Abstract
Severe acute pancreatitis (SAP) is a common critical disease of the digestive system, with high mortality and a lack of effective prevention and treatment measures. Despite mesenchymal stromal cell transplantation having the potential to treat SAP, its clinical application prospect is limited, and the mechanism is unclear. Here, we reveal the therapeutic role of exosomes from TNF-α-preconditioned human umbilical cord mesenchymal stromal cells (HUCMSCs) in attenuating SAP and show that it is partly dependent on exosomal metabolites. Bioactive metabolomics analysis showed that 48 metabolites be significantly differentially expressed between the two groups (Exo-Ctrl group versus Exo-TNF-α group). Then, the further functional experiments indicated that 3,4-dihydroxyphenylglycol could be a key molecule mediating the therapeutic effect of TNF-α-preconditioned HUCMSCs. The animal experiments showed that 3,4-dihydroxyphenylglycol reduced inflammation and oxidative stress in the pancreatic tissue and inhibited acinar cell autophagy in a rat model of SAP. Mechanistically, we revealed that 3,4-dihydroxyphenylglycol activated the mTOR pathway to inhibit acinar cell autophagy and alleviate SAP. In summary, our study demonstrated that exosomes from TNF-α-preconditioned HUMSCs inhibit the autophagy of acinar cells of SAP by shuttling 3,4-dihydroxyphenylglycol and inhibiting the mTOR pathway. This study revealed the vital role and therapeutic potential of metabolite-derived exosomes in SAP, providing a new promising method to prevent and therapy SAP.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-023-04861-1.
Keywords: Severe acute pancreatitis; Mesenchymal stromal cell; Exosome; 3,4-Dihydroxyphenylglycol; Autophagy
Introduction
The global incidence rate of acute pancreatitis (AP), a common disease of the digestive system, is increasing, with the disease reported affecting 13–45 per 100,000 individuals [1, 2]. Furthermore, approximately 30% of the patients with AP develop severe acute pancreatitis (SAP), which is characterized by multiple organ dysfunctions and is associated with poor prognosis and mortality rates up to 30–47% [3, 4]. Despite the recent popularization of the diagnostic and treatment concept of the multidisciplinary team (MDT), individualization, and minimally invasive approaches, the high mortality rate of SAP patients is still a major problem worldwide [4, 5]. Hence, specific and effective treatment methods for SAP remain an urgent medical requirement.
Mesenchymal stromal cells (MSCs) are multipotent cells with self-renewal potential and multi-lineage differentiation properties combined with low immunogenicity. MSCs have been applied to several clinical diseases because of their immunomodulatory and anti-inflammatory properties [6, 7]. Furthermore, some studies have indicated that MSCs can improve pancreatic tissue injury and promote the repair damaged pancreatic tissue in SAP, indicating its clinical potential [8, 9]. However, the underlying mechanism of action remains unclear and is being explored.
MSCs can release numerous extracellular vesicles (EVs), including microvesicles (MV; 100–2000 nm in diameter) and exosomes (30–200 nm in diameter), which may act as mediators for the cross talk between MSCs and target cells [10, 11]. Furthermore, some studies have shown that the exosomes from MSCs can reproduce the biological activity of MSCs and can be used as substitutes for whole-cell treatment [12]. In recent years, it has become evident that different cultural conditions or additional interventions affect the biological functions of MSCs [13, 14]. Increasing data indicate that the exposure of MSCs to cytokines increases their trophic effects and functional properties to respond to the hostile inflammatory environment, which could be due to optimization of the biomolecule expression profiles in the exosomes [13, 15]. Accumulating evidence suggests that the tumor necrosis factor alpha (TNF‐α) can enhance the efficacy of MSC‐based therapies and biological properties can be transferred via the exosome as a carrier [16, 17]. Although the therapeutic potential of exosomes from MSCs for treatment SAP has been reported [18, 19], it remains no clear whether TNF‐α preconditioning can regulate the cargo of MSC‐derived exosomes and enhance their efficacy in SAP.
Exosomes can transfer biomolecules including lipids, carbohydrates, nucleic acids, and proteins from one cell to another, resulting in genetic information exchange, host cell reprogramming, and cellular communication [20–22]. Recently, the concept of “metabolite-protein interactions omics” based on the existing “omics” was introduced and it provides a new idea for the development and expansion of the clinical application of endogenous bioactive metabolites [23]. Autocrine or paracrine metabolites are involved in the regulation of pancreatic endocrine function and insulin resistance [24]. Tryptophan metabolite 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) promotes the differentiation of tumor stem cells and decreases their tumorigenic potential [25]. N-acetyl-l-tyrosine is an endogenous metabolite that promotes stress resistance through the induction of mitohormesis [26]. However, whether bioactive metabolites from MSC-derived exosomes inhibit SAP remains unclear.
Therefore, the current study aimed to evaluate the therapeutic effects of the exosomes from TNF-α-preconditioned human umbilical cord MSCs (HUCMSCs) in SAP, and investigate the effects and molecular mechanism of the bioactive metabolites from the exosomes in SAP.
Methods and materials
Animals
Sprague–Dawley rats (aged 6 weeks and weighing 180–200 g each) were purchased from Shanghai Laboratory Animal Co. Ltd. All animal experiments were carried out according to the guidelines of the Shanghai Laboratory Animal Ordinance and were approved by the Ethics Committee of Shanghai Tenth People’s Hospital, Tongji University School of Medicine (Shanghai, China).
Isolation, culture, identification, and TNF-α preconditioning of HUCMSCs
HUCMSCs were isolated by processing the human umbilical cord matrix [27]. Then, the cells were routinely resuspended in α-minimum essential medium (α-MEM; Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (Gibco/Life Technologies, USA) and 100 U/mL penicillin/streptomycin (Gibco/Life Technologies, USA). The cultures were maintained at 37 °C in 5% CO2 and 95% humidity, and cells at the 3rd–5th passages were used for subsequent experiments. HUCMSCs were identified as described in the Supplementary Materials (Fig. S1).
HUCMSCs were seeded in 10 cm dishes for 24 h to obtain a confluence of 70–80%. Then, the medium was aspirated; the cells were rinsed 2–3 times with PBS and preconditioned with TNF-α in serum-free medium (10 ng/mL, Cell Signaling Technology, USA) or serum-free medium alone as a negative control and then incubated for 48 h before supernatant collection.
Extraction and characterization of exosomes
The cell supernatants were filtered by a 0.22-µm filter to remove large debris and dead cells, centrifuged at 10,000×g (30 min) to remove cellular debris, and then centrifuged at 100,000×g (3 h) at 4 °C. After this step, the obtained pellet primarily contained exosomes. The amount of protein in the exosomes was quantified using the BCA Protein Assay Kit (Thermo Fisher Scientific, USA). Transmission electron microscopy (TEM) (JEM‐200CX, Japan) was performed to observe exosome morphology. The size distribution and concentration of exosomes were measured by nanoparticle tracking analysis (NTA) using a Nanoparticle Characterization System (Malvern Panalytical, Malvern, UK). According to International Society of Extracellular Vesicles (ISEV) recommendations [28], TSG101 (antirabbit, dilution 1:1000, Cat #72312, Cell Signaling Technology, USA), CD81 (antirabbit, dilution 1:1000, Cat #10037, Cell Signaling Technology, USA), and Syntenin-1 (antirabbit, dilution 1:1000, Cat# 22399-1-AP, Proteintech, China) were observed by Western blotting, which are characteristic surface markers of exosomes. To confirm the absence of contamination from other cellular components, the calnexin (endoplasmic reticulum) (antirabbit, dilution 1:1000, Cat# 72312, Cell Signaling Technology, USA) and GM130 (Golgi apparatus) (antimouse, dilution 1:1000, Cat# sc-55591, Santa Cruz Biotechnology, USA) were detected. The exosomes were stored at − 80 °C.
SAP model establishment and treatment
The SAP model was established by the retrograde injection of Na-taurocholate (Na-T) as previously reported [29]. Briefly, all rats were fasted for at least 12 h and water deprive for at least 4 h before SAP was induced. The rats were anesthetized through 3% pentobarbital, followed by retrograde injection of freshly prepared 3% Na-T (Sigma, USA) into the common biliopancreatic duct (1 mL/kg). All operations were performed under sterile conditions and processes. The rats were randomly divided into five groups as follows (n = 5–8): normal control (NC) group, sham group (direct suture after opening abdominal cavity), SAP + Vehicle group (SAP model with vehicle treatment), SAP + Exo-Ctrl group (SAP model treated with the HUCMSC-derived exosomes), and SAP + Exo-TNF-α group (SAP model treated with TNF-α-preconditioned HUCMSC-derived exosomes). The control rats were injected with an equal volume of normal saline in parallel. Six hours after SAP induction, either phosphate-buffered saline (PBS) or exosome (400 µg) in a volume of 0.2 mL was infused into the caudal vein. Then, after 72 h, rats were euthanized, and the pancreas tissues and blood samples were collected.
Bioactive metabolomics analysis of exosomes
Six groups (Exo-TNF-α groups = 3; Exo-Ctrl groups = 3) of exosomal metabolites were successfully extracted and transferred into the detection bottle for liquid chromatography/mass spectrometry (LC/MS) (Thermo Fisher Scientific, USA). The LC/MS raw data were exported by ProteoWizard software (v3.0.8789). Differentially expressed metabolites were subjected to pathway analysis by MetaboAnalyst, which combines results from powerful pathway enrichment analysis with the pathway topology analysis. The identified metabolites in metabolomics were then mapped to KEGG pathways for biological interpretation of higher-level systemic functions.
Cell viability and cytotoxicity
Cell viability and cytotoxicity were detected using a Cell Counting Kit-8 (CCK-8) assay. Rat pancreatic acinar cell line AR42J cells were seeded into 96‐well plates (1.0 × 104 cells/well) with Na-T (5 μM), followed by the addition of different concentrations of the metabolites. After 24, 48, and 72 h of growth, 10 μL of CCK‐8 reagent (Abcam, USA) was added to each well and incubated for 3 h at 37 °C. Thereafter, we measured the absorbance at 450 nm on a microplate reader (BioTek, Winooski, VT, USA).
Histopathology and the wet-to-dry ratio of pancreatic tissue
The pancreatic and lung tissues were fixed with 4% formaldehyde. The paraffin-embedded tissues were sectioned at 5–6 μm and stained with hematoxylin and eosin (H-E) for histological analysis. The severity of pancreatitis was scored based on edema (0–4), inflammation (0–4), vacuolization (0–4), and necrosis (0–4) as previously described. The severity of pulmonary damage was evaluated by a scale for interstitial and intra-alveolar edema, interstitial and intra-alveolar leukocyte infiltration, and fibrosis, as previously described [30]. For each rat, three independent sections were evaluated by two separate observers in a blinded manner. Moreover, the severity of pancreatic edema was evaluated by detecting the wet-to-dry ratio of pancreatic tissue water content.
Biochemical examination
The serum amylase activity was detected using a kit (BioVision, USA), following the manufacturer’s protocols. The levels of cytokines (TNF-α, IL-1β, IL-6, and IL-10) in the serum and cell culture medium supernatant were assayed using ELISA kits (Multisciences, China). The malondialdehyde (MDA) levels, superoxide dismutase (SOD), and catalase (CAT) activity in the pancreatic tissues were detected using specific kits (Abbkine, China). Intracellular reactive oxygen species (ROS) production was measured using a ROS assay kit (Beyotime, China) according to the manufacturer’s instructions.
Western blot analysis
The tissues or cells were lysed in RIPA lysis buffer containing protease inhibitor (EpiZyme, China), and protein concentrations were quantified by BCA protein assay kit (Thermo Scientific, Rockford, IL, USA). Total proteins from the samples were separated by SDS-PAGE, blotted onto nitrocellulose membranes, and incubated with primary antibodies against mTOR (antirabbit, dilution 1:500, Cat# 2983S, Cell Signaling Technology, USA), Phospho-mTOR (p-mTOR) (antirabbit, dilution 1:500, Cat# 5536S, Cell Signaling Technology, USA), and β-actin (antimouse, dilution 1:5000, Cat# ab8226, Abcam, Cambridge, UK) at 4 °C overnight. After incubation with secondary antibodies, the nitrocellulose membranes were analyzed using Odyssey 3.0 software (LI-COR Biosciences, Lincoln, NE, USA). The experiments were performed repeatedly three times.
TEM
The ultrastructure of acinar cells was observed by TEM (JEM 1230, Tokyo, Japan) as previously described [31].
Immunohistochemistry
Immunostaining was performed on 4–5-μm-thick sections after deparaffinization. Antigen retrieval was performed in citric acid, and then, the 3% hydrogen peroxide was used to inhibit endogenous peroxidase activity. After incubation with the primary antibodies against P62 (antirabbit, dilution 1:150, Cat# 23214, Cell Signaling Technology, USA), Beclin-1 (antirabbit, dilution 1:100, Cat# ab62557, Abcam, Cambridge, UK), and LC3A/B (antirabbit, dilution 1:200, Cat# 12741, Cell Signaling Technology, USA) at 4 °C overnight. The sections were treated with biotin-conjugated rabbit anti-rat secondary antibody, followed by visualization with 3,3′-diaminobenzidine (DAB). Finally, the sections were counterstained with hematoxylin after developing with diaminobenzidine tetrahydrochloride substrate for 10 min. The sections were observed and analyzed using a computer image analysis system (Media Cybernetics, Silver Spring, MD, USA). Each section was examined in at least five random fields.
Statistical analysis
All data were shown as means ± standard deviation (SD). Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by post hoc test with Tukey correction for multiple comparisons. A value of P < 0.05 was deemed to indicate significant differences.
Results
Exosomes from TNF‐α preconditioned HUCMSCs show enhanced therapeutic effects in a rat model of SAP
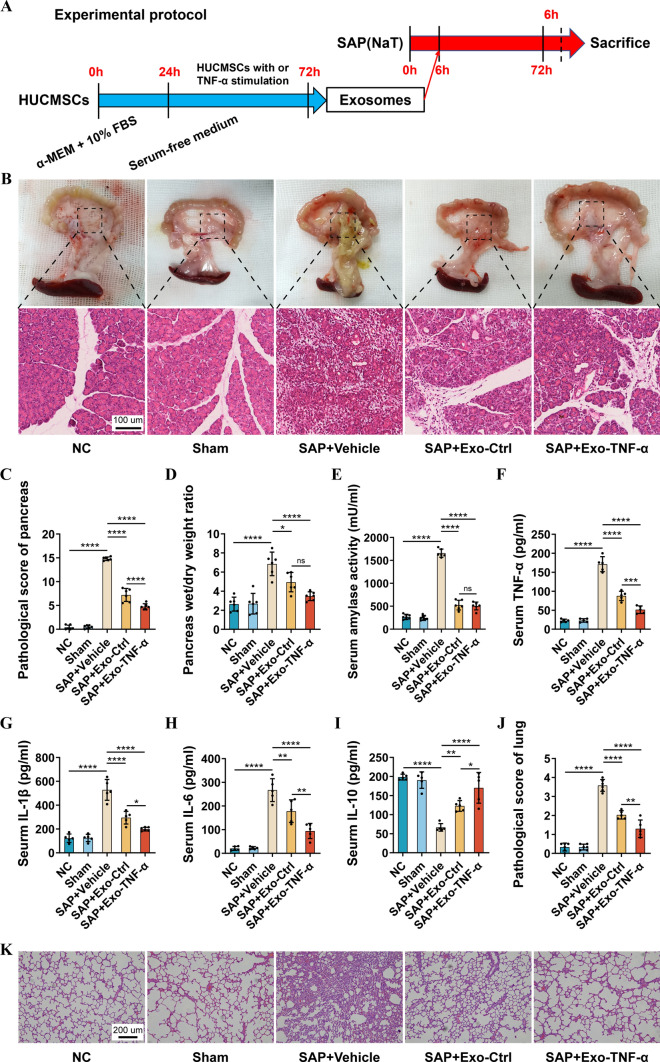
A rat model of SAP was created by retrograde cholangiopancreatic injection of 3% Na-T. To evaluate the role of exosomes from TNF‐α preconditioned HUCMSCs in this model, the exosomes were injected through the caudal vein of the rats within 6–12 h after the successful establishment of the SAP model, and pancreatic tissue and serum were extracted after 72 h (Fig. 1A). The pathological scores of the pancreas, serum amylase activity, and the wet/dry weight ratios of pancreatic tissue were significantly increased after 72 h of induction of SAP (Fig. 1B–D).
Fig. 1.
Exosomes from TNF‐α preconditioned HUCMSCs demonstrate enhanced therapeutic effects in a rat model of SAP. A Experimental protocol. B Histological analysis of pancreas. C Pancreatic histopathological scores. D Wet/dry weight ratio of pancreatic tissue. E Serum amylase levels. F–I Levels of serum inflammatory factors (TNF-α, IL-1β, IL-6, and IL-10) were evaluated using ELISA. J, K Histological analysis of the lung and the histopathological scores. All experiments were independently performed three times. Statistical analysis was performed using one-way ANOVA and post hoc Tukey correction (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n = 5–6)
After the introduction of exosomes from HUCMSCs, the edema and necrosis of pancreatic tissue and the pathological score were reduced (Fig. 1B–D). The level of serum inflammatory factors (TNF-α, IL-1β, IL-6, and IL-10) and amylase was also improved (Fig. 1E–I). Notably, these parameters were more significantly improved in the Exo-TNF-α group compared with that in the Exo-Ctrl group (Fig. 1B–I). Furthermore, the pathological scores related to pulmonary damage in the SAP model were decreased after the exosome treatment (Fig. 1J, K). Notably, these therapeutic effects were more significant in the Exo-TNF-α group compared with that the Exo-Ctrl group (Fig. 1J, K). Thus, we concluded that TNF‐α preconditioned HUCMSCs enhanced the therapeutic effects of exosomes in a rat model of SAP.
Identification of TNF-α preconditioned HUCMSCs-derived exosomes
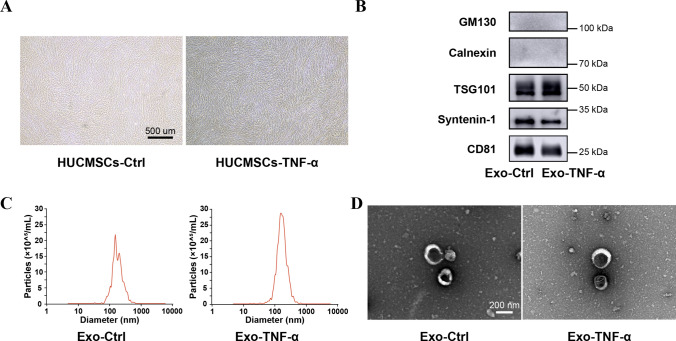
When HUCMSCs were passaged to the fifth generation (P5), the cells were still of uniform size, had fibroblast-like shapes, and formed spiral-like colonies (Fig. 2A). Exosomes were isolated and identified from the supernatants of the culture medium of TNF-α preconditioned HUCMSCs and un-preconditioned HUCMSCs (as control). Measurement of the particle size of the exosomes by NTA indicated that the average diameter of the exosomes in the two groups was 153.1 nm and 159.1 nm, respectively (Fig. 2C), which is consistent with the range of exosome particle sizes. Western blotting revealed that they all expressed TSG101, CD81, and Syntenin-1, and both exosomes were absence of contamination from other cellular components by observing calnexin (endoplasmic reticulum) and GM130 (Golgi apparatus) (Fig. 2B). The typical exosome shape was observed by TEM (Fig. 2D). There were no morphological differences in size, shape, or electron density between the two groups. These results verified that the vesicles isolated from HUCMSCs possess exosome phenotypes.
Fig. 2.
Identification of preconditioned HUCMSC-derived exosomes. A Spindle-shaped HUCMSCs form spiral-like colonies on plastic culture dishes. B The surface markers of exosomes detected using western blotting. C The particle size of the exosome measured using NTA. D The typical exosome shape detected using TEM
Identification of the TNF-α-induced HUCMSC-derived exosomal metabolites
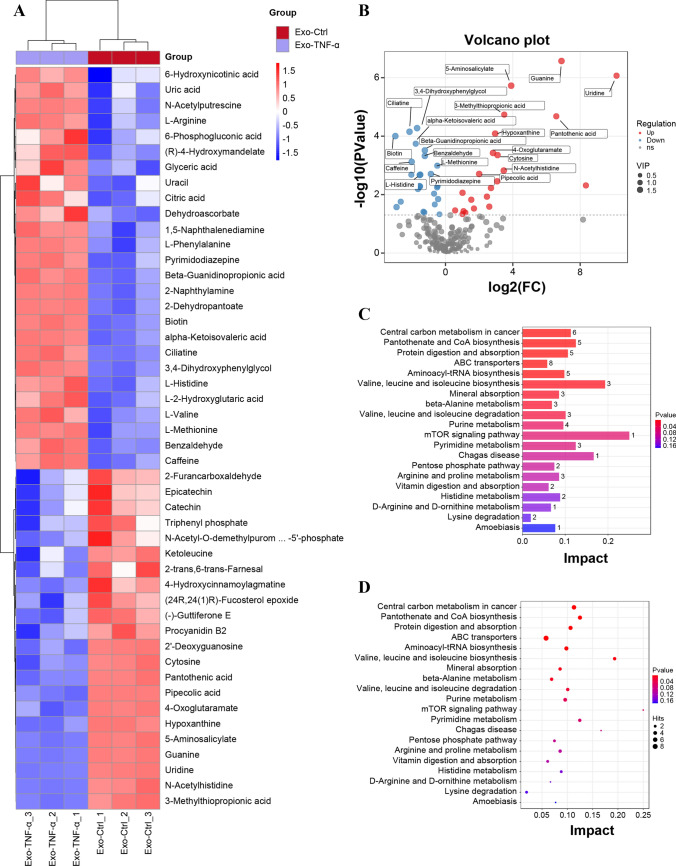
To evaluate the therapeutic effects of the main functional components of the exosomes, we analyzed changes in the metabolite contents of exosomes by bioactive metabolomics analysis. The clustering heatmap showed that TNF-α pre-treatment significantly influenced HUCMSC-derived exosomal metabolite profile, 48 metabolites were found to be significantly differentially expressed between the two groups (Fig. 3A). Among these, 26 and 22 metabolites were up- and down-regulated, respectively, in the Exo-TNF-α group compared with that in the Exo-Ctrl group. The volcano plot in (Fig. 3B) shows the top 10 differential metabolites in the exosomes (Exo-Ctrl group versus Exo-TNF-α group). The differentially expressed metabolites were subjected to GO term and KEGG pathway enrichment analyses to reveal their biological functions. The exosomal metabolites expressed differentially in the Exo-TNF-α group were involved in multiple processes, including carbon metabolism, synthesis and degradation of organic acids, metabolism of amino acids, glucose metabolism and coenzyme A biosynthesis, as well as carbon metabolism and mTOR signaling pathway (Fig. 3C, D). These results indicate that the bioactive metabolites are closely related to acinar cell injury. To further reveal the function of these metabolites in acinar cell injury, the top five differentially expressed metabolites were screened for further functional verification according to the mass spectrometry signal intensity, differential multiple, and structural stability (Table 1).
Fig. 3.
Identification of TNF-α inducing HUCMSC-derived exosomal metabolites. A Heatmap analysis of exosome metabolites in the Exo-TNF-α and Exo-Ctrl groups (n = 3). B Volcano plot showing the differentially expressed metabolites between the Exo-TNF-α and Exo-Ctrl groups. C, D GO term and KEGG pathway analyses of differentially expressed exosomal metabolites between the Exo-TNF-α and Exo-Ctrl groups
Table 1.
Five candidate differential metabolites for exosomes
| Name | MZ | RT(s) | FC (Exo-TNF-α/Exo-Ctrl) | P value | VIP |
|---|---|---|---|---|---|
| Ciliatine | 125.986 | 399.6 | 4.411957172 | 7.05384E−05 | 1.757684463 |
| 3,4-Dihydroxyphenylglycol | 170.0599 | 398 | 3.225708623 | 5.3144E−05 | 1.758781253 |
| Alpha-ketoisovaleric acid | 116.0492 | 363.6 | 3.417089404 | 0.00018134 | 1.504013344 |
| Beta-guanidinopropionic acid | 132.0806 | 398.4 | 2.343048282 | 0.000307048 | 1.743290268 |
| L-2-Hydroxyglutaric acid | 146.9658 | 472.2 | 1.573098792 | 0.003573849 | 1.44229369 |
Identification of the therapeutic effect of exosome-derived metabolites
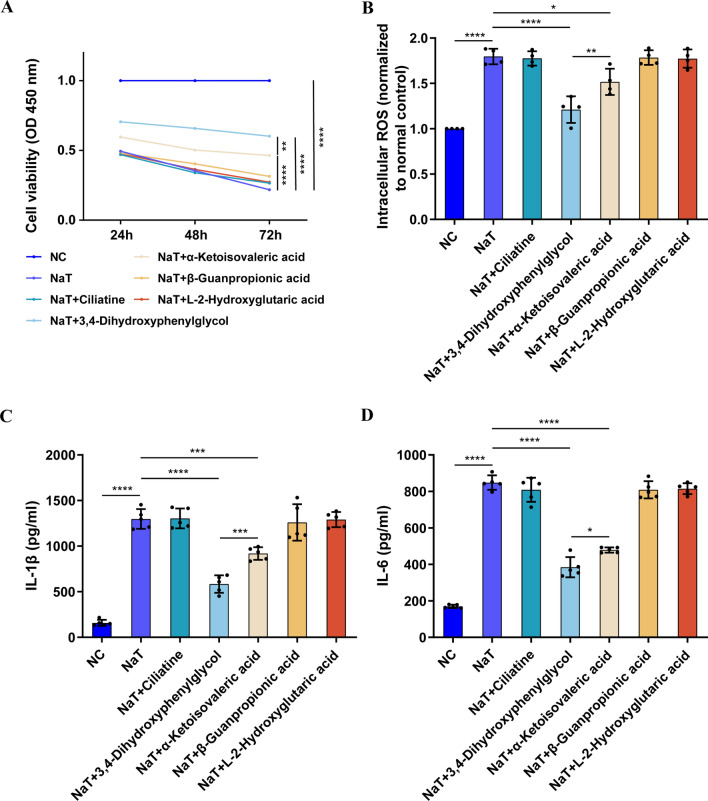
We evaluated the biological functions of five chemically synthesized metabolites in rat pancreatic acinar cell line AR42J cells. The AR42J cells were stimulated using Na-T (5 µM), followed by the addition of the metabolites to the culture supernatant, and subsequent analysis of the cell viability, inflammatory factor release, and intracellular ROS production. The results indicated that the cell viability was inhibited and inflammation and oxidative stress injury were observed under Na-T induction (Fig. 4A–D). Notably, 3,4-Dihydroxyphenylglycol (40 µM) and alpha-Ketoisovaleric acid (40 µM) could improve the viability of the cells and reduce inflammation and oxidative stress injury, while the other metabolites did not show effective protective effects. Furthermore, 3,4-Dihydroxyphenylglycol showed a more significant anti-oxidative stress effect compared with alpha-Ketoisovaleric acid (Fig. 4A–D).
Fig. 4.
Identification of the therapeutic effect of exosome-derived metabolites. A Cell viability detected by CCK-8 assay. B Intracellular ROS levels based on 2ʹ, 7ʹ-dichlorofluorescein-diacetate (DCFH-DA) fluorescence. C, D Levels of inflammatory factors (IL-1β and IL-6) in cell culture supernatant evaluated by ELISA. All experiments were independently performed three times. Statistical analysis was performed using one-way ANOVA and post hoc Tukey correction (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n = 4–5)
3,4-Dihydroxyphenylglycol, also known as DHPG or DOPEG, belongs to the class of organic compounds known as catechols. Looking up the human metabolome database (HMDB) (https://hmdb.ca/), we found that 3,4-Dihydroxyphenylglycol has a stable structure, good hydrophilicity, and easy dissolvability (Supplement material 1). Therefore, we further explored the therapeutic effect of 3,4-Dihydroxyphenylglycol in SAP.
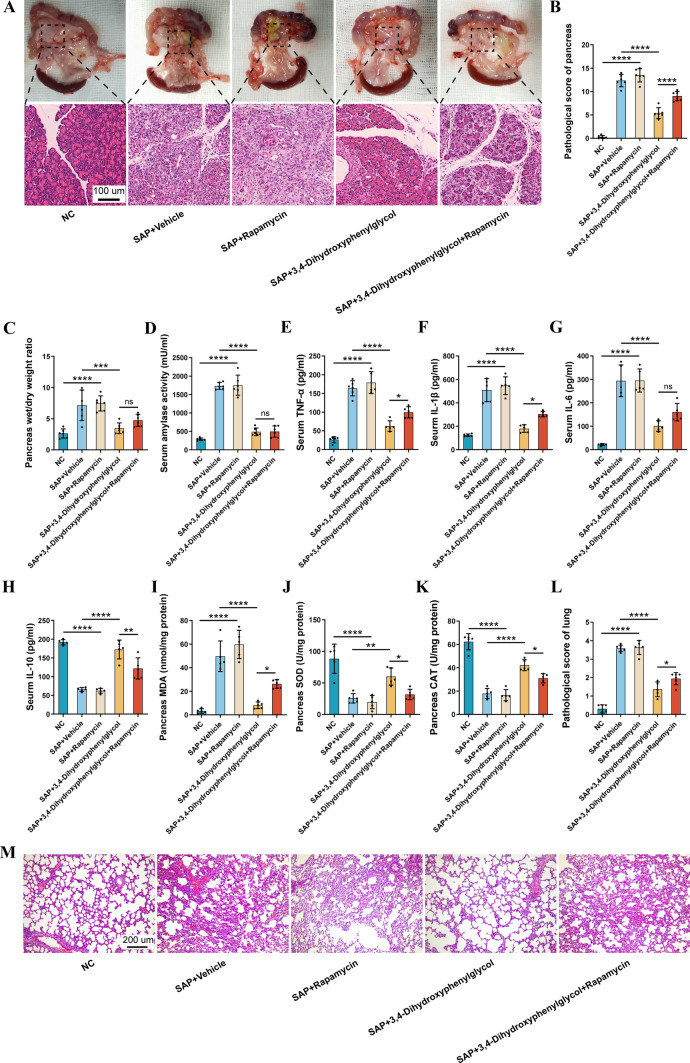
Therapeutic effect of 3,4-Dihydroxyphenylglycol in SAP
The SAP model was created by retrograde cholangiopancreatic injection of 3% Na-T. After the successful establishment of the model, 3,4-Dihydroxyphenylglycol (2 mg/kg) was injected through the caudal vein within 6–12 h, and pancreatic tissue and serum were extracted after 72 h. The results showed that 3,4-Dihydroxyphenylglycol reduced the edema and necrosis of pancreatic tissue, pathological score, and serum amylase activity (Fig. 5A–D), and improved the levels of serum inflammatory factors (TNF-α, IL-1β, IL-6, and IL-10) (Fig. 5E–H). Moreover, the levels of MDA were decreased and CAT activity and SOD activity were increased after treatment with 3,4-Dihydroxyphenylglycol (Fig. 5I–K). In addition, SAP-induced pulmonary damage was decreased by 3,4-Dihydroxyphenylglycol (Fig. 5L, M).
Fig. 5.
The therapeutic effect of 3,4-Dihydroxyphenylglycol in SAP. A Histological analysis of pancreas. B Pancreatic histopathological scores. C Wet/dry weight ratio of pancreatic tissue. D Serum amylase levels. E–H Serum levels of inflammatory factors (TNF- α, IL-1β, IL-6, and IL-10) evaluated using ELISA. I MDA levels in the pancreas. J SOD activity in the pancreas. K CAT activity in the pancreas. L Histological analysis of the lung. M Histopathological scores of the lung. All experiments were independently performed three times. Statistical analysis was performed using one-way ANOVA and post hoc Tukey correction (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n = 5–6)
In summary, our data showed that 3,4-Dihydroxyphenylglycol could significantly reduce inflammation and oxidative stress in damaged pancreatic tissue, and decrease pulmonary damage.
3,4-Dihydroxyphenylglycol regulates the autophagy of acinar cells via the mTOR signaling pathway
The ultrastructure of acinar cells was observed by TEM, and the number of autophagosomes was found to be significantly increased in the SAP + Vehicle groups, while administration of 3,4-dihydroxyphenylglycol reduced the number of autophagosomes (Fig. 6A, E). To evaluate the effect of 3,4-Dihydroxyphenylglycol in SAP, we observed the expression of P62/SQSTM1, Beclin-1, and LC3B in the pancreatic tissue, as these molecules play key roles in the development of autophagy. Immunohistochemistry analysis revealed that administration of 3,4-Dihydroxyphenylglycol could decrease the expression of Beclin-1 and LC3 and increase the expression of P62, while the opposite pattern was observed in the SAP + Vehicle groups (Fig. 6B–D, F–H). These results indicated that 3,4-Dihydroxyphenylglycol could inhibit the autophagy of acinar cells in SAP.
Fig. 6.
3,4-Dihydroxyphenylglycol regulates autophagy of acinar cells via the mTOR signaling pathway. A The percentage of autophagic vacuoles per cytoplasmic area. B–D Immunohistochemical analysis of the proportion of tissue positively stained for P62, Beclin-1, and LC3. E Characteristic images of autophagosomes were detected using TEM in the pancreas (scale bar 2 μm); red arrows indicate the autophagosomes. F–H Immunohistochemical staining of pancreatic LC3, Beclin-1, and P62 protein. I–K Western blot analysis of mTOR and Phospho-mTOR protein levels in the pancreatic tissue. All experiments were independently performed three times. Statistical analysis was performed using one-way ANOVA and post hoc Tukey correction (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n = 4–6)
The mTOR pathway has been shown to play a key role in the regulation of autophagy [32]. Furthermore, in our study, the differentially expressed exosomal metabolites were subjected to GO term and KEGG pathway enrichment analyses, which suggested that the mTOR signaling pathway was regulated (Fig. 3C, D). Therefore, we examined whether 3,4-Dihydroxyphenylglycol could attenuate pancreatic damage by regulating the mTOR pathway. Upon administration of the mTOR inhibitor rapamycin (2 mg/kg), the protective effect of 3,4-Dihydroxyphenylglycol was significantly inhibited (Fig. 5A–M). Moreover, the suppression of autophagy by 3,4-dihydroxyphenylglycol was partially neutralized by rapamycin, suggesting a key role of the mTOR signaling pathway in the therapeutic effect of 3,4-Dihydroxyphenylglycol in SAP (Fig. 6A–H). Finally, we observed that 3,4-Dihydroxyphenylglycol increased the level of p-mTOR, while this was significantly reduced in the SAP + Vehicle groups (Fig. 6I–K).
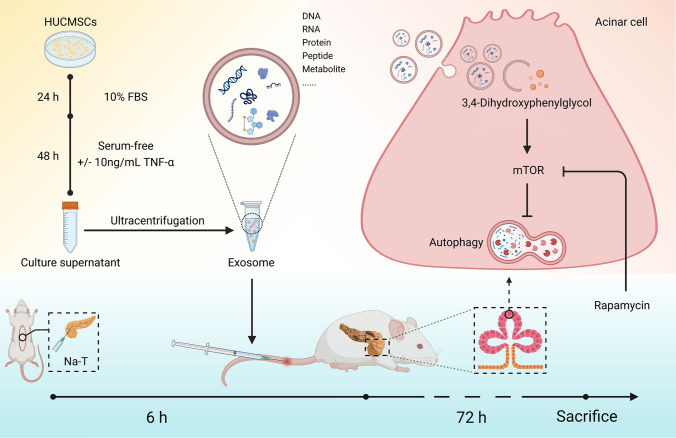
Taken together, we concluded that 3,4-Dihydroxyphenylglycol attenuates SAP by inhibiting the autophagy of acinar cells via regulation of the mTOR signaling pathway (Fig. 7).
Fig. 7.
Proposed therapeutic strategy for SAP by application of exosomes derived from TNF-α-preconditioned HUCMSCs
Discussion
SAP is a common critical digestive disease, with high mortality and a lack of effective prevention and treatment measures [3, 33]. Although a variety of clinical trials and cohort studies have explored this issue, no effective drugs and standard care have been identified to improve the outcomes of SAP [4]. Recently, several studies have revealed that MSCs transplantation has the potential to treat SAP [8, 9]. Although MSCs have good therapeutic effects, there are also several limitations, including difficulty in generating a consistent source of cells with a stable phenotype and maintenance of biological activity, infusion toxicities caused by large cells physically trapped in the lung microvasculature, ectopic tissue formation, tumorigenicity, quantification of bioactive substances, and the logistics of delivery [9, 12]. These limitations might hinder the clinical application of MSCs for SAP. Some previous studies have revealed that most MSC-based treatment effects can be attributed to the MSC-sourced secretomes, which consist of a soluble component and encapsulated microvesicles and exosomes [34]. Notably, some preclinical data have shown that MSC-derived exosome therapeutics might be superior to cell-based therapy in terms of safety and versatility [35–37]. The application of exosomes may present considerable advantages over their cellular counterparts owing to a higher safety profile, lower immunogenicity, the inability to directly form tumors, and the potential to migrate efficiently to the target organ after administration without getting trapped in the lung microvasculature [12, 38, 39]. In recent years, MSCs-derived exosomes have demonstrated significant therapeutic potential in numerous diseases [10]. Furthermore, some studies have also shown that exosomes could alleviate AP [18, 19]. In this study, we have also indicated that MSC-derived exosomes could significantly alleviate SAP. Thus, exosomes will have a good application prospect in SAP.
Previous Liu et al. study revealed that MSCs have short-term memory, which can affect their therapeutic potential [40]. Several recent studies have demonstrated that different conditioned culture conditions or additional treatments might regulate or enhance the biological functions of MSCs [13, 14, 41]. However, this mechanism has not been clarified. That could be because the biomolecule expression profiles contained in exosomes are affected by pre-treatment regimens. Ma et al. successfully constructed an experimental model of pre-treating MSCs with TNF-α and then extracted small RNAs from the exosomes derived from these MSCs. Subsequently, high-throughput sequencing revealed 180 differentially expressed microRNAs in the exosome compartment (TNF-α pre-treatment vs. normal control) [14]. Recently a study found that TNF-α preconditioning enhanced the neuroprotective effects of GMSC-derived exosomes in retinal ischemia–reperfusion injury, and evaluation of the exosomal miRNA expression found that miR-21-5p levels were markedly elevated after stimulation of the GMSCs with TNF-α. The study further indicated that the MEG3/miR-21-5p/PDCD4 axis could play a key role during exosome therapy [42]. Moreover, Ti et al. found that LPS-preconditioned MSC-derived exosomes improved regulatory abilities for macrophage polarization and resolution of chronic inflammation by shuttling let-7b microRNA [15]. Interestingly, a recent study reported that exosomes derived from LPS-preconditioned thymic MSCs promoted the polarization of macrophages to M1-like phenotype, IL-6 and TNF-α secretion, and the pro-inflammatory differentiation of CD4+ T cells into Th17 cells, which enhanced inflammation [43]. Furthermore, modulations of cell activity with cytokine regulation have shown to also be efficient in the treatment of other diseases such as cancer, HIV, and Leishmaniasis [44, 45]. The data in our study also demonstrated that the exosomes from TNF-α-preconditioned MSCs could significantly enhance the therapeutic effects of the exosomes in a rat model of SAP. Taken together, the findings of these studies indicate that MSCs are activated by inflammatory cytokines present within the inflammatory microenvironment, enabling them to affect target cells via exosomes.
MSC-derived exosomes are capable of intercellular communication and carry proteins, mRNA, microRNA, enzymes, cytokines, chemokines, and immunomodulatory and growth factors into the targeted cells [22, 46–50]. Recent study reveals that exosomes promote tumor progression and chemoresistance by delivering cargoes [51–53]. Moreover, levels of biomolecules within exosomes have also been shown to be useful diagnostic and prognostic biomarkers in various diseases [54, 55]. In our study, bioactive metabolomics analysis revealed that 48 metabolites were found to be significantly differentially expressed between the two groups (Exo-Ctrl group versus Exo-TNF-α group). We screened the top five differentially expressed metabolites for further functional verification according to the mass spectrometry signal intensity, differential multiple, and structural stability. The animal experiments found that 3,4-Dihydroxyphenylglycol reduced inflammation and oxidative stress in a rat model of SAP. This might be a key molecule mediating the therapeutic effect of TNF-α-preconditioned MSCs. Therefore, this study provided a direction for future study on the exosome shuttling bioactive metabolites to therapy various diseases.
Notably, 3,4-dihydroxyphenylglycol is also known as an endogenous metabolite of dopamine and possesses specific biological properties [56, 57]. 3,4-Dihydroxyphenylglycol is bioavailable and has antioxidant properties [58, 59]. Previously, Hashimoto et al. revealed that 3,4-dihydroxyphenylethanol has a protective role against oxidative stress-induced cell damage in dopaminergic neurons [60]. Bermúdez-Oria et al. indicated that bioactive 3,4-dihydroxyphenylglycol could significantly affect intestinal health and prevent or improve IBD [59]. Fernández-Prior et al. also showed that 3,4-dyhydroxyphenylglycol has a high potential as an antioxidant and anti-inflammatory agent [61]. Recently a study reported that infusion of 3,4-dihydroxyphenylglycol into a rat model of type 1 diabetes exerted a neuroprotective effect on the brain slices subjected to hypoxia–reoxygenation and on the retina [62]. Here, our study demonstrated that 3,4-dihydroxyphenylglycol could decrease inflammation and oxidative stress in the damaged pancreas and inhibit acinar cell autophagy in SAP.
Autophagy is a lysosomal degradation pathway that plays a key role in survival, differentiation, development, and homeostasis [63]. Remarkably, AP has been associated with autophagy [64–66]. Autophagy induces devastating effects in pancreatic acinar cells via activating trypsinogen to trypsin in the early stage of AP by promoting the delivery of trypsinogen to the lysosome [67]. Autophagy also plays a key role in the progress of AP, and the overactivation of autophagy could aggravate the signs of SAP [68, 69]. Yang et al. found that the severity of SAP could be reduced by inhibiting autophagy [70]. Our previous study indicated that infusion of MSCs could reduce SAP-induced multiple organ damage via inhibiting autophagy [31]. The atypical serine/threonine kinase mTOR is a master regulator of cell growth and metabolism; it promotes anabolic processes such as ribosome biogenesis and protein, nucleotide, fatty acid, and lipid synthesis and inhibits catabolic processes such as autophagy [71, 72]. mTOR kinase blocks autophagy by directly inhibiting the early steps of the process and regulates the lysosomal degradation ability of cells by inhibiting the transactivation of genes encoding structure, and regulatory and catalytic factors [32]. Previous studies have shown that pancreatic fibrosis was decreased via reducing autophagy of pancreatic stellate cells by activating the mTOR signaling pathway in chronic pancreatitis [73, 74]. Recently, Liu et al. reported that pancreatic injury and autophagy of acinar cells could be alleviated by activating the mTOR signaling pathway in the SAP model [75]. Furthermore, our previous studies have also shown that MSCs alleviate SAP by inhibiting autophagy via regulating the mTOR pathway [31, 76]. In our current study, we have revealed that bioactive metabolite 3,4-dihydroxyphenylglycol could alleviate SAP by inhibiting the autophagy of acinar cells via regulating the mTOR signaling pathway. However, the underlying mechanism of this regulation has not been elucidated. Therefore, future studies should investigate the cross talk between mTOR and 3,4-dihydroxyphenylglycol.
In summary, our study demonstrated that the exosomes derived from TNF-α-preconditioned MSCs can significantly alleviate SAP and that 3,4-dihydroxyphenylglycol might be the key molecule mediating this therapeutic effect. Furthermore, we confirmed that 3,4-dihydroxyphenylglycol alleviated SAP by inhibiting the autophagy of acinar cells via regulating the mTOR signaling pathway, which provides a new promising approach to therapy SAP.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Prof. Xingyun Wang from Shanghai Jiao Tong University School of Medicine for his support and guidance on Metabonomic analysis.
Abbreviations
- SAP
Severe acute pancreatitis
- AP
Pancreatitis
- HUCMSCs
Human umbilical cord mesenchymal stromal cells
- MDT
Multidisciplinary team
- MSCs
Mesenchymal stromal cells
- EVs
Extracellular vesicles
- FBS
Fetal bovine serum
- TEM
Transmission electron microscopy
- NTA
Nanoparticle tracking analysis
- ROS
Reactive oxygen species
- MDA
Malondialdehyde
- SOD
Superoxide dismutase
- CAT
Catalase
Author contributions
All authors contributed to the study conception and design. ZM, WX, TL, ZH, and JH performed the experiments and participated in drafting the manuscript. XY, JZ, WW, TY, and ZS performed conception design, data collection, and analysis. SS and JX performed study design, data interpretation, and supervised the review. All authors have read and approved the article.
Funding
This work was supported by the National Natural Science Foundation of China (82200717 and 81670582).
Availability of data and materials
The data generated in this study are available upon request from the corresponding author.
Declarations
Ethics approval
The animal study was approved by the Ethics Committee of Shanghai Tenth People’s Hospital, Tongji University School of Medicine (SHDSYY-2022-2330).
Consent for publication
All authors have read and approved of its submission to this journal.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhilong Ma and Wangcheng Xie contributed equally to this work.
Contributor Information
Jin Xu, Email: xujin@fudanpci.org.
Si Shi, Email: shisi@fudanpci.org.
References
- 1.Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175–184. doi: 10.1038/s41575-018-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, Petrov MS. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45–55. doi: 10.1016/S2468-1253(16)30004-8. [DOI] [PubMed] [Google Scholar]
- 3.Leppaniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, Ball CG, Parry N, Sartelli M, Wolbrink D, van Goor H, Baiocchi G, Ansaloni L, Biffl W, Coccolini F, Di Saverio S, Kluger Y, Moore E, Catena F. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27. doi: 10.1186/s13017-019-0247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726–734. doi: 10.1016/S0140-6736(20)31310-6. [DOI] [PubMed] [Google Scholar]
- 5.Baron TH, DiMaio CJ, Wang AY, Morgan KA. American Gastroenterological Association clinical practice update: management of pancreatic necrosis. Gastroenterology. 2020;158(67–75):e1. doi: 10.1053/j.gastro.2019.07.064. [DOI] [PubMed] [Google Scholar]
- 6.Galipeau J, Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, Nolta J, Phinney DG, Sensebe L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT(R)) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21:1019–1024. doi: 10.1016/j.jcyt.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Jung KH, Song SU, Yi T, Jeon MS, Hong SW, Zheng HM, Lee HS, Choi MJ, Lee DH, Hong SS. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011;140:998. doi: 10.1053/j.gastro.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 9.Ma Z, Zhou J, Yang T, Xie W, Song G, Song Z, Chen J. Mesenchymal stromal cell therapy for pancreatitis: progress and challenges. Med Res Rev. 2021;41:2474–2488. doi: 10.1002/med.21801. [DOI] [PubMed] [Google Scholar]
- 10.Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ha DH, Kim HK, Lee J, Kwon HH, Park GH, Yang SH, Jung JY, Choi H, Lee JH, Sung S, Yi YW, Cho BS. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells. 2020;9:1157. doi: 10.3390/cells9051157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transpl. 2019;54:789–792. doi: 10.1038/s41409-019-0616-z. [DOI] [PubMed] [Google Scholar]
- 13.Liang YC, Wu YP, Li XD, Chen SH, Ye XJ, Xue XY, Xu N. TNF-alpha-induced exosomal miR-146a mediates mesenchymal stem cell-dependent suppression of urethral stricture. J Cell Physiol. 2019;234:23243–23255. doi: 10.1002/jcp.28891. [DOI] [PubMed] [Google Scholar]
- 14.Ma H, Zhang S, Xu Y, Zhang R, Zhang X. Analysis of differentially expressed microRNA of TNF-alpha-stimulated mesenchymal stem cells and exosomes from their culture supernatant. Arch Med Sci. 2018;14:1102–1111. doi: 10.5114/aoms.2017.70878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, Zhao Y, Liu H, Fu X, Han W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su W, Wan Q, Huang J, Han L, Chen X, Chen G, Olsen N, Zheng SG, Liang D. Culture medium from TNF-alpha-stimulated mesenchymal stem cells attenuates allergic conjunctivitis through multiple antiallergic mechanisms. J Allergy Clin Immunol. 2015;136(423–32):e8. doi: 10.1016/j.jaci.2014.12.1926. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, Jiang L, Hu H, Wang H, Wang X, Jiang J, Ma Y, Yang J, Hou Y, Xie D, Zhang Q. Pretreatment of exosomes derived from hUCMSCs with TNF-alpha ameliorates acute liver failure by inhibiting the activation of NLRP3 in macrophage. Life Sci. 2020;246:117401. doi: 10.1016/j.lfs.2020.117401. [DOI] [PubMed] [Google Scholar]
- 18.Yin G, Hu G, Wan R, Yu G, Cang X, Xiong J, Ni J, Hu Y, Xing M, Fan Y, Xiao W, Qiu L, Tang M, Zhao Y, Wang S, Wang X. Role of microvesicles from bone marrow mesenchymal stem cells in acute pancreatitis. Pancreas. 2016;45:1282–1293. doi: 10.1097/MPA.0000000000000694. [DOI] [PubMed] [Google Scholar]
- 19.Wang N, Ma J, Ren Y, Xiang S, Jia R. Secreted klotho from exosomes alleviates inflammation and apoptosis in acute pancreatitis. Am J Transl Res. 2019;11:3375–3383. [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Wang Y, Kong J, Dong M, Duan H, Chen S. Therapeutic efficacy of neural stem cells originating from umbilical cord-derived mesenchymal stem cells in diabetic retinopathy. Sci Rep. 2017;7:408. doi: 10.1038/s41598-017-00298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Jia H, Zhang B, Yin L, Mao F, Yu J, Ji C, Xu X, Yan Y, Xu W, Qian H. HucMSC exosome-transported 14-3-3zeta prevents the injury of cisplatin to HK-2 cells by inducing autophagy in vitro. Cytotherapy. 2018;20:29–44. doi: 10.1016/j.jcyt.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 2019;8:1605. doi: 10.3390/cells8121605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piazza I, Kochanowski K, Cappelletti V, Fuhrer T, Noor E, Sauer U, Picotti P. A map of protein-metabolite interactions reveals principles of chemical communication. Cell. 2018;172(358–372):e23. doi: 10.1016/j.cell.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Husted AS, Trauelsen M, Rudenko O, Hjorth SA, Schwartz TW. GPCR-mediated signaling of metabolites. Cell Metab. 2017;25:777–796. doi: 10.1016/j.cmet.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Cheng J, Li W, Kang B, Zhou Y, Song J, Dan S, Yang Y, Zhang X, Li J, Yin S, Cao H, Yao H, Zhu C, Yi W, Zhao Q, Xu X, Zheng M, Zheng S, Li L, Shen B, Wang YJ. Tryptophan derivatives regulate the transcription of Oct4 in stem-like cancer cells. Nat Commun. 2015;6:7209. doi: 10.1038/ncomms8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumura T, Uryu O, Matsuhisa F, Tajiri K, Matsumoto H, Hayakawa Y. N-acetyl-l-tyrosine is an intrinsic triggering factor of mitohormesis in stressed animals. EMBO Rep. 2020;21:e49211. doi: 10.15252/embr.201949211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hua J, He ZG, Qian DH, Lin SP, Gong J, Meng HB, Yang TS, Sun W, Xu B, Zhou B, Song ZS. Angiopoietin-1 gene-modified human mesenchymal stem cells promote angiogenesis and reduce acute pancreatitis in rats. Int J Clin Exp Pathol. 2014;7:3580–3595. [PMC free article] [PubMed] [Google Scholar]
- 28.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham CB, Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borras FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Gorecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzas EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, de Datta Chaudhuri A, Candia PD, De Santana EF, Wever OD, Portillo HA, Demaret T, Deville S, Devitt A, Dhondt BD, Vizio D, Dieterich LC, Dolo VD, Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekstrom KE, Andaloussi S, Elie-Caille C, Erdbrugger U, Falcon-Perez JM, Fatima F, Fish JE, Flores-Bellver M, Forsonits A-B, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicl. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Z, Song G, Zhao D, Liu D, Liu X, Dai Y, He Z, Qian D, Gong J, Meng H, Zhou BO, Yang T, Song Z. Bone marrow-derived mesenchymal stromal cells ameliorate severe acute pancreatitis in rats via hemeoxygenase-1-mediated anti-oxidant and anti-inflammatory effects. Cytotherapy. 2018;21(2):162–174. doi: 10.1016/j.jcyt.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Yang HH, Jiang HL, Tao JH, Zhang CY, Xiong JB, Yang JT, Liu YB, Zhong WJ, Guan XX, Duan JX, Zhang YF, Liu SK, Jiang JX, Zhou Y, Guan CX. Mitochondrial citrate accumulation drives alveolar epithelial cell necroptosis in lipopolysaccharide-induced acute lung injury. Exp Mol Med. 2022;54(11):2077–2091. doi: 10.1038/s12276-022-00889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song G, Liu D, Geng X, Ma Z, Wang Y, Xie W, Qian D, Meng H, Zhou B, Song Z. Bone marrow-derived mesenchymal stem cells alleviate severe acute pancreatitis-induced multiple-organ injury in rats via suppression of autophagy. Exp Cell Res. 2019;385:111674. doi: 10.1016/j.yexcr.2019.111674. [DOI] [PubMed] [Google Scholar]
- 32.Deleyto-Seldas N, Efeyan A. The mTOR-autophagy axis and the control of metabolism. Front Cell Dev Biol. 2021;9:655731. doi: 10.3389/fcell.2021.655731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hines OJ, Pandol SJ. Management of severe acute pancreatitis. BMJ. 2019;367:l6227. doi: 10.1136/bmj.l6227. [DOI] [PubMed] [Google Scholar]
- 34.Harrell CR, Jankovic MG, Fellabaum C, Volarevic A, Djonov V, Arsenijevic A, Volarevic V. Molecular mechanisms responsible for anti-inflammatory and immunosuppressive effects of mesenchymal stem cell-derived factors. Adv Exp Med Biol. 2019;1084:187–206. doi: 10.1007/5584_2018_306. [DOI] [PubMed] [Google Scholar]
- 35.Bagno L, Hatzistergos KE, Balkan W, Hare JM. Mesenchymal stem cell-based therapy for cardiovascular disease: progress and challenges. Mol Ther. 2018;26:1610–1623. doi: 10.1016/j.ymthe.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18:1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu P, Zhang B, Shi H, Qian H, Xu W. MSC-exosome: a novel cell-free therapy for cutaneous regeneration. Cytotherapy. 2018;20:291–301. doi: 10.1016/j.jcyt.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Liew LC, Katsuda T, Gailhouste L, Nakagama H, Ochiya T. Mesenchymal stem cell-derived extracellular vesicles: a glimmer of hope in treating Alzheimer's disease. Int Immunol. 2017;29:11–19. doi: 10.1093/intimm/dxx002. [DOI] [PubMed] [Google Scholar]
- 39.Borger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, Giebel B. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci. 2017;18:1450. doi: 10.3390/ijms18071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu GY, Liu Y, Lu Y, Qin YR, Di GH, Lei YH, Liu HX, Li YQ, Wu C, Hu XW, Duan HF. Short-term memory of danger signals or environmental stimuli in mesenchymal stem cells: implications for therapeutic potential. Cell Mol Immunol. 2016;13:369–378. doi: 10.1038/cmi.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mead B, Chamling X, Zack DJ, Ahmed Z, Tomarev S. TNFalpha-mediated priming of mesenchymal stem cells enhances their neuroprotective effect on retinal ganglion cells. Invest Ophthalmol Vis Sci. 2020;61:6. doi: 10.1167/iovs.61.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Z, Wen Y, Jiang N, Li Z, Guan J, Zhang Y, Deng C, Zhao L, Zheng SG, Zhu Y, Su W, Zhuo Y. TNF-alpha stimulation enhances the neuroprotective effects of gingival MSCs derived exosomes in retinal ischemia-reperfusion injury via the MEG3/miR-21a-5p axis. Biomaterials. 2022;284:121484. doi: 10.1016/j.biomaterials.2022.121484. [DOI] [PubMed] [Google Scholar]
- 43.Li Q, Li J, Sun L, Sun Y, Zhao F, Liu P, Peng X, Xuan X, Li Y, Wang P, Tan C, Du Y. Exosomes derived from LPS-stimulated human thymic mesenchymal stromal cells enhance inflammation via thrombospondin-1. Biosci Rep. 2021;41:BSR20203573. doi: 10.1042/BSR20203573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Josephs SF, Ichim TE, Prince SM, Kesari S, Marincola FM, Escobedo AR, Jafri A. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. J Transl Med. 2018;16:242. doi: 10.1186/s12967-018-1611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maksoud S, El Hokayem J. The cytokine/chemokine response in Leishmania/HIV infection and co-infection. Heliyon. 2023;9:e15055. doi: 10.1016/j.heliyon.2023.e15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heldring N, Mager I, Wood MJ, Le Blanc K, Andaloussi SE. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum Gene Ther. 2015;26:506–517. doi: 10.1089/hum.2015.072. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Morelli MB, Matarese A, Sardu C, Santulli G. Cardiomyocyte-derived exosomal microRNA-92a mediates post-ischemic myofibroblast activation both in vitro and ex vivo. ESC Heart Fail. 2020;7:284–288. doi: 10.1002/ehf2.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morelli MB, Shu J, Sardu C, Matarese A, Santulli G. Cardiosomal microRNAs are essential in post-infarction myofibroblast phenoconversion. Int J Mol Sci. 2019;21:201. doi: 10.3390/ijms21010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sukma Dewi I, Celik S, Karlsson A, Hollander Z, Lam K, McManus JW, Tebbutt S, Ng R, Keown P, McMaster R, McManus B, Ohman J, Gidlof O. Exosomal miR-142-3p is increased during cardiac allograft rejection and augments vascular permeability through down-regulation of endothelial RAB11FIP2 expression. Cardiovasc Res. 2017;113:440–452. doi: 10.1093/cvr/cvw244. [DOI] [PubMed] [Google Scholar]
- 50.Zheng B, Yin WN, Suzuki T, Zhang XH, Zhang Y, Song LL, Jin LS, Zhan H, Zhang H, Li JS, Wen JK. Exosome-mediated miR-155 transfer from smooth muscle cells to endothelial cells induces endothelial injury and promotes atherosclerosis. Mol Ther. 2017;25:1279–1294. doi: 10.1016/j.ymthe.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Mills J, Capece M, Cocucci E, Tessari A, Palmieri D. Cancer-derived extracellular vesicle-associated MicroRNAs in intercellular communication: one cell's trash is another cell's treasure. Int J Mol Sci. 2019;20:6109. doi: 10.3390/ijms20246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chuang HY, Su YK, Liu HW, Chen CH, Chiu SC, Cho DY, Lin SZ, Chen YS, Lin CM. Preclinical evidence of STAT3 inhibitor pacritinib overcoming temozolomide resistance via downregulating miR-21-enriched exosomes from M2 glioblastoma-associated macrophages. J Clin Med. 2019;8:959. doi: 10.3390/jcm8070959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santos JC, Lima NDS, Sarian LO, Matheu A, Ribeiro ML, Derchain SFM. Exosome-mediated breast cancer chemoresistance via miR-155 transfer. Sci Rep. 2018;8:829. doi: 10.1038/s41598-018-19339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu S, Li Y, Liao Z, Wang Z, Wang Z, Li Y, Qian L, Zhao J, Zong H, Kang B, Zou WB, Chen K, He X, Meng Z, Chen Z, Huang S, Wang P. Plasma extracellular vesicle long RNA profiling identifies a diagnostic signature for the detection of pancreatic ductal adenocarcinoma. Gut. 2020;69:540–550. doi: 10.1136/gutjnl-2019-318860. [DOI] [PubMed] [Google Scholar]
- 55.Gambardella J, Coppola A, Izzo R, Fiorentino G, Trimarco B, Santulli G. Role of endothelial miR-24 in COVID-19 cerebrovascular events. Crit Care. 2021;25:306. doi: 10.1186/s13054-021-03731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 57.Arando A, Delgado JV, Bermudez-Oria A, Leon JM, Fernandez-Prior A, Nogales S, Perez-Marin CC. Effect of olive-derived antioxidants (3,4-dihydroxyphenylethanol and 3,4 dihydroxyphenylglycol) on sperm motility and fertility in liquid ram sperm stored at 15 degrees C or 5 degrees C. Reprod Domest Anim. 2020;55:325–332. doi: 10.1111/rda.13631. [DOI] [PubMed] [Google Scholar]
- 58.de Roos B, Zhang X, Rodriguez Gutierrez G, Wood S, Rucklidge GJ, Reid MD, Duncan GJ, Cantlay LL, Duthie GG, O'Kennedy N. Anti-platelet effects of olive oil extract: in vitro functional and proteomic studies. Eur J Nutr. 2011;50:553–562. doi: 10.1007/s00394-010-0162-3. [DOI] [PubMed] [Google Scholar]
- 59.Bermudez-Oria A, Rodriguez-Gutierrez G, Rodriguez-Juan E, Gonzalez-Benjumea A, Fernandez-Bolanos J. Molecular interactions between 3,4-dihydroxyphenylglycol and pectin and antioxidant capacity of this complex in vitro. Carbohydr Polym. 2018;197:260–268. doi: 10.1016/j.carbpol.2018.05.089. [DOI] [PubMed] [Google Scholar]
- 60.Hashimoto T, Ibi M, Matsuno K, Nakashima S, Tanigawa T, Yoshikawa T, Yabe-Nishimura C. An endogenous metabolite of dopamine, 3,4-dihydroxyphenylethanol, acts as a unique cytoprotective agent against oxidative stress-induced injury. Free Radic Biol Med. 2004;36:555–564. doi: 10.1016/j.freeradbiomed.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Fernandez-Prior A, Bermudez-Oria A, Millan-Linares MDC, Fernandez-Bolanos J, Espejo-Calvo JA, Rodriguez-Gutierrez G. Anti-inflammatory and antioxidant activity of hydroxytyrosol and 3,4-dihydroxyphenyglycol purified from table olive effluents. Foods. 2021;10:227. doi: 10.3390/foods10020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez-Perez MD, Perez de Algaba I, Martin-Aurioles E, Arrebola MM, Ortega-Hombrados L, Verdugo C, Fernandez-Prior MA, Bermudez-Oria A, De La Cruz JP, Gonzalez-Correa JA. Neuroprotective effect of 3',4'-dihydroxyphenylglycol in type-1-like diabetic rats-influence of the hydroxytyrosol/3',4'-dihydroxyphenylglycol ratio. Nutrients. 2022;14:1146. doi: 10.3390/nu14061146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gukovskaya AS, Gukovsky I, Algul H, Habtezion A. Autophagy, inflammation, and immune dysfunction in the pathogenesis of pancreatitis. Gastroenterology. 2017;153:1212–1226. doi: 10.1053/j.gastro.2017.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gukovskaya AS, Gukovsky I. Autophagy and pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G993–G1003. doi: 10.1152/ajpgi.00122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng D, Park O, Radaeva S, Wang H, Yin S, Kong X, Zheng M, Zakhari S, Kolls JK, Gao B. Interleukin-22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway. Int J Biol Sci. 2012;8:249–257. doi: 10.7150/ijbs.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hashimoto D, Ohmuraya M, Hirota M, Yamamoto A, Suyama K, Ida S, Okumura Y, Takahashi E, Kido H, Araki K, Baba H, Mizushima N, Yamamura K. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol. 2008;181:1065–1072. doi: 10.1083/jcb.200712156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kong L, Deng J, Zhou X, Cai B, Zhang B, Chen X, Chen Z, Wang W. Sitagliptin activates the p62-Keap1-Nrf2 signalling pathway to alleviate oxidative stress and excessive autophagy in severe acute pancreatitis-related acute lung injury. Cell Death Dis. 2021;12:928. doi: 10.1038/s41419-021-04227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X, Chu J, Sun H, Zhao D, Ma B, Xue D, Zhang W, Li Z. MiR-155 aggravates impaired autophagy of pancreatic acinar cells through targeting Rictor. Acta Biochim Biophys Sin (Shanghai) 2020;52:192–199. doi: 10.1093/abbs/gmz152. [DOI] [PubMed] [Google Scholar]
- 70.Yang H, Ma S, Guo Y, Cui D, Yao J. Bidirectional effects of pyrrolidine dithiocarbamate on severe acute pancreatitis in a rat model. Dose Response. 2019;17:1559325819825905. doi: 10.1177/1559325819825905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mossmann D, Park S, Hall MN. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer. 2018;18:744–757. doi: 10.1038/s41568-018-0074-8. [DOI] [PubMed] [Google Scholar]
- 73.Xue R, Yang J, Wu J, Meng Q, Hao J. Coenzyme Q10 inhibits the activation of pancreatic stellate cells through PI3K/AKT/mTOR signaling pathway. Oncotarget. 2017;8:92300–92311. doi: 10.18632/oncotarget.21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cui LH, Li CX, Zhuo YZ, Yang L, Cui NQ, Zhang SK. Saikosaponin d ameliorates pancreatic fibrosis by inhibiting autophagy of pancreatic stellate cells via PI3K/Akt/mTOR pathway. Chem Biol Interact. 2019;300:18–26. doi: 10.1016/j.cbi.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 75.Liu MW, Wei R, Su MX, Li H, Fang TW, Zhang W. Effects of Panax notoginseng saponins on severe acute pancreatitis through the regulation of mTOR/Akt and caspase-3 signaling pathway by upregulating miR-181b expression in rats. BMC Complement Altern Med. 2018;18:51. doi: 10.1186/s12906-018-2118-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Song G, Zhou J, Song R, Liu D, Yu W, Xie W, Ma Z, Gong J, Meng H, Yang T, Song Z. Long noncoding RNA H19 regulates the therapeutic efficacy of mesenchymal stem cells in rats with severe acute pancreatitis by sponging miR-138-5p and miR-141-3p. Stem Cell Res Ther. 2020;11:420. doi: 10.1186/s13287-020-01940-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.