Abstract
The composition of the gut microbiome has been shown to influence disease outcome in patients with colorectal cancer (CRC). In a recent Nature Biotechnology article, Wang et al. demonstrate that killing CRC-associated bacteria with a liposomal antibiotic elicits CRC-targeting immune responses of therapeutic relevance as a consequence of epitope mimicry.
Keywords: antigenicity, cancer immunosurveillance, Fusobacterium nucleatum, hypoxia, immune checkpoint inhibitors, liposomes
It is now accepted that cancer is not simply caused by genetic or epigenetic alterations of healthy cells that ultimately acquire a growth advantage and expand beyond control locally and systemically. Rather, cancer results from a myriad of additional factors, including changes in the interaction of (pre)malignant cells with the immune system.1 Although adaptive immunity mainly emerged along with the pathogen-host co-evolution as a system that would fight infections while providing the host with a specific memory to control subsequent challenges by the same microorganisms, innate immunity operates in a less specific manner, largely reacting to general alterations of cell homeostasis that may be associated with the early phases of infection as well as oncogenesis (e.g., endoplasmic reticulum stress).2 Moreover, while it is believed that the immune system cannot react against strictly self-antigens (i.e., non-mutated, human) due to thymic counterselection of potentially autoreactive T cell clones,3 it is now clear that central tolerance is a very leaky process. This leakiness explains why (1) full-blown T cell activation coupled with the acquisition of effector and memory functions requires three distinct signals (i.e., TCR engagement, co-stimulation, and cytokine help),4 and (2) why a number of mechanisms are in place to ensure peripheral tolerance on healthy tissues, such as the existence of antigen-specific immunosuppressive CD4+CD25+FOXP3+ regulatory T (TREG) cells.5 That said, a number of non-mutational events can cause the loss of peripheral tolerance and hence elicit autoreactive conditions. For example, stress-driven changes in the homeostasis of antigen-presenting cells (APCs) can drive qualitative and quantitative alterations in antigen presentation and hence the activation of previously suppressed T-cell clones, in some cases involving a similarity between microbe-derived and self-antigens (i.e., epitope mimicry).6
An expanding literature demonstrates that the abundance and composition of the intestinal microbiome (i.e., the viral, bacterial and eukaryotic species colonizing the human intestine) have major impacts on disease outcome in patients with colorectal carcinoma (CRC). This is due not only to the ability of intestinal microbes to metabolize nutrients and drugs that directly influence CRC biology, but also their impact on the immunological fitness of the host.7 The interconnection between microbes and the immune system in context of CRC were further explored in recent work from Wang and colleagues. In a recent Nature Biotechnology article, these authors demonstrated that killing anaerobic bacteria including Fusobacterium nucleatum (which has previously been associated with poor disease outcome in patients with CRC)8 with a liposomal antibiotic initiates therapeutically relevant CRC-targeting immune responses that involve tumor-specific peptides mimicking bacterial epitopes.9
Wang and colleagues set to interrogate a nationwide pharmaco-epidemiologic database of patients with CRC for the impact of antibiotics targeting anaerobic bacteria, namely nitroimidazole and lincomycin, on disease-free survival (DFS). Interestingly, nitroimidazole or lincomycin usage before (but not after) CRC resection was associated with a significant improvement in DFS, an effect that could not be documented in patients with breast cancer and hence was attributed to alterations in the CRC-microbiota interaction.9
Inspired by the existing link between F. nucleatum and poor disease outcome in patients with CRC,8 Wang and collaborators harnessed mouse CRC CT26 cells expressing luciferase and RFP to investigate the impact of hypoxia on the interaction between CRC cells and F. nucleatum. In line with the clinical observation that F. nucleatum preferentially associates with large and hypoxic tumors,10 reducing oxygen levels to 1% increased CRC cell invasion by F. nucleatum 15-fold, a finding that could be recapitulated not only in 3D CRC spheroids, but also with the facultative anaerobe Escherichia coli Nissle. Confirming a detrimental role for F. nucleatum infection on CRC progression, CT26 tumors established in immunocompetent syngeneic mice infected with F. nucleatum exhibited a 30-fold higher tumor growth ratio compared to their control, uninfected counterparts. This was accompanied by a repolarization of the tumor microenvironment (TME) toward an immunosuppressive state enriched in M2-like macrophages, as well as accrued metastatic dissemination.9
To circumvent the global effects of non-targeted antibiotic delivery and achieve a specific release at hypoxic tumor areas, Wang and co-workers developed nitroimidazole-bearing silver/tinidazole-based liposomes. Such an approach successfully reduced the abundance of anaerobic bacteria both at primary and metastatic CRC sites, with no major effects on the global composition of the gut microbiome. Moreover, the targeted delivery of nitroimidazole to hypoxic CRC areas significantly inhibited primary tumor growth and metastatic dissemination of CT26 tumors established in immunocompetent syngeneic mice. This anticancer effect that was accompanied by a reconfiguration of the TME toward an immunostimulatory state dominated by cytotoxic CD8+ T cells and prolonged mouse survival.9
Interestingly, Wang and collaborators observed that mice efficiently eradicating CT26 lesions upon treatment with nitroimidazole-bearing silver/tinidazole-based liposomes were efficiently protected from a rechallenge with CT26 cells, irrespective of whether the latter were infected with F. nucleatum. Such an effect depended on both CD8+ and CD4+ T cells, as demonstrated with antibody-mediated depletion experiments. In line with this notion, T cells from long-term survivors not only displayed specificity for both infected and uninfected CT26 cells, but were also able to control the growth of CT26 tumors in vivo upon adoptive transfer. Furthermore, this effect was equally manifest against infected and uninfected CT26 lesions.9 These data suggested that killing CRC-associated F. nucleatum with nitroimidazole unleashed an immune response against CRC-specific antigens.
Thorough extensive proteomic analyses and epitope prediction by genome-wide alignment, Wang and colleagues identified F. nucleatum epitopes with sequence homology to CT26 antigens, notably three bacterial epitopes encoded in DnaK that were predicted to be presented on mouse MHC Class I molecules. Confirming in vivo reactivity, splenocytes from mice efficiently eradicating CT26 lesions upon treatment with nitroimidazole-bearing silver/tinidazole-based liposomes reacted ex vivo against these bacterial peptides, as well as against F. nucleatum neoepitopes not sharing homology with mouse peptides, a response that could not be detected in splenocytes from untreated CT26-bearing mice.9 Thus, targeting intracellular F. nucleatum with antibiotics is sufficient for breaking immunological tolerance to non-mutated CRC epitopes as a consequence of epitope mimicry.
In summary, Wang and colleagues invoked the possibility to use targeted antibiotics to kill CRC-associated bacteria and hence unleash CRC-specific immune responses of therapeutic relevance linked emerging from epitope mimicry (Figure 1). Whether this approach can be safely implemented in patients with CRC remains to be formally demonstrated. It is indeed plausible that anaerobic Gram-negative species other than F. nucleatum may be beneficial for patients with CRC, especially in the context of standard therapeutic regimens that are often linked with severe gastrointestinal side effects, calling for the development of highly targeted antibiotic approaches. Along similar lines, the systemic delivery of relatively unselective antibiotics targeting Gram-negative bacterial commensals may be associated with side effects that need to be carefully investigated, especially in mucosal tissues such as the genitourinary trait. Despite these and other unknowns, Wang and collaborators highlighted a novel strategy to target non-eukaryotic components of the human ecosystem to treat CRC and potentially other tumors.
Figure 1. Tumor-targeting immune responses elicited by antigen mimicry.
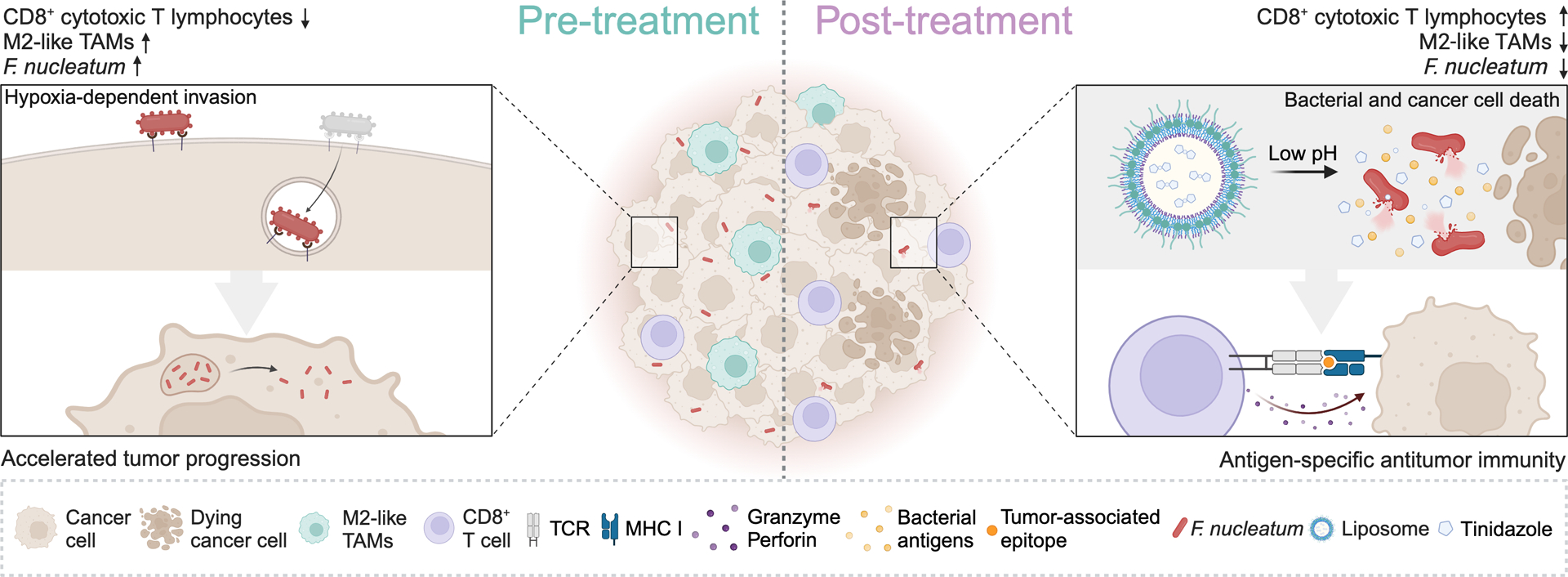
Central tolerance as generated by positive and negative thymocyte selection results in the deletion of useless or strongly autoreactive T cell clones. However, a large number of potentially autoreactive T cells survive thymic selection, but are generally unable to react against self entities thanks to peripheral tolerance mechanisms. In specific settings, including exposure to high amounts of microbial antigens, for instance after eradication of colorectal cancer (CRC)-associated Fusabacterium nucleatum infection by liposome-targeted antibiotic therapy, peripheral tolerance against CRC epitopes that structurally resemble microbial antigens can be lost. At least in some scenarios, this strategy may be employed to elicit tumor-targeting immune responses driven by antigen mimicry. TAM, tumor-associate macrophage; TCR, T-cell receptor. Created with BioRender.com
Acknowledgements.
ZT has been supported by the European Research Council (#786295). LG is/has been supported (as a PI unless otherwise indicated) by one R01 grant from the NIH/NCI (#CA271915), by two Breakthrough Level 2 grants from the US DoD BCRP (#BC180476P1; #BC210945), by a grant from the STARR Cancer Consortium (#I16-0064), by a Transformative Breast Cancer Consortium Grant from the US DoD BCRP (#W81XWH2120034, PI: Formenti), by a U54 grant from NIH/NCI (#CA274291, PI: Deasy, Formenti, Weichselbaum), by the 2019 Laura Ziskin Prize in Translational Research (#ZP-6177, PI: Formenti) from the Stand Up to Cancer (SU2C), by a Mantle Cell Lymphoma Research Initiative (MCL-RI, PI: Chen-Kiang) grant from the Leukemia and Lymphoma Society (LLS), by a Rapid Response Grant from the Functional Genomics Initiative (New York, US), by a pre-SPORE grant (PI: Demaria, Formenti) and a Clinical Trials Innovation Grant from the Sandra and Edward Meyer Cancer Center (New York, US), by startup funds from the Dept. of Radiation Oncology at Weill Cornell Medicine (New York, US), by industrial collaborations with Lytix Biopharma (Oslo, Norway), Promontory (New York, US) and Onxeo (Paris, France), as well as by donations from Promontory (New York, US), the Luke Heller TECPR2 Foundation (Boston, US), Sotio a.s. (Prague, Czech Republic), Lytix Biopharma (Oslo, Norway), Onxeo (Paris, France), Ricerchiamo (Brescia, Italy), and Noxopharm (Chatswood, Australia).
Footnotes
Competing Interests. LG is/has been holding research contracts with Lytix Biopharma, Promontory and Onxeo, has received consulting/advisory honoraria from Boehringer Ingelheim, AstraZeneca, OmniSEQ, Onxeo, The Longevity Labs, Inzen, Imvax, Sotio, Promontory, Noxopharm, EduCom, and the Luke Heller TECPR2 Foundation, and holds Promontory stock options. All other authors have no conflicts to declare.
References
- 1.Kroemer G, Chan TA, Eggermont AMM, and Galluzzi L (2023). Immunosurveillance in clinical cancer management. CA Cancer J Clin, In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroemer G, Galassi C, Zitvogel L, and Galluzzi L (2022). Immunogenic cell stress and death. Nat Immunol 23, 487–500. 10.1038/s41590-022-01132-2. [DOI] [PubMed] [Google Scholar]
- 3.Klein L, Kyewski B, Allen PM, and Hogquist KA (2014). Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat Rev Immunol 14, 377–391. 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, and Flies DB (2013). Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 13, 227–242. 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ElTanbouly MA, and Noelle RJ (2021). Rethinking peripheral T cell tolerance: checkpoints across a T cell’s journey. Nat Rev Immunol 21, 257–267. 10.1038/s41577-020-00454-2. [DOI] [PubMed] [Google Scholar]
- 6.Stern LJ, Clement C, Galluzzi L, and Santambrogio L (2023). Non-mutational antigens in disease. Nat Immunol, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Tekle G, and Garrett WS (2023). Bacteria in cancer initiation, promotion and progression. Nat Rev Cancer 23, 600–618. 10.1038/s41568-023-00594-2. [DOI] [PubMed] [Google Scholar]
- 8.White MT, and Sears CL (2023). The microbial landscape of colorectal cancer. Nat Rev Microbiol. 10.1038/s41579-023-00973-4. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Rousseau B, Qiu K, Huang G, Zhang Y, Su H, Le Bihan-Benjamin C, Khati I, Artz O, Foote MB, et al. (2023). Killing tumor-associated bacteria with a liposomal antibiotic generates neoantigens that induce anti-tumor immune responses. Nat Biotechnol. 10.1038/s41587-023-01957-8. [DOI] [PubMed] [Google Scholar]
- 10.Brennan CA, and Garrett WS (2019). Fusobacterium nucleatum - symbiont, opportunist and oncobacterium. Nat Rev Microbiol 17, 156–166. 10.1038/s41579-018-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


