Abstract
Impulsivity is a common feature of bipolar disorder (BD) with ramifications for functional impairment and premature mortality. This PRISMA-guided systematic review aims to integrate findings on the neurocircuitry associated with impulsivity in BD. We searched for functional neuroimaging studies that examined rapid-response impulsivity and choice impulsivity using the Go/No-Go Task, Stop-Signal Task, and Delay Discounting Task. Findings from 33 studies were synthesized with an emphasis on the effect of mood state of the sample and affective salience of the task. Results suggest trait-like brain activation abnormalities in regions implicated in impulsivity that persist across mood states. During rapid-response inhibition, BD exhibit under-activation of key frontal, insular, parietal, cingulate, and thalamic regions, but over-activation of these regions when the task involves emotional stimuli. Delay discounting tasks with functional neuroimaging in BD are lacking, but hyperactivity of orbitofrontal and striatal regions associated with reward hypersensitivity may be related to difficulty delaying gratification. We propose a working model of neurocircuitry dysfunction underlying behavioral impulsivity in BD. Clinical implications and future directions are discussed.
Keywords: Inhibition, Rapid-response impulsivity, Choice impulsivity, Go/no-go, Stop-signal task, Delay discounting, Functional neuroimaging, Neurocircuitry
1. Introduction
1.1. Background
Bipolar disorder (BD) is a serious psychiatric illness involving extreme shifts in mood that affects 45.5 million individuals worldwide (James et al., 2018). The hallmark characteristic of BD is mania, which comprises symptoms including elated or irritable mood, inflated self-esteem, decreased need for sleep, rapid speech, racing thoughts, distractibility, increased goal-directed activity, and risky behaviors (American Psychiatric Association, 2013). Typically, individuals with BD also experience significant periods in depressive states involving low mood, anhedonia, problems with appetite, sleeping, thinking, and psychomotor activity, loss of energy, inappropriate feelings of guilt, feelings of worthlessness, and suicidal ideation. Manic episodes with mixed features can also be present, during which manic and depressive symptoms co-occur. BD is associated with significant cognitive impairment, functional difficulties, and increased mortality (Lomholt et al., 2019).
Impulsive behavior is a clinical feature of BD and a symptom of mania (American Psychiatric Association, 2013). Impulsivity refers to a general predisposition toward rapid, unplanned reactions to internal or external stimuli with little or no regard for consequences (Moeller et al., 2001). Impulsivity arises from deficits in inhibitory processes co-occurring with strong impulses and modulated by dispositional and situational factors (Bari and Robbins, 2013; Hofmann et al., 2009). Although impulsivity is a transdiagnostic construct, it has significant implications for maladaptive behaviors in BD that contribute to poor functioning and premature mortality in this population, such as problematic gambling, excessive spending, risky sexual behaviors, and substance abuse (Varo et al., 2019). Crucially, impulsivity has been associated with suicidal behaviors in BD (Swann et al., 2005), which carries one of the highest risks for suicide among psychiatric disorders (Baldessarini and Tondo, 2020).
Impulsivity as measured via self-report questionnaires such as the Barratt Impulsivity Scale (BIS-11; Patton et al., 1995) and the Urgency, Premeditation, Perseverance, Sensation Seeking, Positive Urgency, Impulsive Behavior Scale (UPPS-P; Cyders et al., 2007; Whiteside and Lynam, 2001) indicate that high impulsivity is a relatively stable trait in BD across illness phase and mood state (Saddichha and Schuetz, 2014; Strakowski et al., 2010; Swann et al., 2003). Complementary to self-report measures are laboratory behavioral tasks that can provide insight regarding how self-report impulsivity translates to behavioral reactions within situational contexts, or “in-the-moment” behaviors. These tasks permit experimental manipulation and more precise measurement to better understand contextual factors associated with impulsive behavior in BD. When combined with neuroimaging techniques such as blood oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI) and functional near-infrared spectroscopy (fNIRS), brain activity can be mapped to inform neural mechanisms underlying impulsivity.
1.2. Rapid-response impulsivity
Rapid-response impulsivity is the tendency to engage in quick, unplanned actions with concomitant difficulty inhibiting motor responses that are pre-potent or already initiated (Hamilton et al., 2015a; Moeller et al., 2001). Two types of rapid-response impulsivity are refraining from action initiation and stopping an ongoing action (Hamilton et al., 2015a), which have important conceptual and neurobiological distinctions (Cieslik et al., 2015; Hamilton et al., 2015a). Refraining from action initiation involves suppressing a pre-potent response that has not yet been initiated such as responding to one type of stimulus, but inhibiting a response to another stimulus. In contrast, stopping an ongoing action requires an individual to respond to a stimulus, but in some situations to stop an already initiated response once a (stop) signal is presented. The important distinction is that in refraining from action initiation the signal to inhibit a response is given as part of the stimulus property with no delay, which captures action restraint, whereas in stopping an ongoing action, the signal to inhibit is given after the action has already begun, which captures action cancellation.
A prototypical task that measures refraining from action initiation is the Go/No-Go Task (GNG). The standard GNG involves a Go condition that requires individuals to respond (e.g., press a button) quickly for all stimuli of a certain type (the “Go” stimulus) and a No-Go condition that requires individuals to inhibit a response during presentation of a different stimulus (the “No-Go” stimulus). Although the exact ratio of Go to No-Go stimuli differs across study designs, the majority of the stimuli require a Go response, thus creating a pre-potent tendency to respond that must be inhibited when a No-Go stimulus is presented. Non-affective versions of the GNG use neutral stimuli such as letters or shapes while modified affective (or “emotional”) GNG versions use emotional words or faces to examine biases for affective stimuli. The primary outcome measure of impulsivity in the GNG is the number or rate of commission errors (i.e., false alarms) when responses are incorrectly made to No-Go stimuli.
The prototypical task to assess the ability to stop an ongoing action is the Stop-Signal Task (SST). The SST requires an individual to respond (e.g., press a button) quickly to a stimulus, but on some trials, a stop signal (e.g., a tone) is presented after the stimulus requiring the individual to stop the already initiated response. The standard SST adjusts the delay at which the stop signal is presented (i.e., the stop signal delay [SSD]) such that inhibition reaches a success rate of 50% on stop trials. The primary outcome measure of the SST is the stop signal reaction time (SSRT), calculated as the mean go signal reaction time minus the stop signal delay, which represents the “race” between responding to the initial stimulus and successfully inhibiting (i.e., stopping) the motor response when required if the stop signal is subsequently presented. Longer SSRT and lower accuracy rate on stop trials are believed to reflect greater impulsivity.
A meta-analysis of neuroimaging studies identified the inferior frontal gyrus extending into the right anterior insula as brain regions involved in both refraining from action initiation and stopping an ongoing action (Cieslik et al., 2015). Beyond these shared regions, research has shown that additional primary brain regions recruited by the two types of rapid-response impulsivity are distinct. Specifically, refraining from action initiation likely encompasses a right-lateralized network comprising the inferior frontal gyrus, insula, dorsolateral prefrontal cortex and parietal regions to facilitate stimulus-response associations (Miller and Cohen, 2001; Zheng et al., 2008). In contrast, stopping an ongoing action likely recruits the inferior frontal gyrus, insula, pre-supplementary motor area, midcingulate cortex, subthalamic nucleus, and thalamus (Aron et al., 2014; Cieslik et al., 2015).
1.3. Choice impulsivity
In contrast to rapid-response impulsivity, choice impulsivity refers to an inability to delay immediate gratification in favor of larger rewards occurring in the future. In this model the subjective value of a reward (e.g., money) is reduced (i.e., discounted) in proportion to the length of delay in receiving the reward. Choice impulsivity is frequently assessed through delay discounting tasks (DDT) that require the individual to choose between larger, delayed rewards and smaller, immediate rewards. Successive questions are asked until the individual eventually chooses an immediate reward instead of the delayed reward, arriving at an “indifference point” when the immediate and delayed rewards have equal subjective value (Reynolds and Schiffbauer, 2004). Using different delay paradigms, these subjective values can be computed for participants to generate a curve (or other metrics) to quantify discounting rate. Delay discounting ostensibly captures the extent to which rewards lose value over time, with steeper delays hypothesized to reflect an impulsive preference for immediate rewards.
Frontoparietal and limbic systems have been shown to underlie choice impulsivity as measured from delay discounting tasks (McClure, 2004). A meta-analysis of fMRI studies revealed that lower activity in inferior frontal gyrus, ventral striatum, and anterior cingulate cortex/-medial prefrontal cortex are associated with selection and valuation processes that may contribute to steeper delay discounting (Schüller et al., 2019). In contrast, cognitive control required for delaying larger rewards has been linked to greater lateral/ventral prefrontal activity (McClure, 2004; Miller and Cohen, 2001). Connectivity among these regions may also contribute to regulation of delay discounting processes, as evidenced by a study finding that stronger functional coupling between the nucleus accumbens and orbitofrontal cortex was associated with a greater ability to focus away from immediate temptations (Luerssen et al., 2015). Therefore, an imbalance in crosstalk between the ventral striatum (including the nucleus accumbens) and prefrontal cortex and/or their connectivity may contribute to choice impulsivity.
1.4. Rationale and objective of this review
A growing body of self-report, behavioral, and neuroimaging studies have sought to better understand impulsivity in BD. While reviews have been conducted in BD on self-report measures of impulsivity and behavioral performance on inhibition tasks (Ramírez-Martín et al., 2020; Saddichha and Schuetz, 2014), there is no published review to our knowledge of neuroimaging studies that used inhibition tasks to examine the neural correlates of impulsivity in BD. The objective of this systematic review was to examine findings from functional neuroimaging studies using tasks to investigate behavioral and cognitive impulsivity in BD. We review and discuss findings within an integrated framework that includes different types of impulsivity, neural mechanisms, and contextual factors such as mood state. From our findings, a working model of the neural circuitry underlying behavioral impulsivity in BD is described and its clinical significance is discussed. We also identify limitations of current research and offer suggestions for future directions.
2. Methods
A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA; Page et al., 2021). Inclusion criteria were published studies that: 1) included individuals with bipolar disorder and a control comparison group, 2) used a version of the Go/No-Go, Stop-Signal, or Delay Discounting Task during fMRI or fNIRS, and 3) examined differences in regional brain activation between groups. No limits were applied to the publication year. Embase, Pubmed/MEDLINE, and PsycINFO were last searched on September 7, 2022. The full lists of search terms for each database are available in the Supplementary Materials. Two reviewers (authors CCC and PRS) independently screened all titles and abstracts for inclusion, and disagreements were resolved through discussion. CCC and PRS then independently assessed the full article for inclusion and again resolved disagreements through consensus.
Outcome measures were differences between groups in regional activation during task performance. Studies varied in imaging analysis methodology (e.g., contrasts), but we included any differences between the bipolar patient group and a comparison group (e.g., healthy controls, another psychiatric condition). For studies that involved an intervention trial, we examined the results reported for baseline differences. Risk of bias was assessed using a modified version of the Newcastle-Ottawa Scale adapted for case control studies (Wells et al., 2013; see modified scale in Supplementary Materials). Data were synthesized by grouping versions of each task used and the mood state of the BD sample. Because of the multidimensional nature of impulsivity and inconsistent definitions used in studies of BD, we adopted terms, definitions, and measures recommended by the International Society for Research on Impulsivity (Hamilton et al., 2015a, 2015b) as a framework for integrating the literature.
3. Results
3.1. Study Characteristics
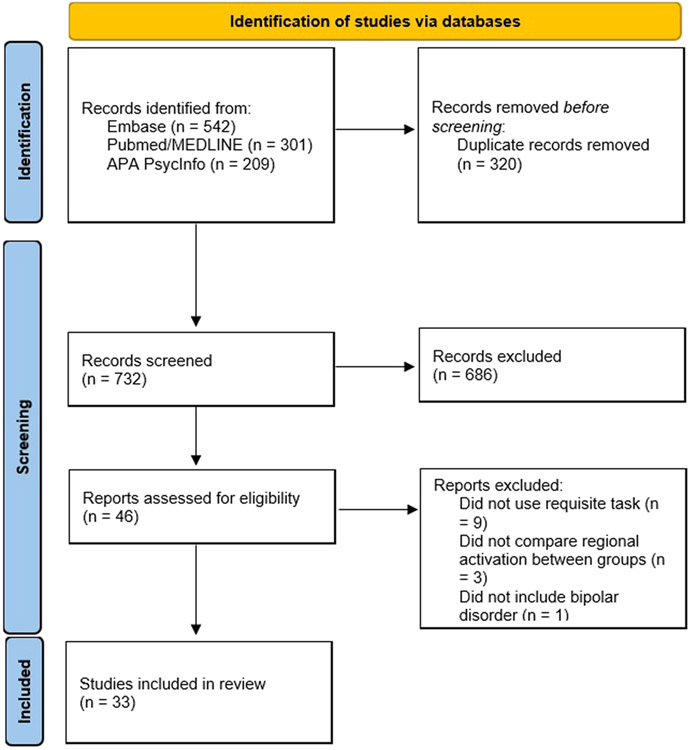
The search returned a total of 1052 records across the three databases. After removal of duplicates, title and abstract screening, and full-text assessment, 33 studies were included in this review (see Fig. 1 for PRISMA flow diagram). A total of 22 studies used a variation of the Go/No-Go paradigm to examine neural correlates of refraining from action initiation. Of the 22 studies, 17 used a non-affective version of the GNG and 5 used an affective GNG. Of the 17 that used a non-affective GNG, 10 examined a BD sample with predominantly euthymic mood (Ajilore et al., 2015; Joshi et al., 2016; Kaladjian et al., 2009b; Metcalfe et al., 2016; Quidé et al., 2018; Singh et al., 2010; Townsend et al., 2012, 2013; Welander-Vatn et al., 2013; Welander-Vatn et al., 2009), 6 examined BD during a mood episode (Altshuler et al., 2005; Diler et al., 2013, 2014; Fleck et al., 2011; Mazzola-Pomietto et al., 2009; Penfold et al., 2015), and one study (Kaladjian et al., 2009a) tested BD patients while in a mood episode and re-tested them during remission. Of the 5 that used an affective GNG, 2 examined a BD sample during predominantly euthymic mood (Wessa et al., 2007; Xiao et al., 2021b), 2 examined BD during a mood episode (Elliott et al., 2004; Xiao et al., 2021a), and 1 compared euthymic, depressed, and manic BD patients (Hummer et al., 2013).
Fig. 1.
PRISMA flow diagram for study selection.
A total of 11 studies used the Stop Signal Task or a variation to examine neural correlates of stopping an ongoing action. Of those, 6 used a SST to examine a BD sample with predominantly euthymic mood (Cao, 2022; Deveney et al., 2012b, 2012a; Leibenluft, 2007; Tsujii et al., 2018; Weathers et al., 2012) and 4 examined BD during a mood episode (Cerullo et al., 2009; Passarotti et al., 2010; Pavuluri et al., 2010; Strakowski et al., 2008), and one examined BD during depression and a subset were re-assessed after remission (Kopf et al., 2019). Our search did not return any study using the Delay Discounting Task with concurrent fMRI or FNIRS to examine neural correlates of choice impulsivity. Individual characteristics of the included studies using the GNG and the SST are summarized in Table 1 and Table 2, respectively. Results of the risk of bias assessment are presented in Supplementary Table 1. Of the 33 included studies, 30 (91%) were given high scores (7–9) and three (9%) were given moderate scores (4–6).
Table 1.
Summary of neuroimaging studies reviewed that used the Go/No-Go Task in bipolar disorder.
| Study | Sample, mood state, subtype [%female; mean (SD) age] |
Task characteristics | Main behavioral findings |
Neuroimaging contrast | Main neuroimaging findings [activation in BD relative to HC unless otherwise specified] |
|---|---|---|---|---|---|
| Nonaffective Go/No-Go Task in BD during euthymia | |||||
| Kaladjian et al. (2009b) | -20 BD-I euthymic [50%; 37.9 (11.4)] -20 HC [50%; 34.6 (10.6)] |
Equiprobable nonaffective GNG | No difference in commission error rate | Correct No-Go trials minus Correct Go trials | -↓left frontopolar cortex (BA 10) and bilateral dorsal amygdala |
| Kaladjian et al. (2009a) * | -10 BD-I euthymic [50%; 40.1 (13.7)] -10 HC [50%; 41.5 (13.4)] |
Equiprobable nonaffective GNG | No difference in commission error rate | Correct No-Go trials minus Correct Go trials | -↓left amygdala and bilateral putamen |
| Welander-Vatn et al. (2009) | -27 BD-II; 15 euthymic, 12 mildly-severely depressed [70.3%; 34.7 (11.4)] -28 HC [60.7%; 38.1 (10.8)] |
Standard GNG (75% Go; 25% No-Go) | No difference in % of correct responses | No-Go condition versus resting condition | -No difference in ROI analysis in dorsal ACC and pre-SMA regions (other brain regions not investigated) |
| Singh et al. (2010) | -26 BD; 18 BD-I, 8 BD-II euthymic [38%; 15.4 (2.7)] -22 HC [41%; 14.3 (2.5)] |
Equiprobable nonaffective GNG | No difference in commission error rate | No-Go condition minus Go condition | -↑right DLPFC |
| Townsend et al. (2012) | -32 BD-I euthymic [34.4%; 37 (13)] -30 HC (43.4%, 37 (13)] |
Equiprobable nonaffective GNG | No difference in accuracy rate | No-Go condition minus Go condition | -↓IFC -↓bilateral putamen, bilateral caudate, bilateral globus pallidus, right thalamus, and right STN -No effect of medication |
| Townsend et al. (2013) | -16 BD-I euthymic without ADHD [25%; 38.1 (12.2)] -16 BD-I euthymic with ADHD [43.75%; 36.4 (14.1)] -16 ADHD without BD [37.5%; 35.7 (10.8)] -30 HC 43.3%; 36.8 (12.6)] |
Standard GNG (75% Go, 25% No-Go) | No difference in commission error rate | No-Go condition minus Go condition | -BD without ADHD had ↓left IFC, left frontopolar region, left DLPFC, bilateral ACC, and left parietal lobe compared with HC -BD with comorbid with ADHD resulted in ↑cingulate and frontal areas compared with HC |
| Welander-Vatn et al. (2013) | -24 BD-I euthymic [58.3%; 35.6 (11.3)] -24 HC 45.8%; 34.5 (9.4)] |
Standard GNG (75% Go, 25% No-Go) | No difference in commission error rate | No-Go condition versus resting condition | -No difference in full brain analysis and ROI (dorsal ACC/pre-SMA) approach (did not investigate No-Go vs Go contrast) |
| Ajilore et al. (2015) | -16 BD-I euthymic [43.75%; 46.7 (11.8)] -16 HC (56.25%; 40.4 (11.2)] |
-Equiprobable GNG | No difference in accuracy rate | No-Go condition minus Go condition | -↓BA 47 (pars orbitalis) activation, particularly in right hemisphere |
| Joshi et al. (2016) | -45 BD-I euthymic [46.7%; 39.9 (12.1)] -45 HC [48.9%; 37.7 (10.5)] |
-Equiprobable GNG -Spiderman picture (Go); non-Spiderman picture (No-go) | No difference in accuracy rate | No-Go condition minus Go condition | -↓IFC, medial frontal gyrus (BA 6), insula, left and right superior frontal gyri, cingulate gyrus (BA 32), and right parietal lobule (BA 40/7) |
| Metcalfe et al. (2016) | -30 BD; 10 BD-I, 10 BD-II, 10 BD-NOS euthymic [57%; 16.8 (1.4)] -20 HC [55%; 16.1 (1.5) |
-GNG (85% Go, 15% No-Go) | No difference in commission error rate | No-Go correct trials minus Go trials No-Go incorrect trials minus Go trials | No-Go correct trials minus Go trials: -↓left OFC and IFG; ↓ right operculum and putamen No-Go incorrect trials minus Go trials: ↑left IFG, middle frontal gyrus, posterior cingulate cortex |
| Quidé et al. (2018) | -56 BD with psychosis -53 HC [43%; 38.1 (9.8)]† |
-GNG Flanker Task (28% congruent trials, 28% incongruent trials, 21% neutral trials, 23% No-Go trials) | No difference in task accuracy | No contrasts specified. | No effect of diagnosis on activation. |
| Nonaffective Go/No-Go Task in BD during a mood episode | |||||
| Altshuler et al. (2005) | -11 BD-I manic or hypomanic [64%; 36 (7.6)] -13 HC [62%; 31 (6.7)] |
Equiprobable standard GNG | No difference in accuracy rate | No-Go condition minus Go condition | -Lack of activation in right lateral OFC region (BA 47), right lateral PFC (BA 45), and right hippocampus -↓left rostral cingulate (BA 24) |
| Mazzola-Pomietto et al. (2009) | -16 BD-I manic [62.5%; 35.8 (12.1)] -16 HC [62.5%; 34.6 (12.6)] |
Equiprobable standard GNG | No difference in commission error rate | Correct No-Go trials minus Correct Go trials | -↓bilateral VLPFC (BA 47) |
| Kaladjian et al. (2009a) * | -10 BD-I manic [50%; 40.1 (13.7)] -10 HC [50%; 41.5 (13.4)] |
Equiprobable standard GNG | No difference in commission error rate | Correct No-Go trials minus correct Go trials | -↓bilateral putamen |
| Fleck et al. (2011) | -8 BD-I mixed [67%; 30 (8)] -10 BD-I depressed [50%; 34 (10)] -10 HC [80%; 33 (8)] |
Standard GNG (83.34% Go; 16.66% No-Go) | Mixed episode BD trended toward more commission errors than HC (p = 0.09) | Correct No-Go, Incorrect Go, and Incorrect No-Go trials vs Correct Go trials | -Compared to HC, mixed BD had ↑right amygdala, right middle frontal gyrus, and left medial frontal gyrus. -Compared to depressed BD, mixed BD had ↑left thalamus, left cerebellum, and right IFC -mixed BD activated right amygdala while HC deactivated right amygdala |
| Diler et al. (2013) | -10 BD depressed; 3 BD-I, 4 BD-II, 3 BD-NOS [80%, 15.6 (0.9)] -10 HC [80%; 15.6 (1.2)] |
Equiprobable standard GNG | No difference in commission error rate | 2 (BD depressed, HC) X 2 (Go block, No-Go block) | -↑bilateral VLPFC during both Go and No-Go conditions (did not investigate No-Go vs Go contrast) |
| Diler et al. (2014) | -12 BD depressed; 6 BD-I, 6 BD-II [83%; 15.5 (1.2)] -10 unipolar major depressive disorder [80%; 15.9 (1.1)] -10 HC [80%; 15.6 (1.2)] |
Equiprobable standard GNG | No difference in commission error rate | 3 (BD depressed, unipolar major depressive disorder, HC) X 2 (Go block, No-Go block) | -↑left superior temporal, left caudate, and ACC in BD during No-Go condition compared with HC (did not investigate No-Go vs Go contrast) |
| Penfold et al. (2015) | -19 BD-II depressed [52.6%; 36.3 (12.2)] -20 HC [50%; 35.6 (11.6)] |
Standard GNG (75% Go, 25% No-Go) | No difference in accuracy rate | No-Go condition minus Go condition | -↓right inferior, middle, and superior frontal gyri, right insula, and bilateral precentral gyrus -↓bilateral middle temporal gyrus, right superior temporal gyrus, left middle occipital gyrus, bilateral cuneus and lingual gyrus |
| Affective Go/No-Go Task in BD during euthymia | |||||
| Wessa et al. (2007) | -17 BD; 10 BD-I, 7 BD-II euthymic [41%; 44.94 (12.7)] -17 HC [35%; 44.94 (11.36)] |
-Affective GNG (66.7% Go; 33.3% No-Go) -8 conditions with different emotional and nonemotional faces |
No difference in commission error rate | Compared different No-Go conditions against one another | -All No-Go conditions vs rest: lack of superior temporal poles activation -Emotional vs nonemotional conditions: ↑right inferior temporal gyrus, middle temporal gyrus, and superior temporal gyrus during emotional GNG -Emotional vs neutral distractors: ↑right medial and left superior OFC, left ACC, right precuneus extending to posterior cingulate cortex, left insula, and caudate |
| Hummer et al. (2013) § | -30 BD manic/hypomanic, 17 BD-I, 13 BD-II [63%; 34 (11)] -30 BD depressed,13 BD-I, 17 BD-II, [60%; 35 (11)] -14 BD euthymic, 10 BD-I, 4 BD-II [71%; 31 (11)] -30 HC [63%; 32 (10)] |
Equiprobable affective GNG | No difference in commission error rate | No-Go condition minus Go condition | -BD across mood states generally had ↑frontal and insular regions during affective conditions of the task than HC, particularly when inhibiting to sad stimuli -Euthymic BD ↑insula, IFG, and OFC |
| Xiao et al. (2021b) | -18 BD; subtype not specified. euthymic [50%; 15.2 (1.7)] -17 HC [59%; 14.2 (1.6)] |
-Affective GNG (66.7% Go; 33.3% No-Go) | No difference in commission error rate | NoGo condition minus rest Emotional vs non-emotional condition Emotional vs neutral distractors |
-Emotional vs non-emotional condition: ↓bilateral DLPFC, DMPFC, right middle cingulate gyrus, and right middle temporal gyrus -Emotional vs neutral distractors: ↑left DLPFC, VLPFC, ACC, inferior parietal lobule, and left insula |
| Xiao et al. (2021a) ¶ | -16 BD, subtype not specified, manic [63%; 14.9 (1.9)] -18 BD subtype not specified, euthymic [50%; 15.1 (1.7)] -17 HC [59%; 14.2 (1.6)] |
-Affective GNG (66.7% Go; 33.3% No-Go) | No difference in commission error rate | Emotional vs neutral distractors | -Euthymic BD had ↑right DLPFC, left inferior parietal lobule, and left insula |
| Affective Go/No-Go Task in BD during a mood episode | |||||
| Elliott et al. (2004) | -8 BD; subtype not specified, 7 manic, 1 hypomanic [50%; 33.5 (SD not reported)] -11 HC [72.7%; 37.6 (9.7)] |
-Equiprobable affective GNG -8 conditions with different emotional and nonemotional words |
No difference in commission error rate | Compared different Go/No-Go conditions against one another | -All conditions vs rest: ↓left middle frontal gyrus. -Emotional vs neutral targets: ↑R inferior occipital gyrus, L OFC, L temporal gyrus, R middle frontal gyrus, and superior frontopolar region -Happy vs sad targets: no difference -Sad vs neutral distractors: ↑right DLPFC and VLPFC -Happy vs neutral distractors: ↑bilateral DLPFC and subgenual cingulate extending to medial OFC. |
| Hummer et al. (2013) § | -30 BD (I/II) manic [63%; 34 (11)] -30 BD (I/II) depressed [60%; 35 (11)] -14 BD (I/II) euthymic [71%; 31 (11)] -30 HC [63%; 32 (10)] |
Equiprobable affective GNG | No difference in commission error rate | No-Go condition minus Go condition | -BD across mood states generally had ↑frontal and insular regions during affective conditions of the task than HC, particularly when inhibiting to sad stimuli -Depressed and manic BD had ↓insula than euthymic BD when inhibiting to happy face and ↑putamen when inhibiting to sad faces -Depressed BD and manic BD had similar activity during affective GNG |
| Xiao et al. (2021a) ¶ | -16 BD, subtype not specified, manic [63%; 14.9 (1.9)] -18 BD subtype not specified, euthymic [50%; 15.1 (1.7)] -17 HC [59%; 14.2 (1.6)] |
-Affective GNG (66.7% Go; 33.3% No-Go) | No difference in commission error rate | Emotional vs neutral distractors | -Manic BD had ↑right DLPFC, left inferior parietal lobule -Manic BD had ↑left superior frontal gyrus compared with euthymic BD |
this study scanned BD patients during mania and again when they remitted
descriptives were reported for individuals with and without childhood trauma separately, present values are calculated pooled mean and standard deviation
This study compared euthymic, depressed, and manic individuals with BD
This study included manic and euthymic children with BD. BD=bipolar disorder; HC=healthy controls; OFC=orbitofrontal cortex; PFC=prefrontal cortex; VLPFC=ventrolateral prefrontal cortex; ACC=anterior cingulate cortex; SMA=supplementary motor area; IFC=inferior frontal cortex; STN=subthalamic nucleus; DLPFC=dorsolateral prefrontal cortex; DMPFC=dorsomedial prefrontal cortex
Table 2.
Summary of neuroimaging studies reviewed that used the Stop-Signal Task in bipolar disorder.
| Study | Sample, mood state, subtype [% female; mean (SD) age] |
Task Characteristics | Main behavioral findings |
Neuroimaging contrast | Main neuroimaging findings [activation in BD relative to HC unless otherwise specified] |
|---|---|---|---|---|---|
| Stop Signal Task in BD during euthymia | |||||
| (Leibenluft, 2007) | -26 children with BD (subtype not specified, 5 hypomanic, 21 euthymic; 15 had comorbid ADHD) [54%; 13.6 (2.6)] -17 HC [47%; 14.6 (1.8)] |
SST, adjusted SSD | No difference in SSRT | -stop incorrect vs go trials -stop correct vs stop incorrect trials | -Stop incorrect vs go: ↓bilateral caudate, putamen, accumbens, and right ventral PFC -Stop correct vs stop incorrect: ↑right ventral PFC -BD with ADHD had ↑right putamen and right ACC than BD without ADHD |
| Weathers et al. (2012) | -16 Child BD (13 BD-I, 3 BD-II; 12 euthymic, 4 hypomanic) [50%; 14.65 (2.19)] -23 Adult BD (14 BD-I, 9 BD-II; 11 euthymic, 8 depressed, 1 hypomanic, 1 mixed) [69.6%; 40.85 (11.81)] -21 Child HC [42.9%;13.79 (1.97)] -29 Adult HC [62.1%; 35.18 (8.06)] |
SST, adjusted SSD | -BD had lower accuracy than HC -No difference in SSRT |
-stop-incorrect vs go -stop-correct vs go -stop-correct vs stop-incorrect |
-Stop-incorrect vs Go: child BD had the lowest activation in ACC; adult BD had ↑left ACC than adult HC -Stop-correct vs go: BD had trend toward ↓nucleus accumbens (p = 0.06 after correction), BD had ↓left VLPFC compared with HC -Stop-correct vs stop-incorrect: no difference between groups |
| Deveney et al. (2012b) | -32 BD; 28 BD-I, 4 BD-II; 19 euthymic, 9 hypomanic, 1 manic, 2 mixed [53%; 14.2 (2.5)] 21 HC [38%; 13.8 (2.0)] |
SST, adjusted SSD | No difference in SSRT or accuracy on stop trials | -stop correct minus go -stop correct minus stop incorrect -stop incorrect minus go |
-stop incorrect minus go: ↓right ACC and right nucleus accumbens |
| Deveney et al. (2012a) | -19 youths with BD euthymic, 17 BD-I, 2 BD-II [63.2%, 14.76 (2.9)] -13 at-familial-risk youths [53.8%; 13.46 (1.8)] -21 HC [38.1%; 13.78 (2.0)] |
SST, adjusted SSD | No difference in SSRT or accuracy on stop trials | -stop incorrect vs stop correct -stop correct vs go -stop incorrect vs go |
-stop correct versus go: no difference between groups -stop incorrect vs stop correct: at risk and BD had ↑left putamen than HC -stop incorrect vs go: at-risk had ↑bilateral putamen compared with HC; at-risk had ↑right putamen compared with BD; BD had ↓right nucleus accumbens compared with HC and at-risk |
| Tsujii et al. (2018) (fNIR study) | -21 BD-I (mean mild HAMD and remitted YMRS) [57.1%; 36.9 (10.2)] -20 SZ [55%; 33.6 (8.7)] -18 HC [55.6%]; 36.6 (10.7)] |
SST, adjusted SSD | -SZ had longer SSRT than HC -No difference between BD and HC -No difference between BD and SZ |
SST vs pre-task | -BD and SZ had ↓bilateral middle frontal, inferior frontal, and superior frontal gyri compared with HC -BD had ↓middle temporal and superior temporal gyri compared with SZ and HC -Greater SSRT was correlated with reduced oxygenated haemoglobin in the right IFC and superior frontal gyrus. |
| (Cao, 2022)(Leibenluft, 2007) | -49 BD-I (mood state not specified) [43%; 35.3 (9.0)] -89 HC [45%; 35.0 (8.1)] |
SST, adjusted SSD | -No difference in % successful stops | -successful stop and unsuccessful stop vs go | -No group difference in activation |
| Kopf et al. (2019) (NIRS study)* | -36 BD (subtype not specified) depressed, 15 of which were re-assessed after remission [47.2%; 42 (11.2)] -30 HC [66.7%; 42.3 (10.7)] |
SST and Go/No-Go Task combined, adjusted SSD | -BD depressed had longer SSRT than HC -When BD remitted, no difference in SSRT from HC |
ANOVA with levels go, no-go, successful inhibition, unsuccessful inhibition | -During successful inhibition, remitted BD had ↓bilateral prefrontal cortex |
| Stop Signal Task in BD during a mood episode | |||||
| Strakowski et al. (2008) | -16 BD-I in first manic or mixed episode [25%; 19 (4)] -16 HC [44%; 20 (4)] |
-SST -no adjustment of SSD |
No difference in % correct stops | correct stops vs nontargets | -↓left ACC, medial dorsal thalamus, precuneus, and left middle temporal gyrus -↑de-activation in bilateral posterior cingulate -BD had prefrontal activation in BA10 that was not observed in HC |
| Cerullo et al. (2009) | -11 adolescent BD-I manic [64%; 14.2 (1.5)] -10 ADHD 30%; 14.0 (2.0)] -13 HC [46%; 14.5 (1.9)] |
CPT-X with stop signal, no adjustment of SSD | No difference in total accuracy | correct stops vs correct nonresponse to nontargets | -↓cerebellar vermis, parahippocampal gyrus, hippocampus, amygdala, postcentral gyrus, precentral gyrus, fusiform gyrus, inferior temporal gyrus -↑middle and superior temporal gyrus -Compared with ADHD, BD had ↓superior temporal gyrus, insula, cuneus, posterior cingulate, middle occipital gyrus, lingual gyrus |
| Passarotti et al. (2010) | -15 BD (5 BD-I manic, 5 BD-1 mixed, 5 BD-II hypomanic) [53%; 13.2 (2.65)] -11 ADHD [45%; 13.09 (2.7)] -15 HC [53%; 14.13 (3.16)] |
-SST -no adjustment of SSD |
BD had lower accuracy on go and stop trials than HC | stop vs go condition | -↓right medial and left middle frontal gyrus, left inferior/middle frontal gyrus, right pregenual ACC -↑left superior temporal gyrus and inferior parietal lobule and right posterior cingulate cortex -Compared with ADHD, BD had ↑bilateral IFC/VLPFC, bilateral middle frontal gyrus and DLPFC, right superior frontal gyrus, right middle temporal cortex, left posterior cingulate gyrus -Compared with ADHD, BD had ↓occipital cortex and left postcentral gyrus |
| Pavuluri et al. (2010) | -13 BD; 8 BD-I, 5 BBD-II, mixed, manic, or hypomanic [23%; 14.4 (2.2)] -13 HC [70%; 14.4 (2.8)] |
-SST -SSD at 250, 350, and 450 ms with equal probability |
-No difference in accuracy for Go or Stop trials | Stop vs go condition | -↓Left IFG, bilateral medial frontal gyrus, right middle frontal gyrus -↑bilateral motor cingulate, right ventral premotor cortex and striatum |
| Kopf et al. (2019) (NIRS study)* | -36 BD (subtype not specified) depressed, 15 of which were re-assessed after remission [47.2%; 42 (11.2)] -30 HC [66.7%; 42.3 (10.7)] |
SST and Go/No-Go Task combined, adjusted SSD | -BD depressed had longer SSRT than HC -When BD remitted, no difference in SSRT from HC |
ANOVA with levels go, no-go, successful inhibition, unsuccessful inhibition | -No difference between depressed BD and HC -During successful inhibition, remitted BD had ↓in left prefrontal cortex than during their depressed state. |
This study followed BD patients during depression and re-assessed them after remission; BD=bipolar disorder; HC=healthy controls; SST=Stop Signal Task; SSD=Stop Signal Delay; NIRS=near infrared spectroscopy; PFC=prefrontal cortex; ACC=anterior cingulate cortex; IFC=inferior frontal cortex; VLPFC=ventrolateral prefrontal cortex; DLPFC=dorsolateral prefrontal cortex
3.2. Refraining from action initiation
Table 1 summarizes studies using the GNG to examine neural correlates of refraining from action initiation in BD. Most analyzed the No-Go condition/trials minus Go condition/trials contrast, which captures brain activity related to response inhibition in contrast to other cognitive and motor processes associated with the task. However, other studies compared No-Go with rest or different affective and nonaffective conditions to one another.
3.2.1. Nonaffective Go/No-Go in BD
Studies using nonaffective GNG comparing No-Go and Go conditions or trials have reported an overall pattern of less activation among euthymic adults with BD compared to healthy controls in key brain regions associated with refraining from action initiation, including the inferior frontal gyrus (Ajilore et al., 2015; Joshi et al., 2016; Townsend et al., 2012, 2013), insula (Joshi et al., 2016), dorsolateral prefrontal cortex (Townsend et al., 2013), and parietal cortex (Joshi et al., 2016; Townsend et al., 2013). Studies have also reported reduced activation in other areas including the amygdala (Kaladjian et al., 2009a, 2009b), putamen (Kaladjian et al., 2009a; Townsend et al., 2012), and cingulate (Joshi et al., 2016). These findings were observed despite comparable task performance between individuals with BD and healthy controls and do not appear to be appreciably altered by psychotropic medications (Townsend et al., 2012). Studies in children have produced mixed findings. One study also found underactivation of the inferior frontal gyrus and putamen, but only when comparing correct No-Go trials minus Go trials (Metcalfe et al., 2016). When comparing incorrect No-Go trials minus Go trials, they found increased activation in the inferior frontal gyrus, dorsolateral prefrontal cortex, and cingulate. Another study in children with BD during euthymia examining the No-Go minus Go contrast found increased dorsolateral prefrontal cortex activation (Singh et al., 2010). Other studies that compared the No-Go condition with rest or neutral conditions reported no activation differences in full brain analysis (Welander-Vatn et al., 2013) and region of interest analysis (Quidé et al., 2018; Welander-Vatn et al., 2013; Welander-Vatn et al., 2009).
Several fMRI studies investigated the relationship between mood state in BD and brain activation during performance of a non-affective GNG. These results suggest that individuals with BD experiencing mania also have less activation compared to healthy controls in prefrontal regions, including the lateral and orbitofrontal cortices, as well as the putamen (Altshuler et al., 2005; Kaladjian et al., 2009a; Mazzola-Pomietto et al., 2009). Similarly, depressed individuals with a diagnosis of BD-II had less activation compared with healthy controls in the inferior frontal gyrus as well as middle frontal gyrus, superior frontal gyrus, insula, and precentral gyrus (Penfold et al., 2015). In contrast, two studies found greater activation in depressed BD: one in bilateral ventrolateral prefrontal cortex during Go and No-Go conditions (Diler et al., 2013) and another in anterior cingulate during No-Go condition (Diler et al., 2014), but neither study investigated No-Go and Go contrasts. Individuals with BD in a mixed mood episode were found in a preliminary study to have increased activity in the frontal lobe and amygdala compared with healthy controls and BD in a depressive episode (Fleck et al., 2011).
3.2.2. Affective Go/No-Go in BD
Studies have also used affective GNG tasks to determine whether emotional stimuli might influence refraining from action initiation in BD. In euthymic BD, investigation of the No-Go minus Go contrast revealed greater activation in the inferior frontal gyrus, insula, and orbitofrontal cortex (Hummer et al., 2013). Comparison of emotional versus nonemotional distractors revealed greater activity in prefrontal cortex, cingulate cortex, parietal cortex and insula (Wessa et al., 2007; Xiao et al., 2021b, 2021a). Examination of mood state effects indicates that individuals with BD during mania and depression also exhibit abnormalities similar to those seen in euthymic BD individuals during the affective GNG (Elliott et al., 2004; Hummer et al., 2013). When comparing groups of individuals with BD in different mood episodes, both depressed and manic BD had lower insula activity compared with euthymic BD when having to inhibit to happy faces and greater activity in the putamen when having to inhibit to sad faces (Hummer et al., 2013). A recent study in children found greater left superior frontal gyrus activation in a group of children with BD in during a manic episode mania compared to a group of children with BD during euthymia when inhibiting emotional versus neutral distractors (Xiao et al., 2021a).
3.2.3. Summary of Go-No/Go in BD
Taken together, findings suggest that in BD the ability to refrain from action initiation involving non-affective stimuli is associated with under-activation of key brain regions implicated in this process, and for affective stimuli these regions may be over-activated. These results are generally consistent across mood states in BD compared with healthy controls. A few studies assessing mood state in BD reported greater activation during mania compared with depression in the thalamus, cerebellum, and right inferior frontal cortex in the non-affective GNG (Fleck et al., 2011). In one study, greater activity in the left superior frontal gyrus in mania compared with euthymia was found when inhibiting to emotional versus neutral distractors (Xiao et al., 2021b) and lower insula activity was found in depression and mania when compared with euthymia when inhibiting to happy faces (Hummer et al., 2013). Overall, these findings are consistent with theoretical and empirical evidence of abnormal functional connectivity between frontal and limbic regions intrinsically and during emotional processing and regulation (Magioncalda and Martino, 2021; Maletic and Raison, 2014; Phillips and Swartz, 2014). Higher activation when inhibiting responses to affective stimuli in BD could potentially represent a compensatory mechanism in an attempt to successfully perform these tasks.
3.3. Stopping an ongoing action
Although an important and distinct component of rapid-response impulsivity, there are substantially fewer studies investigating the ability to stop an ongoing action in BD compared to refraining from action initiation. Similar to studies of refraining from action initiation, neuroimaging studies of stopping an ongoing action using the SST in BD have reported brain activation abnormalities even in the absence of performance deficits (Table 2). In predominantly euthymic BD samples, studies reported lower activation in the ventrolateral prefrontal cortex (Tsujii et al., 2018; Weathers et al., 2012), prefrontal cortex more broadly (Kopf et al., 2019), and nucleus accumbens (Deveney et al., 2012b, 2012a; Leibenluft, 2007; Weathers et al., 2012) compared with healthy controls, with less activation in the right inferior frontal gyrus being related to longer SSRT (Tsujii et al., 2018). On failed inhibitory trials compared with go trials, children with BD demonstrated less right ventral prefrontal and bilateral striatal cortex activation compared to controls (Leibenluft, 2007). There are mixed findings regarding the ACC; one study found higher ACC activity (Weathers et al., 2012) while another found lower activity in that region (Deveney et al., 2012b). Finally, a recent study did not find differences in activation across all voxels in the brain when comparing BD (and other disorders) with healthy controls (Cao, 2022).
Individuals with BD experiencing mania demonstrate less activation in the thalamus (Strakowski et al., 2008), pre- and post-central gyri (Cerullo et al., 2009), inferior frontal gyrus and dorsolateral prefrontal cortex (Passarotti et al., 2010; Pavuluri et al., 2010) concurrent with higher activation in Brodmann Area 10 (Strakowski et al., 2008), temporal regions (Cerullo et al., 2009; Passarotti et al., 2010), parietal cortex (Passarotti et al., 2010), and right ventral premotor cortex and striatum (Pavuluri et al., 2010). Mixed findings have again been reported in the cingulate, with one study finding less activity in anterior and posterior cingulate (Strakowski et al., 2008), another study finding less activity in pregenual ACC, but higher activity in posterior cingulate (Passarotti et al., 2010), and a third study finding higher activity in the motor cingulate (Pavuluri et al., 2010). Thus, stopping an ongoing action in BD during mania may involve compensatory activation in frontopolar and posterior cingulate regions. The one study that examined BD patients while in a depressed state reported no differences from HC, but found that during successful inhibition, depressed BD had higher left prefrontal cortex activity compared to their remitted state (Kopf et al., 2019).
3.4. Choice impulsivity
Our search did not return any fMRI or FNIRS studies in BD that used a task-based DDT, which limits our understanding of the neurocircuitry of choice impulsivity in BD. Studies of reward processing in BD may provide some insight, and suggest abnormal activation in frontal and limbic regions during euthymic and mood states; however, findings are mixed with regard to whether activation is abnormally higher or lower (e.g., Dutra et al., 2015; Mason et al., 2014). There is a complex relationship between reward processing and frontostriatal network activity in BD that may be influenced by differential functional connectivity among frontal and striatal regions. For example, in BD there is lower functional connectivity between the ventral striatum and anterior prefrontal cortex during anticipation of reward (Schreiter et al., 2016) and less suppression of reward-related activation in the ventral striatum when having to reject immediate rewards in favor of a long-term goal (Trost et al., 2014). However, these studies only provide preliminary insights and do not represent choice impulsivity in BD given there is no decision-making between immediate smaller rewards and later larger rewards.
3.5. Neural correlates of impulsivity in BD risk and over the course of BD
Comparison of individuals at risk for BD, youth with BD, and adults with BD can inform the trajectory of impulsivity and the underlying brain mechanisms over the course of BD. One study directly compared unaffected youth at familial risk for BD, euthymic youth with BD, and healthy controls using a Stop Signal Task and fMRI (Deveney et al., 2012a). While groups did not differ on behavioral performance, at-risk youth had higher activation in the putamen compared to HC and BD. Another study also examined youth at risk for BD (Kim et al., 2012), although they used a task that required subjects to change their pre-potent response to another response if a stimulus appeared, which taps into cognitive flexibility, in addition to inhibition. While the task did not meet criteria for formal inclusion in this review, their findings also indicate increased brain activation during inhibition in individuals at risk for BD, particularly in the right inferior parietal lobe, right ventrolateral prefrontal cortex, and striatal areas compared with HC. These findings suggest risk for BD is associated with brain activation abnormalities underlying inhibition, possibly reflecting compensatory activity or a marker of impairment prior to BD onset.
One study directly compared child BD with adult BD (Weathers et al., 2012) by examining children and adults with BD as well as children and adult HCs during a Stop Signal Task. The majority of patient samples were in a euthymic mood state. During inhibition, child BD showed the least activity in anterior cingulate cortex while adult BD showed greater activity in this region than adult HC. There was also less left ventral prefrontal cortex activity in BD compared with HCs across age groups suggesting an abnormality that is consistent over the course of BD. These results suggest that cortical dysfunction is present already in childhood BD and that there may be some changes in neural dysfunction in the anterior cingulate cortex over the course of illness.
Taken together, these data suggest that risk for BD is associated with more activation in inferior frontal gyrus/ventrolateral prefrontal cortex, parietal lobe, and striatum during inhibition, which may reflect compensatory activity and/or otherwise be a risk marker. Abnormalities in the anterior cingulate cortex and ventral prefrontal cortex appear to already be present in childhood BD. It should be acknowledged, however, that longitudinal studies are needed to better characterize neural abnormalities associated with inhibition in BD that follow at-risk youth who subsequently develop BD.
3.6. Effects of BD subtype and medication
Some evidence suggests that BD subtypes have different alterations in brain function (Liu et al., 2022; Thomas et al., 2019), but few studies in the present review investigated differences in BD subtype. One study examined BD-II and did not find any difference in dorsal anterior cingulate cortex or pre-supplementary motor area (Welander-Vatn et al., 2009) using ROI analysis. However, Penfold et al. (2015) did find less activation in frontal and temporal lobes using whole brain analysis in BD-II patients on a nonaffective GNG task, which was similar to findings in BD-I. Several studies with samples that included both BD-I and BD-II participants performed subgroup analyses comparing the two subtypes and none found any differences in brain activation associated with inhibition between the two subtypes (Diler et al., 2014, 2013; Hummer et al., 2013; Wessa et al., 2007). These findings are consistent with a review reporting no evidence of differences between BD-I and BD-II in fMRI studies (McGrath et al., 2004). However, the sample sizes for subtype analyses were small and future studies should use more adequately powered samples to properly address this question.
The effect of medication is an important issue given there is evidence that medications alter brain activation in individuals with BD. An early study found that while unmedicated patients exhibited neural activation abnormalities during response inhibition compared to HC, the medicated patients did not (Leibenluft, 2007). However, this finding has not been replicated in later studies that sought to address this potential confound in various ways. Some studies directly compared a subset of medicated versus unmedicated patients and found no difference in neural activation (Townsend et al., 2012; Welander-Vatn et al., 2009). Other studies compared only a subset of unmedicated BD patients (Altshuler et al., 2005; Townsend et al., 2012) or only a subset of medicated BD patients (Weathers et al., 2012) with HC, and found similar findings as with the overall BD group. Finally, some studies included only unmedicated patients (Cerullo et al., 2009; Hummer et al., 2013; Passarotti et al., 2010, 2010; Penfold et al., 2015) to minimized confounds associated with medications. These studies showed abnormalities largely consistent with medicated or mixed samples that used the same task.
Some studies compared subgroups taking different types of medication and found no difference between those medicated with mood stabilizers, anticonvulsants, antipsychotics, or antidepressants (Kaladjian et al., 2009b; Mazzola-Pomietto et al., 2009; Wessa et al., 2007). Each study found that the individual subgroups showed similar abnormalities that were evident when the medication groups were combined. Two studies did find differences based on medication exposure, but none that overlapped with findings of differential activation from HC (Singh et al., 2010; Strakowski et al., 2008). Finally, one of the studies in this review specifically examined the effect of psychotropic treatment (initially antipsychotics followed by lamotrigine) on neural activation during inhibition and found that medication attenuated the abnormal decrease in prefrontal regions (Pavuluri et al., 2010), demonstrating a normalizing effect of medications.
Overall, studies in this review suggest that there may be general effects of medication exposure on brain activation and normalizing effects on brain activity related to impulsivity in BD. Therefore, medication is unlikely to account for the present findings. Furthermore, the finding that most of the patients in the studies are medicated and nonetheless exhibit abnormal activation during inhibition suggests that the abnormality is robust enough to be detected despite the attenuating effect of medication. This is consistent with reviews that have generally found that medication effects on neuroimaging studies in BD, when present, tend to ameliorate abnormal neuroimaging measures and do not explain the main findings in the bipolar fMRI literature (Hafeman et al., 2012; Phillips et al., 2008).
4. Summary and integration with existing models of BD and impulsivity
Impulsive traits in BD can manifest behaviorally as difficulty refraining from action initiation, stopping an ongoing action, and delaying gratification, all of which have important conceptual and neurobiological distinctions. Refraining from action initiation has been most commonly assessed in BD using the GNG. Although most studies using fMRI did not find impaired behavioral performance, functional neuroimaging studies suggest there are abnormalities in brain activation characterized by under-activation of frontopolar, inferior frontal, parietal, insular, and basal ganglia regions, but over-activation of this circuitry when a GNG task involves emotional stimuli. This pattern is evident among BD individuals while euthymic, depressed, manic or in a mixed mood state. These regions partly comprise the central executive (or lateral frontoparietal) network, which is thought to direct attention to relevant stimuli, weigh choices depending on context, and maintain relevant information, thus requiring working memory and sustained attention (Seeley et al., 2007). There is also evidence of a mood-congruent affective bias in which depressed BD patients exhibit the greatest brain activation when refraining from responding to sad faces compared with inhibiting responses to happy faces. These results suggest a trait-like abnormality in neurocircuitry activation that is differentially affected by the presence of emotional stimuli.
Stopping an ongoing action has been most commonly assessed using the SST in BD. Although a distinct and important aspect of rapid-response impulsivity, there are much fewer studies of stopping an ongoing action than refraining from action initiation in BD. There are mixed results regarding whether individuals with BD exhibit greater difficulty with stopping an ongoing action, with some studies reporting no difference between BD and healthy controls and others reporting greater difficulty, even during euthymia (Table 2). Regardless of behavioral performance, neuroimaging data converge to implicate less activation in BD than healthy controls in brain regions typically associated with stopping an ongoing action, including ventrolateral, striatal, cingulate, and thalamic regions, which is evident even during euthymia. Therefore, as in refraining from action initiation, there appears to be trait-like neurocircuitry abnormalities in BD when trying to stop an ongoing action.
Of note is the consistent finding of comparable behavioral task performance between BD and HC despite abnormal brain activation between groups. There are several reasons for the lack of difference in behavioral performance on tasks used during MRI scanning. When designing these tasks for use in clinical groups, they cannot be overly difficult or else risk having the patients perform very poorly or become too frustrated, thus potentially confounding the BOLD signal. Furthermore, functional neuroimaging studies typically do not include patients who are severely impaired, for whom we might normally see the greatest differences in performance compared with healthy controls. These subjects may move excessively, have difficulty understanding the task instructions, or perform too poorly to reliably interpret their BOLD signal. Also, some tasks are specifically designed to individually tailor the task difficulty so that performance reaches the same specified level on a subject-by-subject basis. For example, the stimulus presentation timing on the SST is often individually tailored so that accuracy is always 50%. A goal of many research teams is to create neuroimaging tasks that lead to roughly equivalent performance across groups so that any between-group differences in BOLD cannot be simply attributed to performance deficits. Rather, any functional brain differences observed despite comparable task performance suggests a difference in the underlying neural mechanism of the disorder itself.
Although some behavioral studies suggest individuals with BD have steeper delay discounting (see Amlung et al., 2019), our search did not yield any studies that used a delay discounting task during functional neuroimaging in BD. The ability to delay gratification relies on a complex neural network that include orbitofrontal regions implicated in cognitive control, the nucleus accumbens of the ventral striatum associated with reward processing, and modulation of these areas via the connecting accumbofrontal white matter tract (Karlsgodt et al., 2015). Despite the lack of search results, studies that examined reward processing during fMRI suggest that in BD there is hyperactivation in orbitofrontal cortex and the nucleus accumbens, and less functional connectivity between ventral striatal and prefrontal regions during reward anticipation and receipt. Disruption of this reward processing network characterized by enhanced sensitivity to rewards and an inability to modulate prefrontal executive control systems for reward valuation may contribute to choice impulsivity in BD.
The neural circuits involved in rapid-response impulsivity and choice impulsivity have significant overlap with those proposed to be disrupted in BD, namely the dorsal and orbitofrontal cortices, inferior frontal gyrus, insular cortex, basal ganglia, thalamus, and cingulate. These brain regions are known to be involved in the regulation of emotion and cognition, and are featured prominently in theoretical and empirical neurobiological models of BD (Magioncalda and Martino, 2021; Phillips and Swartz, 2014). An important mechanistic feature of these models is an emphasis on abnormalities in neural networks and their connecting white matter tracts (Mahon et al., 2010) in contrast to individual brain regions. The prefrontal cortex is essential for cognitive control and modulation of thoughts, emotions, and behavior (Miller and Cohen, 2001; Ott and Nieder, 2019) including the inhibition of automatic behavior when it is discordant with task goals. This type of higher order regulation requires a wide array of functions including representation of goals, maintenance of task rules, and communication with other brain structures (Gratton et al., 2018; Miller and Cohen, 2001). Disrupted prefrontal cortical modulation of subcortical and limbic structures involved in automatic affective and cognitive processes may contribute to behavioral impulsivity in BD. A working model of neural circuits involved in different forms of behavioral impulsivity is provided in Fig. 1.
An important caveat of these models is that the specific mechanism by which the prefrontal cortex mediates inhibition, particularly the role of the inferior frontal gyrus in rapid-response inhibition, is hotly debated. Although there is some evidence for an inhibitory module in the inferior frontal gyrus that initiates stopping behavior (Aron et al., 2003), other data attribute more general roles to this region involving attention, encoding task rules, or monitoring (Xu et al., 2017). Brain regions implicated in refraining from action initiation appear underactive in BD compared to healthy controls consistent with a model of lower prefrontal modulation of subcortical structures resulting in less inhibitory control. In stopping an ongoing action, however, the relative role of the inferior frontal gyrus, subthalamic nucleus, and pre-SMA may be less clear. It has been proposed that the inferior frontal gyrus itself is a brake that triggers the pre-SMA perhaps via the subthalamic nucleus of the basal ganglia (Aron et al., 2014), while others argue that the inferior frontal gyrus signals the pre-SMA to initiate the stopping (Xu et al., 2017) Using dynamic causal modeling, Rae and colleagues (2015) estimated effective connectivity between these regions during a SST and found that the best performing model was one in which the inferior frontal gyrus modulated (via excitation) the excitatory influence of the pre-SMA on the subthalamic nucleus, thus amplifying the inhibitory influence of the subthalamic nucleus on the motor cortex. Under-activation of prefrontal cortical regions in BD assessed while refraining from action initiation and stopping an ongoing action coupled with overactivity of this region in choice impulsivity, points to dysfunction in the cognitive control facet of the top-down modulatory network.
Given substantial mood fluctuations characteristic of BD and the close relationship between cognition, emotion, and their underlying brain systems, the role of emotion may be particularly important for understanding the pathophysiology of behavioral impulsivity in BD. The model of deficient prefrontal modulation of limbic regions is proposed to result in hyperactivation of fronto-limbic regions leading to the affective symptoms seen in BD (Magioncalda and Martino, 2021; Phillips and Swartz, 2014), consistent with higher activation in the frontoparietal network during refraining from action initiation in an affective context. Individuals with BD are hypersensitive to emotional stimuli (Henry et al., 2012) and it is possible that a greater increase in frontal and insular regions during affective GNG performance reflects greater recruitment of control mechanisms to inhibit hypersensitive emotional responses to achieve task goals. This model is also consistent with circuits involved in choice impulsivity in which there is over-activation of fronto-limbic regions including the ventral prefrontal cortex and nucleus accumbens. Potentially increased reward sensitivity in BD is likely associated with the choice to seek reward as soon as possible on delay discounting tasks. At present it is unclear how affective contexts may influence neural activity on stopping an ongoing action and although emotional SST paradigms have been developed (Yang et al., 2021), they have not been used in studies of BD. The over-activation across key regions in choice impulsivity is similar to that observed during affective GNG tasks in BD, suggesting a common mechanism in rewarding or emotionally salient contexts.
The pattern of aberrant neural circuit activation observed during behavioral impulsivity tasks in BD are generally similar across manic and depressive mood states. These findings are consistent with the presence of self-reported trait impulsivity in BD that predisposes individuals to impulsive behavior regardless of mood state. It should be acknowledged, however, that few studies have examined the specific association of mixed episodes with functional imaging paradigms, although there is some preliminary evidence for hyperactivation in fronto-limbic brain regions during a mixed-mood state in contrast to manic and depressive states (Fleck et al., 2011).
5. Comparison of impulsivity in BD and other psychiatric disorders
A critical question is whether the underlying neural basis of impulsivity in BD can be differentiated from those of other psychiatric disorders in which impulsivity is also a hallmark feature, such as borderline personality disorder (PD), attention-deficit/hyperactivity disorder (ADHD), and substance use disorders (SUD). However, this question is complicated by the high rates of comorbidity between BD and both ADHD and SUD, as well as the diagnostic overlap between BD and borderline PD. Nonetheless, findings in borderline PD, ADHD, and SUD compared with BD may have important implications for determining unique neural signatures of BD associated with impulsivity to inform the pathophysiology and treatment of BD.
Some studies in borderline PD during nonaffective rapid-response impulsivity tasks found no difference in brain activation from healthy controls (Jacob et al., 2013; Mortensen et al., 2010), while others found less activation in orbital (Silbersweig et al., 2007) and ventrolateral frontal regions (Ruocco et al., 2021). Jacobs and colleagues (2013) found that after induction of anger, BPD patients had lower activation of the left inferior frontal gyrus and greater activation of sub-thalamic nucleus. During affective rapid-response impulsivity tasks, individuals with borderline PD exhibit activation that is higher in some lateral frontal, parietal, basal ganglia, and medial temporal regions (Silbersweig et al., 2007; Soloff et al., 2015; van Zutphen et al., 2020) and in ventral medial and orbitofrontal cortex (Silbersweig et al., 2007; Soloff et al., 2015). Overall, evidence suggests that dysfunction in neurocircuitry implicated in rapid-response impulsivity may be more ubiquitous across contexts and mood states in BD than in borderline PD. Furthermore, BD is characterized by overactivation of ventral and dorsal lateral prefrontal cortex, anterior cingulate cortex, and parietal regions while borderline PD show heightened activity in the lateral orbitofrontal cortex, ventrolateral and dorsolateral prefrontal cortex, basal ganglia, hippocampus, amygdala, and parietal regions coupled with lower activity in some areas of the medial orbitofrontal cortex and anterior cingulate cortex. There are no studies, to our knowledge that have compared BD and borderline PD directly on these measures, which would be an important topic in future research.
Results from studies that directly compared BD with ADHD have been mixed. Cerullo et al. (2009) found that ADHD had greater activation in the superior temporal gyrus, insula, and posterior cingulate compared with BD. Deveney et al. (2012) also found that ADHD youth had a trend toward higher activation in the ACC compared with BD youth. In contrast, Passarotti et al. (2010) generally found that ADHD patients exhibited less activation than BD patients in prefrontal regions (i.e., inferior, middle, and superior frontal gyri), middle temporal cortex, and posterior cingulate cortex. In a study that included individuals with BD alone, ADHD alone, comorbid BD/ADHD, and healthy control samples, Townsend and colleagues (2013) found that BD adults exhibit lower activation while comorbid BD/ADHD had enhanced activation relative to controls in executive circuitry. Although visual inspection of their data suggests that BD had greater activation than ADHD (see (Townsend et al., 2013), Fig. 1), a firm conclusion cannot be drawn because there was no direct statistical comparison between these two groups. A potentially important neurobiological distinction between BD and ADHD can be made in the inferior frontal cortex. This region appears to be hyperactive in ADHD compared with healthy controls during response inhibition in non-emotional contexts (Lei et al., 2015). In contrast, in BD, inferior frontal cortex appears to be underactive during response inhibition and overactive only during response inhibition when the task involves emotionally-charged information.
Studies in SUD have examined groups of nicotine users (Lesage et al., 2020), alcohol dependent patients (Czapla et al., 2017), abstinent heroin users (Fu et al., 2008), stimulant dependent individuals (Morein-Zamir et al., 2013), and American Indians with any substance use disorder (White et al., 2022). These fMRI studies have largely used neutral stimuli in rapid-response inhibition tasks and consistently report less activation of brain regions involved in inhibition, including the inferior frontal gyrus (Fu et al., 2008; Morein-Zamir et al., 2013; White et al., 2022), dorsolateral prefrontal cortex (White et al., 2022), insula (Czapla et al., 2017; Fu et al., 2008), medial prefrontal cortex (Fu et al., 2008), parietal regions (Czapla et al., 2017; Fu et al., 2008), and anterior cingulate cortex (Fu et al., 2008; Morein-Zamir et al., 2013). One study in alcohol dependent patients also used pictures of different alcoholic beverages and found that for contrasts involving alcohol-related stimuli, these patients showed significantly increased activation in left anterior cingulate cortex and left medial frontal and medial orbital cortex (Czapla et al., 2017). These results are similar to findings in BD of decreased activation in inhibition with neutral stimuli, but higher activation with stimuli that are salient for individuals with BD (i.e., emotional stimuli). Findings suggest common neural correlates associated with impulsivity in BD and SUD, although there are no studies that directly compare these disorders. Fig. 2.
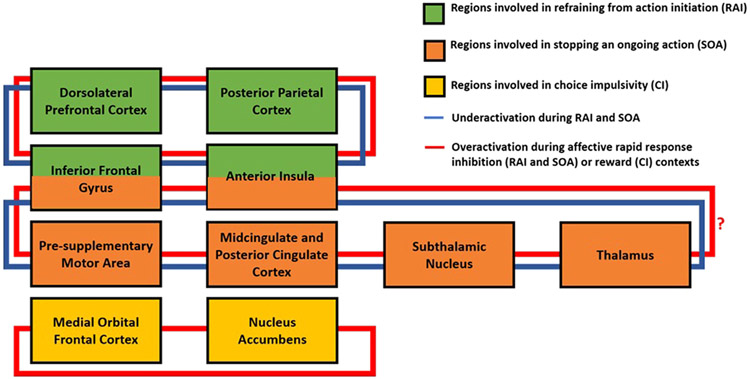
Fig. 2.
Proposed model of key regions in disrupted neural circuits contributing to impulsivity in bipolar disorder (BD) based on findings from task-based functional magnetic resonance imaging studies (see Table 1 and 2). In rapid-response impulsivity, key regions involved in refraining from action initiation resemble those of the executive control network and include dorsolateral prefrontal cortex, inferior frontal gyrus, posterior parietal cortex, and anterior insula. Key regions involved in stopping an ongoing action include the inferior frontal gyrus, anterior insula, pre-supplementary motor area, cingulate cortex, subthalamic nucleus, and thalamus. The circuit involved in refraining from action initiation is largely underactive in BD compared with healthy controls, but becomes overactivated when affective stimuli are presented. We postulate this pattern to also be evident for the neurocircuitry involved in stopping an ongoing action, although this needs to be verified by studies using affective stimuli in tasks of stopping an ongoing action (noted with a question mark). Over-activation to affective stimuli may reflect a “compensatory” mechanism in which more effort is required to engage brain regions responsible for action inhibition in the context of affective stimuli due to hypersensitivity in affective contexts. These functional neuroimaging abnormalities are generally present without associated performance deficits and evident across mood states. Decisional choice impulsivity in BD is thought to be associated with hyperactivation in the frontostriatal reward network involving the medial orbital frontal cortex and ventral striatum (including nucleus accumbens) during reward anticipation and reward receipt. Over-activation in this network may reflect difficulty in prefrontal control systems to modulate reward sensitivity resulting in reward hypersensitivity, which may drive BD individuals to seek reward as soon as possible despite the availability of a later larger reward. Not all connections among regions are provided and not all regions involved in each type of impulsivity is shown.
6. Clinical implications
Impulsivity has strong clinical significance for the phenomenology of BD given its association with destructive behaviors and clinical trajectory of worse functional outcome and high risk of suicide. Situations requiring action restraint may initially require executive control mechanisms involving the dorsolateral prefrontal cortex as part of a top-down process. This appears to be reduced over time, however, after consistent stimulus-stop associations are gradually learned through changes in basal ganglia connections (Guo et al., 2018), and accompanied by a shift toward automatic bottom-up processing involving the inferior frontal gyrus. In contrast, situations requiring action cancellation likely involve a sustained level of top-down processing over the course of the task given that stimulus-stop associations are inconsistent, thus requiring ongoing monitoring from prefrontal executive control functions to perform needed actions on an ongoing basis (Verbruggen and Logan, 2008). A deficit in executive control functions in BD may interfere with successful motor inhibition and concomitant difficulty either restraining or cancelling impulsive behavior. The successful reduction of motor impulsivity in BD may relate to learning how to downregulate overactive bottom-up processing and/or the ability to strengthen top-down functions.
Interventions targeting rapid-response impulsivity could help individuals with BD refrain from initiating an impulsive act and if already begun, to stop the ongoing action to minimize potentially negative consequences. The use of cognitive remediation to enhance consideration of options and increase mental flexibility within cognitive control networks involving the prefrontal cortex might reduce both types of motor impulsivity. Inhibitory control training using the GNG has been used to reprogram motor responses when unhealthy behaviors (e.g., alcohol use, food over-consumption) are paired with responses requiring inhibition. For example, one study showed that training heavy drinking students to repeatedly stop pre-potent responses toward alcohol-related stimuli was effective in reducing weekly alcohol intake (Houben et al., 2011). Although these studies did not examine brain activity, successful behavioral interventions in BD have been linked to changes in frontal regions that play a role in cognitive control and flexibility considered integral to models of rapid-response impulsivity (Favre et al., 2013). In BD, cognitive training that pairs impulsive behaviors (e.g., gambling, substance use) with No-Go or stop signals may have the potential to improve rapid-response impulsivity by altering the associated underlying neural circuitry.
The ability to successfully perform tasks involving action restraint and cancellation (i.e., motor impulsivity) in BD is likely influenced by hypersensitivity to emotionally salient information that could impair the required process of forming stimulus-stop associations. This is particularly relevant given that inhibitory control is often required in the context of emotionally salient information in real world situations. Hyperactivation associated with inhibition during an affective GNG task may, in part, represent compensatory activity to achieve task performance comparable to controls. This is supported by a study demonstrating that treatment with mood stabilizers or antipsychotics normalized brain activity toward “healthy” levels by decreasing overactivation during an attention task that involved emotional stimuli (Strakowski et al., 2016). Thus, treatments focused on reducing bottom-up (over)activity during emotional processing (e.g., in the amygdala) in combination with increasing cognitive control involving top-down functions (e.g., dorsolateral prefrontal cortex) may reduce the need for compensatory overactivity during response inhibition involving emotional content and improve behavioral outcome.
Choice impulsivity may also be an important therapeutic target in the treatment of BD, but has been largely neglected in the literature despite evidence that individuals with BD have the steepest delay discounting compared to other neuropsychiatric disorders (Amlung et al., 2019). Although little is known regarding the neurocircuitry underlying choice impulsivity in BD, there is evidence that impulsive behavior in response to reward may partly reflect hypersensitivity to immediate reward gratification in contrast to the explicit reward, per se. This suggests involvement of early attentional mechanisms prior to processing through the mesocorticolimbic pathway (Mason et al., 2012). Therefore, enhancing top-down prefrontal executive control systems could theoretically constrain reward processing in these dopaminergic midbrain reward networks in situations where the preference for an immediate reward is prioritized.
The application of learning-based experimental manipulations to reduce delay discounting and impulsive behavior has gained increasing attention in disorders of addiction (Rung and Madden, 2018), but applications in BD remain sparse. One potentially fruitful treatment of choice impulsivity is the use of episodic future thinking (Atance and O’Neill, 2001), which involves imagining feelings and emotions related to the future experiences of an event. Among healthy individuals, spontaneous episodic imagery during cue processing predicted changes in individuals’ preferences toward future-minded choice behavior (Peters and Büchel, 2010). Furthermore, reward delay discounting was modulated by episodic future event cues, and this was associated with valuation signals in the anterior cingulate and functional coupling between the anterior cingulate and hippocampus/amygdala complex. This suggests that reducing choice impulsivity in BD may be altered through the use of both positive (Ye et al., 2021) and future (Raucher-Chéné et al., 2021) episodic imagery to alter reward-based decision making involving the ACC to reframe choices between smaller immediate gains vs. larger delayed rewards. This may facilitate more future-oriented choices in BD in contrast to what may appear to feel good or relieve unpleasant emotions in-the-moment.
Approaches for noninvasive stimulation such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) may be used to target brain regions implicated in the neurobiology of motor and choice impulsivity deficits in healthy and psychiatric populations (Teti Mayer et al., 2020). Prior studies indicated that these interventions had a small, but positive effect on tasks of action restraint (Go/No-Go Task) and action cancellation (stop-signal task) in healthy volunteers (de Boer et al., 2021). Similarly, prior studies (Hecht et al., 2013) reported that tDCS of the dorsolateral prefrontal frontal cortex was associated with changes in delay discounting. Although repetitive transcranial magnetic stimulation (rTMS) is considered a treatment for the management of acute mania and bipolar depression (Yatham et al., 2018) in BD, little work has investigated the efficacy of targeting impulsive behavior using noninvasive brain stimulation in BD, but this approach does show promise (e.g., O’Donnell et al., 2021), and will be an important goal of future studies.
Although neurocircuitry dysfunction associated with impulsivity may be at least partly independent of mood episodes, increasing evidence suggests that emotion can impact these experiences. Individuals with BD consistently self-report elevated levels of positive and negative urgency and there is preliminary evidence of increased activation during mixed mood episodes in frontal and amygdala regions. Therefore, it may be reasonable to target emotion regulation in BD as a means toward reducing impulsivity. Although dialectical behavior therapy has efficacy in improving emotion regulation (Goldstein et al., 2015) in BD, it may have limited impact on reducing impulsive behavior overall (Zargar et al., 2019). There is therefore a need to better understand the impact of acute emotional experience on neurocircuitry during in-the-moment impulsive behavior in BD to inform potential treatment strategies.
In combination with clinical data, neural activity during state impulsivity tasks could potentially inform early disease identification and intervention efforts to mitigate clinical symptoms and reduce functional impairment. Although there is significant clinical (e.g., affective instability) and behavioral (e.g., impulsivity) overlap between BD and other highly comorbid disorders, a potentially unique neural signal in BD could serve as a biological marker to potentially assist in differential diagnosis and guide treatment options. We acknowledge that while it is not yet practical to use neuroimaging for differential diagnostic assessment, more studies are needed to compare diagnostic groups and/or utilize transdiagnostic approaches to better clarify overlapping and distinct neurobiological features associated with state impulsivity.
Understanding and targeting the relevant neurocircuitry associated with rapid-response and choice impulsivity has the potential to provide multiple ways of mitigating impulsive behaviors with negative consequences in BD, such as suicidal behavior and substance use. Although many of the approaches described in this section have been well-established in the laboratory, they will need further development to be used in real-life situations among individuals with BD. In this vein, the investigation of several approaches described herein can be easily adapted for use in smart phone applications for ongoing training. The field may also benefit from additional studies investigating distinct types of behavioral impulsivity over the course of illness.
7. Conclusions and future directions
While impulsivity is a clinical characteristic of BD and individuals with BD self-report significant impulsivity, there is increasing research using task-based fMRI to better understand the neurobiological underpinnings of impulsivity in BD. Using tasks that tap rapid-response impulsivity and choice impulsivity, neuroimaging evidence suggests that there are neural activation abnormalities associated with impulsive motor behaviors and choice in BD. These abnormalities are characterized by trait-like (i.e., persisting across mood phases of the illness) under- and over-activation of brain regions implicated in impulsive behavior and the pathophysiology of BD. Evidence suggests that during rapid-response tasks requiring inhibition, individuals with BD exhibit under-activation of key regions involved in rapid-response inhibition including frontal, insular, parietal, cingulate, and thalamic regions, but overactivation of these regions when emotional stimuli are involved in the task. While there is no study in BD using a delay discounting task with fMRI, evidence from the reward processing literature suggests that hyperactivity of orbitofrontal and striatal regions associated with reward hypersensitivity may be associated with difficulty delaying gratification.
Although we present an integrated model to test hypotheses, there are remaining challenges and questions to be addressed by future research. There is little consensus on the domains and subdomains of impulsivity relevant to BD that should be assessed by behavioral tasks. The most common self-report measures indicate at least three (Patton et al., 1995) to four (Whiteside and Lynam, 2001) dimensions of impulsivity and experts from the International Society of Research on Impulsivity have provided recommendations for assessing both rapid-response (Hamilton et al., 2015a) and choice (Hamilton et al., 2015b) impulsivity. However, task-based fMRI studies in BD have only examined rapid-response impulsivity. Inclusion of choice impulsivity tasks in fMRI studies of BD will provide a greater understanding of neurocircuitry underlying poor decision-making that could lead to maladaptive behavior. Ecological momentary assessment or the use of performance-based naturalistic tasks may capture variance in impulsivity and associations with real-world behaviors. Such tasks could provide information regarding associations between type of impulsivity and destructive behavior to target the most relevant ones.
Within rapid-response impulsivity, research indicates conceptual and empirical differences between refraining from action initiation and stopping an ongoing action with each engaging overlapping, but distinct neural systems (Cieslik et al., 2015; Hamilton et al., 2015a). However, studies in BD refer to response inhibition very broadly when using tasks measuring either construct (i.e., GNG and SST). There is a disproportionate number of studies examining refraining from action initiation using the GNG and much fewer studies examining stopping an ongoing action. The field would benefit from future work that directly compares behavioral performance and neural activation during these two types of rapid-response impulsivity in BD to determine whether neurocircuitry dysfunction in rapid-response inhibition is global or specific to certain types of motor inhibition. Furthermore, studies involving stopping an ongoing action that include emotional stimuli, such as one used recently in borderline PD (Yang et al., 2021), are sorely needed to determine whether the effect of emotions is similar across impulsivity tasks.
It should also be acknowledged that individuals with BD are a heterogeneous group regarding medication status, illness severity, and comorbidities. Although some studies attempt to control for the potential effects of these factors, the small sample sizes (Table 1 and 2) are likely insufficiently powered for sub-analyses, thus precluding any systematic evaluation of their potential influence. In the only fMRI study that compared BD with comorbid ADHD, BD without comorbid ADHD, and ADHD on rapid-response impulsivity (Townsend et al., 2013), the results provide evidence that having comorbid ADHD in BD may be associated with differential patterns of brain activation compared to BD alone while refraining from action initiation. Because impulsivity is a transdiagnostic construct future studies would benefit from including large sample sizes for transdiagnostic analyses using identical tasks and neuroimaging methods. Such studies could help clarify neurobiological pathways related to impulsive behavior in BD that might one day be used for differential diagnosis and to inform treatment.
Finally, the specific mechanism by which the purported brain networks are disrupted in BD remains to be clarified. While the predominant hypothesis is that in BD, prefrontal executive control systems are unable to modulate subcortical and limbic structures, it is unclear how this disruption occurs. The mechanism is likely to be complex and involve interactions among multiple regions within the network (Rae et al., 2015) and possibly other closely related circuits. Furthermore, moving beyond the simple examination of activation in discrete brain regions to the investigation of effective connectivity (e.g., Ajilore et al., 2015) and large-scale connectome analysis will provide a more detailed understanding of the neural mechanisms underlying behavioral impulsivity in BD.
Supplementary Material
Role of funding source
This work was supported in part by VA Merit Award grants I01CX002039 to PRS and I01CX002093 to MG and EAH. CCC is supported in part by the VISN2 Mental Illness Research, Education, and Clinical Center (MIRECC) at the James J. Peters VA Medical Center.
EAH is supported by VA CSR&D Research Career Scientist Award 1IK6CX001738.
Footnotes
Declarations of interest
None.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.neubiorev.2023.105109.
References
- Ajilore O, Vizueta N, Walshaw P, Zhan L, Leow A, Altshuler LL, 2015. Connectome signatures of neurocognitive abnormalities in euthymic bipolar I disorder. J. Psychiatr. Res 68, 37–44. 10.1016/j.jpsychires.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL, Bookheimer SY, Townsend J, Proenza MA, Eisenberger N, Sabb F, Mintz J, Cohen MS, 2005. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol. Psychiatry 58, 763–769. 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, ed. American Psychiatric Association. 10.1176/appi.books.9780890425596. [DOI] [Google Scholar]
- Amlung M, Marsden E, Holshausen K, Morris V, Patel H, Vedelago L, Naish KR, Reed DD, McCabe RE, 2019. Delay discounting as a transdiagnostic process in psychiatric disorders: a meta-analysis. JAMA Psychiatry 76, 1176–1186. 10.1001/jamapsychiatry.2019.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW, 2003. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci 6, 115–116. 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA, 2014. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn. Sci 18, 177–185. 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Atance CM, O’Neill DK, 2001. Episodic future thinking. Trends Cogn. Sci 5, 533–539. 10.1016/s1364-6613(00)01804-0. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Tondo L, 2020. Suicidal risks in 12 DSM-5 psychiatric disorders. J. Affect Disord 271, 66–73. 10.1016/j.jad.2020.03.083. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW, 2013. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog. Neurobiol 108, 44–79. 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- de Boer NS, Schluter RS, Daams JG, van der Werf YD, Goudriaan AE, van Holst RJ, 2021. The effect of non-invasive brain stimulation on executive functioning in healthy controls: a systematic review and meta-analysis. Neurosci. Biobehav Rev 125, 122–147. 10.1016/j.neubiorev.2021.01.013. [DOI] [PubMed] [Google Scholar]
- Cao H., 2022. Prefrontal-cerebellar dynamics during post-success and post-error cognitive controls in major psychiatric disorders. Psychol. Med 1–8. 10.1017/S0033291722001829. [DOI] [PubMed] [Google Scholar]
- Cerullo MA, Adler CM, Lamy M, Eliassen JC, Fleck DE, Strakowski SM, DelBello MP, 2009. Differential brain activation during response inhibition in bipolar and attention-deficit hyperactivity disorders. Early Interv. Psychiatry 3, 189–197. 10.1111/j.1751-7893.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Mueller VI, Eickhoff CR, Langner R, Eickhoff SB, 2015. Three key regions for supervisory attentional control: evidence from neuroimaging meta-analyses. Neurosci. Biobehav Rev 0, 22–34. 10.1016/j.neubiorev.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Smith GT, Spillane NS, Fischer S, Annus AM, Peterson C, 2007. Integration of impulsivity and positive mood to predict risky behavior: development and validation of a measure of positive urgency. Psychol. Assess 19, 107–118. 10.1037/1040-3590.19.1.107. [DOI] [PubMed] [Google Scholar]
- Czapla M, Baeuchl C, Simon JJ, Richter B, Kluge M, Friederich H-C, Mann K, Herpertz SC, Loeber S, 2017. Do alcohol-dependent patients show different neural activation during response inhibition than healthy controls in an alcohol-related fMRI go/no-go-task? Psychopharmacol. (Berl. ) 234, 1001–1015. 10.1007/s00213-017-4541-9. [DOI] [PubMed] [Google Scholar]
- Deveney CM, Connolly ME, Jenkins SE, Kim P, Fromm SJ, Brotman MA, Pine DS, Leibenluft E, 2012a. Striatal dysfunction during failed motor inhibition in children at risk for bipolar disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 38, 127–133. 10.1016/j.pnpbp.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveney CM, Connolly ME, Jenkins SE, Kim P, Fromm SJ, Pine DS, Leibenluft E, 2012b. Neural recruitment during failed motor inhibition differentiates youths with bipolar disorder and severe mood dysregulation. Biol. Psychol 89, 148–155. 10.1016/j.biopsycho.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diler RS, Segreti AM, Ladouceur CD, Almeida JRC, Birmaher B, Axelson DA, Phillips ML, Pan L, 2013. Neural correlates of treatment in adolescents with bipolar depression during response inhibition. J. Child Adolesc. Psychopharmacol 23, 214–221. 10.1089/cap.2012.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diler RS, Pan LA, Segreti A, Ladouceur CD, Forbes E, Cela SR, Almeida JRC, Birmaher B, Axelson DA, Phillips ML, 2014. Differential anterior cingulate activity during response inhibition in depressed adolescents with bipolar and unipolar major depressive disorder. J. Can. Acad. Child Adolesc. Psychiatry 23, 10–19. [PMC free article] [PubMed] [Google Scholar]
- Dutra SJ, Cunningham WA, Kober H, Gruber J, 2015. Elevated striatal reactivity across monetary and social rewards in bipolar I disorder. J. Abnorm Psychol 124, 890–904. 10.1037/abn0000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ, 2004. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol. Psychiatry 55, 1163–1170. 10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Favre P, Baciu M, Pichat C, De Pourtalès M-A, Fredembach B, Garçon S, Bougerol T, Polosan M, 2013. Modulation of fronto-limbic activity by the psychoeducation in euthymic bipolar patients, a functional MRI study. Psychiatry Res.: Neuroimaging 214, 285–295. 10.1016/j.pscychresns.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Fleck DE, Kotwal R, Eliassen JC, Lamy M, Delbello MP, Adler CM, Durling M, Cerullo MA, Strakowski SM, 2011. Preliminary evidence for increased frontosubcortical activation on a motor impulsivity task in mixed episode bipolar disorder. J. Affect Disord 133, 333–339. 10.1016/j.jad.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Bi G, Zou Z, Wang Y, Ye E, Ma L, Ming-Fan, null, Yang Z, 2008. Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci. Lett 438, 322–326. 10.1016/j.neulet.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Goldstein TR, Fersch-Podrat RK, Rivera M, Axelson DA, Merranko J, Yu H, Brent DA, Birmaher B, 2015. Dialectical behavior therapy for adolescents with bipolar disorder: results from a pilot randomized trial. J. Child Adolesc. Psychopharmacol 25, 140–149. 10.1089/cap.2013.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Cooper P, Fabiani M, Carter CS, Karayanidis F, 2018. Dynamics of cognitive control: theoretical bases, paradigms, and a view for the future. Psychophysiology 55, e13016. 10.1111/psyp.13016. [DOI] [PubMed] [Google Scholar]
- Guo Y, Schmitz TW, Mur M, Ferreira CS, Anderson MC, 2018. A supramodal role of the basal ganglia in memory and motor inhibition: meta-analytic evidence. Neuropsychologia 108, 117–134. 10.1016/j.neuropsychologia.2017.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML, 2012. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 14, 375–410. 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Littlefield AK, Anastasio NC, Cunningham KA, Fink LHL, Wing VC, Mathias CW, Lane SD, Schütz CG, Swann AC, Lejuez CW, Clark L, Moeller FG, Potenza MN, 2015a. Rapid-response impulsivity: definitions, measurement issues, and clinical implications. Pers. Disord 6, 168–181. 10.1037/per0000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Mitchell MR, Wing VC, Balodis IM, Bickel WK, Fillmore M, Lane SD, Lejuez CW, Littlefield AK, Luijten M, Mathias CW, Mitchell SH, Napier TC, Reynolds B, Schütz CG, Setlow B, Sher KJ, Swann AC, Tedford SE, White MJ, Winstanley CA, Yi R, Potenza MN, Moeller FG, 2015b. Choice impulsivity: definitions, measurement issues, and clinical implications. Pers. Disord 6, 182–198. 10.1037/per0000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht D, Walsh V, Lavidor M, 2013. Bi-frontal direct current stimulation affects delay discounting choices. Cogn. Neurosci 4, 7–11. 10.1080/17588928.2011.638139. [DOI] [PubMed] [Google Scholar]
- Henry C, Phillips M, Leibenluft E, M’Bailara K, Houenou J, Leboyer M, 2012. Emotional dysfunction as a marker of bipolar disorders. Front Biosci. (Elite Ed. ) 4, 2722–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W, Friese M, Strack F, 2009. Impulse and self-control from a dual-systems perspective. Perspect. Psychol. Sci 4, 162–176. 10.1111/j.1745-6924.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- Houben K, Nederkoorn C, Wiers RW, Jansen A, 2011. Resisting temptation: decreasing alcohol-related affect and drinking behavior by training response inhibition. Drug Alcohol Depend. 116, 132–136. 10.1016/j.drugalcdep.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Hummer TA, Hulvershorn LA, Karne HS, Gunn AD, Wang Y, Anand A, 2013. Emotional response inhibition in bipolar disorder: a functional magnetic resonance imaging study of trait- and state-related abnormalities. Biol. Psychiatry 73, 136–143. 10.1016/j.biopsych.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob GA, Zvonik K, Kamphausen S, Sebastian A, Maier S, Philipsen A, van Elst LT, Lieb K, Tüscher O, 2013. Emotional modulation of motor response inhibition in women with borderline personality disorder: an fMRI study. J. Psychiatry Neurosci 38, 164–172. 10.1503/jpn.120029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SL, Abate D, Abate KH, et al. , 2018. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 392, 1789–1858. 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SH, Vizueta N, Foland-Ross L, Townsend JD, Bookheimer SY, Thompson PM, Narr KL, Altshuler LL, 2016. Relationships between altered functional magnetic resonance imaging activation and cortical thickness in patients with euthymic bipolar I disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 1, 507–517. 10.1016/j.bpsc.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin J-M, Nazarian B, Roth M, Anton J-L, Mazzola-Pomietto P, 2009a. Remission from mania is associated with a decrease in amygdala activation during motor response inhibition. Bipolar Disord. 11, 530–538. 10.1111/j.1399-5618.2009.00722.x. [DOI] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin J-M, Nazarian B, Roth M, Mazzola-Pomietto P, 2009b. Reduced brain activation in euthymic bipolar patients during response inhibition: an event-related fMRI study. Psychiatry Res 173, 45–51. 10.1016/j.pscychresns.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, John M, Ikuta T, Rigoard P, Peters BD, Derosse P, Malhotra AK, Szeszko PR, 2015. The accumbofrontal tract: diffusion tensor imaging characterization and developmental change from childhood to adulthood. Hum. Brain Mapp 36, 4954–4963. 10.1002/hbm.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Jenkins SE, Connolly ME, Deveney CM, Fromm SJ, Brotman MA, Nelson EE, Pine DS, Leibenluft E, 2012. Neural correlates of cognitive flexibility in children at risk for bipolar disorder. J. Psychiatr. Res 46, 22–30. 10.1016/j.jpsychires.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf J, Glöckner S, Schecklmann M, Dresler T, Plichta MM, Veeh J, Kittel-Schneider S, Reif A, 2019. Neural correlates of response inhibition in patients with bipolar disorder during acute versus remitted phase. World J. Biol. Psychiatry 20, 637–646. 10.1080/15622975.2018.1428356. [DOI] [PubMed] [Google Scholar]
- Lei D, Du M, Wu M, Chen T, Huang X, Du X, Bi F, Kemp GJ, Gong Q, 2015. Functional MRI reveals different response inhibition between adults and children with ADHD. Neuropsychology 29, 874–881. 10.1037/neu0000200. [DOI] [PubMed] [Google Scholar]
- Leibenluft E., 2007. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am. J. Psychiatry 9. [DOI] [PubMed] [Google Scholar]
- Lesage E, Sutherland MT, Ross TJ, Salmeron BJ, Stein EA, 2020. Nicotine dependence (trait) and acute nicotinic stimulation (state) modulate attention but not inhibitory control: converging fMRI evidence from Go–Nogo and Flanker tasks. Neuropsychopharmacol 45, 857–865. 10.1038/s41386-020-0623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Jiang X, Deng Z, Jia L, Sun Q, Kong L, Wu F, Tang Y, 2022. Altered dynamic amplitude of low-frequency fluctuation between bipolar type I and type II in the depressive state. Neuroimage Clin. 36, 103184 10.1016/j.nicl.2022.103184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomholt LH, Andersen DV, Sejrsgaard-Jacobsen C, Øzdemir CM, Graff C, Schjerning O, Jensen SE, Straszek SPV, Licht RW, Grøntved S, Nielsen RE, 2019. Mortality rate trends in patients diagnosed with schizophrenia or bipolar disorder: a nationwide study with 20 years of follow-up. Int J. Bipolar Disord 7, 6. 10.1186/s40345-018-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luerssen A, Gyurak A, Ayduk O, Wendelken C, Bunge SA, 2015. Delay of gratification in childhood linked to cortical interactions with the nucleus accumbens. Soc. Cogn. Affect Neurosci 10, 1769–1776. 10.1093/scan/nsv068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magioncalda P, Martino M, 2021. A unified model of the pathophysiology of bipolar disorder. Mol. Psychiatry 1–10. 10.1038/s41380-021-01091-4. [DOI] [PubMed] [Google Scholar]
- Mahon K, Burdick KE, Szeszko PR, 2010. A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci. Biobehav Rev 34, 533–554. 10.1016/j.neubiorev.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maletic V, Raison C, 2014. Integrated neurobiology of bipolar disorder. Front Psychiatry 5. 10.3389/fpsyt.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason L, O’Sullivan N, Blackburn M, Bentall R, El-Deredy W, 2012. I want it now! neural correlates of hypersensitivity to immediate reward in hypomania. Biol. Psychiatry 71, 530–537. 10.1016/j.biopsych.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Mason L, O’Sullivan N, Montaldi D, Bentall RP, El-Deredy W, 2014. Decision-making and trait impulsivity in bipolar disorder are associated with reduced prefrontal regulation of striatal reward valuation. Brain 137, 2346–2355. 10.1093/brain/awu152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola-Pomietto P, Kaladjian A, Azorin J-M, Anton J-L, Jeanningros R, 2009. Bilateral decrease in ventrolateral prefrontal cortex activation during motor response inhibition in mania. J. Psychiatr. Res 43, 432–441. 10.1016/j.jpsychires.2008.05.004. [DOI] [PubMed] [Google Scholar]
- McClure SM, 2004. Separate neural systems value immediate and delayed monetary rewards. Science 306, 503–507. 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McGrath BM, Wessels PH, Bell EC, Ulrich M, Silverstone PH, 2004. Neurobiological findings in bipolar II disorder compared with findings in bipolar I disorder. Can. J. Psychiatry 49, 794–801. 10.1177/070674370404901202. [DOI] [PubMed] [Google Scholar]
- Metcalfe AWS, MacIntosh BJ, Scavone A, Ou X, Korczak D, Goldstein BI, 2016. Effects of acute aerobic exercise on neural correlates of attention and inhibition in adolescents with bipolar disorder. Transl. Psychiatry 6, e814. 10.1038/tp.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD, 2001. An integrative theory of prefrontal cortex function. Annu Rev. Neurosci 24, 167–202. 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC, 2001. Psychiatric aspects of impulsivity. Am. J. Psychiatry 158, 1783–1793. 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Morein-Zamir S, Simon Jones P, Bullmore ET, Robbins TW, Ersche KD, 2013. Prefrontal hypoactivity associated with impaired inhibition in stimulant-dependent individuals but evidence for hyperactivation in their unaffected siblings. Neuropsychopharmacology 38, 1945–1953. 10.1038/npp.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen JA, Rasmussen LA, Håberg A, 2010. Trait impulsivity in female patients with borderline personality disorder and matched controls. Acta Neuropsychiatr. 22, 139–149. 10.1111/j.1601-5215.2010.00468.x. [DOI] [PubMed] [Google Scholar]
- O’Donnell CM, Barrett DW, Fink LH, Garcia-Pittman EC, Gonzalez-Lima F, 2021. Transcranial infrared laser stimulation improves cognition in older bipolar patients: proof of concept study, 0891988720988906 J. Geriatr. Psychiatry Neurol. 10.1177/0891988720988906. [DOI] [PubMed] [Google Scholar]
- Ott T, Nieder A, 2019. Dopamine and cognitive control in prefrontal cortex. Trends Cogn. Sci 23, 213–234. 10.1016/j.tics.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D, 2021. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN, 2010. Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Res 181, 36–43. 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES, 1995. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol 51, 768–774. . [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA, 2010. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. J. Clin. Psychiatry 71, 1526–1534. 10.4088/JCP.09m05504yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold C, Vizueta N, Townsend JD, Bookheimer SY, Altshuler LL, 2015. Frontal lobe hypoactivation in medication-free adults with bipolar II depression during response inhibition. Psychiatry Res 231, 202–209. 10.1016/j.pscychresns.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C, 2010. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron 66, 138–148. 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Swartz HA, 2014. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am. J. Psychiatry 171, 829–843. 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ, 2008. Medication effects in neuroimaging studies of bipolar disorder. Am. J. Psychiatry 165, 313–320. 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quidé Y, O’Reilly N, Watkeys OJ, Carr VJ, Green MJ, 2018. Effects of childhood trauma on left inferior frontal gyrus function during response inhibition across psychotic disorders. Psychol. Med 48, 1454–1463. 10.1017/S0033291717002884. [DOI] [PubMed] [Google Scholar]
- Rae CL, Hughes LE, Anderson MC, Rowe JB, 2015. The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. J. Neurosci 35, 786–794. 10.1523/JNEUROSCI.3093-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Martín A, Ramos-Martín J, Mayoral-Cleries F, Moreno-Küstner B, Guzman-Parra J, 2020. Impulsivity, decision-making and risk-taking behaviour in bipolar disorder: a systematic review and meta-analysis. Psychol. Med 50, 2141–2153. 10.1017/S0033291720003086. [DOI] [PubMed] [Google Scholar]
- Raucher-Chéné D, Berna F, Vucurovic K, Barrière S, Van Der Linden M, Kaladjian A, Cuervo-Lombard C, 2021. How to project oneself without positive and integrated memories? Exploration of self-defining memories and future projections in bipolar disorder. Behav. Res Ther 138, 103817 10.1016/j.brat.2021.103817. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Schiffbauer R, 2004. Measuring state changes in human delay discounting: an experiential discounting task. Behav. Process 67, 343–356. 10.1016/j.beproc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Rung JM, Madden GJ, 2018. Experimental reductions of delay discounting and impulsive choice: a systematic review and meta-analysis. J. Exp. Psychol. Gen 147, 1349–1381. 10.1037/xge0000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruocco AC, Rodrigo AH, Lam J, Ledochowski J, Chang J, Wright L, McMain SF, 2021. Neurophysiological biomarkers of response inhibition and the familial risk for borderline personality disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 111, 110115. 10.1016/j.pnpbp.2020.110115. [DOI] [PubMed] [Google Scholar]
- Saddichha S, Schuetz C, 2014. Is impulsivity in remitted bipolar disorder a stable trait? a meta-analytic review. Compr. Psychiatry 55, 1479–1484. 10.1016/j.comppsych.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Schreiter S, Spengler S, Willert A, Mohnke S, Herold D, Erk S, Romanczuk-Seiferth N, Quinlivan E, Hindi-Attar C, Banzhaf C, Wackerhagen C, Romund L, Garbusow M, Stamm T, Heinz A, Walter H, Bermpohl F, 2016. Neural alterations of fronto-striatal circuitry during reward anticipation in euthymic bipolar disorder. Psychol. Med 46, 3187–3198. 10.1017/S0033291716001963. [DOI] [PubMed] [Google Scholar]
- Schüller CB, Kuhn J, Jessen F, Hu X, 2019. Neuronal correlates of delay discounting in healthy subjects and its implication for addiction: an ALE meta-analysis study. Am. J. Drug Alcohol Abus 45, 51–66. 10.1080/00952990.2018.1557675. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci 27, 2349–2356. 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig D, Clarkin JF, Goldstein M, Kernberg OF, Tuescher O, Levy KN, Brendel G, Pan H, Beutel M, Pavony MT, Epstein J, Lenzenweger MF, Thomas KM, Posner MI, Stern E, 2007. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. AJP 164, 1832–1841. 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- Singh MK, Chang KD, Mazaika P, Garrett A, Adleman N, Kelley R, Howe M, Reiss A, 2010. Neural correlates of response inhibition in pediatric bipolar disorder. J. Child Adolesc. Psychopharmacol 20, 15–24. 10.1089/cap.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, White R, Omari A, Ramaseshan K, Diwadkar VA, 2015. Affective context interferes with brain responses during cognitive processing in borderline personality disorder: fMRI evidence. Psychiatry Res.: Neuroimaging 233, 23–35. 10.1016/j.pscychresns.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Cerullo MA, Eliassen JC, Lamy M, Fleck DE, Lee J-H, DelBello MP, 2008. Magnetic resonance imaging brain activation in first-episode bipolar mania during a response inhibition task: response inhibition in first-episode mania. Early Interv. Psychiatry 2, 225–233. 10.1111/j.1751-7893.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Fleck DE, DelBello MP, Adler CM, Shear PK, Kotwal R, Arndt S, 2010. Impulsivity across the course of bipolar disorder: Impulsivity in bipolar disorder. Bipolar Disord. 12, 285–297. 10.1111/j.1399-5618.2010.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Fleck DE, Welge J, Eliassen JC, Norris M, Durling M, Komoroski RA, Chu W-J, Weber W, Dudley JA, Blom TJ, Stover A, Klein C, Strawn JR, DelBello MP, Lee J-H, Adler CM, 2016. fMRI brain activation changes following treatment of a first bipolar manic episode. Bipolar Disord. 18, 490–501. 10.1111/bdi.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Pazzaglia P, Nicholls A, Dougherty DM, Moeller FG, 2003. Impulsivity and phase of illness in bipolar disorder. J. Affect Disord. 73, 105–111. 10.1016/s0165-0327(02)00328-2. [DOI] [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG, 2005. Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. Am. J. Psychiatry 162, 1680–1687. 10.1176/appi.ajp.162.9.1680. [DOI] [PubMed] [Google Scholar]
- Teti Mayer J, Chopard G, Nicolier M, Gabriel D, Masse C, Giustiniani J, Vandel P, Haffen E, Bennabi D, 2020. Can transcranial direct current stimulation (tDCS) improve impulsivity in healthy and psychiatric adult populations? a systematic review. Prog. Neuropsychopharmacol. Biol. Psychiatry 98, 109814. 10.1016/j.pnpbp.2019.109814. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Christensen RE, Schettini E, Saletin JM, Ruggieri AL, MacPherson HA, Kim KL, Dickstein DP, 2019. Preliminary analysis of resting state functional connectivity in young adults with subtypes of bipolar disorder. J. Affect Disord 246, 716–726. 10.1016/j.jad.2018.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JD, Bookheimer SY, Foland-Ross LC, Moody TD, Eisenberger NI, Fischer JS, Cohen MS, Sugar CA, Altshuler LL, 2012. Deficits in inferior frontal cortex activation in euthymic bipolar disorder patients during a response inhibition task. Bipolar Disord. 14, 442–450. 10.1111/j.1399-5618.2012.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JD, Sugar CA, Walshaw PD, Vasquez RE, Foland-Ross LC, Moody TD, Bookheimer SY, McGough JJ, Altshuler LL, 2013. Frontostriatal neuroimaging findings differ in patients with bipolar disorder who have or do not have ADHD comorbidity. J. Affect. Disord 147, 389–396. 10.1016/j.jad.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost S, Diekhof EK, Zvonik K, Lewandowski M, Usher J, Keil M, Zilles D, Falkai P, Dechent P, Gruber O, 2014. Disturbed anterior prefrontal control of the mesolimbic reward system and increased impulsivity in bipolar disorder. Neuropsychopharmacology 39, 1914–1923. 10.1038/npp.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii N, Mikawa W, Adachi T, Hirose T, Shirakawa O, 2018. Shared and differential cortical functional abnormalities associated with inhibitory control in patients with schizophrenia and bipolar disorder. Sci. Rep 8, 4686. 10.1038/s41598-018-22929-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varo C, Murru A, Salagre E, Jiménez E, Solé B, Montejo L, Carvalho A, Stubbs B, Grande I, Martínez-Arán A, Vieta E, Reinares M, 2019. Behavioral addictions in bipolar disorders: a systematic review. Eur. Neuropsychopharmacol 76–97. 10.1016/j.euroneuro.2018.10.012. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD, 2008. Automatic and controlled response inhibition: associative learning in the go/no-go and stop-signal paradigms. J. Exp. Psychol. Gen 137, 649–672. 10.1037/a0013170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers JD, Stringaris A, Deveney CM, Brotman MA, Zarate CA Jr., Connolly ME, Fromm SJ, LeBourdais SB, Pine DS, Leibenluft E, 2012. A developmental study of the neural circuitry mediating motor inhibition in bipolar disorder. AJP 169, 633–641. 10.1176/appi.ajp.2012.11081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welander-Vatn A, Jensen J, Otnaess MK, Agartz I, Server A, Melle I, Andreassen OA, 2013. The neural correlates of cognitive control in bipolar I disorder: an fMRI study of medial frontal cortex activation during a Go/No-go task. Neurosci. Lett 549, 51–56. 10.1016/j.neulet.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Welander-Vatn AS, Jensen J, Lycke C, Agartz I, Server A, Gadmar ØB, Melle I, Nakstad PH, Andreassen OA, 2009. No altered dorsal anterior cingulate activation in bipolar II disorder patients during a Go/No-go task: an fMRI study. Bipolar Disord. 11, 270–279. 10.1111/j.1399-5618.2009.00680.x. [DOI] [PubMed] [Google Scholar]
- Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P, 2013. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. [Google Scholar]
- Wessa M, Houenou J, Paillère-Martinot M-L, Berthoz S, Artiges E, Leboyer M, Martinot J-L, 2007. Fronto-striatal overactivation in euthymic bipolar patients during an emotional Go/NoGo task. AJP 164, 638–646. 10.1176/ajp.2007.164.4.638. [DOI] [PubMed] [Google Scholar]
- White EJ, Demuth MJ, Nacke M, Kirlic N, Kuplicki R, Spechler PA, McDermott TJ, DeVille DC, Stewart JL, Lowe J, Paulus MP, Aupperle RL, T1000 investigators, 2022. Neural processes of inhibitory control in American Indian peoples are associated with reduced mental health problems. Soc. Cogn. Affect. Neurosci nsac045. 10.1093/scan/nsac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR, 2001. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personal. Individ. Differ 669–689. 10.1016/S0191-8869(00)00064-7. [DOI] [Google Scholar]
- Xiao Q, Wu Z, Hui X, Jiao Q, Zhong Y, Su L, Lu G, 2021a. Manic and euthymic states in pediatric bipolar disorder patients during an emotional Go/Nogo task: A functional magnetic resonance imaging study. J. Affect Disord 282, 82–90. 10.1016/j.jad.2020.12.105. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Wu Z, Jiao Q, Zhong Y, Zhang Y, Lu G, 2021b. Children with euthymic bipolar disorder during an emotional go/nogo task: Insights into the neural circuits of cognitive-emotional regulation. J. Affect Disord 282, 669–676. 10.1016/j.jad.2020.12.157. [DOI] [PubMed] [Google Scholar]
- Xu KZ, Anderson BA, Emeric EE, Sali AW, Stuphorn V, Yantis S, Courtney SM, 2017. Neural basis of cognitive control over movement inhibition: human fMRI and Primate Electrophysiology Evidence. e6 Neuron 96, 1447–1458. 10.1016/j.neuron.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Liu Q, Peng W, Liu Z, Chu J, Zheng K, Cao W, Yi J, 2021. Impaired impulse inhibition of emotional stimuli in patients with borderline personality disorder. Sci. Rep 11, 16628. 10.1038/s41598-021-96166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, Sharma V, Goldstein BI, Rej S, Beaulieu S, Alda M, MacQueen G, Milev RV, Ravindran A, O’Donovan C, McIntosh D, Lam RW, Vazquez G, Kapczinski F, McIntyre RS, Kozicky J, Kanba S, Lafer B, Suppes T, Calabrese JR, Vieta E, Malhi G, Post RM, Berk M, 2018. Canadian network for mood and anxiety treatments (CANMAT) and International society for bipolar disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 20, 97–170. 10.1111/bdi.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J-Y, Ding Q-Y, Cui J-F, Liu Z, Jia L-X, Qin X-J, Xu H, Wang Y, 2021. A meta-analysis of the effects of episodic future thinking on delay discounting, 17470218211066282 Q J. Exp. Psychol. 10.1177/17470218211066282. [DOI] [PubMed] [Google Scholar]
- Zargar F, Haghshenas N, Rajabi F, Tarrahi MJ, 2019. Effectiveness of dialectical behavioral therapy on executive function, emotional control and severity of symptoms in patients with bipolar I disorder. Adv. Biomed. Res 8, 59. 10.4103/abr.abr_42_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Oka T, Bokura H, Yamaguchi S, 2008. The key locus of common response inhibition network for no-go and stop signals. J. Cogn. Neurosci 20, 1434–1442. 10.1162/jocn.2008.20100. [DOI] [PubMed] [Google Scholar]
- van Zutphen L, Siep N, Jacob GA, Domes G, Sprenger A, Willenborg B, Goebel R, Tüscher O, Arntz A, 2020. Impulse control under emotion processing: an fMRI investigation in borderline personality disorder compared to non-patients and cluster-C personality disorder patients. Brain Imaging Behav. 14, 2107–2121. 10.1007/s11682-019-00161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.