Abstract
Background and Objectives
Micro-RNAs (miRNAs) are critical for regulating the expression of genes in multiple neurodegenerative diseases, but miRNAs have not been investigated in spinocerebellar ataxia type 2 (SCA2). SCA2, a dominantly inherited progressive neurodegenerative polyglutamine (polyQ) disease, is caused by a CAG repeat expansion in the ataxin-2 (ATXN2) gene. In this study, we determined miRNA transcriptomes in SCA2-BAC-ATXN2[Q72] transgenic mice.
Methods
We assessed the expression of miRNAs in SCA2 transgenic mouse cerebella using the HiSeq Illumina sequencer. We used the miRNA target filter tool in Qiagen Ingenuity Pathway Analysis (IPA) to identify target genes of differentially expressed miRNAs (DEmiRs) within in the SCA2 mouse transcriptomes and then performed pathway analyses.
Results
Our analysis revealed significant changes in the expression levels of multiple miRNAs in mice with SCA2. We identified 81 DEmiRs in mice with SCA2, with 52 miRNAs upregulated and 29 miRNAs downregulated after onset of rotarod deficit. Subsequent IPA processing enabled us to establish connections between these DEmiRs and specific biological regulatory functions. Furthermore, by using the IPA miRNA target filter, we identified target genes of DEmiRs in the SCA2-BAC-ATXN2[Q72] transcriptome data set and demonstrated their significant impact on several biological functional and disease pathways.
Discussion
Our study establishes the role of both DEmiRs and their targets in SCA2 pathogenesis. By expressing mutant ATXN2 under the control of its endogenous regulatory elements in the SCA2-BAC-ATXN2[Q72] mouse model, we identified a set of DEmiRs that are shared across multiple neurodegenerative diseases including other SCAs, Alzheimer disease (AD), Parkinson disease (PD), and amyotrophic lateral sclerosis (ALS). There was a significant overlap of both DEmiRs and their targets of BAC-ATXN2[Q72] transcriptomes in dysregulated pathways that characterize SCA2. This observation also extended to dysregulated pathways in ALS, AD, and PD. DEmiRs identified in this study may represent therapeutic targets for neurodegeneration or lead to biomarkers for characterizing various neurodegenerative diseases.
Introduction
SCA2 is a progressive neurodegenerative polyglutamine (polyQ) disease caused by a CAG repeat expansion (≥33 CAG repeats) in the ataxin-2 (ATXN2) gene.1-3 The SCA2 phenotype includes ataxia of gait and appendicular movements as well as slow saccadic eye movements. Degeneration of cerebellar Purkinje cells (PCs) and other neuronal structures including the pons are prominent.4 Earlier age at onset and greater disease severity are associated with longer CAG repeats.4,5 Meiotic instability results in anticipation of age at disease onset.
Beyond SCA2, ATXN2 intermediate CAG repeat expansions (30–33 CAG repeats) are associated with amyotrophic lateral sclerosis (ALS).6-8 Presence of these intermediate ATXN2 risk alleles also increase cytoplasmic protein aggregates and motor neuron dysfunction and death in patients with C9ORF72 ALS.9,10 Frontotemporal dementia phenotypes in patients with C9ORF72 ALS are modified by intermediated expansions in ATXN2.9,10 Reduction of wild-type ATXN2 in TDP-43 ALS mice by genetic interaction or by treatment with an ATXN2 antisense oligonucleotide (ASO) significantly improved mouse survival.11 We have also shown that lowering the overall expression of ATXN2 in SCA2 mouse models by ATXN2 ASO improved motor, molecular, and neurophysiologic phenotypes.12 These findings suggest ATXN2 is a potential target for treating ALS and SCA2.
Interactions between ATXN2 and ALS-linked TDP-437 led us to identify other potential therapeutic targets by purifying the ATXN2 protein complex. Using immunoprecipitation and mass spectrometry, we identified Staufen1 (STAU1), a double-stranded RNA–binding protein and stress granule component, as an ATXN2 interactor.13 We showed that STAU1 was overabundant in human spinal cord with ALS-C9ORF72 mutation and sporadic ALS and multiple in vitro and in vivo models of neurodegenerative diseases (NDDs) accompanied by abnormal autophagy markers and markers of the unfolded protein response.13-16 Reducing STAU1 levels attenuated in vitro cellular phenotypes, restored SCA2 mouse cerebellar molecular phenotypes and improved their motor behavior, and normalized mTOR activity and autophagy readouts in SCA2 and ALS-TDP43 mice.13-16 These studies suggest that targeting STAU1 may be an effective approach to treating NDDs.
The generation of transgenic mouse models have helped to elucidate the role of ATXN2 in neurodegeneration and in testing experimental therapeutics for SCA2.17-20 We, along with others, have shown changes in cerebellar transcriptomes in SCA2 and SCA1 mouse models, including BAC-ATXN2[Q72], Pcp2-ATXN2[Q127], and ATXN1-Q82 models.17,19,21 In addition, we investigated the modification of gene expression in spinal cord following treatment of SCA2-BAC-ATXN2[Q72] mice with an ATXN2 ASO.20 Differentially expressed genes in SCA2-BAC-ATXN2[Q72] mice demonstrated associations with multiple interconnected pathways, including fatty acid and cholesterol biosynthesis, the complement system, innate immunity, and lysosome/phagosome pathways.20 This supports the idea that lowering ATXN2 expression would be well tolerated with few off-target effects or inflammation.20 All mouse models have shown robust differentially expressed genes (DEGs), suggesting that reliable and meaningful expression changes occur in 3 independent mouse models.
Mutations in neurodegenerative disease genes induce dysregulation of microRNAs (miRNAs or miRs) in the brain during the progression of NDDs including ALS—suggesting miRNAs may be one of these contributing factors.22,23 Similar to observations in other NDDs, human SCA1 brains show changes in steady-state levels of miRNAs, and disrupted miRNA biogenesis in Purkinje neurons can lead to cerebellar degeneration.24-26 These substantial alterations in miRNA expression in NDDs including ALS and SCA1 highlight the significant role of miRNAs in the pathogenesis of disease. However, the investigation of miRNAs in SCA2 remains limited, unlike in other spinocerebellar ataxias.
In this study, we analyzed RNA sequencing data to profile global miRNA expression in the cerebellum of BAC-ATXN2[Q72] transgenic mice17 at 16 weeks of age. Our analysis revealed significant changes in the expression levels of multiple miRNAs in BAC-ATXN2[Q72] mice. Ingenuity Pathway analyses (IPA) identified relevant biological functions defined by the DEmiRs. Furthermore, we demonstrated significant impacts of the DEmiRs on their target genes in BAC-ATXN2[Q72] mice on several biological functional and disease pathways. Our results reveal novel molecular mechanisms underlying the pathogenesis of SCA2 and suggest that DEmiRNAs could potentially offer new therapeutic targets for treating SCA2 and other NDDs.
Methods
Mice with SCA2
BAC-ATXN2[Q22], BAC-ATXN2[Q72] (BAC-Q72), and Pcp2-ATXN2[Q127] mice were previously described.17,18 BAC-ATXN2[Q22] or BAC-ATXN2[Q72] transgenic mice are engineered from a 169-kb BAC (RP11-798L5 BAC clone; Empire Genomics., USA) containing the entire 150 kb human ATXN2 gene (CAG22 or CAG72 repeats) containing all exons, introns, and 16 kb 5′-flanking and 3 kb 3-’ flanking genomic regions.17 Pcp2-ATXN2[Q127] transgenic mouse lines were generated using the pGEM construct containing the full human ataxin-2 cDNA encoding 127 glutamine repeats (Q127) under the control of the Pcp2 promoter.18 BAC-ATXN2[Q72] mice were maintained in an FVB/NJ background, and Pcp2-ATXN2[Q127] mice were maintained in a B6D2F1/J background.
Standard Protocol Approvals, Registrations, and Patient Consents
All mice were bred and maintained under standard conditions in accordance with NIH guidelines and adhered to an approved University of Utah institutional animal care and use committee protocol (IACUC).
miRNA Sequencing
Cerebellar tissues from 16-week-old wild-type (WT) and transgenic BAC-ATXN2[Q72]17 littermate mice (N = 4/group) were used for miRNA sequence analyses. Total RNA was isolated using miRNeasy Mini Kit (Qiagen Inc., USA) according to the manufacturer's protocol. RNA quality was determined using the Bioanalyzer 2100 Pico Chip (Agilent). Samples with an RNA integrity number (RIN) > 8 were used for microRNA library construction. The construction of small RNA sequencing libraries was performed using the NEBNext Multiplex Small RNA Library Prep Set for Illumina (New England Biolabs [NEB], USA; Cat #E7300S/L) according to the manufacturer's protocol. After denaturation, total RNA (1 μg) 3′ and 5′ RNA adapters were sequentially ligated to appropriately modified ends of RNA molecules. Using an RNA primer complementary to the 3′-adapter sequence and Protoscript II Reverse Transcriptase, adapter-ligated RNA was reverse transcribed and adapter sequences were extended with index tags by 12 PCR cycles. The PCR-amplified library was resolved on a 6% 25 Novex TBE PAGE gel (Invitrogen, USA; Cat# EC6265BOX), and the size ranges of adapter-modified small RNA cDNA were excised and purified using the Monarch PCR and DNA kit. Small RNA library molecules were eluted by gel filtration column (Sigma Aldrich, USA; Cat# CLS8162) and eluted fractions were precipitated with sodium acetate and ethanol. Small RNA library pellet was resuspended with TE buffer. To define the size distribution of the sequencing library, an Agilent 2200 Tape Station using a high-sensitivity D1K (Cat# 5067-5363 and 5067-5364) assay was used. Libraries were adjusted to a concentration of 5 nM and then loaded on a HiSeq Illumina sequencer.
RNA Sequence for BAC-ATXN2[Q72] and Pcp2-ATXN2[Q127] Mice
Cerebellar tissues from 8-week-old WT and transgenic BAC-ATXN2[Q72] littermate mice (N = 4/group) and 6-week-old Pcp2-ATXN2[Q127] and wild-type littermates (N = 16/group) were used for RNA sequence analyses. RNA sequencing of BAC-ATXN2[Q72] and Pcp2-ATXN2[Q127] mice was previously described.17 The previously published list of DEGs for both mouse models17 was used in this study.
miRNA Expression Analyses by Quantitative RT-PCR
Total RNA was extracted from mouse cerebella using miRNeasy Mini Kit (Qiagen Inc., USA) according to the manufacturer's protocol. DNAse I treated RNAs were used to synthesize cDNA using Taqman Advanced miRNA cDNA Synthesis kit (ThermoFisher; cat# A28007). Quantitative RT-PCR was performed in QuantStudio 12K (Life Technologies, Inc., USA) at University of Utah core facilities. The TaqMan assay kits include mmu-miR-455-5p (Assay ID: mmu481173_mir), mmu-miR-217-5p (Assay ID: mmu480999_mir), let-7e-5P (Assay ID: 478579_mir), mmu-miR-380-5p (Assay ID: mmu481148_mir), mmu-miR-487b-3p (Assay ID: mmu481184_mir), and mmu-miR-298-5p (Assay ID: mmu481673_mir), obtained from ThermoFisher Scientific, USA. For normalization of the qPCR, the miRNA cDNA was synthesized by TaqMan™ MicroRNA Reverse Transcription Kit (Cat #4366596; ThermoFisher Scientific, USA) and used to measure U6 snRNA levels (Assay ID: 001973).
miRNA Functional Analysis
Ingenuity Pathway Analysis (IPA) was performed using the IPA software package from Qiagen, accessible at the IPA Ingenuity website.27 In this study, we used miRNA target filter and core analysis tools in IPA software package to identify target genes of DEmiRs within in the SCA2 mouse transcriptomes and then performed pathway analyses.
Statistical Analysis
Statistical analyses were conducted using GraphPad Prism 9 software (GraphPad Software Inc., La Jolla, CA). For qRT-PCR analyses, 3–4 animals per group and for comparisons, unpaired t tests were used to establish statistical significance. The levels of significance are indicated as follows: nonsignificant (ns): p > 0.05, *: p ≤ 0.05, **: p ≤ 0.01, and ***: p ≤ 0.001, mean ± standard deviations are shown throughout unless otherwise specified.
Data Availability
Data generated and/or analyzed during the study are available from the corresponding author on request.
Results
Identification of Differentially Expressed miRNAs in BAC-ATXN2[Q72] Mice
We previously analyzed cerebellar and spinal cord transcriptomes of 2 SCA2 mouse models with a focus on steady-state changes in mRNAs.17,19,20 In this study, our focus has shifted to assessing the changes of miRNA expression in cerebellar tissues of WT and transgenic BAC-ATXN2[Q72] mice at 16 weeks of age, a time point when mice are symptomatic. This time point was chosen because BAC-ATXN2[Q72] mice developed progressive motor deficits beginning at 16 weeks of age.17 Small RNA libraries were constructed from 4 biological replicates for each group and subsequently sequenced on an Illumina HiSeq platform. An average of 29.0 million reads was generated from the 8 libraries, with a range of 26–32 million reads. The reads were aligned to the mm10 mouse genome using NovoAlign. Low-quality reads, including those with zero counts (708 miRs) or fewer than 10 reads (675 miRs), were removed from the raw data.
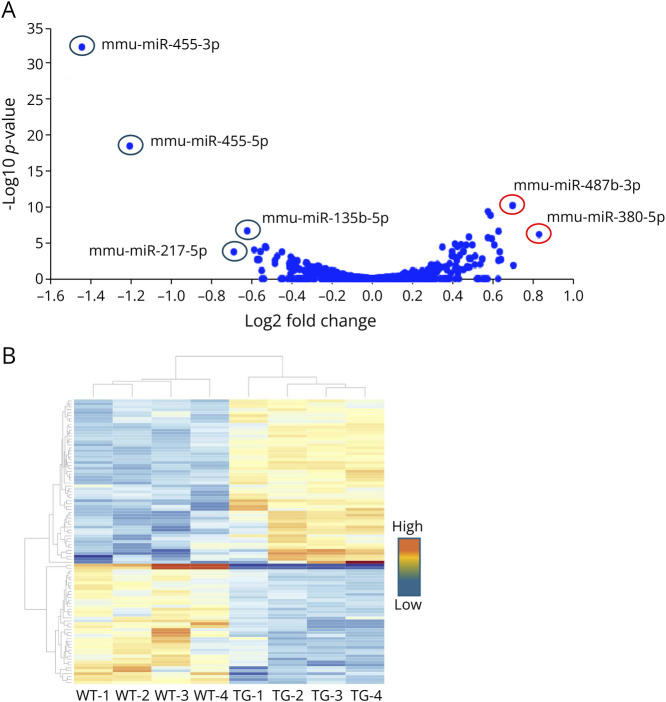
After normalizing the raw data, we identified DEmiRs between the 2 groups. A volcano plot illustrates several upregulated and downregulated DEmiRs (Figure 1A). We also generated a heat map based on miRNA expression across the samples, and cluster analysis showed that the DEmiRs were well clustered (Figure 1B). The dendrogram at the top (columns) shows the clustering of miRNAs in samples (wildtype and transgene), with the distance between 2 links reflecting the relationship between the samples. The dendrogram on the side (rows) shows clustering of DEmiRs across samples. A shorter distance between 2 links indicates a higher degree of similarity.
Figure 1. Sequences Determining the miRNA Expression Profiles in Wild-Type (WT) and SCA2-BAC-ATXN2[Q72] (TG) Mouse Cerebella (16 Weeks of Age; n = 4).
(A) miRNAs in the volcano plots represent the log2 ratio of 0.8-fold upregulated and −1.6-fold downregulated logarithmized miRNAs with statistical significance (p < 0.05). Upregulated miRNAs are represented by red circles, while downregulated miRNAs are represented by blue circles. (B) Heat map of differentially expressed miRNAs. The samples are shown in the columns, and the miRNAs are represented in rows. Red indicates high relative expression, and blue indicates low relative expression. miRNA = micro-RNA.
We defined a significantly DEmiR as one with a log2(fold change) (log2(FC)) ≥ |0.26| and adjusted p value (AdjP) < 0.05 in experimental tissues vs controls. With this definition, we identified 81 DEmiRs in BAC-ATXN2[Q72] mice compared with WT mice, with 52 miRNAs upregulated and 29 miRNAs downregulated (Table 1). Of note, miRNAs were detected as miRNA-5p and miRNA-3p. The -5p and -3p designations indicate the origin of the mature miRNA from either the 5′ arm (guide strand) or 3' arm (passenger strand/miRNA*) of the precursor miRNA molecule, respectively. The miRNA-5p/guide strand is usually more abundant and incorporated into RISC, exerting repression functions on target mRNAs. On the contrary, miRNA-3p is less abundant but may possess unique regulatory functions on target mRNAs.
Table 1.
Changes in miRNA Expression in BAC-ATXN2[Q72] Mouse Data Set
| Name | log2Fold change | padj | Name | log2Fold change | padj |
| mmu-miR-455-3p | −1.446 | 6.2E-33 | mmu-miR-154-5p | 0.363 | 3.52E-02 |
| mmu-miR-455-5p | −1.207 | 4.3E-19 | mmu-miR-1981-3p | 0.369 | 2.98E-02 |
| mmu-miR-217-5p | −0.690 | 2.7E-04 | mmu-miR-376a-5p | 0.384 | 1.18E-02 |
| mmu-miR-135b-5p | −0.623 | 3.1E-07 | mmu-miR-411-3p | 0.395 | 9.87E-04 |
| mmu-miR-212-5p | −0.588 | 1.4E-04 | mmu-miR-127-3p | 0.396 | 2.45E-04 |
| mmu-miR-212-3p | −0.573 | 3.1E-03 | mmu-miR-382-5p | 0.406 | 1.21E-03 |
| mmu-miR-676-5p | −0.564 | 3.1E-03 | mmu-miR-379-5p | 0.410 | 2.04E-05 |
| mmu-miR-129-2-3p | −0.557 | 2.4E-04 | mmu-miR-496a-3p | 0.413 | 2.14E-02 |
| mmu-miR-132-3p | −0.534 | 5.2E-05 | mmu-miR-17-5p | 0.417 | 3.51E-02 |
| mmu-miR-132-5p | −0.529 | 7.1E-05 | mmu-miR-485-3p | 0.419 | 4.01E-03 |
| mmu-miR-350-3p | −0.485 | 1.2E-02 | mmu-miR-434-3p | 0.422 | 5.16E-05 |
| mmu-miR-34a-5p | −0.485 | 4.2E-03 | mmu-miR-431-3p | 0.434 | 8.84E-04 |
| mmu-miR-125a-3p | −0.474 | 1.1E-03 | mmu-miR-136-5p | 0.440 | 2.58E-05 |
| mmu-let-7e-3p | −0.450 | 2.7E-04 | mmu-miR-411-5p | 0.451 | 2.24E-04 |
| mmu-miR-374b-5p | −0.413 | 9.5E-03 | mmu-miR-410-3p | 0.453 | 2.38E-04 |
| mmu-miR-129-1-3p | −0.412 | 1.4E-02 | mmu-miR-1983 | 0.466 | 2.91E-05 |
| mmu-miR-3081-3p | −0.412 | 3.5E-02 | mmu-miR-31-5p | 0.471 | 2.83E-02 |
| mmu-miR-30b-5p | −0.406 | 1.3E-03 | mmu-miR-300-5p | 0.473 | 3.77E-02 |
| mmu-let-7e-5p | −0.397 | 3.1E-03 | mmu-miR-668-3p | 0.480 | 2.11E-06 |
| mmu-miR-99b-3p | −0.392 | 9.3E-03 | mmu-miR-412-5p | 0.481 | 2.07E-03 |
| mmu-miR-374b-3p | −0.377 | 3.5E-02 | mmu-miR-1224-5p | 0.483 | 3.45E-02 |
| mmu-let-7j | −0.374 | 4.7E-03 | mmu-miR-381-3p | 0.490 | 2.16E-03 |
| mmu-miR-125a-5p | −0.331 | 8.9E-03 | mmu-miR-380-3p | 0.497 | 2.80E-05 |
| mmu-miR-192-5p | −0.324 | 1.1E-02 | mmu-miR-539-5p | 0.510 | 1.56E-02 |
| mmu-miR-320-3p | −0.311 | 2.7E-02 | mmu-miR-485-5p | 0.511 | 1.06E-03 |
| mmu-miR-378a-3p | −0.294 | 3.2E-02 | mmu-miR-3059-5p | 0.536 | 1.43E-02 |
| mmu-miR-99b-5p | −0.274 | 3.3E-02 | mmu-miR-543-5p | 0.546 | 2.59E-03 |
| mmu-miR-149-5p | −0.272 | 2.3E-02 | mmu-miR-323-3p | 0.574 | 2.76E-06 |
| mmu-miR-700-3p | −0.265 | 2.6E-02 | mmu-miR-127-5p | 0.574 | 5.91E-10 |
| mmu-miR-370-3p | 0.287 | 3.9E-02 | mmu-miR-370-5p | 0.587 | 2.11E-09 |
| mmu-miR-329-5p | 0.291 | 1.6E-02 | mmu-miR-434-5p | 0.593 | 3.94E-06 |
| mmu-miR-488-3p | 0.303 | 2.7E-02 | mmu-miR-369-5p | 0.606 | 2.91E-05 |
| mmu-miR-337-5p | 0.304 | 1.8E-02 | mmu-miR-412-3p | 0.625 | 2.47E-02 |
| mmu-miR-431-5p | 0.323 | 3.2E-02 | mmu-miR-137-3p | 0.627 | 4.14E-03 |
| mmu-miR-541-5p | 0.323 | 1.4E-02 | mmu-miR-666-5p | 0.627 | 3.48E-07 |
| mmu-miR-669c-5p | 0.326 | 3.5E-02 | mmu-miR-493-5p | 0.633 | 2.58E-04 |
| mmu-miR-92a-3p | 0.327 | 3.2E-02 | mmu-miR-298-5p | 0.639 | 3.08E-03 |
| mmu-miR-335-3p | 0.337 | 4.5E-02 | mmu-miR-487b-3p | 0.697 | 8.38E-11 |
| mmu-miR-376b-5p | 0.338 | 3.2E-02 | mmu-miR-200c-3p | 0.701 | 1.96E-02 |
| mmu-miR-300-3p | 0.348 | 6.0E-05 | mmu-miR-380-5p | 0.829 | 9.52E-07 |
| mmu-miR-409-5p | 0.361 | 3.2E-02 |
Validation of Key Differentially Expressed miRNAs
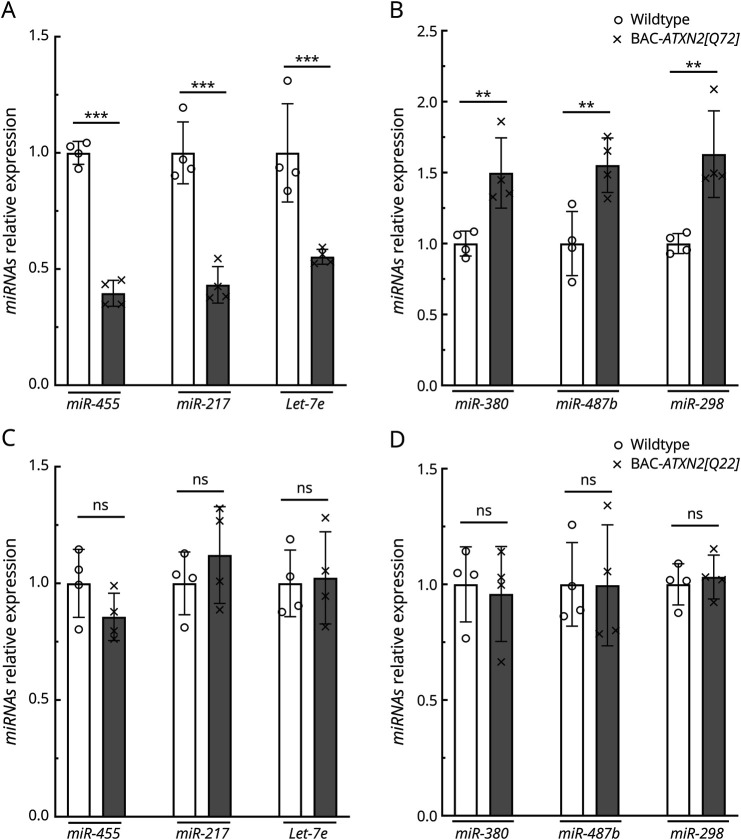
We validated relative expression levels of the top 6 DEmiRs with the greatest altered abundance (see Table 1) using quantitative reverse transcriptase PCR (qRT-PCR) in BAC-ATXN2[Q72]17 mouse cerebellar RNA (Figure 2, A and B). This confirmed the results obtained from miRNAseq, with only minor variation observed between qRT-PCR and miRNAseq. Validated DEmiRs include mmu-miR-455-5p, mmu-miR-217-5p, mmu-let-7e-5p, mmu-miR-380-5p, mmu-miR-487b-3p, and mmu-miR-298-5p. However, the levels of these top 6 dysregulated miRs remained unchanged in BAC-ATXN2[Q22] mice17 (16 weeks of age) when compared with WT mice (Figure 2, C and D). Notably, the BAC-ATXN2[Q22] line did not exhibit a motor or cellular phenotype.
Figure 2. Validation of Key DEmiRs.
(A, B) Validation of key top-ranked miR changes in BAC-ATXN2[Q72] transgenic mice using quantitative RT-PCR. qRT-PCR analyses of cerebellar RNAs from BAC-ATXN2[Q72] and WT littermates (16 wks of age; n = 4) show significant decreased (A) and increased (B) levels of miRs analyzed. (C, D) The top-ranked DEmiR levels are not altered in BAC-ATXN2[Q22] mice. qRT-PCR analyses of cerebellar RNAs from BAC-ATXN2[Q22] and WT littermates (16 wks of age; n = 4) showing no changes in levels of miRs analyzed. miR expression levels were normalized to U6 small nuclear RNA (U6 snRNA). DEmiRs tested are downregulated miRs: miR-455-5p, miR-217-5p, and Let-7e-5p, and upregulated miRs: miR-380-5p, miR-487b-3p, and miR-298-5p. Data are mean ± SD, ns = p > 0.05, **p < 0.01, ***p < 0.001, unpaired Student t tests. DEmiRs = differentially expressed miRNAs; miRNA = micro-RNA.
Analyses of SCA2-BAC-ATXN2[Q72] DEmiRs by IPA
To gather insights into miR-regulated pathways, we used IPA to perform pathway analyses on the DEmiRs (Table 1). IPA is a web-based bioinformatics tool that uses a comprehensive database of curated scientific literature (IPA Ingenuity website27 to analyze gene expression from RNA-Seq or miRNA expression data. This application enables the biologically meaningful comparison of results and enhances the understanding of connections among biological pathways, diseases, genes, and networks of upstream regulators.
Through core analyses, we observed that a significant number of DEmiRs were annotated for several significant pathways. The top disease pathways along with their associated DEmiRs are shown in Figure 3 and summarized in Table 2. eTable 1 summarizes a complete list of pathways and the respective DEmiRs. Perhaps not surprisingly, among the top pathways were neurologic disease with 31 miRNAs, organismal injury and abnormalities with 49 miRNAs, psychological disorders with 24 miRNAs, cancer with 31 miRNAs, and reproductive system disease with 27 miRNAs. Figure 3 provides a graphical representation of highly significant disease and disorders pathways targeted by DEmiRs. Several miRs contribute to multiple disease pathways. Among the DEmiRs were let-7a-5p, miR-100-5p, miR-1224-5p, miR-125b-5p, miR-127-3p, and miR-129-1-3p that are associated across top disease pathways, as described in greater detail in the Discussion section.
Figure 3. Disease and Biological Functional Pathways Targeted by DEmiRs of BAC-ATXN2[Q72] Mouse Data Set Using IPA Platform.
The vertical axis shows the names of the pathways indicated by bars in the chart. The horizontal axis represents the bar length corresponding to the -log(p value) (Fisher exact test p value) for the molecules assigned to each pathway. A higher -log(p value) indicates a pathway that is significantly targeted by DEmiRs. DEmiRs = differentially expressed miRNAs; IPA = Ingenuity Pathway Analysis.
Table 2.
Top Disease Pathways Along With Their Associated DEmiRs in BAC-ATXN2[Q72] Mouse Data Sets
| Category | p Value | Molecules |
| Neurologic disease | 5.7E-24-4.16E-02 | let-7a-5p,miR-100-5p,miR-1224-5p,miR-125a-3p,miR-125b-5p,miR-127-3p,miR-129-1-3p,miR-132-3p,miR-135a-5p,miR-149-5p,miR-17-5p,miR-200b-3p,miR-298-5p,miR-300-3p,miR-30c-5p,miR-320,miR-34a-5p,miR-369-5p,miR-374b-5p,miR-378a-3p,miR-381-3p,miR-382-5p,miR-431-5p,miR-434-3p,miR-455-3p,miR-455-5p,miR-485-3p,miR-485-5p,miR-487b-3p,miR-488-3p,miR-92a-3p |
| Organismal injury and abnormalities | 5.7E-24-4.84E-02 | let-7a-5p,miR-100-5p,miR-1224-5p,miR-125a-3p,miR-125b-5p,miR-127-3p,miR-129-1-3p,miR-132-3p,miR-135a-5p,miR-136-5p,miR-149-5p,miR-17-5p,miR-192-5p,miR-200b-3p,miR-202-3p,miR-217-5p,miR-298-5p,miR-300-3p,miR-30c-5p,miR-31-5p,miR-320b,miR-323-3p,miR-335-3p,miR-344d-3p,miR-34a-5p,miR-369-5p,miR-370-3p,miR-374b-5p,miR-378a-3p,miR-379-5p,miR-380-5p,miR-381-3p,miR-382-5p,miR-411-3p,miR-412-5p,miR-431-5p,miR-434-3p,miR-455-3p,miR-455-5p,miR-485-3p,miR-485-5p,miR-487b-3p,miR-488-3p,miR-493-5p,miR-503-3p,miR-539-5p,miR-668-3p,miR-92a-3p,miR-99a-3p |
| Psychological disorders | 1.35E-22-1.11E-02 | let-7a-5p,miR-100-5p,miR-1224-5p,miR-125a-3p,miR-125b-5p,miR-127-3p,miR-129-1-3p,miR-132-3p,miR-149-5p,miR-17-5p,miR-200b-3p,miR-300-3p,miR-30c-5p,miR-320b,miR-34a-5p,miR-374b-5p,miR-378a-3p,miR-381-3p,miR-382-5p,miR-434-3p,miR-455-3p,miR-487b-3p,miR-488-3p,miR-92a-3p |
| Cancer | 3.7E-13-4.84E-02 | let-7a-5p,miR-100-5p,miR-1224-5p,miR-125b-5p,miR-127-3p,miR-129-1-3p,miR-132-3p,miR-135a-5p,miR-136-5p,miR-149-5p,miR-17-5p,miR-192-5p,miR-200b-3p,miR-202-3p,miR-30c-5p,miR-31-5p,miR-320b,miR-323-3p,miR-335-3p,miR-34a-5p,miR-370-3p,miR-374b-5p,miR-378a-3p,miR-379-5p,miR-381-3p,miR-455-3p,miR-487b-3p,miR-493-5p,miR-503-3p,miR-92a-3p,miR-99a-3p |
| Reproductive system disease | 3.7E-13-3.67E-02 | let-7a-5p,miR-100-5p,miR-1224-5p,miR-125b-5p,miR-127-3p,miR-129-1-3p,miR-132-3p,miR-135a-5p,miR-149-5p,miR-17-5p,miR-192-5p,miR-200b-3p,miR-30c-5p,miR-31-5p,miR-320b,miR-335-3p,miR-34a-5p,miR-369-5p,miR-374b-5p,miR-378a-3p,miR-379-5p,miR-455-3p,miR-455-5p,miR-493-5p,miR-539-5p,miR-92a-3p,miR-99a-3p |
Analyses of DEmiRs Target Genes in the SCA2-BAC-ATXN2[Q72] Transcriptome Data Set
Extant mRNAseq analyses in our SCA2 mouse models provide a unique opportunity to examine whether predicted miRNA targets are indeed changed in abundance in vivo. In a previous study, we showed cerebellar transcriptional changes in BAC-ATXN2[Q72] mice at the onset of 8 weeks of age.17 This time point was chosen for BAC-ATXN2[Q72] mice because morphologic changes begin at 8 weeks.17 Therefore, we aimed to compare the predicted targets of miRs with the levels of mRNAs in the cerebellum of BAC-ATXN2[Q72] mice.
For this analysis, DEGs for BAC-ATXN2[Q72] were selected using a definition of Log2(FC) ≥ |0.3| and a false discovery rate (FDR) of ≥15 (BAC-ATXN2[Q72] transgenic vs wildtype) at 8 weeks of age.17 With these filtering criteria, we identified 728 DEGs, with 487 downregulated and 241 upregulated genes in BAC-ATXN2[Q72] mice (eTable 2). Of the 81 DEmiRs (Table 1), IPA incorporated information from 74 DEmiRs (26 downregulated and 48 upregulated). The analysis did not include the 7 DEmiRs. Of these, 4 DemiRs, miR-132-5p (downregulated), miR-300-3p, miR-485-3p, and miR-434-5p (upregulated), were excluded due to the unavailability of their targeting information. The remaining 3 DemiRs, miR-132-3p, miR-129-1-3p (down regulated), and miR-1983 (upregulated), share either the same miRBase accession number or alias.
Target analyses of DEmiRs within the 728 BAC-ATXN2[Q72] transcripts using the IPA platform identified a total of 365 (50.1%) targets (254 downregulated and 111 upregulated). IPA software uses TargetScan Mouse, TargetScan Human, Ingenuity Expert Findings, TarBase, and miRecords data sources to identify miRNA targets. The list of BAC-ATXN2[Q72] target genes is summarized in eTable 3. Within the BAC-ATXN2[Q72] data set, we observed that 26 downregulated miRNAs targeted 111 upregulated target genes, while 48 upregulated miRNAs targeted 254 downregulated target genes in BAC-ATXN2[Q72] mice. The top ranked differentially expressed target genes (based on Log2fold change) include the following downregulated genes: Stap2, Ca9, Rorc, Cabp2, Il20rb, Serpinb1, Rasal1, Igfbp5, Nab2, and Eomes. Top-ranked upregulated genes were Usp18, Ifi16, Rigi, Cldn14, Sfrp4, Sdk1, Kcnb2, Rnf213, Prokr2, and Pde3a (eTable 3).
Next, we assessed the correlation between the 6 validated dysregulated DEmiRs and their predicted DEG targets identified in BAC-ATXN2[Q72] mouse data set. Subsequent analyses revealed that of a total of 365 DEmiR target genes identified in the BAC-ATXN2[Q72] model, 99 genes (27.1%) (67 downregulated and 32 upregulated) exhibited pairs/correlations with 6 most altered miRs (miR-455, miR-217, let-7e, miR-380, miR-487b, and miR-298) (eTable 4). This observation demonstrated that the 6 most altered DEmiRs predicted targets overlap a significant number of DEGs within BAC-ATXN2[Q72] DEG target data sets. The top-ranked DEGs (based on Log2fold change) include the following downregulated genes: Cabp2, Il20rb, Th, Grm4, and Slc25a29. The top-ranked upregulated genes were Sdk1, Pla2g3, Irf9, Eif3j, and Cebpd (eTable 4).
Identification of Biological Functional and Disease and Disorder Pathways Associated With BAC-ATXN2[Q72] Target Genes
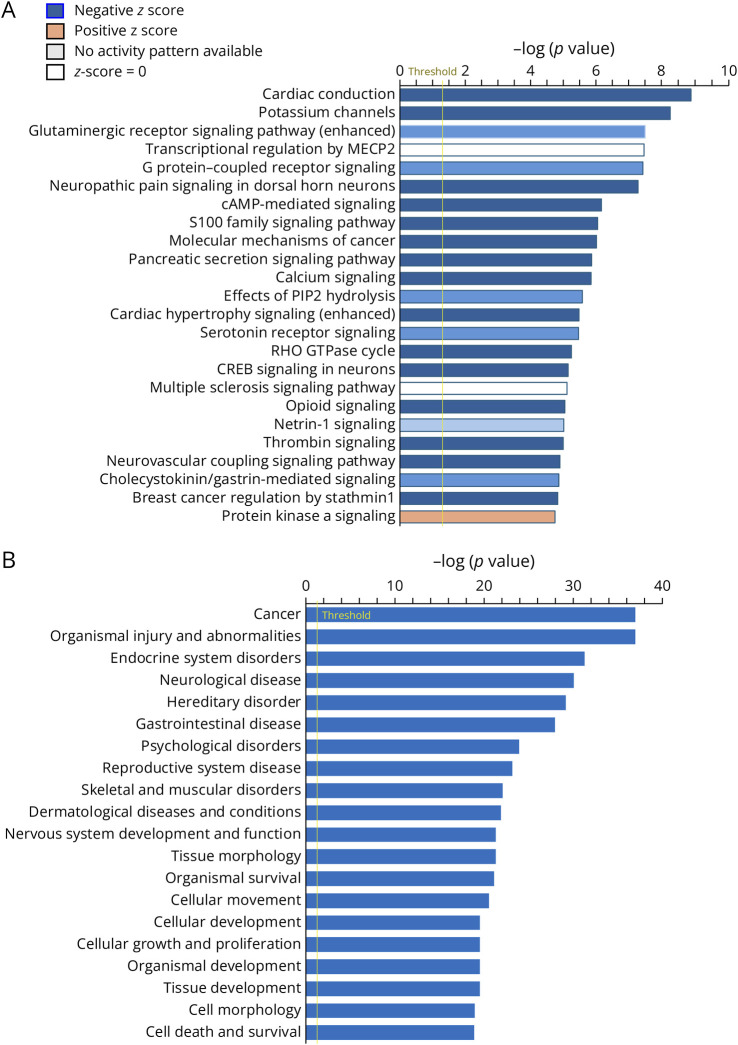
To understand the regulatory functions of differentially expressed miRNA targets in BAC-ATXN2[Q72] mice, we used the IPA platform to perform core analyses on the 365 target genes. Our analysis revealed that several cellular and disease pathways were annotated for BAC-ATXN2[Q72] targets. The top canonical pathways along with their associated genes are summarized in Table 3, and the complete list of pathways and their associated genes are summarized in eTable 5. The top canonical pathways for differentially expressed BAC-ATXN2[Q72] targets included cardiac conduction, potassium channels, glutaminergic receptor signaling pathway (enhanced), transcriptional regulation by MECP2, G protein–coupled receptor signaling, and neuropathic pain signaling in dorsal horn neurons. The complete list of diseases and biological functional pathways along with their associated genes are summarized in eTable 6. The leading disease pathways for differentially expressed BAC-ATXN2[Q72] targets included cancer, organismal injury and abnormalities, endocrine system disorders, neurologic disease, and hereditary disorder. Furthermore, Figure 4, A and B provides a graphical representation, illustrating the highly significant association of DEmiRs target genes across multiple canonical and disease pathways.
Table 3.
Top Pathways Along With Their Associated Genes in BAC-ATXN2[Q72] Mouse Data Sets
| Ingenuity canonical pathways | -log(p value) | z-score | Target molecules in BAC-ATXN2[Q72] mice |
| Cardiac conduction | 8.9 | −2.138 | ATP1A1, ATP1B2, ATP2B4, CAMK2A, FGF11, FXYD6, ITPR2, KCNIP1, KCNIP4, KCNK10, KCNK13, KCNK2, NPR1, SCN3B, SLC8A1 |
| Potassium channels | 8.27 | −1.941 | GABBR1, GABBR2, GNG12, KCNA6, KCNB2, KCNC1, KCNC3, KCNG4, KCNJ9, KCNK10, KCNK13, KCNK2, KCNQ2 |
| Glutaminergic receptor signaling pathway (enhanced) | 7.5 | −1.091 | BDNF, CACNB3, CAMK2A, CAMK4, DGKZ, GABBR1, GABBR2, GRIA1, GRM2, GRM4, GRM5, ITPR2, KCNK10, KCNK2, MAPK12, PLA2G3, PLCB3, PRKCD, SCN3B, STX1B, TGFB3 |
| Transcriptional regulation by MECP2 | 7.47 | 0 | BDNF, CAMK2A, CAMK4, FKBP5, GAMT, GPRIN1, MEF2C, MOBP, RBFOX1, TRPC3 |
| G protein–coupled receptor signaling | 7.43 | −1.061 | ACKR1, ACKR4, ADGRB1, ADRB2, CAMK2A, CAMK4, CCND1, CNR1, CRHR1, DUSP1, FZD7, GABBR1, GABBR2, GALR1, GNG12, GPRC5C, GPRIN1, GRK3, GRK5, GRM2, GRM4, GRM5, HRH3, KCNQ2, MAP3K5, MAPK12, MEF2C, PDE3A, PDE8A, PLCB3, RGS16, VIPR1 |
| Neuropathic pain signaling in dorsal horn neurons | 7.28 | −2.309 | BDNF, CAMK1D, CAMK2A, CAMK4, GRIA1, GRM2, GRM4, GRM5, ITPR2, KCNQ2, PLCB3, PRKCD |
Figure 4. Biological Functional and Disease Pathways Targeted by DEmiRs Target Genes of BAC-ATXN2[Q72] Mouse Data Set.
(A, B) The vertical axis shows the names of the pathways indicated by bars in the chart. The horizontal axis represents the bar length corresponding to the -log(p value) (Fisher exact test p value) for the molecules assigned to each pathway. A higher -Log(p value) indicates a pathway that is significantly targeted by DEmiR target genes of BAC-ATXN2[Q72] mouse data set (upregulated and downregulated). (A) The orange, blue, and gray bars in the chart indicate predicted pathway activation, predicted inhibition, or predicted no activity by BAC-ATXN2[Q72] target DEGs (both the direction), denoted by z score. Positive Z score: pathway activation (orange), negative Z score: pathway inhibition (blue), and zero Z score: predicted no activity (gray). DEmiRs = differentially expressed miRNAs.
Comparing DEmiRs Target Genes of BAC-ATXN2[Q72] in Pcp2-ATXN2[Q127] Transcriptome Data Sets
In contrast to the BAC-ATXN2[Q72] model,17 our Pcp2-ATXN2[Q127] mouse model18 targets the transgene to Purkinje cells. This provides the opportunity to analyze whether a subset of DEGs identified in Pcp2-ATXN2[Q127] mice17 is also a target for miRNAs identified in the BAC-ATXN2[Q72] mouse model. For these analyses, we used cerebellar transcriptome data from Pcp2-ATXN2[Q127] mice at 6 weeks of age because behavioral and morphological changes begin at this time point.18 Prior analyses had indicated that DEGs at the 6-week time point in Pcp2-ATXN2[Q127] mice showed partial overlap with BAC-ATXN2[Q72] DEGs at 8 weeks of age,17mainly in PC-specific transcripts.
We first tested whether the most altered abundance of DEmiRs in the BAC-ATXN2[Q72] mouse model was dysregulated in Pcp2-ATXN2[Q127] mice using qRT-PCR. We observed dysregulation of these DEmiRs in cerebellar RNAs of Pcp2-ATXN2[Q127] mice at 14 weeks of age (Figure 5, A and B). We did not perform a separate complete analysis of DEmiRs specific to the Pcp2-ATXN2[Q127] mouse model. For comparison analysis, DEGs for Pcp2-ATXN2[Q127] mice17 were selected with similar cutoffs as for the BAC-ATXN2[Q72] mouse data set (log2fold change ≥0.3 or ≤ −0.3 and FDR of ≥15). Applying these filtering criteria, we identified a total of 1742 DEGs in Pcp2-ATXN2[Q127] mice, with 1148 downregulated and 594 upregulated genes (eTable 7). We found that among the 365 DEmiR-target genes identified in the BAC-ATXN2[Q72] model, 105 genes overlapped with Pcp2-ATXN2[Q127] mouse transcriptome data sets (Figure 5C and eTable 8). Of these 105 overlapping genes, 93 genes exhibited the same direction of expression patterns in both models (79 down regulated and 14 upregulated). However, the remaining 12 genes showed opposite directions of expression between the 2 data sets. The nonoverlapping target genes specific to BAC-ATXN2[Q72] mice are summarized in eTable 9.
Figure 5. DEmiRs Identified in BAC-ATXN2[Q72] Are Dysregulated in Pcp2-ATXN2[Q127] Mice.
(A, B) Relative expression of top-ranked miRNAs in Pcp2-ATXN2[Q127] transgenic mice analyzed by quantitative RT-PCR. qRT-PCR analyses of cerebellar RNAs from Pcp2-ATXN2[Q127] and WT littermates (14 weeks of age; n = 3) show significant decreased (A) and increased (B) levels of miRNAs analyzed. miRNA expression levels were normalized to U6 small nuclear RNA (U6 snRNA). DEmiRs tested are downregulated miRNAs: miR-455-5p, miR-217-5p, and let-7e-5p, and upregulated miRNAs: miR-380-5p, miR-487b-3p, and miR-298-5p. Data are mean ± SD, ns = p > 0.05, ***p < 0.001, unpaired Student t tests. (C) BAC-ATXN2[Q72] target genes comparison in Pcp2-ATXN2[Q127] mice data set. Venn diagram of DEmiR target genes in BAC-ATXN2[Q72] and DEGs in Pcp2-ATXN2[Q127] mouse data sets. Of 365 target genes of BAC-ATXN2[Q72], 105 genes shared with Pcp2-ATXN2[Q127] mouse transcriptome data sets. DEGs for Pcp2-ATXN2[Q127] were selected with cutoff values: log2fold change ≥0.3 or ≤−0.3 and FDR of ≥15. DEmiRs = differentially expressed miRNAs; miRNA = micro-RNA.
Discussion
miRNAs represent a novel class of RNAs with the potential of playing a major role in the pathogenesis of neurodegenerative diseases. In this study, we integrated the analysis of miRNA and mRNA expression profiles to study the effect of mutant ATXN2 to identify potential miRNA networks and networks of target mRNAs. A previous study showed a small number of dysregulated miRs (16 miRs, all downregulated) in SCA2-Pcp2-ATXN2[Q127] mouse cerebellar tissues at 3 weeks of age.19 It is possible that the Pcp2-ATXN2[Q127] mouse may have been unresponsive to such an early stage of miRNA changes. These findings indicate that DEmiRs may be dynamically regulated and exhibit their functions at different stages of cerebellar pathologies induced by mutant ATXN2 expression. To gain understanding of the impact of miRNAs on SCA2 phenotypes and their progression, we performed miRNA sequence analyses on cerebellar tissues from an SCA2-BAC-ATXN2[Q72] mouse model expressing the full-length human ATXN2-Q72 protein at the symptomatic stage (16 weeks of age),17 a time point BAC-ATXN2[Q72] mice developed progressive motor deficits. Following analysis, we used the DEmiRs for pathway analyses using IPA. Furthermore, we used the DEmiR data set to identify target transcripts in SCA2-BAC-ATXN2[Q72] mouse data set and performed pathway analyses with these DEmiRs target genes using the IPA platform.
There are more than 1,000 miRNAs expressed in the human brain with an expression profile exhibiting regional and spatiotemporal variation. For instance, miR-10b, miR-145, miR-378c, and miR-217 are predominantly expressed in the prefrontal cortex, while miR-205 is expressed in the frontal cortex and miR-21, miR-224, and miR-373 in the substantia nigra.28 Mutations in ALS-associated proteins change stress granule dynamics and alter the localization and dynamics of the miRNA processing enzyme DICER and AGO2 protein, resulting in dysregulated expression of functional mature miRNAs.23 Motor neurons of patients with ALS have shown a significant number of dysregulated miRNAs with an overall downregulation.29,30 Similarly, Alzheimer disease (AD) tissues have showed dysregulated expression of miRNAs: miR-9, miR-124a, miR-125b, miR-128, miR-132, and miR-219.31 Overexpression of miR-219 ameliorated tau-induced toxicity in the Drosophila model.32 In Parkinson disease (PD), mutations in alpha-synuclein (SNCA) or overabundance cause cytoplasmic insoluble aggregates in Lewy bodies, and expression of miRNAs (miR-7 and miR-153) lower the expression of SNCA abundance and attenuate dopaminergic neuron degeneration in PD models.33,34 These substantial alterations in miRNA expression in NDDs including ALS, AD, and PD highlight the significant role of miRNAs in the pathogenesis of these diseases.
Like other NDDs, changes in miRNA expression are observed in multiple Poly Q diseases, including SCA1 and SCA3.24,25,35,36 Despite overlapping disease phenotypes among poly Q diseases, some miRNA changes are disease gene specific. For instance, expression of miR-19a, miR-101, miR-130a, and miR-144 inhibit ATXN1 translation via cooperative binding to the ATXN1 3′-UTR, and inhibition or reduction of these miRNAs expression leads to increased ATXN1 levels.35 Furthermore, miR-150 influences SCA1 pathogenesis by suppressing the expression of Rgs8 and VEGFA through interaction with their 3′UTR in cerebellar Purkinje neurons in an SCA1 mouse model.25 Of interest Rgs8 has also been identified as a highly interconnected or hub gene in SCA2 and SCA7.19,21,37
The 2 SCA disease models (SCA2 and SCA1) provide insight into shared transcriptome changes in neurodegeneration.17,19,21 In a previous study on an SCA1 mouse model, miRNA sequencing was performed on SCA1-ATXN1-Q82 mouse cerebellar tissues, resulting in the identification of 46 annotated miRNAs (12 downregulated and 34 upregulated).25 When comparing SCA2-BAC-ATXN2[Q72] and SCA1-ATXN1-Q82 mouse data sets, there was a modest overlap of 11 differentially expressed miRNAs. Of these, 5 shared miRNAs (miR-376b, miR-376a, miR-335, miR-379, and miR-31) showed same direction (upregulation) of expression patterns in both data sets. However, the remaining 6 shared miRNAs (miR-100, miR-30b, miR-350, miR-129, miR-329, and miR-487b) displayed an opposite expression pattern.
Dysregulated miRNAs have also been identified in SCA3.36,38 Through interaction with the 3′-UTR of ATXN3 gene, miR-25, miR-9, miR-181a, and miR-494 reduce wild-type and mutant ATXN3 protein expression, thereby rescuing SCA3 neuronal cell death and mutant ATXN3 protein aggregation.36,38,39 Simultaneous profiling of DEmiRs and DEGs was conducted in ex vivo SCA6 models with α1ACT overexpression, a CACNA1A transcription factor.40 Among findings was that SCA6 phenotypes were modified by miRNAs that regulate α1ACT.
miRNAs are often clustered in genomes, and multiple miRNAs are produced from the same primary transcript.41 When 2 or more miRNAs are located within a short physical distance (less than 10 kb), they are designated as a miRNA gene cluster.41 These clusters exhibit coexpression and target multiple mRNA transcripts and coordinate the regulation of related cellular pathways.42,43 Nine clusters with 2 downregulated and 7 upregulated miRs were identified in our study. The downregulated clusters contain miR-212/132 and miR-125a/let-7e/99b, while the upregulated clusters include miR-431/127/434/136, miR-412/409/369/410/541/412, miR-411/379/380, miR-485/382/668, miR-154/496a, miR-543/666, and miR-493/337. In addition, pathway analysis unveiled that these miRNAs collectively regulate multiple steps within a pathway, implying intricate control over entire pathways.
We used the IPA platform to analyze cerebellar DEmiRs in BAC-ATXN2[Q72] mice to identify potential associations of DEmiRs with cellular pathways. While no connections with canonical pathways were established through this analysis, the DEmiRs exhibited connections with multiple disease pathways (Figure 3 and Table 2). The neurologic disease pathway (31 molecules) emerged as the leading category followed by organismal injury and abnormalities (49 molecules), psychological disorders (24 molecules), cancer (31 molecules), and reproductive system disease (27 molecules). Some members of neurologic disease pathway include let-7a, miR-100, miR-1224, miR-125a, miR-127, miR-129-1, miR-132, miR-149, miR-320, miR-378a, miR-431, miR-455, miR-485, and miR-487b. These miRNAs were also found to be dysregulated in multiple neurodegenerative diseases.44 There is a significant overlap of DEmiRs among the top disease pathways (Table 2). For instance, the top 6 dysregulated miRs (miR-455, miR-217, let-7e, miR-380, miR-487b, and miR-298) are also annotated in the top disease pathways. In addition to that, several other dysregulated miRs, such as miR-100, miR-1224, miR-125a, miR-125b, miR-127, and miR-129-1 are found to be common among these pathways (Table 2). Let-7 is the most abundant miRNA in the mammalian brain and regulates stability of target mRNAs and their localization in neurites.45 It is worth noting that let-7 is downregulated in many cancer types and acts as a tumor suppressor. Reinstating the normal expression of let-7 has been found to impede the growth of cancer.46 It was not unexpected for us to observe a set of DEmiRs in our data set that are associated with cancer pathways.
Several computational algorithms are in use to predict miRNA-mRNA binding.47-52 In an elegant study, miR-walk 2.0 was used to predict miRNA targets in a model of α1ACT overexpression during cerebellar development, and these miRNA targets were subsequently verified using cerebellar transcriptomes.40 This research revealed that α1ACT coordinated miRNA-mRNA networks that play important functions in neonatal mouse cerebellar development.40 Thirty-one DEmiRs and 2,365 DEmiR/DEGs pairs were modulated by α1ACT overexpression, many of them in a multipaired fashion involved in the regulation of multiple developmental processes.
We used a similar strategy but applied it to uncover DEmiR/DEGs pairs in adult-onset cerebellar degeneration. The availability of deep RNAseq data sets in 2 SCA2 mouse models allowed us to empirically verify that the 81 DEmiRs (Table 1) in aggregate targeted 365 mRNAs (254 downregulated and 111 upregulated) in BAC-ATXN2[Q72] mouse data set (eTable 3), all of which showed significant changes in expression. The 48 upregulated miRNAs targeted 254 mRNAs resulting in reduced expression, and 26 downregulated miRNAs were associated with 111 mRNA targets with increased expression. We cannot exclude that other mechanisms affect steady-state levels of mRNAs such as transcriptional changes or mRNA decay, for example, STAU1-mediated decay.13
Pathway analysis revealed several canonical and disease pathways associated with neurodegeneration (Figure 4, Table 3, and eTables 5–6). The disease pathways are cancer, organismal injury and abnormalities, endocrine system disorders, neurologic disease, and hereditary disorder. The canonical pathways include cardiac conduction, potassium channels, glutaminergic receptor signaling pathway (enhanced), transcriptional regulation by MECP2, G protein–coupled receptor signaling, and neuropathic pain signaling in dorsal horn neurons. Considering that glutamate toxicity is a characteristic feature of neurodegenerations including ALS, we found dysregulation of various glutamate transporters and receptors that were associated with these pathways. These genes include Adrb2, Camk2a, Camk4, Gria1, Gabbr1, Gabbr2, Grm1, Grm2, Grm4, Grm5, Gprc5c, Gprin1, Mapk12, Mef2c, and Rgs16. Our previous studies have shown a close correlation between the fold changes of DEGs in both BAC-ATXN2[Q72] and Pcp2-ATXN2[Q127] transcriptome data sets and the observed experimental qRT-PCR data.17 Consistent with this, it is reasonable to assume that RNA sequence–derived fold changes of DEGs associated with canonical or disease pathways will align with experimental qRT-PCR data.
The regulator of G protein–signaling 8 gene (Rgs8) was abnormally reduced in both SCA2 data sets17,19 consistent with our previous findings of enhanced GRM1/mGluR1–mediated excitatory postsynaptic currents and increased intracellular Ca++ in SCA2 Purkinje neurons.53 Furthermore, Rgs2, Rgs4, and Rgs16 were also found to be deregulated and associated with the G protein–coupled receptor signaling pathway. Some DEmiR target genes in BAC-SCA2-ATXN2[Q72] mice encoding small or circulating peptides or processed proteins merit investigation as biomarkers for ALS. For instance, DEmiRs (miR-380 and miR-487b) predicted target IL33 is highly downregulated in SCA2 mouse cerebellum,17 consistent with a study that found reduced Il33 in SCA2 mouse spinal cord transcriptomes20 and in the CSF from patients with ALS.54 Furthermore, our SCA2-BAC-ATXN2[Q72] mouse cerebellar target transcriptome data identified by DEmiRs in IPA reflect the multiple NDDs including ALS transcriptome data. For instance, these DEGs include Serpinb1, Trim25, Irf9, Uchl1, Aldoc, Gfap, Agt, Dao, Fgfr2, Fgfr2, and Gria1, and these are linked to the progression of ALS.20 Previous study has shown that Trim25, coding for an E3 ubiquitin ligase, is associated with AD and PD through a loss-of-function mechanism, suggesting its potential as a biomarker.55
In contrast to other polyQ proteins, ATXN2 may have a direct and general role in miR action. Following biogenesis, miRNAs are loaded into 2 distinct silencing complexes, Ago1-RISC and Ago2-RISC, to execute either translational repression or degradation of target mRNAs.56,57 The repression of target mRNAs by Ago1-RISC pathway is P-body protein, Gwaky (GW182) dependent, while Ago2-RISC pathway is GW182 independent.57 Atx2, the fly ortholog of ataxin-2, functions in long-term olfactory habituation along with the known miRNA pathway proteins, Ago1 and maternal expression at 31B (Me31B).58 Atx2 binds to the RNA regulatory proteins, polyA-binding protein (PABP) and Me31B, which are required for polyribosome assembly.59-61 Depletion of Atx2 leads to the upregulation of GW182-dependent or Ago1-dependent miRNA reporters, suggesting that Atx2 plays a direct role as a component of Ago1-GW182 RISC pathway.58
By expressing mutant ATXN2 under the control of its endogenous regulatory elements, we identified DEmiRs and their mRNA targets and described their involvement in pathways relevant to SCA2. These DEmiRs and targets are shared with multiple NDDs including AD, PD, ALS, and SCA1, suggesting a role for ATXN2 in the modulation of common neurodegeneration pathways. DEmiRs identified in this study may represent therapeutic targets for neurodegeneration or lead to biomarkers for characterizing various neurodegenerative diseases.
Acknowledgment
The authors thank Kaylee Esparza Dayton for mouse handling. The authors thank Chris Stubben, Cancer Bioinformatics Shared Resource, Huntsman Cancer Institute, and Salt Lake City, Utah, for miRNA sequence analyses. Research reported in this publication used the High-Throughput Genomics and Bioinformatic Analysis Shared Resource at Huntsman Cancer Institute at the University of Utah.
Glossary
- AD
Alzheimer disease
- ALS
amyotrophic lateral sclerosis
- ASO
antisense oligonucleotide
- ATXN2
ataxin-2
- DEG
differentially expressed gene
- DEmiRs
differentially expressed miRNAs
- IPA
Ingenuity Pathway Analysis
- miRNA
micro-RNA
- NDDs
neurodegenerative diseases
- PCs
Purkinje cells
- PD
Parkinson disease
- SCA2
spinocerebellar ataxia type 2
Appendix. Authors
| Name | Location | Contribution |
| Sharan Paul, PhD | Department of Neurology, University of Utah | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Warunee Dansithong, PhD | Department of Neurology, University of Utah | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Mandi Gandelman, PhD | Department of Neurology, University of Utah | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Karla P. Figueroa, MA | Department of Neurology, University of Utah | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Daniel R. Scoles, PhD, FAAN | Department of Neurology, University of Utah | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Stefan M. Pulst, MD, Dr. Med, FAAN | Department of Neurology, University of Utah | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
Study Funding
This work was supported by National Institutes of Neurologic Disorders and Stroke (NINDS) grants R37NS033123 and R56NS33123 to S.M.P., R01NS097903 to D.R.S., and U01NS103883 and R21NS103009 to D.R.S. and S.M.P. S.M.P. also received grant support from the Target ALS Foundation, and D.R.S. received grant support from the Harrington Discovery Institute. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NG for full disclosures.
References
- 1.Pulst SM, Nechiporuk A, Nechiporuk T, et al. . Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet. 1996;14(3):269-276. doi: 10.1038/ng1196-269 [DOI] [PubMed] [Google Scholar]
- 2.Sanpei K, Takano H, Igarashi S, et al. . Identification of the spinocerebellar ataxia type 2 gene using a direct identification of repeat expansion and cloning technique, DIRECT. Nat Genet. 1996;14(3):277-284. doi: 10.1038/ng1196-277 [DOI] [PubMed] [Google Scholar]
- 3.Imbert G, Saudou F, Yvert G, et al. . Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats. Nat Genet. 1996;14(3):285-291. doi: 10.1038/ng1196-285 [DOI] [PubMed] [Google Scholar]
- 4.Scoles DR, Pulst SM. Spinocerebellar ataxia type 2. Adv Exp Med Biol. 2018;1049:175-195. doi: 10.1007/978-3-319-71779-1_8 [DOI] [PubMed] [Google Scholar]
- 5.Fernandez M, McClain ME, Martinez RA, et al. . Late-onset SCA2: 33 CAG repeats are sufficient to cause disease. Neurology. 2000;55(4):569-572. doi: 10.1212/wnl.55.4.569 [DOI] [PubMed] [Google Scholar]
- 6.Neuenschwander AG, Thai KK, Figueroa KP, Pulst SM. Amyotrophic lateral sclerosis risk for spinocerebellar ataxia type 2 ATXN2 CAG repeat alleles: a metaanalysis. JAMA Neurol. 2014;71(12):1529-1534. doi: 10.1001/jamaneurol.2014.2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elden AC, Kim HJ, Hart MP, et al. . Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466(7310):1069-1075. doi: 10.1038/nature09320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sproviero W, Shatunov A, Stahl D, et al. . ATXN2 trinucleotide repeat length correlates with risk of ALS. Neurobiol Aging. 2017;51:178.e1-178.e9. doi: 10.1016/j.neurobiolaging.2016.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sellier C, Campanari ML, Julie Corbier C, et al. . Loss of C9ORF72 impairs autophagy and synergizes with polyQ Ataxin-2 to induce motor neuron dysfunction and cell death. EMBO J. 2016;35(12):1276-1297. doi: 10.15252/embj.201593350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farhan SMK, Gendron TF, Petrucelli L, Hegele RA, Strong MJ. OPTN p. Met468Arg and ATXN2 intermediate length polyQ extension in families with C9orf72 mediated amyotrophic lateral sclerosis and frontotemporal dementia. Am J Med Genet B Neuropsychiatr Genet. 2018;177(1):75-85. doi: 10.1002/ajmg.b.32606 [DOI] [PubMed] [Google Scholar]
- 11.Becker LA, Huang B, Bieri G, et al. . Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice. Nature. 2017;544(7650):367-371. doi: 10.1038/nature22038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scoles DR, Meera P, Schneider MD, et al. . Antisense oligonucleotide therapy for spinocerebellar ataxia type 2. Nature. 2017;544(7650):362-366. doi: 10.1038/nature22044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul S, Dansithong W, Figueroa KP, Scoles DR, Pulst SM. Staufen1 links RNA stress granules and autophagy in a model of neurodegeneration. Nat Commun. 2018;9(1):3648. doi: 10.1038/s41467-018-06041-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul S, Dansithong W, Figueroa KP, Gandelman M, Scoles DR, Pulst SM. Staufen1 in human neurodegeneration. Ann Neurol. 2021;89(6):1114-1128. doi: 10.1002/ana.26069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul S, Dansithong W, Gandelman M, et al. . Staufen impairs autophagy in neurodegeneration. Ann Neurol. 2023;93(2):398-416. doi: 10.1002/ana.26515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandelman M, Dansithong W, Figueroa KP, Paul S, Scoles DR, Pulst SM. Staufen 1 amplifies proapoptotic activation of the unfolded protein response. Cell Death Differ. 2020;27(10):2942-2951. doi: 10.1038/s41418-020-0553-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dansithong W, Paul S, Figueroa KP, et al. . Ataxin-2 regulates RGS8 translation in a new BAC-SCA2 transgenic mouse model. PLoS Genet. 2015;11(4):e1005182. doi: 10.1371/journal.pgen.1005182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen ST, Meera P, Otis TS, Pulst SM. Changes in Purkinje cell firing and gene expression precede behavioral pathology in a mouse model of SCA2. Hum Mol Genet. 2013;22(2):271-283. doi: 10.1093/hmg/dds427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pflieger LT, Dansithong W, Paul S, et al. . Gene co-expression network analysis for identifying modules and functionally enriched pathways in SCA2. Hum Mol Genet. 2017;26(16):3069-3080. doi: 10.1093/hmg/ddx191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scoles DR, Dansithong W, Pflieger LT, et al. . ALS-associated genes in SCA2 mouse spinal cord transcriptomes. Hum Mol Genet. 2020;29(10):1658-1672. doi: 10.1093/hmg/ddaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingram M, Wozniak EAL, Duvick L, et al. . Cerebellar transcriptome profiles of ATXN1 transgenic mice reveal SCA1 disease progression and protection pathways. Neuron. 2016;89(6):1194-1207. doi: 10.1016/j.neuron.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajgor D. Macro roles for microRNAs in neurodegenerative diseases. Noncoding RNA Res. 2018;3:154-159. doi: 10.1016/j.ncrna.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma S, Lu HC. microRNAs in neurodegeneration: current findings and potential impacts. J Alzheimers Dis Parkinsonism. 2018;8(1):420. doi: 10.4172/2161-0460.1000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Persengiev S, Kondova I, Otting N, Koeppen AH, Bontrop RE. Genome-wide analysis of miRNA expression reveals a potential role for miR-144 in brain aging and spinocerebellar ataxia pathogenesis. Neurobiol Aging. 2011;32(12):2316.e17-2316.e27. doi: 10.1016/j.neurobiolaging.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Lebron E, Liu G, Keiser M, Behlke MA, Davidson BL. Altered Purkinje cell miRNA expression and SCA1 pathogenesis. Neurobiol Dis. 2013;54:456-463. doi: 10.1016/j.nbd.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaefer A, O'Carroll D, Tan CL, et al. . Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med. 2007;204(7):1553-1558. doi: 10.1084/jem.20070823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qiagen Digital Insights. Accessed November 12, 2023. ingenuity.com.
- 28.Dong X, Cong S. The emerging role of microRNAs in polyglutamine diseases. Front Mol Neurosci. 2019;12:156. doi: 10.3389/fnmol.2019.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez B, Peplow PV. MicroRNA expression in animal models of amyotrophic lateral sclerosis and potential therapeutic approaches. Neural Regen Res. 2022;17(4):728-740. doi: 10.4103/1673-5374.322431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Figueroa-Romero C, Hur J, Lunn JS, et al. . Expression of microRNAs in human post-mortem amyotrophic lateral sclerosis spinal cords provides insight into disease mechanisms. Mol Cell Neurosci. 2016;71:34-45. doi: 10.1016/j.mcn.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maes OC, Chertkow HM, Wang E, Schipper HM. MicroRNA: implications for alzheimer disease and other human CNS disorders. Curr Genomics. 2009;10(3):154-168. doi: 10.2174/138920209788185252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santa-Maria I, Alaniz ME, Renwick N, et al. . Dysregulation of microRNA-219 promotes neurodegeneration through post-transcriptional regulation of tau. J Clin Invest. 2015;125(2):681-686. doi: 10.1172/JCI78421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Junn E, Lee KW, Jeong BS, Chan TW, Im JY, Mouradian MM. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci USA. 2009;106(31):13052-13057. doi: 10.1073/pnas.0906277106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doxakis E. Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J Biol Chem. 2010;285(17):12726-12734. doi: 10.1074/jbc.M109.086827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y, Samaco RC, Gatchel JR, Thaller C, Orr HT, Zoghbi HY. miR-19, miR-101 and miR-130 co-regulate ATXN1 levels to potentially modulate SCA1 pathogenesis. Nat Neurosci. 2008;11(10):1137-1139. doi: 10.1038/nn.2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Y, Huang F, Tang B, et al. . MicroRNA profiling in the serums of SCA3/MJD patients. Int J Neurosci. 2014;124(2):97-101. doi: 10.3109/00207454.2013.827679 [DOI] [PubMed] [Google Scholar]
- 37.Wu Q-W, Kapfhammer JP. Modulation of increased mGluR1 signaling by rgs8 protects purkinje cells from dendritic reduction and could be a common mechanism in diverse forms of spinocerebellar ataxia. Front Cell Dev Biol. 2020;8:569889. doi: 10.3389/fcell.2020.569889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carmona V, Cunha-Santos J, Onofre I, et al. . Unravelling endogenous microRNA system dysfunction as a new pathophysiological mechanism in Machado-Joseph disease. Mol Ther. 2017;25(4):1038-1055. doi: 10.1016/j.ymthe.2017.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang F, Zhang L, Long Z, et al. . miR-25 alleviates polyQ-mediated cytotoxicity by silencing ATXN3. FEBS Lett. 2014;588(24):4791-4798. doi: 10.1016/j.febslet.2014.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei C, Benzow K, Koob MD, Gomez CM, Du X. The transcription factor, α1ACT, acts through a MicroRNA network to regulate neurogenesis and cell death during neonatal cerebellar development. Cerebellum. 2023;22(4):651-662. doi: 10.1007/s12311-022-01431-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo L, Zhao Y, Zhang H, Yang S, Chen F. Integrated evolutionary analysis of human MiRNA gene clusters and families implicates evolutionary relationships. Gene. 2014;534(1):24-32. doi: 10.1016/j.gene.2013.10.037 [DOI] [PubMed] [Google Scholar]
- 42.Baskerville S, Bartel DP. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA. 2005;11(3):241-247. doi: 10.1261/rna.7240905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He L, Thomson JM, Hemann MT, et al. . A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828-833. doi: 10.1038/nature03552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noronha O, Mesarosovo L, Anink JJ, Iyer A, Aronica E, Mills JD. Differentially expressed miRNAs in age-related neurodegenerative diseases: a meta-analysis. Genes (Basel). 2022;13(6):1034. doi: 10.3390/genes13061034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendonsa S, von Kügelgen N, Dantsuji S, et al. . Massively parallel identification of mRNA localization elements in primary cortical neurons. Nat Neurosci. 2023;3:394-405. doi: 10.1038/s41593-022-01243-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barh D, Malhotra R, Ravi B, Sindhurani P. MicroRNA let-7: an emerging next-generation cancer therapeutic. Curr Oncol. 2010;17(1):70-80. doi: 10.3747/co.v17i1.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riolo G, Cantara S, Marzocchi C, Ricci C. miRNA targets: from prediction tools to experimental validation. Methods Protoc. 2020;4:1. doi: 10.3390/mps4010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krek A, Grun D, Poy MN, et al. . Combinatorial microRNA target predictions. Nat Genet. 2005;37(5):495-500. doi: 10.1038/ng1536 [DOI] [PubMed] [Google Scholar]
- 49.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15-20. doi: 10.1016/j.cell.2004.12.035 [DOI] [PubMed] [Google Scholar]
- 50.Krüger J, Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006;34:W451–W454. doi: 10.1093/nar/gkl243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thadani R, Tammi MT. MicroTar: predicting microRNA targets from RNA duplexes. BMC Bioinformatics. 2006;7(suppl 5):S20. doi: 10.1186/1471-2105-7-S5-S20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nat Genet. 2007;39(10):1278-1284. doi: 10.1038/ng2135 [DOI] [PubMed] [Google Scholar]
- 53.Meera P, Pulst SM, Otis T. A positive feedback loop linking enhanced mGluR function and basal calcium in spinocerebellar ataxia type 2. elife. 2017;6:e26377. doi: 10.7554/eLife.26377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin CY, Pf luger CM, Henderson RD, McCombe PA. Reduced levels of interleukin 33 and increased levels of soluble ST2 in subjects with amyotrophic lateral sclerosis. J Neuroimmunol. 2012;249(1-2):93-95. doi: 10.1016/j.jneuroim.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 55.Gómez-Tortosa E, Baradaran-Heravi Y, Dillen L, et al. . TRIM25 mutation (p.C168*), coding for an E3 ubiquitin ligase, is a cause of early-onset autosomal dominant dementia with amyloid load and parkinsonism. Alzheimers Dement. 2023;19(7):2805-2815. doi: 10.1002/alz.12913 [DOI] [PubMed] [Google Scholar]
- 56.Förstemann K, Horwich MD, Wee L, Tomari Y, Zamore PD. Drosophila microRNAs are sorted into functionally distinct argonaute complexes after production by dicer-1. Cell. 2007;130(2):287-297. doi: 10.1016/j.cell.2007.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iwasaki S, Kawamata T, Tomari Y. Drosophila argonaute1 and argonaute2 employ distinct mechanisms for translational repression. Mol Cell 2009;34(1):58-67. doi: 10.1016/j.molcel.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 58.McCann C, Holohan EE, Das S, et al. . The Ataxin-2 protein is required for microRNA function and synapse-specific long-term olfactory habituation. Proc Natl Acad Sci USA. 2011;108(36):E655-E662. doi: 10.1073/pnas.1107198108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nonhoff U, Ralser M, Welzel F, et al. . Ataxin-2 interacts with the DEAD/H-box RNA helicase DDX6 and interferes with P-bodies and stress granules. Mol Biol Cell. 2007;18(4):1385-1396. doi: 10.1091/mbc.e06-12-1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Satterfield TF, Pallanck LJ. Ataxin-2 and its Drosophila homolog, ATX2, physically assemble with polyribosomes. Hum Mol Genet. 2006;15(16):2523-2532. doi: 10.1093/hmg/ddl173 [DOI] [PubMed] [Google Scholar]
- 61.Ciosk R, DePalma M, Priess JR. ATX-2, the C. elegans ortholog of ataxin 2, functions in translational regulation in the germline. Development. 2004;131(19):4831-4841. doi: 10.1242/dev.01352 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated and/or analyzed during the study are available from the corresponding author on request.