Abstract
Purpose:
This study aims at the development and preliminary evaluation of dexamethasone nanomicelles for treating posterior uveitis. Nanomicelles were formulated using polyoxyl 40 stearate (P40S) and polysorbate 80 (P80), which are approved by the FDA for ocular use.
Methods:
Dexamethasone nanomicelles were prepared and characterized for critical micellar concentration, solubility of dexamethasone, particle size, surface charge, morphology, in vitro drug release, clarity, stability, filtration efficiency, and sterility. Ocular tolerance and the tissue drug distribution of dexamethasone were assessed in rabbits after single and multiple topical administration.
Results:
Dexamethasone nanomicelles (0.1% w/v) were successfully developed and characterized with an optimized composition of P40S/P80=7/3 by weight. The mean diameter of blank and drug-loaded nanomicelles was 13.3±0.4 and 14.5±0.4 nm, respectively. Transmission electron microscopy images revealed the spherical structure of nanomicelles. Nanomicelles were found to be stable with respect to clarity, size and drug content at 4°C and 25°C for up to 6 months. No irritation or redness was observed in the treated eyes as compared with the untreated control rabbit eyes. Therapeutic concentrations of dexamethasone were observed in the retina and choroid after single and multiple topical application in rabbits.
Conclusion:
In conclusion, the nanomicelles of P40S and P80 could efficiently solubilize 0.1% dexamethasone in their cores. The results also indicate that mixed nanomicelles could be utilized as a potential delivery system for delivering dexamethasone to treat the back of the eye diseases such as posterior uveitis after topical application.
Introduction
Uveitis, an autoimmune disease of the eye, is responsible for 10% of all blindness in the United States, with ∼30,000 new cases diagnosed each year. Uveitis patients are estimated to account for more than 5 million eye clinic visits per year, while the care of these patients in the United States is estimated to cost more than $117 million annually. Estimates also indicate that each year, 17.6% of patients with active uveitis experience transient or permanent loss of vision, 12.5% develop glaucoma, and 32% develop at least one complication during the course of a year. This inflammatory, Th1-mediated disease mainly occurs in the 20–50 year age group and can affect 1 or both eyes.1–3
In uveitis, damaging chronic inflammation can occur in the front of the eye (anterior uveitis), the back of the eye/retina (posterior uveitis or choroiditis), the ciliary body (intermediary uveitis or cyclitis), or all layers of uvea (panuveitis). Treatment of posterior and panuveitis is challenging, as the anatomical and physiological properties that effectively protect the eye hinder efficient absorption of drugs.4 Corticosteroids, nonsteroidal anti-inflammatory drugs, and immunosuppressants have shown beneficial effects in uveitis treatment.5 However, delivery of these drugs to the retina is challenging, and ophthalmologists are left with no options other than administering them locally via implants and intravitreal injections, which are highly invasive and are associated with side effects and patient noncompliance. Although intravitreal administration enables accumulation of high drug concentrations in the retina, the risk of drug precipitation (especially for lipophilic drugs), short vitreal half life of injection, and patient noncompliance deters this route of administration.6 Moreover, the chronic nature of uveitis requires multiple and frequent injections that carry the risk of vitreous hemorrhage, retinal detachment, and cataract progression.
Implants (eg, Retisert-a fluocinolone acetonide intravitreal implant) overcome many of the disadvantages associated with intravitreal injections; however, the surgical procedure and risk of drug precipitation may result in undesirable effects.7,8 The FDA has approved intravitreal dexamethasone implant Ozurdex™ (administered via intravitreal injection) for treating posterior uveitis. However, it is associated with side effects such as hyperemia, vitreous hemorrhage, pruritis, implant migration into the anterior chamber with rise in intraocular pressure, and corneal decompensation.8,9 Topical eye drops have the potential to reduce the side effects associated with intraocular implants and intravitreal injections, and to improve patients' quality of life and compliance.
The small molecules used in uveitis therapy are mostly lipophilic in nature with limited water solubility, thereby posing problems in formulating them as aqueous eye drops. To increase the retinal bioavailability of lipophilic drugs after topical application, 2 major issues should be addressed: (1) increasing the solubility of drugs in aqueous eye drops and (2) increasing the permeability of drugs through the conjunctiva and sclera. Recent research has focused on identifying new strategies to deliver small lipophilic molecules to various posterior segment tissues after topical administration. Cyclodextrins have gained some popularity in delivering lipophilic molecules to both anterior and posterior segment tissues.10,11 Dexamethasone/γcyclodextrin (γCD) microparticles have been shown to effectively deliver dexamethasone to the retina and vitreous humor after topical application.10 The same research group has also revealed an improvement in the visual acuity and a decrease in central macular thickness using dexamethasone/γCD eye drops in diabetic macular edema patients.12 Currently, a number of topical therapies, including ATG003, TG100801, difluprednate, OC-10X, and OT-551, are undergoing clinical trials for treating retinal diseases such as age-related macular degeneration.13
Lately, mixed nanomicelles have been used in the retinal delivery of lipophilic drugs after topical administration.14 Mixed nanomicelles enhance the solubility of drugs and increase the penetration across the conjunctival epithelium by temporarily altering the tight junctions.15 Though nanomicelles are known for 3 decades, their potential as vehicles for topical ocular drug delivery has been investigated only recently. A recent study demonstrated that topical application of a mixed nanomicellar solution of 0.1% w/v dexamethasone, prepared using D-alpha-tocopheryl polyethylene glycol 1000 succinate (vitamin E TPGS) and octoxynol-40, resulted in retinal drug concentration in rabbits.16 Velagaleti et al.17 developed a nanomicellar solution of vitamin E TPGS and octoxynol-40 for voclosporin, and ocular distribution studies after topical application in rabbits resulted in therapeutic concentrations of voclosporin in the retina and choroid. However, the mechanism by which nanomicelles deliver the drug to the retina after topical administration is not completely understood.
In the present study, we intend to develop and evaluate a mixed nanomicellar solution using polyoxyethylated amphiphilic compounds [polyoxyl 40 stearate (P40S) and polysorbate 80 (P80)] that are approved by the FDA for ocular use.18 Polyoxyethylated nonionic surfactants are known to be safe in ophthalmic use compared with their ionic counterparts. P40S and P80 have been previously used in commercial ophthalmic products and are generally regarded as nontoxic and nonirritant materials.18 P80 has been proved to be nonirritating to the rabbit eye till a concentration of 10%.19 To the best of our knowledge, mixed nanomicelles of P40S and P80 were not reported in the literature. The objective of this study was to develop and characterize dexamethasone (0.1% w/v) nanomicelles with potential for treating posterior uveitis following topical application.
Methods
Materials
Dexamethasone (Lot C137572) was procured from PCCA. P80 (Lot 20589) was procured from Fisher Scientific. Polyoxyl–40–stearate (Lot 109K0160V) was procured from Sigma Aldrich. Ethanol (Lot B0522876) was supplied by ACROS. Polyvinyl pyrrolidine-K-29/32 (PVP-K-29/32) (Lot 70804) and polyvinyl pyrrolidine-K-90 (PVP-K-90) (Lot 58-3) were procured from GAF. Tryptic soy broth (Soyabean Casein Digest medium-Bacto™, Lot 2030828) and Mueller-Hinton broth (Lot 3240477) were purchased from Fisher Scientific. High-performance liquid chromatography (HPLC) solvents, including acetonitrile (Lot 121151) and methanol (Lot 113904), were supplied by Fisher Scientific. Distilled deionized water was used in the preparation of nanomicellar eye drop solutions.
Animals
New Zealand White (NZW) albino adult male rabbits, weighing between 2.0 and 2.5 kg, were obtained from Robinson Services Incorporated. The animals were housed in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALACI) and U.S. Department of Agriculture (USDA). The animal protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at The University of Toledo (Toledo, OH). Studies were performed as per the Association for Research in Vision and Ophthalmology (ARVO) guidelines.
Preparation of 0.1% dexamethasone mixed nanomicelles
Mixed nanomicelles of dexamethasone were prepared by the rotary evaporation method with an optimized composition of P40S/P80=7/3 by weight. In brief, dexamethasone (10 mg) and P40S (0.42 g) were dissolved in 5 mL of ethanol. Ethanol was then evaporated under a vacuum at 50°C, leaving behind a thin film of dexamethasone and P40S. The film was rehydrated using 10 mL of water containing P80 (0.18 g). The final solution was passed through a 0.22 μm sterile Nylon membrane filter (Millex® Syringe Filter, Sterile, 0.22 μm).
HPLC analysis of dexamethasone
HPLC (Waters Alliance e2695 separation module), equipped with a 2998 PDA detector and reverse-phase C8 column (5 μm, 100 Å; Luna), was used to identify dexamethasone in nanomicelles. The solution was analyzed by an isocratic method with a mobile phase containing water and acetonitrile (50:50) pumped at a flow rate of 1.0 mL/min. The absorbance of dexamethasone was measured at a wavelength of 242 nm. A stock solution of 1 mg/mL of dexamethasone was prepared in methanol. Calibration standards ranging from 1 to 30 μg/mL were prepared in the mobile phase. Each calibration standard was analyzed in triplicate, and the average peak area was plotted against the concentration. The standard curve obtained was linear with an r2 value of 0.9975. The percentage recovery of dexamethasone ranged from 98.82% to 104.43%. The intra- and inter-assay precisions of dexamethasone were satisfactory; the relative standard deviations did not exceed 2%. In addition, forced degradation studies were carried out by exposing dexamethasone to different stress conditions, including heat (70°C), acid (0.1 N hydrochloric acid), base hydrolysis (0.1 N sodium hydroxide solution), oxidation (3.5% hydrogen peroxide solution), and irradiation with UV light (exposure to 365 nm) to ensure that the degradation products did not interfere with pure drug peak. A base line resolution of 1.5 was considered acceptable.20
Characterization of dexamethasone mixed nanomicelles
Critical micellar concentration determination
The critical micellar concentration (CMC) was determined by measuring surface tensions of P80, P40S, and P40S/P80 (7/3) mixture at varying concentrations.21 Dilutions of P80 ranging between 3.04 and 45.6 μM, P40S ranging between 5 and 80 μM, and mixed nanomicelles ranging between 5 and 60 μM were prepared and analyzed for surface tension using the capillary rise method. A small bore capillary was inserted into the liquid whose surface tension is to be determined. The surface tension of the liquid is directly proportional to the height to which the liquid rises in the capillary. The surface tension was measured using the equation given next.22
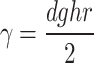 |
where γ is the surface tension, h is the height lifted liquid, g is the acceleration due to gravity, d is the density of the fluid, and r is the radius of the capillary.
Effect of total surfactant concentration on the solubility of dexamethasone
Mixed nanomicelles were prepared by varying the total concentration of P40S and P80 in the 1%–6% w/v solutions. The ratio of P40S/P80 (7/3) by weight is kept constant in all trials. Dexamethasone (10 mg) and a predetermined quantity of P40S were dissolved in 5 mL of ethanol. Ethanol was then evaporated under a vacuum at 50°C, leaving behind a thin film of dexamethasone and P40S. The thin film was rehydrated using 10 mL of water with a predetermined quantity of P80 under continuous sonication for 30 min. The final solution was passed through a 0.22 μm sterile Nylon membrane filter (Millex Syringe Filter, Sterile, 0.22 μm), and the dexamethasone content was analyzed using HPLC.
Determination of particle size and zeta potential
The particle size and zeta potential of blank and drug-loaded nanomicelles were determined by dynamic light scattering (DLS, NICOMP 380 ZLS; Particle Sizing Systems) technique. The sample was taken in a borosilicate glass disposable culture tube and placed in a sample chamber maintained at 23°C. The particle size measurements were done at a scattering angle of 90° with an avalanche photodiode array detector. For the zeta potential measurement, the sample was taken in a standard 1 cm square glass cuvette and placed in a sample chamber maintained at 23°C. The scattering angle was 14.1° with an electric field strength of 15 V/cm. The particle size and zeta potential were determined after filtering the sample through a 0.22 μm sterile Nylon membrane filter (Millex Syringe Filter, Sterile, 0.22 μm). Distilled deiozined water was meant for making dilutions for size and zeta potential measurements. The measurements were done in triplicate.
Transmission electron microscopy
The morphology of mixed nanomicelles was studied using transmission electron microscopy (TEM, HITACHI HD-2300 A, Ultra-thin Film Evaluation System; Hitachi High Technologies America). The TEM sample was prepared by placing a small drop of dexamethasone nanomicelles on a holey carbon 400-mesh copper grid (Ted Pella). Negative staining was done with a 2% phosphotungstic acid solution (Fisher Scientific). The copper grid was air dried overnight. The sample was analyzed, and images were captured using TEM.
Differential scanning calorimetry
Physical state and thermal properties of dexamethasone, blank nanomicelles, and dexamethasone nanomicelles were determined using a differential scanning calorimeter (DSC; 822e Mettler Toledo) equipped with a TS0800GCI gas flow system attached to a nitrogen gas cylinder. The samples (5–10 mg) were placed and sealed in aluminum crucibles using the Mettler MT 5 microbalance. DSC studies were performed at a heating rate of 10°C/min over a wide range (20°C–350°C) using a DSC 822e Mettler Toledo instrument (Mettler Toledo GmbH) fitted with a TSO801RO sample robot and a TSO800GCI Gas control attached to a nitrogen gas cylinder. Star-e software V8.10 was used to obtain the scans. Nitrogen gas was purged at a rate of 20 mL/min.
In vitro drug release study
In vitro release of dexamethasone from nanomicelles was performed using a dialysis bag (Fisherbrand® Dialysis tubing, MWCO: 12,000–14,000 Da). One milliliter of dexamethasone nanomicelles was placed in the dialysis bag and sealed. The dialysis bag was introduced into a vial containing 45 mL of isotonic phosphate buffer, pH 7.4, containing 0.025% (w/v) P80 to maintain sink conditions. The vials were placed in a shaker bath at 37°C±0.5°C and 60 oscillations/min. One milliliter of sample was withdrawn at predetermined time points and replaced with an equal volume of fresh buffer. The study was carried out in triplicate, and the samples were analyzed using HPLC.
Clarity test
The clarity of dexamethasone nanomicelles was measured in the presence of phosphate buffer and viscosity enhancers such as PVP-K-29-32 and PVP-K-90. Samples were prepared by mixing dexamethasone nanomicelles (2×) with an equal volume of phosphate buffer (2×), PVP-K-29-32 (1.8% w/v), and PVP-K-90 (1.2% w/v).23 The clarity of dexamethasone nanomicelles was examined visually using a black and white background and by measuring the absorbance at 400 nm using a UV-visible spectrophotometer (Agilent 8453, UV-Vis Spectroscopy System). Distilled deionized water was used as a blank.
Stability study
Dilution stability of dexamethasone nanomicelles in artificial and simulated tear fluids (STF): Dilution stability of mixed nanomicelles in artificial tears was determined using a UV-visible spectrophotometer in the presence of PVP-K-29-32 (1.8% w/v) and PVP-K-90 (1.2% w/v).23 Each sample was further mixed with different brands of artificial tears such as Refresh Tears® (Lubricant Eye Drops; Equate); Visine Tears® (Lubricant Eye Drops; Johnson & Johnson Healthcare Products); Artificial Tears® (Lubricant Eye Drops; Prestige Brands, Inc.); Gentle® (Lubricant Eye Drops; Equate); and STF in 1:1, 1:5, and 1:10 ratios, respectively. The measurements were taken under ambient conditions. The absorbance of each sample was recorded at 400 nm.
Stability of dexamethasone nanomicelles at various storage conditions: Dexamethasone nanomicelles were transferred into glass vials and then stored at 4°C and 25°C for 6 months. At regular time intervals during the storage period, samples were examined for clarity, changes in particle size, and drug content.
Filtration efficiency
Dexamethasone nanomicelles were filtered through 0.22 μm sterile membrane syringe filters made of polytetrafluoroethylene (PTFE), polyethersulfone (PES), and nylon membranes. The filtered solutions were analyzed for drug content using HPLC.
Sterility test
Sterility testing was carried out for dexamethasone nanomicelles sterilized via the filtration method. Validation of the sterilization method was performed using the direct and plate inoculation technique as per our published protocol.24 Trypsin soy broth (TSB) was used for the direct (tube) inoculation method. For the direct inoculation, a negative control vial, positive control vial, positive sample control vial, and aseptically filtered dexamethasone nanomicelles were prepared. The negative control vial contained 1 mL of sterile water and 9 mL of the uninoculated medium. Liquid culture of Staphylococcus aureus Rosenbach ATCC BAA 1692 was grown in TSB at 37°C for 24 h in a shaking water bath. The liquid culture was diluted to achieve a final concentration of 102 CFU/mL. The positive control vial contained 1 mL of water containing 102 CFU/mL and 9 mL of uninoculated medium. The positive sample control vial contained 1 mL of dexamethasone nanomicelles containing 102 CFU/mL and 9 mL of uninoculated medium. The aseptically filtered dexamethasone nanomicelles vial contained 1 mL of the dexamethasone nanomicelles that was aseptically passed through a 0.22 μm sterile nylon membrane filter (Millex Syringe Filter, Sterile, 0.22 μm) and 9 mL of the uninoculated medium. The vials were incubated at 37°C. For the plate inoculation, samples of 100 μL were withdrawn by the direct inoculation method from each of the vials on days 0, 7, and 14. These samples were transferred and uniformly spread on Mueller Hinton (MH) agar plates. The study was performed under aseptic conditions in a laminar air flow hood. The plates were then incubated at 37°C for 12 h and observed for bacterial growth.
Ocular irritation study after single administration of blank nanomicelles
The ocular irritation potential of blank nanomicelles (pH adjusted to 6.53 using 0.1 M NaOH) was carried out in 3 male NZW rabbits at the NAMSA facility (Toledo, OH). Before administration, all test and control eyes were judged clinically normal for rabbits by gross examination with an auxiliary light source. To detect any pre-existing corneal injury, the eyes were treated with a fluorescein stain, flushed with 0.9% sodium chloride United States Pharmacopeia (USP) solution, and observed with ultraviolet light in a dark room. A 0.1 mL dose was administered into the lower conjunctival sac of 1 eye of each rabbit, and the lid was gently held loose for 1 s. The other eye of each rabbit remained untreated and served as the comparative control. The animals were returned to their cages after treatment. At 24, 48, and 72 h after dosing, the test eye of each rabbit was examined with an auxiliary light source and appropriate magnification, compared with the untreated control eye, and graded for ocular irritation. To detect or confirm corneal injury, the test eye was treated with fluorescein stain, flushed with 0.9% sodium chloride USP solution, and examined in a dark room with an ultraviolet lamp at 24 h after treatment. Reactions were scored in accordance with the FHSA-modified Draize scoring criteria based on any alterations to the cornea (0–4), iris (0–2), and conjunctiva for redness (0–3) and the eyelids for chemosis (0–4).25
Ocular tissue distribution of dexamethasone after single and multiple administrations
Ocular tissue distribution of dexamethasone after a single topical administration of the final nanomicellar solution was carried out in 5 NZW rabbits. Dexamethasone nanomicellar solution for in vivo study was prepared by rehydrating the film (0.1% dexamethasone and 4.2% P40S) with 10 mL of isotonic phosphate containing 1.8% P80 and 0.02% benzalkonium chloride. The final nanomicellar solution was passed through a 0.22 μm sterile Nylon membrane filter. For single administration, the dexamethasone nanomicellar solution (100 μL of the final nanomicellar solution/eye) was administered in the left eye of 5 rabbits. The eye was gently closed for 30 s to prevent the loss of eye drop solution. The right eye was used as a control. After 60 min, rabbits were euthanized using 1 mL of intravenous injection of Beuthanasia® into the marginal ear vein.
Eyeballs were then enucleated immediately within a few seconds and cleaned with cold phosphate buffer to remove any drug adsorbed onto the surface. Aqueous humor was withdrawn by limbal paracentesis, and vitreous humor was aspirated using a 1 mL tuberculin syringe after making a tiny incision in the sclera–limbus junction. The eyeball was dissected and tissues such as the conjunctiva, cornea, iris-ciliary body, lens, retina-choroid, and sclera were collected, dried with Kim wipes®, and stored in preweighed vials. All tissue samples were stored at −80°C before further analysis. Ocular tissue concentrations of dexamethasone were analyzed by a validated LCMS/MS method described earlier from our lab.16,26 Triamcinolone acetonide (10.0 μg/mL) was used as internal standard. A Varian 320-MS LC/MS triple quadruple mass spectrometer was used for analysis. High-performance liquid chromatographic system consisted of Varian 212-LC Chromatography pump, Auto sampler (Varian 469-LC; Varian, Inc.) with a reversed-phase Betasil C18 column (50×4.6 mm i.d, 5 μm; Thermo Scientific) attached to a guard column (Phenomenex). An isocratic mobile phase composed of acetonitrile and 0.1% formic acid in water (40:60) was pumped at a rate of 0.3 mL/min. Dexamethasone was detected with proton adduct at m/z 393.1→355.1, in multiple reaction monitoring positive mode. The extraction recovery of dexamethasone was greater than 85%. The limits of detection and quantification of dexamethasone were found to be 0.78 and 3.125 ng/mL, respectively. The intra- and interday precision (measured by the coefficient of variation, CV%) was less than 3% and 5%, respectively.16,26
For multiple administration, the dexamethasone nanomicellar solution (100 μL of the final nanomicellar solution/eye) was administered twice per day for 7 days in the left eye of the rabbits (n=6). The eye was gently closed for 30 s to prevent the loss of eye drop solution.
The right eye was used as a control. A standard ocular examination was performed 30 min after each dose and scored based on the modified Draize eye test. Sixty minutes after the final dose, rabbits were euthanized and further processing was done as mentioned earlier.
Statistical analysis
All the data presented are expressed as mean and standard deviation (mean±SD) with the number of data replicates in each study. Two-tailed Student's t-test was used to assess the differences between 2 means, and a value of P<0.05 was considered significant.
Results
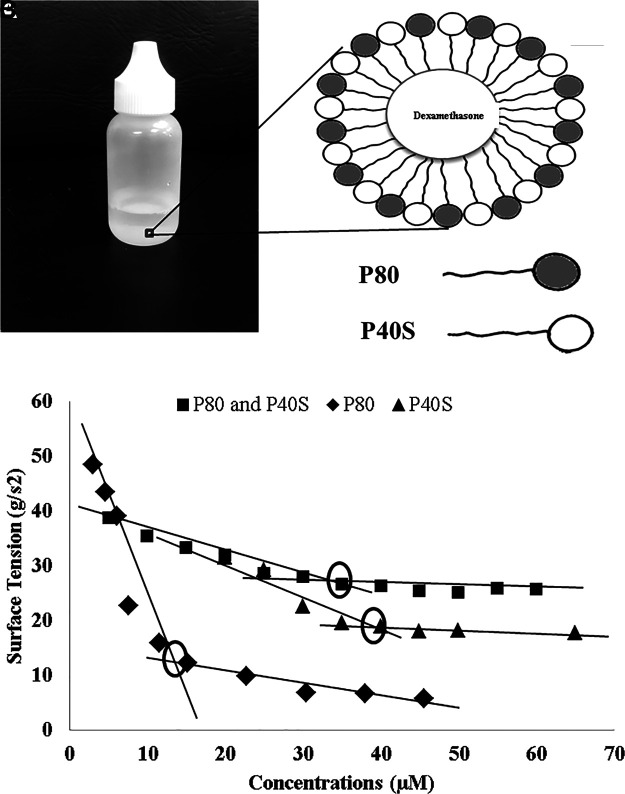
Preliminary studies were carried out to identify the ideal ratio of P40S/P80 for solubilizing 0.1% w/v dexamethasone. Various P40S/P80 ratios (1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9:1) were studied with an overall surfactant concentration of 6% w/v. The ratios of P40S/P80 from 6:4 to 9:1 could efficiently solubilize 0.1% w/v dexamethasone in their cores, while other ratios such as 1:9, 2:8, 3:7, 4:6, and 5:5 failed to solubilize the drug (Table 1). Based on particle size and Chi-square values, dexamethasone nanomicelles formulated with a composition of P40S/P80=7/3 by weight was considered for later studies (Fig. 1A, B). Dexamethasone, a poorly water soluble molecule (∼0.1 mg/mL), demonstrated at least 10 times higher solubility in nanomicelles of P80 and P40S. Mixed nanomicelles prepared by varying the total concentration of P40S and P80 from 1% to 5% w/v resulted in a turbid solution, indicating the incomplete solubility of dexamethasone. The solubility of dexamethasone increased with P40S and P80 concentrations. At a total surfactant concentration of 6% w/v, mixed nanomicelles were able to dissolve 0.1% w/v of dexamethasone. Moreover, 6% concentrations of individual surfactants were unable to produce a clear and stable eye drop formulation. The CMC of surfactants, P80, P40S, and P40S/P80 mixture was performed by measuring the change in surface tension. The CMC values of P80 and P40S were found to be 12 and 40 μM, respectively. The CMC value of P40S/P80 mixture was found to be 35 μM (Fig. 1C). The CMC value of P40S/P80 mixture (7/3) was an intermediate value between individual CMC values of the surfactants.27
Table 1.
Preliminary Trials to Assess the Solubility of 0.1% w/v Dexamethasone at Various P40S and P80 Ratios
| Ratio of P40S/P80 | Clarity | Particle size (nm) (mean±SD) | Chi square values |
|---|---|---|---|
| 1:9 | Hazy | — | — |
| 2:8 | Hazy | — | — |
| 3:7 | Hazy | — | — |
| 4:6 | Hazy | — | — |
| 5:5 | Hazy | — | — |
| 6:4 | Clear | 18.4±11.8 | 12.4 |
| 7:3a | Clear | 13.7±4.7 | 6.6 |
| 8:2 | Clear | 13.0±4.8 | 7.5 |
| 9:1 | Clear | 17.2±9.9 | 17.9 |
Clear solution indicates complete drug solubility in the mixed nanomicellar core. Hazy solution indicates incomplete drug solubility.
The composition of P40S/P80=7/3 by weight that was considered for later studies.
P40S, polyoxyl 40 stearate; P80, polysorbate 80; SD, standard deviation.
FIG. 1.
(A) Dexamethasone nanomicellar solution, (B) structure of a mixed nanomicelle, (C) critical micellar concentration of nonionic surfactants, polyoxyl 40 stearate (P40S), polysorbate 80 (P80), and mixture of P40S and P80 in 7:3 ratio.
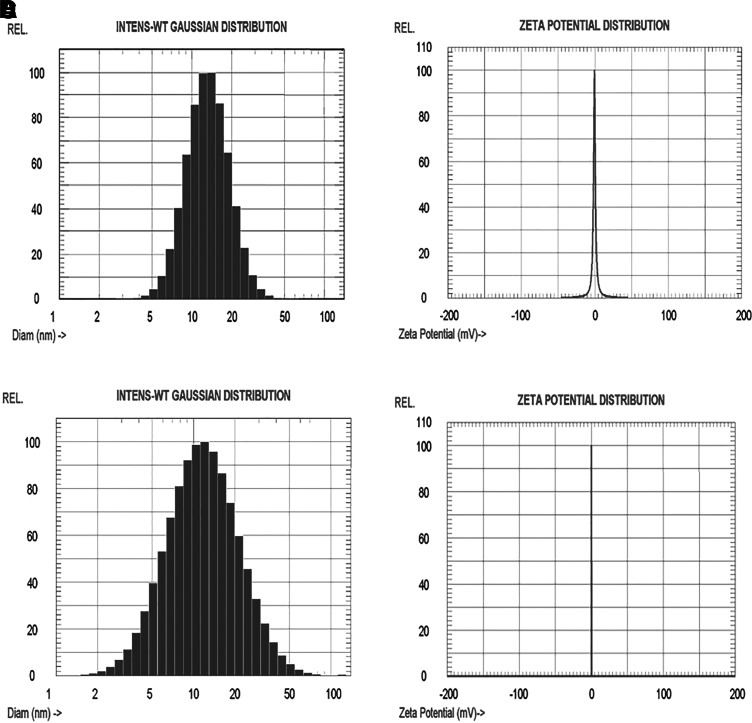
Dexamethasone nanomicelles were further characterized for morphology, size, surface charge, drug content, DSC, and in vitro release. Mixed nanomicelles were dispersed in a 2% (w/v) phosphotungstic acid negative stain, and the morphology was analyzed by TEM. The TEM image revealed the spherical shape of nanomicelles with a size ranging between 15 and 50 nm (Fig. 2). The particle size determination was carried out for blank and drug-loaded mixed nanomicelles. The mean diameters of blank and drug-loaded nanomicelles were 13.3±0.4 and 14.5±0.4 nm, respectively (Figs. 3A, 3C). The average zeta potential values of blank and dexamethasone nanomicelles were found to be −0.30 and 0.23 mV (Figs. 3B, 3D).
FIG. 2.
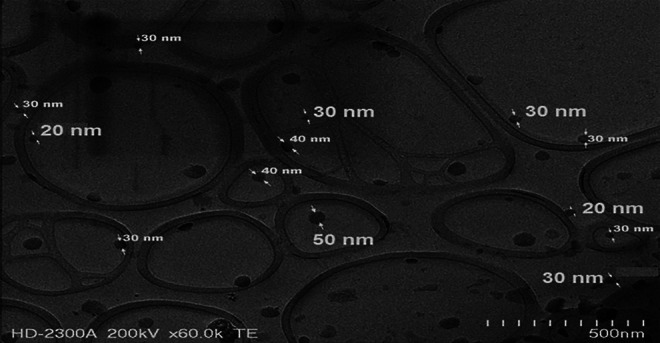
Transmission electron microscopy of dexamethasone nanomicelles.
FIG. 3.
Particle size and zeta potential curves, (A) particle size of blank nanomicelles; (B) zeta potential of blank nanomicelles; (C) particle size of drug-loaded nanomicelles; and (D) zeta potential of drug-loaded nanomicelles
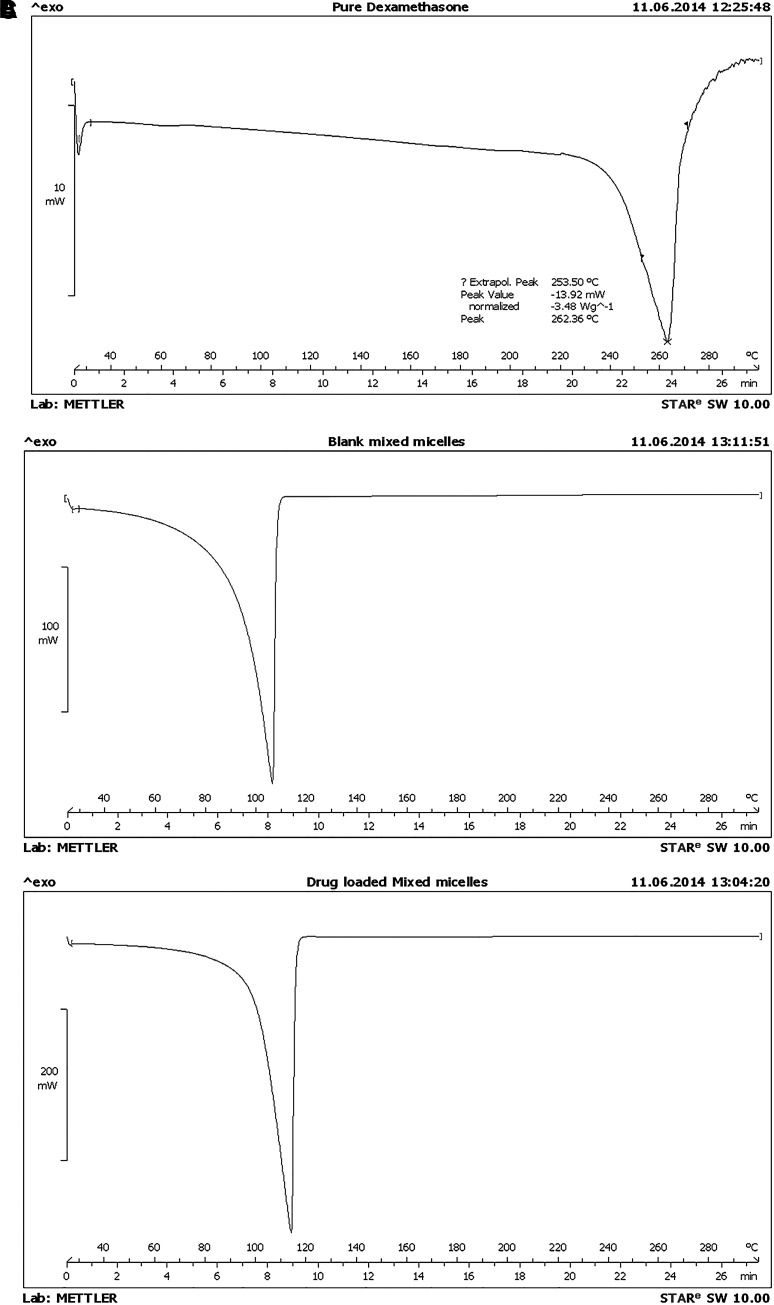
DSC was carried out for dexamethasone, blank nanomicelles, and drug-loaded nanomicelles (Fig. 4). Pure dexamethasone exhibited a characteristic sharp endothermic peak with an onset at 243°C and a peak temperature of 262°C, which corresponds to its melting point.28 Blank nanomicelles showed an endothermic peak with an onset at 90°C and a peak temperature of 100°C, which mostly corresponds to water. Dexamethasone nanomicelles showed an endothermic peak with an onset at 97°C and a peak temperature of 101°C. The characteristic sharp endothermic peak of dexamethasone was completely absent in dexamethasone nanomicelles. This indicated the absence of any un-dissolved dexamethasone in nanomicelles. A HPLC method for the analysis of dexamethasone was successfully established and validated. The retention time of dexamethasone was found to be 2.9 min.
FIG. 4.
Differential scanning calorimetry thermogram of (A) pure dexamethasone, (B) blank mixed nanomicelles, and (C) drug-loaded nanomicelles.
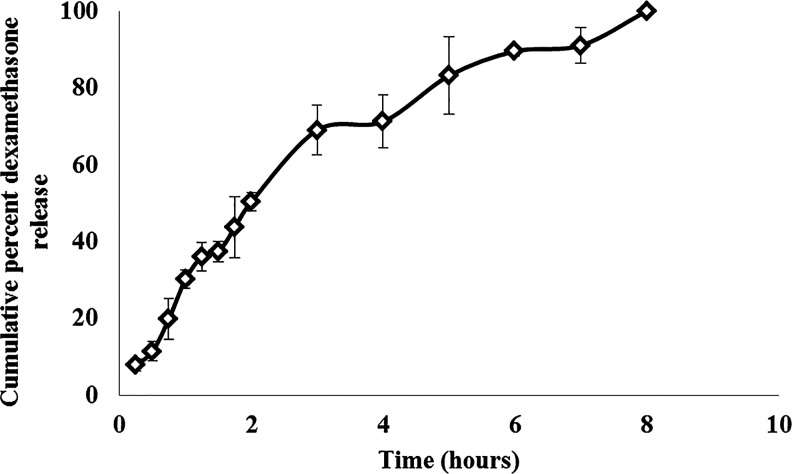
The in vitro release of dexamethasone from nanomicelles was investigated using a dialysis membrane. Sink condition was maintained by the addition of 0.025% w/v P80 in the release medium. Approximately, 80% of dexamethasone was released from nanomicelles within the first 3 h and the remaining drug was gradually released till 8 h (Fig. 5). The clarity of dexamethasone nanomicelles was measured in the presence of phosphate buffer and viscosity enhancers such as PVP-K-29-32 and PVP-K-90. The absorbance of mixed nanomicelles was found to be less than 0.1 at 400 nm (Table 2). This indicated the stability of nanomicelles in the presence of buffer and different grades of PVP, which are commonly used as excipients in eye drops. The effect of dilution on the stability of nanomicelles was studied in the presence of various artificial tears and STF. Dexamethasone nanomicelles were diluted 2×, 5×, and 10× times with various marketed artificial tear fluids and STF along with various grades of PVP (Table 3). No increase in turbidity or precipitation of dexamethasone was observed. This indicated the stability of dexamethasone nanomicelles in the presence of excess tear fluids.
FIG. 5.
In vitro drug release of dexamethasone from nanomicelles.
Table 2.
Clarity Test of Dexamethasone Nanomicelles
| Label and ingredients | Abs(400 nm) |
|---|---|
| Basic formulation (2×)+buffer mixture (2×)+PVP-K-29-32 | 0.0372 |
| Basic formulation (2×)+buffer mixture (2×)+PVP-K-90 | 0.0387 |
Abs, absorbance.
Table 3.
Artificial Tear Dilution Test of Dexamethasone Nanomicelles
| Formulation | Type of tear fluid | Dilution factor | Abs(400 nm) |
|---|---|---|---|
| PVP-K-30 | Refresh Tears® | 2× | 0.0307 |
| 5× | 0.0116 | ||
| 10× | 0.0321 | ||
| Visine Tears® | 2× | 0.0271 | |
| 5× | 0.0279 | ||
| 10× | 0.0257 | ||
| Artificial Tears® | 2× | 0.0387 | |
| 5× | 0.0339 | ||
| 10× | 0.0831 | ||
| Gentle® Lubricant Eye Drops | 2× | 0.0239 | |
| 5× | 0.0426 | ||
| 10× | 0.0609 | ||
| Simulated tear fluids | 2× | 0.0897 | |
| 5× | 0.0374 | ||
| 10× | 0.0726 | ||
| PVP-K-90 | Refresh Tears | 2× | 0.0803 |
| 5× | 0.0448 | ||
| 10× | 0.0424 | ||
| Visine Tears | 2× | 0.0329 | |
| 5× | 0.0671 | ||
| 10× | 0.0305 | ||
| Artificial Tears | 2× | 0.0354 | |
| 5× | 0.0395 | ||
| 10× | 0.0411 | ||
| Gentle Lubricant Eye Drops | 2× | 0.0249 | |
| 5× | 0.0386 | ||
| 10× | 0.0353 | ||
| Simulated tear fluids | 2× | 0.0753 | |
| 5× | 0.0347 | ||
| 10× | 0.0726 |
Dexamethasone nanomicelles were stored at 4°C and 25°C for 6 months. At regular time intervals, nanomicelles were evaluated for changes in physical characteristics such as clarity, particle size, and drug content. A stability indicating HPLC method was used to detect the drug concentrations at each time interval. No changes were observed in the clarity, particle size, and drug content till 6 months at 4°C and 25°C (Table 4). The vials that were turbid at 4°C turned clear at room temperature. No visible change was observed during the 6-month evaluation period in vials stored at 25°C. No precipitation of dexamethasone was observed during the 6-month period. This indicated the physical and chemical stability of dexamethasone nanomicelles at 4°C and 25°C for at least 6 months.
Table 4.
Stability of Dexamethasone Nanomicelles
| Temp. | 0 month | 1 month | 2 month | 4 month | 6 month |
|---|---|---|---|---|---|
| 4°C | |||||
| Visual clarity | Clear | Clear | Clear | Slightly turbid, clear at room temperature | Slightly turbid, clear at room temperature |
| Particle size (nm) | 12.9±6.8 | 7.2±2.9 | 11.4±4.6 | 11.9±5.4 | 10.9±4.7 |
| Drug content remaining (%) | 103.27±0.30 | 103.29±0.30 | 94.62±0.44 | 100.32±0.24 | 93.93±0.05 |
| 25°C | |||||
| Visual clarity | Clear | Clear | Clear | Clear | Clear |
| Particle size (nm) | 11.5±4.6 | 7.4±2.7 | 10.9±3.7 | 10.8±3.8 | 11.0±3.9 |
| Drug content remaining (%) | 97.95±0.04 | 97.95±0.04 | 95.87±1.92 | 103.69±0.14 | 98.33±3.11 |
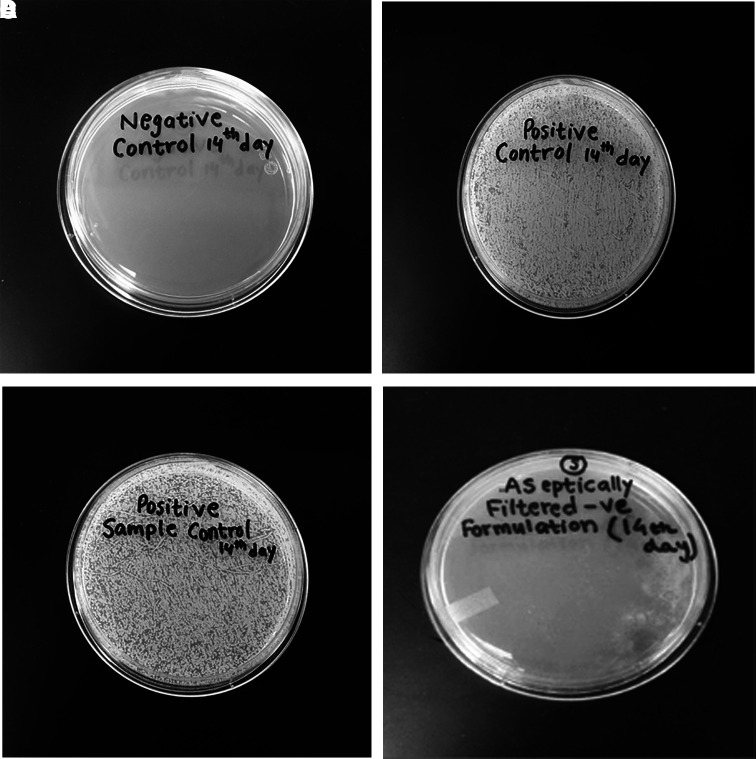
PTFE, PES, and nylon membranes were found to be acceptable for sterilization. The recovery of dexamethasone was found to be acceptable in filtered samples (Table 5). No loss of dexamethasone was observed during the filtration process. Further studies were carried out to test the sterility of the filtered sample. Based on USP guidelines, the sterility of filtered nanomicelles was analyzed using direct inoculation and plate inoculation techniques in a TSB broth. The nanomicelles were aseptically filtered through a 0.2 μm syringe filter. The vials were prepared and stored as per the method described. Except for the positive control and positive sample control vial, other vials for the direct inoculation method were clear throughout the 14-day test period. The samples were withdrawn from the test tubes on days 0, 7, and 14 and were transferred onto MH agar plates for the plate inoculation method. The plates were examined for microbial growth after 24 h of incubation at 35°C, and the presence or absence of microbial growth are shown in Table 6. The positive control test tubes showed turbidity and microbial growth. Plates with negative control sample and the aseptically filtered solution did not show any microbial growth throughout the 14-day test period (Fig. 6).
Table 5.
Filtration Efficiency Test of Dexamethasone Nanomicelles, Values Represented as Mean and Standard Deviation
| Membrane | Drug recovery (%) |
|---|---|
| Polytetrafluoroethylene membrane | 94.7±0.3 |
| Polyethersulfone membrane | 97.6±0.2 |
| Nylon membrane | 97.3±0.3 |
Table 6.
Sterility Validation Test Performed on Mueller Hinton Agar Plates Indicating the Presence (+) or Absence (-) of Microbial Growth on Days 0, 7, and 14
| Day | Negative control | Positive control | Positive sample control | Aseptically filtered solution |
|---|---|---|---|---|
| 0 | - | + | + | - |
| 7 | - | + | + | - |
| 14 | - | + | + | - |
FIG. 6.
Sterility validation test performed on Mueller Hinton Agar plates indicating no bacterial growth at 14 days. (A) One milliliter of sterile water and 9 mL of the uninoculated medium; (B) one milliliter of water containing 102 CFU/mL and 9 mL of uninoculated medium; (C) one milliliter of nanomicellar solution containing 102 CFU/mL and 9 mL of uninoculated medium; and (D) one milliliter of the dexamethasone nanomicellar solution that was aseptically passed through a 0.22 μm sterile nylon membrane.
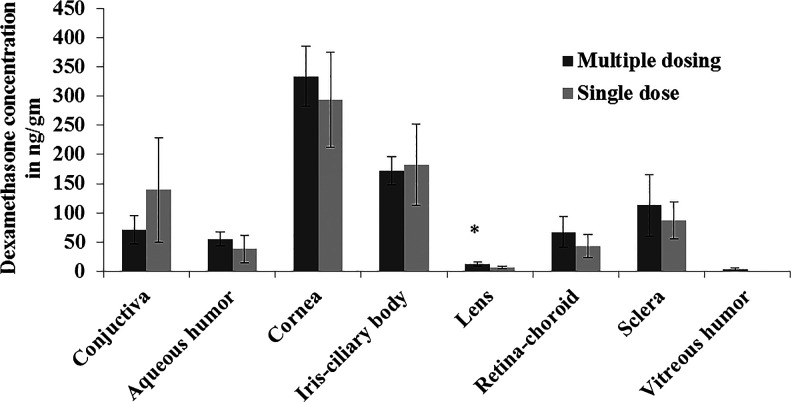
Both blank and dexamethasone-loaded nanomicelles were tested for ocular irritation in rabbits. We initially assessed the irritation of blank nanomicelles on 3 rabbits. One eye of each rabbit was treated with blank nanomicelles. This study was performed to examine any alterations to the cornea, iris, conjunctiva, and eyelids. The rabbit eyes were examined and graded for ocular irritation after 24, 48, and 72 h (Table 7). The treated eyes were compared with untreated eyes to determine any changes. No irritation or redness was observed in the treated eyes as compared with the untreated control rabbit eyes. After a single topical administration, tissue concentrations of dexamethasone (mean±SD, n=5) in the cornea, iris-ciliary body, conjunctiva, aqueous humor, lens, sclera, and retina-choroid were found to be 293.44±81.43, 181.85±69.48, 139.51±89.40, 38.13±23.40, 6.91±2.48, 86.94±31.70, and 43.27±20.21 ng/g of tissue, respectively (Fig. 7).
Table 7.
Ocular Irritation of Blank Nanomicelles in Rabbit Eyes, Values Are Expressed as Mean±Standard Deviation, n=3
| Items' scored | 24 h | 48 h | 72 h | Results |
|---|---|---|---|---|
| Cornea | 0 | 0 | 0 | (−) |
| Iris | 0 | 0 | 0 | |
| Rednessa | 0 | 0 | 0 | |
| Chemosisa | 0 | 0 | 0 | |
| Fluorescein exam | (−) | NA | NA |
Conjunctival tissue.
(−), negative; NA, not applicable.
FIG. 7.
Dexamethasone concentration in ocular tissues after single and multiple topical administrations of dexamethasone nanomicellar solution (n=5–6 animals). Error bars represent the standard deviation (SD), *P<0.05. Two eye drops were applied to the left eye, and the right eye was untreated.
Tissue concentrations of dexamethasone (mean±SD, n=6) in the cornea, iris-ciliary body, conjunctiva, aqueous humor, lens, sclera, retina-choroid, and vitreous humor were found to be 333.83±50.65, 172.15±24.57, 71.13±24.52, 55.13±11.70, 12.88±3.40, 112.75±53.09, 67.32±26.49, and 3.85±1.75 ng/g of tissue, respectively, after multiple administrations (Fig. 7). Dexamethasone appeared to partition into the lens and vitreous humor with repeated administration. Concentration of dexamethasone in lens after repeated administration was significantly higher compared with single administration (P<0.05). All animals were clinically normal throughout the study. No significant corneal lesions, opacity, conjunctival chemosis, redness, discharge, or iris alterations were observed in any of the rabbits. Representative digital images of the test and control eyes are shown in Figs. 8A and 8B. Even after repeated administration, no redness was observed in the treated eyes as compared with the untreated control rabbit eyes.
FIG. 8.
Rabbit eyes treated with (A) dexamethasone nanomicellar solution, and (B) control.
Discussion
Posterior uveitis therapy requires localized delivery of corticosteriods to the choroid layer, retina, and retinal vessels. The delivery of corticosteroids to the back of the eye tissues with minimal systemic exposure and high patient acceptability is a significant challenge. Delivery of steroids for treating posterior uveitis is currently achieved with periocular and intravitreal injections and implants. Despite several limitations, intravitreal injections and implants have gained popularity due to the lack of noninvasive topical methods for treating posterior segment eye diseases.10 Drug delivery to the back of the eye after topical administration still remains an unmet need of the scientific community.
In this study, we have developed and characterized dexamethasone nanomicelles using polyoxyethylated amphiphilic compounds P40S and P80 that are approved by US FDA for ocular use.18 A mixed nanomicellar system is made up of 2 amphiphilic molecules and has superior stability and solubilization potential compared with conventional nanomicelles. Nanomicelles have the potential to improve the solubility of lipophilic drugs and increase the ocular bioavailability by overcoming diffusion barriers.16 Mixed nanomicelles of P80 and P40S significantly increased the solublility of poorly soluble dexamethasone in water by 10 times. Solubility of dexamethasone increased with concentrations of P40S and P80. At a surfactant concentration of 6% w/v, the core of nanomicelles was sufficiently nonpolar compared with the bulk of medium, resulting in the partition of dexamethasone (0.1% w/v) into nanomicelles. The CMC value of P40S/P80 mixture (7/3 ratio) was an intermediate value between individual CMC values of the surfactants.27 This study indicates that P40S and P80 self-assemble into mixed nanomicelles above 35 μM, and such a low CMC value guarantees the formation of nanomicelles even on dilution in tears. TEM studies revealed the spherical shape of nanomicelles. No visible drug particles were observed in the TEM. The sizes of mixed nanomicelles usually range between 10 and 100 nm, and our results were in accordance with literature findings.29 The particle size of blank and drug-loaded mixed nanomicelles was small with a narrow distribution. The higher particle size of drug-loaded nanomicelles as compared with blank nanomicelles could be attributed to the incorporation of dexamethasone (mol. wt.: 392.46 g/mol) within the core of nanomicelles.30 The size of some droplets obtained using TEM was slightly larger than that determined by DLS. This could be attributed to the difference in the sample preparation techniques and experimental conditions in TEM and DLS experiments. In DLS, liquid sample is first placed in a cuvette and the cuvette is then placed in the path of a laser beam. The nanomicelles undergo Brownian motion and causes laser light to be scattered at different intensities. The particle size can be directly related to the variation in the intensity of the scattered light. While in TEM, a drop of nanomicelles is placed on a dimensionally constrained and flat TEM grid. As the water in the external phase evaporates after deposition, low surface tension facilitates spreading and the high surface-free energy of the nanodroplets leads to aggregation. This might result in a slight increase in the particle size with TEM. Similar findings were reported for nanoemulsions in our earlier publication.24
The surface charge of nanomicelles as determined by zeta potential values was close to neutral. The surface charge of nanomicelles depends on the nature of surfactants used in the preparation of nanomicelles. The low zeta potential value of nanomicelles could be attributed to the nonionic nature of P40S and P80, which reduces the absolute zeta potential value.18 The presence of dexamethasone inside nanomicelles nullified the half-width zeta potential distance and resulted in a straight peak at 0.23 mV. This behavior needs further investigation for detailed explanation. The DSC study was carried out to identify the nature of dexamethasone in nanomicelles. The complete disappearance of the melting point peak (243°C) in drug-loaded nanomicelles represents the absence of the free form of dexamethasone. This also indicates the presence of dexamethasone in an amorphous or molecular dispersed form inside the nanomicellar core. Crystalline compounds existing in a molecular dispersion and amorphous compounds do not exhibit sharp endothermic events.24
In vitro release study indicated that nearly 80% of dexamethasone was released from nanomicelles within the first few hours. This could be attributed to the short relaxation time (typically in the range of microseconds to seconds) of nanomicelles and rapid partitioning of dexamethasone in and out of micelles.18 No visual change was observed in nanomicelles on incubation with the release medium at 37°C, which indicated the stability of the drug-loaded nanomicelles in the presence of release medium.
Nanomicelles should also be stable and intact in the presence of formulation excipients such as viscosity enhancers and buffers, commonly used in eye drops. The optical clarity of dexamethasone nanomicelles in the presence of viscosity enhancers and buffers was studied using a UV spectrophotometer at 400 nm.22 At 400 nm, the UV absorbance of dexamethasone is close to zero or negligible. 400 nm was selected for optical clarity study. The absorbance values indicate that ∼93% of UV light has been transmitted through the solution. Destabilization of nanomicelles in the presence of formulation excipients might result in the precipitation of dexamethasone, which, in turn, scatters the UV light increasing absorbance values. This study indicated the stability of nanomicelles in the presence of buffer solution and different grades of PVP, which are commonly used excipients in eye drops.
The stability of nanomicelles was also studied in the presence of various artificial and simulated tear fluids. On topical administration, dexamethasone nanomicelles would be diluted by the tear fluids, which, in turn, might destabilize nanomicelles, resulting in precipitation of dexamethasone in the precorneal area. Precipation of drug in the precorneal area would adversely affect its absorption and ocular bioavilability. The results indicated no increase in turbidity on dilution (2×, 5×, and 10× times) of nanomicelles with artificial tear fluids and STF. This study indicated the stability of nanomicelles to tear fluid dilution with no signs of drug precipitation. Dexamethasone nanomicelles were found to be stable with respect to clarity, particle size, and drug content at 4°C and 25°C for up to 6 months. The drug content of nanomicelles dropped below 90% at higher storage temperature of 40°C (data not presented). This could be attributed to the degradation of P80 at higher temperatures. Further studies, including long-term stability of dexamethasone nanomicelles at 4°C and 25°C along with other formulation excipients used in eye drop formulations are warranted.
Sterility is an important requirement for ophthalmic dosage forms. In this study, dexamethasone nanomicelles were sterilized using sterile syringe filters and the filtration efficiency was studied. Dexamethasone nanomicelles were passed through 0.22 μm PTFE, PES, and nylon filter membranes and the drug content was measured using HPLC. Acceptable recovery of dexamethasone was found in filtered samples. The effectiveness of the filtration technique was validated using direct inoculation and plate inoculation techniques in a TSB broth.
The results obtained after days 0, 7, and 14 successfully validated the aseptic sterilization method used for dexamethasone nanomicelles.
A preliminary ocular irritation study was carried out on 3 rabbits after topical application of blank nanomicelles. The treated eyes were compared with the untreated eyes to determine any changes and redness of the eye. No signs of irritation or redness were observed in the cornea, iris, conjunctiva, and eyelids of treated eyes as compared with the untreated control rabbit eyes after 24, 48, and 72 h. Tissue distribution of dexamethasone was carried out in NZW albino adult male rabbits after single and multiple administrations. Dexamethasone was able to penetrate into the retina-choroid in a required concentration after both single and multiple administrations. For single administration, the rank order in terms of greatest to lowest concentration was cornea>iris-ciliary body>conjunctiva>sclera>retina-choroid>aqueous humor>lens. The lipophilic nature of dexamethasone might have restricted its penetration into the vitreous humor, resulting in vitreal concentrations below the limit of quantification.
Topical application of dexamethasone disodium phosphate (0.1%) is known to produce low dexamethasone concentrations in the posterior segment tissues and hence alternative routes such as subconjunctival injection, peribulbar injection, intravitreal injection, and oral administration are widely used in attaining therapeutic drug concentrations in the retina.31,32 However, with the nanomicellar approach, we were able to achieve therapeutically relevant dexamethasone concentrations in the retina and choroid after topical administration. Similar ocular tissue concentrations of dexamethasone were reported in the literature with 0.1% dexamethasone nanomicelles prepared using D-alpha-tocopheryl polyethylene glycol 1000 succinate (vitamin E TPGS) and octoxynol-40.16 Loftsson et al. studied dexamethasone concentrations in the rabbit retina and vitreous after topical administration of aqueous microparticulate formulation (dexamethasone/γCD) and compared it with an aqueous eye drop solution [dexamethasone/randomly methylated β-cyclodextrin (RMβCD)]. Two hours after a single application of dexamethasone/γCD eye drops, the mean±SD concentrations of dexamethasone in vitreous and retina were found to be 29±16, 57±22 ng·g−1, respectively, while vitreous and retina concentrations from dexamethasone/RMβCD were found to be 22.6±9 and 66±49 ng·g−1, respectively. In comparison with the published data, our results indicate that amphiphilic compounds such as PS40 and P80, approved by the US FDA for ocular use, could be a better choice to dissolve and deliver dexamethasone to the retina in therapeutically relevant concentrations.
For multiple administration, the rank order in terms of concentration was cornea>iris-ciliary body>sclera>conjunctiva>retina-choroid>aqueous humor>lens>vitreous humor, from greatest to lowest concentration. Dexamethasone concentration was observed in vitreous humor after multiple administrations, which indicated the slow partitioning of dexamethasone into the vitreous humor with time. Dexamethasone is rapidly cleared from the vitreous, with an estimated vitreal half life of 5.5 h in humans.33 This could be the reason for low dexamethasone concentration in the vitreous humor as compared with the choroid/retina. Dexamethasone concentrations in the retina and choroid are close to the estimated therapeutic dexamethasone concentrations obtained after subconjunctival injection.34 No signs of irritation or redness were observed in rabbit eyes even after repeated application (2 times daily for 7 days). The amount of dexamethasone in most ocular tissues, except for lens and vitreous humor, after single and multiple administrations was comparable. This was expected, since the elimination half life of dexamethasone is only a few hours and, thus, results in a rapid clearance of the drug without any significant accumulation in ocular tissues.33
In target tissues such as retina/choroid/vitreous, dexamethasone concentrations ranging from 10 to 4,000 ng/mL are required for effective treatment of various inflammatory conditions.6 It is certainly noteworthy that high concentrations of dexamethasone in the back of the eye tissues, that is, choroid and retina, were obtained after single and multiple topical administrations of nanomicelles. The favorable concentrations of dexamethasone in the posterior segment eye tissues support further clinical investigation and suggest that a topical daily dosing regimen may be efficacious for treating the back of the eye inflammations.
Conclusion
Despite some limitations, topical delivery remains attractive due to the ease of application. From a clinical and cost perspective, designing a delivery strategy that allows topical application of existing drug molecules would be a realistic alternative and would overcome the problems associated with posterior uveitis therapy. In this study, we demonstrated that amphiphilic molecules such as P40S and P80, when mixed in proper ratios, form nanomicelles that can efficiently solubilize hydrophobic drugs such as dexamethasone in their core. At a surfactant concentration of 6% w/v, mixed nanomicelles were able to dissolve 0.1% w/v of dexamethasone. No irritation or redness was observed in the treated eyes as compared with the untreated control rabbit eyes after single and repeated topical administration. Ocular distribution studies after topical application in rabbits revealed therapeutic concentrations of dexamethasone in the retina and choroid. This study provides preliminary evidence that nanomicelles could deliver dexamethasone in required concentrations to the choroid and retina after topical administration. The proposed topical therapy would enhance patient compliance and minimize the side-effects associated with intraocular implants and intravitreal injections in posterior uveitis therapy. Further long-term stability studies are necessary to ascertain the chemical stability of nanomicelles and in vivo studies are warranted to evaluate the complete pharmacokinetic profile of dexamethasone and safety nanomicelles.
Acknowledgment
This work was supported by the research start-up funds from The University of Toledo.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gritz D.C., and Wong I.G. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 111:491–500; discussion 500, 2004. [DOI] [PubMed] [Google Scholar]
- 2.de Boer J., Wulffraat N., and Rothova A. Visual loss in uveitis of childhood. Br. J. Ophthalmol. 87:879–884, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suhler E.B., Lloyd M.J., Choi D., Rosenbaum J.T., and Austin D.F. Incidence and prevalence of uveitis in Veterans Affairs Medical Centers of the Pacific Northwest. Am. J. Ophthalmol. 146:890–896.e8, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Janoria K.G., Gunda S., Boddu S.H., and Mitra A.K. Novel approaches to retinal drug delivery. Expert Opin. Drug Deliv. 4:371–388, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Boddu S.H.S. Polymeric nanoparticles for ophthalmic drug delivery: an update on research and patenting activity. Recent Patents Nanomed. 2:96–112, 2012. [Google Scholar]
- 6.Boddu S.H., Jwala J., Vaishya R., et al. . Novel nanoparticulate gel formulations of steroids for the treatment of macular edema. J. Ocul. Pharmacol. Ther. 26:37–48, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arcinue C.A., Cerón O.M., and Foster C.S. A comparison between the fluocinolone acetonide (Retisert) and dexamethasone (Ozurdex) intravitreal implants in uveitis. J. Ocul. Pharmacol. Ther. 29:501–507, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Kompella U.B., and Edelhauser H.F. Drug Product Development for the Back of the Eye. AAPS Advances in the Pharmaceutical Sciences Series. Boston: American Association of Pharmaceutical Scientists, Springer; 2011. [Google Scholar]
- 9.Mateo C., Alkabes M., and Burés-Jelstrup A. Scleral fixation of dexamethasone intravitreal implant (OZURDEX®) in a case of angle-supported lens implantation. Int. Ophthalmol. 34:661–665, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Loftsson T., Hreinsdóttir D., and Stefansson E. Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: aqueous dexamethasone eye drops. J. Pharm. Pharmacol. 59:629–635, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Loftsson T., Sigurđsson H., Konráđsdóttir F., Gisladottir S., Jansook P., and Stefansson E. Topical drug delivery to the posterior segment of the eye: anatomical and physiological considerations. Pharmazie. 63:171–179, 2008. [PubMed] [Google Scholar]
- 12.Loftsson T., Sigurdsson H.H., Hreinsdottir D., Konrádsdóttir F., and Stefánsson E. Dexamethasone delivery to posterior segment of the eye. J. Incl. Phenom. Macrocycl. Chem. 57:585–589, 2007. [Google Scholar]
- 13.Ni Z., and Hui P. Emerging pharmacologic therapies for wet age-related macular degeneration. Ophthalmologica. 223:401–410, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Vadlapudi A.D., and Mitra A.K. Nanomicelles: an emerging platform for drug delivery to the eye. Ther. Deliv. 4:1–3, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trivedi R., and Kompella U.B. Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine (Lond). 5:485–505, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earla R., Boddu S.H., Cholkar K., Hariharan S., Jwala J., and Mitra A.K. Development and validation of a fast and sensitive bioanalytical method for the quantitative determination of glucocorticoids—quantitative measurement of dexamethasone in rabbit ocular matrices by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 52:525–533, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velagaleti P., Anglade E., Khan J., Gilger B., and Mitra A. Topical delivery of hydrophobic drugs using a novel mixed nanomicellar technology to treat diseases of the anterior & posterior segments of the eye. Drug Deliv. Technol. 10:42–47, 2010. [Google Scholar]
- 18.Jiao J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv. Drug Deliv. Rev. 60:1663–1673, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Hazleton L.W. Relation of surface active properties of irritation of the rabbit eye. Proc. Sci. Sect. Toilet Goods Assoc. 17:1–5, 1952. [Google Scholar]
- 20.Boddu S., Bonam S.P., Wei Y., and Alexander K. Preparation and in vitro evaluation of a pluronic lecithin organogel containing ricinoleic acid for transdermal delivery. Int. J. Pharm. Compd. 18:256–261, 2013. [PubMed] [Google Scholar]
- 21.Bondarenko A.I., Malli R., and Graier W.F. The GPR55 agonist lysophosphatidylinositol directly activates intermediate-conductance Ca2+− activated K+ channels. Pflugers Arch. 462:245–255, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prajapati S.T., Joshi H.A., and Patel C.N. Preparation and characterization of self-microemulsifying drug delivery system of olmesartan medoxomil for bioavailability improvement. J. Pharm. 2013:Article ID 728425, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitra A. K., Velagaleti P. R., and Natesan S.: Ophthalmic compositions comprising calcineurin inhibitors or mTOR inhibitors. U.S. Patent 8,435,544. 2013.
- 24.Nesamony J., Kalra A., Majrad M.S., et al. . Development and characterization of nanostructured mists with potential for actively targeting poorly water-soluble compounds into the lungs. Pharm. Res. 30:2625–2639, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Wilhelmus K.R. The draize eye test. Surv. Ophthalmol. 45:493–515, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Boddu S.H., Gupta H., and Bonam S.P. Preclinical evaluation of a ricinoleic acid poloxamer gel system for transdermal eyelid delivery. Int. J. Pharm. 470:158–161, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Li L., and Tan Y.B. Preparation and properties of mixed micelles made of Pluronic polymer and PEG-PE. J. Colloid Interface Sci. 317:326–331, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Gómez-Gaete C., Tsapis N., Besnard M., Bochot A., and Fattal E. Encapsulation of dexamethasone into biodegradable polymeric nanoparticles. Int. J. Pharm. 331:153–159, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Gan L., Han S., Shen J., et al. . Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: improving preocular retention and ocular bioavailability. Int. J. Pharm. 396:179–187, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Song H., Geng H., Ruan J., et al. . Development of Polysorbate 80/Phospholipid mixed micellar formation for docetaxel and assessment of its in vivo distribution in animal models. Nanoscale Res. Lett. 6:1–12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weijtens O., Schoemaker R.C., Romijn F.P.H.T.M., Cohen A.F., Lentjes E.G.W.M., and van Meurs J.C. Intraocular penetration and systemic absorption after topical application of dexamethasone disodium phosphate. Ophthalmology. 109:1887–1891, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Kompella U.B., Amrite A.C., Pacha Ravi R., and Durazo S.A. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog. Retin. Eye Res. 36:172–198, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang-Lin J.-E., Attar M., Acheampong A.A., et al. . Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest. Ophthalmol. Vis. Sci. 52:80–86, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Weijtens O., Feron E.J., Schoemaker R.C., et al. . High concentration of dexamethasone in aqueous and vitreous after subconjunctival injection. Am. J. Ophthalmol. 128:192–197, 1999. [DOI] [PubMed] [Google Scholar]