Abstract
Our aim was to assess the efficacy of adjuvant programmed cell death protein-1 (PD-1) inhibitors and compare the other adjuvant treatments in patients with surgically resected stage III or IV acral melanoma. This study is a multicenter, retrospective analysis. We included 114 patients with stage III or IV acral malignant melanoma who underwent surgery within the past 10 years. We analyzed the effect of adjuvant programmed cell death protein-1 inhibitors on disease-free survival (DFS). The mean follow-up was 40 months, during which 69 (59.5%) patients experienced recurrence. Among the participants, 64 (56.1%) received systemic adjuvant therapy. Specifically, 48.4% received anti–PD-1 therapy, 29.7% received interferon, 14.1% received tezozolomide, and 7.8% received B-Raf proto-oncogene/mitogen-activated protein kinase inhibitors. Patients who received adjuvant therapy had a median DFS of 24 (10.9–37.2) months, whereas those who did not receive adjuvant therapy had a median DFS of 15 (9.8–20.2) months. Multivariate analysis for DFS revealed that the receipt of adjuvant therapy and lymph node metastasis stage were independent significant parameters (P = 0.021, P = 0.018, respectively). No statistically significant difference was observed for DFS between programmed cell death protein-1 inhibitor treatment and other adjuvant treatments. Regarding overall survival (OS), patients who received adjuvant treatment had a median OS of 71 (30.4–111.7) months, whereas those who did not receive adjuvant treatment had a median OS of 38 (16.7–59.3; P = 0.023) months. In addition, there were no significant differences in OS observed between various adjuvant treatment agents (P = 0.122). In our study, we have shown that adjuvant therapy had a positive effect on both DFS and OS in patients with stages III–IV acral melanoma who underwent curative intent surgery. Notably, we found no significant differences between anti–PD-1 therapy and other adjuvant therapies.
Key Words: acral malignant melanoma, adjuvant therapy, nivolumab
Acral melanoma represents a rare subtype of malignant melanoma, distinguished by its clinical characteristics and origin in the glabrous skin of areas such as the palms, soles, and nail beds (subungual regions). Unlike nonacral cutaneous melanoma, which arises from different skin regions, acral melanoma originates from melanocytes in the secretory portion of eccrine glands.1 It is interesting to note that melanocyte precursors in acral skin differ from those in nonglabrous sites, which primarily reside in hair follicles and can differentiate into melanocytes. This unique origin in acral skin, combined with its development in sun-shielded locations, suggests that UV exposure is not a significant factor in its etiology. Consequently, the precise cause of acral melanoma remains elusive. Notably, the lower tumor mutation burden in acral melanoma may explain its reduced responsiveness to immunotherapy. The primary treatment approach for acral melanoma involves surgical intervention, typically consisting of wide excision and sentinel lymph node sampling. However, despite optimal surgical management, acral melanoma exhibits a local recurrence rate that is two to five times higher than cutaneous melanoma.2 This elevated recurrence rate may, in part, be attributed to the discovery of genetically abnormal melanocytes in the normal epidermis adjacent to melanomas on the skin.2 In addition, factors, such as high ulceration rates and a low prevalence of B-Raf proto-oncogene (BRAF) mutations, further contribute to the unfavorable prognosis associated with acral melanoma.3,4
Acral melanoma exhibits distinct gene expression profiles compared with nonacral skin melanoma, which significantly influences the microenvironment and the response to immunotherapy. Notably, one of these distinctions is the observed decrease in CCL27 gene expression—a chemokine produced by keratinocytes that serves to attract T cells to the skin. T cells play a vital role in skin inflammation.5,6 In addition, an association between lower levels of the tumor-suppressor protein, p16(INK4A), and lower density of CD3+ and CD8+ tumor-infiltrating lymphocytes was found in acral melanoma cells, which were associated with worse prognosis.7,8 Furthermore, studies have shown that acral melanoma is characterized by an elevated presence of M2 macrophages in both peritumoral and intratumoral areas. This significant increase in M2 macrophages has been linked to adverse prognostic histopathological features, including tumor thickness, ulceration, mitotic rate, and metastasis, ultimately leading to a worse prognosis.9 Considering the pivotal role of the microenvironment in the development, progression, and therapeutic response of melanoma, it becomes apparent that the unique microenvironmental differences observed in acral melanoma may play a significant role in shaping the response to immunotherapy.
As the origin, etiology, pathogenesis, underlying molecular alterations, and microenvironment of acral melanoma diverge from those of cutaneous melanomas, it becomes evident that the response to immunotherapy may vary significantly. Thus far, studies focusing on metastatic stage acral melanoma have reported limited efficacy of programmed cell death protein-1 (PD-1) inhibitors when compared with cutaneous melanomas.10 In our study, our aim was to analyze the effectiveness of adjuvant PD-1 inhibitors in patients who had undergone surgery for stage 3 or 4 acral melanoma.
PATIENTS AND METHODS
Study Design
This multicenter retrospective observational study included patients from 16 centers across various regions of Turkey. Inclusion criteria encompassed individuals aged at least 18 years who had received a histologic diagnosis of acral malignant melanoma, were operable at stages 3 or 4, and had no residual tumor postoperatively. Patients with mucosal melanoma, nodular melanoma, superficial spreading melanoma, lentigo malignant melanoma, and uveal melanomas were excluded from the study. In addition, individuals lacking sufficient organ reserve or with secondary malignancies were excluded.
The study was conducted in accordance with the principles outlined in the Declaration of Helsinki and received approval from the ethics committees of the participating centers. It was also approved as a multicenter study by the Gaziantep University Faculty of Medicine Ethics Committee (No. 2023/13).
Patients histopathologically diagnosed with acral malignant melanoma between March 2023 and January 2023 were included in the study. Baseline characteristics, treatment regimens, and treatment and recurrence dates were retrospectively examined from patient records and electronic systems. Notably, the use of adjuvant nivolumab in the treatment of malignant melanoma was not covered by the reimbursement agency. However, patients accessed nivolumab through early access programs, private insurance, or self-payment. Nivolumab was administered to patients at a dosage of 240 mg every 2 weeks or 3 mg/kg every 2 weeks for a duration of 1 year. Interferon was administered intravenously at a maximum tolerated dose of 20 MU/m2/day for 1 month, followed by subcutaneous dosing at 10 MU/m2 three times a week for 48 weeks. Temozolomide was administered orally at a dose of 200 mg/m2 once daily for 5 consecutive days within a 28-day treatment cycle, repeated for a total of 6 cycles. Patients with BRAF mutations received dabrafenib orally at a dose of 150 mg twice daily, along with trametinib orally at a dose of 2 mg once daily, for a duration of 1 year. For patients receiving pembrolizumab, the regimen involved a dosage of 200 mg every 3 weeks for 1 year. Disease staging was conducted according to the American Joint Committee on Cancer staging system, specifically the eighth edition. Lymph node status was determined through either sentinel lymph node biopsy or lymph node dissection. All patients underwent postoperative evaluation for recurrence. The evaluation schedule included assessments every 12 weeks for the first 2 years and subsequently every 6 months for up to 5 years. During each evaluation visit, patients underwent a physical examination, computed tomography (chest, abdomen, and pelvis), and other relevant imaging studies, such as brain magnetic resonance imaging, when deemed necessary. Furthermore, we meticulously examined the recurrence patterns (local or distant) among patients who experienced recurrence and documented the treatments they received during these recurrence episodes.
The primary endpoint of our study was disease-free survival (DFS), with the aim of investigating effective adjuvant treatment options. The secondary endpoint was overall survival (OS). DFS was defined as the duration from the commencement of adjuvant therapy to the occurrence of the first instance of recurrence (local, regional, or distant metastasis), death from any cause, or the date of the last follow-up. For patients who did not receive adjuvant treatment, the starting date was determined as the date of surgery. OS was defined as the period from the initiation of adjuvant treatment to the date of death or last follow-up. In cases where adjuvant treatment was not administered, OS was measured from the date of surgery to the date of the last follow-up or death.
Statistical Analyses
The normal distribution of numerical variables was assessed using the Shapiro-Wilk test. Relationships between categorical variables were examined using the χ2 test, with categorical variables presented as counts (n) and percentages (%). Continuous variables were reported as mean ± SD. DFS and OS were estimated by the Kaplan-Meier method and the log-rank test was used to compare the effect of clinicopathological parameters and laboratory parameters on survival for the univariate analysis. All potential predictive factors with a probability value of <0.10 on univariate analyses were included in the multivariate analysis. The Cox proportional hazard model was used for multivariate analysis to determine independent significant factors. A P value of ≤0.05 was considered statistically significant, and statistical analyses were carried out by using the statistical software package SPSS 22.0 (SPSS).
RESULTS
A total of 114 patients with stages III–IV acral melanoma were included in this retrospective analysis. The mean follow-up period was 40 months. The median age was 60 (range: 19–88), and 45.6% of the patients were females. During the follow-up, 69 patients (59.5%) experienced recurrence, and 50 patients (43.9%) passed away. Optimal surgery was performed in 108 patients (94.7%), and 64 (56.1%) of them received systemic adjuvant treatment. Among those who received adjuvant treatment, 48.4% received anti-PD-1 therapy, 29.7% received interferon, 14.1% received temozolomide, and the remaining received BRAF/ mitogen-activated protein kinase (MEK) inhibitors. None of the patients using PD-1 inhibitors had to discontinue treatment due to toxicity. However, 33.3% of the patients using programmed cell death protein-1 inhibitors experienced disease progression. The clinical and pathologic characteristics of the patients are summarized in Table 1.
TABLE 1.
Clinicopathological Features of the Patients
| Parameters | N (%) |
|---|---|
| Age | |
| <65 | 75 (65.8) |
| ≥65 | 39 (34.2) |
| Sex | |
| Female | 52 (45.6) |
| Male | 62 (54.4) |
| Lacalization | |
| Nail bed | 26 (22.8) |
| Sole | 80 (70.2) |
| Palm | 8 (7.0) |
| T stage | |
| 1 | 5 (4.4) |
| 2 | 8 (7.9) |
| 3 | 30 (26.3) |
| 4 | 69 (60.5) |
| Node stage | |
| 1 | 52 (46.6) |
| 2 | 28 (24.6) |
| 3 | 34 (29.8) |
| Lymph node metastasis types | |
| In-transit | 23 (20.2) |
| Satellite | 28 (24.6) |
| Microsatellite | 6 (5.3) |
| Unknown | 57 (50) |
| Stage | |
| IIIA | 10 (8.8) |
| IIIB | 22 (19.3) |
| IIIC | 53 (46.5) |
| IIID | 19 (16.7) |
| IV | 10 (8.8) |
| Ulseration | |
| Presence | 43 (37.7) |
| Absence | 68 (59.6) |
| Performance status | |
| 0 | 67 (58.8) |
| 1 | 32 (28.1) |
| 2–3 | 3 (2.7) |
| Unknown | 12 (10.5) |
| LDH | |
| Above the UNL | 81 (71.1) |
| Below the UNL | 21 (18.4) |
| Unknown | 12 (10.5) |
| BRAF mutation | |
| Mutant | 14 (12.3) |
| Wild type | 77 (67.5) |
| Unknown | 23 (20.2) |
| Adjuvant treatment | |
| Received | 64 (56.1) |
| Not received | 50 (43.9) |
| Type of adjuvant treatment | |
| Anti–PD-1* | 31 (48.4) |
| Temozolomide | 9 (14.1) |
| Interferone | 19 (29.7) |
| Braf-Mek inhibitors | 5 (7.8) |
Nivolumab: 29 patients; pembrolizumab: 2 patients.
BRAF indicates B-Raf proto-oncogene; Mek, mitogen-activated protein kinase; UNL indicates upper normal limit.
Disease-Free Survival
The median DFS was 22.0 months, with a range of 15.5–28.5 months. There was a significant difference in DFS between patients who received adjuvant therapy, with a median DFS of 24.0 months (range: 10.9–37.2), and those who did not receive adjuvant therapy, with a median DFS of 15.0 months (range: 9.8–20.2; P = 0.051). It is worth noting that the median DFS could not be determined for patients who received anti–PD-1 treatment due to a lack of events. For patients who received temozolomide, interferon, and BRAF-MEK inhibitors, the median DFS was 23, 22, and 23 months, respectively.
Factors, such as age, sex, T stage, tumor localization, types of lymph node metastasis, ulceration, eastern cooperative oncology group performance status, stage (III vs IV), lactate dehydrogenase level, and the presence of a BRAF mutation, did not significantly affect DFS. However, we observed a worse DFS with increasing node stage (Table 2). Notably, no significant differences were found in DFS among different adjuvant treatment types (P = 0.481). In a multivariate analysis adjusting for lymph node stage and stage (stage III vs IV), we found that patients who received adjuvant therapy made a significant contribution to DFS compared with those who did not receive it [hazard ratio (HR): 95% CI: 1.79; 1.09–2.95; P = 0.021; Fig. 1].
TABLE 2.
The Effect of Clinicopathological and Treatment-Related Factors on DFS
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Parameters | HR (95% CI) | P | HR (95% CI) | P |
| Age | ||||
| <65 | 1 (reference) | 0.938 | — | — |
| ≥65 | 0.98 (0.60–1.60) | — | — | — |
| Sex | ||||
| Female | 1 (reference) | 0.26 | — | — |
| Male | 1.34 (0.83–2.16) | — | — | — |
| Lacalization | ||||
| Nail bed (subungual) | 1 (reference) | 0.156 | — | — |
| Soles | 1.52 (0.83–2.91) | — | — | — |
| Palms | 0.53 (0.12–2.33) | — | — | — |
| T stage | ||||
| 1 | 1 (reference) | 0.44 | — | — |
| 2 | 0.41 (0.10–1.66) | — | — | — |
| 3 | 0.56 (0.19–1.68) | — | — | — |
| 4 | 0.76 (0.27–2.12) | — | — | — |
| Node stage | — | 0.022 | — | 0.018 |
| 1 | 1 (reference) | 0.456 | 1 (reference) | 0.343 |
| 2 | 1.26 (0.68–2.35) | 0.007 | 1.35 (0.73–2.52) | 0.005 |
| 3 | 2.12 (1.23–3.66) | — | 2.38 (1.30–4.34) | — |
| Lymph node metastasis types | ||||
| In-transit | 1 (reference) | 0.744 | — | — |
| Satellite | 1.30 (0.65–2.60) | — | — | — |
| Microsatellite | 1.02 (0.29–3.59) | — | — | — |
| Stage | ||||
| III | 1 (reference) | 0.087 | 1 (reference) | 0.675 |
| IV | 1.91 (0.91–4.02) | — | 1.19 (0.52–2.72) | — |
| Ulceration | ||||
| Presence | 1.13 (0.69–1.879 | 0.62 | — | — |
| Absence | 1 (reference) | — | — | — |
| ECOG performance status | ||||
| 0 | 1 (reference) | 0.315 | — | — |
| 1 | 1.49 (0.89–2.53) | — | — | — |
| 2-3 | 1.35 (0.32–5.66) | — | — | — |
| LDH | ||||
| Above the UNL | 1.06 (0.57–1.95) | 0.86 | — | — |
| Below the UNL | 1 (reference) | — | — | — |
| BRAF mutation | ||||
| Mutant | 1 (reference) | 0.407 | — | — |
| Wild type | 1.35 (0.66–2.74) | — | — | — |
| Adjuvant treatment | ||||
| Received | 1 (reference) | 0.056 | 1 (reference) | 0.021 |
| Not received | 1.56 (0.99–2.57) | — | 1.79 (1.09–2.95) | — |
| Type of adjuvant treatment | ||||
| Anti–PD-1 | 1 (reference) | 0.481 | — | — |
| Temozolomide | 1.09 (0.42–2.89) | — | — | — |
| Interferon | 1.06 (0.49–2.27) | — | — | — |
| Braf-Mek inhibitors | 0.76 (0.17–3.36) | — | — | — |
| Not received | 1.58 (0.83–2.99) | — | — | — |
BRAF indicates B-Raf proto-oncogene; DFS, disease-free survival; ECOG, eastern cooperative oncology group; HR, hazard ratio; Mek, mitogen-activated protein kinase; UNL, upper normal limit.
FIGURE 1.
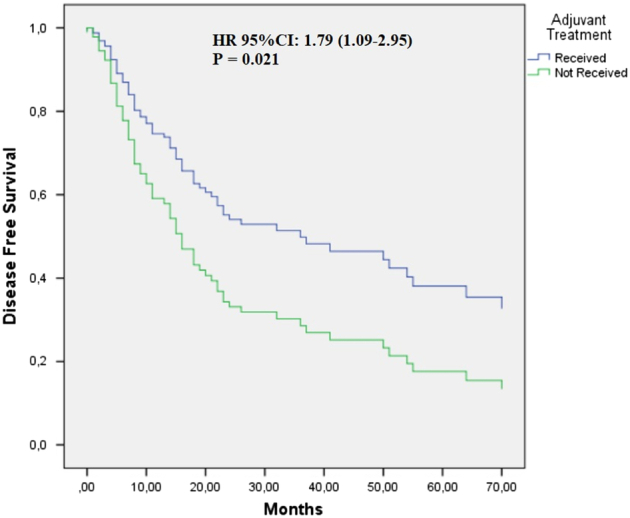
Cox regression graph for disease-free survival comparing patients with acral melanoma who received adjuvant therapy and those who did not. HR was adjusted for node stage and stage III/IV. HR indicates hazard ratio.
Overall Survival
The median OS for patients was 54 months, ranging from 37.6 to 70.4 months. Patients who received adjuvant therapy had a median OS of 71.0 months (30.4–111.7), whereas those who did not receive adjuvant therapy had a median OS of 38.0 months (16.7–59.3).
Factors, such as age, sex, T stage, tumor localization, types of lymph node metastasis, Eastern Cooperative Oncology Group performance status, stage (III vs IV), lactate dehydrogenase level, and the presence of a BRAF mutation, did not significantly affect OS. However, worse OS was observed with increasing node stage (Table 3). Notably, no significant differences were found in OS among different adjuvant treatment types (P = 0.122). In a multivariate analysis adjusting for age, sex, ulceration, lymph node stage, stage (stage III vs IV), and ulceration, patients who received adjuvant therapy made a significant contribution to OS (HR: 95% CI: 2.99; 1.59–5.63; P = 0.001; Fig. 2).
TABLE 3.
The Effect of Clinicopathological and Treatment-Related Factors on OS
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Parameters | HR (95% CI) | P | HR (95% CI) | P |
| Age | ||||
| <65 | 1 (reference) | — | 1 (reference) | 0.514 |
| ≥65 | 1.62 (0.92–2.83) | 0.093 | 1.26 (0.67–2.25) | — |
| Sex | ||||
| Female | 1 (reference) | 0.056 | 1 (reference) | 0.058 |
| Male | 1.75 (0.98–3.10) | — | 1.87 (0.98–3.56) | — |
| Lacalization | ||||
| Nail bed (subungual) | — | — | — | — |
| Soles | — | 0.915 | — | — |
| Palms | — | — | — | — |
| T stage | ||||
| 1 | 1 (reference) | — | — | — |
| 2 | 0.26 (0.16–4.22) | 0.12 | — | — |
| 3 | 1.18 (0.15–9.07) | — | — | — |
| 4 | 1.92 (0.26–14.07) | — | — | — |
| Node stage | — | 0.036 | — | 0.009 |
| 1 | 1 (reference) | 0.043 | 1 (reference) | 0.004 |
| 2 | 2.09 (1.02–4.30) | 0.014 | 3.07 (1.43–6.60) | 0.016 |
| 3 | 2.37 (1.19–4.71) | — | 2.67 (1.20–5.93) | — |
| Lymph node metastasis types | ||||
| In-transit | — | — | — | — |
| Satellite | — | 0.522 | — | — |
| Microsatellite | — | — | — | — |
| Stage | ||||
| III | 1 (reference) | 0.094 | 1 (reference) | 0.776 |
| IV | 1.99 (0.89–4.46) | — | 1.15 (0.43–3.07) | — |
| Ulceration | ||||
| Presence | 1.71 (0.92–3.19) | 0.089 | 1.99 (1.03–3.83) | 0.040 |
| Absence | 1 (reference) | — | 1 (reference) | — |
| ECOG performance status | ||||
| 0 | 1 (reference) | — | — | — |
| 1 | 1.50 (0.80–2.82) | 0.277 | — | — |
| 2-3 | 2.41 (0.56–10.33) | — | — | — |
| LDH | ||||
| Above the UNL | 0.88 (0.42–1.83) | 0.726 | — | — |
| Below the UNL | 1 (reference) | — | — | — |
| BRAF mutation | ||||
| Mutant | 1 (reference) | 0.162 | — | — |
| Wild type | 1.95 (0.77–4.97) | — | — | — |
| Adjuvant treatment | ||||
| Received | 1 (reference) | 0.023 | 1 (reference) | 0.001 |
| Not received | 1.92 (1.09–3.35) | — | 2.99 (1.59–5.63) | — |
| Type of adjuvant treatment | ||||
| Anti–PD-1 | 1 (reference) | 0.122 | — | — |
| Temozolomide | 1.87 (0.59–5.82) | — | — | — |
| Interferone | 0.77 (0.27–2.22) | — | — | — |
| Braf-Mek inhibitors | 0.66 (0.08–5.48) | — | — | — |
| Not received | 1.88 (0.77-4.58) | — | — | — |
BRAF indicate B-Raf proto-oncogene; ECOG, eastern cooperative oncology group; HR, hazard ratio; Mek, mitogen-activated protein kinase; OS, overall survival; UNL, upper normal limit.
FIGURE 2.
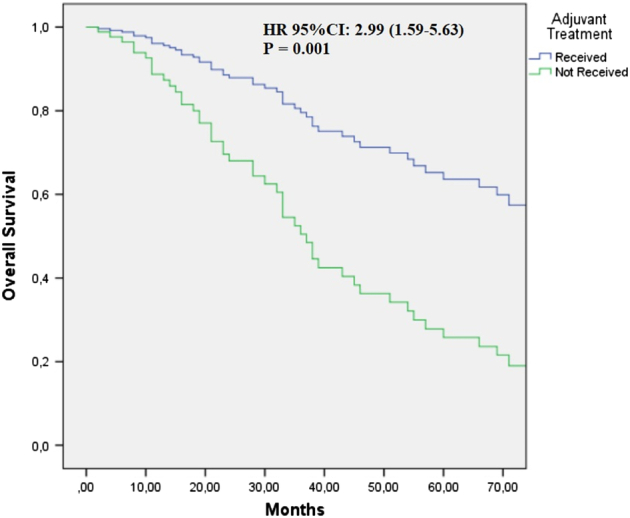
Cox regression graph for overall survival comparing patients with acral melanoma who received adjuvant therapy and those who did not. HR was adjusted for age, sex, ulceration, lymph node stage, stage (stage III vs IV), and ulceration. HR indicates hazard ratio.
Among patients who experienced recurrence, 21.7% had local recurrence, whereas 73.9% had distant metastasis. Surgical treatment was administered to 13% of recurrent patients. In the first-line treatment, 58.6% received temozolomide, 24% received anti–PD-1 therapies, 4 patients received BRAF/MEK inhibitors, 2 patients received ipilimumab, and 2 patients received carboplatin plus paclitaxel. Second-line treatment was given to 35 patients, with 40% receiving nivolumab, 22.8% receiving nivolumab plus ipilimumab, and 8 patients receiving carboplatin plus paclitaxel.
For patients who experienced recurrence, the median survival was 38.0 months (24.6–51.43) for those who received anti–PD-1 treatment at any stage of their treatment (adjuvant, recurrence first line, or later lines) compared with 33.0 months (22.5–43.5) for patients treated with other systemic therapies (temozolomide, carboplatin paclitaxel, and ipilimumab; P = 0.765).
DISCUSSION
In our study, we have shown the favorable effect of adjuvant treatment on both DFS and OS in patients with surgically treated stages III–IV acral melanoma. However, there was no significant difference observed between the effectiveness of anti–PD-1 therapy and other forms of adjuvant therapies, neither for DFS nor OS. Furthermore, when assessing the OS of patients who received anti–PD-1 therapy either in the adjuvant setting or at any metastatic stage, it did not differ significantly from that of those who did not receive it.
When considering the effect of the tumor’s pathologic features on survival, it is well-established that factors, such as Breslow thickness, mitotic index, and the presence of ulceration in the primary tumor, are robust predictors for the development of regional lymph node metastasis. Consequently, these parameters are associated with poor prognostic outcomes in early-stage melanoma.11 The risk of developing systemic metastatic disease in patients with regional lymph node metastasis is further correlated with whether the metastasis is microscopic or palpable, as well as the number of positive nodes12. However, the most significant factor determining prognosis, as observed in our study, remains lymph node metastasis. In our present study, lymph node metastasis type, T stage, and ulceration were not found to be predictive parameters for DFS. In contrast, node stage emerged as a predictive clinical parameter, independent of adjuvant systemic therapy. In addition, the presence of ulceration and node stage were identified as independent prognostic parameters for OS.
When reviewing the history of adjuvant therapies in cutaneous malignant melanoma, interferon was the first approved biological agent in both the USA and the EU as an adjuvant therapy for resected melanoma. It demonstrated a HR for recurrence or death in the range of 0.83–0.85. However, its use as the standard of care was limited due to its high toxicity.13 Subsequently, ipilimumab, a fully human monoclonal antibody that blocks CTLA-4, showed efficacy compared with a placebo in patients with completely resected stage III cutaneous melanoma. It presented a 0.75 HR for recurrence-free survival (RFS; 95% CI: 0.63–0.88; P < 0.001) and a 0.73 HR for OS (95% CI: 0.60–0.89; P = 0.002).14 However, even with dose reduction studies aimed at minimizing toxicity, treatment-related grade ≥3 adverse events were still observed in 37% of patients receiving ipilimumab 3 mg/kg. Although RFS favored ipilimumab over interferon, the difference was statistically insignificant (HR: 0.85; 99.4% CI, 0.66–1.09; P = 0.065)15. First, nivolumab, and later pembrolizumab, demonstrated statistically significant efficacy in terms of RFS and had a more favorable toxicity profile compared with ipilimumab in patients with melanoma.16,17 However, while neither of the anti–PD-1 agents provided an OS advantage over ipilimumab, a cumulative analysis of adjuvant studies involving previous biological treatments indicated a survival benefit with these treatments over a placebo. Considering the safety profile, current guidelines recommend the use of anti–PD-1 treatments for adjuvant melanoma therapy.
Adjuvant therapy studies for acral melanoma are limited. In a randomized phase 2 trial, 158 high-risk Chinese patients with acral melanoma were analyzed for the efficacy of adjuvant interferon for 4 weeks or 1 year. The median RFS was not statistically significant, with 17.9 months and 22.5 months, respectively.18 In our study, 19 patients received high-dose adjuvant interferon. The obtained DFS, at 22 months, was consistent with the results of this phase 2 study and was not statistically different from other adjuvant therapies. The frequency of BRAF mutations, a significant target for adjuvant treatment, in acral melanoma is relatively low. The clinical benefit of BRAF and MEK inhibitors in this patient population has been limited.19 In a retrospective study involving 28 patients with metastatic acral melanoma, the median PFS and OS with BRAF and MEK inhibitors were only 3.6 months (95% CI: 3.0–6.4) and 6.2 months (95% CI: 6.1–12.1), respectively. These survival durations are notably shorter than those observed in cutaneous melanoma.20 In our study, the rate of BRAF mutations was found to be relatively low at 15.4%, consistent with the literature compared with cutaneous melanoma.21 We had 5 patients who received BRAF/MEK inhibitors as adjuvant treatment, and their median DFS was 23 months, similar to other adjuvant treatments. Although the presence of a BRAF mutation is a favorable prognostic biomarker in cutaneous melanoma, our study did not observe a positive prognostic effect on DFS or OS in patients with acral melanoma, aligning with the literature.20 This discrepancy may be attributed to the genomic differences between acral melanoma and cutaneous melanoma.
Temozolomide is commonly employed as a single agent or in combination with other chemotherapeutic drugs in the management of metastatic melanoma. However, there is a lack of phase-3 studies investigating the adjuvant use of temozolomide in malignant melanoma. In a retrospective study, the effectiveness of adjuvant temozolomide after complete resection in patients with recurrent malignant melanoma was examined. In this study, the median relapse-free survival was 12.9 months, and the median OS was 23.6 months.22 In Turkey, temozolomide may be considered as a treatment option when immunotherapy or targeted treatments are not available. In our study, 9 (14.1%) patients with acral malignant melanoma, who received adjuvant treatment, were administered temozolomide. In addition, 34 (58.6%) of the patients who received first-line systemic therapy received temozolomide. Importantly, the DFS and OS of patients receiving adjuvant temozolomide were found to be comparable to those of patients receiving other adjuvant treatments.
In the CheckMate 238 study, which assessed the effectiveness of adjuvant nivolumab, acral and mucosal melanoma subgroups were included alongside cutaneous melanomas, although the number of patients in these subgroups was relatively small. Of the adjuvant biological treatments, only the CheckMate 238 study analyzed this challenging subgroup separately16. In the intention-to-treat population, the median RFS was 52.4 months (95% CI: 42.5–not reached) in the nivolumab group and 24.1 months (16.6–35.1) in the ipilimumab group (HR: 0.71; 95% CI: 0.60–0.86; P = 0.0003). When subgroup analysis was conducted, the HR for RFS was 0.67 (0.54–0.82) in cutaneous melanomas, while it was 1.71 (0.68–4.29) in mucosal melanomas and 1.04 (0.43–1.48) in acral melanomas. For acral melanoma, the advantage of nivolumab over ipilimumab seems to diminish, but it is worth noting that only 34 patients with acral melanoma were included, which accounts for a mere 3.75% of the study population, making it challenging to draw definitive conclusions on this matter. In summary, considering that all biological treatments are more effective than a placebo in terms of RFS for cutaneous melanomas, it can still be argued that anti–PD-1 treatments are the most suitable option, bearing a manageable toxicity profile as observed in our study, even though their effectiveness may diminish. In our study, we analyzed 114 patients with acral melanoma, and of these, 64 received adjuvant treatment. Although adjuvant treatment appears beneficial for acral melanoma, the superiority of anti–PD-1 therapy over other treatments, as observed in the CheckMate 238 study for cutaneous melanoma, was not evident in our study for acral melanoma.
One of the primary limitations of our study is its retrospective design and the relatively small number of patients included. However, considering that adjuvant studies in acral melanoma are limited and have a small number of patients, the results obtained in our study are valuable in shedding light on the potential benefits of adjuvant treatment in acral melanoma.
CONCLUSION
Although programmed cell death protein-1 inhibitors may not demonstrate statistical superiority over other adjuvant treatments in the adjuvant therapy of stages III–IV operable acral melanoma, they remain an effective treatment modality with a low toxicity profile.
Author’s name has been updated post publication ahead of print.
Acknowledgments
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
None reported. All authors have declared that there are no financial conflicts of interest with regard to this work.
Contributor Information
Haci Arak, Email: harak63@hotmail.com.
Suna Erkiliç, Email: Serk52@yahoo.com.
Şendağ Yaslikaya, Email: drysendag@gmail.com.
Eda Eylemer Mocan, Email: edaeylemer@gmail.com.
Gökmen Aktaş, Email: aktas_gokmen@hotmail.com.
Melek Özdemir, Email: melekozdemir@hotmail.com.tr.
Hüseyin Salih Semiz, Email: hsalihsemiz@hotmail.com.
Saadettin kiliçkap, Email: skilickap@yahoo.com.
Faruk Recep Özalp, Email: ozalpfarukrecep@gmail.com.
Özlem Nuray Sever, Email: ozlem.sever@hotmail.com.
Goncagül Akdağ, Email: akdaggoncagul@gmail.com.
Ahmet Burak Ağaoğlu, Email: abagaoglu@hotmail.com.
Melike Özçelik, Email: drmelike.ozcelik@gmail.com.
Murat Sari, Email: drmuratsari@gmail.com.
REFERENCES
- 1. Okamoto N, Aoto T, Uhara H, et al. A melanocyte--melanoma precursor niche in sweat glands of volar skin. Pigment Cell Melanoma Res. 2014;27:1039–50. [DOI] [PubMed] [Google Scholar]
- 2. Basurto-Lozada P, Molina-Aguilar C, Castaneda-Garcia C, et al. Acral lentiginous melanoma: basic facts, biological characteristics and research perspectives of an understudied disease. Pigment Cell Melanoma Res. 2021;34:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tas F, Erturk K. Acral lentiginous melanoma is associated with certain poor prognostic histopathological factors but may not be correlated with nodal involvement, recurrence, and a worse survival. Pathobiology. 2018;85:227–231. [DOI] [PubMed] [Google Scholar]
- 4. Tas F, Erturk K. Limb melanomas: acral melanomas have worse survival. J Dermatolog Treat. 2022;33:1630–1637. [DOI] [PubMed] [Google Scholar]
- 5. Bissonnette R, Suárez-Fariñas M, Li X, et al. Based on molecular profiling of gene expression, palmoplantar pustulosis and palmoplantar pustular psoriasis are highly related diseases that appear to be distinct from psoriasis vulgaris. PLoS One. 2016;11:e0155215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Homey B, Alenius H, Müller A, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–65. [DOI] [PubMed] [Google Scholar]
- 7. Castaneda CA, Castillo M, Torres-Cabala C, et al. Relationship between tumor-associated immune infiltrate and p16 staining over clinicopathological features in acral lentiginous melanoma. Clin Transl Oncol. 2019;21:1127–1134. [DOI] [PubMed] [Google Scholar]
- 8. Park CK, Kim SK. Clinicopathological significance of intratumoral and peritumoral lymphocytes and lymphocyte score based on the histologic subtypes of cutaneous melanoma. Oncotarget. 2017;8:14759–14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zúñiga-Castillo M, Pereira NV, Sotto MN. High density of M2-macrophages in acral lentiginous melanoma compared to superficial spreading melanoma. Histopathology. 2018;72:1189–1198. [DOI] [PubMed] [Google Scholar]
- 10. Mao L, Qi Z, Zhang L, et al. Immunotherapy in acral and mucosal melanoma: current status and future directions. Front Immunol. 2021;12:680407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balch CM, Gershenwald JE, Soong SJ, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28:2452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suciu S, Ives N, Eggermont AM, et al. Predictive importance of ulceration on the efficacy of adjuvant interferon-a (IFN): an individual patient data (IPD) meta-analysis of 15 randomized trials in more than 7,500 melanoma patients (pts). Am Soc Clin Oncol. 2014;15:9067. [Google Scholar]
- 14. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med. 2016;375:1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tarhini AA, Lee SJ, Hodi FS, et al. Phase III study of adjuvant ipilimumab (3 or 10 mg/kg) versus high-dose interferon alfa-2b for resected high-risk melanoma: North American Intergroup E1609. J Clin Oncol. 2020;38:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ascierto PA, Del Vecchio M, Mandalá M, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21:1465–1477. [DOI] [PubMed] [Google Scholar]
- 17. Grossmann KF, Othus M, Patel SP, et al. Adjuvant pembrolizumab versus IFNα2b or ipilimumab in resected high-risk melanoma. Cancer Discov. 2022;12:644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mao L, Si L, Chi Z, et al. A randomised phase II trial of 1 month versus 1 year of adjuvant high-dose interferon α-2b in high-risk acral melanoma patients. Eur J Cancer. 2011;47:1498–1503. [DOI] [PubMed] [Google Scholar]
- 19. Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94–100. [DOI] [PubMed] [Google Scholar]
- 20. Bai X, Mao LL, Chi ZH, et al. BRAF inhibitors: efficacious and tolerable in BRAF-mutant acral and mucosal melanoma. Neoplasma. 2017;64:626–632. [DOI] [PubMed] [Google Scholar]
- 21. Goydos JS, Shoen SL. Acral lentiginous melanoma. Cancer Treat Res. 2016;167:321–329. [DOI] [PubMed] [Google Scholar]
- 22. Tas F, Erturk K. Single-agent temozolomide may be an effective option for late adjuvant therapy in patients with melanoma. J Oncol Pharm Pract. 2021;27:40–45. [DOI] [PubMed] [Google Scholar]


