SUMMARY
Fruit bats are suspected to be natural hosts of filoviruses, including Ebola virus (EBOV) and Marburg virus (MARV). Interestingly, however, previous studies suggest that these viruses have different tropisms depending on the bat species. Here, we show a molecular basis underlying the host-range restriction of filoviruses. We find that bat-derived cell lines FBKT1 and ZFBK13-76E show preferential susceptibility to EBOV and MARV, respectively, whereas the other bat cell lines tested are similarly infected with both viruses. In FBKT1 and ZFBK13-76E, unique amino acid (aa) sequences are found in the Niemann-Pick C1 (NPC1) protein, one of the cellular receptors interacting with the filovirus glycoprotein (GP). These aa residues, as well as a few aa differences between EBOV and MARV GPs, are crucial for the differential susceptibility to filoviruses. Taken together, our findings indicate that the heterogeneity of bat NPC1 orthologs is an important factor controlling filovirus species-specific host tropism.
Graphical Abstract

In Brief
Differential susceptibilities of bats to filoviruses have been suggested. Takadate et al. compare structures of the filovirus receptor among a variety of bat cell lines and discover a molecular mechanism determining their susceptibility to Ebola and Marburg viruses, providing information for understanding the ecology of filoviruses.
INTRODUCTION
Viruses in the family Filoviridae are divided into five genera: Marburgvirus, Ebolavirus, Cuevavirus, Striavirus, and Thamnovirus. There is one known species in the genus Marburgvirus, Marburg marburgvirus, consisting of two viruses, Marburg virus (MARV) and Ravn virus (RAVV). On the other hand, five distinct species are known in the genus Ebolavirus: Zaire ebolavirus, Sudan ebolavirus, TaïForest ebolavirus, Bundibugyo ebolavirus, and Reston ebolavirus, represented by Ebola virus (EBOV), Sudan virus (SUDV), Taï Forest virus (TAFV), Bundibugyo virus (BDBV), and Reston virus (RESTV), respectively (Amarasinghe et al., 2018). A novel ebolavirus species, Bombali ebolavirus, represented by Bombali virus (BOMV), has been proposed recently (Goldstein et al., 2018). The genus Cuevavirus has a single species with one known virus, Lloviu virus (LLOV), whose RNA genome was detected in insectivorous bats in Europe (Kemenesi et al., 2018; Negredo et al., 2011). The other two genera, Striavirus and Thamnovirus, have single species, respectively, with viruses whose genomes were detected in fishes. Recently, a novel filovirus, Mengla virus (MLAV), was found in China, and a new genus (Dianlovirus) has been proposed for this virus (Yang et al., 2019). EBOV, SUDV, TAFV, BDBV, MARV, and RAVV cause severe hemorrhagic fever in humans and nonhuman primates (Feldmann and Geisbert, 2011). Since infectious LLOV, BOMV, and MLAV have never been isolated, nothing is known about the pathogenicity of these viruses in humans and nonhuman primates. Although human filovirus disease has only been reported from central and west African countries (Changula et al., 2014), ecological and epidemiological studies strongly suggest the occurrence of filovirus infection in humans and animals in nonendemic areas in Africa, and even in Asian and European countries (Barrette et al., 2009; Changula et al., 2018; Glynn et al., 2017; Goldstein et al., 2018; Kemenesi et al., 2018; Miranda et al., 1991; Miranda and Miranda, 2011; Negredo et al., 2011; Nidom et al., 2012; Pawęska et al., 2018; Taniguchi et al., 2011; Yang et al., 2019).
7Bats are suspected to be the natural reservoir of filoviruses. Numerous epidemiological studies have suggested that filoviruses infect many bat species, including frugivorous and insectivorous bats, both of which are widely distributed in African, European, and Asian countries (Olival and Hayman, 2014). Viral RNA genomes of EBOV, RESTV, BOMV, LLOV, MLAV, MARV, and RAVV have been detected in bats (Amman et al., 2012; Goldstein et al., 2018; Jayme et al., 2015; Kemenesi et al., 2018; Leroy et al., 2005; Negredo et al., 2011; Pawęska et al., 2018; Swanepoel et al., 2007; Towner et al., 2007, 2009; Yang et al., 2019). However, infectious ebolaviruses LLOV, BOMV, and MLAV have never been isolated from any species of bats (Goldstein et al., 2018; Kemenesi et al., 2018; Leroy et al., 2005; Negredo et al., 2011; Yang et al., 2019), whereas infectious MARV and RAVV were isolated from a particular fruit bat species (i.e., Rousettus aegyptiacus) (Amman et al., 2012; Towner et al., 2009). Interestingly, it has been experimentally demonstrated that MARV, but not ebolaviruses, efficiently infects R. aegyptiacus bats and replicates in multiple organs (Jones et al., 2015), suggesting a different host preference between marburgviruses and ebolaviruses. Our previous study using non-replicating vesicular stomatitis virus (VSV) pseudotyped with filovirus envelope glycoproteins (GPs) (Takada et al., 1997) also demonstrated that a cell line (SuBK12-08) derived from the Schreiber’s bat (Miniopterus schreibersii), the only bat species in which LLOV was detected, might show higher susceptibility to LLOV than other tested bat cells and that a Yaeyama flying fox (Pteropus dasymallus yayeyamae)-derived cell line (FBKT1) might be susceptible to EBOV but not MARV (Maruyama et al., 2014). Thus, we postulated that each virus species has its preferential bat species and sought to identify biological factors that determine susceptibility of bat cells to filoviruses.
Since little is known about the difference in susceptibility of bat-derived cells to filoviruses (Hoffmann et al., 2016; Kühl et al., 2011; Maruyama et al., 2014; Ng et al., 2015), we first compared the susceptibilities of bat cell lines derived from various bat species using pseudotyped VSVs and infectious filoviruses and found that, while FBKT1 was not susceptible to MARV, a straw-colored fruit bat (Eidolon helvum)-derived cell line (ZFBK13-76E) showed remarkably low susceptibility to EBOV. In this study, we determined the molecular basis underlying this host-range restriction of filoviruses by focusing on the interaction between filovirus GPs and a host cell receptor, Niemann-Pick C1 (NPC1) (Carette et al., 2011; Côté et al., 2011; Ng et al., 2014).
NPC1 is a multipass transmembrane protein ubiquitously expressed in many cell types (Carstea et al., 1997; Davies and Ioannou, 2000). It localizes to late endosomes and lysosomes and acts as a lysosomal cholesterol transporter (Carstea et al., 1997; Cruz et al., 2000; Higgins et al., 1999). Loss of NPC1 function is known to cause Niemann-Pick disease, a fatal neurode-generative disorder characterized by lipid accumulation in cellular lysosomes (Carstea et al., 1997). NPC1 has been identified as a filovirus receptor that mediates membrane fusion between viral envelope and cellular membrane and appears to be a key factor of cell susceptibility to filoviruses (Kondoh et al., 2018; Ndungo et al., 2016; Ng et al., 2015). We found that only a few amino acids (aa) of NPC1 and GP were found to be essential for the differential susceptibility of bat cells to EBOV and MARV, suggesting a molecular mechanism involved in the host-range restriction of these filoviruses.
RESULTS
Differential Susceptibility to EBOV and MARV between Bat-Derived Cell Lines FBKT1 and ZFBK13-76E
Using non-replicating VSVs pseudotyped with GPs of EBOV, SUDV, TAFV, BDBV, RESTV, and MARV (VSV-EBOV, -SUDV, -TAFV, -BDBV, -RESTV, and -MARV, respectively), we investigated GP-dependent tropism, which appears to be the principal determinant for the host range-restriction of filoviruses. Vero E6 cells, which are commonly used for filovirus studies, human embryonic kidney (HEK) 293T cells, and eight bat-derived cell lines of different origins were used to compare their susceptibilities (Table S1; Figure 1A) (Maeda et al., 2008; Maruyama et al., 2014, 2016; Ogawa et al., 2017; Sato et al., 2019). We found that Vero E6, HEK293T, and the bat-derived cell lines, except FBKT1 and ZFBK13-76E, were susceptible to all pseudotyped VSVs tested. Consistent with a previous study (Maruyama et al., 2014), FBKT1 was susceptible to VSV-EBOV, -SUDV, -TAFV, -BDBV, and -RESTV but not to VSV-MARV. In contrast, ZFBK13-76E was susceptible to VSV-SUDV, -TAFV, -BDBV, -RESTV, and -MARV but not to VSV-EBOV, indicating that cell lines derived from this bat species might be less susceptible to EBOV (Ng et al., 2015). Next, the impaired GP-dependent susceptibilities of FBKT1 and ZFBK13-76E were confirmed using infectious filoviruses (Table S2). Consistent with the results for pseudotyped VSVs, FBKT1 cells showed susceptibility to infectious EBOV but not to MARV. Although ZFBK13-76E cells were susceptible to both EBOV and MARV, the infectivity of EBOV in ZFBK13-76E cells was significantly lower than in Vero E6, FBKT1, and DemKT1 cells.
Figure 1. Susceptibility of Cell Lines to VSVs Pseudotyped with Filovirus GPs.
(A) Vero E6 and bat-derived cells were infected with VSVs pseudotyped with filovirus GPs (VSV-EBOV, -SUDV, -TAFV, -BDBV, -RESTV, and -MARV).
(B) Vero E6, bat cells (FBKT1 and ZFBK13-76E) expressing exogenous human NPC1, and empty vector-transduced FBKT1 and ZFBK13-76E were infected with VSVs pseudotyped with filovirus GPs. Viral infectious units (IUs) in each cell line were determined by counting the number of GFP-expressing cells as described in STAR Methods. Each experiment was conducted three times, and average and standard deviations are shown. Asterisks represent IUs under the limit of detection (20 IU/mL).
Rescued Susceptibility of FBKT1 and ZFBK13-76E Cells Expressing Exogenous Human NPC1
Since pseudotyped VSVs rely on GP-dependent entry into cells, the interaction between GP and its ligands is likely the crucial step involved in the differential susceptibility of FBKT1 and ZFBK13-76E cells. Thus, we hypothesized that the impaired susceptibility of FBKT1 and ZFBK13-76E cells to particular filoviruses was due to the structural difference of cellular molecules required for filovirus entry into cells and focused on the interaction between GP and the NPC1 receptor. Although several cellular molecules have been identified as filovirus receptors, the NPC1 molecule is thought to be the only essential receptor required for membrane fusion during filovirus entry into cells (Carette et al., 2011; Côté et al., 2011; Ng et al., 2014). To investigate whether introduction of human NPC1 affected the susceptibilities of these bat cells, we generated FBKT1 and ZFBK13-76E cells stably expressing exogenous NPC1 derived from HEK293T and infected them with pseudotyped VSVs. As expected, both FBKT1 and ZFBK13-76E became fully susceptible to all of the pseudotyped VSVs upon the expression of the human NPC1 (Figure 1B). These data indicated that the heterogeneity of NPC1 molecules among FBKT1, ZFBK13-76E, and HEK293T cells was likely involved in the host specificity of MARV and EBOV.
Unique aa Sequences Found in NPC1 of FBKT1 and ZFBK13-76E Cells
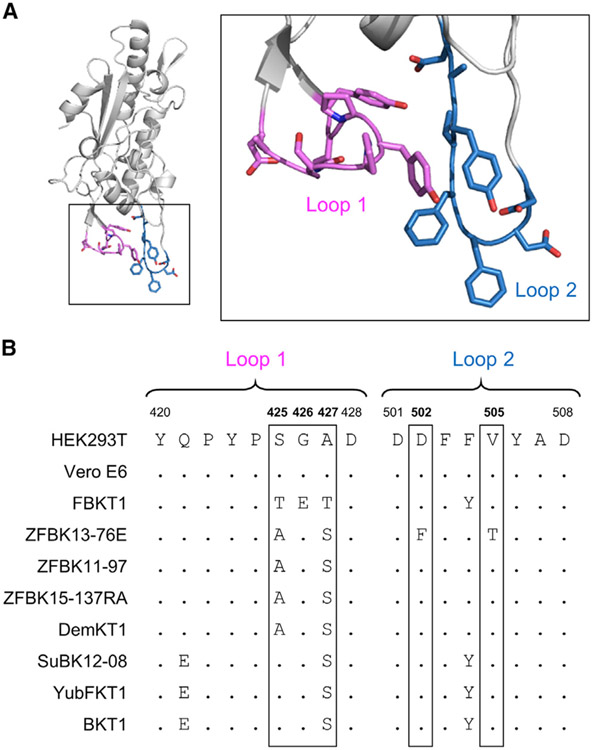
Previously published structural data have shown that some of the aa residues in loop 1 and loop 2 of the NPC1 domain C (NPC1-C) interact with the receptor binding domain (RBD) of EBOV GP (Figure 2A) (Gong et al., 2016; Wang et al., 2016). Thus, we assumed that the loop regions of NPC1-C might have genetic variations that affect susceptibility of FBKT1 and ZFBK13-76E cells to MARV and EBOV infection, respectively. Therefore, we sequenced the NPC1 genes of the bat cell lines and compared the deduced aa sequences of the loop regions of bat NPC1 orthologs. We found that NPC1 proteins of FBKT1 and ZFBK13-76E cells had unique aa sequences: FBKT1 cells with threonine (T), glutamic acid (E), and T at positions 425, 426, and 427, respectively, in loop 1 and ZFBK13-76E cells with phenylalanine (F) and T at positions 502 and 505, respectively, in loop 2, whereas the corresponding aa residues of HEK293T and Vero E6 cells were serine (S), glycine (G), and alanine (A), in loop 1 and aspartic acid (D) and valine (V) in loop 2 (Figure 2B). Among the other bat cell lines examined, shared sequences (AGS or SGS in loop 1 and D and V in loop 2) were found.
Figure 2. Comparison of aa Sequences of the Domain C Loops of Bat NPC1 Orthologs.
(A) The three-dimensional structure of domain C of human NPC1 (PDB: 5F1B) is represented as a ribbon model. GP-interacting regions, loop 1 and loop 2 (indicated in violet and sky blue, respectively), are shown in the boxed regions. Nitrogen and oxygen atoms in side chains are shown in blue and red, respectively.
(B) Deduced aa sequences of the domain C loop regions of NPC1 orthologs are aligned. The aa positions including the unique aa residues observed in FBKT1 (positions 425, 426, and 427 in the loop 1 region) and ZFBK13-76E (positions 502 and 505 in the loop 2 region) are enclosed by rectangles.
Importance of aa Residues in NPC1-C Loop Regions in the Cell Susceptibility to EBOV and MARV Infection
To elucidate the importance of these unique aa residues found in the NPC1-C loop regions of FBKT1 and ZFBK13-76E cells, we generated wild-type and NPC1 mutants in which aa residues at positions 425, 426, and 427 in loop 1 were swapped between HEK293T and FBKT1 cells and those at positions 502 and 505 in loop 2 were swapped between HEK293T and ZFBK13-76E cells (Figures 3A and 3B). Then, we generated Vero E6 cells stably expressing these exogenous NPC1 molecules using an NPC1-knockout cell line, Vero E6/NPC1-KO cl.19 (Kondoh et al., 2018) and compared their susceptibilities to VSVs pseudotyped with filovirus GPs (Figure 3C). We observed significantly lower infectivity of VSV-MARV in Vero E6 cells expressing wild-type FBKT1 NPC1 or human NPC1 having 3 point mutations in loop 1 (HEK293T-NPC1/TET) than in those expressing wild-type human NPC1 (HEK293T-NPC1). Interestingly, converse mutations in FBKT1 NPC1 (FBKT1-NPC1/SGA) significantly increased the infectivity of VSV-MARV. In contrast, these mutations did not affect the infection with VSVs pseudotyped with ebolavirus GPs. We also found that expression of wild-type ZFBK13-76E NPC1 (ZFBK13-76E-NPC1) or human NPC1 having 2 point mutations at positions 502 and 505 (HEK293T-NPC1/FT) resulted in significantly lower infectivity of VSV-EBOV than in wild-type human NPC1 and that F502D and T505V mutations in ZFBK13-76E NPC1 (ZFBK13-76E-NPC1/DV) converted the NPC1 function to efficiently mediate VSV-EBOV infection. No significant differences were observed in the infectivity of the other viruses except VSV-TAFV.
Figure 3. Effects of aa Substitutions in the NPC1-C Loops on Cell Susceptibility to Pseudotyped VSVs, EBOV, and MARV.
(A) Wild-type and mutant NPC1 genes were constructed to assess the importance of the unique aa sequences (shown in boldface) in loop 1 of FBKT1 and loop 2 of ZFBK13-76E.
(B) Locations of the unique aa residues of the loop regions are indicated in light green (loop 1) and orange (loop 2). Nitrogen and oxygen atoms in side chains are shown in blue and red, respectively.
(C and D) Vero E6/NPC1-KO cl.19 cells transduced with exogenous NPC1 genes and control cells (NPC1 knockout and vector control) were infected with pseudotyped VSVs (C) or infectious filoviruses (D). Relative infectivity was determined as described in STAR Methods. Each experiment was conducted three times (C) or in triplicate (D), and averages and standard deviations are shown. For comparison of viral infectivity among NPC1-expressing cells, one-way analysis of variance was performed, followed by Dunnett’s test, and significant differences compared to cells expressing wild-type human NPC1 (HEK293T-NPC1) are shown with asterisks (*p < 0.05).
These changes of cell susceptibility were confirmed using infectious EBOV and MARV (Figure 3D). Although the infectivity of EBOV in cells expressing wild-type FBKT1 NPC1 was lower than in those expressing wild-type human NPC1, the reduction was much more prominent in MARV infection. Taken together, these data suggested that the unique aa residues found in loop 1 and loop 2 in NPC1-C were major determinants for the differential susceptibility of FBKT1 and ZFBK13-76E cells to EBOV and MARV infection.
Comparison and Identification of aa Residues at the GP RBD and NPC1-Binding Interface
The previously determined co-crystal structure of human NPC1-C and EBOV GP has demonstrated that the GP RBD contains key aa residues that directly interact with some of the aa residues identified above in the loop structures of NPC1-C. Particularly, it has been shown that the identified aa motif in loop 1 (i.e., SGA in human NPC1) principally interacts with S at position 142 of GP and that D at position 502 in loop 2 interacts with cysteine (C) at position 147 of GP (Wang et al., 2016) (Figures 4A and 4B). We then compared aa sequences around this region of GP (i.e., positions 141–150; EBOV numbering) among all filovirus GPs (Figure 4C) and found an aa difference at position 142 (EBOV numbering); S in EBOV, TAFV, and BDBV GPs, and glutamine (Q) in SUDV, RESTV, and MARV GPs at the corresponding aa positions. An aa difference (C in EBOV, SUDV, TAFV, BDBV, and RESTV GPs, and H in MARV GP) was also found at position 147 (EBOV numbering).
Figure 4. Effects of aa Substitutions in the GP RBD on the Infectivity of Pseudotyped VSVs in FBKT1 and ZFBK13-76E Cells.
(A and B) In the three-dimensional structure of the complex of EBOV GP and human NPC1-C, the GP-NPC1 interfaces are indicated in the boxed regions. The aa residues at positions 425–427 (S, G, and A) in NPC1-C loop 1 and at position 142 (S) of EBOV GP are shown in light green and pink, respectively (A). The aa residues at positions 502 and 505 (D and V) in NPC1-C loop 2 and at positions 147 and 148 (C and A) of EBOV GP are shown in orange and green, respectively (B). Oxygen atoms in side chains are shown in red (A and B).
(C) Deduced aa sequences of filovirus GPs are aligned. The aa residues at positions 142 and 147/148, which are assumed to interact with the aa at positions at 425–427 in loop 1 and at 502 and 505 in loop 2 of human NPC1-C, respectively, are enclosed by rectangles.
(D and E) Vero E6, FBKT1, and ZFBK13-76E cells were infected with VSVs pseudotyped with wild-type and mutant GPs of EBOV, SUDV, TAFV, BDBV, RESTV, and MARV, whose aa at positions 142 (D) or 148 (E) were substituted (EBOV numbering). Viral infectious units (IUs) in each cell line were determined by counting the number of GFP-expressing cells. Each experiment was conducted three times, and averages and standard deviations are shown. Asterisks represent IUs under the limit of detection (20 IU/mL).
To identify key aa residues on RBD for the ability to infect FBKT1 and ZFBK13-76E cells, we generated VSVs pseudotyped with GP mutants whose aa at positions 142 (142 for EBOV, SUDV, TAFV, and BDBV, 143 for RESTV, and 126 for MARV) or 147 (147 for EBOV, SUDV, TAFV, and BDBV, 148 for RESTV, and 131 for MARV) were substituted, and their infectious units were compared using Vero E6, FBKT1, and ZFBK13-76E cells. Though there was no significant difference among the viruses for the infectivity in Vero E6 cells, VSV-EBOV S142Q failed to infect FBKT1 cells, like VSV-MARV, and VSV-MARV Q126S infected FBKT1 cells at a similar extent to VSV-EBOV (Figure 4D). We tested the other aa differences between MARV and EBOV GPs at the positions that were suggested to potentially interact with NPC1-C (Wang et al., 2016) and found that some of the aa substitutions (e.g., V79P, I113V, K114T, and V141I) also reduced the infectivity of VSV-EBOV in FBKT1, but none of them resulted in complete loss of the infectivity and that the A71G substitution altered the infectivity of VSV-MARV in this cell line (Figure S1). VSV pseudotyped with SUDV, TAFV, BDBV, RESTV, and their GP mutants similarly infected FBKT1 cells. Unexpectedly, VSVs pseudotyped with the EBOV 147 GP C147H mutant lacked the ability to infect Vero E6 and ZFBK13-76E cells (data not shown). Since C147 of EBOV GP has been reported to be important for the folding of the GP structure (Jeffers et al., 2002), the lack of infectivity was likely due to structural misfolding of the GP molecule. We then focused on the aa residue at position 148 (148 for EBOV, SUDV, TAFV, and BDBV, 149 for RESTV, and 132 for MARV), which was assumed to be potentially involved in the interaction between GP and NPC1-C loop 2 based on the co-crystal structures of these molecules (Figure 4E). We indeed found aa differences at this position among the viruses (Figure 4C). Thus, we generated VSVs pseudotyped with GPs whose aa at this position were substituted and compared their infectivity in Vero E6 and ZFBK13-76E cells (Figure 4E). VSV pseudotyped with the A148P GP mutant of EBOV successfully infected ZFBK13-76E cells as well as VSV-SUDV and -RESTV. Interestingly, the P148A mutation did not affect the infectivity of VSV pseudotyped with the SUDV GP mutant, whereas VSV-TAFV and -BDBV P148A GP mutants failed to infect ZFBK13-76E cells similarly to VSV pseudotyped with wild-type EBOV GP. The other aa substitutions at the positions potentially interacting with NPC1-C (Wang et al., 2016) showed limited effects to change the infectivity of VSV-MARV to ZFBK13-76E cells (Figure S1). Altogether, these results suggested that Q126 of MARV GP and A148 of EBOV GP were responsible for the reduced ability of MARV and EBOV to infect FBKT1 and ZFBK13-76E cells, respectively.
Finally, binding activities to FBKT1, ZFBK13-76E, and their mutant NPC1 molecules were compared among EBOV and MARV wild-type and mutant GPs (Figure 5A). We found that the aa substitution of S142Q of EBOV GP reduced the binding activity to FBKT1-NPC1 and HEK293T-NPC1/TET and that the corresponding aa substitution (Q126S) of MARV GP enhanced the binding activity to these NPC1 molecules. No significant difference was found in the binding activities to FBKT1-NPC1/SGA between wild-type and mutant GPs of both viruses. Similarly, the A148P substitution of EBOV GP enhanced the binding activity to ZFBK13-76E-NPC1 and HEK293T-NPC1/FT and that no significant difference was found in the binding activities to ZFBK13-76E-NPC1/DV between wild-type and mutant GPs of both viruses.
Figure 5. Interaction between GP and NPC1 and Predicted Structure of the NPC1 Loops and EBOV GP.
(A) A solid phase immunosorbent assay to detect binding activity of NPC1 and GP was carried out as described in STAR Methods. Each experiment was conducted three times and averages and standard deviations of relative OD values are shown. Significant differences are shown with asterisks (*p < 0.05).
(B and C) The three-dimensional co-crystal structure of domain C of human NPC1 and EBOV GP (PDB: 5F1B) was used as a template. The aa residues G426 or D502 of NPC1 and S142 or A148 of EBOV GP were substituted to E426 or F502 and Q142 or P148 by in silico mutagenesis.
(B) The interface of NPC1 loop 1 and EBOV GP is shown as a ribbon model. G426/E426 of NPC1 and S142/Q142 of GP are shown in light green/purple and pink/yellow, respectively.
(C) Loop 2 of NPC1 is shown as a ribbon model. GP1 (dark gray) and GP2 (light gray) are shown in a surface model. The aa residues forming a hydrophobic cavity of GP1 (i.e., V79, P80, T83, W86, G87, F88, L111, E112, I113, V141, G145, P146, C147, A152, and I170) are colored light cyan. D502/F502 of NPC1 and A148/P148 of GP are shown in orange/dark blue and green/deep red, respectively. Nitrogen and oxygen atoms in side chains are shown in blue and red, respectively (B and C). All mutagenesis procedures were performed using PyMOL (Schrödinger).
DISCUSSION
Bats are suspected to be the natural reservoirs of filoviruses. Although differences in their susceptibilities to each filovirus species have been suggested previously (Changula et al., 2018; Jones et al., 2015; Maruyama et al., 2014; Ng et al., 2015; Olival and Hayman, 2014), the molecular mechanisms for these differences are poorly understood. In this study, we focused on the differential susceptibility of two bat-derived cell lines (FBKT1 and ZFBK13-76E) to MARV and EBOV infection. Site-directed mutagenesis revealed that three and two aa differences in the NPC1-C loop 1 and loop 2 regions, respectively, were essential for the preferred susceptibility of these bat cells either to EBOV or MARV infection (Figure 3), indicating that aa residues in both loop 1 and 2 regions are critical determinants for the host-range restriction of filoviruses.
The loop 1 region of FBKT1 NPC1 has the unique aa residues T, E, and T (TET) at positions 425, 426, and 427, whereas the corresponding aa residues of the other bat and primate cell lines tested in this study are S, G, and A (SGA, AGS, or SGS) (Figure 2B). We demonstrated that wild-type FBKT1 and transduced cell lines expressing NPC1 mutants with the TET residues were susceptible to EBOV GP-mediated, but not to MARV GP-mediated, infection (Figures 1A and 3). Since the co-crystal structure of human NPC1-C and EBOV GP revealed that G426 of NPC1 was in direct contact with S142 of GP (Wang et al., 2016), it is conceivable that both TET and SGA residues of the loop 1 region interact with S142 of EBOV GP but that the TET residues are unable to interact with the corresponding residue (Q126) of MARV GP. We further confirmed this phenomenon using NPC1 mutants with single mutations at positions 426 (G or E) and found that these single mutations also switched the phonotypes of the NPC1-expresisng Vero E6 cells, although the effect on the susceptibility was comparatively lower than 3 point mutations (i.e., SGA/TET) (Figure S2). Indeed, in silico structural analysis using the NPC1 and EBOV GP suggests that steric hindrance caused by the side chains of E426 of NPC1 and Q142 of GP likely impairs the interaction between these aa, which may explain reduced susceptibility of FBKT1 cells to MARV (Figure 5B). On the other hand, SUDV and RESTV GPs, as well as MARV GP, have Q at this position but VSV-SUDV and -RESTV infected FBKT1 cells to an extent similar to VSV-EBOV (Figure 1A). Moreover, the aa substitution of Q142S did not significantly affect the infectivity of VSV-SUDV and -RESTV in FBKT1 cells (Figure 4D). Likewise, the aa substitution of S142Q had little effect on the infectivity of VSV-TAFV and -BDBV in FBKT1 cells (Figure 4D). These observations might suggest that the aa residue at position 142 (EBOV numbering) is less important for SUDV, RESTV, TAFV, and BDBV to infect FBKT1 cells than for EBOV and MARV. Interestingly, there is an aa difference between SUDV/RESTV/TAFV/BDBV and EBOV/MARV GPs (i.e., P in SUDV, RESTV, TAFV, and BDBV GPs, and A in EBOV and MARV GPs at position 148 [EBOV numbering]) (Figure 4C). The co-crystal structure of human NPC1-C and EBOV GP indicates that the position 148 is located adjacent to the GP RBD but at a distance from loop 1 of NPC1-C (Wang et al., 2016). Although the aa residue at position 148 may not directly interact with loop 1, the aa difference at position 148 (A or P) might cause distortion of the conformation of the RBD, resulting in a change of the size and/or shape of the RBD cavity. A similar mechanism has been previously reported—the substitution of aa residue V141A, which may not directly make contact with NPC1 loop 2, restored the NPC1 loop 2-dependent interaction with GP (Pontremoli et al., 2016). This mechanism might also explain the effect of the A71G substitution on the VSV-MARV infectivity.
ZFBK13-76E NPC1 has unique aa residues F and T (FT) at positions 502 and 505 in the loop 2 region and the corresponding aa residues of the other cell lines were D and V (DV). We found that swapping of these aa between HEK293T and ZFBK13-76E NPC1 changed the susceptibility to EBOV and MARV infection (i.e., cells expressing NPC1 with the DV residues were susceptible to both EBOV and MARV and those with the FT residues were less susceptible to EBOV) (Figure 3). This suggests that the DV, but not FT, residues in NPC1 interact with EBOV GP efficiently. Our in silico analysis indicates that V505 of NPC1 interacts with only T144 of EBOV GP through a hydrogen bond between backbone atoms, suggesting that the difference of the side chain between V and T might not affect the interaction with EBOV GP (data not shown). In contrast, a single aa substitution at residue 502 was shown to affect the susceptibility of E. helvum bat-derived cell lines to EBOV (Ng et al., 2015), suggesting that the aa difference at position 502 of NPC1 is a major determinant for the reduced susceptibility of ZFBK13-76E cells to EBOV infection. Since the hydrophobic character of aa residues at this position is substantially different between F and D, this aa difference might change the structure of loop 2, affecting the cell’s susceptibility to EBOV infection. Indeed, point mutation at this position changed the susceptibility of the NPC1-expresisng Vero E6 cells, although its effect was lower than 2 point mutations (i.e., DV/FT) (Figure S2). We demonstrated that the aa substitution of A148P of EBOV GP affected the infectivity of the pseudotyped VSV on ZFBK13-76E cells. The co-crystal structure of human NPC1-C and EBOV GP suggests that A148 directly makes contact with loop 2 and is located adjacent to the RBD (Figure 5C) (Wang et al., 2016). Our in silico mutagenesis suggested that an A148P mutation of GP altered the size and/or shape of the hydrophobic cavity of the GP RBD (Figure 5C), which might restore the interaction between GP and NPC1 having the D502F substitution.
The previous study showed that Niemann-Pick C2 (NPC2), a partner of NPC1 in LDL-derived cholesterol transportation, binds to NPC1 using an interaction interface that is similar to that used by GP (Li et al., 2016), raising the possibility that the competition between GP and NPC1 might be involved in the filovirus host tropism. In our study, we found that aa positions 425, 426, 427, 502, and 505 of NPC1 were important for the interaction with filovirus GP. It was suggested that aa positions 425–427 of NPC1 did not seem to be important for the interaction with NPC2 and that aa position 505 was not involved in the interaction with NPC2. Although it was also suggested that aa position 502 of NPC1 is important for the interaction with NPC2 (aa position 25; lysine), we confirmed that this lysine in NPC2 is conserved among human 293T, FBKT1, and ZFBK13-76E cells. Thus, it is unlikely that NPC2 plays a major role in controlling the filovirus host tropism.
The Yaeyama flying fox, the origin of FBKT1 cells, is one of the subspecies of Ryukyu flying foxes (Pteropus dasymallus) distributed in Asian countries such as Japan, the Philippines, and Taiwan (Vincenot, 2017). In the Philippines, RESTV infection was confirmed in bats, monkeys, and pigs (Barrette et al., 2009; Jayme et al., 2015; Miranda et al., 1999; Miranda and Miranda, 2011). Although filovirus infection of this bat species has never been reported, anti-RESTV antibodies were detected in a large flying fox (Pteropus vampyrus), which is evolutionary related the to the Yaeyama flying fox (Bastian et al., 2002; Jayme et al., 2015). Interestingly, the unique aa motif of loop 1 (i.e., TET found in FBKT1) has also been found in NPC1 of other fruit bat species, including the large flying fox (P. vampyrus) and the black flying fox (Pteropus alecto) (Lowe and Eddy, 1997), both of which are widely distributed in Asian and Oceanian countries (i.e., P. vampyrus in Brunei Darussalam, China, Indonesia, Malaysia, Myanmar, the Philippines, Singapore, Thailand, Timor-Leste, and Vietnam, and P. alecto in Indonesia and Papua New Guinea) (Bates et al., 2008; Roberts et al., 2018). Considering the accumulating seroepidemiological evidence suggesting filovirus infection of wild animals in Asian countries such as China, Singapore, Bangladesh, and Indonesia (He et al., 2015; Laing et al., 2018; Nidom et al., 2012; Olival et al., 2013; Yang et al., 2017, 2019; Yuan et al., 2012), these fruit bat species may play a role in the ecology of ebolaviruses, including yet unknown species, while our data suggest the inability of MARV or MARV-related filoviruses (i.e., filoviruses that have Q at position 142 of GP [EBOV numbering]) to efficiently infect these bat species.
ZFBK13-76E cells are derived from the straw-colored fruit bat (E. helvum), which is widely distributed in sub-Saharan African countries (Mickleburgh et al., 2008). It has been shown that this bat species migrates between the tropical forests of African countries (Richter and Cumming, 2008). Previous studies provided serological evidence of the infection of E. helvum bats with EBOV (Hayman et al., 2010; Ogawa et al., 2015). However, our findings here, as well as our data published previously (Ng et al., 2015), suggest that this bat species may not be highly susceptible to EBOV. Serological cross-reactivity with multiple ebolavirus species or the existence of natural EBOV variants that have P at position 148 of GP may explain this contradictory observation. Alternatively, it is possible to assume that there might be polymorphism of the NPC1 gene in this same bat species and some minor populations of this species might be susceptible to EBOV. In addition, in these bats another factor besides NPC1 could play a role for filovirus infection. This remains to be clarified in future studies.
In this study, we have demonstrated that GP-NPC1 engagement is one of the genetic determinants of the host-range restriction of filoviruses in bat species. Interestingly, R. aegyptiacus bats were not fully susceptible to ebolaviruses when infected experimentally (Jones et al., 2015), whereas cell lines derived from this bat species (e.g., ZFBK15-137RA) were susceptible to VSVs pseudotyped with GPs of ebolaviruses (Figure 1A) and infectious ebolaviruses (Hoffmann et al., 2016; Krähling et al., 2010; Kuzmin et al., 2017; Miller et al., 2016; Ng et al., 2015). Thus, some other host factors (e.g., those involved in the immune system) that interact with viral proteins may play an additional role in determining the susceptibility of bats to filoviruses. Indeed, unique functions of bat interferon (IFN) and IFN-induced proteins, as well as IFN-inhibitory viral proteins, have been reported previously (Feagins and Basler, 2015; Messaoudi et al., 2015; Pavlovich et al., 2018; Sarkis et al., 2018; Zhou et al., 2016). Further biological and bioinformatic analyses with a larger number of bats and bat-derived cell lines, and their genomic sequences are required to better understand the molecular basis of virus-host protein interactions involved in the filovirus host tropism.
STAR★METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and results for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Ayato Takada (atakada@czc.hokudai.ac.jp). All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Vero E6 and HEK293T cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma) supplemented with 10% fetal calf serum (FCS) (Cell Culture Bioscience), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (GIBCO). Bat-derived cell lines were established as described previously (Maeda et al., 2008; Maruyama et al., 2014, 2016; Ogawa et al., 2017; Sato et al., 2019). All of the bat cell lines were grown in Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma) supplemented with 10% FCS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. Origins of these cell lines are shown in Table S1. All cell lines were grown in 37°C. Vero E6 cell is a female monkey cell line and HEK293T cell is a female human cell line. Sex of bat cell lines is not yet identified.
METHOD DETAILS
Viruses
Using VSV containing the green fluorescent protein (GFP) gene instead of the receptor binding VSV glycoprotein (G) gene, pseudotyped viruses with GPs of EBOV (Mayinga), SUDV (Boniface), TAFV (Pauléoula), BDBV (Butalya), RESTV (Pennsylvania), and MARV (Angola) were generated as described previously (Changula et al., 2013; Takada et al., 1997). The amounts of GPs incorporated into VSV particles were measured by western blotting with a mixture of a rabbit anti-BDBV GP antiserum (FS0510), which is produced by immunization with a synthetic peptide corresponding to aa residues 83–97 (TKRWGFRAGVPPKVV) of BDBV GP, and a mouse anti-MARV GP monoclonal antibody (AGP127-8) (Kajihara et al., 2012) and confirmed to be similar among virus species (data not shown). Mutant GP genes were constructed by site-directed mutagenesis and were cloned into the protein expression vector pCAGGS (Niwa et al., 1991). VSVs pseudotyped with filovirus GPs (VSV-EBOV, -SUDV, -TAFV, -BDBV, -RESTV, and -MARV) were preincubated with the anti-VSV G monoclonal antibody VSV-G [N] 1-9 (Nakayama et al., 2011) to abolish the background infectivity of parental VSV. Tenfold diluted pseudotyped VSVs were inoculated onto confluent cell monolayers cultured on 96-well plates, and the infectious unit in each cell line was determined twenty hours later by counting the number of GFP-expressing cells under a fluorescent microscope. Relative infectivity of pseudotyped VSVs in an NPC1-knockout Vero E6 cell line (Vero E6/NPC1-KO cl.19) expressing exogenous NPC1 was determined by setting the GFP-positive cell number of wild-type HEK293T-NPC1-expressing cells infected with each virus to 100%.
Infectious EBOV (Mayinga) and MARV (Musoke) were used for TCID50 assays in Vero E6, FBKT1, ZFBK13-76E, and DemKT1 cells (Table S2). Tenfold diluted stock viruses were inoculated onto cell lines in 96-well plates. Cells were fixed with 10% formalin 3 days postinfection and stained with a mixture of a mouse anti-EBOV GP monoclonal antibody (ZGP42/3.7) (Furuyama et al., 2016) and a mouse anti-EBOV NP monoclonal antibody (ZNP74-7) (Changula et al., 2013) or a mixture of a rabbit anti-MARV GP and NP antisera (FS0505 and FS0608, respectively) (Furuyama et al., 2016) as primary antibodies, and anti-mouse IgG (Jackson ImmunoResearch, 115-095-003) or anti-rabbit IgG (Jackson ImmunoResearch, 711-096-152) conjugated with FITC as secondary antibodies. Infected cells were observed under a fluorescent microscope. TCID50 values were calculated by the Reed and Muench method.
Infectious EBOV-GFP (Mayinga) (Ebihara et al., 2007) and MARV (Angola) were used for focus-forming assays as described previously (Kajihara et al., 2012; Kondoh et al., 2018). These infectious filoviruses were inoculated onto confluent cell monolayers cultured in 96-well plates. After adsorption for 1 hour, the inoculum was replaced with Eagle’s minimal essential medium containing 1.2% carboxymethyl cellulose. After incubation for 3 days, cells were fixed. MARV-infected cells were immunostained with a mixture of rabbit anti-MARV GP and NP (FS0505 and FS0609, respectively) (Furuyama et al., 2016) as primary antibodies followed by anti-rabbit IgG conjugated with Alexa Fluor 488 (A11034, Invitrogen) as a secondary antibody. Focus-forming units of filoviruses were quantified by counting the number of fluorescent foci. Relative infectivity was determined by setting focus forming unit values given by Vero E6 cells expressing wild-type HEK293T-NPC1 to 100%.
Biosafety
Infectious work with wild-type EBOV and MARV was performed in the Galveston National Laboratory biosafety level 4 (BSL-4) laboratory at the University of Texas Medical Branch and in the BSL-4 laboratory at the Integrated Research Facility of the Rocky Mountain Laboratories, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH; Hamilton, MT). Experiments were performed following the standard operating procedures approved by the Institutional Biosafety Committees.
Sequencing of NPC1 genes and plasmid construction
Total RNA was extracted from FBKT1, ZFBK13-76E, ZFBK11-97, ZFBK15-137RA, DemKT1, SuBK12-08, YubFKT1, and BKT1 cells using ISOGEN (Nippongene) and mRNAs were reverse transcribed with Superscript IV (Invitrogen). To amplify NPC1 genes of FBKT1 and ZFBK13-76E, polymerase chain reaction (PCR) was performed with KOD-Plus Neo (TOYOBO) using primer sets designed based on the sequences of P. vampyrus (GenBank : XM_023530841.1) and Miniopterus natalensis bats (GenBank: XM_016211523.1). PCR products were directly sequenced or cloned into TOPO (Invitrogen) or pSP72 (Promega) plasmid vectors followed by sequencing. After sequence confirmation, wild-type and mutant NPC1 genes of HEK293T, FBKT1, and ZFBK13-76E were inserted into the pMXs-puro retroviral vector (Cell Biolabs). The plasmids of mutant NPC1 genes were constructed by site-directed mutagenesis with KOD-Plus Neo. After sequence confirmation, these mutant genes were inserted into the retroviral vector. An In-Fusion cloning kit (BD Clontech) was used for constructing the retroviral vectors carrying NPC1 genes.
Stable cell lines expressing NPC1 proteins
To generate retroviruses carrying NPC1 genes, HEK293T-derived Platinum-GP cells (Cell Biolabs) were co-transfected with pMXs-puro encoding NPC1 genes and the expression plasmid pCAGGS encoding the VSV G using Lipofectamine 2000 (Invitrogen). Empty pMXs-puro was used for vector control cells. Forty-eight hours later, the culture supernatants containing retroviruses were collected, filtered through 0.45-μm filters, and used to infect FBKT1, ZFBK13-76E, and Vero E6/NPC1-KO cl.19. Transduced cells stably expressing exogenous NPC1 were selected with a growth medium containing 6.0 μg/ml (FBKT1), 1.0 μg/ml (ZFBK13-76E), or 10.0 μg/ml (Vero E6/NPC1-KO cl.19) puromycin (Sigma-Aldrich). We examined expression levels and intracellular localization of exogenous NPC1 molecules in western blotting and confocal microscopy and confirmed that similar band intensities and lysosomal localization were uniformly observed in each cell line (Figure S3).
Solid-phase NPC1-GP binding assay
Vero E6/NPC1-KO cells and Vero E6/NPC1-KO cells expressing HA-tagged NPC1 and its mutants (Kondoh et al., 2018) were lysed with CHAPS-NTE buffer (0.5% wt/vol CHAPS, 140 mM NaC1, 10 mM Tris-HC1, 1 mM EDTA; pH7.5) (107 cells/ml). Then, EDTA-free Complete Protease Inhibitor Cocktail (Roche) was added. The cells were sedimented at 10,000 × g for 10 min at 4°C, and the supernatant was harvested. VLPs (4-6 mg/ml in PBS) were treated with thermolysin (Sigma) at 37°C for 90 min. The VLP solution was diluted at 1:10 with 0.05 M carbonate buffer (pH9.6). ELISA plates (Nunc, Maxisorp) were coated with the diluted VLPs, and incubated at 4°C overnight. The VLPs were removed and the plates were blocked with BSA (10 mg/ml in PBS) and incubated at room temperature for 2 hours. After washing the plates once with 0.05% Tween 20 in PBS (PBST), the cell lysate was added to each well and incubated at 4°C overnight. After removal of the lysate, the plates were washed with PBST 3 times, and rat anti-HA antibody 3F10 (Sigma) diluted with PBST containing BSA (5 mg/ml) was added, and then incubated at room temperature for 1 hour. After washing 3 times with PBST, horseradish peroxidase (HRP)-conjugated anti-rat IgG (H+L) (Jackson ImmunoResearch) was added to each well. After incubation at room temperature for 1 hour, the plates were washed 4 times with PBST and the 3,3′,5,5′-Tetramethyl-benzidine (TMB) substrate (Sigma) was added and incubated in the dark at room temperature for 60 min. The OD value at 450 nm was measured after stopping the reaction with 1M phosphoric acid.
Molecular modeling
Three-dimensional models of the NPC1-C and EBOV GP complex were prepared based on a previous study (Wang et al., 2016) (Protein Data Bank [PDB] code 5F1B). Three-dimensional structures shown in the figures of this study were prepared using PyMOL (Schrödinger LLC).
QUANTIFICATION AND STATISTICAL ANALYSIS
All statistical analyses were performed using R software (version 3.5.2) (R Core Team, 2018). For comparison of viral infectivity between NPC1-transduced cell lines, reported in Figures 3 and S2, one-way analysis of variance, was performed, followed by Dunnett’s test. Student t test was used in Figure 5. P-values of less than 0.05 were considered to be significant.
DATA AND CODE AVAILABILITY
The accession numbers for the NPC1 sequences of FBKT1, ZFBK13-76E, ZFBK11-97, ZFBK15-137RA, DemKT1, SuBK12-08, YubFKT1, and BKT1 reported in this paper are GenBank: LC462999, LC462993, LC462994, LC462995, LC462996, LC462997, LC462271, and LC462998, respectively.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-BDBV GP antiserum (FS0510) | This paper | N/A |
| Mouse anti-MARV GP monoclonal antibody (AGP127-8) | Kajihara et al., 2012 | N/A |
| Mouse anti-VSV G monoclonal antibody (VSV-G[N] 1-9) | Nakayama et al., 2011 | N/A |
| Mouse anti-EBOV GP monoclonal antibody (ZGP42/3.7) | Furuyama et al., 2016 | N/A |
| Mouse anti-EBOV NP monoclonal antibody (ZNP74-7) | Changula et al., 2013 | N/A |
| Rabbit anti-MARV GP antisera (FS0505) | Furuyama et al., 2016 | N/A |
| Rabbit anti-MARV NP antisera (FS0608) | Furuyama et al., 2016 | N/A |
| Goat anti-mouse IgG polyclonal antibody conjugated with FITC | Jackson ImmunoResearch | Cat#115-095-003; RRID:AB_2338589 |
| Donkey anti-rabbit IgG polyclonal antibody conjugated with FITC | Jackson ImmunoResearch | Cat#711-096-152; RRID:AB_2340597 |
| Rabbit anti-MARV NP antisera (FS0609) | Furuyama et al., 2016 | N/A |
| Goat anti-rabbit IgG polyclonal antibody conjugated with Alexa Fluor 488 | Invitrogen | Cat#A11034; RRID:AB_2576217 |
| Rat anti-HA monoclonal antibody | Sigma | Cat#11867423001; RRID:AB_390918 |
| Goat anti-rat IgG (H+L) polyclonal antibody conjugated with horseradish peroxidase | Jackson ImmunoResearch | Cat#112-035-003; RRID:AB_2338128 |
| Rabbit anti-NPC1 monoclonal antibody | Abcam | Cat#ab134113; RRID:AB_2734695 |
| Mouse anti-β actin monoclonal antibody | Abcam | Cat#ab6276; RRID:AB_2223210 |
| Goat anti-rabbit IgG polyclonal antibody conjugated with horseradish peroxidase | KPL | Cat#074-1506; RRID:AB_2721169 |
| Goat anti-mouse IgG polyclonal antibody conjugated with horseradish peroxidase | Jackson ImmunoResearch | Cat#115-035-062; RRID:AB_2338504 |
| Donkey anti-rabbit IgG (H+L) polyclonal antibody conjugated with Alexa Fluor 488 | Invitrogen | Cat#A21206; RRID:AB_141708 |
| Rabbit anti-LAMP1 polyclonal antibody | Sigma-Aldrich | Cat#SAB3500285; RRID:AB_10646194 |
| Bacterial and Virus Strains | ||
| VSV, whose genome code the GFP gene instead of the G protein gene | Takada et al., 1997 | N/A |
| EBOV (Mayinga) | The University of Texas Medical Branch, TX, USA | N/A |
| MARV (Musoke) | The University of Texas Medical Branch, TX, USA | N/A |
| EBOV-GFP (Mayinga) | Ebihara et al., 2007 | N/A |
| MARV (Angola) | NIAID/NIH, MT, USA | N/A |
| Deposited Data | ||
| NPC1 sequence of FBKT1 | This paper | GenBank: LC462999 |
| NPC1 sequence of ZFBK13-76E | This paper | GenBank: LC462999w |
| NPC1 sequence of ZFBK11-97 | This paper | GenBank: LC462994 |
| NPC1 sequence of ZFBK15-137RA | This paper | GenBank: LC462995 |
| NPC1 sequence of DemKT1 | This paper | GenBank: LC462996 |
| NPC1 sequence of SuBK12-08 | This paper | GenBank: LC462997 |
| NPC1 sequence of YubFKT1 | This paper | GenBank: LC462271 |
| NPC1 sequence of BKT1 | This paper | GenBank: LC462998 |
| Experimental Models: Cell Lines | ||
| Vero E6 | ATCC | CRL-1586 |
| HEK293T | ATCC | CRL-1573 |
| FBKT1 | Maeda et al., 2008 | N/A |
| ZFBK13-76E | Ogawa et al., 2017 | N/A |
| ZFBK11-97 | Maruyama et al., 2014 | N/A |
| ZFBK15-137RA | Sato et al., 2019 | N/A |
| DemKT1 | Maeda et al., 2008 | N/A |
| SuBK12-08 | Maruyama et al., 2014 | N/A |
| YubFKT1 | Maeda et al., 2008 | N/A |
| BKT1 | Maeda et al., 2008 | N/A |
| FBKT1-HEK293T NPC1 | This paper | N/A |
| FBKT1-empty vector | This paper | N/A |
| ZFBK13-76E-HEK293T NPC1 | This paper | N/A |
| ZFBK13-76E-empty vector | This paper | N/A |
| Vero E6/NPC1-KO cl.19 HEK293T NPC1 | Kondoh et al., 2018 | N/A |
| Vero E6/NPC1-KO cl.19 | Kondoh et al., 2018 | N/A |
| Vero E6/NPC1-KO cl.19 empty vector | Kondoh et al., 2018 | N/A |
| Vero E6/NPC1-KO cl.19 HEK293T NPC1/TET | This paper | N/A |
| Vero E6/NPC1-KO cl.19 FBKT1 NPC1 | This paper | N/A |
| Vero E6/NPC1-KO cl.19 FBKT1 NPC1/SGA | This paper | N/A |
| Vero E6/NPC1-KO cl.19 HEK293T NPC1/FT | This paper | N/A |
| Vero E6/NPC1-KO cl.19 ZFBK13-76E | This paper | N/A |
| Vero E6/NPC1-KO cl.19 ZFBK13-76E/DV | This paper | N/A |
| Vero E6/NPC1-KO cl.19 HEK293T NPC1/G426E | This paper | N/A |
| Vero E6/NPC1-KO cl.19 FBKT1 NPC1/E426G | This paper | N/A |
| Vero E6/NPC1-KO cl.19 HEK293T NPC1/D502F | This paper | N/A |
| Vero E6/NPC1-KO cl.19 ZFBK13-76E NPC1/F502D | This paper | N/A |
| Platinum-GP cells | Cell Biolabs | RV-103 |
| Recombinant DNA | ||
| pCAGGS-EBOV GP | Changula et al., 2013 | N/A |
| pCAGGS-SUDV GP | Changula et al., 2013 | N/A |
| pCAGGS-TAFV GP | Changula et al., 2013 | N/A |
| pCAGGS-BDBV GP | Changula et al., 2013 | N/A |
| pCAGGS-RESTV GP | Changula et al., 2013 | N/A |
| pCAGGS-MARV GP | Changula et al., 2013 | N/A |
| pCAGGS-EBOV GP S142Q | This paper | N/A |
| pCAGGS-SUDV GP Q142S | This paper | N/A |
| pCAGGS-TAFV GP S142Q | This paper | N/A |
| pCAGGS-BDBV GP S142Q | This paper | N/A |
| pCAGGS-RESTV GP Q143S | This paper | N/A |
| pCAGGS-MARV GP Q126S | This paper | N/A |
| pCAGGS-EBOV GP A148P | This paper | N/A |
| pCAGGS-SUDV GP P148A | This paper | N/A |
| pCAGGS-TAFV GP P148A | This paper | N/A |
| pCAGGS-BDBV GP P148A | This paper | N/A |
| pCAGGS-RESTV GP P149A | This paper | N/A |
| pCAGGS-MARV GP A132P | This paper | N/A |
| pCAGGS-EBOV GP V79P | This paper | N/A |
| pCAGGS-EBOV GP P80L | This paper | N/A |
| pCAGGS-EBOV GP T83S | This paper | N/A |
| pCAGGS-EBOV GP G87A | This paper | N/A |
| pCAGGS-EBOV GP L111I | This paper | N/A |
| pCAGGS-EBOV GP E112S | This paper | N/A |
| pCAGGS-EBOV GP I113V | This paper | N/A |
| pCAGGS-EBOV GP K114T | This paper | N/A |
| pCAGGS-EBOV GP V141I | This paper | N/A |
| pCAGGS-EBOV GP T144Q | This paper | N/A |
| pCAGGS-EBOV GP G145N | This paper | N/A |
| pCAGGS-EBOV GP C147H | This paper | N/A |
| pCAGGS-EBOV GP I170M | This paper | N/A |
| pCAGGS-MARV GP P63V | This paper | N/A |
| pCAGGS-MARV GP L64P | This paper | N/A |
| pCAGGS-MARV GP S67T | This paper | N/A |
| pCAGGS-MARV GP A71G | This paper | N/A |
| pCAGGS-MARV GP I95L | This paper | N/A |
| pCAGGS-MARV GP S96E | This paper | N/A |
| pCAGGS-MARV GP V97I | This paper | N/A |
| pCAGGS-MARV GP T98K | This paper | N/A |
| pCAGGS-MARV GP I125V | This paper | N/A |
| pCAGGS-MARV GP Q128T | This paper | N/A |
| pCAGGS-MARV GP N129G | This paper | N/A |
| pCAGGS-MARV GP H131C | This paper | N/A |
| pCAGGS-MARV GP M154I | This paper | N/A |
| pCAGGS-EBOV VP40 | Changula et al., 2013 | N/A |
| pCAGGS-EBOV NP | Changula et al., 2013 | N/A |
| pCAGGS-MARV VP40 | Changula et al., 2013 | N/A |
| pCAGGS-MARV NP | Changula et al., 2013 | N/A |
| pMXs-puro-HEK293T NPC1 | Kondoh et al., 2018 | N/A |
| pMXs-puro-HEK293T NPC1/TET | This paper | N/A |
| pMXs-puro-FBKT1 NPC1 | This paper | N/A |
| pMXs-puro-FBKT1 NPC1/SGA | This paper | N/A |
| pMXs-puro-ZFBK13-76E NPC1 | This paper | N/A |
| pMXs-puro-ZFBK13-76E NPC1/DV | This paper | N/A |
| pMXs-puro-HEK293T NPC1/G426E | This paper | N/A |
| pMXs-puro-FBKT1 NPC1/E426G | This paper | N/A |
| pMXs-puro-HEK293T NPC1/D502F | This paper | N/A |
| pMXs-puro-ZFBK13-76E NPC1/F502D | This paper | N/A |
| Software and Algorithms | ||
| PyMOL | Schrödinger LLC | https://pymol.org/2/ |
| R software (version 3.5.2) | R Core Team, 2018 | https://cran.r-project.org/ |
| ZEN 2.3 Lite | Carl Zeiss | https://www.zeiss.co.jp/microscopy/products/microscope-software/zen-lite.htm |
Highlights.
Some bat cell lines show differential susceptibilities to Ebola and Marburg viruses
Distinctive amino acid sequences exist in the filovirus receptor of some bats
Receptor heterogeneity in bat species controls their susceptibility to filoviruses
Receptor preference is important for differential filovirus tropism to bat cells
ACKNOWLEDGMENTS
We thank Ms. A. Shigeno for technical assistance, Drs. K. Maeda and E. Hondo for providing bat-derived cell lines, and Mr. K. Barrymore for editing the manuscript. This work was supported by KAKENHI (16H02627, 15H01249, and 16H06600); a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT); and the Japanese Initiative for Progress of Research on Infectious Disease for Global Epidemics (J-PRIDE) (JP17fm0208101 and JP18fm0208101) from the Japan Agency for Medical Research and Development (AMED). Funding was also provided in part by the Science and Technology Research Partnership for Sustainable Development (SATREPS) (JP18jm0110019), the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) (JP18fm0108008), the Program for Leading Graduate Schools from MEXT, Japan, and the Intramural Research Program of the NIAID, NIH.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2019.12.042.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Amarasinghe GK, Aréchiga Ceballos NG, Banyard AC, Basler CF, Bavari S, Bennett AJ, Blasdell KR, Briese T, Bukreyev A, Caì Y, et al. (2018). Taxonomy of the order Mononegavirales: update 2018. Arch. Virol 163, 2283–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amman BR, Carroll SA, Reed ZD, Sealy TK, Balinandi S, Swanepoel R, Kemp A, Erickson BR, Comer JA, Campbell S, et al. (2012). Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog. 8,e1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrette RW, Metwally SA, Rowland JM, Xu L, Zaki SR, Nichol ST, Rollin PE, Towner JS, Shieh WJ, Batten B, et al. (2009). Discovery of swine as a host for the Reston ebolavirus. Science 325, 204–206. [DOI] [PubMed] [Google Scholar]
- Bastian ST Jr., Tanaka K, Anunciado RV, Natural NG, Sumalde AC, and Namikawa T (2002). Evolutionary relationships of flying foxes (genus Pteropus) in the Philippines inferred from DNA sequences of cytochrome b gene. Biochem. Genet 40, 101–116. [DOI] [PubMed] [Google Scholar]
- Bates P, Francis C, Gumal M, Bumrungsri S, Walston J, Heaney L, and Mildenstein T (2008). Pteropus vampyrus. In The IUCN Red List of Threatened Species 2008. 10.2305/IUCN.UK.2008.RLTS.T18766A8593657.en. [DOI] [Google Scholar]
- Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, Kuehne AI, Kranzusch PJ, Griffin AM, Ruthel G, et al. (2011). Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 477, 340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, et al. (1997). Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science 277, 228–231. [DOI] [PubMed] [Google Scholar]
- Changula K, Yoshida R, Noyori O, Marzi A, Miyamoto H, Ishijima M, Yokoyama A, Kajihara M, Feldmann H, Mweene AS, and Takada A (2013). Mapping of conserved and species-specific antibody epitopes on the Ebola virus nucleoprotein. Virus Res. 176, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changula K, Kajihara M, Mweene AS, and Takada A (2014). Ebola and Marburg virus diseases in Africa: increased risk of outbreaks in previously unaffected areas? Microbiol. Immunol 58, 483–491. [DOI] [PubMed] [Google Scholar]
- Changula K, Kajihara M, Mori-Kajihara A, Eto Y, Miyamoto H, Yoshida R, Shigeno A, Hang’ombe B, Qiu Y, Mwizabi D, et al. (2018). Seroprevalence of filovirus infection of Rousettus aegyptiacus bats in Zambia. J. Infect. Dis 218 (suppl 5), S312–S317. [DOI] [PubMed] [Google Scholar]
- Côté M, Misasi J, Ren T, Bruchez A, Lee K, Filone CM, Hensley L, Li Q, Ory D, Chandran K, and Cunningham J (2011). Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature 477, 344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JC, Sugii S, Yu C, and Chang TY (2000). Role of Niemann-Pick type C1 protein in intracellular trafficking of low density lipoprotein-derived cholesterol. J. Biol. Chem 275, 4013–4021. [DOI] [PubMed] [Google Scholar]
- Davies JP, and Ioannou YA (2000). Topological analysis of Niemann-Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J. Biol. Chem 275, 24367–24374. [DOI] [PubMed] [Google Scholar]
- Ebihara H, Theriault S, Neumann G, Alimonti JB, Geisbert JB, Hensley LE, Groseth A, Jones SM, Geisbert TW, Kawaoka Y, and Feldmann H (2007). In vitro and in vivo characterization of recombinant Ebola viruses expressing enhanced green fluorescent protein. J. Infect. Dis 196 (Suppl 2), S313–S322. [DOI] [PubMed] [Google Scholar]
- Feagins AR, and Basler CF (2015). Lloviu virus VP24 and VP35 proteins function as innate immune antagonists in human and bat cells. Virology 485, 145–152. [DOI] [PubMed] [Google Scholar]
- Feldmann H, and Geisbert TW (2011). Ebola haemorrhagic fever. Lancet 377, 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama W, Marzi A, Nanbo A, Haddock E, Maruyama J, Miyamoto H, Igarashi M, Yoshida R, Noyori O, Feldmann H, and Takada A (2016). Discovery of an antibody for pan-ebolavirus therapy. Sci. Rep 6, 20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn JR, Bower H, Johnson S, Houlihan CF, Montesano C, Scott JT, Semple MG, Bangura MS, Kamara AJ, Kamara O, et al. (2017). Asymptomatic infection and unrecognised Ebola virus disease in Ebola-affected households in Sierra Leone: a cross-sectional study using a new non-invasive assay for antibodies to Ebola virus. Lancet Infect. Dis 17, 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein T, Anthony SJ, Gbakima A, Bird BH, Bangura J, Tremeau-Bravard A, Belaganahalli MN, Wells HL, Dhanota JK, Liang E, et al. (2018). The discovery of Bombali virus adds further support for bats as hosts of ebolaviruses. Nat. Microbiol 3, 1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Qian H, Zhou X, Wu J, Wan T, Cao P, Huang W, Zhao X, Wang X, Wang P, et al. (2016). Structural insights into the Niemann-Pick C1 (NPC1)-mediated cholesterol transfer and ebola infection. Cell 165, 1467–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman DT, Emmerich P, Yu M, Wang LF, Suu-Ire R, Fooks AR, Cunningham AA, and Wood JL (2010). Long-term survival of an urban fruit bat seropositive for Ebola and Lagos bat viruses. PLoS ONE 5, e11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Feng Y, Zhang H, Xu L, Yang W, Zhang Y, Li X, and Tu C (2015). Filovirus RNA in fruit bats, China. Emerg. Infect. Dis 21, 1675–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ME, Davies JP, Chen FW, and Ioannou YA (1999). Niemann-Pick C1 is a late endosome-resident protein that transiently associates with lysosomes and the trans-Golgi network. Mol. Genet. Metab 68, 1–13. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, González Hernández M, Berger E, Marzi A, and Pöhlmann S (2016). The glycoproteins of all filovirus species use the same host factors for entry into bat and human cells but entry efficiency is species dependent. PLoS ONE 11, e0149651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayme SI, Field HE, de Jong C, Olival KJ, Marsh G, Tagtag AM, Hughes T, Bucad AC, Barr J, Azul RR, et al. (2015). Molecular evidence of Ebola Reston virus infection in Philippine bats. Virol. J 12, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers SA, Sanders DA, and Sanchez A (2002). Covalent modifications of the ebola virus glycoprotein. J. Virol 76, 12463–12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Schuh AJ, Amman BR, Sealy TK, Zaki SR, Nichol ST, and Towner JS (2015). Experimental inoculation of Egyptian Rousette bats (Rousettus aegyptiacus) with viruses of the Ebolavirus and Marburgvirus genera. Viruses 7, 3420–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajihara M, Marzi A, Nakayama E, Noda T, Kuroda M, Manzoor R, Matsuno K, Feldmann H, Yoshida R, Kawaoka Y, and Takada A (2012). Inhibition of Marburg virus budding by nonneutralizing antibodies to the envelope glycoprotein. J. Virol 86, 13467–13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemenesi G, Kurucz K, Dallos B, Zana B, Földes F, Boldogh S, Görföl T, Carroll MW, and Jakab F (2018). Re-emergence of Lloviu virus in Miniopterus schreibersii bats, Hungary, 2016. Emerg. Microbes Infect 7, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh T, Letko M, Munster VJ, Manzoor R, Maruyama J, Furuyama W, Miyamoto H, Shigeno A, Fujikura D, Takadate Y, et al. (2018). Single-nucleotide polymorphisms in human NPC1 influence filovirus entry into cells. J. Infect. Dis 218 (suppl 5), S397–S402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krähling V, Dolnik O, Kolesnikova L, Schmidt-Chanasit J, Jordan I, Sandig V, Günther S, and Becker S (2010). Establishment of fruit bat cells (Rousettus aegyptiacus) as a model system for the investigation of filoviral infection. PLoS Negl. Trop. Dis 4, e802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl A, Hoffmann M, Müller MA, Munster VJ, Gnirss K, Kiene M, Tsegaye TS, Behrens G, Herrler G, Feldmann H, et al. (2011). Comparative analysis of Ebola virus glycoprotein interactions with human and bat cells. J.Infect. Dis 204 (Suppl 3), S840–S849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin IV, Schwarz TM, Ilinykh PA, Jordan I, Ksiazek TG, Sachidanandam R, Basler CF, and Bukreyev A (2017). Innate immune responses of bat and human cells to filoviruses: commonalities and distinctions. J. Virol 91, e02471–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing ED, Mendenhall IH, Linster M, Low DHW, Chen Y, Yan L, Sterling SL, Borthwick S, Neves ES, Lim JSL, et al. (2018). Serologic evidence of fruit bat exposure to filoviruses, Singapore, 2011-2016. Emerg. Infect. Dis 24, 114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, Délicat A, Paweska JT, Gonzalez JP, and Swanepoel R (2005). Fruit bats as reservoirs of Ebola virus. Nature 438, 575–576. [DOI] [PubMed] [Google Scholar]
- Li X, Saha P, Li J, Blobel G, and Pfeffer SR (2016). Clues to the mechanism of cholesterol transfer from the structure of NPC1 middle lumenal domain bound to NPC2. Proc. Natl. Acad. Sci. USA 113, 10079–10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe TM, and Eddy SR (1997). tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25, 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Hondo E, Terakawa J, Kiso Y, Nakaichi N, Endoh D, Sakai K, Morikawa S, and Mizutani T (2008). Isolation of novel adenovirus from fruit bat (Pteropus dasymallus yayeyamae). Emerg. Infect. Dis 14, 347–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama J, Miyamoto H, Kajihara M, Ogawa H, Maeda K, Sakoda Y, Yoshida R, and Takada A (2014). Characterization of the envelope glycoprotein of a novel filovirus, lloviu virus. J. Virol 88, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama J, Nao N, Miyamoto H, Maeda K, Ogawa H, Yoshida R, Igarashi M, and Takada A (2016). Characterization of the glycoproteins of bat-derived influenza viruses. Virology 488, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi I, Amarasinghe GK, and Basler CF (2015). Filovirus pathogenesis and immune evasion: insights from Ebola virus and Marburg virus. Nat. Rev. Microbiol 13, 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickleburgh S, Huston AM, Bergmans W, Fahr J, and Racey PA (2008). Eidolon helvum. In The IUCN Red List of Threatened Species 2008 (African Straw-coloured Fruit-bat, Pale Xantharpy, Staw-coloured Flying Fox, Straw-coloured Fruit Bat). [Google Scholar]
- Miller MR, McMinn RJ, Misra V, Schountz T, Müller MA, Kurth A, and Munster VJ (2016). Broad and temperature independent replication potential of filoviruses on cells derived from old and new world bat species. J. Infect. Dis 214 (suppl 3), S297–S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda ME, and Miranda NL (2011). Reston ebolavirus in humansand animals in the Philippines: a review. J. Infect. Dis 204 (Suppl 3), S757–S760. [DOI] [PubMed] [Google Scholar]
- Miranda ME, White ME, Dayrit MM, Hayes CG, Ksiazek TG, and Burans JP (1991). Seroepidemiological study of filovirus related to Ebola in the Philippines. Lancet 337, 425–426. [DOI] [PubMed] [Google Scholar]
- Miranda ME, Ksiazek TG, Retuya TJ, Khan AS, Sanchez A, Fulhorst CF, Rollin PE, Calaor AB, Manalo DL, Roces MC, et al. (1999). Epidemiology of Ebola (subtype Reston) virus in the Philippines, 1996. J. Infect. Dis 179 (Suppl 1), S115–S119. [DOI] [PubMed] [Google Scholar]
- Nakayama E, Tomabechi D, Matsuno K, Kishida N, Yoshida R, Feldmann H, and Takada A (2011). Antibody-dependent enhancement of Marburg virus infection. J. Infect. Dis 204 (Suppl 3), S978–S985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndungo E, Herbert AS, Raaben M, Obernosterer G, Biswas R, Miller EH, Wirchnianski AS, Carette JE, Brummelkamp TR, Whelan SP, et al. (2016). A single residue in Ebola virus receptor NPC1 influences cellular host range in reptiles. MSphere 1, e00007–e00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negredo A, Palacios G, Vázquez-Morón S, González F, Dopazo H, Molero F, Juste J, Quetglas J, Savji N, de la Cruz Martínez M, et al. (2011). Discovery of an ebolavirus-like filovirus in Europe. PLoS Pathog. 7, e1002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M, Ndungo E, Jangra RK, Cai Y, Postnikova E, Radoshitzky SR, Dye JM, Ramírez de Arellano E, Negredo A, Palacios G, et al. (2014). Cell entry by a novel European filovirus requires host endosomal cysteine proteases and Niemann-Pick C1. Virology 468-470, 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M, Ndungo E, Kaczmarek ME, Herbert AS, Binger T, Kuehne AI, Jangra RK, Hawkins JA, Gifford RJ, Biswas R, et al. (2015). Filovirus receptor NPC1 contributes to species-specific patterns of ebolavirus susceptibility in bats. eLife 4, e11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nidom CA, Nakayama E, Nidom RV, Alamudi MY, Daulay S, Dharmayanti IN, Dachlan YP, Amin M, Igarashi M, Miyamoto H, et al. (2012). Serological evidence of Ebola virus infection in Indonesian orangutans. PLoS ONE 7, e40740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, and Miyazaki J (1991). Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Miyamoto H, Nakayama E, Yoshida R, Nakamura I, Sawa H, Ishii A, Thomas Y, Nakagawa E, Matsuno K, et al. (2015). Seroepidemiological prevalence of multiple species of filoviruses in fruit bats (Eidolon helvum) migrating in Africa. J. Infect. Dis 212 (Suppl 2), S101–S108. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Kajihara M, Nao N, Shigeno A, Fujikura D, Hang’ombe BM, Mweene AS, Mutemwa A, Squarre D, Yamada M, et al. (2017). Characterization of a novel bat adenovirus isolated from straw-colored fruit bat (Eidolon helvum). Viruses 9, E371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olival KJ, and Hayman DT (2014). Filoviruses in bats: current knowledge and future directions. Viruses 6, 1759–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olival KJ, Islam A, Yu M, Anthony SJ, Epstein JH, Khan SA, Khan SU, Crameri G, Wang LF, Lipkin WI, et al. (2013). Ebola virus antibodies in fruit bats, Bangladesh. Emerg. Infect. Dis 19, 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovich SS, Lovett SP, Koroleva G, Guito JC, Arnold CE, Nagle ER, Kulcsar K, Lee A, Thibaud-Nissen F, Hume AJ, et al. (2018). The Egyptian Rousette genome reveals unexpected features of bat antiviral immunity. Cell 173, 1098–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawęska JT, Jansen van Vuren P, Kemp A, Storm N, Grobbelaar AA, Wiley MR, Palacios G, and Markotter W (2018). Marburg virus infection in Egyptian Rousette bats, South Africa, 2013-2014. Emerg. Infect. Dis 24, 1134–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli C, Forni D, Cagliani R, Filippi G, De Gioia L, Pozzoli U, Clerici M, and Sironi M (2016). Positive selection drives evolution at the host-filovirus interaction surface. Mol. Biol. Evol 33, 2836–2847. [DOI] [PubMed] [Google Scholar]
- R Core Team (2018). R: a language and environment for statistical computing (R Foundation for Statistical Computing; ). [Google Scholar]
- Richter H, and Cumming G (2008). First application of satellite telemetry to track African straw-coloured fruit bat migration. J. Zool. (Lond.) 275, 172–176. [Google Scholar]
- Roberts B, Eby P, Tsang SM, and Sheherazade. (2018). Pteropus alecto The IUCN Red List of Threatened Species 2017. 10.2305/IUCN.UK.2017-2.RLTS.T18715A22080057.en. [DOI] [Google Scholar]
- Sarkis S, Lise MC, Darcissac E, Dabo S, Falk M, Chaulet L, Neuveut C, Meurs EF, Lavergne A, and Lacoste V (2018). Development of molecular and cellular tools to decipher the type I IFN pathway of the common vampire bat. Dev. Comp. Immunol 81, 1–7. [DOI] [PubMed] [Google Scholar]
- Sato M, Maruyama J, Kondoh T, Nao N, Miyamoto H, Takadate Y, Furuyama W, Kajihara M, Ogawa H, Manzoor R, et al. (2019). Generation of bat-derived influenza viruses and their reassortants. Sci. Rep 9, 1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanepoel R, Smit SB, Rollin PE, Formenty P, Leman PA, Kemp A, Burt FJ, Grobbelaar AA, Croft J, Bausch DG, et al. (2007). Studies of reservoir hosts for Marburg virus. Emerg. Infect. Dis 13, 1847–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A, Robison C, Goto H, Sanchez A, Murti KG, Whitt MA, and Kawaoka Y (1997). A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. USA 94, 14764–14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi S, Watanabe S, Masangkay JS, Omatsu T, Ikegami T, Alviola P, Ueda N, Iha K, Fujii H, Ishii Y, et al. (2011). Reston Ebolavirus antibodies in bats, the Philippines. Emerg. Infect. Dis 17, 1559–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner JS, Pourrut X, Albarirño CG, Nkogue CN, Bird BH, Grard G, Ksiazek TG, Gonzalez JP, Nichol ST, and Leroy EM (2007). Marburg virus infection detected in a common African bat. PLoS ONE 2, e764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner JS, Amman BR, Sealy TK, Carroll SA, Comer JA, Kemp A, Swanepoel R, Paddock CD, Balinandi S, Khristova ML, et al. (2009). Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 5, e1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenot C. (2017). Pteropus dasymallus. In The IUCN Red List of Threatened Species 2017. 10.2305/IUCN.UK.2017-2.RLTS.T18722A22080614.en. [DOI] [Google Scholar]
- Wang H, Shi Y, Song J, Qi J, Lu G, Yan J, and Gao GF (2016). Ebola viral glycoprotein bound to its endosomal receptor Niemann-Pick C1. Cell 164, 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X-L, Tan CW, Anderson DE, Jiang R-D, Li B, Zhang W, Zhu Y, Lim XF, Zhou P, Liu X-L, et al. (2019). Characterization of a filovirus (Měnglà virus) from Rousettus bats in China. Nat. Microbiol 4, 390–395. [DOI] [PubMed] [Google Scholar]
- Yang XL, Zhang YZ, Jiang RD, Guo H, Zhang W, Li B, Wang N, Wang L, Waruhiu C, Zhou JH, et al. (2017). Genetically diverse filoviruses in Rousettus and Eonycteris spp. bats, China, 2009 and 2015. Emerg. Infect. Dis 23, 482–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Zhang Y, Li J, Zhang Y, Wang LF, and Shi Z (2012). Serological evidence of ebolavirus infection in bats, China. Virol. J 9, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Tachedjian M, Wynne JW, Boyd V, Cui J, Smith I, Cowled C, Ng JH, Mok L, Michalski WP, et al. (2016). Contraction of the type I IFN locus and unusual constitutive expression of IFN-α in bats. Proc. Natl. Acad. Sci. USA 113, 2696–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession numbers for the NPC1 sequences of FBKT1, ZFBK13-76E, ZFBK11-97, ZFBK15-137RA, DemKT1, SuBK12-08, YubFKT1, and BKT1 reported in this paper are GenBank: LC462999, LC462993, LC462994, LC462995, LC462996, LC462997, LC462271, and LC462998, respectively.