Abstract
Efficiency of mosquito-borne disease transmission is dependent upon both the preference and fidelity of mosquitoes as they seek the blood of vertebrate hosts. While mosquitoes select their blood hosts through multi-modal integration of sensory cues, host-seeking is primarily an odor-guided behavior. Differences in mosquito responses to hosts and their odors have been demonstrated to have a genetic component, but the underlying genomic architecture of these responses has yet to be fully resolved. Here, we provide the first characterization of the genomic architecture of host preference in the polymorphic mosquito species, Culex pipiens. The species exists as two morphologically identical bioforms, each with distinct avian and mammalian host preferences. Cx. pipiens females with empirically measured host responses were prepared into reduced representation DNA libraries and sequenced to identify genomic regions associated with host preference. Multiple genomic regions associated with host preference were identified on all 3 Culex chromosomes, and these genomic regions contained clusters of chemosensory genes, as expected based on work in Anopheles gambiae complex mosquitoes and in Aedes aegypti. One odorant receptor and one odorant binding protein gene showed one-to-one orthologous relationships to differentially expressed genes in A. gambiae complex members with divergent host preferences. Overall, our work identifies a distinct set of odorant receptors and odorant binding proteins that may enable Cx. pipiens females to distinguish between their vertebrate blood host species, and opens avenues for future functional studies that could measure the unique contributions of each gene to host preference phenotypes.
Subject terms: Behavioural genetics, Evolutionary genetics
Introduction
Mosquito-borne disease affects 380–500 million people per year (Franklinos et al. 2019; James et al. 2018), and causes nearly 1 million deaths (James et al. 2018). Transmission of disease-causing pathogens and parasites occurs when female mosquitoes feed on vertebrate blood, a behavior required for reproduction in most species. Of the approximately 3500 species of mosquito, high human morbidity and mortality results from disease transmission by relatively few species from the genera Aedes, Anopheles, and Culex (Foster and Walker 2019; Reidenbach et al. 2009). In many cases, the life histories of these mosquitoes are closely linked to that of humans: they rest in human domiciles, use human-generated water sources for oviposition, mate in enclosed spaces, and readily accept humans as vertebrate blood hosts (Besansky et al. 1994; Crawford et al. 2017; Fonseca et al. 2004). As humans have altered the natural landscape in unprecedented ways, new niches and novel selection pressures have emerged, resulting in complex evolutionary interactions that have the potential to shape mosquito genomes (Asgharian et al. 2015; Crutzen 2006; Fouet et al. 2018; McDonnell and Hahs 2015; Moran and Alexander 2014; Otto 2018; Rose et al. 2020; Stone and Gross 2018). Given the disproportionate impact of mosquito blood acquisition on disease transmission to humans, understanding how selection has shaped the genomic architecture of mosquitoes with distinct host-seeking behaviors, and the architecture of the behavior itself, is of strong interest.
Preference for blood feeding on one vertebrate host over others may vary between or within mosquito species. For example, in the domesticated form of Aedes aegypti, preference for human or animal odors varies across sub-Saharan Africa (Christophers 1960; Powell et al. 2018; Rose et al. 2020). This variation is correlated with both environmental conditions (e.g., the intensity of the dry season), as well as human population density (Rose et al. 2020). In the Anopheles gambiae species complex, variation in host-seeking behavior is loosely correlated with the resting behavior of female mosquitoes (Coluzzi et al. 1979; White 1974). An. gambiae sensu stricto and An. arabiensis are both willing to exploit human domiciles as resting habitat, and show strong preferences for human hosts. A closely related species, An. quadriannulatus, shows relatively lower willingness to rest indoors and lacks specialized feeding on humans (Dekker and Takken 1998; Rinker et al. 2013). The consequence of this variation in host preference within and between species is modulation of mosquito host contact rates, which ultimately impacts efficiency of pathogen and parasite transmission between humans (Garrett-Jones 1964).
Culex pipiens is a cosmopolitan mosquito species and vector of multiple vertebrate pathogens and parasites (Barr 1967; Brugman et al. 2018; Farajollahi et al. 2011; Gargan et al. 1983). Across its global distribution, it exists as two behaviorally and physiologically divergent forms: true pipiens and molestus (Harbach 2012; Harbach et al. 1984). While both forms associate with humans, the molestus form is uniquely adapted to exploit below ground human-made structures for resting, mating, and breeding. In contrast, true pipiens mates and breeds above-ground. These distinct bioforms also show divergent host-seeking behaviors (Apperson et al. 2004; Fritz et al. 2015; Hamer et al. 2009; Noreuil and Fritz 2021; Spielman 2001). Above-ground form pipiens shows preference for avian hosts, while below-ground form molestus shows preference for mammalian and often human hosts (Farajollahi et al. 2011; Fonseca et al. 2004; Nelms et al. 2013). It is known that the distinct host preferences of these bioforms are genetically determined, but the underlying genetic architecture of the trait remains unclear (Fritz et al. 2015).
Multi-modal integration of visual and chemosensory cues is important for host detection, yet chemosensory cues are arguably the most important component of host specificity (Raji and DeGennaro 2017; Takken and Verhulst 2013). Odor detection begins when odorants enter pores in the sensilla that cover mosquito sensory appendages, traverse the aqueous sensillum lymph, and bind to odorant receptors (ORs) expressed on the dendrites of olfactory sensory neurons, thereby triggering an action potential. It is generally thought that odorant binding proteins (OBPs) bind and chaperone odorants to the site of their receptor (Guidobaldi et al. 2014; Hansson and Stensmyr 2011; Kaupp 2010; Leal 2013; Renou 2014; Sachse and Krieger 2011; Tegoni et al. 2004), although other roles for OBPs have been proposed (Sun et al. 2018). The importance of olfaction to host attraction and specificity has been demonstrated in both A. aegypti and A. coluzzii by creating mutant mosquitoes that lacked a functional OR co-receptor gene, orco (DeGennaro et al. 2013; Raji and DeGennaro 2017; Sun et al. 2020). Tuning ORs that bind volatile compounds form heteromeric dimers with this essential odorant receptor co-receptor and do not function in its absence (Larsson et al. 2004). For both mosquito species, orco mutants showed reduced attraction to human hosts when no CO2 was present (DeGennaro et al. 2013; Sun et al. 2020). Even in the presence of CO2, A. aegypti were unable to distinguish humans from other vertebrate host species (DeGennaro et al. 2013). Differences in OR expression and amino acid sequence are correlated with divergent host-seeking behaviors described for both A. gambiae complex species (Rinker et al. 2013), as well as the sylvatic and peri-domestic forms of A. aegypti (McBride et al. 2014). Moreover, Or4 has been shown to play an important role in the ability of host-seeking A. aegypti females to detect sulcatone, a chemical compound present in human odor (McBride et al. 2014). Although these studies demonstrate the importance of chemosensory genes to human-seeking behavior in Aedes and Anopheles vectors of public health importance, there is still limited knowledge about specific chemosensory genes and the extent of their role in Culex host-seeking behaviors.
Here, we compare host-seeking behavior and its genomic architecture in the two bioforms of Cx. pipiens, one of which has a life history closely tied to humans. Multi-day behavioral assays were used to estimate individual host preference (avian or human) in three populations of Cx. pipiens: 1 autogenous mammal-seeking population originally collected from a below-ground site, and two anautogenous populations collected from above-ground locations that typically seek avian hosts. We then used a reduced representation genomic sequencing approach to genotype individuals with measured responses to human and avian hosts. We ask what regions of the genome are associated with shifts in host preference, and whether these regions overlap with annotated OBPs and ORs, which are known to play a role in host detection. Identifying the regions associated with host preference in this polymorphic species will provide insight into the mechanisms by which selection may have shaped host-seeking behavior and specialized feeding on humans.
Methods
Mosquito populations
Three mosquito populations previously described in detail by Noreuil and Fritz (2021) were used in this study. A below-ground population (form molestus) of Cx. pipiens was collected in Calumet, IL, in 2009 (hereafter referred to as BG1). Two above-ground populations (form pipiens) of Cx. pipiens were collected in Evanston and Northfield, IL, (referred to as AG2 and AG3, respectively), in 2016. All three populations were maintained in the laboratory following Fritz et al. (2015, 2014) and Noreuil and Fritz (2021) and completed approximately 11 generations per year. Additional details about rearing can be found in the supplementary methods.
Multi-day behavioral assay
To estimate individual and population-level preferences, we used a behavioral testing arena and multi-day testing approach described in Fritz et al. (2015) and Noreuil and Fritz (2021). Mosquitoes were held and tested under L:D photoperiod of 16h:8h. BG1 females were approximately 3 weeks old, while AG2 and AG3 females were approximately 2 weeks old. This 1 week difference in age of AG and BG females is consistent with previous studies (Fritz et al. 2015; Noreuil and Fritz 2021) and accommodates the unique ability of BG1 females to produce eggs without a blood meal in their first gonotrophic cycle. It does not significantly influence host choice (Noreuil and Fritz 2021). For each trial, 5–10 female mosquitoes (from a single population) were simultaneously released into the testing arena and given the choice of a human hand or a 2-week-old White Leghorn domestic chicken (Gallus domesticus). Within the testing arena, hosts rested upon a raised circular platform (17 cm height, 12 cm diameter, and 0.5 cm high rim) with a 1 cm hole to allow flow of CO2 from 50 g of dry ice placed underneath the platform. During testing, the human host’s breath was piped away from the arena, allowing for a more even distribution of CO2, an important activator of host-seeking, throughout the arena (Dekker et al. 2001; Geier et al. 1999; Van Breugel et al. 2015). Each trial lasted for 15 min, or until all mosquitoes had landed on a host and tapped their labellum on the host’s skin. After host selection, but prior to blood feeding, mosquitoes were removed from the arena using a glass dram vial sealed with absorbent cotton. Mosquitoes that responded by landing on a host were re-tested the following two days, and thus each responding mosquito was tested a total of three times. Host position in the arena, and the hand that the human host used, were alternated daily. Phenotyped mosquitoes were stored at −80∘C until genetic analysis. Some BG1 and AG2 individuals tested by Noreuil and Fritz (2021) and held at −80∘C were also incorporated into our genetic analyses. Use of animal subjects for behavioral tests was approved by the University of Maryland Institutional Animal Use and Care Committee under protocol number 1094335.
Statistical analysis of mosquito behavior
A hierarchical Bayesian model implemented in the R package BayesSPref (version 1.0), was used to directly estimate population-level and individual-level host landing probabilities and obtain credible intervals around those estimates (Fordyce et al. 2011) (R version 3.6.3 R Core Team 2021). These host landing probabilities served as our quantitative measures of host preference. Individual counts (i.e., how many times a mosquito landed on a human or avian host) were modeled as P(counts ∣ individual preference). The probability of individual preference is modeled as P(individual preference ∣ population preference), where the prior probability of population preference is not specified, but instead estimated from the data. All parameters of interest were estimated using Markov chain Monte Carlo (MCMC). We ran each model for 15,000 MCMC steps, with a burn-in of 2000 steps. To ensure that a stable sampling distribution had been reached, we checked effective sample sizes and trace plots. Our analysis included data collected as part of this study (n mosquitoes = 74), and also incorporated data from a previously published paper by Noreuil and Fritz (2021) (n mosquitoes = 41, Supplementary Table S1).
GBS library preparation and sequencing
For our genotype-by-sequencing (GBS) library, individual mosquitoes were categorized as having either an avian or human preference if they landed on that specific host more than once, and at no time landed on the alternate host. We categorized individuals as “switchers” if they landed on both the human and avian hosts in our multi-day tests, but we did not distinguish between individuals based on the order of the host switch (i.e., human to avian, or avian to human). Because we included samples from Noreuil and Fritz (2021), behaviors of individuals from BG1 and AG2 were measured in response to one of two possible human hosts, whereas the behaviors of AG3 individuals used in this study were only measured in response to a single human host. In total, we prepared 115 responding individuals from the three laboratory populations into GBS libraries (Table 1).
Table 1.
Number of individuals per population, per host preference category, used in GBS library preparation.
| Host Preference | |||
|---|---|---|---|
| Colony Strain | Avian | Human | Switcher |
| BG1 | 2 | 36 | 10 |
| AG2 | 23 | 13 | 8 |
| AG3 | 16 | 3 | 4 |
Genomic DNA was isolated and purified from the head and thorax of individual mosquitoes using a QIAgen DNeasy Blood and Tissue 96-kit (QIAgen Inc., Valencia, CA, USA) following the manufacturer’s protocol with one minor modification. After tissue lysis, 5 μl of RNase was added to each sample. To ensure DNA concentration was above 5ng/μl, we dried samples using a Savant SpeedVac vacuum concentrator (Thermofisher Scientific Inc., Waltham, Massachusetts, USA) and re-hydrated each sample using 25μl of nuclease-free water. We generated GBS libraries following the protocol of Gompert et al. (2014) and Parchman et al. (2012). Briefly, we digested genomic DNA (6μl per sample) using two restriction enzymes: EcoRI and MseI (New England Biolabs, Ipswich, MA, USA). Double stranded DNA oligos with sequences overlapping restriction enzyme cut sites, and containing 8–10 bp unique barcodes and Illumina adapter sequences were ligated to fragments using T4 DNA ligase (New England Biolabs, Ipswich, MA, USA). We amplified fragments using two polymerase chain reactions (PCRs) with standard Illumina PCR primers (30 cycles with annealing for 30 s at 60∘C, and extension for 30 s at 72∘C followed by a final amplification with 2 min for annealing and 10 min for extension). Barcoded PCR products were pooled, and we size selected fragments between 300–400 bp using a BluePippin (Sage Science, Inc., Beverly, MA) at the North Carolina State University Genomic Sciences Laboratory (NCSU GSL). GBS libraries were sequenced using 1 lane of Illumina NovaSeq SP (100 bp single end reads) at the NCSU GSL.
Illumina read filtering, genome alignment, and variant calling
Illumina sequencing often includes PhiX control sequences, which we removed by aligning reads to the PhiX genome with BOWTIE 2 (version 1.2.2) (Langmead and Salzberg 2012). PhiX genome-aligned reads were excluded from further analysis. Remaining reads were demultiplexed using custom Perl scripts, then aligned to the 3 complete chromosomes from the Cx. quinquefasciatus genome (GCA_015732765.1) with BWA (version 0.7.17-r1188) and the MEM algorithm (Li 2013; Li and Durbin 2009). Cx. pipiens and Cx. quinquefasciatus are both members of the Cx. pipiens assemblage. While they are currently considered separate species (Harbach 2012), feasibility of mapping Cx. pipiens Illumina reads to the Cx. quinquefasciatus genome has already been demonstrated (Noreuil and Fritz 2021; Yurchenko et al. 2020). We ran BWA using a minimum seed length of 19, internal seed length of 28.5, and output alignments with a quality score of ⩽30. SAMtools (version 1.5) was used to compress, sort, and index the alignments (Li et al. 2009), and variants were called on the whole data set using BCFtools (version 1.7). We only called single nucleotide polymorphisms (SNPs) when the posterior probability that the nucleotide was invariant was ⩽0.05 (-p), and set the prior on SNPs to 0.001 (-P). Insertion deletion polymorphisms were excluded from our final data set. We further filtered SNP variants by requiring an average read depth of 2× the total number of individuals (2 × 115 = 230), sequence data for 85% of individuals, a minimum mapping quality of 30, and an allele frequency between 0.05 and 0.95. We removed any variants with absolute values for Wilcoxon rank sum tests of base quality, mapping quality, and read position above 3, 2.5, and 2, respectively (these values are similar to those used in other studies with similar sequencing approaches e.g., Chaturvedi et al. 2020; Gompert et al. 2022; Nice et al. 2021). Finally, we removed SNPs that had excessive sequence coverage (3 standard deviations above the mean) and those that were tightly clustered (within 5 bp), as these may be produced by poor read alignments.
Genomic variation among multi-day tested individuals
We used genotype likelihoods of our SNPs in the Bayesian admixture model in ENTROPY (version 1) (Shastry et al. 2021). From the model, we obtained posterior probability estimates of genotypes and explored patterns of admixture and genetic differentiation among the three populations of Cx. pipiens, as well as between individuals with different host preferences. The model implemented in ENTROPY is similar to the admixture model from STRUCTURE, which provides accurate estimates of recent admixture among populations (Lawson et al. 2018). We chose ENTROPY, however, because it incorporates uncertainty in sequences resulting from variation in coverage, sequencing error, and alignment error (Falush et al. 2003; Gompert et al. 2014; Pritchard et al. 2000; Shastry et al. 2021). This approach to estimating genotype probabilities is appropriate for low to moderate coverage data (Shastry et al. 2021), and has been used in a number of studies, in a number of different biological systems (for example; Faske et al. 2021; Gompert et al. 2014; Jahner et al. 2021; Lucas et al. 2018; Sung et al. 2018). To estimate posterior probability of genotypes, the model incorporates a mixture allele frequency-based prior (across K source populations). Therefore, each individual’s genotype is estimated using a combination of sequence data in the form of genotype likelihoods, and estimates of allele frequencies from K source populations weighted by an individual’s estimated admixture proportions (Gompert et al. 2014; Shastry et al. 2021). To obtain posterior probability distributions for parameters of interest, we used MCMC and ran the model for K = 2 through K = 6. For each model, we ran two chains, for 170,000 steps, saving every 10th step, and a burn-in of 25,000 steps. We examined trace plots and estimated effective sample size and the Gelman-Rubin diagnostic to ensure that a stable sampling distribution had been reached. We used the mean of the posterior distribution for each genotype from the K = 3 model in all downstream analyses.
To visualize patterns of genetic differentiation, we used a principal component analysis (PCA) on centered (but not scaled) genotype probabilities, and produced bar plots of admixture proportions from K = 2 through K = 6. Additional analyses used to characterize population genetic isolation and differentiation included estimation of heterozygosity, Nei’s GST, and linkage disequilibrium (LD) within each population. Genotype probabilities were also used to estimate mean heterozygosity for each SNP, for each population. We calculated genome-wide averaged Nei’s GST (an analog of the standard measure of differentiation, FST) between all three laboratory populations. GST was calculated as the average across all SNPs, and bootstrapped 95% confidence intervals were estimated using 10,000 sampling events. LD (as measured by r2) was used to examine co-inheritance of alleles within populations, as well as for human and avian responding groups (for further details see the supplementary methods).
To explore SNPs that might be associated with differences in host preference we used a partial redundancy analysis (partialRDA). This constrained ordination technique can be used to identify sets of co-varying genetic loci in response to multivariate predictor variables (Capblancq and Forester 2021; Capblancq et al. 2018; Legendre et al. 2011). In our partialRDA, we conditioned the host preference of an individual on population, used host choice (h, c, or s) as the predictor, and centered (but not scaled) genotype probabilities as the response variable. This was conducted using the vegan package in R (version 2.5-7) (Oksanen et al. 2020). To determine statistical significance of the model constraints, we conducted a permutation test (n = 9999) using the anova.cca function in the vegan package. SNPs potentially associated with host preference were identified as those with extreme values when compared to the empirically generated distribution of Mahalanobis distances estimated between each SNP and the center of the RDA space using the 2 RDA axes. Distances were corrected for inflation factor, then transformed to p-values using a χ2 distribution with 2 degrees of freedom. We estimated false discovery rate of these p-values using the q-value package (version 2.26.0) in R (Storey et al. 2021). This approach is based on Capblancq and Forester (2021), and we used the function outlined in the supporting code from their publication. SNPs with q ⩽ 0.05 were considered to be outlier loci associated with host preference in our multi-day behavioral assay. Our statistical approach for outlier detection was chosen because it accommodates the population genomic structure that we know to exist between our laboratory strains. All outlier analyses were conducted in R version 4.1.0 (R Core Team 2021).
After identifying outlier loci, we checked for sources of technical bias with potential to influence our statistical model outcomes. To ensure that identification of outlier loci did not result from shared missing data among individuals with different host preferences, we compared mean coverage per SNP for all outlier SNPs to mean differences in coverage for the whole data set (Supplementary Fig. S1). Our behavioral assay also measured mosquito responses to more than one human host, and differences in human attractiveness are known to exist (Geier et al. 2002). Therefore, we tested whether measuring mosquito responses to different human hosts influenced the SNPs associated with host preference using a correlation analysis. The mean genotype probability for each SNP associated with host preference was calculated separately for human and avian responding mosquitoes tested with either human 1 or human 2. Kendall’s tau was calculated using the mean genotype probabilities at all outlier SNPs for human and avian responding mosquitoes tested with either human 1 or human 2.
Olfactory receptor and odorant binding protein genes associated with divergent host preferences
We used a smoothing spline approach to define genomic windows containing SNPs that were associated with host preference and examined the genes within. Window size selection has the power to influence the outcomes of studies linking organismal phenotypes to their underlying genomic regions (Fuentes-Pardo and Ruzzante 2017; Hoban et al. 2016). Therefore, we adopted a smoothing spline approach because it avoids arbitrary, investigator-driven window size definition and instead allows the data to empirically determine appropriate window size. Smoothed splines were fitted to the −log10 transformed p-values from our partialRDA for all SNPs along each chromosome. This −log10 transformation is commonly used in genome-wide association studies to linearize and capture the magnitude of the differences between small p-values. Spline inflection points defined the window sizes (Beissinger et al. 2015). For each window, we calculated a t-test-like statistic called W (defined in Beissinger et al. 2015). The upper 95% quantile for W contained windows that we considered to be significantly associated with the trait.
We generated one BED file containing significant windows per chromosome. Varying window sizes are produced by this smoothing spline approach, and we therefore measured the correlation between window size and number of SNPs per window using Kendall’s tau. We also compared the distributions of numbers of SNPs per window for windows with W statistics that either exceeded or did not meet our significance threshold. This ensured that low numbers of SNPs per window did not drive which windows were identified as significantly associated with host preference. BEDTools intersect (v. 2.25.0; Quinlan and Hall 2010) identified overlap between genomic windows most strongly associated with host preference and the Cx. quinquefasciatus gene set. We used the VectorBase-58_CquinquefasciatusJHB2020 RefSeq pipeline annotation (GCF_015732765.1; downloaded August 11, 2022). This annotation file was merged with a manually curated OR and OBP gene set (ORs = 159, and OBPs = 117) to produce a gff3 file that contained 28,409 gene transcripts (including splice variants) for the three chromosomes. The orthology groups of ORs and OBPs that overlapped with genomic windows in the 95% W statistic quantile were identified from OrthoMCL DB (https://orthomcl.org/; v. 6.17; updated 12 Jul 2023).
Host selection is primarily an odor-guided behavior, and therefore we hypothesized that genomic windows in the 95% W statistic quantile would be more likely to overlap with one or more ORs or OBPs than should occur by chance alone. To test this hypothesis, we simulated the same number of host preference-associated windows from our empirical dataset (n = 71) with size distributions based on the empirical mean size (in bp) and standard deviation. Simulated windows were randomly placed on the three Cx. quinquefasciatus chromosomes. We scored simulated windows as overlapping with an OR or OBP if they shared even a single nucleotide with a gene from these families. Simulation and scoring were repeated 1000 times, and we quantified the probability with which overlaps occurred as often or more often than observed in our empirical dataset (see supplementary methods for further details).
Results
Multi-day behavioral assay
In keeping with previous work, we found a clear difference in host preference between the below-ground and above-ground Cx. pipiens populations tested in our behavioral assay (Fig. 1 and Table 2). The BG1 population had a median landing probability of 0.8 for human hosts, while AG2 had a probability of 0.3 and AG3 a probability of 0.1 (Table 2). While both AG2 and AG3 showed an overall preference for avian hosts over humans, we observed greater variation in host landing among AG2 individuals. This is reflected in the broader population-level posterior probability distribution estimated using BayesPref (Fig. 1).
Fig. 1. Posterior probability distributions for host preference of Culex pipiens females tested in a multi-day behavioral assay.
Solid lines represent the population-level preferences.
Table 2.
Median, lower, and upper 95% credible intervals from posterior probability distributions for Culex pipiens population level host preference.
| Avian Host | Human Host | |||||
|---|---|---|---|---|---|---|
| Population | Median | Lower 95% CI | Upper 95% CI | Median | Lower 95% CI | Upper 95% CI |
| BG1 | 0.148 | 0.107 | 0.200 | 0.852 | 0.800 | 0.893 |
| AG2 | 0.695 | 0.615 | 0.767 | 0.305 | 0.233 | 0.385 |
| AG3 | 0.856 | 0.788 | 0.903 | 0.144 | 0.097 | 0.212 |
Genomic variation among multi-day tested individuals
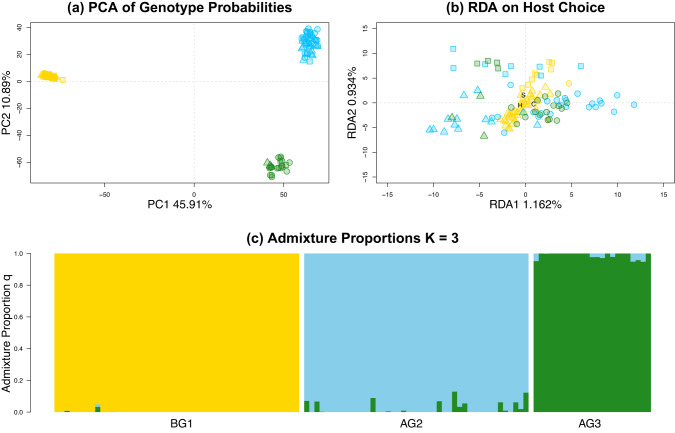
Individual mosquitoes tested in our multi-day behavioral assay were prepared into GBS libraries and sequenced. In total, these sequenced libraries produced 24,529 SNPs spread across the 3 Cx. quinquefasciatus chromosomes. The mean of the median coverage per individual per SNP was 9.6 (s.d. = ±2.5). We used these 24,529 SNPs to quantify patterns of genetic differentiation among the three mosquito populations and among individuals with different host preferences. A PCA on the genotype probabilities showed strong genetic differentiation between all 3 populations (Fig. 2a). PC1 explained 45.91% variation and separated individuals from the below-ground population (BG1), from individuals from the two above-ground populations (AG2 and AG3). PC2 explained 10.89% variation and separated individuals from the two above-ground populations. We did not see any clear patterns of differentiation between individuals with different host preferences on the first three PC axes (Fig. 2a and Supplementary Figs. S3, S4).
Fig. 2. Genomic variation associated with Culex pipiens population and female host preference.
For (a) and (b): data points are coded by host preference (shape) and colony strain (color). S = switchers (squares), A = avian (circles), H = human (triangles). BG1 = yellow, AG2 = blue, AG3 = green. a PC1 and PC2 from a principal component analysis of genotype probabilities for 24,529 SNPs from multi-day tested individuals. Each data point represents combinations of genotype probabilities in an individual mosquito. b Plot showing partialRDA of genotypes for multi-day tested individuals, where host preference was conditioned on population. c Bar plot showing admixture proportions (q) for each individual for K = 3 estimated from the program entropy.
To further explore the relationships between populations and individuals with divergent host preferences, we estimated admixture proportions (q), the proportion of an individual’s genome which is derived from K source populations, using the program ENTROPY (Shastry et al. 2021). We found that for K = 2 the above and below ground populations split from each other, with little evidence of shared ancestry between them (Supplementary Fig. S5). For K = 3, the two above-ground populations split from one another (Fig. 2c), and at increasing values of K, no biologically relevant clusters formed. We found no strong evidence that host preference was associated with increased recent introgression caused by hybridization in the ca. 50 generations these populations were reared in the laboratory. For example, AG2 individuals that chose human hosts or switched hosts did not show introgression from BG1, the typically human-seeking population, which would be observed as increased estimated admixture proportions from BG1 in Fig. 2c (also see Supplementary Fig. S5). However, our approach cannot rule out pre-colonization, or historical introgression of below ground Cx. pipiens alleles into above-ground populations, followed by many generations of back-crossing to explain variation in host-seeking behavior.
We summarized heterozygosity for our SNPs and found that BG1 had the lowest median heterozygosity (median = 0.0004292 (s.d. = ±0.19)), AG2 had the highest (median = 0.2987318 (s.d. = ±0.18)), and AG3 slightly lower than AG2 (median = 0.2488503 (s.d. = ±0.195)) (Supplementary Fig. S6). GST was highest between BG1 and the two above-ground populations (approx. 0.36), and lowest between AG2 and AG3 (0.15) (Supplementary Table S2). LD was found to be relatively low overall for our laboratory populations (range of mean r2 = 0.038–0.07; Table S3), but was higher when individuals were grouped by their response to our behavioral assay (range of mean r2 = 0.05–0.17; Table S3, see supplementary results and discussion for more details).
To explore genomic differences between individuals with distinct host preferences, we used a partialRDA and conditioned host preference on population (Fig. 2b). Host preference explained a very small amount of the genetic variation in our data set (approx. 0.9 %), and population explained a much larger amount (approx. 56.7%). We conducted a permutation test using the anova.cca function in the VEGAN package to test for significance of the model constraints (p < 0.022). Following the methods of Capblancq and Forester (2021), we identified SNPs that showed the strongest association with host preference from the first two axes of the RDA by estimating p-values and q-values. We identified 77 SNPs with a q ⩽ 0.05 (Fig. 3). These 77 SNPs were spread across all 3 chromosomes, with 8 on chromosome 1, 23 on chromosome 2, and 46 on chromosome 3. We calculated the mean difference in number of reads per SNP between individuals that responded to avian and human hosts in our behavioral assay and compared these differences for SNPs with p ⩽ 0.05 to differences in our total SNP set (non-outliers). We found that the majority of outlier SNPs had differences in read number similar to the differences found in our non-outlier SNPs, indicating that shared missing data did not underlie outlier identification (Supplementary Fig. S1). Genotype probabilities for these 77 SNPs were strongly correlated in groups of mosquitoes tested with human host 1 and human host 2 (Supplementary Fig. S7), indicating that within host species variation is not strongly influencing the trait architecture reported here.
Fig. 3. Manhattan plot showing the distribution of −log10(p-values) resulting from a partialRDA of 24,529 SNPs in 115 Culex pipiens females with divergent host preferences.
P-values were generated following the procedure described in Capblancq and Forester (2021), where outlier loci were identified based on their extremeness along a distribution of Mahalanobis distances estimated between each SNP and the center of the RDA space using 2 axes. Distances were corrected for inflation factor then transformed to p-values using a χ2 distribution with 2 degrees of freedom. The dotted gray line represents a p-value of 0.001. SNPs shown in red had a q-value of <0.05. Labeled genes are Culex pipiens assemblage olfactory receptor and odorant binding protein genes found within host preference-associated regions of the genome (identified using the smoothed-spline analysis).
Olfactory receptor and odorant binding protein genes associated with divergent host preferences
Our spline-based approach divided the genome into 1,418 windows spread across the three Cx. pipiens chromosomes. The sizes of these 1418 windows varied, and SNP counts per window were correlated with window size (Supplementary Fig. S8). Seventy-one of these 1418 genomic windows were associated with divergent host preference, and the distributions of SNP counts per window for these 71 windows were similar to those that did not exceed our threshold of statistical significance (Supplementary Fig. S8). The numbers of windows with W scores in the upper 95% quantile were 23, 27, and 21, on chromosomes 1, 2, and 3, respectively. Sizes of these host preference-associated windows ranged from 54.9 Kb to 979.6 Kb, with a mean of 427.8 Kb (s.d. = ± 193.4 Kb). Eighty-four percent of the 77 SNPs with q-values indicating an association with host preference were included in 32 of these 71 defined windows (Supplementary Fig. S9). Yet 39 windows with W scores in the upper 95% quantile did not contain one of the 77 outlier SNPs. This likely resulted from differences in correction for false discovery rate between the two methods. While a formal false discovery rate correction was applied for identification of SNP outliers, no such correction was applied for our spline-based approach, and W was instead calculated from multiple uncorrected SNP p-values across a genomic window.
A total of 473, 759, 687 transcripts could be found within genomic windows associated with divergent host preference along the 3 Cx. quinquefasciatus chromosomes (Supplementary Data 1). These included orco, 19 ORs, and 16 OBPs spread across 10 windows with W scores in the upper 95% quantile (Table 3). Our window simulations revealed that, on average, 5.6 (s.d. = ±2.2) of 71 randomly placed genomic windows should overlap with at least one OR or OBP by a single nucleotide. Yet the probability of 10 or more randomly placed windows overlapping with at least one OR or OBP gene was low (p = 0.043; Supplementary Fig. S10), signaling over-representation of these chemosensory genes in our empirically determined windows associated with host preference.
Table 3.
Culex pipiens assemblage olfactory receptor and odorant binding protein genes within host preference-associated regions of the genome.
| Chromosome | Start | Stop | Strand | Gene | VectorBase ID | Ortho Group |
|---|---|---|---|---|---|---|
| NC_051861.1 | 3852588 | 3853514 | + | Obp92 | CPIJ001690 | OG6_117759 |
| NC_051861.1 | 51920776 | 51921939 | - | Obp2 | CPIJ007617 | OG6_163124 |
| NC_051862.1 | 209436027 | 209437270 | - | Or202 | ||
| NC_051862.1 | 209437564 | 209438850 | - | Or140 | CPIJ016858 | OG6_108769 |
| NC_051862.1 | 209440193 | 209441503 | - | Or201 | ||
| NC_051862.1 | 209441711 | 209442967 | - | Or142 | CPIJ016859 | OG6_108769 |
| NC_051862.1 | 209444125 | 209445381 | - | Or143 | CPIJ016860 | OG6_108769 |
| NC_051862.1 | 209445675 | 209446878 | + | Or144 | CPIJ016861 | OG6_108769 |
| NC_051862.1 | 49806497 | 49807372 | - | Obp82 | CPIJ008161 | OG6_115568 |
| NC_051862.1 | 49809142 | 49810005 | + | Obp81 | CPIJ008160 | OG6_115568 |
| NC_051862.1 | 49810242 | 49811120 | + | Obp78 | CPIJ008159 | OG6_115568 |
| NC_051862.1 | 49811322 | 49812188 | + | Obp77 | CPIJ008158 | OG6_115568 |
| NC_051862.1 | 49813384 | 49814238 | + | Obp76 | CPIJ008157 | OG6_115568 |
| NC_051862.1 | 49814428 | 49815162 | + | Obp80 | CPIJ008156 | OG6_115568 |
| NC_051862.1 | 49819306 | 49820466 | + | Obp79 | CPIJ008155 | OG6_115568 |
| NC_051862.1 | 49820632 | 49821319 | + | Obp83 | CPIJ008154 | OG6_582544 |
| NC_051862.1 | 49821446 | 49822252 | + | Obp120 | ||
| NC_051862.1 | 51249865 | 51251328 | + | Or22 | CPIJ016433 | OG6_163622 |
| NC_051862.1 | 58393834 | 58395729 | - | Or64 | CPIJ006084 | OG6_128920 |
| NC_051862.1 | 58402332 | 58408334 | - | Or65 | CPIJ006217 | OG6_158791 |
| NC_051862.1 | 79148170 | 79148879 | + | Obp22 | CPIJ012721 | OG6_166784 |
| NC_051862.1 | 79151997 | 79152727 | - | Obp21 | CPIJ012720 | OG6_183331 |
| NC_051862.1 | 95233480 | 95235109 | + | Or57 | CPIJ005404 | OG6_583362 |
| NC_051862.1 | 95244528 | 95250839 | + | Or56 | CPIJ005403 | OG6_582306 |
| NC_051862.1 | 95255042 | 95256585 | - | Or55 | CPIJ005401 | OG6_583676 |
| NC_051862.1 | 95260174 | 95261760 | - | Or54 | CPIJ005400 | OG6_151176 |
| NC_051862.1 | 95261771 | 95264830 | - | Or53 | CPIJ005399 | OG6_151176 |
| NC_051862.1 | 95267126 | 95268875 | - | Or52 | ||
| NC_051862.1 | 95292384 | 95293992 | + | Or51 | CPIJ005396 | OG6_158791 |
| NC_051862.1 | 95294779 | 95296245 | + | Or176 | ||
| NC_051863.1 | 143710283 | 143715125 | + | Obp11 | CPIJ006551 | OG6_110106 |
| NC_051863.1 | 143859543 | 143860894 | - | Or208 | ||
| NC_051863.1 | 46700831 | 46713009 | - | Obp14 | CPIJ009586 | OG6_142318 |
| NC_051863.1 | 46861523 | 46863363 | - | Or40 | CPIJ009579 | OG6_126368 |
| NC_051863.1 | 46915625 | 46949854 | - | Orco | CPIJ040865 | OG6_126369 |
| NC_051863.1 | 47120103 | 47120842 | - | Obp8 | CPIJ009568 | OG6_110106 |
VectorBase gene identifiers and orthology groups from OrthoMCL DB are provided, when available, to connect the genes identified here to previous work in Culex and other mosquito species. All listed genes are found in genomic windows in the 95th W-stat percentile, and bolded genes are found in windows that also contain an outlier SNP.
Discussion
The evolution of anthropophilic blood-feeding behavior in mosquitoes has had devastating health-related consequences for humans. Herein, we explored the genetic architecture of host preference in Cx. pipiens populations with differing degrees of adaptation to humans and human landscape alterations. At a population-level, results from our multi-day behavioral assay confirmed that host preferences of females from our below-ground autogenous population (BG1) were biased toward humans, while females of the two above-ground anautogenous populations (AG2 and AG3) showed biased preferences toward avian hosts (Fig. 1). These findings are in line with results from previous laboratory studies (Fritz et al. 2015; Noreuil and Fritz 2021), as well as field observations of the below-ground molestus and above-ground pipiens forms (Apperson et al. 2004; Hamer et al. 2009; Harbach et al. 1984; Shute 1951). Yet, within each population there was inter-individual variation, with some individuals responding to the alternate host rather than the population-level preferred host. Responses by AG2 females demonstrated greater inter-individual variation in behavior in our multi-day assays than did either BG1 or AG3, representing a mild departure from our previous findings of similar inter-individual variation among these three populations (Noreuil and Fritz 2021). To begin to elucidate the genomic architecture of these observed host preferences, we used GBS to generate SNP markers for individuals with measured host preference phenotypes and identified SNPs that showed an association with preference.
Using the 24,529 SNPs in our dataset, we visualized the extent of population genomic structure present among phenotyped individuals with a PCA and observed that individuals clustered tightly into their respective populations. Genomic structure among populations used in our study was also supported by our admixture analysis. When K was equal to 2, BG1 split from AG2 and AG3 as would be expected based on their status as distinct bioforms of Cx. pipens. When K was equal to 3, all three populations appeared to be distinct and this did not change with increasing values of K (Supplementary Fig. S5). Population genomic structure of this nature may result from genetic drift that occurs within laboratory-reared insect populations (Fritz et al. 2016; Munstermann 1994), but is also found between Cx. pipens bioforms in the wild (Yurchenko et al. 2020), due to sexual isolation that is thought to occur between them (Harbach 2012). Interestingly, individuals showing host preferences that diverged from the preferences of their respective populations did not cluster separately from other members of their population, according to PCA (Fig. 2a). Nor did they show different estimates of admixture proportions (Fig. 2c). This suggests that the inter-individual variation in preference observed within our populations was not associated with very recent gene flow (e.g., since the time of colonization) and is not the result of hybridization between our populations in the lab. Results from our genomic analyses could also begin to explain some of the increased inter-individual variation we observed in the host preference of AG2 individuals. AG2 individuals showed greater spread along PC3 than did either BG1 or AG3 in our PCA of individual genotype probabilities (Supplementary Figs. S3, S4). They also had the highest median estimates of heterozygosity (Supplementary Fig. S6). Perhaps this increased genomic variation naturally present among individuals from our AG2 population included regions of the genome underlying host preference, thereby resulting in greater variation around the population-level mean preference.
Identifying genotype-phenotype associations by genome scanning can be challenging when population genomic structure is correlated with inter-population variation in phenotype (Narum and Hess 2011). We took advantage of the variation in host preference that existed within and among populations to identify regions of the genome that showed a statistical association with host preference, while controlling for population genomic structure using a partialRDA. Our GBS dataset contained individuals within each population that only sought human hosts, only sought avian hosts, and switched between them. Because all three phenotypes are shared across all populations, albeit at very different frequencies (Table 1), the first step in our partialRDA was to construct a linear model that conditioned host choice on population (BG1, AG2, or AG3). Conditioning on population allowed for adjustment of the linear effects of host choice on genotype probabilities by partitioning out variation due to population (Capblancq and Forester 2021). This relatively new approach has successfully been used in other study systems to identify genotype-phenotype associations despite existing population genomic structure (Capblancq and Forester 2021; Cheek et al. 2022; Gibson and Moyle 2020; Xuereb et al. 2018). Additionally, use of multivariate approaches like RDA may enhance power to detect weaker, multilocus patterns of genotype-phenotype association, as might be expected from host preference, where more than one gene likely contributes to trait variation (Forester et al. 2018; McBride et al. 2014). Application of partialRDA to our Culex dataset identified 77 SNP outliers (of 24,529 total SNPs) and 71 genomic windows (of 1,418 genomic windows) associated with divergent host preferences. These 71 genomic windows contained 84% of the SNP outliers in our dataset.
Genomic windows could then be explored to identify genes likely to mediate behavioral differences that impact host preference. Although gustation, vision, audition, and thermal detection all play important roles in mosquito host location (Montell and Zwiebel 2016), we focused on genes with roles in olfaction for two reasons: (1) the direct relevance of OR function to host-seeking behavior has been documented in both Aedes and Anopheles species of public health importance (DeGennaro et al. 2013; Sun et al. 2020), and (2) both OR and OBP gene families have been manually curated in the new chromosome-scale Cx. quinquefasciatus genome assembly.
In general, the evolution of insect chemosensory gene families is defined by rapid gene duplication, diversification, and gain or loss of gene function (Sánchez-Gracia et al. 2009), leading to behavioral differences among closely related taxa (e.g., Ambrose et al. 2022; Auer et al. 2022; Dworkin and Jones 2009; Nouhaud et al. 2018; Rinker et al. 2013). This is also true of Cx. pipiens, where chemosensory genes are among the most rapidly diverging gene families in comparisons between the pipiens and molestus bioforms (Price and Fonseca 2015). It is also thought that olfactory genes with major roles in host-seeking should show down-regulated expression patterns following a blood meal. This has been demonstrated in Cx. quinquefasciatus and other mosquitoes, when acquisition of a blood meal inhibits host-seeking behaviors (Klowden 1981) and shifts chemosensory gene expression patterns (Fox et al. 2001; Matthews et al. 2016; Taparia et al. 2017). We expected to see that at least a subset of the OR and OBP genes identified in our analysis would be differentially expressed pre- and post-blood meal according to previous work. Furthermore, previous work has demonstrated that changes in regulation of chemosensory gene expression and allelic variation can interact to influence inter-population variation in mosquito host preference (McBride et al. 2014). Therefore, we also predicted that a subset of the OR and OBP genes uncovered in our study would be differentially expressed in Cx. pipiens populations with unique host preferences, as described in prevous work (Noreuil and Fritz 2021).
Of the 159 annotated ORs and 117 annotated OBPs on the 3 Cx. quinquefasciatus chromosomes, we identified 16–19 genes per gene family within host preference-associated genomic windows. Of these, 6 have previously been shown to be differentially expressed according to blood meal status, population-level host preference or both. Ors 53, 54, and 64 are differentially expressed in Cx. quinquefasciatus antennae when host-seeking and blood fed females are compared (Taparia et al. 2017), and all three genes were present in host preference-associated genomic windows in our analysis. Obp2 (CPIJ007617) is expressed in the antennae of Cx. quinquefasciatus (Taparia et al. 2017), and shows lower expression in the heads of AG2 females relative to BG1 females in a previous differential gene expression study using the same populations tested in the present work (Noreuil and Fritz 2021). Obp8 (CPIJ009568) is highly expressed in the antennae of Cx. quinquefasciatus and is moderately down-regulated in response to a blood meal (Leal et al. 2013; Taparia et al. 2017). Furthermore, expression of Obp8 is higher in the heads of AG2 females relative to BG1 females (Noreuil and Fritz 2021). Finally, Or22 (formerly Or137; CPIJ016433) is highly expressed in Cx. quinquefasciatus antennae (Leal et al. 2013; Taparia et al. 2017), and its expression is significantly down-regulated in response to a blood meal (Taparia et al. 2017). AG2 females have lower Or22 expression in the head than BG1 females. It is also notable that, of these chemosensory genes identified as differentially expressed in previous Culex studies and associated with host choice in the present study, two have one-to-one orthology with differentially expressed genes in anthropophilic A. gambiae and zoophilic A. quadriannulatus(Rinker et al. 2013): Obp2 and Or22. Identifying the volatiles which bind these specific receptors and binding proteins may reveal which odors are important for shifts between human and avian host preference (Syed and Leal 2009).
Limitations and future directions
The work described here represents a first step toward exploring the genomic architecture of host preference among Cx. pipiens assemblage mosquitoes. While we have identified a narrowed set of genomic regions associated with host preference in this important vector species, we acknowledge limitations of our approach that should be considered when interpreting our results. First, our power to detect SNPs associated with host preference may be limited due to the relatively low throughput of our multi-day assay, which resulted in the moderate sample sizes used in our experiments. Secondly, the genomic data that we generated was sparsely distributed due to use of a reduced representation sequencing approach, rather than whole genome sequencing. This means that we may have missed some regions of the genome associated with host preference, due to lack of a nearby SNP marker. Finally, there is genetic structure present among our 3 phenotyped populations, which we addressed by use of a partialRDA. We applied this approach to our dataset because it is able to formally accommodate genomic structure among populations. Supporting evidence for this is that not all differentially expressed genes from a previous study of BG1 and AG2 were identified in our host preference-associated windows (Noreuil and Fritz 2021). We attribute this finding to the ability of our partialRDA analysis to account for general population-level genetic differences that may mediate differences in oviposition, sugar-feeding or mating behaviors that also exist among forms. Yet this approach may produce false negatives due to population genomic structure, particularly for SNPs associated with host preference but predominantly found in only one of the populations. It is also possible that some SNPs identified as ‘outliers’ in our analysis represent false positives and are spuriously associated with host preference. Such false positives may result from correlations between differences in host preference and genetic isolation among populations that cannot be accounted for by our model. To reduce the likelihood that our host preference-associated regions of the genome represent false positives, we (1) applied a false discovery rate correction to our SNP outliers, and (2) compared our SNP outliers to results of a second test (W test; Beissinger et al. 2015) with similar outcomes. If false positives exist in our dataset, they are likely a property of associations between population and host choice that our partialRDA may have been unable to account for when quantifying the effect of host choice on genotype probability.
Although we detected over-representation of ORs and OBPs in our host preference-associated regions of the genome, recent work has also demonstrated the importance of ionotropic receptors (IRs) to successful blood acquisition in An. coluzzii (Ye et al. 2022). It is possible that genes from this family, as well as other chemosensory gene families, play important roles in the divergent Cx. pipiens host-seeking behaviors we observed in our study. For example, one region of genomic divergence among human- and avian-seeking Cx. pipiens appears at the end of Chromosome 2, and yet contained no Ors or Obps. Poor annotation of chemosensory genes other than those from the OR and OBP families in the current Cx. quinquefasciatus assembly prohibited their identification in this analysis. Future manual curation of chemosensory gene families, including, but not limited to, IRs, gustatory receptors, chemosensory proteins, and sensory neuron membrane proteins, will be necessary to reveal their roles in Cx. pipiens host preference.
Finally, using traditional genetic crosses will provide important resolution when identifying regions of the genome associated with host preference in the future. Advanced intercrosses with large sample sizes will likely be needed to narrow the list of candidate genes associated with host preference. Use of this approach will be particularly important for estimating which genes (including those with chemosensory function) have the greatest effect on host preference phenotypes. Although some limitations exist to the approach presented here, our work to identify OBP and OR genes with potential involvement in host preference opens avenues for future quantitative and functional genomic investigations to link specific genes or gene clusters to a mosquito trait of exceptional public health importance.
Supplementary information
Acknowledgements
We thank Mervin Keith Cuadera and Lyra Morina for assistance with mosquito rearing. Gregorio Baek and Rebecca Kaminsky assisted during preference testing. We thank Chris Nice for providing GBS adapters with unique barcodes for use in our library preparation. We thank Texas State University for use of their LEAP computing cluster. We thank Jake Tu and Maria Sharakhova for production of the chromosome-scale publicly available Cx. quinquefasciatus assembly. Rong Guo and the laboratory of Carolyn McBride manually curated the OBP and OR gene families, respectively, without which aspects of this work would not have been possible.
Author contributions
KLB and MLF designed the experiments. AN and KLB conducted the multi-day behavioral assays. KLB prepared the GBS libraries, and KLB, EKM, and MLF analyzed the data. KLB and MLF wrote the manuscript. All authors reviewed and approved the final manuscript.
Funding
Funding for this work was provided by the University of Maryland Brain and Behavior Initiative and National Institutes of Health R01AI125622A to MLF. Partial support for KLB was provided by the Modelscape Consortium with funding from the National Science Foundation (OIA-2019528).
Data availability
Data for this study are available at: http://www.ncbi.nlm.nih.gov/bioproject/1068091. All scripts used to perform these analyses can be found at: https://github.com/katebell1987/CulexMS1 or upon request from corresponding authors.
Competing interests
The authors declare no competing interests.
Ethics
Use of animal subjects for behavioral tests was approved by the University of Maryland Institutional Animal Use and Care Committee under protocol number 1094335.
Footnotes
Associate editor: Gerald Heckel
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Katherine L. Bell, Email: katebell1987@gmail.com
Megan L. Fritz, Email: mfritz13@umd.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41437-024-00675-4.
References
- Ambrose L, Popovic I, Hereward J, Ortiz-Barrientos D, Beebe NW. Comparisons of chemosensory gene repertoires in human and non-human feeding Anopheles mosquitoes link olfactory genes to anthropophily. iScience. 2022;25:104521. doi: 10.1016/j.isci.2022.104521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apperson HK, et al. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the Eastern United States. Vector Borne Zoonotic Dis. 2004;4:71–82. doi: 10.1089/153036604773083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgharian H, et al. Evolutionary genomics of Culex pipiens: global and local adaptations associated with climate, life-history traits and anthropogenic factors. Proc R Soc B Biol Sci. 2015;282:20150728. doi: 10.1098/rspb.2015.0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer TO, Alvarez-Ocana R, Cruchet S, Benton R, Arguello JR. Copy number changes in co-expressed odorant receptor genes enable selection for sensory differences in drosophilid species. Nat Ecol Evol. 2022;6:1343–1353. doi: 10.1038/s41559-022-01830-y. [DOI] [PubMed] [Google Scholar]
- Barr AR. Occurrence and distribution of the Culex pipiens complex. Bull World Health Organ. 1967;37:293. [PMC free article] [PubMed] [Google Scholar]
- Beissinger TM, Rosa GJ, Kaeppler SM, Gianola D, de Leon N. Defining window-boundaries for genomic analyses using smoothing spline techniques. Genet Sel Evol. 2015;47:1–9. doi: 10.1186/s12711-015-0105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besansky NJ, et al. Molecular phylogeny of the Anopheles gambiae complex suggests genetic introgression between principal malaria vectors. Proc Natl Acad Sci. 1994;91:6885–6888. doi: 10.1073/pnas.91.15.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugman VA, et al. The role of Culex pipiens L.(Diptera: Culicidae) in virus transmission in Europe. Int J Environ Res Public Health. 2018;15:389. doi: 10.3390/ijerph15020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capblancq T, Forester BR. Redundancy analysis: a Swiss army knife for landscape genomics. Methods Ecol Evol. 2021;12:2298–2309. doi: 10.1111/2041-210X.13722. [DOI] [Google Scholar]
- Capblancq T, Luu K, Blum MG, Bazin E. Evaluation of redundancy analysis to identify signatures of local adaptation. Mol Ecol Resour. 2018;18:1223–1233. doi: 10.1111/1755-0998.12906. [DOI] [PubMed] [Google Scholar]
- Chaturvedi S, et al. Recent hybrids recapitulate ancient hybrid outcomes. Nat Commun. 2020;11:2179. doi: 10.1038/s41467-020-15641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek RG, et al. Habitat-linked genetic variation supports microgeographic adaptive divergence in an island-endemic bird species. Mol Ecol. 2022;31:2830–2846. doi: 10.1111/mec.16438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophers SR (1960) Aedes aegypti: the yellow fever mosquito. CUP Archive
- Coluzzi M, Sabatini A, Petrarca V, Di Deco M. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- Crawford JE, et al. Population genomics reveals that an anthropophilic population of Aedes aegypti mosquitoes in west Africa recently gave rise to American and Asian populations of this major disease vector. BMC Biol. 2017;15:1–16. doi: 10.1186/s12915-017-0351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutzen PJ (2006) The “Anthropocene”. In: Ehlers E, Krafft T (eds) Earth system science in the Anthropocene, Springer, pp 13–18
- DeGennaro M, et al. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 2013;498:487–491. doi: 10.1038/nature12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker T, Takken W. Differential responses of mosquito sibling species Anopheles arabiensis and An. quadriannulatusto carbon dioxide, a man or a calf. Med Vet Entomol. 1998;12:136–140. doi: 10.1046/j.1365-2915.1998.00073.x. [DOI] [PubMed] [Google Scholar]
- Dekker T, Takken W, Cardé RT. Structure of host-odour plumes influences catch of Anopheles gambiae ss and Aedes aegypti in a dual-choice olfactometer. Physiol Entomol. 2001;26:124–134. doi: 10.1046/j.1365-3032.2001.00225.x. [DOI] [Google Scholar]
- Dworkin I, Jones CD. Genetic changes accompanying the evolution of host specialization in Drosophila sechellia. Genetics. 2009;181:721–736. doi: 10.1534/genetics.108.093419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajollahi A, Fonseca DM, Kramer LD, Kilpatrick AM. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect Genet Evol. 2011;11:1577–1585. doi: 10.1016/j.meegid.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faske TM, et al. Genomic and common garden approaches yield complementary results for quantifying environmental drivers of local adaptation in rubber rabbitbrush, a foundational great basin shrub. Evol Appl. 2021;14:2881–2900. doi: 10.1111/eva.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca DM, et al. Emerging vectors in the Culex pipiens complex. Science. 2004;303:1535–1538. doi: 10.1126/science.1094247. [DOI] [PubMed] [Google Scholar]
- Fordyce JA, Gompert Z, Forister ML, Nice CC. A hierarchical Bayesian approach to ecological count data: a flexible tool for ecologists. PloS One. 2011;6:e26785. doi: 10.1371/journal.pone.0026785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester BR, Lasky JR, Wagner HH, Urban DL. Comparing methods for detecting multilocus adaptation with multivariate genotype–environment associations. Mol Ecol. 2018;27:2215–2233. doi: 10.1111/mec.14584. [DOI] [PubMed] [Google Scholar]
- Foster WA, Walker ED (2019) Mosquitoes (Culicidae). In: Mullen GR, Durden LA (eds) Medical and veterinary entomology, Elsevier, pp 261–325
- Fouet C, Atkinson P, Kamdem C. Human interventions: driving forces of mosquito evolution. Trends Parasitol. 2018;34:127–139. doi: 10.1016/j.pt.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Fox A, Pitts R, Robertson H, Carlson J, Zwiebel L. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down-regulation in response to blood feeding. Proc Natl Acad Sci. 2001;98:14693–14697. doi: 10.1073/pnas.261432998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklinos LH, Jones KE, Redding DW, Abubakar I. The effect of global change on mosquito-borne disease. The Lancet Infectious Diseases. 2019;19:e302–e312. doi: 10.1016/S1473-3099(19)30161-6. [DOI] [PubMed] [Google Scholar]
- Fritz M, Walker E, Miller J, Severson D, Dworkin I. Divergent host preferences of above-and below-ground Culex pipiens mosquitoes and their hybrid offspring. Med Vet Entomol. 2015;29:115–123. doi: 10.1111/mve.12096. [DOI] [PubMed] [Google Scholar]
- Fritz ML, Paa S, Baltzegar J, Gould F. Application of a dense genetic map for assessment of genomic responses to selection and inbreeding in Heliothis virescens. Insect Mol Biol. 2016;25:385–400. doi: 10.1111/imb.12234. [DOI] [PubMed] [Google Scholar]
- Fritz ML, Walker ED, Yunker AJ, Dworkin I. Daily blood feeding rhythms of laboratory-reared North American Culex pipiens. J Circadian Rhythms. 2014;12:1. doi: 10.1186/1740-3391-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Pardo AP, Ruzzante DE. Whole-genome sequencing approaches for conservation biology: advantages, limitations and practical recommendations. Mol Ecol. 2017;26:5369–5406. doi: 10.1111/mec.14264. [DOI] [PubMed] [Google Scholar]
- Gargan TP, Bailey CL, Higbee GA, Gad A, El-Said S. The effect of laboratory colonization on the vector-pathogen interactions of Egyptian Culex pipiens and Rift Valley fever virus. Am J Trop Med Hyg. 1983;32:1154–63. doi: 10.4269/ajtmh.1983.32.1154. [DOI] [PubMed] [Google Scholar]
- Garrett-Jones C. The human blood index of malaria vectors in relation to epidemiological assessment. Bull World Health Organ. 1964;30:241. [PMC free article] [PubMed] [Google Scholar]
- Geier M, Bosch OJ, Boeckh J. Influence of odour plume structure on upwind flight of mosquitoes towards hosts. J Exp Biol. 1999;202:1639–1648. doi: 10.1242/jeb.202.12.1639. [DOI] [PubMed] [Google Scholar]
- Geier M, Bosh O, Steib B, Rose A, Boeckh J (2002) Odour-guides host finding mosquitoes: identification of new attractants on human skin. In Proceedings of the international conference on urban pests, vol. 4. Citeseer, pp 37–46
- Gibson MJ, Moyle LC. Regional differences in the abiotic environment contribute to genomic divergence within a wild tomato species. Mol Ecol. 2020;29:2204–2217. doi: 10.1111/mec.15477. [DOI] [PubMed] [Google Scholar]
- Gompert Z, et al. Admixture and the organization of genetic diversity in a butterfly species complex revealed through common and rare genetic variants. Mol Ecol. 2014;23:4555–4573. doi: 10.1111/mec.12811. [DOI] [PubMed] [Google Scholar]
- Gompert Z, et al. Additive genetic effects in interacting species jointly determine the outcome of caterpillar herbivory. Proc Natl Acad Sci. 2022;119:e2206052119. doi: 10.1073/pnas.2206052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidobaldi F, May-Concha IJ, Guerenstein PG. Morphology and physiology of the olfactory system of blood-feeding insects. J Physiol Paris. 2014;108:96–111. doi: 10.1016/j.jphysparis.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Hamer GL, et al. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am J Trop Med Hyg. 2009;80:268–278. doi: 10.4269/ajtmh.2009.80.268. [DOI] [PubMed] [Google Scholar]
- Hansson BS, Stensmyr MC. Evolution of insect olfaction. Neuron. 2011;72:698–711. doi: 10.1016/j.neuron.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Harbach RE. Culex pipiens: species versus species complex–taxonomic history and perspective. J Am Mosq Control Assoc. 2012;28:10–23. doi: 10.2987/8756-971X-28.4.10. [DOI] [PubMed] [Google Scholar]
- Harbach RE, Harrison BA, Gad AM. Culex (Culex) molestus forskal (Diptera: Culicidae): neotype designation, description, variation, and taxonomic status. Proc Entomol Soc Wash. 1984;86:521–542. [Google Scholar]
- Hoban S, et al. Finding the genomic basis of local adaptation: pitfalls, practical solutions, and future directions. Am Nat. 2016;188:379–397. doi: 10.1086/688018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahner JP, Parchman TL, Matocq MD. Multigenerational backcrossing and introgression between two woodrat species at an abrupt ecological transition. Mol Ecol. 2021;30:4245–4258. doi: 10.1111/mec.16056. [DOI] [PubMed] [Google Scholar]
- James SL, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp UB. Olfactory signalling in vertebrates and insects: differences and commonalities. Nat Rev Neurosci. 2010;11:188–200. doi: 10.1038/nrn2789. [DOI] [PubMed] [Google Scholar]
- Klowden MJ. Initiation and termination of host-seeking inhibition in Aedes aegypti during oöcyte maturation. J Insect Physiol. 1981;27:799–803. doi: 10.1016/0022-1910(81)90071-8. [DOI] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Lawson DJ, Van Dorp L, Falush D. A tutorial on how not to over-interpret structure and admixture bar plots. Nat Commun. 2018;9:3258. doi: 10.1038/s41467-018-05257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal WS. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Ann Rev Entomol. 2013;58:373–391. doi: 10.1146/annurev-ento-120811-153635. [DOI] [PubMed] [Google Scholar]
- Leal WS, Choo Y-M, Xu P, da Silva CS, Ueira-Vieira C. Differential expression of olfactory genes in the southern house mosquito and insights into unique odorant receptor gene isoforms. Proc Natl Acad Sci. 2013;110:18704–18709. doi: 10.1073/pnas.1316059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Oksanen J, ter Braak CJ. Testing the significance of canonical axes in redundancy analysis. Methods Ecol Evol. 2011;2:269–277. doi: 10.1111/j.2041-210X.2010.00078.x. [DOI] [Google Scholar]
- Li H (2013) Aligning sequence reads, clone sequences, and assembly contigs with bwa-mem. arXiv preprint arXiv:1303.3997
- Li H, Durbin R. Fast and accurate short read alignment with burrows–wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, et al. The sequence alignment/map format and samtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas LK, Nice CC, Gompert Z. Genetic constraints on wing pattern variation in Lycaeides butterflies: a case study on mapping complex, multifaceted traits in structured populations. Mol Ecol Resour. 2018;18:892–907. doi: 10.1111/1755-0998.12777. [DOI] [PubMed] [Google Scholar]
- Matthews BJ, McBride CS, DeGennaro M, Despo O, Vosshall LB. The neurotranscriptome of the Aedes aegypti mosquito. BMC genomics. 2016;17:1–20. doi: 10.1186/s12864-015-2239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride CS, et al. Evolution of mosquito preference for humans linked to an odorant receptor. Nature. 2014;515:222–227. doi: 10.1038/nature13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MJ, Hahs AK. Adaptation and adaptedness of organisms to urban environments. Ann Rev Ecol Evol Syst. 2015;46:261–280. doi: 10.1146/annurev-ecolsys-112414-054258. [DOI] [Google Scholar]
- Montell C, Zwiebel L (2016) Mosquito sensory systems. In: Raikhel AS (ed) Advances in insect physiology, vol. 51. Elsevier, pp 293–328
- Moran EV, Alexander JM. Evolutionary responses to global change: lessons from invasive species. Ecol Lett. 2014;17:637–649. doi: 10.1111/ele.12262. [DOI] [PubMed] [Google Scholar]
- Munstermann LE. Unexpected genetic consequences of colonization and inbreeding: allozyme tracking in Culicidae (Diptera) Ann Entomol Soc Am. 1994;87:157–164. doi: 10.1093/aesa/87.2.157. [DOI] [Google Scholar]
- Narum SR, Hess JE. Comparison of FST outlier tests for SNP loci under selection. Mol Ecol Resour. 2011;11:184–194. doi: 10.1111/j.1755-0998.2011.02987.x. [DOI] [PubMed] [Google Scholar]
- Nelms BM, Macedo PA, Kothera L, Savage HM, Reisen WK. Overwintering biology of Culex (Diptera: Culicidae) mosquitoes in the Sacramento valley of California. J Med Entomol. 2013;50:773–790. doi: 10.1603/ME12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nice CC, Fordyce JA, Sotola VA, Crow J, Diaz PH. Geographic patterns of genomic variation in the threatened Salado salamander, Eurycea chisholmensis: Population genetics of the Salado salamander. Conserv Genet. 2021;22:811–821. doi: 10.1007/s10592-021-01364-z. [DOI] [Google Scholar]
- Noreuil A, Fritz ML. Differential gene expression in the heads of behaviorally divergent Culex pipiens mosquitoes. Insects. 2021;12:271. doi: 10.3390/insects12030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouhaud P, et al. Identifying genomic hotspots of differentiation and candidate genes involved in the adaptive divergence of pea aphid host races. Mol Ecol. 2018;27:3287–3300. doi: 10.1111/mec.14799. [DOI] [PubMed] [Google Scholar]
- Oksanen J et al. (2020) vegan: Community Ecology Package. https://CRAN.R-project.org/package=vegan. R package version 2.5–7
- Otto SP. Adaptation, speciation and extinction in the anthropocene. Proc R Soc B. 2018;285:20182047. doi: 10.1098/rspb.2018.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchman TL, et al. Genome-wide association genetics of an adaptive trait in lodgepole pine. Mol Ecol. 2012;21:2991–3005. doi: 10.1111/j.1365-294X.2012.05513.x. [DOI] [PubMed] [Google Scholar]
- Powell JR, Gloria-Soria A, Kotsakiozi P. Recent history of Aedes aegypti: Vector genomics and epidemiology records. Bioscience. 2018;68:854–860. doi: 10.1093/biosci/biy119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DC, Fonseca DM. Genetic divergence between populations of feral and domestic forms of a mosquito disease vector assessed by transcriptomics. PeerJ. 2015;3:e807. doi: 10.7717/peerj.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM. BEDtools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria (2021). https://www.R-project.org/.
- Raji JI, DeGennaro M. Genetic analysis of mosquito detection of humans. Curr Opin Insect Sci. 2017;20:34–38. doi: 10.1016/j.cois.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidenbach KR, et al. Phylogenetic analysis and temporal diversification of mosquitoes (Diptera: Culicidae) based on nuclear genes and morphology. BMC Evol Biol. 2009;9:1–14. doi: 10.1186/1471-2148-9-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renou M. Pheromones and general odor perception in insects. Neurobiol Chem Commun. 2014;1:23–56. doi: 10.1201/b16511-3. [DOI] [PubMed] [Google Scholar]
- Rinker DC, Zhou X, Pitts RJ, Rokas A, Zwiebel LJ. Antennal transcriptome profiles of anopheline mosquitoes reveal human host olfactory specialization in Anopheles gambiae. BMC Genom. 2013;14:1–15. doi: 10.1186/1471-2164-14-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose NH, et al. Climate and urbanization drive mosquito preference for humans. Curr Biol. 2020;30:3570–3579. doi: 10.1016/j.cub.2020.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse S, Krieger J. Olfaction in insects. e-Neuroforum. 2011;17:49–60. doi: 10.1007/s13295-011-0020-7. [DOI] [Google Scholar]
- Sánchez-Gracia A, Vieira F, Rozas J. Molecular evolution of the major chemosensory gene families in insects. Heredity. 2009;103:208–216. doi: 10.1038/hdy.2009.55. [DOI] [PubMed] [Google Scholar]
- Shastry V, et al. Model-based genotype and ancestry estimation for potential hybrids with mixed-ploidy. Mol Ecol Resour. 2021;21:1434–1451. doi: 10.1111/1755-0998.13330. [DOI] [PubMed] [Google Scholar]
- Shute PG. Culex molestus . Trans R Entomol Soc Lond. 1951;102:380–382. doi: 10.1111/j.1365-2311.1951.tb00758.x. [DOI] [Google Scholar]
- Spielman A. Structure and seasonality of Nearctic Culex pipiens populations. Ann N Y Acad Sci. 2001;951:220–234. doi: 10.1111/j.1749-6632.2001.tb02699.x. [DOI] [PubMed] [Google Scholar]
- Stone C, Gross K. Evolution of host preference in anthropophilic mosquitoes. Malar J. 2018;17:1–11. doi: 10.1186/s12936-018-2407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD, Bass AJ, Dabney A, Robinson D (2021) qvalue: Q-value estimation for false discovery rate control. http://github.com/jdstorey/qvalue. R package version 2.26.0.
- Sun H, Liu F, Ye Z, Baker A, Zwiebel LJ, et al. Mutagenesis of the orco odorant receptor co-receptor impairs olfactory functionin the malaria vector Anopheles coluzzii. Insect Biochem Mol Biol. 2020;127:103497. doi: 10.1016/j.ibmb.2020.103497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JS, Xiao S, Carlson JR. The diverse small proteins called odorant-binding proteins. R Soc Open Biol. 2018;8:180208. doi: 10.1098/rsob.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C-J, Bell KL, Nice CC, Martin NH. Integrating Bayesian genomic cline analyses and association mapping of morphological and ecological traits to dissect reproductive isolation and introgression in a Louisiana iris hybrid zone. Mol Ecol. 2018;27:959–978. doi: 10.1111/mec.14481. [DOI] [PubMed] [Google Scholar]
- Syed Z, Leal WS. Acute olfactory response of Culex mosquitoes to a human-and bird-derived attractant. Proc Natl Acad Sci. 2009;106:18803–18808. doi: 10.1073/pnas.0906932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Ann Rev Entomol. 2013;58:433–453. doi: 10.1146/annurev-ento-120811-153618. [DOI] [PubMed] [Google Scholar]
- Taparia T, Ignell R, Hill SR. Blood meal-induced regulation of the chemosensory gene repertoire in the southern house mosquito. BMC Genom. 2017;18:1–9. doi: 10.1186/s12864-017-3779-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegoni M, Campanacci V, Cambillau C. Structural aspects of sexual attraction and chemical communication in insects. Trends Biochem Sci. 2004;29:257–264. doi: 10.1016/j.tibs.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Van Breugel F, Riffell J, Fairhall A, Dickinson MH. Mosquitoes use vision to associate odor plumes with thermal targets. Curr Biol. 2015;25:2123–2129. doi: 10.1016/j.cub.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. Anopheles gambiae complex and disease transmission in Africa. Transactions of the Royal Society of Tropical Medicine and hygiene. 1974;68:278–298. doi: 10.1016/0035-9203(74)90035-2. [DOI] [PubMed] [Google Scholar]
- Xuereb A, Kimber CM, Curtis JM, Bernatchez L, Fortin M-J. Putatively adaptive genetic variation in the giant California sea cucumber (Parastichopus californicus) as revealed by environmental association analysis of restriction-site associated DNA sequencing data. Mol Ecol. 2018;27:5035–5048. doi: 10.1111/mec.14942. [DOI] [PubMed] [Google Scholar]
- Ye Z, et al. Discrete roles of ir76b ionotropic coreceptor impact olfaction, blood feeding, and mating in the malaria vector mosquito Anopheles coluzzii. Proc Natl Acad Sci. 2022;119:e2112385119. doi: 10.1073/pnas.2112385119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenko AA, et al. Genomic differentiation and intercontinental population structure of mosquito vectors Culex pipiens pipiens and Culex pipiens molestus. Sci Rep. 2020;10:1–13. doi: 10.1038/s41598-020-63305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this study are available at: http://www.ncbi.nlm.nih.gov/bioproject/1068091. All scripts used to perform these analyses can be found at: https://github.com/katebell1987/CulexMS1 or upon request from corresponding authors.