Abstract
The cardiotoxicity associated with des-ethyl-dexfenfluramine (norDF) and related agonists of the serotonin receptor 2B (5-HT2B) has solidified the receptor’s place as a traditional “antitarget” in drug discovery. Conversely, a growing body of evidence has highlighted the utility of 5-HT2B antagonists for the treatment of pulmonary arterial hypertension (PAH), valvular heart disease (VHD) and related cardiopathies. In this Perspective, we summarize the link between the clinical failure of fenfluramine-phentermine (fen-phen) with the subsequent research on the role of 5-HT2B in disease progression, as well as the development of drug-like and receptor subtype-selective 5-HT2B antagonists. Such agents represent a promising class for the treatment of PAH and VHD, but their utility has been historically understudied due to the clinical disasters associated with 5-HT2B. Herein, it is our aim to examine the current state of 5-HT2B drug discovery, with an emphasis on the receptor’s role in the central nervous system (CNS) versus the periphery, as well as known and marketed compounds with 5-HT2B antagonist activity as part of their broader polypharmacology.
Graphical Abstract
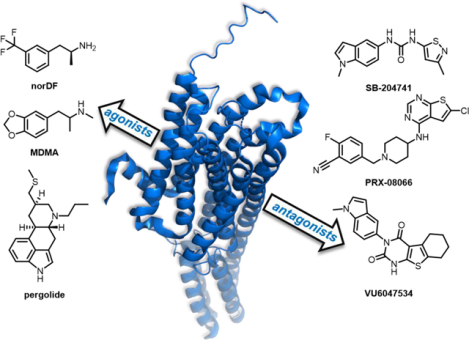
1. INTRODUCTION
1.1. Background and Characterization of 5-HT2B.
Serotonin, 5-hydroxytryptamine (5-HT), is the endogenous ligand for the 5-HT receptor family, where it acts as a neurotransmitter and growth factor through various signaling pathways. Two superfamilies mediate the physiological actions of serotonin: G protein-coupled receptors (GPCRs) and ligand-gated ion channels, comprising fourteen total receptors between both families. The ligand-gated ion channels are currently comprised of one family: 5-HT3. The GPCR superfamily includes 5-HT1, 5-HT2, and 5-HT4–7 (Table 1), and was initially split into two distinct groups: the ‘D’ receptors for their irreversible interaction with the antagonist dibenzyline and the ‘M’ receptors for their ability to be blocked by morphine.1 A 1979 study on brain homogenates identified distinct serotonin receptors: 5-HT1 and 5-HT2. 5-HT1 was reported to have a higher affinity for serotonin, and 5-HT2 had a high affinity for certain antagonists correlating with the ‘D’-type receptors previously described.2 The 5-HT2B subtype was first characterized in an organ bath studying the 5-HT-induced contraction of rat stomach fundus. The receptor was originally known as “5-HT2F” for “stomach fundus” but was later changed to 5-HT2B to match the proposed nomenclature.3 Following the discovery and characterization of 5-HT2B, the receptor has been implicated in many important roles within the cardiovascular system, central nervous system (CNS), and gastrointestinal (GI) tract.
Table 1.
5-HT Receptor Classification.a
| Family | Potential | Type | Mechanism of Action |
|---|---|---|---|
| 5-HT1 | Inhibitory | Gi/Go protein-coupled | Decrease intracellular concentrations of cAMP |
| 5-HT2 | Excitatory | Gq11 protein-coupled | Increase intracellular concentrations of IP3, DAG, and calcium |
| 5-HT3 | Excitatory | Ligand-gated ion channel | Depolarization of plasma membrane |
| 5-HT4 | Excitatory | Gs protein-coupled | Increase intracellular concentrations of cAMP |
| 5-HT5 | Inhibitory | Gi/Go protein-coupled | Decrease intracellular concentrations of cAMP |
| 5-HT6 | Excitatory | Gs protein-coupled | Increase intracellular concentrations of cAMP |
| 5-HT7 | Excitatory | Gs protein-coupled | Decrease intracellular concentrations of cAMP |
Adapted from Reference 4.
1.2. Relationship to Other Serotonin Receptors.
Currently, the seven receptor subtypes are separated by their primary signaling pathways (Table 1).4 The 5-HT2 family is Gq/11-coupled, which activates various signaling molecules and intracellular calcium release from the endoplasmic reticulum. The family is divided into three distinct subtypes: 5-HT2A, 5-HT2B, and 5-HT2C. While 5-HT2A and 5-HT2C are more closely related, 5-HT2B shares similar sequence homology with both 5-HT2A and 5-HT2C, with up to 79% similarity within the transmembrane domain and 50% overall.5 There is high homology between 5-HT2B across species compared to the human receptor: rat (79%), mouse (82%), dog (83%), and pig (95%).6–8
1.3. Roles of 5-HT2B in Physiological Processes.
The primary physiological effects of 5-HT2B are mediated through the canonical Gq11 protein signaling pathway (calcium release and activation of secondary signaling molecules, see Figure 1).9–11 Receptor expression can be found throughout the body, with the highest expression levels in the liver, kidneys, stomach fundus, and gut. 5-HT2B has relatively moderate expression in the cardiovascular system, and low expression within the CNS.12 Within the GI tract, 5-HT2B is responsible for gut motility and hypersensitivity of colonic smooth muscle.13 Within the CNS, 5-HT2B is thought to be involved in sleep initiation as well as regulation of the central respiratory system and blood volume.14,15 Cardiovascular expression and activation of 5-HT2B can lead to myofibroblast proliferation and valvular heart disease (VHD) by increasing valve area and causing poor valve closure, which will be discussed in this Perspective.16 It is because of this expression in the cardiovascular system that 5-HT2B is considered a prototypical “antitarget” in medicinal chemistry programs.
Figure 1.
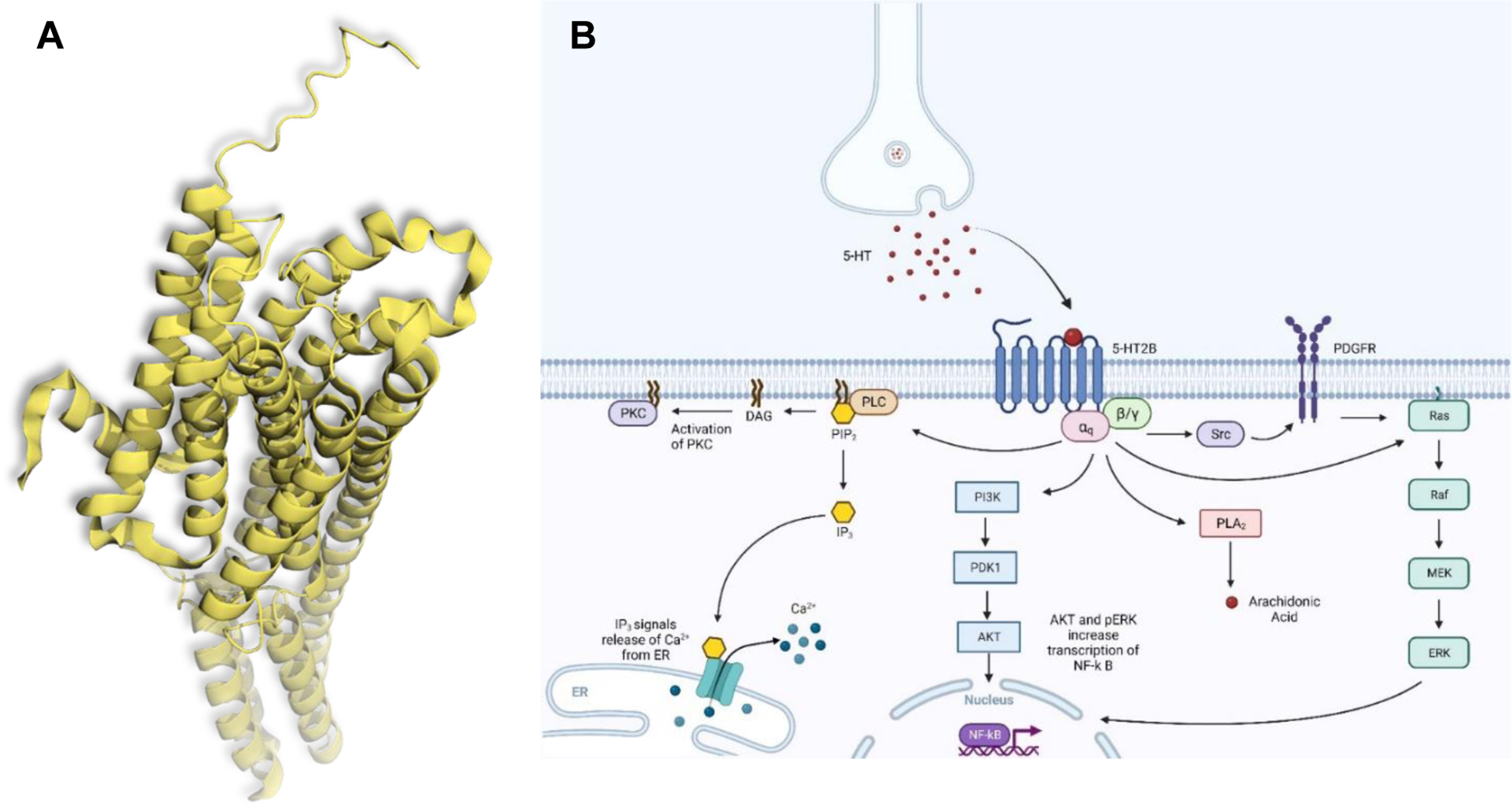
(A) Crystal structure of the human 5-HT2B receptor, PDB: 5TVN. (B) Signaling Pathways Associated with 5-HT2B (Adapted from “Activation of Protein Kinase C (PKC)”, by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates).
2. ANTITARGETS
2.1. The “Antitarget” Designation.
In pharmacology, an “antitarget” is broadly defined as any biological target that “[is] detrimental towards progression of [a] compound towards becoming a drug.”17 A ligand’s activity at an antitarget falls under the broader umbrella of “off-target” activity, which is generally unanticipated at the outset of a drug discovery program (it is not explicitly designed but need not necessarily be detrimental).17,18 While select antitargets garner the majority of attention in the medicinal chemistry literature, and indeed seem to be encountered more frequently in drug discovery programs, the full list of known antitargets is broad and diverse (and certainly not comprehensive).18 In short, any biological target that, upon engagement by a ligand, has the potential to induce adverse drug reactions (ADRs) can be classified as an antitarget.1 As will be further discussed, such a classification is often dependent on the specific mode of pharmacology of the ligand at the target in question (i.e. activation vs. inhibition).17,19
The labelling of a specific biological target as an antitarget also need not be absolute. A given target may be unofficially reexamined and reclassified over time, and an antitarget designation is often program specific. For example, agonist activity at the serotonin receptor 2A (5-HT2A) is associated with visual hallucinations and psychedelic experiences, and indeed many of the classical psychedelics are robust 5-HT2A agonists (lysergic acid diethylamide (LSD), psilocin, etc.).20 However, given the recent resurgence of this class of molecules in the context of psychedelic-assisted therapy,21 in which the overt psychedelic effects are postulated by many to be at least partly responsible for the observed efficacy,22 a blanket classification of 5-HT2A as an antitarget seems inappropriate. For indications unrelated to psychedelic-assisted therapy, however, such effects would almost certainly be undesired.
2.2. Examples of Antitargets.
In addition to 5-HT2A and other GPCRs,23 the current list of biological targets deemed “anti” is broad and includes kinases,24 ion channels,25 cytochrome P450s,26 and efflux pumps.27 Perhaps the most well-known and frequently encountered antitarget in the drug discovery literature is the human Ether-à-go-go-Related Gene, or the hERG channel.28–30 hERG is a potassium ion channel expressed in cardiac tissue, and plays a critical role in the regulation of the heart’s electrical activity.31 Specifically, disruption of hERG is associated with the development of potentially fatal Long QT Syndrome (LQTS), an unnatural lengthening of the QT cardiac repolarization interval.32
hERG has become a canonical antitarget amongst drug discovery scientists.33 Activity at the channel is routinely screened at early stages of the drug discovery pipeline, and strategic medicinal chemistry is implemented (if necessary) to avoid hERG inhibition for next generation molecules. This is due in no small part to the channel’s promiscuity; hERG-biased pharmacophores are routinely encountered in drug-like chemotypes,34 and compounds across a broad range of indications have been pulled from the market following observations of hERG-related cardiac abnormalities.35,36 Other potential antitargets, however, are less ubiquitous and therefore are not always a component of routine counter-screening. Subsequently, many undiscovered or poorly characterized targets likely exist that have the potential to become as notorious as hERG, and conversely, screening may eventually be deprioritized for other putative antitargets as their roles in physiological processes become clearer. A selection of notable biological antitargets, their associated risks, and exemplary ligands is summarized in Table 2.
Table 2.
Selected Antitargets and Associated Ligands.
| Biological Target | Classification | Associated Deleterious Effects | Mode of Pharmacology | Example Ligand(s) |
|---|---|---|---|---|
| 5-HT2A | GPCR | Hallucinations, psychedelic-experiences, changes in perception20–22 | Agonism | LSD Psilocin |
| 5-HT 2B | GPCR | Pulmonary arterial hypertension, valve disease 37,38 | Agonism |
Fenfluramine
Norfenfluramine MDMA |
| μOR | GPCR | Bradypnea, hypoxemia39 | Agonism | Morphine Fentanyl Carfentanil |
| M2 | GPCR | Reduction in heart rate23,40 | Agonism | Oxotremorine M (non-selective) |
| hERG | Ion Channel | LQTS31–36 | Inhibition | Cisapride |
| CaV1.2 | Ion Channel | LQTS, Brugda Syndrome25 | Inhibition | Verapamil (non-selective) |
| CYP3A4 | Cytochrome P40 | Various drug-drug interactions26,41 | Inhibition | Mibefradil |
| MDR1 (ABCB1) | Efflux Pump | Multiple drug resistance (chemotherapy)42,43 | Efflux substrate | Verapamil (inhibitor) Paclitaxel (substrate) |
To reiterate, an antitarget label does not completely preclude the utility of a target (many of our most important and useful drugs target 5-HT2A, calcium channels, and the mu opioid receptor (μOR), Table 2). As will be further discussed, a target’s physiological location (central vs. peripheral tissue) can also be deeply important concerning the manifestation of ADRs.
3. 5-HT2B AS AN ANTITARGET
3.1. Fen-Phen and Related Compounds.
It is now well established that excessive activation of 5-HT2B can lead to an increased risk for a number of cardiopathies including pulmonary arterial hypertension (PAH)37 and valvular heart disease (VHD).38 The wealth of available literature demonstrating this link21,22,44–47 is directly related to the 1997 withdrawal of the combination anti-obesity regimen fenfluramine/phentermine (fen-phen, Figure 2), which was associated with PAH and VHD in humans. In the original press release, the FDA stated that the basis for the withdrawal was “based on new findings from doctors who have evaluated patients taking these two drugs with echocardiograms, a special procedure that can test the functioning of heart valves. These findings indicate that approximately 30 percent of patients who were evaluated had abnormal echocardiograms, even though they had no symptoms. This is a much higher than expected percentage of abnormal test results.”48 The year prior, Connolly et al. identified a patient population of 24 women treated with fen-phen who developed VHD despite no history of cardiac disease.45 Additional studies from around this time demonstrated that a regimen of fenfluramine or its (S)-enantiomer dexfenfluramine (DF, Figure 2), increased the risk of developing PAH by a factor anywhere between 3.7 and 23-fold.49,50 A large population-based study of patients previously taking either fenfluramine, DF, or phentermine revealed several cases of idiopathic valvular disorders in patients taking fenfluramine or DF (with no cases noted for the phentermine population).47 Cumulatively, these studies strongly suggest that fenfluramine (and DF) were the agents responsible for the observed cardiopathies.
Figure 2.
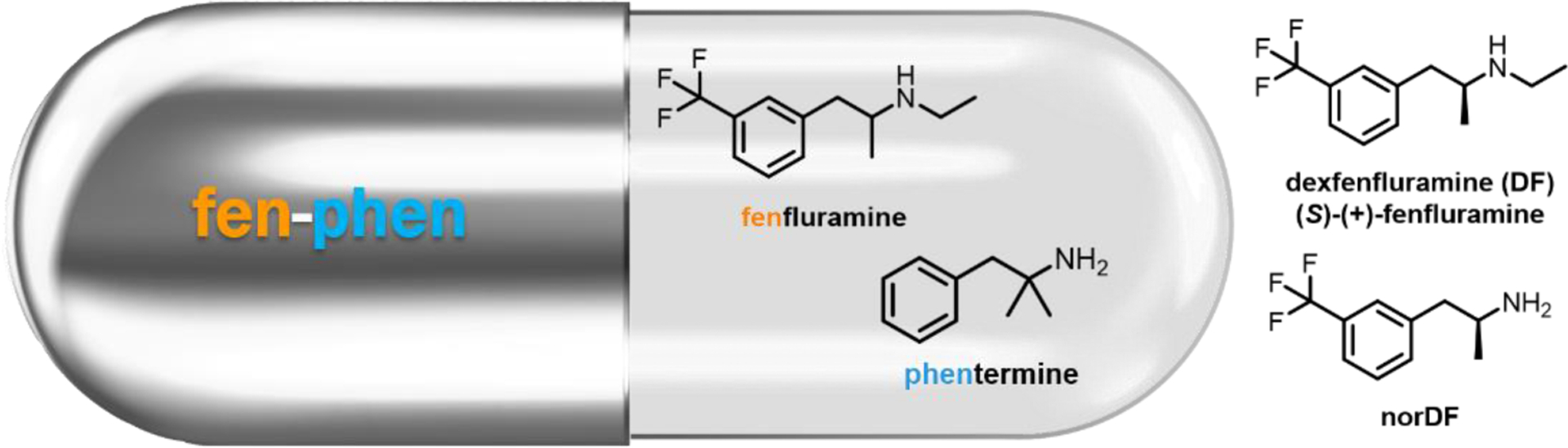
Chemical Structures of Key 5-HT2B Agonists
3.2. Molecular Pharmacology.
Although DF itself binds only weakly to the 5-HT2A, 5-HT2B, and 5-HT2C receptors, its primary metabolite, N-des-ethyl DF (norDF, Figure 2) is a high affinity 5-HT2B ligand with selectivity relative to 5-HT2A and 5-HT2C (5-HT2B Ki = 11.2 ± 4.3 nM).38 In functional assays, norDF is a potent agonist that stimulates phosphoinositide hydrolysis, intracellular Ca2+ levels, and the MAPK cascade (EC50 = 23–24 nM in IP hydrolysis and Ca2+ mobilization assays).38,51 Phentermine, by contrast, has no appreciable 5-HT2B binding affinity up to 10 μM, and is primarily a dopamine-releasing agent.38 A convergent body of evidence indeed suggests that the progression of both PAH and VHD are associated with highly increased 5-HT2B receptor expression levels, and that 5-HT2B activation is essential for disease progression.37,38,46,52,53 Rodent studies have since recapitulated the DF-induced development of PAH observed in human subjects,37,52,53 and additional studies have noted similar cardiopathies, primarily VHD, for other 5-HT2B agonists including MDMA,54 pergolide,54,55 and methysergide38,56. Taken together, these results strongly indicate substantial risks for treatments involving 5-HT2B agonists, and it has been recommended that all serotonergic drugs be screened for this functional profile.38,54 (Several widely used 5-HT2A agonists including DMT, LSD, psilocin and related phenethylamines and tryptamines are relatively non-selective relative to 5-HT2B; the increasingly prevalent use of such compounds will need to be reconciled with the risks associated with 5-HT2B activation).57–59
Unsurprisingly, 5-HT2B is now widely regarded as one of the primary antitargets in drug development pipelines, but it is critical that a compound’s mode of pharmacology at the receptor be fully understood before de-prioritization is initiated. A variety of receptor profiling tools, in silico cheminformatics assays, 5-HT2B functional assays, and suggested safety margins have been recommended toward this end.60 Currently, there is no evidence to suggest a role for 5-HT2B antagonists in the development of PAH and VD, and the paucity of such compounds, particularly those highly selective for 5-HT2B, have not indicated a potential for mechanism-based toxicity (cardiac or otherwise) associated with 5-HT2B inhibition. Moreover, as will be discussed in the following sections, such agents have the potential to be disease-modifying treatment strategies for these and related cardiac disorders.7
4. 5-HT2B ANTAGONISTS AND ANIMAL MODELS
4.1. SB-204741.
Work to elucidate fen-phen’s off-target 5-HT2B agonism led to the hypothesis that 5-HT2B antagonism is a potential therapeutic for cardiopulmonary diseases. Of the multitude of selective and non-selective 5-HT2B antagonists, SB-204741 was the first synthesized (1994) and is widely used; it has a high affinity for 5-HT2B (pKi = 7.95) and high selectivity (>135) compared to 5-HT2C, the receptor most closely matching 5-HT2B in morphology.61,62 One of the first animal studies in which SB-204741 was utilized involved antagonizing individual 5-HT2 receptors to investigate renal sympathoinhibition and mean arterial blood pressure following intracerebroventricular administration of quipazine, a 5-HT2 agonist, in rats15. SB-204741 has been used to study the blockade of 5-HT2B in the context of several cardiopulmonary diseases such as pulmonary hypertension,37,63,64 myocardial infarction,65 and calcific aortic valve disease,66,67 with encouraging results in the prevention of disease progression.
4.2. Gene Editing.
In addition to the administration of antagonists, mouse models that target 5-HT2B through genetic ablation have confirmed the receptor’s role in cardiopulmonary disease. In one report, the 5-HT2B allele was rendered nonfunctional in embryonic stem cells through the interruption of the protein reading frame; this was done by introducing the bacterial neo gene in exon 2 of the 5-HT2B gene sequence.68 Ablation of both copies of the 5-HT2B gene results in viable offspring, with mutant mice growing to adulthood; however, due to its importance in heart development, 5-HT2B mutant mice demonstrate ventricular hypoplasia, myocyte disarray, and ventricular dilation.68,69
The gene editing technology “Cre-Lox” allows for the knockout of both 5-HT2B alleles in a time- and site-specific manner through homologous recombination. It relies upon the recognition of specific DNA sequences called loxP sites by the enzyme Cre recombinase, which is activated by a tissue-specific promoter that itself is induced exogenously by a stimulus, such as tamoxifen or doxycycline.70 Tissue-specific promoters such as Transcription factor 21(Tcf21)Cre, Periostin (Pstn)Cre, and Angiopoietin-1 receptor gene (Tie2)Cre are useful tissue-specific promoters that provide cell-lineage tracing capabilities, as Tcf21 is expressed in resident fibroblasts, Pstn is expressed in myofibroblasts, and Tie2 is expressed in endothelial cells.71–73 5-HT2B conditional knockout models have been used to investigate cardiopulmonary fibrosis in diseases such as myocardial infarction65 and PAH.64
4.3. Relevant Signaling Pathways.
5-HT2B agonism results in downstream activation of the signal transduction pathway Ras/Raf/mitogen-activated protein kinase (MAPK), leading to an increase in rate of cell division and proliferation.9,74 Cytoplasmic tyrosine kinases (e.g., Src) are key mediators of G protein-coupled receptor (GPCRs) signaling to the MAPK pathway.9,75 Additionally, transforming growth factor-β1 (TGF-β1) is a cytokine that mediates fibroblast proliferation, extracellular matrix (ECM) deposition, and myofibroblast differentiation76 through SMADS, the substrates for TGF-β1 receptors. TGF-β1 and its fibrotic activity is upregulated upon 5-HT2B agonism due to signaling pathway crosstalk via Src phosphorylation.67
With 5-HT2B leading to increased stromal fibroblast and myofibroblast proliferation, combined with the increased deposition of collagen into the ECM,77 this provides a rational hypothesis for the pathophysiology of many cardiopulmonary diseases involving 5-HT2B signaling (Figure 3). In PAH, the core etiology is unchecked muscularization of the pulmonary arterioles; the tissue-specific promoter Tie2Cre allows for targeted 5-HT2B ablation in the bone marrow-derived proangiogenic cells (PACs) and results in normalized arteriole compliance.64 Conditional 5-HT2B ablation in myocardial infarction using tissue-specific Cre promoters demonstrated that resident fibroblasts and myofibroblasts were the main culprit of detrimental scar thickness and heart contractility.65 Similarly, calcific aortic valve disease is characterized by fibrotic deposition on aortic valve leaflet cusps by aortic valve interstitial cells (AVICs), and antagonism of 5-HT2B opposed AVIC activation through a myofibroblast, and therefore TGF-β1, mechanism.66,67 Taken together, these studies indicate that 5-HT2B inactivation is an attractive strategy for modifying cardiopulmonary fibrotic disease.
Figure 3.
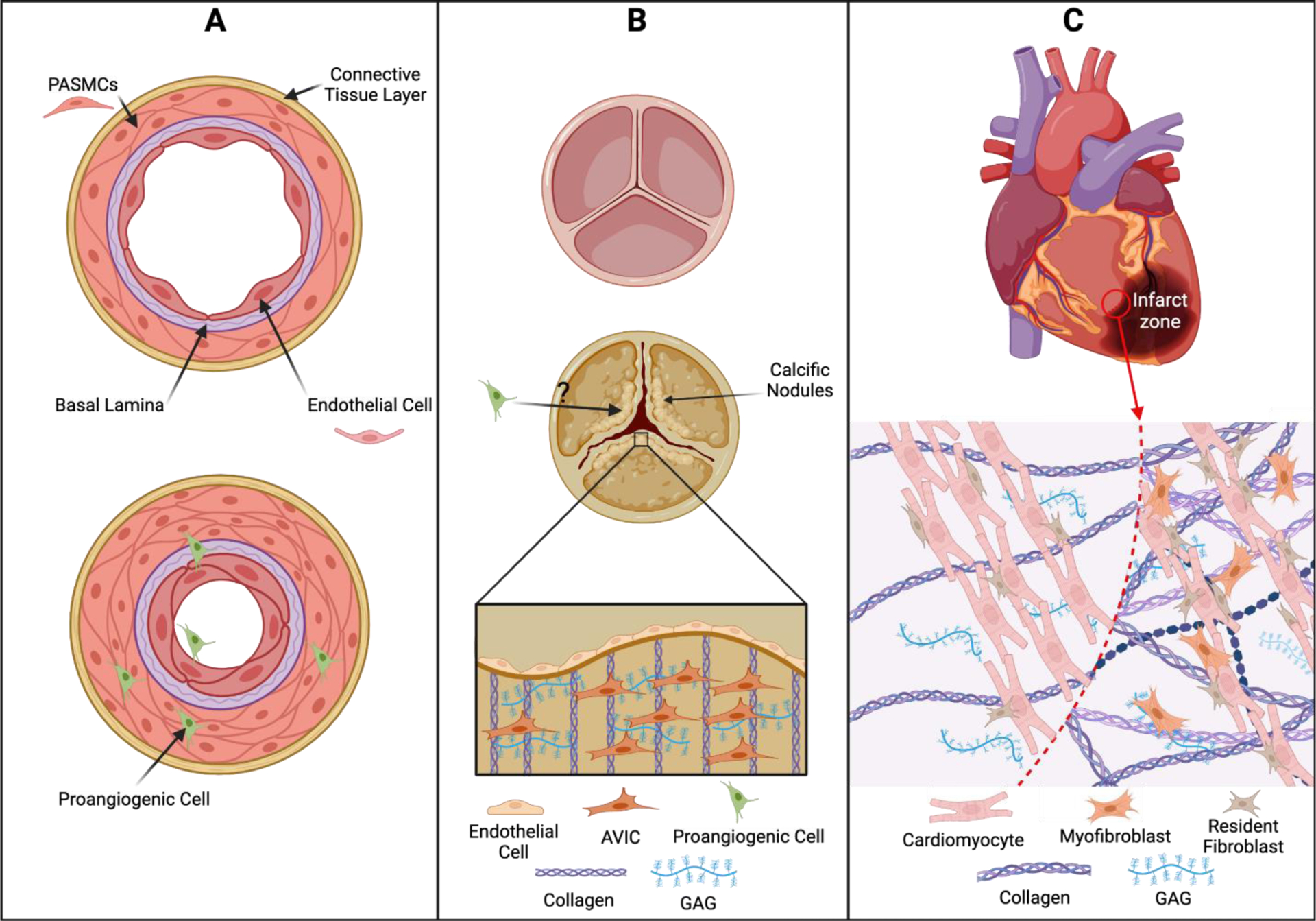
(A) 5-HT2B-mediated recruitment of proangiogenic cells in pulmonary arterial hypertension leads to muscularization of pulmonary arterioles, shown by proliferation of pulmonary artery smooth muscle cells (PASMCs). (B) Valve remodeling in calcific aortic valve disease is driven by aortic valve interstitial cells (AVICs) that increase deposition of collagen and glycosaminoglycans (GAG) into the ECM; this stiffens the aortic valve leaflets and decreases valve compliance. It is unknown whether proangiogenic cells play a role in remodeling the valve ECM. (C) Myofibroblasts are responsible for ECM stiffening and scar tissue formation of the infarct zone in myocardial infarction, causing cardiac tissue deterioration, decreased compliance, and decreased cardiac output. Retrieved from https://app.biorender.com
5. DEVELOPMENT OF NEXT GENERATION 5-HT2B ANTAGONISTS
5.1. Preclinical Compounds.
From a medicinal chemistry perspective, a number of interesting 5-HT2B structure-activity relationship (SAR) studies are reported in the literature, with programs ranging from early lead optimization61,78–81 through clinical development.82,83 In both cases, the optimization for selectivity relative to 5-HT2A and 5-HT2C is a critical parameter, and in many programs has proven challenging to attain for both receptors simultaneously.61,81,84 In the 1990s, a series of reports detailing compounds derived from yohimbine,81 and substituted indoles and indolines61,78–81 described reasonable (~100 fold) selectivity for 5-HT2A and/or 5-HT2C (see Figure 4 for a selection of reported 5-HT2B chemotypes). In the latter class, the previously discussed isothiazole SB-204741 is considered to be the first reported selective 5-HT2B antagonist (Figure 4).61 While these early reports are admirably thorough with respect to SAR and 5-HT2A/2C selectivity profiling, they contain limited information regarding pharmacokinetics (PK) and broader ancillary pharmacology. Bonhaus et al. subsequently reported a series of naphthylpyrimidines, including RS-127445, which displays improved 5-HT2B selectivity (~1,000 fold against a broader off-target profile), albeit with limited oral bioavailability in rats (Figure 4).84 Additional reports of selective 5-HT2B antagonists with more detailed PK profiles have slowly started to emerge in the literature.85,86 More recently, members of the 2-thiazoline pulicatin class of natural products (and synthetic derivatives) were reported to have high selectivity for 5-HT2B relative to a broader panel of serotonin receptor subtypes (Figure 4).87 As before, drug development-enabling PK information is not reported. In 2023, Schieferdecker and Vock reported detailed 5-HT2B pharmacophore models which are likely to aid in the development of next-generation ligands with robust subtype selectivity.88
Figure 4.
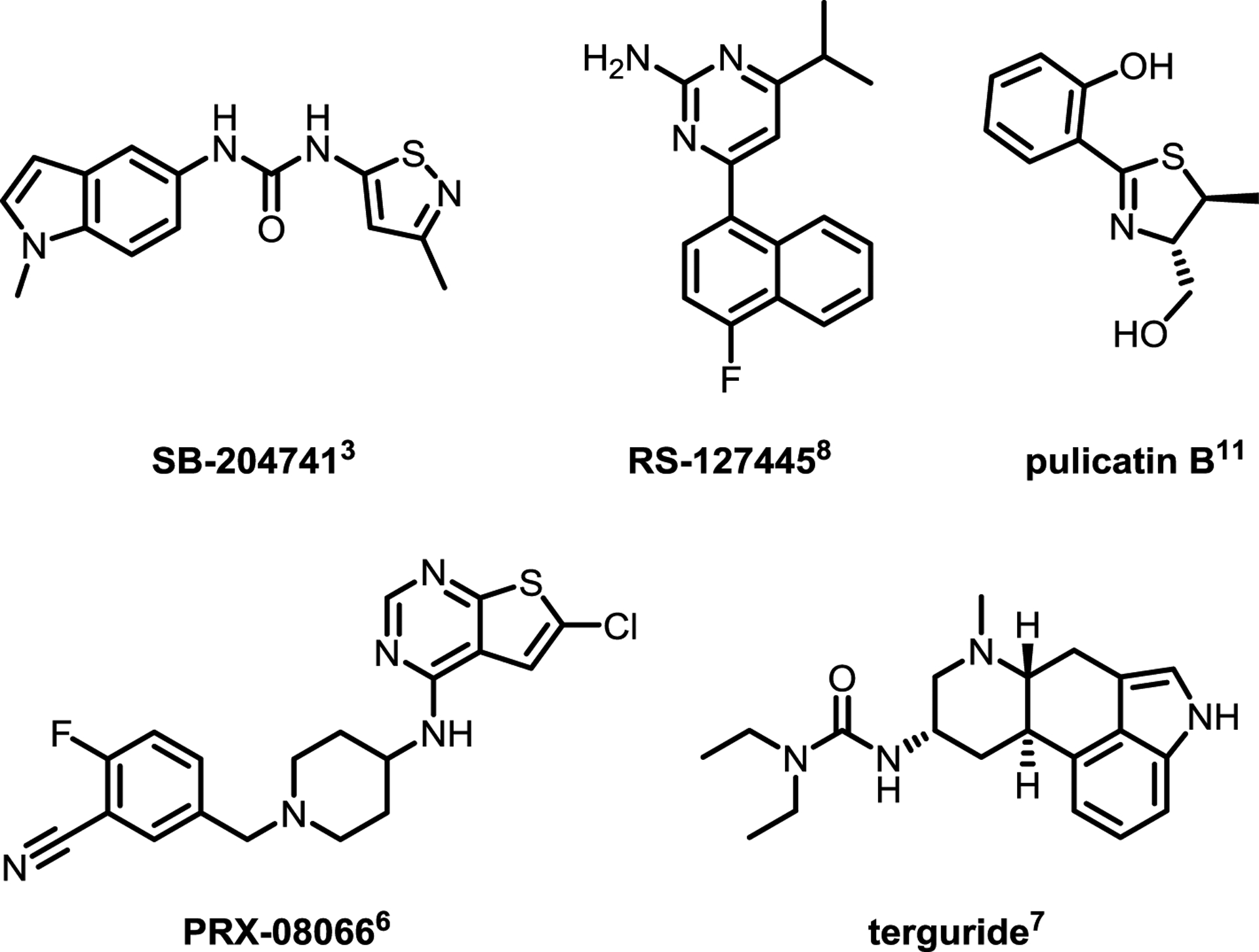
Chemical Structures of Selected 5-HT2B Antagonists
5.2. Clinical Compounds.
With respect to the clinical development of more advanced molecules, thiophenylpyrimidine PRX-08066 (Figure 4) is a potent and selective 5-HT2B antagonist developed by Epix Pharmaceuticals, which was shown to be highly effective in the treatment of drug-induced PAH and VHD in rats.82 As of 2009, a Phase 2, “3-month open label study to evaluate the safety and efficacy of PRX-08066 in patients with pulmonary hypertension and COPD” was terminated for undisclosed reasons.89 Terguride, a potent ergoline 5-HT2A/5-HT2B dual antagonist,83 was granted orphan drug status for PAH treatment as of 2008,90 although clinical development was ultimately discontinued by 2011 due to lack of efficacy.91,92 In the absence of more concrete findings, it is difficult to speculate on the reasons for theses terminations (in the case of terguride the issue is thought to be related to appropriate plasma exposure levels),92 but there is clearly a need for a more thorough discussion on PK, exposure, and tolerability of clinical-stage 5-HT2B antagonists in the literature. These data will be critical for the design of future clinical trials with next generation molecules.
6. 5-HT2B POLYPHARMACOLOGY
6.1. Marketed 5-HT2B Antagonists.
Many currently marketed drugs (as well as widely studied compounds in late stage clinical development) display robust 5-HT2B antagonism in radioligand binding assays as part of their broader polypharmacological profile. This is primarily true of antipsychotic medications, although examples of antidepressants, antihypertensives, antiparkinsonians, and antisedatives with 5-HT2B activity are also known. A search of the National Institute of Mental Health’s Psychoactive Drug Screening Program (NIMH-PDSP)93 Ki Database for 5-HT2B ligands with Ki’s < 100 nM returns over 500 unique results, one of which, aripiprazole, is still among the top 100 pharmaceuticals in terms of yearly sales (Table 3).94 Although many of these compounds are promiscuous with respect to additional CNS receptor targets, it seems clear that 5-HT2B antagonist activity (which should of course be rigorously characterized during development) should not preclude a compound from advancement to the clinic.
Table 3.
Selected 5-HT2B Binding Affinity Data for Notable Psychoactive Compounds
| Compound | Classification | 5-HT2B Ki (nM) | Species | Radioligand |
|---|---|---|---|---|
| Mianserin95 | antidepressant | 9.0 | Human | [3H]-5HT |
| Trazodone38 | antidepressant | 74 | Human | [3H]-5HT |
| Cyproheptadine96 | antihistamine | 1.5 | Human | [3H]-5HT |
| Ketanserin97 | antihypertensive | 2.4 | Bovine | [3H]-ketanserin |
| Lisuride96 | antiparkinsonian | 1.1 | Human | [3H]-5HT |
| Amisulpride98 | antipsychotic | 13 | Human | [3H]-LSD |
| Aripiprazole99 | antipsychotic | 0.36 | Human | [3H]-LSD |
| Asenapine93 | antipsychotic | 0.21 | Human | [3H]-LSD |
| Chlorpromazine97 | antipsychotic | 6.0 | Bovine | [3H]-ketanserin |
| Clozapine95 | antipsychotic | 7.2 | Human | [3H]-5HT |
| Lurasidone93 | antipsychotic | 24 | Human | [3H]-LSD |
| Olanzapine100 | antipsychotic | 12 | Human | [3H]-5HT |
| Quetiapine93 | antipsychotic | 86 | Human | [3H]-LSD |
| Risperidone100 | antipsychotic | 29 | Human | [3H]-5HT |
| Spiperone97 | antipsychotic | 0.8 | Bovine | [3H]-ketanserin |
| Xanomeline101 | antipsychotic | 20 | Human | [3H]-5HT |
| Yohimbine95 | antisedative (veterinary) | 43 | Human | [3H]-5HT |
While many of the examples reported here fall into similar indication classes, a couple of examples warrant further discussion. Lisuride is a potent dopamine agonist and a synthetic ergoline derivative, a class to which cabergoline and pergolide also belong. Of these and related dopamine agonists, only cabergoline and pergolide (5-HT2B agonists) were associated with VHD after long term use; 5-HT2B antagonists (i.e. lisuride) demonstrate no such association. Indeed, lisuride has been prescribed for decades without a single known VHD report.102,103 Although a lack of association does not necessarily demonstrate prevention, examples also exist of marketed drugs with 5-HT2B antagonism as part of their polypharmacology that explicitly reverse drug-induced VHD. Cyproheptadine, a first-generation tricylic antihistamine with potent 5-HT2B antagonist activity (Ki = 1.5 nM)96, has been shown to reverse pergolide-induced valvulopathy in rats.104 Future analyses of patient populations taking one or more of these compounds will be important to further understand the potential for drug repurposing toward the prevention or treatment of VHD and related disorders.
It is worth noting that the compounds in Table 3 represent only molecules that are known to be psychoactive (CNS-penetrant). As will be discussed in the next section, it will be important to understand the potential risks associated with centrally-mediated 5-HT2B antagonism with the development of any next generation therapeutic.
7. NOVEL 5-HT2B ANTAGONISTS FOR PAH TREATMENT
7.1. VU6047534 and Analogs.
Our group has recently disclosed a potent and highly selective 5-HT2B antagonist, VU6047534, which possesses rodent PK properties suitable for proof-of-concept studies (Figure 5). Structurally, VU6047534 is derived from the SB204741-like series of urea-indoles, but is cyclized to give a substituted thieno[2,3-d]pyrimidine-2,4(1H,3H)-dione backbone. Encouragingly, VU6047534 demonstrated robust efficacy in Sugen-hypoxia mouse models of PAH prevention and treatment, as well as the prevention of right ventricle hypertrophy in Sugen-hypoxia and pulmonary arterial banding models. VU6047534 also displayed no significant off-target responses in the Eurofins Panlabs ancillary pharmacology screen of 68 common membrane proteins, ion channels and transporters (including the hERG channel),105 and was clean with respect to cytochrome P450 inhibition across 4 major isoforms (1A2, 2C9, 2D6, 3A4 IC50s >30 μM).106
Figure 5.
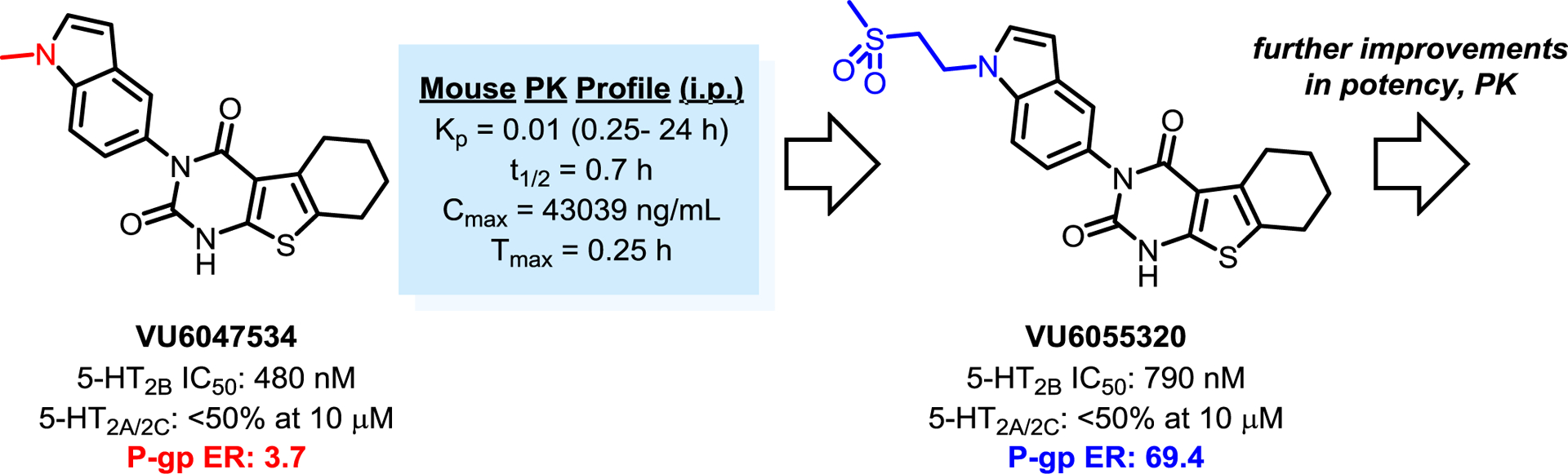
Development of Selective and Peripherally-Restricted 5-HT2B Antagonists for In Vivo Studies
Although VU6047534 displays negligible brain exposure in mice (Figure 5), this compound is predicted to have moderate brain exposure in human subjects due to a relative lack of P-glycoprotein (P-gp)-mediated efflux (3.7 efflux ratio, PappA-B = 18.0 10−6 cm/s). Centrally-mediated 5-HT2B antagonism is thought to be associated with a variety of adverse effects including depression, aggression, impulsivity, and suicidality. The presence of a relatively common 5-HT2B stop codon exclusive to Finnish populations has been associated with these types of psychiatric diseases, highlighting the potential dangers associated with a centrally-penetrant antagonist.107–109
An important caveat, however, is that all of the drugs listed in Table 3 are known to be CNS-active, and many are routinely and safely taken by millions across the globe. While the majority of these compounds tend to be promiscuous with respect to off-target activity at additional CNS receptors, it is tempting to speculate that CNS-penetrant compounds with robust 5-HT2B antagonist activity may be well tolerated with long term use (at least for a majority of the population). Further research in this area is clearly needed to understand this apparent discrepancy.
Subsequent SAR on the VU6047534 scaffold, specifically the exploration of polar indole N-substitutions, yielded next generation molecules with comparable potency and selectivity profiles that are predicted to be robust P-gp efflux substrates (VU6055320; 69.4 efflux ratio, PappA-B = 0.35 10−6 cm/s).106 Further preclinical characterization (and assessment for efficacy in similar rodent models) will be needed for these next generation 5-HT2B antagonists, and such studies are ongoing in our laboratories. Additionally, we have recently disclosed results from a high-throughput screen (HTS) aimed at identifying additional chemical matter for the development of structurally orthogonal 5-HT2B antagonists.110 Our HTS campaign led to the immediate identification of potent and selective compounds (5-HT2B IC50s in the low nanomolar range; <50% inhibition of 5-HT2A/2C at 10 μM). Furthermore, selected compounds from the most potent reconfirmed hits were selected for profiling in the P-gp assay, with exemplary compounds showing a low potential for brain exposure in human subjects.110
8. CONCLUSIONS AND PERSPECTIVES
While 5-HT2B has historically been viewed as an antitarget by medicinal chemists, the assessment of a compound’s mode of pharmacology at the receptor is crucial. The difference between 5-HT2B agonism and antagonism could mean the difference between a cardiotoxic agent (fen-phen) and a disease modifying treatment for PAH, VHD, and related disorders. It is our hope that the chemical scaffolds described herein will provide a useful platform for drug discovery scientists interested in this field, as there still exists an enormous unmet need to develop a 5-HT2B antagonist with the full package of properties suitable for clinical development. Strategies for 5-HT2B inactivation also need not be limited to simple orthosteric antagonists; the development of negative allosteric modulators (NAMs) and 5-HT2B-specific protein degraders could also prove viable. Regardless of the approach, it is our belief that the future for drug discovery at this receptor is bright.
Significance: Antagonists of the serotonin receptor 2B (5-HT2B) are a promising and underexplored potential treatment for pulmonary arterial hypertension (PAH) and valvular heart disease (VHD).
Impact: 5-HT2B antagonists are disease modifying with respect to PAH and VHD in preclinical species, and could translate to a first in class treatment in human subjects.
Innovation: Structurally-novel 5-HT2B antagonists with favorable selectivity and pharmacokinetic (PK) profiles are being profiled for clinical development.
ACKNOWLEDGMENTS
A.M.B. and C.W.L. thank the William K. Warren Family and Foundation for funding the William K. Warren, Jr. Chair in Medicine and support of our programs. We also thank the National Institute of Health (HL135790, HL95797, and HL087738) and Vanderbilt University Discovery Grant. Research undertaken in the Vanderbilt Mouse Neurobehavior Core was supported by the EKS NICHD of the NIH under Award P50HD103537. The Vanderbilt HTS Core (VHTSC) receives support from the Vanderbilt Institute of Chemical Biology and the Vanderbilt Ingram Cancer Center (P30CA68485).
ABBREVIATIONS USED
- 5-HT
5-hydroxytryptamine
- 5-HT2A
serotonin receptor 2A
- 5-HT2B
serotonin receptor 2B
- 5-HT2C
serotonin receptor 2C
- ADRs
adverse drug reactions
- AVICs
aortic valve interstitial cells
- CNS
central nervous system
- DF
dexfenfluramine
- DMT
dimethyltryptamine
- ECM
extracellular matrix
- FDA
Food and Drug Administration
- GI
gastrointestinal
- GPCR
G protein-coupled receptor
- hERG
human ether-à-go-go-related gene
- i.p.
intraperitoneal
- IP
inositol monophosphate
- Ki
inhibition constant
- Kp
total brain:total plasma ratio
- LQTS
long QT syndrome
- LSD
lysergic acid diethylamide
- MAPK
mitogen-activated protein kinase
- MDMA
3,4-methyl enedioxy methamphetamine
- μOR
mu opioid receptor
- NIMH-PDSP
National Institute of Mental Health’s Psychoactive Drug Screening Program
- norDF
des-ethyl-dexfenfluramine
- PACs
proangiogenic cells
- PAH
pulmonary arterial hypertension
- P-gp
P-glycoprotein
- PK
pharmacokinetic
- Pstn
periostin
- SAR
structure-activity relationship
- Tcf21
transcription factor 21
- TGF-β1
transforming growth factor-β1
- Tie2
angiopoietin-1 receptor gene
- VHD
valvular heart disease
Biographies
Aaron M. Bender earned his Ph.D. in medicinal chemistry from the University of Michigan in 2016, and subsequently joined the Warren Center for Neuroscience Drug Discovery (WCNDD) at Vanderbilt University as a postdoctoral scholar. Upon completion of his postdoctoral training, Aaron remained at Vanderbilt and is currently an Assistant Director of Medicinal Chemistry at the WCNDD. His research interests are in medicinal chemistry, particularly the development of therapeutics targeting G protein-coupled receptors, and organic methodology.
Lauren C. Parr received her B.A from the University of Kansas in 2019 and M.S. from the University of Florida in 2022. She is currently a Ph.D. student in Dr. Craig W. Lindsley’s Lab at Vanderbilt University focusing on the molecular mechanisms of kinases and development of small-molecule inhibitors.
William B. Livingston received his B.S. degree from the Jacobs School of Engineering at the University of California San Diego in 2022. He then joined the Merryman Mechanobiology Laboratory in 2022 as a pre-doctoral student in the Biomedical Engineering Department at Vanderbilt University, where he is currently investigating the role of 5-HT2B in hypertrophic cardiomyopathy.
Craig W. Lindsley is the William K. Warren, Jr. Chair in Medicine and a University Professor of Pharmacology, Chemistry and Biochemistry at Vanderbilt University. Dr. Lindsley is also the director of the Warren Center for Neuroscience Drug Discovery (WCNDD) at Vanderbilt, and the Editor-in-Chief of the Journal of Medicinal Chemistry. His research interests include the development of novel treatment strategies for schizophrenia, Parkinson’s disease, and other brain disorders, as well as disorders of the cardiovascular system.
W. David Merryman W. David Merryman received his Ph.D. degree from the University of Pittsburgh in 2007 and is currently the Walters Family Professor in the Department of Biomedical Engineering, and Professor of Pharmacology, Medicine, and Pediatrics at Vanderbilt University. His research interests are cardiopulmonary and renal mechanobiology with a particular focus on developing new therapeutic strategies.
Footnotes
The authors declare no competing financial interest.
Contributor Information
Aaron M. Bender, Warren Center for Neuroscience Drug Discovery, Department of Pharmacology, Vanderbilt University, Nashville, TN
Lauren C. Parr, Warren Center for Neuroscience Drug Discovery, Department of Pharmacology, Vanderbilt University, Nashville, TN
William B. Livingston, Department of Biomedical Engineering, Vanderbilt University
Craig W. Lindsley, Warren Center for Neuroscience Drug Discovery, Department of Pharmacology, Department of Chemistry, Department of Biochemistry, Vanderbilt Institute for Chemical Biology, Nashville TN
W. David Merryman, Department of Biomedical Engineering, Vanderbilt University, Nashville, TN.
REFERENCES
- 1.Gaddum JH; Picarelli ZP Two Kinds of Tryptamine Receptor. Br. J. Pharmacol. Chemother 1957, 12, 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley PB; Engel G; Feniuk W; Fozard JR; Humphrey PP; Middlemiss DN; Mylecharane EJ; Richardson BP; Saxena PR Proposals for the Classification and Nomenclature of Functional Receptors for 5-hydroxytryptamine. 1986. Neuropharmacology, 25, 563–576. [DOI] [PubMed] [Google Scholar]
- 3.Vane JR The Relative Activities of some Tryptamine Analogues on the Isolated Rat Stomach Strip Preparation. Br. J. Pharmacol. Chemother 1959, 14, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pytliak M; Vargová V; Mechírová V; Felšöci M Serotonin Receptors - From Molecular Biology to Clinical Applications. Physiol. Res 2011, 60, 15–25. [DOI] [PubMed] [Google Scholar]
- 5.Schmuck K; Ullmer C; Engels P; Lübbert H Cloning and Functional Characterization of the Human 5-HT2B Serotonin Receptor. FEBS Lett. 1994, 342, 85–90. [DOI] [PubMed] [Google Scholar]
- 6.Bonaventure P; Nepomuceno D; Miller K; Chen J; Kuei C; Kamme F; Tran DT; Lovenberg TW; Liu C Molecular and Pharmacological Characterization of Serotonin 5-HT2A and 5-HT2B Receptor Subtypes in Dog. Eur. J. Pharmacol 2005, 513, 181–192. [DOI] [PubMed] [Google Scholar]
- 7.Brea J; Castro-Palomino J; Yeste S; Cubero E; Párraga A; Domínguez E; Loza MI Emerging Opportunities and Concerns for Drug Discovery at Serotonin 5-HT2B Receptors. Curr. Top. Med. Chem 2010, 10, 493–503. [DOI] [PubMed] [Google Scholar]
- 8.Ullmer C; Schmuck K; Kalkman HO; Lübbert H Expression of Serotonin Receptor mRNAs in Blood Vessels. FEBS Lett. 1995, 370, 215–221. [DOI] [PubMed] [Google Scholar]
- 9.Nebigil CG; Launay JM; Hickel P; Tournois C; Maroteaux L 5-Hydroxytryptamine 2B Receptor Regulates Cell-Cycle Progression: Cross-Talk with Tyrosine Kinase Pathways. Proc. Natl. Acad. Sci. U.S.A 2000, 97, 2591–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karmakar S; Lal G Role of Serotonin Receptor Signaling in Cancer Cells and Anti-Tumor Immunity. Theranostics 2021, 11, 5296–5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masson J; Emerit MB; Hamon M; Darmon M Serotonergic Signaling: Multiple Effectors and Pleiotropic Effects. Wiley Interdiscip. Rev. Membr. Transp. Signal 2012, 1, 685–713. [Google Scholar]
- 12.Nichols DE; Nichols CD Serotonin Receptors. Chem. Rev 2008, 108, 1614–1641. [DOI] [PubMed] [Google Scholar]
- 13.Borman RA; Tilford NS; Harmer DW; Day N; Ellis ES; Sheldrick RL; Carey J; Coleman RA; Baxter GS 5-HT(2B) Receptors Play a Key Role in Mediating the Excitatory Effects of 5-HT in Human Colon in Vitro. Br. J. Pharmacol 2002, 135, 1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Günther S; Maroteaux L; Schwarzacher SW Endogenous 5-HT2B Receptor Activation Regulates Neonatal Respiratory Activity in Vitro. J. Neurobiol 2006, 66, 949–961. [DOI] [PubMed] [Google Scholar]
- 15.Knowles ID; Ramage AG Evidence that Activation of Central 5-HT(2B) Receptors causes Renal Sympathoexcitation in Anaesthetized Rats. Br. J. Pharmacol 2000, 129, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berger M; Gray JA; Roth BL The Expanded Biology of Serotonin. Annu. Rev. Med 2009, 60, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaz RJ; Klabunde T, Eds. Antitargets; Wiley-VCH; Weinheim, 2008. [Google Scholar]
- 18.Peters J-U Polypharmacology – Foe or Friend? J. Med. Chem 2013, 56, 8955–8971. [DOI] [PubMed] [Google Scholar]
- 19.Zakharov AV; Lagunin AA; Filimonov DA; Poroikov VV Quantitative Prediction of Antitarget Interaction Profiles for Chemical Compounds. Chem. Res. Toxicol 2012, 25, 2378–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slocum ST; DiBerto JF; Roth BL Molecular Insights into Psychedelic Drug Action. J. Neurochem 2022, 162, 24–38. [DOI] [PubMed] [Google Scholar]
- 21.Schenberg EE Psychedelic-Assisted Psychotherapy: A Paradigm Shift in Psychiatric Research and Development. Front. Pharmacol 2018, 9, 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yaden DB; Griffiths RR The Subjective Effects of Psychedelics are Necessary for Their Enduring Therapeutic Effects. ACS Pharmacol. Transl. Sci 2021, 4, 568–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruse AC; Weiss DR; Rossi M; Hu J; Hu K; Eitel K; Gmeiner P; Wess J; Kobilka BK; Shoichet BK Muscarinic Receptors as Model Targets and Antitargets for Structure-Based Ligand Discovery. Mol. Pharmacol 2013, 84, 528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munson MC Introduction to Kinase Antitargets. In Antitargets and Drug Safety; Wiley-VCH; Weinheim, 2015; Urban L; Patel VF; Vaz RJ Eds. [Google Scholar]
- 25.Kowalska M; Nowaczyk J; Nowaczyk A KV11.1, NaV1.5, and CaV1.2 Transporter Proteins as Antitarget for Drug Cardiotoxicity. Int. J. Mol. Sci 2020, 21, 8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch T; Price A The Effect of Cytochrome P450 Metabolism on Drug Response, Interactions, and Adverse Effects. Am. Fam. Physician 2007, 76, 391–396. [PubMed] [Google Scholar]
- 27.Broccatelli F; Carosati E; Cruciani G; Oprea TI Transporter-Mediated Efflux Influences CNS Side Effects: ABCB1, from Antitarget to Target. 2010, 29, 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma H; Pagare PP; Li M; Neel LT; Mendez RE; Gillespie JC; Stevens DL; Dewey WL; Selley DE; Zhang Y Structural Alterations of the “Address” Moiety of NAN Leading to the Discovery of a Novel Opioid Receptor Modulator with Reduced hERG Toxicity. J. Med. Chem 2022, ASAP. doi: 10.1021/acs.jmedchem.2c01499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spock M; Carter TR; Bollinger KA; Han C; Baker LA; Rodriguez AL Peng L; Dickerson JW; Qi A; Rook JM; O’Neill JC; Watson KJ; Chang S; Bridges TM; Engers JL; Engers DW; Niswender CM; Conn PJ; Lindsley CW; Bender AM Discovery of VU6028418: A Highly Selective and Orally Bioavailable M4 Muscarinic Acetylcholine Receptor Antagonist . ACS Med. Chem. Lett 2021, 12, 1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W; Lun S; Wang S-S; Cai Y-P; Yang F; Bishai WR; Yu Li.-F. Structure-Based Optimization of Coumestan Derivatives as Polyketide Synthase 13-Thioesterase(Pks13-TE) Inhibitors with Improved hERG Profiles for Mycobacterium tuberculosis Treatment. J. Med. Chem 2022, 65, 13240–13252. [DOI] [PubMed] [Google Scholar]
- 31.Sanguinetti MC; Tristani-Firouzi M hERG Potassium Channels and Cardiac Arrhythmia. Nature 2006, 440, 463–469. [DOI] [PubMed] [Google Scholar]
- 32.Hedley PL; Jørgensen P; Schlamowitz S; Wangari R; Moolman-Smook J; Brink PA; Kanters JK; Corfield VA; Christiansen M Hum. Mutat 2009, 30, 1486–1511. [DOI] [PubMed] [Google Scholar]
- 33.Kalyaanamoorthy S; Barakat KH Development of Safe Drugs: The hERG Challenge. Med. Res. Rev 2017, 38, 525–555. [DOI] [PubMed] [Google Scholar]
- 34.Kratz JM; Schuster D; Edtbauer M; Saxena P; Mair CE; Kirchebner J; Matuszczak B; Baburin I; Hering S; Rollinger JM Experimentally Validated hERG Pharmacophore Models as Cardiotoxicity Prediction Tools. J. Chem. Inf. Model 2014, 54, 2887–2901. [DOI] [PubMed] [Google Scholar]
- 35.Varró A; Baczkó I Cardiac Ventricular Repolarization Reserve: A Principle for Understanding Drug-Related Proarrhythmic Risk. Br. J. Pharmacol 2011, 164, 14–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rampe D; Brown AM A History of the Role of the hERG Channel in Cardiac Risk Assessment. J. Pharmacol. Toxicol. Methods 2013, 68, 13–22. [DOI] [PubMed] [Google Scholar]
- 37.Launay J-M; Hervé P; Peoc’h K; Tournois C; Callebert J; Nebigil CG; Etienne N; Drouet L; Humbert M; Simonneau G; Maroteaux L Function of the Serotonin 5-Hydroxytrypamine 2B Receptor in Pulmonary Hypertension. Nat. Med 2002, 8, 1129–1135. [DOI] [PubMed] [Google Scholar]
- 38.Rothman RB; Baumann MH; Savage JE; Rauser L; McBride A; Hufeisen SJ; Roth BL Evidence for Possible Involvement of 5-HT2B Receptors in the Cardiac Valvulopathy Associated with Fenfluramine and Other Serotonergic Medications. Circulation 2000, 102, 2836–2841. [DOI] [PubMed] [Google Scholar]
- 39.Algera MH; Kamp J; van der Schrier R; van Valzen M; Niesters M; Aarts L; Dahan A; Olosen E Opioid-Induced Respiratory Depression in Humans: A Review of Pharmacokinetic-Pharmacodynamic Modelling of Reversal. Br. J. Anaesth 2019, 122, e168–e179. [DOI] [PubMed] [Google Scholar]
- 40.Vogel WK; Sheehan DM; Schimerlik MI Site-Directed Mutagenesis on the m2 Muscarinic Acetylcholine Receptor: The Significance of Tyr403 in the Binding of Agonists and Functional Coupling. Mol. Pharmacol 1997, 52, 1087–1094. [DOI] [PubMed] [Google Scholar]
- 41.Prueksaritanont T; Ma B; Tang C; Meng Y; Assang C; Lu P; Reider PJ; Lin JH; Baillie TA Metabolic Interactions Between Mibefradil and HMG-CoA Reductase Inhibitors: An In Vitro Investigation with Human Liver Preparations. Br. J. Clin. Pharmacol 2001, 47, 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sui H; Fan Z-Z; Li Q Signal Transduction Pathways and Transcriptional Mechanisms of ABCB1/Pgp-Mediated Multiple Drug Resistance in Human Cancer Cells. Int. J. Med. Res 2012, 40, 426–435. [DOI] [PubMed] [Google Scholar]
- 43.Szakács G; Paterson JK; Ludwig JA; Booth-Genthe C; Gottesman MM Targeting Multidrug Resistance in Cancer. Nat. Rev. Drug Disc 2006, 5, 219–234. [DOI] [PubMed] [Google Scholar]
- 44.Farber HW; Loscalzo J Pulmonary Arterial Hypertension. N. Engl. J. Med 2004, 351, 1655–1665. [DOI] [PubMed] [Google Scholar]
- 45.Connolly HM; Crary JL; McGoon MD; Hensrud DD; Edwards BS; Edwards WD; Schaff HV Valvular Heart Disease Associated with Fenfluramine-Phentermine. N. Engl. J. Med 1997, 337, 581–588. [DOI] [PubMed] [Google Scholar]
- 46.Dempsie Y; Morecroft I; Welsh DJ; MacRitchie NA; Herold N; Loughlin L; Nilsen M; Peacock AJ; Harmar A; Bader M; MacLean MR Converging Evidence in Support of the Serotonin Hypothesis of Dexfenfluramine-Induced Pulmonary Hypertension With Novel Transgenic Mice. Circulation 2008, 117, 2928–2937. [DOI] [PubMed] [Google Scholar]
- 47.Jick H; Vasilakis C; Weinrauch LA; Meier CR; Jick SS; Derby LE A Population-Based Study of Appetite-Suppressant Drugs and the Risk of Cardiac-Valve Regurgitation. N. Engl. J. Med 1998, 339, 719–724. [DOI] [PubMed] [Google Scholar]
- 48.FDA Announces Withdrawal Fenfluramine and Dexfenfluramine (Fen-Phen). www.fda.gov. September 15, 1997.
- 49.Rich S; Rubin L; Walker AM; Schneeweiss S; Abenhaim L Anorexigens and Pulmonary Hypertension in the United States: Results from the Surveillance of North American Pulmonary Hypertension. Chest 2000, 117, 870–874. [DOI] [PubMed] [Google Scholar]
- 50.Abenhaim L; Moride Y; Brenot F; Rich S; Benichou J; Kurz X; Higenbottam T; Oakley C; Wouters E; Aubier M; Simonneau G; Bégaud B Appetite-Suppresant Drugs and the Risk of Primary Pulmonary Hypertension. International Primary Pulmonary Hypertension Study Group. N. Engl. J. Med 1996, 335, 609–616. [DOI] [PubMed] [Google Scholar]
- 51.Fitzgerald LW; Burn TC; Brown BS; Patterson JP; Corjay MH; Valentine PA; Sun JH; Link JR; Abbaszade I; Hollis JM; Largent BL; Hartig PR; Hollis GF; Meunier PC; Robichaud AJ; Robertson DW Possible Role of Valvular Serotonin 5-HT(2B) Receptors in the Cardiopathy Associated with Fenfluramine. Mol. Pharmacol 2000, 57, 75–81. [PubMed] [Google Scholar]
- 52.Launay J-M; Hervé P; Callebert J; Mallat Z; Collet C; Doly S; Belmer A; Diaz SL; Hatia S; Côté F; Humbert M; Maroteaux L Serotonin 5-HT2B Receptors are Required for Bone-Marrow Contribution to Pulmonary Arterial Hypertension. Blood 2012, 119, 1772–1780. [DOI] [PubMed] [Google Scholar]
- 53.Eddahibi S; Adnot S; Frisdal E; Levame M; Hamon M; Raffestin B Dexfenfluramine-Associated Changes in 5-Hydroxytryptamine Transporter Expression and Development of Hypoxic Pulmonary Hypertension in Rats. J. Pharmacol. Exp. Ther 2001, 297, 148–154. [PubMed] [Google Scholar]
- 54.Setola V; Hufeisen SJ; Grande-Allen KJ; Vesely I; Glennon RA; Blough B; Rothman RB; Roth BL 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) Induces Fenfluramine-Like Proliferative Actions on Human Cardiac Valvular Interstitial Cells in Vitro. Mol. Pharmacol 2003, 63, 1223–1229. [DOI] [PubMed] [Google Scholar]
- 55.Schade R; Andersohn F; Suissa S; Haverkamp W; Garbe E Dopamine Agonists and the Risk of Cardiac-Valve Regurgitation. N. Eng. J. Med 2007, 356, 29–38. [DOI] [PubMed] [Google Scholar]
- 56.Bana DS; MacNeal PS; LeCompte PM; Shah Y; Graham JR Cardiac Murmurs and Endocardial Fibrosis Associated with Methysergide Therapy. Am. Heart J 1974, 88, 640–655. [DOI] [PubMed] [Google Scholar]
- 57.Klein AK; Chatha M; Laskowski LJ; Anderson EI; Brandt SD; Chapman SJ; McCorvy JD; Halberstadt AL Investigation of the Structure-Activity Relationships of Psilocybin Analogues. ACS Pharmacol. Transl. Sci 2021, 4, 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kargbo RB Improved 5-HT2 Selective Receptor Modulators for the Treatment of Psychological Disorders. ACS Med. Chem. Lett 2021, 12, 1876–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luethi D; Liechti ME Drugs of Abuse Affecting 5-HT2B Receptors. In 5-HT2B Receptors; Springer Nature Switzerland AG, 2021; Maroteaux L; and Monassier L Eds. [Google Scholar]
- 60.Cavero I; Guillon J-M Safety Pharmacology Assessment of Drugs with Biased 5-HT(2B) Receptor Agonism Mediating Cardiac Valvulopathy. J. Pharmacol. Toxicol. Methods 2014, 69, 150–161. [DOI] [PubMed] [Google Scholar]
- 61.Forbes IT; Jones GE; Murphy OE; Holland V; Baxter GS N-(1-Methyl-5-indolyl)-N′-(3-methyl-5-isothiazolyl)urea: A Novel, High-Affinity 5-HT2B Receptor Antagonist. J. Med. Chem 1995, 38, 855–857. [DOI] [PubMed] [Google Scholar]
- 62.Spampinato U; Cathala A; Devroye C The serotonin2B Receptor and Neurochemical Regulation in the Brain. Handb. Behav. Neurosci 2020, 31, 147–156. [Google Scholar]
- 63.West JD; Carrier EJ; Bloodworth NC; Schroer AK; Chen P; Ryzhova LM; Gladson S; Shay S; Hutcheson JD; Merryman WD Serotonin 2B Receptor Antagonism Prevents Heritable Pulmonary Arterial Hypertension. PLoS One 2016, 11, e0148657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bloodworth NC; Clark CR; West JD; Snider JC; Gaskill C; Shay S; Scott C; Bastarache J; Gladson S; Moore C; D’Amico R; Brittain EL; Tanjore H; Blackwell TS; Majka SM; Merryman WD Bone Marrow-Derived Proangiogenic Cells Mediate Pulmonary Arteriole Stiffening via Serotonin 2B Receptor Dependent Mechanism. Circ. Res 2018, 123, E51–E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Snider JD; Riley LA; Mallory NT; Bersi MR; Umbarkar P; Gautam R; Zhang Q; Mahadevan-Jansen A; Hatzopoulos AK; Maroteaux L; Lal H; Merryman WD Targeting 5-HT2B Receptor Signaling Prevents Border Zone Expansion and Improves Microstructural Remodeling after Myocardial Infarction. Circulation 2021, 143, 1317–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joll JE; Clark CR; Peters CS; Raddatz MA; Bersi MR; Merryman WD Genetic Ablation of Serotonin Receptor 2B Improves Aortic Valve Hemodynamics of Notch1 Heterozygous Mice in a High-Cholesterol Diet Model. PLoS One 2021, 15, e0238407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hutcheson JD; Ryzhova LM; Setola V; Merryman WD 5-HT2B Antagonism Arrests Non-Canonical TGF-β1-Induced Valvular Myofibroblast Differentiation. J. Mol. Cell Cardiol 2012, 53, 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nebigil CG; Choi D-S; Dierich A; Hickel P; Le Meur M; Messaddeq N; Launay J-M; Maroteaux L Serotonin 2B Receptor is Required for Heart Development. Proc Natl. Acad. Sci. U.S.A 2000, 97, 9508–9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nebigil CG; Hickel P; Messaddeq N; Vonesch J-L; Douchet MP; Monassier L; György K; Matz R; Andriantsitohaina R; Manivet P; Launay J-M; Maroteaux. Ablation of Serotonin 5-HT2B Receptors in Mice Leads to Abnormal Cardiac Structure and Function. Circulation 2001, 103, 2973–2979. [DOI] [PubMed] [Google Scholar]
- 70.Kim H; Kim M; Im S-K; Fang S Mouse Cre-LoxP System: General Principles to Determine Tissue-Specific Roles of Target Genes. Lab. Anim. Res 2018, 34, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Acharya A; Baek ST; Huang G; Eskiocak B; Goetsch S; Sung CY; Banfi S; Sauer MF; Olsen GS; Duffield JS; Olson EN; Tallquist MD The bHLH Transcription Factor Tcf21 is Required for Lineagespecific EMT of Cardiac Fibroblast Progenitors. Development (Cambridge) 2012, 139, 2139–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kanisicak O; Khalil H; Ivey MJ; Karch J; Maliken BD; Correll RN; Brody MJ; Lin S-CJ; Aronow BJ; Tallquist MD; Molkentin JD Genetic Lineage Tracing Defines Myofibroblast Origin and Function in the Injured Heart. Nat. Comm 2016. 7, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hughes DP; Marron MB; Brindle NPJ The Antiinflammatory Endothelial Tyrosine Kinase Tie2 Interacts With a Novel Nuclear Factor-κB Inhibitor ABIN-2. Circ. Res 2003, 92, 630–636. [DOI] [PubMed] [Google Scholar]
- 74.Molina JR; Adjei AA The Ras/Raf/MAPK Pathway. J. Thorac. Oncol 2006, 1, 7–9. [PubMed] [Google Scholar]
- 75.Dikic I; Tokiwa G, Lev S; Courtneidge SA; Schlessinger J A Role for Pyk2 and Src in Linking G-Protein-Coupled Receptors with MAP Kinase Activation. Nature 1996. 383, 547–550. [DOI] [PubMed] [Google Scholar]
- 76.Biernacka A; Dobaczewski M; Frangogiannis NG TGF-β Signaling in Fibrosis. Growth Factors 2011, 29, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tripathi M; Billet S; Bhowmick NA Understanding the Role of Stromal Fibroblasts in Cancer Progression. Cell. Adh. Migr 2012, 6, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forbes IT; Ham P; Booth DH; Martin RT; Thompson M; Baxter GT; Blackburn TP; Glen A; Kennett GA; Wood MD 5-Methyl-1-(3-pyridylcarbamoyl)-1,2,3,5-tetrahydropyrrolo[2,3-f]indole: A Novel 5-HT2C/5-HT2B Receptor Antagonist with Improved Affinity, Selectivity, and Oral Activity. J. Med. Chem 1995, 38, 2524–2530. [DOI] [PubMed] [Google Scholar]
- 79.Nozulak J; Kalkman HO; Floerscheim P; Hoyer D; Schoeffter P; Buerki HR (+)-cis-4,5,7a,8,9,10,11,11a-Octahydro-7H-10-methylindolo[1,7- bc][2,6]- naphthyridine: A 5-HT2C/2B Receptor Antagonist with Low 5-HT2A Receptor Affinity. J. Med. Chem 1995, 38, 28–33. [DOI] [PubMed] [Google Scholar]
- 80.Fludzinski P; Wittenauer LA; Schenck KW 2,3-Dialkyl-(dimethylamino)indoles: interaction with 5HT1, 5HT2, and rat stomach fundal serotonin receptors. J. Med. Chem 1986, 29, 2415–18. [DOI] [PubMed] [Google Scholar]
- 81.Audia JE; Evrard DA; Murdoch GR; Droste JJ; Nissen JS; Schenck KW; Fludzinski P; Lucaites VL; Nelson DL; Cohen ML Potent, Selective Tetrahydro-β-carboline Antagonists of the Serotonin 2B (5HT2B) Contractile Receptor in the Rat Stomach Fundus. J. Med. Chem 1996, 39, 2773–2780. [DOI] [PubMed] [Google Scholar]
- 82.Porvasnik SL; Germain S; Embury J; Gannon KS; Jacques V; Murray J; Byrne BJ; Schacham S; Al-Mousily FJ PRX-08066, a Novel 5-Hydroxytryptamine Receptor 2B Antagonist, Reduces Monocrotaline-Induced Pulmonary Arterial Hypertension and Right Ventricular Hypertrophy in Rats. J. Pharm. Exp. Ther 2010, 334, 364–372. [DOI] [PubMed] [Google Scholar]
- 83.Dumitrascu R; Kulcke C; Königshoff M; Kouri F; Yang X; Morrell N; Ghofrani HA; Weissmann N; Reiter R; Seeger W; Grimminger F; Eickelberg O; Schermuly RT; Pullamsetti SS Terguride Ameliorates Monocrotaline-Induced Pulmonary Hypertension in Rats. Eur. Respir. J 2011, 37, 1104–1118. [DOI] [PubMed] [Google Scholar]
- 84.Bonhaus DW; Flippin LA; Greenhouse RJ; Jaime S; Rocha C; Dawson M; Van Natta K; Chang LK; Pulido-Rios T; Webber A; Leung E; Eglen RM; Martin GR RS-127445: A Selective, High Affinity, Orally Bioavailable 5-HT2B Receptor Antagonist. Br. J. Pharmacol 1999, 127, 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moss N; Choi Y; Cogan D; Flegg A; Kahrs A; Loke P; Meyn O; Nagaraja R; Napier S; Parker A; Peterson JT; Ramsden P; Sarko C; Skow D; Tomlinson J; Tye H; Whitaker M A New Class of 5-HT2B Antagonists Possesses Favorable Potency, Selectivity, and Rat Pharmacokinetic Properties. Bioorg. Med. Chem. Lett 2009, 19, 2206–2210. [DOI] [PubMed] [Google Scholar]
- 86.Gabr MT; Abdel-Raziq MS Pharmacophore-Based Tailoring of Biphenyl Amide Derivatives as Selective 5-Hydroxytryptamine 2B Receptor Antagonists. Med. Chem. Comm 2018, 9, 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin Z; Smith MD; Concepcion GP; Haygood MG; Olivera BM; Light A; Schmidt EW Modulating the Serotonin Receptor Spectrum of Pulicatin Natural Products. J. Nat. Prod 2017, 80, 2360–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schieferdecker S; Vock E Development of Pharmacophore Models for the Important Off-Target 5-HT2B Receptor. J. Med. Chem 2023, ASAP. doi: 10.1021/acs.jmedchem.2c01679. [DOI] [PubMed] [Google Scholar]
- 89.An Open-Label Study to Evaluate the Safety and Efficacy of PRX-08066 in Patients With Pulmonary Hypertension and Chronic Obstructive Pulmonary Disease. ClinicalTrials.gov identifier: NCT00677872. Updated July 30, 2009. Accessed January 17, 2023. https://clinicaltrials.gov/ct2/show/NCT00677872?term=PRX-08066&draw=2&rank=2. [Google Scholar]
- 90.Antoniu S Fresh from the Designation Pipeline: Orphan Drugs Recently Designated in the European Union (November 2012 – January 2013). Expert Opin. Orphan Drugs 2013, 1, 499–505. [Google Scholar]
- 91.Ghofrani HA; Al-Hiti H; Vonk-Noordegraaf A; Behr J; Neurohr C; Jansa P; Wilkens H; Hoeper MM; Gruenig E; Opitz C; Speich R; Ewert R; Halank M; Torbicki A; Kaehler C; Olschewski H; Filusch A; Reiter R; Rosenkranz S Proof-Of-Concept Study To Investigate The Efficacy, Hemodynamics And Tolerability Of Terguride Vs. Placebo In Subjects With Pulmonary Arterial Hypertension: Results Of A Double Blind, Randomised, Prospective Phase Iia Study. Am. J. Respir. Crit. Care Med 2012, 185, A2496. [Google Scholar]
- 92.Lythgoe MP; Rhodes CJ; Ghataorhe P; Attard M; Wharton J; Wilkins MR Why Drugs Fail in Clinical Trials for Pulmonary Arterial Hypertension, and Strategies to Succeed in the Future. Pharmacol. Ther 2016, 164, 195–203. [DOI] [PubMed] [Google Scholar]
- 93.Roth BL; Kroeze WK; Patel S; Lopez E The Multiplicity of Serotonin Receptors: Uselessly Diverse Molecules or an Embarrassment of Riches? The Neuroscientist 2000, 6, 252–262. [Google Scholar]
- 94.Top 200 Medicines Annual Report 2021: Reaching New Heights. https://www.pharmalive.com/top-200-medicines-annual-report-reaching-new-heights/ (accessed 2023-01-06).
- 95.Wainscott DB; Sasso DA; Kursar JD; Baez M; Lucaites VL; Nelson DL [3H]Rauwolscine: An Antagonist Radioligand for the Cloned Human 5-Hydroxytryptamine2B (5-HT2B) Receptor. Ach. Pharmacol 1998, 357, 17–24. [DOI] [PubMed] [Google Scholar]
- 96.Bonhaus DW; Weinbardt KK; Taylor M; DeSouza A; McNeeley PM; Szczepanski K; Fontana DJ; Trinh J; Rocha CL; Dawson MW; Flippin LA; Eglen RM RS-102221: A Novel High Affinity and Selective, 5-HT2C Receptor Antagonist. Neuropharmacology 1997, 36, 621–629. [DOI] [PubMed] [Google Scholar]
- 97.McKenna DJ; Peroutka SJ Differentiation of 5-Hydroxytryptamine2 Receptor Subtypes using 125I-R-(−)2,5-Dimethoxy-4-iodo-phenylisopropylamine and 3H-Ketanserin. J. Neurosci 1989, 9, 3482–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abbas A,I; Hedlund PB; Huang X-P; Tran T,B; Meltzer HY; Roth BL Amisulpride is a Potent 5-HT7 Antagonist: Relevance for Antidepressant Actions In Vivo. Psychopharmacology 2009, 205, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shapiro DA; Renock S; Arrington E; Chiodo LA; Liu L-X; Sibley DR; Roth BL; Mailman R Aripiprazole, a Novel Atypical Antipsychotic Drug with a Unique and Robust Pharmacology. Neuropsychopharmacology 2003, 28, 1400–1411. [DOI] [PubMed] [Google Scholar]
- 100.Wainscott DB; Luciates VL; Kursar JD; Baez M; Nelson DL Pharmacologic Characterization of the Human 5-Hydroxytryptamine2B Receptor: Evidence for Species Differences. J. Pharm. Exp. Ther 1996, 276, 720–727. [PubMed] [Google Scholar]
- 101.Watson J; Brough S; Coldwell MC; Gager T; Ho M; Hunter AJ; Jerman J; Middlemiss DN; Riley GJ; Brown AM Functional Effects of the Muscarinic Receptor Agonist, Xanomeline at 5-HT1 and 5-HT2 Receptors. Br. J. Pharmacol 1998, 125, 1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hutcheson JD; Setola V; Roth BL; Merryman WD Serotonin Receptors and Heart Valve Disease – It Was Meant 2B. Pharmacol. Ther 2011, 132, 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roth BL Drugs and Valvular Heart Disease. N. Engl. J. Med 2007, 356, 6–9. [DOI] [PubMed] [Google Scholar]
- 104.Droogmans S; Roosens B; Cosyns B; Degaillier C; Hernot S; Weytjens C; Garbar C; Caveliers V; Pipeleers-Marichal M; Franken PR; Lahoutte T; Schoors D; Van Camp G Cyproheptadine Prevents Pergolide-Induced Valvulopathy in Rats: An Echocardiographic and Histopathological Study. Am. J. Physiol. Heart Circ 2009, 296, H1940–H1948. [DOI] [PubMed] [Google Scholar]
- 105. https://www.eurofinsdiscoveryservices.com .
- 106.Merryman PAH paper (pending acceptance)
- 107.Bevilacqua L; Doly S; Kaprio J; Yuan Q; Tikkanen R; Paunio T; Zhou Z; Wedenoja J; Maroteaux L; Diaz S; Belmer A; Hodgkinson CA; Dell’Osso L; Suvisaari J; Coccaro E; Rose RJ; Peltonen L; Virkkunen M; Goldman D A Population-Specific HTR2B Stop Codon Predisposes to Severe Impulsivity. Nature 2010, 468, 1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pitychoutis PM; Belmer A; Moutkine I; Adrien J; Maroteaux L Mice Lacking the Serotonin Htr2B Receptor Gene Present an Antipsychotic-Sensitive Schizophrenic-Like Phenotype. Neuropsychopharmacology 2015, 40, 2764–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qian Y; Cao Y; Deng B; Yang G; Li J; Xu R; Zhang D; Huang J; Rao Y Sleep Homeostasis Regulated by 5HT2b Receptor in a Small Subset of Neurons in the Dorsal Fan-shaped Body of Drosophila. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bender AM; Valentine MS; Bauer JA; Days E; Lindsley CW; Merryman WD Identification of Potent, Selective and Peripherally Restricted Serotonin Receptor 2B Antagonists from a High-Throughput Screen. Assay Drug Dev. Technol 2023, ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]


