Abstract
In this issue of Cancer Cell, Shiao et al. reveal the counteracting role of bacteria and fungi in antitumoral immune responses to radiation therapy (RT). While bacterial depletion impairs the response, fungal depletion improves efficacy of RT. An interplay between innate and adaptive immunity is implicated and orchestrated by Dectin-1.
Factors that determine the ability to respond to antitumor therapies are the focus of research to improve treatment effectiveness. The gut microbiota have been identified as a key host factor in mediating responses to immunotherapy and chemotherapy (Routy et al., 2018; Viaud et al., 2013). Bacteria depletion has been associated with decreased efficacy of antitumor therapies in cancers like melanoma (Iida et al., 2013), but it has been linked with better outcomes in others like pancreatic ductal adenocarcinoma (PDAC) (Pushalkar et al., 2018). On the other hand, colonization with specific bacteria species can improve therapy responses (Iida et al., 2013; Viaud et al., 2013). However, the role played by gut microbes during the immune responses to radiation therapy (RT) is unknown. It has been revealed that fungi are capable of colonizing the pancreas, specifically a fungus called Malassezia, and can promote pancreatic ductal adenocarcinoma (Aykut et al., 2019). Moreover, like bacterial dysbiosis, changes in the biodiversity of fungal communities are linked to the severity of inflammatory processes as well as tumor development (Bacher et al., 2019).
In this issue of Cancer Cell, Shiao et al. demonstrate that bacterial depletion with antibiotics reduces the efficacy of RT in murine models of breast cancer and melanoma (Shiao et al., 2021). Because bacterial depletion is associated with a marked increase in the fungal populations in the gut, the authors aim to determine if the effect of bacterial depletion may actually depend on the increase in fungi. Upon fungal depletion, the contrasting finding of an increase in RT efficacy is discovered, confirming the deleterious influence of these microorganisms in the response to radiation.
The immune system has a pivotal role in antitumoral responses in different therapeutic settings. Although a high density of tumor-infiltrating cytotoxic CD8 T cells (CTLs) correlates with a favorable clinical outcome, the presence of myeloid-derived suppressor cells and tumor-associated macrophages (TAMs), as well as an abundance of CTL-expressing PD-1 or regulatory T cells, impairs antitumor immunity and is associated with poor prognosis (Chen et al., 2016). Shiao et al. performed flow-cytometry-based immune profiling in order to better understand the influence of bacteria and fungi in shaping the immune response in the context of RT, which itself has immunomodulating capacity. Bacterial depletion with antibiotics resulted in an increase in the population of TAMs with a suppressive phenotype, but without altering the total populations of CD8 or CD4 T cells. However, a decrease in activated CD8 T cells (CD8+CD69+) was observed both with antibiotics alone and in combination with RT. Interestingly, fungal depletion in combination with RT considerably increased both the total CD8 T cell population and the CTLs, while it decreased the exhausted phenotype of T cells as well as suppressive TAMs. Altogether, the data presented suggest that the efficacy of antifungal antibiotics (AF) when combined with RT depends on both CD8 T cells and macrophages. In short, the presence of fungi seems to contribute to the generation of a suppressive tumor microenvironment, and its depletion eliminates this negative effect, improving responses to RT.
To understand microbial networks and determine the influence of these microorganisms on the response to RT, the composition of the bacterial and fungal communities was analyzed using 16S rRNA and ITS-1 gene sequencing, respectively, after their depletion. Interestingly, antibiotic treatment produced the expansion of specific communities of commensal fungi of the Saccharomycetales order, highlighting an increase in the genera Saccharomyces and Candida. To test the hypothesis that fungi can regulate the response to cancer therapy, the authors colonized tumor-bearing mice with Candida albicans, the most prevalent Saccharomycetales commensal present in humans. Strikingly, the experiment revealed that C. albicans overgrowth increases exhausted CD8 T cells, and ultimately impairs the antitumor response to RT and decreases survival. Treatment with fluconazole, a commonly used AF which is capable of decreasing C. albicans burden, reverses the effect, thus increasing responses to RT and survival, with a considerable decrease in exhausted CD8 T cells.
In search of molecular mediators of the fungal effect, Shiao et al. found increased expression of Dectin-1 in breast cancer tissue, mostly on macrophages, and inversely associated with survival. Dectin-1 is a member of the C-type lectin family of pattern-recognition receptors that sense pathogens present in the fungal cell wall and are frequently expressed in innate immune cells that activate the antifungal protective immune pathway (Goodridge et al., 2011). Importantly, mice deficient in Dectin-1 presented enhanced responses to RT, mimicking the administration of AF.
Considering that most patients with breast cancer or melanoma commonly use antibiotics, the knowledge that overgrowth of specific fungal communities could contribute to the generation of an immunosuppressive tumor microenvironment which decreases the efficiency of antitumor therapy could be very relevant. In addition to the presence of fungi, the signaling produced by these microorganisms in the immune system transmits an inhibitory signal that deteriorates the response to RT, and the blocking of these signals can have positive effects on the efficacy of the antitumor response. The interaction of Dectin-1 with galectin-9 has been said to result in the programming of tolerogenic macrophages and immune suppression (Daley et al., 2017); therefore, blocking these pathways appears to be an attractive therapeutic target in cancer.
This study highlights the role of fungi in modulating the antitumor response to RT and further describes a tight regulatory network between the commensal communities of fungi and bacteria and the immune tumor microenvironment. The alteration of some of the bacterial communities can result in expansion of specific fungi which can generate an immunosuppressive microenvironment that contributes to reducing the effectiveness of antitumor therapy (Figure 1). The findings suggest the therapeutic potential of the depletion of fungi or the blocking of the signaling generated in the immune system during RT. Importantly, the discovery of the opposing but dual importance of fungi and bacteria in determining resistance to therapies opens up a novel area of exploration that will require validation in clinical trials using FDA-approved antifungals in combination with RT.
Figure 1. Bacterial and fungal microbiota have a counteracting role in modulating immune responses induced by radiation therapy in the context of cancer.
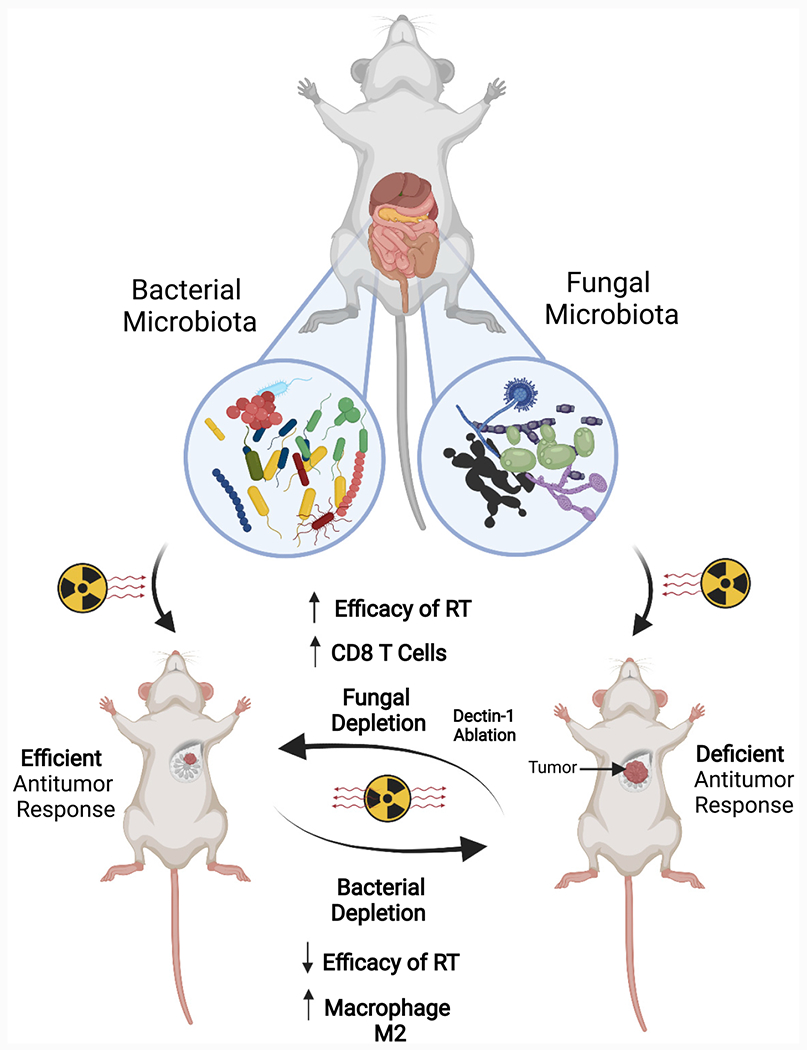
Bacterial ablation with antibiotics increases immune-suppressive populations and favors tumors’ growth while increasing fungal populations. In turn, antifungals increase cytotoxic immune T cell populations and delay tumor growth in response to radiation therapy.
These results also highlight the role of unappreciated factors within the intestinal microbiota, such as fungi, in the response to antitumor therapy. An imbalance in the fungal community may contribute to restructuring of the tumor microenvironment, altering the immune cell populations from the innate and adaptive systems toward a suppressive microenvironment that impairs the antitumor response to RT. This also suggests the importance of keeping these microorganisms under control in any antitumor therapeutic setting. The study ultimately provides attractive new therapeutic targets that may override cancer resistance in the setting of RT.
ACKNOWLEDGMENTS
This work was supported by grants to F.M. from the NCI (1R37CA237384-01A1), CPRIT (RP200173), V Foundation Translational Award, Andrew Sabin Family Fellowship, and Philantropic Moonshot Programs (MDACC). E.R. is supported by FONDECYT-1191526.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Aykut B, Pushalkar S, Chen R, Li Q, Abengozar R, Kim JI, Shadaloey SA, Wu D, Preiss P, Verma N, et al. (2019). The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574, 264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher P, Hohnstein T, Beerbaum E, Röcker M, Blango MG, Kaufmann S, Röhmel J, Eschenhagen P, Grehn C, Seidel K, et al. (2019). Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell 176, 1340–1355.e15. [DOI] [PubMed] [Google Scholar]
- Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, Miller JP, Bassett RL, Gopalakrishnan V, Wani K, et al. (2016). Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov. 6, 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley D, Mani VR, Mohan N, Akkad N, Ochi A, Heindel DW, Lee KB, Zambirinis CP, Pandian GSB, Savadkar S, et al. (2017). Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat. Med 23, 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, Bose N, Chan AS, Magee AS, Danielson ME, et al. (2011). Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature 472, 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, et al. (2013). Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE, et al. (2018). The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 8, 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. (2018). Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97. [DOI] [PubMed] [Google Scholar]
- Shiao SL, Kershaw KM, Limon JJ, You S, Yoon J, Ko EY, Guarnerio J, Potdar AA, McGovern DPB, Bose S, et al. (2021). Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, et al. (2013). The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]


