Abstract
Cancer cells undergoing immunogenic cell death (ICD) can initiate adaptive immune responses against dead cell-associated antigens, provided that (1) said antigens are not perfectly covered by central tolerance (antigenicity), (2) cell death occurs along with the emission of immunostimulatory cytokines and damage-associated molecular patterns (DAMPs) that actively engage immune effector mechanisms (adjuvanticity), and (3) the microenvironment of dying cells is permissive for the initiation of adaptive immunity. Finally, ICD-driven immune responses can only operate and exert cytotoxic effector functions if the microenvironment of target cancer cells enable immune cell infiltration and activity. Multiple forms of radiation, including non-ionizing (ultraviolet) and ionizing radiation, elicit bona fide ICD as they increase both the antigenicity and adjuvanticity of dying cancer cells. Here, we review the molecular determinants of ICD as elicited by radiation as we critically discuss strategies to reinforce the immunogenicity of cancer cells succumbing to clinically available radiation strategies.
Keywords: ATP, calreticulin, HMGB1, immune checkpoint inhibitors, type I IFN, PD-L1
Introduction
Dying cells can initiate antigen-specific immune responses, admitting that cell death occurs in immunocompetent syngeneic hosts, a process that has been dubbed “immunogenic cell death” (ICD).1–3 The ability of regulated cell death (RCD) to elicit adaptive immunity coupled with effector functions and immunological memory relies upon three critical determinants: (1) antigenicity, i.e., the fact that dying (cancer) cells express antigens that are not perfectly covered by central tolerance, implying that T cell clones specific for such antigens are available in the mature T cell repertoire of the host;4 (2) adjuvanticity, i.e., the ability of stressed and dying cells to secrete chemokines and cytokines as well as immunostimulatory damage-associated molecular patterns (DAMPs) that overall recruit and activate professional antigen-presenting cells (APCs) such as dendritic cells (DCs) to sites of cell death;5,6 and (3) microenvironmental features that not only are permissive for the recruitment and activation of APCs or precursors thereof by ICD-associated cytokines and DAMPs (at sites of cell death), but also enable antigen-specific immune effector cells, notably CD8+ cytotoxic T lymphocytes (CTLs) primed by said APCs to reach their targets and execute antigen-specific immune responses (Figure 1).7–10 Importantly, none of these requirements is truly intrinsic to dying cells.1,11 Indeed, not only the composition of the mature T cell repertoire, but also the productive recognition of cytokines and DAMPs as well as the microenvironment of the initiators (dying cells) or target (living cells resisting death) of adaptive immunity is largely dictated by the host.1,11,12 Moreover, the immunogenicity of dying cells is not necessarily dictated by RCD modality.13 Indeed, while caspase-dependent apoptosis generally occurs in an immunologically silent or even tolerogenic manner,14–17 multiple instances of apoptosis have been shown to constitute bona fide cases of ICD.18
Figure 1. Core determinants of ICD.
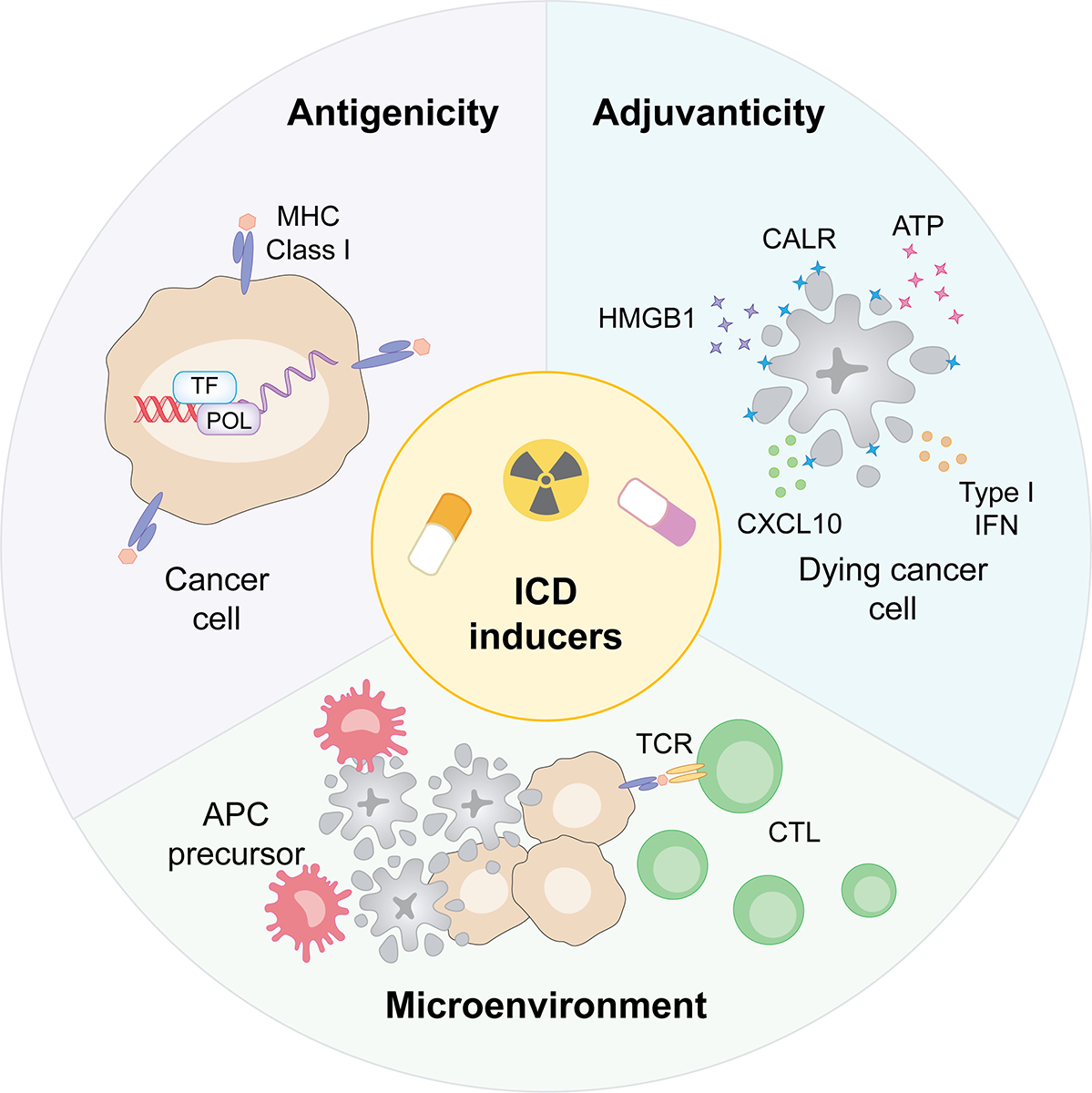
The immunogenicity of regulated cell death (RCD), i.e., the ability of dying cells to elicit antigen-specific immunity coupled with effector and memory functions (as opposed to mere inflammation), relies on three core determinants: (1) antigenicity, i.e., dying cells must express antigenic determinants that can be recognized by circulating T cells; (2) adjuvanticity, i.e., dying cells must emit chemotactic and immunostimulatory signals that enable antigen-presenting cell (APC) recruitment, activation and migration to lymphoid organs for T cell cross-priming; and (3) a permissive microenvironment, i.e., cells must die in an environment that enables APC recruitment and functions. Moreover, cells targeted by immunogenic cell death (ICD)-driven immunity must reside in a microenvironment that is permissive for cytotoxic T lymphocyte (CTL) infiltration and effector functions. CALR, calreticulin; CXCL10, C-X-C motif chemokine ligand 10; DAMP, damage-associated molecular pattern; HMGB1, high mobility group box 1; IFN, interferon.
DAMP emission by dying cells is generally elicited in the context of failing adaptation to stress.3 Thus, cells initially respond to ICD-inducing conditions by activating a panoply of cytoprotective mechanisms aimed at restoring cellular homeostasis, including (but not limited to): (1) the unfolded protein response (UPR) in the context of the so-called “integrated stress response” (ISR),19–22 (2) the DNA damage response,23–25 (3) autophagy,26–28 and (4) the mitochondrial stress response.29–31 Once exposed on the surface of dying cells or released in their extracellular microenvironment, DAMPs are detected by pattern recognition receptors (PRRs) expressed by immune effector cells including APCs and precursors thereof, other myeloid cells and both innate and adaptive lymphoid cell populations.5,32–34
A number of conditions have been shown to elicit bona fide ICD especially (but not exclusively) in cancer cells. These include: (1) numerous intracellular pathogens, notably oncolytic viruses,35,36 (2) a wide panel of conventional chemotherapeutics,37–39 (3) targeted anticancer agents,40–45 (4) oncolytic peptides,46–49 as well as (5) numerous physical stressors encompassing (but not limited to) high hydrostatic pressure,50,51 extracorporeal photochemotherapy,52–54 photodynamic therapy,55–57 nanopulse stimulation,58 near‐infrared photoimmunotherapy,59–62 as well as various forms of ionizing and non-ionizing radiation.63–65
Among these physical agents, radiation therapy (RT) has been consistently investigated for its potential to elicit ICD in cancer cells and hence synergize with commonly employed immunotherapeutics such as immune checkpoint inhibitors (ICIs).66–69 This reflects clinical considerations linked to RT safety12,70 and availability,71 as well as the ability of RT to elicit ICD and other immunostimulatory effects, at least when delivered focally and according to specific dose and fractionation schedules.69,72,73 In line with this notion, mouse cancer cells exposed to multiple forms of RT (including RT with charged particles) ex vivo can be successfully employed to establish prophylactic immunity upon inoculation into immunocompetent syngeneic hosts.63,74,75 Moreover, RT has been shown to synergize with various forms of immunotherapy including immune checkpoint inhibitors (ICIs) in a variety of preclinical tumor models, resulting not only in the inhibition of irradiated lesions, but also in (at least some degree) of immunological control of distant, non-irradiated tumors (the so-called abscopal response).76
Here, we review molecular determinants of ICD as driven by RT and other radiation strategies as we critically discuss potential approaches to boost the immunogenicity of irradiated cancer cells.
CALR
Calreticulin (CALR) is an endoplasmic reticulum (ER) chaperone involved in many biological processes that include (among others) the regulation of calcium homeostasis, the folding of newly synthesized glycoproteins and the trafficking of properly loaded MHC I molecules.77,78 Anthracyclines as well as many other chemical and physical ICD inducers elicit the rapid translocation of CALR to the outer leaflet of the plasma membrane, a process that occurs prior to the apoptosis-related (and generally tolerogenic) externalization of phosphatidylserine.19,38 Surface-exposed CALR operates as a potent pro-phagocytic signal upon binding to LDL receptor related protein 1 (LRP1, best known as CD91) on the surface of immature APCs.79–81 Moreover, externalized CALR has been shown to promote natural killer (NK) cell activation both directly, through natural cytotoxicity triggering receptor 1 (NCR1, best known as NKp46),82 and indirectly, via a mechanism that involves CD11c+CD14high myeloid cells trans-presenting interleukin 15 (IL15).83–86 Supporting the key role of CALR exposure in the immunogenicity of RCD, various genetic and pharmacological strategies blocking CALR exposure and/or preventing its interaction with CD91 have been shown to limit the ability of cancer cells succumbing to ICD inducers to generate prophylactic immunity upon inoculation in immunocompetent syngeneic hosts.3
CALR exposures as elicited by ionizing irradiation in vitro has been documented in mouse mammary adenocarcinoma TS/A cells (RT dose: 2–20 Gy),64 mouse colorectal carcinoma (CRC) CT26 cells (RT dose: 75 Gy),63 mouse melanoma B16 and S91 cells (RT dose: 5Gy),75 human osteosarcoma U2OS cells (RT dose: 5 Gy),75 human prostate cancer LNCaP cells (RT dose: 10 Gy),87 human triple negative breast cancer (TNBC) MD-MBA-231 cells (RT dose: 10 Gy),87 and human lung adenocarcinoma H522 cells (RT dose: 10 Gy).87 Importantly, the ability of irradiated CT26 cells to protect immunocompetent BALB/c (syngeneic) mice from the subsequent inoculation of living cells of the same type could be abrogated by the RNA interference (RNAi)-mediated knockdown of CALR, an effect that could be rescued with recombinant CALR absorption.63
Intriguingly, CALR exposure on the surface of irradiated LNCaP and MD-MBA-231 was accompanied not only by eIF2α phosphorylation as a marker of an ongoing ISR, but also by the upregulation of multiple components of the antigen-presenting machinery including CALR itself as well as low molecular mass protein 2 (LMP2), LMP7 and transporter 2, ATP binding cassette subfamily B member (TAP2), ultimately rendering cancer cells surviving irradiation more susceptible to lysis by CTLs.87,88 While technically more challenging, the ability of RT to drive CALR exposure has also been documented in vivo, both in LNCaP tumors established in immunodeficient mice upon the delivery of a single RT dose of 10 Gy,87 and in patients with metastatic renal cell carcinoma (RCC) receiving stereotactic body radiotherapy (SBRT) in a single fraction of 15 Gy.89
Intriguingly, CALR may also modulate the intrinsic radiosensitivity of cancer cells. Indeed, the transgene-enforced overexpression of CALR has been shown to sensitize radioresistant human glioblastoma U251MG and T98G to ionizing radiation, at least partially via a mechanism that involves reduced pro-survival signaling via AKT serine/threonine kinase 1 (AKT1) coupled with disruption of intracellular Ca2+ homeostasis.90–94
Taken together, these observations suggest that irradiated cancer cells may experience perturbations of reticular homeostasis coupled with the activation of an adaptive response culminating with the upregulation of CALR and its exposure to the cell surface in support of increased adjuvanticity. Moreover, CALR appears to promote intrinsic radiosensitivity, at least in some experimental settings.
ATP
While ATP exists intracellularly at concentrations of 1–10 μM, extracellular ATP levels in healthy tissues are extremely low, at least in part owing to the existence of plasma membrane-associated enzymes that catalyze the sequential conversion of ATP into the immunosuppressive molecule adenosine, including ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1, best known as CD39) and 5’-nucleotidase ecto (NT5E, best known as CD73).95–97 At odds with adenosine, extracellular ATP mediates potent chemotactic and immunostimulatory effects (which culminate with NLRP3 inflammasome activation and consequent IL1B and IL18 secretion) upon binding to purinergic receptor P2Y2 (P2RY2)98–100 and purinergic receptor P2X 7 (P2RX7),101 respectively, on the surface of APCs or their precursors. Importantly, the ICD-associated release of ATP in the extracellular microenvironment appears to require proficient pre-mortem autophagic responses, as these enable dying cells to preserve ATP stores which ultimately are released via a dual mechanism involving lysosomal exocytosis and pannexin 1 (PANX1) channels.26,102–104
In line with the ability of autophagy-dependent ATP release to support danger signaling in the context of ICD, CT26 cells overexpressing CD39 or depleted of key components of the molecular machinery for autophagy including autophagy related 5 (ATG5), ATG7, and beclin 1 (BECN1) fails to provide immunological protection to BALB/c mice when used as a prophylactic vaccine upon exposure to ICD-inducing chemotherapeutics.26,105 Similarly, ATG5-deficient CT26 tumors growing in immunocompetent BALB/c mice partially lose their ability to respond to ICD-inducing chemotherapeutics such as mitoxantrone,26 as well as to RT (delivered as a single dose of 8 Gy),106 two therapeutic approaches that been shown to trigger ATP release from various human and murine cancer cell lines in vitro and/or in vivo.26,64,87 Importantly, such a defect could be rescued by the concomitant administration of a CD39 inhibitor, suggesting it indeed reflected limited ATP release downstream of defective autophagy.106,107 Further corroborating the importance of this pathway for ICD as driven by RT, systemic autophagy activation by alternate-day feeding or caloric restriction,108–112 has been shown to considerably improve the ability of a single RT dose of 6–8 Gy to limit local disease progression and metastatic dissemination of mouse TNBC 4T1 and 67NR lesions established in immunocompetent BALB/c mice.113,114 Altogether, these findings suggest that autophagy activation may support ICD induction by RT.
However, it is important to note that autophagy mediates considerable cytoprotective effects on malignant cells, hence rendering them less sensitive to the cytostatic and cytotoxic activity of RT, as demonstrated in a multitude of in vitro experimental tumor models as well as in vivo, in immunodeficient mice bearing human or mouse malignant lesions.115 Moreover, proficient autophagic responses have been shown to: (1) limited oxidative stress and hence impair ICD-associated CALR exposure as driven by photodynamic therapy,116,117 (2) promote the lysosomal degradation of MHC Class I molecules by cancer cells, hence rendering them poorly visible by the adaptive immune system,118 and (3) inhibit type I interferon (IFN) by malignant cells undergoing ICD in response to RT (see below).74 In line with this notion, genetic signatures of proficient autophagy in diagnostic biopsies correlate with inhibited type I IFN and interferon gamma (IFNG) signaling as well as with poor disease outcomes in patients with breast cancer.74,119 That said, the vast majority of clinical trials testing lysosomal inhibitors such as chloroquine and hydroxychloroquine (which potently inhibit autophagy) along with standard-of-care (SOC) chemotherapy or RT failed to document a clinical benefit for combinatorial regimens over SOC only.115,120 While the reasons underlying these largely negative clinical observations remain to be fully elucidated, it is tempting to speculate that systemic autophagy inhibition may not represent an optimal therapeutic strategy in view of the fact that autophagy is required for the optimal function of many immune cell types, including (but not limited to) DCs, NK cells and CTLs.121
In summary, autophagy appears to influence the immunogenicity of cancer cells succumbing to RT (and other ICD inducers) in a context-dependent manner. The precise reasons underlying such apparently discrepant observations may relate to features of the tumor microenvironment (TME) potentially including baseline infiltration by specific immune cells and/or the expression levels of ATP receptors, extracellular ATP-degrading enzymes and other components of the type I IFN signaling machinery. Additional work is required to deconvolute the contribution of ATP secretion (which is generally promoted by autophagy) vs MHC Class I presentation and type I IFN signaling (which are inhibited by autophagy) in the immunogenicity of RT in specific oncological settings.
HMGB1
High mobility group box 1 (HMGB1) is a non-histone chromatin-binding protein that translocates first from the nucleus to the cytoplasm and then from the cytoplasm to the extracellular microenvironment in the context of multiple RCD instances, including ICD.122 Depending on oxidation status, extracellular HMGB1 can exert mostly chemotactic effects (fully reduced form), upon forming a complex with C-X-C motif chemokine ligand 12 (CXCL12) and binding to C-X-C motif chemokine receptor 4 (CXCR4), mostly immunostimulatory effects (partially oxidized form), upon binding to advanced glycosylation end-product specific receptor (AGER) or Toll-like receptor 4 (TLR4), or be virtually inactive or even tolerogenic (fully oxidized form).123–126 That said, the TLR4-dependent activation of MYD88 innate immune signal transduction adaptor (MYD88) appears to represent the most relevant signaling pathways elicited by HMGB1, ultimately resulting in DC maturation and increased antigen processing and cross-presentation to CTLs.125,127 Indeed, while HMGB1-driven AGER signaling has been implicated in DC activation,128 the perception of anthracycline-driven RCD as immunogenic is largely compromised in Tlr4−/− hosts.125 Moreover, pharmacological TLR4 activation with dendrophilin restores at least some degree of immune control against mouse CRCs and fibrosarcomas expressing low HMGB1 levels.129,130
Akin to CALR exposure and ATP secretion, HMGB1 release has been documented to occur in a dose-dependent when TS/A cells are exposed to ionizing radiation in vitro.64 Similar results have been obtained in human breast and prostate cancer cell lines exposed to a single RT dose of 10 Gy in vitro,87 as well as in a panel of mouse and human cancer cell lines subjected to carbon ion RT in a single dose of 5 Gy.75 Suggesting a relevance for this mechanism in the therapeutic activity of RT, CT26 CRCs as well as mammary TS/A lesions established subcutaneously in immunocompetent BALB/c mice have been shown to exhibit reduced sensitivity to a single RT dose of 10 Gy when developing in Tlr4−/− vs wild-type hosts.125 However, blocking extracellular HGMB1 with a neutralizing antibody failed to influence the control of mouse CRC MC38 lesions subcutaneously developing in immunocompetent syngeneic C57BL/6 mice as enabled by a single RT dose of 20 Gy.131 Similar results were obtained upon the establishment of MC38 tumors in Myd88−/− mice as well as in mice lacking the alternative TLR signal transducer TIR domain containing adaptor molecule 1 (TICAM1, best known as TRIF).131 Whether such an apparent discrepancy relates to tumor type, RT dose or other variables remains to be clarified.
Lending additional support to the clinical relevance of these findings, patients with breast cancer carrying a loss-of-function TLR4 allele experience inferior disease outcome on ICD-inducing chemotherapy or RT than individual carrying wild-type TLR4.125 Along similar lines, circulating HGMB1 levels have been linked with improved disease outcome and/or signs of ongoing anticancer immune responses in patients with breast cancer, rectal cancer, head and neck squamous cell carcinoma (HNSCC) and esophageal squamous cell carcinoma (ESCC) receiving RT alone and/or combined with chemotherapy.132–136 That said, circulating and/or intratumoral levels of HMGB1 have also been associated with poor disease outcome upon irradiation in a variety of clinical cohorts, including patients with bladder carcinoma,137 nasopharyngeal cancer,138 CRC,139 hepatocellular carcinoma,140 HNSCC,141 prostate carcinoma,142 and ESCC.143 In this latter setting, it was found that the RNAi-mediated depletion of HMGB1 increases the radiosensitivity of human ESCC cell lines, both in vitro and in vivo (upon establishment in immunodeficient hosts).143 At least partially, this may reflect the ability of HMGB1 to elicit radioprotective autophagic responses143,144
In conclusion, RT has been consistently shown to drive the relocation of HMGB1 from the nucleus to the cytoplasm and ultimately the extracellular space of cancer cells, a process that is required for RCD to be perceived as immunogenic but may also elicit cytoprotective autophagic responses that limit cell-intrinsic radiosensitivity.
Type I IFN
In human, type I IFN is encoded by a large family of homologous genes encompassing 13 genes coding for IFNα (IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17 and IFNA21), as well as individual genes coding for IFNβ (IFNB1), IFNɛ (IFNE), IFNк (IFNK) and IFNω (IFNW1).145 Besides playing a central role in antiviral immune immunity,146 type I IFN secretion is crucial for cancer cells succumbing to chemotherapy or RT to be perceived as immunogenic.147 In the former setting, type I IFN is initiated downstream of double-stranded RNA (dsRNA) sensing by TLR3, resulting in the abundant secretion of the T cell chemoattractant CXCL10 by cancer cells.148 In the latter setting instead, type I IFN responses appear to be largely mediated by cyclic GMP-AMP synthase (CGAS) and stimulator of interferon response cGAMP interactor 1 (STING1), upon recognition of micronuclear149–151 or mitochondrial DNA (mtDNA),74 both via cancer cell intrinsic mechanisms,74,149,150 or upon the uptake of dying cells or extracellular vesicles therefrom by cross-presenting basic leucine zipper ATF-like transcription factor 3 (BATF3)-dependent DCs.131,152–154
Regardless of source, type I IFN mediates potent immunostimulatory effects upon binding to ubiquitously expressed, generally heterodimeric receptors composed of interferon alpha and beta receptor subunit 1 (IFNAR1) and IFNAR2.155 In particular, type I IFN promotes DC cross-priming,156 boosts the cytotoxic functions of NK cells157–159 promotes the functional competence of naïve T cells,160 triggers the secretion of pro-inflammatory mediators by macrophages161,162 and inhibits the immunosuppressive functions of CD4+CD25+FOXP3+ regulatory T (TREG) cells.163,164
Supporting the clinical relevance of ICD-associated type I IFN signaling, a type I IFN-related transcriptional signature has been shown to predict disease outcome in breast cancer patients treated with neoadjuvant anthracycline-based chemotherapy.148 Similarly, single nucleotide polymorphisms (SNPs) in IFNAR1 have been associated with poor clinical outcome in patients with glioma receiving SOC temozolomide-based chemoradiation.165 That said, transcriptional signature of type I signaling signatures have also been correlated with resistance to chemotherapy and RT in patients with breast carcinoma166–168 and melanoma,169 potentially reflecting the ability of weak, indolent and non-resolving type I IFN responses, as opposed to their robust, acute and resolving counterparts,170,171 to promote cancer stemness and suppress anticancer immunity.155,172,173
Importantly, type I IFN secretion as elicited by RT is under negative control by a number of inducible mechanisms. Specifically, the RT-driven cytosolic accumulation of double-stranded DNA (dsDNA) is actively counteracted by autophagy, which actively disposes of permeabilized and hence mtDNA-spilling mitochondria,74 as well as by the dose-dependent upregulation of three prime repair exonuclease 1 (TREX1), which degrades dsDNA.174 Moreover, the rapid execution of apoptosis by caspase 9 (CASP9) and CASP3 also prevent mtDNA-driven type I IFN secretion in cancer cells by converting dying cells, which retain metabolic functions, into terminally inactive cell corpses.166,175 In line with this notion, various signatures of apoptotic proficiency have been correlated with poor disease outcome in patients with breast cancer.166 In the same setting, though, proficient type I IFN signaling was also linked to detrimental disease outcome,166 pointing to a type I IFN-independent impact of apoptotic defects on the survival of patients with breast cancer.
Taken together, these observations suggest that type I IFN production by irradiated malignant cells and/or tumor-infiltrating immune cells is crucial for the initiation of innate and adaptive anticancer immunity through RT-driven ICD. However, RT also elicits immunosuppressive pathways that need to be targeted to maximize its immunogenicity, as discussed below.
Strategies to boost RT-driven ICD
As amply discussed above, the immunogenicity of RT-driven cell death relies on antigenicity, adjuvanticity and microenvironmental features, all of which are dictated by dying cells as well as by their host.13 This implies that defects in any of these features at least a priori limit the ability of RT to elicit adaptive anticancer immunity via ICD. That said, cancer cells tend to express per se a number of neoantigens not covered by central tolerance, be them genetically encoded or emerging post-transcriptionally/post-translationally.176,177 Moreover, RT is known to boost MHC Class I exposure on cancer cells,178,179 promote the expression of genes encoding neoantigenic determinants,180 and aggravate stress conditions generally linked to the generation of posttranslational neoantigens, such as oxidative stress.181,182 Thus, the immunogenicity of RT-driven ICD is generally limited at the levels of cancer cell adjuvanticity and microenvironment. Accumulating preclinical evidence has defined a number of translationally relevant strategies to circumvent such defects and hence enable superior immune responses to RT (Figure 2).
Figure 2. Strategies to enhance immunogenic cell death induced by RT.
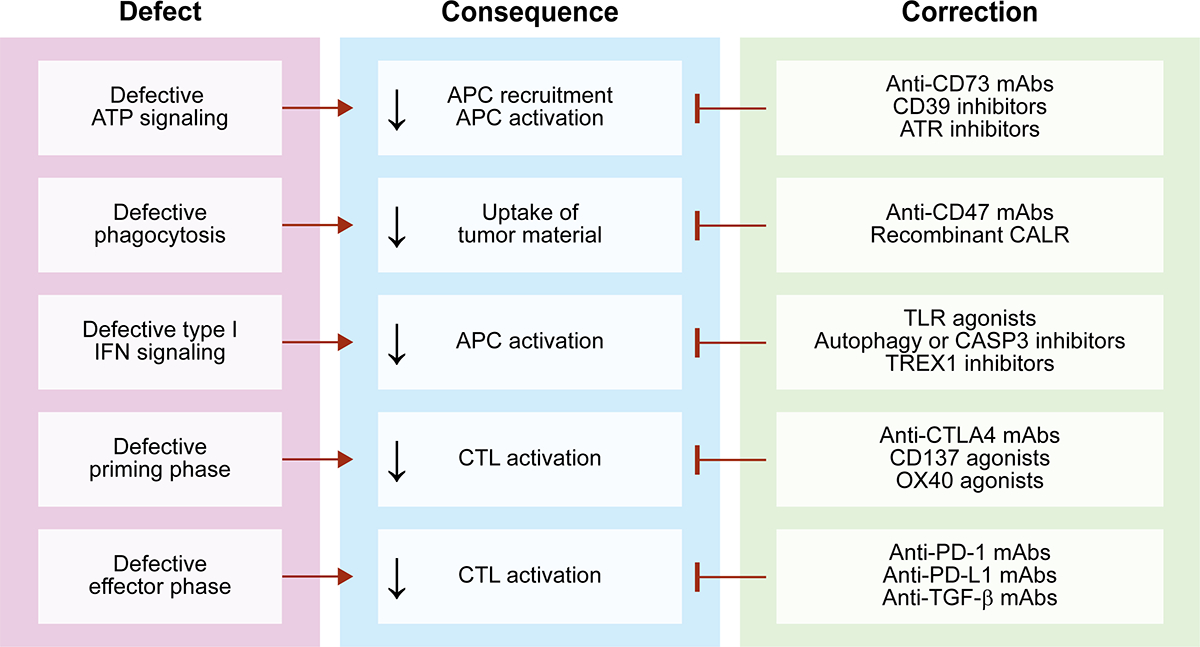
Depending on multiple variables, radiation therapy (RT) may kill cancer cells in the context of suboptimal immunostimulation, resulting in a variant of regulated cell death (RCD) with limited immunogenicity. A number of strategies have been investigated to circumvent these defects and restore superior immunogenic cell death (ICD)-driven adaptive immune responses against non-irradiated or radioresistant cancer cells. APC, antigen-presenting cell; ATR, ATR serine/threonine kinase; CD39 (official name: ENTPD1), ectonucleoside triphosphate diphosphohydrolase 1; CASP3, caspase 3; CD73 (official name: NT5E), 5’-nucleotidase ecto; CD137 (official name: TNFRSF9), tumor necrosis factor receptor superfamily, member 9; CTL, cytotoxic T lymphocyte; CTLA4, cytotoxic T lymphocyte-associated protein 4; mAb, monoclonal antibody; OX40 (official name: TNFRSF4), tumor necrosis factor receptor superfamily, member 4; PD-1 (official name: PDCD1), programmed cell death 1, PD-L1 (official name: CD274); TGF-β, transforming growth factor beta; TLR, Toll-like receptor; TREX1, three prime repair exonuclease 1.
For example, defective phagocytosis of irradiated cells has been efficiently targeted with monoclonal antibodies specific for CD47, which potently suppresses pro-phagocytic signals delivered by CD91.183–185 More specifically, CD47-targeting antibodies have been shown to synergize with RT at the induction of systemic anticancer immunity in mouse models of CRC (RT dose: 5 Gy × 3), an effect that could be potentiated by programmed cell death 1 (PDCD1, best known as PD-1) inhibitors,186,187 glioblastoma, along with an inhibitor of the PD-L1 ligand CD274 (best known as PD-L1),188,189 and small cell lung cancer.190
Poor extracellular ATP accumulation can be efficiently targeted with CD73-specific monoclonal antibodies, which have been shown to improve both the local and the abscopal efficacy of RT in immunocompetent mouse models of rectal cancer (RT dose: 4Gy × 3)191 and mammary adenocarcinoma (RT dose: 8Gy × 3),192 as well as with a CD39 inhibitor, which has been shown to improve the efficacy of RT in immunocompetent mouse models of CRC (RT dose: 8Gy x1).106 Along similar lines, superior ATP (and HMGB1) release after irradiation has been documented in human lung cancer and osteosarcoma cell lines exposed in vitro to a single RT dose of 5 Gy in the presence of ATR serine/threonine kinase (ATR) inhibitors, an effect that (for ATP only) was maximized by caspase inhibition.193 Moreover, short-course (but not prolonged) ATR inhibition has been shown to improve tumor-targeting immunity as elicited by 2 RT fractions of 2 Gy each in mouse immunocompetent models of CRC.194
Limited type I IFN signaling in response to RT has been efficiently restored with TLR3 agonists administered i.t. in wild-type C57BL/6 or BALB/c mice baring subcutaneous MC38, B16 or TS/A lesions (RT dose: 8Gy × 3),195 as well as with TLR9 agonists delivered i.t. in preclinical models of colorectal and lung cancer (RT dose: 12Gy × 3). Importantly, a similar therapeutic strategy has been assessed in patients with lymphoma who were allocated to a single RT fraction of 4 Gy in combination with an intratumorally administered TLR9 agonists, an approach that was safe, elicited systemic signs of anticancer immunity and was associated with at least some efficacy.196–198
While not immediately translatable to clinical settings (see above), both autophagy inhibitors and post-mitochondrial caspase blockers have also been shown to increase the ability of cancer cells to elicit systemic anticancer immunity upon irradiation (RT dose: 8Gy × 3) in immunocompetent mouse models of breast carcinoma.74,166,175 Along similar lines, TREX1 inhibition holds promise as a combinatorial partner for RT-driven RCD to exert maximal immunostimulatory effects,174 but to the best of our knowledge no pharmacological TREX1 inhibitors are currently available to formally address this possibility.
Importantly, a number of preclinical approaches have been successfully tested for their ability to restore microenvironmental conditions permissive for the perception of RT-driven RCD as immunogenic as well as for the execution of the consequence adaptive immune responses. These approaches include (but are not limited to): (1) monoclonal antibodies targeting transforming growth factor beta (TGF-β), as shown in mouse models of TNBC (RT dose: 6Gy × 5 or 8 Gy × 3),199–201 an effect that could be further potentiated with PD-1 blockers plus tumor necrosis factor receptor superfamily, member 9 (TNFRSF9, best known as CD137) agonists;202,203 (2) TNFRSF4 (best known as OX40) agonists, as demonstrated in BALB/c mice bearing syngeneic 4T1 cells (RT dose: 8Gy × 3), which could also be boosted by PD-1 blockade;204,205 as well as (3) conventional, FDA-approved ICIs targeting cytotoxic T lymphocyte-associated protein 4 (CTLA4) and PD-1 signaling, as demonstrated in a wide panel of immunocompetent tumor models.68,206–210 Importantly, while multiple randomized clinical trials combining RT with FDA-approved ICIs have been completed (and many others are ongoing), results have been disappointing in some instances, calling for the careful reconsideration of conventional RT approaches in support of improved cooperativity with ICIs.69,211
Despite these and other obstacles against the rapid implementation of preclinical findings into the clinical practice, multiple strategies that can be harnessed for restoring or reinforcing RT-driven ICD exist, including a large number of approaches with direct translational relevance.
Concluding remarks
In summary, RT is a potent inducer of ICD, an immunostimulatory cell death modality with clinical implications extending largely beyond radiation oncology.212 However, RT (especially when delivered according to standard fractional schedules and to conventional target volumes) can also elicit a number of immunosuppressive mechanisms that ultimately counteract ICD-driven immunostimulation.67,213,214 In line with this notion, while some randomized clinical trials testing RT in combination with FDA-approved ICIs documented a good cooperativity in the absence of unexpected side effects,215–218 many other randomized clinical studies failed to highlight superior therapeutic effects for RT/ICI combinations as compared to SOC RT-based therapeutic regimens.219–222 In this setting, it will be crucial not only to adapt conventional RT approaches to limit local and systemic immunosuppression, but also identify novel, therapeutically relevant targets to extend the intrinsic immunostimulatory effects of RT. Additional work is therefore required to fully harness the ability of RT to elicit ICD for the development of novel, safe and efficient therapeutic strategies against cancer.
Acknowledgements.
CG is supported by a fellowship from the American Italian Cancer Foundation (AICF; #223565-01). VK is supported by a grant from the Luxembourg National Research Fund (FNR) (PRIDE19/14254520/i2TRON). LG is/has been supported (as a PI unless otherwise indicated) by one R01 grant from the NIH/NCI (#CA271915), by two Breakthrough Level 2 grants from the US DoD BCRP (#BC180476P1; #BC210945), by a grant from the STARR Cancer Consortium (#I16-0064), by a Transformative Breast Cancer Consortium Grant from the US DoD BCRP (#W81XWH2120034, PI: Formenti), by a U54 grant from NIH/NCI (#CA274291, PI: Deasy, Formenti, Weichselbaum), by the 2019 Laura Ziskin Prize in Translational Research (#ZP-6177, PI: Formenti) from the Stand Up to Cancer (SU2C), by a Mantle Cell Lymphoma Research Initiative (MCL-RI, PI: Chen-Kiang) grant from the Leukemia and Lymphoma Society (LLS), by a Rapid Response Grant from the Functional Genomics Initiative (New York, US), by a pre-SPORE grant (PI: Demaria, Formenti) and a Clinical Trials Innovation Grant from the Sandra and Edward Meyer Cancer Center (New York, US); by startup funds from the Dept. of Radiation Oncology at Weill Cornell Medicine (New York, US), by industrial collaborations with Lytix Biopharma (Oslo, Norway), Promontory (New York, US) and Onxeo (Paris, France), as well as by donations from Promontory (New York, US), the Luke Heller TECPR2 Foundation (Boston, US), Sotio a.s. (Prague, Czech Republic), Lytix Biopharma (Oslo, Norway), Onxeo (Paris, France), Ricerchiamo (Brescia, Italy), and Noxopharm (Chatswood, Australia).
Competing interests.
LG is/has been holding research contracts with Lytix Biopharma, Promontory and Onxeo, has received consulting/advisory honoraria from Boehringer Ingelheim, AstraZeneca, OmniSEQ, Onxeo, The Longevity Labs, Inzen, Imvax, Sotio, Promontory, Noxopharm, EduCom, and the Luke Heller TECPR2 Foundation, and holds Promontory stock options.
References
- 1.Galluzzi L, Vitale I, Warren S, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yatim N, Cullen S, Albert ML. Dying cells actively regulate adaptive immune responses. Nat Rev Immunol. 2017;17(4):262–275. [DOI] [PubMed] [Google Scholar]
- 3.Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol. 2022;23(4):487–500. [DOI] [PubMed] [Google Scholar]
- 4.Yang K, Halima A, Chan TA. Antigen presentation in cancer - mechanisms and clinical implications for immunotherapy. Nat Rev Clin Oncol. 2023. [DOI] [PubMed] [Google Scholar]
- 5.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20(2):95–112. [DOI] [PubMed] [Google Scholar]
- 6.Harari A, Graciotti M, Bassani-Sternberg M, Kandalaft LE. Antitumour dendritic cell vaccination in a priming and boosting approach. Nat Rev Drug Discov. 2020;19(9):635–652. [DOI] [PubMed] [Google Scholar]
- 7.Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20(11):662–680. [DOI] [PubMed] [Google Scholar]
- 8.Galluzzi L, Chan TA, Kroemer G, Wolchok JD, López-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med. 2018;10(459). [DOI] [PubMed] [Google Scholar]
- 9.Demuytere J, Ernst S, van Ovost J, Cosyns S, Ceelen W. The tumor immune microenvironment in peritoneal carcinomatosis. Int Rev Cell Mol Biol. 2022;371:63–95. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira G, Wu CJ. Dynamics and specificities of T cells in cancer immunotherapy. Nat Rev Cancer. 2023;23(5):295–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Workenhe ST, Pol J, Kroemer G. Tumor-intrinsic determinants of immunogenic cell death modalities. Oncoimmunology. 2021;10(1):1893466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deutsch E, Chargari C, Galluzzi L, Kroemer G. Optimising efficacy and reducing toxicity of anticancer radioimmunotherapy. Lancet Oncol. 2019;20(8):e452–e463. [DOI] [PubMed] [Google Scholar]
- 13.Galluzzi L, Kepp O, Hett E, Kroemer G, Marincola FM. Immunogenic cell death in cancer: concept and therapeutic implications. J Transl Med. 2023;21(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs Y, Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol. 2015;16(6):329–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20(3):175–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vitale I, Pietrocola F, Guilbaud E, et al. Apoptotic cell death in disease-Current understanding of the NCCD 2023. Cell Death Differ. 2023;30(5):1097–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17(7):395–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. [DOI] [PubMed] [Google Scholar]
- 19.Panaretakis T, Kepp O, Brockmeier U, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. Embo j. 2009;28(5):578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humeau J, Sauvat A, Cerrato G, et al. Inhibition of transcription by dactinomycin reveals a new characteristic of immunogenic cell stress. EMBO Mol Med. 2020;12(5):e11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Cubillos-Ruiz JR. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat Rev Cancer. 2021;21(2):71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marciniak SJ, Chambers JE, Ron D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat Rev Drug Discov. 2022;21(2):115–140. [DOI] [PubMed] [Google Scholar]
- 23.Sriram G, Milling LE, Chen JK, et al. The injury response to DNA damage in live tumor cells promotes antitumor immunity. Sci Signal. 2021;14(705):eabc4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klapp V, Álvarez-Abril B, Leuzzi G, Kroemer G, Ciccia A, Galluzzi L. The DNA Damage Response and Inflammation in Cancer. Cancer Discov. 2023;13(7):1521–1545. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Pelaez M, Young L, Vazquez-Chantada M, et al. Targeting DNA damage response components induces enhanced STING-dependent type-I IFN response in ATM deficient cancer cells and drives dendritic cell activation. Oncoimmunology. 2022;11(1):2117321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michaud M, Martins I, Sukkurwala AQ, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334(6062):1573–1577. [DOI] [PubMed] [Google Scholar]
- 27.Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023;24(8):560–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia H, Green DR, Zou W. Autophagy in tumour immunity and therapy. Nat Rev Cancer. 2021;21(5):281–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.West AP, Khoury-Hanold W, Staron M, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520(7548):553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchi S, Guilbaud E, Tait SWG, Yamazaki T, Galluzzi L. Mitochondrial control of inflammation. Nat Rev Immunol. 2023;23(3):159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.West AP, Shadel GS. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol. 2017;17(6):363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McWhirter SM, Jefferies CA. Nucleic Acid Sensors as Therapeutic Targets for Human Disease. Immunity. 2020;53(1):78–97. [DOI] [PubMed] [Google Scholar]
- 33.Vanpouille-Box C, Hoffmann JA, Galluzzi L. Pharmacological modulation of nucleic acid sensors - therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2019;18(11):845–867. [DOI] [PubMed] [Google Scholar]
- 34.Barry ST, Gabrilovich DI, Sansom OJ, Campbell AD, Morton JP. Therapeutic targeting of tumour myeloid cells. Nat Rev Cancer. 2023;23(4):216–237. [DOI] [PubMed] [Google Scholar]
- 35.Boozari B, Mundt B, Woller N, et al. Antitumoural immunity by virus-mediated immunogenic apoptosis inhibits metastatic growth of hepatocellular carcinoma. Gut. 2010;59(10):1416–1426. [DOI] [PubMed] [Google Scholar]
- 36.Palanivelu L, Liu CH, Lin LT. Immunogenic cell death: The cornerstone of oncolytic viro-immunotherapy. Front Immunol. 2022;13:1038226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. [DOI] [PubMed] [Google Scholar]
- 39.Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17(12):725–741. [DOI] [PubMed] [Google Scholar]
- 40.Pozzi C, Cuomo A, Spadoni I, et al. The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat Med. 2016;22(6):624–631. [DOI] [PubMed] [Google Scholar]
- 41.Liu P, Zhao L, Pol J, et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun. 2019;10(1):1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrazzuolo A, Perez-Lanzon M, Liu P, Maiuri MC, Kroemer G. Crizotinib and ceritinib trigger immunogenic cell death via on-target effects. Oncoimmunology. 2021;10(1):1973197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrazzuolo A, Maiuri MC, Zitvogel L, Kroemer G, Kepp O. Trial Watch: combination of tyrosine kinase inhibitors (TKIs) and immunotherapy. Oncoimmunology. 2022;11(1):2077898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petroni G, Buqué A, Zitvogel L, Kroemer G, Galluzzi L. Immunomodulation by targeted anticancer agents. Cancer Cell. 2021;39(3):310–345. [DOI] [PubMed] [Google Scholar]
- 45.Petroni G, Buqué A, Coussens LM, Galluzzi L. Targeting oncogene and non-oncogene addiction to inflame the tumour microenvironment. Nat Rev Drug Discov. 2022;21(6):440–462. [DOI] [PubMed] [Google Scholar]
- 46.Zhou H, Forveille S, Sauvat A, et al. The oncolytic peptide LTX-315 triggers immunogenic cell death. Cell Death Dis. 2016;7(3):e2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie W, Mondragón L, Mauseth B, et al. Tumor lysis with LTX-401 creates anticancer immunity. Oncoimmunology. 2019;8(7):1594555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamazaki T, Wennerberg E, Hensler M, et al. LTX-315-enabled, radiotherapy-boosted immunotherapeutic control of breast cancer by NK cells. Oncoimmunology. 2021;10(1):1962592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vitale I, Yamazaki T, Wennerberg E, et al. Targeting Cancer Heterogeneity with Immune Responses Driven by Oncolytic Peptides. Trends Cancer. 2021;7(6):557–572. [DOI] [PubMed] [Google Scholar]
- 50.Fucikova J, Moserova I, Truxova I, et al. High hydrostatic pressure induces immunogenic cell death in human tumor cells. Int J Cancer. 2014;135(5):1165–1177. [DOI] [PubMed] [Google Scholar]
- 51.Moserova I, Truxova I, Garg AD, et al. Caspase-2 and oxidative stress underlie the immunogenic potential of high hydrostatic pressure-induced cancer cell death. Oncoimmunology. 2017;6(1):e1258505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ventura A, Vassall A, Robinson E, et al. Extracorporeal Photochemotherapy Drives Monocyte-to-Dendritic Cell Maturation to Induce Anticancer Immunity. Cancer Res. 2018;78(14):4045–4058. [DOI] [PubMed] [Google Scholar]
- 53.Garg AD, Vandenberk L, Koks C, et al. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci Transl Med. 2016;8(328):328ra327. [DOI] [PubMed] [Google Scholar]
- 54.Tatsuno K, Yamazaki T, Hanlon D, et al. Extracorporeal photochemotherapy induces bona fide immunogenic cell death. Cell Death Dis. 2019;10(8):578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garg AD, Krysko DV, Verfaillie T, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. Embo j. 2012;31(5):1062–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng Q, Yang J, Ji J, et al. PD-L1 blockade potentiates the antitumor effects of ALA-PDT and optimizes the tumor microenvironment in cutaneous squamous cell carcinoma. Oncoimmunology. 2022;11(1):2061396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kepp O, Kroemer G. A nanoparticle-based tour de force for enhancing immunogenic cell death elicited by photodynamic therapy. Oncoimmunology. 2022;11(1):2098658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skeate JG, Da Silva DM, Chavez-Juan E, Anand S, Nuccitelli R, Kast WM. Nano-Pulse Stimulation induces immunogenic cell death in human papillomavirus-transformed tumors and initiates an adaptive immune response. PLoS One. 2018;13(1):e0191311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moriya T, Hashimoto M, Matsushita H, et al. Near-infrared photoimmunotherapy induced tumor cell death enhances tumor dendritic cell migration. Cancer Immunol Immunother. 2022;71(12):3099–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogawa M, Tomita Y, Nakamura Y, et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget. 2017;8(6):10425–10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagaya T, Friedman J, Maruoka Y, et al. Host Immunity Following Near-Infrared Photoimmunotherapy Is Enhanced with PD-1 Checkpoint Blockade to Eradicate Established Antigenic Tumors. Cancer Immunol Res. 2019;7(3):401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flemming A Boosting cytotoxic T cells for immunotherapy. Nat Rev Immunol. 2022;22(11):655. [DOI] [PubMed] [Google Scholar]
- 63.Obeid M, Panaretakis T, Joza N, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14(10):1848–1850. [DOI] [PubMed] [Google Scholar]
- 64.Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez-Ruiz ME, Vitale I, Harrington KJ, Melero I, Galluzzi L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat Immunol. 2020;21(2):120–134. [DOI] [PubMed] [Google Scholar]
- 66.Petroni G, Cantley LC, Santambrogio L, Formenti SC, Galluzzi L. Radiotherapy as a tool to elicit clinically actionable signalling pathways in cancer. Nat Rev Clin Oncol. 2022;19(2):114–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McLaughlin M, Patin EC, Pedersen M, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20(4):203–217. [DOI] [PubMed] [Google Scholar]
- 68.Grassberger C, Ellsworth SG, Wilks MQ, Keane FK, Loeffler JS. Assessing the interactions between radiotherapy and antitumour immunity. Nat Rev Clin Oncol. 2019;16(12):729–745. [DOI] [PubMed] [Google Scholar]
- 69.Galluzzi L, Aryankalayil MJ, Coleman CN, Formenti SC. Emerging evidence for adapting radiotherapy to immunotherapy. Nat Rev Clin Oncol. 2023;20(8):543–557. [DOI] [PubMed] [Google Scholar]
- 70.De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AWM, Hegi-Johnson F. Radiotherapy toxicity. Nat Rev Dis Primers. 2019;5(1):13. [DOI] [PubMed] [Google Scholar]
- 71.Bates JE, Sanders T, Arnone A, Elmore SNC, Royce TJ. Geographic Density of Linear Accelerators and Receipt of Radiation Therapy for Prostate Cancer. International Journal of Radiation Oncology*Biology*Physics. 2021;111(3, Supplement):e351–e352. [Google Scholar]
- 72.Laurent PA, Morel D, Meziani L, Depil S, Deutsch E. Radiotherapy as a means to increase the efficacy of T-cell therapy in solid tumors. Oncoimmunology. 2023;12(1):2158013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamazaki T, Kirchmair A, Sato A, et al. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat Immunol. 2020;21(10):1160–1171. [DOI] [PubMed] [Google Scholar]
- 75.Zhou H, Tu C, Yang P, et al. Carbon ion radiotherapy triggers immunogenic cell death and sensitizes melanoma to anti-PD-1 therapy in mice. Oncoimmunology. 2022;11(1):2057892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol. 2018;39(8):644–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fucikova J, Spisek R, Kroemer G, Galluzzi L. Calreticulin and cancer. Cell Res. 2021;31(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pishesha N, Harmand TJ, Ploegh HL. A guide to antigen processing and presentation. Nat Rev Immunol. 2022;22(12):751–764. [DOI] [PubMed] [Google Scholar]
- 79.Gardai SJ, McPhillips KA, Frasch SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123(2):321–334. [DOI] [PubMed] [Google Scholar]
- 80.Zhao L, Zhang S, Kepp O, Kroemer G, Liu P. Dendritic cell transfer for cancer immunotherapy. Int Rev Cell Mol Biol. 2022;370:33–64. [DOI] [PubMed] [Google Scholar]
- 81.Boada-Romero E, Martinez J, Heckmann BL, Green DR. The clearance of dead cells by efferocytosis. Nat Rev Mol Cell Biol. 2020;21(7):398–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sen Santara S, Lee DJ, Crespo Â, et al. The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells. Nature. 2023;616(7956):348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fucikova J, Truxova I, Hensler M, et al. Calreticulin exposure by malignant blasts correlates with robust anticancer immunity and improved clinical outcome in AML patients. Blood. 2016;128(26):3113–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Truxova I, Kasikova L, Salek C, et al. Calreticulin exposure on malignant blasts correlates with improved natural killer cell-mediated cytotoxicity in acute myeloid leukemia patients. Haematologica. 2020;105(7):1868–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shide K Calreticulin mutations in myeloproliferative neoplasms. Int Rev Cell Mol Biol. 2021;365:179–226. [DOI] [PubMed] [Google Scholar]
- 86.Huntington ND, Cursons J, Rautela J. The cancer-natural killer cell immunity cycle. Nat Rev Cancer. 2020;20(8):437–454. [DOI] [PubMed] [Google Scholar]
- 87.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5(2):403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021;21(5):298–312. [DOI] [PubMed] [Google Scholar]
- 89.Singh AK, Winslow TB, Kermany MH, et al. A Pilot Study of Stereotactic Body Radiation Therapy Combined with Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma. Clin Cancer Res. 2017;23(17):5055–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okunaga T, Urata Y, Goto S, et al. Calreticulin, a molecular chaperone in the endoplasmic reticulum, modulates radiosensitivity of human glioblastoma U251MG cells. Cancer Res. 2006;66(17):8662–8671. [DOI] [PubMed] [Google Scholar]
- 91.Bustos G, Ahumada-Castro U, Silva-Pavez E, Puebla A, Lovy A, Cesar Cardenas J. The ER-mitochondria Ca(2+) signaling in cancer progression: Fueling the monster. Int Rev Cell Mol Biol. 2021;363:49–121. [DOI] [PubMed] [Google Scholar]
- 92.Groenendyk J, Agellon LB, Michalak M. Calcium signaling and endoplasmic reticulum stress. Int Rev Cell Mol Biol. 2021;363:1–20. [DOI] [PubMed] [Google Scholar]
- 93.Trebak M, Kinet JP. Calcium signalling in T cells. Nat Rev Immunol. 2019;19(3):154–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20(2):74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kepp O, Bezu L, Yamazaki T, et al. ATP and cancer immunosurveillance. Embo j. 2021;40(13):e108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer. 2018;18(10):601–618. [DOI] [PubMed] [Google Scholar]
- 97.Moesta AK, Li XY, Smyth MJ. Targeting CD39 in cancer. Nat Rev Immunol. 2020;20(12):739–755. [DOI] [PubMed] [Google Scholar]
- 98.Elliott MR, Chekeni FB, Trampont PC, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allard B, Allard D, Buisseret L, Stagg J. The adenosine pathway in immuno-oncology. Nat Rev Clin Oncol. 2020;17(10):611–629. [DOI] [PubMed] [Google Scholar]
- 100.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–1178. [DOI] [PubMed] [Google Scholar]
- 102.Martins I, Wang Y, Michaud M, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2014;21(1):79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Michaud M, Xie X, Bravo-San Pedro JM, Zitvogel L, White E, Kroemer G. An autophagy-dependent anticancer immune response determines the efficacy of melanoma chemotherapy. Oncoimmunology. 2014;3(7):e944047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martins I, Michaud M, Sukkurwala AQ, et al. Premortem autophagy determines the immunogenicity of chemotherapy-induced cancer cell death. Autophagy. 2012;8(3):413–415. [DOI] [PubMed] [Google Scholar]
- 105.Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21(6):360–378. [DOI] [PubMed] [Google Scholar]
- 106.Ko A, Kanehisa A, Martins I, et al. Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death Differ. 2014;21(1):92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mullen NJ, Singh PK. Nucleotide metabolism: a pan-cancer metabolic dependency. Nat Rev Cancer. 2023;23(5):275–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bravo-San Pedro JM, Pietrocola F. Fasting and cancer responses to therapy. Int Rev Cell Mol Biol. 2022;373:107–123. [DOI] [PubMed] [Google Scholar]
- 109.Hofer SJ, Kroemer G, Kepp O. Autophagy-inducing nutritional interventions in experimental and clinical oncology. Int Rev Cell Mol Biol. 2022;373:125–158. [DOI] [PubMed] [Google Scholar]
- 110.Di Tano M, Longo VD. Fasting and cancer: from yeast to mammals. Int Rev Cell Mol Biol. 2022;373:81–106. [DOI] [PubMed] [Google Scholar]
- 111.Krstic J, Schindlmaier K, Prokesch A. Combination strategies to target metabolic flexibility in cancer. Int Rev Cell Mol Biol. 2022;373:159–197. [DOI] [PubMed] [Google Scholar]
- 112.Taylor SR, Falcone JN, Cantley LC, Goncalves MD. Developing dietary interventions as therapy for cancer. Nat Rev Cancer. 2022;22(8):452–466. [DOI] [PubMed] [Google Scholar]
- 113.Saleh AD, Simone BA, Palazzo J, et al. Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle. 2013;12(12):1955–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simone BA, Dan T, Palagani A, et al. Caloric restriction coupled with radiation decreases metastatic burden in triple negative breast cancer. Cell Cycle. 2016;15(17):2265–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Galluzzi L, Bravo-San Pedro JM, Demaria S, Formenti SC, Kroemer G. Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. Nat Rev Clin Oncol. 2017;14(4):247–258. [DOI] [PubMed] [Google Scholar]
- 116.Garg AD, Dudek AM, Ferreira GB, et al. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy. 2013;9(9):1292–1307. [DOI] [PubMed] [Google Scholar]
- 117.Irvine DJ, Dane EL. Enhancing cancer immunotherapy with nanomedicine. Nat Rev Immunol. 2020;20(5):321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yamamoto K, Venida A, Yano J, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581(7806):100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gocher AM, Workman CJ, Vignali DAA. Interferon-γ: teammate or opponent in the tumour microenvironment? Nat Rev Immunol. 2022;22(3):158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bonam SR, Wang F, Muller S. Lysosomes as a therapeutic target. Nat Rev Drug Discov. 2019;18(12):923–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clarke AJ, Simon AK. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat Rev Immunol. 2019;19(3):170–183. [DOI] [PubMed] [Google Scholar]
- 122.Tang D, Kang R, Zeh HJ, Lotze MT. The multifunctional protein HMGB1: 50 years of discovery. Nat Rev Immunol. 2023. [DOI] [PubMed] [Google Scholar]
- 123.Venereau E, Casalgrandi M, Schiraldi M, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209(9):1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29(1):21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. [DOI] [PubMed] [Google Scholar]
- 126.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. [DOI] [PubMed] [Google Scholar]
- 127.Man SM, Jenkins BJ. Context-dependent functions of pattern recognition receptors in cancer. Nat Rev Cancer. 2022;22(7):397–413. [DOI] [PubMed] [Google Scholar]
- 128.Messmer D, Yang H, Telusma G, et al. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173(1):307–313. [DOI] [PubMed] [Google Scholar]
- 129.Yamazaki T, Hannani D, Poirier-Colame V, et al. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 2014;21(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lind NA, Rael VE, Pestal K, Liu B, Barton GM. Regulation of the nucleic acid-sensing Toll-like receptors. Nat Rev Immunol. 2022;22(4):224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Deng L, Liang H, Xu M, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41(5):843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bains SJ, Abrahamsson H, Flatmark K, et al. Immunogenic cell death by neoadjuvant oxaliplatin and radiation protects against metastatic failure in high-risk rectal cancer. Cancer Immunol Immunother. 2020;69(3):355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Exner R, Sachet M, Arnold T, et al. Prognostic value of HMGB1 in early breast cancer patients under neoadjuvant chemotherapy. Cancer Med. 2016;5(9):2350–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Suzuki Y, Mimura K, Yoshimoto Y, et al. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 2012;72(16):3967–3976. [DOI] [PubMed] [Google Scholar]
- 135.Clasen K, Welz S, Faltin H, Zips D, Eckert F. Dynamics of HMBG1 (High Mobility Group Box 1) during radiochemotherapy correlate with outcome of HNSCC patients. Strahlenther Onkol. 2022;198(2):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kroemer G, Kepp O. Radiochemotherapy-induced elevations of plasma HMGB1 levels predict therapeutic responses in cancer patients. Oncoimmunology. 2021;10(1):2005859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang GL, Zhang LH, Bo JJ, et al. Increased expression of HMGB1 is associated with poor prognosis in human bladder cancer. J Surg Oncol. 2012;106(1):57–61. [DOI] [PubMed] [Google Scholar]
- 138.Wu D, Ding Y, Wang S, Zhang Q, Liu L. Increased expression of high mobility group box 1 (HMGB1) is associated with progression and poor prognosis in human nasopharyngeal carcinoma. J Pathol. 2008;216(2):167–175. [DOI] [PubMed] [Google Scholar]
- 139.Yao X, Zhao G, Yang H, Hong X, Bie L, Liu G. Overexpression of high-mobility group box 1 correlates with tumor progression and poor prognosis in human colorectal carcinoma. J Cancer Res Clin Oncol. 2010;136(5):677–684. [DOI] [PubMed] [Google Scholar]
- 140.Liu F, Zhang Y, Peng Z, Gao H, Xu L, Chen M. High expression of high mobility group box 1 (hmgb1) predicts poor prognosis for hepatocellular carcinoma after curative hepatectomy. J Transl Med. 2012;10:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu Y, Xie C, Zhang X, et al. Elevated expression of HMGB1 in squamous-cell carcinoma of the head and neck and its clinical significance. Eur J Cancer. 2010;46(16):3007–3015. [DOI] [PubMed] [Google Scholar]
- 142.Zhao CB, Bao JM, Lu YJ, et al. Co-expression of RAGE and HMGB1 is associated with cancer progression and poor patient outcome of prostate cancer. Am J Cancer Res. 2014;4(4):369–377. [PMC free article] [PubMed] [Google Scholar]
- 143.Ma H, Zheng S, Zhang X, et al. High mobility group box 1 promotes radioresistance in esophageal squamous cell carcinoma cell lines by modulating autophagy. Cell Death Dis. 2019;10(2):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thorburn J, Horita H, Redzic J, Hansen K, Frankel AE, Thorburn A. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 2009;16(1):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hardy MP, Owczarek CM, Jermiin LS, Ejdebäck M, Hertzog PJ. Characterization of the type I interferon locus and identification of novel genes. Genomics. 2004;84(2):331–345. [DOI] [PubMed] [Google Scholar]
- 146.McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15(2):87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Borden EC. Interferons α and β in cancer: therapeutic opportunities from new insights. Nat Rev Drug Discov. 2019;18(3):219–234. [DOI] [PubMed] [Google Scholar]
- 148.Sistigu A, Yamazaki T, Vacchelli E, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20(11):1301–1309. [DOI] [PubMed] [Google Scholar]
- 149.Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548(7668):466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mackenzie KJ, Carroll P, Martin CA, et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature. 2017;548(7668):461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xiong H, Xi Y, Yuan Z, et al. IFN-γ activates the tumor cell-intrinsic STING pathway through the induction of DNA damage and cytosolic dsDNA formation. Oncoimmunology. 2022;11(1):2044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Diamond JM, Vanpouille-Box C, Spada S, et al. Exosomes Shuttle TREX1-Sensitive IFN-Stimulatory dsDNA from Irradiated Cancer Cells to DCs. Cancer Immunol Res. 2018;6(8):910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xu MM, Pu Y, Han D, et al. Dendritic Cells but Not Macrophages Sense Tumor Mitochondrial DNA for Cross-priming through Signal Regulatory Protein α Signaling. Immunity. 2017;47(2):363–373.e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71(7):2488–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Boukhaled GM, Harding S, Brooks DG. Opposing Roles of Type I Interferons in Cancer Immunity. Annu Rev Pathol. 2021;16:167–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Papewalis C, Jacobs B, Wuttke M, et al. IFN-alpha skews monocytes into CD56+-expressing dendritic cells with potent functional activities in vitro and in vivo. J Immunol. 2008;180(3):1462–1470. [DOI] [PubMed] [Google Scholar]
- 157.Oh JH, Kim MJ, Choi SJ, et al. Sustained Type I Interferon Reinforces NK Cell-Mediated Cancer Immunosurveillance during Chronic Virus Infection. Cancer Immunol Res. 2019;7(4):584–599. [DOI] [PubMed] [Google Scholar]
- 158.Guillot B, Portalès P, Thanh AD, et al. The expression of cytotoxic mediators is altered in mononuclear cells of patients with melanoma and increased by interferon-alpha treatment. Br J Dermatol. 2005;152(4):690–696. [DOI] [PubMed] [Google Scholar]
- 159.Björkström NK, Strunz B, Ljunggren HG. Natural killer cells in antiviral immunity. Nat Rev Immunol. 2022;22(2):112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jergović M, Coplen CP, Uhrlaub JL, et al. Infection-induced type I interferons critically modulate the homeostasis and function of CD8(+) naïve T cells. Nat Commun. 2021;12(1):5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang L, Tassiulas I, Park-Min KH, et al. ‘Tuning’ of type I interferon-induced Jak-STAT1 signaling by calcium-dependent kinases in macrophages. Nat Immunol. 2008;9(2):186–193. [DOI] [PubMed] [Google Scholar]
- 162.Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21(11):799–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gangaplara A, Martens C, Dahlstrom E, et al. Type I interferon signaling attenuates regulatory T cell function in viral infection and in the tumor microenvironment. PLoS Pathog. 2018;14(4):e1006985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lucca LE, Dominguez-Villar M. Modulation of regulatory T cell function and stability by co-inhibitory receptors. Nat Rev Immunol. 2020;20(11):680–693. [DOI] [PubMed] [Google Scholar]
- 165.Fujita M, Scheurer ME, Decker SA, et al. Role of type 1 IFNs in antiglioma immunosurveillance--using mouse studies to guide examination of novel prognostic markers in humans. Clin Cancer Res. 2010;16(13):3409–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rodriguez-Ruiz ME, Buqué A, Hensler M, et al. Apoptotic caspases inhibit abscopal responses to radiation and identify a new prognostic biomarker for breast cancer patients. Oncoimmunology. 2019;8(11):e1655964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Post AEM, Smid M, Nagelkerke A, et al. Interferon-Stimulated Genes Are Involved in Cross-resistance to Radiotherapy in Tamoxifen-Resistant Breast Cancer. Clin Cancer Res. 2018;24(14):3397–3408. [DOI] [PubMed] [Google Scholar]
- 168.Weichselbaum RR, Ishwaran H, Yoon T, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci U S A. 2008;105(47):18490–18495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Benci JL, Xu B, Qiu Y, et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016;167(6):1540–1554.e1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang X, Wang S, Zhu Y, et al. Double-edged effects of interferons on the regulation of cancer-immunity cycle. Oncoimmunology. 2021;10(1):1929005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vanpouille-Box C, Demaria S, Formenti SC, Galluzzi L. Cytosolic DNA Sensing in Organismal Tumor Control. Cancer Cell. 2018;34(3):361–378. [DOI] [PubMed] [Google Scholar]
- 172.Musella M, Guarracino A, Manduca N, et al. Type I IFNs promote cancer cell stemness by triggering the epigenetic regulator KDM1B. Nat Immunol. 2022;23(9):1379–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.De Martino M, Vanpouille-Box C. Type I interferon induces cancer stem cells-mediated chemotherapy resistance. Oncoimmunology. 2022;11(1):2127274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Han C, Liu Z, Zhang Y, et al. Tumor cells suppress radiation-induced immunity by hijacking caspase 9 signaling. Nat Immunol. 2020;21(5):546–554. [DOI] [PubMed] [Google Scholar]
- 176.Lang F, Schrörs B, Löwer M, Türeci Ö, Sahin U. Identification of neoantigens for individualized therapeutic cancer vaccines. Nat Rev Drug Discov. 2022;21(4):261–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bai P, Li Y, Zhou Q, et al. Immune-based mutation classification enables neoantigen prioritization and immune feature discovery in cancer immunotherapy. Oncoimmunology. 2021;10(1):1868130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Newcomb EW, Demaria S, Lukyanov Y, et al. The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clin Cancer Res. 2006;12(15):4730–4737. [DOI] [PubMed] [Google Scholar]
- 180.Lhuillier C, Rudqvist NP, Yamazaki T, et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J Clin Invest. 2021;131(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Clement CC, Osan J, Buque A, et al. PDIA3 epitope-driven immune autoreactivity contributes to hepatic damage in type 2 diabetes. Sci Immunol. 2022;7(74):eabl3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Clement CC, Nanaware PP, Yamazaki T, et al. Pleiotropic consequences of metabolic stress for the major histocompatibility complex class II molecule antigen processing and presentation machinery. Immunity. 2021;54(4):721–736.e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. 2019;19(10):568–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cao X, Lai SWT, Chen S, Wang S, Feng M. Targeting tumor-associated macrophages for cancer immunotherapy. Int Rev Cell Mol Biol. 2022;368:61–108. [DOI] [PubMed] [Google Scholar]
- 185.Nath PR, Pal-Nath D, Kaur S, et al. Loss of CD47 alters CD8+ T cell activation in vitro and immunodynamics in mice. Oncoimmunology. 2022;11(1):2111909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hsieh RC, Krishnan S, Wu RC, et al. ATR-mediated CD47 and PD-L1 up-regulation restricts radiotherapy-induced immune priming and abscopal responses in colorectal cancer. Sci Immunol. 2022;7(72):eabl9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kroemer G, Kepp O. Small cell lung cancer responds to immunogenic chemotherapy followed by PD-1 blockade. Oncoimmunology. 2021;10(1):1996686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang P, Rashidi A, Zhao J, et al. STING agonist-loaded, CD47/PD-L1-targeting nanoparticles potentiate antitumor immunity and radiotherapy for glioblastoma. Nat Commun. 2023;14(1):1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yamaguchi H, Hsu JM, Yang WH, Hung MC. Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat Rev Clin Oncol. 2022;19(5):287–305. [DOI] [PubMed] [Google Scholar]
- 190.Nishiga Y, Drainas AP, Baron M, et al. Radiotherapy in combination with CD47 blockade elicits a macrophage-mediated abscopal effect. Nat Cancer. 2022;3(11):1351–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tsukui H, Horie H, Koinuma K, et al. CD73 blockade enhances the local and abscopal effects of radiotherapy in a murine rectal cancer model. BMC Cancer. 2020;20(1):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wennerberg E, Spada S, Rudqvist NP, et al. CD73 Blockade Promotes Dendritic Cell Infiltration of Irradiated Tumors and Tumor Rejection. Cancer Immunol Res. 2020;8(4):465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Eek Mariampillai A, Hauge S, Kongsrud K, Syljuåsen RG. Immunogenic cell death after combined treatment with radiation and ATR inhibitors is dually regulated by apoptotic caspases. Front Immunol. 2023;14:1138920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vendetti FP, Pandya P, Clump DA, et al. The schedule of ATR inhibitor AZD6738 can potentiate or abolish antitumor immune responses to radiotherapy. JCI Insight. 2023;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rodriguez-Ruiz ME, Serrano-Mendioroz I, Garate-Soraluze E, et al. Intratumoral BO-112 in combination with radiotherapy synergizes to achieve CD8 T-cell-mediated local tumor control. J Immunother Cancer. 2023;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Frank MJ, Reagan PM, Bartlett NL, et al. In Situ Vaccination with a TLR9 Agonist and Local Low-Dose Radiation Induces Systemic Responses in Untreated Indolent Lymphoma. Cancer Discov. 2018;8(10):1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Brody JD, Ai WZ, Czerwinski DK, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28(28):4324–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jeon D, McNeel DG. Toll-like receptor agonist combinations augment mouse T-cell anti-tumor immunity via IL-12- and interferon ß-mediated suppression of immune checkpoint receptor expression. Oncoimmunology. 2022;11(1):2054758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vanpouille-Box C, Diamond JM, Pilones KA, et al. TGFβ Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res. 2015;75(11):2232–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.De Martino M, Daviaud C, Vanpouille-Box C. Activin A backs-up TGF-ß to promote regulatory T cells. Oncoimmunology. 2021;10(1):1883288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nixon BG, Gao S, Wang X, Li MO. TGFβ control of immune responses in cancer: a holistic immuno-oncology perspective. Nat Rev Immunol. 2023;23(6):346–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rodríguez-Ruiz ME, Rodríguez I, Mayorga L, et al. TGFβ Blockade Enhances Radiotherapy Abscopal Efficacy Effects in Combination with Anti-PD1 and Anti-CD137 Immunostimulatory Monoclonal Antibodies. Mol Cancer Ther. 2019;18(3):621–631. [DOI] [PubMed] [Google Scholar]
- 203.Lee KY, Wong HY, Zeng Q, et al. Ectopic CD137 expression by rhabdomyosarcoma provides selection advantages but allows immunotherapeutic targeting. Oncoimmunology. 2021;10(1):1877459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Han MG, Wee CW, Kang MH, Kim MJ, Jeon SH, Kim IA. Combination of OX40 Co-Stimulation, Radiotherapy, and PD-1 Inhibition in a Syngeneic Murine Triple-Negative Breast Cancer Model. Cancers (Basel). 2022;14(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sturgill ER, Rolig AS, Linch SN, et al. Galectin-3 inhibition with belapectin combined with anti-OX40 therapy reprograms the tumor microenvironment to favor anti-tumor immunity. Oncoimmunology. 2021;10(1):1892265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2 Pt 1):728–734. [PubMed] [Google Scholar]
- 208.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18(5):313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pointer KB, Pitroda SP, Weichselbaum RR. Radiotherapy and immunotherapy: open questions and future strategies. Trends Cancer. 2022;8(1):9–20. [DOI] [PubMed] [Google Scholar]
- 211.Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20(1):25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fucikova J, Moserova I, Urbanova L, et al. Prognostic and Predictive Value of DAMPs and DAMP-Associated Processes in Cancer. Front Immunol. 2015;6:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cytlak UM, Dyer DP, Honeychurch J, Williams KJ, Travis MA, Illidge TM. Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat Rev Immunol. 2022;22(2):124–138. [DOI] [PubMed] [Google Scholar]
- 214.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. [DOI] [PubMed] [Google Scholar]
- 216.Zhou Q, Chen M, Jiang O, et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022;23(2):209–219. [DOI] [PubMed] [Google Scholar]
- 217.Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med. 2021;384(13):1191–1203. [DOI] [PubMed] [Google Scholar]
- 218.Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24(12):1845–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lim M, Weller M, Idbaih A, et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro Oncol. 2022;24(11):1935–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Omuro A, Brandes AA, Carpentier AF, et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: An international randomized phase III trial. Neuro Oncol. 2023;25(1):123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lee NY, Ferris RL, Psyrri A, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22(4):450–462. [DOI] [PubMed] [Google Scholar]
- 222.Maity A, Mick R, Rengan R, et al. A stratified phase I dose escalation trial of hypofractionated radiotherapy followed by ipilimumab in metastatic melanoma: long-term follow-up and final outcomes. Oncoimmunology. 2021;10(1):1863631. [DOI] [PMC free article] [PubMed] [Google Scholar]


