Abstract
Botulinum neurotoxin serotype B (BoNT/B) uses two separate protein and polysialoglycolipid-binding pockets to interact with synaptotagmin 1/2 and gangliosides. However, an integrated model of BoNT/B bound to its neuronal receptors in a native membrane topology is still lacking. Using a panel of in silico and experimental approaches, we present here a new model for BoNT/B binding to neuronal membranes, in which the toxin binds to a preassembled synaptotagmin-ganglioside GT1b complex and a free ganglioside allowing a lipid-binding loop of BoNT/B to interact with the glycone part of the synaptotagmin-associated GT1b. Furthermore, our data provide molecular support for the decrease in BoNT/B sensitivity in Felidae that harbor the natural variant synaptotagmin2-N59Q. These results reveal multiple interactions of BoNT/B with gangliosides and support a novel paradigm in which a toxin recognizes a protein/ganglioside complex.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04527-4.
Keywords: Botulinum neurotoxin type B, Synaptotagmin, Gangliosides, Molecular modelling
Introduction
Botulinum neurotoxins (BoNTs), are a family of potent protein toxins produced by anaerobic gram-positive Clostridia [1, 2]. BoNTs are classified as seven different serotypes from BoNT/A to G, further divided into subtypes with different amino acid sequences, although additional BoNTs are still being discovered, including mosaic toxins derived from a combination of different serotypes [1, 3].
BoNTs are the etiological agents of botulism, a rare but severe disease affecting many vertebrates, which results from the inhibition of acetylcholine release in the peripheral nervous system, causing flaccid paralysis. At the same time, BoNT/A, and to a lesser extent BoNT/B, are widely exploited for therapeutic applications [4–6].
BoNTs intoxicate neurons using a multistep mechanism. BoNTs are structurally similar to AB toxins with a 100-kDa heavy chain (HC) and a 50-kDa catalytic light chain, associated via a disulphide bond and non-covalent interactions. After entering the circulation, BoNTs target high-affinity receptors on peripheral nerve terminals via the HC domain. The amino-terminal domain of the HC then translocates the enzymatic light chain into the cytoplasm where the latter cleaves one of the three intracellular SNARE proteins (VAMP1-3, SNAP-25 or syntaxin 1) necessary for synaptic vesicle fusion and neurotransmitter release [5]. The BoNT carboxyl-terminal sub-domain of HC (HCc), organized into a β-trefoil fold, displays two independent binding pockets, which bind two distinct classes of receptors.
The first receptor to be discovered is a ganglioside localized on the outer surface of the plasma membrane of vertebrate cells [7], typically GT1b or GD1a [8]. These polysialogangliosides are part of a family of glycosphingolipids classified according to the number and position of the sialic acid (sia) linked on the sugar chain [9]. GT1b and GD1a are recognized by the ganglioside-binding site (GBS1) of BoNT/B, conserved in most BoNTs [8]. While the sia 5 and sia 6 present in GD1a and GT1b interact with GBS1, the GT1b-specific sia7 was suggested to reinforce BoNT/B binding [10]. Gangliosides associate with cholesterol in tightly packed lipid domains that are in dynamic equilibrium with less ordered membrane regions and can support lipid and protein-lipid interactions in cis and trans configurations [7, 9]. Moreover, microdomains containing gangliosides provide an entry pathway for several viruses and other pathogens [11–13].
The second receptor is a protein, corresponding to the luminal sequence of a synaptic vesicle protein: synaptotagmin 1 and 2 (SYT) for BoNT/B, G and the mosaic toxin BoNT/DC or SV2 for BoNT/A, D, E, F although the identity of BoNT/D protein receptor is still to be confirmed [2]. BoNT/C has no identified protein receptor, but like BoNT/D [14], harbors an additional ganglioside-binding pocket distinct from GBS1 and termed “sialic-binding site” that overlaps with the SYT-binding pocket of BoNT/B, contributing to toxicity [15, 16].
Besides these two receptors, a solvent-exposed lipid-binding loop (LBL), present in BoNT/B, C, D, G and BoNT/DC is localized between GBS1 and the protein (BoNT/B, G and DC) or sialic acid-binding pocket (BoNT/C and BoNT/D), participates in BoNT toxicity and neuronal membranes recognition [2, 17, 18]. The exceptional neurotropism of BoNT/B is conferred by interaction with the extracellular juxtamembrane domain (JMD) of SYT that is translocated to the plasma membrane by synaptic vesicle fusion [19]. SYT1 and SYT2 have comparable biochemical properties and similar functions, regulating exo-endocytic recycling of synaptic vesicles by interacting with cytosolic proteins such as the adaptor protein AP2, as well as with specific lipids like cholesterol and PIP2 [20–22]. While SYT1 is widely distributed in terminals of autonomic and sensory neurons, as well as in some neuromuscular junctions, SYT2 is the dominant isoform at most neuromuscular junctions [5]. Co-crystallization data indicate that BoNT/B binds SYT1 and SYT2 in a very similar manner using a saddle-shaped pocket interacting with 10–14 SYT JMD residues [6, 10, 23, 24]. The extracellular domain of SYT is not structured in solution, but the JMD of SYT adopts a helical conformation upon binding to BoNT/B [6, 10, 23, 24]. In the absence of gangliosides, BoNT/B displays a much higher affinity for rat SYT2 (40 nM) than for SYT1 (> 4 μM), due to a small difference in primary sequence in the SYT JMD [23]. Although BoNT/B has low affinity for GT1b (µM range) [17, 18], the latter drastically increases BoNT/B affinity (~ 0.4 nM) for membranes containing SYT [25–27]. In detergent, the synergistic effect of GT1b/GD1a requires the presence of the SYT transmembrane domain (TMD) [28, 29] and high-affinity binding is only reached in reconstituted lipids systems containing GT1b/GD1a as well as the transmembrane domain of SYT, suggesting a role for the intramembrane segments in toxin binding [10, 26, 30]. As the available structural data were obtained in the absence of apolar domains of BoNT/B receptors [6, 10, 23, 24], how BoNT/B binds to its receptors in a membrane context remains to be elucidated.
Recently we reported that the transmembrane and JMD of SYT interact with complex gangliosides inducing an α–helical structure [27]. A mutation (SYT1-K52A) that decreases GT1b assembly with SYT1, abolished BoNT/B binding in neuroendocrine cells, suggesting that the preassembly of a GT1b/SYT complex is crucial for BoNT/B interaction. Using a panel of in silico and experimental approaches, we now report that the SYT-binding pocket of BoNT/B can accommodate the preassembled GT1b/SYT complex. We thus propose a new model for BoNT/B-SYT interaction taking into account the membrane topology of neuronal toxin receptors, a parameter that has not been considered in previous structural studies.
Materials and methods
Experimental design
The main objective of this study was to investigate how botulinum neurotoxin serotype B binds to its receptors in a membrane context, since the available structural data were obtained in the absence of apolar domains. The detailed experimental design can be found in supplemental data.
Reagents
BoNT/B (B1 Okra strain) with non-toxic accessory proteins was from Metabiologics (Madison, WI). All peptides, biotinylated or not were synthesized by Genecust. DMPC (1,2-Dimyristoyl-sn-glycero-3-phosphocholine) was from Avanti Polar Lipids. GT1b was from Matreya LLC. Polyclonal anti-SYT1 31–55 region antibodies were generously provided by M. Takahashi. Rabbit anti-SYT2 40–65 polyclonal antibodies were produced by Genecust using a synthetic peptide (rat SYT2 40–65) and purified using protein-A sepharose. All experiments were performed in accordance with French and European guidelines for handling botulinum neurotoxin. GT1b, lyso-lactosylceramide and sphingomyelin were from Matreya LLC. Anti-BoNT/B and anti-SYT1/2 (1D12) antibodies were obtained as described [27]. Alexa-coupled secondary antibodies were from Jackson Immunoresearch. DAPI was from SIGMA-Aldrich. Anti-GT1b monoclonal antibodies were from Merck Millipore (MAB 5608).
Surface Plasmon Resonance (SPR) experiments
SPR were performed with a Biacore T200 apparatus (Cytiva) (see supplemental data).
Langmuir monolayers experiments
Surface pressure measurements revealing peptide-lipid interactions at the air–water interface were studied using the Langmuir film balance technique with a fully automated microtensiometer (µTROUGH SX, Kibron Inc. Helsinki, Finland) as described previously, using an initial pressure of 17.5 mN/m [27, 31, 32].
Immunofluorescence
HEK293 or PC12 cells were cultured on poly-l-Lysine (10 μg/ml) treated coverslips (300,000 cells per well) in DMEM containing 5% FBS, 5% HS and 1% penicillin/streptomycin mixture (complete medium). Cells were transfected with the corresponding plasmids (pIRES-EGFP-SYT2; pIRES-EGFP-K60A-SYT2; pIRES-EGFP-N59Q-SYT2; pIRES-EGFP-SYT1 or pIRES-EGFP-H51G-SYT1) using Lipofectamine 2000 and according to the manufacturer’s procedure. 40 h after transfection, GT1b (10 µg/ml) was added to the wells in DMEM and incubated for 1.5 h at 37 °C followed by one washing step and transfer to complete medium. BoNT/B (10 nM and 1 nM for SYT1 and SYT2 conditions, respectively) was added afterwards and incubated for 30–45 min at 37 °C. After a first wash with the culture medium, additional washes were performed with PBS and cells were fixed in the dark at 4 °C in 4% paraformaldehyde/PBS for 15 min followed by NH4Cl washing steps. Non-specific binding was blocked with 0.2% (w/v) gelatine or 5% (v/v) goat serum in a PBS buffer containing 0.1% saponin. Anti-BoNT/B (0.5 µg/µl) and 1D12 anti-SYT (1 µg/ml) antibodies were then added for 45 min at 22 °C. After subsequent washing, staining was visualized using secondary anti-rabbit Alexa-594 and anti-mouse Alexa-488 antibodies. Nuclei were detected using DAPI.
Image acquisition and analysis
Confocal images were acquired on a Zeiss LSM780 microscope and processed using ImageJ (http://rsb.info.nih.gov/ij/). For quantification, SYT immunolabeling images were thresholded in order to get a binary mask. This binary mask was used to obtain immunoreactivity (IR) values of the regions of interest (ROIs) over SYT and BoNT/B channels. For comparisons of BoNT/B binding to WT vs mutant SYTs, IR values were normalized to WT in every experiment. Results are presented as mean ± SEM. Statistical analysis was performed using Mann–Whitney U test.
Molecular modelling
Molecular modelling studies were performed in vacuo using Hyperchem (http://www.hyper.com), Deep View/Swiss-Pdb viewer (https://spdbv.vital-it.ch) and Molegro Molecular viewer (http://molexus.io/molegro-molecular-viewer) as described in previous studies [27, 33]. The coordinates of the BoNT/B lipid-binding loop (aa 1245–1252) present in PDB 2NM1 were inserted in PDBs files 4KBB and 6G5K. The sugar coordinates of GD1a were then merged with the PDB file 6G5K to reconstitute a trimolecular complex for SYT1. The preassembled complex GT1b/SYT1 and GT1b/SYT2 were docked on the synaptotagmin binding pocket according to the crystal coordinates of SYT1 (PDB 6G5K) and SYT2 (PDB 4KBB). All presented structures were of the BoNT/B1 subtype. The structures of the ceramide part of GD1a and cholesterol were retrieved from the CHARMM-GUI platform and added to the models to obtain a full system in a membrane context. Energy minimization of the complex with BoNT/B aa 1079–1290 was performed with the Polak-Ribière conjugate gradient algorithm, with the Bio + (CHARMM) force field in Hyperchem, typically with 3 × 105 steps, and a root-mean-square (RMS) gradient of 0.01 kcal. Å−1 mol−1 as the convergence condition.
Graphical representation of membrane-embedded complexes
In order to generate a schematic representation of the membrane-embedded protein complexes, the tool “membrane builder” available on CHARMM-GUI was used to generate a patch consisting of 128-DPPC (1,2-dipalmitoylphosphatidylcholine) molecules in a lipid-bilayer topology. Then, the minimized models were inserted according to the orientation proposed by the PPM Web Server. The snapshots were taken using Chimera software [34].
Statistical analysis
Results are presented as mean ± SEM for immunofluorescence quantifications or mean ± SD for Langmuir monolayers and SPR analysis, of n independent experiments. Statistical analysis was performed using either Mann–Whitney U test or One-way ANOVA followed by Bonferroni post-hoc test for means comparisons. All statistical tests were performed using OriginPro 8.0.
Results
BoNT/B binds to SYT pre-assembled with GT1b
The JMD of SYT binds GT1b [27] via a consensus ganglioside-interaction motif that overlaps with the described BoNT/B-SYT interaction domain [10]. We, therefore, addressed the question as to whether BoNT/B can bind to a SYT/GT1b complex. To investigate this point, we developed an SPR-based approach, consisting in capturing a peptide encompassing the JMD of SYT (pSYT1 32–58 (Biot-GEGKEDAFSKLKQKFMNELHKIPLPPW) or pSYT2 40–66 (Biot-GESQEDMFAKLKDKFFNEINKIPLPPW) (Supplemental Fig. 1) on a sensor chip, assembling a SYT/GT1b complex and then evaluating BoNT/B binding. GT1b diluted in running buffer interacted strongly with pSYT1 or pSYT2 immobilized on a sensor chip (Fig. 1a). The interaction was specific, as no binding occurred on a control pSYT9 peptide (Biot-HDSCQDFIYHLRDRARPRLRDPDISVS) (Fig. 1a, Supplemental Fig. 1). Ganglioside binding to pSYT was detected at 10 nM GT1b (Supplemental Fig. 2a), a concentration far below its critical micellar concentration [35] and was dose-dependent (Supplemental Fig. 2b). Estimation of the ganglioside/peptide molar ratio indicated that a mean of 3 molecules of GT1b were bound per peptide (3.04 ± 0.4, n = 7 independent experiments ± SD using pSYT1 or pSYT2). This observation is compatible with ceramide-mediated multimeric self-assembly of gangliosides that occurs in lipid rafts [36]. The interaction of the JMD of SYT with GT1b was further corroborated using antibodies that specifically recognize the JMD of SYT. As shown in Supplemental Figs. 2c, d and 3, GT1b bound to SYT masks the recognition domain of anti-SYT JMD antibodies and inhibits their binding to SYT. Altogether, these results demonstrate that the ganglioside binding site of the JMD of SYT immobilized on a chip can stably capture GT1b. This experimental protocol mimics native conditions where SYT binds one GT1b molecule in a gangliosides cluster allowing BoNT/B to interact with SYT/GT1b (1:1) using the SYT-binding pocket and other SYT-free GT1b molecules using GBS1 and LBL.
Fig. 1.
SPR based on-chip reconstitution of BoNT/B binding to SYT JMD pre-assembled with GT1b. A GT1b binding to pSYT1 (Biot-GEGKEDAFSKLKQKFMNELHKIPLPPW) and pSYT2 (Biot-GESQEDMFAKLKDKFFNEINKIPLPPW). GT1b (200 nM) was injected for 1 min over immobilized pSYT1, pSYT2 and pSYT9 (Biot-HDSCQDFIYHLRDRARPRLRDPDISVS) (240 RU) at 40 µl/min. Representative of > 10 independent experiments. B GT1b bound to SYT induces an increment in BoNT/B binding signal. BoNT/B (30 nM) was injected (first arrow) onto pSYT1 (260 RU) showing a transient interaction that rapidly returns to baseline level (lower dashed line). GT1b (10 nM, second arrow) was then stably immobilized on pSYT1 (ΔGT1b) generating a new baseline (upper dashed line) and BoNT/B (30 nM, third arrow) was then injected again. Black bars highlight the BoNT/B injection phases. Representative of 6 independent experiments. C GT1b potentiation of BoNT/B binding to pSYT1 depends on the amount of GT1b bound to pSYT1. Sensorgrams resulting from the interaction of BoNT/B (30 nM) with pSYT1 (300 RU) pre-assembled with various amounts of GT1b (from 0 to 400 RU) were superposed. Representative of three independent experiments
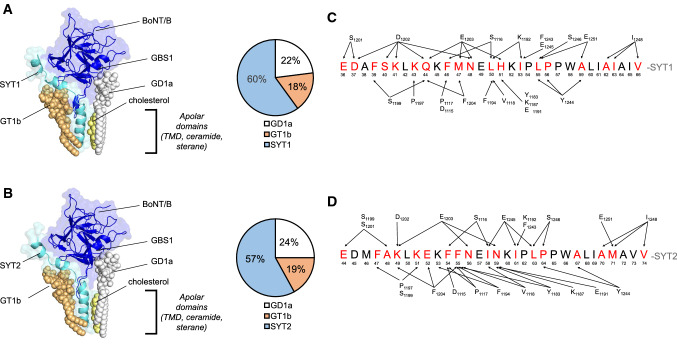
Fig. 2.
Overall structure of the energy-minimized complex between BoNT/B and its membrane ligands. A Model of SYT1-BoNT/B complex (SYT1 aa 34–72, BoNT/B HC aa 1079–1290). B Model of SYT2-BoNT/B complex (SYT2 aa 42–80, BoNT/B HC aa 1079–1290). The models are based on the initial superposition of a preformed synaptotagmin1/2-GT1b complex positioned in the SYT binding site of the toxin, also bound to GD1a, with a cholesterol molecule positioned between SYT-TM and GD1a ceramide. Both BoNT/B and SYT are represented as cartoons (dark and light blue respectively). The gangliosides and cholesterol are represented as spheres (GT1b: light orange, GD1a: white, cholesterol: light yellow). The apolar domains indicated in the models correspond to the sterane and isooctyl chains of cholesterol, the ceramide part of GD1a and GT1b, and the TMD of SYT. The pie charts indicate the relative distribution of the energies of interaction in the complex between BoNT/B and SYT1/2, GT1b, GD1a and between BoNT/B and SYT2, GT1b, GD1a. Note that cholesterol does not interact with the toxin, but with SYT TM and the ceramide part of GD1a. C and D depict mapping of the intermolecular interactions between BoNT/B and SYT. C SYT1 and D SYT2 residues interacting with the toxin are highlighted in red. Arrows indicate BoNT/B-SYT interaction points. A cut off < 3.5 Å was used to select the illustrated residues
We then compared BoNT/B binding to SYT and SYT/GT1b complex. In the absence of GT1b, BoNT/B binding yielded a transient SPR signal on pSYT1 (Fig. 1b first arrow), consistent with its reported low affinity [23, 27]. GT1b was then immobilized on pSYT1 (Fig. 1b second arrow) before a subsequent injection of BoNT/B on the SYT/GT1b complex (Fig. 1b last arrow). Compared to pSYT1 alone, GT1b interaction with pSYT enhanced BoNT/B binding during the association phase, with a slower dissociation kinetic. This increase in BoNT/B affinity due to GT1b association with pSYT1 is similar to the effect of GT1b measured in proteoliposomes containing full-length SYT1 [27]. We previously showed that SYT1-K52A mutation blocked GT1b-induced potentiation of BoNT/B binding to synaptotagmin-expressing cells [27]. The present SPR experiments reproduced this observation as no GT1b enhancement of BoNT/B binding was observed on pSYT1-K52A bound to GT1b (Supplemental Figs. 1 and 3a). The potentiation of BoNT/B binding by GT1b depended on the amount of gangliosides bound to SYT, reaching a plateau (Fig. 1c, Supplemental Fig. 3b). At saturating concentrations of GT1b, BoNT/B binding to pSYT1 increase to 580% ± 43% (n = 3 independent experiments ± SD). Similar results were obtained when GD1a was bound to pSYT1 instead of GT1b (Supplemental Fig. 3c). To rule out the possibility that GT1b alone produces an enhancement of BoNT/B binding independently of SYT, we used a mutant SYT1 peptide (F46A, Supplemental Fig. 1) that is unable to bind BoNT/B, but still interacts with GT1b [24] (Supplemental Fig. 3d). In contrast to pSYT1/GT1b complex, BoNT/B did not interact with pSYT1-F46A/GT1b complex (Supplemental Fig. 3b) in agreement with the low affinity of BoNT/B for gangliosides. BoNT/B binding does not induce GT1b dissociation from pSYT/GT1b complex, as anti-GT1b antibodies detected the same amount of GT1b before and after BoNT/B binding (Supplemental Fig. 3e, f). BoNT/B binding to pSYT2 was also measured when GT1b was bound to SYT2, with the difference that BoNT/B signals were higher on pSYT2 than on pSYT1 in the absence of ganglioside, in accordance with their relative affinity (Fig. 1, Supplemental Fig. 3g). As for SYT1, the presence of GT1b, bound to SYT2, promoted BoNT/B interaction with SYT2, increasing binding affinity mainly by decreasing the dissociation rate of BoNT/B from pSYT2 (Supplemental Fig. 3g). Altogether, these results demonstrate that BoNT/B binds to a preassembled SYT/GT1b complex.
Molecular modeling of BoNT/B bound to a SYT/GT1b complex
In order to obtain molecular insight into the interaction of BoNT/B with SYT1-GT1b and SYT2-GT1b complexes, with specific information on the fate of the LBL upon toxin binding, we developed a molecular modeling strategy that considers membrane topology. Using the initial coordinates of toxin-SYT (PDB 4KBB, 6G5K and 2NM1), we constructed a full system consisting of a SYT-GT1b complex, a GD1a-toxin complex and a cholesterol molecule. After several rounds of energy minimization, a stable complex was obtained for both systems including SYT1 (Fig. 2a) and SYT2 (Fig. 2b). The initial conditions had to be slightly adjusted to take into account the TM domain of SYT and the ceramide part of the GD1a ganglioside which were absent from the crystal structure [10]. These conformational constraints respected the overall geometry of the membrane, except that a gap between GD1a and the TM domain of SYT was filled by a cholesterol molecule [20]. GBS1 of BoNT/B interacts with a SYT-free GD1a and the BoNT/B SYT-binding pocket interacts with a GT1b molecule precomplexed with SYT (~ 25% and ~ 15% of the total energy, respectively) but in both cases the major contribution of toxin binding was due to SYT (~ 60% of the total energy) as shown in the pie chart in Fig. 2. A key feature of our models is the insertion of the LBL loop between GT1b and SYT (Fig. 2, Supplemental Fig. 4b). The overall energy of interaction of BoNT/B-SYT complex is around -600 kJ/mol (− 592 kJ/mole for SYT1 versus − 664 kJ/mole for SYT2). It was previously noted that the presence of ganglioside and SYT in contact with BoNT/B1 does not change its overall structure [10]. This is also the case in our modeling data when a SYT/GT1b complex is bound to the toxin (Supplemental Fig. 5).
Fig. 5.
Measurement of BoNT/B apolar loop (LBL) interaction with GT1b using Langmuir monolayers. A Stable monolayers of GT1b, lyso-LacCer (Lyso-LC), and sphingomyelin (SM) were prepared at the air–water interface at an initial surface pressure of 15–20 mN m−1. After equilibrium of the monolayer, the BoNT/B apolar loop (RFYESGIVFEEYKDY) (aa 1242–1256) was added at a final concentration of 10 µM. The data show the surface pressure increase ∆π induced by the loop as a function of time. The data are representative of three distinct experiments. B Histograms comparing the endpoints in a ± SD of three independent experiments
BoNT/B-SYT interaction
The toxin was found to interact with a significant part of the extracellular regions of SYT1 (E36-W58) and SYT2 (E44-W66) via the SYT binding pocket, including loops Y1183-K1188, P1197-D1202 and K1113-P1117 (Supplemental Fig. 4a, Fig. 2c, d). The SYT helix, pre-conformed by GT1b, extends from E36 to H51 in SYT1 and E44 to N59 in SYT2, whereas a small distortion of the helix was observed in the central part of BoNT/B-SYT interface (Fig. 2a, b, Supplemental Fig. 4a). Almost all amino acids of BoNT/B that interact with SYT in crystal structures (6G5K, 2NP0, 4KBB, 2NM1) were found in contact with SYT and/or GT1b in our models (Supplemental Table 1). However, the conformational adjustment induced by the TM domain of SYT slightly turned the helix, generating a different interaction map with BoNT/B, compared to the BoNT/B-SYT binding interface determined by X-ray diffraction crystallography without membrane constraints. A detailed analysis of the contribution of amino acid residues of SYT and of the toxin revealed the evolution of the complex between the initial crystal conditions and the presented models (Supplemental Tables 1 and 2). SYT1-F46 and SYT2-F54, which initially interacted with GT1b in SYT/GT1b complexes [27], engage interactions with residues 1115–1117 of the toxin while retaining ganglioside association (Figs. 2c, d, 4, Supplemental Fig. 6). A superposition of the crystal structure (6G5K and 2NP0) with our models is shown in Fig. 3 for SYT1-F46 and M47 and its SYT2 counterparts that are described as key energetic hotspot residues in the crystal structure. In the case of SYT1 the aromatic ring of F46 (white) is replaced in the model by the apolar side chain of M47 (Fig. 3a, light blue). Consequently, most of the amino acid residues of the toxin that were in contact with F46 now interact with M47. In the case of SYT2 a similar substitution was evidenced between F54 in the crystal structure (white) and F55 in our model (Fig. 3b, light blue). Interestingly, the aromatic ring of both residues is oriented in a similar way so that the pi-pi network involving residues Y1183, F1194 and F1204 of the toxin was still operative. These models uncover several additional SYT JMD residues compared to crystal data (E36, D37, S40, K41, Q44, N48, H51 in SYT1 and E44, A48, K49, E52, N56, N59 in SYT2) (Fig. 2c, d, Supplemental Table 2). Among them, SYT1-H51 and SYT2-N59 residues are facing the toxin and exhibit a high energy of interaction involving BoNT/B residues Y1183, K1187, E1191 and E1203 (Supplemental Fig. 7). In addition to the SYT/binding pocket, the BoNT/B LBL participates in the toxin-SYT complex by interacting tightly with apolar extramembrane (L50, I53, L55, P56) and membrane-embedded (A59, A62, I63, V66) residues of SYT1 (Fig. 2c, d, Supplemental Table 2a). Similar interactions were observed with homologous residues of SYT2 (Fig. 2c, d, Supplemental Table 2b).
Fig. 4.
Schematic overview of intermolecular interactions between GT1b-BoNT/B and GT1b-SYT. The amino acids of SYT1 (A) and SYT2 (B) are boxed while those of the toxin are circled. A cut off < 3.5 Å was used to select the residues indicated in the figure. Glc glucose, Gal galactose, Gal-Nac N- acetylgalactosamine, Sia sialic acid, Cer ceramide. Only residues with energy ≥ 3 kJ/mol are listed
Fig. 3.
Close-up view of the molecular interface of SYT1-F46-M47 and SYT2-F54-F55. A Superposition of the SYT1-BoNT/B complex from PDB: 6G5K (BoNT/B in green and SYT1 in white) and the present model (BoNT/B in blue and SYT1 in light blue). B Superposition of the SYT2-BoNT/B complex from PDB 2NP0 (BoNT/B in green and SYT2 in white) and the present model (BoNT/B in blue and SYT2 in light blue). Interacting BoNT/B residues are shadowed in gray. Note that the position of BoNT/B residues are conserved between the proposed models and the corresponding crystal structures while their relative partners shift from F46 to M47 in SYT1 and F54 to F55 in SYT2.
SYT-GT1b interaction
GT1b imposes an angle of about 45° between the JMD of SYT and the membrane (Fig. 2a, b). The mapping of the molecular interactions between GT1b and either SYT1 (Fig. 4a) or SYT2 (Fig. 4b) and the toxin revealed that the binding involved the ceramide part of GT1b and the four terminal sugars and sialic acids (Glc1 and Gal2 are not involved in binding). The overall binding energy between SYT and GT1b was conserved upon interaction with BoNT/B (Supplemental Table 2). Interestingly, we noted a rearrangement involving F46 in SYT1 and its homologous F54 in SYT2 that reinforced the interaction with GT1b upon toxin binding (+ 32% and + 90% for SYT1 and SYT2 respectively), involving Gal4, Sia5 and Sia6 (Fig. 4, Supplemental Table 2, Supplemental Fig. 8). In contrast, SYT1-K52, I53, L55, W58 and SYT2-K60, I61, W66 that were interacting initially with the Sia6 and Sia7 of GT1b in the preassembled SYT/GT1b complex, lose energy upon toxin binding (− 92% and − 47%, respectively, Supplemental Table 2, Supplemental Fig. 8), suggesting also a molecular rearrangement in this region.
BoNT/B-GT1b and GD1a interactions
In the minimized complexes, both the LBL and the beta hairpin loop K1113-P1117 that interact with SYT, also bind to sialic acids of GT1b bound to SYT (Fig. 4, Supplemental Table 1b, Supplemental Fig. 9b). The LBL binds to Sia 7, whereas the K1113–P1117 loop binds to Sia 5 and Sia 6 (Fig. 4). Remarkably, as shown in Supplemental Fig. 9b, the BoNT/B loop K1113–P1117 corresponds to conserved β-hairpin loops E1114–V1117, A1126-R1129 and K1143–D1147 of BoNT/D, BoNT/C and tetanus toxin, respectively, which contribute to the sialic acid binding site [14–16, 37]. Our model suggests that the BoNT/B-protein binding pocket has an evolutionarily conserved ability to bind sialic acids that are brought by the SYT-associated ganglioside in the case of BoNT/B. Concerning the canonical ganglioside binding site, the BoNT/B residues interacting with the sugar part of GD1a were globally conserved after minimization compared to structural data (Supplemental Table 3).
Finally, it is worth noting that, in the minimized complex, cholesterol interacts with the TM domain of SYT, occupying a space created by the addition of the ceramide part of GD1a. Cholesterol increases the stability of the complex through a set of London forces with the ceramide part of GD1a and the TMD domain of SYT (Supplemental Table 4). These data raise the interesting notion that cholesterol could play an active role in the initial steps of toxin binding to lipid rafts in agreement with a previous description of SYT/cholesterol interactions [20].
Altogether, these results suggest that BoNT/B interacts with the JMD and TMD domains of SYT along with two ganglioside molecules, one associated with SYT and the other with the ganglioside binding pocket of the toxin, with interconnection of the different intramembrane domains.
The lipid-binding loop of BoNT/B binds to GT1b
Our molecular modeling data suggest that the LBL could physically interact with SYT and GT1b. In order to study in isolation, the LBL binding to GT1b and to corroborate our modeling data, we used the Langmuir monolayer method and recorded surface pressure changes in GT1b monolayers induced by a water-soluble BoNT/B peptide p1242-1256 (RFYESGIVFEEYKDY) encompassing the LBL (Supplemental Fig. 1). As shown in Fig. 5a, b, injection of p1242-1256 underneath a monolayer of GT1b yielded an increase in surface pressure (12.43 ± 1.14 mN/m, n = 3), whereas limited interaction was found with lyso-LacCer (5.2 ± 0.72 mN/m, n = 3), a lipid with an inverted conic shape resembling gangliosides, or sphingomyelin (1.97 ± 0.37 mN/m, n = 3), a major sphingolipid component of lipid rafts. The absence of interaction with the ceramide domain of sphingomyelin indicates that the LBL of BoNT/B interacts preferentially with the sugar part of GT1b.
SYT1H51/K52 and SYT2-N59/K60 are key residues in BoNT/B binding
We have previously shown that the mutation SYT1-K52A abolished BoNT/B binding to PC12 neuroendocrine cells [27]. Our present molecular modeling data predict that, upon toxin binding, a molecular rearrangement occurs in the vicinity of SYT1-K52 and the corresponding SYT2-K60. We thus investigated whether the SYT2-K60 was also an important determinant in BoNT/B binding. Surface Plasmon Resonance analysis indicated that binding of the SYT2-G40-W66 peptide to GT1b-containing liposomes was drastically inhibited by mutating K60 to alanine (Supplemental Figs. 1 and 10), showing that SYT2-K60 residue is involved in the SYT2/GT1b interaction like SYT1-K52 [27]. Immunofluorescence experiments showed that BoNT/B binding was severely impaired in PC12 cells expressing SYT2-K60A, compared to cells expressing SYT2-WT, with a decrease of 64% (Supplemental Fig. 11a, c). A similar degree of inhibition (69%) was obtained using HEK 293 cells (Supplemental Fig. 11b, d). Although this decrease is less than that observed for SYT1-K52A [27], these results indicate that the SYT2-K60A like SYT1-K52, is an important determinant in BoNT/B binding.
According to our model, SYT1-H51 and SYT2-N59 residues adjacent to SYT1-K52 and SYT2-K60, respectively, exhibit a high energy of interaction with BoNT/B (Fig. 2c, d, Supplemental Fig. 7, Supplemental Table 2). To ascertain the functional involvement of SYT1-H51 in BoNT/B binding to SYT1, we mutated SYT1-H51 to a glycine residue and measured by immunofluorescence BoNT/B binding to either WT or SYT1 mutant transfected HEK 293 cells. The mutation SYT1-H51G induced a significant reduction (35%) in the binding of BoNT/B to the cell surface (IR BoNT/B of SYT1-WT: 1.00 ± 0.04 vs. IR BoNT/B of SYT1-H51G: 0.65 ± 0.04) while it did not affect expression levels of SYT1 (IR SYT of SYT1-WT: 1.00 ± 0.02 vs. IR SYT of SYT1-H51G: 1.01 ± 0.02) (Fig. 6). Interestingly, a variant of SYT2-N59 (SYT2-N59Q) occurs naturally in cats, which are known to be resistant to type B botulism [38], while expressing cleavable VAMP1, the predominant VAMP isoform in motor neurons [6] (Supplemental Table 5). Of note, a Q residue at position 59 was present in all Felidae sequences analyzed (Supplemental Table 5). We thus evaluated the potential impact on BoNT/B binding of this naturally occurring mutation. HEK 293 cells were transfected with either SYT2-WT or SYT2-N59Q and BoNT/B binding in the presence of GT1b was investigated by immunofluorescence. As shown in Fig. 6, the N59Q mutation induced a 50% decrease in BoNT/B binding (IR BoNT/B of SYT2-WT: 1.00 ± 0.03 vs. IR BoNT/B of SYT2-N59Q: 0.52 ± 0.02), while the expression of SYT2 remained unaffected (IR SYT of SYT2-WT: 1.00 ± 0.01 vs. IR SYT of SYT2-N59Q: 0.96 ± 0.01).
Fig. 6.
Mutations in the BoNT/B binding interface of SYTs decrease the binding of BoNT/B to HEK 293 cells. A Immunostaining of SYT1 (green) and BoNT/B (red) in HEK 293 cells transfected with either SYT1-WT (top) or H51G-SYT1 (bottom). DAPI signal is shown in blue, and the merge over DIC images indicated. Orthogonal projections of SYT1 labelling (green) in cells transfected with WT-SYT1 or SYT1-H51G (right). Scale bars, 10 µm. B Immunostaining of SYT2 (green) and BoNT/B (red) in HEK 293 cells transfected with either SYT2-WT (top panels) or SYT2-N59Q (bottom panels). DAPI signal is shown in blue, and the merge over DIC images indicated. Orthogonal projections of SYT2 labelling (green) in cells transfected with SYT2-WT or SYT2-N59Q (right). Scale bars, 10 µm. C Quantification of BoNT/B binding (gray and pink) and SYT1 expression (black and red) in cells expressing SYT1-WT or SYT1-H51G. The number of ROIs analyzed is indicated within each column. Normalized immunoreactivity data (IR) are expressed as mean ± SEM. Mann–Whitney U test was used for comparisons. **P < 0.01; n.s. non-significant. SYT1-WT IR to SYT1-H51G IR P = 0.64; BoNT/B SYT1-WT to BoNT/B SYT1-H51G P = 4.42 × 10–12. D Quantification of BoNT/B binding (gray and pink) and SYT2 expression (black and red) in cells expressing SYT2-WT or SYT2-N59Q. The number of ROIs analyzed is indicated within each column. Data are expressed as mean ± SEM. Mann–Whitney U test was used for comparisons. **P < 0.01; n.s. non-significant. SYT2-WT IR to SYT2-N59Q IR P = 0.64; BoNT/B SYT2-WT to BoNT/B SYT2-N59Q P < 0.001
Taken together, these results indicate that both SYT1-H51K52 and SYT2-N59K60 homologous residues constitute a crucial doublet for BoNT/B binding and are consistent with the proposed structure of the SYT/GT1b/GD1a/cholesterol/BoNT-B complex. They also provide an evolutionary explanation for the appearance of mutations (SYT2-N59Q) in animals with a diet at least partially based on carrion.
Discussion
The current view of the BoNT/B binding determinants that anchor the distal tip of BoNT/B C-terminal domain to nerve terminals consists of two closed pockets interacting independently with SYT and gangliosides (GT1b or GD1a) and a lipid-binding loop thought to interact with the cell membrane via hydrophobic interactions. The central role of SYT in BoNT/B toxicity is supported by its relatively high affinity for the toxin, its synaptic localization conferring tissue specificity and by the observation that changes in potency among different BoNT/B subtypes are related to variability in the BoNT/B domain recognizing SYT but not in GBS1 [39].
Functional assays have unambiguously demonstrated that gangliosides (GT1b / GD1a) are also necessary for BoNT/B intoxication and have a drastic synergistic effect on BoNT/B binding to SYT-containing membranes [24, 26, 27, 29]. However, the contribution of gangliosides to neuronal membrane recognition by BoNT/B is not totally understood. The affinity of the toxin for GT1b reconstituted in nanodiscs is weak (30–50 µM) [17, 18] and not sufficient to measure detectable BoNT/B binding and VAMP2 cleavage in SYT knockout hippocampal neurons [40]. Yet, both the canonical ganglioside binding site GBS1 and the LBL appear to participate in GT1b potentiation of BoNT/B binding to SYT, as inactivation of the GBS1 or the LBL abolishes the synergistic effect of GT1b in vitro and causes a strong reduction of toxicity [18, 29].
In a recent study we elucidated an important new mechanism underlying the role of gangliosides by demonstrating that GT1b actually binds to SYT JMD and induces the formation of an alpha-helix from an initially disordered domain [27]. Intriguingly, GT1b overlaps critical residues defined by crystallographic and biochemical experiments in the SYT2-F54-I58 region, raising the question whether and how BoNT/B recognizes a preassembled GT1b/SYT complex and whether this complex dissociates upon BoNT/B binding [27].
Several experimental methods have been used to analyze the synergetic effect of GT1b on BoNT/B binding to SYT, including cultured cells and reconstituted systems (proteoliposomes, ELISA, mixed detergent micelles) [26, 27, 29, 41]. However, these approaches were not adapted to assessing whether during the GT1b potentiation effect, SYT is associated with GT1b. In the present study, we developed a SPR binding assay, with several GT1b molecules engaged in a complex with SYT, ensuring that BoNT/B could recognize SYT complexed to GT1b, along with free GT1b molecules that could also interact with the ganglioside-binding pocket of the toxin. Altogether, the SPR data strongly suggested that the SYT binding pocket of BoNT/B can accommodate SYT bound to GT1b.
We then used molecular modeling to assess how BoNT/B could recognize the SYT/GT1b complex. Compared to GD1a, GT1b is widely used in biochemical experiments as it induces a higher potentiation effect on BoNT/B binding to SYT [24, 30], whereas available crystallographic data were all performed using the oligosaccharide of GD1a [10, 17, 39].
We docked the SYT/GT1b complex into the BoNT/B SYT-binding pocket, based on the co-crystal coordinates of BoNT/B associated with GD1a, together with the membrane-embedded domains of SYT and gangliosides. Cholesterol, which is known to interact with both the TM of SYT and the ceramide moiety of gangliosides [42] was also included in the system. Flexible docking revealed that BoNT/B bound to GD1a can also interact with SYT1 or SYT2 bound to GT1b via the BoNT/B SYT-binding pocket described by structural data. The BoNT/B residues interacting either with SYT or GT1b are overall the same as described in the crystal structure, yet with additional interactions in the N-terminal domain of SYT. The SYT helix pre-conformed by GT1b extends from E44 to N59 and the BoNT/B-SYT interaction is mainly driven by apolar residues, including SYT2-F47, F54, F55 and I58 as previously described [23, 24]. In our present model, the membrane constraints induced by the overall polar and apolar ganglioside/SYT interactions introduce an angle between SYT and the membrane plane, modifying the interaction map between BoNT/B and the SYT helix in comparison to structural data obtained with only the extracellular domain of SYT [10, 23, 24]. It is of note that GT1b rescue of BoNT/B binding to several SYT mutants has revealed the crucial role of SYT2-F54 at the BoNT/B-SYT interface [23, 24, 43]. Interestingly, our proposed models are compatible with this observation since they show that SYT2-F54 and its counterpart SYT1-F46 strongly interact with both GT1b and BoNT/B. Thus, molecular modeling, along with SPR binding experiments, is consistent with the view that the SYT/GT1b complex does not dissociate upon BoNT/B interaction and is recognized by the SYT binding pocket.
After the minimization process, the BoNT/B LBL was found to interact with the sialic acids of GT1b, the extracellular C-terminal part of the SYT JMD, as well as membrane-embedded SYT residues. This positioning of the LBL is allowed by the initial interaction of BoNT/B with the preformed SYT/GT1b complex, which determines the orientation of the toxin so that the LBL is directed toward the glycone part of GT1b and the TM domain of SYT. Consistent with our model, we determined experimentally that LBL directly binds to a monolayer of GT1b, but does not recognize sphingomyelin. This suggests that the LBL preferentially recognizes the polar headgroup of GT1b. The interaction between LBL and GT1b corroborates a report where LBL deletion decreased BoNT/B binding to nanodiscs containing GT1b [18]. From a structural point of view, our findings suggest that BoNT/B LBL reinforces the interaction of BoNT/B with the SYT/GT1b receptor, explaining why its deletion dramatically reduces BoNT/B toxicity [18]. Interestingly, BoNT/C, BoNT/D and tetanus toxin also possess a lipid-binding loop that has been shown to bind sialic acids and it has been reported that the BoNT/C and BoNT/D LBL are structurally close to the corresponding SYT-binding site of BoNT/B [14–16, 44]. We propose that, for BoNT/B, C, D and tetanus toxin, a functional relationship exists between the presence of an LBL and the ability of these toxins to bind free or SYT-associated sialic acids outside of GBS1. As BoNT/G has a similar SYT-binding site to BoNT/B and an LBL, it would be interesting to investigate if this toxin also interacts with a SYT/GT1b complex [43]. Likewise, it would be interesting to address whether a SYT/GM1 complex binds to BoNT/DC. For BoNT/A and BoNT/E, the absence of LBL would be compensated by an interaction with glycosides covalently linked to their receptor, SV2 in this case [1].
In addition to the LBL, the model predicts that BoNT/B loop 1113–1117 also interacts with sialic acids of the GT1b-SYT complex. Interestingly and independently from GBS1, tetanus toxin, BoNT/C and BoNT/D use residues with a similar 3D-position to the BoNT/B 1113–1117 loop to mediate binding to sialic acids. As co-crystallization studies failed to detect the presence of sialyllactose in the BoNT/B SYT binding pocket [45], our model suggests that BoNT/B can bind sialic acid only when the glycoside is presented by SYT. The fact that BoNT/B could bind another ganglioside, in addition to that occupying GBS1, is compatible with the observation that trefoil recognition of carbohydrates is often multivalent [46]. It has been noted that BoNT/B binds SYT using a pocket that is homologous to the sia-binding site of BoNT/C, BoNT/D and tetanus toxin [15], consistent with the view that the so-called sialic acid site of other BoNTs and the SYT binding site of BoNT/B may have a structurally related conserved function [15]. Our model indicates that this pocket has conserved its ability to bind sialic acid associated with SYT in the case of BoNT/B. Thus, instead of the predominant view that BoNTs independently recognize a protein and a ganglioside or two gangliosides (BoNT/C) using two distinct pockets, our data support a new scenario in which a BoNT protein-binding pocket accepts a preformed protein/ganglioside complex. To our knowledge, this is the first description of recognition by a toxin of a protein/ganglioside complex.
Our model significantly extends our understanding of the BoNT/B-SYT binding interface and uncovers additional interacting residues in SYT. Among them SYT2-N59 was predicted to engage strong interactions with BoNT/B in particular with E1191, a key residue that modulates engineered BoNT/B activity for therapeutic purpose [6]. Interestingly, the natural variant SYT2-N59Q found in Felidae shows a decrease in BoNT/B binding. To our knowledge type B botulism has never been reported in cats [38]. These data may suggest that this natural variant could confer partial protection against type B botulism in animals feeding on carrion which can contain high amounts of BoNTs [47].
Our current findings thus highlight a new role for GT1b in BoNT/B binding by its direct interaction with SYT, explaining the poor affinity of BoNT/B for GT1b alone, but potently enhancing binding in the presence of SYT, particularly for SYT1. The very low affinity of BoNT/B for SYT1 has been suggested to be partially due to a steric clash with the toxin, involving SYT1-L50 [24, 48]. BoNT/B has been estimated to have at least 100-fold less affinity for SYT1 than SYT2 while GT1b reduces this difference tenfold [23, 27]. Our present model predicts that SYT1-L50 binds GT1b and, therefore, a preassembled SYT/GT1b complex may facilitate BoNT/B binding to SYT1. Moreover, our results are consistent with the fact that competitive neutralization of BoNT/B toxicity requires a SYT/ganglioside mixture rather SYT alone [28]. Similar effects with GD1a could be expected as the affinity of BoNT/B for SYT/GD1a or SYT/GT1b was previously shown to be comparable in liposomal reconstitution assays [26].
Our data revisit the dual receptor model [49] by uncovering an additional role for GT1b. We propose a new model in which, after the toxin is attracted and concentrated on the membrane by the negative charges of GT1b in lipid rafts [50], a preassembled and structured SYT/GT1b complex is accommodated in the SYT-binding pocket of BoNT/B, concomitantly to the binding of a ganglioside in the conserved ganglioside binding site GBS1. The LBL would then reinforce BoNT/B binding by interacting with GT1b associated with SYT. Accordingly, mutations in the GBS1, lipid binding loop or perturbation of GT1b/SYT interaction result in a loss of BoNT/B affinity and toxicity [18, 27, 29]. After internalization, GT1b would participate in the toxin translocation process [51].
It has been suggested that simultaneous binding to SYT and gangliosides could impose a limited degree of freedom on BoNT/B orientation with respect to the membrane surface [23, 52]. In line with this notion, our data suggest that the intramembrane interactions between SYT and gangliosides could indeed immobilize both co-receptors at an appropriate distance, optimizing binding. The presence of SYT clusters at the neuronal surface has been previously described [53] and lipids can participate in the formation of protein clusters [12, 54]. We speculate that gangliosides in the outer leaflet of the plasma membrane may participate in the organization of hot spot SYT clusters for BoNT/B binding.
In summary, we present here a model of BoNT/B binding to neuronal membranes that considers the specific topology of membrane receptors. BoNT/B has been successfully engineered to increase its affinity in a preclinical model [6]. Our present findings could provide insights into the rational design of recombinant BoNTs for medical applications and for the development of inhibitors [17, 55, 56].
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Raymond Miquelis for constructive discussions.
Abbreviations
- BoNTs
Botulinum neurotoxins
- HC
Heavy chain
- GBS1
Ganglioside-binding site
- SYT
Synaptotagmin
- LBL
Lipid-binding loop
- JMD
Juxtamembrane domain
- TMD
Transmembrane domain
- DMPC
1,2-Dimyristoyl-sn-glycero-3-phosphocholine
- DPPC
1,2-Dipalmitoylphosphatidylcholine
- IR
Immunoreactivity
- ROI
Regions of interest
- RMS
Root-mean-square
- Glc
Glucose
- Gal
Galactose
- Gal-Nac
N-acetylgalactosamine
- Sia
Sialic acid
- Cer
Ceramide
- SPR
Surface Plasmon Resonance
Author contributions
CL, OEF and MS conceived the study. CL and OEF supervised the entire project, the experimental design, data interpretation and manuscript preparation. CL and OEF analyzed and interpreted the data. JRF and CL performed immunofluorescence experiments, JRF acquired the corresponding images and performed treatment and analysis. FA performed molecular modelling and prepared with JRF molecular modelling figures. CL and GF performed SPR experiments. JF performed Langmuir monolayer experiments. FY and MS performed expression plasmids preparation and preliminary expression tests. CL performed SPR experiments. C.L. wrote the original draft of the manuscript. CL, FA, JF, OEF and JRF prepared the figures. MRP was involved in discussion and data analysis. All authors edited and reviewed the manuscript.
Funding
Agence Nationale de la Recherche (ANR) (grant ANR-17-CE16-0022) for the postdoctoral financial support of JRF Ministère des Armées (AID) and Aix-Marseille Université AMU for the PhD thesis of FO.
Data availability
Coordinates of structural Details of BoNT/B/synaptotagmin 1 & 2/gangliosides complexes are available upon request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
All authors approved submission.
Consent to publish
All authors approved publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jorge Ramirez-Franco and Fodil Azzaz contributed equally to this work.
Contributor Information
Christian Lévêque, Email: christian.leveque@univ-amu.fr.
Oussama El Far, Email: oussama.el-far@inserm.fr.
References
- 1.Poulain B, Lemichez E, Popoff MR. Neuronal selectivity of botulinum neurotoxins. Toxicon. 2020;178:20–32. doi: 10.1016/j.toxicon.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Dong M, Masuyer G, Stenmark P. Botulinum and tetanus neurotoxins. Annu Rev Biochem. 2019;88:811–837. doi: 10.1146/annurev-biochem-013118-111654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies JR, Liu SM, Acharya KR. Variations in the botulinum neurotoxin binding domain and the potential for novel therapeutics. Toxins (Basel) 2018 doi: 10.3390/toxins10100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellett S, Yaksh TL, Ramachandran R. Current status and future directions of botulinum neurotoxins for targeting pain processing. Toxins (Basel) 2015;7:4519–4563. doi: 10.3390/toxins7114519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol Rev. 2017;69:200–235. doi: 10.1124/pr.116.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott M, et al. Engineered botulinum neurotoxin B with improved binding to human receptors has enhanced efficacy in preclinical models. Sci Adv. 2019;5:eaau7196. doi: 10.1126/sciadv.aau7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnaar RL. Gangliosides of the vertebrate nervous system. J Mol Biol. 2016;428:3325–3336. doi: 10.1016/j.jmb.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rummel A. Double receptor anchorage of botulinum neurotoxins accounts for their exquisite neurospecificity. Curr Top Microbiol Immunol. 2013;364:61–90. doi: 10.1007/978-3-642-33570-9_4. [DOI] [PubMed] [Google Scholar]
- 9.Sipione S, Monyror J, Galleguillos D, Steinberg N, Kadam V. Gangliosides in the brain: physiology: pathophysiology and therapeutic applications. Front Neurosci. 2020;14:572965. doi: 10.3389/fnins.2020.572965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berntsson RP, Peng L, Dong M, Stenmark P. Structure of dual receptor binding to botulinum neurotoxin B. Nat Commun. 2013;4:2058. doi: 10.1038/ncomms3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuverink M, Barbieri JT. Protein toxins that utilize gangliosides as host receptors. Prog Mol Biol Transl Sci. 2018;156:325–354. doi: 10.1016/bs.pmbts.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fantini J, Yahi N. Brain lipids in synaptic function and neurological disease: clues to innovative therapeutic strategies for brain disorders. 2015. p. 398. [Google Scholar]
- 13.Fantini J, Chahinian H, Yahi N. Synergistic antiviral effect of hydroxychloroquine and azithromycin in combination against SARS-CoV-2: What molecular dynamics studies of virus-host interactions reveal. Int J Antimicrob Agents. 2020;56:106020. doi: 10.1016/j.ijantimicag.2020.106020. [DOI] [PubMed] [Google Scholar]
- 14.Strotmeier J, et al. Botulinum neurotoxin serotype D attacks neurons via two carbohydrate-binding sites in a ganglioside-dependent manner. Biochem J. 2010;431:207–216. doi: 10.1042/BJ20101042. [DOI] [PubMed] [Google Scholar]
- 15.Karalewitz AP, Fu Z, Baldwin MR, Kim JJ, Barbieri JT. Botulinum neurotoxin serotype C associates with dual ganglioside receptors to facilitate cell entry. J Biol Chem. 2012;287:40806–40816. doi: 10.1074/jbc.M112.404244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strotmeier J, et al. The biological activity of botulinum neurotoxin type C is dependent upon novel types of ganglioside binding sites. Mol Microbiol. 2011;81:143–156. doi: 10.1111/j.1365-2958.2011.07682.x. [DOI] [PubMed] [Google Scholar]
- 17.Yin L, et al. Characterization of a membrane binding loop leads to engineering botulinum neurotoxin B with improved therapeutic efficacy. PLoS Biol. 2020;18:e3000618. doi: 10.1371/journal.pbio.3000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stern D, et al. A lipid-binding loop of botulinum neurotoxin serotypes B, DC and G is an essential feature to confer their exquisite potency. PLoS Pathog. 2018;14:e1007048. doi: 10.1371/journal.ppat.1007048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angaut-Petit D, et al. Mouse motor nerve terminal immunoreactivity to synaptotagmin II during sustained quantal transmitter release. Brain Res. 1995;681:213–217. doi: 10.1016/0006-8993(95)00294-z. [DOI] [PubMed] [Google Scholar]
- 20.Thiele C, Hannah MJ, Fahrenholz F, Huttner WB. Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nat Cell Biol. 2000;2:42–49. doi: 10.1038/71366. [DOI] [PubMed] [Google Scholar]
- 21.Park Y, et al. Synaptotagmin-1 binds to PIP(2)-containing membrane but not to SNAREs at physiological ionic strength. Nat Struct Mol Biol. 2015;22:815–823. doi: 10.1038/nsmb.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao J, Kwon SE, Gaffaney JD, Dunning FM, Chapman ER. Uncoupling the roles of synaptotagmin I during endo- and exocytosis of synaptic vesicles. Nat Neurosci. 2011;15:243–249. doi: 10.1038/nn.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin R, Rummel A, Binz T, Brunger AT. Botulinum neurotoxin B recognizes its protein receptor with high affinity and specificity. Nature. 2006;444:1092–1095. doi: 10.1038/nature05387. [DOI] [PubMed] [Google Scholar]
- 24.Chai Q, et al. Structural basis of cell surface receptor recognition by botulinum neurotoxin B. Nature. 2006;444:1096–1100. doi: 10.1038/nature05411. [DOI] [PubMed] [Google Scholar]
- 25.Desplantes R, et al. Affinity biosensors using recombinant native membrane proteins displayed on exosomes: application to botulinum neurotoxin B receptor. Sci Rep. 2017;7:1032. doi: 10.1038/s41598-017-01198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozaki S, Kamata Y, Watarai S, Nishiki T, Mochida S. Ganglioside GT1b as a complementary receptor component for Clostridium botulinum neurotoxins. Microb Pathog. 1998;25:91–99. doi: 10.1006/mpat.1998.0214. [DOI] [PubMed] [Google Scholar]
- 27.Flores A, et al. Gangliosides interact with synaptotagmin to form the high-affinity receptor complex for botulinum neurotoxin B. Proc Natl Acad Sci USA. 2019;116:18098–18108. doi: 10.1073/pnas.1908051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong M, et al. Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J Cell Biol. 2003;162:1293–1303. doi: 10.1083/jcb.200305098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rummel A, et al. Identification of the protein receptor binding site of botulinum neurotoxins B and G proves the double-receptor concept. Proc Natl Acad Sci U S A. 2007;104:359–364. doi: 10.1073/pnas.0609713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishiki T, et al. The high-affinity binding of Clostridium botulinum type B neurotoxin to synaptotagmin II associated with gangliosides GT1b/GD1a. FEBS Lett. 1996;378:253–257. doi: 10.1016/0014-5793(95)01471-3. [DOI] [PubMed] [Google Scholar]
- 31.Fantini J, Garmy N, Yahi N. Prediction of glycolipid-binding domains from the amino acid sequence of lipid raft-associated proteins: application to HpaA, a protein involved in the adhesion of Helicobacter pylori to gastrointestinal cells. Biochemistry. 2006;45:10957–10962. doi: 10.1021/bi060762s. [DOI] [PubMed] [Google Scholar]
- 32.Di Scala C, et al. Mechanism of cholesterol-assisted oligomeric channel formation by a short Alzheimer beta-amyloid peptide. J Neurochem. 2014;128:186–195. doi: 10.1111/jnc.12390. [DOI] [PubMed] [Google Scholar]
- 33.Fantini J, Yahi N, Azzaz F, Chahinian H. Structural dynamics of SARS-CoV-2 variants: a health monitoring strategy for anticipating COVID-19 outbreaks. J Infect. 2021;83:197–206. doi: 10.1016/j.jinf.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pettersen EF, et al. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 35.Ulrich-Bott B, Wiegandt H. Micellar properties of glycosphingolipids in aqueous media. J Lipid Res. 1984;25:1233–1245. doi: 10.1016/S0022-2275(20)34467-9. [DOI] [PubMed] [Google Scholar]
- 36.Fantini J, Garmy N, Mahfoud R, Yahi N. Lipid rafts: structure, function and role in HIV, Alzheimer's and prion diseases. Expert Rev Mol Med. 2002;4:1–22. doi: 10.1017/S1462399402005392. [DOI] [PubMed] [Google Scholar]
- 37.Chen C, Baldwin MR, Barbieri JT. Molecular basis for tetanus toxin coreceptor interactions. Biochemistry. 2008;47:7179–7186. doi: 10.1021/bi800640y. [DOI] [PubMed] [Google Scholar]
- 38.Montecucco C, Rasotto MB. On botulinum neurotoxin variability. MBio. 2015 doi: 10.1128/mBio.02131-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davies JR, Masuyer G, Stenmark P. Structural and biochemical characterization of botulinum neurotoxin subtype B2 binding to its receptors. Toxins (Basel) 2020 doi: 10.3390/toxins12090603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong M, Tepp WH, Liu H, Johnson EA, Chapman ER. Mechanism of botulinum neurotoxin B and G entry into hippocampal neurons. J Cell Biol. 2007;179:1511–1522. doi: 10.1083/jcb.200707184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wild E, et al. In vitro potency determination of botulinum neurotoxin B based on its receptor-binding and proteolytic characteristics. Toxicol In Vitro. 2016;34:97–104. doi: 10.1016/j.tiv.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 42.Fantini J, Di Scala C, Baier CJ, Barrantes FJ. Molecular mechanisms of protein-cholesterol interactions in plasma membranes: functional distinction between topological (tilted) and consensus (CARC/CRAC) domains. Chem Phys Lipids. 2016;199:52–60. doi: 10.1016/j.chemphyslip.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Stenmark P, Dong M, Dupuy J, Chapman ER, Stevens RC. Crystal structure of the botulinum neurotoxin type G binding domain: insight into cell surface binding. J Mol Biol. 2010;397:1287–1297. doi: 10.1016/j.jmb.2010.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emsley P, et al. The structures of the H(C) fragment of tetanus toxin with carbohydrate subunit complexes provide insight into ganglioside binding. J Biol Chem. 2000;275:8889–8894. doi: 10.1074/jbc.275.12.8889. [DOI] [PubMed] [Google Scholar]
- 45.Swaminathan S, Eswaramoorthy S. Structural analysis of the catalytic and binding sites of Clostridium botulinum neurotoxin B. Nat Struct Biol. 2000;7:693–699. doi: 10.1038/78005. [DOI] [PubMed] [Google Scholar]
- 46.Karalewitz AP, et al. Identification of a unique ganglioside binding loop within botulinum neurotoxins C and D-SA. Biochemistry. 2010;49:8117–8126. doi: 10.1021/bi100865f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ortiz NE, Smith GR. The production of Clostridium botulinum type A, B and D toxin in rotting carcasses. Epidemiol Infect. 1994;113:335–343. doi: 10.1017/s0950268800051761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam KH, Yao G, Jin R. Diverse binding modes, same goal: the receptor recognition mechanism of botulinum neurotoxin. Prog Biophys Mol Biol. 2015;117:225–231. doi: 10.1016/j.pbiomolbio.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montecucco C. How do tetanus and botulinum toxins bind to neuronal membranes? Trends Biochem Sci. 1986;11:314–317. doi: 10.1016/0968-0004(86)90282-3. [DOI] [Google Scholar]
- 50.Fogolari F, Tosatto SC, Muraro L, Montecucco C. Electric dipole reorientation in the interaction of botulinum neurotoxins with neuronal membranes. FEBS Lett. 2009;583:2321–2325. doi: 10.1016/j.febslet.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 51.Sun S, et al. Receptor binding enables botulinum neurotoxin B to sense low pH for translocation channel assembly. Cell Host Microbe. 2011;10:237–247. doi: 10.1016/j.chom.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kammerer RA, Benoit RM. Botulinum neurotoxins: new questions arising from structural biology. Trends Biochem Sci. 2014;39:517–526. doi: 10.1016/j.tibs.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Willig KI, Rizzoli SO, Westphal V, Jahn R, Hell SW. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature. 2006;440:935–939. doi: 10.1038/nature04592. [DOI] [PubMed] [Google Scholar]
- 54.van den Bogaart G, et al. Membrane protein sequestering by ionic protein-lipid interactions. Nature. 2011;479:552–555. doi: 10.1038/nature10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rasetti-Escargueil C, Popoff MR. Engineering botulinum neurotoxins for enhanced therapeutic applications and vaccine development. Toxins (Basel) 2020 doi: 10.3390/toxins13010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fonfria E, Elliott M, Beard M, Chaddock JA, Krupp J. Engineering botulinum toxins to improve and expand targeting and SNARE cleavage activity. Toxins (Basel) 2018 doi: 10.3390/toxins10070278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Coordinates of structural Details of BoNT/B/synaptotagmin 1 & 2/gangliosides complexes are available upon request.