Abstract
Purpose
In patients with heatstroke, disseminated intravascular coagulation (DIC) is associated with greater risk of in-hospital mortality. However, time-consuming assays or a complex diagnostic system may delay immediate treatment. Therefore, the present study proposes a new heatstroke-induced coagulopathy (HIC) score in patients with heat illness as an early warning indicator for DIC.
Methods
This retrospective study enrolled patients with heat illness in 24 Chinese hospitals from March 2021 to May 2022. Patients under 18 years old, with a congenital clotting disorder or liver disease, or using anticoagulants were excluded. Data were collected on demographic characteristics, routine blood tests, conventional coagulation assays and biochemical indexes. The risk factors related to coagulation function in heatstroke were identified by regression analysis, and used to construct a scoring system for HIC. The data of patients who met the diagnostic criteria for HIC and International Society on Thrombosis and Haemostasis defined-DIC were analyzed. All statistical analyses were performed using SPSS 26.0.
Results
The final analysis included 302 patients with heat illness, of whom 131 (43.4%) suffered from heatstroke, including 7 death (5.3%). Core temperature (OR = 1.681, 95% CI 1.291 − 2.189, p < 0.001), prothrombin time (OR = 1.427, 95% CI 1.175 − 1.733, p < 0.001) and D-dimer (OR = 1.242, 95% CI 1.049 − 1.471, p = 0.012) were independent risk factors for heatstroke, and therefore used to construct an HIC scoring system because of their close relation with abnormal coagulation. A total score ≥ 3 indicated HIC, and HIC scores correlated with the score for International Society of Thrombosis and Hemostasis -DIC (r = 0.8848, p < 0.001). The incidence of HIC (27.5%) was higher than that of DIC (11.2%) in all of 131 heatstroke patients. Meanwhile, the mortality rate of HIC (19.4%) was lower than that of DIC (46.7%). When HIC developed into DIC, parameters of coagulation dysfunction changed significantly: platelet count decreased, D-dimer level rose, and prothrombin time and activated partial thromboplastin time prolonged (p < 0.05).
Conclusions
The newly proposed HIC score may provide a valuable tool for early detection of HIC and prompt initiation of treatment.
Keywords: Heat illness, Heatstroke, Coagulation disorders, Diagnosis, Disseminated intravascular coagulation
1. Introduction
With the gradual rise of global temperature, heatstroke is quickly becoming a major international public health problem.1,2 In the United States, heatstroke contributes to annual increases in hospital admission and all-cause mortality.3,4 Heatstroke is a potentially fatal disease caused by the imbalance of heat production and dissipation in the body, which can be characterized by an uncontrolled increase in body temperature and rapidly progressing multiple organ dysfunction.5 Patients with heatstroke may experience high fever, coma, mucosal hemorrhage, jaundice, anuria, cyanosis, and even shock.6
Many patients with heatstroke experience heatstroke-induced coagulopathy (HIC): about 35% suffer complications with thrombocytopenia7, and another 21% may experience disseminated intravascular coagulation (DIC)8. In heatstroke, vascular endothelial cells and blood brain barrier are damaged9, which can lead to thrombin hyperactivation, excessive consumption of coagulation factors, platelets destruction and in turn cause HIC10. HIC can progress to DIC as a result of fibrinogen depletion and coagulation failure, remarkably increasing risk of death.11,12 DIC scoring systems proposed by the International Society of Thrombosis and Hemostasis (ISTH)13 or Japanese Association for Acute Medicine (JAAM)14 were preferred to be as diagnostic tools for DIC in heatstroke patients, which might delay immediate diagnosis because of time-consuming and complex diagnostic methods. This highlights the need to detect HIC as early as possible and initiate therapeutic measures.15
Unfortunately, no standardized diagnostic methods exist for HIC because of its nonspecific clinical manifestations and the lack of specific laboratory indicators.16 Consequently, the present multicenter study aimed to develop a HIC score based on blood coagulation and biochemical data as an early warning indicator for DIC.
2. Methods
2.1. Study design and patients
This multicenter study retrospectively recruited 355 patients with symptoms of heat illness due to overtraining, who were admitted to the emergency rooms or intensive care units of 24 general hospitals of Chinese People's Liberation Army from March 2021 to May 2022. This study was carried out in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of all participating hospitals. All patients had signed written consent for their anonymized medical data to be analyzed and published for research purposes.
Patients were diagnosed with heat illness or heatstroke according to the American College of Sports Medicine Expert Consensus Statement on Exertional Heat Illness17 and the Bouchama's heatstroke criteria18. Heat illness was defined as evidence of damage or dysfunction of organs caused by hyperthermia, without any central nervous system (CNS) changes. Heatstroke was defined by meeting the following criteria: (1) medical history of exposure to high temperature, high humidity or high-intensity exercise; (2) core temperature over 40 °C; (3) CNS-related changes, including coma, convulsions, delirium, or abnormal behavior.
Patients were excluded from our study if they were younger than 18 years old, they had a congenital coagulation disorder or chronic disease of the liver, or they were using anticoagulant drugs at the time of enrollment.
2.2. Data collection
Demographic and clinical data were collected from electronic medical records, including age, sex, heart rate and core temperature (rectal temperature). Data were also collected on laboratory indicators before liquid infusion, including counts of white blood cells and platelets; hemoglobin range and hematocrit percentage; levels of alanine aminotransferase, aspartate aminotransferase, total bilirubin, creatinine, and creatine kinase; as well as numerous coagulation indicators, including prothrombin time (PT), international normalized ratio, activated partial thromboplastin time (APTT), thrombin time, fibrinogen levels, D-dimer and fibrin degradation products.
2.3. Statistical analysis
Patients were classified as having heatstroke or not. On the basis of new HIC scoring method developed in this study, patients in the heatstroke group were further divided into those without HIC or with HIC. Furthermore, patients in the HIC group were further divided into those without DIC or with DIC. According to the ISTH criteria, a diagnosis of DIC can be made if a patient has a total score ≥ 5, which is calculated by summing the points for the following 4 parameters: platelet count, PT, D-dimer and fibrinogen level.13
Continuous data with a normal distribution were expressed as mean ± standard deviation, while data with a skewed distribution were expressed as median (Q1, Q3). Categorical data were expressed as counts and percentages. Pairwise comparisons between groups were conducted using Student's t-test in the case of continuous variables showing normal distribution and uniform variance; otherwise, the Mann-Whitney U test was used. Fisher's exact test was used when at least 2 theoretical frequencies were ≥ 1 and < 5, or when the theoretical frequencies were < 1. Pairwise comparisons of count data were conducted using the Chi-squared test, for which significance was defined as p < 0.017.
Comparisons among more than 2 groups were conducted using one-way ANOVA in the case of normally distributed continuous data. Specifically, the least significance difference-test method was used for data showing homogeneous variance; otherwise, Tamhane's T2 method was used. In the case of skewed data, comparisons were conducted using nonparametric Kruskal-Wallis single-factor ANOVA (K-sample). Count data were compared among 3 or more groups using the Chi-squared test, for which α′ was adjusted according to the formula
| α' = 2α / K (K-1) = α / 3 = 0.0167 |
where K referred to the number of groups.
Risk factors of heatstroke were identified using univariate regression. After confirming multicollinearity with linear regression, multivariate analysis was performed using forward stepwise regression. Results were expressed as odds ratios (ORs) and corresponding 95% confidence intervals (CIs). The ability of core temperature, PT and D-dimer levels to diagnose heatstroke was assessed in terms of the area under receiver operating characteristic curves (AUC), based on optimal cut-off values determined from the Youden index. Correlations between parameters were analyzed using Spearman correlation analysis.
All statistical analyses were performed using SPSS 26.0 for Windows (IBM, Chicago, IL, USA), and all analyses were two-sided. Significance was defined as p < 0.05 unless otherwise noted.
3. Results
3.1. Baseline characteristics of patients
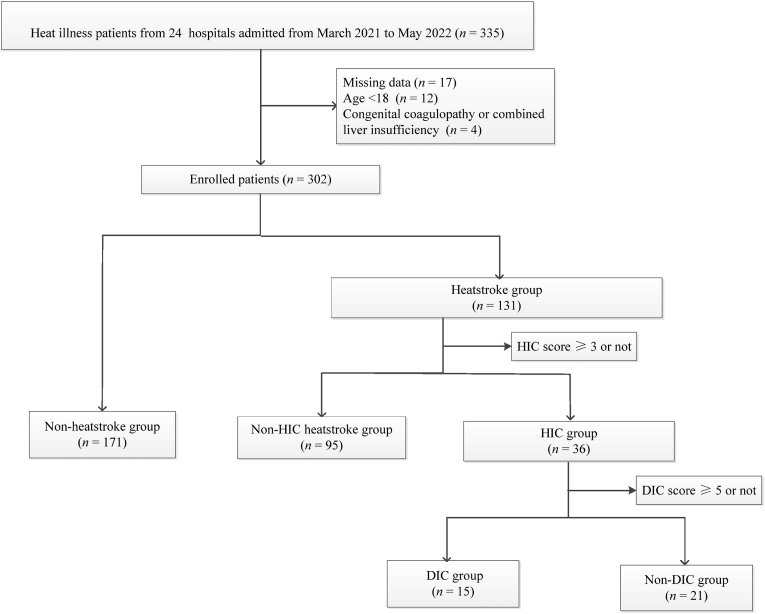
A total of 335 patients with heat illness due to overtraining were assessed for eligibility, of whom 33 were excluded: 17 because missing data, 12 because being < 18 years old, and 4 having a congenital coagulation disorder or liver disease. Finally, 302 patients with heat illness, of whom 299 were men, were included in the study, and their average age was 21 years (a range of 20 – 23 years) (Fig. 1, Table 1). Of the 302 patients, 131 (43.4%) had heatstroke, and all of them were men.
Fig. 1.
Flow chart of patient enrollment.
HIC: heatstroke-induced coagulopathy; DIC: disseminated intravascular coagulation.
Table 1.
Demographic characteristics of patients with heat illness at admission, stratified by heatstroke diagnosis.
| Characteristics | Total (n = 302) | Heatstroke group (n = 131) | Non-heatstroke group (n = 171) | p value |
|---|---|---|---|---|
| Age (year) | 21 (20, 23) | 22 (20, 24) | 21 (20, 23) | 0.155 |
| Male, n (%)a | 299 (99.0) | 131 (100.0) | 168 (98.2) | 0.261 |
| Core temperature (°C) | 38.3 (37.7, 39.6) | 39.7 (38.0, 40.7) | 38.0 (37.6, 38.7) | < 0.001 |
| Heart Rate (beats/min) | 78 (68, 92) | 87 (75, 103) | 73 (64, 84) | < 0.001 |
| WBC (1 × 109/L) | 9.8 (7.8, 13.6) | 11.4 (8.1, 15.2) | 9.4 (7.5, 12.7) | 0.012 |
| Hemoglobin (g/L)b | 139 ± 17 | 140 ± 20 | 138 ± 15 | 0.449 |
| Platelet count (1 × 109/L) | 192 (154, 224) | 185 (129, 222) | 194 (165, 226) | 0.039 |
| Packed cell volume (%)b | 41.2 ± 5.2 | 41.4 ± 6.2 | 41.0 ± 4.4 | 0.536 |
| ALT (U/L) | 23 (17, 45) | 39 (20, 127) | 20 (16, 30) | < 0.001 |
| AST (U/L) | 29 (20, 50) | 41 (24, 107) | 26 (18, 35) | < 0.001 |
| Total bilirubin (μmol/L) | 12.9 (8.8, 19.7) | 14.6 (10.1, 22.3) | 12.2 (8.5, 18.0) | 0.024 |
| Creatinine (mmol/L) | 80 (65, 101) | 96 (72, 125) | 75 (64, 86) | < 0.001 |
| Creatine kinase (U/L) | 239 (115, 728) | 398 (178, 1191) | 180 (83, 470) | < 0.001 |
| Prothombin time (sec) | 13.1 (12.2, 14.3) | 13.9 (12.8, 17.1) | 12.6 (11.9, 13.5) | < 0.001 |
| INR | 1.1 (1.0, 1.2) | 1.2 (1.1, 1.4) | 1.0 (0.9, 1.1) | < 0.001 |
| APTT (sec) | 30.0 (26.8, 33.7) | 31.2 (26.8, 38.1) | 29.8 (26.8, 32.8) | 0.031 |
| Fibrinogen (g/L) | 2.3 (1.9, 2.7) | 2.4 (2.0, 2.8) | 2.1 (1.6, 2.6) | 0.001 |
| D-dimer (μg/mL) | 0.3 (0.2, 1.1) | 0.7 (0.3, 2.7) | 0.2 (0.1, 0.5) | < 0.001 |
| In-hospital mortality (%)a | 7 (2.3) | 7 (5.3) | 0 (0) | < 0.001 |
WBC: white blood cell count; ALT: alanine aminotransferase; AST: aspartate aminotransferase; INR: international normalized ratio; APTT: activated partial thromboplastin time.
Value was presented as n (%).
Values were presented as mean ± standard deviation; and the remaining data were presented as median (Q1, Q3).
Compared with patients without heatstroke, those with heatstroke showed significantly higher core body temperature, heart rate and white blood cell count. They also showed significantly higher levels of alanine aminotransferase, aspartate aminotransferase, total bilirubin, creatinine and muscle creatine kinase, indicating liver and kidney damage (Table 1). The 2 groups of patients showed similar hemoglobin, hematocrit, and platelet count. Heatstroke patients showed significantly lower fibrinogen level, significantly higher D-dimer level, and significantly longer PT and APTT.
Seven patients in the heatstroke group died during hospitalization, but none in the group without heatstroke.
3.2. Predictors of heatstroke in patients with heat illness
We used uni- and multi-variate regression to identify clinical parameters significantly related to heatstroke in patients with heat illness (Table 2). Step-wise regression identified core temperature, PT and D-dimer as independent risk factors for heatstroke. Based on a cutoff of 39.7 °C, core temperature predicted heatstroke with an AUC of 0.723, sensitivity of 50.8%, and specificity of 95.3% (Table 3). Based on a cutoff of 13.8 sec, PT predicted heatstroke with an AUC of 0.721, sensitivity of 52.7%, and specificity of 79.5%. Based on a cutoff of 0.3 μg/mL, D-dimer level predicted heatstroke with an AUC of 0.724, sensitivity of 74.8%, and specificity of 62.6%. The AUC that combines all the 3 of these factors was 0.802 with sensitivity of 65.6%, and specificity of 83.6%.
Table 2.
Logistic regression to identify predictors of heatstroke in patients with heat illness.
| Variables | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Core temperature (°C) | 2.086 (1.686 – 2.581) | < 0.001 | 1.681 (1.291 – 2.189) | < 0.001 |
| PT (sec) | 1.518 (1.301 – 1.773) | < 0.001 | 1.427 (1.175 – 1.733) | < 0.001 |
| D-dimer (μg/mL) | 1.425 (1.196 – 1.697) | < 0.001 | 1.242 (1.049 – 1.471) | 0.012 |
| AST (U/L) | 1.005 (1.002 − 1.008) | 0.001 | 1.004 (1.001 – 1.007) | 0.005 |
| Heart rate (beats/min) | 1.042 (1.027 – 1.057) | < 0.001 | ||
| WBC (1 × 109/L) | 1.081 (1.025 − 1.140) | 0.004 | ||
| Platelet count (1 × 109/L) | 0.994 (0.990 – 0.998) | 0.002 | ||
| ALT (U/L) | 1.002 (1.001 − 1.005) | 0.002 | ||
| Total bilirubin (μmol/L) | 1.022 (1.002 − 1.043) | 0.030 | ||
| Creatinine (mmol/L) | 1.013 (1.007 − 1.019) | < 0.001 | ||
| Creatine kinase (U/L) | 1.000 (1.000 − 1.000) | 0.986 | ||
| INR | 3.733 (1.609 – 8.661) | 0.002 | ||
| APTT (sec) | 1.053 (1.020 – 1.087) | 0.001 | ||
| Fibrinogen (g/L) | 0.867 (0.671 – 1.119) | 0.272 | ||
OR: odds ratios; CI: confidence intervals; PT: prothrombin time; AST: aspartate aminotransferase; WBC: white blood cell count; ALT: alanine aminotransferase; INR: international normalized ratio; APTT: activated partial thromboplastin time.
Table 3.
Ability of core temperature, prothrombin time, or D-dimer to predict heatstroke, based on receiver operating characteristic curves.
| Parameters | AUC | 95% CI | p value | Cut-off value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| Core temperature (°C) | 0.723 | 0.661 − 0.786 | < 0.001 | 39.7 | 50.8 | 95.3 |
| PT (sec) | 0.721 | 0.664 − 0.779 | < 0.001 | 13.8 | 52.7 | 79.5 |
| D-dimer (μg/mL) | 0.724 | 0.666 − 0.782 | < 0.001 | 0.3 | 74.8 | 62.6 |
| Core temperature + PT + D-dimer | 0.802 | 0.750 − 0.855 | < 0.001 | 65.6 | 83.6 |
AUC: area under receiver operating characteristic curves; CI: confidence intervals; PT: prothrombin time.
3.3. Construction of HIC scoring system
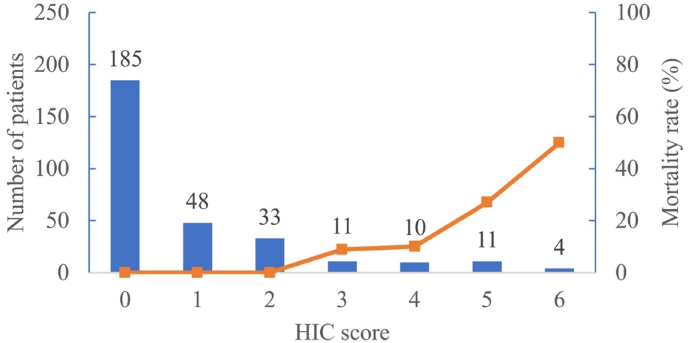
Core temperature, PT and D-dimer were all closely correlated with abnormal coagulation in heatstroke patients. Therefore, the 3 parameters that accurately predicted heatstroke according to multivariate logistic regression, and AUCs were used to construct the HIC scoring system (Table 4). Each parameter was assigned a point value, which increased with symptom severity. All patients with HIC scores ≥ 3 points had HIC. The mortality rate was 9.7% among patients with scores = 3, and the rate increased to 50% for patients with scores = 6 (Fig. 2).
Table 4.
Diagnostic scoring system for heatstroke-induced coagulopathy.
| Parameters | 0 point | 1 point | 2 points |
|---|---|---|---|
| Core temperature (°C) | < 40 | ≥ 40, but < 42 | ≥ 42 |
| D-dimer (μg/mL) | < 2-fold increase over baseline | ≥ 2-fold, but < 5-fold increase over baseline | ≥ 5-fold increase over baseline |
| Prolonged PT (sec) | < 2 | ≥ 2, but < 4 | ≥ 4 |
PT: prothrombin time.
Fig. 2.
Distribution of patients and patient mortality according to HIC score.
HIC: heatstroke-induced coagulopathy.
3.4. Comparison of coagulation function among patients with different HIC scores
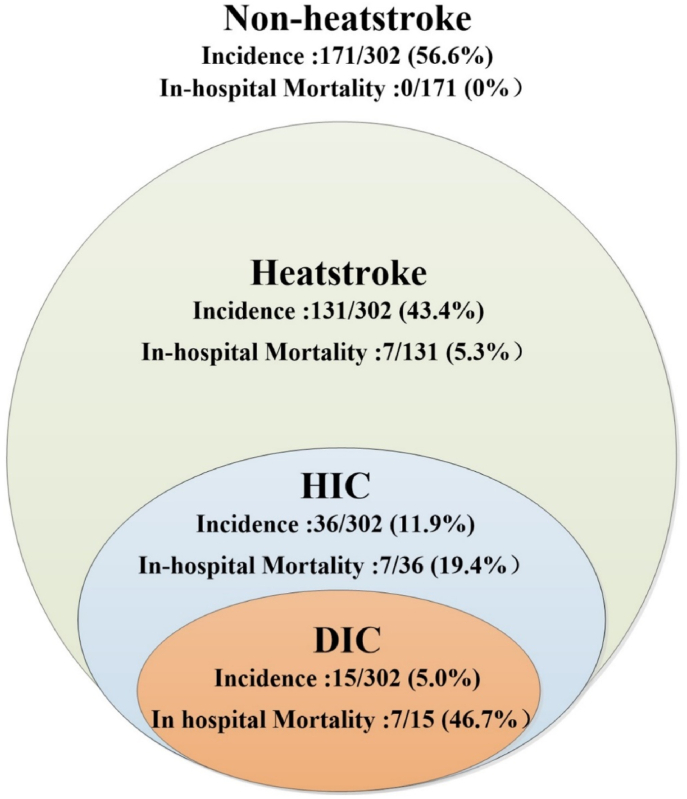
Three groups of patients with heat illness were compared: heatstroke patients with HIC, heatstroke patients without HIC, and patients without heatstroke (Table 5). In all of 302 patients with heat illness, the incidence of heatstroke was 43.4%, HIC was 11.9% and DIC was 5.0% (Fig. 3). Patients with heatstroke but no HIC showed significantly longer PT and higher D-dimer levels than patients without heatstroke. HIC patients showed significantly longer PT and APTT, significantly higher D-dimer levels, and significantly lower platelet count and fibrinogen levels than the other 2 groups. HIC patients also showed significantly higher international normalized ratio than heatstroke patients without HIC.
Table 5.
Comparison of coagulation dysfunction among patients stratified by heatstroke and heatstroke-induced coagulopathy.
| Characteristics | Non-heatstroke group (n = 171) | Heatstroke groups |
Z/χ2 | p value | |
|---|---|---|---|---|---|
| Non-HIC group (n = 95) | HIC group (n = 36) | ||||
| Platelet count (1 × 109/L) | 194 (165, 226) | 202 (158, 236) | 105 (51, 178) ab | 34.351 | < 0.001 |
| PT (sec) | 12.6 (11.9, 13.5) | 13.3 (12.4, 14.3)a | 19.8 (17.0, 27.5) ab | 75.718 | < 0.001 |
| INR | 1.06 (0.98, 1.14) | 1.12 (1.07, 1.22)a | 1.71 (1.41, 2.26) ab | 85.062 | < 0.001 |
| APTT (sec) | 29.8 (26.8, 32.8) | 29.9 (26.5, 32.9) | 39.7 (28.3, 58.6) ab | 23.203 | < 0.001 |
| Fibrinogen (g/L) | 2.4 (2.0, 2.8) | 2.3 (1.9, 2.7) | 1.7 (1.2, 2.3) ab | 21.496 | < 0.001 |
| D-dimer (μg/mL) | 0.2 (0.1, 0.5) | 0.5 (0.2, 0.9)a | 3.6 (2.4, 8.1) ab | 85.815 | < 0.001 |
| HIC score | 0 (0, 0) | 1 (0, 1)a | 4 (3, 5) ab | 141.667 | < 0.001 |
| In-hospital mortality (%) | 0 (0) | 0(0) | 7 (19.4) | 31.072 | < 0.001 |
Values are median (Q1, Q3), unless otherwise indicated.
ap < 0.05 compared with the non-heatstroke group.
bp < 0.05 compared with the non-HIC group.
PT: prothrombin time; INR: international normalized ratio; APTT: activated partial thromboplastin time; HIC: heatstroke-induced coagulopathy.
Fig. 3.
Distribution of patients and the relationship between different stages of heat illness.
HIC: heatstroke-induced coagulopathy; DIC: disseminated intravascular coagulation.
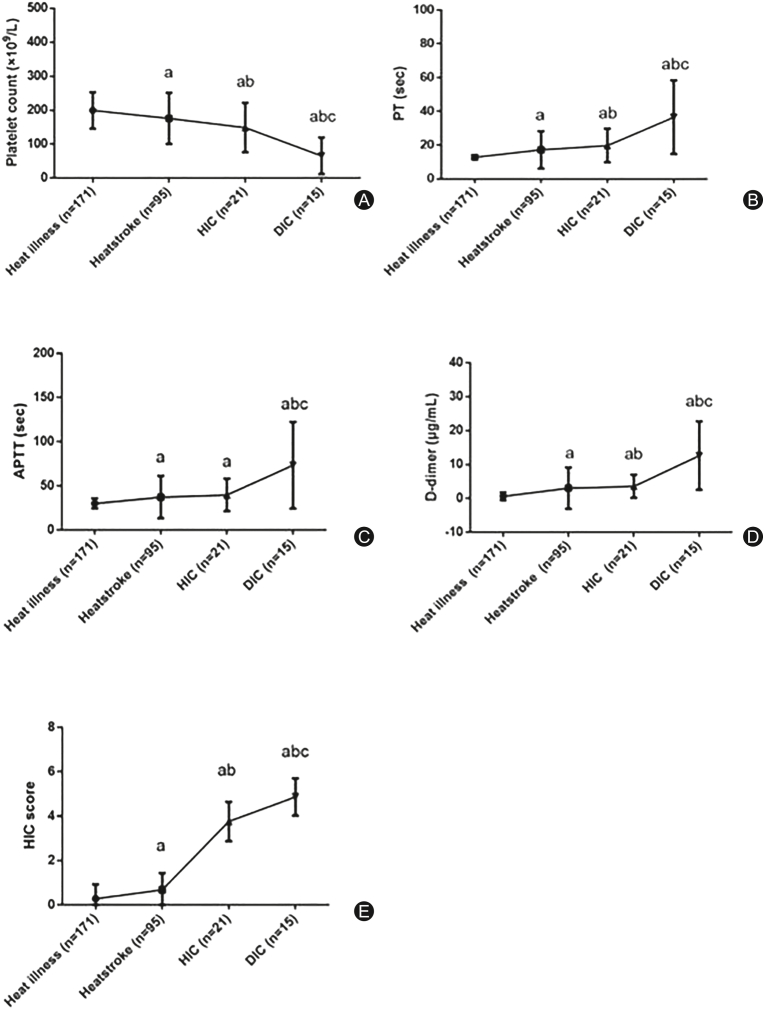
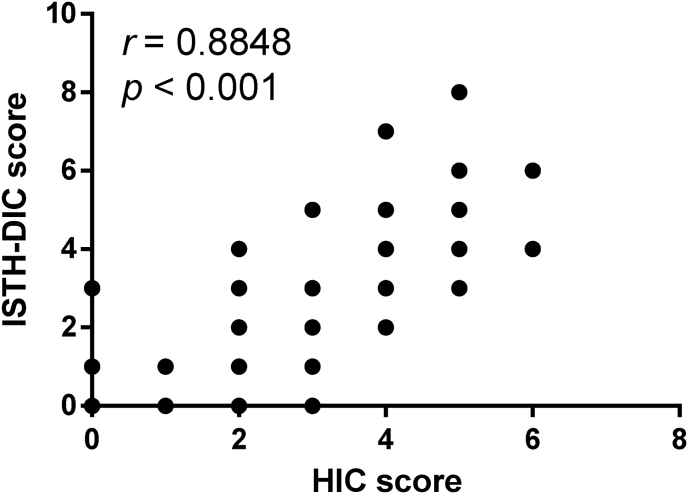
Among HIC patients, those with DIC based on the International Society on Thrombosis and Haemostasis-disseminated intravascular coagulation (ISTH-DIC) criteria were compared to those without DIC. All 7 dead patients suffered from DIC. DIC patients had significantly lower platelet count, higher D-dimer level, and longer PT and APTT (Table 6). Their fibrinogen levels were similar to those of patients without DIC. With the elevation of HIC score and the aggravation of coagulation dysfunction, the patients with heat illness gradually progress to heatstroke without HIC, HIC and even heatstroke-induced DIC (Fig. 4). There was a strong correlation between HIC score and ISTH-DIC score (r = 0.8848, p < 0.001; Fig. 5).
Table 6.
Comparison of coagulation dysfunction between heatstroke-induced coagulopathy patients, stratified by presence of disseminated intravascular coagulation.
| Characteristics | Non-DIC group (n = 21) | DIC group (n = 15) | Z/χ 2 | p value |
|---|---|---|---|---|
| Platelet count (1 × 109/L) | 155 (85, 207) | 53 (25, 68) | −3.274 | < 0.001 |
| PT (second) | 17.6 (13.8, 18.9) | 27.1 (22.2, 44.9) | −3.787 | < 0.001 |
| INR | 1.5 (1.1, 1.7) | 2.2 (1.8, 3.6) | −3.675 | < 0.001 |
| APTT (second) | 32.8 (26.5, 53.7) | 46.2 (39.2, 114.3) | −2.904 | 0.003 |
| fibrinogen (g/L) | 1.7 (1.5, 2.2) | 1.4 (0.6, 2.7) | −1.027 | 0.309 |
| D-dimer (μg/mL) | 2.7 (2.1, 4.0) | 8.1 (3.9, 20.0) | −3.162 | 0.001 |
| HIC scorea | 3.8 ± 0.9 | 4.9 ± 0.8 | −3.770 | 0.001 |
| DIC scorea | 2.6 ± 1.2 | 6.0 ± 1.9 | −10.112 | < 0.001 |
| In-hospital mortality (%) | 0 (0) | 7 (46.7) | 13.847 | 0.001 |
PT: prothrombin time; INR: international normalized ratio; APTT: activated partial thromboplastin time; HIC: heatstroke-induced coagulopathy; DIC: disseminated intravascular coagulation.
Values were presented as mean ± standard deviation and the remaining data were presented as median (Q1, Q3).
Fig. 4.
Changes in coagulation indices at different stages of heat illness.
ap < 0.05 compared with heat illness; bp < 0.05 compared with heatstroke; cp < 0.05 compared with HIC.
PT: prothrombin time; APTT: activated partial thromboplastin time; HIC: heatstroke-induced coagulopathy; DIC: disseminated intravascular coagulation.
Fig. 5.
Correlation between heatstroke-induced coagulopathy score and ISTH-DIC score among 302 patients with heat illness.
ISTH-DIC: International Society on Thrombosis and Haemostasis-disseminated intravascular coagulation; HIC: heatstroke-induced coagulopathy.
4. Discussion
To the best of our knowledge, this study is the first one to put forth a proposed score to evaluate the risk of HIC in patients with heat illness. We found that severity of coagulation dysfunction coincided with severity of heat-related injury and increased mortality.
Some standardized diagnostic criteria exist for heatstroke. For example, the JAAM defines heatstroke14 as a Glasgow coma scale score ≤ 14, total bilirubin or serum creatinine level ≥ 1.2 mg/dL, and JAAM-DIC score ≥ 4. In the present study, we applied criteria from the American College of Sports Medicine and Bouchama's criteria, and both sets of criteria require neurological damage as part of heatstroke. In contrast to heatstroke, no diagnostic standards exist for HIC.5,14 The ISTH-DIC scoring system or JAAM-DIC score is still the main assessment tool for blood coagulation in patients with heatstroke, but DIC seems to be a more advanced state of coagulation dysfunction, so it may not be reliable enough to detect HIC early for ensuring a better prognosis.13,14 Indeed, more than half the patients with HIC in our study did not meet the diagnostic criteria for DIC, illustrating the problem of diagnosing HIC based on DIC score alone.
The HIC scoring system established in our study consists of 3 elements: core body temperature, PT and D-dimer. Core body temperature > 40 °C is a well-established independent predictor of mortality in heatstroke patients verified by a French multicenter observational cohort study enrolled 1456 patients with heat illness19, and a Chinese multicenter respective study including 170 exertional heatstroke patients20. Furthermore, it has been a necessary component for diagnostic criteria developed by Bouchama et al.18,21 since 2002. Increased core temperature exceeding the upper limit of heat tolerance can cause vascular endothelial damage and induce HIC.22,23 In baboons, increasing the core temperature to 42 °C can cause severe microcirculation dysfunction and systemic endotoxemia.24,25 All patients in our study who had core body temperature greater than 42 °C suffered heatstroke, supporting this parameter as useful for early detection of HIC.
PT represents the state of the extrinsic coagulation pathway. After heat stress, inflammatory factors released from injured tissue activate the extrinsic coagulation pathway and prolong PT.26 In fact, prolonged PT has been linked to higher risk of mortality among patients with severe heatstroke.27
D-dimer is a common index used for evaluation of fibrinolytic function. In heatstroke, extensive thrombosis can lead activated plasmin to degrade fibrin, ultimately increasing the levels of D-dimer.28, 29, 30 D-dimer is an independent risk factor for acute renal failure in heatstroke patients, reflecting that microthrombosis can cause heatstroke-induced kidney injury.31,32
Our HIC scoring system showed a positive correlation with the ISTH-DIC score system, and higher scores on both scales were associated with higher mortality rates. Our study suggests that combination of core body temperature, PT and D-dimer can detect HIC before it progresses to DIC, allowing timely initiation of anticoagulation therapy that may prevent such progression. Once heatstroke patients progress to the DIC stage, due to the increased risk of bleeding, transfusion therapy is often required first, followed by an evaluation of the indications for anticoagulant treatment.
This study has certain limitations. First, the HIC scoring system was derived from a retrospective study, albeit a multicenter one, so it should be verified in prospective studies. Second, the results may not be generalizable since a majority of our patients were young male soldiers. The scoring system should be tested on more diverse populations.
A newly proposed HIC score that includes core temperature, PT and D-dimer can detect HIC in patients with heat illness and assess its severity. This tool may will be easy to use and should provide important and novel information to the clinicians.
Funding
The study was funded by Chinese Medicine Education Association (No. 2022KTZ013). The funders were not involved in research design, data collection and manuscript preparation.
Ethical statement
The protocol was approved by the human research ethics committee of all participating hospitals and reported prior to completion of the study. This study was approved by the ethics committee of the 908th Hospital of Chinese PLA Logistic Support Forces. The ethical approval document ID is 908yyLL028.
Declaration of competing interest
The authors declare that they have no competing interests.
Author contributions
Study design: Jing-Chun Song; Acquisition of data: Long-Ping He, Wei Zhang and Qing Song; Statistical analysis: Qing-Wei Lin, Lin-Cui Zhong and Qing-Bo Zeng; Drafting manuscript: Qing-Wei Lin; Manuscript revision: Jing-Chun Song.All authors read and approved the final manuscript.
Acknowledgments
We acknowledge the contribution of the Expert Group of Heatstroke Prevention and Treatment of the People's Liberation Army in the execution of this study.
Members of the Expert Group of Heatstroke Prevention and Treatment of the People's Liberation Army: Tao Wang, Qing Song (Hainan Hospital of PLA General Hospital), Wang Qian (the Third Medical Center of Chinese PLA General Hospital), Shi-Jun Li (General Hospital of Eastern Theater Command of PLA), Qiang Wen (General Hospital of Southern Theatre Command of PLA), Zhong-Zhi Tang (General Hospital of Central Theater Command of PLA), Yan Gao (General Hospital of Northern Theater Command of PLA), Chang-Lin Yin (the First Affiliated Hospital of the Army Medical University), Guo-Qiang Li (Armed Police Characteristic Medical Center), Xin Zhou (General Hospital of Xinjiang Military Region), Wei Zhang (the 900th Hospital of Chinese PLA Logistic Support Forces), Han-Wei Zhao (the 902th Hospital of Chinese PLA Logistic Support Forces), Jing-Chun Song (the 908th Hospital of Chinese PLA Logistic Support Forces), Yan Dou (the 909th Hospital of Chinese PLA Logistic Support Forces) Yi-Xin Li (the 910th Hospital of Chinese PLA Logistic Support Forces), Jun-Tao Xu (the 947th Hospital of Chinese PLA Logistic Support Forces) Zhi Wang (the 964th Hospital of Chinese PLA Logistic Support Forces), Yu-Peng Liu (the 967th Hospital of Chinese PLA Logistic Support Forces) Li Yu (the 969th Hospital of Chinese PLA Logistic Support Forces), Li Shen (the 970th Hospital of Chinese PLA Logistic Support Forces), Hai-Ling Li (the 971th Hospital of Chinese PLA Navy), Tian-Yi Wang (the 980th Hospital of Chinese PLA Logistic Support Forces) Qing-Hua Li (the 990th Hospital of Chinese PLA Logistic Support Forces), Yu-Jing Zhang (the 71st Group Army Hospital of Chinese PLA).
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Weinberger K.R., Wu X., Sun S., et al. Heat warnings, mortality, and hospital admissions among older adults in the United States. Environ Int. 2021;157 doi: 10.1016/j.envint.2021.106834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanda J., Nakahara S., Nakamura S., et al. Association between active cooling and lower mortality among patients with heat stroke and heat exhaustion. PLoS One. 2021;16 doi: 10.1371/journal.pone.0259441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shindell D., Zhang Y., Scott M., et al. The effects of heat exposure on human mortality throughout the United States. Geohealth. 2020;4 doi: 10.1029/2019GH000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberger K.R., Harris D., Spangler K.R., et al. Estimating the number of excess deaths attributable to heat in 297 United States counties. Environ Epidemiol. 2020;4:e096. doi: 10.1097/EE9.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchama A., Abuyassin B., Lehe C., et al. Classic and exertional heatstroke. Nat Rev Dis Prim. 2022;8:8. doi: 10.1038/s41572-021-00334-6. [DOI] [PubMed] [Google Scholar]
- 6.Liu S.Y., Song J.C., Mao H.D., et al. Expert consensus on the diagnosis and treatment of heat stroke in China. Mil Med Res. 2020;7:1. doi: 10.1186/s40779-019-0229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong L., Wu M., Ji J., et al. Association between platelet levels on admission and 90-day mortality in patients with exertional heatstroke, a 10 years cohort study. Front Med. 2021;8 doi: 10.3389/fmed.2021.716058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yezli S., Yassin Y., Ghallab S., et al. Classic heat stroke in a desert climate: a systematic review of 2632 cases. J Intern Med. 2023;294:7–20. doi: 10.1111/joim.13633. [DOI] [PubMed] [Google Scholar]
- 9.Iba T., Helms J., Levi M., et al. The role of platelets in heat-related illness and heat-induced coagulopathy. Thromb Res. 2022;S0049–3848:342–345. doi: 10.1016/j.thromres.2022.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Hemmelgarn C., Gannon K. Heatstroke: clinical signs, diagnosis, treatment, and prognosis. Compend Contin Educ Vet. 2013;35:E3. [PubMed] [Google Scholar]
- 11.He L., Lin Q., Zhong L., et al. Thromboelastography maximum amplitude as an early predictor of disseminated intravascular coagulation in patients with heatstroke. Int J Hyperther. 2022;39:605–610. doi: 10.1080/02656736.2022.2066206. [DOI] [PubMed] [Google Scholar]
- 12.Mustafa K.Y., Omer O., Khogali M., et al. Blood coagulation and fibrinolysis in heat stroke. Br J Haematol. 1985;61:517–523. doi: 10.1111/j.1365-2141.1985.tb02856.x. [DOI] [PubMed] [Google Scholar]
- 13.Taylor F.B., Jr., Toh C.H., Hoots W.K., et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemostasis. 2001;86:1327–1330. [PubMed] [Google Scholar]
- 14.Shimazaki J., Hifumi T., Shimizu K., et al. Clinical characteristics, prognostic factors, and outcomes of heat-related illness (Heatstroke Study 2017-2018) Acute Med Surg. 2020;7:e516. doi: 10.1002/ams2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto H., Takeba J., Umakoshi K., et al. Successful treatment for disseminated intravascular coagulation (DIC) corresponding to phenotype changes in a heat stroke patient. J Intensive Care. 2019;7:2. doi: 10.1186/s40560-019-0359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iba T., Connors J.M., Levi M., et al. Heatstroke-induced coagulopathy: biomarkers, mechanistic insights, and patient management. EClinicalMedicine. 2022;44 doi: 10.1016/j.eclinm.2022.101276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts W.O., Armstrong L.E., Sawka M.N., et al. ACSM Expert consensus statement on exertional heat illness: recognition, management, and return to activity. Curr Sports Med Rep. 2021;20:470–484. doi: 10.1249/JSR.0000000000000878. [DOI] [PubMed] [Google Scholar]
- 18.Bouchama A., Knochel J.P. Heat stroke. N Engl J Med. 2002;346:1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 19.Hausfater P., Megarbane B., Dautheville S., et al. Prognostic factors in non-exertional heatstroke. Intensive Care Med. 2010;36:272–280. doi: 10.1007/s00134-009-1694-y. [DOI] [PubMed] [Google Scholar]
- 20.Yang M.M., Wang L., Zhang Y., et al. Establishment and effectiveness evaluation of a scoring system for exertional heat stroke by retrospective analysis. Mil Med Res. 2020;7:40. doi: 10.1186/s40779-020-00269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo Y., Hifumi T., Shimazaki J., et al. Comparison between the Bouchama and Japanese association for acute medicine heatstroke criteria with regard to the diagnosis and prediction of mortality of heatstroke patients: a multicenter observational study. Int J Environ Res Publ Health. 2019;16:3433. doi: 10.3390/ijerph16183433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proctor E.A., Dineen S.M., Van Nostrand S.C., et al. Coagulopathy signature precedes and predicts severity of end-organ heat stroke pathology in a mouse model. J Thromb Haemostasis. 2020;18:1900–1910. doi: 10.1111/jth.14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall D.M., Buettner G.R., Oberley L.W., et al. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart Circ Physiol. 2001;280:H509–H521. doi: 10.1152/ajpheart.2001.280.2.H509. [DOI] [PubMed] [Google Scholar]
- 24.Roberts G.T., Ghebeh H., Chishti M.A., et al. Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke: a study in baboon model. Arterioscler Thromb Vasc Biol. 2008;28:1130–1136. doi: 10.1161/ATVBAHA.107.158709. [DOI] [PubMed] [Google Scholar]
- 25.Bouchama A., Al-Mohanna F., Assad L., et al. Tissue factor/factor VIIa pathway mediates coagulation activation in induced-heat stroke in the baboon. Crit Care Med. 2012;40:1229–1236. doi: 10.1097/CCM.0b013e3182387bef. [DOI] [PubMed] [Google Scholar]
- 26.Xing L., Liu S.Y., Mao H.D., et al. The prognostic value of routine coagulation tests for patients with heat stroke. Am J Emerg Med. 2021;44:366–372. doi: 10.1016/j.ajem.2020.04.062. [DOI] [PubMed] [Google Scholar]
- 27.Tong H., Wan P., Zhang X., et al. Vascular endothelial cell injury partly induced by mesenteric lymph in heat stroke. Inflammation. 2014;37:27–34. doi: 10.1007/s10753-013-9708-x. [DOI] [PubMed] [Google Scholar]
- 28.Bouchama A., Bridey F., Hammami M.M., et al. Activation of coagulation and fibrinolysis in heatstroke. Thromb Haemostasis. 1996;76:909–915. [PubMed] [Google Scholar]
- 29.Asmara I.G.Y. Diagnosis and management of heatstroke. Acta Med Indones. 2020;52:90–97. [PubMed] [Google Scholar]
- 30.Bouchama A., Kunzelmann C., Dehbi M., et al. Recombinant activated protein C attenuates endothelial injury and inhibits procoagulant microparticles release in baboon heatstroke. Arterioscler Thromb Vasc Biol. 2008;28:1318–1325. doi: 10.1161/ATVBAHA.107.161737. [DOI] [PubMed] [Google Scholar]
- 31.Wu M., Wang C., Liu Z., et al. Clinical characteristics and risk factors associated with acute kidney injury inpatient with exertional heatstroke: an over 10-year intensive care survey. Front Med. 2021;8 doi: 10.3389/fmed.2021.678434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng Q., Zhong L., Zhang N., et al. Nomogram for predicting disseminated intravascular coagulation in heatstroke patients: a 10 years retrospective study. Front Med. 2023;10 doi: 10.3389/fmed.2023.1150623. [DOI] [PMC free article] [PubMed] [Google Scholar]