Abstract
Transthyretin (TTR) is an extracellular protein mainly produced in the liver and choroid plexus, with a well-stablished role in the transport of thyroxin and retinol throughout the body and brain. TTR is prone to aggregation, as both wild-type and mutated forms of the protein can lead to the accumulation of amyloid deposits, resulting in a disease called TTR amyloidosis. Recently, novel activities for TTR in cell biology have emerged, ranging from neuronal health preservation in both central and peripheral nervous systems, to cellular fate determination, regulation of proliferation and metabolism. Here, we review the novel literature regarding TTR new cellular effects. We pinpoint TTR as major player on brain health and nerve biology, activities that might impact on nervous systems pathologies, and assign a new link between TTR and angiogenesis and cancer. We also explore the molecular mechanisms underlying TTR activities at the cellular level, and suggest that these might go beyond its most acknowledged carrier functions and include interaction with receptors and activation of intracellular signaling pathways.
Keywords: Transthyretin, Neuroprotection, Neuronal health, Cell metabolism, Proliferation
Introduction
Transthyretin (TTR) is a highly conserved homotetrameric protein which in humans has a molecular mass of 55 kDa, with each monomer being composed by 127 amino acids which correspond to approximately 14 kDa [1]. The TTR tetramer forms a narrow cylindrical hydrophobic channel running through the center of the molecule which carries two symmetrical binding sites [2, 3], being able to accommodate two molecules of thyroxine (T4), one of the major TTR physiological ligands (Fig. 1A). Besides T4, TTR is also the main carrier of retinol (vitamin A), by establishing a 1:1 molar complex with retinol binding protein (RBP), with binding being limited to a maximum of two RBP molecules per TTR tetramer [4] (Fig. 1B). Accordingly, the main acknowledged physiological function of TTR is the transport of the thyroid hormone (TH) thyroxine and retinol within the body and brain, what is reflected in the name of the protein. The prime sites for TTR synthesis are the liver, from where TTR is released to the blood, and the choroid plexus, which is the origin of cerebrospinal fluid (CSF) TTR [5]. In addition, TTR is also expressed in the endothelial cells of Islets of Langerhans [6], ciliary pigment epithelia [7], retinal pigment epithelium [8, 9], intestine [10], visceral yolk sac [11], and, in fairly minor amounts, stomach, heart, skeletal muscle and spleen [5]. One study also reported expression of TTR in the meninges [12] and, more recently, TTR synthesis has been demonstrated in the human placenta [13]. Importantly, TTR expression has also been suggested to occur in Schwann cells [14] and oligodendrocytes [15], as well as in different neuronal types namely dorsal root ganglia [16], hippocampal neurons [17], motor neurons [18] and cerebellar granule cells [19].
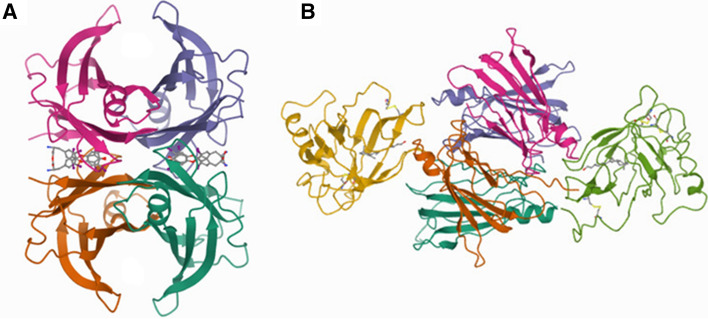
Fig.1.
Structure of human transthyretin (TTR) tetramer in complex with two thyroxine (T4) molecules (stick models) bound in the central hydrophobic channel (A), and with two molecules of retinol-binding protein (RBP) bound to the external surface (green and yellow) (B). Structural data obtained from pdb files (2ROX and 1QAB)
TTR, additionally to the carrier activities, is well recognized due to its association to amyloidosis (ATTR amyloidosis). ATTR amyloidosis might be either sporadic, characterized by the accumulation of wild-type (WT) TTR amyloid deposits (ATTRwt), occurring mainly in cardiac tissue in the elderly [20], or hereditary, caused by TTR variants (ATTRv). ATTRv amyloidosis, for which more than 100 mutations in the TTR gene have been described [21], include TTR-cardiac amyloidosis (ATTR-CM), formerly designated Familial Amyloid Cardiomyopathy (FAC), and ATTR polyneuropathy (ATTR-PN), previously recognized as Familial Amyloid Polyneuropathy (FAP). In ATTR-CM, TTR deposits are present mainly in the heart, leading to cardiomyopathy [22], while in ATTR-PN, deposition initially starts in peripheral nerves, culminating in polyneuropathy [23]. Besides amyloidosis, TTR has been associated to behavior, memory and learning deficits, nerve regeneration and neurodegeneration [24].
The multiple TTR expression sites, the discovery of additional TTR ligands, the impact on the biology of different cell types, and the reported associations to disease, boosted intensive research on unveiling new pathophysiological roles for this protein. In the following sections, we will discuss the established and novel activities ascribed to TTR, focusing on the impact on cell biology, which pinpoint TTR as an admirable study case in health and disease.
Well-known TTR biological functions
An impact of TTR in cell biology has been increasingly addressed in the literature, which might derive from novel TTR activities, or related to its established role as an extracellular transporter protein. In the next section we will revisit the well-known physiological functions of TTR.
Carrier of thyroxine and retinol
As mentioned above, the most recognized physiological role for TTR is the distribution in of T4 and retinol, in the latter case by association with RBP. Although TTR is highly concentrated in human plasma, it has an intermediate affinity for T4 and carries only about 15% of the hormone [25]. In contrast, in the human CSF, TTR is the major T4 distributor, carrying 80% of the hormone [26]. Retinol circulates in the plasma bound to RBP. RBP is synthesized and secreted primarily by hepatocytes and is the sole specific transport protein for retinol in the circulation. In the plasma, RBP-retinol is found in a complex with TTR, which is responsible for the binding of almost all RBP (95%). This association is proposed to facilitate RBP release from its site of synthesis in the endoplasmic reticulum and to prevent its renal filtration [27, 28]. In accordance, with the function of TTR as a distributor, adult TTR knockout (KO) mice exhibited decreased plasma levels of both T4 and retinol [29]. Nevertheless, in the absence of TTR, the levels of T4 were unaltered in tissues, and the TH function appeared normal [30]. Furthermore, TTR KO mice presented unaltered levels of retinol in tissues and did not display any symptoms of vitamin A deficiency [29, 31]. The studies with TTR KO adult mice questioned the relevance of TTR as a distributor of T4 and RBP-bound retinol. Nevertheless, studies with TTR KO mice demonstrating reduced TH bioavailability to neural stem cells in the subventricular zone (SVZ), and delayed bone growth, CNS maturation, and intestine and muscle development at postnatal stage, processes controlled by THs, reinforced the importance of TTR as T4 distributor [32, 33]. In the case of RBP, the relevance of the TTR-RBP-retinol complex is still not clear and awaits further investigation. In terms of the effect of TTR at the cellular level, which is the main focus of this review, the established role of both T4 and retinol on cell biology needs to be taken into account when analysing novel TTR functions, as they might be related to an indirect effect via its ligands.
TTR additional ligands and proteolytic substrates
New roles for TTR were found with the discovery of additional ligands besides T4 and RBP (Table 1). In 2004, further investigation of TTR binding to apolipoprotein A-I (ApoA-I) [34] revealed that TTR has proteolytic activity, being able to cleave the C-terminus of ApoA-I [35], reducing its ability to promote cholesterol efflux and increasing its amyloidogenic potential [36], and, as such, having an impact on the development of atherosclerosis. Later, TTR was defined as a zinc-dependent metalloprotease [37] and additional substrates in the nervous system were described, namely the neuropeptide Y (NPY) [38] and the amyloid β peptide (Aβ) [39], as well as other unidentified nervous system substrates that were suggested to mediate TTR neuritogenic activity [38], as will be further discussed. Concerning NPY, the physiologic relevance of its proteolytic cleavage by TTR was not addressed, however, TTR KO mice present increased NPY levels, supporting a role for TTR in the regulation of the levels of this neuropeptide. In respect to Aβ, which accumulation is a crucial hallmark of Alzheimer’s disease (AD), TTR was described as one of the major Aβ-binding proteins [40]. Moreover, TTR was shown to cleave Aβ, not only its soluble form, but also aggregated Aβ forms in vitro [39], diminishing its fibrillogenesis and toxicity [40] and promoting neuroprotection in vitro [41]. TTR binding and cleavage of Aβ was suggested to underly the reported neuroprotective TTR effect on AD [41]. Additionally, TTR was show to impact on Aβ clearance [42] by facilitating the Aβ efflux from the brain and its internalization by the liver via the Lipoprotein-related receptor 1 (LRP-1) [42].
Table 1.
Transthyretin known ligands and proteolytic substrates
| Carrier |
Thyroid hormones Retinol-binding protein |
| Proteolysis |
Apolipoprotein A-I Amyloid β peptide Neuropeptide Y |
| Other functions |
Norepinephrine oxidation products Lutein Pterin Perlecan Lysosome‐associated membrane protein Metallothionein 2 Endocrine-disrupting chemicals: Diflunisal, Ibuprofen, Heparin, ect |
Additional molecules were described to interact with TTR, namely, norepinephrine (NE) oxidation products, the yellow compounds lutein and pterin, perlecan, lysosome‐associated membrane protein (LAMP‐1), and metallothionein 2 (MT2) [43]. Nevertheless, the physiologic relevance of TTR interaction with the reported ligands is still undiscovered. In addition to the referred TTR endogenous ligands, several exogenous molecules were shown to bind TTR affecting tetrameric stability and the ability to bind T4 and RBP-retinol. These molecules are designated endocrine-disrupting chemicals (EDCs), and were reviewed in [24]. EDCs might enter the human organism in the food, water or aerosols and by affecting TTR distributor activities impact on human health. Some examples of EDCs include Ibuprofen, heparin, 1,4-Tetrachlorophenol, and 2,3-Dichlorophenol, among others.
TTR role on neuronal cell biology
The reported neuroprotective effects of TTR on both peripheral and central neurons were the first evidence of an impact of the protein in cell biology (Fig. 2A) [44–47]. Next, we will describe the established and the novel roles assigned for TTR in neuronal cell biology.
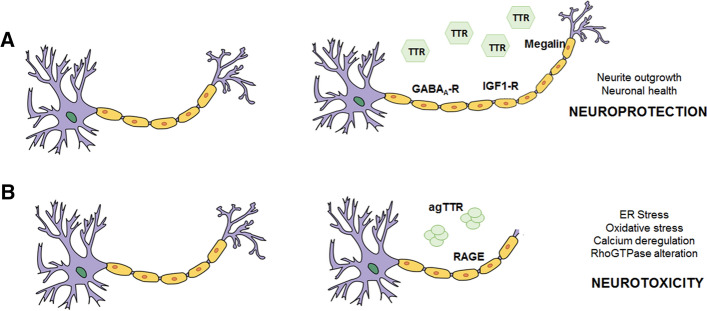
Fig. 2.
Transthyretin (TTR) role on neuronal cell biology. A TTR neuroprotective role. Extracellular TTR controls neuronal health, outgrowth and function via receptors (megalin, GABAA-R and IGF1-R). B Extracellular TTR aggregates (agTTR) cause neurotoxicity via the receptor RAGE. Other unknown receptors might be involved in the pathways impacted by extracellular TTR
Impact on the pathophysiology of peripheral neurons
Research on identifying a TTR function in the PNS was triggered by its association with ATTR-PN, a neurodegenerative disorder where aggregated TTR accumulates in peripheral nerves causing axon degeneration [48, 49]. In physiological conditions, TTR is also present in the PNS. Originally, it was found in the nerve endoneurial fluid, and was proposed to gain access to the PNS through the peripheral blood, crossing the blood-nerve barrier (BNB) [49]. Another suggested potential source of TTR to the PNS was the choroid plexus, due to the communication between the subarachnoid and endoneurial spaces [49]. Besides assess to the PNS, local expression of TTR by the dorsal root ganglia (DRG) was also reported, nevertheless this is a controversial topic, as Sousa et al. showed that TTR is not expressed in DRG [50], while Murakami et al. reported that TTR is expressed in the glia satellite cells of the DRG [16] and in Schwann cells [14]. Regardless of its local or systemic origin, TTR was described as neuroprotective in the PNS, as TTR KO mice were shown to display sensorimotor impairment, and decreased ability to regenerate after sciatic nerve crush [44]. TTR impact on nerve regeneration was linked to a neuritogenic activity in DRG neurons [44], via interaction with the endocytic receptor megalin resulting on TTR internalization [51], and to a role of TTR on axonal transport, as TTR KO DRG neurons showed compromised retrograde transport [51]. These observations unraveled a novel role for TTR on the biology of peripheral neurons, which was suggested to be independent of its transporter function, as a TTR variant with diminished ability to bind T4 and retinol conserved neuritogenic activity [51]. The mechanism underlying TTR impact on neurite outgrowth and axonal transport is still unclear, but some hypothesis can be raised: i) the fact that neuritogenic activity requires TTR internalization might indicate a novel intracellular function in PNS; ii) TTR proteolytic activity might be related, as a proteolytically inactive form of TTR was described to be unable to increase neurite outgrowth of DRG neurons [38], and iii) TTR interaction with megalin might activate an intracellular signaling pathway.
Neuroprotective role on the central nervous system
In the brain, besides being expressed in the epithelial cells of the choroid plexus, TTR was also shown to be expressed in neurons and oligodendrocytes [17–19]. In addition to the function as a TH distributor in the brain, TTR has also been shown to have other roles, namely neuritogenic activity in hippocampal neurons and a neuroprotective role in pathological conditions, such as cerebral ischemia [47, 52] and AD [41, 45, 46]. Moreover, TTR was found diminished in the CSF of schizophrenia and amyotrophic lateral sclerosis (ALS) patients, and in the degenerating ALS motor neurons [53]. All these observations point to TTR as a neuroprotector in the CNS.
The molecular mechanisms by which TTR exerts its neuroprotective effects in CNS are not yet fully understood; however, new reports elucidating these mechanisms are emerging. TTR was found to be determinant for hippocampal neuronal survival and neurite outgrowth and preservation, in in vitro excitotoxic conditions, and in a mouse model of permanent middle cerebral artery occlusion (pMCAO), a model of cerebral ischemia [47]. In support of this, in hippocampal neurons, TTR was found to enhance neurite survival and outgrowth by interacting with the receptor megalin and activating extracellular signal-regulated protein kinase (ERK), protein kinase B (AKT), and tyrosine-protein kinase Src (Src), ultimately leading to the upregulation of the CREB transcription factor, favoring the expression of anti-apoptotic Bcl2 protein family members [47]. TTR was also shown to increase intracellular calcium through NMDA receptors, in a Src/megalin-dependent manner, what could explain the ability of TTR to enhance neurite outgrowth [47]. Besides interacting with megalin, TTR was also shown to upregulate the expression of this receptor [54]. Interestingly, megalin was shown to impact on neurite outgrowth of hippocampal neurons and on synaptic plasticity [54]. As such, we might hypothesize that TTR-megalin interaction underlies TTR impact on CNS.
Besides megalin, TTR also was also shown to bind and positively regulate the expression of insulin-like growth factor 1 receptor (IGF1-R) in hippocampal neurons [55]. TTR, via a synergistic effect with insulin-like growth factor 1 (IGF1), was shown to induce the activation of the IGF1-R-AKT signaling pathway [56]. Similarly to TTR, IGF1 was shown to act as neuroprotector in brain ischemia and AD [57, 58]. Moreover, the IGF1-R signaling pathway was shown to control neuronal plasticity, synaptic activity and neurite outgrowth [59–61]. As such, TTR regulation of IGF1-R may, in part, explain the neuritogenic and neuroprotective effects described for TTR.
Recently, another important receptor in the CNS, the GABA (γ-aminobutyric acid) A receptor (GABAA-R), was described to be regulated by TTR [20]. GABA receptors play a critical role in mediating neural network activity, in particular, in inhibiting neural activity [58]. Deficits in GABAA-R-mediated neurotransmission are involved in epilepsy, anxiety, depression, schizophrenia, and autism [59]. Using yeast two hybrid screening, TTR was identified as an interacting partner of GABAA R´ δ subunit (δ-GABAA-R). More importantly, TTR was found to interact with δ-GABAA-R in primary cerebellar granule neurons, in primary cortical neurons, and in mouse cerebellum extracts. In the same study, TTR was described to regulate δ-GABAA-R, as TTR knockdown in cultured cerebellar granule neurons significantly decreased the receptor expression, whereas overexpressing TTR in cortical neurons increased it. In vivo analysis using TTR knockdown mice revealed a significant decrease in surface expression of δ-GABAA-R in cerebellar granule neurons, confirming TTR as a regulator of these receptors in the brain [20]. This study uncovered the possible role of TTR in mediating neuronal activity through δ-GABAA-R, which can also contribute to the role of TTR on neuronal health.
In summary, in neurons (TTR regulates the expression and interacts with megalin [47], IGF1-R [55], and GABAA-R [19], critical molecules involved in neuronal plasticity, synaptic activity, neurite outgrowth [54, 59] and neuronal activity [62]. Interestingly, in TTR KO mice, the absence of TTR accelerates memory deficits usually associated with ageing [63], and aged memory-impaired rats present decreased TTR expression [64]. The hippocampus plays a crucial role in the formation of memories, and the fact that TTR absence is linked to memory impairment and that TTR can bind to receptors, in hippocampal neurons, that regulate neuronal function, makes TTR a molecule potentially crucial for diseases that involve memory decline, such as AD and other types of dementia. Moreover, focusing on AD, the described impact of TTR on hippocampal neuronal health points to a novel mechanism of neuroprotection independent on TTR binding to Aβ.
Novel roles for TTR on cell biology
New roles for TTR, besides the distribution of T4 and RBP-retinol, were mainly studied in the nervous system and associated with neurologic diseases, as addressed in the previous section. Nevertheless, studies in additional scenarios, such as cancer, revealed novel roles for TTR related to cell fate, proliferation and metabolism (Fig. 3).
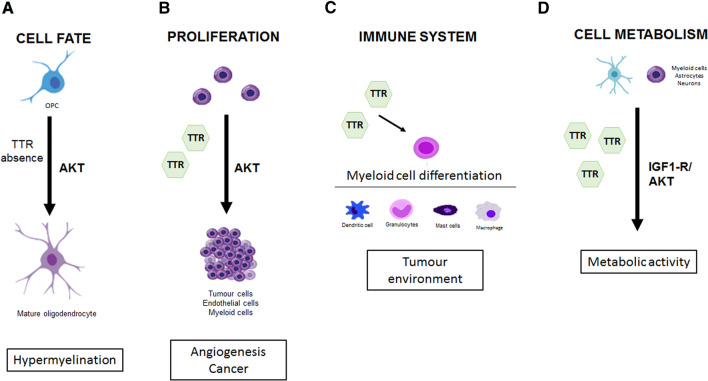
Fig. 3.
Transthyretin (TTR) novel roles in: A cell fate, with TTR absence inducing oligodendrogenesis; B stimulation if cell proliferation via AKT; C immune regulation, by controlling myeloid cell differentiation; and D cell metabolism, via AKT, in difference cell types
Impact on cell fate, proliferation and immune regulation
An impact of TTR on cell fate and proliferation was initially supported by studies in the SVZ. The SVZ, which covers the ventricular walls, is a stem cell niche that sustains lifelong de novo generation of neurons and oligodendrocytes, and is in close contact with CSF, where TTR is highly concentrated. As such, a new role for TTR in neural differentiation in the SVZ region was hypothesized. Brain of TTR KO mice was analysed, and it was shown that the absence of TTR hampers the neuronal differentiation in the SVZ region, resulting in a shift towards oligodendrogenesis at the expense of neurogenesis [15, 65], with an increase in oligodendrocyte number and proliferation leading to hypermyelination of the TTR KO mouse brain [65]. Oligodendroglial cell proliferation, survival, and differentiation rely on the coordinated transduction of phosphatidylinositol 3-kinase (PI3K)/AKT and extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), processes that are essential to CNS myelination [66, 67]. In transgenic mice overexpressing the PI3K/AKT signaling pathway, myelin thickness is increased [68], whereas disruption of the ERK1/2 expression in oligodendrocytes reduces myelin thickness and induces an overall degradation of axonal integrity [69]. How TTR regulates oligodendrocyte maturation and proliferation remains to be elucidated; however, TTR absence was shown to potentiate AKT phosphorylation in oligodendroglial lineage cells, regulating oligodendrogenesis (Fig. 3A), and consequently myelination [15]. Interestingly, TTR was also shown to regulate the levels of 14-3-3 and TTR KO mice presented low levels of this protein [70], which is crucial for neurogenesis [71].
Even though in the SVZ TTR absence potentiates AKT phosphorylation and oligodendrogenesis [15], TTR was shown to bind and positively regulates the IGF1-R/AKT signaling pathway in hippocampal neurons [55], what suggests that TTR may have different roles in different CNS cell types. An important note is that, THs, carried by TTR, were shown to be essential for oligodendrocyte health and CNS myelination [72], what could contradict the observation that TTR absence potentiates oligodendrogenesis [15]. Nevertheless, TTR KO mice were shown to be euthyroid [30]. Additionally, since TTR KO mice present delayed CNS development [33], memory impairment [63, 64], and inferior cognitive performance [73], it would be interesting to understand how hypermyelination in the absence of TTR [15] relates to those phenotypic alterations.
An additional role for TTR on cell proliferation (Fig. 3B) was related to its impact on the regulation of angiogenesis [74, 75], the process of formation of new blood vessels. Angiogenesis is a normal process during growth, development, and wound healing; however, it is also a fundamental step in tumor progression to malignancy [76]. TTR was shown to increase proliferation of lung tumor cells both in vitro and in vivo, by stimulating the formation of capillary-like tubes and increasing the proliferation of endothelial cells, via AKT [75]. Additionally, another study revealed that incubating endothelial cells with recombinant TTRV30M, a prone to aggregate variant [77], induced the down-regulation of several pro-angiogenic genes, such as VEGFR1, VEGFR2, FGF2, TGFB2, and ANGPT2, which are involved in endothelial proliferation, survival, and migration, negatively modulating angiogenesis when comparing to WT TTR [74]. The authors of this study suggested that TTR regulates angiogenesis through the direct regulation of endothelial gene expression [74]. The fact that TTRV30M was not able to induce proliferation and angiogenesis, similarly to WT TTR, and knowing that the TTRV30M variant is less stable that the WT protein [77], suggests that the stability of TTR might be important for its role in regulating angiogenesis.
TTR was also shown to regulate tumor environment and control immune cell function by modulating myeloid cell differentiation [75]. The myeloid lineage cells are the precursors of the granulocytes, macrophages, dendritic cells, and mast cells of the immune system [78]. Hence, it is interesting that the injection of recombinant TTR in mice led to an increase in cell differentiation in the myeloid compartment of the bone marrow [75], suggesting a potential role of TTR in immune system regulation (Fig. 3C).
Overall, TTR has been described as having a role in tumor progression through regulation of proliferation of both endothelial cells and the immune cells surrounding the tumor. In accordance, TTR has been recently described as a biomarker for lung [79] and ovary [80] cancers, as its levels were shown to be elevated in the serum of patients. The biological connection between these types of cancer and TTR is not yet understood, as it is not clear whether the changes in TTR levels are a cause or simply a consequence of the cancer itself. In this respect, lung cancer cells are reported to express TTR, in contrast to normal lung cells [81]. Nevertheless, TTR has been extensively used as a biomarker for mal-nutrition, health status in the elderly and for a broad array of diseases comprising metabolic, inflammatory and septic disorders, as gene expression in the liver was shown to be decreased in those conditions [82, 83]. These observations demonstrate a different response in terms of TTR expression in cases of disease.
In summary, reports are slowly depicting TTR new links to cell fate, in particular neurogenesis and oligodendrogenesis, which for sure impact on brain biology. At the same time, roles in cell proliferation and immune regulation are also emerging relating TTR to angiogenesis and cancer.
Controlling cell metabolism
The same study that presented TTR as a regulator of myeloid cells differentiation and proliferation also showed that TTR was able to induce changes in the metabolism of those cells [75]. Besides activating AKT pathway, ERK, p38, and NF-κB and p65 molecules, TTR enhanced the production of reactive oxygen species (ROS), enabling the cells to become functional myeloid-derived suppressive cells, highly effective in suppressing T-cell response. Thus, the authors suggested an undescribed role for TTR: the control of the metabolic reprogramming of myeloid lineage cells, via AKT [75].
Recently, Zawiślak et al. found that neuron-derived TTR stimulates astrocyte metabolism, in vitro, in a process independent of TTR ligands, as only the hormone-free TTR acted as an activator of glycolysis in astrocytes, and the saturation of TTR with T4 or retinol abolished the glycolytic stimulatory effect of the protein. Moreover, TTR was shown to induce the expression of glycolysis regulators in astrocytes, namely phosphofructokinase P (PFKP) and pyruvate kinase M1/M2 isoforms (PKM1/2), increasing ATP synthesis [84]. TTR effect was restricted to glycolysis, not affecting glucose uptake, as TTR did not affect enzymes of that pathway, such as hexokinase 1 (HK1) [84]. Interestingly, in this scenario, TTR was described to have an antagonistic effect on the PI3K/AKT pathway [84], which is associated with glucose uptake, whereas in neurons PI3K/AKT pathway was described to be stimulated by TTR [55]. This novel report by Zawiślak et al., implicated TTR in astrocytic energy metabolism, and by consequence in brain energetics, glia-neuron interactions, and even in neuronal plasticity.
When considering TTR and cellular metabolism, it is of importance to refer the IGF1-R pathway, which is well known for regulating cell proliferation and differentiation, as well as controlling metabolic activities, being linked, among others, to cancer, neurodegenerative disorders, and metabolic disorders [85]. The IGF1-R controls the activation of two downstream pathways, the MAPK/ERK and PI3K/AKT/mTor pathways, which control various cellular responses, such as differentiation, proliferation, and protection from apoptosis [86]. As described in a previous section, Vieira et. al. revealed TTR as an inducer of the IGF1-R pathway in the CNS. [55]. These observations reinforce the notion that TTR, possibly via IGF1-R, may have pivotal roles in metabolic regulation in different cell types and in maintaining cellular health and function (Fig. 3D). Additionally, the novel reports indicating that TTR stimulates glycolysis in astrocytes, regulating astrocyte metabolism and energy production [84], and the notion that astrocytic degradation of glycogen is a prerequisite for neuron plasticity and memory formation [87], give further support to classify TTR as an important molecule for neuronal function/plasticity and memory formation.
TTR aggregation and cellular toxicity
Additionally to the activity as a carrier protein and to the roles that emerged recently, TTR is also well known due to its involvement in ATTR amyloidosis, a systemic disorder that can result in polyneuropathy, autonomic neuropathy, or cardiomyopathy, and often in a mixture of these clinical manifestations [48]. TTR destabilization due to single amino acid variations precipitates misfolding and aggregation, which leads to TTR extracellular amyloid deposition and tissue dysfunction [48]. Ageing is associated with increased protein aggregation [88], and TTR is no exception to this rule: WT TTR can also be destabilized and aggregate, particularly in the elderly, causing non-familial cases of ATTR amyloidosis [20].
Aggregated TTR has vastly been associated with cytotoxicity and cellular degeneration [48]. The mechanism by which extracellular TTR modulates intracellular molecular pathways is not yet fully understood, although some putative mechanisms were described. In ATTR-PN, aggregated TTR preferentially deposits in the peripheral nervous system (PNS), leading to cytotoxicity and axonal loss (Fig. 2B). TTR aggregates bind to the receptor for Advanced Glycation End Products (RAGE) [89], and possibly to yet unidentified receptors, and activate intracellular pathways, such as endoplasmatic reticulum stress [90], proteosome dysfunction [91], apoptosis [92], inflammation [93, 94] and oxidative stress [95–97].
ATTR-PN is characterized by a dying-back axonal degeneration, what might suggest a disturbance of the distal cytoskeleton as consequence of the TTR deposition. Interestingly, a study using an ATTR-PN Drosophila model expressing the amyloidogenic variant TTRV30M in the fly retina, reported a novel genetic interaction between TTRV30M and members of the Rho GTPase family, well-known regulators of the actin cytoskeleton. The authors have shown that downregulation of Rac1/2, Cdc42, Pak and LIMK suppress TTRV30M-induced axonal defects on the retina of the flies [98]. All these proteins are major players in actin dynamics and implicated in several other neurodegenerative disorders [99], what raised the possibility that Rho GTPases and the actin cytoskeleton are involved in ATTR-PN pathology. The actin cytoskeleton is closely associated with endocytosis, with actin dynamics being critical to remodel the cell surface and vesicular movement [100]. Noticeably, aggregated TTR was found to disrupt the endocytic transport within the cell, as the presence of aggregated TTR decreases transferrin endocytosis in human carcinoma cells and primary liver cells [101]. This observation also supports the idea of a possible link between TTR aggregation and disturbance in the actin cytoskeleton. The question of whether TTR aggregates induce disturbances in neuronal actin regulators and in cytoskeleton dynamics in the distal peripheral axons of ATTR-PN patients is still unanswered. Nevertheless, as cytoskeleton disturbances could be an early event that preceding axonal degeneration, this would be a new pathological pathway with great potential to be a therapeutic target for ATTR-PN.
The deregulation of calcium homeostasis has also been associated to ATTR-PN pathology, as the amyloidogenic variant TTRL55P was shown to induce calcium influx into the growth cones of DRG neurons, in a process involving Nav1.8 voltage-gated sodium channels and transient receptor potential (TRP) M8 channels, whereas WT TTR had no significant effect [102]. In another study, performed in cardiomyocytes, aggregated WT TTR was shown to cause cytotoxicity by deregulating cytoplasmic Ca2+ levels, modifying mitochondrial potential and increasing oxidative stress, leading to the prolongation of the action potential, which may contribute to the development of cellular arrhythmias and conduction alterations often seen in patients with ATTR-CM [103]. The TTR-induced alterations of calcium levels within cells and consequent cytotoxicity may also be a major contributor to the pathophysiology associated with TTR aggregates.
The role of Schwann cells, the PNS glia cells, in ATTR-PN has been the focus of recent studies. In different neurodegenerative disorders, the glia, has been described to have a role in inflammation and in the clearance of aggregates. Thus, in ATTR-PN, where extracellular TTR aggregates cause pathology and inflammation, is reasonable to propose that glia may also have role in the disease. In this respect, the impact of TTR aggregates was analysed in Schwannoma cell lines, and TTR was found to stimulate ROS production and to decrease the levels of endogenous antioxidants, decreasing the overall cellular antioxidant capacity [95]. Additionally, PNS glia (satellite cells and Schwann cells) have been reported to uptake and clear extracellular TTR aggregates, via lysosomal degradation, in a ATTR-PN mouse model and in PNS human tissue [104]. Corroborating this idea, TTR aggregates were found in the cytoplasm of Schwann cells and satellite cells of transgenic mice carrying the human TTR gene bearing the V30M mutation (hTTRV30M mice) [14]. The effect of TTR in Schwann cells health and function surely also have an impact on neuronal health and survival, as glia cells support neurons and maintain their environment. Accordingly, conditioned media from Schwann cells from hTTRV30M mice was shown to inhibit neurite outgrowth of DRG neurons [14], suggesting that Schwann cells have a critical role in the neurodegeneration of sensory neurons. Moreover, sural nerve biopsy specimens from patients with ATTR-PN (TTRV30M variant) were shown to have disrupted blood-nerve barrier, with loss of the tight junctions and the fenestration of endothelial cells and atrophy of Schwann cells [105]. Together, the accumulation of aggregated TTR in Schwann cells, the disruption of clearance mechanisms, and the cytotoxicity triggered in Schwann cells are probably major contributors to the neurodegeneration of sensory neurons observed in ATTR-PN, as neuron-glia interactions are critical for maintaining neuronal function. It is probably the combination of both the direct damage to the peripheral neurons [48] and the damage to the peripheral glia [14], and the consequent impact in glia-neuron interactions, the drivers for the aggregated TTR-associated pathology in the PNS.
Conclusions and future directions
This review sheds light on the evidences of novel roles for TTR in cell biology, which range from neuronal health preservation in both PNS and CNS, to cellular fate determination, and regulation of cell proliferation and metabolism. The overall conclusion of this literature revision is that the physiological functions of TTR are yet to be fully understood and deserve further investigation due to the increasing scenarios where TTR is being shown to be involved. A critical missing point in this subject is to clarify the molecular mechanisms underlying TTR effects on cell biology which, as showed in this review, probably go beyond the distribution of T4 and retinol.
One of the molecules that stands out is megalin, which was shown to mediate an established and solid role for TTR on neuronal cell biology both in the CNS and PNS, as highlighted in this review [47, 51, 54]. In the case of the CNS, TTR was suggested to activate different signaling pathways, crucial in cell biology, in a megalin-dependent way, namely upregulation of intracellular calcium and a Src/ERK/AKT/CREB pathway [47, 54]. TTR was also shown to regulate the receptors IGF1-R [55], in this case leading to activation of an IGF1-R/AKT signaling pathway, and GABAA-R [19], what we suggest that might mediate TTR neuroprotection in the CNS. Besides the impact on neuronal cell biology, novel and less established roles for TTR include the involvement of TTR in cellular fate determination of oligodendrocytes [15], and the regulation of astrocytes metabolism [84],which can directly and importantly affect brain function (Fig. 3A,D). Interestingly, AKT was shown to be involved in those TTR activities [15, 84]. Overall the referred studies support a role for TTR on the biology of the CNS and pinpoints some of the underlying molecular mechanisms, from which megalin and AKT seem to act as key players. Future work should focus on analysing TTR-brain-related activities, as well as, modulation of the mediating molecules, in contexts were TTR was shown to be involved, namely ageing and AD.
In the case of peripheral neurons, the detailed underlying molecular mechanism downstream of TTR-megalin interaction is still unclear and deserves additional research as it could impact on ATTR-PN. In this disease, the mechanism underlying neuronal loss induced by TTR aggregates is still poorly described, but since TTR has a role in neurite outgrowth and axonal transport in peripheral neurons [44, 51], is possible that mutated and unstable TTR, undergoes loss of function, affecting neuronal function and contributing for the pathology observed in ATTR-PN. In this respect, it would be also important to study whether neuronal function, namely axonal transport, which is usually impaired in peripheral neuropathies [106], is affected in peripheral neurons of an ATTR-PN mouse model. Additionally, we refer novel studies, associating actin cytoskeleton alterations to ATTR-PN [98], which constitutes a new concept linking TTR aggregation and cytoskeleton destabilization (Fig. 2B), which could explain axonal loss seen in this pathology.
Here, we also discuss literature pointing to a role of TTR on the proliferation of endothelial cells and on myeloid cell differentiation what links TTR to angiogenesis and cancer (Fig. 3B,C), a subject that also deserves more investigation. In respect to the molecular mechanisms that underly these novel TTR roles, AKT is a molecule that again stands out and as such, we might propose AKT as main mediator of TTR activities.
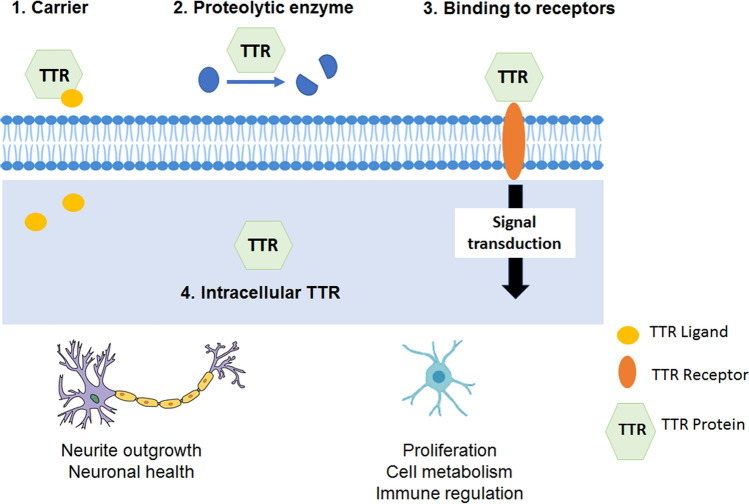
In summary, this review supports the vision of TTR as a multifaced protein. Currently, it is still unclear how TTR is inducing effects on cells, however there are some possibilities: 1) the classic one, supporting that TTR effect is due to its binding and distributor activities, 2) a less explored possibility, TTR proteolytic activity and cleavage of substrates with critical roles on cell biology, and 3–4) the ones suggested by recent literature occurring through protein–protein interaction, 3) extracellular TTR binds to receptors inducing critical intracellular pathways, or 4) intracellular TTR (uptaken or expressed) has a direct effect in intracellular pathways (Fig. 4). Most probably, a combination of these possibilities is on the basis of TTR-reported activities in cell biology. Clarifying the mechanisms underlying TTR novel reported activities will be crucial to define whether those activities represent novel TTR physiological functions. Assigning new functions to TTR is also important concerning the fact that therapies targeting TTR include stabilization or reduction of the protein. Interestingly, long-term studies of the effects of the referred therapies might contribute to a clearer understanding of the biological role of TTR in humans. Overall, we consider research on TTR a significant subject as although there are no humans without the protein, alterations on TTR levels on disease context have been increasingly reported on the literature.
Fig. 4.
Transthyretin (TTR) induces neuroprotection, immune modulation, and impacts in cellular proliferation and cell metabolism. How TTR impacts the cells is still unknown, but at least four pathways have been proposed. (1) TTR as a carrier of hormones, (2) TTR as a proteolytic enzyme, (3) TTR binding to receptors and inducing signal transduction events, and (4) direct effect of intracellular TTR on cellular pathways
Author contributions
JM and JE performed the literature search and wrote the manuscript. JM and MAL conceived the structure and content. MAL critically revised the work.
Funding
The authors were supported by the FEDER – Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020 – Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through FCT – Fundação Para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior in the framework of the project POCI-01–0145-FEDER-028336 (PTDC/MED-NEU/28336/2017). JE is a FCT fellow (SFRH/BD/116343/2016), JM is a research scientist under the project PTDC/MED-NEU/28336/2017. MAL is a FCT Investigator (IF/00902/2015).
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors contributed to the article and approved the submitted version.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Joana Magalhães and Márcia Almeida Liz authors contributed equally to this work.
References
- 1.Kanda Y, Goodman DS, Canfield RE, Morgan FJ. The amino acid sequence of human plasma prealbumin. J Biol Chem. 1974;249(21):6796–6805. doi: 10.1016/S0021-9258(19)42128-5. [DOI] [PubMed] [Google Scholar]
- 2.Blake CC, Geisow MJ, Oatley SJ, Rerat B, Rerat C. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. J Mol Biol. 1978;121(3):339–356. doi: 10.1016/0022-2836(78)90368-6. [DOI] [PubMed] [Google Scholar]
- 3.Blake CC, Geisow MJ, Swan ID, Rerat C, Rerat B. Structure of human plasma prealbumin at 2.5 A resolution. A preliminary report on the polypeptide chain conformation, quaternary structure and thyroxine binding. J Mol Biol. 1974;88(1):1–12. doi: 10.1016/0022-2836(74)90291-5. [DOI] [PubMed] [Google Scholar]
- 4.Monaco HL, Rizzi M, Coda A. Structure of a complex of two plasma proteins: transthyretin and retinol-binding protein. Science. 1995;268(5213):1039–1041. doi: 10.1126/science.7754382. [DOI] [PubMed] [Google Scholar]
- 5.Soprano DR, Herbert J, Soprano KJ, Schon EA, Goodman DS. Demonstration of transthyretin mRNA in the brain and other extrahepatic tissues in the rat. J Biol Chem. 1985;260(21):11793–11798. doi: 10.1016/S0021-9258(17)39100-7. [DOI] [PubMed] [Google Scholar]
- 6.Westermark GT, Westermark P. Transthyretin and amyloid in the islets of Langerhans in type-2 diabetes. Exp Diabetes Res. 2008;2008:429274. doi: 10.1155/2008/429274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawaji T, Ando Y, Nakamura M, Yamamoto K, Ando E, Takano A, Inomata Y, Hirata A, Tanihara H. Transthyretin synthesis in rabbit ciliary pigment epithelium. Exp Eye Res. 2005;81(3):306–312. doi: 10.1016/j.exer.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Cavallaro T, Martone RL, Dwork AJ, Schon EA, Herbert J. The retinal pigment epithelium is the unique site of transthyretin synthesis in the rat eye. Invest Ophthalmol Vis Sci. 1990;31(3):497–501. [PubMed] [Google Scholar]
- 9.Mizuno R, Cavallaro T, Herbert J. Temporal expression of the transthyretin gene in the developing rat eye. Invest Ophthalmol Vis Sci. 1992;33(2):341–349. [PubMed] [Google Scholar]
- 10.Loughna S, Bennett P, Moore G. Molecular analysis of the expression of transthyretin in intestine and liver from trisomy 18 fetuses. Hum Genet. 1995;95(1):89–95. doi: 10.1007/bf00225081. [DOI] [PubMed] [Google Scholar]
- 11.Soprano DR, Soprano KJ, Goodman DS. Retinol-binding protein and transthyretin mRNA levels in visceral yolk sac and liver during fetal development in the rat. Proc Natl Acad Sci U S A. 1986;83(19):7330–7334. doi: 10.1073/pnas.83.19.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blay P, Nilsson C, Owman C, Aldred A, Schreiber G. Transthyretin expression in the rat brain: effect of thyroid functional state and role in thyroxine transport. Brain Res. 1993;632(1–2):114–120. doi: 10.1016/0006-8993(93)91145-i. [DOI] [PubMed] [Google Scholar]
- 13.McKinnon B, Li H, Richard K, Mortimer R. Synthesis of thyroid hormone binding proteins transthyretin and albumin by human trophoblast. J Clin Endocrinol Metab. 2005;90(12):6714–6720. doi: 10.1210/jc.2005-0696. [DOI] [PubMed] [Google Scholar]
- 14.Murakami T, Sango K, Watabe K, Niimi N, Takaku S, Li Z, Yamamura K, Sunada Y. Schwann cells contribute to neurodegeneration in transthyretin amyloidosis. J Neurochem. 2015;134(1):66–74. doi: 10.1111/jnc.13068. [DOI] [PubMed] [Google Scholar]
- 15.Alshehri B, Pagnin M, Lee JY, Petratos S, Richardson SJ. The role of transthyretin in oligodendrocyte development. Sci Rep. 2020;10(1):4189. doi: 10.1038/s41598-020-60699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami T, Ohsawa Y, Sunada Y. The transthyretin gene is expressed in human and rodent dorsal root ganglia. Neurosci Lett. 2008;436(3):335–339. doi: 10.1016/j.neulet.2008.03.063. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Masliah E, Reixach N, Buxbaum JN. Neuronal production of transthyretin in human and murine Alzheimer’s disease: is it protective? J Neurosci. 2011;31(35):12483–12490. doi: 10.1523/JNEUROSCI.2417-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomes JR, Cabrito I, Soares HR, Costelha S, Teixeira A, Wittelsberger A, Stortelers C, Vanlandschoot P, Saraiva MJ. Delivery of an anti-transthyretin nanobody to the brain through intranasal administration reveals transthyretin expression and secretion by motor neurons. J Neurochem. 2018;145(5):393–408. doi: 10.1111/jnc.14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou L, Tang X, Li X, Bai Y, Buxbaum JN, Chen G. Identification of transthyretin as a novel interacting partner for the delta subunit of GABAA receptors. PLoS ONE. 2019;14(1):e0210094. doi: 10.1371/journal.pone.0210094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westermark P, Bergstrom J, Solomon A, Murphy C, Sletten K. Transthyretin-derived senile systemic amyloidosis: clinicopathologic and structural considerations. Amyloid. 2003;10(Suppl 1):48–54. doi: 10.1080/13506129.2003.12088568. [DOI] [PubMed] [Google Scholar]
- 21.Connors LH, Lim A, Prokaeva T, Roskens VA, Costello CE. Tabulation of human transthyretin (TTR) variants, 2003. Amyloid. 2003;10(3):160–184. doi: 10.3109/13506120308998998. [DOI] [PubMed] [Google Scholar]
- 22.Rapezzi C, Quarta CC, Riva L, Longhi S, Gallelli I, Lorenzini M, Ciliberti P, Biagini E, Salvi F, Branzi A. Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol. 2010;7(7):398–408. doi: 10.1038/nrcardio.2010.67. [DOI] [PubMed] [Google Scholar]
- 23.Plante-Bordeneuve V, Said G. Familial amyloid polyneuropathy. Lancet Neurol. 2011;10(12):1086–1097. doi: 10.1016/S1474-4422(11)70246-0. [DOI] [PubMed] [Google Scholar]
- 24.Alshehri B, D’Souza DG, Lee JY, Petratos S, Richardson SJ. The diversity of mechanisms influenced by transthyretin in neurobiology: development, disease and endocrine disruption. J Neuroendocrinol. 2015;27(5):303–323. doi: 10.1111/jne.12271. [DOI] [PubMed] [Google Scholar]
- 25.Bartalena L. Recent achievements in studies on thyroid hormone-binding proteins. Endocr Rev. 1990;11(1):47–64. doi: 10.1210/edrv-11-1-47. [DOI] [PubMed] [Google Scholar]
- 26.Chanoine JP, Braverman LE. The role of transthyretin in the transport of thyroid hormone to cerebrospinal fluid and brain. Acta Med Austriaca. 1992;19(Suppl 1):25–28. [PubMed] [Google Scholar]
- 27.Goodman DS. Vitamin A and retinoids in health and disease. N Engl J Med. 1984;310(16):1023–1031. doi: 10.1056/NEJM198404193101605. [DOI] [PubMed] [Google Scholar]
- 28.Wolf G. Multiple functions of vitamin A. Physiol Rev. 1984;64(3):873–937. doi: 10.1152/physrev.1984.64.3.873. [DOI] [PubMed] [Google Scholar]
- 29.Episkopou V, Maeda S, Nishiguchi S, Shimada K, Gaitanaris GA, Gottesman ME, Robertson EJ. Disruption of the transthyretin gene results in mice with depressed levels of plasma retinol and thyroid hormone. Proc Natl Acad Sci U S A. 1993;90(6):2375–2379. doi: 10.1073/pnas.90.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palha JA, Episkopou V, Maeda S, Shimada K, Gottesman ME, Saraiva MJ. Thyroid hormone metabolism in a transthyretin-null mouse strain. J Biol Chem. 1994;269(52):33135–33139. doi: 10.1016/S0021-9258(20)30107-1. [DOI] [PubMed] [Google Scholar]
- 31.van Bennekum AM, Wei S, Gamble MV, Vogel S, Piantedosi R, Gottesman M, Episkopou V, Blaner WS. Biochemical basis for depressed serum retinol levels in transthyretin-deficient mice. J Biol Chem. 2001;276(2):1107–1113. doi: 10.1074/jbc.M008091200. [DOI] [PubMed] [Google Scholar]
- 32.Richardson SJ. Cell and molecular biology of transthyretin and thyroid hormones. Int Rev Cytol. 2007;258:137–193. doi: 10.1016/S0074-7696(07)58003-4. [DOI] [PubMed] [Google Scholar]
- 33.Monk JA, Sims NA, Dziegielewska KM, Weiss RE, Ramsay RG, Richardson SJ. Delayed development of specific thyroid hormone-regulated events in transthyretin null mice. Am J Physiol Endocrinol Metab. 2013;304(1):E23–31. doi: 10.1152/ajpendo.00216.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sousa MM, Berglund L, Saraiva MJ. Transthyretin in high density lipoproteins: association with apolipoprotein A-I. J Lipid Res. 2000;41(1):58–65. doi: 10.1016/S0022-2275(20)32074-5. [DOI] [PubMed] [Google Scholar]
- 35.Liz MA, Faro CJ, Saraiva MJ, Sousa MM. Transthyretin, a new cryptic protease. J Biol Chem. 2004;279(20):21431–21438. doi: 10.1074/jbc.M402212200. [DOI] [PubMed] [Google Scholar]
- 36.Liz MA, Gomes CM, Saraiva MJ, Sousa MM. ApoA-I cleaved by transthyretin has reduced ability to promote cholesterol efflux and increased amyloidogenicity. J Lipid Res. 2007;48(11):2385–2395. doi: 10.1194/jlr.M700158-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Liz MA, Leite SC, Juliano L, Saraiva MJ, Damas AM, Bur D, Sousa MM. Transthyretin is a metallopeptidase with an inducible active site. Biochem J. 2012;443(3):769–778. doi: 10.1042/BJ20111690. [DOI] [PubMed] [Google Scholar]
- 38.Liz MA, Fleming CE, Nunes AF, Almeida MR, Mar FM, Choe Y, Craik CS, Powers JC, Bogyo M, Sousa MM. Substrate specificity of transthyretin: identification of natural substrates in the nervous system. Biochem J. 2009;419(2):467–474. doi: 10.1042/BJ20082090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa R, Ferreira-da-Silva F, Saraiva MJ, Cardoso I. Transthyretin protects against A-beta peptide toxicity by proteolytic cleavage of the peptide: a mechanism sensitive to the Kunitz protease inhibitor. PLoS ONE. 2008;3(8):e2899. doi: 10.1371/journal.pone.0002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costa R, Goncalves A, Saraiva MJ, Cardoso I. Transthyretin binding to A-Beta peptide–impact on A-Beta fibrillogenesis and toxicity. FEBS Lett. 2008;582(6):936–942. doi: 10.1016/j.febslet.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 41.Silva CS, Eira J, Ribeiro CA, Oliveira A, Sousa MM, Cardoso I, Liz MA. Transthyretin neuroprotection in Alzheimer’s disease is dependent on proteolysis. Neurobiol Aging. 2017;59:10–14. doi: 10.1016/j.neurobiolaging.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Alemi M, Gaiteiro C, Ribeiro CA, Santos LM, Gomes JR, Oliveira SM, Couraud PO, Weksler B, Romero I, Saraiva MJ, Cardoso I. Transthyretin participates in beta-amyloid transport from the brain to the liver–involvement of the low-density lipoprotein receptor-related protein 1? Sci Rep. 2016;6:20164. doi: 10.1038/srep20164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liz MA, Mar FM, Franquinho F, Sousa MM. Aboard transthyretin: from transport to cleavage. IUBMB Life. 2010;62(6):429–435. doi: 10.1002/iub.340. [DOI] [PubMed] [Google Scholar]
- 44.Fleming CE, Saraiva MJ, Sousa MM. Transthyretin enhances nerve regeneration. J Neurochem. 2007;103(2):831–839. doi: 10.1111/j.1471-4159.2007.04828.x. [DOI] [PubMed] [Google Scholar]
- 45.Buxbaum JN, Ye Z, Reixach N, Friske L, Levy C, Das P, Golde T, Masliah E, Roberts AR, Bartfai T. Transthyretin protects Alzheimer’s mice from the behavioral and biochemical effects of Abeta toxicity. Proc Natl Acad Sci U S A. 2008;105(7):2681–2686. doi: 10.1073/pnas.0712197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliveira SM, Ribeiro CA, Cardoso I, Saraiva MJ. Gender-dependent transthyretin modulation of brain amyloid-beta levels: evidence from a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2011;27(2):429–439. doi: 10.3233/JAD-2011-110488. [DOI] [PubMed] [Google Scholar]
- 47.Gomes JR, Nogueira RS, Vieira M, Santos SD, Ferraz-Nogueira JP, Relvas JB, Saraiva MJ. Transthyretin provides trophic support via megalin by promoting neurite outgrowth and neuroprotection in cerebral ischemia. Cell Death Differ. 2016;23(11):1749–1764. doi: 10.1038/cdd.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saraiva MJ, Magalhaes J, Ferreira N, Almeida MR. Transthyretin deposition in familial amyloidotic polyneuropathy. Curr Med Chem. 2012;19(15):2304–2311. doi: 10.2174/092986712800269236. [DOI] [PubMed] [Google Scholar]
- 49.Sousa MM, Saraiva MJ. Neurodegeneration in familial amyloid polyneuropathy: from pathology to molecular signaling. Prog Neurobiol. 2003;71(5):385–400. doi: 10.1016/j.pneurobio.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Sousa MM, Saraiva MJ. Transthyretin is not expressed by dorsal root ganglia cells. Exp Neurol. 2008;214(2):362–365. doi: 10.1016/j.expneurol.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 51.Fleming CE, Mar FM, Franquinho F, Saraiva MJ, Sousa MM. Transthyretin internalization by sensory neurons is megalin mediated and necessary for its neuritogenic activity. J Neurosci. 2009;29(10):3220–3232. doi: 10.1523/JNEUROSCI.6012-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santos SD, Lambertsen KL, Clausen BH, Akinc A, Alvarez R, Finsen B, Saraiva MJ. CSF transthyretin neuroprotection in a mouse model of brain ischemia. J Neurochem. 2010;115(6):1434–1444. doi: 10.1111/j.1471-4159.2010.07047.x. [DOI] [PubMed] [Google Scholar]
- 53.Ranganathan S, Williams E, Ganchev P, Gopalakrishnan V, Lacomis D, Urbinelli L, Newhall K, Cudkowicz ME, Brown RH, Jr, Bowser R. Proteomic profiling of cerebrospinal fluid identifies biomarkers for amyotrophic lateral sclerosis. J Neurochem. 2005;95(5):1461–1471. doi: 10.1111/j.1471-4159.2005.03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomes JR, Lobo A, Nogueira R, Terceiro AF, Costelha S, Lopes IM, Magalhaes A, Summavielle T, Saraiva MJ. Neuronal megalin mediates synaptic plasticity-a novel mechanism underlying intellectual disabilities in megalin gene pathologies. Brain Commun. 2020 doi: 10.1093/braincomms/fcaa135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vieira M, Gomes JR, Saraiva MJ. Transthyretin induces insulin-like growth factor i nuclear translocation regulating its levels in the hippocampus. Mol Neurobiol. 2015;51(3):1468–1479. doi: 10.1007/s12035-014-8824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vieira M, Leal SS, Gomes CM, Saraiva MJ. Evidence for synergistic action of transthyretin and IGF-I over the IGF-I receptor. Biochim Biophys Acta. 2016;1862:797–804. doi: 10.1016/j.bbadis.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Guan J, Bennet L, Gluckman PD, Gunn AJ. Insulin-like growth factor-1 and post-ischemic brain injury. Prog Neurobiol. 2003;70(6):443–462. doi: 10.1016/j.pneurobio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med. 2002;8(12):1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- 59.Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58(5):708–719. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiu SL, Cline HT. Insulin receptor signaling in the development of neuronal structure and function. Neural Dev. 2010;5:7. doi: 10.1186/1749-8104-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nieto-Estevez V, Defterali C, Vicario-Abejon C. IGF-I: a key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front Neurosci. 2016;10:52. doi: 10.3389/fnins.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9(5):331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sousa JC, Marques F, Dias-Ferreira E, Cerqueira JJ, Sousa N, Palha JA. Transthyretin influences spatial reference memory. Neurobiol Learn Mem. 2007;88(3):381–385. doi: 10.1016/j.nlm.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 64.Brouillette J, Quirion R. Transthyretin: a key gene involved in the maintenance of memory capacities during aging. Neurobiol Aging. 2008;29(11):1721–1732. doi: 10.1016/j.neurobiolaging.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Vancamp P, Gothie JD, Luongo C, Sebillot A, Le Blay K, Butruille L, Pagnin M, Richardson SJ, Demeneix BA, Remaud S. Gender-specific effects of transthyretin on neural stem cell fate in the subventricular zone of the adult mouse. Sci Rep. 2019;9(1):19689. doi: 10.1038/s41598-019-56156-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Domenech-Estevez E, Baloui H, Meng X, Zhang Y, Deinhardt K, Dupree JL, Einheber S, Chrast R, Salzer JL. Akt regulates axon wrapping and myelin sheath thickness in the PNS. J Neurosci. 2016;36(16):4506–4521. doi: 10.1523/JNEUROSCI.3521-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ishii A, Furusho M, Dupree JL, Bansal R. Strength of ERK1/2 MAPK activation determines its effect on myelin and axonal integrity in the adult CNS. J Neurosci. 2016;36(24):6471–6487. doi: 10.1523/JNEUROSCI.0299-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28(28):7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishii A, Furusho M, Dupree JL, Bansal R. Role of ERK1/2 MAPK signaling in the maintenance of myelin and axonal integrity in the adult CNS. J Neurosci. 2014;34(48):16031–16045. doi: 10.1523/JNEUROSCI.3360-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vieira M, Saraiva MJ. Transthyretin regulates hippocampal 14–3-3zeta protein levels. FEBS Lett. 2013;587(10):1482–1488. doi: 10.1016/j.febslet.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 71.Toyo-oka K, Wachi T, Hunt RF, Baraban SC, Taya S, Ramshaw H, Kaibuchi K, Schwarz QP, Lopez AF, Wynshaw-Boris A. 14-3-3epsilon and zeta regulate neurogenesis and differentiation of neuronal progenitor cells in the developing brain. J Neurosci. 2014;34(36):12168–12181. doi: 10.1523/JNEUROSCI.2513-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pagnin M, Kondos-Devcic D, Chincarini G, Cumberland A, Richardson SJ, Tolcos M. Role of thyroid hormones in normal and abnormal central nervous system myelination in humans and rodents. Front Neuroendocrinol. 2021;61:100901. doi: 10.1016/j.yfrne.2021.100901. [DOI] [PubMed] [Google Scholar]
- 73.Sousa JC, Grandela C, Fernandez-Ruiz J, de Miguel R, de Sousa L, Magalhaes AI, Saraiva MJ, Sousa N, Palha JA. Transthyretin is involved in depression-like behaviour and exploratory activity. J Neurochem. 2004;88(5):1052–1058. doi: 10.1046/j.1471-4159.2003.02309.x. [DOI] [PubMed] [Google Scholar]
- 74.Nunes RJ, de Oliveira P, Lages A, Becker JD, Marcelino P, Barroso E, Perdigoto R, Kelly JW, Quintas A, Santos SC. Transthyretin proteins regulate angiogenesis by conferring different molecular identities to endothelial cells. J Biol Chem. 2013;288(44):31752–31760. doi: 10.1074/jbc.M113.469858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee CC, Ding X, Zhao T, Wu L, Perkins S, Du H, Yan C. Transthyretin stimulates tumor growth through regulation of tumor, immune, and endothelial cells. J Immunol. 2019;202(3):991–1002. doi: 10.4049/jimmunol.1800736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3(2):65–71. [PubMed] [Google Scholar]
- 77.Longo Alves I, Hays MT, Saraiva MJ. Comparative stability and clearance of [Met30]transthyretin and [Met119]transthyretin. Eur J Biochem. 1997;249(3):662–668. doi: 10.1111/j.1432-1033.1997.00662.x. [DOI] [PubMed] [Google Scholar]
- 78.Herz J, Filiano AJ, Smith A, Yogev N, Kipnis J. Myeloid cells in the central nervous system. Immunity. 2017;46(6):943–956. doi: 10.1016/j.immuni.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ding H, Liu J, Xue R, Zhao P, Qin Y, Zheng F, Sun X. Transthyretin as a potential biomarker for the differential diagnosis between lung cancer and lung infection. Biomed Rep. 2014;2(5):765–769. doi: 10.3892/br.2014.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng X, Chen S, Li L, Liu X, Liu X, Dai S, Zhang P, Lu H, Lin Z, Yu Y, Li G. Evaluation of HE4 and TTR for diagnosis of ovarian cancer: comparison with CA-125. J Gynecol Obstet Hum Reprod. 2018;47(6):227–230. doi: 10.1016/j.jogoh.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 81.Hao S, Sun S, Xiao X, He D, Liu L. Selective expression of transthyretin in subtypes of lung cancer. J Mol Histol. 2016;47(3):239–247. doi: 10.1007/s10735-016-9666-3. [DOI] [PubMed] [Google Scholar]
- 82.Ingenbleek Y. Why should plasma transthyretin become a routine screening tool in elderly persons? J Nutr Health Aging. 2009;13(7):640–642. doi: 10.1007/s12603-009-0175-x. [DOI] [PubMed] [Google Scholar]
- 83.Ingenbleek Y, Bernstein LH. Plasma transthyretin as a biomarker of lean body mass and catabolic states. Adv Nutr. 2015;6(5):572–580. doi: 10.3945/an.115.008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zawislak A, Jakimowicz P, McCubrey JA, Rakus D. Neuron-derived transthyretin modulates astrocytic glycolysis in hormone-independent manner. Oncotarget. 2017;8(63):106625–106638. doi: 10.18632/oncotarget.22542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Werner H. Tumor suppressors govern insulin-like growth factor signaling pathways: implications in metabolism and cancer. Oncogene. 2012;31(22):2703–2714. doi: 10.1038/onc.2011.447. [DOI] [PubMed] [Google Scholar]
- 86.Laviola L, Natalicchio A, Giorgino F. The IGF-I signaling pathway. Curr Pharm Des. 2007;13(7):663–669. doi: 10.2174/138161207780249146. [DOI] [PubMed] [Google Scholar]
- 87.Rich LR, Harris W, Brown AM. The role of brain glycogen in supporting physiological function. Front Neurosci. 2019;13:1176. doi: 10.3389/fnins.2019.01176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trigo D, Nadais A, da Cruz ESOAB. Unravelling protein aggregation as an ageing related process or a neuropathological response. Ageing Res Rev. 2019;51:67–77. doi: 10.1016/j.arr.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 89.Sousa MM, Yan SD, Stern D, Saraiva MJ. Interaction of the receptor for advanced glycation end products (RAGE) with transthyretin triggers nuclear transcription factor kB (NF-kB) activation. Lab Invest. 2000;80(7):1101–1110. doi: 10.1038/labinvest.3780116. [DOI] [PubMed] [Google Scholar]
- 90.Teixeira PF, Cerca F, Santos SD, Saraiva MJ. Endoplasmic reticulum stress associated with extracellular aggregates. Evidence from transthyretin deposition in familial amyloid polyneuropathy. J Biol Chem. 2006;281(31):21998–22003. doi: 10.1074/jbc.M602302200. [DOI] [PubMed] [Google Scholar]
- 91.Santos SD, Cardoso I, Magalhaes J, Saraiva MJ. Impairment of the ubiquitin-proteasome system associated with extracellular transthyretin aggregates in familial amyloidotic polyneuropathy. J Pathol. 2007;213(2):200–209. doi: 10.1002/path.2224. [DOI] [PubMed] [Google Scholar]
- 92.Macedo B, Batista AR, do Amaral JB, Saraiva MJ, Biomarkers in the assessment of therapies for familial amyloidotic polyneuropathy. Mol Med. 2007;13(11–12):584–591. doi: 10.2119/2007-00068.Macedo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Azevedo EP, Guimaraes-Costa AB, Bandeira-Melo C, Chimelli L, Waddington-Cruz M, Saraiva EM, Palhano FL, Foguel D. Inflammatory profiling of patients with familial amyloid polyneuropathy. BMC Neurol. 2019;19(1):146. doi: 10.1186/s12883-019-1369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sousa MM, Du Yan S, Fernandes R, Guimaraes A, Stern D, Saraiva MJ. Familial amyloid polyneuropathy: receptor for advanced glycation end products-dependent triggering of neuronal inflammatory and apoptotic pathways. J Neurosci. 2001;21(19):7576–7586. doi: 10.1523/JNEUROSCI.21-19-07576.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fong VH, Vieira A. Pro-oxidative effects of aggregated transthyretin in human schwannoma cells. Neurotoxicology. 2013;39:109–113. doi: 10.1016/j.neuro.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 96.Fong VH, Vieira A. Transthyretin aggregates induce production of reactive nitrogen species. Neurodegener Dis. 2013;11(1):42–48. doi: 10.1159/000338153. [DOI] [PubMed] [Google Scholar]
- 97.Macedo B, Magalhaes J, Batista AR, Saraiva MJ. Carvedilol treatment reduces transthyretin deposition in a familial amyloidotic polyneuropathy mouse model. Pharmacol Res. 2010;62(6):514–522. doi: 10.1016/j.phrs.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 98.M IOdS, Lopes CS, Liz MA, Transthyretin interacts with actin regulators in a Drosophila model of familial amyloid polyneuropathy. Sci Rep. 2020;10(1):13596. doi: 10.1038/s41598-020-70377-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DeGeer J, Lamarche-Vane N. Rho GTPases in neurodegeneration diseases. Exp Cell Res. 2013;319(15):2384–2394. doi: 10.1016/j.yexcr.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 100.Smythe E, Ayscough KR. Actin regulation in endocytosis. J Cell Sci. 2006;119(Pt 22):4589–4598. doi: 10.1242/jcs.03247. [DOI] [PubMed] [Google Scholar]
- 101.Fong VH, Wong S, Vieira A. Disruption of endocytic transport by transthyretin aggregates. Int J Biochem Cell Biol. 2017;85:102–105. doi: 10.1016/j.biocel.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 102.Gasperini RJ, Hou X, Parkington H, Coleman H, Klaver DW, Vincent AJ, Foa LC, Small DH. TRPM8 and Nav1.8 sodium channels are required for transthyretin-induced calcium influx in growth cones of small-diameter TrkA-positive sensory neurons. Mol Neurodegener. 2011;6(1):19. doi: 10.1186/1750-1326-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sartiani L, Bucciantini M, Spinelli V, Leri M, Natalello A, Nosi D, Maria Doglia S, Relini A, Penco A, Giorgetti S, Gerace E, Mannaioni G, Bellotti V, Rigacci S, Cerbai E, Stefani M. Biochemical and electrophysiological modification of amyloid transthyretin on cardiomyocytes. Biophys J. 2016;111(9):2024–2038. doi: 10.1016/j.bpj.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goncalves NP, Costelha S, Saraiva MJ. Glial cells in familial amyloidotic polyneuropathy. Acta Neuropathol Commun. 2014;2:177. doi: 10.1186/s40478-014-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koike H, Ikeda S, Takahashi M, Kawagashira Y, Iijima M, Misumi Y, Ando Y, Ikeda SI, Katsuno M, Sobue G. Schwann cell and endothelial cell damage in transthyretin familial amyloid polyneuropathy. Neurology. 2016;87(21):2220–2229. doi: 10.1212/WNL.0000000000003362. [DOI] [PubMed] [Google Scholar]
- 106.Prior R, Van Helleputte L, Benoy V, Van Den Bosch L. Defective axonal transport: a common pathological mechanism in inherited and acquired peripheral neuropathies. Neurobiol Dis. 2017;105:300–320. doi: 10.1016/j.nbd.2017.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.