Abstract
Background
Fatty liver disease (FLD) is a common comorbidity of psoriasis and is often referred to as non-alcoholic fatty liver disease (NAFLD). However, the role of inflammation or insulin resistance (IR) in FLD is inconclusive. The study aims to explore whether FLD in psoriasis patients is more related to insulin resistance or systemic inflammation level.
Methods
Data for this study were collected from the Shanghai Psoriasis Effectiveness Evaluation Cohort, a prospective cohort that examines psoriasis characteristics in the Chinese population. IR was assessed using the triglyceride glucose (TyG) and TyG-body mass index (TyG-BMI) indicators. Systemic non-specific inflammation was assessed using the neutrophil-to-lymphocyte ratio (NLR), derived neutrophil-to-lymphocyte ratio (dNLR), and systemic immune inflammation index (SII).
Results
The analysis included a total of 647 patients. Subsequent logistic regression analysis revealed that NLR, dNLR, and SII were not significantly associated with FLD in psoriasis patients, while TyG and TyG-BMI showed significant associations with FLD. Subgroup analysis indicated that in the majority of subgroups, TyG and TyG-BMI were significantly associated with FLD, particularly TyG-BMI. Excluding individuals with methotrexate and acitretin resulted in consistent findings with the main analysis. Further analysis revealed a significantly higher diagnosis rate of metabolic-associated fatty liver disease (MAFLD) compared to NAFLD.
Conclusions
Metabolic factors play a crucial role in FLD in patients with psoriasis, and TyG and TyG-BMI are potential predictors of FLD. Therefore, MAFLD can be recommend as a term to describe FLD in psoriasis patients.
Trial registration
https://www.chictr.org.cn/showproj.html?proj=58256, identifier ChiCTR2000036186. A multi-center clinical study of systemic treatment strategies for psoriasis in Chinese population. Registered 31 August 2020.
Keywords: psoriasis, fatty liver disease, insulin resistance, TyG, TyG-BMI
Introduction
Psoriasis is a chronic systemic inflammatory skin disease that extends beyond the skin and affects the function of many organs throughout the body. Fatty liver disease (FLD) is a common pathophysiological change in patients with psoriasis, and often describes as non-alcoholic fatty liver disease (NAFLD) which is a common comorbid disease of psoriasis and has been a concern for dermatologists (1, 2).In fact, NAFLD is an exclusive disease, there may be many more patients with FLD in patients with psoriasis than there is available evidences. In 2020, an international panel of experts proposed the metabolic-associated fatty liver disease (MAFLD) definition (3). MAFLD is a positive diagnosis that can be compared with other types of FLD, and may be an alternative to NAFLD (3, 4).
The mechanism of FLD in patients with psoriasis is not yet clear, but it is believed that higher systemic inflammation in patients with psoriasis affects insulin resistance (IR), thus aggravating hepatic steatosis (5, 6).Current research is limited to a single inflammatory factor or a single pro-inflammatory factor, and no studies have evaluated the effect of systemic inflammation on FLD in patients with psoriasis.
Indicators of neutrophil-to-lymphocyte ratio (NLR), derived neutrophil-to-lymphocyte ratio (dNLR), and systemic immune inflammation index (SII), which have been shown to correlate with systemic inflammation levels in a variety of diseases, are associated with FLD (6–9). Triglyceride-glucose (TyG) and triglyceride glucose-body mass index (TyG-BMI) are new indicators for evaluating IR, which have been verified in a number of studies to be consistent with or even better than traditional evaluation indicators (10–12). Therefore, the purpose of this study was to explore the association of inflammation or IR in patients with FLD using NLR, dNLR, SII, TyG, and TyG-BMI. Compare the differences between groups based on the diagnostic criteria for NAFLD and MAFLD, and further discuss suitable descriptive terms for psoriatic FLD.
Materials and methods
Study design
The study focused on analyzing patients with psoriasis who were enrolled in the Shanghai Psoriasis Effectiveness Evaluation Cohort (SPEECH) (13). SPEECH is an ongoing observational registry that involves multiple medical centers and aims to explore the unique characteristics of psoriasis in the Chinese population. Additionally, the registry seeks to identify appropriate diagnostic and treatment approaches for this specific demographic. The Ethics Committee of the Shanghai Skin Disease Hospital (#2020-36) thoroughly reviewed this study. All participants provided informed consent, and their privacy and confidentiality were carefully safeguarded throughout the study.
Participants
All study participants were registried in the SPEECH cohort. Inclusion criteria were as follows: 1. Adults who had been diagnosed with moderate-to-severe plaque psoriasis; 2. FLD was assessed using ultrasound, and if the ultrasound was of poor quality or negative, the patient needed to undergo an additional abdominal computerized tomography; 3. Routine blood tests, fasting glucose, and triglyceride tests required venous blood samples to be collected in the early morning. Main exclusion criteria included lack of baseline information, assessment of FLD, or venous blood samples.
Assessment of insulin resistance
The most accurate way to assess IR is the hyperinsulinemic-euglycemic clamp (HIEC) (14), but this method is very complex and difficult to apply in clinical practice outside of endocrinology. In addition, the Homeostasis Model Assessment-IR (HOMA-IR) is widely recognized, but the calculation relies on fasting insulin levels, which are not usually measured in routine diagnosis and treatment of psoriasis. This has led to the use and limitation of HOMA-IR in the psoriasis patient population. Recently, new indices known as TyG and TyG-BMI have been recognized as reliable and direct markers of IR (12, 15). TyG is calculated as follows: Ln [triglyceride (mg/dL) × fasting blood glucose (mg/dL)/2] (16); and TyG-BMI is calculated as TyG x BMI (kg/m2) (12). Therefore, TyG and TyG-BMI were used to assess the levels of IR in this study.
Assessment of non-specific systemic inflammation
Non-specific systemic inflammation was assessed using blood-derived markers, which were measured alongside liver assessments. These included the common NLR, dNLR, and SII. NLR was calculated by dividing the neutrophil count by the lymphocyte count. The dNLR is calculated as the neutrophil count divided by the difference between the white blood cell count and the neutrophil count. The formula for SII is the platelet count multiplied by NLR.
Statistical analysis
The primary method of analysis in this study is cross-sectional analysis. The group was divided into two categories based on the presence or absence of diffuse liver steatosis on imaging. Continuous variables that exhibit a normal distribution are reported as mean ± standard deviation (SD) and evaluated using the student t-test. Non-normally distributed continuous variables were presented with the median (interquartile range [IQR]) and compared using the Mann-Whitney U test. Categorical variables are expressed as frequency (%) and evaluated using Chi-square tests or Fisher’s exact tests.
The patients were subsequently stratified into three average groups based on the NLR, dNLR, SII, TyG and TyG-BMI respectively due to the significant variability in the data. These groups were further categorized as Q1-Q3 according to ascending order. Covariates included age, gender, BMI, smoking history, drinking history, hypertension, diabetes, psoriatic arthritis, duration of psoriasis, family history and PASI score based on previous literature (17). Three models were developed to investigate the correlation: Model 1 did not include any covariates, Model 2 only adjusted for age, gender, smoking history, drinking history, and BMI, and Model 3 included all covariates for analysis. When using TyG-BMI as the target variable, BMI was not used as a covariate. Odds ratios (OR) and 95% confidence intervals (95% CI) for different groups of inflammatory and IR indictors were calculated using univariate logistic regression and multivariate logistic regression. The correlation between continuous variables and FLD was demonstrated using forest plots. The association of inflammation markers and IR indictors with FLD in model 3 was verified by restricted cubic spline diagram (RCS), with knots=4 selected. Subgroup analysis was then conducted to further explore potential interactions and influencing factors. Receiver operating characteristic (ROC) curves were then used to assess the ability of inflammatory markers and IR markers to identify FLD in patients with psoriasis.
Causal mediation analysis was conducted using the ‘mediation’ package in R, and the bootstrap method with 500 simulations was employed to estimate the indirect effects, considering the current mainstream mechanism that believes inflammation leads to psoriatic FLD through the mediating effect of IR in model 3. Probit regression in the “mediation” package was used to analyze the mediating effect as sensitivity analysis. As acitretin and methotrexate (MTX) are the most commonly systemic oral drugs for psoriasis in China and have a significant effect on the liver, another sensitivity analysis was conducted, calculating OR values for patients who have never used MTX or acitretin.
Finally, differences between patients with FLD were compared based on the diagnostic criteria of NAFLD and MAFLD. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using R version 4.2.1.
Results
Patient characteristics
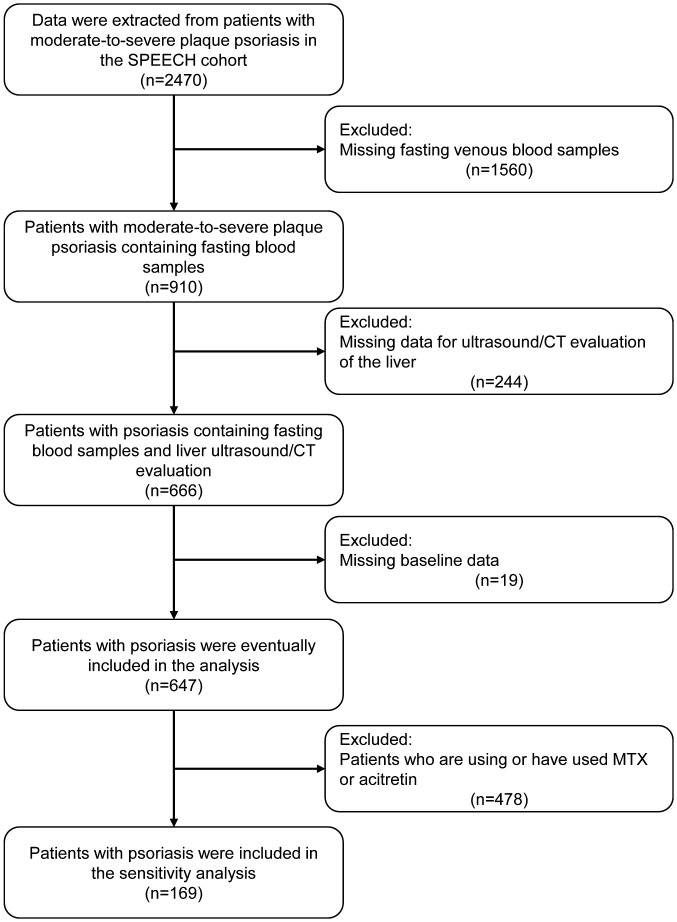
After screening the patient information of the SPEECH cohort according to the exclusion criteria, a total of 674 patients were included in the analysis ( Figure 1 ). Among them, 308 patients (45.7%) were found to have liver steatosis in imaging examinations. Consistent with previous studies (18, 19), patients with FLD had a higher BMI, higher prevalence of hypertension, and higher levels of blood glucose, triglycerides, and IR. Surprisingly, patients with FLD were younger, and no significant differences in inflammatory markers were found between the two groups ( Table 1 ).
Figure 1.
Flow chart of the study.
Table 1.
Baseline demographics and disease characteristics of psoriasis patients.
| Parameter | FLD (n=308) | non-FLD (n=366) | P value |
|---|---|---|---|
| Demographics | |||
| Gender, male, n (%) | 251 (81.5%) | 279 (76.2%) | 0.097 |
| Age, median (IQR) | 55 (40, 65) | 60 (45, 67) | 0.008 |
| Family history of psoriasis, n (%) | 58 (18.8%) | 60 (16.4%) | 0.407 |
| BMI (kg/m²), median (IQR) | 26.1 (23.9, 29.0) | 23.6 (21.2, 25.7) | < 0.001 |
| Smoking, n (%) | 140 (45.5%) | 139 (38%) | 0.050 |
| Drinking, n (%) | 67 (21.8%) | 84 (23%) | 0.710 |
| Comorbidity | |||
| Hypertension, n (%) | 123 (39.9%) | 109 (29.8%) | 0.006 |
| Type 2 diabetes mellitus, n (%) | 56 (18.2%) | 56 (15.3%) | 0.317 |
| PSA, n (%) | 68 (22.1%) | 75 (20.5%) | 0.616 |
| Evaluation of psoriasis | |||
| Duration (years), median (IQR) | 10 (6, 20) | 10 (6, 20) | 0.973 |
| Prior-MTX, n (%) | 135 (43.8%) | 160 (43.7%) | 0.976 |
| Prior-acitretin, n (%) | 160 (51.9%) | 198 (54.1%) | 0.577 |
| BSA (%), median (IQR) | 30 (15, 41) | 25 (14, 40) | 0.167 |
| PASI, median (IQR) | 15.3 (11.6, 21.0) | 15.0 (11.5, 21.0) | 0.318 |
| Hematological results | |||
| Leukocyte (*109/L), median (IQR) | 6.6 (5.6, 7.8) | 6.2 (5.3, 7.7) | 0.004 |
| Thrombocyte (*109/L), median (IQR) | 211.5 (174.8, 263.3) | 208 (170.3, 256.5) | 0.343 |
| Lymphocyte (*109/L), median (IQR) | 1.9 (1.5, 2.3) | 1.8 (1.4, 2.1) | < 0.001 |
| Neutrophil (*109/L), median (IQR) | 3.9 (3.3, 5.0) | 3.9 (3.0, 4.9) | 0.066 |
| NLR, median (IQR) | 2.1 (1.6, 2.9) | 2.2 (1.6, 3.1) | 0.320 |
| dNLR, median (IQR) | 1.5 (1.2, 2.0) | 1.6 (1.2, 2.1) | 0.571 |
| SII, median (IQR) | 449.0 (317.1, 668.7) | 461.8 (305.4, 663.5) | 0.738 |
| Fasting blood glucose (mmol/L), median (IQR) | 5.4 (4.9, 6.4) | 5.1 (4.7, 5.7) | < 0.001 |
| Triglyceride (mmol/L), median (IQR) | 1.7 (1.2, 2.3) | 1.3 (1.0, 1.7) | < 0.001 |
| TyG, median (IQR) | 9.4 (9.0, 9.8) | 9.1 (8.7, 9.5) | < 0.001 |
| TyG-BMI, median (IQR) | 249.8 (222.6, 276.0) | 215.9 (191.6, 235.8) | < 0.001 |
FLD, fatty liver disease; IQR, interquartile range; BMI, body mass index; PSA, psoriatic arthritis; MTX, methotrexate; BSA, body surface area; PSAI, psoriasis area and severity index; NLR, neutrophil-to-lymphocyte ratio; dNLR, derived neutrophil-to-lymphocyte ratio; SII, Systemic Immune Inflammation Index; TyG, triglyceride-glucose; TyG-BMI, triglyceride glucose-body mass index.
* represents the unit of counting.
Association between non-specific indictors of systemic inflammation and insulin resistance and FLD in psoriasis
Previous studies have documented a significant association between systemic nonspecific inflammatory indicators such as NLR and SII and FLD in the population. However, our findings indicate that there is no significant association between NLR, dNLR, and SII and the occurrence of FLD in patients with psoriasis. In contrast, we found that TyG and TyG-BMI were significantly associated with FLD in psoriasis, with a dose-dependent correlation observed in Q2 and Q3 compared to Q1 ( Table 2 ). Compared with TyG, TyG-BMI was more significantly associated with FLD because of its larger OR (TyG-BMI: Q3 OR=7.946, 95% CI=5.138-12.480; TyG: Q3 OR=2.704, 95% CI=1.750-4.206). Additionally, the forest plot demonstrated that, after adjustments for covariates, systemic nonspecific inflammatory indicators exhibited a low association with FLD. In contrast, TyG and TyG-BMI showed a significant positive correlation with FLD in psoriasis patients in all models ( Supplementary Figure 1 ). These results were further confirmed in the RCS ( Supplementary Figure 2 ).
Table 2.
Association between non-specific indictors of systemic inflammation and insulin resistance and fatty liver disease in psoriasis.
| Exposures | Model 1
a
Crude OR (95% CI) |
Model 2
b
Adjusted OR (95% CI) |
Model 3
c
Adjusted OR (95% CI) |
|---|---|---|---|
| NLR | |||
| Q1 | Ref. | Ref. | Ref. |
| Q2 | 1.123 (0.775-1.627) | 1.055 (0.705-1.579) | 1.038 (0.690-1.562) |
| Q3 | 0.881 (0.607-1.279) | 0.932 (0.620-1.401) | 0.897 (0.591-1.360) |
| dNLR | |||
| Q1 | Ref. | Ref. | Ref. |
| Q2 | 1.206 (0.832-1.750) | 1.085 (0.726-1.622) | 1.049 (0.697-1.576) |
| Q3 | 0.916 (0.631-1.329) | 0.933 (0.623-1.399) | 0.887 (0.586-1.343) |
| SII | |||
| Q1 | Ref. | Ref. | Ref. |
| Q2 | 0.843 (0.581-1.222) | 0.780 (0.521-1.165) | 0.767 (0.510-1.150) |
| Q3 | 0.898 (0.620-1.301) | 0.967 (0.646-1.446) | 0.932 (0.618-1.405) |
| TyG | |||
| Q1 | Ref. | Ref. | Ref. |
| Q2 | 1.756 (1.181-2.623) | 1.817 (1.179-2.815) | 1.871 (1.206-2.919) |
| Q3 | 3.131 (2.136-4.624) | 2.621 (1.730-3.999) | 2.704 (1.750-4.206) |
| TyG-BMI | |||
| Q1 | Ref. | Ref. | Ref. |
| Q2 | 2.495 (1.666-3.769) | 2.575 (1.710-3.913) | 2.584 (1.708-3.944) |
| Q3 | 8.189 (5.400-12.603) | 8.300 (5.438-12.860) | 7.946 (5.138-12.480) |
OR, odds ratio; CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; dNLR, derived neutrophil-to-lymphocyte ratio; SII, systemic immune inflammation index; TyG, triglyceride-glucose; TyG-BMI, triglyceride glucose-body mass index; Ref, reference.
No covariates analyzed.
Covariates analyzed adjusted for age, gender, smoking, drinking, and BMI.
All covariates analyzed.
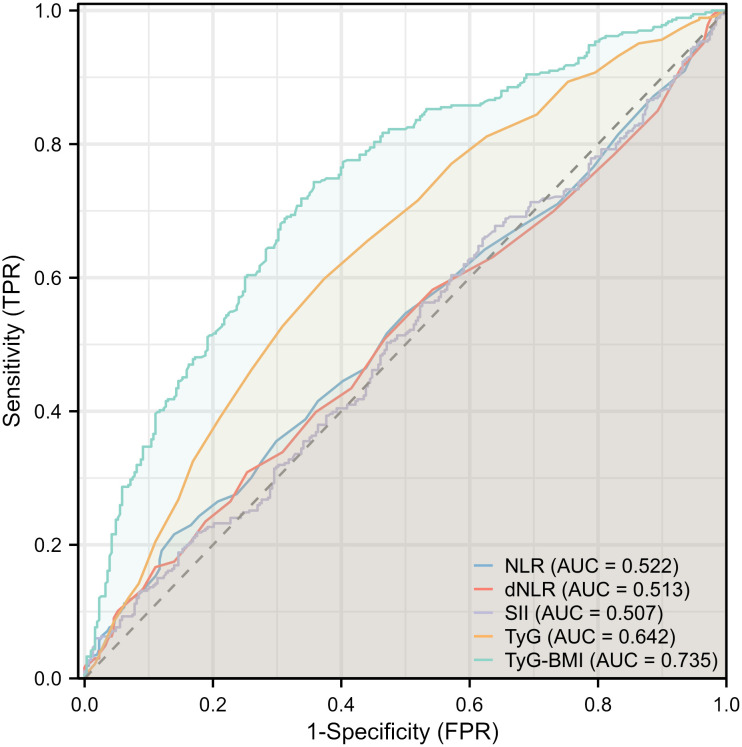
Given the significant association between TyG and TyG-BMI with FLD in patients with psoriasis, further subgroup analysis was conducted to determine the relationship ( Table 3 ). The results of the subgroup analysis indicated that TyG and TyG-BMI remained significantly associated with FLD in the majority of subgroups (P for interaction > 0.05). It is worth noting that in patients with elevated triglycerides, the association between TyG and FLD was weakened, but TyG-BMI still maintained a good correlation. Subsequent ROC analysis revealed that the area under the curve (AUC) for TyG and TyG-BMI was larger than that for NLR, dNLR, and SII. The AUC of TyG-BMI was the largest at 0.735. These findings suggest that TyG-BMI is superior for determining FLD in patients with psoriasis ( Figure 2 ).
Table 3.
Subgroup analysis between indictors and fatty liver disease in psoriasis patients.
| Subgroup | TyG | P value | P for interaction | TyG-BMI | P value | P for interaction |
|---|---|---|---|---|---|---|
| Gender | 0.211 | 0.523 | ||||
| Male | 2.077 (1.578-2.770) | <0.001 | 1.025 (1.015-1.037) | <0.001 | ||
| Female | 2.373 (1.347-4.381) | 0.004 | 1.022 (1.017-1.027) | <0.001 | ||
| Age (years) | 0.012 | 0.176 | ||||
| <45 | 2.800 (1.817-4.496) | <0.001 | 1.025 (1.017-1.033) | <0.001 | ||
| ≥45 | 1.930 (1.417-2.657) | <0.001 | 1.021 (1.015-1.027) | <0.001 | ||
| Family history of psoriasis | 0.234 | 0.453 | ||||
| No | 2.290 (1.677-3.079) | <0.001 | 1.022 (1.017-1.028) | <0.001 | ||
| Yes | 1.790 (1.056-3.149) | 0.035 | 1.025 (1.014-1.038) | <0.001 | ||
| Smoking | 0.031 | 0.026 | ||||
| Never | 2.348 (1.326-3.356) | <0.001 | 1.021 (1.015-1.027) | <0.001 | ||
| Past or current | 1.979 (1.370-2.919) | <0.001 | 1.027 (1.019-1.036) | <0.001 | ||
| Drinking | 0.735 | 0.943 | ||||
| Never | 1.856 (1.406-2.479) | <0.001 | 1.021 (1.016-1.026) | <0.001 | ||
| Past or current | 3.530 (2.063-6.395) | <0.001 | 1.030 (1.019-1.043) | <0.001 | ||
| Type 2 diabetes mellitus | 0.492 | 0.794 | ||||
| No | 2.444 (1.819-3.330) | <0.001 | 1.026 (1.020-1.031) | <0.001 | ||
| Yes | 1.531 (0.899-2.682) | 0.124 | 1.009 (0.999-1.020) | 0.084 | ||
| Hypertension | 0.050 | 0.184 | ||||
| No | 2.397 (1.740-3.359) | <0.001 | 1.027 (1.021-1.033) | <0.001 | ||
| Yes | 1.658 (1.113-2.521) | 0.015 | 1.014 (1.006-1.022) | <0.001 | ||
| BMI (kg/m²) | <0.001 | 0.971 | ||||
| <24 | 2.100 (1.396-3.262) | 0.001 | 1.026 (1.014-1.039) | <0.001 | ||
| ≥24 | 1.804 (1.303-2.534) | 0.001 | 1.020 (1.012-1.027) | <0.001 | ||
| PSA | 0.347 | 0.892 | ||||
| No | 2.213 (1.676-2.964) | <0.001 | 1.023 (1.017-1.028) | <0.001 | ||
| Yes | 2.003 (1.150-3.613) | 0.017 | 1.023 (1.013-1.035) | <0.001 | ||
| Triglyceride a | 0.010 | 0.076 | ||||
| Normal | 1.835 (1.204-2.832) | 0.005 | 1.024 (1.018-1.031) | <0.001 | ||
| Borderline | 0.954 (0.395-2.300) | 0.916 | 1.011 (1.002-1.022) | 0.026 | ||
| HTG | 1.293 (0.699-2.576) | 0.435 | 1.019 (1.007-1.024) | 0.004 |
BMI, body mass index; PSA, psoriatic arthritis; TyG, triglyceride-glucose; TyG-BMI, triglyceride glucose-body mass index; HTG, hypertriglyceridemia.
Fasting plasma triglyceride concentrations were categorized as normal (<150 mg/dL), borderline (150-199 mg/dL), high triglyceride (HTG>200 mg/dL).
Figure 2.
Forest plot depicting multivariate logistic regression results for inflammatory and insulin resistance indicators.
Sensitivity analysis
First, we used causal mediation analysis and probit regression to examine the overall effects of NLR, dNLR, and SII on FLD in psoriasis patients. We then analyzed the indirect effects mediated by TyG and TyG-BMI, as well as the direct effects of inflammatory factors. The analysis revealed that the total effect of NLR on FLD in psoriasis was not significant for both TyG (coefficient -0.02125, p=0.108) and TyG-BMI (coefficient -0.02114, p=0.108). The indirect effects of NLR on FLD through TyG (coefficient -0.00154, P=0.404) and TyG-BMI (coefficient -0.00199, p=0.280) were also not significant. A similar pattern was observed for the direct effects. The proportion mediated was 7.3% (95% CI: -30.2, 94.1) for TyG and 9.4% (95% CI: -30.1, 96.5) for TyG-BMI, indicating a low mediation effect ( Supplementary Table 1 ). Similar results were observed in dNLR and SII.
Given the widespread use of MTX and acitretin in China, even in the biologics era, these drugs continue to be the first-line treatment for patients with moderate-to-severe psoriasis in the country. Therefore, we excluded patients who had previously used MTX and acitretin, leaving only 169 patients in our cohort. In logistic regression analysis, similar to the main analysis, higher levels of TyG and TyG-BMI were found to be significantly associated with FLD in psoriasis, while NLR, dNLR, and SII showed no associations with FLD in psoriasis ( Supplementary Table 2 ). This suggests that the results of the main analysis are robust.
NAFLD and MAFLD
According to the diagnostic criteria for NAFLD and MAFLD ( Supplementary Figure 3 ), 308 patients with hepatic steatosis detected on imaging were diagnosed. In our cohort, a total of 308 patients had radiographic evidence of hepatic steatosis (47.6%), with a diagnosis rate of 39.1% for NAFLD and 46.4% for MAFLD. Therefore, the prevalence of MAFLD was significantly higher than that of NAFLD ( Supplementary Table 3 ). When comparing the characteristics of the two diagnostic populations, patients with MAFLD had a higher history of alcohol consumption and smoking than those with NAFLD. In addition, patients with MAFLD showed higher systemic non-specific inflammation and IR, although there was no statistical difference.
Discussion
Comorbidities of psoriasis affecting multiple systems have received extensive clinical attention, including cardiovascular disease, obesity, diabetes, dyslipidemia, NAFLD, etc (20). FLD is characterized by the accumulation of lipids in the cytoplasm of hepatocytes. Currently, FLD is believed to be closely linked to IR and various inflammatory pathways. Systemic inflammation and metabolic disorders in psoriasis patients can exacerbate IR and worsen hepatic steatosis (21, 22). Our results are consistent with this, as patients with radiographic evidence of hepatic steatosis had higher levels of BMI, hypertension, fasting blood glucose, triglycerides, TyG and TyG-BMI compared to those without FLD.
In this cross-sectional study, we investigated the association of IR and non-specific systemic inflammatory conditions on FLD in patients with moderate-to-severe plaque psoriasis for the first time. Our results based on subgroup analysis and ROC indicate that systemic nonspecific indicators such as SII, NLR, and dNLR showed no significant association with FLD in patients with psoriasis. In contrast, IR-related indicators TyG and TyG-BMI were significantly associated with FLD in patients with psoriasis. Among these, TyG-BMI exhibited a higher AUC value and is expected to be a predictor of FLD in patients with psoriasis. Therefore, we suggest that metabolic factors play a more significant role in FLD in patients with moderate-to-severe plaque psoriasis than systemic inflammation.
IR is characterized by impaired downstream effects of insulin signaling in target tissues, primarily the liver, muscle, and adipose tissue (23). It is implicated in the progression of diseases such as FLD, as well as the advancement of liver fibrosis. Several studies have demonstrated an independent positive association between TyG-BMI and NAFLD (24–26). Our study indicates that TyG and TyG-BMI can be used as potential predictors of FLD in patients with psoriasis. There are some studies that have highlighted the significant value of indices such as BMI, TyG, and TyG-BMI, in identifying the risk of NAFLD and MAFLD. Some studies have proposed that TyG-WC, TyG-WHtR, and TyG-BMI could be used for early screening of NAFLD and MAFLD (24). Furthermore, these three parameters, along with HOMA-IR, were found to be more suitable for assessing metabolic risk and monitoring disease progression in NAFLD patients (24, 27). On comparison with HOMI-IR or the gold standard HIEC, TyG and TyG-BMI offer the advantages of convenience and accessibility, and can be considered as early screening tools for hepatic steatosis in patients with psoriasis.
Previous studies have demonstrated the effectiveness of SII, NLR, and dNLR in evaluating the severity of psoriasis disease activity and treatment effectiveness (28, 29). Some studies also have indicated their association with liver steatosis and NAFLD. Interestingly, our findings suggest that no significant association was found with NLR, dNLR and SII. This study utilized these three indicators to draw novel conclusions, which may be attributed to the fact that previous studies were conducted on a broader population and may not have specifically focused on patients with psoriasis. In addition, hepatic steatosis is closely linked to genetic and environmental factors. Studies have shown that dietary components and dietary habits can increase an individual’s susceptibility to NAFLD (30). Diets high in fat, salt, and sugar can contribute to hepatic steatosis, while dietary flavonoid intake acts as a protective factor (31). The dietary patterns of Chinese individuals differ somewhat from those of Europeans and Americans. Therefore, future studies should fully consider the heterogeneity of population and environmental factors.
It is estimated that the prevalence of NAFLD in the global population is 32.4% (32). Numerous studies have demonstrated heterogeneity in the pathogenesis of NAFLD (33). However, the emphasis of NAFLD on the role of alcohol, viral hepatitis, and other diseases in hepatic steatosis no longer accurately reflects the nature of the disease. In 2020, some scholars proposed the concept of MAFLD, which highlights the significant role of obesity, insulin resistance, dyslipidemia, diabetes, and systemic low inflammatory response in the occurrence and progression of fatty liver disease (4). In our study, the prevalence of NAFLD and MAFLD was 39.1% and 46.4%, respectively, significantly higher than that of the general population. The diagnosis rate of MAFLD was significantly higher than that of NAFLD in patients with psoriasis. As a result, FLD in patients with psoriasis may be significantly underestimated in the current understanding of NAFLD as the primary comorbidity in patients with psoriasis. Based on these results, considering the high incidence of viral hepatitis in China (34), NAFLD may not accurately reflect the specific situation of FLD in psoriasis patients, and MAFLD may be a viable alternative. However, the current evidence for MAFLD in the context of psoriasis is limited, and future high-quality studies with larger sample sizes are necessary to validate our findings.
Overall, our study assessed the association of metabolic and inflammatory factors in FLD in Chinese patients with psoriasis. Furthermore, we utilized the TyG and TyG-BMI to evaluate IR. These indicators are convenient, rapid, practical, and easily promotable in clinical practice. Therefore, our study may contribute to the clinical diagnosis and treatment of psoriasis with FLD.
This study has several limitations. Firstly, FLD was diagnosed solely through imaging and not histology. And the inflammatory indicators evaluated in the patients were non-specific, and did not include IL-17, IL-23, and other specific inflammatory indicators. Future studies should consider including specific indicators for research. Additionally, the patients in this study were predominantly middle-aged to elderly individuals with moderate-to-severe conditions, indicating a potential for selection bias. Furthermore, only TyG and TyG-BMI were used to assess IR, instead of the commonly used the gold standard HIEC. Finally, the cross-sectional nature of this study precludes the ability to make causal inferences. There is a lack of prospective studies on FLD in psoriasis, and future research should focus on larger sample sizes and higher-quality studies to validate our findings.
Conclusion
Our study suggests that metabolic factors play a significant role in the development of FLD in patients with moderate-to-severe psoriasis. In understanding FLD in psoriasis, the term NAFLD may not fully encompass the condition of FLD in psoriasis in China. Therefore, to increase dermatologists’ awareness of psoriasis with FLD, we recommend including metabolic factors in the assessment of hepatic steatosis, and describing comorbidities in psoriasis in terms of MAFLD. We found that both TyG and TyG-BMI showed a significant association with FLD in patients with psoriasis and may serve as potential predictors. A more comprehensive approach to managing psoriasis is necessary in the future, incorporating metabolic assessments, interventions, and dietary modifications to manage or prevent hepatic steatosis in psoriasis.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Ethical Committee of Shanghai Skin Disease Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XZ: Writing – original draft. DH: Conceptualization, Investigation, Visualization, Writing – original draft. RC: Investigation, Writing – original draft. LY: Writing – original draft. RM: Visualization, Writing – original draft. YY: Writing – original draft. YJ: Conceptualization, Writing – original draft. LK: Writing – original draft. JL: Writing – review & editing. YL: Methodology, Resources, Writing – review & editing. YS: Writing – original draft, Writing – review & editing.
Acknowledgments
We gratefully acknowledge the contributions of the SPEECH cohort participants, healthcare professionals, research staff, and supportive friends and family members, whose collective efforts were integral to the successful completion of this study.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82003335, 82073429, 82273510, 82203913), Innovation Program of Shanghai Municipal Education Commission (No.2019-01-07-00-07-E00046), Clinical Research Plan of SHDC (No. SHDC2020CR1014B), Program of Shanghai Academic Research Leader (No. 20XD1403300), Shanghai Municipal Health Commission Project (No.202240369), China Postdoctoral Science Foundation (2023M732651), Shanghai General Hospital Traditional Chinese and Western Medicine Collaborative Guidance Project (no. ZXXT-202212) and Municipal Hospital Diagnosis and Treatment Technology Promotion and Optimization Management Project of SHDC (No. SHDC12023114).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1388967/full#supplementary-material
Receiver operating characteristic analysis comparing hepatic steatosis indicators in psoriasis.
Restricted cubic spline of non-specific inflammatory and insulin resistance indicators and fatty liver disease in psoriasis.
Diagnostic criteria for non-alcoholic fatty liver disease and metabolic-associated fatty liver disease.
References
- 1. Yang JJ, Nguyen KA, Fleischman MW, Aly O, Cheng K. Psoriasis in liver disease: Associations beyond nonalcoholic fatty liver disease. J Am Acad Dermatol. (2022) 86:883–5. doi: 10.1016/j.jaad.2021.03.023 [DOI] [PubMed] [Google Scholar]
- 2. Ruan Z, Lu T, Chen Y, Yuan M, Yu H, Liu R, et al. Association between psoriasis and nonalcoholic fatty liver disease among outpatient US adults. JAMA Dermatol. (2022) 158:745–53. doi: 10.1001/jamadermatol.2022.1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eslam M, Sanyal AJ, George J. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. (2020) 158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312 [DOI] [PubMed] [Google Scholar]
- 4. Gofton C, Upendran Y, Zheng MH, George J. MAFLD: How is it different from NAFLD? Clin Mol Hepatol. (2023) 29:S17–s31. doi: 10.3350/cmh.2022.0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jung I, Kwon H, Park SE, Park CY, Lee WY, Oh KW, et al. Serum lipoprotein(a) levels and insulin resistance have opposite effects on fatty liver disease. Atherosclerosis. (2020) 308:1–5. doi: 10.1016/j.atherosclerosis.2020.07.020 [DOI] [PubMed] [Google Scholar]
- 6. Song Y, Guo W, Li Z, Guo D, Li Z, Li Y. Systemic immune-inflammation index is associated with hepatic steatosis: evidence from NHANES 2015-2018. Front Immunol. (2022) 13:1058779. doi: 10.3389/fimmu.2022.1058779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shavakhi M, Nourigheimasi S, Dioso E, Goutnik M, Lucke-Wold B, Khanzadeh S, et al. Prognostic role of neutrophil to lymphocyte ratio in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Can J Gastroenterol Hepatol. (2022) 2022:1554079. doi: 10.1155/2022/1554079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou Y, Tian N, Li P, He Y, Tong L, Xie W. The correlation between neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with nonalcoholic fatty liver disease: a cross-sectional study. Eur J Gastroenterol Hepatol. (2022) 34:1158–64. doi: 10.1097/MEG.0000000000002439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xie R, Xiao M, Li L, Ma N, Liu M, Huang X, et al. Association between SII and hepatic steatosis and liver fibrosis: a population-based study. Front Immunol. (2022) 13:925690. doi: 10.3389/fimmu.2022.925690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao Y, Zhang J, Chen C, Qin P, Zhang M, Shi X, et al. Comparison of six surrogate insulin resistance indexes for predicting the risk of incident stroke: the Rural Chinese Cohort Study. Diabetes Metab Res Rev. (2022) 38:e3567. doi: 10.1002/dmrr.3567 [DOI] [PubMed] [Google Scholar]
- 11. Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, et al. Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr. (2022) 16:102581. doi: 10.1016/j.dsx.2022.102581 [DOI] [PubMed] [Google Scholar]
- 12. Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PloS One. (2016) 11:e0149731. doi: 10.1371/journal.pone.0149731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu N, Peng C, Zhou J, Gu J, Xu J, Li X, et al. Measurement properties of the patient global assessment numerical rating scale in moderate-to-severe psoriasis. Br J Dermatol. (2023) 189:437–46. doi: 10.1093/bjd/ljad188 [DOI] [PubMed] [Google Scholar]
- 14. Gastaldelli A. Measuring and estimating insulin resistance in clinical and research settings. Obes (Silver Spring). (2022) 30:1549–63. doi: 10.1002/oby.23503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. (2010) 95:3347–51. doi: 10.1210/jc.2010-0288 [DOI] [PubMed] [Google Scholar]
- 16. Liu XC, He GD, Lo K, Huang YQ, Feng YQ. The triglyceride-glucose index, an insulin resistance marker, was non-linear associated with all-cause and cardiovascular mortality in the general population. Front Cardiovasc Med. (2020) 7:628109. doi: 10.3389/fcvm.2020.628109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’Hagan R, Gonzalez-Cantero A, Patel N, Hong CG, Berg AR, Li H, et al. Association of the triglyceride glucose index with insulin resistance and subclinical atherosclerosis in psoriasis: an observational cohort study. J Am Acad Dermatol. (2023) 88:1131–4. doi: 10.1016/j.jaad.2022.08.027 [DOI] [PubMed] [Google Scholar]
- 18. Ndumele CE, Nasir K, Conceiçao RD, Carvalho JA, Blumenthal RS, Santos RD. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol. (2011) 31:1927–32. doi: 10.1161/ATVBAHA.111.228262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Åberg F, Byrne CD, Pirola CJ, Männistö V, Sookoian S. Alcohol consumption and metabolic syndrome: Clinical and epidemiological impact on liver disease. J Hepatol. (2023) 78:191–206. doi: 10.1016/j.jhep.2022.08.030 [DOI] [PubMed] [Google Scholar]
- 20. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. Jama. (2020) 323:1945–60. doi: 10.1001/jama.2020.4006 [DOI] [PubMed] [Google Scholar]
- 21. Tilg H, Adolph TE, Dudek M, Knolle P. Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat Metab. (2021) 3:1596–607. doi: 10.1038/s42255-021-00501-9 [DOI] [PubMed] [Google Scholar]
- 22. Zhu C, Tang S, Xiao J, Zhang Y, Sun L, Zhang J, et al. Insulin resistance, but not obstructive sleep apnea is associated with hepatic steatosis in Chinese patients with severe obesity. Obes Facts. (2023) 16:344–55. doi: 10.1159/000528789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mastrototaro L, Roden M. Insulin resistance and insulin sensitizing agents. Metabolism. (2021) 125:154892. doi: 10.1016/j.metabol.2021.154892 [DOI] [PubMed] [Google Scholar]
- 24. Xue Y, Xu J, Li M, Gao Y. Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: triglyceride glucose index-related parameters. Front Endocrinol (Lausanne). (2022) 13:951689. doi: 10.3389/fendo.2022.951689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sheng G, Lu S, Xie Q, Peng N, Kuang M, Zou Y. The usefulness of obesity and lipid-related indices to predict the presence of Non-alcoholic fatty liver disease. Lipids Health Dis. (2021) 20:134. doi: 10.1186/s12944-021-01561-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang R, Dai L, Zhong Y, Xie G. Usefulness of the triglyceride glucose-body mass index in evaluating nonalcoholic fatty liver disease: insights from a general population. Lipids Health Dis. (2021) 20:77. doi: 10.1186/s12944-021-01506-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peng H, Pan L, Ran S, Wang M, Huang S, Zhao M, et al. Prediction of MAFLD and NAFLD using different screening indexes: a cross-sectional study in U.S. adults. Front Endocrinol (Lausanne). (2023) 14:1083032. doi: 10.3389/fendo.2023.1083032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yorulmaz A, Hayran Y, Akpinar U, Yalcin B. Systemic immune-inflammation index (SII) predicts increased severity in psoriasis and psoriatic arthritis. Curr Health Sci J. (2020) 46:352–7. doi: 10.12865/CHSJ.46.04.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Andersen CSB, Kvist-Hansen A, Siewertsen M, Enevold C, Hansen PR, Kaur-Knudsen D, et al. Blood cell biomarkers of inflammation and cytokine levels as predictors of response to biologics in patients with psoriasis. Int J Mol Sci. (2023) 24(7):6111. doi: 10.3390/ijms24076111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 31. Xie R, Zhang Y. Associations between dietary flavonoid intake with hepatic steatosis and fibrosis quantified by VCTE: Evidence from NHANES and FNDDS. Nutr Metab Cardiovasc Dis. (2023) 33:1179–89. doi: 10.1016/j.numecd.2023.03.005 [DOI] [PubMed] [Google Scholar]
- 32. Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2022) 7:851–61. doi: 10.1016/S2468-1253(22)00165-0 [DOI] [PubMed] [Google Scholar]
- 33. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. (2016) 65:1038–48. doi: 10.1016/j.metabol.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 34. Zhang M, Wu R, Xu H, Uhanova J, Gish R, Wen X, et al. Changing incidence of reported viral hepatitis in China from 2004 to 2016: an observational study. BMJ Open. (2019) 9:e028248. doi: 10.1136/bmjopen-2018-028248 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Receiver operating characteristic analysis comparing hepatic steatosis indicators in psoriasis.
Restricted cubic spline of non-specific inflammatory and insulin resistance indicators and fatty liver disease in psoriasis.
Diagnostic criteria for non-alcoholic fatty liver disease and metabolic-associated fatty liver disease.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.