Abstract
AIM
To investigate the underlying mechanism of dry environment (autumn dryness) affecting the lacrimal glands in rats.
METHODS
Twenty Sprague-Dawley rats were randomly divided into two groups. The rats were fed in specific pathogen free environment as the control group (n=10), and the rats fed in dry environment as the dryness group (n=10). After 24d, lacrimal glands were collected from the rats. The tissues morphology was observed by hematoxylin-eosin (HE) staining. Tandem mass tags (TMT) quantitative proteomics analysis technology was used to screen the differential expressed proteins of lacrimal glands between the two groups, then bioinformatics analysis was performed. Further, the immunohistochemical (IHC) method was used to verify the target proteins.
RESULTS
In dryness group, the lacrimal glands lobule atrophied, the glandular cavities enlarged, the sparse nuclear distribution and scattered inflammatory infiltration between the acinus were observed. The proteomics exhibited that a total of 195 up-regulated and 236 down-regulated differential expressed proteins screened from the lacrimal glands of rats. It was indicated that the biological processes (BP) of differential expressed proteins mainly included cell processes and single BP. The cellular compositions of differential expressed proteins mainly located in cells, organelles. The molecular functions of differential expressed proteins mainly included binding, catalytic activity. Moreover, the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis showed that the differential expressed proteins mainly involved lysosome, complement and coagulation cascade, and ribosome pathway. The IHC result verified that the up-regulated expression proteins of Protein S100A9 (S100A9), Annexin A1 (Anxa1), and Clusterin (Clu) in lacrimal glands of rats in dryness group were higher than control group.
CONCLUSION
The up-regulated expression proteins of S100A9, Anxa1, and Clu may be the potential mechanisms of dry eye symptoms caused by dry environment. This study provides clues of dry environments causing eye-related diseases for further studies.
Keywords: dry eye, lacrimal gland, S100A9, Clu, Anxa1, environment, rats
INTRODUCTION
Dryness can be generalized as a climate environment with low relative humidity as the primary feature[1]. Dryness is the main manifestation in autumn[2]. In the traditional Chinese medicine (TCM) theory, records of the cause of dryness can be traced back to the Internal Medicine Classic of the Yellow Emperor wrote in the Chinese Western Han Dynasty. TCM believed that “dryness always feels astringent for people, is easy to hurt body fluid, and most likely to hurt lung”. When the body cannot tolerate dry environment, the “dryness” in the environment will cause diseases, which was named as “dryness evil” in TCM[3].
The organs exposed to the body surface are the most vulnerable influenced by external environment. As for the eyes and nose, the lacrimal gland secreted tears to keep the eyes surface moist[4]. It also protects the eye surface exposed to the environment from the damage of different pathogens. Tears flow into the nasal cavity through the nasolacrimal duct, which can keep the nasal cavity moist and the humidity of the inhaled air. Tears also play a local immune role in the nasal mucosa to protect the airway[5].
It has reported that lung system (trachea, nose orifices, lung tissue) is influenced by dry environment. The lacrimal glands lobules of rats were slightly atrophic, the connective tissues between the lobules were proliferative, the glandular cavities were expanded, the columnar secretory cells were vacuolized, and the defensin beta-2 of lacrimal gland was “transient” enhancement[5]. In order to further explored such above phenomenon, our present study screened the differential expressed proteins in lacrimal glands based on proteomics and verified the relevant target proteins. It provides a modern scientific evidence for finding the underlying mechanism of dry environment in TCM affecting the eyes symptom.
MATERIALS AND METHODS
Ethical Approval
The study was approved by the Ethics Committee in Animal Experimentation of Xinjiang Medical University [No. SYXK (New) 2011-0004], and protocol was reviewed and approved by Xinjiang Laboratory Animal Ethics Committee (IACUC20160616-05).
Animals Grouping and Intervention
Female SD rats weighing 140-160 g were randomly divided into two groups (n=10/group). The control group was fed in the specific pathogen free standard environment; the dryness group was placed in the artificial climate chamber and interfered with autumn dryness.
Artificial Simulation of Autumnal Dryness
Using the previous method[6] (Table 1), artificial climate chamber (model PRX-1250B, Deyangyibang Company, Shanghai, China) in the laboratory was applied to create dry environment, by adjusting the relative humidity, temperature, wind speed, light intensity to simulate autumn dryness. The temperature parameters of Class I (4°C-24°C) and Class III (10°C-30°C) were set and used in the artificial climate chamber according to the time interval, the average daily temperature difference was ≥20°C; the relative humidity was 5%-45%, the average daily relative humidity was 24.33%±11.29%, the wind speed was 2.1 m/s, and the light intensity was 5-20 light intensity (lx; the ratio of day and night light duration was not less than 10:14). The starting and ending time of the 1st period is 23:00-01:00, and the 12th period is 21:00-23:00. The duration of each period is 2h. The numbered periods 1-12 correspond to 24th of a day.
Table 1. The artificial climate chamber to simulate autumn dryness.
| Time period number | Dryness environment temperature (°C) |
Relative humidity (%) 1-24d | Light intensity (lx) 1-24d | |
| 1-12d | 13-24d | |||
| 1 | 12 | 6 | 30 | 0 |
| 2 | 10 | 4 | 35 | 0 |
| 3 | 12 | 5 | 42 | 0 |
| 4 | 15 | 6 | 40 | 5 |
| 5 | 20 | 8 | 35 | 10 |
| 6 | 24 | 15 | 20 | 15 |
| 7 | 26 | 18 | 15 | 20 |
| 8 | 30 | 24 | 5 | 20 |
| 9 | 26 | 20 | 10 | 15 |
| 10 | 24 | 15 | 15 | 10 |
| 11 | 20 | 10 | 20 | 5 |
| 12 | 15 | 8 | 25 | 0 |
Experimental Specimen Collection
Rats lacrimal glands were collected 24d later, the left lacrimal gland was washed in 0.9% 4°C saline, then placed in lyophilization tubes for liquid nitrogen freezing and stored at -80°C in the refrigerator for test. The right lacrimal gland was fixed with 4% paraformaldehyde, dehydrated routinely, embedded manually into the paraffin for test.
Hematoxylin-eosin Staining for Lacrimal Gland Tissue
The lacrimal gland sections were baked in a 63°C oven, dewaxing routinely, hematoxylin solution (20031605, Zhongshan Company, Beijing, China) staining, washed with distilled water, then differentiation with ethanol hydrochloride for 1s, reverse blue with distilled water, eosin solution (20041701, Zhongshan Company, Beijing) staining, washed with distilled water, dehydration routinely, natural drying at room temperature (23°C-26°C), and then sealed with neutral gum.
Proteomic Testing
The protein concentration of lacrimal gland tissues were determined by bicinchronic acid (BCA) kit (P0011, Biyuntian Company, Shanghai). Pancreatin (SRT220102, Promega, Beijing) decomposed the protease into the peptide segments, and TMT kit (WD330302, Thermo Fisher Scientific, Shanghai) marked the segments. The labeled peptides were graded by applying high performance liquid chromatography (1260, Agilent, Shanghai, China), then mobile phase A [0.1% formic acid (0172192381, Fluka, Germany) and 2% acetonitrile (F22M6E201, Thermo Fisher Scientific, Shanghai) aqueous solution] and mobile phase B (0.1% formic acid and 90% acetonitrile aqueous solution) of high performance liquid chromatography were used for separating, and ionized in the protein electrophoresis instrument (EPS-300, Tianneng Company, Shanghai). Finally, the labeled peptides were analyzed by the mass spectrometer (Q Exactive HF-X, Thermo Fisher Scientific, Shanghai). Data were collected by data dependent acquisition program, and secondary mass spectrometry data were retrieved by Maxquant (v1.5.2.8).
Proteomic Analysis
Protein quantification repeatability was assessed by principal component analysis, relative standard deviation and Pearson's correlation coefficient. The difference significance of the quantified proteins to obtain the P-value was used the two-tail t-test. When P<0.05, the fold change (FC) >1.2 and <1/1.2 were the change thresholds of significant up-regulated and significant down-regulated, respectively. Bioinformatics analysis was performed for differential expressed proteins in dryness group. UniProt-GOA database was used for Gene Ontology (GO) annotation classification and GO enrichment, and Kyoto Encyclopedia of Genes and Genomes (KEGG) database was used for KEGG pathway enrichment.
Immunohistochemical Analysis
The lacrimal gland sections were baked in the 63°C oven, and dewaxing routinely. The sections were soaking in 3% H2O2 and placed in citrate solution, heat them in microwave oven, and put them natural at room temperature. Put the sections into the wet box and drip goat serum solution (60 µL/sample; 20050901, Zhongshan Company, Beijing) to cover all tissues and seal at room temperature. The sections were shaken dry, and incubated with the primary antibodies as Abcam rabbit anti-S100A9 antibody (1:200; 26992-1-AP, Xiangtai Company, China), Abcam rabbit anti-Anxa1 antibody (1:200; Ab-AF5154, Xiangtai Company, China), and Abcam rabbit anti-Clu antibody (1:200; Ab-DF6431, Xiangtai Company, China), and then placed in the refrigerator at 4°C. The next day, the sections were rewarmed at room temperature, washed with phosphate buffer saline (PBS) buffer. The sections were dried with shaking, and added the second antibody (60 µL/sample; PV-9001, Zhongshan Company, Beijing), reacted at room temperature. The sections were developed with diaminobenzidine (DAB) chromogen substrate (K186916P, Zhongshan Company, Beijing, China), and color development was terminated by distilled water. Sections were counterstained with hematoxylin, differentiation in 1% hydrochloric acid ethanol for 1s, dried at room temperature after dehydrated routinely, and then sealed with neutral gum. Under light microscope (LEICA DM3000, Lecia Company, US), The photographs were randomly taken from each section. The integral optical density (IOD) value (IOD/area) was analyzed and calculated by Image-Pro Plus 6.0 software, and the average value of IOD value of six sections were used to represent the protein expression level of the section. The values were obtained as arbitrary units (a. u.).
Statistical Analysis
All data were entered into SPSS 26.0 was used for statistical analysis. Origin Pro 8.5 was used for plotting. Means between the two groups were compared using t-test when the variances were equal; approximate t-test when the variances were not equal. The test level α=0.05, and P<0.05 as the difference was statistically significant.
RESULTS
Effect of Autumn Dryness Intervention on the Histomorphology of Lacrimal Gland in Rats
The hematoxylin-eosin (HE) staining of rats lacrimal gland under autumn dryness intervention were shown in Figure 1. The lacrimal gland acini of rats in control were irregular round and shaped cell islands, with complete structure and tight arrangement, which were divided into lobule by connective tissues, glandular cavities were narrow, cytoplasm of secretory cells was uniform, nuclear distribution was uniform, but a few yellow and pink secretions appeared. In dryness, the connective tissue between the lobules of lacrimal gland proliferated significant, a large number of acini atrophied and fused, the expansion of glandular cavities were large, secretions (pink) appeared inside, a large number of macrophages appeared in the acinar cavity and interstitium, columnar secretory cells arranged closely and numerous vacuoles appeared, the nucleus were scattered and sparse, and local inflammatory infiltration was obvious. It is suggested that the fine structure of lacrimal gland in rats has pathological changes and local inflammatory reaction under the intervention of autumn dryness.
Figure 1. Hematoxylin-eosin staining of lacrimal gland in rats.
n=10.
Differential Expressed Proteins in the Lacrimal Glands of Rats in Dryness
As shown in Figure 2, a total of 3375 proteins were identified, and 431 differential express proteins were screened in the lacrimal gland of rats from dryness group. There were 195 up-regulated proteins, 34 proteins with FC>1.5, and 236 down-regulated proteins, and 5 proteins with FC>0.83.
Figure 2. Volcano plot.

Quantitative volcano map of differential protein. Each dot in the graph represents an assay protein and the scatter colour represents the final screening result, with up-regulated differential expressed proteins in red, down-regulated differential expressed proteins in blue and non-significantly different proteins in grey, n=10.
Major Differential Expressed Proteins in the Lacrimal Glands of Rats in Dryness
The proteomic result of the rat lacrimal gland differential proteins revealed (P<0.05; Table 2) that the higher FC proteins among the up-regulated differential proteins including Odorant-binding protein, S100A9, Reproductive homeobox on X chromosome 12, etc., and the higher FC proteins among the down-regulated differential proteins included isoform CRA_a, long-chain-fatty-acid-CoA ligase 5, Mannose receptor, C type 2, etc.
Table 2. Results of differential protein analysis in the lacrimal glands of rats in dryness.
| Protein number | Name of the protein | Coding genes | FC | Expression modulation |
| Q9QYU9 | Odorant-binding protein | LOC103690319 | 5.708 | Up |
| A0A0H2UHJ1 | Protein S100-A9 | S100A9 | 4.561 | Up |
| Q4TU71 | Reproductive homeobox on X chromosome 12 | Rhox12 | 3.271 | Up |
| Q4FZU2 | Keratin, type II cytoskeletal 6A | Krt6a | 3.106 | Up |
| A0A0G2JXV1 | Coiled-coil domain-containing 196 | Ccdc196 | 2.912 | Up |
| Q63616 | Vacuolar protein sorting-associated protein 33B | Vps33b | 2.782 | Up |
| Q6IFV4 | Keratin, type I cytoskeletal 13 | Krt13 | 2.423 | Up |
| G3V6L1 | Frizzled class receptor 6 | Fzd6 | 2.321 | Up |
| G3V8F9 | Alpha-methylacyl-CoA racemase | Amacr | 2.264 | Up |
| Q4V7D1 | Signal sequence receptor, alpha | Ssr1 | 2.157 | Up |
| D2XZ41 | Androgen-binding protein | Apbh | 2.152 | Up |
| Q6MG73 | Complement C2 | C2 | 1.922 | Up |
| P61149 | Fibroblast growth factor 1 | Fgf1 | 1.916 | Up |
| O08769 | Cyclin dependent kinase inhibitor | Cdkn1b | 1.91 | Up |
| G3V9E3 | Caldesmon 1, isoform CRA_b | Cald1 | 1.725 | Up |
| G3V765 | Envoplakin | Evpl | 1.716 | Up |
| M0R920 | RAN-binding protein 3 | Ranbp3 | 1.708 | Up |
| D3ZZR3 | Cathepsin S | Ctss | 1.698 | Up |
| G3V836 | Clusterin | Clu | 1.694 | Up |
| D4AAE7 | Solute carrier family 31 member 2 | Slc31a2 | 1.693 | Up |
| D4AB17 | Phosphoribosylformylglycinamidine synthase | Pfas | 1.687 | Up |
| D3ZA22 | Similar to Tripartite motif protein 47 | Trim47 | 1.677 | Up |
| D3ZSY4 | Eosinophil peroxidase | Exp | 1.635 | Up |
| D3ZYH8 | Myeloperoxidase | Mpo | 1.621 | Up |
| P08932 | T-kininogen 2 | - | 1.619 | Up |
| Q4QQV0 | Tubulin beta chain | Tubb6 | 1.604 | Up |
| Q5U2V4 | Phospholipase B-like 1 | Plbd1 | 1.599 | Up |
| A0A0G2K6P7 | Keratin, type II cytoskeletal 4 | Krt4 | 1.592 | UP |
| P07150 | Annexin A1 | Anxa1 | 1.588 | UP |
| Q6T5E8 | UDP-glucuronosyltransferase | Ugt1a7c | 1.581 | UP |
| Q91ZN1 | Coronin-1A | Coro1a | 1.559 | UP |
| A0A0H2UHL6 | Pro-cathepsin H | Ctsh | 1.547 | UP |
| A0A0G2JZU8 | Ubiquitin-specific peptidase 9, Y-linked | Usp9y | 1.547 | UP |
| D4ABH2 | Isoform CRA_a | LOC100360933 | 0.833 | Down |
| O88813 | Long-chain-fatty-acid-CoA ligase 5 | Acsl5 | 0.833 | Down |
| F1M495 | Mannose receptor, C type 2 | Pla2r1 | 0.832 | Down |
| Q5RK10 | 60S ribosomal protein L13a | Rpl13a | 0.832 | Down |
| D4A2I4 | ORM1-like protein 2 | Ormdl2 | 0.83 | Down |
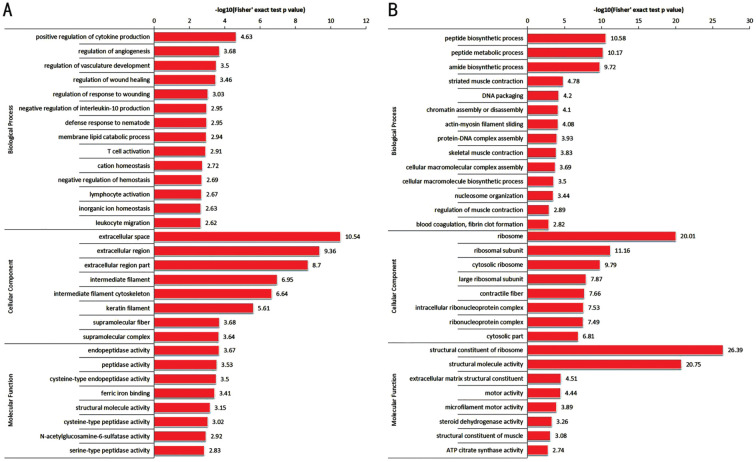
GO Functional Classification and Enrichment of Differential Expressed Proteins in the Lacrimal Gland of Dryness
As shown in Figures 3 and 4, GO functional classification and enrichment of differential expressed proteins in the lacrimal gland of dryness revealed (P<0.05) that the biological processes (BP) of differential expressed proteins were mainly included cellular process, single-organism process, biological regulation, metabolic process, etc. Up-regulated differential expressed proteins were mainly enriched in positive regulation of cytokine production, regulation of angiogenesis, regulation of vasculature development, etc.; down-regulated differential expressed proteins were mainly enriched in peptide biosynthetic process, peptide metabolic process, amide biosynthetic process, etc. The CC of differential expressed proteins were mainly located in cell, organelle, cell membrane, etc. Up-regulated differential expressed proteins were mainly enriched in extracellular space, extracellular region, extracellular region part, etc.; down-regulated differential expressed proteins were mainly enriched in ribosome, ribosomal subunit, cytosolic ribosome, etc. The MF of differential expressed proteins were mainly included binding and catalytic activity. Up-regulated differential expressed proteins were mainly enriched in endopeptidase activity, peptidase activity, cysteine-type endopeptidase activity, etc.; down-regulated differential expressed proteins were mainly enriched in structural constituent of ribosome, structural molecule activity, extracellular matrix (ECM) structural constituent, etc.
Figure 3. GO functional classification of differential expressed proteins.
A: Up-regulated differential proteins; B: Down-regulated differential proteins. GO: Gene Ontology.
Figure 4. Differential protein GO function enrichment.
A: Up-regulated differential proteins; B: Down-regulated differential proteins. The ordinate represents the P-value of the two-tailed t-test with a log-negative base of 10. GO: Gene Ontology.
KEGG Pathway Enrichment of Differential Expressed Proteins in the Lacrimal Gland of Dryness
As shown in Figure 5, KEGG pathway enrichment of lacrimal gland differential protein in dryness revealed (P<0.05) that the up-regulated differential expressed proteins were mainly enriched in the lysosome, complement and coagulation cascades, and antigen processing and presentation and other pathways. The down-regulated differential proteins were mainly enriched in ribosome, ECM-receptor interaction, protein digestion and absorption and other pathways.
Figure 5. Differential protein KEGG pathway enrichment analysis.
A: Up-regulated differential proteins; B: Down-regulated differential proteins. The abscissa is the enrichment degree, and the ordinate is the KEGG path information. The size of the circle indicates the number of differential expressed proteins in the mapping pathway. The larger the circle, the more the number. The color of the circle represents the size of the P-value. The bluer the color is the larger P-value. KEGG: Kyoto Encyclopedia of Genes and Genomes.
Correlation Pathway of Differential Expressed Proteins in the Lacrimal Gland of Dryness
As shown in Figure 6A, the up-regulated differential expressed proteins in the complement and coagulation cascades pathway with the high FC were complement C2, Clu, T-kininogen 2. The following up-regulated differential expressed proteins with the lower FC including C4b binding protein alpha chain, T-kininogen 1. Down-regulated differential expressed proteins in the complement and coagulation cascades pathway were complement C6, fibrinogen gamma chain, fibrinogen beta chain, fibrinogen alpha chain. As shown in Figure 6B, the up-regulated differential expressed proteins in the lysosome pathway with the high FC were Cathepsin S (CTSS) and Cathepsin H. The following up-regulated differential expressed proteins with the lower FC including cathepsin Z, carboxypeptidase, alpha-N-acetylgalactosaminidase, N-acetylglucosamine-6-sulfatase, dipeptidyl peptidase 1, clathrin light chain B, etc. Down-regulated differential expressed proteins in the lysosome pathway were prosaposin and V-type proton ATPase subunit.
Figure 6. Correlation pathway diagram.
The up-regulated differential expressed proteins are in red, down-regulated differential expressed proteins are in green. A: The complement and coagulation cascade pathway; B: The lysosome pathway.
Validation of Key Proteins
Through bioinformatics analysis of differential expressed proteins, the study selected S100A9, Anxa1, and Clu as key targets for IHC verification. As shown in Figure 7, compared with control group, the expression of S100A9, Anxa1, and Clu were enhanced in the lacrimal glands of dryness group (P<0.05), which was consistent with the proteomic findings.
Figure 7. Expression of S100A9, Anxa1, and Clu in lacrimal gland of rats.
A: IHC analysis the expression of S100A9, Anxa1, and Clu under light microscope ×400 Scale 5 µm; B: The results confirmed the expression of S100A9, Anxa1, and Clu in the control were significantly less than the dryness. The data are presented as the mean±SD. aP<0.01, n=10. S100A9: Protein S100-A9; Anxa1: Annexin A1; Clu: Clusterin; IHC: Immunohistochemical.
DISCUSSION
When the “dryness evil” prevailed, people who were attacked by “dryness evil” often have sub-health symptoms such as dryness and discomfort in the mouth, eyes, nose, throat; dry cough, less phlegm, dry skin, astriction, etc[7]. It has been found that indoor dry environment (microclimatic parameters) could cause dry eyes and visual fatigue[8], and at present people had bad living habits such as frequent use of air-conditioning and excessive use of eyes, which made increase the risk of developing the infectious diseases. In this study, the differential expressed proteins in lacrimal gland were analyzed by artificial simulation of autumn dryness in the laboratory, and S100A9, Clu and Anxa1 were selected as target proteins for verification, and hope to provide a scientific basis for dry environment affecting eyes symptom.
S100A9 is found widely distributed in human epithelial tissues, interacted with enzymes, receptors, transcription factors, cytoskeletal proteins, etc[9]. When S100A9 was released into the extracellular environment, it could mediate inflammation and prevent pathogen invasion, also be produced during tear oxidation change reaction. It plays a regulatory role in cell apoptosis, oxidative stress, inflammatory response, and repair processes[10]. Numerous reports have indicated that S100A9 is up-regulated in immune dry eyes, ocular microbial infection, eye redness, corneal abrasion, transient blurred vision and other ocular diseases[9],[11]. Up-regulation of S100A9 may be associated with the dry environment-induced cell apoptosis, mediated ocular inflammation and caused local injury.
Anxa1 refers to an effective anti-inflammatory medium, it can be secreted in different immune cells. For example, Anxa1 could block the activation of eye neutrophils and regulate the migration of leukocytes and the produced of pro-inflammatory mediators in neutrophils[12]. Anxa1 could also mediate the adhesion, chemotaxis, aggregation, and other processes of inflammatory cells by activating multiple anti-inflammatory pathways, and inhibiting the production of inflammatory cytokines[13]–[14]. It could activate the PLC pathway, stimulate mucin secretion from the conjunctival goblet cells, inhibite ERK1/2 phosphorylation, promote the homeostasis of the tear membrane mucin layer, and relieve the inflammatory cell infiltration of eye conjunctiva[15]. In prsent study, the up-regulated expression of Anxa1 demonstrated that there was a possibility of inflammation and ocular surface homeostasis imbalance caused by dry environment.
Complement and coagulation cascades pathway exerted a vital role in activating and maintaining natural immunity[16]. Among them, the complement system, as an inherent component of innate immunity, is the first line of defense for exposing organs, such as eyes, nose, throat, etc. It has been reported that when the complement system was over-activated, it could directly affect the immune function of the eyes, caused local damage such as keratopathy, uveitis, etc[17]–[18]. Clu could participate in the inflammatory reaction process by regulating the complement system. A large number of studies have shown that Clu could be expressed in various organization structures of the eyes, such as cornea, lens, uvea, sclera, etc[19]. It was associated with a various of pathophysiological activities in the eye, including lipids transport, maintenance of membrane integrity, inhibition of cells apoptosis, neurodegenerative changes, etc[20]. The present study speculated that the dry environment affected the lacrimal gland of rats and led a the abnormal expression of Clu, which might be related to the over-activation of complement and coagulation cascade pathway, and caused the inflammatory reaction and affected the immune function.
In addition, we believed that lysosome pathway was also worthy of attention[21]. As known, lysosome is the site of autophagy and endocytosis degradation. Autophagy can clean up the invading pathogens, denatured proteins and damaged organelles in the lysosomal degradation pathway, then maintain cells stability[22]. The autophagy could regulate ocular surface inflammation and reduce the damage of lacrimal gland secretion function and corneal epithelium[23]. Moreover, cathepsin was the main acid hydrolase in lysosomal, it devotes a crucial effect in the degradation of proteins in lysosomal and is inseparable from autophagy[24]. And CTSS is regarded as a ties to regulate the dissolution and remodeling of connective tissue and basement membrane to affect the inflammation and immune response. Up-regulation of CTSS expression will destroy the ocular surface homeostasis, and inhibiting its activity could reduce the inflammatory reaction of lacrimal gland and improve the secretion of tear[25]–[26]. Therefore, it is reasonable to speculate that the activation of lysosome pathway may affect the autophagy level of lacrimal gland tissue, then the related proteins is regulated and it participates in local inflammation and immune response. The pathway and related proteins should be further verified and explored in subsequent studies.
In conclusion, the intervention of dry environment (autumn dryness) caused the tissue morphological changes, local inflammatory infiltration and enhanced the expression of S100A9, Anxa1, and Clu in rats lacrimal gland. It might be related to the low relative humidity of the environment. Such a dry environment affected the micro-environment of the eyes, leading to abnormal pathological links such as inflammation and immune response. Therefore, this study believes that the pathological changes in the fine structure of lacrimal gland in rats and the abnormal expression of S100A9, Anxa1, and Clu proteins are one of the underlying mechanisms of the influence of dry environment on eyes symptoms. It is speculated that long-term exposure to dry environment may lead to xerophthalmia, eye infection, Sjögren's syndrome, etc.
Footnotes
Foundations: Supported by Regional Science Foundation Project of the National Natural Science Foundation of China (No.82060827; No.82260891); The Key Discipline of Universities in the “14th Five-Year Plan” Autonomous Region-Traditional Chinese Medicine at Xinjiang Medical University.
Conflicts of Interest: Sun YL, None; Cui AY, None; Wang LX, None; Zhang WW, None; Shi H, None.
REFERENCES
- 1.Fu YL, Feng Q, Wang ZY. On the reason for the Qin and Han dynasty literature has little discussion on dryness causing disease. Global Tradit Chin Med. 2012;5(7):525–527. [Google Scholar]
- 2.Lu JB, Wu ZB. Discussing Wu Jutong's thoughts on treating autumn dryness based on WenBingTiaoBian. Chin J Ethnomed Ethnopharm. 2021;30(1):6–9. [Google Scholar]
- 3.Yan MM, Yang BA, Huang ZZ. A study on “Autumn Dryness Theory” based on the perspective of hermeneutics. J Tradit Chin Med. 2020;61(15):1307–1310. [Google Scholar]
- 4.Ma BK, Zhou YF, Hu YZ, Duan HY, Sun ZZ, Wang PZ, Li W, Han WL, Qi H. Mapping resident immune cells in the murine ocular surface and lacrimal gland by flow cytometry. Ocul Immunol Inflamm. 2023;31(4):748–759. doi: 10.1080/09273948.2023.2182327. [DOI] [PubMed] [Google Scholar]
- 5.Xu PH, Duan MJ, Hu BH, Jiang J, Shi H. Effects of drought and dryness environment on morphology of lacrimal gland tissues and expression of defensin beta-2 in healthy rats. Chin J Tradit Chin Med Pharm. 2020;35(9):4386–4389. [Google Scholar]
- 6.Shi H, Zhang JF, Hao R, Chan YZ. Exploration on the laboratory simulation of dryness pathogen based on main surface climate indexes in Beijing, Guangzhou, Hetian and other two places. Chin J Tradit Chin Med Pharm. 2019;34(4):1726–1730. [Google Scholar]
- 7.Zheng SY, Ding CH. A literature study on the distribution of TCM with dryness syndrome. Clin Res Tradit Chin Med. 2018;10(16):135–137. [Google Scholar]
- 8.Quinn A, Shaman J. Indoor temperature and humidity in New York City apartments during winter. Sci Total Environ. 2017;583:29–35. doi: 10.1016/j.scitotenv.2016.12.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasus-Jacobi A, Land CA, Stock AJ, Washburn JL, Pereira HA. Antimicrobial peptides derived from the immune defense protein CAP37 inhibit TLR4 activation by S100A9. Invest Ophthalmol Vis Sci. 2020;61(4):16. doi: 10.1167/iovs.61.4.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su JJ, Li HW, Lin BT, Li SM, Zhou XP, Li W, Guo P. Proteomic analysis of meibomian gland secretions in patients with blepharokeratoconjunctivitis. Transl Vis Sci Technol. 2022;11(12):4. doi: 10.1167/tvst.11.12.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong L, Lan WW, Lim RR, Chaurasia SS. S100A proteins as molecular targets in the ocular surface inflammatory diseases. Ocul Surf. 2014;12(1):23–31. doi: 10.1016/j.jtos.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Mimura KK, Tedesco RC, Calabrese KS, Gil CD, Oliani SM. The involvement of anti-inflammatory protein, annexin A1, in ocular toxoplasmosis. Mol Vis. 2012;18:1583–1593. [PMC free article] [PubMed] [Google Scholar]
- 13.Wang XF, Zhang XY, Gao XJ, Liu XX, Wang YH. Proteomic profiling of a respiratory syncytial virus-infected rat pneumonia model. Jpn J Infect Dis. 2016;69(4):285–292. doi: 10.7883/yoken.JJID.2015.244. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Zhang H. Advances in the study of signaling pathways associated with the anti-inflammatory effects of membrane-linked protein A1. Shandong Med. 2020;60(26):102–105. [Google Scholar]
- 15.Lyngstadaas AV, Olsen MV, Bair JA, Hodges RR, Utheim TP, Serhan CN, Dartt DA. Pro-resolving mediator annexin A1 regulates intracellular Ca2+ and mucin secretion in cultured goblet cells suggesting a new use in inflammatory conjunctival diseases. Front Immunol. 2021;12:618653. doi: 10.3389/fimmu.2021.618653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling JY, Tang YL, Ni A, et al. Hepatocellular carcinoma-derived blood-related exosomal components Involved in Natural Immune response in the tumor by the pathway of complement and coagulation cascades. Med Theory Pract. 2020;33(18):2957–2960. [Google Scholar]
- 17.Chrzanowska M, Modrzejewska A, Modrzejewska M. New insight into the role of the complement in the most common types of retinopathy-current literature review. Int J Ophthalmol. 2018;11(11):1856–1864. doi: 10.18240/ijo.2018.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang M, Zhang JJ, Fan W. Complement regulatory protein and ocular disease. Guoji Yanke Zazhi (Int Eye Sci) 2012;12(5):856–860. [Google Scholar]
- 19.Ye Z, Li ZH, He SZ. Action mechanism of clusterin in age-related macular degeneration. Rec Adv Ophthalmol. 2015;35(11):1087–1089, 1093. [Google Scholar]
- 20.Trougakos IP, Gonos ES. Clusterin/apolipoprotein J in human aging and cancer. Int J Biochem Cell Biol. 2002;34(11):1430–1448. doi: 10.1016/s1357-2725(02)00041-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhou C, Pan GY, Liao HT, Tang N. Lysosome related autophagy regulation in cells. Medical Recapitulate. 2021;27(22):4392–4399. [Google Scholar]
- 22.Wang S. Research progress on the mechanism of autophagy in parvovirus infection. Fujian Xumu Shouyi. 2021;44(6):54–60. [Google Scholar]
- 23.Pan YF, Zhang D-W, Lu JF, Zhu LN. Changes of autophagy level and its regulatory effect on inflammatory response in dry eye mice. Linchuang He Shiyan Yixue Zazhi. 2022;21(3):232–235. [Google Scholar]
- 24.Guo YY, Xu M, Tang QZ. The role of lysosomal cathepsin and autophagy in myocardial remodeling. Chin J Geriatr Heart Brain Ves Dis. 2022;24(1):100–102. [Google Scholar]
- 25.Fu RZ, Edman MC, Hamm-Alvarez SF. Rab27a contributes to cathepsin S secretion in lacrimal gland acinar cells. Int J Mol Sci. 2021;22(4):1630. doi: 10.3390/ijms22041630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klinngam W, Janga SR, Lee C, Ju YP, Yarber F, Shah M, Guo H, Wang DD, MacKay JA, Edman MC, Hamm-Alvarez SF. Inhibition of cathepsin S reduces lacrimal gland inflammation and increases tear flow in a mouse model of Sjögren's syndrome. Sci Rep. 2019;9(1):9559. doi: 10.1038/s41598-019-45966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]