Abstract
Background
Biliary complications are frequent in patients with acromegaly. These complications may be secondary either to acromegaly or to somatostatin analogs (SAs). We aimed in this paper to assess the prevalence of biliary complications in patients with acromegaly at diagnosis and after treatment with SAs.
Methods
We conducted an analytical and descriptive retrospective study of 26 patients followed up for acromegaly over 7 years. Biliary complications were screened at diagnosis and follow-up by abdominal ultrasound, biliary magnetic resonance imaging (MRI), and endoscopic ultrasonography (EUS). Data were analyzed using SPSS 21.
Results
The mean age of the patients was 49.6 ± 14 years, with a female predominance (53.8%). The evaluation of biliary complications showed vesicular biliary tract lithiasis and/or sludge in seven patients (29%), including two patients at the time of diagnosis of acromegaly and five patients after an average medical treatment duration of 3 years. Six female patients (24%) had dilation of the bile ducts without the presence of obstruction on biliary MRI and EUS and lithiasis/sludge of the common bile duct, tumor or external compression have been excluded. This condition was discovered incidentally at the diagnosis in five patients and during the follow-up in one patient. The preoperative insulin-like growth factor 1 (IGF-1) levels, disease duration, and female sex were significantly correlated with biliary tract dilation occurrence. Dyslipidemia, the preoperative IGF-1 level, and lanreotide treatment duration were significantly correlated with the occurrence of biliary lithiasis (P < 0.05).
Conclusion
Biliary stones are a frequent biliary adverse effect in patients with acromegaly undergoing SAs treatment. However, primary bile duct dilation has never been reported in acromegaly to the best of our knowledge. This condition could be considered as a complication or a feature of the disease.
Keywords: Acromegaly, Biliary lithiasis, Primary dilation of the biliary tract, Somatostatin analogs, Acute biliary complication
Introduction
Acromegaly is a rare disease resulting from the hypersecretion of growth hormone (GH) leading to overproduction of insulin-like growth factor 1 (IGF-1). It is related in more than 95% of cases to a pituitary GH adenoma arising from somatotrop cells [1]. Chronic exposure to GH and IGF-1 is responsible for systemic complications in particular cardiovascular, metabolic, rheumatologic, respiratory, neoplastic, and digestive complications. These conditions would define the morbidity and mortality of the disease and also affect the quality of life in patients with acromegaly [2]. Acromegaly is most often underdiagnosed due to the insidious progression of the disease. Thus, the diagnosis is usually made 5 to 10 years after its onset, with an average age of diagnosis around 40 to 50 years. This explains the importance of screening for complications and associated comorbidities at the time of the diagnosis and during disease follow-up [3].
The gastrointestinal complications in acromegaly are frequent and can involve several digestive structures. The most documented complications data are the risk of developing digestive tumors including adenomas, hyperplastic polyps, and colorectal cancer compared to the general population with an estimated incidence in acromegalic patients around 0.9-2.4% [4, 5]. Other repercussions have been reported, notably gastroduodenitis, esophagitis, peptic ulcer, intestinal metaplasia, hiatal hernia, and impaired intestinal motility [6, 7].
Patients with acromegaly present a prolonged colonic transit time that is associated with the formation of biliary tract lithiasis [8]. The latter is a complication described particularly as a side effect of somatostatin analogs (SAs) used in several studies [9]. The documented prevalence of biliary tract lithiasis varies between 3.6% and 56% [10]. However, a higher prevalence of gallbladder lithiasis is also reported in patients with acromegaly compared to the general population [10]. This condition can lead to serious acute biliary complications (ABCs) such as pancreatitis, cholecystitis, or lithiasis angiocholitis, especially after medical treatment discontinuation by somatostatin receptor ligands (SRLs) [11].
To date and to the best of our knowledge, no study has assessed biliary complications in our population with acromegaly. Our study aimed to evaluate the prevalence of biliary tract involvement and related complications in patients with acromegaly, including dilation and lithiasis of the biliary tract. We also assessed the associated factors and the impact of SAs on the occurrence of these complications.
Materials and Methods
It was a descriptive and analytical retrospective study of patients referred to the Department of Endocrinology-Diabetology and Nutrition for the management of acromegaly. The study was conducted over 7 years and 9 months (between November 2015 and August 2023).
Study population
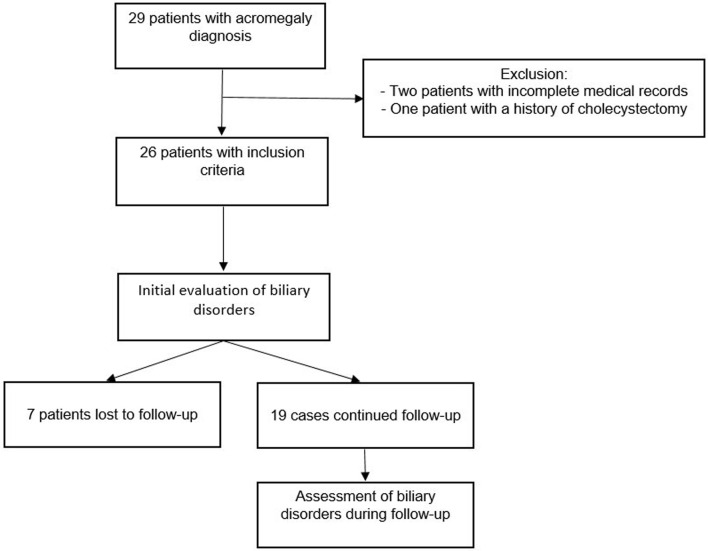
Our study included 26 patients with a confirmed diagnosis of acromegaly related to a somatotropic pituitary adenoma. SAs were indicated in patients with uncontrolled acromegaly or during the preoperative course of pituitary surgery. Lanreotide 120 mg sustained release was the treatment used for the patients in our center. All patients were screened for digestive hepatobiliary disorders at the time of diagnosis and during the follow-up. Seven patients were excluded in the second part of the study because they were lost to follow-up or because they refused medical care. Patients with incomplete clinical data, those with SA duration treatment less than 3 months, and patients with a medical history of cholecystectomy were excluded from the study. Thus, a total of 26 patients were enrolled in the study (Fig. 1).
Figure 1.
Study design.
Study protocol
For each included patient, the following data were recorded: age at diagnosis of acromegaly (years), sex, body mass index (BMI), presence of type 2 diabetes mellitus and hyperlipidemia, history of cholecystectomy or biliary lithiasis, liver enzyme analysis including the assessment of hepatic cytolysis and cholestasis, duration of the disease progression (years), GH and IGF-1 levels at diagnosis, duration of medical treatment with lanreotide (years), and treatment discontinuation.
The diagnosis of biliary complications was based on a clinical evaluation (abdominal pain, biliary colic, and jaundice or subicterus) and imaging. Abdominal ultrasound (US) was performed in all our patients at the onset of diagnosis and during the follow-up to screen for side effects of treatment with SAs. Abdominal computed tomography (CT) scan, biliary magnetic resonance imaging (MRI), and endoscopic ultrasonography (EUS) were used in patients with clinical symptoms or with abdominal US abnormalities. Data on the management of these complications (conservative, endoscopic drainage by endoscopic retrograde cholangiopancreatography (ERCP), or surgery) were also recorded.
Outcomes
The primary outcome was to analyze the demographic, clinical, and biochemical characteristics of the patients included in our study, along with the prevalence of biliary involvement, their acute complications, and their therapeutic management.
The secondary outcome was to study the correlation between the occurrence of these complications, and certain parameters, notably the age at diagnosis, the duration of disease progression, IGF-1 levels, treatment discontinuation, and metabolic parameters.
Statistical analysis
Data were collected on the database from medical records and analyzed using Statistical Package for the Social Sciences Version 21 (SPSS-V21) software. The quantitative results were presented as means ± standard deviations (SDs) and the qualitative results as frequencies and percentages. Pearson correlation and Fisher’s exact tests were used to study the correlations and the relationship between the occurrence of biliary abnormalities in patients with acromegaly (cholelithiasis at the diagnosis of acromegaly, cholelithiasis occurring after medical treatment of acromegaly and the dilation of bile ducts (DBD)) and certain parameters (e.g., age, sex, obesity, dyslipidemia, disease duration, IGF-1 level, SA use duration). A P-value ≤ 0.05 was accepted as statistically significant.
The correlation coefficients and their significance were calculated using the Pearson or the Spearman tests.
Institutional Review Board approval and ethical compliance
The study was approved by the ethical review committee at the Faculty of Medicine, Mohamed the First University of Oujda (CERBO), under the number: Project 22/2020. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Results
The demographic, clinical, biological, and imaging data of the studied population are summarized in Table 1. The mean age of the patients at the time of diagnosis was 49.6 ± 14 years with a slight female predominance (53.8% of cases). The patients had an average diagnostic delay of 7.6 ± 5.5 years since the onset of the first symptoms of acromegaly. The mean IGF-1 and GH levels at the diagnosis were 708 ± 252 ng/mL and 53 ± 83 mIU/L, respectively. Forty-six percent of patients with acromegaly were obese, and 46.1% had diabetes. Moreover, 53.8% had dyslipidemia (Table 1).
Table 1. Demographic, Clinical, Biochemical, and Imaging Characteristics of the Studied Population (n = 26).
| Parameters | N (%) |
|---|---|
| Mean age at diagnosis of acromegaly (years) | 49.6 ± 14 |
| Sex distribution | |
| Female | 14 (53.8) |
| Male | 12 (46.2) |
| Mean IGF-1 levels (ng/mL) | 708 ± 252 |
| Mean GH levels (mIU/L) | 53 ± 83 |
| Surgery | 19 (73) |
| Medical treatment | 16 (61.5) |
| Radiotherapy | 3 (11.5) |
| Pituitary adenoma size | |
| Macroadenoma | 22 (84.6) |
| Microadenoma | 4 (15.4) |
| BMI (kg/m2) | 28.42 ± 3.7 |
| Height | 173.4 ± 13.3 |
| BP | |
| Systolic BP | 130 ± 13.3 |
| Diastolic BP | 69 ± 7.4 |
| Mean HbA1c | 6.8 ± 1.3 |
| TG | 1.41 ± 0.63 |
| LDL-C | 1.12 ± 0.28 |
| HDL-C | 0.4 ± 0.14 |
BMI: body mass index; BP: blood pressure; GH: growth hormone; HbA1c: glycated hemoglobin; HDL-C: high-density lipoprotein-cholesterol; IGF-1: insulin-like growth factor 1; LDL-C: low-density lipoprotein-cholesterol; N: number of patients; TG: triglycerides. Limit ranges of IGF-1 level: 22 - 25 years: 105 - 311 ng/mL, 26 - 30 years: 98 - 290 ng/mL, 31 - 35 years: 87 - 278 ng/mL, 36 - 40 years: 80 - 271 ng/mL, 41 - 45 years: 75 - 249 ng/mL, 46 - 50 years: 64 - 236 ng/mL, 51 - 55 years: 55 - 234 ng/mL, 56 - 60 years: 48 - 241 ng/mL, 66 - 70 years: 32 - 226 ng/mL, 71 - 75 years: 24 - 218 ng/mL. GH levels were analyzed based on international limits: < 1.2 mIU/L.
Acromegaly was integrated into sporadic forms in most of cases (96.4%). A genetic cause of acromegaly has been identified in one case with Mccune-Albright syndrome. The mean volume of pituitary adenoma was 3.2 ± 5 cm3 on preoperative pituitary MRI. Transsphenoidal pituitary surgery was performed on 19 patients as a first-line treatment and seven patients refused medical care and are now lost to follow-up (Fig. 1).
The medical treatment with prolonged-release lanreotide (SA) was indicated in 16 patients with persistent disease activity after surgery or persistence of pituitary tumor. The preoperative SRL therapy was performed on seven patients. Conventional radiotherapy was performed in three cases as a third-line therapy after unsuccessful surgery and medical therapy (Table 1).
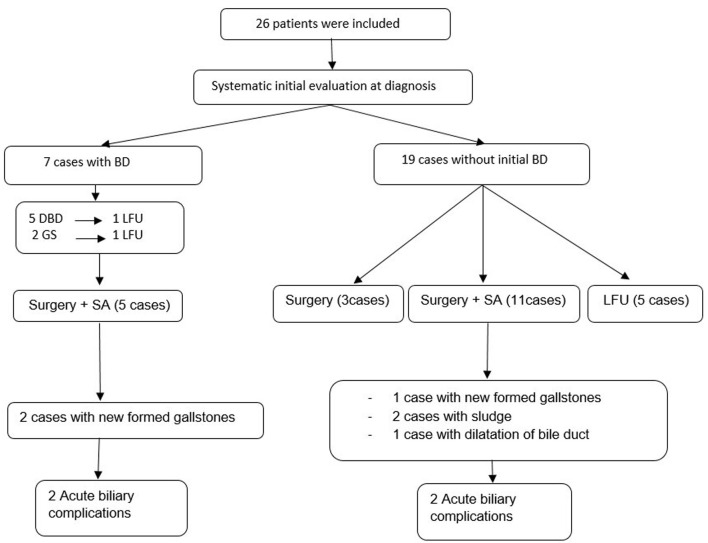
The assessment of biliary tract involvement at diagnosis showed the presence of gallstones (GSs) in two patients (7.7%). The follow-up of 19 patients detected incidentally newly formed GSs in three cases (15.7%) and sludge in two patients (10.5%). This involvement occurred in patients receiving SA treatment after an average of 45 months of lanreotide treatment. This disorder did not lead to discontinuation of treatment (Fig. 2).
Figure 2.
Biliary involvement in selected patients with acromegaly. BDs: biliary disorders; SAs: somatostatin analogs; LFU: lost to follow-up; GS: gallstone; DBD: dilation of the bile duct.
The diagnosis of cholelithiasis was confirmed by annual abdominal US performed during the follow-up. Whereas the biliary MRI and EUS were used to detect microlithiasis not visualized by the abdominal US in one patient presenting with DBD without the presence of obstruction on the abdominal US. The GSs were multiple and millimetric in all patients.
The DBD without the presence of obstruction on US, MRI, and EUS was observed in six female patients and lithiasis or sludge of the common bile duct (CBD), tumor or external compression were the main differential diagnosis and have been excluded (Fig. 3). This condition was discovered incidentally at the diagnosis of acromegaly in five patients on radiological assessment of systemic complications of the disease. The other case of biliary dilation was found on abdominal imaging during the follow-up after 4 years of medical treatment of SA (Fig. 2). Two patients out of the six cases had both GSs and biliary dilation. This later was identified at the diagnosis of the disease and before medical treatment, whereas biliary MRI and EUS did not reveal any obstruction of the bile ducts. In our series, no cases of biliary tract polyps were reported.
Figure 3.
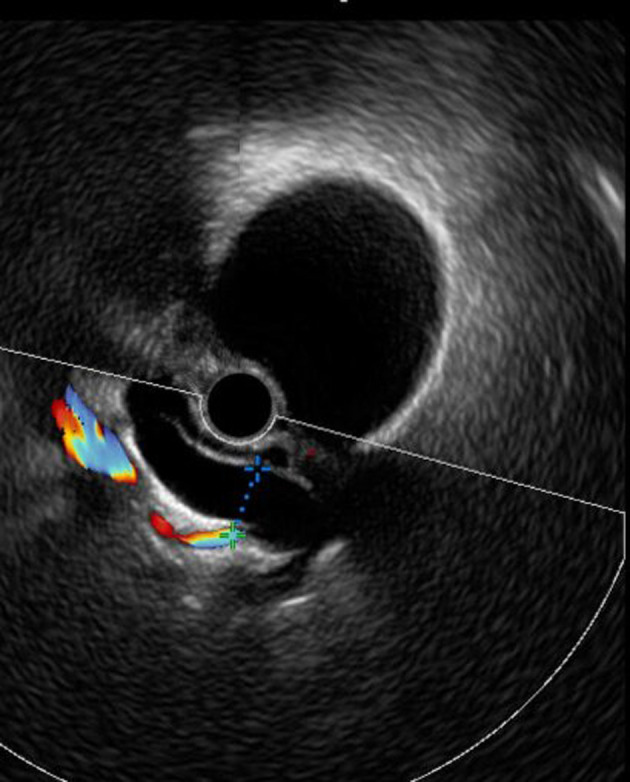
Dilation of common bile duct without the presence of obstruction on EUS. EUS: endoscopic ultrasonography.
During the follow-up of the cases presenting cholelithiasis, four patients (57%) developed ABCs. One patient presented with recurrent atypical epigastralgia related to sludge and microlithiasis of the CBD and microlithiasis of the gallbladder. Two cases were complicated with biliary colic, jaundice, and biological cholestasis. Both patients had microlithiasis of the gallbladder, with associated choledochal lithiasis in one patient (Fig. 4). The other case presented biliary peritonitis with acute cholecystitis during SA treatment. The cholelithiasis in the present four cases occurred after medical treatment in three cases and before starting SA in one case (Table 2).
Figure 4.
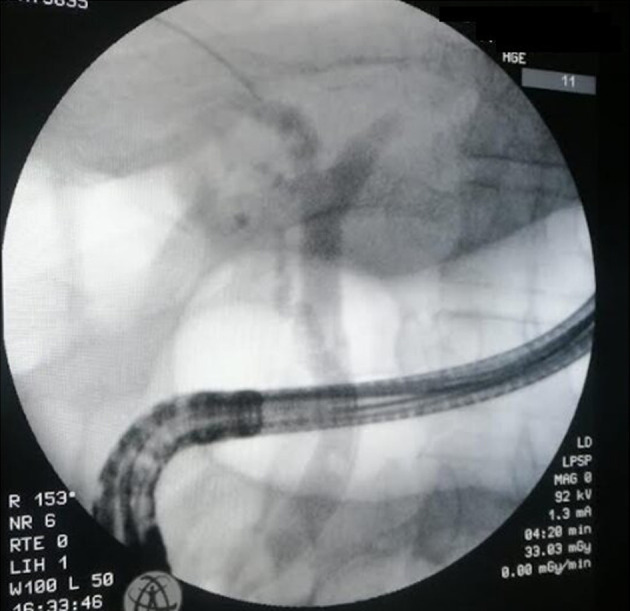
ERCP showing choledochal lithiasis with dilation of common bile duct. ERCP: endoscopic retrograde cholangiopancreatography.
Table 2. Prevalence of Biliary Disorder at the Diagnosis of Acromegaly (n = 26) and During the Follow-Up (n = 19).
| Parameters | N | % |
|---|---|---|
| Prevalence of dilation of bile duct | ||
| At diagnosis of acromegaly | 5/26 | 19.2 |
| After lanreotide treatment | 1/16 | 6.2 |
| Prevalence of GS at acromegaly diagnosis | 2 | 7.7 |
| Prevalence of GS after lanreotide treatment | 3 | 18.7 |
| Prevalence of gallbladder sludge after lanreotide treatment | 2 | 12.5 |
| Duration treatment at the onset of diagnosis of GS/sludge (months) | 45 | - |
GS: gallstone; N: number of patients.
These acute complications occurred after lanreotide treatment discontinuation in three patients (Table 2). The treatment consisted of emergency ERCP with endoscopic sphincterotomy, stone extraction (Fig. 5), and vacuity of the CBD with good clinical and biological evolution. Emergency cholecystectomy with peritoneal lavage was performed in the case of biliary peritonitis. The cholecystectomy was indicated secondarily in the other three patients. However, no cases of pancreatitis occurred in these patients with biliary disorders. The therapeutic management of these associated complications of acromegaly was decided upon by multidisciplinary meetings except for the emergency cases. In addition, the management of associated metabolic disorders, in particular obesity and dyslipidemia, was considered in all patients. Data of these cases are presented in Table 3.
Figure 5.
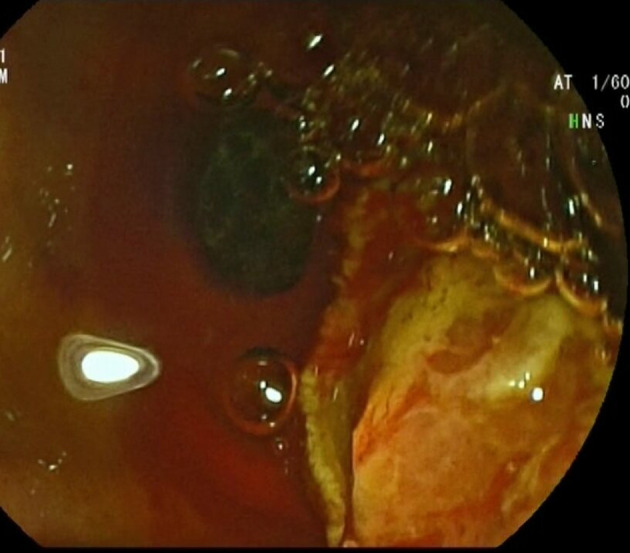
Endoscopic image of balloon-extracted calculus after sphincterotomy.
Table 3. Clinical, Biological, and Imaging Presentation of Cases With Acute Biliary Complications.
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age (years) | 68 | 66 | 68 | 44 |
| History of MD | Diabetes mellitus and hypertension | Diabetes mellitus | Diabetes mellitus and hypertension | None |
| BMI (kg/m2) | 25.3 | 23 | 30 | 24 |
| IGF-1 at diagnosis (ng/mL) | 795 | 774 | 672 | 1,500 |
| IGF-1 at the occurrence of AC (ng/mL) | 389.9 (ULN: 1.9) | 369 (ULN: 1.6) | 299 (ULN: 1.3) | 164 (NR) |
| Disease duration at the onset of the first symptoms (years) | 24 | 21 | 19 | 7 |
| The occurrence of cholelithiasis | After SA treatment | After SA treatment | Cholelithiasis at the diagnosis of acromegaly and before SA treatment | After SA treatment |
| AC-related symptoms | Sub jaundice, biliary colic | Atypical epigastralgia | Sub jaundice, biliary colic | Acute abdominal pain |
| Hepatic cytolysis | No | No | Yes | No |
| Biological cholestasis | TB: 24 mg/L; DB: 15 mg/L; GGT: 856 U/L | TB: 8 mg/L; DB: 3 mg/L; GGT: 36 U/L | TB: 117 mg/L; DB: 86 mg/L; GGT: 1,499 mg/L | TB: 35 mg/L; DB: 11 mg/L; GGT: 181 mg/L |
| Pancreatitis | No | No | No | No |
| Duration of treatment discontinuation before AC event | 2 years | 5 months | 5 months | Occurrence of AC during SA treatment |
| Abdominal US | Microlithiasis of the GB; dilation of CBD (16 mm) | Dilation of CBD (13 mm) | Dilation of the bile ducts (12 mm) | Cholecystitis associated with biliary peritonitis (confirmed on abdominal CT scan) |
| Biliary MRI | - | Not made because of a metal hip prosthesis | Dilation of bile duct (13 mm) | - |
| EUS | - | Dilation of CBD (12 mm) with sludge and microlithiasis; microlithiasis of the GB | Dilation of CBD (14 mm); microlithiasis of the GB | - |
| ERCP | Choledochal lithiasis (Fig. 4); dilation of CBD (20 mm); GS; endoscopic biliary drainage with stone extraction | Dilation of the bile ducts with lithiasis in CBD; endoscopic biliary drainage | Dilation of the bile ducts (17 mm); endoscopic biliary drainage | - |
AC: acute complication; BMI: body mass index; CBD: common bile duct; CT: computed tomography; DB: direct bilirubin; ERCP: endoscopic retrograde cholangiopancreatography; EUS: endoscopic ultrasonography; GB: gallbladder; GGT: gamma-glutamyl transferase; GS: gallstone; IGF-1: insulin-like growth factor 1; MD: metabolic disorder; MRI: magnetic resonance imaging; NR: normal range; SA: somatostatin analog; TB: total bilirubin; ULN: upper limit of normal; US: ultrasound.
Table 4 summarizes all of the studied associated factors to the occurrence of biliary disorders in patients with acromegaly. High preoperative IGF-1 levels, the presence of dyslipidemia, and medical more than 2 years of medical treatment were found to be associated factors for developing cholelithiasis in acromegaly patients treated with lanreotide (P < 0.05). Moreover, the occurrence of cholelithiasis at diagnosis of acromegaly was only correlated with the presence of dyslipidemia (P = 0.017). High preoperative IGF-1 levels, the disease duration, and the female sex were associated with the development of incidental DBD (P < 0.05).
Table 4. Associated Factors to Biliary Abnormalities Occurrence in Patients With Acromegaly.
| Studied parameters | P-value (r) |
||
|---|---|---|---|
| Cholelithiasis at the time of acromegaly diagnosis | Cholelithiasis after medical treatment | Dilation of the bile ductsa | |
| Age ≥ 50 years | 0.48 (r = 0.28) | 0.64 (r = 0.128) | 0.16 (r = 0.35) |
| Sex | 0.48 (r = 0.28) | 0.64 (r = 0.128) | 0.015 (r = 0.54)* |
| BMI | 0.67 (r = -0.093) | 0.5 (r = -0.18) | 0.7 (r = -0.17) |
| Disease duration | 0.06 (r = 0.003) | 0.5 (r = 0.15) | 0.014 (r = 0.3)* |
| Postoperative IGF-1 levels | - | 0.38 (r = -0.28) | - |
| Preoperative IGF-1 levels | 0.84 (r = -0.071) | 0.041 (r = 0.429)* | 0.038 (r = 0.23)* |
| Preoperative GH levels | 1 (r = -0.131) | 0.63 (r = 0.54) | 0.44 (r = -0.39) |
| Preoperative adenoma volume | 0.59 (r = -0.12) | 0.41 (r = 0.219) | 0.34 (r = -0.216) |
| Dyslipidemia | 0.017 (r = 0.47)* | 0.018 (r = 0.48)* | 0.18 (r = 0.34) |
| Diabetes | 1 (r = -0.012) | 0.155 (r = -0.339) | 0.37 (r = -0.21) |
| Lanreotide treatment duration > 2 years | - | 0.005 (r = 0.7)* | - |
aDilation of the bile duct without detected obstruction on abdominal US, MRI, EUS. *Statistically significant values (P < 0.05). The r represents the Pearson correlation coefficient. BMI: body mass index; GH: growth hormone; IGF-1: insulin-like growth factor 1.
Discussion
The present study showed that biliary tract disorders are frequent in patients with acromegaly including the primary DBD and GS formation especially after SAs. The cholelithiasis was significantly correlated to the preoperative IGF-1 levels, lanreotide treatment duration, and the presence of dyslipidemia. DBD was found to be associated with female sex, disease duration, and preoperative IGF-1 levels. In addition, ABCs were a considerable outcome that affected 57% (4/7) of patients with cholelithiasis.
SAs (SRL) are the mainstay of medical treatment in acromegaly. SRLs are generally well tolerated. Their side effects are transient in most cases and include injection site pain and erythema, functional gastrointestinal complaints, glucose metabolism disorders, and biliary sludge or GSs. Biliary abnormalities are the most common side effects of SRLs as reported in several studies. In most cases, they are asymptomatic and occur generally during the first 1 or 2 years of SA treatment [10, 12, 13]. In our study, the prevalence of sludge and microlithiasis (31.2%) in patients treated with lanreotide was comparable to that described in the literature. The documented prevalence of biliary lithiasis varies between 3.6% and 56% in patients with acromegaly undergoing SRL treatment regardless of the molecule use [10, 12, 14-16]. This variability in prevalence suggests the contribution of genetic and environmental factors to GS pathogenesis. According to some authors, acromegaly is a risk factor for biliary lithiasis development with an estimated prevalence of 16-26% [10, 12]. This is suggested to be related to GH and IGF-1 hypersecretion in acromegaly disease [17]. However, in a large retrospective study in tertiary referral centers, the estimated prevalence of GS in acromegaly at the diagnosis in untreated patients was 8.3% [12]. Similarly to our data, the prevalence of biliary lithiasis at the diagnosis of acromegaly was 8% which does not seem to exceed the one found in the general population [18-21].
Several factors related to GS formation in patients with acromegaly undergoing SA treatment were studied in multiple series. According to Attanasio et al [12], GS occurrence was correlated to obesity, dyslipidemia, and treatment duration in 357 patients with acromegaly treated with SA with an average follow-up of 7.1 years. In another large retrospective study including 91 patients treated with SRLs, only IGF-1 levels at baseline were a risk factor for biliary adverse effects, and no difference in the molecule used (lanreotide/octreotide) was found [16]. On the other hand, Sendur and Oguz [15] reported in a retrospective Turkish study that SRL use and BMI were independent predictor risk factors of developing GSs in patients with acromegaly. In our study, preoperative IGF-1 levels, lanreotide treatment duration, and dyslipidemia were associated with the incidence of GS formation.
The biliary side effect does not contraindicate ongoing treatment, and cholecystectomy can be considered in symptomatic patients in addition to adequate management of associated metabolic parameters and radiological monitoring [14]. In our series, SAs were maintained. Cholecystectomy was indicated in a multidisciplinary meeting in cases with clinical symptoms. According to the European Association for the Study of Liver (EASL), a concomitant ursodeoxycholic acid (UDCA) treatment can be considered in patients receiving long-term SA treatment to prevent GS formation because of its effect on cholesterol GS dissolution [21, 22]. UDCA was considered in patients with cholelithiasis undergoing SA treatment in our series; however, it is not yet available in our country.
According to EASL data, ABCs under treatment with SA are higher than in the general population because GS complications occur in 0.1-0.3% of asymptomatic patients [21]. In our series, acute biliary adverse effects occurred in 57% of cases with biliary lithiasis (4/7). As shown in a recent controlled randomized study, lanreotide treatment was associated with serious ABC [11]. This study reported one case of acute pancreatitis that occurred during lanreotide treatment complicated by gastro pancreatic fistula. The other cases of ABC occurred after treatment discontinuation. They included three cases of acute pancreatitis with necrotizing pancreatitis in one case, two cases of cholecystitis, one case of porcelain gallbladder, and two cases of biliary colic [11]. In our series, no case of pancreatitis was reported. However, we reported one case with biliary peritonitis, a recurrent atypical epigastralgia related to sludge, and microlithiasis of the CBD associated with a GS in one patient. The two other cases were complicated with biliary colic, jaundice, and biological cholestasis. Both patients had microlithiasis of the gallbladder, with associated choledochal lithiasis in one patient. This can explain not only the high prevalence of ABC during SA treatment but also the serious associated disorders. In our series, SA discontinuation was decided in three cases presenting ABC with an estimated median time of 11 months between the lanreotide discontinuation and the development of these complications. It has been shown that withdrawal of SA treatment can increase the risk of occurrence of ABC [23]. This can be explained by the resumption of gallbladder contractility inducing the migration of microcalculus at the origin of these complications [24].
In the literature, other gallbladder abnormalities were documented such as gallbladder polyps. Annamalai et al [25] reported an increased prevalence of gallbladder polyps in patients with acromegaly (29%). In our series, this condition was not observed.
To the best of our knowledge, the primary DBD in patients with acromegaly has never been documented in the literature. Before declaring it to be a primary lesion, this anomaly should stress the clinician’s intention to carry out additional tests to rule out differential diagnoses, particularly lithiasis and tumors in this at-risk population. This finding needs to be studied further to comprehend the pathophysiology of this peculiar complication or fortuitous association. Long-term monitoring is necessary before considering this condition as a benign abnormality related to the organomegaly found in acromegaly. However, our study has a major limitation related to the small number of patients. This can be explained by the fact that our study was a single-center study conducted in a relatively young tertiary center.
Conclusion
Patients on long-term SA should be screened for sludge and GSs because of the high occurrence of serious complications. A yearly US assessment is recommended in this population since GS is often asymptomatic and discovered incidentally. The management by a multidisciplinary team with early detection and endoscopic treatment has improved the prognosis of our patients. Primary DBD is an exceptional disorder that has never been reported before in patients with acromegaly. Further studies are needed to assess its risk factors and clarify their therapeutic management.
Acknowledgments
The authors would like to acknowledge the whole team of the Gastroenterology Department for their collaboration and all medical and paramedical staff involved in the management of the patient.
Funding Statement
The authors declare that no financial resources have supported this work.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
All patients provided their informed consent for participation in the study and publication.
Author Contributions
Dr. Lamiae Zarraa wrote the manuscript, Dr. Imane Assarrar participated in the writing, supervised, and revised the manuscript, Dr. Oumaima Magouri participated in collecting data, Pr. Zahi Ismaili supervised the redaction and revised the manuscript, Pr. Siham Rouf helped and supervised the redaction and revised the manuscript, Pr. Hanane Latrech helped in writing, supervised the redaction, revised and approved the final draft for publication. All authors approved the final version of the manuscript.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
Abbreviations
- ABC
acute biliary complication
- BMI
body mass index
- CBD
common bile duct
- CT
computed tomography
- DBD
dilation of bile duct
- EASL
European Association for Study of Liver
- ERCP
endoscopic retrograde cholangiopancreatography
- EUS
endoscopic ultrasonography
- GH
growth hormone
- GS
gallstone
- IGF-1
insulin-like growth factor 1
- MRI
magnetic resonance imaging
- SAs
somatostatin analogs
- SPSS
Statistical Package for the Social Sciences
- SRLs
somatostatin receptor ligands
- UDCA
ursodeoxycholic acid
References
- 1.Melmed S. Medical progress: acromegaly. N Engl J Med. 2006;355(24):2558–2573. doi: 10.1056/NEJMra062453. [DOI] [PubMed] [Google Scholar]
- 2.Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, Wass JA. et al. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933–3951. doi: 10.1210/jc.2014-2700. [DOI] [PubMed] [Google Scholar]
- 3.Vilar L, Vilar CF, Lyra R, Lyra R, Naves LA. Acromegaly: clinical features at diagnosis. Pituitary. 2017;20(1):22–32. doi: 10.1007/s11102-016-0772-8. [DOI] [PubMed] [Google Scholar]
- 4.Capatina C, Wass JA. 60 years of neuroendocrinology: acromegaly. J Endocrinol. 2015;226(2):T141–T160. doi: 10.1530/JOE-15-0109. [DOI] [PubMed] [Google Scholar]
- 5.Gadelha MR, Kasuki L, Lim DST, Fleseriu M. Systemic complications of acromegaly and the impact of the current treatment landscape: an update. Endocr Rev. 2019;40(1):268–332. doi: 10.1210/er.2018-00115. [DOI] [PubMed] [Google Scholar]
- 6.Sisman P, Pekgoz M, Bayrakci I, Sisman M, Cander S, Oz Gul O, Erturk E. et al. Evaluation of upper gastrointestinal system in acromegaly. Ann Endocrinol (Paris) 2019;80(4):196–201. doi: 10.1016/j.ando.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Ezzat S. Hepatobiliary and gastrointestinal manifestations of acromegaly. Dig Dis. 1992;10(3):173–180. doi: 10.1159/000171355. [DOI] [PubMed] [Google Scholar]
- 8.Resmini E, Parodi A, Savarino V, Greco A, Rebora A, Minuto F, Ferone D. Evidence of prolonged orocecal transit time and small intestinal bacterial overgrowth in acromegalic patients. J Clin Endocrinol Metab. 2007;92(6):2119–2124. doi: 10.1210/jc.2006-2509. [DOI] [PubMed] [Google Scholar]
- 9.Giustina A, Barkhoudarian G, Beckers A, Ben-Shlomo A, Biermasz N, Biller B, Boguszewski C. et al. Multidisciplinary management of acromegaly: a consensus. Rev Endocr Metab Disord. 2020;21(4):667–678. doi: 10.1007/s11154-020-09588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasso LF, Auriemma RS, Pivonello R, Colao A. Adverse events associated with somatostatin analogs in acromegaly. Expert Opin Drug Saf. 2015;14(8):1213–1226. doi: 10.1517/14740338.2015.1059817. [DOI] [PubMed] [Google Scholar]
- 11.Aapkes SE, de Haas RJ, Bernts LHP, Blijdorp CJ, Dekker SEI, van Gastel MDA, Meijer E. et al. Incident gallstones during somatostatin analog treatment are associated with acute biliary complications especially after discontinuation. Drugs R D. 2021;21(2):179–188. doi: 10.1007/s40268-021-00342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attanasio R, Mainolfi A, Grimaldi F, Cozzi R, Montini M, Carzaniga C, Grottoli S. et al. Somatostatin analogs and gallstones: a retrospective survey on a large series of acromegalic patients. J Endocrinol Invest. 2008;31(8):704–710. doi: 10.1007/BF03346419. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Shlomo A, Melmed S. Somatostatin agonists for treatment of acromegaly. Mol Cell Endocrinol. 2008;286(1-2):192–198. doi: 10.1016/j.mce.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freda PU. Somatostatin analogs in acromegaly. J Clin Endocrinol Metab. 2002;87(7):3013–3018. doi: 10.1210/jcem.87.7.8665. [DOI] [PubMed] [Google Scholar]
- 15.Sendur SN, Oguz SH. Biliary tract disorders in patients with acromegaly: single-centre experience. Acta Medica Cordoba. 2022;53(2):180–187. doi: 10.32552/2022.actamedica.774. [DOI] [Google Scholar]
- 16.Prencipe N, Bona C, Cuboni D, Parasiliti-Caprino M, Berton AM, Fenoglio LM, Gasco V. et al. Biliary adverse events in acromegaly during somatostatin receptor ligands: predictors of onset and response to ursodeoxycholic acid treatment. Pituitary. 2021;24(2):242–251. doi: 10.1007/s11102-020-01102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montini M, Gianola D, Pagani MD, Pedroncelli A, Caldara R, Gherardi F, Bonelli M. et al. Cholelithiasis and acromegaly: therapeutic strategies. Clin Endocrinol (Oxf) 1994;40(3):401–406. doi: 10.1111/j.1365-2265.1994.tb03938.x. [DOI] [PubMed] [Google Scholar]
- 18.Stinton LM, Myers RP, Shaffer EA. Epidemiology of gallstones. Gastroenterol Clin North Am. 2010;39(2):157–169. doi: 10.1016/j.gtc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Gao X, Zhang L, Wang S, Xiao Y, Song D, Zhou D, Wang X. Prevalence, risk factors, and complications of cholelithiasis in adults with short bowel syndrome: a longitudinal cohort study. Front Nutr. 2021;8:762240. doi: 10.3389/fnut.2021.762240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahja S, Hammoumi W, Figuigui M, Abid H, Yousfi M El, Benajah DA, Abkari M. et al. Epidemiology of biliary lithiasis in a Moroccan rural population: results of a screening survey including 1358 citizens. Saudi J Med. 2022;7(5):272–277. doi: 10.36348/sjm.2022.v07i05.005. [DOI] [Google Scholar]
- 21.European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol. 2016;65(1):146–181. doi: 10.1016/j.jhep.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Avila NA, Shawker TH, Roach P, Bradford MH, Skarulis MC, Eastman R. Sonography of gallbladder abnormalities in acromegaly patients following octreotide and ursodiol therapy: incidence and time course. J Clin Ultrasound. 1998;26(6):289–294. doi: 10.1002/(sici)1097-0096(199807/08)26:6<289::aid-jcu2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Paisley AN, Roberts ME, Trainer PJ. Withdrawal of somatostatin analogue therapy in patients with acromegaly is associated with an increased risk of acute biliary problems. Clin Endocrinol (Oxf) 2007;66(5):723–726. doi: 10.1111/j.1365-2265.2007.02811.x. [DOI] [PubMed] [Google Scholar]
- 24.Shi YF, Zhu XF, Harris AG, Zhang JX, Deng JY. Restoration of gallbladder contractility after withdrawal of long-term octreotide therapy in acromegalic patients. Acta Endocrinol (Copenh) 1993;129(3):207–212. doi: 10.1530/acta.0.1290207. [DOI] [PubMed] [Google Scholar]
- 25.Annamalai AK, Gayton EL, Webb A, Halsall DJ, Rice C, Ibram F, Chaudhry AN. et al. Increased prevalence of gallbladder polyps in acromegaly. J Clin Endocrinol Metab. 2011;96(7):E1120–E1125. doi: 10.1210/jc.2010-2669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.