Abstract
The dramatic evolutionary expansion of the neocortex, together with a proliferation of specialized cortical areas, is believed to underlie the emergence of human cognitive abilities. In a broader phylogenetic context, however, neocortex evolution in mammals including humans is remarkably conservative, characterized largely by size variations on a shared six-layered neuronal architecture. By contrast, the telencephalon in non-mammalian vertebrates, including reptiles, amphibians, bony and cartilaginous fishes, and cyclostomes, features a great variety of very different tissue structures. Our understanding of the evolutionary relationships of these telencephalic structures, especially those of basally branching vertebrates and invertebrate chordates, remains fragmentary and is impeded by conceptual obstacles. To make sense of highly divergent anatomies requires a hierarchical view of biological organization, one that permits the recognition of homologies at multiple levels beyond neuroanatomical structure. Here we review the origin and diversification of the telencephalon with a focus on key evolutionary innovations shaping the neocortex at multiple levels of organization.
In Brief:
Briscoe and Ragsdale review the divergent telencephalon anatomies of the vertebrates and describe the sequential evolutionary origins of neocortex components over 550 million years of evolution.
Introduction
The earliest vertebrates, which roamed the Cambrian seas over 500 million years ago, are believed to have possessed paired sensory organs of the head (including eyes and olfactory bulbs) and a centralized rostral brain [1–3]. In the time since, the ancestral vertebrates and their descendants gave rise to the extant vertebrate classes, from the cyclostomes (jawless vertebrates) to mammals including Homo sapiens. In parallel with the evolutionary radiation of vertebrates, their brains diversified in accordance with the behavioral and ecological pressures of their distinct lifestyles [4,5]. Nowhere is clade-specific evolutionary specialization more evident than in the telencephalon, the paired, rostral-most division of vertebrate brains. Wherever sufficient neuroanatomical data exist, the telencephalon appears to be a center for multimodal sensory integration and, through its output circuitry, to control a rich repertoire of behaviors. A comparison of the telencephalon across the vertebrates should yield insights into how natural selection shapes brains capable of higher cognitive abilities and will help to reconstruct the evolutionary history of the extraordinary human neocortex.
Telencephalon anatomy varies so greatly that neuroanatomists have battled over how to compare mammalian with avian telencephala for more than a century [6,7]. Evolutionary relationships of telencephalic structures across even greater phylogenetic distances, such as from mammals to ray-finned fishes, are unresolved. One illustrative case study is the question of whether ray-finned fishes possess a homolog of the hippocampus [8], a structure in the mammalian dorsal telencephalon (or pallium, see [9]). Researchers have variously proposed that the entire fish pallium is homologous with the hippocampus, that fish do not have a hippocampus homolog, or that a fish hippocampus is found in the medial or the lateral pallium [8]. This example captures in essence the chaos of conflicting telencephalon homologies proposed over the history of comparative neuroanatomy and can be readily extrapolated to other taxa and telencephalon structures.
Why has the comparative anatomy of the telencephalon been such a long-standing problem? More comparative data can be valuable for informing hypotheses of homology, and we will highlight in this review promising future research directions. However, and more importantly, a major problem is a conceptual one: the statements of homology proposed by comparative neuroanatomists often fail to capture where conserved similarities are found. In particular, any proposal of homology requires a stipulation of the level of the homology [9–13]. In the absence of such stipulations, homology at one level of biological organization can be mistakenly conflated with homology at others. We will begin with a discussion of the homology concept and consider how, by comparing features of the human neocortex with similar features in ever more distantly related animals, we can infer the origins of neocortical components at multiple levels of organization: first, neocortex elaboration in recent human evolution; second, the origin of neocortical architecture in stem mammals; third, the origins of neocortical cell types and circuits in stem amniotes; and fourth, the origin of telencephalon molecular patterning in Precambrian invertebrate chordates.
The telencephalon: development and diversity
The developing telencephala first appear as paired outpouchings of the prosencephalon (the anlage of the vertebrate forebrain) near the anterior end of the embryonic neural tube. Each telencephalon comprises initially a thin, seemingly uniform sheet of neuroepithelial stem cells, which, through a sometimes-elaborate series of symmetric and asymmetric cellular divisions, will give rise to the neurons and macroglial elements of the cerebral hemispheres [14,15]. The early neuroepithelial stem cells have a broad developmental potential for telencephalic cell types [16]. Patterning of regional identity in the telencephalon is regulated by organizing centers and their downstream transcription factor targets, mechanisms that are best understood, by far, in the developing mouse embryo [17–20].
In all vertebrates, the telencephalon encompasses two principal subdivisions, the pallium and the ventral telencephalon, or subpallium (Figure 1). Progenitors of the subpallium contribute inhibitory GABAergic neurons to the striatum and pallidum, highly conserved components of basal ganglia circuitry [21–23]. An extensive confluence of data from connections, cellular morphology, neurotransmitter expression, and developmental genetics indicates that a striatum and pallidum are found in all extant vertebrates in a similar topographical arrangement [24–27]. In addition, the subpallium generates GABAergic interneurons that disperse by tangential migration and then integrate into the circuits of the pallium (Figure 1) [28,29]. Similar neuronal migrations have been seen in developing shark and lamprey telencephala, which suggests that such features predate the evolution of vertebrates [30,31]. Comparative studies have now established that the evolution of the telencephalic subpallium is highly conservative with respect to some connectional and functional features, although the striatum and pallidum appear to have become considerably more cell-rich during the transition to the amniotes (mammals, birds, and non-avian reptiles) from their tetrapod ancestors [21].
Figure 1.
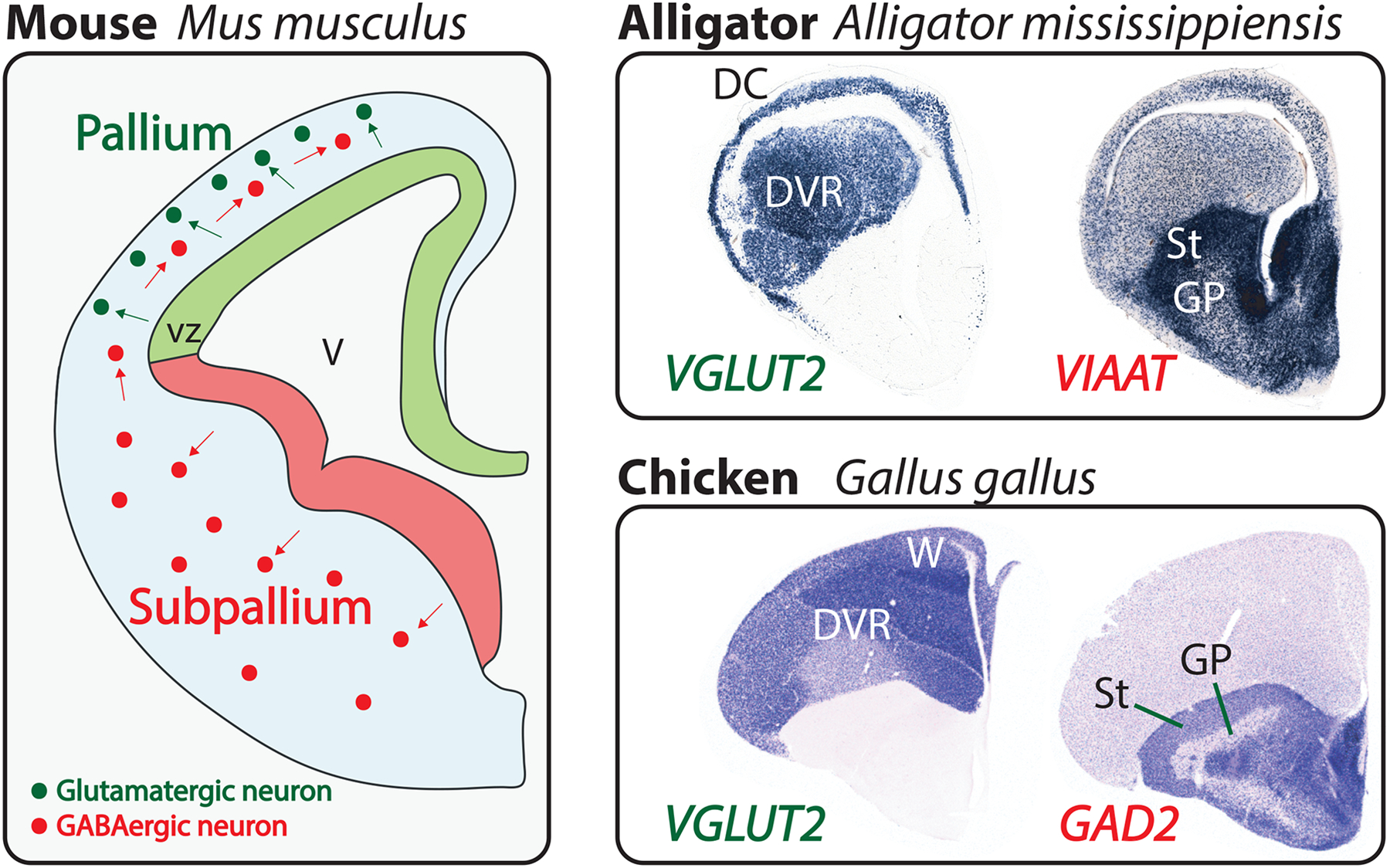
The vertebrate pallium and subpallium.
Left: developing mouse telencephalon at 12.5 days of gestation, shortly after the onset of neocortical neurogenesis. One telencephalic hemisphere is diagrammed in cross-section with medial to the right and dorsal at the top. Neural progenitor cells line the ventricle (V), forming the ventricular zone (vz). Pallial progenitor cells (light green) give rise to excitatory, glutamatergic neurons (dark green), which migrate from their place of birth (green arrows) but remain within the pallium. In contrast, subpallial progenitor cells (light red) produce inhibitory, GABAergic neurons (dark red), which populate the subpallium but also disperse throughout the pallium (red arrows). This developmental pattern is conserved across the vertebrates. Top right: coronal sections through a late-embryonic alligator telencephalon labeled by in situ hybridization for VGLUT2 and VIAAT transcripts, which identify excitatory and inhibitory neurons, respectively [112]. Within the pallium, the three-layered dorsal cortex (DC) and the dorsal ventricular ridge (DVR) are identified. The striatum (St) and globus pallidus (GP) are two broadly conserved subdivisions of the subpallium. Bottom right: coronal sections through the telencephalon of a chicken hatchling labeled for VGLUT2 and GAD2 transcripts. Similar to non-avian reptiles, birds have a prominent DVR in the pallium. However, an additional nuclear structure, the Wulst (W), takes the place of a dorsal cortex. Note the GABAergic neurons scattered throughout the avian and reptilian pallia, as in mammals. Chicken sections from J. Rowell, Ragsdale laboratory.
The pallial progenitor cells, in contrast with those of the subpallium, are believed to produce exclusively glutamatergic, excitatory neurons (Figure 1) [32] (but see [33]). Newborn glutamatergic neurons of the pallium migrate from their place of birth to differentiate and arrange into tissue-scale structures. However, and again in sharp contrast with the striatum and pallidum, the pallium-derived structures are markedly divergent across the vertebrates.
In some groups, such as mammals and some non-avian reptiles, pallial neurons migrate radially to form orderly, stacked layers parallel to the surface of the brain [34]. Layers serve to organize distinct neuronal cell types with specialized circuitries and some excitatory pallial neurons extend apical dendrites perpendicularly across layers in order to sample from and integrate multiple types of information [35]. In neuroanatomical parlance, such a layered structure with apical dendrites is referred to as a cortex [36].
In other vertebrate groups, notably birds, the pallium is dominated by a nuclear architecture in which the different neuronal cell types are found clustered into dense aggregates, or nuclei [6,37]. In yet other groups, including some ray-finned fishes and amphibians, far simpler pallial architecture is present [38,39]. In these animals, neurons are found distributed loosely throughout the telencephalic wall in an organization that cannot be described as either cortical or nuclear. How best to compare the divergent pallial structures with one another, and with the neocortex in particular, has been a persistent problem in comparative neuroscience. Recent research provides a compelling account of relationships in the amniote pallium for one level of homology [40], but for the many anamniote vertebrates nothing resembling an expert consensus presently exists.
Specifying the level of homology
When features of one organism are compared with similar features of another, there is often a statement, sometimes implicit, about whether or not the compared features are ‘the same thing’, that is, whether or not they are homologous. Although homology is arguably the core concept of comparative biology, it has long proven notoriously difficult to define [11,41]. Most researchers have emphasized two essential components of homology: first, homology refers to similarity; and second, homology requires inheritance from a common ancestor [42]. A character (any delineable morphological feature of an organism) in one species is homologous with a similar character in another species if the character was present in their last common ancestor (LCA) and has been maintained continuously in their respective lineages. Importantly, homology can be found at any level of biological organization — including molecules, cell types, embryological domains, and anatomical structures — and levels can be evolutionarily dissociated. In other words, natural selection may preserve similarities at one level of biological organization in two or more diverging species, even while evolutionary change accrues at other levels.
Consider the following proposition: “the human neocortex is homologous with the mouse neocortex”. This may not at first seem a controversial claim, but a closer inspection reveals some difficulties. The human neocortex expresses genes not expressed by the mouse neocortex [43], contains an enormously greater number of cortical areas [44], contains cell types not found in the mouse neocortex [45], develops by divergent signaling mechanisms [46], and is roughly a thousand times larger in surface area and twice as thick [47]. The two characters do, however, share a defining feature, one that allows us to refer to both as ‘neocortex’: the defining feature of neocortex is a distinct cellular architecture, a six-layered cortex located in the pallium [48].
Not all features of the human neocortex and the mouse neocortex are homologous, but some are. More generally, we can state that homologous features always coexist with non-homologous features in a given character in two or more species. Thus, stating simply that human and mouse neocortex are homologous fails to capture what, specifically, is shared between them. We must instead stipulate at what level two characters are homologous: “the human neocortex and the mouse neocortex are homologous at the level of neuroanatomical structure”. Furthermore, this conditional statement contains useful information for describing how the neocortex evolved, including that the LCA of mice and humans had a six-layered neocortex, but that some other neocortical features might have evolved only in their respective lineages. As will become clear in the following sections, when comparing characters in lineages separated by great phylogenetic distance it sometimes becomes necessary to appeal to homologies at levels other than structure in order to formulate a coherent explanation for the evolutionary processes.
Evolution of the human neocortex
An intact adult human brain looks like a giant neocortex (Figure 2). The human brain does in fact contain many parts, some of which, such as basal ganglia components and many diencephalon and brainstem cell populations, are shared with all other vertebrates. Moreover, the human telencephalic pallium contains cell populations in addition to the neocortex, including a number of nuclei (the claustrum and basolateral amygdalar nuclei) and cortices with fewer than 6 layers (olfactory bulb, olfactory cortex, and the hippocampus) [49]. However, the human neocortex, more so than any other human brain structure, has been enlarged and elaborated, such that it buries much of the remaining brain beneath its folds (Figure 2). The question of what makes the human neocortex different from those of our closest relatives has attracted interest from many research groups, yielding exciting insights into the last six million years of human evolution (Figure 3) (reviewed in [50–54]).
Figure 2.
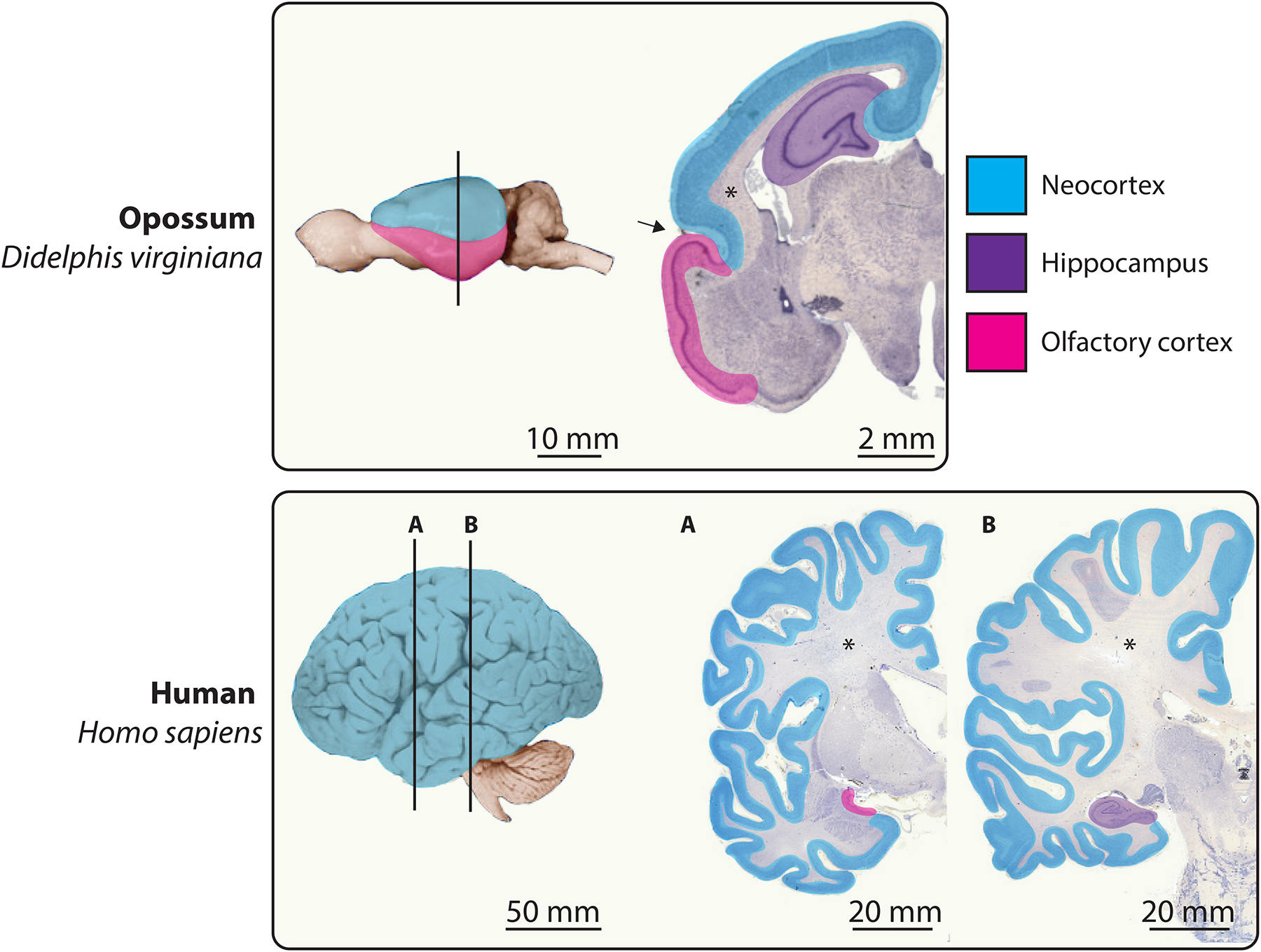
Tangential expansion of the human neocortex.
Whole brains from an opossum and a human are shown in lateral view with anterior to the left. Vertical lines indicate the approximate anteroposterior positions of the adjacent sections. In all mammals, the cerebral cortex includes the 6-layered neocortex (blue) and the 3-layered hippocampal (violet) and olfactory cortices (magenta). Cerebral organization in the opossum, a marsupial (see Figure 3), is thought to be representative of that in the mammalian LCA [95]. The small, smooth opossum neocortex is demarcated from the relatively large olfactory cortex by a deep rhinal sulcus (arrow) [196]. Across the mammals, it is the neocortex that varies most in size. This size variation is principally in the tangential dimension and not in the radial dimension, such that neocortex thickness varies by only about two-fold. The relatively tiny human olfactory and hippocampal cortices are displaced into the temporal lobe by the developmental expansion of the highly folded neocortex. An extensive neocortical white matter (asterisks) of myelinated axons sits below the neuronal cell bodies of the neocortical grey matter. For clarity we have in this Figure grouped the multilayered transitional cortices with the neocortex. Brain images adapted from the University of Wisconsin and Michigan State Comparative Mammalian Brain Collections at http://brainmuseum.org/ (supported by the National Science Foundation and the National Institutes of Health).
Figure 3.
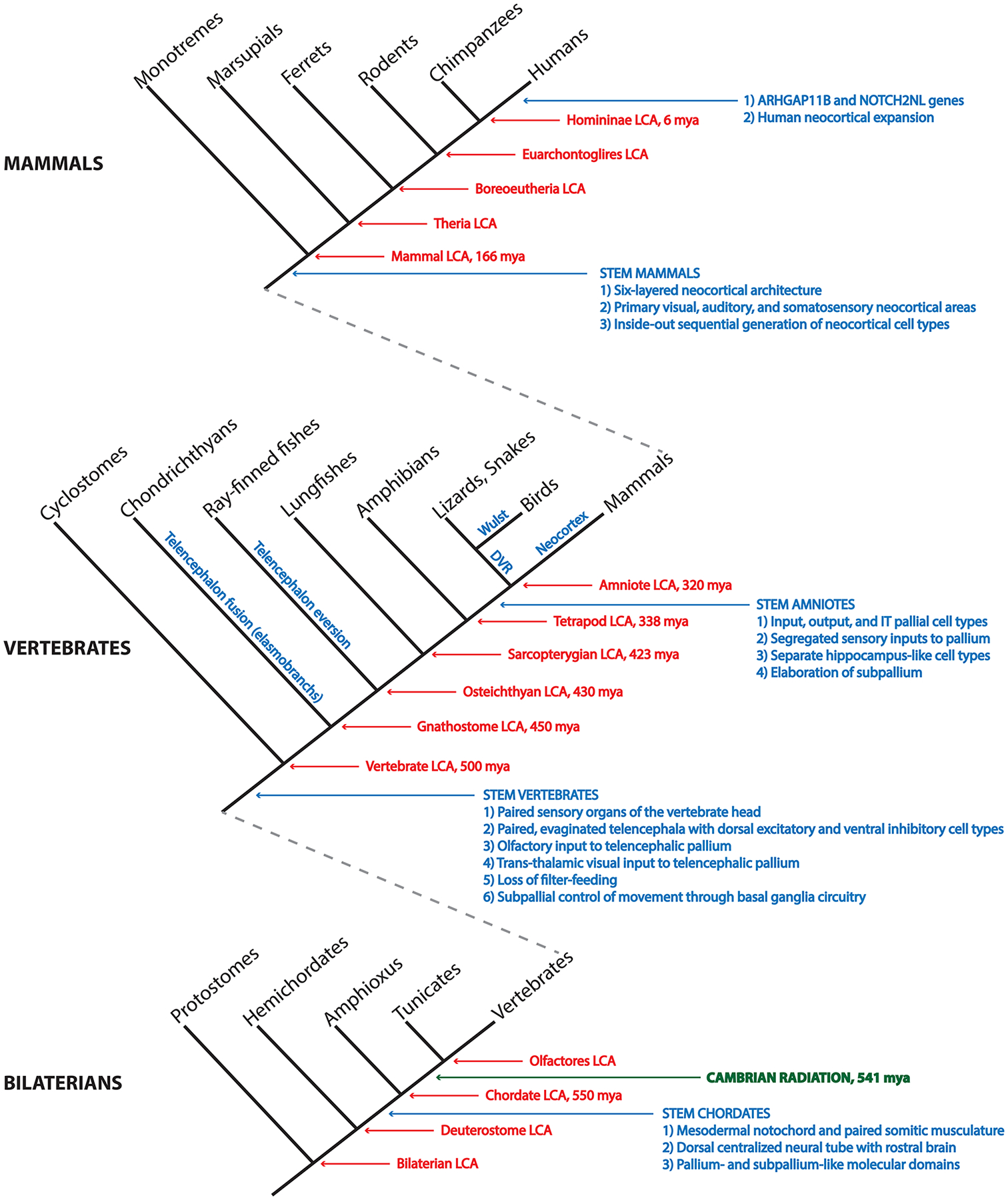
Chordate phylogeny and a synopsis of telencephalon evolution.
A nested cladogram depicts the evolutionary relationships of the chordates and their relatives within the Bilateria, the group of all bilaterally symmetric animals. Red arrows and text identify common ancestors and approximate times of divergence in millions of years before the present (mya). Blue arrows and text identify the emergence of key innovations related to the evolution of the telencephalon and of the neocortex in particular. Blue text along the stems of the vertebrate groups identifies telencephalic morphological innovations of those lineages. Note that our placement of neocortical cell type origins with stem amniotes is a conservative one, based on clades for which extensive molecular data exist and are concordant with connections. See main text for discussion and references.
Species differences in neocortex size can be attributed largely to species differences in the proliferation of neocortical progenitor cells during development [47,55,56]. The human neocortex is approximately three times larger than the neocortex in chimpanzees because more neurons are generated during human embryonic and fetal neurogenesis. One particularly fruitful strategy for explaining human neocortical expansion has been to search for human-specific genomic innovations that may influence neocortical neurogenesis. To this end, a trio of recent studies [57–59] found that gene products of human-specific duplications at the NOTCH2 locus could increase neuronal production during development by promoting self-renewing divisions of neocortical progenitors. These findings supplement studies of ARHGAP11B, an additional human-specific product of gene duplication, which can affect increases in both neocortical thickness and surface area when introduced into ferret neocortical progenitors [60]. Recent improvements to the assemblies and annotations of human and great ape genomes [61], together with insights from the genomes of extinct Homo species [62,63], are likely to accelerate the identification of loci that could have contributed to human neocortex evolution.
The evolutionary increase to the absolute number of neurons in the human neocortex accompanied changes to the proportional allocation of neurons to particular fates. For example, glutamatergic neurons of the upper neocortical layers (layers 2–4), which predominantly form intracortical associative connections, become disproportionately abundant with neocortical thickness increases across mammals [60,64]. Analogous shifts are seen in the relative patterning of the neocortical area map. The human prefrontal cortex, which contains association areas involved in cognitive function, increased in surface area disproportionately relative to the primary sensory areas (Figure 4, middle panel) [65,66]. Moreover, evolutionary refinements to the morphology [67], connectional properties [68,69], and glial support [70,71] of human neocortical neurons suggest strong selective pressures on the functionality of neurons, in addition to their abundance. This evolutionary process, of rapid neocortical innovation and positive selection, may be reflected by the accelerating rates of increase to endocranial volume seen in the hominin fossil record [72,73].
Figure 4.
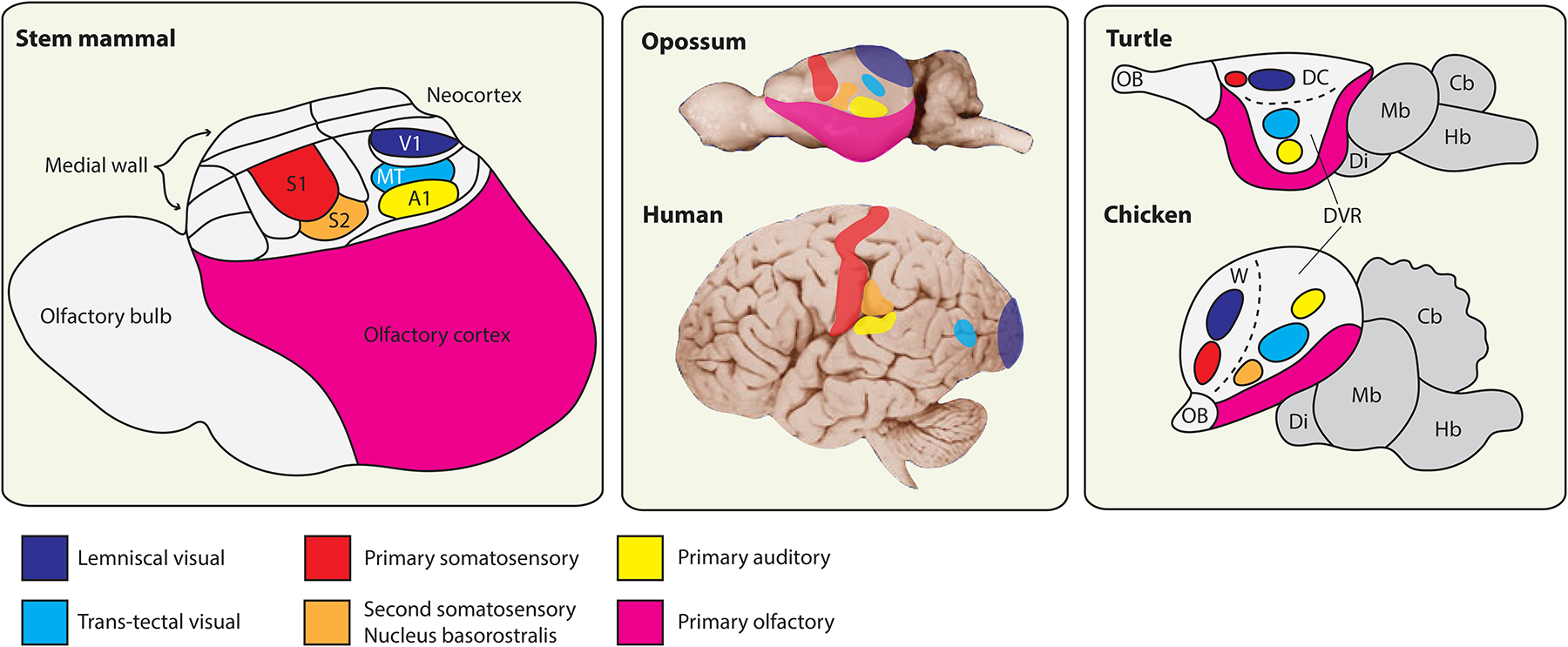
Evolution of telencephalic sensory centers in amniotes.
Left: reconstruction of telencephalon organization in a stem mammal based on a comparative analysis of neocortex in extant mammals, combined with information about brain proportions from early mammalian fossil skull endocasts (adapted from Kaas [95]). This mammal is inferred to have possessed a highly developed olfactory bulb and olfactory cortex, with a compact neocortex located dorsally. This small neocortex nonetheless contained a range of neocortical areas thought to be shared in all extant mammals, a subset of which are identified here (see [95] for further discussion). The primary visual area (V1) receives lemniscal visual input (which relays through the lateral geniculate nucleus of the dorsal thalamus), whereas the middle temporal visual area (MT) receives input from a separate, parallel visual pathway that relays through the optic tectum and then the thalamic lateral posterior nucleus. All mammals additionally share a primary auditory area (A1), a primary somatosensory area (S1) and an adjoining second somatosensory area (S2). Note that this nomenclature of cortical areas does not apply to birds and non-avian reptiles, which lack a neocortex. Middle: the sizes of neocortical sensory areas do not scale linearly with the overall surface area of the neocortical sheet. That is, mammals with a highly expanded neocortex, such as humans, have a larger proportion of non-primary-sensory and higher order association cortex. Differential allocation of cortical surface area is apparent, for example, in the large human frontal cortex rostral to S1. Placement of cortical areas based on [193], MT placement in opossum and human based on [197] and [44], respectively. Right: the core sensory pathways to the mammalian pallium are conserved also in birds and non-avian reptiles, where they target spatially discrete pallial domains. The lemniscal visual channel targets the dorsal cortex (DC) in turtles and the avian Wulst (W), whereas the trans-tectal visual channel targets defined nuclei deep within the dorsal ventricular ridge (DVR) in each species [106]. Primary somatosensory information targets the DC and the Wulst rostral to the lemniscal visual targets. Primary auditory information reaches the DVR in all known sauropsids. Birds possess an additional sensory nucleus in the rostral DVR, the nucleus basorostralis, which receives trigeminal somatosensory information via a direct projection from the hindbrain [6]. This nucleus expresses molecular markers of neocortical input neurons and is conserved in alligators, but possible homologies with mammalian features remain elusive [112]. Turtle and chicken schematics adapted from [114] and [120], respectively. Cb, cerebellum; Di, diencephalon; Hb, hindbrain; Mb, midbrain; OB, olfactory bulb.
Organization and evolution of the neocortex across mammals
Humans are not the only mammalian species to possess a large neocortex. Comparative analyses indicate that neocortical surface area has expanded and contracted numerous times independently across mammalian phylogeny [74–76]. Even between the two surviving families of monotremes, the platypus has a relatively small, smooth neocortex, whereas the neocortex is large and folded in echidnas [77]. It is likely that neocortex evolutionary expansion has been achieved independently by similar strategies. For example, the basal radial glial cells (or outer radial glial cells [78]), specialized progenitor cells in the developing neocortical epithelium, were initially thought to be unique to primates [79]. Subsequent studies have, however, identified comparable progenitor cell types in a range of mammals including ferrets [80–82], marsupials [83], and even mice [84–86]. Evolutionary fine-tuning of radial glial cell abundance could differentially modulate neocortex size, possibly through common pathways including integrin signaling [56,80,87,88] and through common molecular targets, such as the microcephaly-associated gene ASPM [82,89,90]. On the other hand, other mammalian lineages may have acquired expanded populations of neocortical neurons through idiosyncratic gene duplications, as occurred in recent human evolution and throughout primate phylogeny [91–93].
In contrast with the evolutionary plasticity of neocortical surface area, many features of neocortical organization are strikingly constant across humans, non-human primates, and other mammals. The neocortical area map, for example, appears to vary across mammals in complexity while adhering to a common plan [48,94]. All mapped neocortices possess the same set of primary sensory areas in the same relative configuration — a primary visual area in the back, a primary somatosensory area in front of the visual area, and a primary auditory area placed laterally — separated from one another, depending upon the overall size of the neocortex, by varying stretches of higher-order association cortex (Figure 4, middle panel) [48,94]. There is widespread agreement that the primary neocortical sensory areas are homologous structures. Indeed, some authors have suggested that 15–25 neocortical areas are shared across mammals and were likewise present in their LCA (Figure 4, left panel) [94,95]. In addition, the ancestral mammalian pallium contained a medial hippocampus and a lateral olfactory cortex, both of which vary across extant mammals [96,97], but never to the extremes seen for the intervening six-layered neocortex.
Moving beyond neuroanatomical structures, clear homologies exist at the level of neocortical cell types, including both neurons and glia [98]. In the radial dimension of the neocortex, and in all mammals, cortical layers organize similar neuronal cell types and circuitry. One classification scheme, presented here in simplified form, places the excitatory neocortical neurons into high-level categories on the basis of their connections [35]. Intratelencephalic (or IT) neurons send axons only to other telencephalic targets, including the striatum, and are found in all neocortical layers. One major subclass of IT neurons, the layer 4 input neurons, are the main targets of dorsal thalamic axons relaying primary sensory information. The layer 5 output neurons send long-range projections to the brainstem and spinal cord, whereas the layer 6 corticothalamic neurons project to the thalamus but not beyond. Information flow from layer 4 input neurons to layer 5 output neurons through relays in upper layer IT neurons — the canonical microcircuit — is thought to serve as a shared information processing strategy in virtually all neocortical areas and in all extant mammalian species [35].
The relative ease with which we can propose pallial homologies across mammals is testament to the conservative nature of telencephalon evolution within the mammalian class. The basic template for neocortical organization was undoubtedly established already in the mammalian LCA and has been largely preserved over the approximately 166 million years since monotreme mammals diverged from marsupial and placental mammals (Figure 3) [99]. Such conservation suggests that neocortical architecture may be resistant to evolutionary change due to unknown developmental or functional constraints. At the least, it indicates the tremendous selective advantage of neocortical architecture. Outside of mammals, however, pallial architecture has nonetheless adopted radically different forms multiple times independently in the different vertebrate groups.
Neuronal cell type homologies in the amniote pallium
The pallium in sauropsids (birds and non-avian reptiles), the closest living relatives of mammals, does not contain a six-layered neocortex. Instead, non-avian reptiles have a three-layered cerebral cortex positioned above a prominent pallial nuclear territory called the dorsal ventricular ridge (or DVR) (Figure 1) [37]. Birds, as descendants of archosaur reptiles, possess an unmistakably reptilian pallium with notable quantitative and structural modifications. Birds evolved larger brains for their body sizes in comparison with other reptiles [100], and this evolutionary increase to relative brain size in birds is associated with increased numbers of neurons [101], as seen also in large-brained mammals including humans [102]. Many of these additional neurons are found in the avian DVR, which is densely packed with cells and contains morphological subdivisions not readily apparent in many other reptiles such as turtles and snakes [37]. More striking, however, is that the three-layered cerebral cortex so ubiquitous to non-avian reptiles is absent from birds, having been substituted by an additional nuclear complex known as the Wulst (Figure 1, W) [6,40]. This modification does not appear to have precluded the evolution of higher cognitive abilities in birds: despite the absence of a multi-layered cortical structure, some birds, particularly crows, exhibit planning and problem solving skills matching or exceeding those of great apes [103,104].
In order to understand the evolutionary origins of the mammalian neocortex, it is necessary to identify homologous neocortical features shared with sauropsids. The problem is that homology is based upon similarity, and evolution has rendered the amniote pallial structures into utterly dissimilar forms. Comparisons made at the levels of circuitry and cell types, rather than neuroanatomical structure, have provided a wealth of insight into amniote pallium evolution. Recent comparative molecular studies [105–107], together with decades of tract-tracing studies in reptiles and birds, have contributed to a model of pallial neuronal cell type homologies: the neocortical input, output, and IT neurons are homologous characters shared across amniotes and were present in the amniote LCA around 320 million years ago (Figure 3) (reviewed in [9,40]). The distinct structural architectures of the six-layered neocortex, the nuclear DVR, the Wulst, and the reptile cortex are thus evolutionary innovations of their respective lineages which emerged, in part, through the independent reorganizations of pre-existing neuronal cell types [108].
As in mammals, the pallium in sauropsids receives and integrates primary sensory information from at least four distinct channels (Figure 4, right panel) [9]. For example, defined nuclei in the DVR receive primary visual and auditory information reminiscent of sensory input to neurons in layer 4 of mammalian neocortex [109–111]. Furthermore, the pallial input cells of mammals, reptiles, and birds, as identified by connectivity, share the selective expression of multiple transcription factor genes (RORA, RORB, and SATB1) and an ion channel marker gene (KCNH5) [106,107,112,113]. The sauropsid pallial input populations are even distributed in a relative spatial organization equivalent to the neocortical primary sensory areas (Figure 4) [114]. The extent of similarities shared between these characters — by connectivity, gene expression, and location — as well as their presence in all examined amniotes, indicates that independent evolution of the pallial input cell types is exceedingly unlikely. Similarly yoked connectional and molecular data have been found recently for the IT neuronal cell types, which in birds interlink the input with output cells and can be identified by their expression of a conserved set of transcription factors [105,112]. One recent study [113] noted that the pallial excitatory neurons in reptiles and mammals differ in other molecular properties and concluded that the cells are therefore not homologous. However, it is similarity, and not dissimilarity, that forms the basis for judgments of homology. Non-homologous molecular features must be permitted, and even expected, to coexist with homologous features in different amniote pallia given their divergent architectures and 320 million years of independent evolution [40].
Amphibians and evolutionary simplification
Whereas core excitatory neocortical cell types (input, output, and IT) were likely present in the pallium of the amniote LCA, the evolutionary origin of these cell types remains a major unanswered question. Comparisons with anamniote vertebrates may provide some clues, although in most cases relevant experimental data, especially molecular data, are lacking. The extant amphibians, which comprise three orders (the frogs and toads, the salamanders, and the caecilians), form a sister group to amniotes positioned to provide insights into the LCA of tetrapods (Figure 3).
Pallium anatomy in amphibians, at least at the level of structure, is generally very simple (Figure 5, Bullfrog) [38]. There is nothing that resembles either the elaborate layers of the mammalian neocortex or the dense nuclei of the avian DVR. Instead, excitatory neurons of the developing amphibian pallium are thought to migrate little from their place of birth and accumulate in a relatively homogenous periventricular grey matter. The neuronal cell bodies, positioned near the ventricle, extend dendrites into an overlying neuropil that harbors intrinsic and extrinsic telencephalic circuitry [115]. Partially as a consequence of the lack of clear nuclear groups, neuroanatomists have traditionally recognized three or four pallial zones named simply after their topographical relationships: medial, dorsal, lateral, and ventral pallium. Olfactory input to the amphibian lateral pallium is probably a homologous feature shared with the laterally placed olfactory cortices in amniotes, but for other pallial zones possible homologies are less clear.
Figure 5.
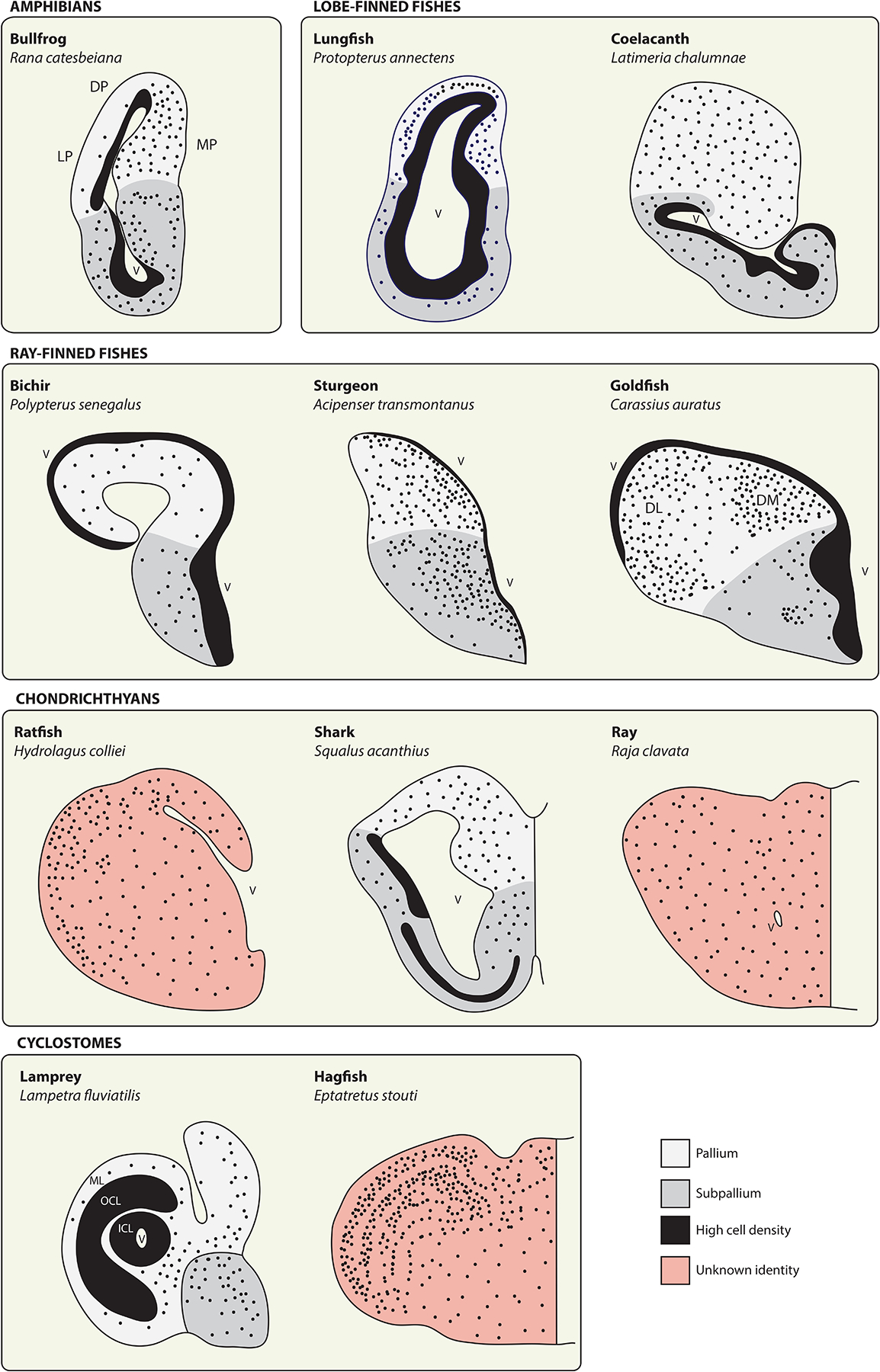
The telencephala of anamniote vertebrates.
Schematics depict telencephalon anatomy from representatives of the anamniote vertebrates. Solid black territories represent regions of particularly high cell density, often along the surface of the lateral ventricle (V), whereas black dots provide a qualitative representation of cellular density and distribution. These illustrations are intended to provide a broad overview of telencephalon morphology in anamniotes and only the subset of neuroanatomical zones referred to in the main text are identified here. In all cases, pallial-subpallial boundaries should be regarded as approximations. See source materials for further details and discussion. Bullfrog adapted from [198] (DP, dorsal pallium; LP, lateral pallium; MP, medial pallium). Lungfish adapted from [24] and coelacanth from [199]. Ray-finned fishes adapted from [130] (DL, dorsolateral area; DM, dorsomedial area). Chondrichthyans adapted from [153]. Lamprey adapted from [175] (ICL, inner cellular layer; OCL, outer cellular layer; ML, molecular layer) and hagfish from [167].
The medial pallium appears to diverge from the general amphibian pallial organization with modest elaboration and cellular migration (Figure 5, MP), and it additionally possesses some interesting connectional properties [115–118]. Tracing studies have reported auditory, somatosensory, and visual input to the frog medial pallium from anterior thalamic nuclei, which suggests that the amphibian pallium, as in amniotes, serves as a center for multimodal sensory integration [117,119]. Whether these thalamopallial sensory projections are homologous with amniote features remains a puzzle. Auditory, somatosensory, and visual pathways target discrete territories in amniote pallia (Figure 4) [120], but recordings from neurons in frog medial pallium have identified only multimodal response properties [121–123]. Based on other features including projections to the subpallial septum, a role in memory formation, and its medial location, previous authors have almost unanimously regarded the amphibian medial pallium as homologous with the mammalian hippocampus [115–117,119].
It will be informative to determine whether excitatory neurons in the amphibian medial pallium express markers for amniote pallial input neurons (see above) or markers for hippocampal cell types [112,113,124]. It is possible that individual medial pallium neurons will be found to exhibit a mixture of hippocampus- and neocortex-like features. Such a result could suggest that stem amniotes evolved increased pallial cell type diversity through subfunctionalization, that is, by distributing features originally found in a single cell type to two or more cell types with more restricted functions [125,126]. Alternatively, the amphibian pallium could have lost cell type diversity through the opposite process, the consolidation of features originally found in multiple distinct cell types into fewer cell types. Indeed, some authors [127] have suggested that neuronal architectures seen in extant amphibians are the result of secondary evolutionary simplification and that the pallium in the tetrapod LCA was more complex. Further studies of lungfishes, the closest living relatives of tetrapods, may help to address this possibility [128,129]. The African lungfish Protopterus annectens appears to possess a cortex-like structure segregated from the pallial ventricular grey matter, but little is known of its intrinsic organization (Figure 5, Lungfish) [24,130].
Telencephalon eversion in ray-finned fishes
Tetrapods, together with their still-aquatic relatives the lungfishes and coelacanths, form the lobe-finned fishes (or sarcopterygians). Their closest relatives are the ray-finned fishes (or actinopterygians), a diverse and species-rich collection of vertebrates (Figure 3) [131]. The lobe- and ray-finned fishes (together, the bony fishes, or osteichthyans) diverged over 430 million years ago, a time span that has allowed for extensive evolutionary innovation in their respective lineages [132]. Molecular evidence strongly suggests that ray-finned fishes possess subpallial homologs of the striatum and pallidum, and that subpallium-derived GABAergic neurons disperse across pallial territories [133–135]. Their pallia, in contrast, feature a diversity of anatomies that mirrors the range of anatomies seen in extant tetrapods. Unsurprisingly, establishing pallial homologies across these groups has been a major challenge and there are to date few firm conclusions.
Ray-finned fishes comprise four extant, sequentially branching superorders with varying degrees of pallium differentiation [130,136,137]. Northcutt [130] referred to this evolutionary pattern as a morphocline: from the most basally branching groups to the most recent, there appears to be a gradual increase in complexity with respect to the numbers of pallial cell groups and their arrangements into higher-order architectures (Figure 5, Ray-finned fishes). The simplest known organization is that of Polypterus, a cladistian ray-finned fish. In the pallium of these animals, much as in amphibians, neuronal cell bodies are found mostly along the ventricular surface deep to a thick neuropil (Figure 5, Bichir). At the other end of the morphocline lie the teleost fishes, some of which have highly developed pallial anatomies with five to seven major cell groups and complex internal circuitry (Figure 5, Goldfish) [135,138–140]. In all ray-finned fishes, however, early telencephalon development is thought to follow a similar, peculiar strategy: the telencephalic neuroepithelia evert from the prosencephalon by a lateral outfolding of its dorsomedial surface, rather than evaginate (inflate outward like a balloon) as in virtually all other vertebrates [137,141].
Telencephalon eversion is suggested to reverse the spatial arrangement of adult pallial cell populations with respect to those in the mammalian pallium. In one scheme, the lateral-most zone in teleost pallium (the dorsolateral division, DL) is homologous with the medially-located mammalian hippocampus, whereas the medial-most teleost zone (DM) is homologous with the laterally-located amygdala [142]. A neocortex homolog, if present, might be found somewhere in the middle [143]. This hypothesis, based on regional identity and developmental origin, is problematic. Such a hypothesis requires evidence from fate-mapping data [9], but the precise developmental origins of the teleost pallial cell populations are not known. The long-standing eversion model has also been challenged by detailed studies of telencephalon morphogenesis in zebrafish embryos, which suggest a different and more complex series of cellular movements [144]. The implications of this revised morphogenesis model for possible pallial homologies are not yet clear.
Other authors have noted detailed similarities across teleosts and mammals at the level of pallial circuitry [139,140,145–147]. The lateral pallial division, DL, receives visual, acoustic, lateral line, somatosensory, and in some species electrosensory input from relays in the preglomerular complex, the major relay in the teleost diencephalon of ascending sensory input [148]. Moreover, recent evidence indicates that the preglomerular complex has some of the molecular and physiological signatures of mammalian dorsal thalamus [148]. However, other features of the teleost lateral pallium, such as its intratelencephalic circuitry [140] and a selective role in spatial memory [149], may more closely resemble the mammalian hippocampus. Consistently, immunohistochemical evidence suggests that zebrafish DL selectively expresses the transcription factor PROX1, a conserved marker for mammalian hippocampal (specifically, dentate gyrus) cell types [150]. Trinh et al. [139] and Elliott et al. [140] previously discussed the apparent conflict posed by these data, which echo the situation described above for the frog medial pallium. Additional comparative data are required to determine whether a combined neocortical input- and dentate gyrus-like cell is an ancestral character for bony fishes, but this interesting possibility warrants further attention. It will be essential in future experiments to combine neuronal tract-tracing with immunostainings for conserved cell type markers. These experiments may benefit from examining more basally branching ray-finned fishes such as gars, which escaped the additional round of genome duplication that complicates molecular studies in teleost species [132].
The big, interesting telencephala of chondrichthyans
The extant cartilaginous fishes, or chondrichthyans, diverged from the bony fishes roughly 450 million years ago (Figure 3) [151]. Chondrichthyans comprise two major lineages: the holocephalans (chimaeras) and the far larger group of elasmobranchs (sharks, skates, and rays) [152]. Again, as in ray- and lobe-finned fishes, the different chondrichthyan groups present a remarkable diversity of telencephalon anatomies consistent with the deep divisions in their lineages (Figure 5, Chondrichthyans) [153]. Some large-brained elasmobranchs even attain relative brain weights comparable to birds and mammals, with the telencephalon accounting for up to half of brain mass [154–156]. The largest chondrichthyan telencephala tend to be found in those species that occupy the most complex habitats, and they may enable some surprising cognitive abilities [155,157]. Although relevant behavioral studies are few, rays have been observed to produce water jets as a tool to remove food from a pipe [158], and both rays and sharks can learn to perform a food-finding task by observing previously trained conspecifics [159,160].
Little is known regarding the evolutionary relationships of chondrichthyan with mammalian pallial structures. Likewise, it is not clear how to compare structures even across chondrichthyan groups, given their great anatomical disparities (reviewed in [153]). The telencephalon in the ratfish Hydrolagus colliei, a holocephalan, appears to consist largely of a bulbous lobe-like structure peppered with neuronal cell bodies (Figure 5, Ratfish). In the spiny dogfish Squalus acanthias, telencephalon morphology resembles that of amphibians, rounded and with a well-developed lateral ventricle but with more extensive cell migration (Figure 5, Shark). Finally, in the ray Raja clavata and in the nurse shark Ginglymostoma cirratum, the two telencephalic hemispheres are almost entirely fused together along the midline, their lateral ventricles are narrowed to pinhole-like slits, and they are filled throughout with neuronal cell bodies—derived features that together give the impression of a single bilateral slab of tissue (Figure 5, Ray) [153,161]. There is no agreement on where to place the division between pallium and subpallium in these animals.
Studies of chondrichthyans will help to reconstruct telencephalon organization in the common ancestor we share, the LCA of all extant jawed vertebrates (or gnathostomes) [162]. The practical advantages offered by their large brains and cartilaginous brain cases make this an especially inviting area for future molecular and neuroanatomical research. Already, molecular studies have begun to suggest some interesting parallels in the developing telencephala of sharks and large-brained amniotes [163]. The little skate Leucoraja erinacea, in particular, is emerging as an exciting model for vertebrate evolutionary developmental biology [164]. However, all extant lineages of bony and cartilaginous fishes are certain to differ from the common ancestor in their own unique ways. It will require broad phylogenetic sampling from multiple holocephalan and elasmobranch clades to identify shared features confidently and to sharpen a model of the ancestral gnathostome telencephalon [162].
Pallial neuronal cell types in cyclostomes
The deepest division in extant vertebrates is between the gnathostomes and the agnathans (or cyclostomes), which diverged around 500 million years ago (Figure 3) [165]. Comparative studies of the pallium in jawed and jawless vertebrates may reveal the pallial organization from which all extant forms are ultimately derived, but the identification of homologies in the cyclostome pallium has been anything but straightforward. The living cyclostomes have been reduced to just two relatively small modern clades, the lampreys and the hagfishes, each with their own distinct telencephalic organizations (Figure 5, Cyclostomes) [166]. Evolutionary interpretations of hagfish neuroanatomy have been especially problematic: their brains are in many ways so unlike those of lampreys and gnathostomes that morphologists long regarded hagfishes as a primitive outgroup to all other vertebrates [166]. For example, and by analogy with telencephalon eversion in actinopterygians and midline fusion in chondrichthyans, the hagfish telencephala undergo an unusual morphogenetic process by fusing anteriorly with the olfactory bulbs and posteriorly with the diencephalon [167]. Despite the strangeness of hagfish neuroanatomy, molecular data [168] and recent fossil evidence [169] tend to support cyclostome monophyly [170]. Consequently, many of the deviant features seen in hagfishes, including the absence of a pineal gland and oculomotor system, could be regarded as derived and are not ancestral to the vertebrates.
Hagfishes, as limbless, jawless, and nearly eyeless deep-sea scavengers, are not known for their cognitive sophistication. Even so, the hagfish pallium exhibits an impressive degree of differentiation compared with most other anamniote vertebrates and is dominated by an expansive multi-layered structure with cytoarchitectural [167], molecular [171], and connectional specializations [172] (Figure 5, Hagfish). This laminar structure receives secondary olfactory input throughout and may help hagfish to locate enticingly pungent carcasses through the generation of spatial maps. However, the hagfish layered pallium is not simply an olfactory cortex because it also receives at least some visual and, possibly, somatosensory input [172]. The layered pallium additionally issues descending output projections to the diencephalon and midbrain tectum [172]. The full extent of cell type diversity in the adult hagfish pallium is unfortunately poorly understood, as are the organizational principles of its elaborate layers.
Lampreys, in contrast with hagfishes, have a tiny telencephalon [173], but it has been recently subjected to extensive connectional and physiological characterization [23,174,175]. In the river lamprey Lampetra fluviatilis, the lateral part of the pallium comprises a three-layered cortex encircling a narrow ventricular space. Like the three-layered dorsal cortex of many reptiles [176], the lamprey cortex contains an outermost molecular layer (with abundant neuropil but few neuronal cell bodies) and two distinct cellular layers with pyramidal excitatory neurons as well as GABAergic interneurons (Figure 5, Lamprey) [175]. Among the excitatory neurons, the lamprey cortex harbors separate populations of primary visual input neurons [175], long-range motor output neurons [174], and IT-like neurons [175]. The highly divergent pallial anatomies seen across the anamniote vertebrates provide little support for a hypothesis that the lamprey cortical architecture is ancestral to vertebrates, but the possibility for pan-vertebrate neuronal cell type homologies is more compelling.
Lampreys and hagfishes [172,175], like most other studied vertebrates [109, 161], receive primary visual input in the pallium via thalamic relays. Thus, at least one thalamopallial primary sensory pathway is likely to be ancestral to extant vertebrates (Figure 3). An ancestral population of pallial input neurons may have served as a template for the introduction of additional sensory modalities to the pallium, and could have given rise to, among other things, the layer 4 neocortical input neurons of mammals and the primary input nuclei of birds and non-avian reptiles [106,112]. Similarly, lampreys [175] now join ray-finned fishes [177], amphibians [178], and all known extant mammals [179] in possessing pallial IT-like neurons with contralateral projections. The remarkable connectional similarities shared by lamprey and mammalian cortices raise the possibility that multiple classes of neocortical-like cell types predate the vertebrates. The application of molecular markers to the lamprey and other anamniote pallia may help to distinguish this scenario from the alternative one of convergent evolution. Whether or not the cyclostome pallial cell types are homologous with mammalian neocortical neurons, it is clear that pallial architecture can be readily molded by evolution into a multilayered structure, as may have occurred independently in lampreys, hagfishes, lungfishes, non-avian reptiles, and mammals.
Non-vertebrate chordates and the origin of the telencephalon
All vertebrates possess a rostral brain that extends paired telencephala and develops from a centralized neuroectoderm. The evolutionary origin of the vertebrate brain, like many other derived vertebrate characters, has long been uncertain [1,180]. The morphological disparities between vertebrates and invertebrates are great, and no vertebrate outgroup presents anything obviously comparable to the vertebrate brain. Indeed, most invertebrate phyla do not have a centralized nervous system at all [181], and a recent large-scale comparative study presented evidence that the brains in annelids and arthropods centralized convergently with vertebrates [182]. Despite these challenges, comparisons across vertebrates and their relatives have revealed remarkable homologies, not at the levels of structure or cell type, but at the level of developmental molecular patterning.
The closest living vertebrate relatives are the non-vertebrate chordates, the amphioxus (cephalochordates) and the tunicates (urochordates), which diverged from the vertebrate lineage around 550 million years ago (Figure 3) [183]. The vertebrates are united with cephalochordates and urochordates into the phylum Chordata by shared-derived characters that include a mesodermal notochord, a post-anal tail, and a dorsal centralized neural tube. The amphioxus is a small, filter-feeding marine animal superficially similar to a vertebrate fish, but without limbs, jaws, or any of the specialized paired sensory organs characteristic of a vertebrate head. In contrast, many tunicates (in particular the ascidians) following a free-swimming larval stage undergo a striking metamorphosis into a sessile, filter-feeding adult form quite unlike amphioxus and other chordates [184]. Despite these morphological features, genomic data revealed that the tunicates, and not amphioxus, are the closest relatives to vertebrates (Figure 3) [185]. Amphioxus, although more distantly related to vertebrates, is likely to retain more ancestral features and to provide clearer insights into the brain of the chordate LCA.
The anterior end of the amphioxus neural tube terminates in a single modest inflation, the cerebral vesicle, which does not display the overt morphological divisions of vertebrate brains. Possible homologies of the amphioxus cerebral vesicle have been puzzling, but multiple authors have concurred that amphioxus lacks anything comparable to the vertebrate telencephalon [186–188]. It now appears, however, that the adult amphioxus cerebral vesicle is molecularly regionalized into domains closely resembling the neuroepithelium in the developing telencephalic pallium and subpallium [189]. FoxG1, which is selectively expressed throughout the telencephalon in vertebrates, is likewise expressed throughout the amphioxus cerebral vesicle. The amphioxus orthologs of vertebrate Pax6 and Emx genes, conserved markers of the pallial neuroepithelium [190], identify a subset of the Foxg1-expressing domain, whereas amphioxus Nkx2.1 and Hh identify a separate, subpallium-like subset of the Foxg1 domain. Together with complementary molecular studies in lampreys and hagfishes [27,191], these data indicate that a complex molecular regionalization of a centralized telencephalon-like territory is ancestral for chordates, and possibly lost in tunicates [192]. Many questions remain, including whether the amphioxus telencephalon-like domain receives primary sensory information, such as from the frontal eye, and whether it is dorsoventrally patterned to generate pallial glutamatergic neurons and subpallial GABAergic neurons — features that together identify the emergence of the telencephalon as a neuroanatomical structure.
Circuitry, behavior, and the path to the neocortex
Neuroanatomy reflects the sensory and behavioral specializations of a given species. This principle is apparent, for example, in the differential allocation of neocortical surface area to particular sensory and motor representations across mammalian species [193]. The duck-billed platypus and the star-nosed mole, two mammalian species with derived, highly developed sensory structures, devote large proportions of their neocortices to their special senses. Other notable examples include the expanded primary auditory cortex in echolocating bats, the somatosensory whisker barrels of rodents, and the large somatosensory hand representations of prehensile raccoons and primates [193]. These anatomical specializations in mammalian neocortices lend themselves readily to straightforward interpretations of their significance.
It is plausible that adaptive tuning of neuroanatomical structure to behavioral and sensory capability occurs broadly throughout vertebrate telencephala in subtle forms that currently defy our understanding. The functional implications of nuclear, layered, and diffuse pallial neuronal architectures are entirely unknown, but it is thought generally that larger, cell-rich pallia with differentiated cytoarchitectures are found in vertebrates with higher cognitive abilities. Similarly, it will be interesting to consider the effects of convergence versus segregation of sensory pathways to the pallium with respect to perceptual and cognitive ability. As described above, multiple sensory pathways converge in the pallium of frogs and teleost fishes, but target discrete territories in amniotes including mammals. It seems intuitive that the latter organization affords, in some sense, enhanced sensory processing, but this idea remains to be tested. In addition, the evolutionary intercalation of increased numbers of pallial IT neurons between sensory input and motor output neurons may allow some vertebrates to extract more useful information from available sensory input or to expand behavioral flexibility [40,105]. The evolution of vertebrate nervous systems is in large part the evolution of neural circuitry — whether through qualitative changes to circuit construction, increase or reduction of neuronal number at particular nodes, or through the reorganization of circuit components in space — with behavior as the target of selection, made manifest through neuronal activity [40,194].
In their influential review, Gans and Northcutt [3] observed that many of the derived features of vertebrates are found in the head, including the paired eyes, ears, and the nose. The development of vertebrate sensory organs and the transition from a filter feeding to a more active, predatory lifestyle may have provided for selective pressures that drove the elaboration of telencephalon anatomy in the different vertebrate classes. In particular, heightened sensory capabilities would enable new opportunities either for predation or for predator avoidance, as well as other complex social interactions likely requiring multimodal sensory-integrative circuitry [195]. The common ancestor of chordates had a rostral neuroepithelial domain with molecular regionalization that presaged the later emergence of vertebrate pallial structures as well as the subpallial striatum and pallidum. This domain would eventually serve as target for ascending sensory pathways to the pallium and would generate the basal ganglia motor circuitry necessary for complex movement [23]. Much of the subsequent history of pallium evolution remains blurry, but we anticipate further attention to the anamniote vertebrates will bring progress.
At least as early as the LCA of amniotes, the core cell types that compose neocortical circuitry emerged in recognizable form [40]. These circuit elements were reorganized, for unknown reasons and by largely unknown mechanisms, into the six-layered structure of the neocortex in early mammals. At this point, mammals appear to have become stuck with a layered architecture resistant to evolutionary reorganization, but highly plastic to tangential variation in size and to functional repurposing when new organs and abilities arise. Throughout early hominin history and accelerating towards the present, the neocortex continued its expansion, adding association cortex and refining the morphological and functional properties of its neurons and glia to meet the increasingly sophisticated behavioral needs of early humans. From its initial form as a tiny centralized Emx-expressing domain in Precambrian chordates, the pallium thus expanded in the human lineage to assimilate multiple sensory pathways, to promote their integration through the labyrinthine circuitry of higher-order cortices, and to subserve the cognitive, motor, and social abilities that characterize the vertebrates and particularly the modern human species.
Acknowledgements
We thank M. Coates for comments, Z-X. Luo and T. White for discussions, and T. Shpigel for help. S. Briscoe is grateful for funding provided by the Alexander von Humboldt Foundation and by the European Molecular Biology Organization (EMBO, ALTF 1014-2018).
References
- 1.Gee H (2018). Across the Bridge: Understanding the Origin of the Vertebrates (Chicago: The University of Chicago Press; ). [Google Scholar]
- 2.Erwin DH, and Valentine JW (2013). The Cambrian Explosion: The Construction of Animal Biodiversity (Greenwood Village, Colorado: Roberts and Company; ). [Google Scholar]
- 3.Gans C, and Northcutt RG (1983). Neural crest and the origin of vertebrates: a new head. Science 220, 268–273. [DOI] [PubMed] [Google Scholar]
- 4.Nieuwenhuys R, Donkelaar H.J.t., and Nicholson C (1998). The Central Nervous System of Vertebrates (New York: Springer; ). [Google Scholar]
- 5.Butler AB, and Hodos W (2005). Comparative Vertebrate Neuroanatomy: Evolution and Adaptation, 2nd Edition. (Hoboken, NJ: Wiley-Interscience; ). [Google Scholar]
- 6.Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, et al. (2004). Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol 473, 377–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Striedter GF (2005). Principles of Brain Evolution (Sunderland, Mass.: Sinauer Associates; ). [Google Scholar]
- 8.Nieuwenhuys R (2009). The forebrain of actinopterygians revisited. Brain Behav. Evol 73, 229–252. [DOI] [PubMed] [Google Scholar]
- 9.Dugas-Ford J, and Ragsdale CW (2015). Levels of homology and the problem of neocortex. Annu. Rev. Neurosci 38, 351–368. [DOI] [PubMed] [Google Scholar]
- 10.Striedter GF, and Northcutt RG (1991). Biological hierarchies and the concept of homology. Brain Behav. Evol 38, 177–189. [DOI] [PubMed] [Google Scholar]
- 11.Hall BK (1994). Homology: The Hierarchical Basis of Comparative Biology (San Diego: Academic Press; ). [Google Scholar]
- 12.Reiner A (1996). Levels of organization and the evolution of isocortex. Trends Neurosci 19, 89–91. [DOI] [PubMed] [Google Scholar]
- 13.Pritz MB (2005). Comparisons and homology in adult and developing vertebrate central nervous systems. Brain Behav. Evol 66, 222–233. [DOI] [PubMed] [Google Scholar]
- 14.Rowitch DH, and Kriegstein AR (2010). Developmental genetics of vertebrate glial-cell specification. Nature 468, 214–222. [DOI] [PubMed] [Google Scholar]
- 15.Taverna E, Gotz M, and Huttner WB (2014). The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu. Rev. Cell. Dev. Biol 30, 465–502. [DOI] [PubMed] [Google Scholar]
- 16.Heins N, Cremisi F, Malatesta P, Gangemi RM, Corte G, Price J, Goudreau G, Gruss P, and Gotz M (2001). Emx2 promotes symmetric cell divisions and a multipotential fate in precursors from the cerebral cortex. Mol. Cell. Neurosci 18, 485–502. [DOI] [PubMed] [Google Scholar]
- 17.Rash BG, and Grove EA (2006). Area and layer patterning in the developing cerebral cortex. Curr. Opin. Neurobiol 16, 25–34. [DOI] [PubMed] [Google Scholar]
- 18.O’Leary DD, Chou SJ, and Sahara S (2007). Area patterning of the mammalian cortex. Neuron 56, 252–269. [DOI] [PubMed] [Google Scholar]
- 19.Hebert JM, and Fishell G (2008). The genetics of early telencephalon patterning: some assembly required. Nat. Rev. Neurosci 9, 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiecker C, and Lumsden A (2012). The role of organizers in patterning the nervous system. Annu. Rev. Neurosci 35, 347–367. [DOI] [PubMed] [Google Scholar]
- 21.Reiner A, Medina L, and Veenman CL (1998). Structural and functional evolution of the basal ganglia in vertebrates. Brain Res. Rev 28, 235–285. [DOI] [PubMed] [Google Scholar]
- 22.Moreno N, Gonzalez A, and Retaux S (2009). Development and evolution of the subpallium. Semin. Cell Dev. Biol 20, 735–743. [DOI] [PubMed] [Google Scholar]
- 23.Grillner S, and Robertson B (2016). The basal ganglia over 500 million years. Curr. Biol 26, R1088–R1100. [DOI] [PubMed] [Google Scholar]
- 24.Reiner A, and Northcutt RG (1987). An immunohistochemical study of the telencephalon of the African lungfish, Protopterus annectens. J. Comp. Neurol 256, 463–481. [DOI] [PubMed] [Google Scholar]
- 25.Northcutt RG, Reiner A, and Karten HJ (1988). Immunohistochemical study of the telencephalon of the spiny dogfish, Squalus acanthias. J. Comp. Neurol 277, 250–267. [DOI] [PubMed] [Google Scholar]
- 26.Reiner A, and Northcutt RG (1992). An immunohistochemical study of the telencephalon of the senegal bichir (Polypterus senegalus). J. Comp. Neurol 319, 359–386. [DOI] [PubMed] [Google Scholar]
- 27.Sugahara F, Pascual-Anaya J, Oisi Y, Kuraku S, Aota S, Adachi N, Takagi W, Hirai T, Sato N, Murakami Y, et al. (2016). Evidence from cyclostomes for complex regionalization of the ancestral vertebrate brain. Nature 531, 97–100. [DOI] [PubMed] [Google Scholar]
- 28.Wamsley B, and Fishell G (2017). Genetic and activity-dependent mechanisms underlying interneuron diversity. Nat. Rev. Neurosci 18, 299–309. [DOI] [PubMed] [Google Scholar]
- 29.Lim L, Mi D, Llorca A, and Marin O (2018). Development and functional diversification of cortical interneurons. Neuron 100, 294–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez-de-la-Torre M, Pombal MA, and Puelles L (2011). Distal-less-like protein distribution in the larval lamprey forebrain. Neuroscience 178, 270–284. [DOI] [PubMed] [Google Scholar]
- 31.Quintana-Urzainqui I, Rodriguez-Moldes I, Mazan S, and Candal E (2015). Tangential migratory pathways of subpallial origin in the embryonic telencephalon of sharks: evolutionary implications. Brain Struct. Funct 220, 2905–2926. [DOI] [PubMed] [Google Scholar]
- 32.Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, and Jones KR (2002). Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci 22, 6309–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letinic K, Zoncu R, and Rakic P (2002). Origin of GABAergic neurons in the human neocortex. Nature 417, 645–649. [DOI] [PubMed] [Google Scholar]
- 34.Naumann RK, and Laurent G (2017). Function and evolution of the reptilian cerebral cortex. In Evolution of Nervous Systems, Volume 1, 2nd Edition, Kaas JH and Striedter G, eds. (Oxford: Academic Press; ), pp. 491–518. [Google Scholar]
- 35.Harris KD, and Shepherd GM (2015). The neocortical circuit: themes and variations. Nat. Neurosci 18, 170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nauta WJH, and Feirtag M (1986). Fundamental Neuroanatomy (New York: W.H. Freeman; ). [Google Scholar]
- 37.Ulinski PS (1983). Dorsal Ventricular Ridge: A Treatise on Forebrain Organization in Reptiles and Birds (New York: John Wiley & Sons; ). [Google Scholar]
- 38.Herrick CJ (1948). The Brain of the Tiger Salamander, Ambystoma Tigrinum (Chicago: The University of Chicago Press; ). [Google Scholar]
- 39.Braford MR Jr., and Northcutt RG (1974). Olfactory bulb projections in the bichir, Polypterus. J. Comp. Neurol 156, 165–178. [DOI] [PubMed] [Google Scholar]
- 40.Briscoe SD, and Ragsdale CW (2018). Homology, neocortex, and the evolution of developmental mechanisms. Science 362, 190–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner GP (2014). Homology, Genes, and Evolutionary Innovation (Princeton: Princeton University Press; ). [Google Scholar]
- 42.Patterson C (1988). Homology in classical and molecular biology. Mol. Biol. Evol 5, 603–625. [DOI] [PubMed] [Google Scholar]
- 43.Ataman B, Boulting GL, Harmin DA, Yang MG, Baker-Salisbury M, Yap EL, Malik AN, Mei K, Rubin AA, Spiegel I, et al. (2016). Evolution of Osteocrin as an activity-regulated factor in the primate brain. Nature 539, 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, et al. (2016). A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sousa AMM, Zhu Y, Raghanti MA, Kitchen RR, Onorati M, Tebbenkamp ATN, Stutz B, Meyer KA, Li M, Kawasawa YI, et al. (2017). Molecular and cellular reorganization of neural circuits in the human lineage. Science 358, 1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lui JH, Nowakowski TJ, Pollen AA, Javaherian A, Kriegstein AR, and Oldham MC (2014). Radial glia require PDGFD-PDGFRβ signalling in human but not mouse neocortex. Nature 515, 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rakic P (1995). A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci 18, 383–388. [DOI] [PubMed] [Google Scholar]
- 48.Brodmann K, and Garey LJ (2006). Brodmann’s Localisation in the Cerebral Cortex (New York: Springer; ). [Google Scholar]
- 49.Nieuwenhuys R, Voogd J, and van Huijzen C (2008). The Human Central Nervous System, 4th Edition. (New York: Springer; ). [Google Scholar]
- 50.Lui JH, Hansen DV, and Kriegstein AR (2011). Development and evolution of the human neocortex. Cell 146, 18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherwood CC, Bauernfeind AL, Bianchi S, Raghanti MA, and Hof PR (2012). Human brain evolution writ large and small. Prog. Brain Res 195, 237–254. [DOI] [PubMed] [Google Scholar]
- 52.Sousa AMM, Meyer KA, Santpere G, Gulden FO, and Sestan N (2017). Evolution of the human nervous system function, structure, and development. Cell 170, 226–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giandomenico SL, and Lancaster MA (2017). Probing human brain evolution and development in organoids. Curr. Opin. Cell Biol 44, 36–43. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell C, and Silver DL (2018). Enhancing our brains: Genomic mechanisms underlying cortical evolution. Semin. Cell Dev. Biol 76, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borrell V, and Calegari F (2014). Mechanisms of brain evolution: regulation of neural progenitor cell diversity and cell cycle length. Neurosci. Res 86, 14–24. [DOI] [PubMed] [Google Scholar]
- 56.Florio M, and Huttner WB (2014). Neural progenitors, neurogenesis and the evolution of the neocortex. Development 141, 2182–2194. [DOI] [PubMed] [Google Scholar]
- 57.Fiddes IT, Lodewijk GA, Mooring M, Bosworth CM, Ewing AD, Mantalas GL, Novak AM, van den Bout A, Bishara A, Rosenkrantz JL, et al. (2018). Human-specific NOTCH2NL genes affect notch signaling and cortical neurogenesis. Cell 173, 1356–1369.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki IK, Gacquer D, Van Heurck R, Kumar D, Wojno M, Bilheu A, Herpoel A, Lambert N, Cheron J, Polleux F, et al. (2018). Human-specific NOTCH2NL genes expand cortical neurogenesis through Delta/Notch regulation. Cell 173, 1370–1384 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Florio M, Heide M, Pinson A, Brandl H, Albert M, Winkler S, Wimberger P, Huttner WB, and Hiller M (2018). Evolution and cell-type specificity of human-specific genes preferentially expressed in progenitors of fetal neocortex. eLife 7, e32332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalebic N, Gilardi C, Albert M, Namba T, Long KR, Kostic M, Langen B, and Huttner WB (2018). Human-specific ARHGAP11B induces hallmarks of neocortical expansion in developing ferret neocortex. eLife 7, e41241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kronenberg ZN, Fiddes IT, Gordon D, Murali S, Cantsilieris S, Meyerson OS, Underwood JG, Nelson BJ, Chaisson MJP, Dougherty ML, et al. (2018). High-resolution comparative analysis of great ape genomes. Science 360, eaar6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prufer K, de Filippo C, Grote S, Mafessoni F, Korlevic P, Hajdinjak M, Vernot B, Skov L, Hsieh P, Peyregne S, et al. (2017). A high-coverage Neandertal genome from Vindija Cave in Croatia. Science 358, 655–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meyer M, Kircher M, Gansauge MT, Li H, Racimo F, Mallick S, Schraiber JG, Jay F, Prufer K, de Filippo C, et al. (2012). A high-coverage genome sequence from an archaic Denisovan individual. Science 338, 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nowakowski TJ, Pollen AA, Sandoval-Espinosa C, and Kriegstein AR (2016). Transformation of the radial glia scaffold demarcates two stages of human cerebral cortex development. Neuron 91, 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smaers JB, Gomez-Robles A, Parks AN, and Sherwood CC (2017). Exceptional evolutionary expansion of prefrontal cortex in great apes and humans. Curr. Biol 27, 714–720. [DOI] [PubMed] [Google Scholar]
- 66.Sneve MH, Grydeland H, Rosa MGP, Paus T, Chaplin T, Walhovd K, and Fjell AM (2018). High-expanding regions in primate cortical brain evolution support supramodal cognitive flexibility. Cereb. Cortex bhy 268, 1–11. [DOI] [PubMed] [Google Scholar]
- 67.Beaulieu-Laroche L, Toloza EHS, van der Goes MS, Lafourcade M, Barnagian D, Williams ZM, Eskandar EN, Frosch MP, Cash SS, and Harnett MT (2018). Enhanced dendritic compartmentalization in human cortical neurons. Cell 175, 643–651.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Charrier C, Joshi K, Coutinho-Budd J, Kim JE, Lambert N, de Marchena J, Jin WL, Vanderhaeghen P, Ghosh A, Sassa T, et al. (2012). Inhibition of SRGAP2 function by its human-specific paralogs induces neoteny during spine maturation. Cell 149, 923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dennis MY, Nuttle X, Sudmant PH, Antonacci F, Graves TA, Nefedov M, Rosenfeld JA, Sajjadian S, Malig M, Kotkiewicz H, et al. (2012). Evolution of human-specific neural SRGAP2 genes by incomplete segmental duplication. Cell 149, 912–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, et al. (2009). Uniquely hominid features of adult human astrocytes. J. Neurosci 29, 3276–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, et al. (2013). Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12, 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller IF, Barton RA, and Nunn CL (2019). Quantitative uniqueness of human brain evolution revealed through phylogenetic comparative analysis. eLife 8, e41250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diniz-Filho JAF, Jardim L, Mondanaro A, and Raia P (2019). Multiple components of phylogenetic non-stationarity in the evolution of brain size in fossil hominins. Evol. Biol 46, 47–59. [Google Scholar]
- 74.Martinez-Cerdeno V, Cunningham CL, Camacho J, Antczak JL, Prakash AN, Cziep ME, Walker AI, and Noctor SC (2012). Comparative analysis of the subventricular zone in rat, ferret and macaque: evidence for an outer subventricular zone in rodents. PLoS ONE 7, e30178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lewitus E, and Huttner WB (2015). Neurodevelopmental lincRNA microsyteny conservation and mammalian brain size evolution. PLoS ONE 10, e0131818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raghanti MA, Munger EL, Wicinski B, Butti C, and Hof PR (2017). Comparative structure of the cerebral cortex in large mammals. In Evolution of Nervous Systems, Volume 2, 2nd Edition, Kaas JH and Herculano-Houzel S, eds. (Oxford: Academic Press; ), pp. 267–289. [Google Scholar]
- 77.Ashwell KWS (2013). Neurobiology of Monotremes: Brain Evolution in Our Distant Mammalian Cousins (Collingwood, Vic: CSIRO Publishing; ). [Google Scholar]
- 78.Martinez-Cerdeno V, and Noctor SC (2016). Cortical evolution 2015: discussion of neural progenitor cell nomenclature. J. Comp. Neurol 524, 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smart IH, Dehay C, Giroud P, Berland M, and Kennedy H (2002). Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb. Cortex 12, 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fietz SA, Kelava I, Vogt J, Wilsch-Brauninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, et al. (2010). OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat. Neurosci 13, 690–699. [DOI] [PubMed] [Google Scholar]
- 81.Reillo I, de Juan Romero C, Garcia-Cabezas MA, and Borrell V (2011). A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb. Cortex 21, 1674–1694. [DOI] [PubMed] [Google Scholar]
- 82.Johnson MB, Sun X, Kodani A, Borges-Monroy R, Girskis KM, Ryu SC, Wang PP, Patel K, Gonzalez DM, Woo YM, et al. (2018). Aspm knockout ferret reveals an evolutionary mechanism governing cerebral cortical size. Nature 556, 370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sauerland C, Menzies BR, Glatzle M, Seeger J, Renfree MB, and Fietz SA (2018). The basal radial glia occurs in marsupials and underlies the evolution of an expanded neocortex in therian mammals. Cereb. Cortex 28, 145–157. [DOI] [PubMed] [Google Scholar]
- 84.Shitamukai A, Konno D, and Matsuzaki F (2011). Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J. Neurosci 31, 3683–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vaid S, Camp JG, Hersemann L, Eugster Oegema C, Heninger AK, Winkler S, Brandl H, Sarov M, Treutlein B, Huttner WB, et al. (2018). A novel population of Hopx-dependent basal radial glial cells in the developing mouse neocortex. Development 145, dev169276. [DOI] [PubMed] [Google Scholar]
- 86.Wang X, Tsai JW, LaMonica B, and Kriegstein AR (2011). A new subtype of progenitor cell in the mouse embryonic neocortex. Nat. Neurosci 14, 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pollen AA, Nowakowski TJ, Chen J, Retallack H, Sandoval-Espinosa C, Nicholas CR, Shuga J, Liu SJ, Oldham MC, Diaz A, et al. (2015). Molecular identity of human outer radial glia during cortical development. Cell 163, 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson MB, Wang PP, Atabay KD, Murphy EA, Doan RN, Hecht JL, and Walsh CA (2015). Single-cell analysis reveals transcriptional heterogeneity of neural progenitors in human cortex. Nat. Neurosci 18, 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fish JL, Kosodo Y, Enard W, Paabo S, and Huttner WB (2006). Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc. Natl. Acad. Sci. USA 103, 10438–10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Montgomery SH, and Mundy NI (2014). Microcephaly genes evolved adaptively throughout the evolution of eutherian mammals. BMC Evol. Biol 14, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arcila ML, Betizeau M, Cambronne XA, Guzman E, Doerflinger N, Bouhallier F, Zhou H, Wu B, Rani N, Bassett DS, et al. (2014). Novel primate miRNAs coevolved with ancient target genes in germinal zone-specific expression patterns. Neuron 81, 1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu J, Liu W, Yang L, Wu Q, Zhang H, Fang A, Li L, Xu X, Sun L, Zhang J, et al. (2017). The primate-specific gene TMEM14B marks outer radial glia cells and promotes cortical expansion and folding. Cell Stem Cell 21, 635–649.e8. [DOI] [PubMed] [Google Scholar]
- 93.O’Neill AC, Kyrousi C, Klaus J, Leventer RJ, Kirk EP, Fry A, Pilz DT, Morgan T, Jenkins ZA, Drukker M, et al. (2018). A primate-specific isoform of PLEKHG6 regulates neurogenesis and neuronal migration. Cell Rep 25, 2729–2741.e6. [DOI] [PubMed] [Google Scholar]
- 94.Krubitzer LA, and Prescott TJ (2018). The combinatorial creature: Cortical phenotypes within and across lifetimes. Trends Neurosci 41, 744–762. [DOI] [PubMed] [Google Scholar]
- 95.Kaas JH (2013). The evolution of brains from early mammals to humans. Wiley Interdiscip. Rev. Cogn. Sci 4, 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klingler E (2017). Development and organization of the evolutionarily conserved three-layered olfactory cortex. eNeuro 4, ENEURO.0193–16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hevner RF (2016). Evolution of the mammalian dentate gyrus. J. Comp. Neurol 524, 578–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Falcone C, Wolf-Ochoa M, Amina S, Hong T, Vakilzadeh G, Hopkins WD, Hof PR, Sherwood CC, Manger PR, Noctor SC, et al. (2019). Cortical interlaminar astrocytes across the therian mammal radiation. J. Comp. Neurol, 1654–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bininda-Emonds OR, Cardillo M, Jones KE, MacPhee RD, Beck RM, Grenyer R, Price SA, Vos RA, Gittleman JL, and Purvis A (2007). The delayed rise of present-day mammals. Nature 446, 507–512. [DOI] [PubMed] [Google Scholar]
- 100.Tsuboi M, van der Bijl W, Kopperud BT, Erritzoe J, Voje KL, Kotrschal A, Yopak KE, Collin SP, Iwaniuk AN, and Kolm N (2018). Breakdown of brain-body allometry and the encephalization of birds and mammals. Nat. Ecol. Evol 2, 1492–1500. [DOI] [PubMed] [Google Scholar]
- 101.Olkowicz S, Kocourek M, Lucan RK, Portes M, Fitch WT, Herculano-Houzel S, and Nemec P (2016). Birds have primate-like numbers of neurons in the forebrain. Proc. Natl. Acad. Sci. USA 113, 7255–7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, and Herculano-Houzel S (2009). Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol 513, 532–541. [DOI] [PubMed] [Google Scholar]
- 103.Clayton NS, and Emery NJ (2015). Avian models for human cognitive neuroscience: a proposal. Neuron 86, 1330–1342. [DOI] [PubMed] [Google Scholar]
- 104.Gruber R, Schiestl M, Boeckle M, Frohnwieser A, Miller R, Gray RD, Clayton NS, and Taylor AH (2019). New caledonian crows use mental representations to solve metatool problems. Curr. Biol 29, 686–692.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Briscoe SD, Albertin CB, Rowell JJ, and Ragsdale CW (2018). Neocortical association cell types in the forebrain of birds and alligators. Curr. Biol 28, 686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dugas-Ford J, Rowell JJ, and Ragsdale CW (2012). Cell-type homologies and the origins of the neocortex. Proc. Natl. Acad. Sci. USA 109, 16974–16979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jarvis ED, Yu J, Rivas MV, Horita H, Feenders G, Whitney O, Jarvis SC, Jarvis ER, Kubikova L, Puck AE, et al. (2013). Global view of the functional molecular organization of the avian cerebrum: mirror images and functional columns. J. Comp. Neurol 521, 3614–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karten HJ (2013). Neocortical evolution: neuronal circuits arise independently of lamination. Curr. Biol 23, R12–R15. [DOI] [PubMed] [Google Scholar]
- 109.Karten HJ (1969). The organization of the avian telencephalon and some speculations on the phylogeny of the amniote telencephalon. Ann. N. Y. Acad. Sci 167, 164–179. [Google Scholar]
- 110.Butler AB (1994). The evolution of the dorsal pallium in the telencephalon of amniotes: cladistic analysis and a new hypothesis. Brain Res. Rev 19, 66–101. [DOI] [PubMed] [Google Scholar]
- 111.Behroozi M, Billings BK, Helluy X, Manger PR, Gunturkun O, and Strockens F (2018). Functional MRI in the Nile crocodile: a new avenue for evolutionary neurobiology. Proc. Royal Soc. B 285, 20180178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Briscoe SD, and Ragsdale CW (2018). Molecular anatomy of the alligator dorsal telencephalon. J. Comp. Neurol 526, 1613–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tosches MA, Yamawaki TM, Naumann RK, Jacobi AA, Tushev G, and Laurent G (2018). Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science 360, 881–888. [DOI] [PubMed] [Google Scholar]
- 114.Reiner A (2000). A hypothesis as to the organization of cerebral cortex in the common amniote ancestor of modern reptiles and mammals. In Evolutionary Developmental Biology of the Cerebral Cortex, Volume 228, Bock GA and Cardew G, eds. (West Susex, England: John Wiley & Sons; ), pp. 83–102. [DOI] [PubMed] [Google Scholar]
- 115.Roth G, Laberge F, Muhlenbrock-Lenter S, and Grunwald W (2007). Organization of the pallium in the fire-bellied toad Bombina orientalis. I: Morphology and axonal projection pattern of neurons revealed by intracellular biocytin labeling. J. Comp. Neurol 501, 443–464. [DOI] [PubMed] [Google Scholar]
- 116.Northcutt RG (1981). Evolution of the telencephalon in nonmammals. Annu. Rev. Neurosci 4, 301–350. [DOI] [PubMed] [Google Scholar]
- 117.Northcutt RG, and Ronan M (1992). Afferent and efferent connections of the bullfrog medial pallium. Brain Behav. Evol 40, 1–16. [DOI] [PubMed] [Google Scholar]
- 118.Schmidt A, and Wake MH (1997). Cellular migration and morphological complexity in the caecilian brain. J. Morphol 231, 11–27. [DOI] [PubMed] [Google Scholar]
- 119.Gonzalez A, Lopez JM, Morona R, and Moreno N (2017). The organization of the central nervous system of amphibians. In Evolution of Nervous Systems, Volume 1, 2nd Edition, Kaas JH and Striedter G, eds. (Oxford: Academic Press; ), pp. 141–170. [Google Scholar]
- 120.Medina L, and Reiner A (2000). Do birds possess homologues of mammalian primary visual, somatosensory and motor cortices? Trends Neurosci 23, 1–12. [DOI] [PubMed] [Google Scholar]
- 121.Supin AY, and Guselnikov VI (1965). Representation of visual, auditory and somatosensory systems in frog forebrain. Fed. Proc. Transl. Suppl 24, 357–62. [PubMed] [Google Scholar]
- 122.Karamian AI, Vesselkin NP, Belekhova MG, and Zagorulko TM (1966). Electrophysiological characteristics of tectal and thalamo-cortical divisions of the visual system in lower vertebrates. J. Comp. Neurol 127, 559–576. [DOI] [PubMed] [Google Scholar]
- 123.Mudry KM, and Capranica RR (1980). Evoked auditory activity within the telencephalon of the bullfrog (Rana catesbeiana). Brain Res 182, 303–311. [DOI] [PubMed] [Google Scholar]
- 124.Abellan A, Desfilis E, and Medina L (2014). Combinatorial expression of Lef1, Lhx2, Lhx5, Lhx9, Lmo3, Lmo4, and Prox1 helps to identify comparable subdivisions in the developing hippocampal formation of mouse and chicken. Front. Neuroanat 8, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arendt D, Musser JM, Baker CVH, Bergman A, Cepko C, Erwin DH, Pavlicev M, Schlosser G, Widder S, Laubichler MD, et al. (2016). The origin and evolution of cell types. Nat. Rev. Genet 17, 744–757. [DOI] [PubMed] [Google Scholar]
- 126.Ebbesson SO (1980). The parcellation theory and its relation to interspecific variability in brain organization, evolutionary and ontogenetic development, and neuronal plasticity. Cell Tissue Res 213, 179–212. [DOI] [PubMed] [Google Scholar]
- 127.Roth G, Nishikawa KC, Naujoks-Manteuffel C, Schmidt A, and Wake DB (1993). Paedomorphosis and simplification in the nervous system of salamanders. Brain Behav. Evol 42, 137–170. [DOI] [PubMed] [Google Scholar]
- 128.Moreno N, Lopez JM, Morona R, Lozano D, Jimenez S, and Gonzalez A (2018). Comparative analysis of Nkx2.1 and Islet-1 expression in urodele amphibians and lungfishes highlights the pattern of forebrain organization in early tetrapods. Front. Neuroanat 12, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Amemiya CT, Alfoldi J, Lee AP, Fan S, Philippe H, Maccallum I, Braasch I, Manousaki T, Schneider I, Rohner N, et al. (2013). The African coelacanth genome provides insights into tetrapod evolution. Nature 496, 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Northcutt RG (2008). Forebrain evolution in bony fishes. Brain Res. Bull 75, 191–205. [DOI] [PubMed] [Google Scholar]
- 131.Ravi V, and Venkatesh B (2018). The divergent genomes of teleosts. Annu. Rev. Anim. Biosci 6, 47–68. [DOI] [PubMed] [Google Scholar]
- 132.Braasch I, Gehrke AR, Smith JJ, Kawasaki K, Manousaki T, Pasquier J, Amores A, Desvignes T, Batzel P, Catchen J, et al. (2016). The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat. Genet 48, 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ganz J, Kaslin J, Freudenreich D, Machate A, Geffarth M, and Brand M (2012). Subdivisions of the adult zebrafish subpallium by molecular marker analysis. J. Comp. Neurol 520, 633–655. [DOI] [PubMed] [Google Scholar]
- 134.Maruska KP, Butler JM, Field KE, and Porter DT (2017). Localization of glutamatergic, GABAergic, and cholinergic neurons in the brain of the African cichlid fish, Astatotilapia burtoni. J. Comp. Neurol 525, 610–638. [DOI] [PubMed] [Google Scholar]
- 135.Kawaguchi M, Hagio H, Yamamoto N, Matsumoto K, Nakayama K, Akazome Y, Izumi H, Tsuneoka Y, Suto F, Murakami Y, et al. (2019). Atlas of the telencephalon based on cytoarchitecture, neurochemical markers, and gene expressions in Rhinogobius flumineus [Mizuno, 1960]. J. Comp. Neurol 527, 874–900. [DOI] [PubMed] [Google Scholar]
- 136.Braford MR Jr. (2009). Stalking the everted telencephalon: comparisons of forebrain organization in basal ray-finned fishes and teleosts. Brain Behav. Evol 74, 56–76. [DOI] [PubMed] [Google Scholar]
- 137.Nieuwenhuys R (2011). The development and general morphology of the telencephalon of actinopterygian fishes: synopsis, documentation and commentary. Brain Struct. Funct 215, 141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Giassi AC, Harvey-Girard E, Valsamis B, and Maler L (2012). Organization of the gymnotiform fish pallium in relation to learning and memory: I. cytoarchitectonics and cellular morphology. J. Comp. Neurol 520, 3314–3337. [DOI] [PubMed] [Google Scholar]
- 139.Trinh AT, Harvey-Girard E, Teixeira F, and Maler L (2016). Cryptic laminar and columnar organization in the dorsolateral pallium of a weakly electric fish. J. Comp. Neurol 524, 408–428. [DOI] [PubMed] [Google Scholar]
- 140.Elliott SB, Harvey-Girard E, Giassi AC, and Maler L (2017). Hippocampal-like circuitry in the pallium of an electric fish: possible substrates for recursive pattern separation and completion. J. Comp. Neurol 525, 8–46. [DOI] [PubMed] [Google Scholar]
- 141.Striedter GF, and Northcutt RG (2006). Head size constrains forebrain development and evolution in ray-finned fishes. Evol. Dev 8, 215–222. [DOI] [PubMed] [Google Scholar]
- 142.Mueller T, and Wullimann MF (2009). An evolutionary interpretation of teleostean forebrain anatomy. Brain Behav. Evol 74, 30–42. [DOI] [PubMed] [Google Scholar]
- 143.Northcutt RG (2011). Do teleost fishes possess a homolog of mammalian isocortex? Brain Behav. Evol 78, 136–138. [DOI] [PubMed] [Google Scholar]
- 144.Folgueira M, Bayley P, Navratilova P, Becker TS, Wilson SW, and Clarke JD (2012). Morphogenesis underlying the development of the everted teleost telencephalon. Neural Dev 7, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yamamoto N, Ishikawa Y, Yoshimoto M, Xue HG, Bahaxar N, Sawai N, Yang CY, Ozawa H, and Ito H (2007). A new interpretation on the homology of the teleostean telencephalon based on hodology and a new eversion model. Brain Behav. Evol 69, 96–104. [DOI] [PubMed] [Google Scholar]
- 146.Ito H, and Yamamoto N (2009). Non-laminar cerebral cortex in teleost fishes? Biol. Lett 5, 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Harvey-Girard E, Giassi AC, Ellis W, and Maler L (2012). Organization of the gymnotiform fish pallium in relation to learning and memory: iv. expression of conserved transcription factors and implications for the evolution of dorsal telencephalon. J. Comp. Neurol 520, 3395–3413. [DOI] [PubMed] [Google Scholar]
- 148.Wallach A, Harvey-Girard E, Jun JJ, Longtin A, and Maler L (2018). A time-stamp mechanism may provide temporal information necessary for egocentric to allocentric spatial transformations. eLife 7, e36769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Broglio C, Rodriguez F, Gomez A, Arias JL, and Salas C (2010). Selective involvement of the goldfish lateral pallium in spatial memory. Behav. Brain Res 210, 191–201. [DOI] [PubMed] [Google Scholar]
- 150.Ganz J, Kroehne V, Freudenreich D, Machate A, Geffarth M, Braasch I, Kaslin J, and Brand M (2014). Subdivisions of the adult zebrafish pallium based on molecular marker analysis. F1000Research 3, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Venkatesh B, Lee AP, Ravi V, Maurya AK, Lian MM, Swann JB, Ohta Y, Flajnik MF, Sutoh Y, Kasahara M, et al. (2014). Elephant shark genome provides unique insights into gnathostome evolution. Nature 505, 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Boisvert CA, Johnston P, Trinajstic K, and Johanson Z (2019). Chondrichthyan evolution, diversity, and senses. In Heads, Jaws, and Muscles: Anatomical, Functional, and Developmental Diversity in Chordate Evolution, Ziermann JM, Diaz RE Jr and Diogo R, eds. (Cham: Springer International Publishing; ), pp. 65–91. [Google Scholar]
- 153.Smeets WJAJ, Nieuwenhuys R, and Roberts BL (1983). The Central Nervous System of Cartilaginous Fishes: Structure and Functional Correlations (Berlin; New York: Springer-Verlag; ). [Google Scholar]
- 154.Bauchot R, Platel R, and Ridet J-M (1976). Brain-body weight relationships in Selachii. Copeia 1976, 305–309. [Google Scholar]
- 155.Yopak KE (2012). Neuroecology of cartilaginous fishes: the functional implications of brain scaling. J. Fish Biol 80, 1968–2023. [DOI] [PubMed] [Google Scholar]
- 156.Northcutt RG (1977). Elasmobranch central nervous system organization and its possible evolutionary significance. Am. Zool 17, 411–429. [Google Scholar]
- 157.Schluessel V (2015). Who would have thought that ‘jaws’ also has brains? Cognitive functions in elasmobranchs. Anim. Cogn 18, 19–37. [DOI] [PubMed] [Google Scholar]
- 158.Kuba MJ, Byrne RA, and Burghardt GM (2010). A new method for studying problem solving and tool use in stingrays (Potamotrygon castexi). Anim. Cogn 13, 507–513. [DOI] [PubMed] [Google Scholar]
- 159.Thonhauser KE, Gutnick T, Byrne RA, Kral K, Burghardt GM, and Kuba MJ (2013). Social learning in Cartilaginous fish (stingrays Potamotrygon falkneri). Anim. Cogn 16, 927–932. [DOI] [PubMed] [Google Scholar]
- 160.Guttridge TL, van Dijk S, Stamhuis EJ, Krause J, Gruber SH, and Brown C (2013). Social learning in juvenile lemon sharks, Negaprion brevirostris. Anim. Cogn 16, 55–64. [DOI] [PubMed] [Google Scholar]
- 161.Cohen DH, Duff TA, and Ebbesson SO (1973). Electrophysiological identification of a visual area in shark telencephalon. Science 182, 492–494. [DOI] [PubMed] [Google Scholar]
- 162.Northcutt RG (1995). The forebrain of gnathostomes: in search of a morphotype. Brain Behav. Evol 46, 275–318. [DOI] [PubMed] [Google Scholar]
- 163.Docampo-Seara A, Lagadec R, Mazan S, Rodriguez MA, Quintana-Urzainqui I, and Candal E (2018). Study of pallial neurogenesis in shark embryos and the evolutionary origin of the subventricular zone. Brain Struct. Funct 223, 3593–3612. [DOI] [PubMed] [Google Scholar]
- 164.Gillis JA, Modrell MS, and Baker CV (2013). Developmental evidence for serial homology of the vertebrate jaw and gill arch skeleton. Nat. Commun 4, 1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Smith JJ, Kuraku S, Holt C, Sauka-Spengler T, Jiang N, Campbell MS, Yandell MD, Manousaki T, Meyer A, Bloom OE, et al. (2013). Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nat. Genet 45, 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Khonsari RH, Li B, Vernier P, Northcutt RG, and Janvier P (2009). Agnathan brain anatomy and craniate phylogeny. Acta Zool 90, 52–68. [Google Scholar]
- 167.Wicht H, and Northcutt RG (1992). The forebrain of the Pacific hagfish: a cladistic reconstruction of the ancestral craniate forebrain. Brain Behav. Evol 40, 25–64. [DOI] [PubMed] [Google Scholar]
- 168.Heimberg AM, Cowper-Sal·lari R, Sémon M, Donoghue PCJ, and Peterson KJ (2010). MicroRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proc. Natl. Acad. Sci. USA 107, 19379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Miyashita T, Coates MI, Farrar R, Larson P, Manning PL, Wogelius RA, Edwards NP, Anné J, Bergmann U, Palmer AR, et al. (2019). Hagfish from the cretaceous tethys sea and a reconciliation of the morphological–molecular conflict in early vertebrate phylogeny. Proc. Natl. Acad. Sci. USA 116, 2146–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shimeld SM, and Donoghue PCJ (2012). Evolutionary crossroads in developmental biology: cyclostomes (lamprey and hagfish). Development 139, 2091–2099. [DOI] [PubMed] [Google Scholar]
- 171.Wicht H, and Northcutt RG (1994). An immunohistochemical study of the telencephalon and the diencephalon in a Myxinoid jawless fish, the Pacific hagfish, Eptatretus stouti. Brain Behav. Evol 43, 140–161. [DOI] [PubMed] [Google Scholar]
- 172.Wicht H, and Northcutt RG (1998). Telencephalic connections in the Pacific hagfish (Eptatretus stouti), with special reference to the thalamopallial system. J. Comp. Neurol 395, 245–260. [DOI] [PubMed] [Google Scholar]
- 173.Pombal MA, Alvarez-Otero R, Perez-Fernandez J, Solveira C, and Megias M (2011). Development and organization of the lamprey telencephalon with special reference to the GABAergic system. Front. Neuroanat 5, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ocana FM, Suryanarayana SM, Saitoh K, Kardamakis AA, Capantini L, Robertson B, and Grillner S (2015). The lamprey pallium provides a blueprint of the mammalian motor projections from cortex. Curr. Biol 25, 413–423. [DOI] [PubMed] [Google Scholar]
- 175.Suryanarayana SM, Robertson B, Wallen P, and Grillner S (2017). The lamprey pallium provides a blueprint of the mammalian layered cortex. Curr. Biol 27, 3264–3277.e5. [DOI] [PubMed] [Google Scholar]
- 176.Naumann RK, Ondracek JM, Reiter S, Shein-Idelson M, Tosches MA, Yamawaki TM, and Laurent G (2015). The reptilian brain. Curr. Biol 25, R317–R321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Giassi AC, Ellis W, and Maler L (2012). Organization of the gymnotiform fish pallium in relation to learning and memory: iii. intrinsic connections. J. Comp. Neurol 520, 3369–3394. [DOI] [PubMed] [Google Scholar]
- 178.Kokoros JJ, and Northcutt RG (1977). Telencephalic efferents of the tiger salamander Ambystoma tigrinum tigrinum (Green). J. Comp. Neurol 173, 613–628. [DOI] [PubMed] [Google Scholar]
- 179.Suárez R, Paolino A, Fenlon LR, Morcom LR, Kozulin P, Kurniawan ND, and Richards LJ (2018). A pan-mammalian map of interhemispheric brain connections predates the evolution of the corpus callosum. Proc. Natl. Acad. Sci. USA 115, 9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pani AM, Mullarkey EE, Aronowicz J, Assimacopoulos S, Grove EA, and Lowe CJ (2012). Ancient deuterostome origins of vertebrate brain signalling centres. Nature 483, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hejnol A, and Lowe CJ (2015). Embracing the comparative approach: how robust phylogenies and broader developmental sampling impacts the understanding of nervous system evolution. Phil. Trans. R. Soc. B 370, 20150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Martin-Duran JM, Pang K, Borve A, Le HS, Furu A, Cannon JT, Jondelius U, and Hejnol A (2018). Convergent evolution of bilaterian nerve cords. Nature 553, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Putnam NH, Butts T, Ferrier DE, Furlong RF, Hellsten U, Kawashima T, Robinson-Rechavi M, Shoguchi E, Terry A, Yu JK, et al. (2008). The amphioxus genome and the evolution of the chordate karyotype. Nature 453, 1064–1071. [DOI] [PubMed] [Google Scholar]
- 184.Horie T, Shinki R, Ogura Y, Kusakabe TG, Satoh N, and Sasakura Y (2011). Ependymal cells of chordate larvae are stem-like cells that form the adult nervous system. Nature 469, 525–528. [DOI] [PubMed] [Google Scholar]
- 185.Delsuc F, Brinkmann H, Chourrout D, and Philippe H (2006). Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439, 965–968. [DOI] [PubMed] [Google Scholar]
- 186.Northcutt RG (2005). The new head hypothesis revisited. J. Exp. Zool. (Mol. Dev. Evol.) 304B, 274–297. [DOI] [PubMed] [Google Scholar]
- 187.Holland LZ (2015). The origin and evolution of chordate nervous systems. Phil. Trans. R. Soc. B 370, 20150048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Albuixech-Crespo B, Lopez-Blanch L, Burguera D, Maeso I, Sanchez-Arrones L, Moreno-Bravo JA, Somorjai I, Pascual-Anaya J, Puelles E, Bovolenta P, et al. (2017). Molecular regionalization of the developing amphioxus neural tube challenges major partitions of the vertebrate brain. PLoS Biol 15, e2001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Benito Gutierrez E, Stemmer M, Rohr SD, Schumacher LN, Tang J, Marconi A, Jekely G, and Arendt D (2018). Patterning of a telencephalon-like region in the adult brain of amphioxus. bioRxiv, 307629. 10.1101/307629 [DOI] [Google Scholar]
- 190.Murakami Y, Ogasawara M, Sugahara F, Hirano S, Satoh N, and Kuratani S (2001). Identification and expression of the lamprey Pax6 gene: evolutionary origin of the segmented brain of vertebrates. Development 128, 3521–3531. [DOI] [PubMed] [Google Scholar]
- 191.Sugahara F, Aota S, Kuraku S, Murakami Y, Takio-Ogawa Y, Hirano S, and Kuratani S (2011). Involvement of Hedgehog and FGF signalling in the lamprey telencephalon: evolution of regionalization and dorsoventral patterning of the vertebrate forebrain. Development 138, 1217–1226. [DOI] [PubMed] [Google Scholar]
- 192.Oda I, and Saiga H (2001). Hremx, the ascidian homologue of ems/emx, is expressed in the anterior and posterior-lateral epidermis but not in the central nervous system during embryogenesis. Dev. Genes Evol 211, 291–298. [DOI] [PubMed] [Google Scholar]
- 193.Krubitzer L (2007). The magnificent compromise: cortical field evolution in mammals. Neuron 56, 201–208. [DOI] [PubMed] [Google Scholar]
- 194.Miller CT, Hale ME, Okano H, Okabe S, and Mitra P (2019). Comparative principles for next-generation neuroscience. Front. Behav. Neurosci 13, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pollen AA, Dobberfuhl AP, Scace J, Igulu MM, Renn SC, Shumway CA, and Hofmann HA (2007). Environmental complexity and social organization sculpt the brain in Lake Tanganyikan cichlid fish. Brain Behav. Evol 70, 21–39. [DOI] [PubMed] [Google Scholar]
- 196.Haberly LB (1983). Structure of the piriform cortex of the opossum. I. Description of neuron types with Golgi methods. J. Comp. Neurol 213, 163–187. [DOI] [PubMed] [Google Scholar]
- 197.Coleman J, and Clerici WJ (1981). Organization of thalamic projections to visual cortex in opossum. Brain Behav. Evol 18, 41–59. [DOI] [PubMed] [Google Scholar]
- 198.Northcutt RG, and Royce GJ (1975). Olfactory bulb projections in the bullfrog Rana catesbeiana. J. Morphol 145, 251–267. [DOI] [PubMed] [Google Scholar]
- 199.Northcutt RG, and Gonzalez A (2011). A reinterpretation of the cytoarchitectonics of the telencephalon of the comoran coelacanth. Front. Neuroanat 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]


