Abstract
Targeted therapy is a new cancer treatment approach, involving drugs that particularly target specific proteins in cancer cells, such as receptor tyrosine kinases (RTKs) which are involved in promoting growth and proliferation, Therefore inhibiting these proteins could impede cancer progression. An understanding of RTKs and the relevant signaling cascades, has enabled the development of many targeted drug therapies employing RTK inhibitors (RTKIs) some of which have entered clinical application. Here we discuss RTK structures, activation mechanisms and functions. Moreover, we cover the potential effects of combination drug therapy (including chemotherapy or immunotherapy agents with one RTKI or multiple RTKIs) especially for drug resistant cancers.
Keywords: Targeted therapy, Immune therapy, RTK inhibitors, Drug resistance, Cancer therapy
Introduction
Cancer describes a variety of diseases which can affect any part of the body, characterized by the rapid growth of abnormal cells beyond their normal boundaries and which can invade other organs [1]. According to the International Agency for Research on Cancer (IARC) GLOBOCAN statistics, data from 185 countries reported about 19.3 million cancers and 10 million deaths worldwide [2]. Distant metastasis is the leading cause of cancer-related death. Overall, liver, lung, and gastric cancers were the most common cause of cancer death [2, 3].
Combination therapy, including chemotherapy plus surgery or radiotherapy, are the most common treatment options [4]. Various types of chemotherapy drugs can be used depending on the cancer type and stage. The majority of tumors eventually become resistant to chemotherapy due to different mechanisms, such as escape from apoptosis, resistance to cell stress conditions, and up-regulation of DNA repair mechanisms [5], and thus the treatment fails [6]. Targeted therapy is a new treatment approach, involving drugs that target characteristic traits in the cells/proteins which promote cancer progression. Targeted therapy uses a drug designed to repress a specific key molecule that is involved in maintaining the growth and proliferation of cancer cells, and thus inhibits tumor progression [4, 7–9].
Signal transduction is a pathway for cell-to-cell communication, by which an external signal (biological, physical, electrical, or chemical) triggers a response in the target cells following binding of an extracellular factor to a receptor. This binding activates an intracellular protein kinase, and consequently triggers a series of biochemical events [10]. Receptor tyrosine kinases (RTKs) are a group of transmembrane receptors characterized by the inherent activity of a tyrosine kinase enzyme (TK) in their cytoplasmic region. RTKs are expressed in all body tissues of multicellular animals during both prenatal and postnatal development, and have an important role in cell survival, proliferation, differentiation, metabolism, regeneration, and cell death pathways [9, 11, 12]. In the last decade, new approaches such as molecular targeting of RTKs, have been shown to improve treatment outcomes for some cancer patients. Many investigations have shown that abnormal RTK activity is involved in different human diseases, especially cancer [4, 7, 13].
A better understanding of RTKs, their signaling pathways, and the effects of RTK inhibitors on cellular functions has enabled the development of new targeted drug therapies, along with improved clinical outcomes [11, 14]. This review article discusses the current RTK-targeted therapies available for cancer patients.
RTK structures, activation mechanisms, and function
RTKs are a type of membrane-bound protein usually activated by the binding of receptor-specific ligands. Their intrinsic catalytic activity allows the phosphorylation of tyrosine residues. This is triggered by the binding of a ligand to the extracellular domain of the RTK protein that helps to stabilize the activation state [10, 15]. The RTK superfamily now has 58 members which are divided into 20 subfamilies/classes (Table 1) [16, 17]. All RTKs have a similar structure which includes an extracellular ligand-binding domain, a transmembrane α-helix spanning the cell membrane, an intracellular tyrosine kinase domain (TKD), a cytoplasmic membrane-assocoated regulatory region, and a carboxy (C)-terminus tail [18]. The extracellular ligand-binding domain varies according to the receptor subfamily. For most RTKs, the specific ligand is a soluble molecule, such as growth factors, cytokines, hormones etc. [10, 16].
Table 1.
The classification of RTK subfamilies
| Class | Subfamily name | Members |
|---|---|---|
| 1 | EGFR | EGFR, ERBB2/3/4 |
| 2 | Ins | INSR IGFR |
| 3 | PDGF | PDGFRα, PDGFRβ, M-CSFR, KIT, FLT3L |
| 4 | VEGF | VEGFR1/2/3 |
| 5 | FGFs | FGFR1/2/3/4 |
| 6 | CCK | CCK4 |
| 7 | NGF | TRKA, TRKB, TRKC |
| 8 | HGF | RON, MET |
| 9 | Eph | EPHA1-6, EPHB1-6 |
| 10 | AXL | AXL, MER, TYRO3 |
| 11 | TIE | TIE, TEK |
| 12 | RYK | RYK |
| 13 | DDRs | DDR1/2 |
| 14 | RET | RET |
| 15 | ROS | ROS |
| 16 | LTK | ALK, LTK |
| 17 | ROR | ROR1/2 |
| 18 | MuSK | MuSK |
| 19 | LMR | AATYK1/2/3 |
| 20 | Undetermined | Undetermined |
EGFR epidermal growth factor receptor; InsR insulin receptor; PDGFR platelet-derived growth factor receptor; VEGFR vascular endothelial growth factor receptor; FGFR fibroblast growth factor receptor; CCK colon carcinoma kinase; NGFR nerve growth factor receptor; HGFR hepatocyte growth factor receptor; EphR ephrin receptor; Axl a Tyro3 PTK; TIE tyrosine kinase receptor in endothelial cells; RYK receptor related to tyrosine kinases; DDR discoidin domain receptor; Ret rearranged during transfection; ROS RPTK expressed in some epithelial cell types; LTK leukocyte tyrosine kinase; ROR receptor orphan; MuSK muscle-specific kinase; LMR lemur
Enzymatically active proteins such as phospholipase Cγ, phosphatidylinositol 3-kinase (PI3K), and cytoplasmic Src superfamily tyrosine kinases are incorporated into RTKs. Moreover, some proteins without enzymatic activity such as scaffold and adaptor proteins have roles as signaling molecules in recruiting and assembling the RTK via additional protein–protein interaction domains [13]. Adaptor proteins and protein kinases containing Src homology 2 (SH2), SH3, and phosphotyrosine-binding (PTB) domains can mediate the phosphotyrosine binding and polyproline region activation. These molecules play significant roles in signaling pathway cascades and activation of other protein kinases [10, 19]. In addition, RTKs can employ other signaling pathways, such as PI3K/V-Akt murine thymoma viral oncogene homolog (Akt), mammalian target of rapamycin (mTOR), phospholipase C gamma 1 or protein kinase C (PLCG1/PKC), and mitogen-activated protein kinase or extracellular signal-regulated kinase (MAPK/ERK).
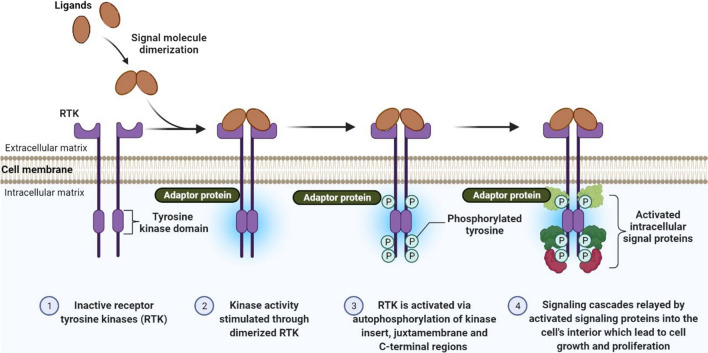
These signaling cascades affect the transcription of genes and produce non-transcriptional alterations that affect the cytoskeletal machinery for cell deformation, adhesion, motility, cell differentiation and progression, and can prevent cellular apoptosis [13, 20]. It should be noted that RTK signaling can occur inside endosomes and also at the surface of cells, involving diverse signaling pathways dependent on the receptor location. In general, the binding of secreted ligands to the RTK extracellular domain can cause three structural changes, i.e. ligand-induced homo- or heterodimerization of the receptor, activation of the cytoplasmic kinase domain leading to tyrosine phosphorylation, and activation of multiple intracellular signaling cascades (Fig. 1) [21].
Fig. 1.
Classical model of RTK activation mechanism. Upon ligand binding, RTKst undergo dimerization and trans-autophosphorylation of the kinase insert. The juxtamembrane domain, activation loop and the C-terminal tail are dislocated away from the kinase activation site, so ATP binding and phosphorylation can take place. This causes secondary transphosphorylations (shown in “RTKIs and cancer therapy” section) in the RTK kinase domains, creating docking sites for the binding of different intracellular signal proteins involved in cell growth, proliferation, and metastasis-associated signaling cascades
Ligand-induced homo or heterodimerization of the receptor
Preliminary studies on RTKs have shown that these receptors are present at the cell surface as monomers, while the reponse to ligand binding involves two monomers converting into an active dimer. Later reports have shown that certain RTKs can exist as dimers (such as Ins) or as oligomers (such as EGFR) on the cell surface before ligand binding. In addition, some RTKs require oligomers to become activated instead of dimers (such as TIE2, Eph). The ligand binding then stimulates tyrosine kinase activity [22, 23].
The dimerization of RTKs can either be ligand-mediated; e.g. TIE2, TRKA, mast/stem cell growth factor receptor kit (c-KIT), and VEGF-R1/FMS related receptor tyrosine kinase 1 (Flt1), or else can be receptor-mediated; e.g., the EGFR family [20]. In the first case, the two RTK monomers within the cell membrane are not in contact with each other, and the binding of the ligand makes them come together. In the second case, the ligand does not form part of the dimer interface, and the binding of the ligand to each receptor monomer causes structural changes in the extracellular domain and exposes sites for dimerization. There are cases where the dimeric junction is formed by both the extracellular receptor region and the ligand, and other cases where the dimer interface is formed by interactions between the receptors, ligand, and an auxiliary molecule (e.g. heparin in the FGFR family) [10, 23].
Activation of the cytoplasmic kinase domains and tyrosine phosphorylation
For activation of the TKDs of RTKs, the N-lobe alpha-C helix and activation loop of TKDs must adopt a specific “active” configuration. It has been pointed out that cis-autoinhibition is necessary for this to happen, which occurs by different mechanisms [24].
In TIR2 and insulin RTKs, tyrosine residues on the C-terminal tail or the activation loop are present in the kinase active site, respectively. This region containing tyrosine autophosphorylation sites blocks the binding of the substrate to the active site, in a type of cis-inhibition mechanism [20, 25]. Tyrosine residues interact with the TKD and its activation loop in some RTKs, including Flt3, c-KIT, Eph family, and MuSK, or in the juxtamembrane region, fixing it in an inactive conformation through juxtamembrane autoinhibition [20]. It has been shown that some mutations in the juxtamembrane autoinhibition mechanism of the KIT/PDGFR family are often associated with various cancers [26]. In all types, ligand-induced dimerization allows the release of the cis-autoinhibition, and therefore permits RTK activity [20].
Some RTKs such as the EGFR/ErbB and RET families are an exception to this rule because they do not require transphosphorylation for activation [27, 28]. The maintenance of EGFR in a non-active conformation is mediated by allosteric interactions within the N-lobe of the TKD of an RTK monomer. However, these interactions are interrupted by the C-lobe of the TKD of another RTK monomer after dimerization, leading to the activation of the TKD receptor. The activation mechanism of these TKDs is not completely understood. It has been theorized that RET may act as a trans-inhibited dimer, and following ligand binding, the inhibitory interactions are reduced [10, 27].
Activation of multiple intracellular signaling cascades
The trans-autophosphorylation pathway is involved in the ability of RTKs to modulate cellular signaling pathways, except for EGFR and RET. In general, autophosphorylation is carried out in two steps. In the first step, trans-autophosphorylation of tyrosine residues reduces the cis-autoinhibition and allows the TKD to adopt an active configuration and to optimize the enzyme kinetics. In the second step, additional tyrosines are phosphorylated in the C-terminal tail, juxtamembrane region, and TKD, forming phosphotyrosine binding sites which can interact with SH2 or PTB domain adapter kinases and proteins. It has also been suggested that the TKD may phosphorylate tyrosines on related docking proteins that lack enzymatic activity themselves, but may provide additional binding sites [20].
These cellular pathways and related RTKs are biologically essential, not only in prenatal development, but also for the function of mature tissues and postnatal homeostasis. For instance, AXL and Mer (AXL subfamily receptor) suppress the immune system by inhibiting the production of TLR-induced cytokines and also enhance the phagocytic uptake of apoptotic cells. The VEGF and PDGF receptors stimulate angiogenesis, vascular remodeling, regulate vasculogenesis, and fibroblast-induced collagen synthesis to accelerate wound healing. It has been reported that mouse embryos with disruption of these signaling pathways undergo death due to abnormal vascular formation and cardiac dysfunction [29]. RET/GDNF (glial cell-line derived neurotrophic factor) signaling is essential for organ development, such as kidneys, intestinal nervous system, and ureters, and is also involved in neuroblastoma tissues. Studies have shown that RET knock-out mutant mice display abnormalities in renal and ureteral development. Nevertheless, RET mutations only occurred in a small percentage of humans with these developmental abnormalities, indicating that these mutations are not critical [30–32]. MuSK is expressed in skeletal muscle cells involved in neuromuscular junction formation and clustering of acetylcholine receptors, and enhances the expression of genes encoding synaptic proteins [33]. The Ins receptor modulates glycogenesis, glucose homeostasis, and lipogenesis in body fat. TrkA/TrkB/TrkC receptors (neural growth factor/brain-derived neurotrophic factor/neurotrophin-3 receptors) and TAM family receptors (e.g. Tyro3, Axl, and MerTK) are necessary for the survival of neurons, as well as the development and function of the central nervous system [34, 35]. These receptors increase the survival and growth of motor neurons and axons [36, 37]. In addition, MerTK and/or Axl expression is associated with increased cancer cell survival, lymph node metastasis, drug resistance, and tumor development in several solid and hematological cancers, including acute myeloid leukemia, non-small-cell lung cancer (NSCLC), breast, liver, and gastric cancer [38, 39]. c-KIT/SCF is involved in spermatogenesis, and leads to overexpression of EphA2 in human breast cancer [40]. TIE2/angiopoietin-1 (ANG1) signaling has an essential role in vascular morphogenesis. It also is important for venous healing and maintenance. Tie2 deficiency causes the death of mice due to disturbed vascular organization, and TIE2 mutations are often seen in patients with venous malformations [41]. EGF receptors (i.e., ErbB1-4) are widely expressed in embryonic tissues, and the lack of EGFR can lead to defects in tissues such as skin, CNS, intestines, liver, lungs, peripheral nervous system, and cranial sensory ganglia myelination. EGFR deficiency also leads to the failure to form cardiac ventricle trabeculae, which results in intrauterine death [42]. Many RTK-ligand systems have essential roles in various tissues, and researchers are constantly discovering new roles for them [29, 43–45].
The roles of RTKs in cancer
Although the activity of RTKs is tightly controlled in normal cells, these receptors may undergo changes that lead to oncogenic activation, tumor metastasis, angiogenesis, and therapy resistance, due to autocrine or paracrine stimulation, mutations, or overexpression [12, 46, 47]. These changes can lead to greater affinity of RTK receptors to their ligands, increased activity of TKD domains, or an increased tendency for dimerization due to the disappearance of cis-inhibitory interactions [48, 49]. Generally, increased RTK signaling can occur through several mechanisms, including gain-of-function mutations in RTK genes, DNA amplification of RTK genes [50], or chromosomal rearrangment of RTK genes such as ROS1 [51]. Increased RTK signaling is associated with uncontrolled cellular proliferation and several hyperproliferative diseases, including cancer [10]. About 30% of all human cancers have been shown to express mutated or overexpressed RTKs [17]. For instance, gain-of-function mutations in FLT3, MET (mesenchymal–epithelial transcription factor), FGFR3, c-KIT, and RET genes can occur in acute myeloid leukemia. In renal cell carcinoma (RCC), bladder and breast cancers, gastrointestinal stromal tumors, and medullary thyroid cancer, RTK activation occurs in a ligand-independent manner along with increased RTK signaling [52–55]. In addition, in some other cancers, gain-of-function mutations have been found, such as v-raf murine sarcoma viral oncogene homolog B (BRAF) V600E substitution mutations in papillary thyroid cancer, melanoma, and lung adenocarcinoma. Other examples are: Akt1 E17K point mutations in ovarian, lung, colorectal, and breast cancer; kirsten rat sarcoma (KRAS) mutations in colorectal, pancreatic, thyroid, lung, and ovarian cancer; PIK3CA mutations in breast, endometrial, colorectal, cervical, and anal cancer [56].
Researchers have detected changes and mutations in some oncogenic driver genes, including KRAS, EGFR, MET, BRAF, anaplastic lymphoma kinase (ALK), c-ROS oncogene 1, neurotrophic receptor tyrosine kinase (NTRK), HER2/neu2, and neuregulin-1 which are associated with NSCLC [57]. It has been suggested that targeting these oncogenes could be a potential therapeutic strategy for the treatment of NSCLC and other cancers.
There have been many reports of RTK involvement in a range of cancers. For instrance, Abdel-Rahman et al. evaluated the expression of AXL RTK in association with tumor protein P53 and its role in tumor metastasis and response to chemotherapy in colon and breast cancer cells. In comparison with AXL-silenced cells, AXL-activated cells, which are affected by p53, showed an increased invasive potential after exposure to chemotherapy. The silencing of AXL demonstrated a minor trend in restoring colon cancer cell sensitivity to chemotherapy, including irinotecan and 5-fluorouracil (5-FU) [58]. In addition, it has been shown that the expression of the RET gene encoding RET-RTK can be used in the diagnosis and prognosis of medullary thyroid carcinoma (MTC) [59]. The c-Met/MET RTK, is upregulated in many cancers, e.g., NSCLC, colorectal cancer (CRC), gastric cancer, and hepatocellular carcinoma (HCC) [60]. Tivantinib is a selective RTK inhibitor targeting the c-Met pathway, which has been evaluated in advanced Met-positive HCC patients [61]. Met binds to HGF to activate intracellular signaling pathways, including MEK, PI3K-Akt, MAPK, STAT3 (signal transducer and activator of transcription 3), β-catenin, and Notch pathways [62–64]. Tivantinib prevents cell growth by inhibiting Met autoactivation and stabilization of the inactive non-phosphorylated kinase configuration [62].
In a study by Abou-Fayçal et al., the role of alternative splicing in cancer growth and RTK-targeted therapy response was evaluated. The bioactivity of RTK alternative splice forms and the upstream signals controlling their expression in cancer was reported. It has been demonstrated that RTK alternative splicing has some biological consequences, for example, the alteration of RTK subcellular distribution and function, the modification of the affinity of RTKs to their ligands, and reprogramming of cells to become more invasive [9].
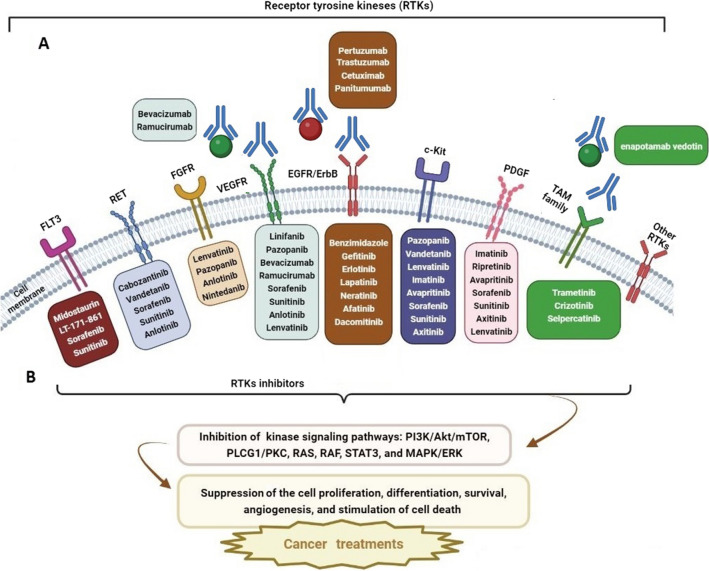
Recently, researchers have suggested a double-edged role for the TAM receptors (Tyro3, Axl and MerTK) in the pathogenesis of cancers, such as glioblastoma. Some TAM receptors are overexpressed in various cancers and can play a major role in tumor development, invasion, and treatment resistance by affecting efferocytosis, immune cell regulation, inflammatory factor secretion, and induction of the epithelial-to-mesenchymal transition (EMT) in the tumor microenvironment. On the other hand, an opposing view proposes there could be an anticancer role of TAM receptors by regulating immune cell functions and angiogenesis inhibition under certain circumstances. It has been reported that these divergent roles of TAM receptors could be due to specific cellular conditions, including the inflammation level, the type of cancer, and immune cell function. In many clinical trials of TAM receptor targeted therapy, different treatment outcomes have been seen after targeting one or more TAM receptors. However, combination therapy with TAM receptor inhibitors plus other immunotherapy agents like anti-PD-L1, could be a good approach for TAM-related cancer therapy [65]. Such findings have motivated the expanded use of RTK inhibitors (RTKIs) in cancer therapy over the past three decades (Fig. 2).
Fig. 2.
An overview of inhibition of cell cycle proliferation, differentiation, survival, angiogenesis, and also enhancement of cell death pathways by receptor tyrosine kinase inhibitors (RTKIs) that target tyrosine kinase receptors. Different kinase inhibitors in each box have been tested in different cancers and are listed according to the specificity and selectivity of the kinase receptor. A Monocolonal antibodies (mAbs) can target RTKs or ligands and inhibit RTK activation. B Small molecule inhibitors can target the ATP-binding site of RTKs in their interacellular domain and inhibit RTK phosphrylation. Therefore, the signaling cascade is blocked. FLT3 FMS-like tyrosine kinase-3; RET rearranged during transfection; FGFR fibroblast growth factor receptor; VEGFR vascular endothelial growth factor receptor; EGFR epidermal growth factor receptor; c-KIT Mast/Stem Cell Growth Factor Receptor Kit; PDGFR platelet-derived growth factor receptor; PI3K phosphoinositide 3-kinase; Akt V-Akt murine thymoma viral oncogene homolog; mTOR mammalian target of rapamycin; PLCG1/PKC phospholipase C gamma 1/protein kinase C; RAS rat sarcoma virus; RAF rapidly accelerated fibrosarcoma; STAT3 signal transducer and activator of transcription 3; MAPK/ERK mitogen-activated protein kinase/extracellular signal-regulated kinase
RTKIs and cancer therapy
As mentioned above, tyrosine kinase proteins are crucial mediators in cancer development and are often overexpressed during carogcinogenesis. Hence, many investigators have suggested that RTKs could be a new target for molecular cancer therapy [66]. In the last three decades, numerous RTK-targeted drugs have been developed, some of which have led to significant clinical advances (Table 2). Diverse approaches have been investigated for inhibiting RTKs, including using antibodies against kinase protein extracellular domains, and antibodies against both transmembrane and intracellular proteins, which can inhibit growth factor binding/receptor dimerization or TKD phosphorylation. Most small molecule RTKIs target and bind to the enzymatic domain and competitively block the ATP binding pocket. The specificity of RTKIs is preserved due to the existence of unique binding pockets [66, 67]. The number of small molecule RTKIs is much higher than RTK-targeted monoclonal antibodies. Following inhibition of RTKs, the proliferation of cancer cells is inhibited, and apoptosis is promoted [10].
Table 2.
RTKIs in preclinical models or clinical trials for cancer treatment
| RTKIs | Functions | Cancer type | References |
|---|---|---|---|
| Linifanib | Antitumor and antiangiogenesis activity by inhibition of VEGF and PGF receptors |
Liver cancer NSCLC, breast cancer, and CRC Human HCC |
[155, 156] |
| Pazopanib | VEGF receptors, c-Kit protein, and inhibiting angiogenesis | RCC | [157, 158] |
| Sunitinib | Inhibition of androgen receptors and protein phosphorylation | RCC | [159] |
| Sorafenib | Target RAF/MEK/ERK serine/threonine pathway and other RTKs for inhibition of tumor cell growth and angiogenesis | Eruptive keratoacanthomas (Grzybowski syndrome), HCC and advanced RCC | [160, 161] |
| Trametinib, crizotinib, or selpercatinib | CSF1R (M-CSF), MEK1/2, ALK, RET inhibition through mutations in genes encoding RTKs | Histiocytosis (clonal hematopoietic disorders) | [162] |
| Pexidartinib | Block the CSF1R and/or KIT proto-oncogene RTK activity | Tenosynovial giant cell tumor | [163] |
| Trastuzumab, pertuzumab | Inhibition of ERBB2 | Breast cancer, HER2-positive metastatic breast cancer | [79, 164] |
| Neratinib, afatinib, dacomitinib | HER2 and ErbB2 inhibition | NSCLC and breast cancer | [165–168] |
| Benzimidazole derivatives | Inhibition of EGFR | HCC | [169, 170] |
| Ripretinib, avapritinib | KIT/PDGFRA kinase inhibitors | Gastrointestinal stromal tumors | [171] |
| Imatinib | Inhibition of PDGF | Pulmonary hypertension and respiratory dysfunction | [172] |
| Gefitinib, erlotinib | Target EGFR | NSCLC | [88] |
| Lapatinib | Inhibition of EGFR and ErbB-1/-2 receptors; alteration of pyruvate kinase type M2 expression | Mammary carcinoma, pancreatic cancer | [97, 173, 174] |
| Trastuzumab | ErbB2 inhibition | Breast cancer | [78] |
| Cetuximab | EGFR inhibition | CCR, Squamous cell carcinomas, head and neck cancer | [175, 176] |
| Cetuximab, panitumumab | EGF/EGFR binding inhibition | Metastatic colon cancer | [81] |
| Bevacizumab | VEGF inhibitor | Lung cancer, CRC | [142] |
| Ramucirumab | VEGFR-2 inhibitor | Gastric, gastro-oesophageal adenocarcinoma | [84, 85] |
| Midostaurin | FLT3 inhibitor | Acute myeloid leukemia, systemic mastocytosis | [101] |
| Almonertinib | Inhibit the EMT and expression of metalloproteinases | NSCLC | [94] |
| Lenvatinib | Antiangiogenic properties | Thyroid cancer, advanced HCC and RCC | [177] |
| Tivozanib, nivolumab | VEGF inhibition | RCC | [178] |
| W2014-S | Disrupted STAT3 dimerization and signaling; suppressed proliferation, survival, migration and invasion of lung cancer cells | NSCLC | [86] |
| Datelliptium | RET transcription inhibitor, suppression of EMT, and thyroid carcinoma metastasis | Medullary thyroid carcinoma | [179] |
| LT-171-861 | FLT3 inhibitor | Acute myeloid leukemia | [100] |
| miR-199b-5p | EMT inhibition | Prostate cancer | [68] |
| Foretinib | c-MET receptor inhibition | Glioblastoma, and Gastric Cancer | [180, 181] |
| Curcumin | Inhibition of RTKs and downstream signaling via MAPK, PI3K/Akt, JAK/STAT, and NF-κB pathways | Different cancers | [4] |
NSCLC non-small cell lung cancer; HCC hepatocellular carcinoma; CRC colorectal cancer; RCC renal carcinoma; CSF1R colony-stimulating factor 1 receptor; ERBB2 Erb-b2 receptor tyrosine kinase 2; HER2 human epidermal growth factor receptor 2; EGFR epidermal growth factor receptor; PDGF platelet-derived growth factor; VEGF vascular endothelial growth factor; EMT epithelial-to-mesenchymal transition; STAT3 signal transducer and activator of transcription 3; RET rearranged during transfection
The non-coding RNA miR-199b-5p, acts as a cancer inhibitor in numerous human cancers, including prostate cancer metastasis. A recent report suggested the role of the miR-199b-5p-DDR1-ERK signaling axis in the treatment of prostate cancer through EMT inhibition [68].
It has been demonstrated that RTK signaling has a critical role in immunosuppression in cancer. The activation and overexpression of some RTKs, such as EGF receptor, c-Kit, ErbB2, ErbB3, and Met, each of which bind to Src homology and collagen A (ShcA), are involved in HER2/neu (human epidermal growth factor receptor 2) positive and basal-like breast cancer pathogenesis [69–72]. ShcA binds to RTKs and can promote extracellular signaling which controls cellular proliferation, invasion, and angiogenesis. Indeed, increased ShcA signaling was associated with higher progression and recurrence in breast cancer patients [72, 73]. Therefore the combination of immune-based therapies with RTK inhibitors could be promising in breast cancer.
On the other hand, other studies have shown that although monotherapy approaches targeting RTKs may not be clinically effective, but the combination of RTKIs attacking several different RTKs at the same time might show better effects in cancer treatment. For instance Crizotinib (a RTKI against c-Met) demonstrated an anticancer effect in breast cancer cells when combined with anti-endocrine drugs through simultaneous downregulation of c-Met and estrogen receptors [74]. In another study, it was shown that the combination of targeting both mTOR and c-Met signaling pathways could have a better effect against epithelioid sarcoma tumors [75]. In another study, Erlotinib plus Tivantinib was more effective than Erlotinib alone in NSCLC [76]. Xu et al. showed that the combined inhibition of both EGFR and c-Met was successful in lung cancer treatment [77].
Some monoclonal antibodies which act as RTKIs, such as trastuzumab and pertuzumab, bind to HER2 to prevent receptor dimerization. These TKIs are effective in both localized and metastatic forms of breast and gastric cancer that overexpress HER2, and can improve the results of breast cancer treatment [78]. Mendes et al. reported that the combination of trastuzumab or pertuzumab with other chemotherapy drugs led to increased overall survival of HER2+ metastatic breast cancer patients, up to 56 months in comparison to chemotherapy alone with only 20 months survival time [79]. Three main strategies for targeting the HER2 signaling pathway involved in breast cancer progression have been described, including TKIs (lapatinib, tucatinib, and neratinib), monoclonal antibodies (pertuzumab and trastuzumab), or antibody–drug conjugates (DS-8201a and T-DM1) [80].
Other monoclonal antibodies against RTKs, such as panitumumab and cetuximab targeting EGFR/EGF have been used in metastatic CRC treatment. It was reported that after combining these antibodies with standard chemotherapy in early CRC, the overall survival of patients was extended by approximately 1.5 months (from 8.5 to 10 months), which could be considered clinically beneficial. This benefit was only found in CRC patients with wild-type RAS, because inhibition of RTKs is ineffective in patients with RAS mutations [81, 82]. In addition, Zhang et al. reported that cetuximab could improve the anticancer effect of AZD6244, a MAPK inhibitor, in CRC cells, and this co-inhibition could be a potential treatment option in CRC patients [83].
Ramucirumab is a monoclonal antibody targeting VEGF/VEGFR2 binding used in patients with advanced or metastatic CRC, gastric, gastro-oesophageal, and NSCLC by inhibition of tumor angiogenesis. Fuchs et al. reported a survival rate of 5.2 months in ramucirumab treated advanced or metastatic gastric and gastroesophageal cancer patients, but in the placebo group it was only 3.8 months, suggesting that VEGFR-2 signal inhibition with ramucirumab could be an important therapeutic option in advanced gastric cancer [84, 85].
Zheng et al. (2021) evaluated the antitumor activity of W2014-S (a STAT3 inhibitor) in NSCLC. STAT3 is an important oncogenic factor leading to acquired resistance to targeted therapy. The results showed that W2014-S inhibited STAT3 dimerization and signaling in NSCLC cell lines, and redued proliferation, metastasis, and survival of cancer cells. In conclusion, W2014-S could be used in the treatment of NSCLC [86].
Lai et al. studied the therapeutic effect of DBPR114, a new FLT3/aurora kinase (AURK) multikinase inhibitor in advanced HCC. They reported that the growth inhibition of HCC cells via DBPR114 was due to increased apoptosis, anti-angiogenic effects, cell cycle arrest, and the formation of polyploid and multinucleated cells. Moreover, they also observed a reduction in AURK phosphorylation levels by DBPR114. As a result, they suggested that targeting FLT3/AURK could be a novel therapeutic approach in HCC patients [87].
Some TKIs, including erlotinib, gefitinib, afatinib [88, 89], osimertinib [90–92], and momelotinib [93] have been approved for metastatic lung adenocarcinoma treatment, leading to longer progression-free survival when combined with standard chemotherapy. Zhang et al. showed that the TKI almonertinib could inhibit proliferation and migration, and increase apoptosis in NSCLC cells (H1975 and PC-9). The proposed mechanism of this effect was inhibition of the EMT and metalloproteinase expression [94]. Osimertinib has been proposed to be the most effective TKI because of its longer progression-free survival compared to other similar drugs. It has been suggested that mutations in EGFR-encoding genes, most frequently an exon 19 deletion/exon 21 substitution, and also a mutation in exon 20, may be important causes of lung adenocarcinoma and metastasis of NSCLC [90, 95].
Expression of chimeric proteins caused by the fusion of NTRK genes with 5' partner genes could lead to NTRK fusion-driven cancers, by ligand-independent kinase activation. Recently larotrectinib has been introduced as a new drug against solid tumors with NTRK fusion and Trk gene translocations [96]. Moreover, lapatinib has been used in treatment of metastatic HER2+ breast cancer [79, 97], and bevacizumab has been tested in advanced NSCLC and breast cancer by inhibition of VEGF/VEGFR binding [98, 99]. LT-171-861 is a new FLT3 inhibitor [100], and midostaurin has been tested in FLT3-mutated positive systemic mastocytosis and acute myeloid leukemia [101]. Other targeted TKI therapies, include dabrafenib [102], vemurafenib [103] and trametinib [104] targeting RAF protein kinases, and inhibiting MEK in melanoma.
In one study, the immunological effects of targeting the AXL RTK using enapotamab vedotin (EnaV), an antibody–drug conjugate, in immunotherapy-resistant cancer models, such as melanoma and lung cancer was assessed. The results showed an induction of inflammatory responses, activation of cytotoxic T cells, and tumor cell killing. The combination of EnaV with tumor-specific T cells increased the cure rate of treatment resistant melanoma and lung cancer [105]. In general, combining RTKI drugs with other common chemotherapy drugs or radiotherapy can increase the therapeutic effectiveness compared to RTKI monotherapy. Combined therapy is generally superior in preventing tumor cell proliferation and inhibiting intracellular signaling cascades (Table 3) [106].
Table 3.
Combination of multiple RTKIs or RTKIs plus chemotherapy or immunotherapy agents to overcome drug resistance
| RTKI + other RTKIs or chemotherapy/immunotherapy agents | Cancer type | References |
|---|---|---|
| Osimertinib + pemetrexed | NSCLC | [127] |
| R428 + temozolomide | Glioblastoma | [143] |
| Sorafenib + DBPR114 | HCC | [87] |
| BMX inhibitor (CHMFL-BMX-078) + vemurafenib | Melanoma | [182] |
| W2014-S + gefitinib + erlotinib | NSCLC | [86] |
| Gefitinib + allogeneic CD8 + CD56 + NKT killer cells | NSCLC | [183] |
| Savolitinib + gefitinib | NSCLC | [184] |
| Anlotinib + gefitinib | NSCLC | [185] |
| Cabozantinib + atezolizumab | Prostate cancer | [186] |
| AXL inhibitor + cisplatin | Ovarian cancer | [187] |
| Camrelizumab + famitinib | Platinum-resistant ROC | [144] |
| Lurasidone + osimertinib | Not mentioned | [188] |
| Anlotinib + RFA | Lung squamous cell carcinoma | [189] |
| Selpercatinib + crizotinib | Lung and thyroid cancers | [145] |
| Osimertinib + siROR1 | Lung cancer | [190] |
| Avelumab + axitinib | Advanced HCC | [191] |
| Foretinib + anti-PD-1 antibody | CRC | [116] |
| Pyrotinib + carboplatin | Breast cancer | [192] |
| Vandetanib + everolimus | Resistant advanced solid tumors | [129] |
| Foretinib + entrectinib | CNS metastases | [193] |
NSCLC non-small cell lung cancer; HCC hepatocellular carcinoma; ROC recurrent ovarian cancer; CRC colorectal cancer; CNS central nervous system; RFA bronchoscope-guided radiofrequency ablation; siROR1 small interfering RNA (siRNA) targeting ROR1
RTKIs in combination with immunotherapy
Recent studies have shown that RTKIs can inactive or normalize almost all components of the tumor microenvironment (TME) that are dysregulated during tumorigenesis, such as stem cells, cancer-associated fibroblasts, endothelial cells, and non-cellular components. RTKIs also have a modulatory effect on many types of immune cells [107]. Generally, the TME is highly immunosuppressive to help cancer cell escape from the immune response [108]. However, RTKIs can modulate different immunosuppressive cells, for instance, tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and regulatory T cells (Treg cells). Indeed, this is an interesting feature of RTKIs, which makes them useful to be combined with immunotherapy [7, 109]. One of the reasons that make the combination of RTKIs with immunotherapy a promising approach for cancer therapy, is that the RTKIs with the most promising clinical effects also show immunomodulatory effects [110–112]. Recent evidence has indicated that some RTKIs can directly inhibit immunosuppressive cells [113]. For example, in vivo and in vitro studies have confirmed that cabozantinib can decrease the proliferation and activity of MDSCs in prostate cancer. MDSCs generally suppress CD4+ and CD8+ T cell proliferation, therefore cabozantinib can block the immunosuppressive activity and restore T cell activity. Interestingly, the use of a cocktail of immune checkpoint inhibitors (ICIs) alone, or just using cabozantinib alone, cannot make a significant impact on prostate tumor masses. However, because cabozantinib affects the TME, its combination with ICIs can synergistically target the growth of primary and metastatic prostate cancer [113]. Furthermore, cabozantinib was demonstrated to successfully target both the innate and adaptive immune systems in models of various cancer types, leading to synergistic benefits when paired with either a cancer vaccine or with ICIs [114]. Interestingly, another study in a murine prostate cancer model revealed that cabozantinib treatment was linked to enhanced release of neutrophil chemotactic factors, resulting in robust neutrophil infiltration into the prostate tumors, despite the fact that the effect of RTKis on immune cell activation (and neutrophils in particular) has only occasionally been described [115].
Fu. et al. studied a novel immunotherapeutic approach for CRC patient treatment. This study evaluated the effect of a combination of the RTKI foretinib plus an anti-PD-1 antibody in CRC mouse models [116]. They showed that this combination therapy suppressed tumor growth, prolonged overall survival, and improved anticancer immunity. T cell infiltration was increased, while tumor-associated macrophages (TAMs) and M2 phenotype TAMs were reduced, which eventually inhibited tumor development. Furthermore, JAK2-STAT1 pathway activation led to increased PD-L1 expression levels. So, the combination of foretinib plus anti-PD-1 antibody could be useful in CRC immunotherapy [116].
Intrinsic and acquired resistance to RTKIs in different cancers
The main obstacle to the effective treatment of many cancers is the development of resistance to chemotherapy drugs by tumor cells. Different mechanisms are involved in this chemotherapy resistance, including the prevention of cell death and apoptosis, and increases in the EMT or DNA damage repair [39]. Furthermore, despite the success of several targeted therapies in different cancers, leading to increased progression-free survival rates and complete responses in some cases, frequent recurrence has been attributed to primary (intrinsic) or secondary (acquired) drug resistance [117]. In primary resistance, the tumor does not respond to the targeted therapy despite displaying molecular changes that suggest the tumor will respond to RTKI treatment. Patients with secondary resistance initially respond to treatment; but the cancer recurs and progresses with restoration of the growth and invasion of the tumor. The mechanisms of primary resistance are mostly unclear, but may result from a small fraction of cells that have amplifications or mutations in the drug target, or have similar changes to those found in secondary resistance [21].
Drug resistance is accepted as a major obstacle in the treatment of cancer and cancer management [118], and the increasing incidence of drug-resistant tumors requires further research before cancer can be cured [12, 118]. Acquired drug resistance in cancer therapy can be caused by genetic or environmental alterations in related metabolic signaling pathways that select for resistant cells [119, 120]. In addition, it has been suggested that mutations in the target proteins which lead to a decrease in the binding between RTKs and drugs, or overexpression of drug efflux transporters resulting in decreased intracellular drug concentrations, are other causes of this resistance [66]. Hence, the identification of molecular drug targets is essential to reduce the survival of cancer cells or prevent their escape from the immune system. In this context, the dysregulated expression of RTKs may play a causal role in drug resistance and can be serve as an important target for drug development [46, 118, 121]. For example, drug resistance in chronic myelogenous leukemia after treatment with imatinib mesylate was caused by mutations in the RTK domain or the overexpression of Bcr-Abl, which is a specific drug target [122].
In addition, acquired resistance to both irreversible and reversible EGFR-TKIs, including osimertinib and gefitinib, has been reported in NSCLC and lung adenocarcinoma due to both EGFR-dependent and EGFR-independent mechanisms. These include mutations in the EGFR kinase domain duplication (EGFR-KDD) [123] or in the EGFR gene [121], mutations in KRAS exon 3 (R68S) [124], and the involvement of hsa_circ_0005576 via miR-512-5p/IGF1R signaling [125]. Acquired drug resistance in EGFR-mutant NSCLC patients is a common reason for the limited long-term efficacy of targeted therapy [126]. Takano. et al. evaluated the synergistic therapeutic effects of EGFR-TKI osimertinib plus pemetrexed (a chemotherapy drug commonly used in pleural mesothelioma and NSCLC) against PC-9 and H1975 NSCLC cell lines. The authors concluded that resistance to chemotherapy could be delayed by treatment with the osimertinib + pemetrexed combination. Since overexpression of the anti-apoptotic gene Polo-like kinase 1 (Plk1) may be a cause of acquired resistance to RTKIs, the combination therapy reduced Plk1 expression in PC-9 and H1975 cells, resulting in more apoptosis and inhibition of proliferation in cancer cells [127]. Furthermore, the overexpression of MUSASHI-2 (MSI2) (an RNA-binding protein) and Nanog (a stemness core protein) has been investigated as a new mechanism for resistance to EGFR-TKIs, including osimertinib or gefitinib [128]. In agreement with this hypothesis, the knockdown of MSI2 and Nanog genes reinstated sensitivity to EGFR-TKIs in NSCLC cells in vivo and in vitro. Finally, combining EGFR-TKIs with an agent targeting the MSI2-Nanog axis could effectively overcome acquired resistance [128].
The enhanced anticancer effects of vandetanib, a TKI targeting RET, VEGFR, and EGFR plus everolimus (a mTOR inhibitor) in refractory advanced solid cancers was reported in one preclinical study [129]. This study assessed the maximum tolerated dose, safety, dose-limiting toxicity, and the recommended phase II dose of vandetanib + everolimus in patients. The combination of vandetanib + everolimus produced some side effects (e.g., rash, fatigue, mucositis, and diarrhea), but the mean progression-free survival and overall survival rates were 4.1 and 10.5 months, respectively, in 80 patients.
The FDA has also approved the use of RTKIs, including sorafenib, sunitinib, pazopanib, and axitinib, for the treatment of some solid tumors, such as metastatic renal cell carcinoma. RTKIs can inhibit the activity of c-Kit, platelet-derived growth factor receptors, and vascular endothelial growth factor receptors [130, 131]. Nevertheless many patients develop resistance to anti-angiogenesis treatment and ultimately relapse [132]. Studies using mouse xenograft models have shown that hypoxia caused by anti-angiogenesis therapy can encourage the emergence of resistance mechanisms like vascular mimicry and stromal cell infiltration, or can increase tumor cell invasion resulting in tumor recurrence [133, 134]. However new multitargeted anti-angiogenesis therapies have been unable to increase patient survival, and we still do not fully understand how tumors react to these agents. Apelin is an endogenous peptide (adipokine) acting as a ligand of the G protein-coupled receptor APJ. According to Uribesalgo et al., inhibition of apelin can alter the TME by inhibiting angiogenesis and significantly slowing tumor progression. Targeting apelin improved vascular function and lowers myeloid derived suppressor cell infiltration. Apelin has been shown to inhibit resistance to anti-angiogenic RTKI treatment in mammary and lung cancers. This approach slowed growth and inhibited angiogenesis in lung and breast cancer models without causing hypoxia within the TME. High apelin levels have been associated with a poor prognosis in patients receiving anti-angiogenesis treatment, and apelin blockade also inhibited metastases caused by RTK inhibitors. These findings point to an anti-angiogenic therapeutic target that may be inhibited by reducing tumor blood vessel density and restoring normalcy to the tumor vasculature [135].
Another mechanism of acquired resistance to RTKIs in cancer cells involves changes in lipid metabolism. It is interesting to note that when therapy with RTKIs is withdrawn, there is a rebound in tumor growth accompanied by increased lipid synthesis and TCA cycle activity. These alterations restore the angiogenesis process, but this may be prevented by inhibiting fatty acid synthase (FASN) activity [136]. The invasion and migration of ovarian, breast, HCC, and CRC cell lines can all be promoted by FASN activity, a reasonably well-studied enzyme [137]. In addition to promoting tumor progression and invasion, FASN-mediated de novo lipid biosynthesis was necessary for glioma stem cell populations to maintain their stemness [138, 139]. According to research in prostate cancer cells, increased FASN levels have been linked to increased PPAR-gamma (peroxisome proliferator-activated receptor gamma) expression and AKT signaling pathways [140]. In a number of preclinical cancer models, FASN inhibition as well as the inhibition of other fatty acid biosynthesis enzymes, including ACC and ATP citrate lyase (ACLY) have been shown to effectively inhibit the proliferation of cancer cells [137].
Wang et al. reported that knockdown of long non-coding RNA (lncRNA)-PCAT-1, an important factor in resistance to gefitinib in NSCLC cells, could improved gefitinib sensitivity, increase apoptosis, and inhibit GSK3 and AKT phosphorylation in H1299/GR cells. Thus this lncRNA could be a potential target to improve gefitinib efficacy [141]. In another study by Satoh et al., combination therapy with erlotinib, bevacizumab plus osimertinib after acquired resistance emerged in NSCLC patients could be an option for these patients if they developed resistance to osimertinib alone [142]. Moreover, in another study, it was reported that the combination of gefitinib plus W2014-S (a STAT3 inhibitor) could overcome acquired resistance to EGFR-TKIs (gefitinib and erlotinib) in NSCLC patients to improve the efficacy of gefitinib in EGFR-TKI resistant lung cancer patients [86].
In a study by Scherschinski et al., the synergistic effects of RTK-AXL plus temozolomide (TMZ) were assessed with regard to tumor progression and drug resistance in the glioblastoma multiforme (GBM) cell lines, U118MG and SF126. They showed that RTX-AXL overexpression resulted in therapy resistance, but combination therapy with TZM + TKI R428 (a selective small molecule Axl inhibitor) increased the therapeutic effect. This study underlined the role of RTK-AXL in intrinsic and acquired therapy resistance [143]. In other cancers such as recurrent ovarian cancer (ROC), combination therapy using an ICI (e.g., camrelizumab) plus a RTKI (e.g., famitinib) was tested against platimun-resistant ROC in a multicenter, open-label, phase 2 clinical trial. This study showed that this combination therapy could improve results in platinum-resistant ROC patients [144].
Intrinsic and acquired resistance to sorafenib in HCC patients could be overcome by using a FLT3/AURK kinase inhibitor (DBPR114). DBPR114 remarkably delayed tumor growth and lengthened survival time compared with regorafenib (another multikinase inhibitor) [87]. The development of acquired resistance to selpercatinib (a RET kinase inhibitor approved for some cancers such as lung and thyroid) was studied by Rosen et al. [145]. They demonstrated that elevated MET expression in RET fusion+ cancer cells could be a cause of resistance to RET-directed therapy in advanced NSCLC. They also suggested that this resistance could be overcome by a combination of selpercatinib plus crizotinib [145]. It has been suggested that highly heterogeneous and phenotypically dynamic cancers should be treated with a combination of multiple drugs (including a chemotherapy drug plus an RTKI, or multiple RTKIs) to overcome drug resistance.
Conclusion
Many studies have focused on mutated or overexpressed RTKs in almost all types of cancer, and have attempted to design and extend RTK inhibitors for improved treatment regimens. Despite the complexity of the relationship between RTK oncogene activation and the development of cancer, several RTK oncogenes have so far been identified in many human cancers. Molecular targeted therapy has considerably improved the treatment outcome of patients with diverse cancers, with fewer treatment-related adverse effects. Compared to conventional chemotherapy, targeted therapy offers a more patient-friendly treatment approach [146].
The use of a combination of low-dose multiple drugs may be preferable to single drugs, but this requires further investigation. First, it is important to understand the signaling pathways involved in tumor progression and metastasis. Second, the combination drug targets must be identified, to achieve effectual suppression of complex networks. Thirdly, the dose of each drug should be as low as possible, and at the same time be non-toxic and effective in combination. Eventually, it must be determined whether the various drug combinations are effective, how many treatments are adequate, and in what order [147]. Overall, in cancers with high heterogeneity and phenotypical traits, combination therapy using several drugs (including chemotherapy drugs plus RTKIs or multiple RTKIs) are likely to be required for effective results in cancer treatment and to overcome drug resistance. One promising approach is the combination of RTKIs with ICIs. RTK inhibitors coupled with mAb-based ICI treatment have shown good results in early phase clinical studies [148].
With more than forty drugs approved by the FDA, RTKIs have transformed the practice of oncology and hematology over the course of the last 20 years. Nevertheless, patients are unlikely to be cured by the use of RTKIs as single agents, with the exception of a few rare instances of chronic myeloid leukemia. The major difficulties facing their use in cancer patients are the development of resistance and treatment-related toxicity, which may result in the decrease of the prescribed dosage or in the suspension of RTKI therapy. Reducing the costs of drug development should be prioritized due to exisiting high prices that restrict access to care, especially in developing countries [149]. A possible strategy is to create novel RTKIs with different modes of action, including covalent inhibitors, inhibitors that can overcome the most common tumor mutations, or inhibitors that cause degradation or internalization of the RTK protein. Another important factor to consider is to decrease the toxicity brought on by “off-target” effects by improving the selectivity of RTKIs. The use of artificial intelligence (AI) might speed up the development of new RTKIs and lower their cost. In fact, machine learning opens up new avenues for the design of new potential drugs, by undertstanding interactions and binding between key molecules, as well as predicting the three-dimensional (3D) structure of a protein from its amino acid sequence [150]. Zhavoronkov et al. reported the first example of the discovery of an RTKI using AI in 2019. In only 21 days, a deep learning approach allowed the discovery of a number of potential discoidin domain receptor 1 inhibitors. Two of these compounds showed positive in vitro results, and one had promising results in a mouse tumor model [151]. New microfluidic methods may speed up the rapid synthesis of the new molecules after their in silico design by AI approaches. For instance, Desai et al. createda range of brand-new ABL inhibitors. The most promising compounds were chosen by algorithms, and automatically synthesized in microfluidic or microarray devices, and then tested by measuring the IC50 values and other criteria. The top candidates could then be rapidly included in pre-clinical research [152, 153]. Combining RTKIs with a compounds from a different family of inhibitors is another interesting strategy. The possibility of increased off-target toxicity and the effects on the TME must be considered in the analysis of such combinations. Innovative in vitro models like organ-on-chip devices, and analysis of massive datasets may be faciliatated by different AI approaches. Organ-on-chip systems consist of microchannels that are continually perfused with culture medium and can be constructed to provide organ-specific, tissue-to-tissue interfaces involving various cell types [154]. The development of cost-effective drugs with lower toxicity will likely benefit from extensive screening of synergistic combinations of RTKIs with other drugs, taking into account the effect of RTKIs on the TME.
Despite many valuable clinical studies, more efforts are required to better understand the molecular changes occurring downstream of dysregulated RTKs. These studies may aid the diagnosis, prediction and treatment of different cancers to lower patient mortality. In addition, these molecular changes may also act as appropriate therapeutic targets for specific new drugs.
Acknowledgements
The authors would like to thank all of those whose fruitful research has contributed in any way to the elucidation of the role of receptor tyrosine kinases in cancer pathogenesis and receptor tyrosine kinase inhibitors in cancer therapy.
Author contributions
NE, and EF designed the review paper and wrote the manuscript, HG, SP, RK, RV, MG, RFT, and, PB contributed to writing and editing the manuscript, AHA, MRH and ARA reviewed and revised the final version of manuscript and supervised the study.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article. MRH was supported by US NIH Grants R01AI050875 and R21AI121700.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
MRH declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc, Cleveland, OH; Hologenix Inc. Santa Monica, CA; Vielight, Toronto, Canada; JOOVV Inc, Minneapolis-St. Paul MN; Sunlighten, Kansas City, MO; Consulting; USHIO Corp, Japan; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany; Klox Asia, Guangzhou, China. Stockholding: Niraxx Light Therapeutics, Inc, Irvine CA; JelikaLite Corp, New York NY. The other authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nasim Ebrahimi and Elmira Fardi shared co-first authorship.
Contributor Information
Amirhossein Ahmadi, Email: ahahmadi@pgu.ac.ir.
Michael R. Hamblin, Email: labhamblin@gmail.com
Amir Reza Aref, Email: amir@xspherabio.com.
References
- 1.Cancer [Internet]. World Health Organization (2022). Available from https://www.who.int/news-room/fact-sheets/detail/cancer
- 2.Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149(4):778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 3.de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8(2):e180–e190. doi: 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 4.Sudhesh Dev S, Zainal Abidin SA, Farghadani R, Othman I, Naidu R. Receptor tyrosine kinases and their signaling pathways as therapeutic targets of curcumin in cancer. Front Pharmacol. 2021;12:772510. doi: 10.3389/fphar.2021.772510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miraghel SA, Ebrahimi N, Khani L, Mansouri A, Jafarzadeh A, Ahmadi A, et al. Crosstalk between non-coding RNAs expression profile, drug resistance and immune response in breast cancer. Pharmacol Res. 2021;176:106041. doi: 10.1016/j.phrs.2021.106041. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho S, Levi-Schaffer F, Sela M, Yarden Y. Immunotherapy of cancer: from monoclonal to oligoclonal cocktails of anti-cancer antibodies: IUPHAR review 18. Br J Pharmacol. 2016;173(9):1407–1424. doi: 10.1111/bph.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleuren EDG, Terry RL, Meyran D, Omer N, Trapani JA, Haber M, et al. Enhancing the potential of immunotherapy in paediatric sarcomas: breaking the immunosuppressive barrier with receptor tyrosine kinase inhibitors. Biomedicines. 2021;9(12):1798. doi: 10.3390/biomedicines9121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu KG, Gupta S, Goel S. Immunotherapy: incorporation in the evolving paradigm of renal cancer management and future prospects. Oncotarget. 2017;8(10):17313–17327. doi: 10.18632/oncotarget.14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abou-Fayçal C, Hatat AS, Gazzeri S, Eymin B. Splice variants of the RTK family: their role in tumour progression and response to targeted therapy. Int J Mol Sci. 2017;18(2):383. doi: 10.3390/ijms18020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wintheiser GA, Silberstein P. Physiology, tyrosine kinase receptors. StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2022. [PubMed] [Google Scholar]
- 11.Moshirfar M, Villarreal A, Ronquillo Y. Tyrosine kinase inhibitor keratitis. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 12.Paul MK, Mukhopadhyay AK. Tyrosine kinase–role and significance in cancer. Int J Med Sci. 2004;1(2):101. doi: 10.7150/ijms.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abella JV, Park M. Breakdown of endocytosis in the oncogenic activation of receptor tyrosine kinases. Am J Physiol Endocrinol Metab. 2009;296(5):E973–E984. doi: 10.1152/ajpendo.90857.2008. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Zhang D, Guo Y, Lu B, Zhao ZJ, Xu X, et al. Tyrosine kinase ROR1 as a target for anti-cancer therapies. Front Oncol. 2021;11:680834. doi: 10.3389/fonc.2021.680834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubbard SR. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997;16(18):5572–5581. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karpov OA, Fearnly GW, Smith GA, Kankanala J, McPherson MJ, Tomlinson DC, et al. Receptor tyrosine kinase structure and function in health and disease. AIMS Biophys. 2015;2(4):476–502. doi: 10.3934/biophy.2015.4.476. [DOI] [Google Scholar]
- 17.Ségaliny AI, Tellez-Gabriel M, Heymann MF, Heymann D. Receptor tyrosine kinases: characterisation, mechanism of action and therapeutic interests for bone cancers. J Bone Oncol. 2015;4(1):1–12. doi: 10.1016/j.jbo.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du Z, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer. 2018;17(1):58. doi: 10.1186/s12943-018-0782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Bryan JP, Songyang Z, Cantley L, Der CJ, Pawson T. A mammalian adaptor protein with conserved Src homology 2 and phosphotyrosine-binding domains is related to Shc and is specifically expressed in the brain. Proc Natl Acad Sci USA. 1996;93(7):2729–2734. doi: 10.1073/pnas.93.7.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander PB, Wang XF. Resistance to receptor tyrosine kinase inhibition in cancer: molecular mechanisms and therapeutic strategies. Front Med. 2015;9(2):134–138. doi: 10.1007/s11684-015-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruyama IN. Mechanisms of activation of receptor tyrosine kinases: monomers or dimers. Cells. 2014;3(2):304–330. doi: 10.3390/cells3020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monast C, Mehta N, Lazzara M. Diversity in dimerization topologies enables differential control of receptor tyrosine kinase phosphorylation dynamics. Cell Mol Bioeng. 2013;7:86. doi: 10.1007/s12195-013-0303-x. [DOI] [Google Scholar]
- 24.Nolen B, Taylor S, Ghosh G. Regulation of protein kinases; controlling activity through activation segment conformation. Mol Cell. 2004;15(5):661–675. doi: 10.1016/j.molcel.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 25.Niu XL, Peters KG, Kontos CD. Deletion of the carboxyl terminus of Tie2 enhances kinase activity, signaling, and function. Evidence for an autoinhibitory mechanism. J Biol Chem. 2002;277(35):31768–31773. doi: 10.1074/jbc.M203995200. [DOI] [PubMed] [Google Scholar]
- 26.Dibb NJ, Dilworth SM, Mol CD. Switching on kinases: oncogenic activation of BRAF and the PDGFR family. Nat Rev Cancer. 2004;4(9):718–727. doi: 10.1038/nrc1434. [DOI] [PubMed] [Google Scholar]
- 27.Knowles PP, Murray-Rust J, Kjær S, Scott RP, Hanrahan S, Santoro M, et al. Structure and chemical inhibition of the RET tyrosine kinase domain. J Biol Chem. 2006;281(44):33577–33587. doi: 10.1074/jbc.M605604200. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125(6):1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011;2(12):1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Futami H, Sakai R. RET protein promotes non-adherent growth of NB-39-nu neuroblastoma cell line. Cancer Sci. 2009;100(6):1034–1039. doi: 10.1111/j.1349-7006.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain S. The many faces of RET dysfunction in kidney. Organogenesis. 2009;5(4):177–190. doi: 10.4161/org.5.4.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeanpierre C, Macé G, Parisot M, Morinière V, Pawtowsky A, Benabou M, et al. RET and GDNF mutations are rare in fetuses with renal agenesis or other severe kidney development defects. J Med Genet. 2011;48(7):497–504. doi: 10.1136/jmg.2010.088526. [DOI] [PubMed] [Google Scholar]
- 33.Herbst R. MuSK function during health and disease. Neurosci Lett. 2020;716:134676. doi: 10.1016/j.neulet.2019.134676. [DOI] [PubMed] [Google Scholar]
- 34.Myers KV, Amend SR, Pienta KJ. Targeting Tyro3, Axl and MerTK (TAM receptors): implications for macrophages in the tumor microenvironment. Mol Cancer. 2019;18(1):94. doi: 10.1186/s12943-019-1022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shafit-Zagardo B, Gruber RC, DuBois JC. The role of TAM family receptors and ligands in the nervous system: From development to pathobiology. Pharmacol Ther. 2018;188:97–117. doi: 10.1016/j.pharmthera.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Artim SC, Kiyatkin A, Lemmon MA. Comparison of tyrosine kinase domain properties for the neurotrophin receptors TrkA and TrkB. Biochem J. 2020;477(20):4053–4070. doi: 10.1042/BCJ20200695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang SS, Kumar NK, Madsen P, Gajjar AA, Gajjar E, Resnick AC, et al. Neurotrophic tyrosine receptor kinase fusion in pediatric central nervous system tumors. Cancer Genet. 2022;262–263:64–70. doi: 10.1016/j.cancergen.2022.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Yan D, Earp HS, DeRyckere D, Graham DK. Targeting MERTK and AXL in EGFR mutant non-small cell lung cancer. Cancers. 2021;13(22):5639. doi: 10.3390/cancers13225639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wium M, Ajayi-Smith AF, Paccez JD, Zerbini LF. The role of the receptor tyrosine kinase Axl in carcinogenesis and development of therapeutic resistance: an overview of molecular mechanisms and future applications. Cancers. 2021;13(7):1521. doi: 10.3390/cancers13071521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao P, Jiang D, Huang Y, Chen C. EphA2: a promising therapeutic target in breast cancer. J Genet Genomics Yi chuan xue bao. 2021;48(4):261–267. doi: 10.1016/j.jgg.2021.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Chu M, Li T, Shen B, Cao X, Zhong H, Zhang L, et al. Angiopoietin receptor Tie2 is required for vein specification and maintenance via regulating COUP-TFII. Elife. 2016;5:e21032. doi: 10.7554/eLife.21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wieduwilt M, Moasser M. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci. 2008;65(10):1566–1584. doi: 10.1007/s00018-008-7440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. TAM receptor signaling in immune homeostasis. Annu Rev Immunol. 2015;33:355–391. doi: 10.1146/annurev-immunol-032414-112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierce AM, Keating AK. TAM receptor tyrosine kinases: expression, disease and oncogenesis in the central nervous system. Brain Res. 2014;1542:206–220. doi: 10.1016/j.brainres.2013.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burden SJ, Yumoto N, Zhang W. The role of MuSK in synapse formation and neuromuscular disease. Cold Spring Harb Perspect Biol. 2013;5(5):a009167. doi: 10.1101/cshperspect.a009167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corcoran C, O'Driscoll L. Receptor tyrosine kinases and drug resistance: development and characterization of in vitro models of resistance to RTK inhibitors. Methods Mol Biol (Clifton, NJ) 2015;1233:169–180. doi: 10.1007/978-1-4939-1789-1_16. [DOI] [PubMed] [Google Scholar]
- 47.Lamorte L, Park M. The receptor tyrosine kinases: role in cancer progression. Surg Oncol Clin N Am. 2001;10(2):271–288. doi: 10.1016/S1055-3207(18)30065-6. [DOI] [PubMed] [Google Scholar]
- 48.Sharma P, Shukla A, Kalani K, Dubey V, Srivastava SK, Luqman S, et al. Water molecules increases binding affinity of natural PI3Kγ inhibitors against cancer. Curr Comput Aided Drug Des. 2015;11(4):304–320. doi: 10.2174/1573409912666151124233847. [DOI] [PubMed] [Google Scholar]
- 49.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 50.Takeuchi K, Ito F. Receptor tyrosine kinases and targeted cancer therapeutics. Biol Pharm Bull. 2011;34(12):1774–1780. doi: 10.1248/bpb.34.1774. [DOI] [PubMed] [Google Scholar]
- 51.Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(8):863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hassanein M, Almahayni MH, Ahmed SO, Gaballa S, El Fakih R. FLT3 Inhibitors for Treating Acute Myeloid Leukemia. Clin Lymphoma Myeloma Leuk. 2016;16(10):543–549. doi: 10.1016/j.clml.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Kato S, Subbiah V, Marchlik E, Elkin SK, Carter JL, Kurzrock R. RET aberrations in diverse cancers: next-generation sequencing of 4,871 patients. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23(8):1988–1997. doi: 10.1158/1078-0432.CCR-16-1679. [DOI] [PubMed] [Google Scholar]
- 54.Chew NJ, Nguyen EV, Su SP, Novy K, Chan HC, Nguyen LK, et al. FGFR3 signaling and function in triple negative breast cancer. Cell Commun Signal. 2020;18(1):13. doi: 10.1186/s12964-019-0486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adashek JJ, Desai AP, Andreev-Drakhlin AY, Roszik J, Cote GJ, Subbiah V. Hallmarks of RET and co-occuring genomic alterations in RET-aberrant cancers. Mol Cancer Ther. 2021;20(10):1769–1776. doi: 10.1158/1535-7163.MCT-21-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Regad T, Targeting RTK. Signaling pathways in cancer. Cancers. 2015;7(3):1758–1784. doi: 10.3390/cancers7030860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chevallier M, Borgeaud M, Addeo A, Friedlaender A. Oncogenic driver mutations in non-small cell lung cancer: past, present and future. World J Clin Oncol. 2021;12(4):217–237. doi: 10.5306/wjco.v12.i4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdel-Rahman WM, Al-Khayyal NA, Nair VA, Aravind SR, Saber-Ayad M. Role of AXL in invasion and drug resistance of colon and breast cancer cells and its association with p53 alterations. World J Gastroenterol. 2017;23(19):3440–3448. doi: 10.3748/wjg.v23.i19.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taccaliti A, Silvetti F, Palmonella G, Boscaro M. Genetic alterations in medullary thyroid cancer: diagnostic and prognostic markers. Curr Genomics. 2011;12(8):618–625. doi: 10.2174/138920211798120835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mo H-N, Liu P. Targeting MET in cancer therapy. Chronic Dis Transl Med. 2017;3(3):148–153. doi: 10.1016/j.cdtm.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao S, Wu W, Jiang H, Ma L, Pan C, Jin C, et al. Selective inhibitor of the c-Met receptor tyrosine kinase in advanced hepatocellular carcinoma: no beneficial effect with the use of tivantinib? Front Immunol. 2021;12:731527. doi: 10.3389/fimmu.2021.731527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woo HY, Yoo SY, Heo J. New chemical treatment options in second-line hepatocellular carcinoma: what to do when sorafenib fails? Expert Opin Pharmacother. 2017;18(1):35–44. doi: 10.1080/14656566.2016.1261825. [DOI] [PubMed] [Google Scholar]
- 63.Qi X-S, Guo X-Z, Han G-H, Li H-Y, Chen J. MET inhibitors for treatment of advanced hepatocellular carcinoma: a review. World J Gastroenterol: WJG. 2015;21(18):5445. doi: 10.3748/wjg.v21.i18.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peters S, Adjei AA. MET: a promising anticancer therapeutic target. Nat Rev Clin Oncol. 2012;9(6):314–326. doi: 10.1038/nrclinonc.2012.71. [DOI] [PubMed] [Google Scholar]
- 65.Zhou Y, Wang Y, Chen H, Xu Y, Luo Y, Deng Y, et al. Immuno-oncology: are TAM receptors in glioblastoma friends or foes? Cell Commun Signal. 2021;19(1):11. doi: 10.1186/s12964-020-00694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.AbbaspourBabaei M, Kamalidehghan B, Saleem M, Huri HZ, Ahmadipour F. Receptor tyrosine kinase (c-Kit) inhibitors: a potential therapeutic target in cancer cells. Drug Des Dev Ther. 2016;10:2443–2459. doi: 10.2147/DDDT.S89114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brizzi MF, Zini MG, Aronica MG, Blechman JM, Yarden Y, Pegoraro L. Convergence of signaling by interleukin-3, granulocyte-macrophage colony-stimulating factor, and mast cell growth factor on JAK2 tyrosine kinase. J Biol Chem. 1994;269(50):31680–31684. doi: 10.1016/S0021-9258(18)31749-6. [DOI] [PubMed] [Google Scholar]
- 68.Zhao Z, Zhao S, Luo L, Xiang Q, Zhu Z, Wang J, et al. miR-199b-5p-DDR1-ERK signalling axis suppresses prostate cancer metastasis via inhibiting epithelial–mesenchymal transition. Br J Cancer. 2021;124(5):982–994. doi: 10.1038/s41416-020-01187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiseman SM, Makretsov N, Nielsen TO, Gilks B, Yorida E, Cheang M, et al. Coexpression of the type 1 growth factor receptor family members HER-1, HER-2, and HER-3 has a synergistic negative prognostic effect on breast carcinoma survival. Cancer. 2005;103(9):1770–1777. doi: 10.1002/cncr.20970. [DOI] [PubMed] [Google Scholar]
- 70.Vijapurkar U, Cheng K, Koland JG. Mutation of a Shc binding site tyrosine residue in ErbB3/HER3 blocks heregulin-dependent activation of mitogen-activated protein kinase. J Biol Chem. 1998;273(33):20996–21002. doi: 10.1074/jbc.273.33.20996. [DOI] [PubMed] [Google Scholar]
- 71.Nalwoga H, Arnes JB, Wabinga H, Akslen LA. Expression of EGFR and c-kit is associated with the basal-like phenotype in breast carcinomas of African women. APMIS Acta Pathol Microbiol Immunol Scand. 2008;116(6):515–525. doi: 10.1111/j.1600-0463.2008.01024.x. [DOI] [PubMed] [Google Scholar]
- 72.Davol PA, Bagdasaryan R, Elfenbein GJ, Maizel AL, Frackelton AR., Jr Shc proteins are strong, independent prognostic markers for both node-negative and node-positive primary breast cancer. Can Res. 2003;63(20):6772–6783. [PubMed] [Google Scholar]
- 73.Ursini-Siegel J, Cory S, Zuo D, Hardy WR, Rexhepaj E, Lam S, et al. Receptor tyrosine kinase signaling favors a protumorigenic state in breast cancer cells by inhibiting the adaptive immune response. Can Res. 2010;70(20):7776–7787. doi: 10.1158/0008-5472.CAN-10-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ayoub NM, Alkhalifa AE, Ibrahim DR, Alhusban A. Combined crizotinib and endocrine drugs inhibit proliferation, migration, and colony formation of breast cancer cells via downregulation of MET and estrogen receptor. Med Oncol (Northwood, Lond, Engl) 2021;38(1):8. doi: 10.1007/s12032-021-01458-1. [DOI] [PubMed] [Google Scholar]
- 75.Imura Y, Yasui H, Outani H, Wakamatsu T, Hamada K, Nakai T, et al. Combined targeting of mTOR and c-MET signaling pathways for effective management of epithelioid sarcoma. Mol Cancer. 2014;13:185. doi: 10.1186/1476-4598-13-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scagliotti G, von Pawel J, Novello S, Ramlau R, Favaretto A, Barlesi F, et al. Phase III multinational, randomized, double-blind, placebo-controlled study of tivantinib (ARQ 197) plus erlotinib versus erlotinib alone in previously treated patients with locally advanced or metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(24):2667–2674. doi: 10.1200/JCO.2014.60.7317. [DOI] [PubMed] [Google Scholar]
- 77.Xu L, Kikuchi E, Xu C, Ebi H, Ercan D, Cheng KA, et al. Combined EGFR/MET or EGFR/HSP90 inhibition is effective in the treatment of lung cancers codriven by mutant EGFR containing T790M and MET. Can Res. 2012;72(13):3302–3311. doi: 10.1158/0008-5472.CAN-11-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haddley K. Trastuzumab emtansine for the treatment of HER2-positive metastatic breast cancer. Drugs Today (Barcelona, Spain, 1998) 2013;49(11):701–715. doi: 10.1358/dot.2013.49.11.2020937. [DOI] [PubMed] [Google Scholar]
- 79.Mendes D, Alves C, Afonso N, Cardoso F, Passos-Coelho JL, Costa L, et al. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer—a systematic review. Breast Cancer Res. 2015;17:140. doi: 10.1186/s13058-015-0648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y. The root cause of drug resistance in HER2-positive breast cancer and the therapeutic approaches to overcoming the resistance. Pharmacol Ther. 2021;218:107677. doi: 10.1016/j.pharmthera.2020.107677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 82.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol Off J Eur Soc Med Oncol. 2014;25(7):1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Q, Xiao H, Jin F, Li M, Luo J, Wang G. Cetuximab improves AZD6244 antitumor activity in colorectal cancer HT29 cells in vitro and in nude mice by attenuating HER3/Akt pathway activation. Oncol Lett. 2018;16(1):326–334. doi: 10.3892/ol.2018.8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chan MM, Sjoquist KM, Zalcberg JR. Clinical utility of ramucirumab in advanced gastric cancer. Biol Targets Therapy. 2015;9:93–105. doi: 10.2147/BTT.S62777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet (Lond, Engl) 2014;383(9911):31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 86.Zheng Q, Dong H, Mo J, Zhang Y, Huang J, Ouyang S, et al. A novel STAT3 inhibitor W2014-S regresses human non-small cell lung cancer xenografts and sensitizes EGFR-TKI acquired resistance. Theranostics. 2021;11(2):824–840. doi: 10.7150/thno.49600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai YL, Wang KH, Hsieh HP, Yen WC. Novel FLT3/AURK multikinase inhibitor is efficacious against sorafenib-refractory and sorafenib-resistant hepatocellular carcinoma. J Biomed Sci. 2022;29(1):5. doi: 10.1186/s12929-022-00788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang CH, Lee CH, Ko JC, Chang LY, Lee MC, Wang JY, et al. Gefitinib or erlotinib in previously treated non-small-cell lung cancer patients: a cohort study in Taiwan. Cancer Med. 2017;6(7):1563–1572. doi: 10.1002/cam4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim YJ, Oremus M, Chen HH, McFarlane T, Fearon D, Horton S. Cost-effectiveness analysis of afatinib, erlotinib, and gefitinib as first-line treatments for EGFR mutation-positive non-small-cell lung cancer in Ontario, Canada. Pharmacoeconomics. 2021;39(5):537–548. doi: 10.1007/s40273-021-01022-9. [DOI] [PubMed] [Google Scholar]
- 90.Zhang LD, Gao H, Qin SM, Zeng Q, Chen QF. Osimertinib is an effective epidermal growth factor receptor-tyrosine kinase inhibitor choice for lung cancer with epidermal growth factor receptor exon 18–25 kinase domain duplication: report of two cases. Anticancer Drugs. 2022;33(1):e486–e490. doi: 10.1097/CAD.0000000000001148. [DOI] [PubMed] [Google Scholar]
- 91.Yoshimatsu Y, Ebi N, Ooi R, Sueyasu T, Nishizawa S, Munechika M, et al. Osimertinib for lung squamous cell carcinoma: a case report and literature review. Intern Med (Tokyo, Jpn) 2021;60(7):1067–1071. doi: 10.2169/internalmedicine.5463-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knetki-Wróblewska M, Kowalski DM, Czyżewicz G, Bryl M, Wrona A, Dziadziuszko R, et al. Effectiveness of osimertinib in patients with lung adenocarcinoma in clinical practice—the Expanded Drug Access Program in Poland. Adv Respir Med. 2020;88(3):189–196. doi: 10.5603/ARM.2020.0130. [DOI] [PubMed] [Google Scholar]
- 93.Padda SK, Reckamp KL, Koczywas M, Neal JW, Kawashima J, Kong S, et al. A phase 1b study of erlotinib and momelotinib for the treatment of EGFR-mutated, tyrosine kinase inhibitor-naive metastatic non-small cell lung cancer. Cancer Chemother Pharmacol. 2022;89(1):105–115. doi: 10.1007/s00280-021-04369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Zhang Y, Niu W, Ge X, Li X, Fan F, et al. Effect of almonertinib on the proliferation, invasion, and migration in non-small cell lung cancer cells. Zhong nan da xue xue bao Yi xue ban J Central South Univ Med Sci. 2021;46(10):1045–1053. doi: 10.11817/j.issn.1672-7347.2021.201009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gregorc V, Lazzari C, Karachaliou N, Rosell R, Santarpia M. Osimertinib in untreated epidermal growth factor receptor (EGFR)-mutated advanced non-small cell lung cancer. Transl Lung Cancer Res. 2018;7(Suppl 2):S165–S170. doi: 10.21037/tlcr.2018.03.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Awada A, Berghmans T, Clement PM, Cuppens K, De Wilde B, Machiels JP, et al. Belgian expert consensus for tumor-agnostic treatment of NTRK gene fusion-driven solid tumors with larotrectinib. Crit Rev Oncol Hematol. 2022;169:103564. doi: 10.1016/j.critrevonc.2021.103564. [DOI] [PubMed] [Google Scholar]
- 97.Awada A, Saliba W, Bozovic-Spasojevic I. Lapatinib ditosylate: expanding therapeutic options for receptor tyrosine-protein kinase erbB-2-positive breast cancer. Drugs Today (Barcelona, Spain, 1998) 2011;47(5):335–345. doi: 10.1358/dot.2011.47.5.1584110. [DOI] [PubMed] [Google Scholar]
- 98.Ai B, Yang Y. [Progress of bevacizumab in the front-line treatment of advanced non-small cell lung cancer] Zhongguo fei ai za zhi Chinese J Lung Cancer. 2020;23(7):626–630. doi: 10.3779/j.issn.1009-3419.2020.101.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nome ME, Euceda LR, Jabeen S, Debik J, Bathen TF, Giskeødegård GF, et al. Serum levels of inflammation-related markers and metabolites predict response to neoadjuvant chemotherapy with and without bevacizumab in breast cancers. Int J Cancer. 2020;146(1):223–235. doi: 10.1002/ijc.32638. [DOI] [PubMed] [Google Scholar]
- 100.Yu Z, Du J, Hui H, Kan S, Huo T, Zhao K, et al. LT-171-861, a novel FLT3 inhibitor, shows excellent preclinical efficacy for the treatment of FLT3 mutant acute myeloid leukemia. Theranostics. 2021;11(1):93–106. doi: 10.7150/thno.46593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weisberg E, Sattler M, Manley PW, Griffin JD. Spotlight on midostaurin in the treatment of FLT3-mutated acute myeloid leukemia and systemic mastocytosis: design, development, and potential place in therapy. Onco Targets Ther. 2018;11:175–182. doi: 10.2147/OTT.S127679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krayem M, Aftimos P, Najem A, van den Hooven T, van den Berg A, Hovestad-Bijl L, et al. Kinome profiling to predict sensitivity to MAPK inhibition in melanoma and to provide new insights into intrinsic and acquired mechanism of resistance. Cancers. 2020;12(2):512. doi: 10.3390/cancers12020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miao B, Ji Z, Tan L, Taylor M, Zhang J, Choi HG, et al. EPHA2 is a mediator of vemurafenib resistance and a novel therapeutic target in melanoma. Cancer Discov. 2015;5(3):274–287. doi: 10.1158/2159-8290.CD-14-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Das I, Gad H, Bräutigam L, Pudelko L, Tuominen R, Höiom V, et al. AXL and CAV-1 play a role for MTH1 inhibitor TH1579 sensitivity in cutaneous malignant melanoma. Cell Death Differ. 2020;27(7):2081–2098. doi: 10.1038/s41418-019-0488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boshuizen J, Pencheva N, Krijgsman O, Altimari DD, Castro PG, de Bruijn B, et al. Cooperative targeting of immunotherapy-resistant melanoma and lung cancer by an AXL-targeting antibody-drug conjugate and immune checkpoint blockade. Can Res. 2021;81(7):1775–1787. doi: 10.1158/0008-5472.CAN-20-0434. [DOI] [PubMed] [Google Scholar]
- 106.Zhou K, Cai X, Wang X, Lan X, Zhang X. Efficacy and safety of WBRT+EGFR-TKI versus WBRT only in the treatment of NSCLC patients with brain metastasis: an updated meta-analysis. Thoracic Cancer. 2022;13(4):563–570. doi: 10.1111/1759-7714.14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tan HY, Wang N, Lam W, Guo W, Feng Y, Cheng YC. Targeting tumour microenvironment by tyrosine kinase inhibitor. Mol Cancer. 2018;17(1):43. doi: 10.1186/s12943-018-0800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garner H, de Visser KE. Immune crosstalk in cancer progression and metastatic spread: a complex conversation. Nat Rev Immunol. 2020;20(8):483–497. doi: 10.1038/s41577-019-0271-z. [DOI] [PubMed] [Google Scholar]
- 109.Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front Immunol. 2020;11:940. doi: 10.3389/fimmu.2020.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gaspar N, Venkatramani R, Hecker-Nolting S, Melcon SG, Locatelli F, Bautista F, et al. Lenvatinib with etoposide plus ifosfamide in patients with refractory or relapsed osteosarcoma (ITCC-050): a multicentre, open-label, multicohort, phase 1/2 study. Lancet Oncol. 2021;22(9):1312–1321. doi: 10.1016/S1470-2045(21)00387-9. [DOI] [PubMed] [Google Scholar]
- 111.Italiano A, Mir O, Mathoulin-Pelissier S, Penel N, Piperno-Neumann S, Bompas E, et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(3):446–455. doi: 10.1016/S1470-2045(19)30825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tang L, Niu X, Wang Z, Cai Q, Tu C, Fan Z, et al. Anlotinib for recurrent or metastatic primary malignant bone tumor: a multicenter, single-arm trial. Front Oncol. 2022;12:811687. doi: 10.3389/fonc.2022.811687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu X, Horner JW, Paul E, Shang X, Troncoso P, Deng P, et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature. 2017;543(7647):728–732. doi: 10.1038/nature21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bergerot P, Lamb P, Wang E, Pal SK. Cabozantinib in combination with immunotherapy for advanced renal cell carcinoma and urothelial carcinoma: rationale and clinical evidence. Mol Cancer Ther. 2019;18(12):2185–2193. doi: 10.1158/1535-7163.MCT-18-1399. [DOI] [PubMed] [Google Scholar]
- 115.Patnaik A, Swanson KD, Csizmadia E, Solanki A, Landon-Brace N, Gehring MP, et al. Cabozantinib eradicates advanced murine prostate cancer by activating antitumor innate immunity. Cancer Discov. 2017;7(7):750–765. doi: 10.1158/2159-8290.CD-16-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fu Y, Peng Y, Zhao S, Mou J, Zeng L, Jiang X, et al. Combination foretinib and anti-PD-1 antibody immunotherapy for colorectal carcinoma. Front Cell Dev Biol. 2021;9:689727. doi: 10.3389/fcell.2021.689727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chong CR, Jänne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med. 2013;19(11):1389–1400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wium M, Ajayi-Smith AF, Paccez JD, Zerbini LF. The role of the receptor tyrosine kinase Axl in carcinogenesis and development of therapeutic resistance: an overview of molecular mechanisms and future applications. Cancers. 2021;13(7):1521. doi: 10.3390/cancers13071521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rosenzweig SA. Acquired resistance to drugs targeting tyrosine kinases. Adv Cancer Res. 2018;138:71–98. doi: 10.1016/bs.acr.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull. 2017;7(3):339–348. doi: 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shaikh M, Shinde Y, Pawara R, Noolvi M, Surana S, Ahmad I, et al. Emerging approaches to overcome acquired drug resistance obstacles to osimertinib in non-small-cell lung cancer. J Med Chem. 2022;65(2):1008–1046. doi: 10.1021/acs.jmedchem.1c00876. [DOI] [PubMed] [Google Scholar]
- 122.Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105(7):2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- 123.He C, Wang Y. Role of the EGFR-KDD mutation as a possible mechanism of acquired resistance of non-small cell lung cancer to EGFR tyrosine kinase inhibitors: a case report. Mol Clin Oncol. 2022;16(2):30. doi: 10.3892/mco.2021.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xiu W, Zhang Q, Yu M, Huang Y, Huang M. Case report: outcome of osimertinib treatment in lung adenocarcinoma patients with acquired KRAS mutations. Front Oncol. 2021;11:630256. doi: 10.3389/fonc.2021.630256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu S, Jiang Z, Xiao P, Li X, Chen Y, Tang H, et al. Hsa_circ_0005576 promotes osimertinib resistance through the miR-512-5p/IGF1R axis in lung adenocarcinoma cells. Cancer Sci. 2022;113(1):79–90. doi: 10.1111/cas.15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qu F, Zhou Y, Yu W. A review of research progress on mechanisms and overcoming strategies of acquired osimertinib resistance. Anticancer Drugs. 2022;33(1):e76–e83. doi: 10.1097/CAD.0000000000001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takano N, Seike M, Sugano T, Matsuda K, Hisakane K, Yoshikawa A, et al. A novel molecular target in EGFR-mutant lung cancer treated with the combination of osimertinib and pemetrexed. Anticancer Res. 2022;42(2):709–722. doi: 10.21873/anticanres.15529. [DOI] [PubMed] [Google Scholar]
- 128.Yiming R, Takeuchi Y, Nishimura T, Li M, Wang Y, Meguro-Horike M, et al. MUSASHI-2 confers resistance to third-generation EGFR-tyrosine kinase inhibitor osimertinib in lung adenocarcinoma. Cancer Sci. 2021;112(9):3810–3821. doi: 10.1111/cas.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cascone T, Sacks RL, Subbiah IM, Drobnitzky N, Piha-Paul SA, Hong DS, et al. Safety and activity of vandetanib in combination with everolimus in patients with advanced solid tumors: a phase I study. ESMO Open. 2021;6(2):100079. doi: 10.1016/j.esmoop.2021.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(22):3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(20):3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 132.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15(3):232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Piao Y, Liang J, Holmes L, Henry V, Sulman E, de Groot JF. Acquired resistance to anti-VEGF therapy in glioblastoma is associated with a mesenchymal transition. Clin Cancer Res Off J Am Assoc Cancer Res. 2013;19(16):4392–4403. doi: 10.1158/1078-0432.CCR-12-1557. [DOI] [PubMed] [Google Scholar]
- 134.Simon T, Gagliano T, Giamas G. Direct effects of anti-angiogenic therapies on tumor cells: VEGF signaling. Trends Mol Med. 2017;23(3):282–292. doi: 10.1016/j.molmed.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 135.Uribesalgo I, Hoffmann D, Zhang Y, Kavirayani A, Lazovic J, Berta J, et al. Apelin inhibition prevents resistance and metastasis associated with anti-angiogenic therapy. EMBO Mol Med. 2019;11(8):e9266. doi: 10.15252/emmm.201809266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sounni NE, Cimino J, Blacher S, Primac I, Truong A, Mazzucchelli G, et al. Blocking lipid synthesis overcomes tumor regrowth and metastasis after antiangiogenic therapy withdrawal. Cell Metab. 2014;20(2):280–294. doi: 10.1016/j.cmet.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 137.Srivastava A, Srivastava P, Mathur S, Abbas S, Rai N, Tiwari S, et al. Lipid metabolism and mitochondria: cross talk in cancer. Curr Drug Targets. 2022;23(6):606–627. doi: 10.2174/1389450122666210824144907. [DOI] [PubMed] [Google Scholar]
- 138.Wang H, Xi Q, Wu G. Fatty acid synthase regulates invasion and metastasis of colorectal cancer via Wnt signaling pathway. Cancer Med. 2016;5(7):1599–1606. doi: 10.1002/cam4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yasumoto Y, Miyazaki H, Vaidyan LK, Kagawa Y, Ebrahimi M, Yamamoto Y, et al. Inhibition of fatty acid synthase decreases expression of stemness markers in glioma stem cells. PLoS ONE. 2016;11(1):e0147717. doi: 10.1371/journal.pone.0147717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ahmad I, Mui E, Galbraith L, Patel R, Tan EH, Salji M, et al. Sleeping beauty screen reveals Pparg activation in metastatic prostate cancer. Proc Natl Acad Sci USA. 2016;113(29):8290–8295. doi: 10.1073/pnas.1601571113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang S, Liu C, Lei Q, Wu Z, Miao X, Zhu D, et al. Relationship between long non-coding RNA PCAT-1 expression and gefitinib resistance in non-small-cell lung cancer cells. Respir Res. 2021;22(1):146. doi: 10.1186/s12931-021-01719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Satoh H, Kagohashi K. Response to erlotinib and bevacizumab combination therapy after acquired resistance to osimertinib in patients with non-small cell lung cancer. Anticancer Drugs. 2022;33(3):320–322. doi: 10.1097/CAD.0000000000001142. [DOI] [PubMed] [Google Scholar]
- 143.Scherschinski L, Prem M, Kremenetskaia I, Tinhofer I, Vajkoczy P, Karbe AG, et al. Regulation of the receptor tyrosine kinase AXL in response to therapy and its role in therapy resistance in glioblastoma. Int J Mol Sci. 2022;23(2):982. doi: 10.3390/ijms23020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xia L, Peng J, Lou G, Pan M, Zhou Q, Hu W, et al. Antitumor activity and safety of camrelizumab plus famitinib in patients with platinum-resistant recurrent ovarian cancer: results from an open-label, multicenter phase 2 basket study. J Immunother Cancer. 2022;10(1):e003831. doi: 10.1136/jitc-2021-003831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rosen EY, Johnson ML, Clifford SE, Somwar R, Kherani JF, Son J, et al. Overcoming MET-dependent resistance to selective RET inhibition in patients with RET fusion-positive lung cancer by combining selpercatinib with crizotinib. Clin Cancer Res Off J Am Assoc Cancer Res. 2021;27(1):34–42. doi: 10.1158/1078-0432.CCR-20-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Abe H, Kamai T. Recent advances in the treatment of metastatic renal cell carcinoma. Int J Urol Off J Jpn Urol Assoc. 2013;20(10):944–955. doi: 10.1111/iju.12187. [DOI] [PubMed] [Google Scholar]
- 147.Yesilkanal AE, Johnson GL, Ramos AF, Rosner MR. New strategies for targeting kinase networks in cancer. J Biol Chem. 2021;297(4):101128. doi: 10.1016/j.jbc.2021.101128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sinha D, Moseley P, Lu X, Wright Q, Gabrielli B, Frazer IH, et al. Repurposing of commercially existing molecular target therapies to boost the clinical efficacy of immune checkpoint blockade. Cancers. 2022;14(24):6150. doi: 10.3390/cancers14246150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pottier C, Fresnais M, Gilon M, Jérusalem G, Longuespée R, Sounni NE. Tyrosine kinase inhibitors in cancer: breakthrough and challenges of targeted therapy. Cancers. 2020;12(3):731. doi: 10.3390/cancers12030731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jones D, Bopaiah J, Alghamedy F, Jacobs N, Weiss HL, de Jong WA, et al. Polypharmacology within the full kinome: a machine learning approach. AMIA Jt Summits Transl Sci Proc. 2018;2017:98–107. [PMC free article] [PubMed] [Google Scholar]
- 151.Zhavoronkov A, Ivanenkov YA, Aliper A, Veselov MS, Aladinskiy VA, Aladinskaya AV, et al. Deep learning enables rapid identification of potent DDR1 kinase inhibitors. Nat Biotechnol. 2019;37(9):1038–1040. doi: 10.1038/s41587-019-0224-x. [DOI] [PubMed] [Google Scholar]
- 152.Desai B, Dixon K, Farrant E, Feng Q, Gibson KR, van Hoorn WP, et al. Rapid discovery of a novel series of Abl kinase inhibitors by application of an integrated microfluidic synthesis and screening platform. J Med Chem. 2013;56(7):3033–3047. doi: 10.1021/jm400099d. [DOI] [PubMed] [Google Scholar]
- 153.Benz M, Molla MR, Böser A, Rosenfeld A, Levkin PA. Marrying chemistry with biology by combining on-chip solution-based combinatorial synthesis and cellular screening. Nat Commun. 2019;10(1):2879. doi: 10.1038/s41467-019-10685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sontheimer-Phelps A, Hassell BA, Ingber DE. Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer. 2019;19(2):65–81. doi: 10.1038/s41568-018-0104-6. [DOI] [PubMed] [Google Scholar]
- 155.Linifanib (2010) Drugs in R&D. 10(2):111–122 [DOI] [PMC free article] [PubMed]
- 156.Adachi Y, Matsuki M, Watanabe H, Takase K, Kodama K, Matsui J, et al. Antitumor and antiangiogenic activities of lenvatinib in mouse xenograft models of vascular endothelial growth factor-induced hypervascular human hepatocellular carcinoma. Cancer Invest. 2019;37(4–5):185–198. doi: 10.1080/07357907.2019.1601209. [DOI] [PubMed] [Google Scholar]
- 157.Méndez-Vidal MJ, Molina Á, Anido U, Chirivella I, Etxaniz O, Fernández-Parra E, et al. Pazopanib: evidence review and clinical practice in the management of advanced renal cell carcinoma. BMC Pharmacol Toxicol. 2018;19(1):77. doi: 10.1186/s40360-018-0264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hutson T, Davis I, Machiels J, De Souza P, Baker K, Bordogna W, et al. Biomarker analysis and final efficacy and safety results of a phase II renal cell carcinoma trial with pazopanib (GW786034), a multi-kinase angiogenesis inhibitor. J Clin Oncol. 2008;26(15 suppl):5046. doi: 10.1200/jco.2008.26.15_suppl.5046. [DOI] [Google Scholar]
- 159.Adelaiye-Ogala R, Damayanti NP, Orillion AR, Arisa S, Chintala S, Titus MA, et al. Therapeutic targeting of sunitinib-induced AR phosphorylation in renal cell carcinoma. Can Res. 2018;78(11):2886–2896. doi: 10.1158/0008-5472.CAN-17-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Abbas MN, Tan WS, Kichenadasse G. Sorafenib-related generalized eruptive keratoacanthomas (Grzybowski syndrome): a case report. J Med Case Rep. 2021;15(1):481. doi: 10.1186/s13256-021-03037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Abdelgalil AA, Alkahtani HM, Al-Jenoobi FI. Profiles of drug substances, excipients, and related methodology. Amsterdam: Elsevier; 2019. Sorafenib; pp. 239–266. [DOI] [PubMed] [Google Scholar]
- 162.Durham BH, Lopez Rodrigo E, Picarsic J, Abramson D, Rotemberg V, De Munck S, et al. Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med. 2019;25(12):1839–1842. doi: 10.1038/s41591-019-0653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tap WD, Singh AS, Anthony SP, Sterba M, Zhang C, Healey JH, et al. Results from phase I extension study assessing pexidartinib treatment in six cohorts with solid tumors including TGCT, and abnormal CSF1 transcripts in TGCT. Clin Cancer Res Off J Am Assoc Cancer Res. 2022;28(2):298–307. doi: 10.1158/1078-0432.CCR-21-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Asgari A, Sharifzadeh S, Ghaderi A, Hosseini A, Ramezani A. In vitro cytotoxic effect of trastuzumab in combination with Pertuzumab in breast cancer cells is improved by interleukin-2 activated NK cells. Mol Biol Rep. 2019;46(6):6205–6213. doi: 10.1007/s11033-019-05059-0. [DOI] [PubMed] [Google Scholar]
- 165.Feldinger K, Kong A. Profile of neratinib and its potential in the treatment of breast cancer. Breast Cancer. 2015;7:147–162. doi: 10.2147/BCTT.S54414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ogoshi Y, Shien K, Yoshioka T, Torigoe H, Sato H, Sakaguchi M, et al. Anti-tumor effect of neratinib against lung cancer cells harboring HER2 oncogene alterations. Oncol Lett. 2019;17(3):2729–2736. doi: 10.3892/ol.2019.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Subramaniam D, He AR, Hwang J, Deeken J, Pishvaian M, Hartley ML, et al. Irreversible multitargeted ErbB family inhibitors for therapy of lung and breast cancer. Curr Cancer Drug Targets. 2015;14(9):775–793. doi: 10.2174/1568009614666141111104643. [DOI] [PubMed] [Google Scholar]
- 168.Zhang Y, Zhang J, Liu C, Du S, Feng L, Luan X, et al. Neratinib induces ErbB2 ubiquitylation and endocytic degradation via HSP90 dissociation in breast cancer cells. Cancer Lett. 2016;382(2):176–185. doi: 10.1016/j.canlet.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 169.Abdullah MN, Ali Y, Abd HS. Insights into the structure and drug design of benzimidazole derivatives targeting the epidermal growth factor receptor (EGFR) Chem Biol Drug Des. 2021;100(6):921–934. doi: 10.1111/cbdd.13974. [DOI] [PubMed] [Google Scholar]
- 170.Abdel-Mohsen HT, Abdullaziz MA, Kerdawy AME, Ragab FAF, Flanagan KJ, Mahmoud AEE, et al. Targeting receptor tyrosine kinase VEGFR-2 in hepatocellular cancer: rational design, synthesis and biological evaluation of 1,2-disubstituted benzimidazoles. Molecules (Basel, Switzerland). 2020;25(4):770. doi: 10.3390/molecules25040770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bauer S, George S, von Mehren M, Heinrich MC. Early and next-generation KIT/PDGFRA kinase inhibitors and the future of treatment for advanced gastrointestinal stromal tumor. Front Oncol. 2021;11:672500. doi: 10.3389/fonc.2021.672500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yoshikawa S, Hara T, Suzuki M, Fujioka M, Taniguchi Y, Hirata KI. Imatinib dramatically improved pulmonary hypertension caused by pulmonary tumor thrombotic microangiopathy (PTTM) associated with metastatic breast cancer. Int Heart J. 2020;61(3):624–628. doi: 10.1536/ihj.19-556. [DOI] [PubMed] [Google Scholar]
- 173.Guan M, Tong Y, Guan M, Liu X, Wang M, Niu R, et al. Lapatinib inhibits breast cancer cell proliferation by influencing PKM2 expression. Technol Cancer Res Treat. 2018;17:1533034617749418. doi: 10.1177/1533034617749418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wu Z, Gabrielson A, Hwang JJ, Pishvaian MJ, Weiner LM, Zhuang T, et al. Phase II study of lapatinib and capecitabine in second-line treatment for metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2015;76(6):1309–1314. doi: 10.1007/s00280-015-2855-z. [DOI] [PubMed] [Google Scholar]
- 175.Kjær I, Lindsted T, Fröhlich C, Olsen JV, Horak ID, Kragh M, et al. Cetuximab resistance in squamous carcinomas of the upper aerodigestive tract is driven by receptor tyrosine kinase plasticity: potential for mAb mixtures. Mol Cancer Ther. 2016;15(7):1614–1626. doi: 10.1158/1535-7163.MCT-15-0565. [DOI] [PubMed] [Google Scholar]
- 176.Goulet DR, Chatterjee S, Lee WP, Waight AB, Zhu Y, Mak AN. Engineering an enhanced EGFR engager: humanization of cetuximab for improved developability. Antibodies (Basel, Switzerland) 2022;11(1):6. doi: 10.3390/antib11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Padda IS, Parmar M. Lenvatinib. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. [Google Scholar]
- 178.Albiges L, Barthélémy P, Gross-Goupil M, Negrier S, Needle MN, Escudier B. TiNivo: safety and efficacy of tivozanib-nivolumab combination therapy in patients with metastatic renal cell carcinoma. Ann Oncol Off J Eur Soc Med Oncol. 2021;32(1):97–102. doi: 10.1016/j.annonc.2020.09.021. [DOI] [PubMed] [Google Scholar]
- 179.Alqahtani T, Alswied A, Sun D. Selective antitumor activity of datelliptium toward medullary thyroid carcinoma by downregulating RET transcriptional activity. Cancers. 2021;13(13):3288. doi: 10.3390/cancers13133288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gortany NK, Panahi G, Ghafari H, Shekari M, Ghazi-Khansari M. Foretinib induces G2/M cell cycle arrest, apoptosis, and invasion in human glioblastoma cells through c-MET inhibition. Cancer Chemother Pharmacol. 2021;87(6):827–842. doi: 10.1007/s00280-021-04242-0. [DOI] [PubMed] [Google Scholar]
- 181.Sohn SH, Kim B, Sul HJ, Choi BY, Kim HS, Zang DY. Foretinib inhibits cancer stemness and gastric cancer cell proliferation by decreasing CD44 and c-MET signaling. Onco Targets Ther. 2020;13:1027–1035. doi: 10.2147/OTT.S226951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jiang S, Jiang T, Huang H, Chen X, Li L, Wang Z, et al. CHMFL-BMX-078, a BMX inhibitor, overcomes the resistance of melanoma to vemurafenib via inhibiting AKT pathway. Chem Biol Interact. 2022;351:109747. doi: 10.1016/j.cbi.2021.109747. [DOI] [PubMed] [Google Scholar]
- 183.Yu W, Ye F, Yuan X, Ma Y, Mao C, Li X, et al. A phase I/II clinical trial on the efficacy and safety of NKT cells combined with gefitinib for advanced EGFR-mutated non-small-cell lung cancer. BMC Cancer. 2021;21(1):877. doi: 10.1186/s12885-021-08590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang JJ, Fang J, Shu YQ, Chang JH, Chen GY, He JX, et al. A phase Ib study of the highly selective MET-TKI savolitinib plus gefitinib in patients with EGFR-mutated, MET-amplified advanced non-small-cell lung cancer. Invest New Drugs. 2021;39(2):477–487. doi: 10.1007/s10637-020-01010-4. [DOI] [PubMed] [Google Scholar]
- 185.Li T, Qian Y, Zhang C, Uchino J, Provencio M, Wang Y, et al. Anlotinib combined with gefitinib can significantly improve the proliferation of epidermal growth factor receptor-mutant advanced non-small cell lung cancer in vitro and in vivo. Transl Lung Cancer Res. 2021;10(4):1873–1888. doi: 10.21037/tlcr-21-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Agarwal N, Azad A, Carles J, Chowdhury S, McGregor B, Merseburger AS, et al. A Phase III, randomized, open-label study (CONTACT-02) of cabozantinib plus atezolizumab versus second novel hormone therapy in patients with metastatic castration-resistant prostate cancer. Future Oncol (Lond, Engl). 2022;18(10):1185–1198. doi: 10.2217/fon-2021-1096. [DOI] [PubMed] [Google Scholar]
- 187.Tian M, Chen XS, Li LY, Wu HZ, Zeng D, Wang XL, et al. Inhibition of AXL enhances chemosensitivity of human ovarian cancer cells to cisplatin via decreasing glycolysis. Acta Pharmacol Sin. 2021;42(7):1180–1189. doi: 10.1038/s41401-020-00546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Suzuki S, Yamamoto M, Sanomachi T, Togashi K, Seino S, Sugai A, et al. Lurasidone sensitizes cancer cells to osimertinib by inducing autophagy and reduction of survivin. Anticancer Res. 2021;41(9):4321–4331. doi: 10.21873/anticanres.15237. [DOI] [PubMed] [Google Scholar]
- 189.Zhou W, Gao Y, Tong Y, Wu Q, Zhou Y, Li Y. Anlotinib enhances the antitumor activity of radiofrequency ablation on lung squamous cell carcinoma. Pharmacol Res. 2021;164:105392. doi: 10.1016/j.phrs.2020.105392. [DOI] [PubMed] [Google Scholar]
- 190.Nakagawa N, Miyake N, Ochi N, Yamane H, Takeyama M, Nagasaki Y, et al. Targeting ROR1 in combination with osimertinib in EGFR mutant lung cancer cells. Exp Cell Res. 2021;409(2):112940. doi: 10.1016/j.yexcr.2021.112940. [DOI] [PubMed] [Google Scholar]
- 191.Kudo M, Motomura K, Wada Y, Inaba Y, Sakamoto Y, Kurosaki M, et al. Avelumab in combination with axitinib as first-line treatment in patients with advanced hepatocellular carcinoma: results from the phase 1b VEGF liver 100 trial. Liver Cancer. 2021;10(3):249–259. doi: 10.1159/000514420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhu W, Wu J, Cui M, Zhang L. Durable clinical benefit from pyrotinib combined with carboplatin in HER2-positive relapsed breast cancer previously treated with taxanes, anthracyclines, and trastuzumab. Ann Palliat Med. 2020;9(5):3684–3689. doi: 10.21037/apm-20-1363. [DOI] [PubMed] [Google Scholar]
- 193.Nishiyama A, Yamada T, Kita K, Wang R, Arai S, Fukuda K, et al. Foretinib overcomes entrectinib resistance associated with the NTRK1 G667C mutation in NTRK1 fusion-positive tumor cells in a brain metastasis model. Clin Cancer Res Off J Am Assoc Cancer Res. 2018;24(10):2357–2369. doi: 10.1158/1078-0432.CCR-17-1623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.