Version Changes
Revised. Amendments from Version 1
In this revision, several enhancements have been made to augment the clarity and comprehensibility of the manuscript while effectively addressing reviewer comments. Textual refinements have been implemented to ensure improved readability throughout the document. An addition comprises the incorporation of additional references and links, notably for resources such as Nextclade, Pangolin, and the precalculated Kraken 2 database on Zenodo. This improves accessibility by providing readers with easily accessible supplementary materials. Also, we appended a list of abbreviations to facilitate smoother comprehension of specialized terminology, addressing a reviewer's comment. Furthermore, the conclusion section has been extended to delineate the potential applications of the methodologies employed in this study to other viruses, thereby broadening the scope and relevance of the research findings. We included a comment elucidating the rationale behind the selection of specific tools, offering insights into the methodological choices made during the research process. The results heading has been removed to streamline the structure and address reviewer feedback, contributing to a more coherent manuscript organization. Lastly, funding sources have been added.
Abstract
Background
Accurate genome sequences form the basis for genomic surveillance programs, the added value of which was impressively demonstrated during the COVID-19 pandemic by tracing transmission chains, discovering new viral lineages and mutations, and assessing them for infectiousness and resistance to available treatments. Amplicon strategies employing Illumina sequencing have become widely established for variant detection and reference-based reconstruction of SARS-CoV-2 genomes, and are routine bioinformatics tasks. Yet, specific challenges arise when analyzing amplicon data, for example, when crucial and even lineage-determining mutations occur near primer sites.
Methods
We present CoVpipe2, a bioinformatics workflow developed at the Public Health Institute of Germany to reconstruct SARS-CoV-2 genomes based on short-read sequencing data accurately. The decisive factor here is the reliable, accurate, and rapid reconstruction of genomes, considering the specifics of the used sequencing protocol. Besides fundamental tasks like quality control, mapping, variant calling, and consensus generation, we also implemented additional features to ease the detection of mixed samples and recombinants.
Results
We highlight common pitfalls in primer clipping, detecting heterozygote variants, and dealing with low-coverage regions and deletions. We introduce CoVpipe2 to address the above challenges and have compared and successfully validated the pipeline against selected publicly available benchmark datasets. CoVpipe2 features high usability, reproducibility, and a modular design that specifically addresses the characteristics of short-read amplicon protocols but can also be used for whole-genome short-read sequencing data.
Conclusions
CoVpipe2 has seen multiple improvement cycles and is continuously maintained alongside frequently updated primer schemes and new developments in the scientific community. Our pipeline is easy to set up and use and can serve as a blueprint for other pathogens in the future due to its flexibility and modularity, providing a long-term perspective for continuous support. CoVpipe2 is written in Nextflow and is freely accessible from {https://github.com/rki-mf1/CoVpipe2}{github.com/rki-mf1/CoVpipe2} under the GPL3 license.
Keywords: SARS-CoV-2, genome reconstruction, whole-genome sequencing, short reads, Illumina, amplicons, WGS, Nextflow pipeline, virus bioinformatics
List of abbreviations
BAM file: Binary Alignment and Map file
BED file: Browser Extensible Data file
BEDPE file: Browser Extensible Data Paired-End file
CCO license: Creative Commons Zero license
CDC: Centers for Disease Control and Prevention
CorSurV: Coronavirus Surveillance Verordnung (eng., Coronavirus Surveillance Regulation)
COVID-19: Coronavirus disease 2019
CSV file: Comma-Separated Values file
DESH: Deutscher Elektronischer Sequenzdaten-Hub (eng., German Electronic Sequence Data Hub)
EBI: European Bioinformatics Institute
EMBL: European Molecular Biology Laboratory
ENA: European Nucleotide Archive
GFF file: General Feature Format file
GISAID: Global Initiative on Sharing All Influenza Data
GPL3 license: GNU General Public License
HPC: High-Performance Computing
HTML: Hypertext Markup Language
ID: Identifier
IMS-SC2: Integrated Molecular Surveillance for SARS-CoV-2
indel: Insertion/Deletion Variant
IUPAC: International Union of Pure and Applied Chemistry
JSON file: JavaScript Object Notation file
NGS:Next-Generation Sequencing
ONT: Oxford Nanopore Technologies
PCR: Polymerase Chain Reaction
QC: Quality Control
RKI: Robert Koch Institute
SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2
SRA: Sequence Read Archive
VCF file: Variant Call Format file
VOC: Variants of Concern
VOI: Variants of Interest
WSL: Windows Subsystem for Linux
Introduction
Since the publication of the first genome sequence of the novel SARS-CoV-2 virus in January 2020 – just 12 days after the initial report of the virus – the international GISAID database 1 – 3 now includes more than 15.5 million SARS-CoV-2 whole-genome sequences (accessed May 23, 2023). The genomic data and metadata collected in GISAID and other resources such as EBI’s COVID-19 Data Portal 4 are pivotal for the largest worldwide genomic surveillance effort ever undertaken to track the evolution and spread of the virus causing the COVID-19 disease. Important viral genome regions have been monitored for mutations, for example, in the S (spike) gene and other immunologically relevant loci. The reconstruction of accurate SARS-CoV-2 genomic sequences is paramount to detect and track substitutions, insertions, and deletions correctly; interpret them in terms of vaccine development, test the efficiency of target regions and antibody binding sites, detect outbreaks and transmission chains, and finally inform public health authorities to consider adjustment of containment measures. 5
According to Ewan Birney, director of EMBL-EBI in Cambridge, U.K., “Genome sequencing is routine in the same way the U.S. Navy routinely lands planes on aircraft carriers. Yes, a good, organized crew does this routinely, but it is complex and surprisingly easy to screw up.”. 6
This quote is no less accurate for genome reconstruction, a crucial step in SARS-CoV-2 genomic surveillance. While sequencing efforts were scaling up rapidly around the globe, several pipelines for the reference-based assembly of SARS-CoV-2 genomes were developed in parallel and in an attempt to rapidly generate the necessary genome sequences ( Table 1). During the first lockdown in Germany in mid-March 2020, the Bioinformatics unit at the Robert Koch Institute (RKI), Germany’s Public Health institute, also started developing a genome reconstruction pipeline, specifically targeting Illumina amplicon sequencing data and amplicon primer schemes. During the development, the pipeline was extensively tested and has gone through continuous improvement due to adjusted wet lab protocols and primer schemes, to accurately call variants in low-coverage regions and near primer sites, to deal with deletions and low-coverage regions correctly, and to robustly reconstruct high-quality SARS-CoV-2 consensus sequences for downstream analyses and genomic surveillance. Due to these features and tests, CoVpipe1 was successfully used in the past at RKI’s sequencing facility and several labs nationwide. 7 – 10 If not addressed appropriately, genotyping errors can lead to wrong consensus sequences and thus impact downstream analyses such as phylogenetic reconstructions and transmission chain tracking in outbreaks. 11
Table 1. A collection of available software for SARS-CoV-2 genome reconstruction.
Here, we mainly focus on open-source pipelines with available source code, specifically targeting the reconstruction of SARS-CoV-2 genomes, also including general pipelines adapted to work with SARS-CoV-2 sequencing data (e.g., V-pipe). Publ. – Publication focusing on the pipeline in a preprint or a peer-reviewed journal, Seq. tech. – focused sequencing technology, Implementation – main software backbone to run the tool (please note that not all pipelines use a workflow management system such as Nextflow 25 or Snakemake 26 ), Dependencies – a list of options to handle necessary software dependencies, Latest release – latest available release version as accessed May 23, 2023. mNGS – sequencing approach not based on amplicons but rather sequencing all available RNA/cDNA (metatranscriptomics/genomics).
| Tool | Publ. | Code | Seq. tech. | Implementation | Dependencies | Latest release |
|---|---|---|---|---|---|---|
| CoVpipe2 | – | github.com/rki-mf1/covpipe2 | Illumina | Nextflow | Conda, Mamba, Docker, Singularity | 0.4.2 (2023-06-09) |
| poreCov | 24 | github.com/replikation/poreCov | Nanopore | Nextflow | Docker, Singularity | 1.8.3 (2023-05-12) |
| viralrecon | 27 | github.com/nf-core/viralrecon | Illumina, Nanopore | Nextflow | Conda, Docker, Singularity, Podman, Shifter, Charliecloud | 2.6.0 (2023-03-23) |
| SIGNAL | 28 | github.com/jaleezyy/covid-19-signal | Illumina | Snakemake | Conda, Mamba, Docker (a single container with all dependencies) | 1.6.2 (2023-05-20) |
| V-pipe | 29 | github.com/cbg-ethz/V-pipe | Illumina | Snakemake | Conda, Docker (a single container with all dependencies) | 2.99.3 (2022-11-02) |
| NCBI SC2VC | – | github.com/ncbi/sars2variantcalling | Illumina, Nanopore, PacBio | Snakemake (multiple pipelines, run via wrapper script) | Docker (a single container with all dependencies) | 3.3.4 (2022-10-28) |
| VirPipe | 30 | github.com/KijinKims/VirPipe | Illumina, Nanopore (mNGS) | Python, Nextflow | Conda and Docker | 1.0.0 (2022-09-24) |
| ViralFlow | 31 | github.com/dezordi/ViralFlow | Illumina | Python | Conda, Singularity, Docker (one single container/environment) | v.0.0.6 (2021-04-18) |
| EDGE COVID-19 | 32 | github.com/LANL-Bioinformatics/EDGE/tree/SARS-CoV2 | Illumina, Nanopore | Python, Perl, available as web tool | Docker (a single container with all dependencies) | 2.4.0 (2020-12-03) |
| Galaxy-COVID19 | 33 | galaxyproject.org/projects/covid19/workflows | Illumina (amplicon & mNGS), Nanopore | Galaxy | Access to a Galaxy instance | different pipelines & versions |
| HAVoC | 34 | bitbucket.org/auto_cov_pipeline/havoc | Illumina | Shell scripts | Conda, Mamba | no release |
| PipeCoV | 35 | github.com/alvesrco/pipecov | Illumina (amplicon & mNGS) | Shell scripts | Docker | no release |
While sequencing intensity increased and turnaround times on variant detection decreased in different countries, there are also major disparities between high-, low- and middle-income countries in the SARS-CoV-2 global genomic surveillance efforts. 12 A recent study also found significant differences between bioinformatics approaches that use the same input data but detect different variants in SARS-CoV-2 samples. 13 In addition, each technology and sequencing approach has its own advantages and limitations, challenging a harmonized genomic surveillance of the virus. 14
In Germany, sequencing efforts increased tremendously in January 2021 following the entry into force of a federal directive (Coronavirus Surveillance Verordnung—CorSurV). Subsequently, a large-scale, decentralized genomic sequencing and data collection system (“Deutscher Elektronischer Sequenzdaten-Hub”, DESH) 15 has been established and was running until June 2023, accompanied by a medium-scale integrated molecular surveillance infrastructure (IMS-SC2) which is still continued at the RKI. 8 By May 22, 2023, 1,227,036 whole-genome SARS-CoV-2 sequences that met the quality criteria were transmitted to the RKI via DESH. Due to their low cost, sensitivity, flexibility, specificity, and efficiency, amplicon-based sequencing designs are broadly used for SARS-CoV-2 sequencing and reference-based genome reconstruction. 16 – 20 From the 1.2 million DESH genomes (publicly available at github.com/robert-koch-institut/SARS-CoV-2-Sequenzdaten_aus_Deutschland), 1.1 million (90.91%) were sequenced with Illumina devices, highlighting the importance of the technology for genomic surveillance ( Table 2). Illumina technology has a lower share at the international level (78.57%), while Oxford Nanopore Technologies (ONT) increased to 12.73% ( Table 2). However, Illumina remains the most widely used approach among the various sequencing technologies, followed by ONT sequencing by a wide margin. A recent benchmark study also showed the advantages of using Illumina MiSeq compared to ONT GridION for SARS-CoV-2 sequencing, resulting in a higher number of consensus genomes classified by Nextclade 21 as good and mediocre. 22 However, these results are, of course, also dependent on the bioinformatics toolchains and could change as ONT becomes more accurate. 23 In addition, although both technologies require the same computational steps for reference-based genome reconstruction (preprocessing, mapping, variant calling, consensus), they need different tools optimized for either short- or long-read data and the associated error profiles to produce high-quality consensus sequences. Therefore, we developed one pipeline, especially for ONT data, 24 and CoVpipe2, specifically targeting SARS-CoV-2 amplicon data derived from short-read Illumina sequencing. CoVpipe2 is a Nextflow re-implementation of CoVpipe1 (written in Snakemake, gitlab.com/RKIBioinformaticsPipelines/ncov_minipipe) and comes with additional features, simplified installation, full container support, and continuous maintenance.
Table 2. Sequencing technologies used for SARS-CoV-2 sequencing of German and international samples.
Data based on 1.2 million and 15.5 million whole-genome sequences submitted to the German DESH portal (“Deutscher Elektronischer Sequenzdaten-Hub”) and to the GISAID database, respectively (accessed: May 22, 2023). To the best of our knowledge, we corrected typos such as Nanonopore, Nasnopore, and Illunima in the GISAID metadata and summarized the available terms into the broader categories shown in this table ( e.g., NextSeq500 ⇒ Illumina). Entries we could not assign to one of the listed sequencing technologies were added to the Other/Unknown category. Please note that most German DESH sequences are also part of the GISAID data set. # – Number of sequences, % – Percentage of sequences on total data set.
| Sequencing technology | # DESH | % DESH | # GISAID | % GISAID |
|---|---|---|---|---|
| Illumina | 1,115,501 | 90.91 | 12,240,675 | 78.57 |
| Nanopore | 75,358 | 6.14 | 1,984,505 | 12.73 |
| SMRT PacBio | 0 | 0 | 573,214 | 3.67 |
| Ion Torrent | 29,450 | 2.40 | 349,001 | 2.24 |
| MGI DNBSEQ | 0 | 0 | 177,480 | 1.13 |
| Sanger | 0 | 0 | 57,820 | 0.37 |
| Other/Unknown | 6,727 | 0.54 | 195,833 | 1.25 |
| Total | 1,227,036 | 100 | 15,578,528 | 100 |
Here we present CoVpipe2, our pipeline engineered over nearly three years of pandemic genome sequencing that accurately reconstructs SARS-CoV-2 consensus sequences from Illumina short-read sequencing data, focusing on challenges associated with amplicon sequencing on a large scale. Besides implementation details, we also highlight pitfalls we discovered and solved during the pipeline development, focusing on variant calling artifacts and how we deal with them in the pipeline.
Methods
Implementation
CoVpipe2 is implemented using the workflow management system Nextflow 25 to achieve high reproducibility and performance on various platforms. The user can choose to use CoVpipe2 with Conda or Mamba support, 36 or containers (Docker, 37 Singularity 38 ) to handle all software dependencies. The Conda/Mamba environments and the container images are preconfigured and have fixed versions of the incorporated tools. The precompiled Docker containers are stored on hub.docker.com/u/rkimf1. Containers and environments are downloaded and cached automatically when executing CoVpipe2. If needed, the Docker images can also be converted into Singularity images by the pipeline. CovPipe2 includes Nextclade 39 and pangolin 40 for lineage assignment. Both tools rely on their latest code and database versions. To address this, we implemented the --update option inspired by poreCov, 24 which triggers an update to the latest available version from anaconda.org/bioconda/pangolin and anaconda.org/bioconda/nextclade, or hub.docker.com/u/rkimf1, respectively. --update is disabled by default; the tool versions can also be pinned manually.
When CoVpipe2 is running on a high-performance computing (HPC) cluster ( e.g., SLURM, LSF) or in the cloud ( e.g., AWS, GCP, Azure), all resources (CPUs, RAM) are pre-configured for all processes but can be customized via a user-specific configuration file. We use complete version control for CoVpipe2, from the workflow itself (releases) to each tool, to guarantee reproducible results. To this end, all Conda/Mamba environments and containers use fixed versions. In addition, each CoVpipe2 release can be invoked and executed individually, and the tool versions used during genome reconstruction and analysis are listed in Nextflow report files.
CoVpipe2 is publicly available under a GPL-3.0 license at github.com/rki-mf1/covpipe2, where details about the implementation and different executions of CoVpipe2 can be found. We use GitHub’s CI for various pipeline tests, particularly a dry-run to check for integrity and an end-to-end test with special attention to the called variants to ensure continuous code quality and robust results.
Operation
As a minimal setup, Nextflow (minimal version 22.10.1, nextflow.io) and either Conda, Mamba, Docker, or Singularity need to be installed for CoVpipe2. Nextflow can be used on any POSIX-compatible system, e.g., Linux, OS X, and on Windows via the Windows Subsystem for Linux (WSL). Nextflow requires Bash 3.2 (or later) and Java 11 (or later, up to 18) to be installed. Initial installation and further updates to the workflow and included tools can be performed with simple commands:
# install (or update) the pipeline
nextflow pull rki-mf1/CoVpipe2
# check available pipeline versions
nextflow info rki-mf1/CoVpipe2
# run a certain release version
nextflow run rki-mf1/CoVpipe2 -r v0.4.1 --help
# test the installation with local execution and Conda
nextflow run rki-mf1/CoVpipe2 -r v0.4.1 -profile local,conda,test --cores 2 --max_cores 4
The pipeline can be executed on various platforms controlled via the Nextflow - profile parameter, which makes it easily scalable, e.g., for execution on an HPC. Each run profile is created by combining different Executors (local, slurm) and Engines (conda, mamba, docker, singularity); the default execution-engine combination (profile) is -profile local, conda.
An overview of the workflow is given in Figure 1. FASTQ files and a reference genome sequence (FASTA) are the minimum required pipeline inputs. If no reference genome sequence is provided, the SARS-CoV-2 index case reference genome with accession number MN908947.3 (identical to NC_045512.2) is used by default (as well as the corresponding annotation GFF), and then only FASTQ files are required. All files can be provided via file paths or defined via a comma-separated sample sheet (CSV); thus, CoVpipe2 can run in batch mode and analyze multiple samples in one run. Optionally, raw reads are checked for mixed samples with the tool LCS. 41 Next, raw reads are quality-filtered and trimmed using fastp 42 and optionally filtered taxonomically by Kraken 2. 43 We provide an automated download of a precalculated Kraken2 database ( doi.org/10.5281/zenodo.6333909) composed of SARS-CoV-2 and human genomes from Zenodo ( zenodo.org). However, the user is free to use a custom database. The reads are aligned to the reference genome using BWA, 44 and the genome coverage is calculated by BEDTools genomecov. 45 Primers can be optionally clipped after mapping with BAMclipper, 46 which is essential to avoid contaminating primer sequences in amplicon data. To locate the primer sequences, a browser extensible data paired-end ( BEDPE) file containing all primer coordinates is required as input. If only a BED file is provided, CoVpipe2 can automatically convert it to a BEDPE file. Users can also choose from the provided popular VarSkip ( github.com/nebiolabs/VarSkip) and ARTIC primer schemes. 47 Next, FreeBayes 48 calls variants (default thresholds: minimum alternate count of 10, minimum alternate fraction of 0.1, and minimum coverage of 20), which are normalized with BCFtools norm. 49 Resulting variants are analyzed and annotated with SnpEff, 50 filtered by QUAL (default 10), INFO/SAP (default disabled), and INFO/MQM (default 40) values, and optionally adjusted for the genotype (default enabled with minimum variant frequency 0.9). Thus, in the case of mixed variants, the predominant variant can be defined as a homozygous genotype if its frequency reaches a certain threshold. With these settings, alternate variants with a more than 90% frequency are set as alternative nucleotides. Furthermore, CoVpipe2 filters out indels below a certain allele frequency, which is, by default, enabled with a minimum allele frequency of 0.6. We create a low coverage mask, without deletions, from the mapping file and the adjusted and filtered VCF file (default coverage cutoff 20), which is then used in the consensus calling with BCFtools consensus. We output an ambiguous consensus sequence with IUPAC characters 51 (RYMKSWHBVDN) and a masked one only containing ACTG and Ns. Then, PRESIDENT ( GitHub) assesses the quality of each reconstructed genome via pairwise alignment to the SARS-CoV-2 index case (NC_045512.2) using pblat 52 with an identity threshold of 0.9 and N threshold of 0.05 per default. pangolin assigns a lineage and Nextclade annotates the mutations; the Nextclade alignment serves as input for sc2rf (original repository from github.com/lenaschimmel/sc2rf, with updates from github.com/ktmeaton/ncov-recombinant) for detection of recombinants. If an annotation file is provided, the reference annotation is mapped to the reconstructed genome with Liftoff. 53
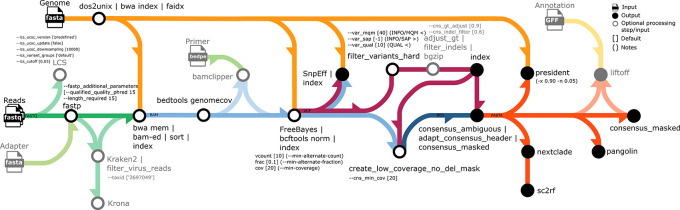
Figure 1. Overview of the CoVpipe2 workflow.
The illustration shows all input (
 ) and output (
) and output (
 ) files as well as optional processing steps and optional input (
) files as well as optional processing steps and optional input (
 ). For each computational step, the used parameters and default values (in brackets [...]) are provided, as well as additional comments ((...)). The arrows connect all steps and are colored to distinguish different data processing steps: green – read (FASTQ) quality control and taxonomy filtering, yellow – reference genome (FASTA) and reference annotation (GFF) for lift-over, blue – mapping files (BAM) and low coverage filter (BED), purple – variant calls (VCF), orange – consensus sequence (FASTA). The icons and diagram components that make up the schematic figure were originally designed by James A. Fellow Yates and
nf-core under a CCO license (public domain).
). For each computational step, the used parameters and default values (in brackets [...]) are provided, as well as additional comments ((...)). The arrows connect all steps and are colored to distinguish different data processing steps: green – read (FASTQ) quality control and taxonomy filtering, yellow – reference genome (FASTA) and reference annotation (GFF) for lift-over, blue – mapping files (BAM) and low coverage filter (BED), purple – variant calls (VCF), orange – consensus sequence (FASTA). The icons and diagram components that make up the schematic figure were originally designed by James A. Fellow Yates and
nf-core under a CCO license (public domain).
All results are summarized via an R Markdown template. The resulting HTML-based report summarizes different quality measures and mapping statistics for each input sample, thus allowing the user to spot low-performing samples even in extensive sequencing runs with many samples. A conditional notification warns the user if samples identified as negative controls (by matching the string ’NK’) show high reference genome coverage (threshold over 0.2).
We carefully selected the bioinformatics tools integrated into CoVpipe2 based on internal benchmarks and in-depth manual reviews of sequencing data, mapping results, and called variants. Based on our hands-on experience with SARS-CoV-2 sequencing datasets during the pandemic, this approach ensured that tools that can robustly detect high allele depth and well-covered variants are used for detection. Despite the primary goal of CoVpipe2 to identify high-confidence variants to reconstruct robust consensus genomes for genomic surveillance, the pipeline also provides flexibility for adaptation to different research environments.
Common variant calling challenges in amplicon-based genome reconstruction and solutions in CoVpipe2
Here, we highlight some implementation decisions that prevent common pitfalls and thus improve the quality of reconstructed SARS-CoV-2 genomes. Although amplicon-based approaches are widely used, these technologies are associated with flaws and limitations that must be considered to ensure that the genotypes obtained, and thus the resulting genome sequences, are reliable. 13 , 54 While some of these implementations might seem obvious and easy to fix, we frequently observed specific errors in the vast amount of consensus genome sequences sent to us via the DESH system.
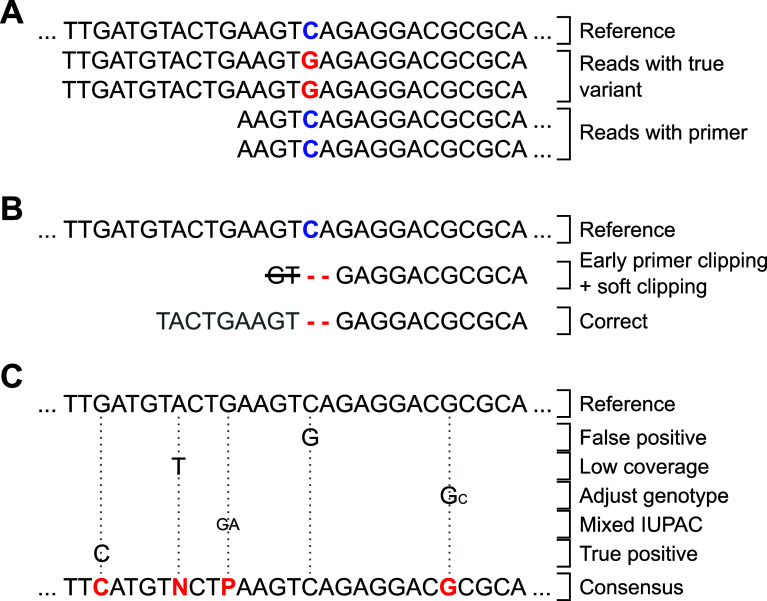
Accurate primer clipping to avoid dilution and edge effects: Primer clipping is an essential step for amplicon sequencing data because primers are inherent to the reference sequence and can disguise true variants in the sample. 13 However, removing primers before the mapping step can result in unwanted edge effects. 55 For example, deletions located close to the end of amplicons may be soft-clipped by the mapping software and hence can not be called as variants subsequently ( Figure 2). Therefore, primer clipping should be performed after mapping to prevent any soft clipping of variants close to the amplicon ends.
Figure 2. Exemplary cases that should be considered during genotyping.
(A) Primer sequences need clipping to call true variants (red) and not mask them via reference bases (blue). (B) Early primer clipping may result in missed deletions due to algorithmic soft clipping (primer sequence in light gray). (C) Genotyping parameters must be carefully set to reliably call different variant cases and represent them in the final consensus. CoVpipe2 puts primer clipping after the mapping step to prevent B) and implements carefully chosen default parameters for robust genotyping also of mixed variants, C).
As an example and worst-case scenario, clipping primer sequences before mapping bears the risk of missing a critical deletion used to define the previous variant of concern (VOC) B.1.1.7, namely deletion HV69/70 in the spike gene (S:H69-, S:V70-). We observed such a misclassification using Paragon CleanPlex amplicon-based sequencing. The kit uses primers similar to the ARTIC protocol V3, where the deletion S:HV69/70 is close to the end of an amplicon. If primer clipping is performed before mapping, the mapping tool might soft clip the amplicon end rather than opening a gap, which is more expensive than masking a few nucleotides ( Figure 2). We checked 151,565 B.1.1.7 sequences obtained via DESH for the characteristic S:H69, S:V70 deletions and found that 139,891 (92.3%) included the deletion. The remaining sequences contained the deletion only partially or lacked it completely. It is unlikely that these B.1.1.7 sequences lost this characteristic deletion. Thus we assume that some of the reconstructed Alpha sequences sent to the RKI from different laboratories in Germany do not account for the described effect and thus miss the detection of this deletion. Unfortunately, we don’t know which bioinformatics pipelines the submitting laboratories used and can only assume an error because of missing or incorrect primer clipping. To avoid this problem, we shift primer clipping after the read mapping step in CoVpipe2; otherwise, a vital feature of an emerging virus lineage might be missed if mutations accumulate close to amplicon ends.
Genotype adjustment to exclude sporadic variant calls: As an additional feature, we provide the option to adjust the genotype for sites where the vast majority of reads support a variant call, but the variant was called heterozygous. By default, the genotype of these locations is set to homozygous if a particular variant call is supported by 90% of the aligned reads.
Deletion-aware masking of low-coverage regions: We ensure that only low-coverage positions that are not deletions are masked. Several pipelines implement a feature to mask low-coverage regions. However, deletions are basically genomic regions with no coverage, and if not appropriately implemented, a pipeline might accidentally mask deletions as low-coverage regions. To prevent this, CoVpipe2 creates a low coverage mask (default minimum coverage 20) from the BAM file with BEDtools genomecov. In the second step, BEDtools subtract removes all deleted sites from the low-coverage mask. Finally, the mask is used in the consensus generation with BCFtools consensus.
IUPAC consensus generation with indel filter: CoVpipe2 generates different consensus sequences based on the IUPAC code. First, an explicit consensus is generated where all ambiguous sites and low-covered regions are hard-masked. Second, a consensus where only low-covered regions are hard-masked. The pipeline includes as much information as possible in the unambiguous consensus sequence by adding low-frequency variants with the respective IUPAC symbol. However, no symbol represents “indel or nucleotide”, so indels are always incorporated into the consensus sequence. As a result, low-frequency or heterozygote indels, often false positives, can introduce frameshifts into the consensus sequence. We overcome this with an indel filter based on allele frequency before consensus generation. Thus, indels below a defined threshold (per default 0.6) are not incorporated in the consensus sequences but can still be looked up in the VCF file.
Additional features beyond genome reconstruction
Over time, we added features beyond genome reconstruction to CoVpipe2 to answer newly emerging questions during the pandemic. The modular design of our implementation makes this seamless.
Mixed infections and recombinants: We included LCS for raw reads. LCS was originally developed for the SARS-CoV-2 lineage decomposition of mixed samples, such as wastewater or environmental samples. 41 In amplicon-based SARS-CoV-2 sequencing, we use LCS results to indicate potential new recombinants, and possible mixed infections (co-infections with different SARS-CoV-2 lineages).
Further, we added sc2rf to detect potential new recombinants via screening the consensus genome sequences. Like other tools and scripts, sc2rf depends on up-to-date lineage and mutation information, specifically, on a manually curated JSON file. We switched to another repository ( JSON GitHub project) than the original one that includes the sc2rf scripts because of more frequent updates on the JSON file.
Keeping up to date with lineage and clade assignments: We implemented an update feature for pangolin and Nextclade. Both tools, especially pangolin, rely on the latest datasets for the lineage assignment of newly designated Pango lineages. 56 Depending on the selected engine, Conda/Mamba or container execution, CoVpipe2 checks for the latest available version from Anaconda or our DockerHub, respectively. The tool versions can also be pinned manually.
Prediction of mutation effects: The called variants are annotated and classified based on predicted effects on annotated genes with SnpEff. SnpEff reports different effects, including synonymous or non-synonymous SNPs, start codon gains or losses, stop codon gains or losses; and classifies them based on their genomic locations. Lastly, CoVpipe2 uses Liftoff to generate an annotation for each sample if a reference annotation is provided.
Selection of benchmark datasets and pipeline evaluation
We compared the results of CoVpipe2 (v0.4.0) with publicly available benchmark datasets for SARS-CoV-2 surveillance 57 ( GitHub CDC data). For our study, we selected from the available benchmark datasets all samples that were sequenced with the ARTIC V3 primer set (according to CDC data, release v0.7.2), ending up with in total 54 samples from three datasets:
-
•
i) 16 samples from variant of interest (VOI)/VOC lineages (dataset 4)
-
•
ii) 33 samples from non-VOI/VOC lineages (dataset 5)
-
•
iii) 5 samples from failed QC (dataset 6)
We stick to the naming scheme (dataset 4, 5, and 6) as declared in the original study. 57 For all 54 samples, we downloaded the raw reads from ENA with nf-core/fetchngs ( v1.9). 58 For 49 samples, we downloaded the available consensus sequences from GISAID 1 – 3 (samples from dataset 4 and 5). We run CoVpipe2 with Singularity, species filtering ( --kraken) and the latest pangolin and Nextclade versions at that point (containers: rkimf1/pangolin:4.2-1.19--dec5681, rkimf1/nextclade2:2.13.1--ddb9e60). We further examined the consensus sequences from dataset 4 and 5, comparing CoVpipe2 and GISAID sequences: We compared lineage information of the reconstructed genomes assigned by pangolin with the indicated lineages from CDC data. Note that the pangolin versions and datasets differ. Also, we run Nextclade (same container version as noted above) to compare the mutation profile, and MAFFT, 59 v7.515 (2023/Jan/15), for comparison on sequence level of the unambiguous IUPAC consensus of CoVpipe2.
Reporting
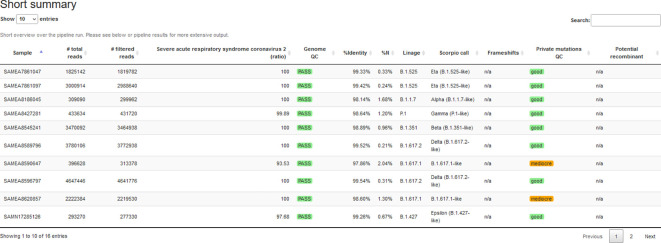
The HTML report consists of several sections and summarizes different results. The first table aggregates critical features for each sample: the number of reads, genome QC, the assigned lineage, and recombination potential, see Figure 3. Furthermore, read properties such as the number of bases and the length of reads (before/after trimming) are summarized. An optional table lists the species filtering results emitted by Kraken 2. 43 The report summarizes reads mapped to the reference genome (number and fraction of input) and the median/standard deviation of fragment sizes, shown as a histogram plot. Genome-wide coverage plots allow users to observe low-coverage regions and potential amplicon drop-outs to optimize primers. We summarize the output for all samples in different tables: i) genome quality from PRESIDENT output, ii) lineage assignments from pangolin output, and iii) variants in amino acid coordinates and detected frameshifts from Nextclade output.
Figure 3. Extract of CoVpipe2’s summary report for dataset 4.
The standalone HTML report summarizes different quality measures and tool results. The overview table can include a conditional notification for negative controls with high reference genome coverage (not shown in this example). All tables are searchable and sortable.
Discussion
CovPipe2 reconstructs consensus genomes matching previously reported SARS-CoV-2 lineages
Here, we compare the results of CoVpipe2 against a selection of available benchmark datasets 57 and their respective consensus genome sequences available from GISAID. 1 – 3 We discuss the observed differences. No software is perfect, and CoVpipe2 may have problems with certain combinations of amplicon schemes and sequencing designs, leading to specific borderline cases in variant detection, which we also highlight and discuss. In addition, in conflicting cases, the real sequence often stays unknown until further sequencing efforts are performed. This makes continuous development and testing of bioinformatics pipelines all the more critical.
Dataset 4, VOI/VOC lineages
Lineages Despite the different pangolin tool versions and lineage definitions that changed over time, all pangolin lineages from CoVpipe2 match the corresponding lineages reported at github.com/CDCgov/datasets-sars-cov-2, see Extended data, Table S1.
Pairwise alignment The sequence identity ranges from 98.44 % to 99.79 % (including Ns) between the corresponding GISAID and CoVpipe2 genome pairs ( Extended data, Table S2). Nine out of 16 sequences are identical when mismatches resulting from gaps are not considered. For one sample, the corresponding GISAID and CoVpipe2 sequences contain three ACGT-nucleotide mismatches. Seven out of 16 GISAID consensus sequences do not contain any Ns, possibly indicating that low coverage regions have been not masked. The respective reconstructed genomes from CoVpipe2 contain Ns located in the first or last 150 nucleotides of the genome sequences, thus masking low coverage regions ( Extended data, Table S3). Due to tiled PCR amplicons, 5′ and 3′ ends of the genome usually have too little coverage or are not sequenced. The genome ends containing Ns seem to be trimmed in 14 GISAID genomes. Overall, the number of Ns in the GISAID and CoVpipe2 genomes is comparable, with CoVpipe2 genomes tending to have more Ns due to the low coverage filter resulting in Ns at genome ends. In addition, CoVpipe2 does not trim Ns from the genome ends and might be more conservative with its default settings, as positions below 20 X coverage ( --cns_min_cov) will be masked with ambiguous N bases in the consensus sequence.
Mutations Although all genomes reconstructed from CoVpipe2 were assigned to the same lineage as previously reported ( CDC data), there are minor differences in Nextclade’s mutation profile, Extended data, Table S4. Three of 16 reconstructed genomes have one or two nucleotide substitutions more than the respective GISAID genome.
Dataset 5, non-VOI/VOC lineages
Lineages Pangolin lineages from CoVpipe2 exactly match the reported lineages at CDC data in 27 of 33 samples, see Extended data, Table S5. For five samples, the pangolin lineage based on the genome sequence reconstructed by Covpipe2 is the parent lineage of the expected sub-lineage. For example, the genome sequence of sample SAMN15919634 was assigned to the B.1.1 lineage after CoVpipe2 reconstruction, whereas the corresponding GISAID sequence was assigned to B.1.1.431 ( CDC data). Since the lineage assignments of Nextclade exactly match for each CoVpipe2-GISAD pair, the different pangolin and pangolin data versions used in CoVpipe2 and Xiaoli and Hagey et al. 57 are most likely the reason for this discrepancy. Different parameter thresholds can also lead to different lineage assignments. For example, the genome sequence of SAMN17571193 was assigned to B.1.1.450 by CoVpipe2 compared to B.1.1.391 in Xiaoli and Hagey et al. 57 The mutation profile differs by two additional SNPs (genome coordinates: C3037T and C3787T) constituting one mutation on amino acid level (ORF1a:D1962E) in the GISAID consensus sequence. Both positions (3037 and 3787) have a read coverage below 20, so they are not eligible for variant calling with default settings in CoVpipe2 and are masked with N. Therefore, this difference in lineage assignment is due to CoVpipe2’s more restrictive approach to integrating the called variants into the consensus.
Pairwise alignment The pairwise sequence identity ranges from 95.96 % to 99.80 % (including Ns) between the GISAID and CoVpipe2’s reconstructed genome sequences ( Extended data, Table S6). Ignoring gap mismatches, 13 out of 33 sequences are identical. No sample contains ACGT mismatches. 18 out of 33 GISAID consensus sequences do not contain any Ns. Sixteen of the respective reconstructed genomes have Ns, all located in the first or last 150 nucleotides ( Extended data, Table S7). Because of tiled PCR amplicons, the 5′ and 3′ ends typically have too little coverage or are not sequenced. The 5′ and 3′ genome ends containing Ns seem trimmed in 30 GISAID genomes. Overall, the number of Ns in the GISAID and CoVpipe2 genomes is comparable, with CoVpipe2 genomes tending to have more Ns. CoVpipe2 does not trim Ns from the genome ends and might be more conservative with its default settings, as positions below 20 ( --cns_min_cov) will be masked with N in the consensus sequence.
Mutations There are minor differences in Nextclade’s mutation profile ( Extended data, Table S8). Five of the 33 reconstructed genomes have one or two more nucleotide substitutions compared to the GISAID genome.
Dataset 6, samples failing quality control
For the five samples from the failed QC dataset by Xiaoli and Hagey et al., 57 CoVpipe2 correctly labeled four samples with failed genome QC. Three samples contain at least one frameshift, whereas two samples, SAMN17486862 and SAMN17822806, were reconstructed by CoVpipe2 without frameshifts ( Extended data, Table S9). The consensus sequence of sample SAMN17486862 passed QC according to CoVpipe2’s genome QC criteria.
All the selected samples from this dataset have originally failed QC because of a VADR 60 alert number greater than one. 57 VADR is part of the TheiaCoV (formerly ‘Titan’) 1.4.4 pipeline, 61 which was used to analyze the samples in Xiaoli and Hagey et al. 57 Among other things, VADR considers frameshifts, which do not occur in two genomes reconstructed with CoVpipe2. However, one of these two consensus genomes (SAMN17822806) contains too many Ns to pass CoVpipe2’s genome QC.
Limitations
The nature of a computational pipeline is that it is a chain of existing individual tools. Especially given the rapid evolution of SARS-CoV-2 during the pandemic, many reference-based tools rely on up-to-date databases and resources to reflect the current situation. For example, LCS depends on a variant marker table and user-defined variant groups. Similarly, sc2rf relies on a list of common variants for each lineage. In addition, Nextclade and pangolin periodically publish up-to-date datasets. Therefore, it is critical for a pipeline, especially in the context of a surveillance tool for rapidly evolving pathogens such as SARS-CoV-2, to allow for regular updates to the underlying data structures. While we have implemented some functionality to update tools such as Nextclade and pangolin automatically, this is not possible for all resources and can only be achieved through the continuous development and maintenance of a pipeline. Furthermore, our default settings may not fit all input data and must be selected carefully.
Finally, there must be enough good-quality input reads to reconstruct a genome successfully. In particular, with amplicon sequencing data, some regions might have a lower coverage due to amplicon drop-outs. Thus, the genome as a whole can be reconstructed with acceptable quality. However, some essential mutations can be missing due to low coverage or variant-calling quality.
Conclusions
Accurate and high-throughput genotyping and genome reconstruction methods are central for monitoring SARS-CoV-2 transmission and evolution. CoVpipe2 provides a fully automated, flexible, modular, and reproducible workflow for reference-based variant calling and genome reconstruction from short-read sequencing data, emphasizing amplicon-based sequencing schemes. Due to the implementation in the Nextflow framework, the setup and automatic installation of the required tools and dependencies is simple and allows the execution on different computing platforms. The comparison with a benchmark dataset showed comparable results where differences could be pinned down to different parameters, filtering thresholds, and tool versions used for lineage assignments. Amplicon-optimized default parameters and the ability to customize critical parameters, combined with comprehensive reporting, ensure the quality of reported SARS-CoV-2 genomes and prevent the inclusion of low-quality sequences in downstream analyses and public repositories. The pipeline is optimized for SARS-CoV-2 amplicon data but can also be used for other viruses and whole-genome sequencing protocols. We used CoVpipe1 and CoVpipe2, which were initially developed for SARS-CoV-2, to reconstruct the genomes of other viruses. By selecting different reference genomes for polio, measles, RSV, and influenza viruses and modifying the analysis processes for the latter two viruses, we were able to demonstrate the workflow’s potential as a universal blueprint for viral genome analysis. This adaptability has been demonstrated by the ability to identify consistent variants and the successful application to different viral characteristics, such as varying genome size and segmentation. However, it should also be noted that for segmented viruses, more customization is required than simply replacing the (non-segmented) reference genome. However, our experience suggests that customization of tools, parameters, and reporting requirements is needed for each virus, pointing to developing a harmonized pipeline for multiple pathogens. CoVpipe2 thus proves to be a robust tool for SARS-CoV-2 and paves the way for broad application in virology research by highlighting its ability to serve as a fundamental framework for building customized pipelines for a wide range of pathogens. CoVpipe2 will therefore form the basis for further genomic surveillance programs at the German Institute of Public Health, which will also extend to other viruses.
Acknowledgements
We gratefully acknowledge all data contributors, i.e., the Authors and their originating laboratories responsible for obtaining the specimens, and their submitting laboratories for generating the genetic sequence and metadata and sharing via the GISAID Initiative, on which this research is based. We also thank all German Electronic Sequence Data Hub contributors, the IMS-SC2 laboratory network, and all data providers, i.e., the originating laboratories responsible for obtaining samples and the submitting laboratories where genetic sequence data were generated and shared. We are incredibly grateful to Petra Kurzendörfer, Tanja Pilz, Aleksandar Radonić, and Aaron Houterman for outstanding sequencing support. We thank Marianne Wedde for manually checking and validating consensus sequences and called variants and Thorsten Wolff and Max von Kleist for fruitful discussions. We thank Ben Wulf, Fabian Rost, and Alexander Seitz – early users of the first version of CoVpipe – for valuable feedback and reporting issues.
Funding Statement
.L. was supported by the European Centre for Disease Control (grant number ECDC GRANT/2021/008 ECD.12222). This work was further supported by the European Health and Digital Executive Agency (grant number 101113012) and Bundesministerium für Wirtschaft und Klimaschutz, Daten- und KI-gestütztes Frühwarnsystem zur Stabilisierung der deutschen Wirtschaft (grant number 01MK21009H).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
Data availability
Underlying data
All SRA accession IDs of raw reads and GISAID IDs of consensus sequences are listed at 57 and github.com/CDCgov/datasets-sars-cov-2; and in our Open Science Framework repository osf.io/26hyx ( Extended data: https://doi.org/10.17605/OSF.IO/MJ6EQ). 62 We used ARTIC V3 samples from dataset 4 (VOI/VOC lineages, 16 samples), dataset 5 (non-VOI/VOC lineages, 33 samples), and dataset 6 (failedQC, 5 samples) from the original study of Xiaoli and Hagey et al. 57
The precalculated Kraken 2 database composed of SARS-CoV-2 and human genomes is available from Zenodo, https://doi.org/10.5281/zenodo.6333909. 63
Extended data
Open Science Framework. Lessons learned: overcoming common challenges in reconstructing the SARS-CoV-2 genome from short-read sequencing data via CoVpipe2, https://doi.org/10.17605/OSF.IO/MJ6EQ. 62
This project contains the following extended data:
-
•
Data folder Accession ID. (SRA and GISAID accession ID lists)
-
•
Data folder Comparison. (Scripts and results of the benchmark comparison)
-
•
Data folder CoVpipe2 results. (Results of CoVpipe2 for each benchmark dataset)
-
•
Data folder Extended data. (Supplementary tables S1-S9)
Software availability
-
•
Software and source code available from: https://github.com/rki-mf1/covpipe2
-
•
Archived source code at time of publication: https://doi.org/10.5281/zenodo.8082695. 64
-
•
License: GNU General Public License v3.0 (GPL3)
References
- 1. Shu Y, McCauley J: GISAID: Global initiative on sharing all influenza data – from vision to reality. Eurosurveillance. 2017;22(13):30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elbe S, Buckland-Merrett G: Data, disease and diplomacy: Gisaid’s innovative contribution to global health. Global Chall. 2017;1(1):33–46. 10.1002/gch2.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khare S, Gurry C, Freitas L, et al. : GISAID Core Curation Team, and Sebastian Maurer-Stroh. Gisaid’s role in pandemic response. 2021. . Reference Source [DOI] [PMC free article] [PubMed]
- 4. Harrison PW, Lopez R, Rahman N, et al. : The COVID-19 Data Portal: accelerating SARS-CoV-2 and COVID-19 research through rapid open access data sharing. Nucleic Acids Res. 2021;49(W1):W619–W623. 10.1093/nar/gkab417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robishaw JD, Alter SM, Solano JJ, et al. : Genomic surveillance to combat COVID-19: challenges and opportunities. Lancet Microbe. 2021;2(9):e481–e484. 10.1016/S2666-5247(21)00121-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Julianna LeMieux Genetic Engineering & Biotechnology News: All Aboard the Genome Express: Is a new generation of DNA sequencing technology about to hit the fast track?last accessed December 01, 2022. Reference Source
- 7. Hufsky F, Lamkiewicz K, Almeida A, et al. : Computational strategies to combat COVID-19: useful tools to accelerate SARS-CoV-2 and coronavirus research. Brief. Bioinform. 2021;22(2):642–663. 10.1093/bib/bbaa232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Djin Ye O, Hölzer M, Paraskevopoulou S, et al. : Advancing precision vaccinology by molecular and genomic surveillance of Severe Acute Respiratory Syndrome Coronavirus 2 in Germany, 2021. Clin. Infect. Dis. 2022;75(Supplement_1):S110–S120. 10.1093/cid/ciac399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baumgarte S, Hartkopf F, Hölzer M, et al. : Investigation of a limited but explosive COVID-19 outbreak in a German secondary school. Viruses. 2022;14(1):87. 10.3390/v14010087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loss J, Wurm J, Varnaccia G, et al. : Transmission of sars-cov-2 among children and staff in german daycare centres. Epidemiol. Infect. 2022;150:e141. 10.1017/S0950268822001194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Maio N, Walker C, Borges R, et al. : Issues with SARS-CoV-2 sequencing data.last accessed November 25, 2022. Reference Source
- 12. Brito AF, Semenova E, Dudas G, et al. : Global disparities in SARS-CoV-2 genomic surveillance. Nat. Commun. 2022;13(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Connor R, Yarmosh DA, Maier W, et al. : Towards increased accuracy and reproducibility in SARS-CoV-2 next generation sequence analysis for public health surveillance. bioRxiv. 2022.
- 14. Chiara M, D’Erchia AM, Gissi C, et al. : Next generation sequencing of SARS-CoV-2 genomes: challenges, applications and opportunities. Brief. Bioinform. 2021;22(2):616–630. 10.1093/bib/bbaa297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robert Koch Institute: Deutscher Elektronischer Sequenzdaten-Hub (DESH).last accessed November 25, 2022. Reference Source
- 16. Grubaugh ND, Gangavarapu K, Quick J, et al. : An amplicon-based sequencing framework for accurately measuring intrahost virus diversity using PrimalSeq and iVar. Genome Biol. 2019;20(1):1–19. 10.1186/s13059-018-1618-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Resende PC, Motta FC, Roy S, et al. : SARS-CoV-2 genomes recovered by long amplicon tiling multiplex approach using nanopore sequencing and applicable to other sequencing platforms. BioRxiv. 2020.
- 18. Brinkmann A, Ulm S-L, Uddin S, et al. : Amplicov: Rapid whole-genome sequencing using multiplex PCR amplification and real-time Oxford Nanopore MinION sequencing enables rapid variant identification of SARS-CoV-2. Front. Microbiol. 2021;12:1703. 10.3389/fmicb.2021.651151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hilaire BGS, Durand NC, Mitra N, et al. : A rapid, low cost, and highly sensitive SARS-CoV-2 diagnostic based on whole genome sequencing. BioRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 20. Gohl DM, Garbe J, Grady P, et al. : A rapid, cost-effective tailed amplicon method for sequencing SARS-CoV-2. BMC Genomics. 2020;21(1):1–10. 10.1186/s12864-020-07283-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hadfield J, Megill C, Bell SM, et al. : Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34(23):4121–4123. 10.1093/bioinformatics/bty407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tshiabuila D, Giandhari J, Pillay S, et al. : Comparison of SARS-CoV-2 sequencing using the ONT GridION and the Illumina MiSeq. BMC Genomics. 2022;23(1):1–17. 10.1186/s12864-022-08541-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo J, Meng Z, Xingyu X, et al. : Systematic benchmarking of nanopore Q20+ kit in SARS-CoV-2 whole genome sequencing. Front. Microbiol. 2022;4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brandt C, Krautwurst S, Spott R, et al. : poreCov – an easy to use, fast, and robust workflow for SARS-CoV-2 genome reconstruction via nanopore sequencing. Front. Genet. 2021;1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Di Tommaso P, Chatzou M, Floden EW, et al. : Nextflow enables reproducible computational workflows. Nat. Biotechnol. 2017;35(4):316–319. 10.1038/nbt.3820 [DOI] [PubMed] [Google Scholar]
- 26. Köster J, Rahmann S: Snakemake – a scalable bioinformatics workflow engine. Bioinformatics. 2012;28(19):2520–2522. 10.1093/bioinformatics/bts480 [DOI] [PubMed] [Google Scholar]
- 27. Patel H, Monzón S, Varona S, et al. : nf-core/viralrecon: nf-core/viralrecon v2.6.0 - Rhodium Raccoon. March 2023. 10.5281/zenodo.7764938 [DOI]
- 28. Nasir JA, Kozak RA, Aftanas P, et al. : A comparison of whole genome sequencing of SARS-CoV-2 using amplicon-based sequencing, random hexamers, and bait capture. Viruses. 2020;12(8):895. 10.3390/v12080895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Posada-Céspedes S, Seifert D, Topolsky I, et al. : V-pipe: a computational pipeline for assessing viral genetic diversity from high-throughput data. Bioinformatics. 2021;37(12):1673–1680. 10.1093/bioinformatics/btab015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim K, Park K, Lee S, et al. : Virpipe: an easy and robust pipeline for detecting customized viral genomes obtained by nanopore sequencing. Bioinformatics. 2023;39:btad293. 10.1093/bioinformatics/btad293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dezordi FZ, Silva Neto AM, Lima Campos T, et al. : Viralflow: a versatile automated workflow for sars-cov-2 genome assembly, lineage assignment, mutations and intrahost variant detection. Viruses. 2022;14(2):217. 10.3390/v14020217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lo C-C, Shakya M, Connor R, et al. : EDGE COVID-19: a web platform to generate submission-ready genomes from SARS-CoV-2 sequencing efforts. Bioinformatics. 2022;38(10):2700–2704. 10.1093/bioinformatics/btac176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maier W, Bray S, Beek M, et al. : Ready-to-use public infrastructure for global SARS-CoV-2 monitoring. Nat. Biotechnol. 2021;39(10):1178–1179. 10.1038/s41587-021-01069-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nguyen PTT, Plyusnin I, Sironen T, et al. : HAVoC, a bioinformatic pipeline for reference-based consensus assembly and lineage assignment for SARS-CoV-2 sequences. BMC Bioinformat. 2021;22(1):1–8. 10.1186/s12859-021-04294-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oliveira RRM, Negri TC, Nunes G, et al. : PipeCov: a pipeline for SARS-CoV-2 genome assembly, annotation and variant identification. PeerJ. 2022;10:e13300. 10.7717/peerj.13300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grüning B, Dale R, Sjödin A, et al. : Bioconda: sustainable and comprehensive software distribution for the life sciences. Nat. Methods. 2018;15(7):475–476. 10.1038/s41592-018-0046-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boettiger C: An introduction to Docker for reproducible research. Oper. Syst. Rev. 2015;49(1):71–79. 10.1145/2723872.2723882 [DOI] [Google Scholar]
- 38. Kurtzer GM, Sochat V, Bauer MW: Singularity: Scientific containers for mobility of compute. PLoS One. 2017;12(5):e0177459. 10.1371/journal.pone.0177459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aksamentov I, Roemer C, Hodcroft EB, et al. : Nextclade: clade assignment, mutation calling and quality control for viral genomes. J. Open Source Softw. 2021;6(67):3773. 10.21105/joss.03773 [DOI] [Google Scholar]
- 40. O’Toole Á, Scher E, Underwood A, et al. : Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool. Virus Evol. 2021;7(2):veab064. 10.1093/ve/veab064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Valieris R, Drummond RD, Defelicibus A, et al. : A mixture model for determining SARS-Cov-2 variant composition in pooled samples. Bioinformatics. 2022;38(7):1809–1815. 10.1093/bioinformatics/btac047 [DOI] [PubMed] [Google Scholar]
- 42. Chen S, Zhou Y, Chen Y, et al. : fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884–i890. 10.1093/bioinformatics/bty560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wood DE, Jennifer L, Langmead B: Improved metagenomic analysis with Kraken 2. Genome Biol. 2019;20:1–13. 10.1186/s13059-019-1891-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li H: Aligning sequence reads, clone sequences and assembly contigs with bwa-mem. 2013.
- 45. Quinlan AR: BEDTools: the Swiss-army tool for genome feature analysis. Curr. Protoc. Bioinformat. 2014;47(1):11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chun Hang A, Ho DN, Kwong A, et al. : BAMClipper: removing primers from alignments to minimize false-negative mutations in amplicon next-generation sequencing. Sci. Rep. 2017;7(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tyson JR, James P, Stoddart D, et al. : Improvements to the ARTIC multiplex PCR method for SARS-CoV-2 genome sequencing using nanopore. BioRxiv. 2020.
- 48. Garrison E, Marth G: Haplotype-based variant detection from short-read sequencing. arXiv preprint arXiv:1207.3907. 2012.
- 49. Danecek P, Bonfield JK, Liddle J, et al. : Twelve years of SAMtools and BCFtools. Gigascience. 2021;10(2):giab008. 10.1093/gigascience/giab008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cingolani P, Platts A, Coon M, et al. : A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly. 2012;6(2):80–92. 10.4161/fly.19695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cornish-Bowden A: Nomenclature for incompletely specified bases in nucleic acid sequences: recommendations 1984. Nucleic Acids Res. 1985;13(9):3021–3030. 10.1093/nar/13.9.3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang M, Kong L: pblat: a multithread blat algorithm speeding up aligning sequences to genomes. BMC Bioinformat. 2019;20(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shumate A, Salzberg SL: Liftoff: accurate mapping of gene annotations. Bioinformatics. 2021;37(12):1639–1643. 10.1093/bioinformatics/btaa1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kubik S, Marques AC, Xing X, et al. : Recommendations for accurate genotyping of SARS-CoV-2 using amplicon-based sequencing of clinical samples. Clin. Microbiol. Infect. 2021;27(7):1036.e1–1036.e8. 10.1016/j.cmi.2021.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Satya RV, DiCarlo J: Edge effects in calling variants from targeted amplicon sequencing. BMC Genomics. 2014;15(1):1073–1077. 10.1186/1471-2164-15-1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rambaut A, Holmes EC, O’Toole Á, et al. : A dynamic nomenclature proposal for sars-cov-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020;5(11):1403–1407. 10.1038/s41564-020-0770-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xiaoli L, Hagey JV, Park DJ, et al. : Benchmark datasets for sars-cov-2 surveillance bioinformatics. PeerJ. 2022;10:e13821. 10.7717/peerj.13821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ewels PA, Peltzer A, Fillinger S, et al. : The nf-core framework for community-curated bioinformatics pipelines. Nat. Biotechnol. 2020;38(3):276–278. 10.1038/s41587-020-0439-x [DOI] [PubMed] [Google Scholar]
- 59. Katoh K, Standley DM: MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30(4):772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schäffer AA, Hatcher EL, Yankie L, et al. : Vadr: validation and annotation of virus sequence submissions to genbank. BMC Bioinformat. 2020;21:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Libuit K, RA P, III, Ambrosio F, et al. : Public health viral genomics: bioinformatics workflows for genomic characterization, submission preparation, and genomic epidemiology of viral pathogens, especially the sars-cov-2 virus. 2022.
- 62. Lataretu M, Hölzer M: Lessons learned: overcoming common challenges in reconstructing the SARS-CoV-2 genome from short-read sequencing data via CoVpipe2.[Dataset].2023, June 27. 10.17605/OSF.IO/MJ6EQ [DOI] [PMC free article] [PubMed]
- 63. Fuchs S, Drechsel O: Kraken 2 Database (Human, SARS-CoV2) (3.0.0).[Data set]. Zenodo. 2022. 10.5281/zenodo.6333909 [DOI]
- 64. MarieLataretu: rki-mf1/CoVpipe2: Version v0.4.3 (v0.4.3). Zenodo. 2023. 10.5281/zenodo.8017744 [DOI]