Abstract
Non-alcoholic fatty liver disease (NAFLD) is related to a dysregulation of mitophagy, a process that is not fully understood. Parkin-related mitophagy can sustain mitochondrial homeostasis and hepatocyte viability. Herein, we report that selenoprotein M (SELENOM) plays a central role in maintaining mitophagy in high-fat diet (HFD)-mediated NAFLD. We show that SELENOM was significantly downregulated in the liver of HFD-fed mice. SELENOM deletion aggravated HFD-mediated hepatic steatosis, inflammation, and fibrosis; accompanied by enhanced fatty acid oxidation and oxidative stress in the liver. Molecular analyses show that lipotoxicity was related to increased mitochondrial apoptosis as evidenced by enhanced mitochondrial ROS production, and attenuation of mitochondrial potential in the liver of HFD-fed SELENOM−/− mice. Additionally, SELENOM deletion reduced mitophagy and aggravated hepatic injury in NAFLD. Mechanistically, SELENOM overexpression activated Parkin-mediated mitophagy to reduce mitochondrial apoptosis and remove HFD-damaged mitochondria. We further found that SELENOM regulates Parkin expression via the AMPKα1–MFN2 pathway; blockade of AMPKα1 prevented SELENOM activation of Parkin-mediated mitophagy. Our work identified SELENOM downregulation as a possible explanation for the defective mitophagy in NAFLD. Thus, targeting SELENOM may be potential new therapeutic modalities for NAFLD treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04385-0.
Keywords: SELENOM, NAFLD, Mitochondria homeostasis, Oxidative stress, Mitophagy
Introduction
Selenoproteins have important regulatory effects on fatty liver and lipid metabolism. Selenoprotein P is involved in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) by regulating lipid accumulation [1]. Hepatic selenoprotein S deficiency caused hepatic steatosis and insulin resistance via regulating hepatic lipid accumulation and insulin action [2]. Selenoprotein M (SELENOM) is one of the selenoproteins in the selenoprotein family. SELENOM, a key thioredoxin-like enzyme present in the endoplasmic reticulum (ER), has been associated with hepatocellular degeneration and carcinoma [3, 4]. SELENOM exerts its physiological effects in many pathways including redox control, fatty acid composition, stress response and cancer cell proliferation [5, 6]. SELENOM could increase antioxidant activity to reduce the levels of oxidative and mitochondria stress in the progression of Alzheimer's disease [7]. Recent data showed that SELENOM is involved in redox signaling and energy metabolism through regulating STAT3 phosphorylation and cytosolic calcium responses [5, 8]. In addition, SELENOM has roles in the regulation of ER and inflammatory stress responses to ameliorate metabolic dysfunction [5, 9]. In liver diseases, selenoproteins have been reported to inhibit oxidative damage and imbalance of mitochondrial dynamic in response to several types of stress [10–12]. Loss of SELENOM could increase obesity by affecting metabolic and endocrine functions [13]. Therefore, SELENOM may be able to alleviate disorders of lipid metabolism and high-fat diet (HFD)-mediated mitochondrial damage in NAFLD.
The incidence of NAFLD is expected to increase with the prevalence of obesity and diabetes globally. NAFLD is the culmination of multiple disorders, including abnormal lipid metabolism, oxidative stress, mitochondrial dysfunction, and lipid peroxidative stress [14]. HFD could contribute to NAFLD, but the mechanisms by which HFD causes excessive accumulation of lipid in hepatocytes are complex, and multiple pathological factors are involved, especially hepatic oxidative stress and mitochondrial dysfunction [15]. NAFLD is often accompanied by lipotoxicity-induced hepatocyte apoptosis and inflammatory response [16]. There is increasing evidence that alterations in the gut microbiota (dysbiosis) influence NAFLD pathological processes [17]. Mitochondrial damage contributes to NAFLD at different levels by impairing fatty liver functions, stimulate the overproduction of reactive oxygen species (ROS), which in turn triggers pro-inflammatory cytokine release, lipid peroxidation and mitochondria-dependent apoptosis to aggravate HFD-mediated liver injury [18, 19]. Thus, restoring mitochondria homeostasis is vital to reverse or retard these NAFLD processes. However, the molecular mechanisms regulating mitochondrial homeostasis for this disease remain unclear.
Mitophagy is a specific type of autophagy that targets damaged mitochondria for degradation by lysosomes in response to mitochondrial stress, to maintain a healthy mitochondrial population [20]. Liver fibrosis has been associated with excess ROS levels and inhibition of mitophagy [21, 22], and mitophagy activation is essential to protect hepatocyte function against mitochondrial apoptosis [23]. The Parkin-dependent mitophagy pathway, including the P62 protein-mediated ubiquitination, is involved in sustaining mitochondrial function to ameliorate HFD-induced NAFLD [24]. Defects in the PTEN-induced kinase 1 (PINK1) protein and Parkin-dependent mitophagy could exacerbate acetaminophen-induced liver injury [25]. Activation of the PINK1/Parkin-mediated mitophagy pathway may also involve AMPKα1 signaling [26], and the AMPK–MFN2 axis may be involved in regulating the mitochondrial-associated ER membrane (MAM) and mitophagy [27, 28]. Clearly, mitophagy is an important pathway in different liver defects. However, the role of mitophagy in HFD conditions and NAFLD remains largely unknown.
In this study, we show that SELENOM expression was markedly reduced in NAFLD induced by HFD in vivo, and in AML12 hepatocytes treated with palmitic acid (PA) in vitro. Although the AMPK–MFN2 axis is involved in the mitochondrial-associated ER membrane (MAM) and autophagy regulation [27, 28], this pathway has not been shown in mitophagy regulation of NAFLD. Accordingly, our study aims to determine how SELENOM regulates the development of NAFLD via mitophagy, whether the role of SELENOM is dependent on AMPKα1–MFN2 signaling, especially focusing on the mitochondrial apoptosis in fatty liver disease.
Methods
Animal treatment
Male SELENOM wild-type (WT) and knockout (SELENOM−/−) C57BL/6 J mice were purchased from Cyagen Biosciences (Cyagen Inc., US). Four-week-old mice (20–25 g) were first housed for 18 weeks under standard conditions (12/12 h of light/dark cycle, 50% ± 4% humidity, 23–25 °C) in individual cages with access to food and water ad libitum. At 4 weeks of age, mice were randomly assigned to receive HFD diet for NAFLD induction, or normal chow diet for another 18 weeks, as previously reported [29]. The composition and energy density of the diets are listed in Table S1. After 18 weeks, fasting blood samples and liver specimens were collected following an overnight fast, and stored at − 80 °C. All animal studies were performed in triplicates.
Histopathological analysis
Liver tissue processing was as previously described [30]. Briefly, liver samples were fixed in 4% paraformaldehyde for 2 h at 4 °C, embedded in paraffin, sectioned at 0.2–0.3 cm and stained with hematoxylin and eosin (H&E, Gibco, USA, 008011), Masson stain or Sirius Red (Gibco, USA, 4351405). Pathological changes were observed with a Nikon optical microscope (Mitsubishi Inc., Japan, S154), and the staining intensity quantified with Image-Pro Plus 6.0 (Media Cybernetics, USA). The average diameter of hepatic vacuolations was measured by Image-Pro Plus 6.0 as previously described [31].
Biochemical evaluation
To evaluate liver injury, the levels of alanine transaminase (ALT), total cholesterol (TC), aspartate transaminase (AST) and triglyceride (TG) (Abcam, UK, ab234579 for ALT, ab282928 for TC, ab105134 for AST, ab178780 for TG) in the serum were determined with commercial kits on the automatic biochemical analyzer HITACHI 7020 (Hitachi, Tokyo, Japan, DS12556).
Transmission electron microscopy (TEM)
The liver tissues were fixed with 3% glutaraldehyde, post fixed with 1% osmium, dehydrated and embedded for ultra-thin sectioning at 70 nm. Sections were stained with uranyl acetate, lead citrate, and dried for imaging at 80 kV to detect changes in autophagy under the TEM (Elibo Biotechnology Co., Ltd, Japan, GEM-1200ES).
Cell culture and treatment
AML12 hepatocytes (ATCC, USA) were cultured in Dulbecco’s modified Eagle’s medium (Gibco, USA, GB12511) supplemented with 10% fetal bovine serum (FBS) at 37 °C in a humidified 5% CO2 atmosphere to 90–100% confluence, with medium changes every 2 days. In some experiments, cells were treated with the palmitic acid (PA, 75 μM; Sigma-Aldrich, G11512) for 24 h to simulate a lipotoxicity. For AMPKα1 inhibition, cells were treated with Compound C (CC, 5 μM, Sigma-Aldrich, St Louis, G13418) for 6 h. For amino acid starvation, cells were treated with Grey’s Balanced Salt Solution (GBSS; Sigma-Aldrich, G9779) for 1–3 h. Autophagic vacuoles were measured using an autophagy detection kit (Abcam, ab139484).
Oil red staining
After fixation with 2 ml fixative solution per well for 30 min, the prepared oil red O working solution (Beyotime, China, C0158M) was stained at room temperature for 10–20 min, and the liver cells washed twice with the washing solution were observed under a microscope (Mitsubishi Inc., Japan, S154).
Immunofluorescence
Immunofluorescence was performed using primary antibodies against Tom20 (1: 200, Abcam, ab283317), LAMP2 (1: 200, Abcam, ab199946), Cyt-c (1: 200, Abcam, ab216971), P-AMPKα1 (1: 200, Wanleibio., China, WL02256) and Parkin (1: 500, Cell Signaling Technology, Inc, 4211). Cells were fixed in 4% paraformaldehyde and permeabilized (Biosharp, BL539A) with 0.1% TritonX-100, blocked with goat serum and incubated with primary antibodies, followed by appropriate secondary antibodies. The following secondary antibodies included Dylight 488-goat anti-rabbit lgG and Alexa Fluor 594-goat anti-mouse lgG. The nuclei were stained using DAPI (ImmunoBioscience, AR-6501–01). Images acquired by an Eclipse Ni-U microscope system (Nikon, N54) and a confocal laser scanning microscopy (Olympus, BX-61).
Oxidative stress biomarker assay
Liver tissues were homogenized in normal saline, and the supernatant collected for the following assays: total antioxidant capacity (T-AOC), glutathione peroxidase (GPX), superoxide dismutase (SOD), malondialdehyde (MDA) and catalase (CAT) (Beyotime, China, S0119 for T-AOC, S0058 for GSH-Px, S0087 for SOD, S0131M for MDA, S0082 for CAT), according to the manufacturer’s protocols as previously described [32]. T-AOC could be used to evaluate the antioxidant capacity of biologically active substances. For MDA measurement, tissues were homogenized in MDA kit extract buffer and assayed according to the manufacturer’s instructions [33].
Mitochondrial function analysis
Mitochondrial membrane potential (△Ψ m) was evaluated with mitochondrial membrane potential probe (JC-1) (Thermo, US, T3168) staining as described previously [34, 35]. Briefly, 3 × 105 cells were stained with 2 μL of JC-1 stock solution (final concentration: 2 μg/mL). Stained cells were observed by fluorescence microscopy at 500 nm (JC-1 monomers) 590 nm (JC-1 aggregates). Apoptosis was assessed with the YO-PRO-1 (green fluorescent) and PI (red fluorescent) apoptosis and necrosis detection kit (Beyotime, China, C2022-0.2 ml). The relative expression of the two dyes determines the level of apoptosis and necrosis. Mitochondrial ROS (mtROS) levels were detected by labeling 2 × 105 cells with the mitoSOX™ red mitochondrial superoxide indicator (Molecular Probes, USA, M36008), and analyzed by a flow cytometry.
RNA interference
For knockdown of SELENOM and Parkin, cells (3 × 105/well) were transfected with siRNA against SELENOM (A04066) or Parkin (A04091) using Lipofectamine (LIP) RNAi MAX Reagent according to the manufacturer’s instruction. To overexpress SELENOM, a pcDNA3.1 vector containing the SECIS element was used according to the method of Gladyshev et al. [36]. The coding regions of mouse SELENOM were amplified by PCR and subcloned into the pcDNA3.1 vector to generate the pcDNA3.1-SELENOM plasmid, followed by verification by sequencing. Cells were transfected with plasmid pcDNA3.1-SELENOM using the LIP 2000 reagent (Invitrogen, USA, 11668030) in an Opti-MEM medium for 24 h.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from liver and AML12 hepatocytes following the manufacturer’s protocol [37, 38]. cDNA was synthesized from 2 to 5 μg total RNA using a ReverTra Ace® qPCR RT Kit & Master Mix (Accurate Biotechnology Co., Ltd., SQ-101). qRT-PCR was performed using the Light Cycler®480 System (Roche, Beijing, China, 015278001) and ChamQ™ Universal SYBR® qPCR Master Mix (Vazyme Biotech Co., Ltd, Nanjing, China, azyme #Q711). The primers for target genes by qRT-PCR are shown in Table S2. GAPDH was used as an internal reference. The relative mRNA abundance was calculated by the 2−∆∆Ct method [39].
Protein extraction and western blot analysis
Samples were lysed with RIPA lysis solution for total protein extraction, and quantification by BCA protein quantitative kit (Beyotime, China, P0012S). Proteins (30–40 μg per sample) were separated by SDS polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. Membranes were blocked with 5% bovine serum albumin (BSA) and incubated with primary antibodies (Table S3), followed by secondary antibodies. Stained proteins were visualized with enhanced chemiluminescent (ECL) substrate (Sigma-Aldrich, Germany, GE3541), imaged and normalized to GAPDH expression.
Quantification and statistical analysis
The GraphPad Prism 9.0 software (Graph Pad Software Inc., San Diego, CA, USA) was used for statistical analysis. Analyzed data are presented as mean ± SEM of triplicate samples (n = 3) for in vitro experiments, and six replicates (n = 6) for in vivo experiments. Statistical analyses were performed one-way ANOVA with Tukey’s post hoc test. All data in this study followed normal distribution. Significance level was set at *P < 0.05. Representative images were quantified by Image-Pro Plus 6.0 software (Media cybernetics Image Technology Co., Ltd, USA) and statistically analyzed by the GraphPad Prism 9.0 software.
Results
SELENOM deletion aggravates HFD-induced NAFLD
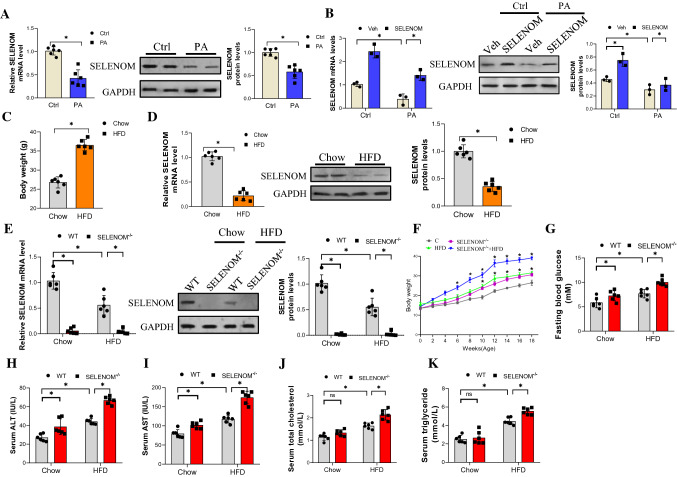
To verify whether SELENOM is involved in NAFLD, an in vitro model of palmitic acid (PA)-mediated lipotoxicity and a mouse model of HFD-induced NAFLD were established, and the level of SELENOM detected with qRT-PCR and western blot. In culture, SELENOM level was significantly reduced in PA-treated hepatocytes at both the mRNA and protein expression levels, compared to the untreated control cells (Fig. 1A). This reduction in SELENOM level by PA treatment can be reversed by overexpression of SELENOM (Fig. 1B). In vivo, mice fed with HFD for 18 weeks showed significantly higher weight (Fig. 1C), and increases in hepatic lipid metabolism parameters including alanine transaminase (ALT), aspartate transaminase (AST), total cholesterol and triglycerides (see Fig. 1H–K wild-type mice data). The liver of these mice showed lower mRNA and protein levels of SELENOM compared to normal chow-fed mice (Fig. 1D). This suggests that HFD downregulates SELENOM expression in the liver. To dive deeper into the role of SELENOM in NAFLD, we generated SELENOM knockout (SELENOM−/−) mice (Fig. S1A) with no expression of SELENOM in the liver (Fig. 1E). Immunohistochemistry result revealed that SELENOM expression was markedly lowered in the cytoplasm of hepatocytes of HFD-fed and SELENOM−/− mice compared with the chow group (Fig. S1B–C). Data related to the mRNA expression of SELENOM in other tissues are shown (Fig. S1D). Comparing the biological characteristics (bodyweight, blood glucose and metabolic parameters) of HFD-fed and SELENOM−/− mice showed that bodyweight (Fig. 1F) and blood glucose content (Fig. 1G) were increased in mice fed with HFD, and were even higher in SELENOM−/− mice. Similarly, the high levels of ALT, AST, total cholesterol and triglycerides in the serum of HFD-fed mice were further aggravated by SELENOM knockdown (Fig. 1H–K). Altogether, these data suggested that HFD downregulates SELENOM, and SELENOM deletion aggravates the development of HFD-induced fatty liver disease.
Fig. 1.
SELENOM is downregulated in livers from HFD-treated mice and related to the development of NAFLD. A The mRNA and protein level of SELENOM in AML12 hepatocytes treated with PA (75 μM) (n = 6; *P < 0.05). B The mRNA and protein level of SELENOM in vitro were detected in Veh, SELENOM, PA and SELENOM + PA (n = 3; *P < 0.05). C Bodyweight of Chow- and HFD-treated mice (n = 6; *P < 0.05). D The mRNA and protein level of SELENOM in livers from Chow- and HFD-treated mice (18 W) (n = 6; *P < 0.05). E The mRNA and protein level of SELENOM was measured in livers from WT, SELENOM−/−, HFD, SELENOM−/− + HFD (n = 6; *P < 0.05). F Bodyweight in mice of WT, SELENOM−/−, HFD and SELENOM−/− + HFD (n = 6; *P < 0.05). G–K Blood glucose levels, ALT, AST, triglyceride, total cholesterol in the blood serum isolated from WT, SELENOM−/−, HFD and SELENOM−/− + HFD mice (n = 6; *P < 0.05). Values represent means ± SEM
SELENOM deletion induces hepatic injury in HFD treatment
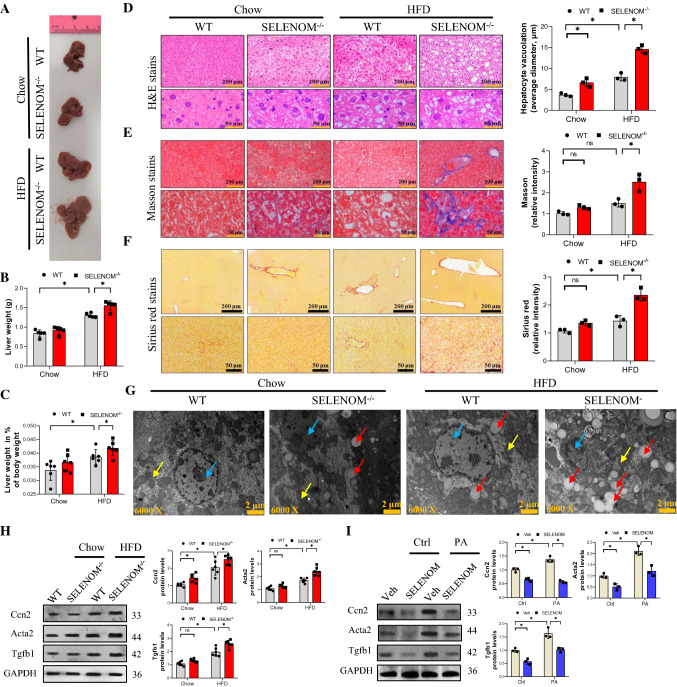
Analysis of the gross liver morphology showed that the liver of HFD-fed mice was significantly larger and heavier than those of chow-fed mice, and SELENOM−/− mice showed further increase in liver weight when fed HFD (Fig. 2A, B). The liver weight in % of body weight was consistent with the trend of body weight (Fig. 2C). H&E staining showed that HFD increased hepatic vacuolization and steatosis, which were further enhanced in the liver of SELENOM−/− mice, particularly with they were fed with HFD (Fig. 2D). Similarly, the increased liver fibrosis caused by HFD, shown by Masson and Sirius Red staining, was also further exacerbated in SELENOM−/− mice (Fig. 2E, F). These increases in steatosis, nuclear atrophy and overall liver degeneration were further validated by transmission electron microscopy (Fig. 2G). At the molecular level, many liver fibrosis-related genes including Cox I, Cox IV, Ccn2, Acta2, Tgfb1, Timp1, Ccn1, Ccn2, Vim and Mmp9 were significantly increased by HFD, and further increased by SELENOM deletion (Fig. S2A). The protein expressions of Ccn2, Tgfb and Acta2 were also similarly increased in vitro (Fig. 2H). These in vivo changes in liver fibrosis-related genes and proteins were recapitulated in PA-induced lipotoxicity cultures, and reversed by SELENOM overexpression in hepatocytes (Figs. 2I and S2B). Together, these results suggest that SELENOM deletion increases HFD-mediated hepatic alveolar steatosis and fibrosis.
Fig. 2.
Mice lacking SELENOM are more susceptible to NAFLD under HFD treatment. A, B The liver weight was measured in HFD-treated livers with SELENOM−/− (n = 6; *P < 0.05). C The liver weight in % of body weight in HFD-treated livers with SELENOM-KO (n = 6; *P < 0.05). D H&E staining shows steatosis in HFD-treated livers with SELENOM−/− (scale bar, 200 and 50 μm). Fields from one representative experiment of three are shown (n = 3; *P < 0.05). E, F Masson and Sirius Red staining show bridging fibrosis (scale bar, 200 and 50 μm). Fields from one representative experiment of three are shown (n = 3; *P < 0.05). G TEM images for liver from WT, SELENOM−/−, HFD and SELENOM−/− + HFD. The blue arrow indicates the nucleus, the yellow arrow indicates the mitochondria and the red arrow indicates the accumulated lipid droplets. Scale bars, 2 μm. Images were chosen from three independent biological samples (n = 6). H The protein levels of Ccn2, Acta2 and Tgfb1 were measured in WT, SELENOM−/−, HFD and SELENOM−/− + HFD (n = 6; *P < 0.05). I Liver fibrosis-related proteins in vitro were detected in Veh, SELENOM, PA and SELENOM + PA (n = 3; *P < 0.05). Values represent means ± SEM
SELENOM deletion increases inflammation response in HFD-induced liver.
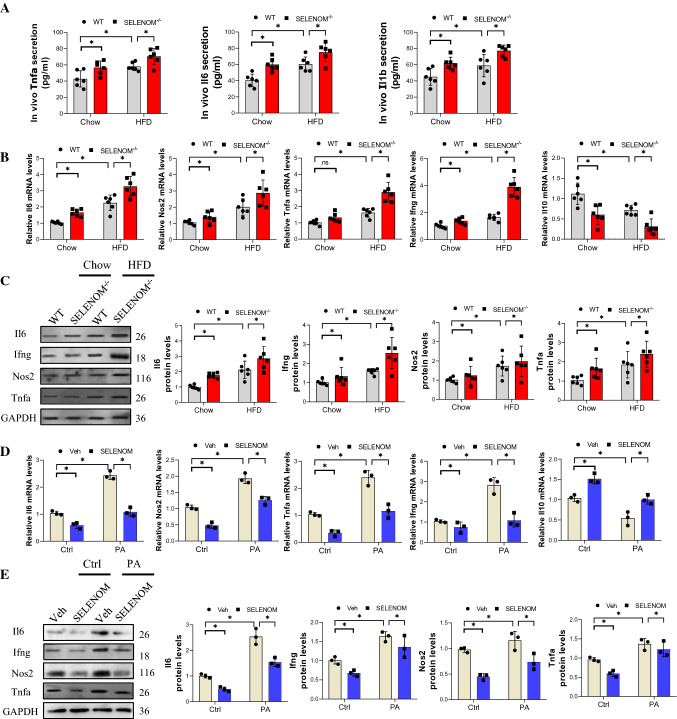
Chronic inflammation is a critical histological change in NAFLD [40, 41], contributing to fibrosis, cirrhosis, liver failure and even hepatocellular carcinoma. Thus, we evaluated the effect of HFD and SELENOM deletion on liver inflammation, and found that both conditions significantly increased the serum levels of Tnfa, Il6, and Il1b compared to the chow-fed group; SELENOM−/− mice fed with HFD showed even higher levels of these proteins (Fig. 3A). In the liver, the gene and protein levels of the pro-inflammatory cytokines Il6, Ifng, Nos2 and Tnfa were similarly increased by HFD and SELENOM deletion (Fig. 3B, C). On the other hand, the mRNA level of the anti-inflammatory factor Il10 was decreased by HFD and SELENOM deletion (Fig. 3B). Again, these observations could be reproduced in vitro with PA treatment, which increased the mRNA and protein levels of Il6, Ifng, Nos2 and Tnfa, and decreased Il10; SELENOM overexpression counteracted these effects (Fig. 3D, E). Taken together, these results support that SELENOM deletion may exacerbate HFD-induced hepatic inflammation.
Fig. 3.
SELENOM−/− increases HFD-mediated inflammation response in liver tissues. A The contents of cytokines (Tnfa, Il6 and Il1b) in blood serum (n = 6; *P < 0.05). B, C The mRNA and protein levels of inflammation response-related genes such as Il6, Il10, Nos2 and Tnfa were determined by qRT-PCR (B) and WB (C) in HFD-treated livers with SELENOM−/− (n = 6; *P < 0.05). D, E The mRNA and protein levels of inflammation response-related genes such as Il6, Il10, Nos2 and Tnfa were detected in Veh, SELENOM, PA and SELENOM + PA (n = 3; *P < 0.05). Values represent means ± SEM
SELENOM−/− increases HFD-mediated oxidative stress and attenuates fatty acid oxidation (FAO) in liver tissues
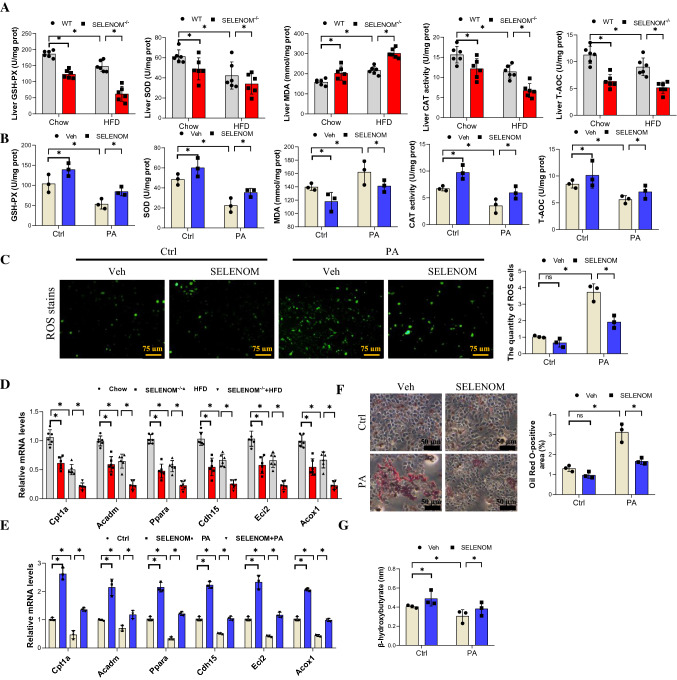
Recent evidence has shown that oxidative stress and FAO play an important role in hepatocyte lipid metabolism and the pathogenesis of NAFLD [42]. Thus, we evaluated these pathways in liver samples and found that HFD reduced the level of CAT, GPX, SOD and T-AOC, and SELENOM deletion further reduced these antioxidant enzymes when combined with HFD (Fig. 4A). By comparison, the lipid peroxidation marker malondialdehyde (MDA) was elevated by HFD, and further enhanced in SELENOM−/− mice fed with HFD. In vitro results show that PA treatment could reduce the levels of CAT, SOD, GPX and T-AOC, and increase the level of MDA; all changes were reversed by SELENOM overexpression (Fig. 4B). In addition, ROS were significantly increased in PA-treated hepatocytes, and this was reduced in hepatocytes with SELENOM overexpression (Fig. 4C). Together, these data indicate that SELENOM deletion increases oxidative stress in the presence of HFD.
Fig. 4.
SELENOM−/− increases HFD-induced hepatic oxidative stress. A Oxidative stress markers of the GSH-PX, SOD, MDA, CAT, T-AOC contents were measured in the livers from WT, SELENOM−/−, HFD and SELENOM−/− + HFD (n = 6; *P < 0.05). B Oxidative stress markers of the GSH-PX, SOD, MDA, CAT, T-AOC contents were measured in hepatocytes of Veh, SELENOM, PA and SELENOM + PA (n = 3; *P < 0.05). Fields from one representative experiment of three are shown. C ROS staining was detected by immunofluorescence with DCFH-DA (green fluorescence, 5 mM) in hepatocytes of Veh, SELENOM, PA and SELENOM + PA (n = 3; *P < 0.05). D The mRNA levels of lipogenic genes such as Cpt1a, Acadm, Ppara, Cdh15, Eci2 and Acox1 in HFD-treated livers with SELENOM−/− (n = 6; *P < 0.05). E The mRNA levels of lipogenic genes such as Cpt1a, Acadm, Ppara, Cdh15, Eci2 and Acox1 in Veh, SELENOM, PA and SELENOM + PA (n = 3; *P < 0.05). F Oil Red O staining in hepatocytes of Veh, SELENOM, PA and SELENOM + PA. Ten fields (Scale bar: 50 μm) were randomly selected for each sample. The positive area in each image was measured (n = 3; *P < 0.05). G β-hydroxybutyrate contents were measured in the livers from WT, SELENOM−/−, HFD and SELENOM−/− + HFD (n = 6; *P < 0.05). Values represent means ± SEM
For the lipid metabolism pathway, we found that the FAO-related genes Cpt1a, Acadm, Ppara, Cdh15, Eci2 and Acox1 were downregulated in HFD-fed mice, and further decreases were seen in SELENOM−/− mice on HFD (Fig. 4D). In vitro results show that PA treatment also decreased the expression of these genes in hepatocytes, and the effect was reversed by SELENOM overexpression (Fig. 4E). Conversely, the levels of lipogenic genes Gpam, Plin1, Scd1, Lipe, Fasn, Acly and Pparg were increased by HFD and further enhanced in SELENOM−/− mice on HFD (Fig. S3A). As expected, SELENOM overexpression could inhibit PA induction of these lipogenic genes (Fig. S3B). In addition, siRNA knockdown of SELENOM enhanced the mRNA level of these lipogenic genes and aggravated PA-induced lipid accumulation (Fig. S3C). Oil red staining showed that SELENOM overexpression could inhibit PA-induced lipid accumulation, and restore the level of beta-hydroxybutyrate, a metabolic product of beta-oxidation (Fig. 4F, G). Together, these data underscore the effects of SELENOM deficiency in decreasing FAO and increasing lipid deposition in HFD-fed mice.
SELENOM attenuates hepatocyte mitochondrial pathway apoptosis
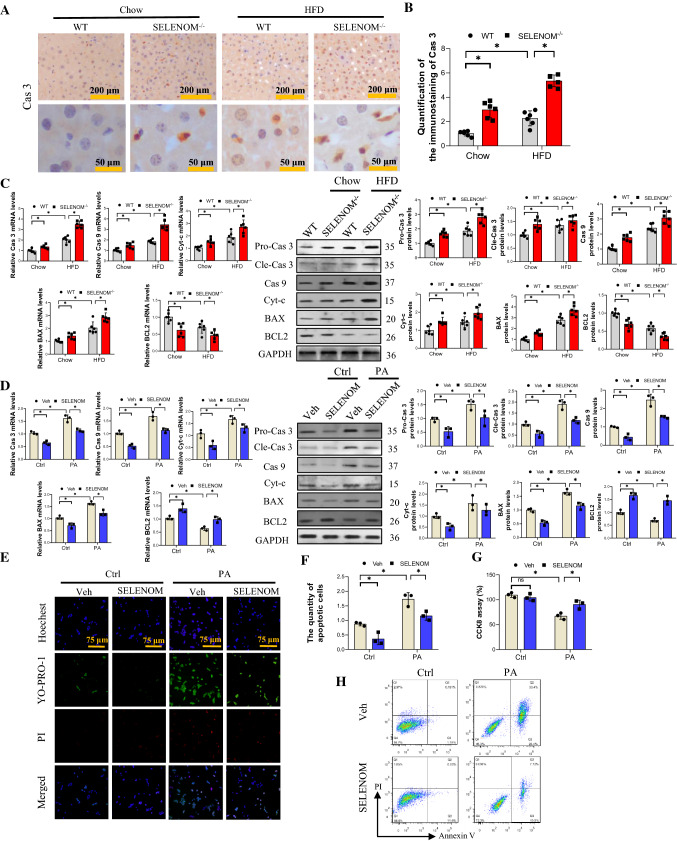
The mitochondrial apoptosis pathway plays an important role in the lipotoxicity of the hepatocyte [43, 44]. Accordingly, we observed that caspase 3 (Cas3) staining was increased in the liver of HFD-treated mice, and further enhanced by SELENOM deletion (Fig. 5A, B). This result was supported with observations of increased mitochondrial pro-apoptotic genes and proteins including Bax, Cyt-c, pro- and cleaved-Cas3 and Cas 9, in HFD-fed mice, an effect that was strengthened by SELENOM deletion (Fig. 5C). In contrast, the level of the anti-apoptotic factor Bcl2 was downregulated. These results were reproducible in vitro with PA-induced hepatocyte apoptosis where the suppression of Bax, Cyt-c, Cas3 and Cas9 expressions, and enhancement of Bcl2 was reversed by SELENOM overexpression (Fig. 5D). Consistent with this, results of YO-PRO-1/PI apoptosis staining, CCK8 cell viability assay and annexin V staining all demonstrate that PA treatment enhanced apoptosis, which was reversed by SELENOM overexpression (Fig. 5E–H). Taken together, these data illustrate that SELENOM deletion augments mitochondrial apoptosis induced by HFD and this can be attenuated by SELENOM overexpression.
Fig. 5.
SELENOM−/− increases HFD-mediated mitochondrial pathway apoptosis in the liver. A, B Cas 3 was measured via immunohistochemistry analysis in livers from WT, SELENOM−/−, HFD and SELENOM−/− + HFD. Six fields (Scale bar: 200 and 50 μm) were randomly selected for each sample. The positive area in each image was measured (n = 6; *P < 0.05). C The mRNA and protein expression levels of mitochondrial pathway apoptosis-related genes Cas 3, Cas 9, Bax and Bcl2 were determined in HFD-treated livers with SELENOM−/− (n = 6; *P < 0.05). D The mRNA and protein expression levels of mitochondrial pathway apoptosis-related genes Cas 3, Cas 9, Bax and Bcl2 were determined in hepatocytes of Veh, SELENOM, PA and SELENOM + PA (n = 3; *P < 0.05). E, F Apoptosis and necrosis staining with YO-PRO-1 and PI for apoptosis detection, and the green nucleus represent an apoptotic cell (n = 3; *P < 0.05). Fields from one representative experiment of three are shown. G CCK8 assay was used to assess the hepatocyte viability under PA treatment (n = 3; *P < 0.05). H Cell viabilities and apoptosis rates were determined by flow cytometry. Healthy (Q1), early apoptotic (Q2), late apoptotic (Q3), and necrotic populations (Q4). Images were chosen from three independent biological samples (n = 3; *P < 0.05). Values represent means ± SEM
SELENOM−/− attenuates hepatocyte mitophagy activity
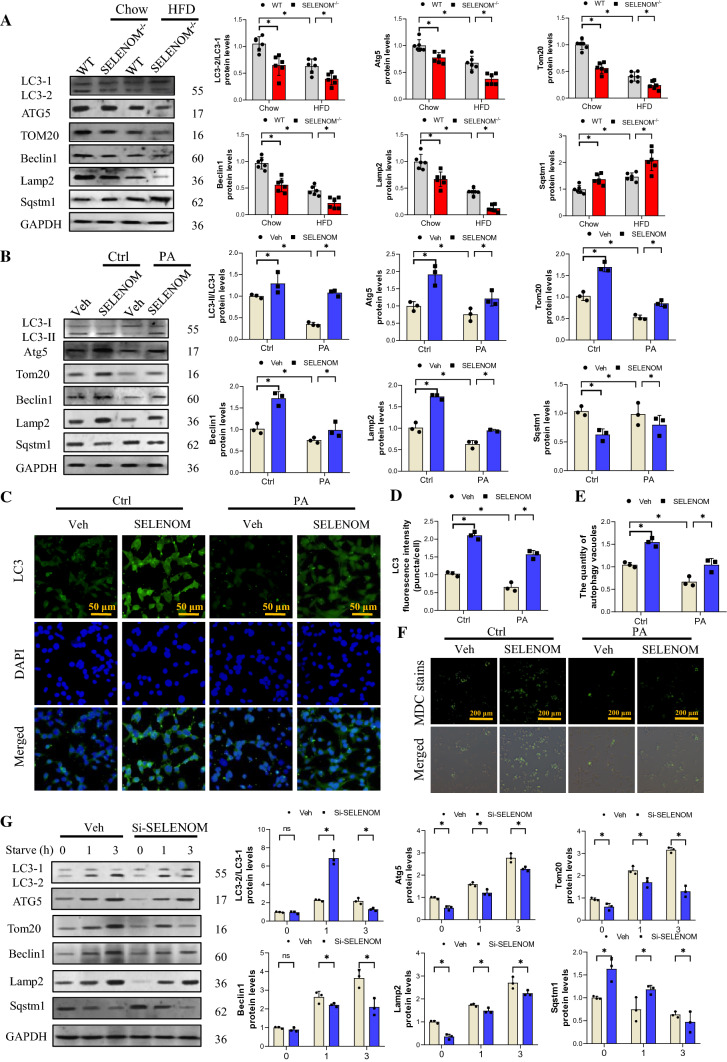
Mitophagy maintains mitochondria quality by removing damaged mitochondria with the help of lysosomes [45]. We evaluated mitophagy-related factors and found that HFD diminished the ratio of LC3-II/LC3-I, and the levels of Beclin1 and Atg5; HFD-fed SELENOM−/− mice exhibited even further reduction (Fig. 6A). Moreover, the protein level of Sqstm1 was elevated in HFD-fed mice and further increased in HFD-fed SELENOM−/− mice. The mitochondrial protein Tom20 and lysosomal protein Lamp2 were downregulated in HFD-fed mice, but the decreases were much greater in SELENOM−/− mice on HFD, indicating that mitophagy was impaired in SELENOM−/− mice under high-fat stress. As expected, mRNA expression levels of these mitophagy-related genes were consistent with the protein expression level (Fig. S4A). Consistent with the in vivo data, PA treatment also decreased the ratio of LC3-II/LC3-I, the levels of Atg5, Tom20, Beclin1 and Lamp2, and increased the level of Sqstm1; SELENOM overexpression reversed the effect (Fig. 6B). The mRNA levels of these mitophagy-related genes were consistent with the protein expression levels (Fig. S4B). Immunofluorescence staining of LC3 was consistent with the western blot data (Fig. 6C, D). Furthermore, we stained autophagy vacuoles with MDC and found that PA treatment reduced the number of vacuoles, and SELENOM overexpression reversed this effect (Fig. 6E, F), indicating that SELENOM may increase the number of autophagy vacuoles.
Fig. 6.
SELENOM involves in HFD-inhibited mitophagy in the liver. A The expression levels of mitophagy-related proteins such as LC3-I, LC3-II, Atg5, Tom20, Beclin1, Lamp2 and Sqstm1 in livers from WT, SELENOM−/−, HFD and SELENOM−/− + HFD (n = 6; *P < 0.05). B The expression levels of mitophagy-related proteins such as LC3-I, LC3-II, Atg5, Tom20, Beclin1, Lamp2 and Sqstm1 in hepatocytes of Veh, SELENOM, PA and SELENOM + PA (n = 3; *P < 0.05). C, D Immunofluorescence assay for mitophagy via assessing LC3 in vitro. Fields from one representative experiment of three are shown (n = 3; *P < 0.05). E, F The quantity of autophagy vacuoles was recorded by MDC staining. Fields from one representative experiment of three are shown (n = 3; *P < 0.05). G Immunoblotting for LC3-I, LC3-II, Atg5, Tom20, Beclin1, Lamp2 and Sqstm1 in AML12 hepatocytes treated with Si-NC or Si-SELENOM were subjected to amino acid starvation for the indicated durations, 0, 1, 3 h, respectively (n = 3; *P < 0.05). Values represent means ± SEM
The autophagy activation model was established with amino acid starvation (AAS) [46] to further determine the role of SELENOM in mitophagy. As shown in Fig. 6G, AAS increased the ratio of LC3-II/LC3-I, levels of Atg5, Tom20, Beclin1, Lamp2, and reduced Sqstm1 expression in a time dependent manner. SELENOM knockdown could decrease the levels of mitophagy induced by AAS of 1 or 3 h (Si-SELENOM; see Fig. S4C, D for the mRNA and protein levels of SELENOM after siRNA treatment). The mRNA levels of mitophagy-related genes were consistent with the protein levels (Fig. S4E). Together, these data indicate that SELENOM may regulate HFD-induced NAFLD via mitophagy pathways.
SELENOM modulates mitochondrial stress via activating the Parkin-related mitophagy
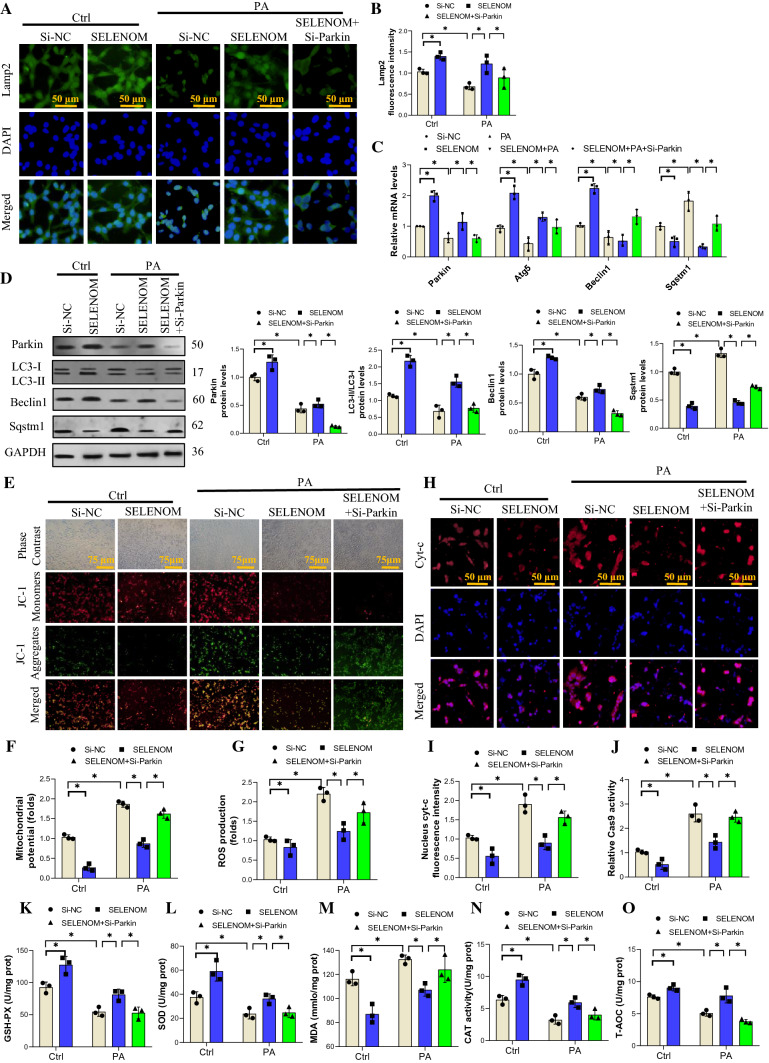
Parkin was found to be a major factor in mitophagy activation [47]. To determine whether Parkin contributes to SELENOM-modulated mitophagy, we first knockdown Parkin (Si-Parkin) in hepatocytes with siRNA and found that SELENOM level did not change (Fig. S5A). Immunofluorescence staining showed that Lamp2 was reduced by PA stress and rescued by SELENOM overexpression; Si-Parkin abolished the effect of SELENOM (Fig. 7A, B). Evaluation of other mitophagy factors showed that PA treatment decreased the mRNA and protein expressions of Parkin, LC3, and Beclin1, and increased the expression of Sqstm1; SELENOM overexpression reversed these effects, and Si-Parkin blocked the action of SELENOM (Fig. 7C, D). We have shown above that PA treatment reduced the number of autophagy vacuoles and the effect was reversed by SELENOM overexpression; Addition of Si-Parkin also counteracted the effect of SELENOM in this setting (Fig. S5B, C). These results show that SELENOM activates mitophagy via enhancement of Parkin expression in response to PA-induced stress.
Fig. 7.
SELENOM regulates in PA-inhibited mitophagy by activating Parkin. A, B The immunofluorescent intensity of Lamp2 was verified via immunofluorescence assay in groups of Si-NC, SELENOM, PA + Si-NC, SELENOM + PA and SELENOM + PA + Si-Parkin (n = 3; *P < 0.05). Si-Parkin (50 nM) was used to block Parkin expression. Fields from one representative experiment of three are shown. C, D The mRNA and protein expression levels of mitophagy-related genes such as Parkin, LC3, Beclin1 and Sqstm1 were determined in hepatocytes of Si-NC, SELENOM, PA + Si-NC, SELENOM + PA and SELENOM + PA + Si-Parkin (n = 3; *P < 0.05). E, F Mitochondrial membrane potential was detected by the JC-1 staining (n = 3; *P < 0.05). Representative images were chosen from three independent biological samples (scale bar, 75 μm). G ROS production stained by DCFH-DA (5 mM) was measured by a fluorescence microplate reader. The data represent the mean ± SEM (n = 3; *P < 0.05). H, I Immunofluorescent intensity of Cyt-c was verified via immunofluorescence assay (n = 3; *P < 0.05). Fields from one representative experiment of three are shown (scale bar, 50 μm). J The mRNA of Cas 9 was determined in hepatocytes of Si-NC, SELENOM, PA + Si-NC, SELENOM + PA and SELENOM + PA + Si-Parkin (n = 3; *P < 0.05). K–O The contents of GSH-PX, SOD, MDA, CAT, T-AOC were measured in hepatocytes of Si-NC, SELENOM, PA + Si-NC, SELENOM + PA and SELENOM + PA + Si-Parkin (n = 3 per group, *P < 0.05 versus the difference between groups). The data represent the mean ± SEM
To further clarify the function of SELENOM in mitochondrial homeostasis, mitochondrial transmembrane potential was assessed by JC-1 staining in vitro. We found that the increased membrane potential induced by PA treatment could be ameliorated by SELENOM overexpression; however, this effect was abrogated by Si-Parkin treatment (Fig. 7E, F). Similarly, we observed that PA-induced up-regulation of mitochondrial ROS was attenuated by SELENOM overexpression in a Parkin-dependent manner as Si-Parkin treatment inhibited the effect of SELENOM (Fig. 7G).
The release of cytochrome C (Cyt-c) from the mitochondrial membrane into the nucleus/cytoplasm is a central step in the mitochondrial apoptosis pathway. Immunofluorescent staining shows that PA treatment increased Cyt-c release, while SELENOM overexpression mitigated this effect; Parkin siRNA transfection again inhabited the action of SELENOM (Fig. 7H, I). Levels of the apoptotic protein Cas 9 were similarly induced by PA treatment, which was reversed by SELENOM overexpression in a Parkin-dependent manner (Fig. 7J). Moreover, the antioxidants GPX, SOD, CAT and T-AOC were all reduced by PA treatment, an effect that was inhibited by SELENOM overexpression, and Parkin deletion blocked the effect of SELENOM; for MDA the reverse is true since PA increased this factor, SELENOM overexpression reduced it, and Si-Parkin treatment abolished the effect of SELENOM (Fig. 7K–O). Altogether, our data indicate that under high-fat pressure, the protective effect of SELENOM overexpression on liver mitochondrial homeostasis and oxidative stress requires Parkin-related mitochondrial autophagy.
SELENOM regulates Parkin via the AMPKα1–MFN2 pathway
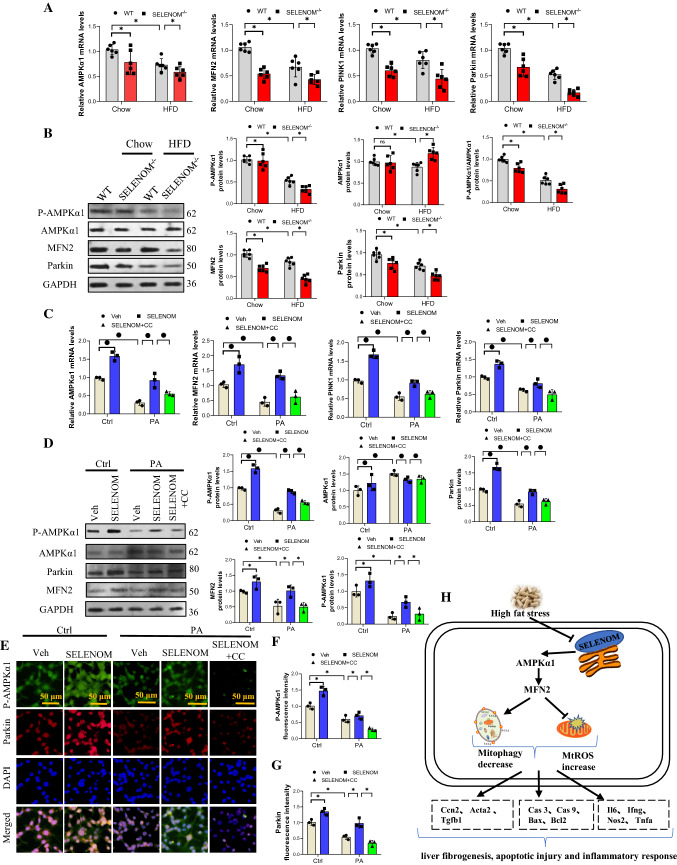
It has been reported that the AMPKα1–MFN2 signaling pathway is involved in mitophagy [27, 28]. We evaluated this pathway in the liver of HFD-fed and SELENOM−/− mice and found that the mRNA levels of AMPKα1, MFN2, PINK1 and Parkin were decreased by HFD and SELENOM deletion alone, but was further decreased in SELENOM−/− mice fed with HFD (Fig. 8A). Similarly, the protein levels of MFN2 and Parkin, and the ratio of P-AMPKα1/AMPKα1 were decreased in HFD-fed and SELENOM−/− mice, and further depleted in SELENOM−/− mice fed with HFD (Fig. 8B). These observations were reproducible in in vitro where PA stress decreased the mRNA and protein expressions of AMPKα1, MFN2, PINK1 and Parkin, and the effects were nullified by SELENOM overexpression. Using an inhibitor of AMPK, Compound C (CC, 5 μM), we further showed that the effect of SELENOM can be abolished (Fig. 8C, D). These findings were also observable with P-AMPKα1 and Parkin immunofluorescent staining (Fig. 8E–G). Together, these data suggest that SELENOM activates Parkin via the AMPKα1–MFN2 pathway in hepatocytes.
Fig. 8.
SELENOM regulates Parkin via the AMPKα1–MFN2 pathway. A, B The mRNA and protein expression levels of AMPKα1, MFN2 and Parkin were determined in HFD-treated livers with SELENOM−/− (n = 6; *P < 0.05). C, D The mRNA and protein levels of AMPKα1, MFN2 and Parkin were determined in hepatocytes of Veh, SELENOM, PA, SELENOM + PA and SELENOM + PA + CC. Compound C (CC) was an inhibitor of AMPKα1 to block the activation of AMPKα1 (n = 3; *P < 0.05). E–G The immunofluorescent intensity of P-AMPKα1 and Parkin was verified via using the immunofluorescence assay in hepatocytes of Veh, SELENOM, PA, SELENOM + PA and SELENOM + PA + CC (n = 3; *P < 0.05). Fields from one representative experiment of three are shown (scale bar, 50 μm). Values represent means ± SEM. H Schematic representation of SELENOM promoting AMPKα1–MFN2 pathway in mitophagy of liver
Discussion
SELENOM has been shown to play a pivotal role in various liver diseases [3]. In this study, we show that SELENOM was downregulated in fatty liver disease and SELENOM deletion increased the susceptibility to fatty liver disease induced by HFD in mice. SELENOM deficiency aggravates HFD-mediated hepatic oxidative stress, mitochondria apoptosis, inflammation, steatosis, and fibrosis. Additionally, SELENOM regulates mitochondrial membrane potential and mitophagy, two routes through which mitochondrial apoptosis may be controlled. Mechanistically, we showed that SELENOM regulates Parkin-mediated mitophagy via the AMPKα1–MFN2 signaling pathway; blockade of the AMPKα1–MFN2 pathway could inhibit mitophagy and abolish the mitochondrial protective effects of SELENOM. Our findings, thus, reveal that manipulating SELENOM provides a potential opportunity for treating NAFLD (Fig. 8H).
Fibrosis is part of the pathological progression in NAFLD and is characterized by increased collagen deposition and inflammation. These features, including the activation of fibrosis markers Ccn2, Tgfb and Acta2 and inflammatory cytokines Tnfa, Il6, Ifng and Nos2, were significantly increased in the absence of SELENOM. Steatosis is another pathological feature of NAFLD and reduction in FAO has been shown to increase lipid deposition and promote NAFLD development [48]. We showed that SELENOM deletion inhibits the expression of FAO-related genes (Ppara, Cpt1α, Cdh15, Acox1 and Acadm), and increases the expression of lipogenic genes (Gpam, Plin1, Scd1, Lipe, Fasn, Acly and Pparg), thus contributes to lipid accumulation and steatosis. Based on these findings, our data suggest that SELENOM inhibition may be one of the reasons that aggravate fatty liver disease, hepatic steatosis, inflammation and liver fibrosis. Further studies are needed to examine the effects of SELENOM-mediated signal transduction on the pathogenesis of NAFLD.
SELENOM and thioredoxin have antioxidant activity and activate hypothalamic leptin signaling in the hypothalamus of mice [49]. SELENOM is mainly located in the brain, and overexpression of SelenoM in rats could improve Alzheimer’s disease by inhibiting the activity of secretase in the brain [50]. Dietary selenium supplementation promotes the development of mature II oocytes in the ovaries of aging mice by increasing the expression of SELENOM [51]. SELENOM knockout mice gained weight and increased white adipose tissue levels, suggesting that SELENOM has a regulatory effect on energy homeostasis in the body [52]. Studies have shown that SELENOM can be expressed in primary murine neuronal cultures, HT-1080 (fibrosarcoma) and MCF-7 (breast adenocarcinoma), hepatocellular carcinoma, adipocytes, neutrophils and so on [53, 54]. SELENOM could prevent the increase in oxidative stress and mitochondria damage in Alzheimer’s disease [7, 55], and reduces chondrocyte apoptosis by regulating oxidative stress and mitochondrial pathways [56]. Similarly, we show that SELENOM deletion in hepatocytes could induce excessive ROS production and unbalance the antioxidant system through decreasing the activity of SOD, GPX and T-AOC, resulting in mitochondrial metabolic dysfunction. Thus, SELENOM plays a key role in maintaining mitochondrial homeostasis in the liver, SELENOM deletion could increase mitochondrial oxidative stress (mtROS) and mitochondrial potential disorder in hepatocytes. The loss of mitochondrial transmembrane potential could be related to the recruitment of the pro-mitophagy factor Parkin [57, 58]. In support of this concept, our results show that SELENOM overexpression could maintain mitochondrial transmembrane potential by activating Parkin-dependent mitophagy. SELENOM also diminishes apoptosis by protecting mitochondria function in chicken chondrocytes [7, 56]. Similarly, we show here that the loss of SELENOM was followed by activation of Cas 3/Cas 9-mediated apoptosis in hepatocytes. High-fat diet-induced liver apoptotic damage may be the root cause of lipid accumulation [59–61]. Accumulation of damaged mitochondria may be a major cause of oxidative stress damage in the liver, which can cause mitochondrial pathway apoptosis to liver cells. We also observed concomitant increase in liver apoptosis and lipid accumulation in SELENOM−/− mice. These data suggest that HFD-mediated hepatocyte injury is caused by mitochondrial dysfunction, and the downregulation of SELENOM may be a pathogenic factor in HFD-induced NAFLD.
Mitophagy defects are thought to induce NAFLD by allowing damaged mitochondria to accumulate [24]. Impaired mitophagy may contribute to the abnormal accumulation of lipid, but the underlying molecular mechanisms are unclear. Our data suggest that SELENOM is the upstream inhibitor of mitochondrial damage, as defective mitophagy was increased by HFD-stress and SELENOM deletion, and further exacerbated in SELENOM−/− mice fed with HFD. Parkin is one of the major activators of mitophagy. Lipid deposition in chronic alcoholic fatty liver is related to the PINK1/Parkin signaling pathway [25], and lipid accumulation induced by mitochondrial damage is associated with NAFLD in mice [62]. Based on these findings, we confirmed that SELENOM deletion may induce the accumulation of damaged mitochondria in NAFLD due to reduced activation of Parkin-related mitochondrial autophagy, leading to liver steatosis and inflammation. Numerous mitophagy-network genes, such as Parkin, Atg5, Tom20, LC3 and Lamp2, are detected under nutrient deprivation [63]. Similarly, we found that SELENOM deficiency suppressed nutrient starvation-induced mitophagy, while SELENOM overexpression effectively rescued the PA-induced mitophagy impairment, suggesting that SELENOM sustains Parkin-related mitophagy activation to decrease hepatocyte apoptosis. Thus, SELENOM is a key activator that mediates the hepatic network involved in Parkin-related mitophagy to maintain liver function.
The activation of AMPK/MFN2 maintains the biological function of mitochondria to regulate the process of energy metabolism [64]. Several previous studies have shown that AMPK could regulate the metabolic impact of pro-inflammatory cytokine and lipogenic genes expression through improvements in mitochondrial biogenesis [65]. Consistent with this, deletion of SELENOM promote the obesity-induced increase lipogenic genes expression in liver via affecting AMPKα1–MFN2 pathway, which also includes increased expression of pro-inflammatory genes involved in NAFLD. Several pieces of evidence indicate a close relationship between AMPKα1 function and Parkin-related mitophagy [66]. The regulation of mitophagy by energy stress is related to AMPKα1 and MFN2, and AMPKα1 regulates the autophagic ability of mouse embryonic fibroblasts via direct interaction with MFN2 [27]. In the present study, we show that the level of AMPKα1 phosphorylation was significantly suppressed in SELENOM-deficient livers in response to HFD treatment along with impaired mitophagy induction. Mechanistically, PA treatment led to the decrease in AMPKα1 phosphorylation and reduced mitophagy, which was abolished SELENOM overexpression. Importantly, AMPKα1 inhibition by Compound C ameliorated the effect of SELENOM, suggesting that SELENOM mediates mitophagy in an AMPKα1-dependent manner. Our study demonstrated that SELENOM could promote Parkin-related mitophagy via interacting with the AMPKα1–MFN2 signal pathway to impede NAFLD development. In addition, the multiple roles of signaling pathways mediated by the SELENOM–AMPKα1–MFN2 axis in the pathogenesis of liver diseases need to be further studied.
Conclusions
In conclusion, our findings show that energy stress-induced downregulation of SELENOM in hepatocytes could aggravate HFD-mediated liver damage. SELENOM deficiency increased oxidative stress, mitochondrial apoptosis, inflammation, steatosis, and fibrosis in HFD-treated livers. SELENOM enhanced Parkin-mediated mitophagy induced by energy stress via activation of the AMPKα1–MFN2 pathway to prevent the pathogenesis of NAFLD. Our findings highlight SELENOM as a molecular target of NAFLD pathogenesis and reveal the importance of SELENOM and Parkin-mediated mitophagy in maintaining mitochondrial homeostasis in hepatocytes.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
JC designed the study and JH wrote the manuscript. JY is the guarantor of this work. XC, HZ and YZ researched data and helped design experiments. QL and ZZ takes responsibility for the integrity of the data and helped design experiments. All authors have read the manuscript and agreed to submit it in its current form for consideration for publication in the Journal.
Funding
This study was supported by the National Natural Science Foundation of China (31872531).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Animal experiments in our study were subject to ethical review by the Animal Care and Use Committee of Northeast Agricultural University (SRM-11), Harbin, China. The investigation conforms to directive 2010/63/EU of the European parliament. All authors agreed to participate.
Consent for publication
All authors read the manuscript and agreed to submission.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Polyzos SA, Kountouras J, Goulas A, Duntas L. Selenium and selenoprotein P in nonalcoholic fatty liver disease. Hormones (Athens) 2020;19:61–72. doi: 10.1007/s42000-019-00127-3. [DOI] [PubMed] [Google Scholar]
- 2.Qiao L, Men L, Yu S, et al. Hepatic deficiency of selenoprotein S exacerbates hepatic steatosis and insulin resistance. Cell Death Dis. 2022;13:275. doi: 10.1038/s41419-022-04716-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerriero E, Accardo M, Capone F, Colonna G, Castello G, Costantini S. Assessment of the Selenoprotein M (SELM) over-expression on human hepatocellular carcinoma tissues by immunohistochemistry. Eur J Histochem. 2014;58:2433. doi: 10.4081/ejh.2014.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou JC, Zhao H, Tang JY, Li JG, Liu XL, Zhu YM. Molecular cloning, chromosomal localization and expression profiling of porcine selenoprotein M gene. Genes Genomics. 2011;33:529. doi: 10.1007/s13258-010-0127-1. [DOI] [Google Scholar]
- 5.Gong T, Hashimoto AC, Sasuclark AR, Khadka VS, Gurary A, Pitts MW. Selenoprotein M Promotes Hypothalamic Leptin Signaling and Thioredoxin Antioxidant Activity. Antioxid Redox Signal. 2019 doi: 10.1089/ars.2018.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson AD, Labunskyy VM, Fomenko DE, et al. NMR structures of the selenoproteins Sep15 and SelM reveal redox activity of a new thioredoxin-like family. J Biol Chem. 2006;281:3536–3543. doi: 10.1074/jbc.M511386200. [DOI] [PubMed] [Google Scholar]
- 7.Chen P, Wang RR, Ma XJ, Liu Q, Ni JZ. Different forms of Selenoprotein M differentially affect Aβ aggregation and ROS generation. Int J Mol Sci. 2013;14:4385–4399. doi: 10.3390/ijms14034385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reeves MA, Bellinger FP, Berry MJ. The neuroprotective functions of selenoprotein M and its role in cytosolic calcium regulation. Antioxid Redox Signal. 2010;12:809–818. doi: 10.1089/ars.2009.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Addinsall AB, Wright CR, Andrikopoulos S, van der Poel C, Stupka N. Emerging roles of endoplasmic reticulum-resident selenoproteins in the regulation of cellular stress responses and the implications for metabolic disease. Biochem J. 2018;475:1037–1057. doi: 10.1042/bcj20170920. [DOI] [PubMed] [Google Scholar]
- 10.Wang P, Lu Z, He M, Shi B, Lei X, Shan A. The effects of endoplasmic-reticulum-resident Selenoproteins in a nonalcoholic fatty liver disease pig model induced by a high-fat diet. Nutrients. 2020 doi: 10.3390/nu12030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma YM, Guo YZ, Ibeanu G, et al. Overexpression of selenoprotein H prevents mitochondrial dynamic imbalance induced by glutamate exposure. Int J Biol Sci. 2017;13:1458–1469. doi: 10.7150/ijbs.21300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Day K, Seale LA, Graham RM, Cardoso BR. Selenotranscriptome network in non-alcoholic fatty liver disease. Front Nutr. 2021;8:744825. doi: 10.3389/fnut.2021.744825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitts MW, Reeves MA, Hashimoto AC, et al. Deletion of selenoprotein M leads to obesity without cognitive deficits. J Biol Chem. 2013;288:26121–26134. doi: 10.1074/jbc.M113.471235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musso G, Cassader M, Paschetta E, Gambino R. Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic Steatohepatitis. Gastroenterology. 2018;155:282–302. doi: 10.1053/j.gastro.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz M, Soto-Alarcón SA, Orellana P, et al. Suppression of high-fat diet-induced obesity-associated liver mitochondrial dysfunction by docosahexaenoic acid and hydroxytyrosol co-administration. Dig Liver Dis. 2020;52:895–904. doi: 10.1016/j.dld.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Nageeb MM, Khatab MI, Abdel-Sameea AA, Teleb NA. Adelmidrol protects against non-alcoholic steatohepatitis in mice. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:777–784. doi: 10.1007/s00210-019-01785-1. [DOI] [PubMed] [Google Scholar]
- 17.Aqel B, DiBaise JK. Role of the Gut microbiome in nonalcoholic fatty liver disease. Nutr Clin Pract. 2015;30:780–786. doi: 10.1177/0884533615605811. [DOI] [PubMed] [Google Scholar]
- 18.Simões ICM, Fontes A, Pinton P, Zischka H, Wieckowski MR. Mitochondria in non-alcoholic fatty liver disease. Int J Biochem Cell Biol. 2018;95:93–99. doi: 10.1016/j.biocel.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Zhao H, Wang Y, Liu Y, et al. ROS-induced hepatotoxicity under Cypermethrin: involvement of the crosstalk between Nrf2/Keap1 and NF-κB/iκB-α pathways regulated by proteasome. Environ Sci Technol. 2021;55:6171–6183. doi: 10.1021/acs.est.1c00515. [DOI] [PubMed] [Google Scholar]
- 20.Palikaras K, Lionaki E, Tavernarakis N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature. 2015;521:525–528. doi: 10.1038/nature14300. [DOI] [PubMed] [Google Scholar]
- 21.Qiu YN, Wang GH, Zhou F, et al. PM2.5 induces liver fibrosis via triggering ROS-mediated mitophagy. Ecotoxicol Environ Saf. 2019;167:178–187. doi: 10.1016/j.ecoenv.2018.08.050. [DOI] [PubMed] [Google Scholar]
- 22.Fan RF, Tang KK, Wang ZY, Wang L. Persistent activation of Nrf2 promotes a vicious cycle of oxidative stress and autophagy inhibition in cadmium-induced kidney injury. Toxicology. 2021;464:152999. doi: 10.1016/j.tox.2021.152999. [DOI] [PubMed] [Google Scholar]
- 23.Wu M, Chen P, Liu F, et al. ONX0912, a selective oral proteasome inhibitor, triggering mitochondrial apoptosis and mitophagy in liver cancer. Biochem Biophys Res Commun. 2021;547:102–110. doi: 10.1016/j.bbrc.2021.02.037. [DOI] [PubMed] [Google Scholar]
- 24.Yamada T, Murata D, Adachi Y, et al. Mitochondrial stasis reveals p62-mediated ubiquitination in Parkin-independent Mitophagy and Mitigates Nonalcoholic fatty liver disease. Cell Metab. 2018;28:588–604.e585. doi: 10.1016/j.cmet.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Ni HM, Chao X, et al. Double deletion of PINK1 and Parkin impairs hepatic mitophagy and exacerbates acetaminophen-induced liver injury in mice. Redox Biol. 2019;22:101148. doi: 10.1016/j.redox.2019.101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He L, Zhou Q, Huang Z, et al. PINK1/Parkin-mediated mitophagy promotes apelin-13-induced vascular smooth muscle cell proliferation by AMPKα and exacerbates atherosclerotic lesions. J Cell Physiol. 2019;234:8668–8682. doi: 10.1016/j.ceb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Hu Y, Chen H, Zhang L, et al. The AMPK-MFN2 axis regulates MAM dynamics and autophagy induced by energy stresses. Autophagy. 2021;17:1142–1156. doi: 10.1080/15548627.2020.1749490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seabright AP, Fine NHF, Barlow JP, et al. AMPK activation induces mitophagy and promotes mitochondrial fission while activating TBK1 in a PINK1-Parkin independent manner. Faseb J. 2020;34:6284–6301. doi: 10.1096/fj.201903051R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang R, Chu K, Zhao N, et al. Corilagin alleviates nonalcoholic fatty liver disease in high-fat diet-induced C57BL/6 mice by ameliorating oxidative stress and restoring autophagic flux. Front Pharmacol. 2019;10:1693. doi: 10.3389/fphar.2019.01693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Verma AK, Rani R, et al. Hepatic deficiency of augmenter of liver regeneration predisposes to nonalcoholic Steatohepatitis and fibrosis. Hepatology. 2020;72:1586–1604. doi: 10.1002/hep.31167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan J, Zhang Y, Yang D, et al. Gastrodin improves nonalcoholic fatty liver disease via activation of the AMPK signaling pathway. Hepatology. 2021 doi: 10.1002/hep.32068. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Xu Y, Chen B, Zhao B, Gao XJ. Selenium deficiency promotes oxidative stress-induced mastitis via activating the NF-κB and MAPK pathways in dairy cow. Biol Trace Elem Res. 2021 doi: 10.1007/s12011-021-02882-0. [DOI] [PubMed] [Google Scholar]
- 33.Song N, Wang W, Wang Y, Guan Y, Xu S, Guo MY. Hydrogen sulfide of air induces macrophage extracellular traps to aggravate inflammatory injury via the regulation of miR-15b-5p on MAPK and insulin signals in trachea of chickens. Sci Total Environ. 2021;771:145407. doi: 10.1016/j.scitotenv.2021.145407. [DOI] [PubMed] [Google Scholar]
- 34.Momcilovic M, Shirihai O, Murphy MP, Koehler CM, Sadeghi S, Shackelford DB. Reply to: in vivo quantification of mitochondrial membrane potential. Nature. 2020;583:E19–e20. doi: 10.1038/s41586-020-2367-9. [DOI] [PubMed] [Google Scholar]
- 35.Miao Z, Miao Z, Shi X, Wu H, Yao Y, Xu S. The antagonistic effect of selenium on lead-induced apoptosis and necroptosis via P38/JNK/ERK pathway in chicken kidney. Ecotoxicol Environ Saf. 2022;231:113176. doi: 10.1016/j.ecoenv.2022.113176. [DOI] [PubMed] [Google Scholar]
- 36.Novoselov SV, Kryukov GV, Xu XM, Carlson BA, Hatfield DL, Gladyshev VN. Selenoprotein H is a nucleolar thioredoxin-like protein with a unique expression pattern. J Biol Chem. 2007;282:11960–11968. doi: 10.1074/jbc.M701605200. [DOI] [PubMed] [Google Scholar]
- 37.Zheng Y, Guan H, Yang J, Cai J, Liu Q, Zhang Z. Calcium overload and reactive oxygen species accumulation induced by selenium deficiency promote autophagy in swine small intestine. Anim Nutr. 2021;7:997–1008. doi: 10.21203/rs.3.rs-118703/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chi Q, Hu X, Liu Z, Han Y, Li S. H2S exposure induces cell death in the broiler thymus via the ROS-initiated JNK/MST1/FOXO1 pathway. Ecotoxicol Environ Saf. 2021;222:112488. doi: 10.1016/j.ecoenv.2021.112488. [DOI] [PubMed] [Google Scholar]
- 39.Liu XJ, Wang YQ, Shang SQ, Xu S, Guo M. TMT induces apoptosis and necroptosis in mouse kidneys through oxidative stress-induced activation of the NLRP3 inflammasome. Ecotoxicol Environ Saf. 2022;230:113167. doi: 10.1016/j.ecoenv.2022.113167. [DOI] [PubMed] [Google Scholar]
- 40.Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15:349–364. doi: 10.1038/s41575-018-0009-6. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Bi M, Yang J, et al. Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine. J Hazard Mater. 2022;421:126704. doi: 10.1016/j.jhazmat.2021.126704. [DOI] [PubMed] [Google Scholar]
- 42.Sangineto M, Bukke VN, Bellanti F, et al. A novel nutraceuticals mixture improves liver Steatosis by preventing oxidative stress and mitochondrial dysfunction in a NAFLD model. Nutrients. 2021 doi: 10.3390/nu13020652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang HR, Zhao FQ, Gai XX, et al. Astilbin attenuates apoptosis induced by cadmium through oxidative stress in carp (Cyprinus carpio L.) head kidney lymphocyte. Fish Shellfish Immunol. 2022;13:S1050–4648. doi: 10.1016/j.fsi.2022.05.021. [DOI] [PubMed] [Google Scholar]
- 44.Paech F, Abegg VF, Duthaler U, Terracciano L, Bouitbir J, Krähenbühl S. Sunitinib induces hepatocyte mitochondrial damage and apoptosis in mice. Toxicology. 2018;409:13–23. doi: 10.1016/j.tox.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 45.Kruppa AJ, Buss F. Actin cages isolate damaged mitochondria during mitophagy. Autophagy. 2018;14:1644–1645. doi: 10.1080/15548627.2018.1486152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eiyama A, Okamoto K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol. 2015;33:95–101. doi: 10.1016/j.ceb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Park HS, Song JW, Park JH, et al. TXNIP/VDUP1 attenuates steatohepatitis via autophagy and fatty acid oxidation. Autophagy. 2020 doi: 10.1080/15548627.2020.1834711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong T, Hashimoto AC, Sasuclark AR, Khadka VS, Gurary A, Pitts MW. Selenoprotein M promotes hypothalamic Leptin signaling and Thioredoxin antioxidant activity. Antioxid Redox Signal. 2021;35:775–787. doi: 10.1089/ars.2018.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang ZH, Song GL. Roles of Selenoproteins in brain function and the potential mechanism of selenium in Alzheimer's disease. Front Neurosci. 2021;15:646518. doi: 10.3389/fnins.2021.646518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qazi IH, Yang H, Wei S, et al. Dietary selenium deficiency and supplementation differentially modulate the expression of two ER-resident selenoproteins (selenoprotein K and selenoprotein M) in the ovaries of aged mice: preliminary data. Reprod Biol. 2020;20:441–446. doi: 10.1016/j.repbio.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Gong T, Berry MJ, Pitts MW. Selenoprotein M: structure, expression and functional relevance. Springer International Publishing; 2016. [Google Scholar]
- 53.Varlamova EG, et al. Protein partners of Selenoprotein SELM and the role of selenium compounds in regulation of its expression in human cancer cells. Dokl Biochem Biophys. 2019;488:300–303. doi: 10.1134/S1607672919050065. [DOI] [PubMed] [Google Scholar]
- 54.Liu Q, Du P, Zhu Y, Zhang X, Cai J, Zhang Z. Thioredoxin reductase 3 suppression promotes colitis and carcinogenesis via activating pyroptosis and necrosis. Cell Mol Life Sci. 2022;79:106. doi: 10.1007/s00018-022-04155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, Zhang W, Zhou P, Tong X, Guo D, Lin H. Selenium deficiency induced apoptosis via mitochondrial pathway caused by Oxidative Stress in porcine gastric tissues. Res Vet Sci. 2022;144:142–148. doi: 10.1134/S1607672919050065. [DOI] [PubMed] [Google Scholar]
- 56.Chi Q, Luan Y, Zhang Y, Hu X, Li S. The regulatory effects of miR-138-5p on selenium deficiency-induced chondrocyte apoptosis are mediated by targeting SelM. Metallomics. 2019;11:845–857. doi: 10.1039/c9mt00006b. [DOI] [PubMed] [Google Scholar]
- 57.Gilkerson RW, De Vries RL, Lebot P, et al. Mitochondrial autophagy in cells with mtDNA mutations results from synergistic loss of transmembrane potential and mTORC1 inhibition. Hum Mol Genet. 2012;21:978–990. doi: 10.1093/hmg/ddr529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miao Z, Miao Z, Wang S, Wu H, Xu S. Exposure to imidacloprid induce oxidative stress, mitochondrial dysfunction, inflammation, apoptosis and mitophagy via NF-kappaB/JNK pathway in grass carp hepatocytes. Fish Shellfish Immunol. 2022;120:674–685. doi: 10.1016/j.fsi.2021.12.017. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka M, Sato A, Kishimoto Y, Mabashi-Asazuma H, Kondo K, Iida K. Gallic acid inhibits lipid accumulation via AMPK pathway and suppresses apoptosis and macrophage-mediated inflammation in hepatocytes. Nutrients. 2020 doi: 10.3390/nu12051479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka S, Hikita H, Tatsumi T, et al. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology. 2016;64:1994–2014. doi: 10.1002/hep.28820. [DOI] [PubMed] [Google Scholar]
- 61.Reinartz A, Ehling J, Leue A, et al. Lipid-induced up-regulation of human acyl-CoA synthetase 5 promotes hepatocellular apoptosis. Biochim Biophys Acta. 2010;1801:1025–1035. doi: 10.1016/j.bbalip.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 62.Wen F, Shi Z, Liu X, et al. Acute elevated resistin exacerbates mitochondrial damage and aggravates liver Steatosis through AMPK/PGC-1α signaling pathway in male NAFLD mice. Horm Metab Res. 2021;53:132–144. doi: 10.1055/a-1293-8250. [DOI] [PubMed] [Google Scholar]
- 63.Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dong H, Zhou W, Xin J, et al. Salvinorin A moderates postischemic brain injury by preserving endothelial mitochondrial function via AMPK/Mfn2 activation. Exp Neurol. 2019;322:113045. doi: 10.1016/j.expneurol.2019.113045. [DOI] [PubMed] [Google Scholar]
- 65.Lyons CL, Roche HM. Nutritional Modulation of AMPK-Impact upon Metabolic-Inflammation. Int J Mol Sci. 2018 doi: 10.3390/ijms19103092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.