Abstract
Homeobox genes encode sequence-specific transcription factors (SSTFs) that recognize specific DNA sequences and regulate organogenesis in all eukaryotes. They are essential in specifying spatial and temporal cell identity and as a result, their mutations often cause severe developmental defects. Pitx genes belong to the PRD class of the highly evolutionary conserved homeobox genes in all animals. Vertebrates possess three Pitx paralogs, Pitx1, Pitx2, and Pitx3 while non-vertebrates have only one Pitx gene. The ancient role of regulating left–right (LR) asymmetry is conserved while new functions emerge to afford more complex body plan and functionalities. In mouse, Pitx1 regulates hindlimb tissue patterning and pituitary development. Pitx2 is essential for the development of the oral cavity and abdominal wall while regulates the formation and symmetry of other organs including pituitary, heart, gut, lung among others by controlling growth control genes upon activation of the Wnt/ß-catenin signaling pathway. Pitx3 is essential for lens development and migration and survival of the dopaminergic neurons of the substantia nigra. Pitx gene mutations are linked to various congenital defects and cancers in humans. Pitx gene family has the potential to offer a new approach in regenerative medicine and aid in identifying new drug targets.
Keywords: Development, Evolution, Disease, Homeobox genes, Pitx
Introduction
Homeobox genes encode sequence-specific transcription factors (SSTFs) recognizing specific DNA sequences that regulate downstream target genes in all eukaryotes and characterized by a conserved 180-bp DNA sequence coding for a 60 amino acid DNA-binding homeodomain. Homeobox genes are essential in specifying cell identity in both spatial and temporal dimensions. Their mutations result in severe developmental defects including loss of specific structures, changes in the identity of one or more body parts or segments, known as homeotic transformations. In fungi, homeobox genes are involved in sexual and fruiting body development [1], cell differentiation and secondary metabolism [2]. In plants, homeobox genes regulate flower patterning [3], leaf development and sensitivity to light [4], and differentiation in the epidermis of the root [5]. In animals, homeobox genes regulate organ patterning and their physiology. Based on the conserved intron–exon structure and unique codomain architectures, 14 homeobox classes have been identified in plants (HD-ZIP I–IV, BEL, KNOX, PLINC, WOX, PHD, DDT, NDX, LD, SAWADEE and PINTOX) [6], and 11 classes are usually recognized in animals (ANTP, PRD, LIM, POU, HNF, SINE, TALE, CUT, PROS, ZF, and CERS) [7].
Pitx genes belong to the PRD class of the homeobox genes that are highly conserved and present in all animals throughout evolution. Vertebrates possess three Pitx paralogs Pitx1, Pitx2, and Pitx3 while non-vertebrates have only one Pitx gene. Due to their early onset of expression during embryogenesis, Pitx genes have been primarily studied in the context of organ and body formation. Pitx1 regulates hindlimb tissue patterning and pituitary development. Pitx2 regulates pituitary, facial, dental, cardiac, intestinal and facial, and skeletal muscle development. Pitx3 regulates lens development and dopaminergic neurons of the substantia nigra. More recently, the involvement of Pitx genes in tumorigenesis and the association of Pitx2 with cardiac arrhythmias shaped them diagnostic markers and potential drug targets. In this review, we provide a holistic view of the Pitx gene family by summarizing recent studies on evolution, expression pattern, organogenesis and associated diseases. We also discuss how Pitx genes could provide a new approach in stem cell biology and cancer-targeted therapy.
Evolutionarily conserved functions of Pitx homeobox genes
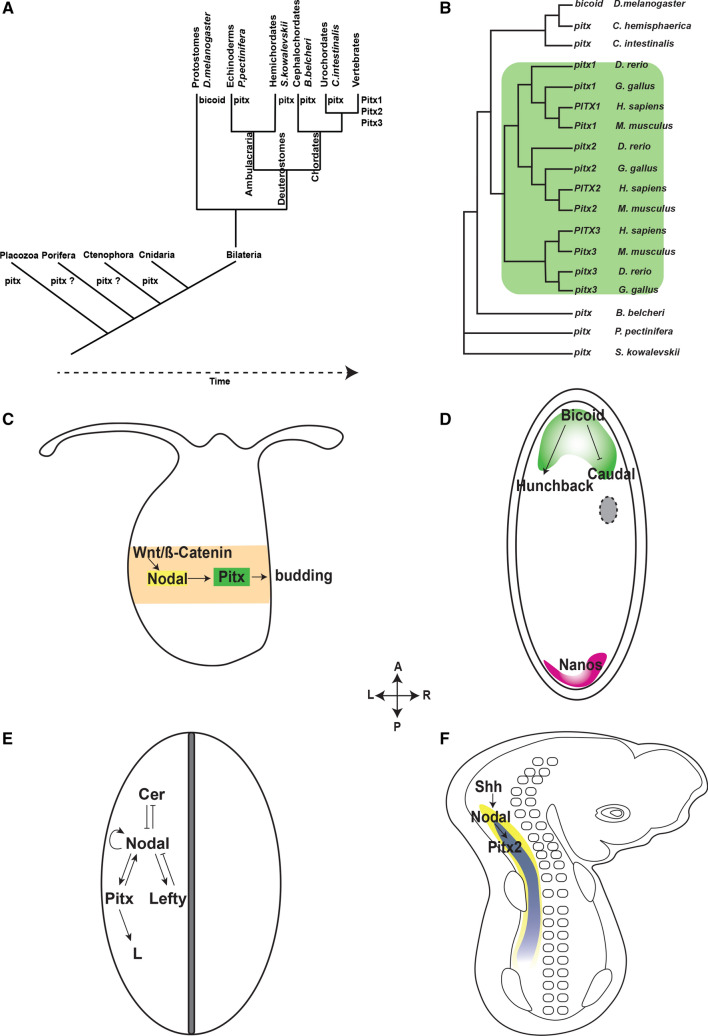
Pitx genes are evolutionarily conserved and have been discovered in almost all members of the animal kingdom (Fig. 1a), from the simplest animals species of Placozoa [8] to very complex humans. In spite of the biological diversity between species in which Pitx genes have been determined, their function in establishing left–right (LR) asymmetry as well as molecular sequence have been conserved throughout the evolution of animals. Molecular phylogeny by Pitx protein sequence alignments shows incredible molecular conservation between different groups of animals (Fig. 1b) implying their essential roles in evolutionary fitness. While invertebrate animals possess only one pitx gene, most vertebrates have three paralogs, pitx1, pitx2, and pitx3 as a result of two gene—duplication events. The first pitx gene duplication gave rise to Pitx3 that enabled vision and the precursor to Pitx1/2. The Pitx1/2 precursor was duplicated again to ultimately become Pitx1 for hindlimb specification and Pitx2 for jaw muscle specification and LR asymmetry. These duplication events must happened around the time that vertebrates were able to explore the world in all dimensions [9].
Fig. 1.
The evolutionarily conserved Pitx gene family. a Pitx genes have been identified in most animal groups from the simplest to the most complex in the animal kingdom. Among the four basal phyla, pitx genes have not been determined in Porifera and Ctenophora. In Placozoa, pitx expression has been evidenced but no associated function has been reported. Pitx genes in members of the Cnidaria and Bilateria have been well characterized. In Protostomes, Bicoid is the homolog of Pitx. Only vertebrates possess Pitx1, Pitx2, and Pitx3. b Phylogenetic tree of the Pitx gene family in animal by protein sequence alignments. All genes are represented in human (H. sapiens), mouse (M. musculus), chick (G. gallus), zebra fish (D. rerio), fruit fly (D. melanogaster), jellyfish (C. hemisphaerica), sea vase (C. intestinalis), amphioxus (B. belcheri), starfish (P. pectinifera), and acorn worm (S. kowalevskii). There is an incredible conservation of PITX protein sequences of species belong to different groups of animals. There are two main duplication events. The first duplication gives rise to Pitx3 and a precursor of Pitx1/2 and the latter of which ultimately becomes Pitx1 and Pitx2 in the second duplication event. c Pitx in molecular pathway that regulates asymmetrical branching in hydra. The initial broad expression of Wnt/ß-Catenin (orange) induces a more localized expression of Nodal (yellow) around the budding site. Nodal activates the transcription of Pitx (green) that is restricted to budding site to initiate branching. d Anterior segment specification by bicoid in fruit fly (D. melanogaster). Two maternal genes that specify the anterior and posterior of the body plan of fruit fly are bicoid (green) and nanos (pink). Before fertilization, bicoid mRNA is most abundant in the anterior while nanos is highly expressed in the opposite pole of the egg. Upon fertilization, bicoid mRNA is translated to protein which enhances the expression of hunchback and other genes associated with the anterior segment while at the same time inhibit the transcription of genes regulating the posterior region. e Left–right asymmetry in non-vertebrate deuterostomes. The molecular pathway that determines the left-side morphology in invertebrates deuterostomes starts with asymmetric expression of Nodal (yellow) that activates the transcription of Pitx (green). PITX proteins then bind to the enhancer regions and activate downstream target genes that are responsible for regulating left–right asymmetry. f Left–right asymmetry in vertebrates. In chick embryo at stage 8, Pitx2 (blue) is expressed broadly in the left lateral plate mesoderm but is completely absent in the right counterpart. Pitx2 acts downstream of Nodal and Shh
The function of pitx genes is not yet well understood in Placozoa, Ctenophora, and Porifera. In Cnidaria Hydra, however, pitx is downstream of Wnt/ß-catenin and Nodal signaling pathways required for biradial asymmetrical bud initiation [10]. The molecular mechanism involves accumulation of ß-catenin at the budding region that subsequently activates the expression of Nodal-related factors (Ndr). The initial broad expression domain of Ndr is eventually restricted to the budding site where Pitx ultimately becomes transcriptionally active (Fig. 1c) [10]. This mechanism represents the same pathway that establishes LR asymmetry in bilaterians.
Pitx genes are homologous to bicoid drosophila maternal gene, which was discovered in 1986 as a regulator of anterior pole development. Before fertilization takes place, bicoid mRNA is most abundant in the anterior [11]. Upon fertilization, bicoid mRNAs are translated into proteins that regulate body arrangement in a concentration-dependent manner. Bicoid is expressed in the anterior pole and nanos is expressed in the posterior pole. Bicoid binds to the enhancer region of hunchback and stimulates its transcription, while inhibits Caudal in the anterior pole. These interactions collectively determine different body segments of the developing Drosophila embryos (Fig. 1d) [11, 12]. Like other homeodomain proteins, bicoid possess the homeodomain that is often found at or near the carboxyl terminal of the encoded protein and is highly homologous across eukaryotic species.
In amphioxus, the earliest diverging chordate lineage, breaking of the symmetry takes place early at neurula stage and is regulated by a regulatory gene network consisted of four genes: Cerberus (Cer), Nodal, Lefty, and Pitx [13]. Located upstream of this gene network is the Hedgehog (Hh) signaling that induces the transcription of Cer on both left and right sides of the embryo prior to the symmetry breaking event [14]. At the onset of asymmetry, expression levels of Cer are depleted on the left side while maintained on the right side as a result of the Nodal expression. The Nodal pathway appears to be at the center of this network that is exclusively expressed on the left side of developing embryo. Nodal is capable of repressing the expression of its upstream repressor Cer and activating its downstream repressor Lefty (Lefty1 and Lefty2) and activator Pitx (Fig. 1e). LR identity of the developing embryo is thus determined by the competition between Lefty and Pitx with Pitx specifying most of the left-side features. Disruption of the Cer–Nodal–Lefty–Pitx gene network can result in embryos with two phenotypic left sides or two phenotypic right sides [13].
Among the three Pitx paralogs possessed by vertebrates, only Pitx2 retains the unique and ancient asymmetric expression pattern during early embryogenesis. Similar to the invertebrate amphioxus (lancelet), the mechanism governing LR asymmetry in most vertebrates is also comprised of Nodal, Lefty, and Pitx2 with the exception of Cer whose molecular involvement remains elusive [15]. This pathway acts downstream of the Sonic Hedgehog (Shh) signaling which exerts the left-sided expression at the first signs of LR asymmetry in the developing chick embryo. Shh indirectly activates the expression of Pitx2 in the left lateral plate mesoderm (LPM) by inducing the asymmetric expression of Nodal which ultimately renders Pitx2 transcriptionally active (Fig. 1f) [16]. Once Nodal is released and cleaved, Nodal homodimer binds to the Transforming Growth Factor ß receptor (TGFRß) TGFRßI/TGFRßII heterodimer that in turn results in phosphorylation and dissociation of R-Smad, followed by the trimerization of two R-Smads and the common partner Smad4 [17, 18]. Signal transduction of Nodal also requires the epidermal growth factor-Cripto-1/FRL-1/Cryptic (EGF-CFC) co-receptors, a family of small cysteine-rich extracellular proteins whose absence results in loss of Nodal signaling activity [19]. The Smad oligomer trans-locates to the nucleus where interacts with the winged-helix transcription factor FoxH1 and/or Mixer homeoproteins on target promoters of Pitx2, Nodal, and Lefty that specify left specificity [20] (Fig. 5b). Nodal is transiently expressed and inhibited by Lefty2 in the left LPM. While Lefty2 remains in the left side, Lefty1 diffuses to the midline and prevents Nodal expression in the right LPM [21–23].Consequently, at mouse embryonic day 8.0 (E8.0), Pitx2 is expressed broadly in the LPM but is absent in the right side. Subsequent studies in mouse further corroborate the necessity of Pitx2 in regulating asymmetric morphogenesis in the developing visceral organs [24–27].
Fig. 5.
Signaling pathways associated with Pitx2. a Pitx2 is induced by the Wnt/Dvl/ß-catenin pathway and is required for effective cell-type-specific proliferation by directly activating specific growth-regulating genes. Upon Wnt3 binding to Frizzled transmembrane protein (FZD), some of ß-catenin is translocated to the nucleus where it forms a complex with Tcf/LEF1 that displaces HDAC1 from the promoter region of Pitx2 and activate its transcription. Regulated exchange of HDAC1/ß-catenin converts Pitx2 from a repressor to activator, to serve as a competence factor required for the temporally ordered and growth factor-dependent recruitment of a series of specific coactivator complexes that prove necessary for induction of the growth control genes Ccnd2, Ccdn1, cjun and cmyc. b The expression of Pitx2 in the LPM is induced by the asymmetric expression of Nodal. Once released and cleaved into mature ligand, Nodal homodimer binds to the TGFRßI/TGFRßII heterodimer and results in a subsequent phosphorylation and dissociation of R-Smad from the TGFßR, followed by the trimerization of two R-Smads and Smad4. Smad trans-locates to the nucleus where it interacts with FoxH1 and/or Mixer on the promoters of Pitx2, Nodal, and Lefty
Genomic loci and alternative splicing
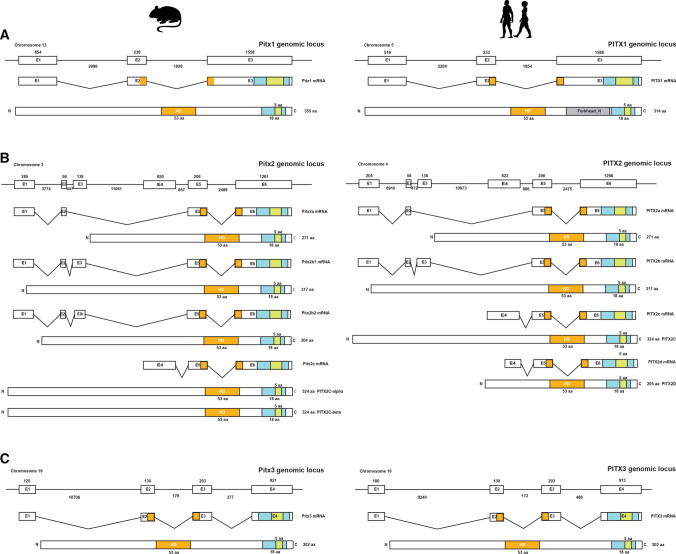
The genomic locus of Pitx1 is located in mouse chromosome 13 and human chromosome 5. Pitx1 gene is 11,147 bp long in mouse and 6,495 bp long in human. Both genes have one transcript of almost 945 bp and harbor three exons, which translate to a PITX1 protein of 315 and 314 amino acids in mouse and human, respectively. In both species, the sequence coding for the homeodomain is shared between exon 2 and exon 3 (Fig. 2a).
Fig. 2.
Genomic loci and alternative splicing of Pitx genes in mouse and human. a The Pitx1 locus is located in mouse chromosome 13 and human chromosome 5. Only one transcript coding for one isoform of PITX1 protein has been identified in both species. b The PITX2 protein possesses several different isoforms as a result of alternative splicing and translation sites. The DNA sequence coding for Pitx2 is located in mouse chromosome 3 and human chromosome 4. c The Pitx3 locus is found in mouse chromosome 19 and human chromosome 10 with only one transcript identified in both species. All PITX proteins possess the homeodomain (orange), OAR-motif (cyan) and a nuclear localization signal (green) located within the OAR
The genomic locus of Pitx2 is located in mouse chromosome 3 and human chromosome 4. Pitx2 gene is 19,717 bp long in mouse and 24,701 bp long in human. Both genes are consisted of 6 exons. The mouse Pitx2 has 3 transcript variants, Pitx2a, Pitx2b, and Pitx2c, in which Pitx2b has an alternative splicing of exon 3 resulting in two additional isoforms Pitx2b1 and Pitx2b2. PITX2C protein has 2 isoforms PITX2c-α and PITX2c-ß produced by alternative translation initiation sites of the Pitx2c mRNA. The human PITX2 gene has 4 transcript variants, Pitx2a, Pitx2b, Pitx2c, and Pitx2d [28]. In both species, the sequence encoding for the homeodomain is shared between exon 5 and exon 6 (Fig. 2b).
The genomic locus of Pitx3 is located in mouse chromosome 19 and human chromosome 10. Pitx3 gene is 13,045 bp long in mouse and 11,324 bp long in human. Both genes consisted of four exons. Pitx3 transcript is 909 bp in both mouse and human and encodes the 302 amino acids in both species (Fig. 2c).
All PITX proteins in mouse and human possess the 15 aa long OAR-motif, a highly conserved motif that was initially discovered in otp, aristaless, rax, cart1, chx10, and s8 (OAR is named using the initials of otp, aristaless and rax). The function of this domain is not yet understood [29]. A 5 amino acid nuclear localization signal located within the OAR-motif is also present in all PITX proteins.
Expression profiling during embryogenesis
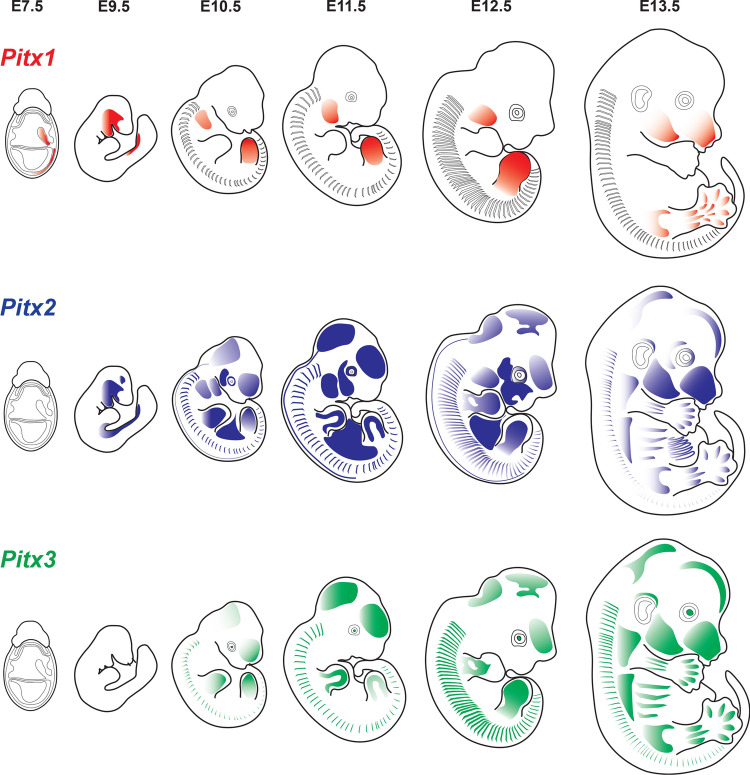
Pitx1 is the earliest gene of the Pitx gene family to be expressed during gastrulation at E6.5 in the posterior and extraembryonic mesoderm (Fig. 3) [30]. From E10.5–E13.5, expression of Pitx1 is restricted to anterior and posterior domains. In the anterior domain, Pitx1 is expressed throughout the stomodeum, maxillary and mandibular epithelium, and the central stripe in the mandibular component of the 1st branchial arch (BA) mesenchyme and its derivatives, the Meckel’s cartilage and the surrounding muscles, the extrinsic muscles of the tongue and the distal end of tongue proper [30, 31]. Epithelial stomodeum-derived organs including the nasopharynx, esophagus, foregut, palate, tongue, adenohypophysis, dental epithelium, submandibular glands maintain Pitx1 expression at least until E13.5. In the posterior domain, Pitx1 expression is first detected at E9.5 in the splanchnic mesoderm and umbilical vessels. By E10.5, its expression domain recess to the posterior LMP. At E11.5, Pitx1 expression is restricted to hindlimb mesenchyme and remains throughout the development of hindlimb. Pitx1 participates in patterning and regulation of various hindlimb tissues, such as bones and muscles [30, 31].
Fig. 3.
Expression patterns of mouse Pitx genes during embryogenesis. The expression patterns of Pitx1 (red), Pitx2 (blue), and Pitx3 (green) are depicted in embryonic day 7.5 (E7.5) to E13.5. Pitx1 is first detected at E7.5 in the allantois and posterior lateral plate mesoderm. As embryogenesis progresses, the expression domain of Pitx1 is restricted to the head and hindlimb. Pitx2 is first detected at E8.0 in the somatopleure and oral cavity. At E9.5, it is strongly expressed in first and second branchial arches (BA) and left lateral plate mesoderm. By E13.5, Pitx2 is expressed in most facial and extraocular muscles, pituitary, tongue, tooth buds, myotomes and skeletal muscles of fore- and hindlimbs. Pitx3 is expressed at E10.5 in the eye lens and somites. By E12.5, Pitx3 expression domain includes the lens, ventral midbrain and their axonal projections, facial muscles and muscles of the trunk and fore- and hindlimbs
Pitx2 expression is first detected at E8.5 in the cephalic mesoderm, the LMP-derived ventral somatopleure, and primordium of first BA (Fig. 3) [32]. Similar to Pitx1, the expression pattern of Pitx2 can be divided into the anterior and posterior domains. In the anterior domain, Pitx2 is prominent in the 1st and 2nd BA by E9.5. At E10.5, Pitx2 is expressed in the ectoderm and mesenchyme of all four BAs. Strong signal of Pitx2 can also be detected in facial and extraocular muscles, tongue, and tooth buds [32]. Pitx1 and Pitx2 are expressed and maintained throughout all stages of pituitary gland development [26, 33]. At E10.5–E14.5, Pitx2 is expressed in a small population of subthalamic nuclei and in the superior colliculus of dorsal midbrain [34]. In the posterior domain, extensive expression of Pitx2 can be observed in various locations of the trunk of developing embryos. It is first expressed in the LPM-derived somatopleure and amnion at E8.5 [32]. About 24 h later, its expression is restricted to the ventrally located somatopleure, which will eventually develop into the body wall. Pitx2 is first expressed in somites at E10.0, and at E10.5 is expressed in the dorsal somatopleure and somites with few Pitx2+ cells migrating to forelimb buds. At E13.5, expression of Pitx2 is well established in fore- and hindlimbs, diaphragm, abdominal wall, and muscles of the back [32, 35]. In the neural tube, Pitx2 is first detected at E11.5–E12.0 and restricted to a small group of interneurons [36]. Pitx2 is also important in regulating LR asymmetry of the heart, lungs, and gastrointestinal tract [24, 26, 37]. Pitx2 expression is detected early in the heart as it undergoes looping. Asymmetric expression of Pitx2 is obvious in the greater curvature (left side) of the rotating stomach from E11.0 to E16.5 as well as the midgut. Pitx2 is also expressed in the caecum at E13.5 and remains until E16.5 [24].
Pitx3 is expressed for the first time at E10.5, in the eye lens and somites (Fig. 3). By E12.5, Pitx3 is well established with strong signals in the lens, ventral midbrain and their axonal projections descending to the striatum and the basal forebrain, head mesenchyme, muscles in the cranial facial region and tongue. Its expression patterns closely resemble those of Pitx2 in developing trunk and limb muscles of developing embryos [35, 38, 39].
Function of Pitx genes during organogenesis
Craniofacial development
Pitx genes are expressed at different developmental stages in the anterior domain of the developing mouse embryo to pattern and specify distinct craniofacial cell lineages. Loss of Pitx1 results in micrognathia, cleft palate, and bifurcated tongue. The mandibular bone is shorter with the proximal part most severely affected (Fig. 4, Table 1) with bone deposition around the Meckel’s cartilage to malleus. Pitx1-null embryos lack gonial bone have smaller tympanic bone and the middle ear cartilage [31], and an abnormal morphology of the mandibular teeth [40]. Loss of Pitx2 leads to defective morphology of the mandibula and maxilla [41], absence of the 1st BA-derived muscles and severe distortion of second BA-derived muscles [42]. At E11.5, the developing jaw of Pitx2-null mice exhibits significantly smaller than normal maxillary and mandibular components and by E13.5 the jaw muscle is missing. Muscle anlage of the 2nd BA-derived mandibular depressor is severely distorted. Pitx2 regulates Tbx1, Tcf21, Msc, and Six2 which in turn activate Myf5 and Myod1 to initiate the myogenic progress in the 1st BA [42]. Thus, Pitx2 is indispensable in specifying myoblasts in the 1st BA.
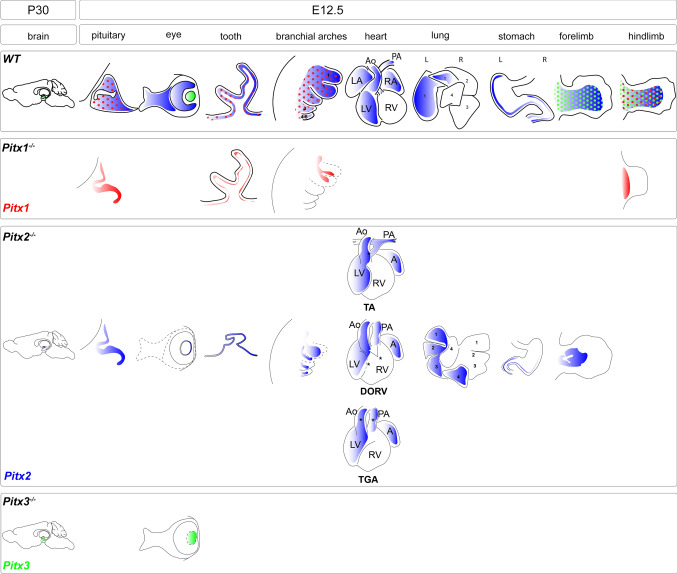
Fig. 4.
Pitx genes in organogenesis. Pitx genes are essential in organogenesis. Pitx expressing adult brain and organs at E12.5 and of wild type and KO mice are depicted. Malformed organs and missing structures in mutants are depicted by a dashed line. Loss of Pitx2 and Pitx3 results in the absence of the subthalamic nuclei and cells of the substantia nigra. Both Pitx1 and Pitx2 are essential for the development of pituitary, tooth, cardiovascular system, and branchial arches-derived tissues. Although all Pitx genes are expressed in the hindlimb, only Pitx1 is crucial for the hindlimb patterning. Pitx2 and Pitx3 are indispensable in eye development. Loss of Pitx2 results in the absence of extraocular muscles, complete loss of the anterior chamber, and lack of corneal epithelium. Loss of Pitx3 causes abnormal lens development. The function of Pitx2 is also critical in heart, lung, stomach, and forelimb muscle development. Pitx2 is asymmetrically expressed in the heart and lungs and its loss results in multiple cardiac phenotypes, right lung isomerism and altered gut rotation. Pitx2 specifies facial myogenesis and regulates skeletal myogenesis
Table 1.
Pitx gene function in organogenesis
| Pitx1 | Pitx2 | Pitx3 | |
|---|---|---|---|
| Mouse | |||
| Mortality aging | Lethal at E14-E15 [55] | Lethal at E12-E14 [25, 26, 37, 41] | Viable and fertile |
| Craniofacial system | Mictognathia;mandibular tooth morphogenesis [40]; cleft palate; abnormal middle ear ossicle; abnormal Meckel’s cartilage [31] | Dental defects, no progression of odontogenesis; cleft palate; small tongue [41, 52] | |
| Nervous system | Induction of midbrain dopaminergic neurons [49] | ||
| Visual system | Ocular development [43]; loss of extraocular muscle [32]; defected lens (aniridia) | Defected lens [47], aphakia [45] | |
| Endocrine system | Diminished somatotropes, thyrotropes and gonadotropes cell lineages [55, 56] | Pituitary development; specification of somatotropes, thyrotropes and gonadotropes cell lineages [26] | |
| Hematopoietic system | Support liver stroma cells [129] | ||
| Cardiovascular system | Non-septated atria; dextrocardia; Truncus Arteriosus (TA); double outlet right ventricle (DORV); transposition of the great arteries (TGA); defected vasculogenesis, thin vascular walls [25, 26, 41, 73]; arrythmogenesis [81, 130] | ||
| Skeletal system | Hindlimb morphogenesis; tibia, fibula, patella, ischium, proximal tarsus [55, 59, 60] | Tendons and skeletal elements of the limbs | |
| Respiratory system | Right pulmonary isomerism [26, 41] | ||
| Alimentary system | Stomach and gut rotation; thin intestinal wall [25, 26, 41, 72, 131] | ||
| Muscular system | Small bifurcated tongue; muscle hypoplasia [87] | Absent jaw muscle [32]; deformed limb musculature; specification of abdominal musculature [63]; myogenesis [66]; maintenance of integrity and energy homeostasis of fetal myofibers [68]; promotion of satellite cells to myogenic state [122], fetal myogenesis [132] | Fetal myogenesis [132] |
| Human | |||
| Craniofacial system | Missing and misplaced teeth [90]; Esophageal Squamous Cell Carcinoma (ESCC) [115] | ||
| Visual system | Axenfeld Rieger Syndrome type 1 (ARS 180,400) [90]; anterior segment dysgenesis 4 (ASSGD4 137,600); ring dermoid of cornea (180,550) [93]; iridogoniodysgenesis syndrome (IGDS); Peters Anomaly [94] | Anterior segment dysgenesis (ASMD); congenital cataract [46] | |
| Endocrine system | Thyroid Cancer [117]; ovarian Cancer [114] | ||
| Cardiovascular system | Tetralogy of Fallot [103]; ventricular and atrial septal defects [102]; transposition of great arteries (TGA) [101]; endocardial cushions defects | ||
| Skeletal system | Club foot [86, 87], Liebenberg syndrome [89] | Short syndrome [97] | |
| Alimentary system | |||
Eye development
Pitx2 is expressed in the periocular mesenchyme at E9.5–10.5. Pronounce expression level of Pitx2 can be observed in the mesenchyme surrounding the retina, presumptive cornea, eyelids and extraocular muscles at E15.5 [37, 41]. Pitx2-null mouse embryos have smaller than normal eyes with irregularly formed pupil and complete loss of the anterior chamber (Fig. 4, Table 1) [37]. The cornea of mutants lacks epithelium and is invaded by a thick layer of undifferentiated mesenchymal cells that express Lmx1b, a marker of periocular mesenchyme [41], while the extrinsic eye muscles fail to form [37]. Though Pitx2 is not expressed in the optic stalk, its expression in the neural crest is crucial for proper development the neural ectoderm [43]. Loss of Pitx2 in neural crest causes misplacement of the eyes deep within the skull medially and directly beneath the brain. Although the lens and retina are present, Pitx2 mutant eyes are attached directly to the ventral hypothalamus and the optic nerve is absent (Table 2) [43]. All three Pitx2 isoforms are expressed in extraocular muscles and each with a distinct temporal pattern. From postnatal day 0 (P0) to P21, Pitx2b expression level is the lowest. Pitx2c expression is the most abundant at P0, which declines significantly by P21. Pitx2-specific ablation in the extraocular muscles results in smaller muscle fibers [44]. Pitx3 is strongly expressed in the developing lens at E10.5 and loss of Pitx3 results in aphakia in adult mice [45, 46]. In Pitx3-null mice, the lenses are formed but do not progress, and by E12.5 only remnant of the lens can be detected (Fig. 4, Table 1). Pitx3 regulates FoxE3 and Prox1 to establish proper proliferation of epithelial cells of the lens [47].
Table 2.
Pitx tissue specific knockout mice and related phenotypes
| Genotype | Tissue | Phenotype |
|---|---|---|
| LhbCre/ + ::Pitx2FL/− | Pituitary (gonadotrophe) | [58] |
| TshbCre/ + ::Pitx2FL/− | Pituitary (thyrotroph) | Decreased body weight, increased TSH level, small thyroid gland [57] |
| Wnt1Cre/ + ::Pitx2FL/FL | Neural crest cells | Misplacement of eyes, absent of optic nerve, partial loss of eye pigmentation [43] |
| NesCre/ + ::Pitx2FL/FL | Embryonic mammillary region | Truncated mammillothalamic tracts [48] |
| MCKCre/ + ::Pitx2FL/FL | Skeletal muscle | Smaller eye muscle fiber sizes [44] |
| MCKCre/ + ::Pitx2FL/Z | Skeletal muscle | Altered integrity and energy homeostasis of fetal myofibers [68] |
| MCKCre/ + ::Pitx2FL/FL | Heart muscle | Impaired tissue regeneration and increased myocardial fat deposit after cardiac injury [85] |
Brain development
Loss of Pitx2 results in the absence of axonal projection in the subthalamic nucleus and in the developing superior colliculus of dorsal midbrain (Fig. 4) [34]. In addition, Pitx2 is required for proper development of the mammillothalamic tract. Conditional KO of Pitx2 in the embryonic mammillothalamic region results in truncation of the mammillothalamic tract (Table 2) [48]. Dopaminergic (DA) neurons in the midbrain composed of the ventral tegmental area (VTA), substantia nigra (SN) and the retrorubral field (RRF). Although Pitx3 is expressed in both DA neurons of the VTA and SN, its function is particularly crucial for development of SN neurons of the midbrain dopaminergic systems [49]. In the absence of Pitx3, SN neurons are depleted by birth (Fig. 4, Table 1) [50, 51].
Tooth development
Tooth development is coordinated by the tightly regulated interaction between Fgf8 and Bmp4 where Fgf8 induces and Bmp4 inhibits dental mesenchyme specification. Both Pitx1 and Pitx2 expressions are restricted to the dental epithelium of developing tooth anlagen. While Pitx2-null mice suffer from severe morphological defects of teeth in both mandible and maxillary bones, loss of Pitx1 function only affects the developing mandibular molars [26, 40]. Loss of Pitx2 disrupts the Fgf8/Bmp4 signaling pathway during odontogenesis. Fgf8 expression fails to maintain in the BA ectoderm after E9.5 [52], while the Bmp4 expression domain is expanded into the oral ectoderm [41]. As a result, mandibular and maxillary dental development arrested at bud and placode stages, respectively (Fig. 4, Table 1) [41, 52]. Pitx1 null mutants have abnormal morphology of the first molar with presence of one cusp instead of two as in WT. Pitx2 expression is not altered in developing mandibular molars of Pitx1 mutants, however, expression levels of Barx1 and Tbx1 are decreased in the dental mesenchyme and epithelium, respectively, in the mandibular molar [40].
Hypothalamus–pituitary axis development
Pitx1 and Pitx2 are expressed early in the invaginating Rathke’s pouch and later in the oral ectoderm-derived anterior lobe [26, 53–56]. Consistent with its expression pattern, Pitx1- and Pitx2-null mice exhibit developmental defects in the anterior pituitary. Anterior pituitary in Pitx1-null mice is smaller with reduced cell number within the gonadotrope and thyrotrope lineages that reflects the lower levels of follicular-stimulating hormone (FSH) and thyroid-stimulating hormone (TSH), respectively [55]. In Pitx2-null mice, the pituitary ceases to progress at E13.5 due to organ arrest and apoptosis [26]. While loss of either Pitx1 or Pitx2 allows normal initiation of the Rathke’s pouch and expression of Lhx3 and Lhx4, Pitx1/Pitx2 double knockout mice fail to activate Lhx3 and initiate invagination of Rathke’s pouch (Fig. 4) [56]. Pitx1 and Pitx2 regulate Lhx3 activation and either one alone is sufficient to initiate proper pituitary development, but both are required for proper proliferation, determination and specification. In addition, Pitx2 can partially compensate for the loss of Pitx1 [57]. Pitx2 is also expressed in the pituitary gonadotrope but its function is not essential to the development of this lineage (Table 2). Ablation of Pitx2 in the pituitary gonadotropes results in no significant changes in anatomy of hormonal physiology [58].
Limb development
Tissues of the vertebrate limbs are of dual origins, the paraxial mesoderm (PM) and LPM. PM is adjacent on both sides of the neural tube, followed by the intermediate mesoderm (IM) and the LPM. PM gives rise to somites, the repetitive, spherical epithelial structures located along both sides of the neural tube. Somites go through an epithelial–mesenchymal transition to become dermomyotome and sclerotome. Dermomyotome further develops into dermatome which gives rise to the skin of limb and myotome which contributes primarily to limb muscles. LPM gives rise to heart, blood vessels and cells and the lining of the body cavities.
Pitx1 is first expressed in the posterior half of the LPM at E6.5. Pitx2 is prominent in the entire left LPM at E8.5. At E10.5, Pitx2 and Pitx3 are expressed in myotomes and embryonic myofibers in both forelimb and hindlimb while the expression of Pitx1 is restricted to the hindlimb area. While Pitx1 is not expressed in the forelimb, it is indispensable in specification and proper patterning of muscle, tendon, cartilage and bones of the hindlimb. Pitx1-null mice possess significantly smaller pelvis, a missing ilium, smaller in size long bones, femurs, tibia, and fibula with the right being more severely affected than the left possibly due to the partial compensation of Pitx2 in the left hindlimb [55, 59, 60]. The diameters of fibula and tibia are more comparable to each other reminiscent the distinct features of ulnar and radius in forelimb. In knee joint, patella and fabella are absent and the fibula directly articulate with the femur resembling the elbow. Hindlimb autopods and digits are not affected [55, 61]. Pitx1 also regulates hindlimb development through regulation of cartilage and muscle development (Fig. 4). Loss of Pitx1 results in significant decreased expression of Foxp4, Snai1, Sox6, Sox8, and Sox9, which regulate cartilage development, and Cxcl12, Eya1, Mef2c, Nfatc2, and Six1, which regulate muscle development [62].
Though Pitx2 is expressed in both fore and hindlimb, its functions have been extensively elucidated in the developing of forelimb muscles. The onset of Pitx2 expression follows Pax3 and Lbx1 in the dermomyotome, myotome, and in the migrating limb muscle precursors [32]. During embryonic and fetal myogenesis, Pitx2 is essential for proper assembly and colonization of muscle anlagen. Loss of Pitx2 results in distortion of muscle anlagen (Fig. 4) [63]. Gene expression profiling of Pitx2-null mouse forelimbs reveals significant reduced expression of genes encoding for cytoskeletal and adhesion proteins which are critical in cell motility [63, 64]. Pitx2 has also been shown to be responsible for extending the time of myoblast progression, promoting formation of sarcomeric structures, and suppressing attachment of neural axons [65]. Pitx2 binds to the core enhancer of Myod1 and activates its expression in parallel with Myf5 [66]. All three Pitx2 isoforms are expressed in mouse limbs. Though its biological implication is not clear, asymmetric expression of Pitx2c has been observed in the developing mouse forelimb [67]. Ablation of Pitx2 in fetal and neonatal myofibers (Pitx2MCK) resulted in smaller in size myofibers and increased number of central nuclei, both signs of pathological fiber repair (Table 2). Mutant fibers exhibit significant reduction of myosin heavy chains normally associated with fast, glycolytic and fetal muscle, and their reduced metabolic activity is a consequence of mitochondria loss due to Foxo3-mediated mitophagy and cell death [68]. Pitx3 follows the Pitx2 expression in fetal stages in both fore and hindlimb [35]. No abnormal limb phenotype has been reported in Pitx3-null mice.
Breaking symmetry—development of heart, lung, and intestinal tract
Pitx2 is critical for proper asymmetric morphogenesis of heart, lung, and gastrointestinal tract. Normal murine lungs are characterized by one single left lobe and four right lobes, and their development is regulated by members of the Fgf and Bmp families [69]. Loss of Pitx2 results in right lung isomerization in which both right and left lungs possess four lobes [26, 37].
Gut originates from the connective tissue and the mesoderm-derived smooth muscles while the epithelial lining is contributed by the endoderm. Its development is a highly complex process beginning with a straight epithelial tube, the primitive gut, located at the midline of the developing embryo. The tube is divided into foregut, midgut, and hindgut along the anterior–posterior axis that will eventually become the stomach, small and large intestines, respectively. Though the detailed mechanisms of gut rotating still remains unclear, it is indisputable that Pitx2 is an important factor regulating this process. Loss of Pitx2 results in improper turning of the stomach with the greater curvature located on the right rather than left [26] (Fig. 4). Pitx2 is expressed in the mesoderm and endoderm of the developing cecum at E12.5 onward. Loss of Pitx2 leads to absence of the mesodermal cecal bud at E13.5 suggesting its critical role in the induction and development of this structure [70]. Pitx2 is also necessary for the development of dorsal mesentery (DM), a major conduit for blood and lymphatic vessels in the gut. Studies in chick have revealed that loss of Pitx2 causes failure of the left dorsal mesentery to condense and LR asymmetry is completely lost resulting in randomized gut looping [71]. Pitx2 directly regulates the chemokine Cxcl12, a ligand for the receptor Cxcr4 whose absence results in defective DM arteriogenesis [72].
Pitx2 is the only member of the Pitx family to be expressed in the heart and is important for correct positioning, proper morphogenesis and function (Fig. 4). Pitx2 is rapidly induced by the Wnt/Dvl/ß-catenin pathway and is required for effective cell-type-specific proliferation in the outflow region of the developing heart by directly activating specific growth-regulating genes (Fig. 5a). Regulated exchange of HDAC1/ß-catenin converts Pitx2 from repressor to activator, to serve as a competence factor required for the temporally ordered and growth factor-dependent recruitment of a series of specific coactivator complexes that prove necessary for induction of the growth control genes Ccnd2, Ccdn1, cjun and cmyc [73–75]. Pitx2 is expressed in the BA-derived secondary heart field and regulates outflow tract development, LR specification of the atria, septation, blood flow and contraction. Pitx2-null mutants display Persistent Truncus Arteriosus (PTA), Dorsal Outlet Right Ventricle (DORV), Transposition of the Great Arteries (TGA) and right atrial isomerism with impaired venous return [26, 37]. Pitx2c is expressed asymmetrically in the heart, primarily within the left atrium [76, 77] and is mostly responsible for the embryonic cardiovascular defects [76]. Pitx2 dictates left atrial cardiomyocytes identity and provides expression signatures for sinoatrial node (SAN) cells [78], which may explain the predisposition to Atrial Fibrillation observed in adult animals with decreased Pitx2 expression [79–81]. Pitx2-null mice display abnormal electrocardiograms with atrioventricular block, irregular R–R intervals and low voltage P waves due to altered activity of genes that encode for calcium handling, gap junctions and ion channels [82]. Pulmonary vein and the sinoatrial node development are severely impaired [80]. Ventricle septa in mutant hearts are dysmorphic in addition to the hypoplastic right ventricle and swelled atrioventricular canal region between the left atrium and left ventricle [37]. Mutant hearts fail to properly develop tricuspid and mitral valves and the leaflets of the vena cava [25, 37, 83]. Pitx2 promotes heart repair by activating an antioxidant response via encoding electron transport chain components after cardiac injury [84], and by maintaining a proper cardiac cellular composition during regeneration via the maintenance of proper mitochondrial structure and function [85].
PITX genes in human diseases
Congenital diseases
Clubfoot, a congenital disorder that mostly affects the skeletal and connective tissues of the right lower limb, though not very common, has been linked to mutation of PITX1 [86] (Table 1). Individuals with clubfoot have reduced muscle and bone volume and abnormal vasculatures that are usually more severe under the knee in the affected foot [87]. Though not as severe, these phenotypical features resemble the musculoskeletal defects present in Pitx1-null mice suggesting a similar pathway that regulates hindlimb and leg morphogenesis in both mouse and human.
Liebenberg syndrome is a rare autosomal dominant congenital disorder associated with a deletion upstream to the PITX1 gene which contains the H2AFY gene. Patients exhibit skeletal malformations of the upper limbs, that resemble the posterior limb features including enlarged elbow that functions more like knee, fused wrist bones and shorter than normal fingers similar to toes size [88]. These skeletal features are identical to mouse with ectopic expression of Pitx1 in the forelimb. The deleted region upstream of PITX1 gene contains a regulatory region that suppresses its expression in the upper limb [89].
PITX2 was first discovered in human in 1996 whose mutation has been determined as one of the causes of Axenfeld–Rieger syndrome (ARS), a rare autosomal dominant genetic disorder [90]. ARS is primarily an eye disorder with the majority of patients present with anterior segment dysgenesis (ASD) that affects the cornea, iris, and lens. The disease’s manifestation varies from one individual to the next with singular or combination of anomalies including corneal opacity, posterior embryotoxon, iris hypoplasia, corectopia or polycoria, and adhesions between the iris and cornea or lens and cornea. In addition, patients affected by ARS often have high risk of developing glaucoma [91]. ARS, however, also affects other craniofacial features, e.g., peg-like incisors, oligodontia (absence of more than six teeth in primary, permanent or both dentitions), microdontia (smaller than normal teeth), underdeveloped jaw, protruding lower lip, and widely-spaced eyes [90]. In addition to ophthalmic and craniofacial malformations, ARS patients are often diagnosed with cardiovascular abnormalities, short stature, developmental delay, Meckel’s diverticulum, anal anomalies, hearing loss, omphalocele, and hypospadias [92].
Several other mutations of PITX2 have been reported to be associated with different ophthalmic conditions. A missense PITX2 mutation was found in patients diagnosed with ring dermoid of the cornea [93]. Peters’ anomaly (combination of corneal opacity, defects in the posterior layers of the cornea, and lenticulo-corneal and/or irido-corneal adhesions) and persistent hyperplastic primary vitreous is known to be associated with a missense mutation of C to A at 649 bp in PITX2 coding region [94]. Iris hypoplasia, a rare disease characterized by an abnormally developed iris stroma and malformations of the eyes and umbilicus, is linked to PITX2 missense mutation in which C is substituted by T at 205 bp [95]. Most recently whole genome sequencing on DNA of two patients from a family with ASD and glaucoma shows mutations of the conserved non-coding elements of PITX2 to be the probable cause of the congenital disorders [96].
SHORT syndrome is a rare human genetic disorder associated with PITX2 mutations. The condition is characterized by short stature, hyperextensibility, hernia, ocular depression, Rieger anomaly [91], teething delay and insulin resistance. Chromosome analysis of affected individuals reveals a translocation that has occurred between chromosome band 1q31.2 and 4q25 where PITX2 is located in the genetic locus 4q25 [97].
PITX2 mutations were discovered in patients with congenital cardiac diseases, including familial atrial fibrillation (AF) [98, 99], atrial septal defects [100], TGA, ventricular septal defect (VSD) [101], endocardial cushion defect (ECD) [102], and tetralogy of Fallot [103] (Table 1). These mutations are caused by altered amino acids that are evolutionarily highly conserved and are transmitted in an autosomal dominant pattern with complete penetrance. Biochemical analyses of PITX2 function reveals significantly reduced transcriptional activity of PITX2 in these patients [98–103].
PITX3 mutations mainly affect the eyes. Several point and chromosomal mutations have been associated with ASD, autosomal dominant congenital cataract (ADCC), and congenital posterior subcapsular cataracts (CPSC) (Table 1). In all these mutations, functional assays revealed that the mutated PITX3 proteins retained their nuclear localization regions but exerted reduced transactivation activity [104–107].
Cancer
While PITX2 hypomorphic mutations lead to developmental defects in various organs, overexpression of PITX2 has recently emerged as a promoter of tumorigenesis. Increased expression level and hypomethylation of PITX2 are associated with advanced progression and poor prognosis of lung adenocarcinoma (LUAD) [108, 109], colorectal cancer [110, 111], ovarian cancer [112–114], esophageal squamous cell carcinoma (ESCC) [115] and thyroid cancer [116, 117]. In LUAD, PITX2 promotes tumorigenesis by transcriptional regulation of WNT3A, and activation of the Wnt/ß-catenin signaling pathway. Elimination of PITX2 in the lung cancer cells inhibits their proliferation and ability to metastasize [109]. The highly invasive nature of ovarian cancers may also be attributed to the overexpression of PITX2 that activates the Transforming Growth Factor ß (TGFß) signaling pathway and activin-A that in turn increase the motility of malignant cells to migrate to other body parts [112]. PITX2 induces CCNA1 and CCND2 in thyroid cancer cells [116, 117]. Decreased expression and hypermethylation of PITX2 in prostate cancer are associated with poor prognosis [118] and thus, PITX2 methylation has been suggested as a biomarker for determination of occurrence and recurrence risk of prostate cancer [119, 120].
Regenerative medicine
Pitx genes are involved in the process of heart and skeletal muscle regeneration. Pitx2-Yap interaction is important for Hippo-deficient cardiac regeneration which cooperatively activates the redox balance and the repair activity in adult cardiomyocytes [84]. In addition, Pitx2 gain-of-function protects mature cardiomyocytes from ischemic injury and promotes heart repair by maintaining proper cardiac cellular composition during heart regeneration via the maintenance of proper mitochondrial structure and function [85]. Adult myogenesis post-injury relies upon the ability of satellite cells to proliferate, migrate, differentiate, and fuse into the existing muscle fibers. Expression of Pitx genes in cultured myoblasts promotes myogenic differentiation and fusion into multinucleated myotubes [121]. Pitx2c accelerates cell proliferation in early activated satellite cells by downregulating miR-15b, miR-106b, miR-23b, and miR-503 subsequently activation of cell cycle genes and expansion of the Myf5+ satellite cell population [122]. Satellite-cells of Pitx2/3 double KO mice exhibit elevated level of reactive oxygen species that leads to regeneration failure suggesting significant functions of Pitx genes in regulating redox state of the regenerating muscle tissue [123].
Conclusion
During evolution, the duplication events that lead to the emergence of the three members of Pitx family possibly coincided with the appearances of animals capable of exploring the world in three dimensions. Pitx genes are involved in the evolution of metazoans and are critical players of organogenesis. Pitx1 patterns the fins/hindlimbs by allowing animals to swim/walk after be initiated by the expression of Tbx5 and Tbx4. Pitx2 breaks the symmetry of several organs by favoring the left side to accommodate complex organ systems to fit in a compact body and forms a blood circulatory system by the asymmetric regression of the aortic arch. Pitx2 specifies the jaw and ocular muscles for better adaptation of the terrestrial animals. Pitx3 specifies their lens by enabling a better vision for hunting and protection from predators. Hindlimb formation requires Tbx4 for its specification [124, 125] and Pitx1 for patterning [31, 126]. Pax6 is required for eye development [127, 128] followed by Pitx2 and Pitx3 for the fine tuning of the muscle movement and lens architecture, respectively [47]. Combinatorial networks might be applied to generate 3D in vitro body parts for the need of aging of disease. The Wnt/ß-catenin/Pitx2/Cnnd pathway [73] is involved in activating postmitotic differentiated cells to enter the cell cycle, a paradigm for cancer, which can be used as a targeted platform for drug discovery.
Acknowledgements
We apologize to our colleagues whose work could not be cited due to space limitations and our focused perspective. We thank Yen Diep for her continuous support. This work was supported by the College of Pharmacy, Oregon State University.
Author contributions
TQT wrote the manuscript; CK reviewed, wrote and edited the manuscript.
Funding
This work was supported by the College of Pharmacy at the Oregon State University.
Availability of data and material
Information provided is based on published works from our team and many others.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vonk PJ, Ohm RA. The role of homeodomain transcription factors in fungal development. Fungal Biol Rev. 2018;32:219–230. doi: 10.1016/j.fbr.2018.04.002. [DOI] [Google Scholar]
- 2.Son S-H, Son Y-E, Cho H-J, Chen W, Lee M-K, Kim L-H, Han D-M, Park H-S. Homeobox proteins are essential for fungal differentiation and secondary metabolism in Aspergillusnidulans. Sci Rep. 2020;10:6094. doi: 10.1038/s41598-020-63300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsumoto N, Okada K. A homeobox gene, PRESSED FLOWER, regulates lateral axis-dependent development of Arabidopsis flowers. Genes Dev. 2001;15:3355–3364. doi: 10.1101/gad.931001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Henriksson E, Söderman E, Henriksson KN, Sundberg E, Engström P. The arabidopsis homeobox gene, ATHB16, regulates leaf development and the sensitivity to photoperiod in Arabidopsis. Dev Biol. 2003;264:228–239. doi: 10.1016/j.ydbio.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks MD, Schiefelbein JW. The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development. 1996;122:1253–1260. doi: 10.1242/dev.122.4.1253. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee K, Brocchieri L, Bürglin TR. A comprehensive classification and evolutionary analysis of plant homeobox genes. Mol Biol Evol. 2009;26:2775–2794. doi: 10.1093/molbev/msp201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holland PW, Booth HAF, Bruford EA. Classification and nomenclature of all human homeobox genes. BMC Biol. 2007;5:47. doi: 10.1186/1741-7007-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava M, Begovic E, Chapman J, Putnam NH, Hellsten U, Kawashima T, Kuo A, Mitros T, Salamov A, Carpenter ML, Signorovitch AY, Moreno MA, Kamm K, Grimwood J, Schmutz J, Shapiro H, Grigoriev IV, Buss LW, Schierwater B, Dellaporta SL, Rokhsar DS. The Trichoplax genome and the nature of placozoans. Nature. 2008;454:955–960. doi: 10.1038/nature07191. [DOI] [PubMed] [Google Scholar]
- 9.Angotzi AR, Ersland KM, Mungpakdee S, Stefansson S, Chourrout D. Independent and dynamic reallocation of pitx gene expression during vertebrate evolution, with emphasis on fish pituitary development. Gene. 2008;417:19–26. doi: 10.1016/j.gene.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe H, Schmidt HA, Kuhn A, Höger SK, Kocagöz Y, Laumann-Lipp N, Özbek S, Holstein TW. Nodal signalling determines biradial asymmetry in Hydra. Nature. 2014;515:112–115. doi: 10.1038/nature13666. [DOI] [PubMed] [Google Scholar]
- 11.Berleth T, Burri M, Thoma G, Bopp D, Richstein S, Frigerio G, Noll M, Nüsslein-Volhard C. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J. 1988;7:1749–1756. doi: 10.1002/j.1460-2075.1988.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frohnhöfer HG, Nüsslein-Volhard C. Organization of anterior pattern in the Drosophila embryo by the maternal gene bicoid. Nature. 1986;324:120–125. doi: 10.1038/324120a0. [DOI] [Google Scholar]
- 13.Li G, Liu X, Xing C, Zhang H, Shimeld SM, Wang Y. Cerberus–Nodal–Lefty–Pitx signaling cascade controls left–right asymmetry in amphioxus. Proc Natl Acad Sci. 2017;114:3684–3689. doi: 10.1073/pnas.1620519114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu G, Li G, Wang H, Wang Y. Hedgehog participates in the establishment of left-right asymmetry during amphioxus development by controlling Cerberus expression. Development. 2017;144:4694–4703. doi: 10.1242/dev.157172. [DOI] [PubMed] [Google Scholar]
- 15.Yoshiba S, Hamada H. Roles of cilia, fluid flow, and Ca2+ signaling in breaking of left-right symmetry. Trends Genet. 2014;30:10–17. doi: 10.1016/j.tig.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Logan M, Pagán-Westphal SM, Smith DM, Paganessi L, Tabin CJ. The transcription factor Pitx2 mediates situs-specific morphogenesis in response to left–right asymmetric signals. Cell. 1998;94:307–317. doi: 10.1016/S0092-8674(00)81474-9. [DOI] [PubMed] [Google Scholar]
- 17.Schier AF, Shen MM. Nodal signalling in vertebrate development. Nature. 2000;403:385–389. doi: 10.1038/35000126. [DOI] [PubMed] [Google Scholar]
- 18.Whitman M. Nodal signaling in early vertebrate embryos: themes and variations. Dev Cell. 2001;1:605–617. doi: 10.1016/S1534-5807(01)00076-4. [DOI] [PubMed] [Google Scholar]
- 19.Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- 20.Shen MM, Schier AF. The EGF–CFC gene family in vertebrate development. Trends Genet. 2000;16:303–309. doi: 10.1016/s0168-9525(00)02006-0. [DOI] [PubMed] [Google Scholar]
- 21.Campione M, Franco D. Current perspectives in cardiac laterality. J Cardiovasc Dev Dis. 2016 doi: 10.3390/jcdd3040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimes DT, Burdine RD. Left–right patterning: breaking symmetry to asymmetric morphogenesis. Trends Genet. 2017;33:616–628. doi: 10.1016/j.tig.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franco D, Sedmera D, Lozano-Velasco E. Multiple roles of Pitx2 in cardiac development and disease. J Cardiovasc Dev Dis. 2017 doi: 10.3390/jcdd4040016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campione M, Steinbeisser H, Schweickert A, Deissler K, van Bebber F, Lowe LA, Nowotschin S, Viebahn C, Haffter P, Kuehn MR, Blum M. The homeobox gene Pitx2: mediator of asymmetric left–right signaling in vertebrate heart and gut looping. Development. 1999;126:1225–1234. doi: 10.1242/dev.126.6.1225. [DOI] [PubMed] [Google Scholar]
- 25.Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, Ohuchi H, Suehiro A, Motegi Y, Nakahara Y, Kondo S, Yokoyama M. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development. 1999;126:5749–5758. doi: 10.1242/dev.126.24.5749. [DOI] [PubMed] [Google Scholar]
- 26.Lin CR, Kioussi C, O’Connell S, Briata P, Szeto D, Liu F, Izpisúa-Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature. 1999;401:279–282. doi: 10.1038/45803. [DOI] [PubMed] [Google Scholar]
- 27.Yoshioka H, Meno C, Koshiba K, Sugihara M, Itoh H, Ishimaru Y, Inoue T, Ohuchi H, Semina EV, Murray JC, Hamada H, Noji S. Pitx2, a bicoid-type homeobox gene, is involved in a lefty-signaling pathway in determination of left–right asymmetry. Cell. 1998;94:299–305. doi: 10.1016/s0092-8674(00)81473-7. [DOI] [PubMed] [Google Scholar]
- 28.Lamba P, Hjalt TA, Bernard DJ. Novel forms of Paired-like homeodomain transcription factor 2 (PITX2): generation by alternative translation initiation and mRNA splicing. BMC Mol Biol. 2008;9:31. doi: 10.1186/1471-2199-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furukawa T, Kozak CA, Cepko CL. rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. Proc Natl Acad Sci. 1997;94:3088–3093. doi: 10.1073/pnas.94.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanctôt C, Lamolet B, Drouin J. The bicoid-related homeoprotein Ptx1 defines the most anterior domain of the embryo and differentiates posterior from anterior lateral mesoderm. Development. 1997;124:2807–2817. doi: 10.1242/dev.124.14.2807. [DOI] [PubMed] [Google Scholar]
- 31.Lanctôt C, Moreau A, Chamberland M, Tremblay ML, Drouin J. Hindlimb patterning and mandible development require the Ptx1 gene. Development. 1999;126:1805–1810. doi: 10.1242/dev.126.9.1805. [DOI] [PubMed] [Google Scholar]
- 32.Shih HP, Gross MK, Kioussi C. Expression pattern of the homeodomain transcription factor Pitx2 during muscle development. Gene Expr Patterns. 2007;7:441–451. doi: 10.1016/j.modgep.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Drouin J, Lamolet B, Lamonerie T, Lanctôt C, Tremblay JJ. The PTX family of homeodomain transcription factors during pituitary developments. Mol Cell Endocrinol. 1998;140:31–36. doi: 10.1016/s0303-7207(98)00026-4. [DOI] [PubMed] [Google Scholar]
- 34.Martin DM, Skidmore JM, Philips ST, Vieira C, Gage PJ, Condie BG, Raphael Y, Martinez S, Camper SA. Pitx2 is required for normal development of neurons in the mouse subthalamic nucleus and midbrain. Dev Biol. 2004;267:93–108. doi: 10.1016/j.ydbio.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 35.L’Honoré A, Coulon V, Marcil A, Lebel M, Lafrance-Vanasse J, Gage P, Camper S, Drouin J. Sequential expression and redundancy of Pitx2 and Pitx3 genes during muscle development. Dev Biol. 2007;307:421–433. doi: 10.1016/j.ydbio.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 36.Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic pre-motor interneurons modulates mouse locomotor activity. Neuron. 2009;64:645–662. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126:4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- 38.Zhao S, Maxwell S, Jimenez-Beristain A, Vives J, Kuehner E, Zhao J, O’Brien C, de Felipe C, Semina E, Li M. Generation of embryonic stem cells and transgenic mice expressing green fluorescence protein in midbrain dopaminergic neurons. Eur J Neurosci. 2004;19:1133–1140. doi: 10.1111/j.1460-9568.2004.03206.x. [DOI] [PubMed] [Google Scholar]
- 39.Smidt MP, van Schaick HSA, Lanctôt C, Tremblay JJ, Cox JJ, van der Kleij AAM, Wolterink G, Drouin J, Burbach JPH. A homeodomain gene Ptx3 has highly restricted brain expression in mesencephalic dopaminergic neurons. Proc Natl Acad Sci USA. 1997;94:13305–13310. doi: 10.1073/pnas.94.24.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitsiadis TA, Drouin J. Deletion of the Pitx1 genomic locus affects mandibular tooth morphogenesis and expression of the Barx1 and Tbx1 genes. Dev Biol. 2008;313:887–896. doi: 10.1016/j.ydbio.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 41.Lu M-F, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left–right asymmetry and craniofacial development. Nature. 1999;401:276–278. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- 42.Shih HP, Gross MK, Kioussi C. Cranial muscle defects of Pitx2 mutants result from specification defects in the first branchial arch. Proc Natl Acad Sci. 2007;104:5907–5912. doi: 10.1073/pnas.0701122104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans AL, Gage PJ. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Genet. 2005;14:3347–3359. doi: 10.1093/hmg/ddi365. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Y, Cheng G, Dieter L, Hjalt TA, Andrade FH, Stahl JS, Kaminski HJ. An altered phenotype in a conditional knockout of Pitx2 in extraocular muscle. Invest Ophthalmol Vis Sci. 2009;50:4531–4541. doi: 10.1167/iovs.08-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varnum DS, Stevens LC. Aphakia, a new mutation in the mouse. J Hered. 1968;59:147–150. doi: 10.1093/oxfordjournals.jhered.a107667. [DOI] [PubMed] [Google Scholar]
- 46.Semina EV, Reiter RS, Murray JC. Isolation of a new homeobox gene belonging to the Pitx/Rieg Family: expression during lens development and mapping to the aphakia region on mouse chromosome 19. Hum Mol Genet. 1997;6:2109–2116. doi: 10.1093/hmg/6.12.2109. [DOI] [PubMed] [Google Scholar]
- 47.Ho H-Y, Chang K-H, Nichols J, Li M. Homeodomain protein Pitx3 maintains the mitotic activity of lens epithelial cells. Mech Dev. 2009;126:18–29. doi: 10.1016/j.mod.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Skidmore JM, Waite MR, Alvarez-Bolado G, Puelles L, Martin DM. A novel TaulacZ allele reveals a requirement for Pitx2 in formation of the mammillothalamic tract. Genesis. 2012;50:67–73. doi: 10.1002/dvg.20793. [DOI] [PubMed] [Google Scholar]
- 49.Martinat C, Bacci J-J, Leete T, Kim J, Vanti WB, Newman AH, Cha JH, Gether U, Wang H, Abeliovich A. Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype. Proc Natl Acad Sci USA. 2006;103:2874–2879. doi: 10.1073/pnas.0511153103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van den Munckhof P, Luk KC, Ste-Marie L, Montgomery J, Blanchet PJ, Sadikot AF, Drouin J. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development. 2003;130:2535–2542. doi: 10.1242/dev.00464. [DOI] [PubMed] [Google Scholar]
- 51.Nunes I, Tovmasian LT, Silva RM, Burke RE, Goff SP. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci. 2003;100:4245–4250. doi: 10.1073/pnas.0230529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu W, Selever J, Lu M-F, Martin JF. Genetic dissection of Pitx2 in craniofacial development uncovers new functions in branchial arch morphogenesis, late aspects of tooth morphogenesis and cell migration. Development. 2003;130:6375–6385. doi: 10.1242/dev.00849. [DOI] [PubMed] [Google Scholar]
- 53.Charles MA, Suh H, Hjalt TA, Drouin J, Camper SA, Gage PJ. PITX genes are required for cell survival and Lhx3 activation. Mol Endocrinol. 2005;19:1893–1903. doi: 10.1210/me.2005-0052. [DOI] [PubMed] [Google Scholar]
- 54.Lanctôt C, Gauthier Y, Drouin J. Pituitary homeobox 1 (Ptx1) Is differentially expressed during pituitary development. Endocrinology. 1999;140:1416–1422. doi: 10.1210/endo.140.3.6549. [DOI] [PubMed] [Google Scholar]
- 55.Szeto DP, Rodriguez-Esteban C, Ryan AK, O’Connell SM, Liu F, Kioussi C, Gleiberman AS, Izpisúa-Belmonte JC, Rosenfeld MG. Role of the Bicoid-related homeodomain factor Pitx1 in specifying hindlimb morphogenesis and pituitary development. Genes Dev. 1999;13:484–494. doi: 10.1101/gad.13.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suh H, Gage PJ, Drouin J, Camper SA. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development. 2002;129:329–337. doi: 10.1242/dev.129.2.329. [DOI] [PubMed] [Google Scholar]
- 57.Castinetti F, Brinkmeier ML, Gordon DF, Vella KR, Kerr JM, Mortensen AH, Hollenberg A, Brue T, Ridgway EC, Camper SA. PITX2 AND PITX1 regulate thyrotroph function and response to hypothyroidism. Mol Endocrinol. 2011;25:1950–1960. doi: 10.1210/me.2010-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charles MA, Mortensen AH, Potok MA. Camper SA (2008) Pitx2 deletion in pituitary gonadotropes is compatible with gonadal development, puberty and fertility. Genes. 2000;46:507–514. doi: 10.1002/dvg.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duboc V, Logan MPO. Pitx1 is necessary for normal initiation of hindlimb outgrowth through regulation of Tbx4 expression and shapes hindlimb morphologies via targeted growth control. Development. 2011;138:5301–5309. doi: 10.1242/dev.074153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nemec S, Luxey M, Jain D, Huang Sung A, Pastinen T, Drouin J. Pitx1 directly modulates the core limb development program to implement hindlimb identity. Development. 2017;144:3325–3335. doi: 10.1242/dev.154864. [DOI] [PubMed] [Google Scholar]
- 61.Lanctot C, Lamolet B, Drouin J. The bicoid-related homeoprotein Ptx1 defines the most anterior domain of the embryo and differentiates posterior from anterior lateral mesoderm. Development. 1997;124:2807–2817. doi: 10.1242/dev.124.14.2807. [DOI] [PubMed] [Google Scholar]
- 62.Wang JS, Infante CR, Park S, Menke DB. PITX1 promotes chondrogenesis and myogenesis in mouse hindlimbs through conserved regulatory targets. Dev Biol. 2018;434:186–195. doi: 10.1016/j.ydbio.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campbell AL, Shih H-P, Xu J, Gross MK, Kioussi C. Regulation of motility of myogenic cells in filling limb muscle anlagen by Pitx2. PLoS ONE. 2012;7:e35822. doi: 10.1371/journal.pone.0035822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campbell AL, Eng D, Gross MK, Kioussi C. Prediction of gene network models in limb muscle precursors. Gene. 2012;509:16–23. doi: 10.1016/j.gene.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh AJ, Gross MK, Filtz TM, Kioussi C. Mapping the chromatin state dynamics in myoblasts. Gene Rep. 2016;3:5–13. doi: 10.1016/j.genrep.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.L’Honoré A, Ouimette J-F, Lavertu-Jolin M, Drouin J. Pitx2 defines alternate pathways acting through MyoD during limb and somitic myogenesis. Development. 2010;137:3847–3856. doi: 10.1242/dev.053421. [DOI] [PubMed] [Google Scholar]
- 67.Shiratori H, Yashiro K, Iwai N, Oki S, Minegishi K, Ikawa Y, Kanata K, Hamada H. Self-regulated left–right asymmetric expression of Pitx2c in the developing mouse limb. Dev Biol. 2014;395:331–341. doi: 10.1016/j.ydbio.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Chang C-N, Singh AJ, Gross MK, Kioussi C. Requirement of Pitx2 for skeletal muscle homeostasis. Dev Biol. 2019;445:90–102. doi: 10.1016/j.ydbio.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hogan BLM. Morphogenesis. Cell. 1999;96:225–233. doi: 10.1016/S0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 70.Nichol PF, Saijoh Y. Pitx2 is a critical early regulatory gene in normal cecal development. J Surg Res. 2011;170:107–111. doi: 10.1016/j.jss.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurpios NA, Ibañes M, Davis NM, Lui W, Katz T, Martin JF, Izpisúa Belmonte JC, Tabin CJ. The direction of gut looping is established by changes in the extracellular matrix and in cell:cell adhesion. Proc Natl Acad Sci USA. 2008;105:8499–8506. doi: 10.1073/pnas.0803578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahadevan A, Welsh IC, Sivakumar A, Gludish DW, Shilvock AR, Noden DM, Kurpios NA. The left-right Pitx2 pathway drives organ-specific arterial and lymphatic development in the intestine. Dev Cell. 2014;31:690–706. doi: 10.1016/j.devcel.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. Identification of a Wnt/Dvl/β-catenin→Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111:673–685. doi: 10.1016/S0092-8674(02)01084-X. [DOI] [PubMed] [Google Scholar]
- 74.Baek SH, Kioussi C, Briata P, Wang D, Nguyen HD, Ohgi KA, Glass CK, Wynshaw-Boris A, Rose DW, Rosenfeld MG. Regulated subset of G1 growth-control genes in response to derepression by the Wnt pathway. Proc Natl Acad Sci. 2003;100:3245–3250. doi: 10.1073/pnas.0330217100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Briata P, Ilengo C, Corte G, Moroni C, Rosenfeld MG, Chen C-Y, Gherzi R. The Wnt/β-catenin→Pitx2 Pathway controls the turnover of Pitx2 and other unstable mRNAs. Mol Cell. 2003;12:1201–1211. doi: 10.1016/S1097-2765(03)00407-6. [DOI] [PubMed] [Google Scholar]
- 76.Liu C, Liu W, Palie J, Lu MF, Brown NA, Martin JF. Pitx2c patterns anterior myocardium and aortic arch vessels and is required for local cell movement into atrioventricular cushions. Development. 2002;129:5081–5091. doi: 10.1242/dev.129.21.5081. [DOI] [PubMed] [Google Scholar]
- 77.Zhang M, Hill MC, Kadow ZA, Suh JH, Tucker NR, Hall AW, Tran TT, Swinton PS, Leach JP, Margulies KB, Ellinor PT, Li N, Martin JF. Long-range Pitx2c enhancer–promoter interactions prevent predisposition to atrial fibrillation. Proc Natl Acad Sci USA. 2019;116:22692–22698. doi: 10.1073/pnas.1907418116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ai D, Liu W, Ma L, Dong F, Lu M-F, Wang D, Verzi MP, Cai C, Gage PJ, Evans S, Black BL, Brown NA, Martin JF. Pitx2 regulates cardiac left–right asymmetry by patterning second cardiac lineage-derived myocardium. Dev Biol. 2006;296:437–449. doi: 10.1016/j.ydbio.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ammirabile G, Tessari A, Pignataro V, Szumska D, Sutera Sardo F, Benes J, Balistreri M, Bhattacharya S, Sedmera D, Campione M. Pitx2 confers left morphological, molecular, and functional identity to the sinus venosus myocardium. Cardiovasc Res. 2012;93:291–301. doi: 10.1093/cvr/cvr314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mommersteeg MTM, Brown NA, Prall OWJ, de Gier-de VC, Harvey RP, Moorman AFM, Christoffels VM. Pitx2c and Nkx2-5 are required for the formation and identity of the pulmonary myocardium. Circ Res. 2007;101:902–909. doi: 10.1161/CIRCRESAHA.107.161182. [DOI] [PubMed] [Google Scholar]
- 81.Wang J, Klysik E, Sood S, Johnson RL, Wehrens XHT, Martin JF. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proc Natl Acad Sci. 2010;107:9753–9758. doi: 10.1073/pnas.0912585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ye T, Min Z, Lele Li, Yan B, Yuefang Z, Moon AM, Kaminski HJ, Martin JF. Pitx2, an atrial fibrillation predisposition gene, directly regulates ion transport and intercalated disc genes. Circ Cardiovasc Genet. 2014;7:23–32. doi: 10.1161/CIRCGENETICS.113.000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma H-Y, Xu J, Eng D, Gross MK, Kioussi C. Pitx2-mediated cardiac outflow tract remodeling. Dev Dyn Off Publ Am Assoc Anat. 2013;242:456–468. doi: 10.1002/dvdy.23934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tao G, Kahr PC, Morikawa Y, Zhang M, Rahmani M, Heallen TR, Li L, Sun Z, Olson EN, Amendt BA, Martin JF. Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury. Nature. 2016;534:119–123. doi: 10.1038/nature17959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li L, Tao G, Hill MC, Zhang M, Morikawa Y, Martin JF. Pitx2 maintains mitochondrial function during regeneration to prevent myocardial fat deposition. Development. 2018 doi: 10.1242/dev.168609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gurnett CA, Alaee F, Kruse LM, Desruisseau DM, Hecht JT, Wise CA, Bowcock AM, Dobbs MB. Asymmetric lower–limb malformations in individuals with homeobox PITX1 gene mutation. Am J Hum Genet. 2008;83:616–622. doi: 10.1016/j.ajhg.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alvarado DM, McCall K, Aferol H, Silva MJ, Garbow JR, Spees WM, Patel T, Siegel M, Dobbs MB, Gurnett CA. Pitx1 haploinsufficiency causes clubfoot in humans and a clubfoot-like phenotype in mice. Hum Mol Genet. 2011;20:3943–3952. doi: 10.1093/hmg/ddr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liebenberg F. A pedigree with unusual anomalies of the elbows, wrists and hands in five generations. South Afr Med J. 1973;47:745–748. [PubMed] [Google Scholar]
- 89.Al-Qattan MM, Al-Thunayan A, AlAbdulkareem I, Al Balwi M. Liebenberg syndrome is caused by a deletion upstream to the PITX1 gene resulting in transformation of the upper limbs to reflect lower limb characteristics. Gene. 2013;524:65–71. doi: 10.1016/j.gene.2013.03.120. [DOI] [PubMed] [Google Scholar]
- 90.Semina EV, Reiter R, Leysens NJ, Alward WL, Small KW, Datson NA, Siegel-Bartelt J, Bierke-Nelson D, Bitoun P, Zabel BU, Carey JC, Murray JC. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 1996;14:392–399. doi: 10.1038/ng1296-392. [DOI] [PubMed] [Google Scholar]
- 91.Reis LM, Semina EV. Genetics of anterior segment dysgenesis disorders. Curr Opin Ophthalmol. 2011;22:314–324. doi: 10.1097/ICU.0b013e328349412b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reis LM, Tyler RC, Volkmann Kloss BA, Schilter KF, Levin AV, Lowry RB, Zwijnenburg PJG, Stroh E, Broeckel U, Murray JC, Semina EV. PITX2 and FOXC1 spectrum of mutations in ocular syndromes. Eur J Hum Genet. 2012;20:1224–1233. doi: 10.1038/ejhg.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xia K, Wu L, Liu X, Xi X, Liang D, Zheng D, Cai F, Pan Q, Long Z, Dai H, Hu Z, Tang B, Zhang Z, Xia J. Mutation in PITX2 is associated with ring dermoid of the cornea. J Med Genet. 2004;41:e129–e129. doi: 10.1136/jmg.2004.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arikawa A, Yoshida S, Yoshikawa H, Ishikawa K, Yamaji Y, Arita R-I, Ueno A, Ishibashi T. Case of novel PITX2 gene mutation associated with Peters’ anomaly and persistent hyperplastic primary vitreous. Eye. 2010;24:391–393. doi: 10.1038/eye.2009.114. [DOI] [PubMed] [Google Scholar]
- 95.Kimura M, Tokita Y, Machida J, Shibata A, Tatematsu T, Tsurusaki Y, Miyake N, Saitsu H, Miyachi H, Shimozato K, Matsumoto N, Nakashima M. A novel PITX2 mutation causing iris hypoplasia. Hum Genome Var. 2014;1:1–3. doi: 10.1038/hgv.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Protas ME, Weh E, Footz T, Kasberger J, Baraban SC, Levin AV, Katz LJ, Ritch R, Walter MA, Semina EV, Gould DB. Mutations of conserved non-coding elements of PITX2 in patients with ocular dysgenesis and developmental glaucoma. Hum Mol Genet. 2017;26:3630–3638. doi: 10.1093/hmg/ddx251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karadeniz NN, Kocak-Midillioglu I, Erdogan D, Bökesoy I. Is SHORT syndrome another phenotypic variation of PITX2? Am J Med Genet A. 2004;130A:406–409. doi: 10.1002/ajmg.a.30206. [DOI] [PubMed] [Google Scholar]
- 98.Qiu X-B, Xu Y-J, Li R-G, Xu L, Liu X, Fang W-Y, Yang Y-Q, Qu X-K. PITX2C loss-of-function mutations responsible for idiopathic atrial fibrillation. Clinics. 2014;69:15–22. doi: 10.6061/clinics/2014(01)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang J, Xin Y-F, Xu W-J, Liu Z-M, Qiu X-B, Qu X-K, Xu L, Li X, Yang Y-Q. Prevalence and spectrum of PITX2c mutations associated with congenital heart disease. DNA Cell Biol. 2013;32:708–716. doi: 10.1089/dna.2013.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yuan F, Zhao L, Wang J, Zhang W, Li X, Qiu X-B, Li R-G, Xu Y-J, Xu L, Qu X-K, Fang W-Y, Yang Y-Q. PITX2c loss-of-function mutations responsible for congenital atrial septal defects. Int J Med Sci. 2013;10:1422–1429. doi: 10.7150/ijms.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wei D, Gong X-H, Qiu G, Wang J, Yang Y-Q. Novel PITX2c loss-of-function mutations associated with complex congenital heart disease. Int J Mol Med. 2014;33:1201–1208. doi: 10.3892/ijmm.2014.1689. [DOI] [PubMed] [Google Scholar]
- 102.Zhao C-M, Peng L-Y, Li L, Liu X-Y, Wang J, Zhang X-L, Yuan F, Li R-G, Qiu X-B, Yang Y-Q. PITX2 loss-of-function mutation contributes to congenital endocardial cushion defect and Axenfeld-Rieger syndrome. PLoS ONE. 2015;10:e0124409. doi: 10.1371/journal.pone.0124409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun Y-M, Wang J, Qiu X-B, Yuan F, Xu Y-J, Li R-G, Qu X-K, Huang R-T, Xue S, Yang Y-Q. PITX2 loss-of-function mutation contributes to tetralogy of Fallot. Gene. 2016;577:258–264. doi: 10.1016/j.gene.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 104.Semina EV, Ferrell RE, Mintz-Hittner HA, Bitoun P, Alward WLM, Reiter RS, Funkhauser C, Daack-Hirsch S, Murray JC. A novel homeobox gene PITX3 is mutated in families with autosomal-dominant cataracts and ASMD. Nat Genet. 1998;19:167–170. doi: 10.1038/527. [DOI] [PubMed] [Google Scholar]
- 105.Verdin H, Sorokina EA, Meire F, Casteels I, de Ravel T, Semina EV, De Baere E. Novel and recurrent PITX3 mutations in Belgian families with autosomal dominant congenital cataract and anterior segment dysgenesis have similar phenotypic and functional characteristics. Orphanet J Rare Dis. 2014;9:26. doi: 10.1186/1750-1172-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu H, Liu H, Tang J, Lin Q, Sun Y, Wang C, Yang H, Khan MR, Peerbux MW, Ahmad S, Bukhari I, Zhu J. Whole exome sequencing identifies a novel mutation in the PITX3 Gene, causing autosomal dominant congenital cataracts in a Chinese family. Ann Clin Lab Sci. 2017;47:92–95. [PubMed] [Google Scholar]
- 107.Wu Z, Meng D, Fang C, Li J, Zheng X, Lin J, Zeng H, Lv S, Zhang Z, Luan B, Zhong Z, Chen J. PITX3 mutations associated with autosomal dominant congenital cataract in the Chinese population. Mol Med Rep. 2019;19:3123–3131. doi: 10.3892/mmr.2019.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dietrich D, Hasinger O, Liebenberg V, Field JK, Kristiansen G, Soltermann A. DNA methylation of the homeobox genes PITX2 and SHOX2 predicts outcome in non-small-cell lung cancer patients. Diagn Mol Pathol Am J Surg Pathol Part B. 2012;21:93–104. doi: 10.1097/PDM.0b013e318240503b. [DOI] [PubMed] [Google Scholar]
- 109.Luo J, Yao Y, Ji S, Sun Q, Xu Y, Liu K, Diao Q, Qiang Y, Shen Y. PITX2 enhances progression of lung adenocarcinoma by transcriptionally regulating WNT3A and activating Wnt/β-catenin signaling pathway. Cancer Cell Int. 2019;19:96. doi: 10.1186/s12935-019-0800-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hirose H, Ishii H, Mimori K, Tanaka F, Takemasa I, Mizushima T, Ikeda M, Yamamoto H, Sekimoto M, Doki Y, Mori M. The significance of PITX2 overexpression in human colorectal cancer. Ann Surg Oncol. 2011;18:3005–3012. doi: 10.1245/s10434-011-1653-z. [DOI] [PubMed] [Google Scholar]
- 111.Semaan A, Uhl B, Branchi V, Lingohr P, Bootz F, Kristiansen G, Kalff JC, Matthaei H, Pantelis D, Dietrich D. Significance of PITX2 promoter methylation in colorectal carcinoma prognosis. Clin Colorectal Cancer. 2018;17:e385–e393. doi: 10.1016/j.clcc.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 112.Basu M, Bhattacharya R, Ray U, Mukhopadhyay S, Chatterjee U, Roy SS. Invasion of ovarian cancer cells is induced byPITX2-mediated activation of TGF-β and Activin-A. Mol Cancer. 2015;14:162. doi: 10.1186/s12943-015-0433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Basu M, Roy SS. Wnt/β-catenin pathway is regulated by PITX2 homeodomain protein and thus contributes to the proliferation of human ovarian adenocarcinoma cell, SKOV-3. J Biol Chem. 2013;288:4355–4367. doi: 10.1074/jbc.M112.409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fung FKC, Chan DW, Liu VWS, Leung THY, Cheung ANY, Ngan HYS. Increased expression of PITX2 transcription factor contributes to ovarian cancer progression. PLoS ONE. 2012;7:e37076. doi: 10.1371/journal.pone.0037076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang J-X, Tong Z-T, Yang L, Wang F, Chai H-P, Zhang F, Xie M-R, Zhang A-L, Wu L-M, Hong H, Yin L, Wang H, Wang H-Y, Zhao Y. PITX2: A promising predictive biomarker of patients’ prognosis and chemoradioresistance in esophageal squamous cell carcinoma. Int J Cancer. 2013;132:2567–2577. doi: 10.1002/ijc.27930. [DOI] [PubMed] [Google Scholar]
- 116.Liu Y, Huang Y, Zhu G-Z. Cyclin A1 is a transcriptional target of PITX2 and overexpressed in papillary thyroid carcinoma. Mol Cell Biochem. 2013;384:221–227. doi: 10.1007/s11010-013-1801-9. [DOI] [PubMed] [Google Scholar]
- 117.Huang Y, Guigon CJ, Fan J, Cheng S, Zhu G-Z. Pituitary homeobox 2 (PITX2) promotes thyroid carcinogenesis by activation of cyclin D2. Cell Cycle. 2010;9:1333–1341. doi: 10.4161/cc.9.7.11126. [DOI] [PubMed] [Google Scholar]
- 118.Vinarskaja A, Schulz WA, Ingenwerth M, Hader C, Arsov C. Association of PITX2 mRNA down-regulation in prostate cancer with promoter hypermethylation and poor prognosis. Urol Oncol Semin Orig Investig. 2013;31:622–627. doi: 10.1016/j.urolonc.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 119.Jiang Q, Xie M, He M, Yan F, Chen M, Xu S, Zhang X, Shen P. PITX2 methylation: a novel and effective biomarker for monitoring biochemical recurrence risk of prostate cancer. Medicine. 2019 doi: 10.1097/MD.0000000000013820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Uhl B, Gevensleben H, Tolkach Y, Sailer V, Majores M, Jung M, Meller S, Stein J, Ellinger J, Dietrich D, Kristiansen G. PITX2 DNA methylation as biomarker for individualized risk assessment of prostate cancer in core biopsies. J Mol Diagn JMD. 2017;19:107–114. doi: 10.1016/j.jmoldx.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 121.Knopp P, Figeac N, Fortier M, Moyle L, Zammit PS. Pitx genes are redeployed in adult myogenesis where they can act to promote myogenic differentiation in muscle satellite cells. Dev Biol. 2013;377:293–304. doi: 10.1016/j.ydbio.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 122.Lozano-Velasco E, Vallejo D, Esteban FJ, Doherty C, Hernández-Torres F, Franco D, Aránega AE. A Pitx2-microrna pathway modulates cell proliferation in myoblasts and skeletal-muscle satellite cells and promotes their commitment to a myogenic cell fate. Mol Cell Biol. 2015;35:2892–2909. doi: 10.1128/MCB.00536-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.L’honoré A, Commère P-H, Negroni E, Pallafacchina G, Friguet B, Drouin J, Buckingham M, Montarras D. The role of Pitx2 and Pitx3 in muscle stem cells gives new insights into P38α MAP kinase and redox regulation of muscle regeneration. eLife. 2018 doi: 10.7554/eLife.32991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rodriguez-Esteban C, Tsukui T, Yonei S, Magallon J, Tamura K, Izpisua Belmonte JC. The T-box genes Tbx4 and Tbx5 regulate limb outgrowth and identity. Nature. 1999;398:814–818. doi: 10.1038/19769. [DOI] [PubMed] [Google Scholar]
- 125.Naiche LA, Papaioannou VE. Loss of Tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development. 2003;130:2681–2693. doi: 10.1242/dev.00504. [DOI] [PubMed] [Google Scholar]
- 126.DeLaurier A, Schweitzer R, Logan M. Pitx1 determines the morphology of muscle, tendon, and bones of the hindlimb. Dev Biol. 2006;299:22–34. doi: 10.1016/j.ydbio.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 127.Zhu J, Palliyil S, Ran C, Kumar JP. Drosophila Pax6 promotes development of the entire eye–antennal disc, thereby ensuring proper adult head formation. Proc Natl Acad Sci. 2017;114:5846–5853. doi: 10.1073/pnas.1610614114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shaham O, Menuchin Y, Farhy C, Ashery-Padan R. Pax6: a multi-level regulator of ocular development. Prog Retin Eye Res. 2012;31:351–376. doi: 10.1016/j.preteyeres.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 129.Kieusseian A, Chagraoui J, Kerdudo C, Mangeot P-E, Gage PJ, Navarro N, Izac B, Uzan G, Forget BG, Dubart-Kupperschmitt A. Expression of Pitx2 in stromal cells is required for normal hematopoiesis. Blood. 2006;107:492–500. doi: 10.1182/blood-2005-02-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chinchilla A, Daimi H, Lozano-Velasco E, Dominguez JN, Caballero R, Delpón E, Tamargo J, Cinca J, Hove-Madsen L, Aranega AE, Franco D. PITX2 insufficiency leads to atrial electrical and structural remodeling linked to arrhythmogenesis. Circ Cardiovasc Genet. 2011;4:269–279. doi: 10.1161/CIRCGENETICS.110.958116. [DOI] [PubMed] [Google Scholar]
- 131.Welsh IC, Kwak H, Chen FL, Werner M, Shopland LS, Danko CG, Lis JT, Zhang M, Martin JF, Kurpios NA. Chromatin architecture of the Pitx2 locus requires CTCF- and Pitx2-dependent asymmetry that mirrors embryonic gut laterality. Cell Rep. 2015;13:337–349. doi: 10.1016/j.celrep.2015.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.L’honoré A, Commère PH, Ouimette JF, Montarras D, Drouin J, Buckingham M. Redox regulation by Pitx2 and Pitx3 is critical for fetal myogenesis. Dev Cell. 2014;29:392–405. doi: 10.1016/j.devcel.2014.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Information provided is based on published works from our team and many others.
Not applicable.