M, Gillis MA, Brand G, Theberge-Julien G, Brodeur MR, et al. The interleukin-1beta modulator gevokizumab reduces neointimal proliferation and improves reendothelialization in a rat carotid denudation model. Atherosclerosis. 2014;236(2):277–285. doi: 10.1016/j.atherosclerosis.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 215.Yan AT, Yan RT, Cushman M, Redheuil A, Tracy RP, Arnett DK, Rosen BD, McClelland RL, Bluemke DA, Lima JA. Relationship of interleukin-6 with regional and global left-ventricular function in asymptomatic individuals without clinical cardiovascular disease: insights from the multi-ethnic study of atherosclerosis. Eur Heart J. 2010;31(7):875–882. doi: 10.1093/eurheartj/ehp454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Amersfoort J, Schaftenaar FH, Douna H, van Santbrink PJ, Kroner MJ, van Puijvelde GHM, Quax PHA, Kuiper J, Bot I. Lipocalin-2 contributes to experimental atherosclerosis in a stage-dependent manner. Atherosclerosis. 2018;275:214–224. doi: 10.1016/j.atherosclerosis.2018.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.