Abstract
Leptospirosis, a zoonosis, is characterized by a wide range of clinical and laboratory findings, varying from self-limiting infection to potentially fatal disease. Leptospirosis-related clinical manifestations, except for Weil’s disease, may often be overlooked due to their nonspecificity in children. Additionally, many health-care professionals lack awareness of leptospirosis. This paper presents a case of leptospirosis that was initially misdiagnosed as staphylococcal toxic shock syndrome. The literature on this topic is also reviewed.
Keywords: Children, leptospirosis, staphylococcal toxic shock syndrome
Introduction
Leptospirosis is a worldwide zoonosis caused by pathogenic spirochetes of the genus Leptospira. It is characterized by a wide range of clinical and laboratory manifestations varying from self-limiting infection to potentially fatal disease. The classical form of Weil’s disease is characterized by jaundice, renal involvement, and hemorrhage. However, some clinical presentations affecting the central nervous and gastrointestinal system, muscle, heart, eye, and skin may often be overlooked because they are nonspecific in children, and most health professionals lack awareness of leptospirosis. Accordingly, the number of cases in studies examining pediatric patients with leptospirosis is very limited.1 This paper presents a case report on leptospirosis, which was initially misdiagnosed as staphylococcal toxic shock syndrome (STSS). Additionally, it reviews the clinical and laboratory manifestations, diagnostic methods, and treatment outcomes in pediatric patients with leptospirosis.
Case Presentation
A 7-year-old boy was referred to our Paediatric Intensive Care Unit with complaints of myalgia and a generalized rash. He had experienced sudden fever, headache, and vomiting for 2 days prior to admission. The initial diagnosis was STSS, and he was being treated with vancomycin. He resided in a rural area. His prenatal, natal, postnatal, and family histories were unremarkable. On admission, the patient’s body weight and height were measured as 19 kg (10th-25th percentile) and 117.5 cm (25th percentile), respectively. He presented with a fever of 38.5°C/101.4 F, low blood pressure (82/60 mm Hg), a rapid heart rate of 169 beats/min, and rapid breathing at 27 breaths/min. The Glasgow Coma Scale score was 11 points, and capillary refill time was 3 seconds. Diffuse erythema, intraoral bleeding, bilateral conjunctival suffusion, tenderness in the calf muscles, hepatomegaly (3 cm below the right costal margin), and splenomegaly (2 cm below the left costal margin) were detected (Figures 1 and 2).
Figure 1.
Generalized erythema of the skin.
Figure 2.

Bilateral conjunctival suffusion.
The initial laboratory studies showed several abnormalities, including mild anemia, metabolic acidosis, hypoglycemia, electrolyte imbalances, hyperbilirubinemia, renal and hepatic function abnormalities, abnormal urine findings, elevated inflammatory markers, and abnormal coagulation profile (Table 1). Culture studies of blood, urine, and throat specimens did not reveal any pathogenic microorganisms. Although the microscopic agglutination test (MAT) was negative, motile spirochetes were observed in the urine sediment through dark-ground microscopy. Additionally, a real-time polymerase chain reaction (PCR) study for leptospires in a urine sample was positive (Figures 3 and 4).
Table 1.
Laboratory Characteristics During the Follow-up Period.
| Markers | On Admission | On the Third Day | On the Sixth Day |
|---|---|---|---|
| Hematologic | |||
| White blood cell (mm3) (4500-13 500) | 12 540 | 10 880 | 15 630 |
| Neutrophil (mm3) (1800-8000) | 11 490 | 6330 | 9240 |
| Lymphocyte (mm3) (1662-3448) | 210 | 3530 | 4500 |
| Monocyte (mm3) (350-400) | 540 | 810 | 1300 |
| Eosinophil (mm3) (<230) | 270 | 130 | 580 |
| Basophil (mm3) (<40) | 30 | 80 | 10 |
| Hb (g/dL) (>12) | 11.7 | 9.4 | 8.7 |
| Platelet (mm3) (150 000-450 000) | 192 000 | 52 000 | 251 000 |
| Venous blood gas | |||
| pH (7.35-7.45) | 7.33 | 7.39 | 7.42 |
| PCO2 (mm Hg) (35-45) | 19.1 | 44.1 | 42 |
| HCO3 (mmol/L) (17-23) | 10 | 26.7 | 28.3 |
| Lactate (mmol/L) (1.1-2.3) | 2.4 | 1.1 | 1.7 |
| Biochemical | |||
| Glucose (mg/dL) (70-100) | 38 | 103 | 95 |
| BUN (mg/dL) (5-18) | 41.5 | 7.9 | 7 |
| Creatinine (≤0.8) | 0.9 | 0.2 | 0.15 |
| Uric acid (mg/dL) (2.5-5.5) | 9.7 | 2.3 | 1.4 |
| Na (mmol/L) (135-145) | 128 | 140 | 137 |
| Cl (mmol/L) (95-108) | 100 | 99 | 96 |
| K (mmol/L) (3.5-5.5) | 3.3 | 2.5 | 4.1 |
| Ca (mg/dL) (8.8-10.8) | 7.2 | 8 | 8.5 |
| P (mg/dL) (4.5-5.5) | 4 | 2.9 | 3.6 |
| Mg (mg/dL) (1.7-2.2) | 2.1 | 1.5 | 1.6 |
| Total bilirubin (mg/dL) (<1.5) | 3.0 | 2.3 | 0.9 |
| Direct bilirubin (mg/dL) (0.0-0.3) | 2.0 | 1.1 | 0.3 |
| Total protein (g/dL) (4.3-7.6) | 3.8 | 5 | 5.8 |
| Albumin (g/dL) (3-5) | 1.9 | 2.8 | 3.0 |
| ALT (U/L) (3-30) | 145 | 73 | 32 |
| AST (U/L) (15-40) | 88 | 66 | 26 |
| LDH (U/L) (60-170) | 425 | 369 | 387 |
| GGT (U/L) (0-23) | 61 | 49 | 29 |
| ALP (U/L) (107-213) | 143 | 144 | 128 |
| CK (U/L) (0-70) | 519 | 263 | 84 |
| CK-MB (U/L) (1-24) | – | 30 | 24 |
| Urine | |||
| Density (1012-1020) | 1012 | – | 1015 |
| Blood (negative) | Trace | – | Negative |
| Protein (negative) | Negative | – | Negative |
| Leucocyte esterase (negative) | Trace | – | Negative |
| Ketone (negative) | +1 | – | Negative |
| Bilirubin (negative) | +1 | – | Negative |
| Inflammatory | |||
| CRP (mg/dL) (0-5) | 260.4 | 220 | 35.8 |
| ESR (mm/h) (0-20) | 40 | – | – |
| Procalcitonin (ng/mL) (<0.5) | 84.3 | – | – |
| Coagulation | |||
| PT (s) (11-16.5) | 30.2 | 16 | 16.7 |
| aPTT (s) (26-34) | 33.9 | 29.5 | 30.5 |
| INR (0.8-1.2) | 2.4 | 1.23 | 1.28 |
| Fibrinogen (mg/dL) (200-400) | 436 | 341 | 350 |
| D-Dimer (ng/mL) (0-500) | 7699 | 6330 | 3596 |
ALT, alanine transaminase; ALP, alkaline phosphatase; aPTT, activated partial thromboplastin time; AST, aspartate transaminase; BUN, blood urea nitrogen; Ca, calcium; Cl, chloride; CK, creatine kinase; CK-MB, creatine kinase, myocardial band; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GGT, gamma glutamyl transferase; Hb, hemoglobin; HCO3, bicarbonate; INR, international normalized ratio; K, potassium; LDH, lactate dehydrogenase; Mg, magnesium; Na, sodium; P, phosphorus; PCO2, partial carbon dioxide pressure; PT, prothrombin time.
Normal values for the patient’s age are provided in parentheses.
Figure 3.
Leptospira on dark-ground microscopy (white arrows).
Figure 4.

Real-time polymerase chain reaction in the urine sample. A. Control. B. Amplification curves indicating the presence of leptospiral DNA (black arrow).
During the first day of hospitalization, the patient received supportive therapies, including saline, adrenaline, fresh frozen plasma, albumin, and calcium gluconate. Additionally, empirical antibiotics, such as vancomycin, meropenem, and clindamycin, were administered to treat STSS and bacterial sepsis. All antibiotics were discontinued on the second day when dark-ground microscopy revealed spirochetes. Cefotaxime was started at 200 mg/kg/day in 3 doses. On the third day of hospitalization, the platelet count reached its lowest level, and most laboratory markers returned to normal. The recovery process continued consistently during the 10-day cefotaxime treatment (Table 1). The patient was discharged after making a full recovery (Figure 5). The release of this information has been authorized by the patient’s parents.
Figure 5.
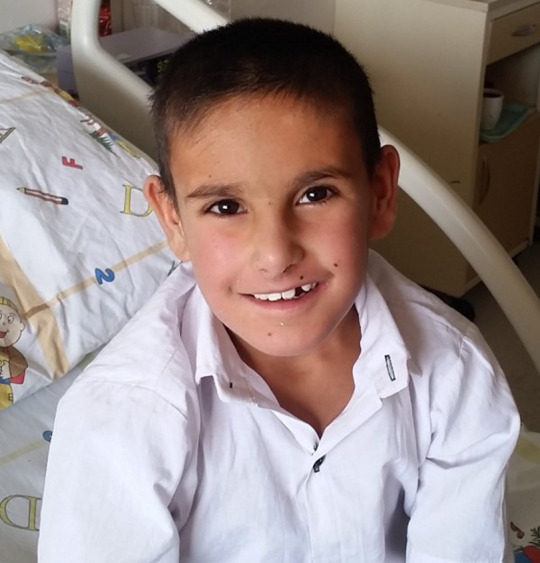
The patient’s discharge appearance.
Discussion
Leptospirosis, also known as Weil’s disease, Weil–Vasiliev disease, Stuttgart disease, swine herder’s disease, canicola fever, rice field fever, waterborne fever, nanukayami fever, cane cutter fever, swamp fever, and mud fever, is a globally important zoonotic disease with very different clinical manifestations. It is caused, so far, by 14 pathogenic species of the genus Leptospira. Leptospira are spiral-shaped, highly motile aerobic spirochetes and contain 18 or more helices per cell.2,3
The main reservoir for spirochetes is rodents, but they can also be carried by other mammals such as horses, dogs, cattle, sheep, goats, and pigs. Leptospira infection in rodents typically occurs during their infancy.4 They can then contaminate the environment, particularly water resources, by excreting the microorganisms in their urine throughout their lives.5 Leptospira can survive in water or muddy soil with a slightly alkaline pH for up to 6 months.3 Leptospira infection in cattle, sheep, goats, and pigs can cause signs of disease, leading to death or spontaneous abortion, although infected animals may be asymptomatic. Humans become infected when their mucous membranes or damaged skin comes into contact with contaminated water, contaminated soil, or infected animal tissues. Individuals in occupations such as farming, ranching, abattoir work, trapping, veterinary medicine, logging, sewage treatment, pet trading, and laboratory work, as well as those who participate in activities such as freshwater swimming, canoeing, kayaking, trail biking, and adventure racing, those who have pet dogs, domesticated livestock, rainwater catchment systems, and skin lesions, and those who walk barefoot through surface water and come into contact with wild rodents, are at risk of contracting leptospirosis.6 Leptospirosis outbreaks have been reported after floods and some triathlons where the swimming portion took place in fresh water.7-9 This information highlights the importance of the freshwater connection in the transmission of spirochetes to humans through contaminated water and mud. Our patient lived in a rural area, and it is possible that outdoor activities were a factor in his exposure to Leptospira.
Leptospirosis can occur sporadically throughout the year, but it frequently breaks out during the rainy season.3 It is reported that the incidence of disease in tropical regions is approximately 10 times higher than in temperate regions.10 However, the fact that leptospirosis is an under-reported disease- makes it difficult to obtain reliable global incidence figures. The Leptospirosis Burden Epidemiology Group of the World Health Organization estimates that there are 873 000 cases of leptospirosis worldwide annually, resulting in 48 600 deaths.11 Clinical reports on pediatric leptospirosis in Turkey have been rarely published. The largest clinical report to date consists of only 5 cases.12 To our knowledge, this is the first pediatric case diagnosed with leptospirosis in Eastern Anatolia. Although farming and cattle breeding are common in the region, the fact that this disease has not been reported in children before is an important finding that should be taken into consideration. All these findings suggest that leptospirosis may be underdiagnosed or misdiagnosed in Turkey. Possible reasons for the underdiagnosis of leptospirosis include the subclinical and self-limiting nature of most cases, lack of awareness among clinicians, and inadequate diagnostic tools in many hospitals. The clinical course of leptospirosis in children and adolescents is highly variable. The disease is usually mild and self-limiting or subclinical, but can sometimes be severe and potentially fatal.13 During the incubation period of 2-26 days (average 10 days), Leptospira multiplies in the blood and tissues. They then bind to the capillary endothelium, causing vasculitis, which is the primary cause of multisystem involvement.14 In pediatric patients, initial symptoms may include an abrupt onset of fever, myalgia, and headache. These symptoms have been reported in a range of 24-100% of cases (Table 2). Conjunctival suffusion, which is characterized by redness of the conjunctiva (Figure 2), is an important but oftenoverlooked sign. It was found in as many as 52% of cases in a pediatric case series.15 As this finding is not commonly seen in other infectious diseases, its presence in a patient with a nonspecific febrile illness should raise the possibility of leptospirosis.16 Gastrointestinal symptoms, including nausea, vomiting, and diarrhea, occur in 6.2-55% of cases (Table 2). Findings such as jaundice, jaundice accompanied by renal failure (Weil’s disease), liver and renal dysfunction, oliguria, hepatomegaly, lymphadenopathy, and meningitis have been reported in 2.9-64% of patients. Less common findings may include hypotension, splenomegaly, skin rash, myocarditis, and shock. Mortality rates in hospitalized pediatric patients range from 1.6% to 8% (Table 2). Although studies from disease-endemic areas have commonly reported that leptospirosis has a milder clinical course in children than in adults,13 our patient experienced critically severe leptospirosis, with acute kidney injury, acute hepatic dysfunction, and hemodynamic instability. Septic shock is defined as persistent hypotension requiring vasopressors to maintain mean arterial pressure ≥65 mm Hg and serum lactate >2 mmol/L (18 mg/dL) despite adequate volume resuscitation, and is very rare in pediatric series.17 The incidence has only been shown to be 9% in one study.18 However, that study did not report the findings related to shock such as hypotension, oliguria, and liver and renal dysfunctions.
Table 2.
Our Case and the Studies Including a Large Number of Pediatric Patients with Leptospirosis.
| Clinical Characteristics | Our Patient, Turkey | Tomari K,15 2018 Japan (n = 44) n (%) | Narayanan R,43 2016 India (n = 35) n (%) | Pérez-García J,44 2016, Colombia (n = 74) n(%) | Guerrier G,45 2013, New Caledonia (n = 60) n(%) | Spichler A,13 2012, Brazil (n = 42) n (%) | Agesilas F,46 2005, Reunion Island (n = 16) n (%) | Rajajee S,18 2002, India (n = 139) n (%) | Cruz ML,47 1994, Brazil (n = 23) n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Age | 7 Years | 0-20 Years | 0-17 Years | 5-17 Years | 6-17 Years | < 18 Years | 9-17 Years | 5-17 Years | 4-12 Years |
| Fever | Yes | 42 (96) | 35 (100) | Unknown | 33 (55) | Unknown | 15 (93.7) | 133 (96) | 23 (100) |
| Headache | Yes | Unknown | 29 (82.9) | Unknown | 43 (71.6) | Unknown | Unknown | 34 (24) | 12 (52.1) |
| Myalgia | Yes | 23 (52) | 22 (62.9) | 18 (24.3) | 43 (71.6) | Unknown | Unknown | 16 (69.5) | |
| Gastrointestinal symptoms | No | 15 (34) | 12 (34.2) | 31 (41.8) | 33 (55) | Unknown | 1 (6,2) | Unknown | 8 (34.8) |
| Respiratory symptoms | No | Unknown | 17 (48.6) | 4 (5.4) | 18 (30) | 13/39 (33) | 1 (6.2) | Unknown | Unknown |
| Conjunctival suffusion | Yes | 23 (52) | 9 (25.7) | Unknown | 13 (21.6) | Unknown | 1 (6.2) | 21 (15) | 3 (13) |
| Hypotension | Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | Unknown | 2 (12.5) | Unknown | Unknown |
| Rash | Yes | 1 (2) | Unknown | 8 (10,8) | Unknown | Unknown | Unknown | Unknown | Unknown |
| Jaundice | No | 6 (14) | 11 (31.4) | 15 (20.2) | 11 (18.3) | 26/41 (64) | 7 (43) | 25 (18) | 11 (47.8) |
| Lymphadenopathy | No | 2 (5) | Unknown | 15 (20.2) | Unknown | Unknown | Unknown | Unknown | Unknown |
| Hepatomegaly | Yes | Unknown | 19 (54.3) | 12 (16.2) | Unknown | Unknown | Unknown | 100 (72) | 1 (4,3) |
| Splenomegaly | Yes | Unknown | Unknown | 1 (1.3) | Unknown | Unknown | Unknown | Unknown | Unknown |
| Oliguria | Yes | Unknown | Unknown | Unknown | 9 (15) | 16/41 (39) | Unknown | Unknown | Unknown |
| Meningitis | No | 4 (9) | 1 (2.9) | Unknown | Unknown | Unknown | 4 (25) | 10 (7) | Unknown |
| Myocarditis | No | Unknown | 3 (8.6) | Unknown | 1 (1.6) | Unknown | 1 (6.2) | 9 (7) | Unknown |
| Liver dysfunction | Yes | 12 (27) | Unknown | Unknown | Unknown | Unknown | 9 (56) | Unknown | Unknown |
| Renal dysfunction | Yes | 11 (25) | Unknown | Unknown | Unknown | Unknown | 8 (50) | Unknown | 4 (17.4) |
| Shock | Yes | 0 (0) | 0 (0) | Unknown | 0 (0) | Unknown | 0 (0) | 12 (9) | Unknown |
| Weil’s disease (jaundice and renal involvement) | Yes | Unknown | Unknown | Unknown | 7 (11.6) | Unknown | 6 (37.5) | Unknown | Unknown |
| Death | No | 0 (0) | 0 (0) | 0 (0) | 1 (1.6) | 2 (5) | 0 (0) | 8 (6) | 0 (0) |
There is currently no literature that specifically describes laboratory findings in children with leptospirosis. Both adult and child reports indicate that routine laboratory tests may lack specificity. The white blood cell (WBC) count can range from 3000 to 26 000/mm3. Thrombocytopenia or pancytopenia may appear as the initial presentation.19,20 An outer membrane protein of Leptospira is thought to interfere with the activity of the Na+–K+–Cl− cotransporter in the thick ascending limb of Henle, resulting in hyponatremia and hypopotassemia that are common in severe leptospirosis.21,22 Proteinuria, pyuria, and granular casts are common findings on urinalysis, with occasional microscopic hematuria.23 Severe leptospirosis can result in renal failure. Elevated creatine kinase levels, which can be a useful indicator of the disease, have been reported in about 50% of patients with leptospirosis.24 Elevations in hepatic transaminases, typically not exceeding 200 IU/mL, may be observed in nearly 40% of patients. In severe cases of leptospirosis, jaundice may be observed, and in some cases, the serum bilirubin concentration may reach as high as 80 mg/dL. The analysis of cerebrospinal fluid may reveal a minimal or moderate increase in protein concentration and a normal or rarely low-glucose concentration, accompanied by a pleocytosis of lymphocytes or neutrophils.25 Oliguria, a WBC count above 12 900/mm3, repolarization abnormalities on an electrocardiogram, and alveolar infiltrates on chest radiography have been associated with an adverse outcome. The patient exhibited nearly all of the laboratory findings mentioned, except for meningeal irritation. Therefore, lumbar puncture was not performed.
Leptospirosis can be diagnosed using a range of tools, including MAT, serological methods, molecular techniques, antigen detection, and microbiologic culture. As the isolation of the organism in culture is only successful in a small percentage of cases (5-50%) and can take several weeks,10 other tests are necessary for early and rapid diagnosis. In suspected cases of leptospirosis, other assays are typically performed first due to the limited accessibility of the MAT. However, the use of serologic tests for acute diagnosis is limited due to the high seropositivity ratio among individuals living in endemic areas.26 So, serum IgM antibody titer should be measured in both acute and convalescent serum samples. A single titer of over 1 : 800 or a fourfold or greater increase in titers is considered reasonable evidence of current or recent infection with Leptospira. However, it should be kept in mind that serologic assays may cross-react in syphilis, relapsing fever, Lyme disease, and legionellosis.27 When a seropositive result is obtained, the most specific test that can be performed to detect the infecting serotype is MAT.28,29 During acute illness, seronegativity has been associated with cross-reactive antibodies. To diagnose leptospirosis in such cases, molecular techniques such as real-time PCR and loop-mediated isothermal amplification can be used. This is because Leptospira DNA can be detected in blood during the initial bacteremic phase of the illness, and in cerebrospinal fluid and urine a few days after the onset of symptoms. These molecular tests do not require samples from the convalescent period and do not cause any delay in the diagnosis.30,31 As there are no other serological tests available in our country, we conducted the MAT test that produced a negative result. This outcome may be due to either a suboptimal level of antibody titer during the acute phase or an infection with a serovar that is not included in the Leptospira test panel.28,29 We detected motile spirochetes in a urine sediment using dark-ground microscopy and also confirmed them with a real-time PCR study in urine. We think that dark-ground microscopy is a fast, easy, simple, and safe method and can be used with the real-time PCR for rapid diagnosis.
Leptospirosis may be difficult to distinguish from other infectious diseases. Acute viral illnesses, Hantavirus infection, malaria, dengue, chikungunya, scrub typhus, rickettsial diseases, typhoid fever, and ehrlichiosis should be considered in the differential diagnosis. Interestingly, our patient was referred to our intensive care unit with a preliminary diagnosis of STSS. The patient’s clinical findings may actually indicate this syndrome, which is characterized by sudden onset of fever, widespread rash, hypotension, and involvement of multiple organ systems. However, the lack of a staphylococcal infection focus in the patient contradicts the diagnosis of STSS. Half of the cases of STSS are associated with menstruation and tampon use, while the other half are related to specific factors such as surgical and postpartum wound infections, mastitis, septorhinoplasty, sinusitis, osteomyelitis, arthritis, burns, and cutaneous and subcutaneous lesions, especially in the extremities, perianal area, and axillae. Additionally, respiratory infections following influenza and enterocolitis have also been linked to STSS.32-38 Furthermore, conjunctival suffusion is a significant but often overlooked indicator of leptospirosis, and is not commonly seen in STSS or other infectious diseases. The presence of this finding in a nonspecific febrile illness should raise the possibility of leptospirosis.16 All this information emphasizes the importance of the medical history and physical examination to distinguish leptospirosis from STSS.
Most cases of leptospirosis have a self-limited course without antimicrobial therapy. However, some patients may experience severe complications that can lead to morbidity and mortality. For children with mild disease, doxycycline (2 mg/kg/day, divided into 2 doses, maximum 200 mg/day, orally for 7 days) or azithromycin (10 mg/kg, once daily, maximum 500 mg/day, on day 1 and followed by 5 mg/kg/day, once daily, maximum 250 mg/day) can be given. Hospitalized children can be treated with penicillin (250 000-400 000 units/kg/day, divided into 4-6 doses, maximum 6-12 million units/day, intravenously), doxycycline (4 mg/kg/day, divided into 2 doses, maximum 200 mg/day, intravenously), ceftriaxone (80-100 mg/kg/day, once daily, maximum 2 g/day), or cefotaxime (100-150 mg/kg/day, divided into 3-4 doses). Azithromycin can be used as an alternative drug for children who experience adverse reactions to other agents. The recommended dosage is 10 mg/kg/day, once daily, with a maximum of 500 mg/day intravenously on day 1, followed by 5 mg/kg/day, once daily, with a maximum of 250 mg/day intravenously on subsequent days. The recommended duration of treatment for severe disease is typically 7 days.39 Doxycycline is contraindicated for children under 8 years of age. Severe illness may require supportive care such as fluid–electrolyte therapy, blood products, ventilatory support, and renal replacement therapy.40 In adult patients, corticosteroids may be administered when there is pulmonary involvement and vasculitis.41,42 The patient received supportive care and was treated with cefotaxime, resulting in an improvement of both clinical and laboratory findings on the sixth day of admission.
The most important measures for protecting against human leptospirosis are rodent control, flood control, and avoiding potential sources of infection such as stagnant water and animal farm water runoff. Public health precautions associated with these conditions should be taken as needed.
Conclusion
Leptospirosis is a disease that is prevalent worldwide and can be fatal. It should be considered as a possible diagnosis for patients who experience a sudden onset of fever, myalgia, and headache. Conjunctival suffusion can be useful in distinguishing it from STSS. The diagnosis of leptospirosis requires serological and molecular tests. Dark-background microscopy may serve as an alternative diagnostic tool. Leptospira is sensitive to penicillins, but it can still cause life-threatening complications. Therefore, it is essential to develop disease prevention strategies for both humans and animals. Increased awareness of leptospirosis among health-care professionals and easy laboratory access are necessary for these reasons.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Informed Consent: Written informed consent was obtained from the patient’s parents who agreed to take part in this review.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – D.H.; Design – D.H., K.H.; Supervision – D.H., K.H.; Resources – K.H., A.H., B.I.H.; Materials – A.H., B.I.H.; Data Collection and/or Processing – A.H., B.I.H., S.B.P.; Analysis and/or Interpretation – D.H., SB.P.; Literature Search – D.H.; Writing – D.H.; Critical Review – D.H., K.H., S.B.P.
Declaration of Interests: The authors have no conflict of interest to declare.
References
- 1. Holk K, Nielsen SV, Rønne T. Human leptospirosis in Denmark 1970-1996: an epidemiological and clinical study. Scand J Infect Dis. 2000;32(5):533 538. ( 10.1080/003655400458839) [DOI] [PubMed] [Google Scholar]
- 2. Mayer J, Donelly TM, eds. Zoonoses. Clinical Veterinary Advisor: Birds and Exotic Pets. MO: Saunders; 2003:690 730. [Google Scholar]
- 3. Lara JM, Von Zuben AV, Costa JV, Donalisio MR, Francisco PMSB. Leptospirosis in Campinas, São Paulo, Brazil: 2007-2014. Rev Bras Epidemiol. 2019;22:e190016. ( 10.1590/1980-549720190016) [DOI] [PubMed] [Google Scholar]
- 4. Saglam YS, Yildirim S, Ozkaraca M, Altun S. Investigation of leptospiral antigen with immunohistochemical and immunofluorescence methods in cattle kidney. Microb Pathog. 2022;164:105434. ( 10.1016/j.micpath.2022.105434) [DOI] [PubMed] [Google Scholar]
- 5. Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7(10):736 747. ( 10.1038/nrmicro2208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chung KJ, Hsiao CT, Liu JW, Lee CH. Case reports of leptospirosis in southern Taiwan. J Formos Med Assoc. 2002;101(7):514 518. [PubMed] [Google Scholar]
- 7. Alderman K, Turner LR, Tong S. Floods and human health: a systematic review. Environ Int. 2012;47:37 47. ( 10.1016/j.envint.2012.06.003) [DOI] [PubMed] [Google Scholar]
- 8. Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593 1599. ( 10.1086/340615) [DOI] [PubMed] [Google Scholar]
- 9. Brockmann S, Piechotowski I, Bock-Hensley O, et al. Outbreak of leptospirosis among triathlon participants in Germany, 2006. BMC Infect Dis. 2010;10:91. ( 10.1186/1471-2334-10-91) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect. 2011;17(4):494 501. ( 10.1111/j.1469-0691.2011.03474.x) [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization. https://www.who.int/home/search-results?indexCatalogue=genericsearchindex1&searchQuery=Leptospirosis&wordsMode=AnyWord. [Google Scholar]
- 12. Aygün FD, Avar-Aydın PÖ, Çokuğraş H, Camcıoğlu Y. Different clinical spectrum of leptospirosis. Turk J Pediatr. 2016;58(2):212 215. ( 10.24953/turkjped.2016.02.015) [DOI] [PubMed] [Google Scholar]
- 13. Spichler A, Athanazio DA, Vilaça P, Seguro A, Vinetz J, Leake JA. Comparative analysis of severe pediatric and adult leptospirosis in Sao Paulo, Brazil. Am J Trop Med Hyg. 2012;86(2):306 308. ( 10.4269/ajtmh.2012.11-0308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Priya SP, Sakinah S, Sharmilah K, et al. Leptospirosis: molecular trial path and immunopathogenesis correlated with dengue, malaria and mimetic hemorrhagic infections. Acta Trop. 2017;176:206 223. ( 10.1016/j.actatropica.2017.08.007) [DOI] [PubMed] [Google Scholar]
- 15. Tomari K, Toyokawa T, Takahashi T, et al. Childhood leptospirosis in an industrialized country: population-based study in Okinawa, Japan. PLOS Negl Trop Dis. 2018;12(3):e0006294. ( 10.1371/journal.pntd.0006294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vanasco NB, Schmeling MF, Lottersberger J, Costa F, Ko AI, Tarabla HD. Clinical characteristics and risk factors of human leptospirosis in Argentina (1999-2005) Acta Trop. 2008;107(3):255 258. ( 10.1016/j.actatropica.2008.06.007) [DOI] [PubMed] [Google Scholar]
- 17. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801 810. ( 10.1001/jama.2016.0287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rajajee S, Shankar J, Dhattatri L. Pediatric presentations of leptospirosis. Indian J Pediatr. 2002;69(10):851 853. ( 10.1007/BF02723704) [DOI] [PubMed] [Google Scholar]
- 19. Chierakul W, Tientadakul P, Suputtamongkol Y, et al. Activation of the coagulation cascade in patients with leptospirosis. Clin Infect Dis. 2008;46(2):254 260. ( 10.1086/524664) [DOI] [PubMed] [Google Scholar]
- 20. Stefos A, Georgiadou SP, Gioti C, et al. Leptospirosis and pancytopenia: two case reports and review of the literature. J Infect. 2005;51(5):e277 e280. ( 10.1016/j.jinf.2005.03.008) [DOI] [PubMed] [Google Scholar]
- 21. Wu MS, Yang CW, Pan MJ, Chang CT, Chen YC. Reduced renal Na+-K+-Cl- co-transporter activity and inhibited NKCC2 mRNA expression by Leptospira shermani: from bed-side to bench. Nephrol Dial Transplant. 2004;19(10):2472 2479. ( 10.1093/ndt/gfh452) [DOI] [PubMed] [Google Scholar]
- 22. Krishnan A, Karnad DR, Medhekar TP. Paralysis due to renal potassium wasting: an unusual presentation of leptospirosis. Nephrol Dial Transplant. 2003;18(11):2454 2455. ( 10.1093/ndt/gfg350) [DOI] [PubMed] [Google Scholar]
- 23. Sanford JP. Leptospirosis--time for a booster. N Engl J Med. 1984;310(8):524 525. ( 10.1056/NEJM198402233100811) [DOI] [PubMed] [Google Scholar]
- 24. Johnson WD, Silva IC, Rocha H. Serum creatine phosphokinase in leptospirosis. JAMA. 1975;233(9):981 982. ( 10.1001/jama.1975.03260090047022) [DOI] [PubMed] [Google Scholar]
- 25. Helmer RE, 3rd, Millsaps RD. Letter: hypoglycorrachia in leptospirosis. Ann Intern Med. 1973;79(6):912. ( 10.7326/0003-4819-79-6-912) [DOI] [PubMed] [Google Scholar]
- 26. Pradutkanchana J, Pradutkanchana S, Kemapanmanus M, Wuthipum N, Silpapojakul K. The etiology of acute pyrexia of unknown origin in children after a flood. Southeast Asian J Trop Med Public Health. 2003;34(1):175 178. [PubMed] [Google Scholar]
- 27. Iroh Tam PY, Obaro SK, Storch G. Challenges in the etiology and diagnosis of acute febrile illness in children in low- and middle-income countries. J Pediatr Infect Dis Soc. 2016;5(2):190 205. ( 10.1093/jpids/piw016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levett PN. Usefulness of serologic analysis as a predictor of the infecting serovar in patients with severe leptospirosis. Clin Infect Dis. 2003;36(4):447 452. ( 10.1086/346208) [DOI] [PubMed] [Google Scholar]
- 29. Smythe LD, Wuthiekanun V, Chierakul W, et al. The microscopic agglutination test (MAT) is an unreliable predictor of infecting Leptospira serovar in Thailand. Am J Trop Med Hyg. 2009;81(4):695 697. ( 10.4269/ajtmh.2009.09-0252) [DOI] [PubMed] [Google Scholar]
- 30. Picardeau M, Bertherat E, Jancloes M, Skouloudis AN, Durski K, Hartskeerl RA. Rapid tests for diagnosis of leptospirosis: current tools and emerging technologies. Diagn Microbiol Infect Dis. 2014;78(1):1 8. ( 10.1016/j.diagmicrobio.2013.09.012) [DOI] [PubMed] [Google Scholar]
- 31. Waggoner JJ, Pinsky BA. Molecular diagnostics for human leptospirosis. Curr Opin Infect Dis. 2016;29(5):440 445. ( 10.1097/QCO.0000000000000295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Broome CV. Epidemiology of toxic shock syndrome in the United States: overview. Rev Infect Dis. 1989;11(S uppl 1):S14 S21. ( 10.1093/clinids/11.supplement_1.s14) [DOI] [PubMed] [Google Scholar]
- 33. Bartlett P, Reingold AL, Graham DR, et al. Toxic shock syndrome associated with surgical wound infections. JAMA. 1982;247(10):1448 1450. ( 10.1001/jama.1982.03320350052030) [DOI] [PubMed] [Google Scholar]
- 34. Ferguson MA, Todd JK. Toxic shock syndrome associated with Staphylococcus aureus sinusitis in children. J Infect Dis. 1990;161(5):953 955. ( 10.1093/infdis/161.5.953) [DOI] [PubMed] [Google Scholar]
- 35. Morrison VA, Oldfield EC, 3rd. Postoperative toxic shock syndrome. Arch Surg. 1983;118(7):791 794. ( 10.1001/archsurg.1983.01390070003001) [DOI] [PubMed] [Google Scholar]
- 36. Paterson MP, Hoffman EB, Roux P. Severe disseminated staphylococcal disease associated with osteitis and septic arthritis. J Bone Joint Surg Br. 1990;72(1):94 97. ( 10.1302/0301-620X.72B1.2298804) [DOI] [PubMed] [Google Scholar]
- 37. Reingold AL, Hargrett NT, Dan BB, Shands KN, Strickland BY, Broome CV. Nonmenstrual toxic shock syndrome: a review of 130 cases. Ann Intern Med. 1982;96(6 Pt 2):871 874. ( 10.7326/0003-4819-96-6-871) [DOI] [PubMed] [Google Scholar]
- 38. MacDonald KL, Osterholm MT, Hedberg CW, et al. Toxic shock syndrome. A newly recognized complication of influenza and influenzalike illness. JAMA. 1987;257(8):1053 1058. ( 10.1001/jama.257.8.1053) [DOI] [PubMed] [Google Scholar]
- 39. American Academy of Pediatrics. Tetracyclines. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Report of the Committ Ee on Infectious Diseases. 31st ed. Itasca, IL: American Academy of Pediatrics; 2018:905. [Google Scholar]
- 40. Wiwanitkit V. Comparison between blood exchange and classical therapy for acute renal failure in Weil’s disease: appraisal on Thai reports. Nephrology 2006;11(5):481. ( 10.1111/j.1440-1797.2006.00677.x) [DOI] [PubMed] [Google Scholar]
- 41. Kularatne SA, Budagoda BD, de Alwis VK, et al. High efficacy of bolus methylprednisolone in severe leptospirosis: a descriptive study in Sri Lanka. Postgrad Med J. 2011;87(1023):13 17. ( 10.1136/pgmj.2009.092734) [DOI] [PubMed] [Google Scholar]
- 42. Azevedo AF, Miranda-Filho B, Henriques-Filho GT, Leite A, Ximenes RA. Randomized controlled trial of pulse methyl prednisolone × placebo in treatment of pulmonary involvement associated with severe leptospirosis. [ISRCTN74625030]. BMC Infect Dis. 2011;11:186. ( 10.1186/1471-2334-11-186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Narayanan R, Sumathi G, Prabhakaran SG, Shanmughapriya S, Natarajaseenivasan K. Paediatric leptospirosis: A population based case-control study from Chennai, India. Indian J Med Microbiol. 2016;34(2):228 232. ( 10.4103/0255-0857.180353) [DOI] [PubMed] [Google Scholar]
- 44. Pérez-García J, Arboleda M, Agudelo-Flórez P. Childhood leptospirosis in patients with febrile syndrome in the Region of Urabá, Colombia. Rev Peru Med Exp Salud Publica. 2016;33(4):745 750. ( 10.17843/rpmesp.2016.334.2561) [DOI] [PubMed] [Google Scholar]
- 45. Guerrier G, Hie P, Gourinat AC, et al. Association between age and severity to leptospirosis in children. PLOS Negl Trop Dis. 2013;7(9):e2436. ( 10.1371/journal.pntd.0002436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Agésilas F, Gey F, Monbrunt A, et al. Acute leptospirosis in children in Reunion Island: a retrospective review of 16 cases. Arch Pediatr. 2005;12(9):1344 1348. ( 10.1016/j.arcped.2005.04.084) [DOI] [PubMed] [Google Scholar]
- 47. Cruz ML, Andrade J, Pereira MM. Leptospirosis in children in Rio do Janeiro. Rev Soc Bras Med Trop. 1994;27(1):5 9. ( 10.1590/s0037-86821994000100002) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a 
