Abstract
Background
Since the introduction of the Swedish back school in 1969, back schools have frequently been used for treating people with low‐back pain (LBP). However, the content of back schools has changed and appears to vary widely today. In this review we defined back school as a therapeutic programme given to groups of people, which includes both education and exercise. This is an update of a Cochrane review first published in 1999, and updated in 2004. For this review update, we split the review into two distinct reviews which separated acute from chronic LBP.
Objectives
To assess the effectiveness of back schools on pain and disability for people with acute or subacute non‐specific LBP. We also examined the effect on work status and adverse events.
Search methods
We searched CENTRAL, MEDLINE, EMBASE, CINAHL, PsycINFO, PubMed and two clinical trials registers up to 4 August 2015. We also checked the reference lists of articles and contacted experts in the field of research on LBP.
Selection criteria
We included randomised controlled trials (RCTs) or quasi‐RCTs that reported on back school for acute or subacute non‐specific LBP. The primary outcomes were pain and disability. The secondary outcomes were work status and adverse events. Back school had to be compared with another treatment, a placebo (or sham or attention control) or no treatment.
Data collection and analysis
We used the 2009 updated method guidelines for this Cochrane review. Two review authors independently screened the references, assessed the quality of the trials and extracted the data. We set the threshold for low risk of bias, a priori, as six or more of 13 internal validity criteria and no serious flaws (e.g. large drop‐out rate). We classified the quality of the evidence into one of four levels (high, moderate, low or very low) using the adapted Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. We contacted study authors for additional information. We collected adverse effects information from the trials.
Main results
The search update identified 273 new references, of which none fulfilled our inclusion criteria. We included four studies (643 participants) in this updated review, which were all included in the previous (2004) update. The quality of the evidence was very low for all outcomes. As data were too clinically heterogeneous to be pooled, we described individual trial results. The results indicate that there is very low quality evidence that back schools are no more effective than a placebo (or sham or attention control) or another treatment (physical therapies, myofascial therapy, joint manipulations, advice) on pain, disability, work status and adverse events at short‐term, intermediate‐term and long‐term follow‐up. There is very low quality evidence that shows a statistically significant difference between back schools and a placebo (or sham or attention control) for return to work at short‐term follow‐up in favour of back school. Very low quality evidence suggests that back school added to a back care programme is more effective than a back care programme alone for disability at short‐term follow‐up. Very low quality evidence also indicates that there is no difference in terms of adverse events between back school and myofascial therapy, joint manipulation and combined myofascial therapy and joint manipulation.
Authors' conclusions
It is uncertain if back schools are effective for acute and subacute non‐specific LBP as there is only very low quality evidence available. While large well‐conducted studies will likely provide more conclusive findings, back schools are not widely used interventions for acute and subacute LBP and further research into this area may not be a priority.
Keywords: Humans, Exercise Therapy, Patient Education as Topic, Program Evaluation, Acute Pain, Acute Pain/therapy, Diathermy, Low Back Pain, Low Back Pain/therapy, Musculoskeletal Manipulations, Randomized Controlled Trials as Topic
Plain language summary
Back schools for acute and subacute non‐specific low‐back pain
Review question
The aim of the review was to assess the effectiveness of back schools on pain, disability, work status and adverse events compared to another treatment, a placebo (sham treatment) or no treatment for acute and subacute non‐specific low‐back pain.
Background
Low‐back pain is a burden in Western societies and causes high costs in terms of healthcare costs and loss of productivity. It is a common disorder that affects 12% to 30% of the population everyday. Back school is a therapeutic programme which includes both education and exercise, and is given to groups of participants and supervised by a healthcare provider. It was introduced in Sweden in 1969 and the content and length of back schools seem now to vary widely.
The target population of this review were people with acute and subacute (between acute and chronic) non‐specific low‐back pain. We defined non‐specific low‐back pain as pain localised below the scapulae (shoulder blade) and above the cleft of the buttocks without any specific cause detectable (e.g. infection, neoplasm, metastasis, osteoporosis, rheumatoid arthritis, fracture or inflammatory process). Acute and subacute pain means that the pain did not last more than six and 12 weeks, respectively. Our primary outcomes were pain and disability, and our secondary outcomes were work status and adverse events.
Study characteristics
We included four studies in this review, which we included in the previous version of this review, which means that we did not identify any new relevant studies for inclusion in this update. The treatment comparisons were too dissimilar to be pooled and half of the studies were at high risk of bias. The quality of the evidence was very low for all outcomes.
One study compared back school with a placebo (sham treatment) and found no difference between groups for pain at short‐term follow‐up. Concerning work status, people in the back school group had a significantly shorter duration of sick‐leave than people in the placebo group at short‐term follow‐up.
Four studies compared back school with another treatment (physical therapies, myofascial therapy, joint manipulations, advice). Overall, there were no differences between groups for pain, disability, work status and adverse events at any time of follow‐up. Only one study showed that back school added to a back care programme was more effective than back school alone for disability at short‐term follow‐up.
Key results
The included studies are insufficient to clearly answer our question and the inclusion of other well‐designed studies is very likely to change the conclusions. However, back schools do not seem to be a treatment widely used nowadays for people with acute and subacute non‐specific low‐back pain and are not endorsed by guidelines.
Quality of the evidence
The quality of the evidence was very low for all the outcomes according to the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach. This was due to poor study designs and imprecision in the results.
Summary of findings
Summary of findings for the main comparison. Back school compared to combined physical therapies for acute or subacute low‐back pain.
| Back school compared to combined physical therapies for acute or subacute low‐back pain | ||||||
| Participant or population: participants with acute or subacute low‐back pain Settings: Volvo factory in Goteborg Intervention: back school Comparison: combined physical therapies | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Combined physical therapies | Back school | |||||
| Pain (short‐term) Pain index1. Scale from: 0 to 70. | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ very low2,3 | The median pain scores were 22/70 for the back school group (N = 14) and 21/70 for the control group (N = 16)5 |
| Duration of sick‐leave Sick‐leave > 21 days | 51 per 100 | 33 per 100 (21 to 51) | RR 0.64 (0.41 to 1.01) | 116 (1 study) | ⊕⊝⊝⊝ very low2,4 | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; GRADE: Grading of Recommendations Assessment, Development and Evaluation; N = number of participants. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1The pain index was based on seven questions with a total score ranging from 0 to 70. The trial authors arbitrarily established the scores. 2Downgraded by two due to the absence of allocation concealment, the lack of blinding, and incomplete outcome data. 3Downgraded by one due to flaws in pain report (pain index and its scores arbitrarily created by the trial authors). There was only one study (N = 30) that examined this outcome, the results would need replication. 4Downgraded by one because there was only one study (N = 116) that examined this outcome, the results would need replication. 5There was no statistically significant difference between groups but the trial did not report any data on dispersion.
Summary of findings 2. Back school compared to short‐wave diathermy at the lowest intensity for acute or subacute low‐back pain.
| Back school compared to short‐wave diathermy at the lowest intensity for acute or subacute low‐back pain | ||||||
| Participant or population: participants with acute or subacute low‐back pain Settings: Volvo factory in Goteborg Intervention: back school Comparison: short‐wave diathermy at the lowest intensity | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short‐wave diathermy | Back school | |||||
| Pain (short‐term) Pain index1. Scale from: 0 to 70. | See comment | See comment | Not estimable | 29 (1 study) | ⊕⊝⊝⊝ very low2,3 | The median pain scores were 22/70 for the back school group (N = 14) and 17/70 for the short‐wave diathermy group (N = 15)5 |
| Duration of sick‐leave Sick‐leave > 21 days | 62 per 100 | 33 per 100 (22 to 50) | RR 0.53 (0.35 to 0.80) | 121 (1 study) | ⊕⊝⊝⊝ very low2,4 | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; ; GRADE: Grading of Recommendations Assessment, Development and Evaluation; N = number of participants. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1The pain index was based on seven questions with a total score ranging from 0 to 70. The trial authors arbitrarily established the scores. 2Downgraded by two due to the absence of allocation concealment, the lack of blinding, and incomplete outcome data. 3Downgraded by one due to flaws in pain report (pain index and its scores arbitrarily created by the trial authors). There was only one study (N = 29) that examined this outcome, the results would need replication. 4Downgraded by one because there was only one study (N = 121) that examined this outcome, the results would need replication. 5There was no statistically significant difference between groups but no data on dispersion were reported.
Summary of findings 3. Back school compared to myofascial therapy for acute or subacute low‐back pain.
| Back school compared to myofascial therapy for acute or subacute low‐back pain | |||||
| Participant or population: participants with acute or subacute low‐back pain Settings: primary care Intervention: back school Comparison: myofascial therapy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Myofascial therapy | Back school | ||||
| Pain (short‐term) Visual analogue scale (VAS). Scale from: 0 to 10. | The mean pain (short‐term) in the control groups was 2.78 | The mean pain (short‐term) in the intervention groups was 0.65 lower (1.49 lower to 0.19 higher) | — | 63 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Pain (intermediate‐term) VAS. Scale from: 0 to 10. | The mean pain (intermediate‐term) in the control groups was 2.99 | The mean pain (intermediate‐term) in the intervention groups was 0.70 lower (1.92 lower to 0.52 higher) | — | 61 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Disability (short‐term) Roland Morris Disability Questionnaire (RMDQ). Scale from: 0 to 24. | The mean disability (short‐term) in the control groups was 5.80 | The mean disability (short‐term) in the intervention groups was 1.54 lower (3.88 lower to 0.80 higher) | — | 63 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Disability (intermediate‐term) RMDQ. Scale from: 0 to 24. | The mean disability (intermediate‐term) in the control groups was 5.06 | The mean disability (intermediate‐term) in the intervention groups was 1.58 lower (4.02 lower to 0.86 higher) | — | 61 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Adverse events Number of events | 8 per 100 | 12 per 100 (4 to 42) | RR 1.59 (0.48 to 5.30) | 99 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; VAS: visual analogue scale; RMDQ: Roland Morris Disability Questionnaire. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
1Downgraded by two due to the absence of allocation concealment and the lack of blinding. 2Downgraded by one because there was only one included study (N = 99), and the results would need replication.
Summary of findings 4. Back school compared to joint manipulation for acute or subacute low‐back pain.
| Back school compared to joint manipulation for acute or subacute low‐back pain | |||||
| Participant or population: participants with acute or subacute low‐back pain Settings: primary care Intervention: back school Comparison: joint manipulation | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Joint manipulation | Back school | ||||
| Pain (short‐term) Visual Analogue Scale (VAS). Scale from: 0 to 10. | The mean pain (short‐term) in the control groups was 2.58 | The mean pain (short‐term) in the intervention groups was 0.45 lower (1.33 lower to 0.43 higher) | — | 59 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Pain (intermediate‐term) VAS. Scale from: 0 to 10. | The mean pain (intermediate‐term) in the control groups was 2.40 | The mean pain (intermediate‐term) in the intervention groups was 0.11 lower (1.39 lower to 1.17 higher) | — | 54 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Disability (short‐term) Roland Morris Disability Questionnaire (RMDQ). Scale from: 0 to 24. | The mean disability (short‐term) in the control groups was 4.42 | The mean disability (short‐term) in the intervention groups was 0.16 lower (2.50 lower to 2.18 higher) | — | 59 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Disability (intermediate‐term) RMDQ. Scale from: 0 to 24. | The mean disability (intermediate‐term) in the control groups was 3.29 | The mean disability (intermediate‐term) in the intervention groups was 0.19 higher (2.30 lower to 2.68 higher) | — | 55 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Adverse events Number of events | 12 per 100 | 12 per 100 (4 to 36) | RR 1.02 (0.35 to 2.94) | 97 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; VAS: visual analogue scale; RMDQ: Roland Morris Disability Questionnaire. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
1Downgraded by two due to the absence of allocation concealment and the lack of blinding. 2Downgraded by one because there was only one included study (N = 97), and the results would need replication.
Summary of findings 5. Back school compared to combined myofascial therapy and joint manipulation for acute or subacute low‐back pain.
| Back school compared to combined myofascial therapy and joint manipulation for acute or subacute low‐back pain | |||||
| Participant or population: participants with acute or subacute low‐back pain Settings: primary care Intervention: back school Comparison: combined myofascial therapy and joint manipulation | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Combined myofascial therapy and joint manipulation | Back school | ||||
| Pain (short‐term) Visual Analogue Scale (VAS). Scale from: 0 to 10. | The mean pain (short‐term) in the control groups was 2.04 | The mean pain (short‐term) in the intervention groups was 0.09 higher (0.68 lower to 0.86 higher) | — | 62 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Pain (intermediate‐term) VAS. Scale from: 0 to 10. | The mean pain (intermediate‐term) in the control groups was 2.24 | The mean pain (intermediate‐term) in the intervention groups was 0.05 higher (1.13 lower to 1.23 higher) | — | 63 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Disability (short‐term) Roland Morris Disability Questionnaire (RMDQ). Scale from: 0 to 24. | The mean disability (short‐term) in the control groups was 3.73 | The mean disability (short‐term) in the intervention groups was 0.53 higher (1.60 lower to 2.66 higher) | — | 62 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Disability (intermediate‐term) RMDQ. Scale from: 0 to 24. | The mean disability (intermediate‐term) in the control groups was 3.56 | The mean disability (intermediate‐term) in the intervention groups was 0.08 lower (2.33 lower to 2.17 higher) | — | 62 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Adverse events Number of events | 13 per 100 | 13 per 100 (5 to 35) | RR 0.93 (0.34 to 2.57) | 100 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; VAS: visual analogue scale; RMDQ: Roland Morris Disability Questionnaire. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
1Downgraded by two due to the absence of allocation concealment and the lack of blinding. 2Downgraded by one because there was only one included study (N = 100), and results would need replication.
Summary of findings 6. Back school + other treatment compared to other treatment alone for acute or subacute low‐back pain.
| Back school + other treatment compared to other treatment alone for acute or subacute low‐back pain | |||||
| Participant or population: participants with acute or subacute low‐back pain Settings: primary care Intervention: back school + other treatment (back care programme) Comparison: other treatment (back care programme) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Back school + other treatment | Other treatment | ||||
| Pain (short‐term) Visual Analogue Scale (VAS). Scale from: 0 to 10. | The mean pain (short‐term) in the control groups was 1.2 | The mean pain (short‐term) in the intervention groups was 0.50 higher (0.07 lower to 1.07 higher) | — | 156 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Pain (intermediate‐term) VAS. Scale from: 0 to 10. | The mean pain (intermediate‐term) in the control groups was 1.2 | The mean pain (intermediate‐term) in the intervention groups was 0.30 higher (0.34 lower to 0.94 higher) | — | 140 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Pain (long‐term) VAS. Scale from: 0 to 10. | The mean pain (long‐term) in the control groups was 1.2 | The mean pain (long‐term) in the intervention groups was 0.20 higher (0.47 lower to 0.87 higher) | — | 141 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Disability (short‐term) Roland Morris Diability Questionnaire (RMDQ). Scale from: 0 to 24. | The mean disability (short‐term) in the control groups was 2.83 | The mean disability (short‐term) in the intervention groups was 1.78 higher (0.52 to 3.04 higher) | — | 156 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Disability (intermediate‐term) RMDQ. Scale from: 0 to 24. | The mean disability (intermediate‐term) in the control groups was 1.90 | The mean disability (intermediate‐term) in the intervention groups was 0.81 higher (0.44 lower to 2.06 higher) | — | 140 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Disability (long‐term) RMDQ. Scale from: 0 to 24. | The mean disability (long‐term) in the control groups was 1.66 | The mean disability (long‐term) in the intervention groups was 0.48 higher (0.65 lower to 1.61 higher) | — | 141 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| Work absence at 30 days Number of participants on sick‐leave | 70 per 100 | 65 per 100 (53 to 81) | RR 0.94 (0.76 to 1.16) | 168 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; VAS: visual analogue scale; RMDQ: Roland Morris Disability Questionnaire. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
1Downgraded by two du to the absence of allocation concealment and the lack of blinding. 2Downgraded by one because only one study (N = 170) examined this outcome, and the results would need to be replicated.
Summary of findings 7. Back school compared to advice for acute or subacute low‐back pain.
| Back school compared to advice for acute or subacute low‐back pain | |||||
| Participant or population: participants with acute or subacute low‐back pain Settings: primary care Intervention: back school Comparison: advice | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Advice | Back school | ||||
| Days of sick‐leave at 1 year | The mean days of sick‐leave at 1 year in the control groups was 39 days | The mean days of sick‐leave at 1 year in the intervention groups was 3.00 lower (29.24 lower to 23.24 higher) | — | 56 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
1Downgraded by two due to the absence of allocation concealment and the lack of blinding. 2Downgraded by one because there was only one included study (N = 56), and results would need to be replicated.
Background
Description of the condition
Low‐back pain (LBP) related disability and work absence accounts for high economic costs in Western societies (Dagenais 2008). Estimates of the cost of back problems in Australia in 2012 were AUD 4.8 billion in healthcare costs per annum (Arthritis and Osteoporosis Victoria 2013) and the estimates in 2003 were AUD 8 billion in lost productivity per annum (Walker 2003). Estimates of the financial burden of LBP in the Netherlands in 2002 indicated that the total costs were almost EUR 6 billion per annum (Lambeek 2011). Although LBP rarely indicates a serious underlying disorder, people with LBP that lasts for longer than one or two months have an elevated risk of developing longer‐term disability and repeated care‐seeking (Koes 2006). To date, many therapeutic interventions have been performed and studied for the treatment of LBP. However, no single treatment has proven to be obviously superior compared to any other. Recommendations from clinical guidelines vary between countries but rarely include information about back schools (Koes 2010). Continuously and systematically summarising the literature provides the best evidence for the treatment of (subgroups of) people with LBP. In this systematic review, we will present the results on the effectiveness of back schools for acute and subacute non‐specific LBP.
Description of the intervention
Zachrisson‐Forsell introduced the original 'Swedish back school' in 1969 (Zachrisson‐Forssell 1980; Zachrisson‐Forssell 1981). The back school consisted of information on the anatomy of the back, biomechanics, optimal posture, ergonomics and back exercises. Four small group sessions were scheduled during a two‐week period, and each session lasted 45 minutes. Since the introduction of the Swedish back school, the content and length of back schools has changed and appears to vary widely today.
How the intervention might work
In its original description in 1980, the back school programme was intended to reduce pain and prevent recurrences of episodes of LBP (Zachrisson‐Forssell 1980). The main purpose was to make subjects active regarding their back problems. They were given the ability and knowledge to cope with pain on their own.
Why it is important to do this review
This is an update of a previously conducted Cochrane review of randomised controlled trials (RCTs) on the effectiveness of back schools (van Tulder 1999), which was updated in 2004 (Heymans 2004). We split the review into two distinct reviews. This review concerns the effectiveness of back schools for acute and subacute non‐specific LBP and a separate review will evaluate back schools for chronic non‐specific LBP. The previous review (Heymans 2004) that included both populations concluded that there was moderate evidence that back schools conducted in an occupational setting were more effective for chronic and recurrent LBP than another treatment, a placebo or a waiting list control for pain, disability and return to work at short and intermediate terms follow‐up. There was limited and conflicting evidence for the acute and subacute LBP population.
Although this review is published after the release of the Furlan 2015 method guidelines, this update was conducted before it was released and therefore we used the previous method guidelines (Furlan 2009).
Objectives
The objective of this review was to determine the effect of back schools on pain and disability for people with acute or subacute non‐specific LBP. In trials that solely recruit workers, we also examined the effect on work status.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) or quasi‐RCTs (e.g. alternation, allocation by date of birth, clinic record number).
Types of participants
We included trials that included participants with acute (less than six weeks) or subacute (six to 12 weeks) non‐specific LBP, and were aged between 18 to 70 years. We defined low‐back pain (LBP) as pain localised below the scapulae and above the cleft of the buttocks; non‐specific indicated that no specific cause was detectable, such as, infection, neoplasm, metastasis, osteoporosis, rheumatoid arthritis, fracture or inflammatory process. We excluded trials including participants with LBP due to pregnancy. When the duration of pain exceeded the subacute definition (e.g. three weeks to six months), we planned to contact the authors to obtain the data for our population of interest only. If this was not possible, we calculated the average duration of the symptoms to include the population either in the acute/subacute LBP review or in the chronic LBP review.
Types of interventions
We included trials in which one of the interventions was back school. We defined back school as an educational and skills acquisition programme, including exercises, in which all lessons were given to groups of participants and supervised by a healthcare provider (Zachrisson‐Forssell 1980). This means that this back school review differs from other LBP Cochrane reviews about exercise (Hayden 2005), patient education (Engers 2008) or multidisciplinary rehabilitation (Karjalainen 2003); back school interventions are given to groups of participants and include exercise sessions (in contrast to exercise or education alone) and are given by a healthcare provider (in contrast to a multidisciplinary team). We included trials that used a clear contrast for the back school intervention, such as, no treatment, waiting list, usual care or other interventions such as exercise therapy or manipulation. Additional interventions were allowed. However, if the back school was part of a larger multidisciplinary treatment programme, we only included the study if a contrast existed for the back school. For example, we included a study that compared a back school plus a fitness programme against a fitness programme, but excluded a study that compared a back school plus a fitness programme against a waiting list. We defined a study as being conducted in an occupational setting when the study population consisted of a working population, and included workers with LBP or who were on sick leave due to LBP at study onset (secondary prevention), and who attended a programme (at their workplace or at a clinic). We excluded trials that studied the effectiveness of back schools in workers or non‐workers without LBP at study onset because these were aimed at the primary prevention of LBP.
Types of outcome measures
We included trials that reported outcomes for short‐term (three months or less), intermediate‐term (three to six months) and long‐term (longer than six months) follow‐up.
Primary outcomes
The primary outcomes were pain and disability.
Secondary outcomes
We collected information about adverse events. In trials that solely recruited workers we considered work status as a secondary outcome.
Search methods for identification of studies
Electronic searches
In the original Cochrane review published in 1999, van Tulder 1999 conducted the following search strategy.
The review authors performed a computer‐aided search of the MEDLINE (from 1966 to 1997) and EMBASE (from 1988 to 1997) databases.
They screened references given in relevant reviews and included RCTs.
They screened CENTRAL, the Cochrane Library 1998, Issue 4, using the search terms ’back pain’ and ’low back pain’.
Heymans 2004 used the same search strategy from January 1998 to May 2003 for the first review update, and also searched the Cochrane Library 2003, Issue 2. The search was up to date in November 2004.
For this update, we conducted the search from November 2004 to 4 August 2015. We based the current searches on the search strategies recommended by the Cochrane Back and Neck Review Group (Furlan 2009). The databases searched were:
Cochrane Central Register of Controlled Trials (CENTRAL,the Cochrane Library, Issue 7).
MEDLINE (OvidSP, 1946 to July Week 4 2015).
MEDLINE In‐Process & Other Non‐Indexed Citations (OvidSP, August 03, 2015).
EMBASE (OvidSP, 1980 to 2015 Week 31).
Cumulative Index to Nursing and Allied Health (CINAHL; EBSCO, 1981 to August 4, 2015).
PsycINFO (2002 to July Week 3 2015).
ClinicalTrials.gov.
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP).
PubMed.
We added PsycINFO and CINAHL to the strategy in 2007, included the clinical trials registries in 2011, and added MEDLINE In‐Process & Other Non‐Indexed Citations in 2015; we searched them from inception to 4 August 2015. We added PubMed in 2015 and searched from 4 March 2014 (the date of the previous search update) to 4 August 2015 to capture studies not yet in MEDLINE using the strategy recommended by Duffy 2014.
We did not use any language restrictions in the search strategy. The Trials Search Co‐ordinator from the Cochrane Back and Neck Review Group updated the literature search. See Appendix 1 for the specific search strategies.
Searching other resources
We screened references given in relevant reviews and included studies and consulted experts in the field of LBP research to identify potentially relevant studies we might have missed.
Data collection and analysis
For each of the following steps, two review authors (NP, CL) independently selected the studies, assessed the risk of bias and extracted the data (using a standardised form). We resolved any disagreements by consensus or consulted a third review author (CM) if disagreement persisted.
Selection of studies
For this update, we first reviewed the included studies from the last update of the review, Heymans 2004, to ensure they still met our revised inclusion criteria. Following the same process as in the original and updated reviews, two review authors (NP, CL) independently screened the titles and abstracts of newly identified search results. We retrieved the full‐text articles of all potentially relevant search results for the final selection of eligible studies. We resolved any disagreements by consensus or consulted a third review author (CM) if disagreements persisted.
Data extraction and management
Two review authors (NP, CL) independently extracted the data (using standardised forms) on population characteristics (participants selection criteria, duration of LBP, LBP characteristics such as radiation to the leg, study setting, gender, age, baseline data on severity and duration of pain), intervention characteristics (description and types of back schools, duration and number of treatment sessions, description of comparison groups, and co‐interventions) and outcome data. We extracted data on the following outcomes: a) pain intensity, b) disability, c) work status and d) adverse events. We defined the short‐term follow‐up as less than three months, intermediate‐term between three and six months, and long term as longer than six months. When several time points fell within the same category, we used the time point closest to six weeks for the short term, four months for the intermediate term, and 12 months for the long‐term follow‐up. We resolved any disagreements on data extraction by consensus, and consulted a third review author (CM) if disagreements persisted.
Assessment of risk of bias in included studies
Two review authors (NP, CL) independently assessed the risk of bias of the included RCTs. We used the Cochrane 'Risk of bias' tool adapted from the Cochrane Handbook for Systematic Reviews of Interventions and recommended by the Cochrane Back and Neck Review Group to assess the risk of bias (Higgins 2011). We have listed the criteria in Appendix 2, along with the operational definitions. We assessed the items as at low risk of bias (+), high risk of bias (‐) or unclear (?). We used a consensus method to resolve disagreements and consulted a third review author (CM) if disagreements persisted. If the article did not contain information on (one or more of) the criteria (score 'unclear'), we contacted the study authors for additional information. We anticipated that study authors might work at places other than those listed in the publications. In that case, we tried to locate their current working address through their last publication in MEDLINE or through the Internet. If we were unable to find a more recent working address, we sent the request for information to the address listed on the paper included in our review. If we could not contact the study authors or if the information was no longer available, we scored the criteria as 'unclear'. We rated a study as being at 'low risk of bias' when it met at least six criteria and the study had no serious flaws (e.g. high drop‐out rate).
Given that the Cochrane 'Risk of bias' tool, Higgins 2011, had been updated since the last review (Heymans 2004), we reassessed the studies from the previous (2004) review, Heymans 2004, for six items of the scale. Two items have been added (items 8 and 13) about reporting and other biases, the definition of three items has changed (items 3, 5 and 12) about the blinding and the timing of outcome assessment and we gave details on how to rate an item (item 11) about compliance. For item 3, a participant was considered as blinded in the previous (2004) review if an attempt was made to blind the participants or if the credibility of the treatments was evaluated and treatments were equally credible and acceptable to participants. The current update considers a participant blinded only if it was not possible to distinguish between the treatments or if the outcome was unlikely to be influenced by lack of blinding. For item 5, it was up to the review author to judge if there was enough information about the blinding of the assessor. The current update specifies that for patient‐centered outcomes, there is a low risk of bias for outcome assessors if there is a low risk of bias for participant blinding. For item 12, the definition in the last review (Heymans 2004) was: "Adequate length of follow‐up ‐ scored positive if an effect measurement is included after 12 months or more". In this review, we used the update definition that specifies that all the important outcome assessments for all intervention groups have to be measured at the same time. For item 11, we defined an acceptable compliance rate as 75%.
Measures of treatment effect
We expressed treatment effects of continuous variables as mean differences (MDs) and 95% confidence intervals (95% CIs). If outcome measures of continuous variables differed between studies, we converted the different outcome measures to a common 0 to 100 scale. We reported treatment effects of dichotomous variables as risk ratios (RR) and 95% CIs.
Assessment of heterogeneity
We evaluated the clinical homogeneity of studies by exploring their similarities and differences, and took into consideration the study population, types of back schools and reference treatments, timing of follow‐up measurements and outcomes and measurement instruments. We formally tested statistical homogeneity for studies that were sufficiently clinically homogenous to pool. We assessed the homogeneity of intervention effects using a Chi² test of the observed differences between studies results. A low P value would be in favour of heterogeneity of intervention effects between studies showing that the variation of effect estimates was beyond chance. We tested inconsistency across studies using the index I² statistic. The results provided the percentage of variability in the effect estimates due to heterogeneity. On the basis of these evaluations, we attempted to statistically pool the data for the outcome measures pain, disability and work status, and recognised that there may be insufficient data to accomplish this. We made these attempts for short‐, intermediate‐ and long‐term follow‐up.
Data synthesis
We planned to perform a meta‐analysis to combine the results of individual trials. The pooling of the data was dependent on the level of statistical heterogeneity between trials. If the I² statistic value was inferior to 50%, we combined the results in a meta‐analysis using a random‐effects model. If the I² statistic value was higher than 50%, we defined studies as too heterogeneous and we did not perform a meta‐analysis. Instead, we synthesised findings narratively reporting descriptive data from each trial.
GRADE and 'Summary of findings' table
We assessed the overall quality of the evidence for each outcome using an adapted Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Higgins 2011), as recommended by the Cochrane Back and Neck Review Group (Furlan 2009). We based the quality of the evidence on a specific outcome on five domains: limitations of the study design, inconsistency, indirectness (inability to generalise), imprecision (insufficient or imprecise data) of results and publication bias across all studies that measured that particular outcome. The quality started at high when at least two high quality RCTs provided results for the outcome, and we reduced the quality of the evidence by a level for each of the domains not met. In situations where only one study with a small sample size measured the outcome, we downgraded the imprecision domain by one level.
High quality evidence: there were consistent findings among at least 75% of RCTs with no limitations of the study design, consistent, direct and precise data and no known or suspected publication biases. Further research was very unlikely to change our confidence in the estimate of effect.
Moderate quality evidence: one of the domains was not met. Further research was likely to have an important impact on our confidence in the estimate of effect and may have changed the estimate.
Low quality evidence: two of the domains were not met. Further research was very likely to have an important impact on our confidence in the estimate of effect and was likely to change the estimate.
Very low quality evidence: three of the domains were not met. There was great uncertainty about the estimate of effect.
No evidence = we did not identify any RCTs that addressed this outcome.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses to investigate if the estimates of effect differed in studies of back school that 1) included participants with LBP with radiation to the leg versus LBP without radiation to the leg and 2) were conducted in an occupational versus another setting.
Sensitivity analysis
We planned to perform sensitivity analyses to see if the overall results on effectiveness between comparison groups changed when we used different definitions of high risk of bias, i.e. if we defined high risk of bias as fulfilling five or more or seven or more criteria respectively, or as having an adequate concealment of treatment allocation.
Results
Description of studies
Results of the search
The electronic searches for this update identified 503 references (Figure 1), which we retrieved after we searched from 2004 to 4 August 2015 in MEDLINE (60 results), EMBASE (97 results), CENTRAL (122 results), ClinicalTrials.gov (44 results) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (11 results), and searched PsycINFO (19 results) and CINAHL (81 results) from their inception to August 2015. We searched PubMed from 4 March 2014 (the date of the previous search update) to 4 August 2015 and retrieved 69 results. After we removed duplicates (129 references) and references already seen (101 references), we screened 273 references for title and abstract selection. Both review authors (NP, CL) agreed that the full text of 12 references should be screened (Chenard 1991; Federwisch 1998; Heymans 2006; Jaromi 2012; Larsen 2002; Mele 2006; Meng 2009; Meng 2011; Ribeiro 2008; Schenk 1996; Sirles 1991; Tavafian 2007).
1.

Study flow diagram
We screened the reference lists of eight systematic reviews (Brox 2008; Di Fabio 1995; Keijsers 1991; Maier‐Riehle 2001; Pengel 2002; Scheer 1995; Turner 1996; Wiese 2009) and did not identify any new relevant study for inclusion.
We also asked experts in the field of research in LBP but did not add any new study.
Finally we included four studies from the previous review in this current update (Bergquist 1977; Hsieh 2002; Leclaire 1996; Lindequist 1984).
Included studies
We have described the characteristics of the four included studies in the 'Characteristics of included studies' table.
Design
One study was a quasi‐randomised controlled trial (RCT) (Lindequist 1984), and the three others were RCTs. Two studies used a two‐group design that compared back school to another treatment (Lindequist 1984) or back school in addition to another treatment to the treatment alone (Leclaire 1996). One study used a three‐group design that compared back school to combined physical therapy and to short‐wave diathermy at the lowest intensity (Bergquist 1977), and one study involved four groups that compared back school to myofascial therapy, to joint manipulation and to combined myofascial therapy and joint manipulation (Hsieh 2002).
Sample size
The sample size of the individual studies ranged from 56 to 217 participants. The median was 185 participants with a total of 643 participants included in the four studies. All included studies reported on the withdrawal or dropout rates, which ranged from 4% (Lindequist 1984) to 80% (Bergquist 1977) for any time of follow‐up. The reason for the high rate of dropouts in Bergquist 1977 is that the trial authors removed participants from data collection as soon as they had recovered.
Setting
Two studies were conducted in an occupational setting (Bergquist 1977; Leclaire 1996), one concerned primary care (Lindequist 1984), and one was conducted in a mixed setting (Hsieh 2002).
Participants
All studies included adults only, aged from 16 to 65 years. The mean or median age of the participants ranged from 32 (Leclaire 1996) to 49 years (Hsieh 2002). Two studies included some participants younger than 18 but the mean age was 37 years for Lindequist 1984 and the median age was 34.5 years for Bergquist 1977. Two studies included age limits in their criteria (Hsieh 2002; Leclaire 1996). Three studies included workers only (Bergquist 1977; Leclaire 1996; Lindequist 1984). Two studies included a homogeneous population of LBP participants without radiation (Hsieh 2002; Leclaire 1996), and two studies included a mixed population of participants with and without radiating symptoms (Bergquist 1977; Lindequist 1984). Three studies reported on acute/subacute LBP (Bergquist 1977; Leclaire 1996; Lindequist 1984), and one study reported on a mixed population of acute and chronic LBP participants (Hsieh 2002). As pre‐specified in the Methods, we used the average duration of the symptoms to decide if we included the study in the review about acute and subacute LBP or in the review about chronic LBP. For Hsieh 2002, the average duration of the symptoms was under 12 weeks in each group. For the three studies including a population with acute and subacute LBP only, there were differences in the time free of pain before onset as specified in the selection criteria. Bergquist 1977 mentioned a pain‐free year before onset, Leclaire 1996 excluded participants with a history of a LBP episode of more than one week duration and Lindequist 1984 did not report any details about previous episodes of pain.
Interventions
The interventions section of the 'Characteristics of included studies' table shows that the back school interventions varied from a single session of information and instructions (Lindequist 1984), to a 'Swedish back school' consisting of four lessons totalling three hours (Bergquist 1977). For the reference treatments, one study compared back school to short‐wave diathermy at the lowest intensity (Bergquist 1977). Three studies compared back school to another treatment and had the following control interventions: physical therapy alone (Bergquist 1977), spinal manipulation (Hsieh 2002), myofascial therapy (Hsieh 2002), a combination of myofascial therapy and joint manipulation (Hsieh 2002), and advice and medication (Lindequist 1984). One study compared back school added to a back care programme (physical therapy combined with medication and rest) to a back care programme alone (Leclaire 1996).
Outcomes
Pain
Three studies reported pain outcomes (Bergquist 1977; Hsieh 2002; Leclaire 1996). Most used the visual analogue scale (VAS) and one study introduced a new scale, the 'pain index' (Bergquist 1977). The 'pain index' was based on seven questions with a total score ranging from 0 to 70. The trial authors had arbitrarily established the scores.
Disability
Two studies reported disability outcomes using the Roland‐Morris Disability Questionnaire (RMDQ) (Hsieh 2002; Leclaire 1996). Leclaire 1996 presented the RMDQ as a 100‐point scale. We converted the data back to a 24‐point scale to enhance comparison.
Work status
Three studies reported the work status of their population and measured the time to return to work (Bergquist 1977; Leclaire 1996; Lindequist 1984). One study reported the reason why participants were sick‐listed (because of LBP or because of other disorders) (Lindequist 1984).
Adverse events
Only one study reported on adverse events (Hsieh 2002).
Timing of outcome assessments
One study reported short‐term (three weeks; Hsieh 2002) and three studies long‐term outcomes (12 months; Bergquist 1977; Leclaire 1996; Lindequist 1984).
Excluded studies
The 2004 review update, Heymans 2004, included 19 studies (27 references) of which 12 studies (19 references) dealt with chronic non‐specific LBP and were excluded (Dalichau 1999; Donchin 1990; Härkäpää 1989; Hurri 1989; Keijsers 1989; Keijsers 1990; Klaber Moffett 1986; Lankhorst 1983; Linton 1989; Lønn 1999; Pentinnen 2002; Postacchini 1988). Seven studies (8 references) included a population with acute or subacute non‐specific LBP (Bergquist 1977; Berwick 1989; Herzog 1991; Hsieh 2002; Indahl 1998; Leclaire 1996; Lindequist 1984). After screening these studies again according to our revised inclusion criteria, we excluded one study, reported in two manuscripts, from the current update due to their definition of the back school programme (Indahl 1998). The description in the published article was unclear on whether the back school programme contained exercise. We sought confirmation from the first trial author who confirmed that it did not, so the study did not fulfil the inclusion criteria and we excluded it from the current update. We also excluded two studies that included a mixed population with acute and chronic pain but did not give any details on the duration of symptoms (Berwick 1989; Herzog 1991). In Berwick 1989, the eligibility criteria specified a duration of pain from two weeks to six months. We considered the median time of this range, which was 14 weeks, to classify the population as having chronic LBP. In Herzog 1991, included participants had sacroiliac pain lasting more than one month, defined as chronic pain. Based on the 'chronic' description used by the trial authors, we considered the population as having chronic pain. The Cochrane review about back schools for chronic non‐specific LBP will include these trials.
We excluded 12 studies from the updated search after full‐text screening. We have described the reasons for exclusion in the 'Characteristics of excluded studies' table. Six studies referred to a population with chronic LBP (Chenard 1991; Heymans 2006; Jaromi 2012; Meng 2009; Ribeiro 2008; Tavafian 2007), two studies compared two types of back schools (Meng 2011; Sirles 1991), two studies were about primary prevention (Larsen 2002; Schenk 1996), and two studies were not RCTs or quasi‐RCTs (Federwisch 1998; Mele 2006).
Risk of bias in included studies
We presented the methodological quality of the four included studies in Figure 2, and provided a summary of the 'Risk of bias' assessment of each item across the trials in Figure 3. We asked the (first) authors of the studies for additional information if one or more of our criteria was 'unclear'. For the current update, we were unable to find the contact details of the authors of the two studies that we needed additional information on (Leclaire 1996; Lindequist 1984). Two studies had six or more criteria at low risk of bias on the Cochrane 'Risk of bias' tool (Hsieh 2002; Leclaire 1996).
2.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
3.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
As pre‐specified in the Methods section, we reassessed four items (3, 5, 11 and 12) for performance and detection bias and assessed for the first time two other items (8 and 13) for reporting and other bias of the previously included studies. We also changed the rating of two items across two studies: we changed the attrition bias for Bergquist 1977 (incomplete outcome data) from low risk of bias to high risk of bias because the trial authors only assessed 20% of the participants at six weeks for the pain index. We amended the selection bias for Lindequist 1984 (allocation concealment) from high risk of bias to unclear risk of bias because the trial authors did not describe the method of allocation.
Allocation
Three studies described an appropriate method of randomisation (Bergquist 1977; Hsieh 2002; Leclaire 1996). One study was a quasi‐RCT (Lindequist 1984), which corresponded to a method of randomisation with a high risk of bias (by date of birth).
All included studies did not describe the method of allocation concealment (Bergquist 1977; Hsieh 2002; Leclaire 1996; Lindequist 1984).
Blinding
None of the included studies blinded participants or care providers. This is due to the nature of the intervention.
None of the studies blinded outcome assessment. This is due to the nature of the pain and disability outcomes measured (self‐reported) and the inability to blind participants. The trials performed an assessment of the work status either by asking the participant or by asking for information to a compensation board or a health insurance service. The first method involved the participant not blinded from the intervention and was at high risk of bias. We also considered the other methods as at high risk of bias as the trial authors did not mention if the external source of data was blinded from the group allocation.
Incomplete outcome data
Most included studies had a good rate of follow‐up, with less than 20% withdrawals and drop‐outs for the short‐term and less than 30% for long‐term follow‐up (Hsieh 2002; Leclaire 1996; Lindequist 1984). One study reported higher rates of withdrawals and drop‐outs at follow‐up, which lead to high risk of bias (Bergquist 1977). Bergquist 1977 excluded the participants from the data collection as soon as they had recovered. This led to a decreasing rate of follow‐up: from 70% at 10 days to 25% at six weeks.
Selective reporting
None of the included studies had a published protocol or could be found on a clinical trial registry. Also, most trials were published before clinical trials registries were created. We scored all studies at unclear risk of bias regarding reporting bias as we could not compare pre‐specified outcomes with the reported ones.
Other potential sources of bias
We considered all studies as having a low risk of other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Statistical pooling
The treatment contrasts in the included studies were all different and therefore we could not pool the data. We considered each study separately and we reported seven treatment comparisons.
Effectiveness of back schools
1. Back schools versus combined physical therapies
One study at high risk of bias compared back school with combined physical therapies in workers (Bergquist 1977). Combined physical therapies included manual therapy based on Cyriax, England, Kaltenborn, Lewit and Janda methods as well as information on lifting techniques and movements to avoid. The study authors reported pain at short‐term follow‐up using the pain index. The median pain scores were 22/70 for the back school group (N = 14) and 21/70 for the control group (N = 16). There was no statistically significant difference between groups but the trial authors did not report data on dispersion to allow presentation on a forest plot. The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (flaws in pain report and only one study (N = 30) assessed the outcome). There is very low quality evidence (one study, 30 participants) that back schools are as effective as combined physical therapies for people with acute and subacute LBP on pain at short‐term follow‐up (Analysis 1.1).
1.1. Analysis.

Comparison 1 Back schools versus combined physical therapies for acute and subacute LBP, Outcome 1 Pain short‐term (pain index 0 to 70).
The results also showed that there was no statistically significant difference between groups in the duration of sick‐leave (RR of sick leave < 21 days: 1.37, 95% CI 1.00 to 1.87; RR of sick leave > 21 days 0.64, 95% CI 0.41 to 1.01). The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 116) examined the outcome). There is very low quality evidence (one study, 116 participants) that back schools are as effective as combined physical therapies for people with acute and subacute LBP on the duration of sick‐leave at short‐term follow‐up (Analysis 1.2; Analysis 1.3).
1.2. Analysis.

Comparison 1 Back schools versus combined physical therapies for acute and subacute LBP, Outcome 2 Work absence < 21 days.
1.3. Analysis.

Comparison 1 Back schools versus combined physical therapies for acute and subacute LBP, Outcome 3 Work absence > 21 days.
2. Back schools versus short‐wave diathermy at the lowest intensity
Bergquist 1977 also compared back school with short‐waves diathermy of the lowest possible intensity in workers. The results showed no statistically significant effect favouring either treatment or short‐wave diathermy in terms of pain at short‐term follow‐up. The median pain score from the pain index was 22/70 for the back school group (N = 14) and 17/70 for the short‐wave diathermy group (N = 15) at six weeks. The trial authors did not report data on dispersion to allow presentation on a forest plot. The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (flaws in pain report and only one study (N = 29) examined the outcome). There is very low quality evidence (one study, 29 participants) that there was no difference in pain at short‐term follow‐up between back schools and short‐wave diathermy at the lowest intensity for people with acute and subacute LBP (Analysis 2.1).
2.1. Analysis.

Comparison 2 Back schools versus short‐wave diathermy at the lowest intensity for acute and subacute LBP, Outcome 1 Pain short‐term (pain index 0 to 70).
In terms of work status, there were no statistically significant differences in the number of sick‐listed and non sick‐listed participants between groups (no quantitative data reported). However, there were significantly more participants with sick‐leave longer than 21 days in the short‐wave diathermy group than the back school group (RR of sick leave > 21 days: 0.53, 95% CI 0.35 to 0.80 favouring back school group). The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 121) examined the outcome). Therefore, there is very low quality evidence (one study, 121 participants) to say that back schools are more effective than short‐wave diathermy at the lowest intensity for people with acute and subacute LBP on the duration of sick‐leave (short‐term follow‐up) (Analysis 2.2).
2.2. Analysis.

Comparison 2 Back schools versus short‐wave diathermy at the lowest intensity for acute and subacute LBP, Outcome 2 Work absence > 21 days.
3. Back schools versus myofascial therapy
One study, Hsieh 2002, compared back school with myofascial therapy. The results showed no statistically significant difference between groups in terms of pain (10 cm visual analogue scale (VAS)) at short‐term follow‐up (MD −0.65, 95% CI −1.49 to 0.19) and intermediate‐term follow‐up (MD −0.70, 95% CI −1.92 to 0.52). The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 99) examined the outcome). There is very low quality evidence (one study, 99 participants) that there is no difference between back schools and myofascial therapy for pain at short and intermediate‐term follow‐up (Analysis 3.1; Analysis 3.2).
3.1. Analysis.

Comparison 3 Back schools versus myofascial therapy for acute and subacute LBP, Outcome 1 Pain short‐term (VAS 0 to 10).
3.2. Analysis.

Comparison 3 Back schools versus myofascial therapy for acute and subacute LBP, Outcome 2 Pain intermediate‐term (VAS 0 to 10).
In terms of disability, the results showed no statistically significant difference between groups at the short‐term follow‐up (MD −1.54, 95% CI −3.88 to 0.80) and intermediate‐term follow‐up (MD −1.58, 95% CI −4.02 to 0.86). The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 99) examined the outcome). There is very low quality evidence (one study, 99 participants) that there is no difference between back schools and myofascial therapy for disability at short and intermediate‐term follow‐up (Analysis 3.3; Analysis 3.4).
3.3. Analysis.

Comparison 3 Back schools versus myofascial therapy for acute and subacute LBP, Outcome 3 Disability short‐term (RMDQ 0 to 24).
3.4. Analysis.

Comparison 3 Back schools versus myofascial therapy for acute and subacute LBP, Outcome 4 Disability intermediate‐term (RMDQ 0 to 24).
This is the only study that reported on adverse events. Most adverse events reported were transient exacerbations of symptoms. One participant had a constant tinnitus in the myofascial therapy group. There were no statistically significant differences between groups in terms of number of adverse events reported when comparing back school to myofascial therapy (RR 1.59, 95% CI 0.48 to 5.30). The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 99) examined the outcome). There is very low quality evidence (one study; 99 participants) that there is no difference between back schools and myofascial therapy in terms of adverse events (Analysis 3.5).
3.5. Analysis.

Comparison 3 Back schools versus myofascial therapy for acute and subacute LBP, Outcome 5 Adverse events.
4. Back schools versus joint manipulation
One study, Hsieh 2002, compared back school with joint manipulation.The results showed no statistically significant difference between groups in terms of pain (10 cm VAS scale) at short‐term follow‐up (MD −0.45, 95% CI −1.33 to 0.43) and intermediate‐term follow‐up (MD −0.11, 95% CI −1.39 to 1.17). The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 97) examined the outcome). There is very low quality evidence (one study; 97 participants) that there is no difference between back schools and joint manipulation for pain at short and intermediate‐term follow‐up (Analysis 4.1; Analysis 4.2).
4.1. Analysis.

Comparison 4 Back schools versus joint manipulation for acute and subacute LBP, Outcome 1 Pain short‐term (VAS 0 to 10).
4.2. Analysis.

Comparison 4 Back schools versus joint manipulation for acute and subacute LBP, Outcome 2 Pain intermediate‐term (VAS 0 to 10).
In terms of disability, the results showed no statistically significant difference between groups at the short‐term follow‐up (MD −0.16, 95% CI −2.50 to 2.18) and intermediate‐term follow‐up (MD 0.19, 95% CI −2.30 to 2.68). The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 97) examined the outcome). There is very low quality evidence (one study, 97 participants) that there is no difference between back schools and joint manipulation in terms of disability at short and intermediate‐term follow‐up (Analysis 4.3; Analysis 4.4).
4.3. Analysis.

Comparison 4 Back schools versus joint manipulation for acute and subacute LBP, Outcome 3 Disability short‐term (RMDQ 0 to 24).
4.4. Analysis.

Comparison 4 Back schools versus joint manipulation for acute and subacute LBP, Outcome 4 Disability intermediate‐term (RMDQ 0 to 24).
Concerning the reported adverse events, they were mostly transient exacerbations of the symptoms. There were no statistically significant differences between groups in terms of number of adverse events reported when comparing back school to joint manipulation (RR 1.02, 95% CI 0.35 to 2.94). The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 97) examined the outcome). There is very low quality evidence (one study, 97 participants) that there is no difference between back schools and joint manipulation in terms of adverse events (Analysis 4.5).
4.5. Analysis.
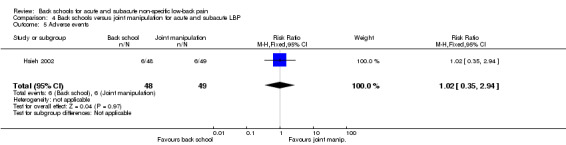
Comparison 4 Back schools versus joint manipulation for acute and subacute LBP, Outcome 5 Adverse events.
5. Back schools versus joint manipulation and myofascial therapy
One study,Hsieh 2002, compared back school with a combination of joint manipulation and myofascial therapy. The results showed no statistically significant difference between groups in terms of pain (10 cm VAS scale) at short‐term follow‐up (MD 0.09, 95% CI −0.68 to 0.86) and intermediate‐term follow‐up (MD 0.05, 95% CI −1.13 to 1.23). The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 100) examined the outcome). There is very low quality evidence (one study, 100 participants) that there is no difference between back schools and a combination of joint manipulation and myofascial therapy for pain at short and intermediate‐term follow‐up (Analysis 5.1; Analysis 5.2).
5.1. Analysis.

Comparison 5 Back schools versus joint manipulation and myofascial therapy for acute and subacute LBP, Outcome 1 Pain short‐term (VAS 0 to 10).
5.2. Analysis.

Comparison 5 Back schools versus joint manipulation and myofascial therapy for acute and subacute LBP, Outcome 2 Pain intermediate‐term (VAS 0 to 10).
In terms of disability, the results showed no statistically significant difference between groups at the short‐term follow‐up (MD 0.53, 95% CI −1.60 to 2.66) and intermediate‐term follow‐up (MD −0.08, 95% CI −2.33 to 2.17). The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 100) examined the outcome). There is very low quality evidence (one study, 100 participants) that there is no difference between back schools and a combination of joint manipulation and myofascial therapy for disability at short and intermediate‐term follow‐up (Analysis 5.3; Analysis 5.4).
5.3. Analysis.

Comparison 5 Back schools versus joint manipulation and myofascial therapy for acute and subacute LBP, Outcome 3 Disability short‐term (RMDQ 0 to 24).
5.4. Analysis.

Comparison 5 Back schools versus joint manipulation and myofascial therapy for acute and subacute LBP, Outcome 4 Disability intermediate‐term (RMDQ 0 to 24).
There were no statistically significant differences between groups in terms of number of adverse events reported (RR 0.93, 95% CI 0.34 to 2.57). These adverse events corresponded in exacerbation of the symptoms. The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 100) examined the outcome). There is very low quality evidence (one study, 100 participants) that there is no difference between back schools and a combination of joint manipulation and myofascial therapy in terms of adverse events (Analysis 5.5).
5.5. Analysis.

Comparison 5 Back schools versus joint manipulation and myofascial therapy for acute and subacute LBP, Outcome 5 Adverse events.
6. Back school added to a back care programme versus back care programme alone
Leclaire 1996 compared back school added to a back care programme including rest, analgesics and daily physiotherapy (hot/cold packs, massage, ultrasounds, transcutaneous nerve stimulation and low‐back exercises) with back care programme alone in workers. The results showed no statistically significant difference between groups for pain (10 cm VAS scale) at short‐term follow‐up (MD 0.50, 95% CI −0.07 to 1.07), intermediate‐term follow‐up (MD 0.30, 95% CI −0.34 to 0.94) and long‐term follow‐up (MD 0.20, 95% CI −0.47 to 0.87). The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 170) examined the outcome). There is very low quality evidence (one study, 170 participants) that there is no difference between back schools added to a back care programme and back care programme alone on pain at short, intermediate and long‐term follow‐up (Analysis 6.1; Analysis 6.2; Analysis 6.3).
6.1. Analysis.

Comparison 6 Back school + other treatment versus other treatment alone for acute and subacute LBP, Outcome 1 Pain short‐term (VAS 0 to 10).
6.2. Analysis.

Comparison 6 Back school + other treatment versus other treatment alone for acute and subacute LBP, Outcome 2 Pain intermediate‐term (VAS 0 to 10).
6.3. Analysis.

Comparison 6 Back school + other treatment versus other treatment alone for acute and subacute LBP, Outcome 3 Pain long‐term (VAS 0 to 10).
The results showed a greater improvement in terms of disability for the control group than for the back school group at short‐term follow‐up (MD 1.78, 95% CI 0.52 to 3.04). The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 170) examined the outcome). There is very low quality evidence (one study, 170 participants) that back schools added to a back care programme is more effective than back care programme alone on disability at short‐term follow‐up (Analysis 6.4).
6.4. Analysis.

Comparison 6 Back school + other treatment versus other treatment alone for acute and subacute LBP, Outcome 4 Disability short‐term (RMDQ 0 to 24).
No difference in disability between groups was found at intermediate‐term follow‐up (MD 0.81, 95% CI ‐0.44 to 2.06) and long‐term follow‐up (MD 0.48, 95% CI ‐0.65 to 1.61). The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 170) examined the outcome). There is very low quality evidence (1 study, 170 participants) that there is no difference between back schools added to a back care programme and back care programme alone on disability at intermediate and long‐term follow‐up (Analysis 6.5; Analysis 6.6).
6.5. Analysis.

Comparison 6 Back school + other treatment versus other treatment alone for acute and subacute LBP, Outcome 5 Disability intermediate‐term (RMDQ 0 to 24).
6.6. Analysis.

Comparison 6 Back school + other treatment versus other treatment alone for acute and subacute LBP, Outcome 6 Disability long‐term (RMDQ 0 to 24).
There was no statistically significant difference between groups for the number of participants on sick‐leave at 30 days (RR 0.94, 95% CI 0.76 to 1.16) and 60 days (RR 0.76, 95% CI 0.32 to 1.84), with a median time to return to work of 33 days in both groups. The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 170) examined the outcome). There is very low quality evidence (one study, 170 participants) that there is no difference between back schools added to a back care programme and back care programme alone on work status at short‐term follow‐up (Analysis 6.7; Analysis 6.8).
6.7. Analysis.

Comparison 6 Back school + other treatment versus other treatment alone for acute and subacute LBP, Outcome 7 Work absence at 30 days.
6.8. Analysis.

Comparison 6 Back school + other treatment versus other treatment alone for acute and subacute LBP, Outcome 8 Work absence at 60 days.
7. Back school versus advice
Lindequist 1984 compared back school with advice (not to strain the back and to use analgesics if needed) in workers. Work status was the only outcome and there was no statistically difference between groups with a mean duration of sick‐leave of 36 days (range 1 to 144) in the back school group and 39 (range 0 to 254) in the control group at one year. The quality of the evidence was downgraded by two due to limitations in the design and implementation (absence of allocation concealment, lack of blinding, incomplete outcome data) and by one due to imprecision (only one study (N = 56) examined the outcome). There is very low quality evidence (one study; 56 participants) that there is no difference between back schools and advice on work status at long‐term follow‐up (Analysis 7.1).
7.1. Analysis.

Comparison 7 Back school versus advice for acute or subacute LBP, Outcome 1 Days of sick‐leave at 1 year.
Subgroup analysis
Due to the small number of included studies, their heterogeneity and our inability to pool the data, it was not possible to perform the planned subgroup analyses.
Sensitivity analysis
Due to the low number of included studies and the inability to pool studies, we were unable to perform a sensitivity analysis.
Discussion
Summary of main results
We included three randomised controlled trials (RCTs) and one quasi‐RCT (643 participants) in this Cochrane review update after splitting the original review, Heymans 2004, into two reviews that focus on acute and subacute LBP, and chronic LBP. The previous review, Heymans 2004, already included these four studies as we could not include any new studies from the updated search. We were unable to pool the data because of the absence of common treatment contrasts, and we reported each study comparison separately. According to the adapted GRADE approach, there was very low quality evidence of no difference between back schools and placebo (or sham or attention control) or comparison treatments (physical therapies, myofascial therapy, joint manipulation, advice) in terms of pain, disability, work status and adverse events for any time of follow‐up. There was very low quality evidence that showed a statistically significant difference between back schools and placebo (or sham or attention control) for return to work at short‐term follow‐up in favour of back school. There was very low quality evidence that showed that back schools added to a back care programme were more effective than back care programme alone for disability at short‐term follow‐up. Only one study reported on adverse events, which made the assessment of benefits against harms unclear. There is uncertainty on the conclusions to draw from the included studies.
Overall completeness and applicability of evidence
Based on the low number of available studies and limited comparison treatments, the overall evidence is incomplete and the comparative effectiveness of back schools versus other contemporary treatments for acute and subacute LBP is unknown. Arguably one of the most revealing findings of our review is the fact that we did not identify any new studies since the last review update 10 years ago (Heymans 2004). The inclusion of future well‐designed studies is very likely to change the conclusions of this review. The international guidelines for the management of acute low‐back pain (LBP) recommend advice and simple medication as first‐line care in addition to self‐care strategies (staying active, early return to work) (Koes 2010). Back schools are supervised group programmes, possibly provided over several weeks. According to the current guidelines and the fact that we were unable to find any new study, back schools seem to be an outdated model of treatment for acute LBP. Consequently, the necessity of further studies in this area has to be discussed and further, the necessity of another review update.
Quality of the evidence
Two of the four included studies had good methodological quality and the rest had less than six criteria at low risk of bias based on the Cochrane 'Risk of bias' tool. Notably, there was no blinding of participants and therapists in any of the included studies due to the nature of the intervention. All included studies lacked allocation concealment. Empirical evidence reports an association between inadequate concealment of treatment allocation and lack of double‐blinding (blinding of participants and observers) with larger treatment effects presumably reflecting bias (Schulz 1995; Schulz 2002).
The risk of bias and the low number of included studies (which contributed to imprecision) meant that the quality of the evidence was very low for all outcomes.
Potential biases in the review process
This Cochrane review is limited by the small number of included studies, and the risk of bias associated with their design and conduct. We were unable to identify any new published or ongoing studies assessing back schools for a population with acute or subacute LBP, so evidence in this area has not progressed since the last review update in 2004 (Heymans 2004). Despite including several sources in our search strategy, we might have missed relevant studies, leading to publication bias (Egger 1998). Screening references of identified trials and systematic reviews may result in an overrepresentation of positive studies in the review, because trials with a positive result are more likely to be referred to in other publications, which can lead to reference bias (Gøtzsche 1987; Thornton 2000).
Another limitation in this review refers to the inclusion of studies with a mixed population with acute and chronic LBP. We were unable to obtain the data for our subgroup of interest only and had to decide to include these studies either in the review for acute LBP or chronic LBP. Of the three concerned studies, we included one in the current review (Hsieh 2002) and the two others in the review about chronic LBP (Berwick 1989; Herzog 1991). As the population included in Hsieh 2002 do not fit exactly our inclusion criteria, this is likely to introduce bias in the results.
Agreements and disagreements with other studies or reviews
The previous version of this review, Heymans 2004, included both participants with acute and chronic LBP and could not conclude on the effects of back schools for acute and subacute non‐specific LBP because of limited evidence. Given that no new studies met the inclusion criteria of this review, the updated results align with those of the previous review version (Heymans 2004). Looking at other systematic reviews assessing back schools for non‐specific LBP, identified through our search strategy, most systematic reviews included participants with acute and chronic pain (Di Fabio 1995; Keijsers 1991; Maier‐Riehle 2001; Turner 1996; Wiese 2009), some focused on participants with acute pain (Pengel 2002; Scheer 1995), and some on participants with chronic pain (Brox 2008). The conclusions of these reviews are in agreement with the current and previous Cochrane review (Heymans 2004). The results for acute, subacute and chronic LBP population appear to differ with insufficient data to conclude on the effectiveness of back schools for people with acute and subacute LBP and more favorable results for people with chronic LBP.
Authors' conclusions
Implications for practice.
There is very low quality evidence that back schools are as effective as a placebo (or sham or attention control) or another treatment (physical therapies, myofascial therapy, joint manipulation, advice) on pain, disability and work status at short‐term, intermediate‐term and long‐term follow‐up. There is very low quality evidence that back schools are more effective than a placebo (or sham or attention control) on work status at short‐term follow‐up. There is very low quality evidence that back schools added to a back care programme are more effective than back schools alone for disability at short‐term follow‐up. The poor quality of these results prevent us from any reliable conclusions in terms of implications for practice.
Implications for research.
Given the scarcity and low quality of evidence in this area, a large well‐designed RCT is very likely to change our conclusions on the effectiveness of back schools for non‐specific acute and subacute LBP. However, back schools do not seem to be a treatment widely used for people with non‐specific acute or subacute LBP nowadays and are not endorsed by guidelines. Further research into this area may not be necessary.
What's new
| Date | Event | Description |
|---|---|---|
| 31 August 2015 | New citation required and conclusions have changed | This review is split from the review 'Back schools for non‐specific low‐back pain', which was last published in 2004. |
| 26 April 2015 | New search has been performed | Three authors joined the review team (Poquet N, Maher C, and Lin C) and one of the original review authors is no longer involved (Bombardier C). We have updated the contact details of the corresponding author. We have made the following methodological changes.
|
Acknowledgements
We thank the Cochrane Back and Neck Review Group for critically reviewing this Cochrane review update. In particular we are grateful to Managing Editors Teresa Marin and Claire Munhall, and Allison Kelly, Administrative Editorial Assistant, for their assistance and support. We also thank Shireen Harbin, Trials Search Co‐ordinator (TSC), who performed the electronic searches in 2014 and 2015, and Rachel Couban, the previous TSC who performed the previous searches.
Appendices
Appendix 1. Search strategies
CENTRAL
We last searched this database on 4 August 2015. We revised the search strategy in 2011 and added back pain to line 3 in 2015.
#1 MeSH descriptor: [Back Pain] explode all trees
#2 dorsalgia
#3 backache or back pain
#4 (lumbar near pain) or (coccyx) or (coccydynia) or (sciatica) or (spondylosis)
#5 MeSH descriptor: [Sciatica] explode all trees
#6 MeSH descriptor: [Spine] explode all trees
#7 MeSH descriptor: [Spinal Diseases] explode all trees
#8 (lumbago) or (discitis) or (disc near degeneration) or (disc near prolapse) or (disc near herniation)
#9 spinal fusion
#10 facet near joints
#11 MeSH descriptor: [Intervertebral Disc] explode all trees
#12 postlaminectomy
#13 arachnoiditis
#14 failed near back
#15 MeSH descriptor: [Cauda Equina] explode all trees
#16 lumbar near vertebra*
#17 spinal near stenosis
#18 slipped near (disc* or disk*)
#19 degenerat* near (disc* or disk*)
#20 stenosis near (spine or root or spinal)
#21 displace* near (disc* or disk*)
#22 prolap* near (disc* or disk*)
#23 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22)
#24 "back school"
#25 (#23 and #24)
January 2009 strategy
#1 MeSH descriptor Back explode all trees
#2 MeSH descriptor Buttocks, this term only
#3 MeSH descriptor Leg, this term only
#4 MeSH descriptor Back Pain explode tree 1
#5 MeSH descriptor Back Injuries explode all trees
#6 MeSH descriptor Low Back Pain, this term only
#7 MeSH descriptor Sciatica, this term only
#8 (low next back next pain)
#9 (lbp)
#10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
#11 (back school):ti,ab,kw
#12 (#10 AND #11), from 2007 to 2009
MEDLINE and MEDLINE In‐Process & Other Non‐Indexed Citations
We last searched this database on 4 August 2015. We added back pain to line 17. In 2014 we added lines 5, 22, 25 and 26.
1 randomized controlled trial.pt.
2 controlled clinical trial.pt.
3 comparative study.pt.
4 clinical trial.pt.
5 pragmatic clinical trial.pt.
6 randomized.ab.
7 placebo.ab,ti.
8 drug therapy.fs.
9 randomly.ab,ti.
10 trial.ab,ti.
11 groups.ab,ti.
12 or/1‐11
13 (animals not (humans and animals)).sh.
14 12 not 13
15 dorsalgia.ti,ab.
16 exp Back Pain/
17 (backache or back pain).ti,ab.
18 (lumbar adj pain).ti,ab.
19 coccyx.ti,ab.
20 coccydynia.ti,ab.
21 sciatica.ti,ab.
22 exp sciatic neuropathy/
23 spondylosis.ti,ab.
24 lumbago.ti,ab.
25 back disorder$.ti,ab.
26 exp Back Muscles/
27 or/15‐26
28 back school.mp.
29 14 and 27 and 28
30 limit 29 to yr=2014‐2015
31 limit 29 to ed=20140304‐20150804
32 30 or 31
The June 2011 search used a different entry date filter to current strategy.
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab,ti.
drug therapy.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐8
(animals not (humans and animals)).sh.
9 not 10
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
exp Low Back Pain/
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
sciatica/
spondylosis.ti,ab.
lumbago.ti,ab.
or/12‐22
back school.mp.
11 and 24 and 23
limit 25 to yr="2009 ‐ 2011"
2009$.ed.
2010$.ed.
2011$.ed.
27 or 28 or 29
25 and 30
26 or 31
In the 26 April 2007 strategy we used a different study design filter to the current strategy.
exp "Clinical Trial [Publication Type]"/
randomized.ab,ti.
placebo.ab,ti.
dt.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐7
Animals/
Humans/
9 not (9 and 10)
8 not 11
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
sciatica/
spondylosis.ti,ab.
lumbago.ti,ab.
exp low back pain/
or/13‐23
back school.mp.
12 and 24 and 25
limit 26 to yr="2004 ‐ 2007"
EMBASE
We last searched this database on 4 August 2015. In March 2014, we changed line 31 from 14 and 30 to 14 or 30, added line 47, and revised the animal study filter (lines 32 to 36) from the June 2011 strategy.
Clinical Article/
exp Clinical Study/
Clinical Trial/
Controlled Study/
Randomized Controlled Trial/
Major Clinical Study/
Double Blind Procedure/
Multicenter Study/
Single Blind Procedure/
Phase 3 Clinical Trial/
Phase 4 Clinical Trial/
crossover procedure/
placebo/
or/1‐13
allocat$.mp.
assign$.mp.
blind$.mp.
(clinic$ adj25 (study or trial)).mp.
compar$.mp.
control$.mp.
cross?over.mp.
factorial$.mp.
follow?up.mp.
placebo$.mp.
prospectiv$.mp.
random$.mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
trial.mp.
(versus or vs).mp.
or/15‐29
14 or 30
exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
human/ or normal human/ or human cell/
32 and 33
32 not 34
31 not 35
dorsalgia.mp.
back pain.mp.
exp BACKACHE/
(lumbar adj pain).mp.
coccyx.mp.
coccydynia.mp.
sciatica.mp.
ischialgia/
spondylosis.mp.
lumbago.mp.
back disorder$.ti,ab.
or/37‐47
back school.mp.
36 and 48 and 49
limit 50 to yr=2014‐2015
limit 50 to em=201409‐201531
51 or 52
The June 2011 strategy used a different animal study and entry date filter.
Clinical Article/
exp Clinical Study/
Clinical Trial/
Controlled Study/
Randomized Controlled Trial/
Major Clinical Study/
Double Blind Procedure/
Multicenter Study/
Single Blind Procedure/
Phase 3 Clinical Trial/
Phase 4 Clinical Trial/
crossover procedure/
placebo/
or/1‐13
allocat$.mp.
assign$.mp.
blind$.mp.
(clinic$ adj25 (study or trial)).mp.
compar$.mp.
control$.mp.
cross?over.mp.
factorial$.mp.
follow?up.mp.
placebo$.mp.
prospectiv$.mp.
random$.mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
trial.mp.
(versus or vs).mp.
or/15‐29
14 and 30
human/
Nonhuman/
exp ANIMAL/
Animal Experiment/
33 or 34 or 35
32 not 36
31 not 36
37 and 38
38 or 39
dorsalgia.mp.
back pain.mp.
exp BACKACHE/
(lumbar adj pain).mp.
coccyx.mp.
coccydynia.mp.
sciatica.mp.
exp ISCHIALGIA/
spondylosis.mp.
lumbago.mp.
exp Low back pain/
or/41‐51
back school.mp.
40 and 52 and 53
limit 54 to yr="2009 ‐ 2011"
2009$.em.
2010$.em.
2011$.em.
56 or 57 or 58
54 and 59
55 or 60
CINAHL
We last searched this database on 4 August 2015. We added back pain to line 27.
In March 2014, we searched CINAHL from inception to May 2007 using the current strategy to ensure records were complete.
S47 S45 OR S46
S46 S44 and EM 20140304‐20150804
S45 S42 AND S43 Limiters ‐ Published Date: 20140301‐20150831
S44 S42 AND S43
S43 back school
S42 S24 and S41
S41 S40 or S39 or S38 or S37 or S36 or S35 or S34 or S33 or S32 or S31 or S30 or S29 or S28 or S27 or S26 or S25
S40 lumbago
S39 (MH "Spondylolysis")
S38 (MH "Spondylolisthesis")
S37 lumbar N2 vertebrae
S36 (MH "Lumbar Vertebrae")
S35 back disorder*
S34 coccydynia
S33 coccyx
S32 sciatica
S31 (MH "Sciatica")
S30 (MH "Coccyx")
S29 lumbar N5 pain
S28 lumbar W1 pain
S27 backache or back pain
S26 (MH "Back Pain+")
S25 dorsalgia
S24 S22 not S23
S23 (MH "Animals+")
S22 S21 or S20 or S19 or S18 or S17 or S16 or S15 or S14 or S13 or S12 or S11 or S10 or S9 or S8 or S7 or S6 or S5 or S4 or S3 or S2 or S1
S21 volunteer*
S20 prospectiv*
S19 control*
S18 followup stud*
S17 follow‐up stud*
S16 (MH "Prospective Studies+")
S15 (MH "Evaluation Research+")
S14 (MH "Comparative Studies")
S13 latin square
S12 (MH "Study Design+")
S11 (MH "Random Sample+")
S10 random*
S9 placebo*
S8 (MH "Placebos")
S7 (MH "Placebo Effect")
S6 triple‐blind
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 randomi?ed controlled trial*
S1 (MH "Clinical Trials+")
For the June 2011 search, Line S3 was changed from "clinical W8 trial" to "clinical W3 trial" and line S21 and S42 were added:
S51 S49 and S50 Limiters ‐ Published Date from: 20090101‐20111231
S50 "back school"
S49 S28 and S48
S48 S35 or S43 or S47
S47 S44 or S45 or S46
S46 "lumbago"
S45 (MH "Spondylolisthesis") OR (MH "Spondylolysis")
S44 (MH "Thoracic Vertebrae")
S43 S36 or S37 or S38 or S39 or S40 or S41 or S42
S42 lumbar N2 vertebra
S41 (MH "Lumbar Vertebrae")
S40 "coccydynia"
S39 "coccyx"
S38 "sciatica"
S37 (MH "Sciatica")
S36 (MH "Coccyx")
S35 S29 or S30 or S31 or S32 or S33 or S34
S34 lumbar N5 pain
S33 lumbar W1 pain
S32 "backache"
S31 (MH "Low Back Pain")
S30 (MH "Back Pain+")
S29 "dorsalgia"
S28 S26 NOT S27
S27 (MH "Animals")
S26 S7 or S12 or S19 or S25
S25 S20 or S21 or S22 or S23 or S24
S24 volunteer*
S23 prospectiv*
S22 control*
S21 followup stud*
S20 follow‐up stud*
S19 S13 or S14 or S15 or S16 or S17 or S18
S18 (MH "Prospective Studies+")
S17 (MH "Evaluation Research+")
S16 (MH "Comparative Studies")
S15 latin square
S14 (MH "Study Design+")
S13 (MH "Random Sample")
S12 S8 or S9 or S10 or S11
S11 random*
S10 placebo*
S9 (MH "Placebos")
S8 (MH "Placebo Effect")
S7 S1 or S2 or S3 or S4 or S5 or S6
S6 triple‐blind
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 "randomi?ed controlled trial*"
S1 (MH "Clinical Trials+")
PsycINFO
We last searched this database on 4 August 2015.
1 clinical trials/
2 controlled trial.mp.
3 RCT.mp.
4 (Random* adj3 trial).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
5 (clin* adj3 trial).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
6 (sing* adj2 blind*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
7 (doub* adj2 blind*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
8 placebo.mp. or exp Placebo/
9 latin square.mp.
10 (random* adj2 assign*).mp.
11 prospective studies/
12 (prospective adj stud*).mp.
13 (comparative adj stud*).mp.
14 treatment effectiveness evaluation/
15 (evaluation adj stud*).mp.
16 exp Posttreatment Followup/
17 follow?up stud*.mp.
18 or/1‐17
19 back pain/
20 lumbar spinal cord/
21 (low adj back adj pain).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
22 (back adj pain).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
23 spinal column/
24 (lumbar adj2 vertebra*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
25 coccyx.mp.
26 sciatica.mp.
27 lumbago.mp.
28 dorsalgia.mp.
29 back disorder*.mp.
30 "back (anatomy)"/
31 ((disc or disk) adj degenerat*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
32 ((disc or disk) adj herniat*).mp.
33 ((disc or disk) adj prolapse*).mp.
34 (failed adj back).mp.
35 or/19‐34
36 back school.mp.
37 18 and 35 and 36
38 limit 37 to yr=2014‐2015
The June 15, 2011 search in Cambridge Scientific Abstracts (CSA)
((KW=(Randomi?ed controlled trial*) OR KW=(clinical trial*) OR KW=(clin* near trail*) OR KW= (sing* near blind*) OR KW=(sing* near mask*) OR (doub* near blind*) OR KW=(doubl* NEAR mask*) OR KW=(trebl* near mask*) OR KW=(trebl* near mask*) OR KW=(tripl* near blind*) OR KW=(tripl* near mask*) OR KW=(placebo*) OR KW=(random*) OR DE=(research design) OR KW=(Latin square) OR KW=(comparative stud*) OR KW=(evaluation stud*) OR KW=(follow up stud*) OR DE=(prospective stud*)OR KW=(control*) OR KW=(prospective*) OR KW=(volunteer*)) AND (DE=(back) OR DE=(back pain) OR DE=(neck))) and(KW=(back school))
ClinicalTrials.gov
We last searched this database on 4 August 2015.
Basic search: “back school” and back pain
Received from 03/04/2014 to 08/04/2015
June 2011 search
Condition: back pain AND Intervention: back school
WHO ICTRP
We last searched this database on 4 August 2015.
Basic search: back school and back pain
June 2011 search
Condition: back pain
AND
Intervention: back school
PubMed
We searched this database on 4 August 2015.
((back pain OR backache OR coccydynia OR sciatica OR back disorder OR lumbago OR spondylosis) AND (back school) AND (random OR randomly OR randomized OR randomised OR placebo OR trial) AND (pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb]))
Publication date from 04/03/2014 to 31/12/2015
Appendix 2. Criteria for assessing risk of bias
Random sequence generation (selection bias)
Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence
There is a low risk of selection bias if the study authors describe a random component in the sequence generation process such as: referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots, minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random).
There is a high risk of selection bias if the study authors describe a non‐random component in the sequence generation process, such as: sequence generated by odd or even date of birth, date (or day) of admission, hospital or clinic record number; or allocation by judgement of the clinician, preference of the participant, results of a laboratory test or a series of tests, or availability of the intervention.
Allocation concealment (selection bias)
Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment
There is a low risk of selection bias if the participants and study authors that enrolled participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; or sequentially numbered, opaque, sealed envelopes.
There is a high risk of bias if participants or study authors enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; or other explicitly unconcealed procedures.
Blinding of participants
Performance bias due to knowledge of the allocated interventions by participants during the study
There is a low risk of performance bias if blinding of participants was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of personnel/care providers (performance bias)
Performance bias due to knowledge of the allocated interventions by personnel/care providers during the study
There is a low risk of performance bias if blinding of personnel was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of outcome assessor (detection bias)
Detection bias due to knowledge of the allocated interventions by outcome assessors
There is low risk of detection bias if the blinding of the outcome assessment was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding, or as follows.
For patient‐reported outcomes in which the patient was the outcome assessor (e.g. pain, disability): there is a low risk of bias for outcome assessors if there is a low risk of bias for participant blinding (Boutron 2005).
For outcome criteria that are clinical or therapeutic events that will be determined by the interaction between patients and care providers (e.g. co‐interventions, length of hospitalisation, treatment failure), in which the care provider is the outcome assessor: there is a low risk of bias for outcome assessors if there is a low risk of bias for care providers (Boutron 2005).
For outcome criteria that are assessed from data from medical forms: there is a low risk of bias if the treatment or adverse effects of the treatment could not be noticed in the extracted data (Boutron 2005).
Incomplete outcome data (attrition bias)
Attrition bias due to amount, nature or handling of incomplete outcome data
There is a low risk of attrition bias if there were no missing outcome data; reasons for missing outcome data were unlikely to be related to the true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data were balanced in numbers, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, the plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size, or missing data were imputed using appropriate methods (if drop‐outs are very large, imputation using even "acceptable" methods may still suggest a high risk of bias) (van Tulder 2003). The percentage of withdrawals and drop‐outs should not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and should not lead to substantial bias (these percentages are commonly used but arbitrary, not supported by literature) (van Tulder 2003).
Selective reporting (reporting bias)
Reporting bias due to selective outcome reporting
There is low risk of reporting bias if the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way, or if the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
There is a high risk of reporting bias if not all of the study's pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Group similarity at baseline (selection bias)
Bias due to dissimilarity at baseline for the most important prognostic indicators.
There is low risk of bias if groups are similar at baseline for demographic factors, value of main outcome measure(s), and important prognostic factors (examples in the field of back and neck pain are duration and severity of complaints, vocational status, percentage of patients with neurological symptoms) (van Tulder 2003).
Co‐interventions (performance bias)
Bias because co‐interventions were different across groups
There is low risk of bias if there were no co‐interventions or they were similar between the index and control groups (van Tulder 2003).
Compliance (performance bias)
Bias due to inappropriate compliance with interventions across groups
There is low risk of bias if compliance with the interventions was acceptable, based on the reported intensity/dosage, duration, number and frequency for both the index and control intervention(s). For single‐session interventions (e.g. surgery), this item is irrelevant (van Tulder 2003).
Intention‐to‐treat analysis
There is low risk of bias if the trial authors reported/analysed all randomised participants in the group to which they were allocated by randomisation.
Timing of outcome assessments (detection bias)
Bias because important outcomes were not measured at the same time across groups
There is low risk of bias if the trial authors measured all important outcome assessments for all intervention groups at the same time (van Tulder 2003).
Other bias
Bias due to problems not covered elsewhere in the table
There is a low risk of bias if the study appears to be free of other sources of bias not addressed elsewhere (e.g. study funding).
Data and analyses
Comparison 1. Back schools versus combined physical therapies for acute and subacute LBP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain short‐term (pain index 0 to 70) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Work absence < 21 days | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.00, 1.87] |
| 3 Work absence > 21 days | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.41, 1.01] |
Comparison 2. Back schools versus short‐wave diathermy at the lowest intensity for acute and subacute LBP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain short‐term (pain index 0 to 70) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Work absence > 21 days | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.35, 0.80] |
Comparison 3. Back schools versus myofascial therapy for acute and subacute LBP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain short‐term (VAS 0 to 10) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐1.49, 0.19] |
| 2 Pain intermediate‐term (VAS 0 to 10) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.92, 0.52] |
| 3 Disability short‐term (RMDQ 0 to 24) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐1.54 [‐3.88, 0.80] |
| 4 Disability intermediate‐term (RMDQ 0 to 24) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐1.58 [‐4.02, 0.86] |
| 5 Adverse events | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.48, 5.30] |
Comparison 4. Back schools versus joint manipulation for acute and subacute LBP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain short‐term (VAS 0 to 10) | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐1.33, 0.43] |
| 2 Pain intermediate‐term (VAS 0 to 10) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐1.39, 1.17] |
| 3 Disability short‐term (RMDQ 0 to 24) | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐2.50, 2.18] |
| 4 Disability intermediate‐term (RMDQ 0 to 24) | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐2.30, 2.68] |
| 5 Adverse events | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.35, 2.94] |
Comparison 5. Back schools versus joint manipulation and myofascial therapy for acute and subacute LBP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain short‐term (VAS 0 to 10) | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.68, 0.86] |
| 2 Pain intermediate‐term (VAS 0 to 10) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐1.13, 1.23] |
| 3 Disability short‐term (RMDQ 0 to 24) | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐1.60, 2.66] |
| 4 Disability intermediate‐term (RMDQ 0 to 24) | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐2.33, 2.17] |
| 5 Adverse events | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.34, 2.57] |
Comparison 6. Back school + other treatment versus other treatment alone for acute and subacute LBP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain short‐term (VAS 0 to 10) | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.07, 1.07] |
| 2 Pain intermediate‐term (VAS 0 to 10) | 1 | 140 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.34, 0.94] |
| 3 Pain long‐term (VAS 0 to 10) | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.47, 0.87] |
| 4 Disability short‐term (RMDQ 0 to 24) | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | 1.78 [0.52, 3.04] |
| 5 Disability intermediate‐term (RMDQ 0 to 24) | 1 | 140 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [‐0.44, 2.06] |
| 6 Disability long‐term (RMDQ 0 to 24) | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.65, 1.61] |
| 7 Work absence at 30 days | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.76, 1.16] |
| 8 Work absence at 60 days | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.32, 1.84] |
Comparison 7. Back school versus advice for acute or subacute LBP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Days of sick‐leave at 1 year | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐29.24, 23.24] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bergquist 1977.
| Methods | Randomised controlled trial (RCT). Each participant was provided with 1 of 8 possible combinations of codes. The 8 combinations of codes were used when randomising the participants to 1 of 3 modes of therapy. Separate tables of random numbers were used for each combined code. |
|
| Participants | 217 participants from the Volvo factory in Goteborg.
Inclusion criteria: acute or subacute low‐back pain (LBP) with or without radiation, duration of pain no longer than 3 months, a pain‐free year before onset of the current episode.
Exclusion criteria: chronic low‐back pain, specific low‐back pain. Age (years): overall median 34.5, range 17 to 64. Sex (female/male): overall 28/189. |
|
| Interventions | Back school treatment (I): Swedish back school: 4 X 45 minutes in 2 weeks (lessons include information on anatomy, causes of LBP, semi‐Fowler position, ergonomics, exercises and advice on physical activity (N = 70). Reference treatment (R1): combined physical therapy: manual therapy according to Cyriax, Kaltenborn, Lewitt, and Janda (N = 72). Reference treatment (R2): 'placebo' short‐wave diathermy at lowest intensity; a maximum of 10 treatments (N = 75). | |
| Outcomes | Timing of assessments: 10 days, 3 weeks, 6 weeks, 3 months, 6 months and 1 year.
The trial authors excluded participants from the assessment as soon as they had recovered Pain: median values of pain index (range 0 to 70) at baseline and after 6 weeks: (I) 43, 22, (R1) 42, 21, (R2) 42, 17. Work status: median number of days of absence from work: (I) 20.5, (R1) 26.5, (R2) 26.5. Significantly more subjects with a shorter duration of sick leave in (I) compared to (R2) |
|
| Notes | Occupational setting. Funding: the Swedish work environment fund and AB Volvo. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "tables of random numbers were used". |
| Allocation concealment (selection bias) | Unclear risk | Comment: the trial authors did not describe the method of allocation. |
| Blinding of participants (performance bias) | High risk | Comment: participant blinding was not possible. |
| Blinding of health care providers (performance bias) | High risk | Comment: therapist blinding was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: patient‐reported outcomes with participants not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | Comment: according to Table 22, only 25% of the participants were assessed at 6 weeks. |
| Intention‐to‐treat analysis (attrition bias) | High risk | Quote: "twenty two patients were excluded from the analysis as they refused treatment". |
| Selective reporting (reporting bias) | Unclear risk | Comment: we did not identify any registered or published protocol. |
| Similarity of baseline characteristics? | Unclear risk | Comment: there was incomplete data to assess the study group comparability at baseline. |
| Co‐interventions avoided or similar? | Unclear risk | Comment: the trial authors did not provide any information on any method used to avoid co‐interventions. |
| Compliance acceptable? | High risk | Comment: participants attended on average 40% of the sessions in the combined physiotherapy group and 50% of the sessions in the placebo group. |
| Timing outcome assessments similar? | High risk | Quote: "patients were reassessed at least five times and at the most seven times during one year". |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Hsieh 2002.
| Methods | RCT. Participants were randomised to group stratified by age, gender, duration of low‐back pain and treatment preference for physical therapy or chiropractic using a computer programme. |
|
| Participants | 200 participants visiting an outpatient physical therapy clinic and centre for spinal chiropractic care, and recruited by public announcements, advertisements in newspapers, radio stations and study brochures.
Inclusion criteria: 18 years of age or older, low‐back pain duration of more than 3 weeks and less than 6 months, a pain‐free period of at least 2 months in the preceding 8 months, agreement for randomisation, consent for treatment.
Exclusion criteria: pregnancy, serious medical problems, neurologic abnormalities in the lower extremities, spine disorders with bony lesions, significant mental disorders, obesity, leg pain with positive nerve root tension test, litigation, automobile injuries, work injuries, inappropriate illness behavior, anticoagulant therapy, history of lumbar surgery, use of study treatments for the current episode. Age [mean (range)] (years): back school 47.9 (34 to 61); myofascial therapy 49.0 (34 to 63); joint manipulation 47.4 (33 to 61); combined group 48.4 (34 to 62). Sex (female/male): back school 19/29; myofascial therapy 17/34; joint manipulation 16/33; combined group 17/35. |
|
| Interventions | Back school treatment (I): 3 once‐per‐week sessions of education (spine anatomy, causes of LBP, body mechanics for daily activities) and practice (exercises for sitting and standing, lumbar flexion, extension, stretching, stabilisation and walking) (N = 48). Reference treatment (R1): myofascial therapy programme, 9 sessions in 3 weeks including intermittent Fluori‐Methane sprays, 5 to 10 stretches of isometric contraction, ischemic compressions, stripping massage, hot packs (N = 51). Reference treatment (R2): joint manipulation, 9 sessions in 3 weeks including high velocity and short‐amplitude manipulations ("diversified" technique) (N = 49). Reference treatment (R3): combined Joint manipulation and myofascial therapy, 9 sessions in 3 weeks (N= 52). | |
| Outcomes | Timing of assessments: 3 weeks and 6 months. Pain: mean (SD) VAS scores at baseline: (I) 4.14 (2.10), (R1) 4.05 (2.15), (R2) 3.66 (1.90), (R3) 3.75 (2.18), 3 weeks (I) 2.13 (1.28), (R1) 4.05 (2.15), 2.78 (1.82), (R2) 2.58 (1.93), (R3) 2.04 (1.35) and 6 months (I) 2.29 (1.98), (R1) 2.99 (2.28), (R2) 2.40 (2.41), (R3) 2.24 (2.01). Disability: mean (SD) Roland Morris Disability Questionnaire (RMDQ) scores at baseline: (I) 7.92 (4.15), (R1) 8.35 (4.57), (R2) 8.40 (5.16), (R3) 7.62 (4.58), 3 weeks (I) 4.26 (3.52), (R1) 5.80 (5.12), (R2) 4.42 (4.92), (R3) 3.73(3.76), and 6 months (I) 3.48 (3.86), (R1) 5.06 (4.78), (R2) 3.29 (4.73), (R3) 3.56 (3.46). Adverse events: number of reports (N): (I) 6, (R1) 4, (R2) 6, (R3) 7. Overall, there were no statistical differences between the 4 treatment groups. |
|
| Notes | Mixed study setting. Funding: grant PHS‐1 R18 AH10004 – 01A1 as part of the Chiropractic Demonstration Project. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised into one of four treatment groups using a computer program designed to balance allocation of patients according to age, gender, duration of LBP, and treatment preference for physical therapy or chiropractic". |
| Allocation concealment (selection bias) | Unclear risk | Comment: the trial authors did not describe the method of allocation. |
| Blinding of participants (performance bias) | High risk | Comment: participant blinding was not possible. |
| Blinding of health care providers (performance bias) | High risk | Comment: therapist blinding was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: patient‐reported outcomes with participants not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Quote: "Of the 200 patients treated, 184 (92%) returned after 3 weeks of care and 178 (89%) returned 6 months later for assessments". Comment: the percentage of withdrawals and drop‐outs didn't exceed 20% at short‐term follow‐up and 30% at long‐term follow‐up. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | Quote: "All statistical analyses were based on an intent‐to‐treat methodology such that all data were analysed regardless of patient compliance". |
| Selective reporting (reporting bias) | Unclear risk | Comment: there was no registered or published protocol. |
| Similarity of baseline characteristics? | Low risk | Comment: according to Table 3, there were no significant differences between groups at baseline for all outcomes. |
| Co‐interventions avoided or similar? | Unclear risk | Comment: the trial authors did not provide any information on any method used to avoid co‐interventions. |
| Compliance acceptable? | Low risk | Quote: "Of the 200 patients treated, 184 (92%) returned after 3 weeks of care and 178 (89%) returned 6 months later for assessments". |
| Timing outcome assessments similar? | Low risk | Comment: the trial authors assessed the participants in all groups at the same time of follow‐up (3 weeks and 6 months). |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Leclaire 1996.
| Methods | RCT. A table of random numbers with blocking in groups of 4. |
|
| Participants | 170 outpatients from a physiatry institute
Inclusion criteria: participants with low‐back pain for < 3 months, aged 18 to 50 years, unable to work and receiving workers compensation.
Exclusion criteria: work history < 1 year with the same employer, previous compensation for low‐back pain, previous low‐back pain longer than 1 week, radiation below knee, herniated disc, cancer, ankylosing spondylitis, stenosis, Paget's disease, spondylolisthesis, serious medical illness including mental illness, pregnancy or plans to become pregnant. Age [mean (range)] (years): back school 31.9 (24 to 39); standard therapy 32.2 (24 to 40). Sex (female/male): back school 35/47; standard therapy 35/51. |
|
| Interventions | Back school (I): same standard programme and daily physiotherapy as (R) plus three 90‐minute back school sessions in 8 weeks with a maximum of 4 participants. Consisting of education (anatomy and pathophysiology of the low‐back, lifestyle changes and coping mechanisms to prevent recurrences) and flexion exercises to do at home (N = 82). Reference treatment (R): rest, analgesics, non‐steroidal anti‐inflammatory drugs as appropriate and daily physiotherapy (hot/cold packs, massage, ultrasound, transcutaneous electrical nerve stimulation (TENS), flexion and isometric hip, back and abdominal exercises, instruction to repeat the exercises each day for the rest of their lives), 30 mins sessions (N = 86). | |
| Outcomes | Timing of assessments: 8 weeks, 6 months and 12 months Pain: mean (SD) VAS scores at baseline: (I) 4.3 (2.1), (R) 4.3 (1.8), 8 weeks: (I) 1.7 (2.0), (R) 1.2 (1.6), 6 months (I) 1.5 (2.1), (R) 1.2 (1.7) and 12 months: (I) 1.4 (22), (R) 1.2 (1.8). Disability: mean (SD) RDQ scores at baseline: (I) 45.9 (18.1), (R) 42.7 (19.1), 8 weeks: (I) 19.2 (17.9), (R) 11.8 (15.2), 6 months: (I) 11.3 (17.1), (R) 7.9 (13.5) and 12 months: (I) 8.9 (15.2), (R) 6.9 (12.9). Work status : median time to return to work: (I) 33 days, (R) 33 days. No significant differences. |
|
| Notes | Occupational setting. Funding: grant RS‐87‐35 from the institut de recherche en santé et en sécurité du travail du Québec (research institute in health and in work security of Quebec). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The schedule of randomisation was based on a table of random numbers with blocking in groups of 4". |
| Allocation concealment (selection bias) | Unclear risk | Comment: the trial authors did not describe the method of allocation. |
| Blinding of participants (performance bias) | High risk | Comment: participant blinding was not possible. |
| Blinding of health care providers (performance bias) | High risk | Comment: therapist blinding was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: patient‐reported outcomes with participants not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Comment: according to Table 3, the percentage of withdrawals and drop‐outs did not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | Quote: "An intention‐to‐treat analysis was done for the outcomes". |
| Selective reporting (reporting bias) | Unclear risk | Comment: there was no registered or published protocol. |
| Similarity of baseline characteristics? | Low risk | Comment: according to Table 3, there were no significant differences between groups at baseline for all outcomes. |
| Co‐interventions avoided or similar? | Unclear risk | Comment: the trial authors did not provide any information on any method used to avoid co‐interventions. |
| Compliance acceptable? | Unclear risk | Comment: the trial authors did not provide any information on the compliance of participants to the treatment. |
| Timing outcome assessments similar? | Low risk | Comment: the trial authors assessed participants in all groups at the same time of follow‐up (8 weeks, 6 months and 12 months). |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Lindequist 1984.
| Methods | Quasi‐RCT. Randomisation by date of birth. |
|
| Participants | 56 consecutive patients visiting a general practitioner.
Inclusion criteria: acute lumbosacral LBP, with or without radiation to the thigh.
Exclusion criteria: chronic pain, earlier back surgery, specific pain caused by infections, tumours and fractures, work absenteeism, pension. Age [mean (range)] (years): back school 37 (16 to 58); control 39 (24 to 65). Sex (female/male): back school 14/10; control 18/14. |
|
| Interventions | Back school treatment (I): postural education "back school type" and training programme supervised by physiotherapist (N = 24). Reference treatment (R): advice not to strain the back and to use analgesics when needed. No physiotherapy (N = 32). | |
| Outcomes | Timing of assessments: 1 week, 3 weeks, 6 weeks and 1 year. Work status: mean (range) days of sick‐leave at baseline and 1 year: (I) 31 (5 to 144), 36 (1 to 144), (R) 29 (4 to 81), 39 (0 to 254). No significant differences. |
|
| Notes | Primary care setting. Funding: N/A. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "were randomised by their date of birth". |
| Allocation concealment (selection bias) | Unclear risk | Comment: the trial authors did not describe the method of allocation. |
| Blinding of participants (performance bias) | High risk | Comment: participant blinding was not possible. |
| Blinding of health care providers (performance bias) | High risk | Comment: therapist blinding was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: patient‐reported outcomes with participants not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Comment: according to Tables II and III, the trial authors assessed 100% of participants at short‐term follow‐up (1, 3 and 6 weeks) and 96% and 97% for the back school and control groups respectively at long‐term follow‐up (1 year). |
| Intention‐to‐treat analysis (attrition bias) | High risk | Comment: the trial authors did not perform an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: there was no registered or published protocol. |
| Similarity of baseline characteristics? | High risk | Quote: "Eighty per cent of the patients in the treatment group, however, had pain when coughing and sneezing compared with 47% in the control group. Even if significant (p<0.05) this pain did not correlate to a longer initial period of sick‐leave (Table 1)." |
| Co‐interventions avoided or similar? | High risk | Quote: "In both groups 37% of the patients had visited a physiotherapist and 9% in the treatment group and 10% in the control group had seen a chiropractor during the past year". |
| Compliance acceptable? | Unclear risk | Comment: the trial authors did not provide any information on the compliance of participants to the treatment. |
| Timing outcome assessments similar? | High risk | Quote: "The follow‐up time for both groups was on an average 11 months (range 9‐12)". |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Abbreviations: RCT = randomised controlled trial; LBP = low‐back pain; SD = standard deviation; N = number of participants.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berwick 1989 | The study included a mixed population with acute and chronic pain. The median time of duration of symptoms as described in the inclusion criteria was 14 weeks (2 weeks to 6 months). The study population was considered as having overall chronic pain. |
| Chenard 1991 | The study population had chronic specific and non‐specific low‐back pain (LBP). |
| Dalichau 1999 | The study population had chronic LBP. |
| Donchin 1990 | The study population had chronic LBP. |
| Federwisch 1998 | The paper was not a randomised controlled trial (RCT). |
| Herzog 1991 | The study included a mixed population with acute and chronic pain. One of the inclusion criteria was to have sacroiliac pain lasting more than 1 month, defined as a chronic pain. On this basis, we considered the study population as having chronic pain. |
| Heymans 2006 | The study population had chronic LBP. |
| Hurri 1989 | The study population had chronic LBP. |
| Härkäpää 1989 | The study population had chronic LBP. |
| Indahl 1998 | The back school programme did not include exercises. |
| Jaromi 2012 | The study population had chronic LBP. |
| Keijsers 1989 | The study population had chronic LBP. |
| Keijsers 1990 | The study population had chronic LBP. |
| Klaber Moffett 1986 | The study population had chronic LBP. |
| Lankhorst 1983 | The study population had chronic LBP. |
| Larsen 2002 | The study was about primary prevention. |
| Linton 1989 | The study population had chronic LBP. |
| Lønn 1999 | The study population had chronic LBP. |
| Mele 2006 | The paper was not a RCT. |
| Meng 2009 | The study population had chronic LBP. |
| Meng 2011 | The study compared 2 back school treatments. |
| Pentinnen 2002 | The study population had chronic LBP. |
| Postacchini 1988 | The study population had chronic LBP. |
| Ribeiro 2008 | The study population had chronic LBP. |
| Schenk 1996 | The study was about primary prevention. |
| Sirles 1991 | The study compared 2 types of back school interventions. |
| Tavafian 2007 | The study population had chronic LBP. |
Abbreviations: RCT = randomised controlled trial; LBP = low‐back pain.
Differences between protocol and review
We made the following methodological changes from the last version of this Cochrane review (Heymans 2004).
We didn't restrict inclusion to RCTs; but also expanded eligible studies to quasi‐RCTs.
The previous review version defined the back school group as supervised by a paramedical therapist or medical specialist. We used a broader definition to include all types of healthcare providers given that the usual professional in charge of the back schools might vary between countries.
In the current update, the primary outcomes were pain and disability and the secondary outcomes were work status and adverse events. The last update defined the primary outcomes as pain, functional status, disability, overall improvement, patient satisfaction with treatment, general well‐being and number of recurrent episodes of back pain. It defined secondary outcomes as physiological outcomes of physical examination, medication use and adverse events. We made changes in order to have a focused clinical question.
We added PsycINFO and CINAHL to the search strategy for this review update, and searched from inception to 4 August 2015. We searched PubMed from 4 March (the date of the previous search update) to 4 August 2015 and also screened ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal.
Contrary to the previous update, we did not score the clinical relevance of included studies.
In the current update we excluded three studies that the previous review version included. In Indahl 1998, the back school programme did not include exercise. In Berwick 1989 and Herzog 1991, the included population had acute and chronic LBP without separate data provided for each subgroup. The average duration of the symptoms was superior to three months and the two studies will be included in the Cochrane review on back schools for chronic LBP.
Contributions of authors
Nolwenn Poquet and Christine Lin updated the searches for new trials, conducted the study selection, quality assessment, data extraction and analysis of all studies. We consulted Chris Maher and Martijn Heymans when disagreements persisted. Bart Koes performed study selection for studies in German. All review authors were involved in developing the protocol for this review. Nolwenn Poquet drafted the final manuscript and all review authors contributed to writing the final manuscript.
Sources of support
Internal sources
The George Institute for Global Health, Sydney Medical School, The University of Sydney, Australia.
External sources
The George Institute for Global Health, Sydney Medical School, The University of Sydney, Australia.
-
Australian Research Council, Australia.
Christopher Maher is funded by a Future Fellowship from the Australian Research Council
-
National Health and Medical Research Council, Australia.
Chung‐Wei Christine Lin is funded by a Career Development Fellowship from the National Health and Medical Research Council, Australia
Dutch Health Insurance Board, Netherlands.
-
Pierre and Marie Curie University, Paris, France.
Nolwenn Poquet was supported by a scholarship from Pierre and Marie Curie University during her MSc internship
Declarations of interest
Two review authors, Maurits van Tulder and Chris Maher, are on the Editorial Board of the Cochrane Back and Neck Review Group. Editors are required to conduct at least one Cochrane review. This requirement ensures that editors are aware of the processes and commitment needed to conduct reviews. None of the editors are first review authors. This involvement does not seem to be a source of conflict of interest in the Cochrane Back and Neck Review Group. Any editor who is a review author is excluded from editorial decisions on the Cochrane review in which they are contributors.
Nolwenn Poquet, Chris Lin, Martijn Heymans, Rosmin Esmail and Bart Koes have no known conflicts of interest.
New
References
References to studies included in this review
Bergquist 1977 {published data only}
- Bergquist‐Ullman M, Larsson U. Acute low‐back pain in industry. A controlled prospective study with special reference to therapy and confounding factors. Acta Orthopaedica Scandinavica 1977;170(Suppl):1‐117. [DOI] [PubMed] [Google Scholar]
Hsieh 2002 {published data only}
- Hsieh CY, Adams AH, Tobis J, Hong CZ, Danielson C, Platt K, et al. Effectiveness of four conservative treatments for subacute low back pain: a randomized clinical trial. Spine 2002;27(11):1142‐8. [DOI] [PubMed] [Google Scholar]
Leclaire 1996 {published data only}
- Leclaire R, Esdaile JM, Suissa S, Rossignol M, Proulx R, Dupuis M. Back school in a first episode of compensated acute low back pain: a clinical trial to assess efficacy and prevent relapse. Archives of Physical Medicine and Rehabilitation 1996;77(7):673‐9. [DOI] [PubMed] [Google Scholar]
Lindequist 1984 {published data only}
- Lindequist SL, Lundberg B, Wikmark R, Bergstad B, Lööf G, Ottermark AC. Information and regime at low‐back pain. Scandinavian Journal of Rehabilitation Medicine 1984;16(3):113‐6. [PubMed] [Google Scholar]
References to studies excluded from this review
Berwick 1989 {published data only}
- Berwick DM, Budman S, Feldstein M. No clinical effect of back schools in an HMO. A randomized prospective trial. Spine 1989;14(3):338‐44. [DOI] [PubMed] [Google Scholar]
Chenard 1991 {published data only}
- Chenard J, Marchand S, Charest J, Li J, Lavignolle B. Evaluation of a behavioral intervention for chronic low‐back pain: The "interactional back school" [Évaluation d'un traitement comportemental de la lombalgie chronique: l'"école interactionnelle du dos"]. Science et Comportement 1991;21(4):225‐39. [Google Scholar]
Dalichau 1999 {published data only}
- Dalichau S, Perrey RM, Solbach T, Elliehausen H‐J. [Erfahrungen bei der Durchführung eines berufsbezogenen Rückenschulmodells im Baugewerbe]. Zbl. Arbeitsmedizin 1998;48:72‐80. [Google Scholar]
- Dalichau S, Scheele K, Perrey RM, Elliehausen H‐J, Huebner J. [Ultraschallgestützte Haltungs‐ und Bewegungsanalyse der Lendenwirbelsäule zum Nachweis der Wirksamkeit einer Rückenschule]. Zbl. Arbeitsmedizin 1999;49:148‐56. [Google Scholar]
Donchin 1990 {published data only}
- Donchin M, Woolf O, Kaplan L, Floman Y. Secondary prevention of low‐back pain. A clinical trial. Spine 1990;15:1317‐20. [DOI] [PubMed] [Google Scholar]
Federwisch 1998 {published data only}
- Federwisch A. A strong back. Does back school help‐or waste time?. HealthWeek 1998;3(1):7. [Google Scholar]
Härkäpää 1989 {published data only}
- Härkäpää K, Järvikoski A, Mellin G, Hurri H. A controlled study on the outcome of inpatient and outpatient treatment of low‐back pain. Part I. Scandinavian Journal of Rehabilitation Medicine 1989;21:81‐9. [PubMed] [Google Scholar]
- Härkäpää K, Mellin G, Järvikoski A, Hurri H. A controlled study on the outcome of inpatient and outpatient treatment of low‐back pain. Part III. Scandinavian Journal of Rehabilitation Medicine 1990;22:181‐8. [PubMed] [Google Scholar]
- Mellin G, Hurri H, Härkäpää K, Järvikoski A. A controlled study on the outcome of inpatient and outpatient treatment of low back pain. Part II. Scandinavian Journal of Rehabilitation Medicine 1989;21:91‐5. [PubMed] [Google Scholar]
- Mellin G, Härkäpää K, Hurri H, Järvikoski A. A controlled study on the outcome of inpatient and outpatient treatment of low back pain. Part IV. Scandinavian Journal of Rehabilitation Medicine 1990;22:189‐94. [PubMed] [Google Scholar]
Herzog 1991 {published data only}
- Herzog W, Conway PJW, Willcox BJ. Effects of different treatment modalities on gait symmetry and clinical measures for sacroiliac joint patients. Journal of Manipulative and Physiological Therapeutics 1991;14(2):104‐8. [PubMed] [Google Scholar]
Heymans 2006 {published data only}
- Heymans MW, Vet HC, Bongers PM, Knol DL, Koes BW, Mechelen W. The effectiveness of high‐intensity versus low‐intensity back schools in an occupational setting: a pragmatic randomized controlled trial. Spine 2006;31(10):1075‐82. [DOI] [PubMed] [Google Scholar]
Hurri 1989 {published data only}
- Hurri H. The Swedish back school in chronic low‐back pain. Part II. Factors predicting the outcome. Scandinavian Journal of Rehabilitation Medicine 1989;21:41‐4. [PubMed] [Google Scholar]
- Hurri H. The Swedish back school in chronic low‐back. Part I. Benefits. Scandinavian Journal of Rehabilitation Medicine 1989;21:33‐40. [PubMed] [Google Scholar]
- Julkunen J, Hurri H, Kankainen J. Psychological factors in the treatment of chronic low back pain. Psychotherapy and Psychosomatics 1988;50:173‐81. [DOI] [PubMed] [Google Scholar]
Indahl 1998 {published data only}
- Indahl A, Haldorsen EH, Holm S, Reikerås O, Ursin H. Five‐year follow‐up study of a controlled clinical trial using light mobilization and an informative approach to low back pain. Spine 1998;23(23):2625‐30. [DOI] [PubMed] [Google Scholar]
- Indahl A, Velund L, Reikeraas O. Good prognosis for low back pain when left untampered. A randomized clinical trial. Spine 1995;20(4):473‐7. [DOI] [PubMed] [Google Scholar]
Jaromi 2012 {published data only}
- Jaromi M, Nemeth A, Kranicz J, Laczko T, Betlehem J. Treatment and ergonomics training of work‐related lower back pain and body posture problems for nurses. Journal of Clinical Nursing 2012;21(11‐12):1776‐84. [DOI] [PubMed] [Google Scholar]
Keijsers 1989 {published data only}
- Keijsers JFEM, Groenman NH, Gerards FM, Oudheusden E, Steenbakkers M. A back school in the Netherlands: Evaluating the results. Patient Education & Counseling 1989;14:31‐44. [DOI] [PubMed] [Google Scholar]
Keijsers 1990 {published data only}
- Keijsers JFME, Steenbakkers WHL, Meertens RM, Bouter LM, Kok GJ. The efficacy of the back school: A randomized trial. Arthritis Care and Research 1990;3:204‐9. [Google Scholar]
Klaber Moffett 1986 {published data only}
- Klaber Moffett JA, Chase SM, Portek I, Ennis JR. A controlled prospective study to evaluate the effectiveness of a back school in the relief of chronic low‐back pain. Spine 1986;11:120‐2. [DOI] [PubMed] [Google Scholar]
Lankhorst 1983 {published data only}
- Lankhorst GJ, Stadt RJ, Vogelaar TW, Korst JK, Prevo AJH. The effect of the Swedish back school in chronic idiopathic low‐back pain. Scandinavian Journal of Rehabilitation Medicine 1983;15:141‐5. [PubMed] [Google Scholar]
Larsen 2002 {published data only}
- Larsen K, Weidick F, Leboeuf‐Yde C. Can passive prone extensions of the back prevent back problems? A randomized, controlled intervention trial of 314 military conscripts. Spine 2002;27(24):2747‐52. [DOI] [PubMed] [Google Scholar]
Linton 1989 {published data only}
- Linton SJ, Bradley LA, Jensen I, Spangfort E, Sundell L. The secondary prevention of low back pain: A controlled study with follow‐up. Pain 1989;36:197‐207. [DOI] [PubMed] [Google Scholar]
Lønn 1999 {published data only}
- Glomsrød B, Lønn JH, SoukupMG, Bø K, Larsen S. Active back school, prophylactic management for low back pain: Three‐year follow‐up of a randomized controlled trial. Journal of Rehabilitation Medicine 2001;33:26‐30. [DOI] [PubMed] [Google Scholar]
- Lønn JH, Glomsrød B, Soukup MG, Bø K, Larsen S. Active back school: Prophylactic management for low back pain. A randomized controlled 1‐year follow‐up study. Spine 1999;24:865‐71. [DOI] [PubMed] [Google Scholar]
Mele 2006 {published data only}
- Mele G, Domenica F, Locati F, Gattoronchieri V, Silingardi M. Rehabilitation, physical therapy or Back School? [Riabilitazione, terapia fisica o back schools?]. Reumatismo 2006;58(Spec No.1):102‐5. [PubMed] [Google Scholar]
Meng 2009 {published data only}
- Meng K, Seekatz B, Rossband H, Worringen U, Faller H, Vogel H. Development of a standardized back school for in‐patient orthopaedic rehabilitation [Entwicklung eines standardisierten Rückenschulungsprogramms für die orthopädische Rehabilitation]. Rehabilitation 2009;48(6):335‐44. [DOI] [PubMed] [Google Scholar]
Meng 2011 {published data only}
- Meng K, Seekatz B, Roband H, Worringen U, Vogel H, Faller H. Intermediate and long‐term effects of a standardized back school for inpatient orthopedic rehabilitation on illness knowledge and self‐management behaviors: a randomized controlled trial. Clinical Journal of Pain 2011;27(3):248‐57. [DOI] [PubMed] [Google Scholar]
Pentinnen 2002 {published data only}
- Penttinen J, Nevala‐PuranenN, AiraksinenO, Jaaskelainen M, Sintonen H, Takala J. Randomized controlled trial of back school with and without peer support. Journal of Occupational Rehabilitation 2002;12:21‐9. [DOI] [PubMed] [Google Scholar]
Postacchini 1988 {published data only}
- Postacchini F, Facchini M, Palieri P. Efficacy of various forms of conservative treatment in low‐back pain. A comparative study. Neuro‐Orthopedics 1988;6:28‐35. [Google Scholar]
Ribeiro 2008 {published data only}
- Ribeiro LH, Jennings F, Jones A, Furtado R, Natour J. Effectiveness of a back school program in low back pain. Clinical and Experimental Rheumatology 2008;26(1):81‐8. [PubMed] [Google Scholar]
Schenk 1996 {published data only}
- Schenk RJ, Doran RL, Stachura JJ. Learning effects of a back education program. Spine 1996;21(19):2183‐9. [DOI] [PubMed] [Google Scholar]
Sirles 1991 {published data only}
- Sirles AT, Brown K, Hilyer JC. Effects of back school education and exercise in back injured municipal workers. AAOHN Journal 1991;39(1):7‐12. [PubMed] [Google Scholar]
Tavafian 2007 {published data only}
- Tavafian SS, Jamshidi A, Mohammad K, Montazeri A. Low back pain education and short term quality of life: a randomized trial. BMC Musculoskeletal Disorders 2007;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Arthritis and Osteoporosis Victoria 2013
- Arthritis, Osteoporosis Victoria. A problem worth solving. A report produced by Arthritis and Osteoporosis Victoria. The rising cost of musculoskeletal conditions in Australia. http://www.arthritisvic.org.au/Research/AOV‐Funded‐Research/Completed/A‐Problem‐Worth‐Solving/APWS.aspx (accessed 16 April 2014).
Boutron 2005
- Boutron I, Moher D, Tugwell P, Giraudeau B, Poiraudeau S, Nizard R, et al. A checklist to evaluate a report of a non pharmacological trial (CLEAR NPT) was developed using consensus. Journal of Clinical Epidemiology 2005;58(12):1233‐40. [DOI] [PubMed] [Google Scholar]
Brox 2008
- Brox JI, Storheim K, Grotle M, Tveito TH, Indahl A, Eriksen HR. Systematic review of back schools, brief education, and fear‐avoidance training for chronic low back pain. The Spine Journal 2008;8(6):948‐58. [DOI] [PubMed] [Google Scholar]
Dagenais 2008
- Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. The Spine Journal 2008;8(1):8‐20. [DOI] [PubMed] [Google Scholar]
Di Fabio 1995
- Fabio RP. Efficacy of comprehensive rehabilitation programs and back school for patients with low back pain: a meta‐analysis. Physical Therapy 1995;75(10):865‐78. [DOI] [PubMed] [Google Scholar]
Duffy 2014
- Duffy S, Misso K, Noake C, Ross J, Stirk L. Supplementary searches of PubMed to improve currency of MEDLINE and MEDLINE In‐Process searches via OvidSP. Kleijnen Systematic Reviews Ltd, York. Poster presented at the UK InterTASC Information Specialists' Sub‐Group (ISSG) Workshop; 9 July 2014; Exeter: UK (2014) [accessed 6.8.14]. Available from: https://medicine.exeter.ac.uk/media/universityofexeter/medicalschool/research/pentag/documents/Steven_Duffy_ISSG_Exeter_2014_poster_1.pdf.
Egger 1998
- Egger M, Davey Smith G. Bias in location and selection of studies. British Medical Journal 1998;316(7124):61‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engers 2008
- Engers AJ, Jellema P, Wensing M, Windt DAWM, Grol R, Tulder MW. Individual patient education for low back pain. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004057.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furlan 2009
- Furlan AD, Pennick V, Bombardier C, Tulder M, Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34(18):1929‐41. [DOI] [PubMed] [Google Scholar]
Furlan 2015
- Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, et al. 2015 updated method guideline for systematic reviews in the Cochrane Back and Neck Group. Spine 2015;40(21):1660‐73. [DOI] [PubMed] [Google Scholar]
Gøtzsche 1987
- Gøtzsche PC. Reference bias in reports of drug trials. British Medical Journal 1987;295(6599):654‐6. [PMC free article] [PubMed] [Google Scholar]
Hayden 2005
- Hayden JA, Tulder MW, Malmivaara A, Koes BW. Exercise therapy for treatment of non‐specific low back pain. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD000335.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Karjalainen 2003
- Karjalainen KA, Malmivaara A, Tulder MW, Roine R, Jauhiainen M, Hurri H, et al. Multidisciplinary biopsychosocial rehabilitation for subacute low‐back pain among working age adults. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD002193] [DOI] [PubMed] [Google Scholar]
Keijsers 1991
- Keijsers JFEM, Bouter LM, Meertens RM. Validity and comparability of studies on the effects of back schools. Physiotherapy Theory and Practice 1991;7(3):177‐84. [Google Scholar]
Koes 2006
- Koes BW, Tulder MW, Thomas S. Diagnosis and treatment of low back pain. British Medical Journal 2006;332(7555):1430‐1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koes 2010
- Koes BW, Tulder M, Lin C, Macedo LG, McAuley J, Maher C. An updated overview of clinical guidelines for the management of non‐specific low back pain in primary care. European Spine Journal 2010;19(12):2075‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambeek 2011
- Lambeek LC, Tulder MW, Swinkels IC, Koppes LL, Anema JR, Mechelen W. The trend in total cost of back pain in The Netherlands in the period 2002 to 2007. Spine 2011;36(13):1050‐8. [DOI] [PubMed] [Google Scholar]
Maier‐Riehle 2001
- Maier‐Riehle B, Härter M. The effects of back schools‐‐a meta‐analysis. International Journal of Rehabilitation Research 2001;24(3):199‐206. [DOI] [PubMed] [Google Scholar]
Pengel 2002
- Pengel HM, Maher CG, Refshauge KM. Systematic review of conservative interventions for subacute low back pain. Clinical Rehabilitation 2002;16(8):811‐20. [DOI] [PubMed] [Google Scholar]
Scheer 1995
- Scheer SJ, Radack KL, O'Brien DR Jr. Randomized controlled trials in industrial low back pain relating to return to work. Part 1. Acute interventions. Archives of Physical Medicine and Rehabilitation 1995;76(10):966‐73. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schulz 2002
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. The Lancet 2002;359(9306):614‐8. [DOI] [PubMed] [Google Scholar]
Thornton 2000
- Thornton A, Lee P. Publication bias in meta‐analysis: its causes and consequences. Journal of Clinical Epidemiology 2000;53(2):207‐216. [DOI] [PubMed] [Google Scholar]
Turner 1996
- Turner JA. Educational and behavioral interventions for back pain in primary care. Spine 1996;21(24):2851‐9. [DOI] [PubMed] [Google Scholar]
van Tulder 2003
- Tulder M, Furlan A, Bombardier C, Bouter L, Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
Walker 2003
- Walker BF, Muller R, Grant WD. Low back pain in Australian adults: the economic burden. Asia Pacific Journal of Public Health 2003;15(2):79‐87. [DOI] [PubMed] [Google Scholar]
Wiese 2009
- Wiese M, Krämer J, Becker C, Nentwig V, Theodoridis T, Teske W. Back school ‐ an update [Ruckenschule heute]. Zeitschrift für Orthopädie und Unfallchirurgie 2009;147(2):194‐8. [DOI] [PubMed] [Google Scholar]
Zachrisson‐Forssell 1980
- Zachrisson‐Forssell M. The Swedish back school. Physiotherapy 1980;66(4):112‐4. [PubMed] [Google Scholar]
Zachrisson‐Forssell 1981
- Zachrisson‐Forssell M. The back school. Spine 1981;6(1):104‐6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Heymans 2004
- Heymans MW, Tulder MW, Esmail R, Bombardier C, Koes BW. Back schools for non‐specific low‐back pain. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD000261.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heymans 2005
- Heymans MW, Tulder MW, Esmail R, Bombardier C, Koes BW. Back schools for nonspecific low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine 2005;30(19):2153‐63. [DOI] [PubMed] [Google Scholar]
van Tulder 1999
- Tulder MW, Esmail R, Bombardier C, Koes BW. Back schools for non‐specific low back pain. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD000261.pub2] [DOI] [PubMed] [Google Scholar]


