Abstract
Maternal cellular and humoral immune responses to the allogeneic fetoplacental unit are a normal part of pregnancy adaptation. Overactive or dysregulated immune responses that often manifest as inflammation are considered a key element for the development of preeclampsia. Infiltration and activation of macrophages, nature killer cells, and T lymphocytes are frequently observed in the decidua and placenta associated with preeclampsia. In addition to local inflammation, systemic inflammatory changes including increased levels of TNF-α and interleukins (ILs) are detected in the maternal circulation. Syncytin-1 is an endogenous retroviral envelope protein that mediates the fusion of trophoblasts to form syncytiotrophoblasts, a cellular component carrying out most of placental barrier, exchange, and endocrine functions. In addition to these well-defined fusogenic functions that are known for their close association with preeclampsia, multiple studies indicated that syncytin-1 possesses nonfusogenic activities such as those for cell cycle and apoptosis regulation. Moreover, syncytin-1 expressed by trophoblasts and various types of immune cells may participate in regulation of inflammation in preeclamptic placenta and decidua. This review concentrates on the triangular relationship among inflammation, syncytin-1 nonfusogenic functions, and preeclampsia pathogenesis. Data regarding the reciprocal modulations of inflammation and poor vascularization/hypoxia are summarized. The impacts of syncytin-A (the mouse counterpart of human syncytin-1) gene knockout on placental vascularization and their implications for preeclampsia are discussed. Syncytin-1 expression in immune cells and its significance for inflammation are analyzed in the context of preeclampsia development. Finally, the involvements of syncytin-1 nonfusogenic activities in neuroinflammation and multiple sclerosis are compared to findings from preeclampsia.
Keywords: Preeclampsia, Syncytin-1, HERV-W, Nonfusogenic activity, Inflammation, Immune response
Introduction
Inflammation, often referring to the excessive responses of innate and acquired immune systems to external stimulation [1], is extensively involved in diverse physio-pathological processes. Inflammation is often detected as quantitative and qualitative alterations of cellular and humoral immunity, e.g., changes in the number or distribution of granulocytes, macrophages and lymphocytes, and changes in the levels of inflammatory cytokines such as TNF-α, interleukins, and antibodies in placental tissues and/or circulation. In pregnancy, certain inflammatory responses are considered a normal part of embedding, placentation and vascularization, immune tolerance, and initiation of delivery [2]. For example, macrophages and natural killer (NK) cells participate in the regulation of trophoblastic invasion, angiogenesis, and spiral artery remodeling [3, 4]. However, excessive inflammation contributes to the pathogenesis of major diseases of pregnancy including preeclampsia (PE), intrauterine growth retardation, infection of placenta-embryo unit, gestational diabetes mellitus, and preterm labor [5–7]. Inflammation can also lead to adverse birth outcomes, perinatal diseases, and could be one of the developmental origins of health and disease (DOHaD) in adulthood [8]. It should be pointed out that during pregnancy, the implantation of the semi-allogeneic embryo-placenta unit, the rapid structural and functional changes in endocrine systems and reproductive tract, and the whole body metabolic adjustments, all occurring in a relative short gestation time, can significantly increase the risk of inflammatory attack.
Preeclampsia is a common hypertensive disease affecting approximately 3–8% pregnancies [9]. With hypertension as the most significant manifestation, if not properly treated, early (before 34 weeks of gestation) or late (at or after 34 weeks of gestation) onset PE can cause stroke, renal and liver failures, neurological disorders, hematological complications, and fetoplacental disorders [10]. Genetic backgrounds and specific gene mutations, malnutrition, unhealthy life styles, hazardous living environments, and poor social-economic status are known etiological risk factors of PE. From the pathological point of view, placental abnormalities are considered a major factor for the development of PE. Restricted blood flow of umbrella vessels, repeated cycles of ischemia and reperfusion, angiogenesis deficiency, and inflammation are well-recognized pathological changes in the fetoplacental unit associated with PE, with inflammation at a center position. Local infiltration and activation of macrophages and granulocytes are readily detected in the decidua and placenta of PE patients [11, 12]. On the maternal side, systemic activation of immune cells and imbalanced differentiation of T-helper cells, and elevated levels of cytokine TNF-α and IL-1β are observed [13]. Indeed, anti-inflammation treatment is effective for the control of blood pressure in PE patients and animal models [14].
Syncytium, a complete layer of syncytiotrophoblasts connected by tight junctions, serves as the fetal–maternal barrier. Syncytiotrophoblasts also carry out fetal–maternal transfer and produce large amounts of steroid and peptide hormones. The multinuclear syncytiotrophoblasts are formed by fusion of mononuclear cytotrophoblasts. Syncytin-1 is the first functional retroviral envelope protein found to bear a defined physiological function. Syncytin-1 molecules on the membranes of cytotrophoblasts mediate cell fusion through binding to their receptors on the membranes of surrounding cytotrophoblasts, to accomplish cell-cell fusion as the terminal differentiation of the trophoblast linage [15]. Findings by numerous in vitro and in vivo studies support that the decreased syncytin expression and the consequential trophoblast fusion deficiency may contribute to placental anomaly and PE pathogenesis. It is noteworthy that the naturally occurring single nucleotide polymorphisms of syncytin-2 gene have been identified in severe PE patients. In vitro studies indicated that these genetic mutations affected the N-glycosylation and fusogenic activity of the gene product [16]. Interestingly, several studies suggested that in addition to the fusogenic activity, syncytin-1 also possesses nonfusogenic activities by regulating the trophoblast proliferation and apoptosis [17, 18]. Also, more and more data indicated a broad expression spectrum of syncytin-1 gene in immune cells such as granulocytes, T lymphocytes, monocytes, glial cell of the brain, and cancer cells. In addition to PE, synctyin-1 has been implicated in the pathogenesis of neuropsychological disorders, angiogenesis, infection, and various malignancies [19]. This review summarizes research findings on the relationship between syncytin-1 regulation/function and PE, with a special attention to the role(s) of syncytin-1 in inflammation. While the detailed molecular mechanisms of these events are often unclear, the possible cellular and molecular pathways are discussed.
Brief overview of syncytin-1 structure and functions
Syncytin-1 protein is encoded by the HERV-W-1 gene located on chromosome 7q21.2. The 73 kDa syncytin-1 is synthesized as a glycosylated peptide containing 538 amino acids. The surface (SU) domain (aa 21–317) and the transmembrane (TM) domain (aa 318–538) are linked by disulfide bonds [20, 21]. The SU and TM domains contain 6 and 1 putative glycosylation sites, respectively, and are N-glycosylated. These precursors form homotrimers through the TM N- and C-terminal heptad repeats, and are cleaved by the furine protease at a consensus cleavage sit (RNKP) to produce the mature gp50 SU domain and gp24 TM domain [20, 22]. The TM domain harbors 3 subdomains of the ectodomain (aa 318–444), the transmembrane domain (aa 445–469), and the cytoplasmic domain (aa 470–538) [23]. The SU N-terminal receptor binding subdomain interacts with the type D mammalian retrovirus receptor ASCT-1/ASCT-2 (sodium-dependent neutral amino acid transporter type 1/2) on cell membranes. Studies suggested that the TM cytoplasmic subdomain is also essential for the fusogenic activity [24–26]. Bjerregaard et al. demonstrated that human breast cancer cells expressing syncytin-1 were capable of fusing with endothelial cells, and the expression level of syncytin-1 was considered a prognostic marker of breast cancer patients [27]. Besides mediating the fusion of trophoblasts, Fei et al. showed that a 58 kDa form of syncytin-1 promoted the formation of polyploidy giant cancer cells through cell fusion [28]. It is unclear if the discrepancy in the molecular size of syncytin-1 (73 verses 58 kDa) may be due to differential glycosylation or alternative cleavage. It is noteworthy that syncytin-2 encoded by the HERV-FRD gene is also expressed in trophoblasts and capable of mediating cell fusion by interacting with a different receptor MFSD2 (major facilitator superfamily domain containing 2). Structural comparison showed that syncytin-2 protein shares a high similarity to syncytin-1 [29], e.g., both contain the TM and SU domains composed of the same subdomains. Since syncytin-1 represents a prototype that has been much better studied than syncytin-2, it will be the focus of this review.
In addition to the well-recognized fusogenic activity, syncytin-1 carries nonfusogenic activities that are involved in the regulation of trophoblast differentiation, proliferation, and apoptosis. Frendo et al. observed that in the primary culture of placental trophoblasts, the cell differentiation and hCG (human chorionic gonadotropin) production was enhanced by treatment with cyclic AMP, and this enhancement was associated with a concomitant upregulation of syncytin-1 expression. In contrast, knockdown of syncytin-1 expression using the syncytin-1-specific antisense oligonucleotides led to an inhibition of trophoblast differentiation as well as a five-fold reduction of hCG level in culture medium [30]. Huang et al. reported that a forced overexpression of syncytin-1 promoted the proliferation of BeWo, a choriocarcinoma cell line that preserves some trophoblastic characters such as the expression of human chorionic somatomammotropin (hCS), hCG, and beta-hydroxysteroid dehydrogenases [18]. Moreover, knockdown of syncytin-1 levels with a specific siRNA resulted in an inhibition of cell growth. Mechanistic studies showed that syncytin-1 deficiency impeded the G1/S transition and inhibited the DNA synthesis. This cell-cycle change was accompanied by an elevated mRNA level of cell-cycle inhibitor p15, and decreased mRNA levels of positive regulators E2F1, PCNA, and c-Myc. Importantly, the forced ectopic expression of syncytin-1 in CHO, a Chinese hamster ovary cell line, promoted the G1-S transition as demonstrated by correspondent changes in the above cell-cycle regulators too. Since CHO cells are deficient of ASCT2 expression and incapable of cell–cell fusion, this observation provided a direct evidence for the existence of a fusion-independent, nonfusogenic activity of syncytin-1. In another study by the same group, the siRNA-mediated knockdown of syncytin-1 expression led to an increased apoptosis of BeWo cells through activation of the non-classic, apoptosis inducing factor (AIF)-mediated pathway [17]. Syncytin-1 knockdown caused an increased expression of AIF and the AIF cleavage enzyme calpain1, resulting in an increase of AIF cleavage products as well as their nuclear translocation, and eventually, more apoptosis events. Closely related to the above findings, there is evidence in support of syncytin-1 modulation of immune cell functions. As discussed later in more details, using a co-culture experimental system, Jonas et al. demonstrated that overexpression of syncytin-1 in choriocarcinoma cells led to a suppressed proliferation of T lymphocytes. These findings bear a high significance because they not only confirmed the syncytin-1-mediated nonfusogenic activity, but also revealed a possibility that syncytin-1 produced by placental trophoblasts could deliver an immunosuppressive effect in T lymphocytes, which could be implicated in the normal as well as PE-complicated pregnancies.
Since ASCT2 is broadly expressed in various cell types, the cell fusion event is largely restricted by the expression of syncytin-1 rather than the presence of its receptor. While syncytin-1 expression is readily detected in human placental trophoblasts as early as first trimester, higher levels of syncytin-1 mRNA and protein are found in the third trimester. Syncytin-1 gene is subject to the regulation of GCM1 (glial cells missing 1), cAMP, and the DNA methylation status of a CpG island located at the 5′ prime region. It is noteworthy that numerous studies have shown that syncytin-1 expression is significantly decreased in placentas of PE patients compared to placentas from normal pregnancy [31, 32]. It was reported that the decreased syncytin-1 expression in preeclamptic placentas was associated with syncytin-1 gene hypermethylation [33]. In a study on placentas of dichorionic twins with discordant fetal growth, significantly higher syncytin-1 mRNA levels were observed in placentas associated with smaller fetuses. Moreover, an inverse correlation between the hypermethylation of syncytin-1 gene and mRNA levels of methyltransferases (DNMT1 and DNMT3) was observed. An altered expression of syncytin-1 is detected in many types of malignancies including endocrine tumors, breast cancer, endometrial cancer, leukemia, and pancreatic cancer. It was also reported that the aberrant expression of syncytin-1 in endometrial cancer tissues was related to the methylation status of the cognate CpG island [34]. While there are data indicating a deep involvement of syncytin-1 function in the modulation of immune cell functions, the mechanisms regarding synctin-1 gene activation in immune cells have not been explored.
The central position of inflammation in preeclampsia pathogenesis
Local and systemic inflammation in preeclampsia
The shallow invasion of placenta into the decidua and the repeated ischemia–reperfusion in fetoplacental unit are important pathological changes of PE. Inflammatory cytokines produced by various types of cells affect placental invasion, spiral artery remodeling and vascularization, and may contribute to hypoxia and excessive ROS, and eventually, PE occurrence [35, 36].
Fu et al. showed that decidual NK (dNK) cells, through their production of IFN-γ, were able to suppress the decidual accumulation of Th17 (T helper 17) cells. The declined number of NK cells and a concomitantly elevated number of decidual Th17 cells were associated with spontaneous abortion [37]. Wallace et al. reported that dNK cells from pregnancies with high PE risk were less active in promoting trophoblast motility, invasion, and chemotaxis than those from normal pregnancies. They also demonstrated that dNK cells-induced trophoblast chemotaxis and invasion was dependent on the ERK1/2 and Akt signaling, and dNK cells from the pregnancies with an impaired placental remodeling were less capable of activating this pathway [38].
Gill et al. demonstrated pro-inflammatory responses in the decidua of PE patients by the detection of an increased M1 subtype of macrophages. M1 macrophages were found on the decidua vessel walls in the decidua basalis with acute atherosis, a characteristic vascular change associated with PE [39]. Moreover, the levels of GM-CSF (granulocyte–macrophage colony stimulating factor) that drives macrophage differentiation, were increased in decidual cells as well as the plasma of PE patients compared with those of the gestational age-matched normal controls [40]. Treg (Regulatory T cell) is known to possess an immunosuppressive function. As part of innate immunity, Treg secrets anti-inflammatory factors such as IL-10 and TGF-β to limit decidual inflammation. Erlebacher et al. showed that the number of Treg was reduced at the fetal–maternal interface as well as in the circulation of PE patients [41, 42], which is consistent with an exaggerated local and systemic inflammation observed in PE patients.
Like what observed in the decidua, a preferential shift of macrophages to the M1 subtype, and an active secretion of TNF-α, IFN-γ, and IL-6, but a decreased secretion of IL-4 and IL-10, were detected in PE placenta [43, 44]. The supernatants of placental extracts from the first trimester were found to be more effective than those from the third trimester to induce the migration of NK cells through a layer of trophoblast cell culture. This difference was associated with an increased expression of CD18 in NK cells along placenta maturation [45]. An immunohistochemistry study revealed the enrichment of CD68+ monocytes and MPO+ neutrophils in the intervillous space of PE placentas. In further experiments, the PE-related local changes in monocytes and neutrophils were confirmed by correspondent alterations of the transcriptional signatures [46].
The association of systemic inflammation with PE was demonstrated by an elevated level of inflammatory cytokines in the circulation of PE patients [47, 48]. Comparing to the normal pregnancy, PE patients have increased serum levels of TNF-α, IL-6, IL-2, IL-8, IL-12, and MCP-1 (macrophage chemoattractant protein-1) [49, 50]. TNF-α is a common regulator of these inflammation effectors, its increased production by macrophages appears to play a central role for the initiation of inflammation. It was also reported that the excessive activation of macrophages and the shift of macrophages towards the M1 phenotype in the maternal circulation was related to adverse outcomes of PE pregnancy [51]. Further investigation is required to validate the significance and prevalence of this shift for the development of PE.
Inflammation contributes to preeclampsia development by affecting local and systemic vascular functions
Placenta undergoes a fast expansion and structural remolding, and active angiogenesis is required from the very beginning of implantation. The deficiency of uteroplacental blood flow is commonly detected in PE patients with the Doppler ultrasound. PE patients also exhibit systemic microvascular endothelial dysfunctions in pregnancy as well as in postpartum phase. These alterations are mediated in part by an increased sensitivity to angiotensin II [52]. Stanhewicz et al. reported that Ang 1–7, an endogenous inhibitor of angiotensin II, was able to attenuate the angiotensin II-mediated vessel constriction via NOS-mediated pathways, indicating that interference of the vascular function could be an effective approach for correcting postpartum symptoms of PE patients [53]. In another study by Kulandavelu et al. endothelial nitric oxide synthase (eNOS) gene knockout mice exhibited deficiencies in uterine blood flow, spiral artery elongation, and placental oxygenation [54]. Mehta et al. demonstrated that in sheep and guinea pig models, application of adenovirus vectors encoding the VEGF-A gene resulted in an increased uteroplacental blood flow and fetal growth [55]. Stepan et al. reported that a decreased VEGF and elevated sFlt1 expression negatively impacted the placental vascular development [56]. The expression of PLGF (placental growth factor), a pro-angiogenic factor of the VEGF family, was decreased in PE. Moreover, this reduction preceded the clinical syndromes of the disease. Indeed, the increased sFlt1/PLGF ratio in serum has been considered a marker for early diagnosis of PE [57].
The importance of inflammatory factors released by immune cells deserves a special attention in the context of vascular functions and PE. Murphy et al. reported that continuous intravenous infusion of a low dose of TNF-α to pregnant rats resulted in hypertension, reduction of renal blood flow and glomerular filtration rate, but an increase of circulating sFLT-1 [58]. In an independent study, Bobek et al. demonstrated that infusion of TNF-α to mice led to hypertension and proteinuria, which was accompanied by an upregulation of TLR-3/TLR-4 and HIF-1α (hypoxia-inducible factor-1α) in placenta [59]. Moreover, they found that the TNF-α-induced preeclamptic features could be reversed by a preemptive treatment with PLGF [60]. It is noteworthy that the decidual NK cells are capable of producing angiogenic factors including VEGF, PLGF, Ang-1, and Ang-2 to modulate the placental vascular development [61, 62]. Endothelin, a circulatory peptide released by endothelial cells, could activate the endothelin receptor type-A of vascular smooth muscle cells to induce vasoconstriction. Interestingly, ABT-627 (Atrasentan hydrochloride), an endothelin receptor type-A antagonist, was found to ease the TNF-α-induced hypertension by decreasing the uteroplacental perfusion pressure in pregnant rats [63], providing a therapeutic model for the treatment of PE. Alison et al. showed that depletion of Treg in mice resulted in increased serum levels of IL-17, MCP-1, and CXCL1 (C-X-C motif chemokine ligand 1). Moreover, in Treg-deficient mice, the uterine arterial pressure became more sensitive to NO, which was accompanied by an increased uterine artery resistance and enhanced production of endothelin-1 in dissected uterine arteries [64]. Thus, inflammatory cytokines, and NK and Treg cells may all contribute to normal placental development as well as PE pathogenesis through local and systemic regulation of vascular function.
Acute atherosis (AA), characterized by the presence of subendothelial lipid-laden foam cells, perivascular leukocyte infiltration and fibrinoid necrosis in the arteries of myometrium, decidual basal and placental basal plate, is closely associated with PE. Alnaes et al. reported that AA lesions were detected in 37% of PE patients, but only in 11% of healthy normotensive pregnant women [65]. Fosheim et al. found that AA was focal lesions with abnormal histological appearance of spiral artery endothelium and associated with the deficiency of endovascular trophoblast invasion [66]. AA shares some morphological similarities with atherosclerosis found in the coronary and other arteries, and both could be related to inflammatory stimulation of vascular endothelium.
Hypoxia aggravates inflammation in preeclampsia development
A large body of evidence from studies on placentas and other organs supports that hypoxia associated with poor vascularization can promote inflammatory responses. The fetoplacental unit normally has a relatively low oxygenation level and an upregulated HIF (hypoxia-inducible factor) expression [67]. Under the PE condition, abnormal vascularization and repeated ischemia–reperfusion, especially the acute surge of oxygen and iron in the reperfusion phase, causes oxidative damage of trophoblast cell membranes [68–70]. It is well established that oxidative stress and tissue damage can elicit inflammatory responses [71]. Hypoxia downregulates the histone demethylase, resulting in epigenetic dysregulation of the von Hippel Lindau tumor suppressor gene, a vital factor for oxygen sensing, and a dysregulation of HIF-1α signaling [72]. Transient upregulation of HIF-1α represents a normal response to hypoxia. However, repeated hypoxic insults and aberrant increase of HIF-1α are related to the placental vessel dysfunctions in PE, especially in early-onset PE [73, 74]. Fang et al. assessed the gene expression profiles in primary cultures of human and murine macrophages, and detected marked increases in the mRNA levels of IL-1β and IL-8, VEGF, CXCR4, and GLUT1 after exposure to hypoxia for 18 h. Macrophage activation by hypoxia also stimulated the expression and phosphorylation of NF-κB signaling proteins [75]. Imtiyaz et al. demonstrated that HIF-1α overexpression induced by oxygen-deprivation promoted neutrophil survival and blood vessel extravasation by modulating β2 integrin expression [76].
While the direct effects of hypoxia and the elevated HIF-1α on local inflammation are understudied in placenta, findings from other tissues concerning the pro-inflammatory effects by ischemia, unstable oxygenation, and increased ROS production could be applicable to preeclamptic placenta. It should be pointed out that inflammatory cell infiltration and increased metabolism during inflammation may aggravate the “relative” or absolute hypoxia in PE [77]. On the other hand, the pro-inflammatory cytokine TNF-α have been shown to increase the accumulation of HIF-1α through stabilization of HIF-1α protein [78]. Thus, hypoxia and inflammation may form a feedback loop to mutually enhance each other along PE development.
The triangular relationship among preeclampsia, inflammation, and syncytin-1
Role(s) of syncytin-1 for vascularization and hypoxia
It is well established that morphological changes in preeclamptic placentas are accompanied by a decreased syncytin-1 expression in placental trophoblasts [79]. Using the TM- and SU-specific antibodies, Holder et al. demonstrated these peptides’ perinuclear and punctate cytoplasmic deposits in placental trophoblasts and co-localization of the two in the BeWo cells with the use of immunostaining assay. Interestingly, the presence of syncytin-1 SU domain but not TM domain, was observed in the vascular endothelium of normal placentas. Moreover, an elevated SU immunoreactivity was detected at the vascular endothelium of PE placentas. Based on the absence of TM subunit, it was suggested that the vascular endothelium may not express the syncytin-1 protein by itself, rather, the detected SU subunit could be released from trophoblast into fetal circulation where it may bind to the vascular endothelium [80]. This observation raised a question if the extracellular syncytin-1 could affect the endothelium function, and be related to the vascularization failure observed in preeclamptic placentas. In this respect, studies using the syncytin-1 knockout mouse model provided key information. Dupressoir et al. generated the syncytin-A gene (the mouse counterpart of human syncytin-1 gene) knockout mice, and observed that the syncytin-A null embryos died between E11.5 and 13.5. Importantly, placental blood vessels from syncytin-A null mice were found to be fewer and narrower, and contained fewer embryonic erythrocytes than those from wild type mice [81]. These findings were confirmed by Qiao et al. in an independent study using the inducible knockout model. The tamoxifen-induced deletion of syncytin-A gene at E11.5 to E17.5 led to decreased size and thickness of placentas, low birth-weight fetuses, and marked vasculature abnormalities in the labyrinth, a specified structure of murine placentas for fetal–maternal exchange function. Following syncytin-A deletion, the mouse placentas were pale, with a reduced number and irregular distribution of fetal microvessels. Moreover, syncytin-A knockout led to an increased expression of sFlt-1 in the labyrinth and an elevated sFlt-1/PLGF ratio in the maternal plasma, a marker for vasculature abnormality in PE patients. While the detailed mechanism remains to be investigated, these findings suggest a fundamental role(s) of syncytin-A for placental vascularization [82].
Restricted blood flow to the fetoplacental unit and ischemia are characteristics of preeclamptic placentas. In vitro experiments have demonstrated significant effects of hypoxia in trophoblastic cell lines. Cultures of BeWo cells at low-oxygen atmosphere exhibited a decreased expression of syncytin-1 and ASCT2, and an inhibition of trophoblast syncytialization [83]. GCM1, a transcriptional activator of syncytin-1 gene, was concomitantly downregulated in preeclamptic placentas [84]. Chiang et al. showed that hypoxia could trigger GCM1 degradation by suppressing the phosphatidylinositol 3-kinase-Akt signaling pathway in choriocarcinoma BeWo and Jar cells. Moreover, it was suggested that the hypoxia-caused syncytin-1 downregulation could attenuate the syncytin-1-mediated immunosuppression and consequently, to enhance inflammation [85]. There are also data suggesting the presence of an alternative pathway by which hypoxia led to upregulation of HIF-1α and inhibition of syncytin-2, another retroviral envelop protein sharing structural and functional similarities with syncytin-1 [86]. Wang et al. reported that hypoxia could inhibit the CPEB2 expression by induction of miRNA210 expression. Since CPEB2 negatively regulated HIF-1α expression, its low levels under hypoxic condition led to an increased HIF-1α and by some unidentified mechanism, a decreased level of syncytin-2. The final effect is an inhibition of trophoblast syncytialization. The downregulation of both syncytin genes by hypoxia could converge to a negative impact on placental functions.
Syncytin-1 modulates inflammation in preeclampsia
A large number of trophoblasts and trophoblastic fragments are normally deported from the syncytium to maternal circulation. It is believed that placental deportation is required for the establishment of immune tolerance and possibly other pregnancy adaptations. Free syncytin-1 molecules as well as those bound to trophoblast membranes will be released into the maternal circulation through deportation [87]. Interestingly, recent studies suggested that syncytin-1 was actively secreted by trophoblasts through an exosomes-mediated mechanism. Tolosa et al. performed the transmission electron microscopy and Western blotting, and the resultant data confirmed the presence of syncytin-1 in exosomes isolated from human placental explants, primary trophoblast cultures or BeWo cells [88]. Importantly, an increased trophoblast deportation was observed under preeclamptic condition [89], raising questions regarding the quantity and significance of extracellular syncytin-1 for PE development.
Several types of viral envelop proteins are known to interfere with immune responses of the hosts. For example, the envelope protein of Human T-cell Lymphocyte Virus 1 (HTLV1) carries an immunosuppressive activity. A synthetic 17-amino acid peptide (CKS-17) containing the HTLV1 immunosuppressive domain was able to promote the Th1/Th2 shift, and to inhibit the production of Th1 cytokines TNF-α, IFN-γ, and IL-2, but to induce the production of Th2 cytokine IL-10. Marianne et al. first reported an immunosuppressive activity of syncytin-2. Transplantation of MCA205 tumor cells into BALB/c mice led to transient tumors or no tumor due to the immune rejection. However, injection of the same cells with stable expression of syncytin-2 led to the growth of large tumors that persisted for a longer time, pointing to an immunosuppressive activity of syncytin-2 [90]. Later studies indicated that both syncytins may have immunosuppressive activities. Tolosa et al. determined the activity of a peptide containing the extracellular part of syncytin-1, which harbors the putative immunosuppressive domain (ISD). Responses to LPS or PHA by mononuclear cells from peripheral blood were examined following pre-treatment for 2 h with the ISD or a control peptide. The results showed that ISD peptide inhibited the production of Th1 cytokines TNF-α and IFN-γ as well as chemokine CXCL10 [88]. Hummel et al. showed that peripheral dendritic cells and T cells from healthy donors expressed syncytin-1 receptors ASCT-1 and -2. In a co-culture system, CHO cells expressing syncytin-1 and -2 did not affect the LPS-driven maturation of dendritic cells or the expansion of T cells, but promoted the release of IL-12 and TNF-α rather than IL-10. In contrast, BeWo cells expressing syncytin-1 suppressed T-cell proliferation and the LPS-inducedIL-12 and TNF-α release. The authors concluded that syncytin-1 might indirectly affect T-cell activation by modulating the dendritic cell activity [91]. It is unclear if the divergence in immune cell responses to syncytin-1 overexpression by CHO and BeWo cells could be related to the differential expression of ASCT in the two cell lines [15].
Lokossou et al. observed the syncytin-2 expression and exosome-mediated secretion in human placenta. The Jurkat T-cell activation was severely inhibited following incubation with placental exosomes. Exosomes isolated from villous cytotrophoblasts transfected by a syncytin-2 siRNA had less suppressive effect on Jurkat T cell compared to exosomes derived from villous cytotrophoblasts transfected with a control siRNA. Syncytins secreted by trophoblasts may participate in the regulation of local as well as systemic inflammation [92]. It is noteworthy that the syncytin expression spectrum covers a far broader range than merely placental trophoblasts. Sun et al. demonstrated the expression of syncytin-1 mRNA in granulocyte, lymphocyte, and malignant cells of leukemia patients, and the higher syncytin-1 expression was associated with a poorer long-term immunity [93]. The syncytin activities in immune cells are likely mediated by nonfusogenic activities similar to those observed in trophoblasts (Section: “Syncytin-1 Structure and Function”). Exactly how the extracellular syncytin and syncytin protein expressed in immune cells may contribute to inflammation and PE pathogenesis remains an issue of further investigation.
Infection and preeclampsia, implication of syncytin-1
Although an intact syncytium serves as a physical and chemical antimicrobial barrier, some intracellular microbes could bypass this natural defense and invade the syncytium. Microbial infection is strongly associated with inflammatory responses, and possibly PE pathogenesis. Urinary tract infection (UTI) is implicated in placental hypoxia and fetal intrauterine growth restriction [94, 95]. Yan et al. performed a meta-analysis, and concluded that the data summary supported the relevance of UTI during pregnancy with the increased risk of PE [96]. Zika virus infection in early pregnancy affects the paracellular permeability of syncytiotrophoblasts by decreasing the expression of the tight junction protein claudin-4 [97]. PE patients with infection of human immunodeficiency virus have increased serum TNF-α and IL-6 levels [98]. Porphyromonas gingivalis (Pg), a Gram-negative anaerobic bacillus often found in periodontal disease, could suppress trophoblast invasion and spiral artery remodeling possibly by reducing the inhibin expression [99, 100].
It is noteworthy that microbial infections can affect syncytin-1 expression in placenta. It was reported that influenza A/WSN/33 virus infection was accompanied by an activation of the syncytin-1 locus in various human cell lines of non-placental origins [101]. Further studies by Li et al. showed that Influenza A virus infection of mouse hippocampus neurons or cortical glial cells led to an increased mRNA levels of GCM1 in vivo. The siRNA-mediated knockdown of GCM1 followed by virus infection resulted in an inhibition of syncytin-1 expression. Influenza A virus infection derepressed the syncytin-1 locus via GCM1 and an unknown factor(s), e.g., a decreased H3K9 trimethylation [102, 103]. A microarray study in glioma cell lines GLiNS1 and G179NS showed that syncytin-1 gene transcription could be activated by infection of CMV, but not infection of Toxoplasma gondii, a bacterial strain often infecting CNS [104]. Epstein-Barr virus could induce syncytin-1 expression in B lymphocytes and monocytes from peripheral blood of multiple sclerosis (MS) patients, and this induction was mediated by the activation of the p65 subunit of NF-κB [105]. These findings suggested that syncytin-1 expression could be activated in various cell types by specific viruses or bacteria. In a preeclamptic placenta, the decreased syncytin-1 expression and the deficiency of trophoblast syncytialization may lead to an increased risk of microbial infection. There seems to be a complicated interplay between microbial infection and dysregulation of syncytin-1 expression during PE pathogenesis.
What we learned from multiple sclerosis
MS is a CNS disorder characterized by chronic inflammation and progressive demyelination of nerve fibers. An elevated secretion of cytokines TNF-α and IL-1β by the circulatory monocytes was observed in MS patients [106]. The MS-Associated Retrovirus (MSRV)-specific mRNA sequences and extracellular synctytin-1 were co-detected in the blood, spinal fluid, and brain samples of MS patients [107]. Komurian et al. detected the syncytin-1-coding sequences when analyzing the mRNA species associated with MSRV particles isolated from MS patient plasma [108]. Schmitt et al. also detected syncytin-1 mRNA in the MS brain lesions as well as the white matter tissues from healthy controls [109, 110]. It was proposed that the enhanced expression of syncytin-1 in the cortical tissues of MS patients could promote the generation of pro-inflammatory cytokines, leading to astrocyte damage, oligodendrocyte cytotoxicity, nitric oxide (NO) synthase production, and demyelination [111]. Importantly, the increased syncytin-1 mRNA and protein levels in the brain tissues of MS patients were found to be associated with the elevation of circulatory TNF-α levels [112]. Studies by Antony et al. specifically demonstrated the upregulation of syncytin-1 gene expression in the astrocyte cells from demyelinated brain regions of MS patients, and syncytin-1 expression in astrocytes induced the release of redox reactants, which were cytotoxic to oligodendrocytes [113]. Transgenic mice with selective syncytin-1 overexpression in glial cells resulted in neuroinflammation, a reduced ASCT-1 expression, and a diminished level of myelin proteins in the corpus callosum, which were consistent with observations in MS patients [114]. These findings points to a deep involvement of syncytin-1 in the initiation of neuroinflammation.
In addition to the syncytin-1 promotion of inflammation, there is also evidence for an inflammation-driven syncytin-1 expression. Fang et al. pointed out that syncytin-1 upregulation may be driven by inflammatory processes involving elevated cytokines, oxidative damage, and the activation of macrophage or microglia in the areas of demyelination [115]. Indeed, Johnston et al. reported that activation of macrophage isolated from the blood of MS patients by phorbol-12-myristate-13-acetate or lipopolysaccharide led to an overexpression of HERV family gene including that of syncytin-1 [112]. Using a cell transfection assay Mameli et al. found that treatment of the human U-87MG astrocyte cell line with MS-related cytokines IL-1β, IL-6, TNF-α, and IFN-γ resulted in the activation of syncytin-1 gene. Particularly, TNF-α could activate syncytin-1 expression by augmenting the NF-κB binding to its cognate responsive element located within the enhancer region of the syncytin-1 gene, providing a mechanism for inflammation-driven syncytin-1 expression in MS lesions [116]. The possible mutual augmentation by the positive feedback between immune activation and syncyin-1 expression may partially explain the lasting and progressive nature of neuroinflammation observed in MS.
The deep involvement of syncytin-1 in the inflammation of MS is an immediate reminiscent of possible syncytin-1 contribution to PE. Paradoxically, in PE syncytin-1 expression displayed a pattern of reduced levels, whereas it is often increased in MS. Data from PE studies support an immunosuppressive and protective role(s) of syincytin-1 for placental function, yet in MS syncytin-1 appears to exert a pro-inflammatory and neurotoxic effect based on data from in vitro experiments or animal models [113, 117]. It seems that syncytin-1 dysregulation in either way may contribute to exaggerated inflammation. With limited knowledge on syncytin-1 actions in immune cells and the downstream pathways, it is difficult to reconcile the seemingly controversial observations from the two diseases. Presumably, the difference among cell types (e.g., trophoblast vs. glial cells) and the divergence of physio-pathological settings (placenta/decidua vs. CNS) involved in the two diseases may need to be cited to explain, and/or to unify, the underlying mechanisms.
Concluding remarks
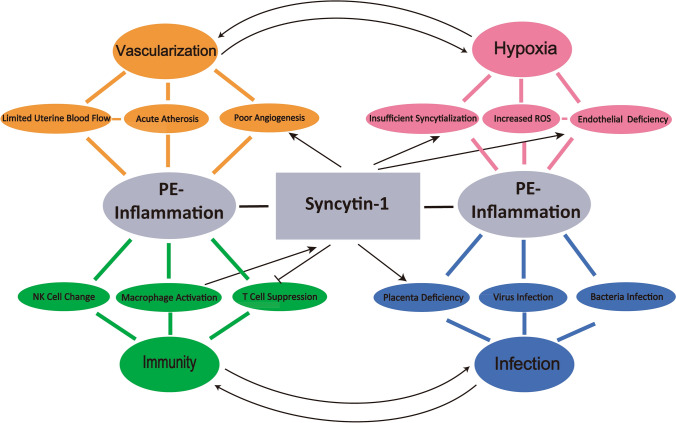
As illustrated in Fig. 1, this review discusses the triangular correlations among inflammation, syncytin-1 expression/function and PE. In addition to the well-recognized contribution to PE by the syncytin-1 fusogenic activity in trophoblast cells, we analyze the following points:
The local as well as systemic inflammation, which explains many preeclamptic manifestations, is considered a central factor for PE development. Immune cells activation and infiltration, shift of Th1/Th2 ratio, and increased cytokine production, are found in PE patients. Inflammation is involved in all the four PE factors of poor vascularization, hypoxia, infection, and dysregulation of immune cell responses. Important references in supports of this view are organized in Table 1.
There is a reciprocal regulation between vascularization and hypoxia: poor vascularity causes ischemia and hypoxia of the fetoplacental units; Vice versa, hypoxia directly induces placental angiogenesis/vascularization, and indirectly, stimulates the production of inflammatory factors to modulate vascularization. While a physiological level of hypoxia is beneficial for placental development, severe hypoxia as observed in PE, is associated with exaggerated inflammation, vascular damages and placental failure.
- Specific evidence in support of syncytin-1 contribution to inflammation during PE pathogenesis includes:
- The SU domain of syncytin-1 binds to placental vascular endothelium and affects placental vascularization. Knockout of the syncytin-A gene in mouse leads to a poor placental vascularization. Hypoxia could downregulate syncytin-1 expression through HIF and/or miRNA210-mediated epigenetic pathways.
- Both syncytin-1 and -2 contain the immunosuppressive domain, and overexpression of syncytin-1 in BeWo cells resulted in the inhibition of T-cell proliferation and the production of TNF-α and IL-12 in a co-culture system, suggesting that extracellular syncytin-1 could regulate immune cell functions.
- Astrocyte cells from MS lesions had increased syncytin-1 but decreased ASCT-1 expression. MS-related cytokines IL-1β, IL-6, TNF-α, and IFN-γ stimulate syncytin-1 expression in the U-87MG astrocytes. Experiments using cell cultures provided evidence for the direct effects of syncytin proteins in trophoblasts as well immune cells. Conceivably, syncytin could contribute to PE pathogenesis by indirect influences, e.g., through the alteration of hormone production by placental trophoblasts. The functions of immune, cardiovascular and other systems can all be affected by these hormones during pregnancy.
- CMV infection of glioma cells, Epstein–Barr viral infection of B cells, or influenza virus infection of neuroepithelioma cells, can all activate syncytin-1 expression, which may contribute to infection-caused inflammation.
Fig. 1.
Schematic illustration of the relationship among syncytin-1, inflammation, and PE. Syncytin-1 participates in regulation of the four major factors contributing to local and systemic inflammation in PE patients: vascularization, hypoxia, immunity, and infection. The close and reciprocal relationship between vascularization and hypoxia as well as between infection and immune responses are indicated with the curved arrows. The major elements through which the four factors modulate inflammation are presented (Limited Uterine Blood Flow, NK Cell Change, etc). Syncytin-1 activation or inhibition of these elements, whenever supported by experimental results, are indicated by strait arrows. Detailed regulatory mechanisms are, respectively, described in the subtitled sections, with the important references listed in Table 1
Table 1.
Summary of key references
| Topic | Title of publication | Journal, author, time | Study subjects/model | Major conclusion | Reference |
|---|---|---|---|---|---|
| Syncytin-1 structure | The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors | J Virol; Lavillette et al.; 2002 | CHO and HEK293T cells | HERV-W Env is capable of mediating syncytium formation by interacting with the human sodium-dependent neutral amino acid transporter type 2 (ASCT2) | [24] |
| Synthesis, assembly, and processing of the Env ERVWE1/syncytin human endogenous retroviral envelope | J Virol; Cheynet et al.; 2005 | BeWo and TELCeB6 cells | The cellular syncytin protein is generated as a glycosylated gp73 precursor, and cleaved into the gp50 SU and the gp24 TM subunits at cleavage site RNKR | [22] | |
| C-Terminal truncations of syncytin-1 (ERVWE1 envelope) that increase its fusogenicity | Biol Chem; Drewlo et al.; 2006 | CHO and HEK293T cells | The C-terminally truncated sequences adjoining to the transmembrane region of syncytin-1 were essential for cell fusion | [23] | |
| Syncytin-1 and vascularization | Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene |
PNAS; Dupressoir et al.; 2007 |
Mouse syncytin-A knockout | Placentas of syncytin-A null mice grew fewer and narrower blood vessels that contained fewer embryonic erythrocytes | [81] |
| Syncytin-1 in the human placenta | Placenta; Holder et al.; 2012 | Human placenta; BeWo cells | Endothelium of PE placental vessels had higher levels of syncytin-1 SU domain | [80] | |
| Inducible knockout of Syncytin-A gene leads to an extensive placental vasculature deficiency, implications for preeclampsia | Clin Chim Acta; Qiao et al.; 2017 | Mouse Syncytin-A knockout | Placentas of syncytin-A knockout mice had decreased size and thickness, and reduced number and irregular distribution of fetal microvessels in labyrinth layer | [82] | |
| Syncytin-1 and hypoxia | Hypoxia alters expression and function of syncytin and its receptor during trophoblast cell fusion of human placental BeWo cells: implications for impaired trophoblast syncytialisation in preeclampsia |
Biochim Biophys Acta; Kudo et al.; 2003 |
BeWo cells | Low oxygen atmosphere led to decreased expression of syncytin-1 and ASCT2, and inhibition of BeWo cell syncytialization | [83] |
| Mechanism of hypoxia-induced GCM1 degradation: implications for the pathogenesis of preeclampsia | J Biol Chem; Chiang et al.; 2009 | BeWo cells | Hypoxia downregulated syncytin-1, attenuated the syncytin-1-mediated immunosuppression, and enhanced inflammation | [84] | |
| A positive feedback self-regulatory loop between miR-210 and HIF-1α mediated by CPEB2 is involved in trophoblast syncytiolization: implication of trophoblast malfunction in preeclampsia | Biol Reprod; Wang et al.; 2019 | Human placenta; BeWo cells | Hypoxia led to upregulation of HIF-1α and inhibition of syncytin-2 by decreasing CPEB expression, which was reversed by inhibition of miR-210 | [85] | |
| Sycytin-1 and immune cell responses | Placental syncytins: Genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins |
PNAS; Mangeney et al.; 2007 |
Tumor zenografts in mouse | Malignant cells expressing syncytin-2 grew larger tumors that persisted longer time, suggesting immunosuppression | [90] |
| The endogenous retroviral envelope protein syncytin-1 inhibits lps/pha-stimulated cytokine responses in human blood and is sorted into placental exosomes | Placenta; Tolosa et al.; 2012 |
Placenta; Primary trophoblast; PBMC |
Syncytin-1 was detected in placental exosomes; Syncytin-1 peptide inhibited production of Th1 cytokines TNF-α and IFN-γ by human blood cells | [88] | |
| Human endogenous retrovirus envelope proteins target dendritic cells to suppress T-cell activation | Eur J Immunol; Hummel et al.; 2015 | CHO/BeWo cells co-culture with DC and T cells | BeWo cells overexpressing syncytin-1 suppressed T-cell proliferation and LPS-induced TNF-α and IL-12 release | [91] | |
| Syncytin-1 and infection | Human cytomegalovirus (HCMV) induces human endogenous retrovirus (HERV) transcription |
Retrovirology; Assinger et al.; 2013 |
Cancer cell lines; Endothelial cells; Monocytes | Syncytin-1 transcription was activated by infection of CMV in human glioma GliNS1 cells | [104] |
| Syncytin-1 and multiple sclerosis | The human endogenous retrovirus envelope glycoprotein, syncytin-1, regulates neuroinflammation and its receptor expression in multiple sclerosis: a role for endoplasmic reticulum chaperones in astrocytes |
J Immunol; Antony et al.; 2007 |
Human brain tissue; Astrocytes; Syncytin-A transgenic mice |
Astrocyte cells from MS lesion had increased syncytin-1 and decreased ASCT-1 expression; Mice overexpressing syncytin-1 displayed neuroinflammation | [114] |
| Regulation of the syncytin-1 promoter in human astrocytes by multiple sclerosis-related cytokines |
Virology; Mameli et al.; 2007 |
Human U-87MG astrocytic cells | IL-1, IL-6, TNF-α and IFN-γ activated syncytin-1 expression by augmenting NF-κB pathway | [116] |
Several key issues deserved a special attention: syncytin-1 is expressed in trophoblasts as well as immune cells. While there are data to support extensive involvements of syncytin-mediated nonfusogenic activities in the regulation of immune cell responses, the specific effects in each cell type and/or the regulatory pathway are poorly understood: How the synctin-1 produced by trophoblasts or immune cells regulate inflammatory responses, and by what molecular pathways; By what mechanism(s) the extracellular syncytin-1 may regulate immune cell functions; How the homeostasis of syncytin-1 levels is maintained in trophoblasts and non-trophoblast cells, respectively; Why both the syncytin-1 downregulation in placenta and upregulation in the brain could cause inflammation and tissue damages.
PE is a leading cause of maternal morbidity and mortality related to pregnancy. To date, the clinical management of PE patients is limited to anti-symptom therapy with vasodilation reagents. Although early termination of pregnancy cues PE, the preterm birth significantly increased the risk of perinatal disorders such as respiratory distress syndrome, jaundice and infection, and can lead to various DOHaD in the later life of neonates. Syncytin gene mutations could be explored for the evaluation of PE risk, and syncytin mRNA/protein could be potential biomarkers for early detection and/or outcome prediction in the managements of preeclamptic pregnancy. It is expected that research efforts on the complicated relationship among preeclampsia, inflammation, and syncytin-1 would be productive and the findings might facilitate the development of new therapeutic models against this common disease.
Acknowledgements
The authors thank their colleagues and reviewers for their productive comments on the points and consents of the review.
Author contributions
Concept and supervision: S-W J and DC; Preparation of first draft: S-W J and CB; All authors contributed to literature search and information collection, discussion, and revision.
Funding
The Leading Talents in Medical and Health Profession Program and the Fenghuanchao Program of the Wuxi Taihu Talent Plan (S-W J); The Jiangsu Shuangchuang Talent Program (S-W J and CB); The Postdoctoral Training Program of Nanjing Medical University (ZW, S-W J, DC).
Data availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chaozhi Bu and Zhiwei Wang contribute equally to this work.
Contributor Information
Daozhen Chen, Email: chendaozhen@163.com.
Shi-Wen Jiang, Email: jiangsw137@163.com.
References
- 1.Ferrero-Miliani L, Nielsen OH, Andersen PS, et al. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007;147(2):227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilman-Sachs A, Dambaeva S, Salazar Garcia MD, et al. Inflammation induced preterm labor and birth. J Reprod Immunol. 2018;129:53–58. doi: 10.1016/j.jri.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 3.Lin C, He H, Cui N, et al. Decreased uterine vascularization and uterine arterial expansive remodeling with reduced matrix metalloproteinase-2 and-9 in hypertensive pregnancy. Am J Physiol Heart Circ Physiol. 2020;318(1):H165–H180. doi: 10.1152/ajpheart.00602.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang P. Decidual vasculopathy and spiral artery remodeling revisited II: relations to trophoblastic dependent and independent vascular transformation. J Matern Fetal Neonatal Med. 2020 doi: 10.1080/14767058.2020.1718646. [DOI] [PubMed] [Google Scholar]
- 5.Weckman AM, Ngai M, Wright J, et al. The impact of infection in pregnancy on placental vascular development and adverse birth outcomes. Front Microbiol. 2019;10:1924. doi: 10.3389/fmicb.2019.01924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S, Zhang Y, Sun Y, et al. Naringenin improves insulin sensitivity in gestational diabetes mellitus mice through AMPK. Nutr Diabetes. 2019;9(1):28. doi: 10.1038/s41387-019-0095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aplin JD, Myers JE, Timms K, et al. Tracking placental development in health and disease. Nat Rev Endocrinol. 2020;16(9):479–494. doi: 10.1038/s41574-020-0372-6. [DOI] [PubMed] [Google Scholar]
- 8.Almeida DL, Pavanello A, Saavedra LP, et al. Environmental monitoring and the developmental origins of health and disease. J Dev Orig Health Dis. 2019;10(6):608–615. doi: 10.1017/s2040174419000151. [DOI] [PubMed] [Google Scholar]
- 9.Jim B, Karumanchi SA. Preeclampsia: pathogenesis, prevention, and long-term complications. Semin Nephrol. 2017;37(4):386–397. doi: 10.1016/j.semnephrol.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Mol BWJ, Roberts CT, Thangaratinam S, et al. Pre-eclampsia. Lancet (London, England) 2016;387(10022):999–1011. doi: 10.1016/s0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 11.Baumwell S, Karumanchi SA. Pre-eclampsia: clinical manifestations and molecular mechanisms. Nephron Clin Pract. 2007;106(2):c72–81. doi: 10.1159/000101801. [DOI] [PubMed] [Google Scholar]
- 12.Brosens I, Puttemans P, Benagiano G. Placental bed research: I the placental bed: from spiral arteries remodeling to the great obstetrical syndromes. Am J Obstet Gynecol. 2019;221(5):437–456. doi: 10.1016/j.ajog.2019.05.044. [DOI] [PubMed] [Google Scholar]
- 13.Giorgi VS, Peracoli MT, Peracoli JC, et al. Silibinin modulates the NF-kappab pathway and pro-inflammatory cytokine production by mononuclear cells from preeclamptic women. J Reprod Immunol. 2012;95(1–2):67–72. doi: 10.1016/j.jri.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 14.LaMarca B, Speed J, Fournier L, et al. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension (Dallas, Tex: 1979) 2008;52(6):1161–1167. doi: 10.1161/hypertensionaha.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blond JL, Lavillette D, Cheynet V, et al. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74(7):3321–3329. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui L, Wang H, Lu X, et al. Effects of individually silenced N-glycosylation sites and non-synonymous single-nucleotide polymorphisms on the fusogenic function of human syncytin-2. Cell Adh Migr. 2016;10(1–2):39–55. doi: 10.1080/19336918.2015.1093720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Q, Chen H, Wang F, et al. Reduced syncytin-1 expression in choriocarcinoma BeWo cells activates the calpain1-AIF-mediated apoptosis, implication for preeclampsia. Cell Mol Life Sci. 2014;71(16):3151–3164. doi: 10.1007/s00018-013-1533-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Q, Li J, Wang F, et al. Syncytin-1 modulates placental trophoblast cell proliferation by promoting G1/S transition. Cell Signal. 2013;25(4):1027–1035. doi: 10.1016/j.cellsig.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu HL, Zhao ZK, Zhu F. The role of human endogenous retroviral long terminal repeat sequences in human cancer (Review) Int J Mol Med. 2013;32(4):755–762. doi: 10.3892/ijmm.2013.1460. [DOI] [PubMed] [Google Scholar]
- 20.Gimenez J, Montgiraud C, Oriol G, et al. Comparative methylation of ERVWE1/syncytin-1 and other human endogenous retrovirus LTRs in placenta tissues. DNA Res: Int J Rapid Publ Rep Genes Genomes. 2009;16(4):195–211. doi: 10.1093/dnares/dsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang C, Chen PT, Chang GD, et al. Functional characterization of the placental fusogenic membrane protein syncytin. Biol Reprod. 2004;71(6):1956–1962. doi: 10.1095/biolreprod.104.033340. [DOI] [PubMed] [Google Scholar]
- 22.Cheynet V, Ruggieri A, Oriol G, et al. Synthesis, assembly, and processing of the Env ERVWE1/syncytin human endogenous retroviral envelope. J Virol. 2005;79(9):5585–5593. doi: 10.1128/jvi.79.9.5585-5593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drewlo S, Leyting S, Kokozidou M, et al. C-Terminal truncations of syncytin-1 (ERVWE1 envelope) that increase its fusogenicity. Biol Chem. 2006;387(8):1113–1120. doi: 10.1515/bc.2006.137. [DOI] [PubMed] [Google Scholar]
- 24.Lavillette D, Marin M, Ruggieri A, et al. The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors. J Virol. 2002;76(13):6442–6452. doi: 10.1128/jvi.76.13.6442-6452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheynet V, Oriol G, Mallet F. Identification of the hASCT2-binding domain of the Env ERVWE1/syncytin-1 fusogenic glycoprotein. Retrovirology. 2006;3:41. doi: 10.1186/1742-4690-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marin M, Lavillette D, Kelly SM, et al. N-linked glycosylation and sequence changes in a critical negative control region of the ASCT1 and ASCT2 neutral amino acid transporters determine their retroviral receptor functions. J Virol. 2003;77(5):2936–2945. doi: 10.1128/jvi.77.5.2936-2945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjerregaard B, Holck S, Christensen IJ, et al. Syncytin is involved in breast cancer-endothelial cell fusions. Cell Mol Life Sci. 2006;63(16):1906–1911. doi: 10.1007/s00018-006-6201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fei F, Li C, Wang X, et al. Syncytin 1, CD9, and CD47 regulating cell fusion to form PGCCs associated with cAMP/PKA and JNK signaling pathway. Cancer Med. 2019;8(6):3047–3058. doi: 10.1002/cam4.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grandi N, Tramontano E. HERV envelope proteins: physiological role and pathogenic potential in cancer and autoimmunity. Front Microbiol. 2018;9:462. doi: 10.3389/fmicb.2018.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frendo JL, Olivier D, Cheynet V, et al. Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol Cell Biol. 2003;23(10):3566–3574. doi: 10.1128/mcb.23.10.3566-3574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vargas A, Toufaily C, LeBellego F, et al. Reduced expression of both syncytin 1 and syncytin 2 correlates with severity of preeclampsia. Reprod Sci (Thousand Oaks, Calif) 2011;18(11):1085–1091. doi: 10.1177/1933719111404608. [DOI] [PubMed] [Google Scholar]
- 32.Langbein M, Strick R, Strissel PL, et al. Impaired cytotrophoblast cell–cell fusion is associated with reduced syncytin and increased apoptosis in patients with placental dysfunction. Mol Reprod Dev. 2008;75(1):175–183. doi: 10.1002/mrd.20729. [DOI] [PubMed] [Google Scholar]
- 33.Zhuang XW, Li J, Brost BC, et al. Decreased expression and altered methylation of syncytin-1 gene in human placentas associated with preeclampsia. Curr Pharm Des. 2014;20(11):1796–1802. doi: 10.2174/13816128113199990541. [DOI] [PubMed] [Google Scholar]
- 34.Lu Q, Li J, Senkowski C, et al. Promoter hypermethylation and decreased expression of syncytin-1 in pancreatic adenocarcinomas. PLoS ONE. 2015;10(7):e0134412. doi: 10.1371/journal.pone.0134412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez-Jaramillo P, Barajas J, Rueda-Quijano SM, et al. Obesity and preeclampsia: common pathophysiological mechanisms. Front Physiol. 2018;9:1838. doi: 10.3389/fphys.2018.01838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cartwright JE, James-Allan L, Buckley RJ, et al. The role of decidual NK cells in pregnancies with impaired vascular remodelling. J Reprod Immunol. 2017;119:81–84. doi: 10.1016/j.jri.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Fu B, Li X, Sun R, et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci USA. 2013;110(3):E231–240. doi: 10.1073/pnas.1206322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace AE, Host AJ, Whitley GS, et al. Decidual natural killer cell interactions with trophoblasts are impaired in pregnancies at increased risk of preeclampsia. Am J Pathol. 2013;183(6):1853–1861. doi: 10.1016/j.ajpath.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gill N, Leng Y, Romero R. The immunophenotype of decidual macrophages in acute atherosis. Am J Reprod Immunol. 2019;81(4):e13098. doi: 10.1111/aji.13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayashi M, Hamada Y, Ohkura T. Elevation of granulocyte-macrophage colony-stimulating factor in the placenta and blood in preeclampsia. Am J Obstet Gynecol. 2004;190(2):456–461. doi: 10.1016/j.ajog.2003.07.032. [DOI] [PubMed] [Google Scholar]
- 41.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13(1):23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 42.Nancy P, Erlebacher A. T cell behavior at the maternal-fetal interface. Int J Dev Biol. 2014;58(2–4):189–198. doi: 10.1387/ijdb.140054ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinheiro MB, Gomes KB, Dusse LM. Fibrinolytic system in preeclampsia. Clin Chim Acta Int J Clin Chem. 2013;416:67–71. doi: 10.1016/j.cca.2012.10.060. [DOI] [PubMed] [Google Scholar]
- 44.Sargent IL, Borzychowski AM, Redman CW. Immunoregulation in normal pregnancy and pre-eclampsia: an overview. Reprod Biomed Online. 2007;14(Spec No 1):111–117. doi: 10.1016/s1472-6483(10)61465-4. [DOI] [PubMed] [Google Scholar]
- 45.Bazhenov DO, Khokhlova EV, Viazmina LP, et al. Characteristics of natural killer cell interaction with trophoblast cells during pregnancy. Curr Mol Med. 2019 doi: 10.2174/1566524019666190808103227. [DOI] [PubMed] [Google Scholar]
- 46.Leavey K, Grynspan D, Cox BJ. Both "canonical" and "immunological" preeclampsia subtypes demonstrate changes in placental immune cell composition. Placenta. 2019;83:53–56. doi: 10.1016/j.placenta.2019.06.384. [DOI] [PubMed] [Google Scholar]
- 47.Haeger M, Unander M, Norder-Hansson B, et al. Complement, neutrophil, and macrophage activation in women with severe preeclampsia and the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 1992;79(1):19–26. [PubMed] [Google Scholar]
- 48.Cornelius DC. Preeclampsia: from inflammation to immunoregulation. Clin Med Insights Blood Disord. 2018;11:1179545x17752325. doi: 10.1177/1179545x17752325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ertas IE, Kahyaoglu S, Yilmaz B, et al. Association of maternal serum high sensitive C-reactive protein level with body mass index and severity of pre-eclampsia at third trimester. J Obstet Gynaecol Res. 2010;36(5):970–977. doi: 10.1111/j.1447-0756.2010.01279.x. [DOI] [PubMed] [Google Scholar]
- 50.Kumar A, Begum N, Prasad S, et al. IL-10, TNF-alpha & IFN-gamma: potential early biomarkers for preeclampsia. Cell Immunol. 2013;283(1–2):70–74. doi: 10.1016/j.cellimm.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 51.Medeiros LT, Peracoli JC, Bannwart-Castro CF, et al. Monocytes from pregnant women with pre-eclampsia are polarized to a M1 phenotype. Am J Reprod Immunol (New York, NY: 1989) 2014;72(1):5–13. doi: 10.1111/aji.12222. [DOI] [PubMed] [Google Scholar]
- 52.Stanhewicz AE, Jandu S, Santhanam L, et al. Increased angiotensin II sensitivity contributes to microvascular dysfunction in women who have had preeclampsia. Hypertension (Dallas, Tex: 1979) 2017;70(2):382–389. doi: 10.1161/hypertensionaha.117.09386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanhewicz AE, Alexander LM. Local Angiotensin 1–7 administration improves microvascular endothelial function in women who have had preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2019 doi: 10.1152/ajpregu.00221.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kulandavelu S, Whiteley KJ, Qu D, et al. Endothelial nitric oxide synthase deficiency reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant mice. Hypertension (Dallas, Tex: 1979) 2012;60(1):231–238. doi: 10.1161/hypertensionaha.111.187559. [DOI] [PubMed] [Google Scholar]
- 55.Mehta V, Abi-Nader KN, Peebles DM, et al. Long-term increase in uterine blood flow is achieved by local overexpression of VEGF-A(165) in the uterine arteries of pregnant sheep. Gene Ther. 2012;19(9):925–935. doi: 10.1038/gt.2011.158. [DOI] [PubMed] [Google Scholar]
- 56.Stepan H, Unversucht A, Wessel N, et al. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension (Dallas, Tex: 1979) 2007;49(4):818–824. doi: 10.1161/01.HYP.0000258404.21552.a3. [DOI] [PubMed] [Google Scholar]
- 57.Eddy AC, Bidwell GL, 3rd, George EM. Pro-angiogenic therapeutics for preeclampsia. Biol Sex Differ. 2018;9(1):36. doi: 10.1186/s13293-018-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy SR, LaMarca BB, Parrish M, et al. Control of soluble fms-like tyrosine-1 (sFlt-1) production response to placental ischemia/hypoxia: role of tumor necrosis factor-alpha. Am J Physiol Regul Integr Comp Physiol. 2013;304(2):R130–R135. doi: 10.1152/ajpregu.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bobek G, Surmon L, Mirabito KM, et al. Placental regulation of inflammation and hypoxia after TNF-alpha infusion in mice. Am J Reprod Immunol (New York, NY: 1989) 2015;74(5):407–418. doi: 10.1111/aji.12417. [DOI] [PubMed] [Google Scholar]
- 60.Chau K, Bobek G, Xu B. Effect of placental growth factor in models of experimental pre-eclampsia and trophoblast invasion. Clin Exp Pharmacol Physiol. 2020;47(1):49–59. doi: 10.1111/1440-1681.13169. [DOI] [PubMed] [Google Scholar]
- 61.Le Bouteiller P. Human decidual NK cells: unique and tightly regulated effector functions in healthy and pathogen-infected pregnancies. Front Immunol. 2013;4:404. doi: 10.3389/fimmu.2013.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12(9):1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 63.Alexander BT, Rinewalt AN, Cockrell KL, et al. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension (Dallas, Tex: 1979) 2001;37(2 Pt 2):485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 64.Care AS, Bourque SL, Morton JS, et al. Reduction in regulatory T cells in early pregnancy causes uterine artery dysfunction in mice. Hypertension (Dallas, Tex: 1979) 2018;72(1):177–187. doi: 10.1161/hypertensionaha.118.10858. [DOI] [PubMed] [Google Scholar]
- 65.Alnaes-Katjavivi P, Lyall F, Roald B, et al. Acute atherosis in vacuum suction biopsies of decidua basalis: an evidence based research definition. Placenta. 2016;37:26–33. doi: 10.1016/j.placenta.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 66.Fosheim IK, Alnaes-Katjavivi P, Redman C, et al. Acute atherosis of decidua basalis; characterization of spiral arteries, endothelial status and activation. Placenta. 2019;82:10–16. doi: 10.1016/j.placenta.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 67.O'Reilly VC, Lopes Floro K, Shi H, et al. Gene-environment interaction demonstrates the vulnerability of the embryonic heart. Dev Biol. 2014;391(1):99–110. doi: 10.1016/j.ydbio.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finsterwalder R, Ganesan MK, Leb H, et al. Hypoxia/reperfusion predisposes to atherosclerosis. PLoS ONE. 2018;13(10):e0205067. doi: 10.1371/journal.pone.0205067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elkin ER, Harris SM, Loch-Caruso R. Trichloroethylene metabolite S-(1,2-dichlorovinyl)-l-cysteine induces lipid peroxidation-associated apoptosis via the intrinsic and extrinsic apoptosis pathways in a first-trimester placental cell line. Toxicol Appl Pharmacol. 2018;338:30–42. doi: 10.1016/j.taap.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vaughan JE, Walsh SW. Oxidative stress reproduces placental abnormalities of preeclampsia. Hypertens Pregnancy. 2002;21(3):205–223. doi: 10.1081/prg-120015848. [DOI] [PubMed] [Google Scholar]
- 71.Michaeloudes C, Abubakar-Waziri H, Lakhdar R, et al. Molecular mechanisms of oxidative stress in asthma. Mol Aspects Med. 2022;85:101026. doi: 10.1016/j.mam.2021.101026. [DOI] [PubMed] [Google Scholar]
- 72.Alahari S, Post M, Rolfo A, et al. Compromised JMJD6 histone demethylase activity affects VHL gene repression in preeclampsia. J Clin Endocrinol Metab. 2018;103(4):1545–1557. doi: 10.1210/jc.2017-02197. [DOI] [PubMed] [Google Scholar]
- 73.Sriyanti R, Mose JC, Masrul M, et al. the difference in maternal serum hypoxia-inducible factors-1alpha levels between early onset and late-onset preeclampsia. Open Access Maced J Med Sci. 2019;7(13):2133–2137. doi: 10.3889/oamjms.2019.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iriyama T, Wang W, Parchim NF, et al. Hypoxia-independent upregulation of placental hypoxia inducible factor-1alpha gene expression contributes to the pathogenesis of preeclampsia. Hypertension (Dallas, Tex: 1979) 2015;65(6):1307–1315. doi: 10.1161/hypertensionaha.115.05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fang HY, Hughes R, Murdoch C, et al. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009;114(4):844–859. doi: 10.1182/blood-2008-12-195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imtiyaz HZ, Simon MC. Hypoxia-inducible factors as essential regulators of inflammation. Curr Top Microbiol Immunol. 2010;345:105–120. doi: 10.1007/82_2010_74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kempf VA, Lebiedziejewski M, Alitalo K, et al. Activation of hypoxia-inducible factor-1 in bacillary angiomatosis: evidence for a role of hypoxia-inducible factor-1 in bacterial infections. Circulation. 2005;111(8):1054–1062. doi: 10.1161/01.cir.0000155608.07691.b7. [DOI] [PubMed] [Google Scholar]
- 78.Zhou J, Schmid T, Brune B. Tumor necrosis factor-alpha causes accumulation of a ubiquitinated form of hypoxia inducible factor-1alpha through a nuclear factor-kappaB-dependent pathway. Mol Biol Cell. 2003;14(6):2216–2225. doi: 10.1091/mbc.e02-09-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roland CS, Hu J, Ren CE, et al. Morphological changes of placental syncytium and their implications for the pathogenesis of preeclampsia. Cell Mol Life Sci. 2016;73(2):365–376. doi: 10.1007/s00018-015-2069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holder BS, Tower CL, Abrahams VM, et al. Syncytin 1 in the human placenta. Placenta. 2012;33(6):460–466. doi: 10.1016/j.placenta.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 81.Dupressoir A, Vernochet C, Bawa O, et al. Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc Natl Acad Sci USA. 2009;106(29):12127–12132. doi: 10.1073/pnas.0902925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qiao S, Wang F, Chen H, et al. Inducible knockout of Syncytin-A gene leads to an extensive placental vasculature deficiency, implications for preeclampsia. Clin Chim Acta; Int J Clin Chem. 2017;474:137–146. doi: 10.1016/j.cca.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 83.Kudo Y, Boyd CA, Sargent IL, et al. Hypoxia alters expression and function of syncytin and its receptor during trophoblast cell fusion of human placental BeWo cells: implications for impaired trophoblast syncytialisation in pre-eclampsia. Biochem Biophys Acta. 2003;1638(1):63–71. doi: 10.1016/s0925-4439(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 84.Chang M, Mukherjea D, Gobble RM, et al. Glial cell missing 1 regulates placental growth factor (PGF) gene transcription in human trophoblast. Biol Reprod. 2008;78(5):841–851. doi: 10.1095/biolreprod.107.065599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chiang MH, Liang FY, Chen CP, et al. Mechanism of hypoxia-induced GCM1 degradation: implications for the pathogenesis of preeclampsia. J Biol Chem. 2009;284(26):17411–17419. doi: 10.1074/jbc.M109.016170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang H, Zhao Y, Luo R, et al. A positive feedback self-regulatory loop between miR-210 and HIF-1alpha mediated by CPEB2 is involved in trophoblast syncytiolization: implication of trophoblast malfunction in preeclampsia. Biol Reprod. 2019 doi: 10.1093/biolre/ioz196. [DOI] [PubMed] [Google Scholar]
- 87.Miura K, Higashijima A, Miura S, et al. Predominantly placenta-expressed mRNAs in maternal plasma as predictive markers for twin–twin transfusion syndrome. Prenat Diagn. 2014;34(4):345–349. doi: 10.1002/pd.4307. [DOI] [PubMed] [Google Scholar]
- 88.Tolosa JM, Schjenken JE, Clifton VL, et al. The endogenous retroviral envelope protein syncytin-1 inhibits LPS/PHA-stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta. 2012;33(11):933–941. doi: 10.1016/j.placenta.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 89.Chen LM, Liu B, Zhao HB, et al. IL-6, TNFalpha and TGFbeta promote nonapoptotic trophoblast deportation and subsequently causes endothelial cell activation. Placenta. 2010;31(1):75–80. doi: 10.1016/j.placenta.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 90.Mangeney M, Renard M, Schlecht-Louf G, et al. Placental syncytins: genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc Natl Acad Sci USA. 2007;104(51):20534–20539. doi: 10.1073/pnas.0707873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hummel J, Kammerer U, Muller N, et al. Human endogenous retrovirus envelope proteins target dendritic cells to suppress T-cell activation. Eur J Immunol. 2015;45(6):1748–1759. doi: 10.1002/eji.201445366. [DOI] [PubMed] [Google Scholar]
- 92.Lokossou AG, Toudic C, Nguyen PT, et al. Endogenous retrovirus-encoded Syncytin-2 contributes to exosome-mediated immunosuppression of T cells. Biol Reprod. 2019 doi: 10.1093/biolre/ioz124. [DOI] [PubMed] [Google Scholar]
- 93.Sun Y, Zhu H, Song J, et al. Expression of leukocytic syncytin-1 in B-cell acute lymphoblastic leukemia and acute myeloid leukemia patients. Clin Lab. 2017;63(10):1567–1574. doi: 10.7754/Clin.Lab.2017.170116. [DOI] [PubMed] [Google Scholar]
- 94.Lin AE, Beasley FC, Olson J, et al. Role of hypoxia inducible factor-1alpha (hif-1alpha) in innate defense against uropathogenic Escherichia coli infection. PLoS Pathog. 2015;11(4):e1004818. doi: 10.1371/journal.ppat.1004818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Christensen B. Which antibiotics are appropriate for treating bacteriuria in pregnancy? J Antimicrob Chemother. 2000;46(Suppl 1):29–34. doi: 10.1093/jac/46.suppl_1.29. [DOI] [PubMed] [Google Scholar]
- 96.Yan L, Jin Y, Hang H, et al. The association between urinary tract infection during pregnancy and preeclampsia: a meta-analysis. Medicine. 2018;97(36):e12192. doi: 10.1097/md.0000000000012192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miranda J, Martin-Tapia D, Valdespino-Vazquez Y, et al. Syncytiotrophoblast of placentae from women with Zika virus infection has altered tight junction protein expression and increased paracellular permeability. Cells. 2019;8(10):1174–1194. doi: 10.3390/cells8101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pillay Y, Moodley J, Naicker T. The role of the complement system in HIV infection and preeclampsia. Inflamm Res. 2019;68(6):459–469. doi: 10.1007/s00011-019-01240-0. [DOI] [PubMed] [Google Scholar]
- 99.Phillips P, Brown MB, Progulske-Fox A, et al. Porphyromonas gingivalis strain-dependent inhibition of uterine spiral artery remodeling in the pregnant rat. Biol Reprod. 2018;99(5):1045–1056. doi: 10.1093/biolre/ioy119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hirohata N, Komine-Aizawa S, Tamura M, et al. Porphyromonas gingivalis suppresses trophoblast invasion by soluble factors. J Periodontol. 2017;88(12):1366–1373. doi: 10.1902/jop.2017.170193. [DOI] [PubMed] [Google Scholar]
- 101.Nellaker C, Yao Y, Jones-Brando L, et al. Transactivation of elements in the human endogenous retrovirus W family by viral infection. Retrovirology. 2006;3:44. doi: 10.1186/1742-4690-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu C, Shen K, Lin M, et al. GCMa regulates the syncytin-mediated trophoblastic fusion. J Biol Chem. 2002;277(51):50062–50068. doi: 10.1074/jbc.M209316200. [DOI] [PubMed] [Google Scholar]
- 103.Li F, Nellaker C, Sabunciyan S, et al. Transcriptional derepression of the ERVWE1 locus following influenza A virus infection. J Virol. 2014;88(8):4328–4337. doi: 10.1128/jvi.03628-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Assinger A, Yaiw KC, Gottesdorfer I, et al. Human cytomegalovirus (HCMV) induces human endogenous retrovirus (HERV) transcription. Retrovirology. 2013;10:132. doi: 10.1186/1742-4690-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mameli G, Poddighe L, Mei A, et al. Expression and activation by Epstein Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: inference for multiple sclerosis. PLoS ONE. 2012;7(9):e44991. doi: 10.1371/journal.pone.0044991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Garcia-Montojo M, Rodriguez-Martin E, Ramos-Mozo P, et al. Syncytin-1/HERV-W envelope is an early activation marker of leukocytes and is upregulated in multiple sclerosis patients. Eur J Immunol. 2020;50(5):685–694. doi: 10.1002/eji.201948423. [DOI] [PubMed] [Google Scholar]
- 107.Mameli G, Madeddu G, Mei A, et al. Activation of MSRV-type endogenous retroviruses during infectious mononucleosis and Epstein–Barr virus latency: the missing link with multiple sclerosis? PLoS ONE. 2013;8(11):e78474. doi: 10.1371/journal.pone.0078474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Komurian-Pradel F, Paranhos-Baccala G, Bedin F, et al. Molecular cloning and characterization of MSRV-related sequences associated with retrovirus-like particles. Virology. 1999;260(1):1–9. doi: 10.1006/viro.1999.9792. [DOI] [PubMed] [Google Scholar]
- 109.Schmitt K, Richter C, Backes C, et al. Comprehensive analysis of human endogenous retrovirus group HERV-W locus transcription in multiple sclerosis brain lesions by high-throughput amplicon sequencing. J Virol. 2013;87(24):13837–13852. doi: 10.1128/jvi.02388-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grandi N, Tramontano E. Type W human endogenous retrovirus (HERV-W) integrations and their mobilization by L1 machinery: contribution to the human transcriptome and impact on the host physiopathology. Viruses. 2017;9(7):162–198. doi: 10.3390/v9070162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Antony JM, Deslauriers AM, Bhat RK, et al. Human endogenous retroviruses and multiple sclerosis: innocent bystanders or disease determinants? Biochem Biophys Acta. 2011;1812(2):162–176. doi: 10.1016/j.bbadis.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johnston JB, Silva C, Holden J, et al. Monocyte activation and differentiation augment human endogenous retrovirus expression: implications for inflammatory brain diseases. Ann Neurol. 2001;50(4):434–442. doi: 10.1002/ana.1131. [DOI] [PubMed] [Google Scholar]
- 113.Antony JM, van Marle G, Opii W, et al. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat Neurosci. 2004;7(10):1088–1095. doi: 10.1038/nn1319. [DOI] [PubMed] [Google Scholar]
- 114.Antony JM, Ellestad KK, Hammond R, et al. The human endogenous retrovirus envelope glycoprotein, syncytin-1, regulates neuroinflammation and its receptor expression in multiple sclerosis: a role for endoplasmic reticulum chaperones in astrocytes. J Immunol (Baltimore, Md: 1950) 2007;179(2):1210–1224. doi: 10.4049/jimmunol.179.2.1210. [DOI] [PubMed] [Google Scholar]
- 115.Li F, Karlsson H. Expression and regulation of human endogenous retrovirus W elements. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2016;124(1–2):52–66. doi: 10.1111/apm.12478. [DOI] [PubMed] [Google Scholar]
- 116.Mameli G, Astone V, Khalili K, et al. Regulation of the syncytin-1 promoter in human astrocytes by multiple sclerosis-related cytokines. Virology. 2007;362(1):120–130. doi: 10.1016/j.virol.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 117.Firouzi R, Rolland A, Michel M, et al. Multiple sclerosis-associated retrovirus particles cause T lymphocyte-dependent death with brain hemorrhage in humanized SCID mice model. J Neurovirol. 2003;9(1):79–93. doi: 10.1080/13550280390173328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.