Abstract
Programmed cell death-1 (PD-1) is a co-inhibitory receptor that plays important roles in regulating T cell immunity and peripheral tolerance. PD-1 signaling prevents T cells from overactivation during acute infections, but it maintains T cell exhaustion during chronic infections. Tumor cells can exploit the PD-1 signaling pathway to evade antitumor immune responses. The PD-1 signaling pathway is also essential for maintaining peripheral tolerance and prevention of autoimmunity. PD-1 expression is strictly and differentially regulated by diverse mechanisms in immune cells. It is activated and repressed by distinct transcription factors in different circumstances. Moreover, epigenetic mechanisms are also involved in regulating PD-1 expression. In this review, we summarize the knowledge of the transcriptional and epigenetic regulation of PD-1 expression during different immune responses.
Keywords: PD-1, Gene expression, Transcriptional regulation, Epigenetic regulation
Introduction
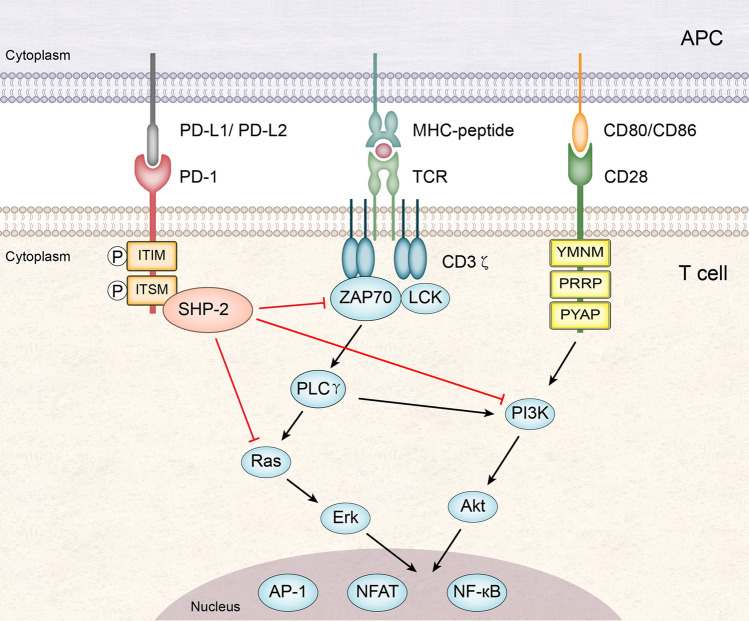
Programmed cell death-1 (PD-1, also known as CD279), which belongs to the CD28 immunoglobulin superfamily, is one of the most important co-inhibitory receptors expressed by immune cells. PD-1 is encoded by the gene Pdcd1. It is a type I transmembrane protein that is composed of an immunoglobulin variable region (IgV)-like domain, a stalk, a transmembrane domain, and an intracellular domain. The intracellular domain contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) and an immunoreceptor tyrosine-based switch motif (ITSM) [1–4]. The PD-1 receptor has two ligands, PD-L1 (CD274) and PD-L2 (CD273) [3–5]. The inhibitory signal delivered via the interaction of PD-1 with a ligand suppresses T cell responses. When PD-1 is engaged with a ligand, it becomes phosphorylated at the tyrosine residues of the ITIM and ITSM in its intracellular domain, which leads to recruitment of the protein tyrosine phosphatase SHP-2 to the ITSM. SHP-2 then dephosphorylates the kinases ZAP70, PI3K, and Ras, resulting in attenuation of T cell receptor (TCR) and CD28 signaling [3, 4, 6–8] (Fig. 1).
Fig. 1.
Suppression of T cell responses by the PD-1 signaling pathway. When PD-1 is engaged with its ligand, PD-L1 or PD-L2 on antigen-presenting cells (APC), it becomes phosphorylated at the tyrosine residues of the ITIM and ITSM in its intracellular domain. PD-1 then recruits the protein tyrosine phosphatase SHP-2 to its ITSM. SHP-2 can inhibit the signaling events triggered by TCR (interacting with the peptide-MHC complex) and CD28 (interacting with CD80 or CD86) via dephosphorylating the kinases ZAP70, PI3K, and Ras. This results in decreased activation of transcription factors such as AP-1, NFAT, and NF-κB, which are important for T cell activation, proliferation, effector functions and survival
The PD-1 signaling pathway plays significant roles in regulating T cell immunity. During acute infections, PD-1 signaling prevents T cells from overactivation, thus maintaining the delicate balance between effective antimicrobial immune responses and immune-mediated tissue damage [1, 2, 6, 9, 10]. Pdcd1 knockout mice infected with adenovirus developed more severe liver damage compared with wild-type mice, but they cleared the virus more rapidly [11]. During chronic infections, PD-1 signaling has a central role in maintaining T cell exhaustion, a state in which T cells lose their effector functions [8, 10, 12, 13]. In human and mouse, antibody blockade of the PD-1 signaling pathway partially reversed T cell exhaustion and resulted in a lower viral load [14–17]. However, PD-1 is not required for initiation of T cell exhaustion during chronic infections [18]. CD8 + T cell exhaustion occurred in the absence of PD-1 during chronic LCMV infection, and some aspects of exhaustion were even more severe from the onset of infection [18]. This suggests that the other co-inhibitory receptor pathways may compensate when PD-1 is missing. Tumor cells can exploit the PD-1 signaling pathway to achieve immune evasion. Signaling through the interaction of PD-1 on tumor-infiltrating T cells with PD-L1 on tumor cells makes tumor-infiltrating T cells exhausted, thus tumor cells can evade host immune attack [6–8, 13]. Currently, antibody blockade of the PD-1 signaling pathway has been widely used in cancer immunotherapies (Fig. 2), and remarkable and durable efficacy has been obtained in a subset of cancer patients [19–22]. Six PD-1/PD-L1 monoclonal antibodies have been approved by the U.S. Food and Drug Administration (FDA) for treatment of a wide range of human cancers [21, 22].
Fig. 2.
Cancer immunotherapies via antibody blockade of the PD-1 signaling pathway. a Signaling through the interaction of PD-1 on tumor-infiltrating T cells with PD-L1 on tumor cells makes tumor-infiltrating T cells exhausted, thus tumor cells can evade host immune attack. b Blockade of the PD-1 signaling pathway via an anti-PD-1 antibody reinvigorates exhausted tumor-infiltrating T cells. Tumor-infiltrating T cells then restore the effector functions to kill tumor cells
The PD-1 signaling pathway is also crucial for maintaining peripheral self-tolerance and prevention of autoimmunity via suppressing autoreactive T cells in the periphery. PD-1 deficiency or antibody blockade of the PD-1 signaling pathway accelerated disease progression in several mouse models of autoimmune diseases [23–27], and mutations in PDCD1 are associated with susceptibility to a couple of human autoimmune disorders [28–31]. Very interestingly, PD-1 can also serve as a tumor suppressor in T cell non-Hodgkin lymphomas (T-NHLs), which originate from peripheral T lymphocytes and are frequently characterized by genetic gain-of-function variants in TCR-signaling molecules. This is achieved via suppressing the oncogenic TCR signaling and increasing the PTEN level in the pre-malignant cells of T-NHLs [32].
PD-1 expression is strictly regulated by diverse mechanisms in immune cells. Activation and repression of Pdcd1 transcription is carried out by distinct transcription factors in different circumstances (Table 1). Epigenetic mechanisms are also involved in regulation of PD-1 expression. Most of the work on regulation of PD-1 expression was conducted in T cells, with a small part performed in other immune cells. Since the PD-1 signaling pathway plays important roles in regulating T cell exhaustion and peripheral tolerance, understanding the mechanisms of regulation of PD-1 expression may be helpful for developing novel immunotherapies for cancers, autoimmune diseases, and chronic infections.
Table 1.
Transcription factors that regulate PD-1 expression
| Transcription factor | Cell types | Infection type | Binding sites locations | Function | References |
|---|---|---|---|---|---|
| NFATc1 | T cells, B cells | Acute | Inside CR-C | Activator | [33] |
| RBP-Jκ | CD8 + T cells | Acute | Upstream of CR-C | Activator | [46, 47] |
| STAT3, STAT4 | T cells | Acute | Inside the enhancers located at − 3.7 kb and + 17.1 kb | Activator | [40] |
| ISGF3 | T cells, macrophages | Acute | Inside CR-C | Activator | [35, 36] |
| FoxO1 | CD8 + T cells | Chronic | Inside CR-C | Activator | [37] |
| c-Fos | Tumor-infiltrating T cells | – | Inside CR-B | Activator | [34] |
| NF-κB | Macrophages, B cells | – | Inside CR-C | Activator | [38] |
| Blimp-1 | T cells | Acute | Downstream of CR-C | Repressor | [49, 54, 55] |
| T-bet | T cells | Chronic | − 0.5 kb | Repressor | [53] |
Gene structure and expression of PD-1
Mouse Pdcd1 is on chromosome 1, whereas human PDCD1 is on chromosome 2. In both species, Pdcd1 is composed of 5 exons. Exon 1 and exon 2 encode a short signal sequence and the IgV-like domain, respectively. Exon 3 codes for the stalk and the transmembrane domain. Exon 4 and exon 5 encode the whole cytoplasmic domain and a long 3′ UTR [1, 3, 10].
PD-1 is transiently expressed in several types of immune cells including T cells, B cells, natural killer (NK) cells, natural killer T (NKT) cells, macrophages, and dendritic cells during their activation [3, 4, 7, 10]. PD-1 is also highly expressed in exhausted T cells [33]. Moreover, PD-1 is differentially expressed in T cells during acute and chronic infections. During acute LCMV infection, PD-1 expression in CD8 + T cells is induced following antigen exposure. It peaks at a certain time point, then it is downregulated and turned off [34, 35]. However, during chronic LCMV infection, PD-1 expression in CD8 + T cells is only slightly downregulated after the peak. Then it is quickly upregulated to a much higher level and sustained during the whole infection process [34].
Transcriptional regulation of PD-1 expression
Important cis-regulatory elements of Pdcd1
Transcription of Pdcd1 is regulated by a couple of cis-regulatory elements. The first identified elements are conserved region B (CR-B) and conserved region C (CR-C), which are highly conserved among mammals [36]. CR-B is located at − 100 bp of Pdcd1 and contains an AP-1-binding site [37]. CR-C is located at − 1.1 kb of Pdcd1 and contains multiple transcription factor-binding sites, including an interferon-stimulated response element (ISRE) [38, 39], an NFAT-binding site [36], a FoxO1-binding site [40], and a p65-binding site [41]. CR-B and CR-C are both hypersensitive to DNase I digestion in EL4 cells that transcribe a high level of Pdcd1 mRNA, but not in A20 cells that do not transcribe Pdcd1 mRNA, suggesting that these two regions play important roles in regulating Pdcd1 transcription [36]. CR-C is crucial for transcriptional activation of Pdcd1. A Pdcd1 luciferase reporter gene construct that lacks CR-C displayed a very low expression level and did not respond to a variety of stimulations [36, 41], and deletion of CR-C in mouse resulted in decreased Pdcd1 transcription during T cell activation [42].
There are also several transcription factor-binding sites adjacent to CR-B and CR-C, including an RBP-Jκ-binding site upstream of CR-C [43], a Blimp-1-binding site downstream of CR-C [44], and a T-bet-binding site at -0.5 kb of Pdcd1 [45]. These transcription factor-binding sites also play important roles in regulation of Pdcd1 transcription.
Two enhancers located at − 3.7 kb and + 17.1 kb of Pdcd1 are also important cis-regulatory elements for Pdcd1 transcription. They both contain a STAT-binding site and interact with the Pdcd1 promoter following cytokine treatment during ex vivo T cell activation [46]. In addition, two insulators located at − 26.7 kb and + 17.5 kb of Pdcd1 can bind CCCTC-binding factor (CTCF) and form a long-range chromatin loop [46]. Since insulators restrain the distant enhancers from promoting the expression of other genes [47], it is very likely that these two cis-elements define the boundaries of the Pdcd1 regulatory locus.
Activation of Pdcd1 transcription
During acute infections, the transcription factor NFATc1 (also known as NFAT2) activates Pdcd1 transcription in T cells [36]. Upon T cell activation, TCR signaling activates the calcineurin pathway, leading to activation and nuclear translocation of NFATc1 [48]. NFATc1 then binds to the NFAT-binding site in CR-C and activates Pdcd1 transcription. The calcineurin inhibitor cyclosporine A and the NFAT-specific inhibitor VIVIT abrogated Pdcd1 transcription in T cells [36]. The Notch signaling pathway, which plays an important role in regulating the effector functions of CD8 + T cells [49–51], is also involved in activating Pdcd1 transcription in CD8 + T cells during acute infections [43, 52]. Activation of the Notch signaling pathway in CD8 + T cells results in formation of the Notch complex composed of the Notch intracellular domain (NICD) and the recombination signal-binding protein for immunoglobulin kappa J region (RBP-Jκ). The complex binds to the RBP-Jκ-binding site upstream of CR-C and promotes Pdcd1 transcription in CD8 + T cells. Blockade of Notch signaling with specific Notch inhibitors decreased Pdcd1 transcription during CD8 + T cell activation [43]. In addition, cytokines IL-6, IL-12, and IFN-α are found to enhance Pdcd1 transcription during T cell activation [39, 46]. IL-6 and IL-12 activate STAT3 and STAT4 in T cells, respectively. Activated STAT3 or STAT4 can bind to the STAT-binding sites in the enhancers located at − 3.7 kb and + 17.1 kb of Pdcd1 and promote its transcription during T cell activation [46]. IFN-α treatment results in formation of the IFN-stimulated gene factor 3 (ISGF3) complex composed of STAT1, STAT2, and interferon regulatory factor 9 (IRF9) in T cells. The complex can bind to the ISRE in CR-C and enhance Pdcd1 transcription during T cell activation [39].
At the T cell activation stage of a chronic infection, Pdcd1 transcription also seems to be activated by NFATc1 in CD8 + T cells, because the PD-1 expression pattern at this stage is similar to that during an acute infection [34]. However, once a chronic infection is established, NFATc1 nuclear translocation is impaired in CD8 + T cells [53]. The high level of Pdcd1 transcription in CD8 + T cells is sustained by the transcription factor FoxO1 thereafter [40]. FoxO1 protein is highly expressed and retained in the nucleus in antigen-specific CD8 + T cells during chronic LCMV infection. It binds to the FoxO1-binding site in CR-C and activates Pdcd1 transcription [40]. During chronic LCMV infection, FoxO1 deficiency remarkably decreased PD-1 expression in antigen-specific CD8 + T cells, whereas overexpression of a constitutively active mutant of FoxO1 in antigen-specific CD8 + T cells increased PD-1 expression [40]. Very intriguingly, although tumor-infiltrating T cells are also exhausted, Pdcd1 transcription is activated by activator protein 1 (AP-1) [37] rather than FoxO1 in these T cells. The c-Fos subunit of AP-1 binds to the AP-1-binding site in CR-B and activates Pdcd1 transcription in tumor-infiltrating T cells [37]. The Pdcd1 mRNA level was significantly reduced in tumor-infiltrating T cells from c-Fos conditional knockout mice, and a knockin mutation of this AP-1-binding site in mice resulted in a decreased level of Pdcd1 mRNA in tumor-infiltrating T cells [37]. Furthermore, both the c-Fos conditional knockout mice and AP-1-binding site mutation knockin mice showed enhanced antitumor immunity [37].
In macrophages, Pdcd1 transcription is activated by nuclear factor kappa B (NF-κB). The NF-κB complex activated by Toll-like receptor (TLR) signaling binds to the p65-binding site in CR-C and activates Pdcd1 transcription during macrophage activation [41]. Inhibition of NF-κB signaling using specific small molecule inhibitors resulted in a total loss of Pdcd1 transcription in macrophages, and mutation of the p65-binding site in CR-C abrogated the expression of a Pdcd1 luciferase reporter gene in RAW267.4 cells (a murine macrophage cell line) [41]. Moreover, IFN-α also enhances Pdcd1 transcription in macrophages, which is also achieved via the ISGF3 complex binding to the ISRE in CR-C [38]. Interestingly, in B cells, Pdcd1 transcription can be activated by both NFATc1 and NF-κB. Activation of either transcription factor induced Pdcd1 transcription in B cells [41].
Repression of Pdcd1 transcription
Most work on repression of Pdcd1 transcription was performed in CD8 + T cells. During acute infections, repression of Pdcd1 transcription in CD8 + T cells is carried out by the transcription factor Blimp-1[44], which is a key regulator of CD8 + T cell terminal differentiation [54, 55]. Blimp-1 binds to the Blimp-1-binding site downstream of CR-C and directly represses Pdcd1 transcription in CD8 + T cells. Moreover, Blimp-1 also represses the expression of NFATc1, which is the transcriptional activator of Pdcd1 in CD8 + T cells during acute infections. Thus, Blimp-1 can turn off Pdcd1 transcription very quickly using a coherent feed-forward circuit in CD8 + T cells [44]. Blimp-1 deficiency resulted in increased and prolonged Pdcd1 transcription in primary CD8 + T cells during ex vivo activation as well as in antigen-specific CD8 + T cells during acute LCMV infection [44]. During chronic infections, Blimp-1 is highly expressed in CD8 + T cells, but it loses the ability to repress Pdcd1 transcription [56]. It is the transcription factor T-bet that represses Pdcd1 transcription in CD8 + T cells during chronic infections. T-bet binds to the T-bet-binding site located at − 0.5 kb of Pdcd1 and represses Pdcd1 transcription, which sustains the immune responses of CD8 + T cells [45]. During chronic LCMV infection, T-bet expression inversely correlated with PD-1 expression. In addition, T-bet deficiency augmented PD-1 expression in exhausted antigen-specific CD8 + T cells, whereas overexpression of T-bet downregulated PD-1 expression and improved the durability of exhausted antigen-specific CD8 + T cells [45].
A small part of work on repression of Pdcd1 transcription was conducted in CD4 + T cells. During acute LCMV infection, overexpression of Blimp-1 significantly decreased PD-1 expression in antigen-specific T follicular helper (TFH) cells [57]. This indicates that it could also be Blimp-1 that represses Pdcd1 transcription in CD4 + T cells during acute infections. During chronic LCMV infection, T-bet deficiency resulted in elevated PD-1 expression in antigen-specific CD4 + T cells [45], suggesting that T-bet is also the repressor of Pdcd1 transcription in CD4 + T cells during chronic infections. Moreover, the Pdcd1 mRNA level was increased in Blimp-1-deficient regulatory T cells (Treg cells) [58], which indicates that Blimp-1 also represses Pdcd1 transcription in Treg cells.
Epigenetic regulation of PD-1 expression
Epigenetic regulation is a type of regulation of gene expression based on nongenetic mechanisms other than changes in the underlying DNA sequence, which include DNA methylation, histone modifications, and alterations in chromatin architecture [59]. PD-1 expression is also regulated by DNA methylation and histone modifications in its regulatory regions.
Regulation of PD-1 expression by DNA methylation
DNA methylation at the CpG sites in the promoter of a gene represses its expression [60]. In T cells, PD-1 expression is regulated by the dynamic changes in DNA methylation in its promoter. In mouse naïve CD8 + T cells that do not express PD-1, both CR-B and CR-C were hyper-methylated [35]. During acute LCMV infection, both CR-B and CR-C displayed a significant loss of DNA methylation in antigen-specific CD8 + T cells when PD-1 was highly expressed. However, CR-B and CR-C both regained DNA methylation in antigen-specific CD8 + T cells after the viruses were cleared and PD-1 expression was turned off, and DNA methylation at CR-B and CR-C in memory CD8 + T cells was restored to a similar level in naïve CD8 + T cells [35]. During chronic LCMV infection, both CR-B and CR-C remained hypo-methylated in antigen-specific CD8 + T cells that expressed a high level of PD-1 [35]. Similarly, in a model of antigen tolerance induced by peptide immunotherapy, tolerant CD4 + T cells that had long-term PD-1 expression showed complete demethylation at CR-C and remarkably reduced DNA methylation at CR-B [61]. In addition, in patients with chronic HIV infection, the PDCD1 promoter was almost completely demethylated in HIV-specific CD8 + T cells that expressed a high level of PD-1, whereas it remained hyper-methylated in naïve CD8 + T cells from the same donor that expressed a very low level of PD-1 [62]. These data of inverse correlation between DNA methylation and PD-1 expression suggests that DNA methylation plays an important role in repressing PD-1 expression in T cells.
Regulation of PD-1 expression by histone modifications
Histone modifications can alter the architecture of chromatin, thus histone modifications in the regulatory regions of a gene affect promoter and enhancer accessibility and lead to changes in gene expression [63, 64]. In T cells, PD-1 expression is also regulated by histone modifications in its regulatory regions. During primary CD8 + T cell ex vivo activation with anti-CD3/CD28 beads, the levels of the active histone marks H3K9Ac, H3K27Ac, H3K4me2, and H3K4me3 were remarkably elevated at both CR-B and CR-C when PD-1 was highly expressed. After PD-1 expression was turned off, the levels of the repressive histone marks H3K9me3, H3K27me3, and H4K20me3 were significantly increased, and the levels of the four active histone marks were markedly decreased at both CR-B and CR-C [44]. The increase of the repressive histone marks and decrease of the active histone marks at CR-B and CR-C should be mediated by Blimp-1, because ectopic overexpression of Blimp-1 in EL4 cells augmented the levels of these repressive histone marks and decreased the levels of these active histone marks at both CR-B and CR-C [44]. Indeed, Blimp-1 has been reported to recruit histone-modifying enzymes, including the histone deacetylases HDAC1 and HDAC2 [65], the histone methyltransferase G9a [66], and the H3K4me2/me1 demethylase lysine-specific demethylase 1 (LSD1), to modify histone H3 of chromatin [67, 68]. Interestingly, the levels of the repressive and active histone marks were low at CR-B and CR-C in naïve CD8 + T cells [35, 44], indicating that silence of PD-1 expression may result from lack of the transcriptional activators as well as the high level of DNA methylation in the PD-1 promoter in naïve CD8 + T cells.
During acute LCMV infection, the levels of H3K9me3 and H3K27me3 were increased at both CR-B and CR-C in antigen-specific CD8 + T cells after the viruses were cleared and PD-1 expression was turned off [35]. However, during chronic LCMV infection, low levels of H3K9me3 and H3K27me3 were maintained at both CR-B and CR-C in antigen-specific CD8 + T cells that express a high level of PD-1 [35]. Similarly, CR-B and CR-C displayed an elevated level of H3K4me3 in the tolerant CD4 + T cells induced by peptide immunotherapy that had long-term PD-1 expression [61].
Histone modifications at the enhancers located at − 3.7 kb and + 17.1 kb of Pdcd1 also regulate PD-1 expression. Poised enhancers are marked by H3K4me1, and they also contain H3K27Ac when they are active [69]. The enhancers located at − 3.7 kb and + 17.1 kb of Pdcd1 are not marked by either of the histone marks when primary CD8 + T cells were ex vivo activated with anti-CD3/CD28 beads, although PD-1 expression was induced by TCR engagement [46], suggesting that these two enhancers do not function without cytokine stimulation. In agreement, treatment of primary CD8 + T cells with IL-6 or IL-12 alone increased the level of H3K4me1 at both enhancers, but it did not have any effect on the level of H3K27Ac at these two enhancers or induce PD-1 expression in primary CD8 + T cells [46]. When primary CD8 + T cells were stimulated with anti-CD3/CD28 beads plus IL-6 or IL-12, the levels of H3K4me1 and H3K27Ac were both significantly increased at these two enhancers. Correspondingly, PD-1 expression in these CD8 + T cells was also remarkably enhanced compared with that in CD8 + T cells treated with only anti-CD3/CD28 beads [46].
Conclusions
PD-1 expression is controlled by distinct transcription factors in different immune cells, and it is regulated by differential mechanisms during acute and chronic infections. Moreover, PD-1 expression is also epigenetically regulated by DNA methylation and histone modifications in its regulatory regions. Since the PD-1 signaling pathway is associated with cancers, autoimmune disorders, and chronic infections, understanding the mechanisms by which PD-1 expression is regulated in immune cells could provide some new perspectives on the treatment of these diseases.
Acknowledgements
This work was supported by Funding for Top-notch Personnel granted to PL from Shandong First Medical University.
Author contributions
PL had the idea for this review and provided the final approval of the version to be published. ZC and YL wrote the manuscript. YY generated the figures. PL and BL revised the manuscript. All the authors have read and approved the final version of this review.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zengde Chi and Yan Lu contributed equally to this work.
References
- 1.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity. 2016;44(5):955–972. doi: 10.1016/j.immuni.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. New Engl J Med. 2016;375(18):1767–1778. doi: 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 6.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18(3):153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 7.LaFleur MW, Muroyama Y, Drake CG, Sharpe AH. Inhibitors of the PD-1 pathway in tumor therapy. J Immunol. 2018;200(2):375–383. doi: 10.4049/jimmunol.1701044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36(4):265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8(3):239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 10.Jubel JM, Barbati ZR, Burger C, Wirtz DC, Schildberg FA. The role of PD-1 in acute and chronic infection. Front Immunol. 2020;11:487. doi: 10.3389/fimmu.2020.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198(1):39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catakovic K, Klieser E, Neureiter D, Geisberger R. T cell exhaustion: from pathophysiological basics to tumor immunotherapy. Cell Commun Signal CCS. 2017;15(1):1. doi: 10.1186/s12964-016-0160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 15.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 16.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203(10):2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 18.Odorizzi PM, Pauken KE, Paley MA, Sharpe A, Wherry EJ. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J Exp Med. 2015;212(7):1125–1137. doi: 10.1084/jem.20142237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017;24(1):26. doi: 10.1186/s12929-017-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilky BA. Immune checkpoint inhibitors: the linchpins of modern immunotherapy. Immunol Rev. 2019;290(1):6–23. doi: 10.1111/imr.12766. [DOI] [PubMed] [Google Scholar]
- 22.Wakabayashi G, Lee YC, Luh F, Kuo CN, Chang WC, Yen Y. Development and clinical applications of cancer immunotherapy against PD-1 signaling pathway. J Biomed Sci. 2019;26(1):96. doi: 10.1186/s12929-019-0588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 24.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ, Auchincloss H, Jr, Sayegh MH. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198(1):63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, Azuma M, Yagita H, Sayegh MH, Khoury SJ. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003;198(1):71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH, Kelley VR. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol. 2008;181(4):2513–2521. doi: 10.4049/jimmunol.181.4.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynoso ED, Elpek KG, Francisco L, Bronson R, Bellemare-Pelletier A, Sharpe AH, Freeman GJ, Turley SJ. Intestinal tolerance is converted to autoimmune enteritis upon PD-1 ligand blockade. J Immunol. 2009;182(4):2102–2112. doi: 10.4049/jimmunol.0802769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prokunina L, Castillejo-Lopez C, Oberg F, Gunnarsson I, Berg L, Magnusson V, Brookes AJ, Tentler D, Kristjansdottir H, Grondal G, Bolstad AI, Svenungsson E, Lundberg I, Sturfelt G, Jonssen A, Truedsson L, Lima G, Alcocer-Varela J, Jonsson R, Gyllensten UB, Harley JB, Alarcon-Segovia D, Steinsson K, Alarcon-Riquelme ME. A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat Genet. 2002;32(4):666–669. doi: 10.1038/ng1020. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen C, Hansen D, Husby S, Jacobsen BB, Lillevang ST. Association of a putative regulatory polymorphism in the PD-1 gene with susceptibility to type 1 diabetes. Tissue Antigens. 2003;62(6):492–497. doi: 10.1046/j.1399-0039.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- 30.Kroner A, Mehling M, Hemmer B, Rieckmann P, Toyka KV, Maurer M, Wiendl H. A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann Neurol. 2005;58(1):50–57. doi: 10.1002/ana.20514. [DOI] [PubMed] [Google Scholar]
- 31.James ES, Harney S, Wordsworth BP, Cookson WO, Davis SJ, Moffatt MF. PDCD1: a tissue-specific susceptibility locus for inherited inflammatory disorders. Genes Immun. 2005;6(5):430–437. doi: 10.1038/sj.gene.6364223. [DOI] [PubMed] [Google Scholar]
- 32.Wartewig T, Kurgyis Z, Keppler S, Pechloff K, Hameister E, Ollinger R, Maresch R, Buch T, Steiger K, Winter C, Rad R, Ruland J. PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature. 2017;552(7683):121–125. doi: 10.1038/nature24649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203(10):2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27(4):670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Youngblood B, Oestreich KJ, Ha SJ, Duraiswamy J, Akondy RS, West EE, Wei Z, Lu P, Austin JW, Riley JL, Boss JM, Ahmed R. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity. 2011;35(3):400–412. doi: 10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 2008;181(7):4832–4839. doi: 10.4049/jimmunol.181.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao G, Deng A, Liu H, Ge G, Liu X. Activator protein 1 suppresses antitumor T-cell function via the induction of programmed death 1. Proc Natl Acad Sci USA. 2012;109(38):15419–15424. doi: 10.1073/pnas.1206370109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho HY, Lee SW, Seo SK, Choi IW, Choi I, Lee SW. Interferon-sensitive response element (ISRE) is mainly responsible for IFN-alpha-induced upregulation of programmed death-1 (PD-1) in macrophages. Biochem Biophys Acta. 2008;1779(12):811–819. doi: 10.1016/j.bbagrm.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, Honjo T. IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol. 2011;186(5):2772–2779. doi: 10.4049/jimmunol.1003208. [DOI] [PubMed] [Google Scholar]
- 40.Staron MM, Gray SM, Marshall HD, Parish IA, Chen JH, Perry CJ, Cui G, Li MO, Kaech SM. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8(+) T cells during chronic infection. Immunity. 2014;41(5):802–814. doi: 10.1016/j.immuni.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bally AP, Lu P, Tang Y, Austin JW, Scharer CD, Ahmed R, Boss JM. NF-kappaB regulates PD-1 expression in macrophages. J Immunol. 2015;194(9):4545–4554. doi: 10.4049/jimmunol.1402550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bally AP, Tang Y, Lee JT, Barwick BG, Martinez R, Evavold BD, Boss JM. Conserved region C functions to regulate PD-1 expression and subsequent CD8 T cell memory. J Immunol. 2017;198(1):205–217. doi: 10.4049/jimmunol.1601464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathieu M, Cotta-Grand N, Daudelin JF, Thebault P, Labrecque N. Notch signaling regulates PD-1 expression during CD8(+) T-cell activation. Immunol Cell Biol. 2013;91(1):82–88. doi: 10.1038/icb.2012.53. [DOI] [PubMed] [Google Scholar]
- 44.Lu P, Youngblood BA, Austin JW, Mohammed AU, Butler R, Ahmed R, Boss JM. Blimp-1 represses CD8 T cell expression of PD-1 using a feed-forward transcriptional circuit during acute viral infection. J Exp Med. 2014;211(3):515–527. doi: 10.1084/jem.20130208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, Wherry EJ. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12(7):663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Austin JW, Lu P, Majumder P, Ahmed R, Boss JM. STAT3, STAT4, NFATc1, and CTCF regulate PD-1 through multiple novel regulatory regions in murine T cells. J Immunol. 2014;192(10):4876–4886. doi: 10.4049/jimmunol.1302750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou C, Zhao H, Tanimoto K, Dean A. CTCF-dependent enhancer-blocking by alternative chromatin loop formation. Proc Natl Acad Sci USA. 2008;105(51):20398–20403. doi: 10.1073/pnas.0808506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 49.Maekawa Y, Minato Y, Ishifune C, Kurihara T, Kitamura A, Kojima H, Yagita H, Sakata-Yanagimoto M, Saito T, Taniuchi I, Chiba S, Sone S, Yasutomo K. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat Immunol. 2008;9(10):1140–1147. doi: 10.1038/ni.1649. [DOI] [PubMed] [Google Scholar]
- 50.Cho OH, Shin HM, Miele L, Golde TE, Fauq A, Minter LM, Osborne BA. Notch regulates cytolytic effector function in CD8+ T cells. J Immunol. 2009;182(6):3380–3389. doi: 10.4049/jimmunol.0802598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuijk LM, Verstege MI, Rekers NV, Bruijns SC, Hooijberg E, Roep BO, de Gruijl TD, van Kooyk Y, Unger WW. Notch controls generation and function of human effector CD8+ T cells. Blood. 2013;121(14):2638–2646. doi: 10.1182/blood-2012-07-442962. [DOI] [PubMed] [Google Scholar]
- 52.Pan T, Liu Z, Yin J, Zhou T, Liu J, Qu H. Notch signaling pathway was involved in regulating programmed cell death 1 expression during sepsis-induced immunosuppression. Mediators Inflamm. 2015;2015:539841. doi: 10.1155/2015/539841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agnellini P, Wolint P, Rehr M, Cahenzli J, Karrer U, Oxenius A. Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection. Proc Natl Acad Sci USA. 2007;104(11):4565–4570. doi: 10.1073/pnas.0610335104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31(2):296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31(2):283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 56.Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, Wherry EJ. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31(2):309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, Belz GT, Smyth GK, Busslinger M, Nutt SL, Kallies A. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12(4):304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 59.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 60.Luo C, Hajkova P, Ecker JR. Dynamic DNA methylation: In the right place at the right time. Science. 2018;361(6409):1336–1340. doi: 10.1126/science.aat6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McPherson RC, Konkel JE, Prendergast CT, Thomson JP, Ottaviano R, Leech MD, Kay O, Zandee SE, Sweenie CH, Wraith DC, Meehan RR, Drake AJ, Anderton SM. Epigenetic modification of the PD-1 (Pdcd1) promoter in effector CD4(+) T cells tolerized by peptide immunotherapy. eLife. 2014 doi: 10.7554/eLife.03416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Youngblood B, Noto A, Porichis F, Akondy RS, Ndhlovu ZM, Austin JW, Bordi R, Procopio FA, Miura T, Allen TM, Sidney J, Sette A, Walker BD, Ahmed R, Boss JM, Sekaly RP, Kaufmann DE. Cutting edge: prolonged exposure to HIV reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J Immunol. 2013;191(2):540–544. doi: 10.4049/jimmunol.1203161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rice JC, Allis CD. Histone methylation versus histone acetylation: new insights into epigenetic regulation. Curr Opin Cell Biol. 2001;13(3):263–273. doi: 10.1016/s0955-0674(00)00208-8. [DOI] [PubMed] [Google Scholar]
- 64.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Yu J, Angelin-Duclos C, Greenwood J, Liao J, Calame K. Transcriptional repression by blimp-1 (PRDI-BF1) involves recruitment of histone deacetylase. Mol Cell Biol. 2000;20(7):2592–2603. doi: 10.1128/mcb.20.7.2592-2603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gyory I, Wu J, Fejer G, Seto E, Wright KL. PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat Immunol. 2004;5(3):299–308. doi: 10.1038/ni1046. [DOI] [PubMed] [Google Scholar]
- 67.Su ST, Ying HY, Chiu YK, Lin FR, Chen MY, Lin KI. Involvement of histone demethylase LSD1 in Blimp-1-mediated gene repression during plasma cell differentiation. Mol Cell Biol. 2009;29(6):1421–1431. doi: 10.1128/MCB.01158-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bally APR, Neeld DK, Lu P, Majumder P, Tang Y, Barwick BG, Wang Q, Boss JM. PD-1 expression during acute infection is repressed through an LSD1-blimp-1 axis. J Immunol. 2020;204(2):449–458. doi: 10.4049/jimmunol.1900601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, Sharp PA, Boyer LA, Young RA, Jaenisch R. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci USA. 2010;107(50):21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]