Abstract
Neuronal ensembles in the medial prefrontal cortex mediate cocaine self‐administration via projections to the nucleus accumbens. We have recently shown that neuronal ensembles in the prelimbic cortex form rapidly to mediate cocaine self‐administration. However, the role of neuronal ensembles within the nucleus accumbens in initial cocaine‐seeking behaviour remains unknown. Here, we sought to expand the current literature by testing the necessity of the cocaine self‐administration ensemble in the nucleus accumbens core (NAcCore) 1 day after male and female rats acquire cocaine self‐administration by using the Daun02 inactivation procedure. We found that disrupting the NAcCore ensembles after a no‐cocaine reward‐seeking test increased subsequent cocaine seeking, while disrupting NAcCore ensembles following a cocaine self‐administration session decreased subsequent cocaine seeking. We then characterized neuronal cell type in the NAcCore using RNAscope in situ hybridization. In the no‐cocaine session, we saw reduced dopamine D1 type neuronal activation, while in the cocaine self‐administration session, we found preferential dopamine D1 type neuronal activity in the NAcCore.
Keywords: acquisition, addiction, learning and memory, reinforcement, self‐administration

1. INTRODUCTION
Substance use disorder (SUD) involves aberrant learning and memory processes. 1 , 2 Within circuits mediating cocaine seeking and reinstatement, neuronal ensembles encode learned associations about cocaine and cocaine‐related cues. Neuronal ensembles are groups of neurons that become synaptically connected through repeated synchronous activation and mediate learned behaviour. Technological advances have made it possible to probe the behavioural relevance of these neuronal ensembles using tools that are dependent on immediate early gene (IEG) expression (e.g., Fos, arc and zif268). 1 , 2
Neuronal ensembles play a functional role in reward seeking, fear learning and spatial navigation. 3 , 4 , 5 , 6 , 7 We recently found that cortical ensembles become behaviourally relevant soon after behavioural acquisition of food, oxycodone and cocaine self‐administration. 8 , 9 , 10 Furthermore, in vivo electrophysiology data support these findings by correlating functional cortical ensemble activity with behavioural output in a Pavlovian learning task. 11 Additionally, calcium imaging studies show a correlation between cortical ensemble activation and cue presentation. 12 Although the literature suggesting when and how cortical ensembles become behaviourally relevant is growing, this process is less clear in the nucleus accumbens core (NAcCore).
Dense reciprocal connections between the medial prefrontal cortex (mPFC) and nucleus accumbens (NAc) play a cooperative role in reward learning. Additionally, NAc ensembles mediate cocaine seeking, conditioned place preference and behavioural sensitization 3 , 13 , 14 ; however, the role of ensembles and how rapidly they form in the NAc after acquisition of motivated operant behaviour is not well understood. We sought to expand this literature by testing if neuronal ensembles in the NAcCore mediate cocaine seeking 1 day after behavioural acquisition, using the Daun02 inactivation procedure. 3 , 10 Additionally, we assess whether the presence of drug reinforcers on induction day changes the outcome of the Daun02 inactivation method in the NAcCore. Finally, we characterize NAc neuronal ensembles activated by reinforced or non‐reinforced cocaine‐seeking after initial acquisition using RNAscope in situ hybridization.
2. EXPERIMENTAL PROCEDURES
2.1. Subjects
We used adult male and female Sprague Dawley wild‐type rats (n = 20) as well as male and female Fos‐LacZ rats 15 (n = 63) with starting weights between 217 and 501 g. We kept rats single housed under a 12 h reverse light–dark cycle (lights off at 10:00 am). Rats had ad libitum access to water throughout the duration of the experiments. One day prior to the beginning of behavioural testing we restricted rats' food to 10–20 g of chow daily and fed them after cocaine self‐administration training each day. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida.
Four rats exhibited stereotypy on the inactive lever on the acquisition day in Experiments 1 and 2. These values were Windsor corrected because their behaviour was otherwise normal, and this did not change the overall interpretation of the experiments. Two significant outliers (Grubb's test) were removed from the %FosD1 and %FosD2 analysis in Experiment 3. This did not change the overall interpretation of the findings. Eleven Fos‐LacZ rats failed to meet the acquisition criteria, and 12 Fos‐LacZ rats had cannula placement outside the NAcCore and were excluded from analysis. Four Fos‐LacZ rats were excluded from Experiments 1 and 2 as significant outliers in active lever presses on induction day or test day (Grubb's test). We excluded seven Fos‐LacZ rats from c‐Fos quantification in Experiments 1 and 2 due to poor staining but included them in the behavioural analysis.
2.2. Surgery
We anaesthetised rats with 3% isoflurane for intracranial guide cannula implantation and jugular catheter implantation. Prior to surgery rats were administered buprenorphine (0.03 mg/kg sc) and meloxicam (5 mg/kg sc) as well as for 3 days post‐surgery. Rats had ad libitum access to chow and water for 4 days after surgery prior to cocaine self‐administration training.
2.3. Intracranial guide cannula implantation
We implanted guide cannula (23‐gauge, Plastics One) bilaterally 1 mm above the NAcCore. The nose bar was set at −3.3 mm, and the coordinates used for the NAcCore were anteroposterior: 1.6 mm, mediolateral: 2.5 mm and dorsoventral: −5.5 mm (10° angle). We used acrylic dental cement and jeweller's screws to create headcaps that secured cannula placement. 9 , 10 , 16
2.4. Intracranial infusions
We used 10 μL Hamilton syringes and a syringe pump (Kent Scientific, Torrington, CT, USA) with polyethylene‐50 tubing connected to 30‐gauge injectors (RWD Life Science, Guangdong, China) that extended 1 mm below the guide cannula to bilaterally infuse 2 μg/0.5 μL/side of Daun02 or sterile 1× PBS over 60 s into the NAcCore 90 min after the beginning of the induction session. Microinfusers were left in place for 60 s to allow diffusion of the Daun02.
2.5. Intravenous catheter implantation
We implanted custom‐built SILASTIC catheters into the jugular vein as described previously. 10 , 16 Jugular catheters are attached to a 22‐gauge cannula and plastic mesh using acrylic dental cement. This cannula protrudes from the midscapular region of the back. We flushed rats daily with 0.2 mL gentamicin in sterile saline (4.25 mg/mL; APP Pharmaceuticals).
2.6. Cocaine self‐administration
We allowed rats 4 days to recover from surgery before behavioural training. Rats trained in Med Associates operant boxes equipped with a house light and two retractable levers that extend at the beginning of each session. Active lever responses resulted in a brief presentation of an auditory and light cue and an infusion of cocaine (0.75 mg/kg/0.1 mL per infusion) on a FR1 schedule. We applied a 10 s timeout after each infusion, during which no further infusions could be earned. Each training session lasted for 3 h and continued for 1–9 days, depending on when individual rats reached our acquisition criteria (defined as ≥30 active lever responses and ≥70% active over inactive lever presses within a single session). We chose this criterion to avoid floor effects on test day, to demonstrate discrimination between the active and inactive levers, and for consistency with previous reports. 9 , 10 , 17 , 18
2.7. Drugs
We used cocaine hydrochloride provided by NIDA's Drug Supply Program. We obtained Daun02 from Medchem Express and dissolved it in a vehicle containing 5% DMSO, 6% Tween 80 and 89% 0.01 M PBS. 4 , 13 , 19
2.8. Induction session
Twenty‐four hours after rats met our acquisition criteria, we placed them in a 30 min no‐cocaine‐seeking session (Experiment 1, cues only) or a 30 min cocaine reward self‐administration session (Experiment 2, cues and cocaine) to induce c‐Fos and ‐Gal in neurons activated during the session. Sixty minutes after the induction session, we bilaterally infused Daun02 or vehicle directly into the NAcCore through implanted guide cannula. These sessions lasted 30 min. The rats remain in the operant boxes for an additional 60 min for Fos and β‐Gal expression to peak prior to Daun02 infusion (Experiments 1 and 2). In Experiment 3, we assigned rats to one of three induction conditions: (1) home cage controls were euthanized directly from their home cages, (2) rats in the no‐cocaine group underwent a 30 min no‐cocaine reward‐seeking session and (3) rats in the cocaine group underwent a 30 min cocaine self‐administration session. Thirty minutes after the beginning of the induction session, we decapitated the rats for RNAscope.
2.9. Cocaine‐seeking test
In Experiments 1 and 2, rats underwent a 30 min no‐cocaine reward‐seeking test 48 h after the induction session. Responses on the active lever resulted in cocaine‐paired cues but not cocaine infusions. Rats were euthanized 90 min after the start of the session for immunohistochemistry.
2.10. Perfusion and c‐Fos immunohistochemistry
In Experiments 1 and 2, we performed immunohistochemistry to quantify and visualize Fos+ nuclei, as previously described. 9 Briefly, we deeply anaesthetised rats with isoflurane and transcardially perfused them with PBS followed by 4% PFA. We cryoprotected and froze brains at −80°C until sectioning. We sectioned the brains into 40 μm slices and washed the brains in PBS. We then blocked the slices with 3% NGS in PBS and 0.25% Triton X‐100 for 1 h and incubated the slices with phospho‐c‐Fos (Ser32) antibody (1:2000 Dilution; Cell Signaling Technology, Danvers, MA; Ref#5348S, RRID:AB 10557109), followed by Alexa Fluor 488 conjugated goat anti‐rabbit secondary antibody for 2 h (1:500 dilution; Invitrogen, Waltham, MA; Reference#A11008). We washed and mounted the sections onto polysine precoated microscope slides before cover slipping with DAPI Fluoromount‐G (Southern Biotech, Birmingham, AL).
We used a Keyence BZ‐X810 microscope at 200X magnification and BZ‐X Analyzer software to capture fluorescent images. Three random coronal sections from each rat were quantified and averaged by blind observers using ImageJ and Image‐based Tool for Counting Nuclei plugin (ITCN) to automatically count Fos+ nuclei.
2.11. RNAscope in situ hybridization
To characterize neuronal cell types in the NAcCore, we performed RNAscope in situ hybridization (Experiment 3). Rats were briefly anaesthetised with isoflurane and decapitated. We extracted and froze their brains in −40°C 2‐methylbutane (Thermo Fisher Scientific, Waltham, MA), then stored the brains at −80°C until sectioning. We took 14 μm coronal sections and placed them directly onto polysine slide adhesion microscope slides (Epredia, Kalamazoo, MI; Reference#P4981‐001) and stored slides at −80°C until use. We used the RNAscope Multiplex Fluorescent Reagent Kit (Advanced Cell Diagnostics, Newark, CA) according to the manufacturer's instructions and as described previously, 20 , 21 using the appropriate probes (Probe‐Rn‐Fos, Ref#403591; Probe‐Rn‐Drd1a‐C2, Ref#317031‐C2; Probe‐Rn‐Drd2‐C3, Ref#315641‐C3). We labelled c‐Fos with Opal 520 (1:1500; Akoya Biosciences; Marlborough, MA; FP1487001KT), Drd1 with Opal 570 (1:1500; Akoya Biosciences, Marlborough, MA; FP1488001KT) and Drd2 with Opal 690 (1:1000; Akoya Biosciences, Marlborough, MA; FP1497001KT). We coverslipped slides using ProLong Gold antifade with DAPI (Invitrogen, Waltham, MA; Ref#P36931).
We imaged slides on a Keyence BZ‐X810 microscope at 200× magnification and used BZ‐X Analyzer software to capture fluorescent images. Three random images were chosen and quantified from each rat, and the results were averaged and counted as n = 1. We used ImageJ and ITCN to automatically quantify c‐fos+, Drd1+ and Drd2+ mRNA separately and subsequently manually counted overlap of c‐fos and Ddrd1 or c‐fos and Drd2 mRNA. Observers (B.S. and S.P.) blind to test conditions quantified images and reached a Person correlation of 0.74.
2.12. Statistical analysis
We used between‐subjects t test, between‐subjects one‐way ANOVA and two‐way mixed ANOVA when appropriate. We ran subsequent Tukey or Bonferroni post hoc analysis when appropriate. Additionally, we used Pearson's correlation in Experiment 3. Outliers were determined by Grubb's test.
3. RESULTS
3.1. Experiment 1: No‐cocaine reward Daun02 inactivation increases cocaine seeking
The goal of Experiment 1 was to determine the role of c‐Fos‐expressing NAcCore ensembles activated during acquisition of cocaine self‐administration in mediating subsequent cocaine seeking. The experiment followed a two‐way mixed design, with two factors: induction day drug (Vehicle × Daun02) and lever (Active × Inactive). We used 32 male and female rats (eight male and eight females per group). We hypothesized that behaviourally relevant ensembles in the NAcCore are recruited and mediate cocaine seeking after behavioural acquisition and predicted NAcCore Daun02 administration would result in ablation of ensembles that mediate cocaine seeking, thus decreasing cocaine seeking in a subsequent no‐cocaine reward‐seeking test relative to vehicle controls.
Figure 1A shows the timeline for Experiment 1. Rats took an average of 2.3 days to reach the acquisition criteria. On the acquisition day, there were no significant differences between the groups in total infusions earned (t (30) = 0.000, p > 0.99) (Figure 1B). A two‐way mixed ANOVA showed no significant interaction between induction day drug and lever (F (1,30) = 0.3287, p = 0.57), a main effect of lever (F (1,30) = 35.07, p < 0.01) and no main effect of induction day drug (F (1,30) = 0.0228, p = 0.88), indicating that on the acquisition day regardless of group assignment, all rats received equivalent cocaine infusions and discriminated between the active and inactive levers (Figure 1C). Vehicle and Daun02 groups did not differ in total training days (t (30) = 1.38, p = 0.18) (Figure 1D) or infusions earned across acquisition days (t (30) = 1.05, p = 0.30) (Figure 1E).
FIGURE 1.

No‐cocaine reward induction session Daun02 inactivation increases subsequent cocaine seeking. (A) Experimental timeline. (B) Total acquisition day infusions between the groups. (C) Total active lever and inactive lever responses on the day of self‐administration acquisition. * indicates a main effect of lever, both groups press the active lever more than the inactive lever on acquisition day, p < 0.05. (D) Total training days across acquisition training. (E) Total cocaine infusions earned across acquisition training by group. (F) Total active and inactive lever responses by group on the induction day. * indicates a main effect of lever, both groups press the active lever significantly more than the inactive lever on the induction day, p < 0.05. (G) Total active and inactive lever responses by group on the test day. * indicates the Daun02 group pressed the active lever significantly more than the vehicle group, p < 0.05. (H) Test day active lever by group and sex. * indicates a main effect of induction day drug, both male and female rats in the Daun02 condition pressed the active lever significantly more than their vehicle controls, p < 0.05. (I) Fos expression by group elicited on test day and representative images of c‐Fos immunohistochemistry. (J) Representative image of cannula placement. Scale bars represent 100 μm. Hollow symbols represent males, while filled in symbols represent females. All data presented as mean ± SEM (n = 15 and 16 per group).
On induction day, a two‐way mixed ANOVA showed no significant interaction between induction day drug and lever (F (1,30) = 0.5656, p = 0.46), a main effect of lever (F (1,30) = 32.67, p < 0.01), but no main effect of induction day drug (F (1,30) = 0.35, p = 0.55). Indicating that prior to vehicle or Daun02, rats did not differ in their induction day behaviour (Figure 1F).
On test day, a two‐way mixed ANOVA revealed a significant interaction between induction day drug and lever (F (1,30) = 11.44, p < 0.01), a main effect of lever (F (1,30) = 31.97, p < 0.01) and no main effect of induction day drug (F (1,30) = 3.304, p = 0.08). A Bonferroni post hoc analysis revealed rats that received Daun02 on induction day pressed the active lever significantly more than vehicle controls (p < 0.01) and more than the inactive lever (p < 0.01) (Figure 1G). A two‐way between‐subjects ANOVA (Sex × Drug) found no significant interaction (F (1,28) = 0.1387, p = 0.71), no main effect of sex (F (1,28) = 0.0077, p = 0.93), with a main effect of drug (F (1,28) = 7.143, p = 0.01), indicating that Daun02 administration on induction day increased active lever presses compared with vehicle controls regardless of sex (Figure 1H). Finally, total c‐Fos expression did not differ between the vehicle and Daun02 groups (Figure 1I) (t (29) = 0.06, p = 0.95).
3.2. Experiment 2: Daun02 inactivation after cocaine self‐administration decreases cocaine seeking
Contrary to our initial prediction, we found that ensemble ablation following an induction session without available cocaine increased test day cocaine seeking. To determine whether withholding cocaine influenced our results, we replicated Experiment 1 but included cocaine reinforcement during the induction. Cocaine was withheld on test day, as in Experiment 1. We followed a two‐way mixed design: induction day drug (Vehicle × Daun02) and lever (Active × Inactive). We used 31 male and female rats (nine male and seven females in the Daun02 condition and nine male and six female in the vehicle condition). We hypothesized that the cocaine‐seeking NAcCore ensemble requires cocaine reinforcement for reactivation. We predicted that NAcCore Daun02 administration after a cocaine self‐administration session would decrease cocaine seeking in the subsequent seeking test.
Figure 2A shows the timeline for Experiment 2. Rats took an average of 4.2 days to reach acquisition criteria. There were no significant differences in total infusions earned (t (29) = 0.74, p = 0.46) on each rat's acquisition day (Figure 2B). A two‐way mixed ANOVA showed no significant interaction (F (1,29) = 0.14, p = 0.71), a main effect of lever (F (1,29) = 154.4, p < 0.01) and no main effect of induction day drug (F (1,29) = 40, p = 0.65) (Figure 2C), indicating that on the acquisition day regardless of group assignment, all rats received equivalent cocaine infusions and discriminated between the active and inactive levers. There were no significant differences between the vehicle or Daun02 groups in total training days (t (29) = 0.21, p = 0.83) (Figure 2D) or in total infusions earned across acquisition days (t (29) = 0.16, p = 0.87) (Figure 2E).
FIGURE 2.

Reinforced induction day Daun02 inactivation decreases subsequent cocaine seeking. (A) Experimental timeline. (B) Total acquisition day infusions between the groups. (C) Total active lever and inactive lever responses on the day of self‐administration acquisition. (D) Total training days across acquisition training. (E) Total cocaine infusions earned across acquisition training. (F) Total induction day infusions. (G) Total active and inactive lever responses by group on the induction day. Rats in the vehicle and Daun02 condition did not press differently from each other in total active lever responses. * indicates a main effect of lever, both groups pressed the active lever significantly more than the inactive lever, p < 0.05. Hollow symbols represent males, while filled in symbols represent females. All data presented as mean ± SEM (n = 15 and 16 per group).
On induction day, there were no significant differences between the vehicle or Daun02 groups in infusions earned (t (30) = 0.76, p = 0.45) (Figure 2F). A two‐way mixed ANOVA showed a significant interaction between induction day drug and lever (F (1,29) = 4.780, p = 0.04), a main effect of lever (F (1,29) = 16.33, p < 0.01) and no main effect of induction day drug (F (1,29) = 0.03, p = 0.85) (Figure 2G). A Bonferroni post hoc analysis revealed no significant difference between the vehicle or Daun02 condition in total active lever responses (p = 0.45). Post hoc analysis revealed that rats in the Daun02 group failed to discriminate between the active and inactive lever (p = 0.39). Importantly, prior to test day, rats in both groups pressed the active lever similarly in both the acquisition day when they are assigned to the group and prior to administration of Daun02 following the induction session. Together, this suggests that the interaction is likely an artefact of lower lever presses potentially because rats titrated their cocaine dose during the brief 30 min session.
On the test day, a two‐way mixed ANOVA revealed a significant interaction between induction day drug and lever (F (1,29) = 8.49, p < 0.01), a main effect of lever (F (1,29) = 105.0, p < 0.01) and no main effect of drug (F (1,29) = 1.6, p = 0.22). A Bonferroni post hoc revealed the interaction was driven by a decrease in active lever presses in the Daun02 condition relative to the vehicle condition (p = 0.04), while both groups discriminated between the active and inactive lever (p < 0.01) (Figure 3A). To determine sex differences in cocaine seeking after Daun02 ablation, we ran a two‐way between‐subjects ANOVA (Sex × Drug) and found no significant interaction (F (1,27) = 0.12, p = 0.73), no main effect of sex (F (1,27) = 0.18, p = 0.67) and a main effect of drug (F (1,27) = 4.26, p < 0.05) (Figure 3B), indicating that Daun02 administration on induction day decreased active lever pressing in the Daun02 condition relative to vehicle controls regardless of sex. There were no significant differences between total c‐Fos expression between the groups (t (23) = 0.89, p = 0.38) (Figure 3C).
FIGURE 3.
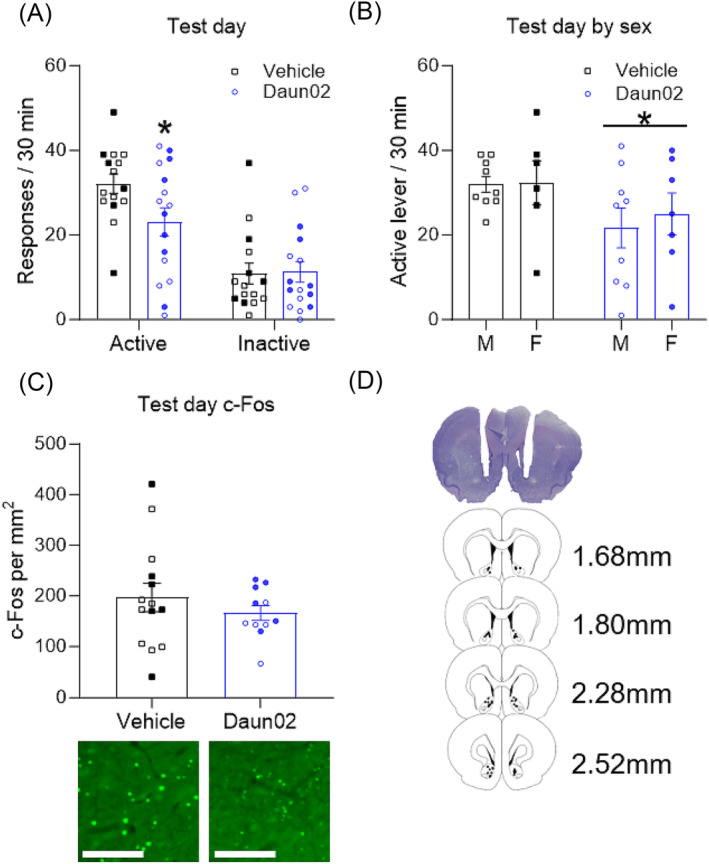
(A) Total active and inactive lever responses by group on the test day. * indicates the Daun02 condition pressed the active lever significantly fewer times than the vehicle group, p < 0.05. (B) Test day active lever by group and sex. * indicates a main effect of induction day drug, both male and female rats in the Daun02 condition pressed the active lever significantly more than their vehicle controls, p < 0.05. (C) Test day Fos expression by group elicited on test day, and representative images of Fos immunohistochemistry. (D) Representative image of cannula placement. Scale bars represent 100 μm. Hollow symbols represent males, while filled in symbols represent females. All data presented as mean ± SEM (n = 15 and 16 per group).
3.3. Experiment 3: Cocaine preferentially recruits D1MSN ensembles while cocaine seeking without cocaine reinforcement preferentially recruits D2MSN ensembles
Due to the results of Experiments 1 and 2, we sought to investigate the cellular phenotype potentially driving these results by using RNAscope in situ hybridization. We replicated Experiments 1 and 2 and added an additional home cage control group for a total of three groups: home cage controls, no‐cocaine on induction day and cocaine on induction day. For the acquisition day data, we used a two by three mixed design, with the factors being lever (Active lever × Inactive lever) and induction day group (home cage, no cocaine and cocaine). We used 20 male and female rats (three to four males and two to four females per group).
Figure 4A shows the timeline for Experiment 3. Rats took an average of 3.3 days to reach acquisition criteria. A one‐way between‐subjects ANOVA revealed no significant difference in total infusions earned on acquisition day (F (2,17) = 1.0, p = 0.39) (Figure 4B). A two‐way mixed ANOVA showed no significant interaction between lever and group (F (2,17) = 0.68, p = 0.52), a main effect of lever (F (1,17) = 49.48, p < 0.01) and no main effect of group (F (2,17) = 0.28, p = 0.76) (Figure 3C). Additionally, there were no significant differences in total infusions earned across training (F (2,17) = 0.01, p = 0.99) (Figure 3D) or in the number of total training days between the groups (F (2,17) = 0.43, p = 0.66) (Figure 3E). On induction day, rats in the cocaine condition earned an average of 9.4 cocaine infusions (Figure 4F). A two‐way between‐subjects ANOVA showed no interaction between lever and group (F (1,12) = 1.419, p = 0.26), no main effect of lever (F (1,12) = 0.08, p = 0.78) and no main effect of group (F (1,12) < 0.001, p = 0.98) (Figure 4G).
FIGURE 4.

RNA scope labelling of non‐reinforced or reinforced cocaine seeking ensembles post‐acquisition in the NAcCore. (A) Experimental timeline. (B) Acquisition day infusion by group. (C) Total active lever and inactive lever responses on the day of self‐administration acquisition. * indicates a main effect of lever, all rats pressed the active lever significantly more than the inactive lever on the acquisition day, p < 0.05. (D) Total cocaine infusions earned across acquisition training by group. (E) Total training days across acquisition training by group. (F) Total infusions earned in the cocaine group on induction day. (G) Total active and inactive lever responses by group on induction day. Hollow symbols represent males, while filled in symbols represent females. All data presented as mean ± SEM (n = 6 and 7 per group).
There were no significant differences between the groups in number of c‐fos+ neurons in the NAcCore (F (2,17) = 2.98, p = 0.07) (Figure 5B), total Drd1 positive cells (F (2,17) = 0.86, p = 0.44) (Figure 5C) or total Drd2 positive cells (F (2,17) = 0.45, p = 0.64) (Figure 5D). A one‐way between‐subjects ANOVA revealed a significant effect of group on total colabelled c‐fos and Drd1 cells per mm2 (F (2,17) = 14.59, p < 0.01). A Tukey post hoc analysis revealed significantly higher c‐fos and Drd1 colabelling per mm2 in the cocaine group compared with the home cage controls (p < 0.01), as well as the no‐cocaine group (p < 0.01), with no significant differences between the home cage controls and the no‐cocaine group (p = 0.99) (Figure 5E). A one‐way ANOVA also revealed a significant effect of group on total colabelled c‐fos and Drd2 cells per mm2 (F (2,17) = 10.90, p < 0.01), a Tukey post hoc analyses revealed significantly higher c‐fos and Drd2 colabelling per mm2 in the home cage controls compared with the cocaine group (p = 0.03), as well as higher c‐Fos and Drd2 colabelling per mm2 in the no‐cocaine group compared with the cocaine group (p < 0.01), with no significant differences between the home cage controls and the no‐cocaine group (p = 0.28) (Figure 5F).
FIGURE 5.

RNA scope labelling of home cage control (HC), no‐cocaine reward‐seeking induction session (NC) or cocaine self‐administration induction session (C) ensembles post‐acquisition in the NAcCore. (A) Representative RNA scope images used for quantification. Panels on the far left are composite images, c‐Fos RNA is represented as green, D1 is represented as magenta and D2 is represented as white. Arrows point to c‐Fos positive neurons that overlap with either D1‐ or D2‐expressing neurons across all panels. The D1/D2 cells that are pointed to are the cells that can be seen overlapping with the c‐Fos+ cells in the composite image. (B) Total c‐fos+ cells per mm2 by group. (C) Total D1dr+ cells per mm2 by group. (D) Total D2dr+ cells per mm2 by group. (E) Total colabelled c‐fos+ and D1dr+ cells per mm2 by group, *p < 0.05 compared with no‐cocaine group and HC controls. (F) Total colabelled c‐Fos+ cells and D2dr+ cells per mm2 by group, *p < 0.05 compared with no‐cocaine group and HC controls. (G) Percentage of c‐fos+ neurons that are either D1+/D2+ or other. Hollow symbols represent males, while filled in symbols represent females. Scale bars represent 100 μm. All data presented as mean ± SEM (n = 6 and 7 per group).
Although we did not reach significance in c‐fos expression between the groups (Figure 5B), it was trending towards significance (p = 0.07). Therefore, we also ran between‐subjects ANOVA on the %c‐Fos‐expressing D1 and %c‐Fos‐expressing D2 to control for potential differences in c‐fos that may bias the prior analysis. A one‐way between‐subjects ANOVA revealed a significant effect of group on the total percentage of c‐Fos/D1dr labelled cells (F (2,16) = 20.62, p < 0.01), a Tukey post hoc analyses revealed significantly higher percentage of c‐fos positive cells expressing D1dr RNA in the cocaine group compared with the no‐cocaine group (p < 0.01) and the home cage controls (p < 0.01), but no difference between the home cage and no‐cocaine group (p = 0.98). A one‐way between‐subjects ANOVA revealed a significant effect of group on the total percentage of c‐fos/D2dr labelled cells (F (2,16) = 24.71, p ≤ 0.01), a Tukey post hoc analyses revealed significantly lower percentage of c‐fos positive cells expressing D2dr RNA in the cocaine group compared with the no‐cocaine group (p < 0.01) and the home cage controls (p < 0.01), but no difference between the home cage and no‐cocaine groups (p = 0.23).
Finally, we looked at the Pearson correlation between cocaine self‐administration and the D1 ensemble and found a strong positive relationship r 2 = 0.63, p = 0.03 in the cocaine group (Figure 6A), but not in the no‐cocaine group r 2 = 0.08, p = 0.55 (Figure 6B). Additionally, we did not see a significant correlation in cocaine seeking and the D2 ensemble in the cocaine group r 2 = 0.00, p = 0.99 (Figure 6C), or in the no‐cocaine group r 2 = 0.02, p = 0.75 (Figure 6D), indicating that only the D1 ensemble was associated with cocaine self‐administration behaviour.
FIGURE 6.

(A) Correlation of the D1 ensemble and test day active lever presses in the cocaine group. (B) Correlation of the D1 ensemble and test day active lever presses in the no‐cocaine group. (C) Correlation of the D2 ensemble and test day active lever presses in the cocaine group. (D) Correlation of the D2 ensemble and test day active lever presses in the no‐cocaine group.
4. DISCUSSION
Here, we show that 24 h after rats acquire cocaine self‐administration, the NAcCore holds neuronal ensembles that mediate cocaine‐related behaviour. We found that under extinction conditions, Daun02 ablation of NAcCore ensembles increased cocaine seeking in a subsequent test of cocaine seeking without contingent cocaine delivery (no‐cocaine reward, Figure 1G). On the other hand, Daun02 ablation following cocaine self‐administration decreased subsequent cocaine‐seeking behaviour (cocaine reward, Figure 3A). We then used RNAscope to characterize the cellular phenotype of c‐Fos‐expressing neurons after a test in which lever presses either did or did not result in cocaine delivery. We found that in the no‐cocaine reward group, D1 ensembles had decreased c‐Fos expression, while during cocaine reward group, c‐Fos expression was increased in D1 neurons (Figure 5D–H). Finally, we found that D1 ensemble activity was strongly correlated with active lever pressing in the cocaine reward group (Figure 6A).
4.1. Cocaine self‐administration, extinction and the role of corticolimbic circuitry
We have previously shown that prelimbic cortex (PL) neuronal ensembles mediate cocaine seeking rapidly after acquisition of cocaine self‐administration. 10 Given the large body of work showing a role for the PL to NAcCore circuit in promoting cocaine seeking during cue‐, drug‐ or stress‐induced reinstatement, 22 , 23 , 24 , 25 , 26 , 27 , 28 we sought to extend our findings to the NAcCore. The chief question under investigation here is how early do neuronal ensembles in NAcCore mediate cocaine self‐administration? To achieve this goal, we began our manipulation immediately after the session in which the rat meets acquisition criteria. While this experimental design uniquely accounts for individual differences in rodent acquisition rates, it is also shorter than most self‐administration procedures. Furthermore, we do not pretrain the rats to self‐administer food, then switch the reinforcer to cocaine, which is commonly used to enhance training. 25 , 26 , 28 Finally, we did not intend to extinguish the behaviour and assess reinstatement as our dependent measure. 22 , 23 , 25 , 27 , 29 These differences in our design enable us to assess how quickly these circuits are recruited, if extinction is necessary for their recruitment, and what role they play early in the learning process. Despite these methodological differences, the results of Experiment 2 were not surprising. Fundamentally, manipulating the NAcCore and observing a deficit in drug‐seeking behaviour have been seen with multiple investigations and methodologies. 24 , 25 , 26 , 28 , 30 , 31 Our findings extend this literature by showing the NAcCore is relevant to cocaine‐seeking behaviour absent extended self‐administration training or extinction training.
While we interpret our findings as demonstrating a deficit in recall after neuronal ensembles ablation, it is not entirely clear whether the deficit we observed was due to a change in recall or some other psychological or motivational state. Indeed, others have shown that rats can acquire cocaine self‐administration even with exocytotic lesions of the NAcCore under a continuous reinforcement schedule, 32 suggesting that the NAcCore may not be necessary for encoding an outcome‐based operant task under a primary reinforcement regiment. Instead, lesioning resulted in deficits under a second order schedule of reinforcement. 32 In other words, conditioned reinforcers (i.e., cocaine associated cues) lost some of their ability to drive and control the drug associated behaviour. Our findings, therefore, may indicate that the NAcCore acquisition ensemble is responsible for the associations between the reinforcer and the cues. 32 , 33
The results of Experiment 1, on the other hand, are more surprising. Although prior studies typically find decreased responding when the NAcCore is compromised, we are not the first to show an increase in seeking following damage to the NAcCore 32 during acquisition. Additionally, lesioning the NAcCore can increase drug‐related locomotor behaviour 32 , 33 , 34 , 35 making it possible that our findings are confounded by overall locomotion driving the increase in active lever responding. This seems unlikely, however, because we only see the increased responding in Experiment 1, despite similar ablations in Experiment 2. Additionally, there was not an increase in inactive lever responding which can act as a proxy for general locomotor behaviour. However, we did not directly measure locomotor behaviour in either experiment so we cannot entirely rule this possibility out.
A final interpretation points to the active role the NAcCore plays in the association between cues and drug reinforcement. Damage to the NAcCore not only increases locomotor output as shown above but also increases impulsivity. 36 Therefore, the increase in cocaine seeking in Experiment 1 may be due to a decrease in the ability of the conditioned cue to control compulsive reward seeking. However, with this interpretation, we are forced to confront the bidirectional outcome of our experiments. This becomes easier to resolve by taking the ensemble literature into account.
4.2. Neuronal ensembles in the NAcCore
Our experiments build on previous work, showing that mPFC neuronal ensembles rapidly mediate operant behaviour after behavioural acquisition, with the vmPFC controlling food and oxycodone seeking, 8 , 9 and the PL mediating cocaine seeking. 10 Additionally, our group has shown distinct ensembles in the mPFC mediate cocaine versus food seeking 37 and appetitive reward seeking versus extinction for both food and cocaine. 16 , 21 Our previous results in the mPFC make our current findings surprising, as withholding the primary reinforcer during the induction session has always been performed, and we have only found an increase in seeking on the test day after ablating the extinction ensemble. This difference in outcome suggests that NAcCore ensembles are more sensitive to extinction conditions in early learning. Cruz et al. found that ablating neuronal ensembles in the NAcShell reduced context induced reinstatement in an ABAA design but found no effect of Daun02 ablation in the NAcCore and instead found a decrease in cocaine seeking in a subsequent reinstatement test when Daun02 was injected into the NAcShell. 13 The authors suggest that extinguishing the conditioned reinforcers (i.e., the cocaine‐paired cues) may have prevented effects in the NAcCore. 13 , 38 They suggest that this may indicate that the NAcCore is more sensitive to the conditioned cues than the context. Our findings agree with this interpretation. Because our induction session is under extinction conditions, it is possible that ablating the ensemble that is encoding the association between the cues and the cocaine may drive an increase in cocaine seeking, perhaps by reducing cue‐controlled compulsive responding. However, others have also shown that the NAcCore plays a role in contextual encoding in Pavlovian related behaviours. 3 , 39
4.3. NAcCore D1 neuronal ensembles drive cocaine seeking
Experiment 1 suggests that, contrary to the mPFC, NAcCore extinction ensembles may be rapidly recruited when cocaine is withheld. However, this interpretation should be taken with caution because it takes many days of extinction training to successfully extinguish cocaine‐seeking behaviour. 16 , 40 In contrast to the results of Experiment 1, our prior studies that differentiated between extinction and appetitive seeking ensembles for food or cocaine in the ventromedial prefrontal cortex (vmPFC) showed that these ensembles were not behaviourally relevant until after at least 7 days of extinction training. 16 , 21 Nevertheless, we did observe significant within trial extinction on induction day in Experiment 1 but not Experiment 2—because cues and context were still paired with cocaine infusions. Our RNAscope findings complement this interpretation nicely, because we saw a significant positive correlation between c‐fos+/D1dr populations and active lever responses in our cocaine group (Figure 6A) but not in our no‐cocaine group (Figure 6B).
Our RNAscope data and our interpretation of that data in the context of our first two experiments are largely in agreement with the literature that suggests bidirectional roles for D1‐ and D2‐expressing MSNs in the context of drug seeking. D1 receptors are critical for cocaine reinforcement, sensitization and seeking, while D2 receptors inhibit cocaine seeking. 14 , 40 , 41 , 42 , 43 , 44 Furthermore, Calipari et al. demonstrated that in the NAcCore, D1 neurons are directly agonized, while D2 neurons are inhibited with cocaine administration, and D2 neurons have higher basal activity relative to D1 neurons. 39 Taken together, it is likely that in Experiment 1 increased drug seeking is driven by ablating an inhibitory D2 ensemble, while the decrease in cocaine seeking in Experiment 2 is driven by targeting an excitatory D1 ensemble.
However, these results may conflict with some previous work. Bobadilla et al. demonstrated that cocaine‐seeking ensembles are primarily composed of D1 ensembles and this is largely consistent with our conclusion. However, they find that the cocaine‐seeking ensemble was observed under extinction conditions (i.e., cocaine‐paired cues were present but not cocaine). These studies differ in key aspects. First, our rats met acquisition criteria in an average of 2–3 days, while they used a longer training regimen for the mice. Additionally, they include at least 10 days of extinction training for the mice. Therefore, it is possible that the differences in training their mice experienced resulted in a cue‐induced reinstatement that is more directly comparable to our cocaine reinforced self‐administration session, as drug‐paired cues become reinforcers. 45 The exceptionally short training regimen that our rats experience may mean that the cues have not developed into full‐fledged reinforcers in our experimental parameters. Nevertheless, we take our findings to be largely consistent with Bobadilla et al., because rats that actively self‐administered cocaine had very similar results in their RNAscope data. Furthermore, the flip in our D1 and D2 ensemble activity in cocaine self‐administration versus extinction conditions also agree with their findings. Similarly, both studies (and others 46 ) observed a significant population of c‐fos+ neurons that were neither D1 nor D2 expressing (Figure 5G). Which raises questions about the phenotype of these neurons, because the majority of neurons within the NAc are expected to be either D1/D2‐expressing MSNs.
4.4. Limitations and future directions
While we believe the limitations of our experiments do not alter our overall conclusions, we acknowledge a few areas that future research should further explore. First, we did not see a decrease in c‐Fos expression in Experiment 1 or 2 after Daun02 ablation. In general, decreased c‐Fos or β‐galactosidase expression is used as an indirect confirmation of neuronal ablation. However, this measure must be interpreted cautiously. We have previously found no changes in this metric after Daun02 ablation despite a robust behavioural response. 16 , 21 Conversely, others saw a decrease in β‐galactosidase with no behavioural effect. 13 While it is unknown why these potentially conflicting findings arise, it has been hypothesized that compensatory activity related to rewiring could explain them. 21 However, given the robust behavioural effects, and this outcome being observed previously, we do not think it changes the overall interpretations of the data. Second, the sample size was likely underpowered to detect sex effects. While we did use both male and female rats in all of our experiments, our RNAscope experiment in particular was not powered to determine if there was any interaction between sex and group. Finally, our interpretation of Experiments 1 and 2 relies on correlational evidence derived from Experiment 3. Therefore, future work should directly test our hypothesis by combining ensemble and cell type‐specific methods to directly excite or inhibit D1‐ and D2‐expressing ensembles during cocaine seeking.
4.5. Conclusion
Here, we show that targeted ablation of Fos‐expressing neurons in the NAcCore after behavioural acquisition of cocaine self‐administration bidirectionally modulates cocaine seeking. A graphical representation of our findings is shown in Figure 7. We hypothesize that the increase in cocaine seeking in Experiment 1 was driven by the ablation of inhibitory populations composed of active D2MSNs in the NAcCore, while the decrease in cocaine seeking in Experiment 2 was driven by the ablation of a cocaine‐seeking ensemble composed primarily of D1MSNs in the NAcCore.
FIGURE 7.

Schematic showing hypothesized mechanism underlying our findings. When cocaine reward is present on induction day, an increased number of D1 neurons are activated. Daun02 ablation of this ensemble leads to an increase in activity of other neurons, potentially D2 neurons, ultimately reducing cocaine‐seeking behaviour. Alternatively, when no‐cocaine reward is present on induction day, there is an increase in D2 cell activity. Daun02 inactivation of this ensemble leads to increased activity of other types of neurons, potentially D1 neurons, which leads to enhanced cocaine seeking behaviour.
AUTHOR CONTRIBUTIONS
Bo W. Sortman and Brandon L. Warren designed the study. Bo W. Sortman performed the surgeries with assistance from Samantha Rakela and Berk Cerci. Behavioural data were collected by Bo W. Sortman, Samantha Rakela, Sarah Paprotna and Berk Cerci. Microscopy and image quantification was done by Bo W. Sortman and Sarah Paprotna. Data analysis was done by Bo W. Sortman. The first draft of the paper was written by Bo W. Sortman with revision by Brandon L. Warren with additional input from Samantha Rakela, Sarah Paprotna and Berk Cerci. All authors received and approved of the final version of the manuscript.
ACKNOWLEDGEMENTS
The authors would like to thank the NIDA Drug Supply Program for providing the drugs used in this study.
Sortman BW, Rakela S, Paprotna S, Cerci B, Warren BL. Nucleus accumbens neuronal ensembles vary with cocaine reinforcement in male and female rats. Addiction Biology. 2024;29 (5): e13397. doi: 10.1111/adb.13397
Funding information B.L.W. received funding from the National Institute on Drug Abuse (NIDA; Grants DA042102 and DA058553) and a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hyman SE, Malenka RC, Nestler EJ. NEURAL MECHANISMS OF ADDICTION: the role of reward‐related learning and memory. Annu Rev Neurosci. 2006;29(1):565‐598. doi: 10.1146/annurev.neuro.29.051605.113009 [DOI] [PubMed] [Google Scholar]
- 2. Robinson TE, Berridge KC. The neural basis of drug craving: an incentive‐sensitization theory of addiction. Brain Res Rev. 1993;18(3):247‐291. doi: 10.1016/0165-0173(93)90013-p [DOI] [PubMed] [Google Scholar]
- 3. Koya E, Golden SA, Harvey BK, et al. Targeted disruption of cocaine‐activated nucleus accumbens neurons prevents context‐specific sensitization. Nat Neurosci. 2009;12(8):1069‐1073. doi: 10.1038/nn.2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bossert JM, Stern AL, Theberge FRM, et al. Ventral medial prefrontal cortex neuronal ensembles mediate context‐induced relapse to heroin. Nat Neurosci. 2011;14(4):420‐422. doi: 10.1038/nn.2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morrison DJ, Rashid AJ, Yiu AP, Yan C, Frankland PW, Josselyn SA. Parvalbumin interneurons constrain the size of the lateral amygdala engram. Neurobiol Learn Mem. 2016;135:91‐99. doi: 10.1016/j.nlm.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 6. Hsiang HL, Epp JR, van den Oever MC, et al. Manipulating a “cocaine engram” in mice. J Neurosci. 2014;34(42):14115‐14127. doi: 10.1523/jneurosci.3327-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanaka KZ, He H, Tomar A, Niisato K, Huang AJY, McHugh TJ. The hippocampal engram maps experience but not place. Science. 2018;361(6400):392‐397. doi: 10.1126/science.aat5397 [DOI] [PubMed] [Google Scholar]
- 8. Quintana‐Feliciano R, Gobin C, Kane L, et al. Food‐seeking behavior is mediated by Fos‐expressing neuronal ensembles formed at first learning in rats. Eneuro. 2021;8(2):ENEURO.0373‐20.2021. doi: 10.1523/eneuro.0373-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gobin C, Sortman B, Rakela S, Quintana‐Feliciano R, Warren BL. Fos‐expressing neuronal ensembles in rat infralimbic cortex encode initial and maintained oxycodone seeking in rats. Addict Biol. 2022;27(2):e13148. doi: 10.1111/adb.13148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sortman BW, Gobin C, Rakela S, Cerci B, Warren BL. Prelimbic ensembles mediate cocaine seeking after behavioral acquisition and once rats are well‐trained. Front Behav Neurosci. 2022;16:920667. doi: 10.3389/fnbeh.2022.920667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takehara‐Nishiuchi K, Morrissey MD, Pilkiw M. Prefrontal neural ensembles develop selective code for stimulus associations within minutes of novel experiences. J Neurosci. 2020;40(43):8355‐8366. doi: 10.1523/jneurosci.1503-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grant RI, Doncheck EM, Vollmer KM, et al. Specialized coding patterns among dorsomedial prefrontal neuronal ensembles predict conditioned reward seeking. Elife. 2021;10:e65764. doi: 10.7554/elife.65764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cruz FC, Babin KR, Leao RM, et al. Role of nucleus accumbens shell neuronal ensembles in context‐induced reinstatement of cocaine‐seeking. J Neurosci. 2014;34(22):7437‐7446. doi: 10.1523/jneurosci.0238-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou Y, Zhu H, Liu Z, et al. A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat Neurosci. 2019;22(12):1986‐1999. doi: 10.1038/s41593-019-0524-y [DOI] [PubMed] [Google Scholar]
- 15. Kasof G, Mandelzys A, Maika S, Hammer R, Curran T, Morgan J. Kainic acid‐induced neuronal death is associated with DNA damage and a unique immediate‐early gene response in c‐fos‐lacZ transgenic rats. J Neurosci. 1995;15(6):4238‐4249. doi: 10.1523/jneurosci.15-06-04238.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Warren BL, Kane L, Venniro M, et al. Separate vmPFC ensembles control cocaine self‐administration versus extinction in rats. J Neurosci. 2019;39(37):7394‐7407. doi: 10.1523/jneurosci.0918-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitchell JM, Cunningham CL, Mark GP. Locomotor activity predicts acquisition of self‐administration behavior but not cocaine intake. Behav Neurosci. 2005;119(2):464‐472. doi: 10.1037/0735-7044.119.2.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taylor TG, Galuska CM, Banna K, Yahyavi‐Firouz‐abadi N, See RE. Response acquisition and fixed‐ratio escalation based on interresponse times in rats. J Exp Anal Behav. 2010;93(2):261‐267. doi: 10.1901/jeab.2010.93-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177‐185. doi: 10.1016/j.neuropharm.2008.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li X, Rubio FJ, Zeric T, et al. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and Trkb, glutamate receptors, and epigenetic enzymes in cue‐activated Fos‐expressing dorsal striatal neurons. J Neurosci. 2015;35(21):8232‐8244. doi: 10.1523/jneurosci.1022-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Warren BL, Mendoza MP, Cruz FC, et al. Distinct Fos‐expressing neuronal ensembles in the ventromedial prefrontal cortex mediate food reward and extinction memories. J Neurosci. 2016;36(25):6691‐6703. doi: 10.1523/jneurosci.0140-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. James MH, McGlinchey EM, Vattikonda A, Mahler SV, Aston‐Jones G. Cued reinstatement of cocaine but not sucrose seeking is dependent on dopamine signaling in prelimbic cortex and is associated with recruitment of prelimbic neurons that project to contralateral nucleus accumbens core. Int J Neuropsychopharmacol. 2017;21(1):89‐94. doi: 10.1093/ijnp/pyx107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McGlinchey EM, James MH, Mahler SV, Pantazis C, Aston‐Jones G. Prelimbic to accumbens core pathway is recruited in a dopamine‐dependent manner to drive cued reinstatement of cocaine seeking. J Neurosci. 2016;36(33):8700‐8711. doi: 10.1523/jneurosci.1291-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stefanik MT, Moussawi K, Kupchik YM, et al. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013;18(1):50‐53. doi: 10.1111/j.1369-1600.2012.00479.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stefanik MT, Kupchik YM, Kalivas PW. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue‐induced transient synaptic potentiation and cocaine‐seeking behavior. Brain Struct Funct. 2016;221(3):1681‐1689. doi: 10.1007/s00429-015-0997-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine‐induced reinstatement of drug‐seeking behavior. J Neurosci. 2003;23(8):3531‐3537. doi: 10.1523/jneurosci.23-08-03531.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Siemsen BM, Barry SM, Vollmer KM, et al. A subset of nucleus accumbens neurons receiving dense and functional prelimbic cortical input are required for cocaine seeking. Front Cell Neurosci. 2022;16:844243. doi: 10.3389/fncel.2022.844243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock‐induced reinstatement of cocaine‐seeking behavior. J Neurosci. 2004;24(7):1551‐1560. doi: 10.1523/jneurosci.4177-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giannotti G, Heinsbroek JA, Yue AJ, Deisseroth K, Peters J. Prefrontal cortex neuronal ensembles encoding fear drive fear expression during long‐term memory retrieval. Sci Rep. 2019;9(1):10709. doi: 10.1038/s41598-019-47095-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McFarland K, Kalivas PW. The circuitry mediating cocaine‐induced reinstatement of drug‐seeking behavior. J Neurosci. 2001;21(21):8655‐8663. doi: 10.1523/jneurosci.21-21-08655.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561‐572. doi: 10.1038/nrn2515 [DOI] [PubMed] [Google Scholar]
- 32. Ito R, Robbins TW, Everitt BJ. Differential control over cocaine‐seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7(4):389‐397. doi: 10.1038/nn1217 [DOI] [PubMed] [Google Scholar]
- 33. Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D‐amphetamine. J Neurosci. 1999;19(6):2401‐2411. doi: 10.1523/jneurosci.19-06-02401.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maldonado‐Irizarry CS, Kelleyt AE. Excitotoxic lesions of the core and shell subregions of the nucleus accumbens differentially disrupt body weight regulation and motor activity in rat. Brain Res Bull. 1995;38(6):551‐559. doi: 10.1016/0361-9230(95)02030-2 [DOI] [PubMed] [Google Scholar]
- 35. Kelsey JE, Willmore EJ. Electrolytic lesions of the nucleus accumbens enhance locomotor sensitization to nicotine in rats. Behav Neurosci. 2006;120(3):600‐611. doi: 10.1037/0735-7044.120.3.600 [DOI] [PubMed] [Google Scholar]
- 36. Cardinal RN, Pennicott DR, Lakmali C, Sugathapala RTW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292(5526):2499‐2501. doi: 10.1126/science.1060818 [DOI] [PubMed] [Google Scholar]
- 37. Kane L, Venniro M, Quintana‐Feliciano R, et al. Fos‐expressing neuronal ensemble in rat ventromedial prefrontal cortex encodes cocaine seeking but not food seeking in rats. Addict Biol. 2021;26(3):e12943. doi: 10.1111/adb.12943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context‐induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl). 2008;200(4):545‐556. doi: 10.1007/s00213-008-1234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Calipari ES, Bagot RC, Purushothaman I, et al. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc National Acad Sci. 2016;113(10):2726‐2731. doi: 10.1073/pnas.1521238113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bobadilla AC, Dereschewitz E, Vaccaro L, Heinsbroek JA, Scofield MD, Kalivas PW. Cocaine and sucrose rewards recruit different seeking ensembles in the nucleus accumbens core. Mol Psychiatry. 2020;25(12):3150‐3163. doi: 10.1038/s41380-020-00888-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Caine SB, Thomsen M, Gabriel KI, et al. Lack of self‐administration of cocaine in dopamine D1 receptor knock‐out mice. J Neurosci. 2007;27(48):13140‐13150. doi: 10.1523/jneurosci.2284-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakamura Y, Longueville S, Nishi A, Hervé D, Girault J, Nakamura Y. Dopamine D1 receptor‐expressing neurons activity is essential for locomotor and sensitizing effects of a single injection of cocaine. Eur J Neurosci. 2021;54(4):5327‐5340. doi: 10.1111/ejn.15394 [DOI] [PubMed] [Google Scholar]
- 43. Heinsbroek JA, Neuhofer DN, Griffin WC, et al. Loss of plasticity in the D2‐accumbens pallidal pathway promotes cocaine seeking. J Neurosci. 2017;37(4):757‐767. doi: 10.1523/jneurosci.2659-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tunstall BJ, Kearns DN. Cocaine versus food cues. Addict Biol. 2016;21(2):282‐293. doi: 10.1111/adb.12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang X, Gallegos DA, Pogorelov VM, et al. Parvalbumin interneurons of the mouse nucleus accumbens are required for amphetamine‐induced locomotor sensitization and conditioned place preference. Neuropsychopharmacology. 2018;43(5):953‐963. doi: 10.1038/npp.2017.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


