Abstract
SARS-CoV-2 causes severe acute respiratory syndrome. mRNA vaccines directed at the SARS-CoV-2 spike protein resulted in development of Abs and protective immunity. To determine the mechanism, we analyzed the kinetics of induction of circulating exosomes with SARS-CoV-2 spike protein and Ab following vaccination of healthy individuals. Results demonstrated induction of circulating exosomes expressing spike protein on day 14 after vaccination followed by Abs 14 days after the second dose. Exosomes with spike protein, Abs and T cells secreting IFN-Ύ and TNFα increased following the booster dose. Transmission electron microscopy of exosomes also demonstrated spike protein antigens on their surface. Exosomes with spike protein and Abs decreased in parallel after 4 months. These results demonstrate an important role of circulating exosomes with spike protein for effective immunization following mRNA based vaccination. This is further documented by induction of humoral and cellular immune responses in mice immunized with exosomes carrying spike protein.
Introduction
The coronavirus disease (COVID-19) pandemic is caused by severe acute respiratory syndrome corona virus 2 (SARS-CoV-2). Most infected individuals with mild symptoms spontaneously recover, but SARS-CoV-2 infection can result in a severe acute respiratory illness requiring mechanical ventilation with about 1% mortality. To induce immunity and reduce the severity of SARS-CoV-2 infection, several categories of vaccines have been developed (1, 2). As per recent updates by Center for Disease Control and prevention (CDC), death rate in SARS-CoV-2 patients are four times higher in people 30–39 years old when compared to 18–29 years old, and 600 times higher in patients 85 years and older. (Resource: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html)
mRNA-based vaccines against the SARS-CoV-2 virus infection developed by Pfizer-BioNTech and Moderna were granted emergency use authorization by the United States Food and Drug Administration (3–5). These vaccines protect recipients from a SARS-CoV-2 infection by developing humoral and cellular immune responses. The beneficial effect of vaccination is often determined by measuring Ab responses to the spike protein (6). In the current study, we analyzed 8 healthy adults who received both doses of the SARS-CoV-2 vaccine (Pfizer-BioNTech). Our results demonstrated the induction of circulating exosomes carrying the SARS-CoV-2 spike protein by day 14, when Abs to the spike protein were not detectable in the sera using an ELISA method developed in our laboratory. Circulating Abs were detectable only after the second booster dose of vaccine (days 14) and the amount of exosomes containing spike protein was increased up to ~12 fold maximum. These results strongly support an important role for exosome induction conferring immune responses following immunization. This is further supported by our finding that immunization of mice with exosomes from the vaccinated individual with the SARS-CoV-2 spike protein resulted in the development of Abs specific to SARS-CoV-2 spike protein.
Materials and Methods
Patient cohort and demographics
We analyzed 8 healthy adult volunteers vaccinated with the mRNA based SARS-CoV-2 vaccine (Pfizer-BIONTech). Blood was collected before vaccination, days 7 and 14 after the first dose, day 14 after the second dose, and 4 months after both the doses. This study was approved by the Institutional Review Boards at St. Joseph’s Hospital (IRB# PHXB16-0027-10-18).
Exosome isolation and nanoparticle tracking analysis
Exosomes were isolated from 500 μl of plasma using Invitrogen exosome isolation kit followed by 0.22-micron filtration (7). All exosomes were analyzed for size by Nanosight NS300 (Malvern, Great Malvern, UK) and the mean size of the paricles used in our experiments was <200 nm (8).
Detection of Abs to SARS-CoV-2 spike protein and nucleocapsid protein from human plasma samples
Development of Abs to SARS-CoV-2 spike antigen was determined using an ELISA developed in our laboratory. In brief, 1 μg/mL of SARS-CoV-2 spike protein (Sino Biologicals) suspended in PBS were coated onto an ELISA plate and incubated overnight at 4°C. Human plasma was added to these plates at 1:750 dilution. Detection was performed using secondary anti-human immunoglobulin G-horseradish peroxidase (1:10,000) and developed using tetramethylbenzidine substrate and read at 450 nm. Plasma samples from individuals infected with SARS-CoV-2 (n=10), and healthy individuals immunized with both doses of vaccine (n=20) were used as positive controls. Healthy individuals with no history of SARS-CoV-2 infection and no vaccination towards SARS-CoV-2 were used as negative controls (n=20). Ab concentration was calculated using a standard curve from known concentrations of respective Abs (ThermoFisher).
Characterization of exosomes using western blot
Total exosome protein (15 μg) was resolved by polyacrylamide gel electrophoresis and the proteins were transferred onto a polyvinylidene difluoride membrane. Western blots were performed as described in (9). The band intensity of the target protein was quantified using ImageJ software and all the blots were normalized with CD9.
Transmission electron microscopy of isolated exosomes for SARS-CoV-2 spike protein
Exosomes were labelled with immunogold, mouse anti-SARS-CoV-2 spike Ab and Corona FIPV3-70 Ab (1:100) was added to the grids. Grids were washed and stained with uranyl acetate and viewed by transmission electron microscopy (JEOL USA, Inc.) (10).
Enzyme-linked immunospot (EliSPOT) for human T cell responses to SARS-CoV-2 spike protein antigen
Blood was obtained from individuals after obtaining informed consent and the study was approved by the Institutional IRB (IRB# PHXB16-0027-10-18). PBMC was isolated by Ficoll-isopaque gradient separation (ICN Biomedicals Inc., Aurora, OH) and cryopreserved. Later, these PBMC`s were processed for EliSPOT assay as described in publications (9, 10).
Immunization of mice with exosomes with SARS-CoV-2 antigen
C57BL/6 mice were immunized subcutaneously with exosomes isolated from vaccinated individuals positive for SARS-CoV-2 spike protein (Exosomes from day 14 after dose 2 of vaccine). Three groups of animals were immunized without any adjuvants, 100ug on days 1, 7 and 21: 1) Control group of animals (N=5); 2) Exosomes isolated from one healthy individual following vaccination (N=5); 3) Exosomes isolated from second healthy individual following vaccination (N=5), and 4) Mice immunized with SARS-CoV-2 spike protein (CSP) (N=5). Animals were sacrificed at day 30 and blood was collected for ELISA and spleens were harvested for T cell responses.
Detection of Abs to SARS-CoV-2 spike protein in mice serum
Development of Abs to SARS-CoV-2 spike antigen was determined using an ELISA as described in previous section.
EliSPOT for murine splenocyte responses to SARS-CoV-2 spike protein antigens
Mice spleens were harvested at D30 post immunization and splenocytes were isolated by Ficoll-Hypaque gradient centrifugation and analyzed by EliSPOT as described previously ((9, 10).
Statistical analysis
Data was analyzed using Prism 8.0 software (GraphPad, Inc.). The Ab levels for SARS-CoV-2 spike protein and the optical density of exosomes containing SARS-CoV-2 spike protein were compared using Wilcoxon Rank test. Results from animal experiments were analyzed using 2 way ANOVA. Data was expressed as mean and standard deviation. P-values <0.05 were considered statistically significant.
Results and Discussion
Exosome Isolation
The mean size of the paricles used in our study were <200 nm, in agreement with the exosome size described by the International Society for Extracellular Vesicles. Representative images for exosomes are given in Figure 1A. There was no significant difference in number of exosomes from different individuals.
Figure 1:
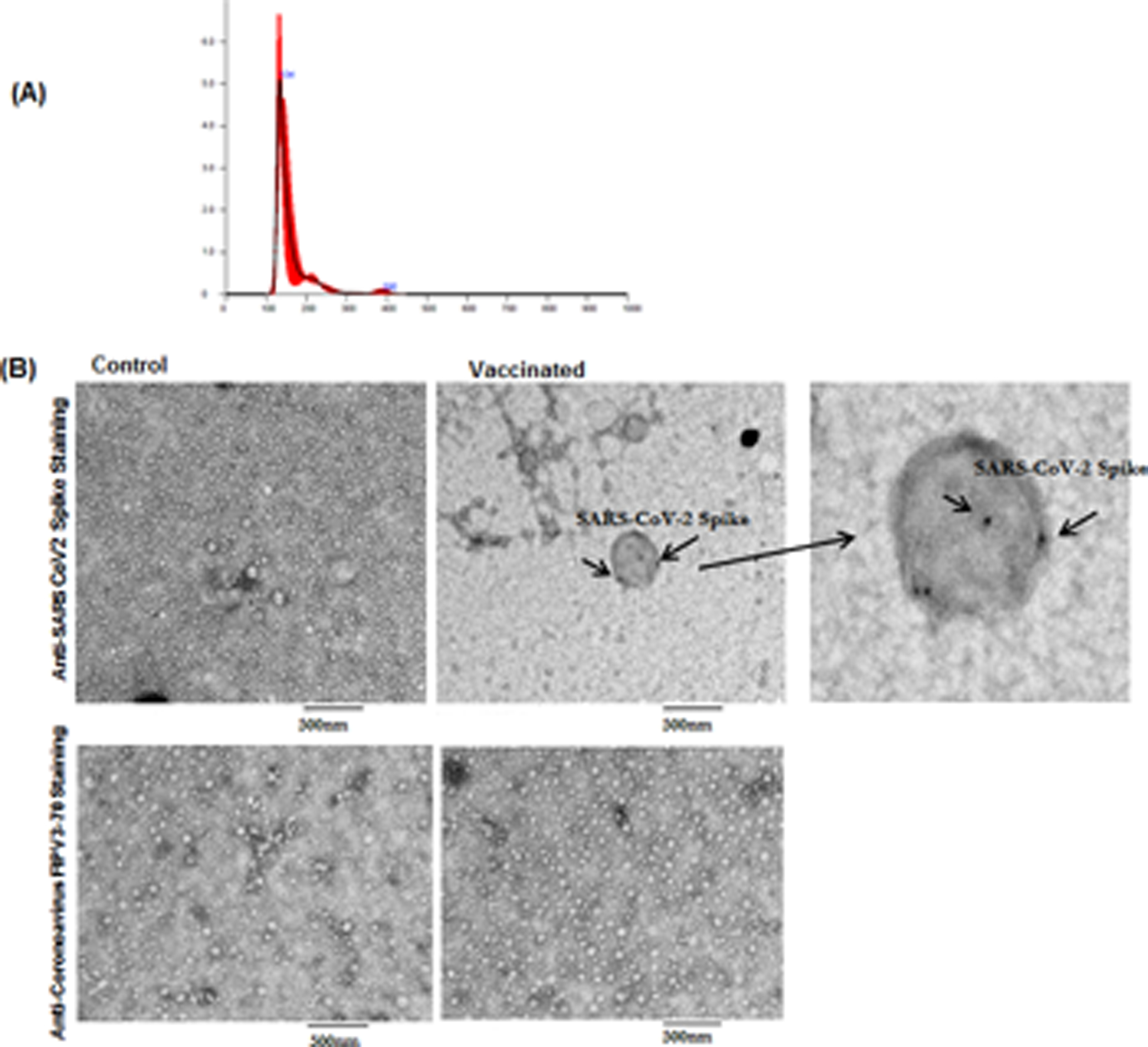
(A) Representative nanosight image for exosomes from vaccinated individuals with mean and median sizes (black thin line in the graph indicates the 3 measurements of the same sample and red line is the average of all 3 lines). (B) Transmission Electron Microscopy Images of SARS-CoV-2 spike antigen on exosomes from control exosomes from control and vaccinated individuals. Arrows indicate SARS-CoV-2 spike positive exosomes. Right side third image is the zoomed image of positive exosome from Vaccinated sample. We have used anti-coronavirus FIPV3-70 Ab as negative control for both the samples.
Transmission electron microscopy demonstrated the surface expression of SARS-CoV-2 spike protein in isolated exosomes from vaccinated individuals
We performed transmission electron microscopy using Abs specific for SARS-CoV-2 spike to demonstrate the presence of SARS-CoV-2 antigens on the surface of exosomes from controls and healthy vaccinated individuals. Exosomes from vaccinated individuals are positive for SARS-CoV-2 antigen (Figure 1B). We have also stained both the exosome samples with Coronavirus FIPV3-70 Ab as negative control and didn`t observe any positive reaction in exosomes (Figure 1B).
Kinetics of development of Abs for SARS-CoV-2 spike protein in vaccinated people
Abs to SARS-CoV-2 spike protein were detected in all the healthy vaccinated individuals after day 14 of the second dose of the vaccine (mean concentration, 2401.25±773.45 ng/ml) with p values <0.0001 when compared to no vaccination and day 14 of the first dose of the vaccine. The Ab levels after 4 months of vaccination had decreased to 1107.38 ± 681.63ng/ml. This decrease is significantly lower as compared to the Abs developed at day 14 following the second shot of vaccine (p=0.0313) (Figure 2A).
Figure 2:
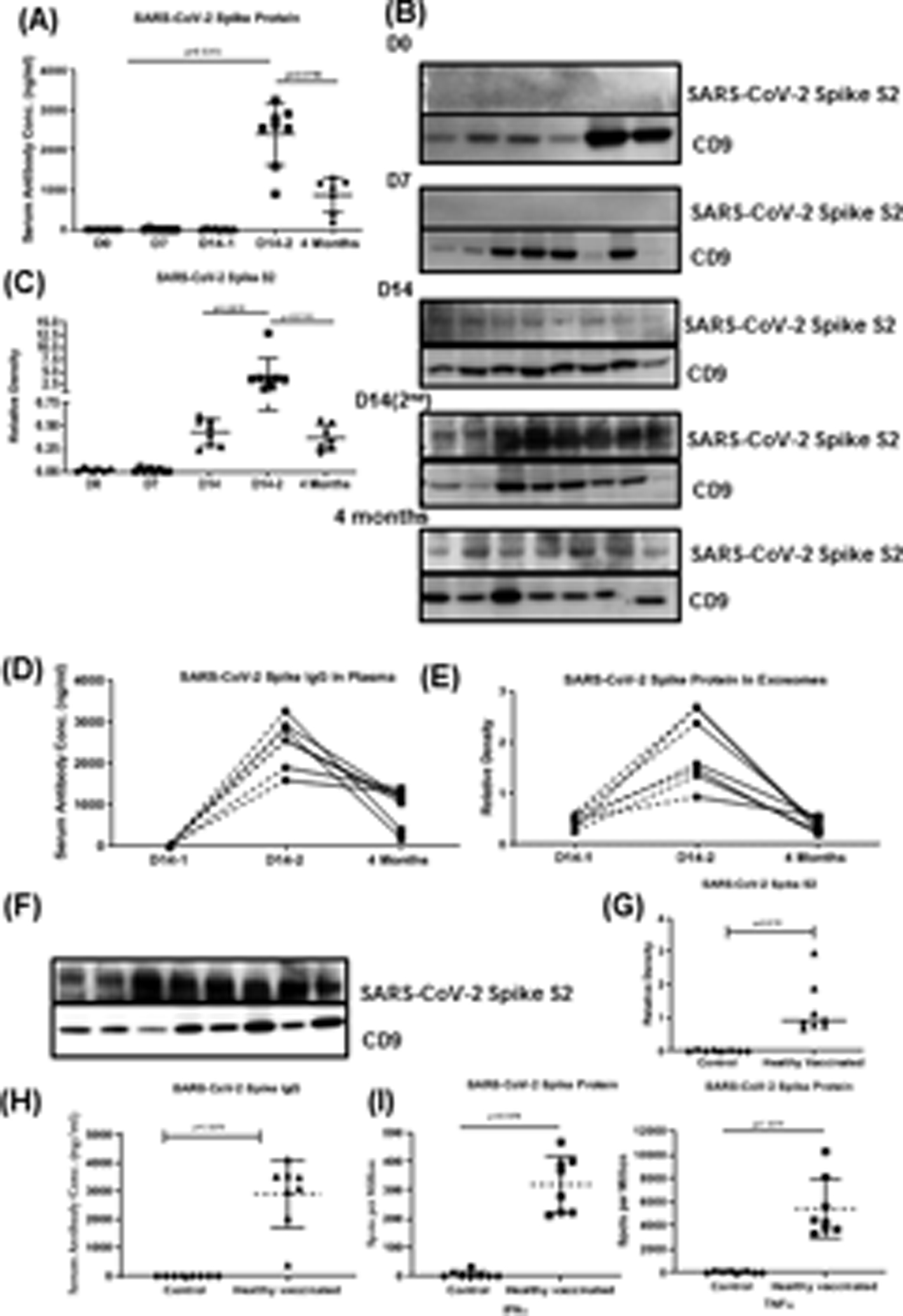
(A) Levels of SARS-CoV-2 spike Ab in vaccinated healthy individuals at D0, D7 and 14 after first dose of vaccine, D14 after second dose of vaccine and 4 months after second dose of vaccine. (B) Western blot of exosome protein SARS-CoV-2 spike protein S2 at D0, D7 and D14 of first vaccination, D14–2 after second dose of vaccine and 4 months after second dose of vaccine. (C) Densitometry and statistical analysis of western blots. (D) Before and after line plot of levels of SARS-CoV-2 spike Ab in vaccinated healthy individuals at D14–1, D14–2 and 4M after second dose of vaccine (E) Before and after line plot of densitometry of western blots for SARS-CoV-2 spike protein in vaccinated healthy individuals at D14–1, D14–2 and 4M after second dose of vaccine. (F) Western blot of SARS-CoV-2 spike protein S2 in exosomes within 14 days after second dose of vaccine in healthy individuals. (G) Densitometry and statistical analysis of western blots. (H) Levels of SARS-CoV-2 spike Ab in vaccinated healthy individuals at 14 days after second dose of vaccine. (I) EliSPOT for cytokine development (TNFα and IFNγ) in response to SARS-CoV-2 spike protein.
*All graphs are represented as scatter dot plots with mean and SD (vertical bar line). CD9 is used to normalize the all the blots. Western blots and Antibody development experiments were performed at least 3 times independently.
Circulating exosomes isolated from vaccinated individuals contained SARS-CoV-2 spike protein antigen S2
We analyzed plasma from vaccinated healthy individuals at days 0, 7 and 14 from the first dose of the vaccine and day 14 of the second dose for the presence of exosomes carrying the SARS-CoV-2 spike protein (Figure 2B &C). The results demonstrated the presence of SARS-CoV-2 spike antigen S2 on exosomes at day 14 of dose 1. There is a significant increase in the concentration of the spike protein at day 14 of dose 2 with a p value of 0.0299. The amount of SARS-CoV-2 spike protein in exosomes after 4 months of both doses of vaccine was significantly decreased as compared to day 14 after the second dose with the p value of 0.0078. Kinetics of Ab development to the spike protein and exosomes with spike protein for each healthy individual at different time points (day 14 after first and second dose and 4 months after second dose) (Figure 2D). Both the kinetics of Ab development and the amount of SARS-CoV-2 spike protein exosomes are in agreement with each other as both are increased following the second booster dose at day 14 Figure 2E. There is a decrease in Ab levels to the SARS-CoV-2 spike protein and the amount of SARS-CoV-2 spike protein in exosomes in each healthy individual from day14 of second booster dose, to 4 months of the second booster dose (Figure 2D & E). Data for each individual for this subset is given as Supplementary Figure S1, S2.
Circulating exosomes isolated from vaccinated healthy individuals contained SARS-CoV-2 spike protein antigen S2
We analyzed exosomes from vaccinated healthy individuals on day 14 following second dose of vaccine. The results demonstrated significant increases in the concentration of exosomes with SARS-CoV-2 spike protein (Figure 2F & G). In parallel, we also found significantly increased levels of Abs to SARS-CoV-2 spike protein in the individuals followed by both the doses of vaccine as compared to healthy controls (Figure 2H).
Higher levels of cytokines IFN-γ and TNFα in healthy vaccinated individuals as compared to controls
T cell responses to SARS-CoV-2 spike protein antigen from healthy vaccinated individuals were analyzed using EliSPOT. Cytokines IFN-γ and TNFα producing cells were significantly higher in vaccinated healthy individuals as compared to healthy controls in response to SARS-CoV-2 spike antigen (p values p=0.0078) (Figure 2I). T cells producing either of the cytokines in response to nucleocapsid antigen were not increased.
Immunization with exosomes from vaccinated individual with spike protein induced higher levels of Abs to SARS-CoV-2 spike protein in mice
We immunized C57BL/6 mice with exosomes isolated from fully vaccinated individuals and controls. However, mice immunized with exosomes from fully vaccinated individuals developed significantly higher Abs to SARS-CoV-2 spike antigen at day 15 controls; (181.49±37.02 vs 64.44±1.7, p<0.0449), at day 21 (352.82±128.82 vs 20.84±2.24, p<0.0001) and at day 30 (372.34±56.08 vs 25.17±1.08 p<0.0001) (Figure 3A). C57BL/6 animals immunized with SARS-CoV-2 spike protein have also shown increased levels of SARS-CoV-2 spike Ab (Figure 3A).
Figure 3:
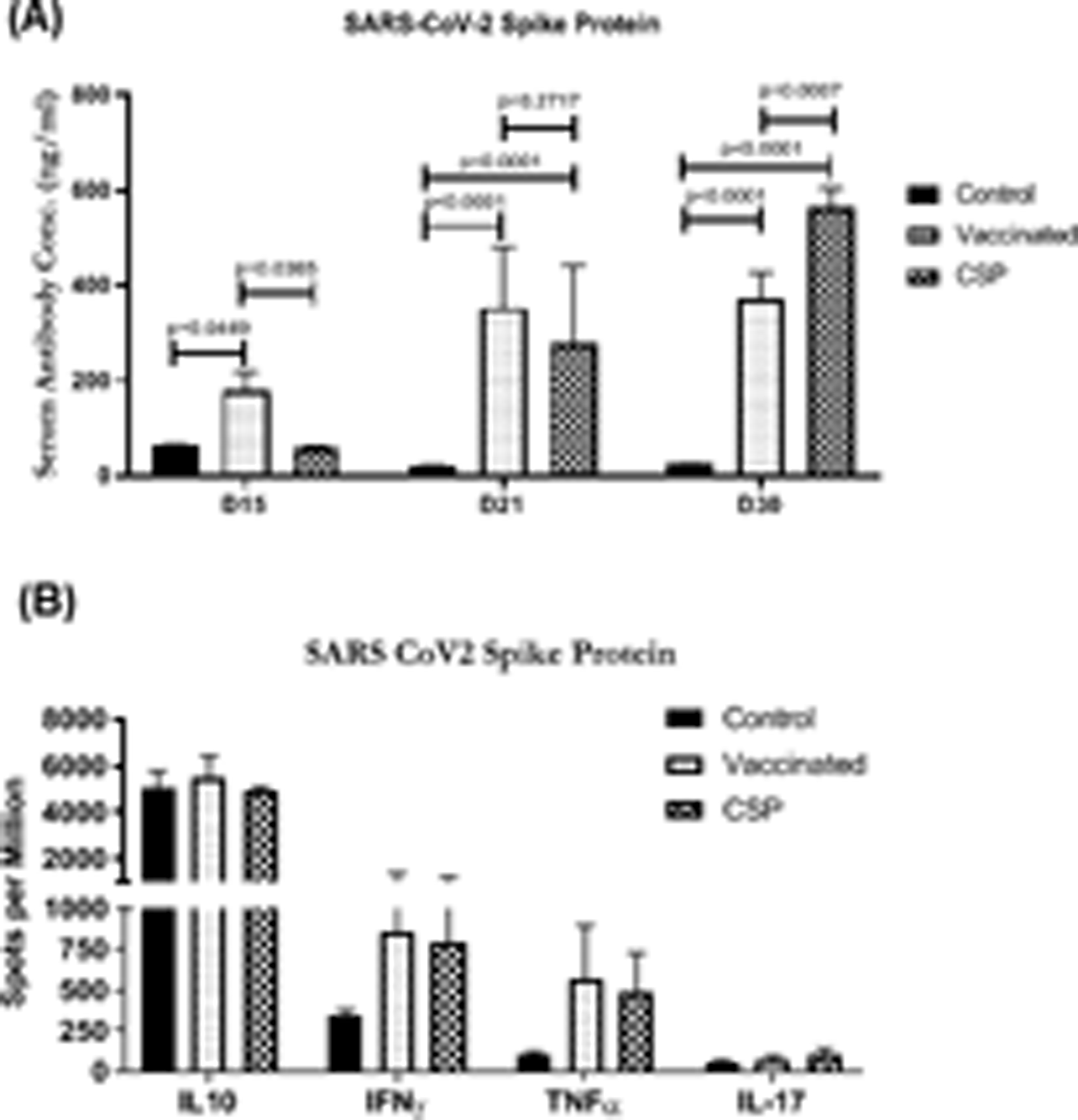
(A) ELISA of serum SARS-CoV-2 spike after immunizing the mice with exosomes from controls, and vaccinated individuals at D15, D21 and D30. (B) EliSPOT for cytokine development (IL10, IL17, TNFα and IFNγ) in splenocytes in response to SARS-CoV-2 spike antigen after immunizing the mice with exosomes from controls, and vaccinated individuals at D30. Graphs are represented as bar graphs with mean and SD (vertical bar line). The mice experiments were performed at least 3 times independently and in each group there were n=3 or n=5 animals.
Higher levels of cytokines (IFN-γ and TNFα) in splenic lymphocytes from mice immunized with exosomes from vaccinated individuals
Splenic lymphocytes from mice immunized with exosomes from vaccinated individuals vs controls demonstrated an increase in the number of cytokine–secreting cells: IFN-γ (853.77±517.84 vs 340.36±38.78), and TNFα (568.25±327.72 vs 102.14±19.06) spots per million but the differences are not statistically significant. Similar results were observed with mice immunized with SARS-CoV-2 spike protein (Figure 3B). Group of mice immunized with SARS-CoV-2 spike protein have also shown increased levels of IFN-γ and TNFα (Figure 3B). In the current study, individuals were administered a mRNA based vaccine developed by Pfizer-BioNTech and our results clearly demonstrate that by 14 days after administering the first dose of vaccine exosomes carrying spike protein to SARS-CoV-2 were induced, followed by spike protein specific Ab response developing by day 14 following booster immunization. Four months post-vaccination the levels of Abs decreased in plasma. This same trend was observed for circulating exosomes with the spike protein. These results support the conclusion that the induction of circulating exosomes with SARS-CoV-2 spike protein is potentially obligatory for effective immunization as a result of mRNA based vaccine administration. We postulate that these exosomes with SARS-CoV-2 spike protein are taken up by the antigen presenting cells which results in immune activation. Immunogenic potential of exosomes in respiratory viral infections has already been reported by us and others (11–13).
We also immunized C57BL/6 animals with circulating exosomes carrying spike protein isolated from vaccinated individuals and demonstrated that these exosomes are immunogenic. These animals developed both Abs to SARS-CoV-2 spike protein and cellular immune responses specific to SARS-CoV-2 spike protein following immunization. Our earlier findings have demonstrated that immunization of mice with human exosomes carrying lung self-antigens resulted in Abs to lung self-antigens. This occurred following the binding of human exosomes to mice antigen presenting cells, leading to immune responses in mice. Therefore, we propose that the mechanism by which immune responses developed following immunization of mice requires binding of exosomes with mice antigen presenting cells leading to development of both humoral and cellular immune responses to the spike protein. It is also of interest that such an immunization strategy resulted in increased frequency of splenic lymphocytes secreting IFN-ƴ and TNFα following antigenic stimulation. Based on these results in murine models we postulate that mRNA based vaccination of healthy individuals will result not only Ab responses but also cellular immunity. In conclusion exosomes carrying the spike protein to SARS-CoV-2 are induced and are detectable on day 14 following the first dose of vaccination and Abs specific to SARS-CoV-2 spike protein were detectable at a later stage following the booster dose. We propose that the induction of circulating exosomes with SARS-CoV-2 spike protein antigen is necessary for effective immunization following mRNA based vaccination of healthy individuals. We also demonstrated that the exosomes from vaccinated individuals were immunogenic and induced Abs to SARS-CoV-2 spike protein as well as T cell responses to spike protein antigen, suggesting that mRNA based vaccination induced exosomes with SARS-CoV-2 spike protein antigen will not only induce humoral immunity but also cellular immune responses.
Supplementary Material
Key Points.
BNT162b2 induces release of exosomes carrying SARS-CoV-2 spike protein.
Antibodies to SARS-CoV-2 develop after detection of circulatory exosomes.
Exosomes with SARS-CoV-2 spike protein immunogenic in mice.
Acknowledgements
We also would like to acknowledge Billie Glasscock and Kristina Nally for their assistance in preparing this manuscript.
Funding sources
This work was supported by grants from the National Institutes of Health AI123034 and funds from St. Joseph foundation (TM).
Footnotes
Disclosure statement
The authors declare no conflict of interest. All authors have reviewed and approved the manuscript and have contributed in a substantial and intellectual manner to the work.
References
- 1.Forni G, Mantovani A, and R. Covid-19 Commission of Accademia Nazionale dei Lincei. 2021. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ 28: 626–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyriakidis NC, Lopez-Cortes A, Gonzalez EV, Grimaldos AB, and Prado EO. 2021. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines 6: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, and C. S. Group. 2021. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 384: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh EE, Frenck RW Jr., Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Tureci O, Tompkins KR, Lyke KE, Raabe V, Dormitzer PR, Jansen KU, Sahin U, and Gruber WC. 2020. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med 383: 2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teo SP 2021. Review of COVID-19 mRNA Vaccines: BNT162b2 and mRNA-1273. J Pharm Pract: 8971900211009650. [DOI] [PubMed] [Google Scholar]
- 6.Meo SA, Bukhari IA, Akram J, Meo AS, and Klonoff DC. 2021. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci 25: 1663–1669. [DOI] [PubMed] [Google Scholar]
- 7.Bansal S, McGilvrey M, Garcia-Mansfield K, Sharma R, Bremner RM, Smith MA, Hachem R, Pirrotte P, and Mohanakumar T. 2020. Global Proteomics Analysis of Circulating Extracellular Vesicles Isolated from Lung Transplant Recipients. ACS Omega 5: 14360–14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, et al. 2018. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7: 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanian V, Ramachandran S, Banan B, Bharat A, Wang X, Benshoff N, Kreisel D, Gelman AE, and Mohanakumar T. 2014. Immune response to tissue-restricted self-antigens induces airway inflammation and fibrosis following murine lung transplantation. Am J Transplant 14: 2359–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, Marrama D, de Silva AM, Frazier A, Carlin AF, Greenbaum JA, Peters B, Krammer F, Smith DM, Crotty S, and Sette A. 2020. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 181: 1489–1501 e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunasekaran M, Bansal S, Ravichandran R, Sharma M, Perincheri S, Rodriguez F, Hachem R, Fisher CE, Limaye AP, Omar A, Smith MA, Bremner RM, and Mohanakumar T. 2020. Respiratory viral infection in lung transplantation induces exosomes that trigger chronic rejection. J Heart Lung Transplant 39: 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crenshaw BJ, Gu L, Sims B, and Matthews QL. 2018. Exosome Biogenesis and Biological Function in Response to Viral Infections. Open Virol J 12: 134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong S, Ruan S, Kubota PG, He M, and McGill JL 2020. Immunogenic potency of engineered exosomes for prevention of respiratory syncytial virus. J Immunol 204: 245.222. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


