Abstract
White matter hyperintensities (WMH), a common feature of cerebral small vessel disease, are related to worse clinical outcomes after stroke. We assessed the impact of white matter hyperintensity changes over 1 year after minor stroke on change in mobility and dexterity, including differences between the dominant and non-dominant hands and objective in-person assessment versus patient-reported experience. We recruited participants with lacunar or minor cortical ischaemic stroke, performed medical and cognitive assessments and brain MRI at presentation and at 1 year. At both time points, we used the timed-up and go test and the 9-hole peg test to assess mobility and dexterity. At 1 year, participants completed the Stroke Impact Scale. We ran two linear mixed models to assess change in timed-up and go and 9-hole peg test, adjusted for age, sex, stroke severity (National Institutes of Health Stroke Scale), dependency (modified Rankin Score), vascular risk factor score, white matter hyperintensity volume (as % intracranial volume) and additionally for 9-hole peg test: Montreal cognitive assessment, hand (dominant/non-dominant), National Adult Reading Test (premorbid IQ), index lesion side. We performed ordinal logistic regression, corrected for age and sex, to assess relations between timed-up and go and Stroke Impact Scale mobility, and 9-hole peg test and Stroke Impact Scale hand function. We included 229 participants, mean age 65.9 (standard deviation = 11.13); 66% male. 215/229 attended 1-year follow-up. Over 1 year, timed-up and go time increased with aging (standardized β [standardized 95% Confidence Interval]: 0.124[0.011, 0.238]), increasing National Institutes of Health Stroke Scale (0.106[0.032, 0.180]), increasing modified Rankin Score (0.152[0.073, 0.231]) and increasing white matter hyperintensity volume (0.176[0.061, 0.291]). Men were faster than women (−0.306[0.011, 0.238]). Over 1 year, slower 9-hole peg test was related to use of non-dominant hand (0.290[0.155, 0.424]), aging (0.102[0.012, 0.192]), male sex (0.182[0.008, 0.356]), increasing National Institutes of Health Stroke Scale (0.160 [0.094, 0.226]), increasing modified Rankin Score (0.100[0.032, 0.169]), decreasing Montreal cognitive assessment score (−0.090[−0.167, −0.014]) and increasing white matter hyperintensity volume (0.104[0.015, 0.193]). One year post-stroke, Stroke Impact Scale mobility worsened per second increase on timed-up and go, odds ratio 0.67 [95% confidence interval 0.60, 0.75]. Stroke Impact Scale hand function worsened per second increase on the 9-hole peg test for the dominant hand (odds ratio 0.79 [0.71, 0.86]) and for the non-dominant hand (odds ratio 0.88 [0.83, 0.93]). Decline in mobility and dexterity is associated with white matter hyperintensity volume increase, independently of stroke severity. Mobility and dexterity declined more gradually for stable and regressing white matter hyperintensity volume. Dominant and non-dominant hands might be affected differently. In-person measures of dexterity and mobility are associated with self-reported experience 1-year post-stroke.
Keywords: mobility, dexterity, white matter hyperintensity, imaging, small vessel disease
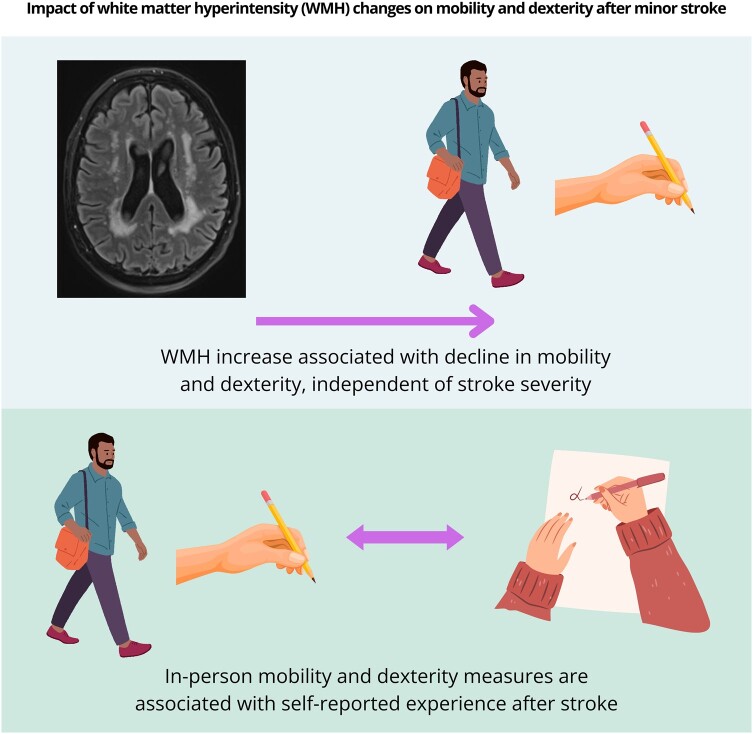
Jochems et al. report that mobility and dexterity are affected by white matter hyperintensity volume longitudinal change after minor ischaemic stroke, independently of stroke severity. Dominant and non-dominant hands might be differently affected. In-person measures of dexterity and mobility are associated with self-reported experience 1 year after stroke.
Graphical Abstract
Graphical abstract.
Introduction
Cerebral small vessel disease (SVD) is a disease of the small perforating blood vessels in the brain. SVD features appear on neuroimaging as subcortical infarcts, lacunes, perivascular spaces, microbleeds and white matter hyperintensities (WMH) of presumed vascular origin.1 WMH are known to progress over time and a recent systematic review and meta-analysis (n = 12 284) showed that WMH can also regress.2
SVD is the most common cause of vascular cognitive impairment and is related to an increased risk of stroke, dementia and death.3 SVD is also associated with worse clinical outcomes after acute stroke. In community-dwelling people, SVD features, and in particular WMH, have been associated with impaired mobility4-12 and dexterity.5,10,13 Gait, mobility14 and dexterity15 are commonly affected after stroke and can negatively affect quality of life,16 daily functioning,17 participation and reintegration into the community after stroke.16,18-20 Worse fine motor dexterity 3 months after stroke is also related to worse cognitive performance.21 However, for both mobility and dexterity, most evidence comes from studies with small sample sizes17,18 and patients with severe stroke16 or varying stroke severities.19,21 Despite the clear links between worsening mobility and dexterity after stroke and WMH in community-dwelling populations, little is known about any additional contribution of WMH to mobility and dexterity outcomes after minor lacunar and cortical ischaemic stroke. Patients with minor strokes might have less apparent physical symptoms that could easily go unnoticed. A previous study showed that mobility and hand function might still be affected 3 years after minor stroke22 and that WMH might have an added effect on impaired hand function after stroke.1,23
We aimed to document the impact of long-term WMH changes on mobility and dexterity over 1 year after a minor ischaemic stroke, as these lesions might have an additive effect after the original stroke. Additionally, we explore whether the dominant and non-dominant hands are affected differently. We also compare objective in-person assessments of mobility and dexterity with a self-reported questionnaire to assess whether objective measures are representative of the experience of patients.
Materials and methods
Participants
Participants were included in the Mild Stroke Study 3,24 a prospective cohort study of patients presenting to the Lothian Stroke Services. Patients were included if they were ≥18 years old and had an acute minor ischaemic stroke (minor defined as National Institute of Health Stroke Scale (NIHSS)25 < 8 and independence in daily living, i.e. a modified Rankin scale (mRS)26 of ≤2) at time of recruitment. In the case of hospital admission, participants might have been seen by a physiotherapist and occupational therapist. However, they did not receive prolonged neurorehabilitation. No participants were in-patients at time of recruitment. Participants were excluded if they had MRI contraindications, severe respiratory, cardiac or neurological conditions. The final stroke diagnosis was made based on symptom classification, as described previously,27 supplemented by additional relevant investigations and diagnostic CT or MRI at time of presenting to stroke services. All participants provided written informed consent. Ethical approval was granted by Southeast Scotland Regional Ethics Committee (reference 18/SS/0044).
Participants attended the baseline visit within 3 months after stroke. They underwent brain MRI, medical, cognitive, mobility and dexterity assessments. As part of the medical assessment, we collected their medical history to establish vascular risk factors including hypertension, hypercholesterolemia, diabetes mellitus and history of smoking. All participants were invited for a follow-up visit 1 year later for medical, cognitive, mobility and dexterity assessments, and underwent further brain MRI.
Assessments
At the baseline visit, we assessed stroke severity with the NIHSS and dependency with the mRS. NIHSS scores range from 0 to 42 with higher scores indicating greater severity. The mRS ranges from 0 (no symptoms) to 6 (death), with scores below two indicating independence in activities of daily living, even when symptoms are present. Higher scores indicate greater dependency.
Global cognition was assessed with the Montreal Cognitive Assessment (MoCA)28 at both visits. Alternative versions of the MoCA were used at subsequent visits to minimize learning effects.29 The MoCA is a cognitive screening test widely used to assess cognitive impairment after stroke, covering several cognitive domains.30 Scores range from 0 to 30, with higher scores related to better cognition. Scores below 26 might indicate cognitive impairment.28 The National Adult Reading Test (NART)31 requires participants to read out a list of 50 irregular English words. Vocabulary knowledge is stable over time and can measure peak cognitive ability32 and is used to establish pre-morbid intelligence.33 The pronunciation of these irregular words can be maintained after stroke.34
Mobility was assessed in-person with the Timed Up and Go (TUG) test.35,36 Participants were asked to stand up from a chair, walk 3 m, turn around, walk back and sit back down on the chair. We recorded the time (seconds) it took to complete the task. Dexterity was recorded with the 9 Hole Peg Test (9HPT).37,38 Participants were asked to place nine pegs, as quickly as possible into nine holes in a board. We recorded the time it takes to complete the task for each hand. We maintained a cut-off of 50 sec.39 Scores were identified separately for the dominant and non-dominant hand.
At the 1-year visit, we repeated the NIHSS, mRS, MoCA, TUG and 9HPT. Before the in-person study visit, participants were asked to fill in the Stroke Impact Scale (SIS)40 by post. The SIS is a questionnaire that evaluates how the stroke has impacted the patient’s life. It covers eight domains: strength in the extremities most affected by the stroke, memory and thinking, emotionality, communication, activities of daily living, mobility, hand function in the hand most affected by stroke, and societal participation, and assesses recovery after stroke on a scale from 0 (no recovery) to 100 (full recovery). In this analysis, we only focussed on the domains of mobility and hand function. For the mobility (scores range from 9 to 45) and hand function domains (ranging scores from 5 to 25), with lower scores indicating worse impact on life.
Imaging acquisition
The full details of the Mild Stroke Study three brain imaging protocols are published.24 Briefly, at both visits participants underwent brain MRI on the same 3T scanner (Siemens Prisma, Erlangen, Germany) with the same sequences. The images were acquired using a 32-channel head coil (Siemens Healthcare, Erlangen, Germany). The MRI protocol included 3D T1-weighted (1 mm3 isotropic; TR/TE/TI = 2500/4.37/1100 ms), T2-weighted (0.9 mm3 isotropic; TR/TE = 3200/408 ms), FLAIR (1 mm3 isotropic; TR/TE/TI = 5000/388/1800 ms), diffusion-tensor imaging (64 diffusion-weighted images (DWI) with b = 1000 s/mm2; 2 mm3 isotropic voxels TR/TE = 4300/74 ms) and 3D proton density (PD) imaging (1.2 mm3 isotropic resolution, TR/TE = 865/1.82 ms). The MRI is monitored with a quality assurance programme to check for scanner performance issues and to maintain consistent scanner function and image quality.
Imaging processing and analysis
The image processing protocol is described elsewhere.24 All image sequences were analysed using validated computational pipelines and were co-registered to the first visit T2-weighted image using FLIRT41 from FSL.42 The intracranial volumes (ICV) were generated computationally from the PD image, and they were checked and manually edited if necessary. WMH were defined according to STRIVE criteria.1 Details on the WMH segmentation and associated pipeline are published elsewhere.43 The pipelines were developed in the course of previous similar studies44,45 and further refined. We acquired WMH volumes (mm3) from FLAIR images and removed false positives in the vicinities of the choroid plexus, aqueduct, third and fourth ventricles, using Freesurfer (https://surfer.nmr.mgh.harvard.edu/). To further exclude any hyperintensities that unlikely reflect pathology, a lesion distribution probabilistic template was applied to the thresholded images,46 and the result was visually checked and manually corrected if still necessary. WMH volumes were normalized for the ICV (mm3) to account for participant’s head size and reported as a percentage of ICV (%ICV) unless otherwise stated.
Old and acute stroke lesions were manually drawn on the FLAIR sequence by an experienced image analyst, guided by other MRI sequences. All cases were discussed with a neuroradiologist and the masks were adjusted if required. Stroke lesions were identified at both visits and excluded from the WMH volumes to avoid erroneous measures of WMH volume. See Fig. 1 for an example of segmented WMH and index stroke lesion at baseline and 1 year. Inter-rater and intra-rater variability in manually rectifying abnormal tissues and ICV have been analysed and reported previously.44
Figure 1.
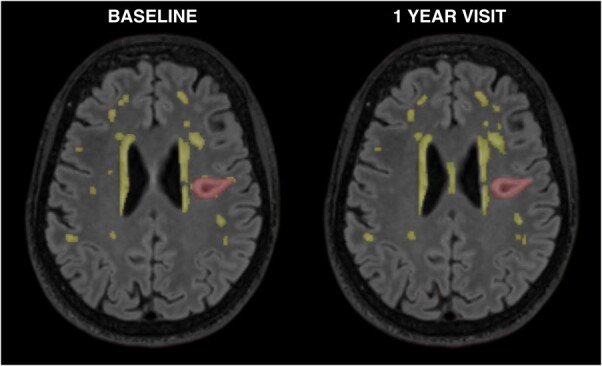
Segmented WMH and index stroke at baseline and 1 year. Example of baseline and 1 year scans of one participant (age 66 at baseline) with WMH segmented (yellow) and index stroke lesion (red). WMH: White matter hyperintensity.
The side of the index lesion was recorded as part of visual MRI assessment. Locations of index lesion include left hemisphere, right hemisphere, both hemispheres, or no visible lesion when no index lesion was visible. All cases were checked by a neuroradiologist (JMW).
Statistical analysis
All statistical analyses were performed with R Studio, using R version 4.2.247 and the ggplot2, lmer, emmeans and MASS packages. To assess longitudinal impact of WMH changes over 1 year we performed repeated measurements linear mixed models for which we reported standardized betas and not the original units of the variables to allow us to compare the influence of the variables. There were no gross violations of the assumptions for all statistical models. We also calculated quintiles of WMH volume change. Volume change, as used in the quintile plots, was defined as the difference in WMH volume between the 1 year visit and the baseline visit.
We performed two repeated measures linear mixed models with TUG and 9HPT as outcomes. The models included a random intercept for the individual participants to account for individual differences in TUG and 9HPT. We included predictors based on relevance to our research question and on the available literature, not on statistical significance. For both models, predictors included age (in years, centred to age at baseline)48 and sex,38,49 NIHSS, mRS, combined vascular risk factor (VRF) score, WMH volume. We used a combined VRF score which summarizes the presence (yes or no) of hypertension, hypercholesterolemia, diabetes mellitus and smoking (current or ex-smoker ≤1 year) and ranges from 0 (no vascular risk factors present) to 4 (all vascular risk factors present).50
For the 9HPT model, we also added a random slope for hand, i.e. whether the dominant or non-dominant hand was used for the test, to allow for differences in the slope for dominant and non-dominant hand. We also added the following predictors: hand (dominant and non-dominant), side of brain of index lesion, MoCA and NART.51,52 Examples of the linear mixed models can be found in the Supplementary material. We obtained the estimated marginal means for handedness (dominant versus non-dominant hand), using the same linear mixed model, to examine any differences in effect between the dominant and non-dominant hand. For both the TUG and 9HPT models, we examined the role of stroke subtype to see whether a cortical or subcortical stroke might be relevant for the outcomes. To further explore whether predictors differently affect the 9HPT scores for the dominant and non-dominant hand, we performed linear mixed models with an interaction term between hand and the predictors: age, sex, NIHSS, mRS, VRF, MoCA, NART, brain side of index lesion and WMH volume. Due to the explorative nature, these analyses were not corrected for multiple corrections and need to be interpreted with caution. As the size of our data did not allow the inclusion of all interaction terms in a single model, we repeated the model including all predictors and just one interaction term at a time and a random intercept for the individual participants. People who indicated they were ambidextrous (n = 2) were excluded from all analyses examining dexterity.
For visualization purposes only, we divided WMH volume change into quintiles. Quintile one reflects largest WMH decrease and quintile five reflects largest WMH volume increase.
SIS mobility and hand function scores were grouped to allow ordinal logistic regressions. SIS mobility was divided in three groups: (1) including maximum score of 45, reflecting participants who experience no difficulties at all; (2) scores 36 to 44, reflecting participants experiencing very few or some difficulties; (3) scores below 36, reflecting participants who experienced difficulties or who could not do activities at all. For the SIS hand domain the groups were as follows: (1) including scores of 25, i.e. no difficulties at all; (2) scores 24 to 20, i.e. some difficulties; (3) 19 or lower, very difficult to use hand or not able to use hand at all. To compare the objective in-person assessments with the self-reported questionnaires, we performed three ordinal logistic regressions, corrected for age and sex, at time of the 1-year visit. One regression was performed with the TUG and SIS mobility domain score, the other two with the dominant and non-dominant 9HPT outcomes and the SIS hand function domain score.
Results
At baseline, 229 participants underwent MRI, the scan of one participant was not useable for analysis due to the quality of the scan (Fig. 2). The mean age at baseline was 65.9 years (standard deviation [SD] = 11.13), 66% were male, 57% had a subcortical stroke, a history of transient ischaemic attacks (TIA) or strokes prior to the index stroke that led to study inclusion was present in 16% (Table 1). There were slightly more index infarcts located in the right hemisphere (45.9%), than in the left hemisphere (40.6%). 66% of the participants had two or more vascular risk factors, the most common risk factors being hypercholesterolemia (75%) and hypertension (69%). At presentation, the median mRS score was 1 (interquartile range [IQR] 1 to 1), and the median NIHSS total score was 1 (IQR 0 to 2). The mean MoCA score was 25.05 (SD = 3.48), mean TUG time was 12.6 sec (SD = 6.28) and the mean dominant hand 9HPT time was 15.9 sec (SD = 5.78) and 17.7 sec for the non-dominant hand (SD = 6.87).
Figure 2.
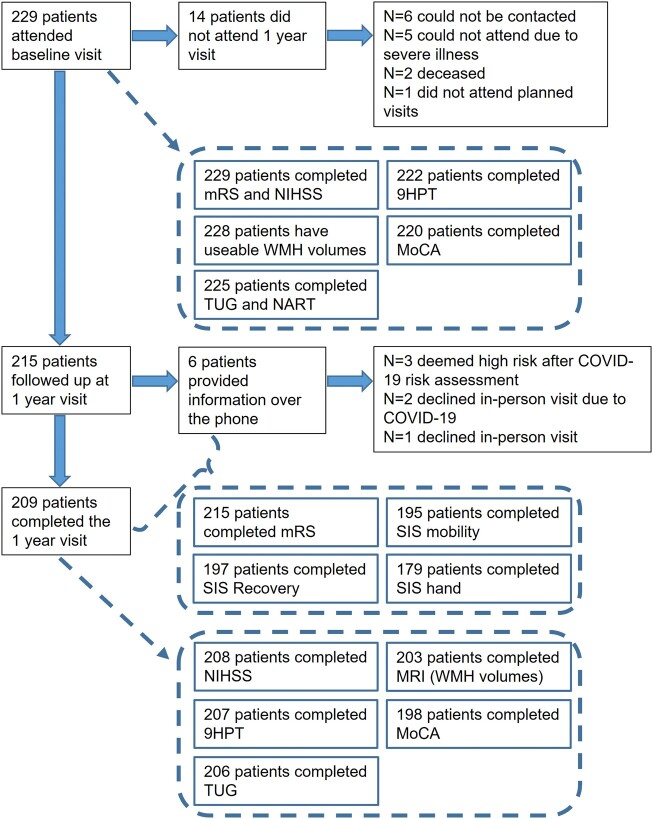
Flow diagram of data collection at baseline and the 1 year visit. Overview of the number of participants attending the baseline and 1 year visits, data completeness and reasons for missing data and non-attendance of the 1 year visit. 9HPT: 9 Hole Peg Test; MoCA: Montreal Cognitive Assessment; mRS: modified Rankin Scale; NART: National Adult Reading Test; NIHSS: National Institutes of Health Stroke Scale; SIS: Stroke Impact Scale; TUG: Timed Up and Go; WMH: White matter hyperintensities.
Table 1.
Demographic characteristics of the mild stroke study 3 participants attending baseline and 1 year visit
| N | Baseline | N | 1 year | |
|---|---|---|---|---|
| Age, mean (SD) | 229 | 65.85 (11.13) | 215 | 66.79 (11.18) |
| Sex, male (%) | 229 | 152 (66) | 215 | 143 (62) |
| Index stroke subtype, N (%) | 229 | 215 | ||
| Subcortical | 130 (57) | 124 (58) | ||
| Cortical | 99 (43) | 91 (42) | ||
| Brain side affected by index stroke, n (%) | 229 | 215 | ||
| Right | 105 (45.9) | 100 (46.5) | ||
| Left | 93 (40.6) | 85 (39.5) | ||
| Both | 16 (7.0) | 16 (7.4) | ||
| Not visible | 15 (6.6) | 14 (6.5) | ||
| Handedness, N (%) | 229 | 215 | ||
| Right | 211 (92) | 198 (92) | ||
| Left | 16 (7) | 15 (7) | ||
| Both | 2 (1) | 2 (1) | ||
| TIA or stroke before index stroke, yes (%) | 229 | 36 (16) | - | - |
| TIA or stroke since baseline, yes (%) | - | - | 228 | 13 (5.7) |
| Incidental DWI + lesion since stroke diagnosis, yes (%) | - | - | 228 | 52 (22.8) |
| Hypertension, yes (%) | 229 | 157 (69) | 215 | 151 (70) |
| Hypercholesterolemia, yes (%) | 229 | 171 (75) | 215 | 160 (74) |
| History of smoking, yes (%) | 229 | 44 (19) | 215 | 35 (16) |
| Diabetes mellitus, yes (%) | 229 | 50 (22) | 215 | 47 (22) |
| VRF combined score (%) | 229 | 215 | ||
| 0 | 21 (9) | 20 (9) | ||
| 1 | 57 (25) | 52 (24) | ||
| 2 | 94 (41) | 90 (42) | ||
| 3 | 51 (22) | 49 (23) | ||
| 4 | 6 (3) | 4 (2) | ||
| mRS, median (IQR), range | 229 | 1 (1–1), 0–2 | 215 | 1 (0–1) 0–3 |
| NIHSS, median (IQR), range | 229 | 1 (0–2), 0–7 | 208 | 1 (0–2) 0–7 |
| TUG time (seconds), mean (SD) | 225 | 12.61 (6.28) | 206 | 12.30 (5.51) |
| 9HPT dominant hand (seconds), mean (SD) | 222 | 15.90 (5.78) | 207 | 16.70 (5.39) |
| 9HPT non-dominant hand (seconds), mean (SD) | 222 | 17.65 (6.87) | 207 | 18.68 (8.16) |
| MoCA total score, mean (SD) | 220 | 25.05 (3.48) | 198 | 25.83 (3.57) |
| NART (total errors), mean (SD) | 225 | 17.37 (9.53) | - | - |
| SIS—mobility (max. 45), median (IQR) | - | - | 195 | 44 (38–45) |
| SIS—hand function (max. 25), median (IQR) | - | - | 179 | 25 (21–25) |
| SIS—recovery (max. 100, i.e. full recovery), median (IQR) | - | - | 197 | 90 (75–95) |
| Fazekas score periventricular WMH (%) | 228 | 203 | ||
| 0 | 8 (3.5) | 11 (5.4) | ||
| 1 | 111 (48.7) | 91 (44.8) | ||
| 2 | 59 (25.9) | 55 (27.1) | ||
| 3 | 50 (21.9) | 46 (22.7) | ||
| Fazekas score deep WMH (%) | 228 | 203 | ||
| 0 | 21 (9.2) | 20 (9.9) | ||
| 1 | 122 (53.5) | 110 (54.2) | ||
| 2 | 59 (25.9) | 45 (22.2) | ||
| 3 | 26 (11.4) | 28 (13.8) | ||
| WMH volume (ml), mean (SD) | 228 | 14.93 (18.19) | 203 | 16.55 (20.59) |
| WMH volume (%ICV), mean (SD) | 228 | 0.934 (1.122) | 203 | 1.032 (1.266) |
9HPT, 9 hole peg test; DWI+, Diffusion weighted imaging positive; ICV, Intracranial volume; IQR, Interquartile range; MoCA, Montreal Cognitive Assessment; mRS, Modified Rankin Score; NART, National Adult Reading Test; NIHSS, National Institutes of Health Stroke Scale; SD, Standard Deviation; SIS, Stroke impact scale; TIA, Transient Ischaemic Attack; TUG, Timed-up and go; VRF, Vascular risk factors; WMH, White matter hyperintensity.
All participants were invited for the 1 year follow-up, 94% (n = 215) returned of whom six had a telephone follow-up (Fig. 2). Of the 14 participants who did not return, 2 were deceased, and 5 had a severe illness and could not attend an in-person or telephone study visit (Fig. 2). After comparison of baseline characteristics of participants who did and did not return for the 1-year visit, only the TUG time and dominant hand 9HPT time differed between the two groups (Supplementary Table 1). The people who did not attend their 1-year visit had faster TUG times and were slightly faster on the 9HPT.
At the 1-year visit, the prevalence of VRF (Table 1) was comparable to baseline as were mRS and NIHSS scores. Mean TUG time was 12.30 sec (SD = 5.51), mean 9HPT time was slightly longer than at baseline with 16.70 sec (SD = 5.39) for the dominant hand and 18.68 sec (SD = 8.16) for the non-dominant hand. Mean MoCA score was 25.83 (SD = 3.57). A total of 13/228 participants (5.7%) had had a clinical stroke or TIA since the baseline visit and 52/288 had an incidental diffusion-weighted positive lesion. Four participants had both.
Most WMH change in any direction, i.e. regression and progression, was seen in participants with most baseline WMH volumes (Supplementary Fig. 1).
Mobility
The linear mixed model shows that, across a 1-year period, the TUG time increased with increasing age (standardized Β [standardized 95% confidence interval (CI)]: 0.124 [0.011 to 0.238], increasing NIHSS scores (0.106, [0.032 to 0.180]), increasing mRS (0.152 [0.073 to 0.231]) and increasing WMH volumes (0.176 [0.061 to 0.291]), Table 2. The change in TUG time was smaller for men than women (−0.306 [−0.533 to −0.078]). More VRF did not contribute to TUG time change, neither did stroke subtype (Supplementary Table 2).
Table 2.
Results of linear mixed model assessing mobility with the timed-up and go
| Predictors | Standardized Beta | Standardized 95% CI | P value |
|---|---|---|---|
| Age | 0.124 | 0.011, 0.238 | 0.032 |
| Sex (male) | −0.306 | −0.533, −0.078 | 0.009 |
| NIHSS | 0.106 | 0.032, 0.180 | 0.005 |
| mRS | 0.152 | 0.073, 0.231 | <0.001 |
| Vascular risk factor (combined score) | 0.027 | −0.074, 0.129 | 0.599 |
| WMH volume (%ICV) | 0.176 | 0.061, 0.291 | 0.003 |
CI, Confidence Interval; ICV, Intracranial volume; mRS, modified Rankin Score; NIHSS, National Institutes of Health Stroke Scale; WMH, White matter hyperintensity.
In the 1-year period between baseline and follow-up, mean TUG time decreased slightly from 12.61 sec (n = 225; Table 1) to 12.30 sec (n = 206). TUG time change per quintile of WMH volume change between baseline and 1-year visit is plotted in Fig. 3. The figure suggests that the mean TUG time (black lines in Fig. 3) decreased slightly between visits in all quintiles, but times were generally longer in individuals with greater WMH volume increase.
Figure 3.
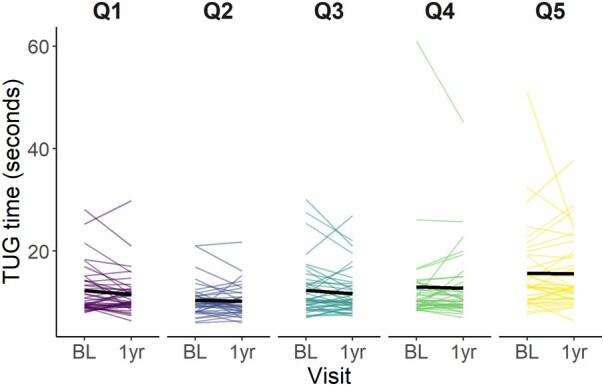
Time on the timed-up and go test between baseline and 1-year visit per quintile of WMH volume (%ICV) change over 1 year. Q1 is greatest WMH volume reduction and Q5 represents the greatest WMH increase. BL: Baseline; ICV: Intracranial volume; Q: Quintile; TUG: Timed- Up and Go; WMH: White matter hyperintensity.
Dexterity
Based on the linear mixed models, increase in 9HPT time over 1 year was larger for the non-dominant hand (Table 3; 0.290 [0.155 to 0.424]) than the dominant hand. 9HPT time increase was associated with older age (0.102 [0.012 to 0.192]), male sex (0.182 [0.008 to 0.356]), increasing NIHSS (0.160 [0.094 to 0.226]), increasing mRS (0.100 [0.032 to 0.169]), decreasing MoCA score (−0.090 [−0.167 to −0.014]) and increasing WMH volume (0.104 [0.015 to 0.193]). The average time for the dominant hand was 16.1 sec (95% CI = 15.2 to 16.9) and for the non-dominant hand the mean time was 18.0 sec (95% CI = 17.0 to 18.9). The estimated difference between the hands was 1.88 sec (t(219) = −4.227, P < 0.001). Stroke subtype did not affect 9HPT time (Supplementary Table 3).
Table 3.
Results linear mixed model assessing dexterity with the 9 hole peg test
| Standardized Beta | Standardized 95% CI | P value | |
|---|---|---|---|
| Hand (non-dominant) | 0.290 | 0.155, 0.424 | <0.001 |
| Age | 0.102 | 0.012, 0.192 | 0.027 |
| Sex (male) | 0.182 | 0.008, 0.356 | 0.040 |
| NIHSS | 0.160 | 0.094, 0.226 | <0.001 |
| mRS | 0.100 | 0.032, 0.169 | 0.004 |
| Vascular risk factor (combined score) | −0.024 | −0.105, 0.057 | 0.562 |
| Side index lesion (both) | 0.071 | −0.260, 0.401 | 0.675 |
| Side index lesion (not visible) | 0.003 | −0.338, 0.343 | 0.988 |
| Side index lesion (right) | −0.017 | −0.193, 0.158 | 0.845 |
| NART (errors) | 0.013 | −0.076, 0.102 | 0.767 |
| MoCA score | −0.090 | −0.167, −0.014 | 0.021 |
| WMH volume (%ICV) | 0.104 | 0.015, 0.193 | 0.023 |
CI, Confidence Interval; ICV, Intracranial volume; MoCA, Montreal Cognitive Assessment; mRS, modified Rankin Score; NART, National Adult Reading Test; NIHSS, National Institutes of Health Stroke Scale; WMH, White matter hyperintensity.
In Figs 3 and 4 the times on the 9HPT are plotted at baseline and 1 year visit per quintile of WMH volume change for the dominant (Fig. 4) and non-dominant hand (Fig. 5). The plots show that for the dominant hand, the mean time on the 9HPT increases over time in all quintiles. In Q5, the mean time might be going down slightly. Mean time for the non-dominant hand also increases over time. Mean time in Q3 shows a slight decrease in time, however, this could be driven by the time of one participant.
Figure 4.
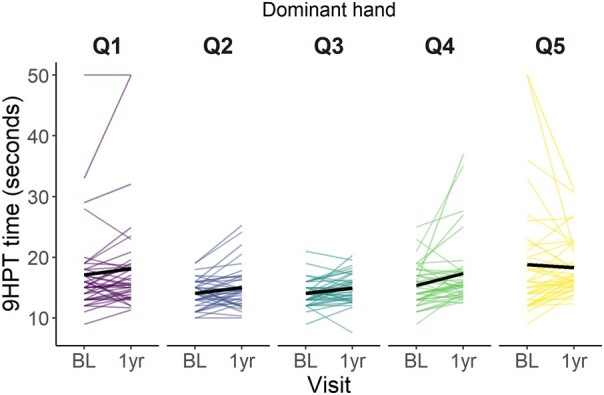
Dominant hand 9 hole peg test time over baseline and 1-year visit per quintile of WMH volume (%ICV) change. Q1 represents the greatest WMH volume reduction, Q5 the greatest WMH volume increase. 9HPT: 9 Hole Peg Test; BL: Baseline; ICV: Intracranial volume; Q: Quintile; WMH: White matter hyperintensity.
Figure 5.
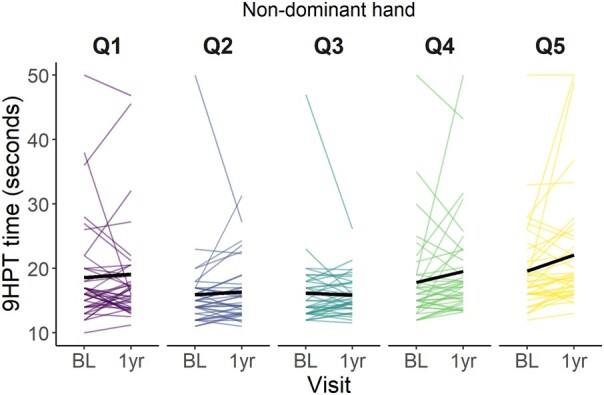
Non-dominant hand 9 hole peg test time over baseline and 1-year visit per quintile of WMH volume (%ICV) change. Q1 reflects the greatest WMH volume reduction, Q5 the greatest WMH volume increase. 9HPT: 9 Hole Peg Test; BL: Baseline; ICV: Intracranial volume; Q: Quintile; WMH: White matter hyperintensity.
We further explored the interaction between predictors and time for the dominant versus non-dominant hand using linear mixed models (Supplementary Tables 2–12). For the 9HPT, increasing NIHSS scores are associated with a greater increase in time for the non-dominant (0.188 [0.080 to 0.295]) than for the dominant hand (0.075 [−0.007 to 0.157]) (Supplementary Table 7). An index infarct in the right hemisphere is also related to increasing 9HPT time for the non-dominant hand (0.492 [0.207 to 0.777]) and time tends to decrease for the dominant hand (−0.191 [−0.393 to 0.011]) (Supplementary Table 9). Other predictors did not suggest an interaction with handedness.
Objective in-person assessment versus self-reported experience via the stroke impact scale
The impact of stroke on mobility and hand function was self-reported with the SIS subdomains of mobility (n = 195) and hand function (n = 179) 1 year after stroke. Higher scores on the domains indicate less impact of the stroke on the specific domain. Overall, participants reported good mobility with a median score of 44/45 (IQR 38 to 45; Table 1). Based on logistic regression analysis, for men, the odds of higher scores on the SIS mobility domain were greater than for women (odds ratio [OR] 1.99 [95% CI 1.04 to 3.85]). For every one-second increase in TUG time, the odds of a lower SIS mobility score increased (OR 0.67 [0.60 to 0.75]). Age was not associated with SIS mobility (OR 0.99 [0.96 to 1.02]).
Participants reported good hand function, median 25/25 (IQR 21 to 25). Logistic regression shows that age was not associated with SIS hand domain scores, for either dominant (OR 1.0 [0.97 to 1.03]) or non-dominant hand (OR 0.99 [0.96 to 1.02]). For the dominant hand, the odds for men having a higher score on the SIS was greater than for women (OR 2.58 [1.36 to 4.94]). Per one-second increase on the 9HPT, the odds for a lower SIS hand function increased (OR 0.79 [0.71 to 0.86]). For the non-dominant hand, the odds of having a higher score on the SIS were greater for men versus women (OR 2.86 [1.52 to 5.42]). Per one-second increase on the 9HPT with the non-dominant hand, the odds for a lower score on the SIS hand domain increased (OR 0.88 [0.83 to 0.93]).
At 1 year, participants (n = 197) reported that they were close to full recovery (median = 95, IQR 75 to 95; Table 1). However, none of the participants reported that they were fully recovered from their stroke.
Discussion
Our longitudinal observational study of people with lacunar or minor cortical ischaemic stroke and features of SVD shows that a decrease in mobility and dexterity is associated with WMH volume increase between baseline and 1 year, independent of stroke severity. A more severe stroke and an index lesion in the right hemisphere might affect hands differently and lead to worse dexterity of the non-dominant hand. In-person objective measurement of mobility relates closely with self-reported change in mobility after stroke, as are the measures of dexterity and self-reported hand function. However, based on self-reported measures, changes in function of the dominant hand might have a bigger impact on life than similar changes in the non-dominant hand, perhaps reflecting that patients are less likely to be affected by deficits in the non-dominant hand even when objective testing shows these to be present.
WMH volume increase contributes to worsening mobility, independently of other variables including sex, stroke severity and disability. This contribution of WMH to declining mobility was previously established in (older) people with sporadic SVD but without stroke.4-8,10 In stroke research, the contribution of WMH might be overlooked, as impaired mobility is often measured in relation to hemiparesis or hemiplegia, which suggests a more severe stroke. WMH might not be taken into account when assessing long-term outcomes. Some people with a minor stroke might experience weakness, but it might be deemed not severe or it improves. While WMH presence and changes are more examined in minor stroke, mobility might not be as well examined as in severe stroke. Some cross-sectional studies with small sample sizes, n = 1253 and n = 82,54 found that patients with minor stroke or TIA can have worse mobility than healthy controls. These findings were supported by the Rotterdam Study55 where gait of 147 community-dwelling participants with a prior ischaemic stroke was compared to gait of stroke-free participants. While the stroke participants did not report any gait problems at time of the stroke, they did have worse gait than the stroke-free participants. WMH and stroke might contemporaneously affect gait and mobility as stroke and sporadic SVD features, such as WMH, disrupt diffuse white matter connections and can affect mobility in the long term.55-58
In our longitudinal analysis, decline in dexterity was more strongly associated with the non-dominant hand compared to the dominant hand, and also with stroke severity, male sex compared to female sex, WMH volume increase during follow-up, older age, worse disability and declining global cognition. Current literature mainly describes cross-sectional associations between dexterity and WMH. WMH were not found to be associated with worse dexterity among population-based adults (mean age 57.2 years).10 However, in healthy adults with a higher risk of vascular disease13 and with sporadic SVD,59 WMH were cross-sectionally associated with worse dexterity. The finding in our longitudinal study that WMH can independently affect dexterity, builds on previous literature evidence that WMH might play a role in extremity motor deficits due to stroke (n = 28).23 While it might be expected that the hand contralateral to the index lesion is worst affected, a small cross-sectional study examining 30 haemorrhagic and ischaemic stroke patients found that dexterity was worst affected in the ipsilateral hand.15 In healthy right-handed participants (n = 44), worse fine motor movements with the non-dominant hand were associated with worse WMH cross-sectionally.60 Again, the dual effects of stroke (lesion) and WMH in minor stroke have not been widely examined in relation to dexterity. When we explored the difference in dexterity of the dominant and non-dominant hand, we found that an index lesion in the right hemisphere leads to worse dexterity of the non-dominant hand 1 year after experiencing the stroke (Supplementary Table 9). This is likely due to 46% of the participants having a right hemisphere lesion and the left hand being the non-dominant hand for 92% of the participants. However, the difference in effect between dominant and non-dominant hand is small. A more severe stroke was also predictive of worse dexterity. This effect was bigger for the non-dominant than the dominant hand. The non-dominant hand might potentially be more sensitive to any disruptions of white matter networks as it is less well trained than the dominant hand.59-61 Left-right differences and white matter networks involved in dexterity is beyond the scope of this paper and would require further research.
In addition to quantitative measures, we did collect self-reported hand function of the hand most affected by stroke, and mobility at 1 year after stroke. We found that the in-person measures and self-reported measures using the SIS are associated. Interestingly, the in-person measures suggest that the non-dominant hand performs worse on the 9HPT than the dominant hand and is more affected by stroke severity. However, the self-reported scores indicate that a worse performance on the in-person objective test (9HPT) for the dominant hand is more related to experienced difficulties in hand function, as reported on the questionnaire, than for the non-dominant hand. This could be explained by the fact that the dominant hand is used most of the time and for more complex fine motor movements and therefore changes are more likely to be noticed than in the non-dominant hand. This points out the importance of the experience of patients in relation to what we can measure. Lai and colleagues62 found that patients (n = 81) who were considered to be recovered 90 days after stroke, still could experience an impact of their stroke on their daily living, physical function, participation in life, and hand function when asked about experience via a questionnaire. Previously, we found among a similar population of minor stroke patients to our current study, that hand function and mobility can still be negatively affected 3 years after stroke.63 This shows that patients with minor stroke with mild symptoms can still experience (physical) difficulties, even when they are considered to be recovered 3 months after stroke,62 a year after stroke64 and even after 3 years.63 These difficulties can still affect their lives65 and our current results suggest that this might be worsened by the presence and progression of WMH.
The strengths of this study are the longitudinal nature of the study, the large sample size and inclusion of minor stroke patients, with 94% of the participants providing data at the 1-year follow-up visit. There is a gap in the literature on mobility and dexterity outcomes after minor stroke in general and particularly in combination with the presence and progression of SVD features which are common in patients with stroke. This study combines this information and not only corrects for WMH but also examines the role of WMH. We also combined data from left- and right-handed people by looking at dominant and non-dominant hands, instead of including only right-handed participants, since the latter limits data and does not represent the general population. Additionally, we take into account how the participants experience their mobility and hand function. This provides valuable information on not just the effect of minor stroke and SVD but indirectly reflects their recovery and raises awareness of potential clinical needs after a minor stroke. However, our explorative analyses on the effects of several factors on dexterity and hand dominance need to be interpreted with caution. Due to the sample size, we were not able to include all interactions in one model and due to the explorative nature of the analyses, no correction for multiple comparisons has been done. The explorative analyses might still be underpowered due to inclusion of the interactions. We did not include the time interval between stroke and baseline visit, which might have an influence on the baseline scores. It might be valuable to include this in future analyses.
Some participants were not able to or chose not to attend the 1-year follow-up visit. Six participants did not attend in person and we gathered their 1-year information via a telephone follow-up. We compared participants (n = 14) without a 1-year visit to participants with an in-person or telephone visit and we only found that they had a shorter TUG time and potentially shorter time on the 9HPT for the dominant hand. There is no clear explanation for this. Perhaps they were not as severely affected by their stroke, or perhaps they were fully recovered and were less inclined to continue. There is no significant difference in baseline NIHSS score between the 1 year attendees and non-attendees, but the IQR (0 to 1) and range (0 to 4) of the baseline NIHSS score might suggest that the non-attendees included people with lower stroke severities than the attendee group (baseline IQR = 0 to 2; range: 0 to 7).
We would encourage further studies on the influence of the presence and progression of all SVD features on mobility, dexterity and functional outcomes after minor ischaemic stroke. As all SVD features, not only WMH, might have a contemporaneous effect on functioning and recovery after stroke. This can be an opportunity to highlight recovery and potential aspects for clinical support and rehabilitation that might currently be overlooked. Mild symptoms at the time of stroke might not necessarily indicate potential for full recovery and can still have an impact on patient’s independence and daily living.
Our findings show that WMH changes are independently associated with worsening mobility and dexterity up to 1 year after minor ischaemic stroke. Non-dominant hand dexterity seems to be affected more by stroke than the dominant hand, but effects of the stroke on the dominant hand appear to have a bigger impact on patients’ daily lives. Measures of mobility and dexterity at 1 year post-stroke are associated with self-reported experiences of mobility and hand function difficulties. Self-reported patient data offers valuable additional information. It also shows that no symptoms to mild symptoms after a minor stroke can still result in difficulties 1 year later, sustained by WMH progression and incidental lesions.
Supplementary Material
Contributor Information
Angela C C Jochems, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom.
Susana Muñoz Maniega, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom.
Francesca M Chappell, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom.
Una Clancy, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom.
Carmen Arteaga, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom.
Daniela Jaime Garcia, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom.
Olivia K L Hamilton, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC/CSO Social and Public Health Sciences Unit, School of Health and Wellbeing, University of Glasgow, G12 8TB Glasgow, United Kingdom.
Will Hewins, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom.
Rachel Locherty, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom.
Ellen V Backhouse, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom.
Gayle Barclay, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; Edinburgh Imaging Facility, Royal Infirmary of Edinburgh, EH16 4TJ Edinburgh, United Kingdom.
Charlotte Jardine, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; Edinburgh Imaging Facility, Royal Infirmary of Edinburgh, EH16 4TJ Edinburgh, United Kingdom.
Donna McIntyre, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; Edinburgh Imaging Facility, Royal Infirmary of Edinburgh, EH16 4TJ Edinburgh, United Kingdom.
Iona Gerrish, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; Edinburgh Imaging Facility, Royal Infirmary of Edinburgh, EH16 4TJ Edinburgh, United Kingdom.
Yajun Cheng, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; Department of Neurology, West China Hospital of Sichuan University, 610041 Chengdu, China.
Xiaodi Liu, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; Department of Medicine, LKS Faculty of Medicine, University of Hong Kong, Hong Kong, China.
Junfang Zhang, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; Department of Neurology, Shanghai General Hospital, Shanghai Jiao Tong University School of medicine, 200080 Shanghai, China.
Agniete Kampaite, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom.
Eleni Sakka, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom.
Maria Valdés Hernández, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom.
Stewart Wiseman, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom.
Michael S Stringer, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom.
Michael J Thrippleton, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; Edinburgh Imaging Facility, Royal Infirmary of Edinburgh, EH16 4TJ Edinburgh, United Kingdom.
Fergus N Doubal, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom.
Joanna M Wardlaw, Centre for Clinical Brain Sciences, University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; MRC UK Dementia Research Institute at the University of Edinburgh, EH16 4SB Edinburgh, United Kingdom; Edinburgh Imaging Facility, Royal Infirmary of Edinburgh, EH16 4TJ Edinburgh, United Kingdom.
Supplementary material
Supplementary material is available at Brain Communications online.
Funding
This study was funded by the UK Dementia Research Institute which receives its funding from Dementia Research Institute Ltd. Funded by the UK Medical Research Council, Alzheimer’s Society and Alzheimer’s Research UK; the Fondation Leducq Network for the Study of Perivascular Spaces in Small Vessel Disease (16 CVD 05); Stroke Association ‘Small Vessel Disease-Spotlight on Symptoms (SVD-SOS)’(SAPG 19n100068); British Heart Foundation Edinburgh Centre for Research Excellence (RE/18/5/34216); The Row Fogo Charitable Trust Centre for Research into Aging and the Brain. A.C.C.J. was funded by Alzheimer’s Society (ref 486 (AS-CP-18b-001)), University of Edinburgh College of Medicine and Veterinary Medicine and the UK Dementia Research Institute which receives funding from UK DRI Ltd. as described above. S.M.M. was funded by Biotechnology and Biological Sciences Research Council, and the Economic and Social Research Council (BB/W008793/1). UC was supported by the Scottish Chief Scientist Office (CAF/18/08). CAR is funded by the Mexican National Council of Science and Technology (CONACYT, 2021-000007-01EXTF-00234), the UK DRI (as above), the Rowling Clinic and Row Fogo Centre. O.K.L.H. was supported by the Medical Research Council (MC_UU_00022/2) and the Scottish Chief Scientist Office (SPHSU17). M.J.T. received funding from NHS Lothian Research and Development Office. SW, FND, EB are supported by The Stroke Association (SA PDF 18\100026, TSA LECT 2015/04, 16 VAD 07 respectively). Y.C. is supported by the China Scholarship Council; X.D.L. received funding from HKU Foundation Postgraduate Fellowship and HKU Research Postgraduate Student Exchange Scheme. The University 3T MRI Research scanner in Royal Infirmary Edinburgh is supported by the Scottish Funding Council through the Scottish Imaging Network, A Platform for Scientific Excellence (SINAPSE) collaboration; the Wellcome Trust (104916/Z/14/Z), Dunhill Trust (R380R/1114), Edinburgh and Lothians Health Foundation (2012/17), Muir Maxwell Research Fund, Edinburgh Imaging, and the University of Edinburgh. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising.
Competing interests
The authors report no competing interests.
Data availability
Examples of the statistical models used can be found in the Supplementary material. Pipelines for WMH segmentation are publicly available.43 Details on the observational cohort study are published elsewhere.24 The data that we used in this study can be made available upon reasonable request to the corresponding author.
References
- 1. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jochems ACC, Arteaga C, Chappell F, et al. Longitudinal changes of white matter hyperintensities in sporadic small vessel disease: A systematic review and meta-analysis. Neurology. 2022;99:e2454–e2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Debette S, Schilling S, Duperron M-G, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of vascular brain injury: A systematic review and meta-analysis. JAMA Neurol. 2019;76:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Callisaya ML, Beare R, Phan T, et al. Progression of white matter hyperintensities of presumed vascular origin increases the risk of falls in older people. J Gerontol A Biol Sci Med Sci. 2015;70:360–366. [DOI] [PubMed] [Google Scholar]
- 5. Hou Y, Li Y, Yang S, Qin W, Yang L, Hu W. Gait impairment and upper extremity disturbance are associated with total magnetic resonance imaging cerebral small vessel disease burden. Front Aging Neurosci. 2021;13:640844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kreisel SH, Blahak C, Bäzner H, et al. Deterioration of gait and balance over time: The effects of age-related white matter change—The LADIS study. Cerebrovasc Dis. 2013;35:544–553. [DOI] [PubMed] [Google Scholar]
- 7. de Laat KF, van Norden AG, Gons RA, et al. Gait in elderly with cerebral small vessel disease. Stroke. 2010;41:1652–1658. [DOI] [PubMed] [Google Scholar]
- 8. Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Srikanth V, Beare R, Blizzard L, et al. Cerebral white matter lesions, gait, and the risk of incident falls. Stroke. 2009;40:175–180. [DOI] [PubMed] [Google Scholar]
- 10. Su N, Zhai F-F, Zhou L-X, et al. Cerebral small vessel disease burden is associated with motor performance of lower and upper extremities in community-dwelling populations. Front Aging Neurosci. 2017;9:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whitman GT, Tang T, Lin A, Baloh RW. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57:990–994. [DOI] [PubMed] [Google Scholar]
- 12. Wolfson L, Wakefield DB, Moscufo N, et al. Rapid buildup of brain white matter hyperintensities over 4 years linked to ambulatory blood pressure, mobility, cognition, and depression in old persons. J Gerontol A Biol Sci Med Sci. 2013;68:1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nyquist PA, Yanek LR, Bilgel M, et al. Effect of white matter lesions on manual dexterity in healthy middle-aged persons. Neurology. 2015;84:1920–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: A systematic review. Lancet Neurology. 2009;8:741–754. [DOI] [PubMed] [Google Scholar]
- 15. Sunderland A, Bowers MP, Sluman S-M, Wilcock DJ, Ardron ME. Impaired dexterity of the ipsilateral hand after stroke and the relationship to cognitive deficit. Stroke. 1999;30:949–955. [DOI] [PubMed] [Google Scholar]
- 16. Cohen JW, Ivanova TD, Brouwer B, Miller KJ, Bryant D, Garland SJ. Do performance measures of strength, balance, and mobility predict quality of life and community reintegration after stroke? Arch Phys Med Rehabil. 2018;99:713–719. [DOI] [PubMed] [Google Scholar]
- 17. Huang P-C, Hsieh Y-W, Wang C-M, Wu C-Y, Huang S-C, Lin K-C. Predictors of motor, daily function, and quality-of-life improvements after upper-extremity robot-assisted rehabilitation in stroke. Am J Occup Ther. 2014;68:325–333. [DOI] [PubMed] [Google Scholar]
- 18. D'Alisa S, Baudo S, Mauro A, Miscio G. How does stroke restrict participation in long-term post-stroke survivors? Acta Neurol Scand. 2005;112:157–162. [DOI] [PubMed] [Google Scholar]
- 19. Singam A, Ytterberg C, Tham K, von Koch L. Participation in Complex and social everyday activities six years after stroke: Predictors for return to Pre-stroke level. PLos One. 2015;10:e0144344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ezekiel L, Collett J, Mayo NE, Pang L, Field L, Dawes H. Factors associated with participation in life situations for adults with stroke: A systematic review. Arch Phys Med Rehabil. 2019;100:945–955. [DOI] [PubMed] [Google Scholar]
- 21. Verstraeten S, Mark RE, Dieleman J, van Rijsbergen M, de Kort P, Sitskoorn MM. Motor impairment three months post stroke implies A corresponding cognitive deficit. J Stroke Cerebrovasc Dis. 2020;29:105119. [DOI] [PubMed] [Google Scholar]
- 22. McHutchison CA, Backhouse EV, Cvoro V, Shenkin SD, Wardlaw JM. Education, socioeconomic status, and intelligence in childhood and stroke risk in later life. Epidemiology. 2017;28:608–618. [DOI] [PubMed] [Google Scholar]
- 23. Hicks JM, Taub E, Womble B, et al. Relation of white matter hyperintensities and motor deficits in chronic stroke. Restor Neurol Neurosci. 2018;36:349–357. [DOI] [PubMed] [Google Scholar]
- 24. Clancy U, Jaime Garcia D, Stringer MS, et al. Rationale and design of a longitudinal study of cerebral small vessel diseases, clinical and imaging outcomes in patients presenting with mild ischaemic stroke: Mild stroke study 3. Eur Stroke J. 2021;6:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke. 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- 26. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 27. Makin SDJ, Doubal FN, Dennis MS, Wardlaw JM. Clinically confirmed stroke with negative diffusion-weighted imaging magnetic resonance imaging. Stroke. 2015;46:3142–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 29. Chertkow H, Nasreddine Z, Johns E, Phillips N, McHenry C. The Montreal cognitive assessment (MoCA): Validation of alternate forms and new recommendations for education corrections. Alzheimers Dement. 2011;7:S157. [Google Scholar]
- 30. Dong Y, Sharma VK, Chan BPL, et al. The Montreal cognitive assessment (MoCA) is superior to the Mini-mental state examination (MMSE) for the detection of vascular cognitive impairment after acute stroke. J Neurol Sci. 2010;299:15–18. [DOI] [PubMed] [Google Scholar]
- 31. Nelson HE, Willison J. National adult Reading test (NART). Nfer-Nelson Windsor; 1991. [Google Scholar]
- 32. Salthouse TA. Selective review of cognitive aging. J Int Neuropsychol Soc. 2010;16:754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Makin SD, Doubal FN, Shuler K, et al. The impact of early-life intelligence quotient on post stroke cognitive impairment. Eur Stroke J. 2018;3:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McHutchison CA, Chappell FM, Makin S, Shuler K, Wardlaw JM, Cvoro V. Stability of estimated premorbid cognitive ability over time after minor stroke and its relationship with post-stroke cognitive ability. Brain Sci. 2019;9:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Podsiadlo D, Richardson S. The timed “up & go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 36. Hafsteinsdóttir TB, Rensink M, Schuurmans M. Clinimetric properties of the timed up and go test for patients with stroke: A systematic review. Top Stroke Rehabil. 2014;21:197–210. [DOI] [PubMed] [Google Scholar]
- 37. Chen H-M, Chen CC, Hsueh I-P, Huang S-L, Hsieh C-L. Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabil Neural Repair. 2009;23:435–440. [DOI] [PubMed] [Google Scholar]
- 38. Wang Y-C, Bohannon RW, Kapellusch J, Garg A, Gershon RC. Dexterity as measured with the 9-hole peg test (9-HPT) across the age span. J Hand Ther. 2015;28:53–60. [DOI] [PubMed] [Google Scholar]
- 39. Sunderland A, Tinson D, Bradley L, Hewer RL. Arm function after stroke. An evaluation of grip strength as a measure of recovery and a prognostic indicator. J Neurol Neurosurg Psychiatry. 1989;52:1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Duncan PW, Bode RK, Min Lai S, Perera S. Rasch analysis of a new stroke-specific outcome scale: The stroke impact scale. Arch Phys Med Rehabil. 2003;84:950–963. [DOI] [PubMed] [Google Scholar]
- 41. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 42. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. [DOI] [PubMed] [Google Scholar]
- 43. Valdés Hernández MDC, Ballerini L, Glatz A, et al. Step-by-step pipeline for segmenting enlarged perivascular spaces from 3D T2-weighted MRI, 2018–2023 [software]; 2023. https://datashare.ed.ac.uk/handle/10283/8501.
- 44. Valdés Hernández MDC, Armitage PA, Thrippleton MJ, et al. Rationale, design and methodology of the image analysis protocol for studies of patients with cerebral small vessel disease and mild stroke. Brain Behav. 2015;5:e00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wardlaw JM, Chappell FM, Valdés Hernández MDC, et al. White matter hyperintensity reduction and outcomes after minor stroke. Neurology. 2017;89:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen L, Tong T, Ho CP, et al. Identification of cerebral small vessel disease using multiple instance learning. Springer International Publishing; 2015:523–530. [Google Scholar]
- 47. R Core Team, R: A language and environment for statistical computing . R Foundation for Statistical Computing; 2022. www.R-project.org.
- 48. Selves C, Stoquart G, Lejeune T. Gait rehabilitation after stroke: Review of the evidence of predictors, clinical outcomes and timing for interventions. Acta Neurol Belg. 2020;120:783–790. [DOI] [PubMed] [Google Scholar]
- 49. Pondal M, del Ser T. Normative data and determinants for the timed “up and go” test in a population-based sample of elderly individuals without gait disturbances. J Geriatr Phys Ther. 2008;31:57–63. [DOI] [PubMed] [Google Scholar]
- 50. Jochems ACC, Blair GW, Stringer MS, et al. Relationship between venules and perivascular spaces in sporadic small vessel diseases. Stroke. 2020;51:1503–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scherder E, Dekker W, Eggermont L. Higher-Level hand motor function in aging and (preclinical) dementia: Its relationship with (instrumental) activities of daily life—A Mini-review. Gerontology. 2008;54:333–341. [DOI] [PubMed] [Google Scholar]
- 52. Vasylenko O, Gorecka MM, Rodríguez-Aranda C. Manual dexterity in young and healthy older adults. 2. Association with cognitive abilities. Dev Psychobiol. 2018;60:428–439. [DOI] [PubMed] [Google Scholar]
- 53. Batchelor FA, Williams SB, Wijeratne T, Said CM, Petty S. Balance and gait impairment in transient ischemic attack and Minor stroke. J Stroke Cerebrovasc Dis. 2015;24:2291–2297. [DOI] [PubMed] [Google Scholar]
- 54. Li N, Li J, Gao T, Wang D, Du Y, Zhao X. Gait and balance disorder in patients with transient ischemic attack or Minor stroke. Neuropsychiatr Dis Treat. 2021;17:305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Heshmatollah A, Darweesh SKL, Dommershuijsen LJ, Koudstaal PJ, Ikram MA, Ikram MK. Quantitative gait impairments in patients with stroke or transient ischemic attack. Stroke. 2020;51:2464–2471. [DOI] [PubMed] [Google Scholar]
- 56. Srikanth V, Phan TG, Chen J, Beare R, Stapleton JM, Reutens DC. The location of white matter lesions and gait—A voxel-based study. Ann Neurol. 2010;67:265–269. [DOI] [PubMed] [Google Scholar]
- 57. Verlinden VJA, de Groot M, Cremers LGM, et al. Tract-specific white matter microstructure and gait in humans. Neurobiol Aging. 2016;43:164–173. [DOI] [PubMed] [Google Scholar]
- 58. de Laat KF, Tuladhar AM, van Norden AGW, Norris DG, Zwiers MP, de Leeuw F-E. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2010;134:73–83. [DOI] [PubMed] [Google Scholar]
- 59. Hannawi Y, Yanek LR, Kral BG, et al. White matter injury is associated with reduced manual dexterity and elevated Serum ceramides in subjects with cerebral small vessel disease. Cerebrovascular Diseases. 2021;50:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Riaz M, Vangberg TR, Vasylenko O, et al. What does hand motor function tell US about our aging brain in association with WMH? Aging Clin Exp Res 2021;33:1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Andersen KW, Siebner HR. Mapping dexterity and handedness: Recent insights and future challenges. Curr Opin Behav Sci. 2018;20:123–129. [Google Scholar]
- 62. Lai S-M, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the stroke impact scale. Stroke. 2002;33:1840–1844. [DOI] [PubMed] [Google Scholar]
- 63. McHutchison CA, Cvoro V, Makin S, Chappell FM, Shuler K, Wardlaw JM. Functional, cognitive and physical outcomes 3 years after minor lacunar or cortical ischaemic stroke. J Neurol Neurosurg Psychiatry. 2019;90:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Walsh ME, Galvin R, Loughnane C, Macey C, Horgan NF. Factors associated with community reintegration in the first year after stroke: A qualitative meta-synthesis. Disabil Rehabil. 2015;37:1599–1608. [DOI] [PubMed] [Google Scholar]
- 65. Turner GM, McMullan C, Atkins L, Foy R, Mant J, Calvert M. TIA and minor stroke: A qualitative study of long-term impact and experiences of follow-up care. BMC Fam Pract. 2019;20:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Examples of the statistical models used can be found in the Supplementary material. Pipelines for WMH segmentation are publicly available.43 Details on the observational cohort study are published elsewhere.24 The data that we used in this study can be made available upon reasonable request to the corresponding author.