Abstract
Background and aims
Sec62 is a membrane protein of the endoplasmic reticulum that facilitates protein transport. Its role in cancer is increasingly recognised, but remains largely unknown. We investigated the functional role of Sec62 in gastric cancer (GC) and its underlying mechanism.
Methods
Bioinformatics, tissue microarray, immunohistochemistry (IHC), western blotting (WB), quantitative polymerase chain reaction (qPCR), and immunofluorescence were used to examine the expression of target genes. Transwell, scratch healing assays, and xenograft models were used to evaluate cell migration and invasion. Transmission electron microscopy and mRFP-GFP-LC3 double-labeled adenoviruses were used to monitor autophagy. Co-immunoprecipitation (CO-IP) was performed to evaluate the binding activity between the proteins.
Results
Sec62 expression was upregulated in GC, and Sec62 upregulation was an independent predictor of poor prognosis. Sec62 overexpression promoted GC cell migration and invasion both in vitro and in vivo. Sec62 promoted migration and invasion by affecting TIMP-1 and MMP2/9 balance. Moreover, Sec62 could activate autophagy by upregulating PERK/ATF4 expression and binding to LC3II with concomitant FIP200/Beclin-1/Atg5 activation. Furthermore, autophagy blockage impaired the promotive effects of Sec62 on GC cell migration and invasion, whereas autophagy activation rescued the inhibitory effect of Sec62 knockdown on GC metastasis. Notably, Sec62 inhibition combined with autophagy blockage exerted a synergetic anti-metastatic effect in vitro and in vivo.
Conclusion
Sec62 promotes GC metastasis by activating autophagy and subsequently regulating TIMP-1 and MMP2/9 balance. The activation of autophagy by Sec62 may involve the unfolded protein response (UPR)-related PERK/ATF4 pathway and binding of LC3II during UPR recovery involving FIP200/Beclin-1/Atg5 upregulation. Specifically, the dual inhibition of Sec62 and autophagy may provide a promising therapeutic strategy for GC metastasis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04143-2.
Keywords: EMT, ER-phagy, ER stress, Protein translocation machinery, Unfolded protein response (UPR)
Introduction
Gastric cancer (GC) is the fourth leading cause of cancer-related mortality worldwide [1]. Owing to the rapid advances in early diagnosis and surgical approaches, the prognosis of patients with early GC has greatly improved. However, a considerable number of GC patients are usually diagnosed at an advanced or metastatic stage, heralding a poor 5-year survival rate of less than 30% [2]. A high incidence of metastasis in GC is a major cause of cancer-related death. Unfortunately, the mechanism underlying GC metastasis remains largely unknown.
Sec62 is an endoplasmic reticulum (ER) transmembrane protein and a component of the protein translocation machinery. Functionally, Sec62 facilitates the transport of proteins into the ER, regulates cellular Ca2+ homeostasis and mediates ER stress-induced unfolded protein response (UPR) recovery by eliminating excess ER, also called ER-phagy or recov-ER-phagy [3–6]. ER stress is a cellular state in which the homeostasis of protein biogenesis, folding and transport at the ER is disturbed by intracellular and extracellular perturbations [7]. To cope with the impact of ER stress, ER-resident sensors such as Bip, IRE1, ATF6 and PERK, consequently trigger the UPR, which is characterised by ER membrane expansion and accumulation of misfolded proteins [8, 9]. To compensate for ER stress and downsize expanded ER, Sec62 can facilitate the elimination of excess ER and inner accumulated misfolded proteins via autophagy [4, 5, 10].
Because tumor growth is under constant stresses such as high metabolic demand, nutrient limitation, hypoxia and oxidative stress, robust ER stress and UPR activation have been recognised in a variety of human cancers. Particularly, UPR activation is reported to be correlated with metastasis [9]. In addition, several studies have demonstrated that Sec62 amplification and overexpression are frequently found in various cancer types, especially in GC. Moreover, Sec62 overexpression was found to be associated with enhanced cell migration and invasion in prostate, lung, and cervical cancer, but the mechanism remains unknown [3, 11–15]. In this study, we investigated the functional role of Sec62 in GC metastasis and its underlying mechanism.
Materials and methods
Patient samples and ethics statement
This study was performed with approval from the Ethics Committee at Xijing Hospital, the first Affiliated Hospital of Air Force Medical University, and complied with the Helsinki Declaration. A tissue microarray with GC and matched adjacent non-tumour tissue samples from 90 patients between January 2010 and November 2016 were purchased from Outdo Biotech Co., Ltd. (Shanghai, China). The clinicopathological characteristics of the included patients are shown in Table 1. Additional histopathologically diagnosed GC samples and matched adjacent non-tumour tissues from the State Key Laboratory of Cancer Biology and National Clinical Research Center for Digestive Diseases, Xijing Hospital of Digestive Diseases, were also obtained. GC diagnosis was based on the NCCN clinical practice guideline [16]. All patients provided informed consent for the use of clinical specimens for medical research.
Table 1.
Correlation of Sec62 expression with clinicopathological features in GC
| Variables | Number | Sec62, n (%) | χ2 | P value | |
|---|---|---|---|---|---|
| Low | High | ||||
| Gender | 0.761 | 0.258 | |||
| Male | 53 | 33(62.3) | 20(37.7) | ||
| Female | 36 | 18(50.0) | 18(50.0) | ||
| Age(years) | 2.069 | 0.152 | |||
| < 50 | 18 | 9(50) | 9(50) | ||
| ≥ 50 | 71 | 42(59.2) | 29(40.8) | ||
| Smoke | 0.157 | 0.631 | |||
| Yes | 64 | 36(56.3) | 28(43.7) | ||
| No | 25 | 15(60.0) | 10(40.0) | ||
| Alcoholism | 2.853 | 0.080 | |||
| Yes | 20 | 8(40.0) | 12(60.0) | ||
| No | 69 | 43(62.3) | 26(37.7) | ||
| Metastasis | 3.170 | 0.036 | |||
| Yes | 26 | 10(38.5) | 16(61.5) | ||
| No | 63 | 41(65.1) | 22(34.9) | ||
| Tumor size | 5.166 | 0.028 | |||
| ≤ 5 | 28 | 11(39.3) | 17(60.7) | ||
| > 5 | 61 | 40(65.6) | 21(34.4) | ||
| Microvascular invasion | 3.029 | 0.040 | |||
| Yes | 29 | 11(37.9) | 18(62.1) | ||
| No | 60 | 40(66.7) | 20(33.3) | ||
| TNM stage | 11.370 | 0.005 | |||
| I | 52 | 37(71.2) | 15(28.8) | ||
| II | 24 | 11(45.8) | 13(54.2) | ||
| III | 13 | 3(23.1) | 10(76.9) | ||
GC gastric cancer, TNM tumor, node, metastasis
Bioinformatics analysis
The GEPIA (http://gepia2.cancer-pku.cn/) database was used to investigate the expression of Sec62 in GC and non-tumor stomach tissues. The relationship between Sec62 expression and GC prognosis was analysed using the Kaplan–Meier Plotter (https://kmplot.com/analysis/index.php?p=service).
Immunohistochemistry (IHC)
IHC was performed as previously described [17] to examine the expression of Sec62 in GC and adjacent non-tumor tissue samples. Quantitative scoring of IHC staining were performed by two independent pathologists. IHC staining intensity was defined as 0 = negative, 1 = weak (light yellow), 2 = medium (yellow), or 3 = strong (brown) and the percentage of positively stained cells in fields of interest was defined as 0% positive tumor cells = 0, 1–25% positive tumor cells = 1, 26–50% positive tumor cells = 2, > 50% positive tumor cells = 3. Final staining scores were determined by multiplying the intensity and percentage scores with a range from 0 to 9. High Sec62 expression was defined as the final score ≥ 5, and low expression was final score < 4.
Cell culture
Human GC cell lines (MKN45, AGS, BGC823, HCG27, SGC7901, SGC7901-M, SGC7901-NM and MKN28) and the immortalised gastric epithelial cell line GES were preserved at our institute. All the cell lines were cultured in DMEM medium (Gibco) supplemented with 10% fetal bovine serum (Gibco) and 100U/ml penicillin–streptomycin (Hyclone) at 37 °C in humidified atmosphere with 5% (vol/vol) CO2. Sec62 protein and RNA were extracted from the cell lines above and measured with Western Blot (WB) and quantitative real-time PCR (qRT-PCT) assays, respectively.
ELISA
Cell culture supernatants were tested using ELISA kits (MULTISCIENCES (LIANKE) BIOTECH, CO., LTD, China). In brief, the standard curves and calculation formulas were obtained using absorbance at 450 nm following the manufacturer’s instructions. The resulting formula was used to calculate the concentrations of target proteins, including TIMP1, 2, 4, and MMP2, 9 in the supernatants by using the absorbances of the samples.
Western blotting (WB)
Protein extraction and WB were performed as previously described [17]. Briefly, total proteins of cultured cells were extracted with lysis buffer (RIPA, Millipore) with 1:500 protease inhibitor (Millipore) and 1:200 phosphatase inhibitor (Millipore) on ice for 20 min. Extracted proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. Then 5% milk was used to block the membrane for one hour. Thereafter, the membranes were incubated with primary antibodies at 4 °C overnight and on the next day incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (goat anti-mouse or anti-rabbit IgG) (Zhong Shan Golden-bridge Biotech, China). At last, target proteins were visualised using Bio-Rad Imaging Systems (Bio-Rad). The primary and secondary antibodies used in the experiment were listed in Supplementary Table 1.
Quantitative polymerase chain reaction (qPCR)
The qPCR assay was performed as previously reported [17]. A TaKaRa MiniBEST Universal Extraction Kit (TaKaRa) was used to extract total RNAs from the above cell lines, frozen GC tissues and the adjacent normal tissues according to manufacturer’s protocols. PrimeScriptTM RT Master Mix (Perfect Real Time) (TaKaRa) was used to synthesise complementary DNA (cDNA). Relative quantitation of target gene expression were measured by the 2−△△Ct method and GAPDH was deemed as a reference. The primer sequences used are as follows (5′ to 3′): GAPDH, GTCTCCTCTGACTTCAACAGCG (forward) and ACCACCCTGTTGCTGTAGCCAA (reverse); Sec62, TCAGTGTGGGTGCAGGCTGTTT (forward) and ACCAAAAGTGGTGCCTTCCTCC (reverse).
Lentivirus construction and transfection of GC cell lines
All recombinant lentiviruses carrying human Sec62 knockdown and overexpression sequences were purchased from Genechem Co., Ltd. (Shanghai, China). Lentivirus transfection was conducted according to the manufacturer’s protocol. Lentiviral vectors carrying human Sec62 overexpression sequences were transfected into MKN45 cells and designated as MKN45 OE. Lentiviral vectors carrying Sec62 knockdown sequences were transfected into AGS cells and designated as Sec62 KD. Two knockdown sequences were used to rule out potential off-target effects. The sequences are listed as follows: control (NC): CGCTTCCGCGGCCCGTTCAA; Sec62-sh1: CTGTGGTTGACTACTGCAAC; Sec62-sh2: ACAGTTGAATCGAAGATACT. In addition, the gastric epithelial cell line (GES) was transfected with Sec62 overexpression sequences as a validation group and designated as GES OE. Empty vectors were used as negative controls and designated as NC. After 24 h of transfection, all transfected cells were selected for 7 days with 3–5 μg/mL puromycin (Solarbio). Finally, the transfection efficiencies were verified by examining Sec62 protein expression using western blotting (WB).
Migration and invasion assays
Cell migration and invasion were assessed using Corning 6.5 mm Transwell chambers with a pore size of 8 um and Corning BioCoat Matrigel Invasion Chambers. Detailed procedures were performed according to the manufacturer’s instructions and were similar to those previously described [18]. Briefly, cells were seeded onto the upper chamber membrane in serum-free Dulbecco's Modified Eagle Medium (DMEM). Complete DMEM containing 20% foetal bovine serum (Gibco) was added to the lower chamber as a chemoattractant. After 30 h, cells that had migrated or invaded through the membrane were fixed with 4% paraformaldehyde (Solarbio) and stained with 0.1% crystal violet (Shanghai Biyuntian Bio-Technology Co., Ltd., China). Finally, more than 10 random views from each chamber were analysed under light microscopy (× 40 magnification), and the numbers of migrated and invaded cells were counted using ImageJ software (National Institutes of Health, USA).
Scratch healing assay
Cell migration was measured using a scratch healing assay. Briefly, cultured cells with at least 90% confluence in 6-well plates were scratched with a 200 ul sterile pipette tip and then cultured for 24 h in serum-free DMEM. The wound closure was photographed under a light microscope (Olympus, Japan) at 0 h and 24 h. Wound closure (%) was calculated using the following formula: (1-(empty area at 24 h/empty area at 0 h)) × 100 using ImageJ software (National Institutes of Health, USA).
Immunofluorescence
Immunofluorescence was performed to measure the expression of epithelial–mesenchymal transition (EMT)-related markers and immunofluorescence co-localisation of Sec62 and LC3, as previously described [19]. Briefly, cultured cells were washed with PBS and fixed with 4% paraformaldehyde for 10 min. After washing twice with PBS, the cells were treated with foetal bovine serum (Gibco) as a blocking solution for 60 min at room temperature. Next, the cells were incubated with the following primary antibodies: anti-E-cadherin (Cell Signalling Technology, CST), anti-vimentin (Abcam), LC3 (CST) or FLAG (Sigma) at 4 °C overnight and then incubated with the corresponding secondary antibodies. Nuclei were counterstained with DAPI (Thermo Fischer Scientific). Immunofluorescence images were captured using a fluorescence microscope (Olympus, Tokyo, Japan). Co-localisation of Sec62 and LC3 was quantified by Image-Pro Plus version 6.0 using Manders Overlap Coefficient (MOC) with background corrected.
MMP2 and 9 zymography assay
Gelatin zymography was used to measure MMP-2 and 9 protease activity in the supernatant medium as previously reported [20]. Briefly, 7.5% SDS–PAGE containing 0.1% gelatin (Applygen Technologies Inc, Beijing, China.) were used to separate the proteins with electrophoresis at 25 mA. After the electrophoresis, renaturing and incubating the gels were conducted according to the manufacturer’s instructions, and the gels were then stained with 0.4% Coomassie blue. Finally, after de-staining, the proteolytic bands visualised as clear bands were captured and analysed.
Transmission electron microscopy
Transmission electron microscopy was used to assess autophagosomes/autolysosomes, as previously described [21]. Briefly, cultured cells were collected and fixed with 4% glutaraldehyde overnight at 4 °C (Solarbio), washed with phosphate buffered saline (PBS), and fixed with 1% OsO4 buffer for 1.5 h at 4 °C. After washing with PBS, the cells were dehydrated in a graded series of ethanol solutions and embedded in Epon812 epoxy resin. Ultra-thin (80 nm) sections were collected on copper grids, double-stained with 1% uranyl acetate and 0.2% lead citrate, and observed using an HT7800 transmission electron microscope (Hitachi, Japan).
mRFP–GFP–LC3 double-labelled adenovirus transfection and confocal laser scanning microscopy
To detect the autophagic flux of GC cell lines, mRFP–GFP–LC3 double-labeled adenovirus was purchased from Hanbio Biotechnology Co., Ltd. (Shanghai, China) and transfected into previously constructed MKN45, AGS, and GES cells. Detailed procedures for mRFP–GFP–LC3 transfection were performed according to the operation manual provided by Hanbio and similar to that previously described [22]. At 24 h after mRFP–GFP–LC3 transfection, the cells were fixed using 4% paraformaldehyde for 10 min. DAPI (Thermo Fischer Scientific) was used to counterstain the nuclei for 20 min. All procedures were performed at room temperature and protected from light. Finally, the treated cells on the slides were imaged using an FV3000 confocal laser scanning microscope (Olympus, Japan).
Animal experiments
All animal experiments were performed in accordance with the guidelines approved by the Animal Care and Use Committee of the Air Force Medical University. Twenty male BALB/c nude mice (six-week-old) were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and used for metastasis observations. The mice were randomly divided into two groups (n = 10/group): Sec62 OE and Sec62 NC. MKN45 cells at a concentration of 2 × 106 in 100 ul of PBS per animal were injected through the tail vein. The mice were anaesthetised and sacrificed 8 weeks after the injection. Histological evaluation of the lungs and livers was performed using haematoxylin and eosin (H&E) staining. Hydroxychloroquine (HCQ) (50 mg/kg) intraperitoneal injection was performed as a rescue experiment to block autophagy in vivo.
Co-Immunoprecipitation (Co-IP)
Co-IP was performed using a Pierce® Co-Immunoprecipitation kit (Thermo Scientific, USA) according to the manufacturer’s protocol.
Statistical analysis
All results are expressed as the mean ± SEM. GraphPad Prism software (La Jolla, USA) and SPSS Statistics 21 (IBM, USA) were used to perform the statistical analyses. The Cox proportional hazard model was used for univariable and multivariable risk factor analyses for survival. The Kaplan–Meier method and log-rank test were used to plot and compare the survival curves. Student’s t test or Mann–Whitney U test was used for comparison between two groups. Multiple group comparisons were performed using one-way analysis of variance (ANOVA) or the Kruskal–Wallis test. Categorical data were compared using the chi-square test or Fisher’s exact test. Statistical significance was set at p < 0.05.
Results
Sec62 overexpression in GC was verified and predicted poor survival
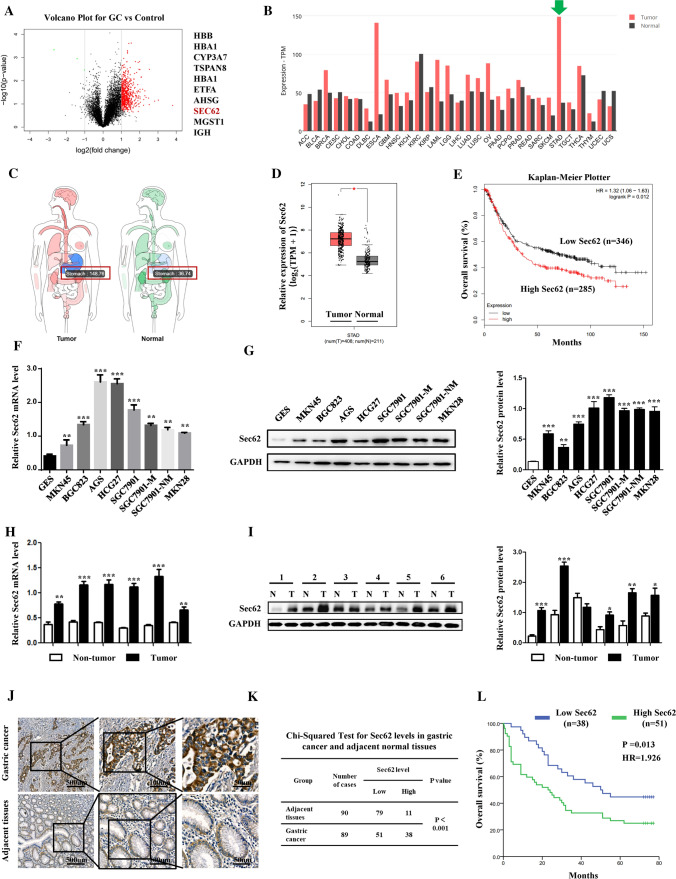
In our latest study [23], mass spectrometry analysis using GC tissues versus controls identified Sec62 as one of the top 10 upregulated proteins (unpublished data) (Fig. 1A). Bioinformatics analysis showed the mRNA expression of Sec62 in GC was significantly higher than that in most other human malignancies (Fig. 1B, green arrow). When compared to adjacent normal tissues, Sec62 expression was also significantly upregulated in GC (Fig. 1C, D). Further, the online database KM-plotter was employed to investigate the correlation of Sec62 with GC patient survival. The data suggested that GC patients with high Sec62 expression had shorter median overall survival (OS) than patients with low Sec62 levels (HR = 1.32, log-rank p = 0.012) (Fig. 1E). To validate these findings, GC cell lines and GC samples from the State Key Laboratory of Cancer Biology and National Clinical Research Center for Digestive Diseases, Xijing Hospital of Digestive Diseases, were obtained and tested for Sec62 expression. Similarly, both qPCR and WB showed significant overexpression of Sec62 in GC compared to normal controls (Fig. 1F–I). Moreover, a GC tissue microarray was used to examine the expression of Sec62 in GC tissues (Fig. 1J, K) and make correlations with the clinicopathological features of GC patients (Table 1). High Sec62 expression was positively correlated with higher metastasis rate (χ2 = 3.170, p = 0.036), larger tumour size (χ2 = 5.166, p = 0.028), higher microvascular invasion rate (χ2 = 3.029, p = 0.040), and higher tumour, node, metastasis (TNM) stage (χ2 = 11.370, p = 0.005). Kaplan–Meier analysis using tissue microarray data revealed that GC patients with high Sec62 expression had significantly shorter median overall survival (OS) than those with low Sec62 expression (HR = 1.926, log-rank p = 0.013) (Fig. 1L). Further, univariable and multivariable analyses suggested that high Sec62 expression was significantly correlated with poor OS and Sec62 upregulation was an independent risk factor for poor prognosis in GC patients (HR = 5.016, p < 0.0001) (Table 2).
Fig. 1.
Upregulation of Sec62 in GC predicts poor survival. Volcano plots of mass spectrometry data showed the top 10 upregulated molecules identified in GC tissues versus controls (A). Sec62 mRNA expression was significantly higher in GC than in other human malignancies (B) and adjacent normal stomach tissues (C, D) in the GEPIA database. Correlation of Sec62 mRNA expression in GC patients with OS in KM-plotter database (E). Quantitative PCR (F) and WB (G) assays suggested that Sec62 expression in GC cell lines was much higher than that in GES. In GC tissues and adjacent normal tissues, qRT-PCR (H) and WB (I) assays also confirmed higher Sec62 levels in GC tissues than in normal controls. IHC assays of Sec62 protein expression in GC and adjacent normal tissue (J). Chi-squared test for Sec62 expression in GC and adjacent normal tissues (K). Kaplan–Meier analysis using tissue microarray data showed a correlation between Sec62 protein levels and the OS of GC patients (L). **p < 0.01, ***p < 0.0001
Table 2.
Univariable and multivariable analysis of clinicopathological factors for overall survival
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender (male/female) | 1.153(0.768–1.890) | 0.751 | ||
| Age (years, < 50 vs ≥ 50) | 2.939(1.419–5.863) | 0.008 | 3.217(1.305–6.921) | 0.005 |
| Smoke (no/yes) | 2.091(1.135–4.589) | 0.027 | 2.109(1.056–4.762) | 0.018 |
| Alcoholism(no/yes) | 1.382(0.639–2.531) | 0. 598 | ||
| Metastasis (no/yes) | 3.409 (1.439–8.757) | 0.010 | 3.602 (1.368–9.583) | 0.020 |
| Tumor size (cm, ≤ 5 vs. > 5) | 2.896 (1.581–6.537) | 0.002 | ||
| Microvascular invasion (no/yes) | 2.758 (1.311–5.278) | 0.010 | ||
| TNM stage (I vs. II & III) | 2.093 (1.596–3.769) | 0.001 | ||
| Sec62 (low vs high) | 4.751 (2.068–11.089) | 0.000 | 5.016 (2.109–12.649) | 0.000 |
TNM tumor, node, metastasis
Sec62 Upregulation promotes GC cells migration and invasion in vitro and in vivo
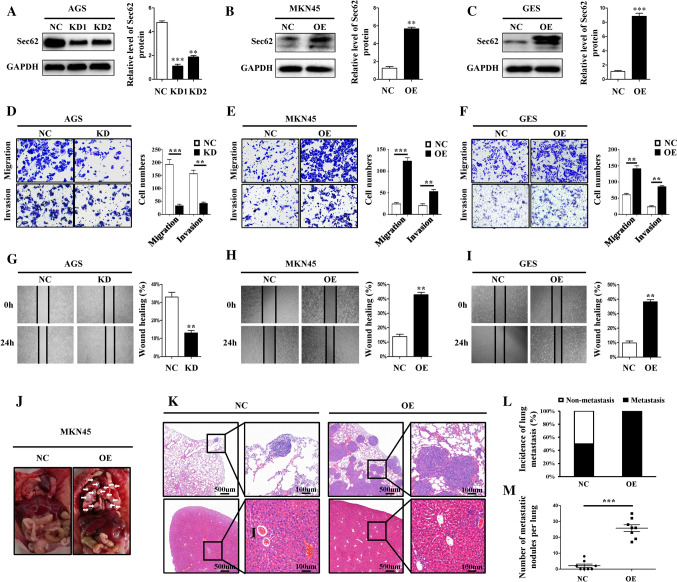
To determine the role of Sec62 in GC cell migration and invasion, three GC cell lines with stable Sec62 overexpression and knockdown were successfully constructed by lentivirus transfection. The three cell lines were AGS (KD and NC), MKN45 (OE and NC), and GES (OE and NC). Transfection efficiency was verified by measuring Sec62 expression using a WB assay (Fig. 2A–C). The invasion and migration ability of GC cells were examined using transwell and scratch healing assays. Transwell assays showed that high Sec62 expression significantly promoted GC cell migration and invasion (Fig. 2D–F). In addition, the promotive ability of Sec62 upregulation on migration was validated by a scratch healing assay (Fig. 2G–I). The nude mice tumour metastasis model also supported Sec62 upregulation promoting GC cell lung metastasis in vivo, although no difference in liver metastasis was identified (Fig. 2J–M). Collectively, these data demonstrate that Sec62 plays a promotive role in GC metastasis. On the other hand, no effect of Sec62 on GC cell proliferation and apoptosis has also been identified (Fig. S1A–K).
Fig. 2.
Upregulation of Sec62 promotes GC cells migration and invasion in vitro and in vivo. After lentivirus transfection, WB assays verified the protein expression of Sec62 in three GC cell lines, namely, AGS (A), MKN45 (B) and GES (C). Transwell assays showed that Sec62 significantly promoted cell migration and invasion in AGS (D), MKN45 (E), and GES (F) cell lines. Scratch healing assays confirmed that upregulation of Sec62 enhanced GC cell migration in GC cell lines (G–I). Representative images of lung tissues from nude mice (J). Hematoxylin and eosin staining of the lungs and liver (K). The incidence of lung metastasis (L) and the number of metastatic nodules in the lungs (M) between the two groups**p < 0.01, ***p < 0.0001
Sec62 upregulated MMP2/9 proteose activity by affecting TIMP-1 and MMP 2/9 balance
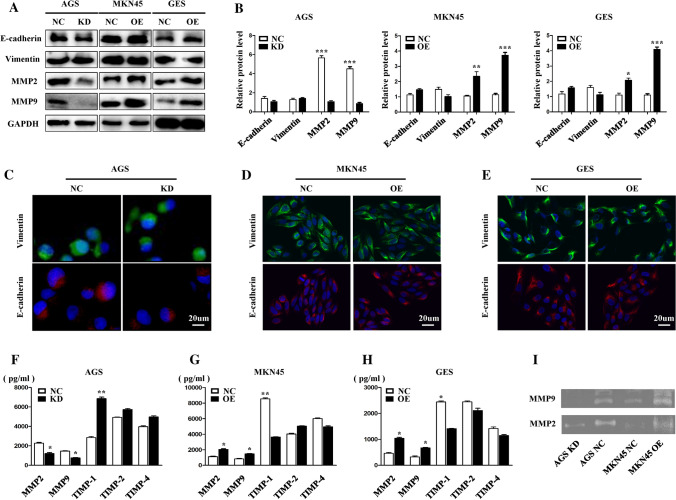
To explore the direct mechanism by which Sec62 promotes GC cell metastasis, EMT markers, including E-cadherin and vimentin, were first detected. Both WB (Fig. 3A, B) and immunofluorescent staining (Fig. 3C–E) assays showed no correlation between Sec62 expression and E-cadherin or vimentin expression. Considering that Sec62 upregulation could promote GC cell invasion in this study, and MMPs are associated with tumour invasion, we subsequently found that Sec62 could affect TIMP-1 and MMP2/9 levels but showed no effect on TIMP-2 and TIMP- 4 (Fig. 3A, B and F–H). Furthermore, gelatin zymography demonstrated Sec62 upregulated the activity of MMP2/9 in the supernatant of cell medium. Taken together, Sec62 may promote GC cell migration and invasion by affecting the TIMP-1 and MMP 2/9 balance and activity of MMP2/9 instead of the EMT pathway.
Fig. 3.
Sec62 affects TIMP-1 and MMP 2/9 expression instead of EMT. Western blotting showed that Sec62 expression was positively associated with MMP2 and MMP9 in cell lysates, while no correlation was found between Sec62 and EMT markers such as E-cadherin or vimentin (A, B). Immunofluorescence staining confirmed no correlations between Sec62 and E-cadherin or vimentin in AGS (C), MKN45 (D), and GES (E) cell lines. ELISA showed that Sec62 expression was positively correlated with the activity of MMP2 and MMP9, and negatively associated with TIMP-1 activity in GC cell culture supernatant (F–H). Gelatin zymography demonstrated Sec62 upregulated the activity of MMP2/9 in the supernatant of cell medium (I). *p < 0.05, **p < 0.01, ***p < 0.0001
Sec62 regulates PERK/ATF4 expression and activates autophagy accompanied by FIP200/Beclin-1/Atg5 upregulation
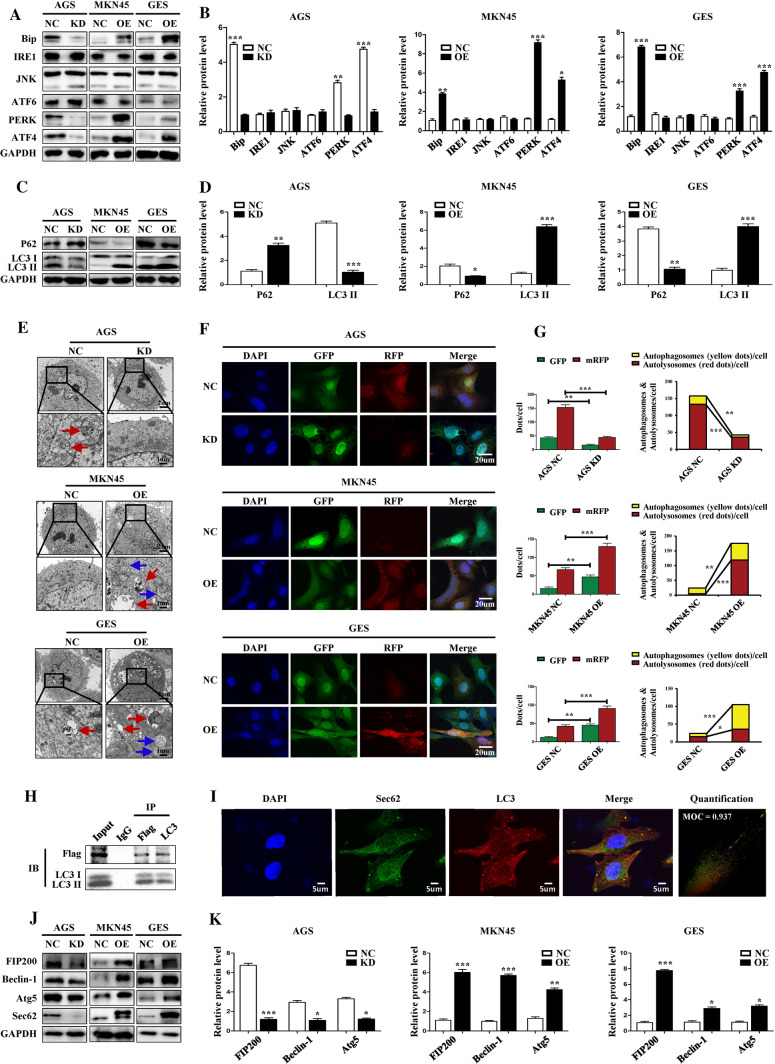
In light of the role of Sec62 in resolving UPR, we first evaluated the association between Sec62 expression and several key molecules in the UPR signalling pathway. The WB assay showed that Sec62 could affect the expression of PERK and ATF4. As for IRE1/JNK and ATF6, there was no correlation with Sec62 (Fig. 4A, B). Given that Sec62 resolves UPR by involving autophagy via binding to LC3II on autophagosomes, and PERK/ATF4 activation can activate autophagy [4, 5, 9, 10], we next explored the association between Sec62 expression and autophagy. We found that high Sec62 expression was correlated with higher LC3II (Fig. 4C, D). Similarly, under transmission electron microscopy, more autophagolysosomes and autophagosomes were observed in GC cells with higher Sec62 expression (Fig. 4E). Furthermore, immunofluorescence assays of GC cells after mRFP–GFP–LC3 adenovirus transfection also showed that the levels of autophagy were positively correlated with Sec62 (Fig. 4F, G). Moreover, a reciprocal Co-IP assay was conducted on MKN45 cell lysates. The data confirmed that exogenous FLAG-tagged Sec62 (the lentivirus bears a FLAG tag) cross-talked with endogenous LC3II directly, suggesting that Sec62 could dock to LC3II compared to IgG (Fig. 4H). In addition, an immunofluorescence co-localisation assay also supported this finding. The Manders Overlap Coefficient (MOC) was 0.937 (Fig. 4I) The WB assay revealed that the expression of several proteins in the autophagy pathway, including FIP200, Beclin-1 and Atg5, was correlated with Sec62 levels (Fig. 4J, K). In brief, Sec62 can activate autophagy via the UPR process involving PERK/ATF4 and binding to LC3II with concomitant upregulation of FIP200/Beclin-1/Atg5.
Fig. 4.
Sec62 regulates PERK/ATF4 expression and activates autophagy accompanied by FIP200/Beclin-1/Atg5 upregulation. The WB assay demonstrated that Sec62 could affect PERK and ATF4 expression and showed no effect on IRE1, JNK, and ATF6 (A, B). Sec62 correlated with autophagy activation indicated by LC3 II, and P62 was identified via WB assays (C, D). Transmission electron microscopy revealed more autophagolysosomes (red arrows) or autophagosomes (blue arrows) in GC cells with higher Sec62 expression (E). Immunofluorescence assays after mRFP–GFP–LC3 double-labeled adenovirus transfection indicated increased LC3II expression (red or yellow dots) in GC cells with higher Sec62 levels (F, G). The CO-IP assay showed that exogenous FLAG-tagged Sec62 (the lentivirus bears a FLAG tag) is associated with endogenous LC3II (H). Immunofluorescence co-localisation assays also supported the relationship between Sec62 and LC3II. The Manders Overlap Coefficient (MOC) was 0.937 (I). Western blotting revealed a positive correlation between Sec62 and FIP200, Beclin-1, and Atg5 expression (J–K). *p < 0.05, **p < 0.01, ***p < 0.0001
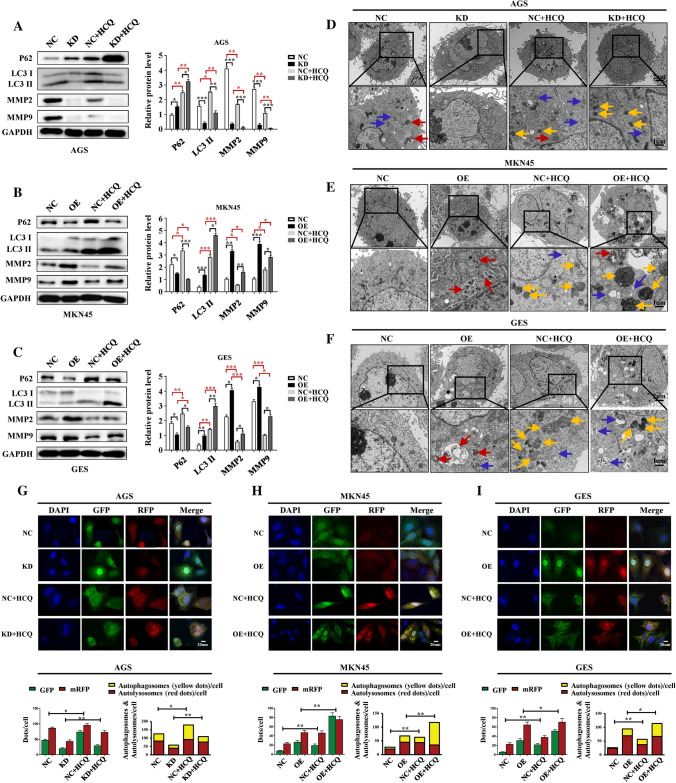
Autophagy blockage by hydroxychloroquine (HCQ) was confirmed and correlated with repressed MMP2 and MMP9 expression
To determine whether the autophagy process affects GC metastasis, HCQ (25 uM) was used to treat the lentivirus-constructed GC cells for 24 h to block autophagy. After 24 h of treatment with HCQ, WB assays showed that both P62 and LC3II were elevated. In particular, both MMP2 and MMP9 expression was inhibited after HCQ treatment (Fig. 5A–C). Transmission electron microscopy revealed that the fusion of autophagosomes and lysosomes was inhibited (Fig. 5D–F) and immunofluorescence assays after mRFP–GFP–LC3 adenovirus transfection also suggested the inhibition of autophagy flux (Fig. 5G–I). Taken together, these results indicated that autophagy was successfully blocked.
Fig. 5.
Autophagy blockage induced by HCQ was confirmed and correlated with repressed MMP2 and MMP9 expression. After HCQ (25 uM) treatment for 24 h, WB blotting showed that autophagy was blocked, as indicated by LC3II and P62. In addition, both MMP2 and MMP9 expression was also downregulated after HCQ treatment (A–C). Transmission electron microscopy showed more autophagosomes (blue arrows) and lysosomes (yellow arrows) in the HCQ-treatment groups (D–F). Immunofluorescence assays demonstrated upregulated LC3II expression (yellow dots) in GC cells after MRFP-GFP-LC3 adenovirus transfection (G–I). HCQ hydroxychloroquine, blue arrows: autophagosomes; red arrows, autophagolysosomes; yellow arrows, lysosomes; *p < 0.05, **p < 0.01, ***p < 0.0001
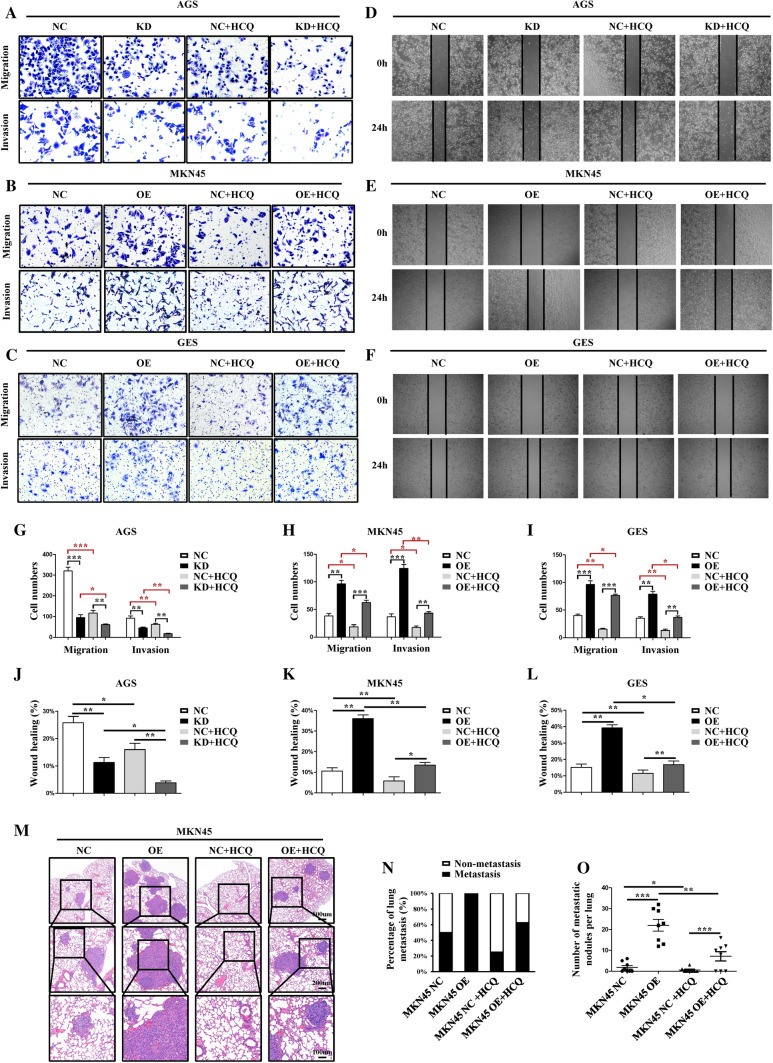
Sec62 inhibition combined with autophagy blockage exerts synergetic anti-metastatic effects in vitro and in vivo
Next, the effect of autophagy blockage on GC cell migration and invasion was evaluated. When the autophagy flux was blocked, migration and invasion of GC cells, as measured by transwell assays (Fig. 6A–C and G–I) and scratch healing assays (Fig. 6D–F and J–L), were found to be inhibited. Autophagy blockage could disturb the promotive effect of Sec62 upregulation on GC cell migration and invasion. In particular, autophagy blockage demonstrated a more obvious inhibitory effect on migration and invasion in cells with lower Sec62 expression, such as AGS KD, MKN45 NC, and GES NC. Moreover, an in vivo rescue experiment using HCQ (50 mg/kg) intraperitoneal injection of nude mice confirmed that autophagy inhibition could impair the promotive effect of Sec62 on GC metastasis (Fig. 6M–O). Collectively, autophagy blockage combined with Sec62 inhibition could exert synergetic anti-metastatic effects on GC metastasis, suggesting a promising therapeutic strategy. Conversely, autophagy activation induced by rapamycin (30 uM, RAPA) treatment enhanced the ability of GC cell migration and invasion and rescued the inhibitory effect of Sec62 knockdown on GC metastasis (Supplementary Fig. S2–S3).
Fig. 6.
Autophagy blockage inhibited GC metastasis in vitro and in vivo. Sec62 inhibition combined with autophagy blockage exerts synergetic anti-metastatic effects on GC cells. Transwell assays demonstrated the inhibitory effects of autophagy blockage induced by HCQ on GC cell migration and invasion (A–C and G–I). Scratch healing assays confirmed that GC cell migration was inhibited after HCQ treatment (D–F and J–L). In vivo rescue experiments using HCQ (50 mg/kg) intraperitoneal injection of nude mice verified that autophagy inhibition could rescue the promotive effect of Sec62 on GC metastasis. Hematoxylin and eosin staining of the lungs (M). The incidence of lung metastasis (N) and the number of metastatic nodules in the lungs (O) in diverse groups. HCQ hydroxychloroquine. *p < 0.05, **p < 0.01, ***p < 0.0001
Discussion
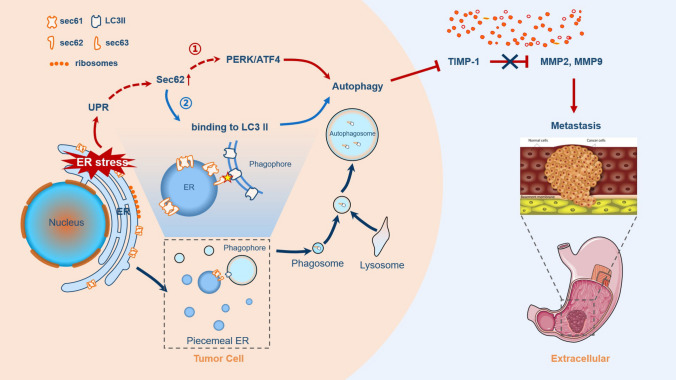
Metastatic GC remains the main cause of cancer-associated mortality. In this study, we found that Sec62 was significantly upregulated in GC tissues and cell lines compared to that in normal controls. Clinically, Sec62 upregulation in GC patients was positively associated with aggressive tumour features such as larger tumours, higher incidence of metastasis and advanced TNM stage. Moreover, high Sec62 expression could be an independent predictor of worse prognosis in patients with GC. Functionally, Sec62 upregulation could promote GC metastasis in vivo and in vitro, and revealed a novel role of Sec62 in autophagy activation via upregulation of the UPR-related PERK/ATF4 pathway and binding of LC3II during UPR recovery involving FIP200/Beclin-1/Atg5 upregulation. Autophagy then promoted GC cell migration and invasion by modulating TIMP-1 and MMP2/9 balance, as depicted in the schematic diagram in Fig. 7. Specifically, dual inhibition of Sec62 and autophagy exerted synergistic anti-metastatic effects in vitro and in vivo, suggesting a promising therapeutic strategy for GC metastasis.
Fig. 7.
Schematic diagram for the pro-metastatic role of Sec62 in GC. Schematic diagram of the role of Sec62 in GC metastasis. Sec62 activation of autophagy may involve two pathways, namely, the UPR-related PERK/ATF4 pathway and binding of LC3II during UPR recovery involving FIP200/Beclin-1/Atg5 upregulation. Autophagy subsequently promotes GC metastasis by regulating the TIMP-1 and MMP2/9 balance
Several studies have reported the upregulation of Sec62 in various tumours, including peripheral blood mononuclear cells from patients with liver cancer [11, 13, 24–29]. In 2019, a preliminary study published in Biochemical and Biophysical Research Communications first reported that Sec62 was highly expressed in gastric cancer which was consistent with our findings [30]. However, this study only explored the expression of Sec62 at the mRNA level. The effect of Sec62 on GC malignant behaviour, especially metastasis, and the underlying mechanism remain unknown.
Functionally, in this study, bioinformatics and tissue microarray analysis suggested that Sec62 upregulation was related to poor prognosis and worse clinicopathological characteristics in GC patients. Similar findings have been reported for other tumours. Linxweiler et al. [13], reported that higher expression levels of Sec62 were correlated with more severe cervical atypical hyperplasia. Greiner et al. [14] found that the upregulation of Sec62 protein was positively correlated with higher Gleason scores in prostate cancer. In addition, in non-small cell lung cancer and head and neck squamous cell carcinoma, a correlation between Sec62 overexpression and shorter survival was also identified [3, 24]. In non-small cell lung cancer, Sec62 upregulation was found to be associated with poor tumour differentiation and high incidence of lymph node metastasis [11]. Collectively, these findings support the role of Sec62 in malignancy.
Furthermore, regarding the effect of Sec62 on GC malignant behaviours, transwell and scratch healing assays showed that the migratory and invasive abilities of GC cells were enhanced when Sec62 was upregulated in this study. Similar findings have been reported for other cancer cells. Sec62 gene knockout has been reported to significantly inhibit tumour cell invasion and migration in cell lines of prostate cancer, non-small cell lung cancer, thyroid cancer, and cervical cancer, but the underlying mechanism remains unknown [11, 13]. In this study, we performed in vivo experiments and demonstrated that upregulation of Sec62 could promote GC cell lung metastasis, while the liver was not affected. This finding can be explained as follows: Nude mouse tail vein injection is an animal model of lung metastases [31]. After the tumour cells are injected through the tail vein, they first enter the inferior vena cava, heart, and capillary network of the lungs in succession, and then into the arterial blood circulation system and liver. Because the tumour cells are relatively viscous and easy to gather into mass, they are generally trapped in the lungs, mainly forming lung metastases, which then may cause metastasis to distant organs in the later stage. However, the effect of Sec62 on tumour cell proliferation remains controversial. Several studies have reported that neither Sec62 knockout nor overexpression affects tumour cell proliferation [11, 13, 28], while others have shown that the loss of Sec62 inhibits the proliferation ability of tumour cells, such as HeLa cells [29, 32].
To date, the mechanism by which Sec62 regulates tumour migration and invasion has been scarcely investigated. Matrix metalloproteinases (MMPs) and EMT are two major mechanisms in regulating tumour metastasis [33]; in particular, MMPs are associated with gastric cancer invasion [34–37]. Thus, when we found that Sec62 promoted GC cell invasion and was not correlated with E-cadherin and vimentin, we subsequently examined the relationship between Sec62 and MMPs and found that Sec62 could affect MMP2 and MMP9 expression. In particular, we simultaneously evaluated the levels of several soluble TIMPs, such as TIMP-1, 2 and 4, in the culture supernatant. Interestingly, we found that Sec62 could affect TIMP-1 level, which is a well-established inhibitor of MMP2 and MMP9 [38]. Moreover, gelatin zymography also supported the up-regulatory effect of Sec62 on MMP2/9 proteose activity. Thus, Sec62 may promote GC metastasis by regulating TIMP-1 and MMP2/9 balance.
As for the modulating mechanism of TIMP-1 and MMP2/9 by Sec62, a link between UPR activation and tumor metastasis has been identified in various malignancies [9, 39, 40]. UPR markers such as IRE1 and PERK have been reported to be important regulatory factors of MMPs. Xia et al. reported that IRE1 upregulation in oesophageal squamous cell carcinoma and glioma is positively related to the expression of MMP9 [41]. In oesophageal squamous cell carcinoma, Zhu et al. found that ATF4, downstream of PERK, promoted tumour migration by regulating MMP2, and was an independent risk factor for poor prognosis [42]. In addition, PERK activation was also found to be associated with MMP2 and MMP9 in oral squamous cell carcinoma and breast cancer [43]. The activation of UPR in GC has also been previously identified and related to the depth of GC invasion, distant metastasis and TNM stage [44]. Based on the above findings, and considering the role of Sec62 in UPR resolution, we propose that Sec62 modulating the TIMP-1 and MMP2/9 balance may involve UPR related molecules. Therefore, we further explored the correlation between Sec62 and several key molecules in UPR signalling pathways and found that Sec62 could affect PERK and ATF4 expression, but had no effect on IRE1, JNK, and ATF6. Collectively, these data suggest that Sec62 regulating TIMP-1-MMP2/9 may involve PERK/ATF4 modulation.
Furthermore, it has been well defined that autophagy is one of the results of the PERK/ATF4 pathway [9] and is implicated in Sec62-mediated UPR resolution. Namely, Sec62 acts as an autophagy receptor and promotes the degradation of excess ER and misfolded proteins. Through its LC3-interacting region (LIR) motif, Sec62 binds to membrane-anchored LC3 II, enabling the inclusion of the ER parts into autophagosomes [4–6, 10, 45, 46]. In addition, autophagy plays a significant role in cancer metastasis [47, 48]. In particular, autophagy has been recognised as an inhibitor of TIMP-1 [49–52] and can promote metastasis by contributing to focal adhesion turnover. MMPs are one of the most important players in regulating focal adhesion turnover [47, 53]. Thus, we surmised that autophagy might be involved in the regulation of the TIMP-1 and MMP2/9 balance by Sec62 and consequently found that Sec62 upregulation was positively correlated with enhanced autophagy. Furthermore, we verified that Sec62 could bind to LC3II and upregulate the expression of FIP200, Beclin-1, and Atg5, which are all key players in the autophagy activation pathway. Therefore, Sec62 activation of autophagy may involve the UPR-related PERK/ATF4 pathway and binding of LC3II during UPR recovery involving FIP200/Beclin-1/Atg5 upregulation, although the exact mechanism remains to be elucidated. Then, autophagy promotes GC metastasis by regulating the TIMP-1 and MMP2/9 balance.
We further explored the effect of autophagy on GC cell migration and invasion by enhancing autophagy with rapamycin and blocking autophagy with HCQ. The data indicated that inhibition of autophagy impaired GC cell migration and invasion, accompanied by downregulation of MMP2 and MMP9. In addition, we performed in vivo rescue experiments by inhibiting autophagy with HCQ and found that autophagy blockage could also inhibit GC metastasis and attenuate the promotive effect of Sec62 on GC metastasis. Conversely, after activation of autophagy, GC cell invasion and migration were enhanced, accompanied by upregulation of MMP2 and MMP9. These results suggest that Sec62 promotes GC invasion and migration by regulating MMP2/MMP9 via autophagy, and dual inhibition of both Sec62 and autophagy may be an effective therapeutic strategy for GC metastasis.
In conclusion, the strength of this study is that we have provided new insights into the mechanism by which Sec62 promotes GC metastasis. Sec62 promotes GC metastasis by activating autophagy and subsequently regulating the TIMP-1 and MMP2/9 balance. The activation of autophagy by Sec62 may involve the UPR-related PERK/ATF4 pathway and binding of LC3II during UPR recovery involving FIP200/Beclin-1/Atg5 upregulation, although the exact mechanism remains to be elucidated. Furthermore, the dual inhibition of Sec62 and autophagy may be an attractive therapeutic strategy for GC metastasis.
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. S1 Sec62 has no effect on GC cell proliferation, apoptosis, and several autophagy associated molecules. The influence of Sec62 on GC cell growth was assessed via Cell Counting Kit (CCK8) (A-C) and colony-formation (D-G). The effect of Sec62 on GC cell apoptosis was evaluated with flow cytometry (H-K). WB assays examined the effect of Sec62 on several autophagy associated molecules (L). ns, no significance. (TIFF 2243 KB)
Fig. S2 Autophagy activation induced by RAPA was confirmed and correlated with upregulated MMP2 and MMP9 expression. After RAPA (30 uM) treatment for 24 h, WB blotting showed that autophagy was enhanced, as indicated by LC3II and P62. Additionally, both MMP2 and MMP9 expression was also upregulated after RAPA treatment (A–C). Transmission electron microscopy showed more autophagolysosomes (red arrows) and autophagosomes (blue arrows) in the RAPA-treatment groups (D–F). Immunofluorescence assays demonstrated enhanced LC3II expression (yellow dots) in GC cells after MRFP-GFP-LC3 adenovirus transfection (G–I). RAPA, rapamycin; blue arrows: autophagosomes; red arrows, autophagolysosomes. *p<0.05, **p < 0.01, ***p < 0.0001. (TIFF 9286 KB)
Fig. S3 Autophagy activation promoted GC cell migration and invasion and partially rescued the inhibitory effect of Sec62 knockdown on. Scratch healing assays confirmed that GC cell migration was promoted after RAPA treatment (A–C). Transwell assays demonstrated the promotive effects of autophagy activation induced by RAPA on GC cell migration and invasion (D–F). RAPA, rapamycin. *p<0.05, **p < 0.01, ***p < 0.0001. (TIFF 8739 KB)
Abbreviations
- Co-IP
Co-immunoprecipitation
- ER
Endoplasmic reticulum
- GC
Gastric cancer
- HCQ
Hydroxychloroquine
- IHC
Immunohistochemistry
- KD
Knockdown
- MMP
Matrix metalloproteinases
- NC
Normal control
- OE
Overexpression
- OS
Overall survival
- qPCR
Quantitative real-time polymerase chain reaction
- RAPA
Rapamycin
- TIMP
Tissue inhibitor of matrix metalloproteinases
- UPR
Unfolded protein response
- WB
Western blot
Author contributions
SS, LJ: designed the work, interpretated results and critically revised the manuscript; SS, S YT; performed the experiments, analysed the data and drafted the manuscript; CY, J MZ, WN, XB, ZH, L JC, J YR and L XF: participated in several parts of experiments; All the authors: approved the final version.
Funding
This work was supported in part by the National Natural Science Foundation of China (Nos. 81772650, 81322037, 81572302, 81421003), National Excellent Doctoral Dissertation of PR China (No. 201182).
Availability of data and material
Data and material will be made available on reasonable request.
Code availability
Not applicable.
Declarations
Conflicts of interest
None.
Ethics approval
This study was performed with approval from the Ethics Committee at Xijing Hospital, the first Affiliated Hospital of Air Force Medical University and complied with the Helsinki Declaration.
Consent to participate
Informed consent was collected from all individual participant included in the study.
Consent for publication
All authors have read and approved the content, and agree to submit for consideration for publication in the journal.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Song Su and Yan-Ting Shi contributed equally to this work.
References
- 1.Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, Bray F(2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin [DOI] [PubMed]
- 2.Wadhwa R, Song S, Lee J, Yao Y, Wei Q, Ajani J. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10:643–655. doi: 10.1038/nrclinonc.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linxweiler M, Schick B, Zimmermann R. Let's talk about Secs: Sec61, Sec62 and Sec63 in signal transduction, oncology and personalized medicine. Signal Transduct Target Ther. 2017;2:17002. doi: 10.1038/sigtrans.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loi M, Raimondi A, Morone D, Molinari M. ESCRT-III-driven piecemeal micro-ER-phagy remodels the ER during recovery from ER stress. Nat Commun. 2019;10:5058. doi: 10.1038/s41467-019-12991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fumagalli F, Noack J, Bergmann TJ, Cebollero E, Pisoni GB, Fasana E, Fregno I, Galli C, Loi M, Solda T, D'Antuono R, Raimondi A, Jung M, Melnyk A, Schorr S, Schreiber A, Simonelli L, Varani L, Wilson-Zbinden C, Zerbe O, Hofmann K, Peter M, Quadroni M, Zimmermann R, Molinari M. Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery. Nat Cell Biol. 2016;18:1173–1184. doi: 10.1038/ncb3423. [DOI] [PubMed] [Google Scholar]
- 6.Molinari M. ER-phagy responses in yeast, plants, and mammalian cells and their crosstalk with UPR and ERAD. Dev Cell. 2021;56:949–966. doi: 10.1016/j.devcel.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Urra H, Dufey E, Avril T, Chevet E, Hetz C. Endoplasmic reticulum stress and the hallmarks of cancer. Trends in cancer. 2016;2:252–262. doi: 10.1016/j.trecan.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Juan RC-R, Sarah EB, Glimcher LH. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell. 2017;168:692–706. doi: 10.1016/j.cell.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14:581–597. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 10.Schuck S. On keeping the right ER size. Nat Cell Biol. 2016;18:1118–1119. doi: 10.1038/ncb3430. [DOI] [PubMed] [Google Scholar]
- 11.Linxweiler M, Linxweiler J, Barth M, Benedix J, Jung V, Kim YJ, Bohle RM, Zimmermann R, Greiner M. Sec62 bridges the gap from 3q amplification to molecular cell biology in non-small cell lung cancer. Am J Pathol. 2012;180:473–483. doi: 10.1016/j.ajpath.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 12.Takacs FZ, Radosa JC, Linxweiler M, Kasoha M, Bohle RM, Bochen F, Unger C, Solomayer EF, Schick B, Juhasz-Boss I (2019) Identification of 3q oncogene SEC62 as a marker for distant metastasis and poor clinical outcome in invasive ductal breast cancer. Arch Gynecol Obstet [DOI] [PubMed]
- 13.Linxweiler M, Bochen F, Schick B, Wemmert S, Al Kadah B, Greiner M, Hasenfus A, Bohle RM, Juhasz-Boss I, Solomayer EF, Takacs ZF. Identification of SEC62 as a potential marker for 3q amplification and cellular migration in dysplastic cervical lesions. BMC Cancer. 2016;16:676. doi: 10.1186/s12885-016-2739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greiner M, Kreutzer B, Jung V, Grobholz R, Hasenfus A, Stohr RF, Tornillo L, Dudek J, Stockle M, Unteregger G, Kamradt J, Wullich B, Zimmermann R. Silencing of the SEC62 gene inhibits migratory and invasive potential of various tumor cells. Int J Cancer. 2011;128:2284–2295. doi: 10.1002/ijc.25580. [DOI] [PubMed] [Google Scholar]
- 15.Muller CSL, Kreie L, Bochen F, Pfuhl T, Smola S, Graber S, Vogt T, Schick B, Linxweiler M. Expression of 3q oncogene SEC62 in atypical fibroxanthoma-immunohistochemical analysis of 41 cases and correlation with clinical, viral and histopathologic features. Oncol Lett. 2019;17:1768–1776. doi: 10.3892/ol.2018.9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ajani J, D'Amico T, Almhanna K, Bentrem D, Chao J, Das P, Denlinger C, Fanta P, Farjah F, Fuchs C, Gerdes H, Gibson M, Glasgow R, Hayman J, Hochwald S, Hofstetter W, Ilson D, Jaroszewski D, Johung K, Keswani R, Kleinberg L, Korn W, Leong S, Linn C, Lockhart A, Ly Q, Mulcahy M, Orringer M, Perry K, Poultsides G, Scott W, Strong V, Washington M, Weksler B, Willett C, Wright C, Zelman D, McMillian N, Sundar H. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw JNCCN. 2016;14:1286–1312. doi: 10.6004/jnccn.2016.0137. [DOI] [PubMed] [Google Scholar]
- 17.Chen D, Xu L, Li X, Chu Y, Jiang M, Xu B, Zhao M, Wang W, Wang H, Kang H, Wang K, Wu K, Liang J, Ren G. Enah overexpression is correlated with poor survival and aggressive phenotype in gastric cancer. Cell Death Dis. 2018;9:998. doi: 10.1038/s41419-018-1031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen D, Liu L, Wang K, Yu H, Wang Y, Liu J, Guo Y, Zhang H. The role of MALAT-1 in the invasion and metastasis of gastric cancer. Scand J Gastroenterol. 2017;52:790–796. doi: 10.1080/00365521.2017.1280531. [DOI] [PubMed] [Google Scholar]
- 19.Du R, Xia L, Ning X, Liu L, Sun W, Huang C, Wang H, Sun S. Hypoxia-induced Bmi1 promotes renal tubular epithelial cell-mesenchymal transition and renal fibrosis via PI3K/Akt signal. Mol Biol Cell. 2014;25:2650–2659. doi: 10.1091/mbc.e14-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Q, Lan F, Wang X, Yu Y, Ouyang X, Zheng F, Han J, Lin Y, Xie Y, Xie F, Liu W, Yang X, Wang H, Dong L, Wang L, Tan J. IL-1β-induced activation of p38 promotes metastasis in gastric adenocarcinoma via upregulation of AP-1/c-fos, MMP2 and MMP9. Mol Cancer. 2014;13:18. doi: 10.1186/1476-4598-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng X, Feng H, Wu H, Jin Z, Shen X, Kuang J, Huo Z, Chen X, Gao H, Ye F, Ji X, Jing X, Zhang Y, Zhang T, Qiu W, Zhao R. Targeting autophagy enhances apatinib-induced apoptosis via endoplasmic reticulum stress for human colorectal cancer. Cancer Lett. 2018;431:105–114. doi: 10.1016/j.canlet.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 22.Lu X, Jin E, Cheng X, Feng S, Shang X, Deng P, Jiang S, Chang Q, Rahmy S, Chaudhary S, Lu X, Zhao R, Wang Y, DePinho R. Opposing roles of TGFβ and BMP signaling in prostate cancer development. Genes Dev. 2017;31:2337–2342. doi: 10.1101/gad.307116.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang M, Wu N, Xu B, Chu Y, Li X, Su S, Chen D, Li W, Shi Y, Gao X, Zhang H, Zhang Z, Du W, Nie Y, Liang J, Fan D. Fatty acid-induced CD36 expression via O-GlcNAcylation drives gastric cancer metastasis. Theranostics. 2019;9:5359–5373. doi: 10.7150/thno.34024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linxweiler M, Schorr S, Schauble N, Jung M, Linxweiler J, Langer F, Schafers HJ, Cavalie A, Zimmermann R, Greiner M. Targeting cell migration and the endoplasmic reticulum stress response with calmodulin antagonists: a clinically tested small molecule phenocopy of SEC62 gene silencing in human tumor cells. BMC Cancer. 2013;13:574. doi: 10.1186/1471-2407-13-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung V, Kindich R, Kamradt J, Jung M, Muller M, Schulz WA, Engers R, Unteregger G, Stockle M, Zimmermann R, Wullich B. Genomic and expression analysis of the 3q25-q26 amplification unit reveals TLOC1/SEC62 as a probable target gene in prostate cancer. Mol Cancer Res MCR. 2006;4:169–176. doi: 10.1158/1541-7786.MCR-05-0165. [DOI] [PubMed] [Google Scholar]
- 26.Wemmert S, Lindner Y, Linxweiler J, Wagenpfeil S, Bohle R, Niewald M, Schick B. Initial evidence for Sec62 as a prognostic marker in advanced head and neck squamous cell carcinoma. Oncol Lett. 2016;11:1661–1670. doi: 10.3892/ol.2016.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weng L, Du J, Zhou Q, Cheng B, Li J, Zhang D, Ling C. Identification of cyclin B1 and Sec62 as biomarkers for recurrence in patients with HBV-related hepatocellular carcinoma after surgical resection. Mol Cancer. 2012;11:39. doi: 10.1186/1476-4598-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greiner M, Kreutzer B, Lang S, Jung V, Cavalie A, Unteregger G, Zimmermann R, Wullich B. Sec62 protein level is crucial for the ER stress tolerance of prostate cancer. Prostate. 2011;71:1074–1083. doi: 10.1002/pros.21324. [DOI] [PubMed] [Google Scholar]
- 29.Hagerstrand D, Tong A, Schumacher SE, Ilic N, Shen RR, Cheung HW, Vazquez F, Shrestha Y, Kim SY, Giacomelli AO, Rosenbluh J, Schinzel AC, Spardy NA, Barbie DA, Mermel CH, Weir BA, Garraway LA, Tamayo P, Mesirov JP, Beroukhim R, Hahn WC. Systematic interrogation of 3q26 identifies TLOC1 and SKIL as cancer drivers. Cancer Discov. 2013;3:1044–1057. doi: 10.1158/2159-8290.CD-12-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He H, Wu W, Sun Z, Chai L. MiR-4429 prevented gastric cancer progression through targeting METTL3 to inhibit m(6)A-caused stabilization of SEC62. Biochem Biophys Res Commun. 2019;517:581–587. doi: 10.1016/j.bbrc.2019.07.058. [DOI] [PubMed] [Google Scholar]
- 31.Jang H, Shin S, Kim C, Won J, Xu R, Kim D, Yim H(2021) PLK1/vimentin signaling facilitates immune escape by recruiting Smad2/3 to PD-L1 promoter in metastatic lung adenocarcinoma. Cell Death Differ [DOI] [PMC free article] [PubMed]
- 32.Abell B, Pool M, Schlenker O, Sinning I, High S. Signal recognition particle mediates post-translational targeting in eukaryotes. EMBO J. 2004;23:2755–2764. doi: 10.1038/sj.emboj.7600281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Ng JM, Wong CC, Ng EKW, Yu J. Molecular alterations of cancer cell and tumour microenvironment in metastatic gastric cancer. Oncogene. 2018;37:4903–4920. doi: 10.1038/s41388-018-0341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubben FJGM, Sier CFM, Hawinkels LJAC, Tschesche H, van Duijn W, Zuidwijk K, van der Reijden JJ, Hanemaaijer R, Griffioen G, Lamers CBHW, Verspaget HW. Clinical evidence for a protective role of lipocalin-2 against MMP-9 autodegradation and the impact for gastric cancer. Eur J Cancer (Oxford, England 19) 2007;43:1869–1876. doi: 10.1016/j.ejca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Wu C-Y, Wu M-S, Chen Y-J, Chen C-J, Chen H-P, Shun C-T, Chen G-H, Huang S-P, Lin J-T (2007) Clinicopathological significance of MMP-2 and TIMP-2 genotypes in gastric cancer. Eur J Cancer (Oxford, England: 1990) 43:799–808. [DOI] [PubMed]
- 37.Shim K-N, Jung S-A, Joo Y-H, Yoo K. Clinical significance of tissue levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in gastric cancer. J Gastroenterol. 2007;42:120–128. doi: 10.1007/s00535-006-1975-y. [DOI] [PubMed] [Google Scholar]
- 38.Eckfeld C, Häußler D, Schoeps B, Hermann CD, Krüger A. Functional disparities within the TIMP family in cancer: hints from molecular divergence. Cancer Metastasis Rev. 2019;38:469–481. doi: 10.1007/s10555-019-09812-6. [DOI] [PubMed] [Google Scholar]
- 39.Lin Y, Jiang M, Chen W, Zhao T, Wei Y. Cancer and ER stress: Mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed Pharmacother. 2019;118:109249. doi: 10.1016/j.biopha.2019.109249. [DOI] [PubMed] [Google Scholar]
- 40.Hsu SK, Chiu CC, Dahms HU, Chou CK, Cheng CM, Chang WT, Cheng KC, Wang HD, Lin IL(2019) Unfolded Protein Response (UPR) in Survival, Dormancy, Immunosuppression, Metastasis, and Treatments of Cancer Cells. Int J Mol Sci 20 [DOI] [PMC free article] [PubMed]
- 41.Xia T, Tong S, Fan K, Zhai W, Fang B, Wang SH, Wang JJ. XBP1 induces MMP-9 expression to promote proliferation and invasion in human esophageal squamous cell carcinoma. Am J Cancer Res. 2016;6:2031–2040. [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu H, Chen X, Chen B, Chen B, Song W, Sun D, Zhao Y. Activating transcription factor 4 promotes esophageal squamous cell carcinoma invasion and metastasis in mice and is associated with poor prognosis in human patients. PLoS ONE. 2014;9:e103882. doi: 10.1371/journal.pone.0103882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Podszywalow-Bartnicka P, Cmoch A, Wolczyk M, Bugajski L, Tkaczyk M, Dadlez M, Nieborowska-Skorska M, Koromilas AE, Skorski T, Piwocka K. Increased phosphorylation of eIF2alpha in chronic myeloid leukemia cells stimulates secretion of matrix modifying enzymes. Oncotarget. 2016;7:79706–79721. doi: 10.18632/oncotarget.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, Takano Y. Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol. 2008;39:1042–1049. doi: 10.1016/j.humpath.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Loi M, Molinari M. Mechanistic insights in recov-ER-phagy: micro-ER-phagy to recover from stress. Autophagy. 2020;16:385–386. doi: 10.1080/15548627.2019.1709767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birgisdottir Å, Lamark T, Johansen T. The LIR motif—crucial for selective autophagy. J Cell Sci. 2013;126:3237–3247. doi: 10.1242/jcs.126128. [DOI] [PubMed] [Google Scholar]
- 47.Dower CM, Wills CA, Frisch SM, Wang HG. Mechanisms and context underlying the role of autophagy in cancer metastasis. Autophagy. 2018;14:1110–1128. doi: 10.1080/15548627.2018.1450020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mowers EE, Sharifi MN, Macleod KF. Functions of autophagy in the tumor microenvironment and cancer metastasis. FEBS J. 2018;285:1751–1766. doi: 10.1111/febs.14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long F, Jiang H, Yi H, Su L, Sun J. Particulate matter 2.5 induced bronchial epithelial cell injury via activation of 5'-adenosine monophosphate-activated protein kinase-mediated autophagy. J Cell Biochem. 2019;120:3294–3305. doi: 10.1002/jcb.27597. [DOI] [PubMed] [Google Scholar]
- 50.Ma J-Q, Sun Y-Z, Ming Q-L, Tian Z-K, Yang H-X, Liu C-M. Ampelopsin attenuates carbon tetrachloride-induced mouse liver fibrosis and hepatic stellate cell activation associated with the SIRT1/TGF-β1/Smad3 and autophagy pathway. Int Immunopharmacol. 2019;77:105984. doi: 10.1016/j.intimp.2019.105984. [DOI] [PubMed] [Google Scholar]
- 51.Liu M-B, Wang W, Gao J-M, Li F, Shi J-S, Gong Q-H. Icariside II attenuates cerebral ischemia/reperfusion-induced blood-brain barrier dysfunction in rats via regulating the balance of MMP9/TIMP1. Acta Pharmacol Sin. 2020;41:1547–1556. doi: 10.1038/s41401-020-0409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen W, Sun Y, Gu X, Hao Y, Liu X, Lin J, Chen J, Chen S. Conditioned medium of mesenchymal stem cells delays osteoarthritis progression in a rat model by protecting subchondral bone, maintaining matrix homeostasis, and enhancing autophagy. J Tissue Eng Regen Med. 2019;13:1618–1628. doi: 10.1002/term.2916. [DOI] [PubMed] [Google Scholar]
- 53.Shi F, Sottile J. MT1-MMP regulates the turnover and endocytosis of extracellular matrix fibronectin. J Cell Sci. 2011;124:4039–4050. doi: 10.1242/jcs.087858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Sec62 has no effect on GC cell proliferation, apoptosis, and several autophagy associated molecules. The influence of Sec62 on GC cell growth was assessed via Cell Counting Kit (CCK8) (A-C) and colony-formation (D-G). The effect of Sec62 on GC cell apoptosis was evaluated with flow cytometry (H-K). WB assays examined the effect of Sec62 on several autophagy associated molecules (L). ns, no significance. (TIFF 2243 KB)
Fig. S2 Autophagy activation induced by RAPA was confirmed and correlated with upregulated MMP2 and MMP9 expression. After RAPA (30 uM) treatment for 24 h, WB blotting showed that autophagy was enhanced, as indicated by LC3II and P62. Additionally, both MMP2 and MMP9 expression was also upregulated after RAPA treatment (A–C). Transmission electron microscopy showed more autophagolysosomes (red arrows) and autophagosomes (blue arrows) in the RAPA-treatment groups (D–F). Immunofluorescence assays demonstrated enhanced LC3II expression (yellow dots) in GC cells after MRFP-GFP-LC3 adenovirus transfection (G–I). RAPA, rapamycin; blue arrows: autophagosomes; red arrows, autophagolysosomes. *p<0.05, **p < 0.01, ***p < 0.0001. (TIFF 9286 KB)
Fig. S3 Autophagy activation promoted GC cell migration and invasion and partially rescued the inhibitory effect of Sec62 knockdown on. Scratch healing assays confirmed that GC cell migration was promoted after RAPA treatment (A–C). Transwell assays demonstrated the promotive effects of autophagy activation induced by RAPA on GC cell migration and invasion (D–F). RAPA, rapamycin. *p<0.05, **p < 0.01, ***p < 0.0001. (TIFF 8739 KB)
Data Availability Statement
Data and material will be made available on reasonable request.
Not applicable.