Abstract
Cholestasis is a key causative factor in alcohol-related liver disease (ALD) and variable degrees of cholestasis occur in all stages of ALD. However, the pathogenetic mechanisms and biomarkers associated with cholestasis are not well characterized. Cholestatic disease is marked by the disruption of bile acids (BA) transport and homeostasis. Consequently, in both human and experimental ALD, the disease shows a direct correlation with an imbalance in BA equilibrium, which in turn may also affect the severity of the disease. Modulation of BA metabolism or signaling pathways is increasingly considered as a potential therapeutic strategy for ALD in humans. In this paper, we highlight the key advances made in the past two decades in characterizing the molecular regulatory mechanisms of BA synthesis, enterohepatic circulation, and BA homeostasis. We summarize recent insights into the nature of the linkage between BA dysregulation and ALD, including the abnormal expression of genes involved in BA metabolism, abnormal changes in receptors that regulate BA metabolism, and disturbance in the gut flora engaged in BA metabolism caused by alcohol consumption. Additionally, we provide novel perspectives on the changes in BAs in various stages of ALD. Finally, we propose potential pharmacological therapies for ALD targeting BA metabolism and signaling.
Keywords: Bile acid homeostasis, Alcoholic liver disease, Intestinal microbiota, Farnesoid X receptor, Enterohepatic circulation, FGF19
Introduction
Alcohol-related liver disease (ALD) comprises a wide range of hepatic disorders, including asymptomatic steatosis, alcoholic steatohepatitis (ASH), alcoholic liver fibrosis and cirrhosis, and alcohol-related hepatocellular carcinoma (HCC). ALD is one of the most prevalent global causes of chronic liver diseases. The mortality and morbidity rates associated with ALD have shown an increasing trend over successive years. According to the World Health Organization's latest Global Status Report on Alcohol, more than two-fifths of the world's population over the age of 15 years consumes alcohol, which accounts for approximately 2.3 billion people. Every year, approximately 35 billion liters of pure ethanol are consumed, which corresponds to three cups of pure ethanol per person per day (33 g of pure ethanol), demonstrating the growing popularity of alcohol [1]. According to epidemiological research, roughly 1,256,900 individuals perish annually due to chronic liver disease. About 27% of all deaths are attributed to alcohol abuse [2]. The pathogenetic mechanisms of ALD are not well characterized. At present, ethanol is believed to cause liver toxicity through modifying hepatocellular lipogenesis and boosting the secretion of endotoxins from the intestine, thereby promoting inflammation and oxidative stress. Furthermore, environmental and genetic variables, including sex, ethnicity, nutrition, liver disease complications, and oxidative stress, may have a fundamental impact on the progression of ALD [3]. While abstinence from alcohol remains the optimal option for ALD, this objective is frequently not met adequately or on time for a large number of patients. As a result, the search for effector chemicals that may be involved in ALD may help identify novel therapeutic targets. Cholestasis occurs in various phases of ALD, and disruption of transport and equilibrium of bile acids leads to the development of cholestatic disease. Alcohol consumption can induce abnormal changes in the levels and content of the BA pool in the human body, which further aggravates ALD. The intestinal microbiota (IM) plays an essential role in the development and progression of ALD. Alcohol intake can lead to changes in the composition and function of the gut microbiota. Microbial functions, especially those related to bile acid synthesis and biotransformation, can modulate alcohol-induced liver damage. Nearly all the proteins required for the synthesis, absorption, metabolism, and transport of BAs have the ability to modify the size and composition of the BA pool and BA signaling, making them desirable drug candidates for ALD. Here, we review the emerging evidence pertaining to the protective effect of BA signaling against ALD by altering several cellular and molecular pathways. Moreover, we summarize the potential mechanisms driving the alcohol-induced abnormal alterations in BAs, as well as the abnormal changes in the size and composition of BA pool observed in various stages of ALD. Finally, we discuss the prospects of use of agents targeting BA signaling in the therapy of ALD (Fig. 1).
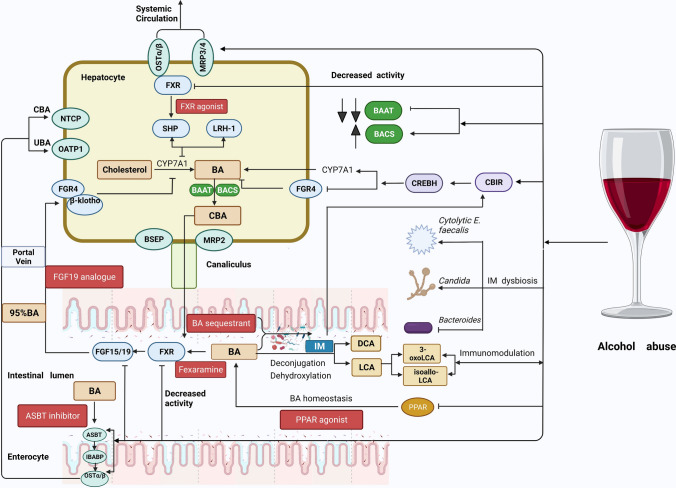
Fig. 1.
The effect of alcohol abuse on bile acid metabolism and the therapeutic target for ALD based on the bile acid signaling pathway. Alcohol abuse increases CYP7A1 transcription while decreasing the expression of fibroblast growth factor receptor 4 (FGFR4). Upon alcohol exposure, the expression levels of the bile acid CoA: amino acid N-acyltransferase (BAAT) are dramatically decreased, whereas bile acid CoA synthetase (BACS) expression is marginally elevated. Chronic alcohol abuse upregulates the transcription of bile acid efflux transporters, such as the multidrug resistance-associated protein ¾ (MRP3/4) and the organic solute transporter α/β (OSTα/β) in the liver, and also upregulates the expression of the OSTα/β and the apical sodium-dependent BA transporter (ASBT) in the ileum. Furthermore, alcohol treatment inhibits the farnesoid X receptor (FXR) activity in the liver and intestine, which results in increased BA synthesis. Finally, chronic heavy drinking causes the dysbiosis of intestinal flora involved in bile acid metabolism. Both alcohol consumption and derivatives of lithocholic acid (LCA) can regulate the intestinal immune response. Beneficial targets for ALD along with the enterohepatic and cholehepatic BA circulation. Enterohepatic drugs acting within the gut–liver axis: FXR agonists and FGF19 analogs. Transport blockers: ASBT inhibitors and BA sequestrants. PPAR agonists. The red box indicates potential effective targets for ALD therapy based on bile acid metabolism. Bile acid transporters are indicated by the green box. Created with BioRender.com. CB1R cannabinoid receptor type 1, CREBH cAMP response element-binding protein hepatocyte-specific, DCA deoxycholic acid, FGF15/19 fibroblast growth factor 15/19, PPAR peroxisome proliferator-activated receptor, SHP small heterodimer partner, LRH-1 liver receptor homolog-1, CBA conjugated primary bile acids, UBA unconjugated bile acids, MRP2 multidrug resistance-associated protein2, BSEP bile salt export pump, IBABP ileum bile acid binding protein, NTCP sodium/taurocholate cotransporting polypeptide, OATP organic anion-transporting polypeptide 1, IM intestinal microbiota
Advances in the regulation of bile acids in ALD
Bile acid homeostasis
Bile acid is a lipophilic steroid having one or more hydroxylation sites and a carboxylic acid-terminated side chain. There are two main pathways for BA synthesis: the classical and alternative routes [4]. Bile acid is a byproduct of hepatic cholesterol metabolism. Primary BAs are synthesized in the hepatocytes and are largely delivered to the enteric cavity as amino acid conjugates. Approximately 95% of BAs are resorbed in the distal ileum and colon before being returned to the liver through the portal vein, a process known as enterohepatic circulation of BA. The remaining 3–5% of BAs excreted into the bile are not reabsorbed in the small intestine and pass through to the colon where they undergo deconjugation and multistep dehydroxylation processes carried out by intestinal microflora. The conjugated primary BAs are further converted to secondary BAs [5].
Under normal circumstances, the BA pool size remains constant. This is accomplished through the several "checkpoints" located across the enterohepatic circulation. Overexpression or underexpression of several transporter genes may result in an increase in BA reprocessing or a decrease in the BA reservoir through excretion. Pharmacological targeting of BA sites and carriers is a fast-emerging frontier in the therapeutics research for ALD.
Bile acid production and transport are controlled in hepatocytes and intestinal epithelial cells via a complex network of hormone-like signaling. Bile acids primarily sense the existing BA reserve in the liver and ileum by combining with the nuclear receptor FXR (farnesoid X receptor) and closely regulating de novo BA synthesis via negative feedback. Bile acid production and enterohepatic circulation are modulated by FXR, a transcription factor that is activated by ligands [6]. Two different pathways are used by FXR to regulate the production of BAs [7, 8]. Bindings of BAs with FXR inhibit the CYP7A1/Cyp7a1 and the CYP8B1/Cyp8b1 gene production in the liver by inducing small heterodimer partners (SHP) to engage with liver receptor homolog-1 (LRH-1) [9]. Bile acids also suppress their own synthesis in the gut via an SHP-independent route involving FGF15 (FGF19 in humans). Bile acids activate FXR, inducing the secretion of fibroblast growth factor 15/19 (FGF15/19) from the gut into the portal circulation. FGF15 phosphorylates fibroblast growth factor receptor 4 (FGR4), which activates the extracellular regulated protein kinases1/2 (ERK1/2) pathway and necessitates the presence of cofactors, β-klotho, to decrease the expression of CYP7A1/CYP7A1 and Cyp8b1/Cyp8b1 genes [10, 11].
Mechanism of abnormal changes in bile acids in ALD
Effect of alcohol consumption on the expression of genes involved in the BA metabolism
First, chronic alcohol intake alters the size of BA pool by upregulating the expression of BA synthesis genes. Chronic heavy alcohol consumption was found to increase Cyp7a1 and Cyp8b1 transcription while decreasing the expression of FGFR4, a CYP7a1 transcriptional inhibitor [12, 13]. This may be attributable to alcohol-induced activation of cyclic AMP-responsive element-binding protein H (CREBH) via stimulation of the hepatic cannabinoid receptor type 1 (CBIR) [14], which is resistant to activated FXR-FGF19 or FXR-SHP pathways. However, a recent study in humanized mice.
revealed that gut microbiota can also trigger CREBH and BA biosynthesis genes via the production of a molecule that activates the hepatocellular CB1R [15]. Collectively, it is still debatable if the modulation of the key enzymes in the production of BA in the hepatocytes is caused by intestinal flora or alcohol consumption. Additionally, CYP7A1 synthesis was discovered to be decreased in patients with alcoholic hepatitis (AH) [16], which may be a compensatory response in these patients. Further studies are required to discern the changes in BA synthesis genes at various stages of ALD and to further illustrate the mechanism of alcohol-induced BA biosynthetic pathways.
Second, chronic alcohol intake affects the composition of BAs by changing the metabolic enzymes that facilitate BA conjugation. Upon alcohol exposure, the expression level of the bile acid CoA: amino acid N-acyltransferase (BAAT) was dramatically decreased, whereas the expression of bile acid CoA synthetase (BACS) was marginally elevated. In rodents, BAAT has a far greater specificity for taurine than it does for glycine, and the bulk of BAs in rodents tend to be taurine conjugated, whereas glycine-conjugated BAs are more common in humans. Therefore, this results in more unconjugated BAs, which are more hydrophobic and more damaging to the hepatocyte membrane. Unconjugated BAs can have a beneficial or detrimental effect on hepatic and gastrointestinal diseases depending on their concentrations [17]. Bile acids can also acquire epimerization on 3-, 7-, and 12-carbon hydroxyl groups by the gut microbiota, altering their hydrophobicity and hence potential cytotoxicity [18].
Third, chronic alcohol intake disrupts BA homeostasis by modulating bile acid transporters. Chronic alcohol abuse increases the transcription of BA efflux transporters, such as bile salt export pump (BSEP), multidrug resistance-associated protein 3/4 (MRP3/4), and organic solute transporter α/β (OSTα/β) in the liver, and downregulates the expression of BA uptake transporters, such as sodium/taurocholate cotransporting polypeptide (NTCP). In the ileum, it upregulates the expression of OSTα/β and apical sodium-dependent BA transporter (ASBT) [13, 19], which may be a compensatory response to cholestasis induced by chronic alcohol exposure. However, the changes in BSEP are highly controversial, and some previous investigations have demonstrated that heavy drinking can significantly increase the expression of BSEP, thereby affecting the basal metabolism and equilibrium of BAs in alcohol-treated mice [19, 20]. In contrast, in patients with alcohol-related hepatocellular carcinoma, alcohol consumption was found to significantly decrease BSEP expression compared with liver cancer patients who did not consume alcohol [23]. Additionally, previous studies have indicated a dramatic decrease in the expression of BSEP in patients with HCC [21]. In addition, the levels of BSEP showed an inverse correlation with the progression and prognosis of liver cancer [22]. All in all, the detailed mechanism of the effect of alcohol on BSEP may change with different stages of ALD and requires further exploration.
Effect of alcohol consumption on the gut microbiota involved in BA metabolism
The mammalian intestinal microbiome comprises germs, fungi, archaea, and viruses. Numerous recent studies have revealed the important role gut flora plays in ALD. For example, Duan et al. used bacteriophages to specifically alter the microbial community, lowering cytolytic E. faecalis and its secreted cytolysin levels in the liver which are associated with the severity of liver disease and mortality, thereby eradicating alcohol-induced liver injury in humanized rats transplanted with fecal germs from cytolysin-positive patients with AH [24]. Additionally, individuals with ALD showed decreased fungal diversity and significantly increased Candida albicans overgrowth, with an increased fraction of Candida in the fecal fungal community. Candida hemolysin is a peptide toxin produced by C. albicans, a commensal intestinal fungus, which can cause dose-dependent injury to primary hepatocytes in vitro and is closely linked with the severity of liver illness and mortality in people with AH. This suggests that Candida hemolysin may be a viable pharmacological target for ALD patients [25]. Patients with AH show considerably higher levels of the anti–Saccharomyces cerevisiae antibodies (ASCA), which act as a systemic immune response against fungal compounds or fungi, such as Candida. Moreover, ASCA levels in conjunction with the Model for end-stage liver disease (MELD) score were found to effectively predict the severity of AH [26].
The numerous microorganisms that are found in the gastrointestinal system play an essential role in BA bioconversion and have a significant impact on the size and composition of the BA pool. Moreover, variations in the composition of the BA pool can adversely affect intestinal flora (Fig. 2). Recent evidence suggests that derivatives of secondary BAs metabolized by intestinal bacteria, such as 3-oxoLCA and isoallo-LCA, found in both humans and rodents directly affect CD4+ T cells [27]: in mice, 3-oxo-LCA was found to directly bind to retinoic acid receptor-related orphan receptor- γ t (RORγt) and inhibit the differentiation of Th17 cells, whereas isoallo-LCA induced the differentiation of Treg cells by stimulating the production of mitochondrial reactive oxygen species (ROS), which resulted in an increase in the forkhead box protein 3 (FOXP3) expression (a major transcriptional modulator of Treg cell differentiation). In addition, mice fed with 3-oxo-LCA or isoallo-LCA showed reduced Th17 cells and increased Treg cell differentiation in the lamina propria of the ileum, suggesting that BA metabolites modify the host immunological responses partly by regulating the function of Treg cells and Th17 cells [28]. Moreover, CD4+ T cells play different roles in the pathogenesis of ALD by producing a characteristic profile of cytokines [29]. For example, Th17 cells not only promote liver inflammation and fibrosis in ALD by producing IL-17 but may also help in liver repair by stimulating IL-22 production [30, 31]. Excessive alcohol drinking also increases T regulator cells, causing broad immunosuppression [32]. In conclusion, bile acid metabolites can control host immune responses by directly modulating the TH17 and Treg cell differentiation. This, in turn, affects the onset and progression of ALD. 3-oxoLCA inhibits IL-17 production by Th17 cells, thereby reducing liver inflammation and fibrosis. Isoallo-LCA enhances Treg cell differentiation, thereby resulting in an increased risk of bacterial infection in patients with ALD. Elderly individuals over the age of 100 years show higher levels of intestinal 3-oxoLCA and isoallo-LCA compared to young individuals. This is in line with the notion that these metabolites are beneficial for human health [33]. The ability of intestinal microflora to biotransform-conjugated primary BAs into unconjugated secondary BAs is at the core of gastrointestinal metabolic homeostasis. The digestive system and alcohol are inseparably related. Alcohol consumption alters the function of intestinal microflora [34], especially those related to BA metabolism in the following two aspects.
Fig. 2.
The relationship between alcohol consumption, intestinal flora, and bile acids. AFLD alcoholic fatty liver disease, ASH alcoholic steatohepatitis. Created with BioRender.com
First, gut microflora responsible for the production of bile salt hydrolase (BSH) is out of balance. It is currently believed that functional BSH exists in all major bacterial and archaeal species in the human intestine, including Lactobacillus, Bifidobacterium, Clostridium, and Bacteroides [35]. BSH can convert taurine-conjugated and glycine-conjugated primary BAs to their corresponding free forms. Chronic alcohol intake is linked to qualitative and quantitative alterations in the intestinal flora. For example, compared with healthy humans, alcohol was found to augment the species richness of Proteus, Enterobacteriaceae, and Streptococcus, while reducing the richness of Bacteroides, Ackermann, and Coccobacteria [36]. The pathophysiology of this biological disorder is not well characterized. Alcohol-induced oxidative stress (which obligate anaerobes, like Bacteroides, do not handle well) and down-regulation of antimicrobial peptides, like defensin expression, are two potential mechanisms. Additionally, another metagenomic investigation revealed that the bacterial DNA coding glycocholic acid hydrolase was more abundant in the gut lumen of rodents administered ethanol than in the control group. According to the sequencing results, glycocholic acid hydrolase is principally synthesized by Lactobacillus, Lactococcus, Bacteroides, and Micrococcus [37]. Accordingly, an important reason is that reduced BA secretion in the intestine during cholestasis of ALD results in bacterial overgrowth in the small bowel because of the direct antibacterial effect of BAs; in addition, BAs also indirectly promote the generation of antimicrobial peptides via their signaling properties. Furthermore, another study of HCC patients and chemical-induced HCC mouse models [38] revealed that decreasing gut BSH-rich microorganisms may promote the progression of HCC by substantially reducing the proportion of serum secondary BAs in the overall BA pool of patients with HCC.
Second, the gut microflora responsible for the dehydroxylation reaction is out of balance. The intestinal flora engaged in the biotransformation of secondary BAs is mainly Clostridium and Eubacterium in thick-walled Helicobacter pylori, especially Clostridium. The primary BAs CA and CDCA can be converted into secondary BAs DCA and LCA, respectively, by 7-dehydroxylase-rich bacteria in the gut [39], which are the most plentiful secondary BAs in human beings, and are recognized to play a significant role in three metabolic pathways: Clostridium difficile outgrowth suppression [40], hepatocellular carcinogenesis promotion [41], and immunological response modulation [27]. For example, accumulation of DCA leads to liver injury. Obesity-induced lipoteichoic acid (LTA) (membrane phosphoteic acid, a common component of gram-positive bacterial cell wall) combines with the intestinal microbial metabolite DCA to enhance the expression of senescence-associated secretory phenotype (SASP) of the hepatic stellate cells (HSCs) and cyclooxygenase-2 (COX-2) via toll-like receptor 2 (TLR2). On one hand, SASP creates a microenvironment that promotes the occurrence of liver cancer. On the other hand, through binding to the prostaglandin E receptor 4 (PTGER4), COX-2-mediated prostaglandin E2 (PGE2)suppresses antitumor immunity, thus promoting the development of HCC [41]. Recent research in mouse models revealed that NKT cells whose expression is regulated by CXCL16 suppress carcinogenesis in the liver. CXCL16 expression was found to be positively correlated with the primary BA CDCA and negatively correlated with the secondary BA GLCA, suggesting that secondary BAs may contribute to the growth of liver tumors [42]. Furthermore, Jin et al. developed the gene-editing tool for the intestinal microbiota and determined the single-gene transfer method of intestinal Firmicutes and Clostridium in the complex gut microbes; they demonstrated that it is more favorable to investigate the role of a single microorganism related to BA metabolism in the complex gut flora [43]. Collectively, these findings indicate progressive advances in obtaining mechanistic insights into the relationships between alcohol consumption and BAs processed by intestinal flora as a result of the development of novel profiling approaches and techniques, including metagenomics and genetic manipulation.
Effect of alcohol consumption on the BA receptor involved in BA metabolism
The role of FXR in ALD
Following alcohol consumption, a small amount is digested promptly in the stomach, while the majority of leftover alcohol is absorbed via the gastrointestinal system and then transferred to the liver through the portal circulation. In the liver, alcohol is metabolized in three distinct ways. First, the majority of alcohol is converted in hepatocytes to acetaldehyde, mostly by alcohol dehydrogenase (ADH), and then to acetate via acetaldehyde dehydrogenase (ALDH). Acetate is quickly eliminated from the body as water and carbon dioxide. In addition to ADH, ethanol can be oxidized by the microsomal ethanol oxidation system (MEOS), which is primarily composed of the cytochrome P450 2E1 enzyme (CYP2E1). Finally, catalase is capable of eliminating alcohol. Due to the fact that it is a tetramer and can be expressed by colonic flora, it may result in the generation of acetaldehyde in the lower gastrointestinal tract [44]. The ADH genes, which are human alcohol metabolizing enzymes, have previously been identified as specific targets of the BA receptor FXR. FXR overexpression was found to increase ADH mRNA levels, whereas silencing of FXR prevented FXR agonists from affecting the ADH transcriptional activity [45]. Furthermore, FXR activation also reduced oxidative stress in hepatocytes by inhibiting ethanol-induced upregulation of the Cyp2e1 gene [12]. However, the effect of FXR on ALDH2 is largely unknown, and more basic and clinical experiments are required to further explore the potential mechanism.
Tissue specificity of FXR in ALD
Emerging evidence has revealed that pharmacological FXR agonists can attenuate liver steatosis and oxidative stress induced by chronic alcohol consumption [46], while whole-body FXR gene knockout was found to aggravate alcohol-induced liver injury [47]. These findings suggest an essential role of FXR in the occurrence and development of ALD. Nevertheless, systemic lack of FXR leads to an increased BA pool, making it hard to assess the separate impacts of FXR and BAs on the severity of ALD. Zhang et al. studied the tissue-specific function of FXR in the onset and development of ALD [48]. They found that hepatocyte-specific FXR knockout mice (FXRhep−/−) showed similar BA pool size as wild-type (WT) mice, which enabled them to explore the direct effect of hepatic FXR deficiency on the development of ALD independent of the increase in BA pool size. After alcohol treatment, hepatic FXR deficiency was not found to aggravate alcoholic liver injury in FXRhep−/− mice compared with WT mice. Specifically, they exhibited similar severity of alcohol-induced hepatic steatosis, inflammation, and fibrosis. Moreover, hepatic FXR deficiency did not alter the expression of genes engaged in alcohol metabolisms, such as ADHs, CYP2E1, and ALDHs. Therefore, hepatic FXR deficiency may only play a minor role in the occurrence of ALD. They then [49] compared the severity of the alcoholic fatty liver disease (AFLD) in WT and gut-specific FXR knockout (FXRInt−/−) mice after either a control or an ethanol administration. They discovered intestinal-specific FXR deficiency worsened the occurrence and development of ALD. In conclusion, FXR deficiency in the whole body and the intestines worsens alcohol-induced injury, but FXR deficiency in the hepatocyte has little effect on the occurrence and development of ALD.
Effect of alcohol consumption on FXR
Chronic alcohol abuse has been demonstrated to suppress FXR activation in previous investigations, and the activation of FXR by the specific agonist WAY-362450 saved FXR activity. Chronic alcohol consumption impairs the interaction between FXR and RXRα (retinoid X receptor-α) by increasing the acetylation of FXR, which leads to the inactivation of FXR. For the reason that chronic ethanol intake upregulates the ratio of [NADH:NAD+] and downregulates the level of NAD+, which may reduce the activity of the NAD + -dependent sirtuins1 (SIRT1) enzyme, resulting in the failure of deacetylation of FXR and an increase in acetylation [50]. Moreover, alcohol abuse may also reduce the activity of the NAD+-dependent (sirtuins5) SIRT5 enzyme in the same way. A recent study of human HCC samples and HCC mouse models found that SIRT5 deficiency can promote the progression of HCC. Lack of SIRT5 in hepatocytes was found to promote the abnormal accumulation of BAs in hepatocyte peroxisomes, forming an immunosuppressive tumor microenvironment [51]. This may also be one of the potential mechanisms by which alcohol consumption leads to hepatocellular carcinoma. Alcohol impairs FXR activity, aggravating the development of ALD from the following five pathophysiological aspects.
First, it worsens alcohol-related liver steatosis Steatosis is the initial response of the liver to chronic alcohol consumption, characterized by the buildup of lipids in hepatocytes. Chronic alcohol consumption increases hepatic lipogenesis and decreases hepatic lipolysis, resulting in hepatic steatosis. In wild-type and intestinal-FXR-deficient mice with normal liver histopathology, ethanol feeding only led to mild liver steatosis in wild-type mice; however, significant vesicular steatosis and bullous steatosis, as well as hepatocyte balloon degeneration and Mallory-Denk bodies, were observed in the latter, which were characterized by spherical lipid droplets of different sizes in the cytoplasm of hepatocytes [49]. This indicated that ethanol-induced impairment of FXR activity further exacerbates alcohol-induced liver steatosis. Deng et al. recently discovered that lack of FXR promotes liver adipogenesis via regulation of the pyruvate dehydrogenase kinase 4 (PDK4). PDK4 is a mitochondrial enzyme that inhibits the conversion of pyruvate to acetyl CoA by preventing the phosphorylation of pyruvate dehydrogenase complex (PDC), resulting in an increase in liver fat. Additionally, inhibiting PDK4 expression was found to alleviate hepatocyte lipid accumulation induced by FXR deficiency, providing additional knowledge of the potential mechanism of action of FXR agonists in the therapy of ALD [52].
Second, it worsens alcoholic steatohepatitis Inflammation in the liver has a profound impact on scarring, fibrosis, and eventually cirrhosis. Chronic ethanol consumption leads to impaired FXR activity, which mainly aggravates liver inflammation from three aspects. First of all, decreased FXR activity leads to increased intestinal permeability [47], leading to the entry of intestinal-derived pathogen-associated molecular patterns (PAMPs) into the liver. PAMPs in collaboration with damage-associated molecular patterns (DAMPs) activate innate immune cell receptors, such as toll-like receptors (TLRs) and nod-like receptors (NLRs). The signals sent by these receptors cause an increase in the expression of pro-inflammatory transcription factors (including NF-κB), which promotes the release of a large number of inflammatory factors and cytokines, resulting in liver inflammation, also known as the "liver inflammatory factor storm" [29]. For example, when lipopolysaccharides cross the intestinal barrier and reach the liver via the portal circulation, these bind to the TLR4 on liver Kupffer cells, causing the production of superoxide and tumor necrosis factor-α, which triggers liver injury [53]. In addition, the decrease in FXR activity upregulates liver lipid peroxidation. The level of malondialdehyde, a byproduct of lipid peroxidation, was found to increase after ethanol feeding in FXR−/− mice [47]. Finally, decreased FXR activity increases alcohol-mediated production of reactive oxygen species, further exacerbating oxidative stress.
Third, it worsens alcoholic liver fibrosis and cirrhosis Hepatic fibrosis is the process of repair of damaged cells and tissues in the liver caused by a variety of factors. Extracellular matrix deposition induced by activation of HSCs is a critical element in the onset and progression of hepatic fibrosis [54]. HSCs can be stimulated not only by chemokines but also by alcohol and its byproducts. The inflammatory reaction and the toxic reaction induced by the increase in BAs caused by the decrease in FXR activity activate HSCs and promote alcoholic liver fibrosis and alcoholic cirrhosis. Garrido et al. reported reduced levels of the microspherule protein 1(MCRS1) and NTCP in individuals with liver cirrhosis. More specifically, deletion of MCRS1 in hepatic cells increased the level of BAs in hepatic sinusoids, thus activating FXR on HSCs and promoting the progression of liver cirrhosis. This was accomplished by enhancing histone lysine acetylation of BA transporter genes, such as NTCP. These findings implied that modulation of the BA-FXR axis in hepatic fibrosis is critical for the progression of liver cirrhosis and is a viable therapeutic target for cirrhosis [55].
Fourth, it worsens the gut mucosal damage The gut mucosal barrier isolates the host from intestinal microorganisms and prevents the flow of bacteria and metabolites out of the enteric cavity. This barrier comprises three components: (1) physical barrier, which includes mucins connected by tight junction proteins and possibly intestinal epithelial cells; (2) biochemical barrier, which includes antimicrobial proteins; and (3) immune barrier, which includes immune cells. Previous experiments have shown that intestinal FXR also contributes to maintaining the integrity of the gastrointestinal wall and inhibiting pathogen overgrowth and translocation [56]. Huang et al. [49] examined the histology of the distal ileum in WT and FXRInt−/− mice fed a normal diet. They found that the ileum of intestinal-specific FXR gene knockout rodents displayed a deformed epithelial cell lining, expanded subepithelial space, degenerative changes, and perforated mucosal lining. Both WT and FXRInt−/− mice exhibited morphological alterations in their villi following alcohol feeding, including broadening and shortening of villi, as well as submucosa and muscular shrinkage. However, these histological abnormalities were more severe in FXRInt−/− mice. These findings suggest that an alcohol-induced decrease in FXR activity exacerbates the gut mucosal damage.
Fifth, it increases intestinal permeability Chronic ethanol intake increases the permeability of the gut, resulting in the impairment of gut barrier function and translocation of microorganisms [57]. E-cadherin is an intercellular adhesion molecule that is essential for the establishment of tight junctions between epithelial cells in the intestine. In the whole-body FXR knockout rats, the expression of E-cadherin is down-regulated, which may increase the likelihood of hepatocellular carcinoma in mice [58]. In a recent study [49], E-cadherin staining was performed on the small intestine of WT and FXRInt−/− mice on control diets. The basic protein level of E-cadherin in the ileal recess in FXRInt−/− mice was found to be markedly lower than that in WT mice. Furthermore, E-cadherin expression was also reduced by alcohol feeding in both strains of mice, but it was significantly reduced in FXRInt−/− mice. The intestinal permeability in vivo was evaluated by measuring the leakage of FITC-dextran in serum after oral administration. The serum FITC-dextran level in FXRInt−/− mice fed on a control diet was dramatically higher than that of WT mice. These findings indicate that decreased FXR activity caused by alcohol abuse can increase intestinal permeability and aggravate the occurrence and development of ALD by reducing the expression of E-cadherin.
Effect of alcohol consumption on the FGF15/19 involved in the bile acid metabolism
Under normal physiological conditions, FGF19 is primarily generated and released in the terminal ileum and shuttled into the portal vein circulation to modulate BA synthesis in hepatic cells. In an experiment conducted in 2013, ethanol administration to non-cirrhotic rats substantially reduced FGF15 expression, presumably explaining the increase in CYP7a1 and BA production [13]. In a study of patients with liver cirrhosis conducted in 2014, on the contrary, FGF19 levels in cirrhosis patients who were heavy drinkers were higher than those in cirrhosis patients who had ceased drinking and the healthy controls. This may be potentially attributable to a distinct modulation of the FXR-FGF19 axis in the setting of cirrhosis with chronic alcohol intake wherein CYP7A1 is not negatively regulated by FGF19 [59]. In a study of patients with alcoholic hepatitis conducted in 2018 [16], consistent with previous studies, the expression of FGF19 in patients with severe AH (SAH) was significantly higher than that in patients with alcohol use disorder and controls. Furthermore, serum FGF19 expression in patients with AH was found to be 100 times higher than that in patients with non-alcoholic steatohepatitis (NASH) or non-alcoholic fatty liver (NAFL), with serum levels of the latter being equal to those seen in normal control. There are two possible explanations for the rise in FGF19 levels in AH. On one hand, AH is characterized by a strong ductal response, and recent studies have shown that FGF19 can also be derived from biliary epithelial cells and ductal cells. On the other hand, systemic and hepatic FGF19 expression was shown to be increased in patients with extrahepatic cholestasis, which decreased after biliary drainage [60]. Moreover, patients with primary biliary cirrhosis (PBC) also have increased FGF19 levels [61], indicating that an increase in FGF19 levels is strongly associated with intrahepatic and extrahepatic cholestasis.
In univariate regression analysis, serum FGF19 demonstrated a significant positive correlation with serum levels of bilirubin and gamma-glutamyl-transferase (GGT) in AH. FGF19 demonstrated a significant negative correlation with the stage of fibrosis and the degree of neutrophil infiltration, suggesting that FGF19 may serve as a potential diagnostic and prognostic marker for AH and also facilitate the differential diagnosis of clinical diseases. In addition, in patients with SAH, FGF19 levels were substantially linked with 30-day mortality [16], and FGF19 may predict mortality in AH similar to the MELD score. Additionally, the expression of FGF19 increased with an increase in the severity of hepatic pathology in AH. Whether this phenomenon is a manifestation of disease activity is not clear. However, considering the advantageous anti-fibrosis and anti-cholestasis effects of FGF19 [62], beneficial metabolic effects [37], and liver regeneration, the compensatory upregulation of FGF19 seems more likely to be a response to the deterioration of liver health.
Abnormal changes in the size and composition of the BA pool in ALD (Table 1)
Table 1.
Abnormal changes in the BA pool in ALD
| TBA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UBA | CBA | |||||||||
| PBA | SBA | PBA | SBA | |||||||
| Normal | CA | CDCA | DCA | LCA | UDCA | GCA | GCDCA | GDCA | GLCA | GUDCA |
| TCA | TCDCA | TDCA | TLCA | TUDCA | ||||||
| ALD | ||||||||||
| AH | TBA CBA TUDCA LCA UDCA ↑ | DCA ↓ | ||||||||
| Cirrhosis | ↓ | ↑ | ||||||||
| ACLF | TBA β-MCA ↑ | CA CDCA ↓ | ||||||||
| HCC | ↑ | ↑ | TCDCA ↑ | TDCA ↓ | TLCA ↓ | |||||
CA cholic acid, CBA conjugated bile acid, CDCA chenodeoxycholic acid, DCA deoxycholic acid, GCA glycocholic acid, GCDCA glycochenodeoxycholic acid, GDCA glycodeoxycholic acid, GLCA glycolithocholic acid, GUDCA glycoursodeoxycholic acid, LCA lithocholic acid, PBA primary bile acid, SBA secondary bile acid, TBA total bile acid, TCA taurocholic acid, TCDCA taurochenodeoxycholic acid, TDCA taurodeoxycholic acid, TLCA taurolithocholic acid, TUDCA tauroursodeoxycholic acid, UBA unconjugated bile acid, UDCA ursodeoxycholic acid, ALD alcoholic liver disease, AH alcoholic hepatitis, ACLF acute-on-chronic liver failure, HCC hepatocellular carcinoma, β-MCA β-muricholic acid
Alcoholic hepatitis
Severe alcoholic hepatitis, identified by Maddrey scoring ≥ 32, has a bad prognosis and is the most severe form of ALD with mortality of 20 to 30% within 1 month after a presentation and 30 to 40% within 6 months after presentation [63, 64]. In a clinical study, patients with alcoholic hepatitis (ABIC score ≥ 9.0) had a higher rate of mortality up to 75% in the first 90 days [65]. The BA profile in AH has been analyzed in five recent investigations [66]. Brandl et al. [16] examined the serum BAs levels of 132 patients with AH and found that total and conjugated BAs levels were considerably higher in patients with AH compared with healthy controls and alcohol use disorders (AUDs). This is due to the fact that during cholestasis, primary BAs mostly enter the bloodstream via MRP3/4. They are secreted far less into the intestines. In addition, although the expression of CYP7A1 genes and the C4 levels, a marker of de novo synthesis of BAs, were notably reduced in patients with AH, the serum total BAs were still increased in AH. However, the limitations of the study included failure to address anomalies in BA levels and their interaction with the gut flora, as well as neglecting the effects of cirrhosis, which was seen in 75% of AH patients. Previous research [67] has demonstrated that patients with SAH have a special dysbiosis, making their hepatocytes more vulnerable to alcohol-related injury. This susceptibility was found by transplanting intestinal microflora from AH patients to mice. Therefore, further exploration of the structure of intestinal microflora in AH patients and its role in BA homeostasis is a key imperative. Ciocan et al. [68] assessed plasma and fecal BA profiles and gut microflora constituents in patients with AH. They found that plasma total BA and total UDCA levels in cirrhotic patients with SAH were higher than in cirrhotic patients without AH(Cir_noAH), and the fecal total BAs and secondary BAs were considerably lower than in Cir_noAH. Moreover, patients with SAH had a higher proportion of Actinobacteria phylum in the gut. Conversely, the proportion of Bacteroidetes phylum was smaller. The increase in the BA pool and its transformation to more hydrophobic and toxic BAs may be the cause of specific changes in intestinal microflora. At present, it is also not clear whether the changes in intestinal microorganisms are caused by the liver disease itself or the etiology of liver disease. However, a limitation of this study was the inclusion of patients with both alcoholic hepatitis and alcoholic cirrhosis. In addition, the study lacked a control group consisting of individuals who consumed excessive amounts of alcohol but did not have an obvious liver injury. In a study in January 2022 by Muthiah et al..[69], aligned with previous research, it was found that plasma-conjugated BAs and secondary BAs UDCA and LCA levels were increased in patients with AH, while DCA was decreased. This study, however, observed a significant rise in 7-HOCA levels (a marker of de novo synthesis of BAs) in patients with AH, consistent with the elevated serum BAs, which suggests that the synthesis of primary BAs in the hepatocytes may increase despite the evidence of cholestasis. This is contrary to the previously reported reduced level of C4 in AH, which needs further research to explore the mechanism. In a recent study of patients with AH [70], the changes in urinary BAs were consistent with those of serum BAs, indicating that urine BAs may be valuable non-invasive markers for early diagnosis of AH and may have a role in predicting the severity of AH.
Alcohol-related fibrosis and cirrhosis
Approximately 10–20% of individuals with ALD develop cirrhosis. Alcohol was implicated in the causation of over 2 million cases of liver cirrhosis in 2017 in the US [71]. The size and composition of BAs in the serum, duodenum, and feces in patients with alcohol-related cirrhosis were markedly different from those in healthy individuals. Kakiyama et al. [59] discovered that serum-conjugated DCA and serum-conjugated CDCA considerably increased and decreased, respectively, in chronic alcoholics (both cirrhotic and non-cirrhotic) compared with the control groups. In feces, secondary BAs and the ratio of secondary BAs to primary BAs were higher in cirrhotic patients without alcohol abstinence than in cirrhotic patients who had abstained from alcohol and did not consume alcohol. Elevated biotransformation of primary BAs to secondary BAs due to changes in the gut microbes, a reduction in colonic transfer time due to heavy alcohol consumption, or a raised "overspill" of secondary BAs into the colonic cavity due to elevated total BA synthesis in the duodenum and ileum as a result of increased alcohol consumption are all possible explanations for the increase in secondary BA levels. Bajaj et al. [72] discovered that the conjugated bile acids in the duodenal fluid and feces of cirrhotic patients who were current alcohol users transformed from relatively harmless taurine-conjugated bile acids to comparatively toxic glycine-conjugated bile acids. It is believed that this is largely due to the dysbiosis reducing the bioavailability of taurine and causing an elevated enterohepatic cycling rate. Moreover, the latest evidence has shown that the shift in the serum BA profile toward a "healthy" composition may not only prevent but also restore already impaired neutrophil dysfunction in cirrhotic patients (alcohol-associated cirrhosis accounts for 50%), which will result in a decrease in bacterial infections and related morbidity and mortality in people with liver cirrhosis [73].
ALD-ACLF
Acute-on-chronic liver failure (ACLF) refers to the development of acute hepatic decompensation in the setting of chronic liver disease. The condition is often accompanied by multiple organ failures and is associated with a high death rate in a short period. Alcohol-related cirrhosis is the background of ALD-ACLF. In a prospective cohort, 65% of patients with severe AH [74] (sAH, Maddrey score ≥ 32) were found to have developed EASL-CLIF ACLF either at the time of diagnosis or within a 6-month follow-up period. However, whether ALD-ACLF is a specific form of ALD or simply a clinical progression of SAH is still uncertain. Previous studies have shown that approximately 33% of hospitalized cirrhotic patients develop ACLF, which is closely linked to an increased mortality rate [75].
In a 1-year prospective cohort study of 143 patients with established cirrhosis (50% of patients with alcohol-related cirrhosis) [76], the more advanced was the organ failure, the higher was the total BA(TBA) level, suggesting that TBA may be a potential indicator of disease severity in patients with ACLF. Second, on receiver operating characteristic (ROC) curve analysis, TBA ≥ 36.9 µmol/L during follow-up was found to be the optimal cut-off level for predicting the new onset of AD (acute decompensation) or ACLF. These findings suggested that total BAs and individual BAs can help predict the new onset of AD or ACLF. Although this was the first research to evaluate the role of serum BAs in the development of AD and ACLF in individuals with liver cirrhosis, this study had some limitations. On one hand, patients with liver cirrhosis caused by cholestatic diseases (such as PBC, PSC, gallstones, and tumors) were excluded from this study. More investigations are required to ascertain whether the BAs profile is different in people with and without basic cholestatic liver disease. Secondly, this study did not assess the serum levels of BAs according to the etiology of liver cirrhosis. The changes in serum BAs in AD/ACLF caused by liver cirrhosis of different etiologies may be different. Additional research should be conducted to determine whether BA levels can be used as non-invasive markers to predict the onset of AD or ACLF in cirrhotic patients. Additionally, future experiments should clarify whether modulating serum BAs with resin or specific BA uptake blockers has an effect on the disease course in individuals with decompensated liver cirrhosis.
In a recent first experimental research on the relationship between the dynamic balance of BAs and acute-on-chronic liver injury (ACLI) [77], the researchers modeled an alcohol-associated ACLI animal model that resembles ACLF based on the Gao-binge model (chronic-plus-binge alcohol feeding) [78] in Abcb4 gene knockout mice (called the Ko-EtOH mouse group) in order to better understand the acute inflammatory response in chronic liver disease. The Ko-EtOH mouse group showed worsened hepatic injury [79]. Compared with the Wt-Cont group, the Ko-EtOH group presented higher β-MCA, and lower CA and CDCA levels. Moreover, the levels of CYP8b1 and CYP7a1 in the liver were significantly inhibited in this model, which is compatible with the transformation of the BA synthesis pathway to an alternative pathway. In addition, alcohol exposure significantly increased FGF21 levels in the livers of WT and KO mice. Some studies have shown that FGF21 is a novel modulator of alcohol desire in rodents and humans, by demonstrating a significant increase in the expression level of FGF21 after acute and chronic drinking [80]. However, there were no discernible differences in the expression levels of FGFR1, FGFR4, FXR, and SHP in the liver and plasma FGF15/FGF19 after alcohol stimulation [77]. This indicates that FGF21 induced by chronic alcohol abuse inhibits the CYP7a1 gene, independent of the FXR-SHP or FXR-FGF19-FGFR4 pathway. Additional research is necessary to identify the relationship between FGF21 and CYP7A1 in different stages of ALD.
Alcohol-related hepatocellular carcinoma
A cohort study showed that approximately 250,000 patients died of alcohol-related liver cancer in 2016, accounting for 30% of all hepatic tumor deaths [81]. Alcohol-induced BA disorder may be one of the essential and direct factors in the progression of hepatocellular carcinoma caused by alcohol abuse. In a recent study [23], the researchers collected tissue samples from HCC patients, including alcoholics and non-alcoholics, and discovered that alcohol abuse significantly disrupted the equilibrium of BAs. The levels of 4 primary BAs (CDCA, GCA, TCA, and TCDCA) were significantly higher, while the levels of 3 secondary BAs (TLCA, TDCA, and HCA) were significantly lower (decrease by 76%) in HCC patients who consumed alcohol; in addition, the level of GDCA (secondary bile acid) was 3.3 times higher in HCC patients who consumed alcohol. Furthermore, a previous study revealed that BA imbalance may lead to the formation and development of HCC through the downregulation of a variety of tumor suppressor genes [21]. In addition, only BSEP expression was dramatically decreased in patients with liver cancer who abused alcohol. Patients with BSEP deficiency were found to be at a higher risk of developing significant hepatobiliary disorders, with approximately 15% of cases progressing to HCC or cholangiocarcinoma [82]. These findings collectively suggest that alcohol can trigger BA dyshomeostasis by reducing the expression of BSEP to promote the progression of HCC.
Other liver diseases
Abnormal changes in BA levels and composition are also observed in other chronic liver diseases. Numerous studies have discovered significant differences in the size and composition of BA pool in mouse models and biological samples from patients with metabolic associated fatty liver disease (MAFLD) or NASH. For example, mice who were fed a methionine-choline-deficient diet (MCD) showed a significant increase in serum levels of Tβ-MCA and TCA [83], and their levels returned to normal after methionine or choline supplementation. In a recent study of patients with MAFLD [84], plasma TCA levels and GCA levels showed a positive correlation with the increase in inflammation and fibrosis, respectively. In patients with HBV infection, all HBV phases showed downregulation of metabolic pathways, such as BA metabolism [85]. In cholestatic diseases, such as PBC and primary sclerosing cholangitis (PSC), the strong inflammatory reaction in the bile duct leads to progressive bile duct fibrosis and cholestasis. Cholestasis leads to increased levels of BAs in the hepatic tissue and circulation [86]. Highly toxic hydrophobic bile acids, such as CDCA, induce Rubicon, a protein that inhibits the normal execution of autophagy. BAs induce Rubicon production and inhibit autophagy through FXR dependence, which weakens the clearance of injured hepatocytes [87]. In a study [21], levels of TCA, GCA, TCDCA, DCA, and TLCA in the liver of the HCC animal model fed with a high-fat diet were considerably higher than those in the healthy control group. In addition, GCDCA has been shown to induce activation of autophagy by targeting the AMPK/mTOR pathway in hepatoma cells to promote the invasion and metastasis of HCC cells. Therefore, it may be a potentially useful prognostic marker in patients with HCC [88].
Advances in the BA-based therapies for ALD
So far, no drug has been found to reverse ALD. Therefore, characterization of the pathogenetic mechanisms of ALD is a key imperative to developing novel drugs. The regulatory roles of BAs and their signaling molecules are increasingly acknowledged as prospective targets for ALD management. Here, various drugs under research are reviewed (Table 2).
Table 2.
BA-based therapies for ALD
| Compound | Therapeutic mechanism | Potential clinical effects | |
|---|---|---|---|
| FXR agonist | OCA | Inhibiting BA synthesis and up-regulating BA transporters |
OCA alleviated steatosis, fibrosis, and portal hypertension [90] OCA reduced bilirubin and ALP [91, 92] OCA improved ALT, AST, FIB-4 levels [93] |
| Fexaramine | FGF15 ↑ CYP7a1 ↓ | Fexaramine improved insulin sensitivity and stabilized the intestinal barrier [98] | |
| FGF19 analogue | NGM282 | Suppression of BA synthesis |
NGM282 improved noninvasive markers of hepatic fibrosis but not ALP in patients with PSC [101] NGM282 mildly improved liver enzyme levels in patients with PBC [103] |
| ASBT inhibitor | GSK2330672 | Inhibition of luminal BA uptake | GSK2330672 reduced pruritus in patients with PBC [107] |
| PPAR agonist | Seladelpar (MBX-8025) | Maintenance of BA balance | MBX-8025 improved cholestasis and reduced ALP [110] |
| Intestinal BA sequestrant | Pectin | Increasing BA secretion | Pectin stabilized the intestinal barrier and reduced liver damage [115] |
ALP alkaline phosphatase, ALT alanine aminotransferase, ASBT apical sodium-dependent bile acid transporter, AST aspartate aminotransferase, BA bile acid, CYP7A1 cholesterol 7α-hydroxylase, FIB-4 fibrosis4Score, FGF15/19 fibroblast growth factor 15/19, FXR farnesoid X receptor, OCA obeticholic acid, PBC primary biliary cholangitis, PPAR peroxisome proliferator-activated receptor, PSC primary sclerosing cholangitis
FXR agonists
Obeticholic acid
Obeticholic acid (also known as INT-747) is a selective FXR agonist and a semi-synthetic derivative of CDCA. Compared with CDCA, OCA (6a-ECDCA) exhibits approximately 100-fold higher FXR agonistic activity. It protects hepatocytes from the toxicity of BAs by inhibiting BA synthesis and up-regulating BA transporters. OCA has been approved by the FDA as a monotherapy or supplemental therapy for PBC in patients who cannot tolerate UDCA or who do not respond adequately to UDCA. OCA has been shown to improve biochemical indicators of cholestasis [89]. In preclinical studies, OCA was found to alleviate steatosis, fibrosis, and portal hypertension [90]. In a clinical trial of PBC, OCA administered in combination with bezafibrate reduced the levels of cholestasis markers, such as bilirubin and alkaline phosphatase (ALP) [91]. In a phase II clinical trial for PSC [NCT02177136], OCA effectively reduced ALP levels [92]. In a multicenter study [NCT02548351], OCA improved ALT, AST, FIB-4 levels, and activity scores in patients with NASH [93]. OCA has now entered phase III clinical trials and is considered as the most effective drug for the treatment of MAFLD and NASH [94]. However, its effect on moderate and severe AH still needs to be confirmed in phase II clinical trial [NCT02039219], as the study was temporarily terminated due to safety concerns. The side effects of OCA include pruritus and gastrointestinal side effects, such as constipation and nausea; elevated total cholesterol and low-density lipoprotein levels; and reduced high-density cholesterol levels [95].
Fexaramine
Fexaramine is an intestinal-restricted FXR agonist. Firstly, fexaramine can increase the expression of FGF15 [96] and transfer it to the liver, thereby reducing the expression of BA synthase CYP7a1 in the liver and protecting the hepatocytes from the toxic effect of BAs. Secondly, fexaramine can alter the composition of intestinal flora by increasing Bacteroides and Acetatifactor [97], which are the dominant bacteria that convert CDCA into LCA, which is a secondary BA identified as a takeda G protein-coupled receptor 5 (TGR5) agonist. The increase in LCA activates TGR5 by inducing the expression of the gene encoding TGR5 to increase the excretion of the glucagon-like peptide-1(GLP1), which can improve insulin sensitivity [98]. Fexaramine has the advantage of intestinal restriction, which potentially avoids the systemic effects of FXR agonists, such as pruritus. In a recent study, administration of fexaramine was found to reduce alcohol-induced liver injury by steadying the gut barrier, which may be another reason for the reduction of liver inflammation observed after ethanol treatment [37]. However, further experimental and clinical studies are required to determine the role of intestinal-specific FXR agonists and antagonists in ALD.
FGF19 analog
NGM282
NGM282 is an analog of FGF19. Previous studies have demonstrated the carcinogenic effect of FGF15/19. Rats with overexpression of the human orthologue FGF19 were found more likely to develop HCC. Moreover, in mice lacking the FGFR4 receptor, the pro-mitogenic and pro-cancerogenic properties of FGF19 were weakened, indicating its role as a potential pharmacological target for the therapy of HCC [99]. NGM282, a N-terminal amino acid modified FGF19 analog, does not promote HCC but completely retains the regulatory activity of BAs, indicating that the effect of FGF19 on BA modulation could be independent of its tumor-promoting activity [100]. In a phase II clinical trial of PSC [NCT02704364], NGM282 effectively inhibited BA synthesis and reduced fibrosis markers without significantly affecting ALP levels [101]. In a phase II clinical trial of NASH [NCT02443116], NGM282 reduced liver adipogenesis and improved fibrosis [102]. Moreover, in a multicenter study of patients with PBC [NCT02026401] [103], NGM282 slightly improved liver enzyme levels. The main adverse reaction of NGM282 was diarrhea. One of the questions that have to be answered is whether high serum FGF19 levels can contribute to downstream signaling resistance. This will be crucial in determining whether FGF19 analogs can be used as a treatment option in ALD patients.
ASBT inhibitor
GSK2330672
GSK2330672 (GSK672) is an ASBT inhibitor. In a recent experiment [104], in a chronic-plus-binge ALD mouse model, GSK2330672 improved hepatocellular steatosis and oxidative stress by blocking the reabsorption of BAs in the gut. These observations not only suggest that inhibiting BAs recycling in the intestine is a potential treatment for ALD, but they also reveal novel mechanistic details about the relationship between BA dyshomeostasis and ALD. GSK672 treatment will, however, increase the amount of BAs in the large bowel, which may have damaging consequences for enterohepatic circulation and intestinal bacteria. Several other ASBT inhibitors are being studied in clinical research on cholestatic diseases [105]. In a NASH preclinical trial, ASBT inhibitors combined with FGF19 analogs minimized BA synthesis and reduced liver inflammation in mice [106]. In a phase II clinical trial of PBC [NCT01899703], the application of GSK2330672 effectively reduced the degree of pruritus [107]. Whether ASBT inhibitors have clinical benefits in patients with ALD remains to be determined.
PPAR agonist
Seladelpar (MBX-8025)
PPAR-α can promote the transport and oxidation of fatty acids. Ethanol inactivates the peroxisome proliferator activated receptor (PPAR), leading to reduced hepatic lipolysis [108]. On one hand, acetaldehyde suppresses the transcriptional activation and DNA binding activities of PPAR. Alcohol, on the other hand, lowers PPAR activity indirectly through oxidative stress mediated by CYP2E1. A recent study [109] demonstrated that seladelpar (MBX-8025), a selective PPAR-δ agonist, protects mice from hepatic injury caused by ethanol administration by recovering intestinal barrier function and BA balance. A phase II clinical trial of seladelpar in the treatment of PBC [NCT02955602] demonstrated improvement in cholestasis status and ALP level of patients [110]. However, in a previous clinical trial [NCT02609048], treatment of PBC with seladelpar was associated with an increased risk of abnormal increase in transaminases [111]. Further clinical trials are required to verify the role of PPAR agonists in the treatment of patients with ALD.
Intestinal bile acid sequestrant
Pectin
Pectin is a kind of fiber that can alter the intestinal microflora and alleviate ALD. Excessive ethanol consumption can result in ALD in humans. However, only a minority of alcoholics develop severe liver damage, indicating that, in addition to the amount of alcohol consumed, there are additional factors that influence the development and progression of ALD. In an experiment [112] to study the relationship between IM (intestinal microflora) and alcohol-induced liver injury in alcohol-sensitive (SENS) or alcohol-resistant (RES) mice, the researchers used two different methods to prevent alcohol-induced changes in IM. The first was pectin treatment, which can lead to significant changes in IM. The pectin comes from apple (Sigma, Saint Quentin Fallavier, France); the second was transplantation of fecal microflora of alcohol-resistant (RES) mice into alcohol-sensitive (SENS) mice. Both methods were found to prevent steatosis, and liver inflammation, and to restore the balance of the intestinal environment. This demonstrates the potential benefit of pectin in the treatment of ALD. A recent study demonstrated the relationship between pectin and BAs [113]. First, due to its physical features, pectin functions as a BA sequestrant because it can form a viscous matrix with cholesterol and BAs in the colon cavity [114], resulting in a reduction of cholesterol and BA levels in the body. In addition, pectin increases the excretion of BAs and alters the overall composition of the BA pool. It is also conducive to the increase of hydrophilic BAs (less toxic) in the liver, plasma, and intestine. This may be due to the abundance of intestinal bacteria containing genes encoding bile acid metabolic enzymes. Finally, patients with SAH have low levels of bacterial tryptophan. Pectin [115] can restore intestinal barrier function by increasing the production of tryptophan metabolites to attenuate alcohol-induced hepatic damage. In conclusion, ingestion of pectin and other fibers can improve alcohol-induced hepatic damage in mice by reshaping the gut microflora and modulating bacterial metabolites, including indoles and BAs. Because patients with ALD often consume minimal fiber, particularly pectin, dietary fiber supplementation as a prebiotic could be a prospective treatment approach for ALD management.
Conclusion
ALD is associated with a high morbidity and mortality worldwide. Firstly, alcohol consumption can alter the composition and size of BA pool by inducing abnormal changes in genes engaged in BAs metabolism, such as up-regulating BAs synthesis genes, changing BAs metabolic enzymes, and regulating BA transporters. Second, alcohol consumption can disrupt BA homeostasis by inducing dysbiosis of intestinal flora involved in BA metabolism. Finally, alcohol consumption causes abnormal alteration of BAs by regulating BA receptors. FXR agonists can alleviate liver steatosis and oxidative stress caused by chronic alcohol consumption, while systemic FXR gene knockout aggravates alcohol-induced hepatic damage. Available evidence suggests a key role of FXR in the occurrence and progression of ALD. Consumption of alcohol suppresses the activity of FXR, which affects the development of ALD by aggravating alcohol-related steatosis, causing mucosal damage, and increasing intestinal permeability, leading to endotoxin translocation and aggravation of liver inflammation and fibrosis. Alcohol abuse will lead to abnormal changes in the size and composition of the BA pool in serum, duodenum, and feces, and the changes in the BAs are markedly distinct in various stages of ALD and other chronic liver diseases. BA signaling may serve as a potential indicator for early diagnosis and prediction of the course of ALD in the foreseeable future, and also in the differential diagnosis of clinical diseases. The current treatment strategy for clinical ALD mainly involves abstinence from alcohol, glucocorticoids, and nutritional support. Recently, FXR agonists, FGF15 analogs, ASBT inhibitors, PPAR agonists, and intestinal BA sequestrants have been shown to alleviate liver injury caused by chronic alcohol consumption. Further clinical studies are required to investigate whether combined or tissue-specific administration can improve the treatment efficacy and reduce treatment-related adverse events in humans. Both BAs and alcohol recycle to the hepatocytes through the portal vein and affect the liver. Firstly, the independent and dual effects of BA dyshomeostasis and alcohol intake on the liver are not clear. More basic and clinical experiments are required to further explore the potential mechanism. Nevertheless, considering the considerable disparities in the makeup of the human and mouse BA profiles, recently, a novel mouse model with human-like BA pools was established [116]. Because Cyp2c70 can convert CDCA to MCA in mice but not in humans, these CYP2c70-deficient mice may be a more appropriate model for exploring BA function in ALD. Secondly, future research should further explore the impact of different types of alcoholic beverages and drinking patterns on the BAs. Finally, the limited patient sample size in the clinical studies does not allow further stratification of patient groups by race, sex, and BMI. Future studies need to evaluate the relationship of demographic characteristics with BA alteration in patients with ALD. Moreover, longitudinal studies are required to accurately evaluate the changes in BAs during the development or regression of ALD. Identification of novel serological biomarkers and therapeutic options for ALD are key research imperatives. Future research should investigate the mechanism between BA metabolism and ALD. This could help us better understand the regulatory role of BAs in the pathogenesis of ALD and the possibility of targeting BA metabolism and signaling as a therapy for ALD.
Acknowledgements
Not applicable.
Abbreviations
- ABCB4
ATP-binding cassette subfamily B member 4
- ACLF
Acute-on-chronic liver failure
- ACLI
Acute-on-chronic liver injury
- AD
Acute decompensation
- ADH
Alcohol dehydrogenase
- AFLD
Alcoholic fatty liver disease
- AH
Alcoholic hepatitis
- ALD
Alcohol-related liver disease
- ALDH
Acetaldehyde dehydrogenase
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- ASBT
Apical sodium-dependent BA transporter
- ASCA
Anti-Saccharomyces cerevisiae antibodies
- ASH
Alcoholic steatohepatitis
- AST
Aspartate aminotransferase
- AUDs
Alcohol use disorders
- BA
Bile acids
- BAAT
Bile acid-CoA: amino acid N-acyltransferase
- BACS
Bile acid-CoA synthetase
- BSEP
Bile salt export pump
- BSH
Bile salt hydrolase
- CA
Cholic acid
- CB1R
Cannabinoid receptor type 1
- CDCA
Chenodeoxycholic acid
- COX-2
Cyclooxygenase-2
- CREBH
CAMP response element-binding protein hepatocyte-specific
- CYP2E1
Cytochrome P450 2E1 enzyme
- CYP7A1
Cholesterol 7α-hydroxylase
- CYP8B1
Sterol 12α-hydroxylase
- DAMPs
Damage-associated molecular patterns
- DCA
Deoxycholic acid
- ERK1/2
Extracellular regulated protein kinases 1/2
- FGF15/19
Fibroblast growth factor 15/19
- FGF21
Fibroblast growth factor 21
- FGR4
Fibroblast growth factor receptor 4
- FIB-4
Fibrosis4Score
- FOXP3
Forkhead box protein 3
- FXR
Farnesoid X receptor
- GCA
Glycocholic acid
- GCDCA
Glycochenodeoxycholic acid
- GDCA
Glycodeoxycholic acid
- GGT
Gamma-glutamyl-transferase
- GLCA
Glycolithocholic acid
- GLP1
Glucagon-like peptide-1
- HCA
Hyocholic acid
- HCC
Hepatocellular carcinoma
- HSC
Hepatic stellate cells
- IM
Intestinal microbiota
- LCA
Lithocholic acid
- LRH-1
Liver receptor homolog-1
- LTA
Lipoteichoic acid
- MAFLD
Metabolic-associated fatty liver disease
- MCD
Methionine-choline-deficient diet
- MCRS1
Microspherule protein 1
- MELD
Model for end-stage liver disease
- MEOS
Microsomal ethanol oxidation system
- MRP3/4
Multidrug resistance-associated protein 3/4
- NAFL
Non-alcoholic fatty liver
- NASH
Non-alcoholic steatohepatitis
- NF-κB
Nuclear transcription factor-κB
- NLRs
Nod-like receptors
- NTCP
Sodium/taurocholate cotransporting polypeptide
- OSTα/β
Organic solute transporter α/β
- PAMPs
Pathogen-associated molecular patterns
- PBC
Primary biliary cirrhosis
- PDC
Pyruvate dehydrogenase complex
- PDK4
Pyruvate dehydrogenase kinase 4
- PGE2
Prostaglandin E2
- PPAR
Peroxisome proliferator-activated receptor
- PSC
Primary sclerosing cholangitis
- PTGER4
Prostaglandin E receptor 4
- ROC
Receiver operating characteristic
- RORγt
Retinoic acid receptor-related orphan receptor- γ t
- ROS
Reactive oxygen species
- RXRα
Retinoid X receptor-α
- SASP
Senescence-associated secretory phenotype
- SHP
Small heterodimer partner
- SIRT1
Sirtuins 1
- SIRT5
Sirtuins 5
- TBA
Total BA
- TCA
Taurocholic acid
- TCDCA
Taurochenodeoxycholic acid
- TDCA
Taurodeoxycholic acid
- TGR5
Takeda G protein-coupled receptor 5
- TLCA
Taurolithocholic acid
- TLR4
Toll-like receptor 4
- TLRs
Toll-like receptors
- Treg
Regulatory T cell
- UDCA
Ursodeoxycholic acid
- β-MCA
β-Muricholic acid
Author contributions
All authors contributed to the study conception and design. YG designed the review outline; YG and YL drafted the manuscript; YG, YL, TL, and XZ revised and edited the paper. All the authors read and approved the final manuscript.
Funding
This work was sponsored by the National Natural Science Foundation of China (Grants nos. 81972265, 82170602), the National Natural Science Foundation of Jilin Province (20200201324JC), and the Project for Health Talents of Jilin Province (JLSWSRCZX 2021-079).
Availability of data and material
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Consent for publication
All authors agreed on the publication of the current version of manuscript.
Ethical approval and consent to participate
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sohi I, Franklin A, Chrystoja B, Wettlaufer A, Rehm J, Shield K. The global impact of alcohol consumption on premature mortality and health in 2016. Nutrients. 2021 doi: 10.3390/nu13093145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell DW. Fifty years of advances in bile acid synthesis and metabolism. J Lipid Res. 2009;50(Suppl):S120–125. doi: 10.1194/jlr.R800026-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111–128. doi: 10.1038/nrgastro.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs CD, Trauner M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat Rev Gastroenterol Hepatol. 2022 doi: 10.1038/s41575-021-00566-7. [DOI] [PubMed] [Google Scholar]
- 7.Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Sun L, Cai J, Gonzalez FJ. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat Rev Gastroenterol Hepatol. 2021;18:335–347. doi: 10.1038/s41575-020-00404-2. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 10.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49:297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu W, Zhu B, Peng X, Zhou M, Jia D, Gu J. Activation of farnesoid X receptor attenuates hepatic injury in a murine model of alcoholic liver disease. Biochem Biophys Res Commun. 2014;443:68–73. doi: 10.1016/j.bbrc.2013.11.057. [DOI] [PubMed] [Google Scholar]
- 13.Xie G, Zhong W, Li H, Li Q, Qiu Y, Zheng X, Chen H, Zhao X, Zhang S, Zhou Z, Zeisel SH, Jia W. Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption. Faseb J. 2013;27:3583–3593. doi: 10.1096/fj.13-231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chanda D, Kim YH, Li T, Misra J, Kim DK, Kim JR, Kwon J, Jeong WI, Ahn SH, Park TS, Koo SH, Chiang JY, Lee CH, Choi HS. Hepatic cannabinoid receptor type 1 mediates alcohol-induced regulation of bile acid enzyme genes expression via CREBH. PLoS ONE. 2013;8:e68845. doi: 10.1371/journal.pone.0068845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang DJ, Hylemon PB, Gillevet PM, Sartor RB, Betrapally NS, Kakiyama G, Sikaroodi M, Takei H, Nittono H, Zhou H, Pandak WM, Yang J, Jiao C, Li X, Lippman HR, Heuman DM, Bajaj JS. Gut microbial composition can differentially regulate bile acid synthesis in humanized mice. Hepatol Commun. 2017;1:61–70. doi: 10.1002/hep4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandl K, Hartmann P, Jih LJ, Pizzo DP, Argemi J, Ventura-Cots M, Coulter S, Liddle C, Ling L, Rossi SJ, DePaoli AM, Loomba R, Mehal WZ, Fouts DE, Lucey MR, Bosques-Padilla F, Mathurin P, Louvet A, Garcia-Tsao G, Verna EC, et al. Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis. J Hepatol. 2018;69:396–405. doi: 10.1016/j.jhep.2018.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen PL, Ghallab A, Vartak N, Reif R, Schaap FG, Hampe J, Hengstler JG. The ascending pathophysiology of cholestatic liver disease. Hepatology. 2017;65:722–738. doi: 10.1002/hep.28965. [DOI] [PubMed] [Google Scholar]
- 18.Doden HL, Ridlon JM. Microbial hydroxysteroid dehydrogenases: from alpha to omega. Microorganisms. 2021 doi: 10.3390/microorganisms9030469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong X, Zhang Q, Ruan Y, Hu M, Liu Z, Gong L. Chronic alcohol consumption increased bile acid levels in enterohepatic circulation and reduced efficacy of irinotecan. Alcohol Alcohol. 2020;55:264–277. doi: 10.1093/alcalc/agaa005. [DOI] [PubMed] [Google Scholar]
- 20.Zinchuk V, Zinchuk O, Akimaru K, Moriya F, Okada T. Ethanol consumption alters expression and colocalization of bile salt export pump and multidrug resistance protein 2 in the rat. Histochem Cell Biol. 2007;127:503–512. doi: 10.1007/s00418-007-0277-7. [DOI] [PubMed] [Google Scholar]
- 21.Xie G, Wang X, Huang F, Zhao A, Chen W, Yan J, Zhang Y, Lei S, Ge K, Zheng X, Liu J, Su M, Liu P, Jia W. Dysregulated hepatic bile acids collaboratively promote liver carcinogenesis. Int J Cancer. 2016;139:1764–1775. doi: 10.1002/ijc.30219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Song X, Valanejad L, Vasilenko A, More V, Qiu X, Chen W, Lai Y, Slitt A, Stoner M, Yan B, Deng R. Bile salt export pump is dysregulated with altered farnesoid X receptor isoform expression in patients with hepatocellular carcinoma. Hepatology. 2013;57:1530–1541. doi: 10.1002/hep.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Zhang Q, Ding M, Yao J, Guo Y, Yan W, Yu S, Shen Q, Huang M, Zheng Y, Lin Y, Wang Y, Liu Z, Lu L. Alcohol triggered bile acid disequilibrium by suppressing BSEP to sustain hepatocellular carcinoma progression. Chem Biol Interact. 2022;356:109847. doi: 10.1016/j.cbi.2022.109847. [DOI] [PubMed] [Google Scholar]
- 24.Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, White RC, Clarke TH, Nguyen K, Torralba M, Shao Y, Liu J, Hernandez-Morales A, Lessor L, Rahman IR, Miyamoto Y, Ly M, Gao B, Sun W, Kiesel R, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575:505–511. doi: 10.1038/s41586-019-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu H, Duan Y, Lang S, Jiang L, Wang Y, Llorente C, Liu J, Mogavero S, Bosques-Padilla F, Abraldes JG, Vargas V, Tu XM, Yang L, Hou X, Hube B, Stärkel P, Schnabl B. The Candida albicans exotoxin Candidalysin promotes alcohol-associated liver disease. J Hepatol. 2020;72:391–400. doi: 10.1016/j.jhep.2019.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang S, Duan Y, Liu J, Torralba MG, Kuelbs C, Ventura-Cots M, Abraldes JG, Bosques-Padilla F, Verna EC, Brown RS, Jr, Vargas V, Altamirano J, Caballería J, Shawcross D, Lucey MR, Louvet A, Mathurin P, Garcia-Tsao G, Ho SB, Tu XM, et al. Intestinal fungal dysbiosis and systemic immune response to fungi in patients with alcoholic hepatitis. Hepatology. 2020;71:522–538. doi: 10.1002/hep.30832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, Ha S, Nelson BN, Kelly SP, Wu L, Zheng Y, Longman RS, Rastinejad F, Devlin AS, Krout MR, Fischbach MA, Littman DR, Huh JR. Bile acid metabolites control T(h)17 and T(reg) cell differentiation. Nature. 2019;576:143–148. doi: 10.1038/s41586-019-1785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paik D, Yao L, Zhang Y, Bae S, D'Agostino GD, Zhang M, Kim E, Franzosa EA, Avila-Pacheco J, Bisanz JE, Rakowski CK, Vlamakis H, Xavier RJ, Turnbaugh PJ, Longman RS, Krout MR, Clish CB, Rastinejad F, Huttenhower C, Huh JR, et al. Human gut bacteria produce T(H)17-modulating bile acid metabolites. Nature. 2022;603:907–912. doi: 10.1038/s41586-022-04480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao B, Ahmad MF, Nagy LE, Tsukamoto H. Inflammatory pathways in alcoholic steatohepatitis. J Hepatol. 2019;70:249–259. doi: 10.1016/j.jhep.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemmers A, Moreno C, Gustot T, Maréchal R, Degré D, Demetter P, de Nadai P, Geerts A, Quertinmont E, Vercruysse V, Le Moine O, Devière J. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646–657. doi: 10.1002/hep.22680. [DOI] [PubMed] [Google Scholar]
- 31.Støy S, Sandahl TD, Dige AK, Agnholt J, Rasmussen TK, Grønbæk H, Deleuran B, Vilstrup H. Highest frequencies of interleukin-22-producing t helper cells in alcoholic hepatitis patients with a favourable short-term course. PLoS ONE. 2013 doi: 10.1371/journal.pone.0055101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortiz V, Wands JR. Chronic ethanol diet increases regulatory t-cell activity and inhibits hepatitis c virus core-specific cellular immune responses in mice. Hepatol Res. 2014;44:788–797. doi: 10.1111/hepr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato Y, Atarashi K, Plichta DR, Arai Y, Sasajima S, Kearney SM, Suda W, Takeshita K, Sasaki T, Okamoto S, Skelly AN, Okamura Y, Vlamakis H, Li Y, Tanoue T, Takei H, Nittono H, Narushima S, Irie J, Itoh H, et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature. 2021;599:458–464. doi: 10.1038/s41586-021-03832-5. [DOI] [PubMed] [Google Scholar]
- 34.Bajaj JS. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:235–246. doi: 10.1038/s41575-018-0099-1. [DOI] [PubMed] [Google Scholar]
- 35.Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Litwinowicz K, Choroszy M, Waszczuk E. Changes in the composition of the human intestinal microbiome in alcohol use disorder: a systematic review. Am J Drug Alcohol Abuse. 2020;46:4–12. doi: 10.1080/00952990.2019.1669629. [DOI] [PubMed] [Google Scholar]
- 37.Hartmann P, Hochrath K, Horvath A, Chen P, Seebauer CT, Llorente C, Wang L, Alnouti Y, Fouts DE, Stärkel P, Loomba R, Coulter S, Liddle C, Yu RT, Ling L, Rossi SJ, DePaoli AM, Downes M, Evans RM, Brenner DA, et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology. 2018;67:2150–2166. doi: 10.1002/hep.29676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen R, Ke L, Li Q, Dang X, Shen S, Shen J, Li S, Liang L, Peng B, Kuang M, Ma Y, Yang Z, Hua Y. Abnormal bile acid-microbiota crosstalk promotes the development of hepatocellular carcinoma. Hepatol Int. 2022;16:396–411. doi: 10.1007/s12072-022-10299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Funabashi M, Grove TL, Wang M, Varma Y, McFadden ME, Brown LC, Guo C, Higginbottom S, Almo SC, Fischbach MA. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature. 2020;582:566–570. doi: 10.1038/s41586-020-2396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang JD, Myers CJ, Harris SC, Kakiyama G, Lee IK, Yun BS, Matsuzaki K, Furukawa M, Min HK, Bajaj JS, Zhou H, Hylemon PB. Bile acid 7α-dehydroxylating gut bacteria secrete antibiotics that inhibit Clostridium difficile: role of secondary bile acids. Cell Chem Biol. 2019;26:27–34.e24. doi: 10.1016/j.chembiol.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, Nakajima-Takagi Y, Iwama A, Koga T, Sugimoto Y, Ozawa T, Nakamura M, Kumagai M, Watashi K, Taketo MM, Aoki T, Narumiya S, Oshima M, Arita M, Hara E, et al. Gut microbiota promotes obesity-associated liver cancer through PGE(2)-mediated suppression of antitumor immunity. Cancer Discov. 2017;7:522–538. doi: 10.1158/2159-8290.Cd-16-0932. [DOI] [PubMed] [Google Scholar]
- 42.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, McCulloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018 doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin WB, Li TT, Huo D, Qu S, Li XV, Arifuzzaman M, Lima SF, Shi HQ, Wang A, Putzel GG, Longman RS, Artis D, Guo CJ. Genetic manipulation of gut microbes enables single-gene interrogation in a complex microbiome. Cell. 2022;185:547–562.e522. doi: 10.1016/j.cell.2021.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang Y, Zhang T, Kusumanchi P, Han S, Yang Z, Liangpunsakul S. Alcohol metabolizing enzymes, microsomal ethanol oxidizing system, cytochrome p450 2E1, catalase, and aldehyde dehydrogenase in alcohol-associated liver disease. Biomedicines. 2020 doi: 10.3390/biomedicines8030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langhi C, Pedraz-Cuesta E, Haro D, Marrero PF, Rodríguez JC. Regulation of human class I alcohol dehydrogenases by bile acids. J Lipid Res. 2013;54:2475–2484. doi: 10.1194/jlr.M039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lívero FA, Stolf AM, Dreifuss AA, Bastos-Pereira AL, Chicorski R, de Oliveira LG, de Souza CE, Fabossi IA, Rabitto IS, Gremski LH, Henneberg R, Telles JE, Oude Elferink RP, Acco A. The FXR agonist 6ECDCA reduces hepatic steatosis and oxidative stress induced by ethanol and low-protein diet in mice. Chem Biol Interact. 2014;217:19–27. doi: 10.1016/j.cbi.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Kong B, Zhang M, Huang M, Rizzolo D, Armstrong LE, Schumacher JD, Chow MD, Lee YH, Guo GL. FXR deficiency alters bile acid pool composition and exacerbates chronic alcohol induced liver injury. Dig Liver Dis. 2019;51:570–576. doi: 10.1016/j.dld.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang M, Kong B, Huang M, Wan R, Armstrong LE, Schumacher JD, Rizzolo D, Chow MD, Lee YH, Guo GL. FXR deletion in hepatocytes does not affect the severity of alcoholic liver disease in mice. Dig Liver Dis. 2018;50:1068–1075. doi: 10.1016/j.dld.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang M, Kong B, Zhang M, Rizzolo D, Armstrong LE, Schumacher JD, Chow MD, Lee YH, Joseph LB, Stofan M, Zhang L, Guo GL. Enhanced alcoholic liver disease in mice with intestine-specific farnesoid X receptor deficiency. Lab Invest. 2020;100:1158–1168. doi: 10.1038/s41374-020-0439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haigis MC, Guarente LP. Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 51.Sun R, Zhang Z, Bao R, Guo X, Gu Y, Yang W, Wei J, Chen X, Tong L, Meng J, Zhong C, Zhang C, Zhang J, Sun Y, Ling C, Tong X, Yu FX, Yu H, Qu W, Zhao B, et al. Loss of SIRT5 promotes bile acid-induced immunosuppressive microenvironment and hepatocarcinogenesis. J Hepatol. 2022 doi: 10.1016/j.jhep.2022.02.030. [DOI] [PubMed] [Google Scholar]
- 52.Deng W, Fan W, Tang T, Wan H, Zhao S, Tan Y, Oware KA, Tan J, Li J, Qu S. Farnesoid X receptor deficiency induces hepatic lipid and glucose metabolism disorder via regulation of pyruvate dehydrogenase kinase 4. Oxid Med Cell Longev. 2022;2022:3589525. doi: 10.1155/2022/3589525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 54.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 55.Garrido A, Kim E, Teijeiro A, Sánchez Sánchez P, Gallo R, Nair A, Matamala Montoya M, Perna C, Vicent GP, Muñoz J, Campos-Olivas R, Melms JC, Izar B, Schwabe RF, Djouder N. Histone acetylation of bile acid transporter genes plays a critical role in cirrhosis. J Hepatol. 2022;76:850–861. doi: 10.1016/j.jhep.2021.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stärkel P, Leclercq S, de Timary P, Schnabl B. Intestinal dysbiosis and permeability: the Yin and Yang in alcohol dependence and alcoholic liver disease. Clin Sci (Lond) 2018;132:199–212. doi: 10.1042/cs20171055. [DOI] [PubMed] [Google Scholar]
- 58.Wolfe A, Thomas A, Edwards G, Jaseja R, Guo GL, Apte U. Increased activation of the Wnt/β-catenin pathway in spontaneous hepatocellular carcinoma observed in farnesoid X receptor knockout mice. J Pharmacol Exp Ther. 2011;338:12–21. doi: 10.1124/jpet.111.179390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kakiyama G, Hylemon PB, Zhou H, Pandak WM, Heuman DM, Kang DJ, Takei H, Nittono H, Ridlon JM, Fuchs M, Gurley EC, Wang Y, Liu R, Sanyal AJ, Gillevet PM, Bajaj JS. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G929–937. doi: 10.1152/ajpgi.00315.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology. 2009;49:1228–1235. doi: 10.1002/hep.22771. [DOI] [PubMed] [Google Scholar]
- 61.Wunsch E, Milkiewicz M, Wasik U, Trottier J, Kempińska-Podhorodecka A, Elias E, Barbier O, Milkiewicz P. Expression of hepatic fibroblast growth factor 19 is enhanced in primary biliary cirrhosis and correlates with severity of the disease. Sci Rep. 2015;5:13462. doi: 10.1038/srep13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou M, Learned RM, Rossi SJ, DePaoli AM, Tian H, Ling L. Engineered fibroblast growth factor 19 reduces liver injury and resolves sclerosing cholangitis in Mdr2-deficient mice. Hepatology. 2016;63:914–929. doi: 10.1002/hep.28257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Jr, Mezey E, White RI., Jr Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. doi: 10.1016/0016-5085(78)90401-8. [DOI] [PubMed] [Google Scholar]
- 64.Dominguez M, Rincón D, Abraldes JG, Miquel R, Colmenero J, Bellot P, García-Pagán JC, Fernández R, Moreno M, Bañares R, Arroyo V, Caballería J, Ginès P, Bataller R. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747–2756. doi: 10.1111/j.1572-0241.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- 65.Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, Gleeson D, MacGilchrist A, Grant A, Hood S, Masson S, McCune A, Mellor J, O'Grady J, Patch D, Ratcliffe I, Roderick P, Stanton L, Vergis N, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619–1628. doi: 10.1056/NEJMoa1412278. [DOI] [PubMed] [Google Scholar]
- 66.Ventura-Cots M, Argemi J, Jones PD, Lackner C, El Hag M, Abraldes JG, Alvarado E, Clemente A, Ravi S, Alves A, Alboraie M, Altamirano J, Barace S, Bosques F, Brown R, Caballeria J, Cabezas J, Carvalhana S, Cortez-Pinto H, Costa A, et al. Clinical, histological and molecular profiling of different stages of alcohol-related liver disease. Gut. 2022 doi: 10.1136/gutjnl-2021-324295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Llopis M, Cassard AM, Wrzosek L, Boschat L, Bruneau A, Ferrere G, Puchois V, Martin JC, Lepage P, Le Roy T, Lefèvre L, Langelier B, Cailleux F, González-Castro AM, Rabot S, Gaudin F, Agostini H, Prévot S, Berrebi D, Ciocan D, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65:830–839. doi: 10.1136/gutjnl-2015-310585. [DOI] [PubMed] [Google Scholar]
- 68.Ciocan D, Voican CS, Wrzosek L, Hugot C, Rainteau D, Humbert L, Cassard AM, Perlemuter G. Bile acid homeostasis and intestinal dysbiosis in alcoholic hepatitis. Aliment Pharmacol Ther. 2018;48:961–974. doi: 10.1111/apt.14949. [DOI] [PubMed] [Google Scholar]
- 69.Muthiah MD, Smirnova E, Puri P, Chalasani N, Shah VH, Kiani C, Taylor S, Mirshahi F, Sanyal AJ. Development of alcohol-associated hepatitis is associated with specific changes in gut-modified bile acids. Hepatol Commun. 2022 doi: 10.1002/hep4.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He L, Vatsalya V, Ma X, Zhang J, Yin X, Kim S, Feng W, McClain CJ, Zhang X. Metabolic profiling of bile acids in the urine of patients with alcohol-associated liver disease. Hepatol Commun. 2021;5:798–811. doi: 10.1002/hep4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singal AK, Mathurin P. Diagnosis and treatment of alcohol-associated liver disease: a review. JAMA. 2021;326:165–176. doi: 10.1001/jama.2021.7683. [DOI] [PubMed] [Google Scholar]
- 72.Bajaj JS, Kakiyama G, Zhao D, Takei H, Fagan A, Hylemon P, Zhou H, Pandak WM, Nittono H, Fiehn O, Salzman N, Holtz M, Simpson P, Gavis EA, Heuman DM, Liu R, Kang DJ, Sikaroodi M, Gillevet PM. Continued alcohol misuse in human cirrhosis is associated with an impaired gut-liver axis. Alcohol Clin Exp Res. 2017;41:1857–1865. doi: 10.1111/acer.13498. [DOI] [PubMed] [Google Scholar]
- 73.Balazs I, Horvath A, Leber B, Feldbacher N, Sattler W, Rainer F, Fauler G, Vermeren S, Stadlbauer V. Serum bile acids in liver cirrhosis promote neutrophil dysfunction. Clin Transl Med. 2022;12:e735. doi: 10.1002/ctm2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michelena J, Altamirano J, Abraldes JG, Affò S, Morales-Ibanez O, Sancho-Bru P, Dominguez M, García-Pagán JC, Fernández J, Arroyo V, Ginès P, Louvet A, Mathurin P, Mehal WZ, Caballería J, Bataller R. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology. 2015;62:762–772. doi: 10.1002/hep.27779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, Arroyo V, Kamath PS. Acute-on chronic liver failure. J Hepatol. 2012;57:1336–1348. doi: 10.1016/j.jhep.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 76.Horvatits T, Drolz A, Roedl K, Rutter K, Ferlitsch A, Fauler G, Trauner M, Fuhrmann V. Serum bile acids as marker for acute decompensation and acute-on-chronic liver failure in patients with non-cholestatic cirrhosis. Liver Int. 2017;37:224–231. doi: 10.1111/liv.13201. [DOI] [PubMed] [Google Scholar]
- 77.Christidis G, Karatayli E, Hall RA, Weber SN, Reichert MC, Hohl M, Qiao S, Boehm U, Lütjohann D, Lammert F, Karatayli SC. Fibroblast growth factor 21 response in a preclinical alcohol model of acute-on-chronic liver injury. Int J Mol Sci. 2021 doi: 10.3390/ijms22157898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karatayli E, Hall RA, Weber SN, Dooley S, Lammert F. Effect of alcohol on the interleukin 6-mediated inflammatory response in a new mouse model of acute-on-chronic liver injury. Biochim Biophys Acta Mol Basis Dis. 2019;1865:298–307. doi: 10.1016/j.bbadis.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 80.Søberg S, Andersen ES, Dalsgaard NB, Jarlhelt I, Hansen NL, Hoffmann N, Vilsbøll T, Chenchar A, Jensen M, Grevengoed TJ, Trammell SAJ, Knop FK, Gillum MP. FGF21, a liver hormone that inhibits alcohol intake in mice, increases in human circulation after acute alcohol ingestion and sustained binge drinking at Oktoberfest. Mol Metab. 2018;11:96–103. doi: 10.1016/j.molmet.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016 (2017). Lancet 390:1151–1210. 10.1016/s0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed]
- 82.Pawlikowska L, Strautnieks S, Jankowska I, Czubkowski P, Emerick K, Antoniou A, Wanty C, Fischler B, Jacquemin E, Wali S, Blanchard S, Nielsen IM, Bourke B, McQuaid S, Lacaille F, Byrne JA, van Eerde AM, Kolho KL, Klomp L, Houwen R, et al. Differences in presentation and progression between severe FIC1 and BSEP deficiencies. J Hepatol. 2010;53:170–178. doi: 10.1016/j.jhep.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tanaka N, Matsubara T, Krausz KW, Patterson AD, Gonzalez FJ. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology. 2012;56:118–129. doi: 10.1002/hep.25630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nimer N, Choucair I, Wang Z, Nemet I, Li L, Gukasyan J, Weeks TL, Alkhouri N, Zein N, Tang WHW, Fischbach MA, Brown JM, Allayee H, Dasarathy S, Gogonea V, Hazen SL. Bile acids profile, histopathological indices and genetic variants for non-alcoholic fatty liver disease progression. Metabolism. 2021;116:154457. doi: 10.1016/j.metabol.2020.154457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Montanari NR, Ramírez R, Aggarwal A, van Buuren N, Doukas M, Moon C, Turner S, Diehl L, Li L, Debes JD, Feierbach B, Boonstra A. Multi-parametric analysis of human livers reveals variation in intrahepatic inflammation across phases of chronic hepatitis B infection. J Hepatol. 2022 doi: 10.1016/j.jhep.2022.02.016. [DOI] [PubMed] [Google Scholar]
- 86.Gulamhusein AF, Hirschfield GM. Primary biliary cholangitis: pathogenesis and therapeutic opportunities. Nat Rev Gastroenterol Hepatol. 2020;17:93–110. doi: 10.1038/s41575-019-0226-7. [DOI] [PubMed] [Google Scholar]
- 87.Panzitt K, Jungwirth E, Krones E, Lee JM, Pollheimer M, Thallinger GG, Kolb-Lenz D, Xiao R, Thorell A, Trauner M, Fickert P, Marschall HU, Moore DD, Wagner M. FXR-dependent rubicon induction impairs autophagy in models of human cholestasis. J Hepatol. 2020;72:1122–1131. doi: 10.1016/j.jhep.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 88.Gao L, Lv G, Li R, Liu WT, Zong C, Ye F, Li XY, Yang X, Jiang JH, Hou XJ, Jing YY, Han ZP, Wei LX. Glycochenodeoxycholate promotes hepatocellular carcinoma invasion and migration by AMPK/Mtor dependent autophagy activation. Cancer Lett. 2019;454:215–223. doi: 10.1016/j.canlet.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 89.Trauner M, Nevens F, Shiffman ML, Drenth JPH, Bowlus CL, Vargas V, Andreone P, Hirschfield GM, Pencek R, Malecha ES, MacConell L, Shapiro D. Long-term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3-year results of an international open-label extension study. Lancet Gastroenterol Hepatol. 2019;4:445–453. doi: 10.1016/s2468-1253(19)30094-9. [DOI] [PubMed] [Google Scholar]
- 90.Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, Elst IV, Windmolders P, Vanuytsel T, Nevens F, Laleman W. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286–2298. doi: 10.1002/hep.26939. [DOI] [PubMed] [Google Scholar]
- 91.Smets L, Verbeek J, Korf H, van der Merwe S, Nevens F. Improved markers of cholestatic liver injury in patients with primary biliary cholangitis treated with obeticholic acid and Bezafibrate. Hepatology. 2021;73:2598–2600. doi: 10.1002/hep.31613. [DOI] [PubMed] [Google Scholar]
- 92.Kowdley KV, Vuppalanchi R, Levy C, Floreani A, Andreone P, LaRusso NF, Shrestha R, Trotter J, Goldberg D, Rushbrook S, Hirschfield GM, Schiano T, Jin Y, Pencek R, MacConell L, Shapiro D, Bowlus CL. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J Hepatol. 2020;73:94–101. doi: 10.1016/j.jhep.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rinella ME, Dufour JF, Anstee QM, Goodman Z, Younossi Z, Harrison SA, Loomba R, Sanyal AJ, Bonacci M, Trylesinski A, Natha M, Shringarpure R, Granston T, Venugopal A, Ratziu V. Non-invasive evaluation of response to obeticholic acid in patients with NASH: results from the regenerate study. J Hepatol. 2022;76:536–548. doi: 10.1016/j.jhep.2021.10.029. [DOI] [PubMed] [Google Scholar]
- 94.Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/s0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 95.Peeraphatdit TB, Simonetto DA, Shah VH. Exploring new treatment paradigms for alcoholic hepatitis by extrapolating from NASH and cholestasis. J Hepatol. 2018;69:275–277. doi: 10.1016/j.jhep.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin Y, Wang M, Gu W, Chen L. Intestine-specific FXR agonists as potential therapeutic agents for colorectal cancer. Biochem Pharmacol. 2021;186:114430. doi: 10.1016/j.bcp.2021.114430. [DOI] [PubMed] [Google Scholar]
- 97.Pathak P, Xie C, Nichols RG, Ferrell JM, Boehme S, Krausz KW, Patterson AD, Gonzalez FJ, Chiang JYL. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology. 2018;68:1574–1588. doi: 10.1002/hep.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chiang JYL, Ferrell JM. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am J Physiol Gastrointest Liver Physiol. 2020;318:G554–G573. doi: 10.1152/ajpgi.00223.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raja A, Park I, Haq F, Ahn SM. FGF19-FGR4 signaling in hepatocellular carcinoma. Cells. 2019 doi: 10.3390/cells8060536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou M, Wang X, Phung V, Lindhout DA, Mondal K, Hsu JY, Yang H, Humphrey M, Ding X, Arora T, Learned RM, DePaoli AM, Tian H, Ling L. Separating tumorigenicity from bile acid regulatory activity for endocrine hormone FGF19. Cancer Res. 2014;74:3306–3316. doi: 10.1158/0008-5472.Can-14-0208. [DOI] [PubMed] [Google Scholar]
- 101.Hirschfield GM, Chazouillères O, Drenth JP, Thorburn D, Harrison SA, Landis CS, Mayo MJ, Muir AJ, Trotter JF, Leeming DJ, Karsdal MA, Jaros MJ, Ling L, Kim KH, Rossi SJ, Somaratne RM, DePaoli AM, Beuers U. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: a multicenter, randomized, double-blind, placebo-controlled phase II trial. J Hepatol. 2019;70:483–493. doi: 10.1016/j.jhep.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 102.Harrison SA, Neff G, Guy CD, Bashir MR, Paredes AH, Frias JP, Younes Z, Trotter JF, Gunn NT, Moussa SE, Kohli A, Nelson K, Gottwald M, Chang WCG, Yan AZ, DePaoli AM, Ling L, Lieu HD. Efficacy and safety of aldafermin, an engineered FGF19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic steatohepatitis. Gastroenterology. 2021;160:219–231. doi: 10.1053/j.gastro.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 103.Mayo MJ, Wigg AJ, Leggett BA, Arnold H, Thompson AJ, Weltman M, Carey EJ, Muir AJ, Ling L, Rossi SJ, DePaoli AM. NGM282 for treatment of patients with primary biliary cholangitis: a multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Commun. 2018;2:1037–1050. doi: 10.1002/hep4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matye DJ, Li Y, Chen C, Chao X, Wang H, Ni H, Ding WX, Li T. Gut-restricted apical sodium-dependent bile acid transporter inhibitor attenuates alcohol-induced liver steatosis and injury in mice. Alcohol Clin Exp Res. 2021;45:1188–1199. doi: 10.1111/acer.14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karpen SJ, Kelly D, Mack C, Stein P. Ileal bile acid transporter inhibition as an anticholestatic therapeutic target in biliary atresia and other cholestatic disorders. Hepatol Int. 2020;14:677–689. doi: 10.1007/s12072-020-10070-w. [DOI] [PubMed] [Google Scholar]
- 106.Matye DJ, Wang H, Luo W, Sharp RR, Chen C, Gu L, Jones KL, Ding WX, Friedman JE, Li T. Combined ASBT inhibitor and FGF15 treatment improves therapeutic efficacy in experimental nonalcoholic steatohepatitis. Cell Mol Gastroenterol Hepatol. 2021;12:1001–1019. doi: 10.1016/j.jcmgh.2021.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hegade VS, Kendrick SF, Dobbins RL, Miller SR, Thompson D, Richards D, Storey J, Dukes GE, Corrigan M, Oude Elferink RP, Beuers U, Hirschfield GM, Jones DE. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114–1123. doi: 10.1016/s0140-6736(17)30319-7. [DOI] [PubMed] [Google Scholar]
- 108.Dhanda A, Atkinson S, Vergis N, Enki D, Fisher A, Clough R, Cramp M, Thursz M. Trace element deficiency is highly prevalent and associated with infection and mortality in patients with alcoholic hepatitis. Aliment Pharmacol Ther. 2020;52:537–544. doi: 10.1111/apt.15880. [DOI] [PubMed] [Google Scholar]
- 109.Chu H, Jiang L, Gao B, Gautam N, Alamoudi JA, Lang S, Wang Y, Duan Y, Alnouti Y, Cable EE, Schnabl B. The selective PPAR-delta agonist seladelpar reduces ethanol-induced liver disease by restoring gut barrier function and bile acid homeostasis in mice. Transl Res. 2021;227:1–14. doi: 10.1016/j.trsl.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bowlus CL, Galambos MR, Aspinall RJ, Hirschfield GM, Jones DEJ, Dörffel Y, Gordon SC, Harrison SA, Kremer AE, Mayo MJ, Thuluvath PJ, Levy C, Swain MG, Neff GW, Sheridan DA, Stanca CM, Berg CP, Goel A, Shiffman ML, Vierling JM, et al. A phase II, randomized, open-label, 52-week study of seladelpar in patients with primary biliary cholangitis. J Hepatol. 2022 doi: 10.1016/j.jhep.2022.02.033. [DOI] [PubMed] [Google Scholar]
- 111.Jones D, Boudes PF, Swain MG, Bowlus CL, Galambos MR, Bacon BR, Doerffel Y, Gitlin N, Gordon SC, Odin JA, Sheridan D, Wörns MA, Clark V, Corless L, Hartmann H, Jonas ME, Kremer AE, Mells GF, Buggisch P, Freilich BL, et al. Seladelpar (MBX-8025), a selective ppar-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol. 2017;2:716–726. doi: 10.1016/s2468-1253(17)30246-7. [DOI] [PubMed] [Google Scholar]
- 112.Ferrere G, Wrzosek L, Cailleux F, Turpin W, Puchois V, Spatz M, Ciocan D, Rainteau D, Humbert L, Hugot C, Gaudin F, Noordine ML, Robert V, Berrebi D, Thomas M, Naveau S, Perlemuter G, Cassard AM. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol. 2017;66:806–815. doi: 10.1016/j.jhep.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 113.Ciocan D, Spatz M, Trainel N, Hardonnière K, Domenichini S, Mercier-Nomé F, Desmons A, Humbert L, Durand S, Kroemer G, Lamazière A, Hugot C, Perlemuter G, Cassard AM. Modulation of the bile acid enterohepatic cycle by intestinal microbiota alleviates alcohol liver disease. Cells. 2022 doi: 10.3390/cells11060968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khorasani AC, Kouhfar F, Shojaosadati SA. Pectin/lignocellulose nanofibers/chitin nanofibers bionanocomposite as an efficient biosorbent of cholesterol and bile salts. Carbohydr Polym. 2021;261:117883. doi: 10.1016/j.carbpol.2021.117883. [DOI] [PubMed] [Google Scholar]
- 115.Wrzosek L, Ciocan D, Hugot C, Spatz M, Dupeux M, Houron C, Lievin-Le Moal V, Puchois V, Ferrere G, Trainel N, Mercier-Nomé F, Durand S, Kroemer G, Voican CS, Emond P, Straube M, Sokol H, Perlemuter G, Cassard AM. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut. 2021;70:1299–1308. doi: 10.1136/gutjnl-2020-321565. [DOI] [PubMed] [Google Scholar]
- 116.Takahashi S, Fukami T, Masuo Y, Brocker CN, Xie C, Krausz KW, Wolf CR, Henderson CJ, Gonzalez FJ. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J Lipid Res. 2016;57:2130–2137. doi: 10.1194/jlr.M071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.