Abstract
Aim
Reducing the blood transfusion volume is important in severe trauma. We hypothesized that carbazochrome sodium sulfonate (CSS) combined with tranexamic acid (TXA) would reduce blood transfusions in severe trauma.
Methods
From April 2017 to March 2023, data were collected from patients (aged ≥16 years) admitted to our hospital for trauma and administered packed red blood cells (pRBC) and plasma transfusions within 12 h postinjury. Patients infused with CSS and TXA (CSS + TXA group) were compared with those infused with TXA alone (TXA group). The outcomes were blood product transfusion volumes within and after 24 h, the number of patients receiving >6 units of pRBC transfusion after 24 h, duration of intensive care unit and in‐hospital stays, and 28‐day in‐hospital mortality.
Results
In total, 138 patients were included in the study. In the univariate analyses, the CSS + TXA group (n = 62) showed a significant reduction in the total pRBC transfusion volume, in‐hospital days, and number of patients receiving >6 units of pRBCs in the delayed phase. Based on the multivariate logistics regression analysis, only the CSS + TXA group had a significantly lower adjusted odds ratio for receiving >6 units of pRBC transfusion after 24 h. During the in‐hospital days, the CSS + TXA group did not experience an increased incidence of major complications when compared with the TXA group.
Conclusion
In patients with trauma, treatment with CSS with TXA may reduce the requirement for blood transfusion after 24 h. Moreover, this treatment can improve admission outcomes without increasing complications.
Keywords: blood transfusions, carbazochrome sodium sulfonate, hemorrhage, injury, tranexamic acid
Combining carbazochrome sodium sulfonate with tranexamic acid potentially decreases blood transfusion needs in patients with trauma. Our retrospective study shows that this combination therapy reduced the number of patients receiving >6 units of packed red blood cell transfusion after 24 h.
INTRODUCTION
Blood transfusion therapy is important to manage coagulopathy in patients with hemorrhage following trauma. However, blood product supplies are limited, and shortages can occur during disasters. Reducing the required blood transfusion volume using drug treatments can reduce exposure to allogeneic blood products and limit the unnecessary use of medical resources. 1 , 2
Tranexamic acid (TXA) is one of the few agents that has elicited beneficial effects in hemorrhage, as evidenced in the CRASH‐2 trial. 3 However, no currently available drugs can reduce the requirement for blood transfusion. Thus, we evaluated the potential of carbazochrome sodium sulfonate (CSS), traditionally used as a hemostatic agent since the 1950s, to address this issue. 4 , 5 , 6 , 7 , 8 , 9 , 10 CSS reportedly increases capillary resistance and reverses endothelial barrier dysfunction. 11 , 12 , 13 However, no previous reports have presented clear evidence indicating CSS effectiveness alone to address all causes of hemorrhage. In trauma medicine, few studies have sufficiently investigated CSS effects combined with TXA therapy. 6 Furthermore, recent studies have explored the potential of this combination in orthopedics. 7 , 8 However, previous investigations primarily focused on hemorrhage during the acute phase. Based on its underlying mechanism of action (capacity to enhance capillary resistance and reverse endothelial barrier dysfunction), CSS could improve bleeding postinjury. Hence, we hypothesized that combination therapy with CSS and TXA would reduce the requirement for blood transfusion 24 h postinjury in patients with severe trauma‐induced hemorrhage. Accordingly, the current study explored this hypothesis, and the results will serve as a basis for future prospective research.
METHODS
Study design
This single‐center, retrospective cohort study was conducted at Juntendo University Shizuoka Hospital. Patient data were collected through a medical chart review, with results presented based on Strengthening the Reporting of Observational Studies in Epidemiology guidelines. 14
Data setting
Since 1981, our institution has provided critical care to patients with trauma in a medical area with a population of 1,500,000 people. In Japan, one unit of blood contains approximately 200 mL of donated blood; the volumes of each whole blood contain 140 mL packed red blood cells (pRBCs), 80 mL fresh frozen plasma, and 20 mL platelet concentrate (PC). In our institution, no protocol for administering TXA or CSS to patients with trauma has been established. TXA administration was determined when clinicians suspected hemorrhage in any part of the body. CSS was administered at the clinicians' discretion. Our institution does not typically undertake viscoelastic testing during clinical trauma care.
Data collection
Data were collected from April 2017 to March 2023. The study population comprised patients with trauma (aged ≥16 years) and admitted to our hospital. Patients who presented with cardiac arrest upon hospital arrival, acquired burns, ingested toxins, experienced asphyxiation, nearly drowned, and attempted suicide by hanging were excluded. The study included patients who received pRBC and plasma transfusions within 12 h postinjury. Patients who were transferred to other hospitals within 3 days of admission, with unclear time courses, with head injuries only, who refused transfusions of any blood products, and who did not receive TXA treatment were also excluded.
Data on demographics, medical history, injury severity score, abbreviated injury scale score (AIS), vital signs (Glasgow Coma Scale, systolic and diastolic blood pressure, heart rate, respiratory rate, and body temperature) upon arrival, results of focused assessment with sonography in trauma, coagulation function status, lactate levels, assessment of blood consumption (ABC) score, trauma‐associated severe hemorrhage (TASH) score, revised trauma score (RTS), probability of survival (Ps) upon admission were collected. Complications that occurred during hospital stay, invasive treatments performed within 24 h, dose and time of CSS and TXA administered, amount of blood products transfused within 24 h/after 24 h/entire in‐hospital stay, number of patients receiving >6 units of pRBC transfusion within 24 h postinjury, duration of ICU and in‐hospital stay, and 28‐day in‐hospital mortality were also recorded.
Data definition
Complications associated with in‐hospital mortality included uncontrolled trauma bleeding, gastrointestinal bleeding, and unexpected bleeding in the operation room, thus requiring blood transfusion. Regarding the timing of blood transfusion, the early phase was described as “within 24 h postinjury,” while the delayed phase was defined as “after 24 h from the time of injury.” AIS >3 means AIS = 4 or higher, excluding AIS = 1, 2, and 3.
Outcomes
Outcomes included the volume of blood products (pRBC, plasma, and PC) transfused in the early and delayed phases, the number of patients receiving 6 units of pRBC transfusion in the delayed phase, the duration of ICU and in‐hospital days, and 28‐day in‐hospital mortality. Additionally, we assessed complications during the hospital stay. The amount of pRBC transfused in the delayed phase, the amounts of plasma transfused in the delayed phase, and the number of patients who received >6 units of pRBC transfusion in the delayed phase were calculated, excluding the patients who died within 24 h postinjury.
Statistical analyses
Upon admission, patients who received both CSS and TXA (CSS + TXA group) were compared with those who received TXA only (TXA group). Data were presented as the median and interquartile range (IQR) or as counts and percentages, as appropriate. The outcomes were compared, and p‐values were calculated. Moreover, complications were compared, and the relative risk (RR) was calculated. Fisher's exact test, Mann–Whitney U‐test, and univariate and multivariate logistics regression analyses were performed to compare the odds ratio (OR) of the incidence of “over 6 units of pRBC transfusion in the delayed phase.” In addition to the presence or absence of CSS, AIS >3 in trunk and/or limbs, shock index >1 upon arrival, emergency operation and/or angioembolization within 24 h, and high amounts of pRBC (>10 units) in the early phase were also examined. We speculated that these variables could be key factors impacting the amount of pRBC transfused during the delayed phase in patients with trauma; evidence on such factors is currently lacking. Sensitivity analyses were performed considering the effect of a head injury. In the first sensitivity analysis, “AIS >2 in the head” was incorporated as an independent variable in the main model. In the second sensitivity analysis, a multivariate logistic regression analysis was performed in the cohort of patients without head injury. Each variable was assessed using an adjusted logistic regression model. All statistical analyses were performed using EZR 1.54 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R 4.0.2 (The R Foundation for Statistical Computing, Vienna, Austria). 15 Statistical significance was set at p < 0.05 or based on the 95% confidence interval (CI).
RESULTS
During the study period, 8834 patients with trauma visited our hospital, but only 206 met the inclusion criteria. After excluding 68 patients, the remaining 138 were divided into the CSS + TXA (n = 62) and TXA (n = 76) groups (Figure 1). Table 1 presents the patient characteristics and treatments performed within 24 h postinjury. The CSS + TXA group tended to experience more severe head injuries than the TXA group. However, AIS, ABC score, TASH score, RTS, and Ps did not differ significantly between the groups. The TXA group tended to have more severe abdominal and limb injuries, undergo emergency angioembolization, and lower systolic blood pressure upon arrival at the emergency room than the CSS + TXA group; however, the groups did not differ significantly in prothrombin time, international normalized ratio, fibrinogen, or fibrinogen degradation products levels.
FIGURE 1.
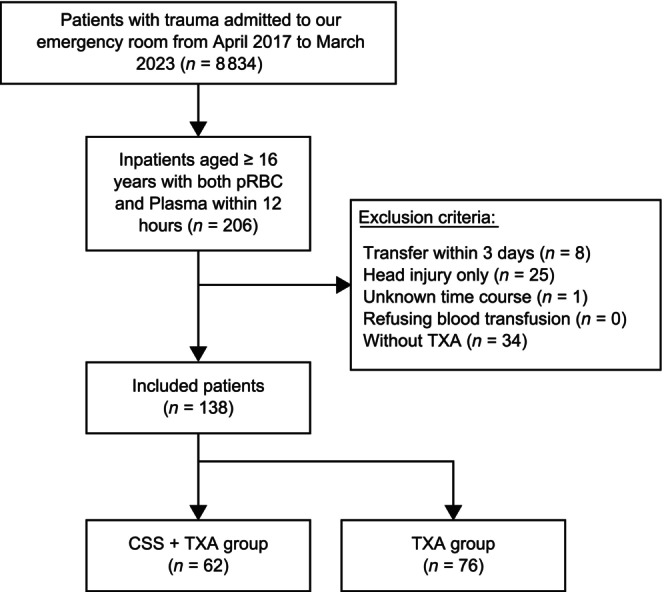
Flowchart of the participant selection process.
TABLE 1.
Participant baseline characteristics.
| Characteristics | CSS + TXA group (n = 62) | TXA group (n = 76) | p‐Value |
|---|---|---|---|
| Age, year | 71 [51.5–81] | 61.5 [41–75] | 0.06 |
| Sex, n (%) | |||
| Female | 21 (33.9) | 20 (26.3) | 0.36 |
| Male | 41 (66.1) | 56 (73.7) | |
| Body mass index | 21.6 [19.4–23.5] | 22.6 [20.3–25.8] | 0.08 |
| Type of injury, n (%) | |||
| Blunt | 60 (96.8) | 75 (98.7) | 0.59 |
| Penetrating | 2 (3.2) | 1 (1.3) | |
| Injury severity score | 27.5 [20–33] | 29 [24–33] | 0.46 |
| Abbreviated injury scale | |||
| Head | 2 [0–4] | 0 [0–2] | <0.001* |
| Face | 0 (0–0) | 0 (0–0) | 0.94 |
| Chest | 3 [0–3] | 3 [0–3] | 0.43 |
| Abdomen | 1 [0–2] | 2 [0–3] | 0.03 a |
| Limbs | 2 [1–3] | 3 [2–4] | 0.003 a |
| Skin | 0 (0–0) | 0 (0–0) | 0.053 |
| Vital signs upon arrival | |||
| Systolic blood pressure, mmHg | 118 [85–144] | 86 [70–110] | <0.001* |
| Heart rate, bpm | 88 [70–115] | 100 [78–120] | 0.13 |
| Respiratory rate, bpm | 22 [18–27] | 24 [20–30] | 0.054 |
| Glasgow coma scale | 13.5 [10–14] | 13.5 [12–14] | 0.68 |
| Body temperature, °C | 36.3 [35.9–36.6] | 36.3 [35.9–36.7] | 0.91 |
| PT‐INR | 1.15 [1.07–1.24] | 1.15 [1.07–1.27] | 0.97 |
| Fibrinogen, mg/dL | 195 [152–247] | 208 [167–241] | 0.34 |
| FDP, μg/dL | 211 [59–572] | 197[101–314] | 0.82 |
| Lactate, mmol/L | 3.4 [2.3–4.7] | 3.8 [2.6–5.8] | 0.13 |
| FAST positive, n (%) | 48 (78.7) | 55 (75.3) | 0.69 |
| ABC score | 1 [0–2] | 1 [0–2] | 0.21 |
| TASH score | 7 [3–11] | 9 [5–12] | 0.12 |
| RTS | 7.11 [6.08–7.84] | 6.82 [5.97–7.55] | 0.11 |
| Ps | 0.88 [0.69–0.94] | 0.82 [0.59–0.90] | 0.18 |
| Comorbidities, n (%) | |||
| Autoimmune disease | 2 (3.2) | 1 (1.3) | 0.59 |
| Chronic heart failure | 2 (3.2) | 4 (5.3) | 0.69 |
| Chronic kidney disease | 2 (4.2) | 7 (11.7) | 0.29 |
| Chronic liver disease | 0 (0.0) | 3 (3.9) | 0.25 |
| Chronic lung disease | 2 (3.2) | 4 (5.3) | 0.69 |
| Diabetes mellitus | 7 (11.3) | 15 (19.7) | 0.24 |
| Malignancy | 2 (3.2) | 4 (5.3) | 0.69 |
| Old myocardial infarction | 1 (1.6) | 6 (7.9) | 0.13 |
| Psychiatric disease | 6 (9.7) | 13 (17.1) | 0.23 |
| Stroke | 9 (14.5) | 4 (5.3) | 0.08 |
| Anticoagulant drugs | 6 (9.7) | 3 (3.9) | 0.30 |
| Antiplatelet drugs | 6 (9.7) | 10 (13.2) | 0.60 |
| Prehospital blood transfusion | 0 (0.0) | 3 (4.1) | 0.25 |
| Prehospital tranexamic acid | 30 (49.1) | 50 (65.7) | 0.14 |
| Treatment received within 24 h upon admission, n (%) | |||
| Massive transfusion protocol | 33 (53.2) | 45 (59.2) | 0.50 |
| Emergency operation | 34 (54.8) | 46 (60.5) | 0.60 |
| Emergency angioembolization | 13 (21.0) | 36 (47.4) | 0.001* |
| Time of Hemostasis intervention, (min) | 210 [191–269] | 185 [131–248] | 0.13 |
| Time >3 h of Hemostasis intervention, n (%) | 21 (67.7) | 25 (50.0) | 0.17 |
| Tracheal intubation | 51 (82.3) | 59 (77.6) | 0.53 |
| REBOA | 3 (4.8) | 3 (3.9) | 1.00 |
| CSS dose, (g) | 50.0 [50.0–50.0] | NA | NA |
| TXA dose, (g) | 2.0 [1.0–2.0] | 2.0 [1.0–2.0] | 0.30 |
| Time of CSS dose, (min) a | 125 [90–163] | NA | NA |
| Time of TXA dose, (min) | 100 [62–153] | 73 [51–106] | 0.01* |
Note: Categorical variables are presented as counts and percentages (%), whereas numerical variables are presented as the median and interquartile ranges.
Abbreviations: ABC, assessment of blood consumption; CSS, carbazochrome sodium sulfonate; FAST, focused assessment with sonography in trauma; FDP, fibrine/fibrinogen degradation products; Ps, probability of survival; PT‐INR, prothrombin time international normalized ratio; REBOA, resuscitative endovascular balloon occlusion aorta; RTS, revised trauma score; TASH, trauma‐associated severe hemorrhage; TXA, tranexamic acid.
There were 17/62 missing data values.
Results with a p‐value of <0.05 were considered significant.
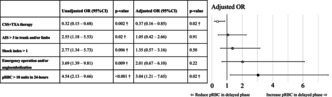
Outcome events are presented in Table 2. In the CSS + TXA group, the total volume of pRBC transfused (CSS + TXA group vs. TXA group, median [IQR]: 12 units [6, 20] vs. 17 units [12, 26], p = 0.004), pRBC transfusion in the delayed phase (2 units [0, 8] vs. 8 units [2, 12], p = 0.004), and the number of patients receiving >6 units of pRBC transfusion in the delayed phase (number [%]; 16/59 [27.1%] vs. 37/70 [52.9%], p = 0.004) decreased significantly, as shown in the univariate analyses. No significant difference was detected in the volume of pRBC transfused in the early phase, or that of plasma in each phase, or other outcomes such as mortality. Results of the univariate and multivariate logistic regression analyses for “the number of patients with >6 units of pRBC transfusion in the delayed phase” are detailed in Table 3. Only the CSS + TXA treatment had a significantly lower unadjusted OR (0.32 [95% CI: 0.15–0.68; p = 0.002]). AIS >3 in trunk and/or limbs (unadjusted OR: 2.55 [95% CI: 1.18–5.53; p = 0.02]), shock index >1 (unadjusted OR: 2.77 [95% CI: 1.34–5.73; p = 0.006]), emergency operation and/or angioembolization (unadjusted OR: 3.69 [95% CI: 1.39–9.81; p = 0.009]), and pRBC >10 units in the early phase (unadjusted OR: 4.54 [95% CI: 2.13–9.66; p < 0.001]) had significantly higher unadjusted ORs. In the multivariate logistic regression analysis, only the CSS + TXA therapy had a significantly lower adjusted OR (0.37 [95% CI: 0.16–0.85; p = 0.02]); pRBC >10 units in the early phase showed a significantly higher adjusted OR (3.04 [95% CI: 1.21–7.65; p = 0.02]).
TABLE 2.
Outcome events.
| Variables | CSS + TXA group (n = 62) | TXA group (n = 76) | p‐Value |
|---|---|---|---|
| 24‐h mortality, n (%) | 3 (4.8) | 6 (7.9) | 0.51 |
| 28‐day mortality, n (%) | 10 (16.1) | 11 (14.5) | 0.82 |
| In‐hospital total pRBC, unit | 12 [6–20] | 17 [12–26] | 0.004* |
| In‐hospital total plasma, unit | 8 [4–14] | 12 [6–21] | 0.08 |
| In‐hospital total PC, unit | 0 [0–10] | 0 [0–10] | 0.22 |
| pRBC transfusion in the early phase, unit | 8 [6–14] | 10 [6–16] | 0.08 |
| Plasma transfusion in the early phase, unit | 8 [4–12] | 10 [4–16] | 0.16 |
| pRBC transfusion in the delayed phase, unit a | 2 [0–8] | 8 [2–12] | 0.004* |
| Plasma transfusion in the delayed phase, unit a | 0 (0–0) | 0 [0–4] | 0.13 |
| Patients with >6 units of pRBC transfusion in the delayed phase, n (%) a | 16/59 (27.1) | 37/70 (52.9) | 0.004* |
| In‐hospital duration, day | 33 [15–49] | 41 [24–57] | 0.09 |
| ICU duration, day | 8 [4–13] | 10 [5–13] | 0.64 |
Note: Categorical variables are presented as counts and percentages (%), whereas numerical variables are presented as the median and interquartile ranges. The early phase is defined as “within 24 h from the time of injury,” whereas the delayed phase is defined as “after 24 h from the time of injury.”
Abbreviations: CSS, carbazochrome sodium sulfonate; ICU, intensive care unit; PC, platelet concentrate; pRBC, packed red blood cell; TXA, tranexamic acid.
The results were calculated excluding the patients who died within 24 h postinjury.
Results with a p‐value of <0.05 were considered significant.
TABLE 3.
Unadjusted and adjusted odds ratio of the number of patients with >6 units of pRBC transfusion in the delayed phase.
| Unadjusted OR (95%CI) | p‐value | Adjusted OR (95%CI) | p‐Value | |
|---|---|---|---|---|
| CSS + TXA therapy | 0.32 (0.15–0.68) | 0.002* | 0.37 (0.16–0.85) | 0.02* |
| AIS >3 in trunk and/or limbs a | 2.55 (1.18–5.53) | 0.02* | 1.05 (0.42–2.66) | 0.91 |
| Shock index >1 | 2.77 (1.34–5.73) | 0.006* | 1.35 (0.57–3.16) | 0.50 |
| Emergency operation and/or angioembolization | 3.69 (1.39–9.81) | 0.009* | 2.01 (0.67–6.10) | 0.22 |
| pRBC >10 units in 24‐h | 4.54 (2.13–9.66) | <0.001* | 3.04 (1.21–7.65) | 0.02* |
Note: The OR of the number of patients with >6 units of pRBC transfusion.
Abbreviations: AIS, abbreviated injury scale score; CI, confidence interval; CSS, carbazochrome sodium sulfonate; OR, odds ratio; pRBC, packed red blood cell; TXA, tranexamic acid.
AIS >3 means AIS = 4 or higher, excluding AIS = 1, 2, and 3.
The results are significant based on the 95% CI, p‐value of <0.05.
During admission, the incidence of major complications did not increase in the CSS + TXA group compared with that in the TXA group (Table 4). The CSS + TXA group had fewer bleeding‐related complications than the TXA group (RR: 0.31 [95% CI: 0.09–1.04]).
TABLE 4.
Complications that occurred during the in‐hospital days.
| Complications | CSS + TXA group | TXA group | RR (95% CI) |
|---|---|---|---|
| Acute kidney injury | 7/58 (12.1) | 15/71 (21.1) | 0.57 (0.25–1.31) |
| ARDS | 9/59 (15.3) | 6/70 (8.6) | 1.78 (0.67–4.71) |
| Arrhythmia | 6/58 (10.3) | 15/71 (21.1) | 0.49 (0.20–1.18) |
| Bleeding | 3/60 (5.0) | 12/74 (16.2) | 0.31 (0.09–1.04) |
| Cardiovascular diseases | 6/58 (10.3) | 7/71 (9.9) | 1.05 (0.37–2.95) |
| Delirium | 18/58 (31.0) | 31/70 (44.3) | 0.70 (0.44–1.11) |
| Infections | 29/58 (50.0) | 36/70 (51.4) | 0.97 (0.69–1.37) |
| Lung edema | 13/59 (22.0) | 21/70 (30.0) | 0.73 (0.40–1.34) |
| Stroke | 3/57 (5.3) | 4/70 (5.7) | 0.92 (0.21–3.95) |
| Venous thromboembolism | 4/57 (7.0) | 8/70 (11.4) | 0.61 (0.20–1.94) |
Note: Categorical variables are presented as counts and percentages (%).
Abbreviations: ARDS, acute respiratory distress syndrome; CSS, carbazochrome sodium sulfonate; RR, relative risk; TXA, tranexamic acid; CI, confidence interval.
The results were considered significant based on the 95% CI.
Considering the first sensitivity analysis, Table 5 presents the results of the multivariate logistic regression analysis. Similar to the results of the main analysis, only the CSS + TXA treatment had a significantly lower adjusted OR (0.29 [95% CI: 0.12–0.70; p = 0.006]), and pRBC >10 units in the early phase (adjusted OR: 2.28 [95% CI: 1.11–7.48; p = 0.03]) and AIS >2 in the head (adjusted OR: 2.02 [95% CI: 1.15–3.55; p = 0.02]) had significantly higher adjusted ORs.
TABLE 5.
Sensitivity analysis: odds ratio adding AIS >2 in head.
| Adjusted OR (95% CI) | p‐Value | |
|---|---|---|
| CSS + TXA therapy | 0.29 (0.12–0.70) | 0.006* |
| AIS >3 in trunk and/or limbs a | 1.44 (0.53–3.87) | 0.47 |
| Shock index >1 | 1.55 (0.64–3.79) | 0.33 |
| Emergency operation and/or angioembolization | 1.96 (0.63–6.14) | 0.25 |
| pRBC >10 units in 24‐h | 2.88 (1.11–7.48) | 0.03* |
| AIS >2 in head a | 2.02 (1.15–3.55) | 0.02* |
Note: OR of the number of patients with >6 units of pRBC transfusion, adding AIS >2 in the head as an independent variable.
Abbreviations: AIS, abbreviated injury scale score; CI, confidence interval; CSS, carbazochrome sodium sulfonate; OR, odds ratio; pRBC, packed red blood cell; TXA, tranexamic acid.
AIS >2 means AIS = 3 or higher, excluding AIS = 1 and 2. As same, AIS >3 means AIS = 4 or higher, excluding AIS = 1, 2, and 3.
The results are significant based on the 95% CI, p‐value of <0.05.
In the second sensitivity analysis, after excluding patients with head injuries (n = 66), 72 patients remained: 22 and 50 in the CSS + TXA and TXA groups, respectively. The results of the multivariate logistic regression analysis are described in Table 6. Similar to observations in the main analysis, only the CSS + TXA treatment had a significantly lower adjusted OR (0.10 [95% CI: 0.02–0.53; p = 0.007]), and pRBC >10 units in the early phase had a significantly higher adjusted OR (4.27 [95% CI: 1.10–16.60; p = 0.04]).
TABLE 6.
Sensitivity analysis: odds ratio in the cohort without head injury.
| Adjusted OR (95% CI) | p‐Value | |
|---|---|---|
| CSS + TXA therapy | 0.10 (0.02–0.54) | 0.007* |
| AIS >3 in trunk and/or limbs a | 0.46 (0.06–3.41) | 0.44 |
| Shock index >1 | 0.87 (0.25–3.02) | 0.83 |
| Emergency operation and/or angioembolization | 3.06 (0.31–30.10) | 0.34 |
| pRBC >10 units in 24‐h | 4.27 (1.10–16.60) | 0.04* |
Note: OR of the number of patients with >6 units of pRBC transfusion, adding AIS >2 in the head as an independent variable.
Abbreviations: AIS, abbreviated injury scale score; CI, confidence interval; CSS, carbazochrome sodium sulfonate; OR, odds ratio; pRBC, packed red blood cell; TXA, tranexamic acid.
AIS >3 means AIS = 4 or higher, excluding AIS = 1, 2, and 3.
The results are significant based on the 95% CI, p‐value of <0.05.
DISCUSSION
CSS is one of the oldest drugs used as a hemostatic agent. 4 , 5 , 6 However, in trauma medicine, only one study has reported CSS use. 6 Compared with this previous study, we only enrolled patients with trauma who required additional blood transfusions, receiving both pRBC and plasma, to rule out bias. Furthermore, to assess CSS + TXA treatment efficacy, we compared the amount of blood products transfused during the early and delayed phases. According to the multivariate logistic regression analysis, CSS + TXA treatment reduced the number of patients receiving >6 units of pRBC in the delayed phase. Thus, CSS + TXA treatment impacted the delayed phase in our cohort, in contrast to the acute phase documented in the previous study, 6 thereby supporting our hypothesis. The result of the sensitivity analysis was similar to that of the main analysis, thus highlighting the robustness of this result.
The most important effect of combined CSS + TXA administration was a reduction in the blood transfusion requirements during the delayed phase. CSS exerts hemostatic effects by reducing vascular hyperpermeability and stabilizing the function of endothelial cells, 11 , 12 , 13 which primarily affects vascular functions. As CSS reinforces endothelial cell function, bleeding incidence tended to be lower in the CSS + TXA group.
In the current study, other CSS‐related effects were expected, including a reduced incidence of complications associated with acute kidney injury, 1 , 16 acute respiratory distress syndrome, and lung edema. 1 , 2 , 17 , 18 However, the incidence of these complications was not reduced in the CSS + TXA group; this observation could be attributed to the small sample size.
CSS is one of the oldest drugs that has been used for various indications, such as in surgical settings, 7 , 8 , 10 , 19 , 20 gastrointestinal bleeding, 4 , 5 trauma, 6 pulmonary hemorrhage, 9 refractory chronic prostatitis 21 , hereditary hemorrhagic telangiectasia, 22 or dengue vascular permeability. 23 , 24 Accordingly, CSS has applications other than as a hemostatic agent. 21 , 22 , 23 , 24 It can be safely used by patients with certain medical conditions. Additionally, CSS is economically beneficial 4 ; 100 mg of CSS costs 88 Japanese Yen, which is <1 USD. 25 Accordingly, the use of this low‐cost drug could reduce the requirement for blood transfusion, making it an attractive option.
This study has some limitations. First, this was a single‐center retrospective cohort study, and there was no protocol for TXA or CSS administration in patients with trauma; this could introduce a potential selection bias. Moreover, we could not eliminate the background bias of the study population. The CSS + TXA group had a higher proportion of patients with more severe head injuries; this trend was similar to that observed previously. 6 In the sensitivity analysis, a patient cohort without head injuries was analyzed, observing the same result as that in the main analysis; however, the possibility of selection bias remains. Additionally, CSS + TXA therapy was shown to potentially reduce the requirement for acute‐phase blood transfusion 6 ; the study may have lacked patients in whom this combination therapy stopped bleeding and abolished the requirement for blood transfusion. In this study, we ensured the accurate extraction of patients with blood transfusion requirements. Although multivariate analysis was performed, the higher severity of abdominal and limb injuries may have increased the requirement for blood transfusion within 24 h postinjury. Second, the doses of CSS and TXA varied, and we were uncertain whether the observed effects on outcomes were related to the effects of this combination therapy. Hence, a prospective study is warranted. Third, the small sample size may have been insufficient to assess certain outcomes. Additionally, some treatment effects may be undetected. Fourth, the duration of CSS‐ and TXA‐mediate effects remains unknown; thus, we could not establish the observation period during which the requirement for blood transfusion would be prolonged. Fifth, there was no protocol for the administration of CSS and TXA, which may also introduce bias. Others, the time and/or kinds of way to hemostasis might potentially affect the coagulation and outcome of patients with trauma. In this study cohort, there was no statistical difference in the hemostasis time between the two groups; however, there were more patients who received urgent angioembolization in the TXA group. The difference in the kinds of hemostasis interventions and the delay of hemostasis intervention might be the risk of coagulopathy. These heterogeneities could not be eliminated in this study design and need to be addressed in future investigations.
Retrospectively, CSS with TXA may reduce the requirement for blood transfusion in patients with trauma after 24 h. This combination therapy may improve outcomes in patients with trauma without increasing the incidence of complications.
CONFLICT OF INTEREST STATEMENT
Youichi Yanagawa is an Editorial Board member of AMS Journal and a co‐author of this article. To minimize bias, they were excluded from all editorial decision‐making related to the acceptance of this article for publication. The authors declare no conflict of interest for this article.
ETHICS STATEMENT
Approval of the research protocol: The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution, and it conforms to the provisions of the Declaration of Helsinki. Committee of Faculty of Medicine, Juntendo University, Approval number: E22‐0341.
Informed consent: Patient consent was not required, and informed consent was waived by the Research Ethics Committee, Faculty of Medicine, Juntendo University, by providing a means to opt out of our institution.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
ACKNOWLEDGMENTS
This work was partly supported by a Grant‐in‐Aid for Special Research in Subsidies for the ordinary expenses of private schools from The Promotion and Mutual Aid Corporation for Private Schools of Japan. We want to thank Editage (www.editage.com) for providing excellent assistance with English‐language editing.
Nagasawa H, Omori K, Ota S, Muramatsu K‐i, Takeuchi I, Ohsaka H, et al. Carbazochrome sodium sulfonate and tranexamic acid combination therapy to reduce blood transfusions after 24 h of injury: A retrospective study. Acute Med Surg. 2024;11:e961. 10.1002/ams2.961
DATA AVAILABILITY STATEMENT
The data of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gelbard RB, Griffin RL, Reynolds L, Abraham P, Warner J, Hu P, et al. Over‐transfusion with blood for suspected hemorrhagic shock is not associated with worse clinical outcomes. Transfusion. 2022;62(Suppl 1):S177–S184. [DOI] [PubMed] [Google Scholar]
- 2. Patel SV, Kidane B, Klingel M, Parry N. Risks associated with red blood cell transfusion in the trauma population, a meta‐analysis. Injury. 2014;45:1522–1533. [DOI] [PubMed] [Google Scholar]
- 3. CRASH‐2 Trial Collaborators , Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH‐2): a randomised, placebo‐controlled trial. Lancet. 2010;376:23–32. [DOI] [PubMed] [Google Scholar]
- 4. Miyamoto Y, Ohbe H, Ishimaru M, Matsui H, Fushimi K, Yasunaga H. The effect of carbazochrome sodium sulfonate in patients with colonic diverticular bleeding: propensity score matching analyses using a nationwide inpatient database. Intern Med. 2020;59:1789–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takahashi K, Sasaki T, Ueno N, Uehara K, Kobayashi Y, Sugiyama Y, et al. Carbazochrome sodium sulfonate is not effective for prevention of post‐gastric endoscopic submucosal dissection bleeding: a retrospective study. Surg Endosc. 2022;36:7486–7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okazaki Y, Takada H, Okada I, Hasegawa E. Effect of carbazochrome sodium sulfonate in addition to tranexamic acid in bleeding trauma patients. Cureus. 2022;14:e22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luo Y, Zhao X, Releken Y, Yang Z, Pei F, Kang P. Hemostatic and anti‐inflammatory effects of carbazochrome sodium sulfonate in patients undergoing total knee arthroplasty: a randomized controlled trial. J Arthroplast. 2020;35:61–68. [DOI] [PubMed] [Google Scholar]
- 8. Luo Y, Releken Y, Yang D, Yue Y, Liu Z, Kang P. Effects of carbazochrome sodium sulfonate combined with tranexamic acid on hemostasis and inflammation during perioperative period of total hip arthroplasty: a randomized controlled trial. Orthop Traumatol Surg Res. 2022;108:103092. [DOI] [PubMed] [Google Scholar]
- 9. Perez‐Moreno CI, Couëtil LL, Pratt SM, Ochoa‐Acuña HG, Raskin RE, Russell MA. Effect of furosemide and furosemide‐carbazochrome combination on exercise‐induced pulmonary hemorrhage in Standardbred racehorses. Can Vet J. 2009;50:821–827. [PMC free article] [PubMed] [Google Scholar]
- 10. Dykes ER, Anderson R. Carbazochrome salicylate as a systematic hemostatic agent in plastic operations. A clinical evaluation. JAMA. 1961;177:716–717. [DOI] [PubMed] [Google Scholar]
- 11. Matsumoto Y, Hayashi T, Hayakawa Y, Shinbo M, Niiya K, Sakuragawa N. Carbazochrome sodium sulphonate (AC‐17) decreases the accumulation of tissue‐type plasminogen activator in culture medium of human umbilical vein endothelial cells. Blood Coagul Fibrinolysis. 1995;6:233–238. [DOI] [PubMed] [Google Scholar]
- 12. Sendo T, Goromaru T, Aki K, Sakai N, Itoh Y, Oishi R. Carbazochrome attenuates pulmonary dysfunction induced by a radiographic contrast medium in rats. Eur J Pharmacol. 2002;450:203–208. [DOI] [PubMed] [Google Scholar]
- 13. Sendo T, Itoh Y, Aki K, Oka M, Oishi R. Carbazochrome sodium sulfonate (AC‐17) reverses endothelial barrier dysfunction through inhibition of phosphatidylinositol hydrolysis in cultured porcine endothelial cells. Naunyn Schmiedeberg's Arch Pharmacol. 2003;368:175–180. [DOI] [PubMed] [Google Scholar]
- 14. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 15. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shashaty MG, Meyer NJ, Localio AR, et al. African American race, obesity, and blood product transfusion are risk factors for acute kidney injury in critically ill trauma patients. J Crit Care. 2012;27:496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674. [DOI] [PubMed] [Google Scholar]
- 18. Chaiwat O, Lang JD, Vavilala MS, Wang J, MacKenzie EJ, Jurkovich GJ, et al. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology. 2009;110:351–360. [DOI] [PubMed] [Google Scholar]
- 19. Basile M, Gidaro S, Pacella M, Biffignandi PM, Gidaro GS. Parenteral troxerutin and carbazochrome combination in the treatment of post‐hemorrhoidectomy status: a randomized, double‐blind, placebo‐controlled, phase IV study. Curr Med Res Opin. 2001;17:256–261. [PubMed] [Google Scholar]
- 20. Squadrito F, Altavilla D, Oliaro Bosso S. Double‐blind, randomized clinical trial of troxerutin‐carbazochrome in patients with hemorrhoids. Eur Rev Med Pharmacol Sci. 2000;4:21–24. [PubMed] [Google Scholar]
- 21. Oh‐oka H, Yamada T, Noto H, Umeyama T, Kadekawa K, Ashitomi K, et al. Effect of carbazochrome sodium sulfonate on refractory chronic prostatitis. Int J Urol. 2014;21:1162–1166. [DOI] [PubMed] [Google Scholar]
- 22. Passali GC, De Corso E, Bastanza G, Di Gennaro L, HHT Gemelli Study Group . An old drug for a new application: carbazochrome‐sodium‐sulfonate in HHT. J Clin Pharmacol. 2015;55:601–602. [DOI] [PubMed] [Google Scholar]
- 23. Tassniyom S, Vasanawathana S, Dhiensiri T, Nisalak A, Chirawatkul A. Failure of carbazochrome sodium sulfonate (AC‐17) to prevent dengue vascular permeability or shock: a randomized, controlled trial. J Pediatr. 1997;131:525–528. [DOI] [PubMed] [Google Scholar]
- 24. Funahara Y, Sumarmo SA, Shirahata A, Harun SR, Setiabudy‐Dharma R, Nathin MA, et al. Protection against marked plasma leakage in dengue haemorrhagic fever by infusion of carbazochrome sodium sulfonate (AC‐17). Southeast Asian J Trop Med Public Health. 1987;18:356–361. [PubMed] [Google Scholar]
- 25. Japan Pharmaceutical Information Center . https://pins.japic.or.jp/pdf/newPINS/00050630.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study are available from the corresponding author upon reasonable request.


