Abstract
The gut microbiota has a crucial effect on regulating the intestinal mucosal immunity and maintaining intestinal homeostasis both in health and in disease state. Many effects are mediated by gut microbiota-derived metabolites and tryptophan, an essential aromatic amino acid, is considered important among many metabolites in the crosstalk between gut microbiota and the host. Kynurenine, serotonin, and indole derivatives are derived from the three major tryptophan metabolism pathways modulated by gut microbiota directly or indirectly. Aryl hydrocarbon receptor (AHR) is a cytoplasmic ligand-activated transcription factor involved in multiple cellular processes. Tryptophan metabolites as ligands can activate AHR signaling in various diseases such as inflammation, oxidative stress injury, cancer, aging-related diseases, cardiovascular diseases (CVD), and chronic kidney diseases (CKD). Accumulated uremic toxins in the body fluids of CKD patients activate AHR and affect disease progression. In this review, we will elucidate the relationship between gut microbiota-derived uremic toxins by tryptophan metabolism and AHR activation in CKD and its complications. This review will provide therapeutic avenues for targeting CKD and concurrently present challenges and opportunities for designing new therapeutic strategies against renal fibrosis.
Keywords: Intestinal flora, Tryptophan metabolites, Chronic kidney disease, Natural products
Introduction
With the development of the economy, environmental pollution has become one of the most serious problems that threaten human health [1, 2]. Some persistent organic pollutants, such as polycyclic aromatic hydrocarbons (PAHs), halogenated aromatic hydrocarbons, and coplanar polychlorinated biphenyls are usually derived from waste incomplete combustion and pyrolysis and as by-products of industrial manufacturing processes. As early as the 1950s, PAHs have been noted to affect the substance metabolism and various physiological functions [3, 4]. Later, aryl hydrocarbon receptor (AHR) was initially reported based on the study of PAHs metabolism [5, 6]. Furthermore, many environmental carcinogens, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), benzo(a)pyrene and hexachlorobenzene, were reported to activate AHR signaling [7–9].
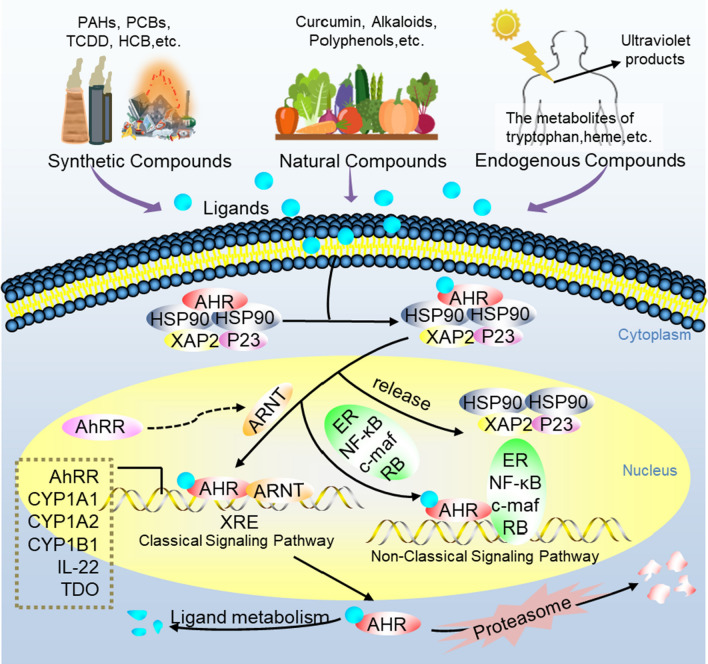
AHR is a member of the family of basic helix-loop-helix transcription factors [10]. Under normal circumstances, the inactive AHR combine with a dimer of the 90 kDa heat shock protein (HSP90), X-associated protein 2 (XAP2), and the co-chaperone p23 to constitute a stable protein complex in the cytoplasm [11]. When the cognate ligands bind to AHR, the complex is activated by a conformational change exposing its amino-terminal nuclear localization sequence and an adjacent nuclear export sequence. After phosphorylated by protein kinase C, the ligand-bound AHR complex is translocated into the nucleus in which it combines with the AHR nuclear translocator (ARNT) through HSP90 displacement. The AHR/ARNT heterodimer is able to bind to the special xenobiotic responsive elements (XREs) or dioxin responsive elements, which locate in the regulatory regions of many AHR target genes [10]. Subsequently, it leads to the transcription of downstream target genes including phase I metabolism enzymes, such as cytochrome P450 family (CYP) 1 enzyme and a few phase II conjugation enzymes [12]. In addition, in the regulatory regions of the above-mentioned target genes, those special DNA enhancer sequences also include AHR repressor protein (AHRR) [13]. AHRR inhibits AHR signal transduction through binding to ARNT or binding to XREs emulously. The expression of AHRR is activated by AHR, which forms a negative feedback loop to regulate AHR conversely [14, 15] (Fig. 1). AHR signaling pathway can interact with other transcriptional factors such as activator protein 1, nuclear factor-erythroid-2-related factor 2 (Nrf2), and nuclear factor kappa B (NF-κB), which boosts the inflammatory response [16–18]. Additionally, AHR in the cytoplasm can activate other cytoplasmic proteins, like Smads, β-catenin, mitogen-activated protein kinase (MAPK) family p38 [19], NADPH oxidase, and extracellular signal-regulated kinase. And the activation of AHR and related pathways would induce and aggravate various diseases such as cancer, cardiovascular disease (CVD), and chronic kidney disease (CKD). In this review, we will discuss gut microbiota-derived tryptophan metabolites (indole metabolites) as AHR ligands to activate AHR signaling and mediate renal fibrosis. Therapeutic potential of natural products as AHR antagonists is also highlighted to provide a new perspective for clinical treatment of CKD.
Fig. 1.
The AHR signaling pathway. Inactive AHR is complexed with HSP90, XAP2, and p23 to maintain stability and keep it in a high-affinity state for its ligands. When the ligands bind to AHR, the complex translocates to the nucleus where it combines ARNT through HSP90 displacement. The AHR-ARNT heterodimer binds to XREs to induce the transcription of downstream target genes, such as CYP1A1, CYP1B1, AHRR, etc. AhRR is also a negative regulator of AHR that can bind to ARNT but does not induce transcription. Besides, the ligands–AHR complex also interacts with other regulator proteins, such as NF-κB, etc
Renal fibrosis
Renal fibrosis, mainly characterized by glomerulosclerosis and tubulointerstitial fibrosis, is the common ultimate pathological feature of CKD [20]. The progression of CKD indicates that patients inevitably reach end-stage renal disease (ESRD) and need renal replacement treatment such as dialysis and transplantation. Renal fibrosis is associated with the activation of renin–angiotensin system, IκB/NF-κB, keap1/Nrf2, AHR, TGF-β/Smad and Wnt/β-catenin signaling pathways as well as the dysregulation of metabolism such as lipid metabolism, purine metabolism, and amino acid metabolism [21–27]. For patients with CKD, the higher risk cannot be completely explained by traditional risk factors such as tobacco use, obesity, high blood pressure, diabetes mellitus, and hypercholesterolemia. The uremic circumstance itself is harmful and uremic toxins have emerged as a key factor to explain CKD and its complication. In CKD patients, uremic toxins contributed to progressive renal fibrosis. Tryptophan metabolism is one of the most important sources of uremic toxins. Several seminal publications have highlighted that a plethora of uremic toxins derived from tryptophan metabolism are produced by host and gut microbiota and were implicated in renal fibrosis [9, 28–30].
Tryptophan metabolism in renal fibrosis
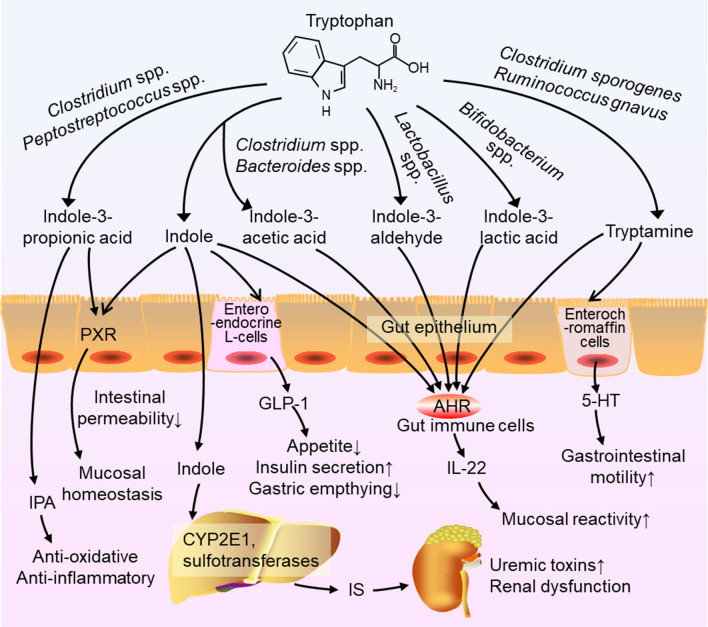
Tryptophan is an essential amino acid that cannot be synthesized in the body but is commonly found in regular diets [9]. Recently, the most compelling evidence has suggested that the intestinal metabolites of tryptophan play a crucial role in AHR activation [31, 32]. Most of tryptophan is metabolized by the kynurenine metabolic pathway, and others are metabolized to melatonin through the serotonin pathway and to indole components through the indolic pathway [29, 33]. First, the kynurenine pathway occurs mainly in the liver, where tryptophan will be metabolized by indoleamine 2,3-dioxygenase (IDO)1, IDO2 and tryptophan 2,3-dioxygenase, producing the intermediate N-formyl kynurenine, and then converting into kynurenine by arylformamidase. Further, kynurenine can be metabolized to kynurenic acid, cinnabarinic acid, xanthurenic acid, picolinic acid, quinolinic acid, and NAD+ [29]. These products in the kynurenine pathway are involved in the regulation of quite a few host biological responses including inflammation, immune response, and neurotransmission. Moreover, kynurenic acid was reported to exert a mucosal protective effect in the gut, which was mediated only by IDO1 [34]. Some studies have focused on the role of kynurenic acid and kynurenine as AHR agonists [35]. Second, in the serotonin pathway, tryptophan is catalyzed by tryptophan hydroxylase to 5-hydroxytryptophan, then metabolized to 5-hydroxytryptamine (5-HT, serotonin) by IDO subsequently converting into melatonin [36]. Serotonin is not an AHR ligand, but it can bind with AHR ligands, as a CYP1A1 substrate, to prevent ligand inactivation and promote sustained AHR signaling [37]. Third, another important pathway occurs in the human gastrointestinal tract, which contains more than 1014 kinds of microorganisms [38]. The diverse and dynamic gut microbiota in human gastrointestinal tract played a critical role in host health and diseases [39, 40]. These intestinal microorganisms can not only regulate host immune response by affecting immune tolerance and autoimmune diseases, but also play an important role in the regulation of gut homeostasis. In the healthy state, the human intestinal flora carries out a variety of activities to the human body. Intestinal flora has a symbiotic relationship with the host, which protects the body against pathogenic bacteria, regulates the immune system, regulates endogenous lipid and carbohydrate metabolism, and maintains the nutritional balance of the body. Many bacterial species have been revealed to metabolize tryptophan into indole and indole derivatives such as tryptamine, indole-3-acetic acid (IAA), indole-3-aldehyde (I3A), indole-3-lactic acid (ILA), and indole-3-propionic acid (IPA) [9]. For instance, tryptophan could be metabolized to I3A by Lactobacillus spp., to ILA by Bifidobacterium spp., to IAA by Bacteroides spp. and Clostridium spp. and to tryptamine by Clostridium sporogenes and Ruminococcus gnavus. In addition, Peptostreptococcus spp. could convert tryptophan into IPA in the gut [9] (Fig. 2). Tryptophan is metabolized by bacterial tryptophan enzymes to produce the precursor indoles, which are absorbed and converted into indoles sulfate (IS) by CYP2E1 and sulfotransferases in the liver [41]. One research had found a tryptophanase gene in Bacteroides thetaiotaomicron and it was a kind of common tryptophanase gene existing in the human gut. And the relative abundance of the tryptophanase gene is closely related to the relative abundance of Bacteroidetes in intestinal flora [41]. Additionally, many types of bacteria in the gastrointestinal tract, such as Escherichia coli, can metabolize tryptophan into indoles, and indoles play a key role in the progress of diseases as an important intercellular and interspecific bacterial signaling molecule [9]. Pathologically, tryptophan is metabolized to indolic compounds, such as indole, IAA and I3A, via intestinal bacteria and then absorbed into blood circulation, which commonly are accumulated in CKD patients [28]. It is also reported that IS and IAA bind with AHR, induce the complex to translocate to nuclear, and upregulate eight genes regulated by AHR, including CYP1A1 and CYP1B1 [42]. Taken together, tryptophan metabolites from gut microbiota have been demonstrated to participate in renal fibrosis as AHR ligands (Fig. 3).
Fig. 2.
Mechanisms of action of microbial tryptophan catabolites on host physiology and disease. Tryptophan in the colonic lumen is converted into various catabolites by the gut microbiota. Indole and indole-3-propionate (IPA) may mediate the pregnane X receptor (PXR) to decrease intestinal permeability and affect mucosal homeostasis. Indole is metabolized to indoxyl sulfate by CYP2E1 and sulfotransferases in the liver leading to the accumulation of IS, which is toxic and associated with renal dysfunction. Indole also induces the release of glucagon-like peptide 1 (GLP-1) in enteroendocrine L-cells, suppressing appetite and insulin secretion and slowing gastric emptying. Several tryptophan catabolites activate AHR in intestinal immune cells to alter innate and adaptive immune responses maintaining mucosal reactivity. In enterochromaffin cells, tryptamine induces the release of 5-HT stimulating gastrointestinal motility by acting on enteric nervous system neurons
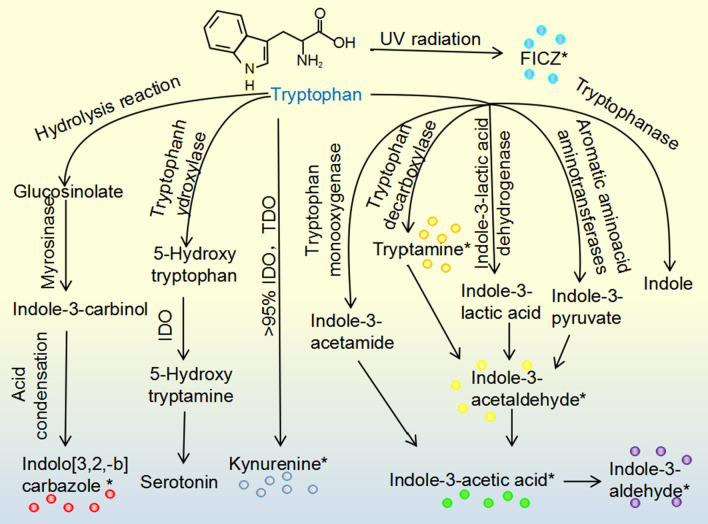
Fig. 3.
AHR ligands from tryptophan metabolism. Tryptophan in cruciferous vegetables produce glucosinolate via hydrolysis reaction, yielding indolo[3,2,-b]carbazole. 95% tryptophan can be metabolized to kynurenine, which is mediated by IDO and TDO. In the gastrointestinal tract, metabolites such as tryptamine, indole-3-acetaldehyde, indole-3-acetic acid, and indole-3-aldehyde are from the microbial metabolic process. Besides, tryptophan can be metabolized to 6-formylindolo-(3,2-b)-carbazole from ultraviolet radiation. Asterisks indicate metabolites with AHR agonistic activity
AHR in renal fibrosis
The roles of AHR have gradually been demonstrated in renal fibrosis. First, activation of AHR can result in progressive glomerular and renal tubular cells damage, which can cause glomerulosclerosis and renal interstitial fibrosis, thereby exacerbating CKD [43]. In the classical AHR signaling pathway, the activated AHR can induce the transcription of downstream target genes including CYP1A1, the main enzyme involved in drug metabolism and biological activation. Second, CKD patients generally have microinflammatory state and lipid metabolism disorder, which would affect each other and accelerate the progression of the disease. CYP1A1 can metabolize many exogenous precancerous substances into electron-friendly compounds, combine with macromolecular substances in vivo to form DNA adductions, and eventually lead to mutation and genotoxicity [44]. Meanwhile, the body also initiates the immune response, when CYPlAl metabolizes endogenous and exogenous substances, which can induce oxidative stress and inflammation. Third, activation of AHR also can explain the high risk of CVD in patients with CKD. AHR activated by uremic toxins increased the expression and activity of cyclooxygenase-2 (COX-2), a key enzyme in the synthesis of prostaglandin and thrombin by arachidonic acid, promoting platelet aggregation in vascular endothelial cells [13]. And in vascular smooth muscle cells, it also induced the increased expression and activity of tissue factor (TF), thus inducing a procoagulant state [45]. Vascular dysfunction caused by AHR activation plays a crucial role in the increased risk of multiple CVD, like peripheral artery disease, myocardial infarction, and stroke in CKD patients [46].
Gut microbiota-derived tryptophan metabolites as AHR ligands in renal fibrosis
IAA as AHR ligand in renal fibrosis
IAA is a kind of tryptophan metabolite mainly produced by Clostridium spp. and Bacteroides spp. in the gut. A growing body of research has demonstrated the deleterious effect of IAA on renal and vascular cells, and AHR was identified as the cellular receptor of toxins [8]. However, IAA could not directly activate the AHR pathway to increase the expression or activity of IF compared with IS. Tawfik et al. found that IAA at 50 μM could activate AHR to induce TF expression but the level of TF was significantly decreased when inhibited the NF-κB pathway, which was suggested that the NF-κB was essential in the process of IAA inducing TF. Moreover, the nuclear translocation of the NF-κB p50 subunit was reduced by p38 MAPK inhibition. It is concluded that AHR did not directly bind to the TF gene promoter, but IAA modulated AHR/p38 MAPK/NF-κB pathway to increase TF in human endothelial cells [47]. Gondouin et al. found that TF levels in human umbilical vein endothelial cells (HUVECs) and peripheral blood mononuclear cells (PBMCs) were increased by 52% when incubated with IAA at a concentration of 50 μM compared with the control group [48]. Further studies found IAA enhanced AHR-dependent gene expression and the level of TF mRNA in endothelial and peripheral blood mononuclear cells by activating AHR. Additionally, indolic uremic solutes could increase TF procoagulant activity [48]. These thrombotic mechanisms induced by toxins include not only increasing the levels of procoagulant microparticles, but also impairing endothelial healing [49]. Also, endothelial dysfunction, oxidative stress, and inflammation induced by uremic solutes could result in increased cardiovascular risk [50]. Dou et al. reported that high levels of IAA (IAA > 3.73 mM)-treated endothelial cells increased COX-2 expression and this raised COX-2 would enhance the synthesis of prostaglandin E2 in endothelial cells, promoting platelet aggregation [51]. Meanwhile, it is verified that IAA increased the mRNA expression of CYP1A1, CYP1B1, and AHRR and increased reactive oxygen species production in endothelial cells. IAA activated the AHR/p38MAPK/NF-κB pathway and upregulate the expression and activity of COX-2 to induce oxidative stress and inflammation, suggesting the prooxidant and proinflammatory effects of the uremic solute IAA.
In diabetic kidney disease (DKD) rats, oxidative stress, metabolic abnormalities, and matrix accumulation lead to glomerular mesangial cell activation and tubular cell function changes. And the activation of AHR has been involved in DKD. Lee et al. found that the chow and water intake, urine output, urine albumin, and albumin to creatinine ratio were obviously increased in streptozotocin-induced diabetic mice, which were alleviated in AHR-knockout mouse [52]. Furthermore, the number of macrophages, macrophage infiltration, and ECM accumulation were significantly higher in the diabetic group than in the control group, and were attenuated in AHR-knockout mouse or AHR inhibitor treatment mouse. Meanwhile, AHR deficiency reduced the levels of COX-2 and prostaglandin E2, lipid peroxidation and oxidative stress. These results suggested that AHR could mediate pathologic alterations of renal function and structure in diabetic mouse [52]. The Nrf2 plays a key role in combating diabetes-induced oxidative stress and inflammation, which are the main causes of diabetes-induced endothelial dysfunction. It is known that endothelial dysfunction is a key first step in the development of diabetic macrovascular complications [53]. Wu et al. found Nrf2 knockout diabetic mice-induced diabetes by streptozotocin showed more severe aortic endothelial oxidative stress, inflammation, and dysfunction than wild-type diabetic mice. And sodium butyrate, an activator of Nrf2, obviously alleviated these effects in the wild-type mice, but not in the Nrf2 knockout mice. Moreover, sodium butyrate could increase Nrf2 mRNA and protein but did not promote Nrf2 nuclear translocation in high glucose-treated aortic endothelial cells. Further, sodium butyrate increased occupancy of AHR and the co-activator P300 at the Nrf2 gene promoter. It was suggested that Nrf2 is necessary to protect against diabetic-induced aortic endothelial dysfunction [53]. Nrf2 and NF-κB signaling pathways are mutually regulated. Nrf2 deficiency up-regulates NF-κB activity and causes proinflammatory factor expression, which in turn regulates Nrf2 transcription and activity. Abnormalities of Nrf2 and NF-κB signaling pathways are closely related to renal fibrosis [24].
Reducing oxidative stress and inflammatory response is important to slow or improve the development of CKD. It is noted that the effects of IAA to induce oxidative stress and endothelial inflammation were significant stimuli of cardiovascular events in clinical studies. Some researchers have studied CKD patients with long-term exposure to urinary toxins and found that elevated TF levels in vessel walls may accelerate atherosclerosis, which probably explains the high cardiovascular mortality observed in this population [54]. It is reported that those patients with higher IAA levels (> 3.73 μM) have an increased risk of cardiovascular disease in CKD patients compared to patients with lower IAA levels (<= 3.73 μM) [51]. Brito et al. also found a positive correlation between AHR protein expression and IAA plasma levels in CKD patients [55]. IAA also can activate the AHR/MAPK pathway to regulate cell proliferation, differentiation, and immune function and induce CVD in ESRD patients [56].
IS as a AHR ligand in renal fibrosis
Indole is the main bacterial tryptophan metabolite, which is generated by Bacteroides spp. and Clostridium spp. in the gut [57]. It has been demonstrated that indole inhibited the upregulation of interleukin-1β, interleukin-6, and chemokine ligand 2 induced by palmitate and lipopolysaccharide to attenuate inflammation and also decreased the secretion of the key oxidative stress inducer protein nitric oxide synthase and NADPH oxidase. Further studies indicated that IS significantly increased the levels of CYP1A1 and CYP1B1 but cannot reduce the upregulation of interleukin-1β and nitric oxide synthase. It is suggested that indole itself, not IS, has anti-inflammatory effects in the liver [58]. Additionally, indole may bind with the pregnane X receptor to reduce the permeability of the intestine and maintain the stability of the intestinal mucosa. It also could induce enteroendocrine L-cell to secrete glucagon-like peptide 1, which is known to stimulate insulin secretion, suppresses appetite, and slow the rate of gastric emptying [9]. Indole is metabolized to IS by CYP2E1 and sulfotransferases in the liver, which is toxic and gradually accumulates in the body. With the aggravation of renal damage, it would develop ESRD and even death. Recently, a great many reviews have suggested that IS plays a crucial role in AHR activation and the progress of CKD. It is noted that IS activated the AHR pathway in cells of CKD mice to upregulate the mRNA levels of CYP1A1 and CYP1B1 [59]. Schroeder et al. determined the expression of CYP1A1, CYP1A2, and CYP1B1 in hepatoma cell lines after IS exposure. They found that IS would increase the levels of CYP1A1, CYP1A2, and CYP1B1 mRNA, and the effects of 100 µM IS and 10 nm TCDD on CYP1B1 gene expression were equivalent [60]. The activation of AHR in CKD may provide a mechanism to explain the harmful effect of toxins on cells. For example, AHR activated by IS can induce podocyte injury and glomerular damage. It is reported that mice exposed to IS for 8 weeks would exhibit progressive glomerular injury and vascular damage along with increased CYP1A1 expression. IS could induce AHR nuclear translocation, increase CYP1A1 levels, and decrease cell size and viability of podocytes in mice [8]. Besides, the level of IS in renal tissue of rats with chronic renal failure was increased by six times, and IS in renal homogenate was up to 71 μM. These results also reflect a significant increase of IS in the damaged kidneys [8]. Indeed, activation of AHR could lead to progressive glomerular injury and the impairment of renal function by podocyte progression. It is demonstrated that macroscopic renal cortex atrophy in IS-treated kidneys may be an end-stage feature due to the sum of glomerular and tubulointerstitial damage [61]. Although a lot of studies support the direct role of IS on podocyte injury, it is still possible that podocytes lesion is a comprehensive consequence of chronic vascular damage by toxins. Hung et al. showed that IS impaired endothelial progenitor cell function in uremia mice by inhibiting the HIF/interleukin-10/vascular endothelial growth factor signaling pathway, thereby inhibiting neovascularization and mediating peripheral arterial disease in CKD [62]. DKD is another primary cause of CKD and leading cause of ESRD, which develop many comorbidities and has a high mortality rate. Zhao et al. observed the increase of serum creatinine and blood urea nitrogen and the decrease of serum albumin in DKD rats. And in the rats treated with Tangshen Formula, the levels of serum creatinine and microalbuminuria were attenuated. They also detected that mesangial matrix was moderately expanded, tubules atrophy and lumens were dilated, and ECM was deposited in glomeruli and tubulointerstitium in the kidney of DKD rats, which were inhibited in Tangshen Formula-treated rats [63]. Additionally, the level of IS in the DKD group increased by four times compared with the sham group and markedly reduced in Tangshen Formula-treated group. The latter group of rats also significantly reduced the mRNA level and expression of AHR and suppressed the nuclear translocation of phosphorylated NF-κB p65 to inhibit activation of NF-κB signaling. It was demonstrated that orally administered Tangshen Formula significantly decreased level of IS and attenuated renal inflammation to inhibit diabetic renal injury [63].
Disturbance of intestinal flora results into the release of proinflammatory bacteria products, leading to insulin resistance, energy consumption, immune disorders, atherosclerosis in diabetic patients, and accelerating the progression of renal disease. Quite a few studies have suggested that DKD is the most severe and one of common long-term complications of diabetes. Kim et al. pointed out that the activity of serum AHR ligands was an important and independent risk factor for DKD based a multiple regression analysis [64]. Recent studies have found decreased tryptophan level and increased levels of tryptophan metabolites, including kynurenic acid, 3-hydroxykynurenine, 5-hydroxytryptophan, 3-hydroxyanthranilic acid, and 5-hydroxyindoleacetic acid in diabetic mellitus patients. Matsuoka et al. divided diabetic patients into higher and lower tryptophan groups. They found higher levels of IAA, kynurenine, kynurenic acid, 5-hydroxytryptophan, and 5-hydroxykynurenine in higher tryptophan group but 5-hydroxyindoleacetic acid was higher in lower tryptophan group [65]. The results indicated that there was more degradation of tryptophan when plasma tryptophan level was high, and more serotonin is converted to 5-hydroxyindoleacetic acid when plasma tryptophan level was low. It was also suggested that diabetic patients are often exposed to stress [65]. Additionally, there have been many clinical studies on the role of IS as an AHR ligand in CKD. IS could activate AHR to mediate the immune response to influence the progression of renal fibrosis. Kim et al. found that the production of TNF-α in macrophages of CKD patients is modulated involving the crosstalk among AHR, NF-κB, and suppressor of cytokine signaling. IS-activated AHR rapidly binds to the NF-κB p65 subunit, resulting in a mutual inhibition of AHR and NF-κB and inhibiting the production of TNF-α early. Afterward, AHR induced the expression of suppressor of cytokine signaling 2 to increase TNF-α production through NF-κB. Meanwhile, co-immunoprecipitation data showed that the direct interaction between AHR and p65 occurred 15-30 min after IS stimulation [66].
Vascular dysfunction caused by AHR activation also explained the high risk of CVD in ESRD patients. Increasing evidence showed that IS was associated with the pathophysiology of cardiovascular and renal dysfunction. More recently, IS has been considered as a vascular toxin [66]. In vascular smooth muscle cells (VSMCs), IS could induce the increased expression and activity of TF, thus inducing a procoagulant state [45]. Shivanna et al. have reported that the levels of IS in ESRD patients were 55 times higher than those in the control group on average. Moreover, the activity of TF in VSMCs increased after exposure to serum of ESRD patients, which was significantly correlated with the level of IS in ESRD patients. IS can activate AHR and then inhibit TF ubiquitination and degradation in VSMCs of patients with renal failure and then promoting thrombosis [67]. Gondouin et al. showed that the production of TF in endothelial cells was increased by 84%, 106%, and 135%, respectively following the treatment of IS at concentrations of 0.1, 0.5, and 1 mM [48]. In addition, the study also demonstrated that IS activated the classical AHR signaling that directly bound to TF, and increased TF stability and induced thrombosis [68]. Abundant evidence has shown that patients with CKD are predisposed to CVD and the emergence and development of CVD are the primary inducements of death in patients treated by dialysis [69]. Therefore, this connection is even more distinct in patients with ESRD, in which the proportion accounts for 50% of all-cause mortality [66]. Cardiovascular mortality of patients with a functioning renal transplant is two to five times higher than general population [70].
There were reports that uremic toxins mediated the vicious cycle between oxidative stress and inflammation aggravating the chronic inflammatory environment, thereby accelerating the progression of CVD in CKD patients [71]. For instance, it has shown that IS can alter the balance between prooxidant and antioxidant mechanisms [72], increase the release of endothelial microparticles [73], and lower the healing ability of endothelial cells, which results in the occurrence of oxidative stress [74]. Reactive oxygen species produced during the metabolism of CYP1A1 can activate NF-κB inducing the expression of proinflammatory factors. Uremic toxin-activated AHR can induce oxidative stress and inflammation increasing the mortality of CVD in patients with CKD [75]. It is reported that NF-κB was activated and transferred into the nucleus to induce the expression of certain proinflammatory factors, thus participating in the pathological process of inflammatory and autoimmune diseases [76]. Another study demonstrated that IS-activated AHR induced monocytes to produce proinflammatory cytokine TNF-α leading to a proinflammatory state of smooth muscle cells in patients with ESRD. But it has also been reported that IS activated AHR to trigger the inflammatory response by mediate activator protein 1 transcriptional activity [77]. Therefore, the mechanism still needs further study.
Other gut microbiota-derived tryptophan metabolites as AHR ligands
Tryptamine, a tryptophan metabolite generated by Clostridium sporogenes and Ruminococcus gnavus, has been considered as AHR ligand with weak potency. It is reported that tryptamine could bind to AHR and induce the expression of AHR target genes to regulate intestinal immune response [78]. Further research showed that tryptamine induced AHR nuclear accumulation and AHR/ARNT promoter recruitment to activate the transcription of CYP1A1 [79]. Besides, I3A has been reported to activate AHR in intestinal immune cells and increase the expression of interleukin-22 to attenuate mucosal inflammation and protect the intestinal epithelial barrier [80]. A recent study found that the combination of I3A and AHR increased the secretion of interleukin-22 by lamina propria lymphocytes and induced the phosphorylation of signal transduction factor and transduction factor 3, thus promoting the intestinal epithelial cells proliferation and restoring the intestinal mucosa injury [81]. Moreover, the treatment of macrophages with I3A reduced the production of TNF-α, interleukin-1β, and monocyte chemoattractant protein-1 induced by lipopolysaccharide to attenuate the inflammatory response obviously. It also regulated the expression of AHR target genes and modulated the hepatocyte metabolism by mediating ligand activation of the nuclear receptor [82]. The study by Yu et al. showed that I3A acted as a ligand of AHR and decreased both the protein and mRNA levels of CYP1A1. It is demonstrated that I3A (100 nM) could suppress the production of thymic stromal lymphopoietin, an important cytokine, reduce inflammation, and enhance antifungal abilities in patients who suffer from atopic dermatitis [83]. In the gut, Bifidobacterium spp. can metabolize tryptophan into ILA, another AHR ligand. It has been reported that ILA was able to inhibit inflammatory T cells and stimulate immunoregulatory T cells through AHR [84]. ILA activated AHR to inhibit the transcription of IL-8 and reduce the interleukin-8 response after interleukin-1β stimulus in immature intestinal enterocytes [85]. ILA also could clear the free radicals and reduce the production of inflammatory mediator interleukin-6 induced by ultraviolet B radiation to exert anti-inflammatory effects in human keratinocytes [86].
As discussed above, accumulated studies showed gut microbiota-derived tryptophan metabolites as AHR ligands exert an adverse influence on the occurrence and progression of renal fibrosis [36]. Moreover, some researchers suggest that AHR activated by different ligands is a bifunctional modulator of the expression of downstream target genes and the progress of many diseases. With these discoveries, we see a promise of AHR as a therapeutic target for diseases and hope to seek therapeutic schedules of CKD to better control the increased rate of CKD based on the regulation of AHR.
The potential role of natural products as AHR antagonists
Identification of potential ligands helps to regulate the activity of AHR and improve CKD and reduce its complications. Traditionally, AHR is considered as a xenobiotic sensor that intermediates the chemical communication between environmental toxins and the homeostasis in the organism [87]. AHR ligands are abundant in many sources, such as diet, yeast and bacteria [88] (Table 1). Recent evidence has identified endogenous AHR ligand candidates, including tryptophan metabolites, indigoid (e.g. indirubin), metabolites of arachidonic acid (e.g. lipoxin 4A), heme metabolites (e.g. bilirubin), lipopolysaccharide, and cyclic AMP (cAMP) [8, 87]. Natural products are rich and important sources for drug discovery and considered as an alternative therapy for improving CKD and inhibiting renal fibrosis [89–93]. It has been demonstrated that several dietary phytochemicals are sources of AHR ligands [94]. Flavonoids derived from dietary materials such as baicalein, apigenin, chrysin, diosmetin, daidzein, genistein, quercetin, and galangin have been recognized as the natural AHR ligands that can activate AHR and regulate downstream target gene expressions in various diseases [87]. Extensive studies have demonstrated that flavonoids were mainly applied to cancer treatment through regulating AHR signaling [94]. However, a few studies have reported that flavonoids regulated CKD via AHR activation. Our latest study identified that endongous metabolite 1-aminopyrene showed strong positive and negative correlation with serum creatinine levels and creatinine clearance, respectively, in the 5/6 nephrectomised rats [21]. Further study demonstrated that mRNA expressions of AHR and its three target genes including CYP1A1, CYP1A2, and CYP1B1 were significantly upregulated in both mice and NRK-52E cells induced by 1-aminopyrene, while this effect was partially weakened in AHR shRNA-treated mice and NRK-52E cells [21]. 1-aminopyrene could lead to renal function decline and fibrosis in the 1-aminopyrene-induced mice. We further showed that treatment with three flavonoids 5,7,3′,4′,5′-pentahydroxy flavanone, barleriside A, and rhoifolin isolated from Semen Plantaginis inhibited 1-aminopyrene-induced upregulation of profibrotic protein expression in NRK-52E cells [21]. Treatment with 5,7,3′,4′,5′-pentahydroxy flavanone and barleriside A ameliorated CKD and renal fibrosis through inhibiting AHR signaling pathway in both 1-aminopyrene-induced mice and 5/6 nephrectomised rats [21]. Structure–function relationship analysis showed that the antagonistic effect of flavonoids on AHR was deeply influenced by the number and location of hydroxyl and glycosyl groups. Previous study has demonstrated that baicalin significantly alleviated aristolochic acid I-mediated kidney toxicity via AHR-dependent CYP1A1 and CYP1A2 induction [95]. In addition, it has been reported that Tanshinone I promoted aristolochic acid I metabolism and prevents aristolochic acid I-mediated kidney injury by the induction of hepatic CYP1A1 and CYP1A2 in vivo [96]. We expect that more flavonoids are identified as AHR ligands to retard CKD and its complication and optimize the treatment of CKD.
Table 1.
Tryptophan metabolites as AHR ligands in CKD
| Ligands | Compounds | Origin | Biological effects | Molecular mechanism | References |
|---|---|---|---|---|---|
| Agonist | Indole | Indolic pathway | Alleviate inflammation and oxidative stress | Decreased IL-1β, IL-6, and chemokine ligand 2 levels | [58] |
| Agonist | IS | Indolic pathway | Induce podocyte injury | Increased CYP1A1 expression | [97] |
| Agonist | IS | Indolic pathway | Inhibit neovascularization, cause peripheral arterial disease | Inhibit TF ubiquitination, down-regulate HIF/IL-10/VEGF signaling pathway | [62, 68] |
| Agonist | IS | Indolic pathway | Induce inflammation | Increased expression of SOCS2 and TNF-α | [75, 77] |
| Agonist | IAA | Indolic pathway | Accelerate vascular dysfunction | Increased TF level via AHR/p38 MAPK/NF-κB pathway | [47, 48] |
| Agonist | IAA | Indolic pathway | Induce oxidative stress and inflammation | Increased CYP1A1,ROS and COX-2 expression | [50, 51, 56] |
| Agonist | Tryptamine | Indolic pathway | Regulate intestinal immune response | Increased CYP1A1 expression | [78, 79] |
| Agonist | Indole-3-aldehyde | Indolic pathway | Reduce mucosal inflammation, protect the intestinal epithelial barrier | Increased IL-22 level and STAT3 phosphorylation, decreased levels of TNF-α and IL-1β | [81, 82] |
| Agonist | Kynurenine | Kynurenine pathway | Accelerate arteriovenous thrombosis | Increased levels of TF and PAI-1 via AHR-TF/PAI-1 axis | [98, 99] |
| Agonist | Kynurenine | Kynurenine pathway | Induce chronic inflammation and oxidative stress | Increased HIF, IL-1β, and IL-6 levels, decreased EPO expression | [100, 101] |
| Antagonist | Indolo[3,2-b] carbazole | Dietary | Protect against oxidative DNA damage | Increased an antioxidant enzyme expression | [102] |
| Antagonist | 6-formylindolo-[3,2-b]-carbazole | UV light | Reduce inflammation | Decreased levels of IL-6 and claudin-2 | [103, 104] |
| Antagonist | 5,7,3′,4′,5′-pentahydroxy flavanone | Semen Plantaginis | Ameliorate CKD and renal fibrosis | Inhibit profibrotic protein expression expression | [21] |
| Antagonist | Barleriside A | Semen Plantaginis | Ameliorate CKD and renal fibrosis | Inhibit profibrotic protein expression expression | [21] |
| Antagonist | Rhoifolin | Semen Plantaginis | Ameliorate CKD and renal fibrosis | Inhibit profibrotic protein expression expression | [21] |
| Antagonist | Baicalein | Scutellaria baicalensis | Alleviate aristolochic acid I (AAI)-mediated kidney toxicity | Induce CYP1A1 and CYP1A2 expression | [95] |
| Antagonist | Tanshinone I | Salvia miltiorrhiza | Alleviate AAI-mediated kidney injury | Increased CYP1A1 and CYP1A2 expression | [96] |
Conclusion
With the improving understanding of the link between AHR and its ligands, the AHR signaling pathway involved in various diseases has become more evident. The deficiency of renal function in CKD patients induces the accumulation of uremic toxins, which increase oxidative stress and proinflammatory cytokine. It is well known that the uremic toxin-AHR pathway exerts multiple adverse biological effects on the development of renal fibrosis. In the cardiovascular system, the deficiency of AHR can result in abnormal cardiac function, hypertension or hypotension, vascular dysfunction, and CVD. A large number of studies have identified gut microbiota-derived uremic toxins as potent endogenous ligands of AHR and investigated the pathophysiological role of the AHR signaling pathway in CKD and CVD. Moreover, DKD induces glomerular mesangial cell activation and tubular cell function changes to promote the progress of renal fibrosis, which has been regarded as primary cause of ESRD with a high mortality rate.
In this review, an advanced understanding of the AHR signaling pathway provides more possibilities to identify novel exogenous and endogenous ligands of AHR. Meanwhile, the integration of mass spectrometry, nuclear magnetic resonance, and other modern analytical techniques, made up of the metabonomics technology platform, had accelerated the identification of AHR ligands. Multiple studies of tryptophan metabolites as AHR agonists and antagonists have led to a clearer understanding of the adverse effects of urinary toxins on patients with CKD. Moreover, due to the importance of flavonoids in the activation of AHR, we could give more attention to study natural products as therapeutic drugs to improve the use of medicines. Importantly, these mechanisms of uremic toxins from tryptophan metabolism activate AHR inspire many new ideas of the activation of AHR signaling pathways and help to develop new treatment strategies targeting AHR and related signaling pathways. Despite advances in our understanding of diseases associated with AHR, the molecular mechanisms of AHR signaling and the crosstalk between AHR signaling and other signaling pathways require further study. Therefore, it is still meaningful for further clinical practice to study the AHR signaling and alternative pathways in the prevention and treatment of CKD.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 81603271, 81673578, 81872985) and National Key Research and Development Project of China (2019YFC1709405).
Abbreviations
- AHR
Aryl hydrocarbon receptor
- AHRR
AHR repressor protein
- ARNT
AHR nuclear translocator
- CKD
Chronic kidney disease
- COX-2
Cyclooxygenase-2
- CVD
Cardiovascular diseases
- CYP
Cytochrome P450 family
- DKD
Diabetic kidney disease
- ESRD
End-stage renal disease
- I3A
Indole-3-aldehyde
- IAA
Indole-3-acetic acid
- IDO
Indoleamine 2,3-dioxygenase
- ILA
Indole-3-lactic acid
- IS
Indoxyl sulfate
- HIF
Hypoxia-inducible transcription factor
- NF-κB
Nuclear factor kappa B
- PAHs
Polycyclic aromatic hydrocarbons
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TF
Tissue factor
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing-Ru Liu and Hua Miao are co-first authors.
Contributor Information
Ping Li, Email: lp8675@163.com.
Ying-Yong Zhao, Email: zyy@nwu.edu.cn.
References
- 1.Harrill JA, Parks BB, Wauthier E, Rowlands JC, Reid LM, Thomas RS. Lineage-dependent effects of aryl hydrocarbon receptor agonists contribute to liver tumorigenesis. Hepatology (Baltimore, MD) 2015;61(2):548–560. doi: 10.1002/hep.27547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pessah IN, Lein PJ, Seegal RF, Sagiv SK. Neurotoxicity of polychlorinated biphenyls and related organohalogens. Acta Neuropathol. 2019;138(3):363–387. doi: 10.1007/s00401-019-01978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corre S, Tardif N, Mouchet N, Leclair HM, Boussemart L, Gautron A, Bachelot L, Perrot A, Soshilov A, Rogiers A, Rambow F, Dumontet E, Tarte K, Bessede A, Guillemin GJ, Marine JC, Denison MS, Gilot D, Galibert MD. Sustained activation of the aryl hydrocarbon receptor transcription factor promotes resistance to BRAF-inhibitors in melanoma. Nat Commun. 2018;9(1):4775. doi: 10.1038/s41467-018-06951-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boström CE, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T, Rannug A, Törnqvist M, Victorin K, Westerholm R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002;110(Suppl 3):451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poellinger L, Lund J, Gillner M, Hansson LA, Gustafsson JA. Physicochemical characterization of specific and nonspecific polyaromatic hydrocarbon binders in rat and mouse liver cytosol. J Biol Chem. 1983;258(22):13535–13542. doi: 10.1016/S0021-9258(17)43947-0. [DOI] [PubMed] [Google Scholar]
- 6.Denison MS, Wilkinson CF. Identification of the Ah receptor in selected mammalian species and induction of aryl hydrocarbon hydroxylase. Eur J Biochem. 1985;147(2):429–435. doi: 10.1111/j.1432-1033.1985.tb08767.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheong JE, Sun L. Targeting the IDO1/TDO2-KYN-AhR pathway for cancer immunotherapy—challenges and opportunities. Trends Pharmacol Sci. 2018;39(3):307–325. doi: 10.1016/j.tips.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Zhao H, Chen L, Yang T, Feng YL, Vaziri ND, Liu BL, Liu QQ, Guo Y, Zhao YY. Aryl hydrocarbon receptor activation mediates kidney disease and renal cell carcinoma. J Transl Med. 2019;17(1):302. doi: 10.1186/s12967-019-2054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9(1):3294. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulte KW, Green E, Wilz A, Platten M, Daumke O. Structural basis for aryl hydrocarbon receptor-mediated gene activation. Structure (London, England: 1993) 2017;25(7):1025–1033. doi: 10.1016/j.str.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Fukunaga BN, Probst MR, Reisz-Porszasz S, Hankinson O. Identification of functional domains of the aryl hydrocarbon receptor. J Biol Chem. 1995;270(49):29270–29278. doi: 10.1074/jbc.270.49.29270. [DOI] [PubMed] [Google Scholar]
- 12.Redaelli C, Gaffarogullari EC, Brune M, Pilz C, Becker S, Sonner J, Jäschke A, Gröne HJ, Wick W, Platten M, Lanz TV. Toxicity of teriflunomide in aryl hydrocarbon receptor deficient mice. Biochem Pharmacol. 2015;98(3):484–492. doi: 10.1016/j.bcp.2015.08.111. [DOI] [PubMed] [Google Scholar]
- 13.Vogel CFA, Ishihara Y, Campbell CE, Kado SY, Nguyen-Chi A, Sweeney C, Pollet M, Haarmann-Stemmann T, Tuscano JM. A protective role of aryl hydrocarbon receptor repressor in inflammation and tumor growth. Cancers. 2019;11(5):589. doi: 10.3390/cancers11050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakurai S, Shimizu T, Ohto U. The crystal structure of the AhRR-ARNT heterodimer reveals the structural basis of the repression of AhR-mediated transcription. J Biol Chem. 2017;292(43):17609–17616. doi: 10.1074/jbc.M117.812974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Pan L, Zhang X, Ji R, Si L, Cao Y. The molecular mechanism of AhR-ARNT-XREs signaling pathway in the detoxification response induced by polycyclic aromatic hydrocarbons (PAHs) in clam Ruditapes philippinarum. Environ Res. 2020;183:109165. doi: 10.1016/j.envres.2020.109165. [DOI] [PubMed] [Google Scholar]
- 16.Wang GZ, Zhang L, Zhao XC, Gao SH, Qu LW, Yu H, Fang WF, Zhou YC, Liang F, Zhang C, Huang YC, Liu Z, Fu YX, Zhou GB. The Aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat Commun. 2019;10(1):1125. doi: 10.1038/s41467-019-08887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaeger C, Tischkau SA. Role of aryl hydrocarbon receptor in circadian clock disruption and metabolic dysfunction. Environ Health Insights. 2016;10:133–141. doi: 10.4137/ehi.S38343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh R, Chandrashekharappa S, Bodduluri SR, Baby BV, Hegde B, Kotla NG, Hiwale AA, Saiyed T, Patel P, Vijay-Kumar M, Langille MGI, Douglas GM, Cheng X, Rouchka EC, Waigel SJ, Dryden GW, Alatassi H, Zhang HG, Haribabu B, Vemula PK, Jala VR. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun. 2019;10(1):89. doi: 10.1038/s41467-018-07859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bao A, Yang H, Ji J, Chen Y, Bao W, Li F, Zhang M, Zhou X, Li Q, Ben S. Involvements of p38 MAPK and oxidative stress in the ozone-induced enhancement of AHR and pulmonary inflammation in an allergic asthma model. Respir Res. 2017;18(1):216. doi: 10.1186/s12931-017-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, Zhao YY. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. doi: 10.1016/j.cbi.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Miao H, Cao G, Wu XQ, Chen YY, Chen DQ, Chen L, Vaziri ND, Feng YL, Su W, Gao Y, Zhuang S, Yu XY, Zhang L, Guo Y, Zhao YY. Identification of endogenous 1-aminopyrene as a novel mediator of progressive chronic kidney disease via aryl hydrocarbon receptor activation. Br J Pharmacol. 2020;177(15):3415–3435. doi: 10.1111/bph.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Yang T, Lu DW, Zhao H, Feng YL, Chen H, Chen DQ, Vaziri ND, Zhao YY. Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed Pharmacother. 2018;101:670–681. doi: 10.1016/j.biopha.2018.02.090. [DOI] [PubMed] [Google Scholar]
- 23.Hu HH, Cao G, Wu XQ, Vaziri ND, Zhao YY. Wnt signaling pathway in aging-related tissue fibrosis and therapies. Ageing Res Rev. 2020;60:101063. doi: 10.1016/j.arr.2020.101063. [DOI] [PubMed] [Google Scholar]
- 24.Feng YL, Chen H, Chen DQ, Vaziri ND, Su W, Ma SX, Shang YQ, Mao JR, Yu XY, Zhang L, Guo Y. Zhao YY (2019) Activated NF-κB/Nrf2 and Wnt/β-catenin pathways are associated with lipid metabolism in CKD patients with microalbuminuria and macroalbuminuria. Biochim Biophys Acta. 1865;9:2317–2332. doi: 10.1016/j.bbadis.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Zhao YY. Metabolomics in chronic kidney disease. Clin Chim Acta. 2013;422:59–69. doi: 10.1016/j.cca.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 26.Feng YL, Cao G, Chen DQ, Vaziri ND, Chen L, Zhang J, Wang M, Guo Y, Zhao YY. Microbiome-metabolomics reveals gut microbiota associated with glycine-conjugated metabolites and polyamine metabolism in chronic kidney disease. Cell Mol Life Sci. 2019;74(24):4961–4978. doi: 10.1007/s00018-019-03155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen DQ, Cao G, Chen H, Argyopoulos CP, Yu H, Su W, Chen L, Samuels DC, Zhuang S, Bayliss GP, Zhao S, Yu XY, Vaziri ND, Wang M, Liu D, Mao JR, Ma SX, Zhao J, Zhang Y, Shang YQ, Kang H, Ye F, Cheng XH, Li XR, Zhang L, Meng MX, Guo Y, Zhao YY. Identification of serum metabolites associating with chronic kidney disease progression and anti-fibrotic effect of 5-methoxytryptophan. Nat Commun. 2019;10(1):1476. doi: 10.1038/s41467-019-09329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen YY, Chen DQ, Chen L, Liu JR, Vaziri ND, Guo Y, Zhao YY. Microbiome-metabolome reveals the contribution of gut-kidney axis on kidney disease. J Transl Med. 2019;17(1):5. doi: 10.1186/s12967-018-1756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolivo DM, Larson SA, Dominko T. Tryptophan metabolites kynurenine and serotonin regulate fibroblast activation and fibrosis. Cell Mol Life Sci. 2018;75(20):3663–3681. doi: 10.1007/s00018-018-2880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen DQ, Cao G, Chen H, Liu D, Su W, Yu XY, Vaziri ND, Liu XH, Bai X, Zhang L, Zhao YY. Gene and protein expressions and metabolomics exhibit activated redox signaling and Wnt/β-catenin pathway are associated with metabolite dysfunction in patients with chronic kidney disease. Redox Biol. 2017;12:505–521. doi: 10.1016/j.redox.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun M, Ma N, He T, Johnston LJ, Ma X. Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR) Crit Rev Food Sci Nutr. 2019 doi: 10.1080/10408398.2019.1598334. [DOI] [PubMed] [Google Scholar]
- 32.Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discovery. 2019;18(5):379–401. doi: 10.1038/s41573-019-0016-5. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Chen DQ, Liu JR, Zhang J, Vaziri ND, Zhuang S, Chen H, Feng YL, Guo Y, Zhao YY. Unilateral ureteral obstruction causes gut microbial dysbiosis and metabolome disorders contributing to tubulointerstitial fibrosis. Exp Mol Med. 2019;51(3):1–18. doi: 10.1038/s12276-019-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T, Yin Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 2018;8:13. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moyer BJ, Rojas IY, Kerley-Hamilton JS, Hazlett HF, Nemani KV, Trask HW, West RJ, Lupien LE, Collins AJ, Ringelberg CS, Gimi B, Kinlaw WB, 3rd, Tomlinson CR. Inhibition of the aryl hydrocarbon receptor prevents Western diet-induced obesity. Model for AHR activation by kynurenine via oxidized-LDL, TLR2/4, TGFβ, and IDO1. Toxicol Appl Pharmacol. 2016;300:13–24. doi: 10.1016/j.taap.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Manzella CR, Ackerman M, Singhal M, Ticho AL, Ceh J, Alrefai WA, Saksena S, Dudeja PK, Gill RK. Serotonin modulates AhR activation by interfering with CYP1A1-mediated clearance of AhR ligands. Cell Physiol Biochem. 2020;54(1):126–141. doi: 10.33594/000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao K, Mu CL, Farzi A, Zhu WY. Tryptophan metabolism: a link between the gut microbiota and brain. Adv Nutr. 2019;11(3):709–723. doi: 10.1093/advances/nmz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luan H, Wang X, Cai Z. Mass spectrometry-based metabolomics: targeting the crosstalk between gut microbiota and brain in neurodegenerative disorders. Mass Spectrom Rev. 2019;38(1):22–33. doi: 10.1002/mas.21553. [DOI] [PubMed] [Google Scholar]
- 40.Jin Q, Black A, Kales SN, Vattem D, Ruiz-Canela M, Sotos-Prieto M. Metabolomics and microbiomes as potential tools to evaluate the effects of the mediterranean diet. Nutrients. 2019;11(1):207. doi: 10.3390/nu11010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yacoub R, Wyatt CM. Manipulating the gut microbiome to decrease uremic toxins. Kidney Int. 2017;91(3):521–523. doi: 10.1016/j.kint.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Vogel CFA, Van Winkle LS, Esser C, Haarmann-Stemmann T. The aryl hydrocarbon receptor as a target of environmental stressors—Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020;34:101530. doi: 10.1016/j.redox.2020.101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milanesi S, Garibaldi S, Saio M, Ghigliotti G, Picciotto D, Ameri P, Garibotto G, Barisione C, Verzola D. Indoxyl sulfate induces renal fibroblast activation through a targetable heat shock protein 90-dependent pathway. Oxid Med Cell Long. 2019;2019:2050183. doi: 10.1155/2019/2050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Li X, Zhang B, Fu Y, Yang A, Zhang H, Zhang H, Niu Y, Nie J, Yang J. CYP1A1 methylation mediates the effect of smoking and occupational polycyclic aromatic hydrocarbons co-exposure on oxidative DNA damage among Chinese coke-oven workers. Environ Health. 2019;18(1):69. doi: 10.1186/s12940-019-0508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trikha P, Lee DA. The role of AhR in transcriptional regulation of immune cell development and function. Biochim Biophys Acta. 2020;1:188335. doi: 10.1016/j.bbcan.2019.188335. [DOI] [PubMed] [Google Scholar]
- 46.Wang M, Chen DQ, Chen L, Cao G, Zhao H, Liu D, Vaziri ND, Guo Y, Zhao YY. Novel inhibitors of the cellular renin-angiotensin system components, poricoic acids, target Smad3 phosphorylation and Wnt/β-catenin pathway against renal fibrosis. Br J Pharmacol. 2018;175(13):2689–2708. doi: 10.1111/bph.14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Addi T, Poitevin S, McKay N, El Mecherfi KE, Kheroua O, Jourde-Chiche N, de Macedo A, Gondouin B, Cerini C, Brunet P, Dignat-George F, Burtey S, Dou L. Mechanisms of tissue factor induction by the uremic toxin indole-3 acetic acid through aryl hydrocarbon receptor/nuclear factor-κB signaling pathway in human endothelial cells. Arch Toxicol. 2019;93(1):121–136. doi: 10.1007/s00204-018-2328-3. [DOI] [PubMed] [Google Scholar]
- 48.Gondouin B, Cerini C, Dou L, Sallee M, Duval-Sabatier A, Pletinck A, Calaf R, Lacroix R, Jourde-Chiche N, Poitevin S, Arnaud L, Vanholder R, Brunet P, Dignat-George F, Burtey S. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 2013;84(4):733–744. doi: 10.1038/ki.2013.133. [DOI] [PubMed] [Google Scholar]
- 49.Addi T, Dou L, Burtey S. Tryptophan-derived uremic toxins and thrombosis in chronic kidney disease. Toxins. 2018;10(10):412. doi: 10.3390/toxins10100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–327. doi: 10.1161/circresaha.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dou L, Sallee M, Cerini C, Poitevin S, Gondouin B, Jourde-Chiche N, Fallague K, Brunet P, Calaf R, Dussol B, Mallet B, Dignat-George F, Burtey S. The cardiovascular effect of the uremic solute indole-3 acetic acid. J Am Soc Nephrol. 2015;26(4):876–887. doi: 10.1681/asn.2013121283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee WJ, Liu SH, Chiang CK, Lin SY, Liang KW, Chen CH, Tien HR, Chen PH, Wu JP, Tsai YC, Lai DW, Chang YC, Sheu WH, Sheu ML. Aryl hydrocarbon receptor deficiency attenuates oxidative stress-related mesangial cell activation and macrophage infiltration and extracellular matrix accumulation in diabetic nephropathy. Antioxid Redox Signal. 2016;24(4):217–231. doi: 10.1089/ars.2015.6310. [DOI] [PubMed] [Google Scholar]
- 53.Wu J, Jiang Z, Zhang H, Liang W, Huang W, Zhang H, Li Y, Wang Z, Wang J, Jia Y, Liu B, Wu H. Sodium butyrate attenuates diabetes-induced aortic endothelial dysfunction via P300-mediated transcriptional activation of Nrf2. Free Radical Biol Med. 2018;124:454–465. doi: 10.1016/j.freeradbiomed.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 54.Assefa EG, Yan Q, Gezahegn SB, Salissou MTM, He S, Wu N, Zuo X, Ying C. Role of resveratrol on indoxyl sulfate-induced endothelial hyperpermeability via aryl hydrocarbon receptor (AHR)/src-dependent pathway. Oxid Med Cell Long. 2019;2019:5847040. doi: 10.1155/2019/5847040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brito JS, Borges NA, Anjos JSD, Nakao LS, Stockler-Pinto MB, Paiva BR, Cardoso-Weide LC, Cardozo L, Mafra D. Aryl hydrocarbon receptor and uremic toxins from the gut microbiota in chronic kidney disease patients: is there a relationship between them? Biochemistry. 2019;58(15):2054–2060. doi: 10.1021/acs.biochem.8b01305. [DOI] [PubMed] [Google Scholar]
- 56.Wu PH, Lin YT, Wu PY, Lee HH, Lee SC, Hung SC, Chen SC, Kuo MC, Chiu YW. Association between circulation indole-3-acetic acid levels and stem cell factor in maintenance hemodialysis patients: a cross-sectional study. J Clin Med. 2020;9(1):124. doi: 10.3390/jcm9010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imhann F, Vich Vila A, Bonder MJ, Fu J, Gevers D, Visschedijk MC, Spekhorst LM, Alberts R, Franke L, van Dullemen HM, Ter Steege RWF, Huttenhower C, Dijkstra G, Xavier RJ, Festen EAM, Wijmenga C, Zhernakova A, Weersma RK. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67(1):108–119. doi: 10.1136/gutjnl-2016-312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beaumont M, Neyrinck AM, Olivares M, Rodriguez J, de Rocca Serra A, Roumain M, Bindels LB, Cani PD, Evenepoel P, Muccioli GG, Demoulin JB, Delzenne NM. The gut microbiota metabolite indole alleviates liver inflammation in mice. FASEB J. 2018 doi: 10.1096/fj.201800544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiang F, Cao X, Shen B, Chen X, Guo M, Ding X, Zou J. Transcriptome profiling reveals indoxyl sulfate should be culpable of impaired T cell function in chronic kidney disease. Front Med. 2020;7:178. doi: 10.3389/fmed.2020.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schroeder JC, Dinatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, Lin JM, Strom SC, Omiecinski CJ, Amin S, Perdew GH. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry. 2010;49(2):393–400. doi: 10.1021/bi901786x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koehler S, Kuczkowski A, Kuehne L, Jüngst C, Hoehne M, Grahammer F, Eddy S, Kretzler M, Beck BB, Höhfeld J, Schermer B, Benzing T, Brinkkoetter PT, Rinschen MM. Proteome analysis of isolated podocytes reveals stress responses in glomerular sclerosis. J Am Soc Nephrol. 2020;31(3):544–559. doi: 10.1681/asn.2019030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hung SC, Kuo KL, Huang HL, Lin CC, Tsai TH, Wang CH, Chen JW, Lin SJ, Huang PH, Tarng DC. Indoxyl sulfate suppresses endothelial progenitor cell-mediated neovascularization. Kidney Int. 2016;89(3):574–585. doi: 10.1016/j.kint.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 63.Zhao T, Zhang H, Yin X, Zhao H, Ma L, Yan M, Peng L, Wang Q, Dong X, Li P. Tangshen formula modulates gut Microbiota and reduces gut-derived toxins in diabetic nephropathy rats. Biomed Pharmacother. 2020;129:110325. doi: 10.1016/j.biopha.2020.110325. [DOI] [PubMed] [Google Scholar]
- 64.Kim JT, Kim SS, Jun DW, Hwang YH, Park WH, Pak YK, Lee HK. Serum arylhydrocarbon receptor transactivating activity is elevated in type 2 diabetic patients with diabetic nephropathy. J Diabetes Investig. 2013;4(5):483–491. doi: 10.1111/jdi.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuoka K, Kato K, Takao T, Ogawa M, Ishii Y, Shimizu F, Masuda J, Takada A. Concentrations of various tryptophan metabolites are higher in patients with diabetes mellitus than in healthy aged male adults. Diabetol Int. 2017;8(1):69–75. doi: 10.1007/s13340-016-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim HY, Yoo TH, Cho JY, Kim HC, Lee WW. Indoxyl sulfate-induced TNF-α is regulated by crosstalk between the aryl hydrocarbon receptor, NF-κB, and SOCS2 in human macrophages. FASEB J. 2019;33(10):10844–10858. doi: 10.1096/fj.201900730R. [DOI] [PubMed] [Google Scholar]
- 67.Shivanna S, Kolandaivelu K, Shashar M, Belghasim M, Al-Rabadi L, Balcells M, Zhang A, Weinberg J, Francis J, Pollastri MP, Edelman ER, Sherr DH, Chitalia VC. The aryl hydrocarbon receptor is a critical regulator of tissue factor stability and an antithrombotic target in uremia. J Am Soc Nephrol. 2016;27(1):189–201. doi: 10.1681/asn.2014121241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chitalia VC, Shivanna S, Martorell J, Balcells M, Bosch I, Kolandaivelu K, Edelman ER. Uremic serum and solutes increase post-vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation. 2013;127(3):365–376. doi: 10.1161/circulationaha.112.118174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sallee M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease. Toxins. 2014;6(3):934–949. doi: 10.3390/toxins6030934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, Fulcher G, Desai M, Li Q, Deng H, Rosenthal N, Jardine MJ, Bakris G, Perkovic V. Cardiovascular and renal outcomes with Canagliflozin according to baseline kidney function. Circulation. 2018;138(15):1537–1550. doi: 10.1161/circulationaha.118.035901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim HY, Yoo TH, Hwang Y, Lee GH, Kim B, Jang J, Yu HT, Kim MC, Cho JY, Lee CJ, Kim HC, Park S, Lee WW. Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD) Sci Rep. 2017;7(1):3057. doi: 10.1038/s41598-017-03130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin YT, Wu PH, Tsai YC, Hsu YL, Wang HY, Kuo MC, Kuo PL, Hwang SJ. Indoxyl sulfate induces apoptosis through oxidative stress and mitogen-activated protein kinase signaling pathway inhibition in human astrocytes. J Clin Med. 2019;8(2):191. doi: 10.3390/jcm8020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shang F, Wang SC, Hsu CY, Miao Y, Martin M, Yin Y, Wu CC, Wang YT, Wu G, Chien S, Huang HD, Tarng DC, Shiu YT, Cheung AK, Huang PH, Chen Z, Shyy JY. MicroRNA-92a mediates endothelial dysfunction in CKD. J Am Soc Nephrol. 2017;28(11):3251–3261. doi: 10.1681/asn.2016111215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang M, Wei R, Wang Y, Su T, Li P, Chen X. The uremic toxin hippurate promotes endothelial dysfunction via the activation of Drp1-mediated mitochondrial fission. Redox biology. 2018;16:303–313. doi: 10.1016/j.redox.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakano T, Katsuki S, Chen M, Decano JL, Halu A, Lee LH, Pestana DVS, Kum AST, Kuromoto RK, Golden WS, Boff MS, Guimaraes GC, Higashi H, Kauffman KJ, Maejima T, Suzuki T, Iwata H, Barabasi AL, Aster JC, Anderson DG, Sharma A, Singh SA, Aikawa E, Aikawa M. Uremic toxin indoxyl sulfate promotes proinflammatory macrophage activation via the interplay of OATP2B1 and Dll4-notch signaling. Circulation. 2019;139(1):78–96. doi: 10.1161/circulationaha.118.034588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiang HC, Lin LX, Hu XF, Zhu H, Li HP, Zhang RY, Hu L, Liu WT, Zhao YL, Shu Y, Pan HL, Li M. AMPK activation attenuates inflammatory pain through inhibiting NF-κB activation and IL-1β expression. J Neuroinflamm. 2019;16(1):34. doi: 10.1186/s12974-019-1411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ito S, Osaka M, Edamatsu T, Itoh Y, Yoshida M. Crucial role of the aryl hydrocarbon receptor (AhR) in indoxyl sulfate-induced vascular inflammation. J Atheroscl Thromb. 2016;23(8):960–975. doi: 10.5551/jat.34462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Islam J, Sato S, Watanabe K, Watanabe T, Ardiansyah Hirahara K, Aoyama Y, Tomita S, Aso H, Komai M, Shirakawa H. Dietary tryptophan alleviates dextran sodium sulfate-induced colitis through aryl hydrocarbon receptor in mice. J Nutr Biochem. 2017;42:43–50. doi: 10.1016/j.jnutbio.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 79.Bhattarai Y, Williams BB, Battaglioli EJ, Whitaker WR, Till L, Grover M, Linden DR, Akiba Y, Kandimalla KK, Zachos NC, Kaunitz JD, Sonnenburg JL, Fischbach MA, Farrugia G, Kashyap PC. Gut microbiota-produced tryptamine activates an epithelial G-protein-coupled receptor to increase colonic secretion. Cell Host Microbe. 2018;23(6):775–785. doi: 10.1016/j.chom.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 81.Hou Q, Ye L, Liu H, Huang L, Yang Q, Turner JR, Yu Q. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018;25(9):1657–1670. doi: 10.1038/s41418-018-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, Yarmush ML, Alaniz RC, Jayaraman A, Lee K. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018;23(4):1099–1111. doi: 10.1016/j.celrep.2018.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu J, Luo Y, Zhu Z, Zhou Y, Sun L, Gao J, Sun J, Wang G, Yao X, Li W. A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J Allergy Clin Immunol. 2019;143(6):2108–2119. doi: 10.1016/j.jaci.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 84.Cervantes-Barragan L, Chai JN, Tianero MD, Di Luccia B, Ahern PP, Merriman J, Cortez VS, Caparon MG, Donia MS, Gilfillan S, Cella M, Gordon JI, Hsieh CS, Colonna M. Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8αα(+) T cells. Science (New York, NY) 2017;357(6353):806–810. doi: 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meng D, Sommella E, Salviati E, Campiglia P, Ganguli K, Djebali K, Zhu W, Walker WA. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr Res. 2020;88(2):209–217. doi: 10.1038/s41390-019-0740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sakurai T, Odamaki T, Xiao JZ. Production of indole-3-lactic acid by Bifidobacterium strains isolated from human infants. Microorganisms. 2019;7(9):340. doi: 10.3390/microorganisms7090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xue Z, Li D, Yu W, Zhang Q, Hou X, He Y, Kou X. Mechanisms and therapeutic prospects of polyphenols as modulators of the aryl hydrocarbon receptor. Food Funct. 2017;8(4):1414–1437. doi: 10.1039/c6fo01810f. [DOI] [PubMed] [Google Scholar]
- 88.Mexia N, Gaitanis G, Velegraki A, Soshilov A, Denison MS, Magiatis P. Pityriazepin and other potent AhR ligands isolated from Malassezia furfur yeast. Arch Biochem Biophys. 2015;571:16–20. doi: 10.1016/j.abb.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen DQ, Feng YL, Cao G, Zhao YY. Natural products as a source for antifibrosis therapy. Trends Pharmacol Sci. 2018;39(11):937–952. doi: 10.1016/j.tips.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 90.Feng YL, Chen DQ, Vaziri ND, Guo Y, Zhao YY. Small molecule inhibitors of epithelial-mesenchymal transition for the treatment of cancer and fibrosis. Med Res Rev. 2020;40(1):54–78. doi: 10.1002/med.21596. [DOI] [PubMed] [Google Scholar]
- 91.Chen YY, Yu XY, Chen L, Vaziri ND, Ma SC, Zhao YY. Redox signaling in aging kidney and opportunity for therapeutic intervention through natural products. Free Radical Biol Med. 2019;141:141–149. doi: 10.1016/j.freeradbiomed.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 92.Chen DQ, Feng YL, Chen L, Liu JR, Wang M, Vaziri ND, Zhao YY. Poricoic acid A enhances melatonin inhibition of AKI-to-CKD transition by regulating Gas6/Axl NFκB/Nrf2 axis. Free Radical Biol Med. 2019;134:484–497. doi: 10.1016/j.freeradbiomed.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 93.Chen DQ, Cao G, Zhao H, Chen L, Yang T, Wang M, Vaziri ND, Guo Y, Zhao YY. Combined melatonin and poricoic acid A inhibits renal fibrosis through modulating the interaction of Smad3 and β-catenin pathway in AKI-to-CKD continuum. Ther Adv Chron Dis. 2019;10:2040622319869116. doi: 10.1177/2040622319869116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang T, Feng YL, Chen L, Vaziri ND, Zhao YY. Dietary natural flavonoids treating cancer by targeting aryl hydrocarbon receptor. Crit Rev Toxicol. 2019;49(5):445–460. doi: 10.1080/10408444.2019.1635987. [DOI] [PubMed] [Google Scholar]
- 95.Wang K, Feng C, Li C, Yao J, Xie X, Gong L, Luan Y, Xing G, Zhu X, Qi X, Ren J. Baicalin protects mice from aristolochic acid I-induced kidney injury by induction of cyp1a through the aromatic hydrocarbon receptor. Int J Mol Sci. 2015;16(7):16454–16468. doi: 10.3390/ijms160716454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feng C, Xie X, Wu M, Li C, Gao M, Liu M, Qi X, Ren J. Tanshinone I protects mice from aristolochic acid I-induced kidney injury by induction of CYP1A. Environ Toxicol Pharmacol. 2013;36(3):850–857. doi: 10.1016/j.etap.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 97.Ichii O, Otsuka-Kanazawa S, Nakamura T, Ueno M, Kon Y, Chen W, Rosenberg AZ, Kopp JB. Podocyte injury caused by indoxyl sulfate, a uremic toxin and aryl-hydrocarbon receptor ligand. PLoS ONE. 2014;9(9):108448. doi: 10.1371/journal.pone.0108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kolachalama VB, Shashar M, Alousi F, Shivanna S, Rijal K, Belghasem ME, Walker J, Matsuura S, Chang GH, Gibson CM, Dember LM, Francis JM, Ravid K, Chitalia VC. Uremic solute-aryl hydrocarbon receptor-tissue factor axis associates with thrombosis after vascular injury in humans. J Am Soc Nephrol. 2018;29(3):1063–1072. doi: 10.1681/asn.2017080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Belghasem M, Roth D, Richards S, Napolene MA, Walker J, Yin W, Arinze N, Lyle C, Spencer C, Francis JM, Thompson C, Andry C, Whelan SA, Lee N, Ravid K, Chitalia VC. Metabolites in a mouse cancer model enhance venous thrombogenicity through the aryl hydrocarbon receptor-tissue factor axis. Blood. 2019;134(26):2399–2413. doi: 10.1182/blood.2019001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Orlando IMC, Lafleur VN, Storti F, Spielmann P, Crowther L, Santambrogio S, Schodel J, Hoogewijs D, Mole DR, Wenger RH. Distal and proximal hypoxia response elements cooperate to regulate organ-specific erythropoietin gene expression. Haematologica. 2019 doi: 10.3324/haematol.2019.236406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garrison AM, Parrott JM, Tuñon A, Delgado J, Redus L, O’Connor JC. Kynurenine pathway metabolic balance influences microglia activity: targeting kynurenine monooxygenase to dampen neuroinflammation. Psychoneuroendocrinology. 2018;94:1–10. doi: 10.1016/j.psyneuen.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Faust D, Nikolova T, Watjen W, Kaina B, Dietrich C. The Brassica-derived phytochemical indolo[3,2-b]carbazole protects against oxidative DNA damage by aryl hydrocarbon receptor activation. Arch Toxicol. 2017;91(2):967–982. doi: 10.1007/s00204-016-1672-4. [DOI] [PubMed] [Google Scholar]
- 103.Wang Q, Yang K, Han B, Sheng B, Yin J, Pu A, Li L, Sun L, Yu M, Qiu Y, Xiao W, Yang H. Aryl hydrocarbon receptor inhibits inflammation in DSS induced colitis via the MK2/pMK2/TTP pathway. Int J Mol Med. 2018;41(2):868–876. doi: 10.3892/ijmm.2017.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mohammadi-Bardbori A, Omidi M, Arabnezhad MR. Impact of CH223191-Induced Mitochondrial Dysfunction on Its Aryl Hydrocarbon Receptor Agonistic and Antagonistic Activities. Chem Res Toxicol. 2019;32(4):691–697. doi: 10.1021/acs.chemrestox.8b00371. [DOI] [PubMed] [Google Scholar]