Abstract
Transfer RNA (tRNA)-derived fragments (tRFs) are an emerging class of conserved small non-coding RNAs that play important roles in post-transcriptional gene regulation. High-throughput sequencing of multiple biological samples have identified heterogeneous species of tRFs with distinct functionalities. These small RNAs have garnered a lot of scientific attention due to their ubiquitous expression and versatility in regulating various biological processes. In this review, we highlight our current understanding of tRF biogenesis and their regulatory functions. We summarize the diverse modes of biogenesis through which tRFs are generated and discuss the mechanism through which different tRF species regulate gene expression and the biological implications. Finally, we conceptualize research areas that require focus to strengthen our understanding of the biogenesis and function of tRFs.
Keywords: tRNA-derived fragments, tRFs, tsRNA, Post-transcriptional regulation, Small RNAs, Non-coding RNAs
Introduction
Transfer RNA (tRNAs) are a class of ubiquitously expressed and conserved class of RNA species that are non-coding in nature. They constitute ~ 10% of the entire cellular RNA and play a fundamental role in protein synthesis. tRNAs function as adapter molecules that deliver amino acids to the translating peptide chain. For decades, the role of tRNAs were thought to be limited to its role in translation. It is becoming increasingly evident that tRNAs, apart from their canonical role in translation, can regulate gene expression through several mechanisms, including that of codon usage bias, their modifications and aminoacylation (charging) levels, or the more recently discovered tRNA-derived fragments (tRFs). Advancements in next-generation, small-RNA sequencing technologies have enabled the identification and characterization of this new class of tRFs that remained ignored for decades. Several recent studies have identified different sizes of these fragments processed from different regions of the parental tRNA. These tRFs have been shown to regulate gene expression through diverse mechanisms in various biological contexts.
Sequencing of various biological samples show that tRFs are conserved across prokaryotes and eukaryotes. tRFs have been identified in bacteria, algae, archaea, protozoa, worms, plants, yeast, Drosophila melanogaster and mammals [1]. The sequence conservation of tRFs suggest that these small RNAs may have evolved earlier than other small RNAs such as micro-RNAs (miRNA). We speculate that the abundant and ubiquitous expression of tRNAs in all organisms could serve as a source for the production of these small RNAs. While several attempts have been made to understand these tRNA-derived small RNAs, the clear rules on how and under what context they are processed, and their biological impact remains poorly understood. There has been a growing interest in tRFs due to their importance in certain biological processes, such as cellular stress, cancer, stem cell differentiation and paternal inheritance. In this review, we summarize the current understanding on the different types of tRFs, their processing and biological relevance.
Transfer RNAs (tRNAs)—One molecule to make them all
Apart from their conventional roles in translation, tRNAs (~ 72 nt) can be processed into smaller biologically active tRNA-derived fragments of size ranging from ~ 18–50 nt. The earliest reports of products derived from tRNAs were from 40 years ago, wherein fragments of tRNAs were observed in cancer patients [2]. Later in 1990s and early 2000s, the phenomenon of tRNA cleavage was established with the identification of endonucleases such as Colicin and Prrc in the cleavage of tRNAs in E.coli [3, 4]. Though tRNAs have been known to undergo cleavage, these products were largely ignored products of non-specific degradation. Since then, several years of intense research has identified several species of small RNAs (miRNA, piRNA, snoRNAs etc.) that are now recognised as biologically ‘active’ molecules with diverse regulatory roles. Joining the family of small RNAs, there is now growing evidence for the presence of tRNA-derived tRFs across diverse biological specimens. tRFs are recognised to be a new species of small RNAs derived from tRNAs that can be classified based on their size and the region of tRNA from which they are derived [5]. Interestingly, depending on the biological context, not all tRNAs are processed into tRFs thereby suggesting some specificity or selectivity in their biogenesis.
Different types of tRNA-derived fragments
tRFs from the 5′ half of the tRNA—tsRNAs/tiRNAs/tRNA halves
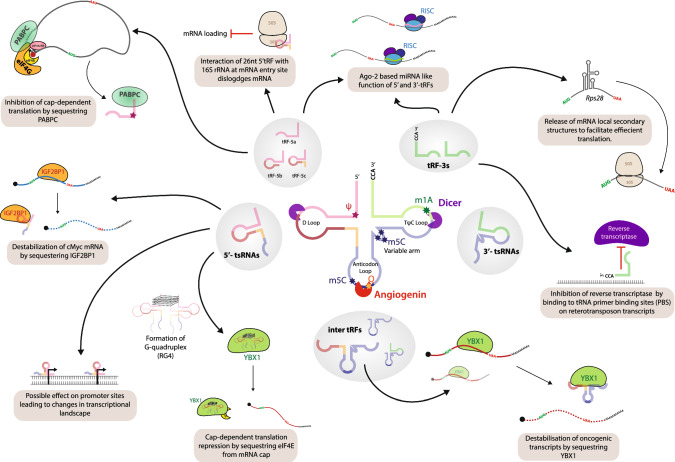
The 5′ half of the tRNA form a hub for some of the most abundant species of tRFs. tRFs ranging from 18—35 nt can be processed from the 5′ arm of the mature tRNA. The most common species of tRFs identified from this specific region are the tRNA-derived small RNA (tsRNAs/tiRNAs/tRNA halves). 5′-tsRNAs are usually the most abundant tRFs species with size ranging from 30–35 nts. 5′-tsRNAs usually encompass the first nucleotide of the tRNAs and typically terminates around the anticodon loop of the tRNA (Fig. 1). These small RNAs are generally produced in response to stress but there is mounting evidence to suggest that they play crucial roles in cancer, stem cell differentiation, transgenerational inheritance etc. [6–9].
Fig. 1.
Biogenesis and the roles of tRNA-derived fragments
Other smaller tRFs have also been identified from this region. These fragments termed as tRF-5s could be further grouped into 3 sub-groups based on their sizes; 18–20 nt long, ~ 25 nt long and the 27–30 nt long tRF-5s [10, 11] (Fig. 1). All these small RNAs have their 5′ end corresponding largely to the 5′ end of the tRNA suggesting that it is the endonucleolytic activity at the 3′ end which determines the size of these fragments.
3′-tsRNAs/3′-half
This group comprises the counterpart of the 5′-tsRNA species, wherein the 3′ ends of these tRFs can be mapped to the 3′ ends of the tRNA. The 5′ ends of these small RNAs map to the anticodon region and they may extend into the CCA region of the mature tRNA (Fig. 1). These tRFs, much like the tRFs-5 s of the 5′ arm, can be categorized based on their size into the 3′-tsRNAs or 3′-halves (35–45 nt species) and the smaller tRF-3s [18–20 nts] [12, 13]. The 3′-tsRNAs (or halves) were shown to be co-processed along with their 5′-counterparts in cellular stress conditions [13].
itRFs and 3′U-tRFs
tRNAs can also give rise to other classes of small RNAs, where the start or end positions do not match with the 5′-or the 3′end of the parent tRNA. These small RNAs are generally termed as itRFs (Fig. 1). These may range from ~ 18 to 50 nt in length and are usually less abundant as compared to the other two groups. These kind of small RNAs have been observed in breast cancer cell lines [14].
The 3′U-tRFs are by-products of tRNA processing. These small RNAs correspond to the 3′ trailer sequence of the premature tRNA that is then processed by RNase Z [15]. The trailer sequence carries a polyuridine tail and hence the name 3′U-tRFs. These small RNAs are ~ 18 -20 nts in size.
Biogenesis of tRNA-derived fragments
The diversity in the generation of differently sized small RNA fragments from tRNAs and the selection of specific tRNAs in the processing of tRFs suggests the existence of multiple intricate processing programs. To comprehend the variability in tRF processing it is essential to understand the processing of mature tRNAs and the features of tRNAs that may regulate their processing into tRFs. Transfer RNAs are transcribed by RNA Polymerase III as a premature tRNA transcript. This premature structure undergoes processing by two endonucleases, namely RNase P and RNaze Z. The by-products (the 5′-leader and the 3′-trailer sequence) of this step can also function as individual tRFs, notably the 3′U-tRFs. Some tRNAs carry intronic sequences in their anticodon loop, that is cleaved by the SEN/TSEN complex and the anticodon loop is ligated to form the tRNA. The trimmed tRNA undergoes several modifications and folding to assume an L shaped 3D conformation. tRNAs can be identified by their characteristic structures, the D-loop, the anticodon loop and the TΨC loop. Several studies suggest that tRFs are indeed processed from mature tRNAs with the exception of 3 ‘U-tRFs. This is evident from the fact that tsRNAs may carry modifications that indicate post transcriptional events during the biogenesis of the parental tRNA [6]. It has been long speculated if the expression of the tRFs is dependent on the expression of the parent tRNA. Unfortunately, addressing this question has been challenging due to the difficulties in sequencing tRNAs. A recent study showed that tissue-specific expression of certain tRNAs drive the tissue-specific expression of tRFs [16]. However, the proteins and factors involved in the processing of tRNAs into tRFs remain poorly understood and requires research focus.
Processing of tsRNAs or tRNA halves
One of the better understood aspects of tRF biogenesis is the stress-induced processing of tsRNAs/tRNA halves by Angiogenin (Ang). Angiogenin is a vertebrate-specific enzyme belonging to the RNase A family. Early studies have established a role for angiogenin in various biological processes such as angiogenesis, oncogenesis etc. Early in vitro studies had shown that angiogenin preferentially cleaves tRNAs [17]. Systematic studies from several research groups showed that angiogenin cleaves tRNAs into 5′ and 3′ halves under stress conditions [13, 18, 19] (Fig. 1). During normal cell homeostasis, Ang is retained in an inhibited state through its interactions with RNH1 (Ribonuclease/Angiogenin Inhibitor 1). This interaction is disrupted under stress conditions leading to Angiogenin-mediated cleavage of the tRNAs [20]. It is also important to note that Angiogenin-associated processing into tsRNAs may be limited to few tRNAs. It must be noted that while Angiogenin is the most widely studied tRNA-processing endonuclease, there may be other endonucleases that could perform similar functions. Processing of tRNAs by Angiogenin results in the formation of 2′-3′-cyclic phosphates at the 3′ end of the tsRNA, which cannot be sequenced using conventional sequencing protocols. Treatment of Angiogenin cleaved tsRNAs with T4 polynucleotide kinase (T4 PNK) converts the cyclic phosphates to 3′-OH, thereby rendering these fragments amenable to sequencing [9]. Furthermore, this technique can also be used to distinguish tsRNAs that are processed in an Angiogenin-dependent versus independent manner. T4PNK treatments of RA-induced differentiating mESCs and cauda sperm RNA followed by sequencing, failed to show any increase in the tsRNA populations observed through conventional sequencing strategies, thereby suggesting the existence of alternate tsRNA processing modules [7, 21]. A recent functional study aimed at understanding angiogenin-mediated tsRNA processing identified tsRNAGly, tsRNAGlu, tsRNALys, tsRNAVal, tsRNAHis, tsRNAAsp and tsRNASeC to undergo Angiogenin-dependent processing upon Ang overexpression [12]. However, in an angiogenin KO background, only the expression levels of tsRNAHis and tsRNAAsp changed, while the others remain unchanged suggesting the presence of additional endonucleases that may be involved in the processing of tsRNAs.
Given that Angiogenin is a vertebrate specific protein, it is not surprising that there may be other nuclease that could cleave tRNA into tsRNAs, since these are ubiquitously expressed across prokaryotes and eukaryotes. Alternate stress related studies in Arabidopsis, S. cerevisae and Tetrahymena identified RNaseT2 in the processing of tRNA halves [22–24]. Similarly, other nucleases implicated in the processing of tRNAs into tsRNAs/tRNA halves are RNase L, RNase I and Endonuclease V [25–27]. While RNase L has been implicated in the production of tsRNAs from tRNAGln, tRNAHis and tRNAPro in human cells; RNase 1 (a secreted endonuclease) seems to be involved in the production tsRNAs in the extracellular environment. The growing catalogue of nucleases involved in the production of tRFs suggests that certain cellular responses may activate specific endonucleases that cleave different sets of tRNAs.
Processing of tRFs
Small RNA sequencing from Hela cell extracts identified tRF-5s that are processed by the miRNA processing enzyme, Dicer. These fragments were 19nt long and were predominantly from tRNAs tRNAGln, tRNALys, tRNAVal, and tRNAArg. In vitro studies with tRNAGln and Dicer extracts conclusively showed that tRNAs are susceptible to endonucleolytic attack from Dicers [28]. The tested tRNAs had certain sequence features, which included a conserved GG dinucleotide at the 18th position preceding a UU dinucleotide at the 20th position. This study also established the interaction of these fragments with Ago2 complex, albeit at lower levels. Furthermore, the Dicer knockout ESCs also revealed the disappearance of a specific tRF populations corresponding to the 18–20 nt tRF-3s implicating its function in the processing of tRNAs [29] (Fig. 1). Although this study showed depletion of tRFs-3 s in the dicer KO condition, the involvement of other endonucleases in the generation of tRFs cannot be ruled out. RNase Z/Elac2, another endonuclease involved in the processing of tRNAs from its premature precursor also generates tRFs [15]. These tRFs have a unique uridine tail, which is typically present at the 3′ ends of premature tRNAs. Therefore, the RNase Z-processed tRNA-fragments are termed as 3′U-tRFs.
tRNA modification and its importance in biogenesis of tRNA-derived small RNAs
tRNAs are one of the most modified RNA species in any cell carrying a multitude of base modifications. Typically, eukaryotic tRNAs carry > 100 different modifications on different nucleotides with an average of 13 modifications per tRNA. While some modifications confer stability to the tRNA, other modifications facilitate tRNA folding, ribosomal interactions and wobble base pairing [30]. In recent years, a handful of studies have highlighted the importance of tRNA modifications in the generation of tRNA-derived fragments [10, 31–33].
One of the well understood modifications that serves as an important determinant in the production of tsRNAs is the 5-methylcytosine (m5C) modification. This modification is catalysed by methyltransferases containing the NOP2/Sun domain and Dnmts [34]. Although m5Cs are present in mRNA and other RNAs, tRNAs contribute to the majority of m5C modifications in the cell. There are seven RNA methyltransferases that have been identified that can methylate the Cytidine residues of RNA; Dnmt2 and the Nsun 1–6 [35]. While some methyltransferases selectively target a subset of tRNAs, others can methylate different RNA species. NSUN2 is a well characterized member of the NSUN family of proteins that methylates the cytidine residues of most tRNAs at the 34th and 48–50th position (Fig. 1). In a study conducted on NSUN2-deficient mice, the authors report that the loss of m5C from the tRNAs render the tRNAs susceptible to endonucleolytic cleavage by Ang resulting in the production of 5′-tsRNAs. Of the Nsun family members, only NSUN2 acts on a variety of different RNA substrates. Other members of the NSUN family methylate specific tRNAs; for example, NSUN6 methylates C72 residue of the tRNAs charged with cysteine and threonine. Similarly, Dnmt2 only methylates the C38 residue of tRNAGlyGCC, tRNAValAAC and tRNAAspGTC. The difference in the extent of tRNA modifications leading to the selectivity of RNA processing enzymes to specific tRNAs can explain the context-specific processing of tRNAs into tsRNA.
Converging studies indicate that the presence of methylation stabilizes the tRNA and prevents endonucleolytic cleavage [31, 34]. However, there are demethylases that could erase these marks making the tRNA vulnerable to nucleases [36]. Recent studies have identified AlkB (alpha-ketoglutarate binding) family of proteins to be involved in the demethylation of tRNAs. Alkbh3 was shown to demethylate the m1A and m3C residues on cytoplasmic tRNA resulting in the formation of 5′-tsRNAs in an angiogenin-dependent manner [33]. The same study also identified tRNAGlyGCC as the only tRNA to be upregulated in an Alkbh3 KO background. Thus, the combination of methylation and demethylation of specific tRNAs may explain the selective processing of tRNAs into tRFs and is pivotal to understand the biogenesis of tsRNAs. Furthermore, many of these proteins also act on different species of RNAs and some are involved in epigenetic regulation, adding to the complexity in understanding their specific effects on tRNA.
Pseudouridylation (Ψ) also critically regulates the tsRNA species in stem cells. In a recent study on the role of PUS7, a pseudouridylation enzyme in human ESCs, the authors found that the loss of PUS7 is concurrent with the loss of ~ 18 nt tRF-5 species suggesting that the presence of this modification may attract endonucleases to process tRNAs into tRF-5s [10] (Fig. 1). While Dicer is generally thought to be responsible for the production of tRF-5s, it remains unclear whether there is a crosstalk between PUS7 or the Ψ modification with Dicer. In the same study, it was also reported that the loss of pseudouridylation in tRNAs resulted in the increase of 5′-tsRNAs. This finding is interesting as it suggests that the presence or absence of pseudouridine may select for different endonuclease and, therefore, produce different species of tRNA fragments (tRF-5s or 5′-tsRNAs) in the context of a specific cell state.
Queuosine (Q) is another important modification that is incorporated at the wobble position of the anticodon. In humans, this particular modification occurs on specific tRNAs carrying GUN in the anticodon loop (Fig. 1). Queuine, the nucleobase that substitutes guanine, is supplied from the gut microbiome as eukaryotes lack the ability to synthesize queuine [37]. This modification of guanosine is catalyzed by QTRT (Queuine TRNA-Ribosyltransferase Catalytic Subunit 1) in eukaryotes. In a study conducted in E.coli, this particular modification was shown to be protective at the C34 position in GUN anticodon carrying tRNAs, preventing RNase L activity and the formation of tsRNAs [25]. A complementary study conducted in Hela cells arrived at the similar conclusion suggesting a conserved role for Q in protecting the tRNAHis and tRNAAsn [32].
It is important to note that many of these tRNA modifying enzymes depend on metabolites as the donor or the cofactors that regulate in their catalytic activity. For example, the methyltransferases depend solely on S-adenosylmethionine (SAM) and methionine as the methyl donor, whereas the demethylase AlkBh3 requires alpha-ketoglutarate as its cofactor. Thus, the availability of these metabolites may dictate the tsRNA/tRF biogenesis. While there is scope for research to establish how the metabolic state of the cells impinge on the methylation of tRNAs, a study in NUSN2 null cells concluded that the loss of NSUN2 methylation resulted in metabolic changes with increased protein degradation and translation arrest [38]. Similarly, it has been observed that the tRFs themselves can inhibit tRNA modifying enzymes. In vitro studies have shown that different species of tRFs can inhibit ADATs (adenosine deaminase acting on tRNAs) in the deamination of Adenosine at the 34th position of tRNAArgACG and tRNAAlaAGC to generate inosines [39]. These results suggest the existence of a feedback mechanism between RNA methylation and metabolism. Another alluring possibility is to understand how the gut microbiome could dictate tsRNA production as queousine requires queuine that is supplied by the microbiome in the body.
tRNA-derived fragments—mechanism of action
Recently, there have been a lot of studies aimed at understanding the biological roles of tRNA-derived small RNAs. The sheer heterogeneity in the types of tRFs under different conditions suggest that these small RNAs could adopt several molecular mechanisms to carry out distinct biological functions. It has been challenging to perform functional studies, because specific perturbation of the function of these small RNAs (through mutations and loss-of-function) remains non-trivial as it could also disrupt the parent tRNA. The lack of proper understanding of endonucleases in the biogenesis of tRFs further compounds this conundrum. Additionally, it is important to note that the effects of these small RNAs can be exerted both in the nucleus and in the cytoplasm making these small RNAs dynamic modulators of gene expression. As of now, most studies have employed the use of antisense oligos (ASOs) to target these molecules to understand their biological roles. Interestingly, the tRFs employ different modes of post-transcriptional regulation to perform their function. To overcome these obstacles, recent studies have described methods to purify endogenous tsRNAs using chromatography and hybridization-based techniques [40, 41]. Interestingly, the endogenous tsRNAs either carried no modifications or carried modifications that were reported in parent tRNAs. The dichotomy in the modifications that tsRNAs carry alludes to an interesting possibility, where in, the functioning of the tsRNAs may be altered by the presence or absence of a particular modifications. Here we attempt to summarize the various strategies that have been used to characterize the function of tRFs.
tsRNA—not lost in translation
One of the better understood roles of these small RNAs lies in the regulation of translation. It has long been speculated that these small RNAs function as regulators of translation. A case for tsRNAs as translational regulators can be made, when considering the fact that most of these fragments carry features from the tRNA that could allow these small RNAs to interact with the translation apparatus. Their role in translational regulation was first reported in stress-associated conditions [13]. As described previously, cellular stress leads to tRNA cleavage by Angiogenin producing 5′ and 3′ tsRNAs (or halves) [12, 18]. These 5′ tsRNAs termed as tiRNA (mainly from tRNAAlaUGC and tRNACysCGA) carrying a terminal-oligoG motif (TOG) at the 5′ end were able to displace the mRNA cap-binding protein, eIF4E, from binding to the m7G cap of mRNAs thus resulted in global translational shutdown [19]. Mechanistically, it was shown that the TOG sequence facilitates G-quadruplex (RG4) formation involving four molecules of tiRNAs [42]. This structure is then bound by YBX1 and this complex of tiRNA-YBX1 dislodges eIF4E from mRNA caps [19] (Fig. 1).
The role of tsRNAs in translation regulation was also independently validated by several groups that employed in vitro rabbit reticulocyte translation system to show that 5′-tsRNAs could indeed arrest translation [7, 43]. Interestingly, the repressive role of 5′-tsRNAs does not seem limited to tsRNAAla and tsRNACys as previously reported. There also apprears to be other features that 5′-tsRNAs carry that may be important for its repressive role. In an in vitro study, it was identified that the GG dinucleotide that is conserved across eukaryotic tRNAs is crucial for its role in translation [43]. The 5′-tsRNAs was shown inhibit translation even if their sizes were reduced to 19 nt compared to their usual ~ 30 nt. However, by mutating the GG dinucleotide resulted in the loss of this inhibitory effect [43]. It remains elusive how the GG dinucleotide interferes with translation. Notably, several studies have also implicated direct interactions of tsRNAs with ribosomes. In a recent study carried out in differentiating mouse ESCs, we showed that 5′-tsRNAs co-sedimented along with the monosomal and polysomal fractions [7]. Furthermore, RNA immunoprecipitation experiments using biotin-tagged 5′-tsRNAs, enriched for several ribosomal proteins. Interestingly, we observed differential association of ribosomal proteins with 5′-tsRNAs in ESCs vs differentiating cells suggesting that 5′-tsRNAs could; a) act on different positions on the ribosomes, or b) interact with select ribosomes that carry certain ribosomal proteins or ribosomal protein modifications. A biochemical study in the archaea, Halofex volcanii, concluded that 5′ tsRNA (tsRNAVal) binds to the 16S rRNA, that corresponds to the mRNA entry site on the 30S subunit of ribosomes thus impairing the mRNA-ribosome association [44] (Fig. 1). While there is a lot of evidence supporting the notion of 5′-tsRNAs interacting with ribosomes, a comprehensive understanding of the nature of these interactions remain to be explored.
Interestingly, tRF-5s were also shown to regulate translation. In a study focused on understanding the roles of tRNA modifications, specifically Ψ that is catalysed by PUS7, the authors identified a role for tRF-5s carrying Ψ modification in translation [10]. The authors observed that knockout of PUS7 led to the reduction in tRF-5 along with a global increase in translation. In this study, the authors present a mechanism, wherein the tRF-5s carrying the Ψ at the 8th position preferentially sequester PABPC, an integral protein responsible for polyA binding and initiation of cap-dependent translation, from the eIF4G/A complex (Fig. 1). The authors conclusively show that the lack of Ψ modifications render the tRFs incapable of sequestering PABPC, thus highlighting the importance of such modifications in the biogenesis as well as biological function(s) of tRNA fragments.
Sequence-complementarity based functions
The ~ 19 nt tRFs that are generated from the tRNAs have long been annotated as miRNAs or piRNAs. Early studies on ~ 21 nt 3′-CCA tRFs revealed a miRNA-like function for these small RNAs. These small RNAs that are processed by Dicer are able to associate with AGO to repress translation through sequence-complementarity [45] (Fig. 1). The authors focused on a single tRF (CU1276) that is derived from the tRNAGlyGCC. This type of miRNA-like regulation was further strengthened in a meta-analysis carried out to identify the targets of tRFs [46]. Analysis of PAR-CLIP samples revealed that both tRF-3s and tRF-5s bind AGO proteins, mainly with AGO1, 3 and 4. Furthermore, in the same study these tRFs were also shown to interact with transcripts in an AGO1-dependent manner. Interestingly, the authors identified a greater representation of tRF-mRNA interactions as compared to miRNAs from their AGO1 CLASH data.
A more comprehensive study was conducted in Drosophila S2 cells by analyzing 495 publicly available small RNA data sets [11]. In this study, the authors report that the majority of the tRNA derived small RNAs in Drosophila were 21–29 nts in length and mapped to the 5′ of parent tRNAs. Using RNA-Seq and Ribo-Seq techniques the authors showed that the transfected tRFs (or tsRNAs as they call it) were able to repress translation of specific mRNAs through sequence complementarity. Interestingly, they showed that these tRFs arise independent of Dicer processing. Bioinformatic analysis of tRF-target complementarity resulted in the authors identifying a conserved 7-mer base pairing. Most of the tRFs targeted the ribosomal proteins and the transfection of tRF mimics resulted in profound inhibition of translation. Interestingly, the studies also revealed that although tRFs appear to function like microRNAs, individually they target distinct sets of mRNAs.
tRFs are also known to associate with Piwi proteins in several species ranging from Tetrahymena to humans. Generally, shorter tRFs associate with Ago1 or Ago2 and larger fragments ranging from 26nt-35nt interact with Ago3 or the Piwi proteins, suggesting a piRNA-like role for these small RNAs. As a deviation from this phenomenon, 18-22nt tRFs of Tetrahymena are found in complexes with the piwi proteins Twil2, that were shown to recruit Xrn1 [47]. In the study that identified miRNA-like functions of tRFs in Drosophila, the authors identified larger fragments of tRFs (~ 26 nts) associated with AGO3/AUB proteins that is involved in the piRNA ping-pong pathway [11]. Recently, we showed that majority of the 5′-tsRNAs expressed in planarians interact with all the three piwi proteins expressed in planarians and important for regeneration [48]. Analysis of RNAs interacting with Piwi proteins in human breast cancer cell lines MDA-MB231 revealed that human piwi2, Hiwi2, interacts mainly with 5′-tsRNAs with sizes ranging from 27–34 nts [49]. Interestingly, comparisons with mouse piwi2 suggested that piwi2-tRNAs association could be limited to human samples. This study also implicates tsRNA-piwi2 interaction in the regulation of translation as this complex co-sediments with polysomal fractions and interacts with proteins involved in translation.
Technical advancements have allowed the identification of large scale intermolecular and intramolecular interactions. Increasing evidence suggests that tRNA fragments can regulate target transcripts through direct, sequence complementarity-based regulation. Apart from miRNA-like functions in association with Ago2, these small RNAs can act directly on the transcript. This was highlighted in a recent report that ascribed a positive regulatory role for tRF-3s. Kim et al. [50], showing that the 3′ tsRNALeuCAG (a 22 nt sequenced derived from the 3′ end of tRNALeuCAG) is complementary to rps28 mRNA. The complementarity observed was within a highly structured region in the rps28 mRNA. This study revealed that the interaction of tRFLeuCAG with rps28 mRNA resolves the secondary structure in the mRNA enabling the efficient translation of rps28, thereby facilitating ribosome biogenesis (Fig. 1). This is one of the first reports that showed tRFs as positive regulators of translation.
This sort of direct association with mRNAs was also observed for 5′-tsRNAs in mouse ESCs. Differential pulldowns with tsRNA baits from lysates with and without proteins revealed that certain mRNAs showed sequence complementarity-based interactions, while others require the presence of adapter proteins [7]. However, the rules for these interactions remain unclear. In-depth bioinformatic analysis paired with functional validation studies will be required to dissect the functional relevance of these two types of tsRNA interactions.
Interaction with ribonucleoproteins
Several studies have aimed to attribute roles to tRFs from the perspective of the proteins that they bind to. Few studies have identified tRFs in various CLIP-Seq datasets and CLASH datasets as previously described, while others have employed the synthetic tsRNAs mimics to be used as bits in pulldown experiments. Each method offers its own set of merits and drawbacks but surely expands our understanding of how these molecules function.
One of the earliest studies to characterize the role of 5′-tsRNAs (tiRNAs) in translational control during stress conditions utilized biotin-tagged candidate tiRNAs to identify the protein interactome of these small RNAs [19]. This method resulted in the identification of several RNA-binding proteins that interact with tiRNAs which paved way to establishing the role YBX1-tiRNA in translation regulation. Several of the proteins identified are involved in RNA metabolism and those that are involved in translation indicating a strong post-transcriptional function for tiRNAs. In this study, the authors also identified interactions of tiRNA with Ago2 suggesting that there could be an interference or overlap with the miRNA pathway, which still remains unexplored. The interaction of YBX1 with a different species of tRNA fragments, the itRFs, in the breast cancer cell line MDAMB231 suggested that these tRFs may act as defence mechanisms against cancer [14]. Specifically, they identified that these tsRNAs (tsRNAGlu, tsRNAAsp, tsRNAGly, and tsRNATyr) were hypoxia induced and shared a common sequence motif “SCUBYC”. The tRFs in this study competitively bound YBX1 thereby resulting the destabilization of YBX1 targets (Fig. 1).
Similarly, in our study that aimed at understanding the roles of 5′-tsRNAs in differentiating mouse embryonic stem cells, we performed pulldown experiments with candidate tsRNAs (tsRNAGlyGCC, tsRNAValCAC, tsRNAGlnCTG, tsRNAGluCTC, tsRNALysTTT) to understand the role of these small RNAs in stem cell differentiation [7]. The analysis of the proteins led to the identification of IGF2BP1, as an important tsRNA interacting-protein that regulates the transcript stability and translation of cMyc (Fig. 1). In the same study, we also identified YBX1 and other ribosomal proteins strongly indicating a function for tsRNAs in translational regulation. Notably, the interaction of tsRNAs with proteins were dynamic between the stem and differentiating conditions suggesting that tsRNAs could preferentially bind to distinct sets of proteins under different developmental conditions [7].
Nuclear functions of tRNA-derived fragments
Many studies have identified tRFs in the nucleus [6, 51]. Since most of the tRNA processing occurs within the nucleus before the mature tRNA is exported out, it is quite possible that certain species of tRFs could be processed in the nucleus. The presence of tRFs in the nucleus could indicate a regulatory role for the small RNAs in transcriptional control.
Two seminal studies on 5′-tsRNAs, revealed an important role for these small RNAs in transgenerational inheritance [6, 8]. The authors showed that diet regulates the tsRNAs levels in sperms that eventually regulate the metabolism in the offspring. Chen et al., showed that 5′-tsRNAs carried in the sperm had sequence complementary to certain promoter regions, which suggested their putative role in shaping the transcriptional landscape (Fig. 1). Interestingly, modifications in the injected tsRNAs, particularly methylation, proved to be crucial for their activity. In a complementary study, the authors also identified DNMT2 to be important for the methylation of sperm tsRNAs [52]. Sharma et al. arrived at similar conclusions in which the offsprings of mice subjected to low-protein diet showed dysregulation in tsRNAGlyGCC levels resulting in the alteration of the retrotransposon MERVL levels. This change in MERVL-levels and its target genes resulted in altered growth of the offspring. However, in this study, they identified that tsRNAGlyGCC that was enriched in the sperm of the low-protein diet parent, was able to downregulate the several ribosomal proteins and the targets of MERVL retrotransposon.
Likewise, 3′ tRFs were also identified as crucial regulators of the retrotransposons in the cell [51]. In this study, the authors observed an enrichment for 3′ tRFs in mouse ESCs that lacked histone 3 lysine trimethylation (responsible for keeping retrotransposons in check). Further analysis, it was revealed that these 3′ tRFs were antisense to the tRNA primer binding site (PBS) on an active retrotransposon. The authors demonstrated a dual check mechanism for the regulation of retrotransposon expression, where in the 18 nt tRF-3s were able to inhibit the reverse transcription of the retrotransposon, and the 22 nt tRF-3s were able to post-transcriptionally silence the retrotransposon transcript (Fig. 1).
Biological roles of tRNA-derived fragments
tRNA-derived fragments are rapidly emerging as one of the largest regulators of gene expression in various studies with far-reaching biological implications. Due to their highly conserved nature and ancient origins, it is possible that tRFs could be involved in regulating several cellular processes. Multiple studies have identified a role for these small RNAs in regulating the simplest to complex biological processes [6, 7, 14, 32, 48, 53, 54]. Abnormal levels of these small RNAs, especially in diseases conditions, have become attractive to researchers interested in developing these small RNAs as biomarkers. Here we describe some of the well-established biological roles of tRNA-derived small RNAs.
Stress
Cells cope with environmental stress conditions by modulating several cellular processes. One of the first responses to environmental stress involves the regulation of translation. Several signalling processes sensitive to environmental stress impinge on translation and result in dramatic changes in transcriptional landscape. The first report of the involvement tRNA-derived fragments in stress came from a study in Tetrahymena. In this study, the authors described the accumulation of tRNA halves (tsRNAs) in response to metabolic stress [55]. Several additional studies have since been published identifying stressors such as oxidative and metabolic stress being responsible for the production of tsRNAs [56]. This cleavage as described earlier has been attributed to Angiogenin and these tsRNAs were termed as tRNA-derived-stress-induced RNAs (tiRNAs) [12, 13, 18]. Since the first response to cellular stress is regulating translation, follow up studies have conclusively shown a primary role for these small RNAs in translational repression [13, 19]. Studies have also shown that these tiRNAs are integral to the formation stress granules, a typical ribonucleoprotein aggregate that is formed under stress conditions resulting in translation repression [42, 57]. Another mechanism by which tsRNAs function under oxidative stress is by forming complexes with Cytochrome C. During oxidative stress, Cytochrome C is released from mitochondria and triggers the apoptotic pathway. Saikia et al. showed that under such conditions, angiogenin-dependent tiRNAs interact with Cytochrome C and thus abolishes the formation of apoptotosomes [58].
This phenomenon has also been observed in plants, such as Arabidopsis, that produced high levels of tsRNAs in response to phosphate starvation [23]. Interestingly, different stress conditions result in the production of tsRNAs in different parts of the plant. While phosphate stress induced high expression of tsRNAs in roots, oxidative stress induced their expression in flowers.
Circulatory tRFs and Cancer
Rapid proliferation in tumorigenic cells often results in deficiency of blood supply leading to hypoxic stress. Reports from several cancer studies have suggested that tumors adapt to a stressful environment by adopting several unique mechanisms. Considering tsRNA production are one of the responses to stress, it is not surprising that several studies have identified dysregulated expression of these small RNAs in a variety of cancers. tRNA-derived fragments have attracted a lot of interest in cancer biology as sequencing of several oncogenic tumors have resulted in the identification of a variety of tRFs as major classes of small RNAs. The first observation of tRFs in cancer dates back to 1970s, where these small RNAs were identified in patients with cancer [2]. This observation is also supported by several follow up studies were specific tRFs have been identified as circulatory molecules. The increased or dysregulated occurrence of tRFs in biofluids such as serum, urine or sperm in various cancer or disease conditions makes them attractive candidates for biomarker development [59–62]. An exciting observation that augments the prospect of developing these small RNAs as biomarkers for cancer is the differential enrichment of tRFs from specific tRNAs under certain cancer conditions [63–66]. Interestingly, it was observed that 5′-tsRNAs, especially tsRNAGly and tsRNAGlu undergo dimerization thus gaining protection from endonucleases and can hence exist freely outside extracellular vesicles [67, 68]. While, the altered levels of tRFs in the serum of cancer patients make them ideal biomarkers, their function and the targets in these scenarios remain unknown. Considering tRFs are found in circulation, it is tempting to speculate a role for these small RNAs in long-range cell signalling.
Many studies have ascribed a functional role for these small RNAs in cancer samples thus evoking the possibility of developing therapies based on targeting these small RNAs. For instance, analysis of cancer cell lines, such as HCT116 and MDAMB231 revealed that tRFs are one of the largest small RNA species in these cells [69]. Many of these fragments function as tumor suppressors in certain cancers inhibiting proliferation and metastasis. This type of tumor suppressive role was exhibited by a 17 nt 3′ tRF from tRNALeu in colorectal cancers [70]. In contrast, a longer version of the same Leu tRF-3s, a 22 nt sequence, showed that this species was important for tumor survival and viability of the cancer cells [71]. Similarly, itRFs were also shown to function as defence molecules as they scavenge YBX1 protein and thereby destabilize oncogenic transcripts [14]. As described earlier, many 5′-tsRNAs are also expressed in certain cancer conditions. Interestingly, Ang is overexpressed in many cancers suggests that there could be a consequential increase in 5′-tsRNA fragments in such cases [72]. This was evidenced by the abundant expression of the angiogenin-dependent tsRNAs in breast and prostate cancer cell lines (positive for estrogen receptor) and also in patient tissues positive for androgen receptor [9]. In other cancer conditions, such as renal cell carcinoma, 5′tsRNAs were found to be downregulated. These contrasting expression and roles for these small RNAs warrants a further investigation focusing on tsRNAs across several cancer lines in order to better understand their expression and their oncogenic potential.
Stem cells and differentiation
Stem cells both human and mouse have shown to abundantly express tsRNAs. It was observed that in mouse ESCs, the 5′-tsRNAs represent one of the largest species of small RNAs comparable to the miRNA population [7]. While the function of tsRNAs in the stem state remains unclear, we demonstrated that RA-induced differentiation of mouse ESCs resulted in the increased expression of tsRNAs derived from specific tRNAs, including tRNAGlyGCC, tRNAGlnCTG, tRNAGluTTC, tRNAValCAC and tRNALysTTT. Functional studies of these tsRNAs during differentiation revealed that these tsRNAs may function to suppress pluripotency genes to facilitate differentiation. Interactome studies in differentiating mESCs revealed that these small RNAs could also be involved in lineage specification [7]. While the interaction with IGF2BP1 seems to be important to regulate the cMyc transcript, it remains unclear as to how these tsRNAs target other transcripts and the fate of these mRNAs.
Embryonic stem cells also express 18nt tRF-5s that are processed based on a Ψ modification on certain tRNAs. Cristian Bellodi’s group showed that knockout of PUS7, an important enzyme that modifies uridines on tRNA, resulted in the concurrent decrease in the expression of tRF-5s [10]. Downregulation of these small RNAs resulted in impaired germ layer formation attributed to increased protein synthesis a mechanism explained earlier in this review. In the same study, the authors also showed that these Ψ carrying tRF-5s were also important for proper differentiation of hematopoietic stem cells. While the authors focussed on the 18nt TOG tRFs, it is interesting to note that knockout of PUS7 resulted in a concomitant upregulation of 5′tsRNAs in mESCs. It is possible that PUS7 may be a key determinant in the processing of tRF-5s versus 5′-tsRNAs.
Intergenerational inheritance
It is becoming increasingly apparent that environmental effects such as stress can lead to altered gene expression in the progeny. Since the earliest events during development are controlled post-transcriptionally without the involvement of the genome, changes in paternal or maternal small RNAs could result in changes in gene expression. A study by Peng et al. first identified specific pools of 29–34 nt 5′-tsRNAs in the mature sperm suggesting that these small RNAs could deliver “paternal” messages [73]. Follow up studies reported that these signatures change when the mice are subjected to a high-fat diet rendering the offspring of these mice vulnerable to metabolic disorders [6, 74]. In a parallel study, Oliver Rando’s group showed similar results with altered 5′-tsRNAs in the sperms of mice subjected to low protein diet [8]. In both these cases, the offspring suffered increased vulnerability to metabolic disorders due to altered gene expression of certain metabolic processes. Follow up studies identified that the sperm gains these 5′ tsRNAs near maturation from extracellular vesicles delivered from the epididymis (called epididymosomes) [21].
Neurological disorders
The clinical implications of tRNA-derived fragments extend beyond stem cells and cancer. These small RNAs have also been found in neurological disorders and neurodegenerative diseases. Most of these neurodegenerative disorders such as amyotrophic lateral sclerosis (ALS) and Parkinson’s are associated defects in Angiogenin and hence the involvement of tsRNAs in these diseases seem plausible [75, 76]. This is strengthened by the observation that G-quadruplex forming tiRNAs (that are angiogenin-dependent products) protects motor neurons from oxidative and excitotoxic stress [77]. However, the fact that only the TOG containing tRNAs such as tRNAAla and tRNACys can form G-quadruplex complex evokes the possibility of treating neuronal stress using these small RNAs. As proof of concept, Ivanov et al. showed that the RNA–DNA counterpart of the tiRNA Ala was able to rescue motor neurons from stress.
Plants
Similar to mammalian systems, prokaryotes and archaea, plants have also been shown to produce tRFs. In an early study, it was shown that Arabidopsis produce tsRNAs, tiRNAs, particularly from tRNAs tRNAHisGTG, tRNAArgCCT, tRNATrpCCA and tRNAGluCTC in response to hydrogen peroxide [53]. While tsRNAGluCTC was found to be expressed in flowers, whereas the rest were identified in seedlings. Plants produce different kinds of tsRNAs from different tRNAs in response to certain stress. Elevated expression of tRFs were observed in plants challenged with low phosphate conditions [23]. Interestingly, while there were elevated tRFs it was also found that the expression of certain tRFs respond positively and negatively to stress.
In recent years, several studies have established the expression of tsRNAs (both 3′ and 5′) across different regions in plants such as roots, flowers, seedlings, leaves etc. [23, 78]. The identification of tsRNAs in the sap suggests that these small RNAs could act as signalling molecules as observed in animal cells. tRNA-derived fragments also seem to play an important role in plant symbiotic relationships, such as nitrogen fixation [79]. In this study, the authors present evidence that the tRF-5s and tRF-3s produced in the rhizobium, Bradyrhizobium japonicum, regulate the soyabean (the host) genes. Using functional studies on three candidate rhizobial tRFs they showed that these tRFs hijack the host AGO1 and function in a miRNA-like manner to silence host transcripts thereby promoting nodulation.
Concluding remarks
tRNA-derived fragments are being increasingly recognized as one of the fundamental and important post-transcriptional regulators of gene expression. The abundance and ubiquitous expression of these small RNAs in almost all the extant species suggests an ancient origin for these small RNAs. This is also strengthened by the heterogeneity seen in the types of small RNAs that are derived from tRNAs. Though these small RNAs have been long neglected as degradation products, recent studies have implicated these small RNAs in several biological process. However, our understanding of the function of these small RNAs is still in its infancy. It is becoming increasingly evident that the modifications on tRNA and possibly tRFs is crucial for the generation and functioning of these small RNAs. The inability to identify the modifications on the tRFs with high confidence have hindered the comprehensive characterization and molecular understanding of the different tsRNAs. Although the role of a few modifications such as methylations have been studied, there is a need to develop protocols to isolate specific small RNAs and study the different modification to gain a more comprehensive understanding of these biomolecules. The identification of circulatory tRFs in the serum of several cancer and diseased individuals evokes a possibility to exploit these small RNAs as potential biomarkers. Given their diversity and versatility in regulating several biological processes, an in-depth research focused on their expression, biogenesis and specific functions inside the cell is imperative to realise the clinical and therapeutic application of tRFs.
Acknowledgements
The authors acknowledge all the research groups who have contributed towards understanding tRNA-derived fragments. DP thanks DST-Swarnajayanti (DST/SJF/LSA-02/2015-16) and inStem core funds. SR is funded through DBT Grant BT/PR31418/BRB/10/1758/2019 and inStem core funds. RD was supported by core funds from A*STAR.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Srikala Raghavan, Email: srikala@instem.res.in.
Ramanuj DasGupta, Email: dasguptar@gis.a-star.edu.sg.
Dasaradhi Palakodeti, Email: dasaradhip@instem.res.in.
References
- 1.Thompson DM, Parker R. Stressing Out over tRNA Cleavage. Cell. 2009 doi: 10.1016/j.cell.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Speer J, Gehrke CW, Kuo KC, Waalkes TP, Borek E. tRNA breakdown products as markers for cancer. Cancer. 1979 doi: 10.1002/1097-0142(197912)44:6<2120::AID-CNCR2820440623>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Tomita K, Ogawa T, Uozumi T, Watanabe K, Masaki H. A cytotoxic ribonuclease which specifically cleaves four isoaccepting arginine tRNAs at their anticodon loops. Proc Natl Acad Sci USA. 2000 doi: 10.1073/pnas.140213797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levitz R, Chapman D, Amitsur M, Green R, Snyder L, Kaufmann G. The optional E. coli prr locus encodes a latent form of phage T4-induced anticodon nuclease. EMBO J. 1990;9:1383. doi: 10.1002/j.1460-2075.1990.tb08253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keam S, Hutvagner G. tRNA-Derived Fragments (tRFs): emerging new roles for an ancient RNA in the regulation of gene expression. Life. 2015;5:1638–1651. doi: 10.3390/life5041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Q, Yan M, Cao Z, Li X, Zhang YY, Shi J, Feng GHG-h, Peng H, Zhang X, Zhang YY, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351:397–400. doi: 10.1126/science.aad7977. [DOI] [PubMed] [Google Scholar]
- 7.Krishna S, Yim DG, Lakshmanan V, Tirumalai V, Koh JL, Park JE, Cheong JK, Low JL, Lim MJ, Sze SK, et al. Dynamic expression of tRNA‐derived small RNAs define cellular states. EMBO Rep. 2019 doi: 10.15252/embr.201947789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, Belleannee C, Kucukural A, Serra RW, Sun F, et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science (80-) 2016;351:391–396. doi: 10.1126/science.aad6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honda S, Loher P, Shigematsu M, Palazzo JP, Suzuki R, Imoto I, Rigoutsos I, Kirino Y. Sex hormone-dependent tRNA halves enhance cell proliferation in breast and prostate cancers. Proc Natl Acad Sci U S A. 2015;112:E3816–E3825. doi: 10.1073/pnas.1510077112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzzi N, Cieśla M, Ngoc PCT, Lang S, Arora S, Dimitriou M, Pimková K, Sommarin MNE, Munita R, Lubas M, et al. Pseudouridylation of tRNA-Derived Fragments Steers Translational Control in Stem Cells. Cell. 2018 doi: 10.1016/j.cell.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Luo S, He F, Luo J, Dou S, Wang Y, Guo A, Lu J. Drosophila tsRNAs preferentially suppress general translation machinery via antisense pairing and participate in cellular starvation response. Nucleic Acids Res. 2018 doi: 10.1093/nar/gky189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su Z, Kuscu C, Malik A, Shibata E, Dutta A. Angiogenin generates specific stress-induced tRNA halves and is not involved in tRF-3-mediated gene silencing. J Biol Chem. 2019 doi: 10.1074/jbc.ra119.009272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamasaki S, Ivanov P, Hu GF, Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol. 2009;185:35–42. doi: 10.1083/jcb.200811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodarzi H, Liu X, Nguyen HCB, Zhang S, Fish L, Tavazoie SF. Endogenous tRNA-derived fragments suppress breast cancer progression via YBX1 displacement. Cell. 2015;161:790–802. doi: 10.1016/j.cell.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YS, Shibata Y, Malhotra A, Dutta A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs) Genes Dev. 2009;23:2639–2649. doi: 10.1101/gad.1837609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres AG, Reina O, Attolini CSO, De Pouplana LR. Differential expression of human tRNA genes drives the abundance of tRNA-derived fragments. Proc Natl Acad Sci USA. 2019 doi: 10.1073/pnas.1821120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxena SK, Rybak SM, Davey RT, Youle RJ, Ackerman EJ. Angiogenin is a cytotoxic, tRNA-specific ribonuclease in the RNase A superfamily. J Biol Chem. 1992;267(30):21982–21986. doi: 10.1016/S0021-9258(19)36710-9. [DOI] [PubMed] [Google Scholar]
- 18.Fu H, Feng J, Liu Q, Sun F, Tie Y, Zhu J, Xing R, Sun Z, Zheng X. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett. 2009;583:437–442. doi: 10.1016/j.febslet.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell. 2011;43:613–623. doi: 10.1016/j.molcel.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pizzo E, Sarcinelli C, Sheng J, Fusco S, Formiggini F, Netti P, Yu W, D’Alessio G, Hu G-F. Ribonuclease/angiogenin inhibitor 1 regulates stress-induced subcellular localization of angiogenin to control growth and survival. J Cell Sci. 2013 doi: 10.1242/jcs.134551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma U, Sun F, Conine CC, Reichholf B, Kukreja S, Herzog VA, Ameres SL, Rando OJ. Small RNAs are trafficked from the epididymis to developing mammalian sperm. Dev Cell. 2018 doi: 10.1016/j.devcel.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen KL, Collins K. Several RNase T2 enzymes function in induced tRNA and rRNA turnover in the ciliate Tetrahymena. Mol Biol Cell. 2012 doi: 10.1091/mbc.E11-08-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh LC, Lin SI, Shih ACC, Chen JW, Lin WY, Tseng CY, Li WH, Chiou TJ. Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol. 2009 doi: 10.1104/pp.109.147280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Megel C, Hummel G, Lalande S, Ubrig E, Cognat V, Morelle G, Salinas-Giegé T, Duchêne AM, Maréchal-Drouard L. Plant RNases T2, but not Dicer-like proteins, are major players of tRNA-derived fragments biogenesis. Nucleic Acids Res. 2019 doi: 10.1093/nar/gky1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donovan J, Rath S, Kolet-Mandrikov D, Korennykh A. Rapid RNase L–driven arrest of protein synthesis in the dsRNA response without degradation of translation machinery. RNA. 2017 doi: 10.1261/rna.062000.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Caballero D, Pérez-Moreno G, Estévez AM, Ruíz-Pérez LM, Vidal AE, González-Pacanowska D. Insights into the role of endonuclease v in RNA metabolism in Trypanosoma brucei. Sci Rep. 2017 doi: 10.1038/s41598-017-08910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nechooshtan G, Yunusov D, Chang K, Gingeras TR. Processing by RNase 1 forms tRNA halves and distinct Y RNA fragments in the extracellular environment. Nucleic Acids Res. 2020 doi: 10.1093/nar/gkaa526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole C, Sobala A, Lu C, Thatcher SR, Bowman A, Brown JWS, Green PJ, Barton GJ, Hutvagner G. Filtering of deep sequencing data reveals the existence of abundant Dicer-dependent small RNAs derived from tRNAs. RNA. 2009;15:2147–2160. doi: 10.1261/rna.1738409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005 doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira M, Francisco S, Varanda AS, Santos M, Santos MAS, Soares AR. Impact of tRNA modifications and tRNA-modifying enzymes on proteostasis and human disease. Int J Mol Sci. 2018 doi: 10.3390/ijms19123738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanco S, Dietmann S, Flores JV, Hussain S, Kutter C, Humphreys P, Lukk M, Lombard P, Treps L, Popis M, et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. EMBO J. 2014 doi: 10.15252/embj.201489282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Matuszek Z, Huang Y, Parisien M, Dai Q, Clark W, Schwartz MH, Pan T. Queuosine modification protects cognate tRNAs against ribonuclease cleavage. RNA. 2018 doi: 10.1261/rna.067033.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z, Qi M, Shen B, Luo G, Wu Y, Li J, Lu Z, Zheng Z, Dai Q, Wang H. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. 2019 doi: 10.1093/nar/gky1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuorto F, Liebers R, Musch T, Schaefer M, Hofmann S, Kellner S, Frye M, Helm M, Stoecklin G, Lyko F. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nat Struct Mol Biol. 2012 doi: 10.1038/nsmb.2357. [DOI] [PubMed] [Google Scholar]
- 35.Frye M, Harada BT, Behm M, He C. RNA modifications modulate gene expression during development. Science. 2018 doi: 10.1126/science.aau1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alemu E, He C, Klungland A. ALKBHs-facilitated RNA modifications and de-modifications. DNA Repair (Amst) 2016 doi: 10.1016/j.dnarep.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fergus C, Barnes D, Alqasem MA, Kelly VP. The queuine micronutrient: charting a course from microbe to man. Nutrients. 2015 doi: 10.3390/nu7042897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gkatza NA, Castro C, Harvey RF, Heiß M, Popis MC, Blanco S, Bornelöv S, Sajini AA, Gleeson JG, Griffin JL, et al. Cytosine-5 RNA methylation links protein synthesis to cell metabolism. PLoS Biol. 2019 doi: 10.1371/journal.pbio.3000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frigolé HR, Camacho N, Coma MC, Fernández-Lozano C, García-Lema J, Rafels-Ybern À, Canals A, Coll M, de Pouplana LR. TRNA deamination by ADAT requires substrate-specific recognition mechanisms and can be inhibited by tRFs. RNA. 2019 doi: 10.1261/rna.068189.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drino A, Oberbauer V, Troger C, Janisiw E, Anrather D, Hartl M, Kaiser S, Kellner S, Schaefer MR. Production and purification of endogenously modified tRNA-derived small RNAs. RNA Biol. 2020 doi: 10.1080/15476286.2020.1733798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akiyama Y, Kharel P, Abe T, Anderson P, Ivanov P. Isolation and initial structure-functional characterization of endogenous tRNA-derived stress-induced RNAs. RNA Biol. 2020 doi: 10.1080/15476286.2020.1732702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyons SM, Gudanis D, Coyne SM, Gdaniec Z, Ivanov P. Identification of functional tetramolecular RNA G-quadruplexes derived from transfer RNAs. Nat Commun. 2017;8(1):1–11. doi: 10.1038/s41467-017-01278-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sobala A, Hutvagner G. Small RNAs derived from the 5’ end of tRNA can inhibit protein translation in human cells. RNA Biol. 2013;10:553–563. doi: 10.4161/rna.24285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gebetsberger J, Wyss L, Mleczko AM, Reuther J, Polacek N. A tRNA-derived fragment competes with mRNA for ribosome binding and regulates translation during stress. RNA Biol. 2017 doi: 10.1080/15476286.2016.1257470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maute RL, Schneider C, Sumazin P, Holmes A, Califano A, Basso K, Dalla-Favera R. TRNA-derived microRNA modulates proliferation and the DNA damage response and is down-regulated in B cell lymphoma. Proc Natl Acad Sci USA. 2013 doi: 10.1073/pnas.1206761110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar P, Anaya J, Mudunuri SB, Dutta A. Meta-analysis of tRNA derived RNA fragments reveals that they are evolutionarily conserved and associate with AGO proteins to recognize specific RNA targets. BMC Med. 2014;12:1. doi: 10.1186/s12915-014-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Couvillion MT, Bounova G, Purdom E, Speed TP, Collins K. A Tetrahymena Piwi Bound to Mature tRNA 3’ Fragments Activates the Exonuclease Xrn2 for RNA Processing in the Nucleus. Mol Cell. 2012 doi: 10.1016/j.molcel.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lakshmanan V, Bansal D, Sujith TN, Shivaprasad PV, Palakodeti D, Krishna S. Comprehensive annotation and characterization of planarian tRNA and tRNA-derived fragments (tRFs) bioRxiv. 2020 doi: 10.1101/2020.08.25.266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keam SP, Young PE, McCorkindale AL, Dang THY, Clancy JL, Humphreys DT, Preiss T, Hutvagner G, Martin DIK, Cropley JE, et al. The human Piwi protein Hiwi2 associates with tRNA-derived piRNAs in somatic cells. Nucleic Acids Res. 2014 doi: 10.1093/nar/gku620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim HK, Fuchs G, Wang S, Wei W, Zhang Y, Park H, Roy-Chaudhuri B, Li P, Xu J, Chu K, et al. A transfer-RNA-derived small RNA regulates ribosome biogenesis. Nature. 2017 doi: 10.1038/nature25005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schorn AJ, Gutbrod MJ, LeBlanc C, Martienssen R. LTR-Retrotransposon Control by tRNA-Derived Small RNAs. Cell. 2017;170:61–71.e11. doi: 10.1016/j.cell.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Zhang X, Shi J, Tuorto F, Li X, Liu Y, Liebers R, Zhang L, Qu Y, Qian J, et al. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat Cell Biol. 2018 doi: 10.1038/s41556-018-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson DM, Lu C, Green PJ, Parker R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA. 2008;14:2095–2103. doi: 10.1261/rna.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karaiskos S, Naqvi AS, Swanson KE, Grigoriev A. Age-driven modulation of tRNA-derived fragments in Drosophila and their potential targets. Biol Direct. 2015 doi: 10.1186/s13062-015-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee SR, Collins K. Starvation-induced cleavage of the tRNA anticodon loop in Tetrahymena thermophila. J Biol Chem. 2005;280:42744–42749. doi: 10.1074/jbc.M510356200. [DOI] [PubMed] [Google Scholar]
- 56.Emara MM, Ivanov P, Hickman T, Dawra N, Tisdale S, Kedersha N, Hu GF, Anderson P. Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J Biol Chem. 2010 doi: 10.1074/jbc.M112.371799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saikia M, Krokowski D, Guan BJ, Ivanov P, Parisien M, Hu GF, Anderson P, Pan T, Hatzoglou M. Genome-wide identification and quantitative analysis of cleaved tRNA fragments induced by cellular stress. J Biol Chem. 2012;285:10959–10968. doi: 10.1074/jbc.M112.371799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saikia M, Jobava R, Parisien M, Putnam A, Krokowski D, Gao X-H, Guan B-J, Yuan Y, Jankowsky E, Feng Z, et al. Angiogenin-Cleaved tRNA Halves Interact with Cytochrome c, Protecting Cells from Apoptosis during Osmotic Stress. Mol Cell Biol. 2014 doi: 10.1128/MCB.00136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeri A, Courtright A, Reiman R, Carlson E, Beecroft T, Janss A, Siniard A, Richholt R, Balak C, Rozowsky J, et al. Total extracellular small RNA profiles from plasma, saliva, and urine of healthy subjects. Sci Rep. 2017 doi: 10.1038/srep44061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Magee RG, Telonis AG, Loher P, Londin E, Rigoutsos I. Profiles of miRNA Isoforms and tRNA Fragments in Prostate Cancer. Sci Rep. 2018 doi: 10.1038/s41598-018-22488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dhahbi JM, Spindler SR, Atamna H, Yamakawa A, Boffelli D, Mote P, Martin DIK. 5’ tRNA halves are present as abundant complexes in serum, concentrated in blood cells, and modulated by aging and calorie restriction. BMC Genomics. 2013 doi: 10.1186/1471-2164-14-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Zhang Y, Shi J, Zhang H, Cao Z, Gao X, Ren W, Ning Y, Ning L, Cao Y, et al. Identification and characterization of an ancient class of small RNAs enriched in serum associating with active infection. J Mol Cell Biol. 2014 doi: 10.1093/jmcb/mjt052. [DOI] [PubMed] [Google Scholar]
- 63.Wu X, Somlo G, Yu Y, Palomares MR, Li AX, Zhou W, Chow A, Yen Y, Rossi JJ, Gao H, et al. De novo sequencing of circulating miRNAs identifies novel markers predicting clinical outcome of locally advanced breast cancer. J Transl Med. 2012 doi: 10.1186/1479-5876-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinez BV, Dhahbi JM, Nunez Lopez YO, Lamperska K, Golusinski P, Luczewski L, Kolenda T, Atamna H, Spindler SR, Golusinski W, et al. Circulating small non coding RNA signature in head and neck squamous cell carcinoma. Oncotarget. 2015 doi: 10.18632/oncotarget.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao C, Tolkach Y, Schmidt D, Kristiansen G, Müller SC, Ellinger J. 5′-tRNA halves are dysregulated in clear cell renal cell carcinoma. J Urol. 2018 doi: 10.1016/j.juro.2017.07.082. [DOI] [PubMed] [Google Scholar]
- 66.Zhao C, Tolkach Y, Schmidt D, Muders M, Kristiansen G, Müller SC, Ellinger J. tRNA-halves are prognostic biomarkers for patients with prostate cancer. Urol Oncol Semin Orig Investig. 2018 doi: 10.1016/j.urolonc.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Tosar JP, Gámbaro F, Darré L, Pantano S, Westhof E, Cayota A. Dimerization confers increased stability to nucleases in 5 halves from glycine and glutamic acid tRNAs. Nucleic Acids Res. 2018 doi: 10.1093/nar/gky495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tosar JP, Segovia M, Castellano M, Gámbaro F, Akiyama Y, Fagúndez P, Olivera Á, Costa B, Possi T, Hill M, et al. Fragmentation of extracellular ribosomes and tRNAs shapes the extracellular RNAome. Nucleic Acids Res. 2020 doi: 10.1093/nar/gkaa674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheong JK, Nguyen TH, Wang H, Tan P, Voorhoeve PM, Lee SH, Virshup DM. IC261 induces cell cycle arrest and apoptosis of human cancer cells via CK1δ/ɛ and Wnt/β-catenin independent inhibition of mitotic spindle formation. Oncogene. 2011;30:2558–2569. doi: 10.1038/onc.2010.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang B, Yang H, Cheng X, Wang D, Fu S, Shen W, Zhang Q, Zhang L, Xue Z, Li Y, et al. TRF/miR-1280 suppresses stem cell-like cells and metastasis in colorectal cancer. Cancer Res. 2017 doi: 10.1158/0008-5472.CAN-16-3146. [DOI] [PubMed] [Google Scholar]
- 71.Shao Y, Sun Q, Liu X, Wang P, Wu R, Ma Z. tRF-Leu-CAG promotes cell proliferation and cell cycle in non-small cell lung cancer. Chem Biol Drug Des. 2017 doi: 10.1111/cbdd.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheng J, Xu Z. Three decades of research on angiogenin: A review and perspective. Acta Biochim Biophys Sin (Shanghai) 2016 doi: 10.1093/abbs/gmv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peng H, Shi J, Zhang Y, Zhang H, Liao S, Li W, Lei L, Han C, Ning L, Cao Y, et al. A novel class of tRNA-derived small RNAs extremely enriched in mature mouse sperm. Cell Res. 2012;22:1609–1612. doi: 10.1038/cr.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sarker G, Sun W, Rosenkranz D, Pelczar P, Opitz L, Efthymiou V, Wolfrum C, Peleg-Raibstein D. Maternal overnutrition programs hedonic and metabolic phenotypes across generations through sperm tsRNAs. Proc Natl Acad Sci USA. 2019 doi: 10.1073/pnas.1820810116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Es MA, Schelhaas HJ, Van Vught PWJ, Ticozzi N, Andersen PM, Groen EJN, Schulte C, Blauw HM, Koppers M, Diekstra FP, et al. Angiogenin variants in Parkinson disease and amyotrophic lateral sclerosis. Ann Neurol. 2011 doi: 10.1002/ana.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greenway MJ, Andersen PM, Russ G, Ennis S, Cashman S, Donaghy C, Patterson V, Swingler R, Kieran D, Prehn J, et al. ANG mutations segregate with familial and ‘sporadic’ amyotrophic lateral sclerosis. Nat Genet. 2006 doi: 10.1038/ng1742. [DOI] [PubMed] [Google Scholar]
- 77.Ivanov P, O’Day E, Emara MM, Wagner G, Lieberman J, Anderson P. G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments. Proc Natl Acad Sci USA. 2014 doi: 10.1073/pnas.1407361111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang S, Sun L, Kragler F. The phloem-delivered RNA pool contains small noncoding RNAs and interferes with translation1[W][OA] Plant Physiol. 2009 doi: 10.1104/pp.108.134767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ren B, Wang X, Duan J, Ma J. Rhizobial tRNA-derived small RNAs are signal molecules regulating plant nodulation. Science (80-) 2019 doi: 10.1126/science.aav8907. [DOI] [PubMed] [Google Scholar]