Abstract
AIM
To introduce the macular hole (MH) hydromassage technique as a potentially beneficial approach for the treatment of large or persistent MH.
METHODS
This retrospective observational case series comprised 16 consecutive patients (17 eyes) diagnosed with MH. Inclusion criteria involved a hole aperture diameter larger than 600 µm or the presence of an unclosed MH larger than 600 µm following the previous vitrectomy. Standard MH repair procedures were administered in all cases, involving the manipulation and aspiration of the hole margin through the application of water flow with a soft-tip flute needle. A comprehensive assessment was conducted for each case before and after surgery, and optical coherence tomography (OCT) images were captured at every follow-up point.
RESULTS
The mean preoperative aperture diameter was 747±156 µm (range 611-1180 µm), with a mean base diameter of 1390±435 µm (range 578-2220 µm). Following surgery, all cases achieved complete anatomical closure of MH, with 13 cases (76.5%) exhibiting type 1 closure and 4 cases (23.5%) demonstrating type 2 closure. No significant differences were observed in the preoperative OCT variables between the two closure types. Eyes with type 1 closure showed a significantly improved visual acuity (0.70±0.10, range 0.50-0.80) compared to those with type 2 closure (0.90±0.12, range 0.80-1.00, P=0.014).
CONCLUSION
The MH hydromassage technique demonstrates promising results, achieving acceptable closure rates in cases of large or persistent MH. This technique may serve as an effective adjunctive maneuver during challenging MH surgery.
Keywords: macular hole, large macular hole, persistent macular hole, optical coherence tomography, surgical technique, hydromassage
INTRODUCTION
Full-thickness macular hole (MH) is an entity of macular disease defined as a complete defect of neurosensory retinal tissue and could lead to significant vision impairment. The most common type of MH is primary, which is caused by the vitreous-retina separation and traction in anteroposterior or tangential directions on the foveal center. In 1991, Kelly and Wendel[1] first reported that vitrectomy could treat MH with a success rate of 58%. In 1998, another milestone was achieved by Olen et al[2], who reported that the inner limiting membrane (ILM) peeling in vitrectomy could further enhance the success rate to around 90%, which had reminded all the investigators of the importance of ILM in the mechanism of the disease. With the current standards of MH repair containing three-port trocar placement (23, 25, or 27 gauge), core vitrectomy, creation of posterior hyaloid separation, peripheral vitrectomy, staining, ILM peeling, fluid-air exchange, and gas tamponade, the closure rate of a primary MH had exceeded 90% at present[3].
Nevertheless, a proportion of patients still suffer from incomplete closure of the MH. In complex cases with multiple risk factors, the success rate of the procedure might be compromised. According to Stec et al[4], the closure rate was related to the preoperative diameter of the holes in chronic cases, and MH larger than 400 µm were less likely to close after a standard pars plana vitrectomy (PPV). Recurrent MH with a history of the previous vitrectomy may also be recalcitrant. According to a study by Valldeperas and Wong et al[5] in 2008, the success rate of reoperation within 2mo of the first surgery after failed MH surgery was only 74%. In this way, various adjunctive maneuvers and agents have begun to be investigated in surgery to improve closure rates, although the effectiveness of some of them may still be controversial. In this study, we introduced a new surgical technique namely MH hydrodynamic massage, performed simultaneously with conventional vitrectomy and ILM stripping. It takes up very little time and can deepen our understanding of MH treatment and surgery, especially in refractory cases.
SUBJECTS AND METHODS
Ethical Approval
The study complies with the tenets of the Declaration of Helsinki revised in 2013. The study was reviewed and approved by Medical Ethics Committee of Peking University People's Hospital. All the patients recruited signed the informed consent.
Participants
This is an uncontrolled retrospective study involving a total of 17 eyes from 16 consecutive patients who were diagnosed with refractory or primary large MH at University People's Hospital Eye Center from 2018 to 2023. The inclusion criteria were as followed: all recruited cases have confirmed the diagnosis of full-thickness MH using spectral domain-optical coherence tomography (OCT) at present or before their first MH repair surgery, with an MH aperture diameter larger than 600 µm on OCT or remained an unclosed MH larger than 600 µm after the last vitrectomy. Cases meeting the following criteria were excluded: 1) high myopia, defined as <-6.00 diopter, or with an axial length (AL) larger than 26.5 mm; 2) secondary to or combined with trauma or other ocular diseases; 3) with an increased intraocular pressure (IOP) at baseline or had a history of glaucoma; 4) poor OCT signals secondary to the reduced refractive interstitial opacity; 5) previous intraocular surgery except for the first MH repair surgery within 6mo; 6) Combined with uveitis or scleritis.
If the preoperative MH diameter was greater than 800 µm, it would be classified into “the very large hole group”; the remaining eyes with a preoperative diameter ranged from 600 to 800 µm were categorized as “the large group”, accordingly. Moreover, all cases with a duration of symptoms of more than 2y would be analyzed separately as another subgroup called “the chronic group”, whereas the remains were defined as “the non-chronic group”. Cases with a previous ordinary MH repair surgery were defined as persistent MH.
Surgical Procedures
All cases received standard three-port vitrectomies (25 gauge), which were performed by one experienced doctor (Liang JH). For those phakic eyes with significant cataracts, phacoemulsification and intraocular lens implant were also combined. Followed by core vitrectomy, a posterior vitreous detachment was made to ensure that the posterior hyaloid was removed entirely. Minimum 2 papillary-diameter (PD) ILM was peeled with the assistant of indocyanine green staining (ICG; approximately 0.2 mL ICG diluted in 5% glucose, which was 1 mg/mL solution). For those who already received a previous MH repair surgery, the range of ILM peeling was extended to 4 PD. Then by using the soft-tip flute needle to massage the hole margin, which is meant to blow and suck gently, to make the retina tissue at the edge of the hole loose and soft through the water flow, thereby creating undulating waves in the hole margin that follow the water's course. Next, make the edges of the hole approximated as far as possible when absorbing with the flute needle. Sulfur hexafluoride (SF6; 20%) or hexafluoroethane (C2F6; 20%) were applied for gas tamponade followed by complete gas-fluid exchange. Finally, patients recruited were asked to maintain a face-down position for one week postoperatively (Video 1, online supplementary).
Main Outcome Measures
All cases received comprehensive assessment before and after the surgery. Baseline characteristics, including the patient's age, sex, duration of symptoms, AL, spherical equivalence (SE), lens status, and vitrectomy history, were recorded. The preoperative and postoperative anatomic parameters of the MH were measured using two-dimensional spectral-domain OCT (Optovue, Fremont, CA, US), with the standardized 0°, 45°, 90°, 135° sections through the center of macula, which were routinely collected at baseline and every follow-up timepoint. The anatomic quantitative data were obtained from built-in processing software. The following variables were gathered at baseline, 1wk, 1, and 3mo, latest follow-up time after surgery, including best-corrected visual acuity (BCVA), the central retinal thickness (CRT), the length of the bare retina and retinal pigment epithelium (RPE) band, the diameter of the ellipsoid zone (EZ) defect. All parameters were measured by one investigator (Cai Y) 3 times, and the mean values were calculated respectively.
After surgery, the morphological closure type of the MH was classified according to the contour of the macula. A complete hole closure, defined as a complete sealing of the RPE layer without any RPE bared, was classified into type 1 closure. When the MH was closed with a bare RPE section remained but absent of any detachment of the edge, it would be classified as a type 2 closure. An unclosed hole with the rim detached was regarded as a failure of the surgery and would be classified as a type 3 closure[6].
Statistical Analysis
Statistical analyses were performed with SPSS Version 25.0 (SPSS, Inc, Chicago, IL, USA). The BCVA was recorded in decimal units, and then converted to the logarithm of the minimal angle of resolution (logMAR). The descriptive data with normal distribution are presented as the means±standard deviations (range). The comparisons of variables between different closure types were performed by independent samples t-test, and the paired samples two-tailed t-test were undertaken to determine differences before and after surgery. Data that did not demonstrate a normal distribution, such as the age, duration of the symptoms, SE, were expressed as median (interquartile range) and was performed using Mann-Whitney U test. Categorical data were expressed as n (%). Differences in categorized data were assessed using Chi-squared tests. In multivariate regression models, variables like age, duration of symptom, preoperative lens status, choice of tamponade, SE, and preoperative visual acuity (VA) were selected for analysis, and forward was used in the selection of independent variables. A P value <0.05 was considered significant.
RESULTS
Totally 17 eyes in 16 patients with the MH aperture diameter larger than 600 µm were enrolled in this retrospective research. Detailed baseline characteristics were presented in Table 1. The 12 (70.6%) cases had a history of a failed MH repair surgery. About the preoperative lens status, there were 7 phakic eyes (41.2%), and during the surgery, 6 of them underwent combined phacoemulsification with intraocular lens implantation.
Table 1. Characteristics of subjects and surgical variables.
| Variables | Value |
| Age (y) | 65±10.1 (36-74) |
| Male, n (%) | 11 (68.8) |
| Right eye, n (%) | 10 (58.8) |
| MH surgery history, n (%) | 12 (70.6) |
| Symptoms duration (y) | 1.19±2.13 (0.08-9) |
| Preop. phakic eyes, n (%) | 7 (41.2) |
| Phaco-vitrectomy, n (%) | 6 (35.3) |
| Axial length (mm) | 24.70±1.25 (23.10-26.50) |
| Choice of tamponade, n (%) | |
| 20% C2F6 | 12 (70.6) |
| 20% SF6 | 5 (29.4) |
| Follow-up duration (d) | 142±82.5 (90-388) |
| SE (D) | -1.91±2.46 (-5.75-0) |
MH: Macular hole; SE: Spherical equivalence.
All eyes enrolled were classified as stage IV MH preoperatively. The overall mean preoperative aperture diameter was 747±156 (611, 1180) µm, and the mean base diameter was 1400±413 (678, 2220) µm. The aperture and base diameter did not differ significantly between the chronic group and the non-chronic group. The overall mean preoperative VA was 0.99±0.23 (range 0.7-1.5) logMAR. There was no statistically significant difference detected between the very large group and the large group (P=0.17), neither between the chronic group and non-chronic group (P=0.10; Table 2).
Table 2. Comparison of OCT parameters and preoperative visual acuity.
| Variables | All cases | Very large group | Large group | P 1 | Chronic group | Non-chronic group | P 2 |
| No. of eyes (%) | 17 | 4 (23.5) | 13 (76.5) | - | 4 (23.5) | 13 (76.5) | - |
| Preop. aperture diameter, µm | 747±156 (611, 1180) | 963±188 (800, 1180) | 681±59.1 (611, 794) | - | 788±265 (632, 1180) | 735±120 (611, 1060) | 0.72 |
| Preop. base diameter, µm | 1400±413 (678, 2220) | 1810±343 (1410, 2220) | 1270±350 (678, 1730) | 0.04 | 1260±735 (678, 2220) | 1400±413 (678, 2220) | 0.60 |
| Preop. VA, logMAR | 0.99±0.23 (0.70, 1.50) | 0.88±0.15 (0.70, 1.00) | 1.03±0.25 (0.70, 1.50) | 0.17 | 0.825±0.189 (0.700, 1.10) | 1.04±0.227 (0.700, 1.50) | 0.10 |
| Postop. VA, logMAR | 0.75±0.13 (0.50, 1.00) | 0.75±0.21 (0.50, 1.00) | 0.75±0.11 (0.60, 1.00) | 0.97 | 0.78±0.15 (0.70, 1.00) | 0.74±0.13 (0.50, 1.00) | 0.68 |
| Gain in VA, logMAR | -0.24±0.21 (-0.700, 0) | -0.13±0.10 (-0.20, 0) | -0.28±0.22 (-0.70, 0) | 0.07 | -0.05±0.06 (-0.10, 0) | -0.30±0.20 (-0.70, 0) | 0.001 |
The very large group is defined as cases with a preoperative aperture diameter larger than 800 µm, while the large group comprises cases with diameters ranging from 600 to 800 µm. The chronic group includes cases with a duration of symptoms exceeding 2y, whereas the non-chronic group is defined as having a symptom duration of less than 2y. P1: Very large group vs large group; P2: Chronic group vs non-chronic group. VA: Visual acuity; OCT: Optical coherence tomography.
mean±SD (range)
After surgery, all the cases received a complete anatomical closure of the MH with whether a type 1 (13/17, 76.5%) or type 2 (4/17, 23.5%) closure. There was no significant difference in preoperative OCT variables between type 1 and type 2 closure (Table 3). Cases with a type 2 closure have a longer mean EZ defect compared with cases with a type 1 closure at the 3-month follow-up point after surgery, but no significance was noted (P=0.825).
Table 3. The comparison of the characteristics, OCT variables, and visual outcomes between MH with type 1 and type 2 closure.
| Parameters | Type 1 closure (n=13) | Type 2 closure (n=4) | P |
| Preop. aperture diameter, µm | 739±153 (611, 1180) | 775±188 (670, 1060) | 0.745 |
| Preop. base diameter, µm | 1430±445 (678, 2220) | 1310±321 (1050, 1700) | 0.583 |
| Axial length, mm | 24.4±1.04 (23.3, 26.5) | 25.5±1.63 (23.1, 26.5) | 0.267 |
| Duration of symptom, y | 0.78±0.76 (0.08, 2.00) | 2.54±4.33 (0.08, 9.00) | 0.477 |
| Spherical equivalent, D | -1.23±2.01 (-5.00, 0) | -4.13±2.77 (-5.75, 0) | 0.124 |
| Ellipsoid zone defect, µm | 1070±496 (315, 1700) | 1160±748 (524, 1960) | 0.825 |
| Preop. VA, logMAR | 0.90 (0.20) | 1.28 (0.43) | 0.014a |
| Postop. VA, logMAR | 0.70 (0.20) | 0.90 (0.20) | 0.030a |
| Gain in VA, logMAR | -0.20±0.12 (-0.45, 0) | -0.38±0.38 (-0.70, 0) | 0.726 |
aP<0.05 based on Mann-Whitney U test. MH: Macular hole; VA: Visual acuity. Continuous variables are expressed as mean±standard deviation or median (interquartile range).
Totally 14 (82.3%) eyes received statistically significant vision improvement 3mo after the operation, which was achieved in 12/13 (92.3%) of the very large group, and 11/13 (84.6%) of the chronic group. Six (35.3%) cases achieved a gain in VA of more than 2 lines, which was achieved in 0/13 of the very large group and 0/13 of the chronic group. In the non-chronic group and very large group, VA improved significantly after the surgery (P=0.041, P=0.002, respectively). However, between the very large group and large group, visual outcomes (including preoperative and postoperative mean VA and gain in VA) did not differ significantly. In comparing the non-chronic group to the chronic group, there was a significant difference in the gain in VA. The non-chronic group exhibited a substantial gain in VA (-0.30±0.20, range -0.70 to 0), while the chronic group showed a smaller change (-0.05±0.06, range -0.10 to 0, P=0.001; Table 2).
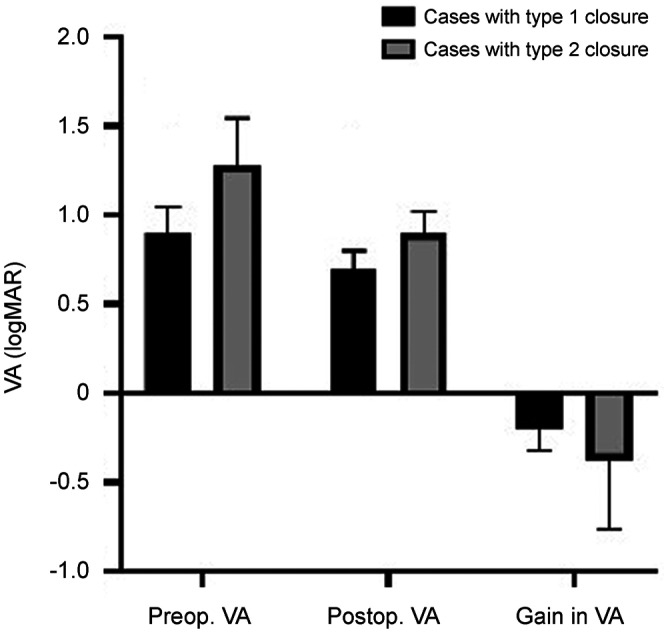
When considering closure types on OCT, eyes with a type 1 closure demonstrated significantly better postoperative VA (0.70±0.10, range 0.50 to 0.80) compared to those with a type 2 closure (0.90±0.12, range 0.80 to 1.00; P=0.03). However, the gain in VA did not differ between the two closure types (P=0.726). Moreover, cases with combined emulsification did not show significant differences in closure type (P=0.978), gain in VA (P=0.219), and EZ defect length (P=0.574) compared to the rest of the cases. The detailed results are presented in Table 3 and Figure 1. OCT images of two cases are illustrated in Figure 2.
Figure 1. Visual outcomes between cases with type 1 and type 2 closure.
The preoperative and postoperative VA differed significantly between the 2 groups (P=0.014, P=0.030, respectively). The gain in VA did not show any significant difference between the 2 groups (P=0.726). Cases with type 1 closure received a significantly improved VA after macular hole repair surgery, however in cases with type 2 closure, no such significance was observed (P=0.001, P=0.347, respectively). VA: Visual acuity.
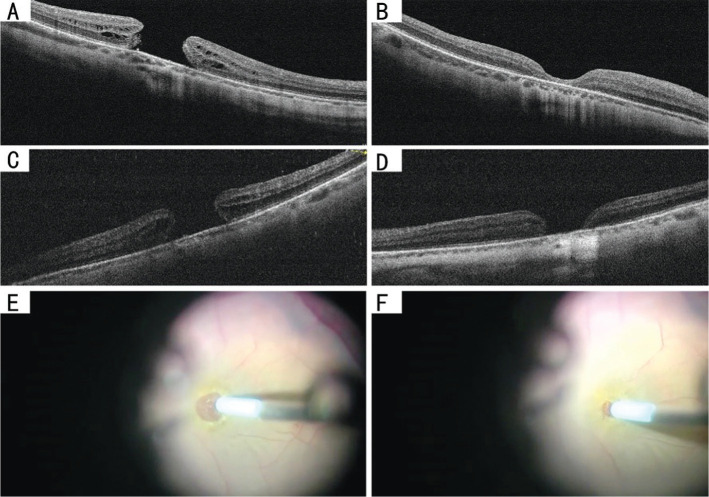
Figure 2. Preoperative and postoperative OCT images of 2 representative cases that underwent the MH repair surgery with the intended hydromassage technique.
Case 1: Sequential Spectral-domain OCT images of a large primary MH of a 41-year-old man were taken at baseline and three months after the surgery. The preoperative OCT image shows the aperture diameter was 702 µm, and the base diameter was 1090.33 µm (A). The postoperative OCT image shows a type 1 closure achieved after surgery, and central retinal thickness of 75 µm (B). The postoperative VA elevated to 0.6 logMAR 3mo after the surgical repair. Case 2: Preoperative OCT of a large primary MH of a 67-year-old man with an aperture diameter of 670.21 µm, the basal diameter of 1064.76 µm (C). Preoperative VA was 1.0 logMAR. Postoperative OCT showing type 2 closure, with a section of bare RPE, remained three months after the surgery (D). His VA was elevated to 0.8 logMAR, three months after the surgery. E, F: Screenshot for hydromassage technique: use the soft-tip flute needle to massage the hole margin, and to blow and suck gently, making the retina tissue at the edge loose and soft through the water flow. Second, make the edges of the hole approximated as far as possible when absorbing with the flute needle. OCT: Optical coherence tomography; MH: Macular hole; VA: Visual acuity; RPE: Retinal pigment epithelium.
DISCUSSION
Although conventional vitrectomy with ILM peeling has been shown to be effective in the treatment of MH, 20% to 60% of large full-thickness MHs still failed to close, and the success of achieving anatomical closure will greatly affect visual outcome[4],[7]–[9]. In this way, it remains a challenge to effectively close the hole. Smiddy et al[10] described the use of transforming growth factor-2 as an adhesive to assist in the closure of the MH. Analogously, autologous serum, platelets have been introduced into the procedure afterward[11]. Materials similar to the ILM for providing a bridge for the proliferation of glial cells are also used as filler, including auto-transplantation of the anterior lens capsule[12] and human amniotic membrane[13]. Adjunctive maneuvers to manipulate the retinal surface have also been proposed, including the inverted ILM peeling technique, defined as creating an incomplete peeling of ILM on macula and invertedly covering the flap over the hole[14]; tapping of MH edges reported by Kumar et al[15]; enlargement of the ILM peeling area and even changing the choice of tamponade[3], etc.
For refractory recurrent holes, hydraulic centripetal macular displacement[16], autologous retinal transplant[17], or induced macular detachment[18] can also be a trial option.
In this study, we presented a novel adjunctive maneuver for large or refractory MH repair procedures. It features using a water stream to assist in softening stiff and puckered hole margins to induce MH margin closure. Its efficacy has been demonstrated both anatomically and functionally: Successful closure was obtained in all cases in this study without any edge detachment, and the overall VA was significantly improved compared to the preoperative VA. Furthermore, 14/17 (82.3%) patients received vision improvement, and 6/17 (35.2%) patients had significantly improved VA by more than 2 lines, especially for eyes with symptoms lasting less than two years. This is in consistency with various previous researches for chronic holes, which are characterized by atrophy of retinal tissue at the MH margin or RPE, and with small increase in VA after MH repair surgery[19]–[20].
Although there were many factors related to the postoperative visual outcomes, including aperture/basal diameter of the hole, previous failed PPV history, chronicity, etc., the contour of the closure was deemed as a significantly relevant factor. According to previous research[21], the postoperative hole closure on OCT was divided into two types according to the morphology. This was a more distinct classification rather than the previous classification system of U type and V type introduced by Imai et al[22], which was ambiguous in the definition to some extent. In this way, we adopted the former classification system in our analysis. It was noted that postoperative VA was highly associated with the contour of the closure. Patients with a type 1 closure had a significantly better postoperative VA compared with cases with a type 2 closure, which is consistent with the previous research[6].
In order to create a type 1 closure with neuroretina restoration, gliosis proliferation, and especially glial cell photoreceptor layer reconstruction on the bare RPE layer[23], As mentioned above, a combination of traditional vitrectomy and a number of adjuncts has been widely used. There were roughly two groups of additive surgical techniques according to the principle: the first group was the plug or elongation of the tissue, including the usage of autologous gluconated blood[24], autologous platelets[11],[25], and inverted ILM flap technique. These plugs provided the scaffold, muller cell remains, or cytokines for introducing the glial cells and photoreceptors to migrate and proliferate[14]. However, many refractory large macular holes had the history of failed vitrectomies and did not have ideal remains to repeat ILM peeling or plugging. Maintaining the location of the flap also requires a degree of surgical skill. Moreover, it was also reported that some chronic MH showed unique morphological characteristics compared with acute MH, such as the squarer, puckered configuration of the margin with stiffness[26]. It is also believed that refractory MHs always have a tight, sometimes pigmented retinal-RPE adhesions[27]. Besides, according to the hydration theory, the liquid vitreous after vitrectomy surgery might get into the margin of MH and would be absorbed by the retinal layers evidence by OCT: the inner retinal layers could get retracted away from each other, and the swelling of the middle and outer retinal layers could cause an increase in base diameter of the hole. Therefore, another key factor for hole closure is isolating the hole from vitreous fluid either by intraocular tamponade or mechanical approximating, which facilitates the functional recovery of the RPE pump[28]. The situations mentioned above could not be solved simply by a plug or elongation. So another group of mechanical maneuvers characterized by lysing the adhesion with RPE, enhancing the retinal compliance, reducing the intrinsic retinal stiffness, thus helping the margin to involute and proximate emerged were proposed, as the technique of tapping the edges of MH[15], iatrogenically creating a limited macular detachment introduced by Claes[29], MH hydro-dissection technique (MHH) proposed by Felfeli and Mandelcorn[30], and hydraulic centripetal macular displacement reported by Ruban et al[16]. Our study belonged to the latter group too. It is a safe way of helping reduce the stiffness of the margin and avoid harming the underlying RPE cells at the same time. As shown in our study, the 100% closure rate was indeed achieved. We speculate that it could be analogous to a soft massage through the water flow, which moves the edges and increases flexibility without unnecessary mechanical damage. In this aspect, it is consistent with the MHH technique proposed by Felfeli and Mandelcorn[27]. Both techniques help to elevate the mobilization of MH edges, allowing them to reapproximate. And our way of soft massage is not intended to process with the mechanical dissection of the retina, so it will reduce the mechanical damage directly towards the retina during water injecting manipulation and minimize the trauma to the RPE.
In our research, 4 eyes achieving type 2 closure (4/17, 23.5%). However, they did not have a significantly larger aperture/basal diameter or longer duration of symptoms compared with other eyes. There may be various factors contributing to it[30]. Among them, the incomplete separation of adhesions may be one of the most important. According to Ishida et al[31], the displacement of the retina around the MH was frequently observed by studying the displacement of the vessels, and the extent of the displacement may be related to the extent of the detachment of the macula. In this situation, the combination with the MHH technique/hydraulic centripetal macular displacement technique mentioned above may have an added value[16],[27]. They can add the volume of the margin tissue through hydration, help to extend the hole margins through the injection of the fluid under the retina, and more adequately alleviate the adhesion around the MH margin at the same time[27]. Therefore, in those recalcitrant large holes with a dislocation adhesion, simply soft massage may be limited effective, a combined hydro-dissection, even iatrogenic limited macular detachment might address the problem.
The advantage of our research is that the manipulate is repeatable and straightforward, with limited damage, and needs less time or practice compared with other types of surgical techniques. It only needs a little time gently massaging the margin of the hole through water flow, in this way it can make the hole edges more compliant, which is favorable in introducing the hole margin proximate. The limitations of our research are that the sample size is too small, and there was no control group designed for comparing the distinct difference, the single non-masked observational nature restricted its conviction. Another weak point is the large variability in follow-up duration. Besides, we only use VA and EZ injury to evaluate the potential damage might cause by the procedure. We will employ various methods in the future to assess the structural and functional impacts resulting from MH surgery. Further, carefully designed clinical experiments and larger study groups remain to be constructed to estimate the validity of this technique.
The MH hydromassage technique could provide an acceptable closure rate in large holes or persistent MH cases. It needs less time and practice, with little damage to the retina tissue and RPE. It may become a preferred adjunct to the MH repair surgery.
Footnotes
Authors' contributions: Liang JH, Li XX, Shi X defined the criteria for inclusion and exclusion. Liang JH performed all the vitrectomy surgery. Wei D, Cai Y, Shi X analyzed and interpreted the patient data, Wei D, Cai Y, Liu WB drafted this manuscript, Deng X and Wei D made the figures and tables. Shi X, Deng X, Li XX, Liang JH, Zhao MW modified the manuscript. All authors read and approved the final manuscript.
Foundation: Supported by National Natural Science Foundation of China (NSFC) fund (No.81970815).
Conflicts of Interest: Cai Y, None; Liu WB, None; Wei D, None; Deng X, None; Li XX, None; Zhao MW, None; Shi X, None; Liang JH, None.
REFERENCES
- 1.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109(5):654–659. doi: 10.1001/archopht.1991.01080050068031. [DOI] [PubMed] [Google Scholar]
- 2.Olsen TW, Sternberg P, Jr, Capone A, Jr, Martin DF, Lim JI, Grossniklaus HE, Aaberg TM., Sr Macular hole surgery using thrombin-activated fibrinogen and selective removal of the internal limiting membrane. Retina. 1998;18(4):322–329. doi: 10.1097/00006982-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Yin L, Liu AQ, Jin X, Jia L, Wang FX. Comparison of outcomes of idiopathic macular holes treated by vitrectomy with air or silicone oil tamponade based on the hole size. Int J Ophthalmol. 2022;15(8):1305–1309. doi: 10.18240/ijo.2022.08.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stec LA, Ross RD, Williams GA, Trese MT, Margherio RR, Cox MS., Jr Vitrectomy for chronic macular holes. Retina. 2004;24(3):341–347. doi: 10.1097/00006982-200406000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Valldeperas X, Wong D. Is it worth reoperating on macular holes? Ophthalmology. 2008;115(1):158–163. doi: 10.1016/j.ophtha.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 6.Gümüş G, Demir G, Tülü Aygün B, Demircan A, Alkın Z, Öztornacı O. Prognostic factors affecting macular hole closure types. Ther Adv Ophthalmol. 2021;13:25158414211009007. doi: 10.1177/25158414211009007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaide RF. Healing mechanisms after macular hole repair suggests process of foveation. Retina. 2023;43(4):539–546. doi: 10.1097/IAE.0000000000003727. [DOI] [PubMed] [Google Scholar]
- 8.Abdul-Kadir MA, Lim LT. Update on surgical management of complex macular holes: a review. Int J Retina Vitreous. 2021;7(1):75. doi: 10.1186/s40942-021-00350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahimy E, McCannel CA. Impact of internal limiting membrane peeling on macular hole reopening: a systematic review and meta-analysis. Retina. 2016;36(4):679–687. doi: 10.1097/IAE.0000000000000782. [DOI] [PubMed] [Google Scholar]
- 10.Smiddy WE, Glaser BM, Green WR, Connor TB, Jr, Roberts AB, Lucas R, Sporn MB. Transforming growth factor beta. A biologic chorioretinal glue. Arch Ophthalmol. 1989;107(4):577–580. doi: 10.1001/archopht.1989.01070010591036. [DOI] [PubMed] [Google Scholar]
- 11.Zhu D, Ma B, Zhang J, Huang R, Liu Y, Jing X, Zhou J. Autologous blood clot covering instead of gas tamponade for macular holes. Retina. 2020;40(9):1751–1756. doi: 10.1097/IAE.0000000000002651. [DOI] [PubMed] [Google Scholar]
- 12.Li D, Ye Q, Li C. Auto-transplantation of the anterior lens capsule and blood for a recurrent large macular hole. Int J Ophthalmol. 2020;13(11):1839–1840. doi: 10.18240/ijo.2020.11.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizzo S, Caporossi T, Tartaro R, Finocchio L, Franco F, Barca F, Giansanti F. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina. 2019;39(Suppl 1):S95–S103. doi: 10.1097/IAE.0000000000002320. [DOI] [PubMed] [Google Scholar]
- 14.Tayyab H, Khan AA, Jahangir S. Efficacy of inverted internal limiting membrane flap for large idiopathic macular holes. Pak J Med Sci. 2019;35(2):315–319. doi: 10.12669/pjms.35.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A, Tinwala SI, Gogia V, Sehra SV. Tapping of macular hole edges: the outcomes of a novel technique for large macular holes. Asia Pac J Ophthalmol (Phila) 2013;2(5):305–309. doi: 10.1097/APO.0b013e31829a1919. [DOI] [PubMed] [Google Scholar]
- 16.Ruban A, Lytvynchuk L, Zolnikova A, Richard G. Efficiency of the hydraulic centripetal macular displacement technique in the treatment of traumatic full-thickness macular holes. Retina. 2019;39(Suppl 1):S74–S83. doi: 10.1097/IAE.0000000000001929. [DOI] [PubMed] [Google Scholar]
- 17.Dhami A, Sharma P, Dhami NB, Dhami GS. To evaluate the functional and anatomical outcomes for autologous retinal autograft with Finesse™ Flex Loop for failed macular holes. Indian J Ophthalmol. 2022;70(8):3033–3037. doi: 10.4103/ijo.IJO_3215_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisina R, Tozzi L, Sabella P, Cacciatori M, Midena E. Surgically induced macular detachment for treatment of refractory full-thickness macular hole: anatomical and functional results. Ophthalmologica. 2019;242(2):98–105. doi: 10.1159/000500573. [DOI] [PubMed] [Google Scholar]
- 19.Roth DB, Smiddy WE, Feuer W. Vitreous surgery for chronic macular holes. Ophthalmology. 1997;104(12):2047–2052. doi: 10.1016/s0161-6420(97)30060-8. [DOI] [PubMed] [Google Scholar]
- 20.Thompson JT, Sjaarda RN, Lansing MB. The results of vitreous surgery for chronic macular holes. Retina. 1997;17(6):493–501. doi: 10.1097/00006982-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Kang SW, Ahn K, Ham DI. Types of macular hole closure and their clinical implications. Br J Ophthalmol. 2003;87(8):1015–1019. doi: 10.1136/bjo.87.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai M, Iijima H, Gotoh T, Tsukahara S. Optical coherence tomography of successfully repaired idiopathic macular holes. Am J Ophthalmol. 1999;128(5):621–627. doi: 10.1016/s0002-9394(99)00200-7. [DOI] [PubMed] [Google Scholar]
- 23.Sisk RA. A technique for closing challenging macular holes. Ophthalmic Surg Lasers Imaging Retina. 2019;50(7):450–452. doi: 10.3928/23258160-20190703-07. [DOI] [PubMed] [Google Scholar]
- 24.Chakrabarti M, Benjamin P, Chakrabarti K, Chakrabarti A. Closing macular holes with “macular plug” without gas tamponade and postoperative posturing. Retina. 2017;37(3):451–459. doi: 10.1097/IAE.0000000000001206. [DOI] [PubMed] [Google Scholar]
- 25.Konstantinidis A, Hero M, Nanos P, Panos GD. Efficacy of autologous platelets in macular hole surgery. Clin Ophthalmol. 2013;7:745–750. doi: 10.2147/OPTH.S44440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yun C, Oh J, Hwang SY, Togloom A, Kim SW, Huh K. Morphologic characteristics of chronic macular hole on optical coherence tomography. Retina. 2012;32(10):2077–2084. doi: 10.1097/IAE.0b013e31825620ba. [DOI] [PubMed] [Google Scholar]
- 27.Felfeli T, Mandelcorn ED. Macular hole hydrodissection: surgical technique for the treatment of persistent, chronic, and large macular holes. Retina. 2019;39(4):743–752. doi: 10.1097/IAE.0000000000002013. [DOI] [PubMed] [Google Scholar]
- 28.Tornambe PE. Macular hole genesis: the hydration theory. Retina. 2003;23(3):421–424. doi: 10.1097/00006982-200306000-00028. [DOI] [PubMed] [Google Scholar]
- 29.Claes CC. Internal repair of very large, myopic and recurrent macular holes by creation of a central retinal detachment and silicone oil tamponade. Retina. 2019;39(Suppl 1):S72–S73. doi: 10.1097/IAE.0000000000001767. [DOI] [PubMed] [Google Scholar]
- 30.Venkatesh R, Mohan A, Sinha S, Aseem A, Yadav NK. Newer indices for predicting macular hole closure in idiopathic macular holes: a retrospective, comparative study. Indian J Ophthalmol. 2019;67(11):1857–1862. doi: 10.4103/ijo.IJO_364_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishida M, Ichikawa Y, Higashida R, Tsutsumi Y, Ishikawa A, Imamura Y. Retinal displacement toward optic disc after internal limiting membrane peeling for idiopathic macular hole. Am J Ophthalmol. 2014;157(5):971–977. doi: 10.1016/j.ajo.2014.01.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.