Abstract
Sertoli cells are essential for spermatogenesis in the testicular seminiferous tubules by forming blood–testis barrier (BTB) and creating a unique microenvironment for spermatogenesis. Many lncRNAs have been reported to participate in spermatogenesis. However, the role of long noncoding RNAs (lncRNAs) in Sertoli cells has rarely been examined. Herein, we found that a high-fat diet (HFD) decreased sperm quality, impaired BTB integrity and resulted in accumulation of saturated fatty acids (SFAs), especially palmitic acid (PA), in mouse testes. PA decreased the expression of tight junction (TJ)-related proteins, increased permeability and decreased transepithelial electrical resistance (TER) in primary Sertoli cells and TM4 cells. Moreover, lncRNA Tug1 was found to be involved in PA-induced BTB disruption by RNA-seq. Tug1 depletion distinctly impaired the TJs of Sertoli cells and overexpression of Tug1 alleviated the disruption of BTB integrity induced by PA. Moreover, Ccl2 was found to be a downstream target of Tug1, and decreased TJ-related protein levels and TER and increased FITC–dextran permeability in vitro. Furthermore, the addition of Ccl2 damaged BTB integrity after overexpression of Tug1 in the presence of PA. Mechanistically, we found that Tug1 could directly bind to EZH2 and regulate H3K27me3 occupancy in the Ccl2 promoter region by RNA immunoprecipitation and chromatin immunoprecipitation assays. Our study revealed an important role of Tug1 in the BTB integrity of Sertoli cells and provided a new view of the role of lncRNAs in male infertility.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04142-3.
Keywords: High-fat diet, Blood–testis barrier, lncRNA, Tug1, Ccl2
Introduction
Obesity has become a pandemic worldwide over the past few decades, and has become a social problem of widespread concern that could trigger a variety of systemic diseases including diseases of the reproductive system [1]. An increasing number of clinical studies have shown that body mass index (BMI) is significantly negatively correlated with male fertility [2–4]. Obesity is closely related to dietary structure. A high intake of saturated and trans fatty acids was positively associated with the odds of having asthenozoospermia and a low sperm concentration [5–7]. Moreover, total cholesterol and triglyceride levels in seminal plasma were negatively correlated with semen quality and positively correlated with the sperm DNA fragmentation index [8], suggesting that abnormal metabolism in the male reproductive system may be one of the factors leading to male infertility.
Human testicular single-cell transcriptomes from nonobstructive azoospermia patients indicated the key role of Sertoli cells in spermatogenesis [9]. High-fat diet (HFD) could affect spermatogenesis by directly impairing the function of Sertoli cells [10]. Sertoli cells near the basal lamina of the seminiferous tubules can form the blood–testis barrier (BTB) and maintain the unique microenvironment for normal spermatogenesis [11]. The BTB is generated by multiple junctions between supporting cells, including tight junctions (TJs), adherens junctions, and other junctional complexes. Proteomic analysis of rats with diet-induced obesity revealed that cytoskeletal remodeling and cell adhesion were the most obviously altered pathways in HFD-induced testicular cells, suggesting that abnormal metabolism may damage BTB function [12]. However, the specific mechanism underlying the metabolic effects affecting BTB function remains unclear.
In the human genome, 98% of RNAs do not have coding functions, of which those larger than 200 nt are called long noncoding RNAs (lncRNAs) [13]. LncRNAs are widely involved in various important regulatory processes such as genomic imprinting, chromatin modification, transcriptional activation, transcriptional interference, and nuclear transport, including the regulation of obesity-related processes [14–16]. Taurine upregulated gene 1 (Tug1) was first discovered in mouse retinal cells [17] and has been found to be involved in various biological processes such as metastasis [18], glycolysis [19] and mitochondrial energy synthesis [20] in different tissues. Several studies have reported that Tug1 can reduce inflammation and enhance insulin sensitivity by regulating the miR-204/SIRT1 pathway in white adipose tissue in obese mice [21]. Moreover, high-fat diet could lead to chronic inflammatory state in multiple systems [22, 23]. These studies indicate that Tug1 may be involved in obesity-induced metabolic disorders. Moreover, tug1−/− locus knockout male mice show severely impaired spermatogenesis and are completely sterile, suggesting that Tug1 is essential for male fertility [24]. Knockdown of Tug1 downregulated the expression of TJ proteins and improved the permeability of the blood–tumor barrier [25]. Therefore, we investigated whether lncRNAs play a role in HFD-induced BTB disruption.
Chemokine ligand 2 (C–C motif chemokine ligand2, Ccl2), formerly also known as MCP-1, is expressed in Sertoli cells of the testis according to the Human Protein Atlas. In brain metastases, the blood–tumor permeability was increased through increased astrocytic secretion of IL-6 and Ccl2 after overexpression of S1P3 [26]. Moreover, Ccl2 was increased with ILA-induced BTB impairment. It remained unclear whether Ccl2 participated in the disruption of the BTB induced by HFD.
In our present study, we constructed a diet-induced obese mice, attempted to detect the composition of fatty acids in the testes and found that HFD induced the accumulation of palmitic acid (PA) in the testes. In addition, we performed RNA-seq of Sertoli cells treated with PA and found that Tug1 expression was downregulated after PA treatment. Functional experiments were performed in mouse primary Sertoli cells (PSCs) and TM4 cell line by overexpressing and silencing Tug1 expression. We found that Tug1 was involved in PA-induced BTB disruption by regulating Ccl2 expression.
Materials and methods
Animals and experimental groups
Eight-week-old ICR mice were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. (Nanjing, CN). Animals were housed in a facility with a light:dark cycle of 12:12 h at a temperature of 23 °C and humidity of 50%–70%. All animal experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Ethics Committee of Nanjing Jinling Hospital. All animals were randomly divided into two groups: 1) the control group, which was fed a standard diet (CD) (1010009, Jiangsu Xietong Pharmaceutical Bioengineering Co., Ltd.); 2) the HFD group, which was fed a high-fat diet (34.9% fat, 26.2% protein, 26.3% carbohydrate by energy, Research Diet, USA) for 16 weeks [27]. Each group contained no less than five mice at the end point of detection. The experiment design of our study is shown in Fig. S1a.
Oral glucose tolerance tests (OGTTs)
For the OGTT, mice were fasted for 12 h followed by glucose administration (2 g/kg body weight) via oral gavage. Blood samples were collected from the tail vein at the indicated time points and glucose concentrations were determined by a glucose tester (Sannuo, China).
Isolation of PSCs and cell culture
Primary Sertoli cells were separated from mouse testes as previously described [28]. Briefly, the testes of more than five mice were decapsulated and digested with 0.5 mg/mL collagenase type I (Millipore Sigma, MO, USA) in DMEM-F12 (Thermo Fisher Scientific) at 37 ℃ for 8 min with gentle shaking. The supernatant was removed, and the remaining part was washed with PBS. Then, the seminiferous tubules were incubated with 0.25% trypsin for 6 min at 37 ℃. The tubules were filtered through screen mesh and the filtrate was collected for centrifugation to obtain PSCs. To investigate the change of Tug1 expression levels in vivo, the mice were randomly divided in two groups (the PA injection group and control group) and the primary Sertoli cells of each group were isolated. For the PA injection group, palmitic acid (PA) was purchased from Sigma (MO, USA) and dissolved in DMEM/F12 containing 2% fatty acid free BSA (Yeasen, Shanghai, CN) to obtain a 10 mM stock solution and was injected intraperitoneally at a dose of 200 mg/kg/day for 35 days according to Xu et al. [29]. The solution without PA was injected intraperitoneally as control group.
Sertoli cells were cultured in DMEM/F12 (Sigma, MO, USA) with 11% penicillin–streptomycin (Life Technologies, NY, USA) and 10% fetal bovine serum (Gibco, CT, USA). The purity of the Sertoli cells was almost 95% and assessed based on IF staining of FSHR, a marker of Sertoli cells (Fig. S2). Sertoli cells were incubated in a humidified atmosphere containing 5% CO2 at 34 °C, and the medium was replaced every day. After 3–4 days in culture, a structure resembling the BTB was established between adjacent Sertoli cells. The TM4 cell line was purchased from iCell Bioscience (Shanghai, CN) and cultured similarly to the PSCs.
For PA and Ccl2 treatment, TM4 and PSCs were cultured in six-well plates. The cells were pretreated with DMEM/F12 for 2 h to allow the state of the cell to remain consistent and then treated with PA or Ccl2 and the appropriate control for 24 h. Ccl2 was obtained from Novoprotein (Shanghai, CN). The concentration of PA treatment was based on our previous study [30] and the concentration of Ccl2 treatment was from Stamatovic et al. [31].
Assessment of BTB in vivo
The integrity of the BTB was assessed using FITC isothiocyanate isomer I (FITC I) tracing assays as previously described [32]. In brief, 5 mg/mL FITC isothiocyanate isomer I (Sigma-Aldrich, MO, USA) (freshly diluted in PBS) was prepared. Then, 200 μL of the solution was injected into the caudal veins of the male mice. Two hours later, the mice were anesthetized and euthanized, and the testes were immediately removed and embedded in optimal cutting temperature (OCT) compound (Sakura, Tokyo, Japan) to obtain frozen sections (thickness of 8 μm). Fluorescence images were taken by a fluorescence microscope (IX73; Olympus Corporation, Tokyo, Japan).
Electron microscopy (TEM)
TEM detection was performed according to a previous study [28]. Briefly, the freshly isolated testes were immersed in 2.5% glutaraldehyde for 12 h. After dehydration and embedding, ultrathin sections were prepared and mounted on copper girds, double stained with uranyl acetate and citrate and examined using a JEM-1200 EX II TEM (JEOL, Tokyo, Japan) operated at 80 kV.
Computer-assisted sperm analysis
Epididymal sperm were prepared by computer-assisted semen analysis as described previously [32]. Briefly, the epididymis was dissected, and the sperm inside were squeezed out with forceps. After incubation in human tubal fluid medium (500 μL per epididymis) at 37 °C for 30 min, sperm were subjected to motility analyses using a Sperm Quality Analyzer. For each measurement, a suspension of spermatozoa was loaded into a microchamber slide with 100 μm depth. 300 spermatozoa were analyzed using the standard setting.
Testicular histopathological and immunofluorescence analysis
Mouse testes were fixed in Bouin’s fixative for > 12 h followed by embedding in paraffin. Paraffin sections were cut into pieces of 5-μm thickness, deparaffinized and hydrated in water. Then slides were stained in hematoxylin solution and eosin solution. Images were obtained by Olympus microscope (IX73; Olympus Corporation, Tokyo, Japan). For immunofluorescence staining, the tissue sections were deparaffinized in xylene and gradient ethanol. Then the slides were incubated in citric acid buffer in a microwave oven for antigen retrieval. After blocking in 3% BSA buffer to block non-specific protein sites, the slides were incubated with antibodies of BTB-related proteins overnight in 4 °C. Then the slides were washed three times by TBST and incubated with anti-rabbit second antibodies in room temperature for 1 h. Finally, the slides were stained by 4′,6-diamidino-2-phenylindole (DAPI, China). Each experiment was repeated three times.
Measurement of FSH levels
At termination, blood samples were collected after anesthesia and serum was obtained by centrifugation for 10 min at 1000 × g. Serum FSH levels were assessed by ELISA using a commercial kit (ml-E2856, MlBio, China). Optical densities (OD) were measured using a microplate reader at 450 nm. The concentrations were calculated according to the standard curves and were expressed in mIU/ml in serum.
Transfection of siRNA and plasmids
Cells (4 × 105 per well) were seeded in six-well plates. After 24 h, cells were washed two times with Opti-MEM (Gibco, Massachusetts, USA) and transfected with siRNA (final concentration 100 nM) and overexpression plasmids of Tug1 using Lipofectamine 3000 (Invitrogen, Carlsbad, USA), according to the manufacturer’s instructions. FBS was added to cells to a final concentration of 10% after 6 h. siTug1 and siEZH2 were obtained from RiboBio (Guangzhou, CN). Tug1 overexpression plasmids were generated by GeneCopoeia (Guangzhou, CN). The empty vector was used as a negative control (null).
Real-time quantitative RT-PCR
Total RNA was isolated from Sertoli cells using a Total RNA Isolation Kit (BEI-BEI Biotech, Zhengzhou, CN). For mRNA detection, cDNA was synthetized from total RNA using PrimeScript® RT Master Mix (TaKaRa) reverse transcriptase according to the manufacturer’s instructions. cDNA was quantified by RT-qPCR using a Roche Light Cycler 96 Real-time PCR system (Roche Diagnostics, Basel, Switzerland). Real-time PCRs were performed in triplicate. β-actin and 18S were used as endogenous controls for mRNA and cytoplasma mRNAs, respectively. Relative expression was calculated using the comparative ∆∆Ct method. The primers are listed in Supplementary Table 1.
Western blotting
Cells were harvested in RIPA lysis buffer (Cell Signaling Technology, Beverley, MA) with a protease inhibitor and phosphatase inhibitor (Sigma, MO, USA). Cell lysates were centrifuged at 12 000 rpm for 10 min at 4 °C and the supernatants were carefully collected. Protein concentration was measured using the BCA protein assay kit (Beyotime Biotechnology, Shanghai, CN) according to the manufacturer’s instructions. Equal amounts of total protein from each sample were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% milk in Tris-buffered saline supplemented with 0.1% Tween-20 for 1 h and then incubated with primary antibodies at 4 °C overnight. After incubation with HRP-conjugated secondary antibodies, protein bands were detected using an ECL Plus Western Blotting Detection System. Anti-ZO-1 and occludin antibodies were purchased from Proteintech (Wuhan, CN). GAPDH was used as loading control for Western blotting. The catalog numbers and dilutions of primary antibodies are listed in Supplementary Table 2.
FITC–dextran permeability assay
The permeability of FITC–dextran (Sigma, MO, USA) across the Sertoli cell monolayer was measured as previously described with modifications [33]. Cells (1 × 104 cells/well) were seeded on Marigel-coated Transwell 24-well Boyden chambers (3413, Corning-Costar, USA) with a 0.4 μm pore size polycarbonate membrane and maintained as a monolayer. FITC–dextran (1.0 mg/mL) was added on the apical side of monolayers after being washed twice with PBS. A 200μL sample in the basal chamber was taken at 120 min. The fluorescence emission at 520 nm was measured with excitation at 490 nm using a Synergy H1 microplate reader.
Transepithelial electrical resistance (TER) measurement
As an indicator of BTB permeability, the TER of Sertoli cells was determined at least every other day in three different areas of the units using a Millicell ERS system (Millipore Corp., Bedford, MA, USA). In brief, Sertoli cells (1 × 104 cells/well) were seeded on Matrigel-coated Transwell 24-well Boyden chambers (3413, Corning-Costar, USA) with a 0.4 μm pore size polycarbonate membrane and maintained as a monolayer. TER across the epithelium of Sertoli cells in the presence or absence of PA or Ccl2 was monitored as previously described [28].
RNA-seq
TM4 cells were treated with 0.4 mM PA for 24 h. Total RNA was extracted using TRIzol reagent. RNA quality was assessed on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) and examined using RNase-free agarose gel electrophoresis. Transcriptome sequencing analysis was performed on an Illumina HiSeq2500 by Gene Denovo Biotechnology Co. (Guangzhou, CN). Raw data were applied to the bioinformatic pipeline. To get high quality clean reads, reads were filtered by fastp(version 0.18.0) and mapped to the reference genome using HISAT2(version 2.1.0). Transcriptome from RNA-seq reads was reconstructed by Stringtie (version 1.3.4). Genes with a false discovery rate (FDR) below 0.05 and absolute fold change ≥ 1.5 were considered differentially expressed genes (DEGs).
GO and KEGG pathway analysis
Gene Ontology (GO) biological process enrichment analysis and KEGG pathway enrichment analysis were performed for all DEGs. For functional enrichment analysis, all DEGs were mapped to terms in the GO databases, and then significantly enriched GO terms were searched for among the DEGs using p < 0.05 as the threshold. GO term analysis was classified into three subgroups, namely biological process (BP), cellular component (CC) and molecular function (MF). All DEGs were mapped to the KEGG database and searched for significantly enriched KEGG pathways at p < 0.05 level.
Medium- and long-chain fatty acid measurements
The testes of mice in the control group and HFD group were collected and medium- and long-chain fatty acid measurements were performed by Shanghai Bioprofile Technology Co., Ltd. The samples were thawed on ice, and 50 µL of each sample was added into a 2-mL glass centrifuge tube. Then, 1 mL of chloroform methanol (2:1 v/v) was added. After ultrasonication for 30 min, 2 mL of 1% sulfuric acid in methanol was added to the supernatant. The mixture was incubated in an 80 °C water bath for 30 min to achieve fatty acid esterification for methyl esterification. Then, 1 mL n-hexane and 5 mL water were added and vortex mixed. The supernatant (500 µL) was spiked with an internal standard (25 µL of 500 ppm methyl salicylate), mixed, and subjected to GC–MS using an Agilent Model 7890A/5975C GC–MS system. To quantify medium- and long-chain fatty acid, Supelco 37-component fatty-acid methyl ester (FAME) mix (Sigma-Aldrich) was used to construct a calibration curve for the concentration range of 0.5–1000 mg/L. The IS was used to correct for injection variability between samples and minor changes in the instrument response.
The samples were separated with an Agilent DB-WAX capillary GC column (30 m × 0.25 mm ID × 0.25 µm). The initial temperature was 50 °C and remained as such for 3 min. The temperature was then increased to 220 °C at 10 °C/min and remained at 220 °C for 20 min. The carrier gas was helium (1.0 mL/min). A quality-control sample was used for testing and evaluating the stability and repeatability of the system. The temperatures of the injection port and transmission line were 280 °C and 250 °C, respectively. The electron energy was 70 eV.
Nucleocytoplasmic separation experiments
A Cytoplasmic and Nuclear RNA Purification Kit (Norgen Biotek Corp., Canada) was used to separate of nuclear and cytosolic fractions of Sertoli cells according to the manual. 18S was used as cytoplasmic control. The levels of Tug1, and 18S in the cytoplasm and nuclear fraction were detected by qPCR. The relative rate of Tug1 and 18S in the cytoplasm or nuclear part is presented as the percentage of the total RNA.
Fluorescence in situ hybridization (FISH)
RNA FISH (RiboBio, Guangzhou, CN) was used to assess the subcellular localization of Tug1 in Sertoli cells and was performed using Ribo™ Fluorescent in situ Hybridization Kit according to the manufacturer’s instructions and a Tug1 probe labeled with the Cy3 fluorescent dye was used.
RNA immunoprecipitation assays (RIP)
TM4 cells were transfected with siTug1 and Tug1 overexpression plasmids for 24 h, and RIP experiments were performed using the Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Catalogue No. 17–701, Millipore, MA, USA) according to the manufacturer’s instructions. To demonstrate that the detected signals were from the RNA that was specifically bound, we simultaneously used total RNA (input controls) and corresponding species IgG controls. Immunoprecipitation of EZH2 was performed using an anti-EZH2 antibody (CST) overnight at 4 °C. RNAs was extracted by the phenol:chloroform:isoamyl alcohol method and subjected to qRT-PCR. The primers are listed in Supplementary Table 3.
Chromatin immunoprecipitation assays (ChIP)
TM4 cells were seeded onto 10 cm2 dishes and transfected with siTug1 for 24 h. ChIP assays were preformed using a commercial kit (P2078, Beyotime Biotechnology, Shanghai, CN) according to the manufacturer’s instructions. DNA was purified and used as templates for qPCR. Relative enrichment of detected proteins was normalized to input DNA using the ΔΔ−Ct method. The primers are listed in Supplementary Table 3.
Statistical analysis
Results are recorded as means ± SD for at least three independent experiments. Different statistical methods were applied depending on the objective and the nature of data under analysis. All the data were analyzed using the software GraphPad Prism 6 (GraphPad Software, Inc.). The Student’s t test was used for continuous variables. One-way ANOVA was adopted to compare TER and FITC–dextran permeability when groups were more than 3. A p value < 0.05 was considered statistically significant.
Results
HFD disrupted BTB integrity and resulted in accumulation of the saturated fatty acids in the mouse testes
After 16 weeks of HFD feeding, the average body weight of the HFD-fed mice was significantly higher than that of the control mice. The results of OGTT indicated that the HFD group became glucose intolerant, whereas the control group did not (Fig. 1a, b). However, no significant difference in testicular weight was observed leading to the organ index (testicular weight/body weight) significantly being decreased in the HFD group (Fig. 1c, d). The sperm concentration and sperm motility of the mice in the HFD group were significantly decreased. All these results suggested that HFD could damage the reproductive system of male mice and reduce sperm quality (Fig. 1e, f). The pathological images showed that compared to those of the control mice, the structure of seminiferous tubules of the HFD-fed mice was loose, and the seminiferous epithelial layers became thinner, which was consistent with the decrease in sperm parameters in the HFD-fed mice (Fig. 1g). BTB integrity was assessed by FITC and exhibited significant damage in the testes of the HFD-fed mice (Fig. 1h). The cell junctions between Sertoli cells in the testes of HFD were loose, broken and structurally disordered as shown by electron microscopy (TEM) (Fig. 1i). The protein and mRNA levels of BTB-related proteins (ZO-1, occludin, claudin-11, claudin-5) in the testes of the HFD-fed mice were significantly decreased (Fig. 1j, k). Moreover, the immunofluorescence staining revealed lower fluorescence intensity of ZO-1 and Claudin-11 in the seminiferous tubules of the HFD-fed mice (Fig. S1b&c). All these results suggest that HFD could disrupt the BTB integrity in mice.
Fig. 1.
HFD decreased sperm quality and impaired BTB integrity (n > 5) a–d Body weight, OGTT, testis weight and organ index of the control (CD) and high-fat diet (HFD) group. e–f Sperm concentration and motility of the CD and HFD group. g The pathological images of the testis of the CD and HFD group. The seminiferous tubules of the HFD group were destroyed and became thinner. h FITC tracing assay of the CD and HFD group. FITC green fluorescence was seen in the lumen of HFD group, which suggested BTB integrity was impaired in HFD group. i The TEM pictures of BTB in the CD and HFD group. The black arrows indicate tight junction (TJ). TJ was disrupted in the HFD group. j The protein levels of BTB-related genes in the CD and HFD group. k The mRNA levels of BTB-related genes in the CD and HFD group
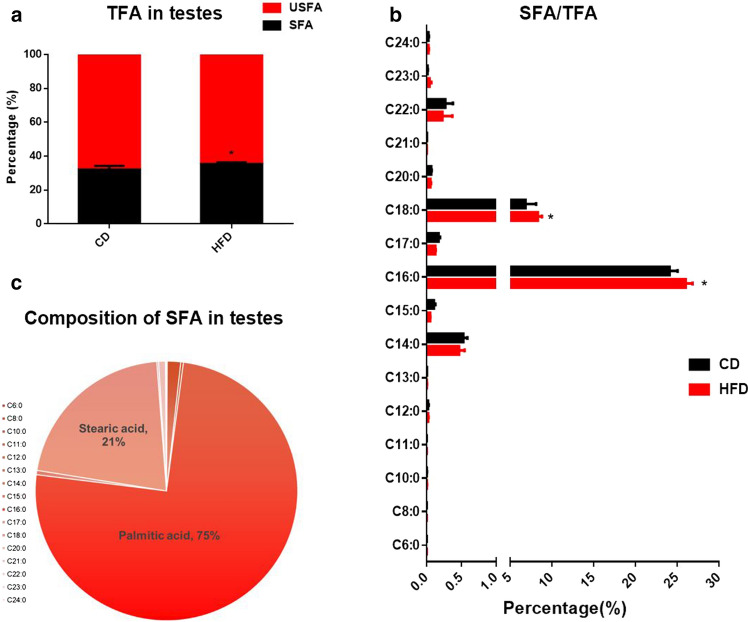
Our previous study showed that the local accumulation of free fatty acids was significantly increased in the testes of HFD-fed mice [30]. To determine the major fatty acids changed in the testes of the HFD-fed mice, we collected the testes and performed medium and long-chain fatty acid analysis. The results showed that the saturated fatty acids were significantly increased in the testes of the HFD-fed mice (Fig. 2). Moreover, among the saturated fatty acids, PA (C16:0, 75%) and stearic acid (SA, C18:0, 21%) accounted for the greatest proportion in the male testes (Fig. 2). As shown in Fig. 2, the proportion of PA (PA/total fatty acids) in the testes of the HFD-fed mice significantly increased. These results suggest that HFD can induce local accumulation of saturated fatty acids, especially PA, in the testis of mice.
Fig. 2.
The accumulation of saturated fatty acids in the HFD testes (n = 5). a Compared with the control group, the ratio of saturated fatty acids to total fatty acids in the testes of HFD group was significantly increased. b The ratio of specific fatty acids to total fatty acids, in which palmitic acid and stearic acid accounted for a significant increase; c The composition of saturated fatty acids, in which palmitic acid accounted for the most (75%). CD control diet, HFD high-fat diet, TFA total fatty acids, USFA unsaturated fatty acids, SFA saturated fatty acids, *p < 0.05
Because the FSH level of the HFD-fed mice decreased compared to that of the control group, we investigated whether the BTB damage of the HFD-fed mice was caused by the decreased function of the HPG axis. Therefore, we intraperitoneally injected highly purified human urinary FSH (6 IU/day) (Metrodin-HP, Serono) into the HFD-fed mice according to Gerard et al. [34]. However, sperm quality and BTB integrity were not rescued by the addition of uFSH (Fig. S2). Therefore, we concluded that the HFD and the local accumulation of fatty acids, but not FSH, contributed to BTB damage and poor sperm quality in mice.
Elevated PA levels induced BTB disruption
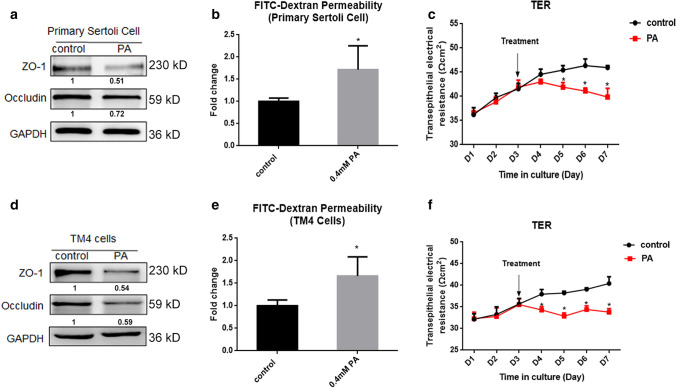
To examine the effect of HFD on the BTB, we established an in vitro BTB model formed by Sertoli cells in Transwell cell culture inserts and employed PA to mimic lipotoxicity. The concentration was from our previous study [30]. After PA treatment, the levels of TJ-related proteins were significantly decreased in Sertoli cells (Fig. 3a), and the FITC–dextran permeability assay in vitro showed more fluorescein leakage in the PA-treated group (Fig. 3b), suggesting that the structure between cells was loose and TJs were destroyed. Moreover, PSCs were plated on Transwell inserts and grown for 3 days followed by PA treatment, and the TER was measured daily. The results showed that the TER was significantly decreased after PA treatment (Fig. 3c). The TM4 cell line is a well-established mouse Sertoli cell line and can provide a useful, steady and reproducible model for testing the male reproductive toxicity and for identifying the underlying mechanism [35]. We treated the TM4 cell line with PA at 0.4 mM and the results resembled those of the PSCs (Fig. 3d–f). Overall, the results suggested that PA treatment could lead to TJ damage in PSCs and the TM4 cell line.
Fig. 3.
Palmitic acid disrupted tight junctions of Sertoli cells in vitro. a The expression of TJ-related proteins of primary Sertoli cell in the control and PA group. b The permeability of FITC–dextran of primary Sertoli cell in the control and PA group. c The TER of primary Sertoli cell in the control and PA group. d The expression and gray value of TJ-related proteins of TM4 cells in the control and PA group. e The permeability of FITC–dextran of TM4 cells in the control and PA group. f The TER of TM4 cells in the control and PA group. The arrow marked the time of PA treatment. Each experiment is replicated at least three times independently. * p < 0.05
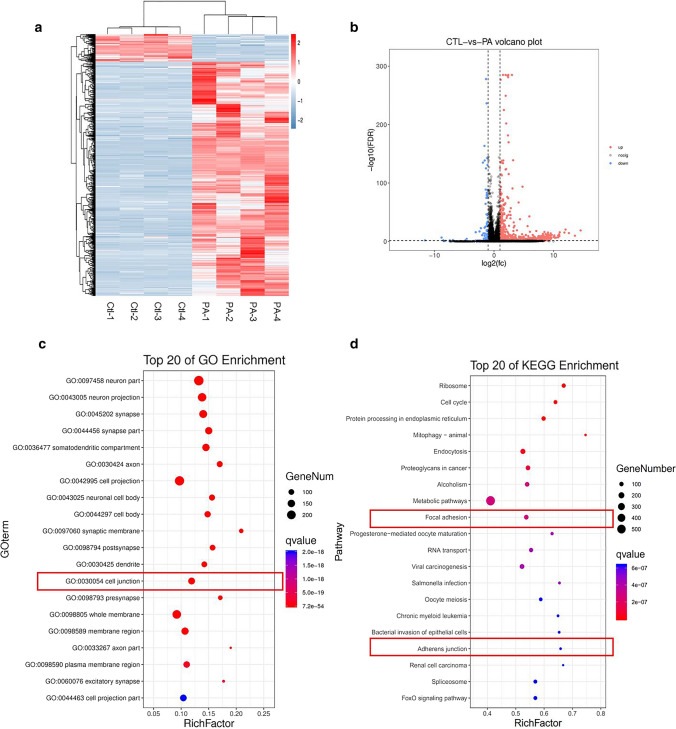
To investigate the underlying molecular mechanism involved in PA-induced Sertoli cell injury, we performed RNA-seq after treating the TM4 cell line with 0.4 mM PA and found a large number of differentially expressed mRNAs in the PA-treated TM4 cells (Fig. 4a). A total of 871 genes had upregulated expression and 103 genes had downregulated expression after PA treatment. GO cluster analysis of differentially expressed mRNAs revealed that “cell junctions” was involved in the altered biological processes after PA treatment (Fig. 4c), and KEGG pathway analysis showed that the mRNAs were enriched in “adherens junction” and “focal adhesion” pathways (Fig. 4d), which are related to BTB integrity. Taken together, the results suggested that elevated PA levels could change the junction proteins between Sertoli cells and disrupt TJ function between cells in vitro.
Fig. 4.
RNA-seq of Sertoli cells with PA treatment. a The differentially expressed genes in control and PA group. b The volcano plot of differentially expressed genes. c GO analysis of the differentially expressed genes in in control and PA group. d KEGG analysis of the differentially expressed genes in in control and PA group
Tug1 was downregulated by PA and was involved in PA-induced BTB disruption.
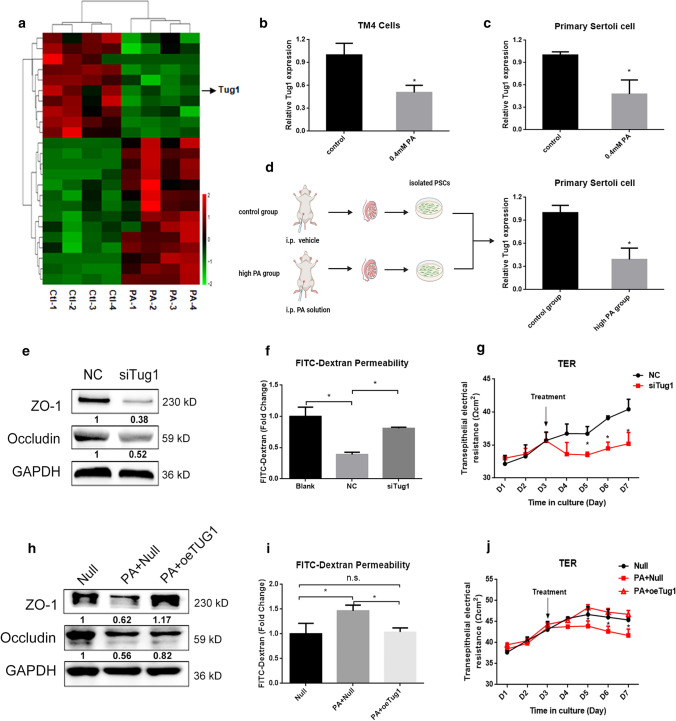
We examined several lncRNAs associated with spermatogenesis [36] and found that Tug1 expression was downregulated significantly after PA treatment (Fig. 5a). A recent study found that tug1-knockout male mice were sterile with underlying defects including low sperm count and abnormal sperm morphology [24]. We verified the downregulation of Tug1 expression in TM4 cells after PA treatment (Fig. 5b). PSCs from normal mouse testes were extracted, and a significant decrease in Tug1 expression was found after treatment with 0.4 mM PA in vitro (Fig. 5c). Then to investigate the alteration of Tug1 expression in vivo, an animal model was constructed by intraperitoneal injection of PA solution [29] and the PSCs were isolated to determine the expression of Tug1 (Fig. 5d). The results showed that the expression of Tug1 was significantly decreased in PSCs of PA injection group (Fig. 5d). Overall, these data showed that PA downregulated lncRNA Tug1 expression both in vivo and in vitro.
Fig. 5.
Tug1 was downregulated by PA and was involved in PA-induced BTB disruption. a The differentially expressed LncRNAs in the RNA-seq in control and PA group. b The expression of Tug1 in TM4 cells after PA treatment. c The expression of Tug1 of primary Sertoli cell after PA treatment. d A simple schematic diagram of animal experiment and the expression of Tug1 in primary Sertoli cell of PA injection group and control group. e The expression levels of TJ-related proteins of Sertoli cell in negative control (NC) and siTug1 group. f The permeability of FITC–dextran of Sertoli cell in NC and siTug1 group. Blank meant Matrigel™-coated culture units without cultured Sertoli cells. g The TER of Sertoli cells in NC and siTug1 group. h The expression levels of TJ-related proteins of Sertoli cells in negative control (Null) with or without PA treatment and overexpression Tug1 (oeTug1) group with PA treatment. i The permeability of FITC–dextran of Sertoli cells in Null with or without PA treatment and oeTug1 group with PA treatment. j The TER of Sertoli cells in Null with or without PA treatment and oeTug1 group with PA treatment. Each experiment is replicated at least three times independently. *p < 0.05
We downloaded the testis RNA-seq data of tug1−/ and wild-type mice from the GEO database (GSE124745 and GSE88819) and performed GO cluster analysis and KEGG pathway enrichment analysis. As shown in Fig. S3, the most enriched pathways included “metabolic pathways”, “focal adhesion”, “tight junction” and “regulation of actin cytoskeleton”. Among genes associated with the TJ pathway, key components (including ZO-1 and occludin) showed downregulated expression, indicating that TJ of the BTB were disrupted in the testes of tug1−/− mice, which may partly account for the decline in sperm concentration of tug1−/− male mice.
To investigate the role of lncRNA Tug1 in the regulation of TJs, overexpression and siRNA silencing experiments were performed. After Tug1 depletion, the expression of TJ-related proteins and TER were significantly decreased and the permeability of FITC–dextran was increased (Fig. 5e–g). To further verify whether Tug1 played a role in PA-induced BTB damage, we constructed a plasmid overexpressing Tug1 and found that overexpression of Tug1 rescued the PA-induced decrease in TJ-related proteins and TER compared with that of transfected controls (null), and reduced permeability of FITC–dextran in the presence of PA (Fig. 5h–j). Taken together, these results indicated that Tug1 plays an important role in PA-induced BTB impairment.
Tug1 depletion induced BTB disruption in a Ccl2-dependent manner.
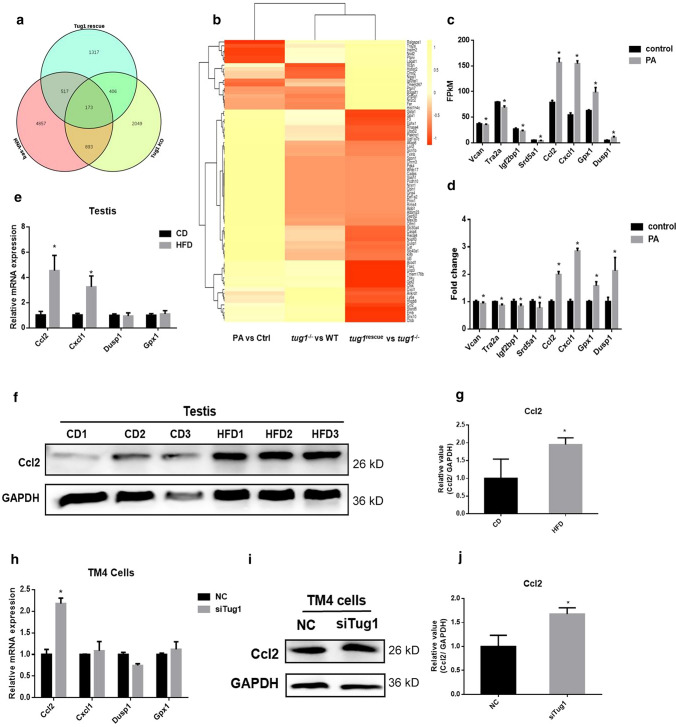
To determine how Tug1 affects TJs in Sertoli cells, we conducted the bioinformatics analysis to identify its potential downstream target genes. Combined the RNA-seq data of testes (tug1−/− and tug1rescue) from the public database which was mentioned above [24] with our RNA-seq data (control vs PA), 173 overlapping genes were involved in all three groups (Fig. 6a). Among them, the genes that were coregulated in the PA and tug1–/– groups and were changed in the opposite direction in the tug1rescue group are shown in Fig. 6b. A total of 18 mRNAs with consistently upregulated expression and 55 mRNAs with consistently downregulated expression in the PA and tug1–/– groups were found, and the changes may be related to Tug1. Among 73 genes, 4 genes with upregulated expression (Ccl2, Cxcl1, Gpx, and Dusp1) and 4 with downregulated expression (Vcan, Tra2a, Igf2bp1, and Srd5a1) were reported to be associated with TJs (Fig. 6c and d). The mRNA and protein levels of Ccl2 in the testicular tissue of mice were significantly increased in the HFD group (Fig. 6e–g). The mRNA and protein levels of Ccl2 in Sertoli cells were also significantly increased after transfection with siTug1 (Fig. 6h–j). These results suggested that Tug1 can regulate the expression of Ccl2 and may be involved in PA-induced Sertoli cell damage.
Fig. 6.
Ccl2 was upregulated by PA and siTug1. a Venn diagram of our RNA-seq data and data from testes of tug1−/− and tug1rescue mice. RNA-seq meant the RNA-seq performed by our lab (Control vs PA). b Heatmap of the overlapped genes. c FPKM value of 4 upregulated and 4 downregulated genes most related to tight junctions in our RNA-seq data. d Fold change of RNA-seq of 4 upregulated and 4 downregulated genes most related to tight junctions in our RNA-seq data. e The mRNA levels of 4 upregulated genes in HFD mice testes. f The protein levels of Ccl2 in HFD mice testes. g The gray value of the western blot. h The mRNA levels of 4 upregulated genes in Sertoli cells after transfection of siTug1. i The protein levels of Ccl2 in Sertoli cells after transfection of siTug1. j The gray value of the western blot. Each experiment is replicated at least three times independently. *p < 0.05
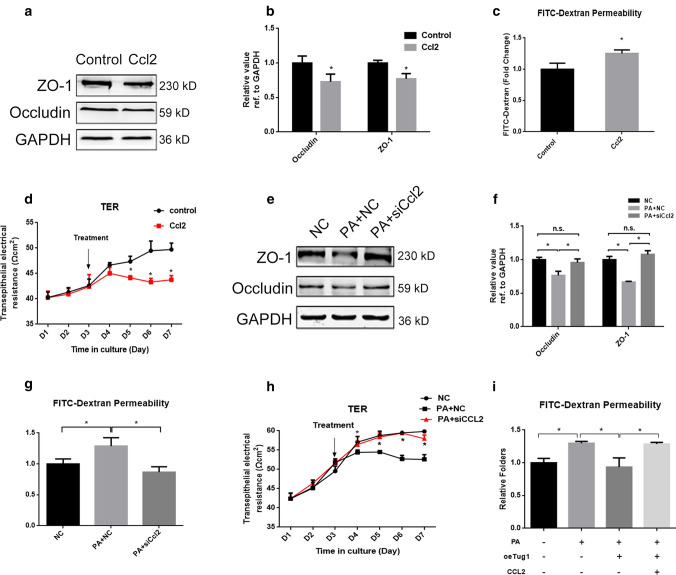
Ccl2 was demonstrated to regulate the expression of TJ-associated proteins in brain epithelial cells and affect blood–brain barrier permeability [31] and its expression was upregulated in IL-17A-induced BTB disruption [37], which suggested that it could participate in the regulation of BTB. To verify whether this molecule can regulate TJs in the BTB, we added Ccl2 cytokines to Sertoli cells. TJ-related protein levels were significantly decreased (Fig. 7a and b), and the permeability of FITC–dextran was significantly increased after the addition of Ccl2 (Fig. 7c). Moreover, TER was reduced in response to Ccl2 (Fig. 7d). These results indicated that Ccl2 could damage the integrity of BTB. Furthermore, we knocked down Ccl2 expression by transfection of siCcl2 and found that knockdown of Ccl2 rescued the PA-induced decrease in TJ-related proteins and TER compared with that of the negative controls and reduced the permeability of FITC–dextran in the presence of PA (Fig. 7e–h). To investigate whether Ccl2 is involved in the TJ damage induced by Tug1 depletion and PA, we performed rescue experiments and the results showed that the permeability of FITC–dextran was increased by Ccl2 addition compared with that of the PA + Tug1 overexpression group (Fig. 7i). The results indicated that Tug1 depletion induced BTB disruption in a Ccl2-dependent manner.
Fig. 7.
Ccl2 was involved in PA-induced TJ disruption. a, b The expression levels and gray value of TJ-related proteins after addition of Ccl2. c The permeability of FITC–dextran after addition of Ccl2. d The TER of Sertoli cells after addition of Ccl2. e, f The expression levels and gray value of TJ-related proteins after knockdown of Ccl2 in the presence of PA. g The permeability of FITC–dextran after knockdown of Ccl2 in the presence of PA. h The TER of Sertoli cells after knockdown of Ccl2 in the presence of PA. i The permeability of FITC–dextran in groups as shown. Each experiment is replicated at least three times independently. *p < 0.05
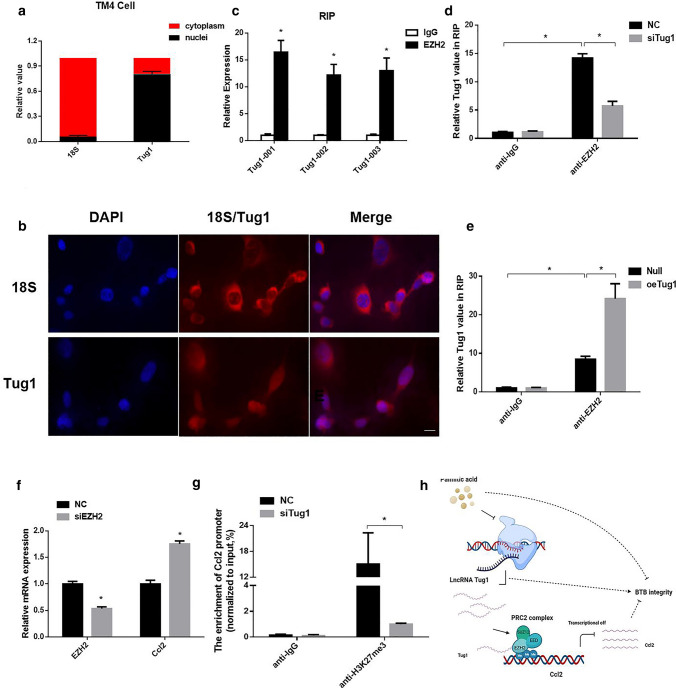
Tug1 epigenetically silenced Ccl2 transcription by interacting with EZH2
The cellular localization of lncRNAs can reflect their specific functions and mechanisms of action to some extent. Therefore, we performed nucleocytoplasmic separation experiments and FISH to investigate the cellular localization of Tug1. Tug1 was distributed in both the nucleus and cytoplasm (Fig. 8a, b). Tug1 was reported to bind to polycomb-repressive complex 2 (PRC2), which catalyzes the mono-, di-, and trimethylation of lysine 27 on histone H3 (H3K27) and epigenetically regulates the expression of downstream target genes [38]. EZH2 is an important catalytic subunit in the PRC2 complex that has methyltransferase activity [39]. Therefore, we conducted RIP experiments and found that Tug1 could directly bind to EZH2 in Sertoli cells (Fig. 8c), and this binding was reduced after knockdown of Tug1 expression and increased after overexpression of Tug1 (Fig. 8d, e). These data confirmed the direct interaction between Tug1 and EZH2. Based on the above results, we inhibited EZH2 with specific siRNAs and found that the expression of Ccl2 was significantly upregulated (Fig. 8f). Hence, we conducted ChIP experiments and found that that knockdown of Tug1 decreased the H3K27me3 levels across the Ccl2 promoter (Fig. 8g). Taken together, these data suggested that Tug1 could recruit EZH2 to epigenetically silence the expression of Ccl2, thereby regulating TJ function and affecting the BTB (Fig. 8h).
Fig. 8.
Tug1 can recruit EZH2 to silence Ccl2 expression. a The relative expression of Tug1 in the cytoplasm and nuclear of TM4 cells, 18S was used as cytoplasmic control. b FISH of Tug1 in TM4 cells, 18S was used as cytoplasmic control. Scale bar = 20 μm. c RIP experiments were performed in TM4 cells. The fold enrichment of Tug1 in anti-EZH2 RIP is relative to its matching anti-IgG control. d–e RIP experiments were performed in TM4 cells after transfection of siTug1 and oeTug1 plasmids. f The expression of Ccl2 in TM4 cells after transfection of siEZH2. g ChIP-qPCR of H3K27me3 binding in the promoters of Ccl2 in TM4 cells treated with nc or siTug1; IgG as a negative control. h Figure abstract of our study (which is drawn in www.biorender.com). Each experiment is replicated independently three times. *p < 0.05
Discussion
In recent years, the population with obesity has been increasing worldwide, and its negative impact on male fertility has become a common concern globally. In this study, we confirmed that HFD impaired BTB and semen quality and led to the local accumulation of saturated fatty acids, especially PA in the testis, which ultimately decreased the expression of Tug1 in Sertoli cells. Moreover, functional experiments suggested that depletion of Tug1 epigenetically regulated Ccl2 through binding to EZH2 and disrupted TJs and BTB integrity.
The semen parameters of the HFD group decreased and BTB integrity of the HFD group was impaired (Fig. 1), which were consistent with the results of previous studies in C57BL/6 mice [40]. Our previous study showed that HFD led to an increase in FFAs in the testis [30] and saturated fatty acid accounted for the majority of FFAs in the testis [22], but the specific type is unknown. In this study, we analyzed the medium and long-chain fatty acids of the testes and found that the proportion of saturated fatty acids was significantly increased and the PA level was the highest (Fig. 2). These results suggest that the damage of HFD may not only act on the whole body through the circulatory system, but also directly lead to the increase of local saturated fatty acids, especially palmitic acid content. These findings provided us with a rationale for further treatment of cells with PA to mimic lipotoxicity in vivo.
We treated mouse Sertoli cells with PA and performed whole transcriptome sequencing to identify possible lncRNAs involved in PA-induced impairment of BTB function (Figs. 3, 4). The expression of Tug1 in Sertoli cells was downregulated by PA both in vivo and in vitro. Moreover, tug1 knockout male mice presented a completely infertile phenotype and the fatty acid metabolic pathway was altered after tug1 knockout, which suggested that Tug1 was associated with fatty acid metabolism [24] and may be involved in HFD-induced damage to male fertility. LncRNAs were involved in a variety of biological events. At present, a large number of abundantly expressed lncRNAs have been found in Sertoli cells, but we have very limited knowledge of them. Therefore, our present study focused on the function of lncRNAs in Sertoli cells.
The BTB is a very important biological barrier that supports spermatogenesis and maintains the immune privileged environment, which contains TJs formed by adjacent Sertoli cells [41]. The results of functional experiments revealed that knockdown of Tug1 damaged the integrity of TJs, while overexpression of Tug1 could mitigate PA-induced TJ damage (Fig. 5). Thus, Tug1 can be involved in the process of PA-induced BTB damage.
Ccl2 was found to be regulated by Tug1 via bioinformatics analysis. Ccl2 has been reported to disrupt TJs in the nervous system [42]. It could induce the degradation or internalization of TJ-related proteins (ZO-1, occludin and claudin-5) through the Rho/PKCα signaling pathway and promote the "opening" of the blood–brain barrier [31]. The addition of Ccl2 induced a decrease of ZO-1 and occludin levels and enhanced blood–brain barrier permeability in vitro [43]. Stefania and his colleagues found that endogenous Ccl2 can induce enhanced blood–spinal cord barrier permeability during peripheral nerve injury [44]. In addition, it has been confirmed that in diabetic retinopathy, Ccl2 can lead to impaired blood–retinal barrier function via using ccl2 −/− knockout mice [45]. During IL17A-mediated disruption of BTB integrity, the expression of Ccl2 in the testes increased significantly [37], suggesting that there may be an association between Ccl2 and BTB function. Ccl2 was elevated in the testes of HFD group. Knockdown of Tug1, similar to the addition of PA, resulted in an increase in Ccl2 expression (Fig. 6). The addition of Ccl2 could induce a disruption of TJs and knockdown of Ccl2 alleviated PA-induced BTB injury (Fig. 7). All these data suggested that Tug1 depletion induced BTB disruption in a Ccl2-dependent manner.
The specific subcellular localization of lncRNAs could reflect their specific mechanism in biological events. Tug1 was localized in the cytoplasm and nucleus, suggesting that it may regulate the expression of Ccl2 at the transcriptional level. Tug1 epigenetically inhibited RND3 by binding to EZH2, thereby affecting trophoblast proliferation [46]. In non-small cell lung cancer, Tug1 could regulate LIMK2b by binding EZH2, one of the elements of PRC2, and affecting the cell cycle and chemoresistance [47]. EZH2 is an important catalytic subunit in the PRC2 complex that has methyltransferase activity, can catalyze H3K27me3 formation, and plays an important role in the initial stage of transcriptional repression [48]. Therefore, we hypothesized that EZH2 might be a key linker protein between Tug1 and Ccl2. To validate our hypothesis, we confirmed direct binding of Tug1 and EZH2 using RIP assay, and ChIP assays demonstrated that knockdown of Tug1 could reduce the binding of H3K27me3 levels on Ccl2 promoter and enhance the transcription of Ccl2 (Fig. 8). These results indicated that Tug1 was essential for TJs of Sertoli cells and epigenetically regulated Ccl2 via EZH2.
Complex endocrine system may be perturbed by many factors, and we paid more attention to the effects of FSH in the present study. However, testosterone is also essential for maintaining the integrity of the blood–testes barrier, thereby ensuring progression of spermatogenesis [49]. A systematic investigation is necessary to figure out whether testosterone supplementation could restore BTB function in the HFD mice. Meanwhile, due to the difficulty of obtaining human Sertoli cells, our study is limited to animal and cellular experiments and the role of Tug1 in human Sertoli cells needs to be further studied. Moreover, knockout and knock-in tug1 mice are required to be constructed by further experiments to determine the role of the Tug1/EZH2/Ccl2 axis in the impairment of BTB integrity in vivo.
In conclusion, we addressed the important role of the Tug1/EZH2/Ccl2 axis in PA-induced impairment of Sertoli cell function and male infertility in the present study. Our study offered essential clues for a better understanding of the role of Tug1 in the BTB integrity formed by Sertoli cells and provided a fresh view to comprehend the role of lncRNAs in male infertility.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Fig. S1 HFD impaired BTB integrity (a) The simple schematic diagram of animal experiment design in this study. (b-c) The immunofluorescence staining of BTB-related genes in the CD and HFD group. Scale bar = 50 μm. * p < 0.05 (TIF 2902 KB)
Supplementary file2 Fig. S2 The immunofluorescence staining of FSHR in primary Sertoli cells (TIF 1083 KB)
Supplementary file3 Fig. S3 FSH supplementation did not rescue the sperm quality and BTB integrity in HFD group (n>5) (a) FSH level in control group, HFD group with or without FSH supplementation. (b-c) Sperm concentration and motility of control group, HFD group with or without FSH supplementation. (d) FITC tracing assay of control group, HFD group with or without FSH supplementation. FITC green fluorescence was seen in the lumen of HFD group and HFD group with FSH supplementation. * p < 0.05 (TIF 1011 KB)
Supplementary file4 Fig. S4 GO and KEGG analysis of different expression genes of the testes of tug1-/- and wild type (WT) mice (a) GO terms of different expression genes of the testes of tug1-/- and WT mice. (b) The top 20 KEGG pathways of differentially expression genes. The right panel showed genes involved in tight junction pathway. (TIF 4240 KB)
Acknowledgements
We thank the other members of Dr. Yao’s laboratory for their discussion and help.
Author contributions
S.W., Z.Q., C.L., L.Z. and K.L. carried out the experiments. R.M., X.G. and J.J. were involved in planning and supervising the work. M.X., L.O. and Y.Z. helped with the animal experiments. S.C., Y.C. and Y.Y. analyzed the data and designed the figures. S.W and Z.Q. wrote the manuscript with support from J.M. and B.Y.
Funding
This work was supported by the National Key Research and Development Program of China (grant no. 2018YFC1004700), the National Natural Science Foundation of China (grant no. 81971373, 82001618, 81901547), the Natural Science Foundation of Jiangsu Province (BK20190252), and the 333 High-level Personnel Training Project of Jiangsu Province (grant no. BRA2019109).
Availability of data and material
All data generated or analysis during this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All animal experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Ethics Committee of Nanjing Jinling Hospital.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuxian Wang and Zhang Qian contributed equally to this work.
Contributor Information
Jinzhao Ma, Email: majinzhao07@163.com.
Bing Yao, Email: yaobing@nju.edu.cn.
References
- 1.Craig JR, et al. Obesity, male infertility, and the sperm epigenome. Fertil Steril. 2017;107(4):848–859. doi: 10.1016/j.fertnstert.2017.02.115. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg ML, et al. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod. 2014;29(2):193–200. doi: 10.1093/humrep/det428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imterat M, et al. Impact of Body Mass Index on female fertility and ART outcomes. Panminerva Med. 2019;61(1):58–67. doi: 10.23736/s0031-0808.18.03490-0. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Ding Z. Obesity, a serious etiologic factor for male subfertility in modern society. Reproduction. 2017;154(4):R123–r131. doi: 10.1530/rep-17-0161. [DOI] [PubMed] [Google Scholar]
- 5.Attaman JA, et al. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod. 2012;27(5):1466–1474. doi: 10.1093/humrep/des065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 2017;23(4):371–389. doi: 10.1093/humupd/dmx006. [DOI] [PubMed] [Google Scholar]
- 7.Eslamian G, et al. Dietary fatty acid intakes and asthenozoospermia: a case-control study. Fertil Steril. 2015;103(1):190–198. doi: 10.1016/j.fertnstert.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Lu JC, et al. Analysis of human sperm DNA fragmentation index (DFI) related factors: a report of 1010 subfertile men in China. Reprod Biol Endocrinol. 2018;16(1):23. doi: 10.1186/s12958-018-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao L, et al. Single-cell analysis of developing and azoospermia human testicles reveals central role of Sertoli cells. Nat Commun. 2020;11(1):5683. doi: 10.1038/s41467-020-19414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo D, et al. High fat diet impairs spermatogenesis by regulating glucose and lipid metabolism in Sertoli cells. Life Sci. 2020;257:118028. doi: 10.1016/j.lfs.2020.118028. [DOI] [PubMed] [Google Scholar]
- 11.Mruk DD, Cheng CY. The mammalian blood–testis barrier: its biology and regulation. Endocr Rev. 2015;36(5):564–591. doi: 10.1210/er.2014-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang XY, et al. Proteomics analysis of testis of rats fed a high-fat diet. Cell Physiol Biochem. 2018;47(1):378–389. doi: 10.1159/000489918. [DOI] [PubMed] [Google Scholar]
- 13.Lander ES, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 14.Ma L, et al. LncBook: a curated knowledgebase of human long non-coding RNAs. Nucleic Acids Res. 2019;47(D1):D128–d134. doi: 10.1093/nar/gky960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao XY, et al. Long noncoding RNA licensing of obesity-linked hepatic lipogenesis and NAFLD pathogenesis. Nat Commun. 2018;9(1):2986. doi: 10.1038/s41467-018-05383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Goede OM, et al. Population-scale tissue transcriptomics maps long non-coding RNAs to complex disease. Cell. 2021;184(10):2633–2648.e19. doi: 10.1016/j.cell.2021.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young TL, Matsuda T, Cepko CL. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr Biol. 2005;15(6):501–512. doi: 10.1016/j.cub.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, et al. LncRNA TUG1 promoted KIAA1199 expression via miR-600 to accelerate cell metastasis and epithelial-mesenchymal transition in colorectal cancer. J Exp Clin Cancer Res. 2018;37(1):106. doi: 10.1186/s13046-018-0771-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Lin YH, et al. Taurine up-regulated gene 1 functions as a master regulator to coordinate glycolysis and metastasis in hepatocellular carcinoma. Hepatology. 2018;67(1):188–203. doi: 10.1002/hep.29462. [DOI] [PubMed] [Google Scholar]
- 20.Long J, et al. Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J Clin Invest. 2016;126(11):4205–4218. doi: 10.1172/jci87927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, et al. LncRNA TUG1 reduces inflammation and enhances insulin sensitivity in white adipose tissue by regulating miR-204/SIRT1 axis in obesity mice. Mol Cell Biochem. 2020;475(1–2):171–183. doi: 10.1007/s11010-020-03869-6. [DOI] [PubMed] [Google Scholar]
- 22.Crisóstomo L, et al. Diet during early life defines testicular lipid content and sperm quality in adulthood. Am J Physiol Endocrinol Metab. 2020;319(6):E1061–e1073. doi: 10.1152/ajpendo.00235.2020. [DOI] [PubMed] [Google Scholar]
- 23.Crisóstomo L, et al. A switch from high-fat to normal diet does not restore sperm quality but prevents metabolic syndrome. Reproduction. 2019;158(4):377–387. doi: 10.1530/rep-19-0259. [DOI] [PubMed] [Google Scholar]
- 24.Lewandowski JP, et al. The Tug1 lncRNA locus is essential for male fertility. Genome Biol. 2020;21(1):237. doi: 10.1186/s13059-020-02081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai H, et al. The long noncoding RNA TUG1 regulates blood–tumor barrier permeability by targeting miR-144. Oncotarget. 2015;6(23):19759–19779. doi: 10.18632/oncotarget.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gril B, et al. Reactive astrocytic S1P3 signaling modulates the blood–tumor barrier in brain metastases. Nat Commun. 2018;9(1):2705. doi: 10.1038/s41467-018-05030-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, et al. High fat diet induced obesity model using four strains of mice: kunming, C57BL/6, BALB/c and ICR. Exp Anim. 2020;69(3):326–335. doi: 10.1538/expanim.19-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, et al. Microcystin-leucine-arginine causes blood–testis barrier disruption and degradation of occludin mediated by matrix metalloproteinase-8. Cell Mol Life Sci. 2018;75(6):1117–1132. doi: 10.1007/s00018-017-2687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu D, et al. Melatonin protects mouse testes from palmitic acid-induced lipotoxicity by attenuating oxidative stress and DNA damage in a SIRT1-dependent manner. J Pineal Res. 2020;69(4):e12690. doi: 10.1111/jpi.12690. [DOI] [PubMed] [Google Scholar]
- 30.Hu X, et al. Effects of saturated palmitic acid and omega-3 polyunsaturated fatty acids on Sertoli cell apoptosis. Syst Biol Reprod Med. 2018;64(5):368–380. doi: 10.1080/19396368.2018.1471554. [DOI] [PubMed] [Google Scholar]
- 31.Stamatovic SM, et al. Protein kinase Calpha-RhoA cross-talk in CCL2-induced alterations in brain endothelial permeability. J Biol Chem. 2006;281(13):8379–8388. doi: 10.1074/jbc.M513122200. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, et al. Omega-3 polyunsaturated fatty acids alleviate hydrogen sulfide-induced blood–testis barrier disruption in the testes of adult mice. Reprod Toxicol. 2020;98:233–241. doi: 10.1016/j.reprotox.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Sun X, et al. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017;24(5):819–831. doi: 10.1038/cdd.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarulli GA, et al. Regulation of testicular tight junctions by gonadotrophins in the adult Djungarian hamster in vivo. Reproduction. 2008;135(6):867–877. doi: 10.1530/rep-07-0572. [DOI] [PubMed] [Google Scholar]
- 35.Ma B, et al. Luteolin ameliorates testis injury and blood–testis barrier disruption through the Nrf2 signaling pathway and by upregulating Cx43. Mol Nutr Food Res. 2019;63(10):e1800843. doi: 10.1002/mnfr.201800843. [DOI] [PubMed] [Google Scholar]
- 36.Joshi M, Rajender S. Long non-coding RNAs (lncRNAs) in spermatogenesis and male infertility. Reprod Biol Endocrinol. 2020;18(1):103. doi: 10.1186/s12958-020-00660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pérez CV, et al. IL17A impairs blood–testis barrier integrity and induces testicular inflammation. Cell Tissue Res. 2014;358(3):885–898. doi: 10.1007/s00441-014-1995-5. [DOI] [PubMed] [Google Scholar]
- 38.Huang MD, et al. Long non-coding RNA TUG1 is up-regulated in hepatocellular carcinoma and promotes cell growth and apoptosis by epigenetically silencing of KLF2. Mol Cancer. 2015;14:165. doi: 10.1186/s12943-015-0431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020;13(1):104. doi: 10.1186/s13045-020-00937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan Y, et al. Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood–testis barrier. PLoS ONE. 2015;10(4):e0120775. doi: 10.1371/journal.pone.0120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng CY, Mruk DD. The blood–testis barrier and its implications for male contraception. Pharmacol Rev. 2012;64(1):16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keaney J, Campbell M. The dynamic blood–brain barrier. FEBS J. 2015;282(21):4067–4079. doi: 10.1111/febs.13412. [DOI] [PubMed] [Google Scholar]
- 43.Song L, Ge S, Pachter JS. Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood. 2007;109(4):1515–1523. doi: 10.1182/blood-2006-07-034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Echeverry S, et al. Peripheral nerve injury alters blood–spinal cord barrier functional and molecular integrity through a selective inflammatory pathway. J Neurosci. 2011;31(30):10819–10828. doi: 10.1523/jneurosci.1642-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rangasamy S, et al. Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood–retinal barrier in diabetic retinopathy. PLoS ONE. 2014;9(10):e108508. doi: 10.1371/journal.pone.0108508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Y, et al. The lncRNA TUG1 modulates proliferation in trophoblast cells via epigenetic suppression of RND3. Cell Death Dis. 2017;8(10):e3104. doi: 10.1038/cddis.2017.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niu Y, et al. Long non-coding RNA TUG1 is involved in cell growth and chemoresistance of small cell lung cancer by regulating LIMK2b via EZH2. Mol Cancer. 2017;16(1):5. doi: 10.1186/s12943-016-0575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laugesen A, Højfeldt JW, Helin K. Molecular mechanisms directing PRC2 recruitment and H3K27 methylation. Mol Cell. 2019;74(1):8–18. doi: 10.1016/j.molcel.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. 2014;30:2–13. doi: 10.1016/j.semcdb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Fig. S1 HFD impaired BTB integrity (a) The simple schematic diagram of animal experiment design in this study. (b-c) The immunofluorescence staining of BTB-related genes in the CD and HFD group. Scale bar = 50 μm. * p < 0.05 (TIF 2902 KB)
Supplementary file2 Fig. S2 The immunofluorescence staining of FSHR in primary Sertoli cells (TIF 1083 KB)
Supplementary file3 Fig. S3 FSH supplementation did not rescue the sperm quality and BTB integrity in HFD group (n>5) (a) FSH level in control group, HFD group with or without FSH supplementation. (b-c) Sperm concentration and motility of control group, HFD group with or without FSH supplementation. (d) FITC tracing assay of control group, HFD group with or without FSH supplementation. FITC green fluorescence was seen in the lumen of HFD group and HFD group with FSH supplementation. * p < 0.05 (TIF 1011 KB)
Supplementary file4 Fig. S4 GO and KEGG analysis of different expression genes of the testes of tug1-/- and wild type (WT) mice (a) GO terms of different expression genes of the testes of tug1-/- and WT mice. (b) The top 20 KEGG pathways of differentially expression genes. The right panel showed genes involved in tight junction pathway. (TIF 4240 KB)
Data Availability Statement
All data generated or analysis during this study are available from the corresponding author upon reasonable request.
Not applicable.