We need to talk about red blood cells
Eukaryotic cells are defined as cells containing distinct nuclei and other membranous organelles. However, red blood cells (RBCs) lack a nucleus and organelles—perhaps to limit the generation of reactive oxygen species (Zhang et al., 2011)—and are filled with hemoglobin (Hb). This oxygen carrier is limited to the cytosol by the cell membrane and cytoskeleton, which maintain cellular integrity and deformability as RBCs pass through narrow capillaries (Gratzer, 1994) to perform their primary function of gas exchange. Moreover, in the absence of mitochondria, RBCs are believed to have a quite simple energy metabolism based on glucose catabolism (van Wijk and van Solinge, 2005). For years, RBCs were viewed as mere hemoglobin carriers and there were—and still are—discussions on whether they should really be considered as classic living cells. RBCs are considered as terminally differentiated cells based on the value of the half-cell reduction potential (Ehc) of the glutathione disulfide (GSSG)/glutathione (GSH) couple, which is linked to the biological status of the cell (proliferation–differentiation–apoptosis), and has been proposed as a key parameter associated with fundamental cellular biology (Schafer and Buettner, 2001). Yet, due to significant variations in human populations, the reported RBC Ehc levels in several cases resemble those of apoptotic cells (van ‘t Erve et al., 2013). Even so, as Walter Gratzer stated, RBCs are “more red than dead” (Gratzer, 1984) and, as complemented by Tibor Greenwalt, “a cell doesn’t have to have a nucleus to be respected” (Greenwalt, 1995). In this context, we will deconstruct the RBC status quo by examining a series of “what ifs”.
Unconventional RBC features
What if RBCs are not so empty after all?
During terminal erythropoiesis, human RBC progenitors extrude their highly condensed nucleus to yield reticulocytes, which then lose their remaining organelles during maturation into RBCs (Mei et al., 2021). While retained in most adult vertebrates except for mammals (Claver and Quaglia, 2009), nucleated RBCs can be found in the circulation of human fetuses but disappear in neonates. Their existence in the peripheral blood of neonates or adults is indicative of pathophysiological conditions (Haase, 2013; Stachon et al., 2007). Additionally, some subpopulations of mature RBCs retain their mitochondria; this phenomenon was reported in several disorders, including sickle cell disease (SCD) (Jagadeeswaran et al., 2017; Moriconi et al., 2022), Rhett syndrome (Sbardella et al., 2021), and systemic lupus erythematosus (SLE) (Caielli et al., 2021). Despite being the minority of circulating RBCs, mitochondria-positive RBCs may have significant effects, having been linked to oxidative stress, lysis phenomena, and immune responses (Caielli et al., 2021; Esperti et al., 2023; Moriconi et al., 2022; Tumburu et al., 2021). The release into the circulation of immature RBC subpopulations, such as nucleated and mitochondria-retaining, is often observed when there is a high demand for RBC production in the bone marrow or when ineffective erythropoiesis occurs (Pikora et al., 2023). Moreover, mitochondrial DNA mutagenesis (Ahlqvist et al., 2015), as well as defects in the ubiquitin (Ub) proteasome system [e.g., in SLE (Caielli et al., 2021)], impair mitochondrial elimination in the erythroid lineage.
Mature RBCs, lacking a nucleus and organelles, were thought to rely on their finite proteome throughout their ~120-day circulatory lifespan. Unexpectedly, a recent study confirmed that mature RBCs retain an, albeit limited, ability to translate not only primarily globin transcripts but also other gene transcripts (e.g., Fos proto-oncogene, FOS; JunB proto-oncogene, JUNB; ZFP36 ring finger protein, ZFP36; ubiquitin B, UBB) (Kumar et al., 2022). Interestingly, the use of translation inhibitors highlighted the importance of this low-level translation in maintaining normal amounts of globin chains (Kumar et al., 2022). It should be noted that this work contained several validation steps to ensure that the observed translation took place in mature RBCs and not reticulocytes, but with regard to non-RBC-specific genes, the results are discussed with caution and should be further validated using extensively leukoreduced samples. RBCs are also equipped with post-translational machinery to respond actively to changes in their environment. Being one of the cell types with the most kinases and phosphatases, it is not surprising that the regulation of RBC cytoskeletal elements and, consequently, membrane flexibility depend upon phosphorylation events (Cilek et al., 2023). These modifications are also implicated in pathological states, as in SCD (Dzandu and Johnson, 1980). Finally, the presence of balanced protein turnover machinery renders the RBC protein life cycle complete. For example, mature RBCs have functional proteasomes, which are hypothesized to be mainly tasked with Hb degradation. An excess of the 20S proteasome core over the 26S holoenzyme (Anastasiadi et al., 2021b) suggests the dominance of a ubiquitin-independent pathway, which may respond to transient or pathological hypoxia (Xu et al., 2022), although this phenomenon is still being elucidated. The discovery of midnolin, a protein that shuttles non-ubiquitinated nuclear proteins to the proteasome (Gu et al., 2023), may shed some light on this pathway, but it remains to be determined if RBCs contain a midnolin-like protein. Notably, translation- and protein control-related molecules seem to differ in RBCs from different pathophysiological backgrounds, such as with glucose-6-phosphate dehydrogenase deficiency (Tzounakas et al., 2022b), beta-thalassemia trait (Anastasiadi et al., 2021b), and hereditary xerocytosis (Caulier et al., 2022), adding complexity to these “simple” cells.
Are RBCs nucleic acid-free?
Although devoid of organelles, mature RBCs do contain nucleic acids. For example, circulating RBCs from splenectomized individuals contain nuclear remnants (“Howell-Jolly bodies”) (Angay et al., 2018). Surprisingly, even healthy RBCs contain nuclear and mitochondrial DNA, and higher DNA levels are observed in patients with particular pathology such as cancer; in the latter setting, RBCs seem to take up DNA from cancer cells, although the underlying mechanism needs further investigation (Liang et al., 2023). DNA uptake by RBCs was also reported for cell-free pathogen and mitochondrial DNA bound to the RBC Toll-like receptor 9; thus, under basal, cell-free DNA conditions, RBCs scavenge these deleterious nucleic acids to prevent inflammation (Hotz et al., 2018). Nonetheless, when there are pathological increases in cell-free DNA, as in sepsis or malaria, RBCs undergo changes to sacrifice themselves through erythrophagocytosis, thereby alerting the immune system by presenting CpG-containing DNA (Lam et al., 2021).
Generally, RBCs contain a large variety of RNA molecules, ranging from microRNAs (miRNAs) to mRNAs to large non-coding RNAs (Doss et al., 2015), and this array is altered in disease (Chen et al., 2008, 2017; Sun et al., 2020; Wu et al., 2023). Single-cell transcriptomic analyses revealed that there is even RNA heterogeneity among RBCs from the same subject; in addition, this RNA cargo is also found in RBC-derived extracellular vesicles (EVs) (Jain et al., 2022; Kerkela et al., 2022). Although it is not yet known which of these molecules are merely remnants and which have noncanonical roles, there are some interesting hypotheses. One example is 7SL, the RNA component of the signal recognition particle, which seems to interact with membrane and cytoskeletal proteins in blood samples, including spectrin, band 3, and protein 4.1 (Talhouarne and Gall, 2018). If this interaction is also true for pure RBC populations, it could imply that 7SL RNA is a dynamic component of unknown ribonucleoprotein complexes (Faoro and Ataide, 2021), aiding in the structural integrity of RBCs, or forming potential docking sites. In this view, it is interesting to note that the highly abundant band 3 is characterized by an N-terminus that is extremely negatively charged, thus potentially serving as a docking site for protein-RNA complexes, since RNA-binding proteins are enriched with basic residues to facilitate the interaction with the negatively charged nucleosides. Another noteworthy finding is that EV-secreted miRNAs from RBCs infected with P. falciparum may communicate with immune cells (Mantel et al., 2013; Regev-Rudzki et al., 2013). Since erythrophagocytosis can reprogram macrophages (Catala et al., 2020), one may speculate that erythrocytic non-coding RNAs—which have modulatory properties—may also be important in this process. Finally, miRNA composition changes during RBC storage and is associated with the “storage lesion” (Mulatie et al., 2023).
What if the RBC’s proteome is not that straightforward?
Classic RBC proteins with unexpected roles
RBCs possess a complex proteome, the interactome of which is dominated by protein homeostasis, redox biology, and cytoskeletal dynamics (Anastasiadi et al., 2021b; Tzounakas et al., 2021). Even their most well-known and abundant proteins are more multifaceted than was initially anticipated, exhibiting various noncanonical properties. For instance, band 3, the most abundant RBC membrane protein, is an anion transporter and a key component of the junctions between the plasma membrane and cytoskeleton that maintain RBC integrity (Reithmeier et al., 2016). Band 3 is also implicated in the recognition of senescent RBCs by the immune system (Badior and Casey, 2018), but its crucial importance in RBC metabolism was only recently deciphered. Thus, band 3 has a cytosolic chain that serves as a docking site for many molecules, implying its involvement in controlling several processes, ranging from energy and redox metabolism to proteostasis (Issaian et al., 2021; Satchwell and Toye, 2021). The RBC channelome, in general, is of high importance for tightly controlling ion permeability, since a slight flux of cations can alter the normal hallmarks of RBC physiology, such as deformability and shape (von Lindern et al., 2022). Interestingly, apart from its implications in RBC shape and lifespan control (Cahalan et al., 2015), the dynamic regulation of K+ transport is essential for maintaining a circadian rhythm in RBCs, in contrast to nucleated cells, which have an intrinsic “clock” mainly based on transcription cycles (Henslee et al., 2017). On another note, glycophorin A (GYPA) provides the RBC surface with a high negative charge, thereby preventing aggregation. GYPA also carries various glycans; given that multiple pathogens use cell surface glycoprotein glycans as receptors for invasion (Imberty and Varrot, 2008), GYPA serves as a potential “decoy receptor.” In this way, pathogens attach to anucleate RBCs instead of their nucleated cell targets (Anderson et al., 2018; Baum et al., 2002; Paulus and van der Hoorn, 2018). Nonetheless, GYPA also acts as a “real” receptor for RBC-targeted pathogens, such as P. falciparum (Jaskiewicz et al., 2019).
While known since the 1980s that RBCs contain non-muscle myosin IIA (NMIIA) molecules (Wong et al., 1985), their role was only quite recently deciphered. Although low in abundance, NMIIA contributes to the control of membrane curvature and RBC deformability, through dynamic interactions with actin (Alimohamadi et al., 2020; Smith et al., 2018). Interestingly, during reticulocyte maturation, NMIIA may participate in vesicle clearance (Moura et al., 2018). Because deformability concerns one of the most vital RBC attributes, allowing RBCs to pass through narrow capillaries, multiple methodologies have been used to elucidate RBC flow properties (Artmann et al., 1997; Dao et al., 2003; Guizouarn and Barshtein, 2020). Indeed, elegant microfluidics approaches showed that, after repeated cycles of deformation, an experimental condition that may simulate the accumulated membrane damage during blood circulation, healthy RBCs exhibited mechanical fatigue, which led to a loss of deformability, and increased membrane shear viscosity and energy dissipation, of a magnitude that could cause dissociation of the cell membrane from its cytoskeleton (Qiang et al., 2019). In the same context, when RBCs from healthy controls and, especially, from patients with SCD were subjected to cyclic hypoxic conditions, similar to Hb’s transition from R to T oxygen states, they exhibited reduced deformability (Qiang et al., 2021). However, NMIIA is not the sole contributor to deformability; rather, deformability is a multiparametric phenotype that depends on a wide range of factors, such as RBC hydration, metabolism, structure, and, even, Hb (Huisjes et al., 2018). Thus, it is not surprising that deformability is altered in various pathophysiological contexts and inherited disorders, which underlines its clinical importance. For example, in SCD, the Hb polymerization is the main issue undermining RBC deformability (Huang et al., 2003), whereas, in pyruvate kinase deficiency, decreased deformability is presumably based on insufficient ATP production leading to deregulation of membrane channel function and impaired ion homeostasis (Rab et al., 2021).
UBB is seemingly among the top-translated transcripts in mature RBCs and Ub is an important part of the Ub proteasome system (UPS), which is crucial not only for degrading defective proteins but also for regulatory purposes. Ubiquitination of membrane transporters affects RBC capacity to adapt to high altitude hypoxia (Xu et al., 2022) and to pathological hypoxia, as in SCD (Song et al., 2022). For example, degradation of bisphosphoglycerate mutase regulates acclimatization to high altitude hypoxia by constraining synthesis of 2,3-bisphosphoglycerate (Xu et al., 2022)—an allosteric modulator of Hb that promotes oxygen off-loading in response to hypoxia (Webb et al., 2021); however, this same process is deleterious in the context of chronic kidney disease (Xu et al., 2022). Similarly, ubiquitination and degradation of the adenosine equilibrative nucleoside transporter ENT1 (SLC29A1) is deleterious and promotes sickling by boosting bisphosphoglycerate synthesis downstream to adenosine signaling via adenosine A2b receptor (ADORA2B) (Song et al., 2022). Nonetheless, the sheer abundance of the 20S proteasome suggests an additional, non-UPS-related role for Ub in RBCs. Thus, its involvement in establishing protein interactions has been explored primarily in the case of spectrin, which possesses an E2/E3 domain and is simultaneously a target of its activity (Goodman et al., 2015). This domain also targets other important cytoskeletal elements (Chang et al., 2004, 2005), rendering ubiquitination an element in the dynamic regulation of protein associations. For instance, in SCD, the absence of spectrin ubiquitination produces excessively stable cytoskeletal complexes contributing to the characteristic rigidity of irreversibly sickled cells (Chang et al., 2005). Because Ub decorates multiple elements of the RBC membrane-cytoskeleton system, it may function as a scaffolding element. For example, when antioxidant and proteostatic components are translocated to the RBC membrane in response to stress (Anastasiadi et al., 2021b), ubiquitination could promote these interactions.
Unanticipated proteins found in RBCs
Surprisingly, multiple unexpected proteins were found in RBCs. As mentioned above, the determination of additional protein synthesis beyond globin chains in mature RBCs has not yet been conclusively ascertained. However, the presence of some reportedly translated transcripts may be plausibly justified, particularly for supporting Hb production. As examples, protein c-Fos and transcriptional factor JunB, members of the activator protein 1 (AP-1) master transcription regulator complex, could be remnants from earlier erythroid differentiation or even serve to enhance transcription of other top-translated genes in RBCs, like globins and ZFP36, which all contain AP-1 positive regulatory elements (Loyd et al., 2003). Moreover, zinc finger protein 36 (ZFP36) is itself a translation regulator, through destabilizing mRNAs by binding AU-rich elements (AREs) of their 3’UTRs (Blackshear and Perera, 2014). Because globin genes lack AREs and are stabilized by C-rich elements (Peixeiro et al., 2011; Waggoner and Liebhaber, 2003), one may speculate that ZFP36 facilitates general mRNA degradation in RBCs, thereby favoring the relative enrichment and translation of globin genes.
RBCs also contain members of the gene-regulating nuclear factor kappa B (NF-κB) pathway: NF-κB subunits p65 and p50, and the upstream elements, inhibitor of κB (IκB) and IκB kinase (IKK) (Ghashghaeinia et al., 2011). Treating RBCs with NF-κB pathway inhibitors induces a clearance-related phenotype (Ghashghaeinia et al., 2011), suggesting NF-κB-dependent pro-survival effects. However, these inhibitors also deplete GSH (Ghashghaeinia et al., 2012), and oxidative overload is linked to RBC clearance. It should be noted that, in oxidatively challenged nucleated cells, pathway components (i.e., IκB, IKKB) are oxidized and glutathionylated, inhibiting further pathway activation (Kanayama et al., 2002; Ogino et al., 2005; Reynaert et al., 2006; Seidel et al., 2011), which points to a multi-layer oxidation-driven blockade. Surprisingly, nitric oxide (NO), another negative regulator of RBC clearance, also inhibits IKKB by nitrosylation (Ghashghaeinia et al., 2017; Reynaert et al., 2004). The inhibitory role of glutathionylation and nitrosylation in pathway activation is especially intriguing in RBCs; however, further investigation is needed to determine if the NF-κB pathway is central to multiple RBC lifespan-controlling mechanisms. On another note, in keeping with the presence of RNAs in mature RBCs, proteomics evidence suggests the presence of potentially functional protein machinery for RNA interference silencing complexes, like Dicer (D’Alessandro et al., 2017a) as a remnant of essential miRNA maturation function at the erythroid stage under stress conditions (Byon et al., 2014).
Another surprising RBC cargo is cytokines, a large superfamily of signaling agents. For example, the Duffy Antigen Receptor of Chemokines (DARC; ACKR1) on the RBC surface is involved in the bioavailability of multiple chemokines, including interleukin 8 (Darbonne et al., 1991), small inducible cytokine A5 (RANTES), monocyte chemotactic protein 1 (MCP-1), and growth-regulated alpha protein (GRO-κ) (Horuk et al., 1994; Papadopoulos et al., 2021a). Because the ligand-DARC interaction on RBCs does not initiate intracellular signaling (Neote et al., 1994), RBCs probably function as a cytokine “sink” for sequestering excess circulating cytokines (Fukuma et al., 2003). Although the “sink hypothesis” assumes a passive role for RBCs, an “active reservoir” hypothesis views RBCs as capable of binding and then releasing their pro-inflammatory cargo as needed (Karsten et al., 2018a); the prolonged bioavailability of externally administered DARC-ligands supports this claim (Fukuma et al., 2003). Timely release is another potential function of this reservoir; supporting this concept, macrophage migration inhibitory factor, a highly abundant cytokine, is functionally active and may be released at sites of hemolysis, along with other RBC contents, thereby exerting a pro-inflammatory effect (Al-Abed et al., 2005; Karsten et al., 2018b).
Lastly, α-synuclein, aggregates of which are molecular hallmarks of Parkinson’s Disease, is highly abundant in RBCs (Barbour et al., 2008). It occurs in a monomeric, intrinsically disordered form (Fauvet et al., 2012), which exists in equilibrium with a membrane-associated multimeric form (Bartels et al., 2011). Although it has an affinity for anionic phospholipids, its involvement in membrane regulation was described in several intriguing reports. First, α-synuclein-null mice exhibit mild anemia, with RBCs of reduced volume (Xiao et al., 2014). Moreover, interactions of α-synuclein are not limited to membrane lipids; in Drosophila melanogaster neurons, it associates with β-spectrin, disrupting the integrity of the spectrin-ankyrin complex (Maor et al., 2023). It would be useful to explore if these interactions occur in human RBCs and contribute to cytoskeletal disorganization. Finally, Hb also interacts with α-synuclein; this was first identified with neuronal globins and then confirmed in RBCs (Yang et al., 2020).
To date, the mechanism through which protein cargo is transferred to RBCs remains mainly unexplored. Notably, as in the case of nucleic acids, there are indications for protein uptake by RBCs either from other cells or from EVs. As recently shown, mechanical stimulation leads to the formation of distinct temporary cord-like structures between RBCs, which seem to enable protein transfer (Hertz et al., 2023). In the same context, earlier works demonstrated sorting of Band 3 to EVs, as well as its intermembrane transfer from EVs to RBCs (Newton et al., 1983). Analogously, lymphocytes can acquire proteins from other immune cells via trogocytosis (Joly and Hudrisier, 2003). Regarding RBCs, protein transfer mechanisms warrant further investigation; they seem to be strictly regulated by cytoskeletal dynamics because, in most cases, the spectrin-actin network inhibits endocytosis phenomena (Gao et al., 2017).
What if Hb does not just carry oxygen?
Hb, the most abundant protein in RBCs, contains four subunits, each of which carries a prosthetic heme group. Oxygen binds reversibly to the iron atom of each heme group so that it can be transported to all tissues (Marengo-Rowe, 2006). Nonetheless, oxygen is not the only molecule that binds to Hb. Nitric oxide (NO) can be sequestered by heme, resulting in its low bioavailability and subsequent effects on vasodilation (Helms and Kim-Shapiro, 2013). In contrast, under hypoxic conditions, when endothelial NO production is compromised, Hb can reduce nitrite to NO (Huang et al., 2005) and can also transport NO by conjugation to cysteine thiols in Hb (Jia et al., 1996), highlighting Hb’s role in regulating NO bioavailability. In general, Hb contains docking sites for several molecules, including GSH and NAD(P)H. GSH is critical for RBC antioxidant defenses, and Hb buffers its levels in an oxygen-dependent manner to enhance antioxidant power (Fenk et al., 2022). Similarly, Hb’s NAD(P)H binding and pseudo-enzymatic activities suppress autoxidation and methemoglobin formation (Yamada et al., 2019). These redox-related roles in RBCs perfectly match the observed antioxidant potential of Hb expressed in cancer cells (Li et al., 2013). Hb was additionally proposed to serve as a murzyme, catalyzing ATP synthesis in RBCs (Parashar et al., 2022). Finally, in mice and rabbits, Hb has other unusual functions, acting as a chemosensory signal (Osakada et al., 2022) and antimicrobial molecule (Patgaonkar et al., 2011), respectively. To our knowledge, these latter roles have not yet been reported in humans.
Is RBC metabolism really that simple?
As highlighted in a recent review (D’Alessandro et al., 2023a), RBC metabolism is far from simple, not only involving glycolysis but also exhibiting an extensive metabolic network, including cytosolic metabolism of tricarboxylic acids (D’Alessandro et al., 2017b), purine (Nemkov et al., 2018b) and arginine (Kalani Roy et al., 2022) metabolism. In addition, RBCs possess receptors and transporters for various important molecules, including insulin, sex and (para)thyroid hormones, catecholamines, neurotransmitters, and multiple drugs (de Almeida and Saldanha, 2010; Gambhir and Agarwal, 1991; Nemkov et al., 2018a; Papadopoulos et al., 2021b). All these render RBCs as a “sink” or transporter for metabolites and signaling molecules, informatively demonstrated in the case of cortisol and aldosterone, both of which seem to be bound and released in a temperature-dependent manner (Papadopoulos et al., 2021b). Activating some of these receptors also induces intracellular signaling cascades. For example, there are indications that shear-induced deformability during blood flow could be regulated by the cyclic AMP/protein kinase A pathway, since inhibitors of this route impair the mechanically induced plasticity of RBCs (Ugurel et al., 2022).
Despite the absence of intracellular compartmentalization in the RBC cytosol, noncanonical metabolism-related microdomains may exist in the RBC membrane (Leo et al., 2020). The most widely discussed such example concerns NO metabolism. Thus, RBCs may possess a “nitrite reductase metabolon” located in lipid rafts and comprised of structural proteins and deoxyHb. DeoxyHb can induce the production of nitrite-derived NO, and the membrane proximity of the implicated reactants and products allows NO to “escape” from Hb’s scavenging properties, and consequently, approach its targets (Gladwin et al., 2005). Additionally, some of the NO produced may react with oxyHb to produce nitrate and metHb, with the latter forming a protective “fence” so that the additional NO produced remains unaffected (Cortese-Krott and Kelm, 2014). RBCs also contain NO synthases (NOS), on the cytoplasmic side of their membrane. Cationic amino acid transporters allow entry of L-arginine into RBCs, which acts as an NOS substrate to produce NO (Cortese-Krott and Kelm, 2014; Gajecki et al., 2022). NOS are also activated during shear stress to regulate vascular properties (Ulker et al., 2009). Taken together, these findings suggest a central role for RBCs in systemic NO metabolism; not just as “pools”, but also as storers, producers, and transporters. Nonetheless, RBCs are not solely mediators in these events, rather NO has been implicated in modulating several RBC properties, including deformability and removal (Brun et al., 2021).
A dynamic perspective on RBC biomarker and drug targeting potential
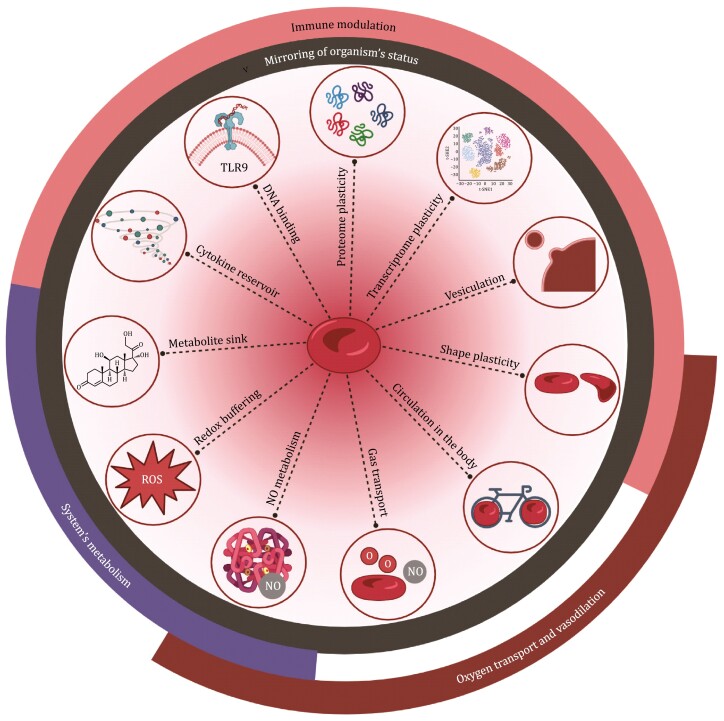
The findings discussed above identify that RBCs have a wide array of abilities and functions (Fig. 1). It is noteworthy that numerous intriguing reports describe unexpected RBC cargoes, highlighting the versatility of RBCs. However, we focus on reviewing studies that isolate RBCs by filtration or sorting techniques and/or those that incorporate validation steps to support the purity of the RBCs being examined, as emphasized by the European Red Cell Society (Minetti et al., 2013). Being the most abundant circulating cell type, it is no surprise that RBCs do not just carry oxygen. Thus, RBCs are not simple. Indeed, anucleate RBCs seem to chart a nucleus-independent course that proves advantageous for the entire organism. They can act as redox and ion buffers and monitors of vascular tone. They sacrifice themselves to alert the immune system to “danger.” Thus, they are more than just simple cells, but, rather, appear to function as an “organ” (Nemkov et al., 2018a). To date, the focus has been on what RBCs are missing; perhaps it is time to focus on what they have, whether as a remnant, or a vital component, or as something acquired later in their lifespan. Therefore, new information and unexpected findings provide food for thought regarding applications of RBCs in medical settings and lead to a provocative question: What if the “blind watchmaker” (Dawkins, 1986), after years of evolution, has already provided the answer to multiple questions? One answer that is hiding in plain sight, maybe in the form of RBCs.
Figure 1.
RBC properties with systemic impact. The versatile properties of RBCs allow them to reflect an organism’s pathophysiological status; in addition, several of them are crucial for regulating systemic metabolism and vasodilation, and for crosstalk with the immune system. NO: nitric oxide. (Created with Biorender).
RBC disease signatures
Altered RBCs are seen in several pathological conditions and can stratify or characterize patients. For example, the percentage of mitochondria-retaining RBCs in SCD was linked to markers of disease severity, such as sickling and hemolysis (Esperti et al., 2023), whereas RBC mir-144 may be associated with anemia in these same patients. The latter is due to mir-144’s effect on modulating antioxidant powers, through targeting nuclear factor erythroid 2-related factor 2 (NRF2) in RBC precursors, making it a remnant that acts as a useful marker of patients who could benefit from antioxidant treatment (Sangokoya et al., 2010). In the context of transfusion-dependent thalassemia, the deformability of the administered RBCs seems to be a potent indicator of the transfusion outcome, since it has been linked to skin blood flow (Barshtein et al., 2016) and Hb increment (Barshtein et al., 2017). Indeed, transfusion of units containing low levels of rigid RBCs increases the time interval between consecutive transfusions in this patient cohort (Barshtein et al., 2017). Of course, hematological diseases are not the only ones reflected in RBC properties. RBCs can “mirror” the whole organism’s homeostasis due to their constant movement through circulatory networks and interactions with all tissues. The information they collect when traveling through the organism can be “imprinted” on them (Nemkov et al., 2018a), making them useful biomarkers (e.g., glycosylated Hb; HbA1c), especially since blood tests are typically minimally invasive, inexpensive, and easily performed. To support this, RBC distribution width (i.e., RDW) is affected in almost every pathological condition, including hematological diseases (e.g., thalassemia, SCD), and others (e.g., neoplastic, autoimmune, and psychiatric disorders) (Lippi and Plebani, 2014). Therefore, many of the paradoxes discussed above, especially RBCs’ impressive plasticity, may be exploited for diagnostic or prognostic purposes.
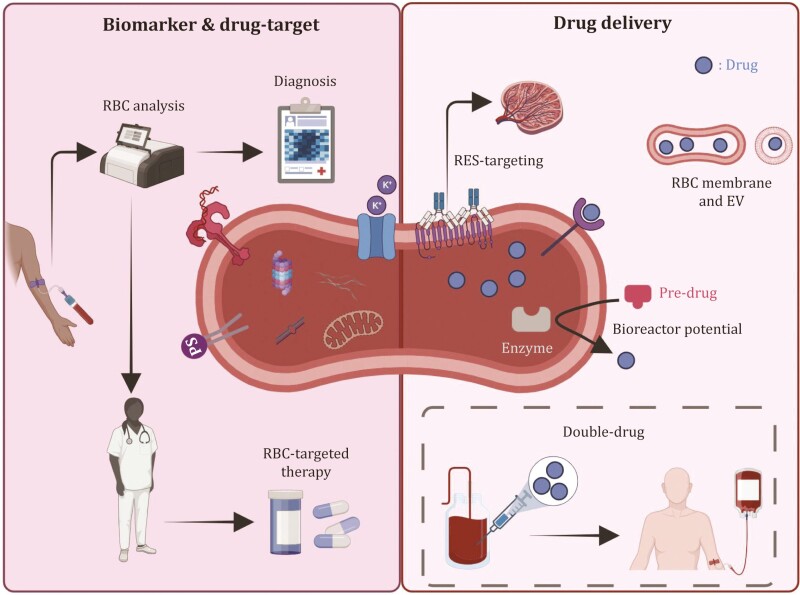
For example, a liquid biopsy might be useful in early-stage lung cancer patients since their mature RBCs seem to contain copies of DNA with genetic mutations derived from the cancer tissue (Liang et al., 2023). Moreover, the presence of nucleated RBCs might be an independent marker of molecular remission failure in chronic myeloid leukemia (Phan et al., 2019). RBCs are also altered in autoimmunity; in addition to retained mitochondria in RBCs from SLE patients (Caielli et al., 2021), the morphology and biophysical membrane properties of RBCs are altered in rheumatoid arthritis, possibly due to the disease itself or to concomitant factors (Olumuyiwa-Akeredolu et al., 2017). Regarding neurological conditions, and having in mind that (i) α-synuclein interacts with lipid membranes (Sarchione et al., 2021) and (ii) RBCs represent the major source of peripheral α-synuclein, RBC levels of the latter were proposed as a biomarker for Parkinson’s Disease, since α-synuclein blood levels differ between healthy individuals and patients (Abd Elhadi et al., 2019; Tian et al., 2019). Analogously, fibrils on the RBC surface, postulated to be comprised of β-amyloid and tau isoforms, are potential biomarkers for early detection of Alzheimer’s disease (Nirmalraj et al., 2021). One key phenomenon in both Alzheimer’s Disease and RBC responses to hypoxia involves protein isoaspartyl-damage arising from dehydration/deamidation-triggering oxidant challenges (e.g., to structural proteins like 4.1 and band 3 in the aging RBC; to tau protein in Alzheimer’s disease), a process that is, in part, counteracted by protein L-isoaspartyl O-methyltransferase both in RBCs and neural cells (D’Alessandro et al., 2023b). Blood testing to classify patients was recently described using plasma tau protein 217, potentially accurately stratifying and detecting Alzheimer’s disease in a cost-effective way (Brum et al., 2023). Perhaps the most obvious link between neurodegenerative diseases and RBCs is found with the rare neuroacanthocytosis syndromes, such as Chorea-acanthocytosis and McLeod syndrome, in which RBC shape and deformability are altered (Zhang et al., 2013a). Although the mechanism of acanthocyte formation is not completely understood, abnormalities in the levels and conformations of membrane lipids and proteins could be responsible (Kay et al., 1990; Sakai et al., 1991). In children affected by autism spectrum disorder (ASD), a specific spectrum of RBC membranes by using hyperspectral dark field microscopy displayed noteworthy characteristics, since it differed from healthy children and correlated with impaired behavior and cognition scores (Giacometti et al., 2017). In accordance, shape abnormalities were found in RBCs from ASD subjects, without evidence of a specific genetic defect in the progenitor cells (Ciccoli et al., 2013). Moreover, RBCs change during viral infections; for example, their elasticity is reversibly affected when exposed to COVID-19 patient plasma (Recktenwald et al., 2022), whereas senescence markers correlate with disease severity (Bouchla et al., 2021). It does not appear to be a coincidence that activation of antiviral interferon responses in COVID-19 patients is accompanied by metabolic markers such as kynurenine (Recktenwald et al., 2022), fragmentation, and oxidation of structural membrane proteins (Thomas et al., 2020), which in turn have been recently associated to increased osmotic fragility, acute phase response protein deposition on RBC membranes and ultimately increased extravascular hemolysis in healthy, older male blood donors with higher body mass indices (Nemkov et al., 2023). Finally, since RBCs can capture exogenous DNA, they can sequester a detectable amount of cell-free nucleic acids, which would otherwise be undetectable in plasma; this is potentially useful for identifying infectious diseases or cancer at earlier stages. These functions were already leveraged for blood donor surveillance during the Zika virus crisis, showing that negative nucleic acid testing and immunoglobulin M (IgM) to immunoglobulin G (IgG) seroconversion in repeat blood donors infected by this flavivirus still coincided with PCR positivity in mature RBCs for up to ~120 days after infection, the average lifespan of a mature RBC (Catala et al., 2022). Overall, RBC screening could potentially complement currently used serum/plasma biomarkers in various settings, thereby enhancing diagnostic sensitivity and specificity (Fig. 2).
Figure 2.
RBC-based biomarkers, drug targets, and drug delivery potential. RBCs contain membrane-associated and cytosolic molecules that could be disease biomarkers or drug targets offering timely and accurate prediction, as well as alternative therapeutic schemes. They can also be bioengineered to function as delivery systems to enhance drug administration and extend the circulatory bioavailability of the therapeutic agent. The potential of a scheme that infuses the selected drug attached to the transfused RBCs should not be excluded. RES: reticuloendothelial system, EV: extracellular vesicle. (Created with Biorender).
The identification of the potential markers described above was significantly propelled by advances in cutting-edge technologies, such as metabolomics, proteomics, and transcriptomics. Especially in the last decade, the elegant approaches of mass spectrometry and next-generation sequencing were applied to RBCs to construct a more precise “map” of RBC biology and consequently played a pivotal role in unraveling molecular signatures associated with various hematological and non-hematological conditions. Additionally, RBC-specific techniques, particularly those involving deformability, membrane/cytoskeleton mechanical properties, and Hb biochemistry, as well as traditional and state-of-the-art methods for RBC morphology, contributed to the detailed exploration of biomarkers. Nonetheless, the translation of these findings into clinical practice requires solid evidence and careful consideration. Moreover, establishing robust methods and incorporating them into routine clinical settings remains an essential challenge.
RBCs in therapy
RBCs as drug targets
Several disease-related RBC alterations and the unique properties of these cells could serve as potential drug targets (Fig. 2). For example, mitophagy agents in SCD mouse models reduced mitochondrial retention in RBCs and increased RBC lifespan, suggesting a promising therapeutic approach (Jagadeeswaran et al., 2017). Similarly, RBC channel blockers and cAMP-pathway modulators were explored in SCD to counteract cell dehydration and deformability issues, respectively (Ataga et al., 2008; Goskel et al., 2023; Toppet et al., 2000). The new knowledge that RBCs perform low levels of protein synthesis could also be therapeutically useful; thus, drugs enabling stop codon readthrough might be useful for patients with beta-thalassemia who have premature stop codons in their beta-globin gene (Kar et al., 2020; Kumar et al., 2022). Similarly, suggestions of channel transfer in RBCs might provide promising therapeutic interventions targeting channelopathies (Hertz et al., 2023). In addition, in malaria, the protective effect of specific RBC miRNAs (Cyrus, 2021), along with the role of RBC GTPases in the invasion process (Paone et al., 2020), suggest novel drug targets.
Despite our focus in the section above on hematological or RBC-related diseases, RBCs may also be drug targets for multiple other disorders. One example is SLE, in which RBCs are considered a key player due to their mitochondrial cargo (Caielli et al., 2021; Wang, 2021). Additionally, the diminished antioxidant powers of RBCs in multiple sclerosis (MS), the role of RBCs as redox buffers, and the improvement of both RBC redox characteristics and MS symptoms by melatonin supplementation imply that targeting RBCs could be therapeutically useful (Groen et al., 2016). Finally, in cancer, tumors can disrupt normal erythropoiesis, leading to the presence of circulating erythroid progenitor cells (EPC), which could suppress immunity and promote neo-angiogenesis, tumor growth, and metastasis (Zhang et al., 2022). Analogously, the presence of extravascular intratumor mature RBCs, resulting from micro-hemorrhages, can be immunosuppressive by multiple immune-metabolic mechanisms (Papadopoulos, 2022; Papadopoulos et al., 2021a). Taken together, this information suggests using drugs that promote EPC differentiation to mature RBCs along with trying to minimize hemorrhage at tumor sites. One could speculate that such therapeutic schemes can complement currently available oncotherapy, by (i) ameliorating anemia (Madeddu et al., 2021), (ii) improving oxygen delivery to the tumor microenvironment to approximate normoxic conditions, and (iii) reversing suppression of immune cells. Interestingly, polyploid giant cancer cells (PGCCs) can generate their own RBCs that bind oxygen with high affinity due to the expression of fetal and embryonic hemoglobins, providing PGCCs with significant survival advantages (Liu et al., 2022; Zhang et al., 2013b). Further examining tumor erythropoiesis and targeting of PGCCs (or the produced RBCs) are essential to the success of novel RBC-implicating therapeutic anticancer approaches.
RBC-based drug delivery
The GYPA decoy theory led to the idea of bioengineering RBCs with decoy viral receptors to prevent invasion of nucleated cells and was examined for several viruses in vitro and in animal models (Asher et al., 2005; Hoffmann et al., 2021). However, this is not the only way to bioengineer RBCs, and some have considered them to be ideal drug delivery systems (DDS). Biological DDS are based on natural cells and their derivatives (e.g., RBC ghosts and EVs) and have the significant advantage of biocompatibility. RBCs have been examined as drug carriers since the 1970s and are felt to be useful for this purpose due to their long circulation lifespan, which ensures sustained drug release over time, and their lack of a nucleus and organelles, which provide “space” for drugs (Chen et al., 2023). They can encapsulate therapeutic molecules or carry them on their surface. In the first case, intraerythrocytic agents can either be a non-enzyme drug (e.g. dexamethasone (Chessa et al., 2014)), or an enzyme for replacement therapy purposes. In the latter case, the RBC can function as a bioreactor for removing the accumulated substrate from the bloodstream (Koleva et al., 2020). Two examples include the encapsulation of asparaginase and thymidine phosphorylase, which are proposed for pancreatic cancer and mitochondrial neurogastrointestinal encephalomyopathy patients, respectively (Bax et al., 2019; Hammel et al., 2020; Robert et al., 2022). Regarding surface loading, the binding of glucose derivative-modified insulin on RBCs was reversible in hyperglycemia and prolonged the therapeutic effects of insulin in diabetic mice (Wang et al., 2017). More recently, a scheme of RBC-leveraged chemotherapy was proposed to combat lung metastasis (Zhao et al., 2019, 2021), suggesting a role for RBC-based cancer therapy. Finally, modifying RBCs to expose antigens favoring their opsonization can target them to the reticuloendothelial system, as exploited in studies of cancer and HIV (Pierige et al., 2017), while the target repertoire can be substantially extended by selecting the proper infusion site (Brenner et al., 2018).
Based on RBCs’ intrinsic characteristics and their versatility, RBC-based delivery systems are expected to have broader applications in the future. However, some factors need to be considered before extensive applications in clinical settings. Indeed, during the process of producing RBC-based drug delivery systems, RBCs become less deformable, while the change in the viscoelasticity of their cytoplasm affects cell dynamics (Chen et al., 2023). A decrease in deformability is also observed during surface coupling of therapeutic molecules to the RBC membrane, along with loss of CD47 (integrin-associated protein) and externalization of phosphatidylserine (Villa et al., 2016), all of which can act as signals for rapid clearance from the circulation (Burger et al., 2012; Nguyen et al., 2011; Sosale et al., 2015). For example, attachment of mesoporous silica nanoparticles to the RBC surface impaired their elasticity and deformability (Zhao et al., 2011). Similarly, RBC rigidity may overpower CD47 cell signaling in phagocytosis (Sosale et al., 2015), influence adherence of platelets to the endothelium (Aarts et al., 1984) and, when combined with increased endothelial potential, induce vascular resistance (Kaul et al., 2008). Taken together, these underline the need for strict control of the mechanical properties of RBCs modified for drug transport to extend their circulation time after administration. The pros and cons of using RBCs or their cellular derivatives as drug carriers exceed the scope of the present work and are extensively reviewed elsewhere (Chen et al., 2023; Tzounakas et al., 2017; Villa et al., 2016). Nonetheless, we believe that RBCs will remain a focus of drug delivery innovation.
Of course, RBCs themselves are a “drug” for patients needing transfusion. The variations in donated RBC physiology and biochemistry are relevant to transfusion medicine and are determined by donor genetics, the donor “exposome” (Nemkov et al., 2021), and the ability of RBCs to absorb information from the whole organism. Because stored RBCs from different donors present different redox and metabolism properties, fragility indices, and senescence signals, and also behave differently post-transfusion, these all form the basis for improving personalized transfusion therapy (Anastasiadi et al., 2021a; D’Alessandro and Hod, 2023; D’Alessandro et al., 2021; Thomas et al., 2023; Tzounakas et al., 2022a). Simultaneously, some blood products fail quality control procedures or pass the expiration date before they are administered to patients. Perhaps blood services could apply for a waste management protocol in which blood products that do not meet eligibility criteria for transfusion, including slightly expired or underweight RBC concentrates and fresh frozen plasma from female donors (Tzounakas et al., 2017), could be used for DDS protocols. If this were the case, every single blood drop would be maximized for use for either classic transfusion purposes or alternative therapeutic protocols. Taking this a step further, it would be interesting to produce RBC units loaded with a drug of interest for patients in whom both transfusion and therapeutic interventions were needed, as was proposed for platelet units (Wu et al., 2016). Hence, novel ideas, such as transfusing asparaginase-loaded RBCs in patients with acute lymphoblastic leukemia (Domenech et al., 2011), could possibly be extended to additional disorders.
We need to keep talking about RBCs
What had started as a basic cell biology and biochemistry quest led to uncovering and rediscovering a multifunctional cell machine with the potential to act as a systemic sensor of disease pathology and as a modulator of physiology. Equipped with a remarkable armamentarium of molecules and cellular properties, RBCs can perform multiple different systemic functions and dedicate their short and strictly controlled lifespan to maintaining organismal homeostasis. Thus, while performing their long-known functions of gas transport and pH buffering in circulation, RBCs can also act as sensors, collecting all sorts of information that are carried by them as extra cargo or as cellular modifications. In this way, we believe that mature RBCs are one of the most promising targets for assessing multiple disease settings, as well as an effective scaffold for designing novel therapies. By applying multi-omics approaches, one can now draw a more complete RBC map and unlock the RBC’s hidden potential to act as a “pathophysiomics” tool itself. Whether the perspectives and opinions provided in this review are biased by the authors’ close encounters with, and appreciation of, this unique cell, or truly represent an opportunity to answer current and future scientific questions, remains to be determined. Until then, we hope you appreciate that “we have a dream” (King, 1963) of an RBC-driven/based biomedicine able to provide new alternatives to health care systems and patients in need.
Contributor Information
Alkmini T Anastasiadi, Department of Biochemistry, School of Medicine, University of Patras, 26504 Patras, Greece.
Vasiliki-Zoi Arvaniti, Department of Biochemistry, School of Medicine, University of Patras, 26504 Patras, Greece.
Krystalyn E Hudson, Department of Pathology and Cell Biology, Columbia University Irving Medical Center, New York City, NY 10032, USA.
Anastasios G Kriebardis, Laboratory of Reliability and Quality Control in Laboratory Hematology (HemQcR), Department of Biomedical Sciences, School of Health & Caring Sciences, University of West Attica (UniWA), 12243 Egaleo, Greece.
Constantinos Stathopoulos, Department of Biochemistry, School of Medicine, University of Patras, 26504 Patras, Greece.
Angelo D’Alessandro, Department of Biochemistry and Molecular Genetics, University of Colorado Anschutz Medical Campus, 13001 Aurora, CO, USA.
Steven L Spitalnik, Department of Pathology and Cell Biology, Columbia University Irving Medical Center, New York City, NY 10032, USA.
Vassilis L Tzounakas, Department of Biochemistry, School of Medicine, University of Patras, 26504 Patras, Greece.
Author contributions
A.T.A., V.Z.A., and V.L.T. prepared the first draft of the manuscript. K.E.H., A.G.K., C.S., A.D., and S.L.S. substantially contributed to the final draft. All authors approved the submitted manuscript.
Funding
A.T.A. was supported by an EHA Research Grant award granted by the European Hematology Association (RG-202212-03039). A.D. was supported by funds by the National Heart, Lung, and Blood Institute (R21HL150032, R01HL146442, R01HL149714, R01HL161004). S.L.S. and A.D. were supported by the National Institutes of Health (NIH R01-HL148151).
Conflict of interest
The authors declare that A.D. is a founder of Omix Technologies Inc and Altis Biosciences LLC. A.D. is a Scientific Advisory Board (SAB) member for Hemanext Inc and Macopharma Inc. S.L.S. is a member of the Scientific Advisory Boards of Hemanext, Inc. and Alcor, Inc. The remaining authors have no conflicts of interest to declare.
Consent to participate
The authors declare their agreement to participate.
Consent for publication
The authors declare their agreement to publish.
References
- Aarts PA, Heethaar RM, Sixma JJ.. Red blood cell deformability influences platelets–vessel wall interaction in flowing blood. Blood 1984;64:1228–1233. [PubMed] [Google Scholar]
- Abd Elhadi S, Grigoletto J, Poli M. et al. alpha-Synuclein in blood cells differentiates Parkinson’s disease from healthy controls. Ann Clin Transl Neurol 2019;6:2426–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlqvist KJ, Leoncini S, Pecorelli A. et al. MtDNA mutagenesis impairs elimination of mitochondria during erythroid maturation leading to enhanced erythrocyte destruction. Nat Commun 2015;6:6494. [DOI] [PubMed] [Google Scholar]
- Al-Abed Y, Dabideen D, Aljabari B. et al. ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. J Biol Chem 2005;280:36541–36544. [DOI] [PubMed] [Google Scholar]
- Alimohamadi H, Smith AS, Nowak RB. et al. Non-uniform distribution of myosin-mediated forces governs red blood cell membrane curvature through tension modulation. PLoS Comput Biol 2020;16:e1007890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadi AT, Paronis EC, Arvaniti VZ. et al. The post-storage performance of RBCs from beta-thalassemia trait donors is related to their storability profile. Int J Mol Sci 2021a;22:12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadi AT, Tzounakas VL, Arvaniti VZ. et al. Red blood cell proteasome in beta-thalassemia trait: topology of activity and networking in blood bank conditions. Membranes (Basel) 2021b;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson HL, Brodsky IE, Mangalmurti NS.. The evolving erythrocyte: red blood cells as modulators of innate immunity. J Immunol 2018;201:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angay O, Friedrich M, Pinnecker J. et al. Image-based modeling and scoring of Howell-Jolly Bodies in human erythrocytes. Cytometry A 2018;93:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artmann GM, Sung KL, Horn T. et al. Micropipette aspiration of human erythrocytes induces echinocytes via membrane phospholipid translocation. Biophys J 1997;72:1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher DR, Cerny AM, Finberg RW.. The erythrocyte viral trap: transgenic expression of viral receptor on erythrocytes attenuates coxsackievirus B infection. Proc Natl Acad Sci USA 2005;102:12897–12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataga KI, Smith WR, De Castro LM. et al. ; ICA-17043-05 Investigators. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood 2008;111:3991–3997. [DOI] [PubMed] [Google Scholar]
- Badior KE, Casey JR.. Molecular mechanism for the red blood cell senescence clock. IUBMB Life 2018;70:32–40. [DOI] [PubMed] [Google Scholar]
- Barbour R, Kling K, Anderson JP. et al. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis 2008;5:55–59. [DOI] [PubMed] [Google Scholar]
- Barshtein G, Pries AR, Goldschmidt N. et al. Deformability of transfused red blood cells is a potent determinant of transfusion-induced change in recipient’s blood flow. Microcirculation 2016;23:479–486. [DOI] [PubMed] [Google Scholar]
- Barshtein G, Goldschmidt N, Pries AR. et al. Deformability of transfused red blood cells is a potent effector of transfusion-induced hemoglobin increment: a study with beta-thalassemia major patients. Am J Hematol 2017;92:E559–E560. [DOI] [PubMed] [Google Scholar]
- Bartels T, Choi JG, Selkoe DJ.. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 2011;477:107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J, Ward RH, Conway DJ.. Natural selection on the erythrocyte surface. Mol Biol Evol 2002;19:223–229. [DOI] [PubMed] [Google Scholar]
- Bax BE, Levene M, Bain MD. et al. Erythrocyte encapsulated thymidine phosphorylase for the treatment of patients with mitochondrial neurogastrointestinal encephalomyopathy: study protocol for a multi-centre, multiple dose, open label trial. J Clin Med 2019;8:1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear PJ, Perera L.. Phylogenetic distribution and evolution of the linked RNA-binding and NOT1-binding domains in the tristetraprolin family of tandem CCCH zinc finger proteins. J Interferon Cytokine Res 2014;34:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchla A, Kriebardis AG, Georgatzakou HT. et al. Red blood cell abnormalities as the mirror of SARS-CoV-2 disease severity: a pilot study. Front Physiol 2021;12:825055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner JS, Pan DC, Myerson JW. et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat Commun 2018;9:2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum WS, Cullen NC, Janelidze S. et al. A two-step workflow based on plasma p-tau217 to screen for amyloid beta positivity with further confirmatory testing only in uncertain cases. Nat Aging 2023;3:1079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun JF, Varlet-Marie E, Myzia J. et al. Metabolic influences modulating erythrocyte deformability and eryptosis. Metabolites 2021;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger P, Hilarius-Stokman P, De Korte D. et al. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood 2012;119:5512–5521. [DOI] [PubMed] [Google Scholar]
- Byon JC, Padilla SM, Papayannopoulou T.. Deletion of Dicer in late erythroid cells results in impaired stress erythropoiesis in mice. Exp Hematol 2014;42:852–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan SM, Lukacs V, Ranade SS. et al. Piezo1 links mechanical forces to red blood cell volume. Elife 2015;4:e07370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caielli S, Cardenas J, De Jesus AA. et al. Erythroid mitochondrial retention triggers myeloid-dependent type I interferon in human SLE. Cell 2021;184:4464–4479.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catala A, Youssef LA, Reisz JA. et al. Metabolic reprogramming of mouse bone marrow derived macrophages following erythrophagocytosis. Front Physiol 2020;11:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catala A, Stone M, Busch MP. et al. Reprogramming of red blood cell metabolism in Zika virus-infected donors. Transfusion 2022;62:1045–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulier A, Jankovsky N, Gautier EF. et al. Red blood cell proteomics reveal remnant protein biosynthesis and folding pathways in PIEZO1-related hereditary xerocytosis. Front Physiol 2022;13:960291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TL, Cubillos FF, Kakhniashvili DG. et al. Band 3 is a target protein of spectrin’s E2/E3 activity: implication for sickle cell disease and normal red blood cell aging. Cell Mol Biol (Noisy-le-grand) 2004;50:171–177. [PubMed] [Google Scholar]
- Chang TL, Kakhniashvili DG, Goodman SR.. Spectrin’s E2/E3 ubiquitin conjugating/ligating activity is diminished in sickle cells. Am J Hematol 2005;79:89–96. [DOI] [PubMed] [Google Scholar]
- Chen SY, Wang Y, Telen MJ. et al. The genomic analysis of erythrocyte microRNA expression in sickle cell diseases. PLoS One 2008;3:e2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PH, Hong J, Chi JT.. Discovery, genomic analysis, and functional role of the erythrocyte RNAs. Curr Pathobiol Rep 2017;5:43–48. [Google Scholar]
- Chen M, Leng Y, He C. et al. Red blood cells: a potential delivery system. J Nanobiotechnology 2023;21:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessa L, Leuzzi V, Plebani A. et al. Intra-erythrocyte infusion of dexamethasone reduces neurological symptoms in ataxia teleangiectasia patients: results of a phase 2 trial. Orphanet J Rare Dis 2014;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccoli L, De Felice C, Paccagnini E. et al. Erythrocyte shape abnormalities, membrane oxidative damage, and beta-actin alterations: an unrecognized triad in classical autism. Mediators Inflamm 2013;2013:432616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilek N, Ugurel E, Goksel E. et al. Signaling mechanisms in red blood cells: a view through the protein phosphorylation and deformability. J Cell Physiol 2023:1–17. [DOI] [PubMed] [Google Scholar]
- Claver JA, Quaglia AIE.. Comparative morphology, development, and function of blood cells in nonmammalian vertebrates. J Exot Pet Med 2009;18:87–97. [Google Scholar]
- Cortese-Krott MM, Kelm M.. Endothelial nitric oxide synthase in red blood cells: key to a new erythrocrine function? Redox Biol 2014;2:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrus C. The role of miRNAs as therapeutic tools in sickle cell disease. Medicina (Kaunas) 2021;57:1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’alessandro A, Hod EA.. Red blood cell storage: from genome to exposome towards personalized transfusion medicine. Transfus Med Rev 2023;37:150750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’alessandro A, Dzieciatkowska M, Nemkov T. et al. Red blood cell proteomics update: is there more to discover? Blood Transfus 2017a;15:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’alessandro A, Nemkov T, Yoshida T. et al. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion 2017b;57:325–336. [DOI] [PubMed] [Google Scholar]
- D’alessandro A, Fu X, Kanias T. et al. ; Recipient Epidemiology and Donor Evaluation Study-III (REDS III). Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity. Haematologica 2021;106:1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’alessandro A, Anastasiadi AT, Tzounakas VL. et al. Red blood cell metabolism in vivo and in vitro. Metabolites 2023a;13:793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’alessandro A, Lukens JR, Zimring JC.. The role of PIMT in Alzheimer’s disease pathogenesis: a novel hypothesis. Alzheimers Dement 2023b;19:5296–5302. [DOI] [PubMed] [Google Scholar]
- Dao M, Lim CT, Suresh S.. Mechanics of the human red blood cell deformed by optical tweezers. J Mech Phys Solids 2003;51:2259–2280. [Google Scholar]
- Darbonne WC, Rice GC, Mohler MA. et al. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J Clin Invest 1991;88:1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins R. The Blind Watchmaker. Essex/England: Harlow, Longman Scientific & Technical, 1986. [Google Scholar]
- De Almeida JP, Saldanha C.. Nonneuronal cholinergic system in human erythrocytes: biological role and clinical relevance. J Membr Biol 2010;234:227–234. [DOI] [PubMed] [Google Scholar]
- Domenech C, Thomas X, Chabaud S. et al. l-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: results of the GRASPALL 2005-01 randomized trial. Br J Haematol 2011;153:58–65. [DOI] [PubMed] [Google Scholar]
- Doss JF, Corcoran DL, Jima DD. et al. A comprehensive joint analysis of the long and short RNA transcriptomes of human erythrocytes. BMC Genomics 2015;16:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzandu JK, Johnson RM.. Membrane protein phosphorylation in intact normal and sickle cell erythrocytes. J Biol Chem 1980;255:6382–6386. [PubMed] [Google Scholar]
- Esperti S, Nader E, Stier A. et al. Increased retention of functional mitochondria in mature sickle red blood cells is associated with increased sickling tendency, hemolysis and oxidative stress. Haematologica 2023;108:3086–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faoro C, Ataide SF.. Noncanonical functions and cellular dynamics of the mammalian signal recognition particle components. Front Mol Biosci 2021;8:679584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvet B, Mbefo MK, Fares MB. et al. alpha-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem 2012;287:15345–15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenk S, Melnikova EV, Anashkina AA. et al. Hemoglobin is an oxygen-dependent glutathione buffer adapting the intracellular reduced glutathione levels to oxygen availability. Redox Biol 2022;58:102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuma N, Akimitsu N, Hamamoto H. et al. A role of the Duffy antigen for the maintenance of plasma chemokine concentrations. Biochem Biophys Res Commun 2003;303:137–139. [DOI] [PubMed] [Google Scholar]
- Gajecki D, Gawrys J, Szahidewicz-Krupska E. et al. Role of erythrocytes in nitric oxide metabolism and paracrine regulation of endothelial function. Antioxidants (Basel) 2022;11:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambhir KK, Agarwal VR.. Red blood cell insulin receptors in health and disease. Biochem Med Metab Biol 1991;45:133–153. [DOI] [PubMed] [Google Scholar]
- Gao X, Yue T, Tian F. et al. Erythrocyte membrane skeleton inhibits nanoparticle endocytosis. AIP Adv 2017;7:065303. [Google Scholar]
- Ghashghaeinia M, Toulany M, Saki M. et al. The NFkB pathway inhibitors Bay 11-7082 and parthenolide induce programmed cell death in anucleated Erythrocytes. Cell Physiol Biochem 2011;27:45–54. [DOI] [PubMed] [Google Scholar]
- Ghashghaeinia M, Toulany M, Saki M. et al. Potential roles of the NFkappaB and glutathione pathways in mature human erythrocytes. Cell Mol Biol Lett 2012;17:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaeinia M, Wesseling MC, Ramos E. et al. Trifluoperazine-induced suicidal erythrocyte death and S-nitrosylation inhibition, reversed by the nitric oxide donor sodium nitroprusside. Cell Physiol Biochem 2017;42:1985–1998. [DOI] [PubMed] [Google Scholar]
- Giacometti G, Ferreri C, Sansone A. et al. High predictive values of RBC membrane-based diagnostics by biophotonics in an integrated approach for Autism Spectrum Disorders. Sci Rep 2017;7:9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin MT, Schechter AN, Kim-Shapiro DB. et al. The emerging biology of the nitrite anion. Nat Chem Biol 2005;1:308–314. [DOI] [PubMed] [Google Scholar]
- Goodman SR, Petrofes Chapa R, Zimmer WE.. Spectrin’s chimeric E2/E3 enzymatic activity. Exp Biol Med (Maywood) 2015;240:1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goskel E, Ugurel E, Nader E. et al. A preliminary study of Phosphodiesterases and Adenylyl Cyclase Signaling Pathway on red blood cell deformability of sickle cell patients. Front Physiol 2023;14:1215835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzer W. Cell biology. More red than dead. Nature 1984;310:368–369. [DOI] [PubMed] [Google Scholar]
- Gratzer WB. From helix to haemolysis. Nat Struct Biol 1994;1:78–79. [DOI] [PubMed] [Google Scholar]
- Greenwalt TJ. The Ernest Witebsky memorial lecture. Red but not dead: not a hapless sac of hemoglobin. Immunol Invest 1995;24:3–21. [DOI] [PubMed] [Google Scholar]
- Groen K, Maltby VE, Sanders KA. et al. Erythrocytes in multiple sclerosis - forgotten contributors to the pathophysiology? Mult Scler J Exp Transl Clin 2016;2:2055217316649981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Nardone C, Kamitaki N. et al. The midnolin-proteasome pathway catches proteins for ubiquitination-independent degradation. Science 2023;381:eadh5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizouarn H, Barshtein G.. Editorial: red blood cell vascular adhesion and deformability. Front Physiol 2020;11:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev 2013;27:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel P, Fabienne P, Mineur L. et al. Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: an open-label, randomized Phase IIb trial. Eur J Cancer 2020;124:91–101. [DOI] [PubMed] [Google Scholar]
- Helms C, Kim-Shapiro DB.. Hemoglobin-mediated nitric oxide signaling. Free Radic Biol Med 2013;61:464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henslee EA, Crosby P, Kitcatt SJ. et al. Rhythmic potassium transport regulates the circadian clock in human red blood cells. Nat Commun 2017;8:1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Flormann D, Birnbaumer L. et al. Evidence of in vivo exogen protein uptake by red blood cells: a putative therapeutic concept. Blood Adv 2023;7:1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann MG, Kieffer C, Bjorkman PJ.. In vitro characterization of engineered red blood cells as viral traps against HIV-1 and SARS-CoV-2. Mol Ther Methods Clin Dev 2021;21:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horuk R, Wang ZX, Peiper SC. et al. Identification and characterization of a promiscuous chemokine-binding protein in a human erythroleukemic cell line. J Biol Chem 1994;269:17730–17733. [PubMed] [Google Scholar]
- Hotz MJ, Qing D, Shashaty MGS. et al. Red blood cells homeostatically bind mitochondrial DNA through TLR9 to maintain quiescence and to prevent lung injury. Am J Respir Crit Care Med 2018;197:470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Hearne L, Irby CE. et al. Kinetics of increased deformability of deoxygenated sickle cells upon oxygenation. Biophys J 2003;85:2374–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Shiva S, Kim-Shapiro DB. et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest 2005;115:2099–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisjes R, Bogdanova A, Van Solinge WW. et al. Squeezing for life - Properties of red blood cell deformability. Front Physiol 2018;9:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imberty A, Varrot A.. Microbial recognition of human cell surface glycoconjugates. Curr Opin Struct Biol 2008;18:567–576. [DOI] [PubMed] [Google Scholar]
- Issaian A, Hay A, Dzieciatkowska M. et al. The interactome of the N-terminus of band 3 regulates red blood cell metabolism and storage quality. Haematologica 2021;106:2971–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeeswaran R, Vazquez BA, Thiruppathi M. et al. Pharmacological inhibition of LSD1 and mTOR reduces mitochondrial retention and associated ROS levels in the red blood cells of sickle cell disease. Exp Hematol 2017;50:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain V, Yang WH, Wu J. et al. Single cell RNA-Seq analysis of human red cells. Front Physiol 2022;13:828700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskiewicz E, Jodlowska M, Kaczmarek R. et al. Erythrocyte glycophorins as receptors for Plasmodium merozoites. Parasit Vectors 2019;12:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Bonaventura C, Bonaventura J. et al. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 1996;380:221–226. [DOI] [PubMed] [Google Scholar]
- Joly E, Hudrisier D.. What is trogocytosis and what is its purpose? Nat Immunol 2003;4:815. [DOI] [PubMed] [Google Scholar]
- Kalani Roy M, La Carpia F, Cendali F. et al. Irradiation causes alterations of polyamine, purine, and sulfur metabolism in red blood cells and multiple organs. J Proteome Res 2022;21:519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama A, Inoue J, Sugita-Konishi Y. et al. Oxidation of Ikappa Balpha at methionine 45 is one cause of taurine chloramine-induced inhibition of NF-kappa B activation. J Biol Chem 2002;277:24049–24056. [DOI] [PubMed] [Google Scholar]
- Kar D, Sellamuthu K, Kumar SD. et al. Induction of translational readthrough across the thalassemia-causing premature stop codon in beta-globin-encoding mRNA. Biochemistry 2020;59:80–84. [DOI] [PubMed] [Google Scholar]
- Karsten E, Breen E, Herbert BR.. Red blood cells are dynamic reservoirs of cytokines. Sci Rep 2018a;8:3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten E, Hill CJ, Herbert BR.. Red blood cells: the primary reservoir of macrophage migration inhibitory factor in whole blood. Cytokine 2018b;102:34–40. [DOI] [PubMed] [Google Scholar]
- Kaul DK, Koshkaryev A, Artmann G. et al. Additive effect of red blood cell rigidity and adherence to endothelial cells in inducing vascular resistance. Am J Physiol Heart Circ Physiol 2008;295:H1788–H1793. [DOI] [PubMed] [Google Scholar]
- Kay MM, Goodman J, Goodman S. et al. Membrane protein band 3 alteration associated with neurologic disease and tissue-reactive antibodies. Exp Clin Immunogenet 1990;7:181–199. [PubMed] [Google Scholar]
- Kerkela E, Lahtela J, Larjo A. et al. Exploring transcriptomic landscapes in red blood cells, in their extracellular vesicles and on a single-cell level. Int J Mol Sci 2022;23:12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King ML. I have a dream; speech at the March on Washington, n.p, 1963. [Google Scholar]
- Koleva L, Bovt E, Ataullakhanov F. et al. Erythrocytes as carriers: from drug delivery to biosensors. Pharmaceutics 2020;12:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SD, Kar D, Akhtar MN. et al. Evidence for low-level translation in human erythrocytes. Mol Biol Cell 2022;33:br21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam LKM, Murphy S, Kokkinaki D. et al. DNA binding to TLR9 expressed by red blood cells promotes innate immune activation and anemia. Sci Transl Med 2021;13:eabj1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo F, Hutzler B, Ruddiman CA. et al. Cellular microdomains for nitric oxide signaling in endothelium and red blood cells. Nitric Oxide 2020;96:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wu Z, Wang Y. et al. Characterization of adult alpha- and beta-globin elevated by hydrogen peroxide in cervical cancer cells that play a cytoprotective role against oxidative insults. PLoS One 2013;8:e54342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang N, Jiao Z, Zhang C. et al. Mature red blood cells contain long DNA fragments and could acquire DNA from lung cancer tissue. Adv Sci (Weinh) 2023;10:e2206361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Plebani M.. Red blood cell distribution width (RDW) and human pathology. One size fits all. Clin Chem Lab Med 2014;52:1247–1249. [DOI] [PubMed] [Google Scholar]
- Liu J, Niu N, Li X. et al. The life cycle of polyploid giant cancer cells and dormancy in cancer: opportunities for novel therapeutic interventions. Semin Cancer Biol 2022;81:132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd MR, Okamoto Y, Randall MS. et al. Role of AP1/NFE2 binding sites in endogenous alpha-globin gene transcription. Blood 2003;102:4223–4228. [DOI] [PubMed] [Google Scholar]
- Madeddu C, Neri M, Sanna E. et al. Experimental drugs for chemotherapy- and cancer-related anemia. J Exp Pharmacol 2021;13:593–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel PY, Hoang AN, Goldowitz I. et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 2013;13:521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maor G, Dubreuil RR, Feany MB.. alpha-Synuclein promotes neuronal dysfunction and death by disrupting the binding of Ankyrin to beta-Spectrin. J Neurosci 2023;43:1614–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengo-Rowe AJ. Structure-function relations of human hemoglobins. Proc (Bayl Univ Med Cent) 2006;19:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y, Liu Y, Ji P.. Understanding terminal erythropoiesis: an update on chromatin condensation, enucleation, and reticulocyte maturation. Blood Rev 2021;46:100740. [DOI] [PubMed] [Google Scholar]
- Minetti G, Egee S, Morsdorf D. et al. Red cell investigations: art and artefacts. Blood Rev 2013;27:91–101. [DOI] [PubMed] [Google Scholar]
- Moriconi C, Dzieciatkowska M, Roy M. et al. Retention of functional mitochondria in mature red blood cells from patients with sickle cell disease. Br J Haematol 2022;198:574–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura PL, Hawley BR, Mankelow TJ. et al. Non-muscle myosin II drives vesicle loss during human reticulocyte maturation. Haematologica 2018;103:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulatie Z, Aynalem M, Getawa S.. MicroRNAs as quality assessment tool in stored packed red blood cell in blood banks. J Blood Med 2023;14:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemkov T, Reisz JA, Xia Y. et al. Red blood cells as an organ? How deep omics characterization of the most abundant cell in the human body highlights other systemic metabolic functions beyond oxygen transport. Expert Rev Proteomics 2018a;15:855–864. [DOI] [PubMed] [Google Scholar]
- Nemkov T, Sun K, Reisz JA. et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica 2018b;103:361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemkov T, Stefanoni D, Bordbar A. et al. ; Recipient Epidemiology and Donor Evaluation Study III Red Blood Cell–Omics (REDS-III RBC-Omics) Study. Blood donor exposome and impact of common drugs on red blood cell metabolism. JCI Insight 2021;6:e146175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemkov T, Stephenson D, Erickson C. et al. Regulation of kynurenine metabolism by blood donor genetics and biology impacts red cell hemolysis in vitro and in vivo. Blood 2023;143:456–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neote K, Mak JY, Kolakowski LF Jr. et al. Functional and biochemical analysis of the cloned Duffy antigen: identity with the red blood cell chemokine receptor. Blood 1994;84:44–52. [PubMed] [Google Scholar]
- Newton AC, Cook SL, Huestis WH.. Transfer of band 3, the erythrocyte anion transporter, between phospholipid vesicles and cells. Biochemistry 1983;22:6110–6117. [DOI] [PubMed] [Google Scholar]
- Nguyen DB, Wagner-Britz L, Maia S. et al. Regulation of phosphatidylserine exposure in red blood cells. Cell Physiol Biochem 2011;28:847–856. [DOI] [PubMed] [Google Scholar]
- Nirmalraj PN, Schneider T, Felbecker A.. Spatial organization of protein aggregates on red blood cells as physical biomarkers of Alzheimer’s disease pathology. Sci Adv 2021;7:eabj2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T, Hosako M, Hiramatsu K. et al. Oxidative modification of IkappaB by monochloramine inhibits tumor necrosis factor alpha-induced NF-kappaB activation. Biochim Biophys Acta 2005;1746:135–142. [DOI] [PubMed] [Google Scholar]
- Olumuyiwa-Akeredolu OO, Soma P, Buys AV. et al. Characterizing pathology in erythrocytes using morphological and biophysical membrane properties: relation to impaired hemorheology and cardiovascular function in rheumatoid arthritis. Biochim Biophys Acta Biomembr 2017;1859:2381–2391. [DOI] [PubMed] [Google Scholar]
- Osakada T, Abe T, Itakura T. et al. Hemoglobin in the blood acts as a chemosensory signal via the mouse vomeronasal system. Nat Commun 2022;13:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paone S, D’alessandro S, Parapini S. et al. Characterization of the erythrocyte GTPase Rac1 in relation to Plasmodium falciparum invasion. Sci Rep 2020;10:22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos C. Immunosuppressive function of intratumor red blood cells: an immune-metabolic perspective. Curr Cancer Ther Rev 2022;18:224–226. [Google Scholar]
- Papadopoulos C, Panopoulou M, Anagnostopoulos K. et al. Immune and metabolic interactions of human erythrocytes: a molecular perspective. Endocr Metab Immune Disord Drug Targets 2021a;21:843–853. [DOI] [PubMed] [Google Scholar]
- Papadopoulos C, Tentes I, Anagnostopoulos K.. Molecular interactions between erythrocytes and the endocrine system. Maedica (Bucur) 2021b;16:489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar A, Jacob VD, Gideon DA. et al. Hemoglobin catalyzes ATP-synthesis in human erythrocytes: a murburn model. J Biomol Struct Dyn 2022;40:8783–8795. [DOI] [PubMed] [Google Scholar]
- Patgaonkar M, Aranha C, Bhonde G. et al. Identification and characterization of anti-microbial peptides from rabbit vaginal fluid. Vet Immunol Immunopathol 2011;139:176–186. [DOI] [PubMed] [Google Scholar]
- Paulus JK, Van Der Hoorn RL.. Tricked or trapped-Two decoy mechanisms in host-pathogen interactions. PLoS Pathog 2018;14:e1006761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixeiro I, Silva AL, Romao L.. Control of human beta-globin mRNA stability and its impact on beta-thalassemia phenotype. Haematologica 2011;96:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TT, Vy HT, Ho TT. et al. Emergence role of nucleated red blood cells in molecular response evaluation for chronic myeloid leukemia. Int J Gen Med 2019;12:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierige F, Bigini N, Rossi L. et al. Reengineering red blood cells for cellular therapeutics and diagnostics. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2017;9:e1454. [DOI] [PubMed] [Google Scholar]
- Pikora K, Kretowska-Grunwald A, Krawczuk-Rybak M. et al. Diagnostic value and prognostic significance of Nucleated Red Blood Cells (NRBCs) in selected medical conditions. Cells 2023;12:1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang Y, Liu J, Dao M. et al. Mechanical fatigue of human red blood cells. Proc Natl Acad Sci USA 2019;116:19828–19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang Y, Liu J, Dao M. et al. In vitro assay for single-cell characterization of impaired deformability in red blood cells under recurrent episodes of hypoxia. Lab Chip 2021;21:3458–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rab ME, Van Oirschot BA, Kosinski PA. et al. AG-348 (Mitapivat), an allosteric activator of red blood cell pyruvate kinase, increases enzymatic activity, protein stability, and ATP levels over a broad range of PKLR genotypes. Haematologica 2021;106:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recktenwald SM, Simionato G, Lopes MGM. et al. Cross-talk between red blood cells and plasma influences blood flow and omics phenotypes in severe COVID-19. Elife 2022;11:e81316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev-Rudzki N, Wilson DW, Carvalho TG. et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 2013;153:1120–1133. [DOI] [PubMed] [Google Scholar]
- Reithmeier RA, Casey JR, Kalli AC. et al. Band 3, the human red cell chloride/bicarbonate anion exchanger (AE1, SLC4A1), in a structural context. Biochim Biophys Acta 2016;1858:1507–1532. [DOI] [PubMed] [Google Scholar]
- Reynaert NL, Ckless K, Korn SH. et al. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci USA 2004;101:8945–8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaert NL, Van Der Vliet A, Guala AS. et al. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci USA 2006;103:13086–13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert M, Laperrousaz B, Piedrahita D. et al. Multiparametric characterization of red blood cell physiology after hypotonic dialysis based drug encapsulation process. Acta Pharm Sin B 2022;12:2089–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Antoku Y, Iwashita H. et al. Chorea-acanthocytosis: abnormal composition of covalently bound fatty acids of erythrocyte membrane proteins. Ann Neurol 1991;29:664–669. [DOI] [PubMed] [Google Scholar]
- Sangokoya C, Telen MJ, Chi JT.. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood 2010;116:4338–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarchione A, Marchand A, Taymans JM. et al. Alpha-synuclein and lipids: the Elephant in the room? Cells 2021;10:2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satchwell TJ, Toye AM.. Band 3, an essential red blood cell hub of activity. Haematologica 2021;106:2792–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbardella D, Tundo GR, Campagnolo L. et al. Author correction: retention of mitochondria in mature human red blood cells as the result of autophagy impairment in Rett Syndrome. Sci Rep 2021;11:12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR.. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 2001;30:1191–1212. [DOI] [PubMed] [Google Scholar]
- Seidel P, Roth M, Ge Q. et al. IkappaBalpha glutathionylation and reduced histone H3 phosphorylation inhibit eotaxin and RANTES. Eur Respir J 2011;38:1444–1452. [DOI] [PubMed] [Google Scholar]
- Smith AS, Nowak RB, Zhou S. et al. Myosin IIA interacts with the spectrin-actin membrane skeleton to control red blood cell membrane curvature and deformability. Proc Natl Acad Sci USA 2018;115:E4377–E4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A, Wen AQ, Wen YE. et al. p97 dysfunction underlies a loss of quality control of damaged membrane proteins and promotes oxidative stress and sickling in sickle cell disease. FASEB J 2022;36:e22246. [DOI] [PubMed] [Google Scholar]
- Sosale NG, Rouhiparkouhi T, Bradshaw AM. et al. Cell rigidity and shape override CD47’s “self”-signaling in phagocytosis by hyperactivating myosin-II. Blood 2015;125:542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachon A, Segbers E, Holland-Letz T. et al. Nucleated red blood cells in the blood of medical intensive care patients indicate increased mortality risk: a prospective cohort study. Crit Care 2007;11:R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Yu Y, Niu B. et al. Red blood cells as potential repositories of MicroRNAs in the circulatory system. Front Genet 2020;11:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouarne GJS, Gall JG.. 7SL RNA in vertebrate red blood cells. RNA 2018;24:908–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Stefanoni D, Dzieciatkowska M. et al. Evidence of structural protein damage and membrane lipid remodeling in red blood cells from COVID-19 patients. J Proteome Res 2020;19:4455–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas TA, Qiu A, Kim CY. et al. Reticulocytes in donor blood units enhance red blood cell alloimmunization. Haematologica 2023;108:2639–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Liu G, Gao L. et al. Erythrocytic alpha-Synuclein as a potential biomarker for Parkinson’s disease. Transl Neurodegener 2019;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppet M, Fall AB, Ferster A. et al. Antisickling activity of sodium cromoglicate in sickle-cell disease. Lancet 2000;356:309. [DOI] [PubMed] [Google Scholar]
- Tumburu L, Ghosh-Choudhary S, Seifuddin FT. et al. Circulating mitochondrial DNA is a proinflammatory DAMP in sickle cell disease. Blood 2021;137:3116–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounakas VL, Karadimas DG, Papassideri IS. et al. Erythrocyte-based drug delivery in Transfusion Medicine: wandering questions seeking answers. Transfus Apher Sci 2017;56:626–634. [DOI] [PubMed] [Google Scholar]
- Tzounakas VL, Anastasiadi AT, Dzieciatkowska M. et al. Proteome of stored RBC membrane and vesicles from heterozygous beta thalassemia donors. Int J Mol Sci 2021;22:3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounakas VL, Anastasiadi AT, Stefanoni D. et al. Beta thalassemia minor is a beneficial determinant of red blood cell storage lesion. Haematologica 2022a;107:112–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounakas VL, Dzieciatkowska M, Anastasiadi AT. et al. Red cell proteasome modulation by storage, redox metabolism and transfusion. Blood Transfus 2022b;20:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurel E, Goksel E, Cilek N. et al. Proteomic analysis of the role of the Adenylyl Cyclase-cAMP Pathway in red blood cell mechanical responses. Cells 2022;11:1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulker P, Sati L, Celik-Ozenci C. et al. Mechanical stimulation of nitric oxide synthesizing mechanisms in erythrocytes. Biorheology 2009;46:121–132. [DOI] [PubMed] [Google Scholar]
- Van ‘T Erve TJ, Wagner BA, Ryckman KK. et al. The concentration of glutathione in human erythrocytes is a heritable trait. Free Radic Biol Med 2013;65:742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wijk R, Van Solinge WW.. The energy-less red blood cell is lost: erythrocyte enzyme abnormalities of glycolysis. Blood 2005;106:4034–4042. [DOI] [PubMed] [Google Scholar]
- Villa CH, Anselmo AC, Mitragotri S. et al. Red blood cells: supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv Drug Deliv Rev 2016;106:88–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lindern M, Egee S, Bianchi P. et al. The function of ion channels and membrane potential in red blood cells: toward a systematic analysis of the erythroid channelome. Front Physiol 2022;13:824478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SA, Liebhaber SA.. Regulation of alpha-globin mRNA stability. Exp Biol Med (Maywood) 2003;228:387–395. [DOI] [PubMed] [Google Scholar]
- Wang M. RBC mitochondria trigger interferon release. Nat Rev Nephrol 2021;17:707. [DOI] [PubMed] [Google Scholar]
- Wang C, Ye Y, Sun W. et al. Red blood cells for glucose-responsive insulin delivery. Adv Mater 2017;29:1606617. [DOI] [PubMed] [Google Scholar]
- Webb KL, Dominelli PB, Baker SE. et al. Influence of high hemoglobin-oxygen affinity on humans during hypoxia. Front Physiol 2021;12:763933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AJ, Kiehart DP, Pollard TD.. Myosin from human erythrocytes. J Biol Chem 1985;260:46–49. [PubMed] [Google Scholar]
- Wu YW, Goubran H, Seghatchian J. et al. Smart blood cell and microvesicle-based Trojan horse drug delivery: merging expertise in blood transfusion and biomedical engineering in the field of nanomedicine. Transfus Apher Sci 2016;54:309–318. [DOI] [PubMed] [Google Scholar]
- Wu Y, Leyk S, Torabi H. et al. Plasmodium falciparum infection reshapes the human microRNA profiles of red blood cells and their extracellular vesicles. iScience 2023;26:107119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Shameli A, Harding CV. et al. Late stages of hematopoiesis and B cell lymphopoiesis are regulated by alpha-synuclein, a key player in Parkinson’s disease. Immunobiology 2014;219:836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Chen C, Zhang Y. et al. Erythrocyte transglutaminase-2 combats hypoxia and chronic kidney disease by promoting oxygen delivery and carnitine homeostasis. Cell Metab 2022;34:299–316.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Matsuhira T, Yamamoto K. et al. Antioxidative Pseudoenzymatic Mechanism of NAD(P)H coexisting with oxyhemoglobin for suppressed methemoglobin formation. Biochemistry 2019;58:1400–1410. [DOI] [PubMed] [Google Scholar]
- Yang W, Li X, Li X. et al. Hemoglobin-alpha-synuclein complex exhibited age-dependent alterations in the human striatum and peripheral RBCs. Neurosci Lett 2020;736:135274. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Cheng J, Xu F. et al. Red blood cell extrudes nucleus and mitochondria against oxidative stress. IUBMB Life 2011;63:560–565. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang S, Lin J.. Clinical and molecular research of neuroacanthocytosis. Neural Regen Res 2013a;8:833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Mercado-Uribe I, Liu J.. Generation of erythroid cells from fibroblasts and cancer cells in vitro and in vivo. Cancer Lett 2013b;333:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wan GZ, Wang YY. et al. The role of erythrocytes and erythroid progenitor cells in tumors. Open Life Sci 2022;17:1641–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sun X, Zhang G. et al. Interaction of mesoporous silica nanoparticles with human red blood cell membranes: size and surface effects. ACS Nano 2011;5:1366–1375. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ukidve A, Gao Y. et al. Erythrocyte leveraged chemotherapy (ELeCt): nanoparticle assembly on erythrocyte surface to combat lung metastasis. Sci Adv 2019;5:eaax9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Ukidve A, Krishnan V. et al. Systemic tumour suppression via the preferential accumulation of erythrocyte-anchored chemokine-encapsulating nanoparticles in lung metastases. Nat Biomed Eng 2021;5:441–454. [DOI] [PubMed] [Google Scholar]