Abstract
BCL2L13 is a BCL2-like protein. It has been discovered for two decades, now on the way to be a hotspot of research with its physiological and pathological meanings found in recent years. Start with the pro-apoptotic activity, there have been reported consecutively that BCL2L13 could also induce mitochondrial fragmentation, inhibit cell death and promote mitophagy. Similar to BNIP3, BCL2L13 cannot be indiscriminately categorized into pro- or anti-apoptotic proteins. It anchors in the mitochondrial outer membrane, and expresses in various cells and tissues. This article reviews for the first time that BCL2L13 functions in physiological processes, such as growth and development and energy metabolism, and its dysregulation participating in pathological processes, including cancer, bacterial infection, cardiovascular diseases and degenerative diseases, suggesting its important roles in these events.
Keywords: BCL-rambo, Autophagy, Apoptosis, Tumor, Cerebrovascular accident, Neurodegenerative disorders
Introduction
Regulated cell death (RCD) plays an important role in biological growth and organismal homeostasis, while is commonly dysregulated during aging and diseases [1–3]. RCD relies on tightly structured regulatory network composed of many molecular effectors [4, 5]. B-cell lymphoma 2 (BCL2) family proteins, which are constitutively expressed in multiple types of cells, regulate the pivotal role of mitochondria mediated cell death pathway [6, 7].
BCL2 was originally identified from the t(14;18) breakpoint in B-cell lymphoma in 1984 [8]. Characterized by the BCL2 homology (BH) domains and grouped by cell death effects, BCL2 family proteins include three sub-families: multi-domain anti-apoptotic members such as BCL2, BCLxL [9], BCLw [10], MCL1 [11] and Caenorhabditis elegans CED-9 [12]; multi-domain pro-apoptotic effector members, e.g., BAX [13] and BAK [14]; BH3-only pro-apoptotic proteins that share only a 15–25 amino acids BH3 motif, like BIK [15], BIM [16], BAD [17], BID [18] and Caenorhabditis elegans EGL-1 [19]. BCL2 family proteins control the mitochondrial outer membrane permeabilization (MOMP) through their interactions. The BH3-only pro-apoptotic proteins could activate BAK or BAX to cause MOMP, either through direct activation [20–22], or through inhibition of the anti-apoptotic members [23], and subsequently release the pro-apoptotic proteins in the mitochondrial intermembrane space into cytosol, such as cytochrome c.
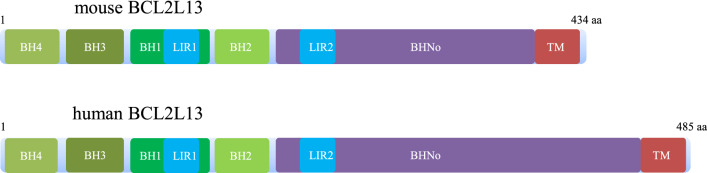
BCL2 like 13 (BCL2L13) also known as BCL-Rambo, is encoded by the BCL2L13 gene on human chromosome 22. BCL2L13 is a BCL2 like protein, has been discovered for twenty years through the screening for sequence homology with BH domain [24]. Unlike some other BCL2 like proteins that only have a BH3 domain, BCL2L13 contains all the four BH domains and a BHNo region, followed by a trans-membrane (TM) motif. The BHNo region contains two pair of tandem repeats, which is embedded between BH1-4 domains and TM motif. The TM motif fixes BCL2L13 in the mitochondrial outer membrane, similar to some other BCL2 family proteins [24, 25].
BCL2L13 has three major isoforms produced by alternative splicing. Isoform 1 (Gly201 mutated to Val, 202–485 amino acids missing) and isoform 3 (1–162 amino acids missing) are both truncated versions, yet no function has been attributed to them at this time [26–28]. The isoform 2 retains the complete sequence 1–485 amino acids, and has been chosen as the canonical sequence here, unless otherwise indicated. Beyond the three major isoforms of BCL2L13, BCL-Rambo-beta is another splice variant consisted only of the BH4 motif, followed by ALU element, the ingredient constitutes approximately 10% of the human genome and often transform into exons [29, 30].
Mouse BCL2L13 is also included in this entry, for its comparable structure and outcome to isoform 2. In this review, we will not only summarize the recent findings of cellular functions of BCL2L13, but also explore its physiological and pathological meanings.
Cellular functions of BCL2L13
Different functions in apoptosis
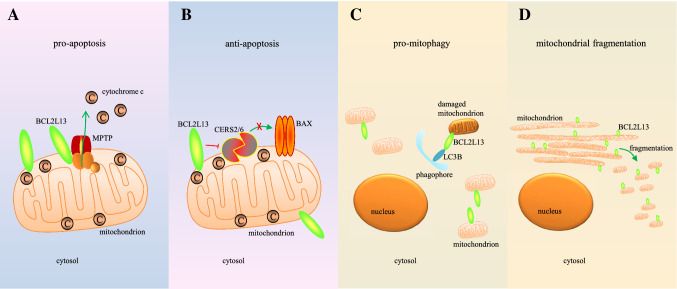
Apoptosis is a type of RCD, which can be mainly divided into two categories: death receptor-mediated and mitochondria-dependent [31, 32]. The first discovered mitochondria-dependent pro-apoptotic activity of BCL2L13 is accompanied by its finding in 2001. Overexpression of BCL2L13 induced apoptosis in HEK293T or Hela cells depending on its BHNo and TM motif instead of the BH domain; while inhibitor of apoptosis proteins (IAP) effectively blocked this cell death [24]. Further in-depth mechanism study was performed in PC3 cells, finding out BCL2L13 interacts with adenine nucleotide translocator (ANT) to allow cytochrome c disperse into cytosol through the conformational transformation of mitochondrial permeability transition pore (MPTP) [33]. Another study indicated, voltage-dependent anion channel (VDAC), which is another component of MPTP same as ANT, correlated with BCL2L13 to facilitate the activation of caspase cascade by releasing cytochrome c in A549 cells [34] (Fig. 1a). On the other hand, BCL-Rambo-beta could be detected in heart, lymph nodes and cervix samples, but not in brain tissues, and promotes etoposide and paclitaxel-induced cell demise in HEK293A or Hela cells [30].
Fig. 1.
Molecular mechanisms proposed for the activities of BCL2L13. (A) BCL2L13 interacts with MPTP to induce cytochrome c disperses into cytosol, which results in the activation of caspase cascade. (B) On the other hand, BAX oligomerization in mitochondrial outer membrane is blocked by the combination of BCL2L13 and CERS2/6 in some tumors, that CERS2 can’t self-dimerize or dimerize with CERS6, inhibiting apoptosis. (C) BCL2L13 binds to LC3B as a mammalian homologue of ATG32, in support the clearance of damaged mitochondria. (D) Mitochondrial fragmentation is potentiated by BCL2L13 under extrinsic pressure, dependent on the integrity of all four BH domains
Beyond expectation, BCL2L13 is characterized as an anti-apoptotic protein in SF767 cells; where BHNo domain binds to ceramide synthase 2 (CERS2) and 6 (CERS6), restricts CERS2/6 homo/hetero-dimers formation, and thereby inhibits BAX oligomerization [35] (Fig. 1b). Thus, under different conditions, BCL2L13 could be either pro-apoptotic or anti-apoptotic.
Promote mitophagy
The exploration process of mouse BCL2L13 is independent of its human counterpart, which was found through homologous sequence screening of yeast mitophagy receptor autophagy-related protein 32 (ATG32) in mouse [36]. The screening was performed because the canonical mammalian mitophagy receptor Parkin is not detectable in a few tissues, for instance, neuronal undifferentiated mouse fetuses, therefore, there might be some other mitophagy receptors exist in these tissues [37, 38].
Although substantial sequence differences exists between mouse BCL2L13 and human BCL2L13, for example, mouse BCL2L13 has a truncation in BHNo domain compared to the human homologue (Fig. 2), however, knockdown of human BCL2L13 also reduced CCCP-induced mitophagy in HEK293 cells, suggesting human BCL2L13 has similar functions [36]. Mouse BCL2L13 was found to induce mitophagy through Parkin-independent pathway in HEK293A cells. There are two WXXL/I motifs found in mouse BCL2L13, while only mutations in the second motif inhibit its mitophagy-inducing ability, suggesting the second one is the LC3-interacting region (LIR2) [36] (Fig. 2). Mutations in the BH domains also inhibit the mitophagy-inducing ability of BCL2L13, suggesting mitochondrial fragmentation occurs prior to mitophagy [36]. Further studies suggested BCL2L13-induced mitophagy involves recruitment of the ULK1 complex [39] (Fig. 1c). A recent study suggested that the LIR domain of human BCL2L13 selectively bound to LC3C, GABARAP and GABARAP-L1 to promote mitophagy [40], indicating its selectivity in mediating mitophagy.
Fig. 2.
Schematic diagrams of BCL2L13. Mouse BCL2L13 (upper) has a truncated BHNo domain and missing some other amino acids when compared to human BCL2L13 (lower). Abbreviation: BH, BCL2 homologous; BHNo, non-BCL2 homologous; LIR, LC3-interacting region; TM, trans-membrane
Mitochondrial fragmentation
Not like canonical BCL2 family proteins, BCL2L13 does not form heterodimers with other BCL2 family members, instead shows a non-canonical role in boosting mitochondrial fragmentation in A549 cells according to intracellular context [34] (Fig. 1d). The pro-fragmentation activity of BCL2L13 is independent of typical mitochondrial fission protein dynamin related protein 1 (DRP1), but requires all its four BH domains; mutations of either one of BH1-4 domains or the TM motif will inhibit this process [36]. Although performing like a prerequisite for BCL2L13 mediated mitophagy, whether the mitochondrial fragmentation ability of BCL2L13 involved in apoptosis is not clear. It is possible that the mitochondrial fragmentation ability is required for its pro-apoptotic activity, because mitochondrial fission proteins, e.g., DRP1, have also been reported involve in apoptosis [41].
As the only BCL2 like protein that has all four BH domains while not belongs to any of the classic three BCL2 sub-families at this time, BCL2L13 is not only implicated in apoptosis signaling pathway, but also participates in regulation of mitophagy and mitochondrial fragmentation. Because of these complicated functions in molecular and cellular level [42], BCL2L13 also exhibits important roles in many physiological procedures and participates in pathological processes.
Physiological roles
Growth and development
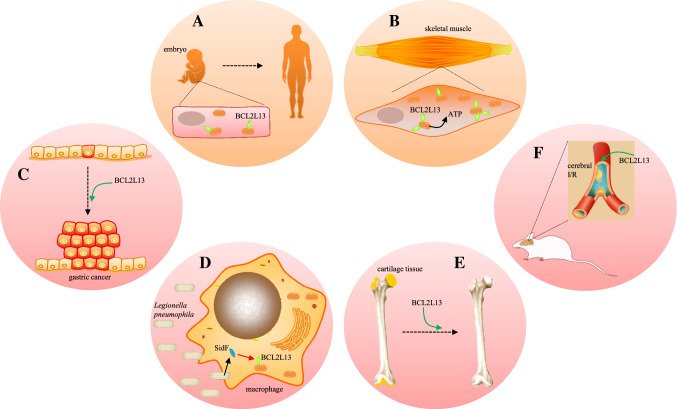
The development and transformation of fertilized egg into early embryo depend on a complex orchestra of genes. BCL2L13 is one of them that constitutively express in primary human embryo, which performs pro-apoptosis activity to help embryonic development [43, 44] (Fig. 3a). It is noteworthy that during the apoptotic process triggered by gambogic acid in oocyte, BCL2L13 changes to peri-nuclear localization from scattered state in light of the stained foci surrounding DNA [44], suggesting its important role in the developmental process. Now we know that most of the time BCL2L13 is moored in mitochondrial outer membrane, and relocates concomitantly with mitochondria, which is highly contextual. During formation of the placenta, BCL2L13 is down-regulated in ruminant chorionic fibroblasts, which is mediated by secreted protein of Ly-6 domain 1 (SOLD1), often acting as a modulator of gene expression in ruminant placenta construction [45].
Fig. 3.
BCL2L13 plays important roles in a variety of physiological and pathological processes. a Constitutive expressing in human embryo, BCL2L13 promotes embryonic development, and maintains organismal homeostasis by its pro-apoptotic ability. b High content of BCL2L13 arising in skeletal muscle after exercise could be beneficial for ATP supply. c BCL2L13 adjusts stomach carcinogenesis conform to certain pathological condition. d SidF from Legionella pneumophila is used to neutralize BNIP3 and BCL2L13 in lung macrophages, resulting in expanding of bacterial reproduction. e Lower BCL2L13 retards age-associated degenerative diseases, like OA. f Elevated BCL2L13 caused by cerebral I/R injury are inclined to formidable brain damage in mice
Balancing cell growth, proliferation and death during biological growth process plays an important role in keeping organism integrity. Ectopic expression of BCL2L13 in Drosophila gives rise to phenotypes with atrophied wing, split thorax and rough eye, together with activated apoptosis [46]. However, small decrease of BCL2L13 expression in active Churg-Strauss syndrome patients doesn't affect eosinophils viability [47]. And single knock out of BCL2L13 gene brings no obvious change in mouse epididymal phenotype, but endows strengthened apoptosis resistance in the cells [48]. It is possible that dose–effect relationship might be responsible for these different outcomes.
Mammals include human, always do not have the ability to regenerate limbs, which could accomplish in some lower organisms, such as amphibian and arthropod [49–51]. MicroRNA-21 is highly up-regulated in blastema of zebrafish caudal fins, bichir pectoral fins or axolotl forelimbs, stimulates appendages regeneration based on sponging their BCL2L13, which can induce cell death in adult animals [52].
Energy metabolism
BCL2L13 influences on energy metabolism through mitochondrial quality control and its interaction with ANT. As a potential candidate protein for adaptive evolution, BCL2L13 is highly increased in skeletal muscle of Mexico Rarámuri, to elevate their physical endurance via stimulating adenosine triphosphate (ATP) supply [53] (Fig. 3b). Exercise training is also possible to facilitate raised content of BCL2L13 in skeletal muscle, while mitochondrial biogenesis is boosted for body’s energy requirements [54, 55]. Preadipocytes predominantly employ oxidative phosphorylation to generate ATP, while preosteoblasts primarily utilize glycolysis [56]. Elevated expression of BCL2L13 is more prone to efficient oxidative phosphorylation, facilitates the adipogenic differentiation of bone marrow stromal cells (BMSCs) and ear mesenchymal stem cells (EMSCs), even browning of white adipose tissue [57, 58].
The physiological activities of BCL2L13 intervened in organismal homeostasis and ATP supply are established on its widely expressing in all tissues of human body, with no conspicuous tissue specificity, only slightly more expressions in pancreas and heart [59]. That is also the fundamental for BCL2L13 underlies miscellaneous human diseases [60, 61].
Pathological implications
Cancer
Tumors of the digestive system
The human digestive system consists of alimentary canal, extramural glands and wastes discharge structures [62, 63]. Tumors of digestive system are neoplasms that take place in the above organs, except oral cavity, pharynx and salivary glands [64, 65]. BCL2L13 expression is significantly enhanced in proximal gastric cancer (PGC) samples than in distal gastric cancer (DGC) samples, which may conduct as a signature of disease progression in stomach neoplasm [66] (Fig. 3c). Other than in disease progression, BCL2L13 expressions also correlate with response. Preoperative chemoradiotherapy (pCRT) is employed as a normative treatment for rectal cancer, though the patients’ responses are always erratic [67]. The down-regulated BCL2L13 corresponds with non-response to pCRT in locally advanced rectal cancer (LARC) patients, providing a potential strategy to predict response to pCRT in LARC cases [68].
Liver is the body's largest digestive gland, which helps digestion by secreting bile [69, 70]. Compared with normal hepatocyte L02 cells, microRNA-222 is overexpressed in HBx-HepG2 liver cancer cells, promoting their growth by negatively regulates BCL2L13 expression through suppression of the 3′-UTR region [71]. End-stage liver disease, including liver cancer, can cause some complications, such as osteoporosis [72]. In advanced cholestatic and end-stage liver disease patients, BCL2L13 is amenable to be positively regulated by the lithocholic acid (LCA), one of the factors involved in liver cancer induced osteroporosis, leading to enhanced bone absorption, which might relate to the pro-apoptotic functions of BCL2L13 in osteoblastic cells [73].
Tumors of the hematopoietic and lymphoid tissues
Leukemia, myeloma and lymphoma occupy the majority of common blood tumors. Since WHO released the classification of lymphoid and myeloid neoplasms and acute leukemia in 2016, many biomarkers were uncovered along the research progresses [74]. Mutations in additional sex combs like 1 (ASXL1) are associated with chronic myelomonocytic leukemia, that ASXL1 knockout mice are constructed to mimic myeloid malignancies, in which BCL2L13 was shown to be up-regulated [75, 76]. Not only related to tumorigenesis, BCL2L13 also showed relation to poor prognosis in leukemia. BCL2L13 is a new prognostic factor in childhood acute lymphoblastic leukemia (ALL), with its high expression in the tumor samples significantly related to poor prognosis [77, 78].
BCL2L13 expressions respond differently when exposed to various factors, suggesting that it executes specific mechanism complies with certain pathological condition. Antimalarial artesunate is STAT3 inhibitor and shows to enhance chronic myeloid leukemia K562 cells apoptosis by interacting with magnetic nanoparticles of Fe3O4 (MNPs-Fe3O4), which was mediated by up-regulation of BCL2L13 [79–81]. MNPs-Fe3O4 could act alone to promote myelodysplastic syndrome (MDS) cell line SKM-1 death, with the same mechanisms [82]. The volatile organic compound benzene belongs to group I carcinogens, and could affect bone marrow damage with no evidence of a threshold [83, 84]. Subchronic benzene inhalation induces bone marrow cells apoptosis in mice, with reduced expression of BCL2L13 mRNA, but does not affect methylation status of its promoter [85]. Cardamonin is a chalcone extracted from Alpiniae katsumadai, applied with the anti-proliferative effect for tumor cells [86]. It induces murine leukemia WEHI-3 cells apoptosis by decreasing expression of BCL2L13 in vitro [87].
Other cancers
Glioblastoma (GBM) is designated by WHO as grade IV astrocytoma, which is the most malignant glioma with genetic and epigenetic variations among heterogeneous cancer cells [88, 89]. BCL2L13 is overexpressed in GBM, inhibits the tumor cells apoptosis by preventing MOMP and caspase-3 activation [35]. ZIC1 is a critical transcription factor for liposarcoma and ZIC1 knockdown results in the decreased expression of downstream molecule BCL2L13, reducing liposarcoma cell lines survivability [90]. While in some other type tumors, such as node-negative breast cancer and lung adenocarcinoma (LUAD), BCL2L13 is reported to be negatively associated with the tumor growth [91, 92]. Mounting pathological complexity will increase the readiness of BCL2L13 in relation to molecule-targeted therapies.
Bacterial infection
Macrophages are derived from myeloid immune cells, which perform as the second line of defense to against pathogen invasion [93], or macrophages burst proactively for resist bacterial replication [94]. On the other hand, bacteria have also developed countermeasures. For example, Legionella pneumophila can neutralize BNIP3 and BCL2L13 with its own virulence factor anhydromevalonyl-CoA transferase (SidF), to prevent lung macrophages from apoptosis and achieve maximum intracellular self-reproduction, although this is not necessary for Legionella pneumophila infection [95, 96] (Fig. 3d).
Cardiovascular disease
Invalid mitophagy for diseased mitochondria is in charge of the development of heart failure [97]. Given that BCL2L13 can ameliorate mitochondrial dysfunction, drugging the mitophagy receptor has become a promising therapeutic strategy to limit the loss of cardiomyocytes [36, 98]. By extension, BCL2L13 circRNA in the heart is generated at the cost of syngeneic linear mRNA [99, 100], competing with the accumulation of linear mRNA, and negatively modulated by RNA binding motif protein 20 (RBM20) [101–103].
Cerebral ischemia reperfusion (I/R) injury is characterized as lethal intracranial hemorrhage and susceptible to cerebrovascular thrombolysis [104], with the pathogenic factors include: free radicals [105, 106], inflammation [107], intracellular calcium overload [108], cellular apoptosis [109], etc. Decreasing microRNA-874-3p level is observed in rat middle cerebral artery occlusion/reperfusion injury or SH-SY5Y cells following oxygen–glucose deprivation/reperfusion, and the liberated BCL2L13 is inclined to promote formidable brain damage in mice [110, 111] (Fig. 3f).
Degenerative disease
Osteoarthritis (OA) or age-related macular degeneration is the degenerative disease that worse over time [112]. Three single nucleotide polymorphisms (SNPs) of BCL2L13, rs1080199, rs2535707 and rs5992088, were significantly associated with serum cartilage oligomeric matrix protein (COMP), the executor that implicated in OA pathogenesis by inhibiting primary chondrocytes death [113, 114]. Long non-coding RNA SNHG15 is antagonist of microRNA-141-3p, while its decline in OA cartilage tissues resulted in synchronous lower expression of BCL2L13, which retards chondrocytes apoptosis, representing the physiological feedback to OA [115, 116] (Fig. 3e).
Neurodegenerative diseases mostly happen with senescence, and more prone to sickness with increasing age [117, 118]. MicroRNA-124 and microRNA-137 are rising rapidly soon after epileptic seizures, where the two microRNAs act synergistically targeting BCL2L13 to thwart caspase activation and maintain the integrity of neuronal cells [119]. Usually disturbed by aberrant mitophagy receptor, impaired degradation of damaged mitochondria is an important causative factor for degenerative disease [120–122]. Lower level of BCL2L13 is predisposed to develop age-related macular degeneration, may resulting from the attenuated mitophagy activity in retinal pigment epithelial cells [123].
As a whole, BCL2L13 has been found to participate in many kinds of diseases, including cancer, cardiovascular diseases and degenerative diseases, but the comprehensive pathogenic mechanism is still not clear. The causal inference of BCL2L13 impressing on these diseases may derive from its influences on cell death, mitochondrial quality control and/or mitochondrial fragmentation. It’s also an immune-related protein, for it is one of the targets of Legionella pneumophila to invade lung macrophages.
Discussions
The research on BCL2L13 has been going on for two decades, and showing an evidence of exponential growth in recent years. As a rising star in BCL2 protein family, BCL2L13 has some unique characteristics: (1) It sheds diverse evidences that BCL2L13 functions at apoptosis regulation, mitochondrial fragmentation and mitophagy promotion (Fig. 1); (2) pro- and anti-apoptotic BCL2 family members often titrate each other by heterodimers formation, accounting for the precise regulation of cell death, whereas the similar interactions haven’t been observed for BCL2L13 [13, 124]; (3) the active BHNo domain is distinct from BH homologous sequences, which introduces an abnormal molecular weight as well as peculiar function to BCL2L13 [24, 35].
BCL2/adenovirus E1B 19-kDa-interacting protein 3 (BNIP3) is a type of BH3-only protein, and behaves some similarity to the function of BCL2L13 [125, 126]. First, they are both single-pass mitochondrion outer membrane proteins. Second, both of them show cell death promoting activity independent of BH3 motif [127]. Third, BNIP3 could also engage as mitophagy enhancer by motivating the accumulation of PINK1 on dysfunctional mitochondria [128]. Heretofore, BIM, BNIP3 and BCL2L13 are the few BCL2 family proteins exhibit cross connections between autophagy and apoptosis [129]. Profiling the shared properties will in support of informative clues for possible therapeutic effects of BCL2L13. In addition, special attention is required for: (1) beyond BNIP3, BCL2L13 still induces mitochondrial fragmentation, depends on the entirety of BH1-4 domains [34, 36]; (2) BCL2L13 couldn’t form heterodimer with anti-apoptotic BCL2, BCLxL, BCLw, MCL1, A1 [130], E1B-19 K [131] and BHRF1 [132] or pro-apoptotic BAX, BAK, BIK, BID, BIM and BAD, while BNIP3 interacts with BCL2 as the antagonist of BECLIN-1 [24, 133–135].
Although physiological and pathophysiological properties of BCL2L13 are the foci of attention in nearly years (Fig. 3), yet there are no targeted drugs and/or drug candidates have been studying on. The molecular pathway of BCL2L13 takes on defined pathological condition remains confused, even the fulfillment is converse in some diseases. BCL2L13 maintains higher expression levels in GBM [35], PGC [66] or childhood ALL [77, 78], but lower in node-negative breast cancer [91], LUAD [92] or OA [115]. And microRNAs also play an important part in the reconciliation of BCL2L13 (Table 1). Therefore, a better grasp of the gene translation and interacting proteins will pave the way for BCL2L13-targeted biotherapy strategies.
Table 1.
MicroRNAs that associate with BCL2L13 mRNA
| Target microRNA | Expression changes |
Locations | Pathological significance | References |
|---|---|---|---|---|
|
microRNA-21 microRNA-181c |
Increased | Blastema | Stimulate appendages regeneration | 52 |
| microRNA-222 | Increased | HBx-HepG2 cells | Promotes tumor cells growth | 71 |
| microRNA-874-3p | Decreased | Brain | Exacerbates cerebral I/R injury | 110 |
| microRNA-141-3p | Increased | Articular cartilage | Ameliorates osteoarthritis | 115 |
|
microRNA-124 microRNA-137 |
Increased | Hippocampus | Against epileptic seizures | 119 |
Author contributions
HD, JX and FM designed the study, edited and approved the final version. FM and NS searched the literature and drafted the manuscript. HD, DL and JJ supervised the study.
Funding
This work is supported by the National Natural Science Foundation of China (No. 31970701, No. 21772201, No.82072807), the co-operative grant from Anhui Medical University and Center of Medical Physics and Technology (No. LHJJ202006, No. LHJJ202007).
Compliance with ethical standards
Conflict of interest
No conflict of interest related to this research.
Ethical approval
No animal experiments or clinical trials were conducted by any of the authors, so we state that there is no ethical problem in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fei Meng and Naitong Sun have equally contributed to this work.
Contributor Information
Jun Xiao, Email: xiaojunpp@126.com.
Haiming Dai, Email: daih@cmpt.ac.cn.
References
- 1.Tower J. Programmed cell death in aging. Ageing Res Rev. 2015;23(Pt A):90–100. doi: 10.1016/j.arr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fearnhead HO, Vandenabeele P, Vanden BT. How do we fit ferroptosis in the family of regulated cell death? Cell Death Differ. 2017;24(12):1991–1998. doi: 10.1038/cdd.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Strasser A, McDunn JE, et al. Cell death. N Engl J Med. 2009;361(16):1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galluzzi L, Bravo-San Pedro JM, Kepp O, et al. Regulated cell death and adaptive stress responses. Cell Mol Life Sci. 2016;73(11–12):2405–2410. doi: 10.1007/s00018-016-2209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinou JC, Youle RJ. Mitochondria in apoptosis: bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21(1):92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correia C, Lee SH, Meng XW, et al. Emerging understanding of Bcl-2 biology: implications for neoplastic progression and treatment. Biochim Biophys Acta. 2015;1853(7):1658–1671. doi: 10.1016/j.bbamcr.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsujimoto Y, Finger LR, Yunis J, et al. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226(4678):1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 9.Boise LH, González-García M, Postema CE, et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 10.Gibson L, Holmgreen SP, Huang DC, et al. bcl-w, a novel member of the bcl-2 family, promotes cell survival. Oncogene. 1996;13(4):665–675. [PubMed] [Google Scholar]
- 11.Zhou P, Qian L, Kozopas KM, et al. Mcl-1, a Bcl-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood. 1997;89(2):630–643. [PubMed] [Google Scholar]
- 12.Xue D, Horvitz HR. Caenorhabditis elegans CED-9 protein is a bifunctional cell-death inhibitor. Nature. 1997;390(6657):305–308. doi: 10.1038/36889. [DOI] [PubMed] [Google Scholar]
- 13.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 14.Chittenden T, Harrington EA, O'Connor R, et al. Induction of apoptosis by the Bcl-2 homologue Bak. Nature. 1995;374(6524):733–736. doi: 10.1038/374733a0. [DOI] [PubMed] [Google Scholar]
- 15.Yang E, Zha J, Jockel J, et al. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80(2):285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 16.Boyd JM, Gallo GJ, Elangovan B, et al. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995;11(9):1921–1928. [PubMed] [Google Scholar]
- 17.Wang K, Yin XM, Chao DT, et al. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10(22):2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor L, Strasser A, O'Reilly LA, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17(2):384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conradt B, Horvitz HR. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell. 1998;93(4):519–529. doi: 10.1016/s0092-8674(00)81182-4. [DOI] [PubMed] [Google Scholar]
- 20.Kuwana T, Bouchier-Hayes L, Chipuk JE, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17(4):525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Dai H, Smith A, Meng XW, et al. Transient binding of an activator BH3 domain to the Bak BH3-binding groove initiates Bak oligomerization. J Cell Biol. 2011;194(1):39–48. doi: 10.1083/jcb.201102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai H, Pang YP, Ramirez-Alvarado M, et al. Evaluation of the BH3-only protein Puma as a direct Bak activator. J Biol Chem. 2014;289(1):89–99. doi: 10.1074/jbc.M113.505701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willis SN, Fletcher JI, Kaufmann T, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315(5813):856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 24.Kataoka T, Holler N, Micheau O, et al. Bcl-rambo, a novel Bcl-2 homologue that induces apoptosis via its unique C-terminal extension. J Biol Chem. 2001;276(22):19548–19554. doi: 10.1074/jbc.M010520200. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann T, Schlipf S, Sanz J, et al. Characterization of the signal that directs Bcl-x(L), but not Bcl-2, to the mitochondrial outer membrane. J Cell Biol. 2003;160(1):53–64. doi: 10.1083/jcb.200210084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Footz TK, Brinkman-Mills P, Banting GS, et al. Analysis of the cat eye syndrome critical region in humans and the region of conserved synteny in mice: a search for candidate genes at or near the human chromosome 22 pericentromere. Genome Res. 2001;11(6):1053–1070. doi: 10.1101/gr.154901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins JE, Wright CL, Edwards CA, et al. A genome annotation-driven approach to cloning the human ORFeome. Genome Biol. 2004;5(10):R84. doi: 10.1186/gb-2004-5-10-r84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ota T, Suzuki Y, Nishikawa T, et al. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat Genet. 2004;36(1):40–45. doi: 10.1038/ng1285. [DOI] [PubMed] [Google Scholar]
- 29.Ule J. Alu elements: at the crossroads between disease and evolution. Biochem Soc Trans. 2013;41(6):1532–1535. doi: 10.1042/BST20130157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi P, Zhang W, Zhai Z, et al. Bcl-rambo beta, a special splicing variant with an insertion of an Alu-like cassette, promotes etoposide- and taxol-induced cell death. FEBS Lett. 2003;534(1–3):61–68. doi: 10.1016/s0014-5793(02)03778-x. [DOI] [PubMed] [Google Scholar]
- 31.Dai H, Meng XW, Kaufmann SH. BCL2 family, mitochondrial apoptosis, and beyond. Cancer Transl Med. 2016;2(1):7–20. [Google Scholar]
- 32.Ichim G, Tait SW. A fate worse than death: apoptosis as an oncogenic process. Nat Rev Cancer. 2016;16(8):539–548. doi: 10.1038/nrc.2016.58. [DOI] [PubMed] [Google Scholar]
- 33.Kim JY, So KJ, Lee S, et al. Bcl-rambo induces apoptosis via interaction with the adenine nucleotide translocator. FEBS Lett. 2012;586(19):3142–3149. doi: 10.1016/j.febslet.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Matsubara H, Tanaka R, Tateishi T, et al. The human Bcl-2 family member Bcl-rambo and voltage-dependent anion channels manifest a genetic interaction in Drosophila and cooperatively promote the activation of effector caspases in human cultured cells. Exp Cell Res. 2019;381(2):223–234. doi: 10.1016/j.yexcr.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Jensen SA, Calvert AE, Volpert G, et al. Bcl2L13 is a ceramide synthase inhibitor in glioblastoma. Proc Natl Acad Sci USA. 2014;111(15):5682–5687. doi: 10.1073/pnas.1316700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murakawa T, Yamaguchi O, Hashimoto A, et al. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun. 2015;6:7527. doi: 10.1038/ncomms8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huynh DP, Dy M, Nguyen D, et al. Differential expression and tissue distribution of parkin isoforms during mouse development. Brain Res Dev Brain Res. 2001;130(2):173–181. doi: 10.1016/s0165-3806(01)00234-6. [DOI] [PubMed] [Google Scholar]
- 38.Pawlyk AC, Giasson BI, Sampathu DM, et al. Novel monoclonal antibodies demonstrate biochemical variation of brain parkin with age. J Biol Chem. 2003;278(48):48120–48128. doi: 10.1074/jbc.M306889200. [DOI] [PubMed] [Google Scholar]
- 39.Murakawa T, Okamoto K, Omiya S, et al. A mammalian mitophagy receptor, Bcl2-L-13, recruits the ULK1 complex to induce mitophagy. Cell Rep. 2019;26(2):338–345.e6. doi: 10.1016/j.celrep.2018.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M, Jia J, Zhang X, et al. Selective binding of mitophagy receptor protein Bcl-rambo to LC3/GABARAP family proteins. Biochem Biophys Res Commun. 2020;530(1):292–300. doi: 10.1016/j.bbrc.2020.07.039. [DOI] [PubMed] [Google Scholar]
- 41.Montessuit S, Somasekharan SP, Terrones O, et al. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142(6):889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fei M, Liwei Z, Hongzhi W, et al. Role of Bcl-rambo in apoptosis and mitophagy. J Cell Signal. 2018;3(3):192. [Google Scholar]
- 43.Boumela I, Assou S, Aouacheria A, et al. Involvement of BCL2 family members in the regulation of human oocyte and early embryo survival and death: gene expression and beyond. Reproduction. 2011;141(5):549–561. doi: 10.1530/REP-10-0504. [DOI] [PubMed] [Google Scholar]
- 44.Boumela I, Assou S, Haouzi D, et al. Developmental regulated expression of anti- and pro-apoptotic BCL-2 family genes during human early embryonic development. Curr Med Chem. 2014;21(11):1361–1369. doi: 10.2174/09298673113206660278. [DOI] [PubMed] [Google Scholar]
- 45.Ushizawa K, Takahashi T, Hosoe M, et al. Cloning and expression of SOLD1 in ovine and caprine placenta, and their expected roles during the development of placentomes. BMC Dev Biol. 2010;10:9. doi: 10.1186/1471-213X-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakazawa M, Matsubara H, Matsushita Y, et al. The human Bcl-2 family member Bcl-rambo localizes to mitochondria and induces apoptosis and morphological aberrations in drosophila. PLoS ONE. 2016;11(6):e0157823. doi: 10.1371/journal.pone.0157823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jakiela B, Szczeklik W, Sokolowska B, et al. Intrinsic pathway of apoptosis in peripheral blood eosinophils of Churg-Strauss syndrome. Rheumatology (Oxford) 2009;48(10):1202–1207. doi: 10.1093/rheumatology/kep209. [DOI] [PubMed] [Google Scholar]
- 48.D’Alonzo D, Hong Z. Apoptosis in Bcl2l13 epididymal cells of mice. Cell Dev Biol. 2017;6:187. [Google Scholar]
- 49.Wang MH, Wu CH, Huang TY, et al. Nerve-mediated expression of histone deacetylases regulates limb regeneration in axolotls. Dev Biol. 2019;449(2):122–131. doi: 10.1016/j.ydbio.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki Y, Chou J, Garvey SL, et al. Evolution and regulation of limb regeneration in arthropods. Results Probl Cell Differ. 2019;68:419–454. doi: 10.1007/978-3-030-23459-1_17. [DOI] [PubMed] [Google Scholar]
- 51.Satoh A, Mitogawa K, Makanae A. Regeneration inducers in limb regeneration. Dev Growth Differ. 2015;57(6):421–429. doi: 10.1111/dgd.12230. [DOI] [PubMed] [Google Scholar]
- 52.King BL, Yin VP. A conserved microRNA regulatory circuit is differentially controlled during limb/appendage regeneration. PLoS ONE. 2016;11(6):e0157106. doi: 10.1371/journal.pone.0157106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ávila-Arcos MC, McManus KF, Sandoval K, et al. Population history and gene divergence in native mexicans inferred from 76 human exomes. Mol Biol Evol. 2020;37(4):994–1006. doi: 10.1093/molbev/msz282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arribat Y, Broskey NT, Greggio C, et al. Distinct patterns of skeletal muscle mitochondria fusion, fission and mitophagy upon duration of exercise training. Acta Physiol (Oxf) 2019;225(2):e13179. doi: 10.1111/apha.13179. [DOI] [PubMed] [Google Scholar]
- 55.Drake JC, Wilson RJ, Yan Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. FASEB J. 2016;30:13–22. doi: 10.1096/fj.15-276337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guntur AR, Gerencser AA, Le PT, et al. Osteoblast-like MC3T3-E1 cells prefer glycolysis for ATP production but adipocyte-like 3T3-L1 cells prefer oxidative phosphorylation. J Bone Miner Res. 2018;33(6):1052–1065. doi: 10.1002/jbmr.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujiwara M, Tian L, Le PT, et al. The mitophagy receptor Bcl-2-like protein 13 stimulates adipogenesis by regulating mitochondrial oxidative phosphorylation and apoptosis in mice. J Biol Chem. 2019;294(34):12683–12694. doi: 10.1074/jbc.RA119.008630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ju L, Chen S, Alimujiang M, et al. A novel role for Bcl2l13 in promoting beige adipocyte biogenesis. Biochem Biophys Res Commun. 2018;506(3):485–491. doi: 10.1016/j.bbrc.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 59.én M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. [DOI] [PubMed]
- 60.Strappazzon F, Cecconi F. The multifaceted mitochondrion: an attractive candidate for therapeutic strategies. Pharmacol Res. 2015;99:425–433. doi: 10.1016/j.phrs.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hartenstein V, Martinez P. Structure, development and evolution of the digestive system. Cell Tissue Res. 2019;377(3):289–292. doi: 10.1007/s00441-019-03102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carpenter GH. The secretion, components, and properties of saliva. Annu Rev Food Sci Technol. 2013;4:267–276. doi: 10.1146/annurev-food-030212-182700. [DOI] [PubMed] [Google Scholar]
- 64.Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Assarzadegan N, Montgomery E. What is new in world health organization (WHO) classification of tumors of the digestive system: review of selected updates on neuroendocrine neoplasms, appendiceal tumors, and molecular testing. Arch Pathol Lab Med. 2019 doi: 10.5858/arpa.2019-0665-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z, Yan Z, Zhang B, et al. Identification of a 5-gene signature for clinical and prognostic prediction in gastric cancer patients upon microarray data. Med Oncol. 2013;30(3):678. doi: 10.1007/s12032-013-0678-5. [DOI] [PubMed] [Google Scholar]
- 67.Cho E, Park IJ, Yeom SS, et al. A multigene model for predicting tumor responsiveness after preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2019;105(4):834–842. doi: 10.1016/j.ijrobp.2019.07.058. [DOI] [PubMed] [Google Scholar]
- 68.Millino C, Maretto I, Pacchioni B, et al. Gene and microRNA expression are predictive of tumor response in rectal adenocarcinoma patients treated with preoperative chemoradiotherapy. J Cell Physiol. 2017;232(2):426–435. doi: 10.1002/jcp.25441. [DOI] [PubMed] [Google Scholar]
- 69.Juza RM, Pauli EM. Clinical and surgical anatomy of the liver: a review for clinicians. Clin Anat. 2014;27(5):764–769. doi: 10.1002/ca.22350. [DOI] [PubMed] [Google Scholar]
- 70.Hofmann AF. Bile acids: trying to understand their chemistry and biology with the hope of helping patients. Hepatology. 2009;49(5):1403–1418. doi: 10.1002/hep.22789. [DOI] [PubMed] [Google Scholar]
- 71.Guifang Y, Shudi C, Xuezhu C, et al. miR-222 enhances HBx-HepG2 cell growth via regulation of BCL2L13 gene. Chin J Pathophysiol. 2016;32(8):1389–1394. [Google Scholar]
- 72.Parés A, Guañabens N. Primary biliary cholangitis and bone disease. Best Pract Res Clin Gastroenterol. 2018;34–35:63–70. doi: 10.1016/j.bpg.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 73.Ruiz-Gaspà S, Guañabens N, Jurado S, et al. Bile acids and bilirubin effects on osteoblastic gene profile Implications in the pathogenesis of osteoporosis in liver diseases. Gene. 2020;725:144167. doi: 10.1016/j.gene.2019.144167. [DOI] [PubMed] [Google Scholar]
- 74.Leonard JP, Martin P, Roboz GJ. Practical implications of the 2016 revision of the world health organization classification of lymphoid and myeloid neoplasms and acute leukemia. J Clin Oncol. 2017;35(23):2708–2715. doi: 10.1200/JCO.2017.72.6745. [DOI] [PubMed] [Google Scholar]
- 75.Nagase R, Inoue D, Pastore A, et al. Expression of mutant Asxl1 perturbs hematopoiesis and promotes susceptibility to leukemic transformation. J Exp Med. 2018;215(6):1729–1747. doi: 10.1084/jem.20171151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang J, Li Z, He Y, et al. Loss of Asxl1 leads to myelodysplastic syndrome-like disease in mice. Blood. 2014;123(4):541–553. doi: 10.1182/blood-2013-05-500272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holleman A, den Boer ML, de Menezes RX, et al. The expression of 70 apoptosis genes in relation to lineage, genetic subtype, cellular drug resistance, and outcome in childhood acute lymphoblastic leukemia. Blood. 2006;107(2):769–776. doi: 10.1182/blood-2005-07-2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang YL, Lin SR, Chen JS, et al. Expression and prognostic significance of the apoptotic genes BCL2L13, Livin, and CASP8AP2 in childhood acute lymphoblastic leukemia. Leuk Res. 2010;34(1):18–23. doi: 10.1016/j.leukres.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 79.Tan M, Rong Y, Su Q, et al. Artesunate induces apoptosis via inhibition of STAT3 in THP-1 cells. Leuk Res. 2017;62:98–103. doi: 10.1016/j.leukres.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 80.Zhang J, Sun X, Wang L, et al. Artesunate-induced mitophagy alters cellular redox status. Redox Biol. 2018;19:263–273. doi: 10.1016/j.redox.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y, Han Y, Yang Y, et al. Effect of interaction of magnetic nanoparticles of Fe3O4 and artesunate on apoptosis of K562 cells. Int J Nanomedicine. 2011;6:1185–1192. doi: 10.2147/IJN.S19723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuqiu L, Bing W, Wence L, et al. MNPs-Fe3O4 mediates malignant hematolpoectic cell apoptosis. J Exp Hematol. 2014;22(6):1649–1655. doi: 10.7534/j.issn.1009-2137.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 83.Capo A, Pennacchio A, Varriale A, et al. The porcine odorant-binding protein as molecular probe for benzene detection. PLoS ONE. 2018;13(9):e0202630. doi: 10.1371/journal.pone.0202630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith MT. Advances in understanding benzene health effects and susceptibility. Annu Rev Public Health. 2010;31:133–148. doi: 10.1146/annurev.publhealth.012809.103646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chong L, Zheng L, Xiuyuan Y, et al. Expressions and methylation analysis of apoptosis-related genes Survivin and Bcl2L13 in mice subchronic exposure to inhaled benzene. J Wenzhou Med Univ. 2014;44(7):480–484. [Google Scholar]
- 86.Jin J, Qiu S, Wang P, et al. Cardamonin inhibits breast cancer growth by repressing HIF-1α-dependent metabolic reprogramming. J Exp Clin Cancer Res. 2019;38(1):377. doi: 10.1186/s13046-019-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liao NC, Shih YL, Chou JS, et al. Cardamonin induces cell cycle arrest, apoptosis and alters apoptosis associated gene expression in WEHI-3 mouse leukemia cells. Am J Chin Med. 2019;47(3):635–656. doi: 10.1142/S0192415X19500332. [DOI] [PubMed] [Google Scholar]
- 88.Basu B, Ghosh MK. Extracellular vesicles in glioma: from diagnosis to therapy. BioEssays. 2019;41(7):e1800245. doi: 10.1002/bies.201800245. [DOI] [PubMed] [Google Scholar]
- 89.Broekman ML, Maas SLN, Abels ER, et al. Multidimensional communication in the microenvirons of glioblastoma. Nat Rev Neurol. 2018;14(8):482–495. doi: 10.1038/s41582-018-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brill E, Gobble R, Angeles C, et al. ZIC1 overexpression is oncogenic in liposarcoma. Cancer Res. 2010;70(17):6891–6901. doi: 10.1158/0008-5472.CAN-10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Petry IB, Fieber E, Schmidt M, et al. ERBB2 induces an antiapoptotic expression pattern of Bcl-2 family members in node-negative breast cancer. Clin Cancer Res. 2010;16(2):451–460. doi: 10.1158/1078-0432.CCR-09-1617. [DOI] [PubMed] [Google Scholar]
- 92.Mao S, Li Y, Lu Z, et al. PHD finger protein 5A promoted lung adenocarcinoma progression via alternative splicing. Cancer Med. 2019;8(5):2429–2441. doi: 10.1002/cam4.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643–675. doi: 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- 94.Orning P, Weng D, Starheim K, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362(6418):1064–1069. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Banga S, Gao P, Shen X, et al. Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family. Proc Natl Acad Sci USA. 2007;104(12):5121–5126. doi: 10.1073/pnas.0611030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Speir M, Vogrin A, Seidi A, et al. Legionella pneumophila strain 130b evades macrophage cell death independent of the effector SidF in the absence of Flagellin. Front Cell Infect Microbiol. 2017;7:35. doi: 10.3389/fcimb.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang B, Nie J, Wu L, et al. AMPKα2 protects against the development of heart failure by enhancing mitophagy via PINK1 phosphorylation. Circ Res. 2018;122(5):712–729. doi: 10.1161/CIRCRESAHA.117.312317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lampert MA, Orogo AM, Najor RH, et al. BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation. Autophagy. 2019;15(7):1182–1198. doi: 10.1080/15548627.2019.1580095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Du WW, Zhang C, Yang W, et al. Identifying and characterizing circRNA-protein interaction. Theranostics. 2017;7(17):4183–4191. doi: 10.7150/thno.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Verduci L, Strano S, Yarden Y, et al. The circRNA-microRNA code: emerging implications for cancer diagnosis and treatment. Mol Oncol. 2019;13(4):669–680. doi: 10.1002/1878-0261.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khan MA, Reckman YJ, Aufiero S, et al. RBM20 regulates circular RNA production from the Titin gene. Circ Res. 2016;119(9):996–1003. doi: 10.1161/CIRCRESAHA.116.309568. [DOI] [PubMed] [Google Scholar]
- 102.Guo W, Schafer S, Greaser ML, et al. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat Med. 2012;18(5):766–773. doi: 10.1038/nm.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aufiero S, van den Hoogenhof MMG, Reckman YJ, et al. Cardiac circRNAs arise mainly from constitutive exons rather than alternatively spliced exons. RNA. 2018;24(6):815–827. doi: 10.1261/rna.064394.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang J, Chen M, Cao RY, et al. The role of circular RNAs in cerebral ischemic diseases: ischemic stroke and cerebral ischemia/reperfusion injury. Adv Exp Med Biol. 2018;1087:309–325. doi: 10.1007/978-981-13-1426-1_25. [DOI] [PubMed] [Google Scholar]
- 105.Chen XM, Chen HS, Xu MJ, et al. Targeting reactive nitrogen species: a promising therapeutic strategy for cerebral ischemia-reperfusion injury. Acta Pharmacol Sin. 2013;34(1):67–77. doi: 10.1038/aps.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Losada-Barreiro S, Bravo-Díaz C. Free radicals and polyphenols: the redox chemistry of neurodegenerative diseases. Eur J Med Chem. 2017;133:379–402. doi: 10.1016/j.ejmech.2017.03.061. [DOI] [PubMed] [Google Scholar]
- 107.Gyoneva S, Davalos D, Biswas D, et al. Systemic inflammation regulates microglial responses to tissue damage in vivo. Glia. 2014;62(8):1345–1360. doi: 10.1002/glia.22686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li LH, Tian XR, Hu ZP. The key target of neuroprotection after the onset of ischemic stroke: secretory pathway Ca2+-ATPase 1. Neural Regen Res. 2015;10(8):1271–1278. doi: 10.4103/1673-5374.162760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu H, Wang L, Weng X, et al. Inhibition of Brd4 alleviates renal ischemia/reperfusion injury-induced apoptosis and endoplasmic reticulum stress by blocking FoxO4-mediated oxidative stress. Redox Biol. 2019;24:101195. doi: 10.1016/j.redox.2019.101195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang D, Sun X, Wang S, et al. Upregulation of miR-874-3p decreases cerebral ischemia/reperfusion injury by directly targeting BMF and BCL2L13. Biomed Pharmacother. 2019;117:108941. doi: 10.1016/j.biopha.2019.108941. [DOI] [PubMed] [Google Scholar]
- 111.Khoshnam SE, Winlow W, Farbood Y, et al. Emerging roles of microRNAs in ischemic stroke: as possible therapeutic agents. J Stroke. 2017;19(2):166–187. doi: 10.5853/jos.2016.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Choi SW, Lee JY, Kang KS. miRNAs in stem cell aging and age-related disease. Mech Ageing Dev. 2017;168:20–29. doi: 10.1016/j.mad.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 113.Coan HB, Curran JE, Dyer TD, et al. Variation in osteoarthritis biomarker serum comp levels in Mexican Americans is associated with SNPs in a region of chromosome 22q encompassing MICAL3, BCL2L13, and BID. Osteoarthritis Cartilage. 2013;21:S172. [Google Scholar]
- 114.Ratnayake M, Reynard LN, Raine EV, et al. Allelic expression analysis of the osteoarthritis susceptibility locus that maps to MICAL3. BMC Med Genet. 2012;13:12. doi: 10.1186/1471-2350-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang X, Huang CR, Pan S, et al. Long non-coding RNA SNHG15 is a competing endogenous RNA of miR-141-3p that prevents osteoarthritis progression by upregulating BCL2L13 expression. Int Immunopharmacol. 2020;83:106425. doi: 10.1016/j.intimp.2020.106425. [DOI] [PubMed] [Google Scholar]
- 116.Liu K, Hou Y, Liu Y, et al. LncRNA SNHG15 contributes to proliferation, invasion and autophagy in osteosarcoma cells by sponging miR-141. J Biomed Sci. 2017;24(1):46. doi: 10.1186/s12929-017-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hou Y, Dan X, Babbar M, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 118.Picca A, Calvani R, Coelho-Junior HJ, et al. Inter-organelle membrane contact sites and mitochondrial quality control during aging: a geroscience view. Cells. 2020;9(3):598. doi: 10.3390/cells9030598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schouten M, Fratantoni SA, Hubens CJ, et al. MicroRNA-124 and -137 cooperativity controls caspase-3 activity through BCL2L13 in hippocampal neural stem cells. Sci Rep. 2015;5:12448. doi: 10.1038/srep12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cameron RB, Beeson CC, Schnellmann RG. Development of therapeutics that induce mitochondrial biogenesis for the treatment of acute and chronic degenerative diseases. J Med Chem. 2016;59(23):10411–10434. doi: 10.1021/acs.jmedchem.6b00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lahuerta M, Aguado C, Sánchez-Martín P, et al. Degradation of altered mitochondria by autophagy is impaired in Lafora disease. FEBS J. 2018;285(11):2071–2090. doi: 10.1111/febs.14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morales I, Sanchez A, Puertas-Avendaño R, et al. Neuroglial transmitophagy and Parkinson's disease. Glia. 2020 doi: 10.1002/glia.23839. [DOI] [PubMed] [Google Scholar]
- 123.Malik D, Hsu T, Falatoonzadeh P, et al. Human retinal transmitochondrial cybrids with J or H mtDNA haplogroups respond differently to ultraviolet radiation: implications for retinal diseases. PLoS ONE. 2014;9(2):e99003. doi: 10.1371/journal.pone.0099003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oltvai ZN, Korsmeyer SJ. Checkpoints of dueling dimers foil death wishes. Cell. 1994;79(2):189–192. doi: 10.1016/0092-8674(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 125.Chen G, Ray R, Dubik D, et al. The E1B 19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein that activates apoptosis. J Exp Med. 1997;186(12):1975–1983. doi: 10.1084/jem.186.12.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Burton TR, Gibson SB. The role of Bcl-2 family member BNIP3 in cell death and disease: NIPping at the heels of cell death. Cell Death Differ. 2009;16(4):515–523. doi: 10.1038/cdd.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ray R, Chen G, Vande Velde C, et al. BNIP3 heterodimerizes with Bcl-2/Bcl-X(L) and induces cell death independent of a Bcl-2 homology 3 (BH3) domain at both mitochondrial and nonmitochondrial sites. J Biol Chem. 2000;275(2):1439–1448. doi: 10.1074/jbc.275.2.1439. [DOI] [PubMed] [Google Scholar]
- 128.Zhang T, Xue L, Li L, et al. BNIP3 protein suppresses PINK1 kinase proteolytic cleavage to promote mitophagy. J Biol Chem. 2016;291(41):21616–21629. doi: 10.1074/jbc.M116.733410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sionov RV, Vlahopoulos SA, Granot Z. Regulation of Bim in health and disease. Oncotarget. 2015;6(27):23058–23134. doi: 10.18632/oncotarget.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vogler M. BCL2A1: the underdog in the BCL2 family. Cell Death Differ. 2012;19(1):67–74. doi: 10.1038/cdd.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Green M, Brackmann KH, Lucher LA, et al. Human adenovirus 2 E1B–19K and E1B–53K tumor antigens: antipeptide antibodies targeted to the NH2 and COOH termini. J Virol. 1983;48(3):604–615. doi: 10.1128/jvi.48.3.604-615.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hickish T, Robertson D, Clarke P, et al. Ultrastructural localization of BHRF1: an Epstein-Barr virus gene product which has homology with bcl-2. Cancer Res. 1994;54(10):2808–2811. [PubMed] [Google Scholar]
- 133.Yasuda M, Theodorakis P, Subramanian T, et al. Adenovirus E1B–19K/BCL-2 interacting protein BNIP3 contains a BH3 domain and a mitochondrial targeting sequence. J Biol Chem. 1998;273(20):12415–12421. doi: 10.1074/jbc.273.20.12415. [DOI] [PubMed] [Google Scholar]
- 134.Niu C, Chen Z, Kim KT, et al. Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway. Autophagy. 2019;15(5):843–870. doi: 10.1080/15548627.2019.1569913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fernández ÁF, Sebti S, Wei Y, et al. Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature. 2018;558(7708):136–140. doi: 10.1038/s41586-018-0162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]