Highlights
-
•
We reported the first MPXV strain hMpxV/China/SZ-SZTH42/2023 isolated in southern China.
-
•
The isolate SZTH42 belongs to C.1 lineage of clade IIb, representing the currently prevalent IIb branch strain worldwide.
-
•
This study provides key resources and technical platforms for the development of antiviral drugs and vaccines.
Dear Editor,
Unlike previous outbreaks of the mpox (previously known as monkeypox), which were localized in Africa and resulted in small numbers of infections due to limited human-to-human transmission, the current outbreak beginning in May 2022, has spread rapidly primarily through human-to-human transmission in non-endemic countries (Gong et al., 2022). As of 27 Sep 2023, 115 countries and regions have reported cases of mpox to the World Health Organization (WHO), bringing the total number of confirmed cases to 90,618 including 125 deaths. Although the number of new cases has decreased in most WHO regions in the second half of 2022, the curves reveal a long outbreak tail in different subregions of the Americas. More worrying, 106 mpox cases were reported in the mainland of China in June 2023, and the total number of new cases surged to 992 within the next two months. Effective drugs, neutralizing antibodies and vaccines were urgently needed for limiting the spread of mpox, and were developed but tested mostly against vaccinia virus (VACV) (Russo et al., 2021; Li et al., 2023; Zhang et al., 2023; Zhou et al., 2023), especially in the mainland of China. The urgent research demand prompted us to isolate the MPXV when the first mpox case was emerged in the city of Shenzhen on June 9, 2023.
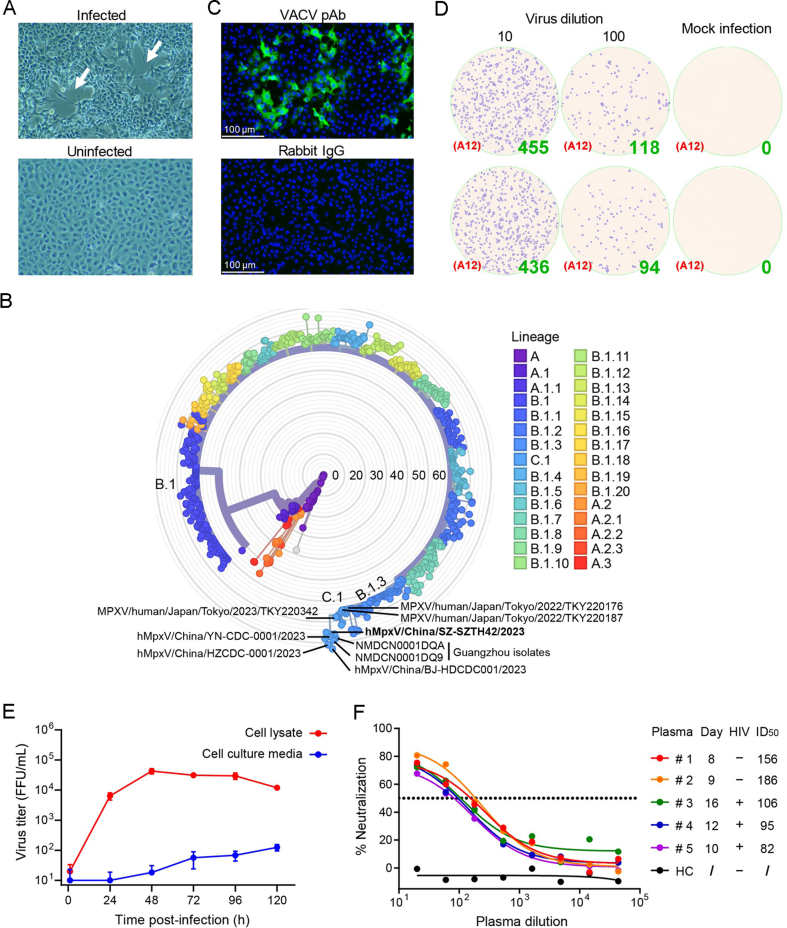
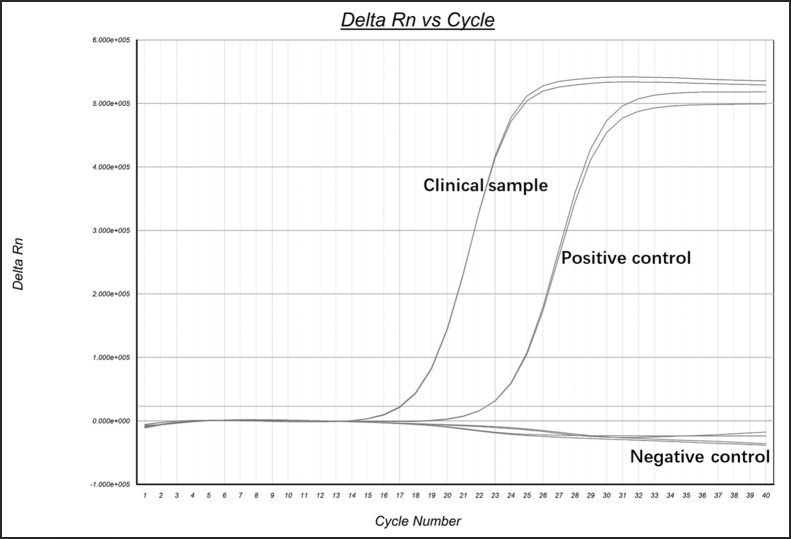
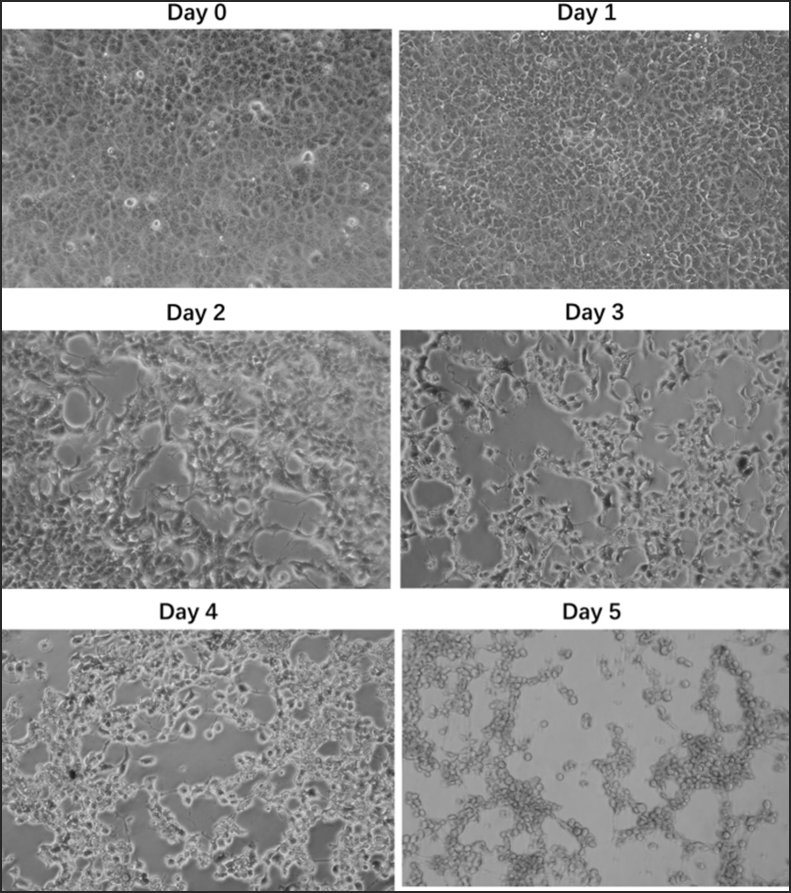
The skin blister fluid sample was collected from a 37-year-old male patient with mpox 5 days after symptom onset in the Shenzhen Third People's Hospital in June 2023 with informed consent. The etiology of the case as MPXV was confirmed by qPCR assay that specifically detect MPXV with DNA from the clinical sample (Supplementary Fig. S1). The specimen was resuspended with cell culture medium, filtered and inoculated onto Vero E6 cell monolayer in a 6-well plate. The virus proliferation-induced cytopathological effects (CPE) were observed daily, and typical plaques occurred within 72 h in the skin blister fluid-incubated cell monolayer (Fig. 1A). The MPXV isolate was harvested by three cycles of freeze-thaw followed by centrifugation to remove cell debris and the virus containing supernatant was aliquoted and stored at −80 °C for passage and further studies.
Fig. 1.
Isolation and characterization the MPXV clinical isolate SZTH42. A MPXV proliferation induced cytopathic effects of in Vero E6 cells. White arrows indicate plaques. B Phylogenetic analysis of representative MPXV isolates from China in 2023 using Nextclade (v2.14.1) online software. C Immunofluorescence analysis of Vero E6 cell infected with MPXV SZTH42 isolate using rabbit derived VACV polyclonal antibody (pAb) or rabbit IgG (negative control). D Quantification of infectious MPXV from the third passage of SZTH42 isolation using focus forming assay in 96-well plate in duplicate. E Vero E6 cells in 24-well plates were infected with the SZTH42 isolate (MOI 0.01) in triplicate. The cell culture media and cell lysate were collected separately at indicated timepoints for titration of infectious virus particles using focus forming assay. Data was represented as mean ± SD. F Neutralizing activity of plasma of mpox patients against MPXV SZTH42 isolate was determined in Vero E6 cells using FRNT assay. The plasma from a healthy donor served as a negative control (HC). Day: time from admission to blood sampling; ID50: half maximal inhibitory dilution.
The Illumina Hiseq sequencing of the isolate resulted in total of 2.88 GB clean data with 100% coverage and ∼7200 × of mean depth. The clean sequencing data was mapped against reference MPXV genome (GenBank accession ID: NC_063383) for genome assembly. The consensus genome obtained had a size of ∼197 KB. The MPXV isolate hMpxV/China/SZ-SZTH42/2023 (hereinafter referred to as SZTH42) was submitted to GISAID (https://gisaid.org/) under accession ID EPI_ISL_18213374. Phylogenetic analysis using Nextclade (https://clades.nextstrain.org/) revealed the SZTH42 isolate belongs to lineage C.1 of clade IIb of MPXV. The SZTH42 isolate was found to form a close group with other recently disclosed China MPXV isolates from Yunnan, Hangzhou, Beijing, and Guangzhou (Jia et al., 2023) along with those from Japan (Fig. 1B). Compared to the reference genome, the isolate SZTH42 has 81 mutations, 35 of which are nonsynonymous mutations, shared with the Guangzhou isolates, but do not have the P569S mutation (OPG025 gene) common to the Guangzhou isolates.
VACV induced antibodies cross-react with MPXV antigens (Yang et al., 2023), so the immunofluorescence assay (IFA) was performed using rabbit-derived VACV-induced polyclonal antibody to confirm MPXV antigen expression. The infected cells were clearly visualized with green fluorescence (Fig. 1C), consistent with previous study in which IFA performed using rabbit antibody against Orthopoxvirus for identification of MPXV infected cells also shown positive signals around the infected foci (Huang et al., 2022). Based on the commercially available cross-reactive antibody, we established a focus forming assay for virus titration. The infectious MPXV titers was about 1 × 105 FFU/mL for the third passage (Fig. 1D).
Orthopoxvirus produces two structurally distinct forms of virions: the intracellular mature virus (IMV) and the extracellular enveloped virus (EEV). The IMV virion can be released upon cell lysis. The EEV on the cell surface is either released (EEV) or retained which is called cell-associated enveloped virus (CEV). The balance between EEV and CEV is influenced by the host cell and strain of virus (Payne, 1979). The growth kinetics of the MPXV isolate was studied in Vero E6 cells. The microscopic observation of the cells revealed progression of CPE at increasing time points (Supplementary Fig. S2). Virus titration of respective time points was performed for estimation of infectious virus particles in cell lysate and cell culture medium. The infectious viral particles in the cell lysate exhibit exponential growth within 24 h, reaching a plateau at 48 h, and maintaining a high level until 96 h (Fig. 1E). The virus particles in the cell culture medium began to grow continuously 24 h post-infection, but remained at a low level, indicating that the proportion of EEV was significantly lower than that of CEV, and the sustained growth of virus particles may be partly due to the release of IMV from the lysed infected cells.
Based on the focus forming assay, the focus reduction neutralization test (FRNT) was established to evaluate the neutralizing activity of antibodies or plasma. The FRNT assay has higher throughput capacity and more time-saving than the plaque reduction neutralization test (PRNT) and therefore was extensively used in the research field of SARS-CoV-2 (Ju et al., 2020; Sun et al., 2021). The neutralizing activity of plasma of five mpox patients (Supplementary Table S1) was assessed using the FRNT assay in the Vero E6 cells with the MPXV SZTH42 isolate (Fig. 1F). The neutralizing activity of 20-fold diluted plasma of the five mpox patients was comparable with that of the Chongqing patient detected by the classical PRNF assay (Huang et al., 2022). Complement plays an important role in the MPXV neutralization assay, especially when used to evaluate the neutralizing activity of specific antibodies targeting EEV membrane proteins. Complement mediates antibody-dependent lysis of the outer membrane of EEV, exposing the inner infectious virus to neutralizing antibodies targeting IMV membrane proteins (Lustig et al., 2004).
In summary, we have reported the first MPXV strain isolated in southern China, which represents the currently prevalent IIb branch strain worldwide. The novel methods developed in this study including IFA, focus forming assay and FRNT will pave the way for further research and the development of antiviral drugs and vaccines against MPXV.
Footnotes
This work was supported by the National Key Plan for Scientific R&D of China (2021YFC2301900), the National Science Fund for Distinguished Young Scholars (82025022), Guangdong Science and Technology Plan Project, Construction of high-level biosafety laboratories (2021B1212030010), Shenzhen Science and Technology Program (ZDSYS20210623091810030), Shenzhen Medical Research Funds (B2302052). All authors declare that they have no conflict of interest. This study was approved by the Ethics Committee of the Shenzhen Third People's Hospital, China (approval number: 2021-030). The participant had provided written informed consent for sample collection and subsequent analysis. All works with infectious MPXV were carried out in Biosafety Level 3 (BSL-3) laboratory.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virs.2024.01.004.
Contributor Information
Fuxiang Wang, Email: Wangfuxiang999@163.com.
Zheng Zhang, Email: zhangzheng1975@aliyun.com.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Supplementary Fig. S1.
Supplementary Fig. S2.
References
- Gong Q., Wang C., Chuai X., Chiu S. Monkeypox virus: a re-emergent threat to humans. Virol. Sin. 2022;37:477–482. doi: 10.1016/j.virs.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Zhao H., Song J., Zhao L., Deng Y., Wang W., Lu R., Wang W., Ren J., Ye F., Tian H., Wu G., Ling H., Tan W. Isolation and characterization of monkeypox virus from the first case of monkeypox - chongqing municipality, China, 2022. China CDC Wkly. 2022;4:1019–1024. doi: 10.46234/ccdcw2022.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H., Sha T., Zhao S., Su W., Liu P., Zhen R., Li P., Zhou L., Xu Y., Wen Y., Chi L., Di B., Li P., Chen H., Qin P. Genomic and epidemiological perspectives on the first local sporadic cases of mpox in China. Emerg. Microb. Infect. 2023;12:2245932. doi: 10.1080/22221751.2023.2245932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., Tang X., Yu J., Lan J., Yuan J., Wang H., Zhao J., Zhang S., Wang Y., Shi X., Liu L., Zhao J., Wang X., Zhang Z., Zhang L. Human neutralizing antibodies elicited by sars-cov-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- Li M., Ren Z., Wang Y., Jiang Y., Yang M., Li D., Chen J., Liang Z., Lin Y., Zeng Z., Xu R., Wang Y., Zhu L., Xiao W., Wu Q., Zhang B., Wan C., Yang Y., Wu B., Peng J., Zhao W., Shen C. Three neutralizing mabs induced by mpxv a29l protein recognizing different epitopes act synergistically against orthopoxvirus. Emerg. Microb. Infect. 2023;12:2223669. doi: 10.1080/22221751.2023.2223669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig S., Fogg C., Whitbeck J.C., Moss B. Synergistic neutralizing activities of antibodies to outer membrane proteins of the two infectious forms of vaccinia virus in the presence of complement. Virology. 2004;328:30–35. doi: 10.1016/j.virol.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Payne L.G. Identification of the vaccinia hemagglutinin polypeptide from a cell system yielding large amounts of extracellular enveloped virus. J. Virol. 1979;31:147–155. doi: 10.1128/jvi.31.1.147-155.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A.T., Grosenbach D.W., Chinsangaram J., Honeychurch K.M., Long P.G., Lovejoy C., Maiti B., Meara I., Hruby D.E. An overview of tecovirimat for smallpox treatment and expanded anti-orthopoxvirus applications. Expert Rev. Anti Infect. Ther. 2021;19:331–344. doi: 10.1080/14787210.2020.1819791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Cai Y., Song T.Z., Pu Y., Cheng L., Xu H., Sun J., Meng C., Lin Y., Huang H., Zhao F., Zhang S., Gao Y., Han J.B., Feng X.L., Yu D.D., Zhu Y., Gao P., Tang H., Zhao J., Zhang Z., Yang J., Hu Z., Fu Y.X., Zheng Y.T., Peng H. Interferon-armed rbd dimer enhances the immunogenicity of rbd for sterilizing immunity against sars-cov-2. Cell Res. 2021;31:1011–1023. doi: 10.1038/s41422-021-00531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Chen Y., Li S., Zhou Y., Zhang Y., Pei R., Chen X., Wang Y. Immunization of mice with vaccinia virus tiantan strain yields antibodies cross-reactive with protective antigens of monkeypox virus. Virol. Sin. 2023;38:162–164. doi: 10.1016/j.virs.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R.R., Wang Z.J., Zhu Y.L., Tang W., Zhou C., Zhao S.Q., Wu M., Ming T., Deng Y.Q., Chen Q., Jin N.Y., Ye Q., Li X., Qin C.F. Rational development of multicomponent mrna vaccine candidates against mpox. Emerg. Microb. Infect. 2023;12:2192815. doi: 10.1080/22221751.2023.2192815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Wang H., Cheng L., Zhao C., Zhou X., Liao X., Ge X., Liu L., Lu X., Ju B., Zhang Z. Two long-lasting human monoclonal antibodies cross-react with monkeypox virus a35 antigen. Cell Discov. 2023;9:50. doi: 10.1038/s41421-023-00556-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.