Abstract
Pancreatic cancer (PC) is a kind of common digestive system cancer with the worst prognosis for its insidious symptoms and high invasiveness. Circular RNAs (circRNAs) are endogenous non-coding RNAs with covalently closed circular structure, which are more stable and conservative than linear RNAs and process major functions of microRNA (miRNA) sponge, RNA binding protein (RBP) sponge and polypeptide translation template. Incremental researches have proved that circRNAs express aberrantly and play a vital role in various types of cancer. Hence, we reviewed the biogenesis, degradation, characteristics, and biological functions of circRNAs and summarized the roles circRNAs played in the proliferation, invasion, metastasis, chemoresistance and exosome-mediated intercellular communication of PC. We then summed up a workflow regarding circRNA research in cancer and relative specific databases and experimental methods. In the future, more efforts ought to be put into circRNAs research in PC, including basic research of discovering and testifying circRNAs centered ceRNA networks, and clinical research of exploiting exosomal or circulating circRNAs as a diagnostic biomarker, chemotherapy sensitivity predictor and prognostic predictor.
Keywords: Circular RNA, Pancreatic cancer, Biomarker, Gemcitabine, Exosome
Background
Pancreatic cancer (PC) is considered to be one of the most lethal malignancies with a poor prognosis on account of its high heterogeneity and rapid progress, whose 5-year related survival rate is merely 10% [54]. Most PC patients have reached advanced stage when diagnosed, and lost the chance to receive radical surgical treatment, which is the only mean with curative possibility [43]. In the new century, with the popularization of screening, the emergence of novel targeted drugs and effective practice of comprehensive therapy, the management for many kinds of malignancies, such as lung cancer, breast cancer and colorectal cancer, has entered a new era. However, lack of effective biomarkers for early diagnosis, debate in neoadjuvant therapy, unsatisfactory response to various chemotherapy regimens and scarcity of targeted drugs are all obstacles in prolonging the survival of PC patients [56]. Hence, it is pivotal to dig more novel molecular mechanisms and regulatory axes of pancreatic cancer so as to develop targeted treatments and provide biomarkers for detection and monitoring.
Tumor is gradually found to be the product of genomic, epigenetic and environmental alterations, among which abnormally expressed RNAs play diverse and vital roles [56]. Circular RNAs (circRNAs), a novel type of non-coding RNAs (ncRNAs), are discovered to play critical roles in the development of various types of cancers. CircRNAs were first observed in eukaryotic cells under an electron microscope in 1976 [50], and then in 1993 they were found in Sry genes of mice [10]. Since 2012, with the help of high-throughput sequencing technology, circRNAs were explored widely and in depth and the cornerstone study of circRNAs in pan-cancer demonstrating their expression profile and major relative signal pathways was published on NATURE in 2013 [15]. Different from linear RNAs, including mRNAs, microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), the structure of circRNAs are covalently closed loop with neither 5'-3' polarity nor poly A tail [7]. Moreover, due to its special structure, circRNAs are considered widespread, conserved, stable, and tissue specific [61, 62]. In previous studies, circRNA_100395 was found to inhibit the progression of ovarian cancer via miR-1228/p53/EMT axis, circRNA hsa_circRNA_0000069 promotes the malignancy of cervical cancer through miR-873-5p/TUSC3 axis [32, 76]. Looking through the researching status of circRNAs, it’s obvious that circRNAs are becoming a novel hot topic and capturing more and more attention in diagnosis and therapy of pancreatic cancer. What’s more, an increasing number of studies have discovered different pathways that circRNAs interact in pancreatic cancer, suggesting the relevance of circRNAs in the tumorigenesis, progression, metastasis and chemoresistance of pancreatic cancer.
Thus, the current review discussed existing knowledge concerning biogenesis, functions and clinical potential of circRNAs in PC and summarized practical research strategies, methods and relevant databases.
Circular RNAs
Biogenesis, regulation and degradation of circRNAs
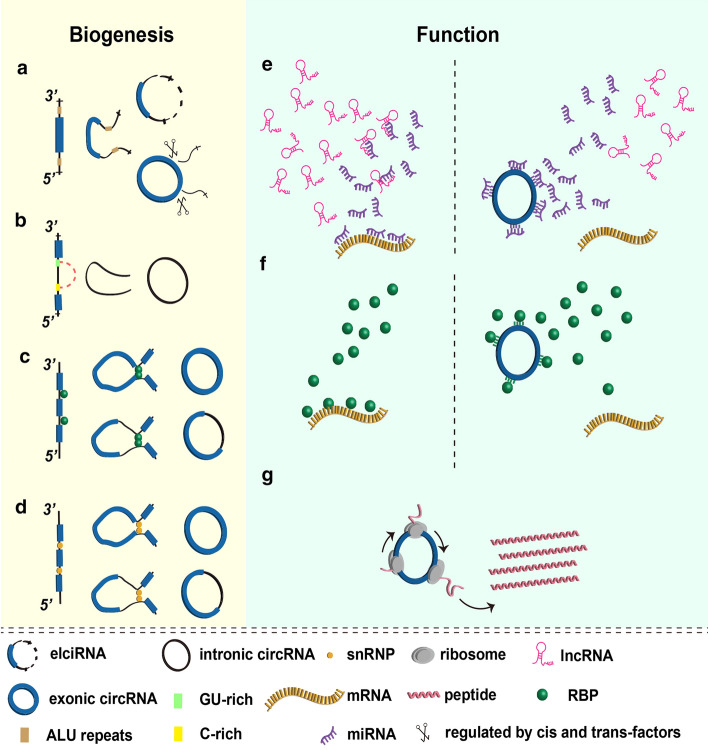
Unlike the classical spicing of linear RNAs, circRNAs have their own unique biogenesis mechanism. Most circRNAs rise from the back-splicing mechanism from widely expressed precursor mRNAs (pre-mRNAs) in eukaryote cells, which are given birth during or after the transcription process of parental genes (Fig. 1) [46, 74]. Different from canonical splicing that tying splice donor site from upstream to downstream orderly, back-splicing mechanism reverses the order and joins a downstream donor site with an upstream one, resulting in a covalently closed circular structure instead [23]. The spliceosomal mechanisms of back-splicing can be led by the following patterns. The form of exonic circRNAs and exon–intron circRNAs (elciRNAs) (arise from both exons and introns) is intron-dependent, where an upstream 3’ splice acceptor is attacked by a 5’ splice donor and leads to a 3’–5’ phosphodiester bond that forms a circular structure [6, 23]. ElciRNAs will be constructed if the introns between the acceptor and the donor are not removed (Fig. 1a). CircRNAs from intronic regions are produced by forming a 2′–5′ linkage which is a branchpoint between the terminal 2′-OH group of the intron and the 5′ splice site after the release of the 3′ exon (Fig. 1b). The mature circRNAs are formed when the 3′ tail is degraded [14]. Furthermore, circularization depends on protein is suggested to facilitate the formation of circRNAs via the mechanism that small nuclear ribonucleoproteins (snRNPs) or RNA-binding proteins (RBPs) can specifically bind to flanking intron sequences, acting as a bridge that narrows the gap between splice donor sites and acception sites sufficiently to promote the formation of circRNAs (Fig. 1c,d) [7, 21].
Fig. 1.
Biogenesis and functions of circRNAs. a Back-splicing mechanism of elciRNA and exonic circRNA biogenesis. b Back-splicing mechanism of intronic circRNA. c RBPs mediated circRNA generation. d snRNPs mediated circRNA generation. e Function as miRNA sponge. f Interact with RBPs. g Translation into proteins or polypeptides
As described before, RNA spliceosome is known to play a critical role in RNA formation and splicing process. RNA spliceosome is a group of enzyme based on RNA, which has a U5 core consisting of 5 snRNAs and dozens of proteins. With the impulse of U6 and U4 promoters, many proteins collaborate on pre-RNA. Similar to mRNA, circular RNA can be modeled from linear pre-RNA by specific RNA spliceosome. Furthermore, a linear RNA can be processed into various types of RNAs, such as lncRNA, circRNA and mRNA via separate splicing events [59]. Besides the regulation of RNA spliceosome, the formation of circRNAs can be regulated by cis-regulatory elements and trans-acting factors, these two molecular tools can balance the competition between canonical splicing and back splicing, in other words, the quantity of circRNA is regulated by the rate of transcription [4]. Another regulation factor of circRNA is Quaking (QKI) protein, which can bind with the special downstream sites of linear pre-RNA, and boost the back-splicing and production of circRNA [7].
Whereas the mechanisms of circRNA biogenesis and regulation are deeply explored, the secret of how circRNAs are degraded remains unclear. Two kinds of possible patterns have been proposed, one of which is circRNAs’ degradation is related to endonuclease, including the combination of exonuclease and endonuclease, the other is that they are degraded by miRNAs and the downstream pathways. Liu et al. explored that circRNAs upon viral infection or Poly(I:C) are significantly downregulated in cells, subsequently, it was found that circRNAs are degraded by RNase L in the aforementioned case [35]. Moreover, a recent research by Fischer et al. suggested that degradation of circRNAs is regulated by two RBPs:UPF-1 and G3BP1, which is termed as structure-mediated RNA decay(SRD) [13].
Functions of circRNAs
Due to its unique features of diversity, abundance, and conservation, circRNAs are widely expressed in tissues and involved in multiple physiological and pathological processes of the human body, therefore, there has been mounting focus on circRNAs’ function in normal or disease circumstance [61]. Accumulating studies have uncovered that circRNAs play vital biological roles, such as miRNA sponge (Fig. 1e), interaction with RBP (Fig. 1f), templates for translation (Fig. 1e), cellular protein scaffold and enhancer of protein function. In the following narrative, we described in detail the main functions of circRNAs. The discovery of these functions not only provides conclusive evidence that circRNAs serve crucial roles in the physiological development and initiation or progression of various diseases but also lays a solid foundation for us to achieve further breakthrough research in target therapy.
miRNA sponge
The first observation of circRNAs acting as miRNA “sponge” was CDR1/miRNA-7 [16]. The reason why circRNAs can sufficiently absorb miRNAs is that there are specific miRNAs complementary binding sites on circRNAs, and we can’t ignore the abundance of binding sites between circRNAs and its targeting miRNAs as highly abundant circRNAs may have enhanced capacity of sponging miRNAs. Several circRNAs have been observed acting their function as miRNA sponge. For instance, circNRIP1 acts as a miRNA-149-5p sponge in gastric cancer, and circRNF20 promotes breast cancer through miR-487a [5, 78]. In this way, both miRNAs and the downstream genes expression of whom can be regulated by circRNAs through a competing endogenous RNA (ceRNA) mechanism and affect the biological process subsequently [27].
Interacting with RBP
RNA-binding proteins (RBPs) are a broad class of proteins involved in transcription and translation and play key roles in the post-transcriptional regulation of RNAs [58]. RBPs are found to participate in almost every stage of circRNAs’ life itinerary including regulation of biogenesis, functional execution, translation and transportation. RBPs bind to the specific franking sequences on both sides of circRNAs and affect its functions [40]. For instance, one-third of circRNAs related to human epithelial-mesenchymal transition (EMT) was dynamically regulated by Quaking, a key RBP in this process. In contrast, circRNAs can also affect the expression of RBPs and alter the consequence of its downstream pathways. CircRNAs, KIRKOS-73, and KIRKOS-71, which are transcribed from the p53 stabilizer WWOX(WW Domain Containing Oxidoreductase) can also regulate p53 generation [44]. Another example of circRNAs interacting with RBPs is circ-Foxo3, which was found to bind with CDK2 and p21, subsequently suppress the cell cycle and serve as a block point in the transition from G1 to S phase [11].
Translating into proteins or peptides
CircRNAs once were considered as a kind of absolute ncRNAs due to the lack of cap-dependent translating essential element, such as the 5’ cap structure and the poly (A) tail [3]. However, a subgroup of circRNAs, ribo-circRNAs was found to translate based on cap-independent mechanism in 2017 by Pamudurti et al, who found circMb13 could translate protein in fly heads [45]. Benefitting from high-throughput technologies, an open reading frame (ORF), which can be translated into proteins via the mechanisms of splicing-dependent and cap-independent was identified in circ-ZNF609 [26]. The existence of internal ribosome entry sites (IRES), a nucleic acid sequence, enables the protein translation to initiate from the 5'cap structure. Zhang et al. explored IRES-driven ORF existing in circ-SHPRH and assisting the translation of SHPRH-146aa, a functional protein in glioma [75]. Additionally, circRNA-encoded peptide was found to show a potential molecular function in cancer and this type of peptide can be transformed into canonical proteins under different conditions [72]. These discoveries gave circRNAs a brand new role in the protein family and served us with more chances to deeply explore the molecular mechanism between circRNAs and others structures intracellular or extracellular.
Other functions
In addition to the functions extensively explored we mentioned above, some circRNAs are found may act as cellular protein scatters or protein function enhancer, such as circ-Foxo3 [73]. Locating in the cytoplasm, circ-Foxo3 acts as a protein scaffold and induces apoptosis in cancer cells via facilitating MDM2-dependent ubiquitylation proteasome-meditated degradation of p53 [77]. CircPAIP2 locates in nuclear works as protein enhancer to regulates the transcription of Pol II positively [38]. Finally, circRNAs are capable of recruiting specific proteins to specific cellular locations. For example as the exonic circular RNA FECR1, which utilizes positive feedback, recruiting TET1 to the promotor region of its host gene FLI1 and coordinating the regulation of DNA methylating and demethylating enzymes to control the process of breast cancer [8].
CircRNAs in pancreatic cancer
CircRNA expression profile of PC
Early on, it has been investigated that circRNAs were expressed aberrantly in diverse cancer types, including breast cancer, hepatic cell carcinoma and colorectal cancer [24]. However, a clear understanding of circRNAs in pancreatic cancer has all through been in its infancy until Li et al. first identified the abnormal expression in pancreatic cancer by analyzing six pairs of pancreatic cancer and paired adjacent normal tissues using a high-throughput circRNA microarray, consisting 5396 circRNAs collected from three published papers, in 2016. They found that 351 circRNAs were differentially expressed with 209 of them up-regulated and 142 of them down-regulated. The expression levels quantified by qRT-PCR of randomly selected seven differentially expressed circRNAs were validated in 20 sets of PDAC tissues and adjacent normal tissues, the result of which was consistent with the microarray data. Guo et al. conducted similar work in 20 pancreatic cancer tissues and corresponding precancerous tissues and found 289 circRNAs were differentially expressed with 128 of them up-regulated and 161 of them down-regulated [12]. Yang et al. identified 173 up-regulated and 105 down-regulated circRNAs based on sequencing of 5 PDAC tissues and paired adjacent non-tumorous tissues [70]. The above three studies laid a solid foundation for further exploration of circRNAs in pancreatic cancer. Inspired by the sequencing data, a growing number of bioinformatics studies have emerged in recent 2 years.
CircRNA-miRNA-mRNA competing endogenous network in PC
Attributing to its structural characteristics, circRNAs have the function of binding miRNAs as miRNA sponge. Meanwhile, miRNAs are capable of binding mRNAs to affect various biological processes of mRNA, the most important of which is translation. Hence, circRNA, miRNA and mRNA can constitute an competing endogenous network to ultimately regulate translation of mRNA and further influence relevant pathways. Several circRNA-miRNA-mRNA competing endogenous networks in pancreatic cancer have been built on the basis of the differential expression data of GSE69362 and GSE79634 from the above two expression profile study (Table 1).
Table 1.
Published circRNA-miRNA-mRNA interaction network in pancreatic cancer based on bioinformatics analysis
| Publish year | Dataset | Network | Predicting tools | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| circRNA dataset | miRNA dataset | mRNA dataset | circRNA | miRNA | mRNA | Hub genes | |||
| 2020 | GSE69362, GSE79634 | GSE60980 | GSE60980 | 4 | 6 | 111 | THBS1, FN1, TIMP3, TFGB2, ITGA1, ITGA3 | Cancer-Specific CircRNA databases, miRTarBase, TargetScan | [21] |
| 2020 | GSE32678, GSE32676 | GSE32678, GSE32676 | 10 | 12 | 118 | CDH1, SERPINE1, IRS1, FYN | CircInteractome, miRWalk | [27] | |
| 2019 | GSE28735 | GSE28735 | 51 | 54 | 123 | DHX9 | Arraystar, miRanda, StarBase, TargetScan | [32] | |
| 2019 | GSE60980 | GSE60980 | 4 | 3 | 149 | CXCR4, HIF1A, ZEB1, SDC1, TWIST1 | Cancer-Specific CircRNA databases, miRDB, miRTarBase, TargetScan | [33] | |
The network constructed by Zhao et al. consisted of four circRNAs, three miRNAs and 149 mRNAs, in which the hub genes were CXCR4, HIF1A, ZEB1, SDC1 and TWIST1 [79]. The first four hub genes in the network were all regulated by circUBAP2 and miR-494. The expression of CXCR4 and HIF1A correlated with the levels of M2 macrophage and T-regulatory cells and the expression of CTLA-4 and PD-1, which suggested that the circUBAP2-mediated ceRNA network modulated PDAC by regulating the infiltration and function of immune cells. Nevertheless, Song et al. obtained a ceRNA network different from that of Zhao using the same GEO datasets of circRNA, miRNA and mRNA by means of different prediction tools [55]. The network of Song included four circRNAs, six miRNAs and 111 mRNAs. Further protein–protein network and module analysis identified six hub genes, namely THBS1, FN1, TIMP3, TGFB2, ITGA1 and ITGA3. The above two studies hinted that bioinformatics results needed to be verified by experiments to come to ultimate practical and instructive conclusions.
CeRNA Network was constructed by Xiao et al. using the same circRNA expression profile datasets and different miRNA and mRNA datasets [64]. Ten differentially expressed circRNAs were picked to build the network, along with twelve miRNA and 118 mRNAs. They finally obtained four hub genes, including CDH1, SERPINE1, IRS1 and FYN.
CircRNAs function as 'cancer suppressor gene'
circNFIB1(hsa_circ_0086375)
CircNFIB1, a novel circRNA which arouse from exons 16–18 of the NFIB gene was identified as downregulated in PDAC tissues and negatively correlated with lymph angiogenesis and lymphatic metastasis of PDAC (Fig. 2; Table 2) [22]. To study the role of circNFIB1 in PDAC, Kong et al. analyzed circNFIB1 expression in 160 PDAC patients and came to the conclusion that the patients with lymphatic metastasis and highly TNM-stage had a lower circNFIB1 level. Following function experiments revealed that knocking circNFIB1 down could effectively promote lymphangiogenesis and lymph node (LN) metastasis in vitro and in vivo. To further explore the mechanism of circNFIB1 suppressing pancreatic cancer progression, Kong et al. did a series assays and came up with circNFIB1/miR-486 5p/PIK3R1/VEGF-C axis. In detail, circNFIB1 functioned as a sponge of miR-486-5p to partially reverse the effect of miR-486-5p. Further, circNFIB1 attenuated the oncogenic effect of miR-486-5p and consequently upregulated PIK3R1 expression, which further downregulated VEGF-C expression through inhibition of the PI3K/Akt pathway to ultimately suppressed lymphangiogenesis and LN metastasis in PDAC.
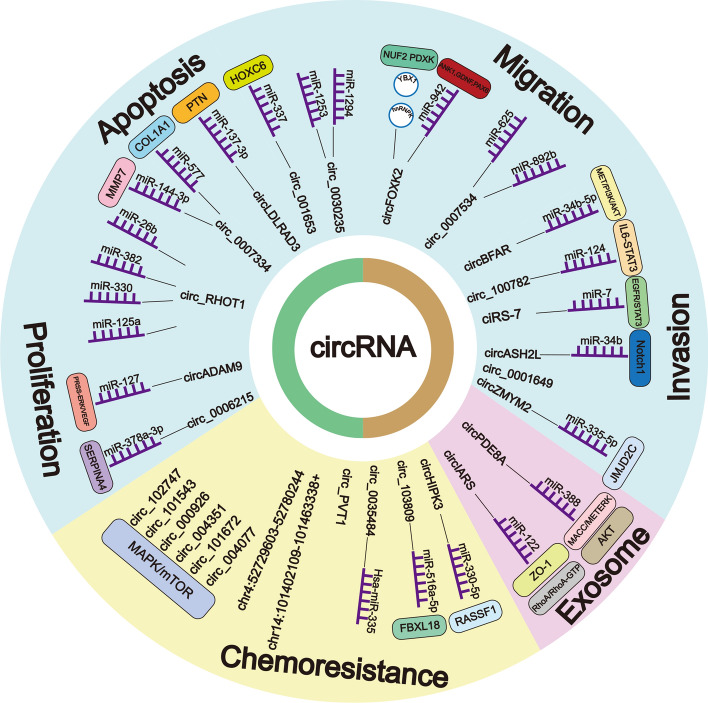
Fig. 2.
CircRNAs in pancreatic cancer. CircRNAs play important roles in a variety of processes in pancreatic cancer through interaction with microRNAs and mRNAs. Such as cancer cell proliferation, apoptosis, migration and invasion, tumor-associated exosomes, and chemoresistance
Table 2.
The role of circRNAs and their bound microRNAs/RBPs in pancreatic cancer
| circRNA | microRNA/RBP | Downstream protein | Phenotype | Reference | |||
|---|---|---|---|---|---|---|---|
| Proliferation and apoptosis | Migration and invasion | Chemoresistance | Exosome-contained | ||||
| circLDLRAD3 | miR-137-3p | PTN | √ | √ | [1, 3] | ||
| circ_0007334 | miR-144-3p | MMP7 | √ | [5] | |||
| miR-577 | COL1A1 | √ | |||||
| circRHOT1 | miR-26b, miR-125a, miR-330, miR382 | / | √ | √ | [8] | ||
| ciRS-7 | miR-7 | EGFR/STAT3 pathway | √ | √ | [10] | ||
| circADAM9 | miR-217 | PRSS-ERK/VEGF pathway | √ | √ | [12] | ||
| circ_0006215 | miR-378a-3p | SERPINA4 | √ | √ | [14] | ||
| circ_001653 | miR-337 | HOXC6 | √ | √ | [17] | ||
| circ_0030235 | miR-1253, miR1294 | / | √ | √ | [20] | ||
| circFOXK2 | miR-942 | ANK1, GDNF, PAX6 | √ | √ | [22] | ||
| YBX1, hnRNPK | NUF2, PDXK | ||||||
| circ_0007534 | miR-625, miR-892b | / | √ | [23] | |||
| circBFAR | miR-34b-5p | MET/PI3K/AKT pathway | √ | √ | [24] | ||
| circ_100782 | miR-124 | IL6-STAT3 pathway | √ | [25] | |||
| circASH2L | miR34b | Notch1 | √ | √ | [26] | ||
| circ_0001649 | / | / | √ | [28] | |||
| circZMYM2 | miR-335-5p | JMJD2C | √ | √ | [30] | ||
| circPDE8A | miR-388 | MACC/MET/ERK or AKT | √ | √ | √ | [6] | |
| circIARS | miR-122 | ZO-1, RhoA, RhoA-GTP | √ | √ | [4] | ||
| circHIPK3 | miR-330-5p | RASSF1 | √ | [15] | |||
| 2 circRNAs* | / | / | √ | √ | [11] | ||
| 6 circRNAs** | / | MAPK or mTOR pathway | √ | [7] | |||
*chr14:101402109-101464448 + , chr4:52729603-52780244 +
**circRNA_101672 and circRNA_003251 (upregulated), circRNA_101543 and circRNA_102747 (downregulated)
circ_0001649
Circ_0001649 is a transcription product of snf2 histone linker phd ring helicase (SHPRH) gene, which regulates tumorigenesis and the development of several kinds of malignancies, such as cholangiocarcinoma, hepatocellular carcinoma and colorectal cancer [18, 47, 49, 67]. In 58 PDAC tissues and paired normal tissues, the expression level of circ_0001649 was significantly decreased in cancer tissues [19]. Patients with low expression of circ_0001649 had an advanced tumor stage, lower differentiation grade and poorer prognosis. Exogenous overexpression of circ_0001649 could inhibit proliferation and promote apoptosis in pancreatic cancer cells. Collectively, these results illustrated that circ_0001649 played a tumor suppressor role and might be a promising prognostic biomarker in PC.
CircRNAs function as 'proto-oncogene'
circRHOT1 (circ_0005397)
CircRHOT1 largely locates in the cytoplasm, was found upregulated in 20 pairs of PDAC tissues [48]. For further investigation, Qu et al. designed two kinds of siRNAs to reduce the expression of circRHOT1 and treated Capan-2 and PANC-1 with the two siRNAs, then selected the siRNA1 for subsequent experiments. To determine the role of circRHOT1 in cellular proliferation, they performed CCK8 and EDU assays in Capan-2 and PANC-1 cell lines, and drew out the conclusion that circRHOT1-siRNA1 inhibited proliferation of cells.
In the later research, circRHOT1 was determined to produce a marked effect via circRHOT1/miRNA(miR-26b-3p,miR-125a-3p,miR-330-5p and miR-382-5p)/MARK, Wnt, Ras signaling pathways in PDAC.
circZMYM2 (circ_0099999)
CircZMYM2 derives from human chromosome 13, was highly expressed in PDAC tissues and cell lines [2]. CircZMYM2 was demonstrated promoting proliferation and invasion while attenuated apoptosis of PDAC cells through related assays in vivo and in vitro. An et al. found the targeting miRNA(miR-335-5p) of circZMYM2 using circular RNA interactome and clarified it with RNA pull-down assay. Subsequently, genes have complementary sequences with miR-335-5p was predicted by TargetScan, among which JMJD2C was found highly expressed in PDAC.
ciRS-7
CiRS-7 is one of the few known circRNAs that inhibit tumor suppressor miR-7 [36]. Liu et al. detected the expression of ciRS-7 and miR-7 by qRT-PCR and assessed the relationship between the two. They found that the expression of cirs-7 in PDAC tissues was higher than precancerous tissues. However, the level of miR-7 showed the opposite trend. Furthermore, the overexpression of cirs-7 caused tumor progression and lymph node metastasis. To explore the downstream pathway of miR-7, Liu et al. using TargetScan and found that EGFR was the possible target of miR-7, and subsequently arrived at a conclusion that ciRS-7 promoted the progression of PDAC by inhibiting miR-7 mediated EGFR/STAT3 signaling pathway.
CircRNA and gemcitabine resistance
Gemcitabine is one of the most important compounds in the chemotherapy regimen of PDAC, resistance to which is a tough issue causing final deaths of pancreatic cancer patients (Drug Resistance Updates 23 (2015) 55–68). CircRNAs participate in the course of inducing gemcitabine resistance. In virtue of a circRNA microarray consisting 12,866 circRNAs, Xu et al. identified 26 upregulated and 55 downregulated circRNAs in SW1990/Gem resistant cell lines comparing SW1990 parental cell lines [66]. Six circRNAs are validated by qRT-PCR and four of them, including circRNA_101672, circRNA_003251, circRNA_101543 and circRNA_102747, are well correlated with the microarray results. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis and Gene Ontology (GO) analysis revealed that the dysregulated circRNAs regulated several chemoresistance-related pathways, such as the mitogen-activated protein kinase (MAPK) and mammalian target of rapamycin (mTOR) signaling pathways. Shao et al. conducted similar work in PANC-1/Gem resistant and PANC-1 parental cell lines and identified silencing of two circRNAs, chr14:101402109-101464448 + and chr4:52729603-52780244 + , could restore the sensitivity of PANC-1/Gem resistant cells to gemcitabine treatment [51]. The following circRNAs are found to be associated with gemcitabine resistance in pancreatic cancer and other kinds of malignancy with the further downstream study.
circHIPK3m
Liu et al. found that the expression level of circHIPK3 was related to gemcitabine resistance in PDAC tissues and cells [37]. Knock circHIPK3 down in PANC-1/Gem and SW1990/Gem could inhibit cell proliferation, invasion, migration, EMT and enhance cell apoptosis. Further study demonstrated that circHIPK3 promoted gemcitabine resistance in PDAC cells by upregulating RASSF1 via sponging miR-330-5p and the interaction within the circHIPK3/miR-330-5p/RASSF1 axis was validated by luciferase reporter assay and RNA pull-down assay. Inconsistent with the above research, the overexpression of circHIPK3 was found to decrease IC50 of gemcitabine and promote gemcitabine's cytotoxicity in bladder cancer cell lines [65].
circPVT1
Gemcitabine combined with cisplatin is a classic regimen in non-small cell lung cancer (NSCLC) [39]. Patients who received the above regimen had a decreased level of circPVT1, and circPVT1 expression in the chemotherapy-resistant patients was higher than that in the chemotherapy-sensitive patients.
circRNA_103809
CircRNA_103809 was highly expressed in bladder cancer (BC) tissues and cell lines, whose high expression was associated with a poor prognosis in BC patients [17]. CircRNA_103809 knockdown impaired the growth and metastasis of BC cells. Furthermore, circRNA_103809 silencing increased the sensitivity of BC cells to gemcitabine treatment, the endogenous mechanism of which was circRNA_103809 acted as sponge for miR-516a-5p and promoted FBXL18 expression via restraining miR-516a-5p activity.
circ_0035483
By high-throughput sequencing of circRNA in renal clear cell carcinoma (RCCC), circ_0035483 was found to facilitate gemcitabine-induced autophagy and enhance the resistance of RCCC to gemcitabine [68]. Hsa-miR-335 is the target regulatory point of circ_0035483. Moreover, circ_0035483 promotes autophagy and tumor growth and enhances gemcitabine resistance in RCCC by regulating hsa-miR-335/CCNB1, and silencing hsa_circ_0035483 can enhance gemcitabine sensitivity in vivo.
CircRNAs as a diagnostic and prognostic biomarker in pancreatic cancer
Lack of effective diagnosis and prognostic biomarkers has always been a disturbing issue in the comprehensive treatment of PC. Since carbohydrate antigen 19-9 (CA19-9) was discovered more than three decades ago, there has almost been no superior biomarker identified. Although glypican-1 (GPC-1) derived from pancreatic cancer secreting exosomes displayed extraordinary diagnostic value with an area under the receiver operating characteristic (ROC) curve (AUC) of 100% in the research performed by Raghu Kalluri, several following studies questioned the authentic diagnostic efficacy of GPC-1 [25, 41, 71]. Various circRNAs were evaluated in terms of diagnostic and prognostic prediction efficacy in PC these years and yielded pleasing results.
CircRNAs within pancreatic cancer cells
circ_LDLRAD3 (circ_0006988)
By means of qRT-PCR, the expression level of circ_LDLRAD3 was quantified in pancreatic cell lines, 30 paired pancreatic cancer tissues and adjacent non-tumorous tissues, 31 plasma samples from patients with pancreatic cancer, and 31 plasma samples from healthy volunteers [69]. Circ_LDLRAD3 was up-regulated in pancreatic cell lines, pancreatic cancer tissues and plasma samples from patients with pancreatic cancer. High expression of circ-LDLRAD3 was associated with venous invasion, lymphatic invasion, and distant metastasis. The AUC of circ-LDLRAD3 in combination with CA19-9 was 0.87, with a sensitivity and specificity of 0.8033 and 0.9355, respectively.
circ_0030235
Circ_0030235 was identified as upregulated in PDAC tissues according to the data of GSE69362 [28]. Xu et al. further detected the expression level of circ_0030235 in 62 paired PDAC tissues and non-cancerous tissues and found that overexpression of circ_0030235 in tumor samples was related to advanced tumor stage and positive lymph node invasion. Kaplan–Meier survival analysis showed that the patients with higher circ_0030235 expression had worse overall survival, which was an independent prognostic factor for overall survival in PDAC patients.
Circ_001569
Circ_001569 is located on the plus strand of human chromosome 16q13.1 and aligns in a sense orientation to the ATP binding cassette subfamily C member 1 (ABCC1) and spans exons33 [57]. ABCC1 gene belongs to the superfamily of ATP-binding cassette (ABC) transporters and associates with drug resistance [53]. Circ_001569 has been found to be upregulated in various types of human gastrointestinal cancer. In the research of Ning Zhou et al., the expression of circ_001569 was detected in 26 tissues samples and 97 plasma samples. The relationship between circ_001569 and clinicopathological parameters was also analyzed. The expression of circ_001569 was elevated in tissues and plasma of patients with pancreatic cancer and positively correlated with lymph node metastasis, clinical stage and venous invasion. Therefore, the expression of circ-001569 is an independent indicator to evaluate the prognosis of pancreatic cancer patients, and patients with high circ_001569 expression have a poor prognosis. In the statistical analysis, the AUC of circ_001569 was 0.716 (95% CI 0.642–0.790), and its sensitivity and specificity were 62.76% and 74.29%, respectively. Therefore circ_001569 may be used as a biomarker for the diagnosis and prognosis of pancreatic cancer [52].
Exosomal circRNAs
As mentioned above, circRNAs can interact with miRNAs and RBPs and then regulate the tumorigenesis, development and metastasis of pancreatic cancer. Besides, increasing evidence suggests that it is possible for circRNAs to act as biomarkers of some cancers to support early diagnosis [42]. Despite the detection techniques of emerging and promising biomarkers, such as circulating tumor cells and circulating tumor DNA, become more and more mature in the last decade, there has been no specific method to detect free circRNAs in blood. However, circRNAs and other ncRNAs have been identified to be enriched and stable in exosomes.
Exosomes are a class of extracellular vesicles about 30–150 nm, containing various growth factors, proteins, lipids and nucleic acids. After being released from cells, exosomes and their contents, including circRNAs, circulate in body fluids such as blood, urine, saliva and breast milk, and elicit a series of biological responses [20]. CircRNAs located in exosomes are verified to be existed as exosomal circRNAs using total transcriptome sequencing in 2015 [33]. Exosomal circRNAs participate in the processes of cell proliferation, epithelial mesenchymal transition and tumor metastasis, which are considered to have the potential of becoming therapy target [60].
Exosomal circRNA expression profile of pancreatic cancer
Li et al. conducted high-throughput whole transcriptome sequencing of exosome contents obtained from 8 PDAC patients and 8 healthy volunteers [31]. A total of 453 significantly differentially expressed circRNAs were identified, 274 of which were up-regulated and 179 were down-regulated. Top 3 up-regulated circRNAs were circ_0002130, circ_0000896 and circ_0101692, and top 3 down-regulated circRNAs were circ_0103896, circ_0006662 and circ_0092763. GO analysis of biological processes showed that the differentially expressed circRNAs were significantly associated with organelle organization, positive regulation of metabolic progress, epidermis development and apoptotic process. Validation was performed in 13 circRNAs and the results confirmed the accuracy of the circRNA-seq data.
Exosomal circPED8A
Exosomal circPED8A has been identified to play an important role in pancreatic ductal adenocarcinoma by Li et al. [34]. Exosome, consisting circPED8A, communication between tumor cells were imaged and tumor secreted exosomes were identified in blood circulation. Exosomal circPED8A was highly expressed in PDAC cell tissues, and the high level of exosomal circPED8A was associated with lymphatic invasion, advanced TNM stage and poor prognosis of PDAC patients. Subsequent research revealed that tumor-released exosomal circPDE8A acted as an 'oncogene' by sponging miR-338 and upregulated MET via circPDE8A/miR-338/MACC1/MET axis. Thus, exosomal circPDE8A may be a useful marker of PDAC diagnosis or progression.
Exosomal circIARS
CircIARS expression was found to be up-regulated both in pancreatic cancer tissues and in plasma exosomes of patients with metastatic disease compared with localized diseases in a cohort of 85 patients [29]. CircIARS entered human microvascular vein endothelial cells (HUVECs) through exosomes and promoted tumor invasion and metastasis, the expression of which was positively correlated with liver metastasis, vascular invasion, and TNM stage and negatively correlated with postoperative survival time. Overexpression of circIARS significantly down-regulated miR-122 and ZO-1 levels, up-regulated RhoA and RhoA-GTP levels, increased F-actin expression and focal adhesion, enhanced endothelial monolayer permeability, and promoted tumor invasion and metastasis. The presence of circIARS in exosomes may be a promising biomarker for early diagnosis, metastasis assessment and prognostic prediction in PDAC.
CircRNA specific methods and databases
The workflow of conducting research on circRNA mainly contains four steps (Fig. 3) [12, 28, 59, 63]. First step, find differentially expressed circRNAs in cancer and confirm target circRNA. We can recur to circRNA microarray or total transcriptome sequencing. Public cancer-specific circRNA databases, such as CSCD (https://gb.whu.edu.cn/CSCD) and MiOncoCirc (https://mioncocirc.github.io/), and published circRNA expression profile, such as GSE79634 and GSE69362, can be utilized.
Fig. 3.
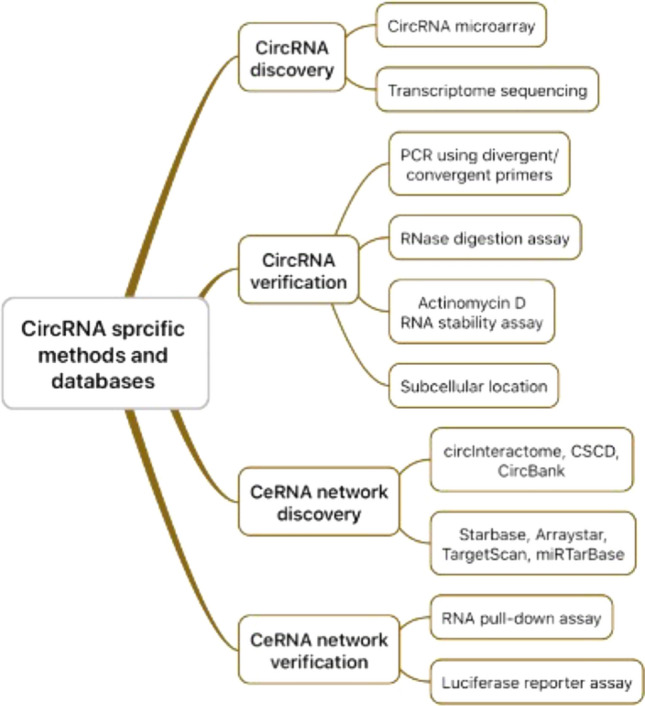
Workflow of conducting circRNA research in cancer and circRNA specific methods and databases
Second step, verify the existence of the target circRNA. As circRNA may share sequences with linear RNA, the circular structure must be verified according to its characteristics. Polymerase chain reaction using divergent and convergent primers can distinguish circRNAs from linear RNAs by positive amplification of divergent primers. RNase digestion assay and actinomycin D RNA stability assay can reveal the stability of circRNAs. Further, subcellular fractionation assay and fluorescence in situ hybridization (FISH) are in need to recognize subcellular location of circRNAs.
Third step, as one of the most important functions of circRNAs is to sponge miRNAs and RBPs, we should explore potential miRNAs or RBPs the target circRNAs bind. CircInteractome is a helpful website enabling the prediction and mapping of the binding sites for miRNAs and RBPs on reported circRNAs. Forward, the interaction between miRNAs and downstream mRNAs can be predicted via computational tools such as Starbase, Arraybase, TargentScan and miRTarBase [1, 9, 30].
Fourth Step, validate the binding and regulation effect of circRNAs on downstream miRNAs and mRNAs. RNA pull-down assay and luciferase reporter assay are generally performed in this step.
Conclusions and perspectives
In this review, we described the molecular biological characteristics of circular RNAs and their relationship with multiple aspects of pancreatic cancer such as tumorigenesis, invasion, chemoresistance and distant metastasis. Pancreatic cancer is one of the most lethal cancers due to its malignant behavior and concealed pathogenesis. Clinically, missed diagnosis and misdiagnosis are not uncommon in patients undergoing pancreatic cancer. Therefore, an effective biomarker for early diagnosis is urgently needed to improve the unsatisfied situation. Circular RNAs, a novel class of non-coding RNAs, are attracting more and more researchers’ attention in recent years, which have been identified to play significant roles in gene expression and various cellular pathways. With the characteristic of stability and tissue-specificity, circular RNAs can be detected in exosomes and blood plasma of pancreatic cancer patients. Consistent with previous evidence, circular RNAs have great potential to be an effective cancer biomarker and a possible therapeutic target of pancreatic cancer. However, the specific mechanisms about circRNAs’ cross-linking with other elements and pathways in pancreatic cancer still need further exploration.
Abbreviations
- PC
Pancreatic cancer
- PDAC
Pancreatic ductal adenocarcinoma
- circRNA
Circular RNA
- miRNA
MicroRNA
- RBP
RNA binding protein
- ncRNA
Non-coding RNA
- lncRNA
Long non-coding RNA
- pre-mRNA
Precursor RNA
- elciRNA
Exon–intron circRNA
- snRNP
Small nuclear ribonucleoproteins
- EMT
Epithelial-mesenchymal transition
- WWOX
WW Domain Containing Oxidoreductase
- ORF
Open reading frame
- IRES
Internal ribosome entry sites
- CA19-9
Carbohydrate antigen 19-9
- GPC-1
Glypican-1
- LN
Lymph node
- BC
Bladder cancer
- RCCC
Renal clear cell carcinoma
Author contribution
BZ and ZL contributed equally to this review. WW, YZ, BZ and ZL raised the concept. BZ and ZL conducted the literature review and wrote the first draft. CQ, TL and HC drew the figures. XY and YW drew the tables. All authors revised and approved the final manuscript.
Funding
WWB received support from the National Natural Science Foundation of China (No. 81773215) and the Chinese Academy of Medical Sciences (No.2019XK320002).
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interests
The authors declared that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bangbo Zhao and Zeru Li contributed equally to this review.
References
- 1.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015 doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An Y, Cai H, Zhang Y, Liu S, Duan Y, Sun D, Chen X, He X. circZMYM2 competed endogenously with miR-335-5p to regulate JMJD2C in pancreatic cancer. Cell Physiol Biochem. 2018;51(5):2224–2236. doi: 10.1159/000495868. [DOI] [PubMed] [Google Scholar]
- 3.Arnaiz E, Sole C, Manterola L, Iparraguirre L, Otaegui D, Lawrie CH. CircRNAs and cancer: biomarkers and master regulators. Semin Cancer Biol. 2019;58:90–99. doi: 10.1016/j.semcancer.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56(1):55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Cao L, Wang M, Dong Y, Xu B, Chen J, Ding Y, Qiu S, Li L, Karamfilova Zaharieva E, Zhou X, Xu Y. Circular RNA circRNF20 promotes breast cancer tumorigenesis and Warburg effect through miR-487a/HIF-1α/HK2. Cell Death Dis. 2020;11(2):145. doi: 10.1038/s41419-020-2336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaichian S, Shafabakhsh R, Mirhashemi SM, Moazzami B, Asemi Z. Circular RNAs: a novel biomarker for cervical cancer. J Cell Physiol. 2020;235(2):718–724. doi: 10.1002/jcp.29009. [DOI] [PubMed] [Google Scholar]
- 7.Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12(4):381–388. doi: 10.1080/15476286.2015.1020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N, Zhao G, Yan X, Lv Z, Yin H, Zhang S, Song W, Li X, Li L, Du Z, Jia L, Zhou L, Li W, Hoffman AR, Hu JF, Cui J. A novel FLI1 exonic circular RNA promotes metastasis in breast cancer by coordinately regulating TET1 and DNMT1. Genome Biol. 2018;19(1):218. doi: 10.1186/s13059-018-1594-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou CH, Shrestha S, Yang CD, Chang NW, Lin YL, Liao KW, Huang WC, Sun TH, Tu SJ, Lee WH, Chiew MY, Tai CS, Wei TY, Tsai TR, Huang HT, Wang CY, Wu HY, Ho SY, Chen PR, Chuang CH, Hsieh PJ, Wu YS, Chen WL, Li MJ, Wu YC, Huang XY, Ng FL, Buddhakosai W, Huang PC, Lan KC, Huang CY, Weng SL, Cheng YN, Liang C, Hsu WL, Huang HD. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018;46(D1):D296–d302. doi: 10.1093/nar/gkx1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cocquerelle C, Mascrez B, Hétuin D, Bailleul B. Mis-splicing yields circular RNA molecules. Faseb j. 1993;7(1):155–160. doi: 10.1096/fasebj.7.1.7678559. [DOI] [PubMed] [Google Scholar]
- 11.Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44(6):2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo S, Xu X, Ouyang Y, Wang Y, Yang J, Yin L, Ge J, Wang H. Microarray expression profile analysis of circular RNAs in pancreatic cancer. Mol Med Rep. 2018;17(6):7661–7671. doi: 10.3892/mmr.2018.8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Wei X, Peng Y. Structure-mediated degradation of CircRNAs. Trends Cell Biol. 2020;30(7):501–503. doi: 10.1016/j.tcb.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Guo Y, Yang J, Huang Q, Hsueh C, Zheng J, Wu C, Chen H, Zhou L. Circular RNAs and their roles in head and neck cancers. Mol Cancer. 2019;18(1):44. doi: 10.1186/s12943-019-1003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 16.Hansen TB, Kjems J, Damgaard CK. Circular RNA and miR-7 in cancer. Cancer Res. 2013;73(18):5609–5612. doi: 10.1158/0008-5472.Can-13-1568. [DOI] [PubMed] [Google Scholar]
- 17.Huang W, Lu Y, Wang F, Huang X, Yu Z. Circular RNA circRNA_103809 accelerates bladder cancer progression and enhances chemo-resistance by activation of miR-516a-5p/FBXL18 Axis. Cancer Manag Res. 2020;12:7561–7568. doi: 10.2147/cmar.S263083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji W, Qiu C, Wang M, Mao N, Wu S, Dai Y. Hsa_circ_0001649: a circular RNA and potential novel biomarker for colorectal cancer. Biochem Biophys Res Commun. 2018;497(1):122–126. doi: 10.1016/j.bbrc.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y, Wang T, Yan L, Qu L. A novel prognostic biomarker for pancreatic ductal adenocarcinoma: hsa_circ_0001649. Gene. 2018;675:88–93. doi: 10.1016/j.gene.2018.06.099. [DOI] [PubMed] [Google Scholar]
- 20.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. The Lancet. 2016;388(10039):73–85. doi: 10.1016/s0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 22.Kong Y, Li Y, Luo Y, Zhu J, Zheng H, Gao B, Guo X, Li Z, Chen R, Chen C. circNFIB1 inhibits lymphangiogenesis and lymphatic metastasis via the miR-486-5p/PIK3R1/VEGF-C axis in pancreatic cancer. Mol Cancer. 2020;19(1):82. doi: 10.1186/s12943-020-01205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 24.Kristensen LS, Hansen TB, Venø MT, Kjems J. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37(5):555–565. doi: 10.1038/onc.2017.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai X, Wang M, McElyea SD, Sherman S, House M, Korc M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017;393:86–93. doi: 10.1016/j.canlet.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade M, Laneve P, Rajewsky N, Bozzoni I. Circ-ZNF609 Is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66(1):22–37.e29. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei M, Zheng G, Ning Q, Zheng J, Dong D. Translation and functional roles of circular RNAs in human cancer. Mol Cancer. 2020;19(1):30. doi: 10.1186/s12943-020-1135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Hao X, Wang H, Liu Z, He Y, Pu M, Zhang H, Yu H, Duan J, Qu S. Circular RNA expression profile of pancreatic ductal adenocarcinoma revealed by microarray. Cell Physiol Biochem. 2016;40(6):1334–1344. doi: 10.1159/000453186. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Li Z, Jiang P, Peng M, Zhang X, Chen K, Liu H, Bi H, Liu X, Li X. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. J Exp Clin Cancer Res. 2018;37(1):177. doi: 10.1186/s13046-018-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v.20: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42(Database issue):D92–97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Geng S, Yuan H, Li Y, Zhang S, Pu L, Ge J, Niu X, Li Y, Jiang H. Circular RNA expression profiles in extracellular vesicles from the plasma of patients with pancreatic ductal adenocarcinoma. FEBS Open Bio. 2019;9(12):2052–2062. doi: 10.1002/2211-5463.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Lin S, Mo Z, Jiang J, Tang H, Wu C, Song J. CircRNA_100395 inhibits cell proliferation and metastasis in ovarian cancer via regulating miR-1228/p53/epithelial-mesenchymal transition (EMT) axis. J Cancer. 2020;11(3):599–609. doi: 10.7150/jca.35041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Yanfang W, Li J, Jiang P, Peng T, Chen K, Zhao X, Zhang Y, Zhen P, Zhu J, Li X. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 2018;432:237–250. doi: 10.1016/j.canlet.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 35.Liu CX, Li X, Nan F, Jiang S, Gao X, Guo SK, Xue W, Cui Y, Dong K, Ding H, Qu B, Zhou Z, Shen N, Yang L, Chen LL. Structure and degradation of circular RNAs regulate PKR activation in innate immunity. Cell. 2019;177(4):865–880.e821. doi: 10.1016/j.cell.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Liu FB, Huang M, Xie K, Xie QS, Liu CH, Shen MJ, Huang Q. Circular RNA ciRS-7 promotes the proliferation and metastasis of pancreatic cancer by regulating miR-7-mediated EGFR/STAT3 signaling pathway. Hepatobiliary Pancreat Dis Int. 2019;18(6):580–586. doi: 10.1016/j.hbpd.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Xia L, Dong L, Wang J, Xiao Q, Yu X, Zhu H. CircHIPK3 promotes gemcitabine (GEM) resistance in pancreatic cancer cells by sponging miR-330-5p and targets RASSF1. Cancer Manag Res. 2020;12:921–929. doi: 10.2147/cmar.S239326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu D, Xu AD. Mini review: circular RNAs as potential clinical biomarkers for disorders in the central nervous system. Front Genet. 2016;7:53. doi: 10.3389/fgene.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu H, Xie X, Chen Q, Cai S, Liu S, Bao C, Luo J, Kong J. Clinical significance of circPVT1 in patients with non-small cell lung cancer who received cisplatin combined with gemcitabine chemotherapy. Tumori. 2020 doi: 10.1177/0300891620941940. [DOI] [PubMed] [Google Scholar]
- 40.Mehta SL, Dempsey RJ, Vemuganti R. Role of circular RNAs in brain development and CNS diseases. Prog Neurobiol. 2020;186:101746. doi: 10.1016/j.pneurobio.2020.101746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P, Wu M. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16(1):94. doi: 10.1186/s12943-017-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. The Lancet. 2020;395(10242):2008–2020. doi: 10.1016/s0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 44.O'Leary VB, Smida J, Matjanovski M, Brockhaus C, Winkler K, Moertl S, Ovsepian SV, Atkinson MJ. The circRNA interactome-innovative hallmarks of the intra- and extracellular radiation response. Oncotarget. 2017;8(45):78397–78409. doi: 10.18632/oncotarget.19228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E, Perez-Hernandez D, Ramberger E, Shenzis S, Samson M, Dittmar G, Landthaler M, Chekulaeva M, Rajewsky N, Kadener S. Translation of CircRNAs. Mol Cell. 2017;66(1):9–21.e27. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patop IL, Wust S, Kadener S. Past, present, and future of circRNAs. EMBO J. 2019;38(16):e100836. doi: 10.15252/embj.2018100836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin M, Liu G, Huo X, Tao X, Sun X, Ge Z, Yang J, Fan J, Liu L, Qin W. Hsa_circ_0001649: a circular RNA and potential novel biomarker for hepatocellular carcinoma. Cancer Biomark. 2016;16(1):161–169. doi: 10.3233/cbm-150552. [DOI] [PubMed] [Google Scholar]
- 48.Qu S, Hao X, Song W, Niu K, Yang X, Zhang X, Shang R, Wang Q, Li H, Liu Z. Circular RNA circRHOT1 is upregulated and promotes cell proliferation and invasion in pancreatic cancer. Epigenomics. 2019;11(1):53–63. doi: 10.2217/epi-2018-0051. [DOI] [PubMed] [Google Scholar]
- 49.Qu Y, Gharbi N, Yuan X, Olsen JR, Blicher P, Dalhus B, Brokstad KA, Lin B, Øyan AM, Zhang W, Kalland KH, Ke X. Axitinib blocks Wnt/β-catenin signaling and directs asymmetric cell division in cancer. Proc Natl Acad Sci U S A. 2016;113(33):9339–9344. doi: 10.1073/pnas.1604520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976;73(11):3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shao F, Huang M, Meng F, Huang Q. Circular RNA signature predicts gemcitabine resistance of pancreatic ductal adenocarcinoma. Front Pharmacol. 2018;9:584. doi: 10.3389/fphar.2018.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen X, Chen Y, Li J, Huang H, Liu C, Zhou N. Identification of Circ_001569 as a potential biomarker in the diagnosis and prognosis of pancreatic cancer. Technol Cancer Res Treat. 2021;20:1533033820983302. doi: 10.1177/1533033820983302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shuaizhang LI, Zhang W, Yin X, Xing S, Xie Q, Cao Z, Zhao B. Mouse ATP-binding cassette (ABC) transporters conferring multi-drug resistance. Anticancer Agents Med Chem. 2015;15(4):423–432. doi: 10.2174/1871520615666150129212723. [DOI] [PubMed] [Google Scholar]
- 54.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 55.Song W, Wang WJ, Fu T, Chen L, Miao DL. Integrated analysis of circular RNA-associated ceRNA network in pancreatic ductal adenocarcinoma. Oncol Lett. 2020;19(3):2175–2184. doi: 10.3892/ol.2020.11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strobel O, Neoptolemos J, Jager D, Buchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16(1):11–26. doi: 10.1038/s41571-018-0112-1. [DOI] [PubMed] [Google Scholar]
- 57.Sun J, Lian M, Ma H, Wang R, Ma Z, Wang H, Zhai J, Meng L, Feng L, Bai Y, Cui X, Fang J. Competing endogenous RNA network analysis of CD274, IL-10 and FOXP3 co-expression in laryngeal squamous cell carcinoma. Mol Med Rep. 2018;17(3):3859–3869. doi: 10.3892/mmr.2017.8307. [DOI] [PubMed] [Google Scholar]
- 58.Tang Q, Hann SS. Biological roles and mechanisms of circular RNA in human cancers. Onco Targets Ther. 2020;13:2067–2092. doi: 10.2147/ott.S233672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao L, Zhang Y, Wu YM, Dhanasekaran SM, Engelke CG, Cao X, Robinson DR, Nesvizhskii AI, Chinnaiyan AM. The Landscape of circular RNA in cancer. Cell. 2019;176(4):869–881.e813. doi: 10.1016/j.cell.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Liu J, Ma J, Sun T, Zhou Q, Wang W, Wang G, Wu P, Wang H, Jiang L, Yuan W, Sun Z, Ming L. Exosomal circRNAs: biogenesis, effect and application in human diseases. Mol Cancer. 2019;18(1):116. doi: 10.1186/s12943-019-1041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Mo Y, Gong Z, Yang X, Yang M, Zhang S, Xiong F, Xiang B, Zhou M, Liao Q, Zhang W, Li X, Li X, Li Y, Li G, Zeng Z, Xiong W. Circular RNAs in human cancer. Mol Cancer. 2017;16(1):25. doi: 10.1186/s12943-017-0598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilusz JE. A 360 degrees view of circular RNAs: from biogenesis to functions. Wiley Interdiscip Rev RNA. 2018;9(4):e1478. doi: 10.1002/wrna.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xia S, Feng J, Chen K, Ma Y, Gong J, Cai F, Jin Y, Gao Y, Xia L, Chang H, Wei L, Han L, He C. CSCD: a database for cancer-specific circular RNAs. Nucleic Acids Res. 2018;46(D1):D925–d929. doi: 10.1093/nar/gkx863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao Y. Construction of a circRNA-miRNA-mRNA network to explore the pathogenesis and treatment of pancreatic ductal adenocarcinoma. J Cell Biochem. 2020;121(1):394–406. doi: 10.1002/jcb.29194. [DOI] [PubMed] [Google Scholar]
- 65.Xie F, Zhao N, Zhang H, Xie D. Circular RNA CircHIPK3 promotes gemcitabine sensitivity in bladder cancer. J Cancer. 2020;11(7):1907–1912. doi: 10.7150/jca.39722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu C, Yu Y, Ding F. Microarray analysis of circular RNA expression profiles associated with gemcitabine resistance in pancreatic cancer cells. Oncol Rep. 2018;40(1):395–404. doi: 10.3892/or.2018.6450. [DOI] [PubMed] [Google Scholar]
- 67.Xu Y, Yao Y, Zhong X, Leng K, Qin W, Qu L, Cui Y, Jiang X. Downregulated circular RNA hsa_circ_0001649 regulates proliferation, migration and invasion in cholangiocarcinoma cells. Biochem Biophys Res Commun. 2018;496(2):455–461. doi: 10.1016/j.bbrc.2018.01.077. [DOI] [PubMed] [Google Scholar]
- 68.Yan L, Liu G, Cao H, Zhang H, Shao F. Hsa_circ_0035483 sponges hsa-miR-335 to promote the gemcitabine-resistance of human renal cancer cells by autophagy regulation. Biochem Biophys Res Commun. 2019;519(1):172–178. doi: 10.1016/j.bbrc.2019.08.093. [DOI] [PubMed] [Google Scholar]
- 69.Yang F, Liu DY, Guo JT, Ge N, Zhu P, Liu X, Wang S, Wang GX, Sun SY. Circular RNA circ-LDLRAD3 as a biomarker in diagnosis of pancreatic cancer. World J Gastroenterol. 2017;23(47):8345–8354. doi: 10.3748/wjg.v23.i47.8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang J, Cong X, Ren M, Sun H, Liu T, Chen G, Wang Q, Li Z, Yu S, Yang Q. Circular RNA hsa_circRNA_0007334 is predicted to promote MMP7 and COL1A1 expression by functioning as a miRNA sponge in pancreatic ductal adenocarcinoma. J Oncol. 2019;2019:7630894. doi: 10.1155/2019/7630894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang KS, Im H, Hong S, Pergolini I, Del Castillo AF, Wang R, Clardy S, Huang CH, Pille C, Ferrone S, Yang R, Castro CM, Lee H, Del Castillo CF, Weissleder R. Multiparametric plasma EV profiling facilitates diagnosis of pancreatic malignancy. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aal3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao F, Huang N, Yang X, Zhao K, Zhou H, Huang S, Xie B, Zhang N. Novel role of FBXW7 Circular RNA in repressing glioma tumorigenesis. J Natl Cancer Inst. 2018;110(3):304–315. doi: 10.1093/jnci/djx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zang J, Lu D, Xu A. The interaction of circRNAs and RNA binding proteins: an important part of circRNA maintenance and function. J Neurosci Res. 2020;98(1):87–97. doi: 10.1002/jnr.24356. [DOI] [PubMed] [Google Scholar]
- 74.Zhang L, Hou C, Chen C, Guo Y, Yuan W, Yin D, Liu J, Sun Z. The role of N(6)-methyladenosine (m(6)A) modification in the regulation of circRNAs. Mol Cancer. 2020;19(1):105. doi: 10.1186/s12943-020-01224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang M, Huang N, Yang X, Luo J, Yan S, Xiao F, Chen W, Gao X, Zhao K, Zhou H, Li Z, Ming L, Xie B, Zhang N. A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis. Oncogene. 2018;37(13):1805–1814. doi: 10.1038/s41388-017-0019-9. [DOI] [PubMed] [Google Scholar]
- 76.Zhang S, Chen Z, Sun J, An N, Xi Q. CircRNA hsa_circRNA_0000069 promotes the proliferation, migration and invasion of cervical cancer through miR-873-5p/TUSC3 axis. Cancer Cell Int. 2020;20:287. doi: 10.1186/s12935-020-01387-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Zhang S, Liao K, Miao Z, Wang Q, Miao Y, Guo Z, Qiu Y, Chen B, Ren L, Wei Z, Lin Y, Lu X, Qiu Y. CircFOXO3 promotes glioblastoma progression by acting as a competing endogenous RNA for NFAT5. Neuro Oncol. 2019;21(10):1284–1296. doi: 10.1093/neuonc/noz128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang X, Wang S, Wang H, Cao J, Huang X, Chen Z, Xu P, Sun G, Xu J, Lv J, Xu Z. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol Cancer. 2019;18(1):20. doi: 10.1186/s12943-018-0935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao R, Ni J, Lu S, Jiang S, You L, Liu H, Shou J, Zhai C, Zhang W, Shao S, Yang X, Pan H, Han W. CircUBAP2-mediated competing endogenous RNA network modulates tumorigenesis in pancreatic adenocarcinoma. Aging (Albany NY) 2019;11(19):8484–8501. doi: 10.18632/aging.102334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.