Abstract
Emotions experienced within sleep mentation (dreaming) affect mental functioning in waking life. There have been attempts at enhancing dream emotions using olfactory stimulation. Odors readily acquire affective value, but to profoundly influence emotional processing, they should bear personal significance for the perceiver rather than be generally pleasant. The main objective of the present sleep laboratory study was to examine whether prolonged nocturnal exposure to self-selected, preferred ambient room odor while asleep influences emotional aspects of sleep mentation and valence of post-sleep core affect. We asked twenty healthy participants (12 males, mean age 25 ± 4 years) to pick a commercially available scented room diffuser cartridge that most readily evoked positively valenced mental associations. In weekly intervals, the participants attended three sessions. After the adaptation visit, they were administered the odor exposure and odorless control condition in a balanced order. Participants were awakened five minutes into the first rapid eye movement (REM) stage that took place after 2:30 a.m. and, if they had been dreaming, they were asked to rate their mental sleep experience for pleasantness, emotional charge, and magnitude of positive and negative emotions and also to evaluate their post-sleep core affect valence. With rs < 0.20, no practically or statistically significant differences existed between exposure and control in any outcome measures. We conclude that in young, healthy participants, the practical value of olfactory stimulation with self-selected preferred scents for enhancement of dream emotions and post-sleep core affect valence is very limited.
Keywords: Dreaming, Hedonic, Olfactory, REM, Smell
Subject terms: Human behaviour, Psychology
Introduction
Mental activity in sleep, also referred to as “sleep mentation”, “mental sleep experience”, or simply “dreaming”1, can profoundly affect daytime mood2,3, mainly when the experience is negatively emotionally valenced4. Emotions appear to be present in a high proportion of recalled dreams5 and, for the most part, tend to be negative rather than positive, even in healthy individuals5,6. Particularly distressing emotions, such as anxiety, fear, terror, anger, sadness, or frustration7, are experienced in bad dreams (which are negatively toned dreams that do not wake the sleeping person up) and in nightmares (which do lead to awakening)8. They may distort sleep architecture9 and hence impair sleep quality10, but also many other aspects of both sleeping and waking life11, including well-being12. The link between mental sleep experiences and functioning in waking life may nevertheless generalize to all dreams13–15.
Given the multifaceted influence of mental sleep experiences on waking life16,17, there have been attempts to modulate emotions experienced while dreaming, e.g., through sensory stimulation18–21. The sensory modality that readily lends itself to this purpose is olfaction because it is tightly interwoven with affective processing22,23. Hedonic value plays a central role in olfactory processing24, and odors have been repeatedly shown to influence affective processing even without conscious perception25,26. This close association between the sense of smell and emotion has been attributed to their shared neural substrates27, which include the amygdala and hippocampus28–30 and the prefrontal cortex31,32. Hence, one notable aspect of olfactory processing is the ease with which odors acquire their affective value. This usually happens through evaluative conditioning33,34, whereby odor stimuli readily assume the tone of the context within which they were experienced35–38. This acquired affective value then translates to their effects on affective and cognitive processing on following exposures39, which can be inferred from neural, physiological, and behavioral responses37,38,40–45.
The capacity of odors to influence mental processing has been utilized in studies of the effects of odor exposure during sleep on sleep mentation. It transpired that odors presented during sleep may affect dream emotions and possibly even dream content3,4,46,47. For instance, in 15 healthy young females in rapid eye movement (REM) sleep, Schredl and colleagues47 found that the emotional tone of sleep mentation (i.e., “dreams” in the broadest sense1) was somewhat more positive upon exposure to generally pleasant phenylethylalcohol (PEA, which has a rose-like odor) compared to generally unpleasant hydrogen sulfide (H2S, which smells of rotten eggs). On the other hand, in depressed female inpatients, Vitinius and colleagues48 observed no significant effect of exposure to PEA on sleep mentation or mood. Another team that used PEA for REM exposure recruited healthy participants who either liked (N = 7, 4 men) or disliked (N = 8, 2 men) its odor46. Their findings were, however, rather surprising in that liking the smell of PEA was linked to a greater degree of negative emotions experienced while asleep under the PEA condition compared to the odorless one. Finally, Sabiniewicz et al.49 randomly assigned their participants to either odor (orange, perfume, or laurinal; delivered via a nose clip) or placebo group and investigated whether condition affected sleep quality over a fortnight of odor exposure/non-exposure and another fortnight post-exposure/non-exposure, controlling for odor pleasantness. They found that reports of sleep and dreaming did not differ with respect to group. Participants who found the odors more pleasant felt more rested on night 28 compared to night 1, while those who rated them as less pleasant tended to feel more rested on night 13 compared to night 2849.
Odor pleasantness tends to exhibit associations with other perceptual properties, such as familiarity [e.g.,50]. Hence, in line with their previous finding, in a later study, Okabe et al.51 showed that adolescents who were grouped according to the rated familiarity of PEA (low/high) reported more negative emotions in their dreams in the PEA condition when its odor was perceived as highly familiar. On the other hand, the latter group experienced more positive dreams than the former when no odor was presented to them51. Another characteristic linked to olfactory hedonic appraisal is odor intensity50. Recently, Okabe and Abe52 observed no differences in dream emotionality and emotional tone between participants who perceived the odor of PEA as intense vs. weak.
Currently, the evidence on the influence of smells presented during sleep on sleep mentation is inconclusive, and the links of perceived odor pleasantness or other related perceptual properties with dream characteristics seem mixed. A potential reason might be that researchers have focused on perceptual pleasantness, disregarding odors’ affective value, which tends to be highly idiosyncratic across people and might lie at the core of olfactory effects on dreaming. Despite this, olfactory exposure studies in which individual odor preference was considered are the exception rather than the rule46,53. In the present investigation of the capacity of ambient odor to modulate affect, we aimed to make full use of the profound individuality of olfactory preferences. This was accomplished by asking participants to choose from a large set of designer room fragrances one scent that most readily evoked positively-valenced mental associations (e.g., reminded them of a beloved significant other, moment of happiness, treasured object, etc.) or conjured up pleasant imagery. Building on the literature on olfactory associative learning and odor primes, the main objectives of the present study were to test whether (1) dream pleasantness, (2) dream emotional charge and magnitude of (3) positive and (4) negative emotions present in dreams differ upon extended, several hour-long exposure to a subjectively salient pleasant ambient odor compared to a time spent in the same room without odorization. Furthermore, assuming continuity between dreaming and waking life [e.g.,14,15], we also tested whether (5) the valence dimension of a person’s post-sleep affect (e.g., feeling happy, optimistic, and relaxed rather than unhappy, pessimistic, and tense) differs between the two conditions. We hypothesized that dreams would be experienced as more emotionally charged, contain a greater degree of positive and a lesser degree of negative emotions, and that the individual’s post-sleep core affect would be more positively valenced upon exposure compared to the odorless condition. Although dreaming is by no means limited to REM sleep54, REM mental processing may qualitatively differ from non-REM55, particularly in the greater involvement of REM sleep in the processing of emotional material56. Therefore, reports were collected upon awakening from REM.
Results
Descriptives
Descriptive statistics of the participant sample are shown in Table 1.
Table 1.
Absolute frequencies and percentages for education, time of going to bed, dream recall and emotional intensity of dreams, and mean ± SD for age, Beck Depression Inventory-II (BDI-II), State-Trait Anxiety Inventory (STAI X-II), University of Pennsylvania Smell Identification Test (UPSIT) scores, monthly consumption of alcohol and stimulants, and sleep duration on weekdays and the weekend.
| Men (N = 12) | Women (N = 8) | Total (N = 20) | |
|---|---|---|---|
| Age | 25.8 ± 4.5 (20–35) | 24.0 ± 1.7 (22–27) | 25.1 ± 3.7 (20–35) |
| Education | |||
| Elementary | 0 | 0 | 0 |
| Secondary | 6 (50%) | 5 (63%) | 11 (55%) |
| Bachelor’s Degree | 3 (25%) | 2 (25%) | 5 (25%) |
| Master’s Degree | 2 (17%) | 1 (13%) | 3 (15%) |
| Doctoral Degree | 1 (8%) | 0 | 1 (5%) |
| BDI-II score | 3.2 ± 3.2 (0–11) | 2.9 ± 2.2 (0–6) | 3.1 ± 2.8 (0–11) |
| STAI X-II score | 37.9 ± 8.2 (24–53) | 32.4 ± 5.7 (23–39) | 35.6 ± 7.6 (23–53) |
| UPSIT score | 31.6 ± 3.6 (25–37) | 34.6 ± 1.8 (32–38) | 32.8 ± 3.32 (25–38) |
| Alcohol | |||
| Soft | 6.3 ± 6.6 (0–18) | 9 ± 10.6 (1–32) | 7.4 ± 8.2 (0–32) |
| Hard | 0.9 ± 1.3 (0–4) | 0.3 ± 0.5 (0–1) | 0.7 ± 1.1 (0–4) |
| Stimulants | |||
| Coffee | 6.5 ± 9.6 (0–28) | 14 ± 23 (0–60) | 9.5 ± 16.2 (0–60) |
| Tea | 17.9 ± 17.9 (0–60) | 18.4 ± 16.9 (0–50) | 18.1 ± 17.1 (0–60) |
| Energy drinks | 0.2 ± 0.6 (0–2) | 0.4 ± 0.7 (0–2) | 0.3 ± 0.6 (0–2) |
| Cigarettes | 4 ± 8.9 (0–30) | 0 | 2.4 ± 7.1 (0–30) |
| Time of going to bed on weekdays | |||
| Before midnight | 10 (83%) | 6 (75%) | 16 (80%) |
| At midnight or later | 2 (17%) | 2 (25%) | 4 (20%) |
| Sleep duration (hours) | |||
| Weekdays | 7.7 ± 0.5 (7–8.5) | 7.6 ± 0.7 (6.5–8.5) | 7.7 ± 0.6 (6.5–8.5) |
| Weekends | 9.0 ± 0.7 (8–10) | 8.9 ± 0.6 (8–10) | 8.9 ± 0.7 (8–10) |
| Self-assessed dream recall | |||
| Once a week | 6 (50%) | 4 (50%) | 10 (50%) |
| More than once a week | 6 (50%) | 4 (50%) | 10 (50%) |
| Emotional intensity of dreams | |||
| Somewhat intense | 8 (67%) | 3 (38%) | 11 (55%) |
| Quite or very intense | 4 (33%) | 5 (62%) | 9 (45%) |
Alcohol units for soft alcohol represent a small beer (0.33 l) or a glass of wine (0.2 l); for hard alcohol a 0.2 l glass. Units for coffee/tea and energy drinks are cups and bottles, respectively.
Main results: differences between the exposure and control condition
Wilcoxon signed-rank tests did not reveal any statistically significant differences between the exposure and control condition in dream pleasantness, emotional charge, the magnitude of positive or negative emotions, or the valence dimension of core affect. With rs < 0.20, the practical significance of most of the effects fell well below the recommended minimum effect size that represents a “practically” significant effect57, although the comparisons of dream emotional charge and mean positive and negative emotions, respectively, closely missed this mark. For complete results, please see Table 2.
Table 2.
Absolute (N) and relative frequencies (percentages) for dream recall and odor presence appraisal, mean ± SD, median, and range for dream pleasantness and emotional charge, mean positive and negative emotions, and the valence dimension of post-sleep core affect.
| Odorless control | Odor exposure | Wilcoxon signed-rank test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Range | Mean ± SD | Median | Range | W | p | r | N | |
| Dream recall (Yes/No) | 15 (75%) | 15 (75%) | ||||||||
| Reported odor presence (Yes/No) | 3 (15%) | 12 (60%) | ||||||||
| Dream pleasantness (1–7) | 4.47 ± 0.92 | 4.00 | 3.00–6.00 | 4.47 ± 1.13 | 4.00 | 3.00–7.00 | 20.50 | 0.722 | 0.07 | 12 |
| Dream emotional charge (1–7) | 3.40 ± 1.96 | 3.00 | 1.00–7.00 | 4.13 ± 1.30 | 4.00 | 2.00–6.00 | 19.00 | 0.391 | 0.17 | 12 |
| Mean positive dream emotions (1–7) | 3.22 ± 1.36 | 3.00 | 1.50–6.25 | 3.58 ± 1.71 | 3.75 | 1.50–6.00 | 49.00 | 0.432 | 0.16 | 12 |
| Median positive dream emotions (1–7) | 3.27 ± 1.70 | 3.00 | 1.00–7.00 | 3.63 ± 1.89 | 4.00 | 1.00–6.00 | 39.50 | 0.562 | 0.12 | 12 |
| Mean negative dream emotions (1–7) | 1.82 ± 1.07 | 1.50 | 1.00–4.75 | 2.15 ± 1.18 | 1.75 | 1.00–5.00 | 43.00 | 0.370 | 0.18 | 12 |
| Median negative dream emotions (1–7) | 1.60 ± 1.17 | 1.00 | 1.00–5.00 | 1.90 ± 1.17 | 1.50 | 1.00–5.00 | 27.50 | 0.546 | 0.12 | 12 |
| Core affect valence index (3–27) | 16.75 ± 3.34 | 16.00 | 12.00–24.00 | 16.59 ± 3.59 | 16.00 | 11.00–23.00 | 35.00 | 0.858 | 0.03 | 14 |
Exploratory comparisons
Differences between the randomization groups
In the control condition, there were no practically and/or statistically significant differences between participants depending on their randomization status (i.e., whether the control session was scheduled for their second or third visit). However, it did matter when exposure occurred in terms of the reported magnitude of negative dream emotions and post-sleep core affect valence. Specifically, participants exposed on their third visit reported lower intensities of negative dream emotions (mean ratings: 2.82 vs. 1.56, respectively; Mann–Whitney U = 10.50, p = 0.038, r = -0.53, N = 7 vs. 8), as well as higher Valence scores (14.78 vs. 18.63; U = 15.00, p = 0.042, r = 0.49, N = 9 vs. 8) than volunteers stimulated on their second visit. In other words, participants whose room was odorized on their third visit experienced less intense negative emotions in their dreams, and felt pleased, glad, and happy to a greater extent than displeased, sad, and depressed, respectively (which were the three adjective pairs loading onto the Valence dimension58).
Gender differences
Several differences emerged between the female and male participants. Women reported greater emotional charge of their dreams (control: 4.83 ± 1.94 vs. 2.44 ± 1.33; U = 8.00, p = 0.022, r = -0.59; exposure: 4.86 ± 1.07 vs. 3.50 ± 1.20; U = 12.00, p = 0.054, r = -0.50). Women also reported higher post-sleep core affect valence under exposure (18.38 ± 3.38 vs. 15.00 ± 3.12; U = 15.50, p = 0.047, r = -0.48). On their third (but not second) visit, women (compared to men) reported a greater emotional charge of their dreams (5.50 ± 1.23 vs. 2.88 ± 1.13; U = 3.00, p = 0.006, r = -0.74). Additional descriptive statistics and exploratory analyses are presented in the Supplementary Material.
Discussion
In the present study, we aimed to investigate whether spending the night in a sleep laboratory bedroom odorized with a self-selected pleasant odor affects dream pleasantness and emotional charge, the magnitude of positive and negative dream emotions, and the valence of the post-sleep affective state. The underlying assumption was that the olfactory modulation of affect would be afforded by the previous association of the self-selected odor with something in the participants’ experience that they explicitly designated as pleasant23,59,60.
We did not find any significant differences in the outcome measures between the odorless control and odor exposure condition. In terms of practical significance, the effect sizes were negligible. However, some emotion-related measures were close to r = 0.20, which some authors the lower limit of what could be called a “small” effect57. Hence, we tentatively suggest that the practical utility of exposing oneself to self-selected pleasant odor stimuli while asleep to enhance dream emotionality and post-sleep core affect valence is very limited in young, healthy adults. This is, however, not to say that sensory-based interventions in general and olfactory exposure in particular are of no practical value. These applications have been investigated predominantly within settings where optimization of arousal is desirable, such as in-patient and community health services61–64, high-stress academic environments65, or even classrooms at lower educational stages66. In particular, there is a growing body of research on the use of sensory design in general and sensory rooms in particular in the mental health and social work settings, which has been reported, e.g., to reduce stress and facilitate self-regulation, instill relaxation and augment positive emotions67–70. Modified sensory environments are employed to achieve a range of outcomes with diverse clinical populations. The use of soothing ambient stimuli to support adaptive emotion regulation has broad applications. Everyone can benefit from self-care practices that enhance sensory comfort to promote well-being66,71. However, we should remember that such olfactory interventions must be highly personalized and that actual exposure likely serves as a mere facilitator of the effects the given individual attributes to the odor in question.
Relatedly, a crucial methodological aspect that is often overlooked is the considerable individual variation in hedonic responses to odors72–75. There has been a notable lack of appreciation for the idiosyncrasies of olfactory perception in general and odor hedonics in particular50. On the one hand, pleasantness is linked to physicochemical structure of molecules24,76,77, with pleasant ones generally being larger and containing oxygen78. Arshamian et al.74 found substantial global consistency in odor liking across a number of cultures, which was predicted by the physicochemical properties. Some odors may be universally liked, such as sweet and floral ones24, although the liking will vary with intensity. The relationship between pleasantness and intensity may be linear for some odors but usually follows an inverted U-curve trend79–81. Yet, for an odor to profoundly influence affective processing and behavior, it needs to be perceived as subjectively—rather than “universally”—pleasant82, but also to have subjective affective value83. This is particularly evident in studies on odor-evoked autobiographical memory and nostalgia (for review, see84).
Olfactory exposure studies in which individual odor preference was considered are the exception rather than the rule46,53. One of the few exceptions was the study by Villemure, Slotnick, and Bushnell53, who reported that mood improved, anxiety decreased, and perceived pain unpleasantness diminished following exposure to a subjectively pleasant odor. In contrast, exposure to one that they designated as unpleasant had the opposite effect.
In the present investigation of the capacity of ambient odor to modulate affect, we aimed to make full use of the profound individuality of olfactory preferences. This was addressed by asking participants to choose from a large set of designer room fragrances one scent that most readily evoked positively-valenced mental associations (e.g., reminded them of a beloved significant other, moment of happiness, treasured object, etc.) or conjured up pleasant imagery. However, the stimuli were selected under a forced-choice paradigm and may not reflect one’s true olfactory preferences, which is one of the current study's limitations. We utilized room scents that were readily available from the odor diffuser manufacturer to keep the perceived intensity at comparable levels across participants and to have complete knowledge of the scents’ chemical composition and odor profile. Also, we only asked the participants to pick the exposure stimuli on their first, adaptation session that was not subject to formal analysis, which was to reduce the effect of the participants’ potential expectancies on the reports collected within the next two sessions that were a week and a fortnight apart from this baseline, respectively. Yet, that may have created a sense of pressure in the participants or feeling overwhelmed at having to deal with a 40-item stimulus set, even though they had been explicitly instructed to take their time as much as they needed and appraise the stimuli at their own pace during the ~ 4-h gap between the check-in and the start of the nocturnal session.
Another issue might have been the prolonged period of continuous olfactory stimulation, which was adopted to mimic the conditions of at-home odor exposure as closely as possible. It may have resulted in olfactory adaptation, which, on the behavioral level, manifests itself as habituation, i.e., reduced responsiveness to continuous or repetitive stimulation85,86. Yet, it is unclear whether olfactory adaptation and habituation pose a significant obstacle to olfactory modulation of sleep mentation. In studies that employed advanced olfactometry to minimize these potentially adverse effects, the rates of incorporating olfactory stimuli in sleep mentation were relatively low46,47,87.
On a related note, it is not entirely clear whether conscious awareness of odor presence during waking is a shortcoming in studies like the present one or whether it is actually desirable. On the one hand, unmasked odors may be used as cues that may influence participants’ retrospective reports about the preceding sleep period in line with beliefs about the odors and their actions. As but one example, the purported effect of essential oils may be modulated by one’s expectancy or belief about their efficacy88,89. On the other hand, reporting odor presence may indicate that the prolonged odor exposure did not result in olfactory adaptation/habituation. There is, nevertheless, no conclusive evidence as to whether these processes interfere with odor-induced modulation of dream emotionality ratings and post-sleep core affect. Conscious odor perception may not be necessary for (some) odors to influence our feelings and actions25,26. In our study, we made no effort to mask the odor presence during waking to achieve a more ecologically valid setting that resembled circumstances under which room odorization would occur at people’s homes. As Table 2 suggests, 12 (60%) participants reported that they perceived an odor in the exposure condition, presumably reflecting their conscious awareness of the self-selected scent. Given the way the question was phrased (“Have you noticed any odor in this room?”), an affirmative response might also indicate that any faint background odor in the room was detected. In other words, an affirmative reply was not necessarily a “hit” in the sense that the participant noticed the self-selected scent. Theoretical debates notwithstanding, there were no statistically significant differences in the outcome measures between “hits” and “misses”. This might, however, be attributed to the small N. With effect sizes rs ranging between 0.05 (for dream emotional charge) and 0.48 (for mean positive dream emotions), appraisal of odor presence/absence, which could not be meaningfully included in the present models due to the limited sample size, warrants consideration in future studies.
Related to this, while we had carried out an a priori sample size computation (as detailed in the Participants section), we unfortunately found ourselves unable to meet the target N. This was because, firstly, we had obtained a lower dream recall rate of 75% compared to our previous study90, which had a rate of 83%. Secondly, we encountered technical issues during the v-PSG recordings, rendering fewer reports available for analysis. The tight lab schedule prevented us from making up for the lost data within the limited time slot dedicated to the present study. Hence, future studies would benefit from accounting for factors that are difficult to predict and are beyond the experimenters’ control, such as the dream recall rate, and increasing the N accordingly.
Materials and methods
Participants
Twenty healthy young adults aged 25.1 ± 3.7 years participated in the study, of which 12 self-identified as male (25.8 ± 4.5 years) and eight as female (24.0 ± 1.7 years). Men and women did not differ in age. The inclusion criteria for participation were (1) being over 18 but less than 35 years of age (which was to reduce the potential confounding effect of olfactory decline that begins towards the end of early adulthood91) and (2) use of hormonal contraceptives in women to minimize fluctuations in the sense of smell across the menstrual cycle92. Exclusion criteria were: (1) self-reported history of neurological, psychiatric or other disorders that are known to affect olfaction93 or sleep94,95, with anxious and depressive tendencies being a prime example; (2) suffering from any medical condition at the present time; (3) a history of head trauma with unconsciousness; (4) taking medication with known effects on olfaction or sleep93; (5) persistent olfactory complaints96,97; (6) mild or more severe depressive tendencies, as evidenced by a score above the minimal range of 0–13 points98 on the Beck Depression Inventory II (BDI-II;99,100); (7) trait anxiety of potential clinical significance, as suggested by a score greater than 39101 on the State-Trait Anxiety Inventory (STAI X-II;102); (8) tobacco dependence beyond light smoking, defined as more than 90 cigarettes a month103; (9) alcohol dependence beyond moderate drinking, defined as more than one drink per day in women and two drinks per day in men, where a “drink” is 0.33 l of beer, 0.2 l of wine, or 0.02 l of liquor104; (10) a history of substance abuse, particularly illicit drug use; and (11) pregnancy or breastfeeding in women. Volunteers were recruited via posts advertising the study on the website and Facebook page of the National Institute of Mental Health (NIMH-CZ) and Faculty of Humanities, Charles University (FHS UK). Compliance with the above-specified criteria was pre-screened with a Qualtrics questionnaire battery (Qualtrics, Provo, UT). Individuals who had indicated their preliminary interest in study participation were e-mailed a link to the anonymous survey, which they completed using a self-generated ID. Eligibility for study participation was evaluated by the researchers and the results were communicated to the respondents within two weeks. Descriptive statistics of these variables are shown in Table 1. Respondents found eligible and confirmed their ongoing interest in study participation were invited to make an appointment with the in-house physician (EM), who confirmed good general health status and referred them as healthy volunteers to the sleep laboratory.
The sample size was calculated a priori based on our previous study with similar design, in which a generally liked and disliked odor was used90. The mean difference between the control and exposure condition in dream pleasantness and emotional charge, averaged positive and negative emotions, and core affect ratings was about 1, with an SD of about 1.5. We aimed for a power (1-β) of 0.8, generally recommended by Cohen105. At a significance level (α) of 5% and power (1-β) of 80% (two-sided test), the estimated sample size was 20 pairs. However, as the actual valid N depends on the dream recall rate, which was 83% in90 but only 75% in the present study, and we encountered technical problems during the v-PSG recordings, fewer reports were available for analysis. Due to time constraints and a tight lab schedule, we could unfortunately not make up for the data loss within the time slot dedicated to this study.
Ethics statement
All of the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and the Helsinki Declaration of 1975, as revised in 2008. The study protocol was approved by the Institutional Review Board of the National Institute of Mental Health as part of a larger project on psychophysiological correlates of dreaming under all-night ambient odor exposure (Approval No. 57/16, amended by Approval No. 5/18). Written informed consent was obtained from the participants. Upon study completion (i.e., on their third and final visit), the participants received a financial compensation of 1500 CZK (app. 58 EUR).
Questionnaires
Health- and sleep-related screening inventory
The pre-screening battery consisted of a brief inventory to evaluate the respondents’ family and personal medical history, including neuropsychiatric and neurological diagnoses, past admissions to medical facilities for treatment, medication used in the previous six months, sleep habits, and consumption and abuse of substances known to affect olfaction and/or sleep (e.g., nicotine, caffeine, alcohol, illicit drug use). This data was used to evaluate compliance with exclusion criteria.
Beck Depression Inventory-II (BDI-II)
Depressive tendencies, which were part of the exclusion criteria, affect olfactory perception106,107 and sleep108,109 and may contribute to the so-called first-night effect110,111, whereby sleep tends to be distorted during the first nocturnal assessment at the sleep laboratory112,113. They are also linked to intense negative reactions to even weak everyday odors114,115.
To briefly evaluate depressive tendencies, we used the 21-item BDI-II100, published in Czech by Preiss and Vacíř99. Within each item, respondents are presented with a list of four graded statements, for example, “I did not have any thoughts of killing myself”; “I have had thoughts of killing myself, but I would not carry them out”; “I would have liked to kill myself”; “I would have killed myself if I had the chance” and asked to select the one that best reflected how they had been feeling in the past two weeks. The statements are always weighted from 0 (not present) to 3 (severe), with higher scores indicating greater severity. Themes assessed by the instrument include sadness, pessimism, past failure, loss of pleasure, guilty feelings, feelings of punishment, self-dislike, self-criticalness, suicidal thoughts or wishes, crying, agitation, loss of interest, indecisiveness, feelings of worthlessness, loss of energy, changes in sleeping patterns, irritability, changes in appetite, difficulties concentrating, tiredness or fatigue, and loss of interest in sex. The individual weights are summed to produce the total score with a theoretical range of 0 to 63 points. The higher the total score, the greater the depressive tendencies. Only respondents scoring 0–13 points were deemed eligible for participation in the present study.
State-Trait Anxiety Inventory (STAI X-II)
Anxiety is another factor that may play a role in the first-night effect116,117. Furthermore, trait anxiety is likewise linked to various alterations and impairments of chemosensory processing118–120 and intense negative responses to common odors115,121.
To screen for anxious tendencies, we employed the validated State-Trait Anxiety Inventory (STAI;122). Its two forms are widely used in research and clinical practice to assess state (form X–I) and trait (form X–II) anxiety. To rule out an anxious disposition that is of clinical interest and might interfere with experimental procedures, we asked our participants to complete the trait scale, which has nonetheless been found also to encompass depression in addition to anxiety123. The trait scale consists of 20 statements, such as “I feel pleasant” or “I wish I could be as happy as others seem to be”, and respondents are asked to rate each of them with regard to how they generally feel. Ratings are provided using a four-point scale (almost never/sometimes/often/almost always). Positive items must be reverse-coded before calculating the total trait anxiety score, which may range between 20 and 80 points. Higher scores are indicative of greater tendencies towards anxiety, with scores over 39 suggesting anxiety of clinical significance101. The Czech translation of the restandardized version was used102.
Dream characteristics and emotions inventory
On awakening, the participants were asked to complete a custom-designed inventory to assess several affective properties of the mental sleep experience they may have had during the preceding sleep period. The participants were first asked whether they had been dreaming (yes/no), and if so, how pleasant and emotionally charged their experience was on a 7-point scale anchored with 1 = “extremely unpleasant” and 7 = “extremely pleasant” and 1 = “not at all” and 7 = “to a great extent”, respectively. Further, they rated the presence of selected positive and negative emotions in their dreams on a 7-point scale (1 = “not at all”, 7 = “to a great extent”). The selection of the four most common positive dream emotions (i.e., joy/happiness, love, contentment, and interest/excitement) was based on the work of Fredrickson124 and that of the negative ones (i.e., anger, apprehension/fear, sadness, and confusion/shock) on Domhoff125. No definition of dreaming or dream emotions was provided, and the participants did not request any clarifications of the terms used. The individual emotion ratings within either category (i.e., positive and negative) were averaged across each participant, with higher mean scores indicating greater magnitude of positive and negative emotions experienced in sleep mentation.
Swedish Core Affect Scale: Valence dimension index
To measure the current affective state, we asked the participants to complete the Swedish Core Affect Scale126. Russell127 defines core affect as “a neurophysiological state, accessible to consciousness as a simple nonreflective feeling: feeling good or bad, feeling lethargic or energized.” It is not synonymous with “emotion”, which begins and ends128; rather, it somewhat resembles “mood” because it lasts and varies over time along with the activity of the autonomic nervous system, cognitive processes, and behavior129. Although authors differ in their views of how many dimensions of core affect there are126,130–132, in subjective perception, these dimensions fuse to produce “a single integral blend”129. One of the main dimensions of core affect is Valence, also referred to as positive—negative hedonic tone, pleasure—displeasure or pain, approach—avoidance133,134. Positive valence means feeling pleased, glad, and happy rather than displeased, sad, and depressed. The Valence dimension comprises three adjective pairs, each rated on a nine-point scale. For instance, if one is feeling as displeased as they can be, they will place their response as close to the negative end of the scale as possible and receive a score of “1” (and similarly for the other two adjective pairs that constitute the valence dimension, i.e., feeling sad—glad and depressed—happy). The outcome is the dimension index, calculated as Σ (weight x adjective scale rating), where the weight equals 1.
Other instruments
The participants were instructed to follow basic sleep hygiene recommendations135. Compliance was verified with a sleep diary, described in detail in Martinec Nováková et al.90. The participants kept the diary throughout the 14 days (from the check-in on their first visit to the check-out on the morning of their third (and final) visit). Relatedly, at the check-in of each visit, they also provided assessments of sleep inertia typically experienced over the past week, which may indicate chronic sleep deprivation136 and is characterized by impaired performance, lowered vigilance, drowsiness, sluggishness, and a desire to go back to sleep137. This was done using the 21-item Sleep Inertia Questionnaire (SIQ;138); see Martinec Nováková et al.90 for a detailed description.
Olfactory assessment
Normal olfactory function was ascertained with the Czech version of the 40-item self-administered University of Pennsylvania Smell Identification Test (UPSIT)139. Being a “scratch and sniff” test, this standardized tool consists of 4 booklets, each containing ten chemically microencapsulated odor patches that release an odor when rubbed with a pencil tip. Four possible verbal labels are provided for each odor, of which one is the target label, and three serve as distractors. For each correct choice, the participant earns one point, and the individual scores are summed to produce the total odor identification score, which may range between 0 and 40. Normative data for the Czech population are not available, but in the US population, scores greater than 31 in men and 30 in women are thought to be indicative of normosmia140. The test–retest reliability of UPSIT exceeds 90%139, and the completion time is about 20 min.
Olfactory stimulation
Each participant was presented with a set of 40 scented cartridges. They were placed at random in a box. The participants were instructed to retrieve them at random, one by one, sniff at each one of them, and finally pick whichever reminded them of something pleasant and intensely positive. They were told to take as much time as needed, proceed at their own pace and complete the task anytime within the circa four-hour time frame (from check-in at 6 p.m. to the start of the session at 10 p.m.). The task was introduced as follows: “This box contains 40 fragrant cartridges. Please smell them all, one by one, and then choose one whose smell evokes intense positive memories of specific people, places, events, periods, etc. If you cannot pick just one of them, please select the one that affects you emotionally the strongest. If none of them elicit any positive memory or emotion, please choose one pleasant enough to consider dispersing in your own bedroom.” The stimuli were designer room fragrance cartridges, manufactured by Mr & Mrs Fragrance or Kartell Fragrances and distributed in the Czech Republic by Joy Fragrances, that were compatible with the home aroma diffuser that was used for odor dispersion (Otello, Mr & Mrs Fragrance). To mimic the real-life context of scented product choice141, we presented the cartridges in their original packaging, allowing the participants to appraise, along with the scent itself, the visual identity of the product (e.g., amber-hued cartridges housed in a sleek gold-and-black paper box), its name (e.g., “Alhambra”), declared scent character (e.g., “citrus, floral, oriental, woody”), and chemical composition (e.g., “tangerine, lime, jasmine, amber, praline, patchouli, cedarwood”). Multiple odor—participant pairings were allowed, and, as Table 3 suggests, several odors were picked by more than one participant. This was even though the position of the individual cartridges within the box was randomized each time before a participant was invited to engage with the stimuli. Next, the participants were instructed to rate the selected scent for pleasantness, intensity, and familiarity. To do so, they were asked to use a 9-point scale, with higher ratings suggesting greater pleasantness, intensity, and familiarity.
Table 3.
Perceptual characteristics (pleasantness, intensity, and familiarity) and mean ratings on the three factors of the Semantic Differential (SDiff), i.e., Evaluation, Potency, and Activity, for the 14 odors used in the study. N > 1 suggests more than one participant selected the odor.
| Odor | Name | Scent composition | N | Perceptual Characteristics | Semantic Differential Factors | Description of mental associations or imagery | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pleasantness | Intensity | Familiarity | Evaluation | Potency | Activity | |||||
| 1 | Hawaiian Poppy | Orange, bergamot, mint, poppy, cyclamen, rose, lilac, woods, musk | 1 | 8 | 3 | 2 | 1.3 | 5.7 | 3.8 | |
| 8 | Florence Talcum Powder | Sicilian lemon, bergamot, pink pepper, iris, violet, rose, cotton plant blossom, cedar wood, vanilla, musk | 1 | 9 | 6 | 9 | 2.1 | 5.0 | 3.8 | Lipstick shared with a friend at high school |
| 19 | Asian Vervain | Mandarin, verveine, lemon blossom, lily of the valley, aromatic herbs, juniper, musk | 1 | 7 | 8 | 7 | 3.1 | 5.3 | 3.8 | |
| 20 | Hokkaido Lavender | Lavender, orange, patchouli, citronella, geranium, tonka bean, sage, fern, musk | 1 | 6 | 7 | 9 | 2.8 | 1.7 | 3.8 | Scent of foaming bath their mother used to prepare for them as young children, bathroom games with brother |
| 31 | Citronella & Flowers | Lemongrass, lemon, elemi, rose, ylang-ylang, guaiac wood | 1 | 8 | 8 | 9 | 2.1 | 1.7 | 4.8 | Working as a tour guide at the Kuks hospital, roaming alone its monumental corridors decorated in frescoes, sunlit and deserted after visiting hours, with a geranium-like floral scent lingering in the air; the smell of its baroque pharmacy and the various herbs, medicines, and ointments of bygone days; a sense of solitude, stillness, and being surrounded by history |
| 32 | Parsley & Tomato | Sparkling parsley, tomato leaves, spring nettle, vegetable garden | 1 | 7 | 7 | 7 | 2.6 | 4.7 | 4.6 | Smell of ex-girlfriend’s place |
| 34 | Citronella & Mint | Lemon, litsea cubeba, mint, lemongrass, orange | 1 | 6 | 8 | 5 | 4.2 | 1.3 | 6.0 | A month-long vacation spent in Australia at the age of 11, scent of the hotel room |
| 37 | Undercover | Plum, jasmine, orange blossom, honey, woody notes, sugar | 1 | 8 | 8 | 7 | 2.8 | 3.3 | 4.4 | |
| 38 | Citrus & Herbs | Citrus, sage, mint, lily of the valley, freesia, sandalwood, musk | 1 | 6 | 6 | 7 | 2.3 | 5.3 | 3.8 | |
| 42 | Lavande Naturelle | Lemon, bergamot, galbanum, basil, clove, lavender, sage, artemisia, oakmoss, patchouli, pine | 2 | 8.5 | 7.5 | 9 | 1.9 | 2.2 | 2.3 | P1: Endless Mediterranean lavender fields, sunny days spent at the sea, sipping lavender lemonade with friends; P2: Herbal scent promoting relaxation, fragrant bath salt used to unwind after a busy day, resting in a scented steam-room, soothing herbal tea, a sense of peace and cleanliness, enjoying “me” time |
| 44 | Temptation Ave | Bergamot, anise, jasmin sambac, rose, sandalwood, amber, vanilla, caramel, tonka beans, musk | 4 | 7.8 | 7.5 | 5.3 | 2.0 | 1.6 | 3.1 | P1: Cotton candy, fair, childhood; P2: Cotton candy, cherry, fair, scented candle gifted by boyfriend, time spent with boyfriend; P3: |
| 46 | Portofino | Bergamot, marine accord, tangerine, green lemon, lavender, rosemary, jasmine, cedarwood, patchouli, amber accord | 1 | 9 | 7 | 9 | 1.6 | 3.3 | 4.2 | Freshly showered boyfriend |
| 47 | Alhambra | Tangerine, lime, jasmine, amber, praline, patchouli, cedarwood | 3 | 8.7 | 5.7 | 4.7 | 2.3 | 3.6 | 2.5 | Some scented product a friend used to wear |
| 48 | Noir | Amber, incense, turkish rose, ylang-ylang, jasmine, cardamom, cumin, atlantic white cedar, oak musk | 1 | 6 | 9 | 7 | 4.4 | 1.3 | 4.2 | Fragrance gifted for Christmas by a high school classmate that was only used on special occasions such as dance classes, prom night, and afterparty. Reminds him of his classmate, then-girlfriend, and lost high-school friends. Evokes a sense of “sweet melancholy” and nostalgia |
The ratings of odors 42, 44, and 47 are averaged scores. Higher perceptual ratings indicate greater pleasantness, intensity, or familiarity. Evaluation SDiff scores ranging between 1 and 3 suggest that the stimulus was rated as fresh, good, happy, harmonious, healthy, beautiful, smooth, clean, and safe rather than stale, bad, sad, unharmonious, unhealthy, ugly, rough, dirty, and dangerous. Evaluation scores in the range of 5–7 suggest the opposite. Potency SDiff scores < 4 indicate that the odor was evaluated as strong, powerful, and harsh rather than weak, powerless, and mild, whereas ratings above 4 suggest the contrary. Activity SDiff ratings ranging between smaller than 4 mean that the stimulus was perceived as more ordered, quiet, relaxing, wet, and muddy than chaotic, noisy, stimulating, dry, and clear, while scores over 4 indicate the opposite. P1, P2, etc. denote Participant 1, Participant 2, etc., reports for odors picked for stimulation more than once.
Semantic Differential
To achieve a more detailed characterization of the stimuli and obtain information about the semantic and affective dimensions that underlie the participants’ mental representations of the odors, we used the Semantic Differential technique (SDiff; 58). Odor SDiff ratings (e.g.,36) are used to capture the complexity of the sensory and affective experience of smelling odors while reducing reliance on verbal descriptions, which are notoriously difficult to provide for odors142. The participants were presented with 17 adjective pairs, which, in the study of Dalton et al.58 loaded onto three dimensions: Evaluation (nine pairs; e.g., good–bad, happy–sad, healthy–unhealthy), Potency (three pairs; strong–weak, powerful–powerless, harsh–mild), and Activity (five pairs; e.g., quiet–noisy, relaxing–stimulating, wet–dry). Each adjective pair was rated on a 7-point scale. A label was placed at each of the scale points, so that the point closest to polar term X was labeled “extremely X”, the next one “quite X”, and the following one “slightly X”. The center of the scale was marked “neither X nor Y; equally X and Y”. Moving towards the opposite polar term Y, the labels followed in reverse order. Ratings on each dimension were averaged across the relevant scales, with lower mean scores suggesting ratings on the given dimension were closer to polar term X than Y. The frequencies of endorsement of the paired polar terms are shown in Fig. 1.
Figure 1.
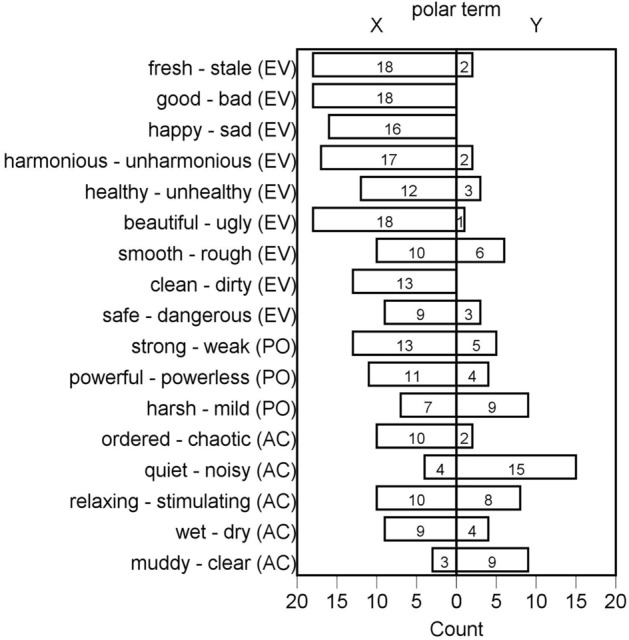
The frequencies of endorsement of the individual polar terms comprising the 17 pairs of adjectives of the olfactory Semantic Differential to evaluate the self-selected, preferred stimuli. Responses “slightly”, “quite”, and “extremely” on the given side of the scale were grouped. Responses “neither X nor Y” and “equally X and Y” are not shown. The left column represents the frequency of the first polar term within the pair, and the right column shows the frequency of the other polar term. For example, eighteen participants felt that the odor of the cartridge they selected was “good” and none thought it was “bad”. AC = Activity, EV = Evaluation, PO = Potency dimension of the olfactory Semantic Differential.
Room odorization and its appraisal
To mimic all-night exposure to ambient odor at home as closely as possible, we utilized a commercial home aroma diffuser (Otello, Mr & Mrs Fragrance). The participant-selected cartridge was used on exposure (stimulation) nights, while on odorless nights, the diffuser operated with no cartridge. Since the diffuser does not generate heat, steam, vibration, etc., and only produces a faint humming sound (about the intensity of a notebook fan), it could be positioned under the bed next to the headboard. The diffuser can be run in several operation modes. One of them affords continuous odor dispersion for up to ten hours. Hence, on stimulation nights, it would be switched on at 10 p.m. and off at the participant’s departure after 7 a.m. to prevent excessive odor dispersion that would require a prolonged ventilation period to get the bedroom ready for subsequent use. On control nights, the empty diffuser was left to run until the staff serving the next shift came to remove it from the bedroom. The air ventilation system in the sleep laboratory was turned off between 10 p.m. and 7 a.m. on both conditions to prevent noise caused by its operation.
The participants were asked to appraise ambient odor in exposure and control conditions. If they indicated that they had noticed any smell in the room, they were asked how pleasant, intense, and familiar it felt on a nine-point scale. In the exposure condition, affirmative responses were considered “hits”; in the control condition, they were regarded as “false alarms”. Negative responses were categorized as “misses” and “correct rejections”, respectively. Nevertheless, the question's phrasing raises the possibility that affirmative responses referring to background odors rather than the stimuli were erroneously classified as “hits”.
Video-polysomnography and sleep stage identification
To identify a waking point from which interim reports could be elicited across all the participants, we asked the participants to undergo overnight video-polysomnography (v-PSG; M&I, Brainscope, Czech Republic). V-PSG is the systematic collection of physiologic parameters during sleep143. We utilized electroencephalography (EEG; F3/A2, F4/A1, C3/A2, C4/A1, O1/A2, and O2/A1 leads), electrooculography (EOG), mental and tibialis anterior electromyography (EMG), electrocardiography (ECG), and pulse oximetry; recorded nasal-oral air flow, oropharyngeal sounds, thoracic and abdominal efforts (via belts), and also obtained synchronized video and audio recordings to determine sleep stages. The sleep stage that was of interest to the present study was the rapid eye movement (REM) stage, which is a physiological state characterized by fast EEG activation with dreaming, cardiovascular changes, muscle atonia, higher arousal threshold, and other features144. Dreaming is by no means limited to REM sleep54. Still, REM mental processing may qualitatively differ from non-REM55, particularly in the greater involvement of REM sleep in processing emotional material56. Sleep stage scoring was performed according to international criteria145 by JB, who was blind to the participants’ randomization status.
Procedure
Eligibility was pre-screened with the health- and sleep-related screening inventory, BDI-II, and STAI X-II. Those who passed the pre-screening were notified within two weeks and invited to schedule an appointment with the in-house physician. The physical examination usually took place several hours before the first night at the sleep laboratory. The physician verified the participants' good health status and referred them to the sleep laboratory.
Meeting the critical but often-overlooked criterion of aromachological research59, namely, that appropriate controls be employed, we required our participants to visit the sleep laboratory repeatedly in weekly intervals on the same day of the week to receive exposure or not. The order of exposure vs. control condition (i.e., on which night the stimulation occurred) was randomized across the participants. Moreover, in sleep studies, it is necessary to also account for the fact that we often experience disrupted sleep in a novel environment146. This is a common sleep disturbance, particularly during the sleep-onset period147, that occurs regardless of the participant’s state anxiety level148. Known as the “first-night effect”112, it is usually addressed by incorporating an extra night in the design that is not analyzed and whose primary purpose is to adapt the participants to the sleep laboratory environment and familiarize them with the procedures149. Therefore, the first night served as an adaptation visit, and the data from that session were not subject to formal analysis.
On each visit, the participants checked in at the medical sleep clinic/laboratory of the National Institute of Mental Health at around 6 p.m. They were required to stay on the premises until the session commenced at 10 p.m. During that time, they spent about 1 hour in the hall completing check-in forms and questionnaires, about 2 hours in the EEG fitting room, and 1 hour relaxing in their bedroom. During this time, they were under constant surveillance. Upon arriving at the sleep laboratory on the first night, the participants were shown around and familiarized with the procedures. Next, they self-administered the UPSIT and were handed the box with scented cartridges for appraisal, stimulus selection, and rating. They were encouraged to go through the box at their own pace, take time, and take as many breaks as needed. This procedure was specific to the first night only. After that, a sequence of procedures was performed on each visit, which involved fitting the v-PSG equipment and completing SIQ for the past week. No pre-sleep affect assessments were made because state affect, i.e., “what one is feeling at any given moment in time”150, and its valence in particular (i.e., negative vs. positive) tends to fluctuate on a momentary and situational basis, and in some people more than in others151. As a result, some people’s ratings of current affective state would have been preponderantly, if temporarily, affected by the procedures they had been subjected to at the sleep laboratory, making it chalenging to select a good point for baseline measurement. Instead, the emotional dimension of SIQ was used to briefly evaluate and control for the participants’ prevailing emotional state specifically with regard to sleep and waking over the past week.
The diffuser was placed under the participants' beds shortly before they retired. V-PSG recordings always started at 10 p.m., when participants went to bed, and the lights went off. For the next eight hours, participants were asked to keep their cell phones, notebooks, and lights in the room and the adjacent bathroom switched off. To request assistance from the sleep laboratory staff (e.g., to get unplugged before using the bathroom), the participants were instructed to sit up on the bed and use the intercom.
REM was the sleep stage we pre-specified for waking to collect interim reports of the affective state. REM is involved in emotional memory processing152 and is thus conducive to collecting reports of sleep mentation46,47,51,87,90, which we then used to control for the effect of dream valence and emotions on post-sleep affect. To this end, after 2:30 a.m., in the monitoring room, the researchers started to visually inspect the v-PSG recording in real time to detect REM. The criteria for REM detection included a desynchronized EEG without spindles or K-complexes accompanied by decreased tonic chin/sub-mental EMG amplitude and rapid eye movements. Once the REM stage hit the five-minute mark, the researchers approached the sleeping participant to wake them up with a pre-arranged signal (e.g., tapping them on the shoulder or calling their name). The participant was temporarily unplugged from the v-PSG equipment and sat at the table with a small lamp to complete the first batch of post-sleep measures. Their completion took about five to ten minutes. The participants first indicated the presence or absence of any sleep mentation and its emotional charge, then assessed their current affective state, and concluded by appraising the ambient atmosphere. All the assessments were made using paper forms (rather than by interviewing participants via the intercom), which, considering the number of administered measures, has previously proven to be practical90. After that, the participant returned to bed, and the v-PSG recording was resumed. It continued until the spontaneous waking in the morning or, if the participant had requested to be woken up, until the pre-arranged waking time. As soon as they got up, the participants again provided evaluations of sleep mentation experienced during the sleep episode that followed the first waking, assessments of post-sleep affect, and appraisal of ambient odor.
A day or two before each scheduled appointment, the participants were reminded of their upcoming visit and asked if they were feeling well. If they had fallen ill, the visit was rescheduled for a later time. On the morning of their third and final visit, the participants were reimbursed with CZK 1,500 in cash. Data collection occurred between September 2018 and March 2019.
Statistical analysis
The exploratory analyses and plots were produced with IBM SPSS 24.0. The normality of the raw data was checked, firstly, by visually examining the individual histograms of all relevant variables, secondly, by producing skewness and kurtosis values and their respective standard errors, from which z-scores were computed and compared to the value of 1.96, as suggested by Field153 and, thirdly, with multiple Shapiro–Wilk’s W tests. Since the data was mostly non-normal and transformations had little effect, non-parametric tests were preferred. To compare the odorless control and odor exposure condition, Wilcoxon signed-rank tests were run. Differences between randomization groups and genders were analyzed using Mann–Whitney U tests. Effect sizes represented by Pearson’s r for Mann–Whitney U and Wilcoxon signed rank tests were computed after Rosenthal154 as follows: .
Supplementary Information
Acknowledgements
The authors would like to express their gratitude to Anna Kernerová for her help with data collection, Radka Vojtušová Mrzílková for her assistance with participant recruitment and administrative work, and Denisa Manková for data digitization. This research was funded by the University of Chemistry and Technology, Prague.
Author contributions
Study conceived and designed by: L.M.N., E.M., M.K., and J.B. Data collection: L.M.N., M.K. and E.M. Data analyzed by: L.M.N. Manuscript drafted by: L.M.N. Final draft reviewed by: L.M.N., E.M., M.K., and J.B.
Data availability
The datasets generated and analysed during this study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-60226-z.
References
- 1.Fagioli I. Mental activity during sleep. Sleep Med. Rev. 2002;6(4):307–320. doi: 10.1053/smrv.2001.0214. [DOI] [PubMed] [Google Scholar]
- 2.Yu CK-C. Emotions before, during, and after dreaming sleep. Dreaming. 2007;17(2):73–86. doi: 10.1037/1053-0797.17.2.73. [DOI] [Google Scholar]
- 3.Wasserman I, Ballif BL. Perceived interactions between the dream and the waking divisions of consciousness. Imagin. Cogn. Personal. 1984;4(1):3–13. doi: 10.2190/3N4G-FFRM-25L9-HCXW. [DOI] [Google Scholar]
- 4.Schredl M, Doll E. Emotions in diary dreams. Conscious. Cogn. 1998;7(4):634–646. doi: 10.1006/ccog.1998.0356. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen TA, Deslauriers D, Baylor GW. Emotions in dream and waking event reports. Dreaming. 1991;1(4):287–300. doi: 10.1037/h0094340. [DOI] [Google Scholar]
- 6.Merritt JM, et al. Emotion profiles in the dreams of men and women. Conscious. Cogn. Int. J. 1994;3(1):46–60. doi: 10.1006/ccog.1994.1004. [DOI] [Google Scholar]
- 7.Zadra A, Pilon M, Donderi DC. Variety and intensity of emotions in nightmares and bad dreams. J. Nerv. Ment. Dis. 2006;194(4):249–254. doi: 10.1097/01.nmd.0000207359.46223.dc. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-5™. 5. American Psychiatric Publishing, Inc; 2013. p. 947. [Google Scholar]
- 9.Simor P, et al. Disturbed dreaming and sleep quality: Altered sleep architecture in subjects with frequent nightmares. Eur. Arch. Psychiatry Clin. Neurosci. 2012;262(8):687–696. doi: 10.1007/s00406-012-0318-7. [DOI] [PubMed] [Google Scholar]
- 10.Paul F, Schredl M, Alpers GW. Nightmares affect the experience of sleep quality but not sleep architecture: An ambulatory polysomnographic study. Borderline Personal. Disord. Emot. Dysregul. 2015;2:3. doi: 10.1186/s40479-014-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly WE. Bad dreams and bad sleep: Relationships between nightmare frequency, insomnia, and nightmare proneness. Dreaming. 2022;32(2):194–205. doi: 10.1037/drm0000203. [DOI] [Google Scholar]
- 12.Blagrove M, Farmer L, Williams E. The relationship of nightmare frequency and nightmare distress to well-being. J. Sleep Res. 2004;13(2):129–136. doi: 10.1111/j.1365-2869.2004.00394.x. [DOI] [PubMed] [Google Scholar]
- 13.Schredl M. The effect of dreams on waking life. Sleep Hypn. 2000;2(3):120–124. [Google Scholar]
- 14.Schredl M, Reinhard I. The continuity between waking mood and dream emotions: Direct and second-order effects. Imagin. Cogn. Personal. 2010;29(3):271–282. doi: 10.2190/IC.29.3.f. [DOI] [Google Scholar]
- 15.Sikka P, et al. Negative dream affect is associated with next-day affect level, but not with affect reactivity or affect regulation. Front. Behav. Neurosci. 2022;16:981289. doi: 10.3389/fnbeh.2022.981289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen MR, Schredl M, Carlsson I. Conscious use of dreams in waking life (nontherapy setting) for decision-making, problem-solving, attitude formation, and behavioral change. Dreaming. 2020;30(3):257–266. doi: 10.1037/drm0000138. [DOI] [Google Scholar]
- 17.Schredl M. Effect of dreams on daytime mood: The effects of gender and personality. Sleep Hypn. 2009;11(2):51–57. [Google Scholar]
- 18.De Koninck J, Brunette R. Presleep suggestion related to a phobic object: Successful manipulation of reported dream affect. J. Gen. Psychol. 1991;118(3):185–200. doi: 10.1080/00221309.1991.9917780. [DOI] [PubMed] [Google Scholar]
- 19.Cartwright RD, et al. Effect of an erotic movie on the sleep and dreams of young men. Arch. Gen. Psychiatry. 1969;20(3):262–271. doi: 10.1001/archpsyc.1969.01740150006002. [DOI] [PubMed] [Google Scholar]
- 20.Goodenough DR, et al. The effects of stress films on dream affect and on respiration and eye-movement activity during Rapid-Eye-Movement sleep. Psychophysiology. 1975;12(3):313–320. doi: 10.1111/j.1469-8986.1975.tb01298.x. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter KA. The effects of positive and negative pre-sleep stimuli on dream experiences. J. Psychol. 1988;122(1):33–37. doi: 10.1080/00223980.1988.10542940. [DOI] [PubMed] [Google Scholar]
- 22.Kontaris I, East BS, Wilson DA. Behavioral and neurobiological convergence of odor, mood and emotion: A review. Front. Behav. Neurosci. 2020;14:35. doi: 10.3389/fnbeh.2020.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadohisa M. Effects of odor on emotion, with implications. Front. Syst. Neurosci. 2013;7:66. doi: 10.3389/fnsys.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan RM, et al. Predicting odor pleasantness from odorant structure: Pleasantness as a reflection of the physical world. J. Neurosci. 2007;27(37):10015–10023. doi: 10.1523/JNEUROSCI.1158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundstrom JN, Olsson MJ. Subthreshold amounts of social odorant affect mood, but not behavior, in heterosexual women when tested by a male, but not a female, experimenter. Biol. Psychol. 2005;70(3):197–204. doi: 10.1016/j.biopsycho.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Lundström JN, et al. Psychological effects of subthreshold exposure to the putative human pheromone 4,16-androstadien-3-one. Horm. Behav. 2003;44(5):395–401. doi: 10.1016/j.yhbeh.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Soudry Y, et al. Olfactory system and emotion: Common substrates. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011;128(1):18–23. doi: 10.1016/j.anorl.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr. Opin. Neurobiol. 2004;14(2):198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 30.Zald DH, Pardo JV. Functional neuroimaging of the olfactory system in humans. Int. J. Psychophysiol. 2000;36(2):165–181. doi: 10.1016/S0167-8760(99)00110-5. [DOI] [PubMed] [Google Scholar]
- 31.Savic I, et al. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26(3):735–745. doi: 10.1016/S0896-6273(00)81209-X. [DOI] [PubMed] [Google Scholar]
- 32.Phan KL, et al. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 33.De Houwer J, Baeyens F, Field AP. Associative learning of likes and dislikes: Some current controversies and possible ways forward. Cogn. Emot. 2005;19(2):161–174. doi: 10.1080/02699930441000265. [DOI] [PubMed] [Google Scholar]
- 34.Levey AB, Martin I. Evaluative conditioning: Overview and further options. Cogn. Emot. 1990;4(1):31–37. doi: 10.1080/02699939008406762. [DOI] [Google Scholar]
- 35.Zucco GM, Paolini M, Schaal B. Unconscious odour conditioning 25 years later: Revisiting and extending ‘Kirk-Smith, Van Toller and Dodd’. Learn. Motiv. 2009;40(4):364–375. doi: 10.1016/j.lmot.2009.05.001. [DOI] [Google Scholar]
- 36.Baeyens F, et al. Toilet rooms, body massages, and smells: Two field studies on human evaluative odor conditioning. Curr. Psychol. 1996;15(1):77–96. doi: 10.1007/BF02686936. [DOI] [Google Scholar]
- 37.Robin O, et al. Emotional responses evoked by dental odors: An evaluation from autonomic parameters. J. Dent. Res. 1998;77(8):1638–1646. doi: 10.1177/00220345980770081201. [DOI] [PubMed] [Google Scholar]
- 38.Robin O, et al. Basic emotions evoked by eugenol odor differ according to the dental experience. A neurovegetative analysis. Chem. Senses. 1999;24(3):327–335. doi: 10.1093/chemse/24.3.327. [DOI] [PubMed] [Google Scholar]
- 39.Herz RS, et al. Influences of odors on mood and affective cognition. In: Laing DG, et al., editors. Olfaction, Taste, and Cognition. Cambridge University Press; 2002. pp. 160–177. [Google Scholar]
- 40.Seubert J, et al. Mood Induction with olfactory stimuli reveals differential affective responses in males and females. Chem. Senses. 2009;34(1):77–84. doi: 10.1093/chemse/bjn054. [DOI] [PubMed] [Google Scholar]
- 41.Weber ST, Heuberger E. The impact of natural odors on affective states in humans. Chem. Senses. 2008;33(5):441–447. doi: 10.1093/chemse/bjn011. [DOI] [PubMed] [Google Scholar]
- 42.Alaoui-Ismaïli O, et al. Basic emotions evoked by odorants: Comparison between autonomic responses and self-evaluation. Physiol. Behav. 1997;62(4):713–720. doi: 10.1016/S0031-9384(97)90016-0. [DOI] [PubMed] [Google Scholar]
- 43.Alaoui-Ismaïli O, et al. Odor hedonics: Connection with emotional response estimated by autonomic parameters. Chem. Senses. 1997;22(3):237–248. doi: 10.1093/chemse/22.3.237. [DOI] [PubMed] [Google Scholar]
- 44.Vernet-Maury E, et al. Basic emotions induced by odorants: A new approach based on autonomic pattern results. J. Auton. Nerv. Syst. 1999;75(2–3):176–183. doi: 10.1016/S0165-1838(98)00168-4. [DOI] [PubMed] [Google Scholar]
- 45.Billot PE, et al. Cerebral bases of emotion regulation toward odours: A first approach. Behav. Brain Res. 2017;317:37–45. doi: 10.1016/j.bbr.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 46.Okabe S, et al. Favorite odor induces negative dream emotion during rapid eye movement sleep. Sleep Med. 2018;47:72–76. doi: 10.1016/j.sleep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 47.Schredl M, et al. Information processing during sleep: The effect of olfactory stimuli on dream content and dream emotions. J. Sleep Res. 2009;18(3):285–290. doi: 10.1111/j.1365-2869.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 48.Vitinius F, et al. Feasibility of an interval, inspiration-triggered nocturnal odorant application by a novel device: A patient-blinded, randomised crossover, pilot trial on mood and sleep quality of depressed female inpatients. Eur. Arch. Otorhinolaryngol. 2014;271(9):2443–2454. doi: 10.1007/s00405-013-2873-6. [DOI] [PubMed] [Google Scholar]
- 49.Sabiniewicz A, et al. Effects of odors on sleep quality in 139 healthy participants. Sci. Rep. 2022;12(1):17165. doi: 10.1038/s41598-022-21371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rouby C, Pouliot S, Bensafi M. Odor hedonics and their modulators. Food Qual. Prefer. 2009;20(8):545–549. doi: 10.1016/j.foodqual.2009.05.004. [DOI] [Google Scholar]
- 51.Okabe S, et al. Presentation of familiar odor induces negative dream emotions during rapid eye movement (REM) sleep in healthy adolescents. Sleep Med. 2020;66:227–232. doi: 10.1016/j.sleep.2019.11.1260. [DOI] [PubMed] [Google Scholar]
- 52.Okabe S, Abe T. Subjectively intense odor does not affect dream emotions during rapid eye movement sleep. Sci. Rep. 2023;13(1):10442. doi: 10.1038/s41598-023-37151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villemure C, Slotnick BM, Bushnell MC. Effects of odors on pain perception: Deciphering the roles of emotion and attention. Pain. 2003;106(1–2):101–108. doi: 10.1016/S0304-3959(03)00297-5. [DOI] [PubMed] [Google Scholar]
- 54.Nielsen TA. A review of mentation in REM and NREM sleep: "Covert" REM sleep as a possible reconciliation of two opposing models. Behav. Brain Sci. 2000;23(6):851–866. doi: 10.1017/S0140525X0000399X. [DOI] [PubMed] [Google Scholar]
- 55.McNamara P, et al. REM and NREM sleep mentation. Int. Rev. Neurobiol. 2010;92:69–86. doi: 10.1016/S0074-7742(10)92004-7. [DOI] [PubMed] [Google Scholar]
- 56.Davidson P, et al. Does sleep selectively strengthen certain memories over others based on emotion and perceived future relevance? Nat. Sci. Sleep. 2021;13:1257–1306. doi: 10.2147/NSS.S286701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferguson CJ. An effect size primer: A guide for clinicians and researchers. Prof. Psychol. Res. Pract. 2009;40(5):532–538. doi: 10.1037/a0015808. [DOI] [Google Scholar]
- 58.Dalton P, et al. The use of semantic differential scaling to define the multidimensional representation of odors. J. Sens. Stud. 2008;23(4):485–497. doi: 10.1111/j.1745-459X.2008.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herz RS. Aromatherapy facts and fictions: A scientific analysis of olfactory effects on mood, physiology and behavior. Int. J. Neurosci. 2009;119(2):263–290. doi: 10.1080/00207450802333953. [DOI] [PubMed] [Google Scholar]
- 60.Engen T. The acquisition of odour hedonic. In: Van Toller S, Dodd GH, editors. Perfumery: The Psychology and Biology of Fragrance. Chapman & Hall; 1988. pp. 79–90. [Google Scholar]
- 61.Sutton D, et al. Optimizing arousal to manage aggression: A pilot study of sensory modulation. Int. J. Ment. Health Nurs. 2013;22(6):500–511. doi: 10.1111/inm.12010. [DOI] [PubMed] [Google Scholar]
- 62.Wright L, Bennett S, Meredith P. ‘Why didn't you just give them PRN?’ A qualitative study investigating the factors influencing implementation of sensory modulation approaches in inpatient mental health units. Int. J. Ment. Health Nurs. 2020;29(4):608–621. doi: 10.1111/inm.12693. [DOI] [PubMed] [Google Scholar]
- 63.Wallis K, Sutton D, Bassett S. Sensory modulation for people with anxiety in a community mental health setting. Occup. Ther. Ment. Health. 2018;34(2):122–137. doi: 10.1080/0164212X.2017.1363681. [DOI] [Google Scholar]
- 64.Hayes C, et al. What nonpharmacological therapeutic interventions are provided to adolescents admitted to general mental health inpatient units? A descriptive review. Int. J. Ment. Health Nurs. 2019;28(3):671–686. doi: 10.1111/inm.12575. [DOI] [PubMed] [Google Scholar]
- 65.Keptner KM, Fitzgibbon C, O’Sullivan J. Effectiveness of anxiety reduction interventions on test anxiety: A comparison of four techniques incorporating sensory modulation. Br. J. Occup. Ther. 2021;84(5):289–297. doi: 10.1177/0308022620935061. [DOI] [Google Scholar]
- 66.Todd C, Smothers M, Colson T. Implementing SEL in the classroom: A practitioner perspective. Clear. House J. Educ. Strateg. Issues Ideas. 2022;95(1):18–25. doi: 10.1080/00098655.2021.2016566. [DOI] [Google Scholar]
- 67.Dorn E, Hitch D, Stevenson C. An evaluation of a sensory room within an adult mental health rehabilitation unit. Occup. Ther. Ment. Health. 2020;36(2):105–118. doi: 10.1080/0164212X.2019.1666770. [DOI] [Google Scholar]
- 68.Bobier C, et al. Pilot investigation of the use and usefulness of a sensory modulation room in a child and adolescent psychiatric inpatient unit. Occup. Ther. Ment. Health. 2015;31(4):385–401. doi: 10.1080/0164212X.2015.1076367. [DOI] [Google Scholar]
- 69.Mills CJ, et al. Evaluating a virtual reality sensory room for adults with disabilities. Sci. Rep. 2023;13(1):495. doi: 10.1038/s41598-022-26100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haig S, Hallett N. Use of sensory rooms in adult psychiatric inpatient settings: A systematic review and narrative synthesis. Int. J. Ment. Health Nurs. 2023;32(1):54–75. doi: 10.1111/inm.13065. [DOI] [PubMed] [Google Scholar]
- 71.Cavanagh B, et al. It's like another world: The perceived beneficial effects of an artistically designed multisensory environment. Med. Humanit. 2019;45(1):52–59. doi: 10.1136/medhum-2018-011492. [DOI] [PubMed] [Google Scholar]
- 72.Ferdenzi C, et al. Variability of affective responses to odors: Culture, gender, and olfactory knowledge. Chem. Senses. 2013;38(2):175–186. doi: 10.1093/chemse/bjs083. [DOI] [PubMed] [Google Scholar]
- 73.Ferdenzi C, et al. Individual differences in verbal and non-verbal affective responses to smells: Influence of odor label across cultures. Chem. Senses. 2017;42(1):37–46. doi: 10.1093/chemse/bjw098. [DOI] [PubMed] [Google Scholar]
- 74.Arshamian A, et al. The perception of odor pleasantness is shared across cultures. Curr. Biol. 2022;32(9):2061–2066.e3. doi: 10.1016/j.cub.2022.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ayabe-Kanamura S, et al. Differences in perception of everyday odors: A Japanese-German cross-cultural study. Chem. Senses. 1998;23(1):31–38. doi: 10.1093/chemse/23.1.31. [DOI] [PubMed] [Google Scholar]
- 76.Kermen F, et al. Molecular complexity determines the number of olfactory notes and the pleasantness of smells. Sci. Rep. 2011;1(1):206. doi: 10.1038/srep00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keller A, et al. Predicting human olfactory perception from chemical features of odor molecules. Science. 2017;355(6327):820–826. doi: 10.1126/science.aal2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zarzo M. Hedonic judgments of chemical compounds are correlated with molecular size. Sensors (Basel) 2011;11(4):3667–3686. doi: 10.3390/s110403667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moskowitz HR, Dravnieks A, Klarman LA. Odor intensity and pleasantness for a diverse set of odorants. Percept. Psychophys. 1976;19(2):122–128. doi: 10.3758/BF03204218. [DOI] [Google Scholar]
- 80.Henion KE. Odor pleasantness and intensity: A single dimension? J. Exp. Psychol. 1971;90(2):275–279. doi: 10.1037/h0031549. [DOI] [PubMed] [Google Scholar]
- 81.Doty RL. An examination of relationships between the pleasantness, intensity, and concentration of 10 odorous stimuli. Percept. Psychophys. 1975;17(5):492–496. doi: 10.3758/BF03203300. [DOI] [Google Scholar]
- 82.Smeets MA, Dijksterhuis GB. Smelly primes: When olfactory primes do or do not work. Front. Psychol. 2014;5:96. doi: 10.3389/fpsyg.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herz RS. The role of odor-evoked memory in psychological and physiological health. Brain Sci. 2016 doi: 10.3390/brainsci6030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hackländer RPM, Janssen SMJ, Bermeitinger C. An in-depth review of the methods, findings, and theories associated with odor-evoked autobiographical memory. Psychon. Bull. Rev. 2019;26(2):401–429. doi: 10.3758/s13423-018-1545-3. [DOI] [PubMed] [Google Scholar]
- 85.Pellegrino R, et al. Habituation and adaptation to odors in humans. Physiol. Behav. 2017;177:13–19. doi: 10.1016/j.physbeh.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 86.Sinding C, et al. New determinants of olfactory habituation. Sci. Rep. 2017;7:41047. doi: 10.1038/srep41047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schredl M, et al. Olfactory stimulation during sleep can reactivate odor-associated images. Chemosens. Percept. 2014;7(3–4):140–146. doi: 10.1007/s12078-014-9173-4. [DOI] [Google Scholar]
- 88.Moss M, et al. Expectancy and the aroma of Roman chamomile influence mood and cognition in healthy volunteers. Int. J. Aromather. 2006;16(2):63–73. doi: 10.1016/j.ijat.2006.04.002. [DOI] [Google Scholar]
- 89.Howard S, Hughes BM. Expectancies, not aroma, explain impact of lavender aromatherapy on psychophysiological indices of relaxation in young healthy women. Br. J. Health Psychol. 2008;13(Pt 4):603–617. doi: 10.1348/135910707X238734. [DOI] [PubMed] [Google Scholar]
- 90.Martinec Nováková L, et al. Effects of all-night exposure to ambient odour on dreams and affective state upon waking. Physiol. Behav. 2021;230:113265. doi: 10.1016/j.physbeh.2020.113265. [DOI] [PubMed] [Google Scholar]
- 91.Doty RL, et al. Smell identification ability: Changes with age. Science. 1984;226(4681):1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- 92.Doty RL, Cameron EL. Sex differences and reproductive hormone influences on human odor perception. Physiol. Behav. 2009;97(2):213–228. doi: 10.1016/j.physbeh.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doty RL. Clinical disorders of olfaction. In: Doty RL, editor. Handbook of Olfaction and Gustation. Wiley; 2015. pp. 375–401. [Google Scholar]
- 94.Jones S, Benca RM. Psychiatric disorders associated with circadian rhythm disturbances. In: Lee-Chiong T, editor. Sleep: A Comprehensive Handbook. Wiley; 2005. pp. 409–414. [Google Scholar]
- 95.Gourineni R, Zee PC. Neurological and medical disorders associated with circadian rhythm disturbances. In: Lee-Chiong T, editor. Sleep: A Comprehensive Handbook. Wiley; 2005. pp. 401–407. [Google Scholar]
- 96.Neuland C, et al. Health-related and specific olfaction-related quality of life in patients with chronic functional anosmia or severe hyposmia. Laryngoscope. 2011;121(4):867–872. doi: 10.1002/lary.21387. [DOI] [PubMed] [Google Scholar]
- 97.Frasnelli J, Hummel T. Olfactory dysfunction and daily life. Eur. Arch. Oto-Rhino-Laryngol. 2005;262(3):231–235. doi: 10.1007/s00405-004-0796-y. [DOI] [PubMed] [Google Scholar]
- 98.Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9) Arthritis Care Res. (Hoboken) 2011;63(Suppl 11):S454–S466. doi: 10.1002/acr.20556. [DOI] [PubMed] [Google Scholar]
- 99.Preiss M, Vacíř K. BDI-II. Beckova sebeposuzovací škála pro dospělé. Psychodiagnostika; 1999. [Google Scholar]
- 100.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; 1996. [Google Scholar]
- 101.Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A) Arthritis Care Res. (Hoboken) 2011;63(11):S467–S472. doi: 10.1002/acr.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heretik A, et al. Restandardizace state-trait anxiety inventory X-2 – úzkostnost jako rys. Českoslov. Psychol. 2009;53(6):587–599. [Google Scholar]
- 103.Husten CG. How should we define light or intermittent smoking? Does it matter? Nicotine Tob. Res. 2009;11(2):111–121. doi: 10.1093/ntr/ntp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dufour MC. What is moderate drinking? Defining "drinks" and drinking levels. Alcohol Res. Health. 1999;23(1):5–14. [PMC free article] [PubMed] [Google Scholar]
- 105.Cohen JE. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- 106.Brand G, Schaal B. Olfaction in depressive disorders: Issues and perspectives. L'Encephale. 2017;43(2):176–182. doi: 10.1016/j.encep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 107.Croy I, Hummel T. Olfaction as a marker for depression. J. Neurol. 2017;264(4):631–638. doi: 10.1007/s00415-016-8227-8. [DOI] [PubMed] [Google Scholar]
- 108.Riemann D, et al. Sleep, insomnia, and depression. Neuropsychopharmacology. 2020;45(1):74–89. doi: 10.1038/s41386-019-0411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dinis J, Bragança M. Quality of sleep and depression in college students: A systematic review. Sleep Sci. 2018;11(4):290–301. doi: 10.5935/1984-0063.20180045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kupfer DJ, Frank E, Ehlers CL. EEG sleep in young depressives: first and second night effects. Biol. Psychiatry. 1989;25(1):87–97. doi: 10.1016/0006-3223(89)90150-9. [DOI] [PubMed] [Google Scholar]
- 111.Rotenberg VS, et al. First night effect in depression: New data and a new approach. Biol. Psychiatry. 1997;42(4):267–274. doi: 10.1016/S0006-3223(96)00343-5. [DOI] [PubMed] [Google Scholar]
- 112.Agnew HW, Jr, Webb WB, Williams RL. The first night effect: An EEG study of sleep. Psychophysiology. 1966;2(3):263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 113.Mendels J, Hawkins DR. Sleep laboratory adaptation in normal subjects and depressed patients ("first night effect") Electroencephalogr. Clin. Neurophysiol. 1967;22(6):556–558. doi: 10.1016/0013-4694(67)90063-6. [DOI] [PubMed] [Google Scholar]
- 114.Tonori H, et al. Anxiety and depressive states in multiple chemical sensitivity. Tohoku J. Exp. Med. 2001;193(2):115–126. doi: 10.1620/tjem.193.115. [DOI] [PubMed] [Google Scholar]
- 115.Johnson D, Colman I. The association between multiple chemical sensitivity and mental illness: Evidence from a nationally representative sample of Canadians. J. Psychosom. Res. 2017;99:40–44. doi: 10.1016/j.jpsychores.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 116.Riedel BW, Winfield CF, Lichstein KL. First night effect and reverse first night effect in older adults with primary insomnia: Does anxiety play a role? Sleep Med. 2001;2(2):125–133. doi: 10.1016/S1389-9457(00)00054-X. [DOI] [PubMed] [Google Scholar]
- 117.Reynolds CF, et al. EEG sleep in outpatients with generalized anxiety: A preliminary comparison with depressed outpatients. Psychiatry Res. 1983;8(2):81–89. doi: 10.1016/0165-1781(83)90094-X. [DOI] [PubMed] [Google Scholar]
- 118.Houghton DC, et al. Odor sensitivity impairment: A behavioral marker of psychological distress? CNS Spectr. 2019;24(4):404–412. doi: 10.1017/S1092852918001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takahashi T, et al. Possible relation between olfaction and anxiety in healthy subjects. Psychiatry Clin. Neurosci. 2015;69(7):431–438. doi: 10.1111/pcn.12277. [DOI] [PubMed] [Google Scholar]
- 120.Fortier-Lebel O, et al. Chemosensation in anxiety: The trigeminal system matters. Chem. Senses. 2023;48:bjad010. doi: 10.1093/chemse/bjad010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zucco GM, Doty RL. Multiple chemical sensitivity. Brain Sci. 2022;12(1):46. doi: 10.3390/brainsci12010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spielberger CD. Manual for the State-Trait Anxiety Inventory: STAI (Form Y) Consulting Psychologists Press; 1983. [Google Scholar]
- 123.Caci H, et al. The Spielberger trait anxiety inventory measures more than anxiety. Eur. Psychiatry. 2003;18(8):394–400. doi: 10.1016/j.eurpsy.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 124.Fredrickson BL. Positive emotions. In: Snyder CR, Lopez SJ, editors. Handbook of Positive Psychology. Oxford University Press; 2002. pp. 120–134. [Google Scholar]
- 125.Domhoff GW. A new neurocognitive theory of dreams. Dreaming. 2001;11(1):13–33. doi: 10.1023/A:1009464416649. [DOI] [Google Scholar]
- 126.Västfjäll D, et al. The measurement of core affect: A Swedish self-report measure derived from the affect circumplex. Scand. J. Psychol. 2002;43(1):19–31. doi: 10.1111/1467-9450.00265. [DOI] [PubMed] [Google Scholar]
- 127.Russell JA. Emotion, core affect, and psychological construction. Cogn. Emot. 2009;23(7):1259–1283. doi: 10.1080/02699930902809375. [DOI] [Google Scholar]
- 128.Scherer KR. Neuroscience projections to current debates in emotion psychology. Cogn. Emot. 1993;7(1):1–41. doi: 10.1080/02699939308409174. [DOI] [Google Scholar]
- 129.Russell JA. Core affect and the psychological construction of emotion. Psychol. Rev. 2003;110(1):145–172. doi: 10.1037/0033-295X.110.1.145. [DOI] [PubMed] [Google Scholar]
- 130.Posner J, Russell JA, Peterson BS. The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev. Psychopathol. 2005;17(3):715–734. doi: 10.1017/S0954579405050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Russell JA. A circumplex model of affect. J. Personal. Soc. Psychol. 1980;39(6):1161–1178. doi: 10.1037/h0077714. [DOI] [Google Scholar]
- 132.Latinjak AT, López-Ros V, Font-Lladó R. A descriptive study of emotion concepts using dimensional models of core affect/Un estudio descriptivo de los conceptos emocionales desde los modelos dimensionales de los estados afectivos. Stud. Psychol. 2015;36(2):413–450. doi: 10.1080/02109395.2015.1026121. [DOI] [Google Scholar]
- 133.Russell JA, Barrett LF. Core affect, prototypical emotional episodes, and other things called emotion: Dissecting the elephant. J. Pers. Soc. Psychol. 1999;76(5):805–819. doi: 10.1037/0022-3514.76.5.805. [DOI] [PubMed] [Google Scholar]
- 134.Kahneman D, Diener E, Schwarz N. Well-Being: The Foundations of Hedonic Psychology. Russell Sage Foundation; 1999. p. 593. [Google Scholar]
- 135.Stepanski EJ, Wyatt JK. Use of sleep hygiene in the treatment of insomnia. Sleep Med. Rev. 2003;7(3):215–225. doi: 10.1053/smrv.2001.0246. [DOI] [PubMed] [Google Scholar]
- 136.Hilditch CJ, McHill AW. Sleep inertia: Current insights. Nat. Sci. Sleep. 2019;11:155–165. doi: 10.2147/NSS.S188911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Trotti LM. Waking up is the hardest thing I do all day: Sleep inertia and sleep drunkenness. Sleep Med. Rev. 2017;35:76–84. doi: 10.1016/j.smrv.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kanady JC, Harvey AG. Development and validation of the sleep inertia questionnaire (SIQ) and assessment of sleep inertia in analogue and clinical depression. Cogn. Ther. Res. 2015;39(5):601–612. doi: 10.1007/s10608-015-9686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania smell identification test: A standardized microencapsulated test of olfactory function. Physiol. Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 140.Doty RL. The Smell Identification Test Administration Manual. Sensonics; 1995. [Google Scholar]
- 141.Herz RS, et al. A three-factor benefits framework for understanding consumer preference for scented household products: Psychological interactions and implications for future development. Cogn. Res. Princ. Implic. 2022;7(1):28. doi: 10.1186/s41235-022-00378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lawless H, Engen T. Associations to odors: Interference, mnemonics, and verbal labeling. J. Exp. Psychol. Hum. Learn. 1977;3(1):52–59. doi: 10.1037/0278-7393.3.1.52. [DOI] [PubMed] [Google Scholar]
- 143.Rundo JV, Downey R. Chapter 25: Polysomnography. In: Levin KH, Chauvel P, editors. Handbook of Clinical Neurology. Elsevier; 2019. pp. 381–392. [DOI] [PubMed] [Google Scholar]
- 144.Gazea M, et al. Chapter 17: Functions and circuits of REM sleep. In: Dringenberg HC, et al., editors. Handbook of Behavioral Neuroscience. Elsevier; 2019. pp. 249–267. [Google Scholar]
- 145.Berry RB, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.2. American Academy of Sleep Medicine; 2015. [Google Scholar]
- 146.Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep. 2005;28(3):303–307. [PubMed] [Google Scholar]
- 147.Tamaki M, et al. Examination of the first-night effect during the sleep-onset period. Sleep. 2005;28(2):195–202. doi: 10.1093/sleep/28.2.195. [DOI] [PubMed] [Google Scholar]
- 148.Tamaki M, Nittono H, Hori T. The first-night effect occurs at the sleep-onset period regardless of the temporal anxiety level in healthy students. Sleep Biol. Rhythms. 2005;3(2):92–94. doi: 10.1111/j.1479-8425.2005.00167.x. [DOI] [Google Scholar]
- 149.Vlahoyiannis A, et al. A critical review on sleep assessment methodologies in athletic populations: Factors to be considered. Sleep Med. 2020;74:211–223. doi: 10.1016/j.sleep.2020.07.029. [DOI] [PubMed] [Google Scholar]
- 150.Thoresen CJ, et al. The affective underpinnings of job perceptions and attitudes: A meta-analytic review and integration. Psychol. Bull. 2003;129(6):914–945. doi: 10.1037/0033-2909.129.6.914. [DOI] [PubMed] [Google Scholar]
- 151.Kritzler S, Krasko J, Luhmann M. Inside the happy personality: Personality states, situation experience, and state affect mediate the relation between personality and affect. J. Res. Personal. 2020;85:103929. doi: 10.1016/j.jrp.2020.103929. [DOI] [Google Scholar]
- 152.Scarpelli S, et al. The functional role of dreaming in emotional processes. Front. Psychol. 2019 doi: 10.3389/fpsyg.2019.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Field A. Discovering Statistics Using IBM SPSS Statistics. 4. Sage; 2013. [Google Scholar]
- 154.Rosenthal R. Meta-Analytical Procedures for Social Research. Sage; 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during this study are available from the corresponding author on reasonable request.


