Abstract
Background
Immune thrombocytopenia (ITP) is an acquired immune-mediated bleeding disorder characterized by isolated thrombocytopenia. Its estimated yearly incidence in the pediatric population is 1.9–6.4/100,000. ITP in children is usually a self-limiting and benign disorder. The clinical management of children with ITP often remains controversial, as robust randomized trials on the management of this disorder are lacking. Treatments vary widely in clinical practice and existing guidelines from hematology societies on clinical management offer indications based largely on expert opinion rather than strong evidence.
Materials and methods
The Coagulative Disorder Working Group of the Italian Association of Pediatric Hematology and Oncology (AIEOP) developed this document to collect shared expert opinions on the management of newly diagnosed ITP, updating previous guidelines and providing recommendations to pediatricians. Each statement has been given a score expressing the strength of evidence, appropriateness and agreement among participants.
Results
Clear-cut definitions of the clinical phases of the disease and clinical response are stated. Recommendations are given regarding the classification of bleeding symptoms, evaluation of bleeding risk, diagnosis, and prognostic factors. Specific recommendations for treatment include indications for first-line (intravenous immunoglobulins, steroids) and second-line (combined therapy, thrombopoietin receptor agonists, immunosuppressive drugs, rituximab) therapeutic agents, as well as hemorrhagic emergency and supportive treatment, including emergency splenectomy. The optimal follow-up schedule, the relation between ITP and vaccines and health-related quality-of-life issues are also discussed.
Discussion
The panel achieved broad consensus on issues related to how to treat children with newly diagnosed ITP, providing a comprehensive review of all relevant clinical aspects.
Keywords: ITP, children, management, quality of life, therapy
INTRODUCTION
Immune thrombocytopenia (ITP) is an acquired immune-mediated disorder characterized by isolated thrombocytopenia, due to platelet destruction and impaired platelet production. The estimated yearly incidence in the pediatric population is 1.9–6.4/100,0001. ITP is usually a self-limiting and benign bleeding disorder in children. Since robust randomized trials on the management of ITP are lacking, treatments vary widely in clinical practice. Indeed, existing guidelines from hematology societies2,3 on the clinical management offer indications based largely on expert opinion rather than strong evidence and the clinical management of children often remains controversial.
The Coagulative Disorder Working Group of the Italian Association of Pediatric Hematology and Oncology (AIEOP) developed this consensus document with the aim of updating previous guidelines4 and providing recommendations to pediatricians. These recommendations are not intended to be standards or fixed rules but rather a tool to support pediatricians in the management of the diagnostic work-up and treatment of children with newly diagnosed ITP.
MATERIALS AND METHODS
The design and methodology were similar to those adopted for AIEOP Consensus Guidelines on acquired aplastic anemia5, autoimmune hemolytic anemia6–7, congenital and acquired neutropenias8, and immune thrombocytopenias9, whose procedures were validated by the AIEOP board. Furthermore, the methodology fulfills the AGREE reporting checklist10.
Twenty-four representatives (22 pediatric hematologists, 1 hematologist, 1 psychologist) from 18 centers participated in the “Acute ITP Committee”. Questions to be addressed in the Recommendations were identified by the whole Committee; they included the diagnostic work-up, various treatment interventions, comparison among different options, possible outcomes and health care context. Each topic was developed by a subgroup in a single document, which included a brief description of the state-of-the-art knowledge, followed by specific recommendations.
For the pre-guideline documents, authors extracted evidence from literature searched for in the Medline and Google Scholar databases (from January 1, 2008 to February 28, 2019, and then updated in December 2022 during the compilation of the final draft). Search terms included: immune thrombocytopenia, immune thrombocytopenic purpura, idiopathic thrombocytopenia, idiopathic thrombocytopenic purpura, autoimmune thrombocytopenia, autoimmune thrombocytopenic purpura, and ITP. The search was also extended to older papers, specifically retrieved following cited references, and to hematology textbooks. The target population consisted of patients with acute ITP aged 0–18 years. Every piece of collected evidence was attributed a strength that was scored using the level of evidence criteria reported in Table I (modified from George JN et al.11).
Table I.
Scoring scale for recommendations
| Literature level of evidence | Study design |
|---|---|
| I (strongest) | Prospective randomized trial with high statistical value |
| II | Prospective randomized trial with low statistical value |
| III | Non-randomized study with concurrent control group |
| IV | Non-randomized study with historical control group |
| V (weakest) | Case report(s), guideline, meta-analysis, reviews |
| Mean Consensus score | |
| 1–3 (weakest) | Inappropriate practice |
| 3.01–6.99 | Uncertain appropriateness |
| 7–9 (strongest) | Appropriate/necessary practice |
| Agreement among Consensus | |
| A (strongest) | Strong agreement (variation more than 1 SD below the average of the variances, in logarithmic scale). |
| B | Moderate agreement (variation less than 1 SD below the average of the variances). |
| C | Moderate disagreement (variation less than 1 SD above the average of the variances). |
| D (weakest) | Strong disagreement (variation more than 1 SD above the average of the variances) |
SD: standard deviation.
Each draft was reviewed by the entire Committee and modified accordingly after exhaustive discussion. The Committee prepared statements that were then subjected to validation during the Consensus Conference, held in Turin on July 4, 2019, during which 24 participants (15 pediatric hematologists, 2 hematologists, 2 pediatric residents, 1 psychologist, 1 pediatric anesthesiologist, 1 pediatric surgeon, 1 nurse, 1 pharmacist) scored the final items. Another 26 participants (19 pediatric hematologists, 4 pediatricians, 2 pediatric oncologists, 1 pediatric resident), who could not attend in presence, scored all the items via an on-line questionnaire. Patients’ associations could not be involved, since there are no national patients’ associations for pediatric ITP.
The strength of each statement is expressed by three domains. The first domain, represented by a Roman number from I (highest) to V (lowest), reports the strength of supporting evidence11; the second, represented by an Arabic number form 1 (lowest) to 9 (highest), reports the mean score expressed by the Consensus; the third domain, represented by a letter from A (highest) to D (lowest), reports the level of agreement among participants, scored by evaluating the distribution of the standard deviations (SD) within each statement. Details of the three indices are reported in Table I.
RESULTS
Definitions
For childhood ITP the consensus panel recommends the following:
adopting the International Working Group (IWG) definition for ITP preserving the acronym ITP, replacing I (idiopathic) by Immune, T and P (purpura) by ThrombocytoPenia, with thrombocytopenia defined as a platelet count below 100×109/L (V- 8.2-A)12;
adopting the IWG definition for phases of ITP: “newly diagnosed” ITP indicating acute ITP within 3 months from onset, “persistent” ITP indicating an ITP lasting between 3 and 12 months and “chronic” ITP indicating ITP lasting more than 12 months (V- 8.6-A)12;
adopting the IWG definition for severity of ITP defining severe ITP based on clinical features, regardless of platelet counts, as: the presence of relevant bleeding at presentation, such to make treatment mandatory, or the appearance of new hemorrhages subsequently, requiring a re-treatment with different drugs or different doses (V- 8.0-A) 12;
-
adopting the IWG definition for response to treatment, based on a quantitative platelet count together with a bleeding outcome, defining:
complete response as resolution of bleeding symptoms, and a platelet count of at least 100×109/L;
partial response as no clinically relevant bleeding and a platelet count between 30 and 100×109/L, at least doubling of the baseline count;
no response as a platelet count lower than 30×109/L or less than doubling baseline count or continued clinically relevant bleeding (V- 7.9-B) 12.
The consensus panel underlines that IWG criteria for defining refractory ITP (failure to respond to splenectomy) cannot be adopted for newly diagnosed and persistent ITP in childhood given the declining rate of splenectomy in pediatric ITP. Splenectomy is very rarely indicated in childhood ITP and should only be considered in children >5 years of age who have failed all available medical therapies, are having thrombocytopenia-related bleeding, and whose life is at risk or whose health-related quality of life (QoL) is substantially impaired.
We therefore suggest at this time defining ITP as “refractory” in a child with more severe and difficult-to-treat disease, i.e., a child with persistent, clinically relevant, active bleeding and persistent low platelet count despite first-line treatments or rescue therapy (other immunosuppressant agents or thrombopoietin receptor agonists [TPO-RA] or splenectomy) or a child who requires frequent courses of therapy to maintain a sustained clinical response, developing worsening disease and medication-induced toxicities (V-7.8-A).
Classification of bleeding symptoms and evaluation of bleeding risk
The identification of possible risk factors can help when deciding whether treatment is indicated.
Reported risk factors for severe bleeding include wet purpura (in the mouth, hematuria, prolonged epistaxis, gastro-intestinal bleeding, or other pronounced mucosal bleeding), trauma, exposure to antiplatelet medications (e.g., aspirin, ibuprofen, other nonsteroidal anti-inflammatory drugs) and anticoagulants (eg, heparin, warfarin), very low platelet count (defined as platelet count <10×109/L) (V-8.1-A)13.
The present consensus panel recommends using a uniform bleeding assessment tool (BAT) at diagnosis and follow-up visits both for grading severity of bleeding and for evaluating response to therapy (V-8.3-A); however, the consensus panel was uncertain on whether to recommend the easy and quick Buchanan-Adix score (V-6.9-C) or the thorough and precise (SMOG) score (V-4.3-C)14,15.
Diagnosis of immune thrombocytopenia
Primary ITP remains a diagnosis of exclusion and the differential diagnosis is often not easy16. A negative family and past medical history and a presentation with a sudden onset of cutaneous bleeding manifestations, most often petechiae, are typical of ITP. A recent infection is often described in pediatric ITP, usually about 2 weeks before the onset17. A history of recent vaccinations, especially against measles, mumps and rubella, can also be found.
There are features that can induce the physician to suspect other causes of thrombocytopenia: constitutional symptoms (fever, weight loss, night sweat), bone pain, recurrent thrombocytopenia, poor treatment response, lymphadenopathy, hepatosplenomegaly, ill-appearing child and signs of chronic disease (Tables II and III).
Table II.
Clinical data and their relation with a diagnosis of immune thrombocytopenia
| Supportive | Not supportive |
|---|---|
| Abrupt onset of symptoms | Fever, recurrent infections, weight loss, fatigue, bone and/or joint pain, skin rash |
| Recent viral infection | Ongoing medications |
| Recent vaccination (particularly live vaccine) | Family history of thrombocytopenia, cataract, deafness, renal failure, myelodysplasia, dysmorphic features |
| Isolated thrombocytopenia, with normal red and white cell counts, except for bleeding-related anemia | Signs related to immune deficiency |
| Previous normal platelet count | Abnormal red and/or white cells |
| Normal or slightly elevated mean platelet volume | Giant or very small platelets |
Table III.
Clinical data suggesting congenital/hereditary thrombocytopenia
| Clinical data |
|---|
| Family history of thrombocytopenia |
| Family history of acute myeloid leukemia |
| No response to steroid and/or intravenous immunoglobulin therapy |
| Thrombocytopenia onset at birth or during the first months of life |
| Lack of previous normal platelet count |
| Casual finding of thrombocytopenia (>20×109/L) |
| Long-term persistence of thrombocytopenia >20×109/L |
| Giant platelets, or very small platelets, as indicated by the mean platelet volume value and/or the blood smear |
| Other non-hematological findings, i.e., short stature, arm/hand malformations, eczema, skin spots, deafness, cataract, kidney impairment |
Diagnostic tests
ITP patients are characterized by isolated thrombocytopenia, expressed by a platelet count <100×109/L, as recently redefined by an international working group, which abandoned the previously considered cut-off of 150×109/L, since non-Caucasian population may have lower platelet levels, without any bleeding risk (V-8.2-A)12.
Evaluation of a peripheral blood smear is indicated in order to exclude inherited and/or secondary thrombocytopenia (V-7.8-B). Some large platelets are typically found, but an excessive number of giant platelets, as well as small platelets, should point to other diagnoses16,18.
Although platelet autoantibodies are thought to be the major underlying cause of ITP19 testing for platelet autoantibodies is not currently recommended for the diagnosis of ITP because of the poor accuracy of the current tests (V-7.7-A)20.
There is no indication for bone marrow aspiration at the onset of ITP in newly diagnosed children with a typical presentation (V-8.2-A). However, this investigation should be considered prior to the administration of steroids, but may safely be omitted in patients with a recent history of complete response to intravenous immunoglobulins (IVIg) (V-7.4-B).
The following tests, aiming at recognizing possible underlying conditions, have been considered not indicated in newly diagnosed ITP, whereas they become appropriate in persistent ITP: anti-ENA and ANA screening (V-8.0-A); screening of thyroid function and/or for anti-thyroid antibodies (V-7.6-A); screening for celiac disease (V-7.6-B) is indicated in patients not responding well to therapy or in older children (>10 years) or if there is a positive family history for autoimmune diseases.
Autoimmune lymphoproliferative syndrome (ALPS)/ALPS-like syndromes and common variable immunodeficiency should be considered as underlying causes of acute ITP in patients with a personal/family history of autoimmunity, lymphoproliferation, or other signs of immune-dysregulation (V-7.9-A).
The diagnostic work-up of patients with acute ITP associated with signs/history of immune dysregulation should include lymphocyte subset count (including T-cell receptor αβ+CD8−CD4− T cells), serum immunoglobulin level, antibody titers to prior vaccinations, and screening for autoimmunity before administration of steroids/IVIg (V-7.8-A).
Patients with newly diagnosed ITP and a history of autoimmune hemolytic anemia and/or neutropenia should be considered as having Evans syndrome and investigated for ALPS/ALPS-like and common variable immunodeficiency (V-8.0-A).
Prognostic factors
The available literature dealing with the identification of prognostic factors capable of predicting the evolution toward a chronic course of ITP is based mainly on case series and retrospective reviews. Identified prognostic factors of chronic ITP in children are female gender, age >10 years at presentation, absence of preceding infection or vaccination, and platelet count >20×109/L at presentation (V-7.9-A)21–24.
Recently, a novel clinical prediction tool has been developed to predict transient versus persistent ITP disease courses in children with newly diagnosed ITP at the time of diagnosis25. Authors recommend its use for research and training purposes only.
Indications for treatment
About 80% of patients with ITP achieve a complete sustained remission within a few weeks to a few months after the initial presentation, irrespective of any given therapy. Consequently, pharmacological treatment is indicated to prevent the risk of severe bleeding, including intracranial hemorrhage that occurs in less than 1% of cases26. The aims of the treatment are to increase the platelet count quickly and stabilize it at a value >20×109/L in those patients who are considered at risk of developing severe mucosal or deep-organ bleeding.
Therefore, the indications for initial treatment depend on the severity:
a) in children with newly diagnosed ITP who have no or mild bleeding only (Buchanan & Adix score 0–2 (equivalent to SMOG S0–3 M0 O0), the Consensus panel proposes the following statements:
• management as an outpatient is favored over admission to hospital, unless conditions such as uncertainty about the diagnosis, social concerns, distance from the hospital, and difficulties for follow-up, make admission to the hospital preferable (V-7.9-A);
• irrespectively of the platelet count, the initial approach should be observation alone (II-7.5-B); however, treatment can be offered to children who may benefit from a higher platelet count, in the case of “special needs”, i.e., toddlers with a high tendency to fall, pubertal girls at risk of menorrhagia, risk behavior in adolescents, upcoming procedures associated with a risk of bleeding, not acceptable traveling distance from hospital, or in the case of a strong wish of parents or patients, after having counselled them regarding risks and benefits of therapy (V-8.2-A).
b) in the case of a Buchanan & Adix score ≥3 (equivalent to SMOG S0-3 M≥2 O≥1), i.e., at least severe spontaneous mucosal bleeding, at onset of ITP, irrespectively of the platelet count, the Consensus panel offers the following statements:
• the patient should receive treatment (V-8.0-A);
• in the case of subsequent appearance of a Buchanan & Adix score ≥3 (equivalent to SMOG S0-3 M≥2 O≥1) (at least mucosal bleeding), patients who were initially observed alone (score 0-2) should be treated (V-8.8-A).
Therapy with first-line agents
Effective treatment strategies are single-dose intravenous immunoglobulin G (IVIg 0.8–1 g/kg) or medium to high-dose corticosteroids administered orally or parenterally, i.e., prednisone 1–2 mg/kg/day for 15 days orally; methyl-prednisolone 5–10 mg/kg/day for 3 days intravenously; dexamethasone 0.6 mg/kg/day for 4 consecutive days3,20. Several randomized studies showed that IVIg, at the dose of 0.8–1 g/kg for 1–2 days or 0.4–0.5 g/kg for 4–5 days, were more effective than steroids, used at various doses and schedules, in achieving a platelet count >20×109/L by 48 hours27; children with mucosal bleeding at diagnosis or treatment with IVIg alone have been recently shown to develop chronic ITP less often28, even though these findings need confirmation in prospective, randomized trials. Therefore:
IVIg is a first-line therapy in children with newly diagnosed ITP (I-8.8-A).
The recommended dose is 0.8 g/kg in a single dose, with possible repetition (II-8.6-A).
When corticosteroids are chosen as initial treatment for ITP, an intermediate-high dose of prednisone or methylprednisolone has the same efficacy (I-8.3-A).
The use of high-dose steroids for a brief period is associated with fewer adverse effects whereas long-term corticosteroids should be avoided in children with ITP because of side effects (V-8.3-A).
Anti-D for ITP is not marketed in Italy and is not available in most if not all European countries: in June 2009 the manufacturer notified the European Medicines Agency of the decision to withdraw all marketing authorizations in Europe because of concerns about the benefit-to-harm balance in ITP therapy.
Therapy with second-line agents
Combined therapy
Combining first-line treatments is appropriate in an emergency setting: prednisone and IVIg are recommended for the emergency treatment of patients with uncontrolled bleeding (V-8.2-A). High-dose methyl-prednisolone may also be useful in this setting29.
Patients who do not have an adequate response to initial therapy with first-line agents may also benefit from additional courses of first-line agents in combination (V-7.6-B)30.
The recommended schedule for combined therapy is IVIg 0.4 g/kg daily on days 1 and 2, and methyl-prednisolone 20 mg/kg daily on days 1–3 (V-7.1-B).
Thrombopoietin receptor agonists
TPO-RA are a highly effective and well-tolerated treatment for achieving hemostatic platelet counts (>50×109/L) in children with ITP31–36.
They are currently approved for patients with chronic ITP, 1 year of age or older who are refractory to other treatments (e.g., corticosteroids, IVIg). Romiplostin is approved for refractory ITP, independently of time, only in adults. In children TPO-RA agents can be used in newly diagnosed ITP, only as off-label therapy, in selected patients with severe bleeding symptoms, refractory to IVIg and steroids (V-7.9-B), and in patients with persistent ITP and a bleeding score >2 or a bleeding score 0–2 in the presence of “special needs” (V-7.9-A).
Immunosuppressive drugs
Immunosuppressive drugs, such as mycophenolate mofetil and sirolimus, offer a treatment option in patients with persistent/chronic ITP thanks to promising results shown in both adult and pediatric populations (52% and 64% response, respectively)37,38. Selected populations of patients with an underlying ALPS-like disorder or with Evans syndrome had an even better outcome (73% and 81%, respectively).
Recently, a randomized controlled study of steroids versus steroid plus mycophenolate mofetil in adults showed a definite benefit from the addition of mycophenolate mofetil in the initial treatment39.
Sirolimus has been shown to be effective in patients with ALPS and other primary or secondary autoimmune cytopenias40–41.
In patients with persistent ITP and a bleeding score >2 or a bleeding score 0–2 in the presence of special needs, mycophenolate mofetil and sirolimus can be considered as treatment options, especially in the context of ALPS/ALPS-like syndromes (V-8.1-A).
Rituximab
Several uncontrolled, open-label studies support the use of rituximab in pediatric patients with chronic ITP, suggesting an initial response rate of approximately 40 to 50%, falling to approximately 25% over a follow-up of 2 to 5 years; adolescent females are more likely than younger children and males to achieve remission42–44.
Mild, transient side effects (urticaria, headache, fever, scratchy throat, and chills) may occur during the infusion. Serum sickness has been reported in 5 to 10% of children with ITP treated with rituximab. Progressive multifocal leukoencephalopathy has been reported as a very rare but extremely serious complication of rituximab therapy, too45,46.
Rituximab can be considered in selected patients with persistent ITP with severe bleeding symptoms, refractory to first-line agents (IVIg and steroids) and TPO-RA (V-7.6-A) or to avoid or postpone splenectomy (V-7.5-B).
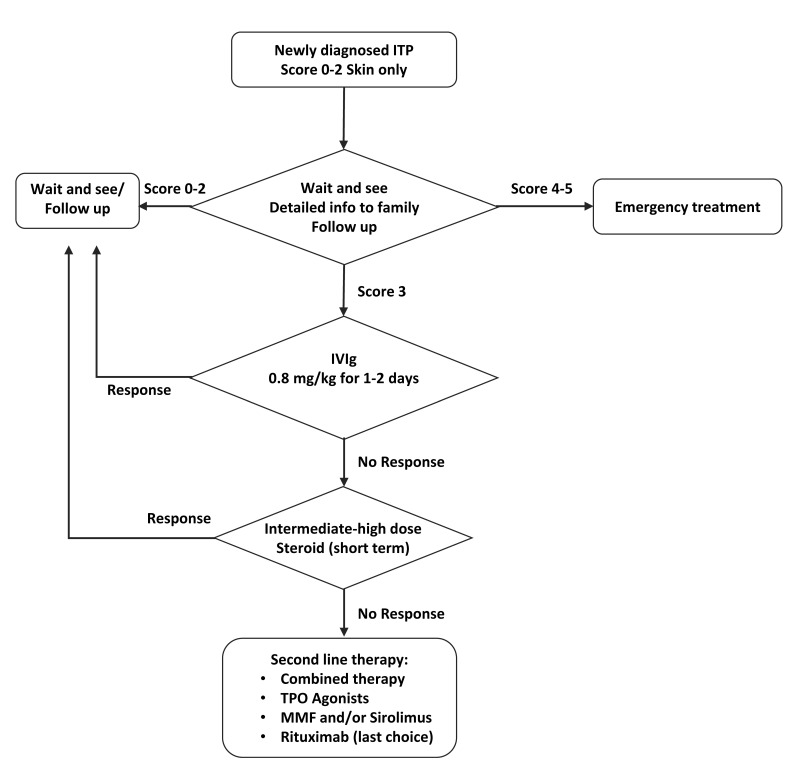
Figure 1 proposes a therapy flow chart, identifying the most suitable treatment options, according to bleeding score.
Figure 1.
Flow chart summarizing the treatment options
ITP: immune thrombocytopenia; IVIg: intravenous immunoglobulin; TPO: thrombopoietin; MMF: mycophenolate mofetil.
Hemorrhagic emergency and supportive treatment
Symptoms or signs suggestive of organ- or life-threatening hemorrhage require action to ensure a prompt increase of circulating platelets.
Recommendations for emergency treatments have already been proposed by AIEOP, the American Society of Hematology and an Indian group with few differences (Table IV)10,20,47.
Table IV.
Previously published recommendations for emergency treatments
| Severe/life-threatening bleeding | AIEOP recommendations | American guidelines | Indian guidelines |
|---|---|---|---|
| First-line therapy | IVIg at a dose of 0.8 g/kg | Immediate IV administration of m-PDN (30 mg/kg, maximum dose 1 g) + platelet transfusion | m-PDN in combination with IVIg or anti-D. m-PDN and IVIg may be repeated for at least 1–2 days, based on response. Platelet transfusions are indicated only in the setting of life-threatening bleeds |
| First-line alternatives | m-PDN at a dose of 15–30 mg/kg/day is administered for 3 days | Combined therapy: after administration of IV m-PDN and platelets, an infusion of IVIg (1 g/kg) |
|
| In selected cases | Combined treatments and add platelet transfusion | Continuous infusion of platelets may be beneficial in selected cases. Emergency splenectomy may also need to be considered | Splenic artery embolization is performed to achieve a prompt rise in platelet count before splenectomy in patients unresponsive to conventional treatment. Recombinant factor VIIa has been used in intracranial hemorrhage refractory to platelet-enhancing agents as “off-label” use at a dose of 90–120 μg/kg |
AIEOP: Italian Association of Pediatric Hematology and Oncology; IVIg: intravenous immunoglobulin; IV: intravenous; m-PDN: methyl-prednisolone.
The consensus panel agrees on the following statements:
IVIg (1–2 g/kg) is proven to have the most rapid onset of action and should be considered along with high-dose corticosteroids with the aim of increasing the platelet count (I-8.4-A).
Platelet transfusions are indicated in the setting of life-threatening bleeds. Survival of transfused platelets may be improved with concurrent IVIg therapy. Continuous infusion of platelets may be beneficial in selected cases. Although the platelet count may not increase substantially, bleeding can often be controlled (V-8.0-A).
Recombinant factor VIIa has been used in patients with intracranial hemorrhage refractory to platelet-enhancing agents as an “off-label” treatment at a dose of 90–120 μg/kg, every 2–3 h, until the cessation of bleeding48–51. Therefore, recombinant factor VIIa can be considered in critical situations (V-6.8-B), but the treatment is “off label” and there is very limited experience with it.
In the case of massive intracranial hemorrhage and raised intracranial pressure, when neurosurgical intervention for evacuation of hematoma or decompression craniotomy may be urgently indicated, splenectomy in the same surgical session may offer the best chance (V-6.8-B).
Control of bleeding from mucosal surfaces, particularly epistaxis, gum bleeding and menorrhagia, can be aided with antifibrinolytic agents. Tranexamic acid is preferred due to its longer half-life, higher potency and lower toxicity. The dose of tranexamic acid is 25–50 mg/kg every 6–8 h. Antifibrinolytic agents are contraindicated during hematuria; formation of clots can result in colic and obstruction of outflow from the renal pelvis (V-8.0-A).
Menorrhagia in adolescents with ITP is commonly managed with oral contraceptive pills and tranexamic acid (V-7.5-B).
Emergency splenectomy
Splenectomy is considered inappropriate for children with newly diagnosed or persistent ITP4.
However, emergency splenectomy may be considered in special circumstances, in the case of severe thrombocytopenia and life-threatening bleeding (V-7.2-B). Emergency splenectomy should be considered heroic, given the dangers of unplanned surgery, lack of immunization, risk of surgical bleeding, and risk of managing bleeding while preparing a patient for major abdominal surgery (V-7.7-B).
If expertise is available, splenic artery embolization could be considered as a faster and safer bridge procedure prior to splenectomy, although data regarding the use of splenic artery embolization in ITP-related life-threatening bleeding are limited52,53.
The risk of infection is the major cause of mortality after splenectomy53. Therefore, measures to prevent infection are recommended. Vaccinations against encapsulated bacteria (Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae type B) should be performed promptly after emergency surgery, at least 2 weeks after splenectomy. Yearly seasonal influenza vaccine is recommended (V-8.3-A).
Prophylactic antibiotics should be administered after splenectomy until the child is 5 years of age, for a minimum of 2 years after splenectomy for children older than 5 years of age and for a longer period if there has been a previous episode of severe infection or there are other infective risks (V-7.9-A). The risk of infection persists throughout life, so it is recommended that patients and families be educated about the risks associated with asplenia, the preventive measures that can be taken and the correct management of post-splenectomy episodes of fever (V-8.7-A).
Follow-up
The management of follow-up in patients with ITP is complex and various aspects such as age, potential co-morbidities and co-medications, activities as well as health-related QoL and psychic, social and economic aspects must be considered in decision making.
The present consensus panel recommends managing the patient with a bleeding score 0–2 and favorable prognostic factors with observation alone, limiting needle sticks after the first-line treatment (V-8.1-A).
Immune thrombocytopenia and vaccines
The consensus panel underlines that all the vaccines included in the pediatric vaccinations’ schedule are recommended even in the case of previous thrombocytopenia (possibly related or not to vaccine) (III-8.3-A).
Although the possibility of ITP onset after measles-mumps-rubella (MMR) vaccination is well recognized, the global incidence of ITP after MMR vaccination has been demonstrated to be lower than that reported after native infection (0.087–4/100,000 per vaccine dose compared to 6 to 1,200/100,000 cases); moreover, ITP after vaccination has been reported to be favorable, with a more self-limited clinical course and complete resolution in >90% of patients after 6 months54–58. In children who have had an episode of thrombocytopenia within 6 weeks after the MMR or MMR-varicella vaccination, the possibility of avoiding the administration of a second dose should be considered, evaluating the anti-measles antibodies (V-7.5-B).
The vaccination is recommended in children who do not have proven seroconversion, as it is considered that the benefit of vaccination outweighs the risk of a possible thrombocytopenia (V-7.9-A).
The consensus panel reminds that in the case of previous use of blood-derived products, it is necessary to postpone live-virus vaccinations (at least 6 months for unwashed red blood cells, 7 months for platelets and 11 months for IVIg) to prevent the eventual interference of passively transmitted antibodies (V-7.5-B).
Health-related quality of life
Health-related QoL issues should be considered while making decisions on management in childhood ITP3,20. Physical symptoms of ITP itself, social limitations, parents’ anxiety and specific medical-related factors (i.e., duration, complications, inconvenience of treatments, need for invasive interventions) may all adversely affect the child’s and family’s quality of life59,60. Fatigue, defined by extreme and persistent tiredness, weakness or exhaustion, possibly associated with decreased functioning, has been reported as an important health-related QoL issue by 54% of children and 62% of adolescents with ITP60.
The consensus panel recommends that QoL be evaluated using specific tools at diagnosis and at defined intervals for each treatment (IV-7.8-A)61. Fatigue should be measured with specifically dedicated tools (V-7.8-A)62. Moreover, the influence of ITP burden on QoL should be considered when deciding on treatment and follow-up for children/adolescents with ITP (V-8.0-A).
CONCLUSIONS
The present report represents a valuable effort to support pediatricians with guidance for the management of diagnosis and therapy of newly diagnosed and persistent ITP in children. The consensus panel achieved broad sharing of issues related to how to treat newly diagnosed children with ITP, yielding a comprehensive review of all relevant clinical aspects.
Table V reports a comparison of current recommendations with previous AIEOP guidelines published in 20004 and international guidelines2,3.
Table V.
Differences between previous and current AIEOP recommendations and international guidelines
| AIEOP 2000 recommendations | AIEOP current recommendations | ASH current guidelines | |
|---|---|---|---|
| Definitions: phase of the disease | Acute ITP <6 months Chronic >6 months |
According to IWG:
|
According to IWG:
|
| Definitions: response to treatment | Not provided | According to IWG:
|
According to IWG:
|
| Indications for treatment | Platelet count bleeding signs | Bleeding signs special needs | Bleeding signs special needs |
| First line – treatments | Steroids or IVIg | IVIg | Steroids |
| Second-line treatments | Not considered | Combined therapy TPO agonists immunosuppressive drugs rituximab | TPO agonists immunosuppressive drugs rituximab |
| Bone marrow biopsy at diagnosis | Mandatory prior to steroid administration | Suggested prior to steroid administration | Not recommended |
| Assessment of HR QoL issues | No | Yes | Yes |
AIEOP: Italian Association of Pediatric Hematology and Oncology; ASH: American Society of Hematology; ITP: immune thrombocytopenia; IWG: International Working Group; PLT: platelets; IVIg: intravenous immunoglobulin; TPO: thrombopoietin; HRQoL: health-related quality of life.
An extensive version of the current recommendations, which includes a detailed description of the background supporting all the statements, can be accessed on the AIEOP website: https://www.aieop.org/web/operatori-sanitari/linee-guida-consensum/.
Information given to parents can be accessed on the AIEOP website: https://www.aieop.org/web/famiglie/schede-malattia/piastrinopenia-immune-itp/.
ACKNOWLEDGMENTS
Acute ITP Committee
Maddalena Casale, Pediatric Hematologist, Department of Women, Children and General and Specialized Surgery, “Luigi Vanvitelli” Universita degli Studi della Campania, Naples, Italy;
Simone Cesaro, Pediatric Hematologist, Pediatric Hematology Oncology, Department of Mother and Child, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy;
Giovanni Del Borrello, Pediatric Hematologist, Pediatric Oncohematology, Pediatrics Department, Hospital Città Della Salute e Della Scienza, University of Turin, Turin, Italy;
Giovanni Carlo Del Vecchio, Pediatric Hematologist, Interdisciplinary Department of Medicine, Pediatric Section, “Aldo Moro” University of Bari, Bari, Italy;
Piero Farruggia, Pediatric Hematologist, Pediatric Hematology and Oncology Unit, ARNAS Ospedale Civico, Palermo, Italy;
Fiorina Giona, Hematologist, Department of Translational and Precision Medicine, Sapienza University of Rome, AOU Policlinico Umberto I, Rome, Italy;
Paola Giordano, Pediatric Hematologist, Interdisciplinary Department of Medicine, Pediatric Section, “Aldo Moro” University of Bari, Bari, Italy;
Chiara Gorio, Pediatric Hematologist, Pediatric Onco-hematology Unit, Children’s Hospital, ASST-Spedali Civili, Brescia, Italy;
Saverio Ladogana, Pediatric Hematologist, Pediatric Onco-hematology Unit, “Casa Sollievo della Sofferenza” Hospital, IRCCS, San Giovanni Rotondo, Italy;
Giuseppe Lassandro, Pediatric Hematologist, Interdisciplinary Department of Medicine, Pediatric Section, “Aldo Moro” University of Bari, Bari, Italy;
Antonio Marzollo, Pediatric Hematologist, Pediatric Hematology, Oncology and Stem Cell Transplant Division, Padua University Hospital, Padua, Italy;
Karolina Maslak, Psychologist, Pediatric Onco-hematology Unit, Azienda Policlinico Rodolico San Marco, Department of Clinical and Experimental Medicine, University of Catania, Italy;
Maurizio Miano, Pediatric Hematologist, Haematology Unit, IRCCS Istituto Giannina Gaslini, Genoa, Italy;
Margherita Nardi, Pediatric Hematologist, Pediatric Hematology Oncology, Bone Marrow Transplant, Azienda Ospedaliero Universitaria Pisana, S. Chiara Hospital, Pisa, Italy;
Lucia Dora Notarangelo, Pediatric Hematologist, Direzione Medica di Presidio, Children’s Hospital, ASST-Spedali Civili, Brescia, Italy;
Giuseppe Palumbo, Pediatric Hematologist, Department of Pediatric Hematology and Oncology Cell and Gene Therapy, Bambino Gesù Children’s Hospital, IRCCS, Department of Systems Medicine, University of Tor Vergata, Rome, Italy;
Emilia Parodi, Pediatric Hematologist, Pediatric and Neonatology Unit, Ordine Mauriziano Hospital, Turin, Italy;
Silverio Perrotta, Pediatric Hematologist, Department of Women, Children and General and Specialized Surgery, “Luigi Vanvitelli” Universita degli Studi della Campania, Naples, Italy;
Ugo Ramenghi, Pediatric Hematologist, Department of Public Health and Pediatric Sciences, Regina Margherita Children’s Hospital, University of Turin, Turin, Italy;
Francesca Rossi, Pediatric Hematologist, Department of Women, Children and General and Specialized Surgery, “Luigi Vanvitelli” Universita degli Studi della Campania, Naples, Italy;
Giovanna Russo, Pediatric Hematologist, Pediatric Onco-hematology Unit, Azienda Policlinico Rodolico San Marco, Department of Clinical and Experimental Medicine, University of Catania, Italy;
Paola Saracco, Pediatric Hematologist, Department of Public Health and Pediatric Sciences, Regina Margherita Children’s Hospital, University of Turin, Turin, Italy;
Marco Spinelli, Pediatric Hematologist, Pediatric Hematology Oncology Unit, Department of Pediatrics, IRCCS San Gerardo dei Tintori Foundation, Monza, Italy;
Alessandra Tolva, Pediatric Hematologist, Pediatric Hematology-Oncology, IRCCS Policlinico San Matteo, Pavia, Italy.
Consensus Conference participants
Rosa Angarano, Pediatric Oncologist, Pediatric Onco-hematology Unit, Azienda Ospedaliero Universitaria Consorziale Policlinico di Bari, Bari, Italy;
Angelica Barone, Pediatric Hematologist, Pediatrics and Pediatric Onco-hematology Unit, Azienda Ospedaliero-Universitaria di Parma, Parma, Italy;
Annamaria Bonutti, Pediatrician, Maternal and Child Department, San Bortolo Ospedale, Vicenza, Italy;
Gianluca Boscarol, Pediatrician, Department of Pediatrics, Central Teaching Hospital of Bolzano/Bozen, Bolzano, Italy;
Maddalena Casale, Pediatric Hematologist, Department of Women, Children and General and Specialized Surgery, “Luigi Vanvitelli” Universita degli Studi della Campania, Naples, Italy;
Teresa Ceglie, Pediatric Resident, Department of Public Health and Pediatric Sciences, Regina Margherita Children’s Hospital, University of Turin, Turin, Italy;
Simone Cesaro, Pediatric Hematologist, Pediatric Hematology Oncology, Department of Mother and Child, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy;
Moreno Crotti Partel, Nurse, Pediatric Onco-hematology Unit, Children’s Hospital, ASST-Spedali Civili, Brescia, Italy;
Giovanni Del Borrello, Pediatric Hematologist, Pediatric Oncohematology, Pediatrics Department, Hospital Città Della Salute e Della Scienza, University of Turin, Turin, Italy;
Giovanni Carlo Del Vecchio, Pediatric Hematologist, Interdisciplinary Department of Medicine, Pediatric Section, “Aldo Moro” University of Bari, Bari, Italy;
Rosanna Di Concilio, Pediatrician, Pediatric Unit, Ospedale Umberto I, Nocera Inferiore, Italy;
Loredana Farinasso, Pediatric Hematologist, Department of Public Health and Pediatric Sciences, Regina Margherita Children’s Hospital, University of Turin, Turin, Italy;
Elena Facchini, Pediatric Hematologist, Pediatric Oncology and Hematology “Lalla Seràgnoli”, Istituto di Ricovero e Cura a Carattere Scientifico, Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy;
Piero Farruggia, Pediatric Hematologist, Pediatric Hematology and Oncology Unit, ARNAS Ospedale Civico, Palermo, Italy;
Giulia Maria Ferrari, Pediatric Hematologist, Pediatric Hematology Oncology Unit, Department of Pediatrics, IRCCS San Gerardo dei Tintori Foundation, Monza, Italy;
Fiorina Giona, Hematologist, Department of Translational and Precision Medicine, Sapienza University of Rome, AOU Policlinico Umberto I, Rome, Italy;
Paola Giordano, Pediatric Hematologist, Interdisciplinary Department of Medicine, Pediatric Section, “Aldo Moro” University of Bari, Bari, Italy;
Chiara Gorio, Pediatric Hematologist, Pediatric Onco-hematology Unit, Children’s Hospital, ASST-Spedali Civili, Brescia, Italy;
Daniela Guardo, Pediatric Resident, Hematology Unit, IRCCS Istituto Giannina Gaslini, Genoa, Italy;
Angela Guarina, Pediatric Hematologist, Pediatric Hematology and Oncology Unit, ARNAS Ospedale Civico, Palermo, Italy;
Grazia Gurdo, Pediatric Hematologist, Pediatric Oncology-Hematology Unit, Santa Maria della Misericordia Hospital, Perugia, Italy;
Giorgio Ivani, Pediatric Anesthesiologist, Pediatric Anesthesia Unit, PO Regina Margherita, AOU Città della Salute e della Scienza, Turin, Italy;
Saverio Ladogana, Pediatric Hematologist, Pediatric Onco-hematology Unit “Casa Sollievo della Sofferenza” Hospital, IRCCS, San Giovanni Rotondo, Italy;
Giuseppe Lassandro, Pediatric Hematologist, Interdisciplinary Department of Medicine, Pediatric Section, “Aldo Moro” University of Bari, Bari, Italy;
Maria Licciardello, Pediatric Hematologist, Pediatric Onco-hematology Unit, Azienda Policlinico Rodolico San Marco, Department of Clinical and Experimental Medicine, University of Catania, Italy;
Laura Longo, Pharmacist, Clinical Pharmacology Unit, Azienda Policlinico Rodolico San Marco, Catania, Italy;
Ilaria Mariotti, Pediatric Hematologist, Pediatric Onco-hematology Unit, Policlinico Modena, Modena, Italy;
Antonio Marzollo, Pediatric Hematologist, Pediatric Hematology, Oncology and Stem Cell Transplant Division, Padua University Hospital, Padua, Italy;
Karolina Maslak, Psychologist, Pediatric Onco-hematology Unit, Azienda Policlinico Rodolico San Marco, Department of Clinical and Experimental Medicine, University of Catania, Italy;
Margherita Mauro, Pediatric Resident, Pediatric Hematology Oncology, Department of Mother and Child, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy;
Maurizio Miano, Pediatric Hematologist, Hematology Unit, IRCCS Istituto “Giannina Gaslini”, Genoa, Italy;
Tommaso Mina, Pediatric Hematologist, Pediatric Hematology-Oncology, IRCCS Policlinico San Matteo, Pavia, Italy;
Rossella Mura, Pediatric Hematologist, Pediatric Onco-hematology Unit, Azienda Ospedaliera Brotzu, Cagliari, Italy;
Margherita Nardi, Pediatric Hematologist, Pediatric Hematology Oncology, Bone Marrow Transplant, Azienda Ospedaliero Universitaria Pisana, S. Chiara Hospital, Pisa, Italy;
Agostino Nocerino, Pediatric Hematologist, Pediatric Unit, AOU Santa Maria della Misericordia, Udine, Italy;
Lucia Dora Notarangelo, Pediatric Hematologist, Direzione Medica di Presidio, Children’s Hospital, ASST-Spedali Civili, Brescia, Italy;
Manuela Pagano, Pediatrician, Department of Public Health and Pediatric Sciences, Regina Margherita Children’s Hospital, University of Turin, Turin, Italy;
Giuseppe Palumbo, Pediatric Hematologist, Department of Pediatric Hematology and Oncology Cell and Gene Therapy, “Bambino Gesù” Children’s Hospital, IRCCS, Department of Systems Medicine, University of Tor Vergata, Rome, Italy;
Emilia Parodi, Pediatric Hematologist, Pediatric and Neonatology Unit, Ordine Mauriziano Hospital, Turin, Italy;
Silverio Perrotta, Pediatric Hematologist, Department of Women, Children and General and Specialized Surgery, “Luigi Vanvitelli” Universita degli Studi della Campania, Naples, Italy;
Angelamaria Petrone, Pediatrician,Pediatric Unit, Ospedale S. Chiara, Trento, Italy;
Paolo Pierani, Pediatric Hematologist, Division of Pediatric Hematology and Oncology, Ospedale G. Salesi, Ancona, Italy;
Cristina Pizzato, Pediatrician, Pediatrics Unit, Ospedale di Treviso, Treviso, Italy;
Ugo Ramenghi, Pediatric Hematologist, Department of Public Health and Pediatric Sciences, “Regina Margherita” Children’s Hospital, University of Turin, Turin, Italy;
Francesca Rossi, Pediatric Hematologist, Department of Women, Children and General and Specialized Surgery, “Luigi Vanvitelli” Universita degli Studi della Campania, Naples, Italy;
Antonio Ruggiero, Pediatric Hematologist, Pediatric Oncology Unit, Fondazione Policlinico Universitario Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy;
Giovanna Russo, Pediatric Hematologist, Pediatric Onco-hematology Unit, Azienda Policlinico Rodolico San Marco, Department of Clinical and Experimental Medicine, University of Catania, Italy;
Laura Sainati, Pediatric Hematologist, Pediatric Hematology, Oncology and Stem Cell Transplant Division, Padua University Hospital, Padua, Italy;
Cristina Santoro, Hematologist, Hematology Unit, “Umberto I” University Hospital, Rome, Italy;
Nicola Santoro, Pediatric Oncologist, Pediatric Onco-hematology Unit, Azienda Ospedaliero Universitaria Consorziale Policlinico di Bari, Bari, Italy;
Paola Saracco, Pediatric Hematologist, Department of Public Health and Pediatric Sciences, “Regina Margherita” Children’s Hospital, University of Turin, Turin, Italy;
Antonella Sau, Pediatric Hematologist, Pediatric Onco-hematology Unit, Oncology and Hematology Department, Azienda Sanitaria Locale di Pescara, Pescara, Italy;
Fabian Schumacher, Pediatric Hematologist, Pediatric Oncohematology Unit, Children’s Hospital, ASST-Spedali Civili, Brescia, Italy;
Marco Spinelli, Pediatric Hematologist, Pediatric Hematology Oncology Unit, Department of Pediatrics, IRCCS San Gerardo dei Tintori Foundation, Monza, Italy;
Elisabetta Teruzzi, Pediatric Surgeon, Pediatric Surgery, Pediatrics Department, Hospital Città Della Salute e Della Scienza, University of Turin, Turin, Italy;
Alessandra Tolva, Pediatric Hematologist, Pediatric Hematology-Oncology, IRCCS Policlinico San Matteo, Pavia, Italy;
Assunta Tornesello, Pediatric Hematologist, Pediatric Onco-hematology Unit, Presidio Ospedliero “Vito Fazzi”, Lecce, Italy;
Federico Verzegnassi, Pediatric Hematologist, Institute of Maternal and Child Health, IRCCS Burlo Garofalo, Trieste, Italy;
Daniele Zama, Pediatric Hematologist, Pediatric Emergency Unit, IRCCS, Azienda Ospedaliero-Universitaria di Bologna, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy.
Footnotes
ETHICAL CONSIDERATION: The manuscript does not involve human or animal subjects or records of human patients. Therefore, no ethical approval was asked for.
AUTHORS’ CONTRIBUTIONS: GR, EP and UR contributed to the conceptualization and design of the study, acquisition and curation of the data, writing, critical appraisal and comments, and reviewing and editing of the manuscript. UR and PG contributed to the acquisition and curation of the data, critical appraisal and comments, and reviewing and editing the manuscript.
PF, LDN, SP, MC, SC, GDB, GCDV, FG, CG, SL, GL, AM, KM, MM, MN, GP, FR, MS, AT, and PS contributed to the acquisition and curation of the data, critical appraisal and comments. All Authors have read and agreed to the published version of the manuscript.
FUNDING: This research received no external funding; the APC was funded by Ibiscus E.T.S., Catania.
CONFLICTS OF INTEREST: All participants were asked to fill out a disclosure form, stating actual and potential conflicts of interest. SP and GR have received honoraria from Novartis as invited speakers; CS has received honoraria from Amgen and Novartis as an invited speaker and has received a contribution from Novartis for medical writing; UR received a contribution from Novartis as support for a Meeting. All the other Authors have no potential conflicts of interest.
REFERENCES
- 1.Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am J Hematol. 2010;85:174–180. doi: 10.1002/ajh.21616. [DOI] [PubMed] [Google Scholar]
- 2.Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, Gernsheimer T, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3:3780–3817. doi: 10.1182/bloodadvances.2019000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neunert CE, Cooper N. Evidence-based management of immune thrombocytopenia: ASH guideline update. Hematology Am Soc Hematol Educ Program. 2018;2018:568–575. doi: 10.1182/asheducation-2018.1.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Mattia D, del Principe D, Del Vecchio GC, Jankovic M, Arrighini A, Giordano P, et al. Acute childhood idiopathic thrombocytopenic purpura: AIEOP consensus guidelines for diagnosis and treatment. Associazione Italiana di Ematologia e Oncologia Pediatrica. Haematologica. 2000;85:420–424. [PubMed] [Google Scholar]
- 5.Barone A, Lucarelli A, Onofrillo D, Verzegnassi F, Bonanomi S, Cesaro S, et al. Diagnosis and management of acquired aplastic anemia in childhood. Guidelines from the Marrow Failure Study Group of the Pediatric Haemato-Oncology Italian Association (AIEOP) Blood Cells Mol Dis. 2015;55:40–47. doi: 10.1016/j.bcmd.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Ladogana S, Maruzzi M, Samperi P, Perrotta S, Del Vecchio GC, Notarangelo LD, et al. Diagnosis & management of newly diagnosed childhood autoimmune haemolytic anaemia. Recommendations from the red cell study group of the paediatric haemato-oncology Italian association. Blood Transfus. 2017;15:259–267. doi: 10.2450/2016.0072-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ladogana S, Maruzzi M, Samperi P, Condorelli A, Casale M, Giordano P, et al. Second-line therapy in paediatric warm autoimmune haemolytic anaemia. Guidelines from the Associazione Italiana Onco-Ematologia Pediatrica (AIEOP) Blood Transfus. 2019;16:352–357. doi: 10.2450/2018.0024-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fioredda F, Onofrillo D, Farruggia P, Barone A, Veltroni M, Notarangelo LD, et al. Diagnosis and management of neutropenia in children: the approach of the Study Group on Neutropenia and Marrow Failure Syndromes of the Pediatric Italian Hemato-Oncology Association (Associazione Italiana Emato-Oncologia Pediatrica-AIEOP) Pediatr Blood Cancer. 2022;69:e29599. doi: 10.1002/pbc.29599. [DOI] [PubMed] [Google Scholar]
- 9.De Mattia D, Del Vecchio GC, Russo G, De Santis A, Ramenghi U, Notarangelo L, et al. Management of chronic childhood immune thrombocytopenic purpura: AIEOP consensus guidelines. Acta Haematol. 2010;123:96–109. doi: 10.1159/000268855. [DOI] [PubMed] [Google Scholar]
- 10.KBrouwers MC, Kerkvliet K, Spithoff K AGREE Next Steps Consortium. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ. 2016;352:i1152. doi: 10.1136/bmj.i1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George JN, Woolf SH, Raskob GE, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88:3–40. [PubMed] [Google Scholar]
- 12.Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 13.Neunert C, Noroozi N, Norman G, Buchanan GR, Goy J, Nazi I, et al. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost. 2015;13:457–464. doi: 10.1111/jth.12813. doi: wiley.com/10.1111/jth.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchanan GR, Adix L. Grading of hemorrhage in children with idiopathic thrombocytopenic purpura. J Pediatr. 2002;141:683–688. doi: 10.1067/mpd.2002.128547. [DOI] [PubMed] [Google Scholar]
- 15.Rodeghiero F, Michel M, Gernsheimer T, Ruggeri M, Blanchette V, Bussel JB, et al. Standardization of bleeding assessment in immune thrombocytopenia: report from the International Working Group. Blood. 2013;121:2596–2606. doi: 10.1182/blood-2012-07-442392. [DOI] [PubMed] [Google Scholar]
- 16.Visweshwar N, Ayala I, Jaglal M, Killeen R, Sokol L, Laber DA, et al. Primary immune thrombocytopenia: a “diagnosis of exclusion”? Blood Coagul Fibrinolysis. 2022;33:289–294. doi: 10.1097/MBC.0000000000001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labarque V, van Geet C. Clinical practice: immune thrombocytopenia in paediatrics. Eur J Pediatr. 2014;173:163–172. doi: 10.1007/s00431-013-2254-6. [DOI] [PubMed] [Google Scholar]
- 18.Lee ACW. Isolated thrombocytopenia in childhood: what if it is not immune thrombocytopenia? Singapore Med J. 2018;59:390–393. doi: 10.11622/smedj.2018089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cines DB, Cuker A, Semple JW. Pathogenesis of immune thrombocytopenia. Presse Med. 2014;43:e49–59. doi: 10.1016/j.lpm.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Neunert C, Lim W, Crowther MA, Cohen A, Solberg Jr L, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 21.Glanz J, France E, Xu S, Hayes T, Hambidge S. A population-based, multisite cohort study of the predictors of chronic idiopathic thrombocytopenic purpura in children. Pediatrics. 2008;121:e506–12. doi: 10.1542/peds.2007-1129. [DOI] [PubMed] [Google Scholar]
- 22.Donato H, Picón A, Martinez M, Rapetti MC, Rosso A, Gomez S, et al. Demographic data, natural history, and prognostic factors of idiopathic thrombocytopenic purpura in children: A multicentered study from Argentina. Pediatr Blood Cancer. 2009;52:491–496. doi: 10.1002/pbc.21872. [DOI] [PubMed] [Google Scholar]
- 23.ElAlfy M, Farid S, Maksoud AA. Predictors of chronic idiopathic thrombocytopenic purpura. Pediatr Blood Cancer. 2010;54:959–962. doi: 10.1002/pbc.22481. [DOI] [PubMed] [Google Scholar]
- 24.Evim MS, Baytan B, Güneş AM. Childhood immune thrombocytopenia: long-term follow-up data evaluated by the criteria of the International Working Group on Immune Thrombocytopenic Purpura. Turk J Haematol. 2014;31:32–9. doi: 10.4274/Tjh.2012.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt DE, Wendtland Edslev P, Heitink-Pollé KMJ, Mertens B, Bruin MCA, Kapur R, et al. A clinical prediction score for transient versus persistent childhood immune thrombocytopenia. J Thromb Haemost. 2021;19:121–130. doi: 10.1111/jth.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iyori H, Bessho F, Ookawa H, Konishi S, Shirahata A, Miyazaki S, et al. Intracranial hemorrhage in children with immune thrombocytopenic purpura. Japanese Study Group on childhood ITP. Ann Hematol. 2000;79:691–695. doi: 10.1007/s002770000219. [DOI] [PubMed] [Google Scholar]
- 27.Beck CE, Nathan PC, Parkin PC, Blanchette VS, Macarthur C. Corticosteroids versus intravenous immune globulin for the treatment of acute immune thrombocytopenic purpura in children: a systematic review and meta-analysis of randomized controlled trials. J Pediatr. 2005;147:521–527. doi: 10.1016/j.jpeds.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 28.Heitink-Polle KM, Nijsten J, Boonacker CW, de Haas M, Bruin MC. Clinical and laboratory predictors of chronic immune thrombocytopenia in children: a systematic review and meta-analysis. Blood. 2014;124:3295–3307. doi: 10.1182/blood-2014-04-570127. [DOI] [PubMed] [Google Scholar]
- 29.Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–186. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 30.Parodi E, Giordano P, Rivetti E, Giraudo MT, Ansaldi G, Davitto M, et al. Efficacy of combined intravenous immunoglobulins and steroids in children with primary immune thrombocytopenia and persistent bleeding symptoms. Blood Transfus. 2014;12:340–345. doi: 10.2450/2014.0185-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarantino MD, Bussel JB, Blanchette VS, Despotovic J, Bennett C, Raj A, et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388:45–54. doi: 10.1016/S0140-6736(16)00279-8. [DOI] [PubMed] [Google Scholar]
- 32.Bussel JB, Buchanan GR, Nugent DJ, Gnarra DJ, Bomgaars LR, Blanchette VS, et al. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. 2011;118:28–36. doi: 10.1182/blood-2010-10-313908. [DOI] [PubMed] [Google Scholar]
- 33.Elalfy MS, Abdelmaksoud AA, Eltonbary KY. Romiplostim in children with chronic refractory ITP: randomized placebo controlled study. Ann Hematol. 2011;90:1341–1344. doi: 10.1007/s00277-011-1172-9. [DOI] [PubMed] [Google Scholar]
- 34.Grainger JD, Kühne T, Hippenmeyer J, Cooper N. Romiplostim in children with newly diagnosed or persistent primary immune thrombocytopenia. Ann Hematol. 2021;100:2143–2154. doi: 10.1007/s00277-021-04590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bussel JB, Hsieh L, Buchanan GR, Stine K, Kalpatthi R, Gnarra DJ, et al. Long-term use of the thrombopoietin-mimetic romiplostim in children with severe chronic immune thrombocytopenia (ITP) Pediatr Blood Cancer. 2015;62:208–213. doi: 10.1002/pbc.25136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giordano P, Lassandro G, Barone A, Cesaro S, Fotzi I, Giona F, et al. Use of eltrombopag in children with chronic immune thrombocytopenia (ITP): a real life retrospective multicenter experience of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Front Med. 2020;7:66. doi: 10.3389/fmed.2020.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor A, Neave L, Solanki S, Westwood JP, Terrinonive I, Mcguckin S, et al. Mycophenolate mofetil therapy for severe immune thrombocytopenia. Br J Haematol. 2015;171:625–630. doi: 10.1111/bjh.13622. [DOI] [PubMed] [Google Scholar]
- 38.Miano M, Ramenghi U, Russo G, Rubert L, Barone A, Tucci F, et al. Mycophenolate mofetil for the treatment of children with immune thrombocytopenia and Evans syndrome. A retrospective data review from the Italian Association of Paediatric Haematology/Oncology. Br J Haematol. 2016;175:490–495. doi: 10.1111/bjh.14261. [DOI] [PubMed] [Google Scholar]
- 39.Bradbury CA, Pell J, Hill Q, Bagot C, Cooper N, Ingram J, et al. Mycophenolate mofetil for first-line treatment of immune thrombocytopenia. N Engl J Med. 2021;385:885–895. doi: 10.1056/NEJMoa2100596. [DOI] [PubMed] [Google Scholar]
- 40.Miano M, Rotulo GA, Palmisani E, Giaimo M, Fioredda F, Pierri F. Sirolimus as a rescue therapy in children with immune thrombocytopenia refractory to mycophenolate mofetil. Am J Hematol. 2018;93:E175–E177. doi: 10.1002/ajh.25119. [DOI] [PubMed] [Google Scholar]
- 41.Miano M, Scalzone M, Perri K, Palmisani E, Caviglia I, Micalizzi C, et al. Mycophenolate mofetil and sirolimus as second or further line treatment in children with chronic refractory primitive or secondary autoimmune cytopenias: a single centre experience. Br J Haematol. 2015;171:247–253. doi: 10.1111/bjh.13533. [DOI] [PubMed] [Google Scholar]
- 42.Patel VL, Mahevas M, Lee SY, Stasi R, Cunningham-Rundles S, Godeau B. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119:5989–5995. doi: 10.1182/blood-2011-11-393975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parodi E, Nobili B, Perrotta S, Rosaria Matarese SM, Russo G, Licciardello M, et al. Rituximab (anti-CD20 monoclonal antibody) in children with chronic refractory symptomatic immune thrombocytopenic purpura: efficacy and safety of treatment. Int J Hematol. 2006;84:48–53. doi: 10.1532/IJH97.E0518. [DOI] [PubMed] [Google Scholar]
- 44.Parodi E, Rivetti E, Amendola G, Bisogno G, Calabrese R, Farruggia P, et al. Long-term follow-up analysis after rituximab therapy in children with refractory symptomatic ITP: Identification of factors predictive of a sustained response. Br J Haematol. 2009;144:552–558. doi: 10.1111/j.1365-2141.2008.07487.x. [DOI] [PubMed] [Google Scholar]
- 45.Arnold DM, Dentali F, Crowther MA, Meyer RM, Cook RJ, Sigouin C, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146:25–33. doi: 10.7326/0003-4819-146-1-200701020-00006. [DOI] [PubMed] [Google Scholar]
- 46.Liang Y, Zhang L, Gao J, Hu D, Ai Y. Rituximab for children with immune thrombocytopenia: a systematic review. PLoS One. 2012;7:e36698. doi: 10.1371/journal.pone.0036698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bansal D, Rajendran A, Singhi S. Newly diagnosed immune thrombocytopenia: update on diagnosis and management [Internet] Indian J Pediatr. 2014;81:1033–1041. doi: 10.1007/s12098-013-1217-2. [DOI] [PubMed] [Google Scholar]
- 48.Larsen OH, Stentoft J, Radia D, Ingerslev J, Sørensen B. Combination of recombinant factor VIIa and fibrinogen corrects clot formation in primary immune thrombocytopenia at very low platelet counts. Br J Haematol. 2013;160:228–236. doi: 10.1111/bjh.12118. [DOI] [PubMed] [Google Scholar]
- 49.Salama A, Rieke M, Kiesewetter H, von Depka M. Experiences with recombinant FVIIa in the emergency treatment of patients with autoimmune thrombocytopenia: a review of the literature. Ann Hematol. 2009;88:11–15. doi: 10.1007/s00277-008-0608-3. [DOI] [PubMed] [Google Scholar]
- 50.Gurion R, Siu A, Weiss AR, Masterson M. Use of recombinant factor VIIa in a pediatric patient with initial presentation of refractory acute immune thrombocytopenic purpura and severe bleeding. J Pediatr Pharmacol Ther. 2012;17:274–80. doi: 10.5863/1551-6776-17.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molica M, Massaro F, Annechini G, Baldacci E, D’Elia GM, Rosati R, et al. Life-threatening autoimmune hemolytic anemia and idiopathic thrombocytopenic purpura. Successful selective splenic artery embolization. Mediterr J Hematol Infect Dis. 2016;8:e2016020. doi: 10.4084/MJHID.2016.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imbach P. When a child with immune thrombocytopenia experiences life-threatening bleeding [Internet] Klin Padiatr. 2010;222:337–339. doi: 10.1055/s-0030-1265161. [DOI] [PubMed] [Google Scholar]
- 53.Nomura S. Advances in diagnosis and treatments for immune thrombocytopenia. Clin Med Insights Blood Disord. 2016;9:15–22. doi: 10.4137/CMBD.S39643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nieminen U, Peltola H, Syrjälä MT, Mäkipernaa A, Kekomäki R. Acute thrombocytopenic purpura following measles, mumps and rubella vaccination. A report on 23 patients. Acta Paediatr. 1993;82:267–270. doi: 10.1111/j.1651-2227.1993.tb12657.x. [DOI] [PubMed] [Google Scholar]
- 55.Jonville-Béra AP, Autret E, Galy-Eyraud C, Hessel L. Thrombocytopenic purpura after measles, mumps and rubella vaccination: a retrospective survey by the French Regional Pharmacovigilance Centres and Pasteur-Mérieux Sérums et Vaccins. Pediatr Infect Dis J. 1996;15:44–8. doi: 10.1097/00006454-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Rajantie J, Zeller B, Treutiger I, Rosthöj S. Vaccination associated thrombocytopenic purpura in children. Vaccine. 2007;25:1838–1840. doi: 10.1016/j.vaccine.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 57.France EK, Glanz J, Xu S, Hambidge S, Yamasaki K, Black SB, et al. Risk of immune thrombocytopenic purpura after measles-mumps-rubella immunization in children. Pediatrics. 2008;121:e687–92. doi: 10.1542/peds.2007-1578. [DOI] [PubMed] [Google Scholar]
- 58.Jadavji T, Scheifele D, Halperin S. Thrombocytopenia after immunization of Canadian children, 1992 to 2001. Pediatr Infect Dis J. 2003;22:119–122. doi: 10.1097/01.inf.0000048961.08486.d1. [DOI] [PubMed] [Google Scholar]
- 59.Hill QA, Newland AC. Fatigue in immune thrombocytopenia. Br J Haematol. 2015;170:141–149. doi: 10.1111/bjh.13385. [DOI] [PubMed] [Google Scholar]
- 60.Grace RF, Klaassen RJ, Shimano KA, Lambert MP, Grimes A, Bussel JB, et al. Fatigue in children and adolescents with immune thrombocytopenia. Br J Haematol. 2020;191:98–106. doi: 10.1111/bjh.16751. [DOI] [PubMed] [Google Scholar]
- 61.Giordano P, Lassandro G, Giona F, Jankovic M, Nardi M, Nobili B, et al. ITP-QoL questionnaire for children with immune thrombocytopenia: Italian version validation’s. Pediatr Hematol Oncol. 2014;31:534–47. doi: 10.3109/08880018.2014.915443. [DOI] [PubMed] [Google Scholar]
- 62.Lassandro G, Palmieri VV, Barone A, Farruggia P, Giona F, Licciardello M, et al. Fatigue perception in a cohort of children with chronic immune thrombocytopenia and their caregivers using the PedsQL MFS: real-life multicenter experience of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Pediatr Blood Cancer. 2021;68:e28840. doi: 10.1002/pbc.28840. [DOI] [PubMed] [Google Scholar]