Abstract
In the past decade, significant progress has been made in understanding the role of protein tyrosine phosphatase as a positive regulator of tumor progression. In this scenario, our group was one of the first to report the involvement of the low molecular weight protein tyrosine phosphatase (LMWPTP or ACP1) in the process of resistance and migration of tumor cells. Later, we and others demonstrated a positive correlation between the amount of this enzyme in human tumors and the poor prognosis. With this information in mind, we asked if LMWPTP contribution to metastasis, would it have an action beyond the primary tumor site. We know that the amount of this enzyme in the tumor cell correlates positively with the ability of cancer cells to interact with platelets, an indication that this enzyme is also important for the survival of these cells in the bloodstream. Here, we discuss several molecular aspects that support the idea of LMWPTP as a signaling hub of cancer hallmarks. Chemical and genetic modulation of LMWPTP proved to shut down signaling pathways associated with cancer aggressiveness. Therefore, advances in the development of LMWPTP inhibitors have great applicability in human diseases such as cancer.
Keywords: Low molecular weight protein tyrosine phosphatase, Signaling hub, Cancer hallmarks, Metabolism reprogramming
Introduction
Eukaryotic cells respond to various stimuli in the microenvironment by modulating signal transduction pathways, which is dependent on post-translational protein modification. Among the different post-translation modifications, phosphorylation/dephosphorylation is the main form of rapid and reversible covalent modulation of proteins [1, 2]. Phosphorylation of a protein can create a new recognition site for protein–protein interactions, control protein stability, and, more importantly, can regulate enzyme/protein activity and localization. Thus, the phosphorylation of tyrosine, serine, and threonine residues, mediated by the balance between the action of protein kinases (PKs) and protein phosphatases (PPs) is recognized as a crucial factor in the generation and regulation of signals necessary for survival, proliferation, cell differentiation, and death [2]. In this context, abnormal changes in the activity of these enzymes can provide serious consequences that include diabetes, obesity, inflammation, immunological, neurodegenerative diseases, and cancers [2–6].
Based on the function, structure, sequence, specificity, sensitivity to activators, and inhibitors, the phosphatases are generally classified into three families: serine/threonine phosphatase, tyrosine phosphatase, and dual-specificity phosphatases. In the human genome, 107 genes from tyrosine phosphatase family have been identified [3, 7] which, based on function, structure, and amino acid sequence on the catalytic domain, can be classified into four groups (I–IV). Low molecular weight protein tyrosine phosphatase (LMWPTP) falls into class II (18 kDa), also known as ACP1. In humans, this enzyme is codified by the ACP1 single gene copy on chromosome 2, whose transcription is derived from four different mRNA by splicings. From four LMWPTP isoforms, the isoform 1 and isoform 2 were described to be catalytically active and identically functional [8]. The isoform 1 was described to play a major role in cancer aggressiveness and chemoresistance [4, 9, 10].
The cellular function of the LMWPTP is the dephosphorylation/regulation of many tyrosine kinase receptors and other molecules involved in signal transduction [11]. The normal function of LMWPTP has been associated with (i) cell motility and spreading coordinated by FAK dephosphorylation on several Tyr sites, in mouse fibroblast model; (ii) immune response modulation by dephosphorylation of Zap-70 Tyr292 (inhibitory site), a member of TCR signaling; (iii) balance between tight modulation of cell–cell contact by co-localization with β-catenin coordinating cell–cell contact, and inhibition of cell–cell adhesion and clustering formation by negative modulation of ICAM-1; (iv) cytoskeletal remodeling by interaction with the EphrinA2 receptor (EphA2) and modulating of Ras-MAPK; (v) decrease of cell proliferation by negative regulation targeting Janus kinase (JAK)-2, signal transducer and activator of transcription (STAT) family members, such STAT-2, -3 and -5, platelet-derived growth factor receptor (PDGFR) and fibroblast growth factor receptor (FGFR)—[10, 12–23]. Under normal cellular aspects, the function of LMWPTP has been extensively characterized in osteoclasts and osteoblasts cell lines. LMWPTP contribution to bone metabolism was first associated with osteoblast differentiation by modulating Src phosphorylation status. Indeed, the LMWPTP expression decreased in time-dependent osteoblastic differentiation. The same pattern of activity was observed in GSH profiles, suggesting the crosstalk between redox status and LMWPTP activity [24, 25]. Besides that, LMWPTP activity also coordinates the adhesion process by transient dephosphorylation of FAK Tyr397 and Src Tyr416, both activators sites, in osteoblasts [26]. Also, FAK plays a major role in bone integrity as its activity was described to be deeply modulated by secreted phosphoprotein 1 (SPP1)-induced LMWPTP expression [27]. Further, keratinocytes cells under hyperosmotic conditions, which provoke cellular architecture modifications, have suggested that LMWPTP selectively targets Src Tyr416 dephosphorylation, instead of Tyr527 (inhibitory site). This mechanism might be associated with LMWPTP phosphorylation on Tyr132 increasing the selectivity to substrates (for LMWPTP modulation, see [5, 28, 29]).
Despite being important in normal processes, LMWPTP has been described to play a role in metabolic diseases, such as obesity, diabetes, and cancer. In metabolic diseases, higher expression of LMWPTP was associated with a protective effect on hypertriglyceridemia [30]. Indeed, the protein tyrosine phosphatases LMWPTP and PTP1B might coordinate lipid overload, and the LMWPTP inhibition provoked lipid-induced apoptosis in the liver [31]. In diabetes, LMWPTP overexpression led to insulin resistance in obese mouse models [32]. Also, LMWPTP knockdown in mice was associated with cardiomyopathy prevention, as decreasing cardiac remodeling, fibrosis, and hypertrophy [33]. In the cancer field, we focused on the recent findings of LMWPTP's major role in cancer progression.
LMWPTP as a signaling hub in cancer
Phosphatases have been considered to be tumor suppressors; however, some of them emerged as having a role in initiating and progression of various types of cancers [34]. LMWPTP influences the phosphorylation of mediators of signaling pathways involved in cancer and, therefore, is postulated to be a tumor-promoting enzyme. Studies have shown that the increase in the expression of LMWPTP is enough to induce cell transformation and that the activity of this enzyme is strongly correlated with the development and progression of tumors in animal models and patients [10, 35]. Accordingly, analyses of human tumors have revealed a high prevalence of the dephosphorylated and oncogenic form of the EphA2 receptor, which has been associated with increased expression of LMWPTP [16, 36].
Malentacchi and colleagues (2005) evaluated the LMWPTP expression levels in samples of breast, colon, lung, and neuroblastoma tumors. The results showed an increase in the expression of LMWPTP in most of the analyzed samples, also indicating the existence of a significant positive correlation between the levels of expression of LMWPTP with the main clinical and pathological characteristics common to each type of cancer. Additionally, the increase in the expression of LMWPTP proved to be indicative of a less favorable prognosis, constituting a marker of tumor aggressiveness [5, 9, 10, 37–39]. Therefore, several intracellular processes are associated with LMWPTP: chemoresistance, energetic metabolism modulation, antioxidant defense, migration, pre-metastatic window partner, and contribution outside cellular signaling.
LMWPTP and resistance to chemotherapeutics
Acquisition of chemotherapeutic resistance is one of the main reasons for the low efficiency of cancer therapy, which can be due to an individual’s genetic differences, called intrinsic and acquired resistance. Acquired resistance occurs by different mechanisms, such as multidrug resistance, cell death inhibition, drug metabolism and DNA repair optimization, epigenetic and drug targets alteration, and gene amplification. We have demonstrated that in chemoresistant human chronic myeloid leukemia cells (Lucena-1), the LMWPTP is much more active (around 20-fold) than in their sensitive counterpart (K562)—[4]. The higher activity and expression of LMWPTP is related to a multidrug resistance profile in chronic myeloid leukemia (CML) including supporting Src kinase and Bcr-Abl activation. The Src kinase family is well known to be upregulated in several cancers. Src was the first proto-oncogene described in animal cells. When it is activated, Src positively regulates survival and proliferation. Also, Src is associated with LMWPTP regulation by Tyr131 and Tyr132 phosphorylation [40]. In CML, the LMWPTP knockdown decreased Src activation, which was associated with sensitizing drug-resistant leukemia cells. LMWPTP and Src down-regulation enhanced the sensitivity through vincristine and imatinib (the last one is the standard treatment for chronic myeloid leukemia). Importantly, the knockdown of LMWPTP in Lucena-1 cells culminated in the inactivation of Bcr-Abl, which is well known to have a relevant contribution in leukemogenesis [4]. The inhibition of LMWPTP by Morin was also associated to chemotherapeutical sensitizer (see more details below) [41, 42].
In solid tumors, LMWPTP was also described to play a major role in tumorigenesis. The LMWPTP knockdown in colorectal cancer cells (HCT116 and Caco-2) improved the sensitivity to chemotherapeutics effect, especially in Caco-2, which presents P-Glycoprotein expression, a multidrug resistance protein 1 (MDR1)—[9]. Furthermore, the LMWPTP inhibition improves chemotherapeutical sensitivity (for more details, see below). All these studies showed the high challenge faced in translational medicine to overcome the chemoresistance led by, in part, due to LMWPTP overexpression.
LMWPTP and mitochondrial function—Warburg effect
Otto Warburg was the first to show that tumor cells substantially metabolize glucose to lactate, even with the availability of oxygen. Under normal conditions, glucose is metabolized to pyruvate by a cascade of enzymatic reactions in the glycolytic pathway, which is subsequently oxidized by the Krebs cycle and respiratory chain, generating CO2, H2O and 32 or 34 molecules of ATP per glucose molecule, while in glycolysis, 2 ATPs/glucose is produced. This change in glucose metabolism depends on the increased transcription of GLUTs, glycolytic enzymes and oncogenes, and increased demand for mitochondrial metabolism for biosynthetic processes [43]. In this scenario, we found out that leukemia cells displaying the high amount of LMWPTP tend to have a predominance of Warburg effect, due to a down-regulation of mitochondrial proteins: PDH1, SDHA, and VDAC and upregulation of GLUT-1 and lactate dehydrogenase, and in turn, improving lactate production [44]. However, Lori and collaborators (2018) have reported the opposite effect of LMWPTP in melanoma metabolic reprogramming. LMWPTP knockdown enhanced glycolytic flux and inhibited the mitochondrial metabolism, and these authors claimed that it was in part, due to promotions of PKM2 translocation from cytosol to nucleus [45].
LMWPTP and antioxidant defense—redox modulation
In this case, under glycolytic activation for energy supply, the cancer cell decreases mitochondrial metabolism, which also contributes to lower ROS production. However, the predominance of glycolytic metabolism also contributes to antioxidant machinery to neutralize the reactive oxygen species (ROS). Cancer cell displays different strategies to decrease the intracellular ROS, such as pentose pathway activation, NADPH, and glutathione peroxidase recruiting, autophagy, and reductases activation [46]. We have found that in resistant chronic myeloid leukemia cells, LMWPTP provides antioxidant advantages via promoting the Warburg effect and expression of SOD and catalase, which in turn, under oxidative stress allows cancer cells to be able to provide a quick defensive response [47]. Therefore, LMWPTP confers survival and growth advantage to tumor cells.
Metastasis promotion
Metastasis is the cause of about 90% of deaths associated with cancer and the mechanisms that govern this process is still poorly understood. During metastatic spread, a cell of a primary tumor performs the following sequence of steps: localized invasion, intravasation, survival in the bloodstream, extravasation, formation of micrometastasis, and colonization [48]. During this process, several environmental challenges and stimuli impact the metastatic potential of tumor cells [49, 50]. As was discussed before, LMWPTP contributes directly to primary tumor progression by positive modulation of chemoresistance phenotype, lower mitochondrial function leading to lower ROS production and efficiency ROS neutralization. On the other hand, the contribution of LMWPTP to the pre-metastatic window is poorly understood. However, the importance of the LMWPTP in cancer progression has also been reported in colorectal cancer. It was demonstrated that the LMWPTP overexpression in colorectal cancer correlated to higher potential to develop liver metastasis. In this case, the higher expression of LMWPTP in colorectal cancer induced a migratory phenotype, suggesting the contribution of this enzyme in metastasis in cell line models and clinical samples. Importantly, it was also demonstrated that the LMWPTP knockdown decreases colorectal cancer cell survival, and sensitizes them to chemotherapy [9].
Poor outcome
It has also been reported that the overexpression of LMWPTP contributes to invasive profile and primary sarcoma formation in nude mice [36]. In this context, a higher amount of LMWPTP (mRNA and protein) was found in primary human prostate cancer in comparison to normal adjacent tissue. Interestingly, the high level of mRNA of LMWPTP was detected in lymph nodes, an indication that this phosphatase takes part in the metastasis process [10]. In the same study, 147 patients out of 481 with prostate cancer presented higher expression of LMWPTP and worse clinical outcomes [10]. Accordingly, the LMWPTP has been categorized as a potential biomarker for recurrence prediction for prostate cancer [51].
Low molecular weight protein tyrosine phosphatase as a druggable target: challenges and advances in inhibitor development
Since the first reports that linked the dysfunction of PTPs with diseases, the search for natural and synthetic inhibitors has attracted widespread interest as drug targets [52–54]. However, some characteristics of PTPs make them difficult for drugs to be designed against them such as:
-
i.
Consensus sequence in the catalytic site—PTPs have the signature motif CX5R at the catalytic site, which forms the P-loop (phosphate-binding loop) [55];
-
ii.
Presence of cysteine residue at the catalytic site—the catalytic mechanism is identical for the majority of PTP members, in which cysteine thiol group takes part in the nucleophilic attack, making the PTP superfamily sensitive to oxidation. Therefore, compounds that either act as an oxidant or through ROS generation, inactivate the majority of PTPs due to their inability to form a cysteinyl-phosphate intermediate at the first step of the catalysis [28, 56–58]. In addition, LMWPTP has two cysteines at the catalytic site (Cys12 and Cys17). Due to this peculiar feature, unique among the PTP family, LMWPTP can form S–S intramolecular bonds that protect the catalytic Cys12 from irreversible oxidation [59, 60].
-
iii.
In general, most competitive inhibitors have a negative charge to favor the electrostatic interaction with a positive-charged environment of the PTP catalytic site. However, this chemical characteristic of inhibitors is responsible for low cell permeability and bioavailability [61].
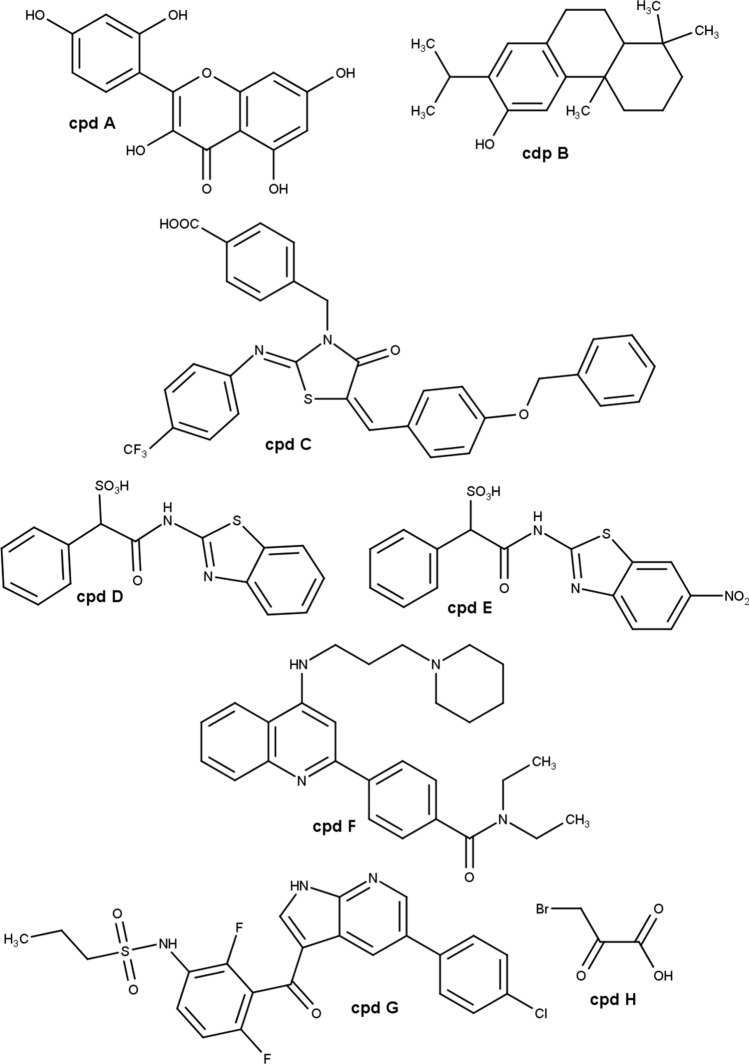
In the literature, there are several examples of natural compounds (from plants, algae, and microorganisms) that can inhibit PTPs [62]. Figure 1 shows the chemical structure of some of them, which will be discussed in this review. Specifically, in the case of LMWPTP, we demonstrated that morin (Fig. 1—cpd A) and ferruginol (Fig. 1—cpd B), at µM concentration range inhibit this enzyme [59, 63]. However, these compounds modulate a broad spectrum of macromolecules including glucose metabolism-related enzymes and DNA repair enzymes, in the case of morin, and inflammation signaling pathway, and apoptotic proteins in the case of ferruginol [64–69]—for anti-tumor activity, see below.
Fig. 1.
Chemical structure of LMWPTP inhibitors. Cpd A morin. Cpd B ferruginol. Cpd C 4-{[5-(4-Benzyloxybenzylidene)-2-(4-trifluoromethylphenylimino)-4-oxo-3-thiazolidinyl]methyl}benzoic acid. Cpd D (1,3-benzothiazol-2-yl)carbamoyl](phenyl)methanesulfonic acid. Cpd E [(6-nitro-1,3-benzothiazol-2-yl)carbamoyl](phenyl)methanesulfonic acid. Cpd F (N,N-diethyl-4-(4-((3-(piperidin-1-yl)propyl)amino)quinolin-2-yl)benzamide. Cpd G Vemurafenib. Cpd H 3-bromopyruvate
Therefore, for many years, the bottleneck has been obtaining specific inhibitors for LMWPTP. This scenario has changed with studies that described the surface topology by crystallization of different PTPs. For instance, Ottanà and collaborators (2012) synthesized 4-[(5-arylidene-2-arylimino-4-oxo-3-thiazolidinyl)methyl]benzoic acid derivatives that displayed IC50 values at sub-µM concentration and acts as competitive and reversible inhibitors of LMWPTP. These authors also validated the inhibitory property of these compounds in differentiated mouse C2C12 myotubes. 4-[(5-arylidene-2-arylimino-4-oxo-3-thiazolidinyl)methyl]benzoic acid derivatives did not affect skeletal muscle cells (C2C12) viability, and importantly, the inhibition of LMWPTP was confirmed via insulin receptor (IR) phosphorylation tracking, one of substrates of this phosphatase. Cells treated with the most potent inhibitor (4-{[5-(4-Benzyloxybenzylidene)-2-(4-trifluoromethylphenylimino)-4-oxo-3-thiazolidinyl]methyl}benzoic acid—Fig. 1—cpd C) displayed a higher level of IR phosphorylated one of the most well-described substrates [70].
A study performed by Ottanà and collaborators (2014), synthesized and validated in vitro a new series of derivative from 4-[(5-arylidene-4-oxo-2-phenylimino/oxothiazolidin-3-yl)methyl] benzoic acids with the ability to inhibit tyrosine phosphatases such as PTP1B, LMWPTP, and T-cell protein tyrosine phosphatase (TCPTP) [71]. The authors provided many optimizations in the central structure of the above-mentioned benzoic acid and docking studies, and they found that many compounds have in vitro activity promoting insulin receptor phosphorylation in mouse C2C12 skeletal muscle cells. The authors claimed a relevant activity of the compounds through non-selective inhibition of PTP1B, TCPTP, and LMWPTP, despite a general preference for human PTP1B. In particular, most compounds selectively inhibited isoform 1 of human LMWPTP opening possibilities to rationale new optimized compounds with specific activity against LMWPTP.
In recent years, the major contributions in the synthetic field of inhibitors targeting LMWPTP came from groups led by Professors Zhang and Bottini. Zhang’s group defined a pipeline from an identification of cefsulodin fragment (SulfoPhenyl Acetic Amide—SPAA) that acts as phospho-tyrosine mimetic, and through detailed mapping of this fragment-LMWPTP interaction, it was possible to capture interactions outside the catalytic site. This finding led them to invest in the SPAA-based synthesis using amines with variable size, charge, and lipophilicity [72]. The products were characterized by kinetic studies, and a set of LMWPTP inhibitors with much higher selectivity for this phosphatase over a panel of PTP family members were identified. From the inhibitor set, compound [(1,3-benzothiazol-2-yl)carbamoyl](phenyl)methanesulfonic acid (Fig. 1—cpd D) was selected to be used for structure-guided optimization, which resulted in compound [(6-nitro-1,3-benzothiazol-2-yl)carbamoyl](phenyl)methanesulfonic acid (Fig. 1—cpd E) that displayed a Ki value around 1.00 μM [60]. Importantly, the inhibitory effect of LMWPTP by Fig. 1—cpd E (at 16 μM dosage) was also validated in HepG2 cell line [61]. Based on that pipeline, Zhang’s group pioneered reported ligand (inhibitor) that induced conformational change in LMWPTP made a tremendous difference for the inhibitor selectivity and potency.
The Bottini group used another strategy, investing in the synthesis of an orthosteric uncompetitive inhibitor of human LMWPTP after a high-throughput screening of NIH chemical library. Quinoline derivatives were produced and their influence in the catalytic mechanism, by acting as uncompetitive inhibitors, was proved by different techniques: isothermal titration calorimetry, nuclear magnetic resonance spectroscopy, X-ray crystallography, hydroxyl radical footprinting, and mutagenesis. In addition to the potency of the inhibitors and oral bioavailability, one inhibitor (N,N-diethyl-4-(4-((3-(piperidin-1-yl)propyl)amino)quinolin-2-yl)benzamide—Fig. 1—cpd F) was able to act as LMWPTP inhibitor. This proposed compound is capable to bind the LMWPTP phospho-cysteine intermediate and fully occupy the active site preventing the access of water molecule required for the hydrolysis in the final catalysis step. The authors claimed an IC50 values around 0.8 µM and performed in vitro and in vivo assays to prove the efficacy of the LMWPTP inhibitor. The treatment of human HepG2 hepatocytes with 10 µM of compound cpd F (Fig. 1) increased insulin receptor phosphorylation after insulin stimulation. In vivo studies showed that cpd F improved glucose tolerance and decreased insulin levels of diabetic mice without body weight loss. Another set of in vivo experiments revealed that 2 weeks of cpd F treatment increased insulin receptor phosphorylation and elevated downstream activation of Akt and Erk signaling pathway in the liver [32].
From now on, we present some examples of compounds that display an inhibitory effect on LMWPTP (purified or recombinant one) and anti-tumor effect.
Morin
The flavonoid morin (Fig. 1—cpd A) presents broad biologic properties (for review, see Caselli, 2016) [68]. As an anti-tumor agent, at µM concentration, morin has been described to play a different role in cancer cell metabolism: (i) the anti-tumor activity is associated to DNA damage protection and ROS controlling [73]; (ii) apoptosis induction by caspase-3 and Bax activation [74]; (iii) inhibition of NFκB and Akt pathway [75–77]. Despite the broad mechanism of action, morin was able to increase the in vitro chemotherapeutical sensitivity, including in chemoresistance melanoma, prostate cancer, leukemia cell lines by decreasing LMWPTP expression [41, 42], the effect observed also in vivo [78]. Morin acted as a non-competitive inhibitor of LMWPTP, at µM range, and triggered transient degradation of LMWPTP through the proteasome-dependent mechanism on the cancer cells [42].
Ferruginol
The abietane diterpene ferruginol (Fig. 1—cpd B) displays an interesting effect on decreasing of reduced glutathione (GSH)/glutathione disulfide (GSSG) ratio, leading to ROS production [69, 79]. Also, Ferruginol showed anti-tumor activity, at µM concentration range, by inducing apoptosis, and inhibiting proliferation and survival signaling of melanoma, thyroid cancer, ovary cancer, and prostate cancer [58, 69, 80, 81]. The treatment with ferruginol against prostate cancer cell (PC3) provoked down-modulation of LMWPTP activity and expression, which it was, in part, due to a change in redox status [58]. The capacity of ferruginol (25 µM) in diminishing the LMWPTP activity indicates the potential of this compound in overcoming the aggressiveness of prostate cancer [10].
Vemurafenib
Vemurafenib (PLX4032—Fig. 1—cpd G) is an ATP analog, which acts as a competitive inhibitor of BRAF kinase and is commonly used on melanoma treatment [82]. BRAF protein belongs to RAF serine–threonine kinase protein family, which is upstream of the mitogen-activated protein kinase (MAPK) pathway that modulates cell functions such as proliferation and survival [83, 84]. Interestingly, although developed to target BRAF, recently we reported that vemurafenib, at µM concentration range, was able to diminish the level of LMWPTP in colorectal cancer cells. As mentioned above, this enzyme has been suggested to be associated with colorectal cancer poor prognosis [85].
Pyruvate analog: 3-bromopyruvate
As for cancer cells, much is known regarding the role of kinase activities in platelets, while phosphatases have been relatively less well studied. We demonstrated for the first time that platelet-containing active LMWPTP enzyme, which is modulated by platelet agonists associated with the adhesion process, arguably plays a role in their physiological activation. In pathological conditions, a higher amount of LMWPTP mRNA was detected in platelets from colorectal, pancreatic, breast, and hepatobiliary cancer patients [86]. In addition, platelets from gastrointestinal cancer patients presented higher sensitivity to agonist aggregation; further, LMWPTP was identified overexpressed in the same hyper-reactive gastrointestinal cancer patients’ platelets, suggesting that the LMWPTP plays a major role in platelet hyperaggregability in cancer [87].
Venous thromboembolism event (VTE) is one of the most common causes of cancer-related mortality [88]. Based on our findings of LMWPTP activity in platelets from healthy and cancer patients, we investigated the ability of 3-Bromopyruvate (3BP—Fig. 1—cpd H) in modulating platelet function, in part, due to LMWPTP inhibition. 3BP is an alkylating agent whose proposed chemical mechanism is a nucleophilic substitution through the SN2 mechanism with a nucleophilic thiol group(s). During the cleavage of bromine (leaving group), an alkylating (electrophilic) center is exposed to nucleophilic targets, such as –SH groups of enzymes [89]. We reported that 3BP also inhibits the enzymatic activity of LMWPTP (100 µM), concomitantly reduces Src activities in platelets from gastrointestinal cancer patients, and in turn, culminating in the lower capacity of tumor cell-induced platelet aggregation, using 3BP subtoxic concentration in ex vivo model. These findings brought out evidence that 3BP might reduce cancer mortality by limiting venous thromboembolism (VTE) in patients [87].
Concluding remarks
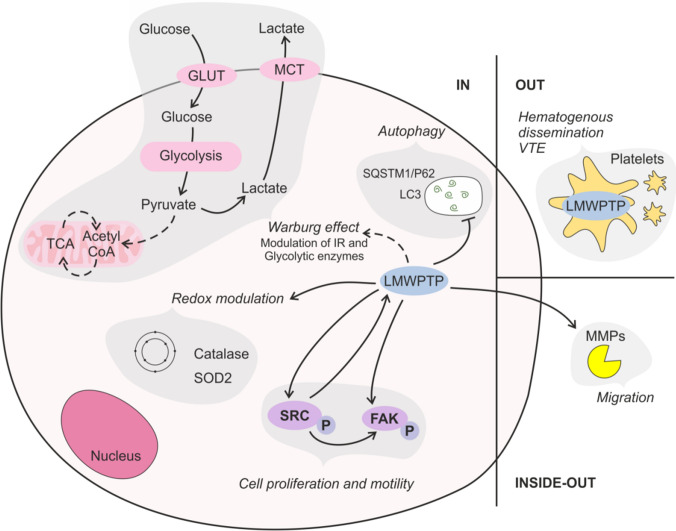
Over the past 2 decades of research on protein tyrosine phosphatases, the field has achieved great progress in scrutinizing the role of these phosphatases in cancer progression. In this review, we pointed out different aspects of the importance of LMWPTP in cancer biology. This phosphatase takes part in a molecular net responsible for providing some capabilities of cancer cells that contribute to the disease complexity: sustaining proliferative signaling, resisting cell death, deregulating cellular energetics, avoiding immune destruction, and activating invasion and metastasis (Fig. 2). Altogether, in our opinion, the rational modulation of LMWPTP has great potential to improve the outcome and life quality of cancer patients. In this aspect, so far, LMWPTP inhibitors developed by the Bottini group that display some desirable characteristics, under pharmacological view: specificity, stability, and bioavailability appear as excellent candidates for improving our understanding about the contribution of LMWPTP for cancer biology and open a new avenue for therapy.
Fig. 2.
LMWPTP acts as a hub in cancer hallmarks. LMWPTP contributes to intracellular metabolic changes such as promoting the Warburg effect by modulating the level of insulin receptor (IR), glycolytic and mitochondrial enzymes, and favoring antioxidant defenses; LMWPTP acts as a positive modulator of Src kinase and FAK, promoting cell proliferation and motility; and as a negative modulator of autophagy in CML. LMWPTP also plays a role outside of the cancer cells: (a) it is overexpressed in platelets from gastrointestinal cancer patients, which supports platelet hyperactivity (a key step of hematogenous dissemination of cancer cell), and a high propensity of venous thromboembolism events (VTE); (b) it favors metalloproteinases activity, key molecules required for cell migration
Acknowledgements
The authors thank Dr. Avram Slovic for revising the manuscript.
Author contribution
All authors contributed equally.
Funding
The study of LMWPTP in cancer biology was supported by the São Paulo Research Foundation under grants SPC (2018/03593-6), AVSF (2017/08119-8), EMBF (2015/11433-0), CVFH (2015/20412-7), National Council for Scientific and Technological Development (CNPq)—Brazil under grants: 141723/2019–0 (HGC) and 303900/2017-2 (CVFH) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Data availability
Not applicable.
Code availability
Not applicable.
Compliance with ethical standards
Conflict of interest
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tamura Y, Simizu S, Osada H. The phosphorylation status and anti-apoptotic activity of Bcl-2 are regulated by ERK and protein phosphatase 2A on the mitochondria. FEBS Lett. 2004;569(1–3):249–255. doi: 10.1016/j.febslet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Jailkhani N, Chaudhri VK, Rao KV. Regulatory cascades of protein phosphatases: implications for cancer treatment. Anticancer Agents Med Chem. 2011;11(1):64–77. doi: 10.2174/187152011794941253. [DOI] [PubMed] [Google Scholar]
- 3.Mustelin T, Vang T, Bottini N. Protein tyrosine phosphatases and the immune response. Nat Rev Immunol. 2005;5(1):43–57. doi: 10.1038/nri1530. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira PA, Ruela-de-Sousa RR, Queiroz KC, Souza AC, Milani R, Pilli RA, Peppelenbosch MP, den Hertog J, Ferreira CV. Knocking down low molecular weight protein tyrosine phosphatase (LMW-PTP) reverts chemoresistance through inactivation of Src and Bcr-Abl proteins. PLoS ONE. 2012;7(9):e44312. doi: 10.1371/journal.pone.0044312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Souza AC, Azoubel S, Queiroz KC, Peppelenbosch MP, Ferreira CV. From immune response to cancer: a spot on the low molecular weight protein tyrosine phosphatase. Cell Mol Life Sci. 2009;66(7):1140–1153. doi: 10.1007/s00018-008-8501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H, Yi JS, Lawan A, Min K, Bennett AM. Mining the function of protein tyrosine phosphatases in health and disease. Semin Cell Dev Biol. 2015;37(1):66–72. doi: 10.1016/j.semcdb.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonso A, Sasin J, Bottini N, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117(6):699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Modesti A, Marzocchini R, Raugei G, Chiti F, Sereni A, Magherini F, Ramponi G. Cloning, expression and characterisation of a new human low Mr phosphotyrosine protein phosphatase originating by alternative splicing. FEBS Lett. 1998;431(1):111–115. doi: 10.1016/s0014-5793(98)00732-7. [DOI] [PubMed] [Google Scholar]
- 9.Hoekstra E, Kodach LL, Das AM, Ruela-de-Sousa RR, Ferreira CV, Hardwick JC, van der Woude CJ, Peppelenbosch MP, Ten Hagen TL, Fuhler GM. Low molecular weight protein tyrosine phosphatase (LMWPTP) upregulation mediates malignant potential in colorectal cancer. Oncotarget. 2015;6(10):8300–8312. doi: 10.18632/oncotarget.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruela-de-Sousa RR, Hoekstra E, Hoogland AM, Souza Queiroz KC, Peppelenbosch MP, Stubbs AP, Pelizzaro-Rocha K, van Leenders GJLH, Jenster G, Aoyama H, Ferreira CV, Fuhler GM. Low-molecular-weight protein tyrosine phosphatase predicts prostate cancer outcome by increasing the metastatic potential. Eur Urol. 2016;69(4):710–719. doi: 10.1016/j.eururo.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 11.Caselli A, Paoli P, Santi A, Mugnaioni C, Toti A, Camici G, Cirri P. Low molecular weight protein tyrosine phosphatase: multifaceted functions of an evolutionarily conserved enzyme. Biochim Biophys Acta. 2016;1864(10):1339–1355. doi: 10.1016/j.bbapap.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Chiarugi P, Cirri P, Raugei G, Camici G, Dolfi F, Berti A, Ramponi G. PDGF receptor as a specific in vivo target for low M(r) phosphotyrosine protein phosphatase. FEBS Lett. 1995;372:49–53. doi: 10.1016/0014-5793(95)00947-8. [DOI] [PubMed] [Google Scholar]
- 13.Chiarugi P, Cirri P, Marra F, Raugei G, Fiaschi T, Camici G, Manao G, Romanelli RG, Ramponi G. The Src and signal transducers and activators of transcription pathways as specific targets for low molecular weight phosphotyrosine-protein phosphatase in platelet-derived growth factor signaling. J Biol Chem. 1998;273(12):6776–6785. doi: 10.1074/jbc.273.12.6776. [DOI] [PubMed] [Google Scholar]
- 14.Stein E, Lane AA, Cerretti DP, Schoecklmann HO, Schroff AD, Van Etten RL, Daniel TO. Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev. 1998;12(5):667–678. doi: 10.1101/gad.12.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bottini N, Stefanini L, Williams S, Alonso A, Jascur T, Abraham RT, Couture C, Mustelin T. Activation of ZAP-70 through specific dephosphorylation at the inhibitory Tyr-292 by the low molecular weight phosphotyrosine phosphatase (LMPTP) J Biol Chem. 2002;277(27):24220–24224. doi: 10.1074/jbc.M202885200. [DOI] [PubMed] [Google Scholar]
- 16.Kikawa KD, Vidale DR, Van Etten RL, Kinch MS. Regulation of the EphA2 kinase by the low molecular weight tyrosine phosphatase induces transformation. J Biol Chem. 2002;277(42):39274–39279. doi: 10.1074/jbc.M207127200. [DOI] [PubMed] [Google Scholar]
- 17.Taddei ML, Chiarugi P, Cirri P, Buricchi F, Fiaschi T, Giannoni E, Talini D, Cozzi G, Formigli L, Raugei G, Ramponi G. Beta-catenin interacts with low-molecular-weight protein tyrosine phosphatase leading to cadherin-mediated cell-cell adhesion increase. Cancer Res. 2002;62(22):6489–6499. [PubMed] [Google Scholar]
- 18.Park EK, Warner N, Mood K, Pawson T, Daar IO. Low-molecular-weight protein tyrosine phosphatase is a positive component of the fibroblast growth factor receptor signaling pathway. Mol Cell Biol. 2002;22(10):3404–3414. doi: 10.1128/mcb.22.10.3404-3414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rigacci S, Rovida E, Dello Sbarba P, Berti A. Low Mr phosphotyrosine protein phosphatase associates and dephosphorylates p125 focal adhesion kinase, interfering with cell motility and spreading. J Biol Chem. 2002;277(44):41631–41636. doi: 10.1074/jbc.M201709200. [DOI] [PubMed] [Google Scholar]
- 20.Giannoni E, Chiarugi P, Cozzi G, Magnelli L, Taddei ML, Fiaschi T, Buricchi F, Raugei G, Ramponi G. Lymphocyte function-associated antigen-1-mediated T cell adhesion is impaired by low molecular weight phosphotyrosine phosphatase-dependent inhibition of FAK activity. J Biol Chem. 2003;278(38):36763–36776. doi: 10.1074/jbc.M302686200. [DOI] [PubMed] [Google Scholar]
- 21.Rigacci S, Talini D, Berti A. LMW-PTP associates and dephosphorylates STAT5 interacting with its C-terminal domain. Biochem Biophys Res Commun. 2003;312(2):360–366. doi: 10.1016/j.bbrc.2003.10.126. [DOI] [PubMed] [Google Scholar]
- 22.Lee JK, Edderkaoui M, Truong P, Ohno I, Jang KT, Berti A, Pandol SJ, Gukovskaya AS. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133(5):1637–1648. doi: 10.1053/j.gastro.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 23.He RJ, Yu ZH, Zhang RY, Zhang ZY. Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol Sin. 2014;35(10):1227–1246. doi: 10.1038/aps.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zambuzzi WF, Granjeiro JM, Parikh K, Yuvaraj S, Peppelenbosch MP, Ferreira CV. Modulation of Src activity by low molecular weight protein tyrosine phosphatase during osteoblast differentiation. Cell Physiol Biochem. 2008;22(5–6):497–506. doi: 10.1159/000185506. [DOI] [PubMed] [Google Scholar]
- 25.de Souza Malaspina TS, Zambuzzi WF, dos Santos CX, Campanelli AP, Laurindo FR, Sogayar MC, Granjeiro JM. A possible mechanism of low molecular weight protein tyrosine phosphatase (LMW-PTP) activity modulation by glutathione action during human osteoblast differentiation. Arch Oral Biol. 2009;54(7):642–650. doi: 10.1016/j.archoralbio.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes GV, Cavagis AD, Ferreira CV, Olej B, Leão Mde S, Yano CL, Peppelenbosch M, Granjeiro JM, Zambuzzi WF. Osteoblast adhesion dynamics: a possible role for ROS and LMW-PTP. J Cell Biochem. 2014;115(6):1063–1069. doi: 10.1002/jcb.24691. [DOI] [PubMed] [Google Scholar]
- 27.Kusuyama J, Bandow K, Ohnishi T, Hisadome M, Shima K, Semba I, Matsuguchi T. Osteopontin inhibits osteoblast responsiveness through the down-regulation of focal adhesion kinase mediated by the induction of low-molecular weight protein tyrosine phosphatase. Mol Biol Cell. 2017;28(10):1326–1336. doi: 10.1091/mbc.E16-10-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.den Hertog J, Ostman A, Bçhmer FD. Protein tyrosine phosphatases: regulatory mechanisms. FEBS J. 2008;275:831–847. doi: 10.1111/j.1742-4658.2008.06247.x. [DOI] [PubMed] [Google Scholar]
- 29.Silva RA, Palladino MV, Cavalheiro RP, Machado D, Cruz BL, Paredes-Gamero EJ, Gomes-Marcondes MC, Zambuzzi WF, Vasques L, Nader HB, Souza AC, Justo GZ. Activation of the low molecular weight protein tyrosine phosphatase in keratinocytes exposed to hyperosmotic stress. PLoS ONE. 2015;10(3):e0119020. doi: 10.1371/journal.pone.0119020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bottini N, MacMurray J, Peters W, Rostamkhani M, Comings DE. Association of the acid phosphatase (ACP1) gene with triglyceride levels in obese women. Mol Genet Metab. 2002;77(3):226–229. doi: 10.1016/s1096-7192(02)00120-8b. [DOI] [PubMed] [Google Scholar]
- 31.Bourebaba L, Łyczko J, Alicka M, Bourebaba N, Szumny A, Fal AM, Marycz K. Inhibition of protein-tyrosine phosphatase PTP1B and LMPTP promotes palmitate/oleate-challenged HepG2 cell survival by reducing lipoapoptosis, improving mitochondrial dynamics and mitigating oxidative and endoplasmic reticulum stress. J Clin Med. 2020;9(5):E1294. doi: 10.3390/jcm9051294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanford SM, Aleshin AE, Zhang V, Ardecky RJ, Hedrick MP, Zou J, Ganji SR, Bliss MR, Yamamoto F, Bobkov AA, Kiselar J, Liu Y, Cadwell GW, Khare S, Yu J, Barquilla A, Chung TDY, Mustelin T, Schenk S, Bankston LA, Liddington RC, Pinkerton AB, Bottini N. Diabetes reversal by inhibition of the low-molecular-weight tyrosine phosphatase. Nat Chem Biol. 2017;13(6):624–632. doi: 10.1038/nchembio.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wade F, Quijada P, Al-Haffar KM, Awad SM, Kunhi M, Toko H, Marashly Q, Belhaj K, Zahid I, Al-Mohanna F, Stanford SM, Alvarez R, Liu Y, Colak D, Jordan MC, Roos KP, Assiri A, Al-Habeeb W, Sussman M, Bottini N, Poizat C. Deletion of low molecular weight protein tyrosine phosphatase (Acp1) protects against stress-induced cardiomyopathy. J Pathol. 2015;237(4):482–494. doi: 10.1002/path.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreira-Halder CV, Clerici SP, Faria AVS, Oliveira PFS, Cordeiro HG, Akagi E (2019) Protein tyrosine phosphatases in tumor progression and metastasis: promotor or protection? Tumor progression and metastasis. IntechOpen
- 35.Marzocchini R, Malentacchi F, Biagini M, Cirelli D, Luceri C, Caderni G, Raugei G. The expression of low molecular weight protein tyrosine phosphatase is up-regulated in 1,2-dimethylhydrazine-induced colon tumours in rats. Int J Cancer. 2008;122(7):1675–1678. doi: 10.1002/ijc.23266. [DOI] [PubMed] [Google Scholar]
- 36.Chiarugi P, Taddei ML, Schiavone N, Papucci L, Giannoni E, Fiaschi T, Capaccioli S, Raugei G, Ramponi G. LMW-PTP is a positive regulator of tumor onset and growth. Oncogene. 2004;23(22):3905–3914. doi: 10.1038/sj.onc.1207508. [DOI] [PubMed] [Google Scholar]
- 37.Malentacchi F, Marzocchini R, Gelmini S, Orlando C, Serio M, Ramponi G, Raugei G. Up-regulated expression of low molecular weight protein tyrosine phosphatases in different human cancers. Biochem Biophys Res Commun. 2005;334(3):875–883. doi: 10.1016/j.bbrc.2005.06.176. [DOI] [PubMed] [Google Scholar]
- 38.Alho I, Costa L, Bicho M, Coelho C. Characterization of low molecular weight protein tyrosine phosphatase isoforms in human breast cancer epithelial cell lines. Anticancer Res. 2013;33(5):1983–1987. [PubMed] [Google Scholar]
- 39.Alho I, Costa L, Bicho M, Coelho C. The role of low-molecular-weight protein tyrosine phosphatase (LMW-PTP ACP1) in oncogenesis. Tumour Biol. 2013;34(4):1979–1989. doi: 10.1007/s13277-013-0784-1. [DOI] [PubMed] [Google Scholar]
- 40.Bucciantini M, Chiarugi P, Cirri P, Taddei L, Stefani M, Raugei G, Nordlund P, Ramponi G. The low Mr phosphotyrosine protein phosphatase behaves differently when phosphorylated at Tyr131 or Tyr132 by Src kinase. FEBS Lett. 1999;456(1):73–78. doi: 10.1016/S0014-5793(99)00828-5. [DOI] [PubMed] [Google Scholar]
- 41.Capitani N, Lori G, Paoli P, Patrussi L, Troilo A, Baldari CT, Raugei G, D'Elios MM. LMW-PTP targeting potentiates the effects of drugs used in chronic lymphocytic leukemia therapy. Cancer Cell Int. 2019;19:67. doi: 10.1186/s12935-019-0786-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lori G, Paoli P, Caselli A, Cirri P, Marzocchini R, Mangoni M, Talamonti C, Livi L, Raugei G. Targeting LMW-PTP to sensitize melanoma cancer cells toward chemo- and radiotherapy. Cancer Med. 2018;7(5):1933–1943. doi: 10.1002/cam4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 44.Faria AVS, Tornatore TF, Milani R, Queiroz KCS, Sampaio IH, Fonseca EMB, Rocha-Brito KJP, Santos TO, Silveira LR, Peppelenbosch MP, Ferreira-Halder CV. Oncophosphosignaling favors a glycolytic phenotype in human drug resistant leukemia. J Cell Biochem. 2017;118(11):3846–3854. doi: 10.1002/jcb.26034. [DOI] [PubMed] [Google Scholar]
- 45.Lori G, Gamberi T, Paoli P, Caselli A, Pranzini E, Marzocchini R, Modesti A, Raugei G. LMW-PTP modulates glucose metabolism in cancer cells. Biochim Biophys Acta Gen Subj. 2018;1862(12):2533–2544. doi: 10.1016/j.bbagen.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020 doi: 10.1038/s41580-020-0230-3.10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 47.Faria AVS, Clerici SP, de Souza Oliveira PF, Queiroz KCS, Peppelenbosch MP, Ferreira-Halder CV. LMWPTP modulates the antioxidant response and autophagy process in human chronic myeloid leukemia cells. Mol Cell Biochem. 2020;466(1–2):83–89. doi: 10.1007/s11010-020-0369-1. [DOI] [PubMed] [Google Scholar]
- 48.Chaffer CL, Weinberg RA. How does multistep tumorigenesis really proceed? Cancer Discov. 2015;5(1):22–24. doi: 10.1158/2159-8290.CD-14-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borensztajn K, Peppelenbosch MP, Spek CA. Factor Xa: at the crossroads between coagulation and signaling in physiology and disease. Trends Mol Med. 2008;14(10):429–440. doi: 10.1016/j.molmed.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Spek CA, Versteeg HH, Borensztajn KS. Anticoagulant therapy of cancer patients: will patient selection increase overall survival? Thromb Haemost. 2015;114(3):530–536. doi: 10.1160/TH15-02-0124. [DOI] [PubMed] [Google Scholar]
- 51.Kurose H, Ueda K, Kondo R, Ogasawara S, Kusano H, Sanada S, Naito Y, Akiba J, Kakuma T, Igawa T, Yano H. Low-molecular-weight protein tyrosine phosphatase is a possible biomarker for predicting postoperative biochemical recurrence in prostate cancer with negative surgical margins. Anticancer Res. 2019;39(2):957–964. doi: 10.21873/anticanres.13199. [DOI] [PubMed] [Google Scholar]
- 52.Tautz L, Pellecchia M, Mustelin T. Targeting the PTPome in human disease. Expert Opin Ther Targets. 2006;10:157–177. doi: 10.1517/14728222.10.1.157. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y, Tian JY, Ye F, Xiao Z. Identification of natural products as selective PTP1B inhibitors via virtual screening. Bioorg Chem. 2020;98:103706. doi: 10.1016/j.bioorg.2020.103706. [DOI] [PubMed] [Google Scholar]
- 54.Stanford SM, Bottini N. Targeting tyrosine phosphatases: time to end the stigma. Trends Pharmacol Sci. 2017;38(6):524–540. doi: 10.1016/j.tips.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang ZY. Structure, mechanism, and specificity of protein-tyrosine phosphatases. Curr Top Cell Regul. 1997;35:21–68. doi: 10.1016/s0070-2137(97)80002-7. [DOI] [PubMed] [Google Scholar]
- 56.Chiarugi P, Buricchi F. Protein tyrosine phosphorylation and reversible oxidation: two cross-talking posttranslation modifications. Antioxid Redox Signal. 2007;9:1–24. doi: 10.1089/ars.2007.9.1. [DOI] [PubMed] [Google Scholar]
- 57.Chiarugi P. PTPs versus PTKs: the redox side of the coin. Free Radic Res. 2005;39:353–364. doi: 10.1080/10715760400027987. [DOI] [PubMed] [Google Scholar]
- 58.Bispo de Jesus M, Zambuzzi WF, Ruela de Sousa RR, Areche C, Santos de Souza AC, Aoyama H, Schmeda Hirschmann G, Rodrguez JA, de Souza M, Brito AR, Peppelenbosch MP, den Hertog J, de Paula E, Ferreira CV. Ferruginol suppresses survival signaling pathways in androgen-independent human prostate cancer cells. Biochimie. 2008;90:843–854. doi: 10.1016/j.biochi.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Chiarugi P. The redox regulation of LMW-PTP during cell proliferation or growth inhibition. IUBMB Life. 2001;52(1–2):55–59. doi: 10.1080/15216540252774775. [DOI] [PubMed] [Google Scholar]
- 60.He R, Wang J, Yu ZH, Zhang RY, Liu S, Wu L, Zhang ZY. Inhibition of low molecular weight protein tyrosine phosphatase by an induced-fit mechanism. J Med Chem. 2016;59(19):9094–9106. doi: 10.1021/acs.jmedchem.6b00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiarugi P, Fiaschi T, Taddei ML, Talini D, Giannoni E, Raugei G, Ramponi G. Two vicinal cysteines confer a peculiar redox regulation to low molecular weight protein tyrosine phosphatase in response to platelet-derived growth factor receptor stimulation. J Biol Chem. 2001;276(36):33478–33487. doi: 10.1074/jbc.M102302200. [DOI] [PubMed] [Google Scholar]
- 62.Ferreira CV, Justo GZ, Souza ACS, Queiroz KC, Zambuzzi WF, Aoyama H, Peppelenbosch MP. Natural compounds as a source of protein tyrosine phosphatase inhibitors: application to the rational design of small-molecule derivatives. Biochimie. 2006;88:1859–1873. doi: 10.1016/j.biochi.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 63.Miranda MA, Okamoto AK, Ferreira CV, Silva TL, Granjeiro JM, Aoyama H. Differential effects of flavonoids on bovine kidney low molecular mass protein tyrosine phosphatase. J Enzyme Inhib Med Chem. 2006;21:419–425. doi: 10.1080/14756360500179523. [DOI] [PubMed] [Google Scholar]
- 64.Vanitha P, Uma C, Suganya N, Bhakkiyalakshmi E, Suriyanarayanan S, Gunasekaran P, Sivasubramanian S, Ramkumar KM. Modulatory effects of morin on hyperglycemia by attenuating the hepatic key enzymes of carbohydrate metabolism and β-cell function in streptozotocin-induced diabetic rats. Environ Toxicol Pharmacol. 2014;37(1):326–335. doi: 10.1016/j.etap.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 65.Dixon M, Woodrick J, Gupta S, Karmahapatra SK, Devito S, Vasudevan S, Dakshanamurthy S, Adhikari S, Yenugonda VM, Roy R. Naturally occurring polyphenol, morin hydrate, inhibits enzymatic activity of N-methylpurine DNA glycosylase, a DNA repair enzyme with various roles in human disease. Bioorg Med Chem. 2015;23(5):1102–1111. doi: 10.1016/j.bmc.2014.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Areche C, Theoduloz C, Yáñez T, Souza-Brito AR, Barbastefano V, de Paula D, Ferreira AL, Schmeda-Hirschmann G, Rodríguez JA. Gastroprotective activity of ferruginol in mice and rats: effects on gastric secretion, endogenous prostaglandins and non-protein sulfhydryls. J Pharm Pharmacol. 2008;60(2):245–251. doi: 10.1211/jpp.60.2.0014. [DOI] [PubMed] [Google Scholar]
- 67.Ho ST, Tung YT, Kuo YH, Lin CC, Wu JH. Ferruginol inhibits non-small cell lung cancer growth by inducing caspase-associated apoptosis. Integr Cancer Ther. 2015;14(1):86–97. doi: 10.1177/1534735414555806. [DOI] [PubMed] [Google Scholar]
- 68.Caselli A, Cirri P, Santi A, Paoli P. Morin: a promising natural drug. Curr Med Chem. 2016;23(8):774–791. doi: 10.2174/0929867323666160106150821. [DOI] [PubMed] [Google Scholar]
- 69.Luo G, Zhou J, Li G, Hu N, Xia X, Zhou H. Ferruginol diterpenoid selectively inhibits human thyroid cancer growth by inducing mitochondrial dependent apoptosis, endogenous reactive oxygen species (ROS) production, mitochondrial membrane potential loss and suppression of mitogen-activated protein kinase (MAPK) and PI3K/AKT signaling pathways. Med Sci Monit. 2019;25:2935–2942. doi: 10.12659/MSM.914348. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Ottanà R, Maccari R, Amuso S, Wolber G, Schuster D, Herdlinger S, Manao G, Camici G, Paoli P. New 4-[(5-arylidene-2-arylimino-4-oxo-3-thiazolidinyl)methyl]benzoic acids active as protein tyrosine phosphatase inhibitors endowed with insulinomimetic effect on mouse C2C12 skeletal muscle cells. Eur J Med Chem. 2012;50:332–343. doi: 10.1016/j.ejmech.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 71.Ottanà R, Maccari R, Mortier J, Caselli A, Amuso S, Camici G, Rotondo A, Wolber G, Paoli P. Synthesis, biological activity and structure-activity relationships of new benzoic acid-based protein tyrosine phosphatase inhibitors endowed with insulinomimetic effects in mouse C2C12 skeletal muscle cells. Eur J Med Chem. 2014;71:112–127. doi: 10.1016/j.ejmech.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 72.He R, Yu ZH, Zhang RY, Wu L, Gunawan AM, Lane BS, Shim JS, Zeng LF, He Y, Chen L, Wells CD, Liu JO, Zhang ZY. Exploring the existing drug space for novel pTyr mimetic and SHP2 inhibitors. ACS Med Chem Lett. 2015;6(7):782–786. doi: 10.1021/acsmedchemlett.5b00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gupta SC, Phromnoi K, Aggarwal BB. Morin inhibits STAT3 tyrosine 705 phosphorylation in tumor cells through activation of protein tyrosine phosphatase SHP1. Biochem Pharmacol. 2013;85:898–912. doi: 10.1016/j.bcp.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 74.Jiang K, Shi J, Shi J. Morin alleviates vincristine-induced neuropathic pain via nerve protective effect and inhibition of NF-κB pathway in rats. Cell Mol Neurobiol. 2019;39(6):799–808. doi: 10.1007/s10571-019-00679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nie ZY, Yang L, Liu XJ, Yang Z, Yang GS, Zhou J, Qin Y, Yu J, Jiang LL, Wen JK, Luo JM. Morin inhibits proliferation and induces apoptosis by modulating the miR-188-5p/PTEN/AKT regulatory pathway in CML cells. Mol Cancer Ther. 2019;18(12):2296–2307. doi: 10.1158/1535-7163.MCT-19-0051. [DOI] [PubMed] [Google Scholar]
- 76.Brown J, O’Prey J, Harrison PR. Enhanced sensitivity of human oral tumours to the flavonol, morin, during cancer progression: involvement of the Akt and stress kinase pathways. Carcinogenesis. 2003;24:171–177. doi: 10.1093/carcin/24.2.171. [DOI] [PubMed] [Google Scholar]
- 77.Kuo HM, Chang LS, Lin YL, Lu HF, Yang JS, Lee JH, Chung JG. Morin inhibits the growth of human leukemia HL-60 cells via cell cycle arrest and induction of apoptosis through mitochondria dependent pathway. Anticancer Res. 2007;27:395–405. [PubMed] [Google Scholar]
- 78.Lori G, Paoli P, Femia AP, Pranzini E, Caselli A, Tortora K, Romagnoli A, Raugei G, Caderni G. Morin-dependent inhibition of low molecular weight protein tyrosine phosphatase (LMW-PTP) restores sensitivity to apoptosis during colon carcinogenesis: studies in vitro and in vivo, in an Apc-driven model of colon cancer. Mol Carcinog. 2019;58(5):686–698. doi: 10.1002/mc.22962. [DOI] [PubMed] [Google Scholar]
- 79.Saijo H, Kofujita H, Takahashi K, Ashitani T. Antioxidant activity and mechanism of the abietane-type diterpene ferruginol. Nat Prod Res. 2015;29(18):1739–1743. doi: 10.1080/14786419.2014.997233. [DOI] [PubMed] [Google Scholar]
- 80.Jia Y, Wu C, Zhang B, Zhang Y, Li J. Ferruginol induced apoptosis on SK-Mel-28 human malignant melanoma cells mediated through P-p38 and NF-κB. Hum Exp Toxicol. 2019;38(2):227–238. doi: 10.1177/0960327118792050. [DOI] [PubMed] [Google Scholar]
- 81.Xiong WD, Gong J, Xing C. Ferruginol exhibits anticancer effects in OVCAR-3 human ovary cancer cells by inducing apoptosis, inhibition of cancer cell migration and G2/M phase cell cycle arrest. Mol Med Rep. 2017;16(5):7013–7017. doi: 10.3892/mmr.2017.7484. [DOI] [PubMed] [Google Scholar]
- 82.Garbe C, Abusaif S, Eigentler TK. Vemurafenib. Recent Results Cancer Res. 2014;201:215–225. doi: 10.1007/978-3-642-54490-3_13. [DOI] [PubMed] [Google Scholar]
- 83.Schreck R, Rapp UR. Raf kinases: oncogenesis and drug discovery. Int J Cancer. 2006;119(10):2261–2271. doi: 10.1002/ijc.22144. [DOI] [PubMed] [Google Scholar]
- 84.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2006;1773(8):1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cordeiro HG, de Sousa V, Faria A, Ferreira-Halder CV. Vemurafenib downmodulates aggressiveness mediators of colorectal cancer (CRC): LMWPTP, PTP1B and TGFβ. Biol Chem. 2020 doi: 10.1515/hsz-2020-0124. [DOI] [PubMed] [Google Scholar]
- 86.Best MG, Sol N, Kooi I, Tannous J, Westerman BA, Rustenburg F, Schellen P, Verschueren H, Post E, Koster J, Ylstra B, Ameziane N, Dorsman J, Smit EF, Verheul HM, Noske DP, Reijneveld JC, Nilsson RJA, Tannous BA, Wesseling P, Wurdinger T. RNA-Seq of tumor-educated platelets enables blood-based Pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell. 2015;28:666–676. doi: 10.1016/j.ccell.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Faria AVS, Andrade SS, Reijm AN, Spaander MCW, de Maat MPM, Peppelenbosch MP, Ferreira-Halder CV, Fuhler GM. Targeting tyrosine phosphatases by 3-bromopyruvate overcomes hyperactivation of platelets from gastrointestinal cancer patients. J Clin Med. 2019;8(7):936. doi: 10.3390/jcm8070936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khorana AA, Carrier M, Garcia DA, Lee AY. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):81–91. doi: 10.1007/s11239-015-1313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.da Silva APP, El-Bacha T, Kyaw N, dos Santos RS, da Silva WS, Almeida FC, Da Poian AT, Galina A. Inhibition of energy-producing pathways of HepG2 cells by 3-bromopyruvate. Biochem J. 2009;417(3):717–726. doi: 10.1042/BJ20080805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.